

Ecomorphology and bone microstructure of Proterochampsia from the Chañares Formation
ANDREA ARCUCCI, ELENA PREVITERA, and ADRIANA C. MANCUSO
Arcucci, A., Previtera, E., and Mancuso, A.C. 2019. Ecomorphology and bone microstructure of Proterochampsia from the Chañares Formation. Acta Palaeontologica Polonica 64 (1): 157–170.
Proterochampsians are a South American endemic group of non-archosaurian archosauriforms with morphological characteristics recollecting Recent crocodilians, and therefore have been proposed as aquatic species. However, this has not been based on careful examination of anatomical and histological features. We provide a review of the morphological and histological evidence present in the skeleton of proterochampsids and discuss its implications for inferring the lifestyles of these organisms. Anatomical features such as a secondary palate, marginal dentition, palatine teeth, morphology of the tail, limb modification, and dermal armor are reviewed, and details of histological structures are described based on bone thin sections. Histological examination reveals a predominance of fibrolamellar bone tissue, suggesting rapid periosteal osteogenesis and therefore overall fast bone growth. The existence of discontinuities (LAGs) demonstrates that these animals responded to changes in their environment. Ecomorphological features do not provide definitive evidence for the lifestyles of proterochampsids, but allow us to propose a terrestrial/amphibious condition. The same is true of the histological features, particularly compactness of the bone.
Key words: Archosauriformes, Proterochampsia, paleobiology, ecomorphology, bone microstructure, Carnian, Argentina.
Andrea Arcucci [andrea.arcucci@gmail.com], Área de Zoología, Universidad Nacional de San Luis, IMIBIO CONICET, Av. Ejercito de los Andes 950, 5700 San Luis, Argentina.
Elena Previtera [elena.previtera@gmail.com] and Adriana C. Mancuso [amancu@mendoza-conicet.gov.ar] (corresponding author), Instituto Argentino de Nivología, Glaciología y Ciencias Ambientales (IANIGLA), CCT-Mendoza, CONICET. Av. Adrián Ruiz Leal s/n, Parque General San Martín CC 330 (CP 5500) Mendoza, Argentina.
Received 24 August 2018, accepted 19 November 2018, available online 6 February 2019.
Copyright © 2019 A. Arcucci et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Proterochampsians are a South American endemic group of non-archosaurian archosauriforms. Their phylogenetic affinities were uncertain for decades, but more recently they have been considered as one of the potential successive sister-taxa of crown group Archosauria (e.g., Sereno and Arcucci 1990; Dilkes and Sues 2009; Ezcurra et al. 2010; Dilkes and Arcucci 2012; Trotteyn et al. 2013; Nessbitt et al. 2013).
Proterochampsians not only have a limited geographic distribution, with taxa exclusively recovered from Argentina and Brazil, but also a restricted temporal distribution, as their origin, diversification and disappearance happened in a very short period of time in the Late Triassic, almost entirely in the Carnian (237–227 Ma). This clade is represented in Argentina by Gualosuchus reigi Romer, 1971, Chanaresuchus bonapartei Romer, 1971, and Tropidosuchus romeri Arcucci, 1990 from the early Carnian Chañares Formation (Marsicano et al. 2016), and by Proterochampsa barrionuevoi Reig, 1959 and Pseudochampsa ischigualastensis Trotteyn and Ezcurra, 2014 from late Carnian–early Norian Ischigualasto Formation (Martínez et al. 2011) from the Ischigualasto-Villa Unión Basin (Fig. 1). In Brazil, the group is represented by Chanaresuchus bonapartei Romer, 1971 from the lower portion of Santa Maria Formation, Rhadinosuchus gracilis Huene, 1938 and Cerritosaurus bindfeldi Price, 1946 from the early Carnian upper portion of the Santa Maria Formation (Langer et al. 2018), and by Proterochampsa nodosa Barberena, 1982 from the early Norian Caturrita Formation of the Paraná Basin (Fig. 1) (Langer et al. 2018).
Proterochampsians are medium-size quadrupedal forms, with an elongated and triangular snout and dorsally flattened head, a distinctive pattern of dermal ornamentation of the skull, and approximately dorsally located orbits and external nares. These morphological characteristics resemble those of Recent crocodilians, which has led several authors to suggest a probable aquatic lifestyle for proterochampsians (Reig 1959; Sill 1967; Bonaparte 1971; Romer 1971; Barberena 1982; Bonaparte 1997; Kischlat 2000; Hsiou et al. 2002).
Ecomorphological studies based on morphological structures, using an integrated combination of form, function, and ecology, have been developed over the last few decades by many authors in various biological disciplines (Bock 1994; Betz 2006). The term ecomorphology was coined in the 1940s (Van der Klaauw 1948) and primarily referred to patterns of resource use by organisms, and their capacity to use resources from their external environment. Ecomorphology centeres then on one fundamental question in ecology: which factors determine the actual resource use of organisms? We use this integrative approach with regard to the form and function of organisms and their relationship to the external environment in the present paper to assess evidence about the habitat (terrestrial or aquatic) of proterochampsians, based on the characteristics of their bone histological morphology and microstructure.
Bone microstructure exhibits many characteristics that reflect physiological or ecological properties of taxa such as locomotor parameters (Casinos 1996), growth trajectory (Castanet et al. 1993; Rimblot-Baly et al. 1995), lifestyle (Buffrènil et al. 1987, 1990a, b; Laurin et al. 2004, 2007; Germain and Laurin 2005), and metabolism (Ricqlès 1983). However, little is currently known about the bone histology of proterochampsians. The only published work includes a short description of the histology of a long bone from Chanaresuchus sp. (Ricqlès et al. 2008) and reports on the histology of some osteoderms of Pseudochampsa ischigualastensis, and Chanaresuchus bonapartei (Cerda et al. 2015; Ponce et al. 2017).
There has been a wealth of recent work on the relationship between bone microstructure and lifestyle (e.g., Fish and Stein 1991; Stein 1989; Wall 1983; Buffrénil et al. 2010; Kriloff et al. 2008; Ricqlès 1974a, b; Germain and Laurin 2005; Nopcsa and Heidsieck 1934; Steyer et al. 2004). Furthermore, some of these studies have analyzed the evolution of bone microanatomy in response to habitat. Most of these studies focus on extant lissamphibian and amniotes, which has confirmed the link between bone microanatomy and lifestyle. A similar approach can be used with extinct species, and the histology of long bones has been used to infer the lifestyle of fossil taxa (Kriloff et al. 2008; Houssaye et al. 2013).
Over the last few years, several important contributions about the anatomy and phylogeny of proterochampsids have been published (e.g., Trotteyn 2011; Dilkes and Arcucci 2012; Trotteyn et al. 2013; Raugust et al. 2013; Trotteyn and Ezcurra 2014; Ezcurra et al. 2015), but paleobiological and paleoecological hypothesis about the group are still scarce. Aquatic or amphibious lifestyles were traditionally accepted for proterochampsians based on convergent/homoplasic characters with extant crocodiles, but this has not been rigorously tested.
We here evaluate the anatomical and ecomorphological features of proterochampians and their relationship with paleoenvironment, and provide detailed descriptions of bone microstructure. Together, these lines of evidence provide new information to infer the lifestyles of proterochampaians.
Institutional abbreviations.—CRILAR-PV, Centro Regional de Investigaciones de La Rioja, Argentina; PULR-V, Museo de Ciencias Antropológicas y Naturales, Universidad Nacional de La Rioja, Argentina.
Other abbreviations.—EFS, External Fundamental System; ICL, inner circumferential lamella; LAG, line of arrested growth.
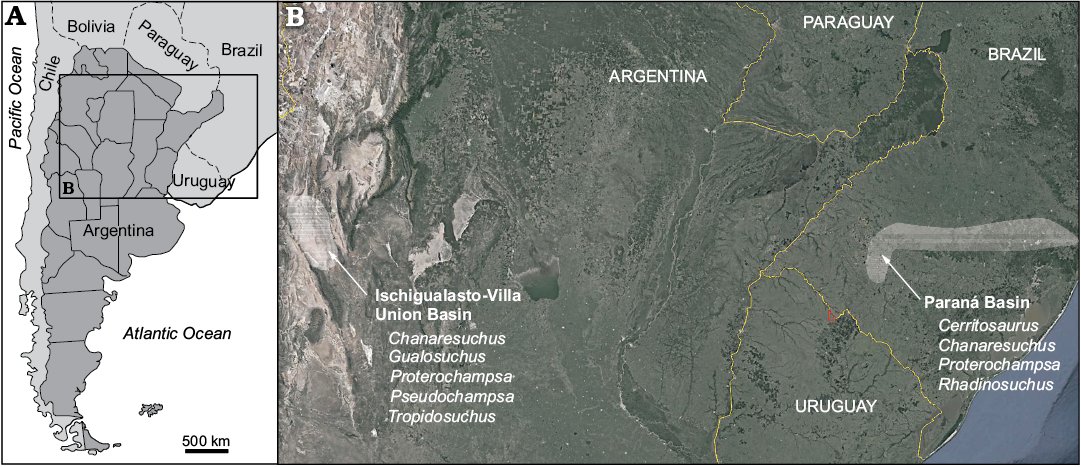
Fig. 1. A. Geographic location of the studied basins. B. Map showing location of Ischigualasto-Villa Union Basin (Argentina) and Paraná Basin (Brazil) and presence of the proterochampsids taxa. Satellite image taken from Google Earth.
Material and methods
The studied material was collected in the last ten years from the Río Gualo locality, Talampaya National Park, La Rioja Province, Argentina (Fig. 2). The specimens were recovered from different stratigraphic levels and different lithologies (Fig. 2), light olive-grey, fine-grained sandstone with horizontal and planar cross stratification and grey fine-grained sandstone and siltstone considered as fining-upwards sequence of channels derived from the hanging wall, and from laterally continuous, structureless, light-grey mudstone strata assigned to littoral low-energy mudflat of the Chañares Formation (Mancuso 2005; Mancuso et al. 2014, 2017). The section with fossiliferous horizons was recently dated in 236.1 ± 0.6 Ma (early Carnian) by Marsicano et al. 2016.
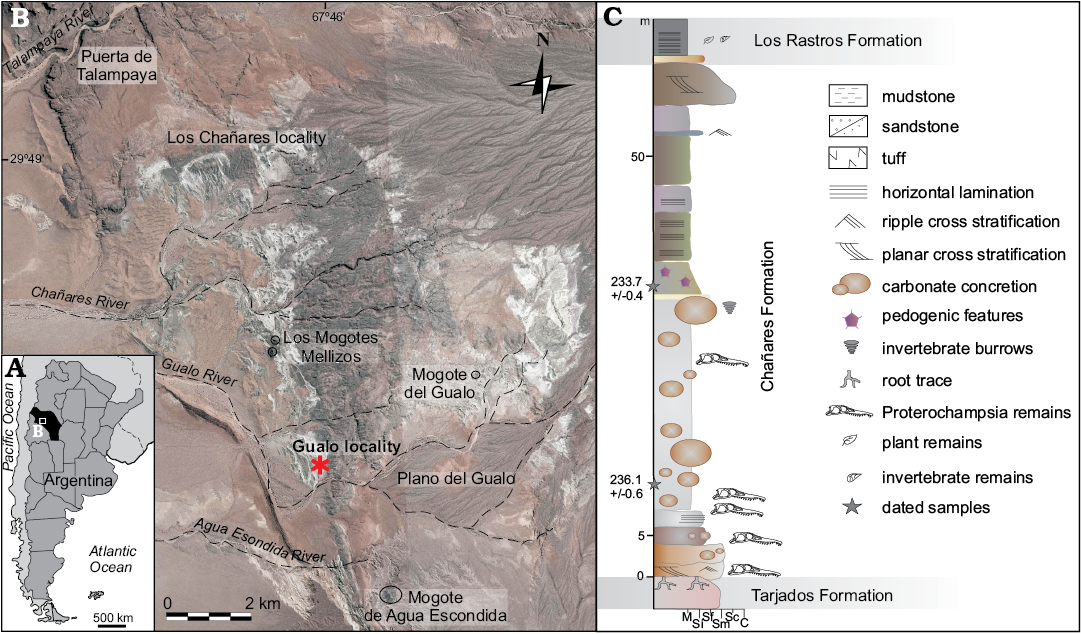
Fig. 2. A. Geographic location of the Río Chañares-Río Gualo area in the La Rioja Province (in black), Argentina. B. Map showing location of the studied area in the Talampaya National Park; Gualo locality asterisked. C. Stratigraphic section of the Chañares Formation at the Gualo locality with the samples locations. Abbreviations: M, mudstone; Si, silstone; Sf, fine-grained sandstone; Sm, medium-grained sandstone; Sc, coarse-grained sandstone; C, conglomerate.
Bone tissue samples were extracted from long bones belonging to proterochampsian specimens PULR-V 117 (fibula); PULR-V 118, CRILAR-PV 603, CRILAR-PV 605 (femora); and PULR-V 117, PULR-V 119, CRILAR- PV 604 (tibiae) from the Chañares Formation. All sections from long bone shafts are transverse. It should be noted that the proximal tibia (PULR-V 117) and proximal fibula (PULR-V 117) were sectioned close to the metaphyseal region. Thin sections were prepared following the method outlined by Chinsamy and Raath (1992). Samples were observed and photographed under a petrographic microscope BX 51-P Olympus both in plane and cross-polarized light. General histological nomenclature and definitions of structures follow Francillon-Vieillot et al. (1990).
The sampled elements are appendicular bones that show proterochampsian diagnostic characters, but they did not display autopomorphies that would allow us to identify particular Chañares taxa. However, the sampled elements are a good representation of the different taxa sizes known from Chañares (Tropidosuchus ~3 kg, Gualosuchus ~10 kg, and Chanaresuchus ~20 kg, see Mancuso et al. 2014).
There is no single protocol to determine the ecomorphology of fossil organisms, but Betz (2006) proposed a general framework in which morphology (structure or form of the organism or parts under study) can be used to interpret the possible function or behaviour of the animal, allowing workers to propose potential resource uses or biological roles for extinct species. We use this approach here, and in doing so, selected possible analogues of key structures of proterochampsians in extant organisms.
Results
Anatomical and ecomorphological features.—As mentioned before, in the particular case of proterochampsids it was apparently obvious for many authors that they were aquatic or at least amphibious organisms (e.g., Price 1946; Reig 1958; Sill 1967; Romer 1971; Bonaparte 1978). Although these authors mention certain cranial features to interpret that way of life in this group, neither of them examined the evidence or discussed the characters either from the skull or from the rest of the skeleton. We made a brief review based on first hand studies on the material (Arcucci 2011) about the morphological features present in the skeleton of proterochampsids (Table 1, Fig. 3) and its pertinence to interpretation a particular mode of life of these organisms.
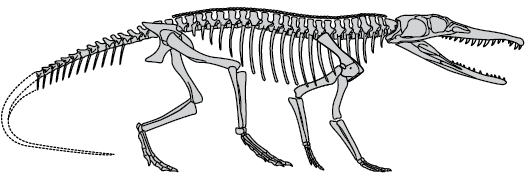
Fig. 3. Reconstruction of the skeleton of Chanaresuchus Romer, 1971 (modified from Mancuso et al. 2014).
Skull (Table 1, Fig. 3): The skull has a triangular shape, with long and acute snout, dorsoventrally flattened, with dorsal orientation of the cranial fenestrae. The palate structure, with posteriorly located choanae, with the development of a secondary bony palate, formed by premaxilla, vomers and pterygoids, reaches the anterior edge of the ectopterygoids. Strong ossification and the ornamentation of the dorsal surface of the skull are anatomical features that are traditionally interpreted as indications of aquatic habit both in Recent and fossil taxa (Romer 1966; Carroll 1985). In proterochampsids, the flattened skull is related to an inclination of the angle of the quadrate to the posterior part of the skull. The extreme condition of this feature is present in Proterochampsa, in which several well preserved articulated skulls show the same remarkable dorsal flattening and dorsal orientation of the orbits and temporal openings (Dilkes and Arcucci 2012). These features have not resulted from a distortion because they are present in all available specimens (Reig 1958; Sill 1967; Arcucci 2011; Trotteyn et al. 2013). In the rest of the proterochampsids (Cerritosaurus, Chanaresuchus, Gualosuchus, Pseudochampsa, Tropidosuchus, and Rhadinosuchus), the inclination of the quadrate is not so strong and this make the skull less flattened (Romer 1971; Arcucci 1990, 2011). The skull openings, including the orbits, are located more laterally in these forms. They kept the proportional length of the snout (premaxilla, maxilla and mandible) and the ornamentation of the dorsal surface of the skull although the pattern of ornamentation is quite different and more subtle, in shape of longitudinal crests instead of the tubercle pattern of Proterochampsa. The marginal dentition of premaxilla and maxilla is formed by numerous conical and laterally compressed and serrated teeth. All these features display resemblance to the ones known form Recent aquatic animals as gavials and interpreted as related to a piscivorous diet (Romer 1966; Weems 1980; Kardong 1999). This type of dentition is present in all proterochampsids, although there is a lesser number of teeth in maxilla and dentary in Proterochampsa in particular (Dilkes and Arcucci 2012). If the high number of teeth is an indication of aquatic habit, then in Proterochampsa it suggests otherwise.
Axial skeleton (Table 1, Fig. 3): In proterochampsids as a group, the most remarkable features are included in the last part of the vertebral column. The caudal series of proterochampsids are known from species of Chanaresuchus and Tropidosuchus. The neural spine in these taxa is moderately low, at approximately the same height of the body of the vertebrae and the transverse processes becomes progressively much longer and wider in the middle part of the tail and reduced again towards its end (Troteyn et al. 2013: fig. 16). This feature makes the profile of the tail much wider than tall, or dorsoventrally flattened.
Appendicular skeleton (Table 1, Fig. 3): In proterochampsids, there are no signs of any kind of reduction or particular enlargement of the limbs, or modification of any part of the general pattern of the basal archosauriomorphs. The posterior limb is approximately one third longer than the anterior limb, as a whole, and is possible to observe this proportions in several available specimens (Arcucci 2011; Troteyn et al. 2013). The femur in particular is approximately 20% longer than the humerus (Arcucci 2011), and the tibia in Chanaresuchus, Gualosuchus, and Proterochampsa (Romer 1971; Trotteyn et al. 2013), but femur and tibia are subequal in Tropidosuchus (Arcucci 1990; Troteyn et al. 2013: fig. 16). The femur have a characteristic dorsoventrally extense laminar fourth trochanter, that projects laterally into a “wing” which indicates strong muscular development for tail movements. The tibia is more robust than the femur and has a thick and short cnemial crest, in all the specimens preserved. The feet is well preserved in Chanaresuchus, Proterochampsa, and Tropidosuchus, but the manus in this group is known only from fragments. The feet of the proterochampsids have unique features among archosauriforms or even among archosaurs. The pattern of the digits is asymmetric where the first and second digits are the shortest and strongest and the fourth digit is the longest of the foot although only for a few millimeters.
Dermal armor (Table 1, Fig. 3): Dermal armor in proterochampsids is poorly developed, or, as in the case of Proterochampsa, it is absent. It consist of a single row of small osteoderms, of approximately rounded shape with approximately two osteoderms located on the top of each vertebrae. The exception is Tropidosuchus (Arcucci 1990; Trotteyn et al. 2013) in which the osteoderms are much bigger in proportion to the body of the animal, and each of them occupies the dorsal part of one vertebrae.
Table 1. Main morphological features of proterochampsids from Chañares and Ischigualasto Formation mentioned in the text (from Arcucci 2011; Troteyn et al. 2013).
| |
Gualosuchus reigi |
Chanaresuchus bonapartei |
Tropidosuchus romeri |
Proterochampsa barrionuevoi |
|
Cranial morphology |
||||
|
triangular skull |
yes |
yes |
yes |
yes |
|
dorsally flattened |
no |
medium |
medium |
yes |
|
dorsally located orbits |
no |
no |
no |
yes |
|
external nares |
no |
no |
no |
yes |
|
choanae location |
medium |
medium |
medium |
medium |
|
secondary palate |
simple |
simple |
simple |
simple |
|
skull ornamentation |
crest and ridges |
crest and ridges |
crest and ridges |
strong tubercles |
|
Post-cranial morphology |
||||
|
caudal region |
low and wide |
low and wide |
low and wide |
low and wide |
|
limb reduction |
no |
no |
no |
no |
|
foot reduction |
no |
no |
no |
no |
|
foot asymmetry |
? |
yes |
yes |
? |
|
dermal scutes |
medium |
smaller than vertebrae |
big than vertebrae |
absent |
Histological descriptions.—This section includes a detailed description of the histology features of each specimen. Because our sample is limited, it cannot represent the variety of tissues deposited in all parts of the skeleton, as well as all stages of growth. However, in the describing histological structures, we offer functional and ontogenetic mechanisms that may explain the presence and development of these structures.
Femur (CRILAR-PV 603, Fig. 4B): The cortex is made of homogenous fibrolamellar tissue with a typical plexiform vascularization. In some places, the plexiform pattern is less regular and has fewer radial canals (sub-plexiform). The dense vascularization decreases towards the periphery. There is no evidence of secondary osteonal development in any area of the element. External Fundamental System (EFS), or similar structures are not observed. A very thin coat of endosteal bone surrounds the free marrow cavity. A faint LAG may be seen at mid-cortical region. The cortex comprises a typically fast-growing tissue deposited continually. This tissue resembles tissues found in large birds and dinosaurs (Horner et al. 2000; Reid 1996).
Femur (PULR-V 118, Fig. 4C): The cortex is entirely fibrolamellar densely vascularized by longitudinal primary osteons. They are scattered in a woven bone matrix of periosteal origin. No LAGs nor annuli are developed in the cortex. There are no secondary osteons in the deep cortex. The large medullary region expands into the deep cortex through a generalized erosion, and is composed of endosteal bony trabeculae and erosion cavities.
Femur (CRILAR-PV 605, Fig. 4D): The compacta is densely vascularized and it is constituted by uninterrupted fibrolamellar bone tissue. Vascular canals are mainly represented by primary osteons, and are longitudinally oriented. These canals are concentrated at the posterolateral portion of the bone, but towards the subperiosteum their number decreases. Osteocyte lacunae are mainly oriented around the primary osteons in the inner and middle cortex at the posterolateral side of the bone. A high density of osteocyte lacunae is present throughout the cortex, however their density slightly decreases from the inner to outer cortex at the posterolateral side of the bone. At least two well-defined LAGs are seen in the outer and mid-cortical region. There are some secondary osteons in the inner cortex. The cortical region is surrounded by a thin layer of endosteally deposited lamellar bone tissue that forms the inner circumferential lamella (ICL). The latter is limited by a large medullary region composed of endosteal bony trabeculae and erosion cavities.
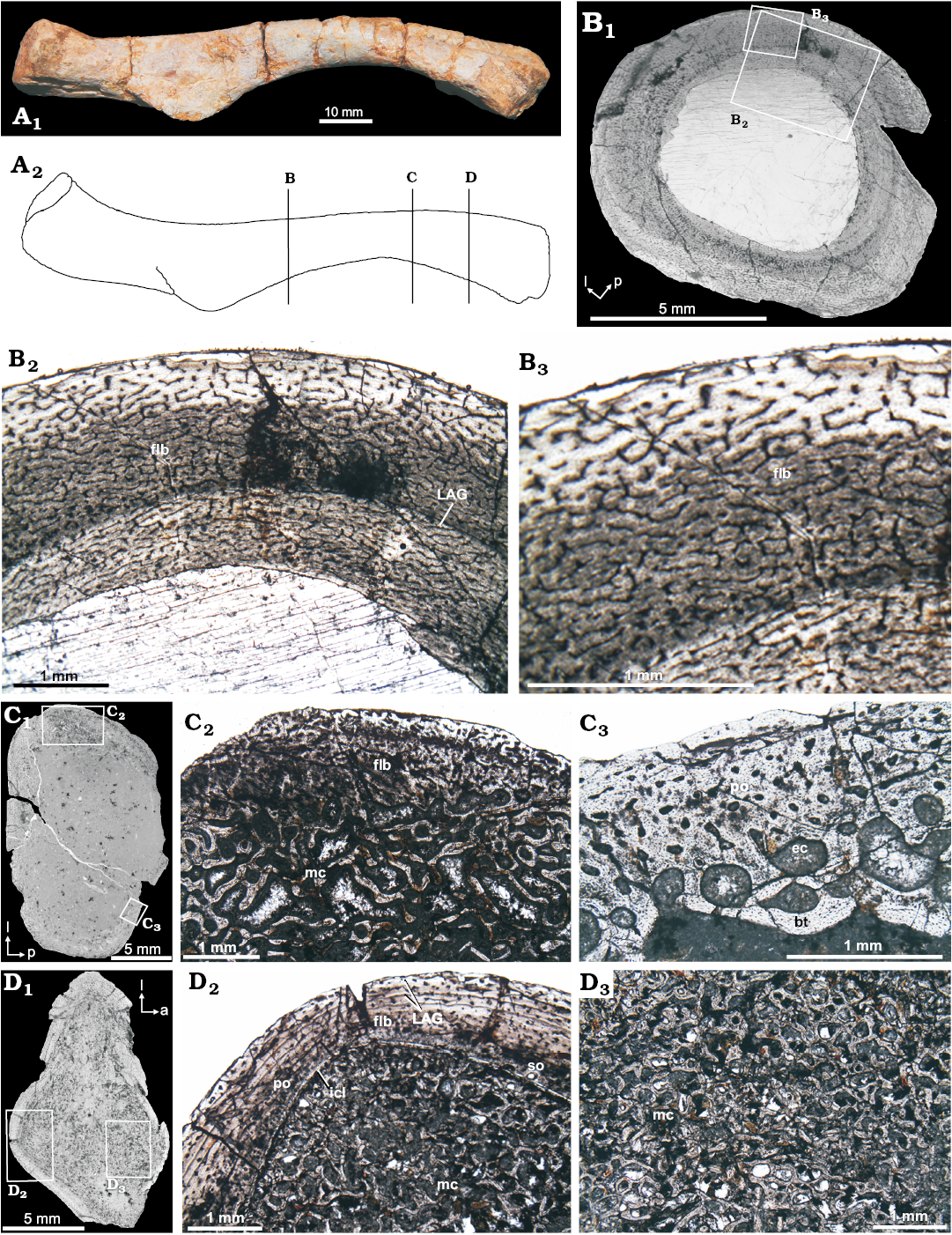
Fig. 4. Femur microstructure of Proterochampsia indet. from the early Carnian Chañares Formation at the Río Gualo locality. A. CRILAR-PV 603 in anterior view; photograph (A1), explanatory drawing (A2); lines indicate the position of the cross-sections (redrawn from Romer 1972). B. CRILAR-PV 603 cross-sections, general view (B1); details (B2, B3), note dense vascularization of the compacta conformed by a fibrolamellar matrix (flb) surrounded by a very thin layer of endosteal bone; one line of arrested growth (LAG) is observed at mid-cortical region; the fibrolamellar cortex with radial anastomoses defining the plexiform organization. C. PULR-V 118, general view (C1); details (C2, C3), showing the fibrolamellar bone (flb) conformed by numerous longitudinal primary osteons that are embedded in a woven matrix is limited by the medullary cavity (mc); primary osteons (po) in the outer cortex, as well as, erosion cavities (ec) and endosteal bony trabeculae (bt) in the medullary region (mc). D. CRILAR-PV 605, general view (D1); details (D2, D3), showing the dense vascularization of the compacta conformed by a fibrolamellar matrix (flb) surronded the inner circumferential layer (icl); primary osteons (po) and secondary osteons (so) are observed in the outer and inner cortex, respectively; two LAGs is observed at mid-cortical region; the medullary region (mc) composed of endosteal bony trabeculae and erosion cavities. Photomicrographs in plane-polarized light (B2, B3, C2, C3, D2, D3); photographs (B1–D1). Abbreviations: a, anterior; l, lateral; p, posterior.
Tibia (PULR-V 119, Fig. 5B): The thick cortical compacta is densely vascularized, and composed entirely of fibrolamellar tissue. Vascular canals are represented by primary osteons are longitudinally and reticularly oriented. Primary osteons are encircled by thin bands of parallel-fibered bone tissue, birrefringent under cross-polarized light that reflects the mainly concentric character of the fibers. LAGs are present in the outer and mid-cortical region. The inner cortex contains secondary osteons easily distinguishable by the cementation lines. The dense vascularization decreases towards the periphery. The subperiosteum is composed of parallel-fibered thin avascular layers, suggesting of some cyclical modulations of bone deposition dynamics (Ricqlès et al. 2003). The medullary cavity is lined by a thin layer of endosteal lamellar bone tissue forming the ICL.
Proximal tibia (PULR-V 117, Fig. 5D): The cross section of tibia shows a peculiar histological structure, perhaps linked to a metaphyseal rather than truly diaphyseal position. Although the tibia is diagenetically altered, it shows a thin cortex formed by a poorly defined fibrolamellar bone with a moderate vascularization that varies locally. Other features of this bone includes extensive development of a secondary endosteal spongiosa in the marrow cavity, irregular subperiosteal periphery, absence of growth marks (annuli and LAGs) and a high cortical porosity.
Tibia (CRILAR-PV 604, Fig. 5E): The cortex is relatively thin and primary in structure. The fibrolamellar tissue is formed by small primary osteons, which are encircled by thin bands of parallel-fibered bone tissue. The deepest cortex is invaded by secondary osteons. Extensive development of a secondary osteons in the inner cortex. The large medullary region expands into the deep cortex through a generalized erosion, and is composed of endosteal bony trabeculae and erosion cavities.
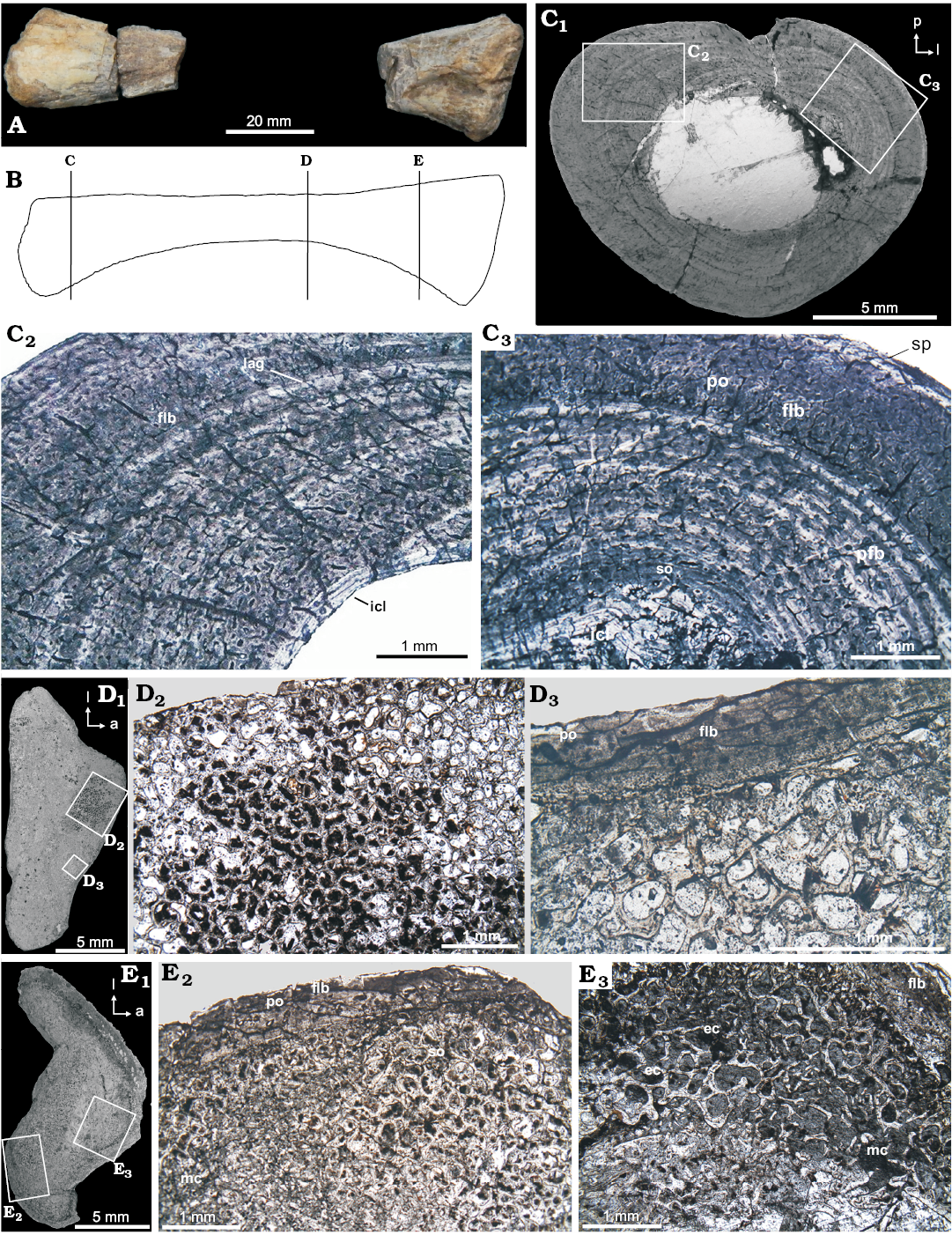
Fig. 5. Tibia microstructure of Proterochampsia indet. from the early Carnian Chañares Formation at the Río Gualo locality. A. PULR-V 119 in anterior view; photograph. B. Idealized explanatory drawing (not to scale), lines indicate the position of the cross-sections (redrawn from Romer 1972). C. PULR-V 119, cross-sections, general view (C1); details (C2, C3) showing the dense vascularization of the compacta conformed by longitudinal canals, embedded in a fibrolamellar matrix (flb). The medullary cavity is delimited by an inner circumferential layer (icl); the fibrolamellar tissue delimited by a thin avascular layer in the subperiosteum (sp) and by an inner circumferential layer (icl). Primary osteons (po) and secondary osteons (so) are observed in the outer and inner cortex, respectively. D. PULR-V 117, cross-sections, general view (D1); details (D2, D3), showing a general view of a region on the proximal “epiphyseal” surface; the fibrolamellar bone (flb) conformed by primary osteons (po). Extensive development of a secondary endosteal spongiosa. E. CRILAR-PV 604, general view (E1); details (E2, E3), showing the compacta conformed by fibrolamellar tissue (flb) with primary osteons (po); extensive development of a secondary osteons (so) in the inner cortex; the medullary region composed of endosteal bony trabeculae and erosion cavities (ec). Photomicrographs in plane-polarized (C2, D2, D3, E2) and cross-polarized light (C3, E3); photographs (C1–E1). Abbreviations: a, anterior; l, lateral; p, posterior.
Proximal fibula (PULR-V 117, Fig. 6): The fibula unfortunately suffered poor histological preservation through intensive diagenesis. Nevertheless, in some local areas, the bone tissue is still preserved. The fibula thin section is likely to belong to the metaphyseal region, as it has characteristics of the proximal tibia (Fig. 5D) of the same taxon. The narrow compact outer cortex is formed by an ill-defined fibrolamellar complex with longitudinal primary osteons, some secondary osteons and an extensive occurrence of resorption cavities in the mid- to inner cortical region. Growth marks are absent.
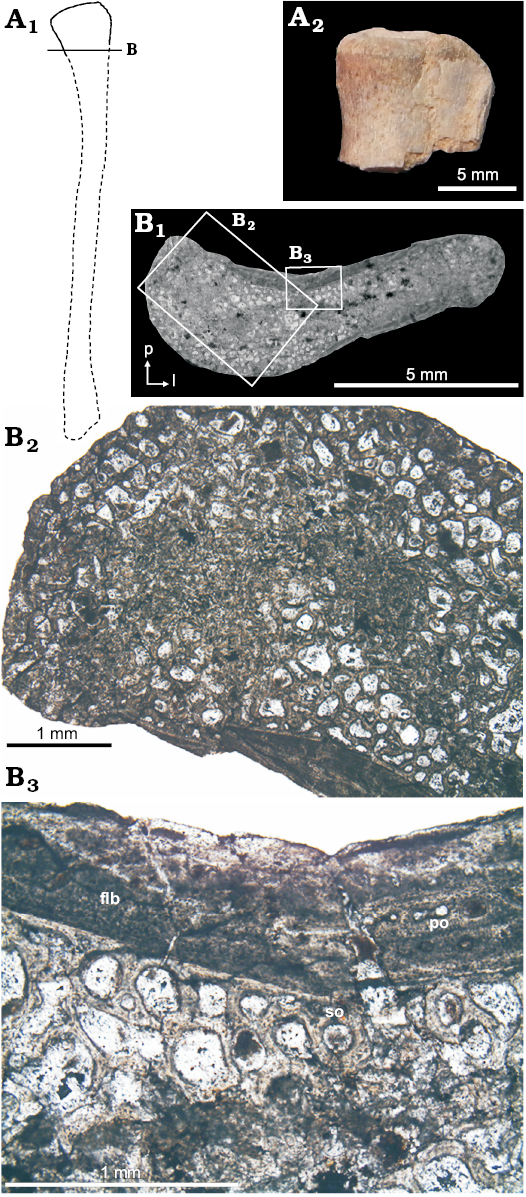
Fig. 6. Fibula microstructure of Proterochampsia indet. from the early Carnian Chañares Formation at the Río Gualo locality. A. PULR-V 117, in anterior view; photograph of proximal part (A2), explanatory drawing (A1); lines indicate the position of the cross-sections (redrawn from Romer 1972). B. PULR-V 117, cross-section in general view (B1); details (B2, B3), photomicrographs in plane-polarized light; note the fibrolamellar bone (fbl) with primary osteons (po) on the anteromedial surface; secondary osteons (so) and resorption cavities in the mid- to inner cortical region. Abbreviations: a, anterior; l, lateral; p, posterior.
Discussion
Histological characteristics.—The protochampsians analyzed here, share several histological features, and display active, continuous growth. Primary osteons occur in all bones, which possess fibrolamellar tissue. In those samples that grew the bone remodelling process was initiated although not completed, which means that the vascular canals or primary osteons are secondarily widened by successive resorption processes, resulting in an overall secondary spongeous tissue (Houssaye et al. 2014). A thick layer of lamellar bone surrounds the widened primary osteons but they are not fully infilled, resulting in incomplete or immature secondary osteons (Klein et al. 2015). Mature secondary osteons are rare, and are restricted mainly to the inner cortex of the tibia samples. The absence of large amounts of mature secondary osteons (Francillon-Vieillot et al. 1990; Currey 2002) in these samples is uncommon and is comparable to the ones recently observed in placodonts (Klein et al. 2015). The local radial trabecular-like architecture observed in the inner cortex of some samples may also indicate anisotropic mechanical properties that depend on the porosity of the specimen as well as the architectural arrangement of the individual trabeculae. The elasticity and strength properties of trabecular bone display substantial heterogeneity with respect to age and health, position in the body, loading direction (with respect to the principal orientation of the trabeculae), and loading mode (Keaveny et al. 2004). Thus, trabecular bone can provide an efficient method to counter repeated forces, as well as replace the primary tissue for lightening the bones (Padian and Lamm 2013).
Fibrolamellar bone is known from modern birds, dinosaurs, most synapsids, and from various extinct marine reptiles such as ichthyosaurs and plesiosaurs (e.g., Chinsamy-Turan 2005, 2011; Wiffen et al. 1995). Therefore, fibrolamellar tissue undoubtedly originated several times within different vertebrate lineages (Klein et al. 2015). In the case here described, the combination of fibrolamellar bone with plexiform organization indicates a high growth rate comparable to that of ichthyosaurs (Buffrénil and Mazin 1990; Houssaye et al. 2014). The samples studied have the typical scaffolding of woven bone surrounded by lamellar bone. However, in one of the samples, the woven bone component is replaced or grades into parallel-fibred bone. This replacement or modification of woven bone by parallel-fibred bone in the fibrolamellar tissue has been described previously for some archosaurs (Ricqlés et al. 2003), the ornithopod dinosaur Gasparinisaura (Cerda and Chinsamy 2012), the titanosaur dinosaur Ampelosaurus (Klein 2012), and recently for placodonts (Klein et al. 2015).
Ecomorphology.—There are several ways to infer the mode of life of fossil vertebrates. One of these approaches, the most traditional, compares the morphology of the animal to modern analogues, in order to identify the probable environment in which it lived. Previously, Sill (1967) compared the skull of Proterochampsa barrionuevoi with Recent crocodiles and suggested that the former had similar aquatic adaptations to the modern species, but his interest was more in trying to relate both groups phylogenetically. Here, we present a brief review of the morphological evidence that may help infer the lifestyle and habitat of proterochampians.
A secondary palate is present in all known proterochampsians. This structure is very simple, consisting in a thin sheet of bone formed by the suture of the right and left premaxillae, palatines, vomers, and pterygoids ventrally along the midline of the palate. There are no pneumatic fossas or foramina that could indicate an inner system of conducts as in archosaurs, particularly in derived Crocodylomorpha. This structure allows the choana to open downward more posteriorly than the nostrils. Although the secondary palate clearly would have allowed the animal to breath during feeding, and thus could be an advantage in an aquatic way of life, this structure is also present in terrestrial crocodylomorphs as Notosuchians and independently has also evolved in mammals, for reasons unrelated to an aquatic lifestyle. Because of its complex anatomy and the difficulty of evaluating homologies, we consider the secondary palate as a possible indicator of an aquatic lifestyle, but not an unambiguous one.
An interesting feature of proterochampsians is that they possess a complex set of teeth on the ventral surface of the secondary palate, on the vomers and pterygoids. This palatal dentition—which complements the marginal dentition—is formed by numerous small teeth, which are covered by enamel and set in alveoli (Dilkes and Arcucci 2012; Trotteyn et al. 2013). Palatal teeth are also present in phylogenetically closely related forms as Mesosuchus (Dilkes 1998) or Euparkeria (Ewer 1965; Sookias and Butler 2013), although in these more primitive forms the teeth are distributed in patches. The palatal teeth of proterochampsians are more consistent with the ones present in Doswellia, Vancleavea (which were interpreted as aquatic animals), or Azendohsaurus, where these teeth were interpreted as a possible supplementary structures for processing food (Weems 1980; Nesbitt et al. 2009; Flynn et al. 2010). In Azendohsaurus, which is interpreted as a terrestrial archosaur, the palatal teeth are similar to the marginal herbivorous teeth, which is also the case in proterochampsians, where the teeth are similar to the maxillary ones. In general, considering the presence of palatal dentition in so many different animals with a variety of locomotion styles and diet, we can not interpret it as an evidence in favor of any particular way of life for proterochampsians.
In many cases, extant aquatic tetrapods display a set of features associated with locomotion in water. Some taxa have developed modifications in some of their limbs, involving important changes in morphology (like soft tissue membranes, or loss or reduction of unguals or entire digits). In other cases, however, there are no important changes of the limbs, but rather modifications are located in the vertebral column, especially in the caudal region, where it is common to find elongated and laterally compressed tails. This morphology is present in aquatic tetrapods that use their tail in lateral undulatory locomotion as ichthyosaurs, mosasaurs, marine crocodylomorphs (as metriorhynchids) and also in extant crocodiles (Carroll 1985). In a study of the swimming capacities of marine Mesozoic reptiles, Massare (1988) categorized marine crocodiles and mosasaurs as “undulatory axial swimmers” explaining that their morphology was well suited for rapid movements like the ones performed in the “ambush prey strategy”. This strategy is also suggested by Nesbitt et al. (2009) to explain the bizarre morphology of the archosauriform Vancleavea, which also exhibits this tail morphology. The morphology of the tail in proterochampsians (Table 1, Fig. 3) is very similar to that in Recent squamates that have a proximal to medial part of the tail wider than tall and thinner, but cylindrical, to its end. Several species of South American iguanids, such as genus Tupinambis, with this kind of tail have strictly terrestrial habits.
The asymmetric foot pattern documented in proterochampsians is also present in the basal diapsids Araeocelis and Youngina (Gauthier 1994) and in extant squamates like Iguana varanus and Tupinambis, which are characterized by an enlargement of the phalanges (Lee 1997). The only digit reduced to the metatarsal is the fifth digit, which is present as a reduced cone, without phalanxes. This morphology of the fifth digit is present also in other diapsids, such as lacertids and terrestrial basal archosaurs like “rauisuchians” and crocodylomorphs, among others (Nessbitt 2011: fig. 47). The tarsal structure of proterochampsians consists of two main tarsal bones: an astragalus that is well developed transversely and a calcaneum with a lateral tuber, which is larger in Tropidosuchus than in the other forms. Unfortunately, the pedal posture of proterochampsians is difficult to interpret: it is not clear if they were plantigrade or digitigrade. This uncertainty stems from the fact that, for most archosaurs and close relatives, their pedal postures are interpreted in relation to their tarsal types (crurotarsal or mesotarsal), and proterochampsians have an intermediate type.
The type of dermal armor reported from proterochampsians is also known in Euparkeria, several “rauisuchids” and sphenosuchids, all of which are derived archosauriforms of terrestrial habits that are not closely related phylogenetically. In other cases, it has been speculated that fossil animals with strongly developed dermal armor, such as Doswellia, Vancleavea or phytosaurs (Weems 1980; Nesbitt et al. 2009), would have a difficult time moving out of the water. Vancleavea is unique also in having a series of enlarged osteoderms located dorsally and ventrally to the tail and supporting the structure of the caudal extremity. The interpretation of a direct relationship between dermal armor and habit is not clear, because there are several cases of terrestrial squamates like the African lizards of the family Cordylidae (armadillo lizards, like Cordylus cathafractus) (Le et al. 2000) that have a very well developed dermal skeleton. The same is also true of terrestrial turtles.
Bone microanatomy and lifestyle.—Lifestyle appears to be reflected in the skeleton at various levels ranging from gross morphology to histological microstructure (Bininda-Emonds et al. 2001; Buffrénil et al. 1987). However, not all aquatic or amphibious vertebrates display noticeable morphological modifications (Carroll 1985); it is thus often difficult to assess the ecological requirements of a taxon on the basis of morphology only, and therefore bone microanatomy may provide additional ecological data. Differences in the bone microstructure between aquatic and terrestrial vertebrates have been known for a long time (Nopcsa 1923), and the relationship between bone texture and lifestyle has been intensively studied in in extant tetrapods (Scheyer and Sander 2007; Kriloff et al. 2008). Generally, the long bones of terrestrial taxa have a large medullary cavity associated with a moderately thick compact cortical area. In contrast, long bones of aquatic vertebrates are massive, with a smaller medullary cavity (if any), which minimizes inertia (Wall 1983; Stein 1989; Fish and Stein 1991; Taylor 1994; Kriloff et al. 2008; Laurin et al. 2011), and the skeleton is either heavier and more compact (pachiostotic/osteosclerotic) or lighter and more spongy (osteoporotic) than in their terrestrial relatives (Ricqlès and Buffrénil 2001).
Thus, in this way of interpretation, bone histology—particularly compactness—can be used as a proxy to infer lifestyle (aquatic, amphibious, terrestrial). Several studies (e.g., Canoville and Laurin 2009; Kriloff et al. 2008; Laurin et al. 2004, 2007, 2011) confirmed higher compactness for aquatic taxa, less compactness for amphibious forms, and much less compactness for terrestrial taxa, but there are some exceptions (turtles, for example, is opposite probably by interference that produce their shell). The bone compactness is defined as the ratio between the surface occupied by bone tissues and the total bone surface, and thus, it can vary along a single bone (the metaphysis is spongier than the diaphysis). Because of this, the mid-diaphysis is thought to be the best place to measure, in the sense that it gives the clearest indication of ecological signal (Laurin et al. 2004).
Increased skeletal mass may result from pachyostosis (a thickening of the cortex by increased deposition of periosteal bone), osteosclerosis (a reduction in resorption of medullary bone) or a combination of both processes. However, these relationships may not hold for all taxa or all bones, and may depend on the evolutionary time available for taxa to adapt to a new ecological niche (Laurin et al. 2004, 2006). In fact, the hindlimb appears to be slightly less informative than the forelimb, possibly because some aquatic taxa have lost the hindlimb or retain only vestiges that are not functional in locomotion (Canoville and Laurin 2010). Recent analyses suggest that the humerus displays a stronger ecological signal than other limb bones. The humeral compactness profile contains a strong ecological signal because it explains 42.9% of the lifestyle variance when covariation with phylogeny excluded (Canoville and Laurin 2010). This is greater than the results from the tibia, which explains only approximately 20% of the lifestyle variance (Kriloff et al. 2008). However, considering the hindlimb, the tibia shows more ecological information than the femur, and their global compactness in amphibious taxa does not differ significantly from that of terrestrial taxa.
Additionally, several studies raise doubts about terrestriality as the ancestral lifestyle of amniotes. The optimizations of lifestyle of extant and extinct amniotes suggest that the first amniotes were either amphibious or terrestrial (Germain and Laurin 2005; Kriloff et al. 2008). Thus, the time at which amniotes (and indeed, vertebrates) became truly terrestrial remains uncertain (Canoville and Laurin 2010).
According to this study, histological examination of proterochampsian bone samples reveals a predominance of fibrolamellar bone tissue suggesting a rapid periosteal osteogenesis (Buffrénil 1980; Reid 1990; Chinsamy 1997), which in turn suggests overall fast bone growth (Ricqlès 1972). This tissue has been reported in other extinct vertebrates like birds; pterosaurs; some pseudosuchians; basal archosauromorphs; captorhinids and pelycosaurs (Ricqlès 1976; Curry 1999; Horner et al. 1999; Ricqlès et al. 2003; Botha-Brink and Smith 2011). The abundance of fibrolamellar cortical tissue and absence of an EFS in the proterochampsian specimens indicates that they were still growing, and not fully-grown adults, when they died (Klein and Sander 2008). On the other hand, the existence of discontinuities in some samples suggest changes in the growth rate. Starck and Chinsamy (2002) have suggested that LAGs are an expression of a high degree of developmental plasticity, which is the capability to respond to changes in the environment by evoking different developmental regimes (Smith-Gill 1983). Although our sampling is limited, it appears that these proterochampsians had higher growth rates than in typical pseudosuchians (e.g., phytosaurs, aetosaurs, Luperosuchus, Poposaurus) and other basal archosauromorphs (Erythrosuchus, Euparkeria), perhaps even commensurate with the pseudosuchian Terrestrisuchus, as well as, dinosauromorphs (e.g., Lewisuchus, Herrerasaurus) and some dinosaurs (e.g., Lesothosaurus, Coelophysis) (Fig. 7).
The histological studies on proterochampsian osteoderms (Cerda et al. 2015; Ponce et al. 2017) obtained ambiguous results, suggesting an aquatic or amphibious life style based on osteoderm compactness but owing the small size and number of osteoderms contradicted the potential buoyancy function of them. The histological features, including compactness, recorded in the specimens we studied, support more strongly an amphibious/terrestrial life style for protoerochampsians.
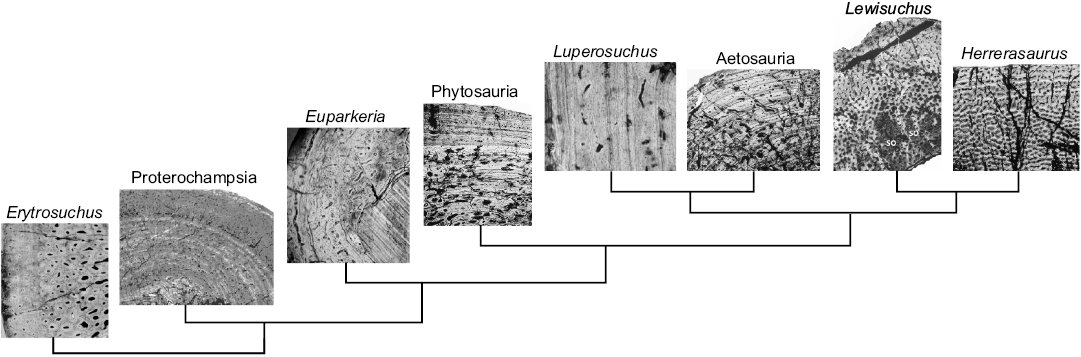
Fig. 7. Phylogenetic interrelationships of Proterochampsia and other archosauriforms showing the histological features in different taxa. Erytrosuchus rib; proterochampsids femur; Euparkeria humerus, Phytosauria femur; Luperosuchus large long bone; Aetosauria humerus; Lewisuchus tibia; Herrerasaurus tibia (modified from Ricqlès et al. 2003, 2008; Garcia Marsà et al. 2017).
Conclusions
The anatomical features recognized in protoerochampsians, such as the secondary palate, marginal dentition, palatal teeth, morphology of the tail, limb modification, and dermal armor, were evaluated and their ecological role was discussed. This suite of characters suggests a terrestrial/amphibious life style.
The histological features, particularly the compactness of the bone and the presence of a reduced medullary cavity, more strongly support an amphibious/terrestrial life style. The histological examination also revealed a predominance of fibrolamellar bone tissue, suggesting rapid periosteal osteogenesis, and therefore overall fast bone growth. The existence of discontinuities (LAGs) shows that proterochampsians had the capability to respond to changes in the environment, and the proterochampsian samples showed higher growth rates than typical for pseudosuchians and basal archosauromorphs, perhaps even similar to dinosauromorphs, as well as some dinosaurs.
Acknowledgements
For access permision we thank Verónica Vargas and Laura Gachón (Dirección de Patrimonio Arqueológico y Paleontológico, Secretaría de Cultura de La Rioja, La Rioja, Argentina) and Administración de Parques Nacionales. We are deeply indebted to the staff of Parque Nacional Talampaya for their constant assistance in the field. For access to specimens in their care, we thank Emilio Vaccari and Gabriela Cisterna (Universidad Nacional de La Rioja, La Rioja, Argentina), Lucas Fiorelli and Francisco Prevosti (CRILAR). Field and laboratory research was supported by the PICT 2013-0805 (ACM). Additional financial support was provided by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
References
Arcucci, A.B. 1990. Un nuevo Proterochampsidae (Reptilia–Archosauriformes) de la fauna local de Los Chañares (Triásico Medio), La Rioja, Argentina. Ameghiniana 27: 365–378.
Arcucci, A.B. 2011. Sistemática y filogenia de los Proterochampsidae (Amniota, Diápsida, Archosauriformes) del Triásico de América del Sur, y sus implicancias en el origen de Archosauria. 302 pp. Ph.D. Thesis, Universidad Nacional de San Juan, San Juan.
Barberena, M.C. 1982. Uma nova espécie de Proterochampsa, P. nodosa sp. nov. do Triássico do Brasil. Anais da Academia Brasileira de Ciências 54: 127–141.
Betz, O. 2006. Ecomorphology: Integration of form, function, and ecology in the analysis of morphological structures. Mitteilungen der Deutschen Gesellschaft für Allgemeine und Angewandte Entomologie 15: 409–416.
Bininda-Emonds, O.R.P., Gittleman, J.L., and Kelly, C.K. 2001. Flippers versus feet: comparative trends in aquatic and non-aquatic carnivores. Journal of Animal Ecology 70: 386–400. Crossref
Bock, W.J. 1994. Concepts and methods in ecomorphology. Journal of Biosciences 19: 403–413. Crossref
Bonaparte, J.F. 1971. Cerritosaurus binsfeldi Price, tipo de una nueva familia de tecodontes (Pseudosuchia–Proterochampsia). Anais da Academia Brasileira de Ciencias 43: 417–422.
Bonaparte, J.F. 1978. El Mesozoico de América del Sur y sus Tetrápodos. Opera Lilloana 26: 1–596.
Bonaparte, J.F. 1997. El Triásico de San Juan-La Rioja Argentina y sus Dinosaurios.190 pp. Museo Argentino de Ciencias Naturales, Buenos Aires.
Botha-Brink, J. and Smith, R.M.H. 2011. Osteohistology of the Triassic archosauromorphs Prolacerta, Proterosuchus, Euparkeria and Erythrosuchus from the Karoo Basin of South Africa. Journal of Vertebrate Paleontology 31: 1238–1254. Crossref
Buffrénnil, V. de 1980. Mise en evidence de l’incidence des conditions de milieu sur la croissance de Crocodylus siamensis (Schneider, 1801) et valeur des marques de croissance squelettiques pour l’evaluation de l’age individual. Archives de Zoologie Experimentale Generale 121: 63–76.
Buffrénil, V. de and Mazin, J.M. 1990. Bone histology of the ichthyosaurs: comparative data and functional interpretation. Paleobiology 16: 435–447. Crossref
Buffrénil, V. de, Mazin J.M., and Ricqlès, A.J. de 1987. Caractères structuraux et mode de croissance du fémur d’Omphalosaurus nisseri, ichthyosaurien du Trias moyen de Spitsberg. Annales De Paleontologie 73: 195–216.
Buffrénil, V. de, Ricqlès, A.J. de, Ray, C.E., and Domning, D.P. 1990a. Bone histology of the ribs of the archaeocetes (Mammalia: Cetacea). Journal of Vertebrate Paleontology 10: 455–466. Crossref
Buffrénil, V. de, Ricqlès, A.J. de, Sigogneau-Russell, D., and Buffetaut, E. 1990b. L’histologie osseuse des champsosauridés: données descriptives et interprétation fonctionnelle. Annales De Paleontologie 76: 255–275.
Buffrénil, V. de, Canoville, A., D’Anastasio, R., Domning, and Daryl P., 2010. Evolution of sirenian pachyosteosclerosis, a model-case for the study of bone structure in aquatic tetrapods. Journal of Mammalian Evolution 17: 101–120. Crossref
Canoville, A. and Laurin, M. 2009. Microanatomical diversity of the humerus and lifestyle in lissamphibians. Acta Zoológica 90: 110–122. Crossref
Canoville, A. and Laurin, M. 2010. Evolution of humeral microanatomy and lifestyle in amniotes, and some comments on paleobiological inferences. Biological Journal of the Linnean Society 100: 384–406. Crossref
Carroll, R.L. 1985. Evolutionary constraints in aquatic diapsid reptiles. Palaeontology 33: 45–155.
Casinos, A. 1996. Bipedalism and quadrupedalism in Megatherium: an attempt at biomechanical reconstruction. Lethaia 29: 87–96. Crossref
Castanet, J., Francillon-Vieillot, H., Meunier, F.J., and Ricqlès, A.J. de 1993. Bone and individual aging. In: B.K. Hall (ed.), Bone Growth, 245–283. CRC Press, London.
Chinsamy, A. 1997. Assessing the biology of fossil vertebrates through bone histology. Palaeontologia Africana 33: 29–35.
Chinsamy, A. and Raath, M.A. 1992. Preparation of fossil bone for histological examination. Palaeontologia Africana 29: 39–44.
Chinsamy-Turan, A. 2005. The Microstructure of Dinosaur Bone. 216 pp. The Johns Hopkins University Press, Baltimore.
Chinsamy-Turan, A. 2011. Forerunners of Mammals: Radiation, Histology, Biology. 352 pp. Indiana University Press, Bloomington.
Cerda, I.A. and Chinsamy, A. 2012. Biological implications of the bone microstructure of the Late Cretaceous ornithopod dinosaur Gasparinisaura cincosaltensis. Journal of Vertebrate Paleontology 32: 355–368. Crossref
Cerda, I.A., Desojo, J.B., Trotteyn, M.J., and Scheyer, T.M. 2015. Osteoderm histology of Proterochampsia and Doswelliidae (Reptilia: Archosauriformes) and their evolutionary and paleobiological implications. Journal of Morphology 276: 385–402. Crossref
Currey, J.D. 2002. Bones: Structure and Mechanics. 456 pp. Princeton University Press, Princeton.
Curry, K.A. 1999. Ontogenetic histology of Apatosaurus (Dinosauria: Sauropoda) new insights on growth rates and longevity. Journal of Vertebrate Paleontology 19: 654–665. Crossref
Dilkes, D. 1998. The Early Triassic rhynchosaur Mesosuchus browni and the interrelationships of basal archosauromorph reptiles. Philosophical Transactions of the Royal Society of London B 353: 501–541. Crossref
Dilkes, D. and Arcucci, A. 2012. Proterochampsa barrionuevoi (Archosauriformes: Proterochampsia) from the Late Triassic (Carnian) of Argentina and a phylogenetic analysis of Proterochampsia. Palaeontology 5: 853–885. Crossref
Dilkes, D. and Sues, H. 2009. Redescription and phylogenetic relationships of Doswellia kaltenbachi (Diapsida: Archosauriformes) from the Upper Triassic of Virginia. Journal of Vertebrate Paleontology 29: 58–79. Crossref
Ewer, R.F. 1965. The anatomy of the thecodont reptile Euparkeria capensis Broom. Philosophical Transactions of the Royal Society of London B 248: 379–435. Crossref
Ezcurra, M.D., Desojo, J.B., and Rauhut, O.W.M. 2015. Redescription and phylogenetic relationships of the proterochampsid Rhadinosuchus gracilis (Diapsida: Archosauriformes) from the early Late Triassic of southern Brazil. Ameghiniana 52: 391–417. Crossref
Ezcurra, M.D., Lecuona, A., and Martinelli, A.G. 2010. A new basal diapsid archosauriform from the Early Triassic of Argentina. Journal of Vertebrate Paleontology 39: 1–18.
Fish, F.E. and Stein, B.R. 1991. Functional correlates of differences in bone density among terrestrial and aquatic genera in the family Mustelidae (Mammalia). Zoomorphology 110: 339–345. Crossref
Francillon-Vieillot, H., Buffrenil, V. de, Castanet, J., Geraudie, J., Meunier, F.J., Sire, J.Y., Zylberberg, L., and Ricqles, A. de 1990. Microstructure and mineralization of vertebrate skeletal tissues. In: J.G. Carter (ed.), Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends, 471–530. Van Nostrand Reinhold 1, New York.
Flynn, J.J., Nesbitt, S.J., Parrish, J.M., Ranivoharimanana, L., and Wyss, A.R. 2010. A new species of Azendohsaurus (Diapsida: Archosauromorpha) from the Triassic Isalo Group of Southwestern Madagascar: cranium and mandible. Palaeontology 53: 669–688. Crossref
Garcia Marsà, J.A., Agnolín, F.L., and Novas, F. 2017. Bone microstructure of Lewisuchus admixtus Romer, 1972 (Archosauria, Dinosauriformes). Historical Biology [published online, https://doi.org/10.1080/08912963.2017.1347646] Crossref
Gauthier, J.A. 1994. The diversification of the amniotes. In: D.R. Prothero and R.M. Schoch (eds.), Major Features of Vertebrate Evolution, 129–159. The Paleontological Society, Knoxville.
Germain, D. and Laurin, M. 2005. Microanatomy of the radius and lifestyle in amniotes (Vertebrata Tetrapoda). Zoologica Scripta 34: 335–350. Crossref
Horner, J.R., Ricqlès, A.J. de, and Padian, K. 1999. Variation in dinosaur skeletochronology indicators: Implications for age assessment and physiology. Paleobiology 25: 295–304. Crossref
Horner, J.R., Ricqlès, A.J. de, and Padian, K. 2000. Long bone histology of the hadrosaurid dinosaur Maiasaura peeblesorum: growth dynamics and physiology based on an ontogenetic series of skeletal elements. Journal of Vertebrate Paleontology 20: 115–129. Crossref
Houssaye, A., Lindgren J., Pellegrini R, Lee A.H., Germain, D., and Polcyn, M.J. 2013. Microanatomical and histological features in the long bones of mosasaurine mosasaurs (Reptilia, Squamata)—implications for aquatic adaptation and growth rates. PLoS ONE 8: e76741. Crossref
Houssaye, A., Scheyer, T.M., Kolb, C., Fischer, V., and Sander, P.M. 2014. A new look at ichthyosaur long bone microanatomy and histology: Implications for their adaptation to an aquatic life. PLoS ONE 9: e95637. Crossref
Hsiou, A., Adbala, F., and Arcucci, A.B. 2002. Novo registro de Proterocampsídeo (Reptilia, Archosauriformes) do Triássico Médio–Superior do Brasil. Revista Brasileira de Palentologia 3: 48–55.
Huene, F. von 1938. Die fossilen Reptilien des südamerikanischen Gondwanalandes. Neues Jahrbuch für Mineralogie, Geologie und Paläontologie 3: 142–151.
Kardong, K.V. 1999. Vertebrados: Anatomía Comparada, Función, Evolución. 732 pp. McGraw-Hill Interamericana, Madrid.
Keaveny, T.M., Morgan, E.F., and Yeh, O.C. 2003. Bone mechanics. In: M. Kurtz (ed.), Standard Handbook of Biomedical Engineering and Design, 8.1–8.23. McGraw-Hill Press, New York.
Klein, N. 2012. Postcranial morphology and growth of the Winterswijk pachypleurosaur Anarosaurus heterodontus (Sauropterygia) from the Lower Muschelkalk of Winterswijk, The Netherlands. Paläontologische Zeitschrift 86: 389–408. Crossref
Klein, N., Houssaye, A., Neenan, J.M., and Scheyer, T.M. 2015. Long bone histology and microanatomy of Placodontia (Diapsida: Sauropterygia). Contributions to Zoology 84: 59–84.
Klein, N. and Sander, M. 2008. Ontogenetic stages in the long bone histology of sauropod dinosaurs. Paleobiology 34: 247–263.
Kischlat, E.E. 2000. Tecodôncios: a aurora dos arcossáurios no Triássico. In: M. Holz and L.F. De Ross (eds.), Paleontologia do Rio Grande do Sul, 273–316. CIGO/UFRGS, Porto Alegre.
Kriloff, A., Germain, D., Canoville, A., Vincent, P., Sache, M., and Laurin, M. 2008. Evolution of bone microanatomy of the tetrapod tibia and its use in palaeobiological inference. Journal of Evolutionary Biology 21: 807–826. Crossref
Langer, M.C., Ramezani, J., and Da Rosa, A.A.S. 2018. U-Pb age constraints on dinosaur rise from south Brazil. Gondwana Research 57: 133–140. Crossref
Laurin, M., Canoville, A., and Germain, D. 2011. Bone microanatomy and lifestyle: a descriptive approach. Comptes Rendu Palevol 10: 381–402. Crossref
Laurin, M., Germain, D., Steyer, J.S., and Girondot, M. 2006. Données microanatomiques sur la conquête de l’environnement terrestre par les vertébrés. Comptes Rendu Palevol 5: 603–618. Crossref
Laurin, M., Girondot, M., and Loth, M.M. 2004. The evolution of long bone microanatomy and lifestyle in lissamphibians. Paleobiology 30: 589–613. Crossref
Laurin, M., Meunier, F.J., Germain D., and Lemoine, M. 2007. A microanatomical and histological study of the paired fin skeleton of the Devonian sarcopterygian Eusthenopteron foordi. Journal of Paleontology 81: 143–153. Crossref
Le, P., Mouton, F.N., Geertsema, H., and Visagie, L. 2000. Foraging mode of a group-living lizard, Cordylus cataphractus (Cordylidae). African Zoology 35: 1–7. Crossref
Lee, M.S.Y. 1997. Reptile relationships turn turtle. Nature 389: 245–246. Crossref
Mancuso, A.C. 2005. Revisión y aportes a la estratigrafía de la sección inferior del Grupo Agua de la Peña (Triásico Medio, Argentina). XVI Congreso Geológico Argentino, La Plata, Argentina, Actas 3: 415–422.
Mancuso, A.C., Gaetano, L.C., Leardi, J.M., Abdala, F., and Arcucci, A.B. 2014. The Chañares Formation: a window to the palaeobiology of a Middle Triassic vertebrate fauna. Lethaia 47: 244–265. Crossref
Mancuso, A.C., Previtera, E., Benavente, C.A., and Hernández del Pino, S. 2017. Evidence of bacterial decay and early diagenesis in a partially-articulated tetrapod from the Triassic Chañares Formation. Palaios 32: 367–381. Crossref
Marsicano, C.A., Irmis, R.B., Mancuso, A.C., Mundil, R., and Chemale, F. 2016. The precise temporal calibration of dinosaur origins. Proceedings of the National Academy of Sciences of the United States of America 113: 509–513. Crossref
Martínez, R.N., Sereno, P.C., Alcober, O.A., Colombi, C.E., Renne, P.R., Montañez, I.P., and Currie, B.S. 2011. A basal dinosaur from the dawn of the dinosaur era in southwestern Pangaea. Science 331: 206–210. Crossref
Massare, J.A. 1988. Swimming capabilities of Mesozoic marine reptiles. Paleobiology 14: 187–205. Crossref
Nesbitt, S. 2011. The early evolution of archosaurs: relationships and the origin of major clades. Bulletin of the American Museum of Natural History 352: 1–292. Crossref
Nesbitt, S., Stocker, M.R., Small, B., and Downs, A. 2009. The osteology and relationships of Vancleavea campi (Reptilia: Archosauriformes). Zoological Journal of the Linnean Society 157: 814–864. Crossref
Nesbitt, S.J., Desojo, J.B., and Irmis, R.B. 2013. Anatomy, phylogeny and palaeobiology of early archosaurs and their kin. Geological Society, London, Special Publications 379: 1–7. Crossref
Nopcsa, F.B. 1923. Vorläufige Notiz über die Pachyostose und Osteosklerose einiger mariner Wirbeltiere. Anatomischer Anzeiger 56: 353–359.
Nopcsa, F.B. and Heidsieck, E. 1934. Uber eine pachyostotische Rippe aus der Kreide Rugens. Acta Zoologica 15: 431–455. Crossref
Padian, K. and Lamm, E.T. 2013. Bone Histology of Fossil Tetrapods: Advancing Methods, Analysis, and Interpretation. 285pp. University of California Press, Berkeley, California. Crossref
Ponce, D.A., Cerda, I.A., Desojo, J.B., and Nesbitt, S.J. 2017. The osteoderm microstructure in doswelliids and proterochampsids and its implications for palaeobiology of stem archosaurs. Acta Palaeontologica Polonica 62: 819–831. Crossref
Price, L.I. 1946. Sobre um novo pseudosuquio do Triássico Superior do Rio Grande do Sul. Boletim Divisão de Geologáa e Mineralogía 120: 1–38.
Raugust, T., Lacerda, M., and Schultz, C.L. 2013. The first occurrence of Chanaresuchus bonapartei Romer 1971 (Archosauriformes, Proterochampsia) of the Middle Triassic of Brazil from the Santacruzodon Assemblage Zone, Santa Maria Formation (Paraná Basin). Geological Society London, Special Publications 379: 303–318. Crossref
Reid, R.E.H. 1990. Zonal growth rings in dinosaurs. Modern Geology 15: 19–48.
Reid, R.E.H. 1996. Bone histology of the Cleveland-Lloyd dinosaurs and of dinosaurs in general, Part I: Introduction: introduction to bone tissues. BrighamYoung University Research Studies. Geology 41: 25–72.
Reig, O.A. 1958. A cerca de la posición sistemática de la Familia Rauisuchidae y del género Saurosuchus (Reptilia–Thecodontia). Publicaciones del Museo Municipal de Ciencias Naturales y Tradicionales de Mar del Plata 1: 73–114.
Reig, O.A. 1959. Primeros datos descriptivos sobre nuevos reptiles arcosaurios del Triásico de Ischigualasto (San Juan, Argentina). Revista de la Asociación Geológica Argentina 13: 257–270.
Ricqlès, A.J. de 1972. Recherches paléohistologiques sur les os longs des Tétrapodes. Part III, Titanosuchiens, Dinocéphales et Dicynodontes. Annales de Paléontologie Vertébrés 58: 17–60.
Ricqlès, A.J. de 1974a. Recherches paléohistologiques sur les os longs des tétrapodes IV. Eothériodontes et pélycosaures. Annales de Paléontologie 60: 1–39.
Ricqlès, A.J. de 1974b. Recherches paléohistologiques sur les os longs des tétrapodes V. Cotylosaures et mésosaures. Annales de Paléontologie 60: 171–216.
Ricqlès, A.J. de 1976. On bone histology of fossil and living reptiles, with comments on its functional and evolutionary significance. In: B. Bellairs Cox (ed.), Morphology and Biology of Reptiles, 126–150. Linnean Society of London Academic Press, London.
Ricqlès, A.J. de 1983. Cyclical growth in the long limb bones of a sauropod dinosaur. Acta Palaeontologica Polonica 28: 225–232.
Ricqlès, A.J. de, and Buffrenil, V. de 2001. Bone histology, heterochronies and the return of tetrapods to life in water: where are we? In: J.M. Mazin and V. de Buffrenil (eds.), Secondary Adaptation of Tetrapods to Life in Water, 289–310. Verlag Dr. Friedrich Pfeil, München.
Ricqlès, A.J. de, Padian, K., and Horner, J.R. 2003. On the bone histology of some Triassic pseudosuchian archosaurs and related taxa. Annales de Paléontologie 89: 67–101. Crossref
Ricqlès, A.J. de, Padian, K., Knoll, F., and Horner, J.R. 2008. On the origin of high growth rates in archosaurs and their ancient relatives: Complementary histological studies on Triassic archosauriforms and the problem of a “phylogenetic signal” in bone histology. Annales de Paléontologie 94: 57–76 Crossref
Rimblot-Baly, F., Ricqlès, A.J. de, and Zylberberg, L. 1995. Analyse paléohistologique d’une série de croissance partielle chez Lapparentosaurus madagascariensis (Jurassique moyen): essai sur la dynamique de croissance d’un Dinosaure Sauropode. Annales de Paléontologie 1: 49–86.
Romer, A.S. 1966. Vertebrate Paleontology. Third Edition, 1–707. University of Chicago Press, Chicago.
Romer, A.S. 1971. The Chañares (Argentina) Triassic reptile fauna. XI: two new long-snouted thecodonts, Chanaresuchus and Gualosuchus. Breviora 379: 1–22.
Scheyer, T.M. and Sander, P.M. 2007. Shell bone histology indicates terrestrial palaeoecology of basal turtles. Proceedings of the Royal Society B 274: 1885–1893. Crossref
Sereno, P.C. and Arcucci, A.B. 1990. The monophyly of the crurotarsal archosaurs and the origin of birds and crocodile ankle joints. Neues Jahrbuch fur Geologie und Paläontologie, Abhandlungen 180: 21–52.
Sill, W.D. 1967. Proterochampsa barrionuevoi and the early evolution of the Crocodilia. Bulletin of the Museum of Comparative Zoology 135: 415–446.
Smith-Gill, J.S. 1983. Developmental plasticity: developmental conversion versus developmental modulation. American Zoologist 23: 47–55. Crossref
Sookias, R.B. and Butler, R.J. 2013. Euparkeriidae. Geological Society London, Special Publications 379: 35–48. Crossref
Starck, J.M. and Chinsamy, A. 2002. Bone microstructure and developmental plasticity in birds and other dinosaurs. Journal of Morphology 254: 232–246. Crossref
Stein, B.R. 1989. Bone density and adaptation in semiaquatic mammals. Journal of Mammalogy 70: 467–476. Crossref
Steyer, J.S., Laurin, M., Castanet, J., and Ricqlès, A.J. de 2004. First histological and skeletochronological data on temnospondyl growth; palaeoecological and palaeoclimatological implications. Palaeogeography, Palaeoclimatology, Palaeoecology 206: 193–201. Crossref
Taylor, L.H. 1994. Stone, bone or blubber? Buoyancy control strategies in aquatic tetrapods. In: L. Maddock, Q. Bone, and J.M.V. Rayner (eds.), Mechanics and Physiology of Animal Swimming, 151–161. Cambridge University Press, Cambridge. Crossref
Trotteyn, M.J. 2011. Material postcraneano de Proterochampsa barrionuevoi Reig, 1959 (Diapsida: Archosauriformes) del Triásico Superior del centro-oeste de Argentina. Ameghiniana 48: 424–446. Crossref
Trotteyn, M.J., Arcucci, A.B., and Raugust, T. 2013. Proterochampsia: an endemic archosauriform clade from South America. Geological Society London, Special Publications 379: 59–90. Crossref
Trotteyn, M.J. and Ezcurra, M.D. 2014. Osteology of Pseudochampsa ischigualastensis gen. et comb. nov. (Archosauriformes: Proterochampsidae) from the early Late Triassic Ischigualasto Formation of northwestern Argentina. PloS ONE 9: e111388. Crossref
Van der Klaauw, C.J. 1948. Ecological studies and reviews. IV. Ecological morphology. Bibliotheory 4: 27–111.
Wall, W. P. 1983. The correlation between high limb-bone density and aquatic habits in recent mammals. Journal of Paleontology 57: 197–207.
Weems, R.E. 1980. An unusual newly discovered archosaur from the Upper Triassic of Virginia, U.S.A. Transactions of the American Philosophical Society 70: 1–53. Crossref
Wiffen, J., Buffrénil, V. de, Ricqlès, A.J. de, and Mazin, J.M. 1995. Ontogenetic evolution of bone structure in Late Cretaceous Plesiosauria from New Zealand. Geobios 28: 625–640. Crossref
Acta Palaeontol. Pol. 64 (1): 157–170, 2019
https://doi.org/10.4202/app.00536.2018