

Quail-thrush birds from the Miocene of northern Australia
JACQUELINE M.T. NGUYEN, MICHAEL ARCHER, and SUZANNE J. HAND
Nguyen, J.M.T., Archer, M., and Hand, S.J. 2018. Quail-thrush birds from the Miocene of northern Australia. Acta Palaeontologica Polonica 63 (3): 493–502.
Quail-thrushes (Passeriformes: Cinclosomatidae: Cinclosoma) are ground-dwelling corvoid songbirds endemic to Australia and New Guinea. Until now, the only known quail-thrush fossils have been from late Quaternary cave deposits in Australia. A new species of quail-thrush, Cinclosoma elachum sp. nov., is described from the early to middle Miocene deposits in the Riversleigh World Heritage Area, Queensland, Australia. A second, larger quail-thrush is identified from the middle Miocene of Riversleigh. The new fossils considerably extend the geographic and temporal ranges of cinclosomatids, and indicate the presence of two species of quail-thrushes in the Miocene of northern Australia, located more than 300 km from the nearest extant member of Cinclosoma. These fossils provide a minimum age of ~18 million years for Cinclosomatidae. They cannot be confidently assigned to the crown group of the genus Cinclosoma, but can be used to calibrate the split between this genus and Ptilorrhoa in molecular dating studies of the Corvides radiation and Passeriformes overall. This material also adds to the growing diversity of songbirds identified from the pre-Pleistocene record of Australia, and reemphasises the global significance of the Riversleigh deposits in developing understanding about the early evolutionary history of passerines.
Key words: Aves, Passeriformes, Cinclosomatidae, Cinclosoma, Miocene, Australia, Riversleigh.
Jacqueline M.T. Nguyen [jacqueline.nguyen@austmus.gov.au], Australian Museum Research Institute, Australian Museum, 1 William Street, Sydney, New South Wales 2010, Australia; PANGEA Research Centre, School of Biological, Earth and Environmental Sciences, UNSW Sydney, Sydney, New South Wales 2052, Australia.
Michael Archer [m.archer@unsw.edu.au] and Suzanne J. Hand [s.hand@unsw.edu.au], PANGEA Research Centre, School of Biological, Earth and Environmental Sciences, UNSW Sydney, Sydney, New South Wales 2052, Australia.
Received 9 April 2018, accepted 22 April 2018, available online 9 July 2018.
Copyright © 2018 J.M.T. Nguyen et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Cinclosomatidae (Passeriformes: Corvides) is an Australo-Papuan oscine (songbird) family that contains eight species of quail-thrushes Cinclosoma and four species of jewel-babblers Ptilorrhoa (Toon et al. 2012; Dickinson and Christidis 2014). These birds are medium-sized, stockily-built ground birds that use their bills to probe sand and leaf litter in search of arthropods and small vertebrates (Schodde and Mason 1999; Boles 2007). The Painted Quail-thrush Cinclosoma ajax (Temminck in Temminck and Meffren Laugier, 1836) and all four species of jewel-babblers are endemic to New Guinean forests from the lowlands to the highlands (Boles 2007; Beehler and Pratt 2016). The remaining seven species of Cinclosoma are confined to Australia and prefer more open habitats with low vegetation, including dry sclerophyll forest and woodland, mallee, dry woodland scrub and other scrublands, and stony plains and tablelands with low scrub cover (Higgins and Peter 2002; Boles 2007). The Spotted Quail-thrush Cinclosoma punctatum (Shaw, 1794) is found in mesic habitats in south-eastern Australia. In contrast, the other Australian species of Cinclosoma occur in the arid and semi-arid zones of the western, central, and southern parts of the continent (Higgins and Peter 2002; Boles 2007).
Quail-thrushes and jewel-babblers are superficially similar in appearance to their English namesakes, thrushes and babblers, but are not closely related to them. These birds were previously grouped into a “scrap basket” (Mayr and Amadon 1951) of terrestrial birds, such as logrunners Orthonyx, rail-babblers Eupetes, whipbirds and wedgebills Androphobus and Psophodes, and melampittas Melampitta (e.g., Deignan 1964; Higgins and Peter 2002; Boles 2007). Molecular studies subsequently demonstrated that Cinclosoma and Ptilorrhoa constitute a separate lineage (Norman et al. 2009; Jønsson et al. 2011) and have accordingly been treated as a distinct family, the Cinclosomatidae (Dickinson and Christidis 2014). A close relationship between Cinclosoma and Ptilorrhoa is also supported by osteological evidence (Baird 1986, 1991). Phylogenetic analyses based on molecular data have variously shown Cinclosomatidae as the sister taxon to all other members of the infraorder Corvides (Selvatti et al. 2015; Moyle et al. 2016); sister taxon to Falcunculidae (shriketits) (Jønsson et al. 2016); sister taxon to a clade comprising Paramythiidae (painted berrypickers), Androphobus, and Psophodes (Jønsson et al. 2011); or in an unresolved position within Corvides (Norman et al. 2009). A phylogenetic study of nuclear loci by Aggerbeck et al. (2014) placed Cinclosoma as the sister taxon to Falcunculus.
Until now, the only known cinclosomatid fossils were from Quaternary cave deposits in Australia. These include Holocene and Late Pleistocene specimens of Cinclosoma punctatum from the Buchan and Glenelg River areas in Victoria (Baird 1991, 1992, 1993), and Holocene specimens of the Nullarbor Quail-thrush C. alisteri Mathews, 1910 from Weekes Cave in South Australia (Baird 1990). Fossils of C. alisteri and Cinclosoma sp. cf. C. alisteri have also been found in Quaternary caves deposits in the Nullarbor region in Western Australia (Baird 1991). Late Quaternary fossils referable to Cinclosoma sp. have been reported from Mair’s Cave in South Australia (Baird 1991). Here we describe a new Miocene species of Cinclosoma and material referable to a second species of the same genus. These new specimens considerably extend the fossil record of cinclosomatids both temporally and geographically, and add to the growing diversity of fossil songbirds identified from the pre-Pleistocene record of Australia.
Institutional abbreviations.—AM, Australian Museum, Sydney, Australia; ANWC, Australian National Wildlife Collection, CSIRO, Canberra, Australia; NMV, Museums Victoria, Melbourne, Australia; QM, Queensland Museum, Brisbane, Australia.
Other abbreviations.—lig., ligamentum; M./m., musculus; proc., processus; tub., tuberculum.
Material and methods
Direct comparisons were made with skeletons of extant cinclosomatid species in the ornithology collections of the AM, ANWC, and NMV. Comparisons were also made with extant species of Psophodes, which are grouped with Cinclosoma, Ptilorrhoa, and Androphobus in a single family Psophodidae by Gill and Donsker (2018). Specimens of the basally-branching Cinclosoma ajax and the monotypic psophodid Androphobus viridis (Rothschild and Hartert, 1911) were unavailable for study.
Cinclosomatidae: Ptilorrhoa leucosticta (Sclater, 1874), Spotted Jewel-babbler (ANWC 24225); Ptilorrhoa caerulescens (Temminck in Temminck and Meffren Laugier, 1836), Blue Jewel-babbler (ANWC PASS-1423); Cinclosoma punctatum (Shaw, 1794), Spotted Quail-thrush (AM O.58023, AM O.67887, AM O.68471, AM O.70739, NMV B.12648, NMV B.12872); Cinclosoma castanotum Gould, 1840, Chestnut Quail-thrush (NMV B.17817); Cinclosoma castaneothorax Gould, 1849, Chestnut-breasted Quail-thrush (NMV B.10899); Cinclosoma cinnamomeum Gould, 1846, Cinnamon Quail-thrush (NMV B.8986, NMB B. 12004).
Psophodidae: Psophodes olivaceus (Latham, 1801), Eastern Whipbird (AM O.58056, AM O.58057, AM O.71374); Psophodes cristatus (Gould, 1838), Chirruping Wedgebill (AM O.65849).
The fossils described here are registered in the Queensland Museum palaeontology collection (QM F). Measurements of specimens were made with digital callipers accurate to 0.01 mm and rounded to the nearest 0.1 mm. Taxonomic nomenclature follows Dickinson and Christidis (2014), with the exception of C. alisteri (sensu Toon et al. 2012) and C. clarum Morgan, 1926 (sensu Dolman and Joseph 2015), which are treated as separate species from C. cinnamomeum and C. castanotum, respectively. Osteological terminology follows Baumel and Witmer (1993). Nomenclature for ligaments and musculature follow Baumel and Raikow (1993) and Vanden Berge and Zweers (1993), respectively.
Systematic palaeontology
Order Passeriformes Linnaeus, 1758
Suborder Passeri Sibley and Ahlquist, 1990
Infraorder Corvides Cracraft, 2014
Family Cinclosomatidae Mathews, 1922
Genus Cinclosoma Vigors and Horsfield, 1827
Type species: Cinclosoma punctatum (Shaw, 1794); region of Port Jackson, New South Wales, Australia; Recent.
Remarks.—The fossils are referred to Cinclosoma because they possess the following combination of features. Carpometacarpus: ventral portion of trochlea carpalis does not protrude far caudally beyond proximo-caudal edge of os metacarpale minus; fovea lig. ventralis large and deep; proc. cranialis located at level with opening situated proximally of proc. intermetacarpalis; depression on caudo-ventral surface of trochlea carpalis and os metacarpale minus deep; sulcus tendinosus wide and shallow; proc. dentiformis long and situated distal to the proximo-distal midpoint of os metacarpale majus, based on proportions of proximal end and os metacarpale majus. Tibiotarsus: stout, shaft immediately proximal to the distal end is medio-laterally wide (compared with, e.g., Psophodes); tuberositas retinaculi extensori medialis well developed and separated from its lateral counterpart by a distance of less than length of former; tuberositas retinaculi extensori lateralis prominent, situated on lateral part of pons supratendineus and extends proximally; bony ridges for attachment of retinaculum m. fibularis extend proximally to about level with tuberositas retinaculi extensori medialis; pons supratendineus length about equal to width; condyli lateralis et medialis about equal in proximal and distal extent; impressio lig. intercondylaris very shallow.
Passerines are osteologically very similar to each other, which can make it challenging to diagnose subclades based on skeletal features. However, species of Cinclosoma can be diagnosed by the following apomorphy: on the carpometacarpus, the proc. cranialis is situated distally of the fovea carpalis caudalis, to about level with the opening located proximally of the proc. intermetacarpalis. In species of Ptilorrhoa and Menura, the proc. cranialis is located distally of the fovea carpalis caudalis but proximally of the opening. In acanthisittids, the proc. cranialis is far distally and is situated at approximately the level of the proc. intermetacarpalis.
The fossils differ from species of Ptilorrhoa by exhibiting the following traits. Carpometacarpus: ventral portion of trochlea carpalis more rounded and does not protrude as far beyond proximo-caudal edge of os metacarpale minus; viewed cranially, proc. extensorius more bulbous and expanded ventrally; fovea carpalis caudalis deeper; viewed dorsally, opening located proximally of proc. intermetacarpalis widely open; proc. dentiformis situated further distally relative to midpoint of os metacarpale majus. Tibiotarsus: tuberositas retinaculi extensori medialis more prominent; bony ridges for retinaculum m. fibularis slightly longer and distally terminate at about level with pons supratendineus, rather than proximally of the pons; condylus medialis less inflected medially; condylus medialis about equal, rather than greater than, in distal extent to condylus lateralis.
Because species of Cinclosoma and Ptilorrhoa are sometimes included in Psophodidae (Gill and Donsker 2018), we have also included here comparisons of the fossils with species of Psophodes. The fossils are distinguished from species of Psophodes in several features. Carpometacarpus: ventral portion of trochlea carpalis only slightly protrudes beyond proximo-caudal edge of os metacarpale minus; proc. cranialis situated further distally; proximal end of sulcus tendinosus is further distally located on os metacarpale majus, rather than distally adjacent of proc. alularis; proc. dentiformis relatively larger and not located at midpoint of os metacarpale majus but further distally; opening situated proximally of proc. intermetacarpalis widely open, rather than a narrow slit; carpometacarpus less curved dorso-ventrally. Tibiotarsus: distal end narrower relative to width of shaft; tuberositates retinaculorum extensorium relatively longer; tuberositas retinaculi extensori lateralis more prominent and occupies the lateral part of pons supratendineus, rather than only the proximo-lateral part of the pons; bony ridges for retinaculum m. fibularis relatively longer; impressio lig. intercondylaris much shallower; distal profile between condyles shallower. Specimens of Androphobus viridis were not available for study, but can be distinguished from C. elachum sp. nov. in its smaller size (Boles 2007).
Cinclosoma elachum sp. nov.
Fig. 1.
Etymology: From Latinised Greek elachum, little; neuter in gender. It is suggested that this new species be informally known as the Little Quail-Thrush.
Type material: Holotype: QM F57949, right carpometacarpus with distal end broken off. Paratype: QM F57951, distal left tibiotarsus from Neville’s Garden Site, Riversleigh World Heritage Area, Queensland, Australia.
Type locality: Wayne’s Wok Site, Riversleigh World Heritage Area, Queensland, Australia.
Type horizon: Wayne’s Wok Site is located on Hal’s Hill in the central section of the D Site Plateau at Riversleigh. Neville’s Garden Site occurs on the northern edge of this plateau (Creaser 1997). Wayne’s Wok and Neville’s Garden Local Faunas are part of Riversleigh’s Faunal Zone B and are interpreted to be early Miocene in age based on biocorrelation, stratigraphy, geochronology, and multivariate analyses (Archer et al. 1989; Creaser 1997; Travouillon et al. 2006; Arena et al. 2015, Woodhead et al. 2016). The two assemblages are interpreted as samples of the same early Miocene Riversleigh palaeocommunity by Myers et al. (2017). The early Miocene age estimate was confirmed by U/Pb radiometric dating of fossil speleothems at Neville’s Garden Site (18.24 ± 0.29 Ma and 17.85 ± 0.13 Ma; Woodhead et al. 2016).
Material.—Type material and tentatively referred specimen (QM F57948), a proximal left humerus from Neville’s Riches Site, Riversleigh World Heritage Area, Queensland, Australia. Neville’s Riches Site is tentatively assigned to Faunal Zone C (middle Miocene) until more taxa from this site are recovered and studied to allow confident biocorrelation (Archer et al. 1989).
Diagnosis.—Slightly smaller in size than Cinclosoma cinnamomeum, the smallest living species of this genus (Higgins and Peter 2002; Boles 2007). The proximal length and width of the carpometacarpus of Cinclosoma elachum sp. nov. is about 15% and 10% smaller, respectively, than the corresponding bone of C. cinnamomeum. The distal width of the tibiotarsus of the new species is about 18% narrower than that of C. cinnamomeum. The new species is distinguished from extant species of Cinclosoma that were examined in this study in the following features. Carpometacarpus: dorsal portion of trochlea carpalis proportionately shorter; fovea carpalis caudalis markedly deeper; sulcus tendinosus terminates further proximally. Tibiotarsus: shaft more cranio-caudally compressed; condyli slightly narrower; distal edge of pons supratendineus at about level with proximal edges of condyles, rather than being slightly proximally located.
Differs from C. punctatum in having a more proximally located proc. cranialis; a larger proc. dentiformis; a relatively shorter fovea lig. ventralis; a shallower sulcus extensoris; a narrower sulcus m. fibularis; and a more distinct ridge on trochlea cartilaginis tibialis.
Differs from C. castanotum in having a deeper fossa infratrochlearis; a deeper fovea lig. ventralis; a larger opening located proximally of proc. intermetacarpalis; longer and more prominent bony ridges for the retinaculum m. fibularis; a tuberositas retinaculi extensori lateralis that extends further proximally beyond the edge of the pons supratendineus; and a shallower impressio lig. intercondylaris.
Differs from C. castaneothorax in having a relatively shorter fovea lig. ventralis; a more developed proc. cranialis; a longer proc. intermetacarpalis; a larger proc. dentiformis; a relatively longer tuberositas retinaculi extensori medialis; longer bony ridges for retinaculum m. fibularis; and a deeper impressio lig. intercondylaris.
Differs from C. cinnamomeum in having a more proximally located proc. cranialis; a larger opening situated proximally of proc. intermetacarpalis; a more prominent proc. dentiformis that is situated slightly more proximally on the os metacarpale majus; more prominent bony ridges for the retinaculum m. fibularis; and a tuberositas retinaculi extensori lateralis that extends further proximally beyond the edge of the pons supratendineus.
Although specimens of C. ajax were not available for study, it is specifically distinct from C. elachum sp. nov. in its larger size (Boles 2007).
Measurements (in mm).—QM F57949, carpometacarpus: preserved length 9.2; proximal width 3.8; proximal length 2.7; length of os metacarpale alulare 2.1. QM F57951, tibiotarsus: preserved length 14.4; distal width c. 3.3; depth of condylus lateralis >2.9; depth of condylus medialis > 3.1.
Description.—QM F57949 (Fig. 1A) is a proximal right carpometacarpus with abrasion to the ventral portion of the trochlea carpalis and the proc. pisiformis and breakage of the distal end. QM F57951 (Fig. 1C) preserves the distal end and shaft of a left tibiotarsus that is partly covered with black mineral dendrites and has minor breakage to the cristae trochleae. The fossils appear to represent adult individuals because they do not show signs of incomplete ossification, such as pitted appearance of the bone surfaces and visible fusion lines, as observed in immature birds (Campbell 1979; Watanabe and Matsuoka 2013). In addition to the traits that characterise cinclosomatids and those used to diagnose the new species, Cinclosoma elachum sp. nov. exhibits the following features.
The proximal end of the carpometacarpus (QM F57949) is broad and bears a moderately deep fossa infratrochlearis. Viewed cranially, the trochlea groove is very shallow. The dorsal portion of the trochlea carpalis is long. Although the apex of the proc. pisiformis is broken, its base indicates that it is broad like in extant species of Cinclosoma examined. The depression on the caudal surface of the os metacarpale minus and caudo-ventral side of the trochlea carpalis is distinctly deep and terminates proximally of the proc. pisiformis. The proc. cranialis at the proximal end of the sulcus tendinosus is low. As in other cinclosomatids, the proc. intermetacarpalis is completely fused to the os metacarpale minus and does not protrude beyond its caudal edge. At about level with the proc. intermetacarpalis is a low protuberance on the os metacarpale minus. The proc. dentiformis is triangular in cranial profile. The os metacarpale minus is incompletely preserved but, based on its proportions and those of the proximal end, the proc. dentiformis appears to be situated well distally of its midpoint.
The cranial and caudal shaft surfaces of the tibiotarsus (QM F57951) are planar. The sulcus extensorius is very shallow. The tuberositas retinaculi extensori medialis is well developed and slightly overhangs the sulcus extensorius. On the cranio-lateral surface of the shaft there are two long ridges that serve as attachment points for the retinaculum m. fibularis. These ridges distally terminate at about level with the pons supratendineus. The lateral bony ridge for this retinaculum is longer than its medial companion. The pons supratendineus is slightly angled disto-laterally. The tuberositas retinaculi extensori lateralis is situated on the lateral part of the pons and extends well proximally beyond the edge of the pons. There is a large foramen immediately proximally of the condylus lateralis. The condyles of the tibiotarsus are long in cranial view. There is a pronounced bump on the condylus medialis, located proximally of the low epicondylus medialis. Viewed laterally, the condylus lateralis is circular in shape and the epidondylus lateralis is indistinct. There is a low but distinct proximo-distal ridge that is centred on the trochlea cartilaginis tibialis. This ridge extends along the caudal and distal surfaces of the trochlea. The presence of this ridge is intraspecifically variable in the extant species of Cinclosoma examined. The cristae trochleae are damaged in the fossil but, from what is preserved, the trochlea appears to have been wide as in extant cinclosomatids. Viewed distally, the condyles converge caudally. The condylus medialis protrudes further cranially than its lateral counterpart.
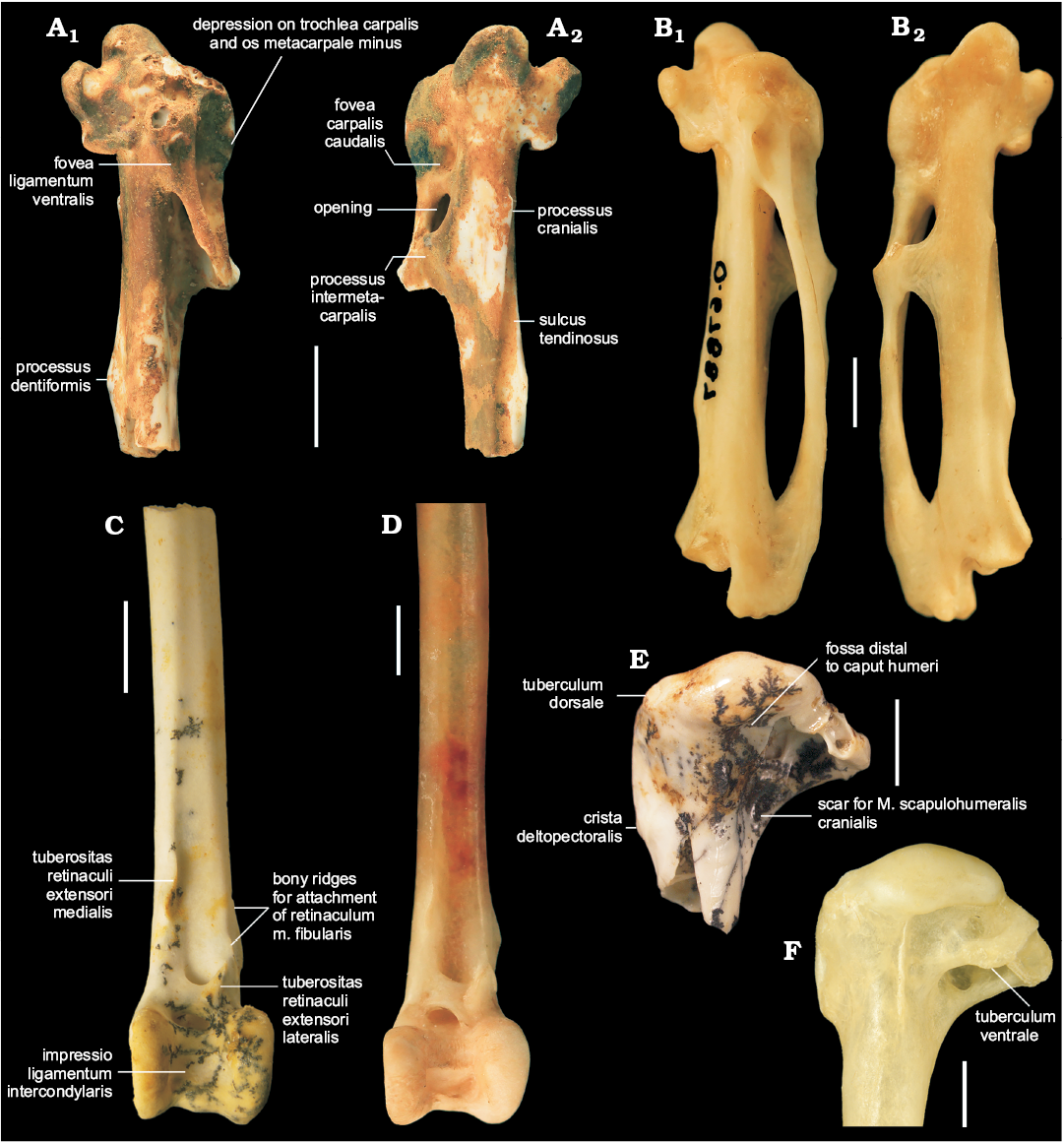
Fig. 1. Quail-thrush bird Cinclosoma elachum sp. nov. (A, C, E) from the Riversleigh World Heritage Area, Australia, compared with the Recent Cinclosoma punctatum (Shaw, 1794) (B, D, F). A. QM F57949 (holotype), early Miocene Wayne’s Wok Site, right carpometacarpus in ventral (A1) and dorsal (A2) views. B. AM O.67887, left carpometacarpus (mirrored) in ventral (B1) and dorsal (B2) views. C. QM F57951 (paratype), early Miocene Neville’s Garden Site, distal left tibiotarsus in cranial view. D. AM O.67887, distal left tibiotarsus in cranial view. E. QM F57948, middle Miocene Neville’s Riches Site, proximal left humerus in caudal view. F. NMV B.12648, proximal left humerus in caudal view. Scale bars 2 mm.
QM F57948 (Fig. 1E) is a proximal left humerus with damage to the tub. ventrale, crus dorsale fossae, and incisura capitis region. The pneumotricipital fossa area is encrusted with black mineral dendrites. The fossil humerus is 8.7 mm long and 7.0 mm wide, and its crista deltopectoralis is 4.0 mm long proximo-distally. QM F57948 shares the following features with species of Cinclosoma: caput humeri broad and dome-like; tub. dorsale large and directed caudo-dorsally; crista deltopectoralis reduced; crista bicipitalis moderately short; ventral pneumotricipital fossa pneumatic; scar for insertion of M. scapulohumeralis cranialis shallow; and shallow fossa distal to the caput humeri and ventrally bound by margo caudalis. In extant species of Cinclosoma studied, the crista bicipitalis extends far ventrally of the tub. ventrale. In the fossil, the tub. ventrale is broken off but the preserved edge of the crista bicipitalis protrudes well ventrally of this region. The proximal profile of the ventral part of the caput humeri is flat in the fossil, but this feature was observed to be intraspecifically variable in specimens of extant species of Cinclosoma.
QM F57948 differs from species of Ptilorrhoa examined in having a less developed margo caudalis; a shallower sulcus lig. transversus; in caudal view, a shallow excavation beneath the caput humeri; and the distal edge of the crista bicipitalis forming a less obtuse angle from the shaft. In QM F57948 the crista deltopectoralis terminates slightly distally of the distal-most point of the crista bicipitalis, whereas in species of Ptilorrhoa it terminates further distally from the crista bicipitalis.
The fossil humerus differs from species of Psophodes in having a better developed crista deltopectoralis; a caudo-dorsally directed tub. dorsale, rather than cranio-dorsally; a proportionately longer crista bicipitalis; a round and shallow scar for M. scapulohumeralis cranialis, rather than an elongate and deep fossa; and a fossa situated ventrally of the margo caudalis and distally to the caput humeri.
Owing to its fragmentary condition, QM F57948 is tentatively assigned to Cinclosoma elachum sp. nov. because of its small size and based on the relative sizes of the fossil carpometacarpus QM F57949 and tibiotarsus QM F57951. It differs from extant species of Cinclosoma studied in having a relatively shorter margo caudalis and a more distinct linea m. latissimi dorsalis. The fossil differs from C. punctatum, C. castaneothorax, and C. cinnamomeum in that the crista deltopectoralis terminates slightly distally, instead of at about level, of the distal-most point of the crista bicipitalis. It differs additionally from C. castanotum in having a shallower fossa ventrally of the margo caudalis.
Stratigraphic and geographic range.—Early and middle Miocene; Wayne’s Wok Site, Neville’s Garden Site, Neville’s Riches Site, Riversleigh World Heritage Area, north-western Queensland, Australia.
Cinclosoma sp.
Fig. 2.
Material.—QM F57969, proximal right tarsometatarsus with most of hypotarsus broken off, from Rick’s Sausage Site, Gag Plateau, Riversleigh World Heritage Area, Queensland, Australia. Based on biocorrelation of mammalian faunas, Rick’s Sausage Site is allocated to Riversleigh’s Faunal Zone C and is interpreted as middle Miocene in age (Archer et al. 1989; Travouillon et al. 2011; Arena et al. 2015).
Measurements (in mm).—Preserved length 9.4; proximal width 5.1; cotyla medialis depth 3.5; cotyla lateralis depth 2.7.
Description.—QM F57969 (Fig. 2A) is referred to Cinclosoma because the tarsometatarsus exhibits the following combination of features: proximal tarsometatarsus stout; arcus extensorius short and approximately perpendicular to shaft long axis; lateral part of arcus extensorius situated well distally of dorsal margin of cotyla medialis; tuberositas m. tibialis cranialis small, centred in sulcus extensorius, and disto-laterally adjacent to arcus extensorius; medial shaft depth shallow. In extant species of Cinclosoma, the crista plantaris lateralis has a large, triangular peroneal foramen that is partially bound plantarly by a long hooked process on the proximal part of the crista plantaris lateralis (Fig. 2B2). In QM F57969 this process is broken off, but the proximal part of the crista plantaris lateralis is shallow and preserves the disto-dorsal edge of the foramen (Fig. 2A2), which indicates that the foramen was similarly large. Of these features, a small tuberositas m. tibialis cranialis that is situated centrally in the sulcus extensorius and disto-laterally adjacent to the arcus extensorius is considered an apomorphy of species of Cinclosoma.
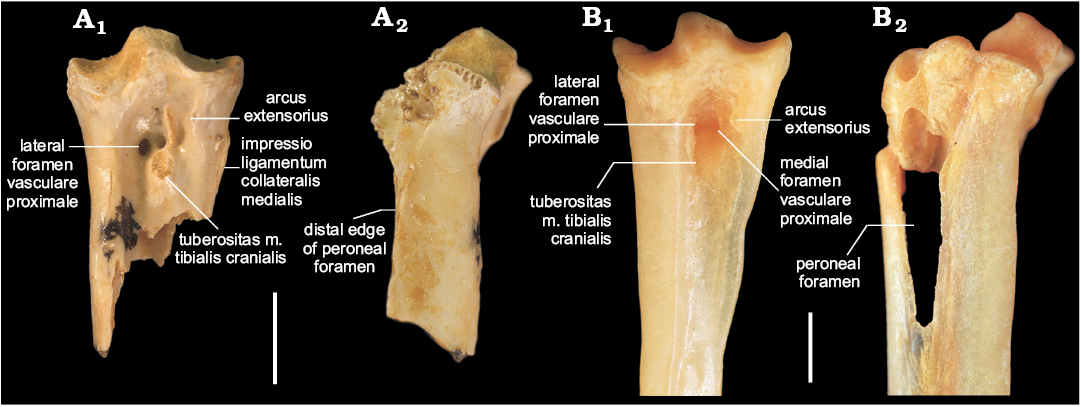
Fig. 2. Proximal right tarsometatarsus of the quail-thrush bird Cinclosoma sp., QM F57969 from the middle Miocene Rick’s Sausage Site, Riversleigh World Heritage Area, Australia (A), compared with a proximal left tarsometatarsus (mirrored) of the Recent Cinclosoma punctatum (Shaw, 1794), NMV B.12648 (B); in dorsal (A1, B1) and lateral (A2, B2) views. Note that the arcus extensorius is broken in NMV B.12648. Scale bars 2 mm.
The fossil tarsometatarsus additionally shares the following features with species of Cinclosoma: eminentia intercotylaris low; fossa on dorso-lateral side of eminentia intercotylaris shallow; fossa infracotylaris deep; foramina vascularia proximalia close together; plantar opening of medial foramen level with crista medialis hypotarsi; impressio lig. collateralis medialis low. Based on what is preserved of the medial, intermediate, and lateral crests of the hypotarsus, the hypotarsal canals for the flexor digitorum longus and flexor hallucis longus tendons are about equal in medio-lateral width, similar to extant species of Cinclosoma studied. The fossil tarsometatarsus differs from the corresponding bone of Ptilorrhoa species examined in being stouter and having less medio-lateral expansion of the cotylae; more elevated impressio lig. collateralis medialis; and a deeper fossa infracotylaris. It differs from species of Psophodes studied in being stouter; having a shorter arcus extensorius; and a relatively smaller tuberositas m. tibialis cranialis that is more circular and centred in the sulcus extensorius, rather than elongate and situated medially. Although specimens of Androphobus viridis were unavailable for study, the fossil can be distinguished from A. viridis in its much larger size (Boles 2007).
QM F57969 differs from extant species of Cinclosoma studied in only a few features. Differs from all species of Cinclosoma studied in having a shallower sulcus ligamentosus. Differs from C. punctatum in having a more pronounced impressio lig. collateralis medialis. Differs from C. castanotum and C. castaneothorax in possessing a shallower groove for the tendon of M. fibularis longus. Differs from C. cinnamomeum in having a less developed protuberance on the proximo-medial corner of the cotyla medialis; and a cotyla medialis larger than the cotyla lateralis, rather than being about equal in size. The fragmentary nature of the fossil and the many morphological similarities it shares with extant species of Cinclosoma preclude its allocation to a species with confidence.
Remarks.—QM F57969 corresponds to the larger end of the size range observed for tarsometatarsi of Cinclosoma punctatum (proximal width 5.1 mm in AM O.68471 and NMV B12872). The proximal tarsometatarsus width of C. cinnamomeum NMV B8986 and NMV B12004 is 4.0 mm, which would be similar to or smaller than that expected for C. elachum sp. nov., based on size comparisons of the carpometacarpus and tarsometatarsus. Therefore, we can infer that QM F57969 represents a species that is considerably larger than C. elachum sp. nov.
Concluding remarks
The fossils described in this paper indicate that two species of quail-thrushes were present in the Miocene of northern Australia: Cinclosoma elachum sp. nov., which was smaller in size than the smallest living quail-thrush C. cinnamomeum; and a larger indeterminate species of Cinclosoma that was similar in size to C. punctatum. Because all of the specimens appear to represent adult individuals, this size difference is not age-related. The carpometacarpus of Cinclosoma elachum sp. nov. has a proc. cranialis that is level with the opening located proximally of the proc. intermetacarpalis, which is an apomorphy for species of Cinclosoma. The fossils represent the first pre-Pleistocene records for cinclosomatids and show that the geographical range for species of family once extended into northern Australia, more than 300 km from the nearest extant congener C. cinnamomeum (Boles 2007; Atlas of Living Australia http://www.ala.org.au; BirdLife Australia Birdata Project http://birdata.birdlife.org.au).
Recent molecular clock analyses have estimated that the divergence between species of Cinclosoma and those of Ptilorrhoa occurred sometime between the early Oligocene and middle Miocene. These molecular estimates range from about 33 Ma (Toon et al. 2012); 27–12 Ma (Jønsson et al. 2011); 21–16 Ma (Jønsson et al. 2016); 24–14 Ma (Selvatti et al. 2015); and 18–11 Ma (Moyle et al. 2016). Cinclosoma elachum sp. nov. provides a minimum age of early Miocene for this genus, which is consistent with these molecular estimates. The paratype tibiotarsus of C. elachum sp. nov. was recovered from Neville’s Garden Site at Riversleigh, which has been U/Pb radiometrically dated to 18.5–17.7 Ma (Woodhead et al. 2016). By providing a minimum age for the split between species of Cinclosoma and those of its sister group, this fossil can be used to calibrate molecular estimates of divergence times between groups within Corvides and within Passeriformes overall (Ho 2007).
Cinclosoma elachum sp. nov. and Cinclosoma sp. indet. add to the diverse passerine avifauna identified from the early and middle Miocene of Riversleigh. Other forest floor dwellers, including the lyrebird Menura tyawanoides Boles, 1995 and logrunner Orthonyx kaldowinyeri Boles, 1993 (Nguyen et al. 2014), have been described from Riversleigh’s Faunal Zone B and C assemblages, as well as Australasian treecreepers (Climacteridae) (Nguyen 2016), honeyeaters (Meliphagidae) (Boles 2005), a sittella Daphoenositta trevorworthyi Nguyen, 2016, an oriolid Longmornis robustirostrata Boles, 1999, a cracticine Kurrartapu johnnguyeni Nguyen, 2013, and a corvid-like songbird Corvitalusoides grandiculus Boles, 2006. The Riversleigh songbirds are significant because, in addition to indeterminate cracticine remains from the early Miocene of New Zealand (Worthy et al. 2007), they are among the oldest crown-group representatives of oscines (Fig. 3). Based on molecular phylogenetic studies, songbirds are thought to have originated in what is now Australia (e.g., Ericson et al. 2002; Barker et al. 2004; Moyle et al. 2016). The new fossils described here re-emphasises the global significance of the Riversleigh deposits in our understanding of the evolutionary history of songbirds, which make up almost half of the world’s living bird species.
Riversleigh’s Faunal Zone B and C assemblages are the best documented fossil vertebrate faunas from the early and middle Miocene of Australia. These assemblages are species-rich and contain abundant sympatric arboreal folivores and other taxa that are typically found in rainforest habitats, including striped possums, cuscuses, musky rat-kangaroos, rhinolophoid bats, and bubble-nesting frogs (Archer et al. 1997; Hand and Archer 2005; Roberts et al. 2007; Black et al. 2012; Bates et al. 2014). Riversleigh’s early Miocene mammalian assemblages alone exhibit significantly greater diversity than those found today in Queensland wet tropical rainforest communities (Archer et al. 1997), and are more comparable to the contemporary mid-montane rainforest communities of New Guinea (Archer et al. 1989).
Radiometric dating provides a date of ~18 Ma for the early Miocene Riversleigh palaeocommunity to which Neville’s Garden and Wayne’s Wok Local Faunas belong (Myers et al. 2017). As noted by Myers et al. (2017), this places their accumulation close to the mid-Miocene climatic optimum (MMCO) and following a 2-million-year period of greenhouse warming. The precise age of Rick’s Sausage Local Fauna is not yet known, but available data (biocorrelation and location of the deposit on the Gag Plateau) suggest it is a Faunal Zone C assemblage and probably middle Miocene in age. On the basis of their fossil vertebrate assemblages the early Miocene, and probably many middle Miocene, palaeoenvironments at Riversleigh are thought to have been closed wet forest (Archer et al. 1989; Travouillon et al. 2009; Black et al. 2012; Myers et al. 2017).
Today, the only species of Cinclosoma that are found in mesic habitats are the Painted Quail-thrush C. ajax and the Spotted Quail-thrush C. punctatum (Boles 2007; Beehler and Pratt 2016). The remaining six species of this genus (C. castanotum, C. clarum, C. marginatum, C. castaneothorax, C. alisteri, and C. cinnamomeum) occur in arid and semi-arid environments in Australia (Boles 2007). Toon et al. (2012) estimated a multilocus species tree with complete taxon sampling of Cinclosoma, which supported sister relationships between the New Guinean C. ajax and all other species of Cinclosoma, and between the mesic zone C. punctatum and the remaining six members of this genus. Their results lend support to the hypothesis that the arid-adapted species of Cinclosoma evolved from forest taxa (Schodde 2006), and that quail-thrushes diversified as a result of the progressive aridification of inland Australia during the Pliocene and Pleistocene (Martin 2006; Byrne et al. 2008, 2011; Dolman and Joseph 2016). The presence of quail-thrushes in the early and middle Miocene forests of Riversleigh also supports a forest-dwelling ancestry for this group of passerines.
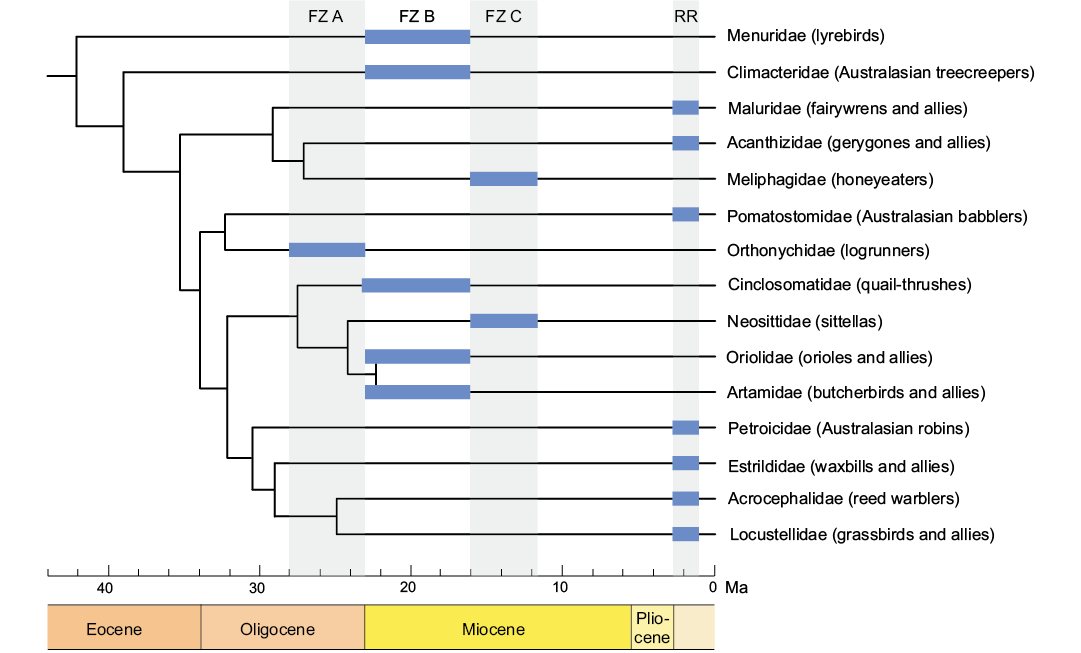
Fig. 3. Passerine groups known from Riversleigh’s Cenozoic assemblages and their interrelationships, based on the time-calibrated phylogeny of Selvatti et al. (2015). Thick horizontal bars indicate the earliest temporal occurrences of passerine groups in the Riversleigh faunal assemblages, based on Boles (1999, 2005), Nguyen et al. (2013, 2014, 2016), and Nguyen (2016). The estimated age ranges of the Riversleigh Faunal Zones and the Rackham’s Roost Local Fauna are from Archer et al. (1989), Creaser (1997), Travouillon et al. (2006), Arena et al. (2015), and Woodhead et al. (2016). Abbreviations: FZ A, Faunal Zone A (late Oligocene, 28.0–23.03 Ma); FZ B, Faunal Zone B (early Miocene, 23.0–16.0 Ma); FZ C, Faunal Zone C (middle Miocene, 16.0–11.6 Ma); RR, Rackham’s Roost Local Fauna, Rackham’s Roost Site, Riversleigh (early Pleistocene, 2.7–1.1 Ma).
Acknowledgements
We thank Sandy Ingleby and Leah Tsang (both AM), Leo Joseph (ANWC), and Karen Roberts (NMV) for facilitating access to comparative specimens. Anna Gillespie, Troy Myers, and Karen Black (all UNSW Sydney, Australia) carried out skilled preparation of fossil material. The Riversleigh project is supported by the Australian Research Council (DP130100197, DE130100467, DP170101420 grants to MA, SH and K. Black); XSTRATA Community Partnership Program (North Queensland); UNSW Sydney; Queensland Parks and Wildlife Service; Environment Australia; the Queensland Museum; the Riversleigh Society Inc.; Phil Creaser and the CREATE Fund at UNSW; Outback at Isa; Mount Isa City Council; private supporters including Alan Rackham and the Rackham family, Ken and Margaret Pettit, Elaine Clark, Margaret Beavis, and Martin Dickson; the Waanyi people of northwestern Queensland; and the field assistance of staff, postgraduate students, and volunteers. Thanks to Walter Boles (AM) for helpful comments that improved an earlier draft of this manuscript, and to Gerald Mayr (Senckenberg Research Institute and Natural History Museum Frankfurt) and an anonymous reviewer for their constructive feedback. Part of this research was conducted while JMTN was supported by a Chadwick Biodiversity Fellowship (AM).
References
Aggerbeck, M., Fjeldså, J., Christidis, L., Fabre, P.H., and Jønsson, K.A. 2014. Resolving deep lineage divergences in core corvoid passerine birds supports a proto-Papuan island origin. Molecular Phylogenetics and Evolution 70: 272–285. Crossref
Archer, M., Godthelp, H., Hand, S.J., and Megirian, D. 1989. Fossil mammals of Riversleigh, northwestern Queensland: preliminary overview of biostratigraphy, correlation and environmental change. Australian Zoologist 25: 29–65. Crossref
Archer, M., Hand, S.J., Godthelp, H., and Creaser, P. 1997. Correlation of the Cainozoic sediments of the Riversleigh World Heritage fossil property, Queensland, Australia. In: J.-P. Aguilar, S. Legendre, and J. Michaux (eds.), Actes du congrès BiochroM’97, Mémoires et Travaux de l’Ecole Pratique des Hautes Etudes 21, 131–152. Institut de Montpellier, Montpellier.
Arena, D.A., Travouillon, K.J., Beck, R.M.D., Black, K.H., Gillespie, A.K., Myers, T.J., Archer, M., and Hand, S.J. 2015. Mammalian lineages and the biostratigraphy and biochronology of Cenozoic faunas from the Riversleigh World Heritage Area, Australia. Lethaia 49: 43–60. Crossref
Baird, R.F. 1986. The Avian Portions of the Quaternary Cave Deposits of Southern Australia and Their Biogeographical and Palaeoenvironmental Interpretations. 811 pp. Unpublished Ph.D. Thesis, Monash University, Melbourne.
Baird, R.F. 1990. The fossil avian assemblage from Weekes Cave (N-15), Nullarbor Plain, South Australia: corrections, additions and reinterpretation. South Australian Ornithologist 31: 29–35.
Baird, R.F. 1991. Avian fossils from the Quaternary of Australia. In: P. Vickers-Rich, J.M. Monaghan, R.F. Baird, and T.H. Rich (eds.), Vertebrate Palaeontology of Australasia, 809–870. Pioneer Design Studio in cooperation with Monash University Publications Committee, Melbourne.
Baird, R.F. 1992. Fossil avian assemblage of pitfall origin form Holocene sediments in Amphitheatre Cave (G-2), south-western Victoria, Australia. Records of the Australian Museum 44: 21–44. Crossref
Baird, R.F. 1993. Pleistocene avian fossils from Pyramids Cave (M-89), eastern Victoria, Australia. Alcheringa 17: 383–404. Crossref
Barker, F.K., Cibois, A., Schikler, P., Feinstein, J., and Cracraft, J. 2004. Phylogeny and diversification of the largest avian radiation. Proceedings of the National Academy of Sciences of the United States of America 101: 11040–11045. Crossref
Bates, H., Travouillon, K.J., Cooke, B., Beck, R.M.D., Hand, S.J., and Archer, M. 2014. Three new Miocene species of musky rat-kangaroos (Hypsiprymnodontidae, Macropodoidea): description, phylogenetics and paleoecology. Journal of Vertebrate Paleontology 34: 383–396. Crossref
Baumel, J.J. and Raikow, R.J. 1993. Arthrologia. In: J.J. Baumel, A.S. King, J.E. Breazile, H.E. Evans, and J.C. Vanden Berge (eds.), Handbook of Avian Anatomy: Nomina Anatomica Avium (second edition). Publications of the Nuttall Ornithological Club 23, 133–188. Nuttall Ornithological Club, Cambridge.
Baumel, J.J. and Witmer, L.M. 1993. Osteologia. In: J.J. Baumel, A.S. King, J.E. Breazile, H.E. Evans, and J.C. Vanden Berge (eds.), Handbook of Avian Anatomy: Nomina Anatomica Avium (second edition). Publications of the Nuttall Ornithological Club 23, 45–132. Nuttall Ornithological Club, Cambridge.
Beehler, B.M. and Pratt, T.K. 2016. Birds of New Guinea: Distribution, Taxonomy, and Systematics. 672 pp. Princeton University Press, Princeton. Crossref
Black, K.H., Archer, M., Hand, S.J., and Godthelp, H. 2012. The rise of Australian marsupials: a synopsis of biostratigraphic, phylogenetic, palaeoecologic and palaeobiogeographic understanding. In: J.A. Talent (ed.), Earth and Life: Global Biodiversity, Extinction Intervals and Biogeographic Perturbations Through Time, 983–1078. Springer, London.
Boles, W.E. 1993. A logrunner Orthonyx (Passeriformes: Orthonychidae) from the Miocene of Riversleigh, north-western Queensland. Emu 93: 44–49. Crossref
Boles, W.E. 1995. A preliminary analysis of the Passeriformes from Riversleigh, northwestern Queensland, Australia, with the description of a new species of lyrebird. Courier Forschungsinstitut Senckenberg 181: 163–170.
Boles, W.E. 1999. A new songbird (Aves: Passeriformes: Oriolidae) from the Miocene of Riversleigh, northwestern Queensland, Australia. Alcheringa 23: 51–56. Crossref
Boles, W.E. 2005. Fossil honeyeaters (Meliphagidae) from the Late Tertiary of Riversleigh, north-western Queensland. Emu 105: 21–26. Crossref
Boles, W.E. 2006. A new songbird (Aves: Passeriformes) from the mid-Cenozoic of Riversleigh, northwestern Queensland. Alcheringa 30: 31–37. Crossref
Boles, W.E. 2007. Family Eupetidae (jewel-babblers and allies). In: J. del Hoyo, A. Elliott, and D.A. Christie (eds.). Handbook of the Birds of the World. Volume 12: Picathartes to Tits and Chickadees, 348–373. Lynx Edicions, Barcelona.
Byrne, M., Yeates, D., Joseph, L., Kearney, M., Bowler, J., Williams, M.A.J., Cooper, S., Donnellan, S.C., Keogh, J.S., Leys, R., Melville, J., Murphy, D.J., Porch, N., and Wyrwoll, K.H. 2008. Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17: 4398–4417. Crossref
Byrne, M., Steane, D.A., Joseph, L., Yeates, D.K., Jordan, G.J., Crayn, D., Aplin, K., Cantrill, D.J., Cook, L.G., Crisp, M.D. and Keogh, J.S. 2011. Decline of a biome: evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota. Journal of Biogeography 38:1635–1656. Crossref
Campbell Jr., K.E. 1979. The non-passerine Pleistocene avifauna of the Talara Tar Seeps, northwestern Peru. Royal Ontario Museum Life Sciences Contributions 118: 1–203.
Cracraft, J. 2014. Avian higher level relationships and classification: Passeriformes. In: E.C. Dickinson and L. Christidis (eds.), The Howard and Moore Complete Checklist of the Birds of the World (fourth edition). Volume 2: Passerines, xvii–xlvi. Aves Press, Eastbourne.
Creaser, P. 1997. Oligocene–Miocene sediments of Riversleigh: the potential significance of topography. Memoirs of the Queensland Museum 41: 303–314.
Deignan, H.G. 1964. Sub-family Orthonychinae. In: E. Mayr and R.A. Paynter Jr. (eds.), Check-list of Birds of the World. Volume 10, 228–240. Museum of Comparative Zoology, Cambridge.
Dickinson, E.C. and Christidis, L. (eds.) 2014. The Howard and Moore Complete Checklist of the Birds of the World (fourth edition). Volume 2: Passerines. 752 pp. Aves Press, Eastbourne.
Dolman, G. and Joseph, L. 2015. Evolutionary history of birds across southern Australia: structure, history and taxonomic implications of mitochondrial DNA diversity in an ecologically diverse suite of species. Emu 115: 35–48. Crossref
Dolman, G. and Joseph, L. 2016. Multi-locus sequence data illuminate demographic drivers of Pleistocene speciation in semi-arid southern Australian birds (Cinclosoma spp.). BMC Evolutionary Biology 16: 226. Crossref
Ericson, P.G.P., Christidis, L., Cooper, A., Irestedt, M., Jackson, J., Johansson, U.S., and Norman, J.A. 2002. A Gondwanan origin of passerine birds supported by DNA sequences of the endemic New Zealand wrens. Proceedings of the Royal Society of London, Series B 269: 235–241. Crossref
Gill, F. and Donsker, D. (eds.) 2018. International Ornithological Committee World Bird List. Version 8.1. http://www.worldbirdnames.org/
Gould, J. 1838. A Synopsis of the Birds of Australia, and the Adjacent Islands. 320 pp. J. Gould, London.
Gould, J. 1840. The Birds of Australia. Volume 4. 436 pp. J. Gould, London. Crossref
Gould, J. 1846. [Untitled]. Proceedings of the Zoological Society of London 1846: 67–71.
Gould, J. 1849. Description of a new species of Cinclosoma. Proceedings of the Linnean Society of London 1848: 139.
Hand, S.J. and Archer, M. 2005. A new hipposiderid genus (Microchiroptera) from an early Miocene bat community in Australia. Palaeontology 48: 371–383. Crossref
Higgins, P.J. and Peter, J.M. (eds.) 2002. Handbook of Australian, New Zealand and Antarctic Birds. Volume 6: Pardalotes to Shrike-thrushes. 1225 pp. Oxford University Press, Melbourne.
Ho, S.Y.W. 2007. Calibrating molecular estimates of substitution rates and divergence times in birds. Journal of Avian Biology 38: 409–414. Crossref
Jønsson, K.A., Fabre, P.H., Ricklefs, R.E., and Fjeldså, J. 2011. Major global radiation of corvoid birds originated in the proto-Papuan archipelago. Proceedings of the National Academy of Sciences of the United States of America 108: 2328–2333. Crossref
Jønsson, K.A., Fabre, P.H., Kennedy, J.D., Holt, B.G., Borregaard, M.K., Rahbek, C., and Fjeldså, J. 2016. A supermatrix phylogeny of corvoid passerine birds (Aves: Corvides). Molecular Phylogenetics and Evolution 94: 87–94. Crossref
Latham, J. 1801. Supplementum Indicis Ornithologici, sive Systematis Ornithologiae. 74 pp. Leigh and Sotheby, London.
Linnaeus, C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Volume 1. Tenth edition, revised. 533 pp. Laurentii Salvii, Holmiae.
Martin, H.A. 2006. Cenozoic climatic change and the development of the arid vegetation in Australia. Journal of Arid Environments 66: 533–563. Crossref
Mathews, G.M. 1910. [Untitled.] Bulletin of the British Ornithologists’ Club 27: 16.
Mathews, G.M. 1921–1922. The Birds of Australia. Volume 9. 652 pp. Witherby, London.
Mayr, E. and Amadon, D. 1951. A classification of recent birds. American Museum Novitates 1496: 1–42.
Morgan, A.M. 1926. Cinclosoma castanotum clarum (Chestnut-backed Ground-bird). South Australian Ornithologist 8: 138–139.
Moyle, R.G., Oliveros, C.H., Andersen, M.J., Hosner, P.A., Benz, B.W., Manthey, J.D., Travers, S.L., Brown, R.M., and Faircloth, B.C. 2016. Tectonic collision and uplift of Wallacea triggered the global songbird radiation. Nature Communications 7: 12709. Crossref
Myers, T.J., Black, K.H., Archer, M., and Hand, S.J. 2017. The identification of Oligo-Miocene mammalian palaeocommunities from the Riversleigh World Heritage Area, Australia and an appraisal of palaeoecological techniques. PeerJ 5: e3511. Crossref
Nguyen, J.M.T. 2016. Australo-Papuan treecreepers (Passeriformes: Climacteridae) and a new species of sittella (Neosittidae: Daphoenositta) from the Miocene of Australia. Palaeontologia Electronica 19.1.1A: 1–13.
Nguyen, J.M.T., Boles, W.E., Worthy, T.H., Hand, S.J., and Archer, M. 2014. New specimens of the logrunner Orthonyx kaldowinyeri (Passeriformes: Orthonychidae) from the Oligo-Miocene of Australia. Alcheringa 38: 245–255. Crossref
Nguyen, J.M.T., Hand, S.J., and Archer, M. 2016. The late Cenozoic passerine avifauna from Rackham’s Roost Site, Riversleigh, Australia. Records of the Australian Museum 68: 201–230. Crossref
Nguyen, J.M.T., Worthy, T.H., Boles, W.E., Hand, S.J., and Archer, M. 2013. A new cracticid (Passeriformes: Cracticidae) from the Early Miocene of Australia. Emu 113: 374–382. Crossref
Norman, J.A., Ericson, P.G.P., Jønsson, K.A., Fjeldså, J., and Christidis, L. 2009. A multi-gene phylogeny reveals novel relationships for aberrant genera of Australo-Papuan core Corvoidea and polyphyly of the Pachycephalidae and Psophodidae (Aves: Passeriformes). Molecular Phylogenetics and Evolution 52: 488–497. Crossref
Roberts, K.K., Archer, M., Hand, S.J., and Godhelp, H. 2007. New genus and species of extinct Miocene ringtail possums (Marsupialia: Pseudocheiridae). American Museum Novitates 3560: 1–15. Crossref
Rothschild, W. and Hartert, E. 1911. Description of new species and subspecies of birds from Mt. Goliath, Dutch New Guinea. Bulletin of the British Ornithologists’ Club 29: 33–35.
Schodde, R. 2006. Australasia’s bird fauna today—origins and evolutionary development. In: J.R. Merrick, M. Archer, G.M. Hickey, and M.S.Y. Lee (eds.), Evolution and Biogeography of Australasian Vertebrates, 413–458. Auscipub, Sydney.
Schodde, R. and Mason, I.J. 1999. The Directory of Australian Birds: Passerines. 851 pp. CSIRO Publishing, Melbourne.
Sclater, P.L. 1874. Characters of new species of birds discovered in New Guinea by Signor d’Albertis. Proceedings of the Zoological Society of London 1873: 690–698.
Selvatti, A.P., Gonzaga, L.P., and de Moraes Russo, C.A. 2015. A Paleogene origin for crown passerines and the diversification of the Oscines in the New World. Molecular Phylogenetics and Evolution 88: 1–15. Crossref
Shaw, G. 1794. Zoology and Botany of New Holland, and the Isles Adjacent. Volume 1: Zoology of New Holland. 69 pp. J. Sowerby, London.
Sibley, C.G. and Ahlquist, J.E. 1990. Phylogeny and Classification of Birds: A Study in Molecular Evolution. 976 pp. Yale University Press, New Haven.
Temminck, C.J. and Meiffren Laugier, G.M.J. 1820–1839. Nouveau recueil de planches coloriées d’oiseaux, pour servier de suite et de complement aux planches enluminées de Buffon. Volume 2. 516 pp. A. Strasbourg, Amsterdam.
Toon, A., Austin, J.J., Dolman, G., Pedler, L., and Joseph, L. 2012. Evolution of arid zone birds in Australia: leapfrog distribution patterns and mesic-arid connections in quail-thrush (Cinclosoma, Cinclosomatidae). Molecular Phylogenetics and Evolution 62: 286–295. Crossref
Travouillon, K.J., Archer, M., Hand, S.J., and Godthelp, H. 2006. Multivariate analyses of Cenozoic mammalian faunas from Riversleigh, north-western Queensland. Alcheringa 30 (S1): 323–349. Crossref
Travouillon, K.J., Legendre, S., Archer, M., and Hand, S.J. 2009. Palaeoecological analyses of Riversleigh’s Oligo-Miocene sites: implications for Oligo-Miocene climate change in Australia. Palaeogeography, Palaeoclimatology, Palaeoecology 276: 24–37. Crossref
Travouillon, K.J., Escarguel, G., Legendre, S., Archer, M., and Hand, S.J. 2011. The use of MSR (Minimum Sample Richness) for sample assemblage comparisons. Paleobiology 37: 696–709. Crossref
Vanden Berge, J.C. and Zweers, G.A. 1993. Myologia. In: J.J. Baumel, A.S. King, J.E. Breazile, H.E. Evans, and J.C. Vanden Berge (eds.), Handbook of Avian Anatomy: Nomina Anatomica Avium (second edition). Publications of the Nuttall Ornithological Club 23, 189–247. Nuttall Ornithological Club, Cambridge.
Vigors, N.A. and Horsfield, T. 1827. A description of the Australian birds in the collection of the Linnean Society; with an attempt at arranging them according to their natural affinities. Transactions of the Linnean Society of London 15: 170–331. Crossref
Watanabe, J. and Matsuoka, H. 2013. Ontogenetic change of morphology and surface texture of long bones in the Gray Heron (Ardea cinerea, Ardeidae). In: U.B. Göhlich and A. Kroh (eds.), Paleornithological Research 2013: Proceedings of the 8th International Meeting of the Society of Avian Paleontology and Evolution, 279–306. Naturhistorisches Museum, Vienna.
Woodhead, J., Hand, S.J., Archer, M., Graham, I., Sniderman, K., Arena, D.A., Black, K.H., Godthelp, H., Creaser, P., and Price, E. 2016. Developing a radiometrically-dated chronologic sequence for Neogene biotic change in Australia, from the Riversleigh World Heritage Area of Queensland. Gondwana Research 29: 153–167. Crossref
Worthy, T.H., Tennyson, A.J.D., Jones, C., McNamara, J.A., and Douglas, B.J. 2007. Miocene waterfowl and other birds from central Otago, New Zealand. Journal of Systematic Palaeontology 5: 1–39. Crossref
Acta Palaeontol. Pol. 63 (3): 493–502, 2018
https://doi.org/10.4202/app.00485.2018