


First phylogenetic analysis of the Miocene armadillo Vetelia reveals novel affinities with Tolypeutinae
DANIEL BARASOAIN, LAUREANO R. GONZÁLEZ RUIZ, RODRIGO L. TOMASSINI, AFREDO E. ZURITA, VÍCTOR H. CONTRERAS, and CLAUDIA I. MONTALVO
Barasoain, D., González Ruiz, L.R., Tomassini, R.L., Zurita, A.E., Contreras, V.H., and Montalvo, C.I. 2021. First phylogenetic analysis of the Miocene armadillo Vetelia reveals novel affinities with Tolypeutinae. Acta Palaeontologica Polonica 66 (Supplement to 3): 31–46.
Vetelia is a Miocene genus of armadillos from Argentina and Chile, traditionally included within the subfamily Euphractinae (Chlamyphoridae, Cingulata, Xenarthra). It includes the species Vetelia puncta (early–middle Miocene), Vetelia perforata (middle–late Miocene), and Vetelia gandhii (late Miocene), mostly known by isolated osteoderms. In this contribution, we provide the first description of the skull for this genus, based on new materials (PVSJ-289 and PVSJ-154) here assigned to V. gandhii. A detailed characterization allows us to amend the diagnosis of the three known species, and to include, for the first time, the genus Vetelia into a morphological phylogenetic analysis. Phylogenetic results reveal a closer affinity to the Tolypeutinae, including the extant genera Priodontes (giant armadillos), Cabassous (naked-tailed armadillos), and Tolypeutes (three banded armadillos), and the fossil genera Pedrolypeutes and Kuntinaru, than to the Euphractinae. More specifically, Vetelia is included within the Priodontini, as sister group of the clade composed by Cabassous + Priodontes. Taking into account the scarce record of fossil Tolypeutinae, this new proposal fills an important temporal gap in the evolutionary history of this linage. Finally, we also provide new information on the diagnostic morphological characters of the Priodontini and Tolypeutini.
Key words: Mammalia, Euphractinae, Tolypeutinae, phylogeny, Neogene, South America.
Daniel Barasoain [danielbarasoain@gmail.com] and Afredo E. Zurita [aezurita74@yahoo.com.ar], Laboratorio de Evolución de Vertebrados y Ambientes Cenozoicos, Centro de Ecología Aplicada del Litoral (UNNE-CONICET) y Cátedra de Paleontología, Facultad de Ciencias Exactas, Naturales y Agrimensura, Universidad Nacional del Nordeste, RP5 3400 Corrientes, Argentina.
Laureano R. González Ruiz [gonzalezlaureano@yahoo.com.ar], Laboratorio de Investigaciones en Evolución y Biodiversidad (LIEB-FCNyCS sede Esquel, UNPSJB) y Centro de Investigaciones Esquel de Montaña y Estepa Patagónica (CIEMEP), CONICET, Universidad Nacional de La Patagonia San Juan Bosco (UNPSJB), Roca 780, 9200 Esquel, Chubut, Argentina.
Rodrigo L. Tomassini [rodrigo.tomassini@yahoo.com.ar], INGEOSUR, Departamento de Geología, Universidad Nacional del Sur-CONICET, Avenida Alem 1253, 8000 Bahía Blanca, Argentina.
Víctor H. Contreras [vcontre@unsj-cuim.edu.ar], Instituto de Geología Dr. Emiliano P. Aparicio, departamentos Geología y Biología, Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de San Juan, Avenida Ignacio de La Rosa y calle Meglioli, Rivadavia, 5400, San Juan, Argentina.
Claudia I. Montalvo [cmontalvolp@yahoo.com.ar], Facultad de Ciencias Exactas y Naturales, Universidad Nacional de La Pampa, Avenida Uruguay 151, 6300 Santa Rosa, Argentina.
Received 28 September 2020, accepted 4 December 2020, available online 2 June 2021.
Copyright © 2021 D. Barasoain et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Xenarthra (early Eocene–Recent) constitutes one of the main clades, and possibly the most basal, among placental mammals (Gelfo et al. 2009; O’Leary et al. 2013). This peculiar group is the result of a long process of endemic evolution during the isolation of South America, which lasted for most of the Cenozoic (Cione et al. 2015). There is general consensus that the origin of Xenarthra is an event posterior to the separation of South America and Africa (see Vizcaíno and Bargo 2014), while molecular evidence suggests that the clade could have arisen ca. 100 Ma (Delsuc et al. 2004).
Xenarthrans encompass both fossil and extant representatives of two different ecologic and morphological monophyletic groups: on the one hand Pilosa, which includes sloths and anteaters, and on the other hand, Cingulata, which includes armadillos, glyptodonts, pampatheres, and pachyarmaterids (Engelmann 1985; O’Leary et al. 2013; Gaudin and Croft 2015; Fernicola et al. 2017). Cingulata (“armored” xenarthrans) is the most ancient (early Eocene; Bergqvist et al. 2004; Gelfo et al. 2009; Woodburne et al. 2014) and diverse clade, with a complex evolutionary history that led to the development of very diverse sizes and morphologies, with distinct taxa occupying very different ecological niches.
Molecular data suggest that living armadillos can be gathered in two families, Dasypodidae and Chlamyphoridae. Among chlamyphorids, three subfamilies are recognized: Chlamyphorinae (fairy armadillos), Euphractinae (yellow and hairy armadillos), and Tolypeutinae (naked-tailed, giant, and three banded armadillos) (Möller-Krull et al. 2007; Delsuc et al. 2012, 2016; Gibb et al. 2016; Mitchell et al. 2016).
Extant tolypeutines are represented by Tolypeutes Illiger, 1811 [T. matacus (Desmarest, 1804) and T. tricinctus (Linnaeus, 1758)], included in the tribe Tolypeutini, and Cabassous McMurtrie, 1831 [C. tatouay (Desmarest, 1804), C. unicinctus (Linnaeus, 1758), C. chacoensis Wetzel, 1980, and C. centralis (Miller, 1899)] and Priodontes Cuvier, 1825 [P. maximus (Kerr, 1792)], included in the tribe Priodontini (Wetzel 1985; McKenna and Bell 1997; Gibb et al. 2016).
Although Tolypeutinae actually presents the highest diversity among living armadillos, the fossil representatives of this subfamily are very scarce and its evolutionary history still remains largely unknown (Billet et al. 2011). Kuntinaru boliviensis Billet, Hautier, Muizon, and Valentin, 2011, from the late Oligocene (~26 Ma; MacFadden et al. 1994) of Bolivia is the oldest record of the subfamily. The age of this taxon is consistent with the results provided by molecular analyses, which suggest that the divergence of tolypeutines occurred before 29 Ma, during the early Oligocene (Delsuc et al. 2004). Pedrolypeutes praecursor Carlini, Vizcaíno, and Scillato-Yané, 1997, from the middle Miocene (~14–12 Ma) of Colombia, is interpreted as a possible ancestor of the extant Tolypeutes, based on similarities observed at the level of the dorsal carapace osteoderms (Carlini et al. 1997). Finally, more modern representatives of Tolypeutinae are Tolypeutes sp. from the late Pliocene of Argentina, T. matacus from the Pleistocene–Holocene of Argentina and Bolivia, T. tricinctus from the Pleistocene–Holocene of Brazil (e.g., Scillato-Yané 1980, 1982; Carlini et al. 1997; McKenna and Bell 1997; Oliveira and Pereira 2009), and Cabassus sp. from the late Pleistocene–early Holocene of Brazil (Oliveira et al. 2014, and references therein).
According to the known findings, tolypeutine armadillos are absent from the fossil record during two long periods, between the late Oligocene and middle Miocene, and between the middle Miocene and late Pliocene/Quaternary transition. With respect to this situation, Billet et al. (2011) considered the tolypeutines as a clear example of a “ghost lineage”, and suggested that it could be related with a historical misidentification of the diagnostic characters of the representatives of this subfamily.
Vetelia Ameghino, 1891, is an armadillo mainly restricted to the Miocene of Argentina and Chile (Fig. 1), represented by the type species Vetelia puncta Ameghino, 1891 (early–middle Miocene) and the referred species Vetelia perforata Scillato-Yané, 1977 (middle–late Miocene) and Vetelia gandhii Esteban and Nasif, 1996 (late Miocene), all of them mostly know by isolated osteoderms of the dorsal carapace (Scillato-Yané 1977, 1982; González Ruiz 2010). This genus has been traditionally included within the subfamily Euphractinae (Scillato-Yané 1982; Esteban and Nasif 1996; Urrutia et al. 2008; González Ruiz 2010). However, Hofstetter (1958) suggested a possible relationship with the Tolypeutinae Priodontini, based on the similarity of dorsal carapace osteoderms between V. puncta and the extant Cabassous and Priodontes. Despite this observation, no studies were performed in order to test this hypothesis.
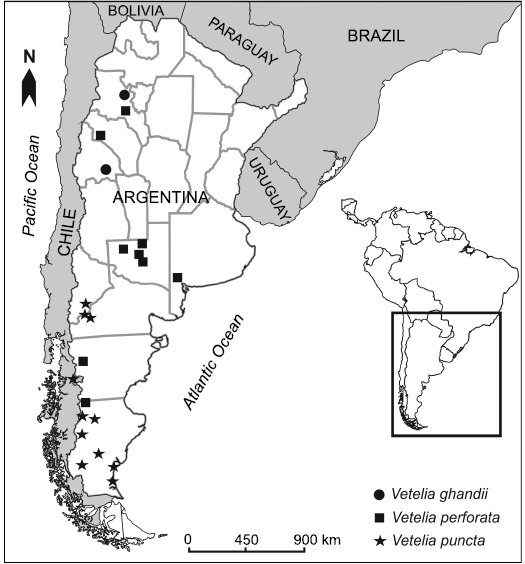
Fig. 1. Map showing the geographic distribution of Vetelia species in Argentina and Chile.
In this contribution, we describe the most complete specimen of Vetelia (i.e., PVSJ-289) from the late Miocene of San Juan Province (Argentina), which allows the first detailed anatomical description of the skull and mandible of this genus. In addition, the diagnosis of the three known species is revised and a morphological phylogenetic analysis is performed to test their relationships with other Euphractinae and Tolypeutinae armadillos. The higher affinity observed with tolypeutines, particularly with the Priodontini Priodontes and Cabassus, provides novel information on the poorly known evolutionary history of this lineage, filling an important temporal gap in the fossil record.
Institutional abbreviations.—AMNH, American Museum of Natural History, New York, USA; CD-UNNE, Colección Didáctica de la Universidad Nacional del Nordeste, Corrientes, Argentina; CML, Colección Mamíferos, Facultad de Ciencias Naturales e Instituto Miguel Lillo, San Miguel de Tucumán, Argentina; FMNH, Field Museum of Natural History, Chicago, USA; INGEO-PV, Instituto de Geología “Dr. Emiliano P. Aparicio”, Universidad Nacional de San Juan, San Juan, Argentina; LEVAC-CO, Colección Laboratorio de Evolución de Vertebrados y Ambientes Cenozoicos, Corrientes, Argentina; MACN, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina; MD-CH, Colección Arroyo Chasicó, Museo Municipal de Ciencias Naturales “Carlos Darwin”, Punta Alta, Argentina; MLP, Museo de La Plata, La Plata, Argentina; MMH-CH, Colección Arroyo Chasicó, Museo Municipal de Ciencias Naturales “Vicente Di Martino”, Monte Hermoso, Argentina; PVL, Colección de Paleontología de Vertebrados, Facultad de Ciencias Naturales e Instituto Miguel Lillo, San Miguel de Tucumán, Argentina; PVSJ, Instituto y Museo de Ciencias Naturales, Universidad Nacional de San Juan, San Juan, Argentina.
Other abbreviations.—SALMA, South American Land Mammal Age. We follow standard convention in abbreviating tooth families as I, C, P, and M, with upper and lower case letters referring to upper and lower teeth, respectively.
Material and methods
This study includes the description and comparison of specimens referred to the three known species of Vetelia (V. puncta, V. perforata, and V. ghandii). We also consider specimens of the extant tolypeutines Priodontes, Cabassous, and Tolypeutes (see SOM 1, Supplementary Online Material available at http://app.pan.pl/SOM/app66-Barasoain_etal_SOM.pdf). Bibliographic information is also considered for the species Kuntinaru boliviensis and Pedrolypeutes praecursor (Billet et al. 2011; Carlini et al. 1997, respectively). The biostratigraphic schemes (e.g., SALMA, regional stages/ages, international stages) considered for the different records vary according to the age and geographical provenance (see below). Skull, mandible, molariforms, and osteoderms measurements (Tables 1–3) were obtained by using a 0.02 mm resolution digital calliper.
Phylogenetic analysis
We carried out a cladistic analysis, based on morphological characters, in order to determine the phylogenetic affinities of Vetelia. Representatives of this genus are included for the first time in a cladistic analysis. In this case, we used V. gandhii and V. perforata. We opted to exclude V. puncta of this analysis because the scarce known specimens (represented only by isolated osteoderms) do not allow the codification of most characters here considered.
The matrix includes 24 taxa and 148 morphological characters (see SOM 2), including both cranial (skull, mandible, and teeth; 1–125) and carapace (dorsal carapace and osteoderms; 126–148) features. All characters were treated as unordered and given the same weight (1.0), according to Gaudin’s (2004) criterion. The matrix (see SOM 3) was created through the software Mesquite 3.04 (Maddison and Maddison 2008). A total of 96 characters were codified as binary, while 52 were multistate (non-additive). Characters that were not preserved or could not be observed were coded as “?”, while non-codifiable characters or characters states were coded as “–”.
The out-group includes three xenarthran taxa: the sloth Bradypus Linnaeus, 1758, the anteater Tamandua Rafinesque, 1815, and the peltephilid armadillo Peltephilus Ameghino, 1887, which were used to root the phylogenetic trees, as they represent all main groups within Xenarthra. The in-group includes 20 genera of both extinct and extant dasypodid and chlamyphorid armadillos, selected to reflect as best as possible their past and present diversity: (i) the Dasypodidae Dasypus Linnaeus, 1758, and †(extinct) Stegotherium Ameghino, 1887 (subfamily Dasypodinae); (ii) the Chlamyphoridae euphractines: †Prozaedyus, Ameghino 1891, †Proeutatus Ameghino, 1891, †Eutatus Gervais, 1867, Zaedyus Ameghino, 1889, Euphractus Wagler, 1830, Chaetophractus Fitzinger, 1871, †Paleuphractus Kraglievich, 1934, †Proeuphractus Ameghino, 1886, and †Macroeuphractus Ameghino, 1887; (iii) the Chlamyphoridae chlamyphorines †Chlamydophractus (Barasoain, Tomassini, Zurita, Montalvo, and Superina, 2020c), Chlamyphorus Harlan, 1825, and Calyptophractus Fitzinger, 1871; and (iv) the Chlamyphoridae tolypeutines †Pedrolypeutes Carlini, Vizcaíno, and Scillato-Yané, 1997, Tolypeutes Illiger, 1811, Priodontes Cuvier, 1825, Cabassous McMurtrie, 1831, and †Kuntinaru Billet, Hautier, Muizon, and Valentin, 2011. Additionally, the in-group also includes the genus here analyzed, Vetelia, including the species V. gandhii and V. perforata.
The character-taxon matrix was evaluated using the software “TNT”, with an “Implicit Enumeration” analysis carried out under the maximum parsimony criteria (Goloboff et al. 2008). For the resulting Most Parsimonious Trees (MPT), the following values were calculated: tree length (TL), Consistency Index (CI), and Retention Index (RI). Additionally, several clade support values for each node were obtained through both absolute and relative Bremer support, and a 1000 replicates “traditional search” Standard Bootstrap analysis and Jacknife resampling (Felsenstein 1985; Bremer 1994; Farris et al. 1996; Goloboff and Farris 2001).
Systematic palaeontology
Xenarthra Cope, 1889
Cingulata Illiger, 1811
Chlamyphoridae Pocock, 1924
Tolypeutinae Gray, 1865
Priodontini Gray, 1873
Genus Vetelia Ameghino, 1891
Figs. 2–5.
Type species: Vetelia puncta Ameghino, 1891, Santa Cruz Formation, mid-early Miocene, Karaiken, Santa Cruz Province, Argentina.
Included species: Vetelia puncta, Vetelia perforata Scillato-Yané, 1977, and Vetelia ghandii Esteban and Nasif, 1996.
Emended diagnosis (after Ameghino 1891).—Large armadillo, comparable in size to Priodontes maximus. Fixed osteoderms with a wide and slightly convex central figure, which does not reach the posterior margin; lateral peripheral figures becoming fused with central figure towards the posterior margin; one to four anterior figures. Fixed osteoderms of the scapular shield more elongated than those of the pelvic shield. Mobile osteoderms with a wide and slightly convex central figure, which starts at the transitional zone and reaches the posterior margin of the osteoderm; undivided and elongated lateral peripheral figures. Mobile osteoderms with similar width to that of fixed osteoderms, but twice longer. Both fixed and mobile osteoderms with piliferous foramina of the posterior margin arranged in a single row. Skull with very wide and low zygomatic arches, and developed olfactory bulbs. Flat cranial case, without sagittal crest, and a strong w-shaped nuchal crest. Wide and broad snout, and presence of premaxillary teeth (observed in V. ghandii). Very robust and massive mandible, with nine ovate and chisel-shaped molariforms. Tooth row extended to the most proximal end of the horizontal ramus, without anterior dental diastema. Condylar process taller than coronoid process; angular process located at the occlusal surface level. Low vertical ramus, with its height equivalent to one third of the horizontal ramus length (observed in V. perforata and V. ghandii).
Stratigraphic and geographic range.—Early Miocene–late Miocene. Pinturas Formation (early Miocene), “Pinturan” (SALMA); Santa Cruz and Boleadoras formations (early Miocene), Santacrucian SALMA; Río Frías Formation (early–middle Miocene), Friasian SALMA; Collón Curá Formation (middle Miocene), Colloncuran SALMA; Río Mayo Formation (middle–late Miocene), Mayoan SALMA; Arroyo Chasicó, Huachipampa and Loma de Las Tapias formations (late Miocene), Chasicoan stage/age; Cerro Azul and Chiquimil formations (late Miocene), Chasicoan/Huayquerian stages/ages; Desencuentro and El Morterito formations (late Miocene), Huayquerian stage/age; and Playa del Zorro Aloformation (late Miocene), Tortonian–Messinian. Santa Cruz, Chubut, Neuquén, Río Negro, Buenos Aires, La Pampa, La Rioja, San Juan, and Catamarca provinces, Argentina; Región Aysén, Chile (Fig. 1).
Vetelia puncta Ameghino, 1891
Fig. 4.
Holotype: MACN-A-2139, fixed and mobile osteoderms.
Type horizon: Santa Cruz Formation, mid-early Miocene.
Type locality: Karaiken, Santa Cruz Province, Argentina.
Material.—Other referred material (see SOM 1).
Emended diagnosis.—Species with similar osteoderm size and ornamentation pattern than V. perforata and V. ghandii, but differing from them by the presence, in both fixed and mobile osteoderms, of numerous piliferous foramina along a main sulcus that delimitate the central figure. Small foramina in the anterior and lateral margins of the osteoderms. Piliferous foramina of the posterior margin similar to the condition in V. ghandii, but larger and less numerous than in V. perforata.
Stratigraphic and geographic range.—Early–middle Miocene. Pinturas Formation (early Miocene), “Pinturan” SALMA; Santa Cruz and Boleadoras formations (early Miocene), Santacrucian SALMA; Río Frías Formation (early–middle Miocene), Friasian SALMA. Santa Cruz Province, Argentina and Región Aysén, Chile (Fig. 1).
Vetelia perforata Scillato-Yané, 1977
Figs. 3, 4.
Holotype: MLP 28-X-11-45, fixed, semimobile, and mobile osteoderms.
Type horizon: Arroyo Chasicó Formation, late Miocene.
Type locality: Arroyo Chasicó, Buenos Aires Province, Argentina.
Material.—Other referred material (see SOM 1).
Emended diagnosis.—Species with similar size and osteoderm ornamentation pattern than V. puncta and V. ghandii, but differing from them by the presence, in both fixed and mobile osteoderms, of smaller and very numerous piliferous foramina located in the posterior margin. Also differs from V. puncta by the absence of foramina in the anterior and lateral margins of the osteoderms, and from V. ghandii by the presence of rounded borders along the entire dorsal carapace margins. Very robust and massive mandible, with nine ovate and chisel-shaped molariforms. Tooth row extended to the most proximal ending of the horizontal ramus, without anterior diastema. Condylar process taller than coronoid process; angular process located at the occlusal surface level. Low vertical ramus, being its height equivalent to one third of the horizontal ramus length. Contact between horizontal and vertical ramus in an obtuse angle (~110º) in contrast to the straight angle (~90º) in V. ghandii.
Stratigraphic and geographic range.—Middle–late Miocene; Río Mayo Formation (middle–late Miocene), Mayoan SALMA; Arroyo Chasicó Formation (late Miocene), Chasicoan stage/age; Cerro Azul and Chiquimil formations (late Miocene), Chasicoan/Huayquerian stages/ages; Desencuentro Formation (late Miocene), Huayquerian stage/age. Santa Cruz, Chubut, Buenos Aires, La Pampa, La Rioja, and Catamarca provinces, Argentina (Fig. 1).
Vetelia gandhii Esteban and Nasif, 1996
Figs. 2–5.
Holotype: PVL 4800, fixed and mobile osteoderms.
Type horizon: Playa del Zorro Alloformation, late Miocene.
Type locality: Santa María, Catamarca Province, Argentina.
Material.—Other referred material (see SOM 1).
Emended diagnosis.—Species with similar size and osteoderm ornamentation pattern to V. puncta and V. perforata. It differs from V. puncta by the absence of foramina in the anterior and lateral margins of the osteoderms, and from V. perforata by the presence of larger and less numerous foramina in the posterior margin of the osteoderms. Dorsal carapace with a reduced scapular shield, including just two rows of fixed osteoderms, and mobile bands composed of at least five rows of osteoderms. Differs from V. perforata in having serrated dorsal carapace edges towards the posterior margin of the pelvic shield. Skull with very wide and low zygomatic arches, and developed olfactory bulbs. Flat cranial case with absence of sagittal crest, and a strong relief w-shaped nuchal crest. Wide and broad snout, and presence of premaxillary teeth. Nine ovate and chisel-shaped molariforms, both in upper and lower dental series. Toothrows anteriorly extended, with absence of both upper and lower anterior diastema, and flat palatines. Contact between horizontal and vertical ramus forms at a straight angle (~90º), differing from the obtuse angle (~110º) in V. perforata.
Remarks.—PVSJ-289, from Loma de Las Tapias Formation, San Juan Province (see SOM 1), described here for the first time, represents the most complete specimen known for the genus.
Stratigraphic and geographic range.—Late Miocene; Loma de Las Tapias Formation (late Miocene), Chasicoan stage/age; El Morterito Formation (late Miocene), Huayquerian stage/age; Playa del Zorro Aloformation (late Miocene), Tortonian–Messinian stages. San Juan and Catamarca provinces, Argentina (Fig. 1).
Results
Skull of Vetelia gandhii.—Descriptions are based on two almost complete skulls (PVSJ-154 and PVSJ-289) here assigned to Vetelia gandhii (Fig. 2A1–A3, B1–B3). These are the first skulls referred to the genus Vetelia. They are large (Table 1), with a similar cranial width to that of Priodontes, but ~33% shorter. This is due to a strong rostral shortening, which differs to that observed in some mainly insectivorous armadillos such as Dasypus, Priodontes, and some species of Prozaedyus (i.e., P. proximus [Ameghino, 1887] and P. exilis [Ameghino, 1887]) (see Smith and Redford 1990; Vizcaíno and Fariña 1994; Barasoain et al. 2020a).
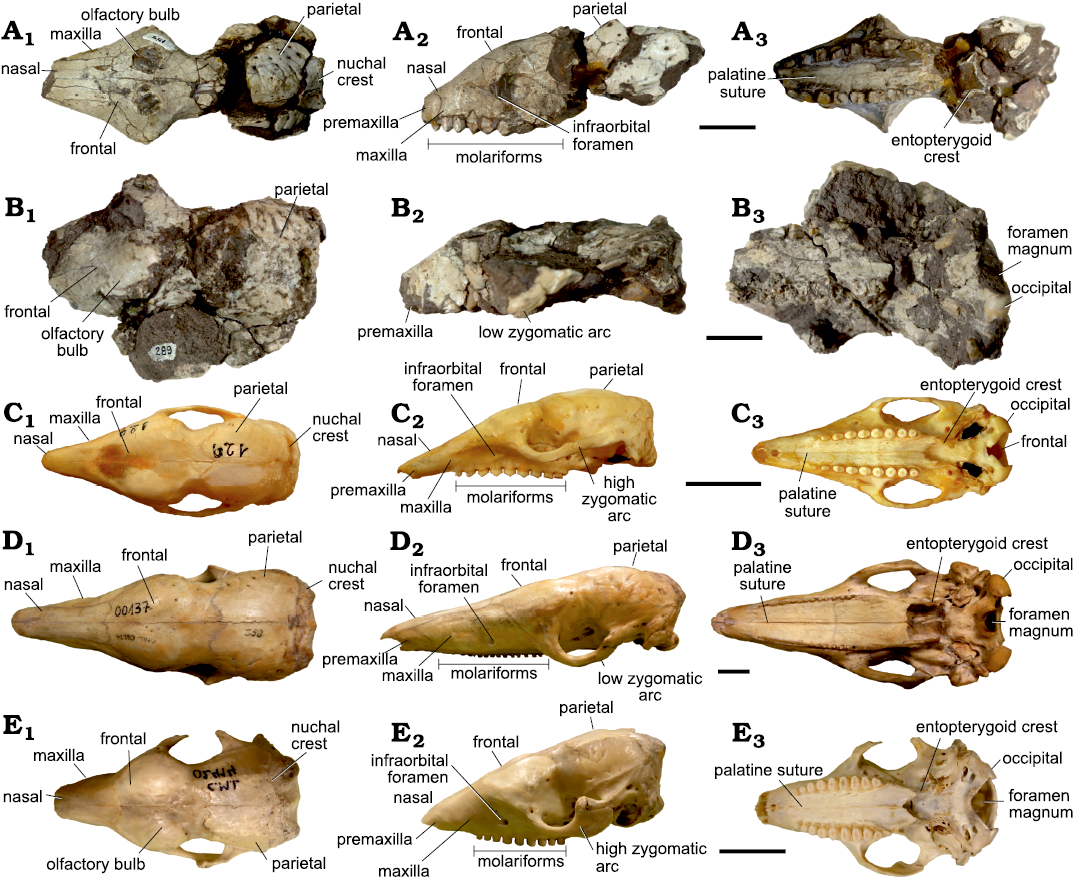
Fig. 2. Skulls of Vetelia ghandii Esteban and Nasiff, 1996, from the late Miocene, Loma de Las Tapias, Argentina (A, B) and extant tolypeutine species (C–E). A. PVSJ-154. B. PVSJ-289. C. Tolypeutes matacus (Desmarest, 1804), CD-UNNE-129. D. Priodontes maximus (Kerr, 1792), CML-00137. E. Cabassus chacoensis Wetzel, 1980, CML-02414. In dorsal (A1–E1), lateral (A2–E2), and ventral (A3–E3) views. Scale bars 20 mm.
Table 1. Cranial measurements (in mm) of Vetelia ghandii specimens.
|
Measurements |
PVSJ-154 |
PVSJ-289 |
|
maximum skull length |
110.71 |
97.85 |
|
maximum skull width at the olfactory bulbs level |
46.42 |
57.14 |
|
minimum interorbitary width |
25.85 |
32.14 |
|
cranial height at Mf1 level |
14.61 |
19.48 |
|
cranial height at Mf4 level |
21.53 |
31.16 |
|
cranial height at Mf9 level |
38.46 |
35.06 |
|
maximum length of the palatine |
62.54 |
64.34 |
|
palatine width at Mf1 level |
8.33 |
12.23 |
|
palatine width at Mf4 level |
16.66 |
20.12 |
|
palatine width at Mf9 level |
16.67 |
21.32 |
|
orbital length |
28.57 |
32.14 |
|
maximum length of the dental series |
58.33 |
54.14 |
|
molariform length |
4.8–8.33 |
5–11 |
|
molariform width |
2.77–6.25 |
3.54–6.43 |
In dorsal view (Fig. 2A1, B1), frontal bones are barely anteriorly projected between the maxillary bones and the suture between frontal and nasal bones is straight as in Tolypeutes (Fig. 2C1–C3), Priodontes (Fig. 2D1–D3), and Cabassous (Fig. 2E1–E3). Contrasting with this condition, in Euphractus, Chaetophractus, and Zaedyus, this suture has an arrow-point morphology towards the beginning of the nasal bones. At the start of the anterior zygomatic process, the frontal bones bear a pair of developed olfactory bulbs, very similar to some species of Cabassous (i.e., C. tatouay [Desmarest, 1804]). Past the anterior zygomatic process, the skull greatly narrows to its minimum width at the level of the orbit. Damaged areas do not allow observing the suture between frontal and parietal bones. The zygomatic arch traces a very broad lateral curve, giving place to a very large orbit area when compared with most armadillos, only similar to that of Macroeuphractus. The cranial case is mainly flattened and does not develop a sagittal crest. The nuchal crest has a marked anteriorly oriented relief, with a w-shaped profile, similar to that of Priodontes and some species of Cabassous (i.e., C. centralis [Miller, 1899]). On the contrary, extant euphractine armadillos and the genera Tolypeutes and Kuntinaru display a nuchal crest with a less pronounced relief and a c-shaped profile.
In lateral view (Fig. 2A2, B2), premaxillary bones are strongly reduced, resulting in a high, wide, and robust rostrum. This morphology generates a less sigmoid dorsal profile of the skull, due to a smaller difference between anterior and posterior cranial heights, than that of most armadillos. The infraorbital foramen is located at the basis of the anterior zygomatic root, a position that is shared with extant euphractine armadillos and Tolypeutes. In Priodontes and Cabassous, this foramen is located more anteriorly. The zygomatic arch is laterally compressed and the curve generated by the jugal bone reaches a low position at the midpoint, reaching the level of the tooth occlusal surface. A similar condition is present in Cabassous and Tolypeutes, while in Priodontes the zygomatic arch reaches an even lower position, its lowest point being at a much lower level than the tooth occlusal level. In extant euphractine armadillos, the zygomatic arch is higher and located at a more dorsal position, with its lowest point at the alveolar level. Towards the posterior half of the zygomatic arch, the jugal bone maintains an approximately uniform width, as in Tolypeutes and Priodontes, and does not develop the mediolaterally compressed process that is observed in Cabassous and euphractines.
In ventral view (Fig. 2A3, B3), the anterior zygomatic processes start to laterally expand at the level of the Mf4, different to extant euphractines, in which the suture between both palatine bones generates a small crest. The whole palatine surface is flat, as occurs in most tolypeutines, with exception of Tolypeutes and some species of Cabassous (i.e., C. unicinctus [Linnaeus, 1758]). The choanae and basicranial areas are damaged in both skulls. However, PVSJ-154 shows the entopterygoid crests sub-parallel and aligned with the dental series; in PVSJ-289, the occipital condyles have a strong ventral development.
Upper dental series are composed of nine massive and robust molariforms (Mf1–Mf9), which are sub-cylindrical, with an anteroposterior main axis. Size increases from Mf1 to Mf6, and then decreases to Mf9 (Table 1). It is noteworthy that the largest molariforms (Mf5–Mf7) are slightly bilobulated. Occlusal surface develops a chisel-shaped morphology, as it is common in armadillo molariforms with little wear. The Mf1 is located in the premaxillary bone. The presence of premaxillary teeth is common in euphractines, but not in tolypeutine armadillos (González Ruiz and MacPhee 2014; González Ruiz et al. 2014, 2017). Tooth rows represent more than half of the length of the skull; they are sub-parallel and extended anteriorly, almost reaching the premaxillary tip. Indeed, armadillos generally develop toothless anterior diastemas (see Thenius 1989). The last two molariforms (Mf8–Mf9) are located posteriorly to the level of the anterior zygomatic root.
Mandibles of Vetelia perforata and Vetelia gandhii.—Descriptions are based on mandibular remains of Vetelia perforata (Fig. 3A, B) (MMH-CH-87-7-109 and MLP-76-VI-12-95) and V. gandhii (Fig. 3C, D) (PVSJ-289 and MLP-64-V1-21-3). MMH-CH-87-7-109 and PVSJ-289 present smaller dimensions than MLP-76-VI-12-95 and MLP-64-V1-21-3 (Table 2), reflecting clear individual variation. Dentary is massive and robust, with a similar morphology in both species. The angle between horizontal and vertical ramus is nearly straight (~90º) in V. ghandii and obtuse (~120º) in V. perforata.
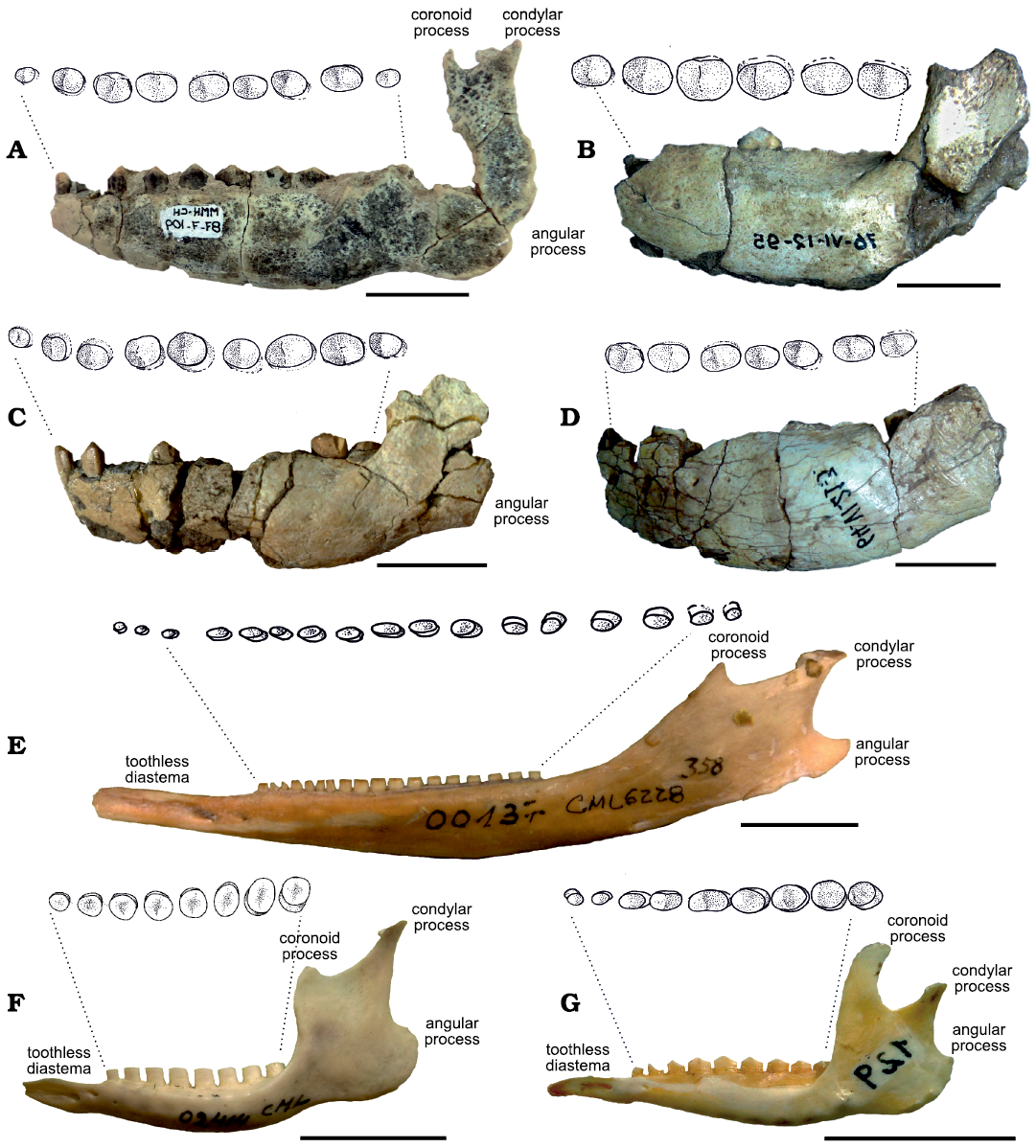
Fig. 3. Hemimandibles (labial views) of Vetelia spp. (A–D) and extant tolypeutine species (E–G), with detail of the molariform occlusal surface. A, B. Vetelia perforata Scillato-Yané, 1977, late Miocene, Arroyo Chasicó, Argentina. A. MMH-CH-87-7-109. B. MLP-76-VI-12-95. C, D. Vetelia ghandii Esteban and Nasiff, 1996. C. PVSJ-289; late Miocene, Loma de Las Tapias, Argentina. D. MLP-64-V1-21-3; late Miocene, Los Berros, Argentina. E. Priodontes maximus (Kerr, 1792), CML-00137; Recent, without provenance data. F. Cabassus chacoensis Wetzel, 1980, CML-02414; Recent, Chaco Province, Argentina. G. Tolypeutes matacus (Desmarest, 1804), CD-UNNE-129; Recent, without provenance data. Scale bars 20 mm.
Horizontal ramus is proportionally much wider than in most armadillos (see Scillato-Yané 1982). Chaetophractus is the extant armadillo with the widest mandible (Squarcia et al. 2009; Sidorkewicj and Casanave 2013), which is approximately a third thinner than in Vetelia. Towards the rostrum, the horizontal ramus becomes slightly thinner, but does not develop the sharpened and narrowed morphology observed in extant armadillos, due to the enlargement of the rostrum. The maximum width of the horizontal ramus is located at the level of the mf8 (Table 2), passing to an abrupt constriction at the level of the mf9. The largest specimens (MLP-76-VI-12-95 and MLP-64-V1-21-3; Fig. 3E, G) show a proportionally wider and thicker horizontal ramus than the smaller specimens. However, to evaluate the significance of this character, a larger sample is necessary to determine possible intraspecific variability (including ontogenetic variability).
The outer surface of the hemimandible is mostly smooth. Two to four mental foramina are located between the mf2 and mf5.
Table 2. Mandibular measurements (in mm) of Vetelia ghandii and Vetelia perforata specimens.
|
Measurements |
Vetelia ghandii |
Vetelia perforata |
||
|
PVSJ-289 |
MLP-64-V1-21-3 |
MMH-CH-87-7-109 |
MLP-76-VI-12-95 |
|
|
horizontal ramus maximum length |
78.56 |
– |
94.54 |
– |
|
vertical ramus maximum length |
– |
– |
49.45 |
– |
|
mandibular height at Mf1 level |
11.5 |
– |
6.54 |
– |
|
mandibular height at Mf4 level |
16 |
18.23 |
15.27 |
17 |
|
mandibular height at Mf9 level |
21.21 |
20.58 |
23.27 |
21 |
|
maximum length of the dental series |
57.5 |
– |
66.66 |
– |
|
molariform length |
3–6.32 |
7.05–8.82 |
3.33–8 |
8.43 |
|
molariform width |
3.5–5.57 |
4.70–5.88 |
4–6.22 |
5 |
Vertical ramus is robust and its height is equivalent to one third of the total length of the horizontal ramus. The vertical ramus is lower in Priodontes (Fig. 3E) and Cabassous (Fig. 3F) compared to Tolypeutes (Fig. 3G) and euphractine armadillos, in which it is equivalent to more than half the total length of the horizontal ramus. Both the coronoid and the condylar processes are located above the level of the occlusal dental surface, while the angular process is approximately at the same level, as it occurs in all extant tolypeutines. In euphractine armadillos, the three processes are located above the occlusal surface level. The presence of a condylar process taller than the coronoid process is shared by Vetelia, Priodontes maximus, and the four species of Cabassous. The angular process is well developed, surpassing the level of the condylear process towards the caudal area, although it is more ventrally expanded in V. perforata. In the internal side of the angular process, a large scarred depression indicates the insertion area of a well-developed masseter muscle. In the internal side of the vertical ramus, the mandibular foramen is located at the level of the alveolar surface of the toothrow.
The lower dental series of both V. perforata and V. ghandii are composed of nine sub-cylindrical, chisel-shaped molariforms. General characteristics are similar to those described for the upper series of V. ghandii. The size increases from mf1 to mf6, and then decreases from mf7 to mf9. The toothrow occupies almost the total length of the horizontal ramus and is anteriorly extended to the most proximal extreme. In fact, the mf1 is anteriorly projected, protruding from the anterior end of the hemimandible. This condition, observed in both V. perforata and V. ghandii, suggests a feature that is unique among armadillos, which generally develop anterior toothless mandibular spouts (see Thenius 1989).
Carapace of Vetelia spp.—Fixed osteoderms: Vetelia (Fig. 4A1, A2, B–D) has osteoderms comparable in size to those of Priodontes, but thicker. They are sub-hexagonal, as in Cabassous (Fig. 4B1–B3, C1–C3) and Priodontes (Fig. 4I1, I2, J–D3, K1–K2, L), in contrast to the typical sub-rectangular osteoderms of euphractine armadillos. Tolypeutes has the most regular hexagonal and isodiametric osteoderms.
All species of Vetelia share a common ornamentation pattern. It includes a wide and slightly convex central figure, surrounded by two lateral peripheral figures and a variable number of anterior minor peripheral figures. The central figure is anteriorly and laterally delimited by a main sulcus that disappears towards the posterior half of the osteoderm, generating a fusion between central and lateral figures. Minor sulci delimit lateral and anterior peripheral figures. Vetelia puncta (Fig. 4B) shows well-developed foramina both in the main and minor sulci, while V. perforata (Fig. 4C) and V. ghandii (Fig. 4D) lack these foramina.
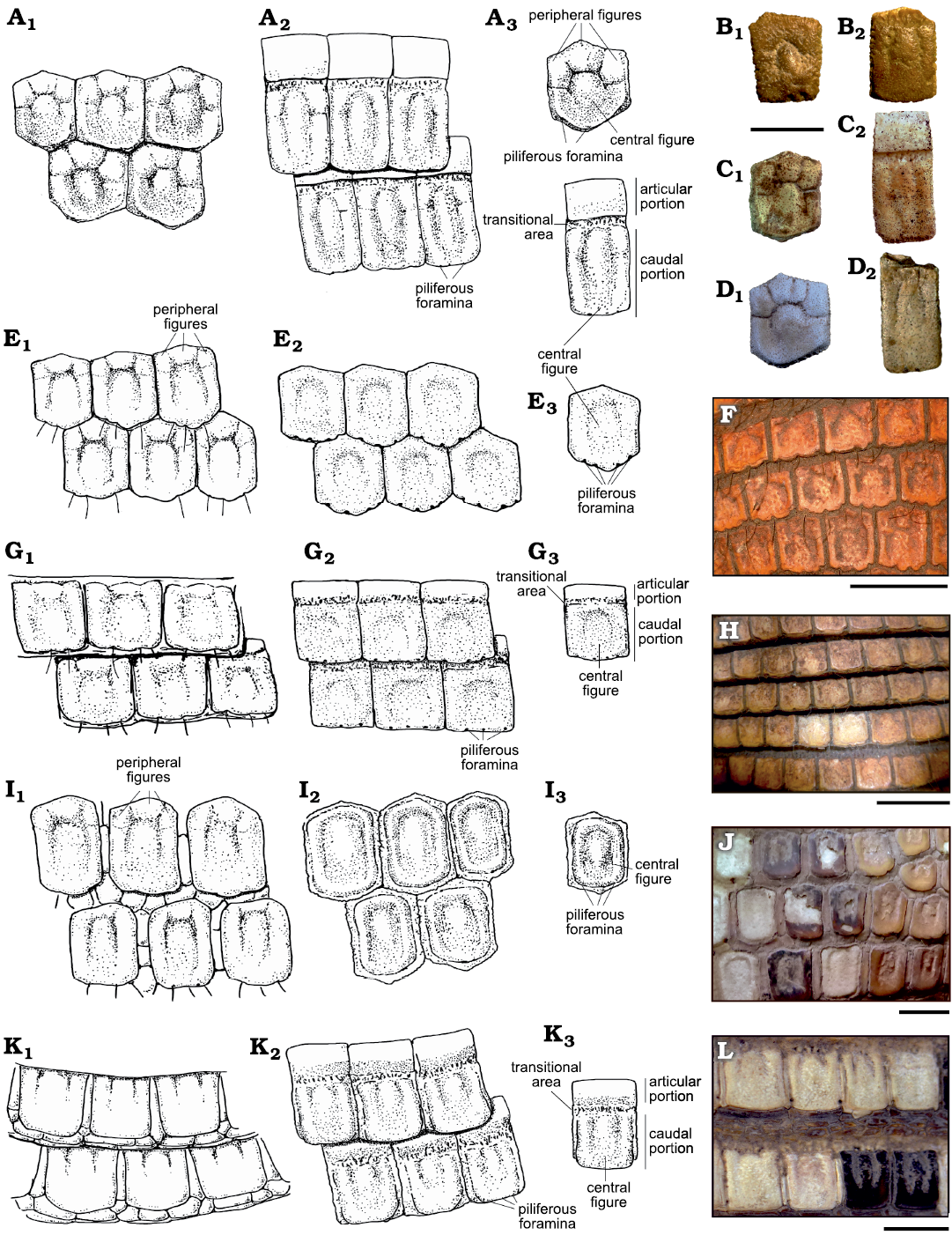
Fig. 4. Osteoderms from Argentina of Vetelia spp. (A–D) and extant Priodontini species (E–L), with detail of the horny scale and bony surface ornamentation pattern (A3, E3, D3, I3, K3). A. A drawing of Vetelia spp. osteoderms: fixed (A1) and mobile (A2). B. Vetelia puncta Ameghino, 1891, MACN-A-2139; middle–early Miocene, Karaiken: fixed (B1) and mobile (B2). C. Vetelia perforata Scillato-Yané, 1977, MD-CH-125; late Miocene, Arroyo Chasicó: fixed (C1) and mobile (C2). D. Vetelia ghandii Esteban and Nasiff, 1996, PVSJ-151; late Miocene, Loma de Las Tapias: fixed (D1) and mobile (D2) . E. A drawing of Cabassous spp. fixed osteoderms: horny scale (E1) and bony surface (E2). F. Cabassous tatouay (Desmarest, 1804), CML-03066, fixed osteoderms of the pelvic shield; Recent, Chaco Province. G. A drawing of Cabassous spp. mobile osteoderms: horny scale (G1) and bony surface (G2). H. Cabassous tatouay (Desmarest, 1804), CML-03066, mobile osteoderms; Recent, Chaco Province. I. A drawing of Priodontes sp. fixed osteoderms: horny scale (I1) and bony surface (I2). J. Priodontes maximus (Kerr, 1792), AMNH-147493, fixed osteoderms of the pelvic shield; Recent, Chaco Province. K. A drawing of Priodontes spp. mobile osteoderms: horny scale (K1) and bony surface (K2). L. Priodontes maximus (Kerr, 1792), AMNH-147493, mobile osteoderms; Recent, Chaco Province. Scale bars 20 mm. A, E, G, I, and K, not to scale.
Anterior peripheral figures vary from one to four, though the most common ornamentation pattern is the presence of three figures. Osteoderms with only one figure, as in the holotype of V. puncta (MACN-A-2139), seem to belong to the posterodorsal region of the pelvic shield. In many armadillos, osteoderms of this region are highly modified due to the presence of odoriferous glands (Scillato-Yané 1982).
A similar ornamentation pattern is observable in Priodontes and Cabassous. However, in many cases, this ornamentation is only preserved in the horny scales that cover the osteoderms during the armadillo’s lifetime, and becomes diffuse or even absent on the bony surface (Fig. 4H, J, L). This is a peculiar phenomenon also recognized in other groups of armadillos, such as the extant representatives of Chlamyphorinae (Barasoain et al. 2020b, c).
The posterior margin of the osteoderm has a single row of piliferous foramina. In V. perforata these foramina are small and numerous (6–14), while in V. puncta and V. ghandii they are larger and less abundant (2–4). Vetelia puncta also develops small foramina in the anterior and lateral margins.
The most complete specimen of V. ghandii (PVSJ-289) shows a very reduced scapular shield, composed of only two rows of fixed osteoderms, located between the nuchal osteoderms and the first row of mobile osteoderms (Fig. 5A). Scapular fixed osteoderms (Fig. 5A) seem to be more elongated than those of the pelvic shield (Fig. 5B–D). Cabassous, Priodontes, and Tolypeutes do not show significant differences between scapular and pelvic fixed osteoderms. In the specimen PVSJ-289, the shape of the fixed osteoderms of the pelvic shield slightly changes towards the lateral margins of the dorsal carapace, becoming sub-square, as occur in Priodontes. Vetelia ghandii has serrated borders in the posterior portion of the pelvic shield, while V. perforata has rounded borders (see Scillato-Yané 1982).
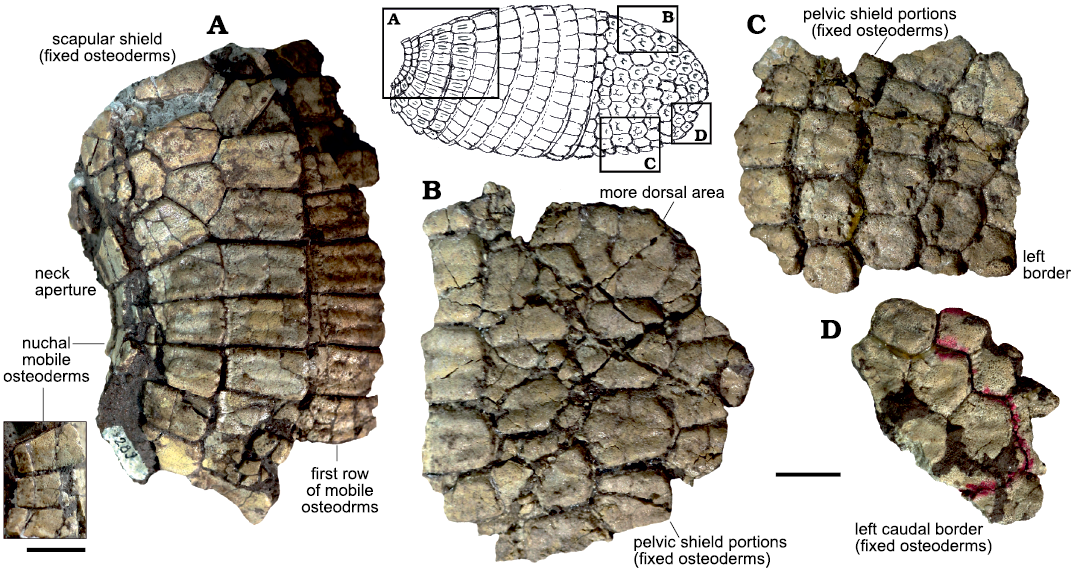
Fig. 5. Features of the different portions of the dorsal carapace of the Vetelia ghandii Esteban and Nasiff, 1996, PVSJ-289 (most complete specimen) from the late Miocene of Loma de Las Tapias, San Juan Province, Argentina. A. Anterior fragment, with detail on the nuchal band. B. Dorsal fragment of the pelvic shield. C. Lateral fragment of the pelvic shield. D. Caudal fragment. Scale bars 20 mm.
Mobile osteoderms: These osteoderms are sub-rectangular, similar in width to fixed osteoderms, but approximately twice as long (Table 3, Fig. 4A2). They are divided into an articular and a caudal portion, separated from each other by a very rugose transitional area. The articular portion has a smooth unornamented surface whose length is equivalent to half the caudal portion. The caudal portion shows a similar ornamentation pattern in all the species of Vetelia. It includes a wide and slightly convex central figure, which begins in the limit with the transitional area and reaches the posterior margin, where it becomes slightly narrower. The central figure is delimited by a shallow and wide main sulcus. Along this sulcus, V. puncta has several piliferous foramina (Fig. 4B), while V. perforata (Fig. 4C) and V. ghandii (Fig. 4D) lack foramina. The central figure is surrounded by two undivided and elongated peripheral lateral figures. The latter widen at the most anterior part of the osteoderms, resulting in minor peripheral figures that are not completely delimitated by sulci. Some osteoderms show well-developed anterior peripheral figures, while these figures are very diffuse in others.
Similar ornamentation pattern is observable in Priodontes and Cabassous species. However, as it occurs in fixed osteoderms, the ornamentation in both extant taxa is generally preserved only in the horny scales that cover the osteoderms, while it is diffuse or absent in the bony surface (Fig. 4H, J, L).
The posterior margin of the osteoderm has a single row of piliferous foramina. In V. perforata, these foramina are smaller and more numerous (5–14) than in V. puncta (2–5) and V. ghandii (2–5). V. puncta also has small foramina in the lateral margins.
Table 3. Osteoderm measurements (in mm) of different Vetelia species.
|
Taxa |
Fixed osteoderms |
Mobile osteoderms |
||
|
length |
width |
length |
width |
|
|
V. puncta |
15.32–20.21 |
13.75–16.87 |
19.23–28.12 |
9.3–17 |
|
V. perforata |
19.46–21.75 |
13.91–20.36 |
20.08–32.75 |
10.03–16.04 |
|
V. ghandii |
15.50–22.14 |
13.32–20.55 |
20.04–27.50 |
11.04–15.34 |
Phylogeny and affinities of Vetelia with Tolypeutinae
The general topology of the obtained most parsimonious tree (Fig. 6) is consistent with previous results of morphological phylogenetic analyses including armadillos (e.g., Gaudin and Wible 2006; Billet et al. 2011; Herrera et al. 2017). Some differences are related to the taxa considered in each analysis.
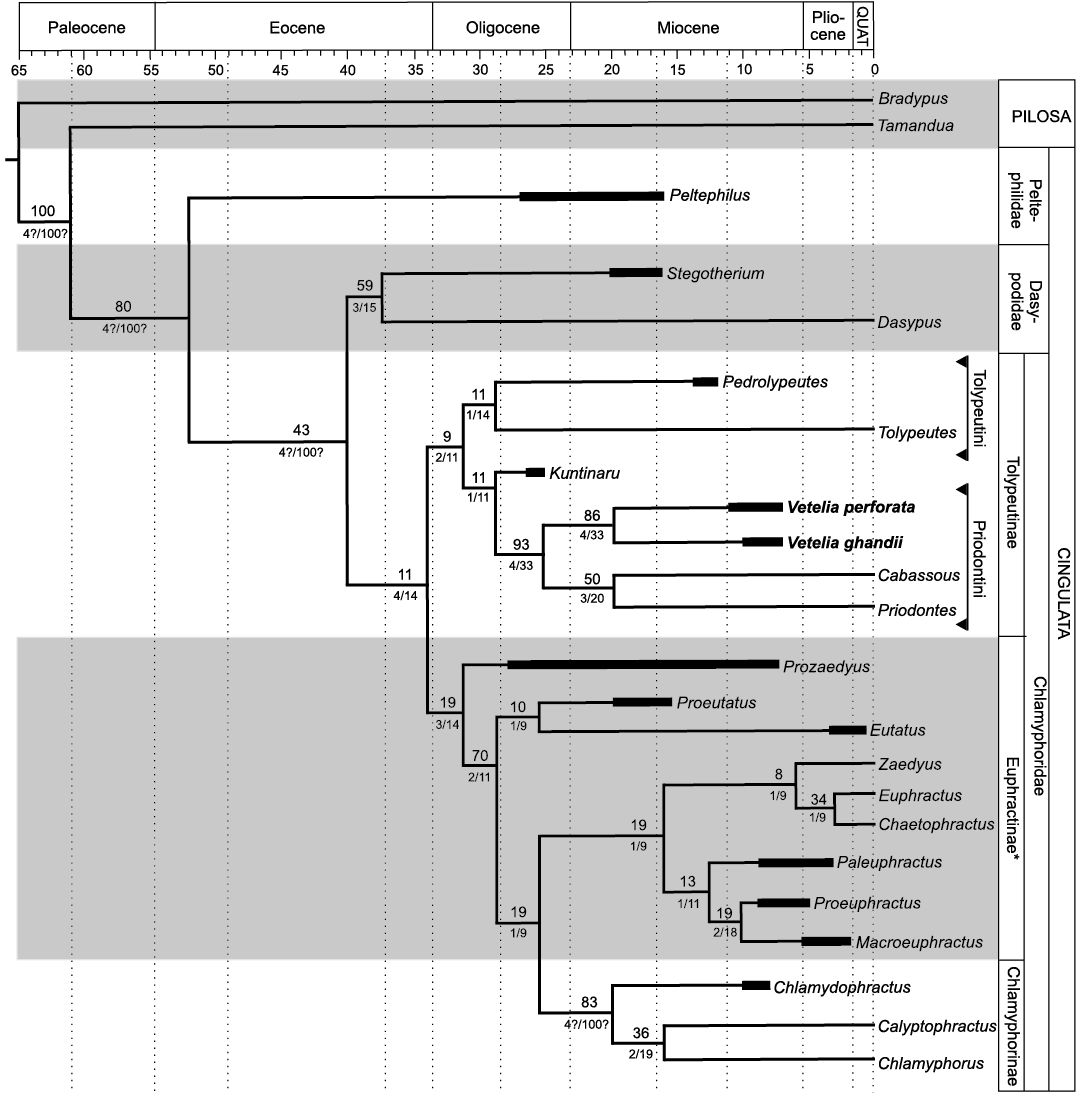
Fig. 6. Most parsimonious tree resulting from the phylogenetic analysis and support values. Numbers above each branch indicate Bootstrap support. Numbers below each branch indicate absolute and relative Bremer support, respectively. Black rectangles are showing the temporal distribution of extinct taxa. *Euphractinae is here recovered as a paraphyletic group.
In our analysis, the armadillos compose a monophyletic group, with the genus Peltephilus as the sister-group of the remaining taxa. The latter are gathered in a large clade that is, in turn, divided in two well-differentiated clades, one formed by Dasypodidae (Dasypus + Stegotherium) and another by Chlamyphoridae (all the remaining taxa). This main division agrees with the latest phylogenetic analyses based on molecular data of extant armadillos (e.g., Delsuc et al. 2002, 2012; Möller-Krull et al. 2007).
The family Chlamyphoridae is divided into two main clades. One of them is composed of representatives of the subfamilies Euphractinae and Chlamyphorinae, while the other gathers the genera of Tolypeutinae.
The extinct euphractine Prozaedyus is recovered as the sister-taxon of the remaining Euphractinae and Chlamyphorinae, which is congruent with previous interpretations (Engelmann 1985; Gaudin and Wible 2006; Billet et al. 2011; Barasoain et al. 2020a–c). The representatives of the tribe Eutatini (i.e., Proeutatus and Eutatus) appear as the sister-group of a clade that clusters the Chlamyphorinae and the representatives of the tribe Euphractini. Though molecular data suggest a close relationship between Chlamyphorinae and Tolypeutinae, results from morphological analyses seem to be disturbed due to the particular and highly derived morphology of the fairy armadillos (see also Barasoain et al. 2020a, b).
Tolypeutinae constitutes a monophyletic group, supported by nine synapomorphies: 16[1], 37[3], 45[0], 48[1], 58[1], 87[1], 112[1], 132[1], and 142[0] (see SOM 2), and is divided in two main clades. One of them clusters Tolypeutes + Pedrolypeutes, and is supported by three synapomorphies: 128[1], 129[0], and 148[1] (see SOM 2, 4); this clade is here recognized as tribe Tolypeutini. The other clade includes Kuntinaru as sister group of the clade composed by the remaining taxa. The latter is here recognized as tribe Priodontini. It clusters the species of Vetelia, Cabassous, and Priodontes, and is supported by 15 synapomorphies: 14[1], 15[1], 35[1], 43[1], 44[0], 51[1], 69[1], 71[2], 96[2], 129[1], 133[1], 136[1], 143[0], 144[1], and 145[0] (see SOM 2 and 4). Within Priodontini, the species of Vetelia (V. ghandii and V. perforata) form the sister group of the clade composed of Cabassous and Priodontes.
Discussion
The subfamily Tolypeutinae is represented by the extant genera Tolypeutes, Priodontes, and Cabassous, plus the extinct Kuntinaru (Billet et al. 2011), Pedrolypeutes (Carlini et al. 1997), and Vetelia (this work). However, the identification and characterization of this clade is relatively recent and its status was not confirmed until molecular analyses were carried out on living armadillos (Delsuc et al. 2002, 2003). Historically, and according to the old systematic schemes, extant tolypeutines were grouped into two different tribes: Tolypeutini, including the two species of Tolypeutes (T. matacus and T. tricinctus), and Priodontini, with Priodontes maximus and the four species of Cabassous (C. tatouay, C. unicinctus, C. chacoensis, and C. centralis).
The most plesiomorphic clade within Tolypeutinae, here assigned to Tolypeutini, is composed of Pedrolypeutes and Tolypeutes. Considering that both genera share two synapomorphic characters (e.g., hexagonal isodiametric osteoderms and presence of small tubercles on the dorsal surface of the osteoderms), the middle Miocene taxon Pedrolypeutes can be proposed as the possible ancestor of Tolypeutes (see Carlini et al. 1997). According to our phylogenetic analysis, Tolypeutini retains a high number of plesiomorphic characters, being morphologically closer to the Euphractinae.
Molecular phylogenetic analysis performed by Delsuc et al. (2002) placed the Tolypeutini Tolypeutes and the Priodontini Cabassous in a sister-group relationship. However, several other phylogenetic analyses have supported a closer relationship between Cabassous and Priodontes, based on their similar external features (Engelmann 1985; Wetzel 1985; Gaudin and Wibble 2006) and spermatozoa morphology (Cetica et al. 1998). According to Delsuc et al. (2002: 12), information obtained from molecular data could be related to a “rapid cladogenesis event that left only short time intervals for molecular synapomorphies to accumulate in these two groups”, which in some cases can lead to an unclear result when using this technique.
Based on a detailed morphological analysis, Gaudin and Wible (2006: 172) considered that “Tolypeutes is more closely allied with euphractan armadillos than with any other dasypodid armadillos”. Many other authors (e.g., Simpson 1945; Hoffstetter 1958; Paula Couto 1979; Scillato-Yané 1982; Wetzel 1985) have previously included Tolypeutes in a distinct subfamily or tribe. Our study supports these previous proposals of a close relationship beteween the Tolypeutini and the Euphractinae. The obtained results reflect several plesiomorphic characters shared between the Tolypeutini (Tolypeutes + Pedrolypeutes) and Euphractinae armadillos: (i) development of a crest along the suture bone on the posterior half palatine suture; (ii) higher central position of the zygomatic arc; (iii) taller mandibular vertical ramus; and (iv) coronoid process taller than condylar process.
The other well-differentiated clade within Tolypeutinae, assigned here to Priodontini, is composed of Vetelia, Cabassous, and Priodontes. This clade includes several autopomorphic characters with respect to the Tolypeutini, and is well supported by diverse cranio-mandibular and carapace (dorsal carapace and osteoderms) synapomorphic characters: (i) absence of a crest along the bone suture on the posterior half palatine; (ii) lower central position of the zygomatic arc; (iii) w-shaped nuchal crest with strong relief; (iv) lower vertical mandibular ramus; (v) condylar process taller than coronoid process; (vi) angular process located at the molariforms occlusal surface level; (vii) common ornamentation pattern of both fixed and mobile osteoderms; and (viii) development of rounded borders in all or almost all the dorsal carapace.
In the clade Priodontini (Fig. 7), Vetelia probably branched before the clade composed of Cabassous and Priodontes. This result is supported by the retention of some plesiomorphic characters in Vetelia, shared with the Tolypeutini and Kuntinaru: (i) proportionally reduced nasal bone (˂25% of the total skull length); (ii) infraorbital foramen placed in a most posterior position; and (iii) less posterior extent of the palatines after the tooth row ending.
Fig. 7. Reconstruction (by Pedro Cuaranta, CECOAL (UNNE-CONICET), Corrientes, Argentina) of Vetelia ghandii Esteban and Nasiff, 1996, based on the most complete known specimens of the genus (PVSJ-154 and PVSJ-289; late Miocene, Loma de Las Tapias, San Juan Province, Argentina). Not to scale.
Previous systematic studies (e.g., Scillato-Yané 1982; Urrutia et al. 2008; González Ruiz 2010) considered Vetelia as an Euphractinae, based on a few fragmentary specimens, mainly represented by isolated osteoderms. The detailed anatomical descriptions provided herein, based on new cranial and mandibular remains and more complete portions of dorsal carapaces, suggest a very close relationship between Vetelia and previously described Tolypeutinae, which is supported by the phylogenetic analysis. This relationship is particularly close with the tribe Priodontini, as previously suggested by Hoffstetter (1958). In fact, all the analyzed characters related to the osteoderms and most of the cranio-mandibular characters reflect the close affinity of Vetelia with Cabassous and Priodontes, which form together the clade Priodontini.
On the other hand, most of the characters shared by Vetelia and the Euphractinae armadillos are related to the dentition: (i) development of premaxillary teeth; (ii) toothrow length; and (iii) position of last lower tooth in relation to the vertical ramus of the mandible. In this sense, the most relevant character is the presence of premaxillary teeth, which is characteristic of the euphractines. However, it is important to note that not all euphractines have premaxillary teeth, as it occurs in two species of Prozaedyus, P. proximus and P. exilis, both interpreted as insectivores (Vizcaíno and Fariña 1994; Barasoain et al. 2020a) and in taxa with very reduced toothrows, such as Stegotherium, with possible myrmecophageal habits (Vizcaíno 1994; Vizcaíno et al. 2006). The presence or absence of premaxillary teeth could be related to feeding habit adaptations rather than to phylogenetic constraints. Among Tolypeutinae, the presence of premaxillary teeth in T. matacus is considered as a rare dental anomaly (González Ruiz and MacPhee 2014).
In the cranio-dental phylogenetic analysis carried out by Billet et al. (2011), Kuntinaru is the sister taxon of the clade composed of all the remaining tolypeutines. In contrast, in our phylogenetic analysis, Kuntinaru is the sister taxon of Priodontini (Vetelia + Cabassous + Priodontes). This result is due to the incorporation of both dorsal carapace characters (unknown for Kuntinaru) and new fossil taxa (Vetelia and Pedrolypeutes). Some cranial features of Kuntinaru (e.g., posterior position of the infraorbital foramen, posterior extent of the palatines, and c-shaped nuchal crest) suggest higher affinities with the Tolypeutini (Pedrolypeutes + Tolypeutes), but the lack of osteoderm and dorsal carapace materials in this taxon prevent more detailed interpretations.
Finally, the inclusion of the Miocene genus Vetelia within Tolypeutinae increases the diversity of this subfamily in the Neogene of southern South America, filling an important temporal gap in its fossil record. Moreover, it also extends the paleobiogeographical distribution of tolypeutines to the Patagonian Argentina and Chile during part of the Miocene. This novel approach to the Tolypeutinae phylogenetic scheme reflects the need for further research focused on this group of armadillos, including a more comprehensive systematic review of known fossil taxa and an improvement of the lists of diagnostic morphological characters for tolypeutine genera and species.
Conclusions
We report the most complete remains belonging to the Miocene armadillo genus Vetelia and provide, for the first time, a detailed anatomical description of the cranial skeletal elements. This study allows a better morphological characterization of the genus, revealing some diagnostic characters among armadillos, such as a shortened rostrum, a very robust dentary with a short vertical ramus, and the absence of an anterior toothless diastema in both upper and lower dental series.
These new anatomical data lead to the proposal of an amended diagnosis for the three known species of Vetelia (V. ghandii, V. perforata, and V. puncta), and the inclusion of this genus, for the first time, into a phylogenetic analysis. A close phylogenetic relationship is determined between Vetelia and the extant Tolypeutinae, more specifically with the representatives of the tribe Priodontini, Cabassous and Priodontes. Several synapomorphic characters support the inclusion of Vetelia within the Tolypeutinae Priodontini rather than within the subfamily Euphractinae, such as the morphology of the condylar and angular processes of the mandible and the development of a common ornamentation pattern of both fixed and mobile osteoderms of the dorsal carapace.
This new proposal fills a temporal gap into the evolutionary history of tolypeutine armadillos, which are very scarce in the fossil record. Both molecular and morphological data show two well characterized natural groups clustered within Tolypeutinae. In this respect, this work also leads to a redefinition and reconceptualization of the old Priodontini and Tolypeutini tribes, which is useful to address the systematics of Tolypeutinae. Finally, we provide new information on the diagnostic morphological characters of the Priodontini and Tolypeutini tribes (see SOM 4).
Acknowledgements
We thank the curators Ricardo Martínez (Museo de Ciencias Naturales, San Juan, Argentina), Ricardo Caputo (MD), Natalia Sánchez (MMH), Marcelo Reguero (MLP), and Laura Chornogubsky (MACN) for the access to the studied specimens. Reconstruction of Vetelia ghandii and drawings were made by Pedro Cuaranta (Centro de Ecología Aplicada del Litoral, Corrientes, Argentina). Romina Mauriño (Universidad Nacional del Nordeste, Corrientes, Argentina) contributed with photography editing. We also thank the editor Olivier Lambert (Institut Royal des Sciences Naturelles de Belgique, Belgium) and both reviewers François Pujos (Instituto Argentino de Nivología, Glaciología y Ciencias Ambientales, Mendoza, Argentina) and Martín Zamorano (Universidad Nacional de La Plata-CONICET, La Plata, Argentina), whose comments greatly improved the manuscript. Esperanza Cerdeño (Instituto Argentino de Nivología, Glaciología y Ciencias Ambientales, Mendoza, Argentina) made a critical review of the manuscript and helped with the English. This research was partially funded by grant PICT 2017 0765, PI QOO2/17, Secretaría General de Ciencia y Tecnología, Universidad Nacional del Sur (PGI 24/H154), project 06-G, Facultad de Ciencias Exactas y Naturales of the Universidad Nacional de La Pampa and CICITCA-UNSJ, E 1151, Universidad Nacional de San Juan, Argentina.
References
Ameghino, F. 1886. Contribución al conocimiento de los mamíferos fósiles de los terrenos terciarios antiguos de Paraná. Boletín de la Academia Nacional de Ciencias de Córdoba 9: 3–226.
Ameghino, F. 1887. Enumeración sistemática de las especies de mamíferos fósiles coleccionados por Carlos Ameghino en los terrenos eocenos de la Patagonia austral y depositados en el Museo de La Plata. Boletín del Museo de la Plata 1: 1–26.
Ameghino, F. 1889. Contribución al conocimiento de los mamiferos fósiles de la Republica Argentina. Actas de la Academia Nacional de Ciencias de Cordoba 6: 1–1027. Crossref
Ameghino, F. 1891. Mamíferos y aves fósiles argentinas. Revista Argentina de Historia Natural 1: 240–288.
Barasoain, D., Contreras, V.H., Tomassini, R.L., and Zurita, A.E. 2020a. A new pygmy armadillo (Cingulata, Euphractinae) from the late Miocene of Andean Argentina reveals an unexpected evolutionary history of the singular Prozaedyus lineage. Journal of South American Earth Sciences 100: 102589. Crossref
Barasoain, D., Tomassini, R.L., Zurita, A.E., Montalvo, C.I., and Superina, M. 2020b. A new fairy armadillo (Cingulata, Chlamyphorinae) from the upper Miocene of Argentina: first fossil record of the most enigmatic Xenarthra. Journal of Vertebrate Paleontology 39 (5): e1716778. Crossref
Barasoain, D., Tomassini, R.L., Zurita, A.E., Montalvo, C.I., and Superina, M. 2020c. Chlamydophractus, new name for Chlamyphractus Barasoain et al., 2020 (Xenarthra, Chlamyphorinae), non Chlamyphractus Castellanos, 1940 (Xenarthra, Glyptodontidae). Journal of Vertebrate Paleontology 40 (2): e1774890. Crossref
Bergqvist, L.P., Abrantes, E.A.L., and Avilla L.S. 2004. The Xenarthra (Mammalia) of São José de Itaboraí Basin (upper Paleocene, Itaboraian), Rio de Janeiro, Brazil. Geodiversitas 26: 326−337.
Billet, G., Hautier, L., De Muizon, C., and Valentin, X. 2011. Oldest cingulate skulls provide congruence between morphological and molecular scenarios of armadillo evolution. Proceedings of the Royal Society B: Biological Sciences 278: 2791–2797. Crossref
Bremer, K.R. 1994. Branch support and tree stability. Cladistics 10: 295−304. Crossref
Carlini, A.A., Vizcaíno, S.F., and Scillato-Yané, G.J. 1997. Armored xenarthrans: a unique taxonomic and ecologic assemblage. In: R.D. Madden, R.L. Cifelli, and J.J Flynn (eds.), Vertebrate Paleontology in the Neotropics. The Miocene Fauna of La Venta, Colombia, 13–226. Smithsonian Institution Press, Washington, DC.
Cetica, P.D., Solari, A.J., Merani, M.S., De Rosas, J.C., and Burgos, M.H. 1998. Evolutionary sperm morphology and morphometry in armadillos. Journal of Submicroscopic Cytology and Pathology 30: 309–314.
Cione, A.L., Gasparini, G.M., Soibelzon, E., Soibelzon, L.H., and Tonni, E.P. 2015 The Great American Biotic Interchange. A South American Perspective. 106 pp. Springer, Dordrecht. Crossref
Cope, E.D. 1889. The Edentata of North America. The American Naturalist 23: 657–664. Crossref
Cuvier, F. 1825. Des dents de mammifères, considérées comme caractères zoologiques. 258 pp. F.G. Levrault, Le Norman, Paris.
Delsuc, F., Scally, M., Madsen, O., Stanhope, M.J., De Jong, W.W., Catzeflis, F.M., Springer, M.S., and Douzery, E.J.P. 2002. Molecular phylogeny of living xenarthrans and the impact of character and taxon sampling on the placental tree rooting. Molecular Biology and Evolution 19: 1656–1671 . Crossref
Delsuc, F., Stanhope, M.J., and Douzery, E.J. 2003. Molecular systematics of armadillos (Xenarthra, Dasypodidae): contribution of maximum likelihood and Bayesian analyses of mitochondrial and nuclear genes. Molecular Phylogenetics and Evolution 28: 261–275. Crossref
Delsuc, F., Vizcaíno, S.F., and Douzery, E.J. 2004. Influence of Tertiary paleoenvironmental changes on the diversification of South American mammals: a relaxed molecular clock study within xenarthrans. BMC Evolutionary Biology 4: 11. Crossref
Delsuc, F., Gibb, G.C., Kuch, M., Billet, G., Hautier, L., Southon, J., Rouillard, J.M., Fernicola, J.C., Vizcaíno, S.F., MacPhee, R.D.E., and Poinar, H.N. 2016. The phylogenetic affinities of the extinct glyptodonts. Current Biology 26: 155−156. Crossref
Delsuc, F., Superina, M., Tilak, M.K., Douzery, E.J., and Hassanin, A. 2012. Molecular phylogenetics unveils the ancient evolutionary origins of the enigmatic fairy armadillos. Molecular Phylogenetics and Evolution 62: 673–680. Crossref
Desmarest, A.G. 1804. Tableau methodique des mammifères. In: J.-F.-P. Deterville (ed.), Nouveau dictionnaire d’histoire naturelle, appliquée aux arts, à l’agriculture, à l’économie rurale et domestique, à la médecine, etc., par une société de naturalistes et d’agriculteurs: avec des figures tirées des trois règnes de la nature. Tome 24, 5–38 pp. Chez Deterville, Paris.
Engelmann, G. 1985. The phylogeny of the Xenarthra. In: G.G. Montgomery (ed.), The Ecology and Evolution of Armadillos, Sloths, and Vermilinguas, 51–64. Smithsonian Institution Press, Washington, DC.
Esteban, G.I., and Nasif, N.L. 1996. Nuevos Dasypodidae (Mammalia, Xenarthra) del Mioceno tardío del Valle del Cajón, Catamarca, Argentina. Ameghiniana 33: 327–334.
Farris, J.S., Albert, V.A., Källersjö, M., Lipscomb, D., and Kluge, A.G. 1996. Parsimony jackknifing outperforms neighbor-joining. Cladistics 12: 99–124. Crossref
Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. Crossref
Fernicola, J.C., Rinderknecht, A., Jones, W., Vizcaíno, S.F., and Porpino, K. 2017. A new species of Neoglyptatelus (Mammalia, Xenarthra, Cingulata) from the late Miocene of Uruguay provides new insights on the evolution of the dorsal armor in cingulates. Ameghiniana 55: 233–253. Crossref
Fitzinger, L.J. 1871. Die naturliche Familie der Gurtelthiere (Dasypodes). Akademie der Wissenschaften in Wien, Sitzungsberichte, Mathematisch-naturwissenschaftliche Klasse, Abteilung 64: 209–276.
Gaudin, T.J. 2004. Phylogenetic relationships among sloths (Mammalia, Xenarthra, Tardigrada): the craniodental evidence. Zoological Journal of the Linnean Society 140: 255–305. Crossref
Gaudin, T.J. and Croft, D.A. 2015. Paleogene Xenarthra and the evolution of South American mammals. Journal of Mammalogy 96: 622–634. Crossref
Gaudin, T.J. and Wible, J.R. 2006. The phylogeny of living and extinct armadillos (Mammalia, Xenarthra, Cingulata): a craniodental analysis. In: M.T. Carrano, T.J. Gaudin, R.W. Blob, and J.R. Wible (eds.), Amniote Paleobiology: Perspectives on the Evolution of Mammals, Birds and Reptiles, 153–198. University of Chicago Press, Chicago.
Gelfo, J.N., Goin, F.J., Woodburne, M.O., and Muizon, C.D. 2009. Biochronological relationships of the earliest South American Paleogene mammalian faunas. Palaeontology 52: 251–269. Crossref
Gervais, P. 1867. Sur une nouvelle collection d’ossementes fossiles de Mammifères recueillé par M. Fr. Seguin dans la Confédération Argentine. Comptes rendus des séances de l’Académie des Sciences 65: 279–282.
Gibb, G.C., Condamine, F.L., Kuch, M., Enk, J., Moraes-Barros, N., Superina, M., Poinar, H.N., and Delsuc, F. 2016. Shotgun mitogenomics provides a reference phylogenetic framework and timescale for living xenarthrans. Molecular Biology and Evolution 33: 621−42. Crossref
Goloboff, P.A. and Farris J.S. 2001. Methods for quick consensus estimation. Cladistics 17: 26–34. Crossref
Goloboff, P.A., Farris, J.S., and Nixon, K.C. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24: 774−786. Crossref
González Ruiz, L.R. 2010. Los Cingulata (Mammalia, Xenarthra) del Mioceno temprano y medio de Patagonia (edades Santacrucense y “Friasense”). Revisión sistemática y consideraciones bioestratigráficas 471 pp. Ph.D. Thesis, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, La Plata.
González Ruiz, L.R. and MacPhee, R.D. 2014. Dental anomalies in Tolypeutes matacus (Desmarest, 1804) (Mammalia, Xenarthra, Cingulata). XXVII Jornadas Argentinas de Mastozoología, Libro de Resúmenes, 94, Esquel, Chubut.
González Ruiz, L.R. and Scillato-Yané, G.J. 2007. El género Vetelia Ameghino (Xenarthra, Dasypodidae). Distribución cronológica y geográfica durante el Mioceno de Patagonia, Argentina. XXIII Jornadas Argentinas de Paleontología de Vertebrados. 21 al 23 de Mayo, Trelew (Chubut), Argentina. Ameghiniana 44 (4): 21R.
González Ruiz, L.R., Aya-Cuero, C.A., and Martin, G.M. 2017. La dentición de Priodontes maximus (Kerr, 1792): fórmula, morfología y anomalías. XXX Jornadas Argentinas de Mastozoología, Libro de Resúmenes, 135. Bahía Blanca, Buenos Aires.
González Ruiz, L.R., Góis, F., Scillato-Yané. G.J., Oliva, C., Contreras, V., and Esteban, G. 2014. New records of Vetelia Ameghino (Mammalia, Xenarthra, Dasypodidae) in the Miocene of Argentina: anatomical and paleobiogeographic implications. 74th International Palaeontological Congress September 28–October 3, Abstract Volume, 722. CCT-CONICET, Mendoza.
González Ruiz, L.R., Ladevèze, S., and MacPhee, R.D. 2014. Dental anomalies in Euphractus sexcinctus Wagler (Mammalia: Xenarthra: Dasypodidae). In: 94 Annual Meeting of the American Society of Mammalogists, Abstract Book, 59–60. Oklahoma City, Oklahoma.
Gray, J.E. 1865. Revision on the genera and species of entomophagus Edentata, founded on examination of the specimens in the British Museum. Proceedings of the Zoological Society of London 33: 359–386. Crossref
Gray, J.E. 1873. Hand-list on the Edentata, Pachydermata and Ruminantia in the British Museum. 174 pp. British Museum (Natural History), London.
Harlan, R. 1825. Description of a new genus of mammiferous quadrupeds, of the order Edentata. Annals of the Lyceum Museum of Natural History 1: 235–245. Crossref
Herrera, C.M., Powell, J.E., Esteban, G.I., and del Papa, C. 2017. A new Eocene dasypodid with caniniforms (Mammalia, Xenarthra, Cingulata) from northwest Argentina. Journal of Mammalian Evolution 24: 275–288. Crossref
Hoffstetter, R. 1958. Xenarthra. In: P. Piveteau (ed.), Traité de Paléontologie, Vol.2 no.6, Mammifères Evolution, 535–636. Masson et Cie, Paris.
Illiger, C.D. 1811. Prodromus systematis mammalium et avium additis terminis zoographicis uttriusque classis. 301 pp. Salfeld, Berlin. Crossref
Kerr, R. 1792. The Animal Kingdom or Zoological System, of the Celebrated Sir Charles Linnaeus. Class I. Mammalia: Contain a Complete Systematic Description, Arrangement, and Nomenclature, of all the Known Species and Varieties of Mammalia, or Animals Which Give Suck To Their Young; Being a Translation of That Part of the Systema Naturae, As Lately Published, With Great Improvements, By Professor Gmelin of Goettingen, Together With Numerous Additions From More Recent Zoological Writers, and Illustrated With Copperplates. 400 pp. A. Strahan, T. Cadell, and W. Creech, Edinburgh.
Kraglievich, L. 1934. La antigüedad pliocena de las faunas de Monte Hermoso y Chapadmalal, deducidas de su comparación con las que le precedieron y sucedieron. 306 pp. Imprenta “El Siglo Ilustrado”, Montevideo.
Linnaeus, C. 1758. Systema naturæ: per regna tria naturaæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tome 1. Editio Decima Reformata. 824 pp. Impensis Direct Laurentii Salvii, Holmiæ. Crossref
MacFadden, B.J., Wang, Y., Cerling, T.E., and Anaya, F. 1994. South American fossil mammals and carbon isotopes: a 25 million-year sequence from the Bolivian Andes. Palaeogeography, Palaeoclimatology, Palaeoecology 107: 257–268. Crossref
Maddison, W.P. and Maddison, D.R. 2008. Mesquite: a modular system for evolutionary analysis. Version 3.04. Available at http://mesquiteproject.org
McKenna, M.C. and Bell, S.K. 1997. Classification of Mammals Above the Species Level. 631 pp. Columbia University Press, New York.
McMurtrie, H. 1831. The Animal Kingdom Arranged in Conformity With its Organization, by the Baron Cuvier. The Crustacea, Arachnides and Insecta, by P.A. Latreille. Translated from the French with Notes and Additions by H. M’Murtrie. 448 pp. G. & C. & H. Carvill, New York.
Miller, G.S. 1899. Notes on the naked-tailed armadillos. Proceedings of the Biological Society of Washington 13: l–8.
Mitchell, K.J., Scanferla, A., Soibelzon, E., Bonini, R., Ochoa, J., and Cooper, A. 2016. Ancient DNA from the extinct South American giant glyptodont Doedicurus sp. (Xenarthra: Glyptodontidae) reveals that glyptodonts evolved from Eocene armadillos. Molecular Ecology 25: 3499–3508. Crossref
Möller-Krull, M., Delsuc, F., Churakov, G., Marker, C., Superina, M., Brosius, J., Douzery, E.J.P., and Schmitz, J. 2007. Retroposed elements and their flanking regions resolve the evolutionary history of xenarthran mammals (armadillos, anteaters, and sloths). Molecular Biology and Evolution 24: 2573−2582. Crossref
O’Leary, M.A., Bloch, J.I., Flynn, J.J., Gaudin, T.J., Giallombardo, A., Giannini, N.P., Goldberg, S.L., Kraatz, B.P., Luo, Z.X., Meng, J., Ni, X., Novacek, M.J., Perini, F.A., Randall, Z.S., Rougier, G.W., Sargis, E.J., Silcox, M.T., Simmons, N., Spaulding, M., Velazco, P.M., Weksler, M., Wible, J.R., and Cirranello, A. 2013. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science 339: 662–667. Crossref
Oliveira, E.V. and Pereira, J.C. 2009. Intertropical cingulates (Mammalia, Xenarthra) from the Quaternary of Southern Brazil: Systematics and paleobiogeographical aspects. Revista Brasileira de Paleontologia 12: 167–178. Crossref
Oliveira, P.V., Ribeiro, A.M., Oliveira, E.V., and Viana, M.S.S. 2014. The dasypodidae (mammalia, xenarthra) from the urso fóssil cave (quaternary), parque nacional de ubajara, state of ceará, brazil: paleoecological and taxonomic aspects. Anais da Academia Brasileira de Ciências 86: 147–158. Crossref
Paula-Couto, C. 1979. Tratado de paleomastozoologia. Academia Brasileira de Ciências 15 (1): 1–590.
Pocock, R.I. 1924. The external characters of the South American edentates. Proceedings of the Zoological Society of London 2: 983–1031. Crossref
Rafinesque, C.S.S. 1815. Analyse de la nature, ou tableau de l’univers et des corps organisés, 224. L’Imprimerie de Jean Barravecchia, Palermo. Crossref
Scillato-Yané, G.J. 1977. Notas sobre los Dasypodidae (Mammalia, Edentata) del Plioceno del territorio argentino I. Los restos de edad Chasiquense (Plioceno inferior) del sur de la Provincia de Buenos Aires. Ameghiniana 14: 133–144.
Scillato-Yané, G.J. 1980. Catálogo de los Dasypodidae fósiles (Mammalia, Edentata) de lal República Argentina. Actas II Congreso Argentino de Paleontología y Bioestratigrafía y I Congreso Latinoamericano de Paleontología. Buenos Aires 1978 3: 7–36.
Scillato-Yané, G.J. 1982. Los Dasypodidae (Mammalia: Edentata) del Plioceno y Pleistoceno de Argentina. 127 pp. Ph.D. Thesis, Facultad de Ciencias Naturales y Museo,Universidad Nacional de La Plata, La Plata.
Sidorkewicj, N.S. and Casanave, E.B. 2013. Morphological characterization and sex-related differences of the mandible of the armadillos Chaetophractus vellerosus and Zaedyus pichiy (Xenarthra, Dasypodidae), with consideration of dietary aspects. Iheringia. Série Zoologia 103: 153–162. Crossref
Simpson, G.G. 1945. The principles of classification and a classification of mammals. Bulletin of the American Museum of Natural History 85: 1–367.
Smith, K. and Redford, K.H. 1990. The anatomy and function of the feeding apparatus in two armadillos (Dasypoda): anatomy is not destiny. Journal of Zoology 222: 27–47. Crossref
Squarcia, S.M., Sidorkewicj, N.S., Camina, R., and Casanave, E.B. 2009. Sexual dimorphism in the mandible of the armadillo Chaetophractus villosus (Desmarest, 1804) (Dasypodidae) from northern Patagonia, Argentina. Brazilian Journal of Biology 69: 347–352. Crossref
Thenius, E. 1989. Zähne und Gebiß der Säugetiere. In: J. Niethammer, H. Schliemann, and D. Starck (eds.), Handbuch der Zoologie. Band VIII Mammalia, 513. Verlag Walter de Gruyter, Berlin. Crossref
Urrutia, J., Montalvo, C., and Scillato-Yané, G. 2008. Dasypodidae (Xenarthra, Cingulata) de la Formación Cerro Azul (Mioceno tardío) de la provincia de La Pampa, Argentina. Ameghiniana 45 (2): 289–302.
Vizcaino, S.F. 1994. Mecánica masticatoria de Stegotherium tessellatum Ameghino (Mammalia, Xenarthra) del Mioceno de Santa Cruz (Argentina). Algunos aspectos paleoecológicos relacionados. Ameghiniana 31: 283–290.
Vizcaíno, S.F. and Bargo, M.S. 2014. Loss of ancient diversity of xenarthrans and the value of protecting extant armadillos, sloths and anteaters. Edentata 15: 27–38. Crossref
Vizcaíno, S.F. and Fariña, R.F. 1994. Caracterización trófica de los armadillos (Mammalia, Xenarthra, Dasypodidae) de Edad Santacrucense (Mioceno temprano) de Patagonia (Argentina). Acta Geologica Leopoldensia 39: 191–200.
Vizcaíno, S.F., Bargo, M.S., Kay, R.F., and Milne, N. 2006. The armadillos (Mammalia, Xenarthra, Dasypodidae) of the Santa Cruz Formation (early–middle Miocene): An approach to their paleobiology. Palaeogeography, Palaeoclimatology, Palaeoecology 237: 255–269. Crossref
Wagler, J. 1830. Naturliches System der Amphibien, mit vorangehender Classification der Saugethiere und Vogel, 354. J.G. Cotta’sche Buchhandlung, München.
Wetzel, R.M. 1980. Revision of the naked-tailed armadillos, genus Cabassous McMurtrie. Annals of the Carnegie Museum 49: 323–357.
Wetzel, R.M. 1985. The identification and distribution of recent Xenarthra (= Edentata). In: G.G. Montgomery (ed.), The Evolution and Ecology of Armadillos, Sloths, and Vermilinguas, 5–21. Smithsonian Institution Press, Washington, DC.
Woodburne, M.O., Goin, F.J., Raigemborn, M.S., Heizler, M., Gelfo, J.N., and Oliveira, E.V. 2014. Revised timing of the South American early Paleogene land mammal ages. Journal of South American Earth Sciences 54: 109–119. Crossref
Acta Palaeontol. Pol. 66 (Supplement to 3): 31–46, 2021
https://doi.org/10.4202/app.00829.2020