

Reproductive phases of Miocene algae from central Paratethys and their bearing on systematics
JURAJ HRABOVSKÝ
Hrabovský, J. 2019. Reproductive phases of Miocene algae from central Paratethys and their bearing on systematic. Acta Palaeontologica Polonica 64 (2): 417–439.
Coralline algae were widespread in shallow-water environments during the middle Miocene in the central Paratethys. Their distribution and diversity patterns are useful in paleoenvironmental and paleoclimatic studies providing that species identifications are accurate and robust. Taxonomy of fossil coralline algae was traditionally based on morphology of bi/tetrasporophytes whereas taxonomy of extant species is additionally supported by characters that can be observed in the remaining life cycle phases. Therefore, investigations of such characters in fossil record are also essential to avoid misidentifications of ancient coralline algae. Here, I analyse morphology of reproductive phases in subfamilies Hydrolithoideae and Mastophoroideae from the middle Miocene of the central Paratethys. Analyses of published and newly collected hydrolithoid and mastophoroid coralline algae allow: (i) identification of three species of the genus Hydrolithon and two species of the genus Lithoporella; (ii) description of complete life cycle phases for extinct Hydrolithon lemoinei, H. corculumis, and Hydrolithon sp. 1 and (iii) description of incomplete life cycles for Lithoporella minus and Lithoporella sp. 1. The results also prove that characters associated with trichocytes can be used in some fossil coralline algae, and bi/tetrasporic pore canal anatomy is reliable diagnostic character also for fossil species, as it is used in extant coralline algae of the order Corallinales.
Key words: Corallinales, Hydrolithon, Lithoporella, reproductive phases, Miocene, central Paratethys, Slovakia, Romania.
Juraj Hrabovský [geoljuhr@savba.sk], Earth Science Institute of the Slovak Academy of Sciences, Dúbravská cesta 9, 84005 Bratislava, Slovakia.
Received 4 December 2018, accepted 15 March 2019, available online 10 May 2019.
Copyright © 2019 J. Hrabovský. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Coralline algae are commonly dominant rock-building organisms of the middle Miocene red-algal limestone (Schaleková 1962, 1978; Pisera 1985, 1996; Studencki 1988a, 1999; Doláková et al. 2008). The presence of coralline algae in analysed fossil associations is of high importance for palaeoecological analyses as their distribution is largely controlled by light and temperature (Adey and McKibbin 1970; Adey et al. 1982; Athanasiadis and Ballantine 2014). The composition of a rich coralline algae limestone is variable in the local and regional scales of the central Paratethys (Baráth 1992; Baráth et al. 1994; Pisera 1996; Riegl and Piller 2000). Recent Adriatic Sea, Tyrrhenian Sea, or Eastern Mediterranean Sea assemblages are perceived as analogues to the late Badenian (early Serravallian) Paratethyan assemblages (Pisera 1985; Seneš and Ondrejčíková 1991; Basso 1998; Basso et al. 2008). In contrast, coral carpets or patch reefs occurring mainly in the early Badenian (Langhian) deposits indicate rather warmer climate conditions during the middle Miocene climatic optimum with analogues in the Recent Red Sea (Riegl and Piller 2000). Despite many analyses from the last century (Schaleková 1969, 1973 and 1978; Schaleková and Čierna 1985; Golonka 1981; Pisera 1985; Pisera and Studencki 1989; Studencki 1988b, Zdražílková 1988), temporal and spatial changes in coralline algae diversity and distribution remain poorly known in the Miocene of the central Paratethys. This insufficiency in our knowledge has several reasons, including: (i) taxonomic changes caused by the type collections revision, new analytic techniques and discrimination of new diagnostic characters were applied to few taxa only (Woelkerling 1988; Braga et al. 1993; Basso et al. 1996); (ii) molecular taxonomy results can sometimes contradict morphology-based taxonomy (Penrose and Woelkerling 1992; Harvey et al. 2006; Bahia et al. 2014; Kato et al. 2011; Rösler et al. 2016); (iii) differences in the treatment of morphological characters between biologists and palaeontologists: classification of fossil coralline algae is typically based on bi/tetrasporophytes morphology, whereas extant species taxonomy is also supported by characters of other reproductive phases (Braga et al. 2005; Athanasiadis et al. 2004; Athanasiadis and Ballantine 2014; Bahia et al. 2015). The recognition and detection of gametangial and carposporangial life cycles stages in fossil coralline algae can solve some of these difficulties (Basso et al. 1996; Basso and Rodondi 2006; Hrabovský et al. 2015; Chelaru and Bucur 2016). Therefore, revision of published data and analyses of the newly collected material from the central Paratethys scoped on gametophyte, carposporophyte bearing plants and bi/tetrasporophyte life phases are necessary.
Life cycle phases morphology of fossil coralline algae were reported only sporadically in the 20th century (Conti 1943; Mastrorilli 1968). However, since the end of the 20th century fossil specimens of the genera Lithophyllum (Basso et al. 1996), Hydrolithon (Woelkerling et al. 2012), Neogoniolithon (Quaranta et al. 2007), Spongites (Chelaru and Bucur 2016), Lithothamnion (Chelaru and Bucur 2016), Phymatolithon (Basso et al. 1997; Hrabovský et al. 2015), Mesophyllum (Hrabovský et al. 2015; Coletti et al. 2018), and in the Sporolithon (Vannucci et al. 2000; Hrabovský et al. 2015; Chelaru and Bucur 2016) have been documented. The mode of the gametangial conceptacles development is comparable to bi/tetrasporangial conceptacles in some coralline algal genera formerly assigned to the subfamily Mastophoroideae Setchell, 1943. Many of these genera were assumed to represent bi/tetrasporophytes (Quaranta et al. 2007; Basso et al. 1996; Basso and Rodondi 2006). Bi/tetrasporophytes with this type of conceptacles inhabit shallow to medium depths in the Recent tropics, subtropics and warm temperate seas (Aguirre et al. 2000; Adey et al. 1982). Therefore, misidentification of gametangial with bi/tetrasporangial conceptacles could distort an information about coralline algae diversity and distribution.
Here, I focus on the genera from the subfamilies Hydrolithoideae Kato and Baba in Kato et al., 2011 and Mastophoroideae Setchell, 1943 from the middle Miocene of the Carpathian Foredeep, Vienna Basin, Danube Basin, Novohrad Basin, and Transylvania Basin. The work purpose is to: (i) describe specimens both in terms of gametangial and carposporangial phases as is possible, (ii) discriminate species among a members belonging to the subfamilies Hydrolithoideae and Mastophoroideae in the fossil record and (iii) to provide the identification key for fossil hydrolithoid and mastophoroid species.
Institutional abbreviations.—NHM, Natural History Museum; VEGA, Vedecká Grantová Agentúra (Scientific Grant Agency) internal grant system of the Ministry of Education, Research and Sport of the Slovak Republic, and Slovak Academy of Sciences.
Other abbreviations.—D, diameter; H, height; L, length; MSi, Miocene Stable isotopes (event); SD, standard deviation; TS, thin section(s); W, wide.
Material and methods
Studied material represents: (i) the collection published in Schaleková (1969, 1973, 1978), Schaleková and Čierna (1985), Novák (1975), and Zdražílková (1988), (ii) new samples from selected localities in the Central Paratethys, including Lopadea Veche in Romania (Transylvania Basin), Maksymivka in Ukraine (Carpathian Foredeep), Modrý Majer, Sazdice, and Veľký Pesek in Slovakia (Danube Basin), Devínska Kobyla, Vývrat, Rohožník, and Stupava, in Slovakia (Vienna Basin) and (iii) personal collections of Daniel Pivko from Department of Paleontology and Geology of the Comenius University in Bratislava. The material published in Pisera (1985) from Roztocze Hills was examined also. Within processing of Roztocze Hills material, I focused only on specimens known as Lithophyllum corculumis Maslov, 1962 and Lithophyllum (Dermatolithon) nataliae Maslov, 1956 and compared it with the type and identified specimens from the other localities to examine the species distribution during the middle Miocene (Fig. 1). Examined material is listed in the Table 1.
Table 1. The list of the studied material. Numbers indicate number of thin sections.
|
Locality |
Reference |
|||
|
this paper |
||||
|
Vienna Basin |
||||
|
Devínska Kobyla |
28 |
|
28 |
44 |
|
Stupava |
4 |
|
|
|
|
Vývrat |
7 |
|
|
|
|
Rohožník |
17 |
|
50 |
|
|
Wolfsthal |
8 |
|
|
|
|
Mikulov |
3 |
|
|
|
|
Hlohovec |
1 |
|
|
|
|
Müllendorf |
2 |
|
|
|
|
Danube Basin |
||||
|
Modrý Majer |
9 |
|
27 |
|
|
Kamenica nad |
|
|
13 |
|
|
Pavlová |
|
|
4 |
|
|
Čierny Hill |
|
|
11 |
|
|
Štúrovo |
4 |
|
|
|
|
Veľký Pesek |
7 |
|
|
|
|
Sazdice |
5 |
|
|
|
|
Novohrad Basin |
||||
|
Kosihovce |
|
|
8 |
|
|
Transylvania Basin |
||||
|
Lopadea Veche |
48 |
|
|
|
|
Carpathian Foredeep |
||||
|
Pratecký Vrch |
|
23 |
|
|
|
Lomnice |
|
7 |
|
|
|
Blučina |
|
4 |
|
|
|
Telnice |
|
1 |
|
|
|
Holubice |
|
2 |
|
|
|
Vyškov |
|
1 |
|
|
|
Ochoz |
|
1 |
|
|
|
Kralice nad Oslavou |
1 |
|
|
|
|
Rebešovice |
3 |
3 |
|
|
|
Hostim |
1 |
|
|
|
|
Hluchov |
1 |
|
|
|
|
Židlochovice |
|
12 |
|
|
|
Maksymivka |
7 |
|
|
|
Fig. 1. Modified geographical and geological map of the studied area with Recent distribution of central Paratethyan deposits, redrawn from Kováč (2000). List of studied sites: 1, Rohožník; 2, Vývrat; 3, Stupava; 4, Devínska Kobyla; 5, Wolfsthal; 6, Müllensdorf; 7, Mikulov; 8, Židlochovice; 9, Blučina; 10, Rebešovice; 11, Telnice; 12, Pratecký Vrch; 13, Holubice; 14, Vyškov; 15, Hluchov; 16, Přemyslovice; 17, Lomnice; 18,- Ochoz; 19, Kralice nad Oslavou; 20, Hostim; 21, Modrý Majer; 22, Čierny Hill; 23, Kamenica nad Hronom; 24, Pavlová; 25, Veľký Pesek; 26,- Sazdice; 27, Štúrovo; 28, Kosihovce; 29, Maksymivka; 30, Lopadea Veche; 31, Weglinek; 32, Zagrody.
The species of Hydrolithon and Lithoporella are identified in newly collected samples and Schaleková’s collection (Schaleková 1969, 1973, 1978; Schaleková and Čierna 1985). All described specimens are stored in Natural History Museum, Bratislava, Slovakia.
VEGA project 2/0122/18 have supported present research where specimens from historical collections and newly collected samples are analysed. Specimens with acronym VEGA in the front of the thin section numbers and assembled during this project will be relocated to NHM after the project is finished.
The observations were performed by light microscope AXIOZEISS scope A1. Figures were produced by AXIOCAM 105 Color. Specimens were measured in AxioVision Microscopy Software. Thin sections displaying specimens of Hydrolithon (Foslie, 1905) Foslie, 1909 and Lithoporella (Foslie, 1902) Foslie, 1909 are listed in Table 2. The two genera are represented by 79 specimens described below and were detected in 56 TS and arranged according to the identification key (Table 3).
Table 2. List of the thin sections containing identified specimens, chronostratigraphy and litostratigraphic units. Abbreviations: n, number of specimens; TS, thin section; “–” not found.
|
Age |
Formation |
Basin |
Locality |
Hydrolithon
corculumis |
Hydrolithon
lemoinei |
Hydrolithon
sp. 1 |
Lithoporella minus n = 34 |
Lithoporella sp. 1 n = 4 |
|
TS |
TS |
TS |
TS |
TS |
||||
|
late |
Vráble |
Danube Basin |
Veľký Pesek |
– |
– |
– |
37913161, 37913261, 37913361, 37913661 |
– |
|
Holíč |
Vienna Basin |
Devínska Kobyla |
– |
– |
– |
7114161, 7114261 |
– |
|
|
Skalica |
Vienna Basin |
Wolfsthal |
– |
– |
– |
381213162, 381213262, 381213362, 381213562, 381213662, 381213762, 381213862 |
– |
|
|
early |
Studienka |
Vienna Basin |
Rohožník |
29111653, 2911353, 29111553, Vz.19, 5100, 5104, 5109 |
5227, |
– |
29711453, 5099, 5102, 5105, 5107, 5111, 5233 |
5233, 5111 |
|
Devínska Kobyla |
406, 422 |
DKc |
– |
Dke, K3b, K3d, ZTK3a |
– |
|||
|
Vývrat |
– |
– |
– |
371011452 |
371111453 |
|||
|
Kosiv |
Carpathian Foredeep |
Maksymivka |
21510253, 21510553 |
– |
21510853, 21510953 |
21510253, 21510153 |
– |
|
|
late |
Jakubov |
Vienna Basin |
Stupava |
– |
– |
– |
– |
31011152 |
|
early |
Gârbova de Sus |
Transylvania Basin |
Lopadea Veche |
184911151, 1849116051, 184911651 |
184911651, 1849115451, 1849113351, 1849114051, 1849114951, 1849114351 |
– |
18491115, 1849115651 |
– |
|
Príbelce |
Novohrad Basin |
Kosihovce |
– |
Vz.20 |
– |
– |
– |
|
|
Bajtava |
Danube Basin |
Kamenica nad Hronom |
6201 |
6174 |
– |
– |
– |
|
|
Pavlová |
– |
IIIb458 |
– |
– |
– |
Table 3. Identification key for the genera Hydrolithon and Lithoporella from the central Paratethys.
|
Bi/tetrasporic conceptacles uniporate with roof formed by cells perpendicularly oriented to the chamber; dimerous construction and primigenous filaments without palisade cells; trichocytes are not arranged in large tightly packed horizontal fields....................Hydrolithon |
|
Bi/tetrasporic conceptacles uniporate with roof formed by cells perpendicularly oriented to the chamber; dimerous construction and primigenous filaments with palisade cells; Trichocytes are not arranged in large tightly packed horizontal fields ..................... Lithoporella |
|
|
|
Bi/tetrasporic conceptacles markedly
project above the thallus surface; they possess long and wide
pore canal lined by papillae |
|
Bi/tetrasporic conceptacles slightly
protrude above thallus surface; short cone-shaped pore canal
is lined by three celled filaments |
|
Bi/tetrasporic conceptacles slightly
project above thallus surface; pore canal is long and
cylindrical not lined by papillae; |
|
|
|
Thallus entirely bistratose;
multicellular postigenous filaments occur only at the margins
of conceptacles or on the places |
|
Thallus mostly multicellular in
section; primigenous filaments are elongated with
height/length ratio that could reach up to 3–6; |
In order to detect specimens fulfilling the modern diagnoses of Hydrolithon and Lithoporella 396 thin sections (excluding Roztocze Hills material) were analysed. Emended diagnoses of these genera include morpho-anatomical characters listed in Kato et al. (2011) and Rösler et al. (2016). The genus Hydrolithon encompasses specimens: (i) producing bi/tetrasporangial conceptacles with roofs formed by filaments peripheral to the fertile area and interspersed among sporangial initials, (ii) dimerous thallus with (iii) primigenous filaments consisting of non-palisade cells, (iv) cells laterally joined with secondary lateral fusions but secondary pits are absent, and (v) trichocytes (if are presented) are not arranged in large tightly packed horizontal fields. The genus Porolithon Foslie, 1909 is similar in many characters to Hydrolithon because it is characterized by bi/tetrasporangial conceptacles formed by initials peripheral and interspersed among sporangial initials and cells laterally joined with fusions while secondary pits are absent. However, these genera differ in the mode of trichocytes arrangement. Porolithon trichocytes are arranged in large tightly packed horizontal fields and Hydrolithon lacks this arrangement (Kato et al. 2011; Rösler et al. 2016). Also, presence of dimerous rather than monomerous construction and single epithallial cells terminating the filaments is characteristic for Hydrolithon (Kato et a. 2011; Rösler et al. 2016). However, some species of Porolithon develop dimerous, entirely bi-stratose thallus (Bahia et al. 2014). Another genus comparable to Hydrolithon is Harveylithon Rösler, Perfectti, Peña, and Braga, 2016. Both possess trichocytes which are not arranged in tightly packed horizontal fields but Harveylithon is mostly of monomerous thallus bearing solitary trichocytes (Rösler et al. 2016).
Genus Lithoporella is characterized by having: (i) bi/tetrasporangial conceptacles with roofs developed from filaments peripheral to fertile area and interspersed among sporangial initials, (ii) the thin thallus with dimerous construction, (iii) palisade cells in primigenous filaments (iv) laterally connected cells with fusions and (v) trichocytes arranged solitary or in groups. Type of the bi/tetrasporangial conceptacle development is the same as in Hydrolithon and Porolithon (Turner and Woelkerling 1982; Kato et al. 2011, Rösler et al. 2016). Considering the primigenous filaments, palisade cells are 2–4 times greater in height than their length (Woelkerling 1988; Harvey et al. 2006). The growth form was described according to Woelkerling et al. (1993). Description of basic morpho-anatomical characters follows Hrabovský et al. (2015).
Vegetative features were measured in the thalli cut longitudinally to the filaments growth axis. Cells dimensions observed in the sections are affected by many factors: (i) cut orientation, (ii) seasonal or lunar cycles, (iii) uneven substrate and (iv) protuberance presence. The cells in the longitudinal sections are mostly rectangular, flattened, square or polygonal while in transverse sections are polygonal and show honeycomb structure. Measurement errors may arise from an improper cuts as well as from ignorance of shortest and longest cells, significantly different from apparent average values. To minimalize statistical error, cells were measured in selected filaments longitudinally sectioned from the base of the filament to its top, wherever possible. The mean values and standard deviation calculated for each character are accompanied with the number of measurements in Systematic Palaeontology chapter. Following Basso et al. (1996) and Irvine and Chamberlain (1994) cells length is length (L) between two primary pit connections, diameter (D) denotes distance between the cell walls given by axis perpendicular to the L. Height (H) measured in primigenous filaments cells is a distance between the cell’s basal and roof wall.
Identification of life cycle phases are going together with palaeontological methods including morpho-anatomical characters comparison of plants bearing different types of the conceptacles (e.g., Basso et al. 1996; Quaranta et al. 2007; Hrabovský et al. 2015). Because of poor fossilization potential of reproductive organs and its absence in studied material also, types and the dimensions of the conceptacles are used for life cycle phase identification. Bi/tetrasporic conceptacles of Hydrolithon and Lithoporella are formed from initials peripheral to and interspersed among sporangial initials. As a result roof cells are perpendicularly oriented to the chamber and longitudinally with the pore canal (Johansen 1981). Male (spermatangial) conceptacles are commonly smaller triangular or of the same dimensions as female (carpogonial) conceptacles and both are smaller than carposporangial in the genera Hydrolithon and Lithoporella (Irvine and Chamberlain 1994; Harvey et al. 2006). Moreover, carpogonial conceptacles can possess central columella—remains of carpogonial branches, while spermatanigal have flat floor (Irvine and Chamberlain 1994; Harvey et al. 2006; Chelaru and Bucur 2016). Excluding bi/tetrasporic conceptacles, the rest are formed from initials peripheral to fertile area. As a result, roof cells run parallel with the chamber and bent upward to develop the pore canal (Johansen 1981).
All samples belong to the Langhian or Serravallian stages corresponding to the Badenian (Langhian to early Serravallian) and the Sarmatian (late Serravallian) of the CP (Fig. 2). According to Kováč et al. (2007) the Badenian base corresponds to the first appearance of Praeorbulina at 16.3 Ma, while end of the Badenian was calibrated on the basis of astronomical cycles and isotope event MSi-3 at 12.7 Ma and marked by different Sarmatian endemic fauna. The Langhian/Serravallian boundary corresponds to the early and late Badenian boundary and is characterized by the last occurrence of Sphenolithus heteromorphus (13.65 Ma). The Serravallian/Tortonian, the Sarmatian/Pannonian boundaries are dated to 11.6 Ma and correspond with glacioeustatic sea-level lowstand of the cycle Transylvania Basin 3.1 (Harzhauser and Piller 2004, 2007).
Fig. 2. Stratigraphical chart with standard chronostratigraphy and regional stages for central Paratethys. Grey area covers the time interval in which studied sites are included.
Systematic palaeontology
Phylum Rhodophyta Wettstein, 1901
Class Florideophyceae Cronquist, 1960
Subclass Corallinophycidae Le Gall and Saunders, 2007
Order Corallinales Silva and Johansen, 1986
Family Hydrolithaceae Townsend and Huisman, 2018
Subfamily Hydrolithoideae Kato and Baba in Kato et al., 2011
Genus Hydrolithon (Foslie, 1905) Foslie, 1909
Type species: Hydrolithon boergesenii (Foslie, 1901) Foslie 1909; St. Crois, S. Virgin Island, Recent.
Hydrolithon corculumis (Maslov, 1962) Braga, Bassi, Zakrevskaya, and Petrovna-Radionova, 2005
Fig. 3A–C, Table 4.
1978 Lithophyllum corculumis Maslov, 1962; Schaleková 1978: 123, pl. 29: 2.
1985 Lithophyllum corculumis Maslov, 1962; Schaleková and Čierna 1985: 45, pl. 2: 2.
1985 Lithophyllum corculumis Maslov, 1962; Pisera 1985: 104, pl. 24: 1–4.
2013 Lithophyllum sp.; Hrabovský 2013: 29, pl. 3: h.
Material.—Bi/tetrasporangial plant (Fig. 3A), NHM B1833/1 (thin section 21510253), early Serravallian reef limestone from Maksymivka, Ukraine (Carpathian Foredeep). Gametophyte bearing spermatangial and carpogonial-carposporangial conceptacles, NHM B1832 (thin section 21510553), the same locality as bi/tetrasporophyte. Carpogonial plant, NHM B1835 (thin section 1849116051), early Langhian, Lopadea Veche, Romania (Transylvania Basin). Other examined specimens are: NHM B1836 (thin section 422), early Serravallian, Devínska Kobyla, Slovakia (Vienna Basin), NHM B1837 (thin section 5100) and NHM B1841 (thin section 29711353), early Serravallian, Rohožník, Slovakia (Vienna Basin), NHM B1840 (thin section 6201), Early Langhian, Kamenica nad Hronom, Slovakia (Danube Basin), NHM B1839/1 (thin section 184911651) and NHM B1839/1 (thin section 184911151), early Langhian, Lopadea Veche, Romania (Transylvania Basin) (Table 4).
Table 4. Comparative table of selected Hydrolithon corculumis (Maslov, 1962) Braga, Bassi, Zakrevskaya, and Petrovna-Radionova, 2005 specimens. * marks gametangial or carpogonial-carposporangial plants. Abbreviations: D, diameter; H, height; L, length; W, wide; nd, no data; “+” presence and “–” absence of character. All measurements in μm.
| |
Transylvania Basin |
Carpathian Foredeep |
Vienna Basin |
Danube Basin |
|||||
|
Specimen number (NHM) |
B1853 |
B1839/1 |
B1833/1 |
B1832 |
B1841 |
B1836 |
B1837 |
B1840 |
|
|
Thin section |
1849116051* |
184911151 |
21510253 |
21510553* |
2911353 |
422 |
5100 |
6201 |
|
|
Habitat |
littoral |
littoral |
littoral |
littoral |
littoral |
littoral |
littoral |
littoral |
|
|
Growth form |
encrusting |
encrusting |
encrusting |
encrusting |
encrusting |
encrusting |
encrusting |
encrusting |
|
|
Applanate branches |
– |
– |
– |
– |
– |
– |
– |
– |
|
|
Thallus thickness |
75–200 |
174–280 |
233–414 |
up to 400 |
up to 500 |
36–220 |
45–596 |
145–269 |
|
|
Primigenous filaments |
|||||||||
|
cells shape |
rectangular flattened square |
rectangular flattened square |
rectangular flattened square |
nd |
nd |
rectangular flattened square |
rectangular flattened square |
flattened |
|
|
H/L ratio |
0.5–1.8 |
0.8–1.6 |
0.8–1.9 |
0.8–1.6 |
0.8–1.7 |
0.5–1.2 |
|||
|
cells |
H L |
7–16 6–13 |
8–20 8–14 |
9–22 7–16 |
9–14 9–13 |
10–18 8–14 |
6–17 9–16 |
||
|
laterall cell fusion |
+ |
+ |
+ |
+ |
+ |
+ |
|||
|
Postigenous filaments |
|||||||||
|
multicellular |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
cells shape |
rectangular flattened |
rectangular flattened |
rectangular flattened |
rectangular square |
rectangular flattened |
rectangular flattened |
rectangular flattened |
rectangular flattened |
|
|
L/D ratio |
0.5–2.9 |
0.7–1.8 |
0.5–2.4 |
0.9–3.1 |
0.8–1.6 |
0.8–1.5 |
0.7–2.3 |
0.8–1.5 |
|
|
cells |
H L |
7–26 9–21 |
8–25 9–25 |
6–26 7–15 |
8–30 6–11 |
9–20(35) 9–13 |
9–22 10–16 |
9–24 8–13 |
11–20 10–18 |
|
laterall cell fusion |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
meristematic cells |
nd |
nd |
nd |
nd |
nd |
nd |
nd |
nd |
|
|
Epithallial cells |
|||||||||
|
number |
nd |
1 |
nd |
nd |
nd |
1 |
nd |
nd |
|
|
cells shape |
flattened rounded |
flattened |
|||||||
|
cells |
L D |
4–6 8–11 |
6–9 8–14 |
||||||
|
trichocytes |
nd |
nd |
solitary |
solitary |
nd |
nd |
nd |
nd |
|
|
Conceptacles |
|||||||||
|
projection |
51 |
30–62 |
– |
– |
– |
– |
– |
61–63 |
|
|
external D |
297 |
578–289 |
– |
– |
– |
– |
– |
300–362 |
|
|
chambers |
D H |
86–103 40–42 |
185–231 72–92 |
231–308 102–130 |
58–134 42–64 |
211–239 78–106 |
124–194 63–65 |
280–330 91–124 |
251–265 77–81 |
|
columellated |
– |
– |
– |
– |
– |
– |
– |
– |
|
|
pore canal |
L W |
55 28–29 |
38 20 |
27–50 33–37 |
68 35 |
34–49 50–76 |
40–45 nd |
39 61 |
51–62 49–60 |
|
cells in the roof filaments |
4–5 |
3 |
2–3 |
8 |
2–3 |
2–3 |
2–3 |
2–3 |
|
|
roof cells elongated |
– |
+ |
+ |
– |
+ |
+ |
+ |
+ |
|
Thin sections with bi/tetrasporophytes from newly collected samples VEGA 29111653 and VEGA 29111553, early Serravallian, Rohožník, Slovakia (Vienna Basin). Schaleková’s (1969, 1973, 1978) material containing H. corculumis including thin sections VEGA VZ19, VEGA 104, VEGA 5109 from Rohožník, Slovakia, VEGA 406 from Devínska Kobyla, Slovakia, both early Serravallian (Vienna Basin) and thin section VEGA 6201 from Kamenica nad Hronom, Slovakia, early Langhian (Danube Basin).
Description.—Hydrolithon corculumis from Slovakian part of central Paratethys inhabited infra- to circalittoral, algal reefs and soft substrates (Schaleková 1978; Seneš and Ondrejčíková 1991; Baráth 1992). Specimens from Maksymivka grew on algal reefs and from Lopadea Veche ones were found on large rhodoliths. Species encrust coralline algae mostly. Growth form is encrusting without applanate branches (Fig. 3A1). Surface is undulate with weak projection of conceptacles.
Thallus is pseudoparenchymatous with dorsiventral internal organisation and dimerous construction consisting two systems of filaments. The primigenous filaments run parallel with the substrate and consist of non-palisade cells (Fig. 3A2). Bistratose thalli, built by primigenous and epithallial cells, could develop on short distances at the thallus margins. These parts are considered as young thalli. Cells are rectangular to flattened with H/L ratio 0.8–1.9 and are 9–22 µm H (mean ± SD: 14 ± 2.7 µm) and 7–16 µm L (mean ± SD: 11 ± 2.4 µm) (n = 29). Cells of postigenous filaments are square to rectangular or flattened with L/D ratio 0.5–2.4 (Fig. 3A2). Postigenous filaments are up to 19 celled. Cells are 6–26 µm L (mean ± SD: 13 ± 4.2 µm) and 7–15 µm D (mean ± SD: 11 ± 2 µm) (n = 55). Cells of adjacent filaments are laterally joined with fusions but cell fusions are rare (Fig. 3A2). Epithallial and meristematic cells were not observed on this specimen but are presented in others. Solitary enlarged cell buried in the thallus is compared with trichocytes (Fig. 3B1). Two additional badly preserved enlarged cells are probably solitary trichocytes as well (Fig. 3B2).
Gametophyte bears two types of conceptacles—smaller triangular spermatangial and larger carpogonial-carposporangial one (Fig. 3B). Both are developed from initials located peripheral to the fertile area and are not columellated. Spermatangial conceptacle is 58 µm D and 42 µm H. Carpogonial-carposporangial conceptacle is 134 µm D and 64 µm H. Pore canal is cylindrical 68 µm L and 35 µm W. Roof cells protrude into the pore canal (Fig. 3B2).
Carpogonial plant observed in Transylvania Basin material possess non-columellated conceptacle with flat floor developed from cells located peripheral to the fertile area (Fig. 3C). Conceptacle protrude 51 mµ above thallus surface external diameter reach 297 µm (Fig. 3C1). Pore canal is cylindrical, 55 µm L and 28–30 µm W at the base. Roof cells protrude into the pore canal (Fig. 3C2). Chambers are ellipsoid in shape 86–103 µm D (mean ± SD: 95 ± 0.5 µm) and 40–42 µm H (mean ± SD: 41 ± 1.7 µm) (n = 2). Conceptacle is smaller than Carpathian Foredeep material and is considered as carpogonial.
Bi/tetrasporangial conceptacles roofs are formed by cells perpendicular to the chamber suggesting their development from cells located peripheral and interspersed among sporangial initials (Fig. 3A). Roof filaments are 2–3 celled. Basal cell or middle cells are often elongated. A roof vanishing is caused by filaments continual growth. Pore canals are more or less cone shaped and bordered by 3 celled filaments (Fig. 3A). They are not protruding into the pore canal. Pore canals are 33–37 µm L and 24–29 µm D at their base (n = 3). Chambers are lens shaped or hemispherical, 231–308 µm D (mean ± SD: 271 ± 27.1 µm) and 102–130 µm H (mean ± SD: 119 ± 9.8 µm) (n = 6). Central columella was not observed.
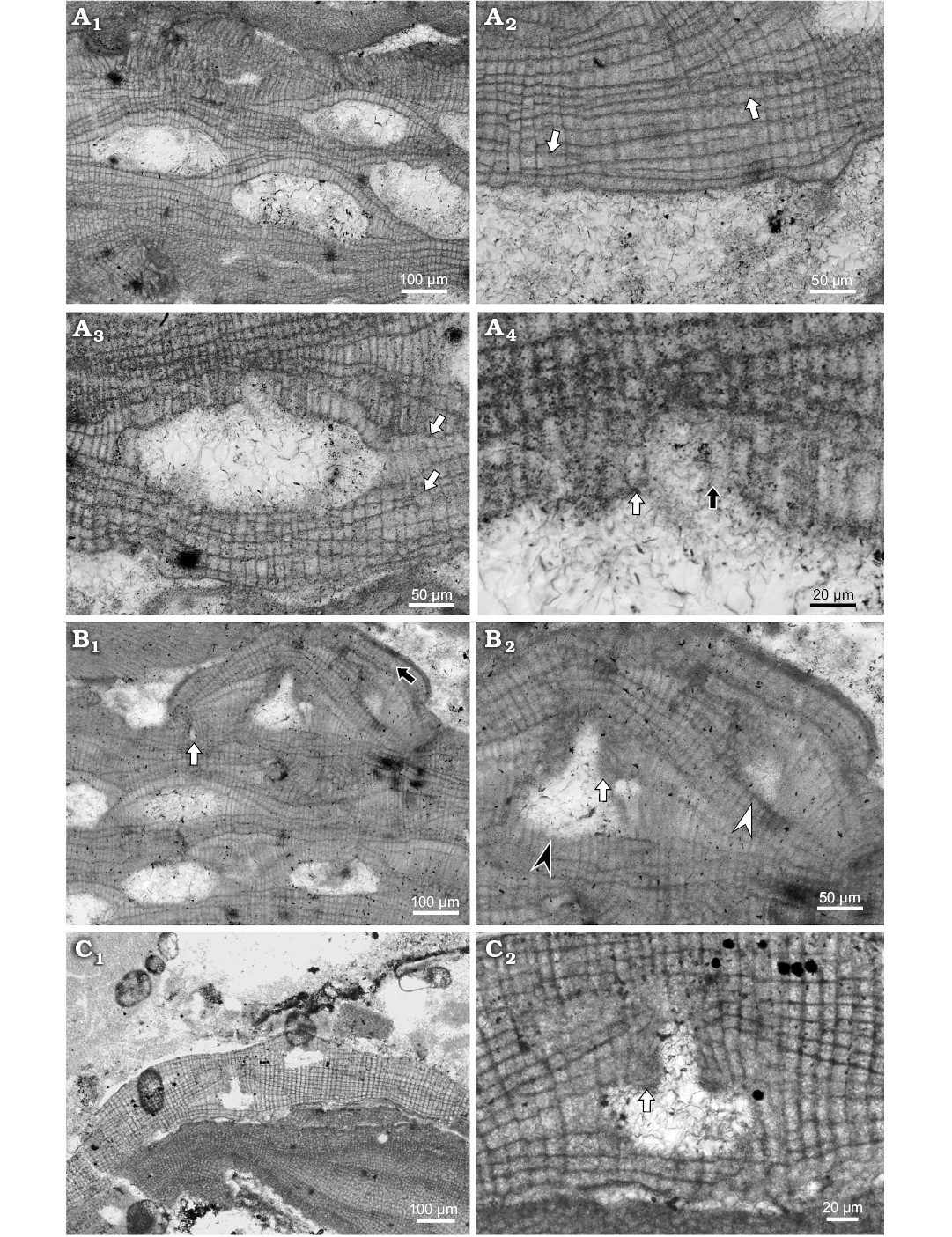
Fig. 3. Coralline alga Hydrolithon corculumis (Maslov, 1962) Braga, Bassi, Zakrevskaya, and Petrovna-Radionova, 2005. A. Bi/tetrasporophyte, NHM B1833/1 (thin section 21510253), early Serravallian, Maksymivka, Ukraine, Carpathian Foredeep. A1. Portion of the thallus with encrusting growth form and several conceptacles; thallus structure suggests conceptacles protruded slightly above thallus surface during their maturity; note conceptacle on the right with cone shaped pore canal. A2. Dorsiventral internal organisation and dimerous thallus construction (arrows point to the lateral fusion of cells in adjacent filaments). A3. Uniporate bi/tetrasporangial conceptacle within dimerous thallus (arrows point to the lateral fusion of the cells in adjacent filaments). A4. Detail of the pore canal (arrows point to the pore canal filaments, white arrow marks three celled filament, black arrow points to the badly preserved pore canal filament). B. Gametangial-carposporangial plant, NHM B1832 (thin section 21510553), early Serravallian, Maksymivka, Ukraine, Carpathian Foredeep. B1. Plant growing above the bi/tetrasporic one; primigenous filaments are not seen (white arrow point to the possible trichocyte, black arrow point to the badly preserved trichocyte. B2. Detail of the spermatangial (white arrowhead) and carposporangial (black arrowhead) conceptacles. Note the flat floor of the spermatangial chamber (arrow point to the roof of the large conceptacle), roof filaments that built the roof and the pore project into the pore canal. C. Carpogonial plant, NHM B1835 (thin section 1849116051), early Langhian, Lopadea Veche, Romania, Transylvania Basin. C1. Encrusting growth form with weak projection of carpogonial conceptacle. C2. Detail of the conceptacle. Note cells forming the roof and the pore canal (arrow); cells of adjacent filaments in surrounding thallus are laterally joined with fusions.
Remarks.—Species was designated as Lithophyllum corculumis by Maslov (1962) on the basis Langhian–early Serravallian material collected in the western Ukraine. Later the type material was reassessed by Braga et al. (2005) and species was positioned into the genus Hydrolithon upon lateral cell fusions, dimerous thallus construction and pore canal anatomy (Braga et al. 2005). However, type of the conceptacles was considered as uncertain either “(tetra) sporangial” or “female/carposporangial” (Braga et al. 2005). Present results suggest that conceptacles of the type are bi/tetrasporangial because carpogonial and carposporangial are different in having roof cells projecting into the pore canal while spermatangial conceptacle is small and triangular.
Described specimen differs in having longer cells of postigenous filaments and slightly larger conceptacles than the type reported by Braga et al. (2005) (Table 4). Nevertheless, important diagnostic features associated with the bi/tetrasporic pore canal and the roof anatomy match the type, hence observed deviations are considered as intraspecific variability. Moreover, comparable vegetative features were reported from Transylvania Basin (Chelaru and Bucur 2016) and Novohrad Basin (Schaleková and Čierna 1985).
Examined material fits within the holotype description. Dimerous thallus construction, cells laterally joined with fusions, bi/tetrasporic conceptacle development, solitary trichocytes are consistent with the Hydrolithon diagnosis (Rösler et al. 2016).
Prior to this study, only two specimens were known from Slovakia (Schaleková and Čierna 1985; Hrabovský 2013). Both occurrences are Langhian, the former was not found in the collection but the second is consistent with the type diagnosis. Other occurrences are in the Polish part of Carpathian Foredeep (Pisera 1985; Studencki 1988b). Material from Roztocze Hills (Pisera 1985) examined in this work prove the species presence in the early Serravallian of Carpathian Foredeep. However, Langhian occurrence was questioned by Braga et al. (2005) because figured specimen does not have cells of adjacent filaments fused. H. corculumis is absent in Czech Republic (Carpathian Foredeep) (Zágoršek et al. 2012; Hrabovský et al. 2015; present study) and in some Croatian sites of Langhian limestone (Basso et al. 2008; Sremac et al. 2016).
Geographical and stratigraphic range.—Langhian–early Serravallian (Middle Miocene) of the central Paratethys.
Hydrolithon lemoinei (Miranda, 1935) Aguirre, Braga, and Bassi, 2011
Fig. 4A–C, Table 5.
1973 Lithophyllum (Dermatolithon) sp.; Schaleková 1973: 218, pl. 74: 2.
Material.—Bi/tetrasporangial plant (Fig. 4A), NHM B1842 (thin section 5227), early Langhian, Rohožník, Slovakia (Vienna Basin). Spermatangial plant (Fig. 4B), NHM B1843 (thin section 5228) from the same locality as bi/tetrasporophyte. Carpogonial-carposporangial conceptacles (Fig. 4C), NHM B1844 (thin section 6174), early Langhian, Kamenica nad Hronom, Slovakia (Danube Basin). Other examined bi/tetrasporophytes (Table 5): NHM B1845 (thin section VZ20), early Langhian, Kosihovce, Slovakia (Novohrad Basin); NHM B1846 (thin section IIIb/458) , early Langhian, Pavlová, Slovakia (Danube Basin); NHM B1847 (thin section DK C), early Serravallian, Devínska Kobyla, Slovakia (Vienna Basin); NHM B1838/2 (thin section 184911651), early Langhian, Lopadea Veche, Romania (Transylvania Basin). Gametophyte, NHM B1838/3 (thin section 184911651), early Langhian, Lopadea Veche, Romania (Transylvania Basin).
Specimens from Schaleková’s collection (Schaleková 1973) bi/tetrasporophyte thin section VEGA 5105, early Serravallian, Rohožník, Slovakia (Vienna Basin). Hydrolithon lemoinei specimens from new samples: both bi/tetrasporophytes and gametophytes, thin section VEGA 184911435; spermatangial gametophyte, thin section VEGA 1849115451; bi/tetrasporophytes, thin sections VEGA 1849113351, VEGA 1849114051, and VEGA 1849114951; all from early Langhian, Lopadea Veche, Romania (Transylvania Basin).
Table 5. Comparative table of selected Hydrolithon lemoinei (Miranda, 1935) Aguirre, Braga, and Bassi, 2011 specimens. * marks gametangial or carpogonial-carposporangial plants. Abbreviations: D, diameter; H, height; L, length; W, wide; nd, no data; “+” presence and “–” absence of character; “±” intermediate. All measurements in μm.
| |
Danube Basin |
Vienna Basin |
Novohrad Basin |
Transylvania Basin |
|||||
|
Specimen number (NHM) |
B1844 |
B1844 |
B1846 |
B1843 |
B1842 |
B1847 |
B1845 |
B1838/2 |
|
|
Thin section |
6174* |
6174* |
IIIb458 |
5228* |
5227 |
DKc |
VZ.20 |
184911651 |
|
|
Habitat |
littoral |
littoral |
littoral |
littoral |
littoral |
littoral |
littoral |
littoral |
|
|
Growth form |
encrusting |
encrusting |
encrusting |
encrusting |
encrusting |
encrusting |
encrusting |
encrusting |
|
|
Applanate branches |
– |
– |
– |
– |
– |
– |
– |
– |
|
|
Thallus thickness |
52–520 |
52–520 |
113–389 |
102–245 |
97–296 |
197–368 |
127–274 |
78–271 |
|
|
Primigenous filaments |
|||||||||
|
cells shape |
polygonal flattened rectangular |
polygonal flattened rectangular |
polygonal square |
polygonal flattened rectangular |
polygonal flattened rectangular |
polygonal flattened rectangular |
polygonal square |
polygonal square |
|
|
H/L ratio |
0.5–1.5 |
0.5–1.5 |
0.5–1.2 |
0.7–1.5 |
0.4–1.5 |
0.6–1.6 |
0.4–0.8 |
0.5–1.2 |
|
|
cells |
H L |
8–20 9–26 |
8–20 9–26 |
11–22 15–30 |
13–29 15–26 |
10–21 12–20 |
16–34 15–27 |
10–17 18–28 |
8–17 12–18 |
|
laterall cell fusion |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
Postigenous filaments |
|||||||||
|
multicellular |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
cells shape |
polygonal rectangular flattened |
polygonal rectangular flattened |
polygonal flattened square |
polygonal rectangular flattened |
polygonal rectangular flattened |
polygonal flattened square |
polygonal flattened square |
polygonal rectangular flattened |
|
|
L/D ratio |
0.4–2 |
0.4–2 |
0.7–1.3 |
0.7–2.3 |
0.9–1.6 |
0.9–1.7 |
1–1.6 |
0.7–2.2 |
|
|
cells |
H L |
6–30 11–20 |
6–30 11–20 |
13–24 16–25 |
13–30 9–21 |
9–31 10–28 |
16–26 13–20 |
14–28 13–23 |
7–28 10–17 |
|
laterall cell fusion |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
Meristematic cells |
|||||||||
|
elongated |
+ |
+ |
± |
± |
+ |
nd |
± |
+ |
|
|
Epithallial cells |
|||||||||
|
number |
1 |
1 |
1 |
1 |
1 |
nd |
1 |
1 |
|
|
shape |
flattened rounded |
flattened rounded |
flattened |
flattened rounded |
flattened rounded |
flattened |
flattened rounded |
||
|
cells |
L D |
4–6 7–12 |
4–6 7–12 |
4–5 10–12 |
4–6 12 |
5–6 5–11 |
6–9 13–19 |
3–5 5–7 |
|
|
trichocytes |
nd |
nd |
nd |
nd |
nd |
nd |
nd |
nd |
|
|
Conceptacles |
|||||||||
|
projection |
up to 131 |
burried |
143 |
148 |
173–174 |
137 |
105–120 |
141 |
|
|
external D |
300–509 |
nd |
491 |
403 |
372–393 |
564 |
404–513 |
380 |
|
|
chambers |
H D |
54–108 23–43 |
272 69 |
305 154 |
65 41 |
214–231 124–134 |
195 152 |
126–195 69–121 |
249 95 |
|
columellated |
± |
± |
– |
– |
– |
– |
– |
– |
|
|
pore canal |
L W |
72–123 13–50 |
nd 45 |
120 58 |
83 25 |
83–102 up to 47 |
132 66 |
102 44 |
70 39 |
|
cells in the roof filaments |
5–9 |
6 |
5 |
5–7 |
6–8 |
5–7 |
5 |
5 |
|
|
roof cells elongated |
– |
– |
– |
– |
– |
– |
– |
– |
|
Description.—Hydrolithon lemoinei occurs in infra- to circalittoral limestone beds (Schaleková 1978; Seneš and Ondrejčíková 1991; Baráth 1992). Species overgrows coralline algae on the soft bottoms and rhodolith beds. It develops thin encrusting non-protuberant thalli (Fig. 4A1). Applanate branches have not been observed. Thallus surface is undulate with distinct projection of conceptacles. Individual thalli are 97–296 μm thick. Superimposed thalli develop 0.5 mm thick crusts. Thallus is pseudoparenchymatous with dorsiventral internal organisation and dimerous construction, consisting of the two systems of filaments (Fig. 4A2). Primigenous filaments consist of non-palisade cells 10–21 μm H (mean ± SD: 15 ± 4.1 μm) and 12–30 μm L (mean ± SD: 22 ± 5.6 μm) (n = 10). Cells are square, flattened, or rectangular to polygonal. Polygonal cells are markedly larger and always produce two cells of postigenous filaments (Fig. 4A2). Postigenous filaments are multicellular and grow perpendicularly to primigenous (Fig. 4A2). However, short bistratose thallus could develop at the margins in young thallus probably. Filaments consist of square or rectangular to polygonal cell that could be of the same dimensions as primigenous filaments cells. Cells are 9–31 μm L (mean ± SD: 16 ± 6.4 μm) and 10–28 μm D (mean ± SD: 13 ± 5.2 μm) (n = 11). Polygonal cells are enlarged and produce always two cells, the same as in primigenous filaments (Fig. 4A2). No alignment of cells was observed. Cells are laterally connected with fusions in both filaments. Meristematic cells are mostly longer than cells subtending them immediately and are 9–18 μm L (mean ± SD: 5 ± 0.5 μm) and 5–11 μm D (mean ± SD: 8 ± 2.4 μm) (n = 5) (Fig. 4A3). Filaments terminate with single epithallial cell (Fig. 4A3).
Spermatangial conceptacles are triangular and developed from initials located peripheral to the fertile area (Fig. 4B). Conceptacle is without central columella. Chamber is 71 μm in diameter and 18 μm in height. Pore canal is 37 μm H and 32 μm W at its base, tapering to the pore opening. Conceptacle protrude 43 μm above thallus surface and its external diameter reach 272 μm.
Gametophytes are dioecious, since carpogonial conceptacles develop on separate thallus than spermatangial (Fig. 4C). They protrude 148 μm above thallus surface and external diameter reach up to 403 μm. The mode of formation is the same as in spermatangial. Chambers may have central columella. Chambers are 65 μm D and 41 μm H. Pore canal is markedly longer than in spermatangial conceptacles and is 103 μm L and 32 μm W. In thin section 6174 from Kamenica nad Hronom are carpogonial and carposporangial conceptacles documented in single plant. Carpogonial conceptacles are triangular and occasionally columellated, possessing broad pore canal (Fig. 4C2). Chambers are 54–108 μm D (mean ± SD: 77 ± 21.8 μm) and are 23–43 μm H (mean ± SD: 31 ± 7.7 μm) (n = 6). Pore canals are long cylindrical. They are 72–123 μm L and 13–50 μm W (n = 6). Carposporangial conceptacle chamber is 271 μm D and 69 μm H without central columella (Fig. 4C3). Bi/tetrasporangial conceptacles are uniporate and markedly protrude over the thallus surface (Fig. 4A4). They protrude up to 173 μm above thallus surface. External diameter is 372–393 μm (n = 2). Conceptacles were developed from initials peripheral and interspersed within sporangial initials, resulting in the more or less perpendicularly oriented roof cells to the chamber (Fig. 4A4). Pore lining cells were not observed. The roof is formed by cells narrower than postigenous and by polygonal cells producing two other cells also. Chambers are rounded to ellipsoid. They are 214–231 μm D (mean ± SD: 222 ± 12.1 μm) and 125–134 μm H (mean ± SD: 129 ± 6.3 μm) (n = 2). Chambers are not columellated. Pore canal is 83–102 μm L and up to 47 μm W.
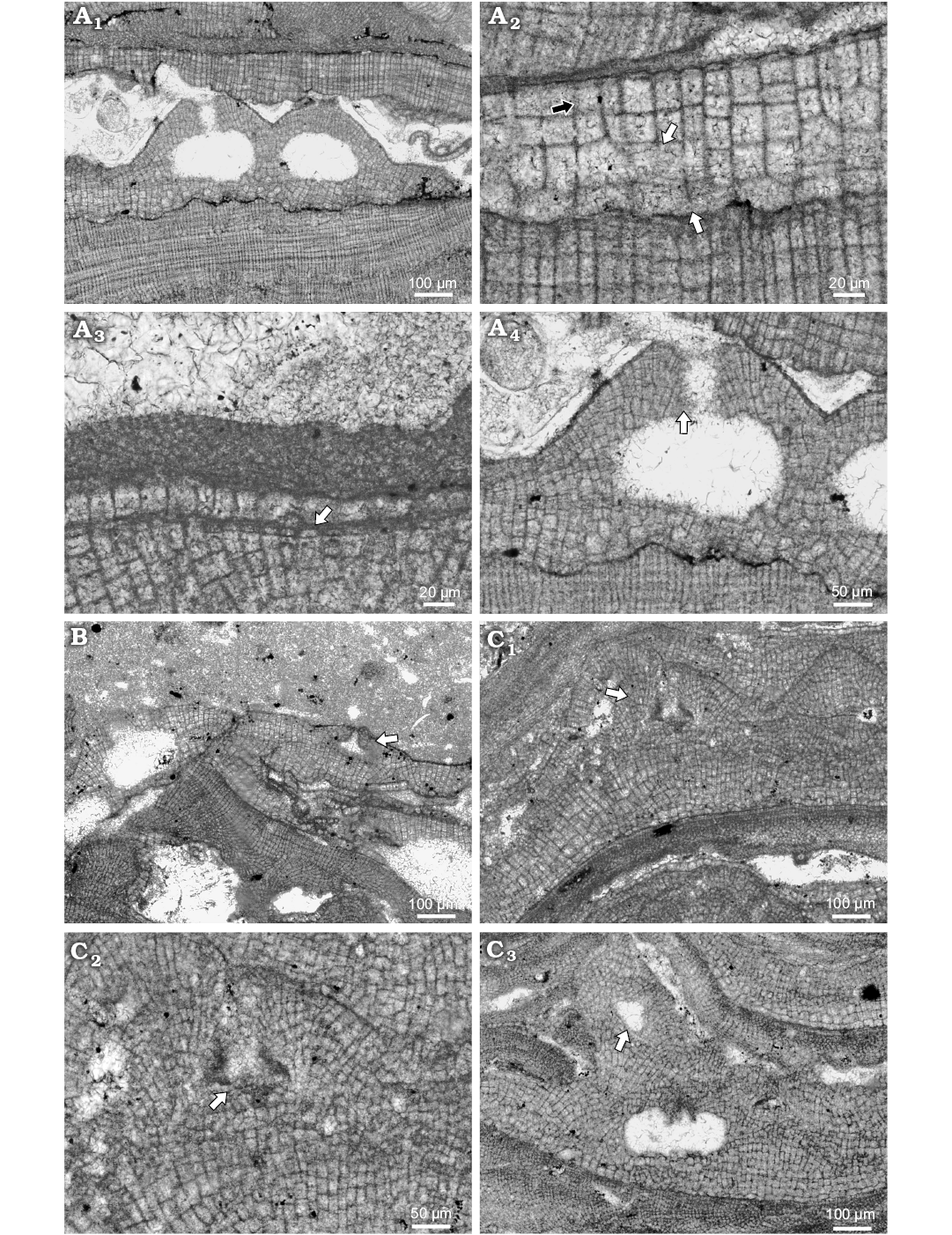
Fig. 4. Coralline alga Hydrolithon lemoinei (Miranda, 1935) Aguirre, Braga, and Bassi, 2011. A. Bi/tetrasporophyte, NHM B1842 (thin section 5227), early Serravallian, Rohožník, Slovakia, Vienna Basin. A1. Encrusting growth form with distinct projection of bi/tetrasporangial conceptacles. A2. Dorsiventral internal organisation and dimerous construction (white arrows point to the lateral fusion of cells in adjacent filaments); note small flattened epithallial cells at the surface and elongated meristematic cells (black arrow). Large polygonal cell producing two long and narrow cells is on the right margin. A3. Dome shaped epithallial cells (arrow). A4. Pore canal filaments and proposed papillae that protrude inside (arrow). B. Male gametophyte, NHM B1843 (thin section R5228), early Serravallian, Rohožník, Slovakia, Vienna Basin; spermatangial conceptacle (arrow) roof and the pore are developed by filaments running parallel with the chamber; note adjacent bi/tetrasporic plant on the left margin of the figure. C. Carpogonial-carposporangial plant, NHM B1844 (thin section 6174), early Serravallian, Kamenica nad Hronom, Slovakia, Danube Basin. C1. Encrusting growth form with prominent conceptacles projections (arrow); carpogonial conceptacle bear wide and long pore canal. C2. Detail of the carpogonial conceptacle (arrow point to the pore canal); note cells projecting into it from the roofs. C3. Another portion of the thallus with both, carpogonial (arrow) and carposporangial conceptacles; carposporangial conceptacle is large with pore canal cells projecting into the pore canal and the chamber.
Remarks.—Species was designated as Melobesia lemoinei by Miranda (1935). Later was reassessed by Aguirre et al. (2011) and positioned to the genus Hydrolithon because of the bi/tetrasporic conceptacle type, presence of lateral cells fusions and thallus construction. Bi/tetrasporophytes fit within the description provided by Aguirre et al. (2011) and gametophytes and carposporangial conceptacles bearing plants possess the same morpho-anatomical features as bi/tetrasporophytes (Table 5).
There is one character briefly discussed in revision of the type material—the pore canal anatomy. All of the studied plants, known plants (Hrabovský et al. 2015: 464, fig. 16; Chelaru and Bucur 2016: 11, fig. 3c) and that ones with the type (Aguirre et al. 2011: 283, fig. 6b) possess conceptacles with pore canals lined by cells projecting into the pore canal. This could be the result of either roof cells projections comparable to Pneophyllum, Neogoniolithon, Mastophora, and Spongites, or presence of papillae lining the pore canal found in Hydrolithon, Porolithon, Lithophyllum, and Titanoderma (Irvine and Chamberlain 1994; Ringeltaube and Harvey 2000; Harvey et al. 2005, 2006; Basso and Rodondi 2006; Rösler et al. 2016). Given close similarity between the papillae (Fig. 4A4, left margin of pore canal) and the type of the conceptacle development, the second option is likely more. Whether the specimen is bi/tetrasporophyte is proved by distinct phases of the complete life cycle presence.
Prior to this study the two occurrences were reported from Slovakia: Lithophyllum (Dermatolithon) sp. (Schaleková 1973: 218, pl. 74: 2) from Rohožník and Lithophyllum (Dermatolithon?) sp. (Schaleková 1969: pl. 21: 2) from Sandberg. The first one represents H. lemoinei but the second one possesses thallus with cells laterally not joined with fusions. Schaleková (1969, 1973, 1978) referred only these two specimens, others from the remaining localities find in her collection during this study are unpublished therefore.
Species has been found in Transylvania, Novohrad, Danube, and Vienna basins. Also, it occurs in the early Langhian Carpathian Foredeep localities from Czech Republic (Hrabovský et al. 2015) and Pinczów Limestone in Poland (Studencki 1988a) but is missing in Maksymivka (Carpathian Foredeep). Occurrence in the early Serravallian Roztocze Hills algal-vermetid reefs documented by Pisera (1985) is not supported. There is another similar species reported under the names of Lithophyllum (Dermatolithon) nataliae possessing comparable morphological features with H. lemoinei found in the Poland (Studencki 1988b: fig. 10; Studencki and Pisera 1989: pl. 12: 1, 2). The type of Lithophyllum (Dermatolithon) nataliae Maslov, 1956 was recently transferred by Bassi et al. (2005) to the extinct genus Karpathia Maslov, 1962. This genus is characterized by: (i) dimerous thallus construction with (ii) cells laterally joined with fusions, (iii) fusion in postigenous filaments are more extensive, (iv) each ventral cell produce one or two cells of postigenous filaments, (v) cells are smaller in size towards the dorsal surface, and (vi) sporangial conceptacle is uniporate (Bassi et al. 2005). Characters (iii) and (v) were not observed in H. lemoinei nor in the figured specimens from Polish Carpathian Foredeep. To the contrary, the examined specimens often terminate with elongated meristematic cells and single epithallial cell.
Geographical and stratigraphical range.—Oligocene to middle Miocene (early Serravallian) of the Mediterranean and central Paratethys.
Hydrolithon sp. 1
Fig. 5A–C, Table 6, SOM: fig. S1 (Supplementary Online Material available at http://app.pan.pl/SOM/app64-Hrabovsky_etal_SOM.pdf).
Material.—Bi/tetrasporophyte (Fig. 5A), NHM B1834/1 (thin section 21510153); gametophytes and carposporangial conceptacle bearing gametophyte (Fig. 5B, C), NHM B1833/2 and NHM B 1833/3 (thin sections 21510253), all from early Serravallian, Maksymivka, Ukraine (Carpathian Foredeep).
Description.—Hydrolithon sp. 1 grows on algal reefs encrusting other coralline algae, polychaetes, and sessile foraminifers, collectively developing large stout columnar structures at the top of the reef. The surrounding deposits infilling the cavities contain late Serravallian (Sarmatian) fauna. Vegetative anatomy is based on bi/tetrasporic plant. Growth form is encrusting without applanate branches. Surface is undulate with weakly projecting conceptacles. Individual thalli are 168–624 μm thick (Fig. 5A1). Thallus is pseudoparenchymatous with dorsiventral internal organisation and dimerous construction (Fig. 5A2). Primigenous filaments consist of non-palisade cells, rectangular to flattened cells, or polygonal cells. Cells are 14–25 μm H (mean ± SD: 14 ± 4.4 μm) and 9–23 μm L (mean ± SD: 16 ± 3.9 μm) (n = 19). Cells of postigenous filaments are 7–31 μm L (mean ± SD: 16 ± 6.3 μm) and 6–21 μm D (mean ± SD: 11 ± 3.3 μm) (n = 81). Large polygonal cells are scattered in the tissue, always producing two smaller cells. Horizontal and vertical cells alignment is missing. Cells are laterally joined with fusions (Fig. 5A3). Meristematic cells are as long as cells immediately subtending them (Fig. 5A4). Epithallial cells are dome shaped, or flattened (Fig. 5A4). Cells are 4–6 μm L (mean ± SD: 4 ± 0.8 μm) and 5–16 μm D (mean ± SD: 8 ± 2.3 μm) (n = 12). Trichocytes are solitare and buried in the thallus (Fig. 5B, SOM: fig. S1).
Spermatangial conceptacle is small and triangular, buried in the thallus (Fig. 5B). Only one conceptacle was observed. Chamber is 125 μm D and 25 μm H with cone shaped pore canal 54 μm L and 37 μm W at its base.
Carpogonial and carposporangial conceptacles occur on the same thallus. Carposporangial is non-columellated, while carpogonial possess weak columella (Fig. 5C1). Carpogonial conceptacles are 109–133 μm D and 76–97 μm H (n = 3). Pore canal is cylindrical 69–96 μm L and 37–42 μm W (Fig. 5C1). Carposporangial conceptacle is 231 μm D and 104 μm H. The roof is 62 μm thick. Roof filaments oriented parallel with the chamber, still remain on some spots (Fig. 5C2).
Bi/tetrasporangial conceptacles are developed from cells peripheral to and interspersed among sporangial initials (Fig. 5A5). Roof consists of cells smaller than other thallus cells. Pore canals are cylindrical or slightly tapering to its top 64–82 μm L and 41–43 D. Pore canal is lined by 7 celled filaments, perpendicularly oriented to the chamber (Fig. 5A5). Chambers could have weak central columella. Chambers are 222–236 μm D (mean ± SD: 229 9.7 μm) and 97–116 μm H (mean ± SD: 107 ± 13.4 μm) (n = 3). External diameter is 239–381 μm and they protrude 50–94 μm above thallus surface.
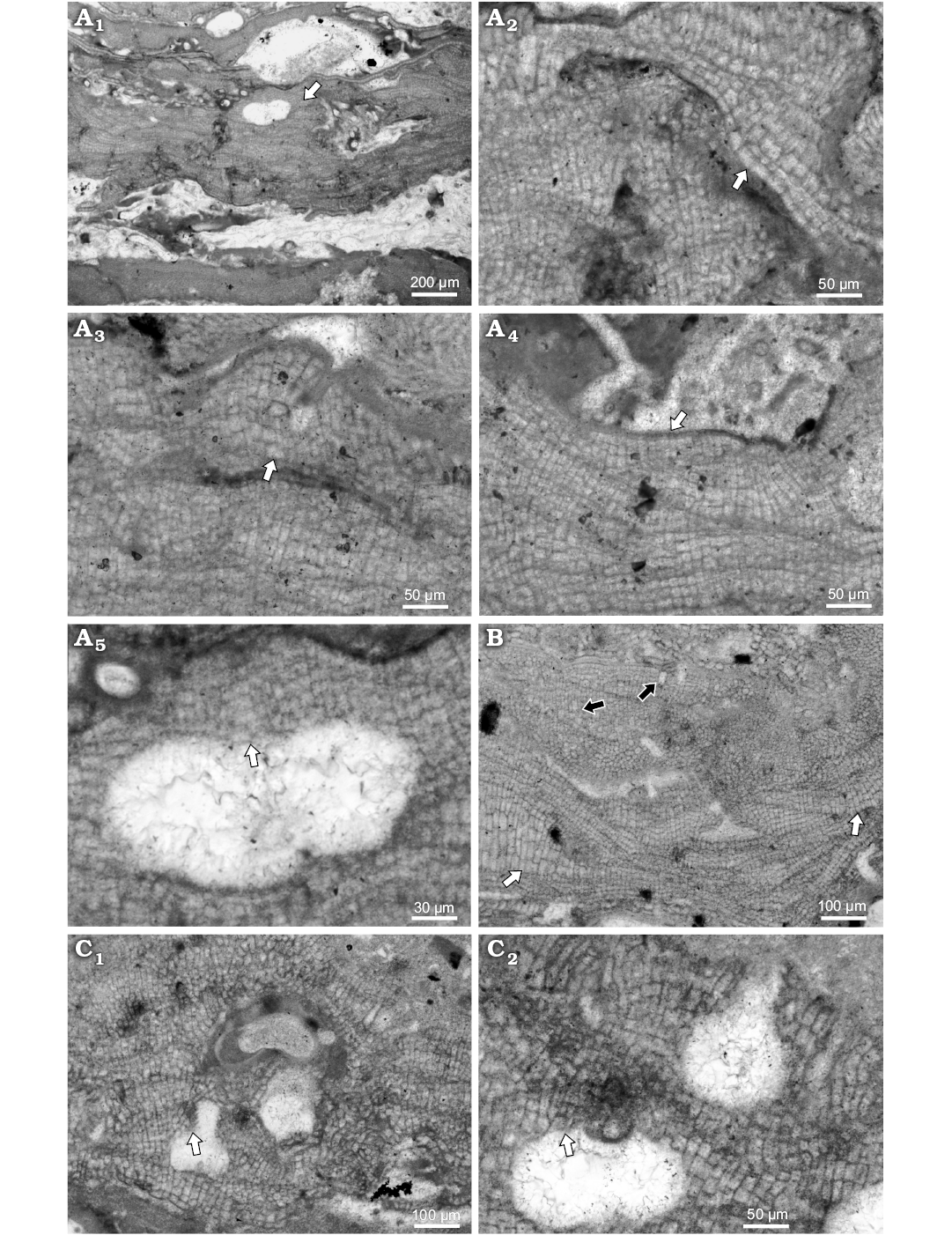
Fig. 5. Coralline alga Hydrolithon sp. 1 from early Serravallian, Maksymivka, Ukraine, Carpathian Foredeep. A. Bi/tetrasporophyte, NHM B1834/1 (thin section 21510153). A1. Encrusting growth form with single slightly projecting bi/tetrasporangial conceptacle (arrow). A2. Dimerous construction with flattened to square cells in primigenous filament (arrow); note the cells dimensions and shape that change from small cells in lower portion and much larger in upper portion of the single thallus. A3. Cell fusions in adjacent cells of postigenous filaments (arrow). A4. Arrow point to single layer of epithallial cells developed above meristematic cells that are elongated or of the same dimensions as cells immediately below them. A5. Enlarged conceptacle from A1. Bi/tetrasporangial conceptacle with roof filaments perpendicular to the chamber (arrow point to the pore canal and pore lining cells oriented perpendicular to the chamber as well). B. Male gametophyte, NHM B1833/2 (thin section 21510253); spermatangial conceptacle slightly projecting above the thallus surface. Note patches of enlarged cells (white arrows) and solitary trichocytes (black arrows). C. Carpogonial-carposporangial plant, NHM B1833/3 (thin section 21510253). C1. Carpogonial conceptacle with large pore canal. Cells are projecting inside the pore canal (arrow). C2. Carpogonial conceptacle (top) and carposporangial conceptacle (bottom). Arrow point to the portion of the conceptacle roof with preserved parallel orientation of the roof filaments with the chamber.
Remarks.—Species was not reported from the central Paratethys, prior to the present work. Its assessment within the genus Hydrolithon is based on dimerous thallus presence, non-palisade cells in primigenous filaments, lateral fusion of cells, type of the bi/tetrasporic conceptacle development and pore lining cells perpendicularly oriented to the chamber, and presence of trichocytes not arranged in large tightly packed horizontal fields (all corresponding with the diagnosis) (Kato et al. 2011; Rösler et al. 2016). Basic characteristics documented on selected specimens are listed in Table 6. This species is known only from the early Serravallian of Maksymivka, Ukraine (Carpathian Foredeep).
Table 6. Comparative table of selected Hydrolithon sp. 1 specimens from Carpatian Foredeep. * marks gametangial or carpogonial-carposporangial plants. Abbreviations: D, diameter; H, height; L, length; W, wide; nd, no data; “+” presence and “–” absence of character; “±” intermediate. All measurements in μm.
|
Specimen number (NHM) |
B1833/3 |
B1833/3 |
B1833/2 |
B1834/1 |
|
|
Thin section |
21510253* |
21510253* |
21510253* |
21510153 |
|
|
Habitat |
littoral |
littoral |
littoral |
littoral |
|
|
Growth form |
encrusting |
encrusting |
encrusting |
encrusting |
|
|
Applanate branches |
– |
– |
– |
– |
|
|
Thallus |
52–520 |
52–520 |
102–245 |
168–624 |
|
|
Primigenous filaments |
|||||
|
cells shape |
polygonal flattend rectangular |
polygonal flattend rectangular |
polygonal flattend rectangular |
polygonal flattend rectangular |
|
|
H/L ratio |
0.5–1.9 |
0.5–1.9 |
0.4–1.4 |
0.4–1.8 |
|
|
cells |
H L |
10–26 9–23 |
10–26 9–23 |
10–29 9–25 |
14–25 9–23 |
|
laterall cell fusion |
+ |
+ |
+ |
|
|
|
Postigenous filaments |
|||||
|
multicellular |
+ |
+ |
+ |
+ |
|
|
cells shape |
polygonal rectangular flattened |
polygonal rectangular flattened |
polygonal rectangular flattened |
polygonal rectangular flattened |
|
|
L/D ratio |
0.5–2.4 |
0.5–2.4 |
0.6–2.3 |
0.5–3 |
|
|
cells |
H L |
6–33 9–21 |
6–33 9–21 |
6–39 6–20 |
7–31 6–21 |
|
laterall cell |
+ |
+ |
+ |
+ |
|
|
Meristematic cells |
|||||
|
elongated |
+ |
+ |
± |
+ |
|
|
Epithallial cells |
|||||
|
number |
1 |
1 |
1 |
1 |
|
|
shape |
flattened rounded |
flattened rounded |
flattened rounded |
flattened rounded |
|
|
cells |
L D |
3–5 8–13 |
3–5 8–13 |
4–6 12 |
4–6 5–16 |
|
trichocytes |
nd |
nd |
nd |
nd |
|
|
Conceptacles |
|||||
|
projection |
up to 131 |
burried |
37 |
50–94 |
|
|
external D |
300–509 |
ND |
54 |
239–381 |
|
|
chambers |
D H |
109–133 |
231 |
125 |
222–236 |
|
76–97 |
104 |
25 |
97–116 |
||
|
columellated |
± |
± |
– |
– |
|
|
pore canal |
L W |
69–96 |
nd |
54 |
64–82 |
|
37–42 |
45 |
37 |
41–43 |
||
|
cells in the |
5–9 |
6 |
5–7 |
7 |
|
|
roof cells |
– |
– |
– |
– |
|
Family Mastophoraceae Townsend and Huisman, 2018
Subfamily Mastophoroideae Setchell, 1943
Genus Lithoporella (Foslie, 1902) Foslie, 1909
Type species: Lithoporella melobesioides (Foslie, 1903) Foslie 1909; South Niladu Island, Maldives, Recent.
Lithoporella minus Johnson, 1964
Fig. 6A, B, Table 7, SOM: figs S2, S3.
Material.—Bi/tetrasporophytes (Fig. 6A) and gametophytes (Fig. 6B), NHM B1850/1 and NHM B1850/2 (thin section 5099), early Serravallian, Rohožník, Slovakia (Vienna Basin). Other examined specimens (Table 7): NHM B1853 (thin section 381213162), late Serravallian, Wolfsthal, Austria (Vienna Basin); NHM B1851/1 (thin section 5111), early Serravallian, Rohožník, Slovakia (Vienna Basin); NHM B1848 (thin section ZTK3a) and NHM B1852 (thin section 711416), both early Serravallian, Devínska Kobyla, Slovakia (Vienna Basin); NHM B1854 (thin section 37913161), late Serravallian, Veľký Pesek, Slovakia (Danube Basin); NHM B1839/2 (thin section 184911151); early Langhian, Lopadea Veche, Romania (Transylvania Basin) and NHM B1833/4 (thin section 21510253), NHM B1834/2 (thin section 21510153), both early Serravallian, Maksymivka, Ukraine (Carpathian Foredeep).
Bi/tetrasporophytes from newly collected samples: thin sections VEGA 1849115651, VEGA 1849115551 from early Langhian, Lopadea Veche, Romania (Transylvania Basin); thin sections VEGA DKe, VEGA K3b, VEGA K3d, early Serravallian, Devínska Kobyla, Slovakia (Vienna Basin); thin section VEGA 371011452, early Serravallian, Vývrat, Slovakia (Vienna Basin); thin section VEGA 29711453, early Serravallian, Rohožník, Slovakia (Vienna Basin); thin sections VEGA 381213262, VEGA 381213362, VEGA 381213562, VEGA 381213662, VEGA 381213762, VEGA 381213862, all late Serravallian, Wolfsthal, Austria (Vienna Basin); thin section VEGA 7114261, late Serravallian, Devínska Kobyla, Slovakia (Vienna Basin); thin sections VEGA 37913261, VEGA 37913361, VEGA 37913661, all late Serravallian, Veľký Pesek, Slovakia (Danube Basin). Bi/tetrasporangial plants from Schaleková’s collection (1973) including L. minus are: thin sections VEGA 5102, VEGA 2105, VEGA 5107, and VEGA 5223, early Serravallian, Rohožník, Slovakia (Vienna Basin).
Table 7. Comparative table of selected Hydrolithon minus Johnson, 1964 specimens. * marks gametangial or carpogonial-carposporangial plants. Abbreviations: D, diameter; H, height; L, length; W, wide; nd, no data; “+” presence and “–” absence of character. All measurements in μm.
| |
Vienna Basin |
Danube Basin |
Transylvania Basin |
Carpathian Foredeep |
|||||
|
Specimen number (NHM) |
B1850/2 |
B1850/1 |
B1853 |
B1848 |
B1852 |
B1854 |
B1839/2 |
B1833/4 |
|
|
Thin section |
5099* |
5099 |
381213162 |
ZTK3a |
711416 |
37913161 |
184911151 |
21510253 |
|
|
Habitat |
littoral |
littoral |
littoral |
littoral |
littoral |
littoral |
littoral |
littoral |
|
|
Growth form |
encrusting |
encrusting |
encrusting |
encrusting |
encrusting |
encrusting |
encrusting |
encrusting |
|
|
Applanate branches |
– |
– |
– |
– |
– |
– |
– |
– |
|
|
Entirely bistratose |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
Primigenous filaments |
|||||||||
|
cells shape |
flattened rounded palisade |
flattened rounded palisade |
flattened rounded palisade |
flattened rounded palisade |
flattened rounded palisade |
flattened rounded palisade |
flattened rounded palisade |
flattened rounded palisade |
|
|
H/L ratio |
0.2–2.6 |
0.5–2.8 |
0.8–2.6 |
0.6–2.2 |
0.5–2.7 |
1.1–3.1 |
0.6–2.4 |
0.5–2.5 |
|
|
cells |
H L |
4–27 7–19 |
7–27 7–18 |
9–25 7–19 |
8–24 9–16 |
6–28 8–17 |
10–21 5–14 |
8–26 9–23 |
9–22 7–19 |
|
laterall cell fusion |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
Epithallial cells |
|||||||||
|
number |
1 |
1 |
1 |
1 |
1 |
nd |
nd |
1 |
|
|
shape |
flattened |
flattened |
flattened |
flattened |
flattened |
flattened |
|||
|
cells |
L D |
3–5 7–13 |
2–6 4–12 |
4–5 8–10 |
4 6–9 |
5 10–11 |
5–7 10–13 |
||
|
trichocytes |
solitary |
nd |
nd |
nd |
nd |
nd |
nd |
nd |
|
|
Conceptacles |
|||||||||
|
projection |
109 |
41–70 |
90 |
65 |
61–85 |
57–68 |
67–83 |
109 |
|
|
external D |
181 |
120–160 |
203 |
190 |
99–150 |
91–120 |
182–238 |
293 |
|
|
chambers |
D H |
118 |
89–109 |
129 |
119–126 |
69–98 |
79–90 |
114–154 |
161 |
|
58 |
65–68 |
59 |
56–67 |
47–64 |
51–56 |
65–71 |
101 |
||
|
columellated |
– |
– |
– |
– |
– |
– |
– |
– |
|
|
pore canal |
L W |
34 |
14 |
22 |
28 |
19–31 |
18 |
27 |
40 |
|
21 |
9 |
12 |
29 |
12.19 |
17 |
6 |
43 |
||
|
cells in the roof filaments |
3 |
3 |
1–2 |
3 |
1–3 |
1–3 |
3 |
2–3 |
|
|
roof cells elongated |
– |
– |
– |
– |
– |
– |
– |
– |
|
Description.—Species was observed in samples from shallow water bioconstructions in Wolfsthal formed by L. minus, Titanoderma pustulatum, melobesioid alga and nubecularid foraminifers. Fragments of thalli are found in micrite sediment, together with ooids and benthic foraminifers. These fragments continue in growth and develop small and irregular rhodoliths less than 1 mm in D. Species from Rohožník grow on coral–coralline-algal–bryozoan reef, or encrust other algae in soft bottom infra- to circalittoral settings (Seneš and Ondrejčíková 1991). Lithoporella minus develops thin thallus with distinct conceptacles projecting above the thallus surface. Growth form is encrusting with applanate branches. Thallus is pseudoparenchymatous with dorsiventral internal organisation and dimerous construction. Specimen is entirely bistratose (Fig. 6A1). Multicellular postigenous filaments are situated near conceptacles only and on the places where thallus applanately branch. Cells of primigenous filaments are palisade in patches, rounded, square, flattened to rectangular (Fig. 6A2). Cells are 7–27 μm H (mean ± SD: 15 ± 5.8 μm) and 7–18 μm L (mean ± SD: 12 ± 3 μm) (n = 30). H/L ratio is 0.5–2.8. Postigenous filaments around conceptacles are of the same dimensions. Epithallial cells are flattened 2–6 μm L (mean ± SD: 3 ± 1.3 μm) and 4–12 μm D (mean ± SD: 6 ± 2.9 μm) (Fig. 6A3) (n = 8). Trichocytes were not observed on bi/tetrasporophyte but occur solitary in gametangial–carposporangial plant.
Gametangial–carposporangial plant possesses uniporate conceptacles projecting above the thallus surface 109 μm with external diameter 224 μm (Fig. 6B1). Conceptacles are of peripheral to fertile area cells and cells of the pore canal projecting inside the canal (Fig. 6B1). Chamber is 122 μm D and 58 μm H. Pore measures 39 μm L and 21 μm W at the base. Dimensions of the conceptacle suggest carposporangial conceptacle. Solitary trichocytes are present in the thallus (Fig. 6B).
Bi/tetrasporangial conceptacle protrudes up to 109 μm above thallus surface and has 181 μm external diameter (Fig. 6A4). Chamber is 118 μm D and 58 μm H. Pore canal is cone shaped 34 μm L and 21 μm W. Single elongated cell lines the pore canal. Cells at the both sides of the cut are elongated and narrower than roof cells (SOM: figs S2, S3). Other specimen could have 1 pore canal cell in the roof formed by two celled filaments next to the pore canal (SOM: fig. S3). The roof filaments can consist of 1–3 cells.
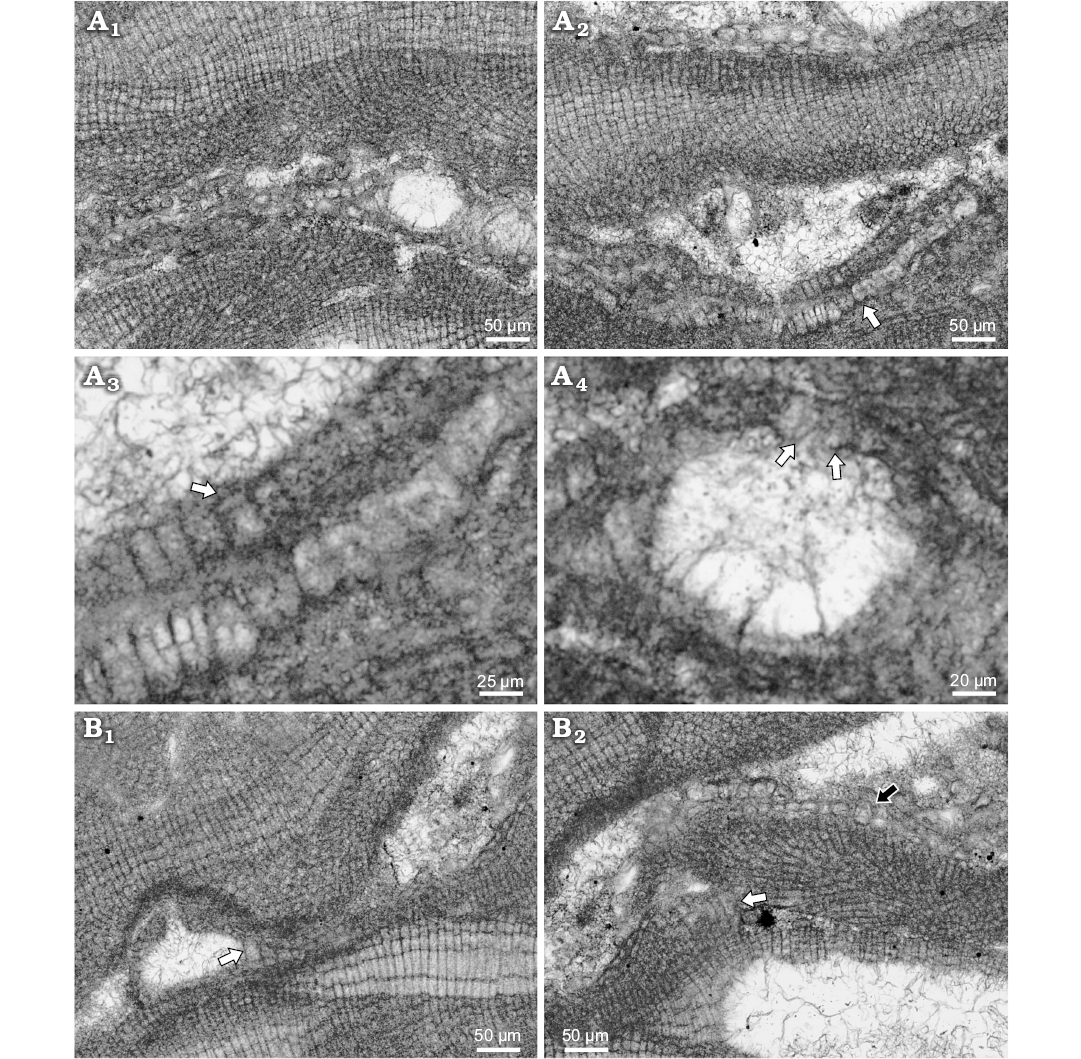
Fig. 6. Lithoporella minus Johnson, 1964 from early Serravallian, Rohožník, Slovakia, Vienna Basin. A. Bi/tetrasporophyte, NHM B1850/1 (thin section 5099). A1. Plant with encrusting growth form and conceptacles projecting above thallus surface. Thallus is entirely bi-stratose. A2. Palisade cells in primigenous filament developed in patches and non-palisade cells are present as well (arrow). A3. Arrow points to flattened not flared epithallial cell; note cell fusions in lower thallus. A4. Enlarged A1. Uniporate conceptacle with preserved pore canal cells (arrows). Cells are narrower and elongated. B. Gametangial-carposporangial plant, NHM B1850/2 (thin section 5099). B1. Projection of the conceptacle above thallus surface; arrow points to the single trichocyte at the side of the conceptacle. B2. Single trichocyte in distant portion of the thallus (black arrow); white arrow points to the palisade cells.
Remarks.—Lithoporella minus has been found in the Langhian and Serravallian (middle Miocene). There is no example that such thallus was published till now from the middle Miocene of Slovakia or Czech Republic. But species known as Melobesia from the Paleocene and Eocene successions, figured in Schaleková´s (1962) correspond with Lithoporella minus (Schaleková 1962: pl. 12: 23). Thus, Melobesia sp. from Sandberg and Rohožník with no figure or reference to thin sections (Schaleková 1969: 100; Schaleková 1973: 218) would most likely represent observed species. Multiporate bi/tetrasporic conceptacles were not proved in any specimen. Species is known from early Langhian Carpathian Foredeep in Poland (Studencki 1988b) and late Serravallian Transylvania Basin (Bucur and Nicorici 1992). However, occurrences from Poland are sterile plants and identifications are based solely on palisade cells presence in entirely bistratose thalli. In contrary, fertile plant from Transylvania Basin known under the name Lithoporella aff. minus fits with characters of the observed specimens (Table 7), but do not match with the type of L. minus (Bucur and Nicorici 1992; Johnson 1964). Johnson (1964) mentioned, in conceptacles chambers description, only mean diameter. This value corresponds with the largest diameter measured in specimens from Central Parathethys. However, it is not known whether all specimens match with the minimum or it is out of the diameter interval of L. minus. Importantly, description of pore canal cells was not provided by Johnson (1964). Presence of palisade cells in primigenous filaments, dimerous thallus construction, lateral cell fusions and the type of bi/tetrasporic conceptacle development correspond with the diagnosis of the genus Lithoporella (Turner and Woelkerling 1982).
Geographical and stratigraphical range.—Eocene to middle Miocene of the Pacific and central Paratethys.
Lithoporella sp. 1
Fig. 7A, B, Table 8.
Material.—Bi/tetrasporic plants from Schaleková’s collection (Schaleková 1973), NHM B1849 and NHM B1851/2 (thin sections 5233 and 5111, respectively), early Serravallian, Rohožník, Slovakia (Vienna Basin). Bi/tetrasporic plants from newly collected samples (Fig. 7A), NHM B1855 (thin section 371111453), early Serravallian, Vývrat, Slovakia (Vienna Basin). Spermatangial gametophyte (Fig. 7B), NHM B1856 (thin section 31011152), late Langhian, Stupava, Slovakia (Vienna Basin).
Description.—Lithoporella sp. 1 was found as overgrow of other coralline algae, geniculate mostly. It creates 63–214 μm thick thallus of encrusting growth form. Description is based on newly collected samples. Applanate branches were not observed. Surface is undulate with protruding conceptacles. Thallus is pseudoparenchymatous with dorsiventral interanal organisation and dimerous construction. Bistratose thallus is presented but with multicelled postigenous filaments predomination (Fig. 7A1). Primigenous filaments consist of palisade cells 9–65 μm H (mean ± SD: 30 ± 16.3 μm) and 7–19 μm L (mean ± SD: 12 ± 2.9 μm) (n = 13). Some cells are flattened to square but palisade predominate. H/L ratio is 0.5–6.2 (Fig. 7A2). Postigenous filaments consist of up to 10 cells. Cells are 11–29 μm L (mean ± SD: 18 ± 5.5 μm) and 9–18 μm D (mean ± SD: 13 ± 2.5 μm) (n = 8). Epithallial cells were not observed on this specimen, but in other are flattened to rounded. Trichocytes were not observed.
Spermatangial conceptacle is more or less triangular, or elliptical, projecting 19 μm above thallus surface (Fig. 7B1). Chamber is 36 μm D and 26 μm H. Pore canal is cylindrical 27 μm L and 11 μm W. The roof consists of 3–4 celled filaments (Fig. 7B2).
Bi/tetrasporangial conceptacle chamber is 139 μm D and 70 μm H (Fig. 7A1). Pore canal is cylindrical 44 μm L and 27 μm W. Pore canal cells are the same as the roof cells. Pore canal filaments are 2 celled (Fig. 7A1).
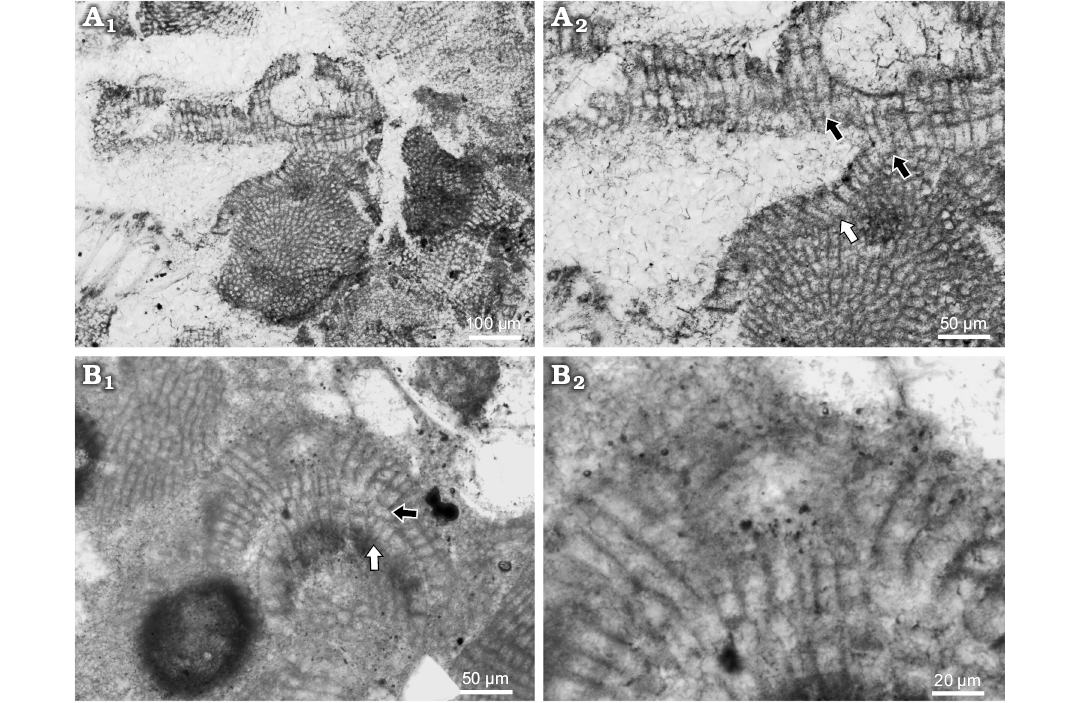
Fig. 7. Coralline alga Lithoporella sp. 1. A. Bi/tetrasporophyte, NHM B1855 (thin section 371111453), early Serravallian, Vývrat, Slovakia, Vienna Basin. A1. Encrusting growth form with prominent conceptacle; note type of the conceptacle with roof cells oriented perpendiculary to the chamber. A2. Palisade cells (white arrow) and lateral fusion of cells in adjacent filaments (black arrows). B. Male gametophyte, NHM B1856 (thin section 31011152), late Langhian, Stupava, Slovakia, Vienna Basin. B1. Spermatangial plant with encrusting growth form; black arrow point to the lateral cells fusions in adjacent filaments; white arrow point to the palisade cells. B2. Detail of the spermatangial conceptacle; note the type of the conceptacle with roof filaments running parallel with the chamber and bending upward to develop the pore canal.
Remarks.—Species occurs in new samples as in Schaleková’s (1973) material from Rohožník. However, it was not described prior to this work by her. Therefore, specimens are considered as unpublished. Species is characteristic in having large palisade cells in primigenous filaments with H/L ratio 2–6, bi/tetrasporic conceptacles development and lateral fusion of cells in adjacent filaments, as well as multicellular thallus. Characters fit to the diagnosis of the genus Lithoporella (Guiry in Guiry and Guiry 2018). Basic characters of known specimens are listed in Table 8.
Table 8. Comparative table of selected Lithoporella sp. 1 specimens from Vienna Basin. * marks gametangial or carpogonial-carposporangial plants. Abbreviations: D, diameter; H, height; L, length; W, wide; nd, no data; “+” presence and “–” absence of character. All measurements in μm.
|
Specimen number (NHM) |
B1856 |
B1855 |
B1851/2 |
B1849 |
|
|
Thin section |
31011152* |
371111453 |
5111 |
5233 |
|
|
Habitat |
littoral |
littoral |
littoral |
littoral |
|
|
Growth form |
encrusting |
encrusting |
encrusting |
encrusting |
|
|
Applanate branches |
– |
– |
– |
– |
|
|
Entirely bistratose |
– |
– |
– |
– |
|
|
Primigenous filaments |
|||||
|
cells shape |
rectangular palisade |
rectangular palisade |
rectangular palisade |
rectangular palisade |
|
|
H/L ratio |
1.4–2.6 |
1.6–4 |
0.4–6.2 |
1–2.5 |
|
|
cells H, L |
16–22 8–15 |
18–36 7–12 |
9–65 7–19 |
13–29 8–15 |
|
|
laterall cell |
+ |
+ |
+ |
+ |
|
|
Postigenous filaments |
|||||
|
multicellular |
+ |
+ |
+ |
+ |
|
|
cells shape |
rectangular flattened |
rectangular flattened |
rectangular flattened |
rectangular flattened |
|
|
L/D ratio |
1.2–2.3 |
1.2–2.7 |
0.6–2.9 |
0.8–1.8 |
|
|
cells |
H L |
12–23 9–11 |
18–33 11–15 |
11–29 9–18 |
9–25 11–16 |
|
laterall cell |
+ |
+ |
+ |
+ |
|
|
Meristematic cells |
|||||
|
elongated |
nd |
nd |
– |
– |
|
|
Epithallial cells |
|||||
|
number |
nd |
nd |
1 |
1 |
|
|
shape |
flattened rounded |
flattened rounded |
|||
|
cells |
L D |
5–6 8–9 |
4–9 11–15 |
||
|
trichocytes |
nd |
nd |
nd |
nd |
|
|
Conceptacles |
|||||
|
projection |
19 |
101 |
56 |
70 |
|
|
external D |
79 |
260 |
194 |
248 |
|
|
chambers |
D H |
36 26 |
139 95 |
139 70 |
159 65 |
|
columellated |
– |
– |
– |
– |
|
|
pore canal |
L W |
27 11 |
38 36 |
44 27 |
40 41 |
|
cells in the |
3–4 |
2 |
2 |
2(3) |
|
|
roof cells |
– |
– |
– |
– |
|
Discussion
Prior to this study, the two species of Hydrolithon, H. corculumis and H. lemoinei were known from the middle Miocene of central Paratethys (Hrabovský et al. 2015; Chelaru and Bucur 2016). Former palaeontologists working in central Paratethys classified these species under the names Lithophyllum corculumis, Lithophyllum (Dermatolithon) nataliae or Lithophyllum (Dermatolithon) sp. (Schaleková 1973; Pisera 1985; Schaleková and Čierna 1985; Studencki 1988b). These species are morphoanatomically coresponding to H. corculumis and H. lemoinei. To the contrary, Lithoporella was known from Carpathian Foredeep and Transylvania Basin (Pisera 1985; Studencki 1988b; Bucur and Nicorici 1992) but it was absent in limestone beds in Slovakian basins. Here morphologically comparable specimens of the genus Melobesia was presented (Schaleková 1969, 1973). Melobesia (Lithoporella) badji Maslov, 1956 was known from the Polish part of Carpathian Foredeep also (Pisera 1985). Some determinations of these species were based on the sterile plants, but most of them consider fertile ones with conceptacles proposed to be bi/tetrasporic (Pisera 1985; Studencki 1988b). Sexual phases were not described for none of these species. This study revealed the presence of three Hydrolithon and two Lithoporella species in selected Paratethyan sites. The first genus is represented by H. corculumis, H. lemoinei, and Hydrolithon sp. 1, all having complete life cycles, including plants with spermatangial, carpogonial–carposporangial and bi/tetrasporangial conceptacles. Lithoporella includes L. minus with plants bearing bi/tetrasporangial conceptacles and possible carpogonial-carposporangial conceptacles and Lithoporella sp. 1 with detected two phases, bi/tetrasporophyte and male gametophyte. Attribution of different phases to each other was, however, based upon morpho-anatomical comparison of plants with different types of the conceptacles, thus raising the question whether identifications are correct. Taxonomy of corallines relies on bi/tetrasporophyte description: calcified sporangial compartments are characteristic for order Sporolithales, multiporate conceptacles for Hapalidiales and uniporate for Corallinales. Bi/tetrasporophytes of the first two differ from their sexual phases. In the last mentioned situation is complicated since pattern of the conceptacles roof and the pore canal development is of the same type in all reproductive phases. The best marks of bi/tetrasporic conceptacles would be bi/tetraspores located within the conceptacles. Bi/tetraspores are found in the fossils sporadically, but in most of the specimens they are missing and unknown (Aguirre and Braga 1998; Braga et al. 2005). The same situation of reproductive organs is within fossil gametophytes. Absence of reproductive structures in central Paratethyan specimens makes clear statements about their nature almost impossible. However, there are few hints for reproductive phases consideration. The first one is the type of the roof and the pore development in proposed types of conceptacles. Bi/tetrasporic plants of Hydrolithon and Lithoporella bear conceptacles with roofs formed by cells perpendicularly oriented to the chamber (Type 2 according Johansen 1981), while gametophytes and carposporangial conceptacles have roofs formed by cells running more or less parallel with the chamber (Type 1 according Johansen 1981). Second hint is in two types of conceptacles presented in the single plant that include larger and smaller conceptacles. Variation of sizes results from sexual reproduction. Enlargement of concaptacles chamber occurs after caryogamy and subsequent development of carposporophyte and carposporangia inside carpogonial conceptacle, associated with the destruction of surrounding cells. As a result, carposporangial conceptacle is larger than carpogonial (e.g., Irvine and Chamberlain 1994). This character was recently studied by Athanasiadis and Ballantine (2014) in genera Mesophyllum and Melyvonnea. Third hint are the same morpho-anatomical features of different plants found in the same site or at least same stratigraphic level (Hrabovský et al. 2015). There are some exceptions because bi/tetrasporic plants were found in Langhian, while gametophyte in Serravallian, in localities close to each other (Lithoporella sp. 1). However, observations give no adequate characters allowing specimens separation. Given above, observed plants from central Paratethys with the same morpho-anatomical characters possessing different conceptacles are life cycle phases, rather than different species.
Approach used here was earlier applied to Lithophyllum racemus (Basso et al. 1996), Neogniolithon conti (Quaranta et al. 2007), and Spongites fruticulosus (Basso and Rodondi 2006). Advantage of this approach is reduction of conspecific species on the basis of well-established coralline-algal taxonomical concept. Subsequently, such improvement led to proper and more consistent palaeobiogeographic and palaeoecological inferences. An example could be found in the ability of gametophyte and bi/tetrasporophyte conceptacles development depending on the sea water temperature (Henriques et a. 2012; Athanasiadis and Ballantine 2014). As a consequence, limestone depositional environment on certain Paratethyan sites and stratigraphic levels favoured full growth of complete cycles. This is important since Lithoporella inhabits Recent tropics and subtropics, while Hydrolithon could be found in tropics to boreal seas (Adey et al. 1982; Irvine and Chamberlain 1994; Ringeltaube and Harvey 2000; Harvey et al. 2005, 2006). However, recent exploration level of fossil corallines from central Paratethys does not allow any palaeoecological interpretation without complete coralline algal assemblage analyses.
Importance of life cycle application is in the improvement of the knowledge of the type material. For example, the H. corculumis type was considered as uncertain life phase, bearing either bi/tetrasporangial or carpogonial-carposporangial conceptacles, although roof cells preferring bi/tetrasporophyte (Braga et al. 2005). The same is true, even not discussed, for H. lemoinei. Although Aguirre et al. (2011) considered the type specimen as bi/tetrasporophyte, its uncertain roof anatomy with roof cells more or less perpendicular to the chamber, brings some doubts. It is worth to note that bi/tetrasporophytes possess prime diagnostic characters for species attribution to higher taxonomic levels. In summary, identification of bi/tetrasorophytes in fossil record would require confirmation via sexual plants at least in some families of the order Corallinales.
Presented finding confirms that type of H. corculumis and H. lemoinei are bi/tetrasporophytes but raises the question whether proposed Lithoporella bi/tetrasporophytes from central Paratethys represent the true bi/tetrasporophytes or gametophytes also. Nevertheless, distinct life phases of L. melobesioides possess conceptacles with clearly differentiated roof anatomy, the situation is different for some Hydrolithon (Ivirne and Chamberlain 1994; Woelkerling and Penrose 1996). Distinct roof anatomy allows inferences that identified specimens from central Paratethys are bi/tetrasporic accompanied with either spermatangial or carpogonial, carposporangial plants.
In summary, H. lemoinei and Hydrolithon sp. 1 gametophytes are dioecious, while H. corculumis is monoecious. The findings are not contradictory to observation of extant species, while both, monoecy and dioecy are known in extant Hydrolithon (Harvey et al. 2006). This character was not used in fossil algae, but is known e.g., in Neogoniolithon conti (Quaranta et al. 2007) and Spongites (Chelaru and Bucur 2016) having dioecious gametophytes preserved. Considering fossil Lithoporella, more specimens have to be found to explore their sexual reproduction.
Excluding reproductive cycles, other characters were used for species determination. These include trichocytes and bi/tetrasporic pore canal anatomy. The discussion whether the trichocytes have any diagnostic value in taxonomical treatment in the genera Hydrolithon and Porolithon proved their importance at least in some species based both, morpho-anatomical and molecular data (e.g., Penrose and Woelkerling 1992; Kato et al. 2011; Basso et al. 2014; Maneveldt and Keats 2014; Rösler et al. 2016). Recently trichocytes were used in the identifications and confirmations of the genera Hydrolithon and Neogoniolithon from the early and late Miocene limestone beds (Basso and Granier 2018; Rösler et al. 2015). Most recent data enable to discriminate: (i) Hydrolithoideae (the genus Hydrolithon), that encompass plants with trichocytes not arranged in large and tightly packed horizontal field and possess dimerous thallus construction (ii) Metagoniolithoideae (genus Harveylithon) including plants with trichocytes solitary and possess monomerosu thallus construction, while (iii) Porolithoideae (genus Porolithon) encompassing plants possessing trichocytes in large and tightly packed horizontal fields and monomerous construction (Rösler et al. 2016). Following the key above, it is most likely that well-established characters in algae palaeontology including dimerous thallus, uniporate bi/tetrasporangial conceptacles with roofs formed by cells perpendicularly (or more, less perpendicularly) oriented to the chamber, would enable determine fossil Hydrolithon, even when trichocytes are absent (e.g., Aguirre at al. 2011). Nevertheless, Hydrolithon sp. 1 and H. corculumis, both with trichocytes solitary scattered in the thallus, fit within the modern genus diagnosis. Situation changes when considering entirely bistratose thalli recently found e.g., in Porolithon improcerum. Absence of trichocytes would favour attribution to the genus Hydrolithon, while their presence in tightly packed horizontal fields would prove Porolithon genus (Harvey et al. 2006; Bahia et al. 2014). However, Hydrolithon nor Porolithon with entirely bistratose thalli are not present in examined material.
Single trichocytes and short patches of palisade cells in primigenous filaments occur in entirely bistratose thallus of the documented Lithoporella species. Trichocytes together with the type of the bi/tetrasporic conceptacles fits within the diagnosis of the genus Lithoporella (Turner and Woelkerling 1982; Guiry and Guiry 2018). Johnson (1964) used length of the palisade cells and dimensions of conceptacles to separate L. melobesioides and L. minus. Studied species of L. minus possesses smaller palisade cells with H/L ratio 2–3 when present, while Lithoporella sp. 1 H/L ratio is often 3–6. Our results show that dimensions of cells, even of the low diagnostic value, can be applied on CP specimens when using shape of the bi/tetrasporic pore canal and the thallus thickness collectively. Given above, palisade cells presence at least in patches allows to distinguish between the Hydrolithon and Lithoporella specimens bearing bistratose thalli.
Considering the bi/tetrasporic pore canal anatomy, few features enable separation of observed species. These are: (i) number of cells per pore canal filament, (ii) length of the pore canal filaments, and (iii) projection of papillae into the pore canal. Excluding the rest of the morpho-anatomical characters, short pore canal filaments consisting of 3 cells without projecting papillae determine H. corculumis, long pore canal filaments with up to 7 pore canal cells and pore canal without projecting papillae determine Hydrolithon sp. 1 and long pore canals lined with projecting papillae characterize H. lemoinei. Determinations could be accompanied by presence of elongated roof cells in H. corculumis, that are absent in the two others. Lithoporella minus possesses conceptacles with single, occasionally two pore canal cells that are elongated and narrow, while in Lithoporella sp. 1 pore canal filaments 2 (3) celled consisting of cells more or less similar to the rest of the roof cells are present.
Generalized identification key (Table 3) based on discussed characters is not a final one, since many sites and specimens have yet to be explored. Also, review of the historical collections from the Paratethys would improve our knowledge of non-geniculate coralline algae. Despite its incompleteness, this key represents a useful tool for coralline algae identification and recognition in the middle Miocene limestone beds.
Conclusions
Three Hydrolithon species with complete life cycles and two Lithoporella with incomplete life cycles were determined in the middle Miocene material from selected Central Paratethys sites. Determined species are Hydrolithon corculumis, Hydrolithon lemoinei, Hydrolithon sp. 1, Lithoporella minus and Lithoporella sp. 1. For the first time, life cycle phases are reported from extinct coralline algae H. corculumis and H. lemoinei and one uncertain species Hydrolithon sp. 1, completing the list of fossil plants with known spermatangial, carpogonial, carposporangial, and bi/tetrasporangial conceptacles. Complete life cycle phases identified in examined material proved presence of bi/tetrasporic plants in the type of H. corculumis and H. lemoinei and represent thus important diagnostic character in the extinct species determination within the order Corallinales. Consequently, H. lemoinei and Hydrolithon sp. 1 possess dioecious and H. corculumis monoecious gametophytes. It is more likely that further research will complete Lithoporella life cycle phases and improve our knowledge on this genus. Despite the absence of full life cycles, both genera are separated on the basis well-established character in palaeontology and biology, palisade cells presence/absence in primigenous filaments.
Emended identification key based on taxonomically important and well-known diagnostic characters is proposed for determination of the central Paratethyan fossil coralline algae. Current level of Paratethyan coralline algae exploration is low and does not allow to treat the key as final but rather as a starting point for further work on other species.
Acknowledgements
Author is thankful to Nela Doláková (Masaryk University, Brno, Czech Republic), Natália Hudáčková and Daniel Pivko (both Comenius University, Bratislava, Slovakia) and Andrzej Pisera (Institute of Paleobiology PAS, Warsaw, Poland) for providing historical and private collections. I would like to express my very great appreciation to Filippo Barattolo (Università degli Studi di Napoli, Italy) and an anonymous referee for expert reports and their time. Also, I would like to thank Ivana Koubová (Earth Science Institute, Slovak Academy of Sciences, Slovakia) for English corrections of the manuscript. Research was supported with the VEGA 2/0122/18 the grant agency for MŠVVaŠ and SAV project.
References
Adey, W.H. and McKibbin, D.L. 1970. Studies on the maerl species Phymatolithon calcareum (Pallas) nov. comb. and Lithothamnion corallioides Crouan in the Ria de Vigo. Botanica marina 13: 100–106. Crossref
Adey, W.H., Townsend, R.A., and Boykins, W.T. 1982. The crustose coralline algae (Rhodophyta: Corallinaceae) of the Hawaiian Islands. Smithsonian Contributions to the Marine Sciences 15: 1–74. Crossref
Aguirre, J. and Braga, J.C. 1998. Redescription of the Lemoine’s types of coralline algal species from Algeria. Palaeontology 41: 489–507.
Aguirre, J., Braga, J.C., and Bassi, D. 2011. Taxonomic assessment of coralline algal species (Rhodophyta; Corallinales and Sporolithales) described by Pfender, Lemoine, and Miranda from northern Spain type localities. Annalen des Naturhistorischen Museums in Wien, Serie A 113: 267–289.
Aguirre, J., Riding, R., and Braga, J.C. 2000. Diversity of coralline red algae: origination and extinction patterns from the Early Cretaceous to the Pleistocene. Paleobiology 26: 651–667. Crossref
Athanasiadis, A. and Ballantine, D.L. 2014. The genera Melyvonnea gen. nov. and Mesophyllum s.s. particularly from the central Atlantic Ocean. Nordic Journal of Botany 32: 385–436. Crossref
Athanasiadis, A., Lebednik, P.A., and Adey, W.H. 2004. The genus Mesophyllum (Melobesioideae, Corallinales, Rhodophyta) on the northern Pacific coast of North America. Phycologia 43: 126–165. Crossref
Bahia, R.G., Amando-Filfo, G.M., Azevedo, J., and Maneveldt, G.W. 2014. Porolithon improcerum (Porolithoideae, Corallinaceae) and Mesophyllum macroblastum (Melobesioideae, Hapalidiaceae): new record of crustose coralline algae for the Southwest Atlantic Ocean. Phytotaxa 1: 38–44. Crossref
Bahia, R.G., Maneveldt, G.W., Amando-Filho, G.M., and Yoneshique-Valentin, Y. 2015. New diagnostic characters for the order Sporolithales (Corallinophycidae; Rhodophyta). Journal of Phycology 51: 1137–1146. Crossref
Baráth, I. 1992. Upper Badenian reef complex on the eastern margin of the Vienna Basin. Knihovníčka ZPN 15: 177–197.
Baráth, I., Nagy, A., and Kováč, M. 1994. Sandberg Member—Upper Badenian marginal deposits of the eastern margin of the Vienna Basin. Geologické práce, Správy 99: 59–66.
Bassi, D., Braga, J.C., Zakrevskaya, E., and Petrovna-Radionova, E. 2005. Re-assessment of the type collections of corallinalean genera (Corallinales, Rhodophyta) described by V.P. Maslov. Palaeontology 48: 929–945. Crossref
Basso, D., Fravega, P., and Vannucci, G. 1996. Fossil and living Corallinaceans related to the Mediterranean endemic species Lithophyllum racemus (Lamarck) Foslie. Facies 35: 275–292. Crossref
Basso, D. 1998. Deep rhodolith distribution in the Pontian Islands, Italy: a model for the paleoecology of a temperate sea. Palaeogeography, Palaeoclimatology, Palaeoecology 137: 173–187. Crossref
Basso, D. and Granier, B. 2018. Johnson & Kaska 1965 fossil coralline algae from Guatemala (revision of the Jasse Harlan Johnson collection, part 4). Rivista Italiana di Paleontologia e Stratigrafia 124: 91–104.
Basso, D. and Rodondi, G. 2006. A Mediterranean population of Spongites fruticulosus (Rhodophyta, Corallinales), the type species of Spongites, and the taxonomic status of S. stalactitica and S. racemose. Phycologia 45: 403–416. Crossref
Basso, D., Caragnano, A., and Rodondi, G. 2014. Trichocytes in Lithophyllum kotchyanum and Lithophyllum spp. (Corallinales, Rhodophyta) from the Indian Ocean. Journal of Phycology 50: 711–717. Crossref
Basso, D., Fravega, P., and Vannucci, G. 1996. Fossil and living Corallinaceans related to the Mediterranean endemic species Lithophyllum racemus (Lamarck) Foslie. Facies 35: 275–292. Crossref
Basso, D., Fravega, P., and Vannucci, G. 1997. The Taxonomy of Lithothamnium ramosissimum (Gümbel non Reuss) Conti and Lithothamnium operculatum (Conti) Conti (Rhodophyta, Corallinaceae). Facies 37: 167–182. Crossref
Basso, D., Vrsaljko, D., and Grgasović, T. 2008. The coralline flora of a Miocene maërl: the Croation “Litavac”. Geologica Croatica 61: 333–340.
Braga, J.C., Bassi, D., Zakrevskaya, E., and Petrovna-Radionova, E. 2005 Reassessment of the type collection of Maslov’s species of Corallinales (Rhodophyta). I. Species originally attributed to Lithophyllum and Melobesia. Revista Española de Paleontologia 20: 207–224.
Braga, J.C., Bosence, D.W.J., and Steneck, R.S. 1993. New anatomical characters in fossil coralline algae and their taxonomic implications. Palaeontology 36: 535–547.
Bucur, I. and Nicorici, E. 1992. Calcareous Algae from the Sarmatian deposits in the Şimleu Basin (Romania). Studia University Babes-Bolyat, Geologia 2: 3–7.
Chelaru, R. and Bucur, I. 2016. The taxonomy of middle Miocene red algae from the Gârbova de Sus Formation (Transylvania Basin, Romania). Carnets de Geologie 16: 307–336. Crossref
Coletti, G., Basso, D., and Corselli, C. 2018. Coralline algae as depth indicators in the Sommières Basin (early Miocene, Southern France). Geobios 51: 15–30. Crossref
Conti, S. 1943. Contributo allo studio delle Corallinaceae de terziario italiano. II: Le Corallinaceae del Miocene del Basino Ligure-Piemontse. Palaeontographica italica 41: 37–61.
Cronquist, A. 1960. The divisions and classes of plants. The Botanical Review 26: 425–482. Crossref
Doláková, N., Brzobohatý, R., Hladilová, Š., and Nehyba, S. 2008. The rea-algal facies of the Lower Badenian limestones of the Carpathian Foredeep in Moravia (Czech Republic). Geologica Carpathica 59: 133–146.
Foslie, M. 1909. Algologiske notiser VI. Kongelige Norske Videnskabers Selskabs Skrifter 1909 (2): 1–63.
Golonka, J. 1981. Glony i biosedymentacja wapieni mioceńskich okolic Rzeszowa. Biuletyn Instytutu Geologicznego 332: 5–46.
Guiry, M.D. and Guiry, G.M. 2018. Algaebase. National University of Ireland, Galway. http://www.algaebase.org [searched on 23 November 2018].
Harvey, A.S., Phillips, L.E., Woelkerling, W.J., and Millar, A.J. 2006. The Corallinaceae, subfamily Mastophoroideae (Corallinales, Rhodophyta) in south-eastern Australia. Australian Systematic Botany 19: 387–429.
Harvey, A.S., Woelkerling, W.J., Farr, T., Neill, K., and Nelson, W. 2005. Coralline algae of central New Zealand: an identification guide to common “crustose” species. NIWA Information Series 57: 1–145. Crossref
Harzhauser, M. and Piller, E.W. 2004. Integrated stratigraphy of the Sarmatian (Upper Middle Miocene) in the western Central Paratethys. Stratigraphy 1: 65–86.
Harzhauser, M. and Piller, E.W. 2007. Benchmark data of a changing sea—Palaeogeography, Palaebiogeography and events in the Central Paratethys during the Miocene. Palaeogeography, Palaeoclimatology, Palaeoecology 253: 8–31. Crossref
Henriques, M.C., Villas-Boas, A., Rodriguez, R.R., and Figueiredo, M.A.O. 2012. New records of rhodolith-forming species (Corallinales, Rhodophyta) from deep water in Espírito Santo State, Brazil. Helgoland Marine Research 66: 219–231. Crossref
Hrabovský, J. 2013. Non-geniculate coralline algae (Corallinales, Sporolithales, Rhodophyta) from lithathamnion limestones of the locality Vrchná Hora at the town of Stupava (Vienna Basin, Slovakia). Mineralia Slovaca 45: 23–34.
Hrabovský, J., Basso, D., and Doláková, N. 2015. Diagnostic characters in fossil coralline algae (Corallinophycidae, Rhodophyta) from the Miocene of Southern Moravia (Carpathian Foredeep, Czech Republic). Journal of Systematic Palaeontology 14: 499–525. Crossref
Irvine, L.M. and Chamberlain, Y.M. 1994. Seaweeds of the British Isles, Volume I Rhodophyta, Part 2B Corallinales, Hildenbrandiales. 276 pp. Pelagic Publishing, London.
Johansen, H.W. 1981. Coralline Algae, A First Synthesis. 239 pp. CRC press, Boca Raton.
Johnson, J.H. 1964. Eocene Algae from Ishigaki-shima Ryūkyū-rettō. 38 pp. United States Government Printing Office, Washington. Crossref
Kato, A., Baba, M., and Suda, S. 2011. Revision of the Mastophoroideae (Corallinales, Rhodophyta) and polyphyly in nongenculate species widely distributed on Pacific coral reefs. Journal of Phycology 47: 663–672. Crossref
Kováč, M. 2000. Geodynamic, Paleogeographic and Structural Development of the Carpathian-Pannonian Region During the Miocene: New Insights on the Slovak Neogene Basins. 202 pp. Veda, Bratislava.
Kováč, M., Andreyeva-Grigorovich, A., Bajraktarević, Z., Brzobohatý, R., Filipescu, S., Fodor, L., Harzhauser, M., Nagymarosy, A., Oszczypko, N., Pavelić, D., Rögl, F., Saftić, B., Sliva, Ľ., and Studencka, B. 2007. Badenian evolution of the Central Paratethyas Sea: paleogeography, climate and eustatic sea-level changes. Geologica Carpathica 58: 579–606.
Le Gall, L. and Saunders, G.W. 2007. A nuclear phylogeny of the Florideophyceae (Rhodophyta) inferred from combined EF2, mall subunit and large subunit ribosomal DNA: establishing the new red algal subclass Corallinophycidae. Molecular Phylogenetics and Evolution 43: 1118–1130. Crossref
Maneveldt, G.W. and Keats, D.W. 2014. Taxonomic review based on new data of the reef-building alga Porolithon onkodes (Corallinaceae, Corallinales, Rhodophyta) along with other taxa found to be conspecific. Phytotaxa 190: 216–249. Crossref
Maslov, V.P. 1956. Fossil calcareous algae of USSR [in Russian]. Trudy Instituta geologičeskih Nauk Akademii Nauk SSSR 160: 1–301.
Maslov, V.P. 1962. Fossil red algae of USSR and their connections with facies [in Russian]. Trudy Geologičeskogo Instituta Akademii Nauk SSSR 53: 1–222.
Mastrorilli, V.I. 1968. Nuovo contributo allo studio delle corallinacee dell’Oligocene Ligure-Piemontese: I reperti della Tavoletta Ponzone. Atti dell’Istituto di Geologia della Università di Genova 5: 154–406.
Miranda, F. 1935. Algas coralináceas fósiles del Terciaro de san Vincente de la Barquera (Santander). Boletin de la Sociedad Española de Historia Natural 35: 279–289.
Novák, Z. 1975. Spodnobádenské vápence karpatské předhlubně. 190 pp. Candidate Dissertation Thesis, University of J.E. Purkyně, Faculty of Sciences, Brno.
Penrose, D. and Woelkerling, W.J. 1992. A reappraisal of Hydrolithon and its relationship to Spongites (Corallinaceae, Rhodophyta). Phycologia 31: 81–88. Crossref
Pisera, A. 1985. Paleoecology and lithogenesis of the Middle Miocene (Badenian) algal-vermetid reefs from Roztocze Hills, south-eastern Poland. Acta Geologica Polonica 35: 89–155.
Pisera, A. 1996. Miocene reefs of the Paratethys: a review. In: E.K, Franseen M. Esteban, W.C. Ward, and J. Rouchy (eds.), Models for Carbonate Stratigraphy from Miocene Reef Complexes of Mediterranean Region, 97–104. SEPM Society for Sedimentary Geology, Tulsa. Crossref
Pisera, A. and Studencki, W. 1989. Middle Miocene rhodoliths from the Korytnica Basin (Southern Poland): environmental significance and paleontology. Acta Paleontologica Polonica 34: 179–209.
Pivko, D., Hudáčková, N., Hrabovský, J., Sládek, I., and Ruman, A. 2017. Palaeoecology and sedimentology of the Miocene marine and terrestrial deposits in the “Medieval Quarry” on Devínska Kobyla Hill (Vienna Basin). Geological Quarterly 61 (3): 549–568. Crossref
Quaranta, F., Vannucci, G., and Basso, D. 2007. Neogoniolithon conti comb. nov. based on the taxonomic re-assessment of Mastrorilli’s original collections from the Oligocene of NW Italy (Tertiary Piedmont Basin). Rivista Italiana di Paleontologia e Stratigrafia 113: 43–55.
Riegl, B. and Piller, W.E. 2000. Biostramal Coral Facies—A Miocene Example from the Leitha Limestone (Austria) and its Actualistic Interpretation. Palaios 15: 399–413. Crossref
Ringeltaube, P. and Harvey, A. 2000. Non-geniculate coralline algae (Corallinales, Rhodophyta) on Heron Reef, Great Barrier Reef (Australia). Botanica Marina 43: 431–454. Crossref
Rösler, A., Perfectti, F., Peña, V., and Braga, J.C. 2016. Phylogenetic relationships of the Corallinaceae (Corallinales, Rhodophyta): Taxonomic implications for reef-building corallines. Journal of Phycology 52: 412–431. Crossref
Rösler, A., Pretković, V., Novak, V., Renema, W., and Braga, J.C. 2015. Coralline algae from the Miocene Mahakam delta (East Kalimantan, Southeast Asia). Palaios 30: 83–93. Crossref
Seneš, J. and Ondrejčíková, A. 1991. Proposal for the terminology of fossil marine benthic shelf ecosystems. Geologica Carpathica 42: 231–240.
Schaleková, A. 1962. Fytogénne vápence mezozoika a terciéru Slovenska. 196 pp. Ph.D. Thesis, Comenius University, Bratislava.
Schaleková, A. 1969. Contribution to the knowledge of red algae in the Leitha Limestone at the locality Sandberg near Devínska Nová Ves (southwestern Slovakia). Acta Geologica et Geographica Universitatis Comenianae 18: 93–102.
Schaleková, A. 1973. Oberbadenische corallinaceen aus dem Steinbruch Rohožník-Vajar an dem Westhang der Kleinen Karpaten. Acta Geologica et Geographica Universitatis Comenianae 26: 211–227.
Schaleková, A. 1978. Riasové (Lithothamniové) vápence v bádene Viedenskej, Dunajskej a Juhoslovenskej panvy Západných Karpát. 150 pp. Doctoral Candidate Dissertation Thesis, Comenius University, Bratislava.
Schaleková, A. and Čierna, E. 1985. Algenflora des unteren Badeniens (Corallinaceae, Bacillariophyceae) aus der Umgebung von Kosihovce (Südslowakisches Becken, Westkarpaten). Acta Geologica et Geographica Universitatis Comenianae, Geologica 39: 35–49.
Setchell, W.A. 1943. Mastophora and the Mastophoreae: genus and subfamily of Corallinaceae. Proceedings of the National Academy of Science of the United States of America 29: 127–135. Crossref
Silva, P.C. and Johansen, H.W. 1986. A reappraisal of the order Corallinales (Rhodophyceae). British Phycological Journal 21: 245–254. Crossref
Sremac, J., Bošnjak Makovec, M., Vrsaljko, D., Karaica, B., Tripalo, K., Fio Firi, K., Majstorović Bušić, A., and Marjanac, T. 2016. Reefs and bioaccumulations in the Miocene deposits of the North Croatian Basin—Amazing diversity yet to be described. Rudarsko-geološko-naftni zbornik 31: 19–29. Crossref
Studencki, W. 1988a. Facies and Sedimentary Environment of the Pińczów limestones (Middle Miocene; Holy Cross Mountains, Central Poland). Facies 18: 1–26. Crossref
Studencki, W. 1988b. Red algae from the Pińczów limestones (Middle Miocene, Swietokrzyskie Mountains, Poland). Acta Paleontologica Polonica 33: 3–57.
Studencki, W. 1999. Red-algal limestones in the Middle Miocene of the Carpathian Foredeep in Poland: facies variability and palaeoclimatic implications. Geological Quarterly 43: 395–404.
Townsend, R.A. and Huisman, J.M. 2018. Coralline algae. In: J.M. Huisman (ed.), Algae of Australia. Marine benthic algae of north-western Australia. 2. Red Algae, 86–97, 105–137, 143–146. CSIRO Publishing, Canberra.
Turner, J.A. and Woelkerling, W.J. 1982. Studies on the Mastophora–Lithoporella complex (Corallinaceae, Rhodophyta). Phycologia 21: 201–217. Crossref
Vannucci, G., Piazza, M., Fravega, P., and Basso, D. 2000. Revision and re-documentation of M. Airoldi’s species of Archaeolithothamnium from the Tertiary Piedmont Basin (NW Italy). Rivista Italiana di Paleontologia e Stratigrafia 106: 191–202.
Wettstein, R. von 1901. Handbuch der systematischen Botanik. Vol. 1. 201 pp. Franz Deuticke, Leipzig.
Woelkerling, W.J. 1988. The Coralline Red Algae: An Analysis of the Genera and Subfamilies of Nongeniculate Corallinaceae. 268 pp. British Museum (Natural History), London and Oxford University Press, Oxford.
Woelkerling, W.J. and Penrose, D.L. 1996. Non-geniculate Corallinales. In: H.B.S. Womersley (ed.), The Marine Benthic Flora of Southern Australia, Rhodophyta. Part BIII, Gracilariales, Rhodymeniales, Corallinales and Bonnemaisoniales, 1–392. Australian Biological Resources Study, Canberra.
Woelkerling, W.J., Campbell, S.J., and Harvey, A.S. 1993. Growth-forms in non-geniculate coralline red algae (Corallinales, Rhodophyta). Australian Systematic Botany 6: 277–293. Crossref
Woelkerling, W.J., Bassi, D., and Iryu, Y. 2012. Hydrolithon braganum sp. nov. (Corallinaceae, Rhodophyta), the first known exclusively fossil semi-endophytic coralline red alga. Phycologia 51: 604–611. Crossref
Zágoršek, K., Nehyba, S., Tomanová Petrová, P., Hladilová, Š., Bitner, M.A., Doláková, N., Hrabovský, J., and Jašková, V. 2012. Local catastrophe caused by tephra input near Přemyslovice (Moravia, Czech Republic) during the Middle Miocene. Geological Quarterly 56: 269–283. Crossref
Zdražílková, N. 1988. Corallinaceae from the Badenian of the Carpathian Foredeep [in Czech]. Časopis pro mineralogii a geologii 33: 187–198.
Acta Palaeontol. Pol. 64 (2): 417–439, 2019
https://doi.org/10.4202/app.00579.2018