

New findings reveal that the Middle Triassic ichthyosaur Mixosaurus cornalianus is the oldest amniote with a dorsal fin
SILVIO RENESTO, CRISTIANO DAL SASSO, FABIO FOGLIAZZA, and CINZIA RAGNI
Renesto, S., Dal Sasso, C., Fogliazza, F., and Ragni, C. 2020. New findings reveal that the Middle Triassic ichthyosaur Mixosaurus cornalianus is the oldest amniote with a dorsal fin. Acta Palaeontologica Polonica 65 (3): 511–522.
Two excellently preserved specimens of Mixosaurus cornalianus from the Anisian layers of the Middle Triassic Formazione di Besano, with soft parts associated with well-articulated skeletal elements, revealed the presence in this species of a dorsal fin and of a well-developed, triangular dorsal lobe of the caudal fin, both stiffened by an array of fibre bundles, as in Jurassic fast-swimming ichthyosaurs. This finding testifies that efficient swimming exaptations were already present in some Middle Triassic ichthyosaurs. Mixosaurus is then the oldest amniote so far known that developed a dorsal fin. The preservation of the fin shapes, scaleless skin, and three-dimensional dermal fibres is remarkable, allowing observation of their microstructure, and ruling out any artefactual interpretation. Stomach contents indicate that both specimens preyed upon cephalopods and small fishes. An internal organ, possibly a tract of the intestine, is also preserved in one specimen, which represents the first documented case in the ichthyosaurs of the Besano Formation, and a rarity in the fossil record of the clade.
Key words: Ichthyosauria, Mixosauridae, soft tissue preservation, functional morphology, Mesozoic, Italy.
Silvio Renesto [silvio.renesto@uninsubria.it, ORCID: https://orcid.org/0000-0002-8845-5717], Department of Theoretical and Applied Sciences, Università degli Studi dell’Insubria, via Dunant 3, Varese Italy.
Cristiano Dal Sasso [Cristiano.DalSasso@comune.milano.it] and Fabio Fogliazza [Fabio.Fogliazza@comune.milano.it], Museo di Storia Naturale di Milano, Corso Venezia 55, Milano, Italy.
Cinzia Ragni [cinziaragni93@gmail.com], Via Serponte 21, Cameri, Novara, Italy.
Received 28 January 2020, accepted 24 April 2020, available online 2 July 2020.
Copyright © 2020 S. Renesto et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Ichthyosaurs are among the most abundant fossil reptiles of the UNESCO World Heritage List (WHL) site of the Besano-Monte San Giorgio area (Lombardy, Italy; and Canton Ticino, Switzerland; Fig. 1), which is one of the richest sites of Middle Triassic palaeobiodiversity (e.g., Furrer 2003) even after the new discoveries in China (e.g., Benton et al. 2013). In fact, as in the present case, fossil preservation in some bituminous layers of the Besano Formation (= “Grenzbitumenzone” of Swiss literature) and of the Meride Limestone can reveal soft tissue preservation not found elsewhere in the world.
After the first reports (Curioni 1847), the Museo di Storia Naturale di Milano arranged several excavations in the surroundings of Besano, collecting a large number of fossils across the last two centuries. This historical collection was destroyed during the Second World War, so that, starting from 1975, the MSNM conducted new fieldwork. The most productive and long-lasting locality (1985–2003) was the “Sasso Caldo” site (Fig. 1), where remarkable material was collected and published (e.g., Dal Sasso and Pinna 1996; Renesto and Avanzini 2002; Nosotti 2007; Renesto 2010; Renesto et al. 2014).
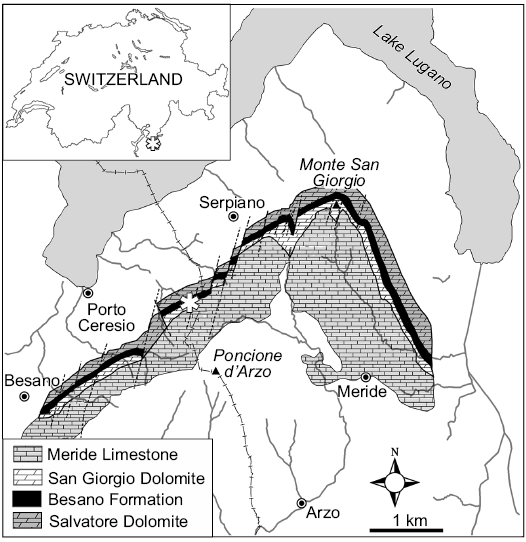
Fig. 1. General location and geological map of the Monte San Giorgio area. The asterisk indicates the position of the Sasso Caldo site.
The specimens described herein were found on February 14th 1993, in the eastern corner of the quarry, within layer 63 of the referred series (Middle Besano Formation sensu Bindellini et al. 2019), which is datable as uppermost Anisian. During preparation, soft tissue anatomical structures with remarkable functional significance never seen before in mixosaurian-grade ichthyosaurs were exposed; their description and interpretation is the aim of the present contribution.
Institutional abbreviations.—BES SC, Besano Sasso Caldo locality, Museo di Storia Naturale di Milano, Milano, Italy; MSNM, Museo di Storia Naturale di Milano, Milano, Italy; SMNS, Staatliches Museum fur Naturkunde Stuttgart, Stuttgart, Gerrmany.
Geological setting
The Middle Triassic carbonate succession of Monte San Giorgio consists of four different formations (Fig. 2) deposited in an intra-platform basin within a carbonate platform on the western margin of the Neo-Tethys (Furrer 1995; Röhl et al. 2001; Etter 2002; Stockar et al. 2012). Above the Anisian Salvatore Dolomite lies the 5–16 m thick Besano Formation (Anisian/Ladinian boundary), from which the greatest part of the well-known vertebrate fauna of Monte San Giorgio has been recovered (Bürgin et al. 1989; Furrer 2003). At the top of the Besano Formation is the San Giorgio Dolomite, which, in turn, grades into the well-bedded Ladinian Meride Limestone, that reaches a total thickness of 400–600 m (Furrer 1995). The Meride Limestone is divided in two parts (Wirz 1945), the Lower Meride Limestone, which is 90–150 m thick (Furrer 1995), and consists of finely laminated limestone with intercalated volcanic ash layers and contains three fossiliferous levels (Cava inferiore, Cava superior, and Cassina), each characterized by different vertebrate assemblages (Sander 1989; Bürgin et al. 1989). The overlying Upper Meride Limestone shows alternating well-bedded limestone and marlstone with an increasing clay content towards the top. Strong seasonal variations of salinity and water level are documented along with the influence of freshwater input from a nearby-emerged area. The uppermost part of the Meride Limestone comprises the so-called “Kalkschieferzone”, which is 120 m thick and represents the final phases of evolution of the intraplatform basin, then followed by the Carnian regressive phase (Pizzella Marls).
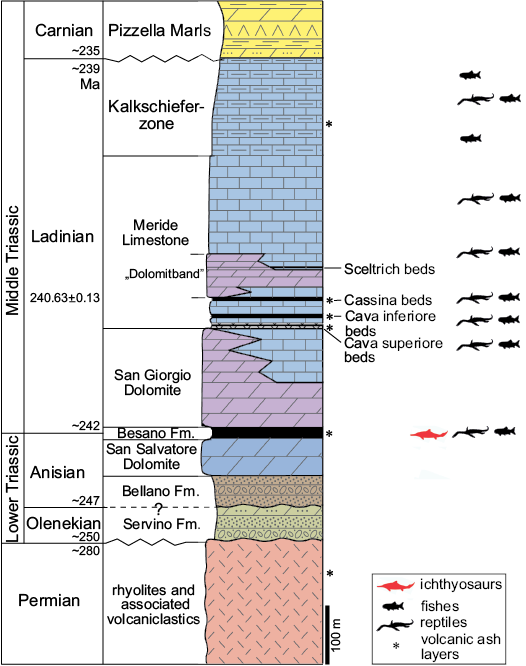
Fig. 2. Middle Triassic stratigraphic units of the Monte San Giorgio area. Stratigraphy modified from Commissione Scientifica Transnazionale Monte San Giorgio (2014; based on Furrer 1995); U-Pb ages after Mundil et al. (2010) and Stockar et al. (2012). The ichthyosaur outline indicates the Besano Formation, in which the specimens were collected.
The Besano Formation consists of an alternation of laminated, bituminous dolomite and bituminous shale, and subordinate cineritic tuff that are dated as late Anisian–early Ladinian (Brack and Rieber 1986, 1993; Brack et al. 2005). Recent biozonation of the Sasso Caldo site, based on index-fossil invertebrates (ammonoids and the bivalve Daonella), indicate that the stratigraphic section cropping out therein is fairly consistent with the most recent biozonation reported by the Swiss authors (Brack et al. 2005) and allows confident correlation with the coeval Swiss localities (Bindellini et al. 2019). In particular, the two mixosaurs here described were collected below the two/three uppermost tuffitic layers and within the N. secedensis Zone (Middle Besano Formation), thus they are of late Anisian age.
The Besano Formation was deposited in a lagoonal basin whose estimated depth should have been around 30–100 m (Bernasconi 1991; Furrer 1995; Röhl et al. 2001; Etter 2002). The upper part of the water column was oxygenated, and a rich fauna, consisting mostly of cephalopods and vertebrates, thrived (Furrer 1995). At the bottom of the basin, water circulation was restricted, thus developing an anoxic environment; however, there is evidence of periodical settling of weak currents that generated dysaerobic conditions (Bernasconi 1994; Furrer 1995; Röhl et al. 2001).
Material and methods
The detailed osteological description is available as SOM (Supplementary Online Material available at http://app.pan.pl/SOM/app65-Renesto_etal_SOM.pdf) and it is only briefly summarized here to testify that the two specimens show a typical combination of skeletal features allowing their assignment to Mixosaurus cornalianus Bassani, 1886 as diagnosed by Maisch and Matzke (1997, 2000), Jiang et al. (2006), and Ji et al. (2016).
Scanning electron microscopy (SEM) images and elemental peaks were obtained by analysing microsamples, taken from carefully selected areas of the specimen, with a Jeol JSM 5610 LV (IXRF Systems Inc.) equipped with an EDS 500 spectrometer.
Systematic palaeontology
Ichthyopterygia Owen, 1840
Ichthyosauria Blainville, 1835
Mixosauridae Baur, 1887
Genus Mixosaurus Baur, 1887
Type species: Mixosaurus cornalianus Bassani, 1886; Besano Formation (Triassic), Monte San Giorgio locality, Italy-Switzerland.
Mixosaurus cornalianus Bassani, 1886
Figs. 3–8.
Material.—Two nearly complete and articulated specimens: BES SC 1000 in right lateral view (Fig. 3A), BES SC 1001 in dorsal view (Fig. 3B). Coded as 20.S288-2.3 and 20.S288-2.4, respectively, in the Inventario Patrimoniale dello Stato (State Heritage Database). From Sasso Caldo site (45°54’03.7” N 8°55’10.6” E, elev. 650 m), Besano, Varese Province, NW Lombardy, N. Italy; Middle Besano Formation (sensu Bindellini et al. 2019), Uppermost Anisian, Nevadites secedensis Zone (sensu Brack et al. 2005), Middle Triassic.
Description.—The premaxilla is caudally pointed, only forming the rostral margin of the external naris; the maxilla shows a high and wide postnarial process contacting the prefrontal, and excluding the lacrimal from the external naris; the latter is very small and slit-like; a long sagittal crest is present on the skull roof, along with a large rostral terrace of the upper temporal fenestra, both reaching the nasals; the nasals are short, not extending caudally beyond the rostral margin of the orbit; the prefrontal and postfrontal are large, each forming about one half of the dorsal margin of the orbit; the upper temporal fenestra is small and slit-like, formed by supratemporal and parietal only; the cheek region is made up of a large supratemporal and a large squamosal. The dentition is subthecodont and isodont; the posterior maxillary and dentary teeth are slender, pointed, and widely spaced; there are 17 maxillary teeth (in BES SC 1000); the maxillary tooth row extends backwards beyond the caudal margin of the postnarial process; the pubis is more than twice the size of the ischium, the neural spines are high and narrow, approximately three times higher than the centra except for the last caudals; the caudal peak occurs at the level of the caudal vertebrae 17–18; the mid caudal vertebrae are larger than the preceding ones; the length of the hindfins is less than two thirds that of the forefins; in the forefins, five metacarpals are present with constricted shafts.
The body size, proportions, and degree of ossification of the two skeletons, compared with a larger mixosaurid sample (Brinkmann 1996, 1997, 2004), indicate they had almost reached a fully adult age.
Soft tissues: Preservation of vertebrate soft tissues has been reported for several specimens from the Grenzbitumenzone/Besano Formation and the Meride Limestone Formation, such as the tanystropheids Macrocnemus (Peyer 1937; Renesto and Avanzini 2002) and Tanystropheus (Renesto 2005; Renesto and Saller 2018), the sauropterygian Neusticosaurus (Sander 1989). and the fish Saurichthys (Renesto and Stockar 2009; Maxwell et al. 2013; Argyriou et al. 2016). Preservation of soft parts is likely due to authigenic mineralization induced by bacterial activity in oxygen-depleted environments, often in fully reducing conditions (Martill et al. 1992), in the presence of organic matter that may also represent the source of the mineralizing phosphates. Microbial activity creates microenvironments within the decaying carcass (Noto 2009; Sagemann et al. 1999). Depending on the microbial activity, phosphatization of soft parts may be limited only to some areas of the carcass, according to local variations of the pH (Briggs and Wilby 1996) which may (or may not) allow precipitation of phosphates that replace integumentary structures or even internal organs (e.g., Dal Sasso and Maganuco 2011).
In both specimens here described, patches of skin are preserved over the body, along with remains of other soft tissue (probably belonging to the stomach) lying inside the rib cage. In BES SC 1000, the upper lobe of the caudal fin is also preserved, along with the trailing edge of the dorsal fin (Fig. 4). In BES SC 1001, a possible tract of intestine is exposed (Fig. 5), and traces of the dorsal lobe of the caudal fin are also present. It is the first time that the presence of these structures is reported for Mixosaurus, and their preservation is good enough to allow a description of their internal microstructure.
Dorsal and caudal fins: In BES SC 1000, a well-delimited, straight, elongate, and apparently stiff structure made by tightly packed fibrous elements is positioned above the longest presacral neural spines, together with its basal portions (Fig. 4B). Given its specific identity, shape, position, and size (68 mm from base to its well-preserved apex), we exclude from being a loose flap of decaying tissue, as seen in rarely preserved cutaneous and subcutaneous tissues of Stenopterygius, which have a clearly wavy, not stiffened aspect (CDS personal observation on SMNS 54051, SMNS 81958). Therefore, the structure seen in BES SC 1000 most likely represents the leading edge of the dorsal fin. It lies above dorsal vertebrae 15–23 and at the level of the trailing edge of the forefins, thus it is placed more cranially than in most of the tentative depictions published so far (e.g., Kuhn-Schnyder 1974; Motani et al. 1996). At a closer look, an array of collagen fibres (sensu Lingham-Soliar and Plodowski 2007; that is, each visible fibre may represent a bundle of packed collagen microfibers) densely packed together is clearly visible under binocular microscope (Fig. 4D). These fibres extend parallel to each other, and are inclined at an angle of approximately 26° with respect to the body axis. All fibrous structures are in relief, giving a near three-dimensional appearance; their nature and microstructure is confirmed by SEM analysis (see below).
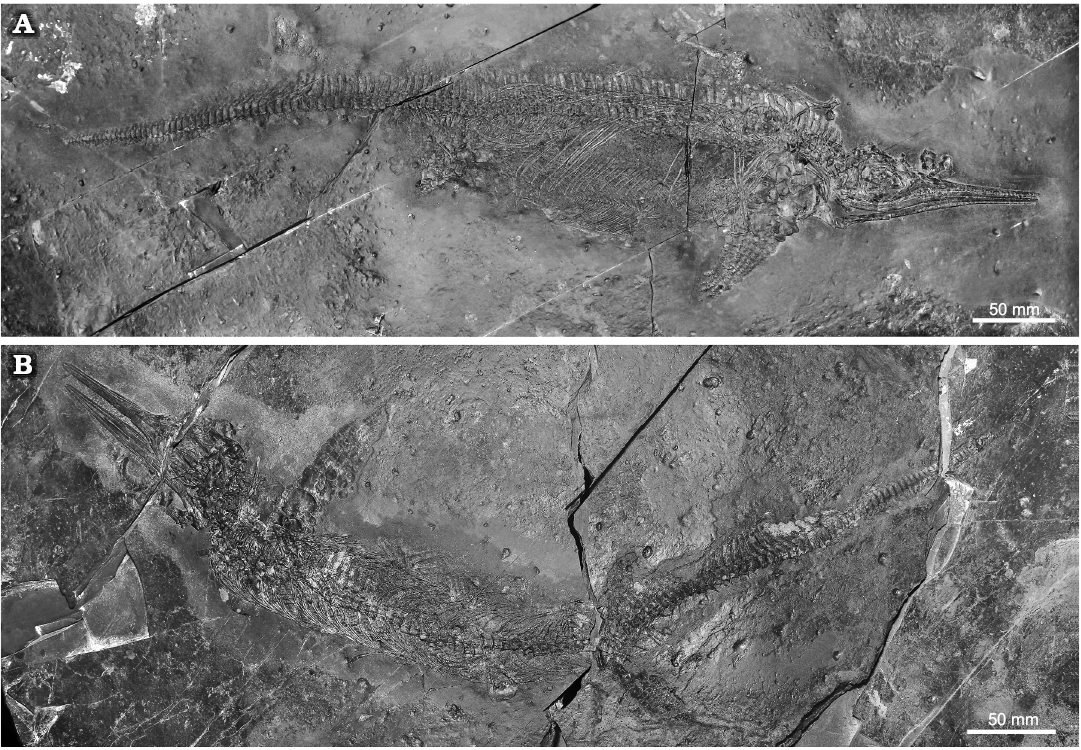
Fig. 3. Mixosaurid ichthyosaur Mixosaurus cornalianus Bassani, 1886, Sasso Caldo quarry, Besano, Italy, upper Anisian. A. BES SC 1000. B. BES SC 1001.
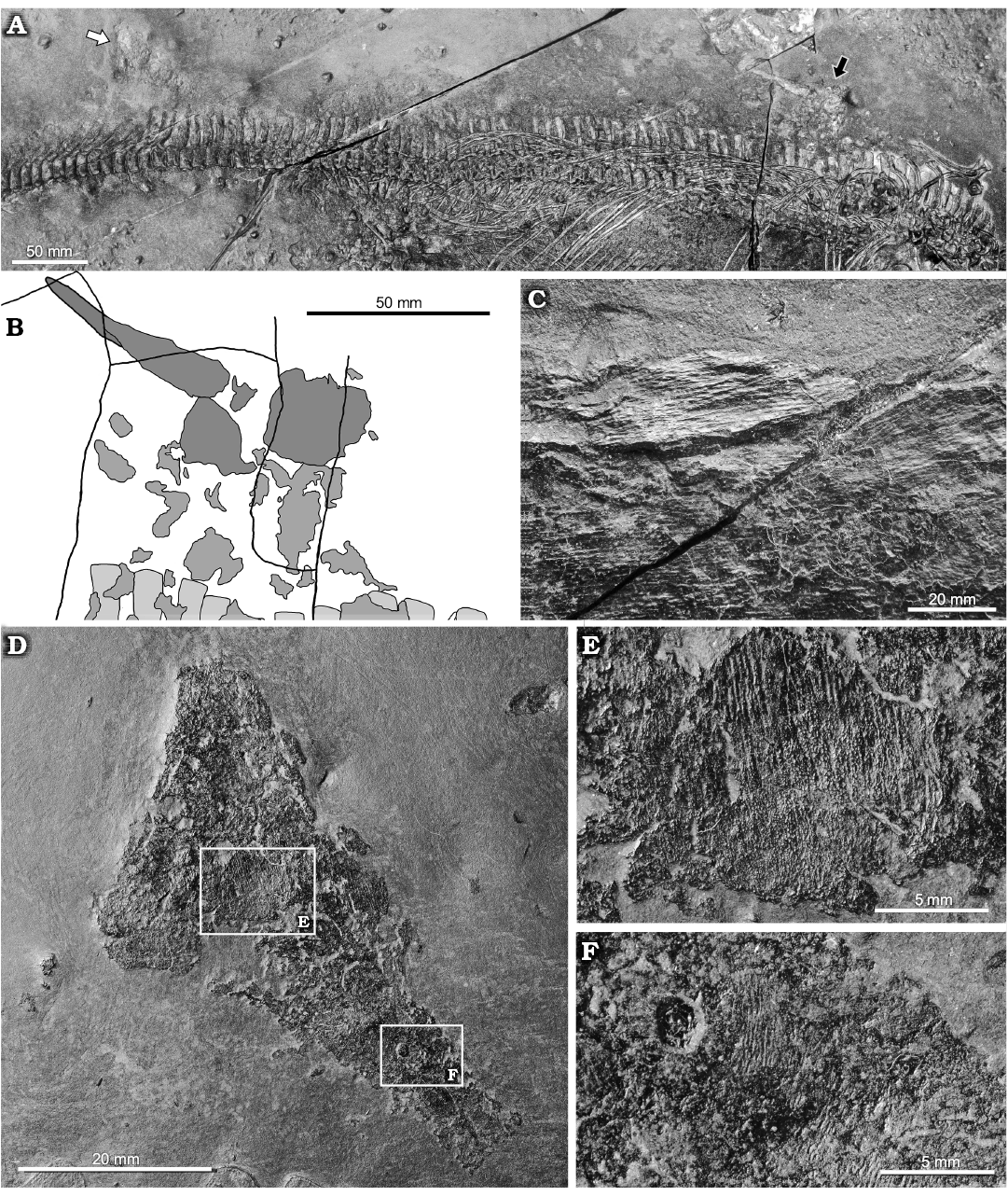
Fig. 4. Mixosaurid ichthyosaur Mixosaurus
cornalianus Bassani, 1886 (BES SC 1000), Sasso Caldo
quarry, Besano, Italy, upper Anisian. A.
General view of the specimen showing the position of the dorsal fin
(black arrow) and of the dorsal lobe of the caudal fin (white
arrow). B. Explanatory
drawing showing the position of the dorsal fin (dark grey), skin
remains (grey), and neural spines (light grey). C.
Close up of the shaft of the dorsal fin showing parallel fibres. D. Dorsal lobe of the caudal fin. E, F.
Close ups of the areas of the dorsal lobe of the caudal fin where
other fibres and skin remains are exposed.
The dorsal lobe of the caudal fin of BES SC 1000 (Fig. 4C) lies at the level of the peak of the caudal vertebrae, just above caudal vertebrae 14–22, it is almost complete and shows a triangular outline. The preserved portion of the fin is 43 mm high (Table 1), the cranial margin is 66 mm long, and the ventral margin (i.e., the base) measures 51 mm. The concave ventral margin mirrors exactly the outline of the caudal peak of the vertebral column, showing the original in vivo position of the fin lobe. In fact, the fin has been set off dorsally and slightly rotated counterclockwise, with post-mortem shifting of a few centimetres. The fossilised skin surface that covers most of the fin seems to retain few details, but in areas where the skin is missing, the underlying array of fibres becomes visible (Fig. 4E, F). As in the dorsal fin, these fibrous structures are in relief. At the cranioventral corner of the base of the fin, fibres can be observed diagonally oriented at an angle of approximately 32° with the body axis. Other almost straight, feebly waved arrays of fibres are visible in the central portion and cranial margin of the fin, with an inclination of 58–53°. A small portion of the dorsal lobe of the caudal fin is present also in BES SC 1001, at the level of caudal vertebrae 19–22, but only few patches of skin are preserved.
Table 1. Selected measurements (in mm) of the soft tissues of the specimens studied herein. Brackets indicate incomplete elements.
| |
|
BES SC 1000 |
BES SC 1001 |
|
Dorsal fin |
length of leading edge |
(68) |
– |
|
Caudal fin |
length of leading edge |
66 |
– |
|
dorsoventral height |
(43) |
– |
|
|
base length |
51 |
– |
|
|
Dorsal and caudal fin |
diameter of a single collagen fibre (in μm) |
60–70 |
– |
|
Intestine |
preserved length along U-shaped arrangement |
– |
(35) |
|
transverse diameter of preserved portion |
– |
9–11 |
Under SEM observation of a tissue microsample from the base of the caudal fin of BES SC 1000, each array consists of parallel fibres with a diameter of 60–70 μm each, which are part of the innermost dermal layer. The lack of transverse segmentation and the size range of the fibres are consistent with the collagen microstructure (e.g., Laurent 2018), unlike somatic muscular myofibres, which are striated and have larger diameters (e.g., Muhl et al. 1976). Apparently homologous and better-preserved fibres, sampled from the trailing edge of the dorsal fin of BES SC 1000, are composed of very fine striations, likely corresponding to collagen fibrils (Fig. 5A–C).
SEM analysis of the microsample from the base of the caudal fin also recovered remnants of skin with an excellent degree of preservation, fossilised through replication in authigenic calcium phosphate (Fig. 5G). No scales or scutes are evident, and the external surface is smooth, as in Stenopterygius (Lindgren et al. 2018). Moreover, the skin of Mixosaurus shows a multi-layered subsurface architecture (Fig. 5D), corresponding to the laminated epidermis and dermis of modern tetrapods, which is also seen in Stenopterygius (Lindgren et al. 2018). In our sample the first layer is smooth and continuous, whereas the second layer appears vacuolar or fine-grained. Theoretically there should be a layer of fibrous connective tissue between the right and left dermal layers, and internal to a thin fat layer (Erin Maxwell, personal communication 2020), which our surficial non-invasive sampling did not penetrate.
In any case, our SEM images and elemental microanalyses clearly demonstrate that the fibres of BES SC 1000 are fossilised anatomical structures, well in relief within the ichthyosaur skin tissue, and not sedimentary cracks or tool marks, as it was questioned recently for similar finds in Jurassic ichthyosaurs (Smithwick et al. 2017).
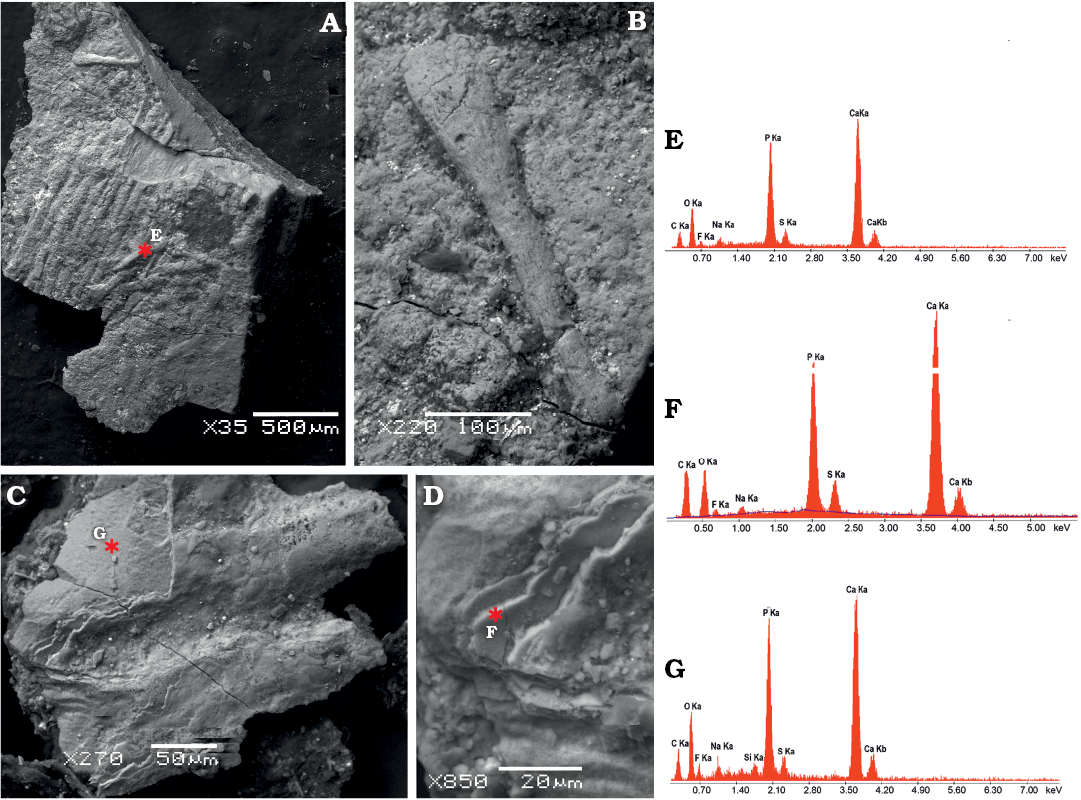
Fig. 5. SEM images of microsampled soft tissues of mixosaurid ichthyosaur Mixosaurus cornalianus Bassani, 1886 (BES SC 1000), Sasso Caldo quarry, Besano, Italy, upper Anisian.. A. Collagen fibres packed in parallel bundles from the leading edge of the dorsal fin. B. Close-up of a single fibre showing very fine striations, interpreted as collagen fibrils. C. A pair of collagen fibres from the base of the caudal fin, still covered by a patch of multi-layered scaleless skin. D. Close-up of the skin layers seen in C. Asterisks indicate locations of the back-scattered electron element microanalysis performed on dorsal fin fibres (E), caudal fin fibres (F), and caudal fin skin (G). The peaks illustrate the relative abundance of each element, with higher intensities indicating greater abundance. Note the enrichment of calcium and phosphorous in the fossilised soft tissue, with some variation in secondary minerals.
Intestine: A finely corrugated structure of apparent soft tissue nature protrudes off the ventral margin of the abdominal region on the right side of BES SC 1001, approximately at the level of the presacral vertebra 40, i.e., cranial to the pelvic girdle (Fig. 6). It shows surficial corrugations and has a U-shaped tubular structure, it measures 31 mm along its long axis, 17 mm perpendicular to it and 9–11 mm in transverse diameter. Secondary ridges, perpendicular to the longitudinal ones, are visible under grazing light and seem to define millimetric internal subdivisions, or contents.
In our opinion, this structure may represent a tract of the Mixosaurus guts, namely a lithified intestinal segment, filled by remains of ingested prey. Some of the granularities glimpsed within the organ look subrectangular in shape and would be compatible in size with the small vertebrae contained in the stomach region; the transverse diameter of the organ is also consistent with the dimensional range of the intestinal tube in a 0.9–1.0 m long reptile (Vitt and Caldwell 2009).
The main corrugations would represent the longitudinal folds of the original external layer of muscular tissue, which might have become fossilised thanks to a prolapse outside the abdominal cavity, under direct contact with the sediment undergoing diagenesis. This event might explain why there is no apparent continuity of gut remains cranial and caudal to this section of the organ, within the abdomen of BES SC 1001, where different chemical conditions likely made the decay of soft tissues prevalent.
Fluorescence induced by UV light did not show similar soft tissue remnants nearby; a microsample of the organ, taken along its broken section and analysed under SEM, did not recover any diagnostic anatomical microstructure. Contrary to the skin and collagen fibres, this organ is extensively colonised by a number of pyrite crystals, and in cross-section it appears amorphous. Fossil preservation, in this case, does not reach the cellular level and is limited to replication of the external morphology. Nevertheless, the organic origin of this structure is confirmed by SEM element microanalysis (Fig. 6B), which gives a chemical signal very similar to skin and collagen of BES SC 1000 (Fig. 6E, G), and also to the visceral tissue fossilised in the theropod dinosaur Scipionyx (Dal Sasso and Maganuco 2011: fig. 143).
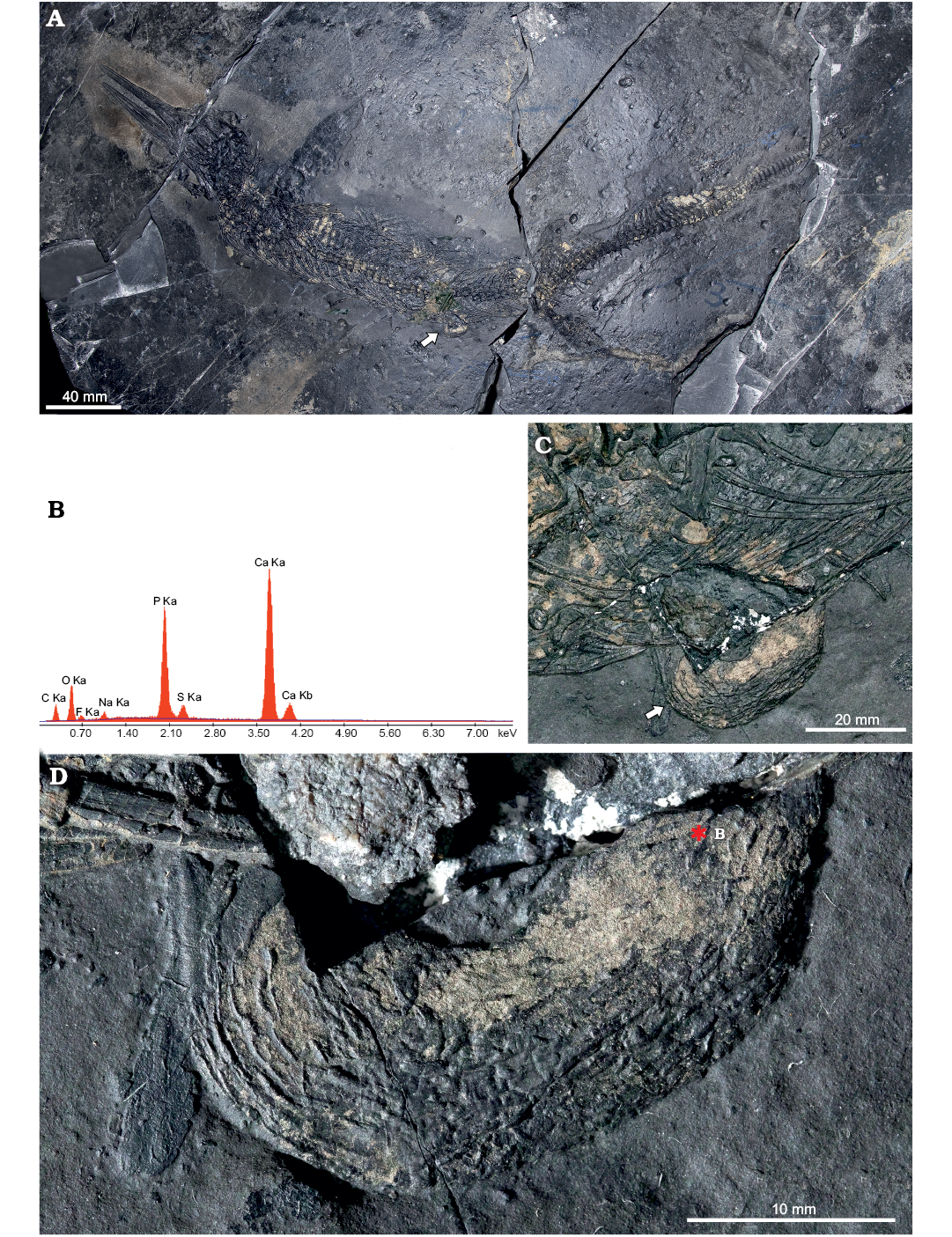
Fig. 6. Mixosaurid ichthyosaur Mixosaurus cornalianus Bassani, 1886 (BES SC 1001), Sasso Caldo quarry, Besano, Italy, upper Anisian. A. General view of the specimen. B. Back-scattered SEM element microanalysis of the possible intestine. C. Close up of the abdominal area with the protruding portion of the organ. D. Close up of the possible intestine, showing the longitudinal folds of the organ (left) and the internal granulosity (right). Arrows (in A, C) point to the organ position; asterisk indicates location of the back-scattered electron element microanalysis of the tissue.
If our interpretation is correct, this find confirms, after indirect hypotheses based on coprolites (e.g., Pollard 1968) that, in ichthyosaurs, the ingested bones could pass through the intestines. Bones are much harder than scales, and more difficult to process throughout the guts. Not all reptiles are able to do that, as the pyloric valve at the end of the stomach can be too narrow to allow transit of solid material (for a complete account see Dal Sasso and Maganuco 2011). In this respect, BES SC 1001 might represent an important addition to our knowledge of digestive physiology in the Ichthyosauria.
Stomach contents: In the thoracic region of BES SC 1000, several hooklets of cephalopods are detectable (Fig. 7A), as is frequent in Mixosaurus specimens; in addition, a finely rough area of dark material, 100 mm long and 40 mm wide, overlies the left ribs and contains a number of tiny, ebony-brownish polygonal to cylindrical elements, whose diameter varies from 0.1 to 0.8 mm. These elements represent vertebral centra (Fig. 7B), often grouped in clusters and embedded in the rough material, whereas the sediment surrounding the Mixosaurus skeleton is devoid of them; thus it is feasible that this area would represent stomach remains and contents. The attribution of these centra is difficult because they differ significantly from the C-shaped or ring-shaped vertebral centra of Triassic actinopterygians, and are also too small and different from vertebral centra of marine reptiles, while there is some resemblance to some Triassic neoselachian vertebrae (Mears et al. 2016: fig. 8). Neoselachian remains are known since the Lower Triassic (Koot et al. 2015), but in the Besano Formation only hybodont sharks have been found so far, thus the attribution to neoselachians must be considered as tentative.
In BES SC 1001, isolated or partially articulated scales (Fig. 7C, D) are preserved in the stomach area. They show a pattern of ridges that suggest they belonged to some actinistian, a group that is well represented in the Besano Formation (Rieppel 1980, 1985; Cavin et al. 2013; Ferrante et al. 2017; Renesto and Stockar 2018).
Given that all other reported gastric contents of mixosaurids from Monte San Giorgio consist exclusively of cephalopod hooklets (Rieber 1970; Brinkmann 1997), the two new specimens testify that adult Mixosaurus also preyed on small fishes, as medium-large ichthyosaurs did (e.g., Pollard 1968; Druckenmiller et al. 2014; Dick et al. 2016).
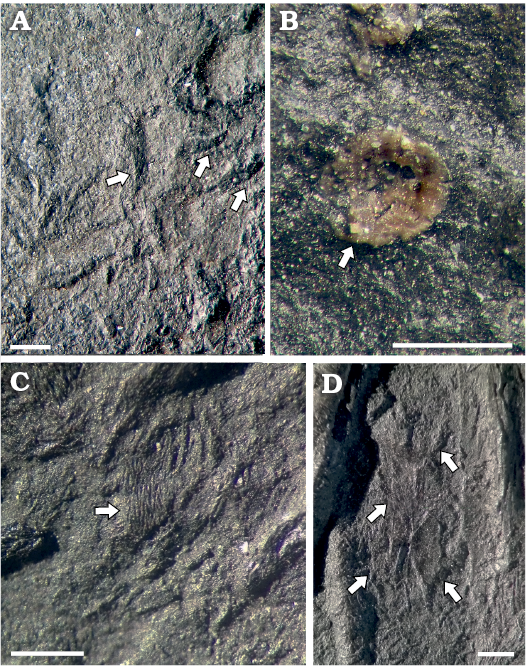
Fig. 7. Stomach contents of mixosaurid ichthyosaur Mixosaurus cornalianus Bassani, 1886 (A, B, BES SC 1000; C, D, BES SC 1001), Sasso Caldo quarry, Besano, Italy, upper Anisian. A. Cephalopod hooklets (arrowed). B. Enigmatic (neoselachian?) vertebral centrum (arrowed) embedded in the dark material of the decayed stomach. C. Isolated actinistian scale (arrowed). D. Semi-articulated actinopterygian scales (arrowed) embedded in the stomach area. Scale bars 1 mm.
Discussion
A large number of fossils of Mixosaurus cornalianus is known (Brinkmann 1998, 2004; Motani 1999; Maisch and Matzke 2000), but the two specimens described here represent the first evidence that this ichthyosaur had both a dorsal fin and a well-developed dorsal lobe of the caudal fin. So far, Mixosaurus cornalianus has been reconstructed either with or without dorsal fins, and the shape and position of the dorsal fin has been conjectural. The specimens described here not only testify that a dorsal fin and a lobate, incipiently semilunate caudal fin were undoubtedly present, but also allow reconstruction of the shape of the fin with remarkable precision (Fig. 8). More importantly, the preservation of the internal structure of the fins, although incomplete, allows us to propose hypotheses about the swimming efficiency of Mixosaurus cornalianus (and possibly other Mixosaurus species).
The fibres preserved in the dorsal and caudal fins of BES SC 1000 (Fig. 5) show striking macroscopic and microscopic similarities to those found in Jurassic ichthyosaurs, including thunniform taxa such as Stenopterygius (Lingham-Soliar and Plodowski 2007). According to these authors, the collagen fibre architecture of Stenopterygius was nearly identical to that of large lamniform sharks like Carcharodon carcharias (Lingham-Soliar 2005a, b) and provided adequate stiffness to allow stability and efficient propulsion during high speed swimming. The dorsal fin in C. carcharias acts as a dynamic stabilizer: during fast swimming the dermal fibres go under tension playing a fundamental role in stiffening the fin against yaw and roll, while during slow swimming the fibres lose tension allowing more flexibility (Lingham-Soliar 2005a, b). In the dorsal and caudal fins of Jurassic ichthyosaurs, bending stresses on the vertical plane are compensated by a mesh of lefthand and righthand bundles of fibres, while bending stresses on the lateral plane are contrasted by fibres that run parallel, or at an acute angle, with respect to the long axis of the ichthyosaur body (Clark and Cowey 1958; Wainwright et al. 1976; Lingham-Soliar 2005a; Lingham-Soliar and Plodowski 2007), and are mainly present at the base and at the tip of the dorsal fin where major torsional stresses are applied.
In BES SC 1000 the preserved portion of the dorsal fin shows a large number of dermal fibre bundles that are tightly grouped together; in the dorsal lobe of the caudal fin, arrays of fibres oriented parallel to the body axis are clearly detectable and follow the pattern described for Jurassic ichthyosaurs, whereas the presence of right/left hand oriented fibres cannot be ascertained. However, the fibres parallel to the body axis of BES SC 1000 are straight as in Stenopterygius, a feature that was considered indicative of a high degree of fibre tension and fin stiffness (Lingham-Soliar and Plodowski 2007). A stiff dorsal fin is essential to stabilize the body during swimming, reducing yaw and roll, and a lobed caudal fin contributes to increase propulsive thrust and fast start acceleration. In extant fast swimming sharks, the main component of stiffness of the control surfaces of the fins is represented by bundles of straight collagen fibres (Lingham-Soliar 2005a, b) and, since straight collagen fibres have a very small stretch when under tension, they represent the main anatomical structure giving stiffness to the fins. Mixosaurus cornalianus shows a dermal architecture similar to Early Jurassic taxa, indicating that this exaptation evolved earlier in the ichthyosaur lineage than high-speed pelagic swimming.
Mixosaurus cornalianus shares some of the characters listed by Webb (1984) as typical of BCF (body/caudal fin) periodic swimmers, that are able to perform sustained swimming, such as a relatively stiff and streamlined anterior body and a large anterior depth and mass, a narrow caudal body region, and fins relatively small with respect to body size. The position of the dorsal fin in Mixosaurus (Fig. 8A) is intriguing since it is placed more cranially than in Jurassic ichthyosaurs and most odontocete cetaceans, somewhat similar to some carchariniform sharks (e.g., the small tail shark, Carcharinus porosus, Fig. 8B). The fin is placed over the base of the pectoral fins, in correspondence to the deepest point of the body, as it occurs, albeit not exclusively, in “sustained swimmers” (Fletcher et al. 2014). On the other hand, Mixosaurus lacked both a lunate tail of high aspect ratio maximising thrust, and a narrow caudal peduncle providing quick oscillation and allowing locally large amplitude displacements, and control of the attack angle. These features that characterize thunniform swimmers appear in Jurassic ichthyosaurs; thus the swimming speed of Mixosaurus was certainly lower. Nevertheless, the stiff dorsal fin acting as a stabilizer might have improved the swimming abilities of Mixosaurus, allowing it to approach those of some carchariniform sharks with a deeply heterocercal tail.
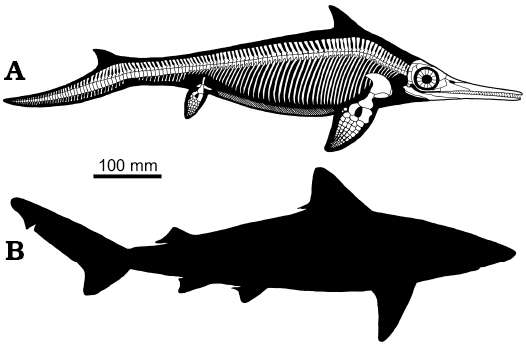
Fig. 8. Reconstruction of the skeleton and body outline of mixosaurid ichthyosaur Mixosaurus cornalianus with the dorsal fin and the dorsal lobe of the caudal fin, as preserved in BES SC 1000 (A) compared to the body outline of the small tail shark Carcharinus porosus (B), which average length is similar to that of Mixosaurus.
Conclusions
The evidence that Mixosaurus cornalianus possessed a dorsal and a caudal fin (Fig. 8), both anatomically well-defined and mechanically efficient, compels a re-examination of evolutionary trends in mixosaurian-grade ichthyosaurs, as well as a reconsideration of their swimming capabilities, as recent research on the matter (e.g., Gutarra et al. 2019) has often used a model that now must be considered definitely obsolete. The dietary preferences of the two specimens examined in this paper also support the idea that M. cornalianus was an efficient open water swimmer, exploiting BCF (body/caudal fin) periodic propulsion (sensu Webb 1984).
The fossilization of portions of the viscera is also remarkable. Traces of fossilised internal organs have been described almost exclusively in Early Jurassic specimens from Holzmaden (Keller 1976; Böttcher 1989, 1990; Lindgren et al. 2018). Pending a definitive organ characterization, BES SC 1001 is certainly the first documented case in the ichthyosaurs of the Besano Formation.
Acknowledgements
We thank Giorgio Teruzzi (MSNM), who directed the Sasso Caldo excavations for several years, and the volunteers of the former “Gruppo paleontologico di Besano”, who unearthed the specimens studied here, and many other exceptional fossils. Some members of this team passed away and cannot read this article, which is thus in memory of Piero Conca, Claudio del Prato, and Sergio Rampinelli. We also thank Michele Zilioli (MSNM) for SEM analysis and macrophotography; the Soprintendenza Archeologica della Lombardia for permissions; Marco Balini and Gabriele Bindellini (both Università degli Studi di Milano, Milano, Italy) for useful discussion; Erin Maxwell (SMNS) and an anonymous referee for constructive criticism, which improved the quality of this paper.
References
Argyriou, T., Calss, M., Maxwell, E., Furrer, H., and Sánchez-Villagra, M. 2016. Exceptional preservation reveals gastrointestinal anatomy and evolution in early actinopterygian fishes. Scientific Reports 6: 18758. Crossref
Benton, M.J., Zhang, Q., Hu, S., Chen, Z.Q., Wen, W., Liu, J., Huang, J., Zhou, C., Xie, T., Tong, J., and Choo, B. 2013. Exceptional vertebrate biotas from the Triassic of China, and the expansion of marine ecosystems after the Permo-Triassic mass extinction. Earth Sciences Review 125: 199–243. Crossref
Bernasconi, S.M. 1994. Geochemical and microbial controls on dolomite formation in anoxic environments: A case study from the Middle Triassic (Ticino, Switzerland). Contributions to Sedimentology 19: 1–109.
Bindellini, G., Balini, M., Teruzzi, G., and Dal Sasso, C. 2019. Ammonoid and Daonella zonation of the Sasso Caldo quarry (Besano Formation, Middle Triassic). Strati 2019 Congress (Abstract book): 14.
Böttcher, R. 1989. Über die Nahrung eines Leptopterygius (Ichthyosauria, Reptilia) aus dem süddeutschen Posidonienschiefer (Unterer Jura) mit Bemerkungen über den Magen der Ichthyosaurier. Stuttgarter Beiträge zür Naturkunde, Serie B (Geologie und Paläontologie) 155: 1–19.
Böttcher, R. 1990. Neue Erkenntnisse über die Fortpflanzungsbiologie der Ichthyosaurier (Reptilia). Stuttgarter Beiträge zur Naturkunde, Serie B (Geologie und Paläontologie) 164: 1–51.
Brack, P. and Rieber, H. 1986. Stratigraphy and ammonoids of the lower Buchenstein beds of the Brescian Prealps and Giudicarie and their significance for the Anisian/Ladinian boundary. Eclogae Geologicae Helvetiae 79:181–225
Brack, P. and Rieber, H. 1993. Towards a better definition of theAnisian/Ladinian boundary: New biostratigraphic data and correlations of boundary sections from the Southern Alps. Eclogae Geologicae Helvetiae 86: 415–527.
Brack, P., Rieber, H., Nicora, A., and Mundil, R. 2005. The global boundary stratotype section and point (GSSP) of the Ladinian Stage (Middle Triassic) at Bagolino (Southern Alps, Northern Italy) and its implications for the Triassic time scale. Episodes 28: 233–244. Crossref
Briggs, D.E.G. and Wilby, P.R. 1996. The role of the calcium carbonate-calcium phosphate switch in the mineralization of soft-bodied fossils. Journal of the Geological Society 153: 665–668. Crossref
Brinkmann, W. 1996. Ein Mixosaurier (Reptilia, Ichthyosauria) mit Embryonen aus der Grenzbitumenzone (Mitteltrias) des Monte San Giorgio (Schweiz, Kanton Tessin). Eclogae Geologicae Helvetiae 89: 1321–1344.
Brinkmann, W. 1997. Die Ichthyosaurier (Reptilia) aus der Mittletrias des Monte San Giorgio (Tessin, Schweiz) und von Besano (Lombardei, Italien) – der aktuelle Forschungsstand. Vierteljahrsschrift der Naturforschenden Gesellschaft in Zürich 142: 68–78.
Brinkmann, W. 1998. Die Ichthyosaurier (Reptilia) aus der Grenzbitumenzone (Mitteltrias) des Monte San Giorgio (Tessin, Schweiz) – neue Ergebnisse. Vierteljahrsschrift der Naturforschenden Gesellschaft in Zürich 143 (4): 165–177.
Brinkmann, W. 2004. Mixosaurier (Reptilia, Ichthyosauria) mit Quetschzähnen aus der Grenzbitumenzone (Mitteltrias) des Monte San Giorgio (Schweiz, Kanton Tessin). Schweizerische Paläontologische Abhandlungen 124: 1–86.
Bürgin, T., Rieppel, O., Sander, P. M., and Tschanz, K. 1989. The fossils of Monte San Giorgio. Scientific American 260 (6): 74–81. Crossref
Cavin, L., Furrer, H., and Obrist, C. 2013. New coelacanth material from the Middle Triassic of eastern Switzerland, and comments of the taxic diversity of actinistians. Swiss Journal of Geosciences 106: 161–177. Crossref
Clark, R.B. and Cowey, J.B. 1958. Factors controlling the change of shape of certain nemertean and turbellarian worms. Journal of Experimental Biology 35: 731–748.
Curioni, G. 1847. Cenni sopra un nuovo saurio fossile dei monti di Perledo sul Lario e sul terreno che lo racchiude. Giornale del Reale Instituto Lombardo di Scienze, Lettre ed Arti 16: 157–170.
Dal Sasso, C. and Maganuco, S. 2011. Scipionyx samniticus (Theropoda: Compsognathidae) from the Lower Cretaceous of Italy. Osteology, ontogenetic assessment, phylogeny, soft tissue anatomy, taphonomy and paleobiology. Memorie della Società Italiana di Scienze Naturali e del Museo di Storia Naturale di Milano 37: 1–282.
Dal Sasso, C. and Pinna, G. 1996. Besanosaurus leptorhynchus n. gen. n. sp., a new shastasaurid ichthyosaur from the Middle Triassic of Besano (Lombardy, N. Italy). Paleontologia Lombarda 4: 3–23.
Dick, D., Schweigert G., and Maxwell E. 2016. Trophic niche ontogeny and palaeoecology of early Toarcian Stenopterygius (Reptilia: Ichthyosauria). Palaeontology 59: 423–431. Crossref
Druckenmiller, P.S., Kelley N., Whalen M.T., McRoberts C., and Carter, J.G. 2014. An Upper Triassic (Norian) ichthyosaur (Reptilia, Ichthyopterygia) from Northern Alaska and dietary insight based on gut contents. Journal of Vertebrate Paleontology 34: 1460–1465. Crossref
Etter, W. 2002. Monte San Giorgio: Remarkable Triassic marine vertebrates. In: D.J. Bottjer, W. Etter, J.W. Hagdorn, and C.M. Tang (eds.), Exceptional Fossil Preservation. A Unique View on the Evolution of Marine Life, 220–242. Columbia University Press, New York.
Ferrante, C., Martini, R., Furrer, H., and Cavin, L. 2017. Coelacanths from the Middle Triassic of Switzerland and the pace of actinistian evolution. Research and Knowledge 3 (2): 59–62.
Fletcher, T., Altringham, J., Peakall, J., Wignall, P., and Dorrell, R. 2014. Hydrodynamics of fossil fishes. Proceedings of the Royal Society B 281: 20140703. Crossref
Fraas, E. 1892. Ueber einen neuen Fund von Ichthyosaurus in Württemberg. Neues Jahrbuch für Mineralogie, Geognosie, Geologie und Petrefaktenkunde 1892: 87–90.
Furrer, H. 1995. The Kalkschieferzone (Upper Meride Limestone;Ladinian) near Meride (Canton Ticino, Southern Switzerland) and the evolution of a Middle Triassic intraplatform basin. Eclogae Geologicae Helvetiae 88: 827–852.
Furrer, H. 2003. Der Monte San Giorgio im Südtessin-vom Berg der Saurier zur fossil-Lagerstätte internationaler Bedeutung. Neujahrsblatt der Naturforschenden Gesellschaft in Zürich 206: 1–64.
Gutarra, S., Moon, B.C., Rahman, I.A., Palmer, C., Lautenschlager, S., Brimacombe, A.J., and Benton, M.J. 2019. Effects of body plan evolution on the hydrodynamic drag and energy requirements of swimming in ichthyosaurs. Proceedings of the Royal Society B 286: 20182786. Crossref
Ji, C., Jiang, D.Y., Motani, R., Rieppel, O., Hao W.C., and Yu, Z. 2016. Phylogeny of the Ichthyopterygia Incorporating Recent Discoveries from South China. Journal of Vertebrate Paleontology 36: e1025956. Crossref
Jiang, S., Schmitz, D.L., Hao, W., and Sun Y. 2006. A new mixosaurid ichthyosaur from the Middle Triassic of China. Journal of Vertebrate Paleontology 26: 60–69. Crossref
Keller, T. 1976. Magen- und Darminhalte von Ichthyosauriern des süddeutschen Posidonienshiefers. Neues Jahrbuch für Geologie und Paläontologie Monatshefte 1976: 253–320.
Koot, M.B., Cuny, G., Orchard, M., Richoz, S., Hart, M.B., and Twitchett, R.J. 2015. New hybodontiform and neoselachian sharks from the Lower Triassic of Oman. Journal of Systematic Paleontology 13: 891–917. Crossref
Kuhn-Schnyder, E. 1974. Neujahrsblatt, Die Triasfauna der Tessiner Kalkalpen. 119 pp. Neujahrsblatt der Naturforschenden Gesellschaft, Zurich.
Laurent, C. 2018. Micromechanics of Ligaments and Tendons. In: J.-F. Ganghoffer (ed.), Multiscale Biomechanics, 489–509. Elsevier, Amsterdam. Crossref
Lindgren, J., Sjövall, P., Thiel, V., Zheng, W., Ito, S., Wakamatsu, K., Hauff, R., Kear, B.P., Engdahl, A., Alwmark, C., Eriksson, M.E., Jarenmark, M., Sachs, S., Ahlberg, P.E., Marone, F., Kuriyama, T., Gustafsson, O., Malmberg, P., Thomen, A., Rodríguez-Meizoso, I., Uvdal., P., Ojika, M., and Schweitzer, M.H. 2018. Soft-tissue evidence for homeothermy and crypsis in a Jurassic ichthyosaur. Nature 564: 359–365. Crossref
Lingham-Soliar, T. 2005a. Caudal fin in the white shark, Carcharodon carcharias (Lamnidae): a dynamic propeller for fast, efficient swimming. Journal of Morphology 264: 233–252. Crossref
Lingham-Soliar, T. 2005b. Dorsal fin in the white shark Carcharodon carcharias: a dynamic stabilizer for fast swimming. Journal of Morphology 263: 1–11. Crossref
Lingham-Soliar, T. and Plodowski, G. 2007. Taphonomic evidence for high-speed adapted fins in thunniform ichthyosaurs. Naturwissenschaften 94: 65–70. Crossref
Maisch, M.W. and Matzke, A.T.1997. Observations on Triassic ichthyosaurs. Part I: Structure of the palate and mode of tooth implantation in Mixosaurus cornalianus (Bassani, 1886). Neues Jahrbuch für Geologie und Palaontologie Monatshefte 1997 (12): 717–732. Crossref
Maisch, M.W. and Matzke, A.T. 2000. The Ichthyosauria, Stuttgarter. Beiträge für Naturkunde B 298: 1–159.
Martill, D.M., Wilby, P.R., and Williams, N. 1992. Elemental mapping: a technique for investigating delicate phosphatized fossil soft tissues. Palaeontology 35: 869–874.
Maxwell, E.E., Furrer, H., and Sánchez-Villagra, M. 2013. Exceptional fossil preservation demonstrates a new mode of axial skeleton elongation in early ray-finned fishes. Nature Communications 4: 2570. Crossref
Mears, E.M., Rossi, V., MacDonald, E., Coleman, G., Davies, T.G., Arias-Riesgo, C., Hildebrandt, C., Thiel, H., Duffin, C.J., Whiteside, D.I., and Benton, M.J. 2016. The Rhaetian (Late Triassic) vertebrates of Hampstead Farm Quarry, Gloucestershire, UK. Proceedings of the Geologists’ Association 127: 478–505. Crossref
Motani, R. 1999. The skull and taxonomy of Mixosaurus (Ichthyopterygia) Journal of Paleontology 73: 924–935. Crossref
Motani, R., You, H., and McGowan, C. 1996. Eel-like swimming in the earliest ichthyosaurs. Nature 382: 347–348. Crossref
Muhl, Z.F., Grimm, A.F., and Glick, P. 1976. Technique for measurements of sarcomere length of striated muscle. Journal of Dental Research 55: 170. Crossref
Mundil, R., Palfy, J., Renne, P.R., and Brack, P. 2010. The Triassic time scale: new constraints and a review of geochronological data. In: S.G. Lucas (ed.), The Triassic Timescale. Geological Society, London, Special Publications 334: 41–60. Crossref
Nosotti, S. 2007. Tanystropheus longobardicus (Reptilia, Protorosauria): re-interpretations of the anatomy based on new specimens from the Middle Triassic of Besano (Lombardy, Northern Italy): Memorie della Società Italiana di Scienze Naturali, e Museo Civico di Storia Naturale Milano 35: 1–88.
Noto, C. 2009. The potential utility of authigenic minerals on modern and fossil bones for environmental and taphonomic analysis. Journal of Vertebrate Paleontology 29 (Supplement 3): 156A.
Peyer, B. 1937. Die Triasfauna der Tessiner Kalkalpen. XII. Macrocnemus bassanii Nopcsa. Schweizerische Paläontologische Abhandlungen 59: 1–140.
Pollard, J.E. 1968. The gastric contents of an ichthyosaur from the Lower Lias of Lyme Regis, Dorset. Palaeontology 11: 376–388.
Renesto, S. 2005. A new specimen of Tanystropheus (Reptilia Protorosauria) from the Middle Triassic of Switzerland and the ecology of the genus: Rivista Italiana di Paleontologia e Stratigrafia 111: 377–394.
Renesto, S. 2010. A new specimen of Nothosaurus from the latest Anisian (Middle Triassic) Besano Formation (Grenzbitumenzone) of Italy. Rivista Italiana di Paleontologia e Stratigrafia 116: 45–160.
Renesto, S. and Avanzini, M. 2002. Skin remains in a juvenile Macrocnemus bassanii Nopcsa (Reptilia, Prolacertiformes) from the Middle Triassic of northern Italy. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 224: 31–48.
Renesto, S. and Saller, F. 2018. Evidences for a semi aquatic life style in the Triassic diapsid reptile Tanystropheus. Rivista Italiana di Paleontologia e Stratigrafia 124: 23–34.
Renesto, S. and Stockar, R. 2009. Exceptional preservation of embryos in the actinopterygian Saurichthys from the Middle Triassic of Monte San Giorgio, Switzerland. Swiss Journal of Geosciences 102: 323–330. Crossref
Renesto, S. and Stockar, R. 2018. First record of a coelacanth fish from the Middle Triassic Meride Limestone of Monte San Giorgio (Canton Ticino, Switzerland). Rivista Italiana di Paleontologia e Stratigrafia 124: 639–653.
Renesto, S., Binelli, G., and Hagdorn, H. 2014. A new pachypleurosaur from the Middle Triassic Besano Formation of Northern Italy. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 271: 151–168. Crossref
Rieber, H.R. 1970. Phragmoteuthis? ticinensis n. sp., ein Coleoidea-Rest aus der Grenzbitumenzone (Mittlere Trias) des Monte San Giorgio (Kt. Tessin, Schweiz). Paläontologische Zeitschrift 44: 32–40. Crossref
Rieppel, O. 1980. A new coelacanth from the Middle Triassic of Monte San Giorgio, Switzerland. Eclogae Geologicae Helvetiae 73: 921–939.
Rieppel, O. 1985. A second actinistian from the Middle Triassic of Monte San Giorgio, Kt. Tessin, Switzerland. Eclogae Geologicae Helvetiae 78: 707–713.
Röhl, H.J., Schmid-Röhl, A., Furrer, H., Frimmel, A., Oschmann, W., and Schwark, L. 2001 Microfacies, geochemistry and palaeoecology of the Middle Triassic Grenzbitumenzone from Monte San Giorgio (Canton Ticino, Switzerland). Geologia Insubrica 6: 1–13.
Sagemann, J., Bale, S.J, Briggs, D.E.G., and Parkes, R.J. 1999. Controls on the formation of authigenic minerals in association with decaying organic matter: an experimental approach. Geochimica Cosmochimica Acta. 63: 1083–1095. Crossref
Sander, P.M. 1989. The large ichthyosaur Cymbospondylus buchseri, sp. nov., from the Middle Triassic of Monte San Giorgio (Switzerland), with a survey of the genus in Europe. Journal of Vertebrate Paleontology 9: 163–173. Crossref
Smithwick, F.M., Mayr, G., Saitta, E.T., Benton, M.J., and Vinther, J. 2017. On the purported presence of fossilised collagen fibres in an ichthyosaur and a theropod dinosaur. Palaeontology 60: 409–422. Crossref
Stockar, R., Baumgartner, P.O., and Condon, D. 2012. Integrated Ladinian bio-chronostratigraphy and geochrononology of Monte San Giorgio (Southern Alps, Switzerland). Swiss Journal of Geosciences 105: 85–108. Crossref
Vitt, L.J. and Caldwell, J.P. 2009. Herpetology: An Introductory Biology of Amphibians and Reptiles. Third Edition. 697 pp. Academic Press, Burlington. Crossref
Wainwright, S.A., Biggs, W.D., Currey, J.D., and Gosline, J.M. 1976. Mechanical Design in Organisms. 423 pp. E Arnold, London.
Webb, P.W. 1984. Form and function in fish swimming. Scientific American 251 (1): 72–82. Crossref
Wirz, A. 1945. Die Triasfauna der Tessiner Kalkalpen. XV. Beiträge zur Kenntnis des Ladinikums im Gebiete desMonte San Giorgio. Schweizerische Paläontologische Abhandlugen 65: 1–84.
Acta Palaeontol. Pol. 65 (3): 511–522,
2020 https://doi.org/10.4202/app.00731.2020