

A new capybara from the late Miocene of San Juan Province, Argentina, and its phylogenetic implications
ESPERANZA CERDEÑO, MARÍA E. PÉREZ, CECILIA M. DESCHAMPS, and VÍCTOR H. CONTRERAS
Cerdeño, E., Pérez, M.E., Deschamps, C.M., and Contreras, V.H. 2019. A new capybara from the late Miocene of San Juan Province, Argentina, and its phylogenetic implications. Acta Palaeontologica Polonica 64 (1): 199–212.
A new hydrochoerine rodent, Cardiatherium calingastaense sp. nov. (Caviidae), is described based on the specimen INGEO-PV 87. It was recovered from the late Miocene Las Flores Formation, cropping out at the Puchuzum locality, San Juan Province, Argentina. The new species is based on a particular combination of characters, among which the following can be highlighted: p4 with internal fissures equally deep as in Cardiatherium paranense, secondary external fissure as in Cardiatherium patagonicum, and lacking the fifth internal fissure and supernumerary internal fissure, as in C. paranense; m3 with a conspicuous labial column in the posterior ramus of the second prism; very deep primary and secondary external fissures in upper cheek teeth, the former producing a labial strong step-shaped profile in M2; sagittal crest on the parietals; bullae small in ventral view; scars of the origin of the masseter medialis muscle with an anterior projection up to the level of the incisive foramen and the maxilla-premaxilla suture. The phylogenetic analysis supports the taxonomic proposal of creating a new species of Cardiatherium and shows C. calingastaense sp. nov. as the sister group of the other species of the genus. The lineage leading to the clade Cardiatherium + largest capybaras would have originated at least during the Chasicoan SALMA (early late Miocene). Cardiatherium calingastaense sp. nov. adds to the previous record of Cardiatherium chasicoense and Cardiatherium paranense in the late Miocene of San Juan and Mendoza provinces, respectively, thus increasing the diversity of capybaras in central-west Argentina.
Key words: Mammalia, Rodentia, Caviidae, Cardiatherium, systematics, phylogeny, Miocene, Argentina.
Esperanza Cerdeño [espe@mendoza-conicet.gob.ar], Instituto Argentino de Nivología, Glaciología y Ciencias Ambientales (IANIGLA), Centro Científico Tecnológico, CONICET Mendoza, Av. Ruiz Leal s/n, 5500, Mendoza, Argentina.
María E. Pérez [mperez@mef.org.ar], Museo Paleontológico Egidio Feruglio, Av. Fontana 140, U9100GYO, Trelew, Argentina.
Cecilia M. Deschamps [ceci@fcnym.unlp.edu.ar], Comisión de Investigaciones Científicas de la provincia de Buenos Aires, Argentina; División Paleontología Vertebrados, Unidades de Investigación Anexo Museo de La Plata, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, 122 y 60, 1900, La Plata, Argentina.
Víctor H. Contreras [vcontre@unsj-cuim.edu.ar], Instituto de Geología Dr. Emiliano P. Aparicio (INGEO) y Departamento Geología, FCEFN, Universidad Nacional de San Juan, Ignacio de la Roza y Meglioli, 5400 Rivadavia, San Juan, Argentina.
Received 5 September 2018, accepted 19 October 2018, available online 26 November 2018.
Copyright © 2018 E. Cerdeño et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Neogene continental outcrops in the San Juan Province, central-west Argentina (Fig. 1), encompass two localities that have yielded important late Miocene vertebrate remains. These localities are Puchuzum (Calingasta Valley), whose faunal assemblage is assigned to the Huayquerian SALMA (late Tortonian–Messinian; see Deschamps et al. 2013, for further details in bio- and chronostratigraphic equivalences), and Loma de Las Tapias, where two different levels are recognized as corresponding to the Chasicoan (Tortonian) and the Huayquerian South American Land Mammal Ages (SALMAs; Contreras et al. 1995; Peralta et al. 1995; Contreras 1996; Cerdeño and Contreras 2000; Ciancio et al. 2006; León and Bracco 2008; Bracco et al. 2011; Contreras and Baraldo 2011; Suvires and Contreras 2011).
Fig. 1. Geographical map showing position of studied area within San Juan Province, central-west Argentina (A) and location of outcrops of the Las Flores Formation (striped area) near the village of Puchuzum (B). The asterisks indicate the fossil locality.
Concerning the fossils from Puchuzum, different levels of the section (distinct architectural elements) have provided remains of molluscs, ostracods, insects, fishes, and palynomorphs (found within lacustrine sediments), on the one hand, and mammals and birds (derived from flood plain sediments), together with footprint tracks, on the other hand (Contreras 1996; Contreras and Peralta 1998; Prámparo et al. 1998; Contreras and Cerdeño 2010; Bracco et al. 2011). Among mammal remains, only the specimens of a Hegetotheriidae (Notoungulata) were studied in detail (Cerdeño and Contreras 2000). However, the faunal assemblage also includes xenarthrans, litopterns and rodents (Contreras and Cerdeño 2010; Bracco et al. 2011). The hegetotheriid Hemihegetotherium achataleptum indicated a Huayquerian SALMA for this fauna (Cerdeño and Contreras 2000), but no absolute age has been obtained for the unit. Herein we present the study of a well-preserved skull and mandible of a capybara, associated with some fragments of postcranial bones.
Capybaras are the largest living rodents, represented by the single genus Hydrochoerus, reaching a body length of 100–130 cm, and adult males weighting up to 91 kg (Mones and Ojasti 1986). They have semiaquatic habits, and live in groups usually ranging in size from two to 30 individuals (Dunnum 2015). They have characteristic euhypsodont, multilaminated cheek teeth (Novak 1991; Deschamps et al. 2007). The phylogenetic relationships of capybaras with other cavioid rodents and the status of hydrochoerids have been revised during the last years (Rowe and Honeycutt 2002; Vucetich and Pérez 2011; Upham and Patterson 2015; Pérez et al. 2018, and references therein). After these authors, capybaras would be within the Family Caviidae as a subfamily, Hydrochoerinae, together with the extinct cardiomyines and the small Kerodon (rock-dwelling mocos), the other living genus of the subfamily (Rowe and Honeycutt 2002; Pérez and Pol 2012; Pérez et al. 2018).
The fossil record of capybaras (that is, hydrochoerines excluding ‘cardiomyines’) goes back to the late Miocene, and many taxa were described since the 19th century (Ameghino 1883a, b; Rovereto 1914; Kraglievich 1930; Rusconi 1933, 1935, 1944; Pascual and Bondesio 1963; Francis and Mones 1965a, b; Bondesio 1985a, b; Mones 1991; among others), but revisions such as those by Mones (1984) and, more recently, Vucetich et al. (2005, 2012, 2014a, b, 2015) and Deschamps et al. (2007, 2009) have evidenced a great taxonomic overestimation due to ontogenetic variation that had not been previously evaluated; consequently, these authors drastically reduced the known diversity of hydrochoerines, especially for the late Miocene–Pliocene interval.
Within the late Miocene, the oldest species is Cardiatherium chasicoense Pascual and Bondesio, 1968 from the Chasicoan SALMA, which is known from the type locality Arroyo Chasicó (Buenos Aires Province) as well as from the lower levels of the Loma de Las Tapias Formation in San Juan Province (Contreras 2000; Contreras et al. 2001; Deschamps et al. 2007, 2009; Contreras and Baraldo 2011). In turn, the Huayquerian SALMA localities with hydrochoerines are more abundant and the material was re-evaluated by Vucetich et al. (2005). These authors described Cardiatherium patagonicum as the southernmost record, from the Puerto Madryn Formation (Chubut Province). At the same time, they proposed that all material from the Ituzaingó Formation (Entre Ríos Province, northeast Argentina) would correspond to only one species, C. paranense, which was later confirmed by Vucetich et al. (2014b). These authors also recognized C. paranense in Tupungato, Mendoza Province (central-west Argentina), from levels of the Río de Los Pozos Formation, referred to the late Miocene (Yrigoyen 1993). Another species assigned to this genus, Cardiatherium orientalis Francis and Mones, 1965b, was recorded in the late Miocene of Uruguay, and tentatively recognized later in the Cerro Azul Formation, La Pampa Province, Argentina, as C. aff. orientalis (Vucetich et al. 2014b). The genus Cardiatherium is also known in the late Miocene of Brazil, Peru and Venezuela (Deschamps et al. 2013 and literature therein).
The present contribution focuses on the description of a new species of Cardiatherium based on well-preserved cranial remains, previously mentioned as Cardiatherium sp. (Contreras and Cerdeño 2010), found in the province of San Juan. On the basis of a cladistic analysis, the phylogenetic position of the new species and the monophyly of the genus Cardiatherium within Caviidae were tested.
Institutional abbreviations.—GHUNLPam, Cátedra de Geología Histórica, Universidad Nacional de La Pampa, Santa Rosa, Argentina; INGEO-PV, Vertebrate Palaeontological collection, Instituto de Geología Dr. Emiliano P. Aparicio, Universidad Nacional de San Juan, San Juan, Argentina; MACN, Museo Argentino de Ciencias Naturales, “B. Rivadavia”, Buenos Aires, Argentina; MLP, Museo de La Plata, Argentina; MMH-CH, Museo Municipal de Ciencias Naturales de Monte Hermoso, Argentina; MMP, Museo Municipal de Ciencias Naturales “Lorenzo Scaglia”, Mar del Plata, Argentina; MNHN-AYO, Ayo-Ayo collection, Muséum national d’Histoire naturelle, Paris, France; MPEF-PV, Palaeontological collection, Museo Paleontológico “Egidio Feruglio”, Trelew, Argentina; PVSJ, Vertebrate Palaeontological collection, Instituto y Museo de Ciencias Naturales, Universidad Nacional de San Juan, San Juan, Argentina; SPV-FHC, Sección Paleontología Vertebrados, Departamento de Paleontología, Facultad de Humanidades y Ciencias, Montevideo, Uruguay.
Other abbreviations.—ch., character; he, external fissure; HFI, fundamental internal fissure; hfe, fundamental external fissure; hi, internal fissure; HPE, primary external fissure; hpi, primary internal fissure; HSE/hse, secondary external fissure; hsi, secondary internal fissure; hsni, internal supernumerary fissure; hti, tertiary internal fissure; M/m, upper/lower molar; P/p, upper/lower premolar; pr, prism; prIIa/b, anterior and posterior lobes of prII; prsa, anterior secondary prism; SALMA, South American Land Mammal Age.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:2A1D2DDE-7100-4BC7-B0B1-8862CC1A064E.
Material and methods
The studied material, INGEO-PV 87, includes a single specimen composed of a well-preserved skull with associated mandible, and some fragmentary postcranial remains of the same individual, housed at the Vertebrate Palaeontological Collection of the INGEO. It was compared with the holotypes and referred materials of Cardiatherium chasicoense, C. paranense, C. orientalis, and C. rosendoi, housed in the following Argentinean museums: IMUSJ, MACN, MLP, MMH, MMP, and MPEF. The specimens of C. patagonicum used for comparisons and the phylogenetic analysis were taken from Vucetich et al. (2005) and Dozo et al. (2010). Cardiatherium aff. orientalis from the Cerro Azul Formation in La Pampa Province was also used in this study. The detailed list of revised remains is in Appendix 1.
Dental nomenclature is given in Fig. 2A–E (flexus/flexid were originally named as fissures by Mones 1991, and are here indistinctly used) and follows partially Vucetich et al. (2005) and modifications according to Pérez et al. (2018). However, some modifications have been introduced in this paper, in order to fit with a new character observed in the p4 of Cardiatherium patagonicum and the new species here described. This new feature is the external flexid of the posterior lobe of prII of p4 (Fig. 2A), here referred to as hse or h2e according to the nomenclature of m1–m3 (h.s.e. sensu Mones 1991; Fig. 2B–D). Hence, the external fissure of the anterior supernumerary prism of p4 (prsa) is named here h3e (h.s.e. or h.2e sensu Mones 1991; Fig. 2A, D). Lobes of caviids are referred to as prisms in hydrochoerines. Cranial nomenclature follows Vucetich et al. (2014b) and Pérez et al. (2018).
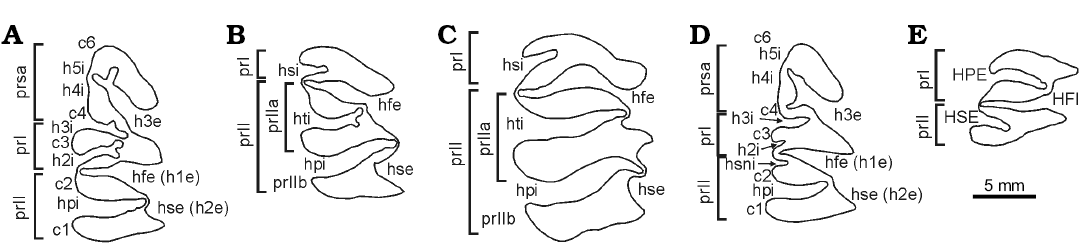
Fig. 2. Dental nomenclature (occlusal view). A–C, E. Cardiatherium calingastaense sp. nov. A. Right p4. B. Right m1. C. Right m3. E. Right M1. D. Cardiatherium patagonicum, right p4. Abbreviations: c, column; he, external fissure (or flexid); HFI, fundamental internal fissure (hypoflexus); hfe, fundamental external fissure; hi, internal fissure; HPE, primary external fissure; hpi, primary internal fissure; HSE/hse, secondary external fissure; hsi, secondary internal fissure; hsni, internal supernumerary fissure; hti, tertiary internal fissure; pr, prism; prIIa/b, anterior and posterior lobes of pr II; prsa, anterior secondary prism.
Skull measurements follow Vucetich et al. (2014b): (i) posterior width of the incisive foramen; (ii) rostral width at the anterior margin of the anterior root of the zygomatic arch (complete or up to the midline in incomplete specimens); (iii) width between the anteriormost points of the scars marking the origin of the masseter medialis muscle; (iv) rostral width at the level of P4, measured at the alveolar margin of prism I; (v) rostral width at the posterior margin of the anterior root of the zygomatic arch (complete or up to the midline in incomplete specimens); (vi) rostral width at the level of M2, measured at the alveolar margin of prII; (vii) distance between the posterior margin of the incisive foramen and P4; (viii) P4–M1 anteroposterior length; and (ix) angle of the beginning of the ventral anterior zygomatic arch and the alveolar series, in lateral view. In order to explore the phylogenetic relationships of the taxon studied, we scored the new anatomical information throughout the last version of the combined Cavioidea matrix (Madozzo Jaén et al. 2018), originally published by Pérez and Pol (2012). The combined dataset of morphological and molecular characters (SOM 1 and 2; Supplementary Online Material available at http://app.pan.pl/SOM/app64-Cerdeno_etal_SOM.pdf) was modified. Some multistate characters are ordered, nine characters have been modified and eight characters are new (see SOM 2). The new dataset was analysed under equally weighted parsimony in TNT (Goloboff and Catalano 2015), using a heuristic search of 1000 replicates of Wagner trees, followed by tree bisection and reconnection branch swapping. Node supports were calculated using absolute GC bootstrap frequencies calculated after 1000 pseudoreplicates. The modified Stratigraphic Manhattan Measure (Pol and Norell 2001) was used to calibrate the most parsimonious phylogenetic trees and the chronostratigraphic information for fossil taxa using TNT.
Geological setting
The specimen herein studied was collected in 2006 in levels of the Las Flores Formation, in the area of Puchuzum in the Calingasta Valley (Fig. 1). This unit is widely developed both geographically and in thickness in the Iglesia valley, where its type locality has been recognized (Wetten 1975; Contreras et al. 2013 and references therein). The stratigraphic section at Puchuzum was previously described by Peralta et al. (1995) (see also Bracco et al. 2011), and corresponds to the Neogene sediments cropping out at both margins of the Candelaria Creek, in the piedmont of the Manrique Mountains, Cordillera Frontal. According to geological and palaeontological studies at Puchuzum (Peralta et al. 1995; Contreras et al. 1995; Contreras 1996; Contreras and Peralta 1998; Cerdeño and Contreras 2000; Bracco et al. 2011; Bogan et al. 2018), the bony remains recorded in this locality, which come from the middle of the exposed section, were assigned to the latest Miocene. This mid-section is characterized by brown-reddish to greyish psammo-pelitic deposits, reddish silty to silty-sandy beds, with some carbonaceous and conglomeratic levels, and with cut and fill structures. Three mantiform interbeddings of bentonitic clay, 3 to 10 m thick, are distinguished by their yellowish coloration. These bentonitic packages clearly correspond to sedimentary events that originated in lakes, evidenced by the recorded fossils: bivalves, gastropods, insects, and fish (Peralta et al. 1995; Contreras 1996; Contreras and Peralta 1998; Bracco et al. 2011; Bogan et al. 2018). In turn, the mammals from this mid-section at Puchuzum represent a Huayquerian faunal association within the late Miocene (Peralta et al. 1995; Contreras 1996; Cerdeño and Contreras 2000).
The general palaeoenvironment of the mid-section of Puchuzum is interpreted as a fluvial system with great development of flood plains and rivers, more or less channelled and strongly sinuous. The lacustrine interbeddings correspond to increasing and decreasing events of the lacustrine basin, influenced by tectonic and hydrologic activity. A similar situation has been interpreted for the Las Flores Formation in the Iglesia Basin (Milana 1994; Jordan et al. 1997).
Systematic palaeontology
Rodentia Bowdich, 1821
Hystricognathi Tullberg, 1899
Cavioidea (Fischer, 1817) sensu Kraglievich 1930
Caviidae (Fischer, 1817) sensu Waterhouse 1839
Hydrochoerinae (Gray, 1825) Gill, 1872
Genus Cardiatherium Ameghino, 1883b
Type species: Cardiatherium doeringi Ameghino, 1883b (= Cardiatherium paranense [Ameghino, 1883a] in Vucetich et al. 2014b); Paraná River cliffs, Entre Ríos, Argentina, Ituzaingó Formation, Late Miocene.
Species included: Cardiatherium patagonicum Vucetich, Deschamps, Olivares, and Dozo, 2005, Cardiatherium orientalis (Francis and Mones, 1965b), Cardiatherium chasicoense (Pascual and Bondesio, 1968), Cardiatherium rosendoi Bondesio, 1985b, Cardiatherium talicei Francis and Mones, 1965a, and Cardiatherium isseli Rovereto, 1914.
Stratigraphic and geographic range.—Late Miocene (Chasicoan and Huayquerian SALMAs) of Argentina, Brazil, Peru, Uruguay, and Venezuela.
Cardiatherium calingastaense sp. nov.
Figs. 3, 4.
2010 Cardiatherium sp.; Contreras and Cerdeño 2010: 8R.
ZooBank LSID: urn:lsid:zoobank.org:act:343DF38E-F84D-43C7-9687-05C91FF26257
Etymology: After the Calingasta Valley, where the locality of Puchuzum is located.
Holotype: INGEO-PV 87, skull and mandible of an adult individual, together with associated fragments of vertebrae and long bones.
Type locality: Right side of the Candelaria Creek, Puchuzum area, Calingasta Valley, San Juan Province, central-west Argentina.
Type horizon: Mid stratigraphic section of Las Flores Formation, late Miocene.
Diagnosis.—Cavioid diagnosed by the following unique combination of characters (autapomorphies marked with an asterisk): euhypsodont cheek teeth; p4 with three prisms and P4 with two-lobed prisms as in other Hydrochoerinae; p4 with hfe, h3e, hpi, h2i, and h3i as in Hydrochoeropsis, Phugatherium, Neochoerus, and Hydrochoerus; h2i and h3i equally deep as in Cardiatherium paranense, Hydrochoeropsis dasseni, but differing from Hydrochoerus and Neochoerus in which h2i is shallower than h3i and its apex is behind the hfe; p4 with hse (h2e) as in C. patagonicum; p4 lacks h5i and hsni, as in C. paranense; hpi, hti, HFI, and hfe of m1/M1–m2/M2 not splitting the teeth, as in other Cardiatherium and differing from Hydrochoeropsis, Phugatherium, Neochoerus, and Hydrochoerus, in which those flexi/ids completely cross the tooth; *m3 with a conspicuous labial column in the posterior ramus of prIIa in contrast to other species; HPE and HSE very deep, the latter shorter than the former but deeper than in other species and oriented backwards; M3 with nine prisms; sagittal crest on the parietals, unlike Hydrochoeropsis, Phugatherium, Neochoerus, and Hydrochoerus, in which the area between both temporal fossae is plane, without forming the sagittal crest; bullae small in ventral view as in Neochoerus and Hydrochoerus; *scars marking the origin of the masseter medialis muscle on the beginning of the anterior zygomatic arch (ventral face), with an anterior projection up to the level of the incisive foramen and the maxilla-premaxilla suture.
Description.—As it is reflected in the diagnosis of the new proposed taxon, the specimen INGEO-PV 87 (Figs. 3, 4) may be referred to the genus Cardiatherium, discarding its assignment to other genera of capybaras such as Phugatherium, Hydrochoeropsis or the modern lineages represented by Hydrochoerus and Neochoerus (see also Phylogenetic analysis and Discussion). Therefore, the following description is focused on the comparison of the studied material with the known species of Cardiatherium.
Skull: It is somewhat mediolaterally deformed and lacks the left zygomatic arch, the left parietal-occipital area, and the left M3 (Fig. 3A1, A2).
In dorsal view (Fig. 3A1), the nasal is anterolaterally broken but the preserved portion indicates that this bone is long. The nasal is a little longer than the frontal in the new species as in C. paranense and the biggest capybaras (Phugatherium, Hydrochoeropsis, Hydrochoerus). Laterally, the nasal is in contact with the dorsal projection of the premaxilla and posteriorly with the frontal. The nasal is dorsally slightly convex, differing from the strongly bulging condition of C. paranense and from Hydrochoerus, in which the nasal is slightly bulky in the middle portion. The posterior border of the nasal is anteriorly slightly concave, differing from the strongly concave border of C. paranense. The nasal and frontal (Fig. 3A1) are proportionately narrower than in C. paranense, Phugatherium, and Hydrochoerus. The frontal and the anterior portion of the parietal are dorsally flat as in most Hydrochoerinae. The posterior portion of the parietal has a temporal fossa posterolaterally concave and the temporal lines join, although not completely fusing, in a short sagittal crest that diverges again posteriorly at the nuchal crest. This condition contrasts with that of the largest capybaras, which present an interposed area between the temporal fossae (which are shallower than in C. calingastaense sp. nov.) instead of the sagittal crest. The postorbital apophyses are incomplete and would have been short.
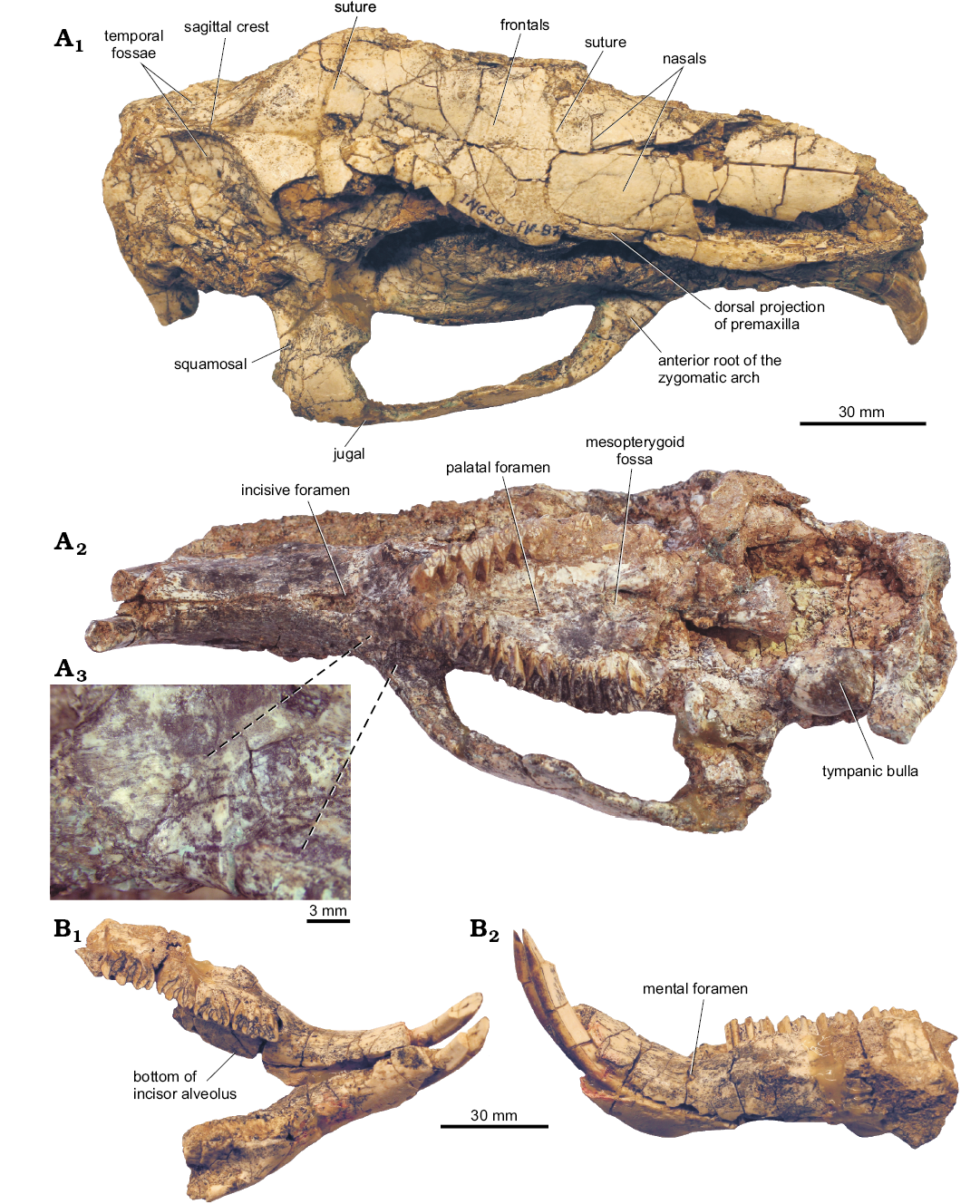
Fig. 3. Holotype (INGEO-PV 87) of the hydrochoerine rodent Cardiatherium calingastaense sp. nov., late Miocene, Puchuzum, San Juan Province, Argentina. A. Skull in dorsal (A1) and ventral (A2) views, and detail of the scars for masseter medialis muscle (A3); correspondence indicated by lines. B. Mandible in occlusal (B1) and left lateral (B2) views.
In right lateral view, the ventral portion of the zygomatic arch is preserved and is similar to the condition of Hydrochoerus. The maxillary anterior root of the arch is dorso-ventrally flattened and narrows posteriorly, being dorso-ventrally elongated like the jugal, which is antero-posteriorly short. The squamosal forms the posterior portion of the arch and is posteriorly extended above the tympanic bulla. The external auditory meatus is above the level of the molar series. The angle formed by the anterior root of the zygomatic arch and the alveolar plane in Lateral view is <20°, similar to C. paranense, but different from C. aff. orientalis from the Cerro Azul Formation, in which it is 24–26° (Vucetich et al. 2014b).
In ventral view (Fig. 3A2), the diastema is longer than the molariform series (Table 1), like in C. paranense, C. patagonicum, H. dasseni, and P. novum. The scars for the insertion of the masseter superficialis muscle are anteriorly convergent as in the other species of Cardiatherium; however, in INGEO-PV 87 there is an additional scar in this area. One is oval, transversely concave, placed between the root of the zygomatic arch and the P4, but extended anteriorly beyond this tooth, as observed in the other species of the genus; and the other one is another smooth surface at an obtuse angle with the first scar, which is directed to the sagittal plane, reaching the border of the incisive foramen, where the premaxilla-maxilla suture is located (Fig. 3A2, A3). The incisive foramen is ovoid and broad as in C. paranense whereas it is narrow in C. aff. orientalis (Table 1). The base of P4 does not project laterally and therefore does not form the bulge observable in ventral view in C. paranense. The ratio between the distance from the base of the incisive foramen up to the middle point between both P4s and the upper tooth series length is close to the minimum values of Cardiatherium, but different from the large capybaras (Phugatherium) and the modern lineages (Hydrochoerus–Neochoerus), in which the tooth series is proportionally longer (Table 1). The palate is rather shallow; from the level of M1 to that of the third prism of M3, there is a medium, narrow crest with a long and narrow palatal foramen at each side (M2–M3 level). The mesopterygoid fossa is narrow in outline, with a rounded anterior margin at the level of the posterior portion of the M3, as in C. paranense and C. patagonicum. The tooth rows are divergent, but with a smooth curvature with M3 aligned with P4–M2, as in C. paranense. In C. patagonicum and C. aff. orientalis, the M3 are nearly parallel. The basicranium is badly preserved, although part of the basisphenoid and the right tympanic bulla are preserved. The bulla is relatively small. The occipital face is incomplete and displaced; the right paraoccipital apophysis is distally incomplete, but it would have been relatively long (Fig. 3A1).
Table 1. Comparison of skull dimensions (in mm, except for 9, 12) of Cardiatherium calingastaense sp. nov. following Vucetich et al. (2014b) with other late Miocene Cardiatherium and Recent Hydrochoerus hydrochaeris. Abbreviations: 1, posterior width of the incisive foramen; 2, rostral width at the anterior margin of the anterior root of the zygomatic arch (complete or up to the midline in incomplete specimens); 3, width between the anteriormost points of the scars marking the origin of the masseter medialis muscle; 4, rostral width at the level of P4, measured at the alveolar margin of prism I; 5, rostral width at the posterior margin of the anterior root of the zygomatic arch (complete or up to the midline in incomplete specimens); 6, rostral width at the level of M2, measured at the alveolar margin of prII; 7, distance between the posterior margin of the incisive foramen and P4; 8, P4–M1 anteroposterior length; 9, angle of the beginning of the ventral anterior zygomatic arch and the alveolar series, in lateral view; 10, length of upper diastema; 11, length of P4–M3; 12, relationship (%) between dimensions 10 and 11; 1 holotype of C. paranense and MLP 71-VI-16-1; 2 GHUNLPam 27389 (the smallest) and GHUNLPam 5274 (the largest).
|
Skull dimension |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
|
|
Cardiatherium calingastaense sp. nov. (INGEO-PV 87) |
5.8 |
29.0 |
13.5/21.8 |
18.1/25.1 |
29.9 |
32.6 |
19.0 |
19.3 |
18 |
71.1 |
56.6 |
75.3 |
|
|
Cardiatherium paranense (N = 12) |
minimum |
3.2 |
22.6 |
–/16.0 |
10.7/– |
21.4 |
24.0 |
9.0 |
12.5 |
18 |
132.0 |
131.0 |
196.8 |
|
maximum |
8.0 |
32.4 |
–/28.0 |
19.4/– |
37.7 |
43.9 |
22.5 |
22.5 |
19 |
175.0 |
159.0 |
178.6 |
|
|
Cardiatherium aff. orientalis (N = 5) |
minimum |
2.5 |
21.8 |
–/16.0 |
12.9/– |
22.1 |
30.5 |
15.1 |
10.2 |
24 |
244.2 |
241.5 |
293.8 |
|
maximum |
3.0 |
29.0 |
–/27.0 |
17.4/– |
28.2 |
36.8 |
18.8 |
18.8 |
26 |
258.0 |
253.0 |
291.3 |
|
|
Cardiatherium patagonicum (MPEF-PV 2521) |
– |
– |
– |
– |
– |
– |
– |
– |
– |
75.0 |
57.0 |
76 |
|
|
Hydrochoerus hydrochaeris (N = 8) |
minimum |
5.0 |
16.2 |
26.2 |
11.0 |
20.2 |
23.6 |
9.2 |
11.6 |
13 |
25.1 |
37.2 |
148.2 |
|
maximum |
10.2 |
40.0 |
34.1 |
24.6 |
43.2 |
52.0 |
24.2 |
27.2 |
17 |
67.9 |
79.3 |
116.8 |
|
Mandible: The mandible preserves the symphysis, with the incisors, most of the left horizontal ramus, with p4–m3, and the anterior part of the right ramus (Fig. 3B). The right cheek teeth were recovered separately, but complete. The symphysis is narrow and procumbent, with a smooth median keel on the labial side; the posterior end of the symphysis does not reach the premolar level. The horizontal rami are well divergent; they are wide and vertically convex; ventrally, the area from the level below m1 to the base of the symphysis is flattened. The mental foramen is located anteriorly to p4 and at the dorso-ventral midpoint of the lateral surface of the dentary. The bottom of the i1 alveolus reaches internally the anterior half of the m2 and widens the lingual side of the ramus (clearly visible on the left side). The posterior border of the symphysis in C. calingastaense sp. nov. is below p4; according to Vucetich et al. (2005) and Deschamps et al. (2007), its position depends on the age of individuals, located anterior of p4 in juveniles and below the midpoint of p4 in larger (older) individuals. Main mandibular measurements are provided in Table 2.
Table 2. Mandibular dimensions (in mm) of Cardiatherium calingastaense sp. nov. (INGEO-PV 87).
|
Symphysis length |
51.5 |
|
Anterior transversal diameter of symphysis |
23.6 |
|
Transversal diameter symphysis at foramen level |
31.9 |
|
Anterior height of symphysis |
c. 23.4 |
|
Height symphysis at foramen level |
24.0 |
|
Transversal diameter between p4s, anterior to alveolus |
30.6 |
|
Height horizontal ramus ahead left p4 |
20.3 |
|
Maximum width anterior to lateral crest |
67.1 |
Dentition: Both upper and lower incisors are narrow (I1, 8.1 mm; i1, 7.4 mm and 7.6 mm) and strongly curved, with bevelled occlusal surface and enamel only on the labial side (Fig. 3B).
Among upper cheek teeth (Fig. 4A1–A4; Table 3), P4, M1, and M2 are formed by two heart-shaped prisms, joined by a short isthmus. As in all species of Cardiatherium, HPE is deeper than HSE, unlike Phugatherium in which these flexi are almost similar in depth (Fig. 5A–E). In INGEO-PV 87, the HPE is very deep and curved backward, in such a way that in M2 it reaches the labial side and produces a deformation of this wall resulting in a strong step-shaped profile (Figs. 4A2, A3, 5A). The HSE is shorter than HPE, but is deeper than in the other species and oriented slightly backward (Fig. 5A–C); only the largest specimen of C. patagonicum has an HSE similar in depth to C. calingastaense sp. nov., but anteriorly concave. P4 has undulated walls of HPE. The HFI is so deep that it almost splits prI from prII. M3 has nine prisms; prI is bilobed with a deep HPE similar to those of the other upper cheek teeth; the other eight prisms are laminar with a shallow, open V-shaped labial fissure in prII to prVII whereas in prVIII there is a barely marked labial concavity; as usual in capybaras, prIX is transversally shorter with a convex posterior margin and no labial fissure (Figs. 4A4, 5A).
Table 3. Comparison of upper tooth dimensions (in mm) of Cardiatherium. a minimum and maximum values after Vucetich et al. (2014b); * M1 and M2 not differentiated; AW, anterior width, L, length; PW, posterior width; W, width; approximate values in parentheses.
| |
P4 |
M1 |
M2 |
M3 |
||||||||
|
L |
AW |
PW |
L |
AW |
PW |
L |
AW |
PW |
L |
W |
||
|
Cardiatherium calingastaense sp. nov. (INGEO-PV-87) |
right |
10.0 |
6.3 |
7.6 |
8.8 |
(8.0) |
8.5 |
8.5 |
7.7 |
7.9 |
28.5 |
11.0 |
|
left |
(9.5) |
(6.8) |
– |
9.2 |
98.4 |
(8.4) |
(8.9) |
(7.4) |
(7.5) |
– |
– |
|
|
Cardiatherium chasicoensea* |
minimum |
8.0 |
7.2 |
7.5 |
8.1 |
6.7 |
7.6 |
|
|
|
12.7 |
5.1 |
|
maximum |
8.6 |
7.2 |
7.6 |
9.2 |
7.6 |
8.3 |
|
|
|
20.1 |
8.0 |
|
|
Cardiatherium paranensea |
minimum |
6.0 |
4.6 |
4.6 |
5.1 |
4.9 |
4.6 |
4.9 |
5.4 |
5.4 |
9.2 |
5.9 |
|
maximum |
12.0 |
8.2 |
9.7 |
11.0 |
8.7 |
9.7 |
9.1 |
6.4 |
7.2 |
37.5 |
10.7 |
|
|
Cardiatherium aff. orientalisa |
minimum |
4.8 |
3.0 |
3.6 |
4.0 |
3.33 |
4.3 |
5.6 |
5.9 |
5.9 |
6.8 |
3.0 |
|
maximum |
9.7 |
5.2 |
6.7 |
8.9 |
7.2 |
7.0 |
8.1 |
7.5 |
7.6 |
21.5 |
7.7 |
|
|
Cardiatherium patagonicuma* |
minimum |
6.8 |
4.8 |
6.1 |
4.4 |
3.8 |
4.3 |
|
|
|
14.1 |
3.6 |
|
maximum |
9.2 |
6.5 |
8.0 |
9.9 |
7.0 |
8.5 |
|
|
|
25.6 |
8.5 |
|
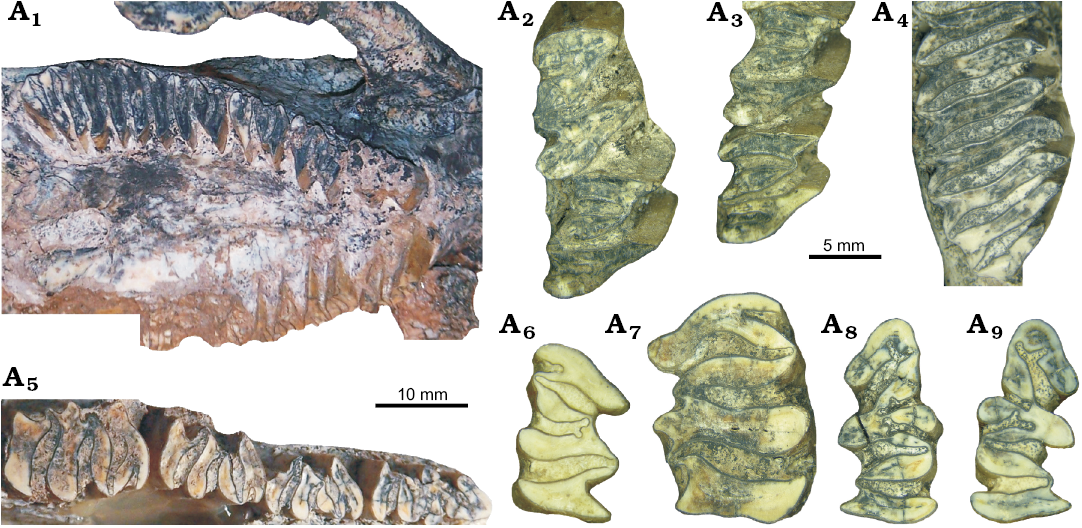
Fig. 4. Occlusal dental details of the holotype (INGEO-PV 87) of the hydrochoerine rodent Cardiatherium calingastaense sp. nov., late Miocene, Puchuzum, San Juan Province, Argentina. Right P4–M3 (A1), left P4–M1 (A2), left M1–M2 (A3), posterior part of right M3 (A4), left p4–m3 (A5), right m1 (A6), left m3 (A7), left p4 (A8), right p4 (A9).
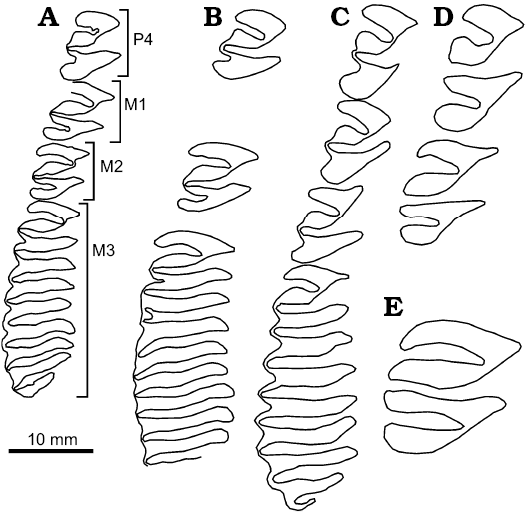
Fig. 5. Schematic occlusal view of upper teeth of Cardiatherium (A–C) and Phugatherium (D–E). A. Cardiatherium calingastaense sp. nov. (late Miocene, Puchuzum, San Juan Province, Argentina), INGEO-PV 87, right P4–M3. B. Cardiatherium patagonicum (late Miocene, Estancia Rincón Chico, Chubut Province, Argentina), MPEF-PV 740/22, reversed left P4; MPEF-PV 740/23, reversed left M1 or M2; MPEF-PV 740/18, right M3. C. Cardiatherium paranense (late Miocene, Ituzaingó Formation, Entre Ríos Province, Argentina), MLP 87-XI-1-27, right P4–M3. D. Phugatherium novum (middle Pliocene, Chapadamalal Formation, Buenos Aires Province, Argentina), MMP 492-S, right P4–M1. E. Phugatherium cataclisticum (late Miocene–Early Pliocene, Monte Hermoso Formation, Buenos Aires Province, Argentina), MLP 15-231a, right M1.
Among lower cheek teeth (Figs. 4A5–A9, 6A; Table 4), p4 has a deep h3e, bifurcated at the end, and anteriorly directed. C. calingastaense sp. nov. lacks h4i and h5i as C. paranense (Fig. 6C), whereas h5i is extended up to 25% of the occlusal surface in C. patagonicum (Fig. 6B), C. orientalis (Fig. 6D) and C. chasicoense (Fig. 6E). C. calingastaense sp. nov. and C. aff. orientalis have no supernumerary internal flexid (hsni) typical of C. orientalis and C. patagonicum (Fig. 6B, D). The hfe is also deep, wide and transversely directed whereas in other species it is oblique. Lingually, the hpi is transversely directed and very deep, reaching almost the labial side, producing a deformation on this margin; at the same time, the third external flexid (h2e) is markedly concave (Figs. 4A8, A9, 6A) whereas in C. patagonicum (also with very deep hpi) the h2e is only incipient (Fig. 6B). The h2i and h3i are long and also bifurcated at the end, and both delimit a conspicuous internal column (c3). In C. patagonicum, h2i is much shallower than h3i; in C. paranense (Fig. 6C) and C. chasicoense (Fig. 6E), these flexids are shallower and similar in depth, those of the latter being the shallowest.
Table 4. Comparison of lower tooth dimensions (in mm) of Cardiatherium. a minimum and maximum values after Vucetich et al. (2005); b values correspond to the holotype MLP 40-XI-15-1; c values after Deschamps et al. (2009), a very small m1/2 of Cardiatherium patagonicum was not here considered; * m1–m2 not differentiated; AW, anterior width; L, length; MW, medium width; PW, posterior width; approximate values in parentheses.
| |
p4 |
m1 |
m2 |
m3 |
|||||||||||
|
L |
AW |
MW |
PW |
L |
AW |
PW |
L |
AW |
PW |
L |
AW |
MW |
PW |
||
|
Cardiatherium calingastaense sp. nov. (INGEO-PV-87) |
left |
14.4 |
5.3 |
7.0 |
6.8 |
11.7 |
7.6 |
7.0 |
12.2 |
7.9 |
8.2 |
16.3 |
10.7 |
11.4 |
9.7 |
|
right |
13.8 |
5.3 |
6.7 |
6.9 |
11.5 |
7.3 |
7.0 |
12.6 |
(8.1) |
8.0 |
17.0 |
10.9 |
11.7 |
– |
|
|
Cardiatherium paranensea |
minimum |
9.7 |
4.0 |
5.1 |
5.1 |
5.7 |
4.0 |
4.1 |
8.9 |
4.9 |
6.4 |
12.6 |
8.0 |
– |
– |
|
maximumb |
19.8 |
6.4 |
7.8 |
8.8 |
15.8 |
9.6 |
10.1 |
17.6 |
9.6 |
11.6 |
21.7 |
10.4 |
– |
– |
|
|
Cardiatherium orientalis (holotype)a |
11.2 |
4.6 |
5.2 |
5.9 |
9.6 |
5.6 |
6.1 |
0.3 |
6.0 |
7.0 |
13.4 |
7.0 |
– |
– |
|
|
Cardiatherium aff. orientalisa |
minimum |
9.9 |
3.8 |
4.8 |
4.9 |
8.0 |
4.5 |
5.1 |
>8 |
5.3 |
>5 |
13.6 |
8.2 |
– |
– |
|
maximum |
23.0 |
8.5 |
10.6 |
11.2 |
18.5 |
12.1 |
10.2 |
20.9 |
>11 |
12.4 |
15.2 |
9.1 |
– |
– |
|
|
Cardiatherium patagonicuma,* |
minimum |
7.2 |
2.8 |
3.3 |
3.8 |
5.6 |
3.3 |
3.2 |
|
|
|
– |
7.2 |
– |
– |
|
maximum |
12.0 |
4.6 |
5.9 |
6.2 |
13.3 |
8.8 |
9.2 |
|
|
|
– |
9.6 |
– |
– |
|
|
Cardiatherium chasicoense* |
minimumc |
6.3 |
2.1 |
3.2 |
3.6 |
6.1 |
3.4 |
5.3 |
|
|
|
9.7 |
4.8 |
6.1 |
5.8 |
|
maximumc |
13.7 |
4.8 |
6.8 |
7.3 |
11.5 |
7.6 |
7.6 |
|
|
|
15.5 |
7.6 |
8.6 |
9.4 |
|
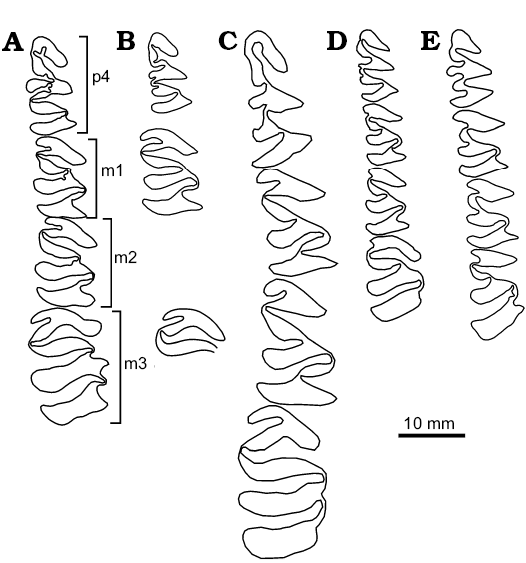
Fig. 6. Schematic occlusal view of lower teeth of Cardiatherium. A. Cardiatherium calingastaense sp. nov. (late Miocene, Puchuzum, San Juan Province, Argentina), holotype, INGEO-PV 87, right p4–m2 and reversed left m3. B. Cardiatherium patagonicum (late Miocene, Estancia Rincón Chico, Chubut Province, Argentina), MPEF-PV 740/9, reversed left p4; MPEF-PV 740/24, right m1–m2; MPEF-PV 740/7, reversed left m3. C. Cardiatherium paranense (late Miocene, Ituzaingó Formation, Entre Ríos Province, Argentina), MLP 40-XI-15-1, right p4–m3. D. Cardiatherium orientalis (late Miocene, Camacho Formation, San Gregorio, Uruguay), SPV-FHC-27-XI-64-20, right p4–m3. E. Cardiatherium chasicoense (late Miocene, Arroyo Chasicó, Buenos Aires Province, Argentina), MMP 300-M, right p4–m2; MMH-CH 88-6-71, reversed left m3.
The m1–m2 are similar in morphology (Figs. 4A6, 6A). The hsi penetrates less than 50% of the width of prI as in the other species of Cardiatherium, whereas hti is deep (crossing the prism but not splitting as in C. patagonicum; Fig. 6B) and Phugatherium) and bifurcated at the end, producing a deformation of the labial margin at this point. The hse is deep and wide. The m3 is the longest molar of the series, and flexids are not bifurcated. This molar has a conspicuous labial column in the posterior ramus of prIIa, delimited by two accessory fissures between hse and hfe (Figs. 4A7, 6A).
The previous comparative description has been focused on the best-known species, but some comparison can also be provided for two other poorly known taxa. In C. isseli (holotype and only specimen MACN 6354; middle Miocene, Río Negro Province; Rovereto 1914), the p4 is quite similar, but the m3 differs because the anterior margin of prI is straight and the hsi is very deep. In C. talicei (holotype SPV-FHC-10-VIII-63-1; Pliocene, San Gregorio, Uruguay; Francis and Mones 1965a; Mones 1991, who adds two other specimens), the flexids are anteroposteriorly wider; the p4 has deeper h4i and no h2e; h2i and h3i are shallower, especially the former; and the m3 shows a deep hsi, more anteriorly directed than in C. calingastaense sp. nov., which is reflected in a longer and more triangular first prism.
Stratigraphic and geographic range.—Late Miocene, middle section of the Las Flores Formation. Right side of the Candelaria Creek, Puchuzum area, Calingasta Valley, San Juan Province, central-west Argentina..
Phylogenetic analysis
We assessed the phylogenetic affinities of C. calingastaense sp. nov. using the dataset and protocols presented in previous studies (Pérez et al. 2018; Madozzo Jaén et al. 2018; see SOM 1–3). The parsimony analysis of the combined dataset (including morphological and molecular partitions) resulted in 333 most parsimonious trees (MPTs) of 3364 steps. The strict consensus of all MPTs is shown in SOM 3. Figure 7 displays the node Hydrochoerinae in the strict consensus.
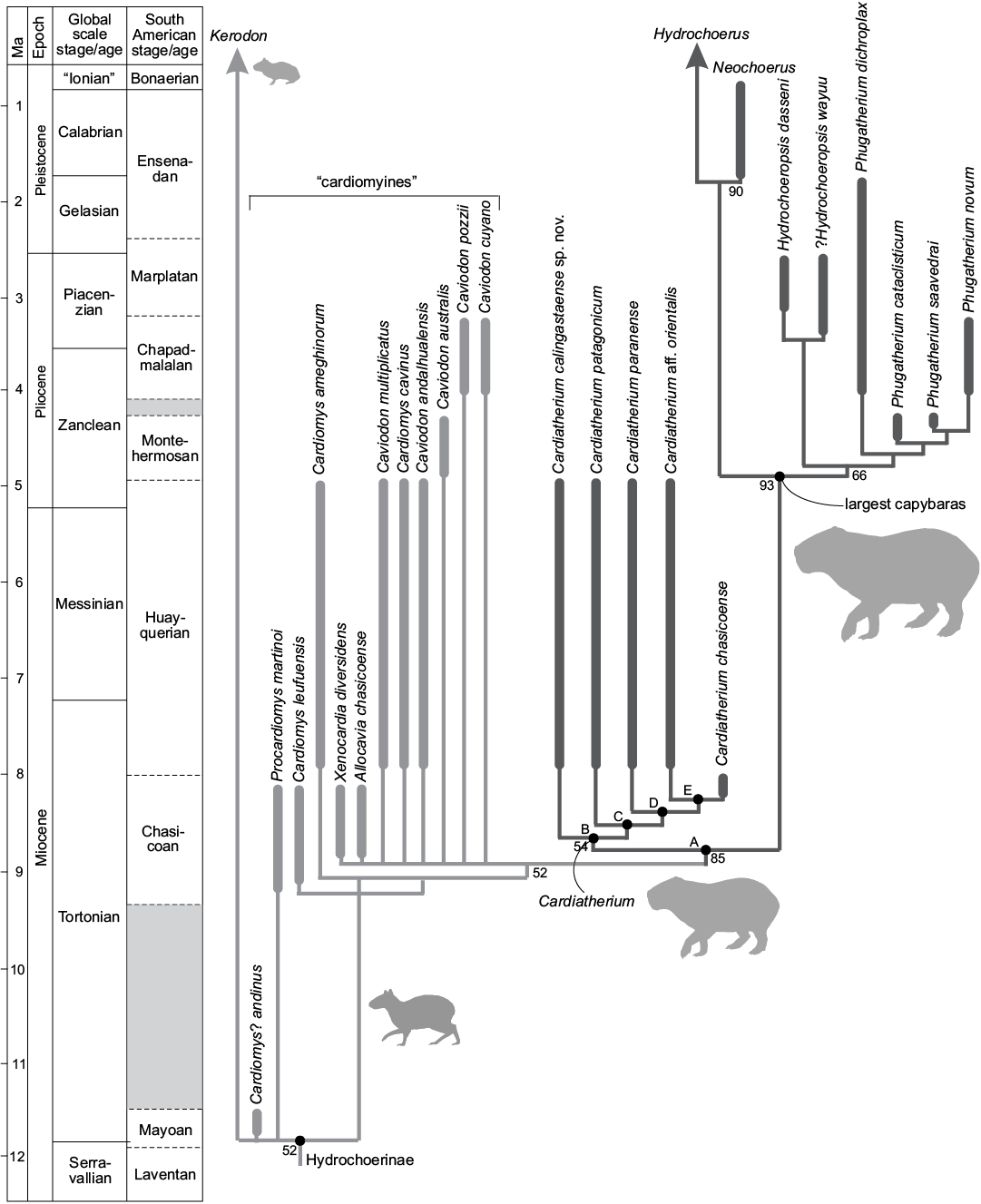
Fig. 7. Stratigraphic adjustment of the strict consensus tree for the phylogenetic analysis of Hydrochoerinae (333 MPTs of 3364 steps). Upper case letters indicate the nodes within Hydrochoerinae, as described in the main text. Silhouettes reflect external aspect and relative size of representatives of the main clades within Hydrochoerinae.
Our phylogenetic analysis recovers the monophyly of Cardiatherium (Fig. 7: node B and SOM 3). At the same time, this clade is the sister group of the largest capybaras (a clade including Hydrochoeropsis, Phugatherium, Neochoerus and Hydrochoerus; Fig. 7). Cardiatherium calingastaense sp. nov. is the sister group of the other species of the genus (Fig. 7: node B). Cardiatherium and the largest capybaras (Fig. 7: node A) share the following unambiguous synapomorphies: root of the lower incisors extending up to the level of the anterior lobe of m2 (ch. 20); concave palatal surface (ch. 55); presence of h2i on p4 (ch. 86); anterior location of h3i (ch. 90); hpi deeper than h5i (ch. 96); c3 present on prI of p4 (ch. 98); oblique h3e (ch. 101); posterior prism of m1–m2 complex heart-shaped (ch. 106); presence of hti on m1–m2 (ch. 109); hsi shallower than hpi on m1 (ch. 114); hpi in m1–m2 reaching the labial end (ch. 115); presence of hse on m1–m2 (ch. 116); p4–m1 shorter than m2–m3 (ch. 121); m1<m2<m3 (ch. 122); m3 complex (ch. 123); HPE deeper than HSE (ch. 138); laminar posterior prisms in M3 with three or more prisms, in which the first prism is heart-shaped (ch. 141); only the first prism is heart-shaped or lanceolate-shaped and the others are laminar in M3 with three or more prisms (ch. 142).
On the other hand, the Cardiatherium clade (Fig. 7: node B) is supported by two unambiguous synapomorphies: antero-posterior length of the posterior portion of the upper diastema more than 20% of the antero-posterior length of the maxilla (measured from the premaxilla-maxilla suture to the posterior border of maxilla at the level of the posterior projection of M3) (ch. 52), and hsi in m1–m2 is extended less than 50% of the occlusal surface (ch. 108). The other species of the genus (C. patagonicum, C. paranaense, C. aff. orientalis, and C. chasicoense; Fig. 7: node C) share a shallow h2i on the occlusal surface of p4 (ch. 88) and a normal development of c3 on p4 (ch. 99), while C. calingastaense sp. nov. and the largest capybaras have a deep h2i on p4 and a long c3 on p4. The clade that includes C. paranaense, C. aff. orientalis and C. chasicoense (Fig. 7: node D) shares hti extended up to 50% of the prism on m1 (ch. 110) and m2 (ch. 111), while in C. calingastaense sp. nov. and C. patagonicum the hti on m1–m2 is crossing the prism but not splitting as in Phugatherium and Hydrochoeropsis; in Neochoerus and Hydrochoerus, the hti on m2 is crossing and dividing the prism. Finally, the clade formed by C. aff. orientalis and C. chasicoense (Fig. 7: node E) is supported by a shallow hsi in complex m3 (ch. 125) and a laminar prI in complex m3 (ch. 126). The other species of Cardiatherium present a deep hsi in complex m3.
The most parsimonious hypothesis places C. calingastaense sp. nov. as the sister group of a clade including all the other species of the genus because it shares some characters with the largest hydrochoerines (ch. 88[1], 99[2], 110[1], 111[1]). When C. chasicoense is forced as the earliest branching species of the genus, the most parsimonious hypothesis costs three more steps.
The analysis with stratigraphic adjustment (Fig. 7) suggests that the lineage leading to the clade Cardiatherium + largest capybaras (Hydrochoeropsis, Phugatherium, Neochoerus, and Hydrochoerus) would have originated at least during the Chasicoan SALMA, in view of the phylogenetic position of C. chasicoense. The evolutionary novelties such as the increasing complexity of molariforms (e.g., presence of h2.i, h5.i, h2.e, c3 on p4, presence of hse on m1–m2, addition of laminar prisms in M3) would have been achieved during the Chasicoan SALMA. In turn, the novel traits that characterize the clade including the largest capybaras (e.g., deepening of flexi/flexids splitting transversally the prisms) would have been acquired at some point in time between the Chasicoan and Montehermosan SALMAs, suggested by the presence of an extensive ghost lineage (Fig. 7; see Pérez et al. 2018 for discussion on basal forms of “cardiomyines”).
Concluding remarks
The specimen INGEO-PV 87 is included in the genus Cardiatherium on the basis of cranial and dental characteristics, as detailed above, some of the latter being close to Cardiatherium patagonicum; however, this specimen shows an exclusive combination of both cranial and dental features that leads to propose the new taxon Cardiatherium calingastaense sp. nov.
Concerning the skull, C. calingastaense sp. nov. is closer to C. paranense than to more derived capybaras, but differs from the former in: nasal dorsally slightly convex (strongly bulging in C. paranense); posterior margin of the nasal slightly concave anteriorly (strongly concave in C. paranense); the scar for the insertion of the masseteric muscle with two differentiated areas (single scar in C. paranense and other species); and absence of bulge at the base of P4 (present in C. paranense).
With respect to the dentition, C. calingastaense sp. nov. is characterized by the great depth of flexi/flexids, which makes it different from most of the species of Cardiatherium. Vucetich et al. (2005) concluded that deeper flexi/ids correlate with larger size, which, in turn, is directly related to age. The great depth observed in flexi/ids of INGEO-PV 87, resulting in a particular morphology with a step-shaped profile, suggests that it would be an adult individual. In addition, the bifurcation of flexi/ids has been also observed in adult (large) specimens of Cardiatherium (e.g., p4, MPEF 740/34, C. patagonicum; m2, MLP 40-XI-15-1, C. paranense; m3, MMH-CH 88-6-41 and MLP 55-IV-28-15, C. chasicoense), as well as in other hydrochoerines (m3, MNHN-AYO 226, Phugatherium saavedrai; p4, MLP 15-232, P. cataclisticum) and some cardiomyines (Caviodon cuyano) (Vucetich et al. 2011, 2014a, b).
Some characters observed in C. calingastaense sp. nov. resemble the molariforms of Pliocene capybaras (such as Phugatherium or Hydrochoeropsis), although the latter are different in many others (see above). One of those characters is the deep HSE slightly oriented backwards and another one is the c3; although this column is present in all the species of Cardiatherium (see Deschamps et al. 2013: fig. 4), in C. calingastaense sp. nov. the c3 is very conspicuous, delimited by very deep h2i and h3i.
A particular character of the studied specimen is the conspicuous labial column in the posterior ramus of prIIa in m3, which is not known in the other species of Cardiatherium. Even though molariforms of hydrochoerines have strong individual variability (Mones 1991), this feature of m3 has not been previously described. Only more material from Puchuzum could reveal the existence of variation on this character within C. calingastaense sp. nov.
The phylogenetic analysis supports the taxonomic proposal of a new species of Cardiatherium and places C. calingastaense sp. nov. as the sister group of a clade including all the other known species of the genus.
Cardiatherium calingastaense sp. nov. increases the known diversity of capybaras in the late Miocene of San Juan Province. Up to now, the only record of capybaras from this area was that of C. chasicoense (the oldest capybara) from the lower levels of Loma de Las Tapias Formation, corresponding to the Chasicoan SALMA. At the same time, the species C. paranense had been recognized in the near Mendoza Province, in levels of Huayquerian age. Therefore, the new taxon corresponds to the first record of capybaras in the Huayquerian SALMA of San Juan Province and increases the general diversity of this group in the late Miocene of central-west Argentina.
Acknowledgements
The specimen INGEO-PV 87 was collected by Mariano Hidalgo (San Juan, Argentina) during a field season at Puchuzum within the project CICIT-UNSJ 21E/649, Universidad Nacional de San Juan. Special thanks are due to Paula Villagra (Mendoza, Argentina) for taking photographs of the fossil; Natalia Mónaco (Centro Científico Tecnológico CONICET, Mendoza, Argentina) prepared Fig. 1, and Santiago Hernández Del Pino (Instituto Argentino de Nivología, Glaciología y Ciencias Ambientales, Mendoza, Argentina) helped with Figs. 3 and 4. The authors thank the editor, Olivier Lambert (Royal Belgian Institute of Natural Sciences, Brussels, Belgium) and reviewers, Álvaro Mones (Augsburg, Germany) and an anonymous reviewer, for their useful comments on the manuscript. This paper contributes to the Research Project CICITCA-UNSJ, Programación 2018–2019: Estudio bio-cronoestratigráfico y correlación regional de las unidades neógenas de Loma de La Dehesa, valle de Matagusano, San Juan, Argentina (V.H. Contreras).
References
Ameghino, F. 1883a. Sobre una colección de mamíferos fósiles del Piso Mesopotámico de la Formación Patagónica recogidos en las barrancas del Paraná por el Profesor Pedro Scalabrini. Boletín de la Academia Nacional de Ciencias de Córdoba 5: 101–116.
Ameghino, F. 1883b. Sobre una nueva colección de mamíferos fósiles recogidos por el Profesor Scalabrini en las barrancas del Paraná. Boletín de la Academia Nacional de Ciencias de Córdoba 5: 257–306.
Bogan, S., Contreras, V.H., Agnolin, F., Tomassini, R.L., and Peralta, S.H. 2018. New genus and species of Anablepidae (Teleostei, Cyprinodontiformes) from the Late Miocene of San Juan province, Argentina. Journal of South American Earth Sciences 88: 374–384. Crossref
Bondesio, P. 1985a. Kiyutherium scillatoyanei n. sp. (Rodentia, Hidrochoeridae, Cardiatheriinae) de la Formación Ituzaingó de la provincia de Entre Ríos (República Argentina). Ameghiniana 21: 275–279.
Bondesio, P. 1985b. Un nuevo roedor Cardiatheriinae (Hidrochoeridae) del Departamento de Belén, provincia de Catamarca. Inferencias paleoambientales. Probable edad de los sedimentos portadores. Ameghiniana 21: 280–284.
Bowdich, T.E. 1821. An Analysis of the Natural Classifications of Mammalia for the Use of Students and Travellers. 115 pp. Smith, Paris.
Bracco, A., León, L., Prámparo, M.B., Cerdeño, E., and Contreras, V.H. 2011. Stratigraphy and sedimentology of the Neogene deposits of Puchuzum, San Juan, Argentina. In: J.A. Salfity and R.A. Marquillas (eds.), Cenozoic Geology of the Central Andes of Argentina, 65–74. SCS Publisher, Salta.
Cerdeño, E. and Contreras, V.H. 2000. El esqueleto postcraneal de Hemihegetotherium (Hegetotheriidae, Notoungulata) del Mioceno superior de Puchuzum, San Juan, Argentina. Revista Española de Paleontología 15: 171–179.
Ciancio, M.R., Krmpotic, C.M., Soibelzon, E., and Urrutia, J.J. 2006. Los Dasypodidae (Mammalia, Xenarthra) de la Formación Loma de Las Tapias, San Juan, Argentina. Implicancias paleobiogeográficas. Ameghiniana 43: 30R.
Contreras, V.H. 1996. Paleontología, Paleoecología y cronoestratigrafía del Neógeno de Puchuzum, provincia de San Juan, Argentina. Ameghiniana 33: 462.
Contreras, V.H. 2000. El Neógeno tardío de Loma de Las Tapias Quebrada de Ullum. Departamentos Ullum, Albardón y Rivadavia, Provincia de San Juan. Relatorio XI Reunión de Campo del Cuaternario, CD Revista Ciencias: 1–11.
Contreras, V.H. and Baraldo, J. 2011. Calibration of the Chasicoan-Huayquerian stages boundary (Neogene), San Juan, western Argentina. In: J.A. Salfity and R.A. Marquillas (eds.), Cenozoic Geology of the Central Andes of Argentina, 111–121. SCS Publisher, Salta.
Contreras, V.H. and Cerdeño, E. 2010. Presencia de Cardiatherium Ameghino, 1883 en la Formación Las Flores, Neógeno del Valle de Calingasta, San Juan, Argentina. Ameghiniana, Suplemento 47: 8R.
Contreras, V.H. and Peralta, S.H. 1998. Primeras evidencias de insectos fósiles en el Neógeno de San Juan, Oeste de Argentina. Aspectos tafonómicos y paleoecológicos relacionados. Acta Geologica Leopoldensia 21: 83–89.
Contreras, V.H., Monetta, A., Baraldo, J., Vallecillo, G., Weidmann, R., and Pesenti, J. 2001. Bioestratigrafía y edad de la Formación Loma de Las Tapias, San Juan, Argentina. 8º Congreso Colombiano de Geología, Actas: 1–15.
Contreras, V.H., Peralta, S.H., and Muñoz, M.A. 1995. Puchuzum, San Juan: una nueva icnofauna de vertebrados en el Neógeno del área occidental de Argentina. II Jornadas Argentinas de Icnología, Actas: 48–73.
Contreras, V.H., Tomassini, R.L., Pérez, M.A., and Oliva, C. 2013. Macrochorobates scalabrinii (Moreno and Mercerat) (Cingulata, Dasypodidae) en el Mioceno tardío de la provincia de San Juan (Argentina). Implicancias biocronoestratigráficas y paleobiogeográficas. Revista Brasileira de Paleontologia 16: 309–318. Crossref
Deschamps, C.M., Olivares, A.I., Vieytes, E.C., and Vucetich, M.G. 2007. Ontogeny and diversity of the oldest capybaras (Rodentia: Hydrochoeridae; late Miocene of Argentina). Journal of Vertebrate Palaeontology 27: 683–692.Crossref
Deschamps, C.M., Vieytes, E.C., Olivares, A.I., and Vucetich, M.G. 2009. Primer registro de Cardiatherium chasicoense (Rodentia, Hydrochoeridae) fuera del área pampeana (Argentina) y su valor bioestratigráfico. Ameghiniana 46: 295–305.
Deschamps, C.M., Vucetich, M.G., Montalvo, C.I., and Zárate, M.A. 2013. Capybaras (Rodentia, Hydrochoeridae, Hydrochoerinae) and their bearing in the calibration of the late Miocene–Pliocene sequences of South America. Journal of South American Earth Sciences 48: 145–158. Crossref
Dozo, M.T., Bouza, P., Monti, A., Palazzesi, L., Barreda, V., Massaferro, G., Scasso, R.A., and Tambussi, C.P. 2010. Late Miocene continental biota in northeastern Patagonia (Península Valdés, Chubut, Argentina). Palaeogeography, Palaeoclimatology, Palaeoecology 297: 100–109. Crossref
Dunnum, J.L. 2015. Family Caviidae. In: J.L. Patton, U.F.J. Pardiñas, and G. D’Elía, (eds.), Mammals of South America, Vol. 2, Rodents, 690–726. University of Chicago Press, Chicago.
Fischer, G. 1817. Adversaria zoologica. Mémoires de la Societé Imperiale des Naturalistes de Moscou 5: 357–428.
Francis, J.C. and Mones, A. 1965a. Sobre el hallazgo de Cardiatherium talicei n. sp. (Rodentia, Hydrochoeridae) en la Playa Kiyú, departamento de San José, República Oriental del Uruguay. Kraglieviana 1: 3–44.
Francis, J.C. and Mones, A. 1965b. Sobre el hallazgo de Kiyutherium orientalis n. g. n. sp. (Rodentia, Hydrochoeridae) en la Formación Kiyú de las barrancas de San Gregorio, departamento de San José, República Oriental del Uruguay. Kraglieviana 1: 45–54.
Gill, T. 1872. Arrangements of the families of mammals with analytical tables. Smithsonian Miscelaneous Collections 11: 1–998.
Goloboff, P.A. and Catalano, S.A. 2016. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32: 221–238. Crossref
Gray, J.E. 1825. An outline of an attempt at the disposition of the Mammalia into tribes and families with a list of the genera apparently appertaining to each tribe. Annals of Philosophy (new series) 10: 337–344.
Jordan, T.E., Kelley, S., Fernández, A., Fernández Seveso, F., Ré, G., and Milana, J.P. 1997. Vinculaciones entre las historias de las cuencas de Iglesia y del Bermejo. Actas de las Jornadas de Geología de Precordillera 2: 142–147.
Kraglievich, L. 1930. Los más grandes carpinchos actuales y fósiles de la subfamilia Hydrochoerinae. Anales de la Sociedad Científica Argentina 110: 233–250.
León, L.I. and Bracco, A. 2008. Evidencias de actividad orgánica en sedimentos fluviales del área de Puchuzum (Neógeno), Departamento de Calingasta, San Juan. XII Reunión Argentina de Sedimentología, Resúmenes: 94.
Madozo Jaén, M.C., Pérez, M.E., Montalvo, C.I., and Tomassini, R. 2018. Systematic review of Neocavia from the Neogene of Argentina: Phylogenetic and evolutionary implications. Acta Palaeontologica Polonica 63: 241–260. Crossref
Milana, J.P. 1994. Secuencias aluviales asociadas a variaciones hidrológicas: consideraciones teóricas y ejemplos. Asociación Argentina de Sedimentología, Revista 1: 103–124.
Mones, A. 1984. Estudios sobre la familia Hydrochoeridae, XIV. Revisión sistemática (Mammalia: Rodentia). Senckerbergiana Biologica 65: 1–17.
Mones, A. 1991. Monografía de la familia Hydrochoeridae (Mammalia: Rodentia). Courier Forschungsinstitut Senckenberg 134: 1–235.
Mones, A. and Ojasti, J. 1986. Hydrochoerus hydrochaeris. Mammalian Species 264: 1–7. Crossref
Novak, R.M. 1991. Walker’s Mammals of the World, Vol. 2. 5th Edition, 642 pp. The John Hopkins University Press, Baltimore.
Pascual, R. and Bondesio, P. 1963. Un nuevo tipo de morfología dentaria en un Cardiatheriinae (Rodentia, Hydrochoeridae) del Plioceno inferior de Huachipampa (San Juan). Ameghiniana 3: 43–49.
Pascual, R. and Bondesio, P. 1968. Los Cardiatheriinae (Rodentia, Caviomorpha) de la Formación Arroyo Chasicó (Plioceno inferior) de la provincia de Buenos Aires. Ameghiniana 5: 237–251.
Peralta, S., Muñoz, M., and Contreras, V.H. 1995. El Neógeno fosilífero de Puchuzum, valle de Calingasta, provincia de San Juan, Argentina. Ciencias 4: 57–62.
Perea, D., Rinderknecht, A., Ubilla, M., Bostelmann, E., and Martínez, S. 2013. Mamíferos y estratigrafía del Neógeno de Uruguay. Publicación Especial de la Asociación Paleontológica Argentina 14: 192–206.
Pérez, M.E. and Pol, D. 2012. Major radiations in the evolution of Caviid rodents: reconciling fossils, ghost lineages, and relaxed molecular clocks. PLoS ONE 7: e48380. Crossref
Pérez, M.E., Deschamps, C.M., and Vucetich, M.G. 2018. Diversity, phylogeny, and biogeography of the South American “cardiomyine” rodents (Hystricognathi, Cavioidea) with a description of two new species. Papers in Palaeontology 4: 1–19. Crossref
Pol, D. and Norell, M.A. 2001. Comments on the Manhattan Stratigraphic Measure. Cladistics 17: 266–281. Crossref
Prámparo, M.B., Papú, O.H., and Contreras, V.H. 1998. Primer registro palinológico en el Neógeno de la localidad de Puchuzum, provincia de San Juan, Argentina. VII Congreso Argentino de Paleontología y Bioestratigrafía, Resúmenes: 27.
Rovereto, C. 1914. Los estratos araucanos y sus fósiles. Anales del Museo Nacional de Historia Natural de Buenos Aires 25: 1–250.
Rowe, D.L. and Honeycutt, R.L. 2002. Phylogenetic relationships, ecological correlates, and molecular evolution within the Cavioidea (Mammalia, Rodentia). Molecular Biology and Evolution 19: 263–277. Crossref
Rusconi, C. 1933. Apuntes preliminares sobre las arenas puelchenses y su fauna. Anales de la Sociedad Científica Argentina 116: 169–193.
Rusconi, C. 1935. Tres nuevas especies de mamíferos del Puelchense de Villa Ballester. Boletín Paleontológico de Buenos Aires 5: 1–3.
Rusconi, C. 1944. Especies nuevas de mamíferos del Puelchense de Buenos Aires. Boletín Paleontológico de Buenos Aires 16: 1–3.
Suvires, G. and Contreras, V.H. 2011. Late Cenozoic geology and geomorphology of Loma de Las Tapias area, San Juan, west central Argentina. In: J.A. Salfity and R.A. Marquillas (eds.), Cenozoic Geology of the Central Andes of Argentina, 111–121. SCS Publisher, Salta.
Tullberg, T. 1899. Uber das System der Nagethiere: eine phylogenetische Studie. Nova Acta Regiae Societatis Scientiarum Upsalensis 3: 1–514.
Upham, N.S. and Patterson, B.D. 2015. Phylogeny and evolution of caviomorph rodents: a complete timetree for living genera. In: A. Vassallo and D. Antonucci (eds.), Biology of Caviomorph Rodents, Diversity and Evolution, 63–120. Sociedad Argentina para el estudio de los Mamíferos, Mendoza.
Vucetich, M.G. and Pérez, M.E. 2011. The putative cardiomyines (Rodentia, Cavioidea) of the middle Miocene of Patagonia (Argentina) and the differentiation of the Family Hydrochoeridae. Journal of Vertebrate Paleontology 31: 1382–1386. Crossref
Vucetich, M.G., Deschamps, C.M., and Pérez, M.E. 2012. Palaeontology, evolution and systematics of capybaras. In: J.R. Moreira, K.M.P.M. de Barros Ferraz, E.A. Herrera, and D.W. Macdonald (eds.), Capybara: Biology, Use and Conservation of a Valuable Neotropical Resource, 39–59. Springer Science and Business Media, New York.
Vucetich, M.G., Deschamps, C.M., and Pérez, M.E. 2015. The first capybaras (Rodentia, Caviidae, Hydrochoerinae) involved in the Great American Biotic Interchange. Ameghiniana 52: 324–333. Crossref
Vucetich, M.G., Deschamps, C.M., Morgan, C.C., and Forasiepi, A.M. 2011. A new species of Cardiomyinae (Rodentia, Hydrochoeridae) from western Argentina. Its age and considerations on ontogeny and diversity of the subfamily. Ameghiniana 48: 556–567. Crossref
Vucetich, M.G., Deschamps, C.M., Olivares, A.I., and Dozo, M.T. 2005. Capybaras, size, shape, and time: a model kit. Acta Paleontologica Polonica 50: 59–272.
Vucetich, M.G., Deschamps, C.M., Pérez, M.E., and Montalvo, C.I. 2014a. The taxonomic status of the Pliocene capybaras (Rodentia) Phugatherium Ameghino and Chapalmatherium Ameghino. Ameghiniana 51: 173–183. Crossref
Vucetich, M.G., Deschamps, C.M., Vieytes, E.C., and Montalvo, C.I. 2014b. Late Miocene capybaras from Argentina: skull anatomy, taxonomy, evolution, and biochronology. Acta Palaeontologica Polonica 59: 517–535.
Waterhouse, G.E. 1839. Observations on the Rodentia, with a view to point out the groups, as indicated by the structure of the crania in this order of Mammals. Magazine of Natural History 3: 90–96, 593–600.
Wetten, C. 1975. Estudio geológico-económico de un yacimiento de diatomitas y análisis de mercado. Congreso Iberoamericano de Geología Económica, Actas 5: 513–529.
Yrigoyen, M. 1993. Los depósitos sinorogénicos terciarios. Geología y recursos naturales de Mendoza. In: V.A. Ramos (ed.), XII Congreso Geológico Argentino y II Congreso de Explotación de Hidrocarburos, Relatorio 1, 123–148. Asociación Geológica Argentina e Instituto Argentino de Petróleo, Buenos Aires.
List of revised material of Cardiatherium.
Cardiatherium chasicoense: MMP 300-M (holotype of Procardiatherium chasicoense), fragment of right mandible with p4–m3; MMH-CH 85-4-40, almost complete right mandible with i1, p4, m1, and m3; Las Barrancas Member (= FSC facies) of the Arroyo Chasicó Formation, Buenos Aires Province, Argentina; PVSJ-537, palate fragment with right and left P4–M3 and associated mandible with p4–m3; Arenisca Albardón Member of the Loma de Las Tapias Formation, San Juan Province, Argentina.
Cardiatherium paranense: MLP 40-XI-15-1 (neotype), right mandibular fragment with p4–m3; MLP 87-XI-1-27 (holotype of Anatochoerus inusitatus), incomplete skull with both P4–M3; and MLP 78-II-27-1 (holotype of Kiyutherium scillatoyanei), right mandibular fragment with p4–m3. “Conglomerado osífero” of the Ituzaingó Formation (= “Mesopotamiense”), Entre Ríos Province, Argentina.
Cardiatherium orientalis: SPV-FHC 27-XI-64-20 (holotype of Kiyutherium orientalis), right mandible with p4–m3; San Pedro Member (or Kiyú Lithofacies) of the Camacho Formation (see Perea et al., 2013); Barrancas de San Gregorio, San José, Uruguay.
Cardiatherium aff. orientalis: GHUNLPam 139, damaged skull fragment with both M1–3; GHUNLPam 2009, skull fragment with both P4–M3; GHUNLPam 5236, anterior fragment of palate with anterior root of the zygomatic arch, both P4 and left M1; GHUNLPam 5274; skull with both P4–M3, rostrum, right orbital area and damaged skull roof; GHUNLPam 8978, small fragment of palate with left M1–2; GHUNLPam 14452, palate with both P4–M3 and posterior portion of rostrum; GHUNLPam 14661, distorted skull with both P4–M3; GHUNLPam 14985, damaged rostrum with right incisor, right P4–M2 and left P4; GHUNLPam 27389, skull with both P4–M3, incisors, a left mandible fragment with p4–m3, and six cervical vertebrae articulated to skull; MLP 62-XII-4-17, left mandible fragment with p4–m3 and palatal fragment with left P4–M3 and right P4–M1 (the palatal fragment is currently lost).
Cardiatherium rosendoi: MLP 29-X-8-26 (holotype and single specimen of Kiyutherium rosendoi), left mandibular fragment with p4, m1 and part of m2; Puerta de Corral Quemado, Andalhuala Formation (“Araucanian”, late Miocene), Catamarca Province, Argentina.
Cardiatherium patagonicum: MPEF-PV 740/1, holotype, right mandibular fragment with m1–m2; MPEF-PV 740/2, isolated right m1 or m2; MPEF-PV 740/9, left p4; MPEF-PV 740/11, incomplete left p4; and MPEF-PV 740/24, isolated right m1 or m2; Estancia Rincón Chico, upper levels of the Puerto Madryn Formation, Península Valdés, Chubut Province. MPEF-PV 2521, complete skull; La Pastosa, Puerto Madryn Formation, Península Valdés, Chubut Province (data after Dozo et al. 2010).
Acta Palaeontol. Pol. 64 (1): 199–212, 2019
https://doi.org/10.4202/app.00544.2018