

New bioerosion traces in rhynchosaur bones from the Upper Triassic of Brazil and the oldest occurrence of the ichnogenera Osteocallis and Amphifaoichnus
LUCCA S. CUNHA, PAULA DENTZIEN-DIAS, and HEITOR FRANCISCHINI
Cunha, L.S., Dentzien-Dias, P., and Francischini, H. 2024. New bioerosion traces in rhynchosaur bones from the Upper Triassic of Brazil and the oldest occurrence of the ichnogenera Osteocallis and Amphifaoichnus. Acta Palaeontologica Polonica 69 (1): 1–21.
New bioerosion traces produced by insects in bones are reported from the Hyperodapedon Assemblage Zone of the Santa Maria Supersequence (Carnian, Brazil). The bones are assigned to a single rhynchosaur Hyperodapedon mariensis individual and among the traces, the ichnogenera Osteocallis (Osteocallis mandibulus, Osteocallis infestans, and Osteocallis isp.) and Amphifaoichnus (Amphifaoichnus isp.) are recognized, along with two morphotypes of indiscrete traces: clusters of grooves and borings. All the traces are assigned to the action of insects exploring the rhynchosaur carcass. Osteocallis and associated clusters of grooves are interpreted as feeding traces, but whether they represent necrophagic or osteophagic behavior is still uncertain. The lack of direct evidence for the ethological interpretation of Amphifaoichnus precludes its sole correlation with osteophagy, and other possibilities, such as the construction of temporary domiciles related to feeding or sediment moisture, are discussed. The traces analyzed here indicate that the insects explored a buried carcass, challenging the automatic association of Osteocallis and prolonged subaerial exposure of bones, placing insects as relevant taphonomic agents that affect the preservation of vertebrate carcasses. Additionally, the first appearance record of Amphifaoichnus is expanded back more than 140 Ma, indicating that complex behaviors employed by insects in bone exploration were already established in the early Late Triassic, shortly after the oldest records of invertebrate bioerosion in bones on continental settings.
Key words: Rhynchosauria, ichnotaxonomy, insect, osteophagy, paleoecology, taphonomy, vertebrate carcass, Triassic, Santa Maria Supersequence, Brazil.
Lucca S. Cunha [lucca98cunha@hotmail.com; ORCID: https://orcid.org/0000-0002-0242-2667 ], Programa de Pós-Graduação em Geociências, Instituto de Geociências, Universidade Federal do Rio Grande do Sul, Av. Bento Gonçalves 9500, Porto Alegre, 91501-970, Brazil.
Paula Dentzien-Dias [pauladentzien@gmail.com; ORCID: https://orcid.org/0000-0001-8708-6799 ] and Heitor Francischini [heitor.francischini@ufrgs.br; ORCID: https://orcid.org/0000-0001-9809-7784 ], Departamento de Paleontologia e Estratigrafia, Instituto de Geociências, Universidade Federal do Rio Grande do Sul, Av. Bento Gonçalves 9500, Porto Alegre, 91501-970, Brazil.
Received 24 July 2023, accepted 29 December 2023, published online 30 January 2024.
Copyright © 2024 L.S. Cunha et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Bioerosion traces produced by insects in bones are of great paleoecological and taphonomical importance. They are direct evidence of interaction between these arthropods and vertebrate skeletal remains, revealing aspects of their biostratinomic history. Not rarely, the environmental conditions of past ecosystems are reconstructed or corroborated based on the information gathered from the bioerosion traces found in bones and the identification of the possible producer and its behavior (e.g., Rogers 1992; Martin and West 1995; Hasiotis et al. 1999; Gatta et al. 2021). Since the 1990s, the number of studies focusing on fossil bioerosion made by insects in bones preserved in continental settings have increased, both in paleontology and archeology (e.g., Rogers 1992; Roberts et al. 2007; Hutchet et al. 2011, 2013; Pirrone and Buatois 2016; Wrobel and Biggs 2018; Backwell et al. 2020). Consequently, a variety of recurrent morphologies have been identified, raising questions and hypotheses about the possible behaviors and producers associated with them, and also allowing for the ichnotaxonomical classification of several traces (Roberts et al. 2007; Xing et al. 2013; Pirrone et al. 2014; Pirrone and Buatois 2016; Xing et al. 2016; Paes Neto et al. 2016; Parkinson 2016; Höpner and Bertling 2017; Serrano-Brañas et al. 2018).
The oldest records of bioerosion trace fossils produced by insects in bones date from the latest Middle Triassic (Paes Neto et al. 2016), revealing that the usage of bones as a substrate by terrestrial species is nearly as old as the origin of some of the groups that show this behavior in the present (e.g., Dermestidae beetles; Zhang et al. 2018). Up until now, however, only two full articles focusing on Triassic insect bioerosion in bones were published (Leal et al. 2002; Paes Neto et al. 2016); other publications that report these traces are either abstracts or articles focusing on other subjects (Müller et al. 2015; Francischini et al. 2018; Pavanatto et al. 2018; Medeiros et al. 2019; Battista et al. 2021). Remarkably, all the mentioned records came from the Middle–Upper Triassic Santa Maria Supersequence (SMS) of Rio Grande do Sul State, southern Brazil. In this contribution, we bring novel and important data from the Brazilian SMS, contributing to the advance of ichnotaxonomical discussions and expanding knowledge about the use of bone substrate by insects in the Triassic.
Institutional abbreviations.—CAPPA/UFSM, Centro de Apoio à Pesquisa Paleontológica da Quarta Colônia, Universidade Federal de Santa Maria, Santa Maria, Brazil; IPR-PUCRS, Instituto do Petróleo e dos Recursos Naturais, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil; UFRGS-PV, Laboratório de Paleontologia de Vertebrados, Departamento de Paleontologia e Estratigrafia, Instituto de Geociências, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
Other abbreviations.—AZ, Assemblage Zone; SMS, Santa Maria Supersequence.
Geological setting
The Paraná Basin covers an area of about 1 500 000 km² of Brazil, Uruguay, Paraguay, and Argentina and comprises a package up to 7000 m thick, ranging from Ordovician to Cretaceous (Fig. 1A, B; Milani et al. 2007). The Triassic deposits of the Paraná Basin are restricted to its southernmost portion, in the state of Rio Grande do Sul (Fig. 1A, B) and are divided into two second-order sequences (Zerfass et al. 2003): the Sanga do Cabral Supersequence (Lower Triassic) and the Santa Maria Supersequence (Middle–Upper Triassic; Fig. 1D). In this latter, four third-order sequences can be recognized from bottom to top (sensu Horn et al. 2014; Fig. 1D): Pinheiros-Chiniquá Sequence (Ladinian–Carnian), Santa Cruz Sequence (lower Carnian), Candelária Sequence (Carnian–Norian) and Mata Sequence (Rhaetian?). Except by this latter, all the SMS sequences yield a rich vertebrate fossil record, which occurs in, at least, four distinct assemblage zones (AZ). The Pinheiros-Chiniquá Sequence comprises the Dinodontosaurus AZ, the Santa Cruz Sequence comprises the Santacruzodon AZ and the Candelária Sequence comprises both the Hyperodapedon AZ (at the base of the sequence) and the Riograndia AZ (at the top).
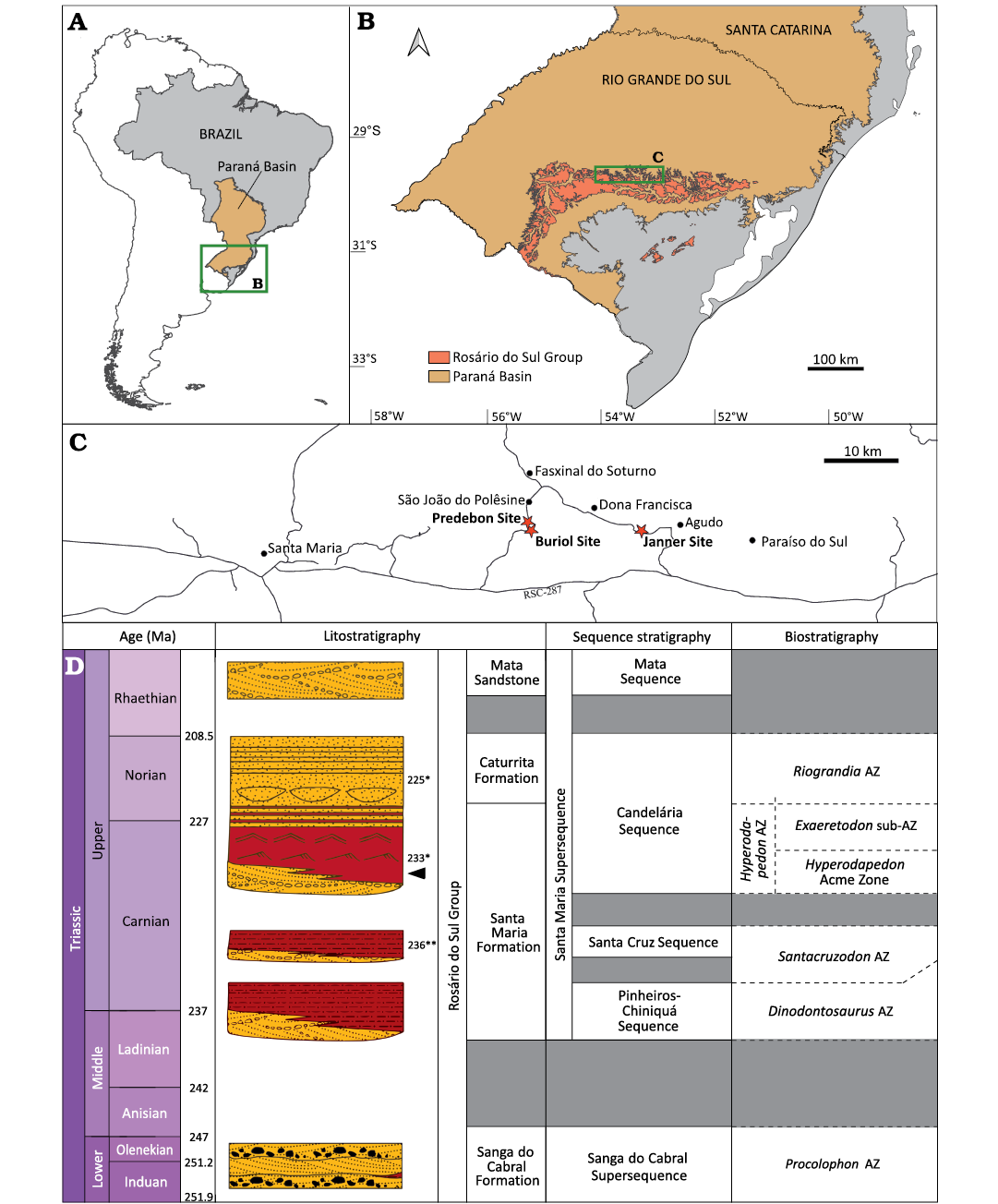
Fig. 1. Geological and geographic context. A. Location of the Paraná Basin in Brazil. B. Limits of the Triassic rocks of Rosário do Sul Group and the Triassic rocks of Paraná Basin in Rio Grande do Sul state. C. Location of the Buriol Site, locality of UFRGS-PV-1581-T, and nearby Predebon and Janner sites. D. Chrono-, lito-, and biostratigraphy of southern Brazilian Triassic (modified from Schultz et al. 2020). Arrow indicates stratigraphical position of UFRGS-PV-1581-T; * refers to absolute ages from Langer et al. (2018); ** refers to absolute ages from Philipp et al. (2018).
The fossil record of the Hyperodapedon AZ, the main focus of this work, is characterized by the presence and abundance of the genera Hyperodapedon, a rhynchosaur, and Exaeretodon, a traversodontid cynodont, and is mostly composed of other cynodonts (e.g., Trucidocynodon, Charruodon, and Alemoatherium) and archosauromorphs (e.g., Aetosauroides, Rauisuchus, Ixalerpeton, Gnathovorax, and Saturnalia) (Schultz et al. 2020). This faunal content is similar to what is found in the Argentinean Ischigualasto Formation (e.g., Desojo et al. 2020). Recently, Schultz et al. (2020) suggested that the Hyperodapedon AZ could be subdivided into a lower portion characterized by the abundance of this rhynchosaur and the lack of the traversodontid cynodont Exaeretodon, and an upper Exaeretodon sub-AZ where the abundance of Hyperodapedon decreases and the dominant taxa are Exaeretodon and the hyperodapedontine rhynchosaur Teyumbaita. The Ischigualasto Formation presents a similar pattern of succession with an abundance of Hyperodapedon in its lower portion and an abundance of Exaeretodon in its upper portion, allowing direct biostratigraphic correlation with the SMS. The U-Pb zircon geochronology study of Langer et al. (2018) indicated an age of 233.23 ± 0.73 Ma for the most iconic outcrop with fossils of the Hyperodapedon AZ, the Cerro da Alemoa site, in Santa Maria City, which would place this biozone in the middle Carnian.
Lithologically, the base of the Candelária Sequence is composed mainly of trough cross bedded sandstones, interpreted as a fluvial system. This facies is overlain by red, massive or laminated mudstones, representing shallow lacustrine deposits and a transition between a lowstand and a transgressive system tract. Upwards, this sequence presents a coarsening trend with decrease in the mudstone content and increase of amalgamated sandstone, which would indicate a progressive replacement of the lacustrine system by a fluvial system (Zerfass et al. 2003).
Material and methods
The materials described here were collected in an area smaller than 2 m2 in the Buriol Site (29º39’10” S, 53º26’35” W), approximately 6 km southeast of the municipality of São João do Polêsine, in the state of Rio Grande do Sul, Brazil (Fig. 1C). It consists of hundreds of pieces of disarticulated or fragmented bones, which can be assigned to a single Hyperodapedon mariensis individual based on: (i) the presence of the combination of the following characters: a single groove on the ventral portion of the maxilla, the presence of teeth in the lingual surface of the dentary blade and the lack of lingual teeth in the maxilla (Langer and Schultz 2000); (ii) size correspondence between the elements; (iii) the lack of bone element duplication; and (iv) the lack of evidence of transportation and reworking. Some of the bone fragments are covered by a concretion, indicating that at least some of the fragmentation occurred prior to diagenesis. The better-preserved skull elements consist of the partial left and right dentaries in articulation, disarticulated partial left and right maxillae and a partial left pterygoid (Fig. 2). No bioerosion traces were found in these elements.
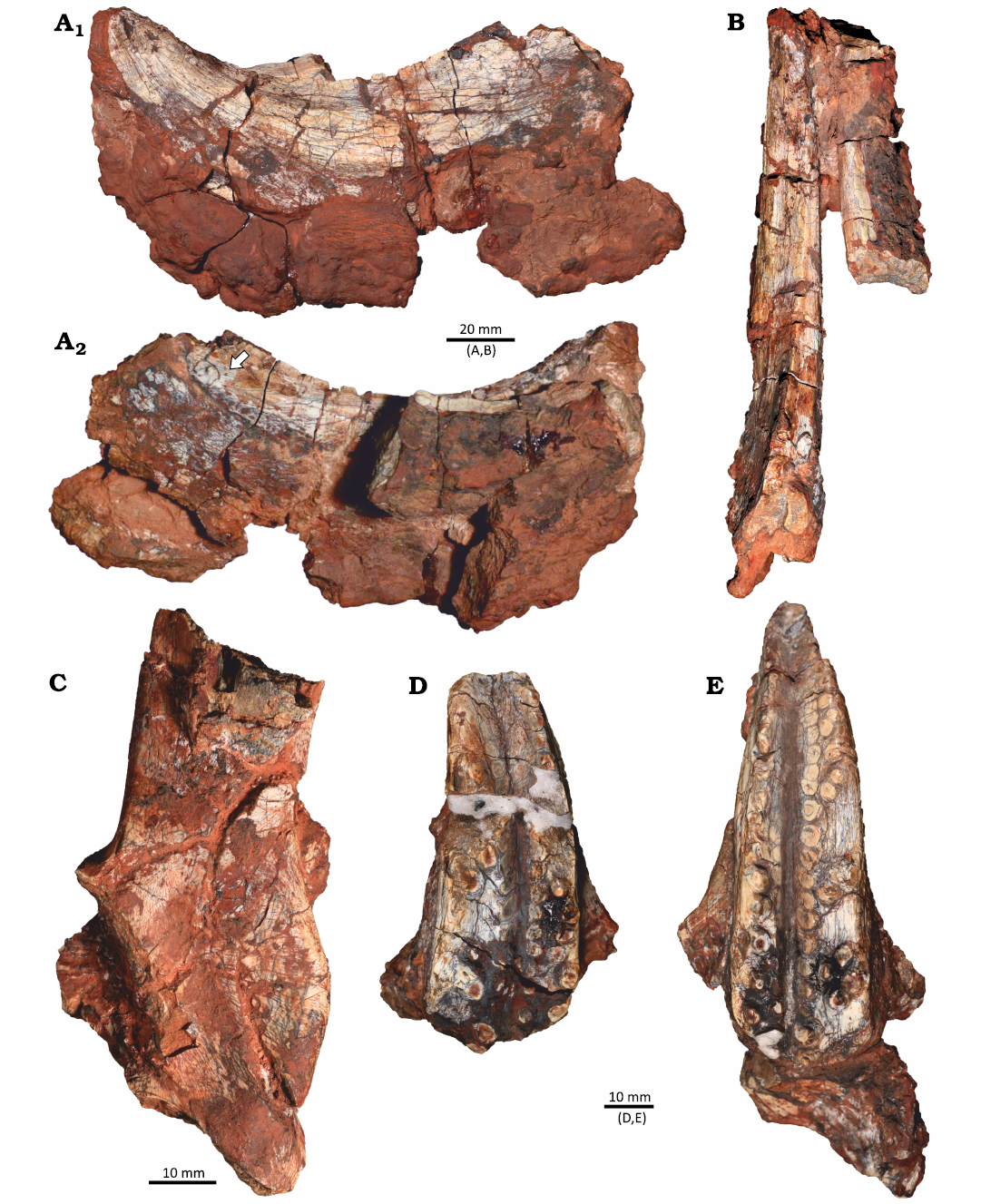
Fig. 2. Identified cranial elements of the rhynchosaurid archosauromorph Hyperodapedon mariensis (Tupi Caldas, 1933) in UFRGS-PV-1581-T from Buriol Site, Brazil, Hyperodapedon AZ, Carnian. A. Left dentary in lateral view (A1) and medial view (A2) showing the dentary blade with at least one lingual tooth (arrow). B. Left and right dentaries in dorsal view. C. Partial left pterygoid in medial view. D. Right maxilla in ventral view. E. Left maxilla in ventral view.
The identification of this material as Hyperodapedon mariensis, along with the faunal content of nearby sites, such as Predebon Site (Fig. 1D), suggests the record represents the lower portion of the Hyperodapedon AZ. Sites in this region have yielded remains of the rhynchosaur Hyperodapedon, the aetosaur Aetosauroides, the sauropodomorph Buriolestes, the lagerpetid Ixalerpeton, and the stereospondyl Compsocerops (Langer et al. 2007; Cabreira et al. 2016; Dias-Da-Silva et al. 2012; Roberto-Da-Silva et al. 2014; Paes Neto et al. 2021), reinforcing our interpretation.
The studied specimen received the collection numbers UFRGS-PV-1581-T and CAPPA/UFSM 0383. Each of the trace-bearing fragments was numbered and will be referred to as UFRGS-PV-1581-T # (Table 1). The fossils were mechanically prepared using standard methods. In total, 520 fragments were analyzed and prospected for traces under an Opticam OPZTS stereomicroscope at magnifications of 10×, 16×, 25×, and 40×. The 29 trace-bearing bone pieces were photographed under different angles of lighting, allowing for the highlight of the traces and more accurate analyzes. For digital measurement of the traces, the software ImageJ 1.53k was used. UFRGS-PV-1581-T #3 was scanned with a SkyScan 1173 CT scan in the IPR-PUCRS. It was used 130 kv and 61 µA and 1800 slices with a voxel size of 21.9 µm were obtained. The slices were analyzed with the software 3D Slicer 5.2.1. The description of the traces followed the nomenclature and ichnotaxobases proposed by Pirrone et al. (2014).
Table 1. List of traces in each bone fragment.
|
Specimen |
Traces |
|
UFRGS-PV-1581-T #1 |
Osteocallis mandibulus |
|
UFRGS-PV-1581-T #2 |
Osteocallis mandibulus |
|
UFRGS-PV-1581-T #3 (Figs. 3A, 4A, 8A) |
Osteocallis mandibulus; Amphifaoichnus isp.; Morphotype 2 (boring) |
|
UFRGS-PV-1581-T #4 (Fig. 3B) |
Amphifaoichnus isp. |
|
UFRGS-PV-1581-T #5 (Fig. 6C) |
Osteocallis
mandibulus; |
|
UFRGS-PV-1581-T #6 (Fig. 6E) |
Morphotype 1 (cluster of grooves) |
|
UFRGS-PV-1581-T #7 (Fig. 8B) |
Morphotype 2 (boring) |
|
UFRGS-PV-1581-T #8 |
Osteocallis
mandibulus; |
|
UFRGS-PV-1581-T #9 (Fig. 8C) |
Morphotype 2 (boring) |
|
UFRGS-PV-1581-T #10 (Fig. 3C) |
Amphifaoichnus isp. |
|
UFRGS-PV-1581-T #11 (Fig. 4D) |
Osteocallis
isp.; |
|
UFRGS-PV-1581-T #12 (Fig. 4C) |
Osteocallis infestans |
|
UFRGS-PV-1581-T #13 (Fig. 7B) |
Morphotype 1 (subcircular cluster of grooves) |
|
UFRGS-PV-1581-T #14 (Fig. 5) |
Osteocallis
mandibulus; |
|
UFRGS-PV-1581-T #15 |
Morphotype 1 (cluster of grooves) |
|
UFRGS-PV-1581-T #16 (Fig. 7C) |
Morphotype 1 (subcircular cluster of grooves) |
|
UFRGS-PV-1581-T #17 (Figs. 6F, 7A) |
Morphotype 1 (subcircular cluster of grooves) |
|
UFRGS-PV-1581-T #18 |
Osteocallis mandibulus |
|
UFRGS-PV-1581-T #19 |
Osteocallis mandibulus |
|
UFRGS-PV-1581-T #20 (Fig. 6A) |
Morphotype 1 (cluster of grooves) |
|
UFRGS-PV-1581-T #21 |
Morphotype 1 (cluster of grooves) |
|
UFRGS-PV-1581-T #22 (Fig. 6B) |
Morphotype 1 (cluster of grooves) |
|
UFRGS-PV-1581-T #23 (Fig. 4B) |
Osteocallis mandibulus; |
|
UFRGS-PV-1581-T #24 |
Morphotype 1 (cluster of grooves) |
|
UFRGS-PV-1581-T #25 |
Morphotype 1 (cluster of grooves) |
|
UFRGS-PV-1581-T #26 (Fig. 6D) |
Morphotype 1 (cluster of grooves) |
|
UFRGS-PV-1581-T #27 |
Morphotype 1 (cluster of grooves) |
|
UFRGS-PV-1581-T #28 |
Morphotype 1 (cluster of grooves) |
|
UFRGS-PV-1581-T #29 |
Morphotype 1 (cluster of grooves) |
Results
From the 520 bone fragments analyzed, only 29 present bioerosion traces. Among them, the ichnogenera Amphifaoichnus and Osteocallis, this latter represented by at least two ichnospecies (O. mandibulus and O. infestans), were recognized due to their discrete morphology. Other traces, however, do not have a clear morphology and, in consequence, do not fall within any known ichnotaxon. Therefore, we opted to describe them using an open nomenclature (morphotypes). They are described properly below.
Systematic palaeoichnology
Ichnogenus Amphifaoichnus Pirrone & Buatois, 2016
Type ichnospecies: Amphifaoichnus seilacheri Pirrone & Buatois, 2016, Plottier Formation, Argentina, Coniacian–lower Santonian (Upper Cretaceous).
Emended diagnosis.—Predominantly horizontal, unbranched, unlined, elongated tubes, circular or subcircular in cross-section, straight or tortuous, located at the interface between bone and sediment, but penetrating the cortical bone tissue and occasionally the trabecular bone tissue; fill consists of both unconsolidated sediment and bone chips; rough texture of the exposed surface (modified from Pirrone and Buatois 2016).
Remarks.—The original diagnosis proposed by Pirrone and Buatois (2016) only mentions the penetration of cortical bone tissue. Because the traces described below also penetrate trabecular bone, we emended the diagnosis of Amphifaoichnus in order to properly accommodate them in the ichnogenus.
Amphifaoichnus isp.
Fig. 3.
Material.—UFRGS-PV-1581-T #3 (Fig. 3A), UFRGS-PV-1581-T #4 (Fig. 3B), and UFRGS-PV-1581-T #10 (Fig. 3C), bone fragments assigned to Hyperodapedon mariensis; from Buriol Site, São João do Polêsine municipality, Rio Grande do Sul State, Brazil; base of the Candelária Sequence (vertebrate remains associated to the lower subunit of the Hyperodapedon AZ), SMS, Paraná Basin; middle Carnian (lower Upper Triassic).
Description.—Elongated, straight to slightly meandering tubes located in the interface between bone and substrate but penetrating cortical and trabecular bone (Fig. 3A3). Tubes are subcircular in cross section and are filled with sediment identical to the rock matrix and bone chips apparently unevenly distributed in the filling (Fig. 3A1, B1). Tube length is 9.6–22.2 mm and the width is 3.9–9.1 mm. At least one tube presents structures indicating a meniscate backfill (Fig. 3A2).
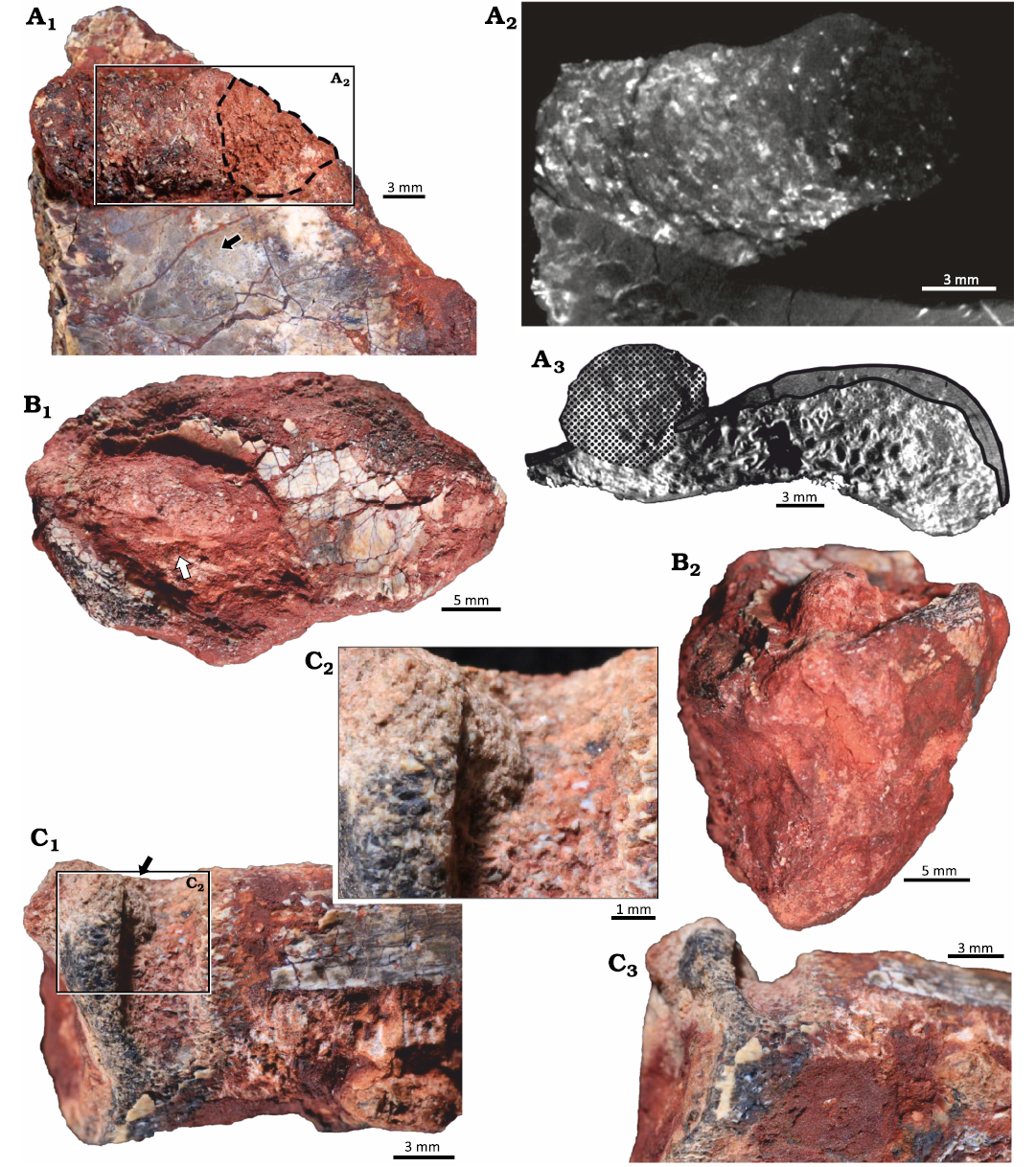
Fig. 3. Arthropod bioerosion trace fossil Amphifaoichnus isp. on bone fragments of the rhynchosaurid archosauromorph Hyperodapedon mariensis (Tupi Caldas, 1933) from Buriol Site, Brazil, Hyperodapedon AZ, Carnian. A. UFRGS-PV-1581-T #3; A1, close up showing Amphifaoichnus isp. (note the bone chips) associated to a perpendicular boring (dashed outline) and feeding traces of Osteocallis mandibulus Roberts et al., 2007 (arrow; see also Fig. 4A1); A2, axial view of µCT scan showing the internal morphology of the tube, meniscate structures and the perpendicular boring; A3, coronal view of µCT scan showing the trace (dotted surface) and the destruction of both cortical (black outline) and trabecular bone. B. UFRGS-PV-1581-T #4; B1, specimen (arrow) showing the uneven distribution of bone chips in the filling; B2, specimen in transversal view showing the rounded morphology of the filling. C. UFRGS-PV-1581-T #10; C1, specimen in negative relief with a small portion of filling still preserved (arrow); C2, close up of filling; C3, specimen in transversal view showing the U-shape of the boring.
Remarks.—In opposition to Amphifaoichnus seilacheri, the tubes described here are not restricted to cortical bone but penetrate to trabecular bone as well. The tubes on UFRGS-PV-1581-T #3 and UFRGS-PV-1581-T #4 are preserved in positive relief, as expected for Amphifaoichnus, but UFRGS-PV-1581-T #10 is preserved in a negative relief (Fig. 3C). Its assignment to Amphifaoichnus is based on the morphological similarities to the other tubes and the presence of a small portion of fill formed by sediment and bone chips at its base and close to its boundary (Fig. 3C2). The tube on UFRGS-PV-1581-T #3 is penetrated by a boring trace, so that one of its extremities is not as well preserved, internally and externally (Fig. 3A1). The presence of meniscate structures on UFRGS-PV-1581-T #3 filling is revealed by µCT scan analysis and the segments are visible only in the termination which was not altered by the second boring (Fig. 3A2). Amphifaoichnus seilacheri is characterized by evenly distributed bone chips in the infill sorted by size, a feature visible only in cross section. On the surface of the filling in UFRGS-PV-1581-T #3 and #4 no pattern of distribution is observed, which could be indicative of unevenly distributed bone chips (Fig. 3A1, B1). However, due to the low number of available specimens of Amphifaoichnus in UFRGS-PV-1581-T, destructive methods were avoided, preventing the analysis of bone chip distribution in cross section. Meniscate structures are not mentioned by Pirrone and Buatois (2016), but the methods used to reveal this structure in UFRGS-PV-1581-T #3 were not applied to A. seilacheri. Another significant difference between the Triassic and Cretaceous specimens comprises the penetration into the trabecular bone, but this feature might be a consequence of differences between the substrates (e.g., thicker cortical bone tissue in the Cretaceous specimens). Considering that Amphifaoichnus specimens are considerably rare (up to now, restricted to the Upper Triassic of Brazil and Upper Cretaceous of Argentina) and that possible influence of taphonomic processes over the trace-bearing bones is still perplexing, new materials (fossil and recent), might bring additional information regarding ichnospecific diversity of Amphifaoichnus traces. A detailed discussion on the ichnotaxonomy of Amphifaoichnus is beyond the scope of this work and will be published elsewhere. Therefore, the traces UFRGS-PV-1581-T #3, #4, and #10 are here assigned to Amphifaoichnus isp.
Ichnogenus Osteocallis Roberts et al., 2007, sensu Paes Neto et al., 2016
Type ichnospecies: Osteocallis mandibulus Roberts et al. 2007, Maevarano Formation, Madagascar, Maastrichtian (Late Triassic).
Diagnosis.—Shallow trail of mandibular grooves bored into external (cortical) bone surfaces. It may present as a single trail or a network of randomly overlapping trails.
Osteocallis mandibulus Roberts et al., 2007
Figs. 4A, B, 5A, B, E.
Diagnosis.—Shallow, meandering trail of arcuate grooves (apparently paired) bored into external (cortical) bone surfaces. Occurs as single trails or network of randomly overlapping trails.
Material.—UFRGS-PV-1581-T #2, #5 (Fig. 6C), #8, #19, and #23 (Fig. 4B) present single trails. UFRGS-PV-1581-T #3 (Fig. 4A) and UFRGS-PV-1581-T #18 present two trails without overlapping. UFRGS-PV-1581-T #1 presents two overlapping trails and UFRGS-PV-1581-T #14 (Fig. 5A, B, E) presents three trails without overlapping. All of these bone fragments are referred to Hyperodapedon mariensis from Buriol Site, São João do Polêsine municipality, Rio Grande do Sul State, Brazil; Base of the Candelária Sequence (vertebrate remains associated to the lower subunit of the Hyperodapedon AZ), SMS, Paraná Basin; middle Carnian (lower Upper Triassic).
Description.—All 14 trails formed by successive mainly arcuate and apparently paired grooves on the bone surface (Figs. 4, 5A, B, E, 6C). In some cases, the grooves overlap, forming long arcuate grooves or crossing each other. Most of the trails present some degree of meandering. Trail length is 1.1–21.5 mm and the width is 0.1–4.4 mm.

Fig. 4. Feeding traces of Osteocallis on bone fragments of the rhynchosaurid archosauromorph Hyperodapedon mariensis (Tupi Caldas, 1933) from Buriol Site, Brazil, Hyperodapedon AZ, Carnian. A. UFRGS-PV-1581 #3; A1, Osteocallis mandibulus Roberts et al., 2007, associated to arthropod bioerosion trace fossil Amphifaoichnus isp.; A2, details of one of the trails. B. UFRGS-PV-1581 #23; B1, Osteocallis mandibulus associated to a cluster of grooves in crescent shape; B2, schematic drawing highlighting the grooves. C. UFRGS-PV-1581-T #12 showing two overlapping Osteocallis infestans Paes Neto et al., 2016. D. UFRGS-PV-1581-T #11 showing Osteocallis isp. (arrow) associated to a cluster of larger grooves.

Fig. 5. A suite of traces in the rhynchosaurid archosauromorph Hyperodapedon mariensis (Tupi Caldas, 1933), UFRGS-PV-1581-T #14, bone fragment from Buriol Site, Brazil, Hyperodapedon AZ, Carnian. A. Two feeding traces of Osteocallis mandibulus Roberts et al., 2007, overlapped by a cluster of larger grooves. B. Natural cast formed by a cover of iron oxide showing the grooves in positive relief. Image mirrored to facilitate comparison. C, D. Clusters of grooves on different surfaces of the same bone fragment. E. Small Osteocallis mandibulus close to the trails shown in A and B.
Remarks.—The grooves that compose the trails are not always arcuate, with some trails also presenting straight grooves. Osteocallis leonardii (Collareta et al. 2023) presents both straight and arcuate grooves as well, but in opposition to the materials referred herein to O. mandibulus, the grooves are predominantly straight. In some of the trails it is difficult to ascertain if the grooves are indeed paired, especially when their overlapping results in long arcuate grooves. This makes the precise measurement of individual grooves nearly impossible. UFRGS-PV-1581-T #14 was found covered by a thin layer of iron oxide that served as a natural cast, allowing for the observation of the traces in a positive relief (Fig. 5A, B). Trails on UFRGS-PV-1581-T #5, #11, #14, and #23 are associated to clusters of grooves (Figs. 4B, D, 5A, B, 6C). In UFRGS-PV-1581-T #14 a cluster is overlapping one of the trails. The only association of trails with other borings occurs in UFRGS-PV-1581-T #3 (Fig. 4A1).
Osteocallis infestans Paes Neto et al., 2016
Fig. 4C.
Diagnosis.—Shallow to moderately deep, meandering trail of generally straight overlapping thick grooves excavated into the cortical bone surfaces. Grooves are randomly orientated, often overlapping other grooves which are either perpendicular or parallel to one another. The intensity of the grooves culminates in an irregular furrow-like morphology for the trail.
Material.—UFRGS-PV-1581-T #12 (Fig. 4C), a bone fragment referred to Hyperodapedon mariensis from Buriol Site, São João do Polêsine municipality, Rio Grande do Sul State, Brazil; Base of the Candelária Sequence (vertebrate remains associated to the lower subunit of the Hyperodapedon AZ), SMS, Paraná Basin; middle Carnian (lower Upper Triassic).
Description.—Two overlapping, moderately deep trails formed by randomly oriented, overlapping grooves culminating in a furrow-like morphology. Isolated grooves outside of the furrow appear to be arcuate, but the intense overlapping prevents the precise observation of groove morphology. The length of the trails ranges from 8.37–14.38 mm and the width ranges from 0.33–1.47 mm.
Remarks.—Both trails described here are considerably narrower than the holotype of O. infestans (which came from the Exaeretodon sub-AZ of the Hyperodapedon AZ) and the grooves are not as thick. As in the holotype, some areas outside of the furrow present isolated grooves. The furrow-like morphology is readily distinguishable from what is seen in O. mandibulus (Fig. 4C).
Osteocallis isp.
Fig. 4D.
Material.—UFRGS-PV-1581-T #11 (Fig. 4D), a bone fragment referred to Hyperodapedon mariensis from Buriol Site, São João do Polêsine municipality, Rio Grande do Sul State, Brazil; Base of the Candelária Sequence (vertebrate remains associated to the lower subunit of the Hyperodapedon AZ), SMS, Paraná Basin; middle Carnian (lower Upper Triassic).
Description.—A trail formed by successive straight and arcuate, mainly non-paired grooves bored on the surface of the bone. Trail length is 3.29 mm and the width ranges from 0.25–1.48 mm.
Remarks.—The preservation of the trail prevents its ichnospecific classification, because it is not possible to ensure that the grooves are mainly arcuate and paired. The grooves do not appear to be organized in parallel rows and regularly spaced as seen in Osteocallis leonardii. The trail is closely associated with an irregular cluster of randomly oriented grooves (Fig. 4D).
Morphotype 1: clusters of grooves
Figs. 6, 7.
Material.—UFRGS-PV-1581-T #5, #6, #8, #11, #13–#15, #17, #20–#29.
Description.—The clusters consist of straight and/or arcuate grooves bored on the surface of the cortical bone without forming discrete trails. In most cases the grooves are randomly oriented and scattered over large areas of the bone surface. When densely concentrated, the grooves give the bone an etched appearance, similar to traces reported both from fossil (Kaiser 2000; Britt et al. 2008; Backwell et al. 2020) and modern (Backwell et al. 2012) bones (Fig. 6B, E). Subparallel grooves appear in at least three different clusters, two of them in UFRGS-PV-1581-T #17 (Fig. 6F).
At least three subcircular concentrations of grooves were observed; they resemble the pits reported by Laudet and Antoine (2004: fig. 2B), Britt et al. (2008: fig. 1D), and Parkinson (2022: fig. 2E, F) but, unlike the pits, they do not form a depression on the bone surface (Fig. 7). The subcircular clusters on UFRGS-PV-1581-T #13 and #16 are formed by grooves with a preferential orientation whereas in the cluster on UFRGS-PV-1581-T #17 the grooves are not easily distinguishable, especially in the center of the concentration (Fig. 7A). The lengths of the subcircular clusters range from 2.90–5.26 mm, being 1.1–1.8×greater than the diameters, which range from 1.74–4.78 mm. The subcircular cluster on UFRGS-PV-1581-T #13 is associated with an irregular cluster of grooves that may be a partial subcircular cluster destroyed by the fragmentation of the bone, since it is located exactly on the edge of the fragment (Fig. 7B). This would be the only case of subcircular clusters occurring together. The subcircular cluster on UFRGS-PV-1581-T #17 is associated with an elongated cluster similar to a trail, but with grooves oriented parallel to the large axis of the cluster, as opposed to Osteocallis, where the grooves are mainly perpendicular to the trail length (Fig. 7A).
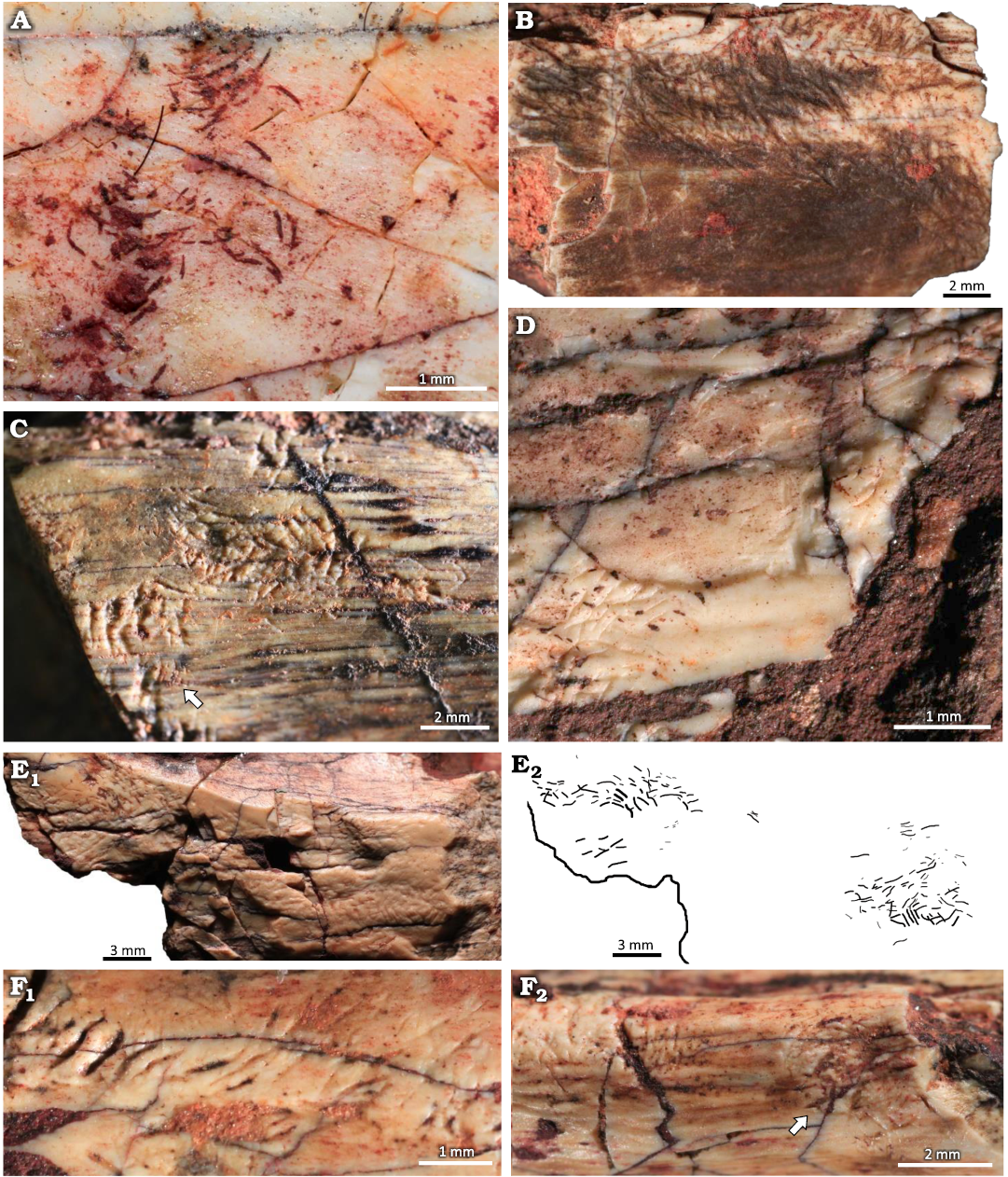
Fig. 6. Clusters of grooves on bone fragments of the rhynchosaurid archosauromorph Hyperodapedon mariensis (Tupi Caldas, 1933) from Buriol Site, Brazil, Hyperodapedon AZ, Carnian. A. UFRGS-PV-1581-T #20, arcuate and paired grooves, similar to feeding traces of Osteocallis mandibulus Roberts et al., 2007, but without forming a trail. B. UFRGS-PV-1581-T #22, densely concentrated grooves, giving the bone surface an etched appearence. C. UFRGS-PV-1581-T #5, straight and arcuate grooves closely associated to an incipient Osteocallis mandibulus (arrow). D. UFRGS-PV-1581-T #26, straight and arcuate grooves and some isolated grooves. E. UFRGS-PV-1581-T #6, dentary fragment; E1, two clusters of grooves; E2, schematic drawing. F. UFRGS-PV-1581-T #17; F1, subparallel grooves; F2, subparallel grooves associated to a subcircular cluster of grooves (arrow).
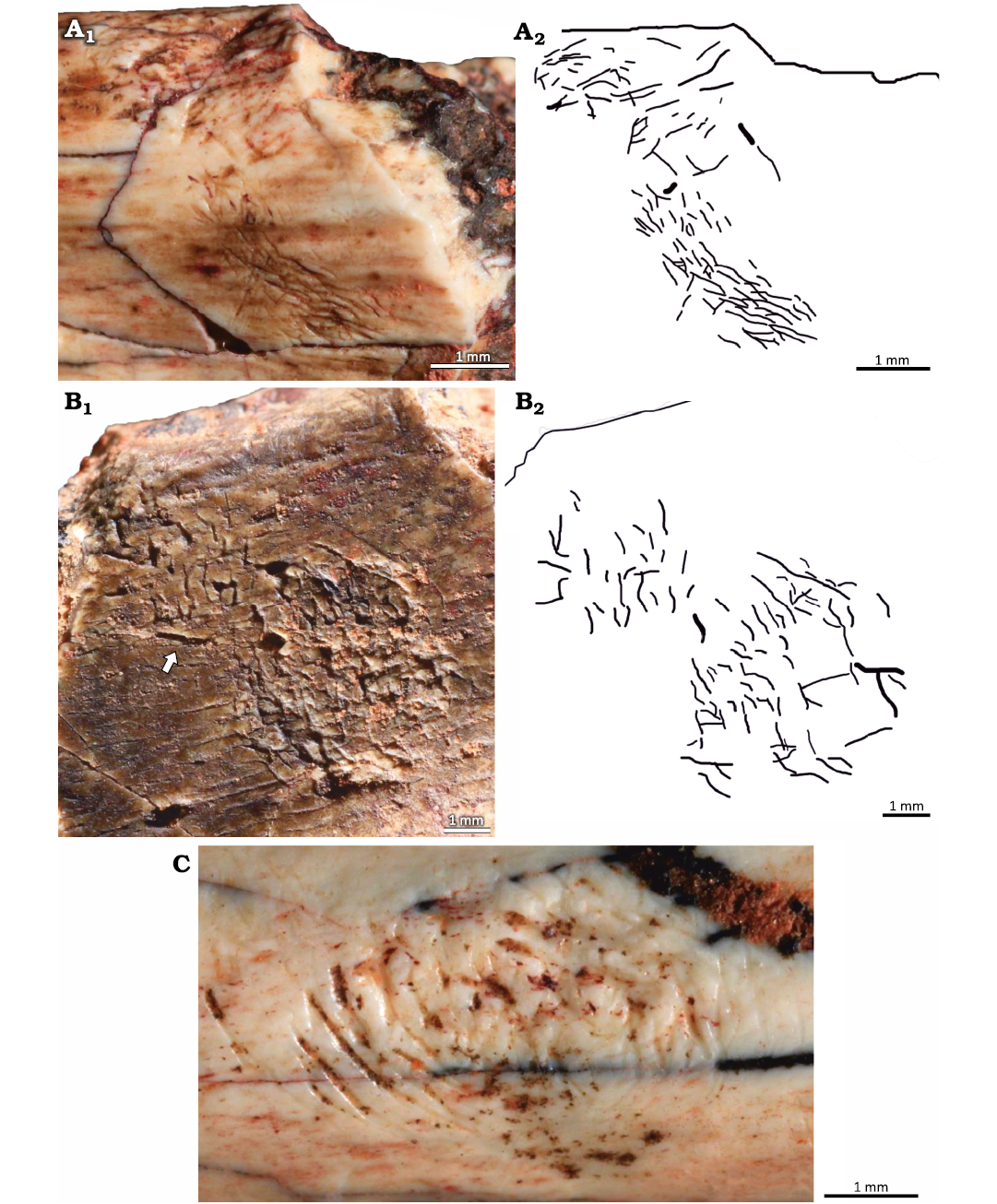
Fig. 7. Subcircular clusters on bone fragments of the rhynchosaurid archosauromorph Hyperodapedon mariensis (Tupi Caldas, 1933) from Buriol Site, Brazil, Hyperodapedon AZ, Carnian. A. UFRGS-PV-1581-T #17; A1, subcircular cluster connected to a cluster of grooves; A2, schematic drawing. B. UFRGS-PV-1581-T #13; B1, subcircular cluster associated to an irregular cluster of grooves (arrow), possibly a partially preserved subcircular cluster; B2, schematic drawing. C. UFRGS-PV-1581-T #16 showing an isolated subcircular cluster of grooves.
Some clusters are formed by arcuate and paired grooves identical to the morphology seen in O. mandibulus, but the lack of a preferential orientation and successiveness prevent their classification as trails and therefore their assignment to this ichnogenus (e.g., UFRGS-PV-1581-T #20; Fig. 6A). Clusters of grooves are associated with trails in UFRGS-PV-1581-T #5, #11, #14, and #23. In UFRGS-PV-1581-T #14, the cluster overlaps a trail and is formed by grooves with a length that reaches over 2 mm and a width of ~0.2 mm, whereas the largest grooves in the trail have a length of ~0.4 mm and a width of ~0.06 mm (Fig. 5A, B). The trail on UFRGS-PV-1581-T #23 is associated with a cluster mainly composed by straight grooves forming a crescent shape that resembles the subcircular clusters described above (Fig. 4B).
Morphotype 2: borings
Fig. 8.
Material.—UFRGS PV-1581-T#3, #7, #9.
Description.—The borings are channel-like structures with at least one rounded termination (Fig. 8). Three borings were identified, their length ranging from 4.34–7.84 mm and their width ranging from 3.0–7.0 mm. They are straight to slightly tortuous with only one defined round termination and U-shaped cross section. The boring on UFRGS PV-1581-T #3 is perpendicular to the Amphifaoichnus trace that occurs in the opposite surface of the bone, but it penetrates it as well (Fig. 8A). The borings on UFRGS PV-1581-T #7 and #9 are not associated with other bioerosion traces (Fig. 8B, C1). Bone chips are scattered over the base of the borings on UFRGS PV-1581-T #3 and #9 (Fig. 8A2, C2). The borings differ from UFRGS PV-1581-T #10 as they are not as elongated, with a length-width ratio varying from 1.09–1.44, whereas in UFRGS PV-1581-T #10 the ratio is 2.43. Also, no indication of fill is present in any of the borings. These morphological differences preclude their classification as Amphifaoichnus. The borings differ from Cuniculichnus variabilis Hopner and Bertling, 2017, for the lack of tapering ends; also, there is no indication of a succession of types from holes to notches and tunnels.
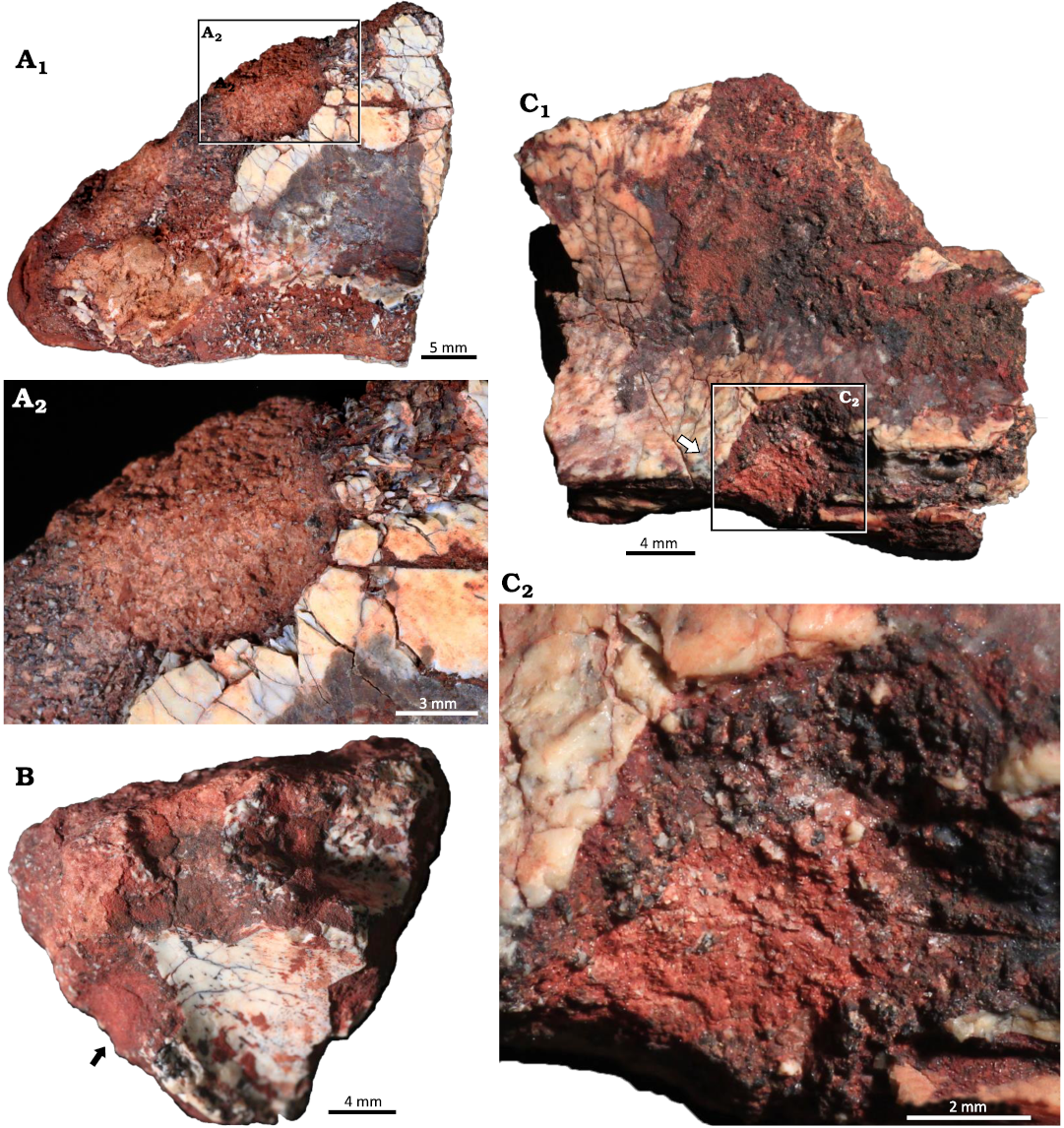
Fig. 8. Indiscrete borings on bone fragments of the rhynchosaurid archosauromorph Hyperodapedon mariensis (Tupi Caldas, 1933) from Buriol Site, Brazil, Hyperodapedon AZ, Carnian. A. UFRGS-PV-1581-T #3; A1, a boring in the opposite face of the arthropod bioerosion trace fossil Amphifaoichnus, but also penetrating it; A2, close up view showing the presence of bone chips in the base of the boring. B. UFRGS-PV-1581-T #7 showing a boring with one rounded termination. C. UFRGS-PV-1581-T #9; C1, an elongated boring with a rounded termination and bone chips scattered on the base; C2, close up view highlighting the bone chips scattered on the base.
Discussion
Tracemaker identity.—The identification of a tracemaker for bioerosion traces in bones allows a series of paleoecological and taphonomic inferences that expand their importance as a source of paleontological information. However, caution is necessary in such identification because different tracemakers may produce identical structures when behaving similarly (Bromley 1996). Furthermore, actualistic experiments focusing on bioerosion in bones are still scarce and when dealing with materials as old as the Triassic, many ethological changes yet undiscovered may have occurred between groups of organisms.
Bioerosion traces made in bones deposited in continental settings are mainly attributed to insects, especially dermestid beetles (e.g., Kitching 1980; Rogers 1992; Martin and West 1995; Britt et al. 2008) and termites (e.g., Watson and Abbey 1986; Kaiser 2000; Fejfar and Kaiser 2005; Backwell et al. 2020). Trace morphologies as those present on UFRGS-PV-1581-T, such as isolated and clustered grooves, have been reproduced in experiments using both termites (Backwell et al. 2012) and dermestids (Parkinson 2022) and, consequently, are not a precise source of information of the identity of the tracemaker. Other traces reported in this contribution (Amphifaoichnus and Osteocallis) do not support less inclusive assignments as well. Pirrone and Buatois (2016) did not assign Amphifaoichnus to a specific producer, but hypothesized that the tracemaker may have been a coleopteran with a heavily sclerotized body and well-developed mandibles capable of destroying bone tissue. This caution is reinforced by the lack of actualistic experiments reproducing Amphifaoichnus structures. Contributions reporting the occurrence of Osteocallis in continental fossil bones do not assign the traces to a specific producer either, only relating them to the action of insects (Roberts et al. 2007; Paes Neto et al. 2016; Perea et al. 2020). The materials referred to Osteocallis leonardii are attributed to the grazing action of polyplacophorans (Collareta et al. 2023), but the lack of evidence for transportation in UFRGS-PV-1581-T makes it highly unlikely that the tracemakers were marine organisms. Even though grooves have been observed in actualistic experiments, both isolated or organized in clusters, trails (as seen in Osteocallis) are yet to be reproduced.
The presence of the two groups of insects usually related to bioerosion in bones on continental settings is also an important point to consider. Molecular phylogenetics suggest that the origin of Coleoptera occurred in the earliest Permian and the divergence of Dermestidae in the Late Triassic (e.g., Zhang et al. 2018). Therefore, dermestid beetles may have been components of the ecosystems contemporaneous to the deposition of the SMS. In contrast, the diversification of termites would have occurred in the Late Jurassic (Bourgignon et al. 2014), thus, their presence in the Carnian strata of the Candelária Sequence would be unlikely. Even though molecular analyses recognized dermestid beetles in Late Triassic ecosystems, their presence by itself is not sufficiently supportive for identifying them as producers of the traces analyzed herein, since the Dermestidae family presents a wide range of feeding strategies and the behavior of Triassic species could differ considerably from what is observed on extant necrophagic species of the genus Dermestes. Also, other still unknown Triassic arthropods could behave similarly to what is observed in dermestids today and produce the traces.
The identification of the tracemakers of UFRGS-PV-1581-T to less inclusive taxonomic levels is difficult, but the morphology of the traces strongly points toward terrestrial arthropods as the producers. In addition, records of Skolithos serratus both in the Buriol (HF and PD-D, personal observation) and Predebon (about 1 km far from the former) sites are interpreted as burrows excavated by tiger beetle larvae (Nascimento and Netto 2019), attesting to the presence of insects in these ecosystems. Therefore, considering the information presented above, we are able to assign the traces found in UFRGS-PV-1581-T to terrestrial insects.
Ethological remarks.—UFRGS-PV-1581-T a wide array of traces with at least four distinct morphotypes identified. This diversity may reflect the presence of more than one biological species exploring the rhynchosaur carcass, a behavioral complexity among the tracemakers, possible ontogenetic changes in the carcass exploitation behavior or even a combination of these possibilities. In order to better understand the possible scenarios behind bone modification in UFRGS-PV-1581-T specimens, an analysis of the conditions in which the traces were produced and their ecological meaning is required.
Grooves and striae are possibly the most recurrent traces produced by insects in bone, occurring isolated, in clusters, forming trails and associated with a variety of other traces (e.g., Kaiser 2000; Laudet and Antoine 2004; Fejfar and Kaiser 2005; Roberts et al. 2007; West and Hasiotis 2007; Britt et al. 2008; Backwell et al. 2012; Paes Neto et al. 2016; Perea et al. 2020; Parkinson 2022). Britt et al. (2008) interpreted grooves (referred to as “scratches”) as probing traces, differing from Osteocallis, which would be a more complex feeding trace. In UFRGS-PV-1581-T grooves are observed isolated, in clusters and forming trails, sometimes with more than one morphotype occurring in association. Most of the clusters are formed by straight and non-paired grooves, but clusters of arcuate and paired grooves are also observed and are likely produced by the same organism as Osteocallis. If we consider the interpretation of Britt et al. (2008), the occurrence of feeding traces (including Osteocallis) would be concentrated on areas of higher nutritional value, while probing traces could occur in any portion of the bones. In the five fragments (UFRGS-PV-1581-T #5, #8, #11, #14, and #23) that present both clusters of grooves and Osteocallis, the grooves forming the clusters are larger and morphologically distinct, being predominantly straight and non-paired. The clusters and the trails target the same bone region, which may be indicative of both being feeding traces. The difference in size and shape of the grooves points to two distinct trace makers, or at least two different ontogenetic states.
Even though Osteocallis is usually interpreted as a feeding trace, it is still debatable if it represents a necrophagic or osteophagic aculate. Hopner and Bertling (2017) stated that the intentionality in the production of Osteocallis is highly speculative, whilst West and Hasiotis (2007) interpreted traces very similar to Osteocallis (referred to as “scallops”) as grazing traces (Pascichnia) of insects feeding on the periosteum rather than the bone. Considering this interpretation, the feeding traces in UFRGS-PV-1581-T (Osteocallis and associated clusters of grooves) could be concentrated in regions of the bone where the periosteum was still preserved. Collareta et al. (2023) relates Osteocallis leonardii to polyplacophoran algal grazing, but consider carrion scavenging and bone consumption as possible scenarios as well. The reproduction of Osteocallis in controlled experiments or its observation in present ecosystems might help elucidate the nature of the feeding behavior it represents.
Amphifaoichnus was assigned to the Fodinichnia ethological group by Pirrone and Buatois (2016) based on the presence of bone chips in the infilling, which would indicate bone consumption. Britt et al. (2008) interpreted bone fragments in the matrix next to bioeroded bones as relicts of frass (insect feces), therefore associating the traces to osteophagy as well. Paik (2000), on the other hand, while attributing the presence of bone fragments in the matrix subjacent to bioeroded bones to the action of carrion insects, did not conclude that they were used for food. The presence of bone chips in the sediment close to bioeroded bones or within the filling of bioerosion traces might not be direct evidence for bone consumption, because the bone chips, even when removed from its source by the action of insects, could have been deposited without being ingested. The frass of insects that feed on wood, for instance, is composed not only of excrements, but also material loosened from the tree without being consumed (Nuorteva and Kinnunen 2008). The excrements differ from the loosened material by being roughly homogeneous in size and shape, and by being enveloped in a thin film (Nuoerteva and Knnunen 2008). Also, excrements of some species of insects might present identifiable surface features, such as the grooves in the excrements of Sphinx pinastri (Lepidoptera) larvae (Nuoerteva and Knuunen 2008: pl. 1B). In addition to the absence of any surface features indicating consumption, bone chips in UFRGS-PV-1581-T present no consistency in shape or sorting by size, meaning that there is no strong evidence for interpreting them as insect frass. Therefore, the osteophagical behavior is not clear for Amphifaoichnus traces.
Other invertebrate traces from nearby sites as well as the Buriol site may help reveal the behavior behind the production of Amphifaoichnus. Specimens of Skolithos serratus resemble UFRGS-PV-1581-T #10, both being circular in cross section and of roughly the same width. The interpretation for Skolithos serratus is that they are burrows excavated by tiger beetle larvae (Coleoptera, Cicindelidae), which prey by positioning themselves in the entrance of the burrow and ambushing other invertebrates (Nascimento and Netto 2019 and references therein). The burrows would also function as domiciles, protecting the beetles in larval stage and their pupae until reaching the adult stage. The difference in substrates of S. serratus (sediment) and Amphifaoichnus (both sediment and bone) is not sufficient to discard the possibility of both traces being produced by the same organism, as Pirrone and Buatois (2016) stated that modes of penetration and behavioral strategies may be independent of the type of substrate. A more significant difference may be the type of filling, since S. serratus is passively filled by sediment and Amphifaoichnus is actively backfilled, presenting a meniscate pattern. This could represent a difference in the purpose of the structures, therefore indicating different tracemakers or different behaviors of the same tracemaker. Another ichnogenus found in Buriol and other sites nearby is Taenidium, which also presents meniscate backfilling. This trace is usually attributed to deposit feeders and is interpreted as Fodinichnia, but Savrda et al. (2000) have discussed the problems with these attributions when no clear evidence indicating feeding behavior is present. For the Taenidium of SMS, Netto (2007) adopted a different explanation, relating the vertical orientation of the burrows to a low water table forcing the organisms to excavate deeply until reaching more humid conditions within the substrate. It is possible that, when excavating the substrate, the organisms would encounter the bones and pass through them, rather than deviating. A similar hypothesis was defended by Rogers (1992), who interpreted bioerosion in dinosaur bones from the Two Medicine Formation (Cretaceous of USA) as the result of chance encounters during subterranean excavations of puparial chambers by dermestid beetles. This hypothesis could be reinforced for UFRGS-PV-1581-T if the orientation of the bone fragments matched those of Taenidium, but this information was not possible to infer during the material collection. Regardless, carcasses tend to increase moisture in the soil (Quagiotto et al. 2019), therefore the buried bones could attract burrowing insects, functioning as a humidity island within the dry sediment.
The occurrence of Amphifaoichnus and Osteocallis in the same bone fragment might be another useful tool for understanding the possible behaviors associated with the traces. Being a feeding trace, Osteocallis would not necessarily be restricted to chance encounters, but instead could be linked to a more recurrent ecological relationship between insects and vertebrate carcasses. In this sense, Amphifaoichnus could represent a temporary domicile used by the insects when feeding on the bones or the remaining soft tissues. In fact, the trails in UFRGS-PV-1581-T #3 are directly connected to Amphifaoichnus, reinforcing the hypothesis that these traces were produced by the same trace maker employing different behaviors.
The lack of direct evidence precludes the certain attribution of any of the above-mentioned behaviors to the production of Amphifaoichnus. Actualistic experiments might help solving the origin of this trace in the future, but at this moment its function and relation to necrophagic or osteophagic behavior is uncertain.
The ethological meaning of subcircular clusters of grooves is also an intriguing question. Similar morphologies reported from the fossil record present, besides the grooves, depressions on the surface of the bones and are interpreted as incipient borings or pits (Laudet and Antoine 2004; Britt et al. 2008; Parkinson 2022). Laudet and Antoine (2004) interpreted these traces as incipient dermestid pupal chambers, whilst for Britt et al. (2008) they are attempts at penetration into the trabecular bone. The only subcircular cluster with a depression is the one in UFRGS-PV-1581-T #17, but it is irregular and considerably different from the incipient borings. It is possible that the subcircular clusters do not reflect a specific behavior, being only a coincident morphology. Another explanation is that they represent an even more incipient stage of abandoned borings or pits.
Taphonomic remarks.—The presence of Osteocallis is usually associated with subaerial exposure of skeletal remains, since dermestid beetles, the insect group most commonly associated with bioerosion in bones, act over exposed carcasses during the dry stage of decomposition. Müller et al. (2015) reported a trail of grooves attributable to Osteocallis in Exaeretodon remains from the Janner Site (Exaeretodon sub-AZ of the Hyperodapedon AZ, Fig. 1C), and used this occurrence as an argument supporting the idea that the bones were subaerially exposed. Francischini et al. (2018), Fontoura et al. (2018) and Perea et al. (2020) also associated the presence of Osteocallis with subaerial exposure of the bony substrate. Paes Neto et al. (2016) defended a pre-burial origin for trails of grooves in bones from the Janner Site as well, but the presence of bioturbation structures in the adjacent sediment raised the possibility that the traces were produced underground and that the trace makers could be soil burying insects like beetles from the families Histeridae, Silphidae, and Staphilinidae.
On the other hand, the compound morphology of Amphifaoichnus indicate that the bony substrate should be at least partially buried when the trace was produced. Therefore, the association between Amphifaoichnus and Osteocallis, as seen in UFRGS-PV-1581-T #3, might be additional evidence supporting a post-burial origin for trails of grooves on the bone surface. Indeed, bioerosion in buried bones has been reported before (Rogers 1992; Huchet et al. 2011) and is strongly supported in the case of dinosaurs from the Djadokhta and Barun Goyot formations (Cretaceous of Mongolia; Saneyoshi 2011), where bioturbation structures are directly connected to borings in bones, sometimes passing through them and continuing in the circumjacent sediment. In these cases, the burrows are lined with fine bone material for a short distance after passing through the bone, demonstrating a clear association of burrows and borings (Kirkland et al. 1998). In the same locality, invertebrate bioturbations are found, including meniscate backfilled burrows (Fastovsky et al. 1997). Roberts et al. (2007) commented that some of the traces associated to Osteocallis in the bones from the Kaiparowits Formation (Cretaceous of USA) are meniscate back-filled burrows. Burrows with meniscate backfilling and bone chips were also reported by Paik (2000) in sediments subjacent to bioeroded bones. Pirrone and Buatois (2016) noted similarities between these traces and Amphifaoichnus, stating, however, that their attribution to this ichnogenus would depend on a reevaluation of the material. Even though burrows are often associated with bored bones, Paik (2000) defended a subaerial scenario for the bioerosions. Based on the evidences of this and previous contributions, the automatic association of Osteocallis and subaerial exposition might be problematic and a post-burial origin for trails of grooves on the bone surface may be considered as a plausible scenario as well (Fig. 9).

Fig. 9. Reconstruction of insect exploration on bone fragments of the rhynchosaurid archosauromorph Hyperodapedon mariensis (Tupi Caldas, 1933) in UFRGS-PV-1581-T. Insect activity is depicted as occurring both in exposed and buried portions of the carcass (see the text for other hypotheses). Reconstuction by Zeinner de Paula.
Adopting a post-burial hypothesis for the origin of Osteocallis, it is worth questioning if the morphology seen for this trace in the fossil record is affected by a preservational bias. As seen in UFRGS-PV-1581-T #10, the sediment filling of Amphifaoichnus might not be preserved, resulting in a simple and concave bioerosion trace on the surface of the bone. Xing et al. (2013) described that, in cases where the convex tunnels of Taoteichnus are detached from the bone, it is possible to observe shallow grooves. Thorne and Kimsey (1983) reported a similar observation in actualistic experiments with termites in Panama. It is possible that Osteocallis originally presented a convex structure covering the trails on the bone surface that was not preserved. In this case, its association with Amphifaoichnus might indicate that both traces would represent a similar behavior with very distinct levels of bone destruction. This hypothesis still lacks clear and direct evidence, but its consideration might guide actions of prospection (both field and collections) and caution in preparation of materials that may eventually lead to a confirmation.
It is important to consider, however, that the presence of Osteocallis and Amphifaoichnus in the same bone fragment is not necessarily indicative of coeval production. The skeletal remains may have been utilized as a substrate in distinct times, both subaerially exposed and/or after burial. This hypothesis would indicate either the presence of at least two phases of the bone taphonomic history or of a certain kind of species able to modify both exposed and buried bones.
Paleoecological and evolutionary remarks.—The succession of organisms exploring terrestrial vertebrate carcasses is well described in the literature and insects are important components in most of the stages of the decomposition process (e.g., Bornemissza 1957; Smith 1986). The abundance and diversity of species occurring in carcasses decrease in the last stages of decomposition as the nutrients become less available and their exploration is more difficult (Payne 1965). Therefore, adaptations for the exploration of the remaining tissues still present in the dry stage of the carcass decay, like fur, skin and other keratinous elements, are important for necrophagous insects competing for resources. In this sense, osteophagy and other uses of the bone substrate would be behaviors capable of prolonging the value of carcasses as a resource, because vertebrate skeletons can remain exposed for more than a decade depending on the environmental conditions (Behrensmeyer 1978). The morphological complexity of Amphifaoichnus, a trace composed by the modification of both bone and sediment, suggests a certain degree of specialization of the tracemaker for bone exploration and, along with the other traces in UFRGS-PV-1581-T, demonstrates that in the ecosystems of the base of the Candelária Sequence the carcasses were explored by insects in latter decomposition stages, where nutrients were scarce and difficult to access. This hypothesis is further strengthened by the evidences of exploration of buried bones, which would expand the availability and duration of resources for organisms with such adaptations.
The record of bioerosion in bones from the SMS indicates that not only rhynchosaur remains were a resource for insects exploring carcasses, but also the remains of dicynodonts (Paes Neto et al. 2016; Medeiros et al. 2019), cynodonts (Müller et al. 2015; Paes Neto et al. 2016; Pavanatto et al. 2018), and dinosaurs (Paes Neto et al. 2016; Francischini et al. 2018). Considering that several groups of tetrapods are represented in the record of bioerosion in bones from the SMS, it is possible to infer that decaying carcasses were an important resource in these Carnian–Norian ecosystems, which would explain the adaptations allowing the exploration of these resources by insects.
To this moment, the fossil record shows that the exploration of bones by insects on continental settings arose during the Middle Triassic (Paes Neto et al. 2016) and was associated with large terrestrial tetrapods. Today, insects are responsible for much of the weight reduction seen in carcasses during the decomposition process (Payne 1965). However, there is much yet to be discovered about the bone destruction promoted by the action of insects during carcass decay, both in past and recent ecosystems. Actualistic studies have mainly focused on establishing identifiable morphologies to aid in the recognition of trace makers for bioerosion in bones (Roberts and Rogers 2003; Backwell et al. 2012; Holden et al. 2013; Parkinson 2022) or on the forensic value of bone modification by insects (Zanetti et al. 2014, 2015a, b, 2019a, b; Charabidzé et al. 2022). Therefore, many taphonomic and paleoecological implications of the emergence of bone modification behavior by insects are yet to be discussed.
Amphifaoichnus shows an elevated level of bone destruction, indicating that the action of insects could be an important factor influencing the preservation of vertebrate skeletons and consequently the fossil record. The burial of bones is an important event aiding in their preservation, since buried bones would be more protected from weathering and the action of organisms (scavenging and trampling). Considering that Amphifaoichnus is probably the result of insects modifying buried bones, even in this favorable scenario the preservation of vertebrate skeletons could be negatively affected. The taphonomical importance of insects in the exceptional preservation of dinosaurs has been recently discussed by Drumheller et al. (2022). In their taphonomic model, invertebrates and microorganisms would be responsible for the removal of internal soft tissues, allowing for the desiccation of dermal tissue and favoring the preservation of both skin and bones. However, if the removal of bone by insects is added to this model, especially in the level seen in Amphifaoichnus, invertebrates might constitute an important factor over the preservation of these carcasses. Therefore, the emergence of the bone modifying behavior by insects in the Late Triassic may have influenced the fossil record of the whole Mesozoic.
UFRFS-PV-1581-T represents the oldest records of the ichnogenera Amphifaoichnus and Osteocallis. Since the specimens of Amphifaoichnus described by Pirrone and Buatois (2016) came from the Upper Cretaceous of Argentina, the records of the SMS extend the occurrence of this ichnogenus in more than 140 Ma, positioning it along with the first records of bioerosion in bones by insects and indicating that this morphology, although complex, emerged shortly after the bone modifying behavior. The records of Osteocallis reported by Paes Neto et al. (2016) come from the same biozone of the Candelária Sequence as UFRGS-PV-1581-T, however, they come from a portion of the Janner Site (Agudo municipality) characterized by the abundance of the cynodont Exaeretodon, representing the Exaeretodon sub-AZ of Schultz et al. (2020), the upper portion of the Hyperodapedon AZ. The Buriol Site, where UFRGS-PV-1581-T was collected, is considered to represent the lower portion of the Hyperodapedon AZ, therefore the records of Osteocallis described here are slightly older. Ecologically, the emergence of new ichnogenera in the early Late Triassic might represent a diversification of behaviors producing bioerosion traces in bones in comparison to the record of the Middle Triassic (Paes Neto et al. 2016), which is mainly composed of indiscrete borings and the only ichnogenus described is Cubiculum.
Conclusions
The material analyzed here represents new records of bioerosion in bones from the SMS and contribute to the knowledge of the diversity and complexity of bone exploration in Late Triassic ecosystems. The record of Amphifaoichnus is expanded back more than 140 Ma, showing that complex bone exploration behavior was present in Carnian insects, as evidenced by tunnels made on bone-sediment interface. This is indicative of the presence of specialized organisms and the importance of tetrapod carcasses as a resource for other components of the fauna. These new records also contribute to the taphonomic discussion regarding bioerosion, indicating that the automatic association between insect traces in bones and prolonged subaerial exposure is problematic due to the existence of evidence pointing to the possibility of bioerosion in buried remains. Furthermore, the level of bone destruction seen in Amphifaoichnus might indicate that insects were relevant taphonomic agents preventing the preservation of vertebrate carcasses even in subsurface conditions. The association of Amphifaoichnus to osteophagic behavior is questioned on the basis of a lack of direct evidence for this behavior. Other possible behaviors, such as the construction of temporary domiciles related to feeding or sediment moisture, are discussed based on the co-occurrence of Amphifaoichnus and Osteocallis and the presence of invertebrate burrows (Taenidium barreti and Skolithos serratus) in the Buriol and Predebon sites. New research might be key to advance the discussions brought here and to comprehend the potential of bioerosion in bones as a tool for studying paleoecology and taphonomy. Therefore, continuing work on bioerosion traces in bone is necessary to help us understand the evolutionary processes that influenced the evolution of bone exploration by insects.
Acknowledgements
We thank Daniel E. Barta (Oklahoma State University, Tahlequah, USA) for assistance during the editorial process. We thank the reviewers Voltaire Paes Neto (Universidade Federal do Pampa, São Gabriel, Brazil) and Christina Shears-Ozeki (Kyoto University, Japan) for their important suggestions that greatly improved the manuscript. We thank Pedro Henrique Fonseca (UFRGS) and Leonardo Kerber (CAPPA) for the useful tips on CT scans. We also thank Luís Flávio Lopes (UFRGS) for the high-quality photographs, Caio Scartezini, Cesar Schultz, and Bianca Mastrantonio (all UFRGS) for helping to identify the bones and Zeinner de Paula (PUC-MG) for the reconstruction in Fig. 9. This work was supported by the Fundação de Amparo à Pesquisa do Estado do RS and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior with grants to LSC (CAPES 88887.616819/2021-00), PD-D (19/2551-0002016-3), and HF (21/2551-0000734-6).
References
Backwell, L., Huchet, J.B., Jashashvili, T., Dirks, P.H.G.M., and Berger, L.R. 2020. Termites and necrophagous insects associated with early Pleistocene (Gelasian) Australopithecus sediba at Malapa, South Africa. Palaeogeography, Palaeoclimatology, Palaeoecology 560: 1–17. Crossref
Backwell, L.R., Parkinson, A.H., Roberts, E.M., d’Errico, F., and Huchet, J.B. 2012. Criteria for identifying bone modification by termites in the fossil record. Palaeogeography, Palaeoclimatology, Palaeoecology 337/338: 72–87. Crossref
Battista, F., Ribeiro, A.M., Ferigolo, J., and Schultz, C.L. 2021. “The Carnian Tales”: the post-mortem adventures of an Exaeretodon riograndensis. Paleodest 37 (76): 62.
Behrensmeyer, A.K. 1978. Taphonomic and ecologic information from bone weathering. Paleobiology 4: 150–162. Crossref
Bornemissza, G.F. 1957. An analysis of Arthropod succession in Carrion and the effect of its aculatesnn on the soil fauna. Australian Journal of Zoology 5 (1): 1–12. Crossref
Bourguignon, T., Lo, N., Cameron, S.L., Šobotník, J., Hayashi, Y., Shigenobu, S., Watanabe, D., Roisin, Y., Miura, T., and Evans, T.A. 2014. The evolutionary history of termites as inferred from 66 mitochondrial genomes. Molecular Biology and Evolution 32: 406–421. Crossref
Britt, B., Scheetz, R. and Dangerfield, A. 2008. A suite of dermestid beetle traces on dinosaur bone from the Upper Jurassic Morrison Formation, Wyoming, USA. Ichnos 15: 59–71. Crossref
Bromley, R.G. 1996. Trace Fossils: Biology, Taphonomy and Applications. Second Ed. 361 pp. Springer Science+Business Media, B.V, London.
Cabreira, S.F., Kellner, A.W.A., Dias-da-Silva, S., Roberto da Silva, L., Bronzati, M., Marsola, J.C. de A., Müller, R.T., Bittencourt, J. De S., Batista, B.J.A., Raugust, T., Carrilho, R., Brodt, A., and Langer, M.C. 2016. A unique Late Triassic dinosauromorph assemblage reveals dinosaur ancestral anatomy and diet. Current Biology 26: 3090–3095. Crossref
Charabidzé, D., Lavieille, V., and Colard, T. 2022. Experimental evidence of bone lesions due to larder beetle Dermestes aculates (Coleoptera: Dermestidae). Biology 11 (9): 1–11. Crossref
Collareta, A., Merella, M., Casati, S., Cencio, A. di, Tinelli, C., and Bianucci, G. 2023. Polyplacophoran feeding traces on Mediterranean Pliocene sirenian bones: Insights on the role of grazing bioeroders in shallow-marine vertebrate falls. Life 13 (327): 1–14. Crossref
Desojo, J.B., Fiorelli, L.E., Ezcurra, M.D., Martinelli, A.G., Ramezani, J., da Rosa, Á.A.S., von Baczko, M.B., Trotteyn, M.J., Montefeltro, F.C., Ezpeleta, M., and Langer, M.C. 2020. The Late Triassic Ischigualasto Formation at Cerro Las Lajas (La Rioja, Argentina): fossil tetrapods, high-resolution chronostratigraphy, and faunal correlations. Scientific Reports 10 (1): 1–34. Crossref
Dias-da-Silva, S., Sengupta, D.P., Cabreira, S.F., and da Silva, L.R. 2012. The presence of Compsocerops (Brachyopoidea: Chigutisauridae) (Late Triassic) in southern Brazil with comments on chigutisaurid palaeobiogeography. Palaeontology 55: 163–172. Crossref
Drumheller, S.K., Boyd, C.A., Barnes, B.M.S., and Householder, M.L. 2022. Biostratinomic alterations of an Edmontosaurus “mummy” reveal a pathway for soft tissue preservation without invoking “exceptional conditions.” PloS ONE 17 (10): 1–25. Crossref
Fejfar, O. and Kaiser, T.M. 2005. Insect bone-modification and paleoecology of Oligocene mammal-bearing sites in the Doupov Mountains, Northwestern Bohemia. Palaeontologia Electronica 8 (1): 1–11.
Fastovsky, D.E., Badamgarav, D., Ishimoto, H., Watabe, M., and Weishampel, D.B. 1997. The paleoenvironments of Tugrikin-Shireh (Gobi Desert, Mongolia) and aspects of the taphonomy and paleoecology of Protoceratops (Dinosauria: Ornithishichia). Palaios 12: 59–70. Crossref
Fontoura, E., Dentzien-Dias, P., and Francischini, H. 2018. Primeiro registro de traços de insetos em ossos do Pleistoceno do Rio Grande do Sul, Brasil. In: L.P.C. Agudelo, A.J.G. Cruz, and M.M. Sánchez (eds.), IV Simposio Latinoamericano de Icnología (SLIC 2018), Resúmenes, 64–65. Universidad de Caldas, Santa Marta.
Francischini, H., Paes Neto, V.D., Dentzien-Dias, P., da Rosa, Á.A.S., Leal, L.A., and Schultz, C.L. 2018. Traços de insetos nos ossos de Unaysaurus tolentinoi (Sauropodomorpha) do Triássico sul-brasileiro. In: L.P.C. Agudelo, A.J.G. Cruz, and M.M. Sánchez (eds.), IV Simposio Latinoamericano de Icnología (SLIC 2018), Resúmenes, 57–59. Universidad de Caldas, Santa Marta.
Gatta, M., Rolfo, M.F., Salari, L., Jacob, E., Valentini, F., Scevola, G., Doddi, M., Neri, A., and Martín-Vega, D. 2021. Dermestid pupal chambers on Late Pleistocene faunal bones from Cava Muracci (Cisterna di Latina, central Italy): Environmental implications for the central Mediterranean basin during MIS 3. Journal of Archaeological Science: Reports 35: 1–11. Crossref
Hasiotis, S.T., Fiorillo, A.R., and Hanna, R.R. 1999. Preliminary report on borings in Jurassic dinosaur bones: Evidence for invertebrate-vertebrate interactions. Miscelaneous Publication Utah Geological Survey 99 (1): 193–200.
Holden, A.R., Harris, J.M., and Timm, R.M. 2013. Paleoecological and taphonomic implications of insect-damaged Pleistocene vertebrate remains from Rancho La Brea, Southern California. PloS ONE 8 (7): 1–9. Crossref
Höpner, S. and Bertling, M. 2017. Holes in bones: Ichnotaxonomy of bone borings. Ichnos 24: 259–282. Crossref
Horn, B.L.D., Melo, T.M., Schultz, C.L., Philipp, R.P., Kloss, H.P., and Goldberg, K. 2014. A new third-order sequence stratigraphic framework applied to the Triassic of the Paraná Basin, Rio Grande do Sul, Brazil, based on structural and paleontological data. Journal of South American Earth Sciences 55: 123–132. Crossref
Huchet, J.B., Deverly, D., Gutierrez, B., and Chauchat, C. 2011. Taphonomic evidence of a human skeleton gnawed by termites in a Moche-civilisation grave at Huaca de la Luna, Peru. International Journal of Osteoarchaeology 21: 92–102. Crossref
Huchet, J.B., le Mort, F., Rabinovich, R., Blau, S., Coqueugniot, H., and Arensburg, B. 2013. Identification of dermestid pupal chambers on Southern Levant human bones: Inference for reconstruction of Middle Bronze Age mortuary practices. Journal of Archaeological Science 40: 3793–3803. Crossref
Kaiser, T.M. 2000. Proposed fossil insect modification to fossil mammalian bone from Plio-Pleistocene hominid-bearing deposits of Laetoli (Northern Tanzania). Annals of the Entomological Society of America 93: 693–700. Crossref
Kirkland, J.I., Delgado, C.R., Chimedtseren, A., Hasiotis, S.T., and Fox, E.J. 1998. Insect? Bored dinosaur skeletons and associated pupae from the Djadokhta Fm. (Cretaceous, Campanian). Journal of Vertebrate Paleontology 18 (Supplement 3): 56A.
Kitching, J.W. 1980. On some fossil arthropoda from the Limeworks, Makapansgat, Potgietersrus. Palaeontologia Africana 23: 63–68.
Langer, M.C. and Schultz, C.L. 2000. A new species of the late Triassic rhynchosaur Hyperodapedon from the Santa Maria Formation of South Brazil. Palaeontology 43: 633–652. Crossref
Langer, M.C., Ramezani, J., and da Rosa, Á.A.S. 2018. U-Pb age constraints on dinosaur rise from south Brazil. Gondwana Research 57: 133–140. Crossref
Langer, M.C., Ribeiro, A.M., Schultz, C.L., and Ferigolo, J. 2007. The continental tetrapod-bearing Triassic of South Brazil. In: S.G. Lucas and J.A. Spielmann (eds.), The Global Triassic. New Mexico Museum of Natural History and Science Bulletin 41: 201–218.
Laudet, F. and Antoine, P.O. 2004. Dermestidae (Insecta: Coleoptera) pupal chambers from a Tertiary mammal bone (phosphorites of Quercy): Taphonomic and palaeoenvironmental implications. Geobios 37: 376–381. Crossref
Leal, L.A., Azevedo, S.A.K., and da Rosa, Á.A.S. 2002. Icnofósseis do Sítio Fossilífero de Água Negra, Formação Caturrita, Neotriássico do Sul do Brasil. Arquivos Do Museu Nacional 60: 217–222.
Martin, L.D. and West, D.L. 1995. The recognition and use of dermestid (Insecta, Coleoptera) pupation chambers in paleoecology. Palaeogeography, Palaeoclimatology, Palaeoecology 113: 303–310. Crossref
Medeiros, T., Francischini, H., Paes Neto, V., and Schultz, C. 2019 Uma nova ocorrência de Osteocallis em ossos de tetrápodes da Zona de Associação de Riograndia. Paleodest 35 (73): 85.
Milani, E.J., Souza, P.A., and Fernandes, L.A. 2007. Bacia do Paraná. Boletim de Geociências Da Petrobras 15: 265–287.
Müller, R.T., Ismael de Araújo-Júnior, H., Sandro Schiller Aires, A., Roberto-da-Silva, L., and Dias-da-Silva, S. 2015. Biogenic control on the origin of a vertebrate monotypic accumulation from the Late Triassic of southern Brazil. Geobios 48: 331–340. Crossref
Nascimento, D.L. do and Netto, R.G. 2019. Skolithos serratus in paleosols: Paleobiological, paleoecological, and paleobiogeographical insights. Palaeogeography, Palaeoclimatology, Palaeoecology 530: 152–162. Crossref
Netto, R.G. 2007. Skolithos-dominated piperock in nonmarine environments: An example from the Triassic Caturrita Formation, Southern Brazil. SEPM Special Publications 88: 109–121. Crossref
Nuorteva, M. and Kinnunen, K.A. 2008. Insect frass in Baltic amber. Bulletin of the Geological Society of Finland 80: 105–124. Crossref
Paes Neto, V.D., Desojo, J.B., Brust, A.C.B., Schultz, C.L., Da-Rosa, A.A.S., and Soares, M.B. 2021. Intraspecific variation in the axial skeleton of Aetosauroides scagliai (Archosauria: Aetosauria) and its implications for the aetosaur diversity of the Late Triassic of Brazil. Anais da Academia Brasileira de Ciências 93 (Supplement 2): 1–36. Crossref
Paes Neto, V.D., Parkinson, A.H., Pretto, F.A., Soares, M.B., Schwanke, C., Schultz, C.L., Kellner, A.W. 2016. Oldest evidence of osteophagic behavior by insects from the Triassic of Brazil. Palaeogeography, Palaeoclimatology, Palaeoecology 453: 30–41. Crossref
Paik, I.S. 2000. Bone chip-filled burrows associated with bored dinosaur bone in floodplain paleosols of the Cretaceous Hasandong Formation, Korea. Palaeogeography, Palaeoclimatology, Palaeoecology 157: 213–225. Crossref
Parkinson, A.H. 2016. Traces of insect activity at Cooper’s D Fossil Site (cradle of humankind, South Africa). Ichnos 23: 322–339. Crossref
Parkinson, A. 2022. Modern bone modification by Dermestes aculates and criteria for the recognition of dermestid traces in the fossil record. Historical Biology 35: 567–579. Crossref
Pavanatto, A.E.B., Pretto, F.A., Kerber, L., Müller, R.T., Da-Rosa, A.A.S., and Dias-da-Silva, S. 2018. A new Upper Triassic cynodont-bearing fossiliferous site from southern Brazil, with taphonomic remarks and description of a new traversodontid taxon. Journal of South American Earth Sciences 88: 179–196. Crossref
Payne, J.A. 1965. A summer carrion study of the baby pig Sus scrofa Linnaeus. Ecology 46: 592–602. Crossref
Perea, D., Verde, M., Montenegro, F., Toriño, P., Manzuetti, A., and Roland, G. 2020. Insect trace fossils in glyptodonts from Uruguay. Ichnos 27: 70–79. Crossref
Philipp, R.P., Schultz, C.L., Kloss, H.P., Horn, B.L.D., Soares, M.B., and Basei, M.A.S. 2018. Middle Triassic SW Gondwana paleogeography and sedimentary dispersal revealed by integration of stratigraphy and U-Pb zircon analysis: The Santa Cruz Sequence, Paraná Basin, Brazil. Journal of South American Earth Sciences 88: 216–237. Crossref
Pirrone, C.A. and Buatois, L.A. 2016. Bioeroded dinosaur bones: Novel signatures of necrophagous a in a Cretaceous continental environment. Ichnos 23: 340–348. Crossref
Pirrone, C.A., Buatois, L.A., and Bromley, R.G. 2014. Ichnotaxobases for bioerosion trace fossils in bones. Journal of Paleontology 88: 195–203. Crossref
Quaggiotto, M.M., Evans, M.J., Higgins, A., Strong, C., and Barton, P.S. 2019. Dynamic soil nutrient and moisture changes under decomposing vertebrate carcasses. Biogeochemistry 146: 71–82. Crossref
Roberto-Da-Silval, L., Desojo, J.B., Cabreira, S.F., Aires, A.S.S., Müller, R.T., Pacheco, C.P., and Dias-Da-Silva, S. 2014. A new aetosaur from the Upper Triassic of the Santa Maria Formation, southern Brazil. Zootaxa 3764: 240–278. Crossref
Roberts, E. and Rogers, R.R. 2003. An experimental approach to identifying and interpreting dermestid (Insecta, Coleoptera) bone modification. Journal of Vertebrate Paleontology 23 (Supplement 3): 89A–90A.
Roberts, E.M., Rogers, R.R., and Foreman, B.Z. 2007. Continental insect borings in dinosaur bone: Examples from the Late Cretaceous of Madagascar and Utah. Journal of Paleontology 81: 201–208. Crossref
Rogers, R.R. 1992. Non-marine borings in dinosaur bones from the Upper Cretaceous Two Medicine Formation, Northwestern Montana. Journal of Vertebrate Paleontology 12: 528–531. Crossref
Saneyoshi, M., Watabe, M., Suzuki, S., and Tsogtbaatar, K. 2011. Trace fossils on dinosaur bones from Upper Cretaceous eolian deposits in Mongolia: Taphonomic interpretation of paleoecosystems in ancient desert environments. Palaeogeography, Palaeoclimatology, Palaeoecology 311: 38–47. Crossref
Savrda, C.E., Blanton-Hooks, A.D., Collier, J.W., Drake, R.A., Graves, R.L., Hall, A.G., Nelson, A.I., Slone, J.C., Williams, D.D., and Wood, H.A. 2000. Taenidium and associated ichnofossils in fluvial deposits, Cretaceous Tuscaloosa Formation, eastern Alabama, southeastern USA. Ichnos 7: 227–242. Crossref
Schultz, C.L., Martinelli, A.G., Soares, M.B., Pinheiro, F.L., Kerber, L., Horn, B.L.D., Pretto, F.A., Müller, R.T., and Melo, T.P. 2020. Triassic faunal successions of the Paraná Basin, southern Brazil. Journal of South American Earth Sciences 104: 1–24. Crossref
Serrano-Brañas, C.I., Espinosa-Chávez, B., and Maccracken, S.A. 2018. Insect damage in dinosaur bones from the Cerro del Pueblo Formation (Late Cretaceous, Campanian) Coahuila, Mexico. Journal of South American Earth Sciences 86: 353–365. Crossref
Smith, K. 1986. A Manual of Forensic Entomology. 205 pp. British Museum (Natural History) and Cornell University Press, London.
Thorne, B.L. and Kimsey, R.B. 1983. Attraction of neotropical Nasutitermes termites to carrion. Biotropica 15: 295–296. Crossref
Watson, J.A.L. and Abbey, H.M. 1986. The effects of termites (Isoptera) on bone: Some archeological implications. Sociobiology 11: 245–254.
West, D.L. and Hasiotis, S.T. 2007. Trace fossils in an Aarchaeological context: Examples from bison aculates, Texas, USA. In: W. Miller (ed.), Trace Fossils: Concepts, Problems, Prospects, 545–561. Elsevier, Amsterdan. Crossref
Wrobel, G.D. and Biggs, J. 2018. Osteophageous insect damage on human bone from Je’reftheel, a Maya mortuary cave site in west-central Belize. International Journal of Osteoarchaeology 28: 745–756. Crossref
Xing, L., Roberts, E.M., Harris, J.D., Gingras, M.K., Ran, H., Zhang, J., Xu, X., Burns, M. E., and Dong, Z. 2013. Novel insect traces on a dinosaur skeleton from the Lower Jurassic Lufeng Formation of China. Palaeogeography, Palaeoclimatology, Palaeoecology 388: 58–68. Crossref
Xing, L., Parkinson, A.H., Ran, H., Pirrone, C.A., Roberts, E.M., Zhang, J., Burns, M.E., Wang, T., and Choiniere, J. 2016. The earliest fossil evidence of bone boring by terrestrial invertebrates, examples from China and South Africa. Historical Biology 28: 1108–1117. Crossref
Zanetti, N.I., Ferrero, A.A., and Centeno, N.D. 2015a. Modification of postmortem wounds by Dermestes maculatus (Coleoptera: Dermestidae) activity: A preliminary study. Journal of Forensic and Legal Medicine 36: 22–24. Crossref
Zanetti, N.I., Ferrero, A.A., and Centeno, N.D. 2019a. Depressions of Dermestes maculatus (Coleoptera: Dermestidae) on bones could be pupation chambers. American Journal of Forensic Medicine and Pathology 40: 122–124. Crossref
Zanetti, N.I., Ferrero, A.A., and Centeno, N.D. 2019b. Scavenging Activity of Dermestes maculatus (Coleoptera: Dermestidae) on Burned Cadaveric Tissue. Neotropical Entomology 48: 1001–1013. Crossref
Zanetti, N.I., Visciarelli, E.C., and Centeno, N.D. 2014. Taphonomic marks on pig tissue due to cadaveric coleoptera activity under controlled conditions. Journal of Forensic Sciences 59: 997–1001. Crossref
Zanetti, N.I., Visciarelli, E.C., and Centeno, N.D. 2015b. Marks caused by the scavenging activity of Necrobia rufipes (Coleoptera: Cleridae) under laboratory conditions. Journal of Forensic and Legal Medicine 33: 116–120. Crossref
Zerfass, H., Lavina, E.L., Schultz, C.L., Garcia, A.J.V., Faccini, U.F., and Chemale, F. 2003. Sequence stratigraphy of continental Triassic strata of Southernmost Brazil: A contribution to Southwestern Gondwana palaeogeography and palaeoclimate. Sedimentary Geology 161: 85–105. Crossref
Zhang, S.Q., Che, L.H., Li, Y., Dan, L., Pang, H., Ślipiński, A., and Zhang, P. 2018. Evolutionary history of Coleoptera revealed by extensive sampling of genes and species. Nature Communications 9 (205): 1–11. Crossref
Acta Palaeontol. Pol. 69 (1): 1–21, 2024
https://doi.org/10.4202/app.01093.2023