

Evidence of external gametophores in puzzling Late Triassic–Early Jurassic dasycladalean green algae
FILIPPO BARATTOLO, ROBERTA ROMANO, and MARC CONRAD
Barattolo, F., Romano, R., and Conrad, M. 2021. Evidence of external gametophores in puzzling Late Triassic–Early Jurassic dasycladalean green algae. Acta Palaeontologica Polonica 66 (4): 901–919.
The mineralized cover preserving soft parts of the Dasycladales, as an external mould, is the only tool allowing to deduce the anatomical parts of these algae. Problems arise when voids within the calcareous skeleton do not reflect accurately the original soft parts due to a loss of mineralization. Based on a rich material collected from the type locality in Languedoc (France), a detailed study was carried out on Chinianella ellenbergeri, the type species of Chinianella. Previous interpretation of voids in the calcified fossil considered the alga to be made of an alternation of fertile and further ramified sterile whorls. Our study shows that large cavities previously interpreted as corresponding to fertile whorls, actually denote empty spaces. Therefore, the alga essentially consists of spaced-out whorls of sterile primary laterals, distally bearing a tuft of three-four phloiophorous secondary laterals. A small number of specimens show the presence of calcified structures interpreted as corresponding to external, subterminal reproductive organs. Consequently, an emended diagnosis is proposed for C. ellenbergeri and for the Chinianella. Present interpretation of Chinianella adds a new step to the understanding of the emergence of external reproductive organs during the geological history of this group of green algae. Chinianella ellenbergeri shows that choristosporate reproduction sensu lato was already well represented and diversified in the Early Jurassic. Other Late Triassic–earliest Jurassic species formerly attributed to Chinianella (namely C. carpatica, C. crosii, C. zanklii, C. micropora, and C. macropora) reveal that the large, fertile primary lateral is a compound pore with a structure like in Cymopolia, i.e., made of a short primary lateral with a terminal gametophore embraced by secondary laterals. Therefore, these species have been referred to the new genus Distefanopolia.
Key words: Dasycladales, Chinianella ellenbergeri, Distefanopolia, green algae, systematic revision, France, Italy, Sicily.
Filippo Barattolo [filippo.barattolo@unina.it], Dipartimento di Scienze della Terra dell’Ambiente e delle Risorse, Università degli Studi di Napoli Federico II, Complesso universitario di Monte Sant’Angelo, via Cintia 21-80126 Napoli, Italy.
Roberta Romano [romanoroberta123@gmail.com], Via Falloppio, 40-35121 Padova, Italy.
Marc André Conrad [sacoluc3712@dfinet.ch], Chemin de Planta 71, 1223 Cologny, Switerland.
Received 22 February 2021, accepted 19 September 2021, available online 14 December 2021.
Copyright © 2021 F. Barattolo et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Dasycladales are well recorded as fossils, thanks to their calcareous cover preserving the soft parts as an external mould. Therefore, the calcareous skeleton is the only tool to deduce the anatomical elements of the alga. Although the pattern of mineralization is considered species specific, the shape and relationships of the soft parts are those basically allowing the taxonomical classification (e.g., Pia 1912, 1920). Problems arise when voids within the calcareous skeleton do not reflect accurately the original soft part by a loss of mineralization (Barattolo 2019). Therefore, such a deficiency in mineralization produces indeterminacy concerning the type and nature of the soft parts, with great impact on systematics.
Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994) Granier, Masse, and Berthou, 1994, is emblematic in this respect. The alga was first described by Lebouché and Lemoine (1963) as “Cylindroporella ellenbergeri” from the upper Sinemurian (Lotharingian) of southern France at Fontcaude, Languedoc (Fig. 1). The name was not validly established because the authors designated six “cotypes” (Lebouché and Lemoine 1963: 94). According to these authors, the alga is characterized by a cylindrical thallus where each whorl of fertile laterals alternates with whorls of sterile laterals, four diverging secondary laterals arising from the distal end of the sterile laterals (Fig. 2).
The voids between whorls occurring in Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994) Granier, Masse, and Berthou, 1994 emend. nov. (Fig. 3) can be variously interpreted: (i) hosting fertile whorls (Fig. 3A), (ii) containing lateral gametophores (Fig. 3B) or (iii) being mere cavities (Fig. 3C).
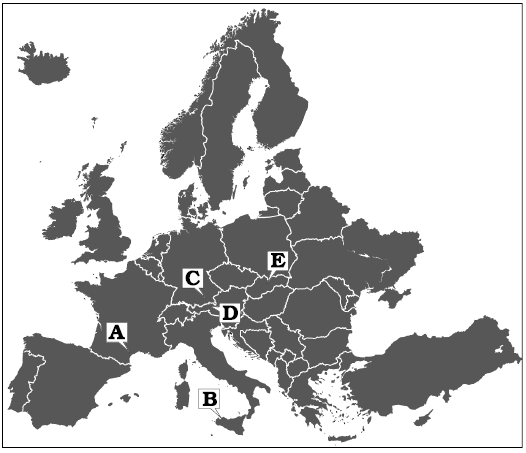
Fig. 1. Map with type localities of the species discussed in the text indicated. A. Chinianella ellembergeri (Lebouché and Lemoine in Granier and Deloffre, 1994) Granier, Masse and Berthou, 1994, emend. nov., upper Sinemurian (Jurassic) of Canders (Languedoc, France). B. Distefanopolia micropora (Di Stefano and Senowbari-Daryan, 1985) and Distefanopolia macropora (Di Stefano, 1981 ex Di Stefano and Senowbari-Daryan, 1985) nov. comb., Norian (Upper Triassic), Cozzo di Lupo (Sicily, Italy). C. Distefanopolia zanklii (Ott, 1967) nov. comb., Norian–Rhaetian (Upper Triassic) of Dürreckberg (Upper Bavaria, Germany). D. Distefanopolia crosii (Ott, 1968) nov. comb., Upper Triassic of Kohlalpen Valley (Kaiser Mountains, Austria). E. Distefanopolia carpatica (Bystrický, 1967) nov. comb., Norian (Upper Triassic) of Muráň Plateau (Slovakia).
The type locality of Chinianella ellenbergeri was re-sampled in March 1992, to restudy Cylindroporella liasica Lebouché and Lemoine in Granier and Deloffre, 1994 (Barattolo and Parente 2000). Rich algal materials were collected, encouraging us to restudy also Chinianella ellenbergeri. Mineralized parts of this alga contain wide empty cavities. These cavities had to be carefully investigated to understand in which degree they correspond to soft parts of the alga. Several characters are taken into consideration and four basic models on the algal structure discussed, with the diagnosis of C. ellenbergeri emended accordingly.
Institutional abbreviations.—LM-DiSTAR, Laboratory of Micropalaeontology, Department of Earth Sciences, Environment and Resources, University of Naples Federico II, Naples, Italy (BA, Barattolo collection); LP-EM Paris, Laboratoire de Paléontologie de l’École des Mines, Paris, France (ALG, Collection Marcel Lemoine); MGG-PA, Gemmellaro Geological Museum, University of Palermo, Italy.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in Plant Fossil Names Registry (PFNR): urn:lsid:plantfossilnames.org:ref:974
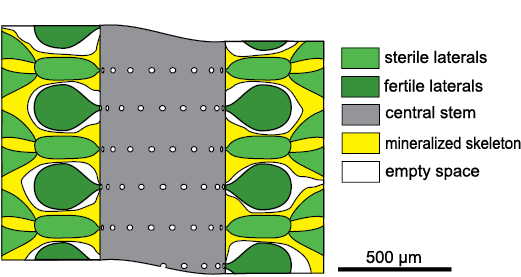
Fig. 2. Structure of dasycladalean alga Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994) according Lebouché and Lemoine (1963).
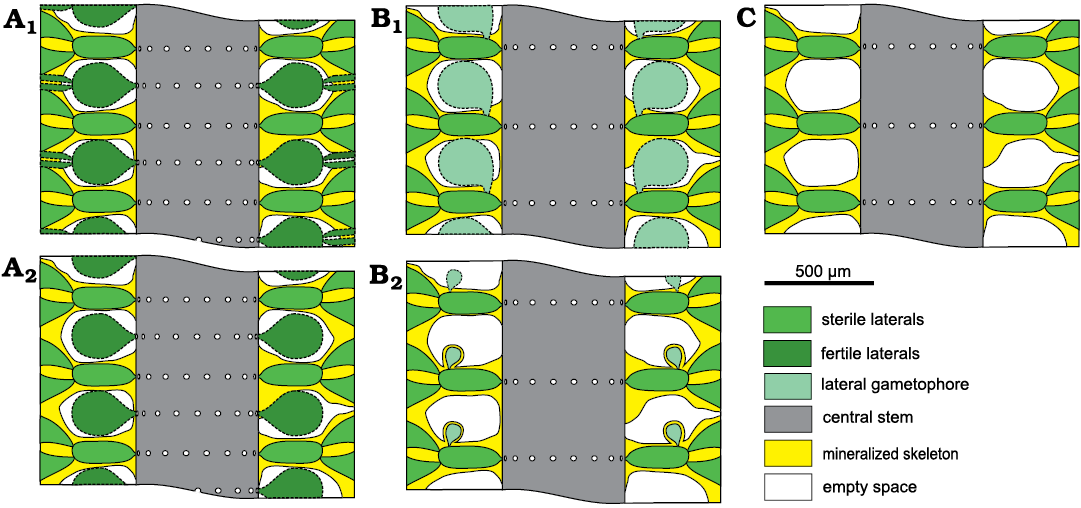
Fig. 3. Possible structural models in dasycladalean alga Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994). A. Alternated whorls of weak/sterile and strong/fertile laterals; sterile and fertile laterals ramified (A1); only sterile laterals ramified (A2). B. Spaced whorls of weak/sterile laterals bearing a laterally attached gametophore (goniospory); large gametophore attached proximally (B1); subterminal gametophore (B2). C. Spaced whorls of weak/sterile laterals with simple interverticillar voids.
Material and methods
The revision of Chinianella ellenbergeri is based on the sample LM-DiSTAR/BA.577, consisting of a large piece of light brown limestone (bioclastic and oolitic grainstone) collected by one of us (FB) in March 1992 at Canders, 2.4 km E of Fontcaude (Hérault Department, southern France; 43°25’07N 3°05’05E). It was sampled in level 5 of Lebouché and Lemoine (1963: fig. 3) and contains dasycladaleans, mainly Chinianella ellenbergeri, frequent Cylindroporella? liasica (Lebouché and Lemoine in Granier and Deloffre, 1994) and occasional Eodasycladus barrabei (Lebouché and Lemoine in Granier and Deloffre, 1994), bivalves, gastropods, echinoids, serpulids, and rare foraminifers.
The algal remains are found as bioclastic component of an oolitic grainstone. The space between vegetative parts of Chinianella ellenbergeri is usually filled with micrite, while the central cavity is filled with ooids and cement. This makes it possible to envisage a transportation from a neighbouring, mud-rich algal habitat. Anyway, the skeletal grains of C. ellenbergeri are commonly fragmented, although long cylindrical pieces can be observed. They do not display severe wearing, but a grain to grain compenetrating contact is often noted.
Two series of thin sections were cut from the same large sample. The first forty-three thin sections labelled as LM-DiSTAR/BA.577.1–BA.577.43 are stored in the Barattolo collection, Department of Earth Sciences, Environment and Resources (DiSTAR), University of Naples Federico II. Other twelve thin sections, labelled as LM-DiSTAR/BA.577.a–BA.577.n, used for a preliminary study, must be considered lost.
The material of Distefanopolia micropora (Di Stefano and Senowbari-Daryan, 1985) nov. comb. is stored in the Gemmellaro Geological Museum (University of Palermo, coll. A66).
Systematic palaeontology
Order Dasycladales Pascher, 1931
Family Bornetellaceae Granier and Bucur in Granier et al., 2013
Tribe Bornetelleae Morellet and Morellet, 1913
Genus Chinianella Ott, 1967 ex Granier, Masse, and Berthou, 1994, emend. nov.
Type species: Cylindroporella ellenbergeri Lebouché and Lemoine, 1963, invalidly published, ≡ Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994) Granier, Masse, and Berthou, 1994 emend. nov.; upper Sinemurian (Lotharingian), Lower Jurassic, Hérault Department, southern France.
Emended diagnosis.—Cylindrical simple thallus. Akrophorous primary laterals arranged in spaced whorls. Phloiophorous secondary laterals, forming a distal cortex. Presence of a single, subterminal gametophore attached to the primary lateral.
Remarks.—According to Ott (1967), his new genus Chinianella is characterized by a cylindrical thallus bearing alternating fertile and sterile whorls, fertile whorls being possibly twofold, while sterile whorls being twofold. Therefore, the structure of the alga fits with models depicted in Fig. 3A. Later on, the same author (Ott 1968) considered his Chinianella to be a junior synonym of Heteroporella Cros and Lemoine, 1967 ex Praturlon, 1967. As coined by Granier and Deloffre (1994: 20), Chinianella was invalidly published because it was based on the invalidly published designation of “Cylindroporella ellenbergeri Lebouché and Lemoine, 1963”, not correctly typified (established on six cotypes). The designation of a lectotype of “Cylindroporella ellenbergeri Lebouché and Lemoine in Granier and Deloffre, 1994”, led to its valid publication as “Heteroporella ellenbergeri Lebouché and Lemoine in Granier and Deloffre, 1994”. Later, Granier et al. (1994) showed that Heteroporella Cros and Lemoine, 1967 ex Praturlon, 1967, is monospecific and does not include “H. ellenbergeri”. That implied the availability of the generic name Chinianella for Chinianella ellenbergeri. Therefore, Granier et al. (1994) preferred reusing Ott’s (1967) name rather than introducing a generic name of their own. They provide a new generic diagnosis stressing on additional characters such as a higher number of laterals in the sterile whorls compared to the fertile whorls, and sterile whorls bearing at least one order of laterals.
Position of the Chinianella changed according to the interpretation of its soft parts. Bassoullet et al. (1978) referred the Heteroporella (and therefore its synonymous Chinianella) to the family Dasycladaceae Kützing, 1843, tribe Cylindroporelleae Pal, 1976. Deloffre (1988) adopted the same hierarchic division proposed by Bassoullet et al. (1978). In turn, Berger and Kaever (1992), included the Heteroporella in the family Triploporellaceae Berger and Kaever, 1992, tribe Cylindroporelleae Pal, 1976, whereas Granier et al. (1994) ascribed Chinianella to the family Acetabulariaceae Hauck, 1885, tribe Uteriae Morellet and Morellet, 1922.
Based on the herein rejected model of Fig. 3C, Chinianella should be ascribed to the family Triploporellaceae, tribe Triploporelleae Pia, 1920, subtribe Linoporellinae Pia, 1927. Following the herein accepted model (Fig. 3B2), the genus could be assigned to the family Dasycladaceae Kützing, 1843, tentatively to the tribe Batophoreae Valet, 1969, if gametophores are interpreted as subterminal. According to this author, the Batophoreae gather plants displaying similar whorls, with laterals ramified up to seventh order, and numerous subterminal gametophores attached to laterals of 1st to 4th order. Alternatively, if gametophores are interpreted as lateral even if shifted outwards, the genus Chinianella could be better ascribed the family Bornetellaceae Granier and Bucur in Granier et al., 2013, tribe Bornetelleae Morellet and Morellet, 1913. Here, Chinianella is assigned tentatively to the tribe Bornetelleae, based on the distal, lateral position of the single reproductive organs. On the other hand, there is no evidence whether reproductive organs were gametangia or gametophores.
Primary laterals bearing secondary laterals and, sideways, a gametophore are characters that Chinianella shares with several genera such as Montiella Morellet and Morellet, 1922, Barattoloporella Parente, 1997, Bornetella Munier-Chalmas, 1877, Conradella Masse and Bucur, 2002, Granieria Barattolo and Romano in Barattolo et al., 2008, Jodotella Morellet and Morellet, 1913, and Bakalovaella Bucur, 1993. Uteria Michelin, 18457, is a moniliform genus whose fertile parts are largely unmineralized so that the algal structure remains unclear. Therefore, comparisons between Uteria and Chinianella will be not discussed. Some dasycladalean genera with primary and secondary laterals are sketched to show differences with Chinianella (Fig. 4).
Bornetella (Fig. 4A) differs from Chinianella (Fig. 4B) because multiple gametophores are set sideways vs a single distal gametophore in Chinianella. Jodotella (Fig. 4C) and Chinianella exhibit the closest affinity (distal gametophores sideways the primary lateral), but Jodotella shows multiple gametophores vs one in Chinianella. Genera of the Granieria group (Fig. 4D) share the occurrence of a single proximal gametophore attached sideways on the primary lateral, vs a single distal gametophore in Chinianella. Genera belonging to the Montiella group (Fig. 4E) differ from Chinianella by the presence of a gametophore terminally located on the primary lateral, and a single secondary lateral, rarely two (Barattoloporella, also characterized by a moniliform thallus).
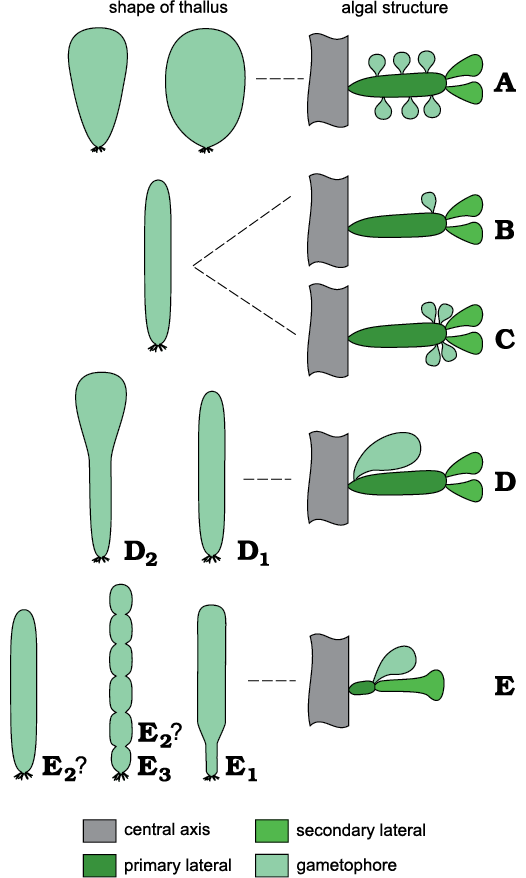
Fig. 4. A comparison of selected dasycladalean genera compared. A. Bornetella Munier-Chalmas, 1877. B. Chinianella Ott, 1967 ex Granier and Deloffre, 1994. C. Jodotella Morellet and Morellet, 1913. D. Granieria group, Conradella Masse and Bucur, 2002 (D1) and Granieria Barattolo and Romano in Barattolo et al., 2008 (D2). E. Montiella group, Bakalovaella Bucur, 1993 (E1), Montiella Morellet and Morellet, 1922 (E2), Barattoloporella Parente, 1997 (E3).
Disregarding the algal reproductive structures, Chinianella compares to numerous other genera ranging from the Paleozoic to the Recent, all with the same kind of vegetative parts, that is a cylindrical thallus bearing whorls of single, tubular primary (first order) laterals, each distally carrying clusters of phloiophorous secondaries. Examples are as follows. In the Triassic to Eocene Dissocladella Pia, 1936, the primaries are club-shaped and the secondaries egg- or pear-shaped. In the Early Jurassic (Hettangian) to Palaeocene-aged genus Steinmanniporella Bucur, Granier, and Schlagintweit, 2010, the thallus shape and vegetative laterals are as in Chinianella, whereas quoting Bucur et al. (2010: 317) “reproductive structures were situated very probably inside the central stem (endosporate)”, contrary to Chinianella.
Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994) Granier, Masse, and Berthou, 1994, emend. nov.
Figs. 6–9, 13.
1963 Cylindroporella ellenbergeri n. sp.; Lebouché and Lemoine 1963: 93–94, pl. 1: 2, pl. 2: 1–6.
1967 Chinianella ellenbergeri (Lebouché and Lemoine 1963) n. gen., n. comb.; Ott 1967: 216–217.
1968 Heteroporella ellenbergeri (Lebouché and Lemoine 1963) n. comb.; Ott 1968: 255.
1972 Heteroporella ellenbergeri (Lebouché and Lemoine, 1963); Fourcade et al. 1972: 239.
1975 Heteroporella ellenbergeri (Lebouché and Lemoine, 1963); Elliott 1975: 362.
1978 Heteroporella ellenbergeri (Lebouché and Lemoine, 1963); Bassoullet et al. 1978: 126–128, pl. 13: 5–6.
1982 Cylindroporella ellenbergeri Lebouché and Lemoine; Lespinasse 1982: 18.
1984 Heteroporella ellenbergeri (Lebouché and Lemoine, 1963); Bernier 1984: 464.
1994 Cylindroporella ellenbergeri Lebouché and Lemoine nom. nud.; Granier and Deloffre 1994: 20.
1994 Heteroporella ellenbergeri (Lebouché and Lemoine ex Granier and Deloffre, 1994); Granier and Deloffre 1994: 22–23.
1994 Chinianella ellenbergeri (Bernet-Rollande and Lemoine, ex Granier and Deloffre, 1994); Granier et al. 1994: 132, pl. 2: 1–3.
2011 Chinianella ellenbergeri Lebouché and Lemoine, 1963; Senowbari-Daryan et al. 2011: 502.
2018 Chinianella ellenbergeri Lebouché and Lemoine in Granier and Deloffre, 1993, non 1963; Granier 2018: 187.
2019 Chinianella ellenbergeri (Lebouché and Lemoine, 1963); Barattolo 2019: 2.
Type material: Lectotype designated by Lebouché and Lemoine in Granier and Deloffre (1994) as oblique section figured in Lebouché and Lemoine (1963: pl. 2: 3), thin section not indicated (ALG. 535 or ALG. 536). Collection Marcel Lemoine, Laboratoire de Paléontologie de l’École des Mines, Paris; thin sections ALG. 535 and ALG. 536 are cotypes indicated by Lebouché and Lemoine (1963).
Type locality: Canders, 2.4 km E of Fontcaude, Department of Hérault, Languedoc, France.
Type horizon: level nr. 5 of Fontecaude section, Lebouché and Lemoine (1963: fig. 3; see also Fig. 1), upper Sinemurian (Lotharingian), Lower Jurassic.
Nomenclatural remarks.—The Shenzhen Code (International Code of Nomenclature for algae, fungi, and plants) introduces some novelty about the specimen for the purpose of typification. As to understand which value must be assigned to “cotypes” used by Lebouché and Lemoine (1963) to designate the species, one of us (FB) submitted on 20th June 2020 a specific query to Giovanni Furnari, a member of the Nomenclature Committee for Algae. Supported by two other colleagues (John Mc Neill and Patrick Herendeen) G. Furnari answered that according to the Code (Art. 40.1 and Art. 40.2) the type can be achieved by reference to an entire gathering, or a part thereof. Therefore the “cotypes” indicated by Lebouché and Lemoine (1963: pl. 2: 1–6), collected from the same locality and the same level, could be considered a gathering and valid the original designation. However, it is not sure the two thin sections (ALG. 535 and ALG. 536) indicated by Lebouché and Lemoine (1963) as cotypes are obtained from a single sample or two different samples. Moreover, it is a matter of fact that the direct transfer of the biological term “gathering” in paleontology probably causes problems and a specific wide debate is needed in the paleontological community. Pending such indeterminacy, in this paper, a prudential solution is followed.
Emended diagnosis.—Cylindrical, simple thallus. Primary laterals set in spaced whorls (euspondyl arrangement). Each whorl is characterized by 16–24 primary laterals. The primary laterals are moderately long, burly, cylindrical, sausage-like, tapered at the inner end, rounded distally. They are set almost perpendicular to the central stem or very slightly inclined upwards. Each primary lateral distally gives rise to four moderately short phloiophorous secondary laterals, flaring out to form a cortical layer. Reproductive organs usually not preserved, rare, forming calcified structures interpreted as corresponding to a single, subterminal gametophore located sideways to the primary laterals. The calcareous skeleton consists of a thin calcareous sheath totally encrusting the surface of the thallus (central stem, primary and secondary laterals). The interverticillar space remains uncalcified. The most significant biometric values are based on 66 specimens found in five thin sections (LM-DiSTAR/BA.577.15–BA.577.20). These are listed in table 1 in SOM, the Supplementary Online Material available at http://app.pan.pl/SOM/app66-Barattolo_etal_SOM.pdf.
Material.—The specimens of Chinianella ellenbergeri illustrated in Figs. 6–8 are based on the sample LM-DiSTAR/BA.577, collected from the Sinemurian of Canders, 2.4 km E of Fontcaude (Hérault Department, southern France; 43°25’07N, 3°05’05E) and was sampled in level 5 of Lebouché and Lemoine (1963: fig. 3). Forty-three thin sections labelled as LM-DiSTAR/BA.577.1–BA.577.43 were obtained from the rock sample. They are stored in the Barattolo collection, Department of Earth Sciences, Environment and Resources (DiSTAR), University of Naples Federico II. Figure 14 originates from a set of twelve thin sections cut from the same sample LM-DiSTAR/BA.577 and labelled as LM-DiSTAR/BA.577.a–BA.577.n. This set was used for a preliminary study, but must be considered lost.
Description.—General features of the calcareous skeleton: the calcareous skeleton is simple, continuous without any trace of intusannulation or annulation in strongly mineralized thalli, largely intusannulated if weakly mineralized. The calcareous skeleton is represented by a calcareous sheath, 0.025–0.040 mm broad around the primary laterals and 0.025–0.11 mm around the central axis, which totally coats the soft parts except the cortical layer and the reproductive organs (perhaps only occasionally calcified). The spacing between whorls gives rise to a large, uncalcified empty space (Fig. 5A).
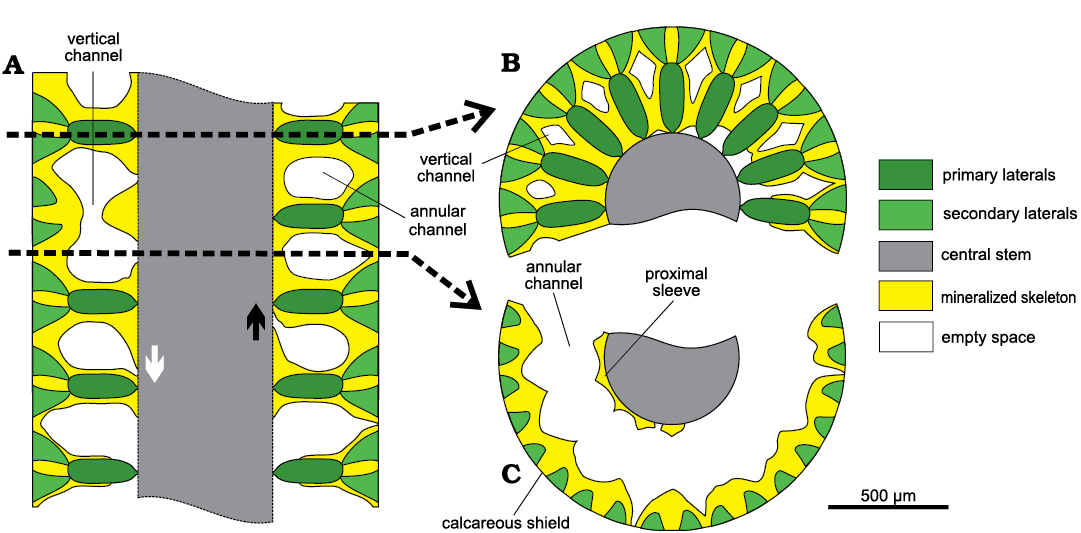
Fig. 5. Scheme of mineralization in dasycladalean alga Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994) Granier, Masse and Berthou, 1994, emend. nov. A. Axial section, lowest whorl is weakly calcified. Trace of transverse sections is indicated with dashed lines. B. Transverse section at whorl level. C. Transverse section through the interverticillar space. The interverticillar empty spaces merge laterally (annular channel) and are connected between whorls (vertical channels). A central pore sometimes leaves in contact the interverticillar void and the central cavity, either shifted downwards (white arrow) or upward (black arrow).
Transversal sections appear different if cutting the calcified whorl (Fig. 5B) or through the space between whorls. In this latter case they display a wide annular channel (Fig. 5C) limited inward by a crenate mineralized wall coating the central stem as shown by some transversal sections in Fig. 6. In oblique sections the crenate surface may appear as an irregular but continuous incision (Figs. 7, 8) . In oblique to tangential section the crenate surface occasionally appears as separated rounded pores. These latter ones constitute voids not related to organic structures (hereby defined as “false pores”) and misinterpreted as corresponding to large fertile laterals (Fig. 8). Outwards, the annular channel is limited by the calcareous shield coating the diverging secondary laterals.
Transversal sections across the whorl display the radially arranged primary laterals enveloped by mineralization up to their inner half part, then more outwards the calcareous wall leaves diamond-shaped empty spaces (Fig. 5B, cf. also transversal-oblique sections in Fig. 9) putting two subsequent interverticillar cavities into communication (vertical channel, Figs. 5A, B, 6F, 7F).
Primary laterals: the primary laterals are almost perpendicular or slightly inclined upward on the main axis, throughout the main part of the thallus. Only near the apical zone, inclination of the laterals increases, so they are oriented almost vertically on the top of the thallus (Fig. 7H, I). The primary laterals are akrophorous, moderately long and sturdy, sausage-like. They are tapered at their proximal end, where they are connected to the central stem by a short peduncle. Their distal end is rounded. In small sized specimens, the primary laterals rather sturdy, but shorter (Fig. 7G, K), compared to those of the large specimens (Fig. 6E, 7L, 8F). The primary laterals are arranged in simple whorls (euspondyl type). Outwards, they are usually perfectly aligned, sometimes with a gently staggered pattern (Figs. 7M, N, 8M), alternating in position in successive whorls (Figs. 7N, 9I) The height between whorl is 0.21–0.44 mm.
By analogy with extant, cylindrical to slightly club-shaped dasycladaleans with phloiophorous or akrophorous laterals (e.g., Neomeris Lamouroux, 1816, and Dasycladus Agardh, 1828), the top of the thallus consists of laterals decreasing upwards in size and inclination, closed like a bud at the apex. No significant change in inclination has been observed along the calcareous skeleton, but the upper end of some oblique to longitudinal sections (Fig. 7H, I) shows laterals bending more and more upward, confirming the presence of a mineralized apex.

Fig. 6. Dasycladalean alga Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994) Granier, Masse and Berthou, 1994, emend. nov., upper Sinemurian (Lotharingian), Canders, 2.4 km E of Fontcaude, (S France). A. LM-DiSTAR/BA.577.16, n. 065, oblique section of a weakly mineralized specimen showing intusannulation. B. LM-DiSTAR/BA.577.21, n. 114, axial section, notice the irregular inner contour. C. LM-DiSTAR/BA.577.24, n. 117, axial section of a weakly mineralized specimen showing intusannulation. D. LM-DiSTAR/BA.577.28, n. 126, oblique section of a weakly mineralized specimen with intusannulation. E. LM-DiSTAR/BA.577.4, n. 003, oblique section of a strongly mineralized specimen, note the regular contour of the inner cavity, interverticillar cavities sometimes communicate with the central cavity by mean of a small pore (see arrow). F. LM-DiSTAR/BA.577.9, n. 018, oblique section showing the annular and vertical channels. G. LM-DiSTAR/BA.577.10, n. 024, transverse-oblique section, a possible gametophore looks attached to primary lateral (see arrow). H. LM-DiSTAR/BA.577.8, n. 014, transverse-oblique section showing the annular and vertical channels. I. LM-DiSTAR/BA.577.15, n. 054, transverse-oblique section, note the annular channel with scalloped surfaces in the proximal sleeve and the calcareous shield, respectively.
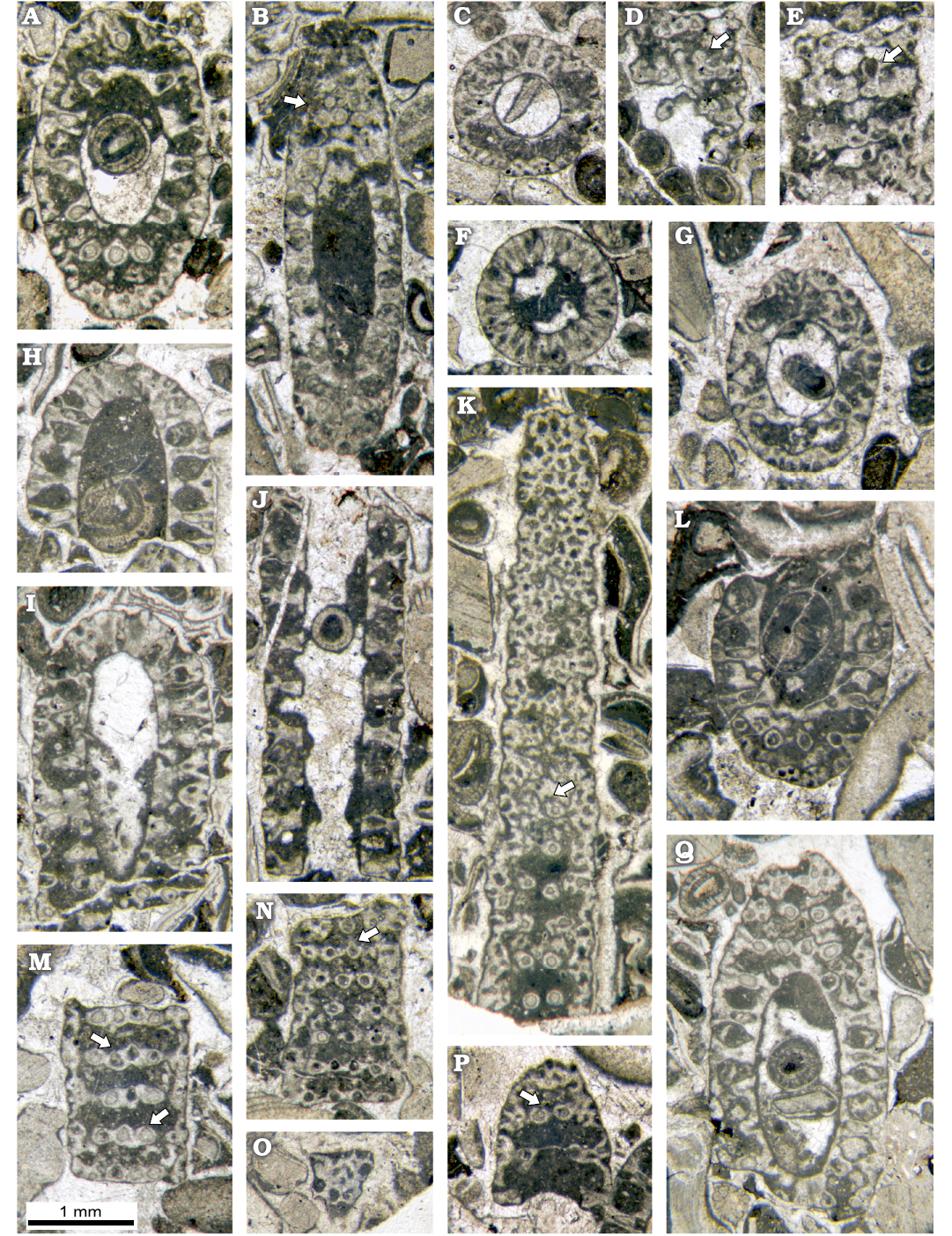
Fig. 7. Dasycladalean alga Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994) Granier, Masse and Berthou, 1994, emend. nov., upper Sinemurian (Lotharingian), Canders, 2.4 km E of Fontcaude (S France). A. LM-DiSTAR/BA.577.27, n. 121, oblique section, note the continuous interverticillar channels. B. LM-DiSTAR/BA.577.15, n. 050, oblique section, mineralized rounded to irregular bodies in all likelihood corresponding to gametophores occur between whorls (see arrow). C. LM-DiSTAR/BA.577.19, n. 093, transversal-oblique section showing a crenate annular channel. D. LM-DiSTAR/BA.577.19, n. 095, tangential section showing calcified rounded bodies attached to a whorl (see arrow). E. LM-DiSTAR/BA.577.14, n. 040, tangential section, with a conceivable gametophore attached to the primary lateral (see arrow). F. LM-DiSTAR/BA.577.5, n. 007, transversal section of a whorl, with vertical channels between primary laterals. G. LM-DiSTAR/BA.577.19, n. 091, oblique section of a small specimen; note the moderately short and large primary laterals. H. LM-DiSTAR/BA.577.30, n. 128, axial section showing the apex of the thallus. I. LM-DiSTAR/BA.577.33, n. 130, oblique section crossing the apical zone. J. LM-DiSTAR/BA.577.15, n. 051, longitudinal section of a weakly mineralized small specimen showing intusannulation; moderately short and large primary laterals can be observed. K. LM-DiSTAR/BA.577.34, n. 132, tangential section cutting primary laterals (below) and secondary laterals; some primary laterals display a small opening (see arrow). L. LM-DiSTAR/BA.577.16, n. 059, oblique section showing slender primary laterals; note a tuft of secondary laterals forming the corners of a parallelogram. M. LM-DiSTAR/BA.577.9, n. 015, tangential section showing primaries with a gently staggered pattern (1st and 2nd whorls from below), some primary laterals display a small opening (see arrow). N. LM-DiSTAR/BA.577.20, n. 108, tangential section; note alternating primary laterals, some primary laterals display a small opening (see arrow). O. LM-DiSTAR/BA.577.18, n. 078, tangential section showing secondary laterals close to the cortex. P. LM-DiSTAR/BA.577.19, n. 082, oblique section; a tuft of cross-arranged secondary laterals is observable, some primary laterals display a small opening (see arrow). Q. LM-DiSTAR/BA.577.1, n. 001, oblique section showing a tuft of cross-arranged secondary laterals (top left).
Secondary laterals: each primary lateral usually holds a tuft of four secondary laterals forming a cross (Fig. 7P, Q) or corresponding to the four corners of a parallelogram (Figs. 7L, 9E). Less frequently, such tufts consist of only three secondaries (Fig. 9F). They quickly diverge from the axis of the primary lateral, with an angle of 50–70°. Tangential sections across this region display an irregular crowding of pores, corresponding to the tufts of secondary laterals and the space between them (Fig. 7K). Outwards. they bend up to set right to the external surface of the thallus, thus the secondaries are in mutual contact, forming a distal cortex. (Figs. 7O, 8L).
Reproductive organs: despite the abundant material, rare evidence of reproductive organ has been observed (see Discussion). Rare sections allow us to consider the presence of gametophores, arranged laterally, next to the top of the primaries. They are drop-like in shape, 0.06–0.12 mm in diameter and 0.11–0.22 mm long. On the other hand, there is no trace of reproductive organs, either in the axial cavity or inside the laterals. Considering the structure of this alga, hesitantly excluding goniospory (gametophores lateral to the primaries) leaves only one possibility, that of the endospory, specifically the presence of reproductive organs inside the axial cavity. However, given the known, general evolution of the Dasycladales from the Paleozoic to the Recent, and the presence of this alga in the Lower Jurassic, this hypothesis may be considered unlikely (cf. Bucur 1985).
Reconstruction of the thallus: reconstructions are based on specimens in thin section (i.e., biometrical values, shape, degree of calcification, character of the apex). Unmineralized parts of the thallus such as the distal, cortical end of the secondary laterals and reproductive organs are mineralized only occasionally, and therefore, are interpreted in the reconstructions. Consequently, despite the vegetative part are well recognized, arrangement of the reproductive organs remains largely uncertain. A reconstruction rendering the “sterile” solution as in Fig. 3C would be more adherent to characters observed, but in this case, an endosporate reproduction must be implicitly inferred. The reconstruction prospected in Fig. 10 assumes the goniosporate solution (Fig. 3B2, see Discussion below).
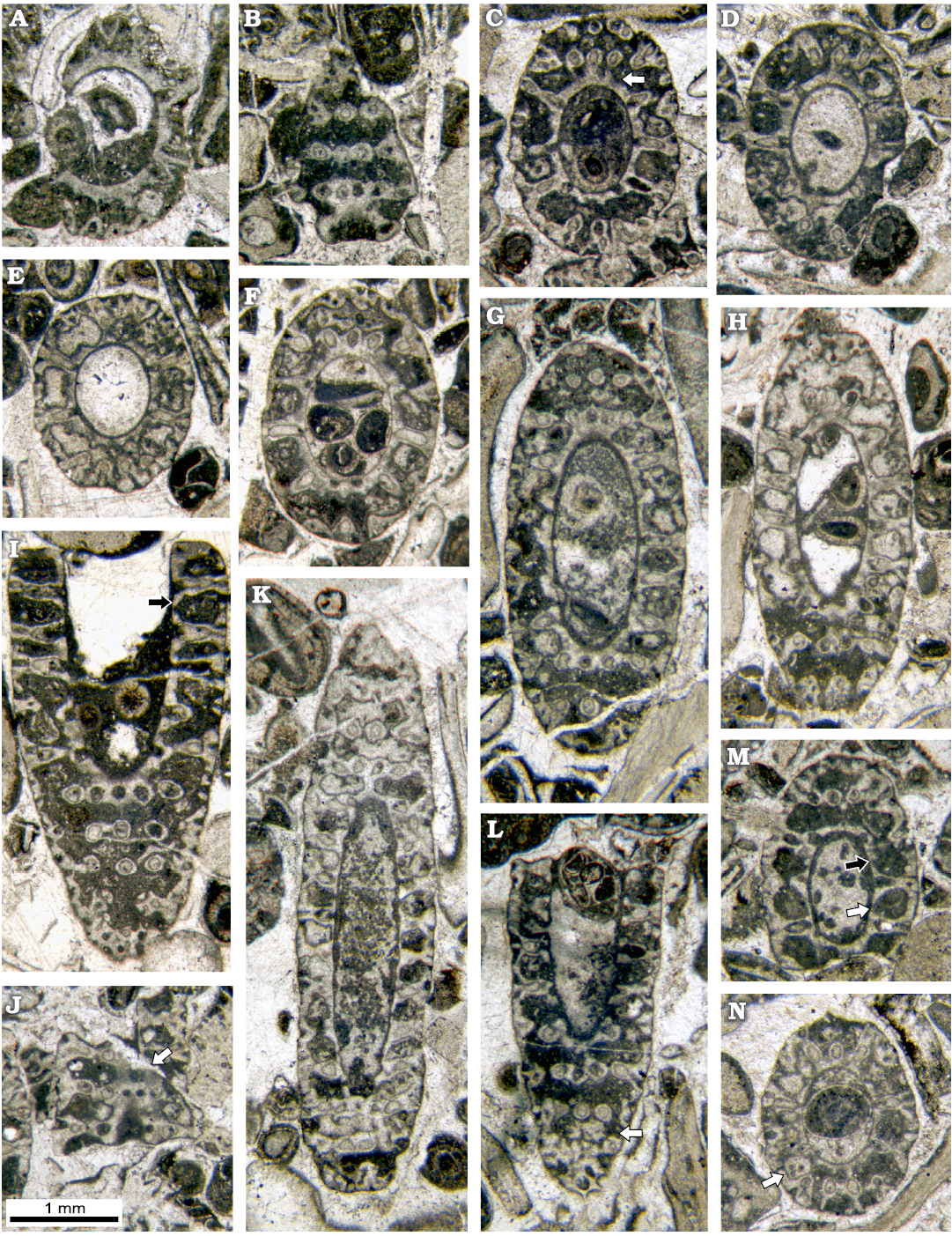
Fig. 8. Dasycladalean alga Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994 ) Granier, Masse and Berthou, 1994, emend. nov., upper Sinemurian (Lotharingian), Canders, 2.4 km E of Fontcaude (S France). A. LM-DiSTAR/BA.577.13, n. 034, oblique section showing a strong proximal sleeve lacking interverticillar pores. B. LM-DiSTAR/BA.577.14, n. 045, tangential-oblique section, note the interverticillar calcification lacking pores (below); a tuft of three secondary laterals is noticeable (up, left). C. LM-DiSTAR/BA.577.16, n. 057, oblique section; false pores can be observed just below the second whorl from the top (see arrow); note the regular inner cavity. D. LM-DiSTAR/BA.577.19, n. 081, oblique section showing a strong proximal sleeve lacking interverticillar pores. E. LM-DiSTAR/BA.577.27, n. 124, oblique section; note the regular interverticillar cavities easy to confound with fertile whorls. F. LM-DiSTAR/BA.577.42, n. 139, oblique section; presence of long akrophorous primary laterals is evident. G. LM-DiSTAR/BA.577.17, n. 069, oblique section. H. LM-DiSTAR/BA.577.7, n. 011, oblique section; note the regular interverticillar cavities (center of the section). I. LM-DiSTAR/BA.577.38, n. 136, oblique section; the interverticillar cavity looks like a large fertile primary lateral bearing three secondaries (see arrow). J. LM-DiSTAR/BA.577.7, n. 010, tangential-oblique section; presence of a large false pore is evident within the proximal calcareous sleeve (see arrow). K. LM-DiSTAR/BA.577.30, n. 129, oblique section. L. LM-DiSTAR/BA.577.21, n. 115, oblique section showing that large pores corresponding to secondary laterals are open (cortex, below), two calcified little bodies are visible between whorls (see arrow). M. LM-DiSTAR/BA.577.19, n. 096, oblique section; regular interverticillar cavities are visible, sometimes verging downward (black arrow) or upward (white arrow). N. LM-DiSTAR/BA.577.30, n. 109, oblique section; calcified, rounded to irregular bodies can be found between whorls (see arrow).
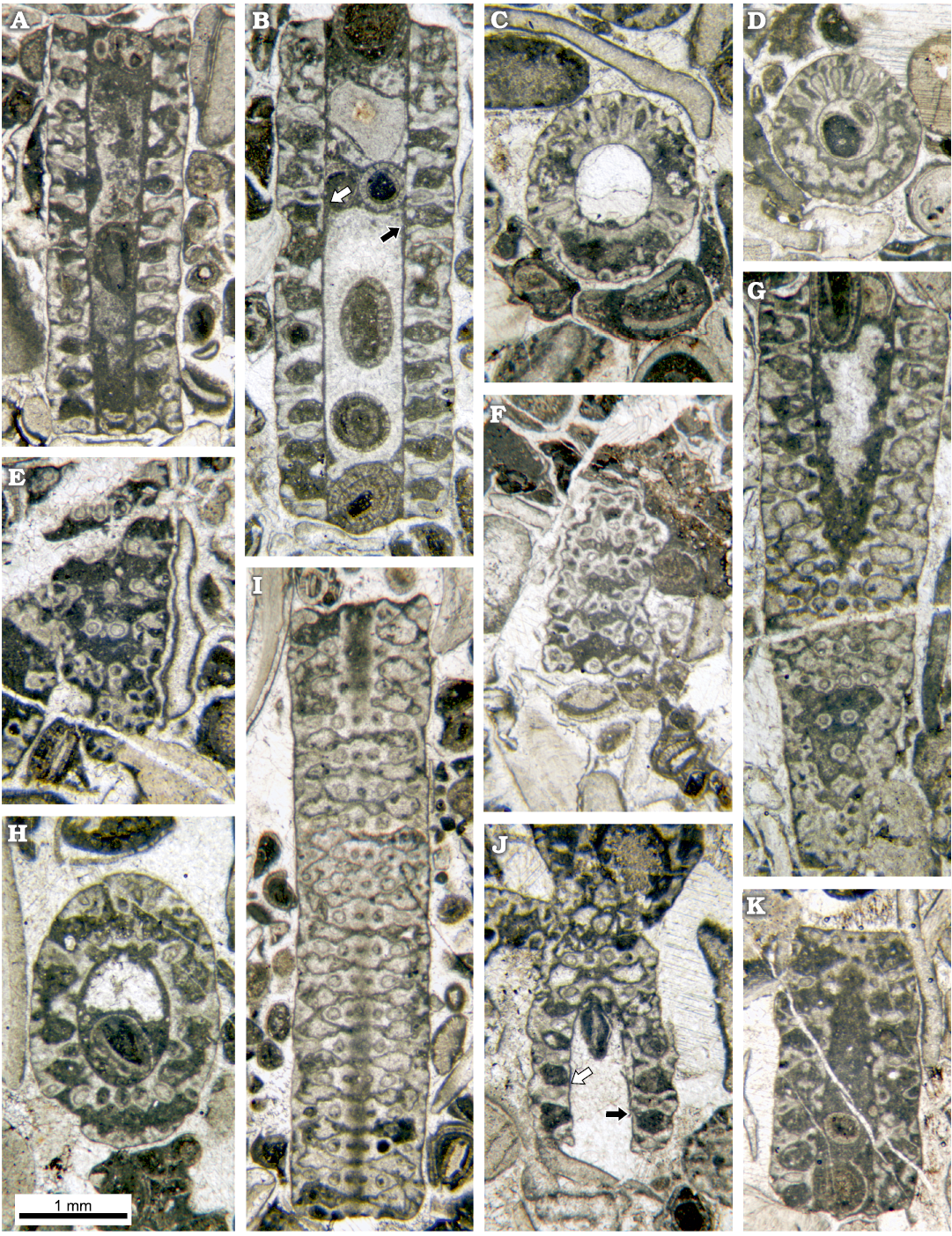
Fig. 9. Dasycladalean alga Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994 ) Granier, Masse and Berthou, 1994, emend. nov., upper Sinemurian (Lotharingian), Canders, 2.4 km E of Fontcaude (S France). A. LM-DiSTAR/BA.577.1, n. 002, longitudinal section; note the regular interverticillar cavities, easily confused with fertile whorls. B. LM-DiSTAR/BA.577.12, n. 029, axial section; note false pores verging downwards (black arrow) and upwards (white arrow). C. LM-DiSTAR/BA.577.17, n. 075, slightly oblique section, with an annular channel (below) and vertical channels between laterals of a whorl (up). D. LM-DiSTAR/BA.577.39, n. 137, transverse-oblique section showing a crenate annular channel and vertical channels between the laterals of a whorl. E. LM-DiSTAR/BA.577.17, n. 070, tangential section; note the irregular spaces between mineralized tufts of secondary laterals, most tufts showing pores forming the corners of a parallelogram. F. LM-DiSTAR/BA.577.34, n. 133, tangential section showing tufts of three to four secondary laterals; adjacent triplets show opposite vergence. G. LM-DiSTAR/BA.577.6, n. 008, oblique section; note false pores, faking fertile whorls alternating with the calcified primaries. H. LM-DiSTAR/BA.577.11, n. 026, oblique section. I. LM-DiSTAR /BA.577.37, n. 135, tangential section; pores of adjacent mineralized whorls alternate in position; space between whorls is either unbroken or crossed by a thin irregular slit. J. LM-DiSTAR/BA.577.16, n. 060, oblique section; note interverticillar cavities sometimes verging downward (black arrow) or upward (white arrow). K. LM-DiSTAR/BA.577.8, n. 012, longitudinal-oblique section; a small false pore is present in calcareous sleeve close to the central cavity (upper part).
Remarks.—The specimens indicated as Cylindroporella ellenbergeri Lebouché and Lemoine, 1963 by Nikler and Sokač (1968: pl. 1: 7, pl. 2: 3) from the Lower Jurassic of Mt. Velebit, Croatia differs from Chinianella ellenbergeri. The oblique section (Nikler and Sokač 1968: pl. 1: 7) shows only large, phloiophorous primary laterals vs elongated primary laterals and phloiophorous secondary laterals. The tangential section (Nikler and Sokač 1968: pl. 2: 3) shows close whorls of large primary laterals vs spaced whorls of elongated primary laterals. Therefore, the occurrence of Chinianella ellenbergeri in the Adria plate is questionable.
In the literature, besides the type species C. ellenbergeri, several species were assigned to Chinianella. Herein, related remarks are as follows:
Chinianella arabica (Elliott, 1957) Raviv and Lorch, 1992, was dated as Late Jurassic, and is a frequently quoted taxon (under the original Cylindroporella arabica). According to the illustrations supplied by the two authors, the species holds sterile primary laterals and gametophore in alternated whorls. The relationship between sterile laterals and gametophores (separated whorls or lateral gametophores) is not evident. However, the attribution to the Chinianella as proposed by Raviv and Lorch (1992) cannot be retained. The species may be tentatively assigned to the Otternstella Granier, 1994. Here, the new combination Otternstella arabica (Elliott, 1957) nov. comb. is proposed.
Chinianella carpatica (Bystrický, 1967) Granier, Masse, and Berthou, 1994 is dated as Late Triassic (Norian– Rhaetian). The species is a junior synonym of Heteroporella zanklii, according to Ott (1968, 1974), and Flügel (1975). However, the original combination Chinianella zanklii from 1967 was considered invalid by Granier et al. (1994), therefore, H. carpatica was considered the senior synonym. Herein, the species is assigned to Distefanopolia gen. nov.
Chinianella crosii (Ott, 1968) Granier, Masse, and Berthou, 1994 is dated as Late Triassic and questionably early Early Jurassic of southern Germany/Austria. The species was originally assigned to the genus Heteroporella Cros and Lemoine, 1966. It is found together with the species originally called Heteroporella zanklii. Herein, the species is assigned to Distefanopolia gen. nov.
Chinianella israelensis Raviv and Lorch, 1992, is dated as Oxfordian; the type specimen(s) are not indicated. Based on the author’s fig. 4 and pl. 8, the species may be provisionally assigned to the Bakalovaella Bucur, 1993.
Chinianella macropora (Di Stefano, 1981 ex Di Stefano and Senowbari-Daryan, 1985) Granier, Masse, and Berthou, 1994, is dated as Late Triassic (Norian), and was originally assigned to the Heteroporella. Quoting excerpts of the original diagnosis “Unsegmented egg-shaped thallus. Central stem with periodical constrictions. Two types of verticils alternate along the thallus axis: the first is composed of fertile globular branches, each supporting 6–7 secondary branches, the second consisting only of primary sterile branches” (Di Stefano and Senowbari-Daryan 1985: 193). Herein, the species is assigned to Distefanopolia gen. nov.
Chinianella micropora (Di Stefano and Senowbari-Daryan, 1985) Granier, Masse, and Berthou, 1994, is dated as Late Triassic (Norian), and was originally assigned to the Heteroporella. Quoting excerpts of the original diagnosis “…egg-shaped, simple thallus with a strongly intusannulated cavity. Two types of uniform verticils alternate along the thallus axis: a) globular, fertile branches with secondary branching and b) sterile branches of one order only” (Di Stefano and Senowbari-Daryan, 1985: 196). Herein, the species is designated as the type species of Distefanopolia gen. nov.
Chinianella scheympflugi Hofmann, 1994, emend. Schlagintweit, 2011, is dated as Kimmeridgian–Berriasian, the thallus is tripartite, with alternating rows of thick (fertile) and slender laterals in the main, fertile part of the thallus. Herein, the species is tentatively assigned to the genus Bakalovaella Bucur, 1993.
Chinianella zanklii Ott, 1967, is herein transferred to Distefanopolia gen. nov. See below.
Stratigraphic and geographic range.—Type locality and horizon (Lebouché and Lemoine, 1963) and related environs (Lespinasse 1982).
Tribe Neomereae Pia, 1920 emend. Bassoullet, Bernier, Conrad, Deloffre, and Jaffrezo, 1979
Genus Distefanopolia nov.
PFNR: PFN002699
Etymology: Dedicated to the colleague Pietro Di Stefano (University of Palermo, Italy) who first described the type species.
Type species: Heteroporella micropora Di Stefano and Senowbari-Daryan, 1985; Norian, Upper Triassic, Cozzo di Lupo, Sicily, Italy.
Species included (original spelling): Heteroporella macropora Di Stefano, 1981 ex Di Stefano and Senowbari-Daryan, 1985, Chinianella zanklii Ott, 1967, Chinianella crosii Ott, 1968, non 1967, Heteroporella carpatica Bystrický, 1967.
Diagnosis.—Cylindrical to egg-shaped thallus. Fertile and sterile whorls alternated. Fertile whorls with primary laterals bearing each a terminal gametophore which is set in the center of a tuft of phloiophorous secondary laterals forming a distal cortex. Sterile whorl with phloiophorous laterals of one or two orders of laterals forming a distal cortex.
Remarks.—Based on location of the reproductive organs and their arrangement relative to the sterile organs, an essential character in the systematics and evolution of the dasycladales, the Upper Triassic Distefanopolia gen. nov., principally resembles three genera, first being Cymopolia Lamouroux, 1816, whose type species is the Recent Cymopolia barbata (Linnaeus, 1758) Lamouroux, 1816, geographically present in the Atlantic. At least 16 species, herein tentatively considered valid, are ascribed to Cymopolia, including eight from the Cretaceous (Barremian–Maastrichtian), and eight from the Cenozoic (Paleocene–Recent). Both genera are choristoporate, with verticils of relatively thick first order laterals, bearing at their distal end a single gametangium (reproductive ampulla devoid of cysts), rather large, surrounded by a tuft of second-order laterals, the latter being distally enlarged and juxtaposed, forming an assimilating cortex. In the case of Distefanopolia gen. nov., the thallus is simple, cylindrical to egg-shaped, with alternated fertile and sterile whorls, while in Cymopolia it is either simple or branched, moniliform in shape. Whorls of sterile laterals are restricted to both ends of each article and fertile whorls do not alternate with those sterile. The second genus is Neomeris Lamouroux, 1816, with a simple thallus, fertile whorls constituted of primary laterals and secondary laterals surrounding a single gametophore (Berger and Kaever 1992). However, the new genus Distefanopolia differs from Neomeris because fertile whorls are alternated with sterile whorls and hold tufts of 4–8 secondary laterals vs characteristically two. The third genus, Eodasycladus Cros and Lemoine in Granier and Deloffre, 1994, comprises three species, all from the Lower Jurassic. Both Distefanopolia and Eodasycladus are choristoporate, with a simple thallus comprising whorls of rather small first-order laterals distally supporting a single gametophore. As shown by the type species Eodasycladus ogilviae Cros and Lemoine in Granier and Deloffre, 1994 (Cros and Lemoine 1966: fig. 9), whorls of sterile laterals are restricted to the lower part of the thallus and do not alternate with fertile whorls. Moreover, the second order laterals surround the gametophore and further ramify to segmented third order laterals forming a cortex at their distal extremity.
In the literature, several species referred to Chinianella share alternating whorls of fertile and sterile laterals, with sterile laterals commonly more numerous than the fertile laterals in their respective whorls (Granier et al. 1994: 132). The fertile whorl is designed as such to be made of an inflated primary lateral bearing several small secondary laterals. The sterile whorl, on the other hand, holds rather thin laterals with at least one order of ramification. In a matter of fact, there is evidence that the inflated primary laterals represent compound pores (sensu Barattolo 2019), such as in the Liassic Eodasycladus alanensis (Sokač, 2001) (Barattolo et al. 2012: fig. 3), denoting voids not related to organic but resulting from a deficiency of calcification. This occurs quite frequently in Chinianella micropora (Di Stefano and Senowbari-Daryan, 1985) but also in Chinianella carpatica (Bystrický, 1967) (Fig. 11). A detailed paleontological revision of the Late Triassic species of Chinianella deserves a specific study, which is out of the scope of this paper. For example, in the two specimens shown in Fig. 11, the “inflated fertile pore” clearly results of a deficiency of mineralization, around a short primary lateral bearing a terminal gametophore, the latter embraced by several secondary laterals, therefore corresponding to a Cymopolia-like structure. As a result, in oblique section, here and there when calcification is stronger, it is possible to observe the secondary laterals encircling a large, rounded gametophore.
The structure of four Late Triassic species formerly ascribed to Chinianella, namely Distefanopolia micropora (Di Stefano and Senowbari-Daryan, 1985) nov. comb., Distefanopolia zanklii (Ott, 1967) nov. comb., Distefanopolia carpatica (Bystrický, 1967) nov. comb. and Distefanopolia crosii (Ott, 1968) nov. comb. is sketched in Fig. 12A–D. The original orthography of “zankli” and “crosi” has been corrected to “zanklii” and “crosii” according to Art. 60.8.b of the Shenzhen Code (International Code of Nomenclature for algae, fungi, and plants). Certain authors (Granier et al. 1994; Granier and Grgasović 2000; Senowbari-Daryan et al. 2011) consider Distefanopolia zanklii (Ott, 1967 nov. comb., Fig. 12B) to be a junior synonym of Distefanopolia carpatica (Bystrický, 1967) nov. comb.; however, sterile whorls of the former species clearly display primary and secondary laterals vs. only primary laterals in the latter species. Consequently, Distefanopolia zanklii (Ott, 1967) is herein considered a valid species, apart from Distefanopolia carpatica (Bystrický, 1967).
Stratigraphic and geographic range.—Norian (Upper Triassic) to questionable lowermost Jurassic of the Tethyan realm (Austria, Czech Republic, Germany, Greece, Italy, Oman, Slovakia).
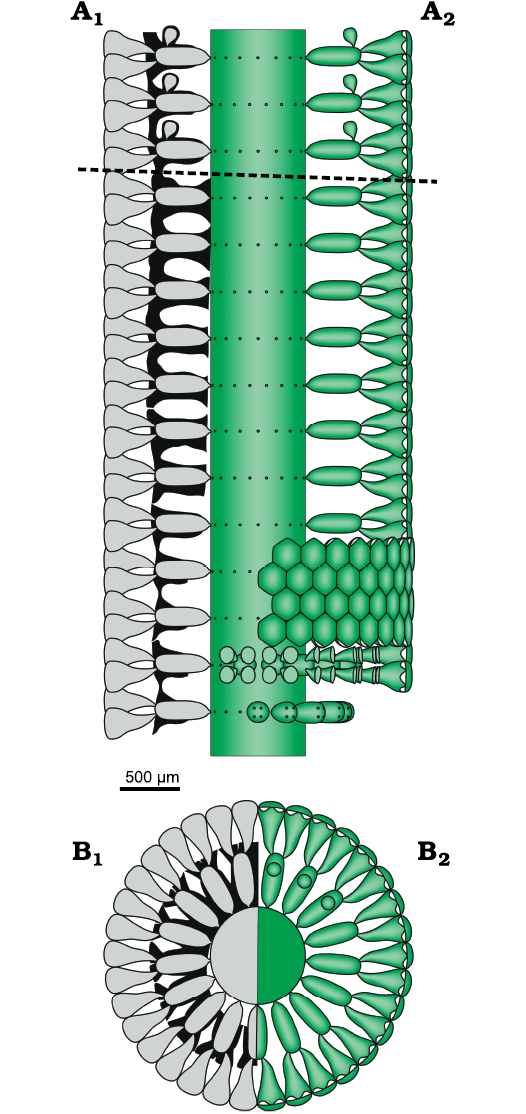
Fig. 10. Dasycladalean alga Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994 ) Granier, Masse and Berthou, 1994, emend. nov. A. Reconstruction of the alga in axial view; A1, calcified skeleton (black) and soft parts (grey) in axial section; A2, axial view of the soft parts (green); dotted line separates the sterile and fertile parts of the thallus. B. Reconstruction of the alga in transverse view. B1, sector of a whorl in transverse section showing the calcified skeleton (black) and reconstruction of the soft parts (grey); B2, upper view of whorl and sector of a whorl also showing gametophores (upper part); soft parts in green.
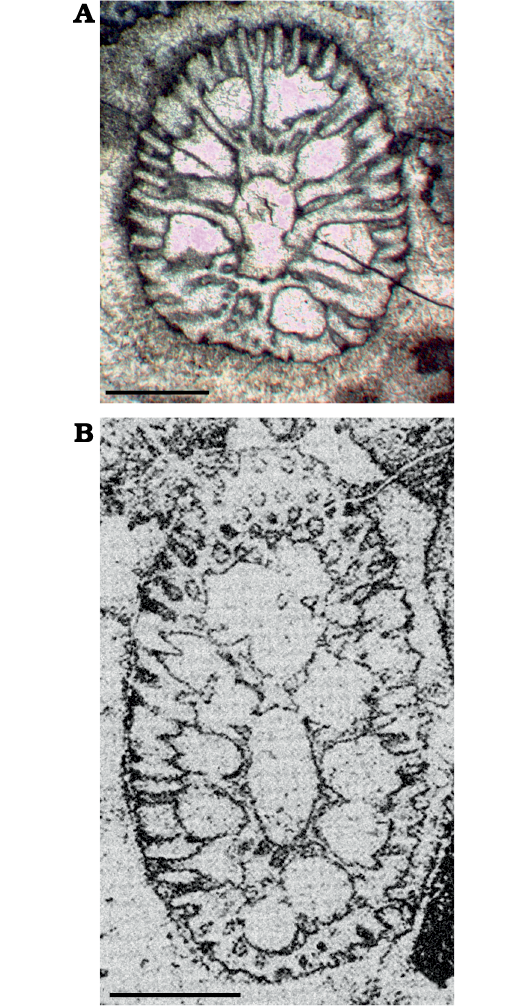
Fig. 11. The structure of the Late Triassic species of dasycladalean algae Distefanopolia gen. nov. A. Distefanopolia micropora (Di Stefano, 1981 ex Di Stefano and Senowbari-Daryan, 1985) nov. comb., MGG-PA/Si24ax, Norian, Cozzo di Lupo (Palermo, Sicily), oblique section; note the first fertile whorl (bottom) showing two tufts of about eight pores, the third pore aside displays the usual inflated shape. B. Distefanopolia carpatica (Bystrický, 1967) nov. comb., Norian, Muráň-PIateau (Gemer, Slovakia), oblique section, holotype, corresponding to Bistricky (1967: pl. 15: 3, thin section Nr. 2237), note the large, rounded gametophore encircled by several secondary laterals (below). Scale bars 1 mm.
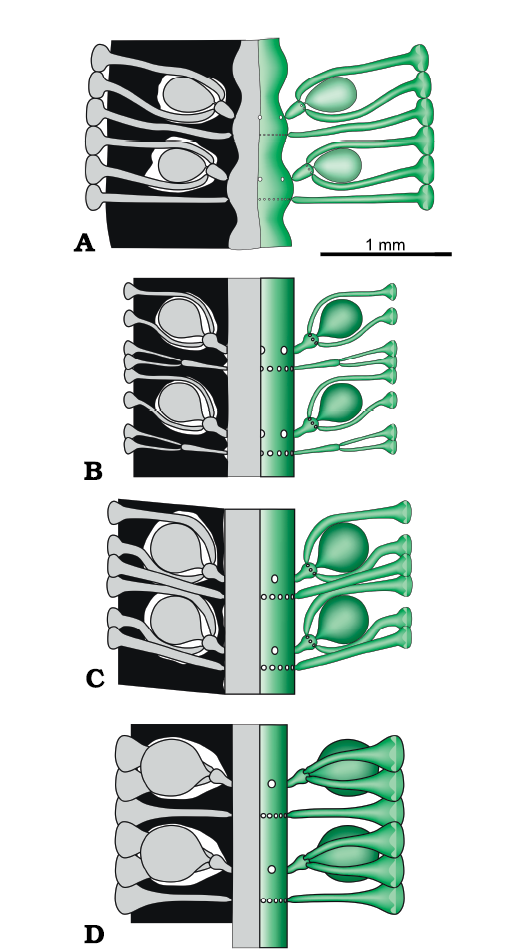
Fig. 12. Late Triassic species of dasycladalean alga Distefanopolia gen. nov. A. Distefanopolia micropora (Di Stefano, 1981 ex Di Stefano and Senowbari-Daryan, 1985) nov. comb. B. Distefanopolia zanklii (Ott, 1968) nov. comb. C. Distefanopolia carpatica (Bistricky, 1967) nov. comb. D. Distefanopolia crosii (Ott, 1968) nov. comb. Calcified skeleton (black) and soft parts (grey and green).
Discussion
Problems in detecting the structure of Chinianella ellenbergeri.—The mineralized skeleton often covers closely the central stem, the akrophorous primary laterals and the phloiophorous secondary laterals (Fig. 5). Cavities, however, may occur between the mineralized spaced whorls, forming irregular channels.
Moreover, different degrees of mineralization can be observed in the samples. Weakly mineralized thalli may display only the distal part of the first order laterals, whereas the secondary laterals are coated by lime (Figs. 5A, 6A–D). In other cases, mineralization is stronger, reaching the central stem, as shown by the regular contour of the inner cavity (Figs. 5A, B, 6E, F). In the space between mineralized whorls, the cavities form wide annular channels (Figs. 5C, 6G, I; see also Lebouché and Lemoine 1963: 93, 94, fig. 6). Often two subsequent annular channels communicate alongside vertical channels, running between laterals of the same mineralized whorl (Fig. 5A, B, 9A–D).
Mineralized parts and possible models.—Considering the mineralized structure of Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre 1994) Granier, Masse, and Berthou, 1994, emend. nov., four possible models are proposed (Fig. 3) and discussed as follows: (i) the alga consists of alternated whorls of weak and strong laterals (to be considered sterile and fertile respectively, Fig. 3A); these models imply the distance between whorls is one half of that between mineralized whorls; (ii) no fertile laterals occur between mineralized whorls (Fig. 3B, C); distance between the whorls is equal to the distance between whorls value. In this case, the voids between mineralized whorls were occupied by lateral gametophores (goniosporate reproduction, Fig. 3B) or they correspond to essentially unmineralized empty spaces (Fig. 3C). Admitting the model of Fig. 3B means that a persistent communication between the primary lateral and the corresponding gametophore must be found. Key sections examined below allow to validate the latter, most likely model.
Secondary laterals on fertile whorls.—In longitudinal and oblique sections, voids look sometimes open toward the exterior by mean of a thinner aperture (Fig. 9A, B). When the calcareous shield (Fig. 5C) is cut tangentially (Figs. 9E, F, 13D) these pores appear as irregular spaces between the mineralized tufts of secondary laterals. In the same sections, the mineralized tufts of secondaries belonging to adjacent whorls are fully in contact, leaving no space between them (Fig. 13B–D). This suggests that these patchy thin apertures result from an incomplete mineralization, rather than from the hypothetical presence of secondary laterals, arranged between the well-mineralized laterals. Yet, this hypothesis was implicitly excluded by Lebouché and Lemoine (1963: fig. 6).
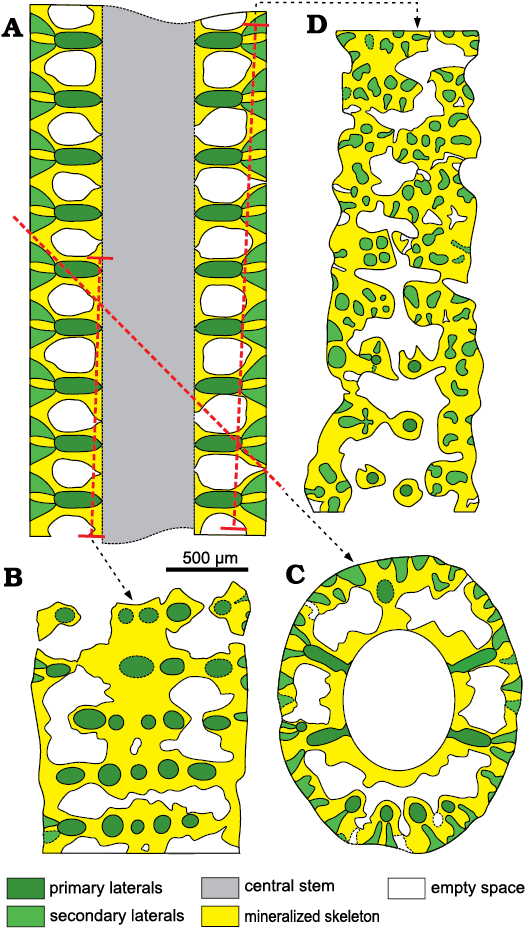
Fig. 13. Interpretation of voids and pores in the sterile specimens of dasycladalean alga Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994) Granier, Masse, and Berthou, 1994, emend. nov.; the meaning of the colors are the same as in Fig. 5. A. Axial view (general axial section). B. Proximal tangential section, first and second interverticillar spaces from the top are lacking pores, based on specimen in Fig. 9I, LM-DiSTAR /BA.577.37, n. 135, upper part. C. Oblique section, the proximal sleeve is missing interverticillar pores; based on specimen in Fig. 8E, LM-DiSTAR/BA.577.27, n. 124. D. Distal tangential section; note the interverticillar, irregular voids; based on specimen in Fig. 7K, LM-DiSTAR/BA.577.34, n. 132, middle–upper part.
Fertile primary laterals.—Several characters of the irregular voids between mineralized whorls apparently speak up for the occurrence of large globular (fertile) laterals in the sense put forward by Lebouché and Lemoine (1963): (i) well defined rounded pores joined to the central stem cavity by mean a small pore (Figs. 6E, 9A, G), which can be observed in longitudinal and oblique sections; (ii) the occurrence of inner and outer crenate surfaces, alternated with thinner mineralized primary laterals (Figs. 6I, 9H; see also Lebouché and Lemoine 1963: fig. 6); (iii) thin slits between mineralized whorls, hypothetically corresponding to the presence of a fertile whorl, as occasionally recorded (Fig. 9I), (iv) thin pores, hypothetically corresponding to the proximal stalk of fertile laterals, displaying a communication between the central stem cavity and the annular channel (Figs. 5C, 6I, 9G, K).
Despite the quite abundant material available, documenting the presence or the absence of fertile first order laterals is not easy for several reasons. Specimens with a thick and well mineralized central stem are rather rare, then, sections do not cut key areas suitably to unravel the problem and finally primary pores of the mineralized whorls are sometimes not well identifiable because filled by sparry calcite, whereas they are characterized by the presence of a thin micritic rim. However, few specimens with a stronger mineralized wall around the central stem display a well calcified interverticillar zone. Transversal-oblique sections and oblique sections cutting a calcified interverticillar tract display no pores, thus disproving the occurrence of fertile laterals in the interverticillar zone (Fig. 8A–D). Integer interverticillar tracts lacking pores are also visible in other oblique and tangential sections (Figs. 8F–H, J, 9I).
Lateral gametophores.—The wide empty spaces between mineralized whorls may hide the presence of a gametophore arising sideways from the mineralized primary whorl (Fig. 3B). Close to the central stem, some specimens (e.g., Figs. 8M, 9B, J) display pores alternately verging toward the nearby upper and lower mineralized whorl (see scheme of Fig. 5A). Assuming these pores represent the inner, proximal stalk of a gametophore attached to mineralized primary laterals (model as in Fig. 3B1), such a pattern cannot be concurrent with a vergence arising from the central stem (Fig. 3A2), the two explanations being mutually exclusive. Conversely, few sections show characters fitting with the model of Fig. 3B2. Mineralized primary laterals sometimes exhibit a small pore, opening in the empty space between whorls (Fig. 7K–N, P). Moreover, mineralized, rounded calcified bodies can be found between whorls (Fig. 7B), others look larger and irregular (Fig. 8L, N). Still, occasional but evident mineralized globular small bodies are attached near the outer end of the primary laterals (Figs. 6G, 7D, E, 14, 15) most likely corresponding to adjacent gametophores, denoting goniospory (Fig. 3B2). Furthermore, in the same specimens traces of gametophores are not ubiquitous (e.g., Fig. 15C).
Possible structure of Chinianella ellenbergeri.—The minimal solution (Fig. 3C) at first glance seems almost acceptable. It is based on factual characters left by the mineralization. This model leaves the type of reproduction undefined (possibly within the primary laterals or the central stem, cladosporate and endosporate, respectively). However, because of the structure of the well mineralized central stem, the model of Fig. 3A2, and consequently also that of Fig. 3A1, are excluded. The model depicted in Fig. 3B2 is well supported, but, objectively rarely applies. The structure shown in Fig. 15B–D is consistent with the presence of a gametophore attached sideways to the outer/distal part of the primary laterals (Fig. 15A). Possible, extant counterparts could be all the species of the genus Batophora Agardh, 1854, or at least all the species of the genus Bornetella Munier-Chalmas, 1877. This is the structure herein watchfully assigned to Chinianella ellenbergeri, along with the corresponding description.
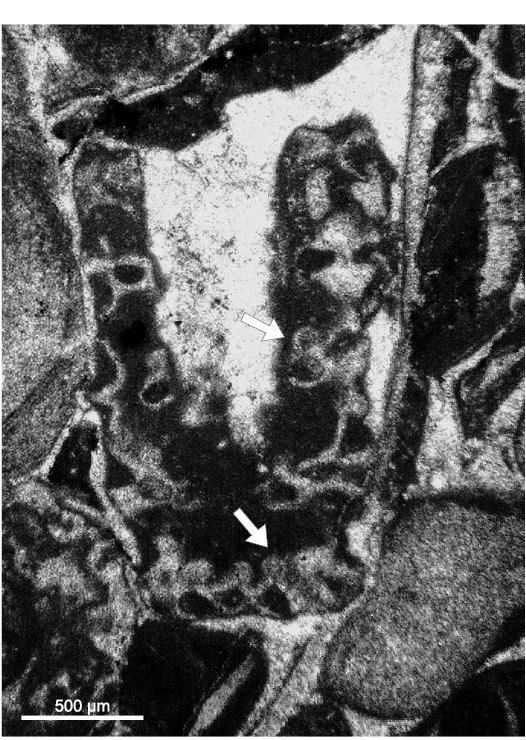
Fig. 14. Dasycladalean alga Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994) Granier, Masse, and Berthou, 1994, emend. nov., upper Sinemurian (Lotharingian), Canders, 2.4 km E of Fontcaude (S France). LM-DiSTAR/BA.577.b, n. 045 (lost specimen), oblique section showing the presence of reproductive structures (see arrows).
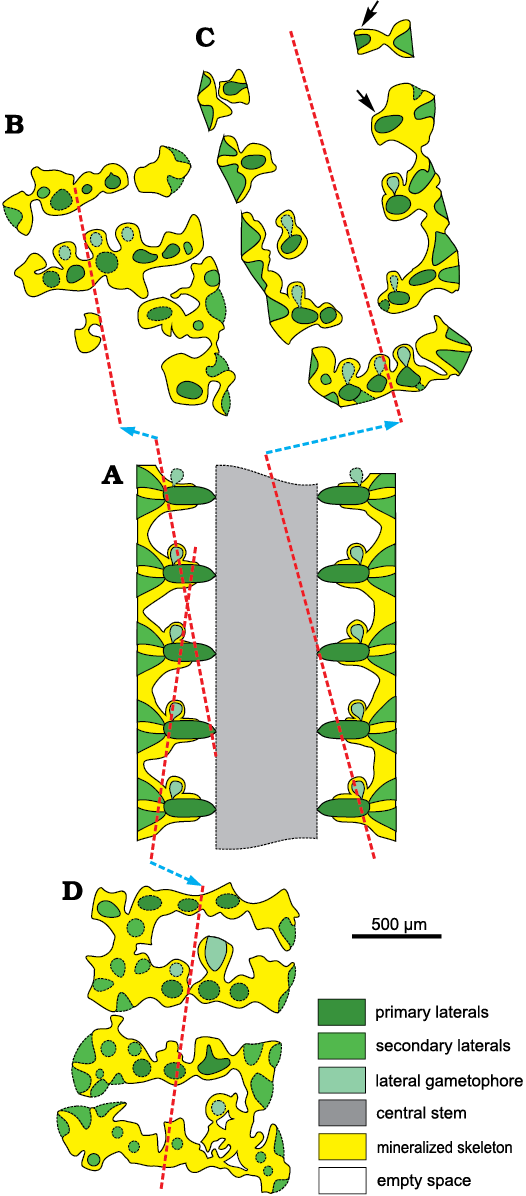
Fig. 15. Interpretation of voids and pores in fertile specimens of dasycladalean alga Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994) Granier, Masse, and Berthou, 1994, emend. nov.; colours as in Fig. 5. A. Axial view (general axial section). B. Tangential oblique section, based on specimen in Fig. 7D, LM-DiSTAR/BA.577.19, n. 095. C. Oblique section showing structures interpreted as reproductive organs, not all whorls display gametophores (see arrow); based on the specimen in Fig. 14, LM-DiSTAR/BA.577.b, n. 045. D. Tangential oblique section, based on specimen in Fig. 7E, LM-DiSTAR/BA.577.14, n. 040.
Conclusions
The mineralized cover preserving soft parts of the Dasycladales as an external mold is the only tool to infer the anatomical elements of these algae. Problems arise when voids within the calcareous skeleton do not reflect perfectly the original soft part, due to a loss of mineralization (Barattolo 2019). Therefore, such a lack of mineralization produces indeterminacy of the type and nature of the soft parts, resulting in a great impact on the corresponding systematics. Based on a rich material collected from the type-locality, a detailed study has been performed on Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994) Granier, Masse, and Berthou, 1994, emend. nov., type species of the Chinianella Ott, 1967 ex Granier, Masse, and Berthou, 1994 emend. nov. An initial interpretation of voids in the mineralization induced Lebouché and Lemoine (1963) to assume the alga was formed by an alternation of fertile and sterile whorls of laterals, four diverging secondary laterals arising from the distal end of the sterile laterals (Fig. 2). Our study shows that the large cavities originally interpreted as corresponding to fertile whorls, actually correspond to unmineralized empty spaces. Therefore, the alga essentially displays spaced whorls of sterile primary laterals, bearing distally a tuft of three-four phloiophorous secondary laterals (Fig. 10). Rare specimens show the occurrence of calcified structures herein accounted for reproductive organs (Figs. 14, 15). As drawn in Fig. 10, the mineralized specimens can be interpreted as corresponding to two different stages, sterile and fertile, of the life cycle of the alga. The occasional occurrence of reproductive organs may depend on several factors such as a low ratio of the fertile vs the sterile tracts and, possibly, a lower degree of mineralization of the gametophores. Consequently, an emended diagnosis is proposed for both the Chinianella Ott, 1967 ex Granier, Masse, and Berthou, 1994 and its type species Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994).
In the history of the Dasycladales (Barattolo 1991), the emergence of external reproductive organs (choristosporate type of reproduction sensu Pia (1920: 161) is a key innovation in the evolution of the Dasycladales. At the time of Pia (1920), this was placed in the Late Cretaceous, Neomeris cretacea Steinmann, 1899, being the classic example. Subsequently, terminal sporangia were discovered in Sinemurian (Early Jurassic) alga Eodasycladus ogilviae Cros and Lemoine in Granier and Deloffre, 1994 (Cros and Lemoine 1966). The same structure was also recorded in other Early Jurassic species previously assigned to Palaeodasycladus (Barattolo et al. 1994, 2012; Fig. 16D). Also, two dasycladaleans with lateral reproductive organs were documented in the Early Jurassic. The first one is Cylindroporella? liasica (Lebouché and Lemoine in Granier and Deloffre, 1994) (Barattolo and Parente 2000; Fig. 16A), and the second is Granieria iberica (Dragastan and Trappe, 1986) Barattolo and Romano in Barattolo et al., 2008 (Fig. 16C). The present, goniosporate interpretation of Chinianella (Fig. 16B) adds a new example, documenting the emergence of external reproductive organs early in the Mesozoic. Chinianella ellenbergeri and the taxa mentioned above confirm that the Early Jurassic was a phase where external reproduction was already well represented and diverse. Moreover, also the Late Triassic–earliest Jurassic species formerly attributed to Chinianella (namely C. carpatica, C. crosii, C. zanklii, C. micropora and C. macropora) reveal that the large, fertile primary lateral is a compound pore with a structure different from Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994) and closer to the Cymopolia Lamouroux, 1816, i.e., made of a short primary lateral with a terminal gametophore embraced by secondary laterals (Fig. 12). Thus, earlier, choristoporate ancestors are found in the Upper Triassic, as shown by the species of the herein introduced new genus Distefanopolia.
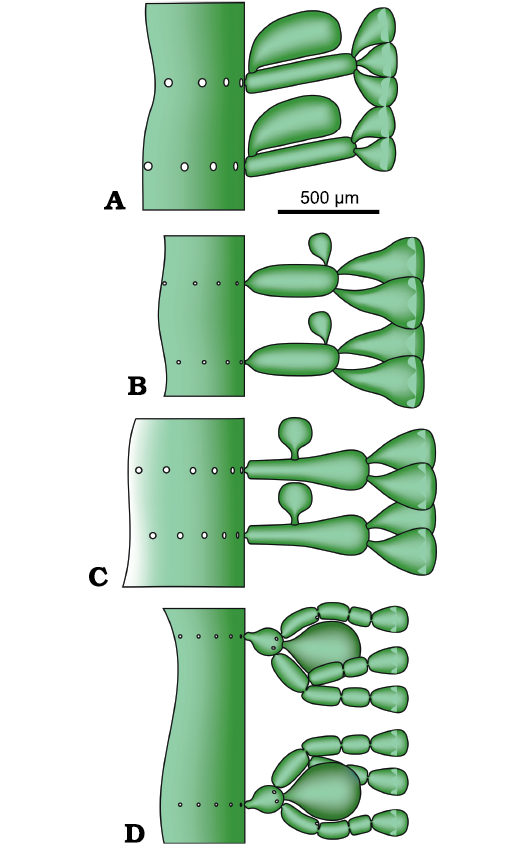
Fig. 16. Early Jurassic goniospore (A–C) and choristospore (D) Dasycladales. A. Cylindroporella? liasica (Lebouché and Lemoine in Granier and Deloffre, 1994), redrawn from Barattolo and Parente (2000). B. Chinianella ellenbergeri (Lebouché and Lemoine in Granier and Deloffre, 1994) Granier, Masse, and Berthou, 1994, emend. nov. C. Granieria iberica (Dragastan and Trappe, 1986) Barattolo and Romano in Barattolo et al., 2008, redrawn from Barattolo et al. (2008). D. Eodasycladus barrabei (Lebouché and Lemoine in Granier and Deloffre, 1994), redrawn from Barattolo et al. (2012).
Acknowledgements
We thank Ioan Bucur (Babeş-Bolyai University, Cluj-Napoca, Romania) and Bruno Granier (Université de Bretagne Occidentale, Brest, France) for their very helpful reviews of the manuscript. A sincere thanks to Giovanni Maria Furnari (Accademia Gioenia, Catania, Italy), John McNeill (Royal Botanic Garden Edinburgh, UK) and Patrick Stephen Herendeen (Chicago Botanic Garden, USA), members of the Nomenclature Committee for Algae of the International Code of Botanical Nomenclature for the useful indications. This work was supported by the University of Naples Federico II.
References
Agardh, C.A. 1828. Species algarum rite cognitae, cum synonymis, differentiis specificis et descriptionibus succinctis. Vol. II. lxxvi + 189 pp. Gryphiae Sumtibus Ernesti Mauritii, Greifswald.
Agardh, J.G. 1854. Nya algformer. Öfversigt af Kongliga Vetenskaps-Akademiens Förhandlingar 11: 107–111.
Barattolo, F. 1991. Mesozoic and Cenozoic marine benthic calcareous algae with particular regard to Mesozoic Dasycladaleans. In: R. Riding (ed.), Calcareous Algae and Stromatolites, 504–540. Springer-Verlag, Berlin. Crossref
Barattolo, F. 2019. Error vacuo, detecting structures in poorly calcified Jurassic Dasycladales. Iranian Journal of Geoscience Museum 1: 1–4.
Barattolo, F. and Parente, M. 2000. New observation on Uragiella liasica (Lebouché & Lemoine) (green algae, Dasycladales) from the type-locality (Canders, Languedoc-Southern France). Bolletino della Società Paleontologica Italiana 39: 123–137.
Barattolo, F., De Castro, P., and Parente, M. 1994. Some remarks on the genera Palaeodasycladus (Pia, 1920) Pia, 1927 and Eodasycladus Cros & Lemoine, 1966 ex Granier & Deloffre, 1994 (Green Algae, Dasycladales). Beiträge zur Paläontologie 19: 1–11.
Barattolo F., Granier, B., Romano, R., and Ferré, B. 2008. Petrascula iberica (Dragastan & Trappe), Tersella genotii Barattolo & Bigozzi, and the relationships of club-shaped dasycladalean algae during Late Triassic–Early Jurassic times. Geologia Croatica 61: 159–176.
Barattolo, F., Shirazi, M.P.N., and Vecchio, E. 2012. The genus Eodasycladus (Lower Jurassic dasycladalean green alga) and its relationship with Palaeodasycladus. Facies 58: 445–455. Crossref
Bassoullet, J.-P., Bernier, P., Conrad, M.A., Deloffre, R. and Jaffrezo, M. 1978. Les Algues Dasycladales du Jurassique et du Crétacé. Geobios, Mémoire Spéciale 2: 1–330.
Berger, S. and Kaever, M. 1992. Dasycladales, an Illustrated Monograph of a Fascinating Algal Order. vii + 247 pp. Georg Thieme Verlag, Stuttgart. Crossref
Bernier, P. 1984. Les formations carbonatées du Kimméridgien et du Portlandien dans le Jura méridional. Stratigraphie, micropaléontologie, sédimentologie. 803 pp. Thèse Doctorat ès Sciences naturelles, Documents des Laboratoires de Géologie de Lyon 92, Lyon.
Bucur, I.I. 1985. Sur la présence des cystes reproducteurs dans les ramifications de Macroporella praturloni et quelques considérations concernant l’évolution de la cladosporie chez les Dasycladales fossiles. In: N. Coman, S. Kiss, and T. Perseca (ed.), Evolutie si Adaptare, volume 2, 101–109. Universitatea Babeș-Bolyai, Cluj-Napoca.
Bucur, I.I. 1993. Some new or poorly known calcareous algae (Dasycladales, Gymnocodiaceae) in the Lower Cretaceous deposits from the Reşiţa Moldova Nouă zone (southern Carpathians, Romania). Revista Española de Micropaleontologia 25: 93–125.
Bucur, I.I., Granier, B., and Schlagintweit, F. 2010. Steinmanniporella, a new dasycladale genus name for “Linoporella” with two orders of laterals. Facies 56: 317–321. Crossref
Bystrický, J. 1967. Die obertriadischen Dasycladazeen der Westkarpaten. Geologický Sborník 18: 285–309.
Cros, P. and Lemoine, M. 1966. Dasycladacées nouvelles ou peu connues du Lias inférieur des Dolomites et de quelques autres régions mediterranéennes (1re partie). Revue de Micropaléontologie 9: 156–168.
Deloffre, R. 1988. Nouvelle taxonomie des Algues Dasycladales. Bulletin du Centre Recherches Exploration-Production Elf Aquitaine 12: 165–217.
Di Stefano, P. 1981. Cozzo di Lupo Reef. Late Triassic depositional model and role of algal communities. In: R. Catalano and B. D’Argenio (eds.), Paleogeographic Evolution of a Continental Margin in Sicily. Penrose Conference on Controls of Carbonate Platform Evolution. Guide book of the field trip in Western Sicily, 70–76, Palermo.
Di Stefano, P. and Senowbari-Daryan, B. 1985. Upper Triassic Dasycladales (green algae) from the Palermo Mountains (Sicily, Italy). Geologica Romana 24: 189–217.
Dragastan, O. and Trappe, J. 1986. Some dasyclad algae of the Sinemurian from the north-western Iberian Chains (Spain). Paläontologische Zeitschrift 60: 169–179. Crossref
Elliott, G.F. 1957. New calcareous algae from the Arabian Peninsula. Micropaleontology 3: 227–230. Crossref
Elliott, G.F. 1975. Transported algae as indicators of different marine habitats in the English Middle Jurassic. Palaeontology 18: 351–366.
Flügel, E. 1975. Kalkalgen aus Riffkomplexen der alpin-mediterranen Obertrias. Verhandlungen der Geologischen Bundesanstalt 1974: 297–346.
Fourcade, É., Jerez, L., Rodriguez, T., and Jaffrezo, M. 1972. El Jurasico terminal y el Cretacico inferior de la Sierra de la Muela (Provincia de Murcia). Consideraciones sobre las biozonas con Foraminiferos del Albense–Aptense del Sureste de España. Revista Española de Micropaleontologia, Numero Extraordinario, XXX Aniversario E.N. Adaro Special volume: 215–248.
Granier, B. 1994. The genus Actinoporella (Gümbel in Alth, 1881) and its representatives: a review. Beiträge zur Paläontologie 19: 113–127.
Granier, B. 2018. Cylindroporella sugdeni Elliott, 1957, an Early Cretaceous Middle Eastern Dasycladalean alga—a revision. Journal of Micropalaeontology 37: 181–190. Crossref
Granier, B. and Deloffre, R. 1994. Inventaire critique des Algues dasycladales fossiles. II partie: Les algues dasycladales du Jurassic et du Cretace. Revue de Paléobiologie 12: 19–65.
Granier, B. and Grgasović,T. 2000. Les algues Dasycladales du Permien et du Trias. Nouvelle tentative d’inventaire bibliographique, géographique et stratigraphique. Geologia Croatica 53: 1–197.
Granier, B., Dias-Brito, D., Bucur, I.I., and Tibana, P. 2013. Brasiliporella, a new mid-Cretaceous dasycladacean genus: the earliest record of the Tribe Batophoreae. Facies 59: 207–220. Crossref
Granier, B., Masse, J.-P., and Berthou, P.-Y. 1994. Heteroporella lepina Praturlon, 1967, revisited (followed by taxonomic notes on the so called “Heteroporella” species). Beiträge zur Paläontologie 19: 129–141.
Hauck, F. 1885. Die Meeresalgen Deutschlands und Oesterreichs. In: A. Grunow, F. Hauck, G. Limpricht, C. Luerssen, P. Richter, and G. Winter (eds.), Dr. L. Rabenhorst‘s Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz. 2, i–xxiii + 1–575. Verlag Eduard Kummer, Leipzig.
Hofman, T. 1994. Chinianella (?) scheympflugi, a new Dasyclad alga (green algae) from the Tithonian Ernstbrunn Limestone in Lower Austria. Beiträge zur Paläontologie 19: 143–147.
Kützing, F.T. 1843. Phycologia Generalis oder Anatomie, Physiologie und Systemkunde der Tange. 458 pp. F.A. Brockhaus, Leipzig.
Lamouroux, J.V.F. 1816. Histoire des Polypiers Coralligènes Flexibles, vulgairement nommés Zoophytes. lxxxiv + 558 pp. F. Poisson, Caën. Crossref
Lebouché, M.C. and Lemoine, M. 1963. Dasycladacées nouvelles du Lias calcaire (Lotharingien) du Languedoc méditerranéen (St-Chinian, Boutenac). Revue de Micropaléontologie 6: 89–101.
Lespinasse, P. 1982. Carte géologique de la France a 1/50000. Narbonne, 25–46. Notice explicative de la feuille Narbonne a 1/50000. 51 pp. Bureau de Recherches Géologiques et Minières, Paris.
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. 824 pp. Laurentii Salvii, Holmiae. Crossref
Michelin, H. 1840. Iconographie zoophytologique. Description par localités et terrains des Polypiers fossiles de France et pays environnants. 348 pp. P. Bertrand, Paris. Crossref
Masse, J.-P. and Bucur, I.I. 2002. Generic reappraisal of Angioporella? bakalovae Conrad & Peybernès (1978): Early Cretaceous Dasycladalean from the Carpatho-Balkanic region. In: I.I. Bucur and S. Filipescu (eds.), Research Advances in Calcareous Algae and Microbial Carbonates. Proceedings of the 4th IFAA Regional Meeting, Cluj-Napoca, 157–165. Cluj-Napoca.
Morellet, L. and Morellet, J. 1913. Les Dasycladacées du Tertiaire parisien. Mémoires de la Société Géologique de France 21, Mémoire 47: 1–43.
Morellet, L. and Morellet, J. 1922. Nouvelle contribution a l’étude des Dasycladacées tertiaires. Mémoires de la Société Géologique de France 25, Mémoire 58: 1–35.
Munier-Chalmas, E.P. 1877. Observations sur les algues calcaires appartenant au groupe des Siphonées verticillées (Dasycladées Harv.) et confondues avec les Foraminifères. Comptes Rendus de l’Académie des Sciences, Paris 85: 814–817.
Nikler, L. and Sokač, B. 1968. Biostratigraphy of the Jurassic of Velebit (Croatia). Geoloski Vjesnik 21: 161–176.
Ott, E. 1967. Dasycladaceen (Kalkalgen) aus der nordalpinen Obertrias. Mitteilungen der Bayerischen Staatssammlung für Paläontologie und Historische Geologie 7: 205–226.
Ott, E. 1968. Zur Nomenklatur obertriadischen Kalkalgen, besonders der Gattungen Heteroporella Praturlon und Poikiloporella Pia (Dasycladaceae). Mitteilungen der Bayerischen Staatssammlung für Paläontologie und Historische Geologie 8: 253–262.
Ott, E. 1974. Algae (Dasycladaceae). Catalogus Fossilium Austriae 17B: 1–64.
Pal, A.K. 1976. The algal family Dasycladaceae its taxonomy and evolution. In: Proceedings of the 6th Indian Colloquium on Micropaleontology and Stratigraphy, 154–181. Department of Geology, Banaras Hindu University, Varanasi.
Parente, M. 1997. Dasycladales from the upper Maastrichtian of Salento peninsula (Puglia, southern Italy). Facies 36: 91–122. Crossref
Pascher, A. 1931. Systematische Übersicht über die mit Flagellaten in Zusammenhang stehenden Algenreihen und Versuch einer Einreihung dieser Algenstämme in die Stämme des Pflanzenreiches. Beihefte zum Botanischen Centralblatt 48: 317–332.
Pia, J. 1912. Neue Studien über die triadischen Siphoneae verticillatae. Beiträge zur Paläontologie und Geologie von Österreich 25: 25–81.
Pia, J. 1920. Die Siphoneae verticillatae vom Karbon bis zur Kreide. Abhandlungen der Zoologisch-Botanischen Gesellschaft in Wien 11: 3–263. Crossref
Pia, J. 1927. Thallophyta. In: M. Hirmer (ed.), Handbuch der Paläobotanik. I: Thallophyta, Bryophyta, Pteridophyta, 31–136. R. Oldenbourg, München.
Pia, J. 1936. Description of the Algae. In: L.R. Rao and J. Pia (eds.), Fossil Algae from the uppermost Cretaceous beds (the Niniyur group) of the Trichinopoly district, S. India. Palaeontologia Indica 21: 13–40.
Praturlon, A. 1967. Heteroporella lepina, new dasyclad species from upper Cenomanian–lower Turonian of Central Apennines. Bollettino della Società Paleontolologica Italiana 5 (for 1966): 202–205.
Raviv, V. and Lorch, J. 1992. Mesozoic Dasycladaceae (Chlorophyceae) from Israel. Israel Journal of Botany 41: 1–54.
Schlagintweit, F. 2011. Morphological specification of Chinianella scheympflugi Hofmann, 1994, a Late Jurassic (?earliest Cretaceous) dasycladalean alga of the western Tethyan domain. Studia Universitatis Babes-Bolyai: Geologia 56: 3–9. Crossref
Senowbari-Daryan, B., Rashidi, K., and Saberzadeh, B. 2011. Dasycladalean green algae and some problematic algae from the Upper Triassic of the Nayband Formation (northeast Iran). Geologica Carpathica 62: 501–517. Crossref
Sokač, B. 2001. Lower and Middle Liassic calcareous algae (Dasycladales) from Mt. Velebit (Croatia) and Mt. Trnovski Gozd (Slovenia) with particular reference to the genus Palaeodasycladus (Pia, 1920) 1927 and its species. Geologia Croatica 54: 133–257.
Sokač, B. and Nikler, L. 1969. O vrstama roda Petrascula iz donjeg lijasa Velebita. Acta Geologica 6: 103–112.
Steinmann, G. 1899. Ueber fossile Dasycladaceen vom Cerro Escamela, Mexico. Botanische Zeitung 8: 137–154.
Valet, G. 1969. Contribution à l’étude des Dasycladales. 2 Cytologie et reproduction. 3 Révision systématique. Nova Hedwigia 17: 551–644.
Acta Palaeontol. Pol. 66 (4): 901–919, 2021
https://doi.org/10.4202/app.00883.2021