

A taxonomic and phylogenetic review of the anhanguerid pterosaur group Coloborhynchinae and the new clade Tropeognathinae
BORJA HOLGADO and RODRIGO V. PÊGAS
Holgado, B. And Pêgas, R.V. 2020. A taxonomic and phylogenetic review of the anhanguerid pterosaur group Coloborhynchinae and the new clade Tropeognathinae. Acta Palaeontologica Polonica 65 (4): 743–761.
Anhanguerids are a particular group of pterodactyloid pterosaurs, characterized mainly by their rostral sagittal crests, well laterally expanded jaw tips and enlarged anterior teeth. Due to the fragmentary nature of most known specimens, including holotypes, the taxonomy of the group has proved particularly difficult and controversial. Coloborhynchinae is a recently proposed clade within the Anhangueridae, and was defined as the most inclusive clade containing Coloborhynchus clavirostris but not Anhanguera or Ludodactylus. Coloborhynchinae was originally thought to include Coloborhynchus, Uktenadactylus, and Siroccopteryx. Here we present a reassessment of the taxonomy and phylogeny of all proposed members of the Coloborhynchinae and Coloborhynchus complex, with new anatomical comparisons and a novel phylogenetic analysis. Several features allow us to establish that coloborhynchines were much more diverse than previously thought, englobing four genera and seven species: Aerodraco sedgwickii gen. et comb. nov., Coloborhynchus clavirostris, Nicorhynchus capito gen. et comb. nov., Nicorhynchus fluviferox gen. et comb. nov., Uktenadactylus rodriguesae sp. nov., and Uktenadactylus wadleighi. Nicorhynchus and Uktenadactylus are considered sister taxa, being distinct on the basis of several rostral characters. Although with a homoplastic flat rostrum surface, Siroccopteryx was recovered out of the Coloborhynchinae, as sister taxon of Tropeognathus, due to similarities on the palatal ridge (which is broad and deep, and starting at the same level) and the relatively stout teeth compared to other anhanguerids. Tropeognathus and Siroccopteryx are further related to the Australian taxa Ferrodraco and Mythunga, which are all grouped in a new clade: the Tropeognathinae. Our analysis suggests that morphological evolution within anhanguerids was quite more complex than previously thought, with coloborhynchines representing the oldest recorded lineage of Anhangueridae, which achieved a worldwide distribution at least from the Aptian to the Cenomanian.
Key words: Pterosauria, Pterodactyloidea, morphology, taxonomy, phylogeny, Cretaceous, USA, United Kingdom.
Borja Holgado [borja.holgado@mn.ufrj.br; borja.holgado@icp.cat], Laboratory of Systematics and Taphonomy of Fossil Vertebrates, Departmento de Geologia e Paleontologia, Museu Nacional/Universidade Federal do Rio de Janeiro, Quinta da Boa Vista s/n, São Cristóvão, 20940-040 Rio de Janeiro, RJ, Brazil; Institut Català de Paleontologia Miquel Crusafont, C/ de les Columnes, Universitat Autònoma de Barcelona, E-08193 Cerdanyola del Vallès, Barcelona, Catalonia, Spain.
Rodrigo V. Pêgas [rodrigo.pegas@hotmail.com], Laboratory of Vertebrate Paleontology and Animal Behavior, Universidade Federal do ABC, Alameda da Universidade, s/n, Anchieta, 09606-045 São Bernardo do Campo, SP, Brazil.
Received 26 March 2020, accepted 13 June 2020, available online 25 September 2020.
Copyright © 2020 B. Holgado and R.V. Pêgas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Back in 1874, Richard Owen described Coloborhynchus clavirostris from a fragment of a pterosaur rostrum coming from the Berriasian–Valanginian Hastings Beds, in the locality of St. Leonards-on-Sea (East Sussex, England). He then proposed a new genus for the new species due to its particular morphology, not previously recognised in any other known pterosaur at that time: “…the foremost pair of teeth projecting forward in the upper jaw from the truncate surface at a higher level than the alveolar border” (Owen 1874: 6). Albeit Owen (1874) referred other two species from the Cambridge Greensand to the genus Coloborhynchus, C. cuvieri, and C. sedgwickii, none of the three was designated as type species.
Forty years later, however, Hooley (1914) proposed that the uncommon placement of the first dental alveoli in Coloborhynchus was a consequence of a full abrasion of the rostral tip. Hooley (1914) then considered Coloborhynchus clavirostris to be a junior synonym of “Criorhynchus” simus (= Ornithocheirus simus) from the Cambridge Greensand. Later, Kuhn (1967) agreed with this view, and finally designated Coloborhynchus clavirostris as the type species of the genus Coloborhynchus.
Although later works accepted such synonymy (e.g., Wellnhofer 1978), Lee (1994) re-validated the genus Coloborhynchus (with the type species C. clavirostris) by describing a new species, Coloborhynchus waddleighi, from the Albian Paw Paw Formation (Texas, USA) on the basis of the following characters: a flattened and triangular anterior margin of the premaxilla; one pair of teeth projecting anteriorly and located above the subsequent teeth; an anterior median depression; and a premaxillary crest beginning near the tip of the skull (Lee 1994; Rodrigues and Kellner 2008). From then on, other authors accepted the re-validation of Coloborhynchus (e.g., Kellner and Tomida 2000; Fastnacht 2001; Unwin 2001; Veldmeijer 2003).
On the basis of the description of a new specimen from the Albian Romualdo Formation (Northeast Brazil), Fastnacht (2001) reconsidered the genus Coloborhynchus and relocated Tropeognathus robustus Wellnhofer, 1987 to this genus. Fastnacht (2001) modified the diagnosis after Lee (1994) adding the following characters: upper jaw laterally expanded in a spoon-shape in dorsal view from the second to the fourth pair of alveoli; first pair of teeth projecting anteriorly from the blunt anterior margin of the upper jaw at a significant elevation above the palate relative to subsequent teeth; second and third pair of alveoli of the upper and lower jaw enlarged relative to other alveoli; lower jaw with medial crest rising from its anterior end; and lower jaw laterally expanded in a spoon-shape from the first to the third pair of alveoli (Fastnacht 2001). This new diagnosis was considered in the subsequent literature by some authors (e.g., Unwin 2001, 2002, 2003; Frey et al. 2003; Veldmeijer 2003, 2006; Lü et al. 2006; Martill and Naish 2006; Fastnacht 2008; Martill and Unwin 2012; Witton 2013; Martill 2015; Martin-Silverstone et al. 2018).
Unwin (2001), accepting the re-validation of Coloborhynchus, followed the works of Lee (1994) and Fastnacht (2001) and further added other species to this genus. Among those, there was one of the species from Cambridge Greensand originally referred to the genus Coloborhynchus by Owen (1874): C. sedgwickii. Unwin (2001) also referred “Ornithocheirus” capito to the genus Coloborhynchus, and several other species from Cambridge Greensand were synonymised to this species (see Supplementary material for further details). Furthermore, Unwin (2001) even suggested the presence of Coloborhynchus out of England and the Americas, referring to this genus Siroccopteryx moroccensis Mader and Kellner, 1999 from the Kem Kem Group (Morocco), as well as a specimen from the Dzun-Bayin Formation, Mongolia (Bakhurina and Unwin 1995; Unwin and Bakhurina 2000). A year later, Unwin (2002) considered Anhanguera piscator as a synonym of Coloborhynchus robustus, what was then followed in subsequent works by some authors (see list above).
Veldmeijer (2003) described a new taxon from the Santana Group as Coloborhynchus spielbergi following the views of Fastnacht (2001) and Unwin (2002). Veldmeijer (2003) also added some characters to the diagnosis of the genus Coloborhynchus: the presence of a robust distal expansion; and maxillae slightly raised in relation to the palate (Veldmeijer 2003).
However, a few years later, Rodrigues and Kellner (2008) reviewed the genus Coloborhynchus and restricted it to the type species, Coloborhynchus clavirostris, with the following revised diagnosis: an oval depression beneath the first pair of dental alveoli, second to fourth pairs of premaxillary alveoli located laterally, fifth and sixth premaxillary alveoli located more medially than the preceding alveoli on the base of the palatal ridge, and anterior part of the palatal ridge bordered by two shallow longitudinally elongated depressions. They relocated the North-American species, Coloborhybchus wadleighi, to a new genus: Uktenadactylus. Concerning the purported records from the Romualdo Formation, Rodrigues and Kellner (2008) referred them to the genus Anhanguera, as A. piscator, A. robustus (following Kellner and Tomida 2000) and A. spielbergi. Furthermore, they accepted the validity of Siroccopteryx as distinct from Coloborhynchus, and excluded “Ornithocheirus” capito and “Coloborhynchus” sedgwickii from the genus Coloborhynchus; thus, raising the need of new generic names for these latter two.
The absence of Coloborhynchus in the Santana Group was accepted by several successive works (Andres 2010; Sayão et al. 2012; Vullo et al. 2012; Wang et al. 2012, 2014a, b; Andres and Myers 2013; Kellner et al. 2013, 2019a, b; Rodrigues and Kellner 2013; Andres et al. 2014; Bantim et al. 2014; Jiang et al. 2014; Manzig et al. 2014; Vila Nova et al. 2014; Upchurch et al. 2015; Pêgas et al. 2016, 2018, 2019; Pinheiro and Rodrigues 2017; Holgado et al. 2019; Jacobs et al. 2019; Zhou et al. 2019). Still, the validity of Siroccopteryx and Uktenadactylus, as well as the exclusion of “Ornithocheirus” capito and “Coloborhynchus” sedgwickii from the genus Coloborhynchus, have remained disputed by some workers (Martill and Unwin 2012).
Later, as a reassessment of the wastebasket genus Ornithocheirus, Rodrigues and Kellner (2013) revised those species once considered within the genus Coloborhynchus in previous works. Following their previous diagnosis (Rodrigues and Kellner 2008), they considered again the genus Coloborhynchus as restricted to the type species, Coloborhynchus clavirostris. Other species were relocated in new genera, as Camposipterus(?) sedgwickii and Cimoliopterus cuvieri, or assigned as nomina dubia (Rodrigues and Kellner 2013: 5–7). “Ornithocheirus” capito, despite having been considered as a valid species and potentially representing a new genus, was referred by the name given in its original description “…until better material comes to light”.
In addition, in the last two decades, other specimens were assigned to the genus Coloborhynchus. On the basis of a highly damaged and large fragment of anterior rostrum from the Cambridge Greensand previously referred to as Coloborhynchus capito (see Unwin 2001) or Coloborhynchus sp. (Martill 2010), Martill and Unwin (2012) described it as a new specimen of Coloborhynchus capito based on the purported presence of a markedly concave anterior margin of the premaxillary crest. Afterwards, Martill (2015) described a specimen attributed to Coloborhynchus sp. from the Wessex Formation (Lower Cretaceous) of the Isle of Wight, which is represented by a fragment of an anterior rostrum. Finally, Jacobs et al. (2019) contributed with the description of a new species, named Coloborhynchus fluviferox, on the basis of a well-preserved anterior rostrum fragment. These authors also discussed on the morphology of several species previously referred to the genus Coloborhynchus, including C. capito, C. clavirostris, and the new C. fluviferox, but also Uktenadactylus wadleighi and Siroccopteryx moroccensis, and included all of them in a phylogenetic analysis. On the same year, Holgado et al. (2019) reassessed the clade Anhangueria and established a new clade within Anhangueridae, which contains Coloborhynchus clavirostris and relatives: the Coloborhynchinae. Following Holgado et al. (2019), coloborhynchines were characterised by the following synapomorphies (based on Rodrigues and Kellner 2008): a quadrangular expansion of the premaxillary tip and a flat anterior surface of the rostrum.
In this work, we focus on the reassessment of the taxonomy and phylogeny of the proposed members of the Coloborhynchinae/Coloborhynchus complex (see SOM: tables S1 and S2 for further details in taxonomical history of this genus, Supplementary Online Material available at http://app.pan.pl/SOM/app65-Holgado_Pegas_SOM.pdf), with new anatomical comparisons and a novel phylogenetic analysis.
Institutional abbreviations.—AODF, Age Of Dinosaurs Fossil, Winton, Queensland, Australia; BSPG, Bayerische Staatssammlung für Palӓontologie und Geologie, Munich, Germany; CAMSM, Sedgwick Museum of Earth Sciences, Cambridge, England, UK; FSAC, Faculté des Sciences Aïn-Chock, Université Hassan II, Casablanca, Morocco; IWCMS, Isle of Wight County Museum Service, Isle of Wight, England, UK; NHMUK, Natural History Museum, London, UK; QM, Queensland Museum, Brisbane, Australia; SMU, Shuler Museum of Paleontology, Southern Methodist University, Dallas, USA.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:84FCB40E-8207-4EDF-BA46-5455101EE488
Material and methods
Here we re-examine all pterosaur specimens associated to the aforementioned Coloborhynchus complex, including several rostra housed in different institutions worldwide (see below for further details). For anatomical nomenclature and orientations, we follow Bennett (2001) as well as Rodrigues and Kellner (2013). We further make use of the term deltoid facet, introduced by Jacobs et al. (2019) in reference to the roughly triangular, upturned anterior palatal surface.
The phylogenetic analysis we present here is based on a data matrix modified from Pêgas et al. (2019), with the inclusion of characters by Jacobs et al. (2019) and several new rostral characters (see the SOM), as well as of the following taxa: Nicorhynchus capito comb. nov. (CAMSM B54625), Nicorhynchus fluviferox comb. nov. (FSAC-KK 10701), Aerodraco sedgwickii gen. et comb. nov. (CAMSM B54422), Uktenadactylus rodriguesae sp. nov. (IWCMS 2014.82), Mythunga camara (QM F18896), and Ferrodraco lentoni (AODF 876).
The modifications on the matrix pointed out above were performed using the software Mesquite 3.6 (Maddison and Maddison 2018). The phylogenetic analysis was performed using the software TNT 1.5 (Goloboff and Catalano 2016). Search for the most parsimonious trees (MPTs) was conducted via Traditional Search (TBR swapping algorithm), 10 000 replicates, random seed, and collapsing trees after search. We also conducted an analysis via New Technology in order to recover the island with the minimum length trees (MLTs). The parameters used in this analysis were Sectorial Search, ratchet (parameters: 20 substitutions made, or 99% swapping completed, six up-weighting prob., six down-weighting prob., and a total number of iterations of 10), tree fusing, Driven search (15 initial addseqs., 15 times of min. length), random seed, and without collapsing trees after search. Subsequently, the results of the New Technology were analysed via Traditional Search (TBR swapping algorithm), starting trees from RAM, and without collapsing trees after search. The latter search aimed for recovering the maximum trees of the island recovered from the first analysis.
Systematic palaeontology
Pterosauria Kaup, 1834
Pterodactyloidea Plieninger, 1901
Ornithocheiroidea Seeley, 1870 sensu Bennett (1994)
Pteranodontoidea Marsh, 1876 sensu Kellner (2003)
Lanceodontia Andres, Clark, and Xu, 2014
Anhangueria Rodrigues and Kellner, 2013
Anhangueridae Campos and Kellner, 1985
Coloborhynchinae Holgado, Pêgas, Canudo, Fortuny, Rodrigues, Company, and Kellner, 2019
Stem-based definition: The most inclusive clade containing Coloborhynchus clavirostris but not Anhanguera blittersdorffi or Ludodactylus sibbicki (Holgado et al. 2019).
Included genera: Aerodraco nov., Coloborhynchus Owen, 1874, Nicorhynchus nov., and Uktenadactylus Rodrigues and Kellner, 2008.
Diagnosis.—Anhanguerids with the anterior end of the rostrum forming a flat surface; a deltoid facet taller than wide; anterior expansion of the premaxilla quadrangular (in occlusal view); a perpendicular angle, in lateral view, between the deflected palatal surface and the rest of the palate; and the 3rd pair of alveoli of the upper jaw double the size of the 4th pair.
Stratigraphic and geographic range.—Late Berriasian–Valanginian, Hastings Beds, St. Leonards-on-Sea, East Sussex, England, UK; Barremian, Wessex Formation, Sudmoor Point, Isle of Wight, England, UK; late Albian, Paw Paw Formation, Fort Worth, Texas, USA; late Albian, Cambridge Greensand, Cambridgeshire, England, UK; Cenomanian, Ifezouane Formation, Kem Kem Group, south-eastern Morocco.
Genus Coloborhynchus Owen, 1874
Type and only species: Coloborhynchus clavirostris Owen, 1874. St. Leonards-on-Sea, East Sussex; late Berriasian–Valanginian.
Coloborhynchus clavirostris Owen, 1874
Holotype: NHMUK PV R1822, an anterior portion of the rostrum displaying the alveoli from the 1st to the 6th pair of teeth (Fig. 1).
Type locality: St. Leonards-on-Sea, East Sussex, England.
Type horizon: Hastings Group (late Berriasian/Valanginian).
Material.—Holotype only.
Diagnosis.—Coloborhynchine distinguished by the following autapomorphies: oval depression on the anterior palatal surface beneath the first pair of alveoli; second, third and fourth pairs of alveoli located laterally; fifth and sixth pairs of alveoli located more medially than the preceding alveoli on the base of the palatal ridge; anterior part of the palatal ridge bordered by two shallow longitudinally elongated depressions (from Rodrigues and Kellner 2008).
Remarks.—Following Rodrigues and Kellner (2008, 2013), who reviewed the taxonomy of the genus Coloborhynchus, we consider Coloborhynchus as restricted to the type species, Coloborhynchus clavirostris. For further details on the taxonomic history of the genus Coloborhynchus and the species Coloborhynchus clavirostris see the Introduction section above.
Genus Nicorhynchus nov.
ZooBank LSID: urn:lsid:zoobank.org:act:36FDC3A2-EA09-4AA3-B738-E79CA3EB6E06
Type species: Ornithocheirus capito Seeley, 1870; see below.
Included species: Nicorhynchus capito (Seeley, 1870), and Nicorhynchus fluviferox (Jacobs, Martill, Ibrahim, and Longrich, 2019).
Etymology: A combination of the Old English nicor, knucker (a kind of water dragon), and Ancient Greek ῥύγχος (rhúgkhos, Latinised as rhynchus), snout; in reference to the fact that the new taxon represents a piscivorous flying reptile from marine and fluvial deposits, represented by fragmentary snouts.
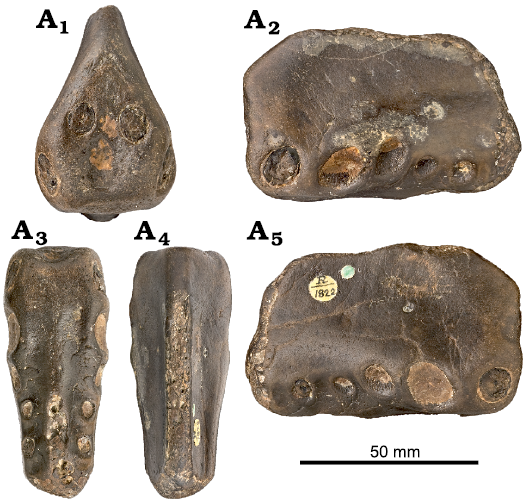
Fig. 1. Anhanguerid pterosaur Coloborhynchus clavirostris Owen, 1874, NHMUK PV R1822, St. Leonards-on-Sea, East Sussex, England, late Berriasian–Valanginian. In anterior (A1), left lateral (A2), palatal (A3), dorsal (A4), and right lateral (A5) views. Photographs from NHMUK data portal collections (https://data.nhm.ac.uk/).
Diagnosis.—Coloborhynchines with a deltoid facet (sensu Jacobs et al. 2019) higher than wide; deltoid facet dorsolateral edges bearing anteriorly-facing ridges; an anteroventral depression between the first and second pairs of upper teeth; and a palatal depression posterior to the second pair of upper teeth.
Remarks.—Rodrigues and Kellner (2008, 2013) were the first workers to suggest that “Coloborhynchus” capito required a new generic name, affirming that referral to the genus Coloborhynchus or any other genus was open to debate. These workers regarded that “C.” capito differed from Coloborhynchus clavirostris in lacking a flat rostral surface; presumably because “C.” capito bears a depression bordered by protruding ridges on the dorsal region of the deltoid facet. Furthermore, Rodrigues and Kellner (2013) noticed that “C.” capito shared with Uktenadactylus wadleighi a dorsal sulcus on the anterodorsal surface of the premaxillary crest, as well as a concave anterodorsal margin of the premaxillary crest in lateral view (Rodrigues and Kellner 2008, 2013); hinting at a possibly closer relationship with Uktenadactylus wadleighi instead of Coloborhynchus clavirostris. However, Rodrigues and Kellner (2008; 2013) prevented from erecting a new genus for “C.” capito until better material would come to light.
Even though the dorsal sulcus on the premaxillary crest could be viewed as a possible sign of abrasion, we agree with Rodrigues and Kellner (2013) that this feature is most likely genuine, given that: (i) Seeley (1881) has affirmed that the bone surface in the holotype of “Ornithocheirus” reedi was smooth and thus most likely not abraded; and (ii) it is similar to the conditions seen in Uktenadactylus wadleighi and Nicorhynchus fluviferox (Rodrigues and Kellner 2008; Jacobs et al. 2019). As for the strongly concave anterodorsal margin of the premaxillary crest, we highlight that the taxonomic relevance of this feature must be viewed with caution, since cranial crest shape is influenced by ontogeny (e.g., Manzig et al. 2014; Pinheiro and Rodrigues 2017).
Subsequently, Jacobs et al. (2019, 2020) described “Coloborhynchus” fluviferox and noticed it shared several traits with “C.” capito (a relatively higher deltoid facet; a palatal depression posterior to upper tooth position two) and, to a lesser extent, Uktenadactylus wadleighi (a sulcus running along the anterodorsal surface of the premaxillary crest; which is confidently not a sign of abrasion at least in the holotype of U. wadleighi and in FSAC-KK 5024; see further below). Even though these workers prevented from presenting a revision of the genus Coloborhynchus, they affirmed that “C.” fluviferox was apparently closely related to Cambridge Greensand coloborhynchines and speculated the possibility that the genus Coloborhynchus sensu Martill and Unwin (2012) could actually be split into further genera (Jacobs et al. 2019).
In this way, supporting the suggestions of Rodrigues and Kellner (2008) as well as of Jacobs et al. (2019), we erect the new genus Nicorhynchus for the reception of N. capito and N. fluviferox (see below). In the present work, we recover a monophyletic Nicorhynchus genus, as the sister-group of a monophyletic Uktenadactylus (see further below).
Stratigraphic and geographic range.—Albian to Cenomanian; Cambridge Greensand, England; Kem Kem Group, Morocco.
Nicorhynchus capito (Seeley, 1870) comb. nov.
Holotype: CAMSM B54625, a fragmented anterior portion of the rostrum displaying the alveoli from the 1st to the 4th pair of the right teeth (Fig. 2).
Type locality: Chesterton, Cambridgeshire, England.
Type horizon: Cambridge Greensand (Cenomanian, fossils Albian in age).
Material.—Holotype of Ornithocheirus reedi (Cambridge Greensand), described by Seeley (1870) and currently lost; and CAMSM B54434 (SOM: fig. S1) from the type locality and horizon.
Emended diagnosis.—Coloborhynchine characterised by the following autapomorphies: anterior margin of the rostrum slightly concave in lateral view; a roughly rhomboidal depression on the deltoid facet above the first pair of upper teeth; angular dorsal margin of the anteroventral depression beneath the first pair of upper teeth.
Remarks.—Rodrigues and Kellner (2008, 2013) considered “Ornithocheirus” capito to be a valid species, but excluded it from the genus Coloborhynchus. Here, we note several similarities between “Ornithocheirus” capito and other coloborhynchines, attesting its placement within the Coloborhynchinae. Firstly, it can be seen from the holotype and referred specimen that, although incomplete, the lateral margins of the lateral expansion of the premaxilla in ventral view are straight and parallel to each other, indicating the presence of a square-shaped expansion as characteristic of the Coloborhynchinae. It further exhibits an abrupt, perpendicular angle between the ventral and anterior faces of the rostral tip, again as in coloborhynchines. Rodrigues and Kellner (2013) did note some similarities between “Ornithocheirus” capito and Uktenadactylus wadleighi, regarding the concave dorsal margin of the premaxillary crest in lateral view and the presence of a sulcus on the anterodorsal surface of the premaxillary crest.

Fig. 2. Anhanguerid pterosaur Nicorhynchus capito gen. et comb. nov. (holotype), CAMSM B54625, Chesterton, Cambridgeshire, England, Albian. In anterior (A1), right lateral (A2), palatal (A3), and left lateral (A4) views. A1 and A3 modified from Rodrigues and Kellner (2013), A2 and A4 photographs by BH.
The holotype of “Ornithocheirus reedi” is herein regarded as conspecific with Nicorhynchus capito, following previous suggestions (Unwin 2001; Rodrigues and Kellner 2013). It shares with the holotype of N. capito a concave anterodorsal margin of the premaxillary crest, a slightly concave anterior margin of the rostrum in lateral view; and a deltoid facet higher than wide, though not extending as far dorsally as in N. fluviferox or N. cf. capito (NHMUK PV R481). Furthermore, this specimen bears a palatal depression (as in N. fluviferox); a roughly rhomboidal depression on the deltoid facet above the first pair of upper teeth; and an angular dorsal margin of the anteroventral depression beneath the first pair of upper teeth. CAMSM B54434 is also regarded as most likely belonging to N. capito. This specimen exhibits a sulcus on the dorsal surface of the rostrum, a slightly higher than wide deltoid facet though not extending as far dorsally as in N. fluviferox or N. cf. capito (NHMUK PV R481); and a faint depression posterior to the second pair of alveoli; thus conforming to Nicorhynchus capito. Its dorsal surface is keeled, though not high, suggesting the presence of an ontogenetically incipient crest.
Stratigraphic and geographic range.—Type locality and horizon only.
Nicorhynchus cf. capito (Seeley, 1870)
Material.—NHMUK PV R481, a highly worn portion of the rostrum displaying the alveoli of, presumably, the 1st and 2nd pairs of teeth (SOM: fig. S2), from Albian fossils in Cenomanian Cambridge Greensand, Cambridgeshire, UK.
Remarks.—NHMUK PV R481 was originally referred to the species “Coloborhynchus” capito based on the purported presence of a markedly concave anterior margin of the premaxillary crest (Unwin 2001; Martill and Unwin 2012), which is a diagnostic feature of the species (Unwin 2001; Rodrigues and Kellner 2008, 2013). Subsequently, such assignment of NHMUK PV R481 to “Coloborhynchus” capito was questioned for the first time by Kellner et al. (2013), who mentioned that a markedly concave anterior margin of the premaxillary crest cannot be confidently seen in NHMUK PV R481. In any case, a markedly concave anterior margin of the premaxillary crest is not exclusive of Nicorhynchus capito, being also present in FSACK-KK 5024 and Uktenadactylus wadleighi. Apart from that, we further note that NHMUK PV R481 displays a remarkably high deltoid facet, which extends dorsally more than twice the level of the first pair of alveoli. This feature cannot be seen in any other coloborhynchine, but is close to Nicorhynchus fluviferox, both specimens (Jacobs et al. 2019, 2020), in which the height of the deltoid facet is roughly twice the level of the first pair of alveoli (but not over as in Nichorhynchus cf. capito). However, such relationship could indicate a continuous variation rather than a discrete character. Considering that the anhanguerid premaxillary crest grows ontogenetically (Pinheiro and Rodrigues 2017), and that NHMUK PV R481 is a much larger specimen than the other coloborhynchines, then this feature could be linked to the ontogenetic growth of the premaxillary crest. In addition, as the rostral surface is highly worn off, it is possible that what is preserved of the presumable first tooth in NHMUK PV R481 would actually be a root cross section. In that case, the root would taper posteriorly and it would be posterodorsally directed. This could be a plausible explanation for its comparatively higher position, as well as its distinctively small first tooth. On the other hand, Jacobs et al. (2019, 2020) have presented a phylogenetic analysis in which NHMUK PV R481 was coded separately from the holotype specimen of “Coloborhynchus” capito, and a sister-group relationship between NHMUK PV R481 and “C”. fluviferox was recovered by their analysis. In any case, they did not present a discussion about this relationship, as a review of the genus Coloborhynchus was beyond their scope (Jacobs et al. 2019). Taking all of the above into account, there is not enough evidence to establish NHMUK PV R481 as a new taxon different from Nicorhynchus capito due to its poor preservation, and consequently we assign it to Nicorhynchus cf. capito.
Nicorhynchus fluviferox (Jacobs, Martill, Ibrahim, and Longrich, 2019) comb. nov.
Holotype: FSAC-KK 10701, an anterior portion of the rostrum displaying the alveoli from the 1st to the 3rd pair of teeth (Fig. 3).
Type locality: South-eastern Morocco, possibly Aferdou N’Chaft, Hassi El Begaa, Er Rachidia Province (see Ibrahim et al. 2010; Martill et al. 2018; Jacobs et al. 2019).
Type horizon: ?Albian–Lower Cenomanian Kem Kem Group, Ifezouane Formation.
Material.—FSAC-KK 5024/SMNK PAL 45833 (cast of a private specimen) from the type locality and horizon.
Diagnosis.—Coloborhynchine distinguished by the following autapomorphies: deltoid facet defining a high isosceles triangle with concave dorsolateral margins in anterior view; deltoid facet with two shallow, sub-circular depressions located dorsal to first upper tooth pair; deltoid facet dorsal region with a shallow sagittal groove defined by low ridges that transitions into a broad rugose anterodorsal margin of the premaxilla; and central point of first upper alveoli level with dorsal border of second tooth pair (modified from Jacobs et al. 2019).
Remarks.—The FSAC-KK 5024 was originally described as a potential second coloborhynchine species from the Kem Kem Group (Jacobs et al. 2020). This specimen shares with the holotype of N. fluviferox the following features: high deltoid facet, over twice the level of the first tooth pair; anteroventral depression below first tooth pair (also shared with N. capito); ridges on the lateral margin (in anterior) extending dorsally onto the crest base (also shared with N. capito); first tooth alveolus ventral border slightly ventral to dorsal border of the second tooth alveolus (unique of N. fluviferox among coloborhynchines); and paired depression dorsal to firth tooth pair (unique of N. fluviferox).
Jacobs et al. (2019) reported on a paired depression for N. fluviferox dorsal to the first tooth pair; and a ventrally bifurcated, single depression for FSAC-KK 5024 (Jacobs et al. 2020). However, in both specimens, the deltoid facet surface is abraded (Jacobs et al. 2019, 2020). The division between left and right depressions in the holotype of N. fluviferox is very discrete and low, and thus may have been lost to abrasion in FSAC-KK 5024. The well-preserved ventral outline of the depression in FSAC-KK 5024 is strongly bifurcated and matches well the condition seen in the holotype of N. fluviferox, in both shape and position. This suggests that the holotype of N. fluviferox and FSAC-KK 5024 bore the same feature.

Fig. 3. Anhanguerid pterosaur Nicorhynchus fluviferox comb. nov., FSAC-KK 10701, Hassi El Begaa, Er Rachidia Province, Morocco, ?Albian–lower Cenomanian. In anterior (A1), posterior (A2), left (A3) and right (A4) lateral, palatal (A5), and dorsal (A6) views. Photographs courtesy of Megan L. Jacobs.
According to Jacobs et al. (2020), FSACK-KK 5024 differed from the holotype of N. fluviferox based on the following features: the slight medial depression on the premaxillary crest dorsal surface (thought of as unique to the specimen) and the lateral margins in anterior view slightly convex, with no narrowing into the premaxillary crest; while the holotype specimen of N. fluviferox exhibits a prominent narrowing and concave margins. The slight medial depression on the dorsal surface of the premaxillary crest (which forms a groove similar to the ones seen in N. capito and Uktenadactylus wadleighi) cannot be assessed in the holotype of N. fluviferox due to incompleteness. The prominent narrowing and concave margins (in anterior view) in the holotype of N. fluviferox are the anterior margins of the premaxillary crest, located dorsal to the deltoid facet. Therefore, the shape of the lateral margins in anterior view (dorsal to the deltoid facet) are intrinsically related to the development of the premaxillary crest, and the reported variation can thus be easily explained by ontogenetic development and/or sexual dimorphism. In fact, if premaxillary crests are unconsidered, then the anterior outline of the holotype of N. fluviferox becomes a match for FSACK-KK 5024, with slightly convex lateral margins without a dorsal narrowing. This feature therefore does not exclude FSACK-KK 5024 from N. fluviferox and we regard the two as most likely conspecific, due to the above mentioned features that they share. The referred specimen adds to the known morphology of N. fluviferox by showing the presence of a groove on the anterodorsal margin of the premaxillary crest, similar to that seen in N. capito and U. wadleighi.
Stratigraphic and geographic range.—Type locality and horizon only.
Genus Uktenadactylus Rodrigues and Kellner, 2008
Type species: Uktenadactylus wadleighi (Lee, 1994); Albian, Texas, USA.
Included species: Uktenadactylus wadleighi (Lee, 1994) and Uktenadactylus rodriguesae sp. nov.
Diagnosis.—Coloborhynchines with a roughly subcircular single depression on the deltoid facet above the first pair of upper teeth; and a palatal bulbous projection between the second pair of upper teeth.
Remarks.—In the present work, we regard the genus Uktenadactylus to contain two species: U. wadleighi and U. rodriguesae sp. nov., based on IWCMS 2014.82, originally described by Martill (2015) and attributed to Coloborhynchus sp. based on the first pair of upper teeth being of anteriorly directed, and a 90° upturn of the palate. These features are herein regarded as general for the Coloborhynchinae (see Discussion). Martill (2015) mentioned the existence of a palatal bulbous projection between the second pair of upper teeth in IWCMS 2014.82, but did not comment on it further. We note here that it is a shared trait with Uktenadactylus wadleighi. We further note that these two taxa share a round depression on the deltoid facet, located above the first pair of upper teeth; which was already reported as present in U. wadleighi (Lee 1994; Rodrigues and Kellner 2008). The surface of the dorsal region of the deltoid facet is flat in Coloborhynchus clavirostris, whereas it bears a pair of lateral ridges in Nicorhynchus.
Jacobs et al. (2019) considered that Nicorhynchus fluviferox shared with Uktenadactylus wadleighi the following feature: a palatal depression posterior to the second pair of upper teeth. However, upon first-hand analysis of the holotype of U. wadleighi, we report here that the condition present in U. wadleighi is not the same that was described for Nicorhynchus fluviferox. In Nicorhynchus fluviferox, a true depression excavates the palatal surface posterior to the second pair of upper teeth, as reported by Jacobs et al. (2019). However, in Uktenadactylus wadleighi, this feature is not present. The palatal surface posterior to the second pair of upper alveoli is not level with the surface between the second pair of upper alveoli, indeed; but this does not happen due to a depression posterior to the second pair of upper alveoli. Instead, it happens due to a bulbous projection between the second pair of upper alveoli, protruding beyond the palatal plane. This feature can only be seen in U. wadleighi and IWCMS 2014.82.
Stratigraphic and geographic range.—Barremian–Albian; Wessex Formation, England; Paw Paw Formation, USA.
Uktenadactylus wadleighi (Lee, 1994)
Holotype: SMU 73058, an anterior portion of the rostrum displaying the alveoli from the 1st to the 8th pair of teeth (Fig. 4).
Type locality: Tarrant County, Texas, USA.
Type horizon: Paw Paw Formation (Albian).
Material.—Holotype only.
Emended diagnosis.—Uktenadactylus species with slightly convex anterodorsal margins of the deltoid facet; a deep and oval (higher than wide) depression above the first tooth pair; a sharp palatal bulbous projection; and second pair of upper alveoli not as lateralised as in U. rodriguesae.
Remarks.—As noted above, Lee (1994) considered this specimen as representing a new species of the genus Coloborhynchus. “Coloborhynchys” wadleighi was reassigned to the newly erected genus Uktenadactylus by Rodrigues and Kellner (2008), whose updated generic attribution was also recently considered by other workers (Witton 2013; Bantim et al. 2014; Jacobs et al. 2019, 2020). For further details see the diagnosis and remarks for the genus.
Stratigraphic and geographic range.—Type locality and horizon only.
Uktenadactylus rodriguesae sp. nov.
ZooBank LSID: urn:lsid:zoobank.org:act:229644AA-6FA5-4C4B-988D-863C20197C14
Etymology: In honour of palaeontologist Taissa Rodrigues, in recognition of her herculean work on the taxonomy of anhanguerians and related forms (e.g., Rodrigues and Kellner 2008, 2013; Rodrigues et al. 2015; Pinheiro and Rodrigues 2017).
Holotype: IWCMS 2014.82, rostrum fragment including tooth positions 1–2 from both sides (Fig. 5).
Type locality: Sudmoor Point, south west coast of the Isle of Wight, England, UK.
Type horizon: Wessex Formation (Barremian), Wealden Group.

Fig. 4. Anhanguerid pterosaur Uktenadactylus wadleighi (Lee, 1994), SMU 73058, Tarrant County, Texas, USA, Albian. In anterior (A1), left lateral (A2), palatal (A3) and right lateral (A4), and dorsal (A5) views. Photographs by BH.
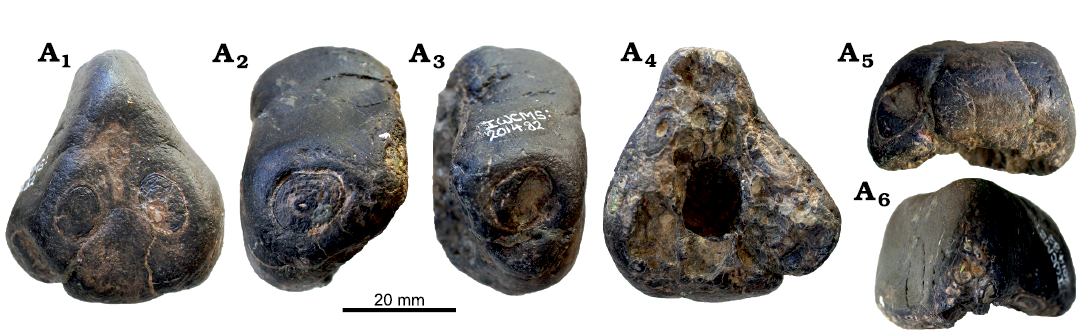
Fig. 5. Anhanguerid pterosaur Uktenadactylus rodriguesae sp. nov., IWCMS 2014.82, Sudmoor Point, Isle of Wight, England, Barremian. In anterior (A1), left (A2) and right (A3) lateral, posterior (A4), and palatal (A5) views. Photographs by BH.
Material.—Holotype only.
Diagnosis.—Uktenadactylus species with strongly concave anterodorsal margins of the deltoid facet; a shallow and subcircular (about as high as wide) depression above the first tooth pair; a smooth palatal bulbous projection; and well lateralised second pair of upper alveoli.
Remarks.—This specimen was originally reported as Coloborhynchus sp. by (Martill 2015), as mentioned above. Such attribution was repeated in subsequent works (Jacobs et al. 2019, 2020). In the present contribution, we note that IWCMS 2014.82 shares with the holotype of Uktenadactylus wadleighi two features: a round depression on the deltoid facet above the first pair of upper teeth, and a palatal bulbous projection between the second pair of upper teeth. These two features are unique within the Anhangueridae as a whole, and are herein regarded as diagnostic for a genus Uktenadactylus comprising U. wadleighi and U. rodriguesae sp. nov.
Even though the specimen is broken, it is three-dimensional and its anterior and lateral surfaces exhibit well-preserved bone compacta. The new species differs from U. wadleighi in that the dorsolateral margins of the deltoid facet are particularly concave (they are straight in U. wadleighi), the second pair of upper alveoli is well lateralised (on both sides, despite some distortion), and that the subcircular depression above the first tooth pair is shallower.
Stratigraphic and geographic range.—Type locality and horizon only.
Genus Aerodraco nov.
ZooBank LSID: urn:lsid:zoobank.org:act:436E9D43-EFBC-4A09-AA FD-C9D12D670A39
Type species: Aerodraco sedgwickii gen. et comb. nov.; see below.
Etymology: From Ancient Greek aero, wind and draco, dragon; in reference to the book Dragons of the Air (Seeley 1901).
Holotype: CAMSM B54422, an anterior portion of the rostrum displaying the alveoli from the 1st to the 7th pair of teeth (Fig. 6).
Type locality: Cambridge, Cambridgeshire, England, UK.
Type horizon: Cambridge Greensand (Cenomanian; fossils Albian in age).
Material.—Holotype only.
Emended diagnosis.—Coloborhynchine with the following autapomorphies: anterior expansion of the rostrum ends abruptly behind the third pair of alveoli; paired row of neurovascular foramina flanking the palatal ridge; third pair of alveoli larger than in any other coloborhynchine relative to the fourth pair (updated from Rodrigues and Kellner 2013).
Remarks.—Owen (1859) assigned eight rostra to the species Pterodactylus sedgwickii, including the holotype CAMSM B54422, and years later he relocated this species to the genus Coloborhynchus Owen, 1874 due to the first pair of premaxillary teeth projecting forward from the truncate surface at a higher level than the alveolar border. After several decades considered as another species within the Ornithocheirus complex (Hooley 1914; Wellnhofer 1978; Lee 1994), Unwin (2001) assigned this species to the genus Coloborhynchus, due to the shape of the rostrum as well as the arrangement and size of the teeth being similar to “Coloborhynchus” capito, differing exclusively from the latter on the basis of the purported lack of a premaxillary crest. This lead Unwin (2001) to suggest that this difference could represent sexual dimorphism (Unwin 2001: 208), but did not synonymise the two species waiting for “…a better understanding of the remaining cranial and postcranial anatomy of these taxa and other coloborhynchids” [sic!] (Unwin 2001). Rodrigues and Kellner (2008: 226) excluded A. sedgwickii from the genus Coloborhynchus, arguing that the anterior end of the rostrum “is round rather than flat” [sic!] as well as a larger spacing between the first and second pairs of alveoli. Posteriorly, Rodrigues and Kellner (2013) tentatively referred it to the genus Camposipterus due to the following combination of features: presence of an expansion of the rostrum, absence of a premaxillary crest, and round rostrum in lateral view. However, none of these features is unique to Camposipterus, and this is why their referral was only tentative (Rodrigues and Kellner 2013). Although the anterior end of the rostrum is round in lateral view (Rodrigues and Kellner 2008, 2013), the anterior and lateral margins in occlusal view are straight, similarly to other coloborhynchines and differently from any other anhanguerians. Concerning the premaxillary crest, its absence may remain unclear since the posterodorsal portion of the rostrum is not preserved (Fig. 6A5). The dorsal surface of the preserved fragment is quite tall and keeled, and thus we regard that an incipient crest was most likely present.

Fig. 6. Anhanguerid pterosaur Aerodraco sedgwickii gen. et comb. nov., CAMSM B54422, Cambridge, Cambridgeshire, England, Albian. In anterior (A1), left lateral (A2), palatal (A3), right lateral (A4), and dorsal (A5) views. Photographs by BH.
Here, we notice that the CAMSM B54422 bears a distinctive paired row of neurovascular foramina flanking the palatal ridge. This feature is absent in anhanguerids for which the palatal bone surface is well-preserved, such as Uktenadactylus wadleighi, Coloborhynchus clavirostris, Tropeognathus mesembrinus, Maaradactylus kellneri, and Anhanguera sp. AMNH 22555 (Wellnhofer 1987, 1991; Rodrigues and Kellner 2008, 2013; Bantim et al. 2014; Pinheiro and Rodrigues 2017).
Stratigraphic and geographic range.—Type locality and horizon only.
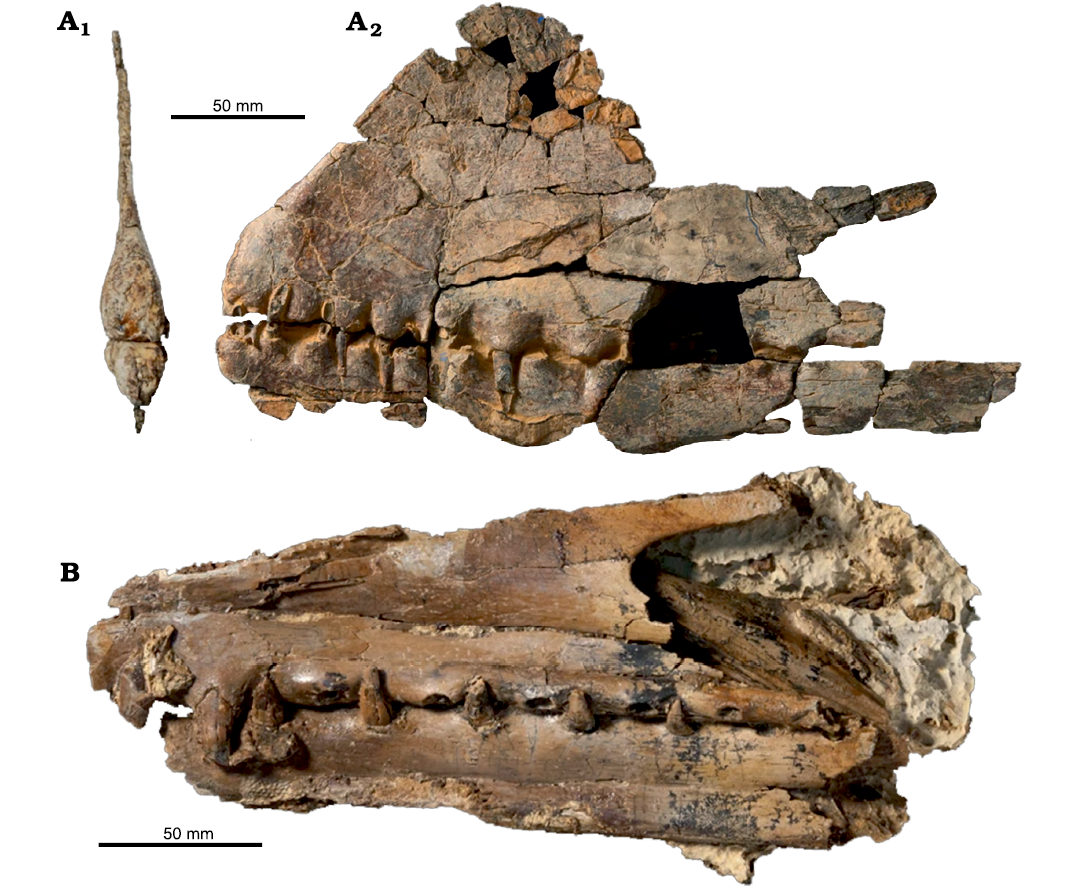
Fig. 7. Australian tropeognathine pterosaurs. A. Ferrodraco lentoni Pentland, Poropat, Tischler, Sloan, and Elliott, 2019, AODF 876, Winton, Queensland, Australia, upper Cenomanian–lower Turonian. In anterior (A1) and left lateral (A2) views. B. Mythunga camara Molnar and Thulborn, 2008, QM F18896, Hughenden, Queensland, Australia, Albian. In left lateral view. Pictures modified from Pentland et al. (2019) under a CC-BY 4.0 license.
Subfamily Tropeognathinae nov.
Zoobank LSID: urn:lsid:zoobank.org:act:0722EB2F-8A40-40E4-9896-E548202E54FE
Stem-based definition: The most inclusive clade containing Tropeognathus mesembrinus but not Coloborhynchus clavirostris or Anhanguera blittersdorffi.
Included genera: Ferrodraco Pentland, Poropat, Tischler, Sloan, Elliott, Elliott, and Elliott, 2019, Mythunga Molnar and Thulborn, 2008, Siroccopteryx Mader and Kellner, 1999, and Tropeognathus Wellnhofer, 1987.
Diagnosis.—Anhanguerids with reduced premaxillary/dentary lateral expansions (under 130% the width of the post-rosette width); relatively short teeth (crown height under 3 times diameter); and premaxillary crests reaching the rostral tip.
Remarks.—Tropeognathus mesembrinus is, alongside Anhanguera blittersdorffi, an internal specifier of a node-based Anhangueridae sensu Kellner (2003). In the present work, we recognize the Tropeognathinae as a clade joining Tropeognathus mesembrinus (Albian, Romualdo Formation, Brazil), Siroccopteryx moroccensis (Albian–Cenomanian, Kem Kem Group, Morocco), Ferrodraco lentoni (Winton Formation, Cenomanian–Turonian, Australia), and Mythunga camara (Albian, Toolebuc Formation, Australia). The relationships of Siroccopteryx moroccensis have been controversial (see Rodrigues and Kellner 2008; Holgado et al. 2019; Jacobs et al. 2019). Siroccopteryx has been previously regarded as a close relative of Coloborhynchus by several works (Rodrigues and Kellner 2008, 2013; Andres et al. 2013, 2014; Holgado et al. 2019). Recently, anatomical comparisons between Siroccopteryx, Coloborhynchus, and Tropeognathus led Jacobs et al. (2019) to, very eloquently, reinterpret for the first time Siroccopteryx as a close relative of Tropeognathus instead of Coloborhynchus. The evidence presented by Jacobs et al. (2019) is taken into account here, leading us to support their conclusions (see further below on Phylogenetic Analysis and Discussion). The close relationship between Ferrodraco + Mythunga and Tropeognathus mesembrinus (see Pentland et al. 2019) is also corroborated here.
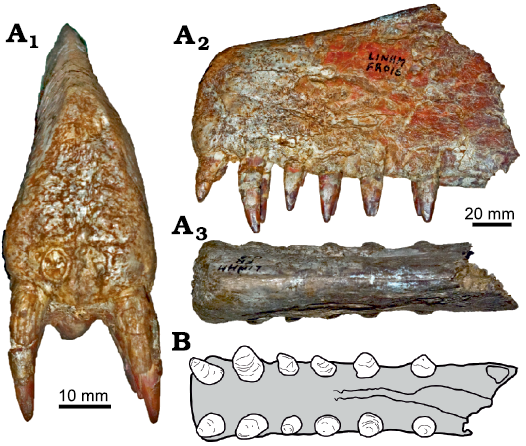
Fig. 8. Tropeognathine pterosaur Siroccopteryx moroccensis Mader and Kellner, 1999, LINHM FR016, Kem Kem beds, Morocco, ?Albian–lower Cenomanian. A. Photograph in anterior (A1), left lateral (A2), and dorsal (A3) views. B. Interpretation of the palatal view following Mader and Kellner (1999). Photographs courtesy of Megan L. Jacobs. Drawing by RVP based on Mader and Kellner (1999).
Concerning the phylogenetic relationships of Tropeognathus mesembrinus and previous analyses, under the works of Holgado et al. (2019) and Pêgas et al. (2019) Tropeognathus was recovered as a monotypic branch within the Anhangueridae, alongside the clades Coloborhynchinae and Anhanguerinae. Jacobs et al. (2019) recovered Tropeognathus mesembrinus as closely related to Ornithocheirus simus and Siroccopteryx moroccensis. Under the analyses of Andres et al. (2014) and Longrich et al. (2018), Tropeognathus mesembrinus was recovered as closely related to Ornithocheirus simus and Coloborhynchus clavirostris.
In the present work, we support a close relationship between Tropeognathus mesembrinus, Siroccopteryx moroccensis, Ferrodraco lentoni, and Mythunga camara, composing the Tropeognathinae (comprised within Anhangueridae). However, this clade does not include Coloborhynchus clavirostris (or any other coloborhynchine) nor Ornithocheirus mesembrinus (regarded as a basal ornithocheiraean). Our systematic proposal and the synapomorphies behind it are further elaborated below.
Stratigraphic and geographic range.—Albian–Cenomanian–Turonian (probably Turonian); Romualdo Formation, Brazil; Toolebuc Formation, Australia; Kem Kem Group, Morocco; Winton Formation, Australia.
Phylogenetic analysis
Our phylogenetic analysis resulted in nine most parsimonious trees, with 417 steps, a consistency index of 0.638 and a retention index of 0.871 (SOM: fig. S3). The strict consensus tree recovers a clade Anhangueridae divided into three main lineages: Tropeognathinae, Coloborhynchinae and Anhanguerinae; comprising a total of 20 species (Fig. 10). The clade Tropeognathinae is supported by three synapomorphies: teeth relatively short (crown height under 3× diameter); premaxillary crest reaching rostrum tip; and premaxillary expansion width reduced (under 130% post-rosette width). The relationship Tropeognathus + Siroccopteryx is based on two synapomorphies: palatal ridge robust forming a strong keel; and palatal ridge restricted posterior to the fifth tooth position. The clade containing Mythunga and Ferrodraco is supported by one synapomorphy: alveoli margins strongly raised.
The node joining Coloborhynchinae and Anhanguerinae, to the exclusion of Tropeognathinae, is supported by the following synapomorphy: fifth pair of upper teeth medially displaced.
The Coloborhynchinae share the following five synapomorphies: premaxilla, anterior expansion, lateral margins straight and subparallel; dorsal deflection of the palate forming 90º angle; teeth, upper jaw, 3rd pair of teeth double size than 4th pair; teeth, upper jaw, first pair of slender elongated teeth, cross-section, elliptical.
The node containing Coloborhynchus, Uktenadactylus, and Nicorhynchus is supported by one synapomorphy: teeth, upper jaw, second pair, position in horizontal plane; aligned with third pair.
The relationship Uktenadactylus + Nicorhynchus is based on the following synapomorphy: anterodorsal surface of premaxillary crest grooved. The Nicorhynchus is supported by four synapomorphies: deltoid facet higher than wide; deltoid facet dorsolateral edges bearing anteriorly-facing ridges; anteroventral depression between the first and second pairs of upper teeth; palatal depression posterior to the second pair of upper teeth.
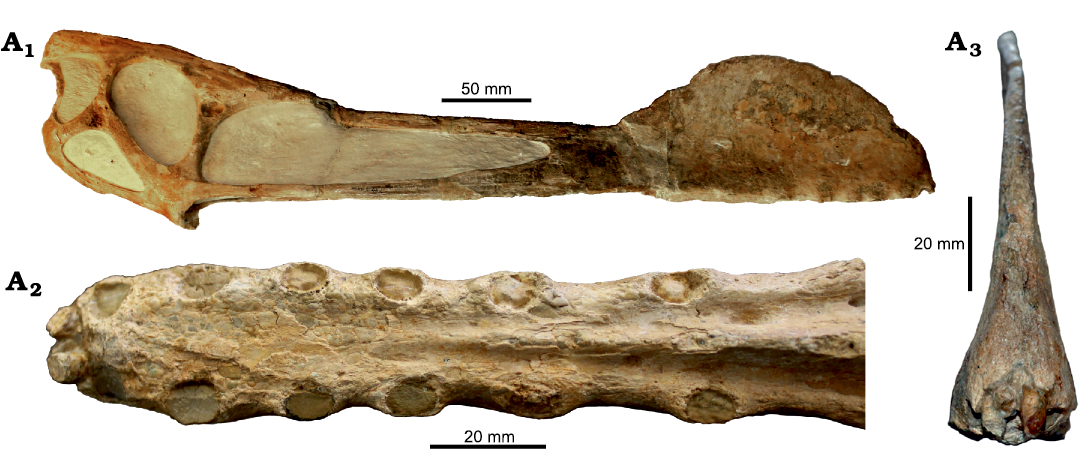
Fig. 9. Tropeognathine pterosaur Tropeognathus mesembrinus Wellnhofer, 1987, BSPG 1987 I 47, Chapada do Araripe, Ceará, Brazil, Albian. In right lateral (A1), palatal (A2), and anterior (A3) views. Photographs by RVP (A1) and BH (A2). A3 modified from Rodrigues and Kellner (2013).
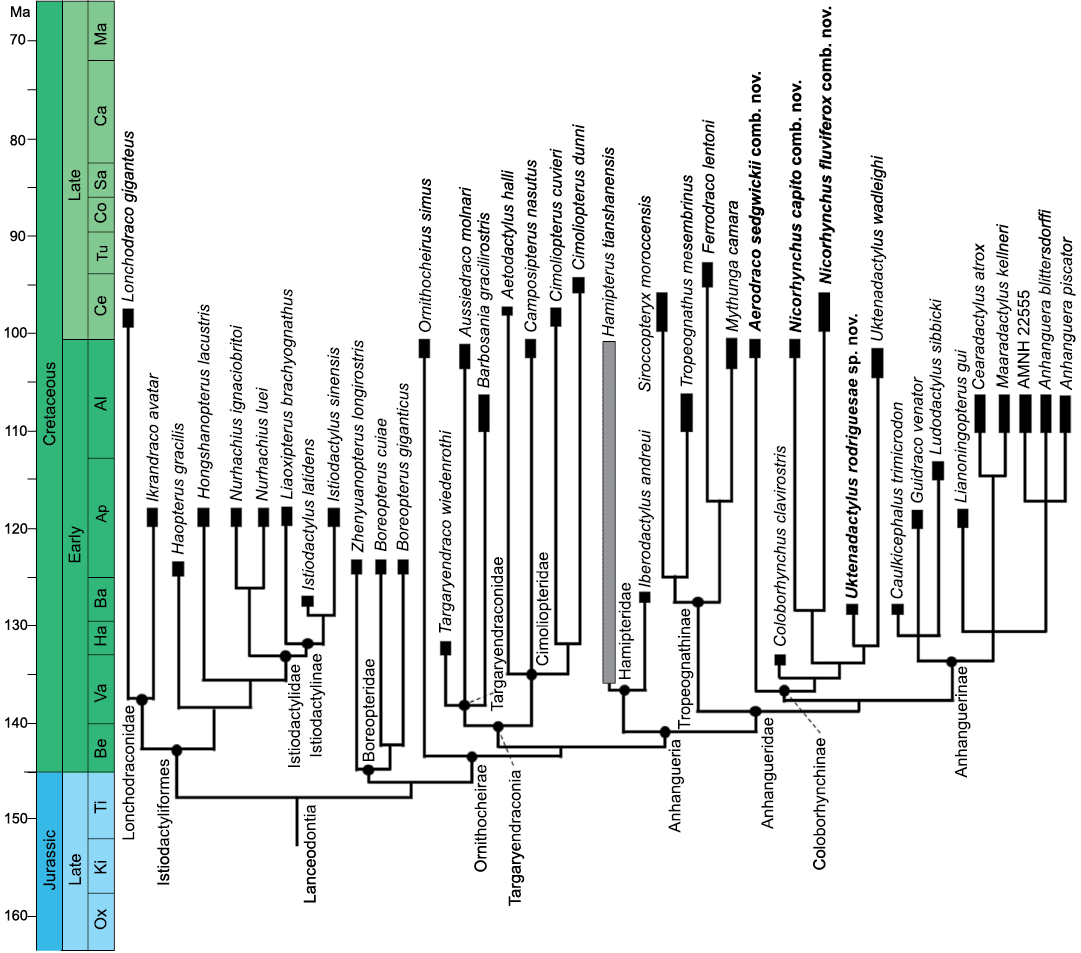
Fig. 10. Phylogenetic relationships of the Coloborhynchinae and Tropeognathinae clade nov. within Lanceodontia. Outgroup relationships are not shown (see SOM: fig. S3 for further details). Gray bar shows uncertain temporal range. Stratigraphic chart modified from Cohen et al. (2013). Drawing by BH. New taxa combinations and species proposed in this paper marked in bold. Abbreviations: Al, Albian; Ap, Aptian; Ba, Barrenian Be, Berriasian; Ca, Campanian; Ce, Cenomanian; Co, Coniacian; Ha, Hauterivian; Ki, Kimmeridgian; Ma, Maastrichtian; Ox, Oxfordian; Sa, Santonian; Ti, Tithonian; Tu, Turonian; Va, Valangian.
Discussion
The composition of the Coloborhynchinae.—The close relationship between Coloborhynchus clavirostris and Uktenadactylus wadleighi has been well-supported by several distinct authors (Lee 1994; Rodrigues and Kellner 2008; Martill and Unwin 2012; Andres et al. 2014; Jacobs et al. 2019), some of which have even kept U. wadleighi under the genus Coloborhynchus (Martill and Unwin 2012; Andres et al. 2014). In our analysis, Coloborhynchus, Uktenadactylus, Nicorhynchus gen. nov., and Aerodraco gen. nov. share the following 5 synapomorphies: rostrum, anterior end, flat surface; premaxilla, anterior expansion, lateral margins straight and subparallel; dorsal deflection of the palate forming 90° angle; teeth, upper jaw, 3rd pair of teeth double size than 4th pair; teeth, upper jaw, first pair of slender elongated teeth, cross-section, elliptical.
As mentioned above, Coloborhynchus has been regarded as closely related to Tropeognathus mesembrinus and Ornithocheirus simus by Andres et al. (2014) and Longrich et al. (2018). However, the purported placement of Ornithocheirus simus as closely related to Tropeognathus mesembrinus is here considered as inadequate, as explored in depth by Rodrigues and Kellner (2013). Ornithocheirus simus lacks a series of anhanguerian and anhanguerid features (Rodrigues and Kellner 2013; Holgado et al. 2019). According to the coding of Longrich et al. (2018), the genera Tropeognathus, Ornithocheirus, Siroccopteryx, and Coloborhynchus would be joined by a premaxillary crest reaching the rostral tip. However, the presence of a premaxillary crest is not confirmed for Ornithocheirus simus (Rodrigues and Kellner 2013; BH personal observations). Under the data matrix of Longrich et al. (2018), Ornithocheirus simus would further share with Coloborhynchus and Siroccopteryx a “strong keel” as a state for the palatal ridge, and also a flat surface for the rostral tip. However, no known specimen of Ornithocheirus simus exhibits a preserved palatal ridge (Rodrigues and Kellner 2013; BH personal observations), and therefore this character is coded here as “?” for this taxon. Furthermore, the rostral tip of Ornithocheirus simus is blunt and not a flat surface (Rodrigues and Kellner 2013; Jacobs et al. 2019; BH personal observations). The flat rostral surface seen in Coloborhynchus is formed by the dorsally deflected palatal surface, or deltoid facet, which is entirely absent in Ornithocheirus simus (see Rodrigues and Kellner 2013) and is a synapomorphy of the clade Anhangueria + Targaryendraconia (Pêgas et al. 2019). This feature was also not taken into account in the data matrices of Andres et al. (2014) and Longrich et al. (2018). Ornithocheirus further lacks a premaxillary expansion (Rodrigues and Kellner 2013; BH personal observations), which is present in all anhanguerians (see Rodrigues and Kellner 2013; Pêgas et al. 2019), but this character was miscoded as present for Ornithocheirus simus in the matrices of Andres et al. (2014) and Longrich et al. (2018). It has been coded as absent in our analysis, as has been done by Rodrigues and Kellner (2013) as well as Jacobs et al. (2019, 2020).
In our analysis, the Coloborhynchinae is regarded as the sister-group of the Anhanguerinae, with which they share the following synapomorphy: upper fifth tooth position medially displaced. The Tropeognathinae lack this feature, as well as hamipterids and targaryendraconians (see Holgado et al. 2019; Pêgas et al. 2019); and tropeognathines are thus set aside from coloborhynchines and anhanguerines, at the base of the Anhangueridae. As such, Tropeognathus mesembrinus is not recovered as closer to Coloborhynchus clavirostris than to Anhanguera blittersdorffi (contra Longrich et al. 2018). Instead, these three species are herein utilised as internal specifiers for three stem-based subgroups for the Anhangueridae: Tropeognathinae, Coloborhynchinae, and Anhanguerinae.
Other species previously referred to the genus Coloborhynchus are also regarded as non-members of the Coloborhynchinae here: Siroccopteryx moroccensis, Amblydectes crassidens, Amblydectes(?) eurygnathus, Anhanguera robustus, Anhanguera araripensis, Anhanguera piscator, Anhaguera spielbergi, and Cimoliopterus cuvieri (see SOM: table S2). The taxonomic status of Anhanguera and Cimoliopterus have already been explored in scrutiny and will not be further addressed here (see Rodrigues and Kellner 2008, 2013; Pinheiro and Rodrigues 2017). The phylogenetic position of Siroccopteryx as a member of the Tropeognathinae is explored further below.
As for the two species formerly attributed to the genus Amblydectes, we consider them here as potentially valid species of non-coloborhynchine anhanguerids. Both species lack a flat surface in the anterior end of the rostrum; a perpendicular angle between the deflected palatal surface and the rest of the palate; and straight anterior and lateral margins of the premaxillary expansion in occlusal view; which are coloborhynchine features. Nevertheless, some features show that the two species are different and most probably not closely related. Amblydectes crassidens is not only a non-coloborhynchine anhanguerid, but also a non-anhanguerine anhnanguerid since they lack an enlarged 4th pair of upper teeth, which is characteristic of the Anhanguerinae (Holgado et al. 2019). Since the only 4th preserved alveolus (the right one) is incomplete, it is not possible to determine if the 3rd pair of teeth is double the size of the 4th pair, which is a synapomorphy of the Coloborhynchinae (see above). In any case, Amblydectes crassidens could be tentatively referred to the Tropeognathinae due to its reduced width of the premaxillary expansion. Still, there is no unambiguous evidence to attribute this anhanguerid species to this clade with much confidence. On the other hand, Amblydectes(?) eurygnathus strongly resembles Anhanguera, with relatively large alveoli and an oval depression between the 1st pair of teeth as well as the presence of a high crest. However, the incompleteness of the specimen, which only preserves the first two pairs of alveoli, does not reveal if Amblydectes(?) eurygnathus is actually an Anhanguerinae or not. Another species involved in the Coloborhynchus complex is Pterodactylus woodwardi (SOM: fig. S4). Originally described by Owen (1861), CAMSM B54433 was referred to as Ornithocheirus woodwardi by Seeley (1870), and later, Hooley (1914) referred the species to the genus “Criorhynchus”. Other authors (Lydekker 1888; Wellnhofer 1978) even considered this species as a synonym of Ornithocheirus (= “Criorhynchus”) simus. Later, Unwin (2001) synonymised “Criorhynchus” woodwardi to Coloborhynchus sedgwickii due to the shape of the rostrum and arrangement of the teeth. Rodrigues and Kellner (2013: 55–57) considered CAMSM B54433 as a nomem dubium since “…several important characters cannot be observed on it”. As observed by Unwin (2001), the anterior and lateral margins of CAMSM B54434 in occlusal view are also roughly straight, which is characteristic of coloborhynchines. Despite the extreme fragmentary nature of CAMSM B54433, it can be confidently assigned to the Coloborhynchinae on the basis of their palatal tip deflection of 90°. However, due to their badly eroded surfaces, they cannot be confidently assigned to, nor excluded from, either Aerodraco or Nicorhynchus. The same rationale can be extended to specimens CAMSM B54435 and B54436 (SOM: fig. S4).
The intrarelationships of the Coloborhynchinae.—According to our phylogenetic analysis, Aerodraco sedgwickii is placed at the base of the Coloborhynchinae. It lacks a second upper tooth pair aligned with the third pair (in palatal view), which is a synapomorphy of the clade comprising Coloborhynchus, Uktenadactylus, and Nicorhynchus. Coloborhynchus, in turn, lacks an anterodorsal surface of premaxillary crest flat/grooved and a premaxillary crest anterior margin strongly concave. These features unite Uktenadactylus and Nicorhynchus.
We recognize that the Coloborhynchinae as herein defined is partially compatible with Coloborhynchus sensu Martill and Unwin (2012), with the exclusion of Siroccopteryx and Anhanguera spp. However, as observed by Jacobs et al. (2019), the clade formed by Coloborhynchus sensu Martill and Unwin (2012) is too diverse. The high level of morphological disparity (Fig. 11), as well as their wide distribution in time and space, led Jacobs et al. (2019) to regard that more than one genus was possibly present; still, they prevented from reviewing the Coloborhynchus complex. As herein defined, the Coloborhynchinae encompass at least seven species, from five different deposits. In summary, in this work, we follow Rodrigues and Kellner (2008; 2013) in restricting the genus Coloborhynchus to its type-species, recognizing Uktenadactylus as a distinct genus, and “C.” capito as a third genus. To the genus Uktenadactylus we add the new species U. rodriguesae. We refer “C.” capito to the new genus Nicorhynchus, which also englobes N. fluviferox. For “O.” sedgwickii, located at the base of the Coloborhynchinae, we erect the new genus Aerodraco.
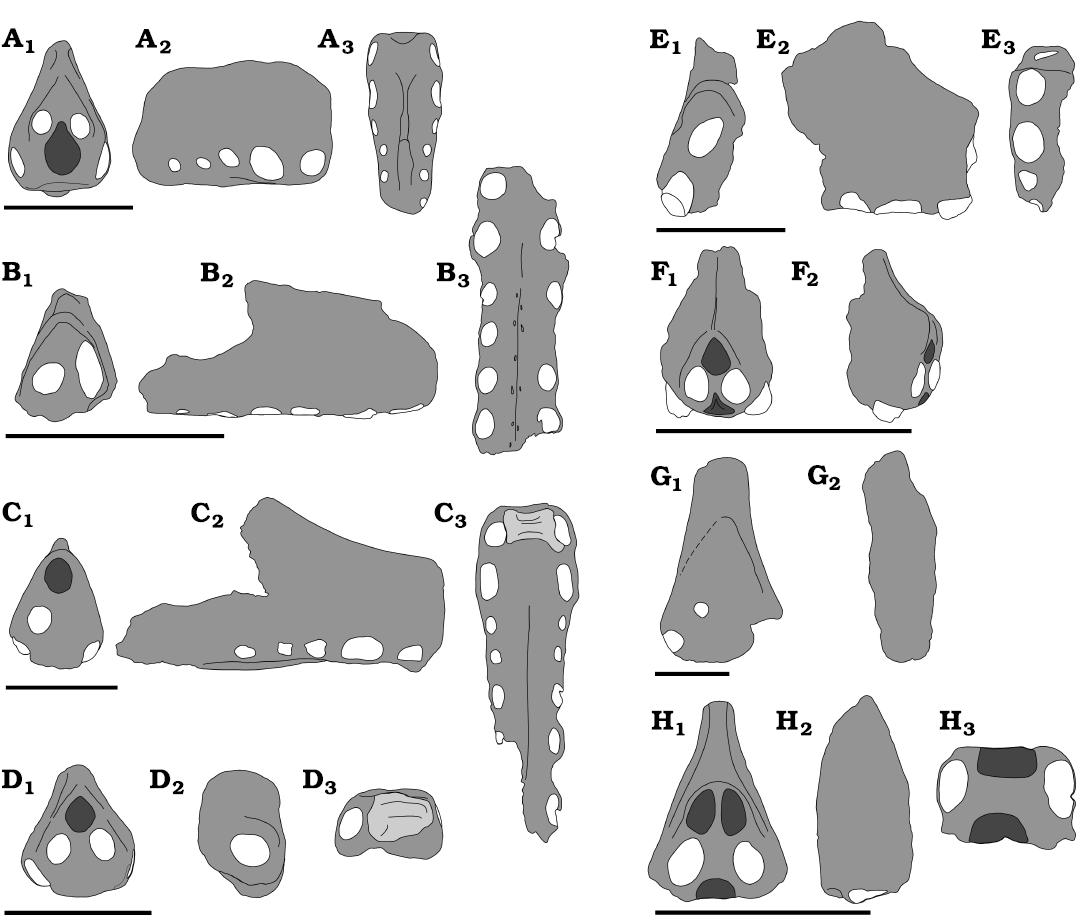
Fig. 11. Coloborhynchinae comparative plate. A. Coloborhynchus clavirostris in anterior (A1), right lateral (A2), and palatal (A3) views. B. Aerodraco sedgwickii in anterior (B1), right lateral (B2), and palatal (B3) views. C. Uktenadactylus wadleighi in anterior (C1), right lateral (C2), and palatal (C3) views. D. Uktenadactylus rodriguesae. in anterior (D1), left lateral (mirrored, D2), and palatal (D3) views. E. Nicorhynchus capito (holotype) in anterior (E1), right lateral (E2), and palatal (E3) views. F. Nicorhynchus capito (referred specimen, originally designated as the holotype of Ornithocheirus reedi, now presumed lost from CAMSM collections) in anterior (F1) and right lateral (F2) views. G. Nicorhynchus cf. capito in anterior (G1) and left lateral (mirrored, G2). H. Nicorhynchus fluviferox (holotype) in anterior (H1), right lateral (H2), and palatal (H3) views. Dark grey represents depressions, lighter gray with lines represents bulbous projections, white represents alveoli. Scale bars 50 mm. Drawings by RVP.
The new clade Tropeognathinae.—The newly named clade Tropeognathinae comprises Tropeognathus mesembrinus, Siroccopteryx moroccensis, Ferrodraco lentoni, and Mythunga camara. The relationships of Tropeognathus mesembrinus and Siroccopteryx moroccensis have not been consensual in the literature and are worthy of note. Tropeognathus mesembrinus has been regarded several times as closely related to Ornithocheirus simus (Unwin 2003; Longrich et al. 2018; Jacobs et al. 2019), but this is based on some misinterpretations as already explored above and demonstrated by Rodrigues and Kellner (2013).
Siroccopteryx moroccensis was originally described as an anhanguerid, though its closer relationships were not investigated (Mader and Kellner 1999). It has been regarded as possibly closely related to Coloborhynchus clavirostris and Uktenadactylus wadleighi by Rodrigues and Kellner (2008), who noted the following similarities: “thickness of the premaxillary crest, the anteriorly-flattened rostrum and the quadrangular anterior expansion of the premaxillae” (Rodrigues and Kellner 2008: 224). However, the relatively broad anterior base of the premaxillary crest can also be found in Tropeognathus mesembrinus and Ferrodraco lentoni (Wellnhofer 1987; Pentland et al. 2019), being herein recovered as symplesiomorphic for anhanguerids. The deltoid facet perpendicular to the remaining palate is herein recovered as a homoplasy between Siroccopteryx moroccensis and the Coloborhynchinae. A quadrangular anterior expansion of the premaxillae is not coded as such for Siroccopteryx moroccensis in our analysis. The premaxillary expansion of Siroccopteryx is superficially similar to those of coloborhynchines because the anterior margin is straight, due to the anteriorly-flattened rostrum. However, the lateral margins of the premaxillary expansion of Siroccopteryx differ from those of coloborhynchines. In Siroccopteryx, the lateral margins gently curve continuously to meet the post-rosette constriction, similarly to Tropeognathus mesembrinus and Ferrodraco lentoni (Fig. 12); while in coloborhynchines the lateral margins are somewhat straight and parallel. In our previous analysis by Holgado et al. (2019), we have coded the premaxillary expansion of Siroccopteryx moroccensis as quadrangular in shape, but this has been rectified in the present analysis. The analysis of Longrich et al. (2018) recovered Siroccopteryx moroccensis as the sister-group of Coloborhynchus clavirostris + Uktenadactylus wadleighi. Later, however, Jacobs et al. (2019) offered for the first time a different proposal for the phylogenetic placement of Siroccopteryx, as more closely related to Tropeognathus mesembrinus and Ornithocheirus simus than to Coloborhynchus clavirostris. Jacobs et al. (2019) noted the following similarities between Tropeognathus mesembrinus and Siroccopteryx moroccensis: a blunt, rounded profile of the rostrum in lateral view; a robust palatal keel, restricted posteriorly; and relatively short teeth.
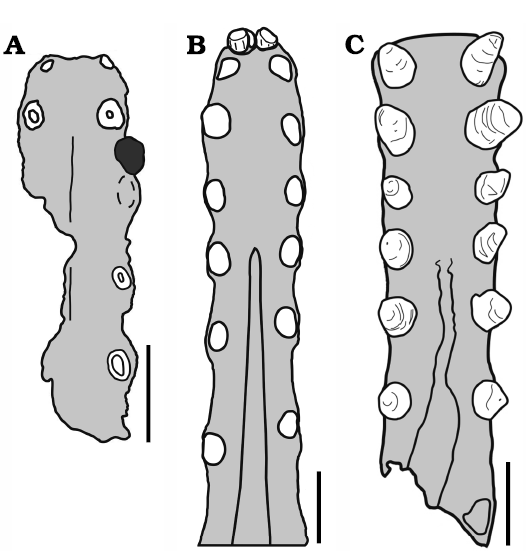
Fig. 12. Tropeognathinae anterior comparative plate. Palates in occlusal views. A. Ferrodraco lentoni. B. Tropeognathus mesembrinus; black area in A represents a lower tooth; dashed line represents the weathered contour of the 3rd alveoli (see Pentland et al. 2019 for further details). C. Siroccopteryx moroccensis. Scale bars 20 mm. Drawings by RVP.
In the present analysis, we have taken into account the similarities noted by Jacobs et al. (2019) and have recovered, accordingly, Tropeognathus mesembrinus and Siroccopteryx moroccensis as sister-taxa. They share as synapomorphies the following features: palatal ridge broad and deep; and palatal ridge posteriorly restricted, posterior to the fifth tooth position (Fig. 12). A deltoid facet abruptly angled at 90° has been recovered as a homoplasy between Siroccopteryx moroccensis and the Coloborhynchinae.
Ferrodraco lentoni, in turn, was originally regarded as the sister-group of Mythunga camara, with Ornithocheirus simus, Coloborhynchus clavirostris, and Tropeognathus mesembrinus as their successive sister-taxa (Pentland et al. 2019) according to an analysis based on the dataset of Longrich et al. (2018). The purported placement of Ornithocheirus simus and Coloborhynchus clavirostris as close to Tropeognathus mesembrinus has already been critically discussed above.
Still, Ferrodraco lentoni shares with Tropeognathus mesembrinus and Siroccopteryx moroccensis the following three features: reduced premaxillary/dentary lateral expansions (under 130% of the post-rosette width), relatively short teeth (crown height under 3× diameter), and premaxillary crests reaching the rostral tip; which are herein recovered as synapomorphies of the Tropeognathinae. In this way, Ferrodraco lentoni is herein recovered as a member of this group. We do express some caution regarding the latter feature (premaxillary crest reaching the rostral tip), since it is also present in the anhanguerine Maaradactylus kellneri (as a homoplasy in our analysis) and an expressive ontogenetic variation can be found in the premaxillary crest of Anhanguera (Pinheiro and Rodrigues, 2017).
Within the Tropeognathinae, Ferrodraco lentoni was recovered in a sister-group relationship with Mythunga camara, corroborating the proposition by Pentland et al. (2019). These two Australian anhanguerids share as a synapomorphy an alveoli border strongly raised (or laterally prominent, with deeply sulcate interalveolar constrictions), a feature that is absent in other anhanguerids such as Tropeognathus, whose jaws are scalloped but are not as deeply sulcate between adjacent alveoli. As such, even though the synapomorphies of the Tropeognathinae cannot be assessed in Mythunga camara (since the rostrum is missing from the holotype and only known specimen), it is still recovered as a member of the group due to this feature that it shares with Ferrodraco lentoni.
The diversity of the clade Anhangueridae through space and time.—Anhanguerids are a diverse clade with a host of recognisable forms and taxa (Fig. 13). As noted by Holgado et al. (2019), the clade Anhangueria could have important ancestral ties in the European archipelago and eastern Asian province. Constricting to the clade Anhangueridae, which is the largest and most diverse clade within anhanguerians, these forms span from the late Berriasian–Valanginian (with the coloborhynchine Coloborhynchus clavirostris) to the early Turonian (with the tropeognathine Ferrodraco lentoni) comprising more than 40 million of years (Fig. 14).
From the late Berriasian–Valanginian to the Barremian, the only known anhanguerid records come from the European archipelago (Fig. 13A), noting especially the nominal species from England. During the Aptian (Fig. 13B), anhanguerids radiated in several forms and lineages, as well as spread over the eastern boundaries of Laurasia and large parts of Gondwana. But it is during the Albian that anhanguerids reached their peak (Fig. 13C): in this stage they spread severely, with taxa having been found over remote places such as Australia and North America (Barrett et al. 2008; Holgado et al. 2011, 2019). In addition, two of the most anhanguerid species-rich faunas, the ones from the Romualdo Formation (NE Brazil) and Cambridge Greensand (England), occurred during the Albian (Fig. 14)—it should be recalled that even though the Cambridge Greesand is regarded as a Cenomanian deposit, its tetrapod fauna is probably reworked from underlying Albian formations (Unwin 2001; Rodrigues and Kellner 2013). During the Late Cretaceous, the recent discoveries from the Kem Kem Group (Morocco) in the early–middle Cenomanian (Fig. 13D) must be emphasised, as they shed light on the high diversity of anhanguerids during this time. As pointed above, the youngest record of an anhanguerid comes from the Winton Formation, in the Cenomanian–Turonian boundary (most likely early Turonian strata) of Queensland, Australia (Figs. 13D, 14). We must remark that, even though the pterosaur fossil record exhibits a strong taphonomic bias (Kellner 1994; Upchurch et al. 2015), the diversity and distribution of the Anhangueridae is clearly greater than previously thought.
We show here that the clade Coloborhynchinae has a prevailing distribution in Laurasia, being Nicorhynchus fluviferox the only known record from northern Gondwana (and from the Late Cretaceous). On the other hand, the clade Tropeognathinae seems to be exclusively distributed in Gondwana, being Siroccopteryx moroccensis the northernmost record (Fig. 13D).
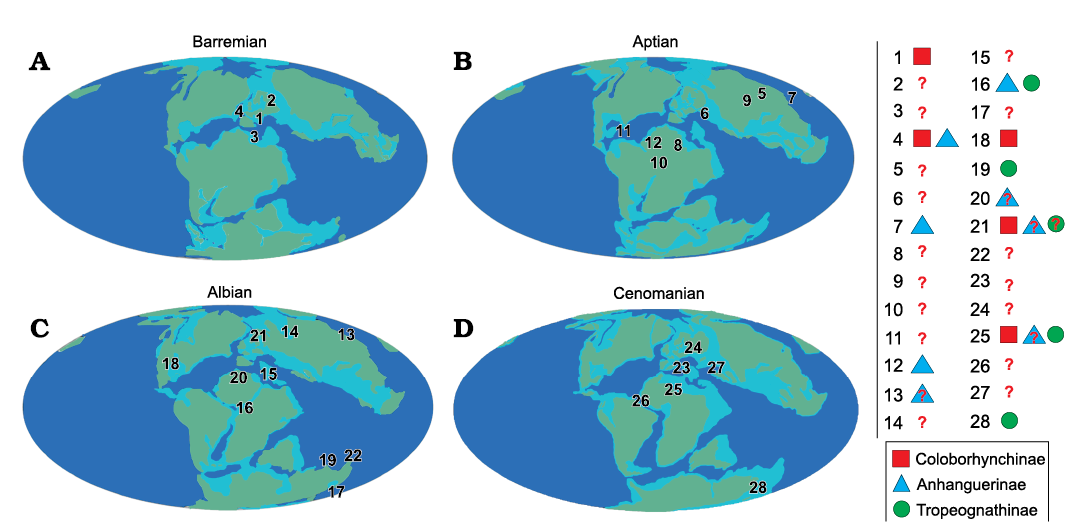
Fig. 13. Paleogeographic maps showing distribution of Anhangueridae. A. Barremian: 1, St. Leonards-on-Sea, East Sussex, Hastings Group (late Berriasian/Valanginian), England; 2, Bol’shoi Kemchug, lower Ilek Formation (?Hauterivian–Barremian) Krasnoyarsk Krai, Russia; 3, Las Hoyas, La Huérgina Formation (Barremian), Cuenca, Spain; 4, Isle of Wight, Wessex Formation (Barremian), England. B. Aptian: 5, Mogoito, Murtoi Formation (Aptian), Buryatia, Russia; 6, Sekmenevka Formation (Aptian), Belgorod Oblast, Russia; 7, Jiufotang Formation (Aptian), Liaoning, China; 8, Elrhaz Formation (Aptian), Niger; 9, Krasnyi Yar, Khilok Formation (Aptian), Buryatia, Russia; 10, Pedra Furada, Recôncavo Basin, Marizal Formation? (Aptian), Bahia, Brazil; 11, Sierra de Perijá, Apón Formation (Aptian), Zulia, Venezuela; 12, Crato Formation (late Aptian), Ceará, Brazil. C. Albian: 13, Khuren-Dukh, Dzun-Bayin Formation (late Aptian–Albian), Mongolia; 14, Sheskatovo, upper Ilek Formation (late Aptian–Albian), Kemerovo Oblast, Russia; 15, Chenini Formation (early Albian), Tunisia; 16, Romualdo Formation (Albian), Ceará, Brazil; 17, Lightning Ridge, Griman Creek Formation (Albian), New South Wales, Australia; 18, Tarrant County, Paw Paw Formation (Albian), Texas, USA; 19, Boulia, Toolebuc Formation (Albian), Queensland, Australia; 20, Cortes de Arenoso, Utrillas Formation (Albian), Valencia, Spain; 21, Cambridge Greensand (Cenomanian, but fossils Albian in age), England; 22, Hughenden, Mackunda Formation (late Albian), Queensland, Australia. D. Cenomanian: 23, Strelitsa Graysand with phosphorite (late Albian–Cenomanian), Voronezh Region, Russia; 24, Pavlovsk (Cenomanian), Voronezh Region, Russia; 25, Kem Kem beds, lower Ifezouane and upper Aoufous Formations (Cenomanian), Dr’a Tafilalt, Southeast Morocco; 26, Laje do Coringa, São Luís-Grajaú basin, Alcântara Formation (Cenomanian), Maranhão, Brazil; 27, Saratov yellow sand with phosphorite (late Cenomanian), Saratov Region, Russia; 28, Belmont Station, Winton, Winton Formation (late Cenomanian–early Turonian), Queensland, Australia. Red question marks refer those specimens tentatively considered Anhangueridae, a red question mark inside one of the above geometric figures means a specimen tentatively attributed to each clade under consideration. World maps modified from the Palaeobiology Database (https://paleobiodb.org/navigator/) under the CC BY 4.0 license. Drawing by BH.
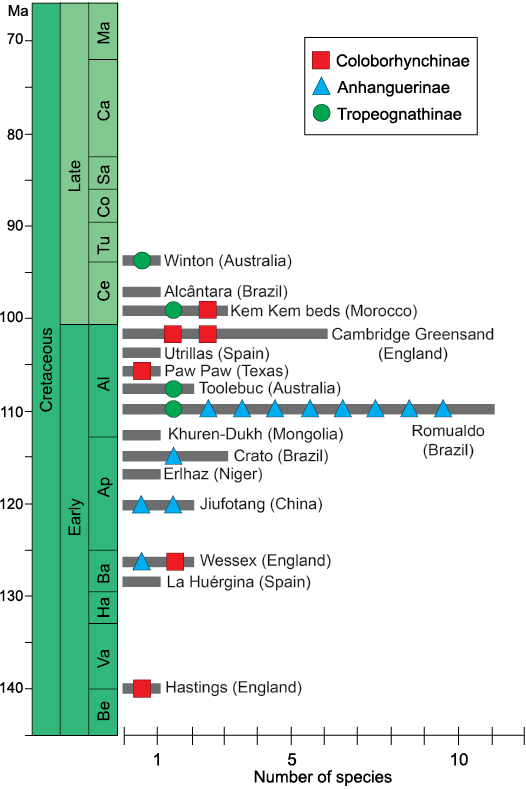
Fig. 14. Histogram showing the distribution of the species and specimens attributed to Anhnagueridae during the Cretaceous. Each bar of the histogram represents the number of diverse species found in each labelled site, whilst the geometric figures identified the species within the three anhangerid clades: Coloborhynchinae, Anhanguerinae, and Tropeognathinae. Drawing by BH. Abbreviations: Al, Albian; Ap, Aptian; Ba, Barrenian Be, Berriasian; Ca, Campanian; Ce, Cenomanian; Co, Coniacian; Ha, Hauterivian; Ma, Maastrichtian; Sa, Santonian; Tu, Turonian; Va, Valangian.
Conclusions
Our anatomical comparisons and phylogenetic analysis lead us to regard that the Coloborhynchinae were more diverse than previously thought. Supporting previous suggestions, we argue that “Ornithocheirus” capito represents a new genus, hereby named Nicorhynchus; that Nicorhynchus capito is closely related to Coloborhynchus fluviferox, which is hereby renamed N. fluviferox; and that Siroccopteryx is more closely related to Tropeognathus than to Coloborhynchus. We also corroborate the view that Ornithocheirus simus is not closely related to Coloborhynchus, Tropeognathus or Anhanguera. We further demonstrate, for the first time, that “Ornithocheirus” sedgwickii represents a new genus, named Aerodraco; that Aerodraco sedgwickii represents a basal coloborhynchine; that IWCMS 2014.82 represents a new species for the genus Uktenadactylus, hereby named U. rodriguesae; and that Uktenadactylus and Nicorhynchus are sister-taxa. Finally, we propose the new clade Tropeognathinae, englobing Tropeognathus, Siroccopteryx, Mythunga, and Ferrodraco.
Acknowledgements
BH thanks Matt Riley (CAMSM), Martin Munt (IWCMS), Michael Day (NHMUK), and Dale Winkler (SMU) for access to specimens under their care. We also thank Oliver Rauhut (BSPG), Alexander Nützel (BSPG), Uiara Cabral, Luciana Carvalho and Alexander Kellner (all Museu Nacional/Universidade Federal do Rio de Janeiro, Brazil) for access to specimens under their care. Special thanks to Megan Jacobs (University of Portsmouth, UK) for pterosaur discussions and for kindly sharing with us photos of Nicorhynchus fluviferox and Siroccopteryx. We acknowledge the Willi Hennig Society for making TNT freely available. We also thank Alexandra Elbakyan for “breaking the barriers of science”, which allows us to access several articles that would not be possible under other conditions. Last but not least, we acknowledge the reviewer contributions by Felipe L. Pinheiro (Universidade Federal do Pampa, Bagé, Rio Grande do Sul, Brazil) and Richard Buchmann (Universidade Federal do Espírito Santo, Vitória, Brazil), which have been very helpful for improving the final version of the manuscript. BH thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazil) for his Ph.D. grant (#140789/2016-2). RVP thanks Fundação de Amparo à Pesquisa do Estado de São Paulo (Brazil) for a Ph.D. scholarship (#2019/10231-6).
References
Andres, B.B. 2010. Systematics of the Pterosauria. 366 pp. Ph.D. Thesis, Yale University, New Haven.
Andres, B. and Myers, S.T. 2013. Lone star pterosaurs. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 103 (3–4): 383–398. Crossref
Andres, B., Clark, J., and Xu, X. 2014. The earliest pterodactyloid and the origin of the group. Current Biology 24: 1011–1016.
Barrett, P.M., Butler, R.J., Edwards, N.P., and Milner, A.R. 2008. Pterosaur distribution in time and space: an atlas. Zitteliana B 28: 61–107.
Bakhurina, N.N. and Unwin, D.M. 1995. A survey of pterosaurs from the Jurassic and Cretaceous of the Former Soviet Union and Mongolia. Historical Biology 10: 197–245. Crossref
Bantim, R.A., Saraiva, A.A., Oliveira, G.R., and Sayão, J.M. 2014. A new toothed pterosaur (Pterodactyloidea: Anhangueridae) from the Early Cretaceous Romualdo Formation, NE Brazil. Zootaxa 3869: 201–223. Crossref
Bennett, S.C. 2001. The osteology and functional morphology of the Late Cretaceous pterosaur Pteranodon. Palaeontographica Abteilung A (Paleozoology, Stratigraphy) 260: 1–153.
Campos, D.A. and Kellner, A.W.A. 1985. Panorama of the flying reptiles study in South America. Anais da Academia Brasileira de Ciências 57: 453–466.
Cohen, K.M., Finney, S.M., Gibbard, P.L., and Fan, J.X. 2013. The ICS International Chronostratigraphic Chart. Episodes 36: 199–204. Crossref
Fastnacht, M. 2001. First record of Coloborhynchus (Pterosauria) from the Santana Formation (Lower Cretaceous) of the Chapada do Araripe, Brazil. Paläontologische Zeitschrift 75: 23. Crossref
Fastnacht, M. 2008. Tooth replacement pattern of Coloborhynchus robustus (Pterosauria) from the Lower Cretaceous of Brazil. Journal of Morphology 269: 332–348. Crossref
Frey, E., Martill, D.M., and Buchy, M.C. 2003. A new crested ornithocheirid from the Lower Cretaceous of northeastern Brazil and the unusual death of an unusual pterosaur. In: E. Buffetaut and J.-M. Mazin (eds.), Evolution and Palaeobiology of Pterosaurs. The Geological Society, London, Special Publications 217: 55–63. Crossref
Goloboff, P.A. and Catalano, S.A. 2016. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32: 221–238. Crossref
Holgado, B., Martínez-Pérez, C., and Ruiz-Omeñaca, J.I. 2011. Revisión actualizada del registro fósil de Pterosauria en la Península Ibérica. In: A. Pérez-García, F. Gascó, J.M. Gasulla, and F. Escaso (eds.), Viajando a Mundos Pretéritos, 183–194. Ayuntamiento de Morella, Morella.
Holgado, B., Pêgas, R.V, Canudo, J.I., Fortuny, J., Rodrigues, T., Company, J., and Kellner, A.W.A. 2019. On a new crested pterodactyloid from the Early Cretaceous of the Iberian Peninsula and the radiation of the clade Anhangueria. Scientific Reports 9: 4940. Crossref
Hooley, R.W. 1914. On the ornithosaurian genus Ornithocheirus, with a review of the specimens from the Cambridge Greensand in the Sedgwick Museum, Cambridge. Annals and Magazine of Natural History 78: 529–557. Crossref
Ibrahim, N., Unwin, D.M., Martill, D.M., Baidder, L., and Zouhri, S. 2010. A new pterosaur (Pterodactyloidea: Azhdarchidae) from the Upper Cretaceous of Morocco. PLoS One 5 (5): e10875. Crossref
Jacobs, M.L., Martill, D.M., Ibrahim, N., and Longrich, N. 2019. A new species of Coloborhynchus (Pterosauria, Ornithocheiridae) from the mid-Cretaceous of North Africa. Cretaceous Research 95: 77–88. Crossref
Jacobs, M.L., Martill, D.M., Unwin, D.M., Ibrahim, N., Zouhri, S., and Longrich, N.R. 2020. New toothed pterosaurs (Pterosauria: Ornithocheiridae) from the middle Cretaceous Kem Kem Group of Morocco and implications for pterosaur palaeobiogeography and diversity. Cretaceous Research [published online https://doi.org/10.1016/j.cretres.2020.104413] Crossref
Jiang, S.X., Wang, X.L., Meng, X., and Cheng, X. 2014. A new boreopterid pterosaur from the Lower Cretaceous of western Liaoning, China, with a reassessment of the phylogenetic relationships of the Boreopteridae. Journal of Paleontology 88: 823–828. Crossref
Kaup, J.J. 1834. Versuch einer Eintheilung der Saugethiere in 6 Stämme und der Amphibien in 6 Ordnungen. Isis von Oken 1834: 311–324.
Kellner, A.W.A. 1994. Remarks on pterosaur taphonomy and paleoecology. Acta Geologica Leopoldensia 39: 175–189.
Kellner, A.W.A. 2003. Pterosaur phylogeny and comments on the evolutionary history of the group. In: E. Buffetaut and J.-M. Mazin (eds.), Evolution and Palaeobiology of Pterosaurs. Geological Society, London, Special Publications 217: 105–137. Crossref
Kellner, A.W.A. and Tomida, Y. 2000. Description of a new species of Anhangueridae (Pterodactyloidea) with comments on the pterosaur fauna from the Santana Formation (Aptian–Albian), northeastern Brazil. National Science Museum Monographs 17: 1–135.
Kellner, A.W.A., Caldwell, M.W., Holgado, B., Dalla Vecchia, F.M., Nohra, R., Sayão, J.M., and Currie, P.J. 2019a. First complete pterosaur from the Afro-Arabian continent: insight into pterodactyloid diversity. Scientific Reports 9: 17875. Crossref
Kellner, A.W.A., Campos, D.A., Sayão, J.M., Saraiva, A.A., Rodrigues, T., Oliveira, G., Cruz, L.A., Costa, F.R., Silva, H.P., and Ferreira, J.S. 2013. The largest flying reptile from Gondwana: a new specimen of Tropeognathus cf. T. mesembrinus Wellnhofer, 1987 (Pterodactyloidea, Anhangueridae) and other large pterosaurs from the Romualdo Formation, Lower Cretaceous, Brazil. Anais da Academia Brasileira de Ciências 85: 113–135. Crossref
Kellner, A.W.A., Weinschütz, L.C., Holgado, B., Bantim, R.A.M., and Sayão, J.M. 2019b. A new toothless pterosaur (Pterodactyloidea) from Southern Brazil with insights into the paleoecology of a Cretaceous desert. Anais da Academia Brasileira de Ciências 91 (Supplement 2): e20190768. Crossref
Kuhn, O. 1967. Die fossile Wirbeltierklasse Pterosauria. 52 pp. Verlag Oeben, Krailling bei München.
Lee, Y.N. 1994. The Early Cretaceous pterodactyloid pterosaur Coloborhynchus from North America. Palaeontology 37: 755–763.
Longrich, N.R., Martill, D.M., and Andres, B. 2018. Late Maastrichtian pterosaurs from North Africa and mass extinction of Pterosauria at the Cretaceous–Paleogene boundary. PLoS Biology 16: e2001663. Crossref
Lü, J.C., Ji, S.A., Yuan, C.X., and Ji, Q. 2006. Pterosaurs from China [in Chinese]. 147 pp. Geological Publishing House, Beijing.
Lydekker, R. 1888. Catalogue of Fossil Amphibia and Reptilia in the British Museum (Natural History). Part 1. 309 pp. British Museum (Natural History), London.
Maddison, W.P. and Maddison, D.R. 2018. Mesquite: a Modular System for Evolutionary Analysis, Version 3.51. Available at http://mesquiteproject.org.
Mader, B.J. and Kellner, A.W.A. 1999. A new anhanguerid pterosaur from the Cretaceous of Morocco. Museo Nacional. Geologia, Nova Série 45: 1–11.
Manzig, P.C., Kellner, A.W.A., Weinschütz, L.C., Fragoso, C.E., Vega, C.S., Guimarães, G.B., Godoy, L.C., Liccardo, A., Ricetti, J.H.C., and Moura, C.C. 2014. Discovery of a rare pterosaur bone bed in a Cretaceous Desert with insights on ontogeny and behavior of flying reptiles. PloS One 9(8): e100005. Crossref
Martill, D.M. 2010. The early history of pterosaur discovery in Great Britain. In: R.T.J. Moody, E. Buffetaut, D. Naish, and D.M. Martill (eds.), Dinosaurs and Other Extinct Saurians: a Historical Perspective. Geological Society Special Publications 343: 287–311. Crossref
Martill, D.M. 2015. First occurrence of the pterosaur Coloborhynchus (Pterosauria, Ornithocheiridae) from the Wessex Formation (Lower Cretaceous) of the Isle of Wight, England. Proceedings of the Geologists’ Association 126: 377–380. Crossref
Martill, D.M. and Naish, D. 2006. Cranial crest development in the azhdarchoid pterosaur Tupuxuara, with a review of the genus and tapejarid monophyly. Palaeontology 49: 925–941. Crossref
Martill, D.M. and Unwin, D.M. 2012. The world’s largest toothed pterosaur, NHMUK R481, an incomplete rostrum of Coloborhynchus capito (Seeley, 1870) from the Cambridge Greensand of England. Cretaceous Research 34: 1–9. Crossref
Martin-Silverstone, E., Sykes, D., and Naish, D. 2018. Does postcranial palaeoneurology provide insight into pterosaur behaviour and lifestyle?: New data from the azhdarchoid Vectidraco and the ornithocheirids Coloborhynchus and Anhanguera. Palaeontology 62 (2): 197–210. Crossref
Owen, R. 1859. On the orders of fossil and recent Reptilia, and their distribution through time. Report of the British Association for the Advancement of Science 29: 153–166.
Owen, R. 1861. A Monograph on the Fossil Reptilia of the Cretaceous Formations. Supplement III. 25 pp. Palaeontographical Society, London. Crossref
Owen, R. 1874. The fossil reptilia of the Mesozoic formations. Palaeontographical Society Monographs 5: 1–118. Crossref
Pêgas, R.V., Costa, F.R., and Kellner, A.W.A. 2018. New information on the osteology and a taxonomic revision of the genus Thalassodromeus (Pterodactyloidea, Tapejaridae, Thalassodrominae). Journal of Vertebrate Paleontology 38 (2): e1443273. Crossref
Pêgas, R.V., Holgado, B., and Leal, M.E.C. 2019. Targaryendraco wiedenrothi gen. nov. (Pterodactyloidea, Pteranodontoidea, Lanceodontia) and recognition of a new cosmopolitan lineage of Cretaceous toothed pterodactyloids. Historical Biology: 1–15 [published online, https://doi.org/10.1080/08912963.2019.1690482]. Crossref
Pêgas, R.V., Leal, M.E.C., and Kellner, A.W.A. 2016. A basal Tapejarine (Pterosauria; Pterodactyloidea; Tapejaridae) from the Crato Formation, Early Cretaceous of Brazil. PLoS One 11 (9): e0162692. Crossref
Pentland, A.H., Poropat, S.F., Tischler, T.R., Sloan, T., Elliott, R.A., Elliott, H.A., Elliott, J.A., and Elliott, D.A. 2019. Ferrodraco lentoni gen. et sp. nov., a new ornithocheirid pterosaur from the Winton Formation (Cenomanian–lower Turonian) of Queensland, Australia. Scientific Reports 9 (1): 13454. Crossref
Pinheiro, F.L., Rodrigues, T. 2017. Anhanguera taxonomy revisited: is our understanding of Santana Group pterosaur diversity biased by poor biological and stratigraphic control? PeerJ 5: e3285. Crossref
Plieninger, F. 1901. Beiträge zur Kenntnis der Flugsaurier. Palaeontographica 48: 65–90.
Rodrigues, T. and Kellner, A.W. 2008. Review of the pterodactyloid pterosaur Coloborhynchus. Zitteliana 117: 219–228. Crossref
Rodrigues, T. and Kellner, A.W.A., 2013. Taxonomic review of the Ornithocheirus complex (Pterosauria) from the Cretaceous of England. ZooKeys 308: 1–112. Crossref
Rodrigues, T., Jiang, S., Cheng, X., Wang, X., and Kellner, A.W. 2015. A new toothed pteranodontoid (Pterosauria, Pterodactyloidea) from the Jiufotang Formation (Lower Cretaceous, Aptian) of China and comments on Liaoningopterus gui Wang and Zhou, 2003. Historical Biology 27: 782e795. Crossref
Sayão, J.M., Vila Nova, B.C., and Romano, P.S. 2012. Considerações sobre os padrões de distribuição geográfica dos Pterodactyloidea (Archosauria, Pterosauria). In: V. Gallo, H.M.A. Silva, P.M. Brito, and F.J. Figueiredo (eds.), Paleontologia de Vertebrados: Relaçoes entre América do Sul e África, 281–300. Editora Interciência, Rio de Janeiro.
Seeley, H.G. 1870. The Ornithosauria: an Elementary Study of the Bones of Pterodactyles, Made from Fossil Remains Found in the Cambridge Upper Greensand, and Arranged in the Woodwardian Museum of the University of Cambridge. 135 pp. Bell, Deighton.
Seeley, H.G. 1881. On evidence of two ornithosaurians referable to the genus Ornithocheirus, from the Upper Greensand of Cambridge, preserved in the collection of W. Reed, Esq., F.G.S. Geological Magazine 8: 13–20. Crossref
Seeley, H.G. 1901. Dragons of the Air. An Account of Extinct Flying Reptiles. 239 pp. Methuen and Co., London.
Unwin, D.M. 2001. An overview of the pterosaur assemblage from the Cambridge Greensand (Cretaceous) of Eastern England. Mitteilungen aus dem Museum für Naturkunde in Berlin, Geowissenschaftliche Reihe 4: 189–221. Crossref
Unwin, D.M. 2002. On the systematic relationships of Cearadactylus atrox, an enigmatic Early Cretaceous pterosaur from the Santana Formation of Brazil. Fossil Record 5: 239–263. Crossref
Unwin, D.M. 2003. On the phylogeny and evolutionary history of pterosaurs. In: E. Buffetaut and J.-M. Mazin (eds.), Evolution and Palaeobiology of Pterosaurs. Geological Society, London, Special Publications 217: 139–190. Crossref
Unwin, D.M. and Bakhurina, N.N. 2000. Pterosaurs from Russia, Middle Asia and Mongolia. In: M.J. Benton, M.A. Shishkin, D.M. Unwin, and E.N. Kurochkin (eds.), The Age of Dinosaurs in Russia and Mongolia, 420–433. Cambrigde University Press, Cambridge.
Upchurch, P., Andres, B., Butler, R.J., and Barrett, P.M. 2015. An analysis of pterosaurian biogeography: implications for the evolutionary history and fossil record quality of the first flying vertebrates. Historical Biology 27: 697–717. Crossref
Veldmeijer, A.J. 2003. Description of Coloborhynchus spielbergi sp. nov. (Pterodactyloidea) from the Albian (Lower Cretaceous) of Brazil. Scripta Geologica 125: 35–139.
Veldmeijer, A.J. 2006. Toothed Pterosaurs from the Santana Formation (Cretaceous; Aptian–Albian) of Northeastern Brazil. A Reappraisal on the Basis of Newly Described Material. 269 pp. Ph.D. Thesis, Utrecht University, Utrecht.
Vila Nova, B.C., Sayão, J.M., Neumann, V.H.M.L., and Kellner, A.W.A. 2014. Redescription of Cearadactylus atrox (Pterosauria, Pterodactyloidea) from the Early Cretaceous Romualdo Formation (Santana Group) of the Araripe Basin, Brazil. Journal of Vertebrate Paleontology 34: 126–134. Crossref
Vullo, R., Marugán-Lobón, J., Kellner, A.W.A., Buscalioni, A.D., Gomez, B., de la Fuente, M., and Moratalla, J.J. 2012. A new crested pterosaur from the Early Cretaceous of Spain: the first European tapejarid (Pterodactyloidea: Azhdarchoidea). PLoS ONE 7 (7): e38900. Crossref
Wang, X., Kellner, A.W., Jiang, S., and Cheng, X., 2012. New toothed flying reptile from Asia: close similarities between early Cretaceous pterosaur faunas from China and Brazil. Naturwissenschaften 99: 249–257. Crossref
Wang, X., Kellner, A.W., Jiang, S., Wang, Q., Ma, Y., Paidoula, Y., Cheng, X., Rodrigues, T., Meng, X., and Zhang, J., Li, N. 2014a. Sexually dimorphic tridimensionally preserved pterosaurs and their eggs from China. Current Biology 24: 1323–1330. Crossref
Wang, X., Rodrigues, T., Jiang, S., Cheng, X., and Kellner, A.W.A. 2014b. An Early Cretaceous pterosaur with an unusual mandibular crest from China and a potential novel feeding strategy. Scientific Reports 4: 6329. Crossref
Wellnhofer, P. 1978. Pterosauria. Handbuch der Paläoherpetologie, Teil 19. 82 pp. Gustav Fischer Verlag, Stuttgart.
Wellnhofer, P. 1987. New crested pterosaurs from the Lower Cretaceous of Brazil. Mitteilungen der Bayerischen Staatssammlung für Palaontologie und Historische Geologie 27: 175–186.
Witton, M.P. 2013. Pterosaurs: Natural History, Evolution, Anatomy. 304 pp. Princeton University Press, Princeton. Crossref
Zhou, X., Pêgas, R.V., Leal, M.E.C., and Bonde, N. 2019. Nurhachius luei, a new istiodactylid pterosaur (Pterosauria, Pterodactyloidea) from the Early Cretaceous Jiufotang Formation of Chaoyang City, Liaoning Province (China) and comments on the Istiodactylidae. PeerJ 7: e7688. Crossref
Acta Palaeontol. Pol. 65 (4): 743–761,
2020 https://doi.org/10.4202/app.00751.2020