


Research history, taphonomy, and age structure of a mass accumulation of the ornithopod dinosaur Dysalotosaurus lettowvorbecki from the Upper Jurassic of Tanzania
TOM R. HÜBNER, CHRISTIAN FOTH, WOLF-DIETER HEINRICH, DANIELA SCHWARZ, and ROBERT BUSSERT
Hübner, T.R., Foth, C., Heinrich, W.-D., Schwarz, D., and Bussert, R. 2021. Research history, taphonomy, and age structure of a mass accumulation of the ornithopod dinosaur Dysalotosaurus lettowvorbecki from the Upper Jurassic of Tanzania. Acta Palaeontologica Polonica 66 (2): 275–300.
The region around Tendaguru Hill in southeastern Tanzania has provided a rich, Late Jurassic dinosaur fauna. Among them, thousands of bones of the basal iguanodontian ornithopod Dysalotosaurus lettowvorbecki have been excavated in the Ig/WJ-quarry northwest of the Tendaguru Hill. The incomplete record of the taphonomic information from the excavation is the main reason for the still inconclusive interpretation of the assemblage as either catastrophic or attritional in origin. In this study, all available historical notes, sketches and field catalogues were analysed and combined with data from CT scans, new taphonomic observations and from bone histological as well as demographic analyses. According to these combined results, the mass accumulation of remains of D. lettowvorbecki consists of four closely associated bonebeds in at least three different stratigraphic levels. The bonebeds are classified as mixed, multitaxic, and monodominant. There is no evidence of abrasion and preburial weathering on the bones suggesting minimal transport distances and duration as well as short preburial exposure times. Although the size/age distributions are bimodally U-shaped, the bonebeds are interpreted as catastrophic in origin due to the restricted spatial extent and the overall uniform preservation of the bones. It is proposed that different herds of D. lettowvorbecki crossed a tidal channel several times during seasonal migration and that each time several to dozens of individuals died. The bodies decomposed and were buried at a barrier or a bend nearby. The demographic conditions as well as the modes of death and burial of D. lettowvorbecki are very similar to the observations made from the modern blue wildebeest in the Masai-Mara Reserve in Kenya.
Key words: Dinosauria, Dysalotosaurus lettowvorbecki, history, demography, taphonomy, Jurassic, Tendaguru, Africa.
Tom R. Hübner [huebner@stiftung-friedenstein.de], Stiftung Schloss Friedenstein Gotha, Schlossplatz 1, 99867 Gotha, Germany.
Christian Foth [christian.foth@gmx.net], Université de Fribourg, Department of Geosciences, Chemin du Musée 6, 1700 Fribourg, Switzerland.
Wolf-Dieter Heinrich [wolf-dieter.heinrich@mfn.berlin] and Daniela Schwarz [Daniela.Schwarz@mfn.berlin], Museum für Naturkunde, Leibniz-Institut für Evolutions- und Biodiversitätsforschung, Invalidenstraße 43, 10115 Berlin, Germany.
Robert Bussert [r.bussert@tu-berlin.de], Technische Universität Berlin, Institut für Angewandte Geowissenschaften, Ernst-Reuter-Platz 1, 10587 Berlin, Germany.
Received 11 October 2019, accepted 21 September 2020, available online 7 June 2021.
Copyright © 2021 T.R. Hübner et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
In 1906, the mining engineer Bernhard Sattler reported on gigantic pieces of bone found at the foot of Tendaguru Hill, which is approximately 60 km from the port of Lindi in southeastern Tanzania (Bussert et al. 2009; Stoecker 2019). One year later Eberhard Fraas visited the site and recovered several dinosaur bones, which he named and described shortly afterwards (Fraas 1908). Between 1909 and 1913, the Museum für Naturkunde (Berlin) organized a large-scale campaign, the so-called German Tendaguru Expedition (GTE), which was documented by Janensch (1914a), Maier (2003), and Heumann et al. (2019). During four seasons of field work 235 metric tons of fossil material was excavated, packed, and shipped to Germany (Maier 2003).
The locality Ig/WJ is one of numerous important dinosaur sites in the hinterland of Lindi (East Africa, southeastern Tanzania), which is located approximately 2.3 km to the north-northwest of Tendaguru Hill close to Kindope village (Fig. 1). In contrast to many other dinosaur localities in the surroundings of Tendaguru Hill, which mainly yielded skeletal remains of sauropods and the stegosaur Kentrosaurus, the site Ig/WJ mainly produced bones and teeth of the small to medium-sized basal iguanodontian ornithopod dinosaur Dysalotosaurus lettowvorbecki. The excavations at the site Ig/WJ were carried out under the direction of Werner Janensch and Edwin Hennig (1909–1911), Hans von Staff (1911), and Hans Reck (1912–1913), who was supported by his wife Ina, née von Grumbkow (Maier 2003). In 1913, more than 14 000 catalogue numbers were assigned at this locality. In 1927, the British Museum East Africa Expedition also carried out excavations at the Ig/WJ-site and sent up to 17 crates of material back to the Natural History Museum in London (Maier 2003: 209, 210).
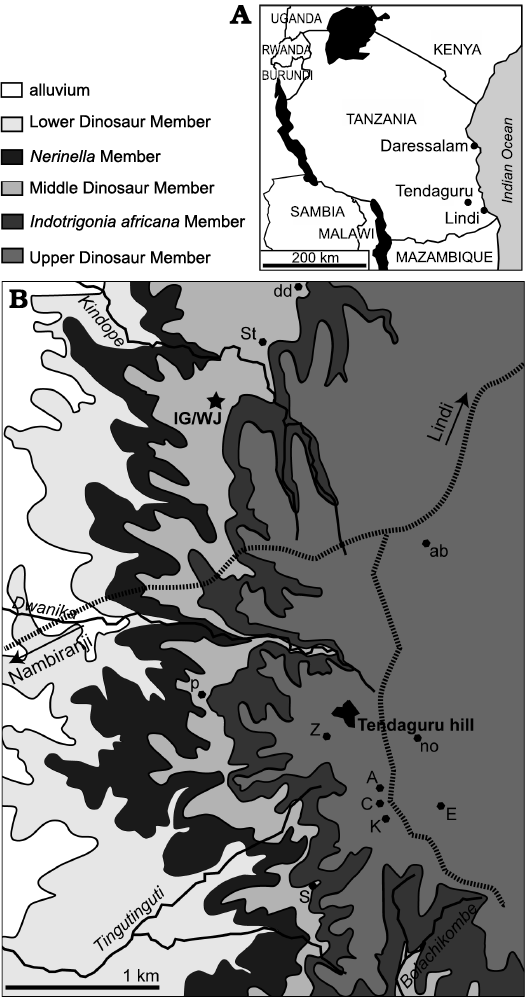
Fig. 1. Location of the Ig/WJ-locality. A. Position of the Tendaguru locality in Tanzania, redrawn from Google Maps and on the basis of locality information of Aberhan et al. (2002). B. Geological map of the Tendaguru area with main stratigraphic units, the position of quarry Jg/WJ is marked with an asterisk, and some other important quarries from the German Tendaguru Expedition (1909–1913) are labelled with their respective letters. Roads are marked by dashed lines. Data are from Janensch (1925b), Heinrich (1999b), and Aberhan et al. (2002). The names of stratigraphic units are from Bussert et al. (2009).
Unfortunately, the reconstruction of the Ig/WJ-quarry excavations is adversely affected by incomplete field records. Substantial parts of these records as well as other documents were lost in Berlin during World War II (WWII), along with already prepared material of D. lettowvorbecki. As a consequence, the primary description of D. lettowvorbecki by Janensch (1955) was based on material that was prepared after WWII. Janensch (1955) described the taxon primarily from isolated material while Galton (1983: 232) designated the skull “dy A” (see below for acronym meaning) as the neotype.
Large numbers of specimens from D. lettowvorbecki were concentrated in several distinct bonebeds at the locality. The reasons for these accumulations have had very different interpretations over the years thereafter. Originally, Janensch (1914b) proposed a mass mortality event of a single herd, basing his view mainly on the mass accumulation of numerous individuals of different ages. He further suggested that there was almost no post-mortem transport of bones due to their excellent preservation and the restricted spatial extent of the bonebeds. According to Janensch (1914b), the adjusted orientation of many long bones was the result of wave action and the high degree of disarticulation the result of a reworking event (strong waves or a tide), which had reopened the first taphocoenosis after the soft parts of the bodies had already decayed (Janensch 1914b). Reck (1925) challenged this view as no evidence for storm deposits or other disturbed sediment layers that would indicate catastrophic events were documented. According to Reck (1925), the sediments maintained a fine clastic character throughout the profile, and the bones were irregularly distributed vertically. Russell et al. (1980) interpreted the mass accumulation of D. lettowvorbecki as the result of attritional mortality during lengthy periods of drought, where the animals had concentrated in areas of more plentiful vegetation and subsequently died en mass by overexploitation. The remains then accumulated through hydraulic flow in concentrated bonebeds once more humid conditions had returned. Heinrich (1999b) also favored the attritional interpretation based on the bimodally U-shaped size distribution of the femora of D. lettowvorbecki, indicating high mortality peaks in juveniles and adults, respectively (Lyman 1994). In addition, an attritional scenario fits the general taphonomic conditions of the sauropod fossil localities from Tendaguru (Heinrich 1999b). Finally, Hübner (2011, 2012) followed the interpretation by Janensch (1914b) that the Ig/WJ-locality resulted from a mass death event of a single D. lettowvorbecki herd. Thus, the taphonomic origin of the Ig/WJ-locality is still not resolved.
The current study collected all available data, including preserved historical notes, sketches, and specimen catalogues. In addition, data derived from new sedimentologic, taphonomic, and demographic analyses incorporating CT scans of yet-unopened packages and recent histological results (Hübner 2012) were also utilised. With these data, we were able to revise the taphonomy and genesis of the Ig/WJ-locality, resulting in a novel interpretation of the D. lettowvorbecki bone assemblage.
Institutional abbreviations.—GPIT, Geologisch-Paläontologisches Institut der Eberhard-Karls-Universität Tübingen, Germany; GZG, Geowissenschaftliches Zentrum der Georg-August-Universität Göttingen, Germany; IZW, Leibniz-Institute for Zoo and Wildlife Research, Berlin, Germany; MfN, Museum für Naturkunde Berlin, Germany; NHMUK, Natural History Museum in London, UK; SMNS, Staatliches Museum für Naturkunde, Stuttgart, Germany.
Other abbreviations.—BB, bonebed; Ig/WJ, excavation site in Tendaguru; GTE, German Tendaguru Expedition; MDM, Middle Dinosaur Member; WWII, World War II.
Material and methods
Excavation records and skeletal remains.—The reconstruction of the course of the excavations at site Ig/WJ is based on published data (e.g., von Branca 1914; Janensch 1914a, b, 1955; Reck 1925; Maier 2003) and unpublished records kept in the Museum für Naturkunde (Berlin). The latter include the GTE field catalogue 1909–1911 (W. Janensch, 1909–1911. GTE field catalogue, Pal. Mus SII, Tendaguru-Expedition 8.5), copies of field diaries of Hennig (1909–1911), handwritten progress reports and letters of Janensch (1909–1911) and Reck (1912–1913, 1914), and the GTE field catalogue 1912–1913 (H. Reck, 1912–1913. GTE field catalogue, Pal. Mus SII, Tendaguru-Expedition 9.3). Further records include sketchbooks with stratigraphic profiles of the Ig/WJ-site, individual pencil and ink drawings of fossil finds, sketches of partial or fairly complete skeletons, and water colours of individual skeletal elements of Dysalotosaurus lettowvorbecki made by Hans Reck and/or his wife Ina in 1912. In addition, copies of Hans Reck’s photos from 1923 and oil paintings made by his wife in 1912 give further impressions of the extent of the excavations at the Ig/WJ-site (Fig. 2). Moreover, data from collection catalogues (MfN, GPIT) were used to reconstruct the locality, including the extent of bonebeds and their spatial relationships.

Fig. 2. Oil painting by Ina Reck (1912), which depicts the excavations at the Ig/WJ-site (from MfN collections, PM_B_VII_9_Reck).
Our observations on the preservation states of specimens as well as their size classes and age structures are based on thousands of specimens housed in Berlin (MfN), Göttingen (GZG), Munich (BSPG), Stuttgart (SMNS), Tübingen (GPIT), and London (NHMUK). In addition, 41 original and unopened bamboo transport corsets in the MfN collection were taken into account, which according to their labeling are filled exclusively with material from quarry Ig/WJ (Fig. 3A–C). As the corsets are still unpacked, a direct assignment of the original field numbers to the specimens was not possible. Unfortunately, no inventory or packing lists exist from the material. Thanks to a scientific cooperation between the MfN and the IZW, however, in 2010 all 41 bamboo corsets were examined using computed tomography (CT) (Fig. 3D). The aim of the CT analysis was to identify the bones stored in the corsets and to gain further information on the taphonomy of the quarry Ig/WJ. CT data were acquired using a high-resolution Multislice CT scanner (Aquilion CX from Toshiba) at the IZW in Berlin. For each bamboo corset, a separate 134 spiral scan with a 0.5 mm interval was made and saved as a DICOM image stack. CT images were taken with a setting of 135 kV and 250 mA. Data reconstruction (bone algorithm) was done with VITREA (Vitrea 2, Version 4.1.2.0, Vital Images, Inc, Minnetonka, Minnesota, USA) and Osirix (Osirix V 3.9.4, 64 Bit) workstations.

Fig. 3. Original, unopened bamboo corsets containing bones of ornithopod dinosaur Dysalotosaurus lettowvorbecki Pompeckj, 1920, from Kimmeridgian, Late Jurassic of Tendaguru, Tanzania, which are housed in the collection of fossil reptiles at the MfN. A. Stored as a stack. B. Bamboo corset in lateral view. C. Showing the labelling on the front side. The bamboo corsets are labelled with the quarry numbers and field numbers as assigned to single fossil blocks. Reference to specimens is not possible, because most of them are unprepared sediment blocks. D. CT slice exposing cross-section through bamboo corset Ig 88, bones are in white whereas lighter materials such as clay, cushioning with grass, and bamboo sticks are displaying around.
The bones were studied using CT section images of the bamboo corsets, by applying either a “bone” or “muscle” filter. Successive virtual deletion of materials allowed visualisation of individual bones, and 3D volume rendering helped to display bones with and without matrix for detailed study. Whereas some of the bamboo corsets contained boxes with multiple contents (e.g., tin cans with many smaller bones, assemblages of vertebrae), most of them revealed a high number of well-preserved bones (vertebrae, pelvic bones, femora, tibiae, metapodials, etc.) of D. lettowvorbecki. All bamboo corsets contained only partially prepared specimens still surrounded by matrix and a protective hull of clay. Corsets and clay jackets often contained clusters of similar bones, e.g., long bones such as femora and tibiae (Fig. 4). The bones in the bamboo corsets thus appeared to have been sorted during packing, particularly into long bones, vertebrae, small unidentified bones and bone fragments. The content in the bamboo corsets might not have reflected the original context and relationships of the bones at the time of their discovery. In 35 of the 41 bamboo corsets studied, hindlimb elements unambiguously belonging to D. lettowvorbecki were identified, including the remains of 27 femora. Some of these bones were distorted or strongly damaged, making correct measurements difficult. Nonetheless, the distal widths of 14 femora were measured (Fig. 4B) and added to the dataset of sizes. The measurements were made directly in the program Oxirix, using the “measurements” tool. For this purpose, the specimens were always oriented in the same view (i.e., from ventrally/distally) to obtain standard dimensions. In addition, the corsets also contained the caudal vertebrae of Kentrosaurus and a cervical rib and a neural arch of Giraffatitan (Brachiosaurus). These specimens corresponded well with the remains of other dinosaur taxa known from Ig/WJ.
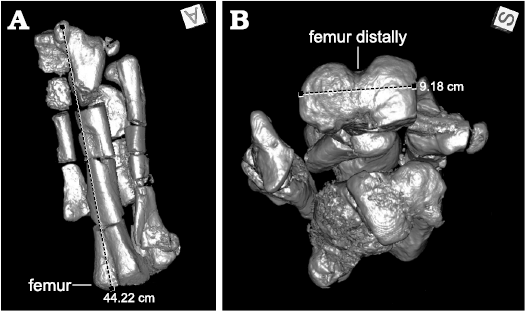
Fig. 4. Measurements of a right femur of ornithopod dinosaur Dysalotosaurus lettowvorbecki Pompeckj, 1920, from Kimmeridgian, Late Jurassic of Tendaguru, Tanzania, visualized from a CT scan of the bamboo corset Ig 133, using the line measurement tool in Osirix. A. Dashed line marks maximum length of femur. B. Dashed line marks distal width of femur (see also Material and methods).
Sedimentology.—The sedimentological findings in this study were based on sedimentological fieldwork in the Tendaguru region in 2000, which included detailed logging of sections of the Tendaguru Formation in the Tingutinguti and Dwanika rivers west of the Tendaguru Hill and the parallel collection of samples. Furthermore, an attempt was made to correlate still recognizable fossil sites of the GTE with these sections. The sediment samples were analysed mineralogically and petrographically at the Technische Universität Berlin using X-Ray Diffraction and thin section analysis. In addition, sediment samples from the Ig/WJ-fossil site, which are still stored in the MfN, were examined using the same methods.
Taphonomic analysis.—Due to the method of block-wise excavation of specimens, originally only a few individual bones were labeled directly with Ig or WJ numbers. Thus, most of the taphonomic observations on single bones could not be directly assigned to a specific bonebed. However, as the bones that could be assigned to one of the bonebeds (see below) did not show any obvious preservational differences, the description of the taphonomic features were generalized for all specimens.
Information on the taphonomy and orientation of bones within bonebeds could only partially be obtained from the literature, field notes, and correspondence of the excavation leaders. A complete count of skeletal elements and their relative abundance was impossible due to the heavy loss of material during WWII. However, a large amount of unprepared material, which was located in the collections in Stuttgart (SMNS), Tübingen (GPIT), Berlin (MfN), and Göttingen (GZG), fortunately escaped destruction and were later prepared. These specimens became the basis for skeletal mounts and for the comprehensive description of the taxon (Janensch 1950, 1955; Galton 1981, 1983; Hübner and Rauhut 2010; Hübner 2018).
Skeletal elements in the collections in Göttingen (GZG) and Tübingen (GPIT) were counted and compared to each other to test whether they could provide a general pattern for the entire assemblage. Nonparametric Mann-Whitney U and Kolmogorov-Smirnov tests were then applied to these data. These tests are more robust for non-normally distributed data (Hammer and Harper 2006). The Mann-Whitney U test estimates whether or not two univariate samples are taken from populations with equal medians, while the Kolmogorov-Smirnov test estimates whether or not the shapes of two univariate distributions are similar (see Hammer and Harper 2006). In all cases, both data sets were regarded as significantly different if the resulting p values were 0.05 or smaller. In addition, the representative skeletal elements of D. lettowvorbecki were counted as percentages of the minimum number of individuals (MNI) in comparison to the percentage of each element in a complete single individual. These percentages were statistically compared with each other by the Mann-Whitney U and Kolmogorov-Smirnov tests, as described above. Since the individual shapes, densities, and sizes of skeletal elements of D. lettowvorbecki were unequal, the sorting of skeletal elements into respective Voorhies groups (Voorhies 1969; Behrensmeyer 1975) was abandoned (see also Eberth et al. 2007; Britt et al. 2009).
The general terminology of taphonomic modes and composition of specimens followed Eberth et al. (2007) and assignment of bone surfaces to stages of weathering and abrasion followed Behrensmeyer (1975) and Fiorillo (1988), respectively.
Demographic analysis.—The large number of specimens of D. lettowvorbecki representing different ontogenetic stages allowed the distribution d(x) of individuals to be assessed with respect to their specific ages x. For this, we used the histological data of the femora collected by Hübner (2011, 2012) as it provided the highest number of histological samples (N = 19). These data were correlated with a proxy of size to extrapolate the age of the remaining femora. Because Hübner (2011, 2012) sampled only incomplete bones, femur length could not be used as a proxy. Instead, we measured the distal width of the femur as a proxy for size and fitted a regression model between the age and width in the program PAST 3.05 (Hammer et al. 2001; see tables S1 and S2 in SOM, Supplementary Online Material available at http://app.pan.pl/SOM/app66-Hubner_etal_SOM.pdf). For extrapolation, a linear regression (y = 0.2568x – 3.936) was chosen as it had the highest R2 value (= 0.9964) and a strongly significant p value (= 0.0001). Using these tests, we were able to estimate the age of a total number of 228 femora preserving their distal ends. For the prepared material, the distal widths were measured using Vernier calipers while the unprepared femora within the original bamboo corsets were measured from the CT data in Osirix.
The femora were then subdivided into left and right elements and tabulated with respect to their age (see also SOM). The number of left and right elements were dissimilar. The minimum number of individuals was estimated using two different methods: (i) we assumed that the higher number of left or right femora within every age class represented the maximum number of individuals per age class (herein termed “total”); (ii) we assumed that the exact age estimation could be erroneous due to intraspecific and intraskeletal variation in growth, as documented by Hübner (2011, 2012). As a consequence of the second method, individuals of similar age classes varied in size. Depending on the range, this variation can result in a potential overlap between the largest and smallest individuals of two successive age classes. In the histological sample of D. lettowvorbecki, such overlap is present, for instance, between individuals of age four and five. To compensate for this effect of age-specific size variation, the numbers of left and right femora for each age-class, were estimated as three-point moving average, containing the sum of the actual number of elements in age class x, the next-younger (x-1) and next-older (x+1) age class (herein termed “average”) divided by three. The values were rounded and, as in the previous method, the left or right femora with the higher preserved numbers were taken as the total number of individuals for each age class.
Based on these data, the age distribution d(x) was calculated for both methods by assigning the number of estimated individuals per age class and comparing them to each other statistically using Mann-Whitney U and Kolmogorov-Smirnov tests (see above). We additionally tested whether or not the age distributions d(x) of the two major bonebeds (see below) were similar to each other. For the latter test, we assigned the femora to one of the two bonebeds on the basis of historical notes of Werner Janensch and Hans Reck, collections data and the remains of the original labels on the bones (see table S3 in SOM). Due to the underrepresentation of hatchlings and early juvenile specimens (x <3 years) in the assemblage, a calculation of survivorship l(x) was avoided.
Geological setting
The Tendaguru Formation consists of six stratigraphic members (Fig. 5), which are from bottom to top: The Lower Dinosaur Member; Nerinella Member; Middle Dinosaur Member; Indotrigonia africana Member; the Upper Dinosaur Member; and the Rutitrigonia bornhardti-schwarzi Member (Bussert et al. 2009; Fig. 5). The dinosaur members consist predominantly of fine-grained sediments of coastal to tidal plain origins and are the main sources for dinosaur remains (e.g., Janensch 1925b; Heinrich 1999b; Aberhan et al. 2002; Bussert et al. 2009). The intercalating members are dominated by sandstone and were deposited in a shallow-marine lagoon environment (e.g., Aberhan et al. 2002; Bussert et al. 2009; Heinrich et al. 2011). Dinosaur remains are also known from these layers but are less common than in the Middle and Upper Dinosaur members. The age of the three transgression-regression cycles of the Tendaguru Formation reach at least from the Middle Oxfordian (early Late Jurassic) to Hauterivian (Early Cretaceous) based on ostracods and dinoflagellate cysts, as well as ammonoids, bivalves, and gastropods (see Bussert et al. 2009 for details). The skeletal remains of Dysalotosaurus lettowvorbecki were embedded in deposits of the Middle Dinosaur Member (MDM) of the Tendaguru Formation, for which a late Kimmeridgian age has been suggested according to the uncovered group assemblage of the dinoflagellate cysts of Endoscrinium attadalense and Ctenidodinium sellwoodi (Bussert et al. 2009: 161–162).
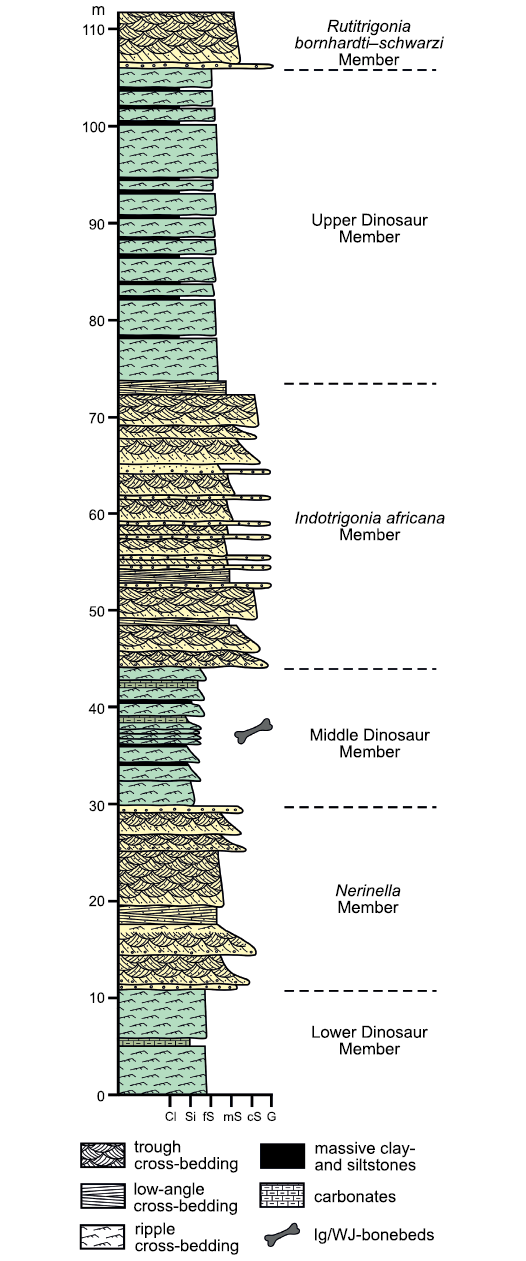
Fig. 5. Simplified composite section of the Tendaguru Formation in the type area (based on Bussert et al. 2009). The position of the Ig/WJ-bonebeds is only approximate, based on the field results of the German-Tanzanian Tendaguru Expedition 2000 (Aberhan et al. 2002). Abbreviations: Cl, clay; cS, coarse-grained sand; fS, fine-grained sand; G, gravel; mS, medium-grained sand; Si, silt.
The MDM is between 13 and 30 m thick in the surroundings of Tendaguru Hill (Fig. 5). It consists of fine-grained heterolithic sediments, mainly of ripple cross-bedded to cross-laminated, fine-grained sandstone and siltstone, as well as massive to crudely ripple cross-laminated or horizontally-bedded siltstone and claystone. The sediments contain varying amounts of calcite. The lower part of the member is mainly composed of lenticular fining-upward sequences that are commonly a few decimeters thick and consist of ripple cross-bedded sandstone and siltstone that grade upward into massive siltstone and claystone and finally into sandy-to-muddy micritic limestone. The lenticular geometry, the presence of fining-upward sequences and the lithofacies architecture of the deposits suggest that they are channel fills and the heterolithic sediment composition likely reflect variable current velocities typical of tidal or tidally influenced channels. A mainly marine depositional environment of the lower to middle part of the MDM is also indicated by the presence of abundant remains of marine bivalves and gastropods and a few dinoflagellate cysts (Schrank 2005). Marine macroinvertebrates also occur in the upper part of the member, but are rare. The upper part of the member consists of similarly fine-grained sediments as the lower part, which also forms small-scale fining-upward sequences. Mud clasts and reworked caliche nodules are partly present at the base of the sequences. Towards the top of the member, increasingly sandy-to-muddy and partly peloidal micrite horizons are intercalated. Besides a variable calcite content, several layers contain dolomite. The peloidal micrite horizons probably result from exposure and early pedogenesis. The presence of dolomite suggests a sabkha-like environment.
The sedimentology and palaeontology of the MDM suggests deposition mainly in a tidally-influenced shallow marine littoral environment. The shallow marine character of the environment is particularly pronounced in the lower part of the member, but decreases in the upper part, where there are indicators of intermittent exposure and early pedogenesis, thus indicating an increased continental influence. The deposition of the MDM probably took place during a sea-level high stand (Bussert et al. 2009). It was characterized by an almost continuous aggradation of mainly shallow marine and minor continental sediments, only slightly interrupted by erosion. The fine-grained sediments indicate relatively low current velocities and deposition in a protected shallow marine environment, e.g., in a wave-protected bight or lagoon on the coast of a low-relief coastal plain.
The precise stratigraphic position of the Ig/WJ-bonebeds in the MDM is uncertain. However, the geographic location of the site Ig/WJ within the outcrop area of the MDM located during the German-Tanzanian Tendaguru Expedition 2000 (Aberhan et al. 2002) suggests that the bonebeds occur in the middle part of the member.
Results
Evaluation of excavation records
Recovery and labeling of the finds.—The excavations of the GTE at site Ig/WJ commenced about mid-October 1910 (Janensch, correspondence, October 22nd, 1910) and were completed on January 19th, 1913 (Reck, 13th report, January 26th, 1913). Some of the bonebeds contained rich mass accumulations of relatively small skeletal remains of Dysalotosaurus lettowvorbecki. As the recovery of individual skeletal elements in the field would have been too time-consuming, the bonebeds were almost exclusively mined in blocks. The weight of these blocks usually ranged from a few kilograms up to about 25 kg. No marks indicating vertical orientations were applied, nor were there any symbols that indicated compass directions. Janensch (1914b: 256) reported that the long limb bones of D. lettowvorbecki, such as the femur, tibia, and fibula, were often aligned parallel to each other in an approximately northwest-southeast direction. Unfortunately, field sketches, photographs or compass directions were never published, possibly due to their destruction during WWII.
In 1910 and 1911, the D. lettowvorbecki locality was designated as “Ig” (derived from “Iguanodonten-Schicht”, or iguanodontid bed), and individual finds and blocks were given this prefix and numbered consecutively with Arabic numerals. In early June 1912, the site name “Ig” was replaced by the acronym “WJ” (derived from Werner Janensch) in order to avoid confusion with previously recovered finds (Reck, 3rd report, June 22, 1912; GTE field catalogue, 1912–1913) (Fig. 6). From then on, all blocks and findings were designated with the prefix “WJ” and also consecutively numbered with Arabic numerals. Finally, it should be noted that Janensch (1955: 107, footnote 1) later unified the site designations “Ig” and “WJ” under the short name “dy” (derived from Dysalotosaurus).

Fig. 6. Scanned image of the first two pages of the field catalogue of Hans Reck from 1912. The first note at the top of the right page says: “19. June 1912 – The quarry Ig will be renamed W.J. due to the ongoing numbering of the bones” (Reck, 1912–1913. GTE field catalogue, Pal. Mus SII, Tendaguru-Expedition 9.3, Archive of the Historical Division of the MfN)
Under the direction of Werner Janensch and Edwin Hennig (1910–1911), and Hans von Staff (1911), two bonebeds were mined at site Ig/WJ (Janensch 1914b: 256). Subsequently, Reck (1912–1913) exploited three bonebeds, one of which had previously yielded skeletal remains of D. lettowvorbecki recovered in 1910 and 1911 (see below). Unfortunately, the skeletal remains and rock samples from the individual bonebeds were not identified according to their origin and were not stored separately during the years of excavation. Therefore, reconstruction of the taphonomic history of the individual bone accumulations at site Ig/WJ was extremely difficult.
Storage of the material.—The blocks with skeletal material of D. lettowvorbecki from site Ig/WJ were first stored in the MfN. Later, parts of the collections were distributed to several other German institutions. According to an unpublished handwritten note of Werner Janensch from the 27th May, 1933, which is housed in the MfN, unprepared material of D. lettowvorbecki was distributed to the following institutions (original catalogue numbers in brackets): Geologisches Staatsinstitut Hamburg (WJ5700–6299), GZG (WJ6300–6799), BSPG (WJ6800–7499), SMNS (WJ7500–8399), and GPIT (WJ8400–10994). It is of note that some catalogue numbers of the GPIT collection exceed the range of numbers given above. This could mean that Janensch’s note was only a preliminary draft for a contract to which additional items were later added. Unfortunately, the original handover protocols were not available. It should also be noted that during WWII substantial parts of the D. lettowvorbecki collections are lost not only in Berlin but also in Göttingen, Munich, and Stuttgart, and the material stored in Hamburg was completely destroyed (Maier 2003).
The bonebeds at the site Ig/WJ
The number of bonebeds.—In the years 1910 and 1911, excavations at site Ig/WJ focused mainly on a bone accumulation, which was referred to as “Mergelschicht” (marl layer) (Janensch 1914b: 256). This bone accumulation was extraordinarily rich in vertebrate fossils and yielded numerous well-preserved skeletal remains of D. lettowvorbecki. Janensch (1914b: 256) reported that the marl layer had a thickness of approximately 10–20 cm. The bone accumulation at the site extended longitudinally approximately in the E-W direction and dipped weakly to the E and NE (Janensch 1914b: 256). Unfortunately, no precise data on the longitudinal extent of this bonebed were given.
In addition, Janensch (1914b: 256–257) briefly described another bonebed from the same site as “zweite Knochenlage” (2nd bonebed), which was exposed NW of and stratigraphically below the marl layer. It also dipped slightly to the E and NE. Janensch (1914b: 256–257) noted that the longitudinal extension of this bonebed was shorter than that of the marl layer. He also reported that skeletal remains were distinctly sparser than in the marl layer (Janensch 1914b: 256–257). The precise thickness and extent of the 2nd bonebed are unknown. The vertical distance between the two bonebeds was only a few decimeters of bone-free sediment (Janensch 1914b: 257).
In contrast to Werner Janensch’s excavations in 1910 and 1911, three superimposed bonebeds were quarried by Reck in 1912 and 1913 (Reck, 9th report, October 7, 1912). At the beginning of the excavations in early June 1912, only one bonebed was exposed at the site Ig/WJ (Reck, GTE field catalogue 1912–1913). It was described using a variety of terms such as “eigentliche Hauptknochenschicht” (actual main bone layer) (Reck, 2nd report, June 12, 1912; 3rd report, June 22, 1912), “Knochenhauptschicht” (main bone layer) (Reck, 4th report, July 5, 1912), “Iguanodontenschicht” (iguanodontid bed) (Reck, 7th report, September 10, 1912) or “Iguanodontidenherde” (iguanodontid herd) (Reck, 8th report, September 15, 1912). Other terms used for this bonebed in the GTE field catalogue (1912–1913) include: “Hauptschicht” (main layer), “Knochenhaupthorizont” (main bone horizon), “Untere Hauptschicht” (lower mainlayer) or only “große Form (gr. F.)” (large form). The latter term refers to the dominance of relatively large iguanodontid-like skeletal remains in this bone accumulation, which were later referred to D. lettowvorbecki.
Later, another bonebed was exposed about half a meter above the main bone layer (iguanodontid bed) (Reck, 2nd report, June 12, 1912; GTE field catalogue 1912–1913). A striking feature of this bone accumulation was the predominance of bones of D. lettowvorbecki, that were often distinctly smaller than those from the underlying iguanodontid bed. It was designated as “große Herde kleiner Saurier” (large herd of small saurians) (Reck, 8th report, September 15, 1912, profile sketch). Other designations are: “obere Schicht” (upper bed), “obere Schicht (kleine Formen) (upper bed, small forms) or only “kleine Form (kl. F.)” (small form) (GTE field catalogue 1912–1913).
The third bonebed, which was referred to as “unterste dritte fossilführende Zone” (lowermost 3rd fossil-bearing zone) (Reck, 9th report, October 7, 1912), was mentioned only once. Information about its thickness, extent and distance to the overlying main bone layer (iguanodontid bed) were not given. Most likely, this bone accumulation only extended over a small area and had probably not reached the dimensions of the two overlying bonebeds. This layer was apparently so insignificant to Hans Reck that it was not mentioned further. It is also missing in a profile sketch of the site by Reck from 1914 (Reck, correspondence, February 10, 1914). In summary, between 1910 and 1913 a total of four different bonebeds were excavated by the GTE at the site Ig/WJ.
Designation and correlation of the bonebeds.—Unfortunately, Hans Reck did not provide any information about the stratigraphic relationships between his three bonebeds (Reck’s reports 1912, 1913) and the two bone accumulations excavated previously (excavated previously by Janensch and Hennig in 1910 and 1911). However, two profile sketches by Reck (Reck, 8th report, September 15, 1912; correspondence, February 10, 1914) provide the basis for the stratigraphic correlation of the bonebeds at site Ig/WJ (Fig. 7). They clearly show that Reck’s main bone layer (iguanodontid bed) is identical to the “marl layer” described by Janensch (1914b: 256; Table 1). The sketches (Fig. 7) also show that the overlying bone accumulation considered attributable to a large herd of small saurians, has no counterpart in the two bonebeds that were previously excavated in 1910 and 1911 (Janensch’s marl layer and 2nd bonebed). This layer is, unambiguously, the uppermost bonebed at the site Ig/WJ (Fig. 7, Table 1).
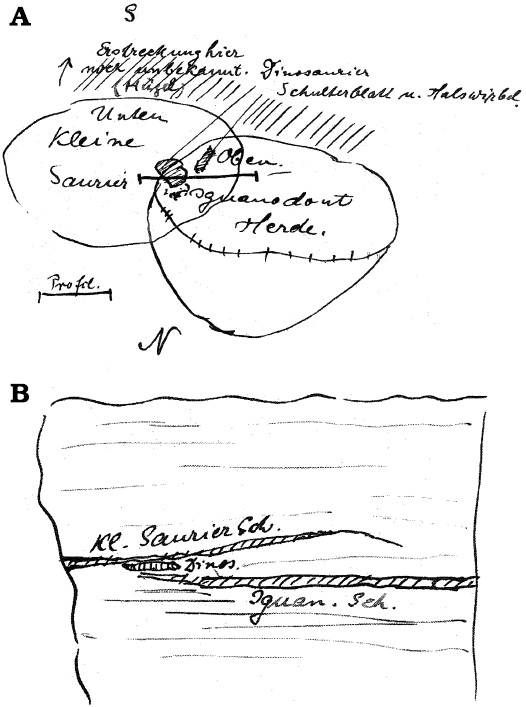
Fig. 7. Field sketches by Hans Reck (Reck, 8th report, September 15, 1912) on the spatial relationships of the two main bonebeds. The two main bonebeds (BB-3 and BB-4) in top (A) and profile (B) views. The indicated large bones in between are labelled with “dinosaur shoulder blade and vertebra” (in German) (A) or simply “Dinos.” (B) and indicate the discovery of sauropod remains in the quarry (H. Reck, 8th report, September 15, 1912; Pal. Mus SII, Tendaguru-Expedition 9.5, Archive of the Historical Division of the MfN).
Table 1. Proposed designations of the Tendaguru bonebeds. Based on the data presented, the following designations of the bonebeds at the Ig/WJ-site are suggested (numbered from bottom to top following the suggested stratigraphic succession). Note that the real sequence of the two lowermost bonebeds is obscure. BB, bonebed.
|
Bonebed |
Selected previous designations |
|
BB-4 |
“(Schicht mit der) große(n) Herde kleiner Saurier” [(Bed with) the large herd of small saurians] (Reck, 8th report, September 15, 1912), “Obere Schicht” [upper bed] and “obere Schicht (kleine Formen)” [upper bed, small forms] (Reck, GTE field catalogue, 1912–1913) |
|
BB-3 |
“Mergelschicht” [marl layer] (Janensch 1914b: 256), “Hauptschicht” [mainlayer] (Reck, GTE field catalogue, 1912–1913), “Knochenhauptschicht” [main bone layer] (Reck, 4th report, July 5, 1912), and “Iguanodontenschicht” [iguanodontid bed] (Reck, 7th report, September 10, 1912) |
|
BB-2 |
“Zweite Knochenlage“ [2nd bonebed] (Janensch 1914b: 256–257) |
|
BB-1 |
Previous designation: “Unterste dritte fossilführende Zone” [lowermost 3rd fossil-bearing zone] (Reck, 9th report, October 7, 1912) |
In contrast to the two upper bonebeds, the relative stratigraphic position of the two lower bonebeds remains obscure. However, there is no doubt that both Janensch’s 2nd bonebed and Reck’s lowermost 3rd fossil-bearing zone were located below the iguanodontid bed (main bone layer). Nevertheless, it is not clear whether or not the lowermost 3rd fossiliferous zone was below or above Janensch’s 2nd bonebed. It is also possible that both bonebeds occurred within the same stratigraphic layer (Fig. 8), but the excavation data noted in the field notes imply that the 3rd fossiliferous zone was not in direct contact to Janensch’s 2nd bonebed.
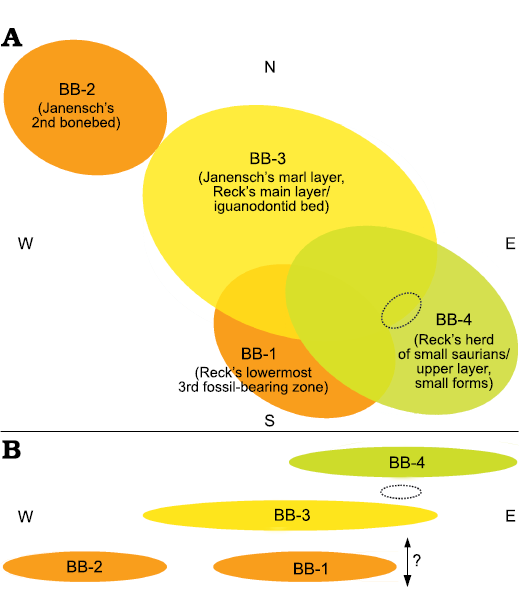
Fig. 8. Tentative reconstruction of the spatial relationships of the four bonebeds of the Ig/WJ-quarry according to the available descriptions and dates of Werner Janensch and Hans Reck. The actual shape and absolute sizes of the bonebeds are schematic and speculative. Top (A) and profile (B) views are in relation to the cardinal points. Note that the actual stratigraphic level of BB-1 in relation to BB-2 is uncertain (double headed arrow). The dotted ellipses show the approximate position of the large, possibly sauropod, bones found in September 1912 within the otherwise bone-free layer in between BB-3 and BB-4 including a scapula and a cervical vertebra. BB, bonebed. See Fig. 7 for comparison.
Allocation of specimens to individual bonebeds.—Specimens from several bonebeds were recovered simultaneously, and typically not labeled with their bonebed of origin. With a few exceptions, most of the specimens registered in the GTE field catalogues lack information about which bone accumulation they come from. However, most of the specimen numbers of Reck’s GTE field catalogue (1912–1913) are dated, so that the time of excavation and registration of specimens at site Ig/WJ can be assessed. These field data, together with the statements in the reports and correspondences of Hans Reck, give approximate dates at which the individual bonebeds were quarried in 1912. The assessment of all these data allows us to allocate at least some of the specimens with some certainty to individual bonebeds within the Ig/WJ-site (Table 2):
Bonebed 1: BB-1 was mentioned only once by Reck (9th report, October 7, 1912) as it was most likely only exposed for a short period of time towards the end of September 1912 or the beginning of October 1912. Due to the lack of adequate information, there are no specimens of D. lettowvorbecki (labeled with “WJ”) that can be assigned with certainty to BB-1.
Bonebed 2: BB-2 (Janensch 1914b) was exposed in 1910 or 1911. It is not known when it was quarried. Because of missing data, D. lettowvorbecki finds labeled with “Ig” are not assignable with certainty to BB-2.
Bonebed 3: BB-3 was first quarried from mid-October 1910 until early autumn 1911 (Janensch 1914b), except during the rainy seasons. Subsequently, Hans Reck continued the recovery of skeletal remains from BB-3 in early June 1912 until the end of September or perhaps the beginning of October 1912 (Reck, 4th report, July 5, 1912; 7th report, September 10, 1912; 8th report, September 15, 1912). Specimens from BB-3 (“large forms”) are mentioned in Reck’s GTE field catalogue, 1912–1913, for the last time on September 21, 1912 (catalogue numbers WJ 4231–4239). However, it is unclear whether or not some of the following registered finds (catalogue numbers > WJ 4239) also belong to BB-3, since information on their provenance is missing. Data on specimens excavated and registered in 1910 and 1911 have a low potential of error and are expected to have little falsifying impact. A mixing of specimens from the underlying BB-2 and BB-1 is possible, but is considered insignificant, as these specimens were located within small localised bone accumulations in which there were few skeletal remains.
Bonebed 4: From the available data, it appears that the topmost bonebed was excavated between mid-June 1912 to early January 1913 (Reck: 2nd report, June 12, 1912; 3rd report, June, 22 1912; 4th report, July 5, 1912; 8th report, September 15, 1912). Since the underlying BB-3 was already exhausted by the end of September or early October 1912, it is most likely that the skeletal remains of D. lettowvorbecki that were recovered afterward came almost entirely from BB-4. Mixing of specimens with BB-1, which was also exposed and exploited towards the end of September or the beginning of October 1912, is considered unlikely, because this bonebed was apparently a small, localised bone accumulation.
Table 2. Assignment of specimens of Dysalotosaurus lettowvorbecki to the two main bonebeds based on original field numbers. Abbreviations: BB, bonebed; Ig, excavation site acronym until 1911; NA, not assignable; WJ, excavation site acronym from 1912 to 1913.
|
Bonebed |
Tentative assignment of specimens |
|
BB-4 |
WJ: 431–474, 507–515, 783–796, 823–861,948–956, 1667–1692, 1697–1707, 1735, 1743, 1873–1882, 1928–2041, 2332–2351, 2441–2499, 2626–2699, 2804–2837, 2880–2892, 3377–3378, 3519–3520, 4163–4164, 4204–4230, 4240–4258, 5700–6299 (lost, Hamburg), 6300–6799, 6800–7499, 7500–8399, 8400–10994, 10995–14482 |
|
BB-3 |
Ig: 01–962, >962; WJ: 01–430, 475–506, 516–712, 740–782, 797–822, 862–947, 957–1152, 1541–1666, 1693–1696, 1708–1734, 1736–1742, 1744–1872, 1883–1927, 2042–2331, 2352–2440, 2500–2625, 2700–2803, 2838–2879, 3376, 4201–4203, 4231–4239 |
|
NA |
WJ: 713–739, 1153–1540, 2893–3375, 3379–3518, 3521–4162, 4165–4200, 4259–5699 |
Taphonomy
Composition, arrangement, and preservation.—The verified taxonomic diversity of the bonebeds of site Ig/WJ consists of four to five dinosaur taxa. Apart from D. lettowvorbecki, only few remains of the dinosaurs Kentrosaurus aethiopicus, Elaphrosaurus bambergi, Giraffatitan brancai, and undetermined sauropod bones are recorded (Janensch 1914b, 1925a, 1955; Hennig 1936; Heinrich 2001; Maier 2003; DS personal observations).
During the last 20 years, during screen washing of more than one ton of matrix from IG/WJ-site housed in Berlin (e.g., Heinrich 1999a), further remains of small, non-dinosaurian vertebrates, in particular pterosaur, crocodile, lizard as well as three genera of mammals from this site were discovered (Heinrich 1998, 1999a, 2001; Broschinski 1999; Unwin and Heinrich 1999). The sampling and collection of specimens differs markedly from other GTE sites, but the low abundance of these micro-vertebrate remains in comparison to the general abundance of D. lettowvorbecki indicates that the latter still accounts for more than 90% of the fossils in terms of number of identifiable specimens (NISP) and the minimum number of individuals (MNI).
Based on the number of right distal femoral ends, a minimum number of 23 D. lettowvorbecki individuals is present in the collections in Göttingen and 39 individuals in Tübingen. Although the percentage of skeletal representation is not the same between both collections, the trend of representation is comparable (Table 3). Accordingly, vertebral centra and the ends of long bones are the most abundant in the collections. However, it should be noted that the vertebrae are still greatly underrepresented (Table 4), as single individuals are thought to have had up to 70 vertebrae (Janensch 1955). Small skeletal elements as well as elements of low overall density and/or of a large area to volume ratio, such as single skull elements, teeth, ribs, vertebral neural arches, sterni, and elements of the hand and feet (excluding the metatarsals) are also underrepresented (Table 4). Among the portions of the skull, the most common elements are the largest and most robust, such as the maxillae, dentaries, frontals, and quadrates. In contrast, most of the long bones (including the metatarsals) as well as the girdle bones of the pelvis are much more often preserved or even overrepresented (Table 4). This bias also holds true for the collections in Berlin, Stuttgart, and London (TH personal observations).
Table 3. Statistical comparison of the preserved number of bones (total count and percentage of Minimum Number of Individuals (MNI) of Dysalotosaurus lettowvorbecki from the collections in GZG and GPIT based on the Mann-Whitney U and Kolmogorov-Smirnov tests.
| |
Mann-Whitney U test |
Kolmogorov-Smirnov test |
||
|
U |
p |
D |
p |
|
|
Total count |
116 |
0.003 |
0.455 |
0.014 |
|
% of MNI |
179 |
0.142 |
0.273 |
0.332 |
Table 4. Representation of skeletal elements of Dysalotosaurus lettowvorbecki in relation to the minimum number of individuals (MNI) in the collections of Göttingen (GZG) and Tübingen (GPIT).
|
Element type |
Number of elements, GZG |
Number of elements, GPIT |
Representation of |
Representation of |
|
skull elements |
7 |
29 |
0.59 |
1.43 |
|
teeth |
10 |
14 |
0.95 |
0.78 |
|
vertebral centra |
235 |
1390 |
14.60 |
50.92 |
|
neural arches |
26 |
86 |
1.61 |
3.15 |
|
ribs |
10 |
78 |
0.74 |
3.39 |
|
scapula |
16 |
49 |
34.78 |
62.82 |
|
coracoid |
8 |
20 |
17.39 |
25.64 |
|
sternum |
1 |
3 |
2.17 |
3.85 |
|
humerus |
28 |
86 |
60.87 |
110.26 |
|
ulna |
8 |
46 |
17.39 |
58.97 |
|
radius |
6 |
13 |
13.04 |
16.67 |
|
manus bones |
0 |
3 |
0 |
0.18 |
|
ilium |
21 |
115 |
45.65 |
147.44 |
|
ischium |
13 |
29 |
28.26 |
37.18 |
|
pubis |
17 |
34 |
36.96 |
43.59 |
|
femur |
71 |
198 |
154.35 |
253.85 |
|
tibia |
57 |
123 |
123.91 |
157.69 |
|
fibula |
7 |
18 |
15.22 |
23.08 |
|
proximal tarsals |
4 |
48 |
4.35 |
30.77 |
|
distal tarsals |
2 |
17 |
2.17 |
10.90 |
|
metatarsals |
70 |
199 |
50.72 |
85.04 |
|
pedal phalanges |
33 |
282 |
5.98 |
30.13 |
The bones were densely packed within the bonebeds, sometimes even stacked and in direct bone-to-bone contact (Fig. 9). According to Janensch (1914b), long bones were often found in parallel orientation to each other in a NW-SE direction, which corresponds approximately to the main axis of BB-3, which has an E-W direction. A vertical sorting of bones within a bonebed by size, skeletal element, or orientation was apparently absent (Reck 1925). The marrow cavity of many broken long bones was partially filled with fine calcareous sediment and authigenic minerals indicating the original orientation of the bones within the bonebed (Fig. 10). In addition, Chinsamy-Turan (2005: 15) noted prismatic calcite often precipitated in a half-moon-shape in the marrow cavities as a possible sign for movement of the bones during crystal growth.
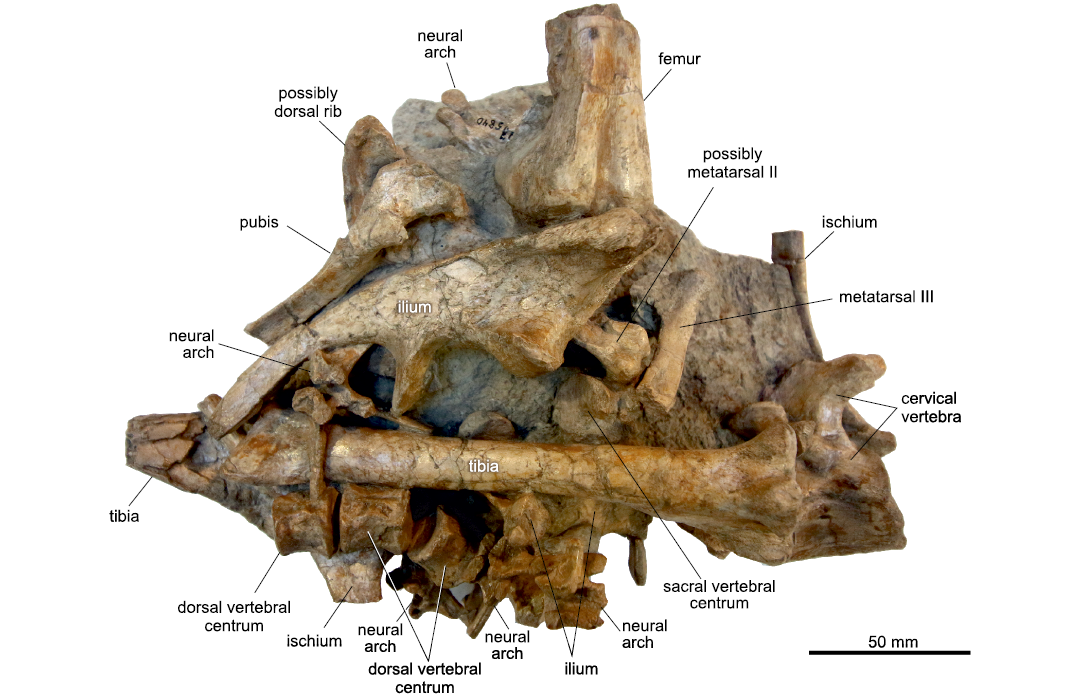
Fig. 9. Fully prepared block MB.R.1910 (WJ5840) of ornithopod dinosaur Dysalotosaurus lettowvorbecki Pompeckj, 1920, from Kimmeridgian, Late Jurassic of Tendaguru, Tanzania, within the bonebeds.

Fig. 10. Thin section of the tibia GPIT/RE/3724 of ornithopod dinosaur Dysalotosaurus lettowvorbecki Pompeckj, 1920, from Kimmeridgian, Late Jurassic of Tendaguru, Tanzania, cut within the lower third of the long bone shaft. Most of the marrow cavity is filled by fine, calcareous marl. Note that the top of the cavity has been filled subsequently by calcite crystals, which indicates that the bone was embedded in the substrate as oriented as in this image.
The majority of specimens consist of isolated skeletal elements. However, there are rare examples of associated and even partially articulated specimens (Hübner 2018) (Fig. 11). These include several short strings of vertebrae, mostly posterior dorsals, sacrals, and anterior caudals representing the most abundant articulated body region (Fig. 12A, B). Furthermore, four partial skulls, which are located in the collections of MfN (dyA, dyB), BSPG (BSPG AS I 834), and SMNS (SMNS 52348) (Hübner and Rauhut 2010) (Fig. 11) and partially articulated feet (Janensch 1955; Galton 1981) are also noteworthy, one of which (GPIT/RE/3452) shows signs of tendinous hyperextension in the toes (Fig. 12D1–D3; Hübner et al. 2014). Articulated hands were originally also present (Pompeckj 1920; Maier 2003), but have since been lost. Most rarely, partially articulated skeletons were uncovered (Hübner 2018). Two of them, most probably lost, were depicted in sketches by either Ina or Hans Reck (see Heinrich 1999ba: fig. 14; Fig. 13). Another partial skeleton, the individual named dyI by Janensch (1955), is today part of the mounted skeleton in the dinosaur hall of the MfN in Berlin (this individual has numerous collection numbers, the axial skeletal elements range MB.R.5025–5055 and the appendicular elements MB.R.5089–5097 and MB.R.5099–5100). The affiliation of most associated and articulated material to a certain bonebed is often unclear, although notes in Reck’s catalogue of specimens make BB-3 and BB-4 the most likely sources. Complete and undamaged bones are in the minority among isolated elements.
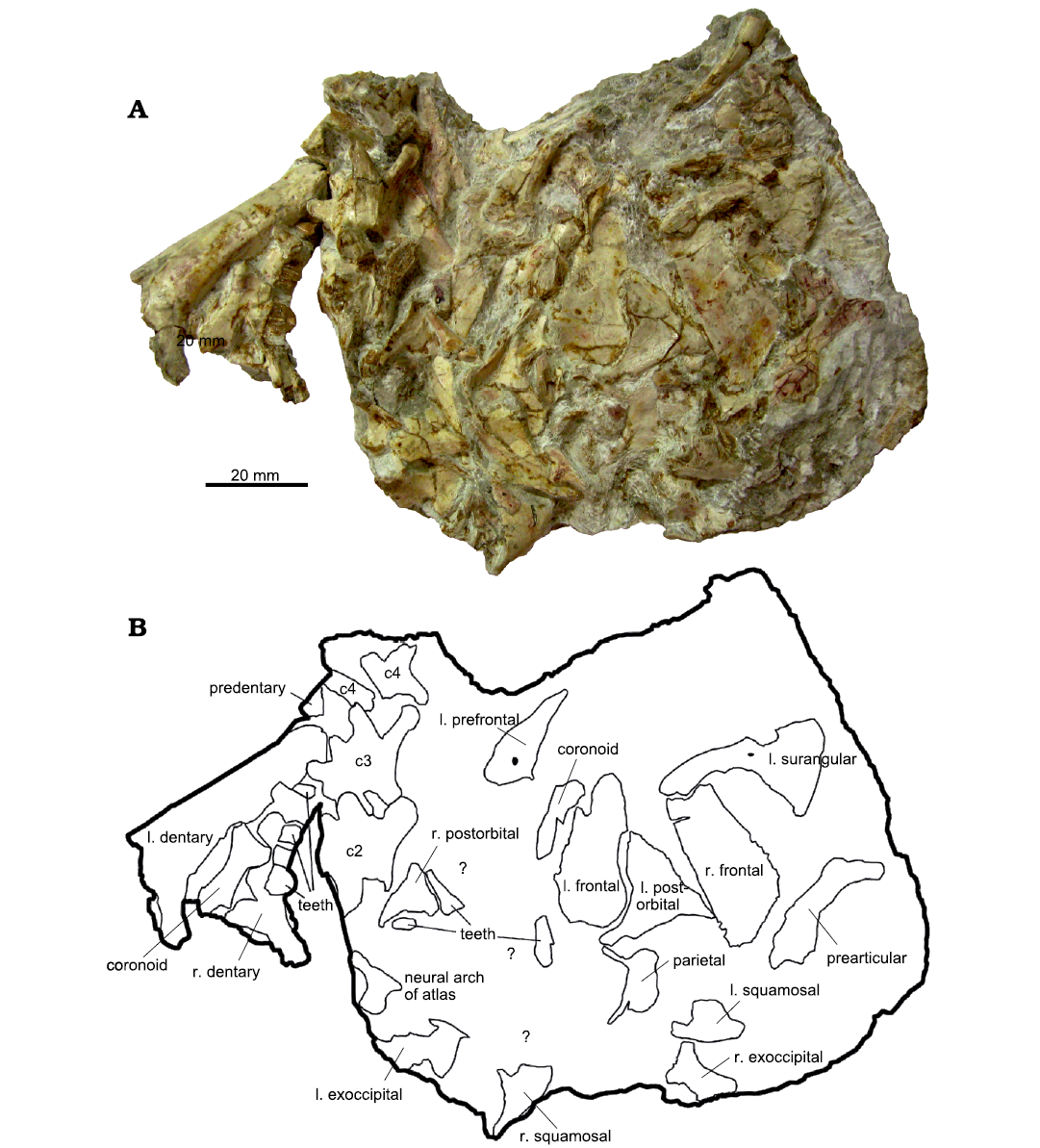
Fig. 11. Associated skull SMNS 52348 of a juvenile individual of ornithopod dinosaur Dysalotosaurus lettowvorbecki Pompeckj, 1920, from Kimmeridgian, Late Jurassic of Tendaguru, Tanzania. A. The lower jaw bones at the left were detached from the specimen during preparation and are reassembled in this image. All currently identifiable elements are framed and labeled. B. Most of the unlabeled elements in the left center between the right postorbital, left exoccipital, left frontal, and left prefrontal (marked by “?”) likely belong to the palate of the skull and may be identifiable after further preparation. Abbreviations: c2–c4, cervical vertebrae 2–4; l., left; r., right.
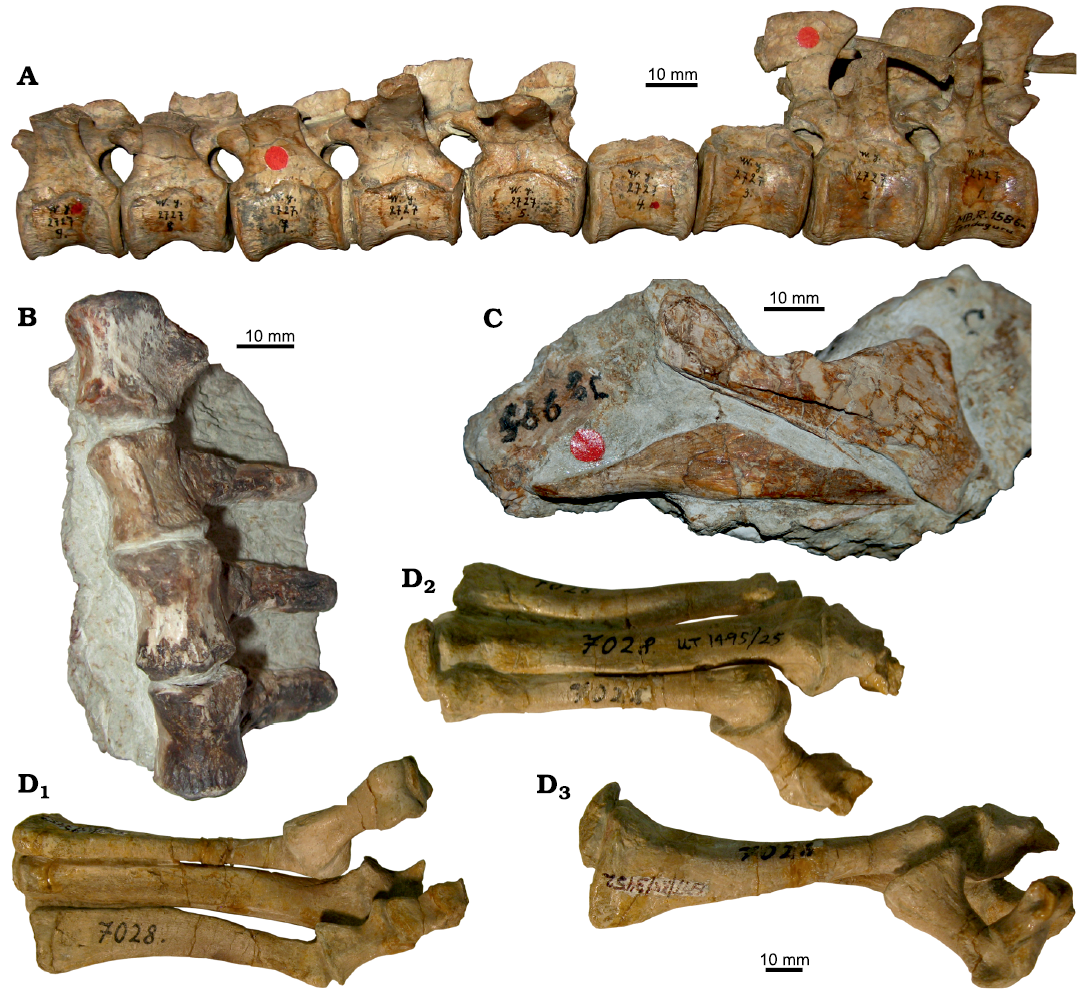
Fig. 12. Examples of articulated or closely associated skeletal elements of ornithopod dinosaur Dysalotosaurus lettowvorbecki Pompeckj, 1920, from Kimmeridgian, Late Jurassic of Tendaguru, Tanzania. A. The 7–15th dorsal vertebrae (anterior to the left) of individual dy II (acronym for individual dy II used by Janensch 1955, today catalogued with the collection numbers MB.R.1586.1–9). B. Unlabeled posterior dorsal vertebrae from the SMNS collections in ventral view. C. Closely associated right angular and surangular MB.R.1335. D. Incomplete articulated left pes GPIT/RE/3452 in ventral (D1), dorsal (D2), and medial (D3) views.
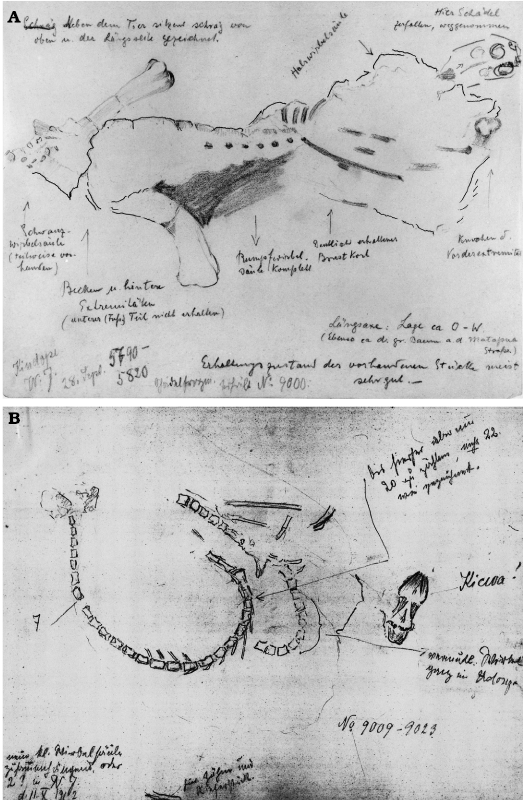
Fig. 13. Sketches by Ina or Hans Reck of articulated partial skeletons found in 1912. According to Table 2, both specimens were found in the uppermost bonebed 4. A. The German notes on the sketch tell correspondingly that this skeleton was lying with its long-axis in W-E-orientation, that it was only missing the lower part of the foot and parts of the tail, and that the skull was broken and removed separately. The skull was catalogued as WJ9000 and the postcranial skeleton as WJ5790-5820 (the latter were lost in Hamburg during WWII) which can be found in H. Reck’s catalogue. The sketch was drawn on the 28th of September. B. The arrow points to a series of at least 20 articulated vertebrae. Another vertebral series, next to it on the right, is still partly covered in clay. Right next to the latter one can see the word Kicwa! (Swahili for skull). At the bottom of the image are noted teeth and a jawbone. According to the note in the lower left corner, the illustrator was unsure whether there were one small vertebral series or two. The numbers WJ9009-9023 are also present in H. Reck’s catalogue. The sketch is dated 2nd of October 1912 (Pal. Mus SII, Tendaguru-Expedition 9.1, Archive of the Historical Division of the MfN).
Most specimens are crushed, distorted, broken and/or incomplete. Although bone fractures are often irregular and also change direction over short distances, some constant patterns can be observed. Fractures occur in long bones more or less perpendicular to the long axis and most often in the thinnest or weakest portions of the bones (Fig. 14A, B). Thus, the majority of long bones consist of isolated distal or proximal ends. For example, femora are often broken proximally or distally with respect to the fourth trochanter (Fig. 14B). Scapulae are often only represented with their scapular blades broken off. Ilia lack their preacetabular processes (Fig. 14C) or have been snapped in two directly above the acetabulum, while ribs are often only preserved as short fragments. The split-up of matrix blocks during excavation may have also contributed to the common breakage, especially of large long bones, but this factor cannot be assessed quantitatively. In the girdle bones, surface fractures with almost net-like patterns are apparent (Fig. 14C). Many fractures are closed by carbonate cement (Fig. 14G). As far as observable, the preservation of the unprepared material stored in the bamboo corsets is similar to the prepared material. Most bones show fractures and cracks, and even bones that were originally complete, are preserved in pieces.
Despite all of this damage, the surfaces of most specimens are well preserved (Fig. 14F). Trample, scratch, bite, or root marks as well as traces of abrasion are lacking. Most sutural surfaces between the cranial bones, between vertebral centra and neural arches, and between scapulae and coracoids are uneroded. Furthermore, the preservation of delicate processes and/or thin edges (e.g., the fourth trochanter of the femur) of otherwise damaged bones is common (Fig. 14B, D, F, G). Distinct longitudinal fractures or flaking of bone surfaces, as a sign of preburial weathering (e.g., Fiorillo 1988), are absent.
The taphonomic deformation of bones is mainly locally distributed, such as the collapse of bone wall areas into the marrow cavity or the compression of bones, sometimes accompanied by shear fractures, reducing their original length (Fig. 14E, H). However, the majority of bones are still preserved in three dimensions and such deformations are far less common than fracturing.

Fig. 14. Examples of states of bone preservation of ornithopod dinosaur Dysalotosaurus lettowvorbecki Pompeckj, 1920, from Kimmeridgian, Late Jurassic of Tendaguru, Tanzania. A. Several tibiae originally labelled with low Ig numbers showing multiple breaks perpendicular to their long axis. B. Isolated shaft of the left femur GPIT/RE/3446 in medial view with its proximal and distal ends broken off but with excellent preservation of the bone surface and of the delicate 4th trochanter. C. The right ilium GPIT/RE/6544 with the usual broken off preacetabular process and net-like surface cracks on the otherwise well-preserved lateral bone surface. D. Dorsal vertebra GPIT/RE/5462 of a juvenile individual in anterior view with plastic deformation of the left diapophysis. The deformation of this side is visible in two additional, potentially associated dorsal vertebrae. E. Unlabeled right humerus from the SMNS collections with well-preserved articular ends but with a distorted and compressed midshaft. F. Excellent preservation of the right calcaneum GPIT/RE/5808 in lateral view. G. The left jugal MB.R.1333 in lateral view with numerous diagenetic cracks which were resealed in situ by calcite. H. Right quadrate MB.R.3478 in lateral view with its cotylar head and upper part of the anterolateral wing broken off and slightly displaced forward whereas the surface of the bone and its delicate processes are generally well preserved. Scale bars 10 mm.
Age distribution of Dysalotosaurus lettowvorbecki.—Both methods (“total” and “average”) for estimating the total number of individuals of D. lettowvorbecki from the locality Ig/WJ show a bimodal distribution of age d(x), resulting in a juvenile and adult grouping with almost the same number of individuals (Fig. 15). Based on the regression, the maximum age for D. lettowvorbecki is 20 years.
The “total” method estimates 138 individuals for site Ig/WJ. The first maximum is found at age six with 15 individuals and the second maximum is found at age 16, with 16 individuals. Between these two maxima, a minimum is present at age 10 with only two individuals. The “average” method estimates 131 individuals for site Ig/WJ with the first maximum found at age six with 11 individuals and the second maximum at age 16 with 12 individuals. At age 10 and 11, a minimum with three individuals each is present (Fig. 15B). Applying the Mann-Whitney U and Kolmogorov-Smirnov test to the numbers of individuals estimated in both methods, resulted in statistically similar results, based on the comparison of the median (U = 240.500; p = 0.981) and the shape of the distribution (D = 0.227; p = 0.563).
On the basis of the allocation of specimens to individual bonebeds (see above) the majority of femora can be assigned to either one of the main bonebeds (ca. 70.3% for the “total” method and ca. 67.9% for the “average” method), allowing the comparison of d(x) for the two main bonebeds. However, due to irregularities in the catalogue, only those femora from the GPIT collection are assigned, which are identifiable by their original field numbers. The results indicate that BB-3 is dominated by sexually mature individuals (x >10 years; see Hübner 2012), while BB-4 is dominated by juveniles (x <10 years). For both methods (“total” and “average”), BB-4 contains more individuals than BB-3.
Applying the “total” method (Fig. 15C), BB-3 contains 45 individuals extending from age one to 20. The data is bimodally distributed with a first maximum at age eight (three individuals) and second maximum at age 15 (eight individuals). The minimum is found at age 9 and 10 (one individual each). BB-4, however, contains 52 individuals extending from age zero to age 18. As in BB-3, the data are bimodally distributed with a first maximum at age four, five and seven (six individuals each) and a second maximum at age 14 (six individuals). The intermediate minimum is located at age 10 and 11 (one individual each).
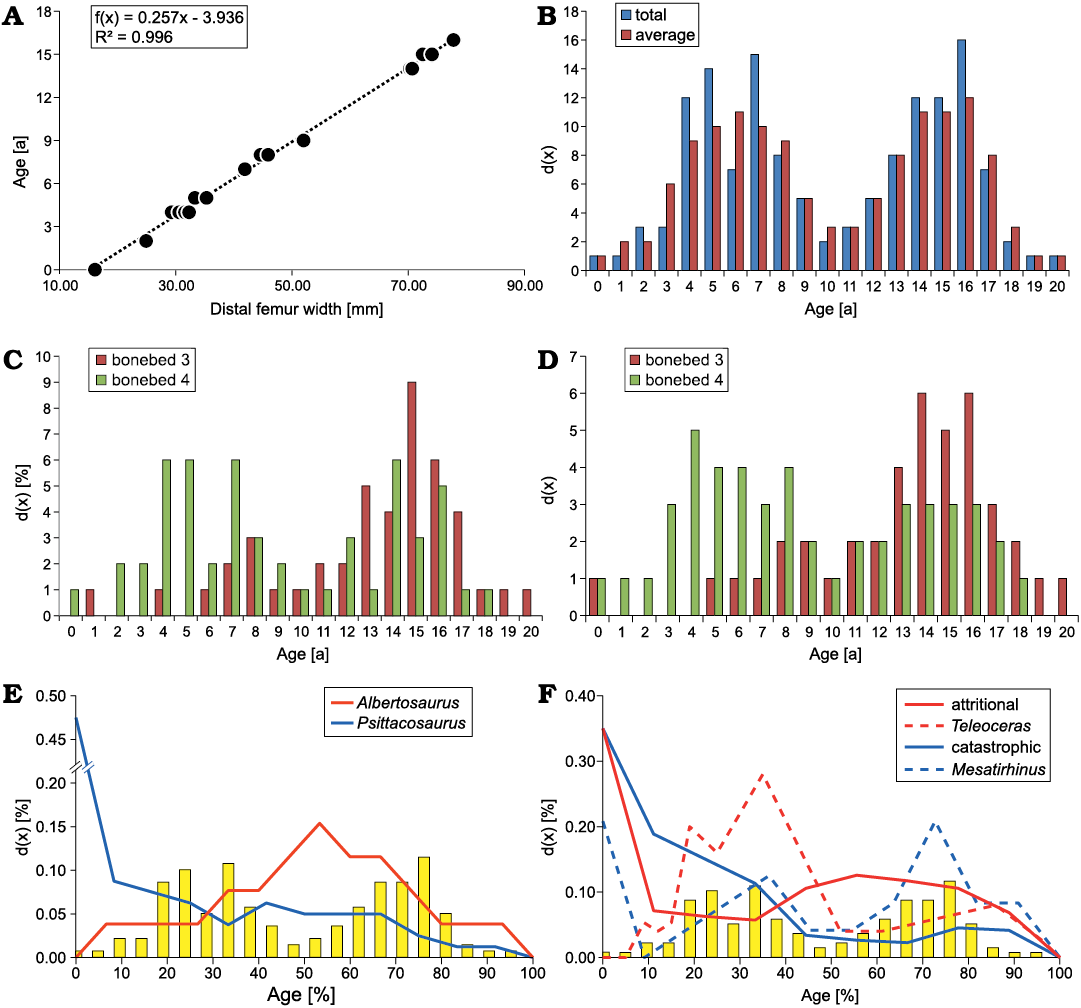
Fig. 15. Age structure d(x) of Dysalotosaurus lettowvorbecki. A. Linear regression between histological age and distal femur width of D. lettowvorbecki (based on Hübner 2012) for estimating the age of the remaining specimens. B. Age distribution d(x) of D. lettowvorbecki from the Ig/WJ-locality showing the “total” (N = 138) and “average” (N = 131) method for estimating the number of deaths per age. C. Age distribution d(x) of D. lettowvorbecki of bonebed 3 (N = 45) and bonebed 4 (N = 52) on basis of the “total” method. D. Similar distribution based on the “average” method (bonebed 3: N = 41; bonebed 4: N = 48. E. Comparison of the age distributions d(x) (in percentage) of D. lettowvorbecki (yellow bars), the Late Cretaceous tyrannosaurid Albertosaurus sarcophagus (red solid line, based on Erickson et al. 2010), and the Early Cretaceous basal ceratopsid Psittacosaurus lujiatunensis (blue solid line, Erickson et al. 2009b). F. Comparison of the age distributions d(x) (in percentage) of D. lettowvorbecki (yellow bars) and large mammals: hypothetical attritional population (red solid line; modified after Klein 1982b), and the Miocene rhinocerotid Teleoceras proterum (red dashed line; based on Mihlbachler 2003); hypothetical catastrophic population (blue solid line; modified after Klein 1982b) and the Eocene hippomorph Mesatirhinus sp. (blue dashed line; based on Turnbull and Martill 1988).
Using the “average” method (Fig. 15D), BB-3 contains 41 individuals extending from age zero to 20. The data shows a single maximum at age 14 to 16 (six individuals each). BB-4 contains 48 individuals extending from age zero to age 18. The data are bimodally distributed with a first maximum at age four (five individuals). The second maximum is found at age 13 to 16 (three individuals each). The minimum is located at age 10 (one individual).
Using both methods, the Mann-Whitney U and Kolmogorov-Smirnov tests show no significant difference when the age distribution d(x) of both bonebeds is compared (Table 5). However, when d(x) is specifically compared for the juvenile group (extending from age zero and 10) both layers significantly differ, while individuals for the adult group (age 11–20) cannot be separated on a significant level (Table 5).
To characterize the remaining, unidentified material from the GPIT collection, we compared its age structure with that of BB-3 and BB-4 (Table 6). Like BB-4, the remaining GPIT material is dominated by juveniles representing 75% of all individuals. However, its age distribution is not significantly different from both BB-3 and BB-4 when the whole lifespan is analysed. Only for adult individuals older than age 10 are significant differences present between the GPIT material and BB-3 (Table 6), which probably resulted from the small number of adult individuals in the GPIT collection. Thus, based on the historical notes and slightly higher statistical similarity, most of the GPIT material probably belongs to BB-4, but because of collection issues, as mentioned above, we avoided the definitive assignment of the material to this bonebed.
Table 5. Statistical comparison of the age distribution d(x) of Dysalotosaurus lettowvorbecki in bonebeds BB-3 and BB-4 based on the Mann-Whitney U and Kolmogorov-Smirnov test and two different approaches (“total” versus “average”, see Material and methods section) for estimating the number of individuals per age. Bold p values indicate significant results.
| |
Mann-Whitney U test |
Kolmogorov-Smirnov test |
||
|
U value |
p value |
D value |
p value |
|
|
Total |
||||
|
all ages |
190.0 |
0.439 |
0.143 |
0.973 |
|
age 0–10 |
24.5 |
0.016 |
0.545 |
0.047 |
|
age 11–20 |
32.0 |
0.175 |
0.300 |
0.675 |
|
Average |
||||
|
all ages |
175.5 |
0.253 |
0.238 |
0.531 |
|
age 0–10 |
19 |
0.005 |
0.545 |
0.047 |
|
age 11–20 |
33.5 |
0.216 |
0.4 |
0.313 |
Table 6. Statistical comparison of the age distribution d(x) of D. lettowvorbecki in the collection of GPIT against BB-3 and BB-4 based on the Mann-Whitney U and Kolmogorov-Smirnov test and two different (“total” vs. “average”, see Material and methods section) for estimating the number of individuals per age. Bold p values indicate significant results. BB, bonebed.
|
Mann-Whitney |
Kolmogorov-Smirnov test |
||||
|
U value |
p value |
D value |
p value |
||
|
Total (N = 36) |
|||||
|
against BB-3 |
all ages |
181.5 |
0.318 |
0.238 |
0.531 |
|
age 0–10 |
46.5 |
0.355 |
0.364 |
0.374 |
|
|
age 11–20 |
16.0 |
0.010 |
0.500 |
0.111 |
|
|
against BB-4 |
all ages |
159.5 |
0.120 |
0.286 |
0.304 |
|
age 0–10 |
49.0 |
0.463 |
0.272 |
0.736 |
|
|
age 11–20 |
32.0 |
0.170 |
0.300 |
0.675 |
|
|
Average (N = 32) |
|||||
|
against BB-3 |
all ages |
187.0 |
0.393 |
0.143 |
0.973 |
|
age 0–10 |
37.0 |
0.118 |
0.455 |
0.147 |
|
|
age 11–20 |
11.0 |
0.002 |
0.600 |
0.031 |
|
|
against BB-4 |
all ages |
151.0 |
0.077 |
0.286 |
0.304 |
|
age 0–10 |
50.5 |
0.523 |
0.272 |
0.736 |
|
|
age 11–20 |
23.0 |
0.038 |
0.500 |
0.111 |
|
Discussion
The Ig/WJ-quarry is a unique dinosaur fossil locality in the Tendaguru Formation in terms of number of specimens, spatial concentration and distribution, and taxonomic affiliation. Most other Tendaguru Formation localities have yielded remains of sauropod dinosaurs and the thyreophoran dinosaur Kentrosaurus aethiopicus. The remains of some non-avian theropods are also known. All of these remains are thought to be the result of miring or from carcasses that had washed in from the hinterland (e.g., Janensch 1914b; Heinrich 1999b; Heinrich et al. 2011). Various authors have considered that the concentration of thousands of remains of Dysalotosaurus lettowvorbecki in closely related bonebeds resulted from one or more mass mortality events (Janensch 1914b; Hübner 2011, 2012) or alternatively from attritional mortality within the population followed by a subsequent concentration of specimens (Reck 1925; Russell et al. 1980; Heinrich 1999b; Heinrich et al. 2011). The patchiness of information on the sedimentology and taphonomy of the Ig/WJ-quarry hampers a thorough analysis of the locality, including, for example, the exact location, count, and orientation of specimens. The available information also seems to be contradictory in some aspects. These include, for example, the common fractures in bones with well-preserved surfaces (e.g., Hübner 2011) or the absence of spatial sorting (Reck 1925; TH personal observations on still unprepared blocks) but with the definitive sorting in terms of element type. A reopening of the quarry for study is currently not feasible, because it has been backfilled and is covered with dense vegetation (WDH personal observations). Therefore, reappraisal of previous taphonomic interpretations cannot be verified on-site and these should be regarded as tentative.
An exceptional Tendaguru locality.—Although at least some skeletal parts of D. lettowvorbecki have the potential to be preserved at sites in the Tendagaru Formation other than the Ig/WJ-quarry, not a single specimen was found outside this locality. This preservational bias is remarkable as even rare dinosaur taxa, such as Elaphrosaurus bambergi, are known from more than one locality (Janensch 1925b; Rauhut and Carrano 2016) and most sauropod taxa, as well as K. aethiopicus, are known from several localities within the Tendaguru Formation (e.g., Hennig 1924; Heinrich 1999b). The most striking difference between the rare dinosaur taxa (D. lettowvorbecki, theropods) and the common taxa is body size and weight. Several partial skeletons of sauropods and of K. aethiopicus were found with their limb bones preserved in an upright position or with their limb and foot bones strongly overrepresented in multi-individual assemblages (Janensch 1914b, 1961; Hennig 1924; Heinrich 1999b; Hübner 2011). This phenomenon forms the basis for the mud trap hypothesis (Janensch 1914b; Heinrich 1999b; Heinrich et al. 2011), which is comparable to similar preservational modes in other formations (see e.g., Sander 1992; Varricchio et al. 2008; Currie et al. 2011). In most fossil-bearing formations, smaller vertebrates are generally less likely to be preserved, and also to be found (e.g., White et al. 1998; Brown et al. 2013) for the following reasons. In general, smaller carcasses are more quickly and completely consumed by scavengers and decomposers and their hard parts more easily destroyed by weathering and diagenetic processes. On the other hand, mud traps have a higher potential to preserve the remains of smaller individuals (Varricchio et al. 2008; Eberth et al. 2010). The general absence of specimens of D. lettowvorbecki outside the Ig/WJ-locality therefore indicates exceptional local circumstances that led to repeated mass accumulations of this small dinosaur taxon.
The sedimentology of the MDM suggests deposition of the bonebeds in a shallow marine environment such as a tidally-influenced sheltered bay or lagoon. Sediments or sedimentary structures that would indicate deposition by catastrophic events such as strong storms or tsunamis, and present in other members of the Tendaguru Formation (Bussert and Aberhan 2004), are missing. Sedimentary indicators of intense wave activity are also missing. Sediment lenses consisting of fining-upward fine-grained sediments are interpreted as tidal channel fills. The field sketches of Hans Reck (Fig. 7) show a similar lens geometry of the two main bonebeds BB-3 and BB-4. The bonebed BB-3 is oriented approximately in an E-W direction. This potentially indicates a deposition in a similarly-oriented channel, which would have been oriented roughly perpendicular to the N-S trending palaeo-shoreline in SE Tanzania during Late Jurassic times (Bussert and Aberhan 2004: fig. 4).
The strongly bimodal size composition of the bonebeds, with a much larger size of the bones in comparison to the fine grain-size of the sediments, contradicts hydrodynamic reasons for the long-distance transport of the bones. The mostly perfect preservation of bone surfaces and the only moderate sorting of bones also implies minimal transport of bones and only limited movement by waves and currents. The concentration of the bones together with mud and calcrete clasts in channels suggest deposition in the form of a lag deposit in places that were largely protected against current or wave activity. These areas of accumulation could have been potholes or point bars.
The absence of bones of D. lettowvorbecki in the fine-grained layers of sediment, several decimetres in thickness, between the bonebeds BB-3 and BB-4 (according to Janensch 1914, also between BB-2 and BB-3) speaks against the origin of the bones in BB-4 from reworking by BB-3. A concentration of bones in BB-4 could only have occurred if bone had been present in the layers that were then eroded by the incision of the tidal channel in which BB-4 was deposited. Since skeletal remains of D. lettowvorbecki are missing in the layers directly below BB-4, this seems unlikely. In contrast, the fine-grained nature of the channel fills suggests normally low flow velocities in these channels and their overall low erosion potential. The lenticular geometry of the channel fills is a further indicator of channels that were relatively fixed and migrated only slightly (Hughes 2012). Furthermore, Hans Reck mentioned a small lens with remains of vertebrae and a scapula of a large dinosaur that were located in the intermediate sediment layer between BB-3 and BB-4. As documented in his field sketches (Reck, 1912–1913. GTE field catalogue, Pal. Mus SII, Tendaguru-Expedition 9.3) (Fig. 7) and GTE field catalogue (9/10 September 1912), it is not clear if these remains belong to K. aethiopicus or a sauropod dinosaur. Nevertheless, the presence of large dinosaur remains in this intermediate layer indicates its deposition between two independent depositional events responsible for the genesis of the bonebeds BB-3 and BB-4. In tidal areas, regularly occuring spring tides can be further intensified by favorable wind conditions. The multiple coincidence of reinforcing winds with spring tides could explain the multiple occurrence of bone beds due to drowning of animal herds in channels, that were rapidly and deeply flooded. Due to subsequent tidal currents and possibly limited wave reworking, we postulate that the carcasses were concentrated in channel areas near where the animals drowned, and were subsequently buried.
The fine-grained, freshly deposited sediment could have been supersaturated with water, making it difficult for animals to negotiate. At high water levels the tidal channels probably had to be crossed by swimming, and animals would have had difficulty exiting the steep and slippery banks. These features would have presented a potential death trap for channel crossing animals.
From death to grave.—Due to the supersaturated conditions at the channel floor, the carcasses could have decomposed quite quickly (Weigelt 1927/1999). Most of the material of D. lettowvorbecki is present in the form of isolated bones. However, the presence of associated and even articulated partial skeletons indicates that the carcasses underwent highly variable rates of decomposition before final burial. For example, the juvenile skull SMNS 52348 (Fig. 11) preserves an associated articulated cervical series, whereas most skull elements were disarticulated but still closely associated. The lower jaws of this specimen were even articulated with each other via the predentary. This indicates that the partial carcass decomposed in largely calm conditions under water. However, the hyperextension of articulated phalanges on other specimens (Fig. 12D1–D3) implies that some carcasses were exposed subaerially and had been desiccated. Thus, the mass accumulation suggests differential rates of decomposition as was similarly observed for a modern-day death assemblage by Weigelt (1927/1999: 183–184, 189, 191). Based on the degree of association between elements, most individuals probably underwent subaqueous decay for more than 50 days before their final burial (see Richter and Wuttke 2012; Syme and Salisbury 2014).
Due to the absence of unambiguous signs of abrasion, even on broken edges, long distance transport between the place of death and the final burial site seems unlikely (see e.g., Fiorillo 1988; Fernandez-Jalvo and Andrews 2003), although the actual transport distance cannot be estimated, because the bonebed sediments are fine-grained and the intensity of abrasion therefore would have been minimal (Bell and Campione 2014). However, the difference in size between the bonebed host sediments and the individual bones and bone fragments can be taken as evidence of short transport distances (Chiba et al. 2015). The stacked and concentrated arrangement of bones, without apparent sorting by size, shape, or vertical gradient may indicate that the carcasses accumulated in a stillwater area or at a barrier within the channel (see e.g., Fiorillo 1991; Capaldo and Peters 1995: 401), where deposition of the finest sediment fraction, such as marlstone, is most likely. However, the nearly NW-SE orientation of many long bones and the half-moon-shaped prismatic calcite in long bone marrow cavities also indicate the repeated influence of water currents, most likely due to tidal activity (Fiorillo 1991; Chinsamy-Turan 2005). These currents might also be responsible for the underrepresentation of smaller and more easily transported skeletal elements, such as phalanges, ribs, teeth, or skull elements.
Besides the fractures, the commonly perfect preservation of bone surfaces (weathering stage 0 sensu Behrensmeyer 1978; Fiorillo 1988) is notable. There are also no traces of bone rot before burial (e.g., Eberth et al. 2014: fig. 30.9; Eberth 2015; Chiba et al. 2015). All observed fractures or cracks are angular to irregular and are typical of fractures in already-permineralised bone (e.g., Zeigler et al. 2005; Gangloff and Fiorillo 2010; Eberth et al. 2014). The corroded regions of bones are interpreted as the result of recent tropical weathering, as they were exposed at or very close to the surface (see Janensch 1914a, b; Hübner 2011). Moreover, the abundance of crushed and distorted bones can partly be explained by the dense bone accumulation and the intraformational compaction of sediment, which is typically high in fine-grained sediments (Ryan et al. 2001; Zeigler et al. 2005). The sediment of the bonebeds has a high clay content that can undergo significant expansion and contraction in volume depending on its water content. The abundant fractures, which are cemented by calcite, suggests that the bones underwent similar expansion and contraction episodes. Spiral fractures, which usually occur in fresh bone due to trampling or scavenging (e.g., Haynes 1983; Fiorillo 1988; Ryan et al. 2001), are not visible in the specimens. Therefore, fracture patterns and weathering stages of the bones indicate that the duration of preburial exposure was probably in the range of weeks to a few months (Richter and Wuttke 2012). This is also supported by the lack of perthotaxic processes, such as bite, gnaw, trample, bore, and root marks on the bone surfaces (see e.g., Fiorillo 1988; Rogers 1990; Britt et al. 2009; Bell and Campione 2014), as well as the degree of bone disarticulation.
Age structure.—Most dinosaur taxa are only known by one or a few, mostly incomplete specimens. Consequently, studies on age distribution d(x) and population biology in dinosaurs are limited to only a handful of taxa, including the tyrannosaurids Albertosaurus sarcophagus, Daspletosaurus torosus, Gorgosaurus libratus, and Tyrannosaurus rex (Erickson et al. 2006; 2010; Steinsaltz and Orzack 2011; Weon 2016), the basal ceratopsid Psittacosaurus lujiatunensis (Erickson et al. 2009a) and the hadrosaurid Maiasaura peeblesorum (Woodward et al. 2015). Because of the large number of individuals with different ages, the age structure of D. lettowvorbecki can be studied and compared with the aforementioned dinosaur taxa, as well as between the D. lettowvorbecki individuals at BB-3 and BB-4.
As mentioned above, the age distribution of D. lettowvorbecki is based on the correlation between distal femur widths and age estimations from histological data. However, the age distribution can only be considered as representative if the growth record of the femora is similar to that of other bones of the skeleton. This is often not the case, histological studies on various specimens of the theropod Allosaurus fragilis from the Cleveland Lloyd Quarry in Utah showed that the humerus has a longer or more complete growth record than the femur (Bybee et al. 2006). A similar pattern was found for the basal sauropodomorph Plateosaurus engelhardti, while the growth pattern between different individuals is also highly variable (Klein 2004; Sander and Klein 2005). In P. lujiatunensis, however, different bones seem to show a comparably long growth record (Zhao et al. 2013). As in A. fragilis and P. engelhardti, the bone histological data of D. lettowvorbecki revealed that the humerus has a longer or more complete growth record (maximum age >20 years) than the tibia (maximum age 17 years) and the femur (maximum age 16 years) (Hübner 2011, 2012). However, by applying a regression analysis we were able to estimate an age of 20 years for the largest preserved femur. Thus, the differences in the growth record of different bones in D. lettowvorbecki is probably an artefact of bone selection and the inherent variation of growth record preservation between skeletal elements.
In contrast to A. sarcophagus, P. lujiatunensis, and Maiasaura peeblesorum, the overall age-distribution d(x) of D. lettowvorbecki has a bimodal U-shape, which results from the small number of individuals at ages 0–3, 9–12, and 18–20. A similarly shaped distribution was previously recognised based on the distal width of the femora and tibiae by Hübner (2011, 2012) and femur length by Heinrich (1999b). The scarcity of early juvenile individuals (0–3 years) in the sample could have resulted from a higher hunting pressure on juveniles by predators (Hone and Rauhut 2010), which reduced the size of the cohort. Furthermore, small individuals appear to have had a lower likelihood of preservation and discovery (Brown et al. 2013), so that these low numbers could be a preservational artefact. A third option is that early juveniles were organized in schools and lived separately from reproductively active adults. Such a behaviour is proposed for P. lujiatunensis (Zhao et al. 2007, 2013) and the basal ornithomimosaur Sinornithosaurus dongi (Varricchio et al. 2008), but the exceptional record of a hadrosaur tracksite from Alaska shows that this behaviour is not omnipresent among dinosaurs (Fiorillo et al. 2014). Since early juvenile individuals are present in the sample, compulsive schooling behavior is rejected for D. lettowvorbecki.
Most speculative is the hypothesis that the youngest age class (<1 year old) was simply not present in the group at the time of death because this event took place outside of the breeding season (see e.g., Hurlburt 1982). A possible hint to such a scenario is the strongly seasonal character of the Jurassic paleoenvironment in the Tendaguru area (Heinrich et al. 2011; Redelstorff et al. 2013). The absence of eggshells at the locality, the age of the youngest recorded individuals of just one year, and the presence of possible medullary bone tissue in two specimens, indicating that the next breeding season might have been imminent (Hübner 2012), are additional potential signs for this scenario. Assuming that D. lettowvorbecki had only one breeding season per year, the youngest age class would already have been about one year of age at the time of the death event.
The small sample of old individuals in the deposit likely resulted from an increased probability of death, which is a natural consequence of aging (Gotelli 2008). More exceptional is the gap between 9–12 years, leading to the bimodal U-shaped age distribution. Interestingly, this gap is associated with a slight decrease in growth rate, starting approximately around the age of 10 years (Hübner 2011, 2012). The simultaneous decrease in growth rate and age distribution in D. lettowvorbecki could be linked to the onset of sexual maturity (Hübner 2012). If this interpretation is correct, the underrepresentation of these age classes in the data set could indicate that individuals reaching sexual maturity were temporarily excluded from the herd, as in Blue Wildebeest (Connochaetes taurinus; Estes 2012). Thus, the underrepresentation of individuals of D. lettowvorbecki of these age classes could represent a demographic bias in the age structure of the herd.
The comparison of the age distribution between BB-3 and BB-4 shows that the latter contains more individuals than BB-3. However, no significant difference in the age distributions could be found when the whole lifespan is analysed, although both bonebeds differ significantly in the composition of individuals younger than 11 years. If all individuals of D. lettowvorbecki were members of one herd that died in a single event, and were subsequently separated into different bonebeds postmortem, the age distribution should reflect a taphonomic separation of the bonebeds in terms of body size. As a consequence of such a scenario, one would expect a positively skewed distribution in BB-4 with a continuous decline in the number of individuals with increasing age, while the age distribution in BB-3 should be negatively skewed. Although both bonebeds differ in the number of juvenile individuals, their age distributions do not show these particular patterns. The high number of old individuals in both layers better supports a scenario where the two bonebeds represent different herds with different compositions of young individuals that died during different events. This is further supported by the absence of skeletal remains from the intervening sedimentary layers, which measure approximately 20–50 cm in thickness. If these layers are the result of simple reworking events, then they should include at least a small number of embedded bones. Such volumes of sediment would also indicate high transport energies that broadly dispersed the carcasses over the deposit. However, the spatial restriction of the bonebeds and the absence of abrasion on the specimens indicate higher energy deposition is not as likely.
The age distribution in BB-4 is very similar to that seen in deposits of the basal ceratopsid P. lujiatunensis (Erickson et al. 2009a) and the hadrosaurid M. peeblesorum (Woodward et al. 2015), which are both dominated by hatchling individuals and early juveniles that reflect catastrophic death assemblages. In contrast, the age distribution in BB-3 appears closer to that of deposits containing D. torosus and T. rex, which are dominated by older individuals and represent attritional death assemblages (i.e., random collection of individuals from a population that differ from each other in time, place and cause of death) (Erickson et al. 2006). The usual deposition of D. torosus and T. rex (Russell 1970; Currie et al. 2005; Larson and Carpenter 2008) is, however, very different from the taphonomic situation of the Ig/WJ-locality and does not allow a direct comparison between the three species. Assuming a similar mode of death (drowning in a tidal channel) for the D. lettowvorbecki bonebeds, the different age structures of the two bonebeds BB-3 and BB-4 most likely do not reflect different types of death assemblages (i.e., attritional and catastrophic), but rather indicate variable herd structures for D. lettowvorbecki, which is also known in the Blue Wildebeest (Estes 2012). Various discoveries of multiple associated specimens, differing in terms of the number of individuals and their age distributions, indicate an analogous pattern in P. lujiatunensis (Meng et al. 2004; Zhao et al. 2007, 2013; Hedrick et al. 2014).
Attritional or catastrophic assemblage.—At least ten taxa of vertebrates were identified at the Ig/WJ-locality (dinosaurs and other vertebrates), but more than 90% of the recorded specimens in all bonebeds belong to D. lettowvorbecki. The bonebeds can therefore be classified as multitaxic and monodominant, with high taxonomic diversity (see Eberth et al. 2007). According to Eberth et al. (2007: 120), monodominant bonebeds occurring at a specific site can be interpreted as either catastrophic, short-term mass death events or as multiple (attritional) death events. However, in most cases monospecific or monodominant bonebeds of dinosaurs are usually interpreted as the result of a mass mortality event (e.g., Hennig 1924; Currie and Dodson 1984; Rogers 1990; Varricchio and Horner 1993; Schwartz and Gillette 1994; Eberth and Getty 2005; Brinkman et al. 2007; Myers and Fiorillo 2009; Scherzer and Varricchio 2010; Bell and Campione 2014; Chiba et al. 2015), with only a small number of dinosaur bonebeds interpreted as being derived from attritional mortality (e.g., Lauters et al. 2008; Gates 2005). Because the bonebeds of the Ig/WJ-locality are part of a tidal channel, mass drowning is a plausible scenario for the assemblages of D. lettowvorbecki, as occurrences in extant wildebeest suggest (see Dechant-Boaz 1982; Capaldo and Peters 1995: 402, table 11).
The distribution of size and age classes in D. lettowvorbecki corresponds to a U-shaped bimodal distribution, which is often interpreted as the result of attritional mortality of the youngest juvenile, youngest mature and oldest-aged animals (e.g., Heinrich 1999b; Lyman 1994; Voorhies 1969; Lauters et al. 2008). In contrast, a catastrophic mass death event is expected to have a rather left-skewed L-shaped size distribution representing a living population or herd due to the superior number of juvenile and subadult individuals with respect to older individuals (e.g., Klein 1982a,b; Lyman 1994; Woodward et al. 2015).
However, the application of shape of size and age distributions can be ambiguous. Klein (1982b) has shown for large-bodied mammals that both the L-shape and the U-shape distribution can fit either catastrophic or attritional models, respectively, depending on the number of offspring of each female per year. If females produce more than one offspring per year, the attritional size distribution becomes more L-shaped, making a clear differentiation between catastrophic and attritional mortality impossible (Klein 1982a). This may also be the case for the Ig/WJ-assemblages, as female dinosaurs, including ornithopods, generally produced more than one offspring per year (Horner 1999; Grigorescu et al. 2010). These problems are also documented in mass assemblages of extinct mammals. For instance, the attritional assemblages of the fossil rhinoceros Teleoceras from the Miocene of Florida are not U-shaped, as expected, but have a peak among the young adults, especially young males (Mihlbachler 2003). On the other hand, some catastrophic mammal assemblages, such as fossil rhinos of the Miocene Poison Ivy Quarry, Nebraska (Voorhies 1985) and titanotheres from the Eocene of Wyoming (Turnbull and Martill 1988), have clearly differing shapes of their size distributions, resembling an attritional age distribution. The former has a U-shaped bimodal distribution (despite of the catastrophic death by volcanic ash) and the latter a trimodal distribution.
As noted above, the underrepresentation of juveniles in many bonebeds, including the Ig/WJ-locality, has likely resulted from higher predation pressure on the living population/herd of animals (Hone and Rauhut 2010) and a smaller likelihood for preservation due to scavenging and hydraulic transport (Brown et al. 2013; Heinrich 1999b; Hulburt 1982; Klein 1982b; Kurten 1953; Mihlbachler 2003; Sander 1992; Varricchio and Horner 1993; Voorhies 1969). However, the herd structure (including cohorts of juveniles) or the specific time of death might also be influential. In addition, the general underrepresentation of smaller and more easily entrained elements in the D. lettowvorbecki bonebeds could indicate that the actual number of juvenile individuals in the assemblages was higher before hydraulic sorting swept smaller carcasses and the skeletal elements of smaller individuals further downstream. A similar observation was made by Capaldo and Peters (1995: 392), who reported the mass drowning of a herd of wildebeest, including numerous calves, in the Serengeti. During a subsequent investigation of the death site, they found only adult remains in the resulting bone assemblage. Thus, by incorporating the possible underrepresentation of juveniles in the bonebeds, the original size-frequency distribution of the D. lettowvorbecki death assemblage might have been much more left-skewed than actually preserved. Consequently, the simple assignment of the shape of a size-frequency distribution to either a catastrophic or attritional mortality pattern might be problematic as taphonomic influence on the assemblage, reproduction strategy, and social behavior can lead to the under- or overrepresentation of single age classes.
Conclusions
Although the four preserved bonebeds are stratigraphically and spatially closely related, it is highly unlikely that they were formed by a single catastrophic event. This view is supported by the bone-free sediment layer of 20–50 cm thickness intercalated between BB-3 and BB-4 that cannot be explained by a single spring tide, as proposed by Hübner (2011). This is also supported by the differing size/age-distributions of BB-3 and BB-4 showing that each layer represents a single herd. Thus, up to four separate events may have triggered the genesis of the bonebeds at the Ig/WJ-locality.
It is likely that the two large bonebeds BB-3 and BB-4 resulted from two independent catastrophic mortality events of Dysalotosaurus lettowvorbecki. The catastrophic origin is supported by the repeated dominance of this dinosaur species in these assemblages, the mixed size/age composition and the consistent preservation of the specimens. The unique U-shape of the age distributions of BB-3 and BB-4 can be explained by taphonomic sorting processes (for the absence of early juvenile individuals) and by social and reproductive behaviour (i.e., herd structure and the onset of sexual maturity) and is consistent with the hypothesis of multiple catastrophic events.
The high concentration of bones in a restricted area, combined with the sedimentological characteristics, suggests that the carcasses were washed in from the place of death into a barrier or bend of the tidal/fluvial channel where they accumulated and decomposed. The generally excellent preservation of specimens at the locality suggests only minor transport duration as well as short preburial exposure time. The observed E-W orientation of long bones as well as the roughly elongate extension of BB-3 (Janensch 1914b) nevertheless indicate the influence of currents on the previously decomposed carcasses, which is further supported by the extensive disarticulation of the skeletons and the postulated influence of wave action during crystallization processes within some of the marrow cavities (Chinsamy-Turan 2005). However, this seems to have happened close to or even at the place of final burial.
The lack of sedimentological data directly from the locality hampers the estimation of the time interval between the deposition of each of the bonebeds, but their close association can be interpreted as the result of repeated drowning events of groups of D. lettowvorbecki in approximately the same area, such as at a crossing of a migration route of this dinosaur. The repeated occurrence of bonebeds in such a restricted area is remarkably similar to observations on the migration pattern of some populations of modern blue wildebeest (Connochaetes taurinus) in Northern Tanzania (Fig. 16). This seasonal migration is triggered by seasonal rainfall and local food availability (Capaldo and Peters 1995; Geraci 2011; Estes 2012). During migration, the herds of blue wildebeest cross lakes and rivers, where several hundreds and sometimes even thousands of individuals may drown each time due to a flood, strong currents, or steep and slippery river banks (e.g., Dechant-Boaz 1982; Capaldo and Peters 1995; Pastino 2007; Cobo-Sanchez et al. 2014). Interestingly, the youngest age class of blue wildebeest is always absent from these migrating groups, because the calves are born elsewhere and have several months to grow during the favorable rainy season before the migration starts again (Estes 2012). Calves of eight months of age or older are also often expulsed and form bachelor herds (Estes 2012), which results in the underrepresentation of this age class in the large female groups.

Fig. 16. A group of blue wildebeest (Connochaetes taurinus) crossing the Mara River, East Africa. Photo by Eric Inafuku, Wikimedia commons (https://commons.wikimedia.org/wiki/File:Connochaetes_taurinus_-Wildebeest_crossing_river_-East_Africa.jpg).
Although the life history between birth and adulthood is much shorter in blue wildebeest than in D. lettowvorbecki and the sex of the latter is indeterminable, apart from two possible exceptions (Hübner 2012: 7, fig. 11), the biological observations made on the blue wildebeest are congruent with the taphonomy and demography found in D. lettowvorbecki at the Ig/WJ-site. It is astounding, therefore, that two totally unrelated vertebrate herbivores, one mammalian and the other dinosaurian, are linked by the same kind of catastrophic death events in approximately the same region of the world, separated in time by 150 million years.
Acknowledgements
This study would not have been possible without the dedication of many African workers who were hired by the German Tendaguru Expedition between 1909 and 1913 to excavate the bones for a comparatively small wage. We would therefore like to use the Acknowledgements section to explicitly thank these men for their profound work and to acknowledge that the fossils we have used for this study are the result of many hours of their hard and undoubtedly exhaustive labour, including the excavation, labelling and packing of this material and its transport to the coast. We are thankful to the current as well as former curators of further collections housing material of Dysalotosaurus lettowvorbecki, who gave us access to the material, namely Rainer Schoch (SMNS), Philip Havlik (formerly GPIT), Sandra Chapman (formerly NHMUK), Oliver Rauhut (BSPG), and Mike Reich (formerly GZG). We also like to thank Guido Fritsch (IZW) for CT scanning of the bamboo corsets from the MfN collections. We are very grateful to Alexander Scheuerlein (University of Greifswald, Germany) for discussion and Gerhard Maier (Calgary, Canada) for his valuable comments on the manuscript. Finally, we thank Matthew Herne and Phil Bell (University of New England, Armidale, Australia) for their valuable comments that greatly improved our manuscript. CF was supported by the German Science Foundation under grant FO 1005/2-1 and the Swiss National Science Fond under grant PZ00P2_174040/1.
References
Aberhan, M., Bussert, R., Heinrich, W.-D., Schrank, E., Schultka, S., Sames, B., Kriwet, J., and Kapilima, S. 2002. Paleoecology and depositional environments of the Tendaguru Beds (Late Jurassic to Early Cretaceous, Tanzania). Mitteilungen aus dem Museum für Naturkunde Berlin, Geowissenschaftliche Reihe 2: 201–205. Crossref
Behrensmeyer, A.K. 1975. The taphonomy and paleoecology of Plio-Pleistocene vertebrate assemblages east of Lake Rudolf, Kenya. Bulletin of the Museum of Comparative Zoology 146: 473–578.
Behrensmeyer, A.K. 1978. Taphonomic and ecologic information from bone weathering. Paleobiology 4: 150–162. Crossref
Bell, P.R. and Campione, N.E. 2014. Taphonomy of the Danek Bonebed: a monodominant Edmontosaurus (Hadrosauridae) bonebed from the Horseshoe Canyon Formation, Alberta. Canadian Journal of Earth Sciences 51: 992–1006. Crossref
von Branca, W. 1914. Kurzer Bericht über die von Dr. Reck erzielten Ergebnisse im vierten Grabungsjahre 1912. Archiv für Biontologie III (1): 59–63.
Brinkman, D.B., Eberth, D.A., and Currie, P.J. 2007. From bonebeds to paleobiology: Applications of bonebed data. In: R.R. Rogers, D.A. Eberth, and A.R. Fiorillo (eds.), Bonebeds—Genesis, Analysis, and Paleobiological Significance, 221–264. The University of Chicago Press, Chicago. Crossref
Britt, B.B., Eberth, D.A., Scheetz, R.D., Greenhalgh, B.W., and Stadtman, K.L. 2009. Taphonomy of debris-flow hosted dinosaur bonebeds at Dalton Wells, Utah (Lower Cretaceous, Cedar Mountain Formation, USA). Palaeogeography, Palaeoclimatology, Palaeoecology 280: 1–22. Crossref
Broschinski, A. 1999. Ein paramacellodides Lacertilier-Fragment aus dem Oberen Jura von Tendaguru/Tansania. Mitteilungen aus dem Museum für Naturkunde Berlin, Geowissenschaftliche Reihe 2: 155–158. Crossref
Brown, C.M., Evans, D.C., Campione, N.E., O’Brien, L.J., and Eberth, D.A. 2013. Evidence for taphonomic size bias in the Dinosaur Park Formation (Campanian, Alberta), a model Mesozoic terrestrial alluvial‐paralic system. Palaeogeography, Palaeoclimatology, Palaeoecology 372: 108–122. Crossref
Bussert, R. and Aberhan, M. 2004. Storms and tsunamis: evidence of event sedimentation in the late Jurassic Tendaguru Beds of southeastern Tanzania. Journal of African Earth Sciences 39: 549–555. Crossref
Bussert, R., Heinrich, W.-D., and Aberhan, M. 2009. The Tendaguru Formation (Upper Jurassic to Lower Cretaceous, southern Tanzania): Definition, palaeoenvironments, and sequence stratigraphy. Fossil Record 12: 141–174. Crossref
Bybee, P.J., Lee, A.H., and Lamm, E.-T. 2006. Sizing the Jurassic theropod dinosaur Allosaurus: assessing growth strategy and evolution of ontogenetic scaling of limbs. Journal of Morphology 267: 347–359. Crossref
Capaldo, S.D. and Peters, C.R. 1995. Skeletal inventories from Wildebeest drownings at Lakes Masek and Ndutu in the Serengeti Ecosystem of Tanzania. Journal of Archaeological Science 22: 385–408. Crossref
Chiba, K., Ryan, M.J., Braman, D.R., Eberth, D.A., Scott, E.E., Brown, C.M., Kobayashi, Y., and Evans, D.C. 2015. Taphonomy of a monodominant Centrosaurus apertus (Dinosauria: Ceratopsia) bonebed from the upper Oldman Formation of southeastern Alberta. Palaios 30: 655–667. Crossref
Chinsamy-Turan, A. 2005. The Microstructure of Dinosaur Bone—Deciphering Biology with Fine-Scale Techniques. 195 pp. Johns Hopkins University Press, Baltimore.
Cobo-Sánchez, L., Aramendi, J., and Domínguez-Rodrigo, M. 2014. Orientation patterns of wildebeest bones on the lake Masek floodplain (Serengeti, Tanzania) and their relevance to interpret anisotropy in the Olduvai lacustrine floodplain. Quaternary International 322–323: 277–284. Crossref
Currie, P.J. and Dodson, P. 1984. Mass death of a herd of ceratopsian dinosaurs. In: W.E. Reif and F. Westphal (eds.), Third Symposium on Mesozoic Terrestrial Ecosystems, Short Papers, 61–66. Attempto Verlag, Tübingen.
Currie, P.J., Badamgarav, D., Koppelhus, E.B., Sissons, R., and Vickaryous, M.K. 2011. Hands, feet, and behaviour in Pinacosaurus (Dinosauria: Ankylosauridae). Acta Palaeontologica Polonica 56: 489–504. Crossref
Currie, P.J., Trexler, D., Koppelhus, E.B., Wicks, K., and Murphy, N. 2005. An unusual multi-individual tyrannosaurid bonebed in the Two Medicine Formation (Late Cretaceous, Campanian) of Montana (USA). In: K. Carpenter (ed.), The Carnivorous Dinosaurs, 313–324. Indiana University Press, Bloomington.
Dechant-Boaz, D. 1982. Modern Riverine Taphonomy: Its Relevance to the Interpretation of Plio-Pleistocene Hominid Paleoecology in the Omo Basin, Ethiopia. 376 pp. Ph.D. Thesis. Department of Anthropology, University of California, Berkeley.
Eberth, D.A. 2015. Origins of dinosaur bonebeds in the Cretaceous of Alberta, Canada. Canadian Journal of Earth Sciences 52: 655–681. Crossref
Eberth, D.A. and Getty, M.A. 2005. Ceratopsian bonebeds: occurrence, origins, and significance. In: P.J. Currie and E.B. Koppelhus (eds.), Dinosaur Provincial Park: A Spectacular Ecosystem Revealed. 501–536, Indiana University Press, Bloomington.
Eberth, D.A., Brinkman, D.B., and Barkas, V. 2010. A centrosaurine megabonebed from the Upper Cretaceous of southern Alberta: implications for behaviour and death events. In: M.J. Ryan, B. Chinnery-Allgeier, and D.A. Eberth (eds.), New Perspectives on Horned Dinosaurs: The Ceratopsian Symposium at the Royal Tyrrell Museum, September 2007, 495–508, Indiana University Press, Bloomington.
Eberth, D.A., Evans, D.C., and Lloyd, D.W.H. 2014. Occurrence and taphonomy of the first documented hadrosaurid bonebed from the Dinosaur Park Formation (Belly River Group, Campanian) at Dinosaur Provincial Park, Alberta, Canada. In: D.A. Eberth and D.C. Evans (eds.), Hadrosaurs, 502–523. Indiana University Press. Bloomington.
Eberth, D.A., Shannon, M., and Noland, B.G. 2007. A bonebeds database: classification, biases, and patterns of occurrence. In: R.R. Rogers, D.A. Eberth, and A.R. Fiorillo (eds.), Bonebeds—Genesis, Analysis, and Paleobiological Significance, 103–220. The University of Chicago Press, Chicago. Crossref
Erickson, G.M., Currie, P.J., Inouye, B.D., and Winn, A.A. 2006. Tyrannosaur life tables: an example of nonavian dinosaur population biology. Science 313: 213–217. Crossref
Erickson, G.M., Currie, P.J., Inouye, B.D., and Winn, A.A. 2010. A revised life table and survivorship curve for Albertosaurus sarcophagus based on the Dry Island mass death assemblage. Canadian Journal of Earth Sciences 47: 1269–1275. Crossref
Erickson, G.M., Makovicky, P.J., Inouye, B.D., Zhou, C.-F., and Gao, K.-Q. 2009a. A life table for Psittacosaurus lujiatunensis: initial insights into ornithischian dinosaur population biology. The Anatomical Record 292: 1514–1521. Crossref
Erickson, G.M., Makovicky, P.J., Inouye, B.D., Zhou, C., and Gao, K. 2009b. ERRATUM: A life table for Psittacosaurus lujiatunensis: initial insights into ornithischian dinosaur population biology. The Anatomical Record 292: 1684. Crossref
Estes, R.D. 2012. The Behavior Guide to African Mammals Including Hoofed Mammals, Carnivores, Primates. 20th Anniversary Edition. 611 pp. University of California Press, Berkeley.
Fernandez-Jalvo, Y. and Andrews, P. 2003. Experimental effects of water abrasion on bone fragments. Journal of Taphonomy 1: 147–163.
Fiorillo, A.R. 1988. Taphonomy of Hazard Homestead Quarry (Ogallala Group), Hitchcock County, Nebraska. University of Nebraska Contributions to Geology 26: 57–97.
Fiorillo, A.R. 1991. Taphonomy and depositional setting of Careless Creek Quarry (Judith River Formation), Wheatland County, Montana, U.S.A. Palaeogeography, Palaeoclimatology, Palaeoecology 81: 281–311. Crossref
Fiorillo, A.R., Hasiotis, S.T., and Kobayashi. Y. 2014. Herd structure in Late Cretaceous polar dinosaurs: A remarkable new dinosaur tracksite, Denali National Park, Alaska, USA. Geology 42: 719–722. Crossref
Fraas, E. 1908. Ostafrikanische Dinosaurier. Palaeontographica 55 (2): 105–144.
Galton, P.M. 1981. Dryosaurus, a hypsilophodontid dinosaur from the Upper Jurassic of North America and Africa. Postcranial skeleton. Paläontologische Zeitschrift 55: 271–312. Crossref
Galton, P.M. 1983. The cranial anatomy of Dryosaurus, a hypsilophodontid dinosaur from the Upper Jurassic of North America and East Africa, with a review of hypsilophodontids from the Upper Jurassic of North America. Geologica et Palaeontologica 17: 207–243.
Gangloff, R.A. and Fiorillo, A.R. 2010. Taphonomy and paleoecology of a bonebed from the Prince Creek Formation, North Slope, Alaska. Palaios 25: 299–317. Crossref
Gates, T. 2005. The Late Jurassic Cleveland–Lloyd dinosaur quarry as a drought-induced assemblage. Palaios 20: 363–375. Crossref
Geraci, G. 2011. “Connochaetes taurinus”, Animal Diversity Web, https://animaldiversity.org/accounts/Connochaetes_taurinus/
Gotelli, N.J. 2008. A Primer of Ecology. 290 pp. Oxford University Press, Sunderland.
Grigorescu, D., Garcia, G., Csiki, Z., Codrea, V., and Bojar, A.-V. 2010. Uppermost Cretaceous megaloolithid eggs from the Haţeg Basin, Romania, associated with hadrosaur hatchlings: Search for explanation. Palaeogeography, Palaeoclimatology, Palaeoecology 293: 360–374. Crossref
Hammer, Ø. and Harper, D.A.T. 2006. Paleontological Data Analysis. 351 pp. Blackwell Publishing, Oxford. Crossref
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4 (1): 1–9.
Haynes, G. 1983. Frequencies of spiral and green-bone fractures on ungulate limb bones in modern surface assemblages. American Antiquity 48: 102–114. Crossref
Hedrick, B.P., Gao, C., Omar, G.I., Zhang, F., Shen, C., and Dodson, P. 2014. The osteology and taphonomy of a Psittacosaurus bonebed assemblage of the Yixian Formation (Lower Cretaceous), Liaoning, China. Cretaceous Research 51: 321–340. Crossref
Heinrich, W.-D. 1998. Late Jurassic mammals from Tendaguru, Tanzania, East Africa. Journal of Mammalian Evolution 5(4): 269–290. Crossref
Heinrich, W.-D. 1999a. First haramiyid (Mammalia, Allotheria) from the Mesozoic of Gondwana. Mitteilungen aus dem Museum für Naturkunde in Berlin, Geowissenschaftliche Reihe 2: 159–170. Crossref
Heinrich, W.-D. 1999b. The taphonomy of dinosaurs from the Upper Jurassic of Tendaguru (Tanzania) based on field sketches of the German Tendaguru Expedition (1909–1913). Mitteilungen aus dem Museum für Naturkunde in Berlin, Geowissenschaftliche Reihe 2: 25–61. Crossref
Heinrich. W.-D. 2001. New records of Staffia aenigmatica (Mammalia, Allotheria, Haramiyida) from the Upper Jurassic of Tendaguru in southeastern Tanzania, East Africa. Mitteilungen aus dem Museum für Naturkunde Berlin, Geowissenschaftliche Reihe 4: 239–255. Crossref
Heinrich, W.D., Bussert, R., and Aberhan, M. 2011. A blast from the past: the lost world of dinosaurs at Tendaguru, East Africa. Geology Today 27 (3): 101–106. Crossref
Hennig, E. 1909–1911. Diaries. Unpublished manuscript deposited at MfN, Pal. Mus SII, Tendaguru-Expedition 8.7.
Hennig, E. 1924. Kentrurosaurus aethiopicus—Die Stegosaurier-Funde vom Tendaguru, Deutsch-Ostafrika. Palaeontographica Supplement VII 1 (2): 100–253.
Hennig, E. 1936. Ein Dentale von Kentrurosaurus aethiopicus Hennig. Palaeontographica Supplement VII 2: 309–312.
Heumann, I., Stoecker, H., Tamborini, M., and Vennen, M. 2018. Dinosaurierfragmente. Zur Geschichte der Tendaguru Expedition und ihrer Objekte, 1906–2018. 311 pp. Wallstein, Göttingen.
Hone, D.W.E. and Rauhut, O.W.M. 2010. Feeding behaviour and bone utilization by theropod dinosaurs. Lethaia 43: 232–244. Crossref
Horner, J.R. 1999. Egg clutches and embryos of two hadrosaurian dinosaurs. Journal of Vertebrate Paleontology 19: 607–611. Crossref
Hübner, T.R. 2011. Ontogeny in Dysalotosaurus lettowvorbecki. 318 pp. Ph.D. Thesis, Ludwig-Maximilians-Universität, München.
Hübner, T.R. 2012. Bone histology in Dysalotosaurus lettowvorbecki (Ornithischia: Iguanodontia)—variation, growth, and implications. PLoS ONE 7 (1): e29958. Crossref
Hübner, T.R. 2018. The postcranial ontogeny of Dysalotosaurus lettowvorbecki (Ornithischia: Iguanodontia) and implications for the evolution of ornithopod dinosaurs. Palaeontographica A: Palaeozoology—Stratigraphy 310 (3–6): 43–120. Crossref
Hübner, T.R. and Rauhut, O.W.M. 2010. A juvenile skull of Dysalotosaurus lettowvorbecki (Ornithischia: Iguanodontia), and implications for cranial ontogeny, phylogeny, and taxonomy in ornithopod dinosaurs. Zoological Journal of the Linnean Society 160: 366–396. Crossref
Hübner, T.R., Foth, C., Heinrich, W.-D., and Bussert, R. 2014. Taphonomy of four associated bonebeds of the ornithopod dinosaur Dysalotosaurus lettowvorbecki from the Upper Jurassic Tendaguru Formation, Tanzania. Journal of Vertebrate Paleontology, 74th Annual Meeting. Program and Abstracts, 150. Society of Vertebrate Paleontology, Berlin.
Hughes, Z. J. 2012. Tidal channels on tidal flats and marshes. In: R.A. Davies Jr. and R.W. Dalrymple (eds.), Principles of Tidal Sedimentology, 269–300, Springer, Dordrecht. Crossref
Hurlburt, R.C., Jr. 1982. Population dynamics of the three-toed horse Neohipparion from the Late Miocene of Florida. Paleobiology 8: 159–167. Crossref
Janensch, W. 1910. Correspondence, October 22, 1910. Pal. Mus SII, Tendaguru-Expedition 5.1.
Janensch, W. 1909–1911. GTE Field Catalogue. Pal. Mus SII, Tendaguru-Expedition 8.5.
Janensch, W. 1914a. Bericht über den Verlauf der Tendaguru-Expedition. Archiv für Biontologie 3 (1): 15–58.
Janensch, W. 1914b. Die Gliederung der Tendaguruschichten im Tendagurugebiet und die Entstehung der Saurierlagerstätten. Archiv für Biontologie 3: 226–261.
Janensch, W. 1925a. Die Coelurosaurier und Theropoden der Tendaguru-Schichten Deutsch-Ostafrikas. Palaeontographica Supplement VII 1: 1–97.
Janensch, W. 1925b. Die Grabungsstellen der Tendaguru-Gegend. Palaeontographica Supplement VII 1: 17–19.
Janensch, W. 1950. Die systematische Stellung des Ornithopoden Dysalotosaurus aus den Tendaguru-Schichten. Neues Jahrbuch für Geologie und Paläontologie Monatshefte 1950: 286–287.
Janensch, W. 1955. Der Ornithopode Dysalotosaurus der Tendaguru-Schichten. Palaeontographica Supplement VII 1 (3): 105–176.
Janensch, W. 1961. Die Gliedmaßen und Gliedmaßengürtel der Sauropoden der Tendaguruschichten. Palaeontographica Supplement VII 1 (3): 177–235.
Klein, R.G. 1982a. Age (mortality) profiles as a means of distinguishing hunted species from scavenged ones in Stone Age archeological sites. Paleobiology 8: 151–158. Crossref
Klein, R.G. 1982b. Patterns of ungulate mortality and ungulate mortality profiles from Langebaanweg (Early Pliocene) Elandsfontein (Middle Pleistocene), south-western Cape Province, South Africa. Annals of the South African Museum 90: 49–94.
Klein, N. 2004. Bone Histology and Growth of the Prosauropod Dinosaur Plateosaurus engelhardti Meyer, 1837 from the Norian Bonebeds of Trossingen (Germany) and Frick (Switzerland). 128 pp. Ph.D. Thesis, Rheinische Friedrich-Wilhelms-Universität, Bonn.
Kurten, B. 1953. On the variation and population dynamics of fossil and recent mammal populations. Acta Zoologica Fennica 76: 1–121.
Larson, P.L. and Carpenter, K. 2008. Tyrannosaurus rex, The Tyrant King. 456 pp. Indiana University Press, Bloomington.
Lauters, P., Bolotsky, Y.L., Vanitterbeeck, J., and Godefroit, P. 2008. Taphonomy and age profile of a latest Cretaceous dinosaur bonebed in far eastern Russia. Palaios 23: 153–162. Crossref
Lyman, R.L. 1994. Vertebrate Taphonomy. 524 pp. Cambridge University Press, Cambridge. Crossref
Maier, G. 2003. African Dinosaurs Unearthed. The Tendaguru Expeditions. 368 pp. Indiana University Press, Bloomington. Crossref
Meng, Q., Liu, J., Varricchio, D.J., Huang, T., and Gao, C. 2004. Parental care in an ornithischian dinosaur. Nature 431: 145–146. Crossref
Mihlbachler, M.C. 2003. Demography of late Miocene rhinoceroses (Teleoceras proterum and Aphelops malacorhinus) from Florida: linking mortality and sociality in fossil assemblages. Paleobiology 29: 412–428. Crossref
Myers, T.S. and Fiorillo, A.R. 2009. Evidence for gregarious behavior and age segregation in sauropod dinosaurs. Palaeogeography, Palaeoclimatology, Palaeoecology 274: 96–104. Crossref
Parkinson, J. 1930. The Dinosaur in East Africa: An account of the giant reptile beds of Tendaguru, Tanganyika Territory, 192 pp. H.F. & G. Witherby, London.
Pastino de, B. 2007. 10,000 Wildebeest Drown in Migration “Pileup”. National Geographic News October 1, 2007 [published online, http://palaeoblog.blogspot.com/2007/10/mass-drowning-of-wildebeest-in-kenya.html].
Pompeckj, J.F. 1920. Das angebliche Vorkommen und Wandern des Parietalforamens bei Dinosauriern. Sitzungsberichte der Gesellschaft Naturforschender Freunde zu Berlin 1920 (3): 109–129.
Rauhut, O.W.M. and Carrano, M.T. 2016. The theropod dinosaur Elaphrosaurus bambergi Janensch, 1920, from the Late Jurassic of Tendaguru, Tanzania. Zoological Journal of the Linnean Society 178: 546–610. Crossref
Reck, H. 1912. 2nd Report, June 12, 1912. Pal. Mus SII, Tendaguru-Expedition 9.5. Crossref
Reck, H. 1912. 3rd Report, June 22, 1912. Pal. Mus SII, Tendaguru-Expedition 9.5.
Reck, H. 1912. 4th Report, July 5, 1912. Pal. Mus SII, Tendaguru-Expedition 9.5.
Reck, H. 1912. 7th Report, September 10, 1912. Pal. Mus SII, Tendaguru-Expedition 9.5.
Reck, H. 1912. 8th Report, September 15, 1912. Pal. Mus SII, Tendaguru-Expedition 9.5.
Reck, H. 1912. 9th Report, October 7, 1912. Pal. Mus SII, Tendaguru-Expedition 9.5.
Reck, H. 1913. 13th Report, January 26, 1913. Pal. Mus SII, Tendaguru-Expedition 9.5.
Reck, H. 1913. GTE Field Catalogue, 1912–1913. Pal. Mus SII, Tendaguru-Expedition 9.3.
Reck, H. 1914. Correspondence, February 10, 1914. Pal. Mus SII, Tendaguru-Expedition 9.3.
Reck, H. 1925. Grabungen auf fossile Wirbeltiere in Deutsch-Ostafrika. Geologische Charakterbilder 31: 1–36.
Redelstorff, R., Hübner, T.R., Chinsamy, A., and Sander, P.M. 2013. Bone histology of the stegosaur Kentrosaurus aethiopicus (Ornithischia: Thyreophora) from the Upper Jurassic of Tanzania. The Anatomical Record 296 (6): 933–952. Crossref
Richter, A. and Wuttke, M. 2012. Analysing the taphonomy of Mesozoic lizard aggregates from Uña (eastern Spain) by X-ray controlled decay experiments. Palaeobiodiversity and Palaeoenvironments 92: 5–28. Crossref
Rogers, R.R. 1990. Taphonomy of three dinosaur bone beds in the Upper Cretaceous Two Medicine Formation of northwestern Montana—evidence for drought-related mortality. Palaios 5 (5): 394–413. Crossref
Russell, D.A. 1970. Tyrannosaurs from the Late Cretaceous of western Canada. National Museum of Natural Sciences, Publications in Palaeontology 1: 1–34.
Russell, D., Beland, F., and McIntosh, J.S. 1980. Paleoecology of the dinosaurs of Tendaguru (Tanzania). Memoires de Societe Geologique de France 59 (139): 169–175.
Ryan, M.J., Russell, A.P., Eberth, D.A., and Currie, P.J. 2001. The taphonomy of a Centrosaurus (Ornithischia: Ceratopsidae) bonebed from the Dinosaur Park Formation (Upper Campanian), Alberta, Canada, with comments on cranial ontogeny. Palaios 16: 482–506. Crossref
Sander, P.M. 1992. The Norian Plateosaurus bonebeds of central Europe and their taphonomy. Paleogeography, Paleoclimatology, Paleoecology 93: 255–299. Crossref
Sander, P.M. and Klein, N. 2005. Developmental plasticity in the life history of a prosauropod dinosaur. Science 310: 1800–1802. Crossref
Scherzer, B.A. and Varricchio, D. 2010. Taphonomy of a juvenile lambeosaurine bonebed from the Two Medicine Formation (Campanian) of Montana, United States. Palaios 25 (12): 780. Crossref
Schrank, E. 2005. Dinoflagellate cysts and associated aquatic palynomorphs from the Tendaguru Beds (Upper Jurassic–Lower Cretaceous) of southeast Tanzania. Palynology 29: 49–85. Crossref
Schwartz, H.L. and Gillette, D.D. 1994. Geology and taphonomy of the Coelophysis quarry, Upper Triassic Chinle Formation, Ghost Ranch, New Mexico. Journal of Paleontology 68: 1118–1130. Crossref
Steinsaltz, D. and Orzack, S.H. 2011. Statistical methods for paleodemography on fossil assemblages having small numbers of specimes: an investigation of dinosaur survival rates. Paleobiology 37: 113–125. Crossref
Stoecker, H. 2019. Maji-Maji-Krieg und Mineralien. In: I. Heuman, H. Stoecker, M. Tamborini, and M. Vennen (eds.), Dinosaurierfragmente, 24–37. Wallstein Verlag, Göttingen.
Syme, C.E. and Salisbury, S.W. 2014. Patterns of aquatic decay and disarticulation in juvenile Indo-Pacific crocodiles (Crocodylus porosus), and implications for the taphonomic interpretation of fossil crocodyliform material. Palaeogeography, Palaeoclimatology, Palaeoecology 412: 108–123. Crossref
Turnbull, W.D. and Martill, D.M. 1988. Taphonomy and preservation of a monospecific titanothere assemblage from the Washakie Formation (Late Eocene), southern Wyoming. An ecological accident in the fossil record. Palaeogeography, Palaeoclimatology, Palaeoecology 63: 91–108. Crossref
Unwin, D. and Heinrich, W.-D. 1999. On a pterosaur jaw remain from the Late Jurassic of Tendaguru, East Africa. Mitteilungen aus dem Museum für Naturkunde Berlin, Geowissenschaftliche Reihe 2: 121–134. Crossref
Varricchio, D.J. and Horner, J.R. 1993. Hadrosaurid and lambeosaurid bone beds from the Upper Cretaceous Two Medicine Formation of Montana: taphonomic and biologic implications. Canadian Journal of Earth Science 30: 997–1006. Crossref
Varricchio, D.J., Sereno, P.C., Zhao, X., Tan, L., Wilson, J.A., and Lyon, G.H. 2008. Mud-trapped herd captures evidence of distinctive dinosaur sociality. Acta Palaeontologica Polonica 53: 567–578. Crossref
Voorhies, M.R. 1969. Taphonomy and population dynamics of an early Pliocene vertebrate fauna, Knox County, Nebraska. University of Wyoming, Contributions to Geology Special Paper 1: 1–69. Crossref
Voorhies, M.R. 1985. A Miocene rhinoceros herd buried in volcanic ash. National Geographic Society Research Reports 19: 671–688.
Weigelt, J. 1999. Rezente Wirbeltierleichen und ihre paläobiologische Bedeutung. 3rd Edition. 312 pp. Verlag Dieter W. Berger, Bad Vilbel. [Reprint from Weigelt 1927]
Weon, B.M. 2016. Tyrannosaurs as long-lived species. Scientific Reports 6: 19554. Crossref
White, P.D., Fastovski, D.E., and Sheehan, P.M. 1998. Taphonomy and suggested structure of the dinosaurian assemblage of the Hell Creek Formation (Maastrichtian), Eastern Montana and Western North Dakota. Palaios 13: 41–51. Crossref
Woodward, H.N., Freedman-Fowler, E.A., Farlow, J.O., and Horner, J.R. 2015. Maiasaura, a model organism for extinct vertebrate population biology: a large sample statistical assessment of growth dynamics and survivorship. Paleobiology 41: 503–527. Crossref
Zeigler, K.E., Heckert, A.B., and Lucas, S.G. 2005. Taphonomic analysis of a fire-related Upper Triassic vertebrate fossil assemblage from north-central New Mexico. In: S.G. Lucas, K.E. Zeigler, V.W. Lueth, and D.E. Owen (eds.), New Mexico Geological Society, 56th Field Conference Guidebook, Geology of the Chama Basin, 341–354. New Mexico Bureau of Geology & Mineral Resources, Socorro.
Zhao, Q., Barrett, P.M., and Eberth, D.A. 2007. Social behaviour and mass mortality in the basal ceratopsian dinosaur Psittacosaurus (Early Cretaceous, People’s Republic of China). Palaeontology 50: 1023–1029.
Zhao, Q., Benton, M.J., Xu, X., and Sander, M.J. 2013. Juvenile-only clusters and behaviour of the Early Cretaceous dinosaur Psittacosaurus. Acta Palaeontologica Polonica 59: 827–833. Crossref
Acta Palaeontol. Pol. 66 (2): 275–300, 2021
https://doi.org/10.4202/app.00687.2019