

Phylogenetic relationships and time-calibration of the South American fossil and extant species of southern beeches (Nothofagus)
BÁRBARA VENTO and FEDERICO A. AGRAÍN
Vento, B. and Agraín, F.A. 2018. Phylogenetic relationships and time-calibration of the South American fossil and extant species of southern beeches (Nothofagus). Acta Palaeontologica Polonica 63 (4): 815–825.
The genus Nothofagus is considered as one of the most interesting plant genera, not only for the living species but also due to the fossil evidence distributed throughout the Southern Hemisphere. Early publications postulated a close relationship between fossil and living species of Nothofagus. However, the intrageneric phylogenetic relationships are not yet fully explored. This work assesses the placement of fossil representatives of genus Nothofagus, using different search strategies (Equal Weight and Implied Weight), and it analyses relationships with the extant species from South America (Argentina and Chile). The relationships of fossil taxa with the monophyletic subgenera Brassospora, Fuscospora, Lophozonia, and Nothofagus and the monophyly of the clades corresponding to the four subgenera are tested. A time-calibrated tree is generated in an approach aiming at estimating the divergence times of all the major lineages. The results support the inclusion of most fossil taxa from South America into the subgenera of Nothofagus. The strict consensus tree shows the following species as closely related: Nothofagus elongata + N. alpina; N. variabilis + N. pumilio; N. suberruginea + N. alessandri; N. serrulata + N. dombeyi, and N. crenulata + N. betuloides. The species N. simplicidens shares a common ancestor with N. pumilio, N. crenulata, and N. betuloides. This contribution is one of the first attempts to integrate fossil and extant Nothofagus species from South America into a phylogenetic analysis and an approach for a time-calibrated tree.
Key words: Fagales, Nothofagus, fossil, extant, phylogeny, time-scaling, Cenozoic, South America.
Bárbara Vento [bvento@mendoza-conicet.gov.ar], Instituto Argentino de Nivología, Glaciología y Ciencias Ambientales, IANIGLA, CCT-CONICET, Adrián Ruíz Leal s/n, Parque General San Martín, 5500 Mendoza, Argentina.
Federico A. Agrain [fagrain@mendoza-conicet.gov.ar], Instituto Argentino de Investigaciones en Zonas Áridas, IADIZA, CCT-CONICET, Adrián Ruíz Leal s/n, Parque. General San Martín, 5500 Mendoza, Argentina.
Received 24 April 2018, accepted 25 September 2018, available online 29 October 2018.
Copyright © 2018 B. Vento and F.A. Agrain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Southern Hemisphere genus Nothofagus Blume, 1851 (southern beech) is one of the most interesting plant genera within the order Fagales due to the austral distribution of living species and the extensive fossil record of leaves, pollen grains, and wood (Ancibor 1991; Romero 1986; Hill 1992; Hill and Jordan 1993; Dutra and Batten 2000; Paull and Hill 2003; Poole 2002; Cantrill and Poole 2005; Poole and Cantrill 2006; Torres et al. 2009). Living and fossil members of this genus are distributed in the southern hemisphere of Antarctica (Dutra and Batten 2000; Cantrill and Poole 2012; Reguero et al. 2013), Australia, New Caledonia, New Guinea, New Zealand, Tasmania (Dettmann et al. 1990; Swenson et al. 2000), and South America (Dusén 1899; Romero 1986; Romero and Dibbern 1985). Vegetation plant reconstructions using fossils provided evidence of past paleoenvironment and paleoclimate as the species of Nothofagus are good indicators of cool-temperate conditions (Cantrill and Poole 2012) during the Cretaceous and Paleogene (Romero 1986; Jordan and Hill 1994; Poole et al. 2001; Hinojosa et al. 2016).
Despite the growing amount of available morphological data in fossils, relationships among extinct species of Nothofagus are not yet fully explored (Hill 1992). Furthermore, there has been little general agreement about their evolutionary history and the classification scheme (Hill and Jordan 1993; Jordan and Hill 1999). Fossil leaves of Nothofagaceae are found in the most southern part of South America from the Late Cretaceous and throughout Paleogene, similar to the present day distribution of extant species (Dusén 1899; Berry 1938; Hünicken 1967; Torres et al. 2009; Acosta and Premoli 2010; Premoli et al. 2011; Torres et al. 2013; Leppe et al. 2016a). The morphological study of the fossil leaves (Romero and Dibbern 1985; Vento et al. 2017) revealed unique features that are also currently present in the extant species of Nothofagus (Jones 1986; Tanai 1986; Premoli 1996; Heenan and Smiseen 2013). The previous comparisons of leaf imprints of fossil representatives of Nothofagus to their living counterparts are based on the description of morphological features and not backed by any phylogenetic analyses (Berry 1937b; Hünicken 1967; Romero and Dibbern 1985; Tanai 1986; Torres et al. 2009).
A systematics of extant species of Nothofagus was established by Steenis (1953) on the basis of the presence of the deciduous or evergreen habit, the vernation and cupular structure with two main divisions recognized: leaves deciduous, with plicate vernation and leaves persistent with no plicate vernation. An evolutionary scheme of leaf margin characters in the genera Nothofagus and Fagus was proposed by Melville (1982) though it was later criticized by Hill and Read (1991), who found some errors in the leaf character coding, probably because the illustrations were not based on cleared leaves methodology for specific details. An exhaustive revision of living and fossil distribution of genus Nothofagus with a detailed explanation about venation patterns and margin type have been published by Tanai (1986). Philipson and Philipson (1988) proposed a taxonomic classification considering its four different types of leaf vernation. Subsequently, Hill and Read (1991) proposed a new and more natural classification incorporating into a phylogenetic analysis characters from the cupule, leaf and cuticle morphology from living Nothofagus species, which resulted in the recognition of four subgenera: Lophozonia, Fuscospora, Brassospora, and Nothofagus. Phylogenetic analyses of extant species of genus Nothofagus (Cracraft 1975; Hill and Jordan 1993; Jordan and Hill 1999; Manos 1997; Premoli et al. 2011; Heenan and Smissen 2013) have shed some light on their evolutionary history. The use of fossil plants to establish better phylogenetic hypotheses has often been promoted with a trend toward using combined analysis of fossil and living taxa (Crane 1985; Nixon and Carpenter 1996; Doyle 2012; Hill 1991; Jordan and Hill 1999, Hill et al. 2015). Nevertheless, relationships between fossil and extant taxa are still poorly understood and only a few paleobotanical records from southern latitudes were incorporated into phylogenetic analyses for a better understanding of the evolutionary history of genus Nothofagus (Hill 1983). Early phylogenetic analyses, based on morphological characters, of this genus have provided a working classification (Hill and Read 1991) and interesting hypotheses about the relationships among species of Nothofagus (Hill and Jordan 1993) for both living and fossil taxa (Jordan and Hill 1999).
The aim of the present contribution is to investigate the relationships of South American (Argentina and Chile) fossil taxa of the genus Nothofagus (Nothofagaceae) with their subgenera: Brassospora, Fuscospora, Lophozonia, and Nothofagus. The monophyly of these four subgenera is also tested, placing Nothofagus fossils in a phylogenetic tree. A time-scaling of the phylogenetic tree is estimated and discussed.
Institutional abbreviations.—BH, L.H. Bailey Hortorium, Ithaca, New York, USA; CPB, Museo de Paleontología, Córdoba, Argentina; IADIZA HRL, Ruíz Leal Herbarium, Mendoza, Argentina; and MACN, Museo Argentino de Ciencias Naturales, Buenos Aires, Argentina.
Other abbreviations.—BC, backbone constraint analysis; EW, equal weight; IW, implied weight; MPTs, Most Parsimonious Trees; FAD, First Appearance Data; LAD, Last Appearance Data.
Material and methods
Fossil and living material.—The fossil material examined in this contribution was mainly collected by Hünicken (1967) and recently re-studied by Vento et al. (2017) and Vento and Prámparo (2018). All specimens are preserved as leaf impressions and housed at CPB. Additional fossil material of Nothofagus from Patagonia was revised at MACN and from the following literature sources: Dusén (1899, 1908); Berry (1937b); Fiori (1939); Romero and Dibbern (1985). Fossil specimens of Nothofagus (Fagus) dicksonii (Dusén, 1899) Tanai, 1986 described from South America (Dusén 1908; Berry 1937b; Fiori 1939) are fragmentary and consequently the descriptions and illustrations are not clear enough to observe diagnostic characters. For all these reasons N. dicksonii is not incorporated into our phylogenetic analyses.
Living species were examined from herbarium sheets held at BH, HRL, and MACN. In order to improve and better understand the phylogenetic relationships among the genera and species of Nothofagaceae, Australasian species were included, based on literature, living material at MACN, and the herbarium catalogue of the Royal Botanical Gardens available online at www.kew.org (accessed on February 2017). Extant hybrids of Nothofagus were not considered in this contribution.
Morphological evidence.—Morphological characters 0–35, including fruit, flower and pollen features of the extant species were coded directly from Heenan and Smissen (2013). Morphological characters based on leaf morphology (36–45) (SOM 1, Supplementary Online Material available at http://app.pan.pl/SOM/app63-Vento_Agrain_SOM.pdf) were coded from South American fossil material and extant species. The fossil characters scored in this contribution were mainly based on the leaf margin and the venation pattern observations utilizing the following characters: type of leaf margin, teeth simple or composite, tooth spacing, teeth size, primary vein, number of secondary veins, secondary vein ending, apex morphology, base morphology and secondary veins, based mainly on the descriptions made by Tanai (1986) and Gandolfo and Romero (1992).
Phylogenetic analysis.—The phylogenetic analysis included both fossil and living taxa of the genus Nothofagus from Southern South America (Argentina and Chile). Representative living species from Southern Australia, New Zealand, and Tasmania were also included. Two outgroups were chosen for the phylogenetic analyses: Fagus Linnaeus, 1753 (Fagaceae) and Betula pendula Roth, 1788 (Betulaceae). Nothofagus species were previously included as members of the Fagaceae (Hill and Jordan 1993; Manos 1997; Jordan and Hill 1999) but were considered later to be more closely related to Betulaceae (Nixon 1982, 1989; Jones 1986; Dutra 1997; Premoli et al. 2011). The unique taxon for which the specimen approach was not applied is Fagus, which was coded as representing the genus as a whole with its polymorphic nature. The data matrix comprises a total of 46 morphological characters. All characters were informative and treated as non-additive. The phylogenetic analysis was performed using TNT v.1.5 parsimony software (Goloboff et al. 2008). The phylogenetic placement of leaf fossils of Nothofagus was tested using three different analytical approaches. We evaluated the results of these approaches and selected our evolutionary hypothesis based on current limitations of each method and considering the congruence of our results with the morphological and molecular available evidence. Firstly, we performed a traditional parsimony analysis under equal weights (EW). As a second alternative, we used a backbone constraint analysis (BC) using a molecular scaffold tree (see Springer et al. 2001 for details). This analysis was performed in order to minimize the negative effects of having too many missing data in parsimony analyses (Manos et al. 2007). The backbone constraint was made taking into account the topology of the consensus trees from Premoli et al. (2011), and Sauquet et al. (2012); the latter to incorporate the subgenus Brassospora. Finally, we used an implied weighting analysis (IW), exploring the tree topologies obtained with different concavity constants (k) (Goloboff 1993). We used the command “xpiwe (*” to avoid that the missing entries generate too much homoplasy of the observed characters during the optimization of the MPTs (i.e., to receive a high fit). This command is comparable to use different values of k for each character according to its percentage of missing entries. Missing entries are assumed to have 50% of the homoplasy of observed entries as part of the extended implied weighting functions of TNT, that allow to assign lower values of k to those characters with more missing data, (i.e., down-weighting homoplasy more strongly), see Goloboff (2014) for details. For the IW analysis we explored the topologies of the strict consensus trees resulting from k = 1 to k = 30. We used IterPCR (Pol and Escapa 2009) in order to identify unstable taxa. This methodology was implemented in TNT under the “pcrprune” command (Goloboff and Szumik 2015). We calculated Bremer support (Bremer 1994; Goloboff and Farris 2001) using up to 10 suboptimal trees, and Jackknife (Lanyon 1985) with removal probability at 0.36 and 500 replicates to measure branch support for the EW and BC analyses. Such measures are indicated as frequency difference. Evaluation of branch support for the IW analysis was performed using Symmetric resampling (Goloboff et al. 2003) with change probability at 0.33 and 500 replicates (values are indicated as a frequency difference). Both, the EW and BC analyses were conducted using implicit enumeration option that finds the MPTs by means of an exact solution. Since implicit enumeration cannot be performed under extended implied weighting, the analysis under IW was performed using a traditional heuristic search. The search was on the base of Wagner trees with 500 random addition sequences, followed by the tree bisection reconnection (TBR) swapping algorithm, saving ten trees per replicate, and collapsing trees after the search. This was followed by a branch and bound search based on these trees from RAM.
Time-scaling estimation.—Divergence times using both fossil and extant taxa were estimated with R (R Core Team 2017) package Strap (Stratigraphic Tree Analysis for Paleontology) designed by Bell and Lloyd (2015). The calibration of the tree uses the first (FAD) and the last appearance datum (LAD) trusted record. The formatting matches that used in the R package paleotree (Bapst 2012) and allows for easy swapping of time-scaled trees between packages (Bell and Lloyd 2015). The minimum and maximum possible age should be considered in a calibrated tree using geochronological information of fossil taxa (Pol and Norell 2006). Therefore, to extract the temporal information from the extinct taxa, the age of each fossil was entered as non-contemporaneous date representing millions of years (Myr) before the present. This age is determined by biostratigraphic or by radiometric isotopic methods in the sequence in which the fossils were derived. The geological age of each fossil (FAD and LAS) was obtained directly from the literature (SOM 2). In order to plot the time-scaling tree against geologic time the function “geoscalePhylo” was used. The geologic time follows Gradstein et al. (2004) or the published time scales by the International Commission on Stratigraphy (Bell and Lloyd 2015). Command “StraPhylloCongruence” was used to assess a congruence of the tree and to calculate the supported measurements (SOM 3).
Results
The EW analysis resulted in six MPT’s of 138 steps. The strict consensus tree presents some polytomies, and the support values for most of the clades are low (SOM 4). The topology of this tree is not congruent with the knowledge of Nothofagus phylogeny, especially when considering the position of the species of subgenus Fuscospora which are nested within Brassospora. IterPCR improved the consensus by pruning the two taxa Nothofagus alpina (Poeppig and Endlicher, 1838) Oersted, 1871 and Nothofagus elongata (Dusén, 1899) Romero and Dibbern, 1985. Conversely, the BC analysis resulted in two MPTs of 506 steps, the strict consensus and branch support are showed in SOM 4. In this case, IterPCR did not find any improvement by pruning taxa from consensus. The topology of the strict consensus tree is in agreement with the current knowledge but the support values are still low. Finally, the analyses using IW analyses, from k = 1 to k = 30, resulted in three MPTs. The topologies of the strict consensus trees obtained from k = 1 to k = 6 are exactly the same, a small change occurs in the topology of strict consensus trees obtained from k = 7 and k = 8 (SOM 4). This change consists of the aggregation of Nothofagus fusca (Hooker, 1844) Oersted, 1871 and Nothofagus solandri (Hooker, 1844) Oersted, 1871, being the latter highly congruent with the results obtained by Premoli et al. (2011). In all the strict consensus trees resulting from k = 9 to k = 30 Fuscospora appears as nested within Brassospora, the latter aggregation being contrary to current molecular and morphological evidence.
There is not an optimal criterion to choose any particular value of k (Goloboff 1993) in the IW analysis. Recently, Goloboff et al. (2018) demonstrated that implied weights outperform other methods such as equally weighted parsimony and even model-based methods. These authors also stated that better results can be obtained when weighting more gently against homoplasy (i.e., using larger values of k). This idea seems to be supported by our data when considering the congruence of the results obtained with IW analyses and the current knowledge of Nothofagus phylogeny. Below, we discuss the results on the base of the strict consensus of the three MPTs with fit = 5.757 obtained with k = 8 (Fig. 1). Our chosen hypothesis combines a satisfactory balance between robustness and resolution that is also coherent with current morphological and molecular evidence. In the EW analysis the consensus tree can be improved by pruning N. alpina and N. elongata Dusén, 1899 as indicated by IterPCR. Our results using the IW analysis recovered the four monophyletic clades corresponding to the four extant subgenera. These are clearly differentiated and close relationships between fossil and extant are observed. We found exclusive synapomorphies supporting all the subgenera of Nothofagus except for Fuscospora because the character granular epidermal cell walls (25-1) is also present in N. obliqua (Mirbel, 1827) Oersted, 1871 (Fig. 1).
The fossil taxa from Southern South America appear related with the subgenera Lophozonia, Fuscospora, and Nothofagus (see dashed lines in Fig. 1). The fossil N. elongata is located in the clade corresponding to the subgenus Lophozonia (SOM 4) and the species Nothofagus subferruginea (Dusén, 1899) Tanai, 1986 is located in the clade of subgenus Fuscospora, this happens both on BC and IW analysis (Fig. 1, SOM 4). Finally, the clade of subgenus Notohofagus includes the placement of fossils Nothofagus simplicidens Dusén, 1899, Nothofagus variabilis Dusén, 1899, Nothofagus serrulata Dusén, 1899, and Nothofagus crenulata Dusén, 1899.
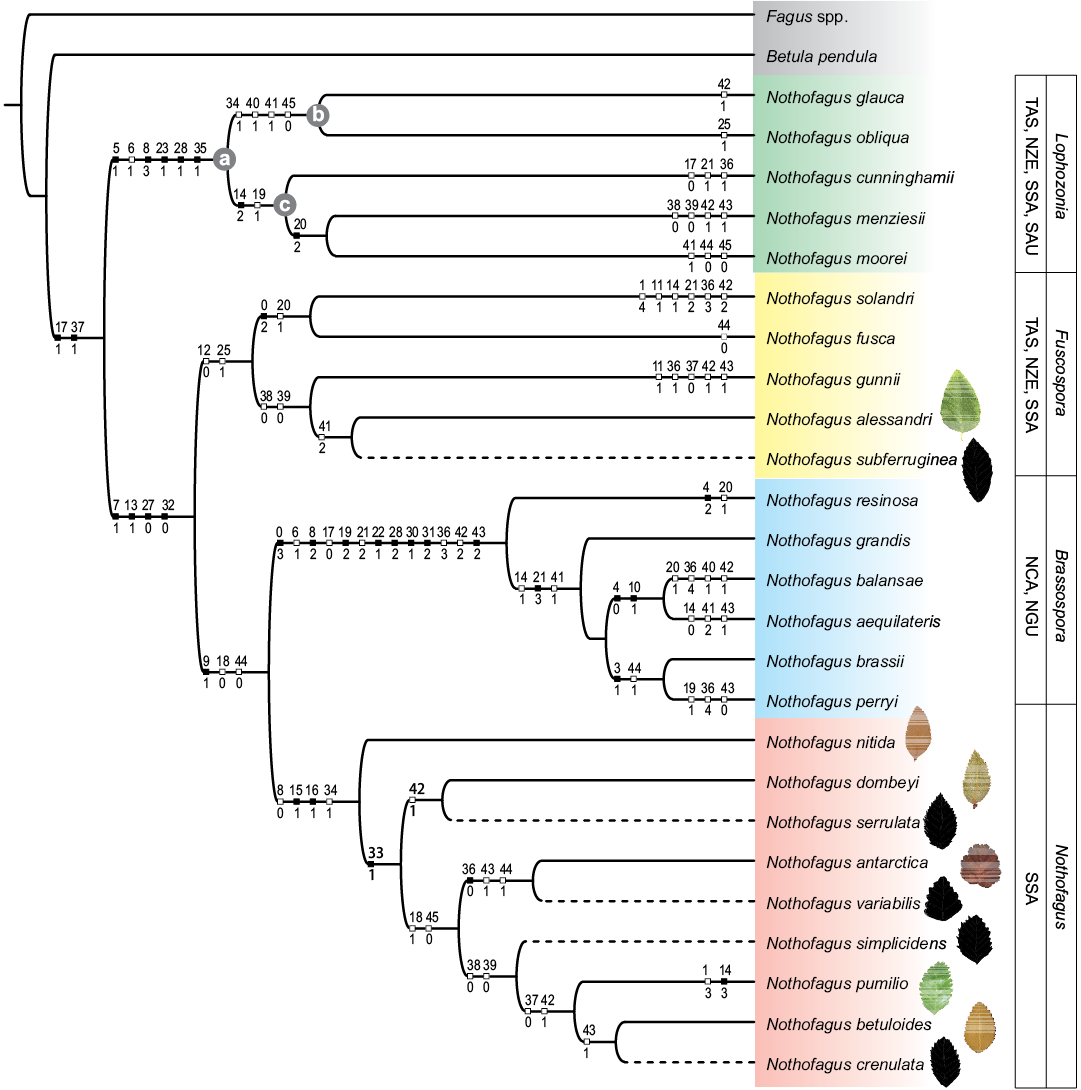
Fig. 1. Strict consensus tree from Implied Weight Analysis (IWA) (k = 8). Dashed cladogram branches indicate fossil taxa. Consistency Index (CI): 0.51; Retention Index (RI): 0.78. Shaded circles (a–c) indicate the three possible placements of the two taxa Nothofagus alpina and Nothofagus elongata before pruning as indicated by IterPCR. Green/brown and black leaves are representatives of living and fossil species, respectively. Abbreviations: NCA, New Caledonia; NGU, New Guinea; NZE, New Zealand; SAU, Southern Australia; SSA, Southern South America; TAS, Tasmania.
Discussion
Extant species of the genera Fagus and Nothofagus have a clear differentiation in their hemispheric distribution, with fossil remains and extant species of Nothofagus distributed in the southern hemisphere (Tanai 1986; Read 1990). A combination of fossil leaves and pollen records likely leads to more precise estimation of the evolutionary history of Nothofagus (Tanai 1986) and occasionally, leaves are the unique available fossil data of Nothofagus together with pollen records (Romero 1986). Variations in venation pattern of leaves within the genus and the relationships between secondary vein termination and leaf margin may be useful in determination of monophyletic clades (Steenis 1953). The species of monophyletic subgenera Brassospora (Fig. 1) share the character secondary vein ending at the margin (42-2) and the entire margin character (36-3), which differentiates it from the monophyletic subgenera Nothofagus (42-1 and 36-1-0).
The results from our phylogenetic analysis support the hypothesis that fossils of genus Nothofagus are closely related to modern species currently distributed in Southern South America (Fig. 1). The intraspecific relationships among extant species of Nothofagus (Fig. 1) are in agreement with the results obtained by Hill and Read (1991), Jordan and Hill (1999), Heenan and Smissen (2013), Vento et al. (2017), and the molecular phylogenetic analysis performed by Setoguchi et al. (1997), Martin and Dowd (1993), Premoli et al. (2011), and Sauquet et al. (2012). Heenan and Smissen (2013) proposed raising the subgenera Fuscospora, Lophozonia, and Nothofagus to the genus rank and renaming Brassospora as Trisyngyne. These authors argued that the morphological and molecular differences are sufficient to recognize four clades and that this classification will be more informative than the currently circumscribed Nothofagus with four subgenera. Nevertheless, this new classification was criticized for not considering fossil species of Nothofagus and how it would complicate the use of the extensive current literature on the genus (Hill et al. 2015). To avoid confusion between the modern taxonomy and the fossil record, the last taxonomic classification made by Heenan and Smissen (2013) is not used in our work. Setoguchi et al. (1997) constructed a molecular phylogeny using pollen grains from extant species but found several polytomies in the tree within the clade of subgenus Nothofagus and the relationship of this clade with subgenus Brassospora. In our cladogram (Fig. 1), the strict consensus tree is resolved (probably as a consequence of some shared homoplastic characters), but the support for most of the nodes is low (SOM 4: fig. C2). The classification made by Hill and Read (1991) incorporated into the phylogenetic analysis characters from the cupule, leaf, and cuticle morphology of living Nothofagus. The cupule morphology better supports the division of the genus on the basis of pollen types, but uncertainty remains regarding the placement of Nothofagus betuloides and Nothofagus pumilio (Poeppig and Endlicher, 1838) Krasser, 1896 (Hill and Read 1991). This issue is resolved in our new analysis where N. betuloides, N. pumilio and the extinct taxa N. crenulata share a common ancestor and belong to the subgenus Nothofagus (Fig. 1). According to Hill and Read (1991) leaf architecture gives the least satisfactory classification, since many species bear little resemblance to others in the same genus. The latter authors proposed that the taxonomy of Nothofagus leaves is difficult to be resolved based solely on the gross leaf morphology and venation pattern, and should involve characters of the cupule, pollen grains, and cuticular pattern. For instance, species N. gunnii (Hooker, 1844) Oersted, 1871 and N. pumilio appear to be very similar on the basis of leaf architecture (Tanai 1986), but the cupule and cuticular morphology of both species are quite distinct (Hill and Read 1991). However, according to the results of our BC analysis (Figs. 1, 2), the inclusion of leaf architecture characters and molecular data, clearly support the monophyly of the four subgenera when fossil taxa and extant species are considered together in a phylogenetic analysis (SOM 4: figs. B1, B2).
Pollen grains of Nothofagus are easily identified due to their characteristic peroblate, stephanocolpate, crassimarginate morphology, with a spinulose exine (Romero 1986). There are three types of grains: “N. fusca”, “N. mensiezii”, and “N. brassii” which are characteristic of each subgenus (Dettman et al. 1990). Living species from Southern South America of subgenus Lophozonia has the “N. mensiezii” pollen type. In contrast, extant representatives from Southern South America of subgenera Fuscospora and Nothofagus have “N. fusca a” and “N. fusca b” pollen type, respectively (Dettman et al. 1990; Hill and Read 1990). “N. brassii” type is characteristic of the living species of subgenus Brassospora (Dettman et al. 1990; Hill and Read 1991).
The consensus tree (Fig. 1) supports a close relationship between extinct taxa and their modern relatives. The subgenus Nothofagus is placed as sister clade to the tropical subgenus Brassospora, and the clade, they form is sister to the subgenus Fuscospora. These results are similar to the phylogenetic studies by Jordan and Hill (1999), Swenson et al. (2000), and Hill (2001). Sauquet et al. (2012) reported the appearance of Brassospora after the Oligocene as it is also revealed by our calibrated tree (Fig. 2). In a preliminary phylogeny that included fossil and extant species from Southern South America, Vento et al. (2017) documented a close relationship between N. variabilis and its modern relative Nothofagus antarctica (Forster, 1789) Oersted, 1871; N. elongata and N. alpina and N. subferruginea with N. alessandri Espinosa, 1928. The fossil taxa N. variabilis + N. serrulata + N. simplicidens + N. crenulata are supported as members of the clade that corresponds to the subgenus Nothofagus (Fig. 1). The fossil taxon N. subferruginea is included in the subgenus Fuscospora and a close relationship between this taxon and the modern N. alessandri is observed (Fig. 1), what is consistent with earlier works (Tanai 1986; Dutra 1997). The fossil taxa N. elongata and its modern relative N. alpina are grouped together in the subgenus Lophozonia as supported by the scaffold tree in our BC analysis. However, IterPCR indicates that the placement of N. alpina and N. elongata is unstable for the EW and IW analyses (i.e., they were pruned from the consensus, and not used for support calculations). For EW and IW approaches these taxa have three slightly different possible placements, all within subgenus Lophozonia (Fig. 1: a–c; SOM 4: dashed branches). Subgenus Lophozonia has been recognized as the most basal clade within Nothofagaceae in majority of studies (Manos 1997; Swenson et al. 2000; Poole 2002; Premoli et al. 2011) except for the cladograms obtained by Hill and Read (1991); Hill and Jordan (1993), and Sauquet et al. (2012). The parasite Cyttaria lineage B (34-1) is present in subgenus Nothofagus except for Nothofagus nitida (Philippi, 1858) Krasser, 1896, which has the synapomorphy: presence of Cyttaria lineage A (33-1). The clade Nothofagus obliqua + Nothofagus glauca (Philippi, 1858) Krasser, 1896 share the presence of the parasite Cyttaria Berkeley, 1842 lineage B (34-1) and the non-exclusive synapomorphies: primary vein curved (40-1), number of secondary veins (41-1) and opposite secondary vein (45-0).
The topology of the IW strict consensus (Fig. 1) shows that fossil and living species of the monophyletic Nothofagus clade have the following synapomorphies: annulate pollen aperture thickening (8-0), T-pieces at stomatal poles present (15-1), the stomatal orientation mostly parallel with the midrib (16-1), being the latter two exclusive synapomorphies of subgenus Nothofagus. Peterson et al. (2010) suggested a co-evolution between Nothofagus and the fungal parasite Cyttaria where the phylogeny of Nothofagaceae would show that species from Southern Australia and Southern South America are more closely related; however, our analysis resulted in a closer relationship between species from Southern Australia and New Zealand as it was found by other authors (Dettmann et al. 1990; Hill and Jordan 1993; Hill and Read 1991; Manos 1997; Martin and Dowd 1993; Setoguchi et al. 1997; Premoli et al. 2011; Heenan and Smissen 2013). The phylogeny of Cyttaria lineage A (33-1) is congruent with the phylogeny of living taxa of the subgenus Nothofagus, which might indicate an ancient association and co-evolution of both lineages.
Nothofagus variabilis and N. antarctica share the leaf type margin (36-0), the apex shape (43-1) and the base morphology (44-1). Nothofagus serrulata and N. dombeyi (Mirbel, 1827) Oerst share the secondary vein ending at the sinus (42-1). Nothofagus crenulata and N. betuloides (Mirbel, 1827) Oersted, 1871 share the apex morphology (43-1) and they are sisters to N. pumilio by sharing the teeth simple (37-0) and the secondary vein ending at the sinus (42-1). Altogether, these taxa share teeth regularly spaced (38-0) and the teeth size (39-0) with the fossil taxa N. simplicidens. Dusén (1899) compared N. crenulata with the extant N. cunninghamii (Hooker, 1844) Oersted, 1871 which is restricted to Australia and Tasmania, while Frenguelli (1941) related it to N. betuloides. Tanai (1986) suggested that it is more similar to N. antarctica because the margin serrate and the secondary vein end at the tooth.
The clade of subgenus Fuscospora contains the fossil N. subferruginea and the modern N. alessandri that share the synapomorphy: number of secondary veins with more than 12 pairs (41-2), and they share a common ancestor with Nothofagus gunnii based on the regular tooth spacing (38-0) and the uniform size of teeth (39-0). A close relationship between N. gunnii and N. pumilio is proposed by Tanai (1986) on the basis of leaf architecture. A phylogenetic relationship cannot be sustained only on leaf architecture (Hill and Read 1991). Our analysis strongly supports the view of Hill and Read (1991) showing these two taxa in different positions: N. gunnii within the subgenus Fuscospora and N. pumilio within the subgenus Nothofagus.
The time-calibrated tree using fossil material from Southern South America (Fig. 2) is an approach to understand the evolutionary history of Nothofagus. The lack of fossil information from Antarctica in the phylogenetic analysis (Fig. 2) limits the discussion about the paleobiogeography of this genus and adding this information will certainly constitute an important future contribution. Areas like Southern Australia, New Zealand, and Southern South America together with Antarctica were connected and formed the supercontinent of Gondwana, and an extensive fossil record indicates that Nothofagus was a main component of the vegetation there since the Late Cretaceous (Dettmann et al. 1990; Hill 1991; Hill et al. 1996; Swenson et al. 2000; Dutra and Batten 2000; Poole and Cantrill 2002). The origin of Nothofagus still remains uncertain. Dutra and Batten (2000) hypothesized that the presence of Nothofagus leaf remains in the Antarctic Peninsula earlier than in the other areas of Gondwana, where only pollen grains have been recorded, may indicate the Antarctic Peninsula as a possible centre of evolution and diversification of Nothofagus during the Late Cretaceous. Antarctica has a good record of pollen grains and impressions of Nothofagus since the Late Cretaceous (Dutra and Batten 2000; Dutra 2004; Hayes et al. 2006; Cantrill and Poole 2012).
The oldest fossil pollen grains were recorded from the Santonian (~85–83 Myr) of the Antarctic Peninsula (Bastos et al. 2013; Reguero et al. 2013), later from the Santonian–Campanian of Southern Australia (Dettman et al. 1990; Dettman 1994) and the Maastrichtian from Southern South America (Archangelsky and Romero 1974). The first mention of fossils leaves of Nothofagus in South America was by Dusén (1899), while the oldest record of the leaves from South America has been recently published by Leppe et al. (2016a) from “Las Chinas” Valley (Chilean Patagonia) dated as the lower Maastrichtian (~68.9–71.4 Myr). Nothofagus imprints in Antarctica are recorded in the upper Campanian (Dutra 1997; Bastos et al. 2013; Leppe et al. 2016b) and recently also from the lower Campanian by Leppe et al. (2016a). Hayes et al. (2006) mentioned the record of nothofagacean from the Coniacian of James Ross Island. In Argentina, Dryophyllum australis Berry, 1937a, with characters similar to Nothofagaceae have been found in the Salamanca Formation (Iglesias 2007) from early to middle Danian (early Paleocene ~65.7–63.5 Myr). Undetermined species of Nothofagus are recorded from late Miocene–early Pliocene in the Navidad Formation of Chile (Tanai 1986; Troncoso 1991; Troncoso and Encinas 2006). Fossils of Nothofagus are also recorded from the late Pliocene–early Pleistocene of Tasmania (Hill 1991; Hill et al. 1996; Jordan 1999).
The morphology of Nothofagus leaves is known to reflect local ecological conditions and climate (Read 1990; Hill 1991; Jordan and Hill 1994; Hinojosa et al. 2016). There are clear differences between environmental and climate requirements of particular species as in the case of the subtropical subgenus Brassospora with entire leaf margin and the remaining clades from cooler climate, which are characterized by serrate margin (Troncoso and Encinas 2006).
Living species of the subgenus Nothofagus are distributed in a cool temperate climate along 35° S in Southern South America (Premoli et al. 2011) with some clear differences in their distribution (Veblen et al. 1996). Representatives of this subgenus have leaves with smaller teeth and fewer secondary veins compared with fossil taxa placed into subgenera Lophozonia and Fuscospora (Romero 1986). Dutra (2004) concluded that bigger leaves suggest warmer conditions. A warm-temperate climate was present from the Late Cretaceous until the beginning of the middle Eocene in the northern Antarctic Peninsula. Smaller leaves are indicative of a deterioration in the climate with cooling conditions during the late Eocene–Oligocene (Dutra 2004; Cantrill and Poole 2012; Reguero et al. 2013). The subgenus Nothofagus includes winter deciduous and evergreen species. Extant species inhabiting Southern South America such as N. nitida, N. betuloides, and N. dombeyi are evergreen species whereas N. antarctica and N. pumilio are deciduous (Hill and Read 1990). It is believed that deciduousness is a primitive character (Hill and Read 1991) and the evergreen habit arose at least three times in the evolution of the genus due to convergent events (Hill and Read 1991; Setoguchi et al. 1997). Currently, species like N. dombeyi, N. pumilio, and N. antarctica thrive at high elevation sites (+1500 m) and species such as N. betuloides, N. obliqua, and N. glauca are distributed from sea level to approximately 1200 m altitude in southern Chile and southwestern Argentina (Veblen et al. 1996). Nothofagus alessandri, an endemic species from Chile, grows on slopes exposed to humid conditions (Amigo and Rodríguez 2011). The patterns of distribution along an altitude gradient indicate differences in the ecological niches and climate requirements for living species (Veblen et al. 1996; Cantrill and Poole 2012). The relationship between fossil and living species as N. pumilio + N. serrulata, N. antarctica + N. variabilis, and N. betuloides + N. crenulata can indicate an altitude differentiation from the Eocene–Oligocene (Figs. 1, 2) and until now.
The genus Nothofagus appears to be extremely conservative and its fossil record has proven useful for reconstructing the genus past distributions and evolutionary events (Hill 1994, 2001; Hayes et al. 2006).
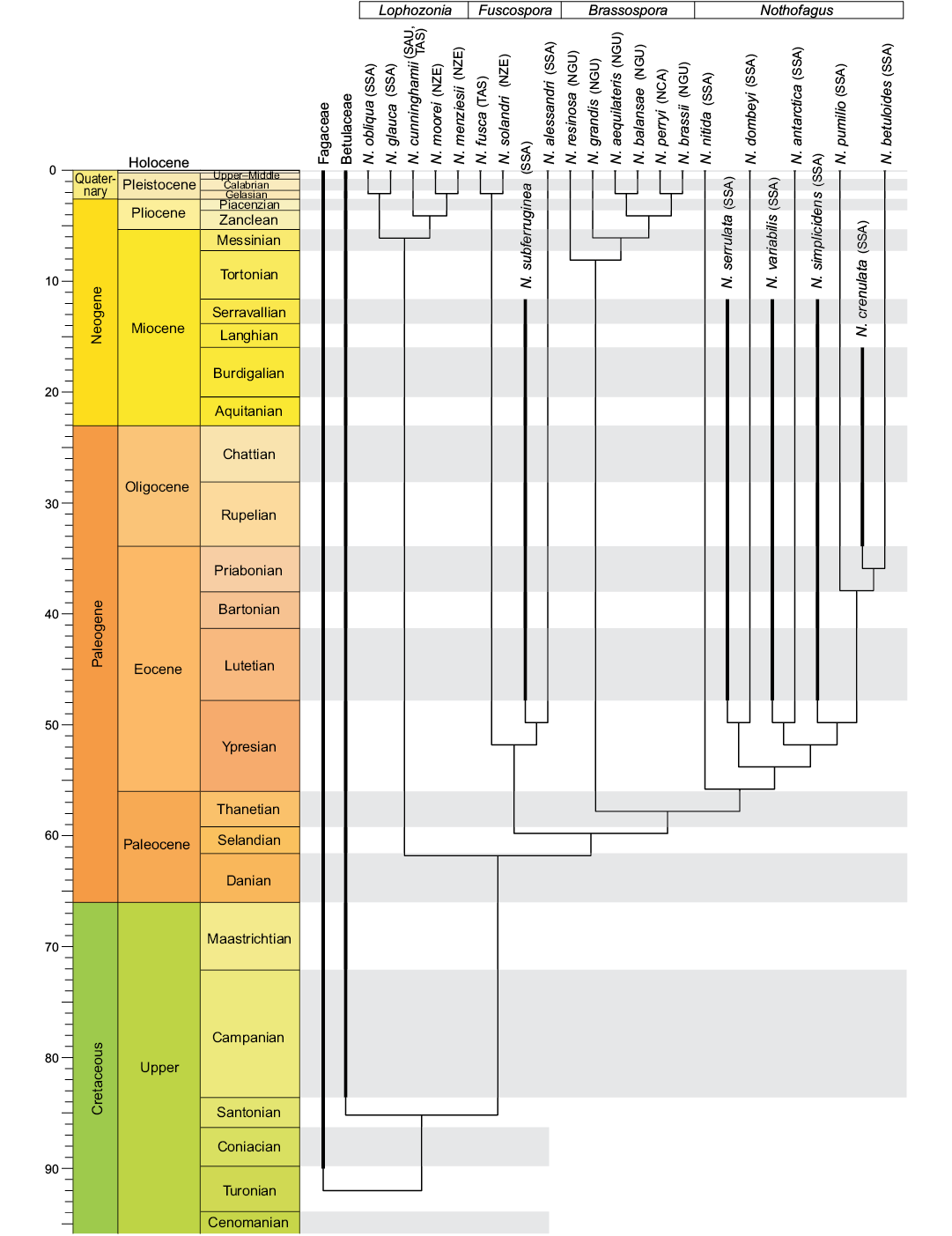
Fig. 2. Time-scaling tree from genus Nothofagus. The tree is time-scaled (Gradstein et al. 2004) using DatePhylo function and timeSliceTree from paleotree package (Bapst 2012). Abbreviations: NCA, New Caledonia; NGU, New Guinea; NZE, New Zealand; SAU, Southern Australia; SSA, Southern South America; TAS, Tasmania.
Conclusions and future directions
In this work we attempted to merge the information from fossils and extant species of Nothofagus from Southern South America into a phylogenetic analysis to refine our understanding of the relationships of this genus. Results of the performed analysis bring new insights on the relationships between modern and fossil species of the genus Nothofagus in Southern South America, supporting its monophyly with all four recognized subgenera of Nothofagus within single clade. There is a close relationship between fossil taxa and extant species with the former being sister taxa of subgenera Lophozonia, Fuscospora, and Nothofagus. The assignment of fossil N. elongata to the subgenus Lophozonia, N. subferruginea to subgenus Fuscospora and N. serrulata, N. variabilis, N. crenulata, N. simplicidens to subgenus Nothofagus, is proposed. Characters such as the venation pattern and the secondary veins ending at the teeth or the sinus and the number of secondary veins are relevant to clarify phylogenetic relationships especially when fossil leaves are available. The fossil record provides evidence for the time of origin and divergence of particular taxa, and it seems that subgenera Lophozonia and Fuscospora in Southern South America may be older representatives than taxa of the subgenus Nothofagus. The climatic conditions for early–middle Eocene were warmer than today. By the late Eocene–early Oligocene the climate probably was similar to the cold climate conditions in southernmost part of Southern South America today. Even though only few morphological characters can be coded from the fossil leaves and most of them can be homoplastic, they still have proven useful in the phylogenetic analysis. Leaf characters have to be considered in the phylogenies especially when no other fossil remains are available. Fossil material from Antarctica has to be included in future phylogenetic analysis for a more accurate interpretation and understanding of the divergence patterns of Nothofagus in Southern South America.
Acknowledgements
Bárbara Vento thanks to Georgina del Fueyo and Luis Lezama, Diego Gutiérrez, (all MACN), Lorena Boujour (HRL) and Adan Tauber (Paleontological Museum, Cordoba, Argentina) for the access to the fossil collections and herbaria. We thank the Willie Hennig Society for making the TNT program freely available and to Mercedes Prámparo (IANIGLA, Mendoza, Argentina) for the comments on the manuscript. Many thanks to Tânia Dutra (Universidade do Vale do Rio dos Sinos, Sao Leopoldo, Brazil) and an anonymous reviewer for helpful comments on the manuscript, and to Gregory Hoke (University of Syracuse, New York, USA) for the final English revision.
References
Acosta, M.C. and Premoli, A.C. 2010. Evidence of chloroplast capture in South American Nothofagus (subgenus Nothofagus, Nothofagaceae). Molecular Phylogenetics and Evolution 54: 235–242. Crossref
Amigo, J. and Rodríguez, M.A. 2011. Bioclimatic and phytosociological dignosis of the species of the Nothofagus genus (Nothofagaceae) in South America. International Journal of Geobotanical Research 1: 1–120. Crossref
Ancibor, E. 1991. Determinación xilológica de la madera fósil de una Fagaceae de la Formación Río Turbio (Eoceno), Santa Cruz, Argentina. Ameghiniana 27: 179–184.
Archangelsky, S. and Romero, E.J. 1974. Los registros más antiguos del polen de Nothofagus (Fagaceae) de Patagonia (Argentina y Chile). Boletín de la Sociedad Botánica de México 33: 13–30.
Bapst, D.W. 2012. Paleotree: an R package for paleontological and phylogenetic analyses of evolution. Methods in Ecology and Evolution 3: 803–807. Crossref
Bastos, B.L., Dutra, T.L., Wilberger, T.P., and Trevisan, C. 2013. Uma flora do final do Cretáceo na Ilha Nelson, Ilhas Shetland do Sul, Península Antártica. Revista Brasileira de Paleontologia 16: 441–464. Crossref
Bell, M.A. and Lloyd, G.T. 2015. Strap: an R package for plotting phylogenies against stratigraphy and assessing their stratigraphic congruence. Palaeontology 58: 379–389. Crossref
Berkeley, M.J. 1842. On an edible fungus from Tierra del Fuego and allied Chilean species. Transactions of the Linnean Society of London 19: 37–43. Crossref
Berry, E.W. 1937a. A Paleocene flora from Patagonia. Johns Hopkins University Studies in Geology 12: 22–50.
Berry, E.W. 1937b. Eocene plants from Río Turbio in the territory of Santa Cruz, Patagonia. Johns Hopkins University Studies in Geology 12: 91–98.
Berry, E.W. 1938. Tertiary flora from Río Pichileufú, Argentina. Geological Society of America, Special Paper 12: 1–149.
Blume, C.L. von 1851. Ord. Cupulifereae. Museum Botanicum Lugduno-Batavum 1: 305–320.
Bremer, K. 1994. Branch support and tree stability. Cladistics 10: 295–304. Crossref
Cantrill, D.J. and Poole, I. 2005. Taxonomic turnover and abundance in Cretaceous to Tertiary wood floras of Antarctica: implications for changes in forest ecology. Palaeogeography, Palaeoclimatology, Palaeoecology 215: 205–219. Crossref
Cantrill, D.J. and Poole, I. 2012. The Vegetation of Antarctica Through Geological Time. 465 pp. Cambridge University Press, Cambridge. Crossref
Cracraft, J. 1975. Historical biogeography and earth history: perspectives for a future synthesis. Annals of Missouri Botanical Garden 2: 227–250. Crossref
Crane, P.R. 1985. Phylogenetic analysis of seed plants and the origin of angiosperms. Annals of the Missouri Botanical Garden 72: 716–793. Crossref
Dettmann, M.E. 1994. Cretaceous vegetation: the microfossil record. In: R.S. Hill (ed.), History of the Australian Vegetation: Cretaceous to Recent, 43–170. Cambridge University Press, Cambridge.
Dettmann, M.E., Pocknall, D.T., Romero, E.J., and Zamaloa, M.C. 1990. Nothofagidites Erdtman ex Potonié, 1960; a catalogue of species with note on the paleogeographic distribution of Nothofagus Bl. (southern beech). New Zealand Geological Survey Paleontological Bulletin 60: 1–79.
Doyle, J.A. 2012. Molecular and fossil evidence on the origin of angiosperms. Annual Review of Earth and Planetary Sciences 40: 301–326. Crossref
Dusén, P. 1899. Über die tertiäre Flora der Magallans-Länder. In: O. Nordenskjöld (ed.), Wissenschaftliche Ergebnisse der Schwedischen Expedition nach den Megallansländer 1895–97. Band I. Geologie, Geographie und Anthropologie, 87–108. Lithographisches Institut des Generalstabs, Stockholm.
Dusén, P. 1908. Über die tertiäre Flora der Seymour Insel. In: O. Nordenskjöld (ed.),Wissenschaftliche Ergebnisse der Schwedischen Südpolar Expedition 1901–1903, Geologie und Paläontologie 3, 1–27. Norstedt and Söner, Stockholm.
Dutra, T.L. 1997. Primitive leaves of Nothofagus (Nothofagaceae) in Antarctic Peninsula: an Upper Campanian record and a betulaceous more than fagaceous morphological character. Congreso Geológico Chileno, Antofagasta, Chile 9: 24–29.
Dutra, T.L. 2004. Paleofloras da Antártica e sua relação com os eventos tectônicos e paleoclimáticos nas altas latitudes do sul. Revista Brasileira de Geociências 34: 401–410. Crossref
Dutra, T.L. and Batten, D.J. 2000. Upper Cretaceous floras of King George Island, West Antarctica, and their palaeoenvironmental and phytogeographic implication. Cretaceous Research 21: 181–209. Crossref
Espinosa, M.R. 1928. Dos especies nuevas de Nothofagus. Revista Chilena Historia de Natural 32: 171–197.
Fiori, A. 1939. Fillite terziarie della Patagonia. II. Fillite del Río Ñirihuau. Giornale di Geologia 13: 1–27.
Forster, G. 1789. Fasciculus Plantarum Magellanicum. Commentationes Societatis Regiae Scientiarum Gottingensis 9: 13–45.
Frenguelli, J. 1941. Nuevos elementos florísticos del Magalleniano de Patagonia austral. Notas del Museo de La Plata 30: 173–202.
Gandolfo, M.A. and Romero, E.J. 1992. Leaf morphology and key to species of Nothofagus Bl. Bulletin of the Torrey Botanical Club 119: 152–166. Crossref
Goloboff, P.A. 1993. Estimating character weights during tree search. Cladistics 9: 83–91. Crossref
Goloboff, P.A. 2014. Extended implied weighting. Cladistics 30: 20–272. Crossref
Goloboff, P.A. and Farris, J.S. 2001. Methods for quick consensus estimation. Cladistics 17: 26–34. Crossref
Goloboff, P.A. and Szumik, C.A. 2015. Identifying unstable taxa: efficient implementation of triplet-based measures of stability, and comparison with Phyutility and RogueNaRok. Molecular Phylogenetics and Evolution 88: 93–104. Crossref
Goloboff, P.A., Farris, J.S., and Nixon, K.C. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24: 774–786. Crossref
Goloboff, P.A., Farris, J.S., Kallersjo, M., Oxelman, B., Ramírez, M.J., and Szumik, C.A. 2003. Improvements to resampling measures of groupsupport. Cladistics 19: 324–332. Crossref
Goloboff, P.A., Torres, A., and Arias, J. 2018. Weighted parsimony outperforms other methods of phylogenetic inference under models appropriate for morphology. Cladistics 34: 407–437. Crossref
Gradstein, F.M., Ogg, J.G., Smith, A.G., Agterberg, F.P., Bleeker, W., Cooper, R.A., Davydov, V., Gibbard, P., Hinnov, I.A., and House, M.R. 2004. A Geologic Time Scale 2004. 589 pp. Cambridge University Press, Cambridge. Crossref
Hayes, P.A., Francis, J.E., Cantrill, D.J., and Crame, J.A. 2006. Palaeoclimate analysis of Late Cretaceous angiosperm leaf floras, James Ross Island, Antarctica. Geological Society, London, Special Publications 58: 49–62. Crossref
Heenan, P.B. and Smissen, R.D. 2013. Revised circumscription of Nothofagus and recognition of the segregate genera Fuscospora, Lophozonia, and Trisyngyne (Nothofagaceae). Phytotaxa 146: 1–31. Crossref
Hill, R.S. 1983. Nothofagus macrofossils from the Tertiary of Tasmania. Alcheringa 7: 169–183. Crossref
Hill, R.S. 1991 Tertiary Nothofagus (Fagaceae) macrofossils from Tasmania and Antarctica and their bearing on the evolution of the genus. Botanical Journal of the Linnean Society 105: 73–112. Crossref
Hill, R.S. 1992. Nothofagus: evolution from a southern perspective. Trends in Ecology and Evolution 7: 190–194. Crossref
Hill, R.S. 1994. History of selected Australian taxa. In: R.S. Hill (ed.), History of the Australian Vegetation: Cretaceous to Recent, 390–419. Cambridge University Press, Cambridge.
Hill, R.S. 2001 Biogeography, evolution and palaeoecology of Nothofagus (Nothofagaceae): The contribution of the fossil record. Australian Journal of Botany 49: 321–332. Crossref
Hill, R.S. and Jordan, G.J. 1993. The evolutionary history of Nothofagus (Nothofagaceae). Australian Systematic Botany 6: 111–126. Crossref
Hill, R.S. and Read, J. 1991. A revised infrageneric classification of Nothofagus (Fagaceae). Botanical Journal of the Linnean Society 105: 37–72. Crossref
Hill, R.S., Hardwood, D.M., and Webb, P.N. 1996. Nothofagus beardmorensis (Nothofagaceae), a new species based on leaves from the Pliocen Sirius group. Trans-antarctic Mountains, Antarctica. Review of Paleobotany and Palynology 94: 11–24. Crossref
Hill, R.S., Jordan, G.J., and Macphail, M.K. 2015. Why we should retain Nothofagus sensu lato? Australian Systematic Botany 28: 190–193. Crossref
Hinojosa, L.F., Gaxiola, A., Pérez, M.F., Carvajal, F., Campano, M.F., Quattrocchio, M., Nishida, H., Uemura, K., Yabe, A., Bustamante, R., and Arroyo, M.T. 2016. Non-congruent fossil and phylogenetic evidence on the evolution of climatic niche in the Gondwana genus Nothofagus. Journal of Biogeography 43: 555–567. Crossref
Hooker, J.D. 1844. Icones plantarum; or figures, with brief descriptive characters and remarks, of new or rare plants, selected from the authors herbarium. Plates 601–690. Baillière, London.
Hünicken, M. 1967. Flora terciaria de los estratos de Río Turbio, Santa Cruz (Niveles plantíferos del Arroyo Santa Flavia). Revista de la Facultad de Ciencias Exactas, Físicas y Naturales de la Universidad de Córdoba. Serie Ciencias Naturales 56: 139–262.
Iglesias, A. 2007. Estudio paleobotánico, paleoecológico y paleoambientalen secuencias de la Formación Salamanca, del Paleoceno Inferior en el sur de la Provincia de Chubut, Patagonia, Argentina. 244 pp. Ph.D. Dissertation, University of La Plata, La Plata.
Jones, J.H. 1986. Evolution of the Fagaceae: the implications of foliar features. Annals of the Missouri Botanical Garden 73: 228–275. Crossref
Jordan, G. 1999. A new Early Pleistocene species of Nothofagus and the climatic implications of co-ocurring Nothofagus fossils. Australian Systematic Botany 12: 757–76. Crossref
Jordan, G. and Hill, R.S. 1994. Past and present variability in leaf length of evergreen members of Nothofagus subgenus Lophozonia related to ecology and population dynamics. New Phytologist 127: 377–390. Crossref
Jordan, G. and Hill, R.S. 1999. The phylogenetic affinities of Nothofagus (Nothofagaceae) leaf fossils based on combined molecular and morphological data. International Journal of Plant Science 160: 1177–1188. Crossref
Krasser, F. 1896. Bemerkungen zur Systematik der Buchen. Annalen des Naturhístoríschen Hofmuseums Wien 11: 155–163.
Lanyon, S.M. 1985 Detecting internal inconsistencies in distance data. Systematic Zoology 34: 397–403. Crossref
Leppe, M., Hinojosa, F., Nishida, H., Dutra, T., Wilberger, T., Trevisan, C., Ortuya, M.J., Pino, J.P., Mansilla, H., and Lobos, V. 2016a. Asyncronic oldest record of Nothofagus leaves in Antarctica and Patagonia. Boletín de la Asociación Latinoamericana de paleobotánica y palinología 16: 1–321.
Leppe, M., Wilbeger T., Ortuya M.J., Ortiz, H. Bastías, J. Mansilla, H., and Dutra, T. 2016b. Oldest record of Nothofagus leaves in Nelson Island, South Shetland Islands, Antarctica: Implicances for biogeography. XXXIV SCAR Meetings and Open Science Conference, Kuala Lumpur, Abstract Book, 603. British Antarctic Survey, London.
Linnaeus, C. 1753. Species plantarum, exhibentes plantas rite cognitas, ad genera relatas, cum differentiis specificis, nominibus trivialibus, synonymis selectis, locis natalibus, secundum systema sexuale digestas. Tomus 1 and 2. xii+1200 pp. Laurentius Salvius, Stockholm.
Manos, P.S.1997. Systematics of Nothofagus (Nothofagaceae) based on rDNA spacersequences (ITS): taxonomic congruence with morphology and plastid sequences.American Journal of Botany 84: 1137–1137. Crossref
Manos, P.S., Soltis, P.S., Soltis, D.E., Manchester, S.R., Oh, S.H., Bell, C.D., Dilcher, D.L., and Stone, D.E. 2007. Phylogeny of extant and fossil Juglandaceae inferred from the integration of molecular and morphological data sets. Systematic Biology 56: 412–430. Crossref
Martin, P.G. and Dowd, J.M. 1993. Using sequences of rbcL to study phylogeny and biogeography of Nothofagus species. Australian Systematic Botany 6: 441–447. Crossref
Melville, R. 1982. The biogeography of Nothofagus and Trigonobalanus and the origin of the Fagaceae. Botanical Journal of the Linnean Society 85: 75–88. Crossref
Mirbel, C.F.B. 1827. Recherches sur la distribution géographique des Végétaux phanérogames de l’Ancien-Monde, depuis l’équateur jusqu’au pôle arctique; suivies de la description de neuf espèces de la famille des Amentacées. Mémoires du Museum d’histoire naturelle de Paris 14: 349–474.
Nixon, K.C. 1982. In support of the Nothofagaceae Kuprianova. Botanical Society of America Miscellaneous Series 162: 102.
Nixon, K.C. 1989. Origins of Fagaceae. In: P.R Crane and S. Blackmore (eds.), Evolution, Systematics, and Fossil History of the Hamamelidae, 23–43. Clarendon Press, Oxford.
Nixon, K.C. and Carpenter, J.M. 1996. On simultaneous analysis. Cladistics 12: 221–241. Crossref
Oersted, S.A. 1871. Bidrag til Kundskab om Egefamilien i Nutid og Fortid.Kongelige Danske Videnskabernes Selskabs Skrifter 9: 31–538.
Paull, R. and Hill, R.S. 2003. Nothofagus kiandrensis (Nothofagaceae subgenus Brassospora), a new macrofossil leaf specie from Miocene sediments at Kienadra, New South Wales. Australian Systematic Botany 16: 549–559. Crossref
Peterson, K.R, Pfister, D.H., and Bell, C. 2010. Cophylogeny and biogeography of the fungal parasite Cyttaria and its host Nothofagus, southern beech. Mycologia 102: 1417–1425. Crossref
Phillipi, R.A. 1858. Plantarum novarum Chilensium: Centuria quarta. Linnaea 29: 1–47.
Philipson, W.R. and Philipson, M.N. 1988. A classification of the genus Nothofagus (Fagaceae). Botanical Journal of the Linnean Society 98: 27–36. Crossref
Poeppig, E.F. and Endlicher, S.F.L. 1838. Nova genera ac species plantarum quas in regno Chilensi Peruviano et in terra Amazonica. Volumen secundum. 74 pp. F. Hofmeister, Leipzig.
Pol, D. and Norell, M.A. 2006. Uncertainty in the age of fossils and the stratigraphic fit to phylogenies. Systematic Biology 55: 512–521. Crossref
Pol, D. and Escapa, I.H. 2009. Unstable taxa in cladistic analysis: identification and the assessment of relevant characters. Cladistics 25: 515–527. Crossref
Poole, I. 2002. Systematics of Cretaceous and Tertiary Nothofagoxylon: implications for Southern Hemisphere biogeography and evolution of the Nothofagaceae. Australian Systematic Botany 15: 247–276. Crossref
Poole, I. and Cantrill, D. J. 2006. Cretaceous and Cenozoic vegetation of Antarctica integrating the fossil wood record. Geological Society, London, Special Publications 258: 63–81. Crossref
Poole, I., Hunt, R.H., and Cantrill, D.J. 2001. A fossil wood flora from King George Island: ecological implications for Antarctic Eocene vegetation. Annals of Botany 88: 33–54. Crossref
Premoli, A.C. 1996. Leaf architecture of South American Nothofagus (Nothofagaceae) using traditional and new methods in morphometrics. Botanical Journal of the Linnean Society 121: 25–40. Crossref
Premoli, A.C., Mathiasen, P., Acosta, M.C., and Ramos, V.A. 2011. Phylogeographically concordant chloroplast DNA divergence in sympatric Nothofagus s.s. How deep can it be? New Phytologist 193: 261–275. Crossref
R Core Team 2017. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. http://www. R-project.org/.
Read, J. 1990. Some effects of acclimation temperature on net photosynthesis in some tropical and extra-tropical Australasian Nothofagus species. The Journal of Ecology 78: 100–112. Crossref
Reguero, M., Goin, F., Acosta Hospitaleche, C., Marenssi, S., and Dutra, T. 2013. The terrestrial biotic dimension of West Antarctica (WANT). In: M. Reguero, F. Goin, C. Acosta Hospitaleche, T. Dutra, and S. Marenssi (eds.), Late Cretaceous/Paleogene West Antarctica Terrestrial Biota and its Intercontinental Affinities, 55–110. Springer, Dordrecht.
Romero, E.J. 1986. Fossil evidence regarding the evolution of Nothofagus Blume. Annals of the Missouri Botanical Garden 73: 276–283. Crossref
Romero, E.J. and Dibbern, M.C. 1985. A review of the species described as Fagus and Nothofagus by Dusén. Palaeontographica Abteilung B 197: 123–137.
Roth, A.W. 1788. Tentamen Florae Germanieae. Vol. 1. 560 pp. I.G. Mülleriano, Leipzig.
Sauquet, H., Ho, S.Y., Gandolfo, M.A., Jordan, G.J., Wilf, P., Cantrill, D., Bayly, M.J., Bromham, L., Brown, G., Carpenter, R., Lee, D., Murphy, D., Kale Sniderman, J.M., and Udovicic, F. 2012. Testing the impact of calibration on molecular divergence times using s fossil-rich group: the case of Nothofagus (Fagales). Systematic Biology 61: 289–313. Crossref
Setoguchi, H., Ono, M., Doi, Y., Koyama, H., and Tsuda, M. 1997. Molecular phylogeny of Nothofagus (Nothofagaceae) based on the atpB-rbcL intergenic spacer of the chloroplast DNA. Journal of Plant Research 110: 469–484. Crossref
Springer, M.S., Teeling, E.C., Madsen, O., Stanhope, M.J., and de Jong, W.W. 2001. Integrated fossil and molecular data reconstruct bat echolocation. Proceedings of the National Academy of Sciences of the United States of America 98: 6241–6246. Crossref
Steenis, C.G.G.J. van 1953. Results of the Archbold expeditions: Papuan Nothofagus. Journal of the Arnold Arboretum 34: 301–374. Crossref
Swenson, U., Hill, R.S., and McLoughlin, S. 2000. Ancestral area analysis of Nothofagus (Nothofagaceae) and its congruence with the fossil record. Australian Systematic Botany 13: 469–478. Crossref
Tanai, T. 1986. Phytogeographic and phylogenetic history of the genus Nothofagus Bl. (Fagaceae) in the southern hemisphere. Journal of the Faculty of Science, Hokkaido University, Series 4: Geology and Mineralogy 21: 505–582.
Torres, T., Cisterna, M., Llanos, A., Galleguillos, H., and Le Roux, J.P. 2009. Nuevos registros de Nothofagus Bl. en Sierras Baguales, Última Esperanza, Patagonia, Chile. XII Congreso Geológico Chileno. Extended Abstracts, 12–19. Universidad de Chile, Santiago de Chile.
Torres, T., Gutiérrez, N.M., Bostelmann, E., Le Roux, J.P., Oyarzún, J.L., Ugalde, R., Otero, R., and Hervé, F. 2013. Exceptionally preserved fossil flora of the Río Leona Formation in Sierra Baguales, Magallanes, Chile: insights into the early Neogene Patagonian ecosystems. Bollettino di Geofisica teorica ed applicata (Supplement B) 54: 352–355.
Troncoso, A. 1991. Paleomegaflora de la Formación Navidad, Miembro Navidad (Mioceno), en el área de Matanzas, Chile central occidental. Boletín Museo Nacional de Historia Natural 42: 131–168.
Troncoso, A. and Encinas, A. 2006. La tafoflora de cerro Centinela (Chile, VI Región): vegetación y clima de Chile central a fines del Mioceno-comienzos del Plioceno. Ameghiniana 43: 171–180.
Veblen T.T., Donoso, C., Kitzberger T., and Rebertus, J. 1996. Ecology of southern Chilean and Argentinean Nothofagus forests. In: T.T. Veblen, R.S. Hill, and J. Read (eds.), The Ecology and Biogeography of Nothofagus Forests, 293–353. Yale University Press, New Haven.
Vento, B., Gandolfo, A., Nixon, K., and Prámparo, M. 2017. Paleofloristic assemblage from the Paleogene Río Guillermo Formation, Argentina: Preliminary results of phylogenetic relationships of Nothofagus in South America. Historical Biology 29: 93–107. Crossref
Vento, B. and Prámparo, M. 2018. Angiosperm association from the Río Turbio Formation (Eocene–?Oligocene) Santa Cruz, Argentina: Revision of Hünicken’s (1955) fossil leaves collection. Alcheringa: An Australasian Journal of Palaeontology 42: 125–153. Crossref
Acta Palaeontol. Pol. 63 (4): 815–825, 2018
https://doi.org/10.4202/app.00493.2018