

A late Paleocene fauna from shallow-water chemosynthesis-based ecosystems, Spitsbergen, Svalbard
KRZYSZTOF HRYNIEWICZ, KAZUTAKA AMANO, MARIA ALEKSANDRA BITNER, JONAS HAGSTRÖM, STEFFEN KIEL, ADIËL A. KLOMPMAKER, THOMAS MÖRS, CRISTINA M. ROBINS, and ANDRZEJ KAIM
Hryniewicz, K., Amano, K., Bitner, M.A., Hagström, J., Kiel, S., Klompmaker, A.A., Mörs, T., Robins, C.M., and Kaim, A. 2019. A late Paleocene fauna from shallow-water chemosynthesis-based ecosystems, Spitsbergen, Svalbard. Acta Palaeontologica Polonica 64 (1): 101–141.
We present a systematic study of late Paleocene macrofauna from methane seep carbonates and associated driftwood in the shallow marine Basilika Formation, Spitsbergen, Svalbard. The fauna is composed of 22 taxa, comprising one brachiopod, 14 bivalves, three gastropods, three crustaceans, and one bony fish. The reported fish remains are among the first vertebrate body fossils from the Paleogene of Spitsbergen. One genus is new: the munidid decapod Valamunida Klompmaker and Robins gen. nov. Four new species are described: the terebratulide brachiopod Neoliothyrina nakremi Bitner sp. nov., the protobranch bivalve Yoldiella spitsbergensis Amano sp. nov., the xylophagain bivalve Xylophagella littlei Hryniewicz sp. nov., and the munidid decapod Valamunida haeggi Klompmaker and Robins gen. et sp. nov. New combinations are provided for the mytilid bivalve Inoperna plenicostata, the thyasirid bivalve Rhacothyas spitzbergensis, the ampullinid gastropod Globularia isfjordensis, and the munidid decapod Protomunida spitzbergica. Thirteen taxa are left in open nomenclature. The fauna contains a few last occurrences of Cretaceous survivors into the Paleocene, as well as first occurrences of Cenozoic taxa. It is composed of chemosymbiotic thyasirid bivalves and background species common in the northern Atlantic and Arctic during the Paleocene. Our results provide no evidence for a Paleocene origin of vesicomyid and bathymodiolin bivalves typical for Eocene and younger seep environments; instead, the Paleocene seeps of the Basilika Formation are more similar to their Late Cretaceous equivalents rich in thyasirids.
Key words: Mollusca, Brachiopoda, Arthropoda, methane seeps, sunken wood, palaeoecology, Cenozoic, Basilika Formation, Spitsbergen.
Krzysztof Hryniewicz [krzyszth@twarda.pan.pl], Maria Aleksandra Bitner [bitner@twarda.pan.pl], and Andrzej Kaim [kaim@twarda.pan.pl], Institute of Paleobiology, Polish Academy of Sciences, ul. Twarda 51/55, 00-818 Warszawa, Poland.
Kazutaka Amano [amano@juen.ac.jp], Department of Geoscience, Joetsu University of Education, 1 Yamayashiki, Joetsu City, Niigata 943-8512, Japan.
Steffen Kiel [steffen.kiel@nrm.se], Jonas Hagström [jonas.hagstrom@nrm.se], and Thomas Mörs [thomas.mors@nrm.se], Swedish Museum of Natural History, Department of Palaeobiology, Box 50007, 104 05 Stockholm, Sweden.
Adiël A. Klompmaker [adielklompmaker@gmail.com], Department of Integrative Biology & Museum of Paleontology, University of California, Berkeley, 1005 Valley Life Sciences Building #3140, Berkeley, CA 94720, USA.
Cristina M. Robins [cristina.robins@gmail.com], Museum of Paleontology, University of California, Berkeley, 1101 Valley Life Science Building, Berkeley, CA 94720, USA.
Received 10 October 2018, accepted 10 December 2018, available online 13 February 2019.
Copyright © 2019 K. Hryniewicz et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Marine ecosystems at hydrothermal vents (Van Dover 2000), cold seeps (e.g., Sibuet and Olu 1998; Levin 2005), vertebrate falls (Smith and Baco 2003), and sunken plant debris (Wolff 1979; Bernardino et al. 2010) are best known for spectacular accumulations of biota fuelled by chemosynthetic rather than by photosynthetic primary production. Chemosynthesis-based ecosystems and associated biota have thrived on Earth throughout most of its recorded history (e.g., Campbell 2006; Kiel 2010b; Sandy 2010; Planavsky et al. 2012); the fossil record of cold seeps tends to be much more continuous than that of the remaining marine chemosyntesis-based ecosystems, and dates back to at least the Silurian (approx. 420 Ma; Barbieri et al. 2004; Jakubowicz et al. 2017). One of the chief interests in ancient seep biotas lies in their reliance on chemosynthetically produced organic matter, making them largely impervious to the effects of disturbances in the trophic chain affecting the remainder of the marine fauna, including mass extinctions (Kiel and Little 2006). There is some evidence that seep biota reacted to those events differently from the remainder of the marine fauna, which derived its nutrition through photosynthesis (e.g., Kiel 2015). However, the incomplete fossil record of seep faunas makes more detailed analyses difficult. The large gaps in the fossil record of seeps comprise, for example, the early Palaeozoic and Permian, from which no conclusive seeps have been discovered thus far (cf. Matos et al. 2017), and the Triassic and Paleocene, with only two conclusive seep faunas known from each (Schwartz et al. 2003; Minisini and Schwartz 2007; Peckmann et al. 2011; Hryniewicz et al. 2016; Kiel et al. 2017; Kiel 2018).
The aims of the present paper are to review and systematically describe the macrofauna of late Paleocene methane seep carbonates and associated driftwood from Spitsbergen (Hryniewicz et al. 2016), except for Conchocele conradii (Rosenkrantz, 1942), which was already discussed by Hryniewicz et al. (2017). We also discuss the composition, palaeoecology, and macroevolutionary significance of the fauna, in particular in relation to stratigraphically adjacent biotic events and the Paleogene diversification of seep faunas.
Institutional abbreviations.—GPIBo, Steinmann’s Institute of Geology, Mineralogy and Palaeontology, University of Bonn, Germany; NRM, Swedish Museum of Natural History (Naturhistoriska riksmuseet), Stockholm, Sweden; ZPAL, Institute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland.
Other abbreviations.—H, height; L, lenght; n, number of data points; W, width.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:E6189ABB-9B6F-4057-BB8D-798C9B0BE388
Geological setting
The fauna discussed in this paper comes from the late Paleocene methane seep carbonates and associated driftwood from the Basilika Formation, Spitsbergen, Svalbard (Hryniewicz et al. 2016). These deposits formed in a semi-enclosed marine embayment in a prodelta to deltafront environment (Nøttvedt 1985; Manum and Throndsen 1986; Dallmann 1999). The presence of glendonite indicates that the Paleocene on Svalbard was a time of a cold climate with near-freezing seawater temperatures (Spielhagen and Tripati 2009). The water column of the Svalbard embayment possibly was stratified (Nagy et al. 2000), likely due to a temperature or salinity gradient, and vegetated areas provided an ample amount of terrestrial plant material preserved in the seep carbonates and in the surrounding siltstone (Hryniewicz et al. 2016).
Material
The fossils from the Basilika Formation come from two museum collections described by Hägg (1925; NRM) and Vonderbank (1970; GPIBo), supported by literature descriptions of the lost material of Gripp (1927). Additional materials used for this study were collected during fieldwork in 2015 in Fossildalen (Hryniewicz et al. 2016) and during fieldwork in 2017 in Zachariassendalen east of Barentsburg. Three localities are discussed briefly below.
Fossildalen, Colesbukta area, Spitsbergen, Svalbard.—The material comes from boulders found in the bed of a small creek in Fossildalen, ~78°5′52.68″ N, 14°31′51.59″ E (Fig. 1). The boulders are composed of seep carbonate, sunken driftwood, and siltstone, and contain a fossil fauna in which the most common species are the thyasirid bivalve Conchocele conradii (Rosenkrantz, 1942), protobranch bivalves, the wood-boring bivalve Xylophagella littlei Hryniewicz sp. nov., the ampullinid gastropod Globularia isfjordensis (Vonderbank, 1970), and the terebratulide brachiopod Neoliothyrina nakremi Bitner sp. nov. The description of the site indicates that the material discussed by Hägg (1925) and Gripp (1927) very likely came from this locality.
Locality 500 m west of Trigonometric point 25, Hollendarbukta area, Spitsbergen, Svalbard.—Trigonometric point 25 is located in the eastern part of Hollendarbukta (Fig. 1). Vonderbank (1970) mentioned that the materials he described were collected from fossiliferous lenses 500 m west of this point. The surrounding strata have been described as belonging to the so-called Grumantdalen Beds (Vonderbank 1970), which is an abandoned unit not mappable in the field (Dallmann 1999). The rocks surrounding the fossiliferous lenses described by Vonderbank (1970) most likely represent the Basilika Formation, which crops out in the eastern Hollendarbukta area. The materials comprise the aporrhaid gastropod Aporrhais cf. gracilis Koenen, 1885, the scaphandrid gastropod Ellipsoscapha sp., the ampullinid gastropod Globularia isfjordensis (Vonderbank, 1970), the mytilid bivalves Inoperna plenicostata (Anderson, 1970) and ?Mytilus hauniensis (Rosenkrantz, 1970), the thyasirid bivalve Rhacothyas spitzbergensis (Anderson, 1970), and crustaceans.
Zachariassendalen, 1.5 km east of Barentsburg, Spitsbergen, Svalbard.—The material comes from a single boulder found in situ on a slope in the upper reaches of Olavbekken rivulet, ~78°3’56.09”N, 14°16’59.77”E (Fig. 1). The boulder is a concretionary limestone with small, rounded, siliciclastic pebbles. It contains abundant plant material including reeds (Phragmites sp.) and unidentified twigs; the most common invertebrates are the thyasirid bivalve Rhacothyas spitzbergensis (Anderson, 1970), protobranch bivalves (including Solemya sp.), rare gastropods, and crustaceans. The carbon isotope signature of five carbonate samples was investigated by us in the same way as described by Hybertsen and Kiel (2018); the lowest measured values reach -48.2‰, clearly indicating that oxidation of biogenic methane played a role in the formation of the carbonate concretion.
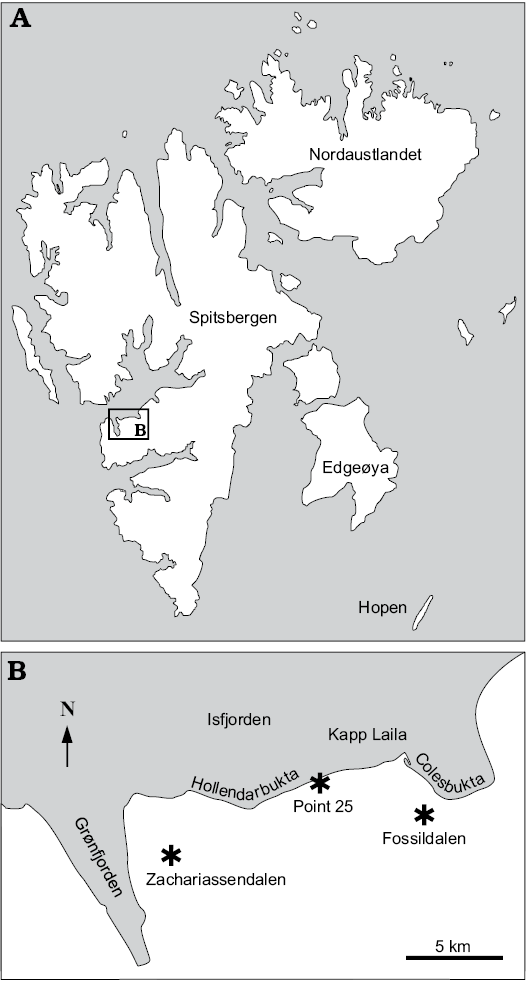
Fig. 1. A. Map of Svalbard showing location of the study area. B. Map of the study area with the fossil localities indicated (asterisks).
Systematic palaeontology
Phylum Brachiopoda Duméril, 1806
Order Terebratulida Waagen, 1883
Suborder Terebratulidina Waagen, 1883
Family Sellithyridae Muir-Wood, 1965
Genus Neoliothyrina Sahni, 1925
Type species: Terebratula obesa Davidson, 1852 (not Sowerby, 1823, see discussion in Popiel-Barczyk 1968: 52); Upper Cretaceous, England.
Neoliothyrina nakremi Bitner sp. nov.
Figs. 2, 3.
?1927 Terebratulina sp.; Gripp 1927: 30, pl. 6: 13–14.
2016 Pliothyrina? sp.; Hryniewicz et al. 2016, table 2.
ZooBank LSID: urn:lsid:zoobank.org:act:C889EAF7-1909-4819-9B0E-9709A1EBA6ED
Etymology: In honour of Norwegian palaeontologist Hans Arne Nakrem, in recognition of his studies on fossil chemosynthesis-based faunas from Spitsbergen.
Type material: Holotype: ZPAL V.48/9-1, decorticated specimen with broken beak (Fig. 3A). Paratypes: ZPAL V.48/9-2–4, decorticated specimens with broken beak (ZPAL V.48/9-2) and anterior part (ZPAL V.48/9-2–3); a set of acetate peels (ZPAL V.48/9-4) (Figs. 2, 3B, C).
Type locality: Fossildalen, Spitsbergen, Svalbard.
Type horizon: Cold seep carbonates from the Basilika Formation, upper Paleocene.
Material.—13 specimens (ZPAL V.48/9-1–13), complete specimens, apart from the holotype all with broken anterior part, all from the type locality and horizon.
Measurements.—Holotype (ZPAL V.48/9-1): L, 25.6 mm; W, 19.9 mm; H, 12.9 mm; other specimens inappropriate for measurements.
Diagnosis.—Small Neoliothyrina with rectimarginate anterior commissure. Outer hinge plates relatively wide, ventrally concave, inner hinge plates narrow and short, not contacting each other. Crural processes short, bluntly pointed. Loop short.
Description.—Shell of medium size, subpentagonal to elongate oval in outline, biconvex with ventral valve deeper. Shell surface smooth with numerous growth lines. Lateral commissure slightly ventrally convex, anterior commissure rectimarginate. Beak suberect with rounded beak ridges. Foramen large, circular, mesothyrid. Symphytium small, only partially visible.
Internal characters investigated in transverse serial sections. Cardinal process distinct, flat, surface with numerous grooves. Inner socket ridges thick, parallel to valve margin. Dental sockets moderately deep. Outer hinge plates relatively wide, ventrally concave, separated from inner hinge plates by distinct crural bases. Inner hinge plates short, narrow, not connecting. Crural processes short, tapering, slightly inwardly curving. Loop short, transverse band not preserved.
Remarks.—Probably the first record of this species is Terebratulina sp. described and figured by Gripp (1927), but the material is missing and the precise identification from the figure alone is impossible. The material studied herein was initially questionably assigned to Pliothyrina Roy, 1980 (see Hryniewicz et al. 2016); however, the investigations of internal structures suggest attribution to Neoliothyrina. Pliothyrina is known from the Oligocene to Pliocene in northern Europe (Cooper 1983), while Neoliothyrina is known from the Upper Cretaceous (Santonian–Maastrichtian) of Great Britain, Germany, and Poland (Steinich 1965; Popiel-Barczyk 1968; Cooper 1983). Both genera are characterized by the presence of inner hinge plates, a feature rarely present in terebratuloids (MacKinnon and Lee 2006). Pliothyrina differs from Neoliothyrina in having very narrow or absent outer hinge plates and its crural processes are unusually long and curved anteromedially (Cooper 1983), whereas the outer hinge plates are well-developed and the crural processes are bluntly pointed in Neoliothyrina. These characters are all observed in the material from Svalbard.
Due to the rectimarginate anterior commissure, very narrow and short inner hinge plate, and small crural processes, Neoliothyrina nakremi Bitner sp. nov. closely resembles the Maastrichtian Neoliothyrina plana Popiel-Barczyk, 1968, from Poland. Neoliothyrina nakremi is, however, more elongate and more pentagonal compared with N. plana. Thus, the present finding extends the stratigraphical and geographical range of this genus into the Paleocene.
The investigated specimens differ strongly from the type species, Neoliothyrina obesa Davidson, 1852. Neoliothyrina obesa is much larger than N. nakremi, having its anterior commissure distinctly biplicate and its inner hinge plates are well developed, contacting or even overlapping each other in many specimens (Steinich 1965; Popiel-Barczyk 1968; Cooper 1983). Also, Neoliothyrina fallax (Lundgren, 1885) has a larger maximum size than the taxon from Svalbard (Brünnich Nielsen 1909; Popiel-Barczyk 1968) and exhibits a more curved beak and biplicate anterior commissure. The species Neoliothyrina fittoni (Hagenow, 1842, assigned to this genus by Steinich 1965), is similar in size to N. nakremi but differs in the character of cardinal process; in N. fittoni, the cardinal process has a distinct cardinal knob that is very poorly developed in other Neoliothyrina species (Steinich 1965; Popiel-Barczyk 1968).
The specimens assigned by Gripp (1927) to Terebratulina d’Orbigny, 1847, undoubtedly belong to smooth, short-looped terebratulides and most probably represent the new species described here. However, the genus Terebratulina is characterized by a ribbed surface, not seen on Gripp’s (1927) figures, and an incomplete foramen with disjunct deltidial plates, absent in the material studied herein.
Stratigraphic and geographic range.—Upper Paleocene cold seep carbonates from the Basilika Formation, Fossildalen, Spitsbergen, Svalbard.
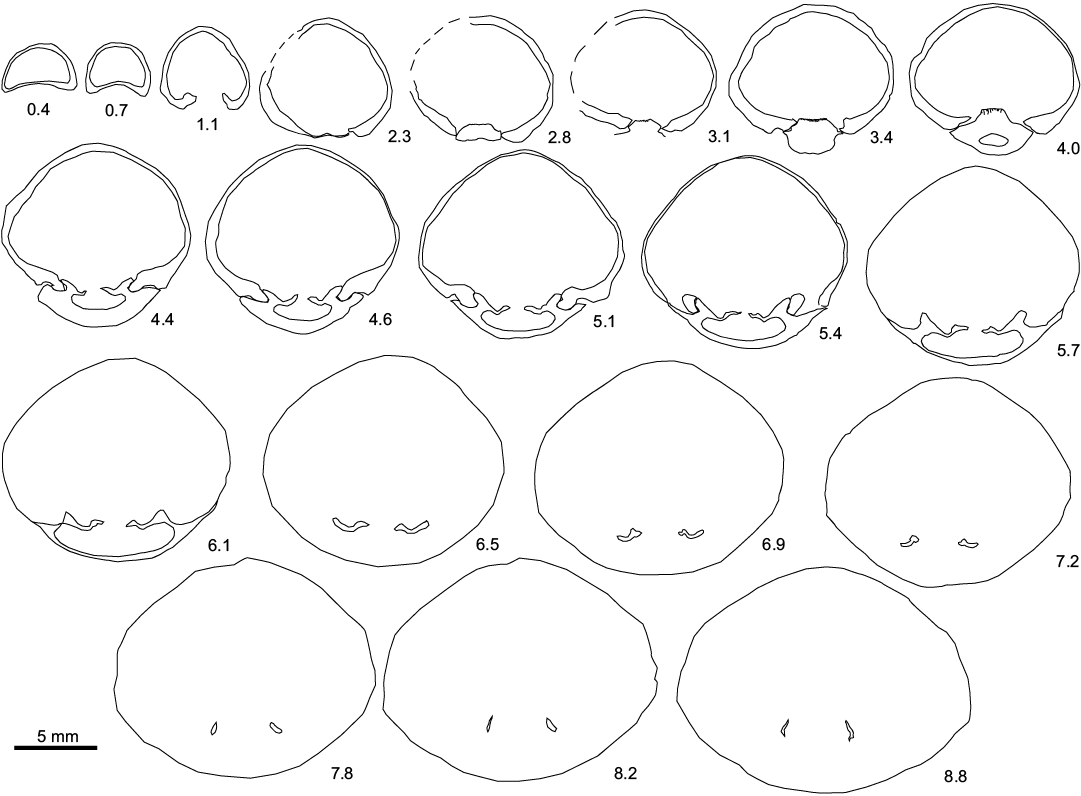
Fig. 2. Transverse serial sections of the sellithyrid brachiopod Neoliothyrina nakremi Bitner sp. nov. paratype (ZPAL V.48/9-4) from the upper Paleocene, Basilika Formation, Fossildalen, Spitsbergen, Svalbard. L >20.2 mm. Numbers indicate distance in mm from the tip of the ventral umbo.
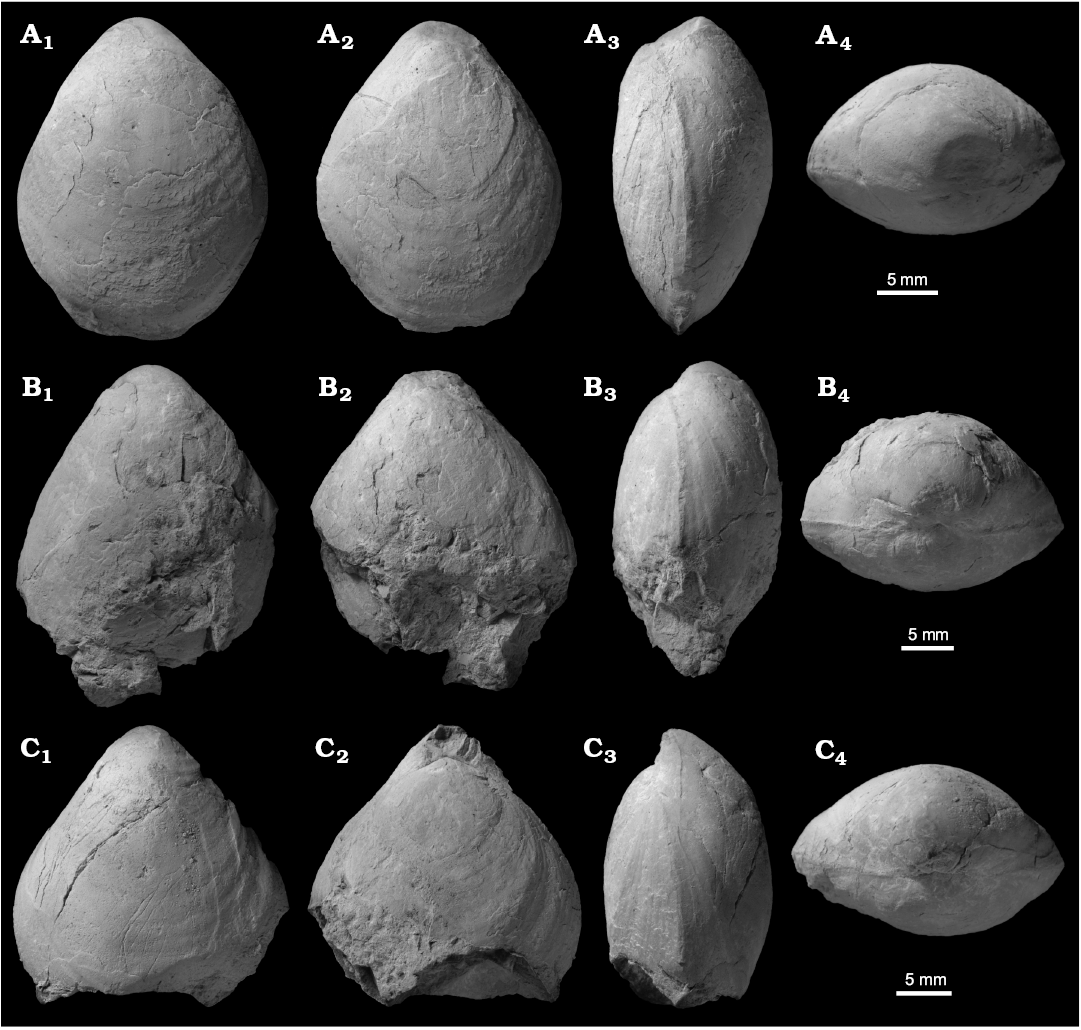
Fig. 3. Sellithyrid brachiopod Neoliothyrina nakremi Bitner sp. nov. from the upper Paleocene, Basilika Formation, Fossildalen, Spitsbergen, Svalbard. A. Holotype, ZPAL V.48/9-1, decorticated shell in ventral (A1), dorsal (A2), and posterior (A4) views; left-lateral view of both valves (A3). B. Paratype, ZPAL V.48/9-2; decorticated shell in ventral (B1), dorsal (B2), and posterior (B4) views; left-lateral view of both valves (B3). C. Paratype, ZPAL V.48/9-3, decorticated shell in ventral (C1), dorsal (C2), and posterior (C4) views; left-lateral view of both valves (C3).
Phylum Mollusca Linnaeus, 1758
Class Gastropoda Cuvier, 1797
Order Caenogastropoda Cox in Knight et al., 1960
Superfamily Stromboidea Rafinesque, 1815
Family Aporrhaidae Gray, 1850
Genus Aporrhais Costa, 1778
Type species: Strombus pespelecani Linnaeus, 1758; Recent, Europe.
Aporrhais cf. gracilis Koenen, 1885
Fig. 4A–C.
?1925 Nassa spec.; Hägg 1925: pl. 3: 5, 5a.
1927 Chenopus (Arrhoges) sp.; Gripp 1927: 32, pl. 6: 8–9.
1970 Chenopus gracilis (Koenen, 1885); Vonderbank 1970: 87, pl. 8: 8–9.
2016 Aporrhaidae sp.; Hryniewicz et al. 2016: fig. 12H.
Material.—Two specimens (GPIBo 116, 117), incomplete adolescent shells from the upper Paleocene, Basilika Formation, locality 500 m west of Trigonometric point 25, Hollendarbukta, Spitsbergen, Svalbard.
Measurements.—GPIBo 117: H, 27 mm; W, 14 mm.
Description.—Shell elongate, largest specimen (GPIBo 117) consists of seven whorls. Whorl flanks moderately inflated with neither keel nor knobs. Ornamentation consists of opisthocline, well-expressed but blunt axial ribs, which disappear at demarcation between lateral flank and base. Demarcation continuous with no sharp angulation. Both lateral flank and base ornamented by numerous delicate spiral ribs. Aperture not preserved, only specimen with piece of single straight digit of outer lip preserved has been illustrated by Gripp (1927: pl. 6: 8, 9), but this specimen was poorly preserved and collection did not survive 2nd World War (Ulrich Kotthoff, personal communication 2015). No trace of ascending whorl has been observed.
Remarks.—The adolescent aporrhaid shells from Isfjorden display characters observed in three Paleogene species, but due to the lack of well-preserved diagnostic characters of the adult expanded outer lip, it is difficult to attribute it to any of these species with confidence. The three species in question are: Aporrhais gracilis Koenen, 1885; A. bowerbankii (Morris, 1852), and A. triangulata Gardner, 1884. The former two are from the Paleocene, while the latter is early Eocene in age. All three have similar early whorls; a tall, elongate teleoconch without keel or knobs and without an ascending last whorl; and differ mostly in the morphology of the terminal whorl and its outer lip, characters not preserved in the aporrhaid from Isfjorden. Vonderbank (1970) attributed the specimens from the Paleocene of Spitsbergen to A. gracilis, which is known to occur in the Selandian of Denmark (Koenen 1885; Schnetler and Nielsen 2018) under the generic name Chenopus Philippi, 1836, which is a junior objective synonym of Aporrhais. It seems indeed that A. gracilis is the most similar species, but due to its fragmentary preservation we prefer to leave the species from the Paleocene of Spitsbergen in open nomenclature. Another similar but poorly preserved aporrhaid-like specimen from Fossildalen has been illustrated and decribed by Hägg (1925) as the neogastropod Nassa sp. This specimen (NRM-PZ Mo149182; Fig. 4C herein) is an inner mould with traces of spiral ornamentation resembling the one of A. cf. gracilis, although it seems to be wider and slightly less elongate. We refrain from any further identification of this specimen and provisionally we leave it as a doubtful aporrhaid Aporrhais cf. gracilis.
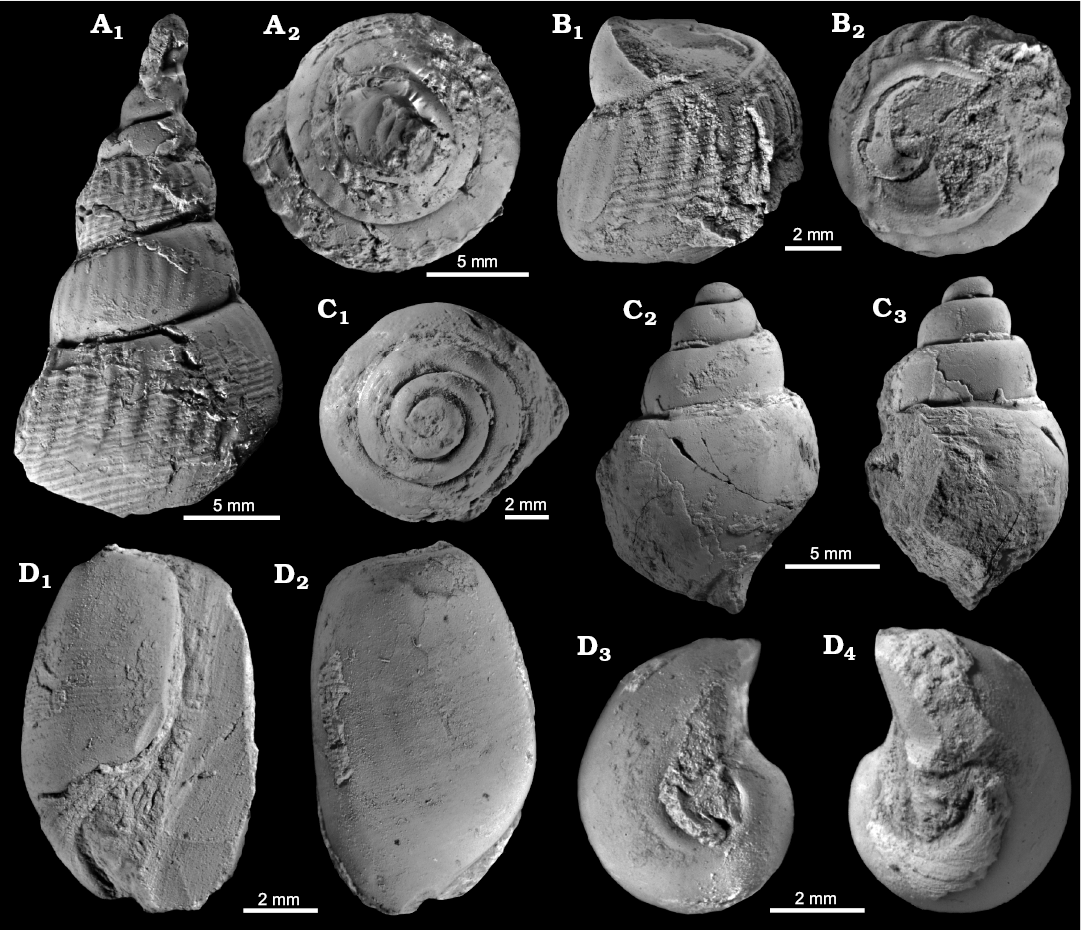
Fig. 4. Aporrhaid and scaphandrid gastropods from the upper Paleocene, Basilika Formation, locality 500 m west from Trigonometric point 25, Hollendarbukta (A, B, D) and Fossildalen (C), Spitsbergen, Svalbard. A, B. Aporrhais cf. gracilis Koenen, 1885, GPIBo 117 (A) and GPIBo 116 (B) in lateral (A1, B1) and apical (A2, B2) views. C. ?Aporrhais cf. gracilis Koenen, 1885, identified by Hägg (1925) as Nassa sp., NRM-PZ Mo 149182 in apical (C1), lateral (C2) and latero-apertural (C3) views. D. Ellipsoscapha sp. GPIBo 115 in apertural (D1), lateral (D2), apical (D3), and abapical (D4) views.
Superfamily Campaniloidea Douvillé, 1904
Family Ampullinidae Cossmann in Cossmann and Peyrot, 1919
Genus Globularia Swainson, 1840
Type species: Ampullaria sigaretina Lamarck, 1804; Eocene, France.
Globularia isfjordensis (Vonderbank, 1970)
Fig. 5.
1925 Natica spec. 1; Hägg 1925: 45, pl. 3: 2.
1925 Natica spec. 2; Hägg 1925: 45, pl. 3: 3.
1925 Natica spec. 3; Hägg 1925: 45, pl. 3: 4.
1927 Natica sp.; Gripp 1927: 32, pl. 6: 11.
1970 Ampullonatica isfjordensis n. sp.; Vonderbank 1970: 87, pl. 8: 1–6.
2016 “Naticiform” gastropod sp.; Hryniewicz et al. 2016: table 1, fig. 12G.
Material.—Ten specimens, poorly to immoderately well preserved shells, including the holotype (GPIBo 111) from Vonderbank’s (1970) collection (four figured GPIBo 110–113) and four specimens from Hägg’s (1925) collection (one figured NRM-PZ Mo 149179); from the upper Paleocene, Basilika Formation, Fossildalen, locality 500 m from Hollendarbukta, Spitsbergen, Svalbard.
Measurements.—The holotype (GPIBo 111): H, 12.5 mm; W, 10.5 mm. NRM-PZ Mo 149179: H, 22 mm; W, 19 mm.
Original diagnosis (from Vonderbank 1970).—Eine Art der Gattung Ampullonatica mit flach eingesenkten Nähten, schwach bogenförmigen Parietalrand und ± offenem Nabel. [A species of the genus Ampullonatica with weakly incised sutures, slightly arched parietal margin and ± open umbilicus].
Description.—Protoconch and juvenile teleoconch not preserved. Shell naticiform, thin-shelled with strongly inflated whorls and moderately incised suture. Ornamentation absent apart from enhanced, orthocline to slightly sinusoidal growth lines. Aperture poorly preserved in all available specimens. No umbilicus observed, contrary to statement by Vonderbank (1970). Parietal callus not developed and no other apertural elaborations preserved.
Remarks.—Vonderbank (1970) classified his new species in the naticid genus Ampullonatica Sacco, 1890a, which is invalid due to its type species being a nomen nudum. In an older account, Hägg (1925) divided the naticiform gastropods from Isfjorden into three species of Natica Scopoli, 1777 in open nomenclature, but provided no description of the species in question. Gripp (1927) also left this form as a species of Natica in open nomenclature, but compared it to a number of naticiform species currently classified as representatives of Ampullinidae (e.g., Ampullospira Harris, 1897, Crommium Cossmann, 1888, Amauropsella Bayle in Chelot, 1885, Euspirocrommium Sacco, 1890b, and Amauropsis Mörch, 1857). We also think that this form is more similar to ampullinids rather than to naticids due to the thin shell, absence of an umbilicus and parietal callus and enhanced growth lines. On the other hand, in molluscan fossils from the studied collection, we noticed several drill holes that could be attributed to predatory gastropods (such as naticids), although another species of predatory gastropod not yet found in Basilika Formation could have been responsible for these holes. Drill holes have been noted previously in fossil cold seep mollusks (Amano 2003; Amano and Jenkins 2007; Amano and Kiel 2007; Kiel et al. 2008, 2016). The species identified by Hägg (1925) as Nassa sp. (Fig. 4C) most likely is a poorly preserved aporrhaid (see above).
Stratigraphic and geographic range.—Upper Paleocene cold seep carbonates from the Basilika Formation, Fossildalen and locality 500 m west of Trigonometric point 25, Hollendarbukta, Spitsbergen, Svalbard.
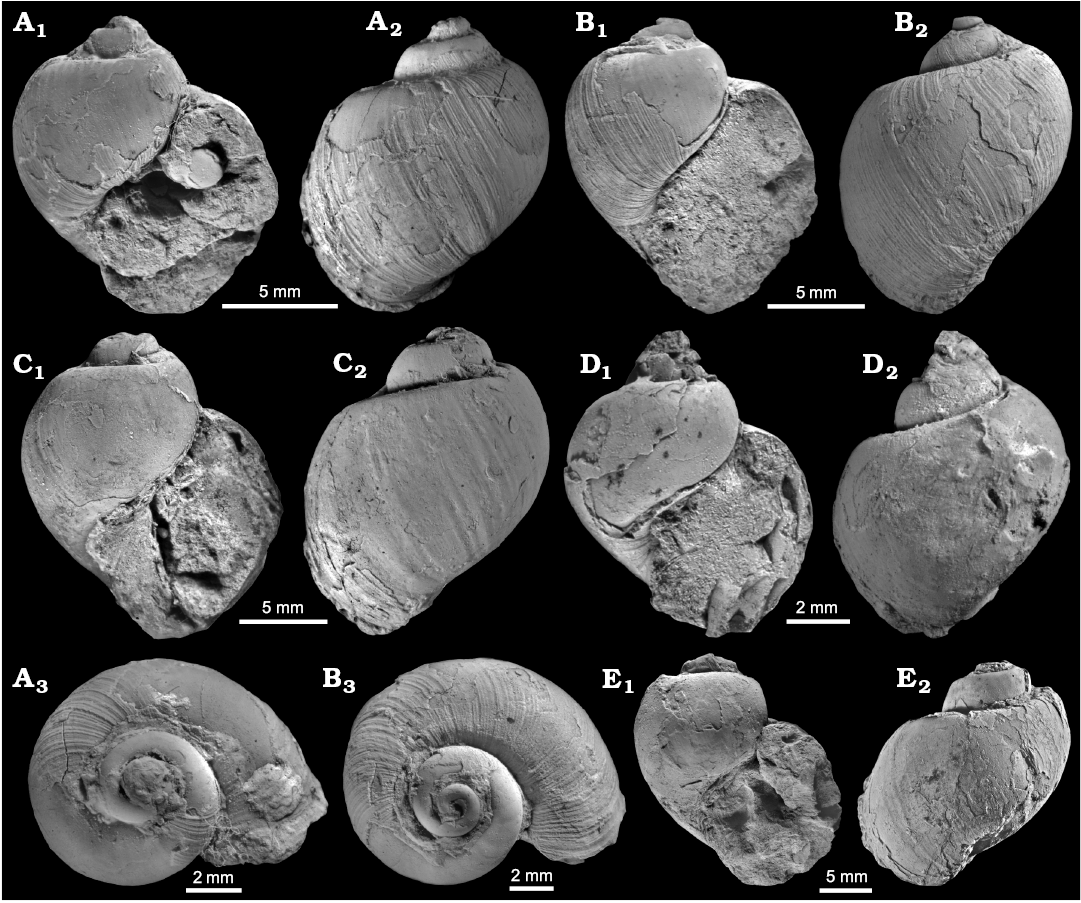
Fig. 5. Ampullinid gastropod Globularia isfjordensis (Vonderbank, 1970) from the upper Paleocene, Basilika Formation, locality 500 m from Trigonometric point 25, Hollendarbukta (A–D) and Fossildalen (E), Spitsbergen, Svalbard. A. GPIBo 111 (holotype). B. GPIBo 112. C. GPIBo 113. D. GPIBo 110. E. NRM-PZ Mo 149179. Apertural (A1, B1, C1, D1. E1), lateral (A2, B2, C2, D2, E2), and apical (A3, B3) views.
Order Cephalaspidea Fischer, 1883
Superfamily Philinoidea Gray, 1850
Family Scaphandridae Sars, 1878
Ellipsoscapha Stephenson, 1941
Type species: Cylichna striatella Shumard, 1861; Cretaceous, USA.
Ellipsoscapha sp.
Fig. 4D.
1970 Cylichna discifera Koenen, 1885; Vonderbank 1970: 88, pl. 8: 7.
2016 Cylichnidae sp.; Hryniewicz et al. 2016: table 1, fig. 12I.
Material.—One specimen (GPIBo 115) of the two mentioned by Vonderbank (1970), poorly preserved shell from the upper Paleocene, Basilika Formation, locality 500 m west of Trigonometric point 25, Hollendarbukta, Spitsbergen, Svalbard.
Measurements.—GPIBo 115: H, 7.0 mm; W, 4.4 mm.
Description.—Shell of small size, cylindrical, slightly widening abapically, apical opening relatively wide, depth obscured by remnants of following shell and/or sediment, protoconch not preserved. Shell layers peeled off in majority of specimen, but remnants display dense spiral ornamentation. Aperture adapically narrow, expanding in abapical direction. No trace of collumellar folds, callus, or umbilical slit; these features seem genuinely to have been absent.
Remarks.—The specimen in question was classified by Vonderbank (1970) as Cylichna discifera Koenen, 1885, known from the Paleocene of Denmark (Koenen 1885; Schnetler and Nielsen 2018); however, the latter species is much more elongate and equally wide over its entire height, being similar to true cylichnids. The specimen from Spitsbergen, in turn, possesses characters of Scaphandridae and in particular the genus Ellipsoscapha, known from the uppermost Cretaceous of the USA (Sohl 1964), especially the shell widening adapically due to the expanding aperture, and the lack of a callus, columellar folds and an umbilical slit. Due to the imperfect preservation we refrain, however, from specific identification. Another Paleocene species of Ellipsoscapha, also in open nomenclature, has been reported from Greenland by Kollmann and Peel (1983). The latter species, however, is slimmer than the one from Spitsbergen and its last whorl reaches high above the apex.
Class Bivalvia Linnaeaus, 1758
Subclass Protobranchia Pelseneer, 1889
Order Solemyida Dall, 1889
Superfamily Solemyoidea Gray, 1840
Family Solemyidae Gray, 1840
Genus Solemya Lamarck, 1818
Type species: Solemya mediterranea (Lamarck, 1818) = Tellina togata Poli, 1795 (by subsequent designation, Children, 1823); Recent, Mediterranean.
Solemya sp.
Fig. 6.
Material.—Two specimens (NRM-PZ Mo 183945, 183946), internal mold with shell partially adhering (NRM-PZ Mo 183945) and butterflied internal mold (NRM-PZ Mo 183946), from the upper Paleocene of Zachariassendalen, Spitsbergen, Svalbard.
Measurements.—NRM-PZ Mo 183945: L, 14.5 mm; H, 6.9 mm; W, 4.4 mm.
Description.—Shell small, thin, very inequilateral, with anterior constituting ca. 75% of length of shell. Umbones nearly undiscernible, dorsal margin straight, passing into slightly oblique and convex anterodorsal margin. Anterior margin rounded, with deepest point above median line. Anteroventral margin convex and oblique, ventral margin very weakly convex. Posterior margin rounded, posterodorsal margin incurved. Posterior adductor muscle scar demarcated anteriorly by ridge. Anterior adductor muscle scar connected with dorsal margin by elongate band. External ornament of ridges radiating from umbo; ridges much broader than their interspaces. Anterior half of shell bearing 12 ridges; total number of ridges unknown due to lack of complete shells. Soft periostracal flange extending a few millimetres beyond margin of the shell.
Remarks.—We have included the present species in Solemya Lamarck, 1818, due to the presence of a faint ridge in front of the posterior adductor muscle scar. Such a ridge is present in some subgenera of Solemya, such as Austrosolemya Taylor, Glover, and Williams, 2008, Petrasma Dall, 1908, Solemyarina Iredale, 1931, and Zesolemya Iredale, 1939. The present species has no internal ligament demipads in front of the umbo that we could identify inequivocally, and so is reminiscent of Petrasma Dall, 1908, but the small number of specimens does not allow us to check whether this is a constant feature. Therefore, we did not assign our species in a subgenus of Solemya.
The conchological features used to subdivide extant species of Solemya are mostly those of the posterodorsal and dorsal shell margin, such as the shape and length of the chondrophore and ligament, shape of the posterior adductor muscle scar and the shape of the anterior ligament extensions (Taylor et al. 2008; Kamenev 2009). These features are not preserved in the material herein, so that identification to the species-level is not possible at present. External conchological characters, perhaps except for the relative position of the umbo in relation to the shell length, are not of great taxonomic significance for solemyids when internal shell features are absent (e.g., Amano and Ando 2011; Hryniewicz et al. 2014) and they should not be used by themselves for species discrimination.
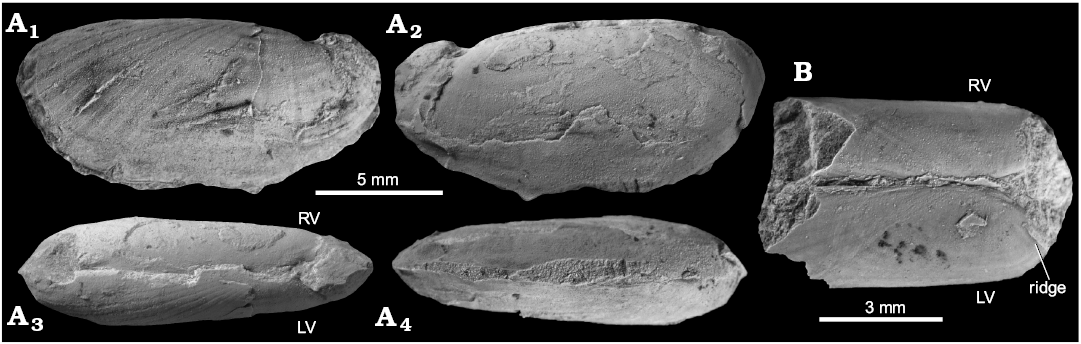
Fig. 6. Solemyid bivalve Solemya sp. from the upper Paleocene, Basilika Formation, Zachariassendalen, Spitsbergen, Svalbard. A. NRM-PZ Mo 183945, shell (LV), internal mold (RV), left (A1) and right (A2) valve, dorsal view of both valves (A3), ventral view (A4). B. NRM-PZ Mo 183946, internal mold, butterflied specimen in dorsal view (LV, left valve; RV, right valve).
Order Nuculida Dall, 1889
Superfamily Nuculoidea Gray, 1824
Family Nuculidae Gray, 1824
Genus ?Nucula Lamarck, 1799
Type species: Arca nucleus Linnaeus, 1758; Recent, northern Atlantic and Mediterranean Sea.
?Nucula sp.
Fig. 7A.
Material.—One specimen (ZPAL V.48/10), poorly preserved internal mold from the upper Paleocene of Fossildalen, Spitsbergen, Svalbard.
Measurements.—ZPAL V.48/10: L, 4.0 mm; H, 2.9 mm; W, 2.2 mm.
Description.—Shell small, well-inflated, ovate, equivalve, inequilateral. Antero-dorsal margin long and straight, passing into rather acute anterior end; postero-dorsal margin also nearly straight, sloping steeply into bluntly pointed posterior end; ventral margin broadly arcuate. Inner side of ventral margin finely crenulate. Posterior adductor muscle scar ovate, but anterior one not visible. Hinge and pallial sinus unknown.
Remarks.—The present species is similar to Nucula nucleus (Linnaeus, 1758) in its shell outline and ventral crenulation. However, attribution to any particular species is not possible because the hinge could not be observed.
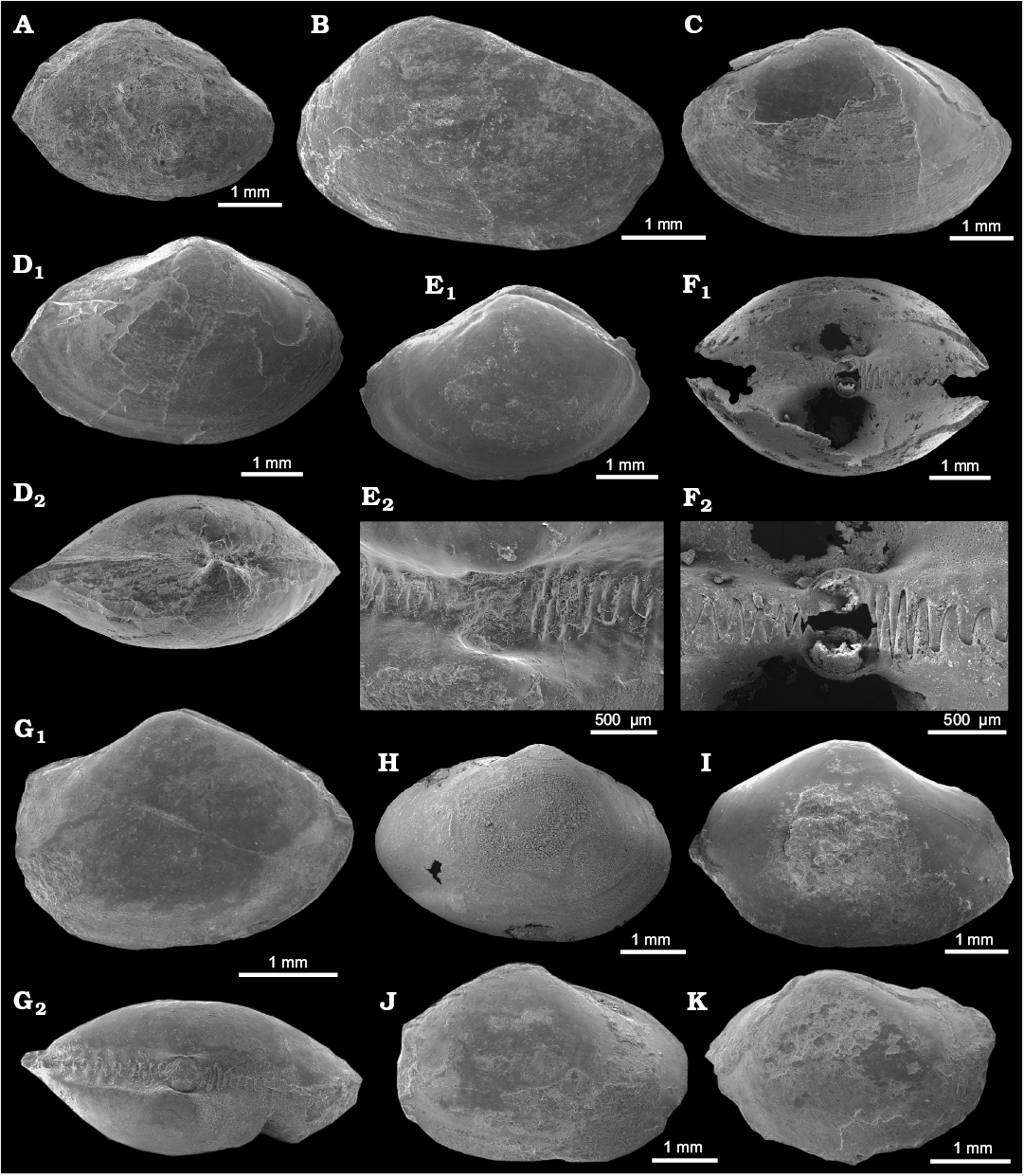
Fig. 7. Non-chemosymbiotic protobranch bivalves from the upper Paleocene, Basilika Formation, Fossildalen (A–E, G, I–K) and Zachariassendalen (F, H), Spitsbergen, Svalbard. A. ?Nucula sp., ZPAL V.48/10, internal mold of right valve. B. ?Malletia sp., ZPAL V.48/11, right valve. C, D. Neilonella sp. C. ZPAL V.48/12, left valve sculptured by fine commarginal ribs. D. ZPAL V.48/13, partial shell, right valve (D1), dorsal view of both valves (D2). E–K. Yoldiella spitsbergensis Amano sp. nov. E. Holotype, ZPAL V.48/17, internal mold, right valve (E1), hinge part (E2). F. Paratype, NRM-PZ Mo 186241, silicified shell, hinge part (F1), outer surface (F2). G. Paratype, ZPAL V.48/18, internal mold, right valve (G1), hinge part (G2). H. Paratype, NRM-PZ Mo 186242, silicified shell, right valve. I. ZPAL V.48/19, internal mold, left valve. J. ZPAL V.48/20, partial shell, left valve. K. ZPAL V.48/21, partial shell, partly preserved outer surface, left valve.
Order Nuculanida Carter, Campbell, and Campbell, 2000
Superfamily Nuculanidea Adams and Adams, 1858
Family Malletiidae Adams and Adams, 1858
Genus ?Malletia Des Moulins, 1832
Type species: Malletia chilensis Des Moulins, 1832; Recent, Pacific off Chile.
?Malletia sp.
Fig. 7B.
Material.—One specimen (ZPAL V.48/11), poorly preserved shell from the upper Paleocene of Fossildalen, Spitsbergen, Svalbard.
Measurements.—ZPAL V.48/11: L, 4.7 mm; H, 3.2 mm; H/L, 0.68.
Description.—Shell small, weakly inflated, elongate-ovate, inequilateral. Surface smooth without fine growth lines. Antero-dorsal margin short, sloping steeply into semicircular anterior margin; postero-dorsal margin long, nearly straight, forming bluntly pointed posterior end with broadly arcuate ventral margin. Beak not very prominent, located at anterior third of shell length.
Remarks.—The investigated specimen is doubtfully classified in Malletia based on its weakly inflated shell and outline. Its outline is very similar to Malletia sp. from the Selandian (middle Paleocene) Katsuhira Formation in eastern Hokkaido, Japan (Amano and Jenkins 2017: fig. 19). However, the present species is smaller and taller than the Katsuhira species (L, 10.4–10.9 mm; H, 5.5–6.4 mm; H/L, 0.53–0.58).
Family Neilonellidae Schileyko, 1989
Genus Neilonella Dall, 1881
Type species: Leda corpulenta Dall, 1881; Recent, Caribbean Sea and Gulf of Mexico.
Neilonella sp.
Fig. 7C, D.
Material.—Five specimens (including two figured: ZPAL V.48/12, 13), partial shells, from the upper Paleocene of Fossildalen, Spitsbergen, Svalbard.
Measurements.—L, 4.5–6.9 mm; H, 3.5–4.9 mm; W, 2.1–3.0 mm; H/L, 0.71–0.80; W/L, 0.44–0.58; n = 5.
Description.—Shell small, moderately inflated, elongate-ovate, equivalve, nearly equilateral. Surface sculptured with fine commarginal ribs. Antero-dorsal margin broadly rounded, passing into semicircular anterior margin; postero-dorsal margin nearly straight, sloping gently into bluntly pointed posterior end; ventral margin broadly arcuate. Escutcheon not demarcated but broad. Beak not very prominent, slightly opisthogyrate, located centrally. Hinge with two series of very small teeth. Pallial sinus narrow, shallow, posterior muscle scar subquadrate.
Remarks.—Although we could not determine the presence of a resilifer, the available specimens are considered to belong in Neilonella because of their elongate-ovate outline, the presence of many fine commarginal ribs on the shell surface and the bluntly pointed posterior end. They resemble Neilonella alleni Amano and Jenkins, 2017, from the Selandian (middle Paleocene) Katsuhira Formation in eastern Hokkaido, Japan, in their small size and in having sculpture of fine commarginal ribs. However, the anteriorly situated and more bluntly pointed beak of N. alleni enables us to separate it from the present species.
Family Yoldiidae Dall, 1908
Genus Yoldiella Verrill and Bush, 1897
Type species: Yoldia lucida Lovén, 1896; Recent, North Atlantic.
Yoldiella spitsbergensis Amano sp. nov.
Fig. 7E–K.
1925 Nuculana sp. 1; Hägg 1925: pl. 3: 6a, 7.
1925 Nuculana sp. 2; Hägg 1925: pl. 3: 8a, b.
1927 Portlandia sp.; Gripp 1927: 31.
1970 Nuculana (Jupiteria) haeggi n. sp.; Anderson 1970: pl. 9: 2a–c (not pl. 9: 1a–c).
ZooBank LSID: urn:lsid:zoobank.org:act:994EA500-2375-482E-AC28-450F0DD0DA11
Etymology: Named after the type locality.
Type material: Holotype: ZPAL V.48/17, internal mold (Fig. 7E). Paratypes: NRM-PZ Mo 186241–186242, silicified shells, ZPAL V.48/18–21, internal molds (Fig. 7F–K).
Type locality: Fossildalen, Spitsbergen, Svalbard.
Type horizon: Cold seep carbonates from the Basilika Formation, upper Paleocene.
Material.—27 specimens, shells, internal molds and silicified shells, including seven figured (ZPAL V.48/14–18; NRM-PZ Mo 186241–186242) from the upper Paleocene of Fossildalen, Spitsbergen, Svalbard.
Measurements.—The holotype (ZPAL V.48/17): L, 4.9 mm; H, 3.6 mm; W, 3.2 mm. Dimensions range: L, 3.3–6.3 mm; H, 2.8–5.0 mm; W, 1.8–3.8 mm; W/L, 0.41–0.65; H/L, 0.59–0.85; n = 14. See Table 1 for full list of measured specimens.
Table 1. List of measured specimens of Yoldiella spitsbergensis Amano sp. nov. Abbreviations: H, height; L, lenght; W, width.
|
Specimen |
Length (mm) |
Height (mm) |
H/L |
Width (mm) |
W/L |
|
ZPAL V.48/17 (holotype) |
4.9 |
3.6 |
0.73 |
3.2 |
0.65 |
|
ZPAL V.48/18 (paratype) |
3.3 |
2.8 |
0.85 |
1.8 |
0.55 |
|
ZPAL V.48/19 (paratype) |
5.3 |
3.9 |
0.74 |
– |
– |
|
ZPAL V.48/20 (paratype) |
3.8 |
2.7 |
0.71 |
2.1 |
0.55 |
|
ZPAL V.48/21 (paratype) |
3.5 |
2.7 |
0.77 |
2.1 |
0.60 |
|
ZPAL V.48/22 |
3.9 |
2.3 |
0.59 |
2.1 |
0.54 |
|
ZPAL V.48/23 |
4.2 |
3.0 |
0.71 |
2.4 |
0.57 |
|
ZPAL V.48/24 |
4.1 |
2.7 |
0.66 |
1.7 |
0.41 |
|
ZPAL V.48/25 |
6.3 |
5.0 |
0.79 |
3.8 |
0.60 |
|
ZPAL V.48/26 |
4.4 |
3.1 |
0.70 |
2.4 |
0.55 |
|
ZPAL V.48/27 |
3.9 |
3.0 |
0.77 |
1.9 |
0.49 |
|
ZPAL V.48/28 |
4.0 |
3.0 |
0.75 |
2.3 |
0.58 |
|
ZPAL V.48/29 |
3.7 |
2.9 |
0.78 |
2.3 |
0.62 |
|
ZPAL V.48/30 |
4.1 |
3.1 |
0.76 |
2.1 |
0.51 |
Diagnosis.—Small Yoldiella with concave postero-dorsal margin, prominent, centrally located beak, taxodont teeth interrupted by rather wide resilifer, ten teeth anteriorly and nine teeth posteriorly, and narrow and very shallow pallial sinus in front of posterior muscle scar.
Description.—Shell small, moderately inflated, elongate-ovate, equivalve, inequilateral. Surface partly preserved, smooth apart from fine growth lines. Antero-dorsal margin nearly straight, passing into narrowly rounded anterior margin; postero-dorsal margin slightly concave, passing into acutely rounded posterior end; ventral margin broadly arcuate. Two fine, weak internal ridges extending from beak to posterior corner, forming shallow depressed area on internal mold between ridges. Beak prominent, swollen, prosogyrate, situated centrally. Hinge with two series of small teeth, interrupted by rather wide resilifer; anterior series consisting of ten teeth; posterior one with nine teeth. Pallial sinus narrow, very shallow, anterior to posterior muscle scar. Anterior adductor scar elongate-subquadrate; posterior adductor scar small, pyriform.
Remarks.—Hägg (1925) described and illustrated Nuculana sp. 1 and N. sp. 2 from the Cenozoic of Spitsbergen. Gripp (1927) referred to them as Portlandia sp. without explanation. Anderson (1970) proposed Nuculana (Jupiteria) haeggi based on two specimens, of which the holotype (GPIBo 150) has a shape resembling that of Nucula, unlike the shape of Hägg’s (1925) Nuculana spp. 1 and 2. Anderson’s (1970) paratype specimen of Nuculana (Jupiteria) haeggi (GPIBo 151), however, is very similar to both species of Nuculana figured by Hägg (1925). Thus, Yoldiella spitsbergensis Amano sp. nov. is proposed here, based on the new material, Hägg’s (1925) Nuculana sp. 1 and N. sp. 2, and Anderson’s (1970) paratype specimen of Nuculana (Jupiteria) haeggi.
Yoldiella philippiana (Nyst, 1843), now living in the northern Atlantic, Mediterranean Sea and around Japan, is the most similar species in its size and outline to Y. spitsbergensis. However, Y. spitsbergensis Amano sp. nov. differs slightly from Y. philippiana by its larger teeth, larger resilifer, and more obtuse posterior end. Yoldiella nielseni was proposed under the genus Portlandia Mörch, 1857, from the Selandian (middle Paleocene) of Copenhagen, Denmark, by Schnetler (2001). This species is similar to Y. spitsbergensis in its small size (L up to 3.6 mm) and numerous taxodont teeth (12 in both anterior and posterior series of teeth), but differs by having a shorter rostrum and a more obtuse posterior end. Moreover, the very small resilifer of Y. nielseni enables us to separate it from the new species. Yoldiella dortea Heinberg, 1989, from the upper Maastrichtian of Copenhagen, Denmark, is another species that is similar to Y. spitsbergensis in having an ovate shell and a prominent umbo. However, the Cretaceous species can be distinguished from Y. spitsbergensis by its much smaller size (L 2.5 mm) and the low commarginal ribs on its surface, in contrast to fine commarginal growth lines in Y. spitsbergensis.
Stratigraphic and geographic range.—Upper Paleocene cold seep carbonates from the Basilika Formation, Fossildalen and Zachariassendalen, Spitsbergen, Svalbard.
Subclass Autobranchia Grobben, 1894
Superorder Pteriomorphia Beurlen, 1944
Order Mytilida Rafinesque, 1815
Family Mytilidae Rafinesque, 1815
Genus ?Mytilus Linnaeus, 1758
Type species: Mytilus edulis Linnaeus, 1758; Northern Atlantic, Recent.
?Mytilus hauniensis (Rosenkrantz, 1920)
Fig. 8.
1920 Modiola hauniensis n. sp.; Rosenkrantz 1920: 37, pl. 2: 15a, b.
1970 Modiolus hauniensis (Rosenkrantz, 1920); Anderson 1970: 92, pl. 9: 5a–c.
2016 Inoperna? sp.; Hryniewicz et al. 2016: table 1 (not fig. 12E1, E2).
Material.—Three specimens, shells, including one from Vonderbank’s collection (GPIBo 154) and two newly collected specimens, with one figured (NRM Mo 183950), from upper Paleocene of Zachariassendalen (NRM-PZ Mo 183950) and locality 500 m west of Trigonometric point 25 (GPIBo 154), Spitsbergen, Svalbard.
Measurements.—L, 6.6–10.9 mm; H, 4.1–5.3 mm; W, 2.4–3.7 mm; n = 3.
Remarks.—?Mytilus hauniensis was erected by Rosenkrantz (1920) on the basis of three mytiliform bivalves from the lower Paleocene Craniakalk, Copenhagen, Denmark. The only figures provided are two illustrations of a left valve (Rosenkrantz 1920: pl. 2: 15a, b), which show a shell with commarginal ornament, a pronounced umbonal carina, a straight dorsal margin and an obtuse posterodorsal shell angle. These features are all present in the material described by Anderson (1970) from the upper Paleocene from locality 500 m west of Trigonometric point 25, Spitsbergen, Svalbard, although the specimens from Svalbard seem to a have less pronounced umbonal carina. The latter is likely caused by the smaller size of the specimens from Svalbard (Lmax 10.9 mm) as compared to those from Denmark (Lmax 36 mm). Anderson (1970) mentioned that 25 specimens were measured, with Lmax reaching 21.3 mm; however, we were able to examine only one specimen from his collection. The terminal umbones of this species and poorly preserved taxodont dentition in the umbonal area suggest placement in Mytilus Linnaeus, 1758, rather than in Modiolus Lamarck, 1799. However, we consider this placement tentative due to the rather poor quality of the material and possible taxodont dentition visible both anteriorly and posteriorly from the umbones instead of just anteriorly as in Mytilus. The species clearly does not belong to Inoperna (cf. Hryniewicz et al. 2016), which has external ornament of commarginal corrugations rather than commarginal growth lines.
Stratigraphic and geographic range.—Lower Paleocene of Denmark (Rosenkrantz 1920) and upper Paleocene cold seep carbonates from the Basilika Formation, Zachariassendalen and locality 500 m west from Trigonometric point 25, Hollendarbukta, Spitsbergen, Svalbard.
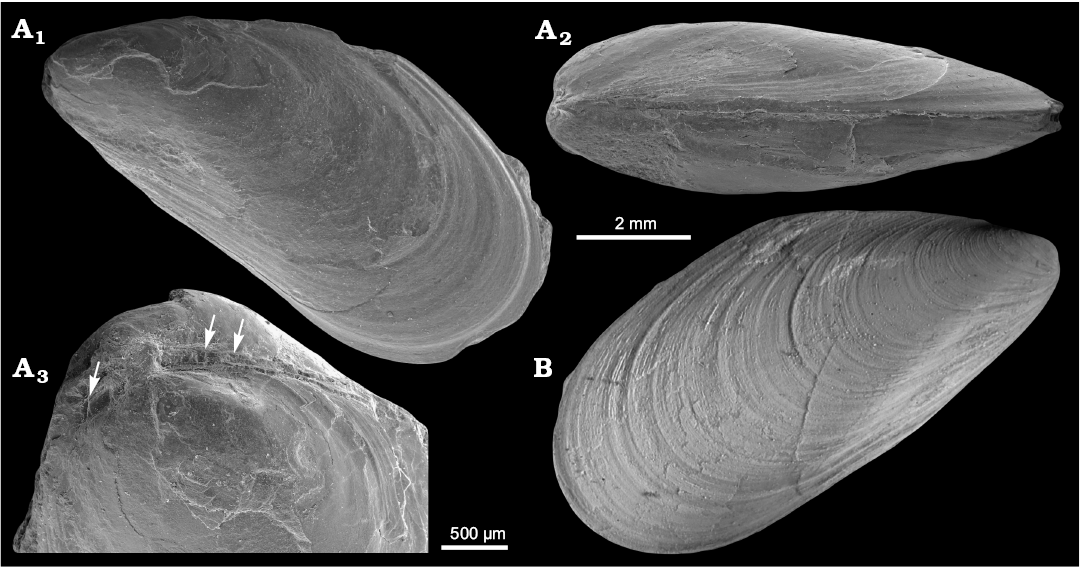
Fig. 8. Mytilid bivalve ?Mytilus hauniensis (Rosenkrantz, 1920) from the upper Paleocene, Basilika Formation, Zachariassendalen (A) and locality 500 m west from Trigonometric point 25, Hollendarbukta (B), Spitsbergen, Svalbard. A. NRM-PZ Mo183950, shell, left valve sculptured with fine commarginal growth lines superimposed on growth halts (A1), dorsal view of both valves (A2), oblique umbonal view showing delaminated prodissoconch, arrows point on poorly preserved taxodont teeth (A3). B. GPIBo 154, shell, right valve.
Genus ?Musculus Röding, 1798
Type species: Mytilus discors Linnaeus, 1767; Northern Atlantic, Recent.
?Musculus sp.
Fig. 9.
1970 ?Arca sp.; Anderson 1970: 92, pl. 9: 4a–b.
2016 Arcidae sp.; Hryniewicz et al. 2016: table 2.
Material.—Two specimens, including one fragmentary right valve (NRM-PZ Mo 183957) from the upper Paleocene of Zachariassendalen, Spitsbergen, Svalbard and one (GPIBo 153) from the upper Paleocene, locality 500 m west of Trigonometric point 25, Spitsbergen, Svalbard.
Description.—Shell small, with fine radial ornament separated by broad interspaces and superimposed on strong commarginal growth lines. Posterior end rounded, posterior lobe inclined ventrally. Details of anterior margin and inner shell surface unknown.
Remarks.—This radially ornamented mytilid was previously figured by Anderson (1970) and misidentified as an arcid, an identification followed by Hryniewicz et al. (2016). Closer examination of the material, supplemented by an additional specimen found during the field season in 2017 in Zachariassendalen, revealed that this species is actually a mytilid. Radial ornament superimposed on commarginal growth lines occurs among several mytilid genera. Radial ribs superimposed on strong commarginal growth lines and fading close to the ventral margin of the mid-shell section, and a median keel visible on the posterior lobe suggest that this species possibly belongs to Musculus Röding, 1798, but the lack of well-preserved specimens makes our assignment tentative. From the ribbed mytilids described from the Paleocene strata of Nuusquaq, northwest Greenland (Petersen and Vedelsby 2000), Brachidontes sp. 1 and Brachidontes sp. 2 have coarser radial ornament, whereas Brachidontes sp. 3 has somewhat similar ornament and a medial keel to those of the current species. Unfortunately, the fragmentary nature of our material does not allow for a more thorough comparison.

Fig. 9. Mytilid bivalve ?Musculus sp. from the upper Paleocene, Basilika Formation, Zachariassendalen, Spitsbergen, Svalbard. NRM-PZ Mo 183957, shell, partially preserved left valve.
Genus Inoperna Conrad, 1875
Type species: Inoperna carolinensis Conrad, 1875; Campanian–Maastrichtian, Upper Cretaceous, southeastern USA.
Remarks.—Inoperna Conrad, 1875, is a poorly known mytilid bivalve genus reported from Mesozoic shallow-water deposits (e.g., Woods 1900; Stephenson 1923; Wade 1926; Cox and Arkell 1948; Hautmann 2001). Specimens assigned to Inoperna are elongate, thin-shelled mytiliform bivalves with subterminal umbones and characteristic ornament of commarginal corrugations, some of which merge into bundles of dorsal commarginal folds. Ten species, including Inoperna plenicostata (Anderson, 1970) discussed herein, have been recorded previously (Stenzel et al. 1957: 74; Vonderbank 1970). Mauricia Harris, 1919, from the middle Eocene Cook Mountain Formation (Claiborne Group) in Texas, USA, is similar to Inoperna with respect to shell shape and ornament and could represent the same lineage (Stenzel et al. 1957).
Inoperna plenicostata (Anderson, 1970)
Fig. 10.
1970 Mytilus plenicostatus n. sp.; Anderson 1970: 93, pl. 9: 6–7.
2016 Inoperna? sp.; Hryniewicz et al. 2016: table 2, fig. 12E.
Material.—Six specimens, all fragmentarily preserved shells, including three (GPIBo 155–156) from the upper Paleocene, locality 500 m west of Trigonometric point 25, Hollendarbukta, Spitsbergen, Svalbard, and three (NRM-PZ Mo 183947–183949) specimens from the upper Paleocene of Zachariassendalen, Spitsbergen, Svalbard.
Measurements.—L, 11.4–~59.8 mm; n = 2. Other measurements difficult to estimate due to the poor quality of the material.
Description.—A description of the species was provided by Anderson (1970: 94). We hereby provide a description of juvenile specimens, which were not studied previously.
Earliest growth stages unknown. Shell shorter than 1.75 mm ornamented with regularly spaced commarginal corrugations with similarly wide depressions separating them. Commarginal growth lines superimposed on fine radial ornament visible in commarginal depressions but diminishing on commarginal corrugations. Possible growth halt visible ~0.75 mm from umbones; second growth halt at ~1.75 mm, roughly coincides with change of ornamentation from regular and strong commarginal ornament superimposed on fine radial ornament to less regular and weaker commarginal currugations and no radial ornament. Latter ornamentation pattern present on remainder of shell; commarginal ornament of largest specimen (GPIBo 155; L ~59.8 mm) grouped in bundles of 3–5, whereas commarginal corrugations on shorter specimens show no obvious grouping. Anterior adductor muscle scar minute, rounded, located ventrally to umbo and separated from pallial line. Pallial line visible only in anteroventral part of shell; remainder unknown.
Remarks.—Some morphological features of this mytilid from Spitsbergen suggest its inclusion in the poorly known genus Inoperna Conrad, 1875. The specimens from locality 500 m west of Trigonometric point 25 are partial shells with a slightly different shape from the specimens from Zachariassendalen. The holotype (GPIBo 155; L ~59.8 mm) is an incomplete mytiliform shell with the posterior lobe broken off; therefore, it was originally larger than the measurement given above. The shell has a terminal umbo and no well-defined carina, whereas the paratype (GPIBo 156; L ≥11.4 mm) has a more complete, modioliform shell and a subterminal umbo. Shell shape changes during mytiloid ontogeny (e.g., Fuller and Lutz 1989; Cosel and Olu 1998; Saether et al. 2010); therefore, the observed shape differences among specimens of different size are likely an ontogenetic feature. In general, the shell shapes observed in the material from Svalbard are similar to those known from other species of Inoperna (reviewed by Stenzel et al. 1957: 74–75). The curved mytiloid shell of I. plenicostata is somewhat reminiscent of some specimens of I. flagellifera Forbes, 1845 (e.g., Forbes 1845: pl. 16: 9; Woods 1900: pl. 17: 1), especially of the specimens from the Santonian (Late Cretaceous) Hochmoos Formation of the Gosau Group in Austria (Dhondt 1987: pl. 1: 13). Most of the species of Inoperna are known mostly from partially preserved specimens (e.g., Stephenson 1923; Wade 1926; Popenoe 1937); therefore, it is difficult to determine their external shapes, and to estimate the degree to which the shell shape of Inoperna is taxonomically or phenotypically controlled.
The holotype (GPIBo 155) of Inoperna plenicostata has a characteristic external ornament composed of commarginal corrugations arranged in groups of three to five, separated by broader furrows devoid of any visible ornament. The paratype (GPIBo 156) has commarginal corrugations not grouped into bundles. We infer that this difference in ornamentation between two type specimens is likely an ontogenetic feature, as the two are of very different size. An ornamentation composed of commarginal corrugations is typical for Inoperna (Woods 1900; Stephenson 1923; Wade 1926; Popenoe 1937). The ornament of I. plenicostata differs from that known from other species of Inoperna because the corrugations do not merge close to the dorsal margin to form broader commarginal folds as seen in I. carolinensis (Stephenson 1923: pl. 62: 14; Wade 1926: pl. 13: 10) and I. flagellifera (e.g., Dhondt 1987: pl. 1: 11–13), and, to some extent, I. bellarugosa (Popenoe 1937: pl. 45: 7 [not fig. 6]). The commarginal corrugations of most of the species are more regular than those of I. plenicostata, but we are unable to determine to what extent this feature is of taxonomic significance.
Stratigraphic and geographic range.—Upper Paleocene cold seep carbonate and associated sunken driftwood from the Basilika Formation, Zachariassendalen and locality 500 m west from Trigonometric point 25, Hollendarbukta, Spitsbergen, Svalbard.
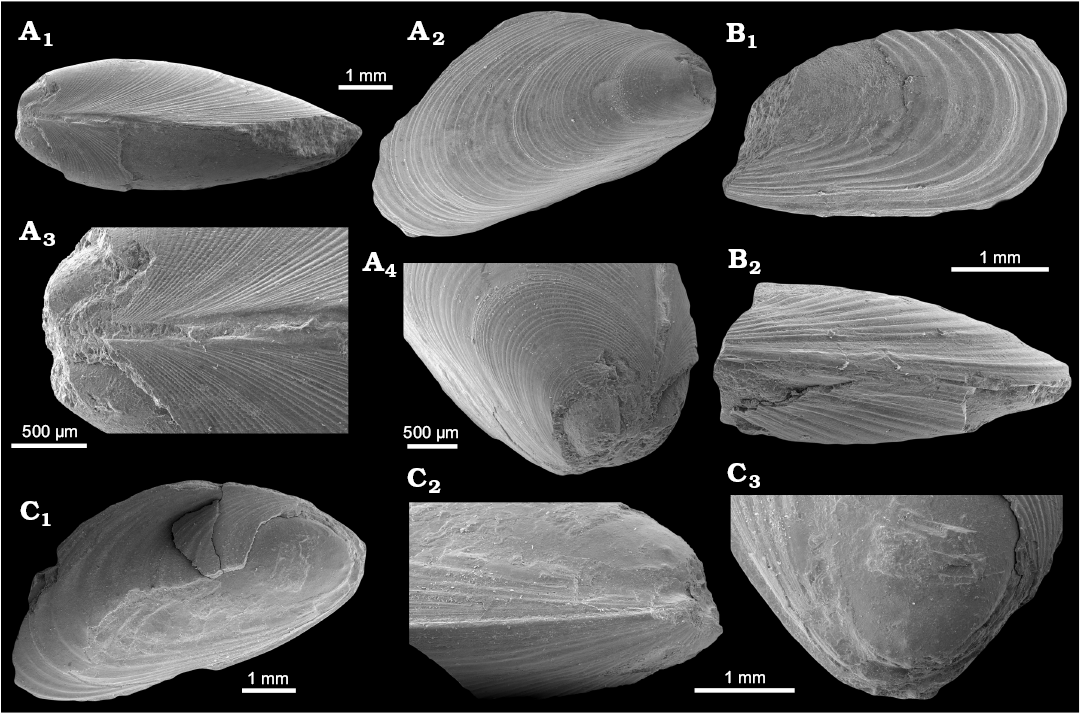
Fig. 10. Mytilid bivalve Inoperna plenicostata (Anderson, 1970) from the upper Paleocene, Basilika Formation, Zachariassendalen, Spitsbergen, Svalbard. A. NRM-PZ Mo 183949, shell, dorsal view of both valves (A1), right valve (A2), enlarged dorsal view of both valves, showing ornamentation of the early growth stages (A3), oblique anterodorsal view (A4). B. NRM-PZ Mo 183947, partial shell, left valve (B1), ventral view of both valves (B2). C. NRM-PZ Mo 183948, partial shell, left valve (C1), ventral view of partially preserved valves (C2), oblique anterior view of left valve, showing anterior adductor muscle scar (C3).
Order Lucinida Gray, 1854
Family Thyasiridae Dall, 1900 (1895)
Genus Rhacothyas Åström
and Oliver in Åström
et al., 2017
Type species: Rhacothyas kolgae Åström and Oliver in Åström et al., 2017; Recent, Northern Atlantic.
Rhacothyas spitzbergensis (Anderson, 1970)
Figs. 11, 12.
1925 Lucina spec.; Hägg 1925: 46, pl. 3: 10a, 11
1927 Thyasira spec. 2: Gripp 1927: 32, pl. 7: 18a.
1970 Anodontia spitzbergensis n. sp.; Anderson 1970: 95, pl. 9: 8a–c.
2016 Myrteinae sp.; Hryniewicz et al. 2016: table 2, fig. 12B1–B3.
Material.—Holotype (GPIBo 157), relatively well preserved shell, from the upper Paleocene, locality 500 m west of Trigonometric point 25, Hollendarbukta, Spitsbergen, Svalbard; 28 specimens, mostly internal molds (including NRM-PZ Mo 149144, 149145, 182205, 182205a, 183970) from the upper Paleocene of Fossildalen, Spitsbergen, Svalbard; and 21 specimens, mostly shells (including NRM-PZ Mo 183958, 183968, 183969, 183970, 183971, 183974, 186243, 186244) from the upper Paleocene of Zachariassendalen, Spitsbergen, Svalbard.
Measurements.—L, 4.7–16.9 mm; H, 4.2–15.3 mm; W, 2.1–10.4 mm; n = 27.
Description.—Shell thin, luciniform, longer than high, of normal inflation, with umbones located centrally or slightly towards anterior. Beaks prosogyrate, anterodorsal margin concave with small, sunken lunule. Anterodorsal margin of some specimens with weak angulation. Anterior shell margin rounded, passing smoothly into convex ventral margin. Posterior margin truncated, with no sinus visible. Posterodorsal margin slightly convex, accompanied by weak sulcus located very close to posterodorsal margin. External ornament of raised, narrowly spaced commarginal lamellae. Hinge edentulous. Anterior adductor muscle scar elongated along pallial line, transversally striated, posterior adductor muscle scar positioned close to lower part of posterodorsal shell margin, transverally striated. Anterior pedal retractor muscle scars small, rounded, separated from anterior adductor muscle scars. Ligament sunken, relatively long, occupying approx. half of length of posterodorsal shell margin.
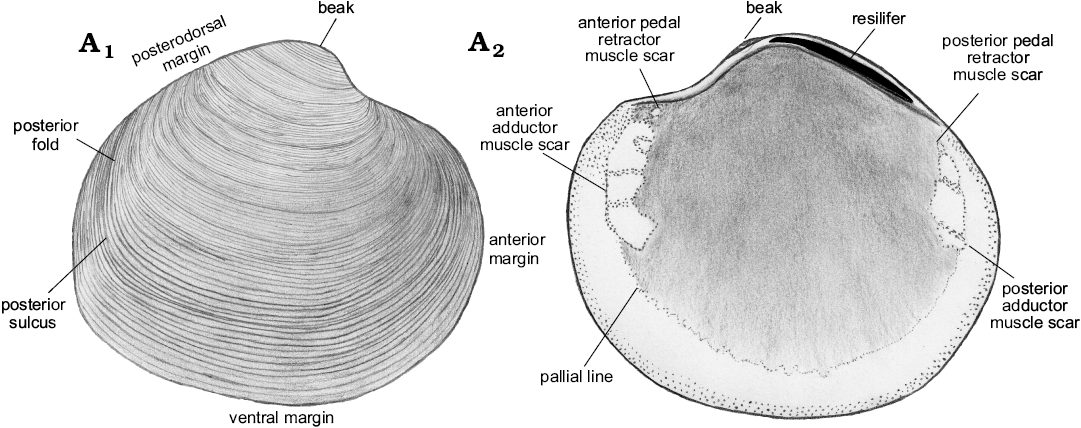
Fig. 11. Schematic illustration of Rhacothyas spitzbergensis (Anderson, 1970) from the upper Paleocene, Basilika Formation, Spitsbergen, Svalbard, showing morphological features discussed. Right valve, outer (A1) and inner (A2) views.
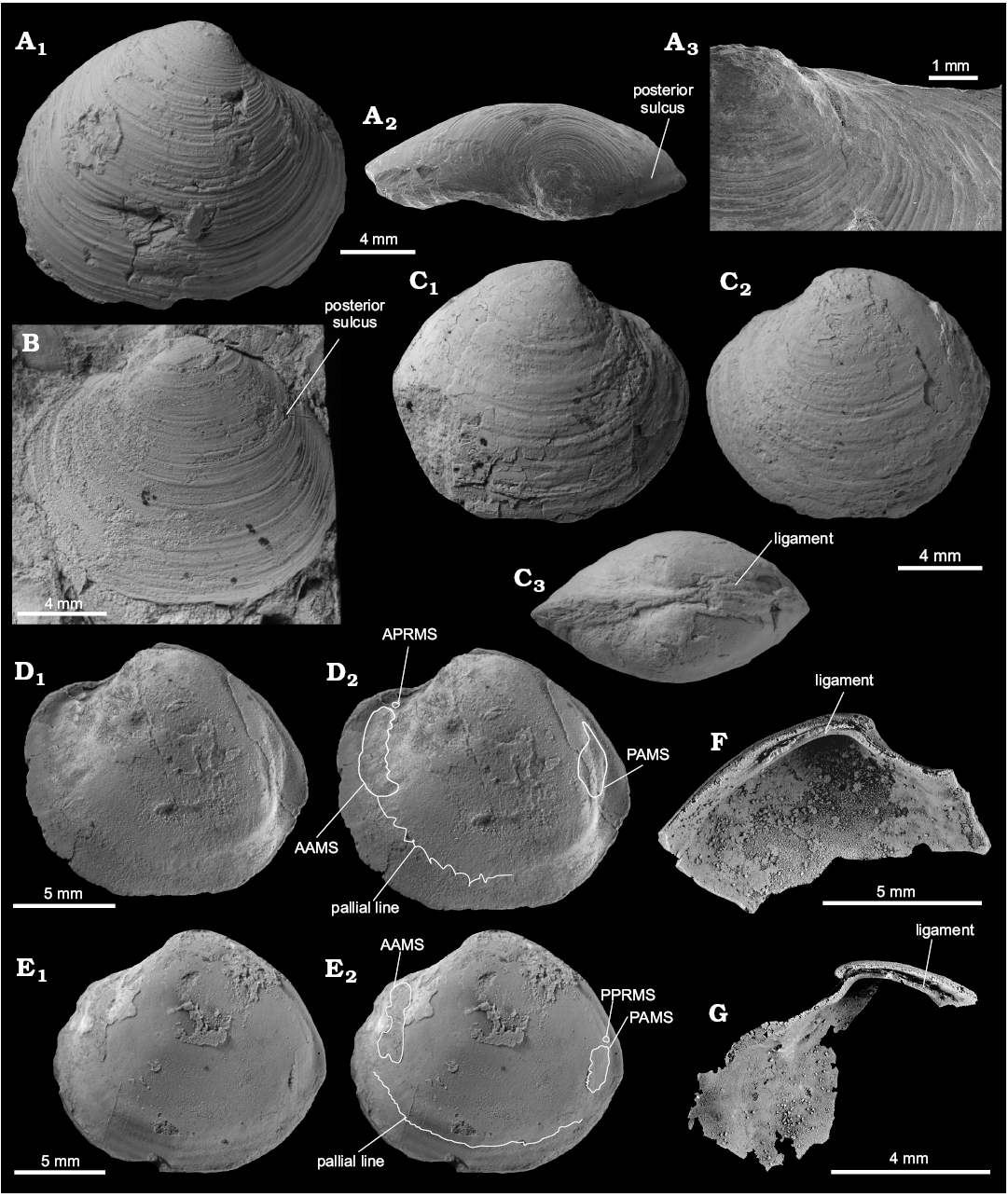
Fig. 12. Thyasirid bivalve Rhacothyas spitzbergensis (Anderson, 1970) from the upper Paleocene, Basilika Formation, Zachariassendalen (A, F, G) and Fossildalen (B–E), Spitsbergen, Svalbard. A. NRM-PZ Mo183968, shell, right valve (A1), dorsal view of a right valve showing posterior sulcus (A2), oblique anterior view showing small lunule (A3). B. NRM-PZ Mo 183970, shell, left valve view. C. NRM-PZ Mo 149144, partial shell, right (C1) and left (C2) valve views, dorsal view, showing partially preserved internal ligament (C3). D. NRM-PZ Mo 182205, internal mold. E. NRM-PZ Mo 182205a, internal mold. Left valves (D1, E1), same valves with outlined positions of anterior (AAMS) and posterior (PAMS) adductor muscle scars, anterior (APRMS) and posterior (PPRMS) pedal retractor muscle scars, and pallial line (D2, E2). F. NRM-PZ Mo 186243, silicified shell, inner view of left valve, showing length of sunken ligament, and no cardinal dentition. G. NRM-PZ Mo 186244, silicified shell, inner view of right valve, showing length of sunken ligament, and no cardinal or anterior lateral dentition.
Remarks.—The species discussed was initially attributed to Lucina by Hägg (1925). In the paper following shortly thereafter, Gripp (1927) included the same species in Thyasira, although Gripp (1927) did not comment on the previous identification by Hägg (1925). The contents of the work of Gripp (1927) show that he was aware of the preceeding paper of Hägg (1925); therefore, it is unclear why he did not comment on the previous identification. Anderson (1970), on the other hand, identified this Paleocene form from Spitsbergen as a new species of the lucinid genus Anodontia Link, 1807, based on the outer shell shape and information on the edentulous hinge provided by Hägg (1925: 46). The species was subsequently classified as an unnamed myrtein lucinid by Hryniewicz et al. (2016), based on overall luciniform shape and the fine, commarginal ornament.
Here we argue that this species, with its luciniform shell, small and sunken lunule, edentulouse hinge, long and sunken ligament, and weak posterior sulcus, belongs to the luciniform thyasirid genus Rhacothyas Åström and Oliver in Åström et al., 2017. The sole modern species of the genus is Rhacothyas kolgae Åström and Oliver in Åström et al., 2017, from the cold seeps off Prins Karls Forland in the North Atlantic (ca. 350 m water depth). Rhacothyas kolgae is somewhat larger (Lmax 27.7 mm), and has a more sloping posterodorsal margin, with a deeper sulcus than the current species, but its general morphology is very similar, with a somewhat stronger posterior sulcus and more incurved umbones than R. spitzbergensis. Other luciniform thyasirid bivalves from chemosynthesis-based ecosystems comprise “Maorithyas” hadalis Okutani, Fujikura, and Kojima, 1999, from a methane seep in the trench off northern Honshu, Japan (7326 m water depth; Fujikura et al. 1999; see Valentich-Scott et al. 2014; Åström et al. 2017, for discussion of the systematic placement of this species), has a weakly defined lunule and a much shorter ligament than the species from Spitsbergen discussed here. Ochetoctena tomasi Oliver, 2014, from the Regab Pockmark seeps in the Gulf of Guinea, equatorial Atlantic (3167 m water depth), the sole species of Ochetoctena Oliver, 2014, is very similar in shape to the Paleocene species from Spitsbergen. However, it has periostracal spines on the outer shell surface not seen in the Spitsbergen material examined, and also less pronounced beaks. Spinaxinus emicatus Oliver in Oliver et al. 2013, from artificial sulfide biogenerators planted on the Louisiana slope (584–643 m water depth) has periostracal spines, less pronounced beaks and a resilifer that broadens towards the posterior. Spinaxinus phrixicus Oliver in Oliver et al. 2013, from hydrothermal vents in the Fiji and Lau Basins, equatorial Pacific (1847–1977 m water depth), in addition to periostracal spines has less pronounced beaks, a resilifer broadening towards the posterior, and commarginal ornament composed of broadly spaced commarginal lamellae, unlike the narrowly-spaced, raised lamellae of R. spitzbergensis. Wallerconcha sarae Valentich-Scott and Powell in Valentich-Scott et al., 2014, from a probable seep in the Beaufort Sea, Arctic Ocean (2358 m water depth), the sole species of Wallerconcha Valentich-Scott and Powell in Valentich-Scott et al., 2014, has a less pronounced umbo and a straight to weakly concave ventral margin, as opposed to the evenly rounded ventral margin of R. spitzbergensis. Wallerconcha sarae also lacks a lunule and has a broad hinge plate with a deeper nymph, whereas the current species possesses a lunule and has a relatively narrow hinge plate. Small, luciniform specimens interpreted as juvenile Conchocele bisecta (Conrad, 1849) from a cold seep off Paramushir Island, Sea of Okhotsk (~800 m water depth; Kharlamenko et al. 2016; see Hryniewicz et al. 2017, for a discussion of nomenclatoral problems of extant species of Conchocele) are very similar in shape to the current species. However, the luciniform thyasirids from the Sea of Okhotsk are much smaller (L 5.5–8.4 mm) than the current species (L 4.7–16.9 mm); at the shell length achieved by R. spitzbergensis, the shell of the thyasirid species from Sea of Okhotsk has a shape more similar to that of typical Conchocele.
Rhacothyas spitzbergensis clearly does not belong in Anodontia, which has a globular shell with short, weakly sloping anterodorsal and posterodorsal margins, and a curved ventral margin forming a deep arch continuous with anterior and posterior margins. In contrast, the Spitsbergen species has relatively long, sloping anterodorsal and posterodorsal margins, and a ventral margin forming a relatively shallow arch unlike that known from extant Anodontia species (Taylor and Glover 2005). There is no reported occurrence of Anodontia older than the Miocene (e.g., Ludbrook 1959, 1978; Olsson 1964; Kiel et al. 2018). Paleogene lucinid bivalves similar to Anodontia have usually been assigned to different genera, such as Eophysema Stewart, 1930. Eophysema encompasses species from the Eocene of the Gulf Coast, USA, and France (see Bretsky 1976; Taylor et al. 2011). It is a genus with relatively small species (L ≤32 mm) with a thin hinge plate; however, it has a globular shell and cardinal dentition, unlike the Paleocene specimen from Spitsbergen herein. Afranodontia marginidentana Adegoke, 1977, from the Paleocene Ewekoro Formation in Nigeria (Adegoke 1977), exhibits cardinal dentition and marginal shell crenulations not known from the Paleocene species of Spitsbergen. Edentulous fossil seep lucinids identified so far comprise Early Cretaceous–Oligocene Amanocina Kiel, 2013, Eocene–Miocene Elongatolucina Gill and Little, 2013, Oligocene–Miocene Nipponothracia Kanie and Sakai, 1997, Oligocene–Recent Elliptiolucina Cosel and Bouchet, 2008, and Miocene–Recent Meganodontia Bouchet and Cosel, 2004. Representatives of all five genera are much larger than the Spitsbergen species, exceeding 130 mm in length (Kelly et al. 2000; Kiel 2013). Among those genera, Elliptiolucina and Elongatolucina have characteristic, elongate shells, very different from the oval shell of Rhacothyas spitzbergensis, whereas the shell of Meganodontia is very large and globular (e.g., Kiel and Taviani 2017; Amano et al. 2018b), also unlike that of the current species. Seep lucinids with a similarly shaped shell with dentition comprise the Late Jurassic genus Beauvoisina Kiel, Campbell, and Gaillard, 2010; the Late Jurassic–Late Cretaceous genus Tehamatea Kiel, 2013, the Early Cretaceous–Oligocene genus Cubatea Kiel, Campbell, and Gaillard, 2010, the Late Cretaceous–Paleocene genus Nymphalucina Speden, 1970, and the Oligocene–Recent genus Lucinoma Dall, 1901. All these genera have cardinal dentition and moderately thick (Beauvoisina, Tehamatea, Cubatea, and Lucinoma) or thick (Nymphalucina) hinge plates (Speden 1970; Kiel 2013; Kiel and Taviani 2017; Amano et al. 2018b), features that are absent from the material studied.
Lucina planistria Koenen, 1885, and Lucina lepis Koenen, 1885, from Paleocene deposits in Copenhagen, Denmark (Koenen 1885) are very similar to Rhacothyas spitzbergensis in shell size and shape, as well as the shape of the resilifer and the apparent lack of dentition, and could potentially represent another fossil occurrence of Rhacothyas. However, the details of muscle scars of both species are unknown, and we refrain from any further comparison with R. spitzbergensis until more data on both L. planistria and L. lepis are available.
Stratigraphic and geographic range.—Upper Paleocene cold seep carbonate and associated sunken driftwood from the Basilika Formation, Fossildalen, Zachariassendalen and locality 500 m west from Trigonometric point 25, Hollendarbukta, Spitsbergen, Svalbard.
Order Anomalodesmata Dall, 1889
Family ?Pleuromyidae Dall, 1900
Genus ?Pleuromya Agassiz, 1842
Type species: Mya gibbosa Sowerby, 1823; Late Jurassic, England.
?Pleuromya sp.
Fig. 13.
1925 Lamellibranchiate 1; Hägg 1925: 48, pl. 5: 21, 21a.
Material.—One specimen (NRM-PZ Mo 149164), internal mold with fragments of the shell preserved, from the upper Paleocene of Fossildalen, Spitsbergen, Svalbard.
Description.—Shell elongate (L 11.7 mm), inequilateral with orthogyrate to opisthogyrate beaks displaced towards anterior. Anteroventral margin straight, anterior margin evenly rounded, ventral margin broadly rounded, passing smoothly into acutely rounded posterior margin. Posterodorsal margin straight, hinge without lateral teeth and apparently edentulous. Opisthodethic part of ligament visible. Posterior adductor muscle scar small, pallial sinus present, deep but not seen completely. External ornament of commarginal growth lines.
Remarks.—This species is most similar to Lyonsia sp. 1 from the Selandian (middle Paleocene) Sonja Lens in Nuussuaq, western Greenland (Petersen and Vedelsby 2000), with respect to the shape, the position of the beak, external ornament, inflation, and size. The available features visible on the material from Spitsbergen, especially a moderately inflated, equivalved shell, deep pallial sinus, and ornament composed of commarginal growth lines, suggest that this species does not belong to Lyonsia Turton, 1822. Lyonsia has a relatively shallow pallial sinus and ornament composed of commarginal lines superimposed on radial rows of spines (Prezant 1981; Coan et al. 2000; Coan and Valentich-Scott 2012). Instead, the specimen is more similar to Pleuromya Agassiz, 1842 (e.g., Duff 1978), which has a deep pallial sinus and ornament composed of commarginal growth lines. The interpretation presented herein should be treated as preliminary because only one specimen was available.

Fig. 13. Pleuromyid bivalve ?Pleuromya sp. from the Paleocene Basilika Formation, Fossildalen, Spitsbergen, Svalbard. NRM-PZ Mo 149164, partial shell, left valve internal mold with partially preserved shell sculptured by commarginal growth lines (A1), dorsal view of partially preserved internal mold of both valves (A2), ventral view of partially preserved internal mold of both valves (A3).
Order Venerida Gray, 1854
Superfamily ?Arcticoidea Newton, 1891
Family ?Arcticidae Newton, 1891
Genus ?Arctica Schumacher, 1817
Type species: Venus islandica Linnaeus, 1767; Recent, Northern Atlantic.
?Arctica sp.
Fig. 14.
1925 Astarte spec.; Hägg 1925: 45, pl. 3: 9.
2016 Astartiidae? sp. 1; Hryniewicz et al. 2016: table 2.
Material.—One specimen (NRM-PZ Mo 149143), shell, from the upper Paleocene of Fossildalen, Spitsbergen, Svalbard.
Measurements.—NRM-PZ Mo 149143: L, 26.9 mm; H, 22.5 mm; W, 12.5 mm.
Description.—Shell medium-sized, oval with prosogyrate, weakly anteriorly incurved umbones. Anterodorsal margin concave, with incised lunular area, anterior margin rounded, continuous with rounded ventral margin. Posterior margin rounded, passing into straight, dorsally inclined posterodorsal margin. Outer surface sculptured with many very fine growth lines (8 per 1 mm). Details of inner shell surface unknown.
Remarks.—After closer examination of the specimen illustrated by Hägg (1925), we conclude it is most likely a species of Arctica sp. based on the external shell shape, position and character of the beak, straight and dorsally inclined posterodorsal shell margin, and numerous very fine commarginal growth lines. The latter character could be related to very slow growth (Morton 2011), known from the extant long-living species Arctica islandica (Linnaeus, 1767). Arctica ovata (Meek and Hayden, 1858) from the Paleocene Cannonball Formation of South and North Dakota (Cvancara 1966) and Danian (lower Paleocene) Prince Creek Formation in Alaska (Marincovich 1993), both USA, is similar to ?Arctica sp. in general shell shape. However, due to the lack of data on the dentition of the Svalbard material, we are unable to compare the two species in more detail. The same applies to Arctica sp. 1 from the lower Danian (Paleocene) Agatdal Formation in Nuussuaq (Petersen and Vedelsby 2000), Greenland.
Fig. 14. Arcticid bivalve ?Arctica sp. from the upper Paleocene, Basilika Formation, Fossildalen, Spitsbergen, Svalbard. NRM-PZ Mo 149143, shell, left (A1) and right (A2) valves, dorsal (A3) and anterodorsal (A4) views, oblique dorsal view of left valve showing very fine commarginal ornament (A5).
Superfamily Tellinoidea Blainville, 1814
Family Tellinidae Blainville, 1814
Genus ?Tellina Linnaeus, 1758
Type species: Tellina radiata Linnaeus, 1758; Recent, western Northern Atlantic.
?Tellina sp.
Fig. 15.
1925 Lamellibranchiate 2; Hägg 1925: 48, pl. 5: 22–22a.
Material.— One specimen (NRM-PZ Mo 149165), internal mold with fragments of the shell preserved, from the upper Paleocene of Fossildalen, Spitsbergen, Svalbard.
Measurements.—NRM-PZ Mo 149165: L, 10.4 mm; H, 5.2 mm; W, 3.5 mm.
Description.—Shell small, elongate, with umbones positioned roughly medially. Anterodorsal and posterodorsal margins straight, with anterior margin slightly truncated, ventral margin broadly rounded, posterior margin weakly pointed. External ornament composed of commarginal growth lines; weak sulcus visible in posterodorsal shell area. Ligament external, short, positioned posterior to umbo. Internal features unknown.
Remarks.—The current species was previously listed as an unnamed bivalve by Hägg (1925). We classify it as a tellinid based on its shell shape and the presence of an external ligament; the elongation and roughly equidistant position of the umbo suggests ascription to Tellina rather than to Macoma Leach, 1819.
Several tellinids have been reported from Paleocene deposits of Greenland (Petersen and Vedelsby 2000). Among those, Tellina sp. 1 is less elongate, whereas Tellina sp. 2 has the umbo positioned closer to the anterior of the shell than the species reported here. Macoma sp. 1 and Macoma sp. 2. are both less elongate than the species reported here. Tellinimera scitula (Meek and Hayden, 1856) from the uppermost Maastrichtian (Upper Cretaceous) Fox Hills Formation in South Dakota, USA, has a less pointed umbo, is less elongate and has more rounded anterodorsal and posterodorsal margins than the Paleocene ?Tellina sp. described here. Tellinimera kauffmani Marincovich, 1993, from the Danian (lower Paleocene) Prince Creek Formation in northern Alaska, USA, is similar in shape to the species reported here, but has a less pointed umbo and weakly convex anterodorsal and posterodorsal margins as opposed to straight in ?Tellina sp.
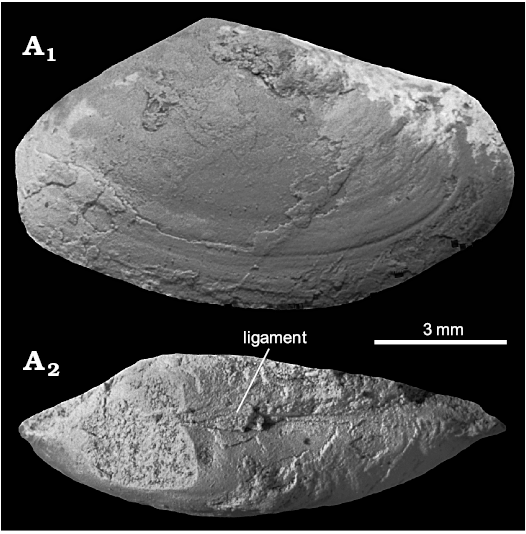
Fig. 15. Tellinid bivalve ?Tellina sp. from the upper Paleocene, Basilika Formation, Fossildalen, Spitsbergen, Svalbard. NRM-PZ Mo 149165, partial shell, left valve view (A1), dorsal view showing partially preserved opisthodethic external ligament (A2).
Order Myida Goldfuss, 1820
Superfamily Pholadoidea Lamarck, 1809
Family Pholadidae Lamarck, 1809
Subfamily Xylophaginae Purchon, 1941
Genus Xylophagella Meek, 1864
Type species: Xylophaga elegantula Meek and Hayden, 1858; Campanian (Late Cretaceous), Taylor Group, Montana, USA.
Xylophagella littlei Hryniewicz sp. nov.
Figs. 16, 17.
1925 Xylophaga spec. n. sp.; Hägg 1925: 48, pl. 3: 13a–c.
2016 Pholadoidea indet.; Hryniewicz et al. 2016: fig. 12c.
ZooBank LSID: urn:lsid:zoobank.org:act:E8836D7A-EAA0-4522-AA 79-639B1E5E3629
Etymology: In honour of the British palaeontologist Crispin T.S. Little, in recognition of his studies on fossil chemosynthesis-based faunas.
Type material: Holotype: ZPAL V.48/5, partial shell (Fig. 17A). Paratypes: ZPAL V.48/6–8, partial shells (ZPAL V.48/6–7), internal mold (ZPAL V.48/8) (Fig. 17B–D) from the upper Paleocene, Fossildalen, Spitsbergen, Svalbard.
Type locality: Fossildalen, Spitsbergen, Svalbard.
Type horizon: Sunken driftwood associated with cold seep carbonates from the Basilika Formation, upper Paleocene.
Material.—Five specimens, mostly partial shells, including four (ZPAL V.48/5–8) from the 2015 field season and one (NRM-PZ Mo 149147); Fossildalen, Spitsbergen, Svalbard.
Measurements.—The holotype (ZPAL V.48/5): W, 11.8 mm; H, 9.7 mm; W, 10.7 mm. Dimensions range: L, 8.1–20.2 mm; H, 7.4–16.9 mm; W, 8.5–23.5 mm; H/L, 0.84–0.98; W/L, 0.88–1.16; n = 5. See Table 2 for full list of measured specimens.
Table 2. List of measured specimens of Xylophagella littlei Hryniewicz sp. nov. Abbreviations: H, height; L, lenght; W, width.
|
Specimen |
Length (mm) |
Height (mm) |
H/L |
Width (mm) |
W/L |
|
ZPAL V.48/5 (holotype) |
11.9 |
9.7 |
0.86 |
10.5 |
0.88 |
|
ZPAL V.48/6 (paratype) |
9.5 |
9.3 |
0.98 |
9.6 |
1.01 |
|
ZPAL V.48/7 (paratype) |
9.6 |
9.3 |
0.97 |
10.3 |
1.07 |
|
ZPAL V.48/8 (paratype) |
8.1 |
7.4 |
0.91 |
8.5 |
1.04 |
|
NRM-PZ Mo 149147 |
20.2 |
16.9 |
0.84 |
23.5 |
1.16 |
Diagnosis.—A species of Xylophagella with inner posterior ridge strong dorsally but diminishing ventrally.
Description.—Shell of normal size for genus (L ≤20.2 mm), thin, globular. Ventral margin convex, deepest around umbonal-ventral groove, rising posteriorly. Faint posteroventral angulation present; posterior margin rounded. Anterior gape wide, posterior gape narrower than anterior one. Prora triangular, occupying ca. 40% of height of anterior slope. Anterior slope short, well-demarcated from prora but poorly demarcated from disc; its dimensions difficult to ascertain. Rasp consists of oblique ridges covered with transverse lamellae; ridges sub-parallel to lower margin of prora, and to anterior margin of anterior slope. Obtuse angle of ridges between prora and anterior slope section of rasp forms well-defined line separating the two shell regions. This angle causes shell thickening, forming internal ridge. Rasp diminishes posteriorly but remains present until umbonal-ventral groove. Umbonal-ventral groove relatively wide, bounded anteriorly and posteriorly by shell thickening, forming two outer and inner ridges. Faint flat-topped outer ridge amidst umbonal-ventral groove. Section of shell posterior to umbonal-ventral groove ornamented with low commarginal folds superimposed on fine, commarginal growth lines. Internal ridge from umbo to posteroventral shell margin prominent dorsally, diminishing ventrally, disappearing completely from later growth stages. Faint muscle scars visible perpendicular to anterior margin; posterior adductor muscle scar faint, formed by multiple oblique attachment scars.
Remarks.—Xylophagella littlei Hryniewicz sp. nov. differs from X. dubius (Stanton, 1901) by its smaller prora and convex ventral margin (as opposed to straight to concave in X. dubius). Xylophagella littlei also has a more posteriorly elongate shell compared to the roughly circular shell of X. dubius. However, we have seen only drawings of the latter species (Stanton 1901: pl. 6: 5–8), and a more detailed comparison of the two species is not possible. Xylophagella littlei has a convex ventral margin with the deepest point around the umbonal-ventral groove, as opposed to a straight ventral margin in X. zonata Casey, 1961 (Woods 1909: pl. 38: 17). Xylophagella elegantula (Meek and Hayden, 1858) has not been figured to our knowledge; therefore, our comparison has to rely on the description (Meek and Hayden 1858: 141), which indicates that the anterior gape of X. elegantula covers ~33% of the shell length. The gape is larger in X. elegantula than in X. littlei, where it occupies 20–25% of the shell length. The description of X. elegantula also indicates that the umbo placed nearly in the middle of the shell length, whereas it is located anteriorly in X. littlei. Xylophagella littlei is more elongate posteriorly as opposed to X. truncata Kelly, 1988, and has a rounded posterior margin as opposed to truncated in X. truncata. The inner umbonal ridge of X. littlei diminishes towards the ventral margin, whereas it is continuous in X. truncata.
Stratigraphic and geographic range.—Upper Paleocene sunken driftwood associated with cold seep carbonates from the Basilika Formation, Fossildalen, Spitsbergen, Svalbard.
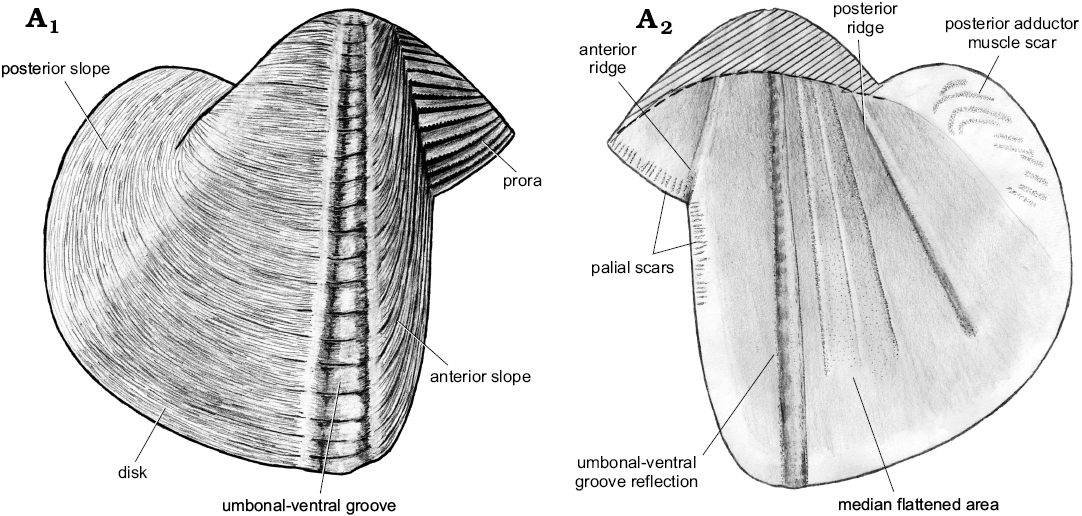
Fig. 16. Schematic illustration of a xylophagain bivalve Xylophagella littlei Hryniewicz sp. nov., from the upper Paleocene, Basilika Formation, Spitsbergen, Svalbard, showing the main morphological features discussed. Outer (A1) and inner (A2) views of right valve. Area above dashed line represents the morphological features we were unable to illustrate due to poor preservation.
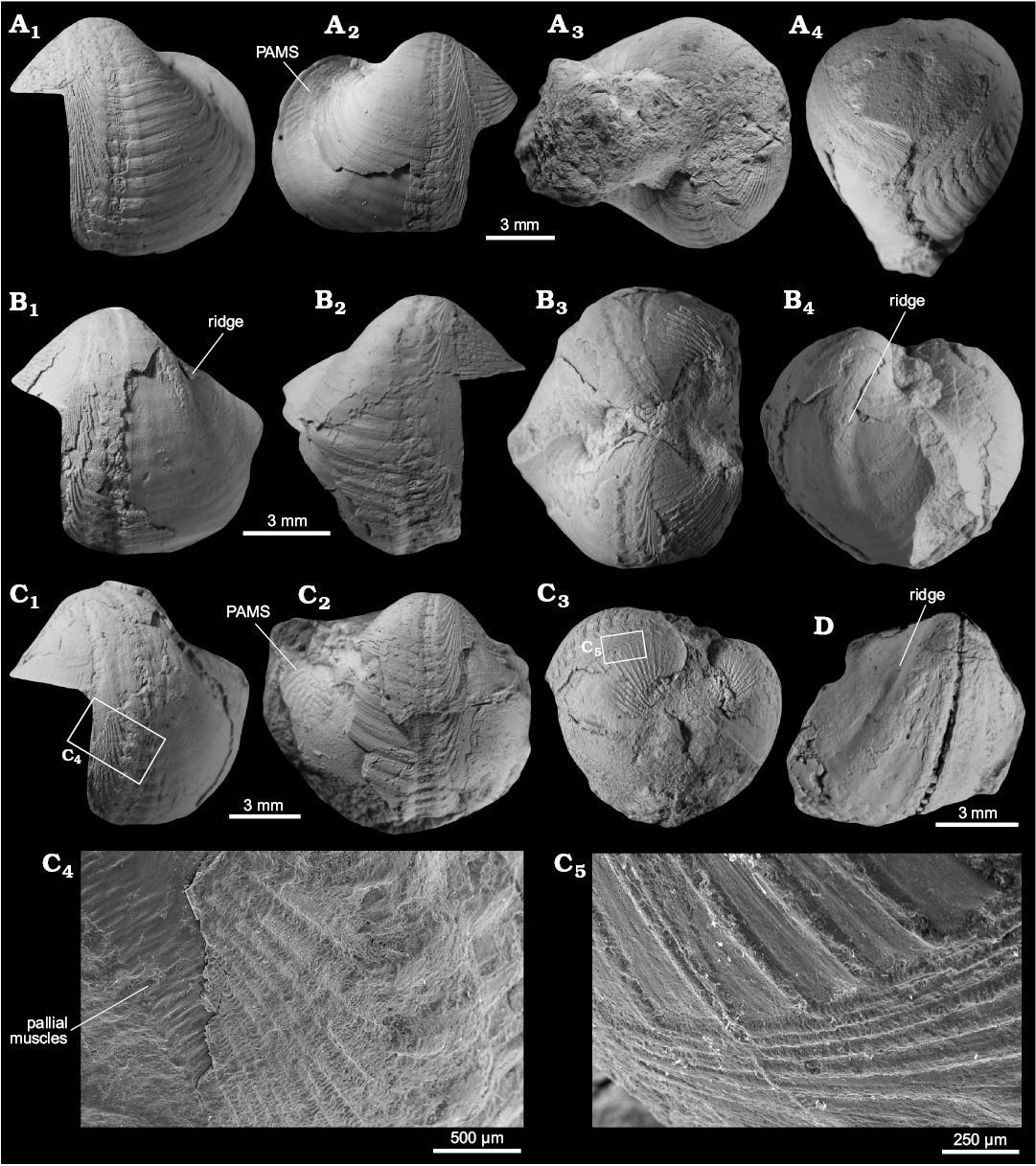
Fig. 17. Xylophagain bivalve Xylophagella littlei Hryniewicz sp. nov. from the upper Paleocene, Basilika Formation, Fossildalen, Spitsbergen, Svalbard. A. Holotype, ZPAL V.48/5, partial shell, left valve (A1), right valve with fragment of posterior adductor muscle scar (PAMS) (A2), dorsal view of both valves (A3), oblique ventral view showing partially overlapping shells (A4). B. Paratype, ZPAL V.48/6, partial shell, left valve, with trace of a ridge on the inner mold (B1), right valve (B2), dorsal view of both valves (B3), oblique posterior view showing internal surface of left valve internal mold with trace of a ridge on inner surface of shell (B4). C. Paratype, ZPAL V.48/7, partial shell, left valve (C1), right valve with fragment of posterior adductor muscle scar (PAMS) (C2), oblique anterior view (C3), enlarged fragment of prora with imprints of pallial muscles perpendicular to the shell edge, rasp composed of raised ridges covered with perpendicular lamellae (C4), enlarged fragment of prora, showing ornament of raised ridges covered with perpendicular lamellae (C5). D. Paratype, ZPAL V.48/8, internal mold, right valve showing trace of a ridge on the inner shell surface.
Order uncertain
Superfamily Hiatelloidea Gray, 1824
Family Hiatellidae Gray, 1824
Genus Cyrtodaria Reuss, 1801
Type species: Mya siliqua Spengler, 1793; Recent, western Northern Atlantic.
Cyrtodaria aff. rutupiensis (Morris, 1852)
Fig. 18.
Material.—One butterflied specimen (NRM-PZ Mo 183943a–b) with valves opened but still attached, from the upper Paleocene, Zachariassendalen, Spitsbergen, Svalbard.
Measurements.—NRM-PZ Mo 183943a–b: L, 43 mm; H, 16.9 mm; W, 5.4 mm.
Description.—Shell elongate, weakly inflated, edentulous, moderately thick, covered with commarginal growth lines. Umbones positioned roughly mid-hinge line, not prominent, dorsal margin composed of two straight, weakly inclined, anterior and posterior sections. Anterior and posterior shell margins rounded, gaping; anterior somewhat more rounded than posterior. Ventral shell margin straight with very weak sinus close to mid-line. Anterior adductor muscle scar teardrop-shaped, elongated along anterodorsal margin. Posterior adductor muscle scar shorter but precise shape unknown.
Remarks.—Elongate bivalves with a medially positioned umbo and a shape similar to the specimen of Cyrtodaria aff. rutupiensis (Morris, 1852) figured herein were previously described and figured from the Paleocene of Fossildalen as belonging to at least three species of Solecurtus Blainville, 1824, by Hägg (1925: pl. 6: 25–27). As those specimens have no visible transverse ornament characteristic of species of Solecurtus (Hägg 1925: pl. 6: 27), they likely do not belong to Solecurtus and could be assigned to Cyrtodaria. Livšic (1974) mentioned Cyrtodaria sp. occurring in the “Storvola Formation”, an obsolete name for the Eocene Aspelintoppen Formation (Dallmann 1999) overlying the Basilika Formation. Since Livšic (1974) did not provide any figures, we are unable to relate our material to his report. Cyrtodaria rutupiensis (Morris, 1852), a common species occurring in Paleocene deposits of western Spitsbergen (Strauch 1972), the Thanet Formation in Kent, UK (Ward 1978), the Prince Creek Formation in northern Alaska (Marincovich 1993), and the Mount Moore Formation of Ellesmere Island in Arctic Canada (Marincovich and Zinsmeister 1991), is very similar to the current species. However, we are unable to perform a more thorough comparison because only one specimen was available. Solecurtus (Macha) sp. from the Paleocene of Fossildalen has been included (Hägg 1925: pl. 6: 26) into the synonymy list of C. rutupiensis by Marincovich (1993), but we are unable to relate Solecurtus (Macha) sp. to our material. Cyrtodaria katieae Marincovich, 1993, from the Paleocene Prince Creek Formation in northern Alaska, USA, is elongated similarly to the Paleocene species from Svalbard; however, the umbo of C. katieae is located nearer to the anterior than in the present species. Cyrtodaria? minuta Speden, 1970, from the Maastrichtian Fox Hills Formation in South Dakota, USA, has a small, thin shell (L 6.5–12 mm) that broadens substantially towards the posterior (Speden 1970: 139, pl. 35: 4–11); in our view, it belongs to Saxicavella Fischer, 1878, rather than to Cyrtodaria.

Fig. 18. Hiatellid bivalve Cyrtodaria aff. rutupiensis (Morris, 1852) from the upper Paleocene, Basilika Formation, Zachariassendalen, Spitsbergen, Svalbard. NRM-PZ Mo 183943a–b, partial shell of a butterflied specimen with left (LV) and partial right valve (RV) preserved, showing external ornament of commarginal growth lines, and ridges on the inner shell surfaces supporting the posterior of anterior adductor muscle scar.
Phylum Arthropoda Siebold, 1848
Subphylum Crustacea Brünnich, 1772
Class Hexanauplia Oakley, Wolfe, Lindgren, and Zaharoff, 2013
Infraclass Cirripedia Burmeister, 1834
Superorder Thoracica Darwin, 1854
Order Scalpelliformes Buckeridge and Newman, 2006
Family Pollicipedidae Leach, 1817
Genus ?Capitulum Gray, 1825
Type species: Lepas mitella Linnaeus, 1758: Recent, tropical Western Pacific.
?Capitulum lailae (Gripp, 1927)
Fig. 19.
Material.—One specimen (ZPAL V.48/22), rostral plates from the upper Paleocene of Fossildalen, Spitsbergen, Svalbard.
Measurements.—L, 6.4 mm, W, 4.2 mm.
Remarks.—The specimen comprises a single plate (a rostrum) that is likely attributable to ?Capitulum lailae (Gripp, 1927), described from the same formation previously by Gripp (1927) and redescribed and refigured by Withers (1953: 112–114, pl. 4.1–4.11), who compared this taxon to Mitella mitella (Linnaeus, 1758) and placed the species in Mitella Oken, 1815, with reservations. However, M. mitella is currently assigned to Capitulum (e.g., Chan et al. 2009), hence the provisional placement of ?C. lailae in that genus. Barnacles have rarely been reported from ancient cold seep-associated deposits so its presence in the Basilika Formation merits a note.
Stratigraphic and geographic range.—Upper Paleocene cold seep carbonates from the Basilika Formation, Fossildalen, Spitsbergen, Svalbard.

Fig. 19. Thoracic barnacle ?Capitulum lailae Gripp, 1927, from the upper Paleocene, Basilika Formation, Fossildalen, Spitsbergen, Svalbard. ZPAL V.48/22, rostral plate.
Class Malacostraca Latreille, 1802
Order Decapoda Latreille, 1802
Infraorder Anomura Milne-Edwards, 1832
Superfamily Galatheoidea Samouelle, 1819
Family Munididae Ahyong, Baba, Macpherson, and Poore, 2010
Included genera (* fossil and modern species; ** exclusively fossil species; no symbol, all modern species): Agononida* Baba and de Saint Laurent, 1996; Anomoeomunida Baba, 1993; Anoplonida Baba and de Saint Laurent, 1996; Austromunida** Schweitzer and Feldmann, 2000; Babamunida Cabezas, Macpherson, and Machordom, 2008; Bathymunida Balss, 1914; Cervimunida Benedict, 1902; Cretagalathea** Garassino, de Angeli, and Passini, 2008; Crosnierita Macpherson, 1998; Eosadayoshia** Beschin, Busulini, and Tessier in Beschin et al., 2016; Enriquea Baba, 2005; Hendersonida Cabezas and Macpherson, 2014; Heteronida Baba and de Saint Laurent, 1996; Juracrista** Robins, Feldmann, and Schweitzer, 2012; Munida* Leach, 1820; Neonida Baba and de Saint Laurent, 1996; Onconida Baba and de Saint Laurent, 1996; Paramunida Baba, 1988; Plesionida Baba and de Saint Laurent, 1996; Pleuroncodes Stimpson, 1860; Protomunida** Beurlen, 1930; Raymunida Macpherson and Machordom, 2000; Sadayoshia* Baba, 1969; Setanida Macpherson, 2006; Tasmanida Ahyong, 2007; Torbenella Baba, 2008; Valamunida** gen. nov.
Remarks.—Munida is very broadly diagnosed (Baba 2005: 88), contains well over 200 species (e.g., De Grave et al. 2009) and is likely to consist of multiple genera (e.g., Cabezas et al. 2008: fig. 2). For comparisons among genera below, we have used its type species, Munida rugosa (Fabricius, 1775). Munidids are known from some modern cold seeps (Olu et al. 1996; Sellanes et al. 2008; Coykendall et al. 2017).
Genus Protomunida Beurlen, 1930
Type species: Galathea munidoides Segerberg, 1900; Paleocene (middle Danian), Faxe Formation, Denmark.
Species included: Protomunida munidoides (Segerberg, 1900); ?P. pentaspina Beschin, Busulini, and Tessier in Beschin et al., 2016; P. primaeva (Segerberg, 1900); P. spitzbergica (Gripp, 1927).
Original diagnosis (translated from Beurlen 1930: 373).—Protomunida is characterized by a smooth, triangular rostrum without teeth, and with two spines near its base. Shape of the carapace and ornamentation as in Galathea. Protomunida thus occupies a pronounced intermediate position between Galathea and Munida.
Emended diagnosis.—Carapace excluding rostrum longer than wide, subrectangular; with three-pronged rostrum, of which one major, moderately narrow, flattened spine on the axis and two smaller accessory spines; forwardly directed spine at outer orbital angle; row of spines or tubercles on epigastric regions; epibranchial regions with tubercles dorsally and spines laterally; other ornamentation oriented transversely; fairly smooth, non-depressed area posterior to cardiac region.
Remarks.—Beurlen (1930: 373) erected Protomunida with Protomunida munidoides (Segerberg, 1900) as the type species, but he also put Munida primaeva Segerberg, 1900, and Galathea spitzbergica Gripp, 1927, in this genus, which was generally not followed by subsequent workers (e.g., Collins and Jakobsen 1994; Jakobsen and Collins 1997; Schweitzer and Feldmann 2000; De Angeli and Garassino 2002; Schweitzer et al. 2010; Robins 2013; Beschin et al. 2016; Klompmaker et al. 2016; Robin et al. 2017; but see Müller and Collins 1991). We here place Munida primaeva (for confirmed images of this rare species see Segerberg 1900: pl. 7.6; Jakobsen and Collins 1997: pl. 2.8; Klompmaker and Boxshall 2015: fig. 1D; Damholt et al. 2010: 22) and Galathea spitzbergica (see below) in Protomunida again after 88 years based on the close similarity to the type species including the three-pronged rostrum, the spine on the outer orbital angle, the oblique ridge of tubercles on the epigastric regions, the overall ornamentation, the fairly smooth, non-depressed area posterior to the cardiac region, the tubercles on the epibranchial regions, and the overall groove pattern. We consider placement of Protomunida pentaspina Beschin, Busulini, and Tessier in Beschin et al., 2016, in Protomunida (see Beschin et al. 2016) provisional because the rostrum is poorly preserved but is wider at the posterior part than in all other congenerics. Other characters match those of Protomunida, but also of many species of Eosadayoshia Beschin, Busulini, and Tessier in Beschin et al., 2016, and Sadayoshia Baba, 1969. Whether ?P. pentaspina has more spines on the anterior carapace region than P. munidoides (see Beschin et al. 2016) cannot be fully confirmed. However, the posterior side of the lateral margin of ?P. pentaspina has more spines than that of P. munidoides. Differences include the cardiac region, which is rectangular in P. spitzbergica and ?P. pentaspina but more triangular in P. munidoides and P. primaeva, the epibranchial regions showing also ridges in addition to tubercles in P. munidoides and ?P. pentaspina, and the wider central rostral spine of P. spitzbergica with tubercles not seen in P. munidoides and P. primaeva. The cervical groove of P. primaeva is more V-shaped than the U-shaped groove in the other species. Given that Protomunida has barely been used since its erection in 1930 and many new genera now classified within the Munididae have been erected without proper comparison to Protomunida, a renewed comparison is warranted. Agononida Baba and de Saint Laurent, 1996, has a very narrow central, needle-like rostral spine (rather than a flattened central projection) and spines around the meta-/urogastric regions not seen in Protomunida. Anoplonida Baba and de Saint Laurent, 1996, and Anomoeomunida Baba, 1993, have smaller accessory rostral spines and epibranchial regions with transverse ridges rather than tubercles/spines. Babamunida Cabezas, Macpherson, and Machordom, 2008, Cervimunida Benedict, 1902, Crosnierita Macpherson, 1998, Enriquea Baba, 2005, and Raymunida Macpherson and Machordom, 2000, have a narrow central, needle-like rostral spine. Austromunida Schweitzer and Feldmann, 2000, has a much narrower central, needle-like rostral spine and no tubercles on the epibranchial regions, the latter of which also applies to Cretagalathea Garassino, de Angeli, and Passini, 2008. The genera Bathymunida Balss, 1914, Heteronida Baba and de Saint Laurent, 1996, Neonida Baba and de Saint Laurent, 1996, Onconida Baba and de Saint Laurent, 1996, Plesionida Baba and de Saint Laurent, 1996, and Tasmanida Ahyong, 2007, all bear a strong forwardly directed ridge ending in a spine occupying the mesogastric region. Hendersonida Cabezas and Macpherson, 2014, has a granular carapace surface rather than transverse ridges. Juracrista Robins, Feldmann, and Schweitzer, 2012, has a much wider rostrum overall, and especially the central projection is wider. Munida Leach, 1820 (type species) and Torbenella Baba, 2008, have a narrow central, needle-like rostral spine and ridges on the epibranchial regions. Paramunida Baba, 1988, differs in that transverse ridges are indistinct and the rostrum is relatively short. Unlike Protomunida, Pleuroncodes Stimpson, 1860, shows the epimeral sutures dorsally and has a narrow central, needle-like rostral spine. Raymunida has a straighter row of epigastric spines. Sadayoshia and Eosadayoshia bear five rostral spines rather than three. Setanida Macpherson, 2006, appears to have a cardiac region reaching the posterior margin and lacks a row of epigastric spines/tubercles. Differences from Valamunida Klompmaker and Robins gen. nov. are described below. Thus, we conclude that Protomunida is a distinct genus within the Munididae.
Protomunida spitzbergica (Gripp, 1927)
Fig. 20.
1927 Galathea spitzbergica n. sp.; Gripp 1927: 33, pl. 7.16,7.17.
1970 Galathea spitzbergica Gripp, 1927; Vonderbank 1970: 78, fig. 25c, pl. 6.1.
2013 Galathea spitzbergica Gripp, 1927; Robins 2013: 38, fig. 8.5.
2016 Munididae; Hryniewicz et al. 2016: fig. 12k.
Material.—Neotype, GPIBo 85, the sole specimen, available thus far, carapace, from the upper Paleocene, Fossildalen and locality 500 m west from Trigonometric point 25, Hollendarbukta, Spitsbergen, Svalbard.
Measurements.—Lmax (without rostrum) 5.1 mm; Wmax 4.6 mm.
Emended diagnosis.—Carapace ~10% longer than wide, widest at meso-metabranchial regions. Relatively wide central rostral spine at base with tubercles. Spines in oblique rows on epigastric regions, near orbital angle, and on anterior part lateral margin. Cardiac region rectangular, poorly delimited laterally, well delimited anteriorly and posteriorly. Epibranchial regions with tubercles. Area posterior to cardiac region without ornamentation, with two tiny elongated tubercles on either side of axis.
Description.—Carapace ~10% longer than wide (excluding rostral length), weakly convex longitudinally, moderately convex transversely, widest at meso-metabranchial regions. Rostrum triangular, flattened dorsally, with weak axial keel, tip and lateralmost parts not preserved, probably with spines adjacent to central rostral spine (Gripp 1927: 33, pl. 7: 17). Orbits not exposed. Frontal margin incompletely preserved, with base of spine near outer orbital angle. Lateral margins moderately convex, rounded. Posterior margin moderately concave, with distinct rim. Epigastric regions with oblique row of bases of spines, followed posteriorly by parallel row of granules. Hepatic region small, depressed, with some tubercles. Proto- and mesogastric regions weakly differentiated, with transverse, interrupted ridges. Mesogastric region triangular with convex base and distinct tip. Epibranchial regions triangular, with tubercles dorsally and three bases of spines laterally. Uro-/metagastric region only delimited anteriorly and posteriorly, containing some transversely elongate tubercles. Confluent meso-metabranchial regions containing transverse ridges best connected laterally; lateral sides without spines except perhaps one near epibranchial regions. Cardiac region rectangular, poorly delimited laterally, well-delimited anteriorly and posteriorly by somewhat sinuous grooves. Area posterior to cardiac region without ornamentation, with two tiny elongate tubercles on either side of axis. Cervical groove distinct, widely U-shaped, curving more anterolaterally in lateralmost part. Lateral groove branching off base cervical groove sinuous. Specimen appears to preserve most of cuticle, partially absent from right epibranchial region. No muscle scars visible. Abdomen, venter, and appendages not preserved.
Remarks.—Since the holotype was destroyed during WWII, Vonderbank (1970) correctly designated a neotype for this species. He gave a five-page description of this species in his monograph. However, only the neotype is conspecific with the illustration of the lost holotype by Gripp (1927). All other carapaces figured by Vonderbank (1970) and the specimen illustrated by Hägg (1925: pl. 6.28, 6.28a) are part of a new species described below.
Placement in the Munididae is with little doubt. Munidids typically have spines directly adjacent to the central rostral spine (e.g., Macpherson 1994; Ahyong et al. 2010; Robins et al. 2012), but this part is not preserved in the sole specimen. However, Gripp (1927: 33, pl. 7.17) did illustrate such adjacent spines and mentioned them in the text. The axial part of the rostrum in munidids is usually a narrow spine rather than a somewhat wider, more flattened surface, but there are some exceptions in munidids including Protomunida primeava (personal observation AAK), P. munidoides (see Jakobsen and Collins 1997), and Juracrista spp. (see Robins et al. 2012). Another character suggesting a munidid affinity are the spines interpreted to be present on the epigastric regions, lateral sides of the epibranchial regions, and at the outer orbital angle. This combination of spines is also seen in extant and other fossil munidids such as Munida, Agononida, Juracrista, Sadayoshia, and P. munidoides (e.g., Ahyong et al. 2010; Robins et al. 2012; Robins 2013; personal observation AAK for P. munidoides), although some galatheids (Lessinigalathea regalis De Angeli and Garassino, 2002, and Acanthogalathea spp. (De Angeli and Garassino, 2002) also exhibit these features.
Whether the ornamentation would look different without the cuticle (see examples in Klompmaker et al. 2015, for various examples and references therein, as well as Robins et al. 2016: 68–70), is difficult to determine as only a minor portion of the cuticle appears gone, exposing the internal mold on the right epibranchial region. In general, however, it appears that differences are limited for galatheoids based on evidence thus far (De Angeli and Garassino 2002: pl. 4.2; Schweitzer and Feldmann 2008: fig. 2; Klompmaker et al. 2012: figs. 7, 10; Robins et al. 2013: figs. 10, 11; Nyborg and Garassino 2015: figs. 1, 2; Robins et al. 2016: figs. 3, 7, 8, 11, 12, 14).
The neotype originates from locality 500 m west from Trigonometric point 25, Hollendarbukta, and not from Fossidalen, interpreted to be the seep and wood fall community of Gripp (1927) by Hryniewicz et al. (2016). The same species was present at Fossildalen as well given the illustration of the lost holotype by Gripp (1927).
Stratigraphic and geographic range.—Upper Paleocene cold seep carbonates from the Basilika Formation, Fossildalen, and locality 500 m west from Trigonometric point 25, Hollendarbukta, Spitsbergen, Svalbard.
Fig. 20. Munidid crustacean Protomunida spitzbergica (Gripp, 1927) from the upper Paleocene, Basilika Formation, locality 500 m west from Trigonometric point 25, Hollendarbukta, Spitsbergen, Svalbard. Neotype, GPIBo 85, dorsal view of carapace (A1), detailed view of incomplete rostrum (A2), right lateral (A3), frontal (A4), and left lateral (A5) views.
Genus Valamunida Klompmaker and Robins nov.
ZooBank LSID: urn:lsid:zoobank.org:act:2BA78BB3-34F8-4686-8C5C-F70F0416A041
Type species: Valamunida haeggi Klompmaker and Robins sp. nov.; by monotypy, see below.
Etymology: From the Norse völva, an ancient seeress who carried a wand, similar to the rostrum on this galatheoid and -munida referring to the family name.
Diagnosis.—As for the type species by monotypy.
Remarks.—The new genus is placed within the Munididae based on a combination of tubercles and spines on the anterior carapace and its lateral margins, the trifid rostrum, and the (bases of) spines at the outer orbital angle. These characters are typical for most Munididae, but this combination of characters is not typical for other galatheoid families (Ahyong et al. 2010).
Valamunida Klompmaker and Robins gen. nov. is established on the basis of a combination of the tubercular ornamentation across the epibranchial and hepatic regions, more transversely elongate transverse ridges on the posterior carapace, an epigastric region with varied ornamentation, lack of a strong, forwardly-oriented spine on the mesogastric region, and a strongly depressed intestinal region posteriorly. Valamunida Klompmaker and Robins gen. nov. differs from all 25 known munidid genera. Tubercular ornamentation on the epibranchial and hepatic regions is uncommon in munidids and can only be found in the fossil genera Juracrista Robins, Feldmann, and Schweitzer, 2012 and Protomunida Beurlen, 1930, whereas it is also present in modern Babamunida Cabezas, Macpherson, and Machordom, 2008, Bathymunida Balss, 1914, Hendersonida Cabezas and Macpherson, 2014, Heteronida Baba and de Saint Laurent, 1996, Paramunida Baba, 1988, Plesionida Baba and de Saint Laurent, 1996, and Tasmanida Ahyong, 2007. All other genera do not exhibit these previously elaborated features and most of them also lack a strongly depressed posterior part of the intestinal region. From the leftover genera, Babamunida, Juracrista, and Protomunida exhibit only rows of tubercles/spines on the epigastric regions, lacking a much stronger spine. They also lack a strongly depressed posterior part of the intestinal region. Unlike the new genus, Bathymunida, Heteronida, and Tasmanida bear a strong mesogastric spine, as does the type species of Plesionida. Plesionida, Paramunida, and Hendersonida have a much more granular, spinose to tubercular posterior carapace than Valamunida Klompmaker and Robins gen. nov. with its transverse ridges. Paramunida and Hendersonida also lack the depression in the intestinal region.
Valamunida haeggi Klompmaker and Robins sp. nov.
Figs. 21, 22.
1925 Galathea sp.; Hägg 1925: 48, pl. 6.28a.
1970 Galathea spitzbergica Gripp, 1927; Vonderbank 1970: 78, fig. 25 [not 25c]; pl. 6.2–6.7.
2013 Galathea spitzbergica Gripp, 1927; Robins 2013: 38, fig. 8.4.
ZooBank LSID: urn:lsid:zoobank.org:act:798CF3CB-27FE-406E-BCFB-F26D76BBE2C9
Etymology: Named after Richard Hägg (1877–1957), who first figured specimens of this species.
Type material: Holotype: NRM-PZ Ar68001A, partial carapace (Fig. 21A). Paratypes: NRM-PZ Ar68001B (Fig. 22C), NRM-PZ Ar68010 (Fig. 22B), and NRM-PZ Ar68011 (Fig. 22A). All represent (partial) carapaces with cuticular layers variably preserved, all from the type locality and horizon.
Type locality: Zachariassendalen, Spitsbergen, Svalbard.
Type horizon: Cold seep carbonates from the Basilika Formation, upper Paleocene.
Material.—Additional material from the upper Paleocene, Zachariassendalen, Spitsbergen, Svalbard: NRM-PZ Ar 68001C, external mold partial branchial region; NRM-PZ Ar 68006, external mold left side carapace; NRM-PZ Ar68007, part of meso-metabranchial and partial cardiac region; NRM-PZ Ar68016, left mid-part of carapace. Additional material from the upper Paleocene, locality 500 m west of Trigonometric point 25, Hollendarbukta, Spitsbergen, Svalbard: GPIBo 86, left part carapace (Vonderbank 1970: pl. 6.2); GPIBo 87, right part carapace (Vonderbank 1970: pl. 6.3); GPIBo 88, central part gastric region (Vonderbank 1970: pl. 6.4); GPIBo 89, central part gastric region (Vonderbank 1970: pl. 6.5); GPIBo 90, left meso-metabranchial region (Vonderbank 1970: pl. 6.6); GPIBo 91, anterior part gastric region and partial rostrum (Vonderbank 1970: pl. 6.7).
Measurements.—Holotype, NRM-PZ Ar68001A: Lmax (without rostrum), 23.3 mm; Wmax, 18.4 mm. NRM-PZ Ar 68001B: L, not available; W, 18.0 mm. NRM-PZ Ar68010: L, not available; W, 8.8 mm. NRM-PZ Ar68011: L, 20.0 mm, W, 15.5 mm. GPIBo 88: L, not available; W (at gastric region), 10.2 mm. Dimensions of paratypes: GPIBo 86, NRM-PZ Ar68002, and additional material cannot be determined due to incomplete preservation.
Diagnosis.—Carapace longer than wide, rostrum trifid, spines on lateral margins of epibranchial anterior of meta-mesobranchial regions, grooves deeply incised. Mesogastric, protogastric, and epigastric divisions pronounced. Epigastric regions with strong spine surrounded by tubercles, anterior ones in row. Hepatic and epibranchial regions slightly inflated, ornamented with tubercles. Cardiac region elevated above remainder of carapace. Intestinal area smooth to less ornamented, deeply depressed near posterior margin.
Description.—Carapace subrectangular (L/W [without rostrum] 1.2–1.3), widens slightly posteriorly. Rostrum trifid, with narrow triangular central spine subsquare in cross-section, and two accessory spines; length and termination unknown. Orbits not exposed. Outer orbital margins bear base of large spine; lateral margins spined at epibranchial (3 bases of spines) and anterior of meta-mesobranchial regions (at least 1 base of spine). Grooves deeply incised; cervical and postcervical groove wide. Gastric region elevated slightly above frontal margin, well-defined, with pronounced mesogastric, protogastric, and epigastric divisions. Epigastric regions with two strong spines surrounded by randomly oriented tubercles posteriorly and laterally, small tubercles form oblique rows anterior to spines (Fig. 22D2). Protogastric areas with transverse ridges, may bear few spines/tubercles. Hepatic and epibranchial regions slightly inflated, ornamented with tubercles. Mesogastric region triangular with transverse ridges, mesogastric process may bear tubercles. Meta-/urogastric region wider than long, not delimited laterally, with interrupted transverse ridges. Cardiac region elevated above remainder of carapace, wider than long, with transverse ridges; anterior margin with straight strong groove, rest delimited by concave forward groove that may be straight posteriorly. Meso-metabranchial region confluent, with transverse ridges that can be interrupted in large specimens. Intestinal area smooth to weakly ornamented, deeply depressed near posterior margin. Posterior margin rimmed, concave, with ridges anterior to it. Cuticle variably present. Articulated appendages, venter, and abdomen not known.
Remarks.—It is possible that Gripp (1927) also studied some specimens conspecific with this species, as he studied four specimens, one of which is Protomunida spitzbergica (see above). The great majority of galatheoid carapaces from the upper Paleocene of Spitsbergen we have seen can be ascribed to the new species.
The marked depression at the anterior part of the intestinal region cannot be explained by post-mortem processes because we have observed this depression in four specimens across a size range. Ontogenetic variation is evident: (i) the largest specimen (holotype) shows more interrupted ridges at the meso-metabranchial regions than smaller specimens, (ii) smaller specimens appear more vaulted transversely, and (iii) the cardiac region appears more swollen in the largest specimen (= holotype). Intraspecific variation is also observed: some of the specimens bear tubercles laterally to the base of the mesogastric process and the there is some variation in the precise course and strength of transverse ridges, not attributable to ontogeny.
As mentioned for the previous species, the presence of cuticle does not seem to affect the presence and strength of ornamentation much for galatheoids. The cuticle is variably present on multiple specimens of the new species and provides an opportunity to evaluate its effect on appearance for this taxon. Upon examination of the specimens, the presence of cuticle indeed does not seem to affect the strength of the ornamentation for each of the figured specimens. The cuticle in the groove branching off the cervical groove of NRM-PZ Ar68010 appears, however, somewhat thicker in the deepest part of the groove (Fig. 22B4). The groove is still present with cuticle preserved, but may be somewhat shallower with cuticle, as previously shown for a raninoid crab (Schweitzer et al. 2009: fig. 8).
Stratigraphic and geographic range.—Upper Paleocene cold seep carbonates from the Basilika Formation, Zachariassendalen and locality 500 m west of Trigonometric point 25, Hollendarbukta, and Fossildalen, Spitsbergen, Svalbard.
Fig. 21. Munidid crustacean Valamunida haeggi Klompmaker and Robins gen. et sp. nov. from the upper Paleocene, Basilika Formation, Zachariassendalen (A) and locality 500 m west from Trigonometric point 25, Hollendarbukta (B, C), Spitsbergen, Svalbard. A. Holotype, NRM-PZ Ar68001a, carapace in dorsal (A1) and right lateral (A2) views, frontal view (note base of outer orbital spine on left) (A3), oblique view on anterior gastric region (arrow indicates upward spine on epigastric region) (A4), external mold (A5), rostral view (arrows indicate incomplete accessory spines of rostrum) (A6). B. GPIBo 88, carapace in dorsal view (note bases of spines on epigastric regions) (B1), right lateral view (note spines on lateral margin and at outer orbital angle) (B2). C. GPIBo 86, carapace in dorsal view. B, C coated with ammonium chloride.
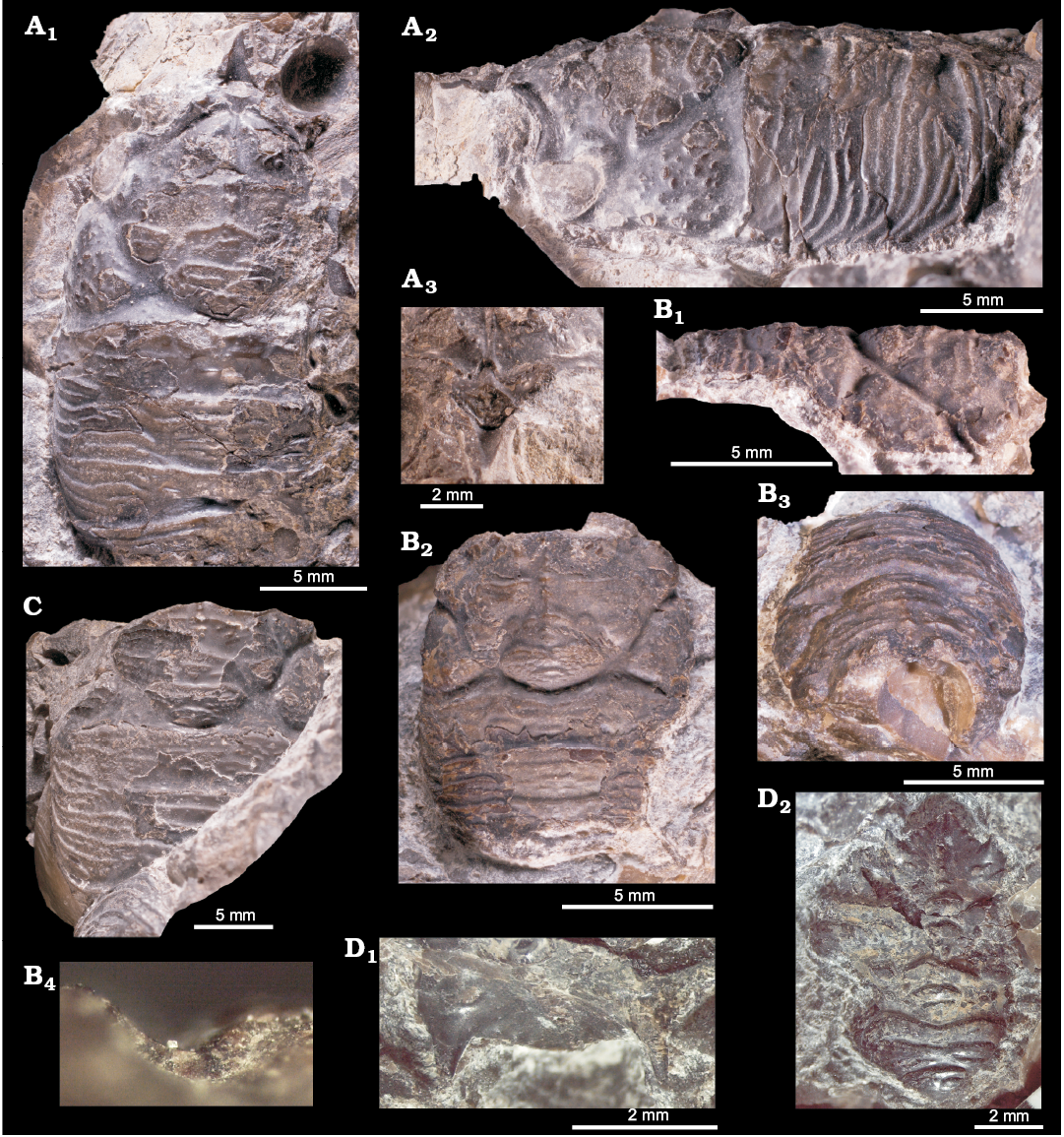
Fig. 22. Munidid crustacean Valamunida haeggi Klompmaker and Robins gen. et sp. nov. from the Paleocene Basilika Formation, Zachariassendalen, Spitsbergen, Svalbard. A. Paratype, NRM-PZ Ar68011, carapace in dorsal view (A1), left lateral view (note bases of lateral spines) (A2), frontal view with cross-section of base central rostral spine (A3). B. Paratype, NRM-PZ Ar68010, carapace in right lateral (B1) and dorsal (B2) views, oblique frontal view (note base of spine on right orbital angle) (B3), view of cross-sectioned cuticle in groove branching off cervical groove on left side (image width ~0.7 mm) (B4). C. Paratype, NRM-PZ Ar68001b, carapace in dorsal view. D. NRM-PZ Ar68002, two epigastric spines in oblique frontal view (D1), dorsal view of gastric region (D2).
Munididae indet.
Fig. 23.
Material.—Various fragments, including ventres, meri, and propodi, GPIBo 92, 93, 101–105, NRM-PZ Ar68002 (2 fragments), NRM-PZ Ar68003 (2 fragments), NRM-PZ Ar68005 (4 fragments), NRM-PZ Ar68006 (6 fragments), NRM-PZ Ar68008+680014 (5 fragments), NRM-PZ Ar 68009, NRM-PZ Ar68010, NRM-PZ Ar68011, NRM-PZ Ar68012, NRM-PZ Ar68013, NRM-PZ Ar68015. From the upper Paleocene of Zachariassendalen (NRM-PZ specimens) and locality 500 m west from Trigonometric point 25 (GPIBo specimens), Hollendarbukta, Spitsbergen, Svalbard.
Remarks.—Gripp (1927) and Vonderbank (1970) ascribed a variety of isolated appendages and venters to what they considered to be P. spitzbergica, but because there are two galatheoid species in the region, those cannot be ascribed to any taxon with certainty. None of those specimens were attached to carapaces. It is likely, however, that most, if not all, can be attributed to Valamunida haeggi Klompmaker and Robins sp. nov. because 15/16 carapaces studied are ascribed to this species and appendages are found in the same rock as some of the carapaces for the ones found in Zachariassendalen (NRM-PZ Ar68002, NRM-PZ Ar68003, NRM-PZ Ar68006, and NRM-PZ Ar68010/Ar68013).
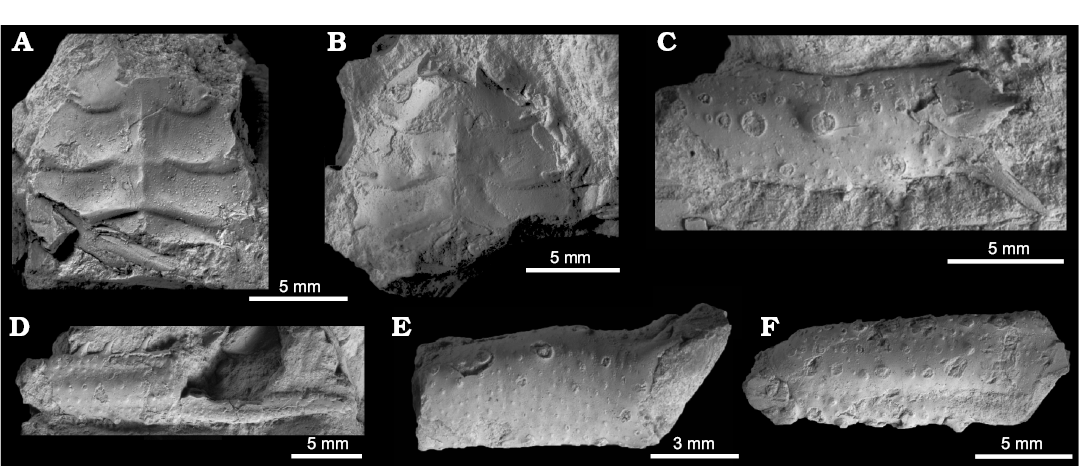
Fig. 23. Non-carapace remains of the Munididae from the upper Paleocene, Basilika Formation, locality 500 m west from Trigonometric point 25, Hollendarbukta, Spitsbergen, Svalbard, likely attributable to Valamunida haeggi Klompmaker and Robins gen. et sp. nov. A. GPIBo 93, venter. B. GPIBo 92, venter. C. GPIBo 102, merus. D. GPIBo 105, propodus of cheliped. E. GPIBo 101, merus. F. GPIBo 104, propodus. Specimens coated with ammonium chloride, re-figured after Vonderbank (1970: pls. 6, 7).
Superclass Actinopterygii Cope, 1887
Infraclass Teleostei Müller, 1845
Teleostei gen. et sp. indet.
Fig. 24.
Material.—NRM-PZ P16479a–e, one complete and one fragmented tooth and three partly fragmented vertebra from the upper Paleocene of Zachariassendalen, Spitsbergen, Svalbard.
Description.—Complete tooth shows translucent cap at apex. Both teeth small, ~1 mm and 2 mm; strongly posteriorly recurved and laterally compressed. Outer surfaces bear very fine longitudinal striations. Both teeth with expanded area of spongy bone at base that marks jaw attachment. Vertebra small, similarly-sized to teeth.
Remarks.—The teleost material reported here represents the second finding of actinopterygian fish from the Cenozoic of Spitsbergen. The first actinopterygian was described as a fossil bowfin, Pseudamia heintzi, by Lehman (1951). According to Thomsen (2013), the specimen was discovered at Cap Dresselhuys, implying that it derives from the late Paleocene (Thanetian) Grumantbyen Formation (Dallmann 2015), which overlies the Basilika Formation. Both bowfin (Amia sp.) and scombroid teleost teeth assigned to Eutrichiurides have been described from the Eocene Eureka Sound Formation on Banks Island in the Canadian High Arctic by Gottfried and Eberle (2018). In addition to its presence in the High Arctic, scombroid teeth are known from many Paleogene localities in Europe, Africa, India, and the USA (Gottfried and Eberle 2018).
Fig. 24. Teleost fish remains from the upper Paleocene, Basilika Formation, Zachariassendalen, Spitsbergen, Svalbard. NRM-PZ P16479a–e, teeth (A1, A2), vertebra (A3–A5).
Discussion
Composition of the fauna.—The late Paleocene fauna from chemosynthesis-based ecosystems of the Basilika Formation is sandwiched between two important biotic events, i.e., Cretaceous/Paleogene (K/Pg) mass extinction (e.g., Alvarez et al. 1980; Schulte et al. 2010), and the extinction associated with the Paleocene/Eocene Thermal Maximum (e.g., Zachos et al. 2015). In the following section, the fauna is subdivided by genus according to their geological ranges relative to the K/Pg and Paleocene/Eocene boundaries. Only species assigned to the genera with certainty are considered. We have also included Conchocele, described previously (Hryniewicz et al. 2017), into the discussion.
The first group comprises genera going back at least to the Late Cretaceous and continuing after the Paleocene (Fig. 25). It comprises all protobranch bivalves: Solemya (Carboniferous–Recent; Pojeta 1988), Neilonella, and Yoldiella (Cretaceous–Recent; Heinberg 1989). The thyasirid bivalve Conchocele is a long-ranging genus, associated predominantly with hydrocarbon seeps, ranging at least from the latest Cretaceous (Maastrichtian) to the Recent (Hryniewicz et al. 2017). The oldest species of Aporrhais are reported from the Late Cretaceous (e.g., Saul 1998) and range until modern times. The ampullinid gastropod Globularia is known already from the Jurassic, but thrives also in the early Cenozoic, declining gradually towards its end along with the entire family Ampullinidae, represented today by a single species (Kase and Ishikawa 2003). The genera in this group are found in both shallow and deep waters (Coan et al. 2000). As the deep sea is more stable over long periods of time, and was also shown to be largely immune to extinctions affecting shallow waters (Kiel and Little 2006; Thuy et al. 2012, 2014), we speculate that this group was either unaffected by the K/Pg extinction or affected only to some degree. Chemosymbiosis could be a factor enhancing the survival of taxa during disturbances of the photosynthetic food chain (Hautmann and Nützel 2005), such as during the K/Pg mass extinction.
The second group comprises genera known from the Mesozoic, but apparently disappearing shortly after the Paleocene. All of these taxa have their last known occurrence in the Basilika Formation (Fig. 25). This group comprises the terebratulide brachiopod Neolithyrina (Late Cretaceous–Paleocene), the mytilid bivalve Inoperna (Triassic?–Paleocene), the wood-boring bivalve Xylophagella (Early Cretaceous–Paleocene), and the gastropod Ellipsoscapha (Campanian–Paleocene). The phenomenon of genera (or rather clades) surviving mass extinctions only to go exinct shortly thereafter has been discussed previously (e.g., Jablonski 2002; Kaim and Nützel 2011), also for the Paleocene (Maricovich 1993; Amano et al. 2018a), but not for the genera mentioned herein.
The third group comprises genera that appear in the Cenozoic for the first time (Fig. 25). This group comprises the thyasirid bivalve Rhacothyas (Paleocene–Recent), the hiatellid bivalve Cyrtodaria (Paleocene–Recent) and the munidid decapod crustaceans Protomunida (Danian–Ypresian) and Valamunida (late Paleocene). Recent Rhacothyas has only been described from the methane seep off western Svalvard by Åström et al. (2017). This study provides the first fossil occurrence of Rhacothyas. This could indicate that the genus evolved during the Paleocene. However, it is equally likely that Rhacothyas has a pre-Paleocene origin, because it is difficult to evaluate the duration of taxa based on fossil occurrences alone (Signor and Lipps 1982), especially in patchy environments such as seeps. The fossil record of thyasirid bivalves during the Mesozoic is far from being understood (Hryniewicz et al. 2017), and there are Cretaceous thyasirid bivalves at seeps that could shed additional light on the evolutionary history of the group during that period (e.g., Nobuhara et al. 2008). The hiatellid Cyrtodaria has been reported from shallow water in the Arctic area since the Paleocene (e.g., Strauch 1972; Marincovich and Zinsmeister 1991; Marincovich 1993). The appearance of Rhacothyas and Cyrtodaria in the Basilika Formation coincides with cold climatic conditions in the Arctic (e.g., Tripati et al. 2001; Spielhagen and Tripati 2009), preferred by the extant representatives of both genera (Nesis 1965; Strauch 1972; Símonarson 1974; Åström et al. 2017). Congenerics of Protomunida spitzbergica have been found only in shallow- and deep-water coral-associated reef deposits thus far (e.g., Segerberg 1900; Jakobsen and Collins 1997; Beschin et al. 2016; Klompmaker et al. 2016). Valamunida is only known from the Basilika Formation.
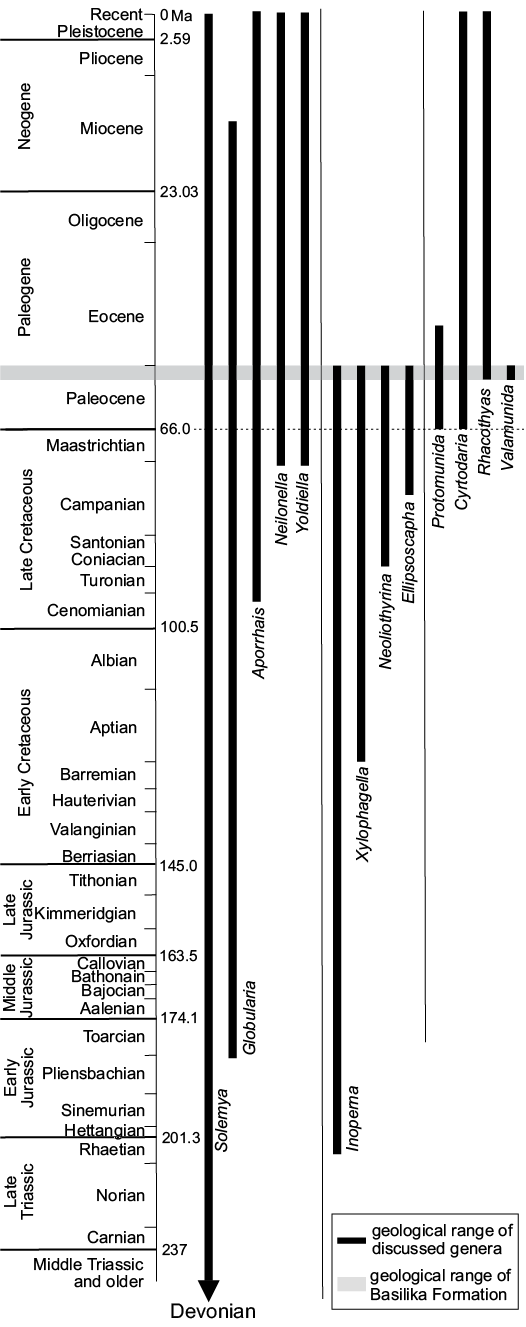
Fig. 25. Geological ranges of the invertebrate genera identified with certainty in this study. Vertical lines determine three groups of taxa according to their geological ranges.
Paleoecological considerations.—The sediments of the Basilika Formation were deposited in a shallow-water prodeltaic setting (e.g., Major and Nagy 1972), in a semi-enclosed marine embayment surrounded by emergent land masses (Nøttvedt 1985). The water column was possibly stratified (Nagy et al. 2000) with sub-freezing temperatures possibly affecting the basin periodically (Spielhagen and Tripati 2009). The associated seep fauna is structured according to the shallow, cold-water environment it lived in, with a high diversity of background species normal for that time (cf. Dando 2010; Kiel 2010a; Hryniewicz et al. 2016). Infaunal bivalve species are both more diverse and more abundant than epifaunal bivalve species, suggesting a soft-bottom environment with few hard substrates for attachment. The infaunal chemosymbiotic thyasirid Conchocele conradii and Rhacothyas spitzbergensis were likely attracted by the presence of sulfide within the sediment. Sulfide is utilized by extant representatives of Conchocele living in symbiosis with sulfide-oxidizing bacteria (Kharlamenko et al. 2016), and is abundant at sites populated by Recent congeners of both thyasirid species (Kharlamenko et al. 2016; Åström et al. 2016). Sulfide can form in marine sediments by microbially mediated anaerobic oxidation of methane above methane seeps (Boetius et al. 2000). This process was likely taking place at Fossildalen and Zachariassendalen, as shown by the lipid biomarker PMI, indicative of the presence of methanotrophic archaea, and the highly depleted carbonate carbon isotope signatures (Hryniewicz et al. 2016, and herein). Therefore, it is likely that the infaunal chemosymbiotic thyasirid bivalves in Fossildalen and Zachariassendalen were at least in part attracted by sulfide accumulating in the sediment above shallow-water methane seeps. Sulfide can also form in marine sediments due to the decomposition of sunken wood or, more generally, the decomposition of terrestrial plants (McLeod et al. 2010). During the decomposition of solid wood, sulfide accumulates chiefly inside the wood rather than in its surroundings (Kalenitchenko et al. 2018). However, if the driftwood that sunk to the seabed was infested by xylophagain bivalves, their fecal pellets may have accumulated in the wood’s surroundings on the sediment surface, impeding gas exchange between marine and pore waters and resulting in sulfide accumulation within the sediment (Turner 2002; Kiel and Goedert 2006; Kiel 2008). As the xylophagain bivalve Xylophagella littlei was living in wood falls at the bottom of the Svalbard embayment during the Paleocene, sulfide-rich conditions could have also developed in the sediments covered with plant litter unrelated to methane seepage. This may have implications for the faunal dispersal between wood falls, methane seeps, and other chemosynthesis-based ecosystems during the Late Cretaceous and Paleocene. Shallow-water wood falls and methane seeps forming in direct proximity, such as the Paleocene methane seeps and wood fall environments from Spitsbergen or their Mesozoic predecessors, could have acted as “evolutionary stepping stones” (cf. Distel et al. 2000; Lorion et al. 2010), allowing faunal exchange between both environments. Indeed, during the Late Cretaceous, the same species characterized deep-sea cold seeps, vertebrate falls and wood falls (Kaim et al. 2008; Kiel et al. 2009; Jenkins et al. 2017), which suggests some faunal exchange between seeps, wood falls, and vertebrate falls was occurring also during the Late Cretaceous. A possible reason for increased faunal similarity between marine chemosynthesis-based ecosystems during the Late Cretaceous are low marine sulphate levels and the related lower intensities of sulphate reduction (Kiel 2015), which has effectively made seeps low sulfide environments comparable to wood and vertebrate falls. We suggest a similar scenario was likely for the Paleocene.
Evolutionary considerations.—The Paleogene was an important time for evolution of seep faunas, witnessing the emergence of vesicomyid clams and bathymodiolin mussels, both of which are key components of extant seep faunas (e.g., Amano and Kiel 2007; Krylova and Sahling 2010; Taylor and Glover 2010; Kiel and Amano 2013). Their appearance in the middle Eocene, as far as we know, was rather sudden and has traditionally been attributed to either paleoceanographic disturbances associated with the Paleocene/Eocene Thermal Maximum (Vrijenhoek 2013) or to the rise of marine sulphate levels and the concomitant development of high sulfidic niches at seeps (Kiel 2015). In addition to the appearance of new groups, the Paleogene was a time of more gradual restructuring of groups already populating seeps. Many shallow- and deep-water lucinid genera present in Mesozoic seeps disappeared during the Paleogene, while new ones emerged (Kiel 2010b, 2013; Natalicchio et al. 2015). In addition, thyasirid faunas composed of Conchocele proliferated (Hryniewicz et al. 2017). The reason for this broad scale Paleogene restructuring of seep faunas remains unclear.
The late Paleocene seep fauna from the Basilika Formation, due to its age and the rarity of Paleocene seep faunas (cf. Schwartz et al. 2003; Kiel 2013; Blouet et al. 2017), could shed more light on the Paleogene history of chemosynthesis-based ecosystems. However, the overall shallow-water character of this fauna does not make it directly comparable to the evolutionarily more meaningful deep-water seep faunas (e.g., Kiel and Little 2006; Kiel 2010a; Kiel et al. 2012; Vrijenhoek 2013). The present fauna lacks vesicomyid clams and bathymodiolin mussels, consistent with the post-Paleocene emergence of these chemosymbiotic bivalves (Amano and Kiel 2007; Kiel and Amano 2013). The fauna also does not include any lucinid bivalves. The present assemblage does, however, contain large numbers of the thyasirid bivalves Conchocele conradii and Rhacothyas spitzbergensis. The accumulation of Conchocele together with the absence of vesicomyid clams and bathymodiolin mussels resembles a fauna in the Maastrichtian seeps of James Ross Basin, Antarctica (Little et al. 2015), although this Antarctic fauna contains some lucinids. There are some other Late Cretaceous seep faunas dominated by thyasirids, such as the Campanian–Maastrichtian Sada Limestone seep deposit of Shikoku, Japan (Nobuhara et al. 2008, 2016). We speculate that there may be trend towards a broader distribution of thyasirid bivalves at seeps during the latest Cretaceous–early Paleogene interval, perhaps aided by the presence of less sulfidic seeps (Kiel 2015; Hryniewicz et al. 2017). If so, Paleocene seep faunas are a continuation of their Late Cretaceous predecessors, and dissimilar from younger Cenozoic seep faunas.
Conclusions
This paper presents a systematic study of Paleocene invertebrate fauna from methane seep and wood fall marine environments in Spitsbergen, Svalbard. The fauna comprises 22 taxa, and is composed predominantly of shallow, cold-water taxa, including some Cretaceous survivor genera such as the brachiopod Neoliothyrina and the bivalves Inoperna and Xylophagella. This Paleocene methane seep and sunken wood fauna from Spitsbergen is of shallow-water character and lived in mutual proximity on a bottom of a marine embayment. The overlap of both seep and sunken wood environments suggests that faunas of both environments could have mutually interchanged in the shallow-water setting some time during the Paleocene and perhaps earlier, which is partially reflected in some older chemosynthesis-based environments known. We did not find any evidence for Paleocene or earlier origins of vesicomyid clams and bathymodiolin mussels so we speculate that Paleocene seep faunas were more reminiscent of their Cretaceous counterparts.
Acknowledgements
The fieldwork on Spitsbergen was registered in the Research in Svalbard database under number RiS 10173 “The influence of Paleocene/Eocene Thermal Maximum on oceanic chemosynthesis-based ecosystems”. TM and JH thank Lars Hansen and the IK Foundation, for the possibility of joining the Bridge Builder Expeditions 2017 Spitsbergen, and the crew of the MS Freya (Gothenburg), who made the fieldwork possible. Authors thank also Grażyna Dziewińska and Marian Dziewiński for macrophotography of the specimens. Special thanks go to journal referees Alan Beu (GNS Science, Lower Hutt, New Zealand), Alfréd Dulai (Hungarian Museum of Natural History, Budapest, Hungary), and Torrey Nyborg (Loma Linda University, Loma Linda, USA) for their carefull reviews which have helped to improve the manuscript. Funding for KH and AK was provided by the Polish National Science Centre (NCN) research grant 2014/15/B/ST10/04886 entitled “The influence of Paleocene/Eocene Thermal Maximum on oceanic chemosynthesis-based ecosystems”. JH was funded by Stiftelsen Olle Engkvist Byggmästare. Funding for KA was provided by the Grants-in-aid for Scientific Research from the Japan Society for Promotion of Science (C, 17K05691, 2017–2019).
References
Adams, H. and Adams, A. 1858. The Genera of Recent Mollusca, Arranged According to Their Organization, Vol. 3. 661 pp. John Van Voost, London.
Adegoke, O.S. 1977. Stratigraphy and paleontology of the Ewekoro Formation (Paleocene) of southwestern Nigeria. Bulletins of American Paleontology 71: 1–379.
Agassiz, L. 1842. Études critiques sur les mollusques fossiles. 2. Monographie des Myes. 141 pp., 48 pls. Imprimerie de Petitpiere, Neuchâtel
Ahyong, S.T. 2007. Decapod Crustacea collected by the NORFANZ Expedition: Galatheidae and Polychelidae. Zootaxa 1593: 1–54.
Ahyong, S.T., Baba, K.J., Macpherson, E., and Poore, G.C.B. 2010. A new classification of the Galatheoidea (Crustacea: Decapoda: Anomura). Zootaxa 2676: 57–68. Crossref
Alvarez, L.W., Alvarez, W., Asaro, F., and Michel, H.V. 1980. Extraterrestrial cause for the Cretaceous–Tertiary extinction. Science 208: 1095–1108. Crossref
Amano, K. 2003. Predatory gastropod drill holes in Upper Miocene cold seep bivalves, Hokkaido, Japan. The Veliger 46: 90–96.
Amano, K. and Ando, H. 2011. Giant fossil Acharax (Bivalvia: Solemyidae) from the Miocene of Japan. The Nautilus 125: 207–212.
Amano, K. and Jenkins, R.G. 2007. Eocene drill holes in cold-seep bivalves of Hokkaido, northern Japan. Marine Ecology 28: 108–114. Crossref
Amano, K. and Jenkins, R.G. 2017. Paleocene protobranch bivalves from Urahoro Town in eastern Hokkaido, northern Japan. Journal of Conchology 42: 283–297.
Amano, K. and Kiel, S. 2007. Fossil vesicomyid bivalves from the North Pacific region. The Veliger 49: 270–293.
Amano, K., Jenkins, R.G., and Kurita, H. 2018a. New and Mesozoic-relict mollusks from Paleocene wood-fall communities in Urahoro Town, eastern Hokkaido, northern Japan. Journal of Paleontology 92: 634–647. Crossref
Amano, K., Little, C.T.S., and Campbell, K.A. 2018b. Lucinid bivalves from Miocene hydrocarbon seep sites of eastern North Island of New Zealand, with comments on Miocene New Zealand seep faunas. Acta Palaeontologica Polonica 63: 371–382. Crossref
Anderson, H.-J. 1970. Pelecypoda. In: K. Vonderbank (ed.), Geologie und Fauna der tertiären Ablagerungen Zentral-Spitzbergens. Norsk Polarinstituts Skrifter 153: 89–101.
Åström, E.K.L., Carroll, M.L., Ambrose Jr., W.L., and Carroll, J. 2016. Arctic cold seeps in marine methane hydrate environments: impacts on shelf microbenthic community structure off Svalbard. Marine Ecology Progress Series 552: 1–18. Crossref
Åström, E.K.L., Oliver, P.G., and Carroll, M.L. 2017. A new genus and two new species of Thyasiridae associated with methane seeps off Svalbard, Arctic Ocean. Marine Biology Research 13: 402–416. Crossref
Baba, K. 1969. Four new genera with their representatives and six new species of the Galatheidae in the collection of the Zoological Laboratory, Kyushu University, with redefinition of the genus Galathea. OHMU, Occasional Papers of the Zoological Laboratory, Faculty of Agriculture, Kyushu University, Fukuoka, Japan 2: 1–32.
Baba, K. 1988. Chirostylid and galatheid crustaceans (Decapoda: Anomura) of the “Albatross” Philippine Expedition 1907–1910. Researches on Crustacea, Special Number 2: 1–203. Crossref
Baba, K. 1993. Anomoeomunida, a new genus proposed for Phylladiorhynchus caribensis Mayo, 1972 (Crustacea: Decapoda: Galatheidae). Proceedings of the Biological Society of Washington 106: 102–105.
Baba, K. 2005. Deep-sea chirostylid and galatheid crustaceans (Decapoda: Anomura) from the Indo-West Pacific, with a list of species. Galathea Reports 20: 1–317.
Baba, K. 2008. Torbenella, a replacement name for Torbenia Baba, 2005 (Decapoda, Galatheidae) preoccupied by Torbenia Libert, 2000 (Insecta, Lepidoptera, Lycaenidae). Crustaceana 81: 1021–1022. Crossref
Baba, K. and de Saint Laurent, M. 1996. Crustacea Decapoda: Revision of the genus Bathymunida Balss, 1914, and description of six new related genera (Galatheidae). In: A. Crosnier (ed.), Résultats des Campagnes MUSORSTOM, v. 15. Mémoires du Muséum National d’Histoire Naturelle 168: 433–502.
Balss, H. 1914. Über einige interessante Decapoden der “Pola”-Expeditionen in das Rote Meer. Anzeiger der Kaiserlichen Akademie der Wissenschaften Mathematisch-Naturwissenschaftlichen Klasse 1914: 133–138.
Barbieri, R., Ori, G.G., and Cavalazzi, B. 2004. A Silurian cold-seep ecosystem from the Middle Atlas, Morocco. Palaios 19: 527–542. Crossref
Benedict, J.E. 1902. Description of a new genus and forty-six new species of crustaceans of the family Galatheidae with a list of the known marine species. Proceedings of the Biological Society of Washington 26: 243–334.
Bernardino, A.F., Smith, C.R., Baco, A., Altamira, I., and Sumida, P.Y.G. 2010. Macrofaunal succession in sediments around kelp and wood falls in the deep NE Pacific and community overlap with other reducing habitats. Deep-Sea Research I 57: 708–723. Crossref
Beschin, C., Busulini, A., Tessier, G., and Zorzin, R. 2016. I crostacei associati a coralli nell’Eocene inferiore dell’area di Bolca (Verona e Vicenza, Italia nordorientale). Memorie del Museo Civico di Storia Naturale di Verona – 2. serie. Sezione Scienze della Terra 9: 1–189.
Beurlen, K. 1944. Beiträge zur Stammesgeschichte der Muscheln. Mathematisch-Naturwissenschaftlichen Abteilung der Bayerischen Akademie der Wissenschaften zu München, Sitzungsberichte 1944 (1–2): 133–145.
Blainville, H.M.D. de 1814. Mémoire sur la classification méthodique des animaux mollusques, et établissement d’une nouvelle considération pour y parvenir. Bulletin des Sciences par la Societé Philomatique de Paris, Zoologie 1814: 175–180.
Blainville, H.M.D. de 1824. Mollusques. Dictionarie des sciences naturelles 32. 392 pp. Levrault, Strassbourg.
Blouet, J.P., Imbert, P., and Foubert, A. 2017. Mechanisms of biogenic gas migration revealed by seep carbonate paragenesis, Panoche Hills, California. American Association of Petroleum Geologists Bulletin 101: 1309–1340. Crossref
Boetius, A., Ravenschlag, K., Schubert, C.J., Rickert, D., Widdel, F., Gieseke, A., Amann, R., Jørgensen, B.B., Witte, U., and Pfannkuche, O. 2000. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407: 623–626. Crossref
Bouchet, P. and Cosel, R. von 2004. The world’s largest lucinid is an undescribed species from Taiwan (Mollusca: Bivalvia). Zoological Studies 43: 704–711.
Bretsky, S.S. 1976. Evolution and classification of the Lucinidae (Mollusca; Bivalvia). Palaeontographica Americana 7: 219–337.
Brünnich, M.T. 1772. Zoologiæ fundamenta praelectionibus academicis accommodate. 254 pp. Fridericus Christianus Pelt, Copenhagen.
Brünnich Nielsen, K. 1909. Brachiopoderne i Danmarks kridtaflejringer. Det Kongelige Danske Videnskabernes Selskabs Skrifter, Naturvidenskabelig og Mathematisk Afdeling 6: 127–178.
Buckeridge, J.S. and Newman, W.A. 2006. A revision of the Iblidae and the stalked barnacles (Crustacea: Cirripedia: Thoracica), including new ordinal, familial and generic taxa, and two new species from New Zealand and Tasmanian waters. Zootaxa 1136: 1–38. Crossref
Burmeister, H. 1834. Beiträge zur Naturgeschichte der Rankenfüsser (Cirripedia). 60 pp. G. Reimer, Berlin.
Cabezas, P. and Macpherson, E. 2014. A new species of Paramunida Baba, 1988 from the Central Pacific Ocean and a new genus to accommodate P. granulata (Henderson, 1885). ZooKeys 425: 15–32. Crossref
Cabezas, P., Macpherson, E., and Machordom, A. 2008. A new genus of squat lobster (Decapoda: Anomura: Galatheidae) from the South West Pacific and Indian Ocean inferred from morphological and molecular evidence. Journal of Crustacean Biology 28: 68–75. Crossref
Campbell, K.A. 2006. Hydrocarbon seep and hydrothermal vent paleoenvironments and paleontology: past developments and future research directions. Palaeogeography, Palaeoclimatology, Palaeoecology 232: 362–407. Crossref
Carter, J.G., Campbell, D.C., and Campbell, M.R. 2000. Cladistic perspectives on early bivalve evolution. In: E.M. Harper, J.D. Taylor, and J.A. Crame (eds.), The Evolutionary Biology of the Bivalvia. The Geological Society of London, Special Publication 177: 47–79. Crossref
Casey, R. 1961. The stratigraphical palaeontology of the Lower Greensand. Palaeontology 3: 487–621.
Chan, B.K.K., Prabowo, R.E., and Kwen-Shen, L. 2009. Crustacean Fauna of Taiwan: Barnacles, Volume 1 Cirripedia: Thoracica, Excluding Pyrgomatidae and Acastinae. 297 pp. National Taiwan Ocean University, Taiwan.
Chelot, M. 1885. Rectifications pour servir à l’étude de la faune éocène du basin de Paris. Bulletin de la Société Géologique de France, série 3 13: 191–203.
Children, J.G. 1823. Lamarck’s genera of shells. Quaterly Journal of Science, Literature & Arts 14: 298–322.
Coan, E.V. and Valentich-Scott, P. 2012. Bivalve seashells of tropical West America. Marine bivalve molluscs from Baja California to Northern Peru. Santa Barbara Museum of Natural History Monographs 4: 1–1258.
Coan, E.V., Scott, P.V., and Bernard, F.R. 2000. Bivalve seashells of Western North America. Marine bivalve molluscs from Arctic Alaska to Baja California. Santa Barbara Museum of Natural History Monographs 2: 1–764.
Collins, J.S.H. and Jakobsen, S.L. 1994. A synopsis of the biostratigraphic distribution of the crab genera (Crustacea, Decapoda) of the Danian (Palaeocene) of Denmark and Sweden. Bulletin of the Mizunami Fossil Museum 21: 35–46.
Conrad, T.A. 1849. Description of new fresh water and marine shells. Proceedings of the Academy of Natural Sciences of Philadelphia 4: 152–156.
Conrad, T.A. 1875. Descriptions of new genera and species of fossil shells of North Carolina in the State Cabinet at Raleigh. In: W.C. Kerr (ed.), Appendix A. Report of the Geological Survey of North Carolina 6: 1–13.
Cooper, A.G. 1983. The Terebratulacea (Brachiopoda) Triassic to Recent: a study of the brachidia (loops). Smithsonian Contributions to Paleobiology 50: 1–445. Crossref
Cope, E.D. 1887. Geology and Paleontology. American Naturalist 21: 1014–1019. Crossref
Cosel, R. von and Bouchet, P. 2008. Tropical deep-water lucinids (Mollusca: Bivalvia) from the Indo-Pacific: essentially unknown, but diverse and occasionally gigantic. Mémoires du Muséum National du Histoire Naturelle 196: 115–213.
Cosel, R. von and Olu, K. 1998. Gigantism in Mytilidae. A new Bathymodiolus from the cold seep areas of the Barbados accretionary prism. Comptes Rendus de l’Academie des Sciences Serie III 321: 655–663.
Cossmann, M. 1888. Catalogue illustré des coquillages fossiles de l’Eocène des environs de Paris, 3. Annales de la Société Royale Malacologique de Belgique 23: 3–328.
Cossmann, M. and Peyrot, A. 1917–1919. Conchologie néogénique de l’Aquitaine. Tome 3, Gastropodes, Scaphopodes et Amphineures. 695 pp. A. Saugnac & E. Drouillard, Bordeaux.
Costa, E.M. da 1778. Historia naturalis testaceorum britanniae or the British Conchology; containing the descriptions and other particulars of natural history of the shells of Great Britain and Ireland: illustrated with figures. 254 pp. Millan, White, Elmsley & Robson, London.
Cox, L.R. and Arkell, W.J. 1948. A survey of the Mollusca of the British Great Oolite Series. Primarily a nomenclatoral revision of the mongraphs by Morris and Lycett (1851–1855), Lycett (1863) and Blake (1905–1907). Monograph of the Palaeontographical Society 102: 1–48. Crossref
Coykendall, D.K., Nizinski, M.S., and Morrison, C.L. 2017. A phylogenetic perspective on diversity of Galatheoidea (Munida, Munidopsis) from cold-water coral and cold seep communities in the western North Atlantic Ocean. Deep-Sea Research II 137: 258–272. Crossref
Cuvier, G. 1797. Table Élémentaire de l’Historie Naturelle des Animaux. 710 pp. Baudouin, Paris.
Cvancara, A.M. 1966. Revision of the fauna of the Cannonball Formation (Paleocene) of North and South Dakota. Contributions from the Museum of Paleontology, The University of Michigan 20: 277–365.
Dall, W.H. 1881. Reports on the results of dredging, under the supervision of Alexander Agassiz, in the Gulf of Mexico and in the Caribbean Sea, 1877–79), by the U.S. Coast Survey steamer “Blake”, Lieut.-Commander C.D. Sigsbee, U.S.N. and Commander J.R. Bartlett, U.S.N. commanding. XV. Preliminary report on the Mollusca. Bulletin of the Museum of Comparative Zoology at Harvard College 9: 33–144.
Dall, W.H. 1889. On the hinge of pelecypods and its development, with an attempt toward a better subdivision of the group. American Journal of Science 38: 445–462. Crossref
Dall, W.H. 1895. Contributions to the Tertiary fauna of Florida with special reference to the Miocene Silex-beds of Tampa and the Pliocene beds of the Caloosahatchie River. Transactions of the Wagner Free Institute of Science 3: 949–1218.
Dall, W.H. 1901. Synopsis of the Lucinacea and of the American species. Proceedings of the US National Museum of Natural History 23: 779–833. Crossref
Dall, W.H. 1908. Reports on the dredging operations off the west coast of Central America to the Galapagos, to the west coast of Mexico, and in the Gulf of California, in charge of Alexander Agassiz, carried on by the U.S. Fish Commission steamer “Albatros”, Lieut.-Commander Z. L. Tanner, U.S.N. Commanding. XXXVII Reports on scientific results of the expedition to the eastern tropical Pacific, in charge of Alexander Agassiz, by the U.S. Fish Commission steamer “Albatros” from October, 1904 to March, 1905, Lieut.-Commander L. M. Garrett, U.S.N. commanding. XIV. The Mollusca and Brachiopoda. Bulletin of the Museum of Comparative Zoology at Harvard College 43: 205–487.
Dallmann, W.K. 1999. Lithostratigraphic Lexicon of Svalbard. Review and Recommendations for Literature Use. Upper Palaeozoic to Quaternary Bedrock. 318 pp. Norsk Polarinstitutt, Tromsø.
Dallmann, W.K. 2015. Geoscience Atlas of Svalbard. 413 Rapportserien 148. 414 pp. Norsk Polarinstitutt, Tromsø.
Damholt, T., Rasmussen, A., and Rasmussen, L. 2010. Fossiler fra Faxe Kalkbrud. 48 pp. Geomuseum Faxe, Faxe.
Dando, P. 2010. Biological communities at marine shallow-water vent and seep sites. In: S. Kiel (ed.), The Vent and Seep Biota. Aspects from Microbes to Ecosystems, 333–378. Springer, Dordrecht. Crossref
Darwin, C. 1854. A Monograph on the Sub-class Cirripedia, with Figures of All Species. The Balanidae, the Verrucidae, etc. 684 pp., 30 pls. Ray Society, London.
Davidson, T. 1852. A Monograph of British Cretaceous Brachiopoda. Part II. Palaeontographical Society Monograph 6: 1–54. Crossref
De Angeli, A. and Garassino, A. 2002. Galatheid, chirostylid and porcellanid decapods (Crustacea, Decapoda, Anomura) from the Eocene and Oligocene of Vicenza (N Italy). Memorie della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano 30: 1–40.
De Grave, S., Pontcheff, N.D., Ahyong, S.T., Chan, T.-Y., Crandall, K.A., Dworschak, P.C., Felder, D.L., Feldmann, R.M., Fransen, C.H.M., Goulding, L.Y.D., Lemaitre, R., Low, M.E.Y., Martin, J.W., Ng, P.K.L., Schweitzer, C.E., Tan, S.H., Tshudy, D., and Wetzer, R. 2009. A classification of living and fossil genera of decapod crustaceans. The Raffles Bulletin of Zoology, Supplement 21: 1–109.
Des Moulins, C. 1832. Description d’un genre de coquille vivante, bivalve, des mers du Chili. Actes de la Société Linnéenne de Bordeaux 5: 83–92.
Dhondt, A.V. 1987. Bivalves from the Hochmoos Formation (Gosau Group, Oberösterreich, Austria). Annalen des Naturhistorischen Museums in Wien A 88: 41–101.
Distel, D.L., Baco, A.R., Chuang, E., Morrill, W., Cavanaugh, C., and Smith, C.R. 2000. Do mussels take wooden steps to deep-sea vents? Nature 403: 725–726. Crossref
d’Orbigny, C.A. 1847. Paléontologie Francaise. Description des Molluques et Rayonnés fossiles. Terrains Crétacés. 4. Brachiopodes. 390 pp. Victor Masson, Paris.
Douvillé, H. 1904. Études géologiques. Partie IV. Paleontologie. Mollusques fossiles. Mission Scientifique en Perse 3: 191–380.
Duff, K.L. 1978. Bivalvia from the English lower Oxford Clay (Middle Jurassic). Palaeontographical Society Monographs 132: 1–137.
Duméril, A.M.C. 1806. Zoologie analytique, ou méthode naturelle de classification des animaux, rendue plus facile à l’aide de tableaux synoptiques. 344 pp. Allais, Paris.
Fabricius, J.C. 1775. Systema Entomologiae, sistens Insectorum Classes, Ordines, Genera, Species, adjectis Sysnonymis, Locis, Descriptionibus, Observationibus. 832 pp. Kortii, Flensburgi et Lipsiae.
Forbes, E. 1845. Report on the fossil Invertebrata from Southern India, collected by Mr. Kaye and Mr. Cunliffe. Transactions of the Geological Society of London. Series 2 7: 97–174. Crossref
Fischer, P. 1878. Essai sur la distribution géographique des brachiopodes et des mollusques du littoral océanique de la France. Actes de la Société Linnéenne de Bordeaux 32: 171–215.
Fischer, P. 1883. Manuel de conchyliologie et de paléontologie conchyliologique, ou Histoire naturelle des mollusques vivants et fossiles. Tome 6, 513–608. Savy, Paris.
Fujikura, K., Kojima, S., Tamaki, K., Maki, Y., Hunt, J., and Okutani, T. 1999. The deepest chemosynthesis-based community yet discovered from the hadal zone, 7326 m deep, in the Japan Trench. Marine Ecology Progress Series 190: 17–26. Crossref
Fuller, S.C. and Lutz, R.A. 1989. Shell morphology of larval and postlarval mytilids from the north-western Atlantic. Journal of the Marine Biological Association of the United Kingdom 69: 181–218. Crossref
Garassino, A., De Angeli, A., and Pasini, G. 2008. New decapod assemblage from the Upper Cretaceous (Cenomanian–Turonian) of Gara Sbaa, southeastern Morocco. Atti della Societa Italiana di Scienze Naturali e del Museo Civico di Storia Naturale in Milano 149: 37–67.
Gardner, J.S. 1884. British Eocene Aporrhaidae. Geological Magazine. Decade III 1: 529–534.
Gill, F.K. and Little, C.T.S. 2013. A new genus of lucinid bivalves from hydrocarbon seeps. Acta Palaeontologica Polonica 58: 573–578.
Goldfuss, G.A. 1820. Handbuch der Zoologie. Dritter Theil, zweite Abtheilung. 512 pp. Johann Leonhard Schrag, Nürnberg.
Gottfried, M.D. and Eberle, J.J. 2018. New records from Banks Island expand the diversity of Eocene fishes from Canada’s western Arctic Greenhouse. Arctic Science 4: 16–24.
Gray, J.E. 1824. A supplement to the Appendix of Captain Perry’s voyage for the discovery of a North West Passage in the years 1819–1820, containing an account of the subjects of natural history. In: E.W. Parry (ed.), Journal of a Second Voyage for the Discovery of a Northwest Passage from the Atlantic to the Pacific, Performed in the Years 1821–22–23, in His Majesty’s Ships Fury and Hecla, under the Orders of Captain William Edward Parry, R.N., F.R.S. Appendix X. Natural History, Shells, 240–246. J. Murray, London.
Gray, J.E. 1825. A synopsis of the genera of Cirripedes arranged in natural families, with a description of some new species. Annals of Philosophy, new series 10: 97–107.
Gray, J.E. 1840. Synopsis of the Contents of the British Museum. 370 pp. British Museum, London.
Gray, J.E. 1850. Figures of Molluscous Animals Selected from Various Authors. Etched for the Use of Students by M.E. Gray. Vol. 4. 219 pp. Longman, Brown, Green & Longmans, London.
Gray, J.E. 1854. A revision of the arrangement of the families of bivalve shells (Conchifera). The Annals and Magazine of Natural History, Series 2 13: 408–418. Crossref
Gripp, K. 1927. Beiträge zur Geologie von Spitzbergen. Abhandlungen des Naturwissenschaftlichen Vereins zu Hamburg 21: 3–38.
Grobben, C. 1894. Zur Kenntniss der Morphologie, der Verwandschaftsverhältnisse und des Systems der Mollusken. Sitzungsberichte der Mathematisch-Naturwissenschaftlichen Klasse der Kaiserlichen Akademie der Wissenschaften 103: 61–86.
Hagenow, F. von 1842. Monographie der Rüg’schen Kreide-Versteinerungen. III. Abteilung: Mollusken. Neues Jahrbuch für Mineralogie, Geognosie, Geologie und Petrefaktenkunde 5: 528–575.
Hägg, R. 1925. A new Tertiary fauna from Spitsbergen. Bulletin of the Geological Institution of the University of Uppsala 20: 39–55.
Harris, G.F. 1897. Catalogue of Tertiary Mollusca in the Department of Geology, British Museum (Natural History). Part 1, The Australasian Tertiary Mollusca. 407 pp. Longman & Co., London.
Harris, G.D. 1919. Pelecypoda of the St. Maurice and Claiborne stages. Bulletins of American Palaeontology 6: 1–202.
Hautmann, M. 2001. Die Muschelfauna der Nayband-Formation (Obertrias, Nor-Rhät) des östlichen Zentraliran. Beringeria 29: 3–181.
Hautmann, M. and Nützel, A. 2005. First record of a heterodont bivalve (Mollusca) from the Early Triassic: Palaeoecological significance and implications for the “Lazarus Problem”. Palaeontology 48: 1131–1138. Crossref
Heinberg, C. 1989. Bivalves from the white chalk (Maastrichtian) of Denmark, IV: Nuculoida. Bulletin of the Geological Society of Denmark 37: 227–236.
Hryniewicz, K., Amano, K., Jenkins, R.G., and Kiel, S. 2017. Thyasirid bivalves from Cretaceous and Paleogene cold seeps. Acta Palaeontologica Polonica 62: 705–728. Crossref
Hryniewicz, K., Bitner, M.A., Durska, E., Hagström, J., Hjálmársdottir H.R., Jenkins, R.G., Little, C.T.S., Miyajima, Y., Nakrem, H.A., and Kaim, A. 2016. Paleocene methane seep and wood-fall marine environments from Spitsbergen, Svalbard. Palaeogeography, Palaeoclimatology, Palaeoecology 462: 41–56. Crossref
Hryniewicz, K., Little, C.T.S., and Nakrem, H.A. 2014. Bivalves from the latest Jurassic–earliest Cretaceous hydrocarbon seep carbonates from Spitsbergen, Svalbard. Zootaxa 3859: 1–66. Crossref
Hybertsen, F. and Kiel, S. 2018. A middle Eocene seep deposit with silicified fauna from the Humptulips Formation in western Washington State, USA. Acta Palaeontologica Polonica 63: 751–768. Crossref
Iredale, T. 1931. Australian Molluscan notes. No. 1. Records of the Australian Museum 18: 201–235. Crossref
Iredale, T. 1939. Mollusca. Part I. Scientific Reports of the Great Barrier Reef Expedition 1928–1929 5: 209–245.
Jablonski, D. 2002. Survival without recovery after mass extinctions. Proceedings of the National Academy of Sciences of the United States of America 99: 8139–8144. Crossref
Jakobsen, S.L. and Collins, J.S.H. 1997. New Middle Danian species of anomuran and brachyuran crabs from Fakse, Denmark. Bulletin of the Geological Society of Denmark 44: 89–100.
Jakubowicz, M., Hryniewicz, K., and Belka, Z. 2017. Mass occurence of seep-specific bivalves in the oldest known cold-seep metazoan community. Scientific Reports 7: 14292. Crossref
Jenkins, R.G., Kaim, A., Sato, K., Moriya, K., Hikida, Y., and Hirayama, R. 2017. Discovery of chemosynthesis-based association on the Cretaceous basal leatherback sea turtle from Japan. Acta Palaeontologica Polonica 62: 683–690. Crossref
Kaim, A. and Nützel, A. 2011. Dead bellerophontids walking—the short Mesozoic history of the Bellerophontoidea (Gastropoda). Palaeogeography, Palaeoclimatology, Palaeoecology 308: 190–199. Crossref
Kaim, A., Kobayashi, Y., Echizenya, H., Jenkins, R.G., and Tanabe, K. 2008. Chemosynthesis-based associations on Cretaceous plesiosaurid carcasses. Acta Palaeontologica Polonica 53: 97–104. Crossref
Kalenitchenko, D., Péru, E., Contreira Pereira L., Petetin, C., Galand, P.E., and Le Bris, N. 2018. The early conversion of deep-sea wood falls into chemosynthetic hotspots revealed by in situ monitoring. Scientific Reports 8: 907. Crossref
Kamenev, G.M. 2009. North Pacific species of the genus Solemya Lamarck, 1818 (Bivalvia: Solemyidae) with notes on Acharax johnsoni (Dall, 1891). Malacologia 51: 233–261. Crossref
Kanie, Y. and Sakai, T. 1997. Chemosynthetic thraciid bivalve Nipponothracia, gen. nov., from the Lower Cretaceous and middle Miocene mudstones in Japan. Venus 56: 205–220.
Kase, T. and Ishikawa, M. 2003. Mystery of naticid predation history solved: Evidence from a “living fossil” species. Geology 31: 403–406. Crossref
Kelly, S.R.A. 1988. Cretaceous wood-boring bivalves from western Antarctica with a review of the Mesozoic Pholadidae. Palaeontology 31: 341–372.
Kelly, S.R.A., Blanc, E., Price, S.P., and Witham, A.G. 2000. Early Cretaceous giant bivalves from seep-related limestone mounds, Wollaston Foreland, Northeast Greenland. In: E.M. Harper, J.D. Taylor, and A. Crame (eds.), The Evolutionary Biology of the Bivalvia, 227–246. Geologial Society, London.
Kharlamenko, V.I., Kamenev, G.M., Kalachev, A.V., Kiyashko, S.I., and Ivin, V.V. 2016. Thyasirid bivalves from the methane seep community off Paramushir Island (Sea of Okhotsk) and their nutrition. Journal of Molluscan Studies 82: 391–402. Crossref
Kiel, S. 2008. Fossil evidence for micro- and macrofaunal utilization of large nekton-falls: examples from early Cenozoic deep-water sediments in Washington State, USA. Palaeogeography, Palaeoclimatology, Palaeoecology 267: 161–174. Crossref
Kiel, S. 2010a. On the potential generality of depth-related ecologic structure in cold-seep communities: evidence from Cenozoic and Mesozoic examples. Palaeogeography, Palaeoclimatology, Palaeoecology 295: 245–257. Crossref
Kiel, S. 2010b. The fossil record of vent and seep mollusks. In: S. Kiel (ed.), The Vent and Seep Biota. Aspects from Microbes to Ecosystems, 255–277. Springer, Dordrecht. Crossref
Kiel, S. 2013. Lucinid bivalves from ancient methane seeps. Journal of Molluscan Studies 79: 346–363. Crossref
Kiel, S. 2015. Did shifting seawater sulfate concentrations drive the evolution of deep-sea methane-seep ecosystems. Proceedings of the Royal Society B 282: 20142908. Crossref
Kiel, S. 2018. Three new bivalve genera from Triassic hydrocarbon seep deposits in southern Turkey. Acta Palaeontologica Polonica 63: 221–234. Crossref
Kiel, S. and Amano, K. 2013. The earliest bathymodiolin mussels: an evaluation of Eocene and Oligocene taxa from deep-sea methane seep deposits in western Washington State, USA. Journal of Paleontology 87: 589–602. Crossref
Kiel, S. and Goedert, J.L. 2006. A wood-fall association from Late Eocene deep-water sediments of Washington State, USA. Palaios 21: 548–556. Crossref
Kiel, S. and Little, C.T.S. 2006. Cold-seep mollusks are older than the general marine mollusk fauna. Science 313: 1429–1431. Crossref
Kiel, S. and Taviani, M. 2017. Chemosymbiotic bivalvies from Miocene methane-seep carbonates in Italy. Journal of Paleontology 91: 444–466. Crossref
Kiel, S., Amano, K., and Jenkins, R.G. 2008. Bivalves from Cretaceous cold-seep deposits on Hokkaido, Japan. Acta Palaeontologica Polonica 53: 525–537. Crossref
Kiel. S., Amano, K., and Jenkins, R.G. 2016. Predation scar frequencies in chemosymbiotic bivalves at an Oligocene seep deposit and their potential relation in inferred sulfide tolerances. Palaeogeography, Palaeoclimatology, Palaeoecology 453: 139–145. Crossref
Kiel, S., Amano, K., Hikida, Y., and Jenkins, R.G. 2009. Wood-fall associations from Late Cretaceous deep-water sediments of Hokkaido, Japan. Lethaia 42: 74–82. Crossref
Kiel, S., Campbell, K.A., and Gaillard, C. 2010. New and little known mollusks from ancient chemosynthetic environments. Zootaxa 2390: 26–48. Crossref
Kiel, S., Krystyn, L., Demirtaş, F., Koşun, E., and Peckmann, J. 2017. Late Triassic mollusk-dominated hydrocarbon-seep deposits from Turkey. Geology 45: 751–754. Crossref
Kiel, S., Sami, M., and Taviani, M. 2018. A serpulid-Anodontia-dominated methane-seep deposit from the Miocene of northern Italy. Acta Palaeontologica Polonica 63: 569–577. Crossref
Kiel, S., Wiese, F., and Titus, A.L. 2012. Shallow-water methane-seep faunas in the Cenomanian Western Interior Seaway: No evidence for onshore-offshore adaptations to deep-sea vents. Geology 40: 839–842. Crossref
Klompmaker, A.A. and Boxshall, G.A. 2015. Fossil crustaceans as parasites and hosts. Advances in Parasitology 90: 233–289. Crossref
Klompmaker, A.A., Feldmann, R.M., and Schweitzer, C.E. 2012. A hotspot for Cretaceous goniodromitids (Decapoda: Brachyura) from reef associated strata in Spain. Journal of Crustacean Biology 32: 780–801. Crossref
Klompmaker, A.A., Hyžný, M., and Jakobsen, S.L. 2015. Taphonomy of decapod crustacean cuticle and its effect on the appearance as exemplified by new and known taxa from the Cretaceous–Danian crab Caloxanthus. Cretaceous Research 55: 141–151. Crossref
Klompmaker, A.A., Jakobsen, S.L., and Lauridsen, B.W. 2016. Evolution of body size, vision, and biodiversity of coral-associated organisms: evidence from fossil crustaceans in cold-water coral and tropical coral ecosystems. BMC Evolutionary Biology 16 (1): 132. doi:10.1186/s12862-016-0694-0 [recommended for online only pubs] Crossref
Knight, J.B., Cox, L.R., Keen, A.M., Smith, A.G., Batten, R.L., Yochelson, E.L., Ludbrook, N.H., Robertson, R., Yonge, C.M., and Moore, R.C. 1960. Treatise on Invertebrate Paleontology. Pt. I. Mollusca. 1. 351 pp. Geological Society of America, Inc., Boulder & University of Kansas Press, Lawrence.
Koenen, A. von 1885. Über eine Paleocäne Fauna von Kopenhagen. Abhandlungen der Königlichen Gesellschaft der Wissenschaften zu Göttingen 32: 1–128.
Kollmann, H.A. and Peel, J.S. 1983. Paleocene gastropods from Nûgssuaq, West Greenland. Grønlands Geologiske Undersøgelse 146: 1–115.
Krylova, E.M. and Sahling, H. 2010. Vesicomyidae (Bivalvia): current taxonomy and distribution. PLoS ONE 5 (4): e9957. Crossref
Lamarck, J.B.P.A. de 1799. Prodrome d’une nouvelle classification des coquilles. Mémoires de la Société d’Histoire Naturelle de Paris 8: 63–91.
Lamarck, J.B.P.A. de 1804. Suite des mémoires sur les fossiles des environs de Paris. Annales du Muséum National d’Histoire Naturelle 5: 28–36.
Lamarck, J.B.P.A. de 1809. Philosophie zoologique, ou exposition des considerations relatives à l’histoire naturelle des animaux; à la diversité de leur organisation et des facultés qu’ils en obtiennent; aux causes physiques qui maintiennent en eux la vie et donnent lieu aux mouvemens qu’ils exécutent; enfin, à celles qui produisent, les unes le sentiment, et les autres l’intelligence de ceux qui en sont doués. 885 pp. The author, Paris.
Lamarck, J.B.P.A. de 1818. Histoire naturelle des animaux sans vertèbres. Vol. 5. 612 pp. Deterville, Paris.
Latreille, P.A. 1802. Histoire naturelle, générale et particulière des crustacés et des insectes. Ouvrage faisant suite à l’histoire naturelle générale et particulière, composée par Leclerc de Buffon, et rédigée par C.S. Sonnini, membre de plusieurs sociétés savantes. Famille naturelles des genres. Tome troisième. 467 pp. F. Dufart, Paris.
Leach, W.E. 1817. Distribution systématique de la classe des Cirripèdes. Journal de Physique 85: 67–69.
Leach, W.E. 1819. Descriptions des nouvelles espèces d’animanux decouvertes par la vaisseau Isabelle dans un voyage au pole boreal. Journal de Physique 88: 462–467.
Leach, W.E. 1820. Galatéadées. In: F. Cuvier (ed.), Dictionnaire des sciences naturelles, dans lequel on trait méthodiquement des différens êtres de la nature, considérés soit en euxmêmes, d’après l’état actuel de nos connoissances, soit relativement a l’utilité qu’en peuvent retirer la médecine, l’agriculture, le commerce et les arts. Suivi d’une biographie des plus célèbres naturalistes. Ouvrage destiné aux médecins, aux agriculteurs, aux commerçans, aux artistes, aux manufacturiers, et à tous ceux qui ont intérêt à connoître les productions de la nature, leurs caractères génériques et spécifi ques, leur lieu natal, leurs propiétés et leurs usages, 49–56. F.G. Levreault, Paris.
Lehman, J.P. 1951. Un nouvel Amiidé de l’Eocène du Spitzberg, Pseudamia heintzi. Tromsö Museums Årshefter 70: 3–11.
Levin, L. 2005. Ecology of cold seep sediments: interactions of fauna with flow, chemistry and microbes. Oceanography and Marine Biology: An Annual Review 43: 1–46. Crossref
Link, H.F. 1807. Beschreibung der Naturalien-Sammlung der Universität Rostock. 3 Abteilung. 101–165. Adlers Erben, Rostock.
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. 824 pp. Laurentius Salvius, Holmiae.
Linnaeus, C. 1767. Systema naturae, Tom. I. Pars II. Editio duodecima, reformata, 533–1327. Laurentius Salvius, Holmiae.
Little, C.T.S., Birgel, D., Boyce, A.J., Crame, A.J., Francis, J.E., Kiel, S., Peckmann, J., Pirrie, D., Rollinson, G.K., and Witts, J.D. 2015. Late Cretaceous (Maastrichtian) shallow water hydrocarbon seeps from Snow Hill and Seymour Islands, James Ross Basin, Antarctica. Palaeogeography, Palaeoclimatology, Palaeoecology 418: 213–228. Crossref
Livšic, Y.Y. 1974. Palaeogene deposits and platform structure of Svalbard. Norsk Polarinstitutt Skrifter 159: 3–50.
Lorion, J., Buge, B., Cruaud, C., and Samadi, S. 2010, New insights into diversity and evolution of deep-sea Mytilidae (Mollusca: Bivalvia). Molecular Phylogenetics and Evolution 57: 71–83. Crossref
Lovén, S.L. 1846. Index Molluscorum litora Scandinaviae occidentalia habitantium. Faune prodromum. Öfversigt af Kongliga Vetenskaps Akademiens Förhandlingar. 50 pp. P.A. Nordstedt et Filii, Holmiae. Crossref
Ludbrook, N.H. 1959. A widespread Pliocene molluscan fauna with Anodontia in South Australia. Transactions of the Royal Society of South Australia 82: 219–233.
Ludbrook, N.H. 1978. Quaternary molluscs of the western part of the Eucla Basin. Geological Survey of Western Australia Bulletin 125: 1–286.
Lundgren, B. 1885. Undersökningar öfver Brachiopoderna i Sveriges Kritsystem. Lunds Universitets Årskrift 20: 1–72.
MacKinnon, D.I. and Lee, D.E. 2006. Loop morphology and terminology in Terebratulida. In: R.L. Kaesler (ed.), Treatise of Invertebrate Paleontology. Part H Brachiopoda revised, Vol. 5, 1974–1993. Geological Society of America, Boulder and University of Kansas, Lawrence.
Macpherson, E. 1994. Crustacea Decapoda: Studies on the genus Munida Leach, 1820 (Galatheidae) in New Caledonia and adjacent waters with descriptions of 56 new species. In: A. Crosnier (ed.), Résultats des Campagnes MUSORSTOM, v. 12. Mémoires du Muséum national d›Histoire naturelle, Paris 161: 421–569.
Macpherson, E. 1998. A new genus of Galatheidae (Crustacea, Anomura) from the western Pacific Ocean. Zoosystema 20: 351–355.
Macpherson, E. 2006. Galatheidae (Crustacea, Decapoda) from the Austral Islands, Central Pacific. In: B. Richer de Forges and J.L. Justine (eds.), Tropical Deep-Sea Benthos, Vol. 24. Mémoires du Muséum national d’Histoire naturelle, Paris 193: 285–333.
Macpherson, E. and Machordom, A. 2000. Raymunida, new genus (Decapoda: Anomura: Galatheidae) from the Indian and Pacific Oceans. Journal of Crustacean Biology 20: 253–258. Crossref
Major, H. and Nagy, J. 1972. Geology of the Adventdalen map area. Norsk Polarinstitut Skrifter 138: 5–58.
Manum, S.B. and Throndsen, T. 1986. Age of Tertiary formations on Spitsbergen. Polar Research 4: 103–131. Crossref
Marincovich, L. Jr. 1993. Danian mollusks from the Prince Creek Formation, northern Alaska, and implications for Arctic Ocean paleogeography. The Paleontological Society Memoir 35: 1–35.
Marincovich Jr., L. and Zinsmeister, W.J. 1991. The first Tertiary (Paleocene) marine mollusks from the Eureka Sound Group, Ellesmere Island, Canada. Journal of Paleontology 65: 242–248. Crossref
Matos, S.A., Warren, L.V., Fürsich, F.T., Alessandretti, L., Assine, M.L., Riccomini, C., and Simões, M.G. 2017. Paleoecology and paleoenvironments of Permian bivalves of the Serra Alta Formation, Brazil: Ordinary suspension feeders or Late Paleozoic Gondwana seep organisms? Journal of South American Earth Sciences 77: 21–41. Crossref
McLeod, R.J., Wing, S.R., and Skilton, J.E. 2010. High incidence of invertebrate-chemoautotroph symbioses in benthic communities of the New Zealand fjords. Limnology and Oceanography 55: 2097–2106. Crossref
Meek, F.B. 1864. Checklist of the invertebrate fossils of North America. Smithsonian Miscellaneous Collections 177: 1–40.
Meek, F.B. and Hayden, F.V. 1856. Descriptions of new species of Acephala and Gasteropoda, from the Tertiary formations of Nebraska Territory with some general remarks on the geology of the country about the sources of the Missouri River. Proceedings of the Academy of Natural Sciences of Philadelphia 8: 111–126.
Meek, F.B. and Hayden, F.V. 1858. Descriptions of new genera of fossils, collected by Dr. F.V. Hayden, in Nebraska territory, under the direction of Lieut. G.K. Warren, US Topographical Engineer; with some remarks on the Tertiary and Cretaceous formations of the north-west, and parallelism of the latter with those of other portions of the United States and Territories. Proceedings of the Academy of Natural Sciences of Philadelphia (for 1857) 9: 117–148.
Milne-Edwards, H. 1832. Extrait d’une Lettre sur les caractères des Crustacés Anomures, adressée à M. Audouin. Annales des Sciences Naturelles, Zoologie et Biologie Animale 26: 255–256.
Minisini, D. and Schwartz, H. 2007. An early Paleocene cold seep system in the Panoche and Tumey Hills, Central California (United States). In: A. Hurst and J. Cartwright (eds.), Sand Injectites: Implications for Hydrocarbon Exploration and Production, 185–197. The American Association of Petroleum Geologists, Tulsa.
Morris, J. 1852. Description of some fossil shells from the lower Thanet Sands. Geological Society of London Quaterly Journal 8: 264–268.
Morton, B. 2011. The biology and functional morphology of Arctica islandica (Bivalvia: Arcticidae): A gerontophilic living fossil. Marine Biology Research 7: 540–553. Crossref
Mörch, O.A.L. 1857. Fortegnelse over Grønlands Bløddyr. In: H. Rink (ed.), Grønland, geographisk og statisk beskrevet, 75–100. A.F. Høst, København.
Muir-Wood, H. 1965. Mesozoic and Cenozoic Terebratulidina. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, Part H, Brachiopoda, vol. 2, 762–816. Geological Society of America, Boulder & University of Kansas Press, Lawrence.
Müller, J. 1845. Über den Bau und die Grenzen der Ganoiden, und über das natürliche System der Fische. Archiv für Naturgeschichte 11: 91–141.
Müller, P. and Collins, J.S.H. 1991. Late Eocene coral-associated decapods (Crustacea) from Hungary. Contributions to Tertiary and Quaternary Geology 28: 47–52.
Nagy, J., Kaminski, M.A., Kuhnt, W., and Bremer, M.A. 2000. Agglutinated foraminifera from neritic to bathyal facies in the Palaeogene of Spitsbergen and the Barents Sea. In: M.B. Hart, M.A. Kaminski, and C.W. Smart (eds.), Proceedings of the Fifth International Workshop on Agglutinated Foraminifera. Grzybowski Foundation Special Publications 7: 333–361.
Natalicchio, M., Peckmann, J., Birgel, D., and Kiel, S. 2015. Seep deposits from northern Istria, Croatia: a first glimpse into the Eocene seep fauna of the Tethys region. Geological Magazine 152: 444–459. Crossref
Nesis, K.N. 1965. Ecology of Cyrtodaria siliqua and history of the genus Cyrtodaria (Bivalvia: Hiatellidae). Malacologia 3: 197–210.
Newton, R.B. 1891. Systematic List of the Frederick E. Edwards Collection of British Oligocene and Miocene Mollusca, with References to the Type Specimens from Similar Horizons. 365 pp. British Museum (Natural History), London.
Nobuhara, T., Onda, D., Kikuchi, N., Kondo, Y., Matsubara, K., Amano, K., Jenkins, R.G., Hikida, Y., and Majima, R. 2008. Lithofacies and fossil assemblages of the Upper Cretaceous Sada Limestone, Shimanto City, Kochi Prefecture, Shikoku, Japan [in Japanese with English abstract]. Fossils, The Palaeontological Society of Japan 84: 47–60.
Nobuhara, T., Onda, D., Sato, T., Aosawa, H., Ishimura, T., Ijiri, A., Tsunogai, U., Kikuchi, N., Kondo, Y., and Kiel, S. 2016. Mass occurrence of the enigmatic gastropod Elmira in the Late Cretaceous Sada Limestone seep deposit in southwestern Shikoku, Japan. Paläontologische Zeitschrift 90: 701–722. Crossref
Nøttvedt, A. 1985. The Askeladden delta sequence (Palaeocene) on Spitsbergen: sedimentation and controls on delta formation. Polar Research 3: 21–48.
Nyborg, T. and Garassino, A. 2015. New fossil squat lobsters (Crustacea: Anomura: Munididae) from the Eastern Pacific. Palaeodiversity 8: 95–101.
Nyst, P.-H. 1843. Description des coquilles et des polypiers fossils des terrains Tertiaries de la Belgique. 676 pp. M. Hayes, Bruxelles.
Oakley, T.H., Wolfe, J.M., Lindgren, A.R., and Zaharoff, A.K. 2013. Phylotranscriptomics to bring the understudied into the fold: monophyletic Ostracoda, fossil placement, and pancrustacean phylogeny. Molecular Biology and Evolution 30: 215–233. Crossref
Oken, L. 1815. Lehrbuch der Naturgeschichte. Theil 3, Zoologie. Abteilung I, Fleischlose Thiere. 850 pp. A. Schmid, Jena.
Okutani, T., Fujikura, K., and Kojima, S. 1999. Two new hadal bivalves of the family Thyasiridae from the plate convergent area of the Japan Trench. Venus 58: 49–54.
Oliver, P.G. 2014. “Tubular gills” Extreme gill modification in the Thyasiroidea with the description of Ochetoctena tomasi gen. et sp. nov. (Bivalvia: Thyasiroidea). Zoosystematics and Evolution 90: 121–132. Crossref
Oliver, P.G., Rodrigues, C.F., Carney, R., and Duperron, S. 2013. Spinaxinus (Bivalvia: Thyasiroidea) from sulfide biogenerators in the Gulf of Mexico and hydrothermal vents in the Fiji Back Arc: chemosymbiosis and taxonomy. Scientia Marina 77: 663–676. Crossref
Olu, K., Sibuet, M., Harmegnies, F., Foucher, J.-P., and Fiala-Médioni, A. 1996. Spatial distribution of diverse cold seep communities living on various diapiric structures of the southern Barbados prism. Progress in Oceanography 38: 347–376. Crossref
Olsson, A.A. 1964. Neogene Mollusks from Northwestern Ecuador. 256 pp. Palaeontological Research Institution, Ithaca.
Peckmann, J., Kiel, S., Sandy, M.R., Taylor, D.G., and Goedert, J.L. 2011. Mass occurrences of the brachiopod Halorella in Late Triassic methane-seep deposits, eastern Oregon. Journal of Geology 119: 207–220. Crossref
Pelseneer, P. 1889. Sur la classification phylogénétique des pélécypodes. Bulletin Scientifique de la France et de la Belgique 20: 27–52.
Petersen, G.H. and Vedelsby, A. 2000. An illustrated catalogue of the Paleocene Bivalvia from Nuusquaq, Northwest Greenland: their paleoenvironments and the paleoclimate. Steenstrupia 25: 25–120.
Philippi, R.A. 1836. Enumeratio Molluscorum Siciliae. 303 pp. S. Schroppii, Berolini.
Planavsky, N.J., Rouxel, O.J., Bekker, A. Hoffman, A., Little, C.T.S., and Lyons, T.W. 2012. The iron isotope composition of some Archean and Proterozoic iron formations. Geochimica et Cosmochimica Acta 80: 158–169. Crossref
Pojeta, J. 1988. The origin and Paleozoic diversification of solemyoid pelecypods. New Mexico Bureau of Mines and Mineral Resources Memoir 44: 201–271.
Poli, G.S. 1795. Testacea utriusque Siciliae eorumque historia et anatome tabulis aenis illustrata. 264 pp. Rusconi & Parma, Chiaje, Neapoli.
Popenoe, W.P. 1937. Upper Cretaceous Mollusca from Southern California. Journal of Paleontology 11: 379–402.
Popiel-Barczyk, E. 1968. Upper Cretaceous terebratulids (Brachiopoda) from the Middle Vistula Gorge. Prace Muzeum Ziemi 12: 3–86.
Prezant, R.S. 1981. Taxonomic re-evaluation of the bivalve family Lyonsiidae. The Nautilus 95: 58–72.
Purchon, R.D. 1941. On the biology and relationships of the lamellibranch Xylophaga dorsalis (Turton). Journal of the Marine Biological Association of the United Kingdom 25: 1–39. Crossref
Rafinesque, C.S. 1815. Analyse de nature, ou tableau de l’univers et des corps organisées. 824 pp. De l’imprimerie de Jean Barravecchia, Palermo.
Reuss, J.D. 1801. Repertorium commentationum a societatis litterariis editarum Scientia Naturalis. Tom I. Historia Naturalis, Generalis et Zoologia. 574 pp. Apud Henricum Dietrich, Gottingae.
Robin, N., Van Bakel, B.W.M., Pacaud, J.-M., and Charbonnier, S. 2017. Decapod crustaceans from the Paleocene (Danian) of the Paris Basin (Vigny stratotype and allied localities) and a limpet palaeoassociation. Journal of Systematic Palaeontology 15: 257–273. Crossref
Robins, C.M. 2013. Systematics and Phylogeny of the Fossil Galatheoidea (Anomura, Decapoda): Uncovering Their Evolutionary Path. 439 pp. Dissertation, Kent State University, Kent.
Robins, C.M., Feldmann, R.M., and Schweitzer, C.E. 2012. The oldest Munididae (Decapoda: Anomura: Galatheoidea) from Ernstbrunn, Austria (Tithonian). Annalen des Naturhistorischen Museums in Wien, Serie A 114: 289–300.
Robins, C.M., Feldmann, R.M., and Schweitzer, C.E. 2013. Nine new genera and 24 new species of the Munidopsidae (Decapoda: Anomura: Galatheoidea) from the Jurassic Ernstbrunn Limestone of Austria, and notes on fossil munidopsid classification. Annalen des Naturhistorischen Museums in Wien, Serie A 115: 167–251.
Robins, C.M., Feldmann, R.M., Schweitzer, C.E., and Bonde, A. 2016. New families Paragalatheidae and Catillogalatheidae (Decapoda: Anomura: Galatheoidea) from the Mesozoic, restriction of the genus Paragalathea, and establishment of 6 new genera and 20 new species. Annalen des Naturhistorischen Museums in Wien, Serie A 118: 65–131.
Röding, P.F. 1798. Museum Boltenianium. Pars secunda continens Conchylia. viii + 199 pp. Trapp, Hamburg.
Rosenkrantz, A. 1920. Craniakalk fra Kjøbenhavn Sydhavn. Danmarks Geologiske Undersøgelse Række II 36: 1–79.
Rosenkrantz, A. 1942. Slægten Thyasiras geologiske Optræden. Meddelelser fra Dansk Geologisk Forening 10: 277–278.
Roy, A. van 1980. Pliothyrina, genre Terebratulide (Brachiopode) nouveau du Neogene. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique 52: 1–9.
Sacco, F. 1890a. I Molluschi dei terreni terziarii del Piemonte e della Liguria. Parte 8. Galeodoliidae, Doliidae, Ficulidae e Naticidae. Bollettino dei Musei di Zoologia ed Anatomia comparata della Reale Universita di Torino 5(86): 21–43.
Sacco, F. 1890b. Catalogo paleontologico del Bacino Terziario del Piemonte. Bollettino della Società Geologica Italiana 9 (2): 185–340.
Saether, K.P., Little, C.T.S., Campbell, K.A., Marshall, B.A., Collins, M., and Alfaro, A.C. 2010. New fossil mussels (Bivalvia: Mytilidae) from Miocene hydrocarbon seep deposits, North Island, New Zealand, with general remarks on vent and seep mussels. Zootaxa 2577: 1–45. Crossref
Sahni, M.R. 1925. Morphology and zonal distribution of some Chalk Terebratulids. Annals and Magazine of Natural History (series 9) 15: 353–385.
Samouelle, G. 1819. The Entomologist’s Useful Compendium, or an Introduction to the Knowledge of British Insects. 496 pp. Thomas Boys, London.
Sandy, M.R. 2010. Brachiopods from ancient hydrocarbon seeps and hydrothermal vents. In: S. Kiel (ed.), The Vent and Seep Biota. Aspects from Microbes to Ecosystems, 279–314. Springer, Dordrecht. Crossref
Sars, G.O. 1878. Bidrag til kundskaben om Norges arktiske fauna, I. Mollusca regionis arcticae Norvegiae. 466 pp. Brøgger, Christiania.
Saul, L.R. 1998. Eight aporrhaid gastropod species from the Cretaceous of the Pacific slope of North America and clarification of the type species of Perissoptera. The Nautilus 111: 119–142.
Schileyko, A.A. [Šilejko A.A.] 1989. Morphological evolution of the superoder Protobranchia (Bivalvia) and peculiarities of abyssal members of the group [in Russian]. Trudy Instituta Okeanologii 123: 81–101.
Schnetler, K.I. 2001. The Selandian (Paleocene) mollusc fauna from Copenhagen, Denmark: the Poul Harder 1920 collection. Geological Survey of Denmark Bulletin 37: 1–85.
Schnetler, K.I. and Nielsen, M.S. 2018. A Palaeocene (Selandian) molluscan fauna from boulders of Kerteminde Marl in the gravel-pit at Gundstrup, Fyn, Denmark. Cainozoic Research 18: 3–81.
Schulte, P., Alegret, L., Arenillas, I. Arz, A.J., Barton, J.P., Bown, R.P., Bralower, T.J., Christeson, G.L., Claeys, P., Cockell, C.S., Collins, G.S., Deutsch, A., Goldin, T.J., Goto, K., Grajales-Nishimura, J.M., Grieve, R.A.F. Gulick, S.P.S., Johnson, K.R., Kiessling, W., Koeberl, C., Kring, D.A., MacLeod, K.G., Matsui, T. Melosh, J. Montanari, A., Morgan, J.V., Neal, C.R, Nichols, D.J., Norris, R.D., Pierazzo, E., Ravizza, G.,Rebolledo-Yieyra, M., Reimold, W.U., Robin, E., Salge, T., Speijer, R.P., Sweet, A.R., Fucugauchi, J.U., Vajda, V., Whalen, M.T., and Willumsen, P.S. 2010. The Chicxulub asteroid impact and mass extinction at the Cretaceous–Paleogene boundary. Science 327: 1214–1218. Crossref
Schumacher, C.F. 1817. Essai d’un nouveau système des habitations des vers testacés. 287 pp. 22 pls. De I’impremerie de Mr. le Directour Schultz, Copenhague.
Schwartz, H., Sample, J., Weberling, K.D., Minisini, D., and Casey Moore, D. 2003. An ancient linked fluid migration system: cold seep deposits and sandstone intrusions in the Panoche Hills, California, USA. Geo-Marine Letters 23: 340–350. Crossref
Schweitzer, C.E. and Feldmann, R.M. 2000. First notice of the Chirostylidae (Decapoda) in the fossil record and new Tertiary Galatheidae (Decapoda) from the Americas. Bulletin of the Mizunami Fossil Museum 27: 147–165. Crossref
Schweitzer, C.E. and Feldmann, R.M. 2008. New Eocene hydrocarbon seep decapod crustacean (Anomura: Galatheidae: Shinkaiinae) and its paleobiology. Journal of Paleontology 82: 1021–1029.
Schweitzer, C.E., Feldmann, R.M., Ćosović, V., Ross, R.L.M., and Waugh, D.A. 2009. New Cretaceous and Eocene Decapoda (Astacidea, Thalassinidea, Brachyura) from British Columbia, Canada. Annals of Carnegie Museum 77: 403–423.
Schweitzer, C.E., Feldmann, R.M., Garassino, A., Karasawa, H., and Schweigert, G. 2010. Systematic list of fossil decapod crustacean species. Crustaceana Monographs 10: 1–230. Crossref
Scopoli, G.A. 1777. Introductio ad historiam naturalem sistens genera lapidum, plantarum, et animalium :hactenus detecta, caracteribus essentialibus donata, in tribus divisa, subinde ad leges naturae. 540 pp. Gerle, Pragae. Crossref
Segerberg, K.O. 1900. De anomura och brachyura dekapoderna inom Skandinaviens Yngre krita. Geologiska Föreningen I Stockholm Förhandlingar 22: 347–394.
Sellanes, J., Quiroga, E., and Neira, C. 2008. Megafauna community structure and trophic relationships at the recently discovered Concepcion Methane Seep Area, Chile, 36°S. ICES Journal of Marine Science 65: 1102–1111. Crossref
Shumard, B.F. 1861. Description of new Cretaceous fossils from Texas. Proceedings of the Boston Society of Natural History 8: 188–205.
Sibuet, M. and Olu, K. 1998. Biogeography, diversity and fluid dependence of deep sea cold-seep communities at active and passive margins. Deep Sea Research II 45: 517–567. Crossref
Siebold, C.T. von 1848. Lehrbuch der vergleichenden Anatomie der Wirbellosen Thiere. Erster Theil. In: C.T. von Siebold and H. Stannius (eds.), Lehrbuch der vergleichenden Anatomie. 679 pp. Verlag von Veit & Comp., Berlin.
Signor III, P.W. and Lipps, J.H. 1982. Sampling bias, gradual extinction patterns, and catastrophes in the fossil record. In: L.T. Silver and P.H. Schultz (eds.), Geological Implications of Impacts of Large Asteroids and Comets on the Earth, 291–296. Geological Society of America, Penrose. Crossref
Símonarson, L.A. 1974. Recent Cyrtodaria and its fossil occurrence in Greenland. Bulletin of the Geological Society of Denmark 23: 65–75.
Smith, C.S. and Baco, A.R. 2003. Ecology of whale falls at the deep-sea floor. Oceanography and Marine Biology: An Annual Review 41: 311–354.
Sohl, N.F. 1964. Neogastropoda, Opisthobranchia and Basommatophora from the Ripley, Owl Creek, and Prairie Bluff Formations. United States Geological Survey Professional Paper 331B: 153–344.
Sowerby, J. de C. 1823. The Mineral Conchology of Great Britain, Vol. 4. pp. 115–160. W. Ardling, London.
Speden, I.G. 1970. The type Fox Hills Formation, Cretaceous (Maestrichtian), South Dakota. Part 2. Systematics of the Bivalvia. Peabody Museum of Natural History, Yale University Bulletin 33: 1–222.
Spengler, L. 1793. II. Beskrivelse over et nyt Slaegt av de toskallede Koknkylier, forben af mig kaldet Chaena, saa og over det Linnéiske Slaegt Mya, Hvilket nøiere bestemmes, og inddeles i tvende Slaegter. Skrivter af Naturhistorieselskabet 3: 16–59.
Spielhagen, R.F. and Tripati, A. 2009. Evidence from Svalbard for near-freezing temperatures and climate oscillations in the Arctic during the Paleocene and Eocene. Palaeogeography, Palaeoclimatology, Palaeoecology 278: 48–56. Crossref
Stanton, T.W. 1901. The marine Cretaceous invertebrates. In: W.B. Scott (ed.), Report of the Princeton University to Patagonia, 1896–1899, Paleontology 1, Part. 1, Vol. 4, 1–43. Princeton University Press and Schweizerbart, Princeton and Stuttgart.
Steinich, G. 1965. Die artikulaten Brachiopoden der Rügener Schreibkreide (Unter-Maastricht). Paläontologische Abhandlungen 2 (1): 1–220.
Stenzel, H.B., Krause, E.K., and Twining, J.T. 1957. Pelecypoda from the type locality of the Stone City beds (Middle Eocene) of Texas. The University of Texas Publication 5704: 3–196.
Stephenson, L.W. 1923. The Cretaceous formations of North Carolina. Part 1. Invertebrate fossils of the Upper Cretaceous formations. North Carolina Geological and Economic Survey 5: 1–408.
Stephenson, L.W. 1941. The larger invertebrate fossils of the Navarro Group of Texas. The University of Texas Publication 4101: 1–641.
Stewart, R.B. 1930. Gabb’s California Cretaceous and Tertiary type lamellibranchs. Academy of Natural Sciences of Philadelphia Special Publications 3: 1–314.
Stimpson, W. 1860. Notes on North American Crustacea, in the Museum of the Smithsonian Institution, No. II. Annals of the Lyceum of Natural History of New York 7: 177–246.
Strauch, F. 1972. Phylogenese, adaptation und migration einiger nordischer mariner Molluskengenera (Neptunea, Panomya, Cyrtodaria und Mya). Abhandlungen der Senckenbergoschen Naturforschenden Gesellschaft 531: 1–211.
Swainson, W. 1840. A Treatise on Malacology or Shells and Shell-fish. 419 pp. Longman, London.
Taylor, J.D. and Glover, E.A. 2005. Cryptic diversity of chemosymbiotic bivalves: A systematic revision of worldwide Anodontia (Mollusca: Bivalvia: Lucinidae). Systematics and Biodiversity 3: 281–338. Crossref
Taylor, J.D. and Glover, E.A. 2010. Chemosymbiotic bivalves. In: S. Kiel (ed.), The Vent and Seep Biota. Aspects from Microbes to Ecosystems, 107–136. Springer, Dordrecht. Crossref
Taylor, J.D., Glover, E.A., and Williams, S.T. 2008. Ancient chemosynthetic bivalves: systematics of Solemyidae from eastern and southern Austalia. Memoirs of the Queensland Museum, Nature 54: 75–104.
Taylor, J.D., Glover, E.A., Smith, L., Dyal, P., and Williams, S.T. 2011. Molecular phylogeny and classification of the chemosymbiotic bivalve family Lucinidae. Zoological Journal of the Linnean Society 163: 15–49. Crossref
Thomsen, E. 2013. Den eneste i verden – en unik fossil fisk fra Spitsbergen. Ottar 298: 41–45.
Thuy, B., Gale, A.S., Kroh A., Kucera, M., Numberger-Thuy, L.D., Reich, M., and Stöhr, S. 2012. Ancient origin of the modern deep-sea fauna. PLoS ONE 7 (10): e46913. Crossref
Thuy, B., Kiel, S., Dulai, A., Gale, A.S., Kroh, A., Lord, A.R., Numberger-Thuy, L.D., Stöhr, S., and Wisshak, M. 2014. First glimpse into Lower Jurassic deep-sea biodiversity: in-situ diversification and resilience against extinction. Proceedings of the Royal Society B281: 20132624. Crossref
Tripati, A., Zachos, J., Marincovich Jr, L., and Bice, K. 2001. Late Paleocene Arctic coastal climate inferred from molluscan stable and radiogenic isotope ratios. Palaeogeography, Palaeoclimatology, Palaeoecology 170: 101–113. Crossref
Turner, R.D. 2002. On the subfamily Xylophagainae (family Pholadidae, Bivalvia, Mollusca). Bulletin of the Museum of Comparative Zoology 157: 223–307.
Turton, W. 1822. Conchylia dithyra Insularum britannicarum. The Bivalve Shells of the British Islands. 279 pp. M.A. Natali, London. Crossref
Valentich-Scott, P., Powell II, C.L., Lorenson, T.D., and Edwards, D.E. 2014. A new genus and species of Thyasiridae (Mollusca, Bivalvia) from deep water, Beaufort Sea, northern Alaska. ZooKeys 462: 11–26. Crossref
Van Dover, C.L. 2000. The Ecology of Deep-sea Hydrothermal Vents. 424 pp. Princeton University Press, Princeton.
Verrill, A.E. and Bush, K.J. 1897. Revision of the genera of Ledidae and Nuculidae of the Atlantic Coast of the United States. American Journal of Science Series 4 3: 51–63. Crossref
Vonderbank, K. (ed.) 1970. Geologie und Fauna der tertiären Ablagerungen Zentral-Spitsbergens. Norsk Polarinstitutt Skrifter 153: 5–119.
Vrijenhoek, R.C. 2013. On the instability and evolutionary age of deep-sea chemosynthetic fauna. Deep-Sea Research II 92: 189–200. Crossref
Waagen, W. 1883. Salt Range fossils, volume 1, Productus Limestone fossils, Brachiopoda. Memoirs of the Geological Survey of India, Palaeontologia Indica 13: 391–546.
Wade, B. 1926. The Fauna of the Ripley Formation on Coon Creek, Tennessee. United States Geological Survey Professional Paper 137: 1–272. Crossref
Ward, D.J. 1978. The Lower London Tertiary (Palaeocene) succession of Herne Bay, Kent. Institute of Geological Sciences Report 78 (10): 1–12.
Withers, T.H. 1953. British Museum (Natural History). Catalogue of the Fossil Cirripedia in the Department of Geology Vol. 3. Tertiary. 396 pp. Bartholomew Press, Dorking.
Wolff, T. 1979. Macrofaunal untilization of plant remains in the deep sea. Sarsia 64: 117–136. Crossref
Woods, H. 1900. A monograph of the Cretaceous Lamellibranchia of England. Part. 2. Palaeontographical Society Monographs 54: 73–112. Crossref
Zachos, J., Röhl, U., Schellenberger, S.A., Sluijs, A., Hodell, D.A., Kelly, C.D., Thomas, E., Nicolo, M., Raffi, I., Lourens, L.J., McCarren, H., and Kroon, D. 2015. Rapid acidification of the ocean during the Paleocene–Eocene Thermal Maximum. Science 308: 1611–1615. Crossref
Acta Palaeontol. Pol. 64 (1): 101–141, 2019
https://doi.org/10.4202/app.00554.2018