

A large hyaenodont from the Lutetian of Switzerland expands the body mass range of the European mammalian predators during the Eocene
FLORÉAL SOLÉ and BASTIEN MENNECART
Solé, F. and Mennecart, B. 2019. A large hyaenodont from the Lutetian of Switzerland expands the body mass range of the European mammalian predators during the Eocene. Acta Palaeontologica Polonica 64 (2): 275–290.
We here present a new hyaenodont genus and species from the Lutetian locality of Egerkingen γ (Switzerland; MP13?): Cartierodon egerkingensis gen. et sp. nov. The new taxon is represented by numerous dental elements, mostly isolated teeth. The molars show typical features of a hypercarnivorous predator such as the strong reduction of the crushing (talonid/protocone) and puncturing (metaconid) structures. The calculation of several dental indices indicates that this hyaenodont may have been a bone-cracking predator. The new taxon differs from all the hyaenodonts previously known in Europe during the Ypresian and Lutetian by its larger size, with an estimated mass of almost 29 kg (the size of the extant African wild dog, Lycaon pictus). Other hyaenodonts known for this period do not exceed 20 kg. Previous authors proposed the hypothesis of an ecological limitation of the body mass, but the description of Cartierodon egerkingensis indicates instead that the European hyaenodonts continuously increased in size throughout the Eocene. We also performed a phylogenetic analysis in order to test the relationships of this new taxon: the new hyaenodont appears to be closely related to the Lutetian hyaenodont Prodissopsalis eocaenicus.
Key words: Mammalia, Hyaenodonta, Cartierodon, ecology, phylogeny, Eocene, Switzerland.
Floréal Solé [floreal.sole@naturalsciences.be], D.O. Earth and History of Life, Royal Belgian Institute of Natural Sciences, Rue Vautier 29, B-1000 Brussels, Belgium.
Bastien Mennecart [mennecartbastien@gmail.com](corresponding author), Natural History Museum Basel, Augustinergasse 2, 4001 Basel, Switzerland; Natural History Museum Vienna, Burgring 7, 1010 Vienna, Austria.
Received 11 December 2018, accepted 30 January 2019, available online 23 May 2019.
Copyright © 2019 F. Solé and B. Mennecart. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The hyaenodonts were the most numerous and diverse carnivorous mammals during the Eocene of Europe (Solé 2014; Solé et al. 2014). Solé (2013) grouped most of these hyaenodonts in the subfamily Proviverrinae. The historical concept of Proviverrinae is complex and has been debated. It was long viewed as a grade-based assemblage that groups the early occurring and morphologically primitive (e.g., talonid bearing three cusps, high metaconid on molars, and transversally aligned paracristid) hyaenodonts such as Prototomus, Arfia (both known in Europe and North America in the Ypresian), and Proviverra (known in the Lutetian of Europe) (Gingerich and Deutsch 1989; Smith and Smith 2001; Van Valen 1965). In this system, the hypercarnivorous subfamilies such as Hyaenodontinae and Hyainailourinae may independently root in the “Proviverrinae” (see the discussion in Polly 1996). Solé (2013), using a cladistic analysis, proposed to restrict this subfamily to some European hyaenodonts that are closely related. This resulted in classifying the proviverrines as an endemic European clade of hyaenodonts that experienced its own evolution. Proviverrinae were thus recorded from the Ypresian of France (Rians, Palette, Fournes, Fordones; Mammal Palaeogene MP7) to the Priabonian of Switzerland (Obergösgen; MP19) (Godinot 1981; Godinot et al. 1987; Morlo and Habersetzer 1999).
However, the recent phylogenetic analyses led by Matthew R. Borths (Borths et al. 2016; Borths and Seiffert 2017; Borths and Stevens 2017a–c) found that Proviverrinae is not monophyletic: the taxa resolved in this clade by earlier studies were actually found in different clades deeply nested among Hyaenodonta. Then, it is clear from these debates that the European hyaenodonts are crucial for understanding the evolution of the Laurasian hyaenodonts.
“Proviverrines” sensu Solé (2013) were restricted to the Southern (= Mesogean) Province (sensu Marandat 1997) during the earliest Eocene (MP7), while the oxyaenids, “sinopines”, and “arfiines”, the two latter being hyaenodont subfamilies (Solé 2013) but see Borths et al. (2016) for a rejection of these subfamilies, were present in the Northern Province (Solé et al. 2013a, 2014). “Proviverrines” dispersed into the Northern Province between the Paleocene–Eocene (PE) III biozone (sensu Hooker 1996, 1998) (~55.12 Mya; Hooker 2010) and the PE IV biozone (≈53–51 Mya; Neal 1996; Duprat 1997; Escarguel 1999; Smith and Smith 2013) when their carnivorous competitors disappeared from Europe (Solé 2013; Solé et al. 2014). This faunal turnover marks the beginning of the European hyaenodont domination of the apex terrestrial carnivore niche. European hyaenodonts were also presumably in competition during the entire Ypresian with the mesonychid Dissacus: the mesonychids are, however, unknown in Europe after the Ypresian/Lutetian transition (Solé et al. 2018a, b). Contrary to the hyaenodonts, the carnivoraforms did not diversify extensively in Europe during the Eocene (Solé 2014; Solé et al. 2018a). As a consequence, one can hypothesize that the European hyaenodonts probably underwent a phylogenetic radiation due to the disappearance of its ecological competitors such as the oxyaenids and mesonychids (Solé et al. 2011, 2013b, 2014, 2017).
The European hyaenodonts occupied a wide array of ecological niches during the Paleogene, and therefore displayed a great diversity of ecomorphs and body size (Solé et al. 2014). However, it has been noted that the body mass of the European hyaenodonts remained very low (Solé et al. 2014). Indeed, the hyaenodonts presently known in the Ypresian and Lutetian never exceed 20 kg; the firsts that reached such a body mass are known only in the beginning of the Lutetian (i.e., MP11). The large hypercarnivorous hyaenodonts from the Lutetian of Europe are morphologically similar to the carnivorous taxa known from North America at the same time (e.g., the oxyaenids Patriofelis and Ambloctonus). However, these hyaenodonts were clearly smaller than their North American relatives during the entire Eocene, it is worth remarking that the large hypercarnivorous predators in North America were oxyaenids. Solé et al. (2014) considered that 20 kg corresponds to the upper limit of the body mass range of the European hyaenodonts during the Ypresian and Lutetian, despite the lack of competitors. Solé et al. (2014) thus hypothesized that differences between the North American environment (mixed landscapes) and the European environment (an archipelago) may have favored different ranges in body mass.
The discovery of a large hyaenodont from the Lutetian of Switzerland allows discussion of the body mass limit of European carnivorous mammals, as well as the ecological radiation of the European hyaenodonts.
Institutional abbreviations.—NMB, Naturhistorisches Museum Basel, Switzerland; UCBL-FSL, Collections of Paleontology, Laboratory of Geology, Lyon 1 University, France.
Other abbreviations.—L, left; MP, Mammal Palaeogene; OTU, Operational Taxonomic Unit; PP, Posterior Probability; R, right; RBL, Relative Blade Length; RPS, Relative Premolar Size.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:78A8F730-F373-4417-8AD1-35 E98B94BB4.
Material and methods
Material.—Most of the specimens presented and described in the present article are housed in the vertebrate collection of the Naturhistorisches Museum Basel (NMB). All these specimens are from the Swiss locality of Egerkingen (Lutetian) (Fig. 1). However, this locality corresponds to karst fillings in an aberrant siderolitic facies. Three fillings have been excavated since 1890: these are designated α, β, and γ. Fillings α and β constitute the reference locality of reference level MP14 (generally noted Egerkingen α + β), while Quarry γ is considered to be slightly older and possibly close to the MP13 reference level (BiochroM’97 1997). The sole information concerning the geographic origin available for some specimens referred to the new species is written on their labels, which indicate they were found in the Quarry γ. The dates of their discovery are 1912–1915 and 1922.
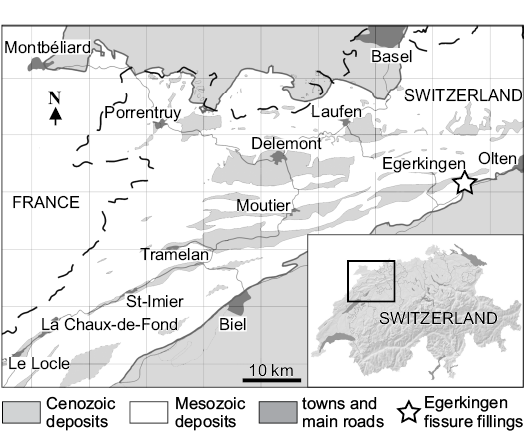
Fig. 1. Geographic position of the fossiliferous locality of Egerkingen. (Close-up on the Northwest Switzerland redrawn from Becker 2003: fig. 3-1).
The unique morphology of some of the fossils referred to Cartierodon egerkingensis was noted by Brigitte Lange-Badré in the 1970s when she studied the collection of the NMB. She even noted the name Cartierodon egerkingensis on some labels, probably planning to publish them one day. In homage to her work, we decided to use the name she planned for this new taxon.
We also discuss herein two premolars from Lissieu (France; Lutetian, ≈ MP14) because they could belong to the same genus (see Systematic palaeontology). Lissieu corresponds to a fissure filling located a few kilometers north of the city of Lyon. The locality was found in the late nineteenth century (Depéret 1894) and no longer exists because of quarry exploitation. The locality yielded a diverse vertebrate fauna. The fossils from this locality are housed in the collections of Paleontology of the Lyon 1 University (France)
Dental measurements and terminology.—We follow the dental terminology of Szalay (1969). The measurements (length × width in mm) follow Gingerich and Deutsch (1989).
Systematic palaeontology
Hyaenodonta Van Valen, 1967
Hyaenodontoidea Leidy, 1869
Hyaenodontidae Leidy, 1869
Genus Cartierodon nov.
ZooBank LSID: urn:lsid:zoobank.org:act:89D67AC0-0E0B-4AC3-85 D6-8DA8C9F029C0
Type species: Cartierodon egerkingensis sp. nov.; monotypic, see below.
Etymology: Dedicated to Pastor Robert Cartier, who excavated the infilling of Egerkingen γ from 1840 to 1884 and gave his collection to the Naturhistorisches Museum Basel; combined with Greek odon, tooth.
Diagnosis.—As for the type and only species.
Cartierodon egerkingensis sp. nov.
Fig. 2.
ZooBank LSID: urn:lsid:zoobank.org:act:9D682E10-CF2A-4C0F-B9 C5-1A00626FB753
Etymology: Refers to the type locality, the filling of Egerkingen γ.
Holotype: NMB.Em.11, right mandible bearing p2, p3, p4, the trigonid of m1, and the alveoli of p1.
Type locality: Egerkingen γ, Gaü, Solothurn, Switzerland.
Type horizon: Unnamed unit of karst fillings in an aberrant siderolitic facies; MP13?, Lutetian, Eocene.
Material.—NMB.Ee.126a, Lp1; NMB.Eh.550, left maxillary bearing P2 and distal root of P1; NMB.Ek.42, RDP4; NMB.En.78, RP2; NMB.En.80, LP3; NMB.En.85, RP4; NMB.En.86, RM2; NMB.En.87, RM2; NMB.En.88, LM; NMB.En.90, RM2; NMB.En.91, Rp2; NMB.En.92, Rp2; NMB.En.93, Rp3; NMB.En.94, Lp3; NMB.En.95, Lp3; NMB.En.96, Lp4; NMB.En.97, Rp4; NMB.En.98, Rm3; NMB.En.99, left mandible bearing m2 and the trigonid of the m3; NMB.En.100, Lm3; NMB.En.134, Rp4; NMB.En.152, Lm3; NMB.En.153, LP3; NMB.En.154, LM1; NMB.En.155, Rdp4; NMB.En.156, Rm2; NMB.En.213, Rdp3; NMB.En.215, Rm1; NMB.En.216, Rm2; NMB.En.217a, Lm2. From the type locality.
Diagnosis.—Differs from all contemporaneous European hyaenodont genera (Oxyaenoides, Proviverra, Cynohyaenodon, Eurotherium, Prodissopsalis, Leonhardtina, Allopterodon, Alienetherium, and Praecodens) by its larger size. It differs from Oxyaenoides by the presence of a metaconid on molars and transversally enlarged premolars. It also differs from Proviverra, Cynohyaenodon, Eurotherium, Leonhardtina, Allopterodon, Alienetherium, and Praecodens by a poorly developed metaconid on molars. It differs from Quercytherium, with which it shares transversally enlarged premolars, by its larger size, poorly developed metaconid on molars, and less squared p2 and p3. It differs from Prodissopsalis eocaenicus, its closest hyaenodont relative, by a second foramen located below the anterior root of the p4, wider lower premolars, mesiodistally shorter talonid on m3, and a protocone area more developed on P3.
Description.—The mandible is deep (≈ 36 mm; Table 1). The symphysis extends below the mesial root of the p3. The first foramen opens below the anterior root of the p2, while the second foramen is located below the anterior root of the p4.
Table 1. Measurements (in mm) of Cartierodon egerkingensis gen. et sp. nov. from Egerkingen γ (MP13?). Dentary depth measured below the m1. Body mass = 28.65 kg (estimated after Morlo 1999).
|
Locus |
Number of specimens |
Observed range |
Mean |
|
|
DP4 |
length |
1 |
14 |
– |
|
width |
1 |
13.1 |
– |
|
|
P2 |
length |
2 |
14.1–15 |
14.55 |
|
width |
2 |
8.1–8.4 |
8.25 |
|
|
P3 |
length |
1 |
14.4 |
– |
|
width |
2 |
10.4–11 |
10.7 |
|
|
P4 |
length |
1 |
16.4 |
– |
|
width |
1 |
12.4 |
– |
|
|
M1 |
length |
1 |
12.4 |
– |
|
width |
0 |
– |
– |
|
|
M2 |
length |
2 |
13.5–15.4 |
14.45 |
|
width |
0 |
– |
– |
|
|
dp3 |
length |
1 |
13.8 |
– |
|
width |
1 |
6.1 |
– |
|
|
dp4 |
length |
1 |
12.1 |
– |
|
width |
1 |
7 |
– |
|
|
p1 |
length |
1 |
9.1 |
– |
|
width |
1 |
6.5 |
– |
|
|
p2 |
length |
2 |
12–14.4 |
13.27 |
|
width |
3 |
8.2–8.7 |
8.43 |
|
|
p3 |
length |
4 |
15.5–16.3 |
15.85 |
|
width |
4 |
7.7–9 |
8.43 |
|
|
p4 |
length |
4 |
14.6–16 |
15.15 |
|
width |
4 |
8.1–9.4 |
8.7 |
|
|
m1 |
length |
1 |
14 |
– |
|
width |
1 |
7.4 |
– |
|
|
m2 |
length |
3 |
13.1–15.2 |
14.13 |
|
width |
3 |
8–8.9 |
8.47 |
|
|
m3 |
length |
2 |
15–17.3 |
16.15 |
|
width |
3 |
10–10.2 |
10.07 |
|
|
dentary depth |
1 |
35.77 |
– |
|
We identified two lower deciduous premolars: one dp3 (NMB.En.213) and one dp4 (NMB.En.155). The two teeth are mesiodistally elongate. The dp3 is characterized by a large but low paraconid, and by individualized hypoconid, hypoconulid, and entoconid. The dp4 is characterized by the absence of contact between the paraconid and metaconid, the two cusps being separated by the protoconid. The metaconid was probably smaller than the paraconid; it is distally located compared to the protoconid. The talonid is mesiodistally short and wide. It bears the three usual talonid cusps. The hypoconulid bears two distinct apexes (i.e., cuspulation).
All the permanent teeth have crenulated enamel. The premolars are characterized by a transversally enlarged but mesiodistally short talonid. The p1 has two roots. The anterior root is curved in lateral view (Fig. 2M2, M3): this may indicate that the p1 was very close to the canine. The paraconid is characterized by a very thin fold in the mesial part of the tooth. A thin transversally aligned crest is visible at the posterior part of the talonid, marking the talonid region. The very small crest bears a cusp (hypoconid?). The p2 is longer and wider than the p1, but it is morphologically similar to the p1. The p3 is obliquely implanted on the holotype (NMB.Em.11; Fig. 2L1). The p3 differs from the p1 and p2 in having an individualized paraconid. The talonid is transversally extended and bears a large hypoconid. The presence of the entoconid is variable: the entoconid is absent on NMB.En.95, while it is present on NMB.En.94; and two cusps are present on the lingual part of the talonid on NMB.En.93. The p4 is very similar to the p3, but differs in having larger paraconid, hypoconid, and entoconid. However, the p4 is slightly mesiodistally shorter than the p3, a feature visible on the holotype NMB.Em.11 (15.63 mm vs. 15.19 mm).
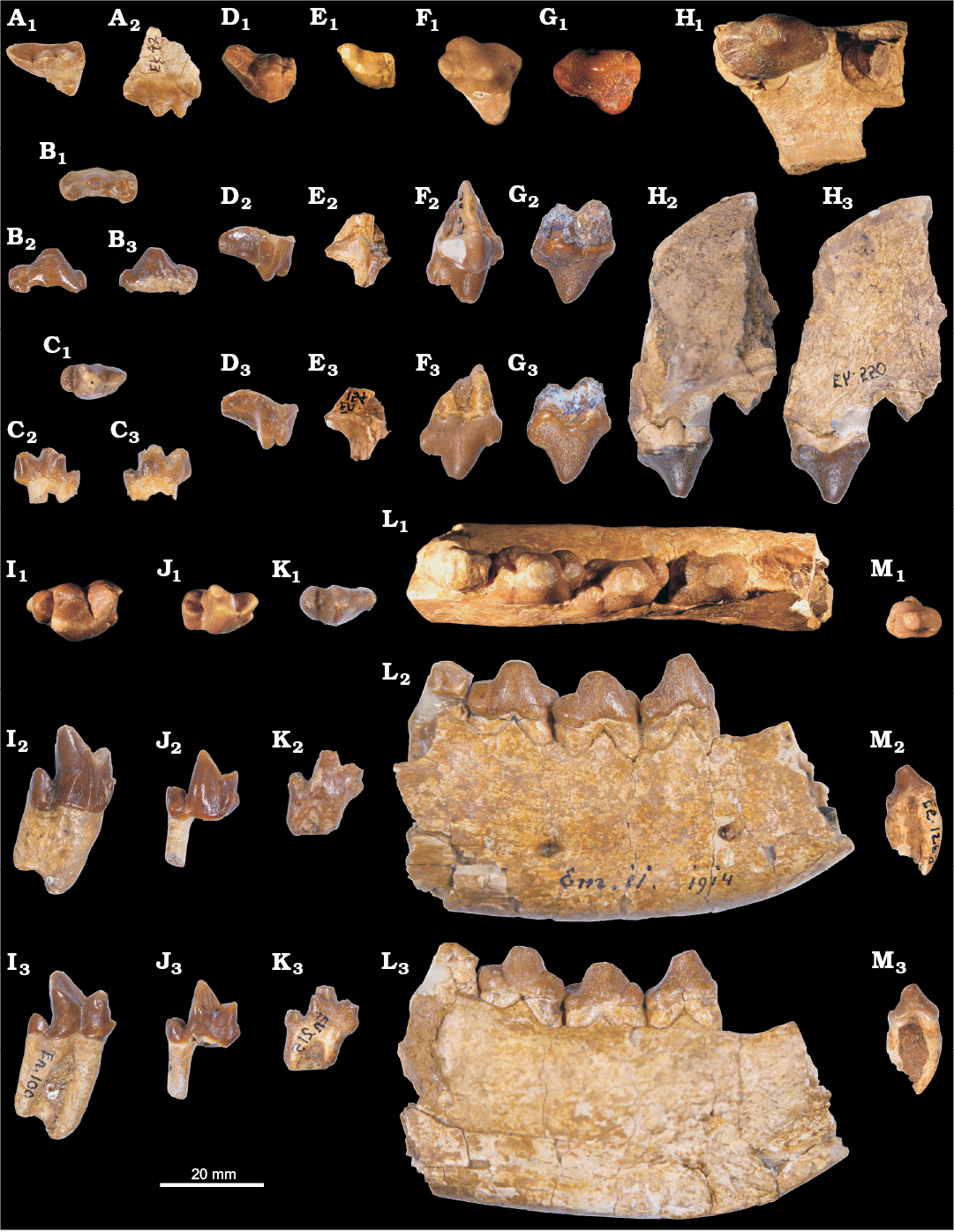
Fig. 2. Hyaenodontid mammal Cartierodon egerkingensis gen. and sp. nov. from Switzerland, Egerkingen γ (MP13?, Lutetian, Eocene). A. NMB.Ek.42, DP4 in occlusal (A1) and labial (A2) views. B. NMB.En.213, dp3 in occlusal (B1), labial (B2), and lingual (B3) views. C. NMB.En.155, dp4 in occlusal (C1), labial (C2), and lingual (C3) views. D. NMB.En.87, M2 in occlusal (D1), lingual (reversed, D2), and labial (D3) views. E. NMB.En.154 in occlusal (reversed, E1), lingual (E2), and labial (reversed, E3) views. F. NMB.En.85, P4; in occlusal (F1), lingual (reversed, F2), and labial (F3) views. G. NMB.En.80, P3 in occlusal (reversed, G1), lingual (G2), and labial (reversed, G3) views. H. NMB.Eh.550, maxillary bearing P2 and distal root of P1 in occlusal (reversed, H1), lingual (H2), and labial (reversed, H3) views. I. NMB.En.100, m3 in occlusal (reversed, I1), labial (reversed, I2), and lingual (I3) views. J. NMB.En.216, m2 in occlusal (J1), labial (J2), and lingual (reversed, J3) views. K. NMB.En.215, m1 in occlusal (K1), labial (K2), and lingual (reversed, K3) views. L. NMB.Em.11, mandible bearing p2, p3, p4 and the trigonid of m1, and the alveoli of p1, in occlusal (L1), labial (L2), and lingual (reversed, L3) views. M. NMB.Ee.126a, p1 in occlusal (reversed, M1), labial (reversed, M2), and lingual (M3) views.
The m1 displays a very small metaconid; the cusp is lower than the paraconid. The paraconid is well mesially located. The paraconid has a sharp mesial crest and possesses a small anterior keel visible on its buccal aspect. The metaconid is distally located compared to the protoconid, but is not as distally located as on the dp4. Moreover, the metaconid and paraconid are not separated by the protoconid. The talonid is mesiodistally short; the postfossid is shallow and narrow. One can observe the entoconid, hypoconid, and hypoconulid on the talonid; the hypoconid is slightly higher than the two other talonid cusps. The precingulid is short but wide. The m2 is similar in morphology to the m1. It differs in being taller and in being longer and wider. Like m1 and m2, the m3 displays a secant trigonid characterized by a mesially located paraconid and small metaconid; the latter is transversally aligned with the protoconid on m3. The protoconid of the m3 is more retroflexed than on m1 and m2. The talonid is mesiodistally shorter and narrower than on the m1 and m2. The specimen NMB.En.100 (isolated m3) is interesting in having only one large root: the anterior and posterior roots are not entirely separated (Fig. 2I2). Moreover, one can note the presence of a single cusp on the talonid.
A DP4 has been identified (Fig. 2A). The tooth is mesiodistally elongated. The paracone and metacone are well separated. The metacone is larger than the paracone. A weak parastyle is present. The metastyle is long and mesiodistally aligned. The protocone is mesiodistally short and narrow. The paraconule and metaconule are present. There is no internal crest in the trigon basin. The metaconule and metastyle are not linked by the metacingulum.
The upper premolars are wide. The P1 is unknown but has two roots based on the alveoli present on NMB.Eh.550 (Fig. 2H). There is a short separation between the P1 and P2. The P2 does not display a parastyle. The metastyle is very small. On NMB.Eh.550, one can notice that the P2 has three roots. The P3 (Fig. 2G), which also has three roots, notably differs from the P2 in having a larger lingual fold. There is no parastyle; the metastyle is larger than on P2. The P4 has an individualized protocone and a strong parastyle. The metastyle is mesiodistally short, high, and secant. It displays two cusps on NMB.En.81 and NMB.En.85. A cingulum links the metastyle and the protocone. The protocone is long but narrow; it does not show any accessory cusp.
The M1 and M2 are incompletely known. They are morphologically very similar; the M2 is longer than the M1 (Table 1). The paracone and metacone are less separated than on the DP4; only the apices of the cusps are separated. The paracone is clearly smaller and mesiodistally shorter than the metacone. The metastyle is as long as the metacone–paracone base. It is distally shifted. The parastyle is more developed on the M2 than on the M1. The protocone is unknown for the molars; because the postfossid on the molars are shallow and narrow, the protocone of the molars could have been narrow and low.
Discussion.—The new taxon is represented mostly by isolated teeth. We consider that they represent the same taxon because they are from the same locality (i.e., Egerkingen), because of their similar morphology (e.g., secant morphology of the molars, transversal enlargement of the premolars), and because of their large size compared to the other hyaenodonts found at Egerkingen.
The new taxon resembles the hyaenodonts recording in the Ypresian and Lutetian of Europe (Proviverra, Cynohyaenodon, Eurotherium, Prodissopsalis, Leonhardtina, Allopterodon, Alienetherium, Quercytherium, and Paracynohyaenodon) because of the presence of a two-rooted p1, of a paraconid and an entoconid on p3 and p4, of a large protocone area on the upper premolars, and of an individualized entoconid on molars.
The transversal enlargement of the premolars and the position of the mental foramina recall the European hyaenodont genus Matthodon. This genus is represented by two species: Matthodon menui from Cuis, Chavot, Monthelon, Mancy, and Grauves (Ypresian, MP10; France) and Matthodon tritens from Geiseltal-Untere Unterkohle (Lutetian, MP11; Germany) (Lange-Badré and Haubold 1990; Solé et al. 2014). The specimens from Egerkingen γ, however, differ in having less bulbous premolars, a larger metaconid, and a two-rooted p1. The presence of the metaconid on molars also distinguishes the Swiss fossils from those of Oxyaenoides (Crochet et al. 1988; Lange-Badré and Haubold 1990; Solé et al. 2014, 2015).
The general morphology of the new fossils is most similar to Prodissopsalis eocaenicus. This species is known from Geiseltal-Untere Mittelkohle (MP12), Geiseltal-Obere Mittelkohle (MP13), Issel (MP14), and Lissieu (MP14) (Matthes 1952; Calas 1969, 1970; Lange-Badré 1972; Lange-Badré and Haubold 1990), see below for a discussion concerning the fossils from Lissieu. The new species and Prodissopsalis eocaenicus actually share the transversally enlarged upper and lower premolars, the p3 as long as the p4, the presence of a poorly developed metaconid on molars, the presence of mesiodistally short but wide talonid on molars, the reduction of the talonid of the m3, and the small size of the paracone.
The taxon described herein differs from Prodissopsalis eocaenicus by a second foramen located below the anterior root of the p4, wider lower premolars (Fig. 2), mesiodistally shorter talonid on m3, and a protocone area more developed on P3.
Surprisingly, the obliquely implanted p3, visible on the holotype (NMB.Em.11), recalls the fossils of Prodissopsalis eocaenicus from Geiseltal-Untere Mittelkohle (MP12) rather than those from Geiseltal-Obere Mittelkohle (MP13) (see Lange-Badré and Haubold 1990 for a discussion concerning this feature). This feature (i.e., obliquely implanted p3) is also visible in the late Eocene Paenoxyaenoides liguritor (Lange-Badré 1979). It possibly traduces a common feature; however, it is possibly related to an anteroposterior shortening of the mandible and thus could represent a convergent feature.
The new taxon shares with Paenoxyaenoides liguritor the transverse enlargement of the lower premolars. However, Paenoxyaenoides differs from the Egerkingen species by the absence of the metaconid on the molars (derived feature), the position of the mental foramina, below p1 and p3, and the P3/p3 distinctly mesiodistally shorter than the P4/p4 (Fig. 3).
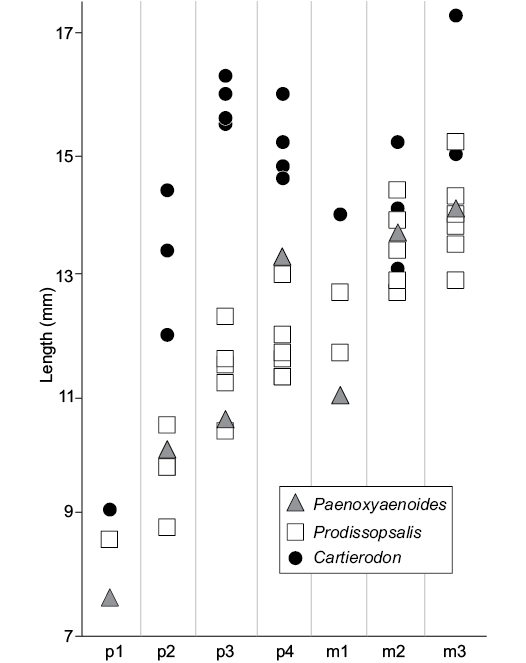
Fig. 3. Comparison of the length of the lower premolars and molars of Cartierodon egerkingensis gen. et sp. nov. from the Eocene of Switzerland, Egerkingen γ (MP13?); Prodissopsalis eocaenicus from the Eocene of Switzerland, Geiseltal-Obere Mittelkohle (MP12) and Geiseltal-Untere Mittelkohle (MP13) (based on Lange-Badré and Haubold 1990: table 3); and Paenoxyaenoides liguritor from the late Eocene of France, Quercy Phosphorites (based on Lange-Badré 1979: table 10).
The fossils here described are also characterized by sizes that are larger than those of the hyaenodonts previously known in the Lutetian of Europe. Until the discovery of this new hyaenodont, the largest European hyaenodonts recorded for this period were Matthodon tritens, Prodissopsalis eocaenicus, and Oxyaenoides schlosseri: the length of the molar row (m1 to m3) for each of these taxa equals 39.5 mm (Lange-Badré and Haubold 1990), 38.8 mm (Van Valen 1965), and 38.12 mm (Solé et al. 2015). Because the molar row of Cartierodon egerkingensis equals roughly 43.5 mm, the new fossils represent the largest hyaenodont ever discovered in the Lutetian of Europe.
As a result, the fossils housed at the NMB correspond to a new hyaenodont taxon. We thus propose to refer all these dental elements to a new genus and new species: Cartierodon egerkingensis.
Stratigraphic and geographic range.—Egerkingen γ (Switzerland; MP13?, Lutetian, Eocene).
Cartierodon cf. egerkingensis sp. nov.
1970 Prodissopsalis sp.; Calas 1970: 226.
1972 Prodissopsalis eocaenicus; Lange-Badré 1972: 2216, fig. 2B,C.
1990 Prodissopsalis eocoenicus; Lange-Badré and Haubold 1990: 615.
Material.—UCBL-FSL 2113, left p3; UCBL-FSL 2114, right p4; both from Lissieu, France; MP14, Lutetian, Eocene.
Description.—See Lange-Badré (1972).
Remarks.—Like Egerkingen, Lissieu yielded a diverse vertebrate fauna. When first mentioned, the premolars from Lissieu, here referred as Cartierodon cf. egerkingensis, were referred to Oxyaenoides schlosseri (formerly described as Hyaenodon schlosseri). However, Lange-Badré (1972) decided the premolars better fit with the premolars of Prodissopsalis; however, she remarked that they differ by being transversally elongated compared with the premolars of Prodissopsalis eocaenicus. Callas (1970) revised the fauna from Lissieu and listed the presence of a taxon that is similar in size to Prodissopsalis eocaenicus but morphologically distinct, we think that this taxon corresponds to the premolars here discussed.
We plotted their sizes (provided by Lange-Badré 1972: table 1) on Fig. 4. As one can see, the ratios width/length measured for the fossils of Lissieu are closer to those of Cartierodon egerkingensis than to those of Prodissopsalis eocaenicus. It is worth noting that the ratios indicate that the premolars of Cartierodon egerkingensis and Cartierodon cf. egerkingensis are more square-shaped than those of Prodissopsalis eocaenicus. We thus believe that the unique features of the premolars from Lissieu noted by Calas (1970) and Lange-Badré (1972) are due to the fact that the fossils from Lissieu belong to Cartierodon. However, we prefer to leave the specimens in open nomenclature (Cartierodon cf. egerkingensis), pending the discovery of better-preserved material.
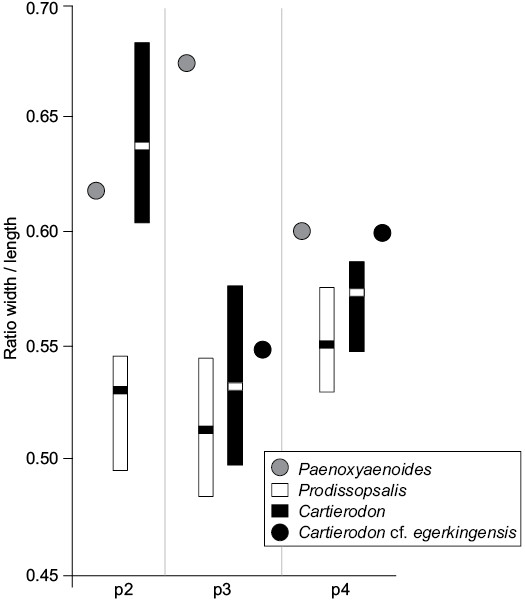
Fig. 4. Comparison of the ratio width/length estimated for the lower premolars of Paenoxyaenoides liguritor from late Eocene of France, Quercy Phosphorites (based on Lange-Badré 1979: table 10), Prodissopsalis eocaenicus from Eocene of Switzerland, Geiseltal-Obere Mittelkohle (MP12) and Geiseltal-Untere Mittelkohle (MP13) (based on Lange-Badré and Haubold 1990: table 3); Cartierodon egerkingensis gen. et sp. nov. from Eocene of Switzerland, Egerkingen γ (MP13?); and Cartierodon cf. egerkingensis from Eocene of France, Lissieu (MP14) (based on Lange-Badré 1972: table 1).
The presence of similar species between Lissieu and Egerkingen is not surprising because Egerkingen α + β and Lissieu are referred to MP14 (BiochroM’97 1997). Moreover, these two localities are separated by 350 km. Recently, Angst et al. (2013) identified the terrestrial carnivorous bird phorusrhacid Eleutherornis cotei in Egerkingen α and Lissieu. However, it is worth keeping in mind that the presence of Cartierodon egerkingensis in Egerkingen α or β is uncertain. Cartierodon is certainly known from Egerkingen γ.
Results
Body mass.—The body mass of Cartierodon egerkingensis was estimated using the methodology provided by Morlo (1999) in his study of the palaeoecology of the “creodonts”. The equation is Log10(P) = [3.5104 × Log10((ΣM)/3)] – 2.6469 where P is the estimated bodymass (in grams) and ΣM the sum of the length of the three lower molars (in mm). We used the means of the three lower molars in order to estimate body mass.
The body mass of the mesonychids are from Solé et al. (2018b). All the values are available in SOM 7 (Supplementary Online Material available at http://app.pan.pl/SOM/app64-Sole_etal_SOM.pdf)
Diet.—Van Valkenburgh (1988) proposed several indices based on 12 dental measurements in order to discriminate among the feeding types of extant Carnivora. The feeding types were: (i) meat, greater than 70% meat in diet; (ii) meat/bone, greater than 70% meat with the addition of large bones; (iii) meat/non-vertebrate, 50–70% meat with the balance fruit and/or insects; and (iv) non-vertebrate/meat-less than 50% meat with fruit and/or insects predominating. We here considered only the three first types. Among the six proposed indices, Van Valkenburgh (1988) remarked that two of the indices, Relative Premolar Size (RPS) and Relative Blade Length (RBL), were able to clearly separate the extant Carnivora into the four feeding types. We thus only estimated these two indices for the selected hyaenodonts. It is worth noting that the first index was adapted to the hyaenodonts by Friscia and Van Valkenburgh (2010). We used here only the European hyaenodonts from the Eocene for which both the RBL and RPS can be estimated. RBL, measured as the ratio of trigonid length to total anteroposterior length of the largest molar; we used the m3 because it is the largest molar in Hyaenodonta that retain this tooth. RPS, maximum width of the largest lower premolar divided by the cube root of body weight.
The RBL tends to discriminate the meat (diet >70% meat) and meat/bone eaters (diet >70% meat with addition of large bones; highest values) from the meat/non-vertebrate eaters (diet 50–70% meat, lowest values), while the RPS discriminates the meat eater (lowest values) from the meat/bone eaters (highest values). All the values are available in SOM 6.
The RPS and RBL of Cartierodon egerkingensis were estimated based on the mean values for the three lower molars.
Taxonomy.—Modifications to the Borths and Stevens (2017b) matrix grouped almost all “proviverrine” taxa sensu Solé (2013) in the same clade (see below). However, hyaenodontines are still included in the “proviverrine” clade, as in previous analyses. This result refutes the monophyly of “Proviverrinae” sensu Solé (2013), resolving “proviverrines” as part of hyaenodontine stem lineages. Because our results agree with those of Borths et al. (2016), we propose to consider the Proviverrinae as a clade that includes the last common ancestor of Proviverra and Parvagula. We here propose to name Hyaenodontoidea the clade that includes the last common ancestor of Proviverra and Hyaenodon. This results in grouping Hyaenodontidae and Proviverrinae among Hyaenodontoidea.
Phylogenetic analysis.—The family Proviverrinae has been resolved as paraphyletic, containing other hyaenodont subfamilies (Polly 1996). Solé et al. (2014) redefined the Proviverrinae and considered it as a subfamily endemic to Europe, which existed only during the Eocene. In the most recent phylogenetic studies (Borths et al. 2016; Borths and Stevens 2017a–c), Proviverrinae is nested at the base of Hyaenodonta. It is worth mentioning that these results also implied a redefinition of Proviverrinae: some “proviverrines”, as defined by Solé et al. (2014), are recovered by Borths et al. (2016) and Borths and Stevens (2017a–c) as stem-taxa of Hyaenodontidae.
Because Cartierodon egerkingensis appears to be related to some European hyaenodonts (see Systematic palaeontology), we included this taxon into a phylogenetic analysis. We did not build a new character-taxon matrix because we considered that the ones recently published are pertinent and exhaustive. However, we have some disagreements with the coding of some European hyaenodontoid in the matrix created by Borths and Stevens (2017b). We thus decided to modify this matrix in order to reflect our interpretations. To do so, we added to the published matrix the new taxon and three hyaenodontoid OTUs (Operational Taxonomic Unit) in order to increase the diversity of the hyaenodontoids represented in the matrix (SOM 1): the species Matthodon menui and Prodissopsalis eocaenicus (the latter being morphologically similar to Cartierodon egerkingensis; see text), and the genus Paracynohyaenodon (considered to be close to Quercytherium and Cynohyaenodon; Solé et al. 2014). We also modified some codings for the European hyaenodontoids (SOM 2). The resulting character-taxon matrix was assembled in Winclada (Nixon 2002), includes 150 characters (SOM 3) and 90 OTUs, and is available in SOM 4. As in Borths and Stevens (2017b), eighteen multistate characters were treated as ordered, and all characters were treated as equally weighted. Phylogenetic analyses were performed using the same Bayesian “tip-dating” phylogenetic method described by Beck and Lee (2014) and applied to hyaenodonts by Borths and Stevens (2017c). This method simultaneously estimates branch length, rate, phylogeny, and support for clades recovered in the analysis, a common approach for palaeontological systematic analyses (e.g., Beck and Lee 2014; Dembo et al. 2015; Borths et al. 2016; Gorscak and O’Connor 2016; Lund et al. 2016; Turner et al. 2017). Bayesian “tip-dating” analyses were performed in MrBayes (Ronquist et al. 2012). MrBayes formatted nexus file, which contains all analytical parameters, is included in SOM 2. SOM 5 can be used to visualize the results (e.g., age estimates, rates of change etc.) in FigTree v1.4.3 (see Borths and Stevens 2017b).
Discussion
Phylogenetic analysis.—The character-taxon matrix that forms the basis of the present analysis has been used, and improved, for several studies of hyaenodonts systematics (Borths et al. 2016; Borths and Seiffert 2017; Borths and Stevens 2017a–c). All the tip-dating Bayesian results of the analysis can be visualized by using SOM 5.
The analysis (“all compat” = all compatible components consensus tree; Fig. 5; SOM 5) recovered all the clades found in the previous phylogenetic analyses. The “all compat” consensus tree shows the monophyly of the Hyainailouroidea (Posterior Probability = 11%), as well as that of the Teratodontinae (PP = 8%), Hyainailourinae (PP = 35%), and Apterodontinae (PP = 94%), three subgroups of the Hyainailouroidea. Because the relationships among Hyainailouroidea have been extensively discussed by Borths et al. (2016), Borths and Seiffert (2017), and Borths and Stevens (2017a, b), we do not discussed them in the present paper. However, it is worth noting that the composition of the nodes that are located between the Galecyon clade and the Hyainailouroidea (Fig. 5) is different from those found in the phylogenetic analyses previously performed (e.g., Borths et al. 2016, Borths and Seiffert 2017, and Borths and Stevens 2017a, b). These differences are due to the position of the Quercytherium, Preregidens, and Boritia among the Hyaenodontidae; in the previous analyses (e.g., Borths and Stevens 2017a–c), they were situated at nodes close to the Hyainailouroidea. Our analysis did recover the Galecyon clade (PP = 95%) (Borths et al. 2016). This clade is closely related to the North America genera Gazinocyon and Pyrocyon (PP = 17%). The two African hyaenodonts from Morocco, Lahimia and Boualitomus, are found together (PP = 96%). This latter clade is close to the Galecyon clade and the genera Gazinocyon and Pyrocyon. This relationship is recovered for the first time; the African genera are generally closer to the origin of hyainailouroids than Galecyon and the North American hyaenodonts. However, the node is poorly supported: the PP equals only 10%.
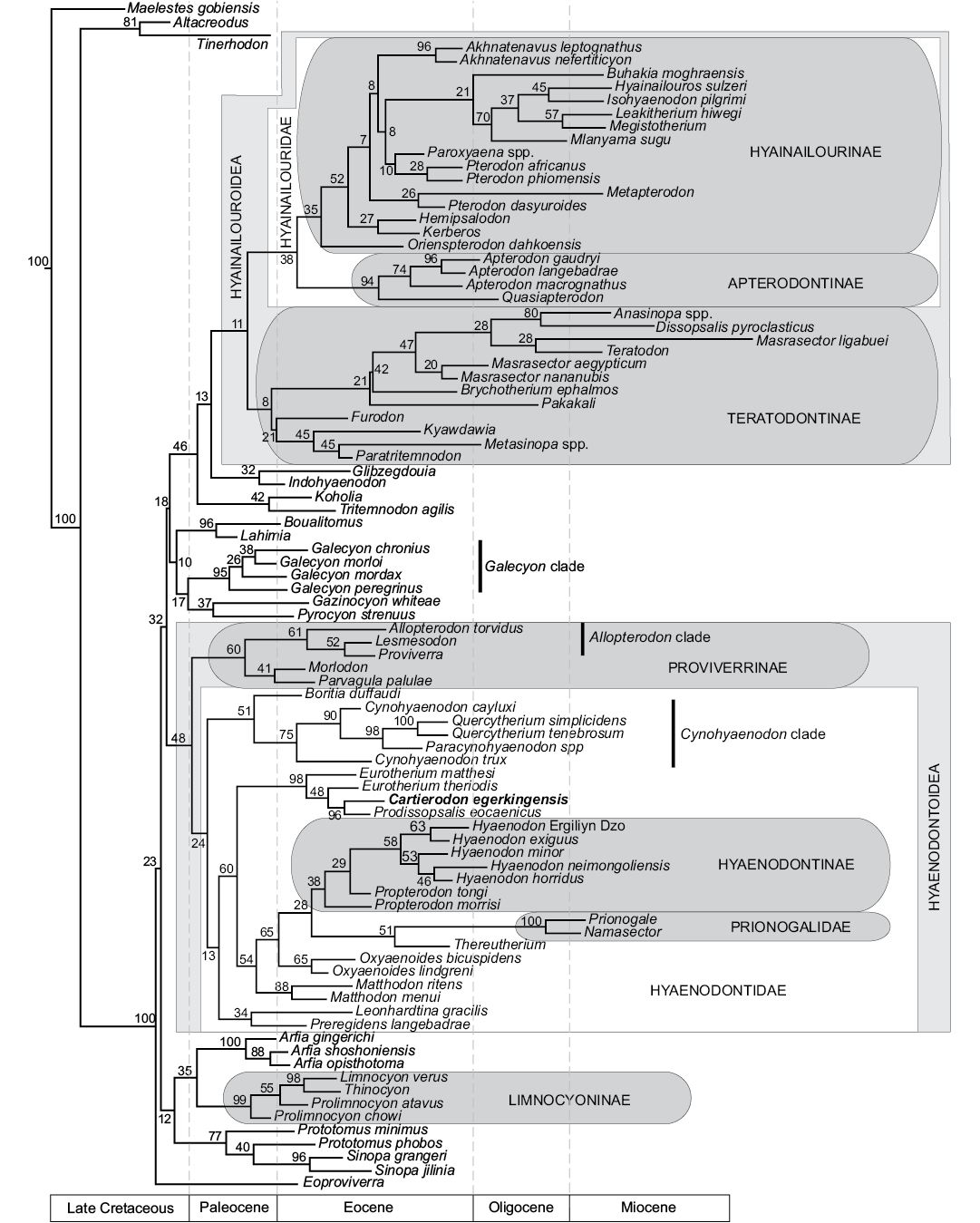
Fig. 5. Results of the phylogenetic analysis of Hyaenodonta character-taxon matrix. Results are visualized as an “all compat” (majority rule plus compatible groups) consensus tree. Major named clades recovered or discussed in this analysis and recovered in other analyses are illustrated.
The Limnocyoninae (PP = 99%), Hyaenodontinae (PP = 38%), and Prionogalidae (PP = 100%) are also recovered.
Contrary to the previous phylogenetic analyses (Borths and Seiffert 2017; Borths and Stevens 2017a–c), some of the “proviverrines” sensu Solé (2013) (i.e., Proviverra, Lesmesodon) are not located at the base of the hyaenodont clade, but are recovered at a more deeply nested node in the Hyaenodonta phylogenetic tree. Moreover, our analysis is significant in that it recovers almost all the “proviverrine” taxa within the same clade (i.e., Hyaenodontoidea), except Eoproviverra (Fig. 5). In the present analysis Eoproviverra is the earliest-diverging hyaenodont (Fig. 5).
In previous analyses (Borths et al. 2016; Borths and Seiffert 2017; Borths and Stevens 2017b, c), the Proviverrinae corresponded to a clade that was located at the base of the Hyaenodonta and that generally included the European genera Proviverra, Eoproviverra, Lesmesodon, Parvagula, and Morlodon. This group also included Allopterodon in Borths et al. (2016), but this genus is closer to the Hyaenodontinae in the other analyses.
We found a clade (PP = 60%) that gathers the European hyaenodont genera Proviverra, Allopterodon, Lesmesodon, Parvagula, and Morlodon. This clade corresponds to the concept of Proviverrinae of Borths et al. (2016). Consequently, we follow Borths et al. (2016) and consider that Proviverrinae is a clade that includes the last common ancestor of Proviverra and Parvagula. However, this clade is not located at the base of Hyaenodonta, but is the sister clade of Hyaenodontidae (PP = 48%). We propose to name Hyaenodontoidea for the clade that gathers Hyaenodontidae and Proviverrinae (Fig. 5).
Among the Proviverrinae (clade including the last common ancestor of Proviverra and Parvagula), the Allopterodon clade (Allopterodon, Proviverra, Lesmesodon) of Solé et al. (2014) is partially recovered (PP = 61%), with only Leonhardtina being missing; the latter is more closely related to Hyaenodontinae and Prionogalidae. The genera Lesmesodon and Proviverra are closely related within this clade (PP = 52%); the similarities between these two genera have been underlined by Morlo and Habersetzer (1999). Morlodon and Parvagula are sister taxa (PP = 41%).
All the other “proviverrine” taxa sensu Solé (2013), Matthodon, Cynohyaenodon, Boritia, Quercytherium, Paracynohyaenodon, Eurotherium, Prodissopsalis, Oxyaenoides, Preregidens, and Leonhardtina, are present among the Hyaenodontidae (clade including the last common ancestor of Hyaenodon and Cynohyaenodon) (PP = 48%). Among this clade, only Thereutherium, the hyaenodontines, and prionogalids are not “proviverrine” taxa sensu Solé (2013). The relationship between the European hyaenodontid taxa, Thereutherium, Hyaenodontinae, and Prionogalidae, is poorly supported (PP = 24%). One can note the presence of a clade (PP = 51%) that includes the genera Boritia, Cynohyaenodon, Paracynohyaenodon, and Quercytherium (Fig. 5). The three later genera form the Cynohyaenodon clade (PP = 75%) (according to Solé et al. 2014); the genus Cynohyaenodon is not monophyletic. Because Quercytherium and Cynohyaenodon were rarely found together in recent analyses (e.g., Borths and Stevens 2017b), one can imagine that the addition of Paracynohyaenodon in our analysis may have influences in the presence of this clade. In this clade, Paracynohyaenodon and Quercytherium are closely related (PP = 98%); Crochet (1991) listed the similarities between the two genera.
Leonhardtina and Preregidens are sister taxa (PP = 34%) and closer to the Hyaenodontinae and Prionogalidae than the other European hyaenodontids previously mentioned. There is a clade (PP = 60%) that contains the Hyaenodontinae, Prionogalidae, Thereutherium, the European hyaenodontids, Eurotherium, Cartierodon, Prodissopsalis, Matthodon, and Oxyaenoides. Eurotherium, Cartierodon, and Prodissopsalis form a clade (PP = 98%) that constitutes the most basal clade of the Hyaenodontidae. One can note that the genus Eurotherium is not monophyletic, E. theriodis is the sister taxon to the clade formed by Prodissopsalis and Cartierodon (PP = 48%). The two latter genera are sister taxa (PP = 96%, Fig. 5). This reinforces the morphological similarities described above for the two genera.
The other hyaenodontids are in a clade that has a PP of 54%. The other node shows the divergence of Matthodon (PP = 88%, Fig. 5). The two Matthodon species are sister taxa. The genus Oxyaenoides (PP = 65%) is the sister taxon to the Prionogalidae and Hyaenodontinae (PP = 65%, Fig. 5). In Borths and Stevens (2017b), this genus was closer to Thereutherium and the Prionogalidae. In some previous analyses, it was close to the Hyaenodontidae (e.g., Borths and Stevens 2017a, c). It is worth noting that the hyaenodontines, Matthodon, Oxyaenoides, Thereutherium, and prionogalids display an hypercarnivorous dentition characterized by the loss of the metaconid and reduction of the talonid.
The close relationship between the Hyaenodontidae and the clade Prionogalidae+Thereutherium (PP = 28%) is surprising and differs from the results of Borths and Stevens (2017b). The Hyaenodontinae, which contains the genera Propterodon and Hyaenodon, are supported by a PP of 38%. As in the recent phylogenetic analyses of Borths and Stevens (2017b), Thereutherium and Prionogalidae are closely related (PP = 51%). The two prionogalids Prionogalidae and Namasector are supported by a PP of 100%. Thereutherium has been considered a limnocyonine for a long time (e.g., Lange-Badré 1979), while the status of the Prionogalidae was ambiguous (e.g., Werdelin and Cote 2010). Despite the modifications of the character-taxon matrix, modifications that deeply affected the relationships of the proviverrines, European Boritia, Preregidens, and Quercytherium, the position of Prionogalidae and Thereutherium has not been modified. To us, this reinforces the recent results of Borths and Stevens (2017b).
Ecological aspects.—The reconstructed body mass of Cartieriodon egerkingensis equals 29 kg; this body mass is close to that of the African wild dog (Lycaon pictus), 20–30 kg (Estes 1992). The diet of extinct predators is more difficult to reconstruct. Some morphological features are useful for constructing dietary hypotheses. The large size of the premolars (i.e., robustness) of Cartierodon, the crowdedness of the premolars (i.e., absence of diastema) relative to the molars, the horizontal abrasion of the premolars, as well as the depth of the mandible, indicate that this hyaenodont would have been capable of powerful slicing and crushing near the molars.
Several indices based on dental measurements can be used to discriminate the diet type of extinct species. Van Valkenburg (1988) developed these indices bases on an extant comparative sample to make inferences about the diets of extinct taxa. Morlo (1999) used these indices to reconstruct the diet of hyaenodonts and oxyaenids. The RPS and RBL were estimated for several hyaenodonts from the Eocene of Europe (Fig. 6). The means estimated for meat, meat/bone, and meat/non-vertebrate among the Carnivora are difficult to apply to hyaenodonts as the two carnivore groups converged on carnivory through different dental adaptations (Van Valkenburgh 1988). Here we do not use an extant sample derived from Carnivora to infer hyaenodont diets. Rather, we partition the European hyaenodonts that comprise our sample into three classes and assign these classes names based on the diets we hypothesize each utilized taxon. For the purposes of this study we hypothesize that hyaenodonts with RBL below 0.7 were meat/non-vertebrate eaters. The hyaenodonts with RBL values above 0.7 were likely meat and meat/bone eaters. The hyaenodonts that were likely strictly meat eaters have RPS values below 2.65, and hyaenodonts with values above 2.65 are assigned to our meat/bone eating category. As a reminder, the mean values established by Van Valkenburgh (1988) were different for the Carnivora: the RBL mean is 0.94 for meat eaters, 0.85 for meat/bone eaters, and 0.61 for meat/non-vertebrate eaters; the RPS mean is 2.14 for meat eaters, 3.79 meat/bone eaters, and 2.18 for meat/non-vertebrate eaters.
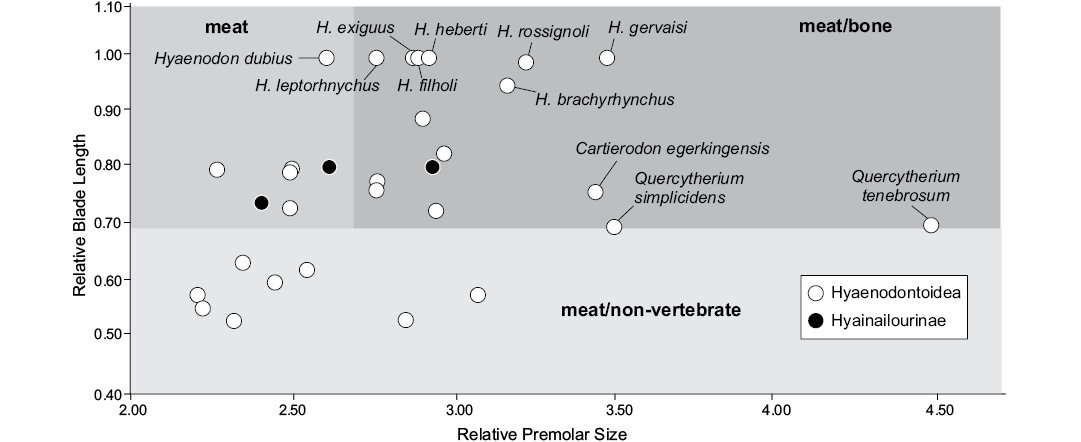
Fig. 6. Bivariate plots representing the Relative Premolar Size (RPS) versus the Relative Blade Length (RBL) for some selected hyaenodonts from the Eocene of Europe.
Quercytherium species have the highest RPS values (Fig. 6; Table 2). This is not surprising based on the strong development of the premolars in this hyaenodonts genus (Lange-Badré 1979). Morlo (1999), in his study of the evolution of the niche structure of the North American and European “creodonts”, suggested that Quercytherium simplicidens might have had a meat/bone diet; however, the molars of this hyaenodonts are not secant as expected in scavenger predators. Thus a durophagous diet (e.g. mollusks, crustaceans), as for the sea otter (Enhydra lutris), can be envisaged for this hyaenodont. The widespread and temporally extensive genus Hyaenodon is characterized by the blade-like morphology of its molars, especially the m3 (Lange-Badré 1979; Bastl 2012). This is supported by its high RBL values (Fig. 6). The range of the species along the RPS axis indicates that some species might have been more capable of bone cracking than the other ones (but the discrimination between these two types is beyond the scope of the present research given the absence of an extant comparison). It is worth noting that Bastl et al. (2012), based on microwear analysis and enamel microstructure, demonstrated tough foods like bone were likely part of the Hyaenodon diet.
The values estimated for the new hyaenodont Cartierodon egerkingensis (RBL = 0.76; RPS = 3.46; Table 2) suggest the taxon possibly had a meat/bone diet. Moreover, this hyaenodont has a high value for RPS; only the Quercytherium species have higher values. Because this is consistent with the robusticity of the premolars and depth of the mandible, we think that Cartierodon can be considered as a scavenging hyaenodont. Among the extant carnivorans that are capable of breaking bones, Van Valkenburgh (2007) recognized two different types: the bone-crackers, which break bones with their premolars (e.g., Crocuta crocuta), and the bone-crushers (e.g., Canis lupus), which break bones with their post-carnassial molars. Because of the robusticity of its premolars, the hyaenodont described herein can be considered as a bone-cracking hypercarnivore.
Table 2. Estimations of the Relative Blade Length (RBL) and Relative Premolar Size (RPS) values of several hyaenodonts from Europe.
Finally, it is interesting to note that Morlo et al. (2010) evidenced that, in the past, the largest carnivorous mammals (e.g., mesonychids, oxyaenids, and hyaenodontines) were meat/bone eater. This structural conformity of the Lutetian carnivorous fauna with the other carnivore palaeo-faunas reinforces the observation of Morlo et al. (2010).
Evolution of the body mass of European hyaenodontoids during the Ypresian and Lutetian.—As previously demonstrated by Solé et al. (2014), the maximum body mass of European hyaenodontoids (“proviverrines” sensu Solé 2013) gradually increases through the Ypresian: the first European hyaenodontoid with a body mass close to 20 kg appeared in MP11. These authors hypothesized 20 kg as the upper limit body mass of European hyaenodontoids, despite the lack of competitors for such body mass. Until the present article, the largest European hyaenodontoid from the Ypresian and Lutetian was Prodissopsalis eocaenicus from MP13 and MP14 reference-levels: its estimated body mass is around 19.8 kg. Based on these data, Solé et al. (2014) hypothesized that European hyaenodontoids were ecologically limited.
Based on a study of energetic requirements of their feeding strategies, Carbone et al. (2007) estimated that mammalian carnivores fall into two broad dietary groups: smaller carnivores (< 20 kg) feed on very small prey (invertebrates and small vertebrates), while larger carnivores (> 20 kg) specialize in feeding on large vertebrates. Consequently, large European hyaenodontoids such as Prodissopsalis would not have hunted large herbivorous mammals such as Lophiodon, but would have rather been feeding on small artiodactyls and perissodactyls.
The new taxon, Cartierodon egerkingensis, represents the largest Lutetian European hyaenodontoid ever discovered in Europe with an estimated mass of 29 kg (Fig. 7). It is interesting to note that Prodissopsalis is close to the large prey limit presented by Carbone et al. (2007) and Cartierodon is well beyond that limit (Fig. 7B). Therefore, Cartierodon likely represents an important step in the ecology of European hyaenodontoids. For the first time, a European hyaenodontoid seems clearly capable of hunting and feeding on large vertebrates.
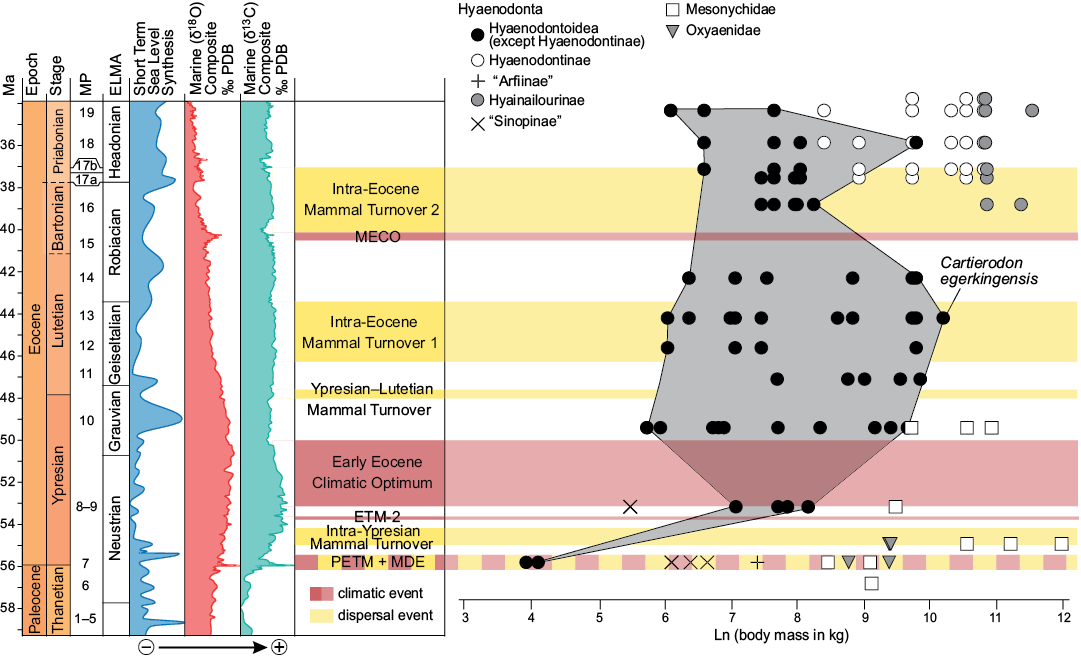
Fig. 7. Values of the body mass (in ln) of oxyaenids, mesonychids, and hyaenodonts (Hyaenodontoidea, “Sinopinae”, “Arfiinae”, Hyainailourinae, and Hyaenodontinae) from MP7 to MP19 with particular attention on the new species from Egerkingen γ (Hyaenodontinae + “Arfiinae”). Values from Table 1 (Cartierodon egerkingensis gen. et sp. nov.) and Solé et al. (2015). Egerkingen γ is here represented to be close to the MP13 reference-level. Abbreviations: ELMA, European Land Mammal Ages; ETM-2, Eocene Thermal Maximum 2; MECO, Middle Eocene Climatic Optimum; MDE, Mammal Dispersal Event; MP, Mammal Palaeogene; PETM, Paleocene–Eocene Thermal Maximum.
The largest European hyaenodontoids are much smaller than the body masses reached by mesonychids in Europe. Some Dissacus species from the end of the Ypresian may have reached up to 60 kg in the South of France and Spain; Dissacus blayaci from Mas de Piquet (Ypresian, France) and Dissacus progressus from Corsà II (Ypresian, Spain) weighed respectively 43 kg and 61 kg (Solé et al. 2018b). Pachyaena gigantea (based on the same methodology) recorded in Vaugirard (MP8+9, early Ypresian; France) may even have reached 165 kg (Solé et al. 2018a). Given the presence of large carnivores in Europe during this interval, it seems that the existence of large European hyaenodontoids was ecologically possible.
The discovery of Cartierodon shows that the maximum body mass of the European hyaenodontoids increased continuously into the MP13–MP14 rather than stopping in MP11, as previously thought by Solé et al. (2014). The body mass of Cartierodon is, however, smaller than many Bartonian (MP16) hyainailourines. Indeed, as presented by Solé et al. (2015), large hyaenodonts appear in MP16 reference-level: Paroxyaena and Kerberos respectively weighted around 47 and 88 kg. These two taxa belong to Hyainailourinae, a hyaenodont subfamily that reached massive body mass. The appearance of hyainailourines in Europe corresponds to the second faunal turnover reconstructed by Franzen (2003) and thus may correspond to ecological changes as open environments expanded in Europe (Solé et al. 2015). However, this disagrees with the presence of large mesonychids during the Ypresian.
Understanding the evolution of the maximum body mass of the European hyaenodontoids in Europe also requires a consideration of other vertebrate competitors in the ecosystem, including the large terrestrial and flightless birds recorded in the Eocene of Europe: the Gastornithidae and Phorusrhacidae. Gastornithidae are known from the middle Paleocene to the middle Eocene in Europe—the latest known representatives of the group appear to be from the Geiseltal-Obere Mittelkohle (MP13). Gastornithidae are notably present in La Borie (Laurent et al. 2010), together with Hyaenodontoidea and the mesonychid Dissacus (Solé et al. 2014, 2015, 2018b). However, recent ecological analyses of Gastornis reconstructed this bird as having an herbivorous diet (Angst et al. 2014). Consequently, this giant bird was likely not in competition with the European hyaenodonts.
The Phorusrhacidae were very briefly present in Europe. Indeed, the recently described phorusrhacid Eleutherornis cotei is only known in Egerkingen α and γ (MP13?–MP14) and Lissieu (MP14). Its appearance may result from a dispersal from Africa, where the group is known in the Eocene; this implies crossing the Tethys Sea (Angst et al. 2013). One can note that this medium-sized carnivorous bird (height about 1.5 m; Angst et al. 2013) is recorded in the same localities as Cartierodon. Terrestrial birds that could have competed with the European hyaenodontoids had a short stratigraphic record in Europe and their ecological impact on the evolution of European hyaenodonts was likely negligible.
One other terrestrial, carnivorous vertebrate group that could have been in competition with European hyaenodonts is the Planocraniidae crocodyliforms (Boverisuchus in Europe and North America, and Planocrania in Asia). These ziphodont, labiolingually compressed serrated teeth, crocodyliforms had blunt, hoof-like unguals, and they are sometimes considered “as an archosaurian attempt to retain the terrestrial predator ecological roles cleared by the disappearance of non-avian theropods” (Brochu 2013: 521). Boverisuchus is known from Messel (MP11), and several horizons at Geiseltal (MP11–MP13) (Brochu 2013). Moreover, it seems possible that planocraniids may have been present in Europe in the Paleocene (Walbeck, Germany; Selandian, MP1–MP5) and also in the Bartonian (Brochu 2013). Whatever the exact stratigraphic distribution of these crocodyliforms, it is clear that they were contemporaries of Cartierodon and were present in Europe when hyaenodontoids radiated on this continent. Therefore, the competition between planocraniids and the European hyaenodonts does not seem to have influenced the evolution hyaenodont body mass.
Once we exclude the impact of the other vertebrate predators (i.e., Planocraniidae, Phorusrhacidae, and Gastornithidae), only one trend seems to explain the evolution of the maximum body mass of hyaenodonts in Europe: maximum body mass tends to increase through time, possibly tracking the opening of European environments, but this increase was slower than observed in the North American carnivorous fauna. European environments were thus favorable for reaching large sizes as seen in mesonychids, and the European hyaenodontoids went to fill this ecological niche during the Lutetian, as evidenced by Cartierodon. The previous hypothesis of an ecological limitation of the maximum body mass proposed by Solé et al. (2014) was thus only due to a lack of data.
Biostratigraphic implications.—As mentioned above, three localities have been excavated since 1890 in Egerkingen area: these are designated α, ß, and γ. Karst fillings α and ß constitute the reference locality of reference level MP14 (generally noted Egerkingen α + ß), while filling γ is considered to be slightly older and possibly close to the MP13 reference level (BiochroM’97 1997). Twenty-one of the 32 specimens that we referred to Cartierodon egerkingensis are from Egerkingen γ, the other fossils have no precise context. Therefore, it seems that this new taxon is unknown from Egerkingen α and ß. Because the new taxon is only known in Egerkingen γ, it is not possible to use it for correlating this locality with closely contemporaneous localities and discussing the difference in age between Egerkingen γ and Egerkingen α + ß. However, a similar taxon is present in Lissieu, a locality considered to be close in age to MP14 reference-level. Therefore, it is possible that C. egerkingensis was present in localities close in age to both MP13 and MP14.
The specific problem of the relative ages of localities temporally close to the MP13 and MP14 reference levels is beyond the scope of the present study and has to be tackled through an investigation that includes localities such as Bouxwiller, La Défense, Saint-Maximin, and Aumelas (BiochroM’97 1997).
Paleobiogeographic implications.—Based on palaeotheres and primates, Franzen (2003) proposed an intra-Eocene faunal turnover in Central Europe (e.g., Egerkingen α and ß); this event may have occurred at 47–44 Ma (MP12–MP14 reference levels). He envisaged that these immigrants may have originated in Southern Europe, notably on the Iberian Peninsula; this hypothesis has been confirmed by Badiola et al. (2009). As discussed by Lange-Badré and Haubold (1990) and Solé et al. (2014, 2015), the hyaenodonts were also affected by this turnover between MP11 and MP14. Lange-Badré and Haubold (1990) demonstrated the replacement of Oxyaenoides bicuspidens, Eurotherium matthesi, and Matthodon tritens (MP11, Geiseltal-Untere Unterkohle) with Prodissopsalis eocaenicus, Leonhardtina gracilis, Cynohyaenodon trux, and Proviverra typica (MP12, Geiseltal-Untere Mittelkohle) in the Geiseltal area.
However, one can note that the genus Leonhardtina is already known in the MP10 of the Paris Basin (Solé et al. 2014) as well as in the South of France around the Ypresian/Lutetian transition (Rouzilhac; Godinot et al. 2018). Moreover, Proviverra is very close to Lesmesodon, a hyaenodont genus known in Messel, the reference-locality of the reference-level MP11 (Germany).
Cartierodon is closely related to Prodissopsalis and Eurotherium matthesi (Fig. 5). Eurotherium matthesi is recorded at Geiseltal-Untere Unterkohle (MP11). As a consequence, one can hypothesize that the appearances of Cartierodon and Prodissopsalis may be the result of local evolution, as for Leonhardtina and Proviverra, rather than a migration from Southern Europe.
Therefore, the turnover among hyaenodonts during the intra-Eocene faunal turnover seems different from that of primates and palaeotheres: hyaenodontoids from Central Europe have not been replaced by immigrants that have originated in Southern Europe. However, it is worth keeping in mind that there is a lack of data concerning the Spanish hyaenodontoids from the Ypresian and Lutetian.
Finally, as underlined by Solé et al. (2015), the presence of hyaenodonts (Oxyaenoides schlosseri and Eurotherium theriodis) from Aigues-Vives 2 (South of France; MP13?) confirms taxonomic homogeneity, at least at the genus level, of the hyaenodont fauna in Europe around the time the faunas close to MP13–MP14 reference-levels got deposited. This has been previously demonstrated with the discovery of Oxyaenoides sp. and Proviverra typica at Saint-Martin-de-Londres (South of France; MP13?) (Crochet et al. 1990), and the presence of Prodissopsalis eocaenicus and Eurotherium theriodis at Lissieu and Issel (South of France, MP14) (Calas 1969, 1970; Lange-Badré and Haubold 1990). The presence of Cartierodon at Lissieu reinforces this pattern.
Conclusions
The description of Cartierodon egerkingensis based on fossils from Egerkingen γ (MP13?) importantly improves our knowledge of the ecology of the Lutetian hyaenodonts. This taxon likely represents a bone-cracking hypercarnivore. Moreover, it is the largest hyaenodont from the Lutetian.
Its body mass clearly shows that the maximum body mass of the European hyaenodontoids increased throughout the Ypresian and Lutetian, possibly in response to the vacated large-size predator niche after the disappearance of oxyaenids (Palaeonictis and Oxyaena) and mesonychids (Dissacus and Pachyaena) during the Ypresian.
However, one can still wonder why European hyaenodonts did not reach 150 kg during the Eocene as some Pachyaena species did during the early Ypresian of Europe. This question needs future study, including the analysis of available prey body masses.
Acknowledgments
This paper is dedicated to the memory of our dear colleague Brigitte Lange-Badré, who first identified Cartierodon egerkingensis. We are extremely grateful to Loïc Costeur (Vertebrate Paleontology Curator at Naturhistorisches Museum Basel, Switzerland) who provided access to the material and permitted the realization of all this study in the Naturhistorisches Museum Basel facilities. Emmanuel Robert (Curator of Paleontological Collections at FSL) is warmly thanked for providing information on the specimens from Lissieu. We warmly thank the editor and the two reviewers, Matthew Borths (Duke Lemur Center, Duke University, Durham, USA) and Michael Morlo (Department of Messel Research and Mammalogy, Senckenberg Research Institute, Frankfurt am Main, Germany), for their constructive comments that greatly improved this manuscript. This work was supported by project Brain Pionnier BR/175/PI/CARNAGES of the Federal Science Policy Office of Belgium. The Swiss National Science Foundation is warmly thanked for granting the SNF project P300P2_161065 and P3P3P2_161066.
References
Angst, D., Buffetaut, E., Lécuyer, C., and Amiot, R. 2013. Terror Birds (Phorusrhacidae) from the Eocene of Europe Imply Trans-Tethys Dispersal. PLOS ONE 8 (11): e80357.
Angst, D., Lécuyer, C., Amiot, R., Buffetaut, E., Fourel, F., Martineau, F., Legendre, S., Abourachid, A., and Herrel, A. 2014. Isotopic and anatomical evidence of an herbivorous diet in the Early Tertiary giant bird Gastornis: Implications for the structure of Paleocene terrestrial ecosystems. Naturwissenschaften 101: 313–322.
Badiola, A., Checa, L., Cuesta, M.A., Quer, R., Hooker, J.J., and Astibia, H. 2009. The role of new Iberian finds in understanding European Eocene mammalian paleobiogeography. Geologica Acta 7: 243–258.
Bastl, K. 2012. The ecomorphology of the European Hyaenodon. 300 pp. Ph.D. Thesis, University of Vienna, Vienna.
Bastl, K., Semprebon, K., and Nagel, D. 2012. Low magnification microwear in Carnivora and dietary diversity in Hyaenodon (Mammalia: Hyaenodontidae) with additional information on its enamel microstructure. Palaeogeography, Palaeoclimatology, Palaeoecology 348–349: 13–20.
Beck, R.M.D. and Lee, M.S.Y. 2014. Ancient dates or accelerated rates? Morphological clocks and the antiquity of placental mammals. Proceedings of the Royal Society B 281: 20141278.
Becker, D. 2003. Paléoécologie et paléoclimats de la Molasse du Jura (Oligo-Miocène): apport des Rhinocerotoidea (Mammalia) et des minéraux argileux. Geofocus 10: 1–331.
BiochroM’97 1997. Synthèses et tableaux de corrélations. In: J.-P. Aguilar, S. Legendre, and J. Michaux (eds.), Actes du Congrèse BiochroM’97. Mémoires et Travaux de l’ Institut de Montpellier de l’Ecole Pratique des Hautes Études 21: 769–805.
Borths, M.R. and Seiffert, E.R. 2017. Craniodental and humeral morphology of a new species of Masrasector (Teratodontinae, Hyaenodonta, Placentalia) from the late Eocene of Egypt and locomotor diversity in hyaenodonts. PLOS ONE 12 (4): e0173527.
Borths, M.R. and Stevens, N.J. 2017a. Deciduous and dental eruption of Hyainailouroidea (Hyaenodonta, Placentalia, Mammalia). Palaeontologia Electronica 20 (3): 1–34.
Borths, M.R. and Stevens, N.J. 2017b. Taxonomic affinities of the enigmatic Prionogale breviceps, early Miocene, Kenya. Historical Biology [published online, https://doi.org/10.1080/08912963.2017.1393075].
Borths, M.R. and Stevens, N.J. 2017c. The first hyaenodont from the late Oligocene Nsungwe Formation of Tanzania: paleoecological insights into the Paleogene–Neogene carnivore transition. PLOS ONE 12 (10): e0185301.
Borths, M.R., Holroyd P.A. and Seiffert, E.R. 2016. Hyainailourinae and Teratodontinae cranial material from the late Eocene of Egypt and the application of parsimony and Bayesian methods to the phylogeny and biogeography of Hyaenodonta (Placentalia, Mammalia). PeerJ 4: e2639.
Brochu, C.A. 2013 Phylogenetic relationships of Palaeogene ziphodont eusuchians and the status of Pristichampsus Gervais, 1853. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 103: 521–550.
Calas, G. 1969. Découverte d’un créodonte dans le Lutétien du bassin d’Aquitaine. Compte Rendu sommaire des Séances de la Société Géologique de France 1969 (8): 299–300.
Calas, G. 1970. Les carnivores du gisement lutétien de Lissieu (Rhône). Compte Rendu sommaire des Séances de la Société Géologique de France 1970 (6): 226–227.
Carbone, C., Teacher, A., and Rowcliffe, J.M. 2007. The costs of carnivory. PLOS Biology 5: e22.
Crochet, J.-Y. 1991. A propos de quelques Créodontes proviverrinés de l’Eocène supérieur du Sud de la France. Neues Jahrbuch für Geologie und Paleontologie Abhandlungen 182: 99–115.
Crochet, J.-Y., Godinot, M., Hartenberger, J.-L., Rémy, J.A., Sigé, B., and Sudre, J. 1988. Découverte dans le bassin de Saint-Martin-de-Londres (Hérault, Sud de la France) d’un gisement à vertébrés continentaux d’âge Eocène moyen. Courier Forschungsinstitut Senckenberg 107: 419–434.
Dembo, M., Matzke, N.J., Mooers, A.Ø., and Collard, M. 2015. Bayesian analysis of a morphological supermatrix sheds light on controversial fossil hominid relationships. Proceedings of the Royal Society B 282: 20150943.
Depéret, C. 1894. Sur un gisement sidérolithique de mammifères de l’Éocène moyen, à Lissieu, près Lyon. Compte Rendus de l’Académie des Sciences, Paris 118: 822–823.
Duprat, M. 1997. Les faciès à mammifères (MP 6 à MP16) dans le Nord-Est du Bassin de Paris (France): argumentation du modèle tectono-sédimentaire des dépôts paléogènes. In: J.-P. Aguilar, S. Legendre, and J. Michaux (eds.), Actes du Congrèse BiochroM’97. Mémoires et Travaux de l’ Institut de Montpellier de l’Ecole Pratique des Hautes Études 21: 315–336.
Escarguel, G. 1999. Les rongeurs de l’Eocène inférieur et moyen d’Europe Occidentale. Systématique, phylogénie, biochronologie et paléobiogéographie des niveaux-repères MP 7 à MP 14. Palaeovertebrata 28: 89–351.
Estes, R. 1992. The Behavior Guide to African Mammals: Including Hoofed Mammals, Carnivores, Primates. 640 pp. University of California Press, Berkeley.
Franzen, J.L. 2003. Mammalian faunal turnover in the Eocene of central Europe. Geological Society of America Special Papers 369: 455–461.
Friscia, A.R. and Van Valkenburgh, B. 2010. Ecomorphology of North American eocene carnivores: Evidence for competition between carnivorans and creodonts. In: A. Goswami and A. Friscia (eds.), Carnivoran Evolution: New Views on Phylogeny, Form, and Function, 311–341. Cambridge University Press, Cambridge.
Gingerich, P.D. and Deutsch, H.A. 1989. Systematics and evolution of early Eocene Hyaenodontidae (Mammalia, Creodonta) in the Clark Fork Basin, Wyoming. Contributions from the Museum of Paleontology, University of Michigan 27: 327–391.
Godinot, M. 1981. Les Mammifères de Rians (Eocène inférieur, Provence). Palaeovertebrata 10: 43–126.
Godinot, M., Crochet, J.-Y., Hartenberger, J.L., Lange-Badré, B., Russell, D.E., and Sigé, B. 1987. Nouvelles données sur les mammifères de Palette (Eocène inférieur, Provence). Münchner Geowissenschaftliche Abhandlungen A 10: 273–288.
Godinot, M., Labarrère, H.-P., Erfurt, J., Franzen, J.L., Lange-Badré, B., Lapparent de Broin, F. de, and Vidalenc, D. 2018. Un nouveau gisement à vertébrés éocènes, Rouzilhac (MP 10–11), dans la série molassique d’Issel (Aude, France). Revue de Paléobiologie 37: 141–333.
Gorscak, E. and O’Connor, P.M. 2016. Time-calibrated models support congruency between Cretaceous continental rifting and titanosaurian evolutionary history. Biology Letters 12 (4): 20151047.
Hooker, J.J. 1996. Mammalian biostratigraphy across the Paleocene–Eocene boundary in the Paris, London and Belgian basins. In: R.W. Knox, R.M. Corfield, and R.E. Dunay (eds.), Correlation of the Early Paleogene in Northwest Europe. Geological Society, London, Special Publications 101: 205–218.
Hooker, J.J. 1998. Mammalian faunal change across the Paleocene–Eocene transition in Europe. In: M.-P. Aubry, S.G. Lucas, and W.A. Berggren (eds.), Late Paleocene–Early Eocene Climatic and Biotic Events in the Marine and Terrestrial Records, 419–441. Columbia University Press, New York.
Hooker, J.J. 2010. The mammal fauna of the early Eocene Blackheath Formation of Abbey Wood, London. Monograph of the Palaeontographical Society London 165: 1–162.
Lange-Badré, B. 1972. A propos de l’origine des Hyaenodon européens. Comptes Rendus hebdomadaires des Séances de l’Académie des Sciences, Série D 275: 2215–2217.
Lange-Badré, B. 1979. Les Créodontes (Mammalia) d’Europe occidentale de l’Eocène supérieur à l’Oligocène supérieur. Mémoires du Muséum National d’Histoire Naturelle 42: 1–249.
Lange-Badré, B. and Haubold, H. 1990. Les créodontes (mammifères) du gisement de Geiseltal (Eocène moyen, RDA). Geobios 23: 607–637.
Laurent, Y., Adnet, S., Bourdon, E., Corbalan, D., Danilo, L., Duffaud, S., Fleury, G., Garcia, G., Godinot, M., Le Roux, G., Maisonnave, C., Métais, G., Mourer-Chauviré, C., Presseq, B., Sigé, B., and Solé, F. 2010. La Borie (Saint-Papoul, Aude): un gisement exceptionnel dans l’Éocène basal du Sud de la France. Bulletin de la Société d’Histoire Naturelle de Toulouse 146: 89–103.
Lund, E.K., O’Connor, P.M., Loewen, M.A., and Jinnah, Z.A. 2016. A new centrosaurine ceratopsid, Machairoceratops cronusi gen. et sp. nov., from the Upper Sand Member of the Wahweap Formation (Middle Campanian), Southern Utah. PLOS ONE 11(5): e0154403.
Marandat, B. 1997. La disparité des faunes mammaliennes du niveau MP7 (Éocène inférieur) des domaines péri-mésogéens et nordique. Investigation d’un provincialisme intra-européen. Newsletters on Stratigraphy 35: 63–82.
Matthes, H.W. 1952. Die Creodontier aus der mitteleozänen Braunkohle des Geiseltales. Hallesches Jahrbuch für Mitteldeutsche Erdgeschicht 1: 201–240.
Morlo, M. 1999. Niche structure and evolution in creodont (Mammalia) faunas of the European and North American Eocene. Geobios 32: 297–305.
Morlo, M. and Habersetzer, J. 1999. The Hyaenodontidae (Creodonta, Mammalia) from the lower Middle Eocene (MP 11) of Messel (Germany) with special remarks on new x-ray methods. Courier Forschungsinstitut Senckenberg 216: 31–73.
Morlo, M., Gunnell, G.F., and Nagel, D. 2010. Ecomorphological analysis of carnivore guilds in the Eocene through Miocene of Laurasia. In: A. Goswami and A. Friscia (eds.), Carnivoran Evolution: New Views on Phylogeny, Form, and Function, 269–310. Cambridge University Press, Cambridge.
Neal, J.E. 1996. A summary of Paleogene sequence stratigraphy in northwest European and the North Sea. In: M.-P. Aubry, S.G. Lucas, and W.A. Berggren (eds.), Late Paleocene–Early Eocene Climatic and Biotic Events in the Marine and Terrestrial Records, 15–42. Columbia University Press, New York.
Nixon, K.C. 2002. WinClada (BETA), version 1.00.08. Published by the author, Ithaca.
Polly, P.D. 1996. The skeleton of Gazinocyon vulpeculus gen. et comb. nov. and the cladistic relationships of Hyaenodontidae (Eutheria, Mammalia). Journal of Vertebrate Paleontology 16: 303–319.
Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A., and Huelsenbeck, J.P. 2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542.
Smith, T. and Smith, R. 2001. The creodonts (Mammalia, Ferae) from the Paleocene–Eocene transition in Belgium (Tienen Formation, MP7). Belgian Journal of Zoology 131: 117–135.
Smith, T. and Smith, R. 2013. A land micro-mammal fauna from the early Eocene marine Egem deposits (NP12, Belgium) and the first occurrence of the peradectid marsupial Armintodelphys outside North America. Geologica Belgica 16: 302–310.
Solé, F. 2013. New proviverrine genus from the early Eocene of Europe and the first phylogeny of late Palaeocene–middle Eocene hyaenodontidans (Mammalia). Journal of Systematic Palaeontology 11: 375–398.
Solé, F. 2014. New carnivoraforms from the early Eocene of Europe and their bearing on the evolution of the Carnivoraformes. Palaeontology 57: 963–978.
Solé, F., Dubied, M., Le Verger, K., and Mennecart, B. 2018a. Niche partitioning of the European carnivorous mammals during the Paleogene. Palaios 33: 514–523.
Solé, F., Falconnet, J., and Laurent, Y. 2014. New proviverrines (Hyaenodontida) from the early Eocene of Europe; phylogeny and ecological evolution of the Proviverrinae. Zoological Journal of the Linnean Society 171: 878–917.
Solé, F., Falconnet, J., and Vidalenc, D. 2015. New fossil Hyaenodonta (Mammalia, Placentalia) from the Ypresian and Lutetian of France and the evolution of the Proviverrinae in southern Europe. Palaeontology 58: 1049–1072.
Solé, F., Gheerbrant, E., and Godinot, M. 2011. New data on the Oxyaenidae from the Early Eocene of Europe; biostratigraphic, paleobiogeographic and paleoecologic implications. Palaeontologia Electronica 14 (2): 46.
Solé, F., Gheerbrant, E., and Godinot, M. 2013a. Sinopaninae and Arfianinae (Hyaenodontida, Mammalia) from the early Eocene of Europe and Asia; evidence for dispersal in Laurasia around the Paleocene/Eocene boundary and for an unnoticed faunal turnover in Europe. Geobios 46: 313–327.
Solé, F., Gheerbrant, E., and Godinot, M. 2013b. The “miacids” (Carnivoraformes Mammalia) from early Eocene locality of Le Quesnoy (MP7, France); first occurrence of Vassacyon in Europe. Comptes Rendus Palevol 12: 191–202.
Solé, F., Godinot, M., Laurent, Y., Galoyer, A., and Smith, T. 2018b. The European Mesonychid Mammals: Phylogeny, Ecology, Biogeography, and Biochronology. Journal of Mammalian Evolution 25: 339–379.
Szalay, F.S. 1969. Mixodectidae, Microsyopidae, and the insectivore-primate transition. Bulletin of the American Museum of Natural History 140: 193–330.
Turner, A.H., Pritchard, A.C., and Matzke, N.J. 2017. Empirical and Bayesian approaches to fossil-only divergence times: a study across three reptile clades. PLOS ONE 12 (10): e0169885.
Van Valen, L. 1965. Some European Proviverrini (Mammalia, Deltatheridia). Palaeontology 844: 638–665.
Van Valkenburgh, B. 1988. Trophic diversity in past and present guilds of large predatory mammals. Paleobiology 14: 155–173.
Van Valkenburgh, B. 1990. Skeletal and dental predictors of body mass in carnivores. In: J. Damuth and B.J. MacFadden (eds.), Body Size in Mammalian Paleobiology: Estimation and Biological Implications, 181–205. Cambridge University Press, Cambridge.
Van Valkenburgh, B. 2007. Déjà vu: the evolution of feeding morphologies in the Carnivora. Integrative and Comparative Biology 47: 147–161.
Werdelin, L. and Cote, S.M. 2010. Prionogalidae (Mammalia Incertae Sedis). In: L. Werdelin and W.J. Sanders (eds.), Cenozoic Mammals of Africa, 567–568. University of California Press, Berkeley.
Acta Palaeontol. Pol. 64 (2): 275–290, 2019
https://doi.org/10.4202/app.00581.2018