

Endocranium and ecology of Eurotherium theriodis, a European hyaenodont mammal from the Lutetian
MORGANE DUBIED, FLOREAL SOLÉ, and BASTIEN MENNECART
Dubied, M., Solé, F., and Mennecart, B. 2021. Endocranium and ecology of Eurotherium theriodis, a European hyaenodont mammal from the Lutetian. Acta Palaeontologica Polonica 66 (3): 545–554.
Hyaenodonts are extinct placental mammals with a carnivorous diet. Their phylogenetic position among mammals and the relationships within Hyaenodonta are at present partially unresolved. The endocranium is a structure that has rarely been studied in this clade. Using 3D tomography, we studied the endocranium of the European hyaenodont Eurotherium theriodis, discovered in Egerkingen (Switzerland, Lutetian, middle Eocene). Eurotherium theriodis has an endocranium morphology that supports an increase in size of the cerebrum relative to the cerebellum over time within the Hyaenodontoidea. The endocranium also supports a complexification of the cerebrum (i.e., at least two furrows per cerebral hemisphere) within the Hyaenodontoidea and allows us to envisage an increase of the encephalization quotient (EQ), over time. Based on morphology, we consider that its endocranium does not depart from that of the hyaenodontoids known in the Lutetian, Bartonian, and Priabonian of Europe, being less complex than that of the hypercarnivorous Hyaenodon. However, the morphology of its olfactory bulbs and turbinates is similar to that observed in Hyaenodon. The large size of the turbinates of E. theriodis is regarded to be the result of a possible scavenger ecology and agrees with the meat/bone diet envisaged based on the analysis of the morphology of the skull and teeth.
Key words: Mammalia, Hyaenodonta, endocranium, phylogeny, ecology, olfaction, Eocene,
Morgane Dubied [morgane.dubied@u-bourgogne.fr], Biogeosciences, UMR 6282 CNRS, EPHE, Université Bourgogne Franche-Comté, 6 bd Gabriel, 21000 Dijon, France.
Floreal Solé [floreal.sole@naturalsciences.be] (corresponding author), D.O. Earth and History of Life, Royal Belgian Institute of Natural Sciences, Rue Vautier 29, B-1000 Brussels, Belgium.
Bastien Mennecart [mennecartbastien@gmail.com], Natural History Museum Basel, Augustinergasse 2, 4001 Basel, Switzerland; Natural History Museum Vienna, Burgring 7, 1010 Vienna, Austria.
Received 18 May 2020, accepted 12 February 2021, available online 7 September 2021.
Copyright © 2021 M. Dubied et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Hyaenodonts are placental mammals that are mostly carnivorous (Rose 2006). Their radiation, which occurred mainly during the Eocene and Oligocene (Lange-Badré 1979; Gunnell 1998; Solé et al. 2014; Borths et al. 2016; Borths and Stevens 2017), precedes that of the Carnivora. However, uncertainties concern interrelationships among the hyaenodonts (Borths et al. 2016; Solé and Mennecart 2019; Solé et al. 2020), as well as the ecology of the different species (Morlo 1999).
The endocranium of hyaenodonts has been studied rarely, being based only on natural casts (Filhol 1877; Gaudry 1878; Gervais 1870; Klinghardt 1934; Lange-Badré 1979; Piveteau 1961; Radinsky 1977; Savage 1973; Scott 1886). The availability of X-ray computed tomography provides access to internal structures without damaging specimens. Only a few tomographic studies are currently available for this group (Pfaff et al. 2016; Borths et al. 2016; Borths and Seiffert 2017; Borths and Stevens 2017; Solé et al. 2018a, b); it is, however, worth mentioning that the first endocast reconstruction of a hyaenodont mammal based on X-ray microtomography has been recently published (Dubied et al. 2019a, b).
The endocranium is the bony capsule enclosing the brain. The latter organ displays various morphological characteristics (including its size) that provide ecomorphological traits. It has been highlighted that an increased encephalization may be related to arboreality (Mace et al. 1981). Information relating to diet (Pirlot and Stephan 1970), or even to diurnal or nocturnal behavior (Mace et al. 1981) have also been studied.
Each structure of the endocranium can help to identify ecological behaviors, since each part of the endocranium is associated with a sensory or motor function (Barone and Bortolomi 2004). The turbinates are located in the frontal sinuses of the muzzle, in the nasal cavity (Barone and Bortolami 2004). Together with the olfactory bulbs, they are linked to olfactory capability (Barone and Bortolomi 2004). Because the turbinates are not part of the encephalon (grouping together cerebellum, cerebrum, and olfactory bulbs), they are often omitted in published drawings. However, some observations on living species indicate that these structures may reflect hunting strategies (Blanton and Biggs 1969; Van Valkenburgh et al. 2014; Negus 1954).
Finally, comparisons of the morphology of the endocranium between species among a mammal group can make it possible to reconstruct the evolution of the brain and encephalization (Dubied et al. 2019a).
Here we describe the endocranium of the holotype of Eurotherium theriodis (Van Valen, 1965). This hyaenodont is from the Lutetian (Eocene) of Europe (Egerkingen, Switzerland, MP13?–MP14; Aigues-Vives 2, France, ?MP13; MP = Mammal Paleogene reference level of the mammalian biochronological scale for the European Paleogene; Aguilar et al. 1997) (Van Valen 1965; Solé et al. 2015; Figs. 1, 2). Eurotherium includes two other species: Eurotherium mapplethorpei Solé, Morlo, Schaal, and Lehmann, 2021, and Eurotherium matthesi Lange-Badré and Haubold, 1990. Eurotherium mapplethorpei is recorded from the locality of Prémontré (late Ypresian, MP10) (Solé et al. 2021). Eurotherium matthesi is known from the Geiseltal-Untere Unterkohle (Lutetian, MP11) (Lange-Badré and Haubold 1990). However, the most recent cladistic analyses have highlighted that E. theriodis and E. matthesi may not be sister species, implying that the genus must be revised (Solé and Mennecart 2019; Solé et al. 2020, 2021). The phylogenetic position of Eurotherium has never been firmly established, oscillating between a basal hyaenodontid (Borths et al. 2016; Borths and Seiffert 2017) and a derived proviverrine (Rana et al. 2015; Morlo et al. 2013; Solé et al. 2014, 2015). Eurotherium is now considered a hyaenodontid that is closely related to the hypercarnivorous European hyaenodontids Oxyaenoides and Matthodon, and hyaenodontines such as Hyaenodon (Solé and Mennecart 2019; Solé et al. 2020, 2021).
The morphological characteristics of the endocranium and turbinates of this fox-sized mammal allow for a discussion regarding its ecological characteristics, such as its hunting strategy, as well as the evolution of the brain and encephalization among hyaenodonts.
Institutional abbreviations.—NMB, Natural History Museum of Basel, Switzerland.
Other abbreviations.—EQ, encephalization quotient; M/m, upper/lower molar; P/p, upper/lower premolar; RBL, relative blade length; RPS, relative premolar size.
Material and methods
Fossil specimen.—The fossil NMB.Em12 is a complete skull, found in the Swiss locality of Egerkingen, probably Egerkingen γ based on the date it was collected (Figs. 1, 2). It is the holotype of Eurotherium theriodis (Van Valen 1965). Egerkingen corresponds to karst fillings in an aberrant siderolitic facies. Three infillings have been excavated since 1890: these are designated α, β, and γ. Quarries α and β constitute the reference locality of reference level MP14 (generally noted as Egerkingen α + β), while Quarry γ is considered to be slightly older and referred with uncertainty to MP13? (Aguilar et al. 1997), possibly close to the MP13 reference level. Therefore, all the infillings are Lutetian (middle Eocene) in age. NMB.Em12 belongs to the old collections of Egerkingen permanently stored at the Natural History Museum of Basel (Naturhistorisches Museum Basel, NMB).
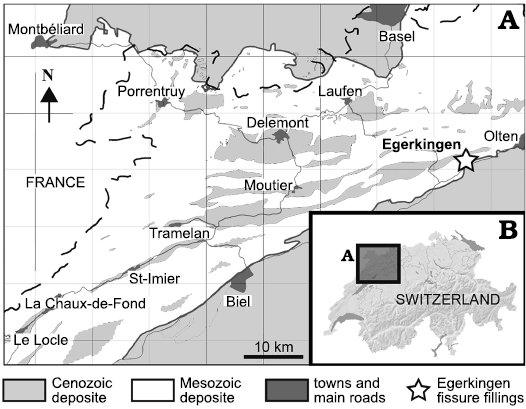
Fig. 1. Geographic position of the fossiliferous locality of Egerkingen (A) in northwest Switzerland (B) (redrawn from Becker 2003: fig. 3-1).
X-ray microtomography.—The X-ray microtomography was performed using a nanoCT® system nanotom® (phoenix X-ray, GE Sensing & Inspection Technologies GmbH, Wunstorf, Germany) hosted at the Biomaterials Science Center of the University of Basel (Switzerland). The scan parameters are 180kV and 30μA for a pixel resolution of 49μm. We digitally segmented the cranium and endocast of NMB.Em12 using AVIZO LITE 9.0. This method allows access to the inner structure of the specimen (herein the endocranium). AVIZO LITE 9.0 was also used for measuring the endocranial structures. The 3D models (skull and endocast) are available at MorphoMuseuM (Dubied et al. 2020).
Nomenclature.—The nomenclature used for the description of the endocranium is based on Denison (1938), Lange-Badré (1979), Barone and Bortolomi (2004), and Orliac et al. (2012).
Body mass.—The body mass has been estimated based on the equation provided by Morlo (1999): Log10(P) = [3.5104 × Log10((ΣM)/3)] – 2.6469. P is the estimated body mass in grams. ΣM is the sum of the length of the three lower molars in mm. To estimate the body mass of E. theriodis, we used the means of the lengths of the three lower molars provided by Van Valen (1965) (Table 1).
Table 1. Encephalization quotients of several hyaenodonts. The values of the body mass have been estimated based on the equation provided by Morlo (1999) (see Material and methods). The values of the endocast volume are from Radinsky (1977) except that for Proviverra typica that is from Dubied et al. (2019a). The encephalization quotients were estimated using the equation provided by Jerison (1973) and Eisenberg (1981) (see Material and methods).
|
Species |
Body |
Endocast volume (cm3) |
Encephalization quotients |
|
|
Jerison (1973) |
Eisenberg (1981) |
|||
|
Tritemnodon agilis |
8893 |
26 |
0.49 |
0.57 |
|
Eurotherium theriodis |
7000 |
26.97 |
0.6 |
0.7 |
|
Proviverra typica |
500 |
>13.45 |
1.74 |
2.46 |
|
Cynohyaenodon cayluxi |
2514 |
12 |
0.53 |
0.66 |
|
Hyaenodon crucians |
9410 |
47 |
0.85 |
0.98 |
|
Hyaenodon horridus |
49233 |
125 |
0.75 |
0.77 |
Encephalization quotient.—The encephalization quotient (EQ) is brain size divided by expected body size for an average mammal of the same body size. As a ratio, it can be used to compare brain sizes among specimens with different body masses (Bertrand et al. 2017). All the EQs herein have first been estimated using the methodology provided by Jerison (1970, 1973); this equation has previously been used to calculate the EQ of Hyaenodon and Cynohyaenodon (Jerison 1973; Radinsky 1977, 1978). The equation is: EQ = E / 0.12 P0.67; where E equals volume of the encephalon in cm3; P, body mass in grams. For the purposes of comparison, we also calculated EQ using the equation provided by Eisenberg (1981): EQ = E / 0.055 P0.74, where E is the volume of the encephalon (in cm3) and P corresponds to body mass (in g). The encephalic volumes of C. cayluxi, H. horridus, H. crucians, and H. brachyrhynchus are based on the estimates made by Radinsky (1977). The cephalic volume of P. typica was estimated by Dubied et al. (2019a).
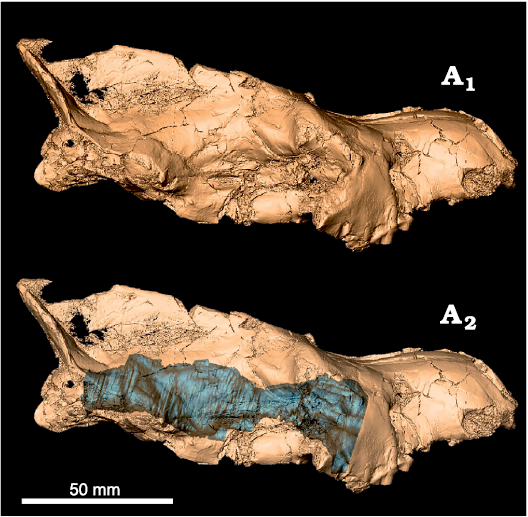
Fig. 2. Digital model of Eurotherium theriodis (Van Valen, 1965), NMB Em12 (holotype) from ?Egerkingen γ (Switzerland), MP13?, with in situ endocast (A2, blue).
Diet.—Based on relative premolar size (RPS) and relative blade length (RBL) the extant Carnivora can be separated into three feeding types (Van Valkenburgh 1988): (i) meat, more than 70% meat in diet; (ii) meat/bone, more than 70% meat with the addition of large bones; (iii) meat/non-vertebrate, 50–70% meat with the balance of fruit and/or insects. The RBL tends to discriminate the meat and meat/bone eaters (highest values) from the meat/non-vertebrate eaters (lowest values), while the RPS discriminates the meat eaters (lowest values) from the meat/bone eaters (highest values). We estimated these two indices for some selected European hyaenodontoids from the Paleogene. The first index was adapted to Hyaenodonta by Friscia and Van Valkenburgh (2010). The method used herein follows that of Solé and Mennecart (2019). However, based on the discrimination generated by the values obtained for European hyaenodontoids, we are able to recognize three distinct diet types: the meat/non-vertebrate eaters have RBL below 0.7; the meat and meat/bone eaters thus have RBL above 0.7; the meat eaters have RPS below 2.65; and the meat/bone eaters have RPS above 2.65.
Systematic palaeontology
Hyaenodonta Van Valen, 1967
Hyaenodontoidea Leidy, 1869
Hyaenodontidae Leidy, 1869
Genus Eurotherium Polly and Lange-Badré, 1993
Type species: Eurotherium theriodis, Van Valen, 1965, ?Egerkingen γ, Switzerland, Lutetian, MP13?.
Eurotherium theriodis (Van Valen, 1965)
Material.—NMB.Em12 (holotype), a complete skull bearing right P3–P4 and M1, left P1–P4 and M1, and roots of right P1–P2; from ?Egerkingen γ, Switzerland, Lutetian, MP13?.
Description.—Endocranium: The olfactory bulbs (maximum length 14 mm, maximum width 20 mm) are tube-shaped (Fig. 3A1). Olfactory tubercles are visible in ventral view (Fig. 3A2). The encephalon is spherical, but its anterior part has no visible structure due to taphonomic modification; for instance, the anterior lobes are not observable due to poor preservation. The cerebrum (maximum length 22 mm, maximum width 34 mm) is similar in width to the cerebellum (maximum length 13 mm, maximum width 37 mm). The cerebrum is divided into two hemispheres (Fig. 3A1). The sagittal sinus is present but weakly marked. A slight protuberance, which may be the result of damage during preservation, is present on the left side of the cerebrum, preventing observation of the different furrows on this side of the cerebrum. On the right side of the cerebrum, the lateral sulci are sigmoidal, with their anterior part concave (Fig. 3A3). The suprasylvian sulci are concavely rounded and become rectilinear in their anterior part (Fig. 3A3). Their exact course is difficult to define given the preservation of the specimen. The cerebellum is transversally wide. However, it is poorly preserved, which prevents a precise description of its morphology. We can only determine the presence of the vermis (Fig. 3A3) and of the medulla oblongata (Fig. 3A1, A2). The preservation of the ventral part of the encephalon does not allow for description of all the nerves and sinuses (Fig. 3A2). The anterior side of the ventral part is the best preserved (Fig. 3A2). In ventral view, the pituitary appears between the canals for cranial nerves III, IV, and V1. Cranial nerve II stands out clearly on the anterior and relatively axial part of the encephalon. Just behind this, cranial nerves III, IV, V1, V2, and VI are located in a single expanded and curved canal (Fig. 3A2). The preservation of the posterior part does not allow for further description of these nerve structures. The cerebellum is broken and heavily restored, preventing any description. The only element that can be distinguished is the vermis, but the latter cannot be described in detail.
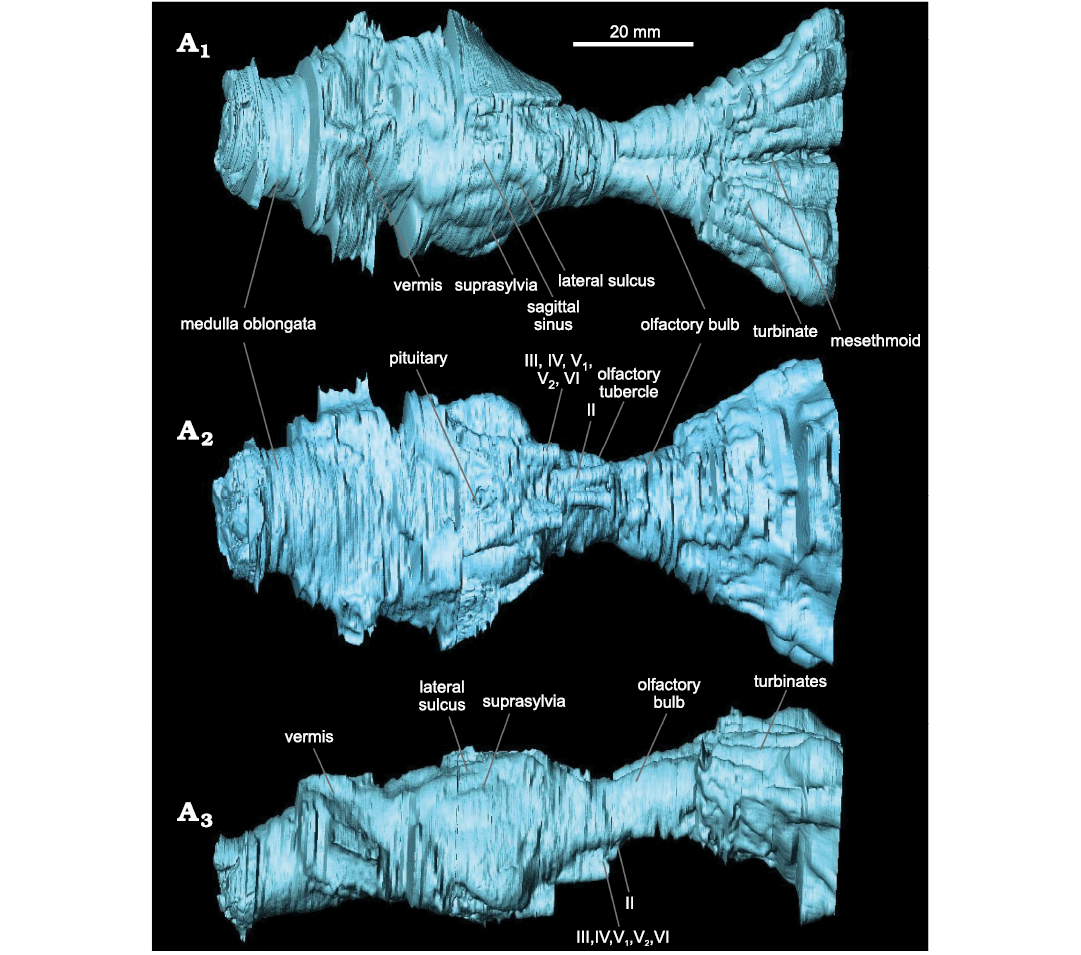
Fig. 3. Labeled endocast of hyaenodont mammal Eurotherium theriodis (Van Valen, 1965) NMB Em12 (holotype) from ?Egerkingen γ (Switzerland), ?MP13. Reconstruction in dorsal (A1), ventral (A2), and right lateral (A3) views. Nerves: II (ophthalmic), III (oculomotor), IV (pathetic), V1 (first branch of the trigeminal nerve), V2 (second branch of the trigeminal nerve), VI (abducens).
Nasal cavity: The study of the nasal cavity allows the recognition of five turbinates on each side of the rostrum; they are visible in dorsal view (Fig. 3A1). The latter are non-rectilinear and are separated from each other. The mesethmoid appears thin and elongated (Fig. 3A1). The turbinates widen and thicken towards the front of the skull, forming two fans (diverging 5–35° from the median axis). They thus form a considerable volume with significant relief; this volume is large compared to the encephalon.
Stratigraphic and geographic range.—Lutetian, ?MP13–M14; Switzerland and France.
Discussion
Comparison of the endocranium of Eurotherium theriodis.—Because Eurotherium belongs to the Hyaenodontoidea (Solé and Mennecart 2019; Solé et al. 2020), the endocranium of Eurotherium theriodis is here mainly compared to the proviverrine Proviverra typica (Dubied et al. 2019a, b) and the hyaenodontids Cynohyaenodon cayluxi (Lange-Badré 1979; Radinsky 1977), Hyaenodon horridus, H. crucians, and H. brachyrhynchus (Radinsky 1977; Lange-Badré 1979; Fig. 4). Gervais (1870) attributed an endocast to the type species of Hyaenodon, H. leptorhynchus, but we have doubt regarding the identification of this specimen considering the recent description (Solé et al. 2018a, b) of the previously unknown skull of that species; it is thus not included in this analysis. Recent phylogenetic analyses (Borths et al. 2016; Borths and Seiffert 2017; Dubied et al. 2019a; Solé et al. 2020) indicate that Eurotherium is more closely related to the hyaenodontine Hyaenodon than to Proviverra and Cynohyaenodon. However, like Cynohyaenodon and Proviverra, it is not a hyaenodontine in the current definition of the clade (Fig. 4). The endocast of the basal hyaenodont Tritemnodon agilis described by Radinsky (1977) is here considered because it may shed light on the polarity of brain character evolution through time.
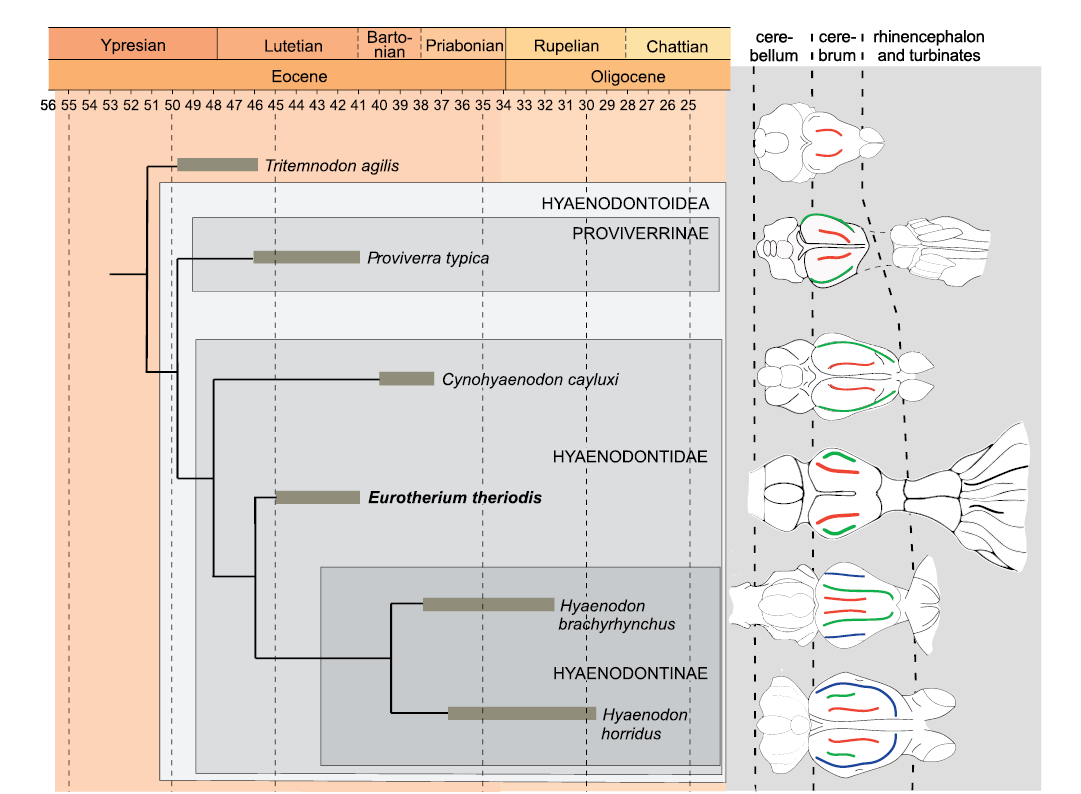
Fig. 4. Schematic evolution through time of the size of the endocast of several hyaenodonts. Red, lateral sulcus; green, suprasylvia; blue, ectosylvia. Endocast morphology has been modified from Radinsky (1977) except that of Proviverra typica Rütimeyer, 1862 (Dubied et al. 2019a) and Eurotherium theriodis (Van Valen, 1965) (present paper). The endocasts are not to scale. The phylogenetic relationships are based on Solé et al. (2020).
Eurotherium theriodis has an EQ that is slightly greater than those of T. agilis and C. cayluxi (Table 1) but it is very low in comparison to P. typica; it is worth remembering that the high EQ of Proviverra is unique among hyaenodonts and may be linked to an allometric effect, as Proviverra is very small (Dubied et al. 2019a). The EQ of E. theriodis is clearly less than to those calculated for H. crucians and H. horridus (Table 1). No obvious trend can be established for the EQ, although an increase over time can be envisaged if P. typica is regarded as a very peculiar case.
In the cerebrum, T. agilis has only one furrow per hemisphere, E. theriodis has two furrows per hemisphere, like P. typica and C. cayluxi, whereas the hyaenodontines have three furrows per hemisphere (Fig. 4). We can also observe that the relative size of the cerebrum in comparison to the cerebellum increases from T. agilis (where the cerebrum is very small), via Proviverra, Cynohyaenodon, and Eurotherium (where the cerebrum and cerebellum are of similar width), to Hyaenodon (where the cerebrum is large).
Tritemnodon agilis, P. typica, and C. cayluxi have drop-shaped olfactory bulbs, while in E. theriodis, the olfactory bulbs are tube-shaped. This olfactory bulb shape is similar to the one observed on the endocranium of some hyaenodontines, especially H. horridus and H. crucians (Radinsky 1977). These species have thick tube-shaped olfactory bulbs.
As indicated in the description, the cerebellum is poorly preserved and cannot be compared.
Eurotherium theriodis presents striking anatomical features at the level of turbinates: they are highly developed and fan-shaped, whereas the turbinates are straight and poorly developed in P. typica and C. cayluxi (Lange-Badré 1979; Dubied et al. 2019a, b). The turbinates of H. brachyrhynchus are also well developed and fan-shaped (Lange-Badré 1979).
To conclude, E. theriodis has an endocranium morphology that supports an increase in size of the cerebrum relative to the cerebellum over time within the Hyaenodontoidea as observed by Dubied et al. (2019a). The endocranium also supports a complexification of the cerebrum (i.e., at least two furrows per cerebral hemisphere) within the Hyaenodontoidea and allows us to envisage an increase of the EQ over time (Proviverra being considered as a special case). The overall morphology of the cerebrum of E. theriodis is like those of Proviverra and Cynohyaenodon and clearly less complex than that of Hyaenodon, despite that Eurotherium is closely related to Hyaenodon (Fig. 4). Its morphology is thus similar to that of the other hyaenodontoids recorded in the Lutetian (Proviverra), Bartonian, and Priabonian (Cynohyaenodon) of Europe. It is worth reminding, however, that Eurotherium shares with Hyaenodon the presence of tube-shaped olfactory bulbs and well-developed and fan-shaped turbinates. The presence of such developed turbinates is unusual among hyaenodonts; however, one must keep in mind that these structures are rarely analyzed among hyaenodonts and this condition cannot be regarded, at this stage, as a diagnostic character either for Hyaenodontidae or Hyaenodontinae.
Ecological aspects.—The reconstructed body mass of E. theriodis is 7 kg, thus the size of a fox. The diet of extinct predators is difficult to reconstruct, but some morphological features provide useful information. In E. theriodis, the sagittal crest (formed by the parietals) is highly developed on the posterodorsal part of the skull (Fig. 5). The strong development of the sagittal crest is similarly observed in some extant hyaenids (Crocuta crocuta, Parahyaena brunnea, and Hyaena hyaena) (Joeckel 1998). One can imagine that the skull of Eurotherium was also similar to these hyaenids in having a vaulted forehead (Fig. 5); this striking morphology, which results from the presence of caudally elongated frontal sinuses that completely overlie the brain cavity, plays a critical role in dissipating stress away from the facial region (Werdelin 1989; Joeckel 1998; Tanner et al. 2008). However, this impression surely results from post-mortem deformation of the skull around the nasal–parietal contact (Fig. 5). When deformation is considered (i.e., when the dentition is aligned with the occipital condyles as usually observed in European hyaenodontoids), the skull of Eurotherium seems morphologically similar to that of other hyaenodontoids known from the Eocene of Europe.
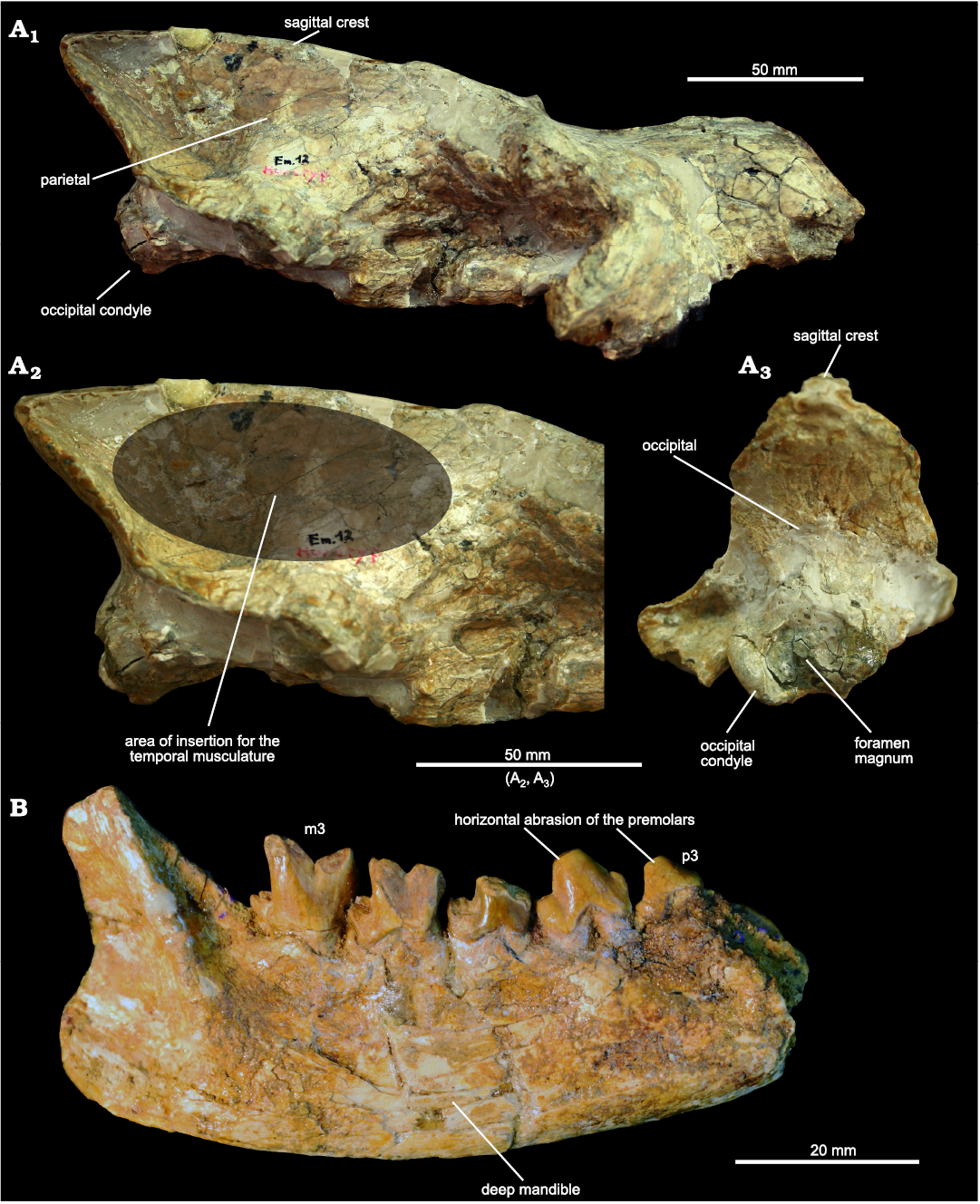
Fig. 5. Hyaeonodont mammal Eurotherium theriodis (Van Valen, 1965) from ?Egerkingen γ (Switzerland), ?MP13. A. NMB.Em12 (holotype) in right lateral (A1), right lateral view with close-up of posterior part (A2), and occipital (A3) views. D. NMB.En120 right mandible bearing p3–p4 and m1–m3 in labial view.
Despite the probable lack of a vaulted forehead, the strong development of the parietals and sagittal crest suggest a durophagous diet, it is worth remembering that extant hyaenids have a broad range of cranial and dental adaptations for durophagy, not just a vaulted forehead (Werdelin and Solounias 1991). Indeed, a long and tall sagittal crest implies strong development of the temporalis muscle, one of the powerful adductor muscles of the mandible (Popowics and Herring 2006).
Concerning the mandible and the lower teeth of Eurotherium theriodis, the transverse width of the premolars (i.e., robustness), the crowded premolars (i.e., absence of diastema), the horizontal abrasion of the premolars, as well as the depth of the mandible (noted by Van Valen 1965), indicate that this hyaenodontid would have been capable of powerful crushing. The secant morphology of the molars, which is characterized by the reduction of the talonid and molars, implies that the crushing function was mainly carried out with the premolars.
Considering feeding habits inferred from the RPS and RBL ratios, the values estimated for E. theriodis (RBL = 0.89; RPS = 2.92; Table 2) support a meat/bone diet hypothesis (Fig. 6). This agrees with the morphology of the sagittal crest, mandible, and premolars. One can note from Fig. 6 that E. theriodis is situated in proximity to genera with which it is united in the Eurotherium clade (as defined in Solé et al. 2020): Cartierodon egerkingensis and Prodissopsalis eocaenicus. However, it is interesting to note that Eurotherium matthesi from Geiseltal is situated far from these taxa. This highlights the necessity to revise the genus Eurotherium. E. theriodis is also close to the three Matthodon species in Fig. 6. These species, which are phylogenetically closer to Hyaenodon than the different genera in the Eurotherium clade (Solé et al. 2020), have been regarded as potentially having a bone/meat diet (Solé et al. 2014).
Table 2. Estimations of the relative premolar size (RPS) and the relative blade length (RBL) values of several hyaenodonts from Europe. The method used for estimated these indexes follows that of Solé and Mennecart (2019) (see Material and methods).
|
Taxon |
RPS |
RBL |
|
Hyaenodontinae |
||
|
Hyaenodon brachyrhynchus |
3.18 |
0.95 |
|
Hyaenodon dubius |
2.62 |
1.00 |
|
Hyaenodon exiguus |
2.89 |
1.00 |
|
Hyaenodon filholi |
2.90 |
1.00 |
|
Hyaenodon gervaisi |
3.49 |
1.00 |
|
Hyaenodon heberti |
2.94 |
1.00 |
|
Hyaenodon leptorhynchus |
2.77 |
1.00 |
|
Hyaenodon rossignoli |
3.24 |
0.99 |
|
Hyainailourinae |
||
|
Kerberos langebadreae |
2.41 |
0.74 |
|
Paroxyaena galliae |
2.95 |
0.80 |
|
Pterodon dasyuroides |
2.62 |
0.81 |
|
Proviverrinae |
|
|
|
Allopterodon bulbosus |
3.09 |
0.58 |
|
Allopterodon minor |
2.21 |
0.58 |
|
Hyaenodontidae |
||
|
Boritia duffaudi |
2.35 |
0.63 |
|
Cartierodon egerkingensis |
3.46 |
0.76 |
|
Cynohyaenodon cayluxi |
2.56 |
0.62 |
|
Cynohyaenodon trux |
2.86 |
0.53 |
|
Eurotherium matthesi |
2.45 |
0.60 |
|
Eurotherium theriodis |
2.92 |
0.89 |
|
Leonhardtina godinoti |
2.33 |
0.53 |
|
Leonhardtina meridiana |
2.76 |
0.58 |
|
Lesmesodon gunnelli |
2.48 |
0.49 |
|
Matthodon menui |
2.96 |
0.73 |
|
Matthodon peignei |
2.92 |
0.86 |
|
Matthodon tritens |
2.77 |
0.76 |
|
Oxyaenoides bicuspidens |
2.50 |
0.79 |
|
Oxyaenoides lindgreni |
2.27 |
0.80 |
|
Oxyaenoides schlosseri |
2.51 |
0.80 |
|
Paenoxyaenoides liguritor |
2.98 |
0.83 |
|
Preregidens langebadrae |
2.50 |
0.73 |
|
Prodissopsalis eocaenicus |
2.77 |
0.78 |
|
Quercytherium simplicidens |
3.52 |
0.70 |
|
Quercytherium tenebrosum |
4.51 |
0.70 |
|
Hyaenodontoidea |
||
|
Minimovellentodon russelli |
2.23 |
0.55 |
The RPS and RBL values also highlight one interesting case for the present discussion, Hyaenodon, because its values are close to those of E. theriodis (Fig. 6). Hyaenodon is a geographically widespread (across the whole of Laurasia) and long-lived genus. It is characterized by robust premolars and the very derived blade-like morphology of the molars, especially in the m3 (Lange-Badré 1979; Bastl 2012). Interestingly, based on microwear analysis and enamel microstructure, Bastl et al. (2012) demonstrated the inclusion of tough food items like bone in Hyaenodon’s diet.
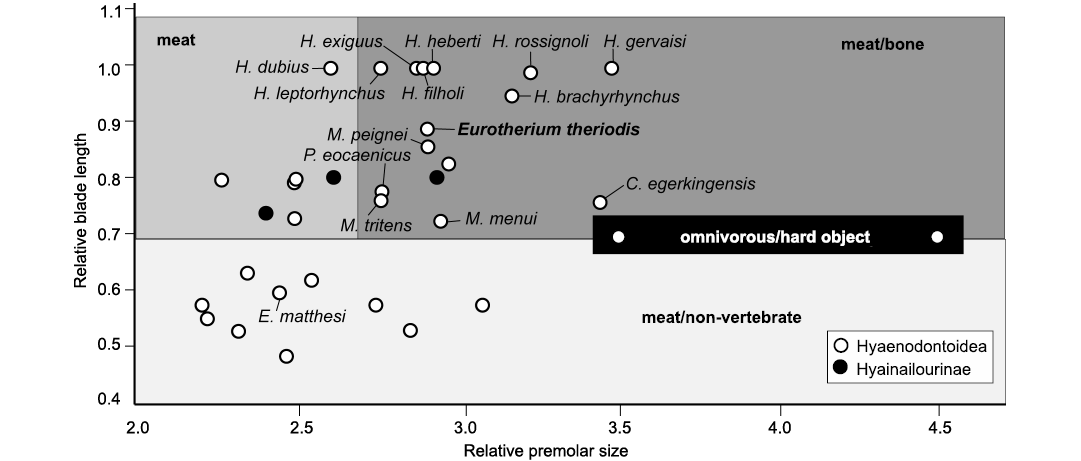
Fig. 6. Bivariate plot representing relative premolar size (RPS) versus relative blade length (RBL) for some selected hyaenodonts from the Eocene of Europe. Abbreviations: C., Cartierodon; E., Eurotherium, H., Hyaenodon, M., Matthodon, P., Prodissopsalis.
Therefore, E. theriodis can be considered as having had a meat/bone diet, probably including carcasses. Due to its fox-size, hyaenas are not the best extant analogs for the ecological reconstruction of E. theriodis. The black-backed jackal (Lupulella mesomelas) is a better analog because its body mass is close to that of E. theriodis. This canid has an opportunistic feeding behavior, being capable of hunting (Krofel 2007), but also being a proficient scavenger (Rowe-Rowe 1983; Hiscocks and Perrin 1987). E. theriodis might thus have been at least partly scavenging on leftovers from other animals’ kills, for instance, from the large hyaenodontid Cartierodon from Egerkingen (Solé and Mennecart 2019, or on carcasses of ungulates such as palaeotheriids (Perissodactyla). However, further analyses, such as a study of the evolution of enamel thickness among hyaenodonts, are necessary to test this hypothesis.
This passive predation strategy could be directly related to the morphology of E. theriodis’ turbinates. A scavenger needs a well-developed sense of smell to detect carrion at a long distance (Stoddart 2012). This is, for instance, the case of the black-backed jackal, which has an acute sense of smell (Fox 1971). Therefore, an olfactory membrane with a large air contact surface is common among scavengers, and some are even nocturnal and have very developed turbinates; this is, for instance, the case in hyaenids (Van Valkenburgh et al. 2014). The turbinates seen in E. theriodis thus testify to a strong expansion of the olfactory membrane of the sinuses. The morphology of its olfactory bulbs could therefore correspond to an adaptation of this hyaenodontid species to a scavenging way of life. It is worth remembering that E. theriodis shares with Hyaenodon the presence of well-developed, fan-shaped turbinates and that the diet of Hyaenodon included tough food items like bone (Bastl et al. 2012).
Conclusions
Comparison of the endocranial anatomy of several hyaenodonts shows that the endocranium of Eurotherium theriodis is not that different from that of some hyaenodontoids recorded in the Lutetian and Bartonian of Europe, such as observed in the genera Proviverra and Cynohyaenodon. The endocranium of E. theriodis is less complex than that of the hypercarnivorous Hyaenodon species in the absence of the ectosylvia. The morphology of the olfactory bulbs and turbinates is, however, similar to those of Hyaenodon species. This could either represent a convergence due to similar ecology or a synapomorphy.
The study of the nasal cavity of E. theriodis provides further information on its ecology. The presence of well-developed turbinates recalls the conditions observed in extant scavenging predators, in agreement with the ecomorphological characters observed on the skull and teeth of E. theriodis, and probably indicates a meat/bone diet. Therefore, E. theriodis could have been a scavenger living similarly to the extant black-backed jackal.
Investigations on a large array of hyaenodont endocrania are in progress and will provide further information on the evolution and ecology of this diverse and successful Paleogene group, as well as on the relationships among the sub-groups thanks to the establishment of phylogenetic characters related to the morphology of the endocranium.
Acknowledgements
Loïc Costeur (NMB) is thanked for providing access to the specimen and for hosting the study. Georg Schulz and Bert Müller (both Department of Biomedical Engineering, University of Basel, Switzerland) are thanked for performing and helping with the CT scans. We warmly thank the editor and the two reviewers, Lars Werdelin (Swedish Museum of Natural History, Stockholm, Sweden) and Michael Morlo (Department of Messel Research and Mammalogy, Senckenberg Research Institute, Frankfurt/M., Germany), for their constructive comments that greatly improved this manuscript. The Swiss National Foundation is warmly thanked for granting the SNF Projects P300P2 161065 and P3P3P2 161066. This work was supported by project Brain Pionnier BR/175/PI/CARNAGES of the Federal Science Policy Office of Belgium.
References
Aguilar, J.-P., et al. 1997. Synthèses et tableaux de corrélations. In: J.-P. Aguilar, S. Legendre, and J. Michaux (eds.), Actes du Congrès BiochroM’97. Mémoires et Travaux de l’EPHE Institut de Montpellier 21, 769–850. École Pratique des Hautes Études-Sciences de la Vie et de la Terre, Montpellier.
Barone, R. and Bortolomi, R. 2004. Anatomie comparée des mammifères domestiques: Tome 6, Neurologie I, Système nerveux central. 652 pp. Vigot Frères, Paris.
Bastl, K. 2012. The Ecomorphology of the European Hyaenodon. 297 pp. Ph.D. Thesis, University of Vienna, Vienna.
Bastl, K., Semprebon, K., and Nagel, D. 2012. Low magnification microwear in Carnivora and dietary diversity in Hyaenodon (Mammalia: Hyaenodontidae) with additional information on its enamel microstructure. Palaeogeography, Palaeoclimatology, Palaeoecology 348–349: 13–20. Crossref
Becker, D. 2003. Paléoécologie et paléoclimats de la Molasse du Jura (Oligo-Miocène): apport des Rhinocerotoidea (Mammalia) et des minéraux argileux. Geofocus 10: 1–331.
Bertrand, O.C., Amador-Mughal, F., and Silcox, M.T. 2017. Virtual endocast of the early Oligocene Cedromus wilsoni (Cedromurinae) and brain evolution in squirrels. Journal of Anatomy 230: 128–151. Crossref
Blanton, P.L. and Biggs, N.L. 1969. Eighteen hundred years of controversy: the paranasal sinuses. Developmental Dynamics 124: 135–147. Crossref
Borths, M.R. and Seiffert, E.R. 2017. Craniodental and humeral morphology of a new species of Masrasector (Teratodontinae, Hyaenodonta, Placentalia) from the late Eocene of Egypt and locomotor diversity in hyaenodonts. PLoS ONE 12: e0173527. Crossref
Borths, M.R. and Stevens, N.J. 2017. The first hyaenodont from the late Oligocene Nsungwe Formation of Tanzania: Paleoecological insights into the Paleogene–Neogene carnivore transition. PLoS ONE 12 (10): e0185301. Crossref
Borths, M.R., Holroyd, P.A., and Seiffert, E.R. 2016. Hyainailourine and teratodontine cranial material from the late Eocene of Egypt and the application of parsimony and Bayesian methods to the phylogeny and biogeography of Hyaenodonta (Placentalia, Mammalia). PeerJ 4: e2639. Crossref
Denison, R.H. 1938. The broad skulled Pseudocreodi. Annals of the New York Academy of Sciences 37: 163–255. Crossref
Dubied, M., Solé, F., and Mennecart, B. 2019a. The cranium of Proviverra typica (Mammalia, Hyaenodonta) and its impact on hyaenodont phylogeny and endocranial evolution. Palaeontology 62: 983–1001. Crossref
Dubied, M., Solé, F., and Mennecart, B. 2019b. 3D model related to the publication: The cranium of Proviverra typica (Mammalia, Hyaenodonta) and its impact on hyaenodont phylogeny and endocranial evolution. MorphoMuseuM 4 [published online, https://morphomuseum.com/articles/view/74] Crossref
Dubied, M., Solé, F., and Mennecart, B. 2020. 3D model related to the publication: Study of the endocranium and ecology of Eurotherium theriodis, a European hyaenodont (Mammalia) from the Lutetian. MorphoMuseuM 7 [published online, https://morphomuseum.com/articles/view/84] Crossref
Eisenberg, J.F. 1981. The Mammalian Radiations: an Analysis of Trends in Evolution, Adaptation, and Behavior. xx + 610 pp. University of Chicago Press, Chicago.
Filhol, H. 1877. Recherches sur les phosphorites du Quercy: étude des fossiles qu’on y rencontre et spécialement des mammifères. 561 pp. Masson, Paris.
Fox, M. 1971. Behaviour of Wolves, Dogs, and Related Canids. 214 pp. Robert E. Krieger Publishing Company Inc., Malabar.
Friscia, A. and van Valkenburgh, B. 2010. Ecomorphology of North American Eocene carnivores: evidence for competition between carnivorans and creodonts. In: A. Goswami and A. Friscia (eds.), Carnivoran Evolution: New Views on Phylogeny, Form, and Function, 311–241. Cambridge University Press, Cambridge. Crossref
Gaudry, A. 1878. Enchaînement du monde animal dans les temps géologiques: Mammifères tertiaires. 293 pp. Savy, Paris. Crossref
Gervais, P. 1870. Mémoire sur les formes cérébrales propres aux Carnivores vivants et fossiles. Nouvelles Archives du Museum d’Histoire naturelle Paris 6: 103–162.
Gunnell, G.F. 1998. Creodonta. In: C.M. Janis, K.M. Scott, and L.L. Jacobs (eds.), Evolution of Tertiary Mammals of North America. Volume 1: Terrestrial Carnivores, Ungulates, And Ungulatelike Mammals, 91–109. Cambridge University Press, Cambridge. Crossref
Hiscocks, K. and Perrin, M.R. 1987. Feeding observations and diet of black-backed jackals in an arid coastal environment. South African Journal of Wildlife Research 17: 55–58.
Joeckel, R.M. 1998. Unique frontal sinuses in fossil and living Hyaenidae (Mammalia, Carnivora): description and interpretation. Journal of Vertebrate Paleontology 18: 627–639. Crossref
Jerison, H.J. 1970. Brain evolution: new light on old principles. Science 170: 1224–1225. Crossref
Jerison, H.J. 1973. Evolution of the Brain And Intelligence. 482 pp. New York Academic Press, New York. Crossref
Klinghardt, F. 1934. Gehirnrelief und Schädelstudien über Hyaenodon und Dinictis. Zeitschrift für Säugetierkunde 9: 76–86.
Krofel, M. 2007. Opportunistic hunting behaviour of black-backed jackals in Namibia. African Journal of Ecology 46: 220–222. Crossref
Lange-Badre, B. 1979. Les créodontes (Mammalia) d’Europe occidentale de l’Éocène supérieur à l’Oligocène supérieur. Mémoires du Muséum national d’Histoire naturelle, nouvelle série, Série C Sciences de la Terre 42: 1–249.
Lange-Badré, B. and Haubold, H. 1990. Les Créodontes (mammifères) du gisement du Geiseltal (Éocène moyen, RDA). Geobios 23: 607–637. Crossref
Leidy, J. 1869. Notice of some extinct vertebrates from Wyoming and Dakota. Proceedings of the Academy of Natural Sciences of Philadelphia 21: 63–67.
Mace, G.M., Harvey, P.H., and Clutton-Brock, T.H. 1981. Brain size and ecology in small mammals. Journal of Zoology 193: 333–354. Crossref
Morlo, M. 1999. Niche structure and evolution in creodont (Mammalia) faunas of the European and North American Eocene. Geobios 32: 397–305. Crossref
Morlo, M., Bastl, K., Wenhao, W., and Schaal, S.F. 2013. The first species of Sinopa (Hyaenodontida, Mammalia) from outside of North America: implications for the history of the genus in the Eocene of Asia and North America. Palaeontology 57: 111–125. Crossref
Negus, V.E. 1954. The function of the paranasal sinuses. Acta Oto-Laryngologica 44: 408–426. Crossref
Orliac, M.J., Argot,C., and Gilissen, E. 2012. Digital cranial endocast of Hyopsodus (Mammalia,“Condylarthra”): a case of Paleogene terrestrial echolocation? PLoS ONE 7: e30000. Crossref
Pfaff, C., Nagel, D., Gunnell, G., Weber, G.H., Kriwet, J., Morlo, M., and Bastl, K. 2016. Palaeobiology of Hyaenodon exiguus (Hyaenodonta, Mammalia) based on morphometric analysis of the bony labyrinth. Journal of Anatomy 230: 282–289. Crossref
Pirlot, P. and Stephan, H. 1970. Encephalization in Chiroptera. Canadian Journal of Zoology 48: 433–444. Crossref
Piveteau, J. 1961. Carnivora. Traité de Paléontologie 6: 641–820.
Polly, P.D. and Lange-Badré, B. 1993. A new genus Eurotherium (Mammalia, Creodonta) in reference to taxonomic problems with some Eocene hyaenodontids from Eurasia. Comptes Rendus de l’Académie des Sciences Paris 2: 991–996.
Popowics, T.E. and Herring, S.W. 2006. Teeth, jaws and muscles in mammalian mastication. In: V. Bels (ed.), Feeding in Domestic Vertebrates: From Structure to Behaviour, 61–83. CABI Publishing, Wallingford. Crossref
Radinsky, L. 1977. Brains of early carnivores. Paleobiology 3: 333–349. Crossref
Radinsky, L. 1978. Evolution of brain size in carnivores and ungulates. The American Naturalist 112: 815–831. Crossref
Rana, R.S., Kumar, K., Zack, S.P., Solé, F., Rose, K.D., Missiaen, P., Singh, L., Sahni, A., and Smith T. 2015. Craniodental and postcranial morphology of Indohyaenodon raoi from the early Eocene of India, and its implications for ecology, phylogeny, and biogeography. Journal of Vertebrate Paleontology 35: e965308. Crossref
Rose, K.D. 2006. The Beginning of the Age of Mammals. 448 pp. John Hopkins University Press, Baltimore.
Rowe-Rowe, D.T. 1983. Black-backed jackal diet in relation to food availability in the Natal Drakensberg. South African Journal of Wildlife Research 13: 17–23.
Savage, R.J.G. 1973. Megistotherium, gigantic hyaenodont from Miocene of Gebel Zelten, Libya. Bulletin of the British Museum (Natural History), Geology 22: 483–511. Crossref
Scott, W.B. 1886. On some new and little known creodonts. Academy of Natural Sciences of Philadelphia 2: 155–185.
Solé, F., Dubied, M., le Verger, K., and Mennecart, B. 2018a. Niche partitioning of the European carnivorous mammals during the Paleogene. Palaios 33: 514–523. Crossref
Solé, F., Dubied, M., le Verger, K., and Mennecart, B. 2018b. 3D models related to the publication: Niche partitioning of the European carnivorous mammals during the Paleogene. MorphomuseumM 5 (1): e63. Crossref
Solé, F., Falconnet, J., and Laurent, Y. 2014. New proviverrines (Hyaenodontida) from the early Eocene of Europe; phylogeny and ecological evolution of the Proviverrinae. Zoological Journal of the Linnean Society 171: 878–917. Crossref
Solé, F., Falconnet, J., and Vidalenc, D. 2015. New fossil Hyaenodonta (Mammalia, Placentalia) from the Ypresian and Lutetian of France and the evolution of the Proviverrinae in southern Europe. Palaeontology 58: 1049–1072. Crossref
Solé, F. and Mennecart, B. 2019. A large hyaenodont from the Lutetian of Switzerland expands the body mass range of the European mammalian predators during the Eocene. Acta Palaeontologica Polonica 64: 275–290. Crossref
Solé, F., Marandat, B., and Lihoreau, F. 2020. The hyaenodonts (Mammalia) from the French locality of Aumelas (Hérault), possible late Ypresian new representatives. Geodiversitas 42: 185–214. Crossref
Solé, F., Morlo, M., Schaal, T., and Lehmann, T. 2021. New hyaenodonts (Mammalia) from the late Ypresian locality of Prémontré (France) support a radiation of the hyaenodonts in Europe already at the end of the early Eocene. Geobios 66–67: 119–141. Crossref
Stoddart, D.M. 2012. The Ecology of Vertebrate Olfaction. 207 pp. Springer Netherlands, Dordrecht.
Tanner, J.B., Dumont, E.R., Sakai, S.T., Lundrigan, B.L., and Holekamp, K.E. 2008. Of arcs and vaults: the biomechanics of bone-cracking in spotted hyenas (Crocuta crocuta). Biological Journal of the Linnean Society 95: 246–255. Crossref
Van Valen, L. 1965. Some european Proviverrini (Mammalia, Deltatheridia). Paleontology 844: 638–665.
Van Valen, L. 1967. New Paleocene insectivores and insectivore classification. Bulletin of the American Museum of Natural History 135: 217–284.
Van Valkenburgh, B. 1988. Trophic diversity in past and present guilds of large predatory mammals. Paleobiology 14: 155–173. Crossref
Van Valkenburgh, B., Pang, B., Bird, D., Curtis, A., Yee, K., Wysocki, C., and Craven, B.A. 2014. Respiratory and olfactory turbinals in feliform and caniform carnivorans: the influence of snout length. The Anatomical Record 297: 2065–2079. Crossref
Werdelin, L. 1989. Constraint and adaptation in the bone-cracking canid Osteoborus (Mammalia: Canidae). Paleobiology 15: 387–401.
Werdelin, L. and Solounias, N. 1991. The Hyaenidae: taxonomy, systematics and evolution. Fossils and Strata 30: 1–104. Crossref
Acta Palaeontol. Pol. 66 (3): 545–554, 2021
https://doi.org/10.4202/app.00771.2020