

A new glimpse on trophic interactions of 100-million-year old lacewing larvae
MARIE K. HÖRNIG, CHRISTINE KIESMÜLLER, PATRICK MÜLLER, CAROLIN HAUG, and JOACHIM T. HAUG
Hörnig, M.K., Kiesmüller, C., Müller, P., Haug, C., and Haug, J.T. 2020. A new glimpse on trophic interactions of 100-million-year old lacewing larvae. Acta Palaeontologica Polonica 65 (4): 777–786.
Larvae of lacewings (Neuroptera) are known to be fierce predators. According to the morphology of fossil forms this seems to have been the case already in the Early Cretaceous. While being predators, lacewing larvae are also food items for other organisms. Here we report two pieces of amber from Myanmar providing instances of such cases. In one amber piece several isolated stylets of lacewing larvae are preserved closely associated together. The most likely interpretation is that a predator preying on lacewing larvae has regurgitated or defecated these non-digestible pieces, yet the identity of the predator remains unclear. The other amber piece preserves a larva resembling modern day larvae of split-footed lacewings (Nymphidae). The larva has projections on its trunk, allowing it to wear a camouflaging cloak. In the head region, a mite (Acari) is attached to the larva; more precisely, the entire anterior body region of the mite is apparently inserted into the lacewing larva. The mite is smaller than the larva. It is known from the modern fauna that stage 1 larvae of Ascalaphidae can be attacked also by rather small predators, such as ants. The mite can therefore well be interpreted as a true predator instead of a parasite, especially considering the unusual mode of attachment. We briefly review interactions of lacewing larvae with other organisms represented in amber from Myanmar and add two new pieces to the puzzle of reconstructing the trophic interactions in the 100-million-year old amber forest.
Key words: Neuroptera, Nymphidae, food-web reconstruction, Burmese amber, larvae, syninclusions, Cretaceous, Myanmar.
Marie K. Hörnig [marie.hoernig@palaeo-evo-devo.info] and Christine Kiesmüller [c.kiesmueller@gmx.de], University of Greifswald, Zoological Institute and Museum, Cytology and Evolutionary Biology, Soldmannstr. 23, D-17489 Greifswald, Germany.
Patrick Müller [pat14789@web.de], Friedhofstr. 9, 66894 Käshofen, Germany.
Carolin Haug [carolin.haug@palaeo-evo-devo.info] and Joachim T. Haug [joachim.haug@palaeo-evo-devo.info], Ludwig-Maximilians-Universität München (LMU), Biocenter, Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany and GeoBio-Center, Ludwig-Maximilians-Universität München, Richard-Wagner-Straße 10, 80333 München, Germany.
Received 12 September 2019, accepted 27 July 2020, available online 3 December 2020.
Copyright © 2020 M.K. Hörnig et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Understanding the structure of food webs has become more important in recent years as it has direct impact for identifying keystone species within the trophic structure of a community (e.g., Bond 1994; de Ruiter et al. 2005; Knight et al. 2005; Jordan 2009; Valls et al. 2015). Identifying such keystone species is of prime importance in the protection of biodiversity and conservation biology.
There are many different types of trophic interactions in biological systems (e.g., Lafferty and Kuris 2002). A simplified list of such interactions among animals includes:
(i) Predator-prey interactions. Predation, is meant here in the narrow sense, i.e., an organism, the prey, is killed and at least partly consumed by another organism, the predator, during the interaction; the time of interaction is relatively short (excluding “micropredation”, see point iii)
(ii) Parasitoid-host interactions. Similar to predation, an organism, the host, is at least partly consumed and killed during the interaction by the other organism, the parasitoid; yet, unlike in the case of predation, the time of interaction is quite long.
(iii) Temporary parasite-host interactions. Unlike in the cases mentioned before, the host organism is not killed (on purpose), yet similar to predation the time of interaction between the temporary parasite and the host is rather short. Typical examples are mosquitoes and ticks. Note that many authors refer to this type of interaction as “micro-predation” because only a small part of the host is consumed (e.g., Lafferty and Kuris 2002). Yet, in many fields of biology the prefix “micro” refers to a specific fraction of organisms with a small body size. Hence, the term “micro-predator” could well refer to small-sized predators that prey on likewise small prey as raptorial waterfleas or raptorial mites. Therefore, the term “micro-predator” may be easily misinterpreted, while “temporary parasite” appears more specific and is used in the following here.
(iv) Permanent parasite-host interactions. In this type of interaction the host is not killed by the other organism, the parasite, and the time of interaction is long.
Apart from these very direct types of interaction in which one individual interacts with another one, there are also more indirect types of interactions. For example, some animals depend on remains of other animals, such as e.g., dung beetles (Hanski and Cambefort 2014) or larvae of some representatives of flies (e.g., Sepsidae; Hafez 1948; or some species of Muscidae, Kutty et al. 2014). In this way, the producer of the faeces will not have direct contact to the other individual, yet the consumer has a direct interaction with the faeces. Hence, this interaction is asymmetric. Also partly indirect is, for example, a scavenger-carcass interaction.
In modern-day communities, the direct identification of interactions, as the ones discussed above, can be more or less challenging depending on many factors, such as size. In other words, for example in the case of predator-prey interaction, it is much more straightforward to observe a lion preying on a zebra, compared to observing a tiny beetle preying on a mite. Yet, luckily quite a large variety of methods can be used in modern-day communities to reconstruct the food web within a community (e.g., Mouchet et al. 2010; Morales-Castilla et al. 2015; Casey et al. 2019).
More challenging is the reconstruction of trophic interactions within fossil communities. Still, also here we can use different approaches for identifying trophic interactions based on fossils:
Actuopalaeontology.—If we observe a certain trophic interaction within a modern community, or even better in several modern communities, and we find the same two components in a fossil community, we can suggest a similar trophic interaction also within the fossil community at least with a certain (though not absolute) confidence. This approach appears to have been used in two different frames:
(i) a phylogenetic frame, i.e., if all representatives of one monophyletic group interact with representatives of another monophyletic group, and if we have representatives of both these groups in the fossil community, the presence of a similar interaction can be inferred (see also concept of extant phylogenetic bracketing; Witmer 1995);
(ii) a functional morphological frame, i.e., organisms with a specific morphological structure are interpreted as representatives of a specific functional group (“guild”) and are hence interpreted as interacting with certain other organisms according to the assumed guild. The second frame is definitely the weaker argumentation scheme, yet especially in older communities, for example, early Palaeozoic ones, this is often the only applicable frame (e.g., Zhu et al. 2004; Vannier 2007).
Direct interaction of two organisms.—This is a type of so-called “frozen behaviour”, i.e., two (or more) fossil organisms are preserved while interacting (Arillo 2007; Boucot and Poinar 2010). As an example, a predator may be preserved while directly interacting with its prey. The famous fighting dinosaurs may be the most generally known case of such a type of fossilisation (Kielan-Jaworowska and Barsbold 1972). Yet, as discussed above, predators and temporary parasites interact only for a short amount of time with the prey, hence such occurrences are rare. Permanent parasites that attach to their host for longer, or scavengers sitting on a carcass for quite some time seem much more likely to be found as fossils (e.g., Weitschat and Wichard 1998; Arillo 2007; Boucot and Poinar 2010: 27–71; Gröhn 2015).
Remains of the prey or host.—If the prey has been consumed by the predator, or parts of the host by the parasite or parasitoid, it will leave vestiges behind. We can therefore have various types of remains of a prey or host organism:
(i) Damaged hard shelled organisms, as for examples molluscan shells that have been attacked by a predator or parasite (e.g., Boucot and Poinar 2010: 79; Bicknell and Paterson 2018; Vinn 2018), yet, such traces can be difficult to interpret and differentiate from other factors influencing preservation.
(ii) Stomach and gut content. While it is rarely possible to find the stomach or gut content sufficiently well preserved to indeed identify these remains, there are few exceptional cases (also termed “gastrolites”, fossilized stomach contents, “enterospira”, contents preserved in the valvular intestine, and “cololites”, non-valvular intestinal contents; Hunt 1992; Northwood 2005; Hunt and Lucas 2012). This is an even better, more reliable type of data than observing the direct interaction between two organisms. In the latter case we still have to assume that the supposed predator would later consume the proposed prey, and a supposed parasite might just use the supposed host for phoresis. In the case of stomach or gut content preservation we can directly observe that the prey or parts of the host have been consumed (e.g., Wilby and Martill 1992; Kriwet et al. 2007; Vannier 2012).
(iii) Coprolites. Fossil faeces may also be preserved well enough to allow the identification of its components. This is in principle comparable to gut content preservation, yet usually we do not know the producer, as coprolites are only very rarely preserved together with their producer. Still, also finds in which the producer remains unknown can be very informative, for example, showing that one type of predator has consumed different other components of one community (e.g., Richter and Baszio 2001; Northwood 2005; Vannier and Chen 2005; Qvarnström et al. 2016). (iv) Very similar to coprolites, but often containing better preserved pieces, is regurgitated material or “Speiballen” (also termed “regurgitalites”, Hunt 1992; Northwood 2005; Hunt and Lucas 2012). Conclusions based on these are comparable to those on coprolites, yet they often contain the harder parts that are easier identifiable (e.g., Hattin 1996; Witton 2018; Salamon et al. 2020).
Fossils preserved in amber have a very high potential to provide such different types of preservation. Within the nearly 100 million years old amber forest represented by Burmese amber, we have quite a number of fierce predators preserved (e.g., Grimaldi and Ross 2004; Poinar and Buckley 2012; Vršanský and Bechly 2015; Delclòs et al. 2016; Bai et al. 2016, 2018; Yin et al. 2018). Among them are the predatory larvae of lacewings (Wang et al. 2016; Liu et al. 2016, 2018; Badano et al. 2018; Haug et al. 2019a, b). We present here new fossils from Burmese amber that provide the “other side” of interactions involving lacewing larvae, in which the latter do not play the role of the predator.
Institutional abbreviations.—BUB, Burmese amber collection of Patrick Müller, Käshofen, Germany; SNSB-BSPG, Staatliche Naturwissenschaftliche Sammlungen Bayerns- Bayerische Staatssammlung für Paläontologie und Geologie, Munich, Germany.
Material and methods
Two specimens presented here come from the Hukawng Valley, Kachin State, Myanmar. Specimens are deposited in the Staatliche Naturwissenschaftliche Sammlungen Bayerns-Bayerische Staatssammlung für Paläontologie und Geologie in Munich (Germany) under the repository numbers SNSB-BSPG 2020 XCIII 18 and SNSB-BSPG 2020 XCIII 19.
Each raw amber piece was first cut with a Dremel 3000. Afterwards it was polished with wet sandpaper, first grade 200, and then subsequently with grade 600, 1000, and 5000. The final polishing was performed with Sidol metal polish.
Burmese amber (locality in the southwest corner, Noije Bum hill) of the Hukawng Valley in Kachin State, Myanmar has first been assigned to the Eocene (Chhibber 1934; Grimaldi et al. 2002; Cruickshank and Ko 2003; Shi et al. 2012) to Miocene (Noetling 1893; Cruickshank and Ko 2003) due to its embedding in probably Eocene clay, calcite and pyrite rock matrix (compare also Zherikhin and Ross 2000). However, this assumption was questioned already by Cockerell (1917).
At present, Burmese amber is unanimously considered to be of mid-Cretaceous based on insect groups present (specifically Turonian–Cenomanian; 90–100 mya; Grimaldi et al. 2002), ammonite (Yu et al. 2019) and crinoid inclusions (Salamon et al. 2019) or uranium-lead radiometric zircon dating (approx. 98 mya; Shi et al. 2012), but also due to the potential Cretaceous age of sediments surrounding Burmese amber (Cruickshank and Ko 2003).
The Hukawng Valley locality is also the only major Lagerstätte of Cretaceous amber in Southeastern Asia; its palaeolatitude is also the most southerly (with Lebanese amber) of all major Cretaceous amber deposits (Grimaldi et al. 2002). The palaeoenvironment of Burmese amber has been postulated to be subtropical to tropical (Grimaldi et al. 2002), potentially nearshore, marine or lagoon (Cruickshank and Ko 2003; Yu et al. 2019; Salamon et al. 2019) and potential part of the past “supercontinent” Gondwana (Poinar 2018).
Another amber locality is known from the Northern Myanmar, near the Nam Sakhaw stream, 90 km southwest of the Noije Bum hill which has also been proposed to be Cretaceous (Cruickshank and Ko 2003 and references therein).
Each specimen was documented with composite imaging under different white light conditions, as well as under autofluorescence. The white-light microscopic images were recorded with a Keyence VHX-6000 equipped with a 20–2000× objective, either under ring illumination or under coaxial cross-polarised illumination. Black and white background colour was used. To achieve an optimal result, every image was recorded with different exposure times (HDR).
Each image detail was documented as a stack, with the single images of the stack (frames) being recorded in different focal levels in z-axis to overcome limitations in depth of field. The frames of each stack were fused to achieve an entirely sharp image detail. Several adjacent stacks were recorded in x-y-axis to overcome limitations in field of view. All image details were stitched to a final panorama image (e.g., Haug et al. 2008; Kerp and Bomfleur 2011).
Description follows the basic approach of Haug et al. (2012; see also Hörnig et al. 2018 for further details), but is presented as plain text. As Insecta is a well accepted in-group of Crustacea sensu lato, neutral crustacean terminology is provided alongside special terminology in squared brackets.
Results
Description of SNSB-BSPG 2020 XCIII 19.—The amber piece (Fig. 1) contains at least five (presumably up to eight) isolated insect mouth parts, which appear to represent neuropteran stylets formed by mandibles and maxillae (appendages of post-ocular segments 3 and 4; Fig. 1A2, A3, see comparison to specimen shown in Fig. 1B). Two of them are preserved in large parts, further three clearly identifiable, but largely concealed by other enclosed material in the amber. Presumable remains of three further stylets are indicated, but cannot be clearly identified (Fig. 1A2, A3).
The preserved stylets seem different in original size, even if the exact original length is not determinable in most cases due to the incomplete preservation. The two best preserved stylets are about 1 mm (including curved part) and 1.25 mm long, with a width of about one tenth of the length at the widest point, and with seven (Fig. 1A3: 2) or six (Fig. 1A3: 1) teeth preserved. All preserved remains of stylets are strongly sclerotised; curved and tapering distally.
Beside the stylet remains, a representative of Hymenoptera (not further determined) is preserved within the amber piece (Fig. 1A1, A4).
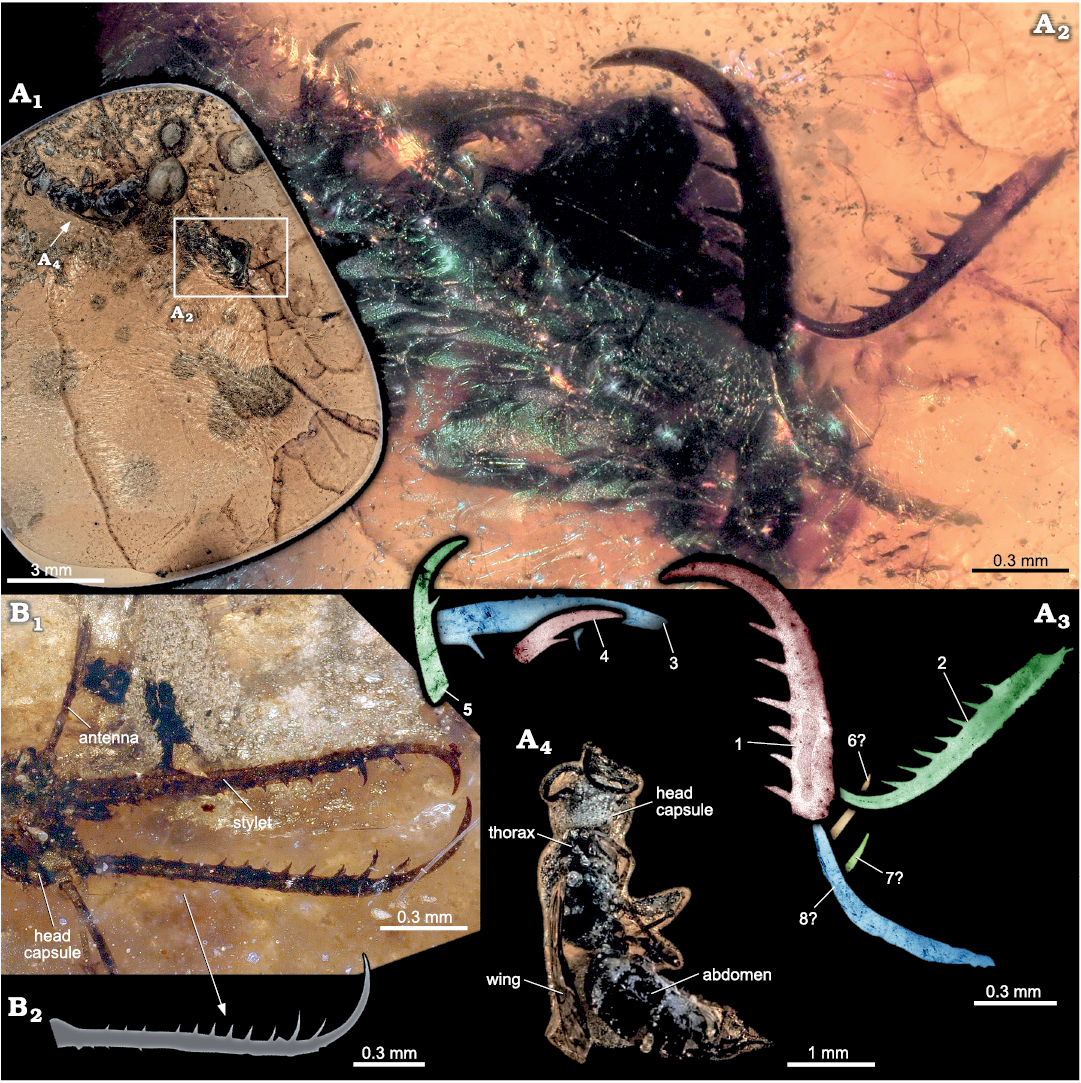
Fig. 1. Neuropteran larvae from Hukawng Valley, Kachin State, Myanmar; Turonian–Cenomanian, Cretaceous, 90–100 mya. A. SNSB-BSPG 2020 XCIII 19 with assemblage of neuropteran stylets; A1, overview; A2, close-up image of enclosed stylets; A3, stylets, with five clearly visible (1–5) and possibly three additional stylets (6?–8?); A4, close-up image of representative of Hymenoptera. B. SNSB-BSPG 2020 XCIII 26 (previously BUB 033 in Haug et al. 2019c); B1, head of neuropteran larva with stylets pair in situ; B2, drawing of a single stylet of the specimen in B1; note the high number of teeth, similar to that of stylets 1 and 2 in A3.
Description of SNSB-BSPG 2020 XCIII 18.—The amber piece contains two inclusions: a neuropteran larva, and a mite attached to it (Figs. 2, 3A1, A2). The visible part of the mite, mostly the posterior trunk (idiosoma), has a length of about 0.3 mm. Anterior body and associated structures are not accessible. The entire anterior region (gnathosoma) appears inserted in the antero-lateral region of the head of the neuropteran larva (Fig. 3A4, A5). Several legs are apparent, at least one appears to have been ripped off from the body of the mite. The larva is surrounded by several pieces of putative plant remains.
The neuropteran larva has a body length of about 1.6 mm (without stylets). Body organised distinctly into head and trunk with (presumably) 20 segments. Ocular segment and post-ocular segments 1–5 form the head capsule.
The head capsule is about 0.5 mm long and 0.75 mm wide, trapezoidal in dorsal/ventral view, reaching maximum width far posteriorly. Surface (at least laterally) equipped with several homogeneously arranged setae, posterior ones longer than anterior ones (Fig. 3A2).
Post-ocular segment 1 recognisable by its appendages, antennae [antennula]. Antennae arising antero-laterally from head capsule. Subdivision into elements not recognisable (possible due to preservation).
No structures of post-ocular segment 2 (intercalary segment) externally visible.
Post-ocular segments 3 and 4 recognisable by their appendages, mandibles and maxillae [maxillulae]. Mandibles and maxillae forming a pair of prominent stylets. Stylets preserved in widely spread position, right stylet orientated forward (prognath), left stylet orientated widely backward (Fig. 3A2). Insertions of stylets well separated. Stylets very long, more than one third of entire body length; strongly inward curved (from approximately the apical one third of it), tapering distally. Mandibular part of stylets with one tooth each, tooth longer than stylet width (Fig. 3A3).
Post-ocular segment 5 recognisable by its appendages, conjoined forming labium [maxillae]. Only distal parts recognisable, palps [endopod] (Fig. 3A2). Labial palps arising medially to stylets; with several tubular elements (palpomeres; exact number not determinable). Palps slightly thicker than antennae, similar in length.
Region posterior to head, neck region (cervix), sclerotised; tubular (cylindrical) in shape, slightly wider than long.
The entire trunk about 0.9 mm long, maximum width about 0.75 mm (at about half the length of the trunk), overall round in shape. Surface overall equipped with long setae and protrusions of cuticle (Fig. 3A2). Borders of segments of thorax (post-ocular segments 6–8; pro-, meso-, and metathorax) and abdomen (post-ocular segments 9–20) not recognisable; region of thorax only indicated by the presence of appendages (legs) of post-ocular segments 6–8. Thorax represents about two thirds of length of trunk.
Each thorax segment equipped with a pair of walking appendages (legs) ventrally and (presumably) one pair of tubercles dorsally (Fig. 3A1, A2). Tubercles elongate (about 0.4 mm) and equipped with setae. Walking appendages similar in length, each composed of five main elements. Most proximal element, coxa [basipod?], relatively short, slightly conical, slightly longer than wide at proximal rim (exact length is not determinable). Element 2, trochanter [endopod element 1?], similar in dimensions to visible part of coxa. Element 3, femur [endopod element 2?], more elongate, tube-shaped and equipped with setae. About the same diameter as coxa and trochanter, but longer, more than 3 times. Element 4, tibia [endopod element 3?], similar to femur in size and shape. Element 5, tarsus [endopod element 4?], slightly thinner and shorter than femur and tibia, longer than wide, about 4 times. Distally with a pair of curved claws (Fig. 3A2).
Individual segments of abdomen (post-ocular segments 9–20) not recognisable. Surface is equipped with numerous protrusions. Terminal end drawn out into lobe-like extension with numerous setae (Fig. 3A2).
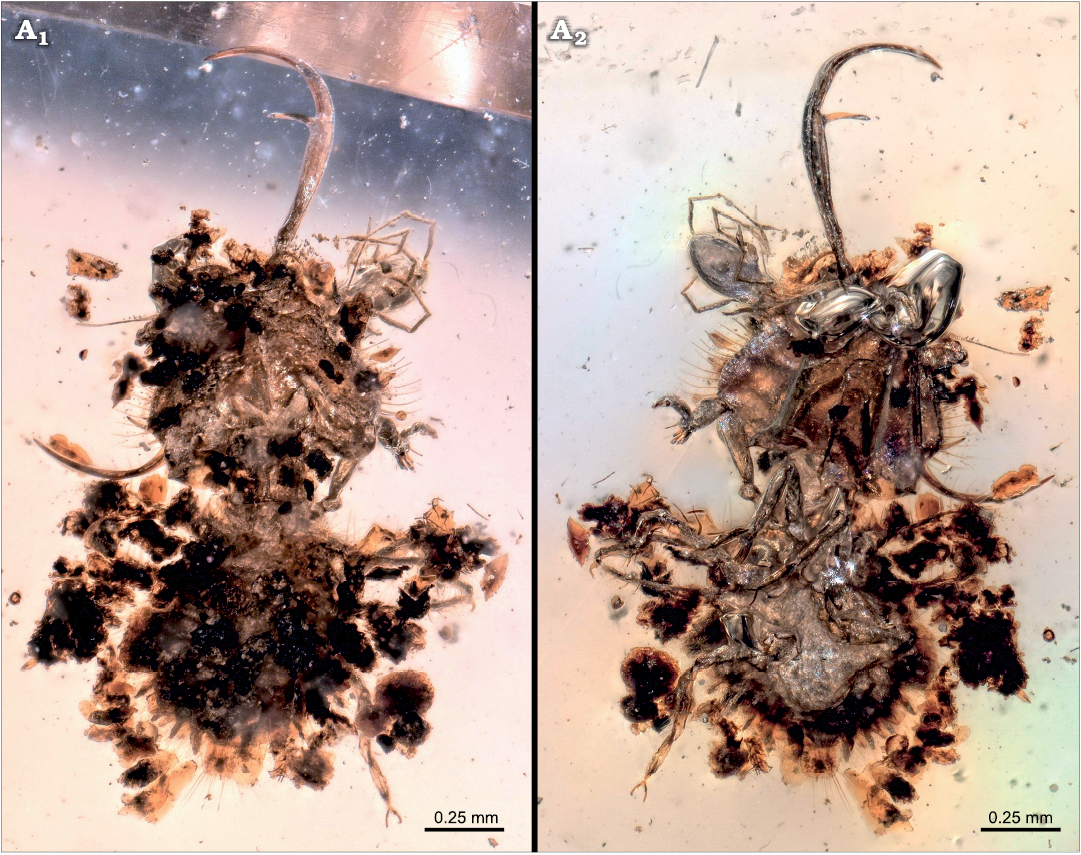
Fig. 2. SNSB-BSPG 2020 XCIII 18 containing a neuropteran larva with attached mite from Hukawng Valley, Kachin State, Myanmar; Turonian–Cenomanian, Cretaceous, 90–100 mya; in dorsal (A1) and ventral (A2) views.
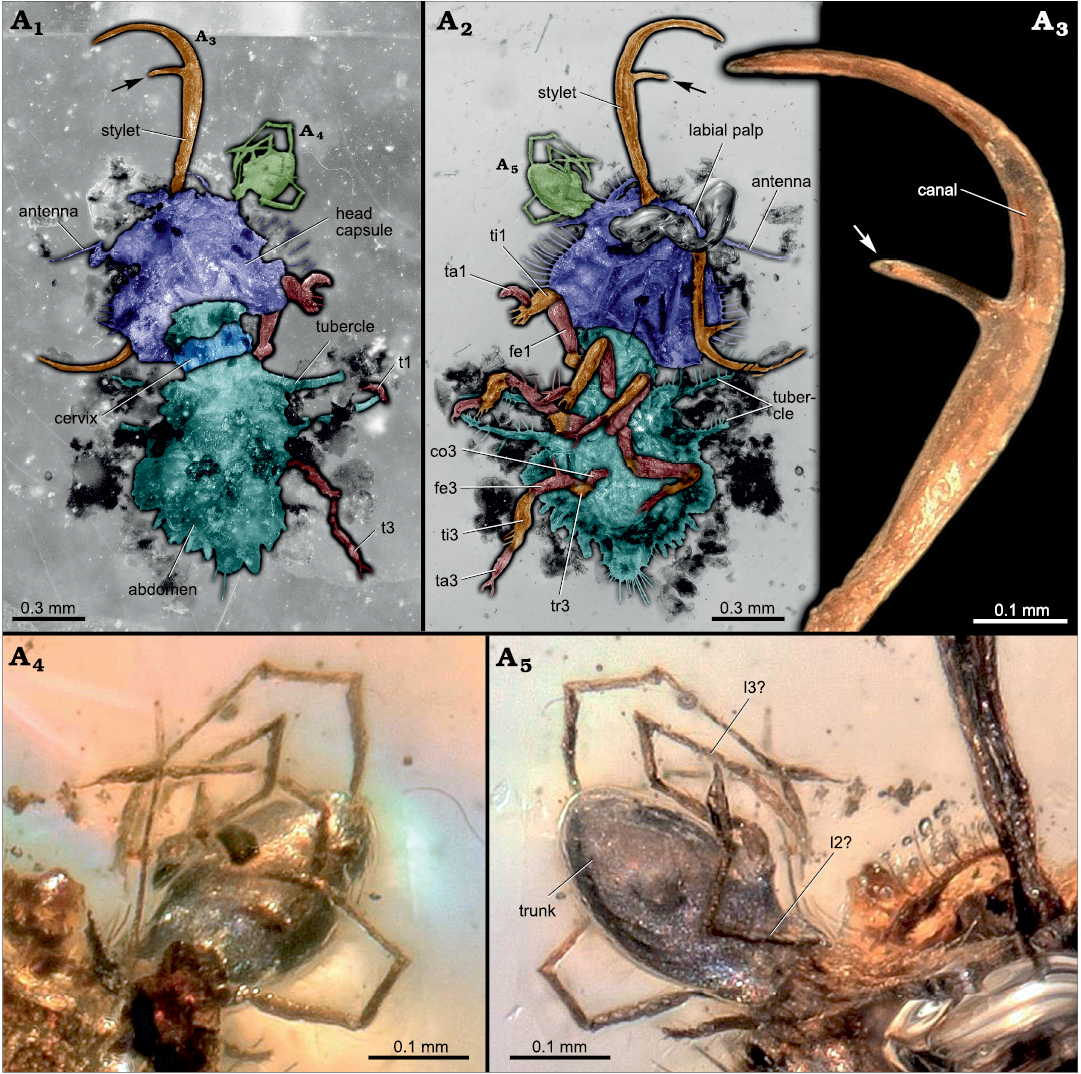
Fig. 3. SNSB-BSPG 2020 XCIII 18 containing a
neuropteran larva with attached mite from Hukawng Valley, Kachin
State, Myanmar; Turonian–Cenomanian, Cretaceous, 90–100 mya. Labeled
version of Fig. 2, dorsal (A1)
and ventral (A2)
views (colour-marked version online, mite marked in light green);
arrows point to single tooth in stylet. Close-up of one of the
stylets (A3);
arrow points to single tooth. Close-up on attached mite, dorsal (A4) and ventral (A5) views. Abbreviations:
co3, coxa of thorax appendage 3; fe1, 3, femur of thorax appendage
1, 3; l2?, 3?, presumed (walking) leg 2?, 3?; t1, 3, thorax
appendage 1, 3; ta1, 3, tarsus of thorax appendage 1, 3; ti1, 3,
tibia of thorax appendage 1, 3; tr3, trochanter of thorax appendage
3.
Discussion
Interpretation of SNSB-BSPG 2020 XCIII 19.—The amber piece includes at least five objects that strongly resemble parts of the stylets (possibly mostly the mandibles) of myrmeleontiformian lacewing larvae as well as three further, possible parts of stylets. More precisely, the two larger and best preserved objects strongly resemble the mouth parts of another larva (Fig. 1B; nicknamed “superfang larva” by Haug et al. 2019c) also known from Burmese amber, being rather long and bearing numerous teeth. It seems therefore likely that these originally belonged to a larva more or less similar to the “superfang larva” of Haug et al. (2019c).
Other objects are less well preserved. Objects 3–5 also show the presence of some teeth, yet it remains unclear whether there were more than two such teeth (Fig. 1A3: 3–5). Many myrmeleontiformian lacewing larvae preserved in Burmese amber have only two such teeth. These larvae are often interpreted as closely related to owlflies and antlions (e.g., Wang et al. 2016; Badano et al. 2018). These forms may therefore represent remains of a species related to extant Ascalaphidae and/or Myrmeleontidae.
Other objects appear even less completely preserved. Two shorter objects (Fig. 1A3: 6, 7) may represent pieces of smaller mandibles, yet they could also represent remains of the maxillary part of a stylet of a neuropteran larva.
Hence, there are at least five objects that appear to represent remains of mandibles, some of the other objects may represent maxillary remains. This would mean that the remains of at least three lacewing larvae are enclosed together. Neuropteran larvae are solitary predators that act aggressively against other such larvae (e.g., Duelli 1981; Devetak 2000 and references therein). It is therefore unlikely that we see here remains of at least three such larvae in one piece of amber, by having three larvae accidentally dying close to each other. Furthermore, as the remains differ in size and morphology, it is not plausible that these fragmentary preserved mouthparts came from individuals of the same stage (e.g., of not aggressive hatchlings) or even the same species. It seems likely that this conglomerate is a “Speiballen”, i.e., the regurgitated remains of prey previously consumed by a predator or faeces content of an insectivorous animal (but the latter seems to be less likely, as no further remains of excrement are attached at least at some of the preserved stylets). Given the fact that there are several of these mouth parts, it seems that a predator actively searched and consumed predatory lacewing larvae. Beside the stylet remains, also a hymenopteran representative is preserved in the amber piece. There is no indication that there is any causal link to the isolated stylets.
Literature on predators of modern lacewing larvae is scarce. Certain species of owlflies are known to perform group defence behaviours against larger predators (Henry 1972; Aspöck and Aspöck 2007). This behaviour is only shown in the time after hatching and before the larvae become aggressively solitary. Among the possible predators, Henry (1972) mentioned ants, wasps and caterpillars. None of these are likely candidates to produce a kind of Speiballen or excrements in this size. Ants, for example, could leave remains of a prey piece. Yet, these would either be expected to represent remains of a single animal if the animal would have been cut into pieces by ant workers, or include many more and also rather different pieces if the conglomerate would represent a kind of trash pile of a nest.
Hence many aspects of this find currently remain unclear. We should assume the presence of a predator, actively preying on lacewing larvae in the Burmese amber forest about 100 million years ago. Yet, given the limited knowledge on such predators in the modern day fauna, we cannot even provide an educated guess for the identity of the predator.
Interpretation of SNSB-BSPG 2020 XCIII 18.—The second case is an example of “frozen behaviour”. The mite appears inserted with its anterior body into the myrmeleontiformian lacewing larva, the latter possibly being a split-footed lacewing (Nymphidae) due to its general appearance, or at least closely related to these. An insertion of the gnathosoma of the mite, and with no further parts attached to the larva, indicates that the mite preyed on the neuropteran larvae and contradicts an interpretation as a case of phoresy, were the interaction would have been restricted to transportation of the mite. Furthermore, as stationary ambush predator (e.g., Eltz 1997; Guillette et al. 2009; Tauber et al. 2009; Klokočovnik and Devetak 2014), it would be not reasonable to choose a neuropteran larva for travelling even short distances.
Given the fact that the mite is smaller than the lacewing larva one might suggest that the mite is in fact not a predator in the strict sense, but a “micro-predator” or better a temporary parasite. Typical examples for this ecological role are mosquitoes and also ticks, hence an in-group of Acari, the group of mites. Could the mite preserved here represent a temporary parasite? The mite is clearly smaller than the lacewing larva, but about half as long as the head of larva. Yet, temporary parasites are significantly smaller than their hosts. The size of the mite is difficult in this aspect while being pretty large if interpreted as a temporary parasite it would be rather small for a true predator (in the strict sense). As stated above, we do not know much about modern day predators or parasites of myrmeleontiformian lacewing larvae. We therefore know of no example of a mite preying on lacewing larvae. The astonishing question is, as the mite is attached at the head right next to the massive stylets, why would the lacewing larva not turn the table and prey on the mite as it comes in reach?
Possible explanations could be, that the mite is simply too small for grasping and piercing it with the large massive stylets or could have come up behind the larva. One could also speculate that the lacewing larva was already partly trapped in the fresh, highly liquid resin and the mite would have preyed on the immobilised lacewing larva before it became also trapped in the liquid resin. Yet, then we would expect to see discrete layering in the amber, resulting from successive resin flows (Martínez-Delclòs et al. 2004), which is not the case. Hence the two organisms seem to have interacted before any of them became trapped. We cannot exclude that the mite preyed on an accidentally deceased lacewing larva and thus the mite could be interpreted as a scavenger. Based on the observable details, we presume that the mite could be best understood as being somewhere in the predator-parasite limbo. Henry (1972) mentioned that stage 1 larvae of owlflies (Ascalaphidae) cannot defend themselves even against smaller ants. Therefore, it seems that there are cases that smaller representatives of Euarthropoda may prey on the likewise predatory larvae of lacewings. While this is not quite the same as a mite, it is in fact the only recent mentioning of such an interaction. Modern biology seems sometimes to forget that observations in the field also need to get published to increase the availability of such type of information. Hence we are left with the information about interactions of ants. Based on size comparison, this makes it at least plausible to understand the mite as a true predator.
Camouflaging of the lacewing larva.—The enclosed lacewing larva features several long tubercles on the dorsal side of the thorax. These structures are also known from several extant larvae of neuropteran species and were found recently also in fossil representatives in Burmese amber (Wang et al. 2016; Badano et al. 2018). The tubercles can bear material such as plant or animal remains, small stones or similar (e.g., Henry 1976). This results in a camouflaging cloak, by which the neuropteran larva is scarcely recognisable for other predators (passive camouflage), but also for their potential prey (aggressive camouflage). The specimen indeed carries small pieces of debris on its back. These pieces are also apparent around the specimen. This may provide additional shelter, especially if the body surface is covered by e.g., small pieces of stone. The preying mite might therefore have been forced to attack the head as there is no protective camouflaging cloak in this body region.
Aspects of the food web in the Burmese amber forest entailing lacewing larvae.—The two amber pieces provide two aspects of data about trophic interactions in the community of the ca. 100 million-year-old fauna represented by the Burmese amber fossils. So far, we knew quite a variety of lacewing larvae in this community including forms that performed various different feeding strategies also well-known from modern day equivalent forms. These include:
(i) A larva most likely parasitising an immature spider female, and potentially later feeding on the eggs of this female, similar to modern day larvae of mantis lacewings (Mantispidae; Haug et al. 2018).
(ii) Predatory behaviour, more precisely ambush predators that used particles of different sources for camouflaging as in modern forms of, for example, green lacewings (Chrysopidae), owlflies (Ascalaphidae), and split-footed lacewings (Nymphidae) and now extinct groups (e.g., Wang et al. 2016; Badano et al. 2018).
(iii) Digging (“fossorial”) predators comparable to modern derived antlion larvae (Myrmeleontidae; Badano et al. 2018).
(iv) Highly specialised predators without camouflaging, but possessing a long neck as in many modern larvae of thread-winged lacewings (Crocinae) (Haug et al. 2019a).
(v) Possible semi-aquatic predators with straight mandibles as in modern larvae of lance lacewings (Osmylidae) (Haug et al. 2019b).
(vi) Possible fully aquatic predators as larvae of dragon lacewings (Nevrorthidae) (Wichard 2017).
(vii) Not fully understood strategies not represented in the modern fauna, involving mimesis (Liu et al. 2018), possible preying on web spiders within their web (Liu et al. 2016), or very large mouth parts (Haug et al. 2019c).
The strategies of these predators have been largely based on comparative functional aspects in comparison to modern forms. So far we also cannot clearly identify possible prey of most of these neuropteran predators. Based on the new fossils reported here we can add some data for the other side of the food web, i.e., presence of animals that fed on some of these predators.
Conclusions
Trophic interactions within fossil communities are only very fragmentarily known so far. This is also due to the circumstance that reconstructions of food webs based on fossils are quite challenging and different approaches have to be carefully considered in the interpretation of the fossils (see above). Lacewing larvae (Neuroptera) today are known to be fierce predators, which we can assume also for lacewing larvae living in the Cretaceous based on the morphology of fossil representatives. Some rare fossil findings indicate that these performed various different feeding strategies also well-known from modern day equivalent forms. However, lacewing larvae are not only predators but they are also prey items for other organisms. About lacewing larvae as prey items is less known in the literature, not only regarding fossils, but also in today living communities. The here described two findings of lacewing larvae in Burmese amber represent examples with indications of lacewing larvae as prey. The first case, with several isolated parts of stylets, likely represents regurgitated remains of prey previously consumed by an unknown predator (“Speiballen” or “regurgitalites”; see discussion for further interpretations). The second case is a single lacewing larva (Nymphidae or closely related) together with a mite. The mite appears inserted with its anterior body into the lacewing larva. We discuss whether this could reflect a case of parasitism or predation. Furthermore, the lacewing larva features several long tubercles on the dorsal side of the thorax, which are known in extant and extinct forms for bearing material such as plant remains or small stones. The resulting camouflaging cloak can hamper the recognition by potential prey (aggressive camouflage), but also by other predators, and may be used also as kind of physical armour.
Although, many aspects concerning these two examples remain unclear, they represent rare fossil cases with indications of trophic interactions. For reconstructions, fossils like these can add further pieces in the fragmentary knowledge of food webs in the Cretaceous amber forest.
Acknowledgements
We highly appreciate the effort of all people involved in providing open access, open source and low cost software. We like to acknowledge also Vladimir Makarkin (Federal Scientific Center of the East Asia Terrestrial Biodiversity, Vladivostok, Russia) and one anonymous reviewer for their helpful comments. MKH, CK, JTH and CH thank J. M. Starck (Ludwig-Maximilians-University Munich, Germany), and S. Harzsch (University of Greifswald, Germany), for their continuous support. The Volkswagen Foundation kindly funds JTH with a Lichtenberg Professorship. CK is kindly funded by the Landesgraduiertenförderung Mecklenburg-Vorpommern. This is LEON publication #12.
References
Arillo, A. 2007. Paleoethology: fossilized behaviours in amber. Geologica Acta: An International Earth Science Journal 5: 159–166.
Aspöck, U. and Aspöck, H. 2007. Verbliebene Vielfalt vergangener Blüte. Zur Evolution, Phylogenie und Biodiversität der Neuropterida (Insecta: Endopterygota). Denisia 20, Kataloge der Oberöstereichischen Landesmuseen Neue Serie 66: 451–516.
Badano, D., Engel, M.S., Basso, A., Wang, B., and Cerretti, P. 2018. Diverse Cretaceous larvae reveal the evolutionary and behavioural history of antlions and lacewings. Nature Communications 9: 3257. Crossref
Bai, M., Beutel, R.G., Klass, K.D., Zhang, W., Yang, X., and Wipfler, B. 2016. † Alienoptera—a new insect order in the roach-mantodean twilight zone. Gondwana Research 39: 317–326. Crossref
Bai, M., Beutel, R.G., Zhang, W., Wang, S., Hörnig, M.K., Gröhn, C., Yan, E., Yang, X., and Wipfler, B. 2018. A new Cretaceous insect with a unique cephalo-thoracic scissor device. Current Biology 28: 438–443. Crossref
Bicknell, R.D. and Paterson, J.R. 2018. Reappraising the early evidence of durophagy and drilling predation in the fossil record: implications for escalation and the Cambrian Explosion. Biological Reviews 93 (2), 754–784. Crossref
Bond, W.J. 1994. Keystone species. In: E.-D. Schulze and H.A. Mooney (eds.), Biodiversity and Ecosystem Function, 237–253. Springer, Berlin. Crossref
Boucot, A.J. and Poinar, G.O., Jr. 2010. Fossil Behavior Compendium. 424 pp. CRC Press, Boca Raton. Crossref
Casey, J.M., Meyer, C.P., Morat, F., Brandl, S.J., Planes, S., and Parravicini, V. 2019. Reconstructing hyperdiverse food webs: Gut content metabarcoding as a tool to disentangle trophic interactions on coral reefs. Methods in Ecology and Evolution 10: 1157–1170. Crossref
Chhibber, H.L. 1934. The Mineral Resources of Burma. 309 pp. MacMillan and Co., London.
Cockerell, T.D.A. 1917. Arthropods in Burmese amber. American Journal of Science 44: 360–368. Crossref
Cruickshank, R.D. and Ko, K. 2003. Geology of an amber locality in the Hukawng Valley, northern Myanmar. Journal of Asian Earth Sciences 21: 441–455. Crossref
Delclòs, X., Peñalver, E., Arillo, A., Engel, M.S., Nel, A., Azar, D., and Ross, A. 2016. New mantises (Insecta: Mantodea) in Cretaceous ambers from Lebanon, Spain, and Myanmar. Cretaceous Research 60: 91–108. Crossref
De Ruiter, P.C., Wolters, V., Moore, J.C., and Winemiller, K.O. 2005. Food web ecology: playing Jenga and beyond. Science 309: 68–71. Crossref
Devetak, D. 2000. Competition in larvae of two European ant-lion species (Neuroptera: Myrmeleontidae). Journal of Neuropterology 3: 51–60.
Duelli, P. 1981. Is larval cannibalism in lacewings adaptive? (Neuroptera: Chrysopidae). Population Ecology 23: 193–209. Crossref
Eltz, T. 1997. Foraging in the ant-lion Myrmeleon mobilis Hagen, 1888 (Neuroptera: Myrmeleontidae): Behavioral flexibility of a sit-and-wait predator. Journal of Insect Behavior 10: 1–11. Crossref
Grimaldi, D.A. and Ross, A.J. 2004. Raphidiomimula, an enigmatic new cockroach in Cretaceous amber from Myanmar (Burma) (Insecta: Blattodea: Raphidiomimidae). Journal of Systematic Palaeontology 2: 101–104. Crossref
Grimaldi, D.A., Engel, M.S., and Nascimbene, P.C. 2002. Fossiliferous Cretaceous amber from Myanmar (Burma): its rediscovery, biotic diversity, and paleontological significance. American Museum Novitates 2002: 1–71. Crossref
Gröhn, C. 2015. Einschlüsse im Baltischen Bernstein. 424 pp. Wachholtz Verlag-Murmann Publishers, Hamburg.
Guillette, L.M., Hollis, K.L., and Markarian, A. 2009. Learning in a sedentary insect predator: antlions (Neuroptera: Myrmeleontidae) anticipate a long wait. Behavioural Processes 80: 224–232. Crossref
Hafez, M. 1948. Ecological and biological observations on some coprophagous Sepsidae (Diptera). Proceedings of the Royal Entomological Society of London 23: 99–104. Crossref
Hanski, I. and Cambefort, Y. 2014. Dung Beetle Ecology. 481 pp. Princeton University Press, Princeton, New Jersey. Crossref
Hattin, D.E. 1996. Fossilized regurgitate from Smoky Hill Member of Niobrara Chalk (Upper Cretaceous) of Kansas, USA. Cretaceous Research 17: 443–450. Crossref
Haug, C., Herrera-Flórez, A.F., Müller, P., and Haug, J.T. 2019a. Cretaceous chimera—an unusual 100-million-year old neuropteran larva from the “experimental phase” of insect evolution. Palaeodiversity 12: 1–11. Crossref
Haug, J.T., Briggs, D.E.G., and Haug, C. 2012. Morphology and function in the Cambrian Burgess Shale megacheiran arthropod Leanchoilia superlata and the application of a descriptive matrix. BMC Evolutionary Biology 12: 162. Crossref
Haug, J.T., Haug, C., and Ehrlich, M. 2008. First fossil stomatopod larva (Arthropoda: Crustacea) and a new way of documenting Solnhofen fossils (Upper Jurassic, Southern Germany). Palaeodiversity 1: 103–109.
Haug, J.T., Müller, P., and Haug, C. 2018. The ride of the parasite: a 100-million-year old mantis lacewing larva captured while mounting its spider host. Zoological Letters 4: 31. Crossref
Haug, J.T., Müller, P., and Haug, C. 2019b. A 100-million-year old predator: a fossil neuropteran larva with unusually elongated mouthparts. Zoological Letters 5: 29. Crossref
Haug, J.T., Müller, P., and Haug, C. 2019c. A 100-million-year old slim insectan predator with massive venom-injecting stylets—a new type of neuropteran larva from Burmese amber. Bulletin of Geosciences 94: 431–440. Crossref
Henry, C.S. 1972. Eggs and rapagula of Ululodes and Ascaloptynx (Neuroptera: Ascalaphidae): a comparative study. Psyche: A Journal of Entomology 79: 1–22. Crossref
Henry, C.S. 1976. Some aspects of the external morphology of larval owlflies (Neuroptera: Ascalaphidae), with particular reference to Ululodes and Ascaloptynx. Psyche: A Journal of Entomology 83: 1–31. Crossref
Hörnig, M.K., Haug, C., Schneider, J.W., and Haug, J.T. 2018. Evolution of reproductive strategies in dictyopteran insects—clues from ovipositor morphology of extinct roachoids. Acta Palaeontologica Polonica 63 (1): 1–24. Crossref
Hunt, A.P. 1992. Late Pennsylvanian coprolites from the Kinney Brick Quarry, central New Mexico, with notes on the classification and utility of coprolites. New Mexico Bureau of Mines and Mineral Resources, Bulletin 138: 221–229.
Hunt, A.P. and Lucas, S.G. 2012. Classification of vertebrate coprolites and related trace fossils. New Mexico Museum of Natural History and Science Bulletin 57: 137–146.
Jordan, F. 2009. Keystone species and food webs. Philosophical Transactions of the Royal Society B: Biological Sciences 364: 1733–1741. Crossref
Kerp, H. and Bomfleur, B. 2011. Photography of plant fossils—new techniques, old tricks. Review of Palaeobotany and Palynology 166: 117–151. Crossref
Kielan-Jaworowska, Z. and Barsbold, R. 1972. Narrative of the Polish-Mongolian Paleontological Expeditions, 1967–1971. Palaeontologica Polonica 27: 5–13.
Klokočovnik, V. and Devetak, D. 2014. Pit-builder vs non-pit-builder: advantage of trap building strategy in antlion larvae does not mean greater behaviour diversity. Behaviour 151: 653–668. Crossref
Knight, T.M., McCoy, M.W., Chase, J.M., McCoy, K.A., and Holt, R.D. 2005. Trophic cascades across ecosystems. Nature 437: 880.Crossref
Kriwet, J., Witzmann, F., Klug, S., and Heidtke, U.H. 2007. First direct evidence of a vertebrate three-level trophic chain in the fossil record. Proceedings of the Royal Society B: Biological Sciences 275: 181–186. Crossref
Kutty, S.N., Pont, A.C., Meier, R., and Pape, T. 2014. Complete tribal sampling reveals basal split in Muscidae (Diptera), confirms saprophagy as ancestral feeding mode, and reveals an evolutionary correlation between instar numbers and carnivory. Molecular Phylogenetics and Evolution 78: 349–364. Crossref
Lafferty, K.D. and Kuris, A.M. 2002. Trophic strategies, animal diversity and body size. Trends in Ecology & Evolution 17: 507–513. Crossref
Liu, X., Shi, G., Xia, F., Lu, X., Wang, B., and Engel, M.S. 2018. Liverwort mimesis in a Cretaceous lacewing larva. Current Biology 28: 1475–1481. Crossref
Liu, X., Zhang, W., Winterton, S.L., Breitkreuz, L.C., and Engel, M.S. 2016. Early morphological specialization for insect-spider associations in Mesozoic lacewings. Current Biology 26: 1590–1594. Crossref
Martı́nez-Delclòs, X., Briggs, D.E., and Peñalver, E. 2004. Taphonomy of insects in carbonates and amber. Palaeogeography, Palaeoclimatology, Palaeoecology 203: 19–64. Crossref
Morales-Castilla, I., Matias, M.G., Gravel, D., and Araujo, M.B. 2015. Inferring biotic interactions from proxies. Trends in Ecology & Evolution 30: 347–356. Crossref
Mouchet, M.A., Villéger, S., Mason, N.W., and Mouillot, D. 2010. Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Functional Ecology 24: 867–876. Crossref
Noetling, F. 1893. On the occurrence of Burmite, a new fossil resin from Upper Burma. Records of the Geological Survey of India 26 (1): 31–40.
Northwood, C. 2005. Early Triassic coprolites from Australia and their palaeobiological significance. Palaeontology 48: 49–68. Crossref
Poinar Jr, G. 2018. Burmese amber: evidence of Gondwanan origin and Cretaceous dispersion. Historical Biology 31: 1304–1309. Crossref
Poinar Jr, G. and Buckley, R. 2012. Predatory behaviour of the social orb-weaver spider, Geratonephila burmanica n. gen., n. sp. (Araneae: Nephilidae) with its wasp prey, Cascoscelio incassus n. gen., n. sp. (Hymenoptera: Platygastridae) in Early Cretaceous Burmese amber. Historical Biology 24: 519–525. Crossref
Qvarnström, M., Niedźwiedzki, G., and Žigaitė, Ž. 2016. Vertebrate coprolites (fossil faeces): an underexplored Konservat-Lagerstätte. Earth-Science Reviews 162: 44–57. Crossref
Richter, G. and Baszio, S. 2001. Traces of a limnic food web in the Eocene Lake Messel—a preliminary report based on fish coprolite analyses. Palaeogeography, Palaeoclimatology, Palaeoecology 166: 345–368. Crossref
Salamon, M.A., Brachaniec, T., and Gorzelak, P. 2020. Durophagous fish predation traces versus tumbling-induced shell damage—a paleobiological perspective. Palaios 35: 37–47. Crossref
Salamon, M.A., Lin, J.P., Duda, P., Gorzelak, P., Ausich, W.I., and Oji, T. 2019. Paleoenvironmental and biostratigraphic implications of echinoderm ossicles trapped within Burmese amber. Palaios 34: 652–656. Crossref
Shi, G., Grimaldi, D.A., Harlow, G.E., Wang, J., Wang, J., Yang, M., Lei, W., Li, Q., and Li, X. 2012. Age constraint on Burmese amber based on U-Pb dating of zircons. Cretaceous Research 37: 155–163. Crossref
Tauber, C.A., Tauber, M.J., and Albuquerque, G.S. 2009. Neuroptera: lacewings, antlions. In: V.H. Resh and R.T. Cardé (eds.), Encyclopedia of Insects, 695–707. Academic Press. San Diego. Crossref
Valls, A., Coll, M., and Christensen, V. 2015. Keystone species: toward an operational concept for marine biodiversity conservation. Ecological Monographs 85: 29–47. Crossref
Vannier, J. 2007. Early Cambrian origin of complex marine ecosystems. In: M. Williams, A.M. Haywood, F.J. Gregory, and D.N. Schmidt (eds.), The Micropalaeontological Society, Special Publications. Deep-time Perspectives on Climate Change: Marrying the Signal From Computer Models And Biological Proxies, 81–100. The Geological Society, London. Crossref
Vannier, J. 2012. Gut contents as direct indicators for trophic relationships in the Cambrian marine ecosystem. PloS ONE 7: e52200. Crossref
Vannier, J. and Chen, J. 2005. Early Cambrian food chain: new evidence from fossil aggregates in the Maotianshan Shale biota, SW China. Palaios 20: 3–26. Crossref
Vinn, O. 2018. Traces of predation in the Cambrian. Historical Biology 30: 1043–1049. Crossref
Vršanský, P. and Bechly, G. 2015. New predatory cockroaches (Insecta: Blattaria: Manipulatoridae fam. n.) from the Upper Cretaceous Myanmar amber. Geologica Carpathica 66: 133–138. Crossref
Wang, B., Xia, F., Engel, M.S., Perrichot, V., Shi, G., Zhang, H., Chen, J., Jarzembowski, E.A., Wappler, T., and Rust, J. 2016. Debris-carrying camouflage among diverse lineages of Cretaceous insects. Science Advances 2: e1501918. Crossref
Weitschat, W. and Wichard, W. 1998. Atlas der Tiere und Pflanzen im Baltischen Bernstein. 256 pp. Dr. Friedrich Pfeil, München.
Wichard, W. 2017. Family Nevrorthidae
(Insecta, Neuroptera) in mid-Cretaceous Burmese amber. Palaeodiversity
10: 1–6. Crossref
Wilby, P.R., and Martill, D.M. 1992. Fossil fish stomachs: a microenvironment for exceptional preservation. Historical Biology 6: 25–36. Crossref
Witmer L.M. 1995. The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In: J.J. Thomason (ed.), Functional Morphology in Vertebrate Paleontology, 19–33. Cambridge University Press, New York.
Witton, M.P. 2018. Pterosaurs in Mesozoic food webs: a review of fossil evidence. Geological Society, London, Special Publications 455: 7–23. Crossref
Yin, Z.W., Parker, J., Cai, C.Y., Huang, D.Y., and Li, L.Z. 2018. A new stem bythinine in Cretaceous Burmese amber and early evolution of specialized predatory behaviour in pselaphine rove beetles (Coleoptera: Staphylinidae). Journal of Systematic Palaeontology 16: 531–541. Crossref
Yu, T., Kelly, R., Mu, L., Ross, A., Kennedy, J., Broly, P., Xia, F., Zhang, H., Wang, B., and Dilcher, D. 2019. An ammonite trapped in Burmese amber. Proceedings of the National Academy of Sciences 116: 11345–11350. Crossref
Zherikhin, V.V. and Ross, A.J. 2000. A review of the history, geology and age of Burmese amber burmite. Bulletin of the Natural History Museum Geology Series. London (Geology) 56: 3–10.
Zhu, M.Y., Vannier, J., Iten, H.V., and Zhao, Y.L. 2004. Direct evidence for predation on trilobites in the Cambrian. Proceedings of the Royal Society of London. Series B: Biological Sciences 271 (Supplement 5): S277–S280. Crossref
Acta Palaeontol. Pol. 65 (4): 777–786,
2020 https://doi.org/10.4202/app.00677.2019