

Systematic review of Neocavia from the Neogene of Argentina: Phylogenetic and evolutionary implications
M. CAROLINA MADOZZO-JAÉN, M. ENCARNACIÓN PÉREZ, CLAUDIA I. MONTALVO, and RODRIGO L. TOMASSINI
Madozzo-Jaén, M.C., Pérez, M.E., Montalvo, C.I., and Tomassini, R.L. 2018. Systematic review of Neocavia from the Neogene of Argentina: Phylogenetic and evolutionary implications. Acta Palaeontologica Polonica 63 (2): 241–260.
Caviidae is one of the groups of rodents with the greatest ecomorphological disparity, and with currently three known lineages: Caviinae (cuises), Dolichotinae (maras), and Hydrochoerinae (capybaras). Caviinae include small caviids represented by three extant genera (Microcavia, Cavia, and Galea) and three fossils forms (Dolicavia, Palaeocavia, and Neocavia). In Argentina, the fossil record of Caviinae is continuous and abundant since the late Miocene. Neocavia, specifically, is represented by different species recorded in the late Miocene–Pliocene. Here, we describe a new species of Neocavia from the late Miocene–early Pliocene of the Cerro Azul Formation (La Pampa Province, Argentina), and provide a re-description of already known species (Neocavia lozanoi and “Neocavia depressidens”). Also, we perform a more comprehensive review of the genus and include the Neocavia species in a phylogenetic context within Caviinae. We analyze the main patterns of the evolution of the tympanic bullae within Caviidae, and infer about a possible occasional fossorial habit of Neocavia. The morphological and phylogenetic analyses indicate that Neocavia is more closely related to Dolicavia and Microcavia than to the other Caviinae, and confirm the monophyly of the genus, with at least two clearly differentiable species. Since this study cannot confirm the systematic position and validity of “N. depressidens”, we suggest not to use this taxon as a biostratigraphic indicator.
Key words: Rodentia, Caviidae, Neocavia, phylogeny, evolution, Miocene, Pliocene, Argentina.
M. Carolina Madozzo-Jaén [cmadozzojaen@mef.org.ar], Museo Paleontológico Egidio Feruglio (MEF), Av. Fontana 140, Trelew, U9100GYO Chubut, Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET); Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán, Miguel Lillo 205, T4000JFE, San Miguel de Tucumán, Argentina.
M. Encarnación Pérez [mperez@mef.org.ar], Museo Paleontológico Egidio Feruglio (MEF), Av. Fontana 140, Trelew, U9100GYO Chubut, Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET); Associate Researcher Field Museum of Natural History (FMNH), Chicago, IL, USA.
Claudia I. Montalvo [cmontalvo@exactas.unlpam.edu.ar], Facultad de Ciencias Exactas y Naturales, UNLPam, Uruguay 151, 6300, Santa Rosa, La Pampa, Argentina.
Rodrigo L. Tomassini [rodrigo.tomassini@yahoo.com.ar], INGEOSUR-CONICET, Departamento de Geología, Universidad Nacional del Sur. Avenida Alem 1253, Bahía Blanca, 8000, Argentina.
Received 31 January 2018, accepted 21 March 2018, available online 17 May 2018.
Copyright © 2018 M.C. Madozzo-Jaén et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Caviidae is one of the most striking groups of South American hystricognath rodents in terms of ecomorphological disparity (Mares and Ojeda 1982; Wood 1985; Vucetich and Verzi 1995; Dunnum 2015), and is currently represented by three main lineages: Dolichotinae (maras), Caviinae (cuises), and Hydrochoerinae (capybaras; Rowe and Honeycutt 2002; Vucetich et al. 2011; Pérez and Pol 2012; Dunnum 2015; Álvarez et al. 2017). The oldest reports of this family correspond to the late middle to early late Miocene (Mayoan South American Land Mammal Age, SALMA) of Patagonia (Vucetich and Pérez 2011), but its fossil record is continuous and abundant since the late middle Miocene (Fields 1957; Ubilla and Rinderknecht 2003; Pérez and Vucetich 2011; Pérez and Pol 2012; Vucetich et al. 2015; Pérez et al. 2018).
Caviinae are currently represented by 15 species and several subspecies gathered in three genera (Galea, Cavia, and Microcavia), with very different cranial and dental morphologies (Dunnum 2015). Members of this subfamily are distributed throughout most of South America; from humid lowlands to arid highlands, primarily associated with grasslands, and they can also inhabit forest edges, rocky scrub areas, and swamplands (Mares and Ojeda 1982; Woods and Kilpatrick 2005; Dunnum 2015). They are terrestrial, diurnal herbivores, and their size ranges from 200 g (Microcavia and Galea) to 1200 g (Cavia), thus being the smallest species within the Caviidae family (Dunnum 2015). In addition to their current diversity, these genera are also represented in the fossil record of South America, particularly in the late Cenozoic of Argentina.
Microcavia includes four extinct species (M. chapalmalensis, M. reigi, M. robusta, and M. criolloensis) and it is abundant since the Pliocene. Isolated molars found in the Aconquija Formation (Catamarca Province), assigned to the late Miocene–early Pliocene, possibly represent the oldest record of this genus (Quintana 1996; Ubilla et al. 1999; Nasif et al. 2007). The oldest record of Cavia (C. cabrerai) was found in the Andalhuala Formation (Catamarca Province), in levels corresponding to the early Pliocene (Candela and Bonini 2017). Other species of Cavia (C. galileloi, C. vates, C. lamingae, and C. tschudii) were documented for the Pleistocene and Holocene of Argentina and Brazil (Ortiz 2003; Verzi and Quintana 2005; Hadler et al. 2008), while Cavia sp. was mentioned for the Pleistocene of Uruguay (Ubilla and Alberdi 1990; Ubilla et al. 2009). The fossil record of Galea is scarce and based mainly on fragmentary remains. Galea orthodonta is the oldest record and was found in the late Pleistocene of Uruguay and the Pleistocene of Bolivia (Ubilla and Rinderknecht 2001, 2014). Remains of G. leucoblephara were found in the late Holocene of La Pampa Province, Argentina (Montalvo et al. 2017). Other specimens of G. leucoblephara, reported as G. tixiensis sensu Quintana (2001), were recovered in Holocene deposits of Buenos Aires Province, Argentina (Teta and Campo 2017). Specimens referred as G. musteloides were found in micromammal assemblages accumulated by owls from the late Pleistocene–Holocene of northwestern Argentina (Ortiz and Pardiñas 2001; Ortiz and Jayat 2007; Sampietro Vattuone et al. 2016).
The extinct genera Palaeocavia, Neocavia, and Dolicavia were recorded in the late Miocene and Pliocene, mainly in central and northern Argentina (Kraglievich 1932; Quintana 1997; Montalvo and Rocha 2003; Tomassini and Montalvo 2013; Montalvo et al. 2016). Palaeocavia is the most diverse genus, with the widest geographical and temporal distribution; it was found in central and northern Argentina from the late Miocene to the late Pliocene (Ameghino 1908; Tomassini and Montalvo 2013; Cruz et al. 2017; Madozzo-Jaén 2017; Madozzo-Jaén and Pérez 2017). Dolicavia minuscula is the only species of this genus, known from numerous and well-preserved remains, and its distribution is restricted to the late Pliocene of Buenos Aires Province, Argentina (Quintana 1997). Neocavia is represented by two species recorded in Argentina: N. lozanoi (Kraglievich 1932) from Catamarca Province, and N. depressidens (Parodi and Kraglievich 1948) from Buenos Aires Province (Fig. 1).
Neocavia lozanoi is represented only by the holotype, which consists of associated skull and mandible (Kraglievich 1932, 1948), recovered from the “Araucanense” (levels without accurate stratigraphic, which encompass the entire stratigraphic column of Chiquimil and Andalhuala formations, late Miocene–early Pliocene; Bossi and Muruaga 2009) of Andalhuala locality, Santa María Valley (Fig. 1A). Additional cranial specimens from the Cerro Azul Formation (late Miocene–early Pliocene) of Caleufú locality, La Pampa Province (Fig. 1B), were assigned to Neocavia cf. N. lozanoi (Montalvo and Rocha 2003). Neocavia depressidens is also known only by the holotype, a currently lost hemimandible (see Montalvo and Rocha 2003), from the Monte Hermoso Formation (early Pliocene) of Farola Monte Hermoso locality (Fig. 1C). The original illustration and description by Parodi and Kraglievich (1948) does not allow precise comparisons with other specimens, due to the lack of details. This taxon has been used to define the “Biozone of Neocavia depressidens” (early Pliocene) at the Pampean Region of Argentina (Cione and Tonni 1995, 2005; but see Tomassini et al. 2013), assigning a relevant biostratigraphic value to this species. Additionally, a hemimandible assigned to Neocavia sp. was also recorded in the Monte Hermoso Formation; according to Tomassini (2012: 90), this material displays differences compared to the other members of this genus, but detailed descriptions were not provided.
In this context, the aim of this work is to revise the genus Neocavia, and describe a new species based on abundant specimens from the Cerro Azul Formation (La Pampa Province). In addition, we test the phylogenetic affinities of Neocavia within Caviinae, and the validity of N. depressidens as a biostratigraphic indicator. The evolutionary implications of the large tympanic bullae of Caviidae are also discussed.
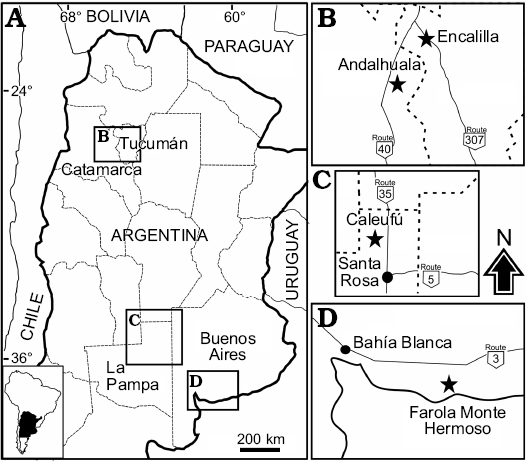
Fig. 1. A. Location map indicating the geographic distribution of Neocavia localities in Argentina. B. Encalilla and Andalhuala localities, Santa María Valley, Tucumán, and Catamarca provinces, respectively. C. Caleufú locality, La Pampa Province. D. Farola Monte Hermoso locality, Buenos Aires Province.
Institutional abbreviations.—GHUNLPam, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de La Pampa, Santa Rosa, La Pampa Province, Argentina; MACN-Ma, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Colección Nacional de Mastozoología, Ciudad Autónoma de Buenos Aires, Buenos Aires Province, Argentina; MACN-Pv, Museo Argentino de Ciencias Naturales Bernardino Rivadavia, Colección Paleontología de Vertebrados, Ciudad Autónoma de Buenos Aires, Buenos Aires Province, Argentina; MD-FM, Museo Municipal de Ciencias Naturales “Carlos Darwin”, Punta Alta, Buenos Aires Province, Argentina; MLP, Museo de La Plata, La Plata, Buenos Aires Province, Argentina; PVL, Colección Paleontología de Vertebrados Lillo, San Miguel de Tucumán, Tucumán Province, Argentina.
Other abbreviations.—APB, anteromedial-posterorlateral length of tympanic bullae; APL, length of premaxillary-maxillary suture to anterior border of foramen magnum; Bo, basioccipital; df, dorsal fossa; Fit, infratympanic fenestra; Hf/hf, hypoflexus/ hypoflexid; h.p.i., primary internal flex; H.S.E., external secondary flex; mae, external auditory meatus; Mx, maxillary; nMpi, masseter medialis pars infraorbitalis muscle; Oc, occipital condyle; Pet, epitympanic of petrous; Pmp, mastoid of petrous; Pt, palatine; rMx, ridge of maxillary.
Material and methods
Measurements were taken with a 0.01 mm resolution digital caliper. To calculate the size between lobules, the difference of the length to width ratio index between the anterior and posterior lobe of the first lower molar (m1) was graphed. The size of the tympanic bullae was compared based on the relationship between the length from the premaxillary-maxillary suture to the anterior point of the foramen magnum (APL), and the anteromedial-posterolateral length of the tympanic bulla (APB). We follow standard convention in abbreviating tooth families as I, P, and M, with upper and lower case letters referring to upper and lower teeth, respectively. Figure 2 shows terminology for description.
Anatomical and dental nomenclature.—The cranial nomenclature follows that of Cherem and Ferigolo (2012); the mandibular nomenclature follows that of Woods and Howland (1979) and Pérez (2010a, b); and the dental nomenclature follows Pérez (2010a).
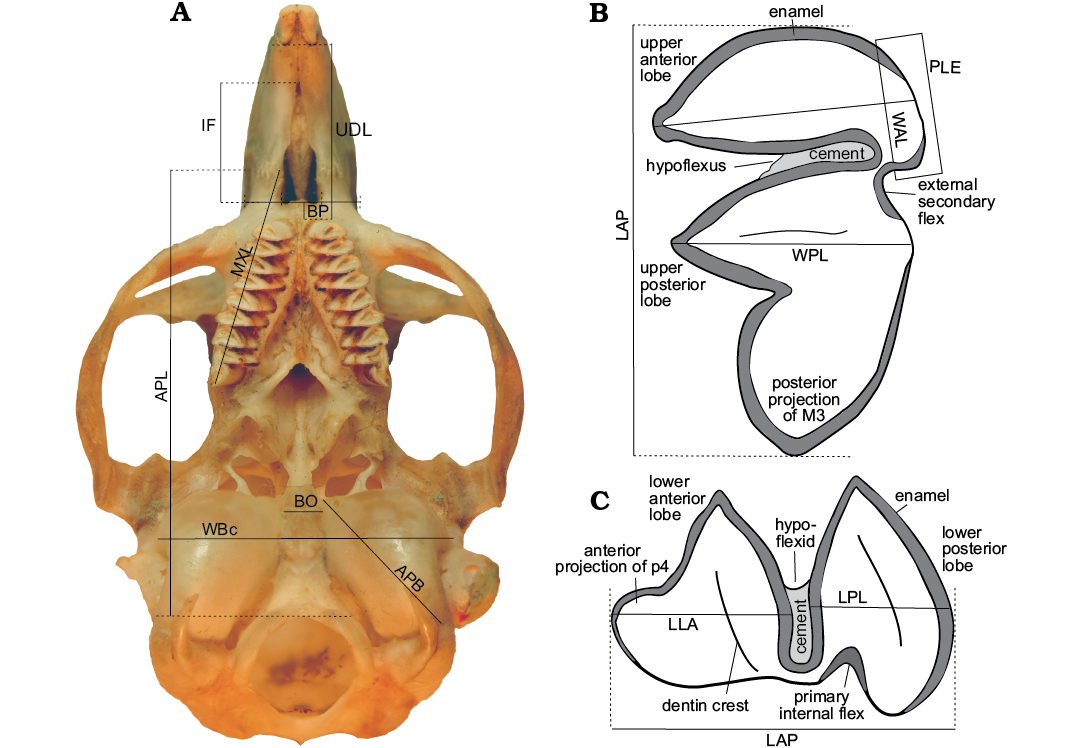
Fig. 2. Measurements used in the comparative analysis A. Skull of Microcavia australis, Recent. B. Third left upper molar (M3) C. First right lower premolar (p4; anterior to left). Abbreviations: Cranial measurements: APB, anteromedial-posterorlateral length of tympanic bullae; APL, length of premaxillary-maxillary suture to anterior border of foramen magnum; BO, width of the anterior half of the basioccipital; BP, anteroposterior length of the posterior part of the diastema; IF, length of incisive foramina; MXL, length from the premaxillary-maxillary suture to the posterior portion of the M3 projection; UDL, upper diastema length from alveolar posterior margin of incisor to alveolar anterior margin of P4; WBc, width of basicranial. Dental measurements: LAP, anteroposterior length of molariforms; LLA, anteroposterior length of anterior lobe of molariforms; LPL, anteroposterior length of posterior lobe of molariforms; PLE, posterolabial extension of anterior lobes; WAL, mediolateral length of anterior lobe of molariforms; WPL, mediolateral length of posterior lobe of molariforms.
Phylogenetic analysis.—To test the affinities between the different species of Neocavia, within Caviidae, a cladistic analysis was performed using a modified version of the combined dataset of Pérez et al. (2018). Cavia cabrerai (Candela and Bonini 2017), C. porcellus, and three species of Neocavia and Neocavia sp., as well as eight new morphological characters were added to the dataset. The combined matrix (see SOM 2, Supplementary Online Material available at http://app.pan.pl/SOM/app63-Madozzo_etal_SOM.pdf) resulted in 65 taxa, 140 morphological characters, and 4014 characters from DNA sequences (see SOM 1). The equally weighted parsimony analysis was conducted using TNT 1.1 (Goloboff and Catalano 2016), performing a heuristic search of 1000 Wagner tree replicates, followed by TBR branch swapping, collapsing zero-length branches under the strictest criterion. Unstable taxa were detected with the Pruned option in TNT, by calculating the pruned consensus. Support values were calculated using bootstrap and jackknife.
Systematic palaeontology
Rodentia Bowdich, 1821
Hystricognathi Tullberg, 1899
Cavioidea Fischer de Waldheim, 1817
Caviidae Fischer de Waldheim, 1817
Caviinae Fischer de Waldheim, 1817
Genus Neocavia Kraglievich, 1932
Type species: Neocavia lozanoi Kraglievich, 1932; Andalhuala locality, Santa María Valley, Catamarca Province, Argentina, “Araucanense” (late Miocene–early Pliocene).
Emended diagnosis.—Cavioid diagnosed by the following unique combination of characters (synapomorphies marked with an asterisk): dental series anteriorly convergent; euhypsodont and double heart-shaped molariforms; presence of a constriction in the apex of each lobe; dentine crest in the middle of occlusal surface in each lobe; external secondary flex and primary internal flex present; hypoflexus/id funnel-shaped; cement present; enamel continuous around the entire crown, interrupted on the labial side in upper molariforms, and on the lingual side in lower molariforms, except in flexus/ids. *Molariforms posteriorly increase in size, differing from Microcavia, Cavia, Dolicavia, and Palaeocavia. The anterior lobe of the upper molariforms is anteroposteriorly smaller than the posterior lobe, and *has a round posterolabial extension that reaches the anterior margin of the posterior lobe, as in Microcavia and Dolicavia. Anterior lobe of lower premolars with absent, *incipient, or developed anterior projection. Presence of a groove for the passage of the infraorbital nerve in the maxilla, with its *lateral ridge less developed dorsoventrally than in Microcavia, Cavia, and Dolicavia. Palatine more dorsally located than the maxilla, forming a ridge in the palate, as in Microcavia and Dolicavia. The notch for the insertion of the tendon of the masseter medialis pars infraorbitalis muscle has a lateral ridge attached to a well-developed horizontal crest, as in other caviids; the mylohyoid crest is present; and the *lower incisor extends up to the level of the anterior lobe of the first molar.
Stratigraphic and geographic range.—Late Miocene–early Pliocene of Argentina: Andalhuala Formation, “Araucanense”, Santa Maria Valley in Andalhuala locality, Catamarca Province and Encalilla locality, Tucumán Province; Cerro Azul Formation, Caleufú locality, Rancul Department, La Pampa Province. Early Pliocene of Argentina: Monte Hermoso Formation, Farola Monte Hermoso locality, Buenos Aires Province (Marshall and Patterson 1981; Strecker et al. 1989; Montalvo and Rocha 2003; Verzi et al. 2008; Tomassini et al. 2013 and references therein).
Neocavia lozanoi Kraglievich, 1932
Figs. 3, 4A, 5A, B, F.
Holotype: MACN-Pv 8400, skull with left P4–M3 and right P4–M2, right mandibular fragment with incisor, p4–m2 and m3 broken (Figs. 3, 4A, 5B).
Type locality: Andalhuala locality, Santa María Valley, Catamarca Province, Argentina.
Type horizon: “Araucanense” (late Miocene–early Pliocene).
Material.—PVL 7057, maxillary fragment with left and right P4–M2 (Figs. 3B, 5A) from Andalhuala Formation (late Miocene–early Pliocene) of Encalilla locality, Santa María Valley, Tucumán Province, Argentina (Fig. 1A); MACN-Pv 8415, left mandibular fragment with p4–m2 (Figs. 3C, 5B2) from the type locality.
Emended diagnosis.—The adults individuals of Neocavia lozanoi are characterized by a unique combination of characters: the primary internal flex is narrower than that of the N. pampeana sp. nov., and deeper than in N. depressidens; the anteroposterior length of the anterior and posterior lobes of the lower molariforms is similar, differing from the N. pampeana sp. nov., in which the posterior lobe is smaller than the anterior lobe. The posterior border of the upper diastema is vertical, as in Microcavia australis and Dolicavia minuscula; the tympanic bulla is larger than in M. australis, Dolicavia, G. musteloides, and Cavia aperea; the parietal and occipital regions are strongly convex; the external auditory meatus is more ventral respect to the occlusal dental series, as in M. australis and Dolicavia.
Description.—Skull: The skull is only known from the holotype, but a maxillary fragment is also assigned herein (PVL 7057; Fig. 3A1–A4, B). The skull is poorly preserved, lacking the most anterior part of the rostrum and the zygomatic arch (Kraglievich 1932, 1948). It is similar in size to M. australis and smaller than in Dolicavia.
In dorsal view (Fig. 3A1), the posterior portion of the nasal, the anterior portion of the frontal, a small fragment of the left parietal, and the endocast are partially conserved. The interorbital width (narrower width of the right frontal in the orbit) is narrower than the braincase, a condition that resembles that of other caviines (e.g., C. aperea, M. australis, Dolicavia), and contrasts with the condition of Dolichotinae and Hydrochoerinae. The preserved portion of the endocast at the level of the parietals is posterodorsally convex, similar to M. australis and Dolicavia, but the parietal-occipital suture and the temporal fossa are not differentiated.
In ventral view (Fig. 3A2, B1), the posterior margin of the incisive foramina is wide, as in M. australis and Dolicavia. On the posterior wall of the diastema, the suture between both maxillae forms a small bulge, similar to that of M. australis and Dolicavia; and the anterior ventral root of the zygomatic arch begins anteriorly to P4 (Fig. 3A2–A4, B1). The Pt is more dorsal than the Mx, forming a ridge in the palate, resembling M. australis and Dolicavia, and contrasting with C. aperea and G. musteloides, in which the Mx and Pt are at the same level in the palate.
The braincase is severely broken, but the basioccipital, occipital condyle, and part of the tympanic bullae are relatively partly preserved. The Bo has a small medial crest as in M. australis, C. aperea, and G. musteloides, but differs from the condition of Dolicavia in that it has a medial concavity. The Oc is elongated and anteromedially-posterolaterally oblique; the cast of the foramen magnum is preserved (Fig. 3A2).
In lateral view (Fig. 3A3, A4, B2), the posterior border of the upper diastema is vertically oriented, as in M. australis and Dolicavia. The length of the posterior border is shorter than in C. aperea and G. musteloides, and longer than in M. australis and Dolicavia. The maxilla has a groove for the passage of the infraorbital nerve and artery, and the rMx is dorsoventrally shorter than in other caviines (e.g., C. aperea, M. australis, Dolicavia). At the level of the first molar, the specimen PVL 7057 has a small alveolar foramen with an oval section that is posteriorly open (Fig. 3B2).
The tympanic bullae are ovoid and strikingly prominent (Fig. 3A2–A4). The anteromedial-posterorlateral length of tympanic bullae (APB) is proportionally larger than that of the other Caviidae, and probably all Cavioidea (Fig. 2A; Table 1). The length of the APB is approximately 50% of the length from the length of premaxillary-maxillary suture to anterior border of foramen magnum (APL), as in Microcavia and Dolicavia, whereas in Galea these proportions are slightly smaller. In C. aperea the APB/APL proportion is even smaller, as in Kerodon and Dolichotinae. Furthermore, in the more derived Hydrochoerinae (e.g., Hydrochoerus hydrochaeris and Phugatherium novum) this ratio is the smallest among Caviidae (Fig. 6A; Table 1).
Table 1. Relative size (in mm) of tympanic bullae. Relationship between the mean of premaxillary-maxillary suture until anterior point of magnum foramen length (APB) and the mean of anteromedial-posterolateral length of the tympanic bulla (APL) in Caviidae.
|
Neocavia lozanoi |
50.53 |
|
Microcavia australis |
40.01 |
|
Microcavia chapalmalensis |
39.91 |
|
Dolicavia minuscula |
37.78 |
|
Galea musteloides |
37.23 |
|
Galea spixii |
37.26 |
|
Cavia aperea |
28.54 |
|
Orthomyctera chapadmalense |
26.32 |
|
Dolichotis patagonum |
29.95 |
|
Dolichotis salinicola |
31.26 |
|
Kerodon rupestris |
30.55 |
|
Hydrochoerus hydrochaeris |
17.25 |
In lateral view, the shape of the epitympanic portion of the epitympanic of petrous seems to be anteroposteriorly shorter and more dorsoventrally developed than in M. australis, resembling C. aperea. The external auditory meatus (mae) is detected by a concavity in the center of the tympanic bulla, and is more ventral than the level of the dental series. The position of mae respect to the dental series resembles that of M. australis and Dolicavia, but contrasts with C. aperea and G. musteloides, in which the mae is at the same level as the dental series, or above. The circular infratympanic fenestra (fit) is anteroventral to the mae, and smaller, as in Dolicavia. The mastoid of petrous (Pmp) forms the mastoid sinus, laterally and posteriorly exposed. The Pmp has an anteroventral-posterodorsal orientation (Fig. 3A3, A4) and differs from other caviines, in which the Pmp seems to be more vertical (e.g., C. aperea, G. musteloides, M. australis, and Dolicavia). The right paraoccipital process is much broken and is only preserved up to the level of the inferior border of the occipital condyle.
Dentary: The preserved mandibles (Fig. 3A5–A7, C) conserve the anterior portion of the dentary, part of the incisors, and p4–m2. The mental foramen is located anteriorly to p4 at half the dorsoventral heigt of the lateral surface of the dentary, as in other caviids. The notch for the insertion of the tendon of the nMpi is at the level of m1–m2, as in M. australis, and the horizontal crest is well-developed, forming a laterally projected shelf as in other caviids. The fossa for the insertion of the masseter medialis muscle (dorsal to the horizontal crest) is deeper than the nMpi, as in other caviines (Fig. 3A7), except for Galea, in which the nMpi is deeper than df. The lateral crest arises at the level of the anterior lobe of m2 and is straight, as in M. australis and G. musteloides (Fig. 3A5, C1). The ventral margin of the dentary exhibits a conspicuous alveolar protuberance, similar to that of Dolicavia, and more developed than in M. australis. In lingual view (Fig. 3A6, C2), the lower incisor extends up to the level of the anterior lobe of m1, and the mylohyoid crest is present, similar to Dolicavia, C. aperea, and Palaeocavia impar.
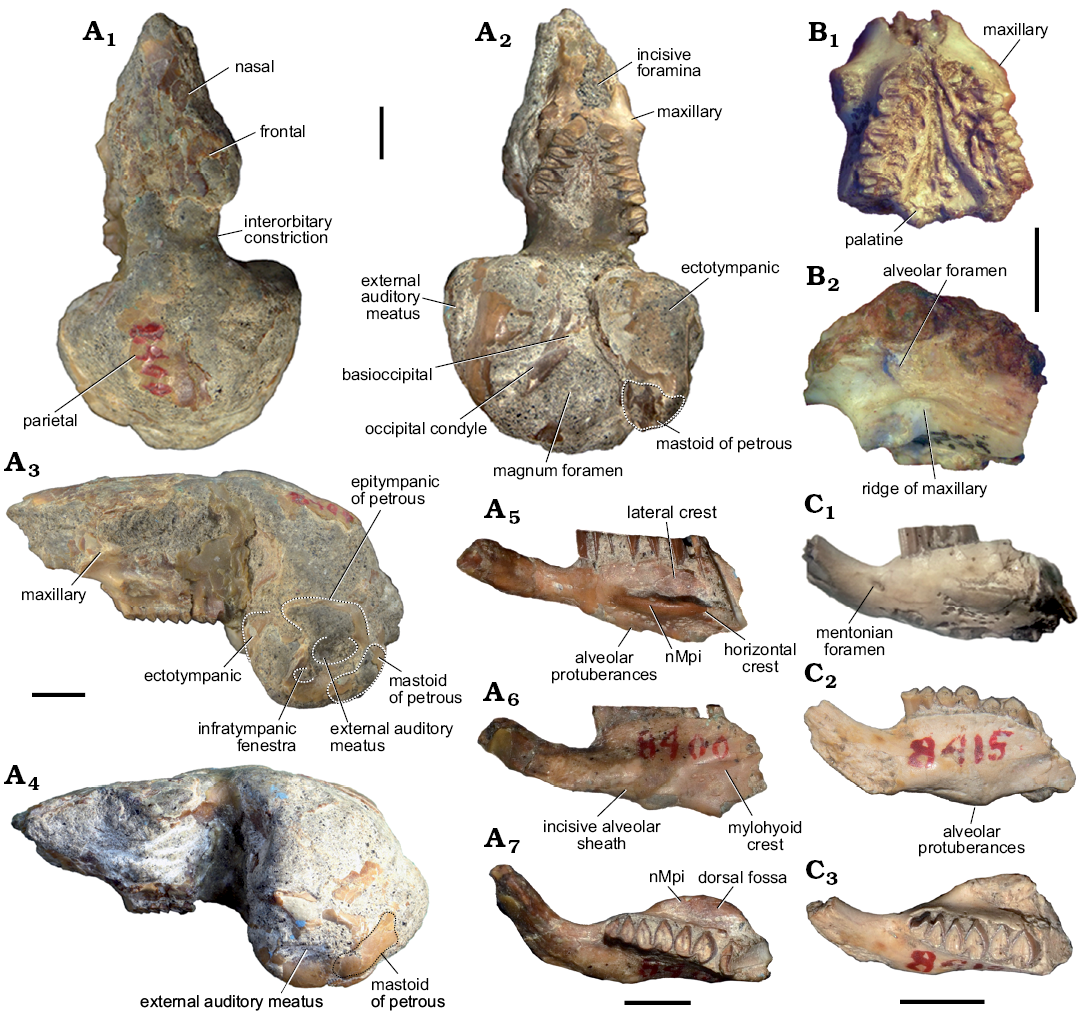
Fig. 3. Caviid rodent Neocavia lozanoi Kraglievich, 1932 from the late Miocene–early Pliocene of northwest Argentina, Andalhuala Formation, “Araucanense”: Andalhuala locality, Santa María Valley, Catamarca Province, Argentina (A, C) and Encalilla locality, Santa María Valley, Tucumán Province (B). A. MACN-Pv 8400, skull with left P4–M3 and right P4–M2 (A1–A4), right mandibular fragment with incisor p4–m2, and m3 broken (A5–A7). B. PVL 7057, maxillary fragment with left and right P4–M2. C. MACN-Pv 8415, left mandibular fragment with p4–m2. In dorsal (A1), ventral (A2, B1), lateral (A3, A4, B2), labial (A5, C1), lingual (A6, C2), and occlusal (A7, C3) views. Abbreviations: nMpi, notch for the insertion of the tendon of the masseter medialis pars infraorbitalis muscle. Scale bars 5 mm.
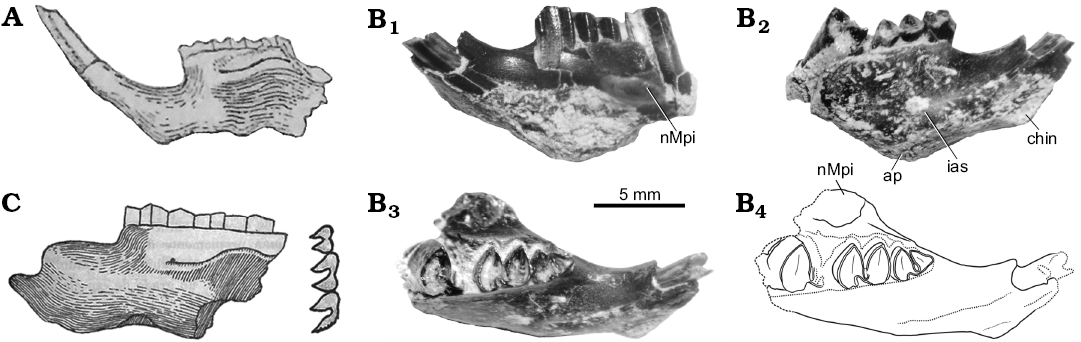
Fig. 4. Mandibles of the caviid rodent Neocavia from the Neogene of Argentina. A. Neocavia lozanoi Kraglievich, 1932 from the “Araucanense”, late Miocene–early Pliocene, Andalhuala locality, Santa María Valley, Catamarca Province. MACN-Pv 8400, mandible in lateral view (from Kraglievich 1948). B. Neocavia sp. from the lower levels of the Monte Hermoso Formation, Montehermosan Stage/Age, early Pliocene, Farola Monte Hermoso locality, Buenos Aires Province. MD-FM-17-01, mandible fragment in labial (B1), lingual (B2), and occlusal (B3; B4, explanatory drawing) views. C. “Neocavia despressidens” Parodi and Kraglievich, 1948 from upper? levels the Monte Hermoso Formation, early Pliocene, Farola Monte Hermoso locality, Buenos Aires Province. MLP 46-V-13-53, mandible in lateral view and molariform series in occlusal view (from Parodi and Kraglievich 1948). Abbreviations: ap, alveolar protuberances; chin, mandibular symphysis; ias, incisive alveolar sheath; nMpi, notch for the insertion of the tendon of the masseter medialis pars infraorbitalis muscle.
Molariforms: The molariforms are euhypsodont, double heart-shaped, with a constriction in the apex of the lobes, and with a transverse dentine crest on the middle of the occlusal surface at each lobe. The Hf/hf is funnel-shaped and has cement (Fig. 5A, B). The enamel is continuous around the entire crown, but is interrupted on the labial side in the upper molariforms, and on the lingual side in the lower molariforms, except in the h.p.i. and the H.S.E., as in other caviines. The length of the molariforms (Tables 2, 3) increases anteroposteriorly (P4/p4 are smaller than M1/m1, M1/m1 are smaller than M2/m2, and M2 is smaller than M3–m3 are not preserved), the lower molariforms pattern is shared with Microcavia, Galea, and P. impar. The anterior and posterior lobes of the lower molariforms are approximately similar in anteroposterior length (Fig. 6B).
Upper teeth: The anterior lobes have a rounded posterolabial extension that reaches the anterior margin of the posterior lobe (Fig. 2B). This characteristic differs from that of M. australis and Dolicavia, in which the projection is posteriorly pointed. The anteroposterior length of the anterior upper lobes is lesser than in the posterior lobes. The shallow H.S.E. (Fig. 5A, B1) is similar to the condition of Dolicavia and M. australis. The M3 is the largest tooth and has an elongated posterior projection similar to that of Dolicavia.
Lower teeth: The h.p.i. seems to be deeper than in “Neocavia depressidens” (Fig. 2C; Table 3). The incisor is broken and the presence of an anterior lobe of p4 is variable (Fig. 5B2, F): the assigned specimen (MACN-Pv 8415) has an anterior projection, absent in the holotype (MACN-Pv 8400). In the holotype, the anterior lobe is oblique in relation to the anteroposterior axis of the mandible, while in the assigned specimen (MACN-Pv 8415) the anterior projection is well developed (Fig. 5B2, F).
Remarks.—The anatomic analyses here performed reveal certain discrepancies with respect to the description of Kraglievich (1932, 1948), mainly due to the poor preservation of the holotype (MACN-Pv 8400). According to the author, “los huesos nasales presentan una convexidad transversal moderada” [the nasal bones show a moderate transverse convexity] (Kraglievich 1948: 143). This characteristic cannot be confirmed because only a small portion of the nasal is preserved in the anterior portion of the skull. Moreover, the author described “...adelante de los premolares unos amplios forámenes palatinos” [wide palatine foramens in front of the premolars] (Kraglievich 1948: 143). Nevertheless, following Cherem and Ferigolo (2012), the foramen anterior to the premolars is the incisive foramen, which is only preserved in the posterior portion without a medial septum. In addition, Kraglievich (1948) described that the rostrum is inclined respect to the basioccipital, as in Dolicavia, but this characteristic is shared by all Caviinae.
The dentary was broken, probably after the description of Kraglievich (1932, 1948; Fig. 4A). In this sense, the specimen has been imprecisely repaired (Fig. 3A5), so that the most anterior part is laterally rotated, and the posteroventral projection of the posterior end of the mandibular symphysis is not present. However, it is illustrated in a posterior paper of Kraglievich (1948). According to Kraglievich (1932, 1948), and Parodi and Kraglievich (1948), the masseteric crest in the dentary is much developed and begins at the level of m1. However, following Pérez (2010a, b), the portion at the level of m1 corresponds to the nMpi; the crest corresponds to the horizontal crest, and the portion of dentary with the masseteric crest is not preserved.
Stratigraphic and geographic range.—Late Miocene–early Pliocene of Argentina, “Araucanense”, Andalhuala Formation; Santa María Valley in Andalhuala locality, Catamarca Province and Encalilla locality, Tucumán Province.
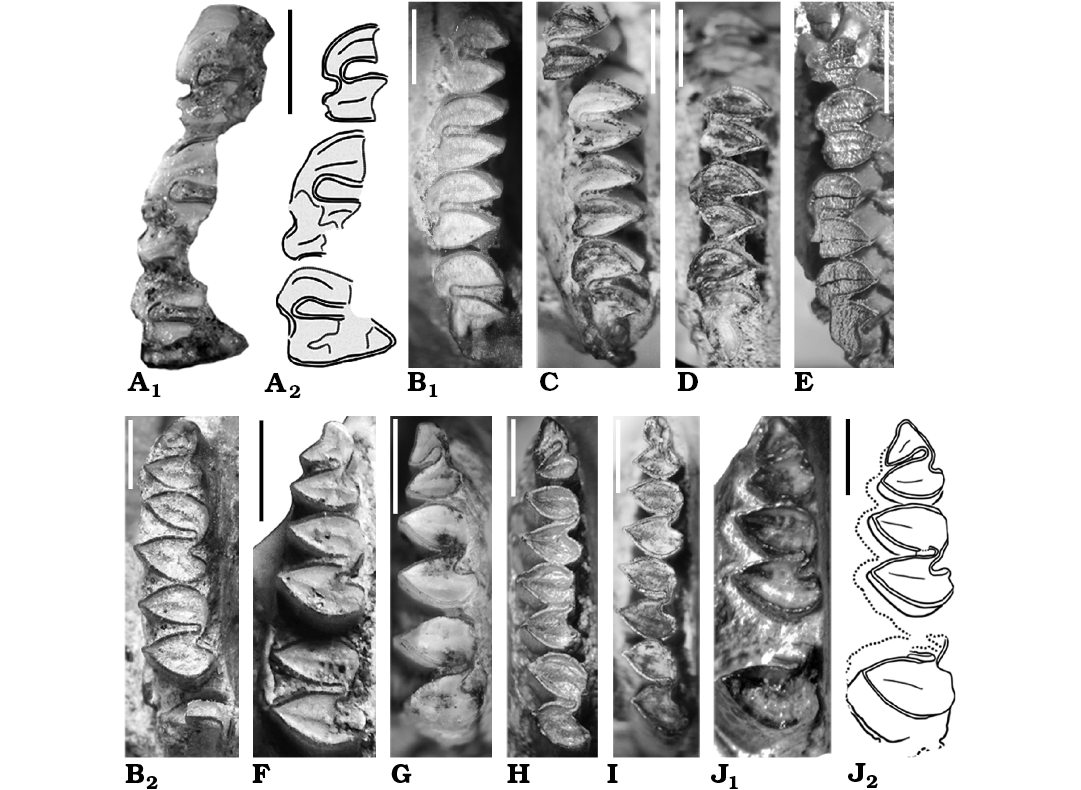
Fig. 5. Caviid rodent Neocavia from the Neogene of Argentina; upper (A, B1, C–E) and lower (B2, F–J) molariforms in occlusal views. A, B, F. Neocavia lozanoi Kraglievich, 1932 from Andalhuala Formation, late Miocene–early Pliocene, Encalilla locality, Santa María Valley, Tucumán Province (A) and “Araucanense”, Andalhuala locality, Santa María Valley, Catamarca Province (B, F). A. PVL 7057, right P4–M2; photograph (A1) and explanatory drawing (A2). B. MACN-Pv 8400 (reflected), right P4–M3 (B1) and right p4–m2 (B2). F. MACN-Pv 8415, left p4–m2. C–E, G–I. Neocavia pampeana sp. nov. from Cerro Azul Formation, late Miocene–early Pliocene, Caleufú locality, La Pampa Province. C. GHUNLPam 21351 (reflected), left P4–M3. D. GHUNLPam 21854, rigth P4–M3. E. GHUNLPam 21286, rigth DP4/P4–M3. G. GHUNLPam 19582, left p4–m2. H. GHUNLPam 19559, left p4–m3. I. GHUNLPam 21288, rigth p4–m2. J. Neocavia sp. from the lower levels of the Monte Hermoso Formation, Montehermosan Stage/Age, early Pliocene, Farola Monte Hermoso locality, Buenos Aires Province, MD-FM-17-01, left p4–m1 and posterior lobe of m2; photograph (J1) and explanatory drawing (J2). Scale bars 2 mm.
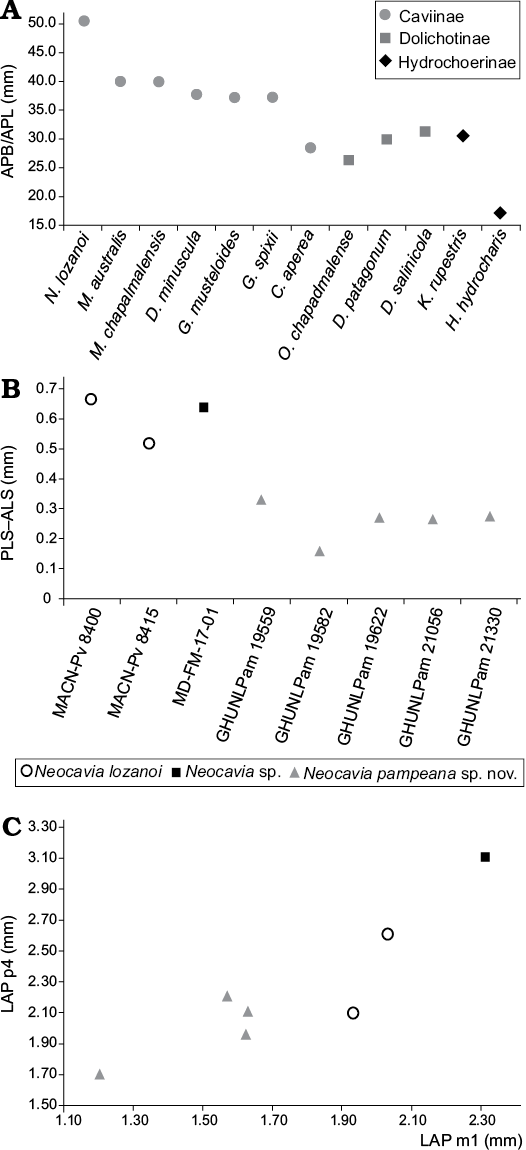
Fig. 6. Graphic measurements of Caviidae. A. Relative size of tympanic bullae in Caviidae (in mm), relationship between the mean length from the premaxillary-maxillary suture to the anterior point of the foramen magnum (APB) and the mean anteromedial-posterolateral length of the tympanic bulla (APL). B. Difference between length×width of anterior (ALS) and posterior (PLS) lobe in m1. C. Bivariant plot of anteroposterior length (LAP) of p4 and m1.
“Neocavia depressidens” Parodi and Kraglievich, 1948
Fig. 4C.
Holotype: MLP 46-V-13-53, left mandibular fragment with p4–m2 (Fig. 4C).
Type locality: Farola Monte Hermoso locality, Buenos Aires Province, Argentina (Fig. 1C).
Type horizon: Upper? levels of the Monte Hermoso Formation, early Pliocene, Monteherrmosan Stage/Age (Parodi and Kraglievich 1948; Tomassini et al. 2013).
Diagnosis (from Parodi and Kraglievich 1948).—“Cavino pequeño con los molares constituidos por dos prismas separados externamente por un surco amplio; internamente ofrecen un surco pequeño poco insinuado colocado un poco atrás con relación al externo. El primer prisma del p4 no tiene prolongamiento anterior y es menor que el segundo; las caras externas anteriores y posteriores de los prismas son algo deprimidas en el centro; la cresta masetérica arranca en la parte más anterior del m1”. [Small caviine with molars consisting of two prisms separated externally by a broad groove; internally showing a small, slightly insinuated groove, located a little back with respect to the external one. The first lobe of p4 has no anterior extension and is smaller than the second one; the anterior and posterior external faces of the prisms are somewhat depressed in the center; the masseteric ridge starts at the most anterior part of m1] (Parodi and Kraglievich 1948: 66–67).
Remarks.—“Neocavia depressidens” (Fig. 4C) was diagnosed, described, and figured by Parodi and Kraglievich (1948). The holotype and only known specimen is currently lost. The original drawing possibly does not accurately reflect all the diagnostic characters given by the authors, and even other characters (e.g., constriction in the apex of each lobe of molariforms) have been drawn, but were not described. Additionally, here we interpret that “por el punto de arranque de la cresta masetérica” [by the starting point of the masseteric crest] (Parodi and Kraglievich 1948: 67) refers to the anterior border of the nMpi (sensu Pérez 2010a, b), and that “el surco ... colocado un poco atrás con relación al externo” [the groove ... located a little behind in relation to the external] (Parodi and Kraglievich 1948: 67) refers to h.p.i. (sensu Mones 1991), which is located posteriorly to the hypoflexid, and which, in this case, would be superficial.
Due to the loss of type material, and the result of phylogenetic analysis (see Phyogenetic analysis below), we decided to use a quotation mark to name “Neocavia depressidens”, until corroborating the validity of this taxon.
Neocavia pampeana sp. nov.
Figs. 5C–E, G–I, 7, 8A.
2003 Neocavia cf. N. lozanoi; Montalvo and Rocha 2003: 501–504, figs. 2, 3.
Etymology: In reference to the La Pampa Province, Argentina, where the holotype and other assigned specimens were collected.
Holotype: GHUNLPam 19559, fragment of left mandible with p4–m3 (Figs. 5H, 7F).
Type locality: Caleufú, La Pampa Province, Argentina (Fig. 1B).
Type horizon: Cerro Azul Formation, late Miocene–early Pliocene, Huayquerian Stage/Age (Montalvo and Rocha 2003; Verzi and Montalvo 2008).
Material.—GHUNLPam 19550: left mandibular fragment with posterior lobe of m1, m2, and anterior lobe of m3; GHUNLPam 19566: right mandibular fragment with m2–m3; GHUNLPam 19581: left mandibular fragment with broken i1 and p4; GHUNLPam 19582: left mandibular fragment with p4–m2 (Fig. 5G); GHUNLPam 19588: right mandibular fragment with m1–m2; GHUNLPam 19589: left mandibular fragment with m1–m2; GHUNLPam 19622: left mandibular fragment with p4–m1; GHUNLPam 21056: left mandibular fragment with p4–m1; GHUNLPam 21286: maxillary and palatine fragment with both dental series, complete left DP4–M3 (Fig. 5E) and right P4–M3; GHUNLPam 21288: right mandibular fragment with complete dental series (Fig. 5I); GHUNLPam 21330: right mandibular fragment with p4–m2; GHUNLPam 21332: right mandibular fragment with p4–m1; GHUNLPam 21351: fragment of maxilla with right P4–M3 and left P4–M1 (Fig. 5C); GHUNLPam 21353: maxillary and palatine fragment with left P4 and right P4, M2, and broken M1; GHUNLPam 21854: fragment of maxilla with complete dental series (Fig. 5D).
Diagnosis.—Neocavia pampeana sp. nov. differs from the other species of the genus because the primary internal flex is wider, and the anterior lobe of lower m1–m2 is proportionally anteroposteriorly longer than the posterior lobe, contrasting with the condition of N. lozanoi.
Description.—Palate: Several specimens include fragments of the palate with a portion of the maxilla and palatine. The most complete specimen (GHUNLPam 21351, Fig. 7A1) has the posterior wall of the diastema with a wide posterior margin of the incisive foramina, similarly to M. australis and Dolicavia. The suture between Mx (in the posterior wall of the diastema) forms a small bulge as in N. lozanoi, M. australis, and Dolicavia. The anterior ventral root of the zygomatic arch begins anteriorly to P4 (Fig. 7A1, B2, C). The Pt is more dorsally located than the Mx, forming a ridge in the palate, as in N. lozanoi, Microcavia, and Dolicavia (Fig. 7A1, B2, C). In lateral view (Fig. 7A2, B1), the posterior border of the upper diastema has a vertical orientation, resembling M. australis and Dolicavia. The groove and ridge for the passage of the infraorbital nerve and artery is present, as in N. lozanoi, but is dorsoventrally shorter than in Cavia aperea, M. australis, and Dolicavia.
Dentary: The preserved fragments include mandibular bodies, lacking any portion of the diastema and the posterior process (Fig. 7D–F). In lateral view, the nMpi is located between m1 and m2, as in M. australis and N. lozanoi. The horizontal crest is only known by its anterior part in GHUNLPam 19559 (Fig. 7F3). The straight lateral crest originates at the level of the anterior lobe of m2 (Fig. 7D3), as in N. lozanoi, M. australis, and Dolicavia. In occlusal view (Fig. 7D3, F3), the dorsal fossa for the insertion of the masseter medialis muscle is deeper than the nMpi, as in N. lozanoi, M. australis, Dolicavia, and C. aperea. In lingual view (Fig. 7D2, E2, F2), the lower incisor extends to the level of the anterior lobe of m1, as in N. lozanoi, and the mylohyoid crest is present, differing from M. australis.
Molariforms: The molariforms are euhypsodont, double heart-shaped, with a constriction in the apex of each lobe, and with a transverse dentine crest in the middle of the occlusal surface in each lobe, as in other caviines (Fig. 5C–E, G–I). The Hf/hf is funnel-shaped and has cement; the enamel is continuous around the entire crown, but is interrupted on the labial side in the upper molariforms and on the lingual side in the lower molariforms, except in H.S.E. and h.p.i., as in other caviines. The anteroposterior length of the molariforms increases posteriorly in the molariform series (P4/p4 are smaller than M1/m1, M1/m1 are smaller than M2/m2, and M2/m2 are smaller than M3/m3) as in N. lozanoi; this pattern is shared with the lower molariforms of Microcavia, Galea, and Palaeocavia impar.
Upper teeth: The anterior lobes have a round posterolabial extension that reaches the anterior margin of the posterior lobe (Fig. 5C–E), as in N. lozanoi, but different from M. australis and Dolicavia, in which the projection is posteriorly pointed. The anteroposterior length of the anterior lobes is smaller than that of the posterior lobes (Table 2). The M3 is the largest molariform and has an elongated posterior projection, with an orientation similar to that of Dolicavia.
Lower teeth: The h.p.i. of the molariforms is wider than that of N. lozanoi, “N. depressidens”, and Neocavia sp.; it has the same depth as in N. lozanoi (Table 3). The anterior lobe of p4 has an incipient anterior projection (Fig. 5G–I), differing from N. lozanoi, which has a well-developed projection when it is present. In addition, it seems that the hypoflexid of p4 is more obliquely oriented, posterolaterally, than in the latter species. A unique characteristic of N. pampeana sp. nov. is that the anteroposterior length of the anterior lobe of m2 is larger than that of the posterior lobe; differing from N. lozanoi, in which both lobes are similar in length. The difference between the surfaces of the anterior and posterior lobe in m1–m2 in N. pampeana sp. nov. is smaller than in N. lozanoi and Neocavia sp. (Fig. 6B), but we cannot corroborate this condition in “N. depressidens”.
Ontogeny: Molariforms of caviines have a simplified occlusal surface, so in the absence of deciduous teeth or other attributes that provide clues for the ontogenetic stage, such as exposed areas of trabecular bone (see Montalvo 2004), it is difficult to estimate the age of the individuals.
The sample from Caleufú includes two juvenile individuals. GHUNLPam 19581 is a mandible fragment with immature bone, a fragment of incisive, and p4. The p4 is slightly conical and the h.p.i. is anteroposteriorly wider than in adult individuals. GHUNLPam 21286 includes maxilla and palatine fragments with complete molariform series; a fragment of right deciduous premolar (DP4), and emergent P4 (Figs. 5E, 7C, 8A). The DP4 is bilobed with cement in the hypoflexus, and the enamel is continuous around the entire crown but is interrupted on the labial side, except in H.S.E., as in the DP4 of juvenile M. australis (Fig. 8B). These molariforms probably developed roots, as in “eocardiids” (e.g., Luantus, Eocardia; Kraglievich 1930; Pérez 2010a, b). The lobes of DP4 are smaller and anteroposteriorly more compressed than molars, as in the DP4 of juvenile M. australis. The base of the DP4 is smaller than the occlusal surface. On the labial side, the partially broken anterior lobe of DP4 has a subtle square projection. The H.S.E. is very shallow and is located a little anteriorly in the posterior lobe, respect to adult individuals. The emerging P4 is cone-shaped, a common condition in erupting euhypsodont cheek teeth, with their apices conspicuously smaller than the bases (Vucetich et al. 2005). GHUNLPam 21286 has a shallower H.S.E., and the posterior projection of M3 is shorter than in adult individuals. Others characteristics (e.g., wide margin of incisive foramina) are similar to the conditions of adult individuals.
In addition, GHUNLPam 21288 is a right dentary fragment with p4–m3 (Figs. 5I, 7D). The p4 is smaller than molars, and its anterior lobe has a rounded outline and a poorly developed apex. The posterior lobe is heart-shaped but the h.p.i. is incipiently developed. Due to the absence of dp4 (confirmed with X-rays analysis) and the presence of immature bone, this specimen would correspond to a subadult individual.
Most of the caviids are born with all cheek teeth already erupted, even with occlusal wear (Kraglievich 1930; Vucetich et al. 2005). Until now, Microcavia was the only identified Caviinae with postnatal dental replacement (Kraglievich 1930; Cabrera 1953; Contreras 1964), but the specimens here reported confirm that Neocavia would have a later replacement of premolars.
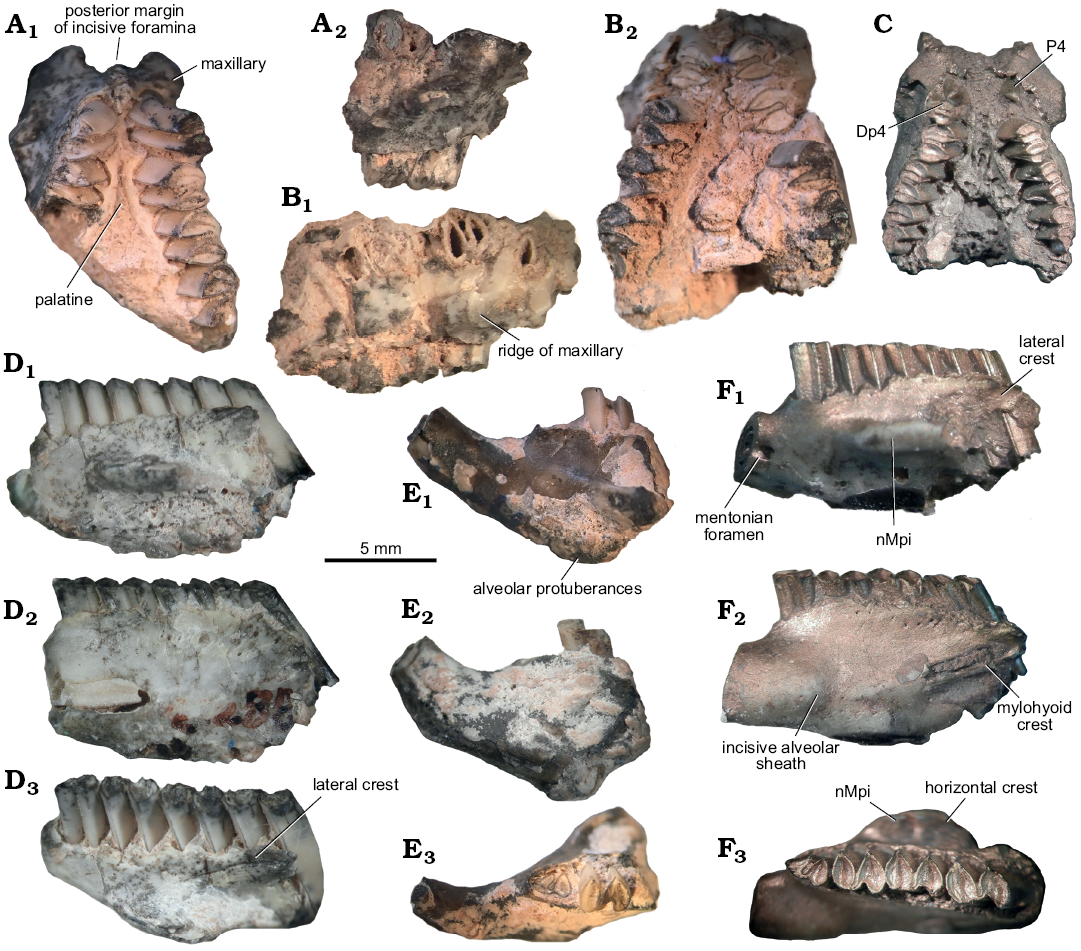
Fig. 7. Caviid rodent Neocavia pampeana sp. nov. from Cerro Azul Formation, late Miocene–early Pliocene, Caleufú locality, La Pampa Province, Argentina. A. GHUNLPam 21351, fragment of palate. B. GHUNLPam 21854, fragment of palate. C. GHUNLPam 21286, fragment of palate. D. GHUNLPam 21288 (reflected), fragment of mandible. E. GHUNLPam 19622 (reflected), fragment of mandible. F. GHUNLPam 19559, holotype (reflected), fragment of mandible. In ventral (A1, B2, C), lateral (A2, B1), labial (D1, E1, F1), lingual (D2, E2, F2), and occlusal (D3, E3, F3) views. Abbreviation: nMpi, notch for the insertion of the tendon of the masseter medialis pars infraorbitalis muscle.
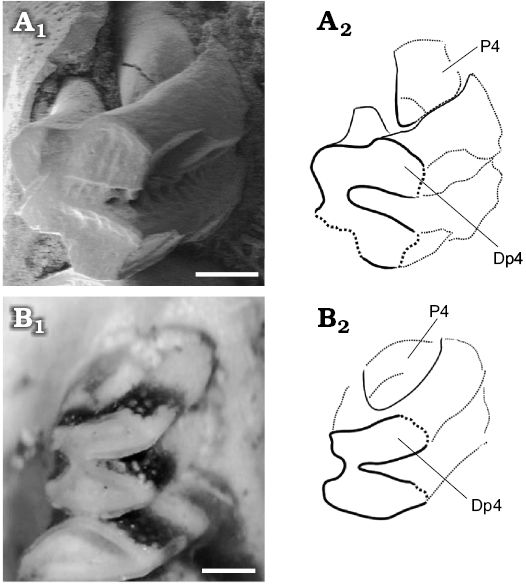
Fig. 8. Juvenile caviid rodents; molariforms (DP4 and P4) in occlusal views. A. Neocavia pampeana sp. nov. (GHUNLPam 21286) from Cerro Azul Formation, late Miocene–early Pliocene, Huayquerian Stage/Age, Calufú locality, La Pampa Province, Argentina. B. Microcavia australis Gervais and Ameghino, 1880 (MACN-Ma.34-12, reversed), Recent, from La Rioja Province. Photographs (A1, B1) and explanatory drawings (A2, B2). Scale bars 0.5 mm.
Remarks.—Montalvo and Rocha (2003) described several fossil remains recorded in the Cerro Azul Formation (Caleufú) and they assigned the sample to Neocavia cf. N. lozanoi. In the present revision, we reassign some of this material (see above) to N. pampeana sp. nov. Among the other specimens of the sample, we have identified ?Microcavia (GHUNLPam 21346), Palaeocavia sp. (GHUNLPam 19568), and two indeterminate caviids (GHUNLPam 21355 and 21356).
GHUNLPam 21346 consists in a maxillary fragment with left and right P4–M3. The molariforms are similar in morphology to those of Microcavia and the posterolabial extension of the anterior lobe is posteriorly more pointed than in Neocavia; however, the palatine is located approximately at the same level to the maxilla, differing from Microcavia which has a well-marked unevennes. GHUNLPam 19568 is a maxillary fragment with left and right P4–M2. The molariforms are similar to those of Palaeocavia, the shape of the anterior lobe is more lanceolate than in Neocavia, and it has no a posterolabial extension of anterior lobes (synapomorphy of Neocavia); the hypoflexus is similar to that of Palaeocavia, anteroposteriorly wider than in Neocavia, and the labial apex of hypoflexus has a bevel contour similar to that of Palaeocavia. The specimens GHUNLPam 21355 and 21356 consist in maxillary fragments with M3 and they have not enough characters to be identified at generic or specific level.
Stratigraphic and geographic range.—Late Miocene–early Pliocene, Cerro Azul Formation, Caleufú locality, La Pampa Province, Argentina.
Neocavia sp.
Figs. 4B, 5J.
Material.—MD-FM-17-01, fragment of left mandible with p4–m1, and broken m2 (Figs. 4B, 5J) from the lower levels of the Monte Hermoso Formation, early Pliocene, Montehermosan Stage/Age (see Tomassini et al. 2013), of Farola Monte Hermoso, Buenos Aires Province, Argentina (Fig. 1C).
Description.—Dentary: This specimen is a little more robust than other specimens of the genus (Table 3). In labial view (Fig. 4B1), only the anterior part of the nMpi has been preserved, which is located between m1 and m2 (Fig. 4B3) as in N. pampeana sp. nov. and N. lozanoi; the condition of “N. depressidens” is unknown. In lingual view (Fig. 4B2), the lower incisor extends posteriorly up to the level of the anterior lobe of m1, as in N. lozanoi and N. pampeana sp. nov. In ventral view, the alveolar protuberance of m1 is well-developed.
Lower teeth: The molariforms (Fig. 5J) are double-heart shaped, and the hypoflexid is funnel-shaped. The anteroposterior length of the molariforms is larger than in other species of Neocavia and both lobes of m1 are similar in anteroposterior length, as in N. lozanoi. Neocavia sp. differs from N. pampeana sp. nov. because the anteroposterior length of the anterior lobe is greater than the posterior lobe (Fig. 6B, C; Table 3). The h.p.i. is narrower than in N. pampeana sp. nov. and N. lozanoi, and differs from “N. depressidens” because it is very shallow (Fig. 5J). The anterior lobe of p4 has an incipient anterior projection, as in N. pampeana sp. nov.; on the contrary, in N. lozanoi, when present, the anterior projection is well-developed, whereas it is absent in “N. depressidens”.
Remarks.—Here we opted for retaining an open taxonomy for Neocavia sp. because there is only one specimen with a combination of few characters; for this reason is not possible to check if the larger size and the morphological differences (i.e., narrower h.p.i., both lobes of m1 similar in anteroposterior length, and incipient anterior projection of p4) correspond to intra or interspecific variability. In addition, the comparison with “N. depressidens”, also recovered in the Monte Hermoso Formation, is inaccurate because the holotype (and the only specimen known) is missing (see above).
Table 2. Upper molariforms measurements (in mm) of Neocavia. Abbreviations: LAP, anterposterior length of molariforms; LLA, anteroposterior length of anterior lobe of molariforms; LPL, anteroposterior length of posterior lobe of molariforms; WAL, length mediolateral of anterior lobe of molariforms; WPL, length mediolateral of posterior lobe of molariforms.
| |
Neocavia lozanoi |
Neocavia pampeana sp. nov. |
|||||
|
MACN 8400 |
PVL 7057 |
GHUNLPam |
|||||
|
21286 |
21351 |
21854 |
|||||
|
P4 |
LAP |
right |
2.02 |
1.87 |
0.96 |
1.74 |
1.73 |
|
left |
1.92 |
|
|
1.62 |
1.6 |
||
|
LLA |
right |
0.88 |
0.77 |
0.43 |
0.82 |
0.77 |
|
|
left |
0.81 |
|
|
0.71 |
0.71 |
||
|
LLP |
right |
0.94 |
0.97 |
0.41 |
0.83 |
0.83 |
|
|
left |
0.91 |
|
|
0.81 |
0.75 |
||
|
WLA |
right |
1.59 |
|
|
1.52 |
1.69 |
|
|
left |
1.34 |
|
|
|
1.48 |
||
|
WLP |
right |
1.73 |
|
|
1.57 |
1.52 |
|
|
left |
|
|
|
|
1.55 |
||
|
M1 |
LAP |
right |
1.92 |
|
1.57 |
1.63 |
1.84 |
|
left |
1.89 |
2.27 |
|
1.67 |
1.75 |
||
|
LLA |
right |
0.89 |
1.05 |
0.68 |
0.79 |
0.81 |
|
|
left |
0.87 |
1.03 |
|
0.76 |
0.78 |
||
|
LLP |
right |
0.91 |
1.11 |
0.74 |
0.67 |
0.88 |
|
|
left |
0.91 |
1.13 |
|
0.75 |
0.85 |
||
|
WLA |
right |
1.94 |
|
1.32 |
1.64 |
1.36 |
|
|
left |
1.85 |
|
|
1.76 |
1.73 |
||
|
WLP |
right |
|
|
1.26 |
1.54 |
|
|
|
left |
1.81 |
|
|
1.68 |
1.66 |
||
|
M2 |
LAP |
right |
2.12 |
1.96 |
1.64 |
|
1.85 |
|
left |
2.10 |
1.94 |
|
1.88 |
2.01 |
||
|
LLA |
right |
0.91 |
0.82 |
0.7 |
|
0.83 |
|
|
left |
0.95 |
0.88 |
|
0.83 |
0.86 |
||
|
LLP |
right |
1.06 |
1.01 |
0.77 |
|
0.87 |
|
|
left |
1.05 |
0.98 |
|
0.98 |
1.04 |
||
|
WLA |
right |
1.92 |
|
|
|
1.68 |
|
|
left |
1.96 |
|
|
1.72 |
1.76 |
||
|
WLP |
right |
|
|
1.18 |
|
|
|
|
left |
1.77 |
|
|
1.6 |
1.59 |
||
|
M3 |
LAP |
right |
|
|
1.91 |
|
2.65 |
|
left |
2.92 |
|
|
2.65 |
|
||
|
LLA |
right |
|
|
0.7 |
|
0.84 |
|
|
left |
0.96 |
|
|
0.96 |
0.89 |
||
|
LLP |
right |
|
|
1.09 |
|
1.69 |
|
|
left |
1.74 |
|
|
1.51 |
|
||
|
WLA |
right |
|
|
1.21 |
|
1.47 |
|
|
left |
1.73 |
|
|
1.67 |
|
||
|
WLP |
right |
|
|
0.98 |
|
1.23 |
|
|
left |
1.60 |
|
|
1.24 |
|
||
Table 3. Lower molariforms measurements (in mm) of Neocavia. Abbreviations: LAP, anterposterior length of molariforms; LLA, anteroposterior length of anterior lobe of molariforms; LPL, anteroposterior length of posterior lobe of molariforms; WAL, length mediolateral of anterior lobe of molariforms; WPL, length mediolateral of posterior lobe of molariforms. * from Parodi and Kraglievich (1948).
|
|
Neocavia lozanoi |
“Neocavia depressidens” |
Neocavia sp. |
Neocavia pampeana sp. nov. |
|||||||
|
MACN |
MLP 46-V-13-53* |
MD-FM-17-01 |
GHUNLPam |
||||||||
|
8400 |
8415 |
19559 |
19582 |
19622 |
21056 |
21330 |
|||||
|
p4 |
LAP |
right |
2.03 |
1.93 |
2 |
|
|
|
|
|
1.19 |
|
left |
|
|
|
2.31 |
1.62 |
1.63 |
1.57 |
1.20 |
|
||
|
LLA |
right |
0.8 |
0.87 |
|
|
|
|
|
|
0.49 |
|
|
left |
|
|
|
1.11 |
0.71 |
0.67 |
0.77 |
0.48 |
|
||
|
LPL |
right |
0.97 |
0.98 |
|
|
|
|
|
|
0.57 |
|
|
left |
|
|
|
1.18 |
0.76 |
0.77 |
0.72 |
0.62 |
|
||
|
WAL |
right |
1.38 |
1.11 |
1 |
|
|
|
|
|
0.66 |
|
|
left |
|
|
|
1.41 |
0.95 |
0.96 |
1.08 |
0.83 |
|
||
|
WPL |
right |
1.74 |
1.39 |
2 |
|
|
|
|
|
0.82 |
|
|
left |
|
|
|
1.75 |
1.10 |
1.20 |
1.33 |
0.88 |
|
||
|
m1 |
LAP |
right |
2.61 |
2.10 |
|
|
|
|
|
|
2.10 |
|
left |
|
|
|
3.10 |
1.95 |
2.1 |
2.20 |
1.69 |
|
||
|
LLA |
right |
1.10 |
0.88 |
|
|
|
|
|
|
0.46 |
|
|
left |
|
|
|
1.32 |
0.84 |
0.98 |
0.94 |
0.72 |
|
||
|
LPL |
right |
1.30 |
1.09 |
|
|
|
|
|
|
0.65 |
|
|
left |
|
|
|
1.51 |
1.03 |
1.01 |
1.11 |
0.88 |
|
||
|
WAL |
right |
2.03 |
1.90 |
|
|
|
|
|
|
0.90 |
|
|
left |
|
|
|
2.30 |
0.85 |
0.91 |
1.43 |
1.22 |
|
||
|
WPL |
right |
2.23 |
2.01 |
|
|
|
|
|
|
1.06 |
|
|
left |
|
|
|
2.43 |
1.01 |
1.03 |
1.45 |
1.30 |
|
||
|
m2 |
LAP |
right |
2.76 |
2.33 |
|
|
|
|
|
|
|
|
left |
|
|
|
|
2.20 |
2.28 |
|
|
|
||
|
LLA |
right |
1.14 |
1.11 |
|
|
|
|
|
|
0.66 |
|
|
left |
|
|
|
|
1.07 |
1.11 |
|
|
|
||
|
LPL |
right |
1.28 |
1.22 |
|
|
|
|
|
|
0.45 |
|
|
left |
|
|
|
1.66 |
1.00 |
0.99 |
|
|
|
||
|
WAL |
right |
2.10 |
1.80 |
2 |
|
|
|
|
|
1.09 |
|
|
left |
|
|
|
|
1.44 |
1.56 |
|
|
|
||
|
WPL |
right |
2.15 |
1.96 |
|
|
|
|
|
|
1.04 |
|
|
left |
|
|
|
2.791 |
1.24 |
1.65 |
|
|
|
||
|
m3 |
LAP |
right |
|
|
|
|
|
|
|
|
|
|
left |
|
|
|
|
2.40 |
|
|
|
|
||
|
LLA |
right |
|
|
|
|
|
|
|
|
|
|
|
left |
|
|
|
|
1.12 |
|
|
|
|
||
|
LPL |
right |
|
|
|
|
|
|
|
|
|
|
|
left |
|
|
|
|
1.19 |
|
|
|
|
||
|
WAL |
right |
|
|
|
|
|
|
|
|
|
|
|
left |
|
|
|
|
1.58 |
|
|
|
|
||
|
WPL |
right |
|
|
|
|
|
|
|
|
|
|
|
left |
|
|
|
|
1.58 |
|
|
|
|
||
Phylogenetic analysis
The combined analysis resulted in a total of 2724 most parsimonious trees (MPTs) of 3334 steps, and a strict consensus of all trees was calculated (Fig. 9A). The list of synapomorphies of the phylogenetic analysis is detailed in the SOM 3. In this analysis, the Subfamily Caviinae was recovered as a monophyletic group, with Galea as the most basal taxon. The subfamily is supported by ten unambiguous synapomorphies of morphological characters (see Supplementary Online Material 3). The Neocavia species are nested within Caviinae (node A) (Fig. 9A), and in the strict consensus they collapse in a polytomy (node C) with Dolicavia, Microcavia and the node E (Palaeocavia and Cavia species).
The polytomy in node C is generated by the instability of “N. depressidens”, which takes a different position in the MPTs (Fig. 9B). Two alternative topologies can be proposed for the analysis of the MPTs for Neocavia species (see the stars in Fig. 9B).
Topology A.—Neocavia is recovered as a monophyletic group in MPTs, although the internal relationships are not resolved (“N. depressidens”, N. lozanoi, N. pampeana sp. nov., and Neocavia sp. collapse in node I). The single unambiguous synapomorphy that supported the monophyly of Neocavia for all MPTs is that lower incisors extend up to the level of the anterior lobe of m1 (C. 20.4). Four unambiguous synapomorphies supported some MPTs, including the ridge through which the maxillary artery and the infraorbital nerve are developed (C.47.1), the incipient projection in the anterior lobe of p4 (C.82.1), the labial, round-shaped projection in M1–M2 (C.128.1), and the premolar smaller than M1, with M1 smaller than M2 (C.132.0).
Topology B.—“N. depressidens” takes a basal position with respect to the node of Palaeocavia and Cavia species (node E) in MPTs. This is supported by one unambiguous synapomorphy for all MPTs: the absence of an anterior projection in the anterior lobe of p4 (C.82.0) (see Taxonomy affinities).
In A and B alternative topologies, and when “N. depressidens” is ignored, the node G (Microcavia + Neocavia) is supported by two unambiguous synapomorphies in the MPTs (Fig. 9B): p4–m1 shorter than m2–m3 (C.61.0), and width of basioccipital up to 20% of the width of the braincase (C.119.0). The node H (Dolicavia, Neocavia, and Microcavia) is supported by seven unambiguous synapomorphies in all the MPTs (Fig. 9B): the area between the temporal fossae has interposed plane (C.43.0); the external auditory meatus is below the level of the occlusal surface of the dental series (C.50:1); the posterior border of the upper diastema is vertical (C.51.1); the posterior border of the upper diastema extends up to 10% respect to the anteroposterior length of the maxilla (C.52.0); the maximum width of the posterior margin of the incisive foramina is wide (> 50%) with respect to the maximum width of the maxilla at the same level (C.54.1); the palatal surface is uneven (C.55.3); and the anterior lobe of M1–M2 has a labial projection (C.127.1). Figure 9C shows the time calibrated tree resulting from phylogeny analysis ignoring the fossil taxon “N. depressidens”, and the boxes indicate changes in the different states of the tympanic bulla size (character 60) (see Evolution of the size of the tympanic bullae).

Fig. 9. Trees of phylogenetic analysis. A. Strict consensus from the 2724 most parsimonious trees shows Caviinae node (node A) and polytomy among Neocavia species, Dolicavia, Microcavia, Palaeocavia, and Cavia (node C). Upper case letters indicate nodes within Caviinae. B. Different positions of “Neocavia depressidens” within the Caviidae node in the most parsimonious trees. Stars show the two possible positions of “N. depressidens” in the most parsimonious trees. Numbers in bold indicate jackknife support values, numbers in italics represent bootstrap values. C. Adjusted in time phylogeny on phylogenetic analysis ignoring the fossils taxon “N. depressidens”. Boxes indicate percentage changes in the state of the character 60 (tympanic bullae size).
Concluding remarks
Taxonomic affinities.—Neocavia Kraglievich, 1932 is a valid genus and this new evaluation corroborates its position within Caviinae. Some of the features shared with other representatives of this subfamily include its small size, its very narrow interorbital region, the anterior portion of the parietals being strongly convex and with shallow temporal fossae, and its dental characters. Neocavia is more closely related to Dolicavia and Microcavia than to the other Caviinae (Fig. 9B) (Kraglievich 1932; Pascual 1962; Ortega Hinojosa 1963; see Phylogenetic analysis above). Dolicavia, Microcavia, and Neocavia share the labial projection in the anterior lobe of M1–M2, the wide posterior margin of the incisive foramina, the vertical posterior border of the upper diastema, and the gap between the palatal bones. Also, in these taxa (Dolicavia, Microcavia, and N. lozanoi) the temporal fossae do not merge on the middle line; the external auditory meatus is below the level of the occlusal surface of the dental series, and the tympanic bullae are the largest among caviids.
We confirm that Neocavia is a monophyletic genus with at least two species (N. lozanoi and N. pampeana sp. nov.), and also confirm that the specimen of the Monte Hermoso Formation belongs to Neocavia (see Tomassini et al. 2013), but keeping an open taxonomy (Neocavia sp.) until new and more complete remains are found. We cannot confirm or discard the assignment of “N. depressidens” to Neocavia, because in the phylogenetic analysis its position within the genus was not supported in all the MPTs (see A and B alternative topologies in Phylogenetic analysis). The coding of “N. depressidens” was made from the original description and drawing, because the holotype (and only specimen assigned) has been lost. We decided to keep the name “N. depressidens” until new data allow for a more exhaustive analysis of alpha taxonomy.
Stratigraphic considerations.—Neocavia lozanoi is restricted to the late Miocene–early Pliocene (Marshall et al. 1979; Georgieff et al. 2017) of northwest Argentina. The specimens MACN-Pv 8400 and MACN-Pv 8415 come from the Andalhuala locality, Santa María Valley, Catamarca Province, but without precise stratigraphic provenance. A dating of the lower third of the Andalhuala Formation, cropping out at Entre Ríos (Santa María Valley, Catamarca Province), yielded an age of 6.02 ± 0.04 Ma (Marshall et al. 1979). The upper limit of this formation was also dated in this locality, yielding an age of 4.78 ± 0.70 Ma (Strecker et al. 1989). Moreover, in the southern area of Santa María Valley, datings between 3.4 Ma and 4.85 Ma were obtained close to the top of the Andalhuala Formation (Strecker et al. 1989; see Georgieff et al. 2017).
The specimens PVL 7057, assigned here to N. lozanoi, were recovered in the upper levels of the Andalhuala Formation, cropping out at Encalilla (Santa María Valley, Tucumán Province) (see Alonso-Muruaga et al. 2017). Armella et al. (2016) assigned the faunal assemblage of Encalilla to the Abundance Biozone of Vassallia maxima–Pseudoplataeomys–Pithanotomys, proposed for the upper third of the Andalhuala Formation in the northern sector of the Hualfín-Belén Valley (Corral Quemado locality, Catamarca Province), with an age of 5.64–3.66 Ma (Latorre et al. 1997; Esteban et al. 2014). However, taking into account the diachronism proposed for the continental Cenozoic units of the Andean foreland of northwest Argentina (see Spagnuolo et al. 2015; Georgieff et al. 2014, 2017), we prefer to assign the levels of Encalilla to the late Miocene–early Pliocene, until more accurate radiometric datings of the area are provided.
Neocavia pampeana sp. nov. comes from the late Miocene–early Pliocene (Huayquerian Stage/Age) of the Cerro Azul Formation, exposed at Caleufú (Montalvo and Rocha 2003; Verzi and Montalvo 2008; Verzi et al. 2008). Remains assigned to Neocavia have not been found in other outcrops with older faunal assemblages of the Cerro Azul Formation (see Verzi et al. 2008; Sostillo et al. 2014). This result provides support for the biostratigraphic and biochronological interpretation for different outcrops of Cerro Azul Formation in previous papers (e.g., Verzi et al. 2008).
Neocavia sp. comes from the early Pliocene (Montehermosan Stage/Age; < 5.28 Ma – 4.5/5.0 Ma, sensu Tomassini et al. 2013) of the Monte Hermoso Formation, Farola Monte Hermoso. “Neocavia depressidens” was also recovered in the Monte Hermoso Formation, but its precise stratigraphic provenance is unknown (see Tomassini et al. 2013).
Based on the available evidence, the occurrence of Neocavia would be restricted to the late Miocene–early Pliocene. However, the specimens were recorded in fossiliferous localities without radiometric datings (e.g., Caleufú, Farola Monte Hermoso, Encalilla), their chronological assignments are based on the associated fauna, and some of them even lack precise stratigraphic provenance (e.g., MACN-Pv 8400, N. lozanoi; MLP 46-V-13-53, “N. depressidens”). For these reasons, a clear temporal correlation among the different species of Neocavia is currently not possible; therefore, its use as a biostratigraphic indicator should be revised (see section below).
Validity of Neocavia depressidens Biozone.—Cione and Tonni (2005, see also references therein) recognized two biostratigraphic units for the Monte Hermoso Formation: Trigodon gaudryi Biozone, biostratigraphic basis of the Montehermosan Stage/Age (late Miocene–early Pliocene, according to these authors), and Neocavia depressidens Biozone, biostratigraphic basis of the lower Chapadmalalan Stage/Age (early Pliocene). The latter was defined based on the record (holotype only) of “N. depressidens”. Tomassini et al. (2013), suggested that the faunistic variations between the different levels of the Monte Hermoso Formation are not significant, and do not support the validity of these two biozones (see also Deschamps et al. 2012). On this basis, the authors defined a new single biostratigraphic unit for this formation, the Eumysops laeviplicatus Biozone, corresponding to the Montehermosan Stage/Age and assigned to the early Pliocene, <5.28 Ma and 4.5/5.0 Ma. Recent biostratigraphic studies performed in different fossiliferous localities of the Quequén Salado river basin (Buenos Aires Province, Argentina), based on paleontological, sedimentological, and magnetostratigraphic data, allow assigning an age between 4.98–4.29 Ma for the Eumysops laeviplicatus Biozone (Beilinson et al. 2017). In this paper we cannot confirm the taxonomic status of “N. depressidens” and neither can we identify Neocavia sp. (MD-FM-17-01) at the species level, thus we endorse the proposal of Tomassini et al. (2013), who suggest not to use this taxon as a biostratigraphic indicator until its taxonomic status is resolved.
Evolution of the size of tympanic bullae.—Throughout the evolutionary history of mammals, the middle ear has undergone remarkable changes. Body size, phylogenetic relationships, and acoustic environment, a play a role in shaping ear structure and function (Lay 1972; Webster and Webster 1980; Ravicz et al. 1992). Cavioidea is one of the most divergent lineages within the South American Hystricognathi; thus, it is interesting to evaluate morphological changes in the evolutionary history of the group (e.g., Pérez 2010a, b; Pérez and Vucetich 2011, 2012), such as size variation of the tympanic bullae.
Three size categories of tympanic bullae (large, middle, and small) were recorded throughout the evolutionary history of Caviidae. An intermediate size (plesiomorphic state) is present in Cavia aperea and C. porcellus, Dolichotinae, the most basal forms of Hydrochoerinae (e.g., Kerodon), and some sister groups of Caviidae (e.g., Guiomys) (Fig. 9C). This plesiomorphic state is acquired at least in the middle Miocene, whereas the more derived conditions originated once the ecomorphological differentiation of the different groups occurred (Vucetich et al. 2015; Álvarez et al. 2103, 2017). A first small reduction of the size of the tympanic bullae is recorded in the late Miocene Caviodon andalhualensis (“Cardiomyinae”) (Pérez et al. 2018), whilst the most derived taxa of Hydrochoerinae (e.g., Hydrochoeropsis dasseni and Phugatherium novum) acquired the smallest tympanic bullae during the Pliocene (Vucetich et al. 2015). Within Caviinae, Galea, Dolicavia, Microcavia, and N. lozanoi, acquired the largest tympanic bullae during the late Miocene–early Pliocene.
The degree of auditory specialization has been widely studied, mainly in rodents. Most studies postulate that rodents inhabiting deserts or with subterranean habits modify the size, volume, and internal structure of the tympanic bulla in a way that increase their sensitivity to low frequencies (Lay 1972; Webster and Webster 1980; Ravicz et al. 1992; Francescoli 1999; Schleich and Vassallo 2003; Alhajeri 2014; Alhajeri et al. 2015). This adaptation is necessary for predator avoidance in open environments, where sound tends to dissipate quickly. In subterranean contexts, the transmission of high-frequency sounds is not possible, due to the rapid attenuation and dissipation in short distances (Francescoli 1999; Schleich and Vassallo 2003).
During the deposition time of Andalhuala Formation (late Miocene–early Pliocene), where N. lozanoi was recorded, South America suffered a general trend of aridization and general deterioration of climate, with short-term warmer climatic fluctuations. The eastern Antarctic glaciation in the late Miocene, the marine ingression “Paranean Sea”, extending up to northern Patagonia, and the Andean Orogeny (Quechua and Diaguita Phase) took place, with the uprising of the eastern orographic systems of Argentina, including the Puna and the Cordillera Oriental, which resulted in more arid and dry conditions in the inter-andean valleys (Muruaga 1998; Zachos et al. 2001; Ortiz-Jaureguizar and Cladera 2006; Esteban et al. 2014). The rodent fauna recorded in Encalilla (Armella et al. 2016), with the exception of cf. Microcavia, Phitanotomys, and Pseudoplataeomys, is not representative of more arid conditions. In this context, the size of bullae as a proxy for indicated arid conditions may be doubtful for the levels of Andalhuala Formation.
The ecomorphology of caviid groups is well differentiated since the late Miocene–Pliocene (Vucetich et al. 2015; Álvarez et al. 2017). In this study, a phylogenetic correlation between the size of the tympanic bullae and the habits of the Caviidae is proposed. The extant hydrochoerines (e.g., H. hydrochaeris), which have the smallest sizes of tympanic bullae are cursorial, with some adaptations to semiaquatic habits (Elissamburu and Vizcaíno 2004; Rocha-Barbosa et al. 2015; García-Esponda and Candela 2016); they use the water bodies mainly as a refuge, but most of their normal activity is on land (Schaller 1976; Vucetich et al. 2010). The extant Dolichotinae species (Dolichotis patagonum and D. salinicola), with an intermediate tympanic bullae size, are terrestrial with highly cursorial specializations (García-Esponda and Candela 2016); they commonly take refuge in vegetation, but can also occupy abandoned burrows of other mammals or build simple burrows for breeding (Campos et al. 2001; Elissamburu and Vizcaíno 2004; Rocha-Barbosa et al. 2015). Within the extant Caviinae, Cavia is the most generalized form and the size of their tympanic bullae has a plesiomorphic condition; different species use shelters in brush and clumps of vegetation, but they do not dig burrows. Among the caviines that have the largest size of tympanic bullae, Microcavia and Galea can build burrows (Elissamburu and Vizcaíno 2004). Additionally, the remains of the extinct Dolicavia are often found inside paleoburrows or in association with them (see Elissamburu et al. 2011). On this way, fossorial habits could be inferred for the fossil taxa Dolicavia and N. lozanoi.
Acknowledgements
The authors thank Olivier Lambert (Institut royal des Sciences naturelles de Belgique, Brussels, Belgium) and two reviewers Martín Ubilla (Facultad de Ciencias, Montevideo, Uruguay) and Cecilia Deschamps (MLP) for their helpful comments on the manuscript. We thank the curators Alejandro Kramarz, Stella M. Alvarez, Pablo Teta, and Sergio Lucero (all MACN); Monica Díaz, Rubén Barquez, and Fernanda Lopez Berrisbeistía (all Colección Mamíferos Lillo, San Miguel de Tucumán, Argentina), and Jaime E. Powell, Pablo E. Ortiz and Rodrigo F. Gonzalez (all PVL) for the access to material under their care. We thank Rebeca Sostillo (CONICET-Universidad Nacional de La Pampa, Santa Rosa, Argentina) and Pablo Teta for helping with the photographs, A. Costa (Centro Odontológico Costa, Santa Rosa, Argentina) for the X-rays analysis, Ana Andruchow Colombo and Andres Elgorriaga (both Museo Paleontológico Egidio Feruglio, Trelew, Argentina) for program management. Daniel A. García-López, Norma L. Nasif, and Matias A. Armella (all Instituto Superior de Correlación Geológica, San Miguel de Tucumán, Argentina) provided valuable assistance during fieldwork and Sergio Georgieff (Facultad de Ciencias Naturales e Instituto Miguel Lillo, San Miguel de Tucumán, Argentina), Matias A. Armella and Daniel García-López, contributed with valuable comments that improved the final version of the manuscript. This research was supported by the scientific project 06-G, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de La Pampa (CIM), G513 Consejo de Investigaciones de la Universidad Nacional de Tucumán (MCM-J), and PICT 1483-2012 (MEP).
References
Alhajeri, B.H. 2014. Adaptation, Diversification, and Desert Ecology of the Most Diverse Order of Mammals (Mammalia, Rodentia). 375 pp. Unpublished Ph.D. Thesis, Florida State University, Tallahassee.
Alhajeri, B.H., Hunt, O.J., and Steppan, S.J. 2015. Molecular systematics of gerbils and deomyines (Rodentia: Gerbillinae, Deomyinae) and a test of desert adaptation in the tympanic bulla. Journal of Zoological Systematics and Evolutionary Research 53: 312–330. Crossref
Alonso-Muruaga, P.J., Alonso, G.E., and García-López, D.A. 2017. Análisis de facies e interpretación paleoambiental de los depósitos de la Formación Andalhuala (Mioceno–Plioceno) en la localidad de Encalilla, provincia de Tucumán. In: P.E. Ortiz and N.L. Nasif (eds.), IV Simposio del Mio-Pleistoceno del Centro y Norte de Argentina, 6–7. Actas of XX Congreso Geológico Argentino, San Miguel de Tucumán.
Álvarez, A., Moyers Arévalo, R.L., and Verzi, D.H. 2017. Diversification patterns and size evolution in caviomorph rodents. Biological Journal of the Linnean Society 121: 907–922. Crossref
Álvarez, A., Pérez, S.I., and Verzi D.H. 2013. Ecological and phylogenetic dimensions of the cranial shape diversification in South American caviomorph rodents (Rodentia: Hystricomorpha). Biological Journal of the Linnean Society 110: 898–913. Crossref
Ameghino, F. 1908. Las formaciones sedimentarias de la región litoral de Mar del Plata y Chapadmalal. Museo de Historia Natural de Buenos Aires 3: 343–428.
Armella, M.A., Madozzo-Jaén, M.C., Nasif, N.L., García-López, D.A., Esteban, G., Ortiz, P.E., González, R., Babot, J., and Georgieff, S. 2016. Vertebrados de la Formación Andalhuala en la Localidad de Encalilla, Tucumán, Argentina. III Simposio del Mio-Pleistoceno del Centro y Norte de Argentina. Ameghiniana Suplemento Resúmenes 53: 4.
Beilinson, E., Gasparini, G.M., Tomassini, R.L., Zárate, M.A., Deschamps, C.M., Barendregt, R.W., and Rabassa, J. 2017. The Quequén Salado river basin: Geology and biochronostratigraphy of the Mio-Pliocene boundary in the southern Pampean plain, Argentina. Journal of South American Earth Sciences 76: 362–374. Crossref
Bossi G. and Muruaga C.M. 2009. Estratigrafía e inversión tectónica del “rift” neógeno en el Campo del Arenal, Catamarca, NO Argentina. Andean Geology 36: 311–341.
Cabrera, A. 1953. Los roedores argentinos de la familia “Caviidae”. Ministerio de Educación. Facultad de Agronomía y Veterinaria, UBA, Escuela de Veterinaria, Publicación 6: 1–90.
Campos, B.C.M., Tognelli, M.F., and Ojeda, R.A. 2001. Dolichotis patagonum. Mammalian Species 8: 1–5. Crossref
Candela, A.M., and Bonini, R.A. 2017. A new guinea pig (Rodentia, Caviomorpha) from northwestern Argentina: implications for the origin of the genus Cavia. Journal of Vertebrate Paleontology 37 (4): 1–10. Crossref
Cherem, J.J., and Ferigolo, J. 2012. Descrição do sincrânio de Cavia aperea (Rodentia, Caviidae) e comparação com as demais espécies do gênero no Brasil. Papéis Avulsos de Zoologia 52 (3): 21–50.
Cione, A.L. and Tonni, E.P. 1995. Chronostratigraphy and “land-mammal ages” in the Cenozoic of Southern South America: principles, practices, and the “Uquian” problem. Journal of Paleontology 69: 135–159. Crossref
Cione, A.L. and Tonni, E.P. 2005. Bioestratigrafía basada en mamíferos del Cenozoico superior de la provincia de Buenos Aires, Argentina. In: R.D. de Barrio, R.O. Etcheverry, M.F. Caballé, and E. Llambías (eds.), Geología y Recursos Minerales de la Provincia de Buenos Aires. Relatorio del XVI Congreso Geológico Argentino, 183–200. Asociación Geológica Argentina, Buenos Aires.
Contreras, J.R. 1964. Datos acerca de la variación intrapoblacional de la morfología de los molares de entidades de los géneros Galea y Microcavia (Rodentia, Caviidae). Ameghiniana 3: 235–255.
Cruz, L.E., Fernicola, J.C., and Carignano, C.A. 2017. New vertebrates of the Brochero Formation (Córdoba, Argentina): A review of the Pliocene of central Argentina. Journal of Mammalian Evolution [published online, doi: 10.1007/s10914-017-9390-0]. Crossref
Deschamps, C.M., Vucetich, M.G., Verzi, D.H., and Olivares, A.I. 2012. Biostratigraphy and correlation of the Monte Hermoso Formation (early Pliocene, Argentina): the evidence from caviomorph rodents. Journal of South American Earth Sciences 35: 1–9. Crossref
Dunnum, J.L. 2015. Family Caviidae. In: J.L Patton, U.F.J Pardiñas, and G. D’Elía (eds.), Mammals of South America, Volume 2: Rodents, 690–726. The University of Chicago Press, Chicago.
Elissamburu, A. and Vizcaíno, S.F. 2004. Limb proportions and adaptations in caviomorph rodents (Rodentia: Caviomorpha). Journal of Zoology 262: 145–159. Crossref
Elissamburu, A., Dondas, A., and De Santis, L. 2011. Morfometría de las paleocuevas de la Fm. Chapadmalal y su asignación a Actenomys (Rodentia), Paedotherium (Notoungulata) y otros mamíferos fósiles hospedantes. Mastozoología Neotropical 18: 227–238.
Esteban, G., Nasif, N., and Georgieff, S.M. 2014. Cronobioestratigrafía del Mioceno tardío-Plioceno temprano, Puerta de Corral Quemado y Villavil, Catamarca. Argentina. Acta Geológica Lilloana 26: 165–192.
Fields, R.W. 1957. Hystricomorph rodents from the late Miocene of Colombia, South America. Geological Science 32: 273–404.
Francescoli, G. 1999. A preliminary report on the acoustic communication in Uruguayan Ctenomys (Rodentia, Octodontidae): basic sound types. Bioacoustics 10: 203–218. Crossref
García-Esponda, C.M. and Candela, A.M. 2016. Hindlimb musculature of the largest living rodent Hydrochoerus hydrochaeris (Caviomorpha): Adaptations to semiaquatic and terrestrial styles of life. Journal of morphology 277: 286–305. Crossref
Georgieff, S.M., Ibañez, L.M., Vides, M.E., Anis, K.B., and Nieva, S.M. 2014. Paleógeno y Neógeno de Tucumán: estratigrafía y paleoambientes sedimentarios. In: M.S. Moyan, M.E. Puchulu, D.S. Fernández, M.E. Vides, S. Nieva, and G. Aceñolaza (eds.), Geología de Tucumán, Publicación Especial, 106–123. Colegio de Graduados de Ciencias Geológicas de Tucumán, Tucumán.
Georgieff, S.M., Muruaga, C.M., Ibañez, L.M., Spagnuolo, C., Bonini, R., Esteban G., Nasif N., and Del Pero, M.A. 2017. Estilos de deformación, cronoestratigrafía y evolución paleoambiental de las unidades neógenas de las Sierras Pampeanas Noroccidentales de Catamarca y Tucumán, Argentina. In: C.M. Muruaga and P. Grosse (eds.), Ciencias de la tierra y recursos naturales del NOA. Relatorio del XX Congreso Geológico Argentino, 1–16. Asociación Geológica Argentina, Tucumán.
Goloboff, P.A. and Catalano, S.A. 2016. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32: 221–238. Crossref
Hadler, P., Verzi, D.H., Vucetich, M.G., Ferigolo, J., and Ribeiro, A.M. 2008. Caviomorphs (Mammalia, Rodentia) from the Holocene of Rio Grande do Sul State, Brazil: systemtics and paleonenvironmental context. Revista Brasileira de Paleontologia 11 (2): 97–116. Crossref
Kraglievich, L. 1930. Diagnosis osteológico-dentaria de los géneros vivientes de la subfamilia Caviinae. Anales del Museo Nacional de Historia Natural de Buenos Aires 36: 59–95.
Kraglievich, L. 1932. Diagnosis de nuevos géneros y especies de roedores cávidos y eumegámidos fósiles de la Argentina. Anales de la Sociedad Científica Argentina CXIV: 211–237.
Kraglievich, L.J. 1948. Sobre Neocavia lozanoi. Anales de Ciencia Argentina, E. III 145: 141–145.
Latorre, C., Quade, J., and McIntosh, W.C. 1997. The expansion of the C4 gases and global changes in the late Miocene: Stable isotope evidence from the Americas. Earth and Planetary Science Letters 146: 83–96. Crossref
Lay, D.M. 1972. The anatomy, physiology, functional significance and evolution of specialized hearing organs of gerbilline rodents. Journal of Morphology 138: 41–120. Crossref
Madozzo-Jaén, M.C. 2017. Los Caviinae (Mammalia, Rodentia, Caviidae) del Cenozoico tardío del noroeste de Argentina: evolución y relaciones filogenéticas. 240 pp. Unpublished Ph.D. Thesis, Facultad de Ciencias Naturales, Universidad Nacional de Tucumán, Tucumán.
Madozzo-Jaén, M.C. and Pérez, M.E. 2017. The most ancient caviine rodent (Hystricognathi, Cavioidea) comes from the late Miocene of Northwest Argentina (South America). Historical Biology 29: 376–383. Crossref
Mares, M.A., and Ojeda, R.A. 1982. Patterns of diversity and adaptation in South American hystricognathi rodents. In: M.A. Mares and H. Genoways (eds.), Mammalian Biology in South America. Pymatuning Laboratory of Ecology Special Publication 6: 393–432.
Marshall, L.G. and Patterson, B. 1981. Geology and geochronology of the mammal-bearing Tertiary of the Valle de Santa María and Río Corral Quemado, Catamarca Province, Argentina. Fieldiana Geology 9: 1–80.
Marshall, L.G., Butler, R.F., Drake, R.E., Curtis, G.H., and Tedford, R.H. 1979. Calibration of the Great American Interchange. A radioisotope chronology for late Tertiary interchange of terrestrial faunas between the Americas. Science 204: 272–279. Crossref
Mones, A. 1991. Monografía de la Familia Hydrochoeridae (Mammalia, Rodentia). Sistemática-Paleontología-Bibliografía. Courier Forschungs-Institut Senckenberg 134: 1–235.
Montalvo, C.I. 2004. Paleobiología de la asociación faunística de Caleufú (La Pampa, Formación Cerro Azul, Mioceno superior–Plioceno inferior) a través de análisis tafonómicos. 251 pp. Unpublished Ph.D. Thesis, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Buenos Aires.
Montalvo, C.I. and Rocha, A.M. 2003. Presence of Neocavia Kraglievich (Rodentia, Caviidae) in the Cerro Azul Formation (late Miocene–early Pliocene?) in La Pampa, Argentina. Ameghiniana 40: 501–504.
Montalvo, C.I., Fernández, F.J., Bargo, M.S., Tomassini, R.L., and Mehl, A. 2017. First record of a Late Holocene fauna associated with an ephemeral fluvial sequence in La Pampa Province, Argentina. Taphonomy and paleoenvironment. Journal of South America Earth Science 76: 225–237. Crossref
Montalvo, C.I., Tomassini, R.L., and Sostillo, R. 2016. Leftover prey remains: a new taphonomic mode from the late Miocene (Cerro Azul Formation) in central Argentina. Lethaia 49: 219–230. Crossref
Muruaga, C.M. 1998. Estratigrafía y sedimentología del Terciario superior de la Sierra de Hualfín, entre las localidades de Villavil y San Fernando. Provincia de Catamarca 263 pp. Unpublished Ph.D. Thesis, Facultad de Ciencias Naturales, Universidad Nacional de Tucumán, Tucumán.
Nasif, N.L., Esteban, G., and Georgieff, S.M. 2007. Nuevo registro de vertebrados para la Formación Aconquija, provincia de Catamarca, Noroeste de Argentina. Implicancias cronoestratigráficas y consideraciones paleoambientales. Acta Geológica Lilloana 20: 99–112.
Ortega Hinojosa, E.J. 1963. Dos nuevos Caviidae de la región de Chapadmalal, datos complementarios a las diagnosis de otros Caviinae poco conocidos Ameghiniana 3: 21–28.
Ortiz-Jaureguizar, E. and Cladera, G.A. 2006. Paleoenvironmental evolution of southern South America during the Cenozoic. Journal of Arid Environments 66: 498–532. Crossref
Ortiz, P.E. 2003. Fossil record and distribution of Cavia tschudii (Caviidae, Rodentia) in Argentina. Mammalia 67: 607–611.
Ortiz, P.E. and Jayat, J.P. 2007. Sigmodontinos (Rodentia: Cricetidae) del límite Pleistoceno–Holoceno en el Valle de Tafí (Tucumán, Argentina): taxonomía, tafonomía y significación paleoambiental. Ameghiniana 44: 641–660.
Ortiz, P.E. and Pardiñas, U.F.J. 2001. Sigmodontinos (Mammalia, Rodentia) del Pleistoceno tardío del Valle de Tafí (Tucumán, Argentina): taxonomía, tafonomía y reconstrucción paleoambiental. Ameghiniana 38: 3–26.
Parodi, L.J., and Kraglievich, L.J. 1948. Un roedor cavino del Plioceno de Monte Hermoso. Anales de la Sociedad Científica Argentina 145: 65–69.
Pascual, R. 1962. Un nuevo Caviinae (Rodentia, Caviidae) de la Formación Arroyo Chasicó (Plioceno inferior) de la provincia de Buenos Aires. Ameghiniana 9: 169–174.
Pérez, M.E. 2010a. A new rodent (Cavioidea, Hystricognathi) from the middle Miocene of Patagonia, mandibular homologies, and the origin of the crown group Cavioidea sensu stricto. Journal of Vertebrate Paleontology 30: 1848–1859. Crossref
Pérez, M.E. 2010b. Sistemática, ecología y bioestratigrafía de Eocardiidae (Rodentia, Hystricognathi, Cavioidea) del Mioceno temprano y medio de Patagonia. 357 pp. Unpublished Ph.D. Thesis, Universidad Nacional de La Plata, Buenos Aires.
Pérez, M.E. and Pol, D. 2012. Major radiations in the evolution of caviid rodents: reconciling fossils, ghost lineages, and relaxed molecular clocks. Plos One 7 (10): e48380. Crossref
Pérez, M.E. and Vucetich, M.G. 2011. A new extinct genus of Cavioidea (Rodentia, Hystricognathi) from the Miocene of Patagonia (Argentina) and the evolution of cavioid mandibular morphology. Journal of Mammalian Evolution 18: 163–183. Crossref
Pérez, M.E. and Vucetich, M.G. 2012. A revision of the fossil genus Phanomys Ameghino, 1887 (Rodentia, Hystricognathi, Cavioidea) from the early Miocene of Patagonia (Argentina) and the acquisition of euhypsodonty in Cavioidea sensu stricto. Paläontologische Zeitschrift 86: 187–204. Crossref
Pérez, M.E., Deschamps, C.M., and Vucetich, M.G. 2018. Diversity, phylogeny and biogeography of the South American “Cardiomyine” rodents (Hystricognathi, Cavioidea) with a description of two new species. Papers in Palaeontology 4 (1): 1–19. Crossref
Quintana, C. 1996. Diversidad del roedor Microcavia (Caviomorpha, Caviidae) de América del Sur. Mastozoología Neotropical 3: 63–86.
Quintana, C. 1997. El roedor Dolicavia minuscula (Caviomorpha: Caviidae) del Plioceno superior de la provincia de Buenos Aires. Historia Animalium 3: 55–71.
Quintana, C. 2001. Galea (Rodentia, Caviidae) del Pleistoceno Superior y Holoceno de las sierras de Tandilia Oriental, Provincia de Buenos Aires, Argentina. Ameghiniana 38: 399–408.
Ravicz, M.E., Rosowski, J.J., and Vogt, H.F. 1992. Sound-power collection by the auditory periphery of the Mongolian gerbil Meriones unguiculatus: I. Middle-ear input impedance. Journal Acoustics Societies American 92: 157–177. Crossref
Rocha-Barbosa, O., Fiuza de Castro Longuercio, M., Casinos, A., Silva Climaco das Chagas, K., and Abreu dos Santos, J. 2015. Ecomorphological and locomotor diversity in caviomorph rodents with emphasis on cavioids. In: A.I. Vassallo and D. Antonucci (eds.), Biology of Caviomorph Rodents, Diversity and Evolution, 139–166. SAREM, Buenos Aries.
Rowe, D. and Honeycutt, R. 2002. Phylogenetic relationships, ecological correlates, and molecular evolution within the Cavioidea (Mammalia, Rodentia). Molecular Biology and Evolution 19: 263–277. Crossref
Sampietro Vattuone, M.M., Peña Monné, J.L., Báez, W., Ortiz, P., and Aguirre, M.G. 2016. Unidades morfosedimentarias holocenas en la quebrada de La Angostura (Valle de Tafí, Noroeste Argentino). In: M.M. Sampietro Vattuone and J.L. Peña Monné (eds.), Geoarqueología de los Valles Calchaquíes, 3–22. Laboratorio de Geoarqueología, Universidad Nacional de Tucumán, Tucumán.
Schaller, N. 1976. The mouse that barks. Internal Wildlife 6 (5): 12–16.
Schleich, C.E. and Vassallo, A.I. 2003. Bullar volume in subterranean and surface-dwelling caviomorph rodents. Journal of Mammalogy 84: 185–189. Crossref
Sostillo, R., Montalvo, C.I., and Verzi, D.H. 2014. A new species of Reigechimys (Rodentia, Echimyidae) from the Late Miocene of central Argentina and the evolutionary pattern of the lineage. Ameghiniana 51: 284–294. Crossref
Spagnuolo, C.M., Georgieff, S.M., and Rapalini, A.E. 2015. Magnetostratigraphy of the Miocene Las Arcas Formation, Santa María Valley, northwestern Argentina. Journal of South American Earth Sciences 63: 101–113. Crossref
Strecker, M.R., Cerveny, P., Bloom, A.L., and Malizia, D. 1989. Late Cenozoic tectonism and landscape development in the foreland of the Andes: Northern Sierras Pampeanas (26–28 S), Argentina. Tectonics 8: 517–534. Crossref
Teta, P. and Campo, D.H. 2017. Is Galea tixiensis Quintana, 2001 a synonym of G. leucoblephara Burmeister, 1861? Therya 8: 209–216.
Tomassini, R.L. 2012. Estudio tafonómico y bioestratigráfico de los vertebrados de la Formación Monte Hermoso (Plioceno) en su localidad tipo, provincia de Buenos Aires. 300 pp. Unpublished Ph.D. Thesis, Universidad Nacional del Sur, Buenos Aires.
Tomassini, R.L. and Montalvo, C.I. 2013. Taphonomic modes on fluvial deposits of the Monte Hermoso Formation (early Pliocene), Buenos Aires province, Argentina: Palaeogeography, Palaeoclimatology, Palaeoecology 369: 282–294. Crossref
Tomassini, R.L., Montalvo, C.I., Deschamps, C.M., and Manera, T. 2013. Biostratigraphy and biochronology of the Monte Hermoso Formation (early Pliocene) at its type locality, Buenos Aires Province, Argentina. Journal of South American Earth Sciences 48: 31–42. Crossref
Ubilla, M. and Alberdi, M.T. 1990. Hippidion sp. (Mammalia, Perissodactyla, Equidae) en sedimentos del Pleistoceno Superior del Uruguay (Edad Mamífero Lujanense). Estudios geológicos. Museo Nacional de Ciencias Naturales 46: 453–64.
Ubilla, M. and Rinderknecht, A. 2001. El género Galea Meyen, 1831 (Rodentia, Caviidae) en el Pleistoceno de Uruguay, primeros registros y descripción de una nueva especie extinguida. Boletín Real Sociedad. Española Historia Natural, Serie Geológica 96: 111–122.
Ubilla, M. and Rinderknecht, A. 2003. A late Miocene Dolichotinae (Mammalia, Rodentia, Caviidae) from Uruguay, with comments about the relationships of some related fossil species. Mastozoología Neotropical 10: 293–302.
Ubilla, M. and Rinderknecht, A. 2014. Comparative analysis of Galea (Rodentia, Caviidae) and expanded diagnosis of Galea ortodonta Ubilla and Rinderknecht, 2001 (late Pleistocene, Uruguay). Geobios 47: 255–269. Crossref
Ubilla, M., Piñeiro, G., and Quintana, C. 1999. A new extinct species of the genus Microcavia (Rodentia, Caviidae) from the Upper Pleistocene of the Northern Basin of Uruguay, with paleobiogeographic and paleoenvironmental comments. Studies on Neotropical Fauna and Environment 34: 141–149. Crossref
Ubilla, M., Perea, D., Rinderknecht, A., and Corona, A. 2009. Pleistocene mammals from Uruguay: biostratigraphic, biogeographic and environmental connotations. In: A.M, Ribeiro, S.G. Bauermann, and C.S. Scherer (eds.), Quaternário do Rio Grande do Sul, 217–230. Sociedade Brasileira de Paleontologia, Porto Alegre.
Verzi, D.H. and Montalvo, C.I. 2008. The oldest South American Cricetidae (Rodentia) and Mustelidae (Carnivora): late Miocene faunal turnover in central Argentina and the Great American Biotic Interchange. Palaeogeography, Palaeoclimatology, Palaeoecology 267: 284–291. Crossref
Verzi, D.H. and Quintana, C. 2005. The caviomorph rodents from the San Andrés Formation, east-central Argentina, and global late Pliocene climatic change. Palaeogeography, Palaeoclimatology, Palaeoecology 219: 303–320. Crossref
Verzi, D.H., Montalvo, C.I., and Deschamps, C.M. 2008. Biostratigraphy and biochronology of the late Miocene of central Argentina: Evidence from rodents and taphonomy. Geobios 41: 145–155. Crossref
Vucetich, M.G. and Pérez, M.E. 2011. The putative cardiomyines (Rodentia, Cavioidea) of the middle Miocene of Patagonia (Argentina) and the differentiation of the family Hydrochoeridae. Journal Vertebrate of Paleontology 31: 1382–1386. Crossref
Vucetich, M.G. and Verzi, D.H. 1995. Los roedores caviomorfos. In: M.T. Alberdi, G. Leone, and E.P. Tonni (eds.), Evolución biológica y climática de la Región Pampeana durante los últimos cinco millones de años. Un ensayo de correlación con el Mediterráneo Occidental, 211–226. Museo Nacional de Ciencias Naturales CSIC, España.
Vucetich, M.G., Arnal, M., Deschamps, C.M., Pérez, M.E., and Vieytes, E.C. 2015. A brief history of caviomorph rodents as told by the fossil record. In: A. Vassallo and D. Antonucci (eds.), Biology of Caviomorph Rodents, Diversity and Evolution, 11–62. SAREM, Buenos Aires.
Vucetich, M.G, Carlini A.A., Aguilera O., and Sánchez-Villagra, M.R. 2010. The tropics as reservoir of otherwise extinct mammals: The case of rodents from a new Pliocene faunal assemblage from Northern Venezuela. Journal of Mammalian Evolution 17: 265–273. Crossref
Vucetich, M.G., Deschamps, C.M., Morgan, C.C., and Forasiepi, A. 2011. A new species of Cardiomyinae (Rodentia, Hydrochoeridae) from western Argentina. Its age and considerations on ontogeny and diversity of the subfamily. Ameghiniana 48: 556–567. Crossref
Vucetich, M.G., Deschamps, C.M., Olivares, A.I., and Dozo, M.T. 2005. Capybaras, shape size and time: A model kit. Acta Paleontologica Polonica 50: 259–272.
Webster, D.B. and Webster, M. 1980. Morphological adaptations of the ear in the rodent family heteromyidae. Integrative and Comparative Biology 20: 247–254. Crossref
Wood, A.E. 1985. The relationships, origins and dispersal of the hystricognathous rodents. In: W.P. Luckett and J.L. Hartenberger (eds.), Evolutionary Relationships Among Rodents: A Multidisciplinary Analysis, 515–548. Plenum Press, New York. Crossref
Woods, C.A. and Howland, E.B. 1979. Adaptive radiation of Capromyid rodents: anatomy of the masticatory apparatus. Journal of Mammalogy 60: 95–116. Crossref
Woods, C.A. and Kilpatrick, C.W. 2005. Infraorder Hystricognathi Brandt. In: D.E. Wilson and D.M. Reeder (eds.), Mammal Species of the World: A Taxonomic and Geographic Reference, 1538–1600. Smithsonian Institution Press, Washington DC.
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K. 2001. Trends, rhythms, and aberrations in global climate 65Ma to Present. Science 292: 686–693. Crossref
Acta Palaeontol. Pol. 63 (2): 241–260, 2018
https://doi.org/10.4202/app.00464.2018