

A new early Pliocene murine rodent from the Iberian Peninsula and its biostratigraphic implications
PEDRO PIÑERO and DIEGO H. VERZI
Piñero, P. and Verzi, D.H. 2020. A new early Pliocene murine rodent from the Iberian Peninsula and its biostratigraphic implications. Acta Palaeontologica Polonica 65 (4): 719–731.
In the last years, a murine identified as Paraethomys aff. abaigari has been repeatedly recognized in several early Pliocene localities of the Iberian Peninsula. We have revised these occurrences, as well as other samples of similar morphology, and propose that all these records correspond to a new species. We diagnose Paraethomys baeticus sp. nov. based on a large sample from the early Ruscinian site of Baza-1 (Guadix-Baza Basin, Spain). It is a medium-sized representative of the genus, with relatively well-developed longitudinal connections both in upper and lower molars, but incomplete stephanodonty. M1 displays high tubercles t6–t9 and low t4–t8 connections. M1 and, to a lesser extent, M2 have posterior spurs on t1 and t3. The t9 and t12 are absent in M2. In m1, a round islet of enamel between the anteroconid and the protoconid-metaconid is regularly observed, as well as a general lack of medial anteroconid, moderate labial cingulum with large posterior accessory cuspid, and longitudinal spur. The new species differs from other European representatives of the genus Paraethomys mainly in its size, which is intermediate between that of the small-sized Paraethomys meini and the large-sized Paraethomys abaigari. In addition, both the size and relative width are smaller than those of Paraethomys jaegeri. The frequency of posterior spurs on t1 and t3 of M1 and M2 is higher than that of P. meini and lower than that of P. jaegeri. The new species here described represents a key biochronological and biostratigraphic marker, since it is restricted to levels of late early Ruscinian age. Thus, this species is useful for establishing divisions within the early Ruscinian, and becomes a characteristic taxon for the early Pliocene in the terrestrial record of the Iberian Peninsula. Magnetobiostratigraphic correlations suggest a chronological range for P. baeticus sp. nov. between ca. 4.6 and 4.3 Ma.
Key words: Mammalia, Murinae, biochronology, diversification pattern, early Ruscinian, Europe.
Pedro Piñero [ppinero@fcnym.unlp.edu.ar; pinerogarciap@gmail.com] and Diego H. Verzi [dverzi@fcnym.unlp.edu.ar], CONICET, Sección Mastozoología, Museo de La Plata, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Paseo del Bosque s/n, B1900FWA La Plata, Buenos Aires, Argentina.
Received 5 April 2020, accepted 14 August 2020, available online 24 November 2020.
Copyright © 2020 P. Piñero and D.H. Verzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Murine rodents, commonly known as Old World rats and mice, are the most diverse and abundant of modern mammal subfamilies, comprising over 650 species (Denys et al. 2017). The first unambiguous member of the subfamily Murinae belongs to the genus Antemus (Jacobs et al. 1990; Jacobs and Flynn 2005), whose first record is in the Siwalik (Potwar, Pakistan) about 13.8 Ma (Kimura et al. 2013a, b). This group increased its relative abundance from its first appearance, and then it dominated over the cricetids and successfully replaced them in their ecological niches when the stem murine Progonomys emerged about 12 Ma (Jacobs et al. 1989; Lazzari et al. 2010). Because of their diversity, abundance, and rapid evolution, murines represent an essential tool for the description of biochronological units in the late Miocene and Pliocene, and therefore for the dating of continental sediments of this time interval (Van de Weerd 1976; Mein et al. 1990; Fahlbusch 1991; Martín-Suárez and Freudenthal 1998; among others). The high evolutionary rates and rapid diversification of this successful group, its cosmopolitanism and record in a wide variety of environments make it an excellent tool for biochronological correlations. Morphological evolutionary changes among species of murines are frequently reflected in the molars (Lazzari et al. 2008), so they can be traced in the fossil record.
The Paraethomys is based on Paraethomys filfilae Petter, 1968, from the Pleistocene of Jebel Filfila (Algeria). It was a widely distributed murine rodent both in northern Africa and southwestern Europe since the late Miocene. The populations on both shores of the Mediterranean underwent divergent evolutionary processes after their split as early as the end of the Messinian Salinity Crisis (De Bruijn 1973; Jaeger et al. 1975; Coiffait and Coiffait 1981; Aguilar et al. 1984; Geraads 1998; Renaud et al. 1999; among others). The North African lineage is the most diversified and longest-lived, reaching the late middle Pleistocene, whereas the European representatives of the genus became extinct during the late Pliocene (late Ruscinian).
In Europe, and particularly in the Iberian Peninsula, there is some diversification of the Paraethomys. At present, five valid European species, other than the new one here described, are recognized: Paraethomys meini (Michaux, 1969), Paraethomys jaegeri Montenat and De Bruijn, 1976, Paraethomys abaigari Adrover, Mein, and Moissenet, 1988, Paraethomys belmezensis Castillo Ruiz, 1992, and Paraethomys balearicus Torres-Roig, Piñero, Agustí, Bover, and Alcover, 2019. Paraethomys meini is the oldest representative of the genus in Europe and has a long record, reaching the late Ruscinian (early Pliocene). Paraethomys anomalus De Bruijn, Dawson, and Mein, 1970, and Paraethomys miocaenicus Jaeger, Michaux, and Thaler, 1975, have been reported from a number of European localities (De Bruijn et al. 1970, 1975; Mein et al. 1978, 1990; Brandy 1979; Agustí et al. 1985; Antunes and Mein 1989; Rook 1992; Opdyke et al. 1997; Agustí and Llenas 1996; among others). However, these two latter species are considered synonyms of P. meini by several authors (Montenat and De Bruijn 1976; Van de Weerd 1976; Adrover 1986; Martín-Suárez 1988; Freudenthal and Martín-Suárez 1999; Minwer-Barakat et al. 2005, 2009a, b; García-Alix et al. 2008a; Mansino et al. 2016; Piñero et al. 2017a; Piñero and Agustí 2019; among others). Paraethomys meini dispersed into Europe probably from northern Africa during the late Turolian (late Miocene), prior to the Messinian Salinity Crisis, at about 6.2 Ma (Garcés et al. 1998; Agustí et al. 2006; Gibert et al. 2013). Paraethomys became more diversified during the Pliocene, when larger species such as Paraethomys jaegeri, Paraethomys abaigari, Paraethomys belmezensis, and Paraethomys balearicus occurred.
In the last years, a medium-sized murine identified as Paraethomys aff. abaigari has been repeatedly recognized in several early Pliocene localities of the Iberian Peninsula (García-Alix et al. 2008a; Mansino et al. 2013, 2015a, b; Piñero et al. 2017a; Piñero and Agustí 2019). Biometric and morphological features of these collections described through open nomenclature justify the definition of a new species. In addition, other samples attributed to Paraethomys sp. 1, P. cf. meini, P. meini, and P. anomalus (De Bruijn 1974; Ruiz Bustos et al. 1984; Martín-Suárez 1988; Adrover et al. 1993) show characteristics similar to those of P. aff. abaigari, so they should be transferred to the new taxon. These samples, clearly distinct from other murine species, are distributed over a wide geographical range in the Iberian Peninsula, including the basins of Granada (García-Alix et al. 2008a), Guadix-Baza (De Bruijn 1974; Ruiz Bustos et al. 1984; Martín-Suárez 1988; Piñero et al. 2017a), Fortuna (Piñero and Agustí 2019), Alcoy (Mansino et al. 2013, 2015a), Cabriel (Mansino et al. 2015b) and Teruel (Adrover et al. 1993) (Fig. 1). As the novel species is restricted to levels of similar age, it has significant biochronological value. This fact was recognized in previous works when P. aff. abaigari was used to name and define biozones from different Iberian basins (Granada Basin, García-Alix et al. 2008b; Alcoy Basin, Mansino et al. 2017; Guadix-Baza Basin, Piñero et al. 2018).
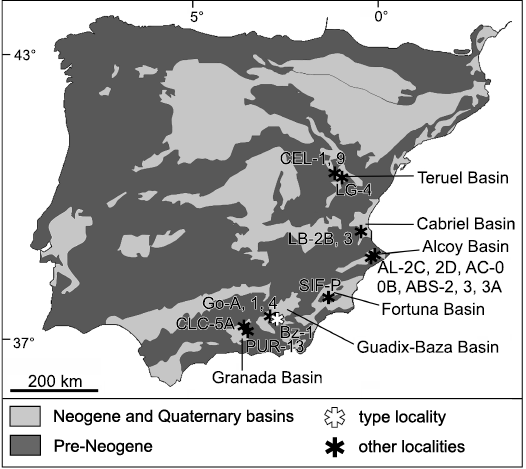
Fig. 1. Simplified geological map of the Iberian Peninsula, indicating the location of the sites where Paraethomys baeticus sp. nov. has been reported. The main Neogene and Quaternary basins referred to in the text are also shown. Abbreviations: ABS, Alcoi Barranc Sud (Mansino et al. 2015a); AC, Alcoi Cristian (Mansino et al. 2015a); AL, Alcoy (Mansino et al. 2013); Bz, Baza (Piñero et al. 2017a); CEL, Celadas (Adrover et al. 1993); CLC, Calicasas (García-Alix et al. 2008a); Go, Gorafe (De Bruijn 1974; Agustí and Martín-Suárez 1984; Ruiz Bustos et al. 1984; Martín-Suárez 1988); LB, La Bullana (Mansino et al. 2015b); LG, La Gloria (Adrover et al. 1993); PUR, Purcal (García-Alix et al. 2008a); SIF-P, Sifón Pista (Piñero and Agustí 2019).
The aim of this paper is to give a clear description of the new species based on the largest and most complete sample from Baza-1 (Piñero et al. 2017a). In addition, we discuss the significance of this new taxon for understanding the biochronology and biostratigraphy of the Pliocene continental deposits from the Iberian Peninsula.
Institutional abbreviations.—IPHES, Institut de Paleoecologia Humana i Evolució Social, Tarragona, Spain; IPS, Institut de Paleontologia de Sabadell (currently named Institut Català de Paleontologia Miquel Crusafont), Barcelona, Spain.
Other abbreviations.—L, length; M1–M3, upper molars; m1–m3, lower molars; t1–t12, numbered tubercles from 1 to 12; tma, medial anteroconid; W, width.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:act:469E1CBA-4E2F-45BD-8900-0A1F804C0F5A
Material and methods
The sample from Baza-1 assigned to Paraethomys aff. abaigari by Piñero et al. (2017a) comprised 155 isolated molars. New provisions of sediment from Baza-1 have yielded 19 unpublished specimens attributable to that same taxon. Both samples are used here to characterize Paraethomys baeticus sp. nov., comprising a total of 174 isolated teeth (minimum number of individuals = 24). Fossils are owned by the Junta de Andalucía and will be housed in the Museo Arqueológico de Granada, but currently part of the collection (122 specimens) is stored at the Institut de Paleoecologia Humana i Evolució Social (IPHES; Tarragona, Spain), whereas the other part (52 specimens) is kept at the Institut Català de Paleontologia Miquel Crusafont (ICP; Sabadell, Barcelona, Spain). The dental nomenclature and measuring methods for the studied material follow Van de Weerd (1976) and Martín-Suárez and Freudenthal (1993), respectively (see Fig. 2). Homologies of “medial anteroconid”, “posterior cingulum”, and “labial anteroconid” (in m2 and m3) follow López-Antoñanzas et al. (2019). Measurements of previously published material have been taken again using the same devise as for the novel material. All the measurements are expressed in millimetres and were taken with the software DinoCapture 2.0, using photographs from the Digital Microscope AM4115TL Dino-Lite Edge. Measurements of each specimen are listed in SOM 1: table S1 (Supplementary Online Material available at http://app.pan.pl/SOM/app65-Pinero_Verzi_SOM.pdf). The illustrations of fossils were made by means of micrographs taken with the environmental scanning electron microscopy (ESEM) of the Servei de Recursos Científics i Tècnics de la Universitat Rovira i Virgili (Tarragona, Spain). Additional archives of digital photographs are included in SOM 2: figs. S1, S2.
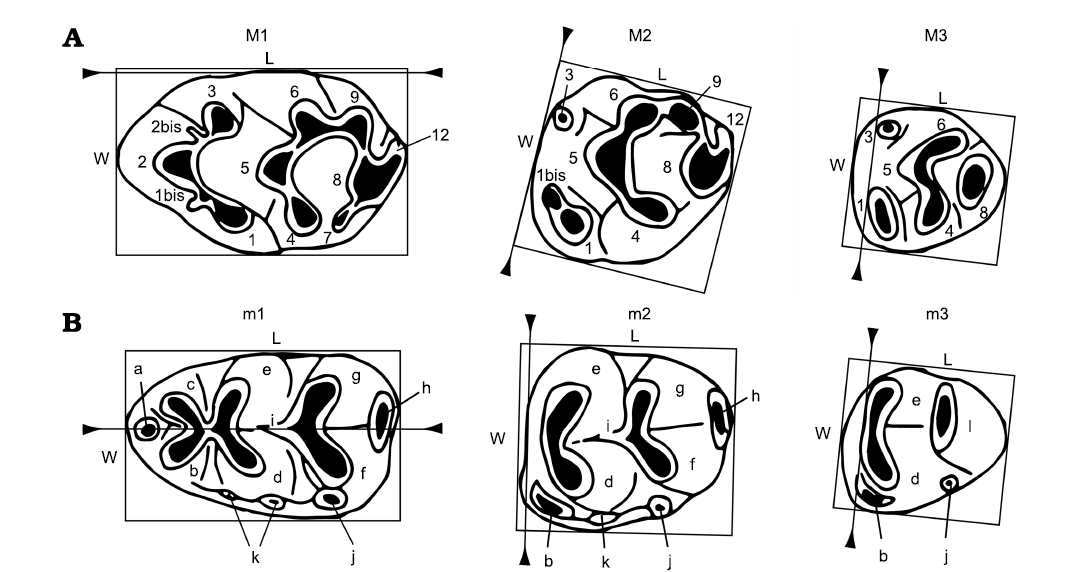
Fig. 2. Nomenclature used in the descriptions of dental elements (modified from Van de Weerd 1976; López-Antoñanzas et al. 2019) and measuring methods (Martín-Suárez and Freudenthal 1993). Length (L) and width (W) are measured as the smallest circumscribed rectangle with sides parallel and perpendicular to the reference line (indicated by triangles). A. Upper dentition (M1–M3): 1–12, tubercle (1–12); 1, anterostyle; 2, lingual anterocone; 1bis, 2bis, accessory cusps; 3, labial anterocone; 4, enterostyle; 5, protocone; 6, paracone; 7, posterostyle; 8, hypocone; 9, metacone; 12, posterior cingulum. B. Lower dentition (m1–m3): a, medial anteroconid (= tma); b, labial anteroconid; c, lingual anteroconid; d, protoconid; e, metaconid; f, hypoconid; g, entoconid; h, posterior cingulum; i, longitudinal spur; j, posterior accessory cuspid; k, accessory cuspids on the labial cingulum; l, posterior complex.
Systematic palaeontology
Order Rodentia Bowdich, 1821
Family Muridae Illiger, 1811
Subfamily Murinae Illiger, 1811
Genus Paraethomys Petter, 1968
Type species: Paraethomys filfilae Petter, 1968; Late Pleistocene, Algeria.
Paraethomys baeticus sp. nov.
Fig. 3; SOM 2: figs. S1, S2.
ZooBank LSID: urn:lsid:zoobank.org:act:469E1CBA-4E2F-45BD-89 00-0A1F804C0F5A
1974 Paraethomys sp. 1; De Bruijn 1974: 438: pl. 2: 7; text-figs. 1, 3.
1984 Paraethomys meini (Michaux, 1969); Agustí and Martín-Suárez 1984: 277.
1984 Paraethomys cf. meini (Michaux, 1969); Ruiz Bustos et al. 1984: 236, pl. 2: 7, 8.
1988 Paraethomys meini (Michaux, 1969); Martín-Suárez 1988: 80, pl. 1: 14.
1993 Paraethomys anomalus Bruijn, Dawson, and Mein, 1970; Adrover et al. 1993: 74, pl. 11: 1–5.
2008 Paraethomys aff. abaigari Adrover, Mein, and Moissenet, 1988; García-Alix et al. 2008a: 195, pl. 3: AE–AJ.
2013 Paraethomys aff. abaigari Adrover, Mein, and Moissenet, 1988; Mansino et al. 2013: 271, pl. 3: 18–23.
2015 Paraethomys aff. abaigari Adrover, Mein, and Moissenet, 1988; Mansino et al. 2015a: 560, pl. 3: E–I.
2015 Paraethomys aff. abaigari Adrover, Mein, and Moissenet, 1988; Mansino et al. 2015b: 280, pl. 3: 18–22.
2017 Paraethomys aff. abaigari Adrover, Mein, and Moissenet, 1988; Piñero et al. 2017a: e1338294-9, pl. 5: M–R.
2019 Paraethomys aff. abaigari Adrover, Mein, and Moissenet, 1988; Piñero and Agustí 2019: 308, pl. 11: I–M, pl. 12: F, M.
Etymology: From Latin masculine adjective baeticus [ba.e’ti.cus], in reference to the Roman province Baetica, partially corresponding to modern Andalusia, the place of the type locality.
Type material: Holotype: BA1-2001-R7/2, isolated left M1. Paratypes: 25 M1 (BA1-2001-R7/1, BA1-2001-R7/3–16, BA1-2018-R1/1–5, IPS 89269, IPS89270, IPS89272, IPS89273, IPS89274), 28 M2 (BA1-2001-R7/17–31, BA1-2018-R1/6, IPS89275–89286), 12 M3 (BA1-2001-R8/17–20, BA1-2018-R1/8, IPHES-Bz-1-R8/22, IPS89287, IPS89288, IPS89292–89295), 37 m1 (BA1-2001-R7/32–54, BA1-2018-R1/9–12, IPS8297–82306), 44 m2 (BA1-2001-R7/55–81, BA1-2018-R1/13–16, BA1-2018-R1/18, IPS89308–89319), 27 m3 (BA1-2001-R8/1–16, BA1-2001-R8/21, BA1-2018-R1/19–21, IPS89320, IPS89323, IPS89325–89329). All from type locality and horizon.
Type locality: Baza-1 (37º29’00”N and 2º47’05”W), Guadix-Baza Basin, Granada Province, Andalusia, Spain (Piñero et al. 2017a; Ros-Montoya et al. 2017).
Type horizon: Paraethomys baeticus Zone (previously referred as Paraethomys aff. abaigari Zone by Piñero et al. 2018), 4.6–4.3 Ma, Baza Formation, early Ruscinian, early Pliocene.
Diagnosis.—Medium-sized species of Paraethomys with marked longitudinal connections both in upper and lower molars, but incomplete stephanodonty. Teeth intermediate in size between the small-sized Paraethomys meini and the large-sized Paraethomys abaigari and Paraethomys jaegeri. M1 and, to a lesser extent, M2 with posterior spurs on t1 and t3 directed towards the t4–t5 and t5–t6 intersections, respectively. M1 with high t6–t9 and low t4–t8 connections and small t12. t9 and t12 absent in M2. Regular presence of a funnel between the anteroconid and the protoconid-metaconid in m1, as well as absent or greatly reduced tma, moderate labial cingulum with large posterior accessory cuspid, and longitudinal spur.
Paraethomys baeticus sp. nov. differs from Paraethomys meini in: larger size (Fig. 4); M1 with higher frequency of distal spurs on t1 and t3, somewhat stronger connection between t6 and t9, and mostly smaller t12; complete absence of t9 on M2; higher frequency of occurrence of longitudinal spur on m1. Differences with Paraethomys abaigari are mainly biometrical, P. baeticus sp. nov. being on average smaller than P. abaigari (Fig. 4). Distinct from Paraethomys jaegeri in: smaller size (Fig. 4); molars relatively narrower; M1 and M2 with less developed distal spurs on t1 and t3. Distinguishable from Paraethomys belmezensis in: better developed connections among cusps in the molars; M1 with t6 connected to the t9; larger t3 on M2; m1 with absent or greatly reduced tma. Differing from Paraethomys balearicus in: lower-crowned molars; complete absence of t9, and t1 and t3 never connected on M2; complete absence of a funnel between the posterior accessory cuspid and hypoconid, and lower accessory labial cuspids on m1. Distinct from Paraethomys lissasfensis Geraads, 1998 in: larger size; M1 with high t2–t3 connection.
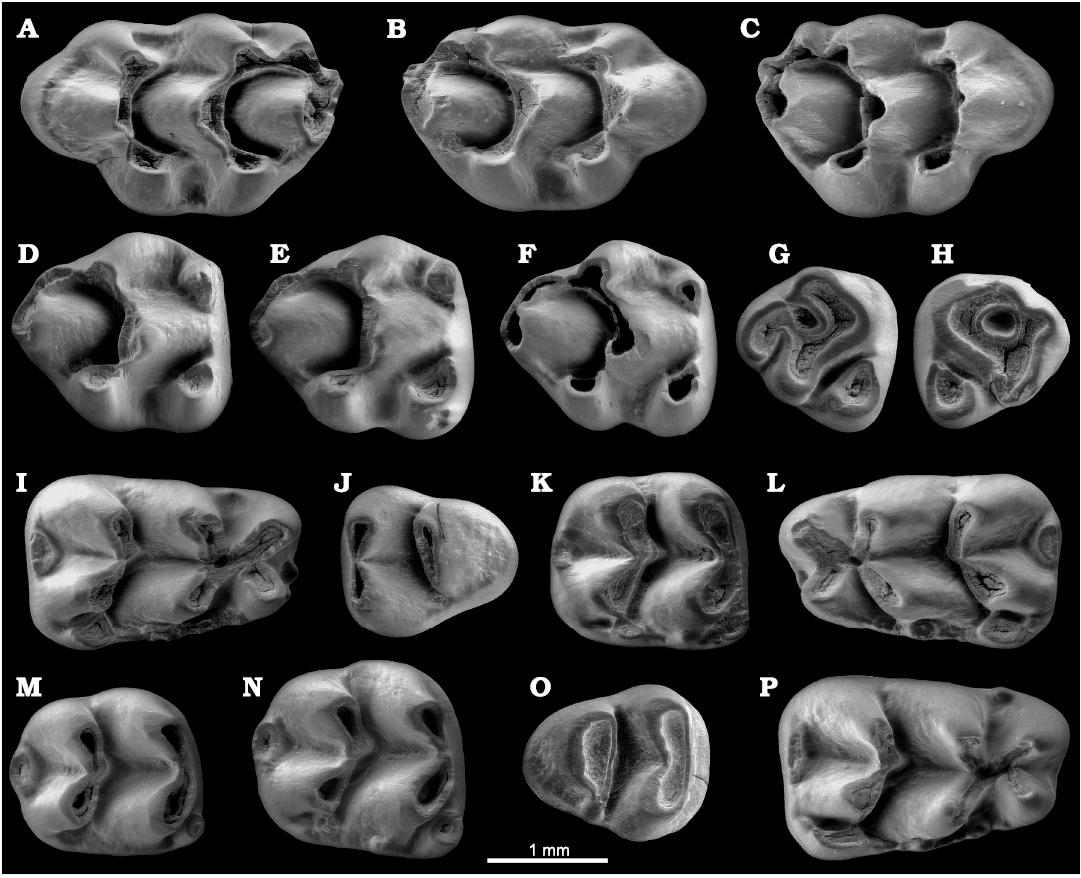
Fig. 3. Upper and lower dentition of the murid rodent Paraethomys baeticus sp. nov. from Baza-1, early Pliocene, Guadix-Baza Basin, Spain, in occlusal view. A. BA1-2001-R7/2, left M1 (holotype). B. BA1-2001-R7/5, right M1. C. BA1-2001-R7/16, right M1. D. BA1-2001-R7/22, right M2. E. BA1-2001-R7/24, right M2. F. BA1-2001-R7/25, right M2. G. BA1-2001-R8/20, right M3. H. BA1-2001-R8/17, left M3. I. BA1-2001-R7/32, right m1. J. BA1-2001-R8/12, left m3. K. BA1-2001-R7/62, right m2. L. BA1-2001-R7/43, left m1. M. BA1-2001-R7/73, right m2. N. BA1-2001-R7/78, right m2. O. BA1-2001-R8/3, right m3. P. BA1-2001-R7/48, right m1.
Measurements.—See Table 1 and SOM 1: table S1.
Table 1. Measurements (in mm) of the teeth of Paraethomys baeticus sp. nov. from Baza-1,
early Pliocene, Guadix-Baza Basin, Spain.
σ, standard deviation.
|
Element |
Length |
|
Width |
|
||||||
|
N |
min |
mean |
max |
σ |
N |
min |
mean |
max |
σ |
|
|
M1 |
19 |
2.47 |
2.61 |
2.76 |
0.086 |
21 |
1.68 |
1.76 |
1.84 |
0.049 |
|
M2 |
26 |
1.79 |
1.92 |
2.03 |
0.052 |
28 |
1.65 |
1.73 |
1.85 |
0.043 |
|
M3 |
10 |
1.25 |
1.32 |
1.42 |
0.056 |
12 |
1.18 |
1.28 |
1.40 |
0.060 |
|
m1 |
33 |
2.24 |
2.40 |
2.57 |
0.079 |
34 |
1.38 |
1.53 |
1.69 |
0.069 |
|
m2 |
39 |
1.64 |
1.74 |
1.86 |
0.057 |
42 |
1.44 |
1.57 |
1.68 |
0.069 |
|
m3 |
26 |
1.39 |
1.50 |
1.63 |
0.053 |
26 |
1.21 |
1.33 |
1.43 |
0.047 |
Description.—Paraethomys baeticus sp. nov. from Baza-1 was first described in detail by Piñero et al. (2017a), who referred it as Paraethomys aff. abaigari. Subsequent sampling in that site has made it possible to enlarge the original sample attributable to that species. On the basis of the original and new teeth we emend the description as follows:
The M1 (n = 26) is antero-posteriorly elongate, wider posteriorly than anteriorly. The t1 is somewhat displaced backwards with respect to the t3. The union between t2 and t3 is higher than the union between t1 and t2. There is neither a t1bis nor a t2bis. A round and low extra cusp between the t2 and t3 is present in one specimen (BA1-2001-R7/15; SOM 2: fig. S1D). All the teeth have a distal spur on the t1 connected basally to the t4–t5 intersection. A distal spur on t3 directed to the t5–t6 intersection is also present. It lies on the valley between the t5 and t6 in eight out of 25 cases in which this part of the tooth is well preserved (BA1-2001-R7/1, BA1-2001-R7/5, BA1-2001-R7/11, BA1-2001-R7/15, BA1-2018-R1/1, BA1-2018-R1/2, IPS89272, IPS89273; Fig. 3B; SOM 2: fig. S1D, E, J, K, L). The t4–t6, t9, t8 are united, the connection between t4 and t8 being much lower than the rest. The t6–t9 connection is high. There is a small or medium-sized t12 in all the specimens. There are three major roots (one anterior, one posterior and one lingual).
The M2 (n = 28) has a nearly rounded occlusal outline, expanded anteriorly and narrower and rounder posteriorly. It has a large, oval or comma-shaped t1. A low posterior spur on the t1 directed towards the t4–t5 intersection is present in 20 out of 28 specimens (Fig. 3E), and absent in the remaining eight (BA1-2001-R7/19, BA1-2001-R7/21, BA1-2001-R7/22, BA1-2001-R7/25, BA1-2001-R7/26, IPS89278, IPS89280, IPS89285; Fig. 3D, F). The small, isolated t3 can be rounded or comma-shaped. It develops a reduced distal spur directed to the t5–t6 intersection in seven out of 28 individuals (BA1-2001-R7/20, BA1-2001-R7/25, BA1-2001-R7/30, BA1-2018-R1/6, IPS89276, IPS89279, IPS89284; see Fig. 3F; SOM 2: fig. S1R, T, Y). The t9 is reduced to a crest connecting t6 and t8. It is slightly inflated only in 14% of specimens (BA1-2001-R7/21, BA1-2001-R7/22, IPS89276, IPS89281; see Fig. 3D; SOM 2: fig. S1U, V). The t4–t5 connection is weak and low. The t4 is connected basally to t8. The t12 is absent. There are three roots (anterior, posterior, and lingual).
In occlusal view, the M3 (n = 12) is subtriangular. The large t1 is isolated. The t3 is absent. The t4–t6, t8 are connected forming a central depression (see Fig. 3G, H). This depression is generally closed, but it is open lingually between t4 and t8 in four specimens (BA1-2001-R8/22, BA1-2018-R1/8, IPS89292, IPS89293; SOM 2: fig. S1AB, AD), and open labially between t6 and t8 in three other teeth (BA1-2001-R8/20, IPS89287, IPS89295; see Fig. 3G; SOM 2: fig. S1AC, AF). Three roots are present (anterior, posterior, and lingual).
The m1 (n = 37) is broader posteriorly than anteriorly. The lingual anteroconid is slightly more anteriorly extended than the labial anteroconid. The protoconid and hypoconid are somewhat displaced posteriorly with respect to the metaconid and entoconid. The tma is absent in 29 specimens (Fig. 3L, P), and greatly reduced and low in the remaining six (BA1-2001-R7/32, BA1-2001-R7/36, BA1-2001-R7/38, BA1-2001-R7/46, BA1-2018-R1/12, IPS89304; see Fig. 3I and SOM 2: fig. S2B). A round islet of enamel between the anteroconid and the protoconid-metaconid complexes is present in 50% of specimens (Fig. 3I, L, P). There is a longitudinal spur (not reaching the protoconid-metaconid intersection) in 22 out of 34 individuals (Fig. 3I, P). As wear advances, the longitudinal spur is usually more pronounced (SOM 2: fig. S2A). The labial cingulum is moderately developed. The large, subtriangular posterior accessory cuspid is connected to the labial face of the hypoconid by a low crest in all but two specimens (BA1-2001-R7/47, BA1-2001-R7/51; SOM 2: fig. S2D). There is occasionally another spur in contact with the posterior face of the hypoconid (Fig. 3P). There are up to three other accessory labial cuspids (see SOM 2: fig. S2I). The medium-sized posterior cingulum can be oval (Fig. 3L) or compressed (Fig. 3P). It is transversally elongated and displaced lingually. Two well-developed roots (anterior and posterior) are present.
The m2 (n = 44) displays a rather square occlusal outline, somewhat wider anteriorly than posteriorly. The moderate-sized labial anteroconid can be round or oval, and is connected basally to the weak or moderate labial cingulum. There is a small posterior accessory cuspid in 23 out of 41 specimens preserving this part (Fig. 3K, N). It can be oval (SOM 2: fig. S2R, T), round (Fig. 3N) or subtriangular (Fig. 3K; SOM 2: fig. S2O) and is connected to the hypoconid in most cases. A longitudinal spur not reaching the protoconid-metaconid intersection is discernible in 49% of specimens (Fig. 3K); it is more pronounced as wear advances. The variable posterior cingulum is displaced lingually. It can be small or large, and oval (Fig. 3M, N) or compressed (Fig. 3K; SOM 2: fig. S2O). There are two roots (anterior and posterior).
The m3 (n = 27) has a subtriangular occlusal outline. The labial anteroconid is absent (except for BA1-2001-R8/11, in which it is very low and reduced; SOM 2: fig. S2AE). The posterior complex (hypoconid-entoconid pair) is separated from the protoconid-metaconid pair. The labial cingulum is weak or absent. A much reduced posterior accessory cuspid attached to the labial side of the posterior complex (hypoconid) is present in eight out of 27 specimens (BA1-2001-R8/4, BA1-2001-R8/10, BA1-2001-R8/11, BA1-2001-R8/12, BA1-2018-R1/20, IPS89326, IPS89327, IPS89328; see Fig. 3O; SOM 2: fig. S2Z, AB, AH). Two roots (anterior and posterior) are present.
Stratigraphic and geographic range.—Early Pliocene (MN14) of the Iberian Peninsula: Baza-1, Gorafe-1, Gorafe-A, Gorafe-4 (Guadix-Baza Basin); Purcal-13, Calicasas-5A (Granada Basin); Alcoy-2C, Alcoy-2D, Alcoi Barranc Sud-2, Alcoi Barranc Sud-3, Alcoi Barranc Sud-3A, Alcoi Cristian-0, Alcoi Cristian-0B (Alcoy Basin); Sifón-P (Fortuna Basin); La Bullana-2B, La Bullana-3 (Cabriel Basin); Celadas-1, Celadas-9, La Gloria-4 (Teruel Basin).
Discussion
The material from Baza-1 displays the common traits of the genus Paraethomys: union between t6 and t9 in M1, reduced or absent t9 in M2, general absence of tma in m1, weak or moderate labial cingulum in the lower molars, common presence of a small funnel between the anteroconid complex and the protoconid-metaconid pair in m1, and reduced or absent labial anteroconid in m3.
De Bruijn (1974) recognized for the first time two European Paraethomys forms larger than Paraethomys anomalus (= P. meini), although the author did not define any new species and referred to them as Paraethomys sp. 1 and Paraethomys sp. 2. The latter, found in Gorafe-2 (Guadix-Baza Basin), represented the largest form and was defined two years later as P. jaegeri (see Montenat and De Bruijn 1976). However, Paraethomys sp. 1 from Gorafe-1, intermediate in size between P. meini and P. jaegeri, was not formally defined. Although the measurements were greater, Agustí and Martín-Suárez (1984) assigned the material from Gorafe-1 to P. meini. Years later, Adrover et al. (1988) erected P. abaigari from Villalba Alta Río (Teruel Basin), a species smaller than P. jaegeri and larger than P. meini. Paraethomys sp. 1 from Gorafe-1 shows features close to those of P. abaigari from its type locality, but the teeth are slightly smaller. Similarly, the large-sized Paraethomys from Purcal-13, Calicasas-5A (García-Alix et al. 2008a), Alcoy-2C, Alcoy-2D (Mansino et al. 2013), Alcoi Barranc Sud-2, 3, 3A, Alcoi Cristian-0, 0B (Mansino et al. 2015a), La Bullana-2B and 3 (Mansino et al. 2015b), Baza-1 (Piñero et al. 2017a) and Sifón-P (Piñero and Agustí 2019) share their main characteristics with P. abaigari from Villalba Alta Río (Adrover et al. 1988): presence of distal spurs on t1 and t3, large t1 with posterior spur in M2, presence of longitudinal spur in m1, moderate labial cingulum, large posterior accessory cuspid and moderate posterior cingulum in m1. However, these samples differ from P. abaigari in their size, being slightly smaller than the material from Villalba Alta Río, La Judería and Celadas 6 (Adrover et al. 1988; Mein et al. 1990). As a result, all these collections were determined as P. aff. abaigari in their respective works (García-Alix et al. 2008a; Mansino et al. 2013, 2015a, b; Piñero et al. 2017a; Piñero and Agustí 2019). Based on morphological similarities, García-Alix et al. (2008a) considered that the material from La Gloria 4 and Celadas 9, originally ascribed to P. anomalus by Adrover et al. (1993), also belonged to P. aff. abaigari. All mentioned collections determined as P. aff. abaigari are now ascribed to P. baeticus sp. nov. Paraethomys anomalus from Celadas-1 (Adrover et al. 1993), P. cf. meini from Gorafe-A (Ruiz Bustos et al. 1984) and P. meini from Gorafe-4 (Martín-Suárez 1988) are morphologically very similar to the sample from Baza-1, their size being consistent with P. baeticus sp. nov. rather than with P. meini.
All samples transferred to P. baeticus sp. nov. agree in size with that from the type locality, Baza-1 (see SOM 1: table S2). The same features as those from Baza-1 are observed in the specimens from Celadas-9, La Gloria-4 (Adrover et al. 1993), Gorafe-A (Ruiz-Bustos et al. 1984), Gorafe-1 (De Bruijn 1974), Gorafe-4 (Martín-Suárez 1988), Alcoy-2B, Alcoy-2C (Mansino et al. 2013), Alcoi Barranc Sud-2, 3, 3A, Alcoi Cristian-0, 0B (Mansino et al. 2015a), La Bullana-3 and La Bullana-2B (except for the presence of a reduced t12 in two M2) (Mansino et al. 2015b). When compared in detail, the teeth from Baza-1 and those from Purcal-13 (García-Alix et al. 2008a) show certain morphological differences: M1 and M2 from Purcal-13 show a lesser development of distal spurs on t1 and t3, and M2 presents a reduced t12 which is absent in M2 from Baza-1. We compared directly the collection from Baza-1 with that from Sifón-P (currently stored at ICP), and found that the specimens from Sifón-P exhibit some traits that can be considered more archaic: moderately lower frequency of distal spurs on t1 and t3, and slightly larger t12 in M1, weaker union between the anteroconid complex and the protoconid-metaconid pair in m1, and better-developed labial cingulum in m3. Moreover, the specimens from Baza-1 are a bit larger, which is coherent with the slightly older age of Sifón-P (Piñero and Agustí 2019).
Morphological features of P. baeticus sp. nov. are close to those of P. abaigari from Villalba Alta Río (see above). Regarding size, the maximum values of P. baeticus sp. nov. from Baza-1 generally match with the minimum values of P. abaigari from Villalba Alta Río and La Judería (Adrover et al. 1988) (Fig. 4). However, these collections cannot be ascribed to a single taxon as the overlap is only partial in M2 and the lower teeth. There is no overlap in the measurements of M1 and M3. The average size is smaller in P. baeticus sp. nov. than in P. abaigari for all the teeth, but differences are especially marked in the case of the M1 (Fig. 4). The slight overlap in size and similarities in morphology can be explained by the probable ancestor-descendant relationship between both species.
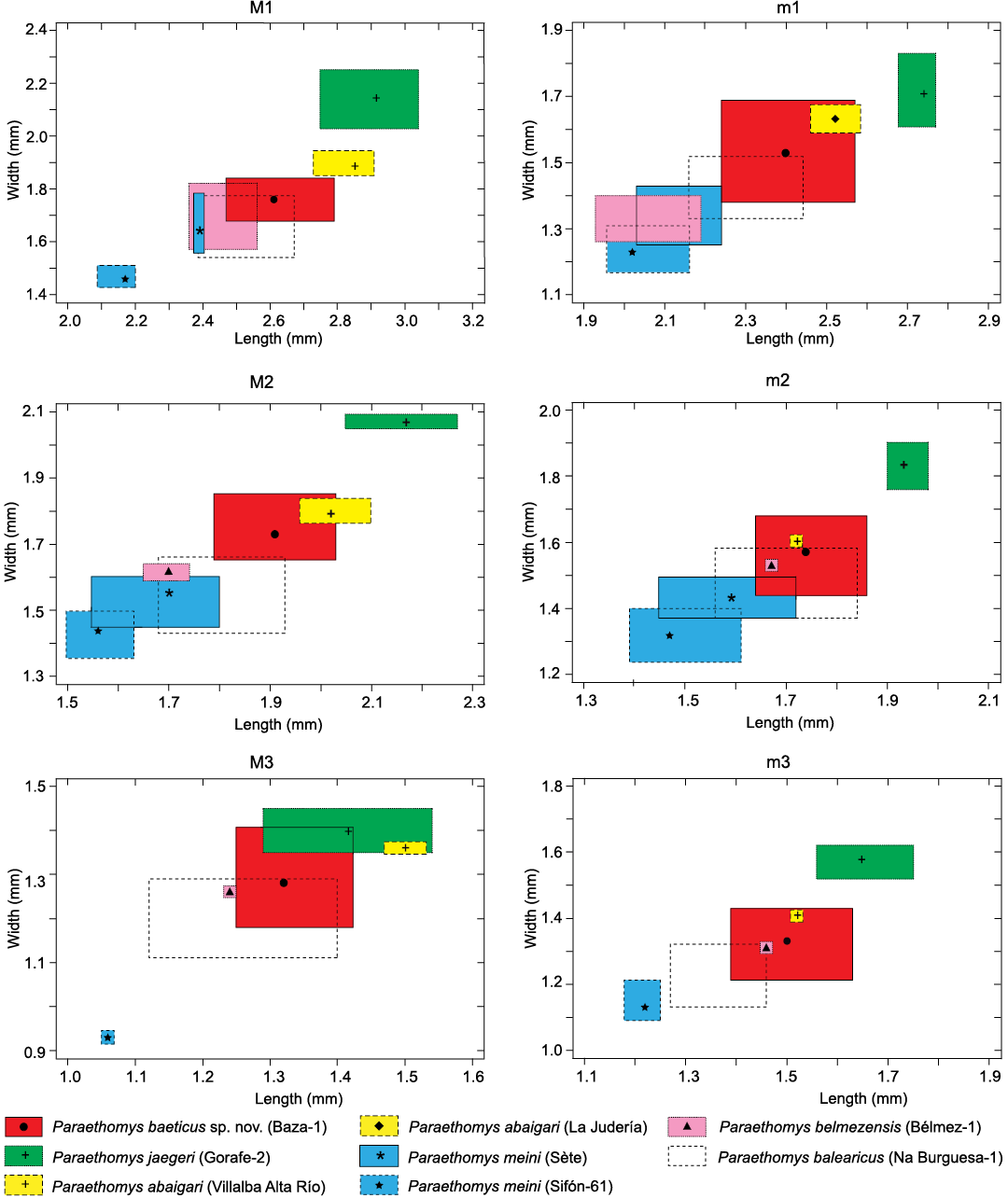
Fig. 4. Ranges of size variation (in mm) in the upper (M1–M3) and lower (m1–m3) teeth of Paraethomys baeticus sp. nov. from Baza-1 (type locality; this paper), Paraethomys jaegeri from Gorafe-2 (type locality; Montenat and De Bruijn 1976), Paraethomys abaigari from Villalba Alta Río (type locality) and La Judería (Adrover et al. 1988), Paraethomys meini from Sète (type locality; Michaux 1969; Adrover 1986) and Sifón-61 (Piñero and Agustí 2019), Paraethomys belmezensis from Bélmez-1 (type locality; Castillo Ruiz 1992), and Paraethomys balearicus from Na Burguesa-1 (type locality; Torres-Roig et al. 2019). Mean sizes are marked by symbols inside the boxes.
The association of a large-sized lineage of Paraethomys (P. baeticus sp. nov.–P. abaigari–P. jaegeri) with a small-sized one (P. meini) has been reported from a number of Pliocene localities in southwestern Europe (Fig. 5). Paraethomys meini and P. baeticus sp. nov. coexist at Baza-1 (Guadix-Baza Basin; Piñero et al. 2017a), Celadas-1 and 9, La Gloria-4 (Teruel Basin; Adrover et al. 1993), Purcal-13, Calicasas-5A (Granada Basin; García-Alix et al. 2008a), Alcoy-2C and 2B (Alcoy Basin; Mansino et al. 2013), La Bullana-2B (Cabriel Basin; Mansino et al. 2015b), Alcoi Barranc Sud-2, 3, 3A, Alcoi Cristian-0, 0B (Alcoy Basin; Mansino et al. 2015a), and Gorafe-1 (Guadix-Baza Basin; De Bruijn 1974; this work). The coexistence of P. abaigari and P. meini has been identified in Villalba Alta Río (Teruel Basin; Adrover et al. 1988). Paraethomys meini (or Paraethomys cf. meini) co-occurs with P. jaegeri in Villalba Alta 1 (Teruel Basin; Adrover et al. 1988), Lomas de Casares (Teruel Basin; Mein et al. 1990), La Juliana (Murcia, Spain; Montenat and De Bruijn 1976), Asta Regia 3 (Jerez Basin; Castillo and Agustí 1996), Sète (Hérault, France; Michaux 1969; Adrover 1986), Mont-Hélène (eastern Pyrenees, France; Aguilar et al. 1986), Lo Fournas 13 (southern France; Bachelet 1990), and Perpignan (southern France; Adrover 1986; Bachelet 1990).
Mein et al. (1990) noted that the two abovementioned lineages of Paraethomys tend to increase in size through time. Further observations showed that there is a much more pronounced size increase along the line P. baeticus sp. nov.–P. abaigari–P. jaegeri than in that of P. meini (Adrover et al. 1993; García-Alix et al. 2008a; Mansino et al. 2016; Piñero and Agustí 2019). The origin of the large-sized lineage has been discussed in previous works. According to García-Alix et al. (2008a), P. abaigari could evolve from P. meini or may be an inmigrant taxon. Piñero and Agustí (2019) proposed that P. baeticus sp. nov. (referred to therein as P. aff. abaigari) originated from P. meini as the result of an evolutionary process marked by a size increase and better development of distal spurs on t1 and t3 in the upper teeth, occurring during the early Pliocene.
The coexistence of P. meini and its descendant lineage can be explained as the result of a branching speciation event that occurred via asymmetric speciation (budding speciation), where an ancestral species gives rise to a new species and both persist after the speciation event (Foote 1996; Stadler et al. 2018: fig. 1ii). This cladogenetic event would have presumably given rise to P. baeticus sp. nov., which after branching off from the P. meini lineage experienced progressive increase in size and in the development of longitudinal connections along the Pliocene, leading to P. abaigari and then to P. jaegeri through an anagenetic evolutionary process.
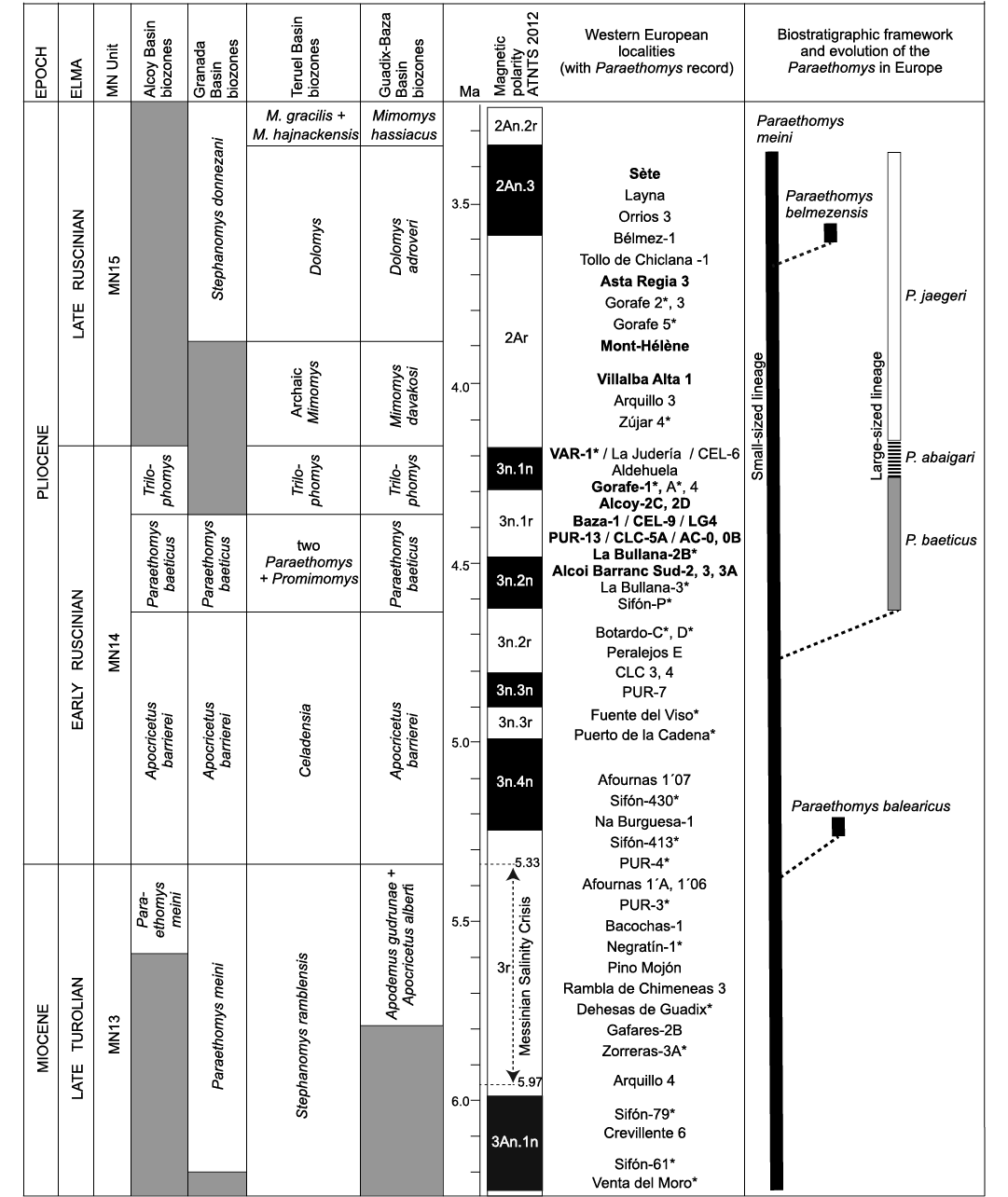
Fig. 5. Biostratigraphic distribution and evolution of the European Paraethomys species, showing their chronological ranges. The localities marked with an asterisk are calibrated with paleomagnetic data (Opdyke et al. 1997; Martín-Suárez et al. 1998, 2000; Oms et al. 1999; Gibert et al. 2013; Garcés et al. 1998, 2001; Hüsing et al. 2010; Mansino et al. 2015b; Piñero et al. 2017b, 2018). The sites with two species of Paraethomys in coexistence are in bold. Abbreviations: AC, Alcoi Cristian; ATNTS, Astronomically Tuned Neogene Time Scale; CEL, Celadas; CLC, Calicasas ELMA, European Land Mammal Ages; LG, La Gloria (Adrover et al. 1993); M., Mimomys; MN, Mammal Neogene; P., Paraethomys; PUR, Purcal; VAR, Villalba Alta Río.
Biochronology and biostratigraphy
The first recorded representative of the genus Paraethomys in Europe is P. meini, whose stratigraphic range extends from the late Turolian (MN13 unit, late Miocene) to the late Ruscinian (MN15 unit, Pliocene). The first occurrence of this species in Europe is recorded in the Iberian basins of Fortuna (Sifón-61; Agustí et al. 2006; Piñero and Agustí 2019) and Cabriel (Venta del Moro; Mansino et al. 2016) at about 6.2 Ma, prior to the Messinian Salinity Crisis (Garcés et al. 1998, 2001; Gibert et al. 2013) (Fig. 5). The youngest samples of P. meini are found in deposits of late Ruscinian age from Spain and France such as Asta Regia 3 (Jerez Basin, Spain; Castillo and Agustí 1996), Tollo de Chiclana 1 (Guadix Basin, Spain; Minwer-Barakat et al. 2005), Layna and Orrios 3 (Teruel Basin, Spain; Adrover 1986), Mont-Hélène (eastern Pyrenees, France; Aguilar et al. 1986) and Sète (Hérault, France; Adrover 1986).
The other European members of the genus Paraethomys have been recorded from Pliocene strata. Paraethomys balearicus is an endemic species from the earliest Pliocene of Mallorca (Balearic Islands) related to the latest Miocene populations of P. meini (see Torres-Roig et al. 2019). Paraethomys meini probably dispersed into Mallorca from eastern Iberia during the peak of the Mediterranean desiccation. After being isolated due to the refilling of the Mediterranean Sea at 5.33 Ma, the mainland ancestor started to evolve under insular conditions, resulting in P. balearicus. So far it has only been identified in its type locality (Na Burguesa-1), suggesting that this species became extinct soon after its arrival on the island (Torres-Roig et al. 2019).
Paraethomys belmezensis is only known from the Pliocene fissure filling of Bélmez-1 (late Ruscinian, MN15) in Córdoba (southern Spain) (Castillo Ruiz 1992). This species is related to P. meini (Castillo Ruiz 1992), and probably represents an endemic divergence from this latter taxon.
Paraethomys baeticus sp. nov. is usually associated to taxa considered to be typical of the early Ruscinian continental stage (MN14), such as the cricetid Apocricetus barrierei, and the murids Stephanomys cordii and Castillomys gracilis (Mein 1975; Fejfar and Heinrich 1990; García-Alix et al. 2008b; Minwer-Barakat et al. 2012; Piñero and Agustí 2019). The oldest known evidences of P. baeticus sp. nov. are those of the levels of Sifón-P (Piñero and Agustí 2019) and La Bullana-3 (Mansino et al. 2015b), in the later part of the early Ruscinian. This species has an exclusively MN14 distribution (Adrover et al. 1993; García-Alix et al. 2008b; Mansino et al. 2013, 2015a, b; Piñero et al. 2017a; Piñero and Agustí 2019), evolving into P. abaigari during the late early Ruscinian (Adrover et al. 1988; Mein et al. 1990) (Fig. 5). Thus, P. baeticus sp. nov. is useful for establishing divisions within the early Ruscinian, and becomes a characteristic taxon for the early Pliocene in the terrestrial record of the Iberian Peninsula.
In terms of magnetostratigraphy, Sifón-P was assigned to C3n.2n, at about 4.6 Ma (Garcés et al. 2001). La Bullana-3 and La Bullana-2B were preliminarily correlated with C3n.4n (5.235–4.997 Ma) and C3n.3r (4.997–4.896 Ma), respectively (Mansino et al. 2015b). Botardo-D represents one of the youngest localities in which two Paraethomys species are not yet distinguished (Piñero and Agustí 2020). However, La Bullana-3 and 2B reported two coexisting Paraethomys species, which suggests a younger age than Botardo-D, a locality included in C3n.2r (4.799–4.631 Ma) (Piñero et al. 2018; Piñero and Agustí 2020). In addition, the occurrence of the gerbil Debruijnimys cf. julii in La Bullana-2B is a further argument to indicate an older age than Botardo-D, where Debruijnimys sp., ancestor of D. julii, is present (Piñero and Agustí 2020). This inconsistency in the magnetostratigraphic correlations is best explained if the normal interval identified at La Bullana-3 corresponds to the younger C3n.2n (4.631–4.493 Ma), and La Bullana-2B to C3n.1r (4.493–4.300 Ma). The origin of P. baeticus sp. nov. has to be placed sometime between the deposition of Botardo-D and Sifón-P, roughly 4.6 Ma. The youngest evidences of P. baeticus sp. nov. are those from Gorafe-1 (De Bruijn 1974), Gorafe-A (Ruiz-Bustos et al. 1986), Alcoy-2C and Alcoy-2D (Mansino et al. 2013). Both Gorafe-1 and Gorafe-A were included in the base of C3n.1n (Piñero et al. 2018), at about 4.30 Ma. Thereby, Paraethomys baeticus sp. nov. is recorded in the time span between ca. 4.6 and 4.3 Ma (Fig. 5).
Paraethomys abaigari has only been identified in the Teruel Basin at the localities of Villalba Alta Río-1, Celadas-6 and La Judería (Adrover et al. 1988; Mein et al. 1990). The stratigraphic range of this species is restricted to a short interval at the top of the MN14 unit, so it represents an excellent indicator of the end of the early Ruscinian (Fig. 5).
The first occurrence of P. jaegeri is attested at the base of the Zújar section, in the site of Zújar-4 (~4.19 Ma), Guadix-Baza Basin (Oms et al. 1999; Agustí et al. 2001; Piñero et al. 2018). This locality also recorded the first occurrence of the arvicolid Mimomys in the Guadix-Baza Basin, which marks the onset of the MN15 unit (late Ruscinian). The stratigraphic record of P. jaegeri extends over the late Ruscinian (Montenat and De Bruijn 1976; Adrover 1986; Aguilar et al. 1986; Martín-Suárez 1988; Bachelet 1990; Mein et al. 1990; Castillo and Agustí 1996; Agustí et al. 2001; Piñero et al. 2018). The latest populations of P. jaegeri are found in Asta Regia 3 (Castillo and Agustí 1996), Mont-Hélène (Aguilar et al. 1986) and Sète (Adrover 1986), where this species co-occurs with the latest representatives of P. meini. All evidences indicate that P. jaegeri vanished at the same time as P. meini towards the end of the Ruscinian (Fig. 5), which involved the disappearance of the genus Paraethomys from Europe. The youngest locality assigned to the MN15 in southern Spain is Tollo de Chiclana-1B, where the arvicolid Mimomys hassiacus is recorded (Minwer-Barakat et al. 2008). In this locality, despite the abundance of micromammal remains, Paraethomys is not identified (Minwer-Barakat et al. 2005). Therefore, this genus must have disappeared at some moment slightly before the Ruscinian/Villanyian boundary.
In the Neogene basins of the Iberian Peninsula, there is a rich and continuous record of rodents. In this context, a significant number of biostratigraphic schemes have been proposed for the upper Miocene and lower Pliocene (Mein et al. 1990; García-Alix et al. 2008b; Mansino et al. 2017; Piñero et al. 2018; among others). Within the lower Ruscinian, the first record of two coexisting Paraethomys species marks the lower limit of different local correlatable biozones in four different basins, whereas the first presence (or first regular presence) of Trilophomys marks their upper limit. Such is the case of the Paraethomys aff. abaigari Zone from the basins of Granada (García-Alix et al. 2008b), Alcoy (Mansino et al. 2017), and Guadix-Baza (Piñero et al. 2018), as well as the Two Paraethomys + Promimomys Zone from the Teruel Basin (Mein et al. 1990). The lower limit of all these biozones is indeed marked by the first occurrence of P. baeticus sp. nov., which coexists with P. meini. Therefore, the biozones previously named “P. aff. abaigari”, should now be called “Paraethomys baeticus” Zone (Fig. 5). This highlights the biochronological and biostratigraphic value of the new species.
Conclusions
We erect a new murine species, Paraethomys baeticus sp. nov., from the early Pliocene of Spain, based on material from Baza-1 (Guadix-Baza Basin, SE Spain). Its distribution range includes southern and eastern Iberian Peninsula (basins of Granada, Guadix-Baza, Fortuna, Alcoy, Cabriel, and Teruel). This species would have diverged from the small-sized Paraethomys meini through asymmetric speciation. Morphological and biostratigraphic evidence suggests that the novel species is the first representative of the large-sized phyletic lineage P. baeticus sp. nov.–P. abaigari–P. jaegeri, which is characterized by an increase of size and a progressive development of longitudinal connections in the molars along the Pliocene. Paraethomys baeticus sp. nov. represents an excellent biostratigraphic marker of the upper part of the MN14 unit (lower Ruscinian continental stage), as well as a characteristic species for the early Pliocene in the continental record of the Iberian Peninsula. Its oldest records are those from Sifón-P and La Bullana-3 (at about 4.6 Ma), whereas the last appearances before it evolves into P. abaigari are recorded in Gorafe-1 and Gorafe-A (at about 4.3 Ma). This new species can contribute significantly to the establishment of a more precise biostratigraphic scheme for the Neogene in the entire Iberian Peninsula.
Acknowledgements
We dedicate this paper to all those researchers who, through their work on the Iberian murine rodent record, have enabled us to define this new species. Here we would like to express our thankfulness to the co-directors of the current excavations in the site of Baza-1: Sergio Ros Montoya (University of Málaga, Spain), Bienvenido Martínez Navarro (Institució Catalana de Recerca i Estudis Avançats [ICREA]-IPHES, Tarragona, Spain) and Francisco Javier Luengo Gutiérrez (IPHES, Universitat Rovira i Virgili, Tarragona, Spain). The curators of the Institut Català de Paleontologia Miquel Crusafont deserve our gratitude for granting access to materials under their care. We are especially grateful to the referees Raef Minwer-Barakat Requena (University of Granada, Spain) and Carolina Castillo Ruiz (University of La Laguna, Spain), and the editor Olivier Lambert for their constructive comments and suggestions on the manuscript. This research has been supported by the projects CGL2016-80000-P (Ministerio de Economía y Competitividad, Spain) and PICT 2016-2881 (Agencia Nacional de Promoción Científica y Tecnológica, Argentina). One author (Pedro Piñero) is beneficiary of a postdoctoral fellowship from the Argentinian Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
References
Adrover, R. 1986. Nuevas faunas de roedores en el Mio-Plioceno continental de la región de Teruel (España). Interés Bioestratigráfico y Paleoecológico. 423 pp. Instituto de Estudios Turolenses, Teruel.
Adrover, R., Mein, P., and Moissenet, E. 1988. Contribución al conocimiento de la fauna de roedores del Plioceno de la región de Teruel. Revista del Instituto de Estudios Turolenses 79: 89–151.
Adrover, R., Mein, P., and Moissenet, E. 1993. Roedores de la transición Mio-Plioceno de la región de Teruel. Paleontologia i Evolució 26–27: 47–84.
Aguilar, J.P., Brandy, L.D., and Thaler, L. 1984. Les Rongeurs de Salobreña (Sud de l’Espagne) et le problème de la migration messinienne. Paléobiologie continentale 14: 3–17.
Aguilar, J.P., Calvet, M., and Michaux, J. 1986. Description des rongeurs Pliocènes de la faune du Mont-Hélène (Pyrénées-Orientales, France), nouveau jalon entre les faunes de Perpignan (Serrat-d’En-Vaquer) et de Sète. Palaeovertebrata 16: 127–144.
Agustí, J. and Llenas, M. 1996. The late Turolian muroid rodent succession in eastern Spain. Acta Zoologica Cracoviensia 39: 47–56.
Agustí, J. and Martín-Suárez, E. 1984. El Plioceno continental de la depresión Guadix-Baza (Prov. Granada) y su fauna de micromamíferos. Nota preliminar. Acta geológica hispánica 19: 277–281.
Agustí, J., Garcés, M., and Krijgsman, W. 2006. Evidence for African-Iberian exchanges during the Messinian in the Spanish mammalian record. Palaeogeography, Palaeoclimatology, Palaeoecology 238: 5–14. Crossref
Agustí, J., Moyà Sola, S., Gibert, J., Guillén, J., and Labrador, M. 1985. Nuevos datos sobre la bioestratigrafía del Neógeno continental de Murcia. Paleontologia i Evolució 18: 83–94.
Agustı́, J., Oms, O., and Remacha, E. 2001. Long Plio-Pleistocene terrestrial record of climate change and mammal turnover in southern Spain. Quaternary Research 56: 411–418. Crossref
Antunes, M.T. and Mein, P. 1989. Petits mammifères du Miocène terminal du basin de Alvalade (Portugal); comparaisons avec des faunes de l’Espagne et du Maghred. Bolletino della Societá Paleontologica Italiana 28: 161–170.
Bachelet, B. 1990. Muridae et Arvicolidae (Rodentia, Mammalia) du Pliocène du Sud de la France: systématique, évolution, biochronologie. 211 pp. Unpublished Ph.D. Thesis, Université de Montpellier II, France.
Bowdich, T.E. 1821. An Analysis of the Natural Classification of Mammalia for the Use of Students and Travellers. 115 pp. J. Smith, Paris.
Brandy, L.D. 1979. Etude de rongeurs muroides du néogène supérieur et du quaternaire d’Europe, d’Afrique du nord et d’Afghanistan: évolution, biogéographie, correlations. 190 pp. Ph.D. Thesis, Université de Montpellier, France.
Castillo Ruiz, C. 1992. Paraethomys belmezensis nov. sp. (Rodentia, Mammalia) du pliocène de Córdoba (Espagne). Geobios 25: 775–780. Crossref
Castillo, C. and Agustí, J. 1996. Early Pliocene rodents (Mammalia) from Asta Regia (Jerez Basin, Southwestern Spain). Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen 99: 25–43.
Coiffait, B. and Coiffait, P.E. 1981. Découverte d’un gisement de micromammifères d’âge Pliocène dans le Bassin de Constantine (Algérie): présence d’un Muridé nouveau, Paraethomys athmeniae n. sp. Palaeovertebrata 11: 1–15.
De Bruijn, H. 1973. Analysis of the data bearing upon the correlation of the Messinian with the Succession of Land Mammals. In: C.W. Drooger (ed.), Messinian Events in the Mediterranean, 260–262. Koninklijke Nederlandse Akademie van Wetenschappen, Amsterdam.
De Bruijn, H. 1974. The Ruscinian rodent succession in Southern Spain and its implications for the biostratigraphic correlation of Europe and North Africa. Senckenbergiana lethaea 55: 435–443.
De Bruijn, H., Dawson, M.R., and Mein, P. 1970. Upper Pliocene Rodentia and Insectivora (Mammalia) from the Isle of Rhodes (Greece). Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen B 73: 568–584.
De Bruijn, H., Mein, P., Montenat, C., and Van de Weerd, A. 1975. Correlations entre les gisements de Rongeurs et les formations marines du Miocene terminal d’Espagne meridional (Prov. d’Alicante et de Murcia). Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen B 78: 1–32.
Denys, C., Taylor, P.J., and Aplin, K.P. 2017. Family Muridae. In: D.E. Wilson, T.E. Lacher Jr., and R.A. Mittermeier (eds.), Handbook of the Mammals of the World. Rodents II, 536–597. Lynx Edicions, Barcelona.
Fahlbusch, V. 1991. The meaning of MN-Zonation: considerations for a subdivision of the European continental Tertiary using mammals. Newsletters on Stratigraphy 24: 159–173. Crossref
Fejfar, O. and Heinrich, W.D. 1990. Muroid rodent biochronology of the Neogene and Quaternary in Europe. In: E. Lindsay, E.V. Fahlbusch, and P. Mein (eds.), European Neogene Mammal Chronology, 91–117. Springer US, New York. Crossref
Foote, M. 1996. On the probability of ancestors in the fossil record. Paleobiology 22: 141–151. Crossref
Freudenthal, M. and Martín-Suárez, E. 1999. Family Muridae. In: G.E. Rössner and K. Heissig (eds.), The Miocene Land Mammals of Europe, 401–409. Verlag Dr. Riedrich Pfeil, Munchen.
Garcés, M., Krijgsman, W., and Agustı́, J. 1998. Chronology of the late Turolian deposits of the Fortuna basin (SE Spain): implications for the Messinian evolution of the eastern Betics. Earth and Planetary Science Letters 163: 69–81. Crossref
Garcés, M., Krijgsman, W., and Agustí, J. 2001. Chronostratigraphic framework and evolution of the Fortuna basin (Eastern Betics) since the Late Miocene. Basin Research 13: 199–217. Crossref
García-Alix, A., Minwer-Barakat, R., Martín-Suárez, E., and Freudenthal, M. 2008a. Muridae (Rodentia, Mammalia) from the Mio-Pliocene boundary in the Granada Basin (southern Spain). Biostratigraphic and phylogenetic implications. Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen 248: 183–215. Crossref
García-Alix, A., Minwer-Barakat, R., Martín, J.M., Martín-Suárez, E., and Freudenthal, M. 2008b. Biostratigraphy and sedimentary evolution of Late Miocene and Pliocene continental deposits of the Granada Basin (southern Spain). Lethaia 41: 431–446. Crossref
Geraads, D. 1998. Rongeurs du Mio-Pliocène de Lissasfa (Casablanca, Maroc). Geobios 31: 229–245. Crossref
Gibert, L., Scott, G.R., Montoya, P., Ruiz-Sánchez, F.J., Morales, J., Luque, L., Abellá, J., and Lería, M. 2013. Evidence for an African-Iberian mammal dispersal during the pre-evaporitic Messinian. Geology 41: 691–694. Crossref
Hüsing, S.K., Oms, O., Agustí, J., Garcés, M., Kouwenhoven, T.J., Krijgsman, W., and Zachariasse, W.J. 2010. On the late Miocene closure of the Mediterranean-Atlantic gateway through the Guadix basin (southern Spain). Palaeogeography, Palaeoclimatology, Palaeoecology 291: 167–179. Crossref
Illiger, C. 1811. Prodromus Systematis Mammaliurn et Avium Additis Terminis Zoographicis Utriusque Classis. 301 pp. C. Salfeld, Berlin. Crossref
Jacobs, L.L. and Flynn, L.J. 2005. Of mice again: the Siwalik rodent record, murine distribution, and molecular clocks. In: D.E. Lieberman, R.J. Smith, and J. Kelley (eds.), Interpreting the Past: Essays on Human, Primate, and Mammal Evolution in Honor of David Pilbeam, 63–80. American School of Prehistoric Research Monographs, Brill Academic Publishers, Boston.
Jacobs, L.L., Flynn, L.J., and Downs, W.R. 1989. Neogene rodents of southern Asia. Papers on Fossil Rodents in Honor of Albert Elmer Wood. Natural History Museum of Los Angeles County, Science Series 33: 157–177.
Jacobs, L.L., Flynn, L.J., Downs, W.R., and Barry, J.C. 1990. Quo vadis, Antemus? The Siwalik muroid record. In: E.H. Linday, V. Fahlbusch, and P. Mein (eds.), European Neogene Mammal Chronology, 743–586. Plenum Press, New York. Crossref
Jaeger, J.J., Michaux, J., and Thaler, L. 1975. Présence d’un rongeur muridé nouveau, Paraethomys miocaenicus n. sp., dans le Turolien supérieur du Maroc et d’Espagne. Implications paléogéographiques. Comptes Rendus de l’Académie des Sciences Paris 280: 1673–1676.
Kimura, Y., Jacobs, L.L., and Flynn, L.J. 2013a. Lineage-specific responses of tooth shape in murine rodents (Murinae, Rodentia) to Late Miocene dietary change in the Siwaliks of Pakistan. PLoS One 8: e76070. Crossref
Kimura, Y., Jacobs, L.L., Cerling, T.E., Uno, K.T., Ferguson, K.M., Flynn, L.J., and Patnaik, R. 2013b. Fossil mice and rats show isotopic evidence of niche partitioning and change in dental ecomorphology related to dietary shift in Late Miocene of Pakistan. PLoS One 8: e69308. Crossref
Lazzari, V., Aguilar, J.-P., and Michaux, J. 2010. Intraspecific variation and micro-macroevolution connection: illustration with the late Miocene genus Progonomys (Rodentia, Muridae). Paleobiology 36: 641–657. Crossref
Lazzari, V., Tafforeau, P., Aguilar, J.P., and Michaux, J. 2008. Topographic maps applied to comparative molar morphology: the case of murine and cricetine dental plans (Rodentia, Muroidea). Paleobiology 34: 46–64. Crossref
López-Antoñanzas, R., Renaud, S., Peláez-Campomanes, P., Azar, D., Kachacha, G., and Knoll, F. 2019. First levantine fossil murines shed new light on the earliest intercontinental dispersal of mice. Scientific Reports 9: 11874. Crossref
Mansino, S., Fierro, I., Montoya, P., and Ruiz-Sánchez, F.J. 2015a. Micromammal faunas from the Mio-Pliocene boundary in the Alcoy Basin (SE Spain): biostratigraphical and palaeoecological inferences. Bulletin of Geosciences 90: 555–576. Crossref
Mansino, S., Fierro, I., Ruiz-Sánchez, F.J., and Montoya, P. 2013. The fossil rodent faunas of the localities Alcoy 2C and 2D (Alcoy Basin, Spain). Implications for dating the classical locality of Alcoy-Mina. Journal of Iberian Geology 39: 261–284. Crossref
Mansino, S., Fierro, I., Tosal, A., Montoya, P., and Ruiz-Sánchez, F.J. 2017. Micromammal biostratigraphy of the Alcoi Basin (eastern Spain): remarks on the Pliocene record of the Iberian Peninsula. Geologica Acta 15: 121–134.
Mansino, S., Ruiz-Sánchez, F.J., De Luque, L., Montoya, P., Gibert, L., Morales, J., Abella, J., Crespo, V.D., and Scott, G.R. 2015b. First Early Pliocene micromammal faunas from Venta del Moro (Cabriel Basin, Spain): New data on the Messinian dispersal of Debruijnimys. Journal of Iberian Geology 41: 273–293. Crossref
Mansino, S., Ruiz-Sánchez, F.J., Fierro, I., and Montoya, P. 2016. Mio-Pliocene rodent assemblages from Alcoi Forn (Alcoy Basin, Eastern Spain). Biostratigraphical and palaeoclimatical inferences. Historical Biology 28: 1050–1065. Crossref
Martín-Suárez, E. 1988. Sucesiones de micromamíferos en la Depresión de Guadix-Baza (Granada, España). 241 pp. Unpublished Ph.D. Thesis, Universidad de Granada, Granada.
Martín-Suárez, E. and Freudenthal, M. 1993. Muridae (Rodentia) from the lower Turolian of Crevillente (Alicante, Spain). Scripta Geologica 103: 65–118. Crossref
Martín-Suárez, E. and Freudenthal, M. 1998. Biostratigraphy of the continental upper Miocene of Crevillente (Alicante, SE Spain). Geobios 31: 839–847. Crossref
Martín-Suárez, E., Freudenthal, M., Krijgsman, W., and Fortuin, A.R. 2000. On the age of the continental deposits of the Zorreras Member (Sorbas Basin, SE Spain). Geobios 33: 505–512. Crossref
Martín-Suárez, E., Oms, O., Freudenthal, M., Agustí, J., and Parés, J.M. 1998. Continental Mio-Pliocene transition in the Granada Basin. Lethaia 31: 161–166. Crossref
Mein, P. 1975. Résultats du Groupe de Travail des Vertébrés. In: J. Senes (ed.), Report on Activity on the RCMNS Working Groups (1971–1975), 78–81. Regional Committee of the Mediterranean Neogene Stratigraphy, Bratislava.
Mein, P., Moissenet, E., and Adrover, R. 1990. Biostratigraphie du Néogène Supérieur du bassin de Teruel. Paleontologia i Evolució 23: 121–139.
Mein, P., Moissenet, E., and Truc, G. 1978 Les formations continentales du Néogène supérieur des vallées du Júcar et du Cabriel au ne d’Albacète (Espagne). Biostratigraphie et environnement 72: 99–148.
Michaux, J. 1969. Muridae (Rodentia) du Pliocène supérieur d’Europe et du Midi de la France. Palaeovertebrata 3: 1–25. Crossref
Minwer-Barakat, R., García-Alix, A., Agustí, J., Martín Suárez, E., and Freudenthal, M. 2009a. The micromammal fauna from Negratín-1 (Guadix Basin, Southern Spain): new evidence of African-Iberian mammal exchanges during the Late Miocene. Journal of Paleontology 83: 854–879. Crossref
Minwer-Barakat, R., García-Alix, A., Martín-Suárez, E., and Freudenthal, M. 2005. Muridae (Rodentia) from the Pliocene of Tollo de Chiclana (Granada, south-eastern Spain). Journal of Vertebrate Paleontology 25: 426–441. Crossref
Minwer-Barakat, R., García-Alix, A., Martín Suárez, E., and Freudenthal, M. 2008. The latest Ruscinian and early Villanyian Arvicolinae from southern Spain re-examined: Biostratigraphical implications. Journal of Vertebrate Paleontology 28: 841–850. Crossref
Minwer-Barakat, R., García-Alix, A., Martín Suárez, E., and Freudenthal, M. 2009b. Late Turolian micromammals from Rambla de Chimeneas-3: considerations on the oldest continental faunas from the Guadix Basin (Southern Spain). Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen 251: 95–108. Crossref
Minwer-Barakat, R., García-Alix, A., Martín-Suárez, E., Freudenthal, M., and Viseras, C. 2012. Micromammal biostratigraphy of the Upper Miocene to lowest Pleistocene continental deposits of the Guadix basin, southern Spain. Lethaia 45: 594–614. Crossref
Montenat, C. and De Bruijn, H. 1976. The Ruscinian rodent faunule from La Juliana (Murcia); its implication for correlation of continental and marine biozones. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen B 79: 245–255.
Oms, O., Dinarès-Turell, J., Agustı́, J., and Parés, J.M. 1999. Refinements of the European mammal biochronology from the magnetic polarity record of the Plio-Pleistocene Zújar section, Guadix-Baza basin, SE Spain. Quaternary Research 51: 94–103. Crossref
Opdyke, N., Mein, P., Lindsay, E., Perez-Gonzales, A., Moissenet, E., and Norton, V.L. 1997. Continental deposits, magnetostratigraphy and vertebrate paleontology, late Neogene of Eastern Spain. Palaeogeography, Palaeoclimatology, Palaeoecology 133: 129–148. Crossref
Petter, F. 1968. Un muridé Quaternaire nouveau d’Algerie, Paraethomys filfilae. Ses rapports avec les Muridés actuels. Mammalia 32: 54–59. Crossref
Piñero, P. and Agustí, J. 2019. The rodent succession in the Sifón de Librilla section (Fortuna Basin, SE Spain): implications for the Mio-Pliocene boundary in the Mediterranean terrestrial record. Historical Biology 31: 279–321. Crossref
Piñero, P. and Agustí, J. 2020. Rodents from Botardo-D and the Miocene–Pliocene transition in the Guadix-Baza Basin (Granada, Spain). Palaeobiodiversity and Palaeoenvironments 100: 903–920. Crossref
Piñero, P., Agustí, J., and Oms, O. 2018. The late Neogene rodent succession of the Guadix-Baza Basin (south-eastern Spain) and its magnetostratigraphic correlation. Palaeontology 61: 253–272. Crossref
Piñero, P., Agustí, J., Oms, O., Blain, H.A., Laplana, C., Ros-Montoya, S., and Martínez-Navarro, B. 2017a. Rodents from Baza-1 (Guadix-Baza Basin, southeast Spain): filling the gap of the early Pliocene succession in the Betics. Journal of Vertebrate Paleontology 37 (4): e1338294. Crossref
Piñero, P., Agustí, J., Oms, O., Fierro, I., Montoya, P., Mansino, S., Ruiz-Sánchez, F.R., Alba, D.M., Alberdi, M.T., Blain, H.-A., Laplana, C., Van der Made, J., Mazo, A.V., Morales, J., Murelaga, X., Pérez-García, A., Pérez-Valera, F., Pérez-Valera, J.A., Sevilla, P., Soria, J.M., and Romero, G. 2017b. Early Pliocene continental vertebrate fauna at Puerto de la Cadena (SE Spain) and its bearing on the marine-continental correlation of the late Neogene of Eastern Betics. Palaeogeography, Palaeoclimatology, Palaeoecology 479: 102–114. Crossref
Renaud, S., Benammi, M., and Jaeger, J.J. 1999. Morphological evolution of the murine rodent Paraethomys in response to climatic variations (Mio-Pleistocene of North Africa). Paleobiology 25: 369–382. Crossref
Rook, L. 1992. Italian Messinian localities with vertebrate faunas. Paleontologia i Evolució 24: 141–148.
Ros-Montoya, S., Martínez-Navarro, B., Espigares, M.P., Guerra-Merchán, A., García-Aguilar, J.M., Piñero, P., Rodríguez-Rueda, A., Agustí, J., Oms O., and Palmqvist, P. 2017. A new Ruscinian site in Europe: Baza-1 (Baza basin, Andalusia, Spain). Comptes Rendus Palevol 16: 746–761. Crossref
Ruiz Bustos, A., Sesé, C., Dabrio, C.J., Peña Ruano, J.A., and Padial, J.M. 1984. Geología y fauna de micromamíferos del nuevo yacimiento del Plioceno inferior de Gorafe-A (Depresión de Guadix-Baza, Granada). Estudios Geológicos 40: 231–241. Crossref
Stadler, T., Gavryushkina, A., Warnock, R.C., Drummond, A.J., and Heath, T.A. 2018. The fossilized birth-death model for the analysis of stratigraphic range data under different speciation modes. Journal of Theoretical Biology 447: 41–55. Crossref
Torres-Roig, E., Piñero, P., Agustí, J., Bover, P., and Alcover, J.A. 2019. First evidence of endemic Murinae (Rodentia, Mammalia) in the early Pliocene of the Balearic Islands (western Mediterranean). Geological Magazine 156: 1742–1750. Crossref
Van de Weerd, A. 1976. Rodent faunas of the Mio-Pliocene continental sediments of the Teruel-Alfambra region, Spain. Utrecht Micropaleontological Bulletin, Special Publication 2: 1–217.
Acta Palaeontol. Pol. 65 (4): 719–731, 2020
https://doi.org/10.4202/app.00755.2020