


Stable isotope analysis of middle Miocene mammals from the Siwalik sub-Group of Pakistan
MUHAMMAD TAHIR WASEEM, ABDUL MAJID KHAN, JAY QUADE, ANTHONY KRUPA, DAVID L. DETTMAN, AMTUR RAFEH, and RANA MANZOOR AHMAD
Waseem, M.T., Khan, A.M., Quade, J., Krupa, A., Dettman, D.L., Rafeh, A., and Ahmad, R.M. 2021. Stable isotope analysis of middle Miocene mammals from the Siwalik sub-Group of Pakistan. Acta Palaeontologica Polonica 66 (Supplement to 3): 123–132.
Stable isotope analysis is pivotal for investigating the paleodiet and paleoecology of past mammals. In this paper, we analyzed thirty fossil enamel samples belonging to the families Suidae, Rhinocerotidae, and Deinotheriidae for δ13Cenamel and δ18Oenamel composition to investigate paleodiet and paleoecology of middle Miocene mammals of the Siwalik sub-Group of Pakistan. The three mammalian groups, when combined together, yielded an average δ13Cenamel value of -12.2 ± 2‰, indicating a pure to nearly pure C3 diet. Suids show slightly higher δ13Cenamel values of -11.2 ± 1.4‰ when compared to rhinocerotids and deinotheres (-12.3 ± 0.8‰ and -12.5 ± 1.3‰, respectively), which could be explained by selective foraging on new leaf shoots or feeding from open spaces. Alternatively, the differences in δ13Cenamel could be due to different digestive physiologies and different enamel-diet enrichment factors. Members of all three families showed significant differences in δ18Oenamel values, where suids yielded higher δ18O values of -8.2 ± 1.2‰ compared to rhinocerotids and deinotheres (-11.4 ± 1.8‰ and -10.4 ± 1.7‰, respectively). Based upon these results, we assume that these mammals inhabited subtropical forests similar that of mid-Miocene of the Siwalik Group, India and Nepal. The modern analogues of such vegetation system are present in East and South of Myanmar, Nepal, and Malaya where precipitation is enough to support evergreen C3 forests. By contrast, today’s floodplain environments in Pakistan are dominated by C4 grasses, and C3 vegetation is only present in non-floodplain settings.
Key words: Mammalia, paleoclimate, paleodiet, Miocene, Chinji Formation, Pakistan.
Muhammad Tahir Waseem [tahirmuhammad1213@gmail.com], Abdul Majid Khan [majid.zool@pu.edu.pk] (corresponding author), and Amtur Rafeh [amturrafeh@gmail.com], Department of Zoology, University of the Punjab, Lahore 54590, Pakistan.
Jay Quade [quadej@email.arizona.edu], Anthony Krupa [akrupa@email.arizona.edu], and David L. Dettman [dettman @email.arizona.edu], Department of Geosciences, University of Arizona, Tucson AZ 85721, USA.
Rana Manzoor Ahmad [manzoor.zoology@uo.edu.pk], Department of Zoology, University of Okara, Punjab 56300, Pakistan.
Received 28 June 2020, accepted 23 October 2020, available online 24 May 2021.
Copyright © 2021 M.T. Waseem et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The temporal and spatial records of climate archived in thick Neogene and Quaternary continental deposits in northern Pakistan provide an exceptional opportunity to explore the patterns of evolution and adaptation of mammals over the time span of million years (Badgley et al. 2008; Morgan et al. 2009; Patnaik et al. 2019). Stable isotope (SI) analysis of carbon and oxygen of enamel from mammals, both expressed in the familiar delta (δ) notation in per mil (‰) according to V-PDB (Vienna Pee Dee Belemnite) standards, allow us to reconstruct the past diets (δ13Cdiets) and paleoenvironmental meteoric water (δ18Omw), which in turn reflect paleovegetation and paleoclimate during the Neogene and Quaternary (Quade et al. 1992, 1995a, b; Cerling et al. 1997a, 2015; White et al. 2009; Sanyal et al. 2005, 2010; Uno et al. 2011; Ben-David and Flaherty 2012; Khan et al. 2020; Waseem et al. 2020a). The Siwalik sub-Group of northern Pakistan contains a high-resolution fossil record spanning the Neogene time period (Quade et al. 1989; Barry et al. 2002, 2013; Dennell et al. 2006; Flynn et al. 2016; Fig. 1). Morgan et al. (1994), Uno et al. (2011), and Hynek et al. (2012) interpreted elevated δ13C values in fossil herbivore enamel (δ13Cenamel) to reflect a component of C4 vegetation in the mid-Miocene (~11–10 Ma) across several continents. However, the major C4 radiation in the Siwalik sub-Group has been documented to occur after ~10 Ma (Barry et al. 1982, 2002; Quade et al. 1995a, b; Morgan et al. 2009). In this study, we explore the isotopic composition of enamel from three families of large herbivores to reconstruct paleoenvironments and check for evidence of C4 dietary intake in the middle Miocene (14.2–11.2 Ma) of the Siwalik sub-Group of Pakistan. Enamel was selected for the analysis due to its high resistance to diagenesis and large crystal size and low porosity, which makes it the best material for paleodietary reconstruction in ancient ecosystems (Quade et al. 1992; Kohn et al. 1996, 2002; Kohn and Cerling 2002).
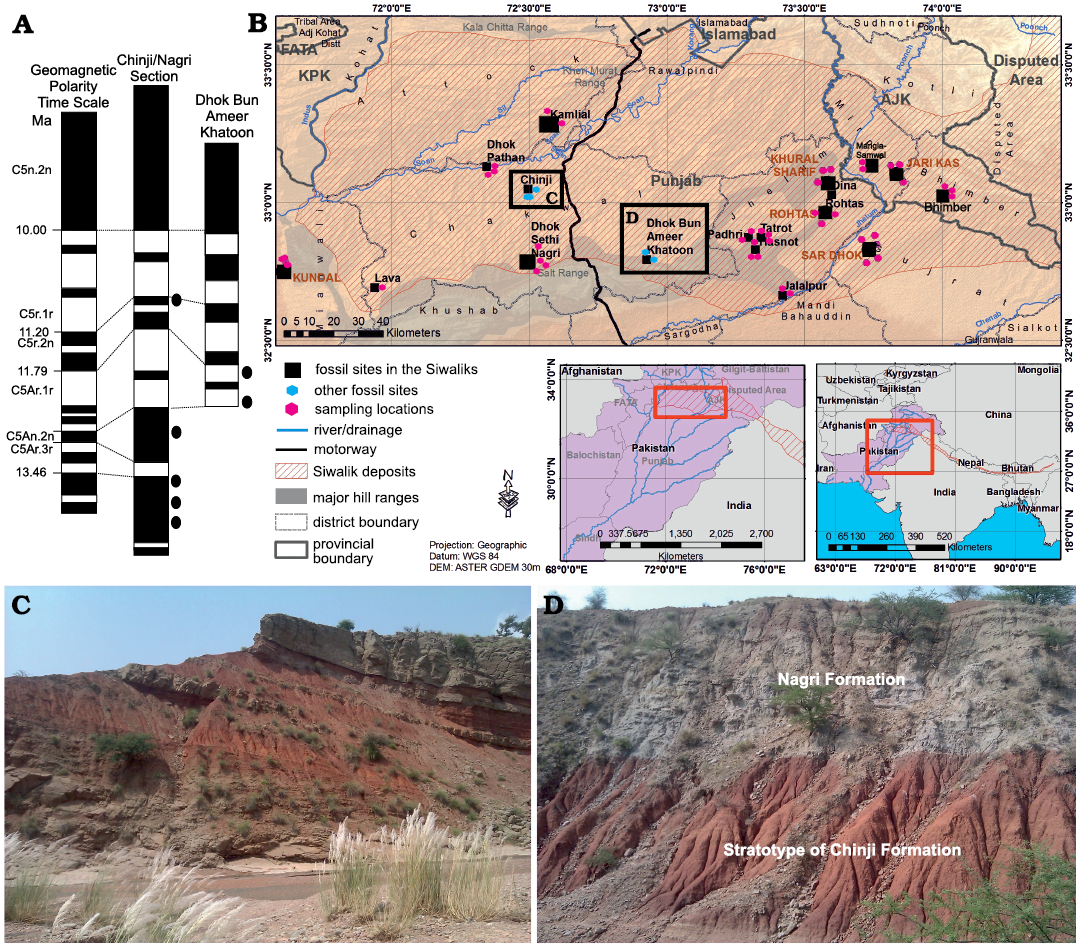
Fig. 1. Map of the Siwaliks indicating the middle Miocene localities from where fossil material for this study was recovered. A. Geomagnetic polarity time scale (Gradstein et al. 2012). B. Map of the Siwalik sub-Group of Pakistan along with the localities and studied area. C. Overview of Chinji-Nagri section. D. Overview of Dhok Bun Ameer Khatoon (DBAK) section.
There are three metabolic pathways through which carbon is incorporated in the plants. Trees, nearly all shrubs, forbs, ferns, sedges, and most non-tropical or high altitude grasses utilize the C3 pathway of photosynthesis in which δ13Cdiet ranges from -34 to -22‰ (Koch 1998; Pérez-Crespo et al. 2017), and average around -28.2 ± 6‰ under the present δ13CCO2 = -8‰, or -6.7‰, pre-industrially (Kohn 2010). The C4 pathway is found in most of the warm region-grasses and some sedges with a range of δ13Cdiet values from -14 to -10‰ (Cerling 1999). The abundance of C3/C4 vegetation in an ecosystem is governed by many factors, including the mean annual temperature (MAT), mean annual precipitation (MAP), and partial pressure of carbon dioxide in the atmosphere (pCO2) (Kohn 2010; Waseem et al. 2020b). The comparatively cooler areas (MAT <25°C) with precipitation higher than 1–2 m/year show a dominance of C3 vegetation while warmer areas (MAT >25°C) with drier conditions (MAP <1–2 m/year) show a dominance of C4 grasses (Nelson 2007). Furthermore, the C4 pathway can work efficiently under lower pCO2 and lower humidity levels compared to C3 pathway (Medrano and Flexas 2000; Bellasio et al. 2018) due to the fact that carbon is fixed twice in C4 pathway as compared to C3 pathway (Kohn and Cerling 2002). The δ13C values of plants are governed by other factors such as low light intensity, saline soil, water availability, and local microhabitat conditions (Ehleringer et al. 1987; Codron et al. 2005; Bibi 2007).
These averages and ranges in δ13C value for modern plants were ~1.3‰ higher during the middle Miocene in fossil C3 and C4 plants, due largely to the effect of fossil fuel burning in decreasing δ13CCO2 of the atmosphere (see Tipple et al. 2010). Thus, we use the δ13CCO2 (-5.2‰) as in Tipple et al. (2010) for pre-10 Ma time span. The third photosynthetic pathway, crassulacean acid metabolism (CAM), is found in succulent plants which are mostly present in deserts or epiphytic plants in closed forests (Passey et al. 2005; Andrade et al. 2007) and were probably negligible in the Siwaliks.
Upon feeding on plants, the carbon is incorporated in the herbivores tooth enamel during the period of tooth formation. When carbon isotopes are incorporated in the enamel of herbivores, δ13Cenamel values are fractionated by an enrichment factor (eenamel-diet):
eenamel-diet = (aenamel-diet - 1) × 1000 (1), where: a enamel-diet = (denamel + 1000)/(ddiet + 1000)
Thus, middle Miocene animals consuming C3 vegetation outside of rainforests and deserts with δ13Cdiet values from -28.1 to -23.1‰ should show δ13Cenamel values ranging from about -14 to -9‰, whereas middle Miocene animals feeding on C4 vegetation should show δ13Cenamel values ranging from ~-2 to ~+2‰. The values between ~-9 to ~-2‰ are considered to reflect mixed feeding on C3/C4 vegetation (MacFadden and Cerling 1996). In this study, we assume that δ13Cenamel values ≤-16.1‰ denote browsing under closed-canopy forests, -16 to -14‰ for sub-canopy browsing, -14 to -12‰ for browsing in forests, and -12 to -8‰ for browsing in woodlands (Cerling and Harris 1999; Domingo et al. 2012). The δ13Cenamel between -8 to -3‰ will be considered as mixed feeding on C3/C4 vegetation while values >-2‰ can be interpreted as grazing on dominantly C4 grasses (Cerling et al. 1997a, b, 2004; Kohn 2010). However, eenamel-diet does not solely depend upon one factor. Tejada-Lara et al. (2018) argue that a single value of eenamel-diet of ~14.1‰ does not account for all the mammals from monkeys to elephants by overlooking the other metabolic and physiological effect on carbon fractionation. For example, different mammals show different eenamel-diet depending on body mass and digestive physiology (Tejada-Lara et al. 2018). Combining all factors, we assume an average eenamel-diet of ~+14.1‰ for large-bodied rhinocerotids and deinotheres and ~+13.1‰ for suids according to Codron et al. (2011) and Tejada-Lara et al. (2018).
Oxygen enters an animal’s body in three main ways: (i) from drinking water, (ii) water derived from food, and (iii) inhalation, and leaves the body through sweat, exhalation, and urination (Sponheimer and Lee-Thorp 1999; Koch 2007; Sánchez 2005; Blumenthal et al. 2017, 2018). Of these sources, the majority of oxygen isotopes incorporated into enamel are from drinking water and diet, and δ18O values of local meteoric water (δ18Omw) are affected by environmental temperature, amount of precipitation, latitude, and altitude (Dansgaard 1964). The general trend of δ18Oenamel values is set by the ecology as the animals living under closed habitat and in humid conditions (forests) tend to show lower δ18Oenamel values compared to the animals living in open and arid (savannah or grasslands) areas (Feranec and MacFadden 2006). Levin et al. (2006) categorized the animals under two broad categories on the basis of their physiology and behavior. Evaporation sensitive (ES) taxa tend to obtain most of their water from their diet (leafy plants) and generally are ruminants (δ18O values of their enamel is correlated to the evaporation of leaf water), whereas evaporation insensitive (EI) taxa are mostly non-ruminants that depend on surface water for their body water requirements (δ18Oenamel is correlated to local drinking water sources). In this study, all the families sampled can be categorized as EI taxa on the basis of the physiology of the modern analogues, providing better insights into the δ18O value of palaeo-precipitation (Levin et al. 2006; Blumenthal et al. 2017; Faith 2018).
The fossil fauna of Chinji Formation includes Rhinocerotidae, Suidae, Tragulidae, Bovidae, Giraffidae, Deinotheriidae, Gomphotheriidae, and other taxa including Hominidae, Rodentia, and Carnivora (Pilgrim 1937; Khan et al. 2013; Barry et al. 2013; Flynn et al. 2016). We focus here on the paleodiets, paleoenvironments, and niche partitioning among Rhinocerotidae, Suidae, and Deinotheriidae from the middle Miocene of northern Pakistan. We selected these three EI taxa over non-EI taxa as δ18Oenamel value of EI taxa is correlated to their water source (Faith 2011, 2018). Furthermore, we selected one specialized browser (deinotheres), one taxon (i.e., Hyotherium pilgrimi) which disappeared from the Siwalik record during the late Miocene, and one taxon (rhinocerotid, Chilotherium intermedium) which shifted towards a C4 diet during the late Miocene (Badgley et al. 2008; Morgan et al. 2009) to evaluate whether these taxa ate C3 or C4 plants during the middle Miocene time span in the Siwalik sub-Group of Pakistan.
The rhinocerotid, Chilotherium intermedium, was widespread in the Siwalik sub-Group of India and Pakistan, and existed up to the late Miocene (~7 Ma) in China. This species is characterized by low-crowned cheek teeth (brachydonty) with thin enamel folding and less complexity, indicating a diet composed of soft and less-gritty vegetation, preferably leaves of plants and trees (Khan 2009). C. intermedium was comparatively smaller (height 1.5 m and weight 1–2 tons) than modern black rhinos (Diceros bicornis) (height up to 1.8 m and weight up to 2.2 tons) (Decher 1999; Deng 2005).
Deinotherium pentapotamiae and D. indicum (Deinotheriidae) (body weight up to 11 tons and height up to 5 m, Larramendi 2016) was widespread in the Siwalik Group and contemporaneous African sites for most of the middle Miocene, and it persisted as late as ~9 Ma in the Indian sub-continental region (Sarwar 1977). Two hooked shaped lower tusks were present in the species of Deinotherium with a flat skull, unlike other proboscideans, along with low-crowned teeth, indicating a browsing diet (Sarwar 1977; Shoshani and Tassy 2005).
Hyotherium pilgrimi (Suidae) was a species with bunodont (rounded cusps) and brachyodont cheek teeth which existed during the middle Miocene time span in the Siwaliks. It had a range of body weight from 150–200 kg and height up to 1.1 m (Sorkin 2008). The mesowear and morphological studies (low hypsodonty index of 1.1, simple bunodont dentition with thick enamel) show that this species browsed on leaves and fruits (Barry et al. 2002; Tariq 2010; Aslam 2018). In general, all the middle Miocene mammals of the Siwalik sub-Group of Pakistan show morphological characters indicating browsing diets in forests and woodland settings, like their Indian counterparts (Patnaik et al. 2019).
Institutional abbreviations.—EB, Environmental Biology Laboratory, Institute of Zoology, University of the Punjab, Lahore, Pakistan.
Other abbreviations.—CAM, crassulacean acid metabolism; EI, evaporation insensitive; ES, evaporation sensitive; MAP, mean annual precipitation; MAT, mean annual temperature; SI, stable isotopes; V-PDB, Vienna Pee Dee Belemnite.
Geological setting
The middle Miocene is represented by the Chinji Formation in the Siwalik sub-Group of Pakistan (Fig. 1), which spans 14.2–11.2 Ma (Barry et al. 2002). The thickness and near-continuous deposition of the strata have permitted the development of a robust chronostratigraphic framework for the Chinji Formation on the basis of paleomagnetic dating. The samples for this study were collected from the Chinji-Nagri and Dhok Bun Ameer Khatoon sections (Fig. 1). The Chinji-Nagri section (Fig. 1B, C) is present in Chakwal District, Punjab (32°39´N, 72°22´E), whereas the Dhok Bun Ameer Khatoon section (DBAK) (Fig. 1B, D) is located 50 km north of Chinji Village (32°47´N, 72°55´E). The boundary between Chinji and Nagri formations can be placed at 11.2 Ma (Fig. 1A), between magnetic zones C5r.3r and C5n.3n (Johnson et al. 1982, calibrated according to Gradstein et al. 2012). The lower boundary of the Chinji Formation with the underlying Kamlial Formation (Fig. 1A) can be placed between C5ACr and C5ADn at 14.2 Ma (Channell et al. 2013). Siltstone and sandstone deposited in an overall fluvial environment dominate the general lithology of the Siwalik sub-Group sediments. Most of the samples come from reddish to gray mudstone (Fig. 1C, D) marginal to large-river paleochannels. The ages are assigned as the mid points following Barry et al. (2002).
Material and methods
For SI analysis, thirty samples from three mammalian families were selected across the middle Miocene of the Siwalik sub-Group of Pakistan. The samples were identified to the species level and further processed for SI analysis. Statistical analysis was conducted using Statistical Package for Social Studies (SPSS) version 20.0.
For the extraction of enamel, a rotary dental drill with carbide burrs was used. Around 15–20 mg of enamel was extracted from each tooth (only molars and premolars were included). Enamel was extracted along one single transect from cervix to crown apex (longitudinal) from buccal surface of upper teeth and lingual surface of lower teeth.
Powdered enamel was further pre-treated with 10 ml of 2% NaOCl for one hour to remove organic matter and the solution was decanted and rinsed with distilled water three times. The samples were than treated with 10 ml of 0.1% acetic acid for 1 hour to remove exogenous carbonates and rinsed with water (Nelson 2007). Samples were then oven dried before isotope analysis (Koch et al. 1997).
δ18Oenamel and δ13Cenamel values were measured at the University of Arizona, USA, using an automated carbonate preparation device (KIEL-III) coupled to a gas-ratio mass spectrometer (Finnigan MAT 252). Powdered samples were reacted with dehydrated phosphoric acid under vacuum at 70°C. The isotope ratio measurement is calibrated based on repeated measurements of NBS-19 and NBS-18, and precision is 0.11‰ for δ18O (V-PDB) and 0.08‰ for δ13C (V-PDB).
Results
Stable Carbon Isotopes.—The average δ13Cenamel across all the families was found to be -12.2 ± 2.2‰. The Suidae, Rhinocerotidae, and Deinotheriidae returned average values of δ13Cenamel of -11.2 ± 1.4‰ (n = 10), -12.3 ± 0.8‰ (n = 10), and -12.5 ± 1.3‰ (n = 10), respectively (Table 1). Normality tests indicate that data were normally distributed. One-way ANOVA along with the post-hoc (Tukey’s HSD) test show no significant differences in δ13C values between Rhinocerotidae and Deinotheriidae (p = 0.061), whereas Suidae showed significant differences compared to Deinotheriidae (p = 0.039) and Rhinocerotidae (p = 0.041).
Stable Oxygen Isotopes.—The average δ18Oenamel (V-PDB) across all the families was -9.9 ± 3‰, wherein Suidae, Rhinocerotidae, and Deinotheriidae yielded average values for δ18Oenamel of -8.2 ± 1.2‰ (n = 10), -11.4 ± 1.8‰ (n = 10), and -10.4 ± 1.7‰ (n = 10), respectively (Table 1). The data were normally distributed. One-way ANOVA along with post-hoc test indicated that the δ18Oenamel of suids is significantly different from the δ18Oenamel of rhinos and deinotheres (p = 0.041) (Table 1, Fig. 2).
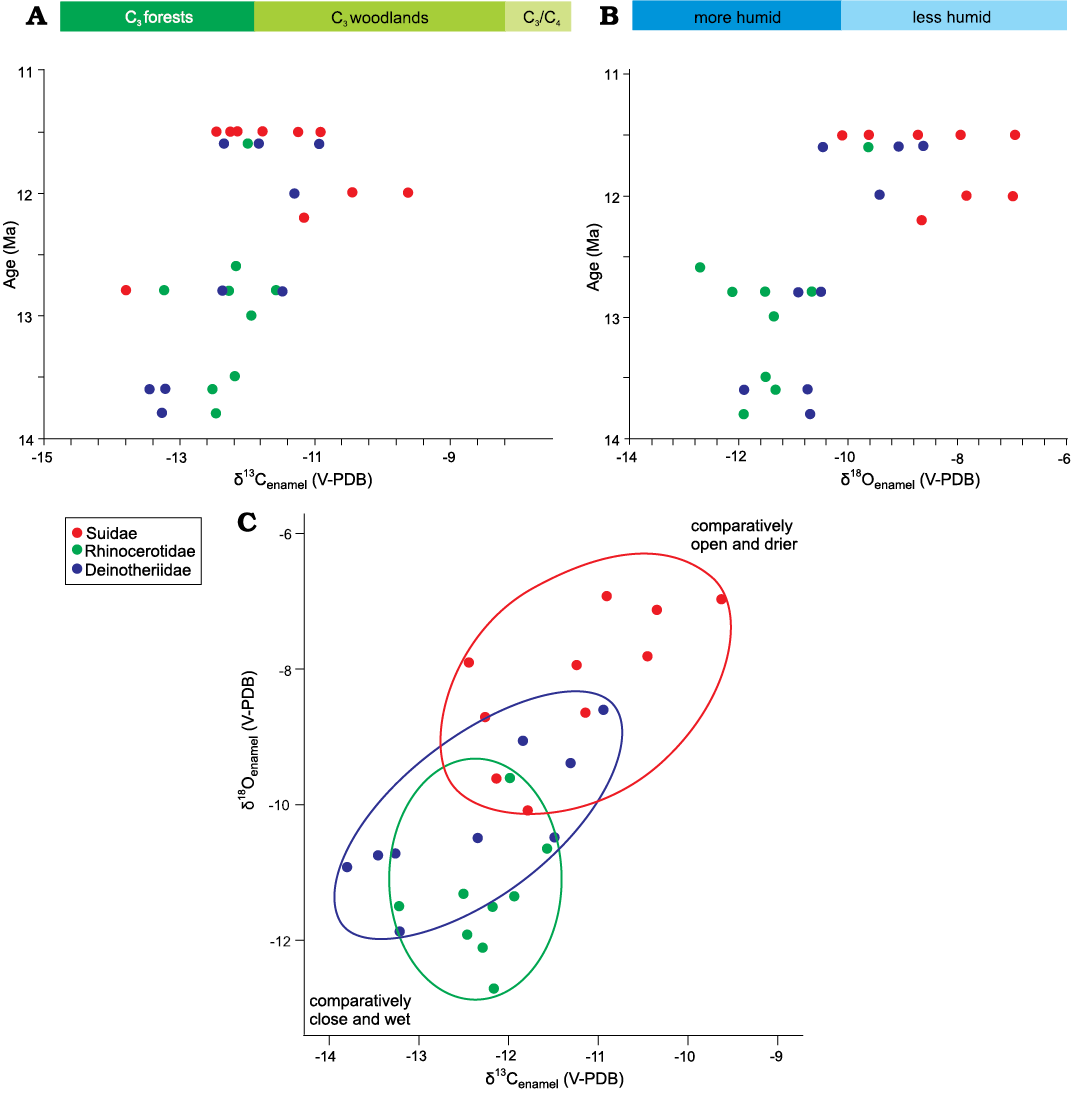
Fig. 2. δ13Cenamel and δ18Oenamel of all results on three herbivore families from the Chinji Formation. A. δ13Cenamel values versus age (Ma) showing close and forest ecology of middle Miocene. B. δ18Oenamel values versus age (Ma) indicating the moisture conditions in middle Miocene. C. The niche partitioning in δ18Oenamel versus δ13Cenamel space among the suids, rhinocerotids, and deinotheres.
Discussion
The average δ13Cenamel value of middle Miocene mammals (-12.2 ± 2‰) in our data overall indicates a diet composed of C3 vegetation, which was likely present in the form of forests and woodlands (Kohn and Cerling 2002; Kohn 2010). Patnaik et al. (2019) report the range of δ13Cenamel for large herbivores from -13.9 to -11.6‰ for middle Miocene Siwalik sub-Group of India, which matches our results. The lowest δ13Cenamel value in our results (-13.9‰) was recorded at ~13 Ma interval, whereas the highest value (-9.6‰) was recorded at ~12 Ma (Fig. 2, Table 1). This shows that the lower Chinji Formation was dominated by C3 vegetation, likely present in the form of forestland, whereas the upper Chinji was dominated by C3 open woodlands (Kohn and Cerling 2002; Kohn 2010). Thus, vegetation and habitat appear to have changed towards more open ecological settings in the upper Chinji Formation. The shift to open woodland is also apparent in the suid δ13Cenamel results, which return an estimate for suid diet of -22.7‰, assuming eenamel-diet = +13.1‰. (Kohn 2010; Cerling et al. 2015; Table 1). Moreover, the possibility of consumption of C3 grasses cannot be ruled out as extant suids in eastern Africa also consume C3 grasses but show a low-crowned dental morphology (Harris and Cerling 2002; Souron 2017).
Table 1. δ13Cenamel and δ18Oenamel values for thirty enamel samples of fossil mammals from the middle Miocene Siwaliks of Pakistan. The ages of the samples have been calibrated according to Gradstein et al. (2012). Abbreviations: l, left; M/m, upper/lower molar; PM, upper premolar; r, right; V-PDB, Vienna Pee Dee Belemnite.
|
Sample ID |
Family |
Species |
Horizon |
Type of tooth |
Age (Ma) |
δ13Cenamel V-PDB (‰) |
δ18Oenamel V-PDB (‰) |
|
EB 470 |
Suidae |
Hyotherium pilgrimi |
upper Chinji |
lM2 |
12.0 |
-10.3 |
-7.1 |
|
EB 471 |
Suidae |
Hyotherium pilgrimi |
upper Chinji |
lM2 |
12.2 |
-9.6 |
-7.0 |
|
EB 472 |
Suidae |
Hyotherium pilgrimi |
upper Chinji |
rP3 |
11.5 |
-11.1 |
-8.6 |
|
EB 473 |
Suidae |
Hyotherium pilgrimi |
upper Chinji |
rm3 |
11.5 |
-12.1 |
-9.6 |
|
EB 474 |
Suidae |
Hyotherium pilgrimi |
upper Chinji |
rm2 |
12 |
-12.25 |
-8.7 |
|
EB 475 |
Suidae |
Hyotherium pilgrimi |
upper Chinji |
rM2 |
11.5 |
-11.8 |
-10.1 |
|
EB 476 |
Suidae |
Hyotherium pilgrimi |
upper Chinji |
rM2 |
11.5 |
-10.4 |
-7.8 |
|
EB 477 |
Suidae |
Hyotherium pilgrimi |
upper Chinji |
rM2 |
11.5 |
-12.45 |
-7.9 |
|
EB 478 |
Suidae |
Hyotherium pilgrimi |
upper Chinji |
lM3 |
12.0 |
-11.2 |
-7.9 |
|
EB 479 |
Suidae |
Hyotherium pilgrimi |
upper Chinji |
lM3 |
12.0 |
-10.9 |
-6.9 |
|
EB 480 |
Rhinocerotidae |
Chilotherium intermedium |
lower Chinji |
lm2 |
13.6 |
-12.5 |
-11.3 |
|
EB 481 |
Rhinocerotidae |
Chilotherium intermedium |
lower Chinji |
lM3 |
13.6 |
-12.2 |
-11.5 |
|
EB 482 |
Rhinocerotidae |
Chilotherium intermedium |
lower Chinji |
lM3 |
12.6 |
-12.2 |
-12.7 |
|
EB 483 |
Rhinocerotidae |
Chilotherium intermedium |
upper Chinji |
P4 |
11.6 |
-12.0 |
-9.6 |
|
EB 484 |
Rhinocerotidae |
Chilotherium intermedium |
lower Chinji |
lM3 |
13.6 |
-12.5 |
-11.3 |
|
EB 485 |
Rhinocerotidae |
Chilotherium intermedium |
lower Chinji |
rm2 |
13.8 |
-12.45 |
-11.9 |
|
EB 486 |
Rhinocerotidae |
Chilotherium intermedium |
lower Chinji |
rM2 |
13.0 |
-11.9 |
-11.3 |
|
EB 487 |
Rhinocerotidae |
Chilotherium intermedium |
lower Chinji |
lM2 |
12.8 |
-13.2 |
-11.5 |
|
EB 488 |
Rhinocerotidae |
Chilotherium intermedium |
lower Chinji |
lM2 |
12.8 |
-11.6 |
-10.65 |
|
EB 489 |
Rhinocerotidae |
Chilotherium intermedium |
lower Chinji |
rm3 |
12.8 |
-12.3 |
-12.1 |
|
EB 490 |
Deinotheriidae |
Deinotherium pentapotamiae |
upper Chinji |
M2 |
11.6 |
-11.8 |
-9.1 |
|
EB 491 |
Deinotheriidae |
Deinotherium pentapotamiae |
upper Chinji |
M3 |
11.6 |
-12.3 |
-10.45 |
|
EB 492 |
Deinotheriidae |
Deinotherium indicum |
lower Chinji |
lm1 |
12.8 |
-13.8 |
-10.9 |
|
EB 493 |
Deinotheriidae |
Deinotherium indicum |
lower Chinji |
lm2 |
12.8 |
-11.5 |
-10.5 |
|
EB 494 |
Deinotheriidae |
Deinotherium indicum |
lower Chinji |
rM3 |
13.6 |
-13.2 |
-11.9 |
|
EB 495 |
Deinotheriidae |
Deinotherium indicum |
lower Chinji |
rm3 |
13.6 |
-13.4 |
-10.7 |
|
EB 496 |
Deinotheriidae |
Deinotherium pentapotamiae |
upper Chinji |
lm3 |
12.0 |
-11.3 |
-9.4 |
|
EB 497 |
Deinotheriidae |
Deinotherium pentapotamiae |
upper Chinji |
lm3 |
11.6 |
-10.9 |
-8.6 |
|
EB 498 |
Deinotheriidae |
Deinotherium indicum |
lower Chinji |
lm3 |
13.8 |
-13.25 |
-10.7 |
|
EB 499 |
Deinotheriidae |
Deinotherium pentapotamiae |
lower Chinji |
lm3 |
13 |
-12.3 |
-10.5 |
These findings are supported by evidence presented in Quade and Cerling (1995), who reported δ13Cpaleosol carbonate values from Kamlial and Chinji formations ranging from -12.9 to -9.5‰, indicating a forestland and woodland environment. Further afield, Quade et al. (1995b) from Pasalar, Turkey, and Uno et al. (2016) from eastern Africa report the δ13C values from paleosol and ungulate enamel, respectively. Both studies report a dominance of C3 vegetation with very little or no C4 vegetation during the middle Miocene time span.
The higher δ13Cenamel values in suids compared to rhinocerotids and deinotheres indicate that this family preferred more open areas for their feeding or browsed on a leafy vegetation. Tariq (2010) reports a frugivorous component to diets in suids on the basis of mesowear analysis. The mesowear analysis coupled with the morphological features of suid teeth (bunodont teeth with thick enamel) is in agreement with the hypothesis, based upon our δ13Cenamel data, that suids of middle Miocene utilized C3 plants in open habitats, including fruits, as their dietary resource and inhabited relatively more open areas (Tariq 2010; Aslam 2018; Waseem et al. 2020b).
On the basis of average δ13Cenamel and δ18Oenamel values, we may hypothesize that middle Miocene climate was sub-humid and animals drank from shaded water holes or rivers (Sanyal et al. 2010; Tada et al. 2016). If we use the equation for MAP calculation on the basis of δ13Cdiet following Kohn (2010), we can say that in middle Miocene of the Siwalik sub-Group of Pakistan MAP was lower than ~700 mm/year (see Kohn 2010: fig. 3). The average value of δ18Oenamel (-9.9 ± 3‰) of all non-ruminant taxa is low, indicating sub-humid conditions (Fig. 2B) during the middle Miocene time span in the Siwalik sub-Group of Pakistan, which is consistent with Indian contemporaneous sites (Patnaik et al. 2019). The low δ18Oenamel values also probably indicate that in the middle Miocene, animals drank from relatively unevaporated water holes or rivers.

Fig. 3. Reconstruction of the middle Miocene ecology of the Siwaliks and relative habitat partitioning among three mammalian families based on the stable isotope data of carbon and oxygen. Deinotheres (A), rhinocerotids (B), and suids (C).
The δ18Oenamel and δ13Cenamel values increase gradually from ~14 Ma to ~11 Ma (Fig. 2A, B), culminating in more open environments supporting a woodland or perhaps minor C4 vegetation (0–18% cover). However, the Hyotherium pilgrimi samples show higher δ13Cenamel values in our data which may also imply that this response may be species specific. Even if we consider the values as species specific, the open ecology towards the end of middle Miocene is still supported as we consider that the studied suid species occupied more open areas similar to the late Miocene suids. However, suids in the late Miocene showed feeding in both open and closed environments (Nelson 2005, 2007).
Niche partitioning among middle Miocene mammals
The studied middle Miocene mammals show some degree of niche partitioning (Fig. 3). The deinotheres and rhinocerotids from middle Miocene of Indian Siwaliks show an average δ13Cenamel values of -12.5‰ and -12.8‰, respectively, very close to the ranges in our results for these taxa (Patnaik et al. 2019), pointing to overall similar ecological and dietary preferences. The suids (average δ13Cenamel = -11.2 ± 1.4‰) inhabited a woodland/forestland setting where they fed on C3 vegetation consisting of plants, including fruits. They may have drunk from less shaded water holes as represented by their elevated δ18Oenamel values (-8.1‰) compared to the other middle Miocene taxa (Fig. 2B, C). The rhinocerotids and deinotheres show similarly low (p >0.05) δ13C values (-12.2‰ and -12.4‰, respectively), indicating that both families inhabited a forestland setting and browsed from understory vegetation (Fig. 2A, C), as reported by Patnaik et al. (2019) for contemporaneous Indian sites. However, the δ18Oenamel values significantly differ between these two families (-11.3 ± 1.8‰ and -10.4 ± 1.7‰; Fig. 2B). Heissig (1972, 2003) reports that middle Miocene rhinocerotids of Pakistan preferred a habitat close to water bodies and Biasatti et al. (2018) report the same behavior for middle Miocene rhinocerotids of China. Thus, we may assume that middle Miocene rhinocerotids lived in habitats close to water, resulting in the lowest δ18Oenamel values among all the middle Miocene taxa analyzed in this study. Deinotheres preferred a forested habitat where they had access to water but they did not live very close to the water bodies like rhinocerotids as indicated by the δ18Oenamel values (Fig. 3).
Conclusions
We analyzed thirty specimens belonging to three families of middle Miocene Siwalik sub-Group of Pakistan. On the basis of our results, we conclude that these middle Miocene species inhabited dense forests and woodlands where they fed on the C3 vegetation and drank from shaded water resources like ponds or rivers which were frequently refilled by precipitation (Fig. 3). We did not find clear evidence of significant C4 vegetation in their diet. The δ18Oenamel results reveal that the environment was more humid at ~14 Ma and became less humid towards the upper Chinji Formation (12–11.2 Ma). In general, the middle Miocene Siwaliks of Pakistan were dominated by forests and woodlands with precipitation supporting a more evergreen ecology as in the middle Miocene of eastern Africa, India, and Nepal and present-day vegetation of Myanmar (Quade et al. 1995a, b; Uno et al. 2016).
Acknowledgements
The authors are thankful to Antoine Souron (University of Bordeaux, France) and anonymous reviewers for their constructive commentary and very helpful comments which enhanced the quality of the manuscript. This work has been supported by the Higher Education Commission indigenous fellowship to MTW for Ph.D. studies vide pin no. 315-28107-2BS3-243. We also acknowledge the support in sample analysis from Desert Laboratory, University of Arizona, Tucson, USA.
References
Andrade, J.L., De La Barrera, E., Reyes-García, C., Ricalde, M.F., Vargas-Soto, G., and Cervera, C.J. 2007. El metabolismo ácido de las crasuláceas: diversidad, fisiología ambiental y productividad. Boletín de la Sociedad Botánica de México 87: 37–50. Crossref
Aslam, S. 2018. Dental and Cranial Morphological Studies Based on New Collection of Neogene Suids from the Siwalik Hills of Pakistan. 206 pp. Unpublished Ph.D. Thesis, University of the Punjab, Lahore.
Badgley, C., Barry, J.C., Morgan, M.E., Nelson, S.V., Behrensmeyer, A.K., Cerling, T.E., and Pilbeam, D. 2008. Ecological changes in Miocene mammalian record show impact of prolonged climatic forcing. Proceeding of National Academy of Science USA 105: 12145–12149. Crossref
Barry, J.C., Behrensmeyer, A.K., Badgley, C.E., Flynn, L.J., Peltonen, H., Cheema, I.U., Pilbeam, D., Lindsay, E.H., Raza, S.M., Rajpar, A.R., and Morgan, M.E. 2013. The Neogene Siwaliks of the Potwar Plateau, Pakistan. In: X. Wang, L.J. Flynn, and M. Fortelius (eds.), Fossil Mammals of Asia: Neogene Biostratigraphy and Chronology, 373–399. Columbia University Press, New York. Crossref
Barry, J.C., Lindsay, E.H., and Jacobs, L.L. 1982. A biostratigraphic zonation of the middle and upper Siwaliks of the Potwar Plateau of Northern Pakistan. Palaeogeography, Palaeoclimatology, Palaeoecology 37: 95–130. Crossref
Barry, J.C., Morgan, M., Flynn, L.J., Pilbeam, D., Behrensmeyer, A.K., Raza, S.M., Khan, I., Badgely, C., Hicks, J., and Kelley, J. 2002. Faunal and environmental change in the L=late Miocene Siwaliks of Northern Pakistan. Palaeobiology 28:1–72. Crossref
Bellasio, C., Quirk, J., and Beerling, D.J. 2018. Stomatal and non-stomatal limitations in savanna tress and C4 grasses grown at low, ambient and high atmospheric CO2. Plant Science 274: 181–192. Crossref
Ben-David, M. and Flaherty, E.A. 2012. Stable isotopes in mammalian research: a beginner’s guide. Journal of Mammalogy 93: 312–328. Crossref
Biasatti, D., Yang, W., and Deng, T. 2018. Paleoecology of Cenozoic rhinos from northwest China: a stable isotope perspective. Vertebrata PalAsiatica 56: 45–68.
Bibi, F. 2007. Dietary niche partitioning among fossil bovids in late Miocene C3 habitats: consilience of functional morphology and stable isotope analysis. Palaeogeography, Palaeoclimatology, Palaeoecology 253: 529–538. Crossref
Blumenthal, S.A., Levin, N.E., Brown, F.H., Brugal, J.P., Chritz, K.L., and Cerling, T.E. 2018. Diet and evaporation sensitivity in African ungulates: A comment on Faith (2018). Palaeogeography, Palaeoclimatology, Palaeoecology 506: 250–251. Crossref
Blumenthal, S.A., Levin, N.E., Brown, F.H., Brugal, J.-P., Chritz, K.L., Harris, J.M., Jehle, G.E. and Cerling, T.E. 2017. Aridity and hominin environments. Proceedings of the National Academy Sciences 114: 7331–7336. Crossref
Cerling, T.E. 1999. Paleorecords of C4 plants and ecosystems. In: R.F. Sage and R.K. Monson (eds.), C4 Plant Biology, 445–469. Academic Press, London. Crossref
Cerling, T.E. and Harris, J.M. 1999. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia 120: 347–363. Crossref
Cerling, T.E., Andanje, S.A., Blumenthal, S.A., Brown, F.H., Chritz, K.L., Harris, J.M., Hart, J., Kirera, F., Kaleme, P., Leakey, L., Meave, L., Levin, N.E., Manthi, F., Passey, B.H., and Uno, K.T. 2015. Dietary changes of large herbivores in the Turkana Basin, Kenya from 4 to 1 Ma. Proceeding of National Academy of Science USA 112: 11467–11472. Crossref
Cerling, T.E., Harris, J.M., Ambrose, S.H., Leakey, M.G., and Solounias, N. 1997a. Dietary and environmental reconstruction with stable isotope analyses of herbivore tooth enamel from the Miocene locality of Fort Ternan, Kenya. Journal of Human Evolution 33: 635–650. Crossref
Cerling, T.E., Harris, J.M., MacFadden, B.J., Leakey, M.G., Quade, J., Eisenmann, V., and Ehleringer, J.R. 1997b. Global vegetation change through the Miocene–Pliocene boundary. Nature 389:153–158. Crossref
Cerling, T.E., Hart, J.A., and Hart, T.B. 2004. Stable isotope ecology in the Ituri Forest. Oecologia 138: 5–12. Crossref
Channell, J.E.T., Ohneiser, C., Yamamoto, Y., and Kesler, M.S. 2013. Oligocene–Miocene magnetic stratigraphy carried by biogenic magnetite at sites U1334 and U1335 (equatorial Pacific Ocean). Geochemistry, Geophysics, Geoecosystem 14: 265–282. Crossref
Codron, J., Codron, D., Lee-Throp, J.A., Sponheimer, M., Bond, W.J., de Ruiter, D. and Grant, R. 2005. Taxonomic, anatomical, and spatiotemporal variations in the stable carbon and nitrogen isotopic compositions of plants from African savanna. Journal of Archaeological Science 32: 1757–1772. Crossref
Codron, D., Hull, J., Brink, J S., Codron, J., Ward, D., and Clauss, M. 2011. Effect of competition on niche dynamics of syntopic grazing ungulates: contrasting the predictions of habitat selection models using stable isotope analysis. Evolutionary Ecology Research 13 (3): 217–235.
Dansgaard, W. 1964. Stable isotopes in precipitation. Tellus 16: 436–468. Crossref
Decher, J. 1999. Book review: “The Kingdon Field Guide to African Mammals” by J. Kingdom. Journal of Mammalogy 80 (2): 692–693. Crossref
Deng, T. 2005. New discovery of Iranotherium morgani (Perissodactyla, Rhinocerotidae) from the late Miocene of the Linxia Basin in Gansu, China, and its sexual dimorphism. Journal of Vertebrate Paleontology 25: 442–450. Crossref
Dennell, R.W., Coard, R., and Turner, A. 2006. The biostratigraphy and magnetic polarity zonation of the Pabbi Hills, northern Pakistan: an Upper Siwalik (Pinjor Stage) upper Pliocene–Lower Pleistocene fluvial sequence. Palaeogeography, Palaeoclimatology, Palaeoecology 234: 168–185. Crossref
Domingo, L., Prado, J.L., and Alberdi, M.T. 2012. The effect of paleoecology and paleobiogeography on stable isotopes of Quaternary mammals from South America. Quaternary Science Reviews 55: 103–113. Crossref
Ehleringer, J.R., Lin, Z.F., Field, C.D., Sun, G.L., and You, L.Y. 1987. Leaf isotope ratios of plants from a subtropical monsoon forest. Oecologia 72: 109–114. Crossref
Faith, J.T. 2011. Late Quaternary dietary shifts of the Cape grysbok (Raphicerus melanotis) in Southern Africa. Quaternary Research 75: 159–165. Crossref
Faith, J.T. 2018. Paleodietary change and its implications for aridity indices derived from δ18O of herbivore tooth enamel. Palaeogeography, Palaeoclimatology, Palaeoecology 490: 571–578. Crossref
Feranec, R.S. and MacFadden, B.J. 2006. Isotopic discrimination of resource partitioning among ungulates in C3-dominated communities from the Miocene of Florida and California. Paleobiology 32: 191–205. Crossref
Flynn, L.J., Pilbeam, D., Barry, J.C., Morgan, M.E., and Raza, S.M. 2016. Siwalik synopsis: A long stratigraphic sequence for the later Cenozoic of South Asia. Comptes Rendus Palevol 15: 877–887. Crossref
Gradstein, F.M., Ogg, J.G., and Hilgen, F.J. 2012. On the Geologic Time Scale. Newsletters on Stratigraphy 45: 171–188. Crossref
Harris, J.M. and Cerling, T.E. 2002. Dietary adaptations of extant and Neogene African suids. Journal of Zoology 256: 45–54. Crossref
Heissig, K. 1972. Paläontologische und geologische Untersuchungen im Tertiär von Pakistan, 5. Rhinocerotidae (Mamm.) aus den unteren und mittleren Siwalik-Schichten. Bayerische Akademie der Wissenschaften Mathematisch Naturwissenschaftliche Klasse, Abhandlungen, Neue Folge 152: 1–112.
Heissig, K. 2003. Change and Continuity in Rhinoceros Faunas of Western Eurasia from the Middle to the Upper Miocene. 89 pp. EEDEN, Stará Lesná.
Hynek, S.A., Passey, B.H., Prado, J.L., Brown, F.H., Cerling, T.E., and Quade, J. 2012. Small mammal carbon isotope ecology across the Miocene–Pliocene boundary, northwestern Argentina. Earth and Planetary Science Letters 321–322: 177–188. Crossref
Johnson, N.M., Opdyke, N.D., Johnson, G.D., Lindsay, E.H., and Tahirkheli, R.A.K. 1982. Magnetic polarity stratigraphy and ages of Siwalik group rocks of the Potwar Plateau, Pakistan. Palaeogeography, Palaeoclimatology, Palaeoecology 37: 17–42. Crossref
Khan, A.M. 2009. Taxonomy and Distribution of Rhinoceroses from the Siwalik Hills of Pakistan. 182 pp. Unpublished Ph.D. Thesis, University of the Punjab, Lahore.
Khan, M.A., Batool, A., Nayyer, A.Q., and Akhtar, M. 2013. Gazella lydekkeri (Cetartiodactyla: Ruminantia: Bovidae) from the Middle Siwaliks of Hasnot (late Miocene), Pakistan. Pakistan Journal of Zoology 45: 981–988.
Khan, A.M., Iqbal, A., Waseem, M.T., Ahmad, R.M., and Ali, Z. 2020. Palaeodietary and palaeoclimatic interpretations for herbivore fauna from late Pliocene to early Pleistocene Siwaliks of Pakistan. Journal of Animal and Plant Sciences 30: 355–363. Crossref
Koch, P.L. 1998. Isotopic reconstruction of past continental environments. Annual Review of Earth and Planetary Science 26: 573–613. Crossref
Koch, P.L. 2007. Isotopic study of the biology of modern and fossil vertebrates. In: R.H. Michener and K. Lajtha (eds.), Stable Isotopes in Ecology and Environmental Science, 99–154. Blackwell Publishing, Boston. Crossref
Kohn, M.J. 2010. Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proceedings of the National Academy Sciences 107: 19691–19695. Crossref
Kohn, M.J. and Cerling, T.E. 2002. Stable Isotope Compositions of Biological Apatite. In: M.J. Kohn, J. Rakovan, and J.M. Hughes (eds.), Phosphates—Geochemical, Geobiological, and Materials Importance. Reviews in Mineralogy and Geochemistry 48: 455–488. Crossref
Kohn, M.J., Schoeninger, M.J., and Valley, J.W. 1996. Herbivore tooth oxygen isotope compositions: effects of diet and physiology. Geochimica et Cosmochimica Acta 60: 3889–3896. Crossref
Koch, P.L., Tuross, N., and Fogel, M.L. 1997. The effects of sample treatment and diagenesis on the isotopic integrity of carbonate in biogenic hydroxylapatite. Journal of Archaeological Science 24: 417–429. Crossref
Larramendi, A. 2016. Shoulder height, body mass, and shape of proboscideans. Acta Palaeontologica Polonica 61: 537–574. Crossref
Levin, N.E., Cerling, T.E., Passey, B.H., Harris, J.M., and Ehleringer, J.R. 2006. A stable isotope aridity index for terrestrial environments. Proceedings of the National Academy Sciences 103: 11201–11205. Crossref
MacFadden, B.J. and Cerling, T.E. 1996. Mammalian herbivore communities, ancient feeding ecology, and carbon isotopes: a 10 million-year sequence from the Neogene of Florida. Journal of Vertebrate Paleontology 16:103–115 Crossref
MacFadden, B.J. and Higgins, P. 2004. Ancient ecology of 15-million-year old browsing mammals within C3 plant communities from Panama. Oecologia 140: 169–182. Crossref
Medrano, H. and Flexas, J. 2000. Fotorrespiración y mecanismos de concentración del dióxido de carbono. In: J. Azcón-Bieto and M. Talón (eds.), Fundamentos de Fisiología Vegetal, 187–201. McGraw-Hill Interamericana, Madrid.
Morgan, M.E., Behrensmeyer, A.K., Badgley, C., Barry, J.C., Nelson, S., and Pilbeam, D. 2009. Lateral trends in carbon isotope ratios reveal a Miocene vegetation gradient in the Siwaliks of Pakistan. Geology 37: 103–106. Crossref
Morgan, M.E., Kingston, J.D., and Marino, B.D. 1994. Carbon isotopic evidence for the emergence of C4 plants in the Neogene from Pakistan and Kenya. Nature 367: 162–165. Crossref
Nelson, S.V. 2005. Paleoseasonality inferred from equid teeth and intra-tooth isotopic variability. Palaeogeography, Palaeoclimatology, Palaeoecology 222: 122–144. Crossref
Nelson, S.V. 2007. Isotopic reconstructions of habitat change surrounding the extinction of Sivapithecus, a Miocene hominoid, in the Siwalik Group of Pakistan. Palaeogeography, Palaeoclimatology, Palaeoecology 243: 204–222. Crossref
Patnaik, R., Singh, N.P., Paul, D., and Sukumar, R. 2019. Dietary and habitat shifts in relation to climate of Neogene–Quaternary and associated mammals of Indian Subcontinent. Quaternary Science Reviews 224: 105968. Crossref
Passey, B.H., Robinson, T.F., Ayliffe, L.K., Cerling, T.E., Sponheimer, M., Dearing, M.D., Roeder, B.L., and Ehleringer, J.R. 2005. Carbon isotope fractionation between diet, breath CO2, and bioapatite in different mammals. Journal of Archaeological Science 32: 1459–1470. Crossref
Pérez-Crespo, V.A., Carranza y Castañeda, O., Arroyo-Cabrales, J., Morales-Puente, P., Cienfuegos-Alvarado, E., and Otero, F.J. 2017. Diet and habitat of unique individuals of Dinohippus mexicanus and Neohipparion eurystile (Equidae) from the late Hemphillian (Hh3) of Guanajuato and Jalisco, central Mexico: stable studies isotopes. Revista Mexicana de Ciencias Geológicas 34: 38–44. Crossref
Pilgrim, G.E. 1937. Siwalik antelopes and oxen in the American Museum of Natural History. Bulletin of American Museum of Natural History 72: 729–874.
Quade, J. and Cerling, T.E. 1995. Expansion of C4 grasses in the late Miocene of northern Pakistan: Evidence from stable isotopes in paleosols. Palaeogeography, Palaeoclimatology, Palaeoecology 115: 91–116. Crossref
Quade, J., Cater, J.M.L., Adam, J., and Harrison, T.M. 1995a. Late Miocene environmental change in Nepal and the northern Indian subcontinent: Stable isotopic evidence from paleosols. GSA Bulletin 107: 1381–1397. Crossref
Quade, J., Cerling, T.E., and Bowman, J.R. 1989. Development of Asian monsoon revealed by marked ecological shift during the latest Miocene in northern Pakistan. Nature 342: 163–166. Crossref
Quade, J., Cerling, T.E., Andrews, P., and Alpagut, B. 1995b. Paleodietary reconstruction of Miocene faunas from Paşalar, Turkey, using stable carbon and oxygen isotopes of fossil tooth enamel. Journal of Human Evolution 28: 373. Crossref
Quade, J., Cerling, T.E., Barry, J., Morgan. M.M., Pilbeam, D.R., Chivas. A.R., Lee-Thorp, J.A., and Van der Merwe, N.J. 1992. A 16 Ma record of paleodiet using carbon and oxygen isotopes in fossil teeth from Pakistan. Chemical Geology 94:182–192. Crossref
Sánchez, B. 2005. Reconstrucción del ambiente de mamíferos extintos a partir del análisis isotópico de los restos esqueléticos. In: P. Alcorno, R. Redondo, and J. Toledo (eds.), Nuevas técnicas metodológicas aplicadas al estudio de los sistemas ambientales: los isótopos estables, 49–64. Universidad Autónoma de Madrid, Madrid.
Sanyal, P., Bhattacharya, S.K., Kumar, R., Ghosh, S.K., and Sangode S.J. 2005. Palaeovegetational reconstruction in Late Miocene: a case study based on early diagenetic carbonate cement from the Indian Siwalik. Palaeogeography, Palaeoclimatology, Palaeoecology 228: 245–259. Crossref
Sanyal, P., Sarkar, A., Bhattacharya, S.K., Kumar, R., Ghosh, S.K., and Agarwal, S. 2010. Intensification of monsoon, microclimate, and asynchronous C4 appearance: isotopic evidence from the Indian Siwalik sediments. Palaeogeography, Palaeoclimatology, Palaeoecology 296: 165–173. Crossref
Sarwar, M. 1977. Taxonomy and distribution of the Siwalik Proboscidea. Bulletin Department of Zoology University of the Punjab Lahore 10: 1–172.
Shoshani, J. and Tassy, P. 2005. Advances in proboscidean taxonomy & classification, anatomy & physiology, and ecology & behavior. Quaternary International 126–128: 5–20. Crossref
Sorkin, B. 2008. A biomechanical constraint on body mass in terrestrial mammalian predators. Lethaia 41: 333–347. Crossref
Souron, A. 2017. Diet and ecology of extant and fossil wild pigs. In: M. Melletti and E. Meijaard (eds.), Ecology, Conservation and Management of Wild Pigs and Peccaries, 29–38. Cambridge University Press, Cambridge. Crossref
Sponheimer, M. and Lee-Thorp, J. A. 1999. Oxygen isotopes in enamel carbonate and their ecological significance. Journal of Archaeological Science 26: 723–728. Crossref
Tada, R., Zheng, H., and Clift, P.D. 2016. Evolution and variability of the Asian monsoon and its potential linkage with uplift of the Himalaya and Tibetan Plateau. Progress in Earth and Planetary Science 3: 4. Crossref
Tariq, M. 2010. Palaeoenvironmental Study of Pakistan Siwaliks, 78–122. Unpublished Ph.D. Thesis, Government College University, Lahore.
Tejada-Lara, J.V., MacFadden, B.J., Bermudez, L., Rojas, G., Salas-Gismondi, R., and Flynn, J.J. 2018. Body mass predicts isotope enrichment in herbivorous mammals. Proceedings of the Royal Society B 285 (1881), 20181020. Crossref
Tipple, B.J., Meyers, S.R., and Pagani, M. 2010. Carbon isotope ratio of Cenozoic CO2: a comparative evaluation of available geochemical proxies. Paleoceanography 25: PA3202. Crossref
Uno, K.T., Cerling, T.E., Harris, J.M., Kunimatsu, Y., Leakey, M.G., and Nakatsukasa, M. 2011. Late Miocene to Pliocene carbon isotope record of differential diet change among East African herbivores. Proceedings of National Academy of Science 18: 1–6.
Uno, K.T., Polissar, P.J., Jackson, K.E., and de Menocal, P. 2016. Neogene biomarker record of vegetation change in Africa. Proceedings of National Academy of Science 113: 6355–6363. Crossref
Waseem, M.T., Khan, A.M., Ghaffar, A., Iqbal, A., and Ahmad, R.M. 2020a. Palaeodietary and palaeoclimatic reconstruction for late Miocene hipparionines from the Siwaliks of Pakistan. Pakistan Journal of Zoology [published online, https://dx.doi.org/10.17582/journal.pjz/ 20180314070354]. Crossref
Waseem, M.T., Khan, A.M., Quade, J., Krupa, A., and Dettman, D.L. 2020b. Stable isotope analysis of middle Miocene faunal communities from the Siwaliks (Pakistan). In: E. Vlachos, D. Crespo, C. Martinez-Perez, H.G. Ferron, J.L. Herraiz, A. Gamonal, F.A.M. Arnal, F. Gasco, and P. Citton (eds.), 2nd Palaeontological Virtual Congress, 199. Crossref
White, T.D., Ambrose, S.H., Suwa, G., Su, D.F., DeGusta, D., Bernor, R.L., Boisserie, J.-R., Brunet, M., Delson, E., Frost, S., Garcia, N., Giaourtsakis, I.X., Haile-Selassie, Y., Howell, F.C., Lehmann, T., Likius, A., Pehlevan, C., Saegusa, H., Semprebon, G., Teaford, M., and Vrba, E. 2009. Macrovertebrate paleontology and the Pliocene habitat of Ardipithecus ramidus. Science 326: 67–93.Crossref
Acta Palaeontol. Pol. 66 (Supplement to 3): 123–132, 2021
https://doi.org/10.4202/app.00788.2020