

New cleroid beetles from the Middle–Late Jurassic of China
JIŘÍ KOLIBÁČ and DIYING HUANG
Kolibáč, J. and Huang, D. 2019. New cleroid beetles from the Middle–Late Jurassic of China. Acta Palaeontologica Polonica 64 (1): 143–155.
Four specimens of fossil beetles recorded from the Middle and Late Jurassic boundary beds of Inner Mongolia, China are assigned to the melyrid lineage of Cleroidea. Three of them are described as Sinomelyris praedecessor gen. et sp. nov. and constitute the earliest known representatives of the family Melyridae. The new species habitually resembles some extant beetles of the subfamily Melyrinae but it is distinctly smaller and possesses four elytral carinae, the pronotum without a pair of the dorsolateral carinae, and the tarsal claw simple. The fourth specimen Juraniscus majeri gen. et sp. nov., is classified as Cleroidea incertae sedis. The new species has the symmetrical antennomeres including three terminal ones, the tarsomeres 1–4 with the lobes, the tarsal claw simple, and the aedeagus with the separated parameres. It is considered to be a member of the melyrid lineage and its relations to the relevant families Mauroniscidae, Rhadalidae, Dasytidae, and Malachiidae are discussed. A complete list of fossil species in the melyrid lineage described to date is provided.
Key words: Coleoptera, Cleroidea, Melyridae, Mesozoic, Inner Mongolia, Daohugou.
Jiří Kolibáč [jkolibac@mzm.cz], Moravian Museum, Department of Entomology, Brno, Czech Republic.
Diying Huang [dyhuang@nigpas.ac.cn], State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing, China.
Received 19 September 2018, accepted 11 December 2018, available online 4 March 2019.
Copyright © 2019 J. Kolibáč and D. Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The volcanic deposits in which the fossils described herein were found, are located close to the village of Daohugou, Wuhua Township, Ningcheng County, Inner Mongolia, north-eastern China and very near the border of the provinces of Hebei and Liaoning. The fossil assemblage, obtained from the Lagerstätte, belongs to the Yanliao Biota (Hong 1983) with an early assemblage known as the Daohugou Biota, a term introduced by Ji and Yuan (2002), the age of which has been a subject of discussion in recent decades. Invertebrate workers (e.g., Ren et al. 2010; Selden and Huang, 2010; Giribet et al. 2012) regard the strata as equivalent to the Haifanggou Formation of Liaoning, also known as the Jiulongshan Formation of Hebei, dated to as early as the latest Middle Jurassic (Callovian; 166.1–163.5±4.0 Ma) (Chen et al. 2004; Liu et al. 2004). Recently, some vertebrate palaeontologists (Ji et al. 2006) have concurred with this age, whereas others have considered the Daohugou strata possibly as late as Middle–Late Jurassic (Zhang et al. 2008; Wang and Li 2008; Evans and Wang 2009) or still later than that (for an overview of the issue see Sullivan et al. 2014). Such discussions have resulted in applying a reserved and somewhat younger dating scale, including the Callovian and Oxfordian stages, to major palaeoentomological surveys in recent years (e.g., McKenna et al. 2015; Smith and Marcot 2015; Toussaint et al. 2017). Following the latest opinions based on new data from isotope geochronology (40Ar-39Ar dating) (Liu et al. 2006; Yang and Li 2008; Chang et al. 2014; Huang 2019), which indicates that the lower tuff layer in the Daohugou bed was formed at 165.0±1.2 Ma (Yang and Li 2008), the current authors incline towards a somewhat older age for the strata, i.e., to the boundary of the Middle and Late Jurassic (Huang 2019). The age of the four fossil specimens described herein probably corresponds to the upper boundary of the Callovian, i.e., approximately 163 Ma.
The Yanliao Biota contains a large number of exceptionally well-preserved fossils, including e.g., insects, crustaceans, spiders, millipedes, molluscs, fishes, salamanders, lizards, pterosaurs, dinosaurs, mammals, plants, pollens, spores, and fungi (Huang 2016; Xing et al. 2016). Fossiliferous layers are distributed to the north of the Yellow River and west of Xinjiang, extending to Central Asia. Huang (2015) divided the Yanliao Biota into an early assemblage called the Daohugou biota (Shihezi Stage: Bathonian to lower Oxfordian) and a late assemblage called the Linglongta biota (Manas Stage: mid-upper Oxfordian to lower Kimmeridgian). Some fossils found in the bottom section of the Daohugou bed are related to the Yaopo Formation, thus indicating that the origin of the Yanliao Biota may be traced back to older strata (Huang et al. 2015). The forests of the Daohugou biota were dominated by gymnosperm trees such as ginkgopsids, conifers, and cycads (Liu et al. 2006, 2018). The Middle Jurassic of China is characterised by the Coniopteris–Phoenicopsis floral assemblage (Czekanowskiales), which appeared in the mid-Early Jurassic and achieved its greatest prosperity in the Middle Jurassic, declining in the mid-Late Jurassic (ca. 155 Ma). It was followed by a series of drought events in the Northern Hemisphere. With the advent of arid climate, the Yanliao Biota declined rapidly around the middle of the Late Jurassic, while certain relict groups migrated southwards (Huang 2019).
Based on the plants found in the Haifanggou Formation, Zhang and Zheng (1987), Kimura et al. (1994), and Wang et al. (2006) concluded that the climate in Liaoning during the Middle Jurassic was subtropical to temperate, warm and humid. From the finding of Phoenicopsis in Daohugou biota, Huang et al. (2016) deduced the occurrence of deciduous forests of the temperate-warm climate growth-type in the area. On the other hand, Selden and Huang (2010), in the light of plectreurid spider records, determined the climate as comparable to modern conditions in the Caribbean islands.
The fossils of terrestrial insects in the locality are often associated with freshwater crustaceans (especially Conchostraca, or clam shrimps). It is supposed that, during rainy periods, insects fell from vegetation into temporary pools or freshwater basins and sank to a fine-grained sediment, an ideal substrate for fossilization. However, fine volcanic ash produced by frequent volcanic activity in the region of the Yanliao Biota is believed to be the key factor in the exceptional preservation of the fossils (Huang 2016, 2019; Xing et al. 2016). The composition of Coleoptera in the locality displays extreme diversity, with beetles among the predominant insect fossils. Daohugou representatives of Cleroidea identified to date have been classified within the families Prionoceridae (Liu et al. 2015), Trogossitidae (Yu et al. 2012, 2014), and Cleridae (Kolibáč and Huang 2016). Mesozoic cleroid fossils also include two findings of the species-poor but clearly-distinguished family Thanerocleridae (Yu et al. 2017; Cai and Huang 2018) from the Burmese amber of the Cretaceous. The species-rich families Rhadalidae, Melyridae, Dasytidae, and Malachiidae are the last of larger cleroid families previously unknown from the Daohugou biota of the Mesozoic. Four species of Rhadalidae (Majer 1998), two of Dasytidae (Wickham 1912) and eight of Malachiidae (Wickham 1912, 1917; Théobald 1937; Kubicz 2001; Kirejtshuk and Nel 2008; Chernyshev 2012, 2016) have been described from the lower Oligocene to upper Eocene. (Spahr 1981a, b; Ponomarenko and Kirejtshuk 2003–2015). Acanthocnemoides sukatshevae Zherikhin, 1977 as yet the only described Mesozoic cleroid species, from the Albian–Cenomanian amber of Siberia (Zherikhin and Sukatsheva 1973; Zherikhin 1977), might belong to the melyrid lineage. However, two specimens of the latter species, well-preserved, need further study to clarify their systematic position. A detailed list of all fossil species belonging to the melyrid lineage is given in Table 1.
Here we report two fossil species of the melyrid lineage of the superfamily Cleroidea. The both present the oldest findings of the beetle group and one of them is the first Mesozoic record of the large cleroid family Melyridae.
Institutional abbreviations.—MMBC, Moravian Museum, Brno, Czech Republic; MMBC-SC, Moravian Museum, systematic collection; NIGP, Nanjing Institute of Geology and Palaeontology, Nanjing, China.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:59D7E8F0-32D0-4718-919F-FFD25A15C044
Table 1. List of fossils described within the melyrid lineage of the superfamily Cleroidea. Species within particular families are ordered by age of the deposit († denotes genera containing exclusively fossil species).
|
Taxon/author |
Locality |
Age |
Classification |
Note |
|
Acanthocnemoides sukatshevae Zherikhin, 1977† |
Russia: N Siberia, Taimyr Penninsula |
Cretaceous amber, Santonian |
Acanthocnemidae? |
|
|
Idgiaites jurrasicus Liu, Ślipiński, Leschen, Ren, and Pang, 2015† |
China: Inner Mongolia Daohugou |
Middle Jurassic, Callovian–Oxfordian |
Prionoceridae |
|
|
Prionocerites tattriei Lawrence, Archibald, and Ślipiński, 2008† |
Canada: British Columbia |
Eocene |
Prionoceridae |
larva |
|
Aploceble berendti Majer, 1998† |
Germany |
Baltic amber, Eocene–Oligocene |
Rhadalidae |
|
|
Aploceble fuscipes Majer, 1998† |
Germany |
Baltic amber, Eocene–Oligocene |
Rhadalidae |
|
|
Aploceble kunowi Majer, 1998† |
Germany |
Baltic amber, Eocene–Oligocene |
Rhadalidae |
|
|
Aploceble viridiaeneus Chernyshev, 2012 |
Russia: Kaliningrad |
Baltic amber, Eocene–Oligocene |
Rhadalidae |
|
|
Eudasytes listriformis Wickham, 1912† |
USA: Colorado |
Florissant shales, upper Miocene |
Dasytidae |
revised by Mawdsley (1999) |
|
Eutrichopleurus miocenicus (Wickham, 1912) |
USA: Colorado |
Florissant shales, upper Miocene |
Dasytidae |
revised by Mawdsley (1999) |
|
Malachius immurus Wickham, 1917 |
USA: Colorado |
Florissant shales, upper Miocene |
Malachiidae |
unrevised classification |
|
Colotes constantini Kirejtshuk and Nel, 2008 |
France |
Oise amber, lower Eocene |
Malachiidae |
|
|
Colotes impexus Kirejtshuk and Nel, 2008 |
France |
Oise amber, lower Eocene |
Malachiidae |
|
|
Colotes sambicus Kubicz, 2001 |
Poland |
Baltic amber, Eocene–Oligocene |
Malachiidae |
|
|
Protocephaloncus
perkovskyi |
Ukraine |
Rovno amber, upper Eocene |
Malachiidae |
|
|
Palpattalus baltiensis Chernyshev, 2016† |
? |
Baltic amber, Eocene–Oligocene |
Malachiidae |
|
|
Palpattalus eocenicus Chernyshev, 2016† |
? |
Baltic amber, Eocene–Oligocene |
Malachiidae |
|
|
Troglops punctatissima Théobald, 1937 |
France |
upper Miocene |
Malachiidae |
unrevised classification |
Material and methods
Four fossil specimens are well-preserved in the finely laminated, pale grey tuffaceous lacustrine deposit. They come from localities near Daohugou (for details of the locality, see Huang et al. 2006). All the material is more-or-less three-dimensionally preserved and partly covered with a dark carbon film; the counterparts were not found. Preparation consisted of separating off the tuff layers with a sharp blade. Photomicrographs of the fossils were taken with a Zeiss Discovery V20 microscope equipped with an AxioCam HSc camera, then refined in Photoshop (Adobe CS6). Body parts were measured by means of AxioVision 4.8 software. Some photographs were taken with the specimens under alcohol for better detail definition and others at lower angles of light to allow for three-dimensional acquisition. Helicon Focus 4.21 software was also used to stack certain images. All drawings were based on both photographs and the original specimens. Blurred contours are indicated by broken lines. The dorsal habitus photographs of Recent, specimens were taken by a Canon EOS 6D with an MP-E 65 mm macro lens and Leica Z16Apo. LAS 3.6.0 software was used to stack the images. Adobe CS6 (Illustrator 16.0.3 and Photoshop 13.0.1) was used for reconstruction of the both species.
Systematic palaeontology
Order Coleoptera Linnaeus, 1758
Superfamily Cleroidea Latreille, 1802
Remarks.—The melyrid “stock” is a term introduced by Majer (1994a) for the cleroid families Acanthocnemidae, Mauroniscidae, Prionoceridae, Dasytidae, Melyridae, Malachiidae, and Attalomimidae. Bocakova et al. (2011) presented a molecular phylogenetic analysis of the lineage, established the monophyletic Rhadalidae, and confirmed Dasytidae, Malachiidae, Melyridae, Prionoceridae, and Mauroniscidae as separate cleroid families. Recently, the comprehensive phylogeny of Cleroidea based on 4-gene analyses (Gimmel et al. 2019) has supported Rhadalidae and Mauroniscidae as the most basal clades of the melyrid lineage, a diversification of which was followed by separation of Prionoceridae and Melyridae sensu lato (incl. Dasytinae and Malachiinae). On the other hand, morphologically-based modern phylogenies (i.e., Lawrence et al. 2011), as summarized by Bouchard et al. (2011), recognise Rhadalinae, Dasytinae, and Malachiinae as subfamilies within Melyridae sensu lato. Two further related families, Attalomimidae and Gietellidae, established by Majer (1994a, b) were generally included in Malachiidae and Dasytidae respectively, although Gietella Constantin and Menier, 1987 has been found as a derived apterous group of Rhadalidae (Gimmel et al. 2019). Crowson (1960, 1964), Lawrence and Newton (1982), and Majer (1994a) proposed closer relationships among Acanthocnemidae, Phycosecidae, Prionoceridae, and Melyridae sensu lato. Recent molecular phylogenies of beetles (Bocak et al. 2014; McKenna et al. 2015) classify Acanthocnemidae as one of basal groups of all Cleroidea, confirm a position for Phycosecidae at the very base of the melyrid lineage, and establish Prionoceridae as actually part of the lineage (cf. Majer 1987 who proposed a relation of Prionoceridae with Cleridae). Thus, the melyrid lineage includes the families Phycosecidae, Rhadalidae, Mauroniscidae, Prionoceridae, Melyridae, Dasytidae, and Malachiidae (see Crowson 1964; Peacock 1987; Majer 1994a, 1995, 2002; Bocakova et al. 2011; Bocak et al. 2014; McKenna et al. 2015). This concept is applied throughout this paper.
Family Melyridae Leach, 1815
Subfamily Melyrinae Leach, 1815
Genus Sinomelyris nov.
ZooBank LSID: urn:lsid:zoobank.org:act:CF56C67A-FD59-42CC-A 38F-3ABC467C0D99
Type species: Sinomelyris praedecessor sp. nov.; by monotypy, see below.
Etymology: From the Latin sina, referring to China, and the nominotypical genus Melyris, associated the name with the family Melyridae. Masculine in gender.
Diagnosis.—As for the type species by monotypy.
Sinomelyris praedecessor sp. nov.
Figs. 1, 2A, 4A.
ZooBank LSID: urn:lsid:zoobank.org:act:5A512392-F673-480C-ADBB-52916821AB66
Etymology: From a Latin praedecessor, ancestor.
Type material: Holotype: NIGP168476 (Fig. 1B); sex unknown, counterpart absent; perfect dorsal side of specimen preserved, elytra open, meso- and metathorax and abdomen visible. Paratypes: NIGP168477 (Fig. 1A); sex unknown, counterpart absent; perfect dorsal side of specimen preserved, elytra partly open, antennae missing, legs partly visible; NIGP168478 (Fig. 2A); sex unknown, counterpart absent; perfect dorsal side of specimen preserved incl. fragment of antenna. All from the type locality.
Type locality: Daohugou Village, Shantou Township, Ningcheng County, Inner Mongolia, China.
Type horizon: Haifanggou Formation, Callovian, Middle Jurassic.
Diagnosis.—Relatively small species (body length 6.2–6.8 mm); body bare; pronotum with single pair of carinae; anterior corners of prothorax obtuse; each elytron with four carinae; tarsomere 1 approximately as long as tarsomere 2 in all pairs of legs; tarsal claw simple.
Description.—Body length (from elytral apex to clypeus): holotype 6.2 mm, paratypes 6.8 and 6.4 mm. For other measurements of visible body parts see Table 2.
Table 2. Measurements (in mm) of visible body parts in Sinomelyris praedecessor gen. et sp. nov.
|
Measurement |
NIGP168476 (holotype) |
NIGP168477 (paratype) |
NIGP168478 (paratype) |
|
|
Body |
length (elytral apex to clypeus) |
6.23 |
6.76 |
6.38 |
|
maximum width |
2.05 |
~ 2.6 |
2.18 |
|
|
Head |
length (base to clypeus) |
– |
– |
0.95 |
|
maximum width (including eyes) |
1.10 |
1.26 |
1.19 |
|
|
minimum width (between eyes) |
– |
– |
0.68 |
|
|
Eye (longitudinal diameter) |
– |
– |
0.45 |
|
|
Antenna (scape to pedicel) |
– |
– |
0.28 |
|
|
Pronotum |
length |
1.36 |
1.38 |
1.45 |
|
minimum width |
1.72 |
2.25 |
2.06 |
|
|
approx. anterior width |
– |
1.36 |
– |
|
|
approx. posterior width |
– |
1.82 |
– |
|
|
Mesoventrite length |
~ 0.6 |
– |
– |
|
|
Metaventrite length |
~ 0.9 |
– |
– |
|
|
Elytron |
width (at base) |
1.02 |
1.12 |
1.09 |
|
maximum width |
1.21 |
1.32 |
1.09 |
|
|
maximum length |
3.65 |
4.35 |
3.90 |
|
|
Metatibia length |
1.00 |
– |
– |
|
|
Metafemur length |
0.67 |
– |
– |
|
|
Metatarsus length (including claw) |
0.83 |
– |
– |
|
|
Metatarsal claw length |
0.18 |
– |
– |
|
|
Abdominal ventrites 1–5 |
2.01 |
– |
– |
|
Coloration and sculpture: body elongate, rather flat; entire dorsal surface either unicolorous or each elytron with two colour patches, in anterior and posterior thirds. Head coarsely, densely punctate (punctures touch one another; interspaces not larger than half of puncture diameter, mostly reduced to border between neighbouring punctures); pubescence not observed. Pronotum very coarsely and densely punctate (interspaces less than diameter of punctures, most of which touch one another); punctures much larger than those on head, their floors flat, rounded or polygonal in shape; some punctures with short seta growing from the centre. Elytron very coarsely sculptured; punctures large, irregularly shaped, touching one another without distinct interspaces; dorsal surface including humeral margin bare, elytron without pubescence. Ventral surface, antenna and legs without coarse punctation or distinct sculpture and without pubescence.
Head: frons distinctly flat; clypeus distinct, extended anteriorly but frontoclypeal suture absent; antennal socket visible from above; eye relatively large (space between eyes approximately two times as wide as eye diameter), distinctly elevated, not emarginate, elliptic, finely facetted; head distinctly narrowed behind eyes.
Mouthparts: labrum oblong, not emarginate anteriorly; mandible robust, apical tooth acuminate.
Antenna: scape robust, as large as three following antennomeres together; antennomeres progressively dilated from fourth one (only 8 antennomeres recognised in the paratype NIGP168478); whole antenna would be approximately as long as head.
Prothorax: pronotum transverse, approximately 1.4–1.6 times as wide as long; anterior margin straight, not emarginate, anterior corners not projecting, rounded; lateral edge present, evenly rounded; carina along lateral margin of pronotum absent; sides of posterior margin of pronotum sinuate.
Mesothorax: mesonotum transverse; mesocoxal cavities narrowly separated. Each elytron with four distinctly elevated carinae, suture also elevated; interspaces between carinae with two or three rows of punctures.
Metathorax: metaventrite flat and wide, weakly transverse, narrowed towards anterior portion; discriminal line (discrimen) conspicuous along whole length; metacoxal cavities narrowly separated. Membranous wing fully developed.
Legs: mesocoxae oval, metacoxae extended to lateral margin of metathorax; femora weakly clavate; tibiae without row of spines along outer margin, apical spines not observed; tarsomere 1 conspicuous, approximately as long as tarsomere 2; tarsomere 4 approximately as long as 3; tarsomere 5 as long as, or slightly longer than, 1–4 combined; tarsal lobes absent but ventral side of tarsomeres setose; claws large, without denticles; empodium probably small (not observed in well-preserved metatarsus of paratype NIGP168477); tarsal formula unknown, metatarsi 5-segmented.
Abdomen: five ventrites distinctly visible; pygidium (sex unknown) nearly straight along apical margin.
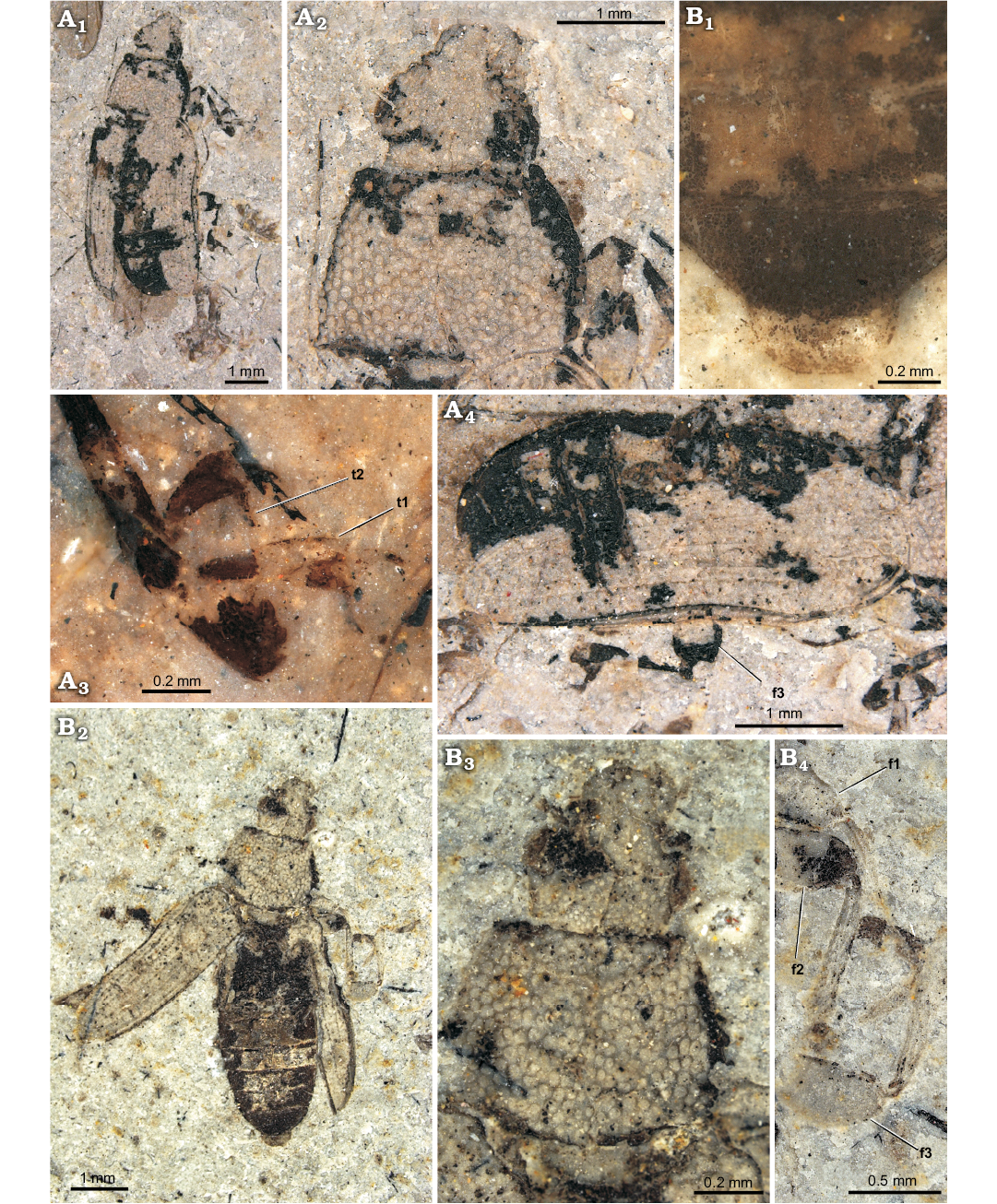
Fig. 1. Melyrid beetle Sinomelyris praedecessor gen. et sp. nov. from the Middle Jurassic Haifanggou Formation of Daohugou Village, Inner Mongolia, China. A. Paratype, NIGP168477; dorsal habitus (A1), pronotum and head dorsally (A2), detail of front and middle legs (A3), right elytron and hind leg dorsally (A4). B. Holotype, NIGP168476; abdominal apex dorsally (B1), dorsal habitus (B2), pronotum and head dorsally (B3), detail of front, middle and hind legs (B4). Abbreviations: f1–3, femora of 1st to 3rd pair of legs; t1–2, tarsi of 1st to 2nd pair of legs.
Remarks.—Habitus as well as body size in the monotypic genus similar to that of extant melyrids of the subfamily Melyrinae (or Melyrini sensu Bouchard et al. 2011), such as, for example, Melyris Fabricius, 1775 or Falsomelyris Pic, 1913 (Fig. 3A1, B2). Pronotum with posterior (basal) margin sinuate at the sides (cf. Figs. 2A2, 3A); punctation of head and pronotum composed of very large punctures with flat floors and a short seta at centre (a pattern denoted as the granular punctation by Kolibáč and Huang [2016]; cf. Figs. 1A2, B3, 3B1); elytron with sharp, distinct carinae (cf. Figs. 1A4, B2, 3B2); elytral sculpture very coarse, irregular, resembling extant members of Melyris; fifth tarsomere as long as tarsomeres 1–4 together; tarsal claw simple, without denticles, as in Melyrodes Gorham, 1882 (Majer 1994a; Constantin 2008). The two carinae running along lateral margins of pronotum in most of the extant species of Melyris (Fig. 3A2) absent in the fossils as well as in Chalchas Blanchard, 1845, and other melyrines. Antennomeres from fourth to eighth progressively dilated (Fig. 2A1); in the extant melyrines antenna serrate from fourth antennomere.
Stratigraphic and geographic range.—NE China: Inner Mongolia, Daohugou; Middle Jurassic, Callovian.

Fig. 2. Melyrid beetle Sinomelyris praedecessor gen. et sp. nov. (A) and cleroid beetle Juraniscus majeri gen. et sp. nov. (B) from the Middle Jurassic Haifanggou Formation of Daohugou Village, Inner Mongolia, China. A. Paratype, NIGP168478; head (A1), dorsal habitus (A2). B. Holotype, NIGP168479; mouth parts dorsally (B1), metatarsus dorsally (B2), dorsal habitus (B3), antenna dorsally (B4). Abbreviations: f1, front femur; l, labrum; m, mandible; p, terminal palpomere of maxillary palp.
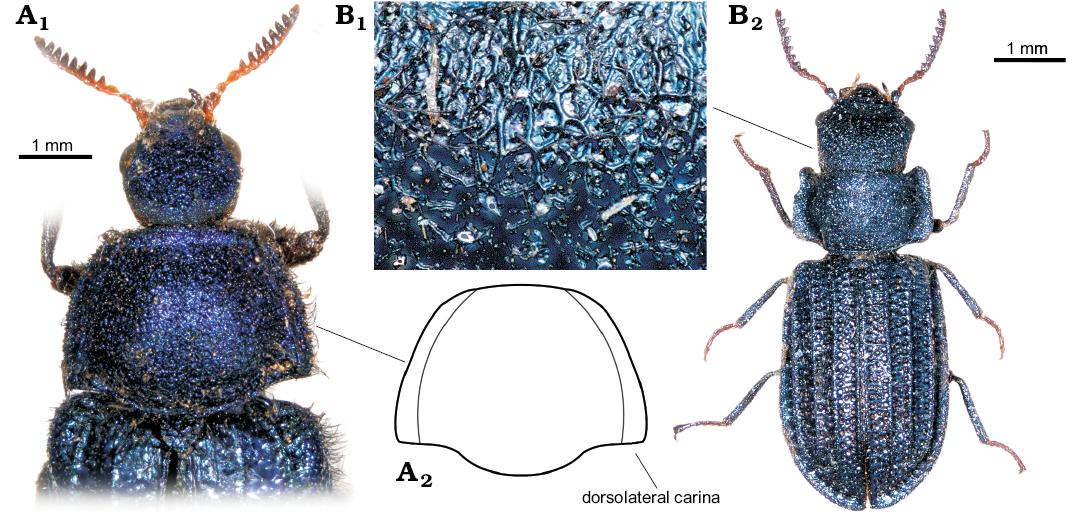
Fig. 3. Extant melyrid beetles Melyris abdominalis Fabricius, 1787 from Senegal and Falsomelyris granulatus Fabricius, 1792 (A) and from Hammoum, Algeria (B). A. MMBC-SC; base of elytra, pronotum, and head dorsally (A1), outline of pronotum (A2). B. MMBC-SC; detail of sculpture of head (B1), dorsal habitus (B2).
Superfamily Cleroidea Leach, 1815
Melyrid lineage, family indet.
Remarks.—As explained below, the new species is a member of the melyrid lineage but it cannot be unambiguously classified within one of the families Mauroniscidae, Rhadalidae, Dasytidae, and Malachiidae. Therefore, the new species is formally classified as Cleroidea incertae sedis.
Genus Juraniscus nov.
ZooBank LSID: urn:lsid:zoobank.org:act:ECBE11A9-9E38-4D59-AAC2-9774EADEF2DD
Type species: Juraniscus majeri sp. nov., by monotypy, see below.
Etymology: A combination of Jura-, referring to the Jurassic period, and the -niscus derived from the generic name Mauroniscus, the genus that, of all the extant melyrid lineage members, resembles the new fossil genus the most. Masculine in gender.
Diagnosis.—As for the type species by monotypy.
Juraniscus majeri sp. nov.
Figs. 2B, 4B.
ZooBank LSID: urn:lsid:zoobank.org:act:6E188F79-0922-46BE-88CB- 748A6ECBA438
Etymology: Named in memory of Karel Majer (1950–2000), an eminent researcher of the melyrid lineage of Cleroidea, for his contribution to the systematics and morphology of the group.
Type material: Holotype NIGP168479; male, counterpart absent; perfect dorsal side of specimen preserved, elytra semitransparent (abdomen and metathorax partly visible).
Type locality: Daohugou Village, Shantou Township, Ningcheng County, Inner Mongolia, China.
Type horizon: Haifanggou Formation, Callovian, Middle Jurassic.
Diagnosis.—Antennomeres symmetrical including three terminal ones, progressively dilated from the fifth onwards; pronotum approximately 1.4 times as wide as long; anterior corners of pronotum weakly projecting; meso- and metatarsi with tarsomere 1 conspicuous, slightly shorter than tarsomere 2; tarsal lobes present in tarsomeres 1–4; tarsal claws without denticles.
Description.—Body length (from elytral apex to clypeus): 4.25 mm. For other measurements of visible body parts see Table 3.
Table 3. Measurements (in mm) of visible body parts of Juraniscus majeri gen. et sp. nov.
|
Measurement |
NIGP168479 (holotype) |
|
|
Complete length |
5.56 |
|
|
Body |
length (elytral apex to clypeus) |
4.25 |
|
maximum width |
2.52 |
|
|
Head |
length (base to clypeus) |
~0.8 |
|
maximum width (including eyes) |
0.86 |
|
|
minimum width (between eyes) |
~0.5 |
|
|
Eye longitudinal diameter |
~0.2 |
|
|
Antenna length |
1.45 |
|
|
Scape length |
~0.17 |
|
|
Pedicel length |
~0.13 |
|
|
Pronotum |
length |
0.81 |
|
maximum width |
1.18 |
|
|
approx. anterior width |
~0.8 |
|
|
approx. posterior width |
~0.9 |
|
|
Elytron |
width (at base) |
0.77 |
|
maximum width |
1.26 |
|
|
maximum length |
2.94 |
|
|
Metafemur length |
~0.9 |
|
|
Metatibia length |
~0.9 |
|
|
Metatarsus length (including claw) |
~0.8 |
|
|
Mesotibia length |
~0.8 |
|
|
Mesotarsus length (including claw) |
~0.6 |
|
|
Abdominal ventrites 1–6 |
~2.0 |
|
Coloration and sculpture: body elongate, weakly convex; body surface unicolorous, without distinct colour stripes or patches, merely remnants of dark pigmentation. Head with very fine sculpture composed of round punctures; interspaces between punctures as large as puncture diameter. Pronotum with similar fine sculpture but interspaces larger than puncture diameter; pronotal punctures without seta at centre. Elytron without carinae, with sparse sculpture composed of small, round punctures; interspaces the same size as, or larger than, puncture diameter; punctation appears irregular. Body completely without pubescence, short, sparse setae observed only on tibiae and labrum.
Head: frons flat; clypeus not extended anteriorly, distinctly separated by arcuate frontoclypeal suture; antennal socket visible from above; eye relatively large (space between eyes approximately three times eye diameter), distinctly elevated, not emarginate, elliptic, finely facetted; head inconspicuously narrowed behind eyes.
Mouthparts: mandibles medium-sized (not unidentate, slender and sharp), bidentate, acuminate; labrum oblong, weakly emarginate along anterior margin; maxillary palps 3-segmented, terminal palpomere slender, conical.
Antenna: 11-segmented; scape longer and wider than pedicel but not larger than two following antennomeres together; antennomeres symmetrical, progressively dilated from the fifth onwards; whole antenna reaches approximately as far as posterior margin of prothorax. Both antennae recognised in the fossil specimen.
Prothorax: pronotum weakly transverse, approximately 1.4 times as wide as long; anterior margin straight, not emarginate, anterior corners weakly projecting; lateral edge present, evenly rounded; carina along lateral margin of pronotum absent; posterior margin of pronotum arcuate, posterior corners conspicuous but not projecting.
Mesothorax: mesonotum transverse; mesocoxal cavities narrowly separated; membranous wing present.
Metathorax: metaventrite flat and wide, transverse; metacoxal cavities narrowly separated. Elytron with rounded humeral corner, without carinae.
Legs: mesocoxae distinctly oval, metacoxae extended to lateral margin of metathorax; femora very weakly clavate. Meso- and metatibia and meso- and metatarsus clearly visible: tibia without row of distinct spines along outer margin but with conspicuous edge along outer side; metatibia with two apical spines (not visible in mesotibia); tarsomere 1 conspicuous, slightly shorter than tarsomere 2; tarsomere 4 approximately as long as 3; tarsomere 5 as long as 1–4 combined; tarsal lobes distinctly present in tarsomeres 1–4; claws without denticles; empodium probably small (not observed in well-preserved tarsi); tarsal formula unknown, meso- and metatarsi 5-segmented.
Abdomen: six ventrites distinctly visible; cucujoid tegmen with separated parameres present.
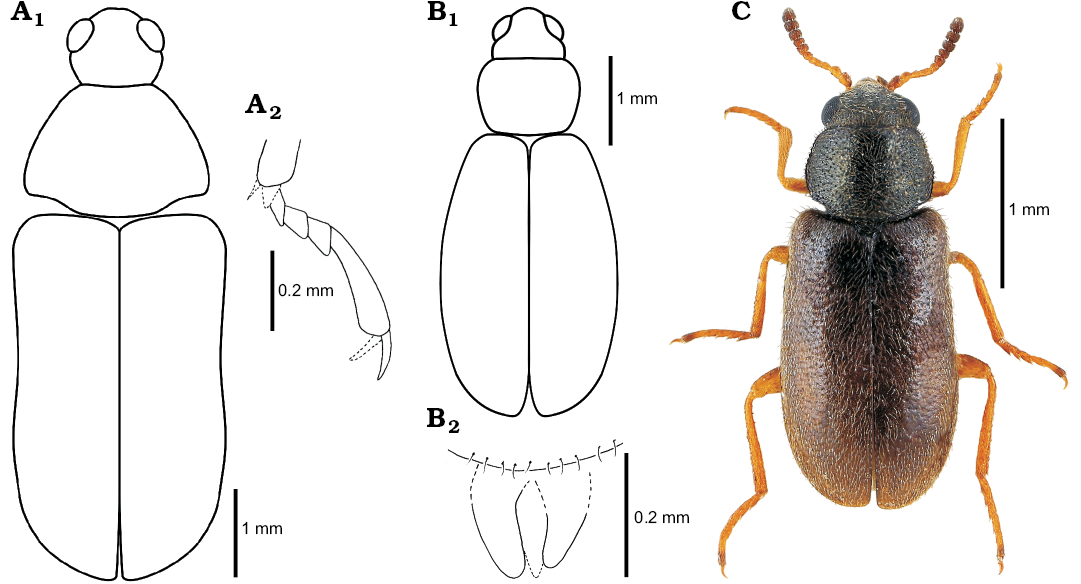
Fig. 4. Melyrid beetle Sinomelyris praedecessor gen. et sp. nov. (A), cleroid beetle Juraniscus majeri gen. et sp. nov. (B) from the Middle Jurassic, Haifanggou Formation of Daohugou Village, Inner Mongolia, China, and extant mauroniscid beetle Mauroniscus titschacki Pic, 1954 (C) from Cuzco, Peru. A. NIGP168477; reconstruction of body shape (A1), mesotarsus laterally (A2). B. NIGP168479; reconstruction of body shape (B1), assumed apex of aedeagus dorsally (B2). C. MMBC-SC, dorsal habitus.
Remarks.—Habitus similar to that of Mauroniscidae (Fig. 4C), Rhadalidae, Dasytidae, and Malachiidae, with tendency to soft-bodiedness. Sparse sculpture of elytra irregular, composed of fine punctures as in members of above mentioned families. Structure of antenna (Fig. 2B4) closely comparable with non-serrate but progressively dilated antennomeres in Mauroniscidae (e.g., Amecomycter Majer, 1995, Mauroniscus Bourgeois, 1911, Scuromanius Majer, 1995), Dasytidae: Danaceinae (e.g., Danacaea Laporte de Castelnau 1836, Hylodanacaea Pic, 1926), and Dasytidae: Chaetomalachiinae (e.g., Dasytiscus Kiesenwetter, 1859, and Eutrix Majer, 1996). Antennomeres from fourth or fifth distinctly asymmetrical in dasytids and weakly asymmetrical in mauroniscids (cf. Majer 1994a, 1995, 1996, 1999). Tarsomeres 1–4 with small, non-membranous lobes and long, distinct setae directed forwards as in Prionoceridae and some Dasytidae and Malachiidae. Claws simple, without denticles as in mauroniscids; membranous appendages of claws absent or not preserved. Abdomen with six ventrites as in all members of the melyrid lineage apart from Phycosecidae (ventrites I and II connate in Rhadalidae, see Peacock [1987], but their suture is clearly visible). Tegmen with separated parameres as in mauroniscids (Majer 1995) and exceptionally also in dasytids (Constantin and Menier 1987; Majer 1999).
Stratigraphic and geographic range.—NE China: Inner Mongolia, Daohugou; Middle Jurassic, Callovian.
Discussion
Beetles of the melyrid lineage differ from other cleroid families in their combination of the following morphological characters: (i) head with protracted clypeus (or “clypeal area”, since the frontoclypeal suture is usually inconspicuous); (ii) eyes large, finely facetted, elevate, not emarginate; (iii) mandible bidentate; (iv) antennomeres progressively dilated from 4th or 5th, without 3-segmented club (distinct 1-segmented club present only in Phycosecidae); (v) tarsal claws usually with denticles and membranous appendages (excepting Phycosecidae, Mauroniscidae, some Prionoceridae); (vi) elytral punctation irregular (excepting some Phycosecidae); (vii) aedeagus inverted, consisting of a single part; (viii) parameres not separated from phallobase, usually mutually coalescent (excepting Prionoceridae, Mauroniscidae, Phycosecidae); (ix) tendency to soft-bodiedness (Bocakova et al. 2016). Both taxa described herein agree in all observed characters with such delineation of the melyrid lineage excluding inverted/uninverted position and internal structure of the aedeagus which is not visible. Although the feature cannot be perfectly proved in fossils, the Sinomelyris praedecessor sp. nov. body does not show a tendency to soft-bodiedness: its elytra braced with carinae and coarsely sculptured head and pronotum seem as robust and hard as in the extant melyrines.
Sinomelyris gen. nov. is similar to the extant beetles of the genus Melyris, from which it can be distinguished by a unique combination of features visible in the three fossils: (i) number of elytral carinae (four instead of three), (ii) absence of pair of dorsolateral pronotal carinae and (iii) simple tarsal claws. The two latter characters can be considered plesiomorphic and are also known in other melyrids, for example Melyrodes. The very characteristic type of cranial and pronotal sculpture, composed of large, mutually connected flat punctures, each with a central seta growing from small tubercle (“granular punctation”; Figs. 1B3, 3B1) also occurs in the Korynetes-group of Cleridae as well as in the earliest Middle Jurassic clerid species Protoclerus korynetoides Kolibáč and Huang, 2016. The structure confirms the hypothesised sister-relationship between the melyrid and clerid lineages proposed by Kolibáč and Huang (2016) and supported by a certain amount of molecular evidence (Hunt et al. 2007; Bocakova et al. 2011, 2016; Robertson et al. 2015). The evident similarity between Sinomelyris gen. nov. and Melyris indicates that the latter extant genus has changed only slightly over the course of approximately 163 Ma. In the light of the biology of more than fifty extant species of Melyris, Sinomelyris praedecessor gen. et sp. nov. may well have been floricolous. Considering up to date palynological analysis of the Haifanggou Formation (Zhang et al. 2018) showing a dominancy of gymnosperm pollen, we can suppose feeding on the pollen grains and pollination of cycads (cf. Labandeira 2010; Renner 2011; Tang et al. 2018) in S. praedecessor sp. nov. It has been suggested for Palaeoboganium Liu, Ślipiński, Lawrence, Ren, and Pang, 2017 (Liu et al. 2018) and Parandrexis Martynov, 1926 (Kirejtshuk 1994; Kolibáč 2012) of the same fossil assemblage as well. Melyrid larvae are predatory and tend to dwell under the bark of trees and shrubs, a mode of life similar to its relatives in Cleridae and Trogossitinae (Kolibáč 2010; Kolibáč and Leschen 2010). In modern times, Melyridae are distributed worldwide in tropical to temperate zones, except for the Oriental and Australian regions (Majer 1994a). Melyris and its close relative Falsomelyris are widespread in warm or temperate areas of Europe, northern to southern Africa, western to central Asia, and from the warm areas of North America to tropical South America. However, both genera are absent from modern Asia east of Karakoram mountains (Majer 1994a; Mayor 2007) where the fossils of Sinomelyris praedecessor gen. et sp. nov. were found. Wider distribution of some beetle groups in Mesozoic and their later extinction in eastern Asia have already been mentioned for example by Kolibáč and Huang (2016), Liu et al. (2018), and Yu et al. (2017). The reason why some widespread extant taxa are absent in eastern and southeastern Asia has not been explained so far, however, it is known that a large part of the Recent insect fauna of the region is of Gondwanan origin (Sklenarova et al. 2013; Kolibáč 2012; Poinar 2018). We can only presume that diversity of the original Mesozoic insect fauna of the present eastern Asia was reduced during Late Cretaceous–early Cenozoic climatic changes and intense volcanism in combination with processes which later formed Himalayas and Tibetan plateau (Briggs 2003; Karanth 2006; Raven and Axelrod 1974; Sahni 1984).
Juraniscus gen. nov. cannot be unambiguously classified within any of families of the melyrid lineage because its extant representatives, namely Mauroniscidae, Rhadalidae, Dasytidae, and Malachiidae, are similar to each other in: (i) general body shape and size, (ii) tendency to soft-bodiedness, (iii) elevate fine-facetted eye, (iv) anteriorly extended clypeal area, and (v) absence of antennal club. The broadly oval elytra, distinctly wider than the pronotum at their broadest part, occur often in Mauroniscidae (Amecomycter Majer, 1995, Mecomycter Horn, 1882) and Malachiidae (e.g., Colotes Erichson, 1840, Ebaeus Erichson, 1840, Laius Guérin-Méneville, 1838), sparsely in Dasytidae (e.g., Danaceothrix Majer, 1989) and never in Rhadalidae. Other distinguishing characters of Juraniscus majeri gen. et sp. nov. are in agreement with those of the mauroniscids; however, these are presumably plesiomorphic, rendering, their phylogenetic value doubtful. The fossil may constitute a basal group within some of the other families mentioned above. The plesiomorphic character states are: (i) tarsal claw simple, without denticle; (ii) dilated antennomeres symmetrical (weakly asymmetrical in Mauroniscidae, distinctly asymmetrical in other families); (iii) aedeagus, if the authors’ interpretation of the fossil is correct, with separated parameres (very seldom present in Rhadalidae [Kubanius Majer, 1983], unknown in Dasytidae and Malachiidae). The larvae of all the four families mentioned are presumed to be predatory. They often feed on eggs of other insects or hunt for small insect larvae or adults (Kolibáč et al. 2005). Adults frequently dwell on thin branches, leaves, needles and the cones of trees and shrubs as well as the stems and leaves of herbs. They are, however, mostly floricolous, either predatory or pollinivorous or both (Crowson 1964; Peacock 1987; Kolibáč et al. 2005). Such a mode of life may also be proposed for Juraniscus majeri gen. et sp. nov. Extant Mauroniscidae are distributed only in North and South America while other relevant families are widespread: Rhadalidae in all biogeographical regions excluding the Austral realm, Dasytidae and Malachiidae worldwide. If Juraniscus majeri gen. et sp. nov. is actually a relative of the mauroniscids, a wider distribution of the beetle family in the Mesozoic may be assumed. Similar conclusions about the wider distribution of recently American cleroid groups in the past may also be drawn from findings of Mesozoic Cleridae: Epiclininae (Wangweiella calloviana; Inner Mongolia; Kolibáč and Huang 2016) and Thanerocleridae: Zenodosinae (Archaeozenodosus bellus; Myanmar; Yu et al. 2017).
Conclusions
The melyrid lineage with more than 5000 extant species forms the largest group of Cleroidea (>10 000 extant species). Although a few cleroid representatives of all major families have already been described from Mesozoic, no records from the species-rich families Rhadalidae and Melyridae sensu lato were hitherto known. Sinomelyris praedecessor gen. et sp. nov. constitutes the first Mesozoic record and earliest known representative of Melyridae. Three fossils of the species can be safely associated within the family and show surprisingly similar habitus and outer features with some extant melyrine genera (for example Melyris Fabricius, 1775, Falsomelyris Pic, 1913). Considering the biology of extant species of Melyris, Sinomelyris praedecessor gen. et sp. nov. adults may well have been floricolous, living on cycads or early flowering angiosperms. The second described cleroid beetle, Juraniscus majeri gen. et sp. nov., may be also associated within the melyrid lineage, however, its family assignment is not perfectly clear. Therefore the fossil is classified as Cleroidea incertae sedis although most of its outer morphological characters as well as a structure of the aedeagus are in agreement with the recent species-poor American family Mauroniscidae. Considering a distribution of the Recent Melyridae and Mauroniscidae, which are absent in eastern Asia, wider distribution of the both families in the Jurassic is supposed.
Acknowledgements
We would like to extend our thanks to the colleagues who helped to improve a draft of this work and to all reviewers and editors for their valuable comments. Tony Long (Svinošice, Czech Republic) helped work up the English. The project was supported by the National Key R&D Program of China (2016YFC0600406), Strategic Priority Research Program (B) (XDB18000000) and the programme “Macroevolutionary Processes and Palaeoenvironments of Major Historical Biota” (XDPB05) of the Chinese Academy of Sciences, and by the National Natural Science Foundation of China (41688103). This publication also appears through financial support provided to the Moravian Museum by the Ministry of Culture of the Czech Republic, as part of its long-term conceptual development program for research institutions (ref. MK000094862).
References
Blanchard, M.C. 1845. Histoire des Insectes, traitant de leurs moeurs et le leurs métamorphoses en général, et comprenant une nouvelle classification fondée sur leurs rapports naturels. Tom 2. 398 + 524 pp. Didot, Paris.
Bocak, L., Barton, C., Crampton-Platt, A., Chester, D., Ahrens, D., and Vogler, A.P. 2014. Building the Coleoptera tree-of-life for >8000 species: composition of public DNA data and fit with Linnaean classification. Systematic Entomology 39: 97–110. Crossref
Bocaková, M., Bocak, L., Gimmel, M.L., Motyka, M., and Vogler, A. 2016. Aposematism and mimicry in soft-bodied beetles of the superfamily Cleroidea (Insecta). Zoologica Scripta 45: 9–21. Crossref
Bocaková, M., Constantin, R., and Bocak, L. 2011. Molecular phylogenetics of the melyrid lineage Coleoptera: Cleroidea). Cladistics 27: 1–13.
Bourgeois, J. 1911. Mission géodésique de l’Équateur. Collections recueillies par le Dr. P. Rivet. Coléoptères: Mélyrides. Bulletin du Muséum d’Histoire Naturelle Paris 17: 207–218.
Bouchard, P., Bousquet, Y., Davies, A.E., Alonso-Zarazaga, M.A., Lawrence, J.F., Lyal, C.H.C., Newton, A.F., Reid, C.A.M., Schmitt, M., Slipinski, S.A., and Smith, A.B.T. 2011. Family-group names in Coleoptera (Insecta). ZooKeys 88: 1–972. Crossref
Briggs, J.C. 2003. The biogeographic and tectonic history of India. Journal of Biogeography 30: 381–388. Crossref
Cai, C. and Huang, D. 2018. First fossil thaneroclerid beetle from mid-Cretaceous Burmese amber (Coleoptera: Cleroidea: Thanerocleridae). Alcheringa 42: 115–119. Crossref
Chang, S.C., Zhang, H., Hemming, S.R., Mesko, G.T., and Fang, Y. 2014. 40Ar/39Ar age constraints on the Haifanggou and Lanqi Fms: When did the first flowers bloom? Geological Society, London, Special Publications 378: 277–284. Crossref
Chen, W., Ji, Q., Liu, D., Zhang, Y., Song, B., and Liu, X. 2004. Isotope geochronology of the fossil-bearing beds in the Daohugou area, Ningcheng, Inner Mongolia [In Chinese with English abstract]. Regional Geology of China 23: 1165–1169.
Chernyshev, S.E. 2012. Aploceble (Chalcoaploceble) viridiaeneus Tshernyshev—new subgenus and species of dasytid beetles (Coleoptera, Dasytidae) in Baltic Amber. Euroasian Entomological Journal 11: 212–218. Crossref
Chernyshev, S.E. 2016. New taxa of soft-winged flower beetles (Coleoptera, Malachiidae) in Baltic and Rovno Amber. Paleontological Journal 50: 953–962.
Constantin, R. 2008. Description of a new species of Melyrodes Gorham, 1882 from Ecuador (Coleoptera, Melyridae). Biodiversity of South America I. Memoirs on Biodiversity (Verona) 1: 465–468.
Constantin, R. and Menier, J.J. 1987. Étude d’un remarquable Melyridae aptère des Iles Canaries: Gietella fortunata, n. gen., n. sp., type d’une sous-famille nouvelle Gietellinae (Coleoptera,Cleroidea). Revue française d’Entomologie (Nouvelle Series) 9: 53–63.
Crowson, R.A. 1960. The phylogeny of Coleoptera. Annual Review of Entomology 5: 111–134. Crossref
Crowson, R.A. 1964. A review of the classification of Cleroidea (Coleoptera), with descriptions of two genera of Peltidae and of several new larval types. Transaction of the Royal Entomological Society of London 116: 275–327.
Erichson, W.F. 1840. Entomographien, Untersuchungen in dem Gebiete der Entomologie, mit besonderer Benutzung der königl. Sammlung zu Berlin. 180 pp. F.H. Morin, Berlin. Crossref
Evans, S.E. and Wang, Y. 2009. A long-limbed lizard from the Upper Jurassic/Lower Cretaceous of Daohugou, Ningcheng, Nei Mongol, China. Vertebrata PalAsiatica 47: 21–34.
Fabricius, J.C. 1775. Systema entomologiae, sistens insectorum classes, ordines, genera, species adiectis synonymis, locis, descriptionibus, observationibus. 832 pp. Korte, Flensburgi.
Gimmel, M.L., Bocakova, M., Gunter, N.L., and Leschen, R.A.B. 2019. Comprehensive phylogeny of the Cleroidea (Coleoptera: Cucujiformia). Systematic Entomology [published online 10.1111/syen.12338]. Crossref
Giribet, G., Tourinho, A.L., Shih, C.K., and Ren, D. 2012. An exquisitely preserved harvestman (Arthopoda, Arachnida, Opiliones) from the Middle Jurassic of China. Organisms, Diversity and Evolution 12: 51–56. Crossref
Gorham, H.S. 1882. Insecta, Coleoptera, Lycidae, Lampyridae, Telephoridae, Lymexylonidae, Melyridae. In: F.D. Godman and O. Salvin (eds.), Biologia Centrali-Americana, Volume 3, Part 2, 1–112. Porter, London.
Guérin-Méneville, F.É. 1838. Insectes. In: L.I. Duperrey (Ed.), Voyage autour du monde, Exécuté par Ordre du Roi, sur La Corvette de Sa Majesté, La Coquille, pendant les années 1822, 1823, 1824 et 1825, Sous le Ministère et conformément aux Instructions de S.E.M. le Marquis de Clermont-Tonnerre, Ministre de la Marine; Et publié sous les auspices de son Excellence Mgr le Cte de Chabrol, Ministre de la Marine et des Colonies, par M.L.I. Duperrey, Capitaine de Frégate, Chevalier de Saint-Louis et Membre de la Légion d’Honneur, Commandant de l’Expedition. Zoologie, par M. Lesson, Tome Second, 2e Partie, i–xii, 9–319, 1–155. Arthus Bertrand, Libraire Editeur, Imprimerie de Firmin Didot, In-4°, Paris [plates 1 and 2 of “Insectes” published in 1830].
Hong, Y. 1983. Middle Jurassic Fossil Insects in North China [in Chinese]. 223 pp. Geological Publishing House, Beijing.
Horn, G.H. 1882. Notes on some little known genera and species of Coleoptera. Transactions of the American entomological Society 10: 113–126, 164.
Huang, D. 2015. Yangliao biota and Yanshan movement. Acta Palaeontologica Sinica 54: 501–546.
Huang, D. 2016. Daohugou Biota. 332 pp. Shanghai Scientific and Technical Publishers, Shanghai.
Huang, D. 2019. Jurassic integrative stratigraphy and timescale of China. Science China, Earth Sciences 62: 223–255. Crossref
Huang, D., Cai, C., Jiang, J., Su, Y., and Liao, H. 2015. Daohugou bed and fossil record of its basal conglomerate section. Acta Palaeontologica Sinica 54: 351–357.
Huang, D., Nel, A., Shen, Y., Selden, P., and Lin, Q. 2006. Discussions on the age of the Daohugou fauna—evidence from invertebrates. Progress in Natural Science, Special Issue 16: 308–312.
Huang, W., Sun, C., Wang, H., Na, Y., Li, Y., and Li, T. 2016. New Phoenicopsis leaves (Czekanowskiales) from the Middle Jurassic Daohugou Biota, China and their roles in phytogeographic and paleoclimatic reconstruction. Palaeoworld 25: 388–398. Crossref
Hunt, T., Bergsten, J., Levkanicova, Z., Papadopoulou, A., St. John, O., Wild, R., Hammond, P.M., Ahrens, D., Balke, M., Caterino, M.S., Gomez-Zurita, J., Ribera, I., Barraclough, T.G., Bocakova, M., Bocak, L., and Vogler, A.P. 2007. A Comprehensive Phylogeny of Beetles Reveals the Evolutionary Origins of a Superradiation. Science 318: 1913–1916. Crossref
Ji, Q. and Yuan, C.X. 2002. Discovery of two kinds of protofeathered pterosaurs in the Mesozoic Daohugou Biota in the Ningcheng Region and its stratigraphic and biologic significances [in Chinese]. Geological Review 48: 221–224.
Ji, Q., Luo, Z., Yuan, C., and Tabrum, A.R. 2006. A swimming mammaliaform from the Middle Jurassic and ecomorphological diversification of early mammals. Science 311: 1123–1127. Crossref
Karanth, K.P. 2006. Out-of-India Gondwanan origin of some tropical Asian biota. Current Science 90: 789–792.
Kiesenwetter, E.A.H. 1859. Beiträge zur Käferfauna Griechenlands. V–VI. Melyridae. Berliner entomologische Zeitschrift 3: 30–34, 160–161, 163–185, 191.
Kimura, T., Ohana, T., Zhao, L., and Geng, B. 1994. Pankuangia haifanggouensis gen. et sp. nov., a fossil plant with unknown affinity from the Middle Jurassic Haifanggou Formation, Western Liaoning, Northeast China. Bulletin of Kitakyushu Museum of Natural History 13: 255–261.
Kirejtshuk, A.G. 1994. Parandrexidae fam. nov., Jurassic beetles of the Infraorder Cucujiformia (Coleoptera, Polyphaga). Paleontological Journal 28: 69–78.
Kirejtshuk, A.G. and Nel, A. 2008. New beetles of the suborder Polyphaga from the Lowermost Eocene French amber (Insecta: Coleoptera). Annales de la Société Entomologique de France 44: 419–442. Crossref
Kolibáč J. 2010. Cleridae Latreille, 1802. In: R. Leschen, R.G. Beutel, and J.F. Lawrence (eds.), Handbuch der Zoologie/Handbook of Zoology. Band 4: Arthropoda, 2. Halfte: Insecta. Part 39: Coleoptera, Beetles Vol. 2, 257–261. W. de Gruyter, Berlin.
Kolibáč, J. 2012. The oldest fossil Cleroidea and Cucujoidea. In: Abstracts of XXIV International Congress of Entomology, August 19–25 2012, Daegu, Korea, 134. Entomological Society of South Korea, Daegu.
Kolibáč, J. and Huang, D. 2016. The oldest known clerid fossils from the Middle Jurassic of China, with a review of Cleridae systematics (Coleoptera). Systematic Entomology 41: 808–823. Crossref
Kolibáč, J. and Leschen, R.A.B. 2010. Trogossitidae Fabricius, 1801. In: R. Leschen, R.G. Beutel, and J.F. Lawrence (eds.), Handbuch der Zoologie/Handbook of Zoology. Band 4: Arthropoda, 2. Halfte: Insecta. Part 39: Coleoptera, Beetles Vol. 2, 241–247. W. de Gruyter, Berlin.
Kolibáč, J., Majer, K., and Švihla, V. 2005. Cleroidea. Beetles of the Superfamily Cleroidea in the Czech and Slovak Republics and Neighbouring Areas. 186 pp. Clarion, Praha.
Kubicz, D. 2001. Beetles in the collection of the Museum of Amber Inclusions, University of Gdańsk, with description of Colotes sambicus sp. n. (Coleoptera: Melyridae). Polskie Pismo Entomologiczne 70: 259–265.
Labandeira, C.C. 2010. The pollination of mid Mesozoic seed plants and the early history of long-proboscid insects. Annals of the Missouri Botanical Garden 97: 469–513. Crossref
Laporte de Castelnau, F.L. 1836. Études entomologiques, ou descriptions de insectes nouveaux et observations sur la synonymie. Revue entomologique 4: 5–60.
Latreille, P.A. 1802. Histoire naturelle, générale et particuliere des crustacés et des insectes. Ouvrage faisant suite a l’histoire naturelle générale et particuliere, composée par Leclerc de Buff on, et rédigée par C.S. Sonnini, membre de plusieurs sociétés savantes. Familles naturelles des genres. Tome troisieme. xii + 13–467 + [1] pp. F. Dufart, Paris.
Lawrence, J.F. and Newton, A.F., Jr. 1982. Evolution and classification of beetles. Annual Revue of Ecology and Systematics 13: 261–290. Crossref
Lawrence, J.F., Archibald, S.B. and Ślipiński, A. 2008. A new species of Prionoceridae (Coleoptera: Cleroidea) from the Eocene of British Columbia, Canada. Annales Zoologici 58: 689–693. Crossref
Lawrence, J.F., Ślipiński, A., Seago, A.E., Thayer, M.K., Newton, A.F., and Marvaldi, A.E. 2011. Phylogeny of the Coleoptera based on morphological characters of adults and larvae. Annales Zoologici 61: 1–217. Crossref
Leach, W.E. 1815. Entomology. In: D. Brewster (ed.), Brewster’s Edinburgh Encyclopedia. Volume IX [part I], 57–172. W. Blackwood, J. Waugh, etc., Edinburgh.
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. 824 pp. Salvius, Stockholm.
Liu, Y., Liu, Y., Ji, S., and Yang, Z. 2006. U-Pb zircon age for the Daohugou Biota at Ningcheng of Inner Mongolia and comments on related issues. Chinese Science Bulletin 51: 2634–2644. Crossref
Liu, Y., Liu, Y., Li, P., Zhang, H., Zhang, L., Li, Y., and Xia, H. 2004. Daohugou biota-bearing lithostratigraphic succession on the southeastern margin of the Ningcheng basin, Inner Mongolia, and its geochronology [in Chinese with English abstract]. Geological Bulletin of China 23: 1180–1185.
Liu, Z., Ślipiński, A., Lawrence, J.F., Ren, D., and Pang, H. 2018. Palaeoboganium gen. nov. from the Middle Jurassic of China (Coleoptera: Cucujoidea: Boganiidae): the first cycad pollinators? Journal of Systematic Palaeontology 16: 351–360 [published online 4 April 2017]. Crossref
Liu, Z., Ślipiński, A., Leschen, R.A.B., Ren, D., and Pang, H. 2015. The oldest Prionoceridae (Coleoptera: Cleroidea) from the Middle Jurassic of China. Annales Zoologici 65: 41–52. Crossref
Majer, K. 1983. A new genus and species of Dasytinae from Central Asia (Coleoptera, Melyridae). Acta Entomologica Bohemoslovaca 80: 385–389.
Majer, K. 1987. Comparative morphology and proposed major taxonomy of the family Melyridae (Insecta, Coleoptera). Polskie Pismo Entomologiczne 56: 719–859.
Majer, K. 1989. Generic classification of the tribe Chaetomalachiini (Coleoptera, Melyridae). Polskie Pismo Entomologiczne 58: 745–774.
Majer, K. 1994a. A review of the classification of the Melyridae and related families (Coleoptera, Cleroidea). Entomologica Basiliensia 17: 319–390.
Majer, K. 1994b. Gietellidae, full family status for Melyridae: Gietellinae (Coleoptera: Cleroidea). Entomological Problems 25: 65–72.
Majer, K. 1995. Revision of the family Mauroniscidae (Insecta: Coleoptera: Cleroidea). Entomologische Abhandlungen 57: 57–89.
Majer, K. 1996. Complementary generic revision of the subfamily Chaetomalachiinae. Annalen des Naturhistorischen Museums in Wien 98B: 435–500.
Majer, K. 1998. Rhadalinae from the Baltic Amber (Coleoptera, Dasytidae). Deutsche Entomologische Zeitschrift 45: 255–264. Crossref
Majer, K. 1999. Generic synopsis of Neotropical Danaceinae and a revision of the genus Hylodanacaea Pic, 1926 (Coleoptera: Dasytidae). Boletín del Museo Nacional de Historia Natural Chile 48: 77–137.
Majer, K. 2002. Subfamilial classification of the family Malachiidae (Coleoptera, Cleroidea). Entomologica Basiliensia 24: 179–244.
Mayor, A. 2007. Family Melyridae Leach, 1815. In: I. Löbl and A. Smetana (eds.), Catalogue of Palaearctic Coleoptera. Vol. 4, 386–388. Apollo Books, Stenstrup.
Mawdsley, J.R. 1999. Review of the extant and fossil Dasytinae (Coleoptera: Melyridae) of Colorado, U.S.A. Transactions of the American Entomological Society 125: 251–267.
McKenna, D.D., Wild, A.L., Kanda, K., Bellamy, C.L., Beutel, R.G., Caterino, M.S., Farnum, C.W., Hawks, D.C., Ivie, M.A., Jameson, M.L., Leschen, R.A.B, Marvaldi, A.E., McHugh, J.V., Newton, A.F., Robertson, J.A., Thayer, M.K., Whiting, M.F., Lawrence, J.F., Ślipiński, A., Maddison, D.R., and Farrell, B.D. 2015. Beetle tree of life reveals that Coleoptera survived end-Permian mass extinction to diversify during the Cretaceous terrestrial revolution. Systematic Entomology 40: 835–880. Crossref
Peacock, E.R. 1987. A review of the Rhadalinae (= Aplocneminae) (Coleoptera: Melyridae). Bulletin of the British Museum of natural History (Entomology) 56: 129–170.
Pic, M. 1913. Notes diverses, description et diagnoses. Échange. Revue Linéenne 28: 25–26.
Pic, M. 1926. Malacodermes exotique. Échange. Revue Linéenne 42: 21–24, 25–28, 29–32, 33–36.
Ponomarenko, A.G. and Kirejtshuk, A.G. 2003–2015. Taxonomic List of Fossil Beetles of the Suborder Scarabaeina (Part 3 of catalogue). http://www.zin.ru/Animalia/Coleoptera/eng/paleosy2.htm [accessed March 2018]
Poinar, G., Jr. 2018. Burmese amber: evidence of Gondwanan origin and Cretaceous. Historical Biology [published online]. Crossref
Raven, P.H. and Axelrod, D.I. 1974. Angiosperm biogeography and past continental movements. Annals of the Missouri Botanical Garden 61: 539–673. Crossref
Ren, D., Shih, C.K., Gao, T., Yao, Y., and Zhao, Y. 2010. Silent Stories—Insect Fossil Treasures from Dinosaur Era of the Northeastern China. 322 pp. Science Press, Beijing.
Renner, S.S. 2011. Living fossil younger than thought. Science 334: 766–799. Crossref
Robertson, J.A., Ślipiński, A., Moulton, M., Shockley, F.W, Giorgi, A., Lord, N.P., McKenna, D.D., Tomaszewska W., Forrester, J., Miller, K.B., Whiting, M.F., and McHugh, J.V. 2015. Phylogeny and classification of Cucujoidea and the recognition of a new superfamily Coccinelloidea (Coleoptera: Cucujiformia). Systematic Entomology 40: 745–778. Crossref
Sahni, A. 1984. Cretaceous–Paleocene terrestrial faunas of India: Lack of endemism during drifting of the India plate. Science 226: 331–443. Crossref
Selden, P.A. and Huang, D. 2010. The oldest haplogyne spider (Araneae: Plectreuridae), from the Middle Jurassic of China. Naturwissenschaften 97: 449–59. Crossref
Sklenarova, K., Chesters, D., and Bocak, L. 2013. Phylogeography of poorly dispersing net-winged beetles: A role of drifting India in the origin of afrotropical and oriental fauna. PLoS ONE 8 (6): e67957. Crossref
Smith, D.M. and Marcot, J.D. 2015. The fossil record and macroevolutionary history of the beetles. Proceedings of the Royal Society B 282 [published online]. Crossref
Spahr, U. 1981a. Bibliographie der Bernstein- und Kopal-Kafer (Coleoptera). Stuttgarter Beiträge zur Naturkunde, Serie B 72: 1–21.
Spahr, U. 1981b. Systematischer Katalog der Bernstein- und Kopal-Kafer (Coleoptera). Stuttgarter Beiträge zur Naturkunde, Serie B 80: 1–107.
Sullivan, C., Wang, Y., Hone, D.W.E., Wang, Y., Xu, X., and Zhang, F. 2014. The vertebrates of the Jurassic Daohugou Biota of Northeastern China. Journal of Vertebrate Paleontology 34: 243–280. Crossref
Tang, T., Xu, G., O’Brien, C.W., Calonje, M., Franz, N.M., Johnston, M.A., Taylor, A., Vovides, A.P., Pérez-Farrera, M.A., Salas-Morales, S.H., Lazcano-Lara, J.C., Skelley, P., Lopez-Gallego, C., Lindström, A., and Rich, S. 2018. Molecular and morphological phylogenetic analyses of new world cycad beetles: What they reveal about cycad evolution in the new world. Diversity 10 (38): 1–26. Crossref
Théobald, N. 1937. Les Insectes fossiles des Terrains oligocenes de France. Mémoires de la Société des Sciences de Nancy (Nouvelle Series) 2: 1–473.
Toussaint, E.F.A., Seidel, M., Arriaga-Varela, E., Hájek, J., Král, D., Sekerka, L., Short, A.E.Z., and Fikáček, M. 2017. The peril of dating beetles. Systematic Entomology 42: 1–10. Crossref
Wang, Y. and Li, J. 2008. Squamata. In: J. Li, X. Xu, and F. Zhang (eds.), The Chinese Fossil Reptiles and Their Kin, 115–137. Science Press, Beijing.
Wang, Y., Ken, S., Zhang, W., and Zheng, S. 2006. Biodiversity and palaeoclimate of the Middle Jurassic floras from the Tiaojishan Formation in western Liaoning, China. Progress in Natural Science 16: 222–230.
Wickham, H.F. 1912. A report of some recent collections of fossil Coleoptera from the Miocene shales of Florissant. Bulletin from the Laboratory National History (State University Iowa) 6: 1–38.
Wickham, H.F. 1917. New species of fossil beetles from Florissant, Colorado. Proceedings U.S. National Museum 52 (2189): 463–472. Crossref
Xing, X., Zhou, Z., Sullivan, C., Wang, Y., and Ren, D. 2016. An updated review of the middle–late Jurassic Yanliao Biota: Chronology, taphonomy, paleontology and paleoecology. Acta Geologica Sinica 90: 2229–2243. Crossref
Yang, W. and Li, S. 2008. Geochronology and geochemistry of the Mesozoic volcanic rocks in Western Liaoning: Implications for lithospheric thinning of the North China Craton. Lithos 102: 88–117. Crossref
Yu, Y., Deng, C., Kolibáč, J., Ślipiński, A., Ren, D., Jin, J., and Pang, H. 2017. The first record of Cretaceous thaneroclerids (Insecta: Coleoptera) from the Burmese amber. Annales Zoologici 67: 549–554. Crossref
Yu, Y., Leschen, R.A.B., Ślipinski, A., Ren, D., and Pang, H. 2012. The first fossil bark-gnawing beetle from the Middle Jurassic of Inner Mongolia, China (Coleoptera: Trogossitidae). Annales Zoologici 62: 245–252. Crossref
Yu, Y., Ślipiński, A., Leschen, R.A.B., Ren, D., and Pang, H. 2014. Enigmatic Mesozoic bark-gnawing beetles (Coleoptera: Trogossitidae) from the Jiulongshan Formation in China. Annales Zoologici 64: 667–676. Crossref
Zhang, F., Zhou, Z., Xu, X., Wang, X., and Sullivan, C. 2008. A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature 455: 1105–1108. Crossref
Zhang, Q., Li, J., and Huang, D. 2018. Palynological assemblage of the Middle–Upper Jurassic Haifanggou Formation in Daohugou, Ningcheng, Inner Mongolia, China [in Chinese with English abstract]. Acta Micropalaeontologica Sinica 35: 190–199.
Zhang, W. and Zheng, S.L. 1987. Early Mesozoic fossil plants in western Liaoning, Northeast China. In: X.H. Yu, W.L. Wang, X.T. Liu, W. Zhang, S.L. Zheng, J.H. Yu, F.Z. Ma, G.Y. Dong, and P.Y. Yao (eds.) Mesozoic Stratigraphy and Palaeontology of Western Liaoning [in Chinese with English abstract], 239–338. Geological Publishing House, Beijing.
Zherikhin V.V. [Žerihin V.V.] 1977. Mesozoic beetles [in Russian]. Trudy Paleontologičeskogo Instituta 161: 1–204.
Zherikhin, V.V. [Žerihin V.V.] and Sukatsheva, I.D. [Sukaŝeva, I.D.] 1973. On the Cretaceous insectiferous “ambers” (retinits) in the North Syberia [in Russian]. In: E.P. Narčuk (ed.), Doklady na 24-m ežegodnom čtenii pamâti N.A. Holodkovskogo, 1–2. aprelâ 1971. Voprosy paleontologii nasekomyh, 3–48. Nauka, Leningrad.
Acta Palaeontol. Pol. 64 (1): 143–155, 2019
https://doi.org/10.4202/app.00550.2018