

Biodiversity of museum and bulk field samples compared: The Eocene Chiampo sponge fauna, Lessini Mountains, Italy
VIVIANA FRISONE, ANDRZEJ PISERA, NEREO PRETO, and WOLFGANG KIESSLING
Frisone, V., Pisera, A., Preto, N., and Kiessling, W. 2018. Biodiversity of museum and bulk field samples compared: The Eocene Chiampo sponge fauna, Lessini Mountains, Italy. Acta Palaeontologica Polonica 63 (4): 795–805.
The sponge body fossils from the Lutetian (Eocene) of Chiampo Valley in north-eastern Italy, Lessini Mountains, exhibit a high diversity. The fauna, comprising 32 species, was recently described in a systematic study based on museum material. Here we compare diversity measures and rank-abundance distributions between the museum material and new material from random surface collection at the original sampling site. Not surprisingly, we find that selectively collected museum material tends to have greater diversity and evenness than bulk field samples. Nevertheless, abundance rank-orders are maintained between samples. Bulk field sampling revealed hexactinellids to be strongly dominant over lithistids, which suggests a deep-water setting of greater than 200 m water depth.
Key words: Demospongiae, Hexactinellida, field sampling, museum collection, diversity, Eocene, Italy, Lessini Mountains.
Viviana Frisone [museo.scienze@comune.montecchio-maggiore.vi.it], Museo di Archeologia e Scienze Naturali “G. Zannato”, Piazza Marconi 17, 36075 Montecchio Maggiore, Italy.
Andrzej Pisera [apis@twarda.pan.pl], Institute of Paleobiology, Polish Academy of Science, ul. Twarda 51/55, 00-818 Warsaw, Poland.
Nereo Preto [nereo.preto@unipd.it], Dipartimento di Geoscienze, Università degli Studi di Padova, via Gradenigo 6, 35131 Padua, Italy.
Wolfgang Kiessling [wolfgang.kiessling@fau.de], GeoZentrum Nordbayern, Universität Erlangen, Loewenichstraße 28, 91054 Erlangen, Germany.
Received 9 March 2018, accepted 25 September 2018, available online 9 November 2018.
Copyright © 2018 V. Frisone et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The sponge body fossils from the middle Eocene (Lutetian) of Chiampo Valley in northeastern Italy, Lessini Mountains, are rich and diversified (Matteucci and Russo 2005). A systematic study by Frisone et al. (2016) identified 32 species (Fig. 1), 15 hexactinellid species (47% of the total), among which one (3%) belongs to the order Lyssacinosida, seven to Hexactinosida (22%) and seven to Lychniscosida (22%). 17 species belong to the class Demospongiae (53%), of which 15 are lithistids (47%) and two (Astrosclera sp. Lister, 1900 and Vaceletia progenitor Pickett, 1982) are hypercalcifying demosponges (“ceractinomorphs” sensu Buckeridge et al. 2013) (6%). Most of the studied sponges have fused or articulated spicules. As non-lithistid (soft) demosponges (e.g., Keratosa) have a low fossilization potential, Frisone et al. (2016) interpreted their absence as a taphonomical effect rather than a real attribute of the fauna. Those species counts are based on museum material and may be affected by selective sampling for well-preserved sponges and previously unrecorded species. Here we assess this potential bias by comparing the quantitative information in the museum material with results from random surface collection at the original sampling site. We compare rank-abundance distributions and diversity measures and find that museum material tends to have greater diversity and evenness than bulk field samples. Although bulk field surface collection is prone to its own biases, ecological diversity may be better assessed by bulk sampling in the field rather than museum material.

Fig. 1. Sponge fossils from the Eocene of Chiampo. A. The lyssacinosid Stauractinella eocenica Frisone, Pisera, and Preto, 2016. B. The lychniscosid Callicylix eocenicus Pisera and Busquets, 2002. C. The lithistid demosponge Platychonia sp. D. The hexactinosid Laocoetis patula Pomel, 1872. Scale bars 10 mm. Photos S. Castelli.
Geological setting
The studied area is located in the eastern Lessini Mountains, a portion of the Prealps of Northern Italy, on the west side of Chiampo Valley, Veneto Province (Fig. 2). The Lessini Mountains belonged to the Cenozoic Lessini Shelf, a carbonate platform with scattered reefs, lagoons, islands and volcanoes delimited northwards by land and by deeper water settings in other directions (e.g., Bosellini 1989; Bosellini and Papazzoni 2003; Bassi et al. 2008). The Chiampo site was located within a NNW-trending extensional structure known as the Alpone-Agno or Alpone-Chiampo graben (Barbieri et al. 1982, 1991), bounded to the west by the Castelvero normal fault. Large volumes of mainly mafic subaqueous volcanics and their contemporaneous reworking products (hyaloclastites and tuffites) accumulated in the graben during the Paleogene. The Chiampo sponges were originally collected in two adjacent quarries, Lovara and Cengio dell’Orbo. They occurred in a volcaniclastic horizon intercalated in Eocene nummulitic limestone, which is widespread in the western part of the Veneto region (e.g., Fabiani 1915). In Chiampo Valley, this nummulitic limestone is called “Chiampo limestone”, which is traditionally divided into two members (lower and upper) separated by the volcaniclastic horizon, which is also known as “Lophoranina tuff” (sensu Beschin et al. 1991; see also Matteucci and Russo 2005). The name derives from Lophoranina marestiana (König, 1825), a decapod crustacean which is widespread in this horizon. In the sponge-bearing rocks of Chiampo, the associated fauna is composed by foraminifers (planktonic, small benthic and larger foraminifera including Nummulites and Discocyclina), crustaceans (decapods and ostracods), echinoids, bryozoans, molluscs (gastropods, especially pteropods, rare cephalopods, bivalves), red algae and rare corals (Frisone et al. 2016). Beccaro et al. (2001) found that locally there are accumulations of planktonic foraminifera and that the sponge-bearing rocks exhibit sedimentary structures indicative of mass transport. Therefore, they suggested an outer ramp sedimentary environment for Lovara and Cengio dell’Orbo quarries.
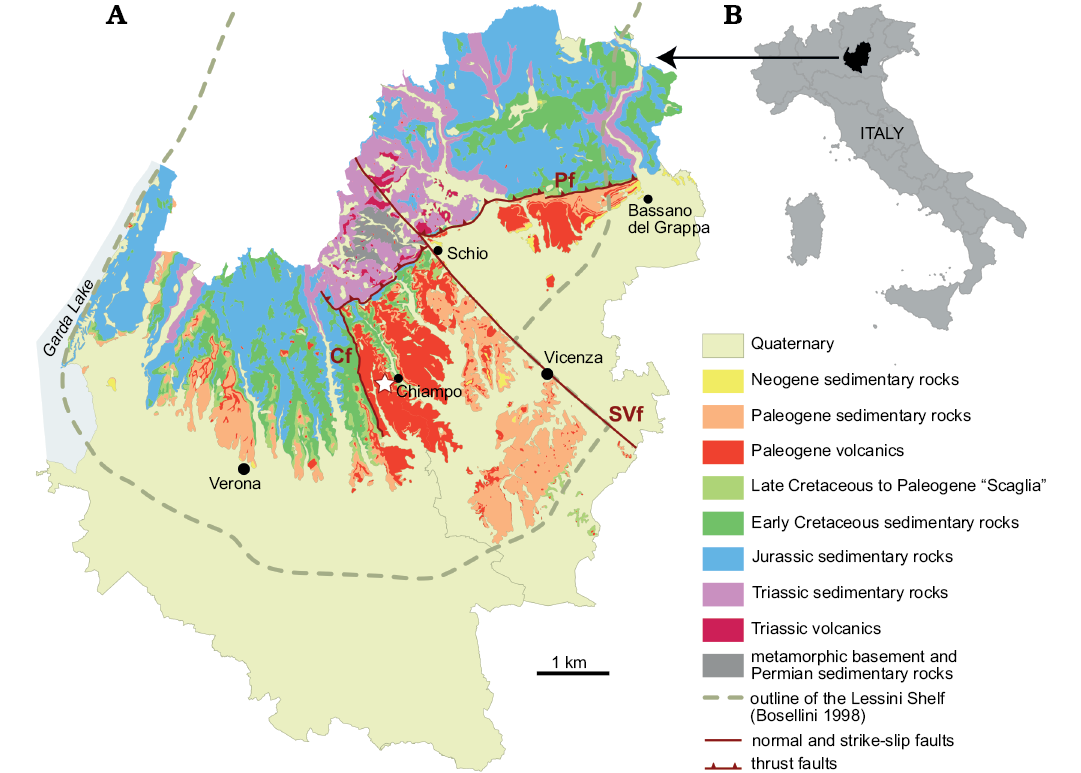
Fig. 2. Location (B) and geological map (A) of the study area (Verona and Vicenza provinces, Northern Italy), modified from http://gisgeologia.regione.veneto.it/, author: Regione Veneto, Sezione Geologia e Georisorse, and released under the Italian Open Data License 2.0 (https://www.dati.gov.it/content/italian-open-data-license-v20). Outline of the Lessini Shelf during the Eocene modified from Bosellini (1989). The sponge-bearing outcrop is indicated by the white star near Chiampo. Cf, Castelvero fault; SVf, Schio-Vicenza fault; Pf, Pedemontana thrust fault.
Material and methods
The sponge material studied by Frisone et al. (2016) stems from two adjacent quarries, which are both located in the municipality of Chiampo (Vicenza): Cengio dell’Orbo (45°32’ 25.56” N, 11°15’44.47” E) and Lovara (45°32’11.87” N, 11°15’ 58.92” E). The sponge fauna was collected from a tuffitic horizon with arenaceous grain size, generally in situ in both sections (Menin 1972; Agostini 1991; Matteucci and Russo 2005; Frisone et al. 2016). The age of the tuffitic sponge horizon is Lutetian (Matteucci and Russo 2005; Frisone et al. 2016). The “Lophoranina tuff” can be traced throughout the Chiampo Valley (e.g., Fabiani 1915, 1930; Beschin et al. 2016). Based on proximity and very similar faunal content (Menin 1972; Agostini 1991; Beccaro 2003; Matteucci and Russo 2005), we argue that the tuffitic beds are identical at Lovara and Cengio dell’Orbo. Therefore, we treat the material from the two original sites as one assemblage.
The quarries have been abandoned since the 1990s and the sponge-bearing horizon is now inaccessible. At the end of 2013, clearing work resulted in the accumulation of debris from the Lovara and Cengio dell’Orbo quarries (Antonio De Angeli, personal communication 2014). Fossil sponges were visible on the surface of the debris heap, which was selected for a quantitative bulk sampling effort. Bulk surface collections were gathered annually from March 2014 to April 2017. All specimens appearing on the surface were randomly picked by two of the authors (VF, WK) and a group of students for one hour per sampling event (Fig. 3). Overall, 436 sponge specimens were collected.
The specimens were washed in water and brushed. As the siliceous sponges were calcified during diagenesis, no etching was performed to remove attached volcaniclastics. The fossils were determined using hand lens, caliber and binocular microscope. Classification was done by the first author of this study (VF) and follows Frisone et al. (2016). The approximate minimum size of identified and counted sponge specimens was one centimeter. The specimens that were unrecognizable with reasonable confidence at class level (e.g., no diagnostic characters were visible) were classified as indeterminate sponges and they were not used for this study. The specimens were generally fragmented and partly covered by sediment. When we found fragments that looked as belonging to the same specimen, we counted them as one individual.
For Guettardiscyphia thiolati (d’Archiac, 1846) and Pleuroguettardia iberica Pisera and Busquets, 2002 (two homeomorphic species/genera very similar in general morphology but belonging to different families, having different canalization patterns), assignment was possible only if at least the outer surface was visible. In our material it was only possible to identify G. thiolati with confidence. The other specimens with outer surface covered by sediment were classified as G. thiolati as well, even though we could not exclude that some specimens may belong to P. iberica. To be consistent, we merged P. iberica and G. thiolati as one species group in both datasets. As a consequence, in the museum data, we considered in this study 31 taxa instead of the 32 described in Frisone et al. 2016.
Of the 436 bulk-sampled specimens, 71 were unrecognizable with reasonable confidence. The other 365 specimens were identified at the species level, whenever possible. Otherwise open nomenclature was used. The abundant use of open nomenclature for the Chiampo fauna kept in museum collections is explained in Frisone et al. (2016). Although the material is three-dimensionally preserved, it generally shows a poor preservation: spicules are calcified and many specimens lost important characters of the outer surface (e.g., rim around canal openings, small outgrowths, papillae) during preparations to remove attached volcaniclastics.
The sponges were counted and classified into the respective categories (classes, orders, families, genera, species). The standard error of the proportions of counts was approximated with a binomial distribution, SE = ±√p(1-p)/n, where p is the proportion of a sponge species and n the total number of individuals in the sample. Alpha-diversity indices (Shannon index and Evenness) were calculated with the program PAST© (Hammer et al. 2001). Shannon index (H) and Evenness (J) are expressed by the equations (n = number of individuals, S = number of taxa) H = -∑ni/n ln ni/n and J = H/ln(S). Rank-abundance distributions were assessed with the R package “vegan” (Oksanen et al. 2014). Rarefaction curve was computed with random subsampling in R (R Development Core Team 2018).

Fig. 3. Bulk collection of Eocene sponges at Lovara Quarry. Some of the sponge specimens are indicated by white arrows.
Results
Species richness in bulk field samples.—Thirteen species belong to class Hexactinellida (62%) and eight to class Demospongiae (38%). Among the 13 hexactinellid species, one (4% of total fauna) belongs to the order Lyssacinosida, six to Hexactinosida (29%) and six to Lychniscosida (29%). Eight species belong to the class Demospongiae and all of them are lithistids (38%). No “ceractinomorphs” or other non-lithistid demosponges were collected.
Species abundance.—Rarefaction suggests that sufficient specimens have been sampled to characterize diversity (Fig. 4). The rarefaction curve already flattens at 200 specimens. In the bulk-sampled material, the distribution of specimens is more skewed among higher taxonomic units than that of species. For example, 92.3±1.4% of the sampled individuals belong to Hexactinellida while only 7.7±1.4% belong to Demospongiae. On the other hand, when we consider richness, hexactinellid species (62%) are less dominant over demosponge species (38%) (Fig. 5).
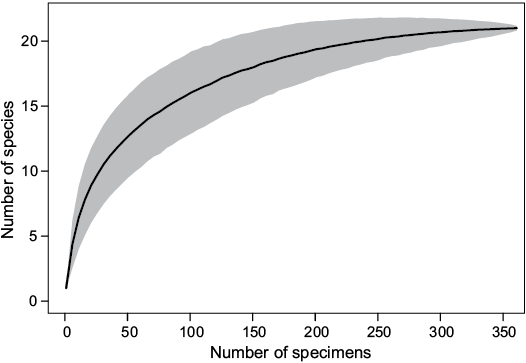
Fig. 4. Rarefaction curve (line in the middle) of the Chiampo sponge fauna at Lovara. Grey area demarcate 95% confidence intervals of diversity estimates.
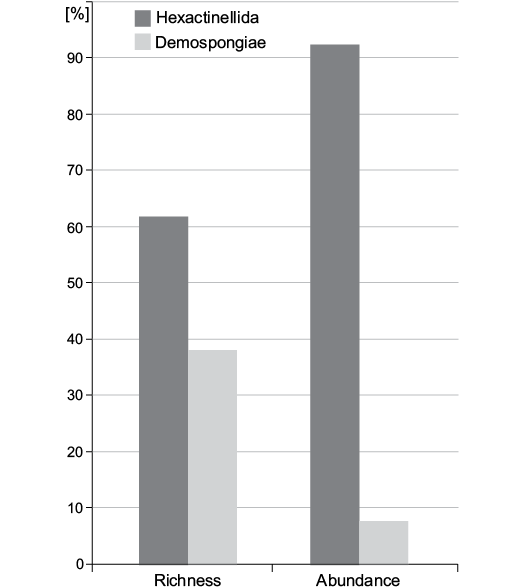
Fig. 5. Distribution of species richness (number of taxa) and species abundance (number of individuals per taxon) among classes.
A comparison of frequencies in museum collections from Frisone et al. (2016) (N = 269) versus those obtained from bulk field sampling (N = 365) is provided in Table 1.
Table 1. Comparison of taxa frequencies (± standard error) between specimens collected at Lovara quarry with bulk field sampling (this study) and identified specimens in the museum collections studied by Frisone et al. (2016). N, numbers of individuals.
|
Class |
Order |
Family |
Species |
Bulk field |
Museum |
||
|
N |
frequency [%] |
N |
frequency [%] |
||||
|
Hexactinellida |
Hexactinosida |
Craticulariidae |
Laocoetis patula Pomel, 1872 |
91 |
24.9±2.3 |
30 |
11.2±1.9 |
|
Hexactinellida |
Hexactinosida |
Cribrospongiidae |
Guettardiscyphia thiolati (d’Archiac, 1846) |
73 |
20.0±2.1 |
22 |
8.2±1.7 |
|
Hexactinellida |
Hexactinosida |
?Auloplacidae |
Rigonia plicata Frisone, Pisera, and Preto, 2016 |
37 |
10.1±1.6 |
2 |
0.7±0.5 |
|
Hexactinellida |
Hexactinosida |
Tretodictyidae |
Hexactinella clampensis Frisone, Pisera, and Preto, 2016 |
35 |
9.6±1.5 |
5 |
1.9±0.8 |
|
Hexactinellida |
Lychniscosida |
Callodictyonidae |
Callicylix eocenicus Pisera and Busquets, 2002 |
35 |
9.6±1.5 |
32 |
11.9±2.0 |
|
Hexactinellida |
Lychniscosida |
Neoaulocystidae |
Cavispongia scarpai Frisone, Pisera, and Preto, 2016 |
19 |
5.2±1.2 |
18 |
6.7±1.5 |
|
Hexactinellida |
Lyssacinosida |
Stauractinellidae |
Stauractinella eocenica Frisone, Pisera, and Preto, 2016 |
16 |
4.4±1.1 |
34 |
12.6±2.0 |
|
Hexactinellida |
Hexactinosida |
Craticulariidae |
Laocoetis ?emiliana (Malfatti, 1901) |
13 |
3.6±1.0 |
4 |
1.5±0.7 |
|
Demospongiae |
“Lithistida” |
Platychoniidae |
Platychonia sp. |
10 |
2.7±0.9 |
2 |
0.7±0.5 |
|
Hexactinellida |
Lychniscosida |
?Diapleuridae |
Coronispongia confossa Frisone, Pisera, and Preto, 2016 |
6 |
1.6±0.7 |
8 |
3.0±1.0 |
|
Hexactinellida |
Lychniscosida |
Camerospongiidae |
Toulminia italica Frisone, Pisera and Preto, 2016 |
4 |
1.1±0.5 |
5 |
1.9±0.8 |
|
Demospongiae |
“Lithistida” |
?Siphoniidae |
?Siphonia sp. |
4 |
1.1±0.5 |
9 |
3.3±1.1 |
|
Demospongiae |
“Lithistida” |
Azoricidae |
Stachyspongia sp. |
4 |
1.1±0.5 |
4 |
1.5±0.7 |
|
Hexactinellida |
Lychniscosida |
Camerospongiidae |
Camerospongia visentinae Frisone, Pisera, and Preto, 2016 |
3 |
0.8±0.5 |
22 |
8.2±1.7 |
|
Hexactinellida |
Lychniscosida |
Camerospongiidae |
Camerospongia tuberculata Frisone, Pisera, and Preto, 2016 |
3 |
0.8±0.5 |
7 |
2.6±1.0 |
|
Demospongiae |
Sphaerocladina |
Vetulinidae |
Ozotrachelus conicus (Roemer, 1841) |
3 |
0.8±0.5 |
16 |
5.9±1.4 |
|
Hexactinellida |
Hexactinosida |
Tretodictyidae |
Anomochone sp. |
2 |
0.5±0.4 |
1 |
0.4±0.4 |
|
Demospongiae |
“Lithistida” |
?Siphoniidae |
?Rhoptrum sp. |
2 |
0.5±0.4 |
2 |
0.7±0.5 |
|
Demospongiae |
“Lithistida” |
Azoricidae |
Bolidium bertii Frisone, Pisera, and Preto, 2016 |
2 |
0.5±0.4 |
3 |
1.1±0.6 |
|
Demospongiae |
“Lithistida” |
uncertain lithistid family |
Indeterminate rhizomorine sp. A |
2 |
0.5±0.4 |
3 |
1.1±0.6 |
|
Demospongiae |
“Lithistida” |
Corallistidae |
Corallistes multiosculata Frisone, Pisera, and Preto, 2016 |
1 |
0.3±0.3 |
12 |
4.5±1.3 |
|
Hexactinellida |
Lychniscosida |
Ventriculitidae |
?Ventriculites sp. |
0 |
0 |
1 |
0.4±0.4 |
|
Demospongiae |
“Lithistida” |
Corallistidae |
?Corallistes sp. |
0 |
0 |
1 |
0.4±0.4 |
|
Demospongiae |
“Lithistida” |
Azoricidae |
Jereopsis clavaeformis (Pomel, 1872) |
0 |
0 |
1 |
0.4±0.4 |
|
Demospongiae |
“Lithistida” |
Scleritodermatidae |
Verruculina ambigua (Pomel, 1872) |
0 |
0 |
7 |
2.6±1.0 |
|
Demospongiae |
“Lithistida” |
uncertain lithistid family |
Indeterminate rhizomorine sp. B |
0 |
0 |
1 |
0.4±0.4 |
|
Demospongiae |
“Lithistida” |
uncertain lithistid family |
Indeterminate rhizomorine sp. C |
0 |
0 |
3 |
1.1±0.6 |
|
Demospongiae |
“Lithistida” |
uncertain lithistid family |
Indeterminate rhizomorine sp. D |
0 |
0 |
8 |
3.0±1.0 |
|
Demospongiae |
“Lithistida” |
uncertain lithistid family |
Indeterminate lithistid sp. A |
0 |
0 |
1 |
0.4±0.4 |
|
Demospongiae |
“ceractinomorphs” |
Astroscleridae |
Astrosclera sp. |
0 |
0 |
3 |
1.1±0.6 |
|
Demospongiae |
“ceractinomorphs” |
Verticillitidae |
Vaceletia progenitor Pickett, 1982 |
0 |
0 |
2 |
0.7±0.5 |
|
Total |
365 |
|
269 |
|
|||
At the ordinal level (Fig. 6) 68.8±2.4% of the specimens belong to Hexactinosida in bulk sampling while the most abundant order in museum collection is Lychniscosida with 34.6±2.9%. At family level (Fig. 7) bulk sampling reveals Craticulariidae as the most abundant family (28.5±2.4%), followed by Cribrospongiidae (20.0±2.1%). Abundance distribution in the museum collections yield three dominant families: Craticulariidae, Stauractinellidae, and Camerospongiidae (all with 12.6±2.0%). The following families, recorded in museum collection, are absent in the bulk field sampling: Ventriculitidae, Astroscleridae, Scleritodermatidae, Verticillitidae.
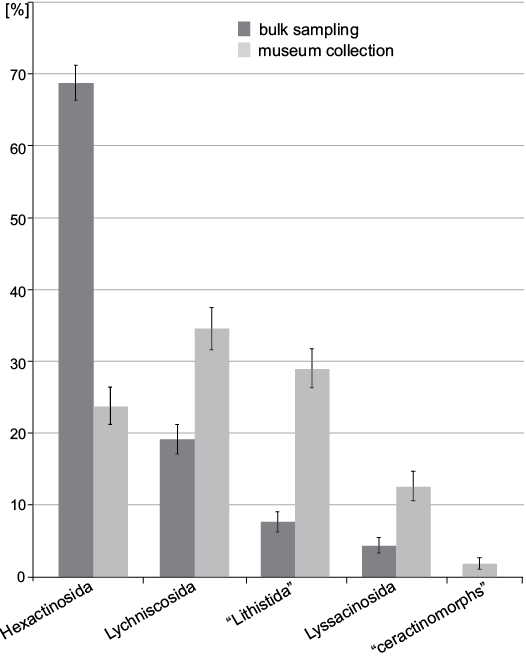
Fig. 6. Comparison between frequency of museum collections (data from Frisone et al. 2016) and bulk field sampling (this study) at the ordinal level. Error bars denote binomial standard errors of percentages.
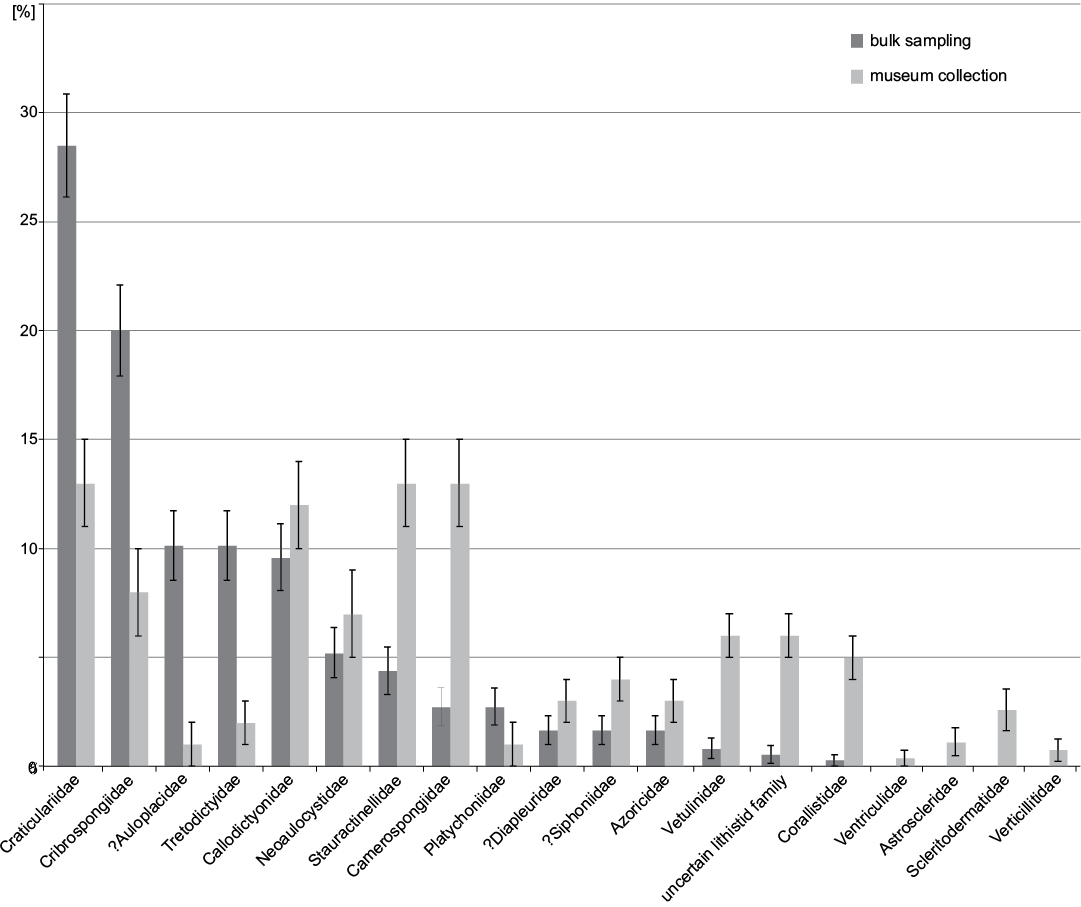
Fig. 7. Families and relative abundances frequencies from museum collections (data from Frisone et al. 2016) and bulk sampling (this study). Error bars denote binomial standard errors of percentages.
At the genus level, Laocoetis Pomel, 1872 is the most abundant genus in bulk sampling (28.5±2.4%) (Fig. 8) followed by Guettardiscyphia de Fromentel, 1860 (20.0±2.1%) and the hexactinosan Rigonia Frisone, Pisera, and Preto, 2016 (10.1±1.6%). In the museum collection, the most abundant genera are Stauractinella Zittel, 1877 and Laocoetis (both 12.6±2.0%), Callicylix Schrammen, 1912 (11.9±2.0%) and Camerospongia d’Orbigny, 1849 (10.8±1.9%).
In bulk field sampling, the most abundant species are all hexactinosan (Fig. 9). Laocoetis patula Pomel, 1872 is the most abundant species in the total assemblage (24.9±2.3%), followed by G. thiolati (20.0±2.1%) and Rigonia plicata Frisone, Pisera and Preto, 2016 (10.1±1.6%). Hexactinella clampensis Frisone, Pisera, and Preto, 2016 and the lychniscosan Callicylix eocenicus Pisera and Busquets, 2002 are equally frequent with both 9.6±1.5%. Ten taxa described in Frisone et al. (2016) were not observed in the bulk samples. Of these unobserved taxa, only one is a lychniscosidan hexactinellid (?Ventriculites sp.). All the others are demosponges: ?Corallistes sp., Jereopsis clavaeformis (Pomel, 1872), Verruculina ambigua (Pomel, 1872), Indeterminate rhizomorine sp. B, C, D, Indeterminate lithistid sp. A, Astrosclera sp. and Vaceletia progenitor. In museum collections, the most abundant species is the lyssacinosid Stauractinella eocenica Frisone, Pisera, and Preto, 2016 (12.6±2.0%), followed by the lychniscosid Callicylix eocenicus 11.9 ±2.0% and Laocoetis patula 11.2±1.9%. Overall, bulk sampling resulted in lower diversity and evenness estimates than museum materials. In terms of richness, 21 species were found in surface bulk samples, whereas 31 were found in the museum materials. The Shannon index (H) is also lower for bulk sampling (H = 2.32) than for museum collections (H = 2.91). Evenness (J) is similarly reduced in the bulk samples (J = 0.76) compared with the museum material (J = 0.84). Spearman’s rank-order correlation between bulk and museum species abundance is moderately high, but strongly significant (0.6, p < 0.001). The percent similarity (sum of minimum percentages, also known as Bray-Curtis similarity) between both samples is 53% and the Jaccard similarity (based on presence absence data) is 68%. The similarity between bulk and museum collections does not increase substantially with taxonomic level until the comparison is made at the level of classes. The Jaccard similarity is always greater than the Bray-Curtis similarity (Fig. 10). This suggests that comparisons between bulk sampling and museum are more reliable for presence-absence data than for relative abundance data.
When plotting the species abundance data in a rank-abundance (Whittaker) plot (Fig. 11), the distribution suggests a moderately complex community structure with a Mandelbrot distribution characterized by a relatively long tail of rare species (Magurran 2004). Although the museum data suggest an even simpler community structure (preemption = geometric series), the fit is similar for both model distributions based on Akaike’s Information Criterion (Akaike 2011).
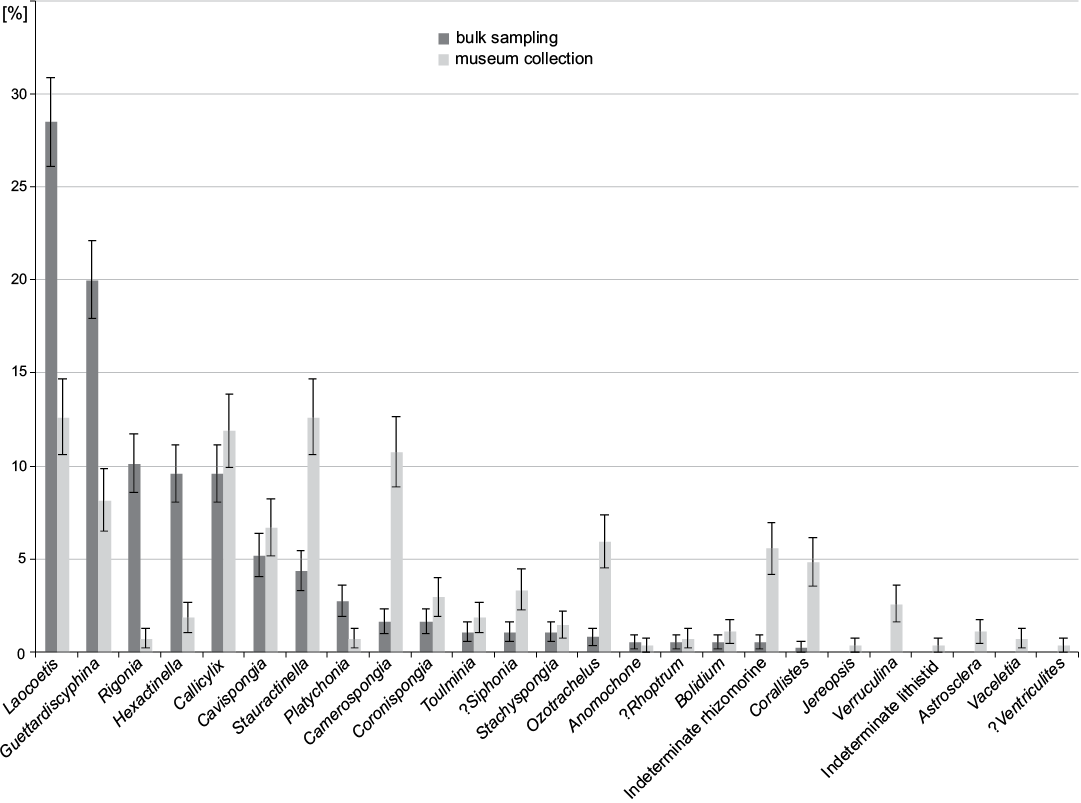
Fig. 8. Distribution of sponge genera from museum collections (data from Frisone et al. 2016) and bulk sampling (this study). Error bars denote binomial standard errors of percentages.
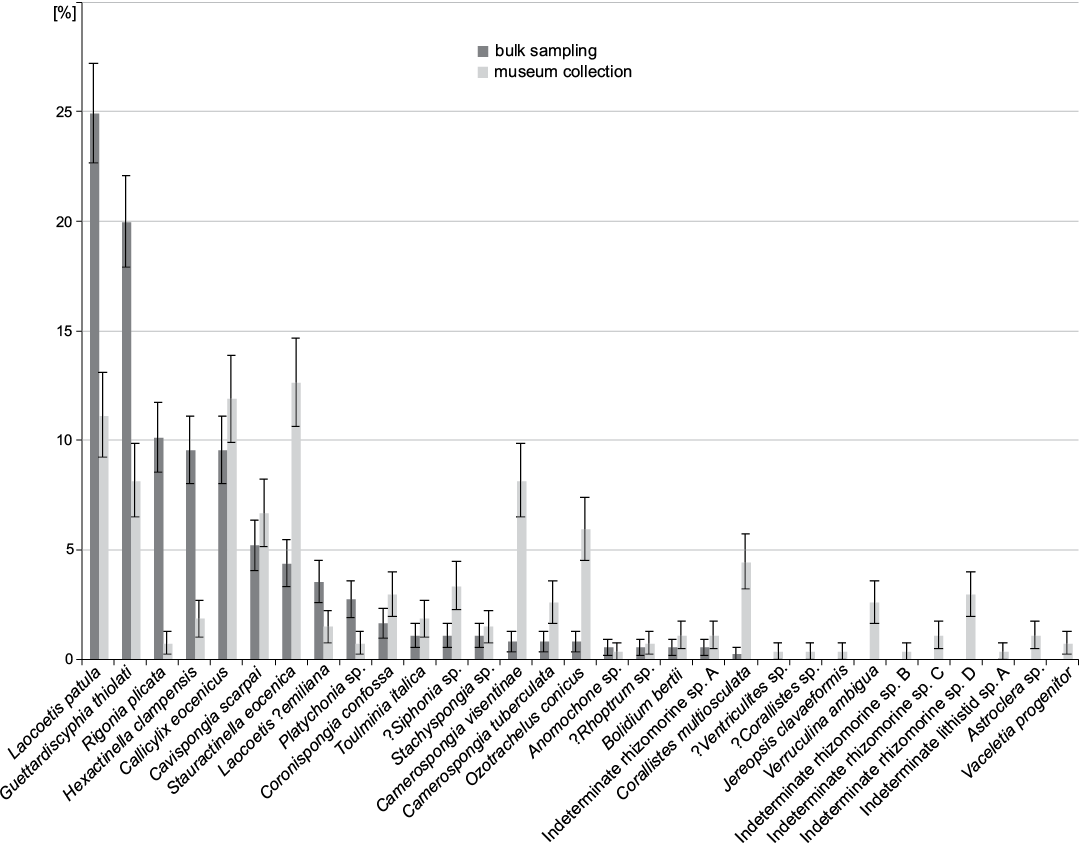
Fig. 9. Comparison between species frequency of museum collections (data from Frisone et al. 2016) and bulk sampling (this study). Error bars denote binomial standard errors of percentages.
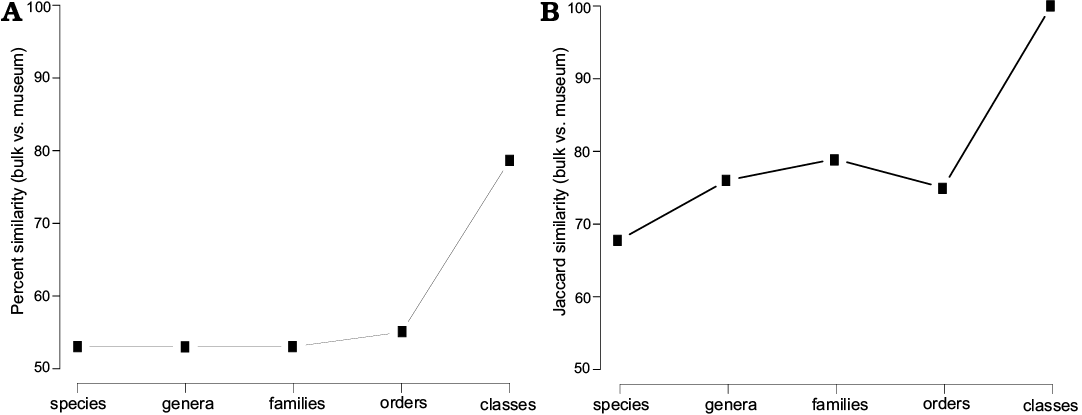
Fig. 10. Percent similarity (A) and Jaccard similarity (B) measurements between bulk and museum collection material at different taxonomic levels.
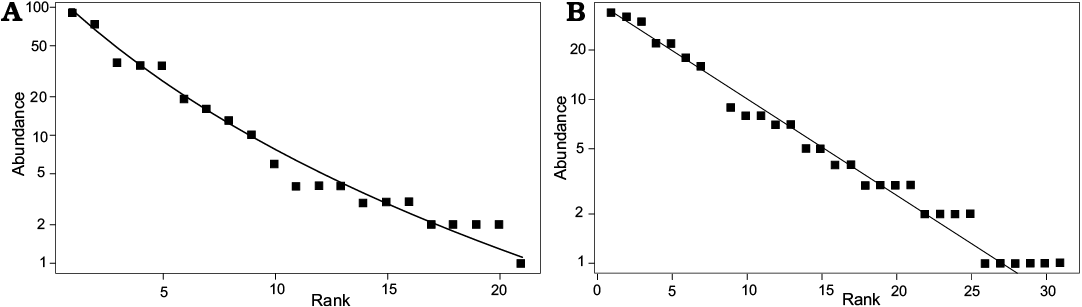
Fig. 11. Rank-abundance distribution of bulk samples (A) and museum collection (Whittaker plots) (B). Y axis shows species abundance; X axis ranks each species in order from most to least abundant. Black dots represent species.
Discussion
Effects of sampling bias.—Bias affects both bulk sampling and museum collections. For example, we suspect that there is a possible sampling bias, both in bulk sampling and museum collections, towards Laocoetis patula as its characteristic morphology with canal openings in quadrate arrangement is easier to recognize in the field (Fig. 12). In bulk sampling, L. patula is the most abundant species (24.9%) and in museum collection is the third common, with 11.2% relative abundance. On the contrary, in bulk sampling, lithistid sponges were difficult to spot in the field as their sponge nature is hardly recognizable in the scree as they may be confused with rubble and other bioclasts such as rhodoliths. Moreover, it is very hard to recognize desmas in unprepared hand specimens. For these reasons, we think that lithistid abundance may be underestimated in the bulk sampling. In museum collections, the most abundant species (12.6%) is the generally large (up to 20 cm wide) lyssacinosid Stauractinella eocenica. Probably its rounded shape and the large size particularly attracted collectors’ attention in the field. The second most abundant species in the museum collection (11.9%) is the lychniscosid Callicylix eocenicus, which is also quite large (up to 12 cm wide) and its globose shape and meandriform openings make it easily recognizable in the field. These observations suggest that unconscious selectivity towards larger taxa may have occurred during sampling of the museum material.
Museum material tends to have greater diversity and evenness than bulk samples. This could be explained by the fact that museum collections were formed by several years of sampling, generally from the 1970s to 1990s, when the quarries were still active and new material was continuously exposed at the surface. Sponge collectors for the museum material may also have focused on rare species that have not been collected before. This is a likely explanation of the greater richness of the museum fauna. For example, non-lithistid demosponges of the shallow water group “ceractinomorphs” (sensu Buckeridge et al. 2013), such as Vaceletia and Astrosclera, were probably not found in the bulk sampling because they are much rarer than suggested by the counts of museum specimens. Moreover, museum material comes from two quarries (Lovara and Cengio dell’Orbo) and was generally collected directly on the fossil horizon. The bulk sampling was instead performed at only one site, where the debris from the two quarries was possibly mixed with other debris. Despite these potential biases, the rank-order abundance distributions match significantly between the bulk sampling and museum collection efforts. The presence-absence data generally match better than the species-abundance data.

Fig. 12. In situ Eocene specimen of Laocoetis patula from Lovara Quarry, Italy; showing its characteristic morphology with canal openings in quadrate arrangement, indicated by the white arrow. Coin for scale is 22 mm in diameter.
Species abundance and paleoenvironmental interpretation.—With 62% of the species belonging to Hexactinellida, 38% to lithistids and no “ceractinomorphs”, the species counts in bulk sampling are more similar to Matteucci and Russo’s (2005) preliminary study of Chiampo sponges (74% Hexactinellida, 26% lithistids, and 0% “ceractinomorphs”) than to museum material (Frisone et al. 2016), with 45%, 48%, and 7%, respectively.
The bulk sampling results are also more in line with previous environmental interpretations based on sedimentology, associated fauna and sponge autoecology (Beccaro et al. 2001; Márton et al. 2011; Frisone et al. 2016). The most common extant sponge taxa at Chiampo inhabit rather deep water today: e.g., Laocoetis (24.9% in bulk sampling) and Hexactinella Carter, 1885 (9.6%). Laocoetis perion Lévi, 1986 is known from 250–750 m water depth in the Southern Indian Ocean (Lévi 1986; Tabachnick and Lévi 1997; Pisera and Tabachnick 2014). Hexactinella has a depth range of approximately 200–1200 m (Reiswig et al. 2008; Tabachnick 1990; Reiswig and Kelly 2011). Therefore, the Chiampo sponges probably inhabited a deeper shelf environment. The presence of the lyssacinosid Stauractinella further strengthens this hypothesis. With few exceptions, Recent lyssacinosids live in the bathyal or even abyssal zone (e.g., Janussen et al. 2004; Van Soest et al. 2007; Janussen and Reiswig 2009) and this environment is generally confirmed in the fossil record (among others Pisera and Busquets 2002; Świerczewska-Gładysz and Jurkowska 2013). The associated fauna of the embedding sediment is a mix of organisms both from the inner-middle and outer ramp. We suggest that the mixed shallow and deep-water character of the whole fauna in the sponge-rich horizons of Chiampo could be explained by the rapid burial of in situ deep-water sponges by skeletal-rich sediment, in part of shallow water provenance. Therefore, the dominance of hexactinellids in the in situ sponge fauna, together with the pelagic nature of some of the associated fauna (planktonic foraminifera, pteropods, shark teeth; Beccaro et al. 2001) point to an outer carbonate ramp depositional environment.
Our interpretation is at odds with the traditional interpretation of a shallow-water environment in Chiampo (e.g., Fabiani 1915; De Zanche 1965; Bosellini 1989) and hence the notion that the sponge assemblages from Val di Chiampo developed in a shallow-water paleoenvironment (Matteucci and Russo 2005, 2011).
A deeper water setting is, however, plausible because the Chiampo assemblage inhabited the Alpone-Agno graben, which was substantially deeper than the surrounding area and the studied sites have been interpreted to represent an outermost carbonate ramp (Beccaro et al. 2001). Both the nummulitic limestone and the volcaniclastic material show evidence of reworking in debris and turbidity flows (Márton et al. 2011). Our results strengthen the hypothesis of a deep-water setting.
We propose the following paleoenvironmental setting for the sponge assemblage: (i) the setting was the distal, oligophotic to aphotic part of a carbonate slope or ramp. (note that a ramp setting has been proposed by other authors (Beccaro et al. 2001; Márton et al. 2011)); (ii) the shallow water fauna observed in the sponge-horizon has been transported from the inner ramp and redeposited.
Conclusions
The sponge fossils from the Lutetian (middle Eocene) of Chiampo Valley show a high diversity. The diversity recorded by Frisone et al. (2016), however, based on museum collections, is likely affected by selective sampling for larger sponges. To explore the rank-abundance distribution of the sponge fauna more rigorously, we performed repeated surface bulk sampling near the original collection sites. We gathered 365 specimens that could be assigned to 21 taxonomic entities, usually species. The abundances in the bulk sampling collection differ from the museum-based material, which suggests that quantitative information from museum collections should be treated with caution. For instance, the most abundant taxa in museum collections are large and rounded. Museum-based material showed a greater evenness, together with higher values of Shannon diversity indexes. Despite differences, the sampling identity seems to be maintained, especially in its qualitative state.
In terms of abundance, bulk sampling revealed hexactinellids to be strongly dominant over lithistids, which points to a deep-water setting. Additional evidence supports an outer carbonate ramp depositional environment of the assemblage: (i) the most common extant genera dwell today in rather deep water; (ii) shallow water “ceractinomorphs” (sensu Buckeridge et al. 2013) non-lithistid demosponges are absent; (iii) sedimentological evidence and composition of the associated fauna (e.g., planktonic foraminifera and pteropods) also point to a deep-water environment.
Acknowledgements
We are deeply indebted to the following professional and amateur palaeontologists who made this study possible: Antonio De Angeli, Claudio Beschin, Ermanno Quaggiotto (all Associazione Amici del Museo “G. Zannato”, Montecchio Maggiore, Italy), and Valentina Carpanese (Museo Padre Aurelio Menin, Chiampo, Italy). We also highly appreciate the help of many colleagues: Paolo Mietto (local geology expertise), Claudia Agnini (nannofossil expertise), Luca Giusberti (benthic foraminifera expertise), Stefano Castelli (photos) (all Padova University, Italy) and Andrea Papazzoni (larger foraminifera expertise) (Modena and Reggio Emilia University, Italy). We thank Nadia Spaliviero and students from GeoZentrum Nordbayern Erlangen, Germany for their help in bulk field sampling. The manuscript also benefitted from the constructive comments of Ewa Świerczewska-Gładysz (University of Łódź, Poland) and Michelle Kelly (National Institute of Water and Atmospheric Research, Auckland, New Zealand). Comune di Montecchio Maggiore gave financial support to VF for attending the 10th Word Sponge Conference, National University of Ireland, Galway, 25–30 June 2017.
References
Agostini, A. 1991. La spongiofauna eocenica della Valle di Chiampo (Lessini orientali, Vicenza). 78 pp. Unpublished MS thesis, Università degli Studi di Modena, Modena.
Akaike, H. 2011. Akaike’s information criterion. In: M. Lovric (ed.), International Encyclopedia of Statistical Science, 25. Springer, Berlin. Crossref
Barbieri, G., De Zanche, V., and Sedea, R. 1991. Vulcanesimo paleogenico ed evoluzione del semigraben Alpone-Agno (Monti Lessini). Rendiconti della Società Geologica Italiana 14: 5–12.
Barbieri, G., De Zanche, V., Medizza, F., and Sedea, R. 1982. Considerazioni sul vulcanismo terziario del Veneto occidentale e del Trentino meridionale. Rendiconti della Società Geologica Italiana 4: 267–270.
Bassi, D., Bianchini, G., Mietto, P., and Nebelsick, J. H. 2008. Southern Alps in Italy: Venetian Pre-Alps. In: T. McCain (ed.), The Geology of Central Europe. Volume 2, Mesozoic and Cenozoic, 1087–1092. The Geological Society of London, London.
Beccaro, L. 2003. Revisioni stratigrafiche nel Paleocene del Veneto occidentale. 200 pp. Unpublished Ph.D. Thesis, Università degli Studi di Padova, Padova.
Beccaro, L., Fornaciari, E., Mietto, P., and Preto, N. 2001. Analisi di facies e ricostruzione paleoambientale dei ‘Calcari nummulitici’ Eocene (Monti Lessini orientali – Vicenza): dati preliminari. Studi Tridentini di Scienze Naturali – Acta Geologica 76 (for 1999): 3–16.
Beschin, C., Busulini, A., De Angeli, A., Tessier, G., and Ungaro, S. 1991. Due nuovi generi di Raninidae dell’Eocene del Veneto (Italia). Lavori – Società Veneziana di Scienze Naturali 16: 187–212.
Beschin, C. De Angeli, A., Checchi, A., and Zarantonello, G. 2016. Crostacei decapodi del “tufo a Lophoranina” (Luteziano Inferiore) della Valle del Chiampo (Vicenza – Italia Nordorientale). 92 pp. Città di Montecchio Maggiore, Museo di Archeologia e Scienze naturali “G. Zannato”, CTO, Vicenza.
Bosellini, A. 1989. Dynamics of Tethyan Carbonate Platform. In: P.D. Crevello, L.W. James, J.F. Sarg, and J.F. Read. (eds.), Controls on Carbonate Platform and Basin Platform. Society of Economic Paleontologists and Mineralogists, Special Publication 44: 3–13. Crossref
Bosellini, F.R. and Papazzoni, C.A. 2003. Palaeoecological significance of coral-encrusting foraminiferan associations: a case-study from the Upper Eocene of northern Italy. Acta Palaeontologica Polonica 48: 279–292.
Buckeridge, J.S., Kelly, M., Janussen, D., and Reiswig, H.M. 2013. New Palaeogene sponges from the Red Bluff Tuff, Chatham Island, New Zealand. New Zealand Journal of Geology and Geophysics 56 (3): 171–185. Crossref
De Zanche, V. 1965, Le microfacies eoceniche nella Valle del Chiampo tra Arzignano e Mussolino (Lessini orientali). Rivista Italiana di Paleontologia e Stratigrafia 71: 925–948.
Fabiani, R. 1915. Il Paleogene del Veneto. Memorie dell’Istituto di Geologia della Regia Università di Padova 3: 1–336.
Fabiani, R. 1930. Le risorse del sottosuolo della provincia di Vicenza. 150 pp. Industria della stampa G. Peronato, Vicenza.
Frisone, V., Pisera, A., and Preto, N. 2016. A highly diverse siliceous sponge fauna (Porifera: Hexactinellida, Demospongiae) from the Eocene of northeastern Italy: systematics and palaeoecology. Journal of Systematic Palaeontology 14: 949–1002. Crossref
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: 1–9.
Janussen, D. and Reiswig, H.M. 2009. Hexactinellida (Porifera) from the ANDEEP III Expedition to the Weddell Sea, Antarctica. Zootaxa 2136: 1–20.
Janussen, D., Tabachnick, K.R., and Tendal, O.S. 2004. Deep-sea Hexactinellida (Porifera) of the Weddell Sea. Deep Sea Research Part II: Topical Studies in Oceanography 51: 1857–1882. Crossref
Lévi, C. 1986. Laocoetis perion nov. sp., Spongiaire Hexactinellide Craticulariidae de l’océan Indien. Bulletin du Muséum National d’Histoire Naturelle de Paris , Section A , Zoologie, biologie et écologie animales 8: 437–442.
Magurran, A.E. 2004. Measuring Biological Diversity. 256 pp. Blackwell, Oxford.
Márton, E., Zampieri, D., Kázmér, M., Dunkl, I., and Frisch, W. 2011. New Paleocene–Eocene paleomagnetic results from the foreland of the Southern Alps confirm decoupling of stable Adria from the African plate. Tectonophysics 50: 89–99. Crossref
Matteucci, R. and Russo, A. 2005. The Middle Eocene siliceous sponges from Val di Chiampo (Lessini Mountains, northen Italy). Annali dell’Università di Ferrara, Sezione Museologia Scientifica e Naturalistica Volume speciale: 51–62.
Matteucci, R. and Russo, A. 2011. The Italian Cenozoic siliceous sponges: a review, with a revision of the Catullo (1856) collection. Journal of Mediterranean Earth Sciences 3: 33–43.
Menin, A. 1972. Silicosponge dell’Eocene medio della Valle del Chiampo (M. Lessini, Vicenza). Annali dell’Università di Ferrara (nuova serie), Scienze Geologiche e Paleontologiche 5: 63–69.
Oksanen, J., Blanchet, F.G., Kindt, R., Legendre, P., Minchin, P.R., O’Hara, R.B., Simpson, G.L., Solymos, P., Henry, M.H. Stevens, and Wagner, H. 2014. Vegan: Community Ecology Package. R package version 2.2-0. http://CRAN.R-project.org/package=vegan. Accessed 12 April 2017.
Pisera, A. and Busquets, P. 2002. Eocene siliceous sponges from the Ebro Basin (Catalonia, Spain). Geobios 35: 321–346. Crossref
Pisera, A. and Tabachnick, K. 2014. The sponge genus Laocoetis Pomel, 1972 (Hexactinosida, Craticulariidae): a living fossil? In: E. Cerdeño (compiler), 4th International Paleontological Congress, The history of life: A view from the Southern Hemispere, Mendoza Sept. 28–Oct. 3, 2014. 936 pp. IANIGLA, CCT-CONICET, Mendoza.
R Development Core Team. 2018. R: A Language and Environment for Statistical Computing, Version 3.5.1. The R Foundation for Statistical Computing, Vienna, https://www.R-project.org/.
Reiswig, H.M. and Kelly, M. 2011. The Marine Fauna of New Zealand: Hexasterophoran Glass Sponges of New Zealand (Porifera: Hexactinellida: Hexasterophora): Orders Hexactinosida, Aulocalycoida and Lychniscosida. NIWA Biodiversity Memoir 124: 1–176.
Reiswig, H.M., Dohrmann, M., Pomponi, S., and Wörheide, G. 2008. Two new tretodictyids (Hexactinellida: Hexactinosida: Tretodictyidae) from the coast of North America. Zootaxa 1721: 53–64.
Świerczewska-Gładysz, E. and Jurkowska, A. 2013. Occurrence and paleoecological significance of lyssacinosid sponges in the Upper Cretaceous deposits of southern Poland. Facies 59: 763–777. Crossref
Tabachnick, K.R. 1990. Hexactinellid sponges from the South-East Atlantic Ocean. Transactions of the Institute of Oceanology, Academy of Sciences of the USSR 126: 67–73.
Tabachnick, K.R. and Lévi, C. 1997. Les Craticulariidae sont des spongiaires Hexactinellida Scopularia. Zoosystema 19: 7–14.
Van Soest, R.W.M., Van Duyl, F.C., Maier, C., Lavaleye, M.S.S., Beglinger, E., and Tabachnick, K.R. 2007. Mass Occurrence of the hexactinellid Rossella nodastrella Topsent in bathyal coral reefs of Rockall Bank, W Of Ireland (Lyssacinosida, Hexactinellida). In: M.R. Custódio, G. Lôbo-Hajdu, E. Hajdu, and G. Muricy (eds.), Porifera Research: Biodiversity, Innovation and Sustainability, 6–13. Museu Nacional, Rio de Janeiro.
Acta Palaeontol. Pol. 63 (4): 795–805, 2018
https://doi.org/10.4202/app.00474.2018