

Aysheaia prolata from the Utah Wheeler Formation (Drumian, Cambrian) is a frontal appendage of the radiodontan Stanleycaris
STEPHEN PATES, ALLISON C. DALEY, and JAVIER ORTEGA-HERNÁNDEZ
Pates, S., Daley, A.C., and J. Ortega-Hernández, J. 2017. Aysheaia prolata from the Utah Wheeler Formation (Drumian, Cambrian) is a frontal appendage of the radiodontan Stanleycaris. Acta Palaeontologica Polonica 62 (3): 619–625.
Aysheaia prolata, was described as the only lobopodian from the Drumian (Cambrian) Wheeler Formation in Utah, USA, and the sole representative of this genus besides the type species Aysheaia pedunculata, from the Cambrian (Stage 5) Stephen Formation, British Columbia. A redescription of Aysheaia prolata reveals previously overlooked morphological features, including segmental boundaries between putative lobopods, and curved terminal spines on the putative anterior end. These observations undermine lobopodian affinities of Aysheaia prolata, and instead we interpret this specimen as an isolated radiodontan frontal appendage. The presence of 11 podomeres, five of which possess elongate and anteriorly recurved ventral blades with auxiliary spines, together with shorter robust dorsal spines, identify the specimen as Stanleycaris. This represents the first report of Stanelycaris outside of the Cambrian Stage 5 thin Stephen Formation in British Columbia, expanding its palaeobiogeographic and stratigraphic range. Aysheaia is left as a monotypic genus endemic to the Burgess Shale. The Spence Shale luolishaniid Acinocrinus stichus is currently the only lobopodian known from the Cambrian of Utah.
Key words: Euarthropoda, Radiodonta, Hurdiidae, Cambrian, United States.
Stephen Pates [stephen.pates@zoo.ox.ac.uk], Department of Zoology, University of Oxford, Oxford, OX1 3PS, UK.
Allison C. Daley [allison.daley@unil.ch], Institute of Earth Sciences, University of Lausanne, Géopolis, CH-1015, Lausanne, Switzerland.
Javier Ortega-Hernández [jo314@cam.ac.uk], Department of Zoology, Downing Street, University of Cambridge, Cambridge, CB2 3EJ, UK.
Received 8 March 2017, accepted 11 May 2017, available online 14 August 2017.
Copyright © 2017 S. Pates et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Radiodontans, a diverse group of large nektonic predators, are among the most recognizable faunal components in Lower Palaeozoic Burgess Shale-type biotas (e.g., Daley et al. 2009, 2013a, b; Daley and Edgecombe 2014; Van Roy et al. 2015; Cong et al. 2016; Zeng et al. 2017). Despite their fundamentally soft-bodied construction, there is a comprehensive record of radiodontan diversity owing to the preferential preservation of their sclerotized frontal appendages (e.g., Caron et al. 2010; Daley and Budd 2010; Daley and Peel 2010; Wang et al. 2013; Lerosey-Aubril et al. 2014; Vinther et al. 2014). Recent work has expanded the palaeobiogeographic and stratigraphic occurrence of radiodontan taxa previously known only from the Cambrian (Stage 5) Burgess Shale in British Columbia, including new reports from the Cambrian (Series 3) of Utah (Pates et al. in press). Here we demonstrate that Aysheaia prolata Robison, 1985, widely regarded for over 30 years as the only lobopodian from the Wheeler Formation (Drumian), is actually a radiodontan frontal appendage, and discuss implications for the known diversity of middle Cambrian radiodontans and lobopodians from Utah, USA.
Institutional abbreviations.—KUMIP, University of Kansas Natural History Museum, Lawrence, Kansas, USA; ROM, Royal Ontario Museum, Toronto, Canada; USNM, Smithsonian Institution National Museum of Natural History, Washington D.C., USA.
Other abbreviations.—Pn, podomere n; sag., sagittal; trans., transverse.
Material and methods
The only known specimen (KUMIP 153923) is housed at the University of Kansas Natural History Museum. A Canon EOS 500D DSLR camera, with Canon EF-S 60 117 mm Macro Lens, controlled for remote shooting with EOSUtility 2 was used for photography. Photographs were taken wet, dry, under cross-polarized and non-polarized light. Lengths and angles were measured from photographs using ImageJ 2. Photoshop CS6 was used to convert photographs to black and white and manipulate colour channels.
Systematic palaeontology
Phylum Euarthropoda Lankester, 1904
Order Radiodonta Collins, 1996
Family Hurdiidae Vinther, Stein, Longrich, and Harper, 2014
Genus Stanleycaris Caron, Gaines, Mángano, Streng, and Daley, 2010
Type species: Stanleycaris hirpex Caron, Gaines, Mángano, Streng, and Daley, 2010; Stephen Formation (Cambrian Series 3, Stage 5), near Stanley Glacier (Kootenay National Park), British Columbia, Canada.
Remarks.—Stanleycaris was originally described from the thin Stephen Formation near Stanley Glacier (Cambrian Stage 5), British Columbia. 37 specimens of the type species, S. hirpex, have been described: disarticulated assemblages including frontal appendages, oral cones, and carapace material; no post cephalic remains have yet been found.
Stratigraphic and geographic range.—Thin Stephen Formation (Cambrian Series 3, Stage 5), British Columbia, Canada (Caron et al. 2010); Wheeler Formation (Cambrian Series 3, Drumian), Utah, USA (this study).
Aysheaia prolata
Robison, 1985 nomen dubium
= Stanleycaris sp.
Figs. 1A, 2, 3A.
1985 Aysheaia prolata sp. nov.; Robison 1985: 226–235, text-fig. 3.
1989 Aysheaia? prolata Robison, 1985; Dzik and Krumbiegel 1989: 175.
1995 Aysheaia? prolata Robison, 1985; Hou and Bergström 1995: 13.
1998 Aysheaia prolata Robison, 1985; Ramsköld and Chen 1998: 108, 134, 138–139, 143–144.
2001 Aysheaia? prolata Robison, 1985; Bergström and Hou 2001: 238, 245.
2004 Aysheaia? prolata Robison, 1985; Hou et al. 2004: 238.
2009 Aysheaia? prolata Robison, 1985; Whittle et al. 2009: 562.
2011 Aysheaia? prolata Robison, 1985; Ou et al. 2011: 587.
2011 Aysheaia? prolata Robison, 1985; Gámez Vintaned et al. 2011: 211.
2015 Aysheaia? prolata Robison, 1985; Robison et al. 2015; 27, fig. 56.
2016 Aysheaia prolata Robison, 1985; Foster and Gaines 2016: 299, 303, 330, fig. 9H.
Material.—KUMIP 153923, isolated frontal appendage, preserved flattened in light grey shale collected from locality 114 of Robison and Richards (1981: 3), Utah, USA, approximately 100 m below the top of the Wheeler Formation (Cambrian Series 3, Drumian; Robison 1985).

Fig. 1. A. Radiodontan euarthropod Stanleycaris sp., KUMIP 153923 from the Wheeler Formation (Cambrian Series 3, Drumian), Utah, USA; non-polarized light (A1), cross-polarized light, red and yellow channels reduced (A2), cross polarized light (A3), cross-polarized light, red and yellow channels enhanced (A4). B. Stanleycaris hirpex Caron, Gaines, Mángano, Streng, and Daley, 2010, ROM 59944 (holotype) from the Stephen Formation (Cambrian Series 3, Stage 5), British Columbia, Canada; cross-polarized light (B1), black and white (B2).
Description.—The appendage measures 26.5 mm in length (sag.), and displays 11 podomeres, indicated by the preservation of serially repeated segmental boundaries. P1 differs from the other podomeres in having a subrectangular outline (length [sag.] ca. 7.0 mm; width [trans.] ca. 4.6 mm). P2 to P11 are shorter (sag.) and wider (trans.). A layer of sediment between P1 and P2 suggests that P1 has shifted forwards to cover the posterior border of P2 (Fig. 2), making the precise dimensions of P2 unclear. P3 to P8 are similar lengths (sag. ca. 2 mm). P3 to P6 maintain a stable width (trans. ca. 3.5 mm), whereas P7 to P11 taper distally (P7 ca. 3.2 mm; P11 ca. 2.2 mm). P9 to P11 are flexed laterally towards the rock surface, slightly overlapping one another. It is not possible to measure the dimensions of these podomeres owing to their orientation. The armature consists of well-developed spinose ventral blades with auxiliary spines, and short dorsal spines. P1 lacks ornamentation altogether. P2 to P6 possess ventral blades (“lobopods 1–5” in Robison 1985: fig. 3.2), which emerge approximately perpendicular to the limb axis. The most complete of the blades (on P4, Fig. 3A2), has a length (sag.) of approximately 8 mm and possesses two distally-pointing auxiliary spines. The tip curves sharply towards the distal end of the appendage, giving the blade a crescent-shape. A ventral blade on P7 is approximately a third of the length (sag.) of the others, with no visible auxiliary spines. Dorsal spines are observed on P4, P6, and P7. The most complete dorsal spine is on P6; it has a subtriangular outline with a robust base (“lobopod 1” in Robison 1985: fig. 3.2), projects at an angle of 71° from the main limb axis, and shows no evidence for more than a single-point. P4 and P7 show poorly preserved dorsal spine bases (“anterior appendage” in Robison 1985: fig. 3.2). Armature is absent from P8 to P10. P11 preserves two recurved terminal spines (Fig. 3A1; “papillae?” in Robison 1985: fig. 3.2).
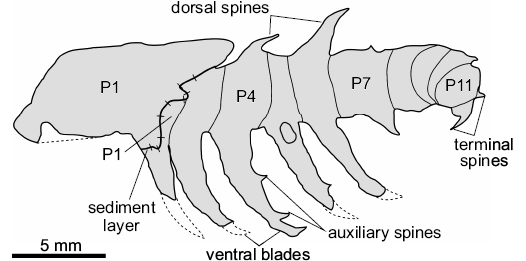
Fig. 2. Explanatory drawing of radiodontan euarthropod Stanleycaris sp., KUMIP 153923 from the Wheeler Formation (Cambrian Series 3, Drumian), Utah, USA. Hachure direction indicates lower sediment level. Dotted lines indicate expected path of incomplete ventral blades.
Remarks.—The recognition of podomere boundaries, together with the occurrence of ventral, dorsal, and terminal spines, support the reinterpretation of KUMIP 153923 as an isolated radiodontan appendage. Robison’s (1985) original lobopodian interpretation was largely based on the similar outline of this specimen to Aysheaia pedunculata preserved in lateral view (Fig. 4; Whittington 1978). Although A. pedunculata frequently displays compaction wrinkles that may resemble segmental boundaries (e.g., Fig. 3D; Ma et al. 2014: fig. 5B), these are generally restricted to the lobopodous limbs. The features identified as podomere boundaries in KUMIP 153923 occur along the main axis of the fossil, rather than on the putative limbs. Furthermore, the podomere boundaries cross the whole width of the specimen, and are more widely spaced than annulations on the trunk of A. pedunculata (Fig. 4). The putative lobopods and oral papillae of A. prolata described by Robison (1985) are best regarded as ventral blades and terminal spines respectively. The ventral blades are curved and bear auxiliary spines pointing to the anterior end, as also observed in in Stanleycaris hirpex (compare Fig. 3A2 and 3C). The ventral blades lack compaction wrinkles often preserved on limbs of A. pedunculata (Figs. 3D, 4; Ma et al. 2014: fig. 5B). The terminal spines at the anterior end of the specimen are short, recurved, and have a sharp termination. This morphology is similar to that of the spines in S. hirpex, and quite unlike the straight and less robust oral papillae of A. pedunculata (compare Fig. 3A1 and 3B). It should be noted that Robison (1985) studied the specimen before the extent of radiodontan frontal appendage morphological diversity was known (e.g., Daley and Budd 2010; Caron et al. 2010), and so lacked the appropriate context for correctly interpreting KUMIP 153923.

Fig. 3. Comparison between terminal spines and vetral blades of Stanleycaris and oral papillae and lobopodous limbs of Aysheaia. A. Stanleycaris sp., KUMIP 153923 from the Wheeler Formation (Cambrian Series 3, Drumian), Utah, USA; anterior end showing recurved terminal spines (A1), ventral blade (A2). B. Aysheaia pedunculata Walcott, 1911, USNM 189199 from the Stephen Formation (Cambrian Series 3, Stage 5), British Columbia, Canada; anterior end showing anterior appendages and oral papillae. C, D. Stanleycaris hirpex Caron, Gaines, Mángano, Streng, and Daley, 2010 from the Stephen Formation (Cambrian Series 3, Stage 5), British Columbia, Canada. C. ROM 59975 (paratype), ventral blade. D. USNM 83942, trunk section and lobopods. Abbreviation: Pn, podomere n.
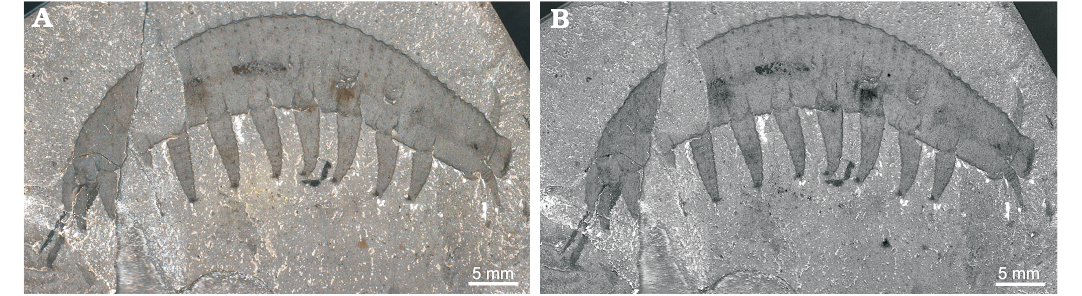
Fig. 4. Lobopodian Aysheaia pedunculata Walcott, 1911, USNM 365608 from the Stephen Formation (Cambrian Series 3, Stage 5), British Columbia, Canada; in cross-polarized light (A), cross-polarized light, red and yellow channels reduced (B).
Within the known diversity of radiodontan frontal appendages, the presence of five elongated ventral blades with auxiliary spines on P2 to P6, and differentiated distal podomeres, support a more specific classification as a hurdiid (e.g., Daley and Budd 2010; Daley et al. 2013a). Within hurdiids, KUMIP 153923 most closely resembles Stanleycaris hirpex (Figs. 1B, 5): both have 11 podomeres, including an elongated P1; long, curved ventral blades with auxiliary spines on P2–P6; and a distally tapering P7–P11. The only significant difference is that the dorsal spine preserved on P6 of KUMIP 153923 appears to be single-pointed, whereas dorsal spines of Stanleycaris from Stanley Glacier are double-pointed (Figs. 1B, 5; Caron et al. 2010). The lack of a double-point may be the result of incomplete preservation in KUMIP 153923, as the size of the dorsal spine relative to podomere width (trans.), robustness, and angle relative to the dorsal surface of the podomere all conform to the morphology of dorsal spines in Stanleycaris from Stanley Glacier (Figs. 1B, 5). Additionally KUMIP 153923 fits within the size-range described for Stanley Glacier specimens (12.5–30.3 mm length [sag.]).
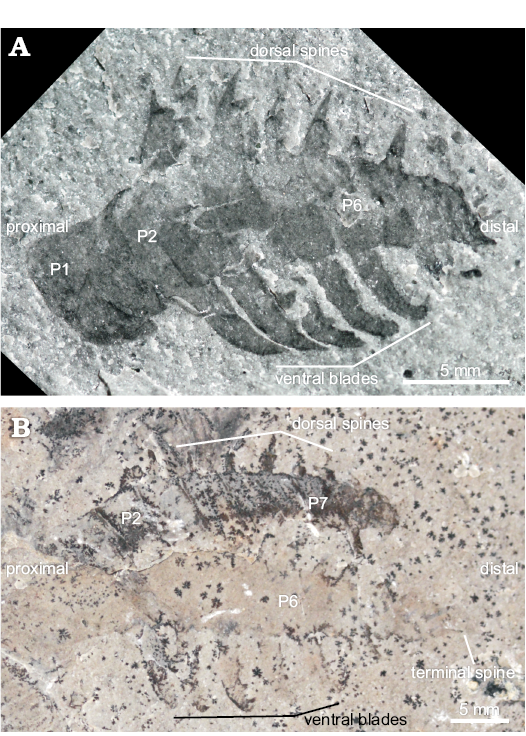
Fig. 5. Radiodontan euarthropod Stanleycaris hirpex Caron, Gaines, Mángano, Streng, and Daley, 2010 from the Stephen Formation (Cambrian Series 3, Stage 5), British Columbia, Canada. A. ROM 59975 (paratype). B. ROM 59976 (paratype). Abbreviation: Pn, podomere n.
KUMIP 153923 differs from Hurdia frontal appendages in the possession of 11 podomeres and more than one terminal spine; Hurdia usually displays nine podomeres (rarely 10 or 11) and only one terminal spine (Daley et al. 2013a). KUMIP 153923 differs from Peytoia frontal appendages in the presence of a small ventral spine on P7; Peytoia displays no small ventral spines (Daley et al. 2013a). Furthermore, KUMIP 153923 has a large dorsal spine which projects at a 71° angle to the surface of P6, contrasting with the smaller, more inclined, dorsal spines of both Hurdia and Peytoia appendages (Daley et al. 2013a).
Aysheaia prolata should be considered a nomen dubium due to the lack of distinguishing characters. As the incomplete preservation of KUMIP 153923 precludes a more detailed comparison with the type material of Stanleycaris hirpex (Figs. 1B, 5; Caron et al. 2010), we regard KUMIP 153923 as Stanleycaris sp. (see Table 1 for comparison between the two species). Additional material from the Wheeler Formation is necessary to determine if the presence of single-pointed dorsal spines in Stanleycaris sp. is a legitimate morphological feature or a preservational artefact.
Table 1. Comparison between Stanleycaris sp. and Stanleycaris hirpex.
| |
Stanleycaris sp. |
Stanleycaris hirpex |
|
Occurrence |
Wheeler Formation |
Stephen Formation |
|
Number of specimens |
1 |
37 |
|
Number of podomeres |
11 |
11 |
|
Length |
26.5 mm |
12.5–30.3 mm |
|
Number of large ventral blades |
5, on P2–P6 |
5, on P2–P6 |
|
Morphology of large ventral blades |
elongate, curved, with auxiliary spines |
elongate, curved, with auxiliary spines |
|
Number of short ventral blades |
1, on P7 |
1, on P7 |
|
Number of dorsal spines |
evidence for dorsal spines on P4, P6, P7, but dorsal surface poorly preserved |
9, on P2–P10 |
|
Morphology of dorsal spines |
robust, single pointed (?) |
robust, double pointed |
|
Angle of projection of dorsal spines |
71°, for dorsal spine on P6 |
45–90° |
|
Morphology of terminal spines |
recurved, pointed |
recurved, pointed |
|
Reference |
this study |
Concluding remarks
The recognition of KUMIP 153923 as a radiodontan frontal appendage directly impacts the known diversity of these extinct organisms for the lower Palaeozoic of Laurentia. Stanleycaris can now be added to the list of radiodontan taxa have been reported from the Cambrian (Series 3) of Utah and Canada (Table 2). Peytoia nathorsti and Hurdia victoria (Briggs et al. 2008; Pates et al. in press) are known in both regions from frontal appendages, mouthparts, and carapace material. Anomalocaris has also been described from post cephalic remains in both the Spence Shale and Wheeler Formation (Briggs et al. 2008), and isolated frontal appendages in the younger (Guzhangian) Weeks Formation (Lerosey-Aubril et al. 2014). The Wheeler Formation specimen is the youngest stratigraphic occurrence of Stanleycaris, expanding its temporal range from the Bathyuriscus–Elrathina Zone to the Ptychagnostus atavus Zone.
Table 2. Diversity of Cambrian radiodontans from Canada and Utah. A, appendage; C, carapace element; O, oral cone; P, post cephalic remains.
| |
Burgess Shale (Stage 5) |
Stanley Glacier (Stage 5) |
Spence Shale (Stage 5) |
Wheeler Formation |
Marjum Formation (Drumian) |
Weeks
Formation |
|
Anomalocaris canadensis |
A, C, O, P |
A |
|
|
|
|
|
Anomalocaris sp. |
|
|
P |
P |
|
A |
|
Hurdia victoria |
A, C, O, P |
A, C |
A, C, O |
|
|
|
|
Hurdia triangulata |
A, C, O, P |
|
|
|
|
|
|
Hurdia indet. sp. |
|
|
|
C |
|
|
|
Caryosyntrips serratus |
A, C |
|
|
|
|
|
|
Peytoia nathorsti |
A, O, P |
|
|
A, O |
O, P |
|
|
Amplectobelua stephenensis |
A |
|
|
|
|
|
|
Stanleycaris hirpex |
|
A, C, O |
|
|
|
|
|
Stanleycaris sp. |
|
|
|
A |
|
|
|
References |
Daley
et al. 2009, 2013a, b; |
Briggs et al. 2008; Pates et al. in press; this study |
Aysheaia prolata is here shown to be an invalid lobopodian taxon, reducing the known diversity of these organisms for the Cambrian of Laurentia. For over three decades, A. prolata was regarded as the first lobopodian to be discovered from the numerous Cambrian (Series 3) Konservat-Lagerstätten exposed in Utah (Robison 1985) and was widely referenced as such in subsequent studies (e.g., Dzik and Krumbiegel 1989; Hou and Bergström 1995; Ramsköld and Chen 1998; Bergström and Hou 2001; Hou et al. 2004; Whittle et al. 2009; Ou et al. 2011; Gámez Vintaned et al. 2011; Robison et al. 2015; Foster and Gaines 2016). Our reappraisal of KUMIP 153923 leaves the luolishaniid Acinocricus stichus (Conway Morris and Robison 1988) from the Spence Shale as the only Cambrian lobopodian currently known from the USA (Ramsköld and Chen 1998; García-Bellido et al. 2013; Yang et al. 2015).
Acknowledgements
Bruce S. Lieberman and Paul A. Selden (KUMIP) provided access to the facilities and use of photographic equipment, respectively. We thank Greg D. Edgecombe (Natural History Museum, London, UK) and an anonymous reviewer for their valuable comments and suggested improvements. SP was funded by a Palaeontological Association Sylvester-Bradley Award (PA-SB201503) and is supported by an Oxford-St Catherine’s Brade-Natural Motion Scholarship. ACD was funded by the Oxford University Museum of Natural History. JOH is supported by a Herchel Smith Postdoctoral Fellowship in Biological Sciences held at the Department of Zoology, University of Cambridge.
References
Bergström, J. and Hou, X.G. 2001. Cambrian Onychophora or xenusians. Zoologischer Anzeiger—A Journal of Comparative Zoology 240: 237–245. Crossref
Briggs, D.E., Lieberman, B.S., Hendricks, J.R., Halgedahl, S.L., and Jarrard, R.D. 2008. Middle Cambrian arthropods from Utah. Journal of Paleontology 82: 238–254. Crossref
Caron, J.B., Gaines, R.R., Mángano, M.G., Streng, M., and Daley, A.C. 2010. A new Burgess Shale-type assemblage from the “thin” Stephen Formation of the southern Canadian Rockies. Geology 38: 811–814. Crossref
Collins, D. 1996. The “evolution” of Anomalocaris and its classification in the arthropod class Dinocarida (nov.) and order Radiodonta (nov.). Journal of Paleontology 70: 280–293. Crossref
Cong, P., Daley, A.C., Edgecombe, G.D., Hou, X., and Chen, A. 2016. Morphology of the radiodontan Lyrarapax from the early Cambrian Chengjiang biota. Journal of Paleontology 90: 663–671. Crossref
Conway Morris, S. and Robison, R.A. 1988. More soft-bodied animals and algae from the Middle Cambrian of Utah and British Columbia. University of Kansas Paleontological Contributions 122: 1–48.
Daley, A.C. and Budd, G.E. 2010. New anomalocaridid appendages from the Burgess Shale, Canada. Palaeontology 53: 721–738. Crossref
Daley, A.C. and Edgecombe, G.D. 2014. Morphology of Anomalocaris canadensis from the Burgess Shale. Journal of Paleontology 88: 68–91. Crossref
Daley, A.C. and Peel, J.S. 2010. A possible anomalocaridid from the Cambrian Sirius Passet Lagerstätte, north Greenland. Journal of Paleontology 84: 352–355. Crossref
Daley, A.C., Budd, G.E., and Caron, J.B. 2013a. Morphology and systematics of the anomalocaridid arthropod Hurdia from the Middle Cambrian of British Columbia and Utah. Journal of Systematic Palaeontology 11: 743–787. Crossref
Daley, A.C., Budd, G.E., Caron, J.B., Edgecombe, G.D., and Collins, D. 2009. The Burgess Shale anomalocaridid Hurdia and its significance for early euarthropod evolution. Science 323: 1597–1600.
Daley, A.C., Paterson, J.R., Edgecombe, G.D., García-Bellido, D.C., and Jago, J.B. 2013b. New anatomical information on Anomalocaris from the Cambrian Emu Bay Shale of South Australia and a reassessment of its inferred predatory habits. Palaeontology 56: 971–990.
Dzik, J. and Krumbiegel, G. 1989. The oldest “onychophoran” Xenusion: a link connecting phyla? Lethaia 22: 169–181. Crossref
Foster, J.R. and Gaines, R.R. 2016. Taphonomy and paleoecology of the “Middle” Cambrian (Series 3) formations in Utah’s West Desert: Recent finds and new data. In: J.B. Comer, P.C. Inkenbrandt, K.A. Krahulec, and M.L. Pinnell (eds.), Resources and Geology of Utah’s West Desert. Utah Geological Association Publication 45: 291–336.
Gámez Vintaned, J.A., Liñán, E., and Zhuravlev, A.Y. 2011. A new Early Cambrian lobopod-bearing animal (Murero, Spain) and the problem of the ecdysozoan early diversification. In: P. Pontarottia (ed.), Evolutionary Biology—Concepts, Biodiversity, Macroevolution and Genome Evolution, 193–219. Springer, Berlin.
García-Bellido, D.C., Edgecombe, G.D., Paterson, J.R., and Ma, X. 2013. A “Collins’s monster”—type lobopodian from the Emu Bay Shale Konservat-Lagerstätte (Cambrian), South Australia. Alcheringa 37: 474–478. Crossref
Hou, X. and Bergström, J. 1995. Cambrian lobopodians—ancestors of extant onychophorans? Zoological Journal of the Linnean Society 114: 3–19. Crossref
Hou, X.G., Ma, X., Zhao, J., and Bergström, J. 2004. The lobopodian Paucipodia inermis from the lower Cambrian Chengjiang fauna, Yunnan, China. Lethaia 37: 235–244. Crossref
Lankester, E.R. 1904. The structure and classification of Arthropoda. Quarterly Journal of Microscopical Science 47: 523–582.
Lerosey-Aubril, R., Hegna, T.A., Babcock, L.E., Bonino, E., and Kier, C. 2014. Arthropod appendages from the Weeks Formation Konservat-Lagerstätte: new occurrences of anomalocaridids in the Cambrian of Utah, USA. Bulletin of Geosciences 89: 269–282. Crossref
Ma, X., Edgecombe, G.D., Legg, D.A., and Hou, X. 2014. The morphology and phylogenetic position of the Cambrian lobopodian Diania cactiformis. Journal of Systematic Palaeontology 12: 445–457. Crossref
Ou, Q., Liu, J., Shu, D., Han, J., Zhang, Z., Wan, X., and Lei, Q. 2011. A rare onychophoran-like lobopodian from the Lower Cambrian Chengjiang Lagerstätte, Southwestern China, and its phylogenetic implications. Journal of Paleontology 85: 587–594. Crossref
Pates, S., Daley, A.C., and Lieberman, B.S. (in press). Hurdiid radiodontans from the middle Cambrian (Series 3) of Utah. Journal of Paleontology. Crossref
Ramsköld, R. and Chen, J. 1998. Cambrian lobopodians: morphology and phylogeny. In: G. Edgecombe (ed.), Arthropod Fossils and Phylogeny, 107–150. Columbia University Press, New York.
Robison, R.A. 1985. Affinities of Aysheaia (Onychophora), with description of a new Cambrian species. Journal of Paleontology 59: 226–235.
Robison, R.A. and Richards, B.C. 1981. Larger bivalved arthropods from the Middle Cambrian of Utah. University of Kansas Paleontological Contributions 106: 1–28.
Robison, R.A., Babcock, L.E., and Gunther, V.G. 2015. Exceptional Cambrian fossils from Utah—a window into the age of trilobites. Utah Geological Survey Miscellaneous Publication 15: 1–97.
Van Roy, P., Daley, A.C., and Briggs, D.E. 2015. Anomalocaridid trunk limb homology revealed by a giant filter-feeder with paired flaps. Nature 522: 77–80. Crossref
Vinther, J., Stein, M., Longrich, N.R., and Harper, D.A. 2014. A suspension-feeding anomalocarid from the Early Cambrian. Nature 507: 496–499. Crossref
Wang, Y.Y., Huang, D.Y., and Hu, S.X. 2013. New anomalocardid frontal appendages from the Guanshan biota, eastern Yunnan. Chinese Scientific Bulletin 10: 1–6. Crossref
Whittington, H.B. 1978. The lobopod animal Aysheaia pedunculata Walcott, Middle Cambrian, Burgess Shale, British Columbia. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 16: 165–197. Crossref
Whittle, R.J., Gabbott, S.E., Aldridge, R.J., and Theron, J. 2009. An Ordovician lobopodian from the Soom Shale Lagerstätte, South Africa. Palaeontology 52: 561–567. Crossref
Yang, J., Ortega-Hernández, J., Gerber, S., Butterfield, N.J., Hou, J.B., Lan, T., and Zhang, X.G. 2015. A superarmoured lobopodian from the Cambrian of China and early disparity in the evolution of Onychophora. Proceedings of the National Academy of Sciences 112: 8678–8683. Crossref
Zeng, H., Zhao, F., Yin, Z., and Zhu, M. 2017. Morphology of diverse radiodontan head sclerites from the early Cambrian Chengjiang Lagerstätte, south-west China. Journal of Systematic Palaeontology [published online]. Crossref
Acta Palaeontol. Pol. 62 (3): 619–625, 2017
https://doi.org/10.4202/app.00361.2017