


Discovery of chemosynthesis-based association on the Cretaceous basal leatherback sea turtle from Japan
ROBERT G. JENKINS, ANDRZEJ KAIM, KEI SATO, KAZUHIRO MORIYA, YOSHINORI HIKIDA, and REN HIRAYAMA
Jenkins, R.G., Kaim, A., Sato, K., Moriya, K., Hikida, Y., and Hirayama, R. 2017. Discovery of chemosynthesis-based association on the Cretaceous basal leatherback sea turtle from Japan. Acta Palaeontologica Polonica 62 (4): 683–690.
We report a Late Cretaceous chemosynthetic community fueled by decomposing basal leatherback sea turtle on the ocean floor in the western Pacific. The fossil association representing this community has been recovered from the matrix of a concretion containing a single carapace of Mesodermochelys sp. from Late Cretaceous outer shelf to upper slope deposit of northern Hokkaido, Japan. The carapace displays boreholes most likely performed by boring bivalves, and is associated with molluscan shells, mainly Provanna cf. nakagawensis and Thyasira tanabei. Since this association is similar to fauna already known from Late Cretaceous hydrocarbon seeps, sunken wood, and plesiosaur-falls in Hokkaido, it is suggested that all types of chemosynthesis-based communities in the Late Cretaceous of western Pacific may have belonged to the same regional pool of animals and were not yet fully differentiated into three independent types of communities as it is known today. This finding also indicates that the sulfophilic stage of the vertebrate-fall communities was supported not only by plesiosaur carcasses, which were previously reported, but also by sea turtle carcasses. It highlights the possibility of surviving vertebrate-fall communities through the end-Cretaceous mass extinction event on carcasses of sea turtles which are the only large marine vertebrates surviving this event.
Key words: Dermochelyoidae, Provannidae, Thyasiridae, vertebrate-fall, chemosynthesis-based ecosystem, Cretaceous, Japan.
Robert G. Jenkins [robertgj@staff.kanazawa-u.ac.jp], College of Science and Engineering, Kanazawa University, Kanazawa 920-1192, Japan.
Andrzej Kaim [kaim@twarda.pan.pl], Institute of Paleobiology, Polish Academy of Sciences, ul. Twarda 51 ⁄ 55, 00-818 Warszawa, Poland.
Kei Sato [keisato@kueps.kyoto-u.ac.jp], Faculty of Science, Kyoto University, Kitashirakawaoiwake-cho, Sakyo-ku, Kyoto 606-8502, Japan.
Kazuhiro Moriya [kazuhiro-moriya@asahi-koh.co.jp], Asahi Kohmatsushiryo LTD. Partnership Shin-Takine Mine, Ohgoe, Tamura-city, Fukushima 963-4111, Japan.
Yoshinori Hikida [nmhikida@coral.ocn.ne.jp], Nakagawa Museum of Natural History, Yasukawa 28-9, Nakagawa Town, Hokkaido 098-2626, Japan.
Ren Hirayama [renhirayama@gmail.com], School of International Liberal Studies, Waseda University, Nishiwaseda 1-6-1, Shinjuku-ku, Tokyo 169-8050, Japan.
Received 29 June 2017, accepted 10 August 2017, available online 20 October 2017.
Copyright © 2017 R.G. Jenkins et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Vertebrate-falls (e.g., whale-falls) on the sea floor support unique macrofaunal communities during its decaying and burial processes (Smith et al. 1989, 2015). Smith et al. (2002) and Smith and Baco (2003) proposed four ecologic stages in the development of modern whale-fall communities, i.e., mobile-scavenger, enrichment-opportunistic, sulfophilic, and reef stages. Vertebrate-fall communities attracted the attention of marine biologists after their discovery in 1989 partially because close relatives of organisms associated with enrichment-opportunistic and sulfophilic stages have also been found at deep-sea hydrothermal vents and/or hydrocarbon seeps. Hence, it is hypothesized that the vertebrate-fall habitats may have an important role in progressive adaption of organisms inhabiting hydrothermal vent and hydrocarbon seep environments, i.e., they serve as evolutionary stepping stones (Distel et al. 2000; Miyazaki et al. 2010; Lorion et al. 2013; Smith et al. 2015), although Kiel (2016) argues that biogeographic connectivity among chemosynthetic communities does not support dispersal stepping stone hypothesis. Fossil record of vertebrate-fall communities is essential in addressing this debate, regrettably it is still rather sparse, especially in the Mesozoic times when whales had not yet evolved. During that time only marine reptiles would have been important sources of large organic-falls on the deep-sea floor. According to Kaim et al. (2008b), who discovered chemosynthetic communities associated with two plesiosaurian carcasses from Upper Cretaceous Yezo Group, northern Japan, marine reptile carcasses can also support chemosynthetic life in a similar way to modern and ancient whale carcasses. However, majority of the large marine reptiles (plesiosaurs and mosasaurs) became extinct at or even before the K/Pg boundary (Motani 2009). Since whales first appeared in Eocene (Thewissen et al. 1994), there is ca. 15 Myr gap between plesiosaur extinction and whale appearance. Sea turtles are one of a few groups of large marine organisms which survived the Cretaceous/Paleogene extinction event (Motani 2009). Other groups, suggested to sustain chemosynthesis-based associations during that period, are large fishes (Dell 1987; Kiel 2008) and large sea-birds (Kiel et al. 2011). Lorion et al. (2009) deployed several possible substrates, including sea turtle bones, to check their suitability for development of vertebrate-fall communities. They found that bathymodiolins, which have chemosynthetic bacteria in their gills, had successfully colonized deployed turtle bones. In addition, Marshall (1994) reported a single specimen of the limpet Osteopelta cf. mirabilis from a middle Eocene leatherback turtle Dermochelys sp. from New Zealand which strongly resembles O. mirabilis from modern whale-falls. Recently, Danise and Higgs (2015) reported traces of bone-eating Osedax worm, considered to be heterotrophic (Tresguerres et al. 2013), from mid-Cretaceous plesiosaur and sea turtle bones. Thus, although it has been known since some time that sea turtle-falls could sustain vertebrate-fall communities in the geological past, details of chemosynthetic invertebrate associations with sea turtle-falls in the Mesozoic Era are still largely unknown. Here we present an example of chemosynthesis-based community on Cretaceous sea turtle Mesodermochelys sp. from Late Cretaceous Yezo Group in Hokkaido, Japan.
Institutional abbreviations.—ESKU, Earth Science Course, Kanazawa University, Kanazawa City, Ishikawa Prefecture, Japan; NMV, Nakagawa Museum of Natural History Vertebrate collections, Nakagawa Town, Hokkaido, Japan.
Material and methods
The Cretaceous sea turtle Mesodermochelys sp. (NMV 50; Dermochelyidae; Chelonioidea; Sato et al. 2012) and associated fauna were found in a large carbonate nodule (ca. 600 mm in diameter) by an amateur fossil collector, Fujiyuki Endo in the year 2001 at the tributary of Nio River in Nakagawa Town, northern Hokkaido, Japan where only the Osoushinai Formation of Yezo Group is cropping out (Fig. 1; Hashimoto 1967; Takahashi et al. 2003, 2007). The turtle fossil was extracted from the nodule with assistance from Nakagawa Museum of Natural History volunteers using chemical (formic acid) and mechanical preparations. To prevent dissolution by formic acid, the bones were frequently coated by paraloid (plastic) dissolved in acetic acid solvent. Along with most of the concretion matrix, it is likely that several molluscan fossils were also dissolved during the chemical procedure. However, the mechanical procedures that were carried out simultaneously with the chemical procedures, enabled recovery of some molluscan fossils from the subdivided concretions. The samples were first observed by naked-eyes and then the extracted molluscan fossils were studied with the aid of binocular and scanning electron microscopes (JSM-6010LV, JEOL) at the ESKU.
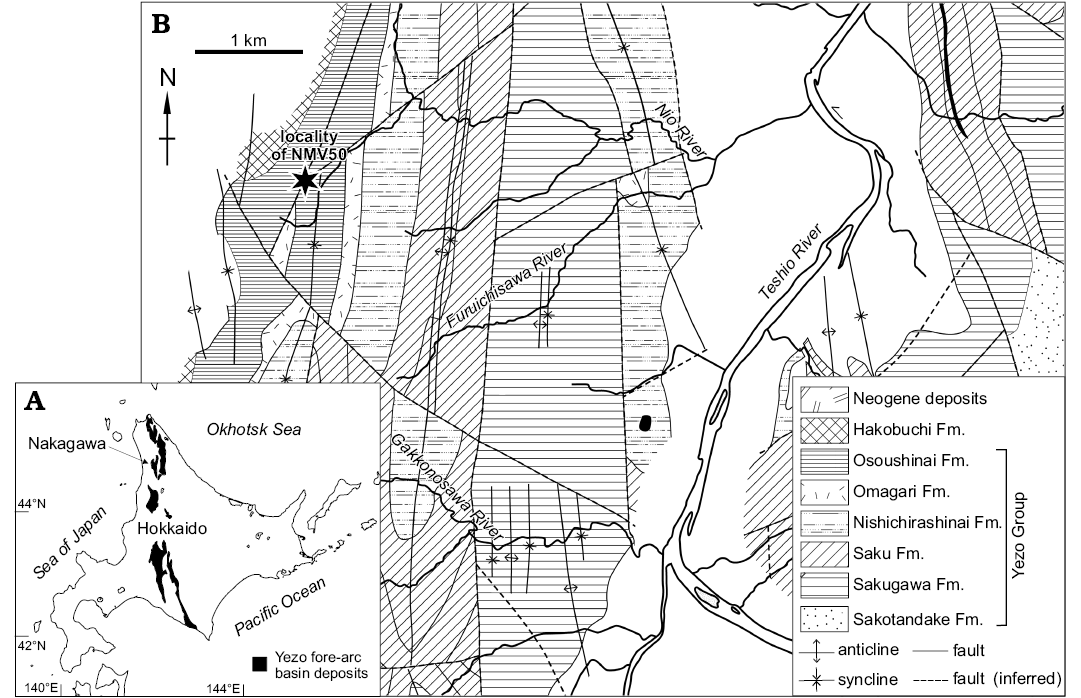
Fig. 1. Geology of the locality where the nodule containing Mesodermochelys sp. (NMV50) and associated invertebrate fossils were found. A. Map of Hokkaido with distribution of Yezo Group, Cretaceous fore-arc basin deposits. B. Detailed geological map of the Nakagawa Area, after Hashimoto et al. (1967).
Geological setting
The Yezo Group consists of Cretaceous marine clastic sediments (Takashima et al. 2004) formed in the Yezo fore-arc basin along the western margin of a subduction zone in the northwestern Pacific. The deposits of Yezo Group are distributed along a meridional belt in Hokkaido, Japan. The upper part of the Yezo Group and the Hakobuchi Formation, which unconformably overlie the Yezo Group, are distributed along the Nio River where the sea turtle was discovered. The upper part of the Yezo Group in this area is subdivided into the Sakugawa, Saku, Nishichirashinai, Omagari, and Osoushinai formations, in order of descending age. Among them, only the Osoushinai Formation crops out along the turtle-yielding tributary of Nio River. The Osoushinai Formation is composed of grey to dark grey siltstone with intercalations of sandstone. The underlying Omagari Formation is composed of mudstone with frequent intercalations of turbidite sandstone beds with many slump structures which were deposited on the landward slope of the trench. The overlying Hakobuchi Formation is mainly composed of sandy shallow water deposits with distinct hummocky cross-stratification. The Osoushinai Formation is located between these two types of deposits and thus most likely formed in the outer shelf to the upper continental slope setting (Jenkins et al. 2007; Takahashi et al. 2007; Kaim et al. 2009; Kiel et al. 2009). The age of Osoushinai Formation is assigned to Campanian, Late Cretaceous (Takahashi et al. 2003, 2007).
Results
Preservation of sea turtle carapace.—Nearly half of the large carapace of Mesodermochelys sp. (at least ca. 445 mm wide and ca. 435 mm long; Sato et al. 2012) was preserved in the investigated nodule (Fig. 2). Estimated total carapace length is ca. 700 mm. The carapace bears several bore holes, each hole is one to few centimeters in diameter, and the holes are distributed both on the external and internal surfaces. Several holes contain invertebrate shells, probably boring bivalves. However, due to the fragile nature of the carapace, it would have been difficult to extract these shells without damaging it. Hence, we refrained from extracting these invertebrate shells. The cancellous bones, which are below the external cortex layer of the carapace, were partially exposed. Some of the exposed areas were due to the action of boring mollusks. Other parts of the carapace were exposed by the action of formic acid at the time of sample preparation. In addition, selected sections of the carapace exhibited cancellous bones covered in a layer of sediments without a cortex layer; the cortex layer has apparently been removed prior to burial.
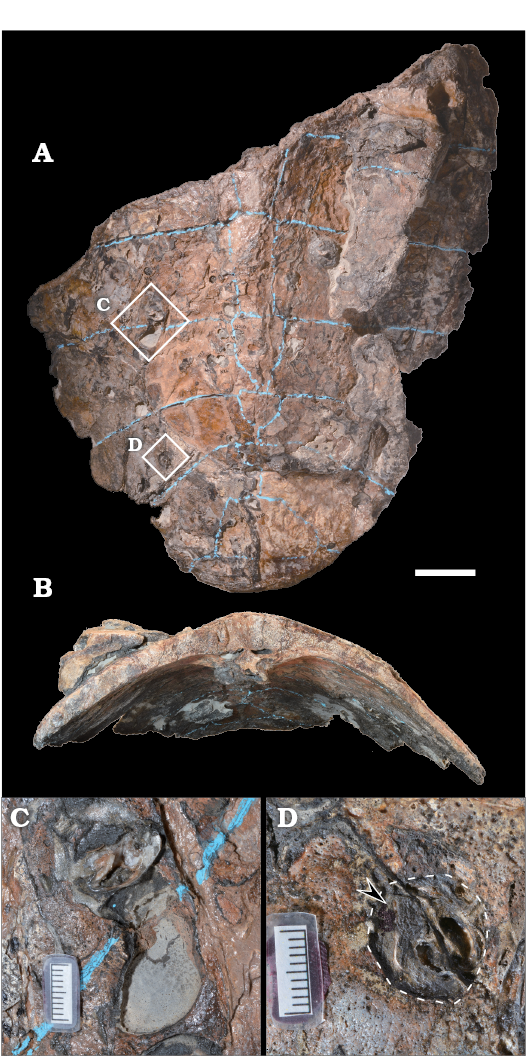
Fig. 2. Basal leatherback sea turtle Mesodermochelys sp. (NMV50) from Nio Creek, Nakagawa, Japan, Campanian, Upper Cretaceous. Carapace with drill holes on its surface, in dorsal (A) and anterior (B) views. Examples of drill holes (C, D), showing probable bivalve (arrow) in the hole (dashed line). Scale bars A, B, 50 mm; C, D, 10 mm.
Mollusks associated with sea turtle fall.—In total 12 molluscan fossils were found in the matrix surrounding Mesodermochelys sp. (NMV50; Fig. 3). The exact positions of the pieces of matrix containing the mollusc fossils within the nodule are not known. However, considering the size of the nodule, these fossils were distributed within a 150 mm radius of the sea turtle carapace. Most of the molluscan fossils were preserved with the original shell mineralogy and have not been subjected to significant diagenesis. The shell substance preservation state is much better than fossils from Late Cretaceous hydrocarbon seeps in Hokkaido (e.g., Jenkins et al. 2007; Kaim et al. 2008a, 2009) and appears to be pristine aragonite. Among the fossils associated with the turtle, abyssochrysoid gastropod Provanna cf. nakagawensis (Fig. 3A–C) is the most common species (three specimens), while Thyasira tanabei (Fig. 3H, I) is the second common species (two articulated specimens). The remaining taxa are represented by single specimens and have been identified preliminarily as Hikidea cf. yasukawensis (Fig. 3E), ?Gyrodes sp. (Fig. 3F), ?cephalaspid gastropod (Fig. 3G), ?abyssochrysoid gastropod (Fig. 3D). Additionally, we noticed one unidentified bivalve and two other shell fragments. P. cf. nakagawensis and H. cf. yasukawensis found in this study are very similar to Provanna nakagawensis and H. yasukawensis from Late Cretaceous Omagari and Yasukawa seeps in the Nakagawa, respectively (Kaim et al. 2008a, 2009). P. nakagawensis from the Omagari and Yasukawa seeps are characterized by spiral ornament consisting of two median prominent ribs and blunt nodes present at the intersections of spiral and axial ribs (Kaim et al. 2009). Strength of spiral ribs and nodes are variable among the specimens from seeps (Kaim et al. 2009), and P. cf. nakagawensis (Fig. 3A–C) reported in this paper shows stronger ribs and nodes than the P. nakagawensis from the Omagari and Yasukawa seeps. Hikidea yasukawensis displays variable strengths of spiral ornaments; the H. cf. yasukawensis reported in this paper has strong spiral ornament with faint ribs similar to H. yasukawemsis from the Yasukawa seep (Kaim et al. 2009: fig. 5B, C). Identification of other gastropods is still not clear at this moment and pending further study in the future. Thyasira tanabei is commonly found in the Cretaceous seeps in Japan (Kiel et al. 2008; Hryniewicz et al. 2017).
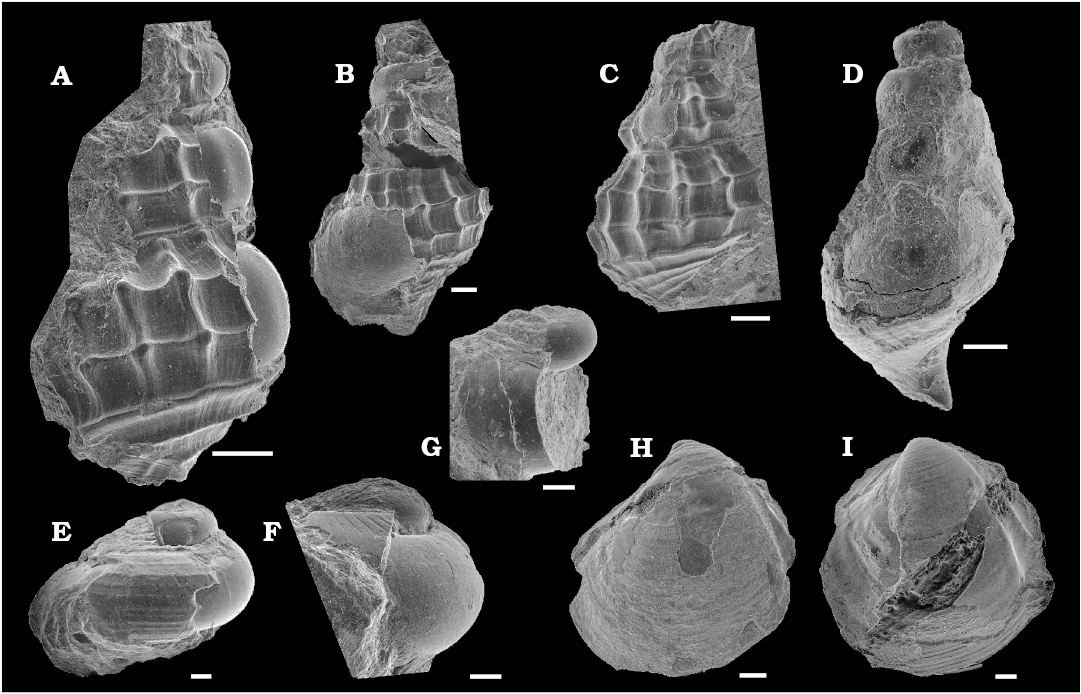
Fig. 3. SEM images of invertebrate fossils associated with the basal leatherback sea turtle Mesodermochelys sp. from Nio Creek, Nakagawa, Japan, Campanian, Upper Cretaceous. A–C. Abyssochrysoid gastropod Provanna cf. nakagawensis Kaim, Jenkins, and Warén, 2008a (A, NMV50-M01; B, NMV50-M04; C, NMV50-M09). D. ?Abyssochrysoid gastropod (NMV50-M11). E. Trochoid gastropod Hikidea cf. yasukawensis (Kaim, Jenkins, and Hikida, 2009) (NMV50-M03). F. ?Campaniloid gastropod ?Gyrodes sp. (NMV50-M10). G. ?Cephalaspid gastropod (NMV50-M06). H, I. Thyasirid bivalve Thyasira tanabei Kiel, Amano, and Jenkins, 2008 (H, NMV50-M02; I, NMV50-M12). Scale bars 500 μm.
Discussion
The combination of Provanna, Hikidea, and Thyasira associated with the Late Cretaceous sea turtle Mesodermochelys sp. from the Nakagawa area indicates that sea turtle dead-falls supported chemosynthetic life in the Late Cretaceous. All three taxa are known to be members of other chemosynthesis-based ecosystems. Species of abyssochrysoid Provanna constitute one of the most common groups of gastropods in seeps, vertebrate-falls and sunken woods, at least since the Late Cretaceous (Johnson et al. 2010; Kaim et al. 2008a, b; Kiel et al. 2009). Some extant large-sized taxa of abyssochrysoids (Alviniconcha and Ifremeria), classified so far jointly with Provanna, host endosymbiotic sulfur-oxidizing and/or methanotrophic bacteria in their bodies. However, according to both molecular and fossil data, it seems that large-sized abyssochrysoids most likely diverged from small-sized ones already in Jurassic/Early Cretaceous (compare Johnson et al. 2010; Kaim et al. 2014; Chen et al. 2016). The small-sized abyssochrysoids are unknown to host symbiotic bacteria; they are rather grazers on microbial mats (Warén and Bouchet 1986; Desbruyères et al. 2006). The species found in the studied turtle-fall are tiny and possess tall shells reminiscent of modern, grazing abyssochrysoids. This type of abyssochrysoids is known also from ancient vertebrate-falls, e.g., Cenozoic whale-falls and Cretaceous plesiosaur-falls (Kiel and Goedert 2006; Kaim et al. 2008b). Kaim et al. (2008b) illustrated plesiosaur bones corroded by dense aggregations of microborings and impregnated by pyrite crystals, and concluded that the abyssochrysoid gastropods fed on the microbial mats composed of sulfur-oxidizing bacteria covering the bones. Since we refrained from sectioning the single carapace at our disposal we do not have at this point any evidence of microbial activity on the carapace of Mesodermochelys sp. reported herein. Nevertheless, judging from the exclusive occurrences of Provanna in chemosynthetic environments (Kaim et al. 2012), where it grazes on microbial mats, we expect that Provanna cf. nakagawensis reported in this paper most likely also grazed on microbial mats developed on bones of Mesodermochelys sp. In addition, Provanna sp. was also found in a Late Cretaceous wood-fall (Kiel et al. 2009). Thus, this finding reinforces the idea that several taxa of abyssochrysoid gastropods were already adapted to seep, vertebrate-fall and sunken-wood environments in the Cretaceous (Johnson et al. 2010). Due to the limited numbers and incomplete specimens found so far, it is difficult to make a species level comparison between these communities, however we hope that the increasing number of records of Cretaceous chemosynthesis-based communities in Hokkaido, and especially in Nakagawa region, will allow us to compare them at species level in the near future. Hikidea is a genus recently established by Kaim et al. (2014) for small-sized taxa similar to the Recent Cantrainea which also occurs at seeps. Hikidea now consists of four nominal species and all are restricted to hydrocarbon seeps (Kaim et al. 2014, 2017). Recently Kiel et al. (2017) reported a Hikidea-like gastropod from Late Triassic seep in Turkey, however, it needs further investigations to confirm this identity because of its poor preservation (Kaim et al. 2017). In Nakagawa area, H. yasukawensis and H. omagariensis abundantly occur in Yasukawa and Omagari seeps, respectively (Jenkins et al. 2007; Jenkins and Hikida 2011; Kaim et al. 2009). In addition, Hikidea sp. and Hikidea-like species occurred in Late Cretaceous hydrocarbon seeps in Nakagawa and California (Kaim et al. 2009, 2014), a plesiosaur fall (Kaim et al. 2008b), and a sunken wood (Kiel et al. 2009) jointly with the abyssochrysoid gastropods, and has not yet been reported from non-chemosynthesis-based ecosystems. Thyasirids are a well-known infaunal bivalve group that lives in chemosynthetic environments. Although the detailed taxonomy of thyasirids is still poorly understood, the species described herein clearly belongs to Thyasira tanabei known from Cretaceous seeps and a sunken wood in Japan (Kiel et al. 2008, 2009; Hryniewicz et al. 2017). Dufour (2005) pointed out that many Recent large-sized species of thyasirids host chemosymbiotic bacteria. The shell length of T. tanabei is moderate, around 10 mm (Kiel et al. 2008), thus, it is difficult to judge whether T. tanabei hosted chemosynthetic bacteria. In total, at least half of the species found next to the investigated turtle carapace are known to be members of Late Cretaceous chemosynthetic communities. The remaining specimens are pending further taxonomic work, which may prove to be difficult due to their incomplete preservation. However the Provanna–Hikidea–Thyasira association alone points out to the development of chemosynthesis-based community on the Late Cretaceous sea turtle Mesodermochelys sp.
Since chemosynthesis-based associations were reported from the Late Cretaceous plesiosaur carcasses (Kaim et al. 2008b), it was hypothesized that other marine reptiles could also have supported vertebrate-fall communities (Vrijenhoek et al. 2009). Among marine reptiles, sea turtles are one of the rare groups to survive K/Pg boundary, and thus it is important to confirm their ability to support vertebrate deadfall communities. It is also worth to note that modern leatherback sea turtles have high oily subcutaneous insulation (Zug and Parham 1996) and decomposing of these fats may generate additional amounts of hydrogen sulfides by sulfate-reducing bacteria. This may make carcasses of these turtles particularly attractive to chemosynthesis-based communities in spite of their smaller sizes (in comparison to plesiosaurs and whales). Danise and Higgs (2015) reported traces of the bone-eating Osedax, which consumes lipids within the bones, in the Cretaceous sea turtles highlighting the susceptibility of turtle carcasses to develop the enrichment opportunistic stage. The finding in this paper confirms that the sea turtle-fall could also support the sulfophilic stage in the development of vertebrate-fall community as early as in the Cretaceous in a similar way to plesiosaur-falls (Kaim et al. 2008b; Danise and Higgs 2015). As suggested in several papers (e.g., Danise and Higgs 2015; Smith et al. 2015) the lipid content in the bone is crucial to develop the sulfophilic stage. Majority of marine reptiles had (and still have) cancellous bones―which are an adaptation to aquatic environment―with the lipids filling its marrow space (Houssaye 2013; Nakajima et al. 2014; Danise and Higgs 2015). Therefore, the other Mesozoic marine reptiles could potentially have had capability to develop such vertebrate-fall communities. In the case of ichthyosaur from the Late Jurassic of the UK, Danise et al. (2014) found the mobile scavenger, enrichment opportunistic and reef stages of a vertebrate-fall community, but no sulfophilic stage. Danise et al. (2014) argued that the absence of sulfophilic stage in their ichthyosaur-fall is due to its small size (ca. 3 m long) or too shallow depositional environment. Estimated carapace length of the Mesodermochelys reported herein is ca. 700 mm, therefore, its total length would be around 1 m. Although the amount of lipids within these bones and the total body weight are unknown, the size of the Mesodermochelys sp. is definitely smaller than the ichthyosaur from the UK. Thus, we think that the size alone would not be a reason for the absence of sulfophilic stage in the Late Jurassic ichthyosaur. The more plausible explanation stems from the shallow water setting of its deposition, where animals of photosynthetic communities may outcompete animals typical of sulfophilic stage (Danise et al. 2014), or perhaps inverse correlation between abundance of Osedax and chemosynthetic microbial mats with epifaunal assemblages suggested by Alfaro-Lucas et al. (2017). Dick (2015) also reported ichthyosaur-fall from Posidonia shale which did not sustain chemosynthetic community, but instead displayed increased biodiversity and biomass of background taxa around the carcass.
Another plausible explanation may come from the absence of macro bone eaters and/or drilling organisms on the ichthyosaur-falls described by Danise et al. (2014) and Dick (2015). In the modern oceans, activity of sulfate-reducing bacteria and resulting amount of released hydrogen sulfide is increased when the hard surface compact cortical layer of bone is mechanically torn off or drilled through, e.g., by Osedax worms, allowing sufficient amount of sulfate containing sea water to penetrate lipid rich cancellous bones (Deming et al. 1997; Treude et al. 2009), although excessive degradation of bones and organic matter within the bones by Osedax may also decrease the production of hydrogen sulfide due to higher oxygen content hampering the activity of sulfate reducing bacteria (Alfaro-Lucas et al. 2017). Vrijenhoek (2009) estimated splitting age of Osedax from other siboglinids during the Eocene or Cretaceous based on different molecular clock calibrations. Fossil evidence of the Osedax traces found from mid Cretaceous sea turtle and plesiosaur bones (Danise and Higgs 2015) supports the slower clock calibration for estimation of splitting age of Osedax form other siboglinid worms. Thus, the bone degradation has been accelerated since the mid Cretaceous and the sulfophilic stage of the vertebrate-fall community might have occurred more frequently since then.
In the case of Late Cretaceous Mesodermochelys carcass, there are many drill holes in the carapace which could have been made by boring bivalves (Fig. 2C, D). This kind of borings performed by bivalves on whale bones is known also from mammal and fish bones (Belaústegui et al. 2012). We do not know exact ecological impact on the formation of chemosynthetic community by these borings at this moment, however, they could facilitate the exposure of the cancellous layer of the bone before burial, and therefore, seawater could penetrate into the bones accelerating bacterial degradation of lipids within the bones. Subsequently, the hydrogen sulfide could have been released and the chemosynthesis-based organisms could flourish on the Late Cretaceous sea turtle-fall.
In addition, Kiel (2015) pointed out that the lowering sulfate concentrations in the Late Cretaceous sea water resulted in fewer emission of sulfides at seeps. The same phenomenon could result in a limited reaction of microbial sulfate-reduction in bones of vertebrate-falls in the Late Cretaceous (Kiel 2015). This hypothesis can explain why there are only few records of chemosynthetic communities on Cretaceous vertebrate-falls and no large-scaled (ca. < 100 mm length) chemosynthetic fauna in them.
Conclusions
One-meter sized Late Cretaceous basal leatherback sea turtle Mesodermochelys sp. and associated molluscan fauna were recovered from the Late Cretaceous outer shelf to slope deposits in northern Hokkaido, Japan. The molluscan fossils are represented mainly by abyssochrysoid gastropod Provanna and thyasirid bivalves. Similar composition was revealed in the Late Cretaceous plesiosaur-fall associations in Hokkaido and Cretaceous seeps worldwide. Modern relatives of these mollusks are also well known members of chemosynthetic communities. Thus, the molluscan fossils associated to the Late Cretaceous Mesodermochelys sp. can be interpreted as the chemosynthetic community developed on decaying Late Cretaceous sea turtle. This is the first certain record of chemosynthesis-based community associated with a sea turtle in geologic time, and it shows that sea turtle falls had the capability to sustain chemosynthesis-based communities during the Late Cretaceous equally well as plesiosaur falls. It is worth to stress that sea turtles are one of the very few groups of marine reptiles that survived the K/Pg mass extinction event. Thus, sea turtle falls could form a link in the evolutionary history of vertebrate falls between Cretaceous plesiosaur falls and Eocene-to-Recent whale-falls.
Acknowledgements
We thank Fujiyuki Endo (Nakagawa, Japan) who found the carapace and for his (and other volunteers at NMV) help in preparation of the specimen. The journal referees Silvia Danise (University of Georgia, Athens, USA) and Steffen Kiel (Swedish Museum of Natural History, Stockholm, Sweden) are thanked for helpful comments. We also thank for the linguistic adjustments of Prerna Chand (Kanazawa University, Japan). The research of RGJ was supported by JSPS KAKENHI Grant Number 26287131, 15H04412 and 16H05740 and Grant for Program to Disseminate Tenure Tracking System (JST). AK was supported by the research grant no. 2012/07/B/ST10/04189 from National Science Centre, Poland.
References
Alfaro-Lucas, J.M., Shimabukuro, M., Ferreira, G.D., Kitazato, H., Fujiwara, Y., and Sumida, P.Y.G. 2017. Bone-eating Osedax worms (Annelida: Siboglinidae) regulate biodiversity of deep-sea whale-fall communities. Deep-Sea Research Part II [published online]. Crossref
Belaústegui, Z., de Gibert, J.M., Domènech, R., Muñiz, F., and Martinell, J. 2012. Clavate borings in a Miocene cetacean skeleton from Tarragona (NE Spain) and the fossil record of marine bone bioerosion. Palaeogeography, Palaeoclimatology, Palaeoecology 323–325: 68–74. Crossref
Chen, C., Ogura, T., Hirayama, H., Watanabe, H.K., Miyazaki, J., and Okutani, T. 2016 First seep-dwelling Desbruyeresia (Gastropoda: Abyssochrysoidea) species discovered from a serpentinite-hosted seep in the Southeastern Mariana Forearc. Molluscan Research 36: 277–284. Crossref
Danise, S. and Higgs, N.D. 2015. Bone-eating Osedax worms lived on Mesozoic marine reptile deadfalls. Biology Letters 11 (4): 20150072. Crossref
Danise, S., Twitchett, R.J., and Matts, K. 2014. Ecological succession of a Jurassic shallow-water ichthyosaur fall. Nature Communications 5: 4789. Crossref
Dell, R.K. 1987. Mollusca of the family Mytilidae (Bivalvia) associated with organic remains from deep water off New Zealand, with revisions of the genera Adipicola Dautzenberg, 1927 and Idasola Iredale, 1915. National Museum of New Zealand Records 3: 17–36.
Deming, J.W., Reysenbach, A.L., Macko, S.A., and Smith, C.R. 1997. Evidence for the microbial basis of a chemoautotrophic invertebrate community at a whale fall on the deep seafloor: Bone-colonizing bacteria and invertebrate endosymbionts. Microscopy Research and Technique 37: 162–170. Crossref
Desbruyères, D., Segonzac, M., and Bright, M. 2006. Handbook of Deep-Sea Hydrothermal Vent Fauna. 544 pp. Biologiecentrum der Oberösterreichische Landesmuseum, Linz.
Dick, D.G. 2015. An ichthyosaur carcass-fall community from the Posidonia Shale (Toarcian) of Germany. Palaios 30: 353–361. Crossref
Distel, D.L., Baco, A.R., Chuang, E., Morrill, W., Cavanaugh, C., and Smith, C.R. 2000. Do mussels take wooden steps to deep-sea vents? Nature 403: 725–726. Crossref
Dufour, S.C. 2005. Gill anatomy and the evolution of symbiosis in the bivalve family Thyasiridae. Biological Bulletin 208: 200–212. Crossref
Hashimoto, W., Nagao, S., Kanno, S., Asaga, M., Otomo, R., Koyakai, T., Tono, S., Kitamura, K., Taira, K., and Wajima, M. 1967. Geology and Underground Resources in Nakagawa-cho, Hokkaido [in Japanese]. 48 pp. Nakagawa-cho, Nakagawa.
Houssaye, A. 2013. Bone histology of aquatic reptiles: what does it tell us about secondary adaptation to an aquatic life? Biological Journal of the Linnean Society 108: 3–21. Crossref
Hryniewicz, K., Amano, K., Jenkins, R.G., and Kiel, S. 2017. Thyasirid bivalves from Cretaceous and Paleogene cold seeps. Acta Palaeontologica Polonica 62: 705–728. Crossref
Jenkins, R.G. and Hikida, Y. 2011. Carbonate sediments microbially induced by anaerobic oxidation of methane in hydrocarbon-seeps. In: V. Tewari and J. Seckbach (eds.), Stromatolites: Interaction of Microbes with Sediments, 591–605. Springer, Dordrecht. Crossref
Jenkins, R.G., Kaim, A., Hikida, Y., and Tanabe, K. 2007. Methane-flux-dependent lateral faunal changes in a Late Cretaceous chemosymbiotic assemblage from the Nakagawa area of Hokkaido, Japan. Geobiology 5: 127–139. Crossref
Johnson, S.B., Warén, A., Lee, R.W., Kano, Y., Kaim, A., Davis, A., Strong, E.E., and Vrijenhoek, R.C. 2010. Rubyspira, new genus and two new species of bone-eating deep-sea snails with ancient habits. The Biological Bulletin 219: 166–177. Crossref
Kaim, A., Hryniewicz, K., Little, C.T.S., and Nakrem, H.A. 2017. Gastropods from the Late Jurassic–Early Cretaceous seep deposits in Spitsbergen, Svalbard. Zootaxa 4329: 351–374. Crossref
Kaim, A., Jenkins, R.G., and Hikida, Y. 2009. Gastropods from Late Cretaceous Omagari and Yasukawa hydrocarbon seep deposits in the Nakagawa area, Hokkaido, Japan. Acta Palaeontologica Polonica 54: 463–490. Crossref
Kaim, A., Jenkins, R.G., and Warén, A. 2008a. Provannid and provannid-like gastropods from the Late Cretaceous cold seeps of Hokkaido (Japan) and the fossil record of the Provannidae (Gastropoda: Abyssochrysoidea). Zoological Journal of the Linnean Society 154: 421–436. Crossref
Kaim, A., Jenkins, R.G., Tanabe, K., and Kiel, S. 2014. Mollusks from late Mesozoic seep deposits, chiefly in California. Zootaxa 3861: 401–440. Crossref
Kaim, A., Kobayashi, Y., Echizenya, H., Jenkins, R.G., and Tanabe, K. 2008b. Chemosynthesis-based associations on Cretaceous plesiosaurid carcasses. Acta Palaeontologica Polonica 53: 97–104. Crossref
Kaim, A., Tucholke, B.E., and Warén, A. 2012. A new Late Pliocene large provannid gastropod associated with hydrothermal venting at Kane Megamullion, Mid-Atlantic Ridge. Journal of Systematic Palaeontology 10: 423–433. Crossref
Kiel, S. 2008. Fossil evidence for micro- and macrofaunal utilization of large nekton-falls: Examples from early Cenozoic deep-water sediments in Washington State, USA. Palaeogeography, Palaeoclimatology, Palaeoecology 267: 161–174. Crossref
Kiel, S. 2015. Did shifting seawater sulfate concentrations drive the evolution of deep-sea methane-seep ecosystems? Proceedings of the Royal Society B 282: 20142908. Crossref
Kiel, S. 2016. A biogeographic network reveals evolutionary links between deep-sea hydrothermal vent and methane seep faunas. Proceedings of the Royal Society B 283: 20162337. Crossref
Kiel, S., Amano, K., and Jenkins, R.G. 2008. Bivalves from Cretaceous cold-seep deposits on Hokkaido, Japan. Acta Palaeontologica Polonica 53: 525–537. Crossref
Kiel, S., Amano, K., Hikida, Y., and Jenkins, R.G. 2009. Wood-fall associations from Late Cretaceous deep-water sediments of Hokkaido, Japan. Lethaia 42: 74–82. Crossref
Kiel, S. and Goedert, J.L. 2006. Deep-sea food bonanzas: early Cenozoic whale-fall communities resemble wood-fall rather than seep communities. Proceedings of the Royal Society B-Biological Sciences 273: 2625–2631. Crossref
Kiel, S., Kahl, W.-A., and Goedert, J.L. 2011. Osedax borings in fossil marine bird bones. Naturwissenschaften 98: 51–55. Crossref
Kiel, S., Krystyn, L., Demirtaş, F., Koşun, E., and Peckmann, J. 2017. Late Triassic mollusk-dominated hydrocarbon-seep deposits from Turkey. Geology 45: 751–754. Crossref
Lorion, J., Duperron, S., Gros, O., Cruaud, C., and Samadi, S. 2009. Several deep-sea mussels and their associated symbionts are able to live both on wood and on whale falls. Proceedings of the Royal Society of London B: Biological Sciences 276: 177–185. Crossref
Lorion, J., Kiel, S., Faure, B., Kawato, M., Ho, S.Y.W., Marshall, B., Tsuchida, S., Miyazaki, J.-I., and Fujiwara, Y. 2013. Adaptive radiation of chemosymbiotic deep-sea mussels. Proceedings of the Royal Society of London B: Biological Sciences 280: 20131243. Crossref
Marshall, B.A. 1994. Deep-sea gastropods from the New Zealand region associated with recent whale bones and an Eocene turtle. Nautilus 108: 1–8.
Miyazaki, J.-I., Martins, L.d.O., Fujita, Y., Matsumoto, H., and Fujiwara, Y. 2010. Evolutionary process of deep-sea Bathymodiolus mussels. Plos One 5 (4): e10363. Crossref
Motani, R. 2009. The evolution of marine reptiles. Evolution: Education and Outreach 2: 224–235. Crossref
Nakajima, Y., Houssaye, A., and Endo, H. 2014. Osteohistology of the Early Triassic ichthyopterygian reptile Utatsusaurus hataii: Implications for early ichthyosaur biology. Acta Palaeontologica Polonica 59: 343–352.
Sato, T., Konishi, T., Hirayama, R., and Caldwell, M. 2012. A review of the Upper Cretaceous marine reptiles from Japan. Cretaceous Research 37: 319–340. Crossref
Smith, C.R. and Baco, A.R. 2003. Ecology of whale falls at the deep-sea floor. Oceanography and Marine Biology 41: 311–354.
Smith, C.R., Baco, A.R., and Glover, A.G. 2002. Faunal succession on replicate deep-sea whale falls: time scales and vent-seep affinities. Cahiers de Biologie Marine 43: 293–297.
Smith, C.R., Glover, A.G., Treude, T., Higgs, N.D., and Amon, D.J. 2015. Whale-fall ecosystems: recent insights into ecology, paleoecology, and evolution. Marine Science 7: 571–596. Crossref
Smith, C.R., Kukert, H., Wheatcroft, R.A., Jumars, P.A., and Deming, J.W. 1989. Vent fauna on whale remains. Nature 341: 27–28. Crossref
Takahashi, A., Hikida, Y., Jenkins, R.G., and Tanabe, K. 2007. Stratigraphy and megafauna of the Upper Cretaceous Yezo Supergroup in the Teshionakagawa area, northern Hokkaido, Japan. Bulletin of the Mikasa City Museum, Natural Science 11: 25–59.
Takahashi, A., Hirano, H., and Sato, T. 2003. Stratigraphy and fossil assemblage of the Upper Cretaceous in the Teshionakagawa area, Hokkaido, northern Japan [in Japanese with English abstract]. Journal of the Geological Society of Japan 109: 77–95. Crossref
Takashima, R., Kawabe, F., Nishi, H., Moriya, K., Wani, R., and Ando, H. 2004. Geology and stratigraphy of forearc basin sediments in Hokkaido, Japan: Cretaceous environmental events on the north-west Pacific margin. Cretaceous Research 25: 365–390. Crosssref
Thewissen, J.G., Hussain, S., and Arif, M. 1994. Fossil evidence for the origin of aquatic locomotion in archaeocete whales. Science 263: 210–211. Crossref
Tresguerres, M., Katz, S., and Rouse, G.W. 2013. How to get into bones: proton pump and carbonic anhydrase in Osedax boneworms. Proceedings of the Royal Society B: Biological Sciences 280: 20130625. Crossref
Treude, T., Smith, C.R., Wenzhöfer, F., Carney, E., Bernardino, A.F., Hannides, A.K., Krüger, M., and Boetius, A. 2009. Biogeochemistry of a deep-sea whale fall: sulfate reduction, sulfide efflux and methanogenesis. Marine Ecology Progress Series 382: 1–21. Crossref
Vrijenhoek, R.C., Johnson, S.B., and Rouse, G.W. 2009. A remarkable diversity of bone-eating worms (Osedax; Siboglinidae; Annelida). BMC Biology 7 (1): 74. Crossref
Warén, A. and Bouchet, P. 1986. Four new species of Provanna Dall (Prosobranchia, Cerithiacea?) from East Pacific hydrothermal sites. Zoologica Scripta 15: 157–164. Crossref
Zug, G.R. and Parham, J.F. 1996. Age and growth in leatherback turtles, Dermochelys coriacea (Testudines: Dermochelyidae): a skeletochronological analysis. Chelonian Conservation and Biology 2: 244–249.
Acta Palaeontol. Pol. 62 (4): 683–690, 2017
https://doi.org/10.4202/app.00405.2017