

A new dolphin from the early Miocene of Patagonia, Argentina: Insights into the evolution of Platanistoidea in the Southern Hemisphere
MARIANA VIGLINO, MÓNICA R. BUONO, CAROLINA S. GUTSTEIN, MARIO A. COZZUOL, and JOSÉ I. CUITIÑO
Viglino, M., Buono, M.R., Gutstein, C.S., Cozzuol, M.A., and Cuitiño, J.I. 2018. A new dolphin from the early Miocene of Patagonia, Argentina: Insights into the evolution of Platanistoidea in the Southern Hemisphere. Acta Palaeontologica Polonica 63 (2): 261–277.
The contents of the superfamily Platanistoidea, an early-diverging lineage comprising extinct species and a single extant representative of South Asian river dolphin (Platanista gangetica), remain controversial. We describe here a partial skull and associated tympano-periotic bones identified as a new genus and species, Aondelphis talen gen. et sp. nov., collected in the lower levels of the Gaiman Formation (early Miocene), in Patagonia (Chubut Province, Argentina). Aondelphis is the first Patagonian platanistoid species named in almost a century. Phylogenetic analyses suggest Aondelphis talen gen. et sp. nov. and a taxon from New Zealand (cf. Papahu ZMT-73) are basal Platanistoidea sensu lato. Unambiguous synapomorphies related to the ear bones allowed us to determine its phylogenetic position. Aondelphis talen markedly differs from the other well-known early Miocene Patagonian platanistoid Notocetus, suggesting the coexistence of at least two different morphotypes that may have occupied different ecological niches at that time. The putative close relationship with a species from New Zealand indicates there was a rapid diversification and widespread distribution of the group in the Southern Hemisphere during the early Miocene. The description of new species and revision of historical records of Patagonian platanistoids can help shedding light on cetacean assemblages of the Patagonian sea during this epoch.
Key words: Mammalia, Platanistoidea, evolution, Miocene, Gaiman Formation, Argentina, Chubut Province.
Mariana Viglino [viglino@cenpat-conicet.gob.ar]; Mónica R. Buono [buono@cenpat-conicet.gob.ar], and José I. Cuitiño [jcuitino@cenpat-conicet.gob.ar], Instituto Patagónico de Geología y Paleontología, CONICET, Puerto Madryn, U9120ACD, Chubut, Argentina.
Carolina S. Gutstein [sgcarolina@gmail.com], Red Paleontológica U.Chile, Laboratorio de Ontogenia y Filogenia, Departamento de Biología, Facultad de Ciencias, Universidad de Chile, Ñuñoa, 8320000, Santiago, Chile. Área Patrimonio Natural, Consejo de Monumentos Nacionales, Providencia, 7500000, Santiago, Chile.
Mario A. Cozzuol [mario.cozzuol@gmail.com], Departamento de Zoologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, 31270-910, Pampulha, Belo Horizonte, Minas Gerais, Brazil.
Received 8 November 2017, accepted 9 February 2018, available online 20 March 2018.
Copyright © 2018 M.Viglino et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Platanistoidea is one of the earliest diverging lineages of odontocetes, which includes a morphologically and taxonomically diverse group of dolphins. The unique extant genus Platanista (e.g., Muizon 1987, 1991, 1994; Fordyce and Muizon 2001; Barnes 2006; Barnes et al. 2010), can be split into one or three species (e.g., Reeves and Martin 2009; Shostell and Ruiz-García 2010). Although the definition of Platanistoidea still remains controversial, as it has been traditionally recognized as paraphyletic (e.g., Muizon 1987, 1991; Nikaido et al. 2001; McGowen et al. 2009), some stable groups have been identified in recent phylogenetic analyses: Otekaikea, Waipatiidae, Squalodelphinidae and Platanistidae (e.g., Muizon 1994; Fordyce 1994; Tanaka and Fordyce 2014, 2015a, b, 2016, 2017; Lambert et al. 2014). It should be noted that the contents of family Squalodelphinidae vary among different phylogenetic analyses (e.g., Lambert et al. 2014, 2017; Tanaka and Fordyce 2014, 2015a, b, 2016, 2017; Boersma and Pyenson 2016; Boersma et al. 2017). The inclusion of the family Allodelphinidae among platanistoids was tested in a few studies (Barnes et al. 2010; Boersma and Pyenson 2016; Boersma et al. 2017). The fossil record of platanistoids dates back to the late Oligocene–early Miocene, when the group achieved its maximum radiation, followed by a decrease in its diversity during the middle–late Miocene (Muizon 1987, 1994; Fordyce and Muizon 2001; Barnes et al. 2010). Platanistoids are particularly diverse in late Oligocene marine beds from New Zealand, with many new and well preserved taxa recently described (e.g., Tanaka and Fordyce 2014, 2015a, b, 2016, 2017). However, the early Miocene history of this group remains poorly recorded, with only some taxa known (e.g., Moreno 1892; Muizon 1988; Barnes and Reynolds 2009; Lambert et al. 2014).
The eastern Atlantic coast of South America has promising outcrops to expand the Miocene record of platanistoids, as it possesses one of the few early Miocene marine beds globally (i.e., Gaiman Formation; Scasso and Castro 1999; Cuitiño et al. 2017). For this epoch, the Gaiman Formation holds a remarkably rich and well-preserved assemblage of cetaceans (e.g., Cuitiño et al. 2017 and references therein). In Patagonia (Argentina), platanistoid dolphins are represented by three species: (i) Notocetus vanbenedeni Moreno, 1892, which is the most common cetacean in the Gaiman Formation (Cione et al. 2011); (ii) Phoberodon arctirostris Cabrera, 1926; and (iii) Prosqualodon australis Lydekker, 1894 (Cozzuol 1996), all of which are currently in revision by some of the authors (MV, MRB). Here, we describe a partial skull and associated tympano-periotic bones identified as a new genus and species, collected in the lower levels of the Gaiman Formation, Chubut Province (Argentina). This specimen is the first Patagonian platanistoid named in almost a century. This new record increases the platanistoid diversity known locally from the early Miocene and helps understand the evolutionary history of the superfamily; it also expands the record of odontocetes from the early Miocene in Southern oceans.
Institutional abbreviations.—AMNH, American Museum of Natural History, New York, USA; CNPMAMM, Laboratorio de Mamíferos Marinos, Centro Nacional Patagónico, Puerto Madryn, Argentina; LACM, Natural History Museum of Los Angeles County, Los Angeles, USA; MACN, Colección de Mastozoología, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina; MPEF-PV, Museo Paleontológico “Egidio Feruglio”, Trelew, Argentina; MLP, Departamento de Paleontología de Vertebrados, Museo de La Plata, La Plata, Argentina; NMNZ, Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand; OM-GL, Otago Museum, Dunedin, New Zealand; OU, Geology Museum, University of Otago, Dunedin, New Zealand; USNM, Department of Paleobiology and Department of Vertebrate Zoology, National Museum of Natural History, Smithsonian Institution, Washington D.C., USA; ZMT, Fossil mammals catalogue, Canterbury Museum, Christchurch, New Zealand.
Other abbreviations.—BIZYG, bizygomatic width; CI, consistency index; K, concavity values of implied weights; m., musculus; MPTs, most parsimonious trees; OTU, operational taxonomic unit; RI, retention index.
Nomenclatural acts.—The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature, and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be re-solved and the associated information viewed through any standard web browser by appending the LSID to the pre-fix “http://zoobank.org/”. The LSID for this publication is: urn:lsid:zoobank.org:pub:CE9D1F40-AD00-496C-8FE7-627807554BB8. The electronic edition of this work was published in a journal with an eISSN 1732-2421, and has been archived and is available from the following digital repository: http://www.app.pan.pl/article/item/app004412017.html
Material and methods
Specimens and terminology.—The description is based on the specimen MPEF-PV 517, collected by MAC and Pablo Puerta in Bryn Gwyn, Chubut River Valley in January 1990. The specimen is deposited in the Museo Paleontológico “Egidio Feruglio” in Trelew, Chubut Province, Argentina. Data from the literature and specimens held in scientific collections were used here for comparative and phylogenetic studies (see SOM 1, Supplementary Online Material available at http://app.pan.pl/SOM/app63-Viglino_etal_SOM.pdf).
For the skull and tympano-periotic complex, morphological terms followed Mead and Fordyce (2009), except when explicitly noted. Descriptions are based either on the right or left side, whichever was more informative, with differences between them mentioned only if directional asymmetry was evident. Measurements were taken following Perrin (1975) and Kasuya (1973), using digital calipers. Photographs were taken with a Canon PowerShot G16 camera in manual mode. In order to obtain images with greater depth of focus, an image-stacking technique (Bercovici et al. 2009) was used. With this technique, a well-focused image is obtained by merging several images captured at slightly different focal planes. Each resulting stacked image was checked for possible artifacts.
Phylogenetic analysis.—The phylogenetic analysis was based on the matrix published by Tanaka and Fordyce (2016) and it was edited using Mesquite 3.2 (Maddison and Maddison 2011). The morphological dataset was expanded through the incorporation of MPEF-PV 517 and the addition of one new character. As it is beyond the scope of this paper to conduct a thorough analysis of the phylogenetic relationships within Platanistoidea, we have maintained the taxonomic sampling of the original matrix (for example, there are no allodelphinid species included). Modifications to character description and codings are listed in SOM 2 and matrix is available in nexus format in SOM 8.
The resulting matrix is composed of 84 taxa and 287 characters (224 craneo-mandibular, 32 postcranial, and 31 soft-tissue characters). MPEF-PV 517 has 77% of data missing (including soft-tissue characters). For the purpose of coding the characters related to the periotic and tympanic bulla, views used here were the same as for the description (see below).
Heuristic parsimony analysis of the dataset was performed in TNT version 1.5 (Goloboff and Catalano 2016) using the traditional search under equal and implied weights. We used K values ranging from 3 (more stringent weight against homoplasy) to 23 (less stringent weight against homoplasy), which allowed us to examine potential effects of homoplasious characters on MPTs. All characters were treated as unordered. The analysis was performed using 1000 replicates of Wagner trees (using random addition sequences), tree bisection reconnection branch swapping holding 10 trees per replicate. The trees obtained at the end of the replicates were subjected to a final round of tree bisection reconnection branch swapping. The resulting MPTs were summarized using strict consensus trees with zero-length branches collapsed (i.e., “rule 1” of Coddington and Scharff 1994). To estimate branch support, jackknife resampling analysis (with p = 0.30 and 1000 pseudoreplicates; Farris et al. 1996; Goloboff et al. 2003) and Bremer support were performed.
After the analyses were performed, species in the more-diverse families crown-ward of Papahu, Squaloziphius, and Xiphiacetus were merged for ease of illustration. The full cladograms that show all OTUs are provided in SOM 3–6.
For the present work, we follow Tanaka and Fordyce’s (2017) definition of Platanistoidea sensu stricto (Waipatiidae + Awamokoa + Otekaikea + “Squalodelphinidae” + Platanistidae), as well as Platanistoidea sensu lato (Squalodon + Waipatiidae + Awamokoa + Otekaikea + “Squalodelphinidae” + Platanistidae).
Geological setting
The holotype MPEF-PV 517 was collected from the sediments of the early Miocene Gaiman Formation (Haller and Mendía 1980), five meters above the conformable contact with the underlying terrestrial Sarmiento Formation (Fig. 1). The Gaiman Formation is 70 m-thick in Bryn Gwyn and is composed by marine tuffaceous mudstone and sandstone beds. The basal stratum of the Gaiman Formation is a thin transgressive lag with some gravels, bones and teeth from marine vertebrates (Cione 1978; Haller and Mendía 1980; Scasso and Castro 1999). The marine sediments overlying this basal stratum are composed of white, tuffaceous, thoroughly bioturbated mudstone and fine sandstone with occasional mollusk molds and thin oyster horizons, deposited in a shallow shelf (Haller and Mendía 1980; Scasso and Castro 1999; Lech et al. 2000). The Gaiman Formation on Bryn Gwyn is covered by 28 m of shallow marine, estuarine to terrestrial deposits of the late Miocene Puerto Madryn Formation (Scasso and Castro 1999).
The early Miocene age of the Gaiman Formation is based on stratigraphic correlations to other absolutely-dated sections in Patagonia and biostratigraphic data. In southern Patagonia, chronologically equivalent units were dated by isotopic methods as early Miocene (Parras et al. 2012; Cuitiño et al. 2012; Cuitiño et al. 2015a). Equivalent beds in the Comodoro Rivadavia region (Chubut Province) span from the early Miocene (Burdigalian) to the middle Miocene (early Langhian) (Cuitiño et al. 2015b). The horizon containing the MPEF-PV 517 of Bryn Gwyn is part of the lower part of the Gaiman Formation and can be considered as the initial phase of the marine cycle. Based on regional correlations, an early Miocene age is proposed for these cetacean bearing beds. An early Miocene age for the lower part of the Gaiman Formation was also suggested by the Colhuehuapian mammal fauna recovered from the underlying Trelew Member of the Sarmiento Formation (Flynn and Swisher 1995; Dunn et al. 2013). In addition, evidences of marine vertebrates in the Gaiman Formation (i.e., fishes and penguins) (Cione et al. 2011), as well as a palynological assemblage recovered from the study area (Palazzesi et al. 2006) also indicated an early Miocene age.
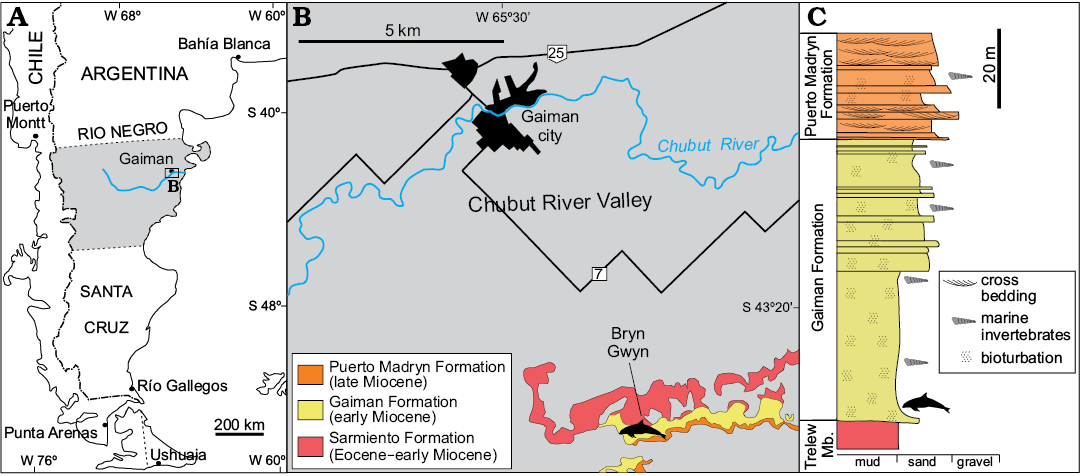
Fig. 1. Map and stratigraphic section of Bryn Gwyn, the type locality of Aondelphis talen gen. et sp. nov. A. Regional map of the study area. B. Detailed map with stratigraphic information of the type locality (dolphin outline). C. Simplified stratigraphic section of Bryn Gwyn, modified from Scasso and Bellosi (2004). Dolphin outline indicates type horizon of Aondelphis talen gen. et sp. nov. Trelew Mb., Trelew Member of Sarmiento Formation.
Systematic palaeontology
Cetacea Brisson, 1762
Odontoceti Flower, 1867
Platanistoidea Simpson, 1945 sensu Muizon, 1987
Genus Aondelphis nov.
Etymology: From the Tehuelche language aone, south; to indicate the geographic provenance; and from Latin delphis, dolphin.
Type species: Aondelphis talen sp. nov., see below.
Diagnosis.—As for the type species by monotypy.
Aondelphis talen sp. nov.
Figs. 2–6, Table 1.
Etymology: From the Tehuelche language t’alen, small; in reference to small size.
Holotype: MPEF-PV 517; incomplete skull including basioccipital, exoccipitals, squamosals, a portion of the sphenoids, almost complete left tympanic bulla, and complete left periotic.
Type locality: Bryn Gwyn (= Loma Blanca), southern cliff of Chubut River Valley, 8 km southeast of the town of Gaiman, in Chubut Province, Patagonia, Argentina (Fig. 1).
Type horizon: Gaiman Formation, early Miocene.
Diagnosis.—Aondelphis talen gen. et sp. nov. differs from all other Platanistoidea (sensu lato; see Phylogenetic analysis section) in the unique autapomorphy: convex dorsal surface of periotic (character 182).
Aondelphis talen differs from cf. Papahu ZMT-73 in the absence of a ridge on the inside of the tympanic bulla (character 221). Aondelphis talen differs from Squalodon calvertensis in the concave ventral surface of the posterior process of the periotic (character 201). Aondelphis talen differs from cf. Papahu ZMT-73 and S. calvertensis in the development of an anteroposterior ridge on the dorsal side of the anterior process and body of periotic (character 174). Aondelphis talen differs from Platanistoidea sensu stricto (see Phylogenetic analysis section) in the anteroposteriorly wide and squared-off postglenoid process of the squamosal (character 118); no obvious lateral groove on profile of the periotic (character 172); from the Platanistoidea sensu stricto (except Platanista gangetica) in the tubular fundus of the internal acoustic meatus of the periotic (character 189); from Platanistoidea sensu stricto (except Awamokoa tokarahi and Notocetus vanbenedeni) in the weakly-curved parabullary sulcus (character 176). Aondelphis talen differs from S. calvertensis and Platanistoidea sensu stricto in the excavated dorsal margin of the involucrum of the tympanic bulla at mid-length (character 220); in the absence of an articular rim in the periotic (character 196); from S. calvertensis and Platanistoidea sensu stricto (except A. tokarahi and P. gangetica) in the slit-like external auditory meatus (character 157); from S. calvertensis and Platanistoidea sensu stricto (except P. gangetica) in the wide angle between the posterior process and pars cochlearis of the periotic (character 199); from the S. calvertensis and Platanistoidea sensu stricto (except Pomatodelphis inaequalis) in the high lateral wall of the internal acoustic meatus (character 191). Aondelphis talen further differs from S. calvertensis, and Platanistoidea sensu stricto (except Otekaikea marplesi; unknown in Phocageneus venustus) in the deep emargination of the neck muscle fossa in the zygomatic process of the squamosal (character 114).
Aondelphis talen differs from cf. Papahu ZMT-73 and Platanistoidea sensu stricto in the rounded profile of the cochlear aqueduct (character 194); and in the absence of a subcircular fossa (character 152); from cf. Papahu ZMT-73, Squalodelphinidae and Platanistidae in the poorly defined ventromedial keel of the tympanic bulla (character 222).
Aondelphis talen differs from Squalodon calvertensis, Awamokoa tokarahi, Otekaikea, Notocetus vanbenedeni, and Platanistidae in the absence of a fossa for articular rim of the periotic (character 287); from S. calvertensis, Waipatia maerewhenua, A. tokarahi, Squalodelphis fabianii, and N. vanbenedeni in the distinctly shorter inner posterior prominence of the tympanic bulla with respect to the outer posterior prominence (character 219); from S. calvertensis and N. vanbenedeni in the smoothly deflected anterior process of the periotic (character 173). Aondelphis talen differs from S. calvertensis, N. vanbenedeni and Platanistidae in the shallow posterior portion of the periotic fossa (character 155). Aondelphis talen differs from S. calvertensis, Phocageneus venustus, N. vanbenedeni, and Platanistidae in the presence of a posterodorsal edge of the stapedial muscle fossa in the periotic (character 184); and a long posterior process of the periotic (character 203). MPEF-PV 517 differs from N. vanbenedeni and Zarhachis flagellator in the open jugular notch (character 164). Aondelphis talen differs from Platanistidae in the triangular tympanosquamosal recess medial to the postglenoid process (character 148); and in the absence of an excavation of the tegmen tympani at the base of the anterior process (character 188).
Description.—Physical maturity and body size: Due to the fragmentary condition of this specimen, it is difficult to determine the age of MPEF-PV 517. The lack of punctate texture on the occipital condyles suggests that at least it is not a juvenile specimen (Aguirre-Fernandez and Fordyce 2014). Even though the zygomatic processes are incomplete, an estimated bizygomatic width was measured in order to infer the total length of this species. We applied the formula proposed by Pyenson and Sponberg (2011) for stem Platanistoidea: Log(L) = 0.92 × (log(BIZYG) – 1.51) + 2.49. The BIZYG for Aondelphis talen gen. et sp. nov. is 21.55 cm, giving a reconstructed body length of 2.13 m. It is of similar size to Waipatia maerwhenua (BIZYG: 24.4 cm, total length 2.4 m; Fordyce 1994), Huaridelphis raimondii (BIZYG: 20.7 cm, total length 2.05 m; Lambert et al. 2014) and adult males of Platanista gangetica (2.2 m; Jefferson et al. 2008). However, Aondelphis talen gen. et sp. nov. is smaller than the remaining platanistoids, such as Zarhachis flagellator (BIZYG: 26.8 cm, total length 2.6 m; Kellogg 1924), Squalodelphis fabianii (BIZYG: 25.6 cm, total length 2.5 m; Dal Piaz 1917) and Otekaikea marplesi (BIZYG: 25.7 cm, total length 2.5 m; Tanaka and Fordyce 2014).
Exoccipital (Fig. 2): The occipital condyles are gently convex, with a smooth articular face and a short but clearly delimited pedicle that projects them posteriorly (greatest occipital breadth 91.98 mm). The ventral condyloid fossa is not clearly delimited. Ventrally, the paroccipital process is wide but eroded. There is a shallow fossa on the anterior face of this process, dorsal to the paroccipital process. In odontocetes, there are at least two bony correlates of the pterygoid sinus system in the exoccipital: one corresponds to the posterior sinus (in the anteroventral surface of the paroccipital process) and the other one to the posterolateral extension of the peribullary sinus (ventral surface of paroccipital process; Mead and Fordyce 2009). The identification of these bony correlates is confusing in the literature, as the posterior sinus fossa is variably developed (Fordyce 1994; Fraser and Purves 1960). Based on the anatomical location of the fossa observed in Aondelphis talen gen. et sp. nov. on the anterior surface of the paroccipital process, it is tentatively identified as a posterolateral sinus fossa. Another interpretation is that the concavity on the ventral surface of the paroccipital process corresponds to the point of articulation with the stylohyal (Fraser and Purves 1960; Mead and Fordyce 2009; Marx et al. 2016). There is a large fissure, similar to what was described for Otekaikea (Tanaka and Fordyce 2014) and Awamokoa (Tanaka and Fordyce 2017) on the exoccipital-squamosal suture anterior to the latter fossa (Fig. 2B).
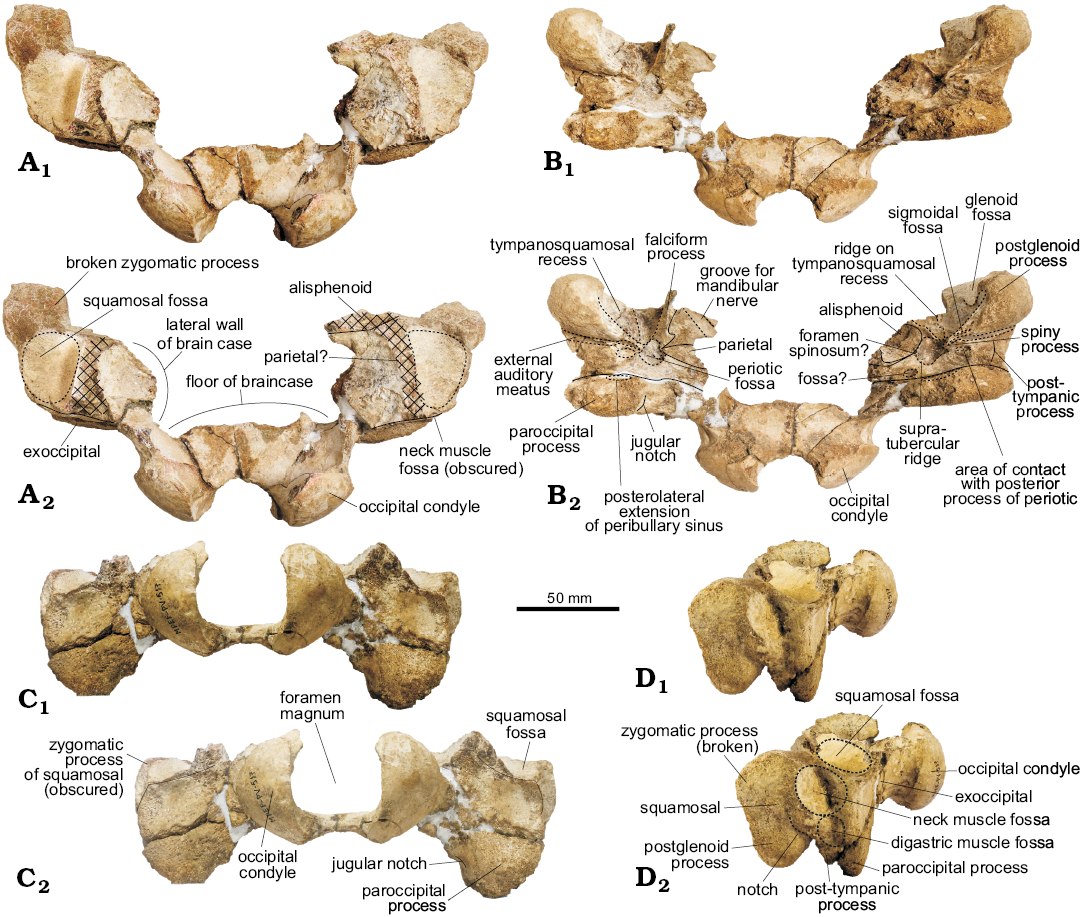
Fig. 2. Partial skull of the holotype of the platanistoid dolphin Aondelphis talen gen. et sp. nov. (MPEF-PV 517) from the early Miocene Gaiman Formation, Bryn Gwyn, Patagonia, Argentina; in dorsal (A), ventral (B), posterior (C), and lateral (D) views. Continuous lines indicate sutures, whilst dashed lines indicate outlines of a particular structure. Hatched outlines show broken areas of the specimen. Photographs (A1–D1), photographs with explanations (A2–D2).
Basioccipital (Fig. 3): The basioccipital is ventrally trapezoideal (greatest length 85.3 mm), with no clear sutures with the basisphenoid. Dorsally, there is a distinct rounded pontine impression on the anterior portion of the basioccipital. Just posterolaterally, there are two dorsolateral projections followed by a deep elliptical fossa that is longer mediolaterally than dorsoventrally deep. They mark the anterior limit of the cerebellar lobe (sensu Anderson 1878). In ventral view, the basioccipital crest is transversely thick with a strong laterally projection on its posterior portion. Medially, the posterior portion of the basioccipital has a well-developed muscular tubercle for the insertion of the m. rectus capitis ventralis. The posteroventral margin of the basioccipital crest has a distinct and narrow depression, oriented dorsolaterally. A shallow depression on the lateral surface of the crest (Fig. 3F) indicates the probable medial extension of the peribullary sinus.
Parietal (Fig. 2B): There appears to be a small exposure of the parietal in ventral view, medial to the squamosal and posterior to the alisphenoid, at the basicranium, similar to what was described in Waipatia (Fordyce 1994; Tanaka and Fordyce 2015b), Otekaikea (Tanaka and Fordyce 2014, 2015a), cf. Papahu ZMT-73 (Tanaka and Fordyce 2016) and Papahu taitapu (Aguirre-Fernández and Fordyce 2014). No distinctive features could be recognized.
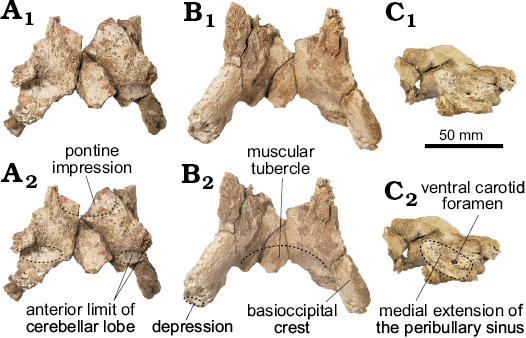
Fig. 3. The portion of basioccipital of the holotype of the platanistoid dolphin Aondelphis talen gen. et sp. nov. (MPEF-PV 517) from the early Miocene Gaiman Formation, Bryn Gwyn, Patagonia, Argentina; in dorsal (A), ventral (B), and lateral (C) views. Dashed lines indicate specific structures. Photographs (A1–C1), photographs with explanations (A2–C2).
Squamosal (Figs. 2, 4): Laterally, the short postglenoid process is robust and blunt, ventrally oriented. On the lateral surface of the zygomatic process (bizygomatic width: 215.5+ cm), dorsal to the external auditory meatus, there is a long circular rugose-surfaced neck muscle fossa (sensu Fordyce 1981); the posterior margin is formed by the exoccipital, like in cf. Papahu ZMT-73 (Tanaka and Fordyce 2016). Like this latter species, there is a small fossa (probably for the digastric muscle) on the lateral surface of the post-tympanic process. A sigmoideal notch dorsal to the external auditory meatus is more visible on the left side. The same condition occurs in cf. Papahu ZMT-73 (Tanaka and Fordyce 2016). The posteroventral portion of the temporal fossa (floor of temporal fossa or squamosal fossa sensu Lambert et al. 2015) is preserved in dorsal view and of small size.
In ventral view, the glenoid fossa is shallow. The tympano-squamosal recess is wide and very deep, delimited laterally by a distinct crest and medially by the broken base of the falciform process. It is longer anteroposteriorly and narrow lateromedially, and extends posteriorly at about the postglenoid process. The surface presents multiple striae anterolaterally to posteromedially oriented, and there is a low but distinct anteroposteriorly oriented crest that divides the tympano-squamosal recess in two portions; the medial portion is the smallest. Mesoplodon europaeus, M. mirus, and M. stejnegeri show a similar condition. Posteriorly, there is a shallow but distinct oval-shaped sigmoid fossa (sensu Geisler et al. 2005). The spiny process is broken, but the base is oval-shaped and descends to the start of the falciform process. The falciform process is better preserved on the right side; it is very thin and presents a sigmoidal shape, with a distinct notch just anterior to the spiny process. The falciform process is ventrally oriented, skewing slightly medially. The squamosal-alisphenoid suture is not very clear.
The periotic, when in situ, lies posterior to the falciform process, medial to the external auditory meatus, anterior to the posterior sinus fossa and lateral to the foramen ovale. The periotic fossa is triangular-shaped, apparently formed only by the squamosal. The low supratubercular ridge, more distinct on the medial area of the fossa, divides it in shallow anterior and posterior portions. A circular foramen spinosum opens anteriorly, on the medial margin of the anterior portion of the periotic fossa, just lateral to the most posterior portion of the alisphenoid-squamosal suture. It resembles the condition found in Waipatia maerewhenua (Fordyce 1994). A distinct and wide path for the mandibular nerve (V3) is observed on the alisphenoid on the right side, which runs mediolaterally at an oblique angle. Unfortunately, the foramen ovale was not preserved. Posteromedial to the periotic fossa, there is a concave surface (Fig. 2B2: fossa?) of unknown homology or function.
The external auditory meatus is long, slit-like and deep (similar to Platanista gangetica; Anderson 1878), slightly wider laterally and delimited by distinct anterior and posterior meatal crests. Posterior to the external auditory meatus is the post-tympanic process (better preserved on the right side), which provides an area of contact with the posterior process of the tympanic bulla. There is also a small fossa just medial to this process, here interpreted as the area of contact with the posterior process of the periotic. The post-tympanic process is shorter lateromedially than anteroposteriorly.
Basisphenoid (Fig. 3): Only a portion of this bone was preserved, including the large oval ventral carotid foramen on the lateral surface of the basiooccipital crest. The foramen appears to have been covered by the peribullary sinus. There is no visible suture with the basioccipital and thus, its extension cannot be inferred.
Alisphenoid (Fig. 2B): There is a small portion preserved in the basicranial region, lateral to the squamosal and anterior to the parietal. On the left side of the skull the groove for the mandibular nerve is preserved. The exposure of this bone is longer anteroposteriorly than lateromedially wide.
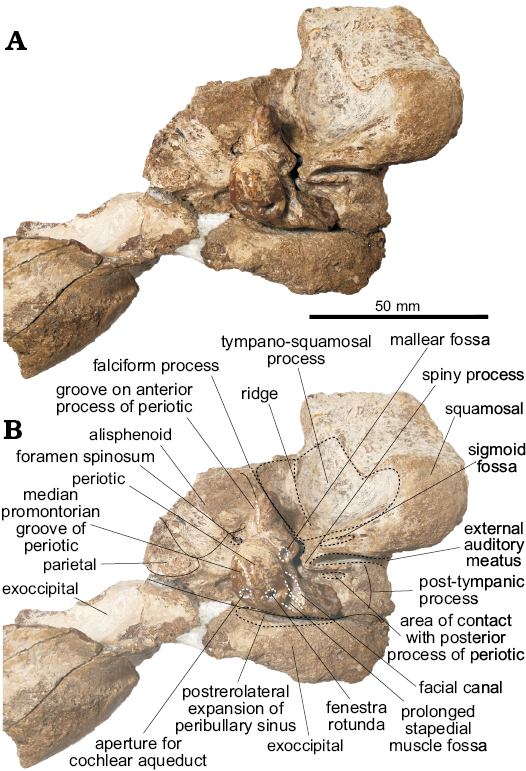
Fig. 4. Partial skull and periotic in situ of the holotype of the platanistoid dolphin Aondelphis talen gen. et sp. nov. (MPEF-PV 517) from the early Miocene Gaiman Formation, Bryn Gwyn, Patagonia, Argentina; left side in ventral view. Dashed lines indicate specific structures (black, skull; white, periotic), whilst continuous lines indicate sutures. It should be noted that the position of the periotic does not take into account the volume that may have occupied the peribullary sinus and other soft tissue structures. Photograph (A), photograph with explanations (B).
Periotic (Figs. 4–5): For description purposes, the isolated periotic was placed sitting in stable position on a flat surface with the internal acoustic meatus facing dorsally, to produce a dorsal view. The periotic has a short and wide anterior process, wider posterior process, a dorsoventrally inflated pars cochlearis and in dorsal view, the periotic has a crescentic outline. When in place on the skull, the anterior process is roughly parallel with the anteroposterior axis, whilst the posterior process is posterolaterally oriented.
The anterior process of the periotic is anteroposteriorly short, with a narrow apex (similar to Waipatia maerewhenua and cf. Papahu ZMT-73) but wider at its base (Table 1). It is anteriorly oriented with a strongly concave anterodorsal angle and an oval-shaped anterior bullar facet. In ventral view, the sigmoidal parabullary sulcus (sensu Tanaka and Fordyce 2014) is deep and more elongated on its posterior portion. When articulated (Fig. 4), the parabullary sulcus is medial to the falciform process. Posterior to this is the shallow anteroexternal sulcus, which does not reach the dorsal crest. Between the anteroexternal sulcus and the lateral tuberosity is a small circular fossa (Fig. 5B2: fossa?) that might represent an area of contact of the sigmoid process of the tympanic bulla. The small and shallow anterior bullar facet has an elliptical outline with low but distinct margins. The fovea epitubaria is a rounded and deep depression placed between the mallear fossa and the anterior bullar facet. The mallear fossa is rounded and deep, medial to the rounded lateral tuberosity. The latter, though not markedly inflated, projects outside the outline of the periotic in dorsal view, like W. maerewhenua (Fordyce 1994), Otekaikea (Tanaka and Fordyce 2014, 2015a), Papahu taitapu (Aguirre-Fernández and Fordyce 2014) and Awamokoa tokarahi (Tanaka and Fordyce 2017). When the periotic is articulated, the lateral tuberosity lies medial to the notch on the base of the falciform process of the squamosal.
In dorsal view, the body of the periotic has a vestige of a dorsal crest, that extends anteriorly up to the base of the anterior process; a concave surface (Fig. 5A2: concave surface) is medial to this crest and anterior to the anterior incisure. In Otekaikea and Waipatia maerewhenua the dorsal crest is more conspicuous (Fordyce 1994; Tanaka and Fordyce 2014, 2015a). The anterior incisure (groove for the tensor tympani muscle) is a shallow and narrow sulcus between the anterior process and the pars cochlearis, extending posteriorly on the ventral surface towards the fenestra ovalis like in W. maerewhenua and Otekaikea (Fordyce 1994; Tanaka and Fordyce 2014, 2015a). It also extends dorsally towards the dorsal crest. Posteriorly, there is a small hiatus Fallopii for the exit of the major petrosal nerve. The apex of the anterior process presents a marked groove, of unknown homology or function (Fig. 5A, C, E: groove on anterior process).
The pars cochlearis is dorsoventrally thin, longer anteroposteriorly than mediolaterally, with a rounded anterior margin and a straight posterior margin (Table 1). The internal acoustic meatus is piriform and wider posteriorly, and opens slightly anteriorly in dorsal view (Fig. 5C). It comprises four foramina, which open deeply: (i) the area cribosa media, (ii) the spiral cribiform tract, (iii) the foramen singulare and (iv) the proximal opening of the facial canal. The latter is separated from the elliptical foramen singulare by a short but distinct transverse crest. The spiral cribiform tract is the largest of the four foramina and is separated from the foramen singulare by a thin and high crest (Fig. 5C2: crest), as also observed in Otekaikea (Tanaka and Fordyce 2014, 2015a). The large and subcircular aperture for the cochlear aqueduct (anteroposterior length 1.93 mm, width 2.36 mm) opens dorsomedially on the posterior portion of the pars cochlearis. The small and oval-shaped aperture for the vestibular aqueduct (anteroposterior length 1.37 mm, width 1.76 mm) is widely separated from the former. A shallow median promontorial groove is observed on the medial margin of the pars cochlearis. In posterior view, the fenestra rotunda is small and has a trapezoid-like outline, with a short fissure on its medial edge towards the aperture of the cochlear aqueduct. Ventrally, and medial to the prolonged stapedial muscle fossa, there is a concave surface (Fig. 5F2: fossa? 3) of unknown homology or function. There is no caudal tympanic process.
In ventral view, the round fenestra ovalis has some portion of the stapes preserved. Laterally, the rounded distal opening of the facial canal has a facial sulcus extending posteriorly on the medial margin of the posterior bullar facet. This sulcus is delimited laterally by the facial crest, followed by the parafacial sulcus (sensu Tanaka and Fordyce 2016), a deep groove that widens posteriorly. The fossa incudis is shallow and elliptical. The deep fossa for the stapedial muscle has a rhomboidal outline, extending posteriorly on the dorsomedial surface of the posterior process. The wide epitympanic hiatus has a small and deep fossa (Fig. 5B2: fossa? 2) posteriorly, along the anterior margin of the posterior process. When in situ, this fossa housed the spiny process of the squamosal.
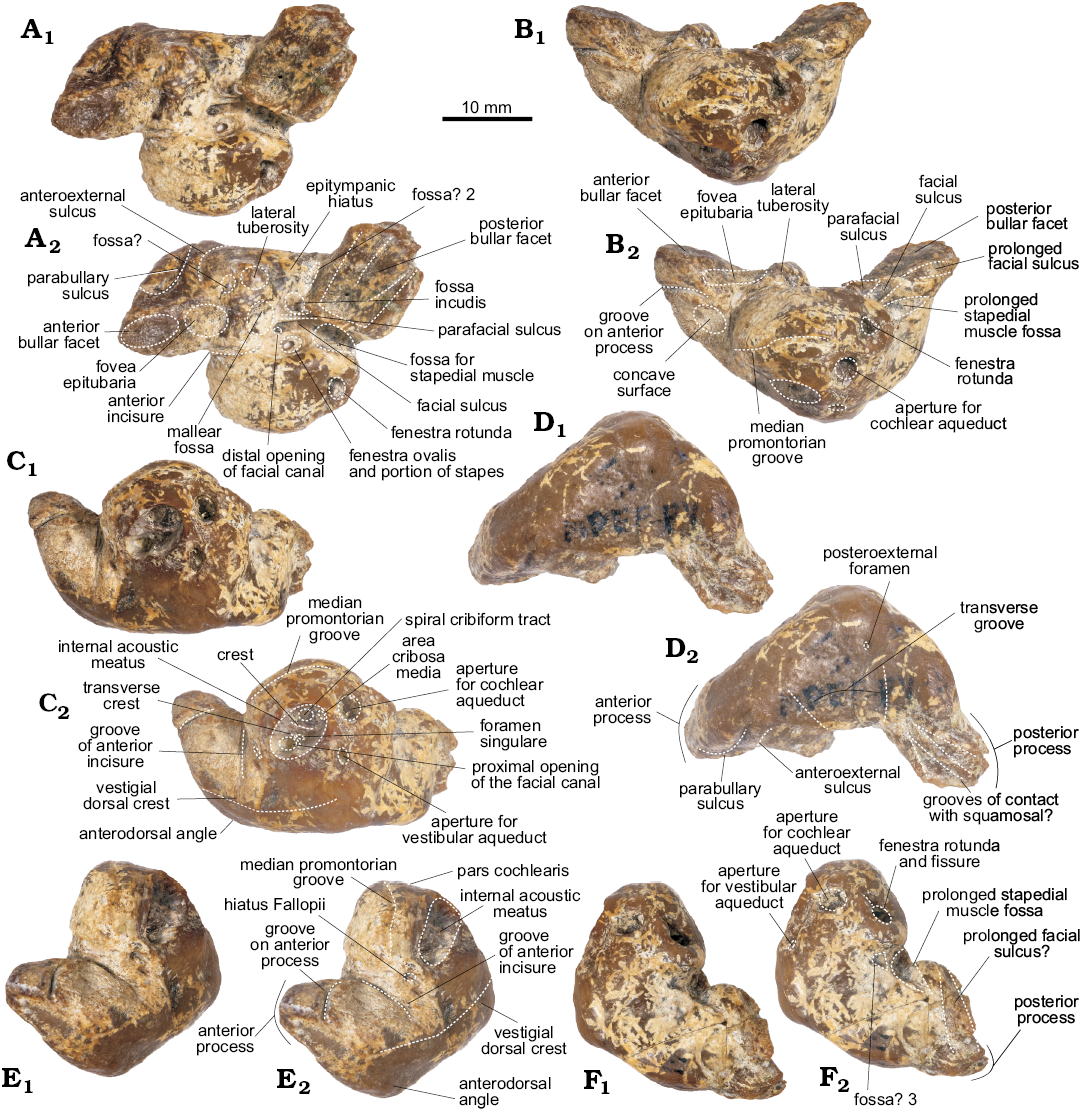
Fig. 5. Left periotic of the holotype of the platanistoid dolphin Aondelphis talen gen. et sp. nov. (MPEF-PV 517) from the early Miocene Gaiman Formation, Bryn Gwyn, Patagonia, Argentina; in ventral (A), medial (B), dorsal (C), lateral (D), anterior (E), and posterior (F) views. Dashed lines indicate specific structures. Photographs (A1–F1), photographs with explanations (A2–F2).
The posterior process has a rectangular outline (Table 1) and it is posterolaterally oriented. In ventral view, the posterior bullar facet has a smooth and wide surface, deeper anteromedially and with a few shallow grooves. On the lateral surface of this process, there are two deep grooves separated by a sharp crest; we infer that this area presumably articulated with the post-tympanic process of the squamosal (see above). Similar structures are present in one specimen of Pomatodelphis cf. inequalis (USNM 13768), referred to Zarhachis cf. flagellator by Muizon (1987: fig. 6, 14d), and in Otekaikea huata (Tanaka and Fordyce 2015a). Anteromedially, there is a minute posteroexternal foramen, more anteriorly located than in Waipatia (Fordyce 1994) and Otekaikea (Tanaka and Fordyce 2014, 2015a). No articular rim (sensu Muizon 1987) could be recognized and the transverse groove is almost indistinct.
Tympanic bulla (Fig. 6): For the purpose of description, the dorsal view is defined as the position of the tympanic bulla when the ventral face is sitting on a flat surface. In general, the tympanic bulla is wider posteriorly and narrows anteriorly in dorsal view, with a heart-shaped outline and a thin involucrum. It is incomplete, with two additional detached fragments.
In dorsal view, the anterior portion of the tympanic tapers slightly anteriorly, which could suggest the presence of an anterolateral convexity and notch but is not possible to ascertain its condition because this area was not completely preserved. The medial profile is straight and flattened in posterior view. The involucrum is nearly straight, narrower anteriorly and dorsoventrally short, with a marked depression approximately 6mm anterior to the base of the inner posterior prominence. The involucrum presents smooth transverse ridges on its dorsal surface (like in Waipatia maerewhenua and Otekaikea marplesi; Fordyce 1994; Tanaka and Fordyce 2014). Medially, a low but distinct crest marks the dorsal limit of the peribullary sinus (Fraser and Purves 1960; Mead and Fordyce 2009). In dorsal view, a small tubercle projects medially from the involucrum, but the expected ridge that would divide the tympanic cavity is absent. Anteriorly, the involucrum gently tapers into the tympanic cavity, which is relatively large.
In posterior view, the tympanic bulla is bilobed, with a deep and medially oriented interprominential notch. The lateral margin of the tympanic bulla is slightly convex. Because the area is not properly preserved, no inferences can be made regarding the size and shape of the elliptical foramen. In ventral view, the inner posterior prominence is narrow mediolaterally and slightly longer dorsoventrally, whilst the outer posterior prominence is wider and projects further posteriorly (Table 1). There is no horizontal ridge between the two prominences. Ventrally, from the interprominential notch, the median furrow extends anteriorly up to the center of the ventral surface of the tympanic bulla (Fig. 6B), with a sigmoid profile as seen in Waipatia maerewhenua (Fordyce 1994) and Platanista gangetica (Anderson 1878). Both the furrow and ventral surface are rugose. The ventral keel is almost indistinct.
In dorsal view, the posterior process is posterolaterally oriented (Table 1) and three articular surfaces may be recognized. Dorsally, the smooth surface for articulation with the posterior process of the periotic is the largest, delimited by two thin crests. Anterolaterally, there is a rugose and deep surface, possibly for articulation with the posterior meatal crest of the squamosal. The most lateral portion of this process has a surface of articulation with the post-tympanic process of the squamosal. This contact is inferred due to erosion of the region.
There are two loose fragments of tympanic bulla (Fig. 6G, H). One fragment contains the region from the sigmoid process to the accessory ossicle (greatest length 27.08 mm; greatest width 9.45 mm). The sigmoid process, though broken, has a squared profile (greatest width 8.42 mm; greatest height 7.11 mm) and thick edges. Just anteriorly, is a short mallear ridge (greatest length 3.45 mm), with the apex broken. The accessory ossicle is anteroposteriorly elongated and small (greatest length 5.98 mm; greatest width 4.43 mm). It has a minute oval-shaped foramen on its dorsal surface, and another one on its anterior surface. A small concave surface anterolateral to the accessory ossicle could correspond to the most-dorsal part of the lateral furrow. The other fragment of tympanic bulla preserved appears to correspond to the outer lip, although no contact surface was found.
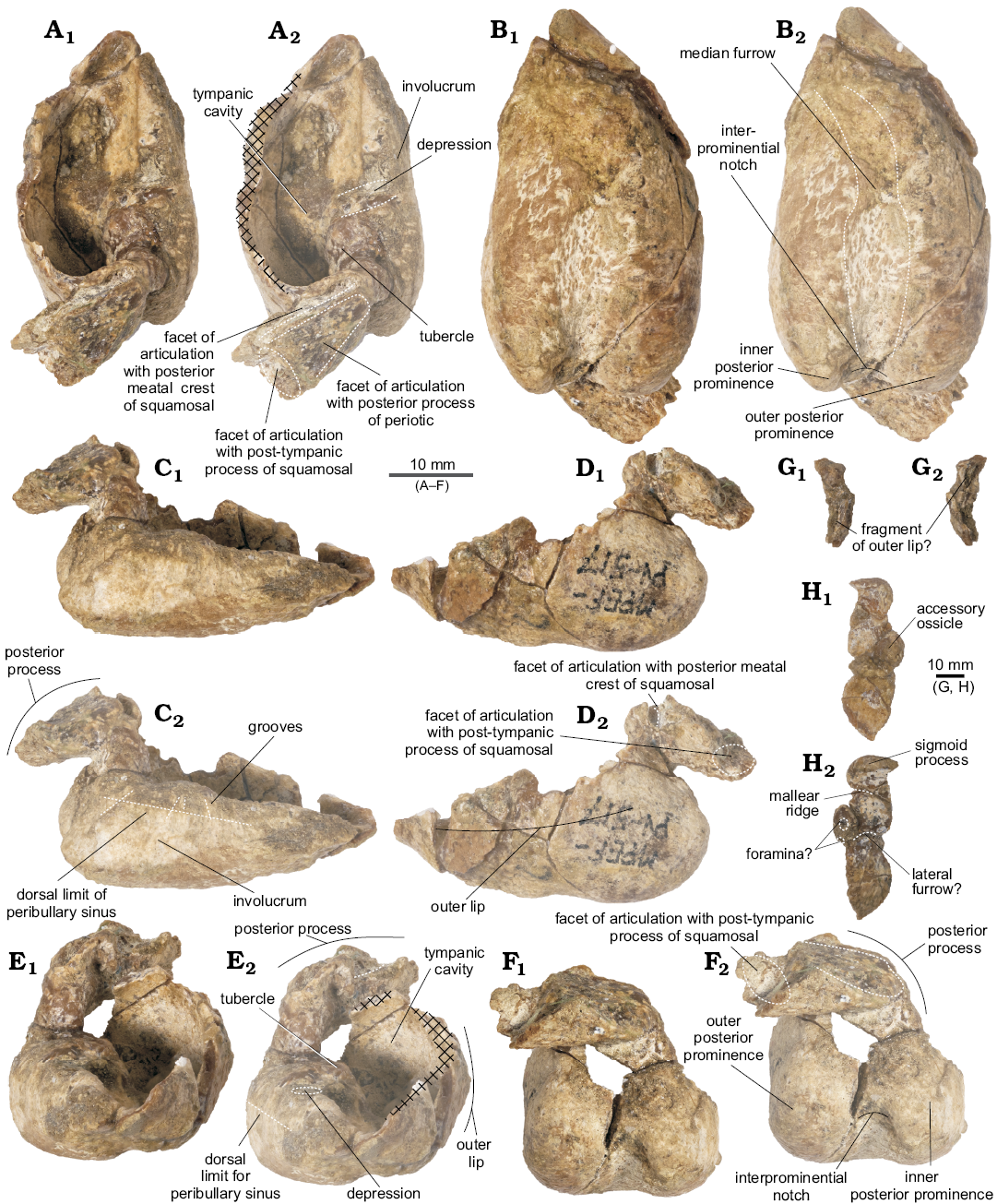
Fig. 6. Left tympanic bulla of the holotype of the platanistoid dolphin Aondelphis talen gen. et sp. nov. (MPEF-PV 517) from the early Miocene Gaiman Formation, Bryn Gwyn, Patagonia, Argentina; in dorsal (A), medial (B), ventral (C), lateral (D), anterior (E), and posterior (F) views. Isolated fragments sigmoid process (G) and a portion of outer lip (H); in posterior (G1, H1) and anterior (G2, H2) views. Dashed lines indicate specific structures. Hatched outlines show broken areas of the specimen. Photographs (A1–F1), photographs with explanations (A2–F2, G, H).
Table 1. Measurements (in mm) of left tympanic
bulla and periotic of Aondelphis talen gen.
et sp. nov., MPEF-PV 517 (after Kasuya 1973).
+ nearly complete; ? uncertainty in the measurement taken.
|
Tympanic bulla |
|
|
Distance from anterior tip to posterior end of outer posterior prominence |
+40 |
|
Distance from anterior tip to posterior end of inner posterior prominence |
+38 |
|
Width across inner and outer posterior prominence |
19.5 |
|
Greatest depth of interprominential notch |
5? |
|
Maximum length of posterior process |
19 |
|
Maximum width |
23 |
|
Maximum width of involucrum |
13 |
|
Periotic |
|
|
Standard length of periotic, from tip of anterior process to posterior end of posterior process, measured on a straight line parallel with cerebral border |
32 |
|
Width of periotic across cochlear portion and superior process, at the level of upper tympanic aperture |
19 |
|
Least distance between the margins of fundus of internal auditory meatus and of aperture of ductus endolymphaticus |
2 |
|
Least distance between the margins of fundus of internal auditory meatus and of aperture of aqueduct cochleae |
1.7 |
|
Length of articular facet of the posterior process of the periotic for the posterior process of tympanic bulla |
13 |
|
Antero-posterior diameter of cochlear portion |
14 |
|
Dorsoventral depth at fovea epitubiaria |
4 |
|
Anteroposterior diameter of facial canal |
7 |
|
Maximum width of anterior process at base |
11 |
|
Transverse width of pars cochlearis from internal edge to fenestra ovalis |
11 |
|
Length of posterior process |
14 |
|
Length of anterior process from anterior apex to level of posterior of mallear fossa |
13 |
|
Maximum width of posterior process |
12 |
|
Anteroposterior length of aperture for cochlear aqueduct |
2 |
|
Width of aperture for cochlear aqueduct |
2 |
|
Anteroposterior length of aperture for vestibular aqueduct |
1 |
|
Width of aperture for vestibular aqueduct |
2 |
Phylogenetic analysis
We have conducted two different parsimony analyses with equal weights and implied weights. Results of both analyses are reported and discussed below.
The analysis under equal weight resulted in 164 MPTs of 1834 steps (CI = 0.24, RI = 0.64; Fig. 7). The strict consensus showed Aondelphis talen gen. et sp. nov. forming a clade with cf. Papahu ZMT-73 (Tanaka and Fordyce 2016), supported by six unambiguous synapomorphies: deep emargination of posterior edge of zygomatic process by neck muscle fossa (character 114); slit-like external auditory meatus (character 157); abruptly ventrally deflected anterior process of periotic (character 173); pars cochlearis of the periotic dorsoventrally thin (character 193); posterior edge of inner posterior prominence of the tympanic bulla distinctly anterior to the posterior edge of outer posterior prominence (character 219); dorsal margin of involucrum of tympanic bulla excavated at mid-length (character 220). Squalodon calvertensis was recovered as the sister taxon to a clade including Aondelphis talen + cf. Papahu ZMT-73 based on one unambiguous synapomorphy: poorly defined ventromedial keel of the tympanic bulla (character 222; except cf. Papahu ZMT-73 which presents a different state). Additional ambiguous synapomorphies supporting this clade were: small contact area between the anterior process of periotic and squamosal (character 178); tubular fundus of the internal acoustic meatus of periotic (character 189); and narrow and long tympanic bulla (character 208). Even though none of these nodes have high support (Fig. 7), their synapomorphies (see above) are related to the earbones, which are highly phylogenetically informative bones (Fraser and Purves 1960; Kasuya 1973) and thus, we are confident in our interpretations. Platanistoidea sensu stricto (Awamokoa + Otekaikea + Waipatiidae + “Squalodelphinidae” + Platanistidae) appeared as the sister lineage to other crown Odontoceti, based on the following six ambiguous synapomorphies: wide premaxillae at the base of rostrum (character 53, except in Zarhachis flagellator and unknown in Waipatia hectori, Awamokoa tokarahi, Otekaikea huata, OU 22670, Phocageneus venustus, and Platanista gangetica); alisphenoid-squamosal suture along groove for mandibular nerve or just posterior to it (character 151, unknown in Waipatia, Awamokoa tokarahi, OU 22670, “Squalodelphinidae”, Pomatodelphis inaequalis and Z. flagellator); sigmoidal profile of periotic (character 172, unknown in W. hectori, OU 22670, and Squalodelphis fabianii); anteroposterior ridge developed on the anterior process and body of periotic (character 174, unknown in W. hectori, OU 22670, and S. fabianii); sub-rectangular aperture for cochlear aqueduct (character 194, except P. venustus and Notocetus vanbenedeni and unknown in W. hectori, OU 22670, S. fabianii, P. inaequalis, and Z. flagellator); and small articular rim (character 196, except Platanistidae and unknown in W. hectori, OU 22670, and S. fabianii). The internal relationships of Platanistoidea sensu stricto were resolved, with two major clades: one including Waipatia + Awamokoa + Otekaikea, and another with OU 22670 + “Squalodelphinidae” + Platanistidae. Some relationships within crown Odontoceti were not fully resolved in the present analysis (see SOM 3), and we believe this could be related to the different settings we used for the phylogenetic analysis (no molecular constraint with respect to Tanaka and Fordyce 2016) and also might be related to the matrix modifications conducted here (see SOM 2 and 8 for more details). A modern and thorough revision of the character and codings of some delphinoid taxa is needed but beyond the scope of the present contribution.
For the analysis under implied weights, we only discuss here the MPTs of K = 3, K = 4, and K = 9 as their topology is different between themselves and from the strict consensus of the analysis under equal weights.
With K = 3, we recovered three MPTs (fit = 136.9, 1880 steps; Fig. 7; SOM 4). In the strict consensus, Aondelphis talen gen. et sp. nov. formed a basal clade with cf. Papahu ZMT-73 and sister to Squalodon calvertensis + Platanistoidea sensu stricto. However, relationships within “Squalodelphinidae” were not resolved in this analysis. Aondelphis talen + cf. Papahu ZMT-73 clade was supported by four of the six unambiguous synapomorphies under equal weights (characters 114, 173, 193, and 219). In turn, the clade comprising A. talen + cf. Papahu ZMT-73 + S. calvertensis + Platanistoidea sensu stricto was supported by three ambiguous synapomorphies: enlarged tympanosquamosal recess (character 148, except Platanistidae and unknown in Awamokoa tokarahi, OU 22670 and Phocageneus venustus); weakly curved parabullary sulcus of the periotic (character 176, except in Otekaikea, Waipatia maerewhenua, and Zarhachis flagellator, and unknown in Waipatia hectori, Platanista gangetica, Pomatodelphis inaequalis, OU 22670, P. venustus, and Squalodelphis fabianii); and poorly defined ventromedial keel of the tympanic bulla (character 222, except in cf. Papahu ZMT-73, Platanistidae and “Squalodelphinidae”, and unknown in Otekaikea huata, W. hectori, and OU 22670). Physeteroidea + Ziphiidae appeared as the earliest diverging clade of crown Odontoceti, sister to Platanistoidea + the remaining odontocetes.
With K = 4, the analysis recovered one tree (fit = 122.87, 1868 steps; Fig. 7; SOM 5) with ZMT-73 as the earliest diverging Platanistoidea sensu lato followed by Aondelphis talen gen. et sp. nov. as the sister taxon to the Platanistoidea sensu stricto clade. The position of Aondelphis talen was supported only by one synapomorphy: developed anteroposterior ridge on anterior process and body of periotic (character 174). Unlike the analyses above, Squalodon calvertensis was placed as the most basal Platanistoidea sensu lato, sister to the clade including the remaining platanistoids (cf. Papahu ZMT-73 + Aondelphis talen + Platanistoidea sensu stricto). The latter clade was supported by four ambiguous synapomorphies: deep emargination of the posterior edge of zygomatic process by the neck muscle fossa (character 114, except in Otekaikea huata, Awamokoa tokarahi, Waipatia, OU 22670, Squalodelphis fabianii, Notocetus vanbenedeni, and Platanistidae and unknown in Phocageneus venustus); shallow posterior portion of periotic fossa (character 155, except in N. vanbendeni and Platanistidae, and unknown in Awamokoa tokarahi, OU 22670, and P. venustus); abruptly ventrally deflected anterior process of periotic (character 173, except in N. vanbenedeni and unknown in Waipatia hectori, OU 22670, S. fabianii, P. venustus, and Platanistidae); and dorsoventrally thin pars cochlearis of the periotic (character 193, except in Awamokoa tokarahi and Waipatia maerewhenua and unknown in Waipatia hectori, OU 22670, S. fabianii, Pomatodelphis inaequalis, and Zarhachis flagellator).
With K = 9, weighting more against homoplasy in the matrix, the implied weights analysis recovered one tree (fit = 82.90, 1841 steps; Fig. 7). The phylogenetic position of Aondelphis talen gen. et sp. nov., cf. Papahu ZMT-73, Squalodon calvertensis and Platanistoidea sensu stricto was the same as with equal weights (see SOM 6). The synapomorphies for the clades of interest were the same as the analysis under equal weights.
Even though analyses under implied weights seem to deal better with homoplasy for morphological data sets (Goloboff et al. 2008, 2017), our discussion was based on the equal weights phylogenetic hypothesis. This is because the implied weights method has been criticized by having some inconsistencies and lack of accuracy (Congreve and Lamsdell 2016; Puttick et al. 2017)

Fig. 7. Strict consensus trees of the phylogenetic analysis under equal weights (A), and implied weights with K = 3 (B) and K = 4 (C) obtained in the present study. Numbers above branches indicate Bremer support, whilst number below branches indicate jackknife support using p = 0.30 and 1000 pseudoreplicates. Delphinida sensu Geisler et al. (2011) were collapsed for ease of illustration.
Discussion
Phylogenetic relationships.—The superfamily Platanistoidea sensu Muizon (1987) has always been a problematic group from a phylogenetic point of view, as the majority of its members are extinct and there is only one extant representative, Platanista gangetica. Recent morphological and phylogenetic studies (e.g., Barnes 2006; Barnes et al. 2010; Bianucci et al. 2014; Lambert et al. 2014; Tanaka and Fordyce 2014, 2015a, b, 2016, 2017; Boersma and Pyenson 2016; Kimura and Barnes 2016; Boersma et al. 2017) have improved our knowledge of the group and understanding of their evolutionary history. In Patagonia, the fossil record of platanistoids is well represented, but some taxa are poorly known and require up-to-date revisions (i.e., Notocetus and Phoberodon). In this paper, we focus on reporting a new genus and species of an early Miocene platanistoid from Patagonia, which expands our knowledge about the diversity of this group during a critical time in its evolution.
Our phylogenetic analyses consistently showed Platanistoidea as a monophyletic clade (Fig. 7) but with some differences in relationships among species compared to previous analysis. In accordance to Tanaka and Fordyce (2014, 2015a, b, 2017), but in contrast to Lambert et al. (2014) and Boersma and Pyenson (2016), we recovered a Platanistoidea clade sensu stricto including Waipatiidae + Awamokoa tokarahi + Otekaikea + OU 22670 + Squalodelphinidae + Platanistidae. Also, our results showed Squalodontidae as a sister lineage to Platanistoidea sensu stricto, different from previous analyses by Barnes (2006), Barnes et al. (2010), Tanaka and Fordyce (2016), and Lambert et al. (2017). Our results also corroborated two of the three synapomorphies for Platanistoidea obtained by Tanaka and Fordyce (2014) under equal weights, with the addition of four new synapomorphies, and only one of the synapomorphies reported by Tanaka and Fordyce (2015b) under implied weights. Platanistoidea according to Boersma and Pyenson (2016) was supported by seven synapomorphies; three of which were also recovered in the present analysis (character 154, 171, and 172). Similar to previous studies (e.g., Tanaka and Fordyce 2014, 2015a, b, 2016, 2017; Boersma and Pyenson 2016; Lambert et al. 2017), we also recovered a paraphyletic “Squalodelphinidae” group (sensu Muizon 1987), with the earliest diverging Squalodelphis fabianii sister to the clade formed by Notocetus vanbenedeni + Phocageneus venustus. However, these results were in contrast with the analyses by Lambert et al. (2014) and Boersma et al. (2017), which showed Squalodelphinidae as a monophyletic clade, yet with unresolved relationships. Currently, N. vanbenedeni is the focus of a comprehensive revision by one of the authors (MV), which might help to resolve the relationships among this family. In contrast to Boersma et al. (2017), but in agreement with Tanaka and Fordyce (2014, 2015a, b, 2016, 2017), we recovered a monophyletic clade comprising Waipatia + Otekaikea. The species in the family Platanistidae were the same than in previous analyses (Barnes 2006; Barnes et al. 2010; Bianucci et al. 2014; Lambert et al. 2014, 2017; Tanaka and Fordyce 2014, 2015a, b, 2016, 2017; Boersma and Pyenson 2016; Kimura and Barnes 2016; Boersma et al. 2017). The differences observed might be related to the different datasets used in the previous analyses, the incorporation of new taxa, as well as matrix modifications we have introduced. Descriptions of new and historical taxa and further phylogenetic analyses using the same morphological matrix will help improve the characters used. This may help understand the relationships among the members of the superfamily. For example, Boersma and Pyenson (2016) and Boersma et al. (2017) recovered Platanistoidea including the family Allodelphinidae, which was not included in our original matrix. In addition, the homology of some characters (see below) needs a careful revision and consideration in future phylogenetic analyses of Platanistoidea.
Both phylogenetic analyses consistently recovered Aondelphis talen gen. et sp. nov. and cf. Papahu ZMT-73 in a basal position within Platanistoidea sensu lato (except in implied weights under K = 3) either forming a clade or as successive branches. Both species share a combination of synapomorphic characters, such as a deep emargination of the neck muscle fossa, slit-like external acoustic meatus, anteroposteriorly wide squared-off postglenoid process, dorsoventrally thin pars cochlearis, abruptly ventrally deflected anterior process of periotic and a shorter inner posterior prominence than outer posterior prominence of the tympanic bulla. Aondelphis talen also exhibits some derived characters in the morphology of the earbones, such as a small contact of the anterior process of periotic with squamosal, developed anteroposterior ridge on anterior process and body of periotic, tubular fundus and circular outline of internal acoustic meatus of periotic and poorly defined ventromedial keel of the tympanic bulla. Aondelphis talen + cf. Papahu ZMT-73 also exhibit a combination of some plesiomorphic characters, which also supported their basal position within Platanistoidea, such as: the absence of a lateral groove affecting profile of the periotic and a tear-drop fenestra rotunda. We observed unambiguous synapomorphies in the ear bones of A. talen, structures that have a strong phylogenetic signal in cetaceans (e.g., Kasuya 1973; Ekdale et al. 2011; Tsai and Fordyce 2016). In addition, A. talen was consistently observed in a basal position among platanistoids in all the different analyses performed in this work.
Aondelphis talen gen. et sp. nov. is markedly different to the other well-known platanistoid from the early Miocene of Patagonia, Notocetus vanbenedeni. These differences include: an abruptly deflected anterior process of the periotic; dorsally convex dorsal surface of periotic; presence of a posterodorsal edge of the stapedial muscle fossa; absence of an articular rim; narrow angle between the posterior process of periotic and long axis of pars cochlearis; long posterior process of periotic; median furrow reaching beyond the lateral furrow of the bulla; shorter inner posterior prominence of bulla; excavated dorsal margin of involucrum; and absence of a ridge inside of the bulla. Unfortunately, no ear bones of Phoberodon are available for comparison.
Aondelphis talen adds to the diversity of platanistoids in Patagonia during the early Miocene, demonstrating the coexistence of at least two different morphotypes: one characterized by a more plesiomorphic morphology (i.e., Aondelphis), and another one with a more derived Platanista-like morphology (i.e., Notocetus). Similar patterns were also observed in platanistoids from Peru (e.g., Lambert et al. 2014, 2017). These suggest that during the early Miocene platanistoids were morphologically diverse and may have occupied different ecological niches. Finally, a close relationship between A. talen and another early Miocene platanistoid from New Zealand (ZMT-73) implies a rapid diversification and spread distribution of the group in the Southern Hemisphere during a period of only 10 Ma.
Parabullary sulcus.—The parabullary sulcus was defined recently by Tanaka and Fordyce (2014) as a sulcus on the anterior process of the periotic, associated with the anteroexternal sulcus. This sulcus has been proposed by Tanaka and Fordyce (2014) as a characteristic of platanistoids, although not shown as a synapomorphy in their phylogenetic analysis. In contrast, Boersma and Pyenson (2016) recovered the parabullary sulcus as one of the ambiguous synapomorphies in their definition of Platanistoidea. Here, the presence of a parabullary sulcus (character 176) was recovered as an ambiguous synapomorphy of Platanistoidea sensu lato under implied weights analysis (K = 3). As mentioned by Tanaka and Fordyce (2017), other taxa such as Papahu taitapu (Aguirre-Fernández and Fordyce 2014) and some eurhinodelphinids (e.g., Lambert 2005) also present this feature. When looking at the evolutionary trend of this character, the parabullary sulcus appeared no earlier than the Platanistoidea sensu lato node, reversed to an absent state in Platanista gangetica and also in the Delphinida clade. Since some authors have proposed Eurhinodelphinidae as closely related to the Squalodontidae (e.g., Barnes et al. 1985) or even Platanistoidea (Muizon 1991), a thorough revision of eurhinodelphinids should test if the parabullary sulcus is a potential synapomorphy of Platanistoidea or a convergent feature among early-diverging odontocetes.
Suprameatal pit and fossa for the articular rim.—Muizon (1987: 5) observed a fossa in the squamosal of Notocetus that encloses the hook-like articular process of the periotic, located “at the base of the post-meatal process (= post-tympanic process)”. This author did not formally name this structure (see below), but also described a structure that was named as subcircular fossa, located “dorsal to the spiny process and above the periotic” and stated that “the function of this structure has not been elucidated, although it could represent a simple extension of the peribullary sinus which partially surrounds the periotic and the tympanic”.
On the other hand, Geisler and Sanders (2003: 50) mentioned that: “The feature herein termed the suprameatal pit of the squamosal was briefly mentioned by Muizon (1987) as the fossa that receives the hook-like articular process of the petrosal in Platanista and Zarhachis. The pit probably houses an expansion of the peribullary sinus because (1) it is much larger than the hook-like articular process, and (2) some taxa that do not have the articular process do have a suprameatal pit (e.g., Xenorophus). The suprameatal pit occurs immediately dorsolateral to the spiny process of the squamosal and ventrolateral to the subcircular fossa of the squamosal”.
In this sense, Geisler and Sanders (2003) definition of the suprameatal pit, its location and function partially overlaps the anatomical location and function of the two fossae that Muizon (1987) described. Also, the subcircular fossa (= suprameatal pit) lies in a different position, being anatomically impossible to house the articular process of the periotic, as the latter is located on the dorsal surface of the posterior process of the periotic, close to the post-tympanic process of the squamosal. The spiny process, on the other hand, is located anterior to the periotic fossa, posterior to the falciform process and medial to the external acoustic meatus. In conclusion, in the present study, the subcircular fossa was defined as the fossa located dorsolateral to the spiny process of the squamosal and medial to the external auditory meatus, thus the term suprameatal pit should be avoided.
In our study, the subcircular fossa (character 153) appeared to have evolved independently in several clades, such as cf. Papahu ZMT-73, Platanistoidea sensu stricto, Papahu taitapu, Physeter catodon, some ziphiids, some inioids; it is also variably developed in some species of Delphinoidea (see matrix in SOM 8). These results are in contrast with the suggestion of Muizon (1994) that the presence of a subcircular fossa is a synapomorphy of Squalodelphinidae + Platanistidae. Muizon (1994) also mentioned the presence of a homologous fossa in Squalodontidae. Prior to resolving the distribution of this character, it is imperative to resolve the problems of homology of the subcircular fossa, which are still a matter of debate. A possible homology between the foramen spinosum and the subcircular fossa has been proposed by Fordyce (1994), as this feature is probably related to the circulatory system (Muizon 1994). On the other hand, the fossa present in the squamosal of Recent Delphinidae has been interpreted as not homologous to the subcircular fossa, but rather related to the development of an enlarged cranial hiatus in this clade (Muizon 1994). Future comprehensive anatomical studies in extant and extinct species are needed to test these homologies, hence the distribution of this character is still debatable.
As mentioned before, Muizon (1987: 5) described a fossa that receives the hook-like articular process of the periotic for Notocetus (not figured). In this study, this fossa has been named “fossa for the articular rim” (new term) and has been incorporated in our phylogenetic analysis as a new character (character 287). When the periotic is in situ in Platanista gangetica (USNM 23456; SOM 7), the articular rim of the periotic is actually contacting a concave surface on the medial side of the squamosal just posterodorsal to the external acoustic meatus (previously described by Fraser and Purves 1960: 43). This feature is also present in other platanistoid species, such as Awamokoa tokarahi (Tanaka and Fordyce 2016), Otekaikea (Tanaka and Fordyce 2014, 2015a), Zarhachis flagellator (USNM 13768), Pomatodelphis inaequalis (USNM 187414) and Squalodon calvertensis (USNM 328343). The presence of a bony contact between the periotic and squamosal was previously mentioned by Muizon (1994) as a characteristic exclusive of platanistoids (with the possible exception of some squalodontids), with the derived state of a tight connection in Platanista gangetica. Even though the evolutionary trend among Odontoceti is the loss of bony contacts between ear bones and the skull (e.g., Heyning 1989, 1997; Fraser and Purves 1960), platanistoids appear to have retained this rare condition.
The echolocation abilities of Platanista gangetica are complex and very different from other odontocetes. It presents a unique extension of the pterygoid sinus on the maxillary crests, a different pattern of sound emission (Anderson 1878; Pilleri 1990) and a narrow-band pattern for emitted frequencies. These frequencies have only one peak of best-frequency, in contrast to the bimodal pattern of the modern marine dolphins (e.g., Morisaka and Connor 2007). In addition, Gutstein et al. (2014) identified the particular morphology of the periotic of Platanista (e.g., large size, oval pars cochlearis and a rounded internal acoustic meatus), as well as the cochlear shape (type I) related with the riverine environment and the narrow-band frequency pattern. The retention of a tight contact between the periotic and the skull, and the particular morphology of the periotic and cochlea described here, might be related to the development of this particular system of a unique echolocation system, as observed in Platanista. More studies on the anatomy and evolutionary history of echolocation in odontocetes will help elucidate their acoustic abilities.
Conclusions
A new Platanistoid species Aondelphis talen gen. et sp. nov. from the early Miocene of Patagonia is presented here, following a thorough anatomical description. The phylogenetic analysis recovered A. talen as closely related to the unnamed specimen cf. Papahu ZMT-73 from the early Miocene of New Zealand, in a basal clade sister to Platanistoidea sensu stricto. Aondelphis talen exhibits some plesiomorphic characters that support its basal position within Platanistoidea sensu lato, but also some derived characters, especially in the morphology of the ear bones. A new character (fossa for the articular rim) was proposed in the phylogenetic analysis and some modifications of some other key characters were also discussed. This new taxon increases the diversity of this superfamily in Patagonia, and suggests the coexistence of at least two different morphotypes that might have occupied different ecological niches. The close relationship of platanistoids from Patagonia and New Zealand indicates diversification and spread distribution of the group during the early Miocene in the Southern Hemisphere. Future research will improve our knowledge on the morphology of other important and poorly known platanistoids from Patagonia (i.e., Notocetus and Phoberodon). This will shed light on the evolution of platanistoids and help us understand Miocene cetacean assemblages in Patagonia.
Acknowledgements
We thank to Santiago Bessone (Instituto Patagónico de Geología y Palentología-CENPAT, Puerto Madryn, Argentina) for specimen preparation. To the following curators for access to the collections under their care: Eduardo Ruigomez (Museo Paleontológico “Egidio Feruglio”, Trelew, Argentina), Pablo Teta and Sergio Lucero (both MACN), Marcelo Reguero (MLP), Nestor García and Enrique Crespo (both CNPMAMM), Nicholas Pyenson (USNM), Nancy B. Symmons (AMNH) and Anton van Helden (NMNZ). We also would like to thank Yoshihiro Tanaka (Numata Fossil Museum, Numata, Japan), R. Ewan Fordyce (OU) and Damián Perez (MACN) for useful discussions regarding the matrix and phylogenetic analysis. We thank Oscar Lehmann (Universidad de Buenos Aires, Buenos Aires, Argentina) for lending the script used for analysis of multiple implied weights. We thank Carolina Loch (University of Otago, Dunedin, New Zealand) for English revision. This contribution used TNT version 1.5, a program made freely available thanks to a subsidy by the Willi Hennig Society. R.E. Fordyce and Daniela del Castillo (Universidad de Buenos Aires, Buenos Aires, Argentina) are thanked for access to their photographs of cetacean specimens revised. To Verónica Domínguez (Instituto Patagónico de Ciencias Sociales y Humanas-CENPAT, Puerto Madryn, Argentina) and Ana Fernández Garay (Universidad de Buenos Aires) for their help with the Tehuelche words used to nominate this new species. We also want to thank the editor Olivier Lambert (Royal Belgian Institute of Natural Sciences, Brussels, Belgium) and two reviewers, Travis Park (National History Museum, London, UK) and Rachel Racicot (Natural History Museum of Los Angeles County, Los Angeles, USA) for their comments, which greatly improved this manuscript. We would like to thank the following organizations for financial support: Cetacean Society International to MV and MRB; Learner-Gray Grant from the AMNH to MRB; Sociedad Argentina para el Estudio de Mamíferos to MV; Smithsonian Institution (Remington Kellogg Fund) to MRB; Society for Marine Mammalogy to MV; Agencia Nacional de Promoción Científica y Tecnológica (grant number PICT 0792) to MB and JC. MV would like to thank Felipe Busker (Instituto Patagónico de Geología y Paleontología-CENPAT, Puerto Madryn, Argentina) for his continuous support.
References
Anderson, J. 1878. Anatomical and Zoological Researches: Comprising An Account of the Zoological Results of the Two Expeditions to Western Yunnan in 1868 and 1875 and a Monograph of the Two Cetacean Genera, Platanista and Orcella. Vol. 1 and 2. 1042 pp. Quaritch, Londres.
Aguirre-Fernández, G. and Fordyce, R.E. 2014. Papahu taitapu, gen. et sp. nov., an Early Miocene Stem Odontocete (Cetacea) from New Zealand. Journal of Vertebrate Paleontology 34: 195–210. Crossref
Barnes, L.G. 2006. A phylogenetic analysis of the superfamily Platanistoidea. Beiträge zur Paläontologie 30: 25–42.
Barnes, L.G. and Reynolds, R.E. 2009. A new species of Early Miocene allodelphinid dolphin (Cetacea, Odontoceti, Platanistoid) from Cajon Pass, Southern California, USA. Museum of Northern Arizona Bulletin 65: 483–507.
Barnes, L.G., Domning, D.P., and Ray, C.E. 1985. Status of studies on fossil marine mammals. Marine Mammal Science 1: 15–53.Crossref
Barnes, L.G., Kimura, T., and Godfrey, S.J. 2010. The evolutionary history and phylogenetic relationships of the Superfamily Platanistoidea. In: M. Ruiz-García and J.M. Shostell (eds.), Biology, Evolution and Conservation of River Dolphins within South America and Asia, 445–488. Nova Science Publishers, New York.
Bercovici, A., Hadley, A., and Villanueva-Amadoz, U. 2009. Improving depth of field resolution for palynological photomicrography. Palaeontologia Electronica 12: 1–12.
Bianucci, G., Urbina, M., and Lambert, O. 2014. A new record of Notocetus vanbenedeni (Squalodelphinidae, Odontoceti, Cetacea) from the Early Miocene of Peru. Comptes Rendus Palevol 14: 5–13. Crossref
Boersma, A.T. and Pyenson, N.D. 2016. Arktocara yakataga, a new fossil odontocete (Mammalia, Cetacea) from the Oligocene of Alaska and the antiquity of Platanistoidea. PeerJ 4: e2321. Crossref
Boersma, A.T., McCurry, M.R., and Pyenson, N.D. 2017. A new fossil dolphin Dilophodelphis fordycei provides insight into the evolution of supraorbital crests in Platanistoidea (Mammalia, Cetacea). Royal Society Open Science 4: 170022. Crossref
Cione, A.L. 1978. Aportes paleoictiológicos al conocimiento de la evolución de las paleotemperaturas en el área austral de América del Sur durante el Cenozoico. Aspectos zoogeográficos y ecológicos conexos. Ameghiniana 15: 183–208. Crossref
Cione, A.L., Cozzuol, M.A., Dozo, M.T., and Acosta Hospitaleche, C. 2011. Marine vertebrate assemblages in the southwest Atlantic during the Miocene. Biological Journal of the Linnean Society 103: 423–440. Crossref
Coddington, J.A. and Scharff, N. 1994. Problems with zero-length branches. Cladistics 10: 415–423. Crossref
Congreve, C.R. and Lamsdell, J.C. 2016. Implied weighting and its utility in palaeontological datasets: A study using modelled phylogenetic matrices. Palaeontology 59: 447–462. Crossref
Cozzuol, M.A. 1996. The record of aquatic mammals in southern South America. Munchner Geowissenschaftliche Abhandlungen 30: 321–342.
Cuitiño, J.I., Dozo, M.T, del Río, C.J., Buono, M.R., Palazzesi, L., Fuentes, S., and Scasso, R.A. 2017. Miocene marine transgressions: Paleoenvironments and paleobiodiversity. In: P. Bouza and A. Bilmes (eds.), Springer Earth System Sciences, Late Cenozoic of Península Valdés, Patagonia, Argentina, 47–84. Springer International Publishing AG.
Cuitiño, J.I., Pimentel, M.M., Santos, R.V., and Scasso, R.A. 2012. High resolution isotopic ages for the early Miocene “Patagoniense” transgression in Southwest Patagonia: Stratigraphic implications. Journal of South American Earth Sciences 38: 110–122. Crossref
Cuitiño, J.I., Scasso, R.A., Ventura Santos, R., and Mancini, L.-H. 2015a. Sr ages for the Chenque Formation in the Comodoro Rivadavia region (Golfo San Jorge Basin, Argentina): Stratigraphic implications. Latin American Journal of Sedimentology and Basin Analysis 22: 3–12.
Cuitiño, J.I., Ventura Santos, R., Alonso Muruaga, P.J., and Scasso, R.A. 2015b. Sr-stratigraphy and sedimentary evolution of early Miocene marine foreland deposits in the northern Austral (Magallanes) Basin, Argentina. Andean Geology 42: 364–385.
Dal Piaz, G. 1917. Gli odontoceti del Miocene bellunese Parte terza. Premiata Societá Cooperativa Tipográfica 3: 34.
Dunn, R.E., Madden, R.H., Kohn, M.J., Schmitz, M.D., Strömberg, C.A.E., Carlini, A.A., Re, G.H., and Crowley, J. 2013. A new chronology for middle Eocene–early Miocene South American land mammal ages. Geological Society of America Bulletin 125: 539–555. Crossref
Ekdale, E.G., Berta, A., and Deméré, T.A. 2011. The comparative osteology of the petrotympanic complex (ear region) of extant baleen whales (Cetacea: Mysticeti). PloS One 6: e21311. Crossref
Farris, J.S., Albert, V.A., Källersjo, M., Lipscomb, D., and Kluge, A.G. 1996. Parsimony jackknifing outperforms neighbor-joining. Cladistics 12: 99–124. Crossref
Flynn, J.J. and Swisher, C.C. 1995 Cenozoic South American land mammal ages: Correlation to global geochronologies. SEPM Society for Sedimentary Geology 54: 317–333. Crossref
Fordyce, R.E. 1981. Systematics of the Odontocete whale Agorophius pygmaeus and the Family Agorophidae (Mammalia: Cetacea). Journal of Paleontology 55: 1028–1045.
Fordyce, R.E. 1994. Waipatia maerewhenua, new genus and new species (Waipatiidae, new family), an archaic Late Oligocene dolphin (Cetacea: Odontoceti: Platanistoidea) from New Zealand. Proceedings of the San Diego Society of Natural History 29: 147–176. Crossref
Fordyce, R.E. and Muizon, C. de 2001. Evolutionary history of cetaceans: a review. In: J.-M. Mazin and V. de Buffrénil (eds.), Secondary Adaptation of Tetrapods to Life in Water, 169–233. Verlag Dr. Friederich Pfeil, München.
Fraser, F.C. and Purves, P.E. 1960. Hearing in cetaceans. Evolution of the accessory air sacs and the structure and function of the outer and middle ear in recent cetaceans. Bulletin of the British Museum of Natural History 7: 1–140.
Geisler, J.H. and Sanders, A.E. 2003. Morphological evidence for the phylogeny of Cetacea. Journal of Mammalian Evolution 10: 23–129. Crossref
Geisler, J.H., McGowen, M.R., Yang, G., and Gatesy, J. 2011. A supermatrix analysis of genomic, morphological, and paleontological data from crown Cetacea. BMC Evolutionary Biology 11: 1–112.
Geisler, J.H., Sanders, A.E., and Luo, Z.-X. 2005. A new protocetid whale (Cetacea: Archaeoceti) from the Late Middle Eocene of South Carolina. American Museum Novitates 3480: 1–65.
Goloboff, P.A. and
Catalano, S.A. 2016. TNT version 1.5, including a full implementation
of phylogenetic morphometrics. Cladistics
32: 221–238. Crossref
Goloboff, P.A., Carpenter, J.M., Arias, J.S., Miranda, D.R., and Miranda-Esquivel, D.R. 2008. Weighting against homoplasy improves phylogenetic analysis of morphological data sets. Cladistics 24: 758–773. Crossref
Goloboff, P.A., Farris, J.S., Källersjö, M., Oxelman, B., and Szumik, C.A. 2003. Improvements to resampling measures of group support. Cladistics 19: 324–332. Crossref
Goloboff, P.A., Torres, A., and Arias, J.S. 2017. Weighted parsimony outperforms other methods of phylogenetic inference under models appropriate for morphology. Cladistics [published online, doi: 10.1111/cla.12205]. Crossref
Gutstein, C.S., Figueroa-Bravo, C.P., Pyenson, N.D., Yury-Yañez, R.E., Cozzuol, M.A., and Canals, M. 2014. High frequency echolocation, ear morphology, and the marine–freshwater transition: A comparative study of extant and extinct toothed whales. Palaeogeography, Palaeoclimatology, Palaeoecology 400: 62–74. Crossref
Haller, M.J. and Mendía, J.E. 1980. Las sedimentitas del ciclo Patagoniano en el litoral atlántico norpatagónico. In: J.E. Mendía and A. Bayarsky (eds.), Estratigrafía del Terciario en el Valle inferior del Río Chubut. Actas 8º Congreso Geológico Argentino 3: 593–606.
Heyning, J.E. 1989. Comparative facial anatomy of beaked whales (Ziphiidae) and a systematic revision among the families of extant Odontoceti. Contributions in Science 405: 1–64.
Jefferson, T.A., Webber, M.A., and Pitman, R.L. 2008. Marine Mammals of the World: A Comprehensive Guide to Their Identification. 573 pp. Academic Press, Oxford.
Kasuya, T. 1973. Systematic consideration of recent toothed whales based on the morphology of the tympano-periotic bone. The Scientific Reports of the Whales Research Institute 25: 1–103.
Kellogg, R. 1924. A fossil porpoise from the Calvert Formation. Proceedings of the United States National Museum 63: 1–39. Crossref
Kimura, T. and Barnes, L.G. 2016. New Miocene fossil Allodelphinidae (Cetacea, Odontoceti, Platanistoidea) from the North Pacific Ocean. Bulletin of the Gunma Museum of Natural History 20: 1–58.
Lambert, O. 2005. Phylogenetic affinities of the long-snouted dolphin Eurhinodelphis (Cetacea, Odontoceti) from the Miocene of Antwerp, Belgium. Palaeontology 48: 653–679. Crossref
Lambert, O., Bianucci, G., and Urbina, M. 2014. Huaridelphis raimondii, a new early Miocene Squalodelphinidae (Cetacea, Odontoceti) from the Chilcatay Formation, Peru. Journal of Vertebrate Paleontology 34: 987–1004.
Lambert, O., Muizon, C. de, and Bianucci, G. 2015. A new archaic homodont toothed cetacean (Mammalia, Cetacea, Odontoceti) from the early Miocene of Peru. Geodiversitas 37: 79–108. Crossref
Lambert, O., Muizon, C. de, Malinverno, E., Di Celma, C, Urbina, M., and Bianucci, G. 2017. A new odontocete (toothed cetacean) from the Early Miocene of Peru expands the morphological disparity of extinct heterodont dolphins. Journal of Systematic Palaeontology [published online, doi: 10.1080/14772019.2017.1359689]. Crossref
Lech, R., Aceñolaza, F., and Grizinik, M. 2000. Icnofacies Skolithos–Ophiomorpha en el Neógeno del Valle inferior del Río Chubut, provincia de Chubut, Argentina. In: F.G. Aceñolaza and R. Herbst (eds.), El Neógeno de Argentina, 147–161. Instituto Superior de Correlación Geológica, Tucumán.
Maddison, W.P. and Maddison, D.R. 2011. Mesquite: A Modular System for Evolutionary Analysis. Available at http://mesquiteproject.org.
Marx, F.G., Bosselaers, M.E.J., and Louwye, S. 2016. A new species of Metopocetus (Cetacea, Mysticeti, Cetotheriidae) from the Late Miocene of the Netherlands. PeerJ 4: e1572. Crossref
McGowen, M.R., Spaulding, M., and Gatesy, J. 2009. Divergence date estimation and a comprehensive molecular tree of extant cetaceans. Molecular Phylogenetics and Evolution 53: 891–906. Crossref
Mead, J.G. and Fordyce, R.E. 2009. The therian skull: a lexicon with emphasis on the odontocetes. Smithsonian Contributions to Zoology 627: 1–248. Crossref
Moreno, F.P. 1892. Lijeros apuntes sobre dos géneros de cetáceos fósiles de la República Argentina. Revista del Museo de La Plata 3: 393–400.
Morisaka, T. and Connor, R.C. 2007. Predation by killer whales (Orcinus orca) and the evolution of whistle loss and narrow-band high frequency clicks in odontocetes. Journal of Evolutionary Biology 20: 1439–1458. Crossref
Muizon, C. de 1987. The affinities of Notocetus vanbenedeni, an Early Miocene Platanistoid (Cetacea, Mammalia) from Patagonia, Southern Argentina. American Museum Novitates 2904: 1–27. Crossref
Muizon, C. de 1988. Le polyphylétisme des Acrodelphidae, Odontocètes longirostres du Miocène européen. Bulletin Du Muséum d’Histoire Naturelle, Paris 10: 31–88.
Muizon, C. de 1991. A new Ziphiidae (Cetacea) from the Early Miocene of Washington State (USA) and phylogenetic analysis of the major groups of odontocetes. Bulletin du Muséum national d’Histoire Naturelle 4: 279–326.
Muizon, C. de 1994. Are the squalodonts related to the platanistoids? Proceedings of San Diego Society of Natural History 29: 135–146.
Nikaido, M., Matsuno, F., Hamilton, H., Brownell, R.L., Cao, Y., Ding, W., Zuoyan, Z., Sheldock, A.M., Fordyce, R.E., Hasegawam, M., and Okada, N. 2001. Retroposon analysis of major cetacean lineages: the monophyly of toothed whales and the paraphyly of river dolphins. Proceedings of the National Academy of Sciences 98: 7384–7389. Crossref
Palazzesi, L., Barreda, V.D., and Scasso, R.A. 2006. Early Miocene spore and pollen record of the Gaiman Formation (Northeastern Patagonia, Argentina): correlations and paleoenvironmental implications. 4th Latin American Congress on Sedimentology and 11th Argentinean Meeting of Sedimentology, Abstracts, 161. Sociedad Argentina de Sedimentología, Bariloche.
Parras, A., Dix, G.R., and Griffin, M. 2012. Sr-isotope chronostratigraphy of Paleogene–Neogene marine deposits: Austral Basin, southern Patagonia (Argentina). Journal of South American Earth Sciences 37: 122–135. Crossref
Perrin, W.F. 1975. Variation of spotted and spinner porpoise (genus Stenella) in the eastern Pacific and Hawaii. Bulletin of the Scripps Institution of Oceanography 21: 1–206.
Pilleri, G. 1990. Adaptation to water and evolution of echolocation in the Cetacea. Ethology, Ecology and Evolution 2: 135–163. Crossref
Puttick, M.N., O’Reilly, J.E., Tanner, A.R., Fleming, J.F., Clark, J., Holloway, L., Lozano-Fernandez, J., Parry, L.A., Tarver, J.E., Pisani, D., and Donoghue, P.C.J. 2017. Uncertain-tree: discriminating among competing approaches to the phylogenetic analysis of phenotype data. Proceedings of the Royal Society B Biological Sciences 284: 20162290. Crossref
Pyenson, N. and Sponberg, S. 2011. Reconstructing body size in extinct crown Cetacea (Neoceti) using allometry, phylogenetic methods and tests from the fossil record. Journal of Mammalian Evolution 18: 269–288. Crossref
Reeves, R.R. and Martin, A.R. 2009. River dolphins. In: W.F. Perrin, B. Würsig, and J.G.M. Thewissen (eds.), Encyclopedia for Marine Mammals, 2nd edition, 976–979. Academic Press, New York. Crossref
Scasso, R.A. and Bellosi, E.S. 2004. Cenozoic continental and marine trace fossils at the Bryn Gwyn Paleontological Park, Chubut. In: R.A. Scasso and E.S. Bellosi (eds.), Bryn Gwyn Guidebook, 1–19. 1st International Congress on Ichnology, Trelew.
Scasso, R.A. and Castro, L.N. 1999. Cenozoic phosphatic deposits in North Patagonia, Argentina: Phosphogenesis, sequence-stratigraphy and paleoceanography. Journal of South American Earth Sciences 12: 471–487. Crossref
Shostell, J.M. and Ruiz-Garcia, M. 2010. Biology, Evolution, and Conservation of River Dolphins within South America and Asia. 504 pp. Nova Science Publisher, New York.
Tanaka, Y. and Fordyce, R.E. 2014. Fossil dolphin Otekaikea marplesi (Latest Oligocene, New Zealand) expands the morphological and taxonomic diversity of Oligocene cetaceans. PloS One 9: e107972. Crossref
Tanaka, Y. and Fordyce, R.E. 2015a. A new Oligo-Miocene dolphin from New Zealand: Otekaikea huata expands diversity of the early Platanistoidea. Palaeontologia Electronica 18: 1–71. Crossref
Tanaka, Y. and Fordyce, R.E. 2015b. Historically significant late Oligocene dolphin Microcetus hectori Benham 1935: a new species of Waipatia (Platanistoidea). Journal of the Royal Society of New Zealand 45: 135–150. Crossref
Tanaka, Y. and Fordyce, R.E. 2016. Papahu-like fossil dolphin from Kaikoura, New Zealand, helps to fill the Early Miocene gap in the history of Odontoceti. New Zealand Journal of Geology and Geophysics 59: 551–567. Crossref
Tanaka, Y. and Fordyce, R.E. 2017. Awamokoa tokarahi, a new basal dolphin in the Platanistoidea (late Oligocene, New Zealand). Journal of Systematic Palaeontology 15: 365–386. Crossref
Tsai, C.-H. and Fordyce, R.E. 2016. Archaic baleen whale from the Kokoamu Greensand: earbones distinguish a new late Oligocene mysticete (Cetacea: Mysticeti) from New Zealand. Journal of the Royal Society of New Zealand 46: 117–138. Crossref
Acta Palaeontol. Pol. 63 (2): 261–277, 2018
https://doi.org/10.4202/app.00441.2017