


Exceptionally well-preserved Orsten-type phosphatocopid crustaceans from the Cambrian of Poland
EWA OLEMPSKA, ANDREAS MAAS, DIETER WALOSZEK, and MATS E. ERIKSSON
Olempska, E., Maas, A., Waloszek, D., and Eriksson, M.E. 2019. Exceptionally well-preserved Orsten-type phosphatocopid crustaceans from the Cambrian of Poland. Acta Palaeontologica Polonica 64 (1): 19–39.
Phosphatocopids are a group of small extinct marine crustaceans, which occur widespread in Cambrian dysoxic benthic lithofacies. Whereas the anatomy of the earliest ontogenetic stages of phosphatocopids, from the head larva with four pairs of appendages onward, is well documented, that of more advanced or adult growth stages has remained almost unknown (except for the external shields). Here we describe a three-dimensionally preserved specimen of a late growth stage of Cyclotron angelini from the Furongian strata (~490 Ma) of northern Poland, which possesses six pairs of appendages (2nd–7th pairs; antennula missing), much of setation, sternum with paragnath humps, and the trunk end with the putative anus. Based on comparisons with known phosphatocopids, the cephalic feeding system of this late larva comprises the antennae with their gnathobase-like median structures on their syncoxa and the mandible with a likewise strong but oblique gnathic coxal endite. Both appendages have a short, specialized endopod bearing a prominent, dentate, stout median spine on its proximal portion. The antennulae are missing but, as in other phosphatocopids, they are not expected to have contributed to the feeding and locomotory system like they do in crustacean stem-lineage representatives and the Eucrustacea, especially in the early larval phase. The lack of an antennal exopod and strong reduction in size of the mandibular exopod of C. angelini suggest that these appendages had lost their function as locomotory and sweeping devices, in contrast to other phosphatocopids (and eucrustaceans). Therefore, they may have served mainly for food gathering in the vicinity of the mouth, which may represent an autapomorphy of this phosphatocopid species. Furthermore, in this developmental stage of C. angelini the production of water currents and movement of food particles toward the median food path was likely achieved by the large, paddle-shaped setiferous exopods of the serially developed post-mandibular limbs. For comparisons, we studied additional phosphatocopid specimens from the Dębki 2 borehole belonging to other taxa and representing different ontogenetic stages. This is also the first report of exceptionally preserved cuticular apodemes in phosphatocopids, extending internally and still bearing partly preserved, putative muscle bundles. As known from extant eucrustaceans, these apodemes most likely served as attachment sites for appendage muscles.
Key words: Crustacea, Phosphatocopida, Orsten-type preservation, phosphatization, apodemes, Cambrian, Poland.
Ewa Olempska [olempska@twarda.pan.pl], Institute of Paleobiology, Polish Academy of Sciences, ul. Twarda 51/55, PL-00-818 Warszawa, Poland.
Andreas Maas [maas.blaustein@freenet.de], Galgenackerweg 25, D-89134 Blaustein, Germany.
Dieter Waloszek [dieter.waloszek@geol.lu.se] and Mats E. Eriksson [mats.eriksson@geol.lu.se], Department of Geology, Lund University, Sölvegatan 12, S-223 62 Lund, Sweden.
Received 25 September 2018, accepted 11 January 2019, available online 7 February 2019.
Copyright © 2019 E. Olempska et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Phosphatocopida Müller, 1964, is a group of small crustaceans having a secondarily phosphatized bivalved dorsal shield, which covered the entire body but was attached only to the head and the anterior two or three trunk segments. Phosphatocopid shields occur abundantly in residues etched by diluted acetic acid from so-called Orsten limestone nodules. The shields are often still attached dorsally and some specimens still possess three-dimensionally preserved ventral soft parts that are replicated by calcium phosphate, presumably having impregnated and preserved the remains shortly after death (e.g., Müller 1979, 1982, 1985; Maas et al. 2003, 2006; Siveter et al. 2003; Dong et al. 2005).
Besides phosphatocopids, other crustaceans and arthropods (Müller 1985), various sponge spicules (Castellani et al. 2012), phosphatized small shelly fossils, many different conodonts (e.g., Szaniawski 1971; Müller and Hinz 1991) and other metazoan fossils of unclear affinities, and even strands of cyanobacterians (Castellani et al. 2018) were found in the residues obtained from Orsten nodules or similar limestone. In Scandinavia, and best known from Sweden, these nodules are surrounded by bitumen-rich dark grey to black shale, the so-called alum shale (Martinsson 1974; Andersson et al. 1985; Müller 1985; Buchardt et al. 1997; Nielsen and Schovsbo 2007, 2013). Because coprolites, forming a considerable part of the components in the “Orsten” material (Maeda et al. 2011; Eriksson and Terfelt 2007), contain large quantities of mineral phosphate it has been suggested that this faecal matter was the source of phosphorous promoting the exceptional fossil preservation (e.g., Maeda et al. 2011).
The phosphatocopids were the first arthropods reported in the specific Orsten-type preservation (Müller 1979). This means that the specimens still possess 3D-preserved ventral, “soft” cuticular details such as appendages and the inner cuticle of the shield (called the inner lamella; illustrated but not specifically addressed already by Müller in 1964). Fossils of this type were first discovered from the Cambrian Series 3 (Agnostus pisiformis Biozone = oldest zone of the upper Cambrian in the former Cambrian stratigraphy) up to Furongian strata from southern Sweden (e.g., Müller 1979, 1982; Müller and Walossek 1991; Maas et al. 2003, 2006; Maas and Waloszek 2005; Eriksson et al. 2012, 2016; Haug et al. 2013, 2014). Subsequently, phosphatocopids with soft-tissue structures have been reported also from the Cambrian of Poland (Maas et al. 2006), England (Williams and Siveter 1998; Siveter et al. 2001, 2003), Siberia (Müller et al. 1995), North America (Siveter and Williams 1997) and the Cambrian of Australia (Walossek et al. 1993, see discussion below).
More recently, they have also been etched out of limestone rocks from the Cambrian of China (Hou et al. 2002; Dong et al. 2005; Zhang and Dong 2009; Liu and Dong 2009, 2010; Zhang et al. 2011a, b; Zhang et al. 2012; Zhang and Pratt 2012). Additionally, phosphatocopids known only from their empty shields have been reported from various localities (see Hinz-Schallreuter and Schallreuter 2009, and references therein). At the beginning of the latest Guzhangian and in the succeeding Furongian phosphatocopids appear to have undergone a major radiation and became distributed worldwide. Even more remarkably, the entire group disappeared completely from the fossil record before the end of the Cambrian, in the Peltura Biozone (see Williams et al. 2011: fig. 4).
Phosphatocopids are generally considered to be part of the Cambrian benthic to nectobenthic meiofauna adapted to seawater hypoxia and occupying marine offshore environments (Waloszek 2003; Maas et al. 2003, 2006; Williams et al. 1994, 2011). They appear to have tolerated oxygen-poor environments and form abundant and diverse faunal elements in Guzhangian and Furongian strata, especially black shale, organic-rich mudstone, and bituminous nodular limestone (e.g., Maas et al. 2003, 2006; Williams et al. 2011; Nielsen and Schovsbo 2013). The largest specimens reached 10 mm in length (Hinz-Schallreuter 1993, 2000), but most specimens were considerably smaller. Phosphatocopids presumably lived epibenthically, i.e., close to the bottom, feeding on particulate matter. Exceptions may have been taxa with an extremely large mandibular gnathobasic cutting plate at their syncoxa (Maas et al. 2003) and/or forms which lacked exopods on all post-antennular appendages (Walossek et al. 1993).
The systematics of phosphatocopids has long been based on the morphology of the shields, however, soft parts (basically all structures except for the dorsal, shield-like cuticle) proved crucial for assessing phylogenetic relationships, as demonstrated in the first detailed study by Maas et al. (2003) (see also Zhang and Pratt 2012).
Traditionally, phosphatocopids were regarded, mainly because of their bivalve shield, as ostracod maxillopodan (eu)crustaceans (e.g., Müller 1964, 1982; Hinz-Schallreuter 1993, 2000). Besides this superficial similarity, phosphatocopids share various features with the Eucrustacea (crown group including descendants of all living groups), particularly those associated with the anterior cephalic feeding apparatus.
These features include: (i) the morphology of the second appendage (antenna) and third appendage (mandible) having two basal portions with median gnathobases (named coxa and basipod, the carrier of the two rami; plesiomorphic state, only a setiferous proximal endite plus the prominent basipod); (ii) the labrum at the rear of the (phylogenetically older) hypostome; (iii) the recession of the mouth behind the labrum (plesiomorphic state exposed); (iv) the sternites of the third and fourth cephalic segment being fused to a sternum plate; (v) paragnath humps on mandibular portion of sternum; (vi) all parts around the mouth covered with fine hairs of a few microns in length, and a specific setal armature on all medially pointing endites of the basal limb portions and up to the first two endopodal portions (= setae and spines in three sets).
Collectively, this indicates a close relationship between phosphatocopids and eucrustaceans. On the other hand, in phosphatocopids the first post-mandibular appendage is not modified into a (cephalic) mouthpart (termed maxillula in eucrustaceans), but it looks similar to all more posterior appendages (fifth cephalic limb and thoracopods). This and the possession of a “head larva” as the first larval stage (which has four pairs of appendages by contrast to the postembryonic phase of Eucrustacea, which starts with a nauplius larva with only three appendage pairs) are plesiomorphic features demonstrating that the Phosphatocopida cannot be an in-group of Eucrustacea, but is recognized as its sister group (see also Siveter et al. 2001, 2003).
According to Maas et al. (2003), phosphatocopids also possess various autapomorphic features. For example, the rather short antennula with few distal setae (see Maas et al. 2003: pls. 7B, 10A, 11A), which arises on the anterolateral slope on the firmly sclerotized hypostome from a small articulation area completely surrounded by firm cuticle (plesiomorphic state: more and long setae, free, soft articulation, internally, muscles run toward bar-like extensions, wings, of the hypostome; see Müller and Walossek 1988 for the euarthropod Agnostus pisiformis). By contrast, among Eucrustacea, ostracods have, plesiomorphically, a well-developed antennula (see above for phosphatocopids), but a post-mandibular limb modified as a mouthpart = maxillula and a nauplius larva.
Another characteristic feature of phosphatocopids is the massive mandibular gnathobase, which is extended into a shovel-like, oblique “grinding plate” with a rim carrying a row of tooth-like spines. It looks like a coxa as in eucrustaceans, but it represents the fusion product of the coxa and basipod during development, called “syncoxa” in Maas et al. (2003: pl. 25A). Early during ontogeny both structures, coxa and basipod, are still well separated (see also Siveter et al. 2001, 2003 for this structure in Klausmuelleria salopensis). No similar structure is known from other euarthropod groups.
Another potential autapomorphy of phosphatocopids is their large, all-enclosing dorsal shield. This shield has a straight dorsal line, where the narrow but much elevated body is attached to the dorsal midline (Maas et al. 2003: fig. 9, pl. 11C). Segmental fusion to the shield includes the anterior three cephalic and more than four post-mandibular segments (see Maas et al. 2003 for more details). Therefore, phosphatocopids have a cephalothoracic shield. Similarly, also several extant eucrustacean groups have shields which are fused to more than the cephalic segments, but these are simply in-group phenomena and do not count for phylogenetic comparisons. By contrast to other euarthropods with a large bivalve shield, in phosphatocopids the median body proper is attached to the dorsal midline along a narrow area and forms an elevated rod-shaped structure (Maas et al. 2003: pl. 11C). Only in the area of the mandibular segment the dorsal area of the body widens due to the lateral extension of the massive syncoxa, which ends laterally in a joint. An adductor muscle and, likewise muscle scars on the inner side of the shield valves, as in ostracods (often these show external impressions too), are consequently lacking in phosphatocopids. The postmandibular appendages insert in oval to spindle-shaped articulations along the lateral sides of the body proper. Ample joint membranes facilitate that they can be held into the shield cavity.
Characters retained from the basic equipment (ground pattern) of Crustacea in the wider sense include the specialized three anterior head appendages for food intake and locomotion, as said here the first autapomorphically reduced, exopods with many annulations, each annulus carrying a sweeping seta (e.g., Müller 1982: figs. 5, 6) and proximal endites on postantennular appendages (see Müller and Walossek 1990; Walossek 1999; Maas et al. 2003; Waloszek 2003; Haug et al. 2013 for the phylogenetic significance of the proximal endite).
With these sets of own characters, synapomorphies with Eucrustacea, but also those plesiomorphies, which have been further advanced in the latter group (maxilla and nauplius), phosphatocopids can be recognized as the sister group to Eucrustacea within the taxon Labrophora (Walossek 1999; Siveter et al. 2003; Maas et al. 2003; Maas and Waloszek 2005).
Walossek and Szaniawski (1991) were the first to describe three-dimensionally preserved, secondarily phosphatized arthropods from the Cambrian of northern Poland. They established the new species Cambrocaris baltica Walossek and Szaniawski, 1991 from the Furongian of the borehole Hel IG 1, and furthermore described a complete trunk of Skara minuta Müller and Walossek, 1985 from the Agnostus pisiformis Biozone of the Żarnowiec IG 1 borehole.
In this study we describe in detail a specimen of the phosphatocopid Cyclotron angelini (Linnarsson, 1875) from the Furongian of northern Poland (Fig. 1). This specimen apparently represents a late growth stage and reveals new features of phosphatocopid appendage morphology (Figs. 2–6). In addition, we report the presence of segmental apodemes with remains of attached appendage muscles, demonstrated for the first time in Phosphatocopida (Fig. 7). Furthermore, the limb morphology of C. angelini is compared with that of specimens of earlier growth stages from the Furongian of Poland (Fig. 8) and other localities.
Institutional abbreviations.—ZPAL, Institute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland.
Material and methods
The described specimen (ZPAL Cr.11/ph060) of Cyclotron angelini with preserved soft parts and comparative phosphatocopid material were found in the section from the Dębki 2 borehole, situated at the village Dębki, close to the Baltic sea shore. The village of Dębki is situated approximately 50 km north-west of Gdańsk, northern Poland (Fig. 1). In the Dębki 2 borehole, the Cambrian Series 3 (Agnostus pisiformis Biozone) and Furongian shale and limestone beds occur in the depth interval between 2660.3 to 2675.0 m and include the Agnostus pisiformis, Olenus, and Peltura biozones (Bednarczyk and Turnau-Morawska 1975; Bednarczyk 1979b, 1984).
The specimen of C. angelini and comparative material are from a depth between 2662.3 m to 2663.3 m. These strata belong to the Słowińska Formation, and the biostratigraphical interval of the Olenus Biozone of the Furongian (Bednarczyk 1979a; Olempska and Wacey 2016). Unfortunately, no detailed profile of the Cambrian part of this borehole has been published and the drill core from Dębki 2 is no longer available. The specimens were extracted from the lime mudstone using standard acetic acid maceration techniques (see Müller 1985). The picked specimens were coated with platinum and photographed using a Philips XL 20 scanning electron microscope (SEM), equipped with an energy spectrometer EDAX-Dx4i, Genesis, at ZPAL.
The fourth appendage (first post-mandibular limb) of specimen ZPAL Cr.11/ph060 is visible on the left side of the specimen in median view (Fig. 5A1); the corresponding right limb broke off during preparation of the specimen for SEM. It could, however, be saved for separate imaging (Fig. 5A2, A3, A4).
The terminology applied to the body and appendage structures follows that of Maas et al. (2003) and Haug et al. (2013), for terms of more general use see also Walossek (1993). Additionally, we applied the term “apodeme” to structures, which are apparently homologous to the apodemes of Recent arthropods (e.g., Brusca and Brusca 2003).
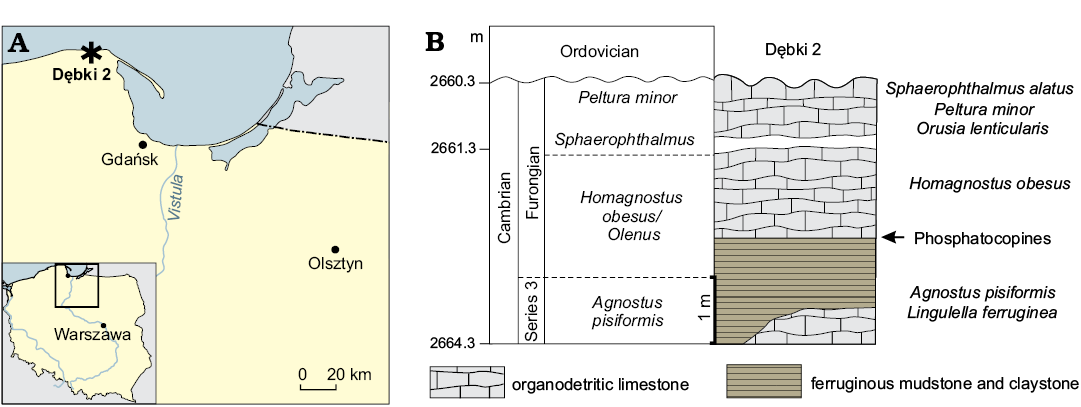
Fig. 1. A. Geographic location of study area in northern Poland, Dębki (asterisk). B. Stratigraphic column showing location of the sample with Phosphatocopida.
Systematic palaeontology
Phylum Arthropoda von Siebold, 1848
Class Crustacea Brünnich, 1772
Order Phosphatocopida Müller, 1964
Family Hesslandonidae Müller, 1964
Genus Cyclotron Rushton, 1969
Type species: Polyphyma lapworthi Groom, 1902; Malvern Hills, England; Cambrian, Furonian, Olenus Biozone (Groom 1902).
Remarks.—Phosphatocopid taxonomy was for a long time based on external shield features, such as the occurrence and location of lobes and spines, and the general outline of the shield (e.g., Maas et al. 2003). Although minute details also of the ventral appendage morphology have been described (Müller 1979, 1982; Maas et al. 2003; Maas and Waloszek 2005; Zhang et al. 2010), such features remain unknown for most species.
Cyclotron angelini (Linnarsson, 1875)
Figs. 2–6.
Material.—One specimen with preserved soft parts (ZPAL Cr.11/ph060), Furongian, Olenus Biozone, Dębki 2 borehole, northern Poland.
Description.—The incompletely preserved specimen ZPAL Cr.11/ph060 described herein possesses two small nodes in the antero-dorsal position on the left shield, a centrally located circular lobe and a shield outline suggesting that it belongs to Cyclotron angelini (Linnarsson, 1875). Centrally, the shield is drawn out into a shallow circular lobe. An interdorsum, a characteristic cuticular bar separating the left and right shields dorsally by two longitudinal furrows (not a hinge), is present (Fig. 6A1).
The right valve is preservationally absent, and the left valve is slightly incomplete antero-ventrally and posteriorly. The shield length measures 1520 µm but is estimated to originally have been approximately 1600 µm.
This specimen is possibly the largest one of the hitherto known phosphatocopids with preserved soft parts. Yet, it still seems to represent an immature ontogenetic stage, as specimens of C. angelini from the Olenus Biozone of Sweden may have grown to a shield length of up to 3.5 mm in length (Williams et al. 1994). The morphology described below is based on observations of specimen ZPAL Cr.11/ph060.
Soft parts: Cyclotron angelini comprises a flat-tubular body proper with differentiated head and trunk appendages and a large shield comprising left and right valves that completely surround the rest of the body. The body proper is connected to the shield only along a very narrow dorsal area, approaching not more than almost the width of the interdorsum. The number of post-mandibular segments involved in the shield formation is not clear but might include at least four segments—as known from other late ontogenetic stages of phosphatocopids, i.e., two segments more than the head composition at the level of Labrophora (= a cephalothoracic shield). Accordingly, it is also unclear how much of the trunk is missing in the specimen at hand and how many segments are free from the shield.
In the antero-ventral part of the body, a tube-like hole, around 50 µm in diameter is located anterio-medially of the antennal insertions (Fig. 3A2: arrow). Since it is located too far anteriorly for representing the actual mouth opening underneath the labrum, which should extend posteriorly to the level of the antennal spines, it might represent the turn of the esophagus into the head with the transition to the midgut running backward (a feature known from specimens of other Orsten taxa, such as the two species of Skara, see Müller and Walossek 1985).
The anterior ventral soft parts of C. angelini (Figs. 2–6), such as the hypostome-labrum complex, are not preserved. However, the void in the anterior part of the body suggests its previous location. The entire region at the antero-lateral edges of the hypostome including the insertions of the first pair of appendages, the antennulae, is not preserved, only phosphatic granules mark their original place, which means that the antennulae are lacking. Further posteriorly, the sternum with its pair of paragnaths (on the mandibular portion of the sternum), covered by fine setulae or denticles (Figs. 3A2, 6A5) is present, which also includes the fourth head segment with a pair of appendages similar to the more posterior ones. The body posterior of the sternum is undifferentiated. An opening is located posterior to the terminal limbs, presumed to represent the anus (Fig. 6A4: arrow). In all, six pairs of post-antennular appendages are present: antennae and mandibles and four post-mandibular limbs.
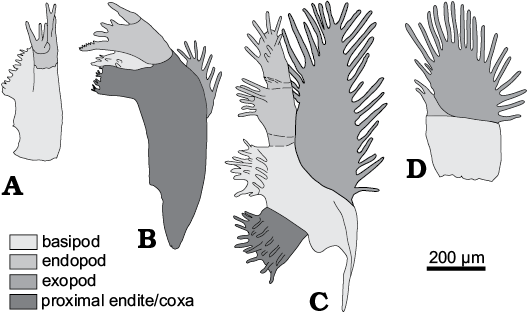
Fig. 2. Terminology of limb morphology of Cyclotron angelini (Linnarson, 1875). A. Antenna. B. Mandibula. C. 1st post-mandibular limb. D. Terminal limb.
Antennula: Not preserved in the specimen at hand.
Antenna: The second appendage (antenna) is uniramous, lacking an exopod and measures c. 220 µm from the basal joint to the beginning of the terminal endopodal spine (Fig. 3A2–A5). The limb includes a prominent trapezoidal, antero-posteriorly compressed, uniform stem portion that slightly curves medially; its proximo-distally extending median margin is wedge-shaped and runs out into a row of spines, in which short and longer spines alternate. These spines point towards the mouth opening at the rear of the hypostome (Figs. 3A2, A3, 4A2). Proximo-laterally, the limb stem exhibits a c. 17 µm long cuticular plate, interpreted as a sclerotized part of the arthrodial membrane that extends proximally into the body proper (Fig. 3A3–A5)—this structure has not been previously observed in any other phosphatocopid species. A separation of the limb stem into a proximal coxa and a distal basipod is not obvious, hence the antennal limb stem is referred to as a syncoxa.
The two-divided endopod (Fig. 3A3, A7) arises latero-distally from the apex of the syncoxa. Its proximal podomere is elongated like an oblique shovel with a row of 6–7, proximo-distally extending and spine-like, setae (Fig. 3A7), originally, they might have pointed around the caudal end of the labrum toward the mouth, which is not preserved in this specimen. The short hump-like distal endopodal portion arises far laterally and bears an antero-posteriorly extending set of three small setae, arranged at a right angle against the spine row of the proximal endite of the endopod (Fig. 3A7). Here, the terminal spine is the central one and the smaller originally median and lateral setae are situated anteriorly and posteriorly (Fig. 3A1, A3, A7). The exopod is apparently absent. At its expected position (as obtainable from other phosphatocopid species; see Maas et al. 2003), a small hump is present (Fig. 3A4: arrow). The slight concavity of the anterior side of the antenna (Fig. 3A1) is due to the oblique attachment of this limb at the sides of the labrum and it was presumably used for pushing food into the mouth area. Accordingly, the arthrodial membrane, which articulates the appendage with the body, is ample, recognizable by the largely poorly preserved area there (Fig. 3A4).

Fig. 3. Phosphatocopid crustacean Cyclotron angelini (Linnarsson, 1875), ZPAL Cr.11/ph060 from the Olenus Biozone, Furongian, Dębki 2 borehole, northern Poland. Stereo-pair in ventro-lateral view side (A1); ventral view exhibiting pairs of antennae and mandibles, first right post-mandibular limb and remains of second and third post-mandibular limbs, putative gut tube indicated by arrow, anterior to the left (A2); pairs of antennae in anterior view, mandibles with reduced exopods partly visible (A3); BSE image highlighting proximal part of the left antenna with ring-like structure at its base (arrow) (A4); BSE image depicting proximal part of the right antenna in anterior view, with basal ring-like cuticular plate (arrow) (A5); left mandible and antenna in postero-lateral view (A6); left antenna and mandible in ventral view (A7). Abbreviations: an, antenna; en, endopod; ex-md, exopod of the mandible; lst, limb stem; md, mandible; st, sternum.
Mandible: The third appendage (mandible) measures around 445 µm, thus being twice as long as the antenna, again excluding the distal endopodal spine (Figs. 3A1–A6). The mandible inserts laterally to the paragnaths and bears a large coxa, c. 280 µm long in medio-lateral extension (Fig. 3A6). Medially, the coxa is drawn out into a wide, flattened and slightly obliquely tilted gnathobase pointing towards the midline (Fig. 4A1, A6). The median extension of the gnathobase is largest proximally (anteriorly), decreasing towards its distal end (posteriorly). The proximal margin of the gnathobase is armed with a prominent spine proximally flanked by a seta. A row of five tuft-like short serrated spinules with a main tip and a smaller adjacent tip arise from the gnathobasic edge between the proximal and distal end of the gnathobase (Fig. 4A2, A4, A6). The anterior ends of the opposing gnathal edges of the right and left mandibles approach each other medially. They are located close to each other and parallel to the surface of the paragnaths and appear to be fairly symmetrical (Fig. 3A3).
Distal to the coxal gnathobase, a wedge-shaped setose endite with a circular cross section more distally, extends medially (Fig. 4A5). Its tip bears a prominent spine, which is flanked by a set of 5–6 irregularly arranged setae anteriorly and posteriorly, respectively (Fig. 4A5). On the right mandible this element is broken off, however, the empty space between the coxa and the endopod suggests its previous location (Fig. 4A6). In the terminology of Maas et al. (2003), this structure is the “basipodal endite”, or the strongly reduced basipod (Haug et al. 2013), characteristic of the morphogenesis of phosphatocopids, where a well-developed basipod is present only in the youngest ontogenetic stages (Maas et al. 2003).
The mandibular endopod is subdivided into two portions and arises latero-distally on the apex of the coxa (Fig. 4A5, A6). The proximal portion/podomere is club-shaped and drawn out medially into a robust, medially pointing tooth slightly dentate at its distal margin. The spine is slightly directed towards the oral region. Distal to the spine, two setae are located, one medio-distally, the second one weakly inclined anteriorly (Fig. 4A5). Additional small setae occur also on the club of the proximal portion of the endopod (Fig. 4A5). The smaller distal portion of the endopod arises latero-distally on the proximal portion of the endopod and bears an anterior retention seta, a strong median spine. A posterior, possibly sieving seta is broken off close to its base (Fig. 4A2, A5, A6). The boundary between the limb stem and the endopod on the lateral side of the limb is well defined (Fig. 4A5, A6).
Laterally on the mandibular limb stem (= on the outer side), the exopod arises about half way between the transition to the arthrodial membrane and the insertion of the endopod (Figs. 3A1, A3, 4A1). The exopod arises latero-medially on the coxa and is a less than 100 µm long and 30 µm wide (Figs. 3A5, 4A3). It is unusually small and short compared to that of most known phosphatocopid species (see Maas et al. 2003). The arthrodial membrane of the exopod is slightly bent up into a ring-like structure with a diameter of around 6 µm (Fig. 4A3: arrow). Its free margin, i.e., the median, lateral and proximal, is armed with a row of more than 12 setae, of which two proximal ones seem to be longest, measuring around 60 µm (Figs. 3A5, 4A3). The marginal row of at least ten long setae is interrupted by setae less than half as long, irregularly inserting and very slightly displaced from the marginal ridge (Fig. 3A5).
Antenna and mandible are located closely together in an oblique antero-lateral to postero-median position at the flanks of the supposed position of the hypostome-labrum complex, their median armature with setae and spines pointing orally (Figs. 3, 4).
The mandible is followed by four appendages, which appear serial in design; only the endopod seems to be smaller in more posterior limbs compared to the anterior ones. Since the first post-mandibular limb is much better preserved than the succeeding ones we give details on the morphology of the remaining trunk limbs when they differ from the first post-mandibular limb.
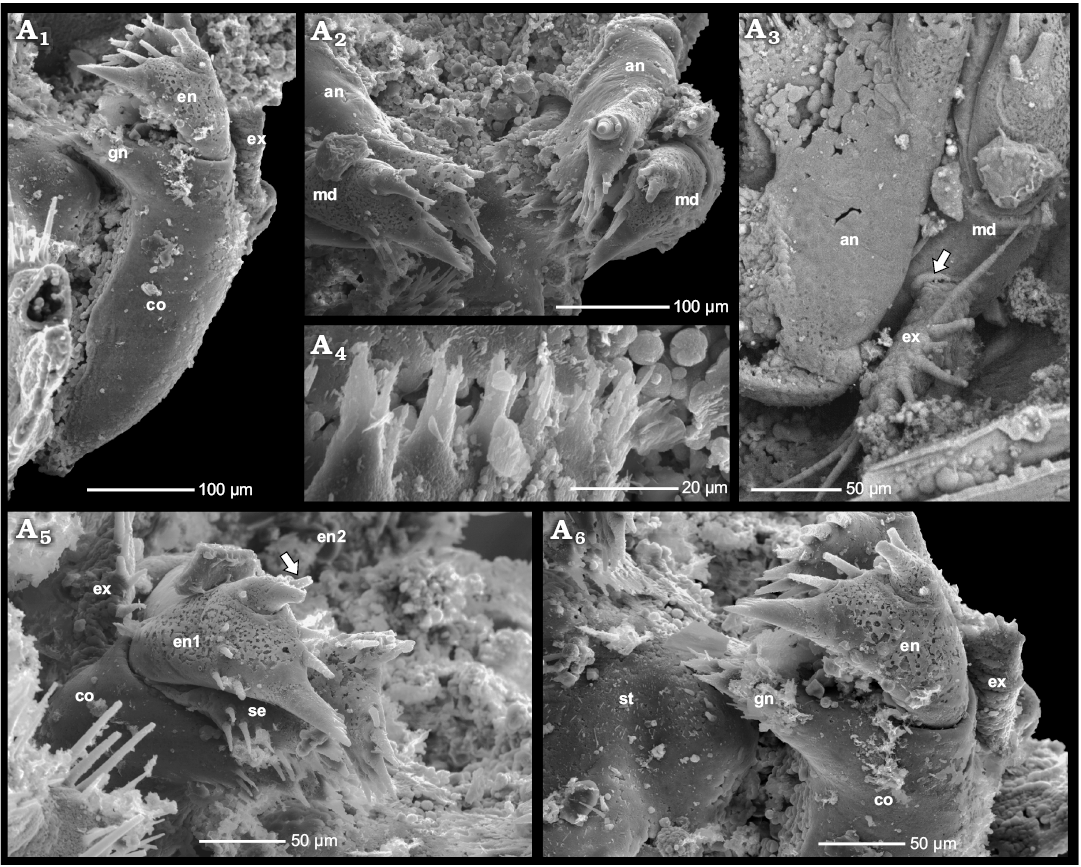
Fig. 4. Phosphatocopid crustacean Cyclotron angelini (Linnarsson, 1875), ZPAL Cr.11/ph060 from the Olenus Biozone, Furongian, Dębki 2 borehole, northern Poland. Left mandible in posterior view, note the empty space left by the setose endite (“basipodal endite”) broken off between gnathobase of coxa and endopod (A1); pairs of antennae and mandibles in oblique ventral view (A2); proximal part of right antenna in anterior view; note coxa of mandible with the circular cuticular structure around the exopod (arrow) (A3); margin of left coxal gnathobase with tuft-like setae (A4); distal part of right mandible with part of coxa, setose endite (“basipodal endite”) and two-divided endopod (arrow) (A5); distal part of left mandible with preserved coxa and endopod, note sternum with left paragnath; setose endite (“basipodal endite”) is not preserved (A6). Abbreviations: an, antenna; co, coxa; en, endopod; ex, exopod; gn, gnathobase; md, mandible; se, setose endite; st, sternum.
First post-mandibular limb: The fourth appendage, i.e., the first post-mandibular limb (Fig. 5A1–A4, A8), is approximately 490 µm long, from its insertion to the tip of the endopod, excluding the distal endopodal spines. Since it is morphologically similar to the following appendages, it is not regarded as a maxillula. The fourth limb appears slightly compressed in antero-posterior aspect (Fig. 5A1) and inserts almost laterally at the narrow body proper. Its stem portion consists of the separate proximal endite and the basipod, which carries the endopod and exopod.
As in many other phosphatocopids, the basipod is subtriangular with a wide excavation basally in its posterior cuticular surface, which is covered by membranous cuticle (Fig. 5A2), permitting a wide posterior flexure of the limb. Laterally, the basipod has a curved, narrow and possibly pointed extension toward the body surface, which seems to be rather rigid (Fig. 5A2). As based on other phosphatocopids (Maas et al. 2003), this extension anchors the limb far dorsally at the body as a pivot, so that the limb can be turned around this pivot while the ample soft median arthrodial membrane (Fig. 5A1, A3, A4) allows for a wide array of swinging motion anteriorly and posteriorly. The weakly humped oval median surface of the single basipodal endite bears a group of 5–6 robust spines and a few finer marginal spines. The median surface is surrounded by a set of around 20 setae, which almost form a complete circle, only the distal two are much thinner than the others (Fig. 5A1, A8 and 5A6, A7 for second post-mandibular limb). This is noteworthy because on enditic surfaces of the limbs of other phosphatocopids and Cambrian eucrustaceans the setae are more clearly subdivided into two distinct sets, in which the setae are arranged in vertical or crescentic rows around the central group. The posterior set is almost vertical and appears to rise from small sockets (Fig. 5A8), whether these were movable remains unclear. The anterior set is arranged in a more crescentic row. Three pores possibly occur on the posterior side of the basipodal endite (Fig. 5A8: arrow) and on the proximal endite. They are located in a row just below the circularly arranged setae. On the anterior side, they are absent or not preserved. Similar pores have also been discovered in several other Orsten eucrustaceans (e.g., see Walossek 1993 for Rehbachiella kinnekullensis on appendages and the furcal rami) and are most likely of sensory function. Accordingly, these setae might also have served this function to monitor the motion of the posterior raking setae.
The proximal endite, inserting clearly separately below the endite of the basipod, extends above the surface of the sternum (Fig. 5A1, A2). Both the proximal endite and the basipodal endite are clearly separated from each other. The median surface of the proximal endite is two-partite, the proximal part is a rounded hump with a few shorter spines or setae, whereas the distal oval surface bears several robust and smaller spines. Around the two enditic parts an oval circle of at least 24 setae is present around the outer margin of the enditic protrusion (Fig. 5A1, A2). These setae are very thin and acute around the proximal surface and slightly longer distally. In all, the arrangement though similar to that of the basipodal endite, appears much more fragile and brush-like.
The two-segmented endopod, around 110 µm in length, inserts medio-distally on the basipod (Fig. 5A1–A4). Its proximal podomere is rod-like and medially extended into a medio-distally pointing endite armed with numerous setae (Fig. 5A1). Here the enditic armature is distinctly divided into three sets, the anterior and posterior rows of setae, 5 in the posterior row, and a few median spine-like setae on the central hump. The cuticle below the endite is also membranous so that the joint membrane is made of two parts, one from the basipod and one from the endopodal podomere. The distal segment is of similar length as the proximal one, but smaller in diameter and designed as a rounded cone or tube with the setation arising from an oval mediodistal area. The arrangement is again circular, with fine setae around the proximal curve, progressively more rigid setae towards the tip and the most robust spine-like seta terminally (Fig. 5A1–A3). Laterally on the outer slope there seems to be another short spine. Only two or three finer setae arise from the area between the setal circle. The two endopodal podomeres are separated by membranous cuticle, preserved as granular-like surfaces (Fig. 5A1, A3, A4).
The outer edge of the basipod is rather narrow and steeply sloping. From this ridge the leaf- or paddle-shaped exopod, c. 110 µm in length, arises at the level between the joint of the first endopodal podomere and the proximal setae of the enditic armature (Fig. 5A1, A2). The basipod-exopod joint is rather poorly developed anteriorly, but sharply defined posteriorly. In anterior or posterior aspect, the shape of the exopod is elongated and triangular, as the surface narrows toward the distal end of the exopod, receiving more of a characteristic design with short annuli, but the joints between them are effaced. Only the median setae with their sockets reflect a subdivision into approximately 4 annuli. Due to this uniform surface, the marginal setation forms a continuous row from medially around the tip and distally to the joint with the basipod. It is further noteworthy that the length of the limb is almost the same from the body to the tip of the endopod and the exopod.
Enditic setae of the anterior and posterior sets as well as the exopod setae bear fine setules densely arranged in two rows (Fig. 5A1, A3). This arrangement is known also from other phosphatocopids (Maas et al. 2003), and eucrustacean taxa, but it is unknown from taxa derived from the earlier evolutionary lineage of Crustacea (e.g., Walossek 1993, see also Haug et al. 2013 on the evolution of crustacean appendages).
Second post-mandibular limb: This limb (Fig. 5A6, A7) is rather poorly preserved and details of its morphology remain unclear in specimen ZPAL Cr.11/ph060. The second post-mandibular limb looks similar to the first one. Preserved details include the setiferous proximal endite and fragments of the basipod together with the basipodal endite (Figs. 3A1, 5A6, A7). On the lateral basipodal margin of the left limb (Fig. 4A5) and the right limb (Fig. 5A6), three tiny slender setulose setae rise in a row, the distal one is the longest and measures c. 85 µm, the two proximal setae are c. 35 µm long (Fig. 5A5). By contrast, no such setae occur on the first post-mandibular limb.
The fifth appendage, or second post-mandibular limb, is the last cephalic appendage in the taxon Labrophora, which includes Phosphatocopida and Eucrustacea. Yet, in phosphatocopids there is no clear cephalic boundary, and at least two more segments are associated with the shield, forming a larger unit; the cephalothorax, convergent to several taxa within Eucrustacea and Euarthropoda.

Fig. 5. Phosphatocopid crustacean Cyclotron angelini (Linnarsson, 1875), ZPAL Cr.11/ph060 from the Olenus Biozone, Furongian, Dębki 2 borehole, northern Poland. First right post-mandibular limb in postero-median view (A1); first left post-mandibular limb in anterior view, note large paddle-shaped exopod with few distal annuli, note arthrodial membrane between endopodal segments (A2); detailed view of A2 concentrating on two-divided endopod and arthrodial membrane between endopodal segments (A3, A4); lateral basipodal margin of the second right post-mandibular limb, bearing three setae (arrows) (A5); partly preserved second left post-mandibular limb in posterior view with three setae (arrows) on lateral margin of basipod in posterior view (A6); and anterior view indicating place left by proximal endite broken off (arrow) (A7); detailed view of A1, with setation and glandular openings (arrows) on posterior side of basipodal endite (A8). Abbreviations: am, arthrodial membrane; ba, basipod; en, endopod; ex, exopod; pe, proximal endite.
Third post-mandibular limb: The sixth appendage, third post-mandibular limb, is known only from the poorly visible external part of the basipod with the basipodal endite of the left limb and part of the basipod of the right limb (Fig. 3A1). It is presumed that the general morphology largely mimics that of the preceding limbs.
Fourth post-mandibular limb: The seventh appendage, fourth post-mandibular limb, is located at the posterior end of the body (Fig. 6A1–A3), and is ventrally directed. The right limb is well preserved, whereas the left is mostly masked by matrix, except for its setae, which are partly visible. The right limb is around 230 µm long, excluding the terminal setae on the exopod. The stem is formed by an undivided, sub-rectangular basipod with a few medio-distal setae. The proximal endite could not be observed in the specimen at hand.
The endopod rises medio-distally on the basipod, as in the preceding limbs, but it is undivided and carries one terminal long and two short anterior and posterior setae (Fig. 6A1–A3). The exopod rises on the distal margin of the basipod. It is paddle-shaped, but not annulated, and directed ventrally. The exopod bears 21 setulose setae around its entire free margin, most setae at the outer margin are broken off close to their base. The folded arthrodial membrane, which links left and right limbs with the ventral body proper, is well-developed (Fig. 6A3).
Stratigraphical and geographical range.—Furongian, Olenus Biozone, Sweden (see Williams et al. 1994 and Hinz-Schallreuter 2000) and Poland.
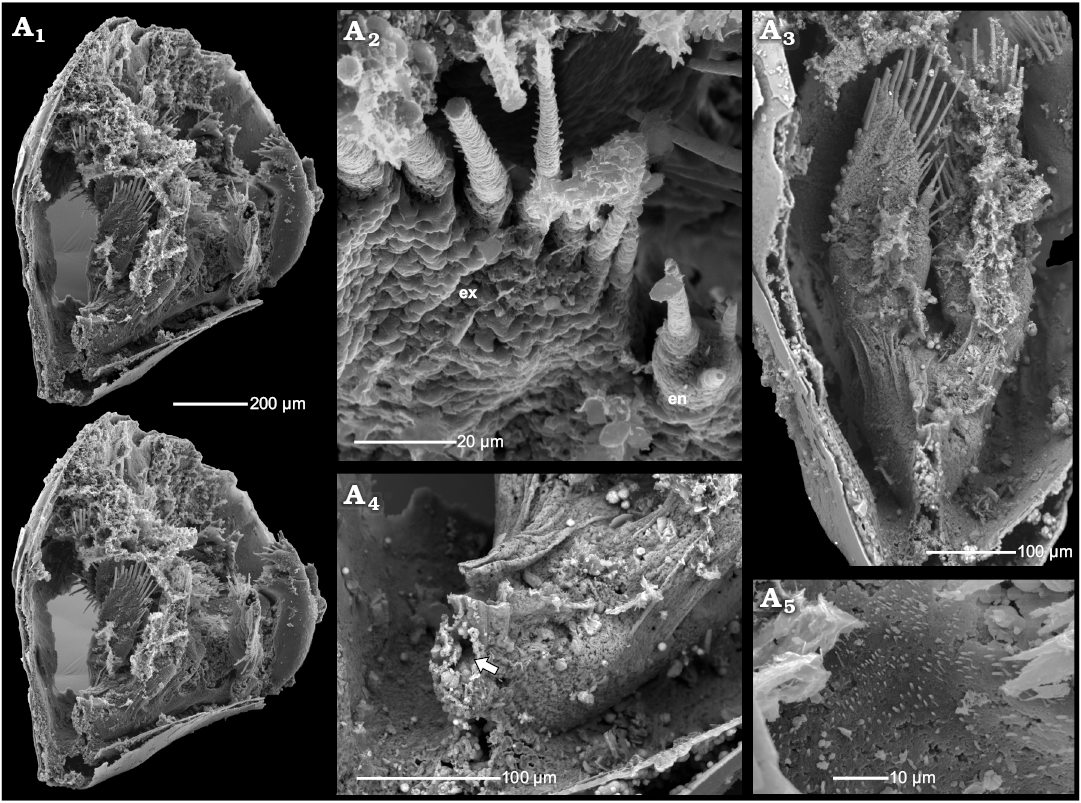
Fig. 6. Phosphatocopid crustacean Cyclotron angelini (Linnarsson, 1875), ZPAL Cr.11/ph060 from the Olenus Biozone, Furongian, Dębki 2 borehole, northern Poland. Stereo-pair in postero-lateral view with 7th limb (A1); detailed view of distal part of 7th limb in poster-ventral view, exhibiting membranous wall and setulose setae of the exopod, note tiny endopod in postero-ventral view (A2); details of 7th limb in posterior view, note arthrodial membrane in distal part (A3); posterior part of the body proper with possible anus (arrow) (A4); detailed view of sternum surface covered with fine setules (A5). Abbreviations: en, endopod; ex, exopod.
Cyclotron sp.
Fig. 7.
Material.—Two separated valves (ZPAL Cr.11/ph095, ZPAL Cr.11/ph102), Furongian, Olenus Biozone, Dębki 2 borehole, northern Poland.
Description.—The specimens of Cyclotron sp. occur as single valves with partly preserved shell layer covered by the inner lamella (here meaning the lower cuticle or surface of the shield expanding between margin and body proper). In the dorsal part of the inner lamella, several small rod-like internal invaginations occur (Fig. 7) bearing attached remains of putative muscle strands. They are interpreted here as the apodemes, serving as a site of insertion for muscles running into the limbs. In extant arthropods, apodemes are infoldings of the cuticle and may occur as rods, flanges or nodes (e.g., Manton 1964; Moore and McCormick 1969; Coleman 2002; Brusca and Brusca 2003; Oliveira and Meyer 2013). In some cases, as on the distal ends of the appendages of onychophorans, a small slit-like pore still indicates the invagination (Oliveira and Mayer 2013).
Right and left phosphatocopid valves with apodemes have been found separately in the same sample. The sizes of the valves are identical and the apodemes are in corresponding positions. Hence, the valves are interpreted as belonging to the same specimen or at least to the same ontogenetic stage. In both valves, apodeme pairs occur in the same position, corresponding to the limb insertions (Fig. 7A1–A3, B1–B3). Possibly they represent attachment places of the antenna, mandible and three post-mandibular limbs. In both valves at the point of the mandible muscles insertion, only a relatively wide proximal part of the cuticle invagination is preserved (Fig. 7A2, B2). There are also two smaller extensions of the cuticle close to the posterior margins of the valves without preserved apodemes, which may also represent the places where additional limbs arise. The insertion of the antennula is not preserved, as it inserts on the antero-lateral slope of the hypostome.
The apodemes are approximately 100–110 µm long extending medially with rounded hook-like apices. All apodemes are oriented almost perpendicular to the internal surface of the cuticle and are gently inclined posteriorly. On the dorsal apodeme surfaces there are narrow keels (Fig. 7A3, B1). On the proximal part of the ventral surfaces small cavities, c. 20–23 µm in diameter, occur (Fig. 7A4, A5, B3–B7). The fragmentarily preserved, inferred muscles from the appendages are attached inside these cavities (Fig. 7A4, A5, B4–B7). Each muscle bundle is composed of numerous muscle fibres of nanomicron-scale (Fig. 7A4, A5, B4–B7). Some muscles are partly covered by a putative phosphatised bacterial film (Fig. 7B6).
The two sides of the apodemes differ morphologically; whereas their anterior sides are smooth (Fig. 7B1, B3), their posterior sides exhibit small elongate transversal grooves (Fig. 7A3, B8).
In addition, in the antero-dorsal part of the valves, small structures (remains of labral muscles?) occur that probably belong to the hypostome-labrum complex (Fig. 7A6, B9).
On the inner surface of the cuticle layer, close to the dorsal marginal area and between the apodeme structures, two scar-like structures are developed as small nodes (Fig. 7A2, A3, B2).

Fig. 7. Phosphatocopid crustacean Cyclotron sp. from Olenus Biozone, Furongian, Dębki 2 borehole, northern Poland. A. ZPAL Cr.11/ph095. Internal view of right valve depicting shell, inner lamella and partly preserved cuticle with apodemes in dorsal part, anterior to the right (A1); detailed view of A1 exhibiting apodemes (numbers of supposed limbs: antenna (2nd), mandible (3rd) and three post-mandibular limbs (4th–6th) and structures interpreted as remains of muscles of hypostome/labrum complex; anterior is up, two nodes, possibly representing muscle scars (arrows) (A2); enlarged posterior part of valve with two apodemes (4th and 5th) and two nodes, possibly representing muscle scars (arrows) in oblique dorso-posterior view, anterior to the right (A3); enlarged proximal part of apodeme (2nd) with muscle fibers attached inside (A4); detailed view of apodeme (4th) with attached putative muscle (A5); detailed view of supposed right proximal part of hypostome-labrum structures, anterior is up (A6). B. ZPAL Cr.11/ph102. Left valve depicting shield, internal lamella cuticle and body cuticle wall with preserved apodemes (B1); detailed view of B1 exhibiting apodemes (2nd–6th), two nodes, possibly representing muscle scars (arrows) (B2); apodemes in oblique latero-ventral view (B3); detailed view of putative muscle fibers within apodeme (4th) (B4); apodeme (4th) in internal view showing putative muscle attached in proximal depression (B5); detailed view depicting proximal part of apodeme (5th) with putative muscle fibers (B6); apodeme (5th) in median view (B7); apodeme (4th) in posterior view (B8); detailed view of supposed proximal hypostome-labrum structures (arrow), in dorsal view (B9). Abbreviations: in, inner lamella cuticle; lst, limb stem; sh, shield.
Discussion
Phosphatocopid specimens in Orsten-type preservation, i.e., with three-dimensionally preserved soft parts, range in shield length from around 100 to 1000 µm. However, the best preserved specimens are those ranging between 100 and 200 µm (see Maas et al. 2003 for material from Sweden). Thus, larger specimens (later growth stages) with soft parts are lacking for most species, even in the abundant collections of well-preserved Orsten material from Sweden (Maas et al. 2003). The species known in most detail is Hesslandona unisulcata Müller, 1982 from the Agnostus pisiformis Biozone of Sweden, with specimens representative of eight ontogenetic stages (Maas et al. 2003). However, several other species have yet to be studied in detail, e.g., Vestrogothia spinata Müller, 1964 and Hesslandona necopina Müller, 1964.
It is assumed that the smallest specimens of phosphatocopids, with the ocular and antennular somite, the segments of three further functional limbs and a short undifferentiated trunk bud, represent the first post-embryonic hatching stage of the taxon (e.g., Haug et al. 2014). In fact, except for the large, all-enclosing shield, this morphology is characteristic of the so-called “head-larva” of Euarthropoda, in accordance with the terminology of Walossek and Müller (1990; see also Walossek 1993 for a detailed discussion of the ontogeny of Eucrustacea). Such a larva represents an autapomorphy in the ground pattern of Euarthropoda and, accordingly, is retained as a plesiomorphy in the taxon Crustacea (Maas et al. 2003; Haug et al. 2014).
Comparisons of the appendage morphologies
The ontogenetic development of the limbs of Cyclotron angelini cannot be assessed because the earlier growth stages are lacking. Therefore, comparisons of limb morphologies can only be made with other taxa (and then, by default, it pertains to smaller specimens).
We do know however, for example, that exopods as well as endopods may be reduced to even lacking in some species (e.g., Walossek et al. 1993). Also the intra and interspecific variability of different structures remains poorly known. An example is the basal part of the hypostome, which may be heart-shaped, as in specimen ZPAL Cr. 11/ph 222 (Fig. 8B1) from the Furongian of Poland, or sub-rectangular, as in the undetermined specimen ZPAL Cr. 11/ph 202 (Fig. 8C). Since this feature is almost unknown from other species and is not preserved in C. angelini, its diagnostic/taxonomic significance remains unexplored. However, the hypostome-labrum complex is a phylogenetically highly valuable character set within euarthropod and crustacean evolution and for arthropod terminology. As the phylogenetically older structure, the hypostome, was already present in the character set (ground pattern) of the euarthropod stem species. Accordingly it was also present, as plesiomorphic retention, in all major in-group taxa, such as in trilobites, chelicerates, tracheates, and crustaceans, as developed in the various Orsten taxa derived from the early evolutionary lineage of Crustacea (e.g., Martinssonia elongata, Müller and Walossek 1986; Cambropachycope clarksoni, Goticaris longispinosa, and Henningsmoenicaris scutula, Walossek and Müller 1990; Oelandocaris oelandica, Stein et al. 2005, 2008; for or a detailed analyses of several of the named species see Haug et al. 2009a, b, 2010). According to our knowledge of the different Orsten taxa and the different taxa within the Eucrustacea, we can conclude that the labrum is phylogenetically much younger and is exclusive/autapomorphic only to Phosphatocopida and Eucrustacea, thus characteristic for the taxon Labrophora (Siveter et al. 2003). All above mentioned derivatives from the early crustacean evolutionary lineage lack a labrum, which, together with retention of an exposed mouth opening at the rear of the hypostome and other features, indicates a less elaborate or at least different feeding mode. Hence, the possession of a hypostome only or a hypostome-labrum complex is significant for both phylogenetic and terminological reasons.
Comparisons of the antenna.—One of the most important differences between the appendages of C. angelini and those of other phosphatocopid taxa (Fig. 8A1–A3) is the morphology of the proximal part of the antenna and the lack of its exopod (Fig. 3A3–A6).
In Klausmuelleria salopensis Siveter, Waloszek, and Williams, 2003 from the uppermost early Cambrian of the UK. there is a limb stem of the antenna subdivided into coxa and basipod. Because this is the situation in all early larval stages of Eucrustracea too, it has been suggested that it represents a plesiomorphic state for Labrophora, hinting at a sister-group relationship of K. salopensis to all remaining phosphatocopids (Siveter et al. 2001, 2003: figs. 4–6; Haug et al. 2014). In known phosphatocopid taxa with preserved soft-parts, the limb stem forms a single unit, syncoxa, or the basipod remains as a tiny endite squeezed into a gap between the coxa and endopod, which came to rest on the coxal “shoulder”, from which the exopod rises (cf. Maas et al. 2003: fig. 59). In the phosphatocopid Dabashanella sp., apparently a very young growth stage, from the early Cambrian of China, the proximal part of the antenna consists of a coxa with set of setae and a subtriangular basipod bearing a median endite with seta (Zhang and Pratt 2012). By contrast, in the phosphatocopids from the Agnostus pisiformis Biozone and from the Furongian, the limb stem of the antenna is undivided and considered to represent the fusing of the coxa and basipod during ontogeny, because earlier stages still possessed a subdivided stem (e.g., Maas et al. 2003; Maas and Waloszek 2005; Dong et al. 2005; Liu and Dong 2009; Zhang et al. 2011a).
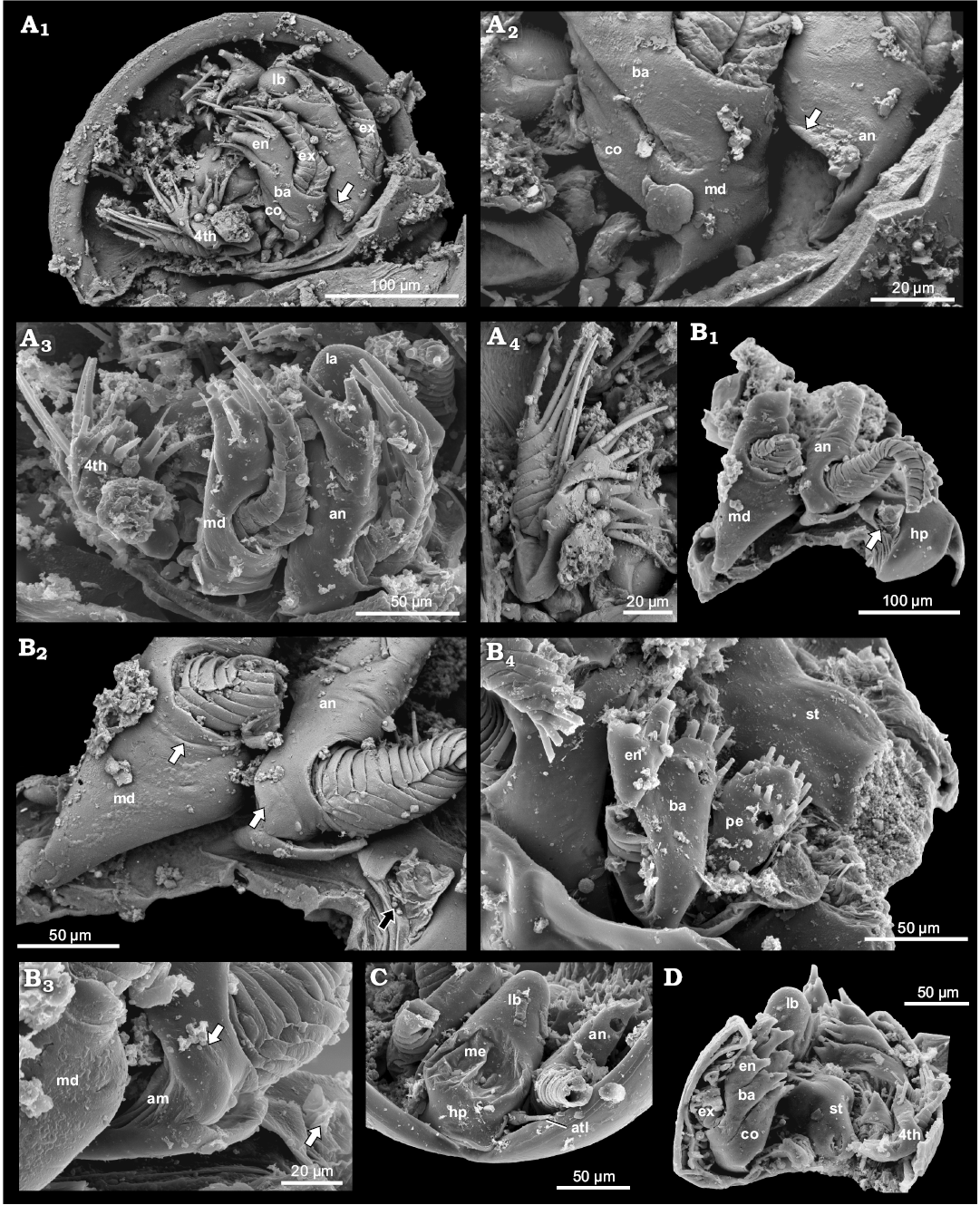
Fig. 8. Examples of phosphatocopids with soft parts preserved from the Olenus Biozone, Furongian, Dębki 2 borehole, northern Poland. A. ZPAL Cr.11/ph414, head larvae stage of Hesslandona unisulcata Müller, 1982, ventral is up; BSE image, lateral view of right side exhibiting antenna, mandible and first post-mandibular limb, small triangle-like cuticular structure at base of antenna (arrow) (A1); BSE image, detailed view of proximal part of antenna, small triangle-like cuticular structure at base of antenna (arrow), and mandible with coxa and basipod, note poorly visible border line between coxa and basipod in lateral part of limb (A2); antero-lateral view depicting antenna (arrow), mandible and first post-mandibular limb, distal part of labrum partly visible (A3); BSE image, exhibiting first post-mandibular limb, proximal endite masked by matrix (note the elongate depression on the lateral part of basipod and exopod inserting on its distal part) (A4). B. ZPAL Cr.11/ph222, an undetermined phosphatocopid; antero-lateral view of the right side depicting heart-like hypostome, inserting place of antennula (arrow); distal parts of antenna and mandible partly broken off (B1); BSE image, detailed view of proximal parts of antenna, note swollen proximal area of limb stem possibly representing lateral part of coxa (white arrow), and possible basipod with arising exopod on its distal part; mandible, note possible remains of border line (white arrow) between coxa and strongly reduced basipod; inserting place of antennula with preserved arthrodial membrane (black arrow) (B2); detailed view of proximal part of antenna exhibiting swollen part (arrow) and arthrodial membrane (B3); postero-lateral view depicting first left post-mandibular limb with relatively large setose proximal endite, basipod with inserting place of exopod developed as long depression and with partly preserved exopod and partly preserved endopod (B4). C. ZPAL Cr.11/ph202, an undetermined phosphatocopid in anterior view, representing early growth stage depicting hypostome-labrum with median eyes, pair of antennulae (arrow), and partly preserved antennae. D. ZPAL Cr.11/ph498, an undetermined phosphatocopid in view from behind, representing early growth stage exhibiting labrum, sternum, mandibles consisting of separate coxa and basipod, and right 1st post-mandibular limb. Abbreviations: atl, antennula; an, antenna; co, coxa; ba, basipod; en, endopod; ex, exopod; hp, hypostome, lb, labrum; lst, limb stem; me, median eye; md, mandible; pe, proximal endite; st, sternum.
According to Haug et al. (2013: 42, fig. 2.3.F), phosphatocopids show an “unclear condition of a coxa-basis border in the second appendage” meaning that a two-part condition is recognizable in the earlier larval stages, but a clear division is not present in all taxa in subsequent growth stages. This implies that the subdivided state represents a primary condition, whereas the secondary/derived state is the fused one, or the one with a reduced basipod stem portion. Because there clearly is no coxa in all presumed pre-labrophoran crustacean “stem taxa” and other Eucrustacea, our suggestion is that the coxa-basipod subdivision with the formation of a new joint and reorganisation of the basal limb musculature is autapomorphic to Labrophora. In Eucrustacea, the plesiomorphic condition of a subdivision was originally retained, but subsequently also modified in many ways; the extreme modification being the retention of the coxa only (see Walossek 1993 for an extensive discussion of eucrustacean taxa). Importantly, it has been demonstrated that the mandibular coxa of eucrustacean taxa develops ontogenetically from the proximal endite (Walossek and Müller 1990; Walossek 1993; Haug et al. 2013). Again, the proximal endite is the evolutionary precursor to the coxa, at least for the mandible (e.g., Walossek 1993). Hence, the presence of a coxa excludes the presence of a proximal endite and vice versa.
However, there are still some details left to be investigated. For example, the evolutionary significance and fate of the gnathobasic seta, a seta associated with the proximal endite and coxa and significantly being involved in food transport (Walossek 1993). Also, the division of the coxal median edge into originally three setal rows, the main spines terminally, flanked by an anterior and a posterior row (a situation extending into basipod and endopodal enditic armature; see Maas et al. 2003: fig. 66A). This feature is often abandoned by many extant crustaceans, which exhibit a two-division of the coxal median edge (cf. Walossek 1993), especially the malacostracan limb morphology is still incompletely understood in the absence of key fossil data (Haug et al. 2013).
The cuticular area on the proximo-lateral margin of the antenna of C. angelini (Fig. 3A3–A5) is somewhat similar to a structure occurring at the same position in the antenna of an undetermined phosphatocopid specimen (ZPAL Cr.11/ph222), from the Dębki 2 borehole. This latter specimen bears at least five limbs and represents a later growth stage (Fig. 8B1–B3). The swollen structure at the base of the antenna (Fig. 8B3) merges into the arthrodial membrane and may indicate the extension of the coxal body. Additionally, the well-preserved specimen ZPAL Cr.11/ph414, identified as H. unisulcata Müller, 1982, from the same borehole, most likely representing a head-larva or very early instar, exhibits a small triangular proximo-lateral structure separated from the rest of the limb by a clear boundary (Fig. 8A1–A4). All these structures are not setose and appear immovable. It cannot be excluded that this structure represents the remains of the coxa. Because of its location we exclude a direct association with the proximal endite in its initial extension being located medially underneath the basipod. A medio-proximal setiferous endite is present in young stages of various entomostracan crustaceans on post-antennal limbs. In advanced stages it is still present on post-mandibular limbs whereas the mandibular proximal endite has turned into the coxa (Walossek 1993; Haug et al. 2013). Until more details are known, we refer to this structure as a cuticular plate on the proximo-lateral margin of a limb.
The antenna of C. angelini differs significantly from almost all known phosphatocopid species, i.e., in lacking the exopod. The only exception is an undetermined species from the Cambrian Series 3, Devoncourt Limestone of Australia (Walossek et al. 1993). In that species, from which only isolated appendages were recovered, remarkably, not only the antennal exopods are lacking, but also the exopods on all known appendages (Walossek et al. 1993: fig. 3A, B), i.e., the animal may have been completely uniramous. In this Australian form, the basal portion of the antenna is as undivided as in other phosphatocopids and medially elongated. Its shovel-like end rests in a similar median surface of the mandible, but it is not well enough preserved for assessing other details (Walossek et al. 1993: fig. 2B). Medio-distally a short, two-segmented endopod rises. The proximal podomere is drawn out into a spine-like projection and surrounded by several short spines or setae; the terminal portion is a conical hump with a terminal spine-like seta flanked by one spine on either side, but, different from those of other phosphatocopids, turned 90° inward toward the hypostome. According to Walossek et al. (1993), the lack of exopods of head and trunk limbs may be due to adaptations to a benthic lifestyle. The lack of exopods in the antennae likely is connected also to the significant involvement of the antennae in food gathering rather than locomotion, rendering the exopod obsolete. A connection with the bivalved morphology, in which a long annulated exopod hinders complete closure of the valves, seems to be a weak argument since that pertains to all phosphatocopids, including those with well-developed exopods. Based on the many specimens preserved with gaping shields (“butterfly mode”), it seems that phosphatocopids did not completely close their shields for much of their lives but rather carried them like a roof, obliquely outward. Yet, completely enclosed specimens are also well-known from the fossil record (e.g., Maas et al. 2003; Eriksson et al. 2016), but it remains uncertain how such closure functioned as there is no space for a closing muscle in phosphatocopids. The opening of the shields may have occurred by pressing the limbs laterally; the prominent limb apodemes with presumably well-developed limb musculature supports this assumption.
Comparisons of the mandible.—The morphology of the mandible of phosphatocopids in the early ontogenetic stages differs from that of advanced stages and still is a matter of debate (see Haug et al. 2013). Nonetheless, detailed structural information is available for the head larva stage (or at least early larval instars) of several species, e.g., Klausmuelleria salopensis from the Cambrian Series 3 of England, Hesslandona angustata Maas, Waloszek, and Müller, 2003 from the Furongian (Paibian Stage) Bitiao Formation of South China (Zhang et al. 2011a: fig. 1; 2012: fig. 1), Hesslandona suecica Maas, Waloszek, and Müller, 2003 from Sweden and China (Maas et al. 2003: fig. 59B; Waloszek et al. 2007: fig. 2D; Liu and Dong 2010: fig. 1c), Hesslandona necopina from China (Zhang et al. 2011b), and Vestrogothia spinata from the Furongian Peltura Biozone of Sweden (Maas and Waloszek 2005: fig. 2A) and the late Cambrian of South China (Liu and Dong 2009: fig. 3). In all these taxa, the limb stem of the mandible of the earliest growth stage comprises a clearly separated coxa and basipod of similar size, and the exopods of the mandibles extend from the proximo-lateral part of the basipod.
Here, we can add the specimens ZPAL Cr.11/ph414 (Fig. 8A) and ZPAL Cr.11/ph498 (Fig. 8D) from Poland, representing head larva stages that possess a coxa and basipod being distinctly separated and medially developed as long setose endites. Antero-laterally, the border between the coxa and basipod is less well developed than posteriorly. The exopod of the mandible in these specimens likewise extends from the proximo-lateral part of the basipod.
By contrast, the rigid proximal portion of the mandible of advanced ontogenetic stages, including the C. angelini specimen described herein, represents an undivided “limb stem” or syncoxa, interpreted as the product of fusion of the coxa and basipod (e.g., Maas et al. 2003; Dong et al. 2005).
In the second growth stage of Hesslandona necopina Müller, 1964 from the Furongian of South China, the coxa and basipod are still separated on the mandible (Zhang et al. 2011b: fig. 1). However, in the third growth stage the coxa and basipod are partly fused and the “basipodal endite” is developed as a wedge-shaped portion inserting between the coxa and endopod, a condition that was described also by Maas et al. (2003) for early stages of H. unisulcata. The fusion of the coxa and basipod in H. necopina is identical to that described for V. spinata by Maas et al. (2003).
Again, the morphological condition of a prominent proximal limb portion being medially extended into an obliquely oriented spinose gnathobase with median setation (= coxal endite representing the evolutionary older proximal endite) and a distally located setose endite (basopodal endite) (see also Maas et al. 2003: fig. 57B; Maas and Waloszek 2005) is similar in at least euphosphatocopid species. According to Haug et al. (2013: 49) the proximal gnathobase, in fact representing the coxa alone, is an “extreme variation of the proximal endite”. The remainder of the basipodal endite indicates that only the lateral part of the basipod became fused to the coxa (forming a so-called syncoxa), thus obscuring the original extension of the basipod in this specimen.
The reduced lateral part of the basipod with connection to the median “basipod endite” is still present in an advanced growth stage of Vestrogothia spinata Müller, 1964 from Sweden, comprising a small structure surrounding the base of the exopod (Maas et al. 2003: fig. 59C; Waloszek 2003: fig. 3.5; Haug et al. 2013: 54). The ring-shaped structure developed around the insertion of the exopod in C. angelini (Fig. 4A3), may likewise represent a strongly reduced basipod. Because specimen ZPAL Cr.11/ph060 represents a later growth stage, the reduction of the basipod without connection to the “basipodal endite” has advanced further than in the V. spinata mandible presented by Maas et al. (2003). Such proximal rings around the exopods in early growth stage specimens from Poland (and other specimens described so far) have not been observed. However, the rings can be interpreted as the blown-up exopodal arthrodial membrane. Furthermore, this structure may come from an even older condition described from early euarthropods (see, e.g. Liu et al. 2007 for the early, coxa-less, chelicerate Leanchoilia illecebrosa (Hou, 1987), in which the exopod is divided into a proximal and a distal portion).
The endopod in the late ontogenetic stages arises laterally on the limb stem. By contrast to the post-mandibular limbs, the exopod of the mandible “arises” on the antero-lateral part of the mandibular limb stem. The mandible of C. angelini differs from that of all other known phosphatocopids from Europe in having an exopod that is strongly reduced in size and lacks annulation (Figs. 3A1, A5, A6, 4A1, A3). By contrast, the exopod of the mandible of ZPAL Cr.11/ph222 is strongly developed and multiannulated (Fig. 8B2). Exopods in young larvae are tubular, multiannulated and each annulus is carrying a long seta on the median part (Fig. 8A1) (see also Maas et al. 2003 for the same morphology in various species; e.g., their pl. 24A for Hesslandona suecica).
Setae of different length arise in a row around the free margin of the mandibular exopod from proximo-medially to medially (Fig. 3A5), more similar to the exopod of the maxillula of Swedish Orsten eucrustaceans such as Rehbachiella kinnekullensis Müller, 1983 (Walossek 1993). Compared to the Australian phosphatocopid specimen, in which the exopod is lacking on all limbs (Walossek et al. 1993), the situation in C. angelini indicates that the life style of some phosphatocopids allowed for a reduction in size and function of the mandibular exopod until its complete loss. The exopod in C. angelini was apparently jointed and functional, but most likely less suited for strong water propulsion.
Comparisons of post-mandibular limbs.—The first post-mandibular limb of C. angelini (Fig. 5) is overall similar to that of many Cambrian phosphatocopids from Sweden and China. However, some differences exist, e.g., some have a two-segmented endopod, as in the case of C. angelini, instead of a three-segmented one, and the former species has much shorter enditic protrusions of all portions of the post-mandibular limbs than the advanced growth stages of other species, e.g., compare with Hesslandona unisulcata Müller, 1982 (Maas et al. 2003: pls. 11D–F, 12B, G).
The second post-mandibular limb of C. angelini differs in having three setae located at the proximo-lateral margin of the basipod (Fig. 5A5, A6). A single seta occurs in a corresponding position on the second post-mandibular limb of Hesslandona curvispina from Sweden (Maas et al. 2003: pl. 30D). These setae are situated in a position corresponding to that of epipods occurring in some eucrustaceans (Maas et al. 2009; Waloszek et al. 2014). Based on the occurrence, morphology and ontogeny of epipods in Eucrustacea Maas et al. (2009) concluded that these structures develop ontogenetically from the base of an original seta. Setae at a corresponding position may therefore be interpreted as possible ontogenetic initials of epipods. However, first, no further growth stages are known of C. angelini, and second, the material known is already advanced and should possibly bear more differentiated epipods by those stages. Therefore, this question must be left open until additional material can demonstrate more clearly the ontogenetic and evolutionary occurrence of the epipods in the early evolutionary lineage of Crustacea.
The post-mandibular limbs of C. angelini and H. suecica from the Cambrian of Sweden, likewise known from advanced growth stages (Maas et al. 2003: pl. 24A, B), are morphologically similar. Yet, those of C. angelini differ in having fewer annuli at the tip of the exopod, short protrusions on the proximal endite and basipod, and in possessing a two-divided rather than three-divided endopod. The morphology of the series of post-mandibular limbs suggests that in C. angelini they mainly served for locomotion and food gathering, unlike most other phosphatocopids, where the antennae and mandibles played a much larger, if not principle role also in locomotion, both equipped with long, multiannulated exopods (see also Müller 1982: figs. 5, 6), whereas the post-mandibular series swept the nutrient-rich water along the inner side of the valves and transported it inward anteriorly again by means of the median setation and spines (see also Maas et al. 2003.).
Further notes on the soft body morphology.—Although it is not present in the material investigated herein, it is important to briefly highlight the forehead including the visual system of Phosphatocopida. The forehead is a prominent joint structure, which is made of the phylogenetically older box-shaped hypostome and the likewise firm backward pointing labrum (this entire structure was referred to as the hypostome-labrum complex by Maas et al. 2003: 29, pls. 6B, 21A; see also Fig. 8C), which in later growth stages is more distally raised. In eucrustaceans the hypostome is rather short and narrow, whereas the labrum is generally a soft, bulging structure, often filled with glands that open posteriorly to slime-in food. In fact also in phosphatocopids the posterior surface of the labrum, which steeply declines toward the deeply recessed mouth opening, the flanks are covered with fine short setae, as e.g. in extant cirripedes, and the median surface bears sensilla and openings (e.g., Müller 1979: fig. 3C; Maas et al. 2003: fig. 15), which may be the pores of glands to slime-in food as known from various small eucrustaceans.
Phosphatocopids possess a prominent eye structure with three cups surrounded by a more or less prominent ring wall, which is located medially at the anteriorly sloping surface of the hypostome (Maas et al. 2003: pls. 9A, 11C, 21A; the two anterolateral cups being more elaborate than the single posteriomedian cup, naupliar eye clearly externally. Similar external median eyes have been reported for several other “Orsten” eucrustaceans, such as Rehbachiella kinnekullensis Müller, 1983 (Walossek 1993), Bredocaris admirabilis Müller, 1983 (Müller and Walossek 1988) and Wujicaris muelleri Zhang, Maas, Haug, Siveter, and Waloszek, 2010; at least an anteromedian eye made of two cups was reported for one of the derivatives of the early stem lineage of Labrophora, Oelandocaris oelandica Müller, 1983 (see Stein et al. 2005, 2008).
We interpret this structure as the median eye; a plesiomorphic feature apparently retained from the ground pattern of Euarthropoda. It had developed in addition to the paired compound eye, which is located anterolaterally and belongs to the first known segment of Arthropoda sensu stricto (e.g., Waloszek et al. 2007). A median eye is known first from euarthropods and is present in chelicerates (paired external eye cups) and, among tracheates in insects, visible externally on the front of the head as a three-cupped structure in insects. Therefore, we regard all anteromedially located structures at the anterior end of the hypostome, including those of all phosphatocopids, as having the same evolutionary origin. In Eucrustacea this structure is called nauplius eye because it can be present already in the hatching stage in all major in-groups. It may persist into the adult stage. In eucrustaceans the nauplius eye is always an internal structure with the eye cups below the cuticle. However, in the Cambrian fossils this structure is external (with the possible exception of one taxon, Skara sp. where two weak impressions hint at an internal location; see Müller and Walossek 1985: pl. 5: 1–3). This contrasts with the interpretation of Zhang and Pratt (2012) and we disagree with their morphological explanation concerning the eye and its location on an extra anterior body segment, not least in light of a strikingly similar morphology in the early larva of Wujicaris muelleri (Zhang et al. 2010). If not the eye itself, possibly the specific arrangement of the three cups, two larger bulging anterolateral ones and a smaller to slightly inwardly pressed medioposterior one surrounded by a cuticular ridge (e.g., Müller 1982: pls. 2: 2–4, 3: 1, 4: 1, 2; Maas et al. 2003: pl. 37A, B) can be understood as another feature to unite Phosphatocopida (autapomorphy), but the images of Dabashanella sp. in Zhang and Pratt (2012) give no clear clues for this feature, and we recommend any further researchers to pay special attention not only to the eye but the complete antero-ventral body region of Euarthropoda and Crustacea.
The status of the compound eyes remains unclear. Eucrustacea possess, plesiomorphically, compound eyes (a feature retained from Arthropoda sensu stricto; see Waloszek et al. 2005), and these are missing in phosphatocopids. Compound eyes have been validated only for one of the known taxa that derived from the early evolutionary lineage of Labrophora, so it is likely that phosphatocopids lost these eyes early during their evolution.
Musculature and apodemes
The musculature of phosphatocopids is virtually unknown, as holds true also for most Orsten-type arthropods. The exception includes muscles detected within limbs and the trunk of Furongian pentastomid parasites from Öland, Sweden (Andres 1989) and muscle scars, and putative antennal muscles, reported from the Orsten eucrustacean Skara Müller, 1983 (Eriksson et al. 2012). Paired labral muscles have been recorded also by synchrotron radiation X-ray tomographic microscopy in Hesslandona species; these muscles are interpreted as being used for controlling the labral movement (Eriksson et al. 2012, 2016), similar as was described for the eucrustacean Skara anulata and S. minuta by Müller and Walossek (1985: pls. 4.2, 4.3 for the external impression of these muscles).
Phosphatocopids lack muscles for closing and opening their valves and hence adductor muscle scars are absent (cf. ostracods; Smith 1971; Anderson 1974; Olempska 2008; Yamada and Matzke-Karasz 2011). Two nodes preserved on the inner walls of the cuticle layer close to the dorsal marginal area in both valves of Cyclotron sp. may represent scars of limb muscles.
The large medio-proximal opening into the interior of the mandibular coxa of C. angelini (Fig. 4A1) may suggest that it served as the path for the large mandible muscles operating the limb-stem. The absence of apodemes in places of the mandible insertion suggests that the system of its insertion was similar to that of other limbs, but probably the apodeme of the mandible was much larger than the others and was broken off or not phosphatized. There are close similarities in the type and possible function of the apodemes in phosphatocopids and modern crustaceans (see, e.g., Hessler 1964 for the muscular system of extant cephalocarid Entomostraca). An occurrence of apodemes in phosphatocopids is expected because apodemes were suggested to be present in the ground pattern of Euarthropoda, hence, being plesiomorphic in Crustacea, Phosphatocopida, and Eucrustacea. Recent studies on onychophorans, derivatives of the earliest phase of arthropod evolution before sclerotization of the cuticle took place, revealed that apodemes as cuticular muscle attachment cites are even older than that (Oliveira and Mayer 2013). Although crustacean apodemes are well known, they have never been investigated in an evolutionary or phylogenetic context.
Functional morphology
In Cyclotron angelini the antennae without exopods and the mandible, with a rather short, undivided and rod-shaped exopod, were probably mainly involved in food gathering and passing food particles toward the mouth. By contrast, in earlier larval stages, as known particularly from many specimens of the Swedish taxa, these appendages had long, multi-articulated exopods with long swimming setae. Therefore, they were used in parallel for locomotion and sweeping in food (see e.g., Maas et al. 2003 for Hesslandona unisculcata). Size reduction of the exopods in later ontogenetic stages likely corresponds to a shift of the locomotory function to the more posterior limbs. The function of the apparently reduced exopod of the mandible is unclear, possibly it served as for cleaning rather than production of water currents. In early growth stages the exopods of both antenna and mandible are multi-annulated with a prominent marginal setation. Presumably, phosphatocopids of advanced growth stages with serially similar post-mandibular limbs were able to create water currents around the body by synchronized movement of these limbs in anterior and posterior directions by large paddle-shaped exopods armed with long setae. As in many extant crustacean larvae, the locomotory apparatus developed in the anterior body in young growth stages and shifts successively towards the posterior as body segments are added and differentiation of post-mandibular limbs occur (Haug et al. 2013). Hence, the anterior limbs (antenna and mandible) are released from the locomotory apparatus and become involved in feeding (see especially Walossek 1993 for a detailed analysis of feeding and locomotory strategies in combination with the ontogeny in Crustacea).
The endopodal robust dentate tooth of the C. angelini mandible, which probably served as a cutting tooth, and the gnathobase together with the gnathobase-like structure of the antenna were involved in the manipulation and food transport to the mouth. The proximal endite of the first post-mandibular limb of the undetermined specimen ZPAL Cr.11/ph222, is much larger relative to the basipodal endite (Fig. 8B4) compared to that of C. angelini.
According to Haug et al. (2014), the anterior three appendages in the head larva of Crustacea sensu lato mainly work in conjunction for locomotion and feeding, and the fourth limb may also have assisted in locomotion (see also Waloszek et al. 2007; Perrier et al. 2015). The proximal endites and endopodal segments of the post-mandibular limbs armed with many setae may also be active in catching food particles by endite spines, transporting them toward the mouth and preventing their backflow (see also Waloszek et al. 2007; Haug et al. 2014). In addition, the membranous areas present between segments of post-mandibular limbs in Cyclotron angelini (Fig. 5A1, A3, A4) suggest great manoeuvrability of the limbs and thus allow for effective food transport toward to the mouth via gnathobases of the mandible and antenna. Similar membrane areas occur in the post-mandibular limb of H. unisulcata from Sweden (Maas et al. 2003: pl. 10B).
In summary, in connection with the different morphologies of limbs in phosphatocopids, with or without exopods, long and short endopodal protrusions, it is obvious that their mode of food intake was very diverse. Modes of feeding and locomotion remain speculative for a number of phosphatocopid species.
Phylogenetic remarks
Monophyly of Phosphatocopida can be based on a number of autapomorphies (see above and Maas et al. 2003; Siveter et al. 2003; Maas and Walossek 2005).
The first internal phylogeny relationship of Phosphatocopida was presented by Maas et al. (2003). They suggested that Vestrogothia spinata and Falites fala constitute a paraphyletic group, Vestrogothiina, whereas the authors regarded Hesslandonina and Hesslandonidae as monophyla, respectively. Hinz-Schallreuter and Schallreuter (2009) proposed a new phosphatocopid phylogeny based on the evolution of the dorsal shield structures. Their analysis differs considerably from that of Maas et al. (2003), e.g., with regards to the position of Vestrogothia situated deeply within the taxon. In a subsequent analysis by Zhang et al. (2011a), Vestrogothia and Falites did not come up as close relatives and Hesslandonidae did not show up as a monophyletic taxon (see also Zhang and Dong 2009). The latter result was reinforced in a subsequent study by Zhang et al. (2011b), in which Hesslandona unisulcata appears to be more closely related to Trapezilites minimus (Kummerow, 1931), than to any other Hesslandona species. Again, the position of Vestrogothia together with Falites was proposed as a monophyletic group with a sister-group relationship to a monophyletic Hesslandonidae, hence much in accordance with the analysis of Maas et al. (2003). The position of the species studied herein, Cyclotron angelini, may be close to the monophylum comprising Cyclotron lapworthi (Groom, 1902), Waldoria rotundata, and Veldotron bratteforsa (Müller, 1964) (cf. Zhang et al. 2011b).
More details, particularly of the appendage morphology of different growth stages and species are, however, needed in order to establish a more firmly validated phylogeny for C. angelini.
Conclusions
Investigation of the soft-part morphology of Cyclotron angelini yielded a combination of morphological features, previously unknown from any other phosphatocopid species. These features include: (i) absence of the exopod on the antenna (a feature previously known only from one unnamed species from the Cambrian of Australia, Walossek et al. 1993); (ii) a cuticular area at the lateral margin of the antenna, possibly the coxal portion of the otherwise uniform limb stem; (iii) very small, and not annulated, exopod for the mandible (in the Australian form it lacks completely, forms from Sweden exhibit a prominent, multi-annulated exopod); (iv) a triplet of very similar setae at the distal endopod segments of the antenna and mandible; (v) difference between proximal endopodal segment of the antenna and that of the mandible; (vi) the exopod of post-mandibular limbs progressively increasing in size and armature from the anterior to the posterior (exopod absent in the Australian species); (vii) a two-segmented endopod of the first post-mandibular limb (not preserved in 2nd and 3rd limbs; three-segmented in all other known taxa with preserved soft parts); (viii) the presence of apodeme structures, most likely functioning for muscle attachment and operation of the limbs.
The range of specific features of C. angelini from Poland in combination with its morphological deviation from the many described species from Sweden indicates a much larger morphological diversity of phosphatocopid species than previously thought (cf. Maas et al. 2003).
Acknowledgements
EO thanks Wiesław Bednarczyk (Institute of Geological Sciences PAS, Warsaw, Poland) for placing geological samples from Dębki 2 at her disposal. Renate Matzke-Karasz (Ludwig-Maximilians-University of Munich, Germany), Mark Williams (University of Leicester, UK) and two anonymous reviewers are thanked for their constructive reviews of our manuscript. MEE acknowledges the Swedish Research Council (grant 2015-05084) for funding.
References
Andersson, A. 1974. Musculature and muscle scars in the cytherid ostracode Cytheridea papillosa (Bosquet). Zoologica Scripta 3: 83–90. Crossref
Andersson, A., Dahlmann, B., Gee, D.G., and Snäll, S. 1985. The Scandinavian Alum Shales. Sveriges Geologiska Undersökning Ca 56: 1–50.
Andres, D. 1989. Phosphatisierte Fossillien aus dem unteren Ordoviz von Südschweden. Berliner geowissenschaftliche Abhandlungen A 106: 9–19.
Bednarczyk, W. 1979a. On the occurrence of Bradorina Raymond, 1935 ostracods in the Upper Cambrian of NW Poland. Bulletin de L’Académie Polonaise des Sciences, Série des sciences de la terre 26: 215–219.
Bednarczyk, W. 1979b. Upper Cambrian to Lower Ordovician conodonts of Łeba elevation, NW Poland, and their stratigraphic significance. Acta Geologica Polonica 29: 409–442.
Bednarczyk, W. 1984. Biostratigraphy of the Cambrian deposits in the Łeba area. Acta Geologica Polonica 34: 95–110.
Bednarczyk, W. and Turnau-Morawska, M. 1975. Cambrian formations in the Łeba area (Northern Poland) [in Polish]. Acta Geologica Polonica 25: 537–566.
Brusca, R.C. and Brusca, G.J. 2003. Invertebrates. Second edition. 936 pp. Sinauer Associates, Inc., Sunderland.
Buchardt, B., Nielsen, A.T., and Schovsbo, N.H. 1997. Alunskiferen i Skandinavien. Norsk Geologisk Tidsskrift 3: 1–30.
Castellani, C., Maas, A., Eriksson, M.E., Haug, J.Y., Haug, C., and Waloszek, D. 2018. First record of Cyanobacteria in Cambrian Orsten deposits of Sweden. Palaeontology 61: 855–880. Crossref
Castellani, C., Maas, A., Haug, C., Hayg, J.T., and Waloszek, D. 2012. Isolated sponge spicules from the late Cambrian Alum Shale Formation (“Orsten” nodules) of Sweden. Bulletin of Geosciences 87: 443–460. Crossref
Coleman, C.O. 2002. The transverse apodeme bridge from the cephalothorax of Amphipoda (Crustacea) and its significance for systematic. Journal of Natural History 36: 37–49. Crossref
Dong, X.P., Donoghue, P.C.J., Liu, Z., Liu, J., and Peng, F. 2005. The fossils of Orsten-type preservation from Middle and Upper Cambrian in Hunan, China. Chinese Science Bulletin 50: 1352–1357. Crossref
Eriksson, M.E. and Terfelt, F. 2007. Anomalous facies and ancient faeces in the latest middle Cambrian of Sweden. Lethaia 40: 69–84. Crossref
Eriksson, M.E., Terfelt, F., Elofsson, R., and Marone, F. 2012. Internal soft-tissue anatomy of Cambrian “Orsten” arthropods as revealed by synchrotron X-ray tomographic microscopy. PloS ONE 7(8): e42582. Crossref
Eriksson, M.E., Terfelt, F., Elofsson, R., Maas, A., Marone, F., Lindskog, A., Waloszek, D., Schmitz, B., and Stampanoni, M. 2016. Baring it all: undressing Cambrian “Orsten” phosphatocopine crustaceans using synchrotron radiation X-ray tomographic microscopy. Lethaia 49: 312–326. Crossref
Groom, T. 1902. On Polyphyma, a new genus belonging to the Leperditiade, from the Cambrian shales of Malvern. The Quarterly journal of the Geological Society of London 58: 83–88. Crossref
Haug, C., Haug, J.T., Maas, A., and Waloszek, D. 2014. Fossil larvae (head larvae, nauplii, and others) from the Cambrian in Orsten preservation. In: J.W. Martin, J. Olesen, and J.T. Høeg (eds.), Atlas of Crustacean Larvae, 17–21. The Johns Hopkins University Press, Baltimore.
Haug, J.T., Maas, A., and Waloszek, D. 2009a. Ontogeny of two Cambrian stem crustaceans,†Goticaris longispinosa and †Cambropachycope clarksoni. Palaeontographica Abteilung A 289: 1–43. Crossref
Haug, J.T., Maas, A., and Waloszek, D. 2009b. † Henningsmoenicaris scutula, †Sandtorpia vestrogothiensis gen. et sp. nov. and heterochronic events in early crustacean evolution. Transactions of the Royal Society of Edinburgh: Earth and Environmental Science 100: 311–350. Crossref
Haug, J.T., Maas, A., Haug, C., and Waloszek, D. 2013. Evolution of crustacean appendages. In: L. Watling and M. Thiel (eds.), The Natural History of the Crustacea. Vol. 1. Functional Morphology and Diversity, 34–73. Oxford University Press, Oxford. Crossref
Haug, J.T., Waloszek, D., Haug, C., and Maas, A. 2010. High-level phylogenetic analysis using developmental sequences: The Cambrian †Martinssonia elongata, †Musacaris gerdgeyeri gen. et sp. nov. and their position in early crustacean evolution. Arthropod Structure & Development 39: 154–173. Crossref
Hessler, R.R. 1964. The Cephalocarida: comparative skeletomusculature. Memoirs of the Connecticut Academy of Arts and Science 16: 1–97.
Hinz-Schallreuter, I. 1993. Cambrian ostracodes mainly from Baltoscandia and Marocco. Archiv für Geschiebekunde 1: 385–448.
Hinz-Schallreuter, I. 2000. Baltoscandian Phosphatocopes. Archiv für Geschiebekunde 2: 841–896.
Hinz-Schallreuter, I. and Schallreuter, R. 2009. Phylogeny of Phosphatocopa. Memoirs of the Association of Australasian Palaeontologists 37: 151–164.
Hou, X.G., Siveter, D.J., Williams, M., and Feng, H.H. 2002. A monograph of the Bradoriid arthropods from the Lower Cambrian of SW China. Transactions of the Royal Society of Edinburgh: Earth Sciences 92: 347–409.
Linnarsson, J.G.O. 1875. Öfversigt af Nerikes öfvergångsbild-ningar. Öfversigt af Kongliga Vetenskaps-Akademiens Förhandlingar 1875: 3–48.
Liu, Y., Hou, X.G., and Bergström, J. 2007. Chengjiang arthropod Leanchoilia illecebrosa (Hou, 1987) reconsidered. Geologiska Föreningen 129: 263–272.
Liu, Z. and Dong, X.P. 2009. Vestrogothia spinata (Phosphatocopina, Crustacea), fossils of Orsten-type preservation from the Upper Cambrian of western Hunan, South China. Acta Geologica Sinica 83: 471–478. Crossref
Liu, Z. and Dong, X.P. 2010. The developmental trend of labrum and median eyes of Orsten-type preserved Phosphatocopina (Crustacea). Science China, Earth Sciences 53: 18–26. Crossref
Maas, A. and Waloszek, D. 2005. Phosphatocopina—ostracode-like sister group of Eucrustacea. Hydrobiologia 538: 139–152. Crossref
Maas, A., Braun, A., Dong, X.P., Donoghue, P.C.J., Müller, K., Olempska, E., Repetski, J.E., Diveter, David J., Stein, M., and Waloszek, D. 2006. The “Orsten”—more than a Cambrian Konservat-Lagerstätte yielding exceptional preservation. Palaeoworld 15: 266–282. Crossref
Maas, A., Haug, C., Haug,J.T., Olesen, J., Zhang, X.G., and Waloszek, D. 2009. Early Crustacean evolution and the appearance of epipods/gills. Arthropod Systematics and Phylogeny 67: 255–273.
Maas, A., Waloszek, D., and Müller, K.J. 2003. Morphology, ontogeny and phylogeny of the Phosphatocopina (Crustacea) from the Upper Cambrian “Orsten” of Sweden. Fossils and Strata 49: 1–238.
Maeda, H., Tanaka, G., Shimobayashi, N., Ohno,T., and Matsuoka, H. 2011. Cambrian Orsten Lagerstätte from the Alum Shale Formation: fecal pellets as a probable source of phosphorus preservation. Palaios 26: 225–231. Crossref
Manton, S.M. 1964. Mandibular mechanisms and the evolution of arthropods. Philosophical Transactions of the Royal Society of London B 737: 1–183.
Martinsson, A. 1974. The Cambrian of Norden. In: C.H. Holland (ed.), Lower Palaeozoic Rocks of the World, Vol. 2, Cambrian of the British Isles, Norden, and Spitsbergen, 185–283. Wiley-Interscience, London.
Moore, R.C. and McCormick, L. 1969. General features of Crustacea. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, Part R, Arthropod 4, 57–120. Geological Society of America, Boulder and University of Kansas Press, Lawrence.
Müller, K.J. 1964. Ostracoda (Bradorina) mit phosphatischen Gehäusen aus dem Oberkambrium von Schweden. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 121: 1–46.
Müller, K.J. 1979. Phosphatocopine ostracodes with preserved appendages from the Upper Cambrian of Sweden. Lethaia 12: 1–27. Crossref
Müller, K.J. 1982. Hesslandona unisulcata sp. nov. with phosphatised appendages from Upper Cambrian “Orsten” of Sweden. In: R.H. Bate, E. Robinson, and L.M. Sheppard (eds.), Fossil and Recent Ostracods, 276–304. Ellis Horwood, Chichester.
Müller, K.J. 1985. Exceptional preservation in calcareous nodules. Philosophical Transactions of the Royal Society of London 311: 67–73. Crossref
Müller, K.J. and Hinz, I. 1991. Upper Cambrian conodonts from Sweden. Fossils and Strata 28: 1–153.
Müller, K.J. and Walossek, D. 1985. Skaracarida, a new order of Crustacea from the Upper Cambrian of Västergötland, Sweden. Fossils and Strata 17: 1–65.
Müller, K.J. and Walossek, D. 1986. Martinssonia elongata gen. et sp. n., a crustacean-like euarthropod from the Upper Cambrian “Orsten” of Sweden. Zoologica Scripta 15: 73–92. Crossref
Müller, K.J. and Walossek, D. 1988. External morphology and larval development of the Upper Cambrian maxillopod Bredocaris admirabilis. Fossils and Strata 23: 1–70.
Müller, K.J. and Walossek, D. 1990. Morphology, ontogeny, and life habit of Agnostus pisiformis from the Upper Cambrian of Sweden. Fossils and Strata 19: 1–124.
Müller, K.J. and Walossek, D. 1991. Ein Blick durch das Orsten-Fenster in die Arthropodenwelt vor 500 Millionen Jahre. Verhandlungen der Deutschen Zoologischen Gesellschaft 84: 281–294.
Müller, K.J., Walossek, D., and Zakharov, A. 1995. “Orsten” type phosphatized soft-integument preservation and a new record from the Middle Cambrian Kuonamka Formation in Siberia. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 191: 101–118. Crossref
Nielsen, A.T. and Schovsbo, N.H. 2007. Cambrian to basal Ordovician lithostratigraphy in southern Scandinavia. Bulletin of the Geological Society of Denmark 53: 47–92.
Nielsen, A.T. and Schovsbo, N.H. 2013. The Cambro-Ordovician Alum Shale revisited: depositional environment, sea-level changes and transient isostatic disturbances. In: A. Lindskog and K. Mehlqvist (eds.), Proceedings of the 3rd IGCP 591 Annual Meeting—Lund, Sweden, 9–19 June 2013, 249–250. Lund University, Lund.
Olempska, E. 2008. Soft body-related features of the carapace and lifestyle of Paleozoic beyrichioidean ostracodes. Journal of Paleontology 82: 717–736. Crossref
Olempska, E. and Wacey, D. 2016. Ambient inclusion trails in Palaeozoic crustaceans (Phosphatocopina and Ostracoda). Palaeogeography, Palaeoclimatology, Palaeoecology 441: 949–958. Crossref
Oliveira, I.S. and Mayer, G. 2013. Apodemes associated with limbs support serial homology of claws and jaws in Onychophora (velvet worms). Journal of Morphology 274: 1180–1190. Crossref
Perrier, V., Williams, M., and Siveter, D.J. 2015. The fossil record and palaeoenvironmental significance of marine arthropod zooplankton. Earth-Science Reviews 146: 146–162. Crossref
Siveter, D.J. and Willams, M. 1997. Cambrian Bradoriid and Phosphatocopid arthropods of North America. Special Papers in Palaeontology 57: 1–69.
Siveter, D.J., Waloszek, D., and Williams, M. 2003. An Early Cambrian phosphatocopid crustacean with three-dimensionally preserved soft parts from Shropshire, England. Special Papers in Palaeontology 70: 9–30.
Siveter, D.J., Williams, M., and Waloszek, D. 2001. A phosphatocopid crustacean with appendages from the Lower Cambrian. Science 293: 479–481. Crossref
Smith, R.N. 1971. The frontal muscle scars of cytherid ostracodes. Micropaleontology 17: 167–180. Crossref
Stein, M., Waloszek, D., and Maas, A. 2005. Oelandocaris oelandica and its significance to resolving the stemlineage of Crustacea. In: S. Koenemann and R. Vonck (eds.), Crustacea and Arthropod Relationships. Crustacean Issues 16: 55–71. Crossref
Stein, M., Waloszek, D., Maas, A., Haug, J.T., and Müller, K.J. 2008. The stem crustacean Oelandocaris oelandica re-visited. Acta Palaeontologica Polonica 53: 461–484.
Szaniawski, H. 1971. New species of Upper Cambrian conodonts from Poland. Acta Palaeontologica Polonica 16: 401–413.
Walossek, D. 1993. The Upper Cambrian Rehbachiella and the phylogeny of Branchiopoda and Crustacea. Fossils and Strata 32: 1–202. Crossref
Walossek, D. 1999. On the Cambrian diversity of Crustacea. In: F.R. Schram and J.C von Vaupel Klein (eds.), Crustaceans and the Biodiversity Crisis, Proceedings of the Fourth International Crustacean Congress, Amsterdam, The Netherlands, July 20–24, 1998, Vol. 1, 3–27. Brill Academic Publishers, Leiden.
Walossek, D. and Müller, K.J. 1990. Upper Cambrian stem-lineage crustaceans and their bearing upon the monophyletic origin of Crustacea and position of Agnostus. Lethaia 23: 409–427. Crossref
Walossek, D. and Szaniawski, H. 1991. Cambrocaris baltica n. gen. n. sp., a possible stem-lineage crustacean from the Upper Cambrian of Poland. Lethaia 24: 363–378. Crossref
Walossek, D., Hinz-Schallreuter, I., Shergold, J.H., and Müller, K.J. 1993. Three-dimensional preservation of arthropod integument from the Middle Cambrian of Australia. Lethaia 26: 7–15. Crossref
Waloszek, D. 2003. The “Orsten” window—a three-dimensionally preserved Upper Cambrian meiofauna and its contribution to our understanding of the evolution of Arthropoda. Paleontological Research 7: 71–88. Crossref
Waloszek, D., Chen, J.-Y., Maas, A., and Wang, X.-Q. 2005. Early Cambrian arthropods—new insights into arthropod head and structural evolution. Arthropod Structure and Development 34: 189–205. Crossref
Waloszek, D., Maas, A., Chen, J., and Stein, M. 2007. Evolution of cephalic feeding structures and the phylogeny of Arthropoda. Palaeogeography, Palaeoclimatology, Palaeoecology 254: 273–287. Crossref
Waloszek, D., Maas, A., Olesen, J., Haug, C., and Haug, J.T. 2014. A eucrustacean from the Cambrian “Orsten” of Sweden with epipods and maxillary excretory opening. Palaeontology 57: 909–930. Crossref
Williams, M. and Siveter, D.J. 1998. British Cambrian and Tremadoc Bradoriid and Phosphatocopid arthropods. Monograph of the Palaeontographical Society 152: 1–49.
Williams, M., Siveter, D.J., Rushton, A.W.A., and Berg-Madsen, V. 1994. The Upper Cambrian bradoriid ostracod Cyclotron lapworthi is a hesslandonid. Transactions of the Royal Society of Edinburgh: Earth Sciences 85: 123–130. Crossref
Williams, M., Vannier, J., Corbari, L., and Massabuau, J.-Ch. 2011. Oxygen as a driver of early arthropod micro-benthos evolution. PloS ONE 6 (12), e28183.
Yamada, S. and Matzke-Karasz, R. 2011. Skeleto-musculature of the mandible and its function in podocopid ostracodes exemplified by Loxoconcha pulchra (Cytheroidea: Loxoconchidae) and Fabaeformiscandona tyrolensis (Cypridoidea: Candonidae). Journal of Morphology 272: 1342–1352. Crossref
Zhang, H.Q. and Dong, X.P. 2009. Two new species of Vestrogothia (Phosphatocopina, Crustacea) of Orsten-type preservation from the Upper Cambrian in western Hunan, South China. Science in China Series D: Earth Sciences 52: 784–796. Crossref
Zhang, H.Q., Dong, X.P., and Maas, A. 2011a. Hesslandona angustata (Phosphatocopida, Crustacea) from the Upper Cambrian of western Hunan, South China, with comments on phosphatocopid phylogeny. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 259: 157–175. Crossref
Zhang H.Q., Dong, X.P., and Xiao, S.H. 2011b. Two species of Hesslandona (Phosphatocopida, Crustacea) from the Upper Cambrian of western Hunan, south China and phylogeny of Phosphatocopida. Journal of Paleontology 85: 770–788. Crossref
Zhang H.Q., Dong, X.P., and Xiao, S.H. 2012. Three head-larvae of Hesslandona angustata (Phosphatocopida, Crustacea) from the Upper Cambrian of western Hunan, South China and the phylogeny of Crustacea. Gondwana Research 21: 1115–1127. Crossref
Zhang, X.G. and Pratt, B.R. 2012. The first stalk-eyed phosphatocopine crustacean from the Lower Cambrian of China. Current Biology 22: 2149–2154. Crossref
Zhang, X.G., Maas, A., Haug, J.T., Siveter, D.J., and Waloszek, D. 2010. A eucrustacean metanauplius from the Lower Cambrian. Current Biology 20: 1075–1079. Crossref
Acta Palaeontol. Pol. 64 (1): 19–39, 2019
https://doi.org/10.4202/app.00553.2018