

The evolution of early Spermophilus in eastern Europe and the antiquity of the Old World ground squirrels
MAXIM V. SINITSA and NATALIYA V. POGODINA
Sinitsa, M.V. and Pogodina, N.V. 2019. The evolution of early Spermophilus in eastern Europe and the antiquity of the Old World ground squirrels. Acta Palaeontologica Polonica 64 (3): 643–667.
Spermophilus is the most diverse and abundant genus of Eurasian sciurids. Even though many species have been described, the early evolution of the group is poorly understood. Here we present a review of the evolution and taxonomy of early Spermophilus, based on analysis of more than 1500 specimens from the late Pliocene and Early–Middle Pleistocene of Ukraine and European Russia, representing the most complete and continuous fossil record of the early Old World ground squirrels known to date. In addition to documenting previously unpublished specimens of Spermophilus nogaici, we describe a new species, Spermophilus praecox sp. nov., the oldest member of the genus, from the late Pliocene and Early Pleistocene (middle Villanyian to earliest Biharian) of southern Ukraine. It is intermediate in size between a somewhat smaller Spermophilus nogaici and larger Spermophilus polonicus and Spermophilus primigenius. Morphologically, the new species can be distinguished from all known Spermophilus by the retention of primitive traits including a small P3; rudimentary lingual metaloph; large metaconule and mesostyle; presence of P4–M2 endoloph; and premolariform p4 lacking a lingual metalophid. The patterns of dental evolution in eastern European early Spermophilus throughout 2.15 My indicates the existence of a single gradually evolving S. praecox–S. nogaici lineage characterized by a decrease of molar size but enlargement of premolars; the tendency toward high-crowned teeth with more expanded anterior and reduced posterior lobes; well-developed transverse ridges and anterostyles; reduced endolophs, anteroconules, metaconules, and mesostyles of P4–M2; molariform p4; complete m1–m2 metalophids; and stronger m3 hypoconid, hypoconulid, and entoconulid. Dental character regression suggests that Spermophilus evolved from a relatively large-sized sciurid having generalized Otospermophilus-like dentition, probably an unknown North American member of stem Marmotina.
Key words: Mammalia, Rodentia, Sciuridae, Xerinae, evolution, Pliocene, Pleistocene, eastern Europe.
Maxim V. Sinitsa [sinitsamax@gmail.com], Institute of Natural Sciences and Mathematics, Ural Federal University, 19 Mira str., Ekaterinburg, 620002, Russia; and Department of Paleontology, National Museum of Natural History, National Academy of Sciences of Ukraine, 15 Bogdan Khmelnitsky str., Kiev, 01030, Ukraine.
Nataliya V. Pogodina [N.V.Pogodina@urfu.ru], Institute of Natural Sciences and Mathematics, Ural Federal University, 19 Mira str., Ekaterinburg, 620002, Russia.
Received 3 February 2019, accepted 28 March 2019, available online 31 May 2019.
Copyright © 2019 M.V. Sinitsa and N.V. Pogodina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Old World ground squirrels, Spermophilus Cuvier, 1825, comprise a group of medium-sized, short-tailed terrestrial sciurids that are endemic to Eurasia (Ognev 1947; Nowak 1999; Thorington and Hoffmann 2005; Thorington et al. 2012). All twelve living species are confined to temperate zones throughout central Europe to eastern Asia, where they inhabit a variety of steppe, semi-desert, and grassland biomes (Gromov et al. 1965; Kryštufek and Vohralík 2012; Thorington et al. 2012). The taxonomic diversity of the group was formally recognized in 2009 when the paraphyletic Holarctic genus Spermophilus sensu lato was restricted to a dozen Eurasian species (Helgen et al. 2009). This restriction followed evidence from molecular studies that Spermophilus constitutes a much more distinct clade than previously thought (Harrison et al. 2003; Herron et al. 2004).
The Old World ground squirrels fossil record extends back to the latest Pliocene, 2.6 Ma, revealing an increasingly diverse group known from a plethora of fossil localities scattered across most of Europe and temperate Asia (Gromov 1961; Gromov et al. 1965; Topachevsky et al. 1998; Kowalski 2001). For many years, the earliest record of Spermophilus consisted only of Spermophilus nogaici (Topachevsky, 1957), a medium-sized species with unusually long temporal range extending from the late Pliocene through the Middle Pleistocene of southeastern Europe (Topachevsky 1957; Gromov et al. 1965; Topachevsky and Nesin 1989; Topachevsky et al. 1998; Stadnik and Dema 2007). Since its original description, S. nogaici, as well as two other early European spermophiles, Spermophilus primigenius (Kormos, 1934) and Spermophilus polonicus (Gromov, 1965), have been allied with Urocitellus Obolenskij, 1927 based on apparent primitive dental features, and classified as a species of the former subgenus (Gromov et al. 1965; Topachevsky 1965, 1973; Black 1972; Black and Kowalski 1974; Kowalski 2001; Popov 2004) or the genus Urocitellus (Kryštufek and Vohralík 2013). However, a recent cladistic analysis of cranial traits (Sinitsa et al. 2019) failed to group S. nogaici with Urocitellus, but clearly indicated Spermophilus affinities of the species by placing it as a crown-group member of subgenus Spermophilus, sister to extant Spermophilus citellus (Linnaeus, 1766) and Spermophilus taurensis Gündüz et al. 2007. Despite the importance of the early Spermophilus fossils for elucidating the evolution of the group and the wealth of information that has been obtained from the cranial data of S. nogaici, there remain significant gaps in our knowledge of fossil Spermophilus, particularly with respect to their dental morphology and the early evolutionary history of the genus.
Here, we describe the largest and the most continuous series of samples of the early Spermophilus ever published. Collectively, these samples include more than 1 500 late Pliocene and Early to Mid-Pleistocene specimens from Ukraine and European Russia. Previous studies of ground squirrels from this region were based on significantly fewer fossils (Topachevsky 1957, 1965, 1973; Gromov et al. 1965; Stadnik and Dema 2007). Thus, our data make it possible to address several questions that have not been answered before. First, it allows us to provide detailed morphological descriptions of teeth based on large samples, redescribe S. nogaici, and recognize a new species, which marks the oldest record of the genus. Second, well-stratified dental remains make it feasible to track the evolutionary changes in early Spermophilus across a two-million-year interval at a finer scale of temporal resolution than has previously been possible. Finally, based on this new evidence we discuss the ancestry and evolutionary biogeography of the Old World ground squirrels.
Institutional abbreviations.—GIN, Geological Institute, Russian Academy of Sciences, Moscow, Russia; HNHM, Hungarian Natural History Museum, Budapest, Hungary; NMNHU-P, Department of Paleontology, National Museum of Natural History, National Academy of Sciences of Ukraine, Kiev, Ukraine; MF, Institute of Systematics and Evolution of Animals of the Polish Academy of Sciences, Kraków, Poland; NMNHU-Z, Department of Zoology, National Museum of Natural History, National Academy of Sciences of Ukraine, Kiev, Ukraine; ZIN, Zoological Institute, Russian Academy of Sciences, Saint Petersburg, Russia; ZMMU, Zoological Museum of Moscow University, Moscow, Russia; ZMUU, Zoological Museum of Ural Federal University, Ekaterinburg, Russia.
Other abbreviations.—We follow standard convention in abbreviating premolars and molars as P and M, with upper and lower case letters referring to upper and lower teeth, respectively.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:82088FB1-501F-4B33-B9A2-05648F35E85F
Material and methods
Material described in this work is deposited in the fossil collections of the Department of Paleontology, National Museum of Natural History, Academy of Sciences of Ukraine (Kiev, Ukraine); the Geological Institute of the Russian Academy of Sciences (Moscow, Russia); and the Zoological Institute of the Russian Academy of Sciences (Saint Petersburg, Russia). The specimens were collected from sixteen Villanyian and Biharian localities in southeastern Ukraine and southwestern Russia (Fig. 1). The stratigraphic positions of the localities and their absolute ages are from Tesakov (2004) and Krokhmal and Rekovets (2010). Detailed information for Kryzhanovka 2 (late Pliocene, late Piacenzian, middle Villanyian), Kotlovina 2, Kotlovina 3, Yuzhny (all Early Pleistocene, Gelasian, late Villanyian), Morskoy, Zhevakhova Gora 1, Tarkhankut, Nogaisk, Babakh-Tarama 1, Moiseevo 1, Tsymbal, Cherevichnoe 1, Karai-Dubina (Early Pleistocene, Calabrian, early–middle Biharian), Tihonovka 1, Bolshevik 1, and Bolshevik 2,I (early Middle Pleistocene, early Ionian, late Biharian) can be found in Schevtschenko (1965), Topachevsky (1965), Topachevsky (1973), Topachevsky and Skorik (1977), Topachevsky et al. (1987), Topachevsky and Nesin (1989), Tesakov (2004), Krokhmal and Rekovets (2010), Tesakov and Sinitsa (2012), and Markova (2014).
For comparative purposes, we examined original specimens of a variety of ground squirrels across the ground squirrel tribe Xerini (see Appendix 1 for details), including all extinct and extant species of Spermophilus (except S. taurensis) housed at the Zoological Institute, Russian Academy of Sciences; the National Museum of Natural History, Academy of Sciences of Ukraine; the Zoological Museum of Moscow University (Moscow, Russia); and the Zoological Museum of Ural Federal University (Ekaterinburg, Russia).
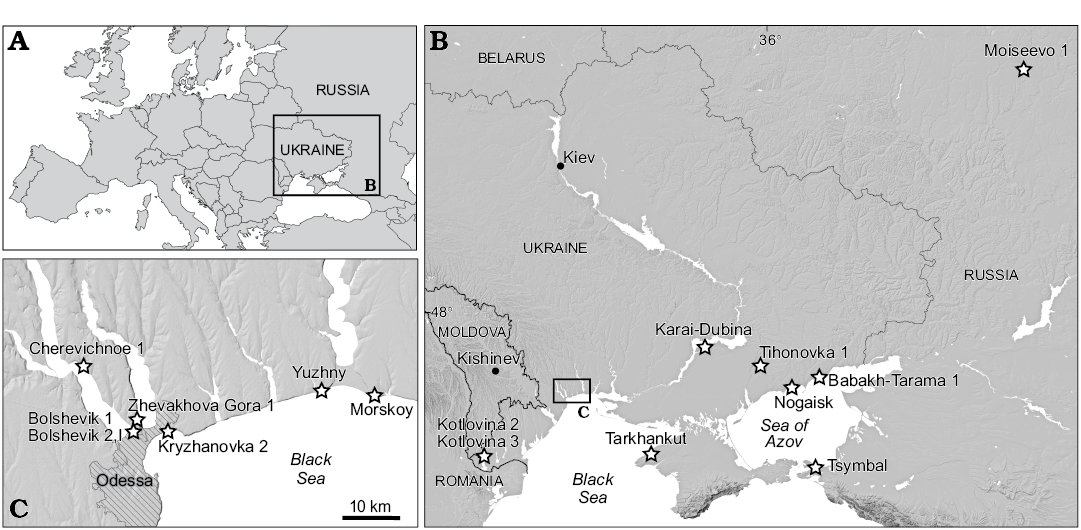
Fig. 1. Geographic location of Spermophilus-bearing fossil localities (stars) discussed in the text.
Measurements of fossil and Recent specimens were taken to the nearest 0.01 mm with a Leica EZ 4 binocular microscope. Dimensions of P4–M3 and p4–m3 were measured at the occlusal surface, as described by Van de Weerd (1976); the P3 measurements represents greatest crown dimensions taken parallel (width) and perpendicular (length) to protoloph. The photographic images were taken by the first author from high-resolution resin casts using a Canon MP-E 65mm macro lens with APS-C DSLR camera and rendered in a focus stacking program. The specimens were coated with ammonium chloride prior to photography.
Terminology for dental morphology follows Marivaux et al. (2004), Comte et al. (2012), Vianey-Liaud et al. (2013), and Maridet et al. (2017), with modifications (Fig. 2). For the terminology that applies to the upper teeth stylar cusps of Spermophilus, that of Popova (2016) is employed here, except for her “hypostyle” and “hypostyle 2”, which we term “hypocone” and “hypostyle”, respectively. To denote a syncline between the posterior bases of hypoconid and posterolophid, not reflected in the listed nomenclatures, the term postflexid (sensu Sulimski 1964) is applied here. Generic- and subtribal-level systematics of Marmotini follow Helgen et al. (2009) and Sinitsa (2018), respectively.
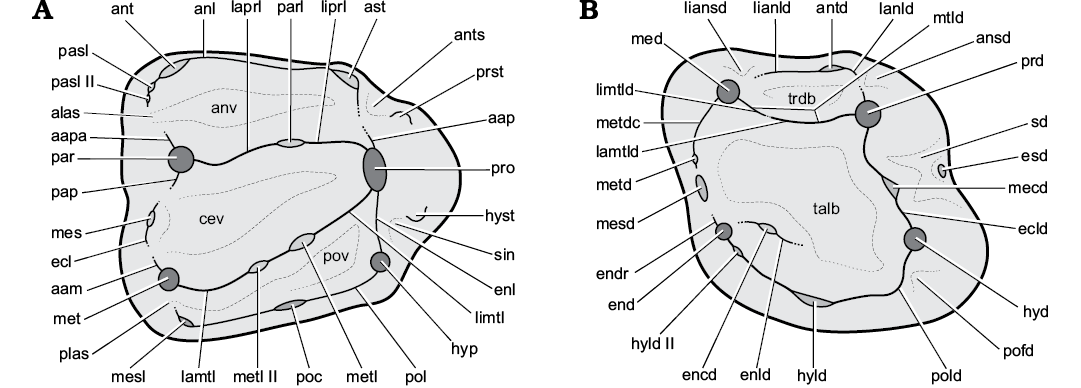
Fig. 2. Upper (A) and lower (B) sciurid cheek teeth illustrating the terminology employed here (after Marivaux et al. 2004; Comte et al. 2012; Vianey-Liaud et al. 2013; and Maridet et al. 2017; with modifications). Abbreviations: aam, anterior arm of metacone (premetacrista sensu Comte et al. 2012; Vianey-Liaud et al. 2013); aap; anterior arm of protocone; aapa, anterior arm of paracone; alas, anterolabial sinus; anl, anteroloph; ansd, anterosinusid; ant, anterocone; ants, antesinus; ast, anterostyle; antd, anteroconulid; anv, anterior valley; cev, central valley; ecl, ectoloph; ecld, ectolophid; encd, entoconulid; end, entoconid; endr, entoconid ridge; enl, endoloph; enld, entolophid; esd, ectostylid; hyd, hypoconid; hyld, hypoconulid; hyld II, hypoconulid II (second hypoconulid); hyp, hypocone (hypostyle sensu Popova 2016); hyst, hypostyle (hypostyle 2 sensu Popova 2016); lamtl, labial metaloph; lamtld, labial metalophid; lanld, labial anterolophid; laprl, labial protoloph; lianld, lingual anterolophid; liansd, lingual anterosinusid; limtl, lingual metaloph; limtld, lingual metalophid; liprl, lingual protoloph; mecd, mesoconid; med, metaconid; mes, mesostyle; mesd, mesostylid; mesl, metastyle; met, metacone; metd, metastylid; metdtc, metastylid crest; metl, metaconule; metl II, metaconule II (second metaconule); mtld, metalophid; pap, posterior arm of paracone (postparacrista sensu Comte et al. 2012; Vianey-Liaud et al. 2013); par, paracone; parl, paraconule; pasl, parastyle; pasl II, parastyle II (second parastyle); plas, posterolabial sinus; poc, posterocone; pofd, postflexid; pol, posteroloph; pold, posterolophid; pov, posterior valley; prd, protoconid; pro, protocone; prst, protostyle; sd, sinusid; sin, sinus; talb, talonid basin; trdb, trigonid basin.
Systematic palaeontology
Order Rodentia Bowdich, 1821
Family Sciuridae Fischer, 1817
Subfamily Xerinae Osborn, 1910
Tribe Marmotini Pocock, 1923
Genus Spermophilus Cuvier, 1825
Type species: Mus citellus Linnaeus, 1766; Recent.
Spermophilus nogaici (Topachevsky, 1957)
Figs. 3, 4.
1957 Citellus (Urocitellus) nogaici sp. nov., Topachevsky 1957: 204–208, figs. 1–13.
1965 Citellus nogaici W. Topacevski, 1957; Topachevsky 1965: 38–39.
1965 Citellus (cf. Urocitellus) nogaici Topatschevky, 1957; Gromov 1965: 178–181 (in part), fig. 36: 1–6.
1973 Citellus (Urocitellus) nogaici W. Topačevski, 1957; Topachevsky 1973: 13–21.
1987 Citellus nogaici Topachevsky, 1957; Topachevsky et al. 1987: 57–58.
Lectotype: NMNHU-P 27-190 (formerly NMNHU-P 27-189), right maxilla with P4 (Topachevsky 1957: 204, fig. 3).
Type locality: Nogaisk, Zaporozhye Region, Ukraine.
Type horizon: Early Pleistocene, Calabrian, middle Biharian.
Material.— From Early Pleistocene, early–middle Biharian, Zhevakhova Gora 1: NMNHU-P ZG1-1–2, DP4; NMNHU-P ZG1-3, P4; NMNHU-P ZG1-4–14, M1–M2; NMNHU-P ZG1-16–19, M3; NMNHU-P ZG1-20–22, dp4; NMNHU-P ZG1-1-22–24, p4; NMNHU-P ZG1-1-25–34, m1–m2; NMNHU-P ZG1-25–36, m3; NMNHU-P ZG1-1-37, mandible with p4–m2. Tarkhankut: NMNHU-P 50-29/5–16, NMNHU-P 50-31/8–13, P3; NMNHU-P 50-29/68–72, NMNHU-P 50-31/14, DP4; NMNHU-P 50-29/17–67, NMNHU-P 50-31/15–21, P4; NMNHU-P 50-27/1–122, NMNHU-P 50-28/1–30, NMNHU-P 50-31/23–45, M1–M2; NMNHU-P 50-28/31–89, NMNHU-P 50-31/46–56, NMNHU-P 50-31/97, M3; NMNHU-P 50-29/73–75, dp4; NMNHU-P 50-26/61–128, NMNHU-P 50-31/57–68, p4; NMNHU-P 50-25/1–145, NMNHU-P 50-31/69–89, m1–m2; NMNHU-P 50-26/1–60, NMNHU-P 50-31/90–96, m3; NMNHU-P 50-29/1, NMNHU-P 50-31/3, maxilla with P4–M2; NMNHU-P 50-31/2, maxilla with P4; NMNHU-P 50-29/2, mandible with m1–m3; NMNHU-P 50-29/3, mandible with m2–m3; NMNHU-P 50-29/4, mandible with p4; NMNHU-P 50-31/4, mandible with m1–m2; NMNHU-P 50-31/5, mandible with m2; NMNHU-P 50-31/6, mandible with m3. Nogaisk: NMNHU-P 27-227–233, P3; NMNHU-P 27-123–132, NMNHU-P 27-235–249, P4; NMNHU-P 27-85, NMNHU-P 27-110–119, NMNHU-P 27-148–158, NMNHU-P 27-250–259, NMNHU-P 27-487–518, M1–M2; NMNHU-P 27-120–122, NMNHU-P 27-518–535, M3; NMNHU-P 27-104–109, NMNHU-P 27-538–552, p4; NMNHU-P 27-133–147, NMNHU-P 27-176–188, NMNHU-P 27-571–638, m1–m2; NMNHU-P 27-159–175, NMNHU-P 27-639–667, m3; NMNHU-P 27-191, maxilla with M1–M2; NMNHU-P 27-224, mandible with p4–m3; NMNHU-P 27-738, mandible with p4–m2; NMNHU-P 27-733, mandible with p4. Babakh-Tarama 1: NMNHU-P BaT1-1–4, M1–M2; NMNHU-P BaT1-5, M3; NMNHU-P BaT1-6–8, m1–m2. Moiseevo 1: ZIN 105151/2–6, P4; ZIN 105151/7–21, M1–M2; ZIN 105151/23–27, M3; ZIN 105151/28, dp4; ZIN 105151/29–37, m1–m2; ZIN 105151/38, m3. Tsymbal: ZIN 105150/1–9, P3; ZIN 105150/10–15, DP4; ZIN 105150/16–26, P4; ZIN 105148/1–30, M1–M2; ZIN 105150/27–46, M3; ZIN 105149/1, dp4; ZIN 105149/2–9, p4; ZIN 105149/10–46, m1–m2; ZIN 105149/47–54, m3. Cherevichnoe 1: NMNHU-P Che1-1–4, P3; NMNHU-P Che1-5–6, DP4; NMNHU-P Che1-7–14, P4; NMNHU-P Che1-15–32, M1–M2; NMNHU-P Che1-33–40, M3; NMNHU-P Che1-41–43, dp4; NMNHU-P Che1-44–46, p4; NMNHU-P Che1-47–58, m1–m2; NMNHU-P Che1-59–70, m3. Karai-Dubina: NMNHU-P KaD-1, P3; NMNHU-P KaD-2–6, P4; NMNHU-P KaD-7–14, M1–M2; NMNHU-P KaD-15–22, M3; NMNHU-P KaD-23, NMNHU-P KaD-49–50, dp4; NMNHU-P KaD-24–28, p4; NMNHU-P KaD-29–43; m1–m2; NMNHU-P KaD-44–46; m3; NMNHU-P KaD-47, mandible with m1–m3; NMNHU-P KaD-48; mandible with m3.
From early Middle Pleistocene, late Biharian, Tihonovka 1: NMNHU-P 29-206–212, P3; NMNHU-P 29-213–218, P4; NMNHU-P 29-4037–4050, M1–M2; NMNHU-P 29-4051–4063, M3; NMNHU-P 29-4064, dp4; NMNHU-P 29-4065–4078, p4; NMNHU-P 29-4079–4093, m1–m2; NMNHU-P 29-4094–4104, m3; NMNHU-P 29-4036, maxilla with P3–M3; NMNHU-P 29-220, mandible with m2–m3. Bolshevik 1: NMNHU-P 45a-54, a damaged skull with P3–M3 and incisors; ZIN 105153/1–5, P3; ZIN 105153/6, P4; ZIN 105153/7–8, M1–M2; ZIN 105153/9, M3; ZIN 105153/10–12, p4; ZIN 105153/13–14, m1–m2. Bolshevik 2,I: NMNHU-P Bol2/1-1–14, P3; NMNHU-P Bol2/1-15, DP4; NMNHU-P Bol2/1-16–24, P4; NMNHU-P Bol2/1-25–44, M1–M2; NMNHU-P Bol2/1-45–48, M3; NMNHU-P Bol2/1-49–65, p4; NMNHU-P Bol2/1-66–95, m1–m2; NMNHU-P Bol2/1-96–107, m3.
Emended diagnosis.—Medium-sized species of Spermophilus with elongated, low, and slightly domed skull having a shortened, evenly tapered anteriorly rostrum, a broad posterodorsal process of premaxilla, a perpendicularly oriented frontoincisive suture, elongated incisive foramen occupying about 45% of the diastemal length, broad hard palate, and small, knob-like facial tuberosity. The braincase is rounded with no sagittal crest and U-shaped temporal lines. The lower diastema is shorter than the lower dentition. The M3 exhibits a metaconule, metaloph, and metacone; the p4 trigonid is subequal or slightly (10–20%) narrower than the talonid and lacks a lingual anterolophid; the m3 possesses a prominent entoconulid and entolophid.
Medium-sized Spermophilus, larger than S. alashanicus, S. citelloides, S. citellus, S. dauricus, S. pygmaeus, S. suslicus, S. taurensis, and S. xanthoprymnus, and smaller than S. fulvus, S. major, S. polonicus, S. primigenius, S. ralli, S. relictus, S. superciliosus, and S. tologoicus. Differs from all species of the genus other than S. polonicus, S. praecox, and S. primigenius in the following combination of features: broad posterodorsal process of premaxilla that exceeds the width of the nasal at its midlength; subparallel upper toothrows; a relatively long posterior valley of M1–M2 that is only two times shorter than the central valley, more pronounced and tall hypocone, and the presence of a rudimentary endoloph of P4–M2; a well-developed M3 metaconule; narrower trigonid of p4 that generally lacks the lingual anterolophid; and salient entoconulid and entolophid of m3 delimited labially by a deep valley. Differs from S. polonicus, S. praecox, and S. primigenius in a larger P3 that has the anterior valley and prominent protocone; more expanded anterior valley, smaller metaconule, weaker endoloph, lower hypocone, and stronger lingual metaloph of P4–M2; lack of mesostyle on P4; presence of the metaloph on M3; more trapezoidal p4 in occlusal outline, less pronounced anteroconulid, and stronger anterolophid of this tooth; a tendency to have more pronounced metalophids of p4–m3; and stronger m3 entoconulid and entolophid. Further differs from S. polonicus in the presence of prominent protocone and paracone of P3; a metaloph on M3; and in having less massive main cusps of P4–M3 and p4–m3.
Description.—The P3 is large (Table 1), generally slightly longer than wide, and circular in occlusal outline (Fig. 3A–E, Z). About two-thirds of the specimens are gently flattened along the posterolabial face of the crown, where the tooth abuts P4. The crown is dominated by the paracone, protocone, and protoloph that extends transversally (Fig. 3A, B) or slightly obliquely across the crown (Fig. 3C–E) delimiting the smaller anterior valley from the larger posterocentral valley. In most specimens the anteroloph is present, but variably expressed; when present it occurs as either an extremely short or faintly elongated low crest, which exceeds two-thirds of the total crown width. The concavity of the anterior valley is discernible in most specimens. The paracone and protocone are nearly equal in size in the specimens from the mid- and late Biharian localities Tsymbal, Cherevichnoe 1, Karai-Dubina, Tihonovka 1, Bolshevik 1, Bolshevik 2,I (Fig. 3D, E); in those from stratigraphically older Tarkhankut and Nogaisk, however, the protocone appears to be one and a half times smaller than the paracone (Fig. 3A–C). The lingual side of the paracone is continued into a massive protoloph that tapers lingually, terminating before the contact with the lingual arm of the protocone. The contact between these structures is defined by a sharp constriction; in heavily worn teeth the paracone and protocone become fused thus forming an asymmetric B-shaped wear facet. A long and prominent posteroloph defines the posterocentral valley. The ridge follows the entire posterior lobe of the tooth from the posterolingual wall of the protocone and gradually diminishes in width and height before reaching the base of the paracone. A small hypocone swelling at the lingual arm of the posteroloph is observable in three out of eight specimens from Nogaisk (Fig. 3B), in eight of seventeen teeth from Tarkhankut, in three of four teeth from Tsymbal, two of the four from Cherevichnoe 1, one of the five P3s from Tihonovka 1, six of the thirteen teeth from Bolshevik 2,I, and the only specimen from Karai-Dubina. The tooth has one labiolingually compressed root with a deep longitudinal groove on its labial surface.
Table 1. Dental measurements (in mm) of Spermophilus nogaici.
|
Locality |
n |
Length |
Width |
Length/Width |
||
|
range |
mean |
range |
mean |
|||
|
Zhevakhova Gora 1 |
||||||
|
DP4 |
2 |
1.88; 2.21 |
– |
1.19; 2.13 |
– |
0.99; 1.04 |
|
P4 |
1 |
2.07 |
– |
2.57 |
– |
0.80 |
|
M1–M2 |
10 |
2.13–2.41 |
2.22 |
2.72–3.24 |
2.94 |
0.72–0.79 |
|
M3 |
4 |
2.52–3.17 |
2.75 |
2.67–2.99 |
2.82 |
0.91–1.06 |
|
dp4 |
1 |
1.68 |
– |
1.76 |
– |
0.95 |
|
p4 |
4 |
2.16–2.27 |
2.20 |
2.23–2.45 |
2.34 |
0.88–0.97 |
|
m1–m2 |
12 |
2.02–2.48 |
2.22 |
2.72–3.46 |
3.05 |
0.63–0.81 |
|
m3 |
2 |
2.59; 2.97 |
– |
2.83; 3.30 |
– |
0.90; 0.91 |
|
Tarkhankut |
||||||
|
P3 |
17 |
1.56–1.96 |
1.74 |
1.51–1.78 |
1.64 |
0.98–1.16 |
|
DP4 |
9 |
1.40–2.21 |
1.81 |
1.39–2.32 |
1.92 |
0.82–1.02 |
|
P4 |
52 |
1.99–2.48 |
2.26 |
2.40–3.16 |
2.75 |
0.74–0.91 |
|
M1–M2 |
161 |
2.01–2.79 |
2.30 |
2.11–3.57 |
3.06 |
0.65–1.01 |
|
M3 |
58 |
2.45–3.09 |
2.80 |
2.52–3.28 |
2.90 |
0.88–1.07 |
|
dp4 |
1 |
2.21 |
– |
2.59 |
– |
0.85 |
|
p4 |
78 |
1.89–2.62 |
2.30 |
2.06–3.01 |
2.59 |
0.71–1.14 |
|
m1–m2 |
164 |
1.90–2.82 |
2.29 |
2.57–3.71 |
3.11 |
0.61–0.89 |
|
m3 |
64 |
2.53–3.37 |
3.01 |
2.70–3.45 |
3.07 |
0.87–1.13 |
|
Nogaisk |
||||||
|
P3 |
8 |
1.59–1.81 |
1.71 |
1.52–1.90 |
1.68 |
0.89–1.10 |
|
P4 |
46 |
1.94–2.51 |
2.27 |
2.35–2.88 |
2.64 |
0.79–0.94 |
|
M1–M2 |
83 |
2.10–3.49 |
2.48 |
2.77–3.67 |
3.27 |
0.67–0.83 |
|
M3 |
21 |
2.47–3.24 |
2.81 |
2.67–3.25 |
2.89 |
0.89–1.03 |
|
p4 |
23 |
1.91–2.39 |
2.18 |
2.21–2.80 |
2.45 |
0.75–1.04 |
|
m1–m2 |
122 |
1.94–2.55 |
2.23 |
2.73–3.61 |
3.24 |
0.60–0.77 |
|
m3 |
46 |
2.54–3.39 |
3.02 |
2.56–3.38 |
3.05 |
0.85–1.09 |
|
Babakh-Tarama 1 |
||||||
|
M1–M2 |
4 |
2.27–2.39 |
3.32 |
2.82–3.07 |
3.00 |
0.74–0.82 |
|
M3 |
1 |
2.65 |
– |
2.87 |
– |
0.93 |
|
m1–m2 |
3 |
1.98–2.37 |
2.23 |
3.01–3.51 |
3.32 |
0.66–0.68 |
|
Moiseevo 1 |
||||||
|
P4 |
5 |
2.00–2.23 |
2.16 |
2.48–2.80 |
2.71 |
0.75–0.87 |
|
M1–M2 |
13 |
2.10–2.59 |
2.31 |
2.85–3.31 |
3.10 |
0.70–0.79 |
|
M3 |
5 |
2.83–3.06 |
2.94 |
2.96–3.20 |
3.07 |
0.93–1.00 |
|
dp4 |
1 |
1.66 |
– |
1.57 |
– |
1.06 |
|
m1–m2 |
7 |
1.90–2.21 |
2.08 |
2.76–3.01 |
2.93 |
0.68–0.74 |
|
m3 |
1 |
3.01 |
– |
3.25 |
– |
0.92 |
|
Tsymbal |
||||||
|
P3 |
8 |
1.65–1.99 |
1.81 |
1.62–1.88 |
1.76 |
0.98–1.07 |
|
P4 |
11 |
2.04–2.50 |
2.31 |
2.70–2.89 |
2.78 |
0.74–0.92 |
|
M1–M2 |
16 |
2.05–2.60 |
2.25 |
2.70–3.17 |
2.98 |
0.69–0.83 |
|
M3 |
20 |
2.52–3.21 |
2.86 |
2.53–3.21 |
2.96 |
0.94–1.02 |
|
p4 |
7 |
2.00–2.39 |
2.22 |
2.19–2.66 |
2.42 |
0.85–0.96 |
|
m1–m2 |
30 |
1.97–2.55 |
2.30 |
2.83–3.73 |
3.34 |
0.61–0.77 |
|
m3 |
13 |
2.90–3.42 |
3.17 |
2.92–3.45 |
3.22 |
0.90–1.08 |
|
Locality |
n |
Length |
Width |
Length/Width |
||
|
range |
mean |
range |
mean |
|||
|
Cherevichnoe 1 |
||||||
|
P3 |
7 |
1.57–2.00 |
1.82 |
1.76–2.06 |
1.87 |
0.83–1.04 |
|
DP4 |
1 |
2.34 |
– |
2.56 |
– |
0.92 |
|
P4 |
7 |
1.96–2.41 |
2.21 |
2.39–2.79 |
2.64 |
0.80–0.88 |
|
M1–M2 |
12 |
2.01–2.39 |
2.21 |
2.69–3.24 |
2.98 |
0.70–0.84 |
|
M3 |
12 |
2.78–3.23 |
3.00 |
2.54–3.05 |
2.88 |
0.96–1.11 |
|
dp4 |
1 |
1.88 |
– |
2.20 |
– |
0.85 |
|
p4 |
13 |
1.96–2.41 |
2.18 |
2.26–2.83 |
2.61 |
0.80–0.88 |
|
m1–m2 |
16 |
1.87–2.29 |
2.07 |
2.77–3.57 |
3.09 |
0.62–0.78 |
|
m3 |
12 |
2.73–3.37 |
3.14 |
2.90–3.44 |
3.14 |
0.93–1.06 |
|
Karai-Dubina |
||||||
|
P3 |
1 |
1.54 |
– |
1.47 |
– |
1.05 |
|
P4 |
4 |
2.00–2.34 |
2.16 |
2.26–2.68 |
2.43 |
0.85–0.89 |
|
M1–M2 |
8 |
2.07–2.29 |
2.15 |
2.74–3.04 |
2.85 |
0.73–0.77 |
|
M3 |
8 |
2.61–3.21 |
2.85 |
2.66–2.85 |
2.77 |
0.95–1.13 |
|
p4 |
5 |
2.06–2.24 |
2.15 |
2.19–2.44 |
2.36 |
0.85–0.98 |
|
m1–m2 |
13 |
1.92–2.33 |
2.05 |
2.77–3.16 |
2.95 |
0.67–0.74 |
|
m3 |
5 |
2.66–3.05 |
2.80 |
2.97–3.29 |
2.98 |
0.88–1.03 |
|
Tihonovka 1 |
||||||
|
P3 |
7 |
1.57–2.00 |
1.82 |
1.76–2.06 |
1.87 |
0.83–1.04 |
|
DP4 |
1 |
2.34 |
– |
2.56 |
– |
0.92 |
|
P4 |
7 |
1.96–2.41 |
2.21 |
2.39–2.79 |
2.64 |
0.80–0.88 |
|
M1–M2 |
12 |
2.01–2.39 |
2.21 |
2.69–3.24 |
2.98 |
0.70–0.84 |
|
M3 |
12 |
2.78–3.23 |
3.00 |
2.54–3.05 |
2.88 |
0.96–1.11 |
|
dp4 |
1 |
1.88 |
– |
2.20 |
– |
0.85 |
|
p4 |
13 |
1.96–2.41 |
2.18 |
2.26–2.83 |
2.61 |
0.80–0.88 |
|
m1–m2 |
16 |
1.87–2.29 |
2.07 |
2.77–3.57 |
3.09 |
0.62–0.78 |
|
m3 |
|
2.73–3.37 |
3.14 |
2.90–3.44 |
3.14 |
0.93–1.06 |
|
Bolshevik 1 |
||||||
|
P3 |
5 |
1.58–1.86 |
1.74 |
1.59–1.69 |
1.66 |
0.99–1.11 |
|
P4 |
1 |
2.08 |
– |
2.47 |
– |
0.84 |
|
M1–M2 |
2 |
2.05; 2.17 |
– |
2.89 |
– |
0.71 |
|
M3 |
2 |
2.85 |
– |
2.71; 2.82 |
– |
1.05 |
|
p4 |
3 |
2.04–2.19 |
2.11 |
2.40–2.49 |
2.43 |
0.85–0.91 |
|
m1–m2 |
4 |
1.86–2.13 |
2.02 |
2.48–3.29 |
2.81 |
0.61–0.77 |
|
Bolshevik 2,I |
||||||
|
P3 |
12 |
1.58–1.91 |
1.76 |
1.48–1.96 |
1.72 |
0.97–1.07 |
|
DP4 |
1 |
1.84 |
– |
1.90 |
– |
0.98 |
|
P4 |
9 |
1.99–2.28 |
2.16 |
2.41–2.79 |
2.60 |
0.78–0.91 |
|
M1–M2 |
19 |
1.95–2.32 |
2.09 |
2.57–3.10 |
2.87 |
0.68–0.79 |
|
M3 |
4 |
2.79–2.99 |
2.88 |
2.75–2.82 |
2.78 |
0.99–2.06 |
|
p4 |
15 |
1.86–2.25 |
2.01 |
2.11–2.45 |
2.28 |
0.76–0.99 |
|
m1–m2 |
28 |
1.75–2.38 |
2.02 |
2.59–3.33 |
3.00 |
0.61–0.72 |
|
m3 |
11 |
2.56–3.11 |
2.88 |
2.80–3.14 |
2.98 |
0.90–1.01 |
The DP4 is proportionally narrower labiolingually and distinctly smaller than the permanent premolar. The protocone, paracone, and metacone are distinct, with their apices substantially higher than the main lophs (Fig. 3F, G). The hypocone is indistinct. The anterior valley is strongly expanded anteriorly and lingually, which gives the crown a widely triangular to rectangular occlusal outline. The valley is bounded anteriorly by a well-developed and high anteroloph crowned with a barely distinct anterocone. The labial and lingual arms of the anteroloph do not extend to the anterior wall of the protoloph leaving both the antesinus and anterolabial sinus open in all examined specimens. A tiny cusplet on the labial end of the ridge, topographically equivalent to the parastyle, is evident in one of the two specimens from Zhevakhova Gora 1 (Fig. 3F), in three of the six DP4s from Tarkhankut, in three of the five teeth from Tsymbal, and one DP4 from Cherevichnoe 1. The anterostyle is discernible in most specimens as a minute isolated swelling on the anterolingual face of the protocone, level with the bottom of the anterior valley (Fig. 3G). The teeth from Zhevakhova Gora 1 do not show any traits of the cusplet (Fig. 3F). Protoloph and metaloph are both complete, roughly straight, and parallel to each other. The metaloph is slightly longer, but always lower than the protoloph, and bears one, rarely two constrictions that delimit the metaconule swelling labially and lingually. The metaconule is relatively larger in DP4s from the early Biharian localities and reduced to anteroposteriorly compressed swelling in those from the mid- and late Biharian assemblages. Two specimens (NMNHU-P 50-29/70, ZIN 105150/14) present a small, albeit well-defined, second metaconule sitting on the medial portion of the labial metaloph. The central valley is symmetrical and trapezoidal; in three specimens from Tarkhankut (NMNHU-P 50-29/71), Tsymbal (ZIN 105150/13), Cherevichnoe 1 (NMNHU-P Che1-5), and the teeth from Zhevakhova Gora 1 its sinus is occupied by a small, knoblike mesostyle (Fig. 3F). The posterior valley is short and shallow due to a reduction of the posteroloph. The endoloph is observable in one tooth from Zhevakhova Gora 1 (Fig. 3F), two from Tarkhankut, and in two of the five teeth from Tsymbal. The crown is supported by three widely spaced, divergent, and thin roots.
The P4 is triangular in occlusal outline, with a gently rounded to nearly flattened posterior lobe, and vast, asymmetrical anterior lobe (Fig. 3H–M, Z). The labiolingual width of the anterior lobe is greater than three-fourths of the total width of the crown. The only exception is the specimen from the early Biharian of Zhevakhova Gora 1 (NMNHU-P ZG1-3), which possesses a slightly narrower anterior valley reaching approximately two-thirds of the crown width. A crescentic anterocone dominates the anterior lobe; the anterocone is about twice the height of the posterior lobe. An elongated lingual arm and short labial arm of the anteroloph descend posterolingually and posteriorly respectively from the anterocone to enclose the anterior valley. The anterostyle is variably present at the lingualmost portion of the anteroloph: it is moderately developed in the teeth from Zhevakhova Gora 1, Tarkhankut, Nogaisk, Moiseevo 1, and Tsymbal (Fig. 3H–K); and it is larger in P4s from Cherevichnoe 1, Karai-Dubina, Tihonovka 1, Bolshevik 1, and Bolshevik 2,I (Fig. 3L, M, Z). The cusplet is tightly appressed to the base of the protocone in most specimens. About half of P4s from the whole sample possess a tiny parastyle at the opposite, labial end of the anteroloph. The protoloph and metaloph are nearly equal in length and height. A slight constriction of the lingual metaloph is evident in the specimens from Zhevakhova Gora 1, Tarkhankut, Nogaisk, and Moiseevo 1 (Fig. 3H–K); in other localities, however, the metaloph of P4 is a massive ridge fused with the protocone (Fig. 3L, M, Z). Approximately halfway between the protocone and metacone is a metaconule swelling that varies in size from moderate (Zhevakhova Gora 1, Tarkhankut, Nogaisk, Moiseevo 1) to small and almost indistinct, subsumed into the metaloph (Tsymbal, Cherevichnoe 1, Karai-Dubina, Tihonovka 1, Bolshevik 1, Bolshevik 2,I). A small to moderately pronounced second metaconule is occasionally present in about one-third of the teeth (Fig. 3H, J, L, Z). The lingual metaloph narrows lingually, thus appearing somewhat thinner when compared with the same portion of the protoloph. A space between them, the central valley, is anteroposteriorly short, about 10–20% shorter than the anterior valley. The sinus is free from accessory cusplets, apart from two specimens (NMNHU-P 50-31/15, NMNHU-P 27-123) that bear a tiny, knob-like mesostyle (Fig. 3J). Along the rear margin of the crown is a distinct, low, and evenly smooth posteroloph with a faint hypocone swelling on its lingual end. The posterior valley is labiolingually broader, but anteroposteriorly shorter than the central one. A low, reduced endoloph is present in the specimens from Zhevakhova Gora 1, seven out of 15 teeth from Tarkhankut, ten of the 18 from Nogaisk, one of the four from Moiseevo 1, three of six from Tsymbal, two of the five from Cherevichnoe 1, one of the five from Karai-Dubina, two of the nine from Bolshevik 2,I, and one of the four P4s from Bolshevik 1. The tooth has three roots: a stronger lingual root, more slender anterolabial root, and the thinnest posterolabial root.
The M1 and M2 are rectangular to roughly trapezoidal in occlusal outline (Fig. 3N–S, Z); the labial portion of the tooth is slightly longer anteroposteriorly, on average, than its lingual counterpart, although the difference is negligible in some specimens (Fig. 3N, P, Q). The anterior lobe is wide labiolingually, and forms about two-thirds of the anterior face of the tooth. The anterior valley, as in P4, is rimmed by a well-developed, salient anteroloph. The lingual portion of the anteroloph in unworn specimens protrudes farther lingually than the apex of the protocone. In 97% of the M1–M2s the anteroloph forms a teardrop-shaped anterostyle separated from the protocone base by the antesinus, which shows a tendency to deepening and widening in geologically younger specimens: from a barely discernible, diminutive depression in the early Biharian teeth from Zhevakhova Gora 1 (Fig. 3N) to a deeply excavated groove in those form the late Biharian localities. The anterolabial corner of the crown is accentuated by a sharp and high, anteroposteriorly compressed anterocone. In half of the M1–M2s the short labial anteroloph forms a diminutive, conical parastyle. Three specimens (NMNHU-P 50-28/27, NMNHU-P 50-31/32, NMNHU-P 27-489) possess a second parastyle lying at the bottom of the anterolabial sinus. The trigon is narrowly V-shaped; it is formed by a well developed, high metaloph and protoloph, which converge lingually at an acute angle of 7–13°. The paracone is higher than the metacone. The protoloph is complete and evenly smooth with almost flat anterior and posterior walls. The metaloph, in contrast, is slightly restricted lingually and labially to the metaconule. The metaconule appears as a moderately developed, anteroposteriorly compressed cusp in most specimens. Those from Moiseevo 1, Cherevichnoe 1, Tihonovka 1, Bolshevik 1, and Bolshevik 2,I have a somewhat less developed metaconule. About 7–12% of M1–M2s bear the second metaconule sitting on the labial metaloph, adjacent to the metaconule. The percentage of the teeth with a mesostyle ranges from 65% in the early Biharian samples to 12% in late Biharian ones. The posteroloph is labiolingually short, about 20% shorter than the anteroloph, and similar to the posteroloph in P4. Its lingual arm may be free, separated from the protocone by the lingual posterosinus or may join with the protocone via a low endoloph (Fig. 3N, P), as seen in 40% of M1–M2s from Zhevakhova Gora 1, 37% from Tarkhankut, 35% from Nogaisk, 50% from Moiseevo 1, 38% from Tsymbal, 35% from Cherevichnoe 1, 10% from Tihonovka 1, 11% from Bolshevik 2,I, two of the four from Babakh-Tarama 1, one of the eight M1–M2 from Karai-Dubina, and one of the two specimens from Bolshevik 1. The hypocone is small to indistinct. Viewed posteriorly, the cusp appears much lower than the main labial cusps, and occurs at the same level as the bottom of the central valley. The root structure is as in P4, differing only in a more slender anterolabial root, comparable to the posterolabial root in size and morphology.
In occlusal outline, the M3 is a rounded triangle, approximately as long as wide, with a well-defined, gently tapering posterior lobe (Fig. 3T–Y, Z). The anterior lobe of the tooth is similar to the same structure of M1–M2; but in the M3 this lobe is less cuspate, slightly wider labiolingually, with anteroposteriorly longer labial portion. The anterostyle is smaller than in M1–M2. The antesinus, separating the anterostyle from the protocone is present in seven out of the 56 M3 from Tarkhankut, in eight of twenty from Nogaisk, in two of four from Moiseevo 1, in two of twelve from Tsymbal, if five of seven from Cherevichnoe 1, in five of six from Karai-Dubina, in four of six teeth from Tihonovka 1, in three of four from Bolshevik 2,I, and in the only M3 specimen from Bolshevik 1. The anterocone is less pronounced than in M1–M2. In about 10% of the specimens the cusp is paired by a faint parastyle (Fig. 3W). The protocone is the tallest cusp on the crown. It is joined to the second tallest cusp, the paracone, by a strong protoloph ridge. The metacone is present in all teeth, except for one aberrant specimen from Tarkhankut (NMNHU-P 50-28/33), but the cusp is substantially smaller than the paracone. A shallow ectoloph extends between the paracone and metacone. The mesostyle is observable in 5–12% of the teeth. The labial metaloph is variably developed. It is mostly incomplete in the majority of the specimens from early and Biharian assemblages and in about half of the mid Biharian teeth (Fig. 3T, W, X). In contrast, the loph is essentially complete, oriented posterolabially, and slightly convex anteriorly in M3s from the late Biharian localities (Fig. 3Y, Z). The metaconule is a swollen cusp connected by a compressed and short lingual metaloph to the posterior arm of protocone. Most specimens from Nogaisk and Tarkhankut, four from Karai-Dubina, five of the 21 M3s from Tsymbal, four from Tihonovka 1, and two from Bolshevik 2,I have a prominent anterior projection of the metaconule that, nevertheless, fails to reach the protoloph (Fig. 3V–Y). Three specimens from Tarkhankut possess a similar, but posterolingually directed projection of the metaconule that almost crosses the posterior valley to delimit it into two nearly equal-sized smaller basins. A prominent sinus indents the labial side of the crown between the protocone and hypocone. The hypocone is a diminutive conule in virtually all specimens. In about 65% of M3s from Tarkhankut, Nogaisk, and Moiseevo 1, in approximately 45% from Cherevichnoe 1, and in 70–90% of teeth from Tsymbal, Tihonovka 1, and Bolshevik 2,I the protocone and hypocone are connected by a narrow endoloph. A low, irregular posteroloph is extended along the posterior margin of the tooth; its labial end is usually free and does not merge with metacone in unworn and lightly worn specimens. The posteriormost apex of the loph is swollen and tends to form a cusp-like bulge (posterocone) that expands anteriorly in about 35% of the M3s to occupy the bottom of the posterior valley (Fig. 3T, U, X).
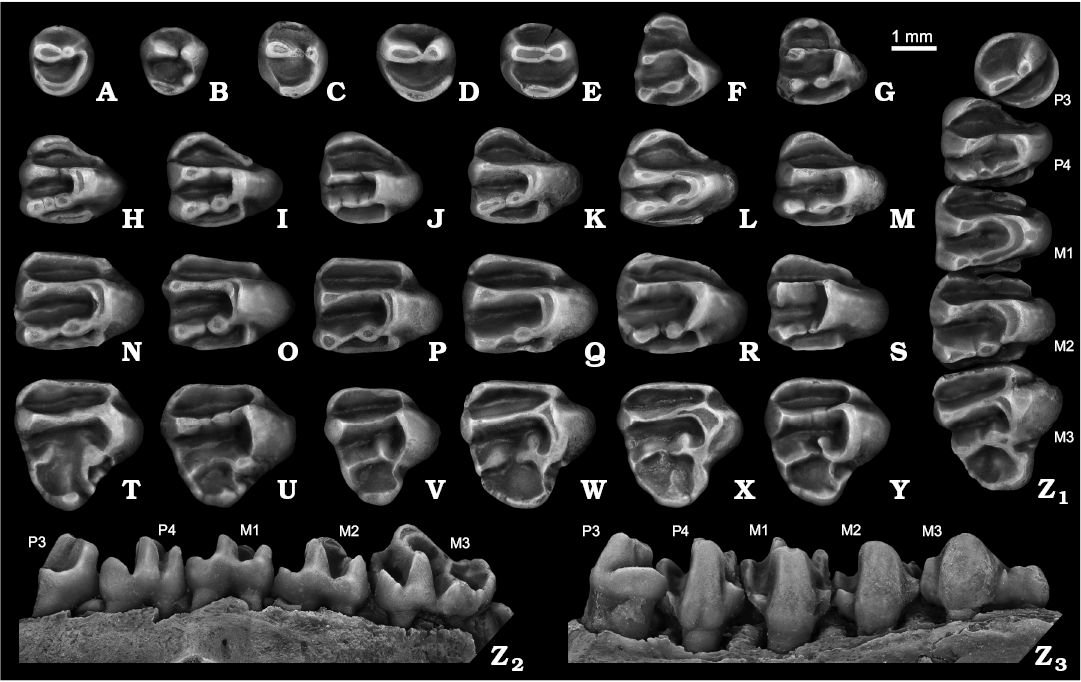
Fig. 3. Ground squirrel Spermophilus nogaici (Topachevsky, 1957) from the Early and Middle Pleistocene of southern Ukraine and southwestern Russia: Tarkhankut (A, B, G, H, I, O, U), Zhevakhova Gora 1 (F, N, T), Nogaisk (C, J, K, P, V, W, X), Moiseevo 1 (Q), Tihonovka 1 (D, L, R, S, Y, Z), Bolshevik 2,I (M); upper cheek teeth (A–E, P3; F, G, DP4; H–M, P4; N–S, M1–M2; T–Y, M3; Z, P3–M3), in occlusal (A–Z1), labial (Z2), and lingual (Z3) views. A. NMNHU-P 50-29/10. B. NMNHU-P 50-29/11. C. NMNHU-P 27-230. D. NMNHU-P 29-212. E. NMNHU-P Bol2/1-4. F. NMNHU-P ZG1-1. G. NMNHU-P 50-29/69. H. NMNHU-P 50-29/25. I. NMNHU-P 50-29/27. J. NMNHU-P 27-123. K. NMNHU-P 27-239. L. NMNHU-P 29-216. M. NMNHU-P Bol2/1-24. N. NMNHU-P ZG1-13. O. NMNHU-P 50-27/5. P. NMNHU-P 27-110. Q. ZIN 105151/12. R. NMNHU-P 29-4037. S. NMNHU-P 29-4043. T. NMNHU-P ZG1-17. U. NMNHU-P 50-28/42. V. NMNHU-P 27-535. W. NMNHU-P 27-532. X. NMNHU-P 27-121. Y. NMNHU-P 29-4059. Z. NMNHU-P 29-4036. A–C, D, H–J, P, Q, T, X (inverted).
The deciduous p4 of S. nogaici is known only from fourteen specimens, nine of which are from Zhevakhova Gora 1, Tarkhankut, and Cherevichnoe 1. The tooth is characterized by a labiolingually narrow trigonid and wide, simple talonid, giving the crown a clearly triangular occlusal outline (Fig. 4A, B). A large metaconid and a somewhat smaller protoconid are closely spaced and separated by a narrow, anteriorly opened trigonid basin, with a well-developed metalophid between them. The anteroconulid is present in all dp4s as a rounded cusp; the specimen from Tihonovka 1 (NMNHU-P 29-4064) shows an anterolophulid, which partly encloses the trigonid basin anteriorly. The ectolophid is a low, narrow crest occasionally (NMNHU-P ZG1-20, NMNHU-P Che1-41) bearing a gentle mesoconid swelling at its central part (Fig. 4A). The posterior portion of the tooth crown is represented by a strong, labially expanded hypoconid, solid posterolophid with indistinct hypoconulid, and a weak entoconid, which may be either isolated (NMNHU-P ZG1-20; NMNHU-P 50-29/74) (Fig. 4A) or fused with posteroloph (Fig. 4B). The tooth lacks any signs of ecto- and mesostylids. Two roots are present: a massive, anteriorly directed anterior root, and weaker posterior root.
The p4 varies in occlusal outline from being nearly triangular to almost rectangular; most specimens possess a relatively narrow and high trigonid that is about two times higher and on average about 7–3% narrower than the talonid (Fig. 4C–M, Z). The protoconid is slightly broader and lower than the metaconid, which is the tallest cusp in unworn specimens. The high labial anterolophid forms the anterolabial margin of a deeply excavated, asymmetrical trigonid basin. This lophid thickens into a weak anteroconulid defined in moderately worn p4s by an almond-shaped, compressed wear facet. The lingual anterolophid is absent in one (NMNHU-P ZG1-1-22) of the three teeth from Zhevakhova Gora 1. When present, it varies from partially incomplete and low, in 36–62% of the teeth (Figs. 4C, D, H, J), to a strong ridge that closes the depression of the trigonid anterolingually. The posterior wall of the trigonid basin is formed by both a pronounced labial metalophid and a somewhat weaker lingual metalophid. The junction between these two is defined as a well-marked constriction roughly at the midline of the trigonid basin. In three specimens (NMNHU-P 50-26/122, NMNHU-P 50-29/27, ZIN 105149/2-9) the ridges fail to connect leaving the basin open posteriorly (Fig. 4F). The ectolophid is a strong, albeit low, anteriorly tapering ridge comparable to the metalophid in width. The mesoconid is usually indistinct, although in two p4s from Tarkhankut (NMNHU-P 50-26/65; NMNHU-P 50-31/59) a swelling of the ectolophid may represent this cuspid (Fig. 4D). The hypoconid ranges in shape and amount of labial expansion from a relatively large, moderately expanded cusp seen in the majority of the teeth from early and mid Biharian localities (Fig. 4C–E, J, Z), to a more derived condition with reduced hypocone that only slightly protrudes labially observed in the specimens from Bolshevik 1, three of eleven from Nogaisk, two of eight from Tsymbal, and two of 13 teeth from Tihonovka 1 (Fig. 4F, H, I, K–M). The entoconid is fully merged with the posterolophid. The posterolophid is straightened or slightly bowed posteriorly and labially, and lacks a distinct hypoconulid. The mesostylid is variably present midway between the metaconid and entoconid: it is observable in all p4 from Zhevakhova Gora 1, in about 55–70% of the specimens from Tarkhankut, Nogaisk, Cherevichnoe 1, and Tsymbal; and in about half of the teeth from Karai-Dubina, Tihonovka 1, Bolshevik 1, and Bolshevik 2,I (Fig. 4C, D, F, G, J, L, M, Z). NMNHU-P 50-26/119 has an ectostylid-like projection on the anterolabial wall of the hypocone. The tooth is two-rooted in most cases: a roughly cylindrical anterior root supporting the trigonid, and a labiolingually widened posterior root beneath the talonid. In most p4s, the lingual side of the posterior root is complicated by a remnant of an additional root. In about two-thirds of the specimens from the late Biharian assemblages and about 10% of those from Nogaisk and Tarkhankut this structure is evident as a solitary, albeit underdeveloped, posterolingual root.
The m1 and m2 are virtually identical in size and morphology, so, similar to the upper counterparts, they are included in one category here. The only questionable distinction between m1 and m2 is a stronger metalophid on the first molar. The occlusal outline of the tooth is rectangular (Fig. 4N–S, Z). The two major trigonid cusps are subequal in size, the metaconid being slightly larger and taller than the protoconid. The trigonid is much taller than the talonid cusps, the hypoconid and reduced entoconid. A massive anterolophid continues labially along the anterior side of the tooth crown, where it bends posteriorly to fuse with the anterior wall of the protoconid. The anteroconulid is indistinct; some 5% of the m1–m2s from Nogaisk and Tarkhankut demonstrate a barely discernible anteroconulid expansion on the anterolabial corner of the anterolophid. The metalophid, either incomplete or complete, is present in six of the nine teeth from the early Biharian of Zhevakhova Gora 1; 81 of the 154 from the mid Biharian of Tarkhankut, 92 of the 115 from Nogaisk, one of the three from Babakh-Tarama 1, five of the eight from Moiseevo 1, 24 of the 30 from Tsymbal, eight of the 12 from Cherevichnoe, 11 of the 14 from Karai-Dubina; and 15 of the 16 from the late Biharian of Tihonovka 1, four of the five specimens from Bolshevik 1, and 23 of the 29 m1–m2 from Bolshevik 2,I (Fig. 4O–S, Z). The ectolophid is prominent, low, and uniform throughout its length. The talonid portion of m1–m2 is marked by a well-developed hypoconid, a weak to indistinct entoconid, and a posteriorly convex posterolophid between them. The bottom of the lingual sinus bears a small to moderately developed mesostylid evident in 70–89% of the specimens; about 30% of specimens with the mesostylid show remnants of the metastylid crest (Fig. 4O, S) connecting the mesostylid to the base of metaconid. In three m1–m2s from Tarkhankut and two from Nogaisk there is a tiny cuspid sitting on the lophid, which we interpret as a metastylid (Fig. 4Z). The ectostylid-like structures are discernible in about one-third of m1–m2s (Fig. 4O, R, Z). The basins are covered with smooth rugosities, which became more pronounced on the posterolabial corner of talonid basin. The tooth has four roots: larger posterolabial and anterolingual roots, a smaller anterolabial, and the smallest posterolingual root.
The m3 is large and triangular to rectangular in occlusal outline with labially expanded hypoconid (Fig. 4T–Y, Z). The trigonid differs from that of m1–m2 in being slightly lower and having a straighter, more uniform anterolophid with no signs of the anteroconulid. The metalophid is short in 40–65% of m3s (Fig. 4U–X, Z), moderately developed and incomplete in 40–65% (Fig. 4Y), or long and complete in the rest of the specimens. One tooth from Nogaisk (NMNHU-P 27-164) and Tarkhankut (NMNHU-P 50-31/93) lack the metalophid. The ectolophid forms a wide angle of 96–115° with the anterolophid. This ridge is massive, tapers both anteriorly and posteriorly, and shows a large, anterolingually expanded wear facet in moderately worn specimens. The sinusid is narrow, slit-like, and oriented anterolabially. About 25% of m3s demonstrate a low ectostylid on the anterolabial wall of a large, rounded hypoconid (Fig. 4V, Z). The mesostylid resembles that of m1–m2. There is a large, swollen hypoconulid on the posteriormost side of the crown, mostly isolated from the hypoconid by a strong postflexid. The posterolophid differs slightly in height and expression among the specimens. Most (about 75–80%) have a complete posterolophid enclosing the talonid posteriorly (Fig. 4V, W, Y, Z); others, however, possess a weaker lophid, with a sharp constriction at the base of the entoconid (Fig. 4T, U, X). The bottom of the talonid basin is uneven. The main part of the basin is occupied by a salient structure consisting of a massive, blunt entoconulid and a smoothened entolophid, which connects the entoconulid with a lophate entoconid. The entoconulid attains its greatest development in the specimens from Tihonovka 1 and Bolshevik 2,I in which it tends to fuse with the ectolophid, thus dividing the talonid basin into a larger anterior and smaller posterior depressions (Fig. 4U). A deep, crescentic valley delimits the lingual extension of the entoconulid from the hypoconid and hypoconulid. There are four roots similar to those in m1–m2.
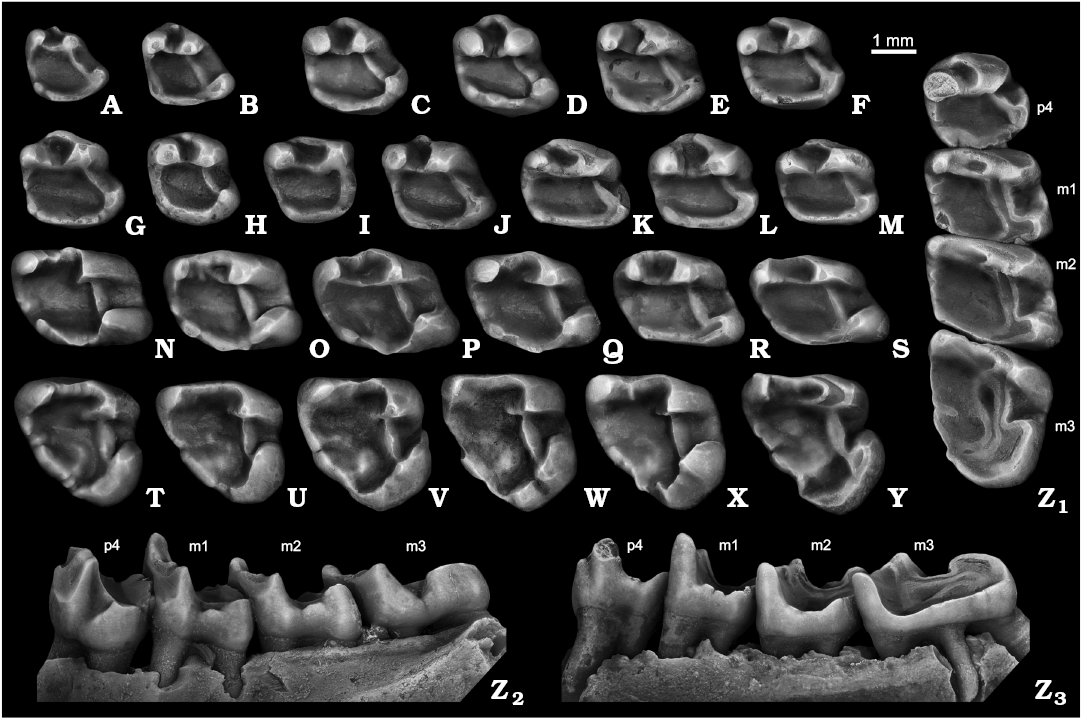
Fig. 4. Ground squirrel Spermophilus nogaici (Topachevsky, 1957) from the Early and Middle Pleistocene of southern Ukraine and southwestern Russia: Zhevakhova Gora 1 (A, C, N), Tarkhankut (D–F, O, T), Nogaisk (G–J, P, U–W, Z), Moiseevo 1 (Q), Cherevichnoe 1 (B, X), Tihonovka 1 (K, L), Bolshevik 2,I (M), lower cheek teeth (A, B, dp4; C–M, p4; N–S, m1–m2; T–Y, m3; Z, p4–m3), in occlusal (A–Z1), labial (Z2), and lingual (Z3) views. A. NMNHU-P ZG1-20. B. NMNHU-P Che1-41. C. NMNHU-P ZG1-24. D. NMNHU-P 50-26/65. E. NMNHU-P 50-26/110. F. NMNHU-P 50-26/122. G. NMNHU-P 27-106. H. NMNHU-P 27-540. I. NMNHU-P 27-541. J. NMNHU-P 27-543. K. NMNHU-P 29-4066. L. NMNHU-P 29-4075. M. NMNHU-P Bol2/1-64. N. NMNHU-P ZG1-25. O. NMNHU-P 50-25/131. P. NMNHU-P 27-575. Q. ZIN 105151/33. R. NMNHU-P 29-4079. S. NMNHU-P Bol2/1-73. T. NMNHU-P 50-26/56. U. NMNHU-P 27-161. V. NMNHU-P 27-163. W. NMNHU-P 27-172. X. NMNHU-P Che1-68. Y. NMNHU-P 29-4099. Z. NMNHU-P 27-224. B, D, H, L, T, U, Y (inverted).
Remarks.—The initial description of the lower dentition of S. nogaici was largely based on its holotype, a well-preserved right mandible with p4–m3 from Nogaisk (NMNHU-P 27-189). Currently, the exact location of this specimen is unknown, and it appears to be lost or destroyed. Nevertheless, the alpha taxonomy needs to be stabilized by selection of a lectotype from what remains of the topotypic material. We were able to find a right maxillary fragment with P4 (NMNHU-P 27-190) listed by Topachevsky (1957: 204) as a cotype and here designate this specimen as the lectotype of S. nogaici.
The species was named based on 85 specimens from the Nogaisk (Topachevsky 1957, 1965). Later, Gromov et al. (1965) documented additional specimens from the type locality, which have been supplemented by the descriptions of more abundant S. nogaici fossils from Tarkhankut (Topachevsky 1973) and Cherevichnoe 1 localities (Topachevsky et al. 1987). Many new dental fossils have been discovered since those reports, including previously undocumented deciduous premolars, bringing the total sample from a few hundred teeth to almost one and a half thousand teeth.
This larger sample allows us to assess patterns of variability in dental traits suggested by previous workers to be characteristic of S. nogaici based on smaller samples. Topachevsky (1957, 1973) and Gromov et al. (1965) highlighted the following features of the upper dentition of S. nogaici as distinguishing the species from other early Spermophilus: relatively small size; anterior valley and lingual metaloph of P4 are more strongly developed than in S. primigenius; and M3 is shorter, with either interrupted or, rarely, complete metaloph and weak metacone (originally “marginal tubercle”). Spermophilus nogaici remains the smallest early Pleistocene species of Spermophilus, although this difference is less marked compared to S. praecox sp. nov., which significantly overlaps in size with S. nogaici (Tables 1, 2). However, S. nogaici is somewhat larger than the specimens of Spermophilus aff. primigenius from the late Biharian of Somssich Hill 2 locality (Pazonyi et al. 2018), southern Hungary (MVS, personal observation), which has smaller upper and lower premolars. Larger sample sizes do not support Gromov et al. (1965) observations of reduced metaloph and metacone on M3. The size of the metacone on this tooth varies from quite small in the specimens pertaining to the early Biharian assemblages to only slightly lower and narrower than the paracone in those from the late Biharian localities, and in most teeth it is not substantially more reduced than in S. primigenius, and appears to be consistently larger than in S. polonicus. Although nine specimens of S. nogaici from Nogaisk have an interrupted metaloph on M3, all other known teeth possess a complete crest connecting metaconule and metaloph (11 out of 20 M3s; 55%). The percentage of specimens possessing a complete metacone tends to increase in time, thus showing a clear evolutionary tendency toward the acquisition of a strong and even crest (Fig. 3T–Y, Z). Despite these problems, other diagnostic characters of S. nogaici listed by Gromov et al. (1965) appear to be valid for distinguishing this species from other early members of the genus.
Table 2. Dental measurements (in mm) of Spermophilus praecox sp. nov.
|
Locality |
n |
Length |
Width |
Length/Width |
||
|
range |
mean |
range |
mean |
|||
|
Kryzhanovka 2 |
||||||
|
M1–M2 |
2 |
2.16; 2.35 |
– |
2.86; 2.95 |
– |
0.76; 0.79 |
|
M3 |
1 |
2.81 |
– |
2.98 |
– |
0.94 |
|
m3 |
2 |
2.76; 2.78 |
– |
3.08; 3.29 |
– |
0.84; 0.90 |
|
Kotlovina 2 |
||||||
|
P4 |
3 |
2.38–2.44 |
2.40 |
2.49–2.96 |
2.79 |
0.81–0.96 |
|
M1–M2 |
7 |
2.25–2.86 |
2.55 |
3.09–3.34 |
3.20 |
0.73–0.85 |
|
M3 |
2 |
2.72; 3.05 |
– |
3.04; 3.29 |
– |
0.90–0.93 |
|
p4 |
3 |
2.13–2.51 |
2.33 |
2.11–2.72 |
2.47 |
0.86–1.01 |
|
m1–m2 |
4 |
2.42–2.74 |
2.59 |
3.24–3.49 |
3.38 |
0.72–0.80 |
|
m3 |
1 |
3.21 |
– |
3.42 |
– |
0.94 |
|
Kotlovina 3 |
||||||
|
P4 |
1 |
2.31 |
– |
2.67 |
– |
0.87 |
|
M1–M2 |
4 |
2.18–2.46 |
2.28 |
2.83–3.23 |
2.02 |
0.72; 0.80 |
|
M3 |
1 |
2.75 |
– |
3.10 |
– |
0.89 |
|
p4 |
2 |
2.30; 2.42 |
– |
2.38; 2.52 |
– |
0.96 |
|
m1–m2 |
7 |
2.32–2.69 |
2.44 |
2.82–3.52 |
3.15 |
0.71–0.83 |
|
m3 |
3 |
2.87–2.98 |
2.93 |
3.14–3.45 |
3.28 |
0.85; 0.92 |
|
Yuzhny |
||||||
|
P3 |
1 |
1.56 |
– |
1.48 |
– |
1.06 |
|
P4 |
3 |
2.00–2.20 |
2.12 |
2.50 |
– |
0.86 |
|
M1–M2 |
6 |
2.25–2.57 |
2.39 |
2.95–3.14 |
3.02 |
0.77–0.80 |
|
M3 |
3 |
2.64–2.83 |
2.74 |
2.91–3.06 |
3.00 |
0.86–0.94 |
|
m1–m2 |
5 |
2.08–2.57 |
2.31 |
2.82–3.42 |
3.05 |
0.73–0.83 |
|
Morskoy |
||||||
|
P3 |
4 |
1.38–1.74 |
1.59 |
1.30–1.48 |
1.38 |
1.06–1.21 |
|
P4 |
7 |
2.12–2.49 |
2.27 |
2.25–2.74 |
2.58 |
0.82–1.11 |
|
M1–M2 |
17 |
2.09–2.60 |
2.39 |
2.86–3.38 |
3.16 |
0.74–0.81 |
|
M3 |
3 |
2.73–2.78 |
2.75 |
2.70–2.87 |
2.81 |
0.96–1.01 |
|
p4 |
10 |
2.12–2.55 |
2.32 |
2.04–2.73 |
2.47 |
0.88–1.04 |
|
m1–m2 |
14 |
2.00–2.56 |
2.31 |
2.87–3.70 |
3.31 |
0.67–0.72 |
|
m3 |
5 |
2.68–2.10 |
2.93 |
2.77–3.16 |
3.04 |
0.85–1.05 |
The westernmost occurrences of S. nogaici are limited to southeastern Ukraine and Moldova. Several putative S. nogaici-like specimens from central Europe have been reported in the literature. Popov (2017) illustrated nineteen isolated teeth of a moderately-sized ground squirrel from the Early Pleistocene (late Biharian) of Futjova Cave in northern Bulgaria that he tentatively referred to “Spermophilus cf. nogaici”. Apart from slightly narrower p4s and somewhat shorter M1–M2s these specimens fall within the size variation of S. nogaici from Nogaisk. Their morphology, however, is not fully consistent with identification as S. nogaici. The p4, in particular, has an anteroposteriorly elongated crown with relatively wide trigonid, and a small, rudimentary hypoconid reminiscent of that in most living species of the genus. Although present in some specimens of S. nogaici, such a combination of features is unusual for this species, but is evident in a putative S. primigenius sample from the late Early Pleistocene of Somssich Hill 2. Other characters supporting the identification of Futjova Cave squirrels as S. primigenius include a weak entoconulid of m3 and weak hypocone of M1–M2. Furthermore, “Spermophilus cf. nogaici” from Futjova and Spermophilus aff. primigenius from Somssich Hill 2 appear to share the same structure of the M3 talon, with a small posterior lobe and weak metaloph bearing no signs of metaconule, although based on figures and the brief description (Popov 2017) it is difficult to tell whether these features are real or artifactual. Given these observations, Spermophilus fossils from Futjova Cave are tentatively attributed here to Spermophilus aff. primigenius. It is quite possible that these late Biharian specimens of middle-sized Spermophilus from central Europe (including those from Somssich Hill 2) belong to a different yet undescribed species of Spermophilus that would fill the apparent stratigraphical gap between the typical S. primigenius and Late Pleistocene Spermophilus citelloides Kormos, 1916. The discovery of more complete material is necessary to allow clarification of this taxon’s relationship to the mentioned species.
Stratigraphic and geographic range.—Early to Middle Pleistocene (Biharian) of southern Ukraine, Moldova, and southwestern Russia.
Spermophilus praecox sp. nov.
Figs. 5–7.
ZooBank LSID: urn:lsid:zoobank.org:act:9EC89A65-A40E-42FC-8418- 55A8DA818088
1965 Citellus (cf. Urocitellus) nogaici Topatschevky, 1957; Gromov et al. 1965: 178–181 (in part), fig. 36: 7.
1989 Citellus (Urocitellus) cf. nogaici Topachevski, 1957; Topachevsky and Nesin 1989: 23–25, fig. 5.
2007 Spermophilus cf. nogaici Topachevski, 1957; Stadnik and Dema 2007: 361–364.
Etymology: From the Latin praecox, premature or precocious; in reference to the early occurrence of the species in the fossil record.
Holotype: NMNHU-P 41-5598, left M1–M2.
Type locality: Kotlovina 2, Reni District, Odessa Region, Ukraine.
Type horizon: Early Pleistocene, Gelasian, late Villanyian.
Material.—From late Pliocene, middle Villanyian, Kryzhanovka 2: ZIN 105160/1, P3; ZIN 105160/2, P4; ZIN 105160/3–5, M1–M2; ZIN 105160/6, M3; ZIN 105160/7–8, m3.
From Early Pleistocene, late Villanyian, Kotlovina 2: NMNHU-P 41-5588–5590, P4; NMNHU-P 41-5591–5600, M1–M2; NMNHU-P 41-5601–5602, M3; NMNHU-P 41-5603–5605, p4; NMNHU-P 41-5606–5608, NMNHU-P 41-5610, m1–m2; NMNHU-P 41-5609, m3; ZIN 105163/2, M1–M2; ZIN 105163/3, m1–m2; ZIN 105163/4, m3; Kotlovina 3: NMNHU-P 41 5611, P4; NMNHU-P 41-5612–5615, M1–M2; NMNHU-P 41-5616, M3; NMNHU-P 41-5617–5618, p4; NMNHU-P 41-5619–5625, m1–m2; NMNHU-P 41-5626–5628, m3; ZIN 105163/1, mandible with p4–m3; Yuzhny: GIN 1166/1, P3; GIN 1166/2–3, P4; GIN 1166/4–9, M1–M2; GIN 1166/10–12, M3; GIN 13–17, m1–m2; GIN 1166/18, m3.
From Early Pleistocene, early Biharian, Morskoy: NMNHU-P MoT-1–4, P3; NMNHU-P MoT-6–10, P4; NMNHU-P MoT-11–30, M1–M2; NMNHU-P MoT-31–33, M3; NMNHU-P MoT-34–44, p4; NMNHU-P MoT-45–57, m1–m2; NMNHU-P MoT-58–61, m3; NMNHU-P MoT-62, mandible with p4–m3; NMNHU-P MoT-63, mandible with incisor, NMNHU-P MoT-64, edentulous mandible.
Diagnosis.—Medium-sized, primitive species of the genus with small P3 having a diminutive protocone and lacking the anteroloph; P4–M2 that consistently exhibit the endoloph and mesostyle, large metaconule, and rudimentary lingual metaloph; M3 is simple with smoothened metacone, metaconule, and labial metaloph; p4 trigonid is 12–23% labiolingually narrower than talonid, lacking lingual anterolophid and lingual metalophid; and m1–m2 with weak, shortened metalophid.
Cheek teeth similar in size to S. nogaici, smaller than S. fulvus, S. major, S. polonicus, S. primigenius, S. ralli, S. relictus, S. superciliosus, and S. tologoicus, but larger than S. alashanicus, S. citelloides, S. citellus, S. dauricus, S. pygmaeus, S. suslicus, S. taurensis, and S. xanthoprymnus. Differs from all species of the genus other than S. polonicus and S. primigenius in retaining relatively lower crowned premolars and molars; small, simple P3 with rudimentary protocone and anterior valley; weak anterior valley, anterostyle, metaloph of P4–M2 accompanied by enlarged posterior valley, salient and circular metaconule, more inflated hypocone, and consistently present endoloph and mesostyle; primitively shortened metalophid of p4–m3; and elongated p4 with labiolingually compressed trigonid, cuspate anteroconulid, weak to indistinct lingual anterolophid and lingual metalophid. Differs from S. polonicus and S. primigenius in being smaller; having stronger endolophs and more globular metaconules of P4–M2; larger M3 metaconule and wider metaloph; and possessing a p4 hypoconulid. Further differs from S. polonicus in having lightly-built, less cuspate dentitions with more trenchant ridges; longitudinally oriented endolophs of P4–M2; more pronounced M3 metacone; stronger metalophid of p4–m3; and posteriorly tapered talonid of m3 that lacks a hypoconulid and postflexid.
Description.—Mandible: Four partial mandibles referable to S. praecox sp. nov. have been recovered from Kotlovina 2 (ZIN 105163/1) and Morskoy (NMNHU-P MoT-62, NMNHU-P MoT-63, NMNHU-P MoT-64), preserving the main portion of the mandibular body with most of the ramus and diastema being missing (Fig. 5). The body is very deep and narrowed mediolaterally and the diastemal portion is robust. The diastema seems to be relatively shallow (Fig. 5A), and forms no acute transition between the horizontal and vertical edges of the diastemal depression seen in most Spermophilus. Dorsally, the depression is marked by a well-defined shelf for the attachment of the anterior mandibular part of the buccinator muscle (buccinator ridge) that extends anteriorly from the lingual rim of the p4 posterior alveolus along the dorsal margin of the bone (Fig. 5A2). The area of the mental foramen is missing in all specimens. The preserved posterior rim of the foramen, preserved in ZIN 105163/1 (Fig. 5A1), suggests that the species had a relatively small mental foramen placed roughly at the middle of the diastema, about 2.41 mm anterior to p4. The masseteric fossa is a vast area with widely rounded anterior edge terminating below the posterior root of p4 (Fig. 5A1, B1, C1). The anterior extent of the fossa is not marked by either a prominent crest or bulging for the anterior fibers of the anterior deep masseter muscle observable in some primitive marmotines (Sinitsa 2018). The fossa is floored by a pronounced lower masseteric ridge, although the ridge does not project strongly laterally. The upper masseteric ridge is smooth but well-elevated above the lateral surface of the bone. The ascending ramus rises gradually opposite the m1–m2 embrasure. The medial surface of the mandibular body is noticeable for an ellipsoidal mylohyoid depression, the anterior and dorsal edge of which is rimmed by a strong mylohyoid line (Fig. 5A2, B2, C2). The ventral part of the depression bears five to seven diminutive venous foramina and a set of irregular grooves marking the paths of accessory veins on the bony surface. As seen in NMNHU-P MoT-64 (Fig. 5C) the angular process of S. praecox was relatively large and only slightly inflected inwards.
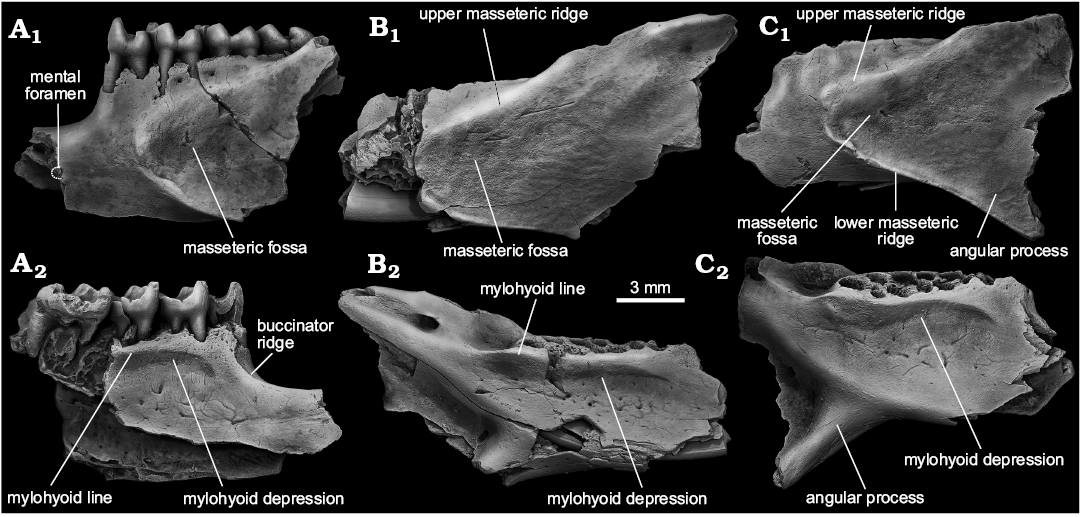
Fig. 5. Ground squirrel Spermophilus praecox sp. nov. from the Early Pleistocene of southern Ukraine: Kotlovina 3 (A) and Morskoy (B, C); mandibles in lateral (A1–C1), and medial (A2–C2) views. A. ZIN 105163/1. B. ZIN NMNHU-P MoT-63 (inverted). C. NMNHU-P MoT-64.
Dentition: The P3 is a small peg-like tooth with simple, gently ellipsoidal crown (Fig. 6A–D). The posterolabial flattening, marking the contact with P4, is very slight, presumably due to the small size of the tooth. As in S. nogaici and other derived marmotines, the crown is formed by two major cusps, the protocone and paracone. In contrast to S. nogaici and all extant species of the genus, however, the protocone in S. praecox sp. nov. is a diminutive cusp, more than three times smaller than the paracone. The protoloph appears to be a massive, uninterrupted ridge when unworn, which is particularly the case of the P3s from Morskoy (Fig. 6B–D), showing nearly fused protocone and paracone connected by a massive protoloph. A small anterocone, forming a recessed anterior valley, is present at the anteriormost margin of the crown. The anteroloph is not defined in all but one specimen (NMNHU-P MoT-3) whose anterior portion bears an extremely short, cuspate anteroloph. The posterior valley is simple, surrounded by a crescent-shaped posteroloph with no recognizable cusps. The tooth possesses a strong endoloph between the protocone and posteroloph. The crown is narrowed toward its base and continues into a simple root with a deep longitudinal groove on the labial surface.
The P4 is a rounded triangle in occlusal outline (Fig. 6E–I). The anterior lobe is relatively small, always narrower labiolingually than half of the total crown width. The anteriormost edge of the lobe is topped with a well-developed, conical or, rarely (NMNHU-P MoT-6, NMNHU-P MoT-10), comblike anterocone (Fig. 6H, I). The lingual arm of anteroloph consists of two sections of nearly equal length: the higher and bulky labial portion adjacent to the anterocone, and the much lower and narrower lingual portion. In most specimens the anteroloph fails to connect with the base of the protocone, thus leaving the anterior valley opened lingually. Apart from three specimens (NMNHU-P MoT-8–10) the anterostyle is weak to nearly absent. On the opposite, labial side of the anterior valley, the labial arm of the anteroloph extends posterolabially from the anterocone and terminates abruptly at the bottom of the labial anterosinus. The parastylar cusplet is present in all but two P4s (NMNHU-P MoT-8, NMNHU-P MoT-10). The protocone is the tallest principal cusp followed by the metacone and paracone. The paracone and metacone are less closely spaced than in S. nogaici, resulting in a much anteroposteriorly longer central valley, which exceeds the anterior valley in both length and width. There is a prominent mesostyle at the central part of the central sinus. The protoloph is straight, uninterrupted, and decreases in width and height at its midlength. The metaloph, in contrast, is slightly longer, narrower, and about two to three times lower than the protoloph in unworn specimens. A salient, large, rounded to slightly anteroposteriorly compressed metaconule is well-bounded from the metaloph by abrupt constrictions; it is closely appressed to the lingual wall of the protocone but separated from it by a strong groove. On NMNHU-P MoT-9 the labial metaloph bears a faint second metaconule. The posterior valley is roughly subequal in size to the central one (Fig. 6E–G), or slightly shorter in the specimens from Yuzhny and Morskoy (Fig. 6H, I). The endoloph is a strong longitudinally oriented crest that extends posteriorly from the protocone to the hypocone, from which also extends a strong posteroloph. The rudimentary hypocone appears well elevated above the base of the crown: in direct posterior aspect the apex of the cusp is aligned with the tip of the metacone.
The M1–M2 is triangular in outline (Fig. 6J–P). The labial part of the crown is markedly longer than the lingual part. The anterior lobe, as in P4, is relatively short labiolingually, constituting 65.5–76.9% (mean 71.6%) of the total width of the crown. A moderately developed anterostyle is present on the anterior slope of the protocone in most specimens and defines the lingual end of the anteroloph that runs across the anterior lobe, ending labially just before reaching the deepest point of the anterolabial sinus. A shallow indentation on the anterolingual side of the crown, just between the anterostyle and protocone, represents a rudimentary antesinus. The anterocone is pointed but weak; posterior to it a diminutive parastyle is observable in one of the three from Kryzhanovka 2, five of seven M1–M2s from Kotlovina 2, two of four teeth from Kotlovina 3, one of five from Yuzhny, and nine of the 18 from Morskoy (Fig. 6K, M, O). The central valley, formed by a complete and straight protoloph, and rudimentary metaloph is substantially longer anteroposteriorly than the anterior valley. At the labial side of the central valley is a knob-like, pointed mesostyle that partially dams the exit of the central sinus. The angle between the protoloph and metaloph ranges from 8.5 to 11.7°. The rudimentary and narrow metaloph with restricted lingual and labial portions is dominated by a large metaconule. The cusp appears to be more pronounced and globular in the specimens from Kotlovina 2 and 3 (Fig. 6K), and slightly compressed anteroposteriorly in those from Kryzhanovka 2, Yuzhny, and Morskoy (Fig. 6N, O). The posterior valley is somewhat anteroposteriorly shorter than the central valley. It is rimmed posteriorly by a massive, albeit low, posteroloph that extends labially from a high hypocone bulging and fades out before the contact with the base of the metacone. The endoloph is consistently present in all specimens, while showing some signs of reduction in those from Morskoy.
The M3 is slightly (7–14%) shorter than wide and resembles a rounded triangle in occlusal outline (Fig. 6Q–W). The posterior lobe is relatively small and weakly defined. The anteroloph is low lingually but continuous from the anterior wall of the protocone to the narrow anterolabial sinus, closing off a vast anterior valley labially. Both the anterostyle and antesinus are weak to indistinct. The anterocone is a small and pointed cusplet barely discernible from the anteroloph. One specimen from Yuzhny (GIN 1166/10) possesses a very faint parastyle. The protocone and metacone are nearly equal in height and connected by a massive, straight protoloph. The posterior and central basins are partially separated by a moderately developed metacone, from the lingual side of which departs a short, but prominent labial metaloph terminating roughly at the center of the tooth crown. In one M3 (NMNHU-P 41-5602) the crest is complete, albeit shallow, and continuous from the metacone to the metaconule (Fig. 6R). The metaconule is a salient but low cusp just labial to the posterolabial side of the protocone. There is no hypocone or true endoloph distinguishable. The posteroloph generally runs from the metaconule along the posterolingual margin of the crown to the voluminous posterocone, then curves around the posterolingual edge of the tooth, and diminishes gradually before reaching the metacone. On the lightly worn specimens from Morskoy (NMNHU-P MoT-31, NMNHU-P MoT-32) the loph fails to connect to the metaconule, leaving the posterior valley opened lingually (Fig. 6V, W). The three roots are ellipsoidal to circular in cross section, with the posterior root being only slightly larger than the anterolabial and, especially, anterolingual roots.
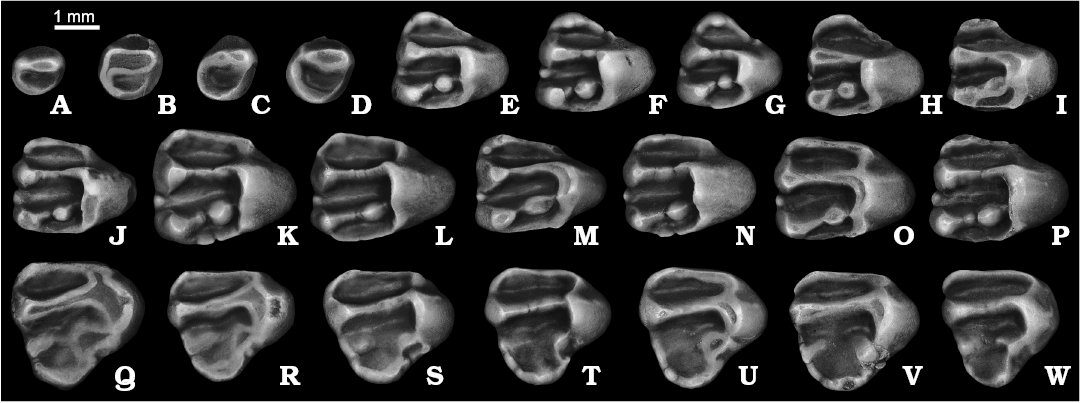
Fig. 6. Ground squirrel Spermophilus praecox sp. nov. from the late Pliocene and Early Pleistocene of southern Ukraine: Kryzhanovka 2 (A, J), Kotlovina 2 (E, F, K, L, Q, R), Kotlovina 3 (G, M, S), Yuzhny (B, N, T, U), Morskoy (C, D, H, I, V, W); upper cheek teeth (A–D, P3; E–I, P4; J–P, M1–M2; Q–W, M3), in occlusal views. A. ZIN 105160/1. B. GIN 1166/1. C. NMNHU-P MoT-2. D. NMNHU-P MoT-4. E. NMNHU-P 41-5588. F. NMNHU-P 41-5589. G. NMNHU-P 41-5611. H. NMNHU-P MoT-6. I. NMNHU-P MoT-10. J. ZIN 105160/5. K. NMNHU-P 41-5598 (holotype). L. NMNHU-P 41-5599. M. NMNHU-P 41-5613. N. GIN 1166/4. O. NMNHU-P MoT-14. P. NMNHU-P MoT-16. Q. NMNHU-P 41-5601. R. NMNHU-P 41-5602. S. NMNHU-P 41-5616. T. GIN 1166/11. U. GIN 1166/12. V. NMNHU-P MoT-31. W. NMNHU-P MoT-32. C, J, K, O, Q, S (inverted).
The p4 of S. praecox sp. nov. is clearly premolariform and similar to the deciduous lower premolars of S. nogaici in overall proportions (Fig. 7A–G, Z). The trigonid is moderately compressed labiolingually, about 12–23% narrower than the talonid, which gives the tooth crown a subtriangular to subrectangular occlusal outline. The two anterior cusps, metaconid and protoconid, are the tallest of the main cusps and are closely appressed; the metaconid is slightly taller than the protoconid. The metaconid is anterolingual in position, and is distinctly separated from the protoconid by a slit-like posterior portion of the trigonid basin. A widely triangular anterior side of the crown bears a weak anteroconulid, which is aligned with a shallow and smoothened labial anterolophid. There is no lingual anterolophid in any of the p4s. The metalophid is thin, tapering lingually, and low. All examined specimens, except two teeth from Morskoy (NMNHU-P MoT-34, NMNHU-P MoT-43) lack the lingual metalophid (Fig. 7D, G) seen in more derived members of the genus, including S. nogaici. A low ectolophid is present along the labial margin of the tooth, in four out of 18 specimens displaying a small mesoconid at the central part of the ridge (Fig. 7B, D, F, Z1). The hypoconid protrudes more anterolabially than it does in S. nogaici and other Spermophilus, thus causing the posterolingual expansion of the sinusid. The entoconid is distinct as a labiolingually compressed ridge-like cuspid. A transition from the entoconid to posterolophid is well defined due to the presence of a massive hypoconulid that forms the entire posterior wall of the crown. A small mesostylid is connected to the base of the metaconid by a faint metastylid crest. The tooth possesses two roots supporting the trigonid and talonid; the posterior root is thicker and expanded lingually.
The m1–m2 is a rectangle with a slightly narrower anterior portion and a gently curved posterolingual angle (Fig. 7H–S, Z). The trigonid is about one and a half times the height of the talonid. Both the metaconid and protoconid are narrow and pointed in unworn teeth. The metaconid is notably taller but appears to be narrower at its base than the protoconid. The anterolophid connects the labial base of the metaconid to the anteriormost side of the protoconid, forming the anterior wall of the tooth crown. The anteroconulid is missing in all examined specimens. An elongated metalophid that at least partially dams the trigonid basin posteriorly is observable in three out of the seven m1–m2s from Kotlovina 2, in four of the seven teeth from Kotlovina 3, in three of the five specimens from Yuzhny, and in seven of 15 m1–m2s from Morskoy, suggesting that the ridge was present in all m1s (Fig. 7M, N, Q, S), while being deeply reduced to absent in m2s (Fig. 7Z1). A small to moderately developed mesostylid is variably present immediately posterior to the metaconid. Three of six teeth from Kotlovina 2, three of four from Kotlovina 3, one of three specimens from Yuzhny, and five of 17 teeth from Morskoy demonstrate a weak metastylid crest between these two cusps (Fig. 7J, K, N–P, R, Z1). The ectolophid is a straightened ridge that joins the posterior part of the protoconid and the anterolingual base of the hypoconid. It varies slightly in shape among specimens from evenly smooth and uniformly thick to slightly tapered and gradually narrowed both anteriorly and posteriorly. Some specimens (Fig. 7K, O, Q, R) appear to retain a more primitive morphology with a narrow ectolophid that gently arcs lingually. The hypoconid is only slightly lower than the protoconid, and about 20–40% taller than the rudimentary, lophate entoconid. The crescentic posterolophid is as high as the primary lophids; it continues along the posterior margin of the crown to merge with the entoconid, and encloses the talonid basin. Two specimens (NMNHU-P 41-5608, NMNHU-P 41-5621) show a diminutive hypoconulid on the posteriormost extent of the crest (Fig. 7J). The root system consists of four slender roots, of which the posterolabial one is the longest and the strongest root.
The m3 has a distinctly triangular crown with narrow talonid due to a weak labial expansion of the hypocone (Fig. 7T–Y, Z). The trigonid is about 25% taller than the talonid. The metaconid and protoconid are of similar size, and the two cusps are connected by a continuous anterolophid. A very faint anteroconulid sitting directly anterior to the protoconid is present in two teeth (NMNHU-P MoT-58 and NMNHU-P MoT-60; Fig. 7V, X). The metalophid is strongly reduced: it is completely absent in two out of the four specimens from Kotlovina 2, two of the three teeth from Kotlovina 3, and three of the five m3s from Morskoy (Fig. 7U–W, Z1). The other m3 specimens possess an extremely shortened crest evident as a lingual protrusion of the protoconid. The ectolophid is a straight or slightly arcuate crest that extends from the anterolingual wall of the hypoconid to the posterolingual base of the protoconid. The anterior and posterior constrictions, seen in most species of the genus, are not as pronounced, and become evident only in heavily worn specimens. A poorly-defined ectostylid, partially incorporated with the hypocone, is present in about half of m3s (Fig. 7T, V, Z1). The hypoconid and a somewhat lower entoconid are located at the posterolabial and posterolingual extremes of the crown, respectively, and dominate the talonid portion of the tooth. The hypoconid varies in size from a rather small cusp, comparable to the protoconid in the teeth from Kryzhanovka 2, Kotlovina 2, and 3 (Fig. 7U, V, T, Z1), to a more voluminous, labially expanded cusp in those from Yuzhny and Morskoy (Fig. 7W–Y). The entoconid is very shallow and reduced with barely discernible entoconulid and entolophid; it fades out gradually labially. The groove between the entoconulid and the hypoconid is only barely discernible in m3s from Kotlovina and Yuzhny (Fig. 7U, V, Z1); whereas the groove is more pronounced in the two molars from Kryzhanovka 2 and Morskoy (Fig. 7T, W–Y). Apart from NMNHU-P MoT-59 showing a sign of postflexid barely separating the remnant hypoconid from the main portion of the posterolophid, there is no sign of either the postflexid or the hypoconulid in any of the m3s. The root structure is similar to that in S. nogaici with four distinct roots that diminish in size from the largest posterolabial to the smallest posterolingual root.
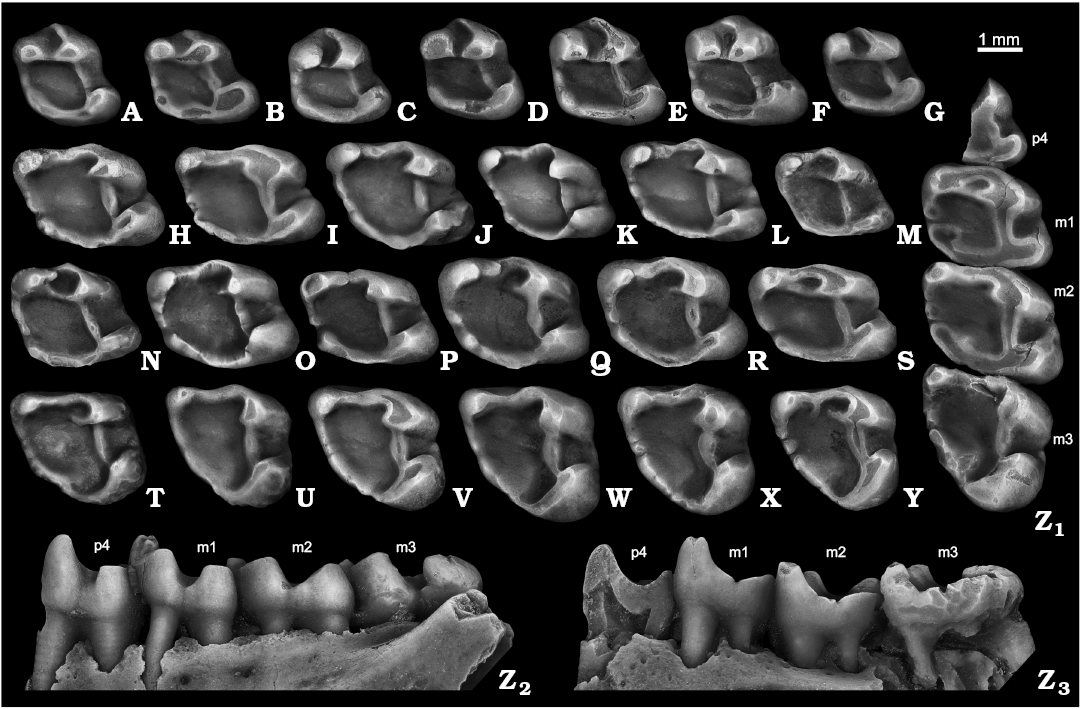
Fig. 7. Ground squirrel Spermophilus praecox sp. nov. from the late Pliocene and Early Pleistocene of southern Ukraine: Kotlovina 2 (A, B, H–J), Kotlovina 3 (C, J–L, U, V, Z), Kryzhanovka 2 (T), Yuzhny (M–O), Morskoy (D–G, P–S, W–Y); lower cheek teeth (A–G, p4; H–S, m1–m2; T–Y, m3; Z, p3–m3), in occlusal (A–Z1), labial (Z2), and lingual (Z3) views. A. NMNHU-P 41-5603. B. NMNHU-P 41-5604. C. NMNHU-P 41-5617. D. NMNHU-P MoT-34. E. NMNHU-P MoT-36. F. NMNHU-P MoT-39. G. NMNHU-P MoT-43. H. NMNHU-P 41-5606. I. NMNHU-P 41-5610. J. NMNHU-P 41-5621. K. NMNHU-P 41-5622. L. NMNHU-P 41-5624. M. GIN 1166/13. N. GIN 1166/15. O. GIN 1166/16. P. NMNHU-P MoT-48. Q. NMNHU-P MoT-47. R. NMNHU-P MoT-50. S. NMNHU-P MoT-57. T. ZIN 105160/7. U. NMNHU-P 41-5627. V. NMNHU-P 41-5626. W. NMNHU-P MoT-58. X. NMNHU-P MoT-60. Y. NMNHU-P MoT-61. Z. ZIN 105163/1. B, G, Q, R, X, Y (inverted).
Remarks.—The
recognition of this new species helps to resolve the dilemma faced in
previous studies of late Pliocene and Early Pleistocene Spermophilus
from eastern Europe (Gromov et al. 1965; Topachevsky
and Nesin 1989; Stadnik and Dema 2007), the
extensive variability in dental morphology among Villanyian and Biharian
specimens attributed to S. nogaici.
Presumably because of the lack of diagnostic specimens, primarily the
P3s, Topachevsky and Nesin (1989) cited Spermophilus
cf. nogaici as occurring at Kotlovina 2 and
3, noting that these specimens were among the earliest records of this
taxon, slightly differing from the type material by having more
pronounced mesostyles, mesoconids, and mesostylids. Subsequently, Spermophilus cf. nogaici
has also been reported from the middle Villanyian of Zhevakhova Gora 4
(originally Zhevakhova Gora 15) in southern Ukraine (Stadnik
and Dema 2007). Based on the limited sample available to them, the
authors noted that the specimens of Spermophilus
cf. nogaici may have represented a new
species, which is described here as S. praecox.
It is perhaps not surprising that among the species of the genus S. praecox sp. nov. shows closest resemblance to
S. nogaici in known parts of the dentition,
and it seems obvious that these two species are closely related. In
contrast to S. nogaici, however, S.
praecox has substantially smaller P3, while being slightly
larger in most other tooth dimensions (Tables 1–3), and retaining what
appears to be a more primitive dental morphology.
Table 3. Summary statistics and dental measurements (in mm) of Spermophilus nogaici (Topachevsky, 1957) and S. praecox sp. nov. CV, coefficient of variation; SD, standard deviation; SE, standard error.
|
Species |
n |
Length |
Width |
||||||
|
mean |
SE |
SD |
CV |
mean |
SE |
SD |
CV |
||
|
Spermophilus nogaici (Topachevsky, 1957) |
|||||||||
|
P3 |
61 |
1.74 |
0.01 |
0.12 |
6.64 |
1.68 |
0.02 |
0.14 |
8.45 |
|
DP4 |
11 |
2.06 |
0.05 |
0.16 |
7.83 |
2.09 |
0.06 |
0.19 |
9.20 |
|
P4 |
123 |
2.25 |
0.01 |
0.13 |
5.68 |
2.69 |
0.02 |
0.17 |
6.26 |
|
M1–M2 |
356 |
2.25 |
0.01 |
0.12 |
5.50 |
2.99 |
0.01 |
0.15 |
4.86 |
|
M3 |
137 |
2.83 |
0.01 |
0.17 |
6.02 |
2.85 |
0.01 |
0.15 |
5.17 |
|
dp4 |
8 |
1.84 |
0.07 |
0.19 |
10.43 |
2.12 |
0.10 |
0.26 |
12.10 |
|
p4 |
142 |
2.19 |
0.01 |
0.15 |
6.77 |
2.49 |
0.02 |
0.19 |
7.63 |
|
m1–m2 |
367 |
2.18 |
0.01 |
0.15 |
6.83 |
3.16 |
0.01 |
0.22 |
6.87 |
|
m3 |
171 |
2.98 |
0.01 |
0.19 |
6.46 |
3.09 |
0.01 |
0.17 |
5.67 |
|
Spermophilus praecox sp. nov. |
|||||||||
|
P3 |
6 |
1.52 |
0.08 |
0.20 |
13.12 |
1.38 |
0.04 |
0.09 |
6.80 |
|
P4 |
14 |
2.24 |
0.04 |
0.17 |
7.38 |
2.61 |
0.06 |
0.20 |
7.84 |
|
M1–M2 |
35 |
2.40 |
0.03 |
0.18 |
7.42 |
3.11 |
0.03 |
0.15 |
4.67 |
|
M3 |
10 |
2.78 |
0.03 |
0.11 |
3.88 |
2.99 |
0.05 |
0.16 |
5.35 |
|
p4 |
14 |
2.33 |
0.04 |
0.15 |
6.44 |
2.47 |
0.06 |
0.22 |
9.02 |
|
m1–m2 |
33 |
2.40 |
0.04 |
0.20 |
8.38 |
3.24 |
0.04 |
0.24 |
7.49 |
|
m3 |
11 |
2.93 |
0.05 |
0.16 |
5.34 |
3.16 |
0.06 |
0.19 |
6.06 |
The more primitive traits of S. praecox sp. nov., as compared with S. nogaici, involve both upper and lower premolars and molars. The P3 of S. praecox, apart from its smaller size, possesses a relatively smaller protocone and larger paracone, and lacks a distinct anterior valley. The P4 is less molariform, with smaller anterior valley hardly reaching two-thirds of the transversal width of the tooth; it has a more cuspate anterocone, weaker anterostyle, stronger endoloph, proportionally larger central valley, consistently present mesostyle, larger metaconule, and more rudimentary metaloph mostly lacking its lingual portion. The M1–M2 of S. praecox differ from those of S. nogaici in possessing a primitively smaller anterior valley that, viewed occlusally, appears to be narrower transversally than the posterior valley; it also lacks a furrow-like antesinus and enlarged anterostyle. As in P4, the M1–M2s of S. praecox are notable in having a mesostyle, larger central valley, complete and strong endoloph with elevated hypocone, and a much weaker and reduced metaloph that yields a voluminous metaconule. Similarly, the M3s of S. praecox are separable from the same teeth of S. nogaici in having a less expanded anterior valley with extremely shallow antesinus and indistinct anterostyle; and a weaker metacone, metaloph, and metaconule, with the latter closely appressed to the protocone.
Compared to S. nogaici, the p4 of S. praecox is more longitudinally elongated and trapezoidal with distinctly narrower trigonid, consistently present anteroconulid, more massive hypocone and hypoconulid, stronger mesoconid, and fainter anterolophid that consists only of a rudimentary labial portion and lacks the lingual portion. The m1–m2 and m3 of S. praecox and S. nogaici are similar in most respects. However, in S. praecox the metalophids are lacking on m2–m3; and the talonid of m3 is relatively weaker and is characterized by a small hypoconid not shifted labially, a weak entoconulid and posterolophid, and no distinct hypoconulid or postflexid.
The dentition of Spermophilus praecox shows resemblances to the cheek teeth of S. primigenius and, especially, S. polonicus in possessing a small P3 with rudimentary anterior valleys, less molariform P4 and p4, inflated metaconules, and stronger mesostyles on P4–M2. Similarities to S. polonicus are certainly plesiomorphic retentions, indicating that S. praecox is more primitive than S. nogaici, in agreement with its earlier occurrence.
Despite these similarities, however, S. praecox clearly differs from S. polonicus in having relatively lightly-built cheek teeth with slender lophs, less stout, cone-shaped major cusps, and more inflated conules (Gromov et al. 1965; Black and Kowalski 1974; MVS, personal observation). In particular, the upper third premolar of S. praecox, although represented by only a few specimens, has less elongated and narrowed anteriorly occlusal surface, with much larger paracone separated from the protocone by the protoloph (Fig. 6A, C). The P4 and M1–M2 are proportionally shorter anteroposteriorly, less cuspate, and show somewhat larger anterior and posterior valleys. The metaconules of these teeth are salient, more globular, and buttressed by a relatively weaker metaloph with deeply reduced lingual portions. Furthermore, the mesostyles and endolophs appear to be stronger in S. praecox than in S. polonicus. The unique configuration of the trigon in S. polonicus, with a more labially positioned hypocone relative to the protocone, further distinguishes this species from S. praecox, which shows a nearly longitudinally oriented endoloph. In contrast to S. polonicus the M3 of S. praecox possesses a salient metaconule, metacone, posterocone, and a distinct, albeit low, metaloph.
Perhaps the most pronounced differences between S. praecox and S. polonicus are in the lower dentition. The p4–m3 of S. praecox are less cuspate, and have much stronger metalophids, particularly in p4 and m1, contrasting with the more cuspate lower cheek teeth of S. polonicus, with no clearly discernible metalophids. The p4 of S. praecox has a stronger anteroconulid, mesoconid, and hypoconulid. The m3 of S. praecox differs noticeably from that of S. polonicus in having a lighter talonid that does not exhibit a clear hypoconulid, postflexid, or the labial expansion of hypoconid seen in S. polonicus. Finally, the bases of the protoconid and hypoconid of m1–m3 in S. praecox are not so closely spaced as they are in S. polonicus.
Specific distinction of S. praecox from S. primigenius (Kormos 1934; Gromov et al. 1965; MVS personal observations) is supported by its smaller size, larger posterior valleys, stronger endolophs with more elevated hypocones, larger metaconules, narrower anterior valleys, and incomplete metalophs of P4–M2. The M3 of S. praecox has larger metaconules and relatively broader, albeit constricted, metalophs. Differences in the lower dentition are equally apparent; for example, in comparison to S. primigenius, the p4 of S. praecox is notable in being clearly premolariform, having a labiolingually narrow trigonid, exhibiting strongly appressed metaconid and protoconid, and displaying a weaker anterolophid topped by a cuspate anteroconulid. Further, the p4 of S. praecox possesses a mesoconid and strong hypoconid, which are lacking in S. primigenius.
The dentition of S. praecox also differs from that of S. tologoicus (Gromov et al. 1965; Erbajeva and Pokatilov 1966; MVS personal observations), the most poorly known early Old World ground squirrel from the Early Pleistocene (Gelasian) of the western Transbaikal area in eastern Asia (Alexeeva and Erbajeva 2008). Compared with S. tologoicus, the teeth of S. praecox are substantially smaller in size and are more cuspate; the P3 is weaker with a reduced anterior valley and protocone; the P4–M2 exhibit anteroposteriorly shorter anterior valleys; stronger endolophs, mesostyles, and metaconules; the p4 is less molariform with labiolingually narrower trigonid; and the m1–m2 exhibit shorter metalophids.
In summary, comparisons between S. praecox and the other early species of Spermophilus reveal that S. praecox is one of the most, if not the most primitive member of the genus, which is consistent with its status as the oldest known member of the genus, documented from the middle Villanyian and earliest Biharian, ca. 2.6–1.7 Ma.
Stratigraphic and geographic range.—Late Pliocene (middle Villanyian) to Early Pleistocene (earliest Biharian) of southern Ukraine.
Concluding remarks
Dental evolution in the Spermophilus praecox sp. nov.–Spermophilus nogaici lineage.—Several factors contribute to the difficulty of diagnosing extinct species of Spermophilus, foremost among them being highly variable but conservative dental morphology and overlapping size ranges. The problem, in turn, is complicated by the scarcity and incompleteness of the fossil record for Spermophilus, which is particularly the case for earliest members of the genus. However, larger sample sizes of eastern European early Spermophilus now available allow us to make progress on systematics and evolution of early Spermophilus. We recognize two species of Spermophilus from the late Pliocene–early Middle Pleistocene of Ukraine and southwestern Russia, the geologically older S. praecox sp. nov. and the younger S. nogaici.
Spermophilus praecox sp. nov., the earliest species of the genus, first appears in the late Pliocene, middle Villanyian (some 2.7–2.5 Ma) of Kryzhanovka 2, Cherevichnoe 2, and Zhevakhova Gora 4 localities in southern Ukraine, presumably as an immigrant (see below). In these earliest sites, fossils of Spermophilus are scarce, consisting of rare isolated teeth. We assign them to S. praecox by default; however, due to a lack of many tooth positions in the fossil samples, it is simply not known whether these earliest ground squirrels resembled S. praecox in morphology and size of most tooth positions. The known specimens do possess the traits believed to be primitive for the genus: very small P3, short anteroloph, weak metaloph lacking its lingual portion, unreduced metaconule, endoloph, and mesostyle (Fig. 8).
More complete materials of S. praecox are known from the late Villanyian of Yuzhny, Kotlovina 2 and 3 localities. Although still somewhat limited in numbers, they are united by the common possession of a small P3 with reduced protocone; less molariform premolars; weak anterior lobes and lingual metalophs but strong metaconules, mesostyles, and endolophs of P4–M2; rudimentary mesolophs and metaconules of M3; generalized lower dentition with anteroposteriorly elongated, trapezoidal p4 having a narrowed trigonid, massive hypoconid, cuspate anteroconulid, and weak anterolophid; the m2–m3 lacking metalophids; and small hypoconid, weak entoconulid, and posterolophid of m3. These traits also characterize the stratigraphically youngest and the most abundant sample of S. praecox from early Biharian of Morskoy and serve to distinguish the species from subsequent samples of S. nogaici.
Throughout its existence, spanning about 1.3 My, S. praecox remains fairly conservative in dental size and morphology, which could also be a result of poor sampling and the relative rarity of fossils from the middle Villanyian strata. It is possible, however, to identify the principal traits that vary temporarily within the entire sample of the species. The late Villanyian specimens tend to be relatively low-crowned and to have a small P3 with very simple crown structure, and extremely weak to absent anterior valley (Figs. 6A, 8). Villanyian P4s are characterized by consistent possession of a parastyle, conical anterocone, extremely weak anterostyle and lingual metaloph, circular metaconule, and evenly smooth, strong endoloph. Similarly, the M1–M2s from these samples show a very small to almost absent anterostyle and antesinus; deeply reduced lingual metaloph bearing a voluminous, rounded metaconule; and a massive endoloph connecting the protocone with relatively tall hypocone, reaching the height of the metacone or even higher. The lower dentition retains generalized morphology in being low-crowned; having narrow trigonid of p4 whose width is 7.9–21.4 % (mean 15.1%) less than the width of the talonid; and distinctly triangular m3 lacking the anteroconulid and a pronounced groove delimiting the hypoconid from the entoconulid.
In contrast, the specimens from the earliest Biharian assemblage of Morskoy appear to be more advanced than those from Villanyian localities in displaying a somewhat larger and, presumably, more complex P3; a P4 with stronger anterostyle and lingual metaloph, slightly anteroposteriorly compressed anteroconid and metaconule, and weaker endoloph (being partially constricted in one of the seven P4s). The M1–M2 from the Biharian is more square-shaped due to a relatively stronger anterostyle and antesinus and more posterolabially protruded hypocone; the lingual metaloph of these teeth is comparatively higher, the metaconule is weaker, and the endoloph is somewhat more reduced, causing the initial isolation of the posterior lobe from the rest of the tooth. When seen posteriorly, the hypocone of M1–M2 from Morskoy appears to be slightly lower than those of Villanyian S. praecox. The differences of lower dentitions are a slightly more high-crowned p4 with less pointed anteroconulid and more pronounced lingual metalophid; and a slightly more rectangular m3 having a labially expanded hypoconid, salient entoconulid and entolophid, and deeply excavated groove between these two.
The oldest fossils that can be identified as S. nogaici are known from the early Biharian of Zhevakhova Gora 1 locality in southern Ukraine, dated to ca. 1.6 Ma. These specimens exhibit a morphology intermediate between S. praecox and a typical S. nogaici and come from the stratigraphic interval between the latest definitive S. praecox specimens at ca. 1.77 Ma and the first undoubted S. nogaici at approximately 1.29 Ma (Fig. 8). Although attribution of these intermediates to S. nogaici is supported by the presence of a suite of derived dental characters not known in S. praecox (expanded anterior lobes, complete lingual metalophs, strongly compressed metaconules, absent mesostyles, and constricted endolophs of P4–M2; shortened crown, small, teardrop-shaped anteroconulid, the presence of lingual anterolophid of p4; and enlarged entoconulid of m3 accentuated labially by a pronounced groove), fossils from Zhevakhova Gora 1 combine features of S. praecox and middle Biharian S. nogaici, which makes their taxonomic assignment problematical. Specifically, the anterior lobe of P4 is narrower; the hypocone of P4–M2 is more inflated, with a less steep transition between this cusp and the protocone than in S. nogaici from middle Biharian assemblages. Similarly, the metaloph of M3 appears to be slightly weaker. Relative to talonid width the trigonid of p4 is narrower and the lingual anterolophid of this tooth is much weaker than in the typical S. nogaici, being reduced on two of the four teeth. The m3 is wider posteriorly and the hypoconid is more expanded than in S. praecox, but is not as substantial as is typical in S. nogaici. Finally, the entoconulid of m3 is less pronounced than in the latter. These intermediate early Biharian specimens foreshadowing the typical S. nogaici are interpreted here as representing a transitional population in a continuously evolving lineage, which suggests the possibility of a relatively gradual anagenetic transition between sympatric S. praecox and S. nogaici that lasted approximately 0.20–0.15 My.
Specimens more confidently assigned to S. nogaici appear in the middle Biharian of Tarkhankut, followed by specimens from the type locality of the species from Nogaisk (Fig. 8). Morphological changes in successive samples of S. nogaici continue the morphocline begun in S. praecox and culminate in the specimens form Tihonovka 1, Bolshevik 1, and Bolshevik 2,I (Fig. 8). This morphocline is reflected in a gradual change in dental morphology toward more high-crowned dentitions with expanded anterior and reduced posterior lobes of P4–M2; taller, trenchant transverse ridges (particularly, the anterolophs of P3–M3, protolophs and metalophs of P4–M3, anterolophids and metalophids of p4–m3, and entolophid of m3); strongly reduced endolophs of P4–M2; enlarged protocone of P3; weaker and more lophate anteroconules and metaconules, but stronger anterostyles of P4–M3; more pronounced hypoconid, hypoconulid, and entoconulid of m3; and proportionally larger and anteroposteriorly compressed (molariform) p4s having massive anterior portions and a complex root system. Based on the last character, namely the acquisition of the posterolingual root of p4, the populations of S. nogaici appear to fall into two barely distinct groups: those retaining two-rooted p4s and those with three-rooted p4s. None of the known populations, however, possesses consistent two- or three-rooted morphologies, but rather demonstrate a gradual shift from predominantly two-rooted p4s with incipient splitting of the posterior root in the sample from Zhevakhova Gora 1 to predominantly three-rooted premolars in the samples from Tsymbal, Tihonovka 1, Bolshevik 1, and Bolshevik 2,I. This suggests that the transition was slow, occurring during the entire existence of the species in the fossil record.
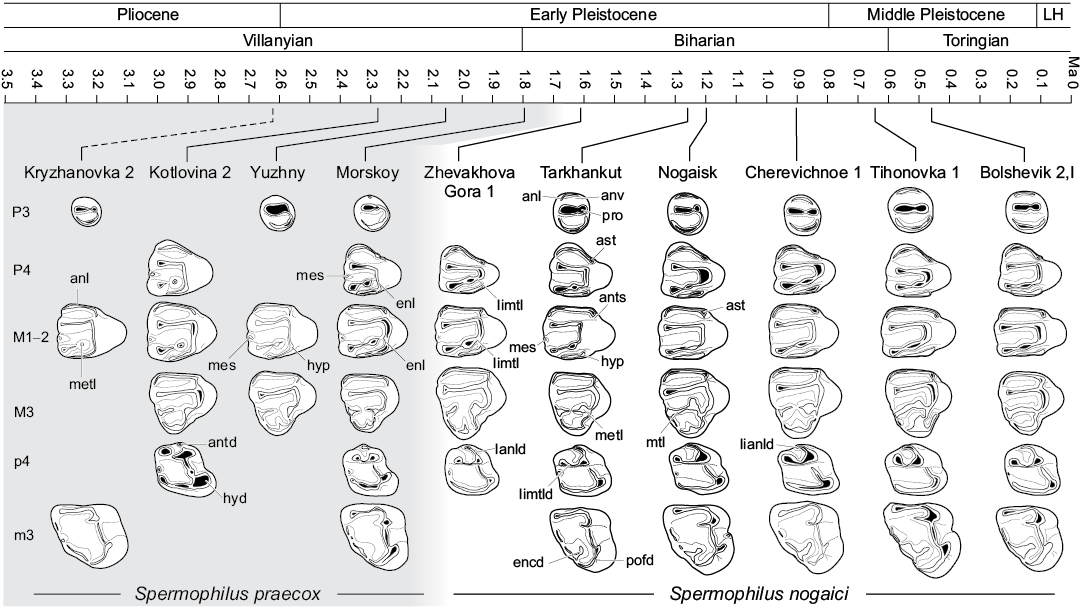
Fig. 8. Temporally-calibrated dental variation in P3, P4, M1–M2, M3, p4, and m3 of Spermophilus praecox sp. nov. and Spermophilus nogaici (Topachevsky, 1957). Abbreviations: anl, anteroloph; ants, antesinus; ast, anterostyle; antd, anteroconulid; anv, anterior valley; encd, entoconulid; enl, endoloph; hyd, hypoconid; hyp, hypocone; lanld, labial anterolophid; LH, Late Pleistocene–Holocene; lianld, lingual anterolophid; limtl, lingual metaloph; limtld, lingual metalophid; mes, mesostyle; metl, metaconule; pofd, postflexid; pro, protocone.
The continuously evolving nature of successively sampled populations of S. nogaici is underscored not only by the gradual transformation of qualitative morphological traits, but also by morphometric analysis. When viewed in stratigraphic context, the species exhibits a modest but more or less continuous trend toward enlargement of P3 (Fig. 9A) and increase in the proportional width of the p4 trigonid (Fig. 9B) accompanied by a decrease of M1–M2 (Fig. 9C) and m1–m2 sizes (Fig. 9D), along with labiolingual narrowing of m3. The only exceptions to this may be the specimens from Tsymbal, which are somewhat larger than the expected value for the middle Biharian populations of S. nogaici.

Fig. 9. Stratigraphic record of P3 (A), p4 (B), M1–M2 (C), and m1–m2 (D) sizes of Spermophilus praecox sp. nov. (circles) and Spermophilus nogaici (Topachevsky, 1957) (triangles). Abbreviations: L, length; W, width; tal.W, talonid width; tri.W, trigonid width.
In the late Biharian–earliest Toringian interval, two slightly different size groups appear. The first, exemplified by the specimens from the eastern locality Tihonovka 1, consists of P3s that are somewhat larger, M3s and m3s with longer and narrower crowns, and p4s that are relatively wider transversally than occurs in the mid Biharian samples (Table 1). The second size group, from localities further to the west (Bolshevik 2,I and possibly, Bolshevik 1), possess a moderately enlarged P3 that continues the size trend observable in the previous assemblages of the species, and smaller P4, M1–M2, M3, p4, m1–m2, and m3, which, nonetheless, still fall within the size range of S. nogaici from Nogaisk and Tarkhankut (Table 1). The existence of these two groups could reflect an increased distinction between “western” and “eastern” populations of S. nogaici not long before the species disappeared from the fossil record, approximately at the end of the early Toringian.
Although restricted, Spermophilus nogaici is still, temporarily and, especially, geographically, among the most wide-ranging extinct species of the genus, having been recorded from more than twenty mid to late Biharian small mammal localities of eastern Europe, from southern Ukraine and Moldova in the west to central regions of European Russia in the east. Coefficients of variation of the main dental dimensions (Table 3) are comparable to those in living ground squirrels and other similarly sized mammals (Simpson et al. 1960), thus suggesting these specimens generally fall within the range expected for a single, albeit polymorphic, species.
Evolutionary biogeography of early Spermophilus.—The extinct species of Spermophilus are known from a vast area throughout temperate Europe (Kormos 1916; Tobien 1972; Sutcliffe and Kowalski 1976; Jánossy 1986; Jánossy and Topál 1990; Kowalski 2001; Reumer and Van den Hoek Ostende 2003; Popova et al. 2018) and Asia (Gromov et al. 1965). The Pleistocene range of the genus greatly exceeds the distribution of the extant members of the genus, whose current westernmost distribution only reaches central Europe. Although some early Old World ground squirrels have been reported from eastern Asia (Gromov et al. 1965; Alexeeva and Erbajeva 2008), four of the five currently known late Neogene and Early Pleistocene species are found in Europe. The oldest known Spermophilus, S. praecox sp. nov. is restricted to southeastern Europe. The species, however, was clearly an immigrant to this area. Its first appearance was preceded by a vast period of European “marmotine vacuum” spanning around 2.2 My, and coincided with the arrival of jerboas of the genus Plioscirtopoda Gromov and Schevtschenko, 1961, which dispersed into southeastern Europe from the east.
The Asian Old World ground squirrel fossil record, in turn, is remarkably poor and provides no reliable evidence of early Spermophilus. The oldest and only known Early Pleistocene Asian species of the genus is S. tologoicus (Gromov et al. 1965; Erbajeva and Pokatilov 1966), which existed some 1.4–1.3 My after the first appearance of S. praecox. Furthermore, S. tologoicus possesses a suite of derived dental and cranial characters, indicating its more derived position compared to contemporaneous European taxa (Sinitsa et al. 2019). This implies the presence of a “ghost lineage” of early Spermophilus from the late Neogene in Asia, a time span for which no Old World ground squirrels or their plausible ancestors record are known. Scarce remains of putative “Urocitellus” fossils reported from the late Villanyian localities of southwestern Siberia (Zazhigin 2009) may provide tantalizing clues to cryptic diversity of the early Old World ground squirrels from Asia. To date, however, it is impossible to draw more precise conclusions about Spermophilus biogeography and origins. Any definite statements in support of either Asia or eastern Europe as a ‘place of origin’ for the genus are not yet supported by the current early fossil record of Spermophilus.
Furthermore, the exclusively Eurasian modern geographical distribution of the genus does not necessarily imply a Eurasian origin of the group. Therefore, absence of a putative common ancestor for Spermophilus and apparent disparity between Early Pleistocene Spermophilus from Europe and Asia might be explained by a Nearctic origin for the genus, followed by a rapid extinction of basal stem Spermophilus in North America. Confirmation or refutation of this pattern will await better sampling of the stem marmotine fossil record, particularly in the middle–late Pliocene of eastern Asia and northwestern North America.
Comments on the phylogenetic origin of Spermophilus.—The ancestry of Spermophilus, both in respect to the group of marmotines from which the first definite Spermophilus was derived and to basalmost species of Spermophilus, is totally unknown. The fossil record of Eurasian and North American Neogene marmotines related to Spermophilus is frustratingly poor, notwithstanding the magnificent record of stem marmotines from Europe (De Bruijn 1999; Sinitsa 2018), Mongolia (Daxner-Höck et al. 2017), China (Qiu 1991, 1996, 2015), and North America (Bryant 1945; Black 1963; Goodwin and Hayes 1994; Goodwin 2008; Goodwin and Martin 2017). The fact that there are no taxa that could be called a plausible ancestor of Spermophilus in the Eurasian fossil record is almost certainly due to the lack of well-sampled high-latitude fossil assemblages from the Pliocene strata of eastern Asia and North America. As such, hypotheses of Spermophilus ancestry can be proposed based primarily on a comparative morphological analysis of the oldest and most primitive members of the genus.
The first and, to date, the only attempt to address the ancestry of Spermophilus (including the former subgenus Urocitellus) was that of Black (1972), who proposed a Nearctic origin for the genus and hypothesized derivation of Eurasian Spermophilus from the late Pliocene (Hemphillian) North American “Spermophilus” mckayensis (Shotwell, 1956). Although the hypothesis of a Nearctic center of origin for many marmotine taxa gains more support as more fossils come to light, the placing of “S.” mckayensis in an ancestral position relative to Spermophilus is in conflict with the morphology of the earliest Old World ground squirrels. These three Villanyian species, namely S. praecox, S. polonicus, and S. primigenius, are united by the retention of plesiomorphic dental characters and acquisition of a few apomorphic traits suggesting their recent common ancestry. Based on the trends in their dental morphology we conclude that the last common ancestor for Spermophilus probably had dentitions with large teeth (the estimated length of upper toothrow is 11.5–13.5 mm). We further infer that upper teeth were characterized by the following combination of characters: moderately low-crowned molars and premolars; conical main cusps and blunt major ridges; relatively small (about 2.5–3 times shorter than P4) and simple P3 lacking the protocone and anterior valley; P4–M2 lacking the posterolingual sinus and having a shallowly depressed antesinus, pronounced tall anteroloph, endoloph, and posteroloph, circular and salient metaconule separated from a cuspate protocone by a deep valley, but connected to a cuspate metacone by a moderately developed, complete, albeit blunt, labial metaloph; the mesostyle and the anterior arm of the protocone on P4–M2 are consistently present; the anterior lobe of P4 is unexpanded and confined to the anterolabial side of the crown; and the M3 is rounded to triangular in occlusal outline with a small posterior lobe and simple basins bearing, a well-elevated anteroloph, blunt protoloph, and lacking any signs of metaloph, metacone, and metaconule. Finally, we infer the characters of the lower dentition of ancestral Spermophilus as: a p4 trigonid that is approximately one and a half times narrower than the trigonid, with a diminutive anteroconulid, remnants of a mesoconid, and lacking anterolophid and metalophid; m1 trigonid probably narrower than the talonid; both m1 and m2 with very short to nearly absent metalophid and complete anterolophid, the metaconid being only slightly taller than the protoconid, and the protoconid almost equal in height to the hypoconid; and m3 is relatively short and triangular with extremely reduced metalophid and smooth talonid basin rimmed by a complete posterolophid, and lacking the entoconulid and entolophid.
The only suitable candidate for this hypothetical ancestor of Spermophilus is an unknown marmotine with generalized Otospermophilus-like dentition. All putative Nearctic “Spermophilus”, including “S.” mckayensis promoted by Black (1963) as ancestral to Spermophilus and Urocitellus, appear too advanced and may be excluded because of dental apomorphies such as slender, lophate, and tall cusps, trenchant ridges, elongated m3 with labially displaced hypoconid, and incomplete posterolophid recessed at the contact with entoconid, and well-developed metalophids on lower molars. Until the discovery of additional material, particularly upper dentition and skull, “S.” mckayensis appears to be a dentally derived ground squirrel with no specific relation to the ancestry of Spermophilus s. str.
On the other hand, the extinct members of “Otospermophilus”, which represent a paraphyletic grouping of stem North American marmotines (Goodwin 2008), are also unsatisfactory in certain aspects of their morphology. Although retaining a rather generalized dentition resembling those of the primitive Spermophilus, the presence of stout and swollen main cusps, anterosinusids, metalophids, primitively arched ectolophids, and cuspate anteroconulids on m1–m3 make them unlikely candidates for the ancestors of the Old World ground squirrels.
A logical next step in tracing the phylogenetic origin of Spermophilus necessitates cladistic analysis, a procedure recently attempted for fossil marmotines (Goodwin 2008; Sinitsa 2018). Given the obvious limitations of a phylogenetic analysis of taxa characterized exclusively by dental characters accompanied by the conservative nature of sciurid masticatory apparatus (Bryant 1945; Black 1963; Gromov et al. 1965; De Bruijn 1999; Goodwin 2008) such an attempt would likely be meaningless considering that most significant species are known by dentitions alone. We hope that future collecting efforts in the Pliocene of North America and Asia will produce additional non-dental elements of stem marmotins and early Spermophilus that will allow the phylogenetic origin of the Old World ground squirrels and related taxa to be recovered.
Acknowledgements
We would like to thank Dmitry V. Ivanoff, Oleksandr M. Kovalchuk, Vadym O. Yanenko (all National Museum of Natural History, Kiev, Ukraine), Olga V. Makarova (Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia), Mihály Gasparik and Piroska Pazonyi (both Hungarian Museum of Natural History, Budapest, Hungary) for access and guidance during MVS’s visits to their respective institutions. We are also grateful to Alexey S. Tesakov (Geological Institute of the Russian Academy of Sciences, Moscow, Russia) for discussions on fossil ground squirrels and southern Ukraine stratigraphy. Finally, we thank Thomas H. Goodwin (Andrews University, Berrien Springs, USA), William W. Korth (Rochester Institute of Vertebrate Paleontology, New York, USA), and the Editor, Olivier Lambert (Royal Belgian Institute of Natural Sciences, Brussels, Belgium) for their constructive comments that greatly improved the manuscript. The research was supported by Act 211 of the Government of the Russian Federation, contract No. 02.A03.21.0006; and by INQUA Project 1606:‘Ground Squirrels on the March: Expansion and Speciation in the Quaternary of the Circum-Pontic Area and Surroundings’.
References
Alexeeva, N.V. and Erbajeva, M.A. 2008. Diversity of Late Neogene–Pleistocene small mammals of the Baikalian region and implications for paleoenvironment and biostratigraphy: an overview. Quaternary International 179: 190–195.Crossref
Bennett, E.T. 1835. Observations of several mammalia from Trebizond and Erzeroum, including a new species of rat (Mus latipes) and of marmot (Citellus xanthoprymna). Proceedings of the Zoological Society of London 3: 89–90.
Black, C.C. 1963. A review of North American Tertiary Sciuridae. Bulletin of the Museum of Comparative Zoology 130: 113–248.
Black, C.C. 1972. Holarctic evolution and dispersal of squirrels (Rodentia: Sciuridae). Evolutionary Biology 6: 305–322. Crossref
Black, C.C, and Kowalski, K. 1974. The Pliocene and Pleistocene Sciuridae (Mammalia, Rodentia) from Poland. Acta Zoologica Cracoviensia 19: 461–485.
Bowdich, T.E. 1821. An Analysis of the Natural Classifications of Mammalia for the Use of Students and Travellers. 115 pp. J. Smith, Paris.
Brandt, J.F. 1841. Note sur deux espèces nouvelles de sousliks de Russe. Bulletin Scientifique de l’Académie Impériale des Sciences de Saint-Pétersbourg 9: 43–44.
Brandt, J.F. 1844 [1843]. Observations sur les différentes espèces de sousliks de Russie, suivies de remarques sur l’arrangement et la distribution géographique du genre Spermophilus, anséque sur la classification de la familie des ecureuils (Sciurina) en général. Bulletin Scientifique de l’Académie Impériale des Sciences de Saint Pétersburg 2: 357–382.
Bryant, M.D. 1945. Phylogeny of Nearctic Sciuridae. American Midland Naturalist 33: 257–390. Crossref
Büchner, E. 1888. Zoologischer Theil I, Saugethiere I. In: N.M. Przewalski (ed.), Wissenschaftliche Resultate der von N. M. Przewalski nach Central-Asien Unternommenen Reisen, 1–48. Kayserlichen Academie der Wissenschaften, St. Petersburg.
Comte, B., Sabatier, M., Marandat, B., and Vianey-Liaud, M. 2012. Les rongeurs de Chéry-Chartreuve et Rocourt-Saint-Martin (est du bassin de Paris; Aisne, France), leur place parmi les faunes de l’Eocène Moyen d’Europe. Palaeovertebrata 37: 167–271. Crossref
Cuvier, F. 1825. Des dents des mammifères, considérées comme caractères zoologiques. 258 pp. Levrault, Le Normant, Paris.
Daxner-Höck, G., Badamgarav, D., Barsbold, R., Bayarmaa, B., Erbajeva, M., Göhlich, U.B., Harzhauser, M., Höck, E., Höck, V., Ichinnorov, N., Khand, Y., López-Guerrero, P., Maridet, O., Neubauer, T., Oliver, A., Piller, W., Tsogtbaatar, K.H., and Ziegler, R. 2017. Oligocene stratigraphy across the Eocene and Miocene boundaries in the Valley of Lakes (Mongolia). In: G. Daxner-Höck and U. Göhlich (eds.), The Valley of Lakes in Mongolia, a Key Area of Cenozoic Mammal Evolution and Stratigraphy. Palaeobiodiversity and Palaeoenvironments 97 (1): 111–218 Crossref
De Bruijn, H. 1999. Superfamily Sciuroidea. In: G.E. Rössner and K. Heissig (eds.), The Miocene Land Mammals of Europe, 271–280. Verlag Dr. Friedrich Pfeil, Munich.
Erbajeva, M.A. and Pokatilov, A.G. 1966. A new species of Eopleistocene souslik from Tologoi (western Transbaikalia) [in Russian]. Paleontological Journal 1: 162–164.
Fischer, G. 1817. Adversaria zoologica, fasciculus primus. Mémoires de la Société impériale des naturalistes de Moscou 5: 357–446.
Goodwin, H.T. 2008. Sciuridae. In: C.M. Janis, G.F. Gunnell, and M.D. Uhen (eds.), Evolution of Tertiary Mammals of North America, Volume 2: Small Mammals, Xenarthrans, and Marine Mammals, 355–376. Cambridge University Press, Cambridge. Crossref
Goodwin, H.T. and Hayes, F.E. 1994. Morphologically derived ground squirrels from the Borchers Local Fauna, Meade County, Kansas, with a redescription of ?Spermophilus cragini. Journal of Vertebrate Paleontology 14: 278–291.
Goodwin, H.T. and Martin, R.A. 2017. Ground squirrels (Rodentia, Sciuridae) of the late Cenozoic Meade Basin sequence: diversity and paleoecological implications. Journal of Paleontology 91 (6): 1244–1257. Crossref
Gromov, I.M. 1961. The quaternary sousliks of western Palearctic, 1. Large sousliks of the subgenus Colobotis Brandt [in Russian]. Trudy Zoologičeskogo Instituta AN SSSR 29: 22–80.
Gromov, I.M. and Schevtschenko, A.I. [Ševčenko, A.I.] 1961. Jerboas (Rodentia, Dipodidae) from the Kuyalnik Strata of the South of the Ukraine [in Russian]. Doklady Akademii nauk SSSR 139 (4): 976–979.
Gromov, I.M., Bibikov, D.I., Kalabukov, N.I., and Meier, M.N. 1965. Ground squirrels (Marmotinae) [in Russian]. In: E.N. Pavlovskij (ed.), Fauna SSSR 3 (2). 467 pp. Nauka, Moskva.
Güldenstaedt, J.A. 1770. Mus suslica nov. sp. Novi Commentarii Academiae Scientiarum Imperialis Petropolitanae 14: 389–402.
Gündüz, I., Jaarola, M., Tez, C., Yeniyurt, C., Polly, P.D., and Searle, J.B. 2007. Multigenic and morphometric differentiation of ground squirrels (Spermophilus, Scuiridae, Rodentia) in Turkey, with a description of a new species. Molecular Phylogenetics and Evolution 43: 916–935. Crossref
Harrison, R.G., Bogdanowicz, S.M., Hoffmann, R.S., Yensen, E., and Sherman, P.W. 2003. Phylogeny and evolutionary history of the ground squirrels (Rodentia: Marmotinae). Journal of Mammalian Evolution 10: 249–276. Crossref
Helgen, K.M., Cole, F.R., Helgen, L.E., and Wilson, D.E. 2009. Generic revision in the Holarctic ground squirrel genus Spermophilus. Journal of Mammalogy 90: 270–305. Crossref
Herron, M.D., Castoe, T.A., and Parkinson, C.L. 2004. Sciurid phylogeny and the paraphyly of Holarctic ground squirrels (Spermophilus). Molecular Phylogenetics and Evolution 31: 1015–1030. Crossref
Jánossy, D. 1986. Pleistocene Vertebrate Faunas of Hungary. 208 pp. Akadémiai Kiadó, Budapest.
Jánossy, D. and Topál, G. 1990. Allophaiomys-Fauna vom Újlaki-hegy (Budapest). Annales Historico-naturales Musei Nationalis Hungarici 81: 17–26.
Kashkarov, D.N. [Kaškarov, D.N.], Korovin, A., and Kurbatov, V. 1923. Rodents of the western Tian-Shan Mountains [in Russian, with English summary]. Trudy Turkestanskogo Naučnogo Obsčestva pri Sredne-Aziatskom Gosudarstvennom Universitete 1: 175–220.
Kormos, T. 1916. Die Saugetiere der Felsnische Pilisszántó in systematischer, zoogeographischer und phylogenetischer Hinsicht. Mitteilungen aus dem Jahrbuche der Königlichen Ungarischen Geologischen Reichsanstalt 23: 331–523.
Kormos, T. 1934. Neue Insektenfresser, Fledermäuse und Nager aus dem Oberpliozän der Villanyer Gegend. Földtani Közlöny 64: 296–321.
Kowalski, K. 2001. Pleistocene rodents of Europe. Folia Quaternaria 72: 1–389.
Krokhmal, A.I. and Rekovets, L.I. 2010. Mestonahoždenia melkih mlekopitaûŝih Pleistocena Ukrainy i sopredel’nyh territorij. 330 pp. LAT&K, Kiev.
Kryštufek, B. and Vohralík, V. 2012. Taxonomic revision of the Palaearctic rodents (Rodentia). Part 1. Sciuridae: Xerinae 1 (Eutamias and Spermophilus). Lynx 43: 17–111.
Kryštufek, B. and Vohralík, V. 2013. Taxonomic revision of the Palaearctic rodents (Rodentia). Part 2. Sciuridae: Urocitellus, Marmota and Sciurotamias. Lynx 44: 27–138.
Lichtenstein, H. 1823. Naturhistorischer Anhang. In: E. Eversmann (ed.), Reise von Orenburg nach Buchara, 112–147. Christiani, Berlin.
Linnaeus, C. 1766. Systema naturae per regna tria naturae: secundum classes, ordines, genera, species cum characteribus, differentiis, synonymis, locis. Tomus 1. Editio duodecima, reformata. 532 pp. Laurentii Salvii, Stockholm. Crossref
Maridet, O., Daxner-Höck, G., López-Guerrero, P., and Göhlich, U.B. 2017. The record of Aplodontidae (Rodentia, Mammalia) in the Oligocene and Miocene of the Valley of Lakes (Central Mongolia) with some comments on the morphologic variability. Paleobiodiversity and Paleoenvironments 97: 25–49. Crossref
Marivaux, L., Vianey-Liaud, M., and Jaeger, J.J. 2004. High-level phylogeny of early Tertiary rodents: dental evidence. Zoological Journal of the Linnean Society 142: 105–134. Crossref
Markova, A.K. 2014. European small mammal faunas of the end of the Early Pleistocene—the beginning of the Middle Pleistocene. Proceedings of the Russian Academy of Sciences, Geological Series 5: 83–98. Crossref
Nowak, R.M. 1999. Family Sciuridae. In: R.M. Nowak (ed.), Walker’s Mammals of the World, 6th Edition, 1246–1306. Johns Hopkins University Press, Baltimore.
Obolenskij, S.I. 1927. A preliminary review of the Palaearctic sousliks (Citellus and Spermophilopsis). Doklady Akademii nauk SSSR A 11: 188–193.
Ognev, S.I. 1947. Zveri SSSR i priležaŝih stran. 809 pp. Izdayel’stvo Akademii nauk SSSR, Moskva.
Osborn, H.F. 1910. The Age of Mammals in Europe, Asia and North America. 635 pp. Macmillan, New York. Crossref
Pallas, P.S. 1778. Zoographia Rosso-Asiatica. Tomus primus. 568 pp. Offcina Caes. Academiae Scientarium Impress, St. Petersburg.
Pazonyi, P., Virág, A., Gere, K., Botfalvai, G., Sebe, K., Szentesi, Z., Mészáros, L., Botka, D., Gasparik, M., and Korecz, L. 2018. Sedimentological, taphonomical and palaeoecological aspects of the late early Pleistocene vertebrate fauna from the Somssich Hill 2 site (South Hungary). Comptes Rendus Palevol 17 (4–5): 296–309. Crossref
Pocock, R.I. 1923. The classification of the Sciuridae. Proceedings of the Zoological Society of London 1923: 209–246. Crossref
Popov, V.V. 2004. Pliocene small mammals (Mammalia, Lipotyphla, Chiroptera, Lagomorpha, Rodentia) from Muselievo (North Bulgaria). Geodiversitas 26: 403–491.
Popov, V.V. 2017. Early Pleistocene small mammals (Eulipothyphla, Chiroptera, Lagomorpha and Rodentia) from Futjova Cave, North Bulgaria. Acta Zoologica Bulgarica 69: 263–282.
Popova, L.V. 2016. Occlusal pattern of cheek teeth in extant Spermophilus: a new approach to the identification of species. Journal of Morphology 277: 814–825. Crossref
Popova, L.V., Maul, L.C., Zagorodniuk, I.V., Veklych, Y.M., Shydlovskiy, P.S., Pogodina, N.V., Bondar, K.M., Strukova, T.V., and Parfitt, S.A. 2018. “Good fences make good neighbours”: concepts and records of range dynamics in ground squirrels and geographical barriers in the Pleistocene of the Circum-Black Sea area. Quaternary International [published online, https://doi.org/10.1016/j.quaint.2018.03.023] Crossref
Qiu, Z.-D. 1991. The Neogene mammalian faunas of Ertemte and Harr Obo in Inner Mongolia (Nei Mongol), China. 8. Sciuridae (Rodentia). Senckenbergiana Lethaea 71: 223–255.
Qiu, Z.-D. 1996. Middle Miocene Micromammalian Fauna from Tunggur, Nei Mongol. 216 pp. Science Press, Beijing.
Qiu, Z.-D. 2015. Revision and supplementary note on Miocene sciurid fauna of Sihong, China. Vertebrata PalAsiatica 53: 219–237.
Reumer, J.W.F. and van den Hoek Ostende, L.W. 2003. Petauristidae and Sciuridae (Mammalia, Rodentia) from Tegelen, Zuurland, and the Maasvlakte (The Netherlands). Deinsea 10: 455–467.
Satunin, K.A. 1903. Neue Nagetiere aus Centralasien. Annuaire du Musée Zoologique de l’Académie Impériale des Sciences de St. Pétersbourg 7: 549–589.
Schevtschenko, A.I. [Ševčenko, A.I.] 1965. Key complexes of small mammals from Pliocene and lower Antropogene of the south-western part of the Russian Plain [in Russian]. In: K.V. Nikiforova (ed.), Stratigrafičeskoe značenie Antropogenovyh faun melkih mlekopitaûŝih, 7–59. Nauka, Moskva.
Shotwell, J.A. 1956. Hemphillian mammalian assemblage from Northeastern Oregon. Bulletin of the Geological Society of America 67: 717–738. Crossref
Simpson, G.G., Roe, A., and Lewontin, R.C. 1960. Quantitative Zoology. 440 pp. Harcourt, Brace, New York.
Sinitsa, M.V. 2018. Phylogenetic position of Sinotamias and the early evolution of Marmotini (Rodentia, Sciuridae, Xerinae). Journal of Vertebrate Paleontology 38 [published online: https://doi.org/10.1080/02724634.2017.1419251]. Crossref
Sinitsa, M.V., Pogodina, N.V., and Kryuchkova, L.Y. 2019. The skull of Spermophilus nogaici (Rodentia: Sciuridae: Xerinae) and the affinities of the earliest Old World ground squirrels. Zoological Journal of the Linnean Society [published online: https://doi.org/10.1093/zoolinnean/zly092]. Crossref
Stadnik, O.I. and Dema, L.P. 2007. The ground squirrels (Spermophilus, Rodentia) of the late Pliocene of Ukraine [in Ukrainian with English summary]. In: P.F. Gozhik (ed.), Paleontologični doslidženniâ v Ukraini: istorija, sučasnyj stan ta perspektyvy. Zbirnyk naukovyh prats’ IGN NAN Ukrainy, Kiev 29: 361–364.
Sulimski, A. 1964. Pliocene Lagomorpha and Rodentia from Węże 1 (Poland). Acta Palaeontologica Polonica 9: 149–244.
Sutcliffe, A.J. and Kowalski, K. 1976. Pleistocene rodents of the British Isles. Bulletin of the British Museum (Natural History), Geology Series 27: 1–147.
Tesakov, A.S. 2004. Biostratigraphy of the Middle Pliocene–Eopleistocene of Eastern Europe [in Russian]. Trudy GIN RAN 554: 1–247.
Tesakov, A.S. and Sinitsa, M.V. [Sinica, M.V.] 2012. Yuzhny—a new late Pliocene (late Kuyalnikian, Gelasian) small mammal locality in Odessa Region [in Russian]. In: P.F. Gožik (ed.), Paleontologični doslidžennja v udoskonalenni stratygrafičnyh shem Fanerozojs’kyh vidkladiv. Zbirnyk naukovyh prats’ IGN NAN Ukrainy, Kiev 34: 108–109.
Thorington, R.W. Jr. and Hoffmann, R.S. 2005. Family Sciuridae. In: D.E. Wilson and D.M. Reeder (eds.), Mammal Species of the World. A Taxonomic and Geographic Reference. 3rd Edition, 754–818. Johns Hopkins University Press, Baltimore.
Thorington, R.W. Jr., Koprowski, J.L., Steele, M.A., and Whatton, J.F. 2012. Squirrels of the World. 459 pp. Johns Hopkins University Press, Baltimore.
Tobien, H. 1972. Citellus (Rodentia) und Lagomorpha aus den älteren Aufsammlungen an der pleistozänen Lokalität Stránská skála bei Brno (ČSSR). Anthropos 20: 137–146.
Topachevsky, V.O. 1957. New species of souslik from the Upper Pleistocene deposits of Azov region, Ukraine [in Ukrainian]. Proceedings of the Academy of Sciences of USSR 2: 204–208.
Topachevsky, V.A. [Topačevskij, V.A.] 1965. Nasekomojadnye i gryzuny Nogajskoj pozdnepliocenovoj fauny. 163 pp. Naukova Dumka, Kiev.
Topachevsky, V.A. [Topačevskij, V.A.] 1973. Gryzuny Tamanskogo faunističeskogo kompleksa Kryma. 234 pp. Naukova Dumka, Kiev.
Topachevsky, V.A. [Topačevskij, V.A.] and Nesin, V.A. 1989. Gryzuny Moldavskogo i Haprovskogo faunističeskih kompleksov Kotlovinskogo razreza. 133 pp. Naukova Dumka, Kiev.
Topachevsky, V.A. [Topačevskij, V.A.], Nesin, V.A., and Topachevsky, I.V. [Topačevskij, I.V.] 1998. Biozonal microtheriological scheme (stratigraphic distribution of small mammals—Insectivora, Lagomorpha, Rodentia) of the Neogene of the northern part of the eastern Paratethys [in Russian]. Vestnik Zoologii 1–2: 76–87.
Topachevsky, V.A. [Topačevskij, V.A.] and Skorik, A.F. 1977. Gryzuny Rannetamanskoj fauny Tiligulskogo razreza. 252 pp. Naukova Dumka, Kiev.
Topachevsky, V.A. [Topačevskij, V.A.], Skorik, A.F., and Rekovets, L.I. [Rekovec, L.I. ] 1987. Gryzuny Verhneneogenovyh i Ranneantropogenovyh otloženij Hadžibejskogo limana. 208 pp. Naukova Dumka, Kiev.
Van de Weerd, A. 1976. Rodent faunas of the Mio-Pliocene continental sediments of the Teruel-Alfambra region, Spain. Utrecht Micropaleontological Bulletin Special Issue 2: 1–217.
Vianey-Liaud, M., Gomes Rodrigues, H., and Marivaux, L. 2013. Early adaptative radiation of Aplodontoidea (Rodentia, Mammalia) on the Holartic region: systematic, and phylogenetic and paleobiogeographic implications. Paläontologische Zeitschrift 87: 83–120. Crossref
Zazhigin, V.S. [Zažigin, V.S.] 2009. Complexes of the small mammals of the late Pliocene–Early Pleistocene of the south of the west Siberia [in Russian]. In: A.E. Kontorovič (ed.), Doklady VI Vserossijskogo sovesčanija po izučeniju Četvertičnogo perioda “Fundamental’nye problemy kvartera: itogi izučeniâ i perspektivy dal’nejsčih issledovanij”, Novosibirsk, 218–220. Izdatel’stvo SO RAN, Novosibirsk.
Comparative sample of specimens examined.
Spermophilus alashanicus (Büchner, 1888), Bayankhongor, Mongolia, ZIN 54804, 54805; Mongolia, ZIN 54801, 54802.
Spermophilus brevicauda (Brandt, 1843), Taldykorgan, Kazakhstan, ZMMU 64538, 64563, 70158, 70159, 70160.
†Spermophilus citelloides, Pilisszánto, Hungary, ZIN 50993, 50994.
Spermophilus citellus (Linnaeus, 1766), Austria, ZIN 40610, 40611, Chernovtsy, Ukraine, NMNHU-Z 820, 821, 2858, Gevgelija, Macedonia, ZIN 33845, Leskovac, Serbia, ZIN 33827, Orhei, Moldova, ZIN 44192, Skopje, Macedonia, ZIN 33830, Sremska Mitrovica, Serbia, ZIN 33832, Vinnitsa, Ukraine, NMNHU-Z 822, 5693, 14587, ZIN 33635, Zrenjanin, Serbia, ZIN 33848.
Spermophilus dauricus Brandt, 1843, Hohhot, China, ZIN 2871, 2873, 6924, Liaoning, China, ZIN 54922.
Spermophilus erythrogenys Brandt, 1841, Barnaul, Russia, ZMMU 10198, Jambyl, Kazakhstan, NMNHU-Z 9962, 9965, Novosibirsk, Russia, ZIN 67374, 67394, 67409, 67410, ZMMU 22400, 22402, Omsk, Russia, ZMMU 10517.
Spermophilus fulvus (Lichtenstein, 1823), Aktobe, Kazakhstan, NMNHU-P 6348, Balkanabat, Turkmenistan, ZMMU 143035, 143037, 143045, Bukhara, Uzbekistan, ZMMU 4508, Kyzylorda, Kazakhstan, ZMMU 6443, Navoiy, Uzbekistan, ZMMU 6563, Samarkand, Uzbekistan, ZMMU 3332, Tashkent, Uzbekistan, NMNHU-P 387, 396, 398, 399, 406, 408.
Spermophilus major (Pallas, 1778), Aktobe, Kazakhstan, ZIN 54765, 54766, 54767, Astrachan, Russia, NMNHU-P 4405, 4406, Kazan, Tatarstan, Russia, ZMMU 73249, 73256, 73258, Kostanay, Kazakhstan, ZMUU 3000/5, 3000/12, 3000/13, 3000/15, 3000/16, 3000/18, 3000/22, 3000/23, 3000/26, 3000/27, 3000/28, 3000/29, 3000/30, 3000/31, 3000/32, 3000/34, 3000/41, 3000/42, 3000/47, 3000/48, 3000/54, 3000/56, 3000/58, 3000/59, 3000/63, 3000/64, 3000/68, 3000/69, 3000/72, 3000/73, 3000/74, 3000/78, 3000/79, 3000/85, 3000/90, 3000/95, 3000/97, 3000/99, Orenburg, Russia, ZIN 17679, 17680, 54717, 54718, ZMMU 11305, 11326, 54681, 54683, 54686, 54687, Saratov, Russia, ZIN 66781, 66784, 67559, 67562, 67565, Ufa, Bashkortostan, Russia, ZMMU 75749, Ekaterinburg, Russia, ZMUU 3000/1, 3000/6, 3000/7, 3000/11, 3000/19, 3000/21, 3000/24, 3000/33, 3000/35, 3000/37, 3000/38, 3000/39, 3000/40, 3000/43, 3000/45, 3000/49, 3000/52, 3000/55, 3000/57, 3000/62, 3000/66, 3000/67, 3000/71, 3000/77, 3000/84, 3000/89, 3000/91, 3000/94, 3000/98.
Spermophilus pallidicauda (Satunin, 1903), Altai, Mongolia, ZMMU 192431, 135156, Bayankhongor, Mongolia, ZIN 103313, Dalanzadgad, Mongolia, ZMMU 192432, 146477, 146478, Uliastai, Mongolia, ZIN 54786, 54787.
†Spermophilus polonicus, Kamyk, Poland, MF/83-1–9; MF/83-60.
†Spermophilus primigenius, Villány 3, Hungary, HNHM 67 249, HNHM 3948.
Spermophilus pygmaeus (Pallas, 1778), Atyrau, Kazakhstan, ZIN 23658, 23662, 53762, 53763, Dagestan, Russia, NMNHU-Z 992, 994, Oral, Kazakhstan, ZIN 23625, 23626, 23661, 23662-1, 27478, Rotsov-on-Don, Russia, NMNHU-P 2527/741, 2527/ 744, ZIN 85653, 85657, Saratov, Russia, ZIN 66850, 66851, Volgograd, Russia, ZIN 54541, 54542, Zaporozhye, Ukraine, NMNHU-P 6319, 6328, 6345.
Spermophilus relictus (Kaschkarov, 1923), Karakol, Kyrgyzstan, ZIN 66803, Nurafshon, Uzbekistan, ZIN 66858, 66932, Taldykorgan, Kazakhstan, ZIN 39172, 39173, 39174, 39175, 39176, 39179, 39180, 39219, 39220, 39221, 39248, Tokmok, Kyrgyzstan, ZIN 53734.
Spermophilus suslicus (Güldenstaedt, 1770), Cherkasy, Ukraine, ZIN 449-1926, 54881, Lutsk, Ukraine, NMNHU-P 3501, 3511, ZIN 36937, Minsk, Belarus, ZIN 8-Z, 54874, Odessa, Ukraine, NMNHU-Z 454, 505, ZIN 6683, 6684, 6718, 6722, 6728, Vinnitsa, Ukraine, NMNHU-P 6348.
†Spermophilus tologoicus (Erbajeva and Pokatolov, 1965), Tologoi, Russia, ZIN 50106.
Spermophilus xanthoprymnus (Bennett, 1835), Erzurum, Turkey, ZIN 10277, 10278, Gyumri, Armenia, ZIN 26081, 26089, 26174, 26176.
Acta Palaeontol. Pol. 64 (3): 643–667, 2019
https://doi.org/10.4202/app.00605.2019