

Distinguishing Quaternary glyptodontine cingulates in South America: How informative are juvenile specimens?
CARLOS A. LUNA, IGNACIO A. CERDA, ALFREDO E. ZURITA, ROMINA GONZALEZ, M. CECILIA PRIETO, DIMILA MOTHÉ, and LEONARDO S. AVILLA
Luna, C.A., Cerda, I.A., Zurita, A.E., Gonzalez, R., Prieto, M.C., Mothé, D., and Avilla, L.S. 2018. Distinguishing Quaternary glyptodontine cingulates in South America: How informative are juvenile specimens? Acta Palaeontologica Polonica 63 (1): 159–170.
The subfamily Glyptodontinae (Xenarthra, Cingulata) comprises one of the most frequently recorded glyptodontids in South America. Recently, the North American genus Glyptotherium was recorded in South America, in addition to the genus Glyptodon. It has been shown that both genera shared the same geographic distribution in central-north and eastern areas of South America (Venezuela and Brazil, respectively). Although some characters allow differentiation between adult specimens of both genera, the morphological distinction between these two genera is rather difficult in juvenile specimens. In this contribution, a detailed morphological, morphometric and histological survey of a juvenile specimen of Glyptodontinae recovered from the Late Pleistocene of northern Brazil is performed. The relative lower osteoderms thickness, the particular morphology of the annular and radial sulci and the distal osseous projections of the caudal osteoderms suggest that the specimen belongs to the genus Glyptotherium. In addition, the validity of some statistical tools to distinguish between different ontogenetic stages and in some cases between genera is verified. The osteoderm microstructure of this juvenile individual is characterized by being composed of a cancellous internal core surrounded by a compact bone cortex. Primary bone tissue mostly consists of highly vascularized, woven-fibered bone tissue. Unlike that observed in adult Glyptodontinae, both the Sharpey fibers and the fibrous and laminar parallel bone tissue as secondary bone are absent. This study enhances our knowledge about those morphological and histological changes that occur through the ontogeny in glyptodonts.
Key words: Mammalia, Cingulata, Glyptodontinae, osteoderm histology, ontogeny, Pleistocene, South America.
Carlos A. Luna [carlosaluna@hotmail.com], Alfredo E. Zurita [aezurita74@yahoo.com.ar], and Romina Gonzalez [romigonzl95@gmail.com], Centro de Ecología Aplicada del Litoral (CECOAL), CONICET-UNNE, Ruta 5, km 2.5, CC 128, (3400) Corrientes, Argentina.
Ignacio A. Cerda [nachocerda6@yahoo.com.ar], Universidad Nacional de Río Negro, CONICET, Instituto de Investigaciones en Paleobiología y Geología, Museo Provincial “Carlos Ameghino”, Belgrano 1700, Paraje Pichi Ruca (predio Marabunta), CP8300 Cipolletti, Río Negro, Argentina.
M. Cecilia Prieto [prietomariacecilia@gmail.com], Facultad de Ciencias Agropecuarias (UNC), IMBIV-CONICET, Ing. Agr. Felix A. Marrone 746, 5000 Córdoba, Argentina.
Dimila Mothé [dimothe@hotmail.com] and Leonardo S. Avilla [leonardo.avilla@gmail.com], Laboratório de Mastozoologia, Departamento de Zoologia, Instituto de Biociências, Universidade Federal do Estado do Rio de Janeiro, Av. Pasteur, 458, 501, Urca, CEP 22290-240, Rio de Janeiro, Brazil; and Programa de Pós-graduação em Biodiversidade Neotropical, Instituto de Biociências, Universidade Federal do Estado do Rio de Janeiro, Av. Pasteur, 458, 501, Urca, CEP 22290-240, Rio de Janeiro, Brazil.
Received 5 July 2017, accepted 10 October 2017, available online 28 February 2018.
Copyright © 2018 C.A. Luna et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Glyptodontidae (Mammalia, Xenarthra) have been one of the most important clades among megamammals from the Quaternary of South America, both for the abundance of records as well as their taxonomic diversity (McKenna and Bell 1997). Among them, the subfamily Glyptodontinae (middle Miocene–Early Holocene, ca. 12–0.0011 MyBP) is characterized by its wide latitudinal distribution, with records from Argentina to Colombia and Venezuela (Carlini et al. 2008; Rincón et al. 2008; Zurita et al. 2012), being possibly the only clade that actively participated in the Great American Biotic Interchange (GABI) (Carlini et al. 2008; Webb 2006; Woodburne 2010; Gillette et al. 2016). Until recently, in South America all the Quaternary records of this group were assigned to the genus Glyptodon Owen, 1839; however, Carlini et al. (2008), Carlini and Zurita (2010), and Oliveira et al. (2009, 2010) proposed the presence of the North American genus Glyptotherium Osborn, 1903 in the northern and northeastern regions of South America (i.e.,Venezuela and Brazil).
In recent years, some contributions have focused on clarification of the systematics of the clade in order to infer the real diversity of the Glyptodontinae. Some of these are related to changes in the morphology of the osteoderms of the dorsal carapace and its relation to the ontogenetic stages of the specimens (see Rinderknecht 2000; Luna and Krapovickas 2011; Zurita et al. 2011b; Gillette et al. 2016). The results of these contributions have shown that the morphological variation at different ontogenetic stages has been used for identification of genera and species now recognized as juveniles that cannot be accurately diagnosed. In fact, some genera, such as Neothoracophorus Ameghino, 1889 (N. elevatus), “Boreostracon” Simpson, 1929 or “Chlamydotherium” Bronn, 1838, must be regarded as nomina dubia and/or species inquirendae, all of them belonging to juvenile specimens of Glyptodontinae. In particular, Zurita et al. (2011b) have pointed out that the osteoderms of juveniles of Glyptodontinae share the same morphology at the level of the external surface, so it is almost impossible to make a generic distinction between Glyptodon and Glyptotherium on the basis of osteoderm osteology.
On the other hand, another group of contributions involves the incorporation of microanatomical characters in the description of the osteoderms of different genera of Glyptodontidae (Hill 2006; Wolf 2007; Chimento et al. 2009; Da Costa Pereira et al. 2014; Asakura et al. 2017). According to some authors, histology could be used to characterize and differentiate both Quaternary genera of Glyptodontinae, Glyptodon and Glyptotherium (see Da Costa Pereira et al. 2014). However, all studies have been performed on adult specimens; in consequence, there are no contributions concerning the histological analysis in juvenile Glyptodontidae.
In the late Pleistocene of Brazil and according to Oliveira et al. (2010), the Glyptodontinae are represented by Glyptotherium, which is recorded in the northeast and southeast areas, while Glyptodon would be restricted to the more southerly regions. Dantas et al. (2011) proposed the presence of Glyptodon in the intertropical region of Brazil, although Zurita et al. (2012) indicated that this record is very doubtful mainly due to the poor preservation of the osteoderms. Beyond this, some authors have also indicated the possibility that, in some areas of Brazil, both genera could have had the same geographical distribution (although not necessarily coexist), configuring a complex paleobiogeographical scenario (see Zurita et al. 2011b, 2012).
One of the sites that have provided an important diversity of Quaternary vertebrates in the last years are the caves located at Aurora do Tocantins, in northern Brazil (see among others, Oliveira et al. 2011; Hsiou et al. 2013; Rodriguez et al. 2013, 2014; Gasparini et al. 2016; Rocha-dos-Santos et al. 2017). In this assemblage, the Cingulata is one of the most abundant groups, mainly due to the large number of recovered osteoderms (Avilla et al. 2011). According to Soibelzon et al. (2015), species represented in these deposits include the Dasypodidae Euphractus sexcinctus Linnaeus, 1758, Dasypus novemcinctus Linnaeus, 1758, Propaopus sulcatus (Lund, 1842) and the Cingulata incertae sedis Pachyarmatherium brasiliense Porpino, Fernicola, and Berqvist, 2009. Meanwhile, the Glyptodontidae are represented by osteoderms belonging to Glyptodontinae that came from the Gruta do Urso cave; the morphology of the external surface indicates that they belong to a juvenile specimen (see Zurita et al. 2011b).
In this contribution we carry out a detailed morphological, morphometric and histological description of these osteoderms with the purpose of taxonomic assignment of the material under study; in addition, we provide, for the first time, new characters for the differentiation of the juvenile specimens of both genera (Glyptodon and Glyptotherium) and new insights on the ontogenetic development of the osteoderms in Quaternary Glyptodontinae.
Institutional abbreviations.—CPRM, Companhia de Pesquisa de Recursos Minerais, Brazil; DGEO-UFPE, Departamento de Geologia, Universidade Federal de Pernambuco, Recife, Brazil; MAMM-PV, Colección de Paleontología de Vertebrados, Museo de Ciencias Naturales de la Región de Ansenuza “Aníbal Montes”, Miramar, Argentina; MCA, Museo Carlos Ameghino, Mercedes, Buenos Aires, Argentina; MCNC-PV, Paleovertebrados, Museo Provincial de Ciencias Naturales “Dr. Arturo U. Illia”, Córdoba, Argentina; MDVS-PV, Paleovertebrados, Museo Dr. Dalmacio Vélez Sarsfield, Amboy, Córdoba, Argentina; UNIRIO-PM, Fossil Mammal Collection, Universidade Federal do Estado do Rio de Janeiro, Brazil.
Material and methods
The osteoderms analyzed in this study are housed in the Paleontological Collection of the Laboratório de Mastozoologia of the Universidade Federal do Estado do Rio de Janeiro, Brazil. The characters considered in the morphometric analysis correspond to those proposed by Rinderknecht (2000) and Luna and Krapovickas (2011): (i) general morphology, diameter and thickness; (ii) form and diameter of the central figure in the external surface; (iii) concavity of the internal surface; (iv) development or absence of peripheral figures; (v) number of large foramina in the external surface. The measurements have been taken using digital caliper with 0.02 mm error and are expressed in millimeters. The terminology used for morphological description of the osteoderms mainly follows Hill (2006) and Krmpotic et al. (2009). Regarding the relative locations of specific structures within the osteoderm, it adheres to the most accurate convention “external” and “basal” proposed by Scheyer and Sander (2004). These terms are synonyms of “superficial⁄deep” used by Hill (2006). The systematics partially follow McKenna and Bell (1997) and Fernicola (2008). Comparisons have been performed directly on specimens house dat the institutions mentioned above (Table 1), as well as on literature (Gillette and Ray 1981; Zurita et al. 2011b; Gillette et al. 2016).
Table 1. List of specimens considered in the comparative analysis.
|
Specimen |
Species |
Age |
Locality |
|
MCNC-PV 246 |
Glyptodon sp. |
juvenile |
Embalse Rio Tercero, Córdoba, Argentina |
|
MAMM-PV 036 |
Glyptodon sp. |
juvenile |
Laguna Mar Chiquita, Córdoba, Argentina |
|
MDVS-PV 064 |
Glyptodon sp. |
young adult |
Atos Pampa, Córdoba, Argentina |
|
MCA-2013 |
Glyptodon sp. |
young adult |
Mercedes, Buenos Aires, Argentina |
|
MCNC -PV 284 |
Glyptodon sp. |
adult |
Lozada, Córdoba, Argentina |
|
DGEO-UFPE 5915/16, 5924/25, 5929–5931 |
Glyptotherium sp. |
adult |
Fazenda Nova, Pernambuco, Brazil |
The diameter and thickness values of dorsal carapace osteoderms were analyzed statistically, and compared to values of Glyptodon and Glyptotherium specimens in different ontogenetic stages (juvenile, young adult, and adult). Some of the data were previously published (see Gillette et al. 2016), while other are unpublished values obtained from specimens deposited in cited institutions, which were used to perform dispersion graphs. In addition, the diameter/thickness ratio was calculated for each osteoderm that, together with the values of diameter and thickness, was used to perform a cluster analysis. This analysis was performed using the Unweighted Pair Group Method with Arithmetic mean (UPGMA) and the Gower distance (sqrt[1-S]). A Multivariate Analysis of Variance (MANOVA) using all parameters mentioned above as variables and the clustering as classification criterion was carried out. Statistical analyses were made using the software InfoStat (Di Rienzo et al. 2008).
Three osteoderms (identified from here and elsewhere as I, II, and III) of the dorsal carapace were selected for histological analyses, following the methodology of Chinsamy and Raath (1992). Whereas the osteoderm I corresponds to the medial portion of the carapace, elements II and III belong to the lateral portion. The histological sections were prepared at the Laboratorio de Tratamiento Integral de Muestras Geológicas (LabGeo), Facultad de Ciencias Exactas, Físicas y Naturales of the Universidad Nacional de Córdoba, Argentina. Because this is a destructive method, all osteoderms were photographed and measured before sectioning. The osteoderms were transected along the major axis. The sections were fixed to slides, polished to variable thicknesses less than 30 microns, according to the characteristics of each osteoderm. The thin sections were observed under normal and polarized light using a petrographic microscope. The bone histological terminology follows Francillon-Vieillot et al. (1990) and Ricqlès et al. (1991). To quantify the degree of compactness, we measured the ratio between the compact bone and the total area (expressed as percentage) from thin sections using Image J software (Abramoff et al. 2004).
Geological setting
The material comes from a limestone cave, known as Gruta do Urso (12°35’0.08” S 46°30’58.39” W), located in the municipality of Aurora do Tocantins, State of Tocantins, Brazil (Fig. 1). The specimen was found in a thick sediment level that fills the corridor of the cave. The limestone of the Aurora do Tocantins region is a part of the Speleological Province of the Bambuí Group, the area were the greatest number of caves in Brazil have been found (Zampaulo and Ferreira 2009). However, the area still lacks more detailed geological studies. Online notes of CPRM about the geology of the municipality of Aurora do Tocantins reported carbonate deposits of the Bambuí Group, which is Neoproterozoic in age (CPRM 2006). The lower portion is represented by the Sete Lagoas Formation, which is composed of thick deposits of pelite, limestone, and dolomite bearing stromatolites. The Sete Lagoas Formation is covered by siltstone and laminate siltstone of the Serra de Santa Helena Formation. These carbonate rocks are superimposed by dark calcarenite and marl, with organic material from the Lagoa do Jacaré Formation. Superimposed on the Bambuí Group are Cretaceous sediments of the Urucuia Formation. In summary, the ages of the deposits containing the osteoderms are inferred by representative taxa recorded in stratigraphic association, such as Equus neogeus Lund, 1840 and Propraopus sulcatus (Lund, 1842). These remains were found at the same stratigraphic level as the osteoderms, suggesting a Late Pleistocene age (Cione and Tonni 1999, 2005). Accordingly, the other known eastern Brazilian caves are also Late Pleistocene in age (Auler et al. 2009). The fossil bearing level is composed of laminated reddish-grey (5YR5/2) loess-like sediment.
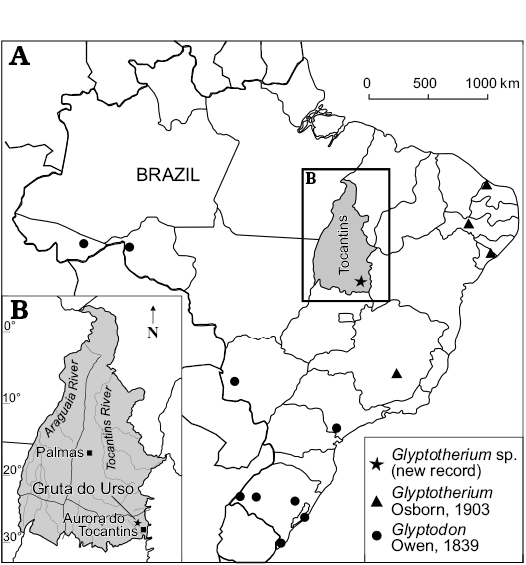
Fig. 1. Map showing the geographic distribution of Glyptodontinae recorded in Brazil (A) and location of studied area within State of Tocantins (B).
Systematic palaeontology
Magnorder Xenarthra Cope, 1889
Order Cingulata Illiger, 1811
Suborder Glyptodontia Ameghino, 1889
Superfamily Glyptodontoidea Gray, 1869
Family Glyptodontidae Gray, 1869
Subfamily Glyptodontinae Gray, 1869
Tribe Glyptotheriini Castellanos, 1953
Genus Glyptotherium Osborn, 1903
Type species: Glyptotherium texanum Osborn, 1903; Llano Estacado, Texas, USA; Late Pliocene–Early Pleistocene.
Glyptotherium sp.
Fig. 2.
Material.—UNIRIO-PM 6231, osteoderms of dorsal carapace and caudal armor.
Description.—External morphology: The material analyzed consists of 118 isolated osteoderms belonging to one individual, from which 86 (72.9%) correspond to the dorsal carapace, while the others belong to the caudal armor. Except for some particular morphological features, most characters are shared with juvenile Glyptodon, as previously postulated (see Zurita et al. 2011b; Luna and Krapovickas 2011). The dorsal carapace osteoderms are mainly hexagonal, followed in frequency by the pentagonal shape, and others are approximately rectangular (Fig. 2A–H). The osteoderm diameter ranges 13.5–25.4 mm, with thickness 7.6–17.1 mm. The ratio between these two variables (diameter/thickness) ranges 1.31–2.46 (Fig. 3), being in most of the osteoderms larger than those of juvenile Glyptodon (1.00–1.88). The external surface of each osteoderm is uniformly punctuated by small foramina that define a rough appearance. In some osteoderms, these foramina have the morphology of sinuous grooves. The central figure is circular and prominent, more elevated than the general surface and occupying from 20% in those from dorsal region (as those observed in morphotype 1 of the juvenile Glyptodon described by Luna and Krapovickas 2011) to the almost complete external surface in some osteoderms of the lateral regions. The central area of the central figure shows a clear depression in approximately 60% of the osteoderms; however, in those osteoderms where the central figure occupies most of the osteoderm, the surface is flat. Peripheral figures are absent or poorly defined in most of the osteoderms, except in those in which the radial and annular sulci are well defined; in these cases up to eight peripheral figures can be observed. In general, compared to juvenile Glyptodon, both central and peripheral figures are less prominent. The annular and radial sulci have a variable morphology: they are broad and shallow in those osteoderms in which the peripheral figures are scarcely defined, and narrower and deeper in those where the figures are better defined, although always less than in juvenile Glyptodon. At the intersection between the annular and radial sulci, it is possible to observe up to seven piliferous foramina with a diameter of about 1 mm (Fig. 2A–G). The articular area between osteoderms has some bony projections, although most osteoderms are eroded. The basal surface shows a mostly spongy aspect with respect to the external surface, being mainly concave, with small neurovascular foramina measuring less than 0.5 mm in diameter (Fig. 2A). These foramina are generally located in the central region; however, in some osteoderms, they are distributed throughout the whole surface and they may also be connected. The osteoderms of the border of the dorsal carapace have a prominent conical shape and their surfaces have a rough appearance (Fig. 2I).
The preserved caudal osteoderms correspond to proximal and distal rings. The former are approximately hexagonal in shape and the antero-posterior diameter ranges 17.8–30.4 mm, while thickness ranges 6.6–11.9 mm. The posterior margin presents a weakly developed projection (Fig. 2J). In turn, the osteoderms of the distal rings are larger, with an antero-posterior diameter ranging 22.5–42.4 mm; the thickness ranges 14.9–31.3 mm, increased by the development of the projection of the posterior margin, which is pronounced and conical (Fig. 2K), although less acute than in the juvenile Glyptodon. The external surfaces of osteoderms of both morphotypes are uniformly punctuated, whereas the basal surface is characterized by large neurovascular foramina with a diameter ranging 0.5–1.0 mm, like the osteoderms of the dorsal carapace.
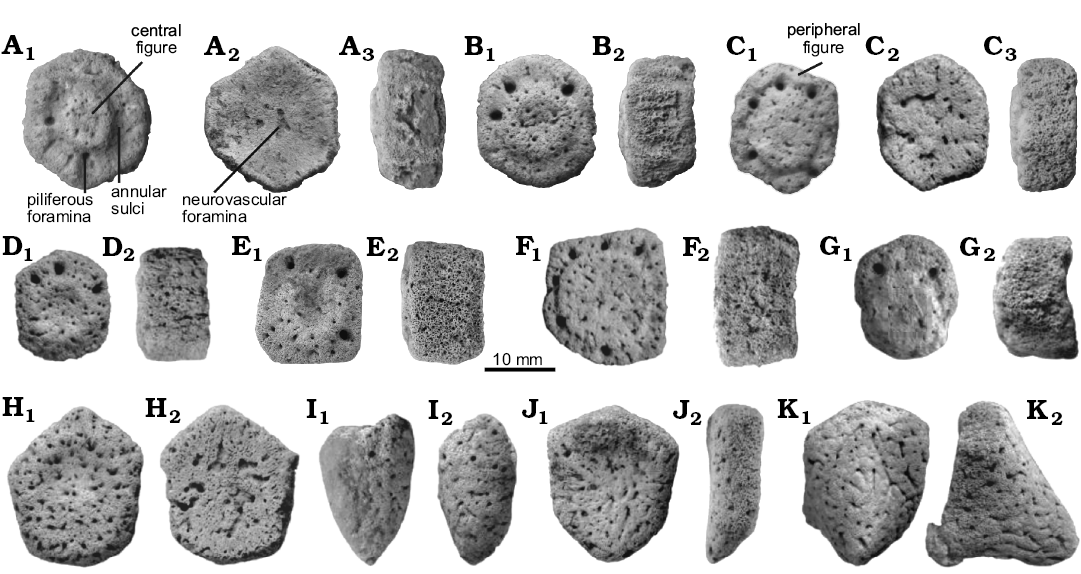
Fig. 2. Juvenile. Osteoderms of the dorsal caparace (A–I) and caudal armor (J–K) of juvenile glyptodont Glyptotherium sp. (UNIRIO-PM 6231) from the Late Pleistocene of Aurora do Tocantins, Brazil; in external (A1–K1), internal (A2, C2), and lateral (A3, B2, C3, D2–K2) views.
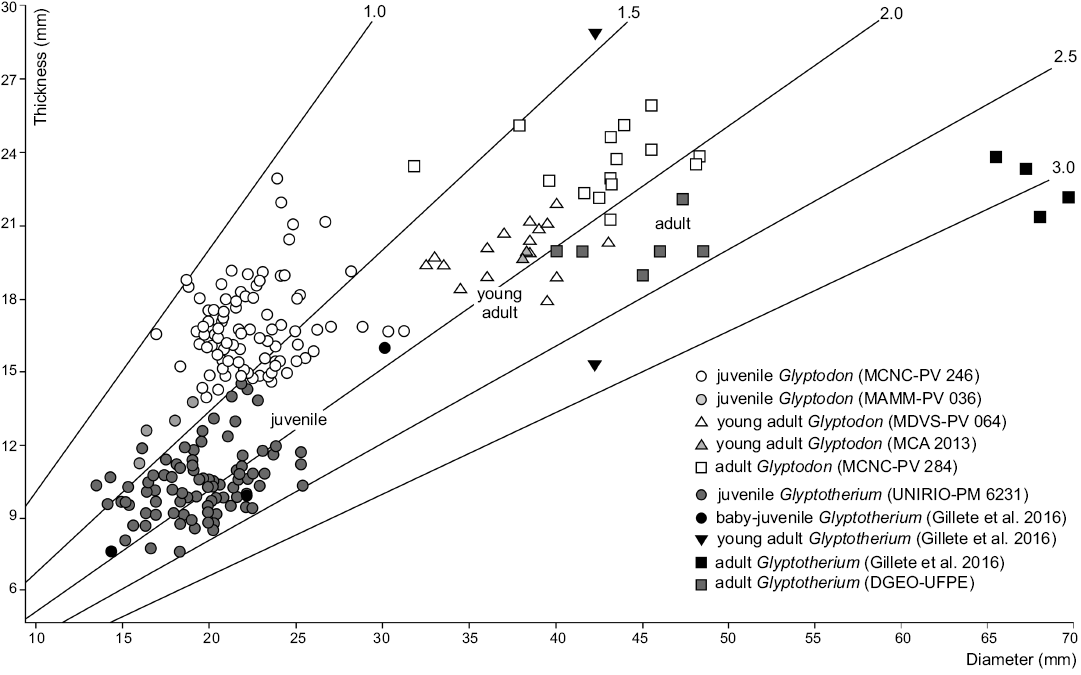
Fig. 3. Dispersion graph including values of thickness and diameter of osteoderms from dorsal carapace of Glyptodon and Glyptotherium in different ontogenetic stages.
Statistical analysis: The cluster analysis (Fig. 4) performed on the osteoderms of dorsal carapace from different specimens of Glyptodon and Glyptotherium resulted in two main clusters, differentiated mainly by the ontogenetic stage (juveniles and young adults/adults). In the former case, the clustering between specimens from the same genus is remarkable, grouping even the samples with scarce osteoderms (e.g., MAMM-PV 036). Within the young adults/adults group, the two taxa are not clearly distincted as in the juvenile group. The adult specimen of Glyptotherium (G. texanum) from North America, described by Gillette et al. (2016), and one group including an adult Glyptodon and two other smaller clusters are differentiated. The first of the latter is formed by the osteoderms of adult specimens of Glyptotherium (G. cylindricum) from South America and the young adult specimen of G. texanum, and the second by the young adult Glyptodon. The cluster analyses showed a high level of fit (cophenetic correlation index = 0.887). The Multivariate analysis of variance (MANOVA) shows that the main groups obtained in the cluster analysis were recognized to be statistically different (P < 0.0001).
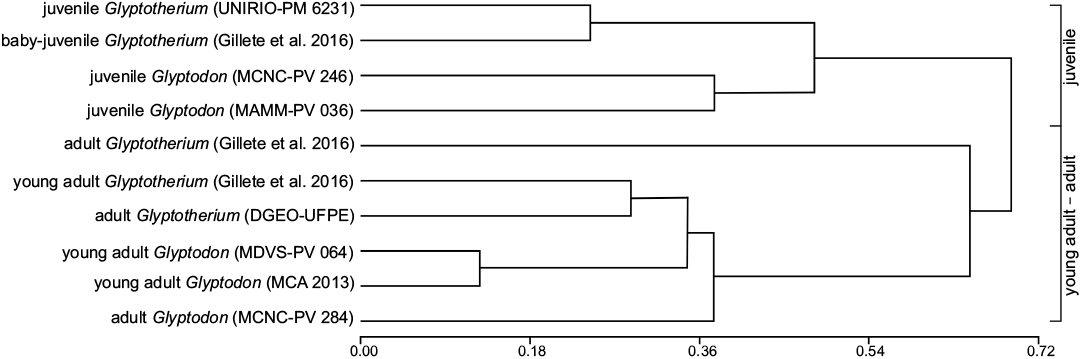
Fig. 4. UPGMA cluster analysis among different specimens of Glyptodon and Glyptotherium with different ontogenetic stages.
Histological analysis.—In general terms, all the osteoderms of Glyptotherium analyzed are composed of a cancellous internal core surrounded by a compact bone cortex. Nevertheless, they present some differences in the bone microanatomy. In the osteoderm of the medial zone of the dorsal carapace (Fig. 5A), the external cortex is thicker than the basal cortex. The cancellous bone in the inner core occupies approximately 40% of the osteoderm thickness. The transition between the compact and the cancellous bone is distinctive and the inter-trabecular spaces are large and show irregular shapes. Different from the osteoderm from the medial zone of the dorsal caparace, elements from lateral regions (Fig. 5B, C) exhibit a more compact appearance (cancellous bone occupies less than 25% of the total area). Also, the transition between compact and cancellous bone is gradual and the inter-trabecular spaces are comparatively smaller than the spaces observed in the dorsal osteoderm.
Despite the microanatomical differences reported between the sampled osteoderms, they share most of the histological features: the predominant extracellular cortical matrix is woven-fibred bone with a haphazard spatial arrangement of the collagen fibers and randomly distributed osteocyte lacunae (Fig. 6A–D); osteocyte lacunae are abundant and they have irregular or elongated shape (Fig. 6B–E); vascular spaces are mostly simple, with only a few primary osteons; and large neurovascular canals passing through the entire osteoderm are visible in all the sampled elements.
However, in the different regions of the osteoderms there are some histological particularities. In osteoderm II (Fig. 5B), the external cortex is characterized by abundant vascular canals of small size (0.05–0.1 mm), which are organized in a reticular pattern. The most external region exhibits several radially oriented vascular spaces, most of them open to the surface (Fig. 6A, B). The extent of vascularization in the external cortex is notably higher in this osteoderm than in the others, which exhibit a more compact appearance. In the marginal cortex of osteoderms I and II, the intrinsic fibers of the woven-fibered matrix tend to be slightly ordered and oriented toward the marginal surface in some areas (Fig. 6C). Vascular spaces in this region are larger than in the external and basal cortices (0.1–0.3 mm), giving a more porous (finely cancellous) aspect to this area. On the other hand, in the osteoderm III, which presents a more compact aspect, the intrinsic fibers and vascular spaces do not show a particular orientation in the marginal area (Fig. 6D). The inner core of all osteoderms is composed of cancellous bone, which results from local processes of resorption. Bony trabeculae mainly consist of remains of woven-fibered bone tissue. Centripetally deposed secondary lamellar bone is mostly absent around the resorption cavities (Fig. 6E). Finally, the basal cortex is only well preserved in the osteoderm III. Here, as occurs in the external cortex, the compact bone is composed of woven-fibered tissue (Fig. 6F). Bone cell lacunae are dense, have elongated or irregular shape, do not follow a particular orientation and show a rather convoluted organization. Vascular spaces are small and mostly longitudinally oriented.
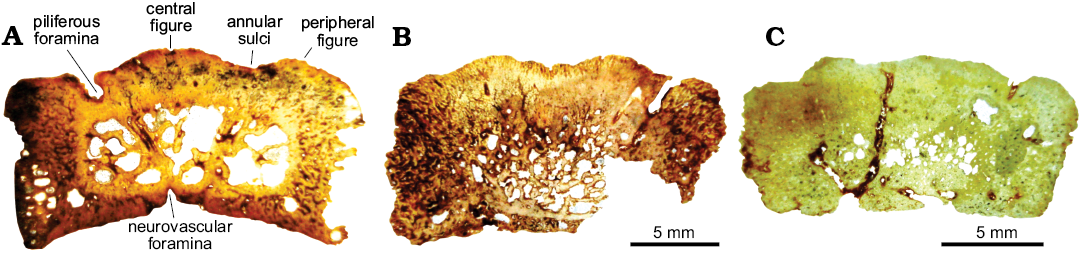
Fig. 5. Osteoderm microanatomy of juvenile glyptodont Glyptotherium sp. (UNIRIO-PM 6231) from the Late Pleistocene of Aurora do Tocantins, Brazil. General view of the complete sections. A. Osteoderm from the medial zone of the dorsal carapace (I). B, C. Osteoderms from lateral regions of the dorsal carapace (II and III).
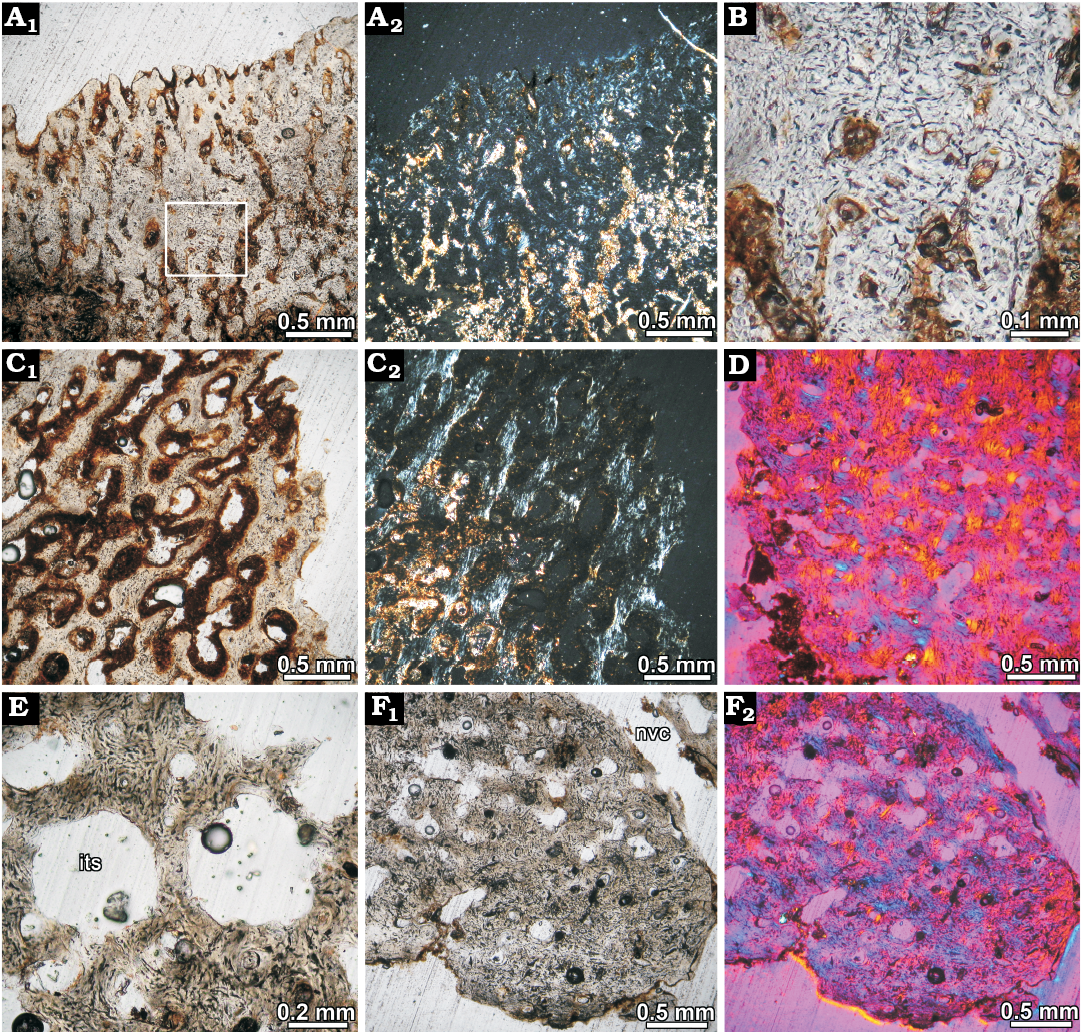
Fig. 6. Osteoderm histology of juvenile glyptodont Glyptotherium sp. (UNIRIO-PM 6231) from the Late Pleistocene of Aurora do Tocantins, Brazil. A. Osteoderm from lateral regions of the dorsal carapace (II), showing highly vascularized superficial cortex composed of woven fibered bone; several vascular canals are opened to the surface. Natural light photograph (A1), under polarized light (A2). Note the monorefrigent nature of the cortical tissue. B. Close up of the woven fibered bone matrix; bone cell lacunae are abundant and they are haphazardly arranged. C. Detail of the cortical bone at the marginal region of the osteoderm; the vascular spaces are larger than in the superficial cortex of the same specimen. Patches of slightly birefringent tissue are present, indicating preferential fiber orientation in some areas (C2). Natural light photograph (C1), under polarized light (C2). D. Osteoderm from lateral regions of the dorsal carapace (III); showing woven fibered bone in the marginal region of the osteoderm viewed under polarized light with lambda compensator. E. Resorpion cavities in the inner core. The bone trabeculae are composed of woven fibered bone. F. Cortical bone at deep cortex. Natural light photograph (F1), under polarized light with lambda compensator (F2). Abbreviations: its, inter-trabecular spaces; nvc, neurovascular canals.
Discussion
The morphological analysis of the Glyptodontidae osteoderms recovered from Gruta do Urso cave reveals characteristics that allow us to propose them as belonging to the subfamily Glyptodontinae, such as the rosette-shaped ornamentation pattern and clearly rough and punctate external surface (Ameghino 1889; Hoffstetter 1958; Gillette and Ray 1981; Soibelzon et al. 2006; Carlini et al. 2008). In particular, the presence of conical osteoderms in the margins of the carapace (Fig. 2I) has been considered as one of the main synapomorphies of the subfamily (Carlini et al. 2008; Zurita et al. 2010, 2013).
As mentioned above, in the Quaternary of South America two genera of this group have been recorded, Glyptodon and Glyptotherium, which are characterized and differentiated by some morphological features, especially on the skull, dorsal carapace and on the external surface of the osteoderms (Gillette and Ray 1981; Zurita et al. 2008, 2012; Carlini et al. 2008; Oliveira et al. 2010). Additionally, Da Costa Pereira et al. (2014) recently indicated that these genera also present differences in the histology of their osteoderms, which could potentially be used with taxonomic value. However, both morphological and histological characters have been analyzed only in adult specimens.
Regarding the juveniles specimens of Glyptodontinae, a set of characters of early ontogenetic stages has been defined in Glyptodon (Rindercknecht 2000; Zurita et al. 2009; Luna and Krapovickas 2011), which includes among others: (i) clearly marked external surface; (ii) prominent central figure with peripheral figures absent or replaced by radial grooves; (iii) remarkable piliferous foramina; (iv) poorly sutured osteoderms, (v) concave basal surface. These characters have been considered by Zurita et al. (2011b) not only belonging to the Pampean genus, but also to be more broadly distributed in the subfamily. This suggests that the juvenile specimens of both genera should have been practically identical. Concerning Glyptotherium, recently Gillette et al. (2016) have described a set of characters in osteoderms of a juvenile individual assigned to the species G. texanum. As the Pampean genus, it is characterized by a relatively large central figure, convex, with a slight depression in the central region, and indistinguishable peripheral figures.
In this context, the material analyzed here presents many of the juvenile characters shared by both genera; however, some differences resulting from the morphological comparison with juvenile specimens of Glyptodon (e.g., MCNC-PV 246, MAMM-PV 036) are highlighted. One of these is related to the development of sulci, which in the specimen under study are wider, shallower and with fewer slopes on their walls. This observed morphology determines that the border of the central figure is softer and the peripheral figures are less convex than in most juvenile Glyptodon osteoderms. As indicated by Zurita et al. (2012), one of the most prominent characters of the osteoderms of Glyptotherium is a less evident development of the annular and radial sulci. Another difference lies in the posterior margin projection in the osteoderms of the distal caudal rings, which is less acute than in juvenile Glyptodon. This has also been cited by different authors (Gillette and Ray 1981; Carlini et al. 2008; Oliveira et al. 2010) as one of the main differences between adult individuals of both genera. Meanwhile, Gillette et al. (2016) indicated that differences on the development of these projections could be mainly related to sexual dimorphism.
Another interesting aspect regards the diameter/thickness ratio of the dorsal carapace osteoderms. Different authors (Castellanos 1951; Luna and Krapovickas 2011; Zurita et al. 2011a; Gillette et al. 2016) have mentioned the importance of this relationship, which can be used with ontogenetic value within the same genus, or systematic value among adult individuals of different genera. The osteoderms of the juvenile specimens of both genera are characterized by a lower diameter/thickness ratio than the adults because, with the development of the individual, the osteoderms present a greater growth rate in diameter than in thickness, and thus the relation between these variables increases progressively with age. On the other hand, among adult individuals of both genera, this ratio is larger in Glyptotherium because the osteoderms are thinner compared to those of Glyptodon (Oliveira et al. 2010; Zurita et al. 2011a). However, it is important to note that, in this type of analyses, a set of osteoderms that include different regions of the dorsal carapace is necessary to obtain confident results.
In order to test the validity of this relationship in the studied material, diameter and thickness values of osteoderms from juveniles and adults of both genera (Glyptodon and Glyptotherium) have been statistically analyzed and compared (Table 1). The material analyzed here has a ratio similar to that of the Glyptotherium baby-juvenile osteoderms studied by Gillette et al. (2016) and higher than that of the juvenile Glyptodon specimens (MCNC-PV 246 described by Luna and Krapovickas 2011, MAMM-PV 036). As it can be seen in the Dispersion Graphs, most of the osteoderms exhibit a similar diameter. Nevertheless, it is evident that the thickness is markedly lower in the specimen from northern Brazil (Fig. 3). Taking into account that the mentioned thickness differences also occur between the osteoderms of adult individuals of both genera (Oliveira et al. 2010; Zurita et al. 2011a, b), such a relationship is here considered as an important parameter to distinguish between juvenile individuals of both genera.
It is necessary to indicate that, considering that there is a variation in the thickness of the osteoderms in the different regions of the carapace (see Duarte 1997), it would be possible to indicate that this observed difference between specimens could correspond to each of the specimens being represented by osteoderms from different regions of the carapace. However, from the morphological analysis, it is possible to indicate that in the samples of the main individuals (UNIRIO-PM 6231 and MCNC-PV 246) there are osteoderms of practically all the regions of the dorsal carapace, a reason why this explanation is rejected.
The results obtained by Cluster Analysis (Fig. 4) indicate that this test can also be used as another tool to carry out a morphometric distinction between different ontogenetic stages (juvenile, young adult, and adult), and within some of them, to distinguish between genera (for example in juveniles) or even between species (e.g., between adults of different species of Glyptotherium). Although it is necessary to have a larger sample than that analyzed in this contribution, the different clusters observed indicate that morphometric differentiation in Glyptodontinae would begin at the genus level in the earlier ontogenetic stages reaching the specific level only in more advanced stages of development.
The microanatomical and histological descriptions of the osteoderms performed here are the first for juvenile Glyptodontidae and provide new criteria for the analysis of ontogenetic variation within the subfamily Glyptodontinae. As expected, the bone microstructure exhibits several features related to its early stage of development. In this regard, osteoderm microstructure consists entirely of a rather simple, homogeneous woven fibred matrix, which is typical of the early stages of development in osteoderms of xenarthrans and other vertebrates (Vickaryous and Hall 2006, 2008). For example, in embryos of Dasypus novemcintus the osteoderms are composed of woven fibered bone (Vickaryous and Hall 2006). In addition, the absence of periosteal lamellar and parallel fibred matrices, as well as secondary lamellar bone in both cancellous and compact bone, is also typical of bones in early stages of development, as these are typically deposited late during the ontogeny. The absence of extrinsic fibres (i.e., Sharpey’s fibres) in our sample (which are commonly described in other Glyptodontidae) was also reported in the early stages of the formation of osteoderms in Dasypus novemcintus (Vickaryous and Hall 2006).
The osteoderm histology of the juvenile specimen analysed here exhibits important differences in comparison with other Glyptodontidae specimens previously studied, including Glyptodon and Glyptotherium. Previous reports reveal that Glyptodontidae osteoderms exhibit a typical diploe microanatomy, with two cortical layers framing a cancellous core. The deep cortex is composed of large fibre bundles (structural fibre bundles sensu Scheyer and Sander 2004) oriented in multiple directions (Hill 2006; Scheyer 2007; Wolf et al. 2012; Da Costa Pereira et al. 2014). Cancellous bone is secondary in origin, with abundant trabeculae composed of lamellar bone (Hill 2006; Scheyer 2007; Wolf et al. 2012; Da Costa Pereira et al. 2014; Asakura et al. 2017). The superficial cortex shows more histological variation than the deep cortex and the cancellous bone. In this regard, several types of bone matrices (woven fibered, parallel fibered, lamellar) have been described. Cortices constituted almost entirely of woven fibered bone were described for Glyptodon sp. (Wolf et al. 2012), Neuryurus sp. (Asakura et al. 2017) and Glyptotherium floridanum (Hill 2006). In the latter, size and morphological characteristics of the external surface suggest that it also belongs to a juvenile specimen (Hill 2006: fig. 16A). The degree of secondary remodelling is also variable, ranging from poorly developed (e.g., Glyptodon sp. described by Wolf et al. 2012) to intense (e.g., Panochthus frenzelianus described by Hill 2006). A common feature reported in the Glyptodontidae osteoderm superficial cortices (but absent in our sample) is the presence of abundant, short Sharpey’s fibres oriented perpendicularly to the outer surface.
In this contribution, it is considered that the histological differences between our sample and the previously studied Glyptodontidae osteoderms are mainly due to ontogenetic variations during the osteoderm development. Similar variation has been reported in Dasypus novemcintus, in which the rather simple histological organization of the osteoderms in the early stages of development later changes into a more complex structure (Vickaryous and Hall 2006). Taking into account the histological changes reported in previous studies, it is possible that several microstructural variations reported in Glyptodontidae (and other xenarthrans) are due to ontogenetic variation.
As previously mentioned, some authors have suggested that the microanatomy and histology of osteoderms can be used with a systematic value, demonstrating differences between taxa (Hill 2006; Wolf et al. 2012; Da Costa Pereira et al. 2014). In this way, Da Costa Pereira et al. (2014) indicated that the differences between the layers of compact bone and the packages of mineralized fibres allow a clear distinction between Glyptotherium from north-eastern Brazil and Glyptodon reticulatus specimens. However, considering that the histological characteristics of the osteoderms studied in this contribution correspond to the first detailed description of a juvenile Glyptodontinae (which is assigned to the genus Glyptotherium), and that there are no detailed descriptions of juvenile Glyptodon, an adequate comparison to analyse the characters that allow a recognition between both taxa in early ontogenetic stages is not possible for the moment.
Finally, the record of this juvenile Glyptotherium specimen in the Late Pleistocene of the Aurora do Tocantins, northern Brazil, increases the known diversity of cingulates in this region, which includes some species of Dasypodidae and the enigmatic Pachyarmatherium (Soibelzon et al. 2015), incorporating for the first time glyptodonts into this association. In addition, from a paleobiogeographic point of view, this record of Glyptotherium could expand the migration route proposed by Carlini et al. (2008), Oliveira et al. (2010), and Zurita et al. (2012), according to which this genus, after its entry into South America during the Great American Biotic Interchange (GABI), may have followed a corridor parallel to the Caribbean Sea first and to the Atlantic Ocean later.
Conclusions
A juvenile Glyptotherium specimen from the Late Pleistocene of South America is described for the first time, providing elements for its distinction compared to juvenile Glyptodon specimens. Although most of the morphological characters of the osteoderms are shared between both genera, some of them, such as the relatively lower thickness, the characteristics of the annular and radial sulci, and the distal osseous projections of the caudal osteoderms indicate that these characters, which are used in the distinction between adult specimens, appear early in ontogeny. In addition, the validity of the diameter/thickness ratio of the dorsal carapace osteoderms and of the Cluster Analysis as a tool to distinguish between different ontogenetic stages (and in some cases between genera) are verified. From a microstructural point of view, the histological differences with previously studied Glyptodontidae are mainly due to ontogenetic variation occurring during osteoderm development.
Acknowledgements
We are grateful to Wagner Moura (Aurora do Tocantins, Brasil) and Aurora do Tocantins municipality, for the assistance and hospitality during fieldwork. The authors are also grateful to Maximiliano Medina, Alvar Sobral, and Edgardo Baldo (all Laboratorio de Tratamiento Integral de Muestras Geológicas, UNC, Córdoba, Argentina) and Marcelo Calvi (Cátedra de Métodos de Investigación Mineral, UNC, Córdoba, Argentina) for assistance in the preparation of histological sections, and to Daniel Alvarez (Museo Historico Regional Dr. Dalmacio Velez Sarsfield, Córdoba, Argentina) and Edison Vicente Oliveira (Universidade Federal de Pernambuco, Brazil) for the measurements of the osteoderms of Glyptodon and Glyptotherium, respectively. In addition, we thank the institutions mentioned above for allowing us the access to the collections under their care. David D. Gillette (Museum of Northern Arizona, Flagstaff, USA), an anonymous reviewer and Editor Olivier Lambert (Institut royal des Sciences naturelles de Belgique, Brussels, Belgium) are also thanked for providing constructive suggestions and editorial assistance that improved the manuscript.The fieldtrips were supported by Universidade Federal do Estado do Rio de Janeiro (UNIRIO) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (401812/2010-3, Edital MCT/CNPq32/2010 and Fortalecimento da Paleontologia Nacional/Edital 32/2010-Faixa B and 552975/2011-Apoio a Projetos de Pesquisa /Chamada MCTI/CNPq Nº 23/2011-Apoio Técnico para Fortalecimento da Paleontologia Nacional). AEZ thanks CONICET (PIP 0150) and PI 001/13 (SGCyT-UNNE) for financial support and DM thanks CNPq for Post-doctoral fellowship (153536/2016-0).
References
Abramoff, M., Magalhaes, P., and Ram, S. 2004. Image processing with ImageJ. Biophotonics International 11 (7): 36–42.
Ameghino, F. 1889. Contribución al conocimiento de los mamíferos fósiles de la República Argentina. Actas de la Academia Nacional de Ciencias de Córdoba 6: 1–1027.
Auler, A.S., Smart, P.L., Wang, X., Pijó, L.B., Edwards, R.L., and Cheng, H. 2009. Cyclic sedimentation in Brazilian caves. Mechanisms and palaeoenvironmental significance. Geomorphology 106: 142–153. Crossref
Avilla, L.S., Soibelzon, E., Castro, M., Oliveira, E.V., and Zurita, A.E. 2011. Os cingulados (Xenarthra: Mammalia) fósseis registrados nas cavernas do sudeste do estado do Tocantins: taxonomia, aspectos climáticos e ambientais no Pleistoceno Superior do norte do Brasil. In: T. Rodrigues (ed.), Caderno de Resumos Paleo RJ/ES 2011, Alegre, 1–8. UFES, Alegre.
Asakura, Y., Da Costa Pereira, P.V.L.G., Oliveira, E.V., and Lopes Da Silva, J.L. 2017. Comparative paleohistology in osteoderms of Pleistocene Panochthus sp. Burmeister, 1886 and Neuryurus sp. Ameghino, 1889 (Xenarthra, Glyptodontidae). Comptes Rendus Palevol 16 (7): 795–803. Crossref
Bronn, H.G. 1838. Lethaea geognostica, oder Abbildugen und Beschreibungen der für die Gebirgs-Formationen bezeichnendsten Versteinerungen. Vol. 2: 545–1346. E. Schweizbart, Stuttgart.
Carlini, A.A. and Zurita, A.E. 2010. An introduction to Cingulate evolution and their evolutionary history during the Great American Biotic Interchange: biogeographical clues from Venezuela. In: M. Sanchez-Villagra, O. Aguilera, and A.A. Carlini (eds.), Urumaco and Venezuelan Paleontology, 233–255. Indiana University Press, Bloomington.
Carlini, A.A., Zurita, A. E., and Aguilera, O. 2008. North American Glyptodontines (Xenarthra, Mammalia) in the Upper Pleistocene of northern South America. Paläontologische Zeitschrift 82: 139–152. Crossref
Castellanos, A. 1951. Acotaciones al género Neothocoraphorus Ameghino. Revista de la Asociación Geológica Argentina 6: 63–82.
Castellanos, A. 1953. Anotações e retificações ao gênero Glyptodon Owen e à Subfamília Glyptodontinae. Anais da Academia Brasileira de Ciências 25: 391–410.
Chimento, N.R., Carlini, A.A., Zurita, A.E., and Krmpotic, C. 2009. Morfología comparada de osteodermos en Glyptodontidae (Xenarthra, Cingulata). Ameghiniana 46 (Suplemento Resúmenes): 69R–70R.
Chinsamy, A. and Raath. M.A. 1992. Preparation of fossil bone for histological examination. Palaeontologia Africana 29: 39–44.
Cione, A.L. and Tonni, E.P. 1999. Biostratigraphy and chronological scale of upper most Cenozoic in the Pampean Area, Argentina. In: J. Rabassa and M. Salemme (eds.), Quaternary of South America and Antarctic Peninsula, 23–52. A.A. Balkema, Rotterdam.
Cione, A.L. and Tonni, E.P. 2005. Bioestratigrafía basada en mamíferos del Cenozoico superior de la provincia de Buenos Aires, Argentina. In: R.E. Barrio, R.O. Etcheverry, M.F. Caballé, and E. Llambias (eds.), Geología y recursos minerales de la Provincia de Buenos Aires. Relatorio XVI Congreso Geológico Argentino 9: 183–200. Quick Press, Buenos Aires.
CPRM, Companhia de Pesquisa de Recursos Minerais 2006. Rochas Carbonáticas do Grupo Bambuí na região Nordeste do Estado de Goiás. Available from: http://www.cprm.gov.br/publique/cgi/cgilua.exe/sys/start.htm?infoid=261&sid=32 (accessed 5.12.2010).
Cope, E.D. 1889. The Edentata of North America. American Naturalist 23: 657–664. Crossref
Da Costa Pereira, P.V.L.G., Victer, G.D., Porpino, K.O., and Bergqvist, L.P. 2014. Osteoderm histology of Late Pleistocene cingulates from the intertropical region of Brazil. Acta Palaeontologica Polonica 59: 543–552.
Dantas, M.T., Melo França, L., Cozzuol, M.A., and Rincón, A.D. 2011. About the occurrence of Glyptodon sp. in the Brazilian Intertropical Region. Quaternary International 305: 206–208. Crossref
Di Rienzo, J.A., Casanoves, F., Balzarini, M.G., Gonzalez, L., Tablada, M., and Robledo, C.W. 2008. InfoStat, versión 2008. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina.
Duarte, R.G. 1997. Gliptodontes del Pleistoceno tardío de Agua de las Palomas, Campo del Pucará, Catamarca, Argentina. Variaciones morfológicas del caparazón de Glyptodon reticulatus Owen, 1845. Ameghiniana 34 (3): 345–355.
Fernicola, J.C. 2008. Nuevos aportes para la Sistemática de los Glyptodontia Ameghino 1889 (Mammalia, Xenarthra, Cingulata). Ameghiniana 45: 553–575.
Francillon-Vieillot, H., Buffrénil, V. de, Castanet, J., Géraudie, J., Meunier, F.J., Sire, J.Y., Zylberberg, L., and Ricqlès, A. de 1990. Microstructure and mineralization of vertebrate skeletal tissues. In: J.G. Carter (ed.), Skeletal Biomineralization: Patterns, Process and Evolutionary Trends, 471–548. Van Nostrand Reinhold, New York.
Gasparini, G.M., Holanda, E.C., Araújo-Júnior, H.I., and Avilla, L.S. 2016. A Quaternary very young juvenile Tapirus Brisson, 1762 (Mammalia, Perissodactyla) from a cave deposit in northern Brazil: taxonomy and taphonomy. Historical Biology 28 (6): 803–811. Crossref
Gillette, D.D. and Ray, C.E. 1981. Glyptodonts of North America. Smithsonian Contributions to Paleobiology 40: 1–255. Crossref
Gillette, D.D., Carranza-Castañeda, O., White R.S. Jr,, Morgan, G.S., Thrasher, L.C., McCord, R., McCullough, G. 2016.Ontogeny and Sexual Dimorphism of Glyptotherium texanum (Xenarthra, Cingulata) from the Pliocene and Pleistocene (Blancan and Irvingtonian NALMA) of Arizona, New Mexico, and Mexico. Journal of Mammalian Evolution 23 (2):133–154. Crossref
Gray, J.E. 1869. Catalogue of Carnivorous, Pachydermatous and Edentates Mammalia in the British Museum. vii + 398 pp. British Museum of Natural History, London.
Hill, R.V., 2006. Comparative anatomy and histology of xenarthran osteoderms. Journal of Morphology 267: 1441–1460. Crossref
Hoffstetter, R. 1958. Xenarthra. In: J. Piveteau (ed.), Traité de Paléontologie, 535–636. Masson et Cie, Paris.
Hsiou, A.S., Winck, G.R., Schubert, B.W., and Avilla, L. 2013. On the presence of Eunectes murinus (Squamata, Serpentes) from the Late Pleistocene of Northern Brazil. Revista Brasileira de Paleontología 16 (1): 77–82. Crossref
Illiger, C. 1811. Prodromus systematis mammalium et avium. 301 pp. Sumptibus C. Salfeld, Berlin.
Krmpotic, C., Ciancio, M., Barbeito, C., Mario, R.C., and Carlini, A.A. 2009. Osteoderm morphology in recent and fossil euphractine xenarthrans. Acta Zoologica 90: 339–351. Crossref
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis,locis. Editio decima. 824 pp. Laurentius Salvius, Holmiae.
Luna, C.A. and Krapovickas, J.M. 2011. Primer registro de un ejemplar juvenil de Glyptodon sp. (Cingulata, Glyptodontidae) del Cuaternario de la provincia de Córdoba. Mastozoología Neotropical 18: 135–141.
Lund, P.W. 1840. Blik paa Brasiliens Dyreverden för Sidste Jordomvaeltning. Treide Afhandling: Forsaettelse af Pattedyrene. Det Kongelige Danske Videnskabernes Selskbas Naturvidenskabeligeog Mathematiske Afhandlinger 8: 217–272.
Lund, P.W. 1842. Blik paa Brasiliens Dyreverden för Sidste Jordomvaeltning. Fjerde Afhandling: Forstsaettelse af Pattedyrene. Det Kongelige Danske Videnskabernes Selskabs Naturnvidenskabeligeog Mathematiske Afhandlinger 9: 137–208.
McKenna, M.C. and Bell, S.K. 1997. Classification of Mammals above the Species Level. 631 pp. Columbia University Press, New York.
Oliveira, E.V., Barreto, A.M.F., and Alves, R.S. 2009. Aspectos sistemáticos, paleobiogeográficos e paleoclimáticos dos mamíferos fósseis do Quaternário de Fazenda Nova, Pernambuco, nordeste do Brasil. Gaea 5: 75–85. Crossref
Oliveira, E.V., Porpino, K., and Barreto, A.F. 2010. On the presence of Glyptotherium in the Late Pleistocene of Northeastern Brazil, and the status of “Glyptodon” and “Chlamydotherium”. Paleobiogeographic implications. Neues Jahrbuchfür Geologie und Paläontologie 258 (3): 353–363. Crossref
Oliveira, E.V., Villa Nova, P., Goin, F.J., and Avilla, L.S. 2011. A new hyladelphine marsupial (Didelphimorphia, Didelphidae) from cave deposits of northern Brazil. Zootaxa 3041: 51–62.
Osborn, H.F. 1903. Glyptotherium texanum, a New Glyptodont, from the Lower Pleistocene of Texas. Bulletin of the American Museum of Natural History 19 (17): 491–494.
Owen, R. 1839. Description of a tooth and part of the skeleton of the Glyptodon, a large quadruped of the edentate order, to which belongs the tessellated bony armour figured by Mr CLIFT in his memoir on the remains of the Megatherium, brought to England by Sir Woodbine PARISH, F.G.S. Proceedings of the Geological Society of London 3: 108–113.
Porpino, K.O., Fernicola, J.C., and Berqvist, L.P. 2009. A new cingulated (Mammalia: Xenarthra), Pachyarmatherium brasiliense sp. nov. from the late Pleistocene of northeastern Brazil. Journal of Vertebrate Paleontology 29: 881–893. Crossref
Ricqlès, A. de, Meunier, F.J., Castanet, J., Francillon-Vieillot, E. 1991. Comparative microstructure of bone. In: B.K. Hall (ed.), Bone. Volume 3: Bone Matrix and Bone Specific Products, 1–78. CRC Press, Boca Raton.
Rincón, A.D., White, R.S., and McDonald, H.G. 2008. Late Pleistocene cingulates (Mammalia: Xenarthra) from Mene de Inciarte Tar Pits, Sierra de Perijá, Western Venezuela. Journal of Vertebrate Paleontology 28: 197–207. Crossref
Rinderknecht, A. 2000. Estudios sobre la familia Glyptodontidae Gray, 1869. II. Variación morfológica en la coraza de Glyptodon sp. juvenil (Mammalia: Cingulata). Revista de la Sociedad Uruguaya de Geología 7: 32–35.
Rocha-dos-Santos, B.C.d A., Avilla, L.S., and Scherer, C.S. 2017. The fossil Camelidae (Mammalia: Cetartiodactyla) from the Gruta do Urso cave, northern Brazil. Quaternary International 436: 181–191. Crossref
Rodrigues, S., Avilla, L.S., Soibelzon, L.H., and Bernardes, C. 2014. Late Pleistocene carnivores (Carnivora: Mammalia) from a cave sedimentary deposit in northern Brazil Anais da Academia Brasileira de Ciências 86 (4): 1641–1655. Crossref
Rodriguez, S.G., Soibelzon, L.H., Rodrigues, S., Morgan, C.C., Bernardes, C., Avilla, L.S., and Lynch, E. 2013. First record of Procyoncancrivorus (G. Cuvier, 1798) (Carnivora, Procyonidae) in stratigraphic context in the Late Pleistocene of Brazil. Journal of South American Earth Sciences 45: 1–5. Crossref
Scheyer, T.M. 2007. Skeletal histology of the dermal armor of Placodontia: the occurrence of “postcranial fibro-cartilaginous bone” and its developmental implications. Journal of Anatomy 211: 737–753. Crossref
Scheyer, T.M. and Sander, P.M. 2004. Histology of ankylosaur osteoderms: implications for systematics and function. Journal of Vertebrate Paleontology 24: 874–893. Crossref
Simpson, G.G. 1929. Pleistocene Mammalian Fauna of the Seminole Field, Pinellas County, Florida. Bulletin of the American Museum of Natural History 56 (8): 561–599.
Soibelzon, E., Avilla, L.S., and Castro, M. 2015.The cingulates (Mammalia: Xenarthra) from the late Quaternary of northern Brazil: Fossil records, paleoclimates and displacements in America. Quaternary International 377: 118–125. Crossref
Soibelzon, E., Zurita, A.E., and Carlini, A.A. 2006. Glyptodon munizi Ameghino (Mammalia, Cingulata, Glyptodontidae): redescripción y anatomía. Ameghiniana 43: 377–384.
Vickaryous, M.K. and Hall, B.K. 2006. Osteoderm morphology and development in the nine banded armadillo, Dasypus novemcinctus (Mammalia, Xenarthra, Cingulata). Journal of Morphology 267: 1273–1283. Crossref
Vickaryous, M.K. and Hall, B.K. 2008. Development of the dermal skeleton in Alligator mississippiensis (Archosauria, Crocodylia) with comments on the homology of osteoderms. Journal of Morphology 269: 398–422. Crossref
Webb, S.D. 2006. The Great American Biotic Interchange: patterns and processes. Annals of the Missouri Botanical Garden 93: 245–257. Crossref
Wolf, D. 2007. Osteoderm histology of extinct and recent Cingulata and Phyllophaga (Xenarthra, Mammalia): Implications for biomechanical adaptation and systematics. Hallesches Jahrbuch für Geowissenschaften 23: 145–151.
Wolf, D., Kalthoff, D.C., and Sander, P.M. 2012.Osteoderm histology of the Pampatheriidae (Cingulata, Xenarthra, Mammalia): implications for systematics, osteoderm growth, and biomechanical adaptation. Journal of Morphology 273: 388–404. Crossref
Woodburne, M.O. 2010. The Great American Biotic Interchange: Dispersals, Tectonics, Climate, Sea Level and Holding Pens. Journal of Mammalian Evolution 17: 245–264. Crossref
Zampaulo, R.A. and Ferreira, R.L. 2009. Terrestrial cave invertebrate diversity in nine caves in the municipality of Aurora do Tocantins (TO). In: M.A. Rasteiro and R.L. Sarmento (eds.), 30° Congresso Brasileiro de Espeleologia, 267–274. Sociedade Brasileira de Espeleologia, Montes Claros. Available from: http://www.sbe.com.br/anais30cbe/ 30cbe_267-274.pdf (accessed 5.12.2010).
Zurita, A.E., Carlini, A.A., and Gillette, D. 2008. Glyptotherium–Glyptodon (Xenarthra, Glyptodontidae, Glyptodontinae): Anatomy and palaeobiogeography. Journal of Vertebrate Paleontology 28 (Supplement 3): 165.
Zurita, A.E., Carlini, A.A., Gillette, D., and Sanchez, R. 2011a. Late Pliocene Glyptodontinae (Xenarthra, Cingulata, Glyptodontidae) of South and North America: Morphology and paleobiogeographical implications in the GABI. Journal of South American Earth Sciences 31: 178–185. Crossref
Zurita, A.E., González-Ruiz, L., Gómez-Cruz, A., and Arenas-Mosquera, J.E. 2013. The most complete known Neogene Glyptodontidae (Mammalia, Xenarthra, Cingulata) from northern South America: taxonomic, paleobiogeographic and phylogenetic implications. Journal of Vertebrate Paleontology 33: 698–706. Crossref
Zurita, A.E., Miño-Boilini, A.R., Francia, A., and Arenas-Mosquera, J.E. 2012. The Pleistocene Glyptodontidae Gray, 1869 (Xenarthra: Cingulata) of Colombia and some considerations about the South American Glyptodontinae. Revista Brasileira de Paleontología 15: 273–280. Crossref
Zurita, A.E., Miño-Boilini, A.R., Soibelzon, E., Carlini, A. A., and Paredes-Ríos, F. 2009. The diversity of Glyptodontidae (Xenarthra, Cingulata) in the Tarija Valley (Bolivia): systematic, biostratigraphic and paleobiogeographic aspects of a particular assemblage. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 251–252: 225–237. Crossref
Zurita, A.E., Oliveira, E., Toriño, P., Rodriguez-Bualó, S., Scillato-Yané, G.J., Luna, C., and Krapovickas, J. 2011b. On the taxonomic status of some Glyptodontidae (Mammalia, Xenarthra,Cingulata) from the Pleistocene of South America. Annales de Paleontologie 97: 63–83. Crossref
Zurita, A.E., Soibelzon, L.H., Soibelzon, E., Gasparini, G.M., Cenizo, M.M., and Arzani, H. 2010. Accessory protection structures in Glyptodon Owen (Xenarthra, Cingulata, Glyptodontidae). Annales de Paleontologie 96: 1–11. Crossref
Acta Palaeontol. Pol. 63 (1): 159–170, 2018
https://doi.org/10.4202/app.00409.2017