

The oldest record of Juniperoxylon, a cupressaceous fossil wood from the Middle Triassic of Argentina
DANIELA P. RUIZ and JOSEFINA BODNAR
Ruiz, D.P. and Bodnar, J. 2019. The oldest record of Juniperoxylon, a cupressaceous fossil wood from the Middle Triassic of Argentina. Acta Palaeontologica Polonica 64 (3): 481–488.
One of the oldest species of fossil woods with affinities to Cupressaceae is Cupressinoxylon zamunerae from the Ladinian, Middle Triassic of Argentina, but the generic assignment of this species was a subject of debate. The main character that is in conflict with Cupressinoxylon is the presence of ray tracheids in C. zamunerae, a character absent in the original diagnosis of the genus. In order to clarify this issue, original microscopic slides of the type materials were restudied herein, and also new sections and SEM samples were observed. The supposed ray tracheids turned out to be parenchyma cells. A new combination is established, relating C. zamunerae to Juniperoxylon due to the markedly pitted ray parenchyma cells walls. This fossil species is the first mention of Juniperoxylon from the Mesozoic of Gondwana and the oldest record worldwide until date.
Key words: Coniferales, Cupressaceae, Juniperoxylon, wood anatomy, Triassic, Cortaderita Formation, Argentina.
Daniela P. Ruiz [ruizdaniela8@gmail.com], Museo Argentino de Ciencias Naturales-CONICET. 470, Ángel Gallardo Av., C1405DJR, Buenos Aires, Argentina.
Josefina Bodnar [jbodnar@fcnym.unlp.edu.ar], Facultad de Ciencias Naturales y Museo, CONICET, Paseo del Bosque s/n, B1900FWA La Plata, Buenos Aires, Argentina.
Received 11 January 2019, accepted 18 March 2019, available online 23 July 2019.
Copyright © 2019 D.P. Ruiz and J. Bodnar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Triassic record of fossil woods with affinities to the family Cupressaceae is very scarce, with reports of Cupressinoxylon zamunerae Bodnar, Ruiz, Artabe, Morel, and Ganuza, 2015 and Protojuniperoxylon ischigualastense (Bonetti, 1966) Bodnar and Artabe, 2007, both from Argentina (Bonetti 1966; Bodnar and Artabe 2007; Bodnar et al. 2015), Protochamaecyparixylon klitzschii Giraud in Giraud and Hankel, 1985 from Chile (Lutz et al. 1999), and Juniperoxylon sp. and Cupressinoxylon sp. from France (Lemoigne 1967). From these, Protochamaecyparixylon klitzschii is of doubtful assignment and, both Juniperoxylon sp. and Cupressinoxylon sp. from France probably correspond to Protocupressinoxylon according to Barale (1987). In addition, the sediments in which the French specimens were found were assigned to the Lower Jurassic (Barale 1987).
Cupressinoxylon zamunerae is the oldest taxon related to the family, and constitutes the earliest record of the genus Cupressinoxylon Göppert, 1850 until today. However, the assignment of the species to this fossil-genus has been questioned by some authors (i.e., Brea et al. 2016; Ruiz et al. 2017) because the characters described in the specific diagnosis do not fit with those in the original generic diagnosis of Göppert (1850).
In order to clarify this controversy, the type material of Cupressinoxylon zamunerae is reanalyzed, the specific diagnosis is emended, and the generic assignment is changed and discussed. Furthermore, this revision provides more details about the systematic affiliation of this species.
Institutional abbreviations.—PBSJ, Palaeobotanical Collection of the Museo de Ciencias Naturales de San Juan, San Juan, Argentina.
Other abbreviations.—Cp, contiguity percentage index; RLS, radial longitudinal section; Si, seriation index; TLS, tangential longitudinal section; TS, transversal section.
Material and methods
The studied material corresponds to the type specimens of Cupressinoxylon zamunerae described by Bodnar et al. (2015). The samples are fossil trunks collected from the Cortaderita Formation (Middle Triassic, Sorocayense Group) at La Tinta creek, 8 km east of Barreal city, located in the western Precordillera, San Juan Province, central-western Argentina, between W 69°26´12.2´´ S 31°37´45.8´´ and W 69°23´52.1´´ S 31°39´00.1´´ (Fig. 1).
The samples are five permineralized woods housed at the Palaeobotanical Collection of the Museo de Ciencias Naturales de San Juan under the acronyms PBSJ 827 to PBSJ 831. Previous microscopic thin sections were revised and also new sections were made. Thin sections were studied using Leica DC 150 light microscopy. Small fragments of all of the woods were observed at SEM, mostly in longitudinal radial view.
A minimum of 30 measurements of each character were made. The measurements are expressed as the mean followed by the range between parentheses. The description was made following the terminology of Philippe (1995) and IAWA Committee (2004), and the Si and Cp indices of Pujana et al. (2016). For the suprageneric assignment the classification scheme of Anderson et al. (2007) was followed.
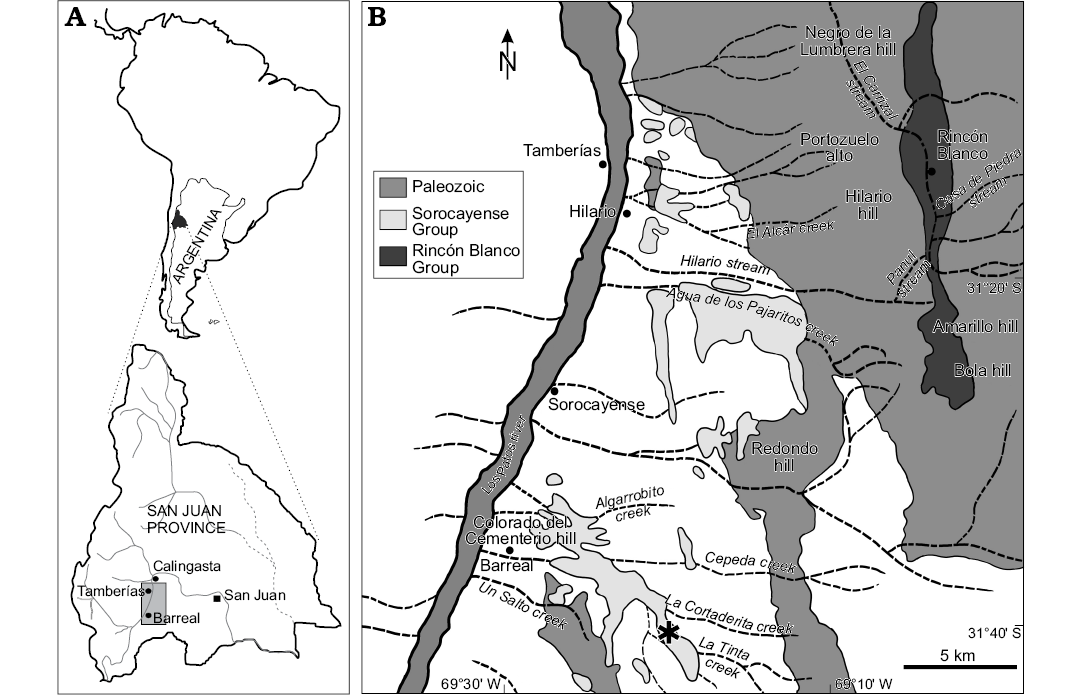
Fig. 1. A. Geographic location of study area at San Juan Province, Argentina. C. Geologic map showing the sampled locality (asterisk) at Cortaderita and La Tinta creeks, near Barreal town. Taken from Bodnar et al. (2018).
Systematic palaeontology
Order Pinales (= Coniferales) Gorozhankin, 1904
Family Cupressaceae Gray, 1822
Genus Juniperoxylon (Houlbert, 1910) Kräusel, 1949
Type species: Juniperoxylon turonense Houlbert, 1910; Miocene, Touraine, France.
Juniperoxylon zamunerae (Bodnar, Ruiz, Artabe, Morel, and Ganuza, 2015) comb. nov. emend.
Fig. 2.
2015 Cupressinoxylon zamunerae; Bodnar at al. 2015: 149–150, fig. 5A−O.
Type material: Five pieces of different trunks and their thin sections. Holotype: PBSJ 828. Paratypes: PBSJ 827; PBSJ 829−831. There are three thin sections for each of the three sections (RLS, TLS, TS) used for anatomical studies.
Type locality: La Tinta creek, Barreal area, Barreal-Calingasta depocenter, Cuyo Basin, San Juan Province, Argentina.
Type horizon: EF6 and EF7, lower member of Cortaderita Formation, Ladinian (Middle Triassic) (Bodnar et al. 2019).
Emended diagnosis.—Abrupt transition from earlywood to latewood, narrow band of latewood. Abietinean radial pitting. Conspicuous torus. Axial parenchyma scarce, with smooth or irregularly thickened transverse end walls. Cross-fields with 1−2 oculipores of cupressoid type in one radial row. Homocelullar, mainly uniseriate rays of medium height.
Description.—Homoxylic and pycnoxylic seconday xylem. Transversal section: growth rings boundaries are distinct, with 1−3 cells of latewood (abrupt transition) (Fig. 2A1). Tracheids are quadrangular in outline (Fig. 2A1). Tangential diameter of tracheids is 26.5 µm (10.9−48.9 µm), and tracheid walls are 7.4 µm (4.2−10.9 µm) thick. Tracheid radial diameter is 34.5 µm (15.7−61 µm) in earlywood. Axial parenchyma is scarce (Fig. 2A1). Rays are separated from each other by 3 (1−6) rows of tracheids.
Radial longitudinal section: the wood has abietinean radial pitting, pits are circular, bordered, spaced (Cp = 21.4) and predominantly uniseriate (Si = 1), with occasional biseriation (Fig. 2A3, A4). Only a few contiguous pits were observed. When biseriation occurs pits are opposite. Pits are 18.9 µm (13.1−25.5 µm) in radial diameter and 17.9 µm (14.9−22.6 µm) in vertical diameter, and pit apertures are 3.9 µm (3.2−4.6 µm) in vertical diameter. Torus is clearly seen (Fig. 2A2, A12).
Rays are homocellular and parenchyma cells are rectangular (procumbent) with distinctly pitted horizontal and end walls (nodular) (Fig. 2A8, B). In some parts of the samples this character is hardly visible because of the poor preservation. End walls are straight (vertical or oblique) or occasionally concave or convex (“indented” in the original diagnosis). In some rays, marginal ray parenchyma cells have an irregular shape giving the appearance of ray tracheids (Fig. 2A9, A10). These cells are higher, 24.3 µm (20.7−31.2 µm), than the other ray cells which are 14.9 µm (7.3−21.3 µm) high, but they are still of procumbent type. There are one or two (up to four) oculipores of the cupressoid type per cross-field (sensu IAWA 2004) (Fig. 2A5−A7, A13), with an oblique aperture of 5.8 µm (3.9−7.8 µm) in vertical diameter.
Tangential longitudinal section: rays are mostly uniseriate (93.3%) but some of them are partially biseriate (6.7%) (Fig. 2A11). Rays are 10 µm (1−34) cells and 210 µm (52−751) high. Ray cells are circular in tangential view and the lumen has a width of 11.1 µm (5.3−18.8 µm), with walls of 3.5 µm (2.3−4.8 µm) thick. Axial parenchyma is scarce and diffuse. The transverse end walls of the axial parenchyma are smooth or irregularly thickened (Fig. 2A11).
Stratigraphic and geographic range.—Ladinian, Middle Triassic, San Juan Province, Argentina.
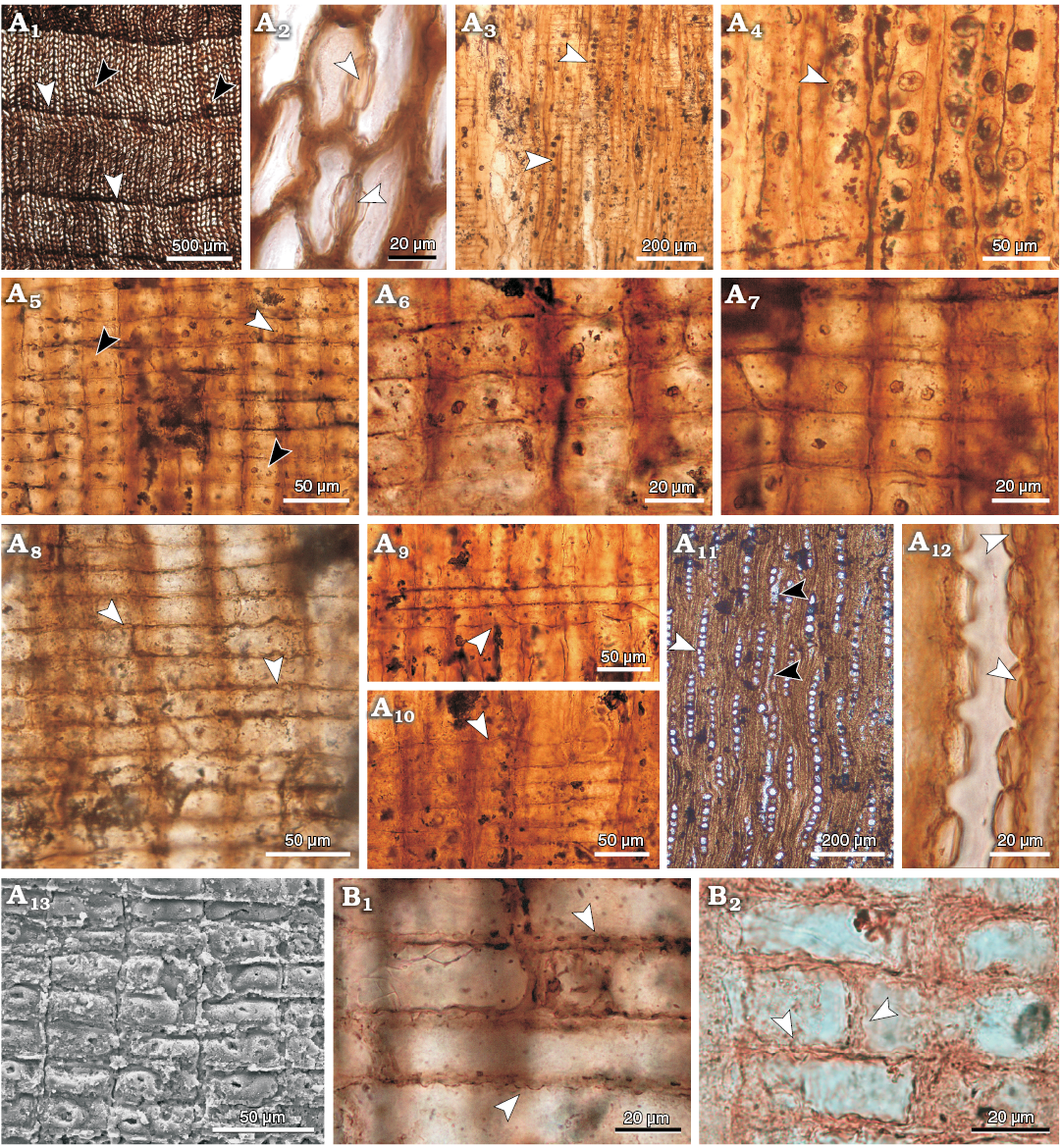
Fig. 2. Cupressaceous wood Juniperoxylon zamunerae (Bodnar, Ruiz, Artabe, Morel, and Ganuza, 2015) comb. nov. (holotype PBSJ 828, A; PBSJ 829, B), Cortaderita Formation, Middle Triassic of Argentina. A1, growth rings (white arrowheads) and axial parenchyma (black arrowheads), TS; A2, torus (arrowheads), TS; A3, A4, uniseriate or rarely biseriate radial pitting on tracheid walls (arrowheads), RLS; A5, cupressoid cross-field pits (black arrowheads) and nodular end walls of ray parenchyma (white arrowheads), RLS; A6, A7, detail of cross-field pits, RLS; A8, general view of ray parenchyma cells walls distinctly pitted (arrowheads), RLS; A9, A10, different ray parenchyma cells (arrowheads), RLS; A11, occasional biseriation on part of a ray (white arrowhead) and axial parenchyma (black arrowhead), TLS; A12, radial pits with torus (arrowheads), TLS; A13, cross-field with cupressoid pits, RLS. B1, B2, ray parenchyma walls distinctly pitted (arrowheads), RLS.
Discussion
Generic assignment and comparisons.—According to Philippe and Bamford (2008), the fossil wood Cupressinoxylon is characterized by an usually narrow latewood with thick-walled flattened tracheids latewood and wide earlywood with thin-walled tracheids; pits round, uni- to quadriseriate and opposite, sometimes also on tangential walls; presence of axial parenchyma; low and uniseriate homocellular rays composed of pitted parenchyma cells; cupressoid cross-field with spaced oculipores in the early-wood usually ordered in rows and columns. However, several authors adopted a wider use of Cupressinoxylon (e.g., Müller-Stoll and Schultze-Motel 1990; Wang et al. 1996; Bamford et al. 2002) as a fossil-genus comprising those woods of Cupressaceae type which cannot be assigned to a more precise fossil-genus (sensu Vaudois and Privé 1971), considering it as a “wastebasket” taxon. The original diagnosis (Bodnar et al. 2015: 149) of Cupressinoxylon zamunerae characterized the species as it follows “Pycnoxylic homoxylic secondary wood with distinct growth rings of variable width […] Tracheid pits in radial walls bordered, circular and spaced, predominantly uniseriate, with circular pore and conspicuous torus. Axial parenchyma scarce and diffuse. Cross-fields with 1−2 oculipores of cupressoid type, in one row. Heterocellular, mainly uniseriate, and 4−33 cells high rays. Ray cells with pitted horizontal walls, and thickened and indented end walls”. This combination of characteristics led Bodnar et al. (2015) to assign the studied samples to Cupressinoxylon in the wider sense of Vaudois and Privé (1971).
Some authors have put in doubt the assignment of the studied species to Cupressinoxylon based on the presence of heterocellular rays (Brea et al. 2016; Ruiz et al. 2017). In the present revision of the type material of Cupressinoxylon zamunerae, the putative rays tracheids are considered as larger parenchyma cells with irregular shape but of the same type of the other ray cells (see description above), because they have identical walls and pits (Fig. 2A9, A10). As a result, rays are herein reinterpreted as homocellular.
The character “indented” end walls on ray parenchyma cells, mentioned in the diagnosis of Bodnar et al. (2015), is not included in the present specific emendation in order to avoid confusion with the term “indentures” used in IAWA (2004).
Another trait that drew attention is the presence of ray parenchyma cells with terminal and horizontal walls distinctly pitted. This feature was included in the specific diagnosis of Bodnar et al. (2015), but only the horizontal walls of the ray cell were indicated as pitted. As seen in the Fig. 2B2, the end walls of ray parenchyma cells are also distinctly pitted (i.e., nodular). Two other fossil-genera of homoxylic wood with cupressoid cross-fields are characterized by the occurrence of nodular walls of ray parenchyma cells: i.e., Protojuniperoxylon and Juniperoxylon (Philippe and Bamford 2008). The abietinean pattern of radial tracheid pitting excludes the analyzed wood from Protojuniperoxylon which possesses mixed tracheid pitting (Table 1). As a consequence, we transferred Cupressinoxylon zamunerae to Juniperoxylon herein.
Houlbert (1910) erected the genus Juniperoxylon but he did not provide an appropriate diagnosis. Therefore, the protologue of Kräusel (1949) is in common use. It reads as follows: “Wood, similar to Cupressinoxylon. Ray cell walls, at least the terminal ones, more or less strongly pitted (“Juniperustüpfelung”), traumatic wood never with resin pockets” (Kräusel 1949: 177 [translated from the German]).
Approximately 19 species of Juniperoxylon have been described, mainly from Europe. From these, seven species were reassigned to another genera: Juniperoxylon uralense Jarmolenko, 1934b to Cupressinoxylon (Kräusel 1949), Juniperoxylon turoviense Kostyniuk, 1967, and Juniperoxylon lusaticum Kostyniuk, 1967 to Taxodioxylon (Süss and Rathner 1998; Kłusek 2014); Juniperoxylon glyptostroboides Kostyniuk, 1967 to Glyptostroboxylon (Dolezych and Van der Burgh 2004), Juniperoxylon sibiricum Nastschokin, 1962 to Cedroxylon (Süss and Rathner 1998); Juniperoxylon lignieri Grambast, 1954 to Prototaxodioxylon (Süss and Rathner 1998); and Juniperoxylon barbaricinum Charrier, 1961 to Brachyoxylon (Philippe et al. 2004). On the other hand, Juniperoxylon silesiacum (Prill, 1913) Kräusel, 1920, Juniperoxylon pauciporosum (Prill, 1913) Kräusel, 1920, Juniperoxylon kalickiji Jarmolenko, 1934a, and Juniperoxylon neosibiricum (Schmalhausen, 1890) Selling, 1944 were synonimized with Juniperoxylon pachyderma (Göppert, 1850) Kräusel, 1949 (Kräusel 1949; Kłusek 2014). In this manner, only eight species are currently accepted within Juniperoxylon. In comparison with these taxa, Juniperoxylon zamunerae (Bodnar, Ruiz, Artabe, Morel, and Ganuza, 2015) comb nov. is distinguishable due to the ray height (up to 34 cells) and the scarce axial parenchyma. The only species with similar ray height (up to 40 cells) is J. rhenanurn Van der Burgh, 1973 from the Miocene of Germany, but it differs from the Argentinean wood because of the presence of crassulae and occasional ray tracheids (Table 1).
The record of Juniperoxylon is mainly restricted to Cenozoic, with only two reports from the Mesozoic: Juniperoxylon sp. from the Early Jurassic of France (Lemoigne 1967; Barale 1987) (of doubtful assignment), and Juniperoxylon pottoniense (Stopes, 1915) Kräusel, 1949 from the Lower Cretaceous of England (Stopes 1915; Kräusel 1949). The only species described as a Juniperoxylon from Gondwanan is Juniperoxylon barbaricinum Charrier, 1961, from Sardinia, Italy, but the determination was rejected by Bamford and Philippe (2001) and Philippe et al. (2004), because it presents mixed tracheid pitting and lacks of pits or thickenings on the end walls of ray cells. Thus, J. zamunerae (Bodnar, Ruiz, Artabe, Morel, and Ganuza, 2015) comb. nov. would be the first record of Juniperoxylon for the Mesozoic of Gondwana, and the earliest record of the genus worldwide until date.
Table 1. Comparison with the accepted species of Juniperoxylon and Protojuniperoxylon. References: Houlbert (1910), Stopes (1915), Eckhold (1923), Kräusel (1920, 1949), Stockmans and Wileière (1934), Grambast (1954), Bonetti (1966), Huard (1966), Van der Burgh (1973), Watari and Nishida (1973), Süss and Rathner (1998), Bodnar and Artabe (2007), Kłusek (2014), Dolezych (2016). Abbreviations: AP, axial parenchyma; C, crassulae; CFPT, cross field pit type; GR, distinct growth rings; RH, ray height (number of cells); RPEW, nodular ray parenchyma end walls; RPHW, ray parenchyma horizontal walls; RT, ray tracheids; RTP, radial tracheid pits; RW, ray width; +, present; –, absent; ± occasional; () characters which appear occasionally; ? unknown.
|
Species |
Age and distribution |
GR |
Serration of RTP |
Spacing of RTP |
Arrangement of bi- or multi- |
AP |
AP transverse end walls |
RH |
RW |
RT |
RPHW cells |
RPEW cells |
CFPT |
C |
|
Juniperoxylon breviparenchymatosum Watari and Nishida, 1973 |
Eocene; Japan |
+ |
uniseriate. biseriate |
spaced |
opposite |
abundant, diffuse and zonate |
smooth or irregularly thickened |
1–14 |
uniseriate, rarely partly biseriate |
– |
smooth or rarely pitted |
+ |
2–6, |
– |
|
Juniperoxylon juniperoides (Kownas, 1951) Huard, 1966 |
Miocene; Germany |
+ |
uniseriate, biseriate |
spaced |
opposite |
abundant, diffuse and zonate |
nodular |
1–12 |
uniseriate |
– |
distinctly pitted |
+ |
1–4 (6), |
+ |
|
Juniperoxylon pachyderma (Göppert, 1850) Kräusel, 1949 |
Oligocene–Miocene; Germany,
Belgium, |
+ |
uniseriate, rarely biseriate triseriate |
spaced |
opposite |
abundant, diffuse and zonate |
smooth or nodular |
1–15 |
uniseriate, rarely partly biseriate |
– |
smooth |
+ |
1–6, |
+ |
|
Juniperoxylon pottoniense (Stopes, 1915) Kräusel, 1949 |
Early Cretaceous– Eocene; England, Denmark |
+ |
uniseriate |
spaced? |
? |
abundant, zonate |
smooth? |
1–12 |
uniseriate |
– |
distinctly pitted |
+ |
cupressoid |
– |
|
Juniperoxylon rhenanurn Van der Burgh, 1973 |
Miocene; Germany |
+ |
uniseriate, biseriate |
spaced |
opposite |
present, diffuse and zonate |
nodular |
1–40 |
uniseriate, occasionally biseriate |
± |
distinctly pitted |
+ |
1–3, |
+ |
|
Juniperoxylon schneiderianum Dolezych, 2016 |
Miocene; Germany |
+ |
uniseriate, biseriate |
spaced |
opposite |
abundant, diffuse |
nodular |
2–18 |
uniseriate, occasionally biseriate |
– |
sparsely pitted |
+ |
2–4, taxodioid, |
+ |
|
Juniperoxylon turonense Houlbert, 1910 |
Eocene–Miocene; France |
+ |
? |
spaced? |
? |
present, diffuse |
? |
? |
uniseriate |
–? |
distinctly pitted? |
+? |
cupressoid or taxodiod? |
–? |
|
Juniperoxylon wagneri Süss and Rather, 1998 |
Miocene; Germany |
+ |
uniseriate, rarely biseriate |
spaced |
opposite |
abundant, diffuse |
nodular |
1–5 |
uniseriate |
– |
smooth |
+ |
2–8, |
– |
|
Juniperoxylon zamunerae (Bodnar, Ruiz, Artabe, Morel, and Ganuza, 2015) Ruiz and Bodnar, 2019 (this paper) |
Middle Triassic; Argentina |
+ |
uniseriate, rarely biseriate |
spaced |
opposite |
scarce, diffuse |
smooth or irregularly thickened |
2–34 |
uniseriate, rarely partly biseriate |
– |
distinctly pitted |
+ |
1–2 (3–4), |
– |
|
Protojuniperoxylon
|
Late Triassic; Argentina |
+ |
uniseriate, biseriate |
spaced, contiguous |
alternate, |
present, diffuse |
smooth |
1–45 |
uniseriate, less frequent partly bi- or triseriate |
+ |
distinctly pitted |
+ |
2–4, |
– |
|
Protojuniperoxylon
|
Lower Cretaceous; England |
+ |
uniseriate |
spaced, contiguous |
? |
absent or scarce, diffuse |
? |
1–20 |
uniseriate, rarely partly biseriate |
– |
smooth? |
+ |
4–6, |
+ |
Systematic affiliation.—Juniperoxylon has been related to the Cupressaceae (Vaudois and Privé 1971; Bodnar and Artabe 2007; Kłusek 2014; Dolezych 2016) due to the presence of abietinean tracheid pitting, distinctly pitted ray cell walls, axial parenchyma and cupressoid cross-fields, and the absence of normal resin canals and helical thickenings.
In particular, Juniperoxylon zamunerae (Bodnar, Ruiz, Artabe, Morel, and Ganuza, 2015) comb. nov. shows characters which relate it to different genera of living Cupressaceae (Table 2). The distinctly pitted end and horizontal walls of ray parenchyma cells relate this fossil wood to genera of Cupressaceae sensu stricto (i.e., Calocedrus, Chamaeocyparis, Cupressus, Fitzroya, and Juniperus), while the ray height (1−34 cells high) link it to genera belonging to the basal Cupressaceae (formerly Taxodiaceae) (i.e., Sequoiadendron and Taxodium) (Table 2). If J. zamunerae (Bodnar, Ruiz, Artabe, Morel, and Ganuza, 2015) comb. nov. corresponds to one of the oldest fossil related to the Cupressaceae, its characteristics could indicate that the earliest representatives of the family would have shared wood traits with both basal and derived Cupressaceae.
Table 2. Comparison of Juniperoxylon zamunerae (Bodnar, Ruiz, Artabe, Morel, and Ganuza, 2015) comb. nov. with selected extant genera of Cupressaceae sensu lato. Abbreviations: GR, growth rings; AT, axial tracheid; ATTP, axial tracheid tangential pitting; AP, axial parenchyma; RW, ray width; RH, ray height; RPEW, ray parenchyma cells end walls; RPHW, ray parenchyma horizontal walls; IN, indentures; CFPT, cross field pit type, CFPN, cross field pit number; RT, ray tracheids; + present; – absent; () characters which appear occasionally. From Román-Jordán et al. (2017) and Nunes et al. (2019).
|
Taxa |
Distribution |
GR |
AT cross |
AT |
ATTP |
AP |
AP |
AP |
RW |
RH |
RPEW |
RPHW |
IN |
CFPT |
CFPN |
RT |
|
Calocedrus |
USA, China, Thailand, Birmania,
Laos, |
well or slightly defined |
polygonal |
uniseriate, (biseriate) |
+ |
+ |
irregularly thickened, nodular |
diffuse, tangentially zonate or marginal |
uniseriate and partially biseriate |
1–15 |
smooth or nodular |
pitted or unpitted |
+ |
cupressoid |
1–4 (7) |
– |
|
Chamaecyparis |
USA, Japan, Taiwan |
well defined |
circular or polygonal |
uniseriate |
– or + |
– or + |
smooth or nodular |
diffuse or tangentially zonate |
uniseriate and partially biseriate |
1–15 (25) |
smooth or nodular |
pitted or unpitted |
– or + |
cupressoid or taxodioid |
1– 4 (7) |
– or + |
|
Cupressus |
temperate regions of Northern Hemisphere |
well or slightly defined |
circular or polygonal |
uniseriate |
+ |
scarce or + |
smooth or irregularly thickened, nodular |
diffuse, tangentially zonate or marginal |
uniseriate and partially biseriate |
1–15 (45) |
smooth or nodular |
pitted or unpitted |
+ |
cupressoid |
1–4 (6) |
– or + |
|
Fitzroya |
Argentina, Chile |
well defined |
polygonal |
uniseriate |
+ |
+ |
(smooth), irregularly thickened, nodular |
diffuse or tangentially zonate |
uniseriate |
1–15 (19) |
smooth or nodular |
pitted or unpitted |
+ |
cupressoid |
1–4 (8) |
– or (+) |
|
Juniperus |
Northern Hemisphere |
well defined |
circular or polygonal |
uniseriate, (biseriate) |
+ |
scarce or + |
smooth or irregularly thickened, nodular |
diffuse, tangentially zonate or marginal |
uniseriate and partially biseriate |
1–14 (24) |
smooth or nodular |
pitted or unpitted |
+ |
cupressoid |
1–4 (8) |
– |
|
Sequoiadendron |
USA |
well defined |
polygonal |
uniseriate (biseriate) |
+ |
+ |
smooth or irregularly thickened, nodular |
diffuse or marginal |
uniseriate and partially biseriate |
130 |
smooth |
pitted or unpitted |
– |
cupressoid and (taxodioid) |
1–2 |
– |
|
Taxodium |
USA, Mexico |
well defined |
polygonal |
uniseriate, biseriate |
+ |
+ |
(smooth) nodular |
diffuse or tangentially zonate |
uniseriate and partially biseriate |
1– 30 |
smooth |
(pitted) or unpitted |
– |
cupressoid and taxodioid |
1–4 (8) |
– |
|
Juniperoxylon zamunerae |
Argentina |
well defined |
polygonal (quadrangular) |
uniseriate, (biseriate) |
– |
scarce |
smooth or irregularly thickened |
diffuse |
uniseriate (partially biseriate) |
1–34 |
nodular |
pitted |
– |
cupressoid |
1–2 (4) |
– |
Conclusions
The systematic revision of the type specimens of Cupressinoxylon zamunerae revealed that: (i) the ray cells previously interpreted as tracheids are actually parenchyma cells with a different morphology than the others; and (ii) distinctly pitted walls of ray parenchyma cells indicate that the fossil wood is accurately assigned to Juniperoxylon. With the new combination, this species constitutes the first record of Juniperoxylon from the Mesozoic of Gondwana and the oldest record worldwide until today. Juniperoxylon zamunerae (Bodnar, Ruiz, Artabe, Morel, and Ganuza, 2015) comb. nov. has a combination of anatomical characters that allow us to assign it to the Cupressaceae, and probably represents one of the earliest record of the family.
Acknowledgments
We thank to Roberto R. Pujana (Museo Argentino de Ciencias Naturales-CONICET, Buenos Aires, Argentina) for the help in the preparation of the new microscopic thin sections and the comments on the manuscript, and to the electronic microscopy service of the Museo Argentino de Ciencias Naturales-CONICET. The editor and two anonymous reviewers are specially thanked for their helpful comments and suggestions. This study was supported by the Agencia Nacional de Promoción Científica y Tecnológica (PICT 2014-2751) and Universidad Nacional de La Plata (Project N807).
References
Anderson, J.M., Anderson, H.M., and Cleal, C.J. 2007. Brief History of the Gymnosperms: Classification, Biodiversity, Phytogeography and Ecology. 280 pp. South African National Biodiversity Institute, Pretoria.
Bamford, M. and Philippe, M. 2001. Gondwanan Jurassic–Early Cretaceous homoxylous woods: a nomenclatural revision of the genera with taxonomical notes. Review of Palaeobotany and Palynology 113: 287–297. Crossref
Bamford, M., Ziljstra, G., and Philippe, M. 2002. Proposal to conserve the name Cupressinoxylon Göppert (Fossil, Gymnospermae, Coniferales) against Retinodendron Zenker (Fossil, Gymnospermae, Coniferales), with a conserved type. Taxon 51: 205−206. Crossref
Barale, G. 1987. Les Cheirolépidiacées du Jurassique inférieur de Saint-Fromond, bassin de Carentan (Manche–France). Bulletin de la Société Botanique de France 134: 19–37. Crossref
Bodnar, J. and Artabe, A.E. 2007. Estudio sistemático y paleodendrológico del leño de una Cupressaceae triásica de la Formación Ischigualasto, provincia de San Juan, Argentina. Ameghiniana 44: 303–319.
Bodnar, J., Drovandi, J.M., Morel. E., and Ganuza, D.G. 2018. Middle Triassic dipterid ferns from west-central Argentina and their relationship to palaeoclimatic changes changes. Acta Palaeontologica Polonica 63: 397–416. Crossref
Bodnar, J., Iglesias, A., Colombi, C.E., and Drovandi, J.M. 2019. Stratigraphical, sedimentological and paleofloristic characterization of Sorocayense Group (Triassic) in Barreal Area, San Juan Province, Argentina. Andean Geology 46 [published online, http://dx.doi.org/10.5027/andgeo%25x] Crossref
Bodnar, J., Ruiz, D.P., Artabe, A.E., Morel, E.M., and Ganuza, D. 2015. Voltziales y Pinales (= Coniferales) de la Formación Cortaderita (Triásico Medio), Argentina, y su implicancia en la reconstrucción de las coníferas triásicas. Revista Brasileira de Paleontologia 18: 141−160. Crossref
Bonetti, M.I.R. 1966. Protojuniperoxylon ischigualastensis sp. nov. del Triásico de Ischigualasto (San Juan). Ameghiniana 4: 211–218.
Brea, M., Bellosi, E.S., Umazano, A.M., and Krause, J.M. 2016. Aptian–Albian Cupressaceae (sensu stricto) woods from Cañadón Asfalto Basin, Patagonia Argentina. Cretaceous Research 58: 17−28. Crossref
Charrier, G. 1961. Nuova segnalazione di un orizzonte lacustre a legni di conifera presso Nuraghe Mamucone (Urzulei) alla basa dela serie transgressiva giurese del Golfo di Orosei (Sardegna centroorientale) – studio stratigrafico e paleoxilologico. Bollettino della Società Geologica Italiana 80: 207–225.
Dolezych, M. 2016. A remarkable extinct wood from Lusatia (central Europe)—Juniperoxylon schneiderianum sp. nov. with affinity to Cupressospermum saxonicum Mai. Palaeontographica B 295: 5–31. Crossref
Dolezych, M. and Van der Burgh, J. 2004. Xylotomische Untersuchungen an inkohlten Hölzern aus dem Braunkohltegebau Berzdorf (Oberlausitz, Deutschland). Feddes Repertorium 115: 397–342. Crossref
Eckhold, W. 1923. Die Hoftüpfel bei rezenten und fossilen Coniferen. Jahrbuch der preußischen geologischen Landesanstalt zu Berlin 42: 472–505.
Giraud, B. and Hankel, O. 1985. Bois fossiles des dépôts du Karoo du Bassin du Luwegu (Tanzanie Méridionale). Annales de Paléontologie 71: 159 –185.
Göppert, J.H.R. 1850. Monographie der fossilen Coniferen. 286 pp. Arnz & Comp., Leiden.
Gorozhankin, I.N. [Gorožankin, I.N.] 1904. Lektsii po morfologii i sistematike archegonialnyh rastenij. II, Pteridophyta, I, Archispermae. 104 pp. A.I. Mamontov, Moskva.
Grambast, L. 1954. Un Juniperoxylon particulier de l’Eocène inférieur du Bassin de Paris. Archives du Muséum National du Paris 3: 1–21.
Gray, S.F. 1822. Natural Arrangement of British Plants, According to Their Relation to Each Other. Vol. 2. 760 pp. Baldwin, Cradock, and Joy, London.
Houlbert, C. 1910. Les bois des faluns de Touraine. Feuille des jeunes Naturalistes 40: 70–76.
Huard, J. 1966. Étude anatomique des bois de conifères des couches à lignite néogènes des Landes. Mémoires de la Société géologique de France 105: 1–85.
IAWA Comittee 2004. International Association of Wood Anatomists list of microscopic features for softwood identification. IAWA Journal 25: 1–70. Crossref
Jarmolenko, A. [Ârmolenko, A.] 1934a. On the fossil woods from the Cheleken Islands [in Russian]. Trudy neftânogo naučno-issledovatel’skogo geologorazvedočnogo instituta (VNIGRI) 48: 20–22.
Jarmolenko, A. 1934b. [Ârmolenko, A.] The significance of fossil woods for stratigraphy and sedimentology [in Russian]. Sovetskaâ botanika 2: 62–69.
Kłusek, M. 2014. Miocene coniferous woods of the Polish Carpathian Foredeep. Acta Palaeontogica Polonica 59: 697–708.
Kownas, S. 1951. Trzeciorzędowe drewna z Dobrzynia nad Wisłą. Studia Societatis Scientiarum Toruniensis 1: 67–121.
Kostyniuk, M. 1967. Pnie drzew iglastych z górnego pokładu węgla brunatnego w Turowie. Prace Muzeum Ziemi 10: 3–96.
Kräusel, R. 1920. Nachträge zur Tertiärflora Schlesiens II. Braunkohlenhölzer. Jahrbuch der preußischen geologischen Landesanstalt 39: 418–460.
Kräusel, R. 1949. Die fossilen Koniferen-Hölzer (Unter Ausschluß von Araucarioxylon Kraus). II: Kritische Untersuchungen zur Diagnostik lebender und fossiler Koniferen-Hölzer. Palaeontographica B 89: 83–203.
Lemoigne, Y. 1967. Paléoflore à Cupressales dans le Trias-Rhétien du Cotentin. Comptes Rendus hebdomadaires des séances de l’Académie des sciences 264: 715–718.
Lutz, A.I., Crisafulli, A., and Herbst, R. 1999. Gymnospermous woods from the Upper Triassic of Northern Chile. The Palaeobotanist 48: 31–38.
Müller-Stoll, W.R. and Schultze-Motel, J. 1990. Gymnospermen-Hölzer des deutschen Jura. Teil 3: Abietoid (modern) getüpfelt Hölzer. Zeitschrift der deutschen deologischen Gesellschaft 141: 61–77.
Nastschokin,V.-D. [Naŝokin,V.-D.] 1962. Fossil woods from Cretaceous sediments [in Russian]. Trudy sibirskogo naučno-issledovate’skogo instituta geologii 22: 161–164.
Nunes, C.I., Bodnar, J., Escapa, I.H., Gandolfo, M.A. and Cúneo, N.R. 2019. A new cupressaceous wood from the Early Cretaceous of Central Patagonia reveals possible clonal growth habit. Cretaceous Research 99: 133–148. Crossref
Philippe, M. 1995. Bois fossiles du Jurassique de Franche-Comté (nord-est de la France): systématique et biogéographie. Palaeontographica B 236: 325–343.
Philippe, M. and Bamford, M.K. 2008. A key to morphogenera used for Mesozoic conifer-like woods. Review of Palaeobotany and Palynology 148: 184–207. Crossref
Philippe, M., Bamford, M., McLoughlin, S., Alves, L.S.R., Falcon-Lang, H.J., Gnaedinger, S., Ottone, E.G., Pole, M., Rajanikanth, A., Shoemaker, R.E., Torres, T., and Zamuner, A. 2004. Biogeographic analysis of Jurassic–Early Cretaceous wood assemblages from Gondwana. Review of Palaeobotany and Palynology 129: 141–173. Crossref
Prill, W. 1913. Beiträge zur Kenntnis schlesischer Braunkohlenhölzer II. 68 pp. Inaugural Dissertation, W.G. Korn, Breslau.
Pujana, R.R., Ruiz, D.P., Martínez, L.C.A., and Zhang, Y. 2016. Proposals for quantifying two characteristics of tracheid pit arrangement in gymnosperm woods. Revista del Museo Argentino de Ciencias Naturales 18: 117–124. Crossref
Román-Jordán, E., Esteban, L.G., de Palacios, P., and Fernández, F.G. 2017. Comparative wood anatomy of the Cupressaceae and correspondence with phylogeny, with special reference to the monotypic taxa. Plant Systematics and Evolution 303: 203–219. Crossref
Ruiz, D.P., Brea, M., Raigemborn, M.S., and Matheos, S.D. 2017. Conifer woods from the Salamanca Formation (early Paleocene), Central Patagonia, Argentina: paleoenvironmental implications. Journal of South American Earth Sciences 76: 427–445. Crossref
Schmalhausen, J. 1890. Tertiäre Pflanzen der Insel Neusibirien. Mémoires
de l’Académie Impériale des Sciences de St. Pétersbourg, Series 7 37: 1–22.
Selling, O.H. 1944. On cupressoid root remains of Mesozoic age from the Arctic. Arkiv for Botanik 31A: 1–20.
Stockmans, F. and Willière, Y. 1934. Notes sur des bois fossiles récoltés en Belgique. Bulletin du Musée royal des sciences naturelles de Belgique 10: 1–8.
Stopes, M.C. 1915. Catalogue of the Mesozoic Plants in the British Museum (Natural History). Part II: Lower Greensand (Aptian) Plants of Britain. xxxvi + 360 pp. Trustees of British Museum, London.
Süss, H. and Rathner, U. 1998. Ein neues fossiles Holz, Juniperoxylon wagneri sp. nova, aus der miozänen Braunkohle von Wetro (Oberlausitz/Sachsen, Deutschland). Feddes Repertorium 109: 15–24. Crossref
Van der Burgh, J. 1973. Hölzer der niederrheinischen Braunkohlenformation. 2. Hölzer der Braunkohlengruben “Maria Theresia” zu Herzogenrath, “Zukunft West” zu Eschweiler und “Victor” (Zülpich-Mitte) zu Zülpich. Nebst einer systematisch-anatomischen Bearbeitung der Gattung Pinus L. Review of Palaeobotany and Palynology 15: 73–275. Crossref
Vaudois, N. and Privé, C. 1971. Révision des bois fossiles de Cupressaceae. Palaeontographica B 134: 61–86.
Wang, R.F., Wang, Y.-F., and Chen, Y.-Z. 1996. Cupressinoxylon jiayinense, a new species of the Late Cretaceous from Heilongjiang Province, China. IAWA Journal 17: 319–326. Crossref
Watari, S. and Nishida, M. 1973. A Juniperoxylon from the Tertiary of Hokkaido. Journal of Japanese Botany 48: 154–159.
Acta Palaeontol. Pol. 64 (3): 481–488, 2019
https://doi.org/10.4202/app.00597.2019