

Systematics, morphology, and appendages of an Early Ordovician pilekiine trilobite Anacheirurus from Fezouata Shale and the early diversification of Cheiruridae
FRANCESC PÉREZ-PERIS, LUKÁŠ LAIBL, MURIEL VIDAL, and ALLISON C. DALEY
Pérez-Peris, F., Laibl, L., Vidal, M., and Daley, A.C. 2021. Systematics, morphology, and appendages of an Early Ordovician pilekiine trilobite Anacheirurus from Fezouata Shale and the early diversification of Cheiruridae. Acta Palaeontologica Polonica 66 (4): 857–877.
Pilekiines are the earliest diverging members of the successful trilobite family Cheiruridae. The pilekiine genus Anacheirurus is characterized by sub-quadratic to sub-oval glabella, pitted genae, and a distinct trunk with elongated pleural spines in its posterior part. Anacheirurus adserai is a common component of the Fezouata Shale (Lower Ordovician, Morocco), where it was intially included into several species of the genus Lehua. This assignment and taxonomic over-splitting created confusion, overestimated cheirurid diversity at this locality, and simultaneously underestimated morphological variability within A. adserai. In this contribution we examine new material of A. adserai from the Fezouata Shale, clarifying its morphology and systematics. A detailed re-description of the species shows that Anacheirurus is distinct from Lehua, the latter being a more derived member of Cheiruridae. The comparison of Anacheirurus with other pilekiines shows that morphological variability within this subfamily is mostly constrained to the trunk region. Exceptionally preserved specimens of A. adserai from the Fezouata Shale show details of appendages, revealing the endopodite and exopodite morphologies in early members of Cheiruridae. The endopodite of A. adserai is unique among trilobites in possessing comparatively longer distal podomeres 5 and 6, but otherwise, it has the same general morphology as other described trilobite endopodites. The exopodite morphology of A. adserai shows characters typical of some Cambrian species but differs in several aspects from those known in post-Cambrian taxa. It is concluded that trilobite exopodite morphology was probably more variable than the endopodite morphology, which remains rather conservative across different taxa. Morphological diversity of trilobite exopodites in post-Cambrian taxa might be related to ecological escalations during the Ordovician biodiversification and the transition between Cambrian and Ordovician trilobite faunas.
Key words: Trilobita, Cheiruridae, Pilekiinae, biodiversification, ecology, endopodite, exopodite, Ordovician, Morocco.
Francesc Pérez-Peris [francesc.perezperis@unil.ch; ORCID: 0000-0002-4526-9308], Allison C. Daley [allison.daley@unil.ch; ORCID: 0000-0001-5369-5879], Institute of Earth Sciences, University of Lausanne, Géopolis, CH-1015 Lausanne, Switzerland.
Lukáš Laibl [lukaslaibl@gmail.com; ORCID: 0000-0001-9049-3811], Czech Academy of Sciences, Institute of Geology, Rozvojová 269, 165 00 Prague 6, Czech Republic; Institute of Geology and Palaeontology, Faculty of Science, Charles University, Albertov 6, Prague, 12843, Czech Republic.
Muriel Vidal [muriel.vidal@univ-brest.fr; ORCID: 0000-0003-3699-2083], Université de Brest, CNRS, IUEM Institut Universitaire Européen de la Mer, UMR 6538 Laboratoire Géosciences Océan, Place Nicolas Copernic, 29280 Plouzané, France.
Received 4 May 2021, accepted 9 September 2021, available online 25 November 2021.
Copyright © 2021 F. Pérez-Peris et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
With 440 Ordovician species, the family Cheiruridae Hawle and Corda, 1847, is one of the most diverse trilobite families during the Ordovician (Adrain 2013). The first record of Cheiruridae comes from Furongian (late Cambrian) of Siberia (Rosova 1960), while the last members went extinct during Givetian (Middle Devonian; Lane 1971; Přibyl et al. 1985; Feist 1991; Adrain 2013). Cheiruridae is currently sub-divided into eight subfamilies (Acanthoparyphinae, Cheirurinae, Cyrtometopinae, Deiphoninae, Eccoptochilinae, Heliomerinae, Pilekiinae, and Sphaerexochinae; Adrain 2013). Their validity is still under discussion and only Acanthoparyphinae, Deiphoninae, Sphaerexochinae, and Eccoptochilinae have been subjected to phylogenetic analyses (Adrain 1998; Congreve and Lieberman 2010, 2011; Gapp et al. 2012). The subfamily Pilekiinae Sdzuy, 1955, is stratigraphically the oldest group within cheirurid trilobites, with their record going as far back as Furongian (Rosova 1960), and surviving until the Darriwilian (Middle Ordovician; Fortey 1980; Edgecombe et al. 1999). Pilekiinae is the only cheirurid subfamily present in the Tremadocian strata and has a worldwide distribution. This subfamily represents one or several early-diverging lineages within Cheirurina (Reed 1896; Kobayashi 1934; Ross 1951; Harrington in Moore 1959; Lane 1971; Demeter 1973; Přibyl et al. 1985; Jell 1985; Peng 1990; Lee and Chatterton 1997) making them crucial for understanding the early evolutionary history and Ordovician biodiversification of Cheirurina.
The trilobite-rich sediments of the Fezouata Shale stratigraphically range from Tremadocian to Floian, Lower Ordovician (Lefebvre et al. 2018), and offer a perfect opportunity to explore the first steps of the Ordovician biodiversification of trilobites, including cheirurids. Of the 26 trilobite species belonging to 21 different genera that have been recorded in the Fezouata Shale (Martin et al. 2016b), one of the most common is the pilekiid Anacheirurus adserai (Vela and Corbacho, 2007). The species was originally described as Lehua adserai by Vela and Corbacho (2007), and during the following years several other species of Lehua Barton, 1915, from the same locality were erected (Vela 2007; Corbacho 2008; Corbacho and Vela 2011). Such an approach resulted in taxonomic over-splitting, with a total of seven species erected and, therefore, an overestimation of cheirurid diversity in Fezouata Shale. In their study of the trilobite assemblages from Fezouata, Martin et al. (2016b) found only one species of the taxon belonging to Lehua and assigned it to the Anacheirurus Reed, 1896. This approach was based on the work done by Whittard (1967), who considered Lehua as a junior synonym of Anacheirurus. However, several other authors disagreed and continued to consider them as two different genera (e.g., Lane 1971; Přibyl et al. 1985). Herein, we describe new material and advocate the generic assignment of Martin et al. (2016b), while also presenting a more detailed discussion of the differences between Lehua and Anacheirurus.
Another important aspect of the Fezouata Shale is that some levels preserve non-biomineralized fossils and body parts (e.g., Van Roy et al. 2010, 2015a, b; Saleh et al. 2020a, b). Among trilobites and trilobitomorphs, not only hard biomineralized parts are preserved, but also appendages and digestive structures are found (Martin et al. 2016b; Gutiérrez-Marco et al. 2017; Pérez-Peris et al. 2021). This offers a unique opportunity to understand the morphological diversification of trilobite soft parts during the Ordovician biodiversification. Unlike the dorsal parts of the exoskeleton, trilobite appendages were only slightly sclerotized (Hughes 2003) and for that reason, they are extremely rare in the fossil record. One of the first reports of trilobite appendages was made by Billings (1870) who found appendages in the genus Isotelus Dekay, 1824. Since then, numerous works have described appendages (e.g., Beecher 1893; Walcott 1918; Raymond 1920; Broili 1930; Størmer 1939, 1951; Seilacher 1962; Bergström 1972, 1973; Stürmer and Bergström 1973; Cisne 1975, 1981; Whittington 1975, 1980; Whittington and Almond 1987; Bruton and Haas 1999; Gutiérrez-Marco et al. 2017; Holmes et al. 2020) in several trilobite species (see Zeng et al. 2017: table 1 for a comprehensive summary). Although the general appendage morphology is comparatively well known, several questions remain unresolved. From all the work carried out on trilobite appendages during more than one century, there is information available for only about 30 trilobite species, from more than 19 000 erected species (cf. Adrain 2011; Zeng et al. 2017; Holmes et al. 2020). Moreover, 18 of the species with appendages preserved are known solely from Cambrian Konservat-Lagerstätten with Burgess Shale-type preservation of carbonaceous compressions (Butterfield 2003; Gaines et al. 2008; Daley et al. 2018). Data from post-Cambrian taxa are much rarer, as they were usually preserved under different conditions than during the Cambrian (Butterfield 1995; Daley et al. 2018). Ceraurus pleurexanthemus Green, 1832, from the Trenton Group (Upper Ordovician, New York State, USA) is the only member of the family Cheiruridae with appendages preserved in the fossil record (Størmer 1939, 1951), despite the huge diversity of the family. C. pleurexanthemus is also one of the few trilobites from the Ordovician with a more complete appendage record, together with Triarthrus eatoni (Hall, 1838) and Cryptolithus bellulus (Ulrich, 1879). This knowledge gap exists not only for cheirurid trilobites but for all Ordovician trilobites in general. New information about trilobite appendages is required to understand the post-Cambrian evolution and ecology of the group.
Here, we present a first comprehensive description of the morphology of the pilekiine Anacheirurus adserai from the Fezouata Shale, based on numerous well-preserved specimens. The late post-embryonic development of this species is also briefly described. We clarify the taxonomy of A. adserai and describe the morphology of its appendages. This work provides new insight into the morphological variability, biodiversification, and appendage evolution within Pilekiinae, and helps to clarify the early evolutionary steps of Cheiruridae.
Institutional abbreviations.—GMSB, Geological Museum of the Seminary of Barcelona, Spain; JV, Czech Geological Survey, Prague, Czech Republic; MGB, Museum of Natural Sciences of Barcelona, Spain; MGL, Musée cantonal de Géologie de Lausanne, Switzerland; ML, Natural History Museum of Lyon, France; NM L, National Museum, Prague, Czech Republic; YPM, Yale Peabody Museum of Natural History, New Haven, USA.
Other abbreviations.—exsag., exsagittal; L1, L2, L3, glabellar lobes one to three; SO, S1, S2, S3, occipital furrow and glabellar furrows one to three, respectively; sag., sagittal; tr., transversal; Tr3, third stage slice of the Tremadocian.
Geological setting
The Lower Ordovician of the Anti-Atlas region in Morocco belongs to the Outer Feijas Group. The Outer Feijas Group is subdivided into the Lower and Upper Fezouata formations, the Zini Formation, and the Tachilla Formation, and comprise a succession stratigraphically ranging from Tremadocian to lower Darriwilian (Choubert et al. 1947; Destombes et al. 1985). In the Zagora region, the Lower and Upper Fezouata formations are grouped into a single unit called the Fezouata Shale, because the boundary between both units is unclear (Martin et al. 2016b). The Fezouata Shale is an approximately 850 m thick succession composed of argillites with sandy mudstone and siltstone beds (Destombes et al. 1985). The depositional environment facilitated a rapid burial of autochthonous communities in an open shallow marine environment (Martin et al. 2016a), ranging from offshore to the foreshore with a depth range from 50 to 150 m (Vaucher et al. 2016; Martin et al. 2016a).
The Fezouata Shale contains a Burgess Shale-type Konservat-Lagerstätte, the Fezouata Biota, that is renowned for its exceptional preservation of soft, cuticularized, and lightly sclerotized parts of a highly diverse marine biocenosis (Van Roy et al. 2010, 2015a, b: Saleh et al. 2020b). The Fezouata Biota provides a critical link between the Cambrian Explosion and the Great Ordovician Biodiversification Event, due to its temporal position between both events (Servais et al. 2010; Landing et al. 2018; Servais and Harper 2018). Exceptional preservation in the Fezouata Shale is restricted to two intervals (Martin et al. 2016a; Lefebvre et al. 2018), the lower of which is about 70 m thick and situated 260–330 m above the Cambrian/Ordovician contact, and the second of which is about 50 m thick and 570–620 m above the Cambrian/Ordovician contact. The lower interval with exceptional preservation is situated mostly within the Araneograptus murrayi Zone and lowermost parts of the Hunnegraptus copiosus Zone, which both correspond to the third slice of the Tremadocian stage (or Tr3, see Gutiérrez-Marco and Martin 2016; Lefebvre et al. 2018). This age is further corroborated by acritarchs and conodonts (Lehnert et al. 2016; Nowak et al. 2016). The upper interval with exceptional preservation most likely belongs to the ?Baltograptus jacksoni Zone, which is of Floian age (Lefebvre et al. 2018), but is stratigraphically less well understood.
Material and methods
Material examined includes over 50 specimens of Anacheirurus adserai from the Fezouata Shale. All specimens come from the lower fossiliferous interval of the Araneograptus murrayi Zone (upper Tremadocian, Lower Ordovician). Specimens are held at the Musée cantonal de Géologie de Lausanne, Switzerland, the University of Lyon (France), the Natural History Museum of Lyon (France), the University of Brest (France), and the Yale Peabody Museum of Natural History (New Haven, USA). Figured specimens are held in the Musée cantonal de Géologie de Lausanne, Switzerland (MGL 102153, 102170, 102172, 102179, 102225, 103863, 104146, 104533), Yale Peabody Museum of Natural History (YPM 226573, 517074, 522182, 525125, 530933) and the Natural History Museum of Lyon (ML20-269198). Specimens of Lehua vinculum (Barrande, 1852) from the Dobrotivá Formation (upper Darriwilian–lowermost Sandbian, Middle–Upper Ordovician) of the Czech Republic that are examined and figured here are held in the National Museum, Prague (NM L 19066 and 19075) and the Czech Geological Survey (JV 1607).
Specimens were photographed with a Canon EOS 800D camera with an associated CANON MACRO LENS MP-E 65 mm 1:2.8 1-5Xlens. The lens was equipped with a polarizing filter to reduce reflections, and a second polarizer on the light source created crossed polarization to increase contrast. Specimens were photographed with low-angle NW lighting or high light from directly above the specimens. Some specimens were covered by ammonium chloride before photography, to enhance their topography. Specimens MGL 102172, 102225, and 103863, were photographed submerged in ethanol to improve the appendages contrast. The images were processed in Adobe Photoshop CC 19.0, to enrich brightness, contrast, shadows, highlights, and saturation. Line drawings were made directly from photographs using Adobe Illustrator CC 22.01, like a digital camera lucida (Antcliffe and Brasier 2011).
Systematic palaeontology
Order Phacopida Salter, 1864
Suborder Cheirurina Harrington and Leanza, 1957
Family Cheiruridae Hawle and Corda, 1847
Subfamily Pilekiinae Sdzuy, 1955
Genera included.—Anacheirurus Reed, 1896, Chashania Lu and Sun in Zhou et al., 1977, Courtessolium? Přibyl and Vaněk in Přibyl et al., 1985, Emsurina Sivov in Egorova et al., 1955, Eocheirurus Rozova, 1960, Koraipsis Kobayashi, 1934, Landyia Jell, 1985, Macrogrammus Whittard, 1966, Metapilekia Harrington, 1938, Metapliomerops Kobayashi, 1934, Parapilekia Kobayashi, 1934, Pilekia Barton, 1915, Pseudopliomera Lu and Qian in Yin and Li, 1978, Seisonia Kobayashi, 1934, Sinoparapilekia Peng, 1990, Tesselacauda Ross, 1951, Tienshihfuia Lu in Lu et al., 1976, Victorispina Jell, 1985, Yinaspis Zhang and Fan, 1960.
Emended diagnosis.—Cheiruridae with thoracic pleura divided into anterior and posterior bands by a deep, long (tr.) pleural furrow; broad (tr.) proximal part of thoracic pleura with fulcrum situated distally 2/3 from the axis; pygidium with at least two first pleural tergites displaying a deep pleural furrow that divide the pleura into anterior and posterior bands, as in the thoracic pleura.
Remarks.—Pilekiinae has been considered as a subfamily of Pliomeridae (Harrington in Moore 1959; Vaněk 1965; Demeter 1973; Peng 1984; Mergl 1984), as a subfamily of Cheiruridae (Fortey 1980; Přibyl et al. 1985; Edgecombe et al. 1999; Adrain 2013; Adrain and Karim 2019) or as a separate family Pilekiidae (Sdzuy 1955; Whittington 1961; Jell 1985; Peng 1990; Lee and Chatterton 1997; Ebbestad 1999; Jell and Adrain 2002; Pärnaste 2004; Mergl 2006). In this paper, we consider pilekiids as a subfamily of Cheiruridae. This suggestion is based on Adrain and Karim (2019), who have shown that pilekiids and the rest of cheirurids share a similar morphology of the rostral plate and the hypostome.
Genus Anacheirurus Reed, 1896
Type species: Cheirurus (Eccoptochile) frederici Salter, 1864; Tremadocian Slates; Wales, upper Tremadocian, Upper Ordovician.
Species included: Anacheirurus frederici (Salter, 1864); Anacheirurus adserai (Vela and Corbacho, 2007); Anacheirurus nanus (Mergl, 1984); Anacheirurus bohemicus (Růžička, 1926); Anacheirurus? plutonis Bulman and Rushton, 1973; Anacheirurus? atecae (Hammann, 1971); Anacheirurus? sougyi (Destombes in Destombes et al., 1969).
Diagnosis.—A genus of Pilekiinae with glabella sub-quadratic to sub-oval in shape, with smooth surface; pleura deeply furrowed, with posterior band extended distally in a spine of variable length; posterior tergites of the trunk bear elongated spines directed backward; pygidium with two to three axial rings, and with one to three pair of pygidial spines; pygidial pleurae furrowed with a deep furrow as in the thoracic pleurae, pygidial spines oriented mainly backward.
Remarks.—Anacheirurus has been recognized as bearing three pairs of pygidial spines (Bulman and Rushton 1973; Fortey 1980; Peng 1990; Mergl 2006). However, the type species of the genus, Anacheirurus frederici, probably bears only two pairs of pygidial pleural spines (Lane 1971). Anacheirurus adserai, a species otherwise morphologically nearly identical to A. frederici, bears only one pair of pygidial pleural spines. The number of pleural spines allocated within pygidium seems to be a highly variable character in early members of Cheiruridae, and consequently of little use when defining genera or even higher taxa. On the other hand, both A. frederici and A. adserai share the same overall morphology of the trunk, with the last seven pairs of spines elongated. Considering the uniqueness of this character within Pilekiinae and morphological similarity between these two species, it seems likely they are both form a monophyletic group. For that reason, we suggest using these elongated posterior spines as a diagnostic character of Anacheirurus, being aware this might restrict the original concept of the genus (and consequently the number of species assigned to it). Except for A. frederici and A. adserai, the remaining species assigned to Anacheirurus lack the thoracic information. We decided to keep them within the genus until the thoracic information is available.
Anacheirurus adserai (Vela and Corbacho, 2007)
Figs. 1, 2A, B, 3–7.
?1969 Parapilekia sougyi n. sp.; Destombes in Destombes et al. 1969: 192, text-fig. 6, pl. 4: 7–11.
1985 Parapilekia sp.; Destombes in Destombes et al. 1985: 189.
2006 Parapilekia sp.; Destombes 2006: pl. 2: 5.
2007 Anacheirurus frederici (Salter, 1864); Vela 2007: 27.
2007 Lehua adserai n. sp.; Vela and Corbacho 2007: text-figs. 3–6.
2007 Lehua corbachoi n. sp.; Vela 2007: 28, text-figs. 2, 3.
2007 Lehua colli n. sp.; Vela 2007: 29, text-fig. 4.
2007 Lehua ponti n. sp.; Vela 2007: 30, text-figs. 5–7.
2008 Anacheirurus frederici (Salter, 1864); Corbacho 2008: 4.
2008 Lehua adserai Vela and Corbacho, 2007; Corbacho 2008: 4.
2008 Lehua corbachoi Vela, 2007; Corbacho 2008: 4.
2008 Lehua colli Vela, 2007; Corbacho 2008: 4.
2008 Lehua ponti Vela, 2007; Corbacho 2008: 4.
2008 Lehua velai n. sp.; Corbacho 2008: 5, text-figs. 1–3.
2008 Lehua iannacconnei n. sp.; Corbacho 2008: 6, text-fig. 4.
2008 Lehua tahirii n. sp.; Corbacho 2008: 7, text-fig. 7.
2010 Lehua vinculum (Barrande, 1852); Bonino and Kier 2010: 290, pl. 84: a.
2010 Lehua sp.; Bonino and Kier 2010: 290, pl. 84: b.
2010 Lehua corbachoi Vela, 2007; Bonino and Kier 2010: 290, pl. 84: c.
2010 Lehua velai Corbacho, 2008; Bonino and Kier 2010: 290, pl. 84: d.
2010 Lehua ponti Vela, 2007; Bonino and Kier 2010: 290, pl. 84: e.
2011 Lehua adserai Corbacho and Vela, 2007; Corbacho and Vela 2011: 48, pl. 1: 1; 49, pl. 2: 6.
2011 Lehua corbachoi Vela, 2007; Corbacho and Vela 2011: 48, 49, pl. 1: 2; pl. 2: 2, 4, 7.
2011 Lehua tahirii Corbacho, 2008; Corbacho and Vela 2011: 48, 49, pl. 1: 3; pl. 2: 8, 9.
2011 Lehua velai Corbacho 2008; Corbacho and Vela 2011: 48, 49, pl. 1: 4; pl. 2: 1, 3, 5.
2015 Lehua adserai Corbacho and Vela, 2007; Van Roy et al. 2015a: 546.
2015 Lehua corbachoi Vela, 2007; Van Roy et al. 2015a: 546.
2015 Lehua tahirii Corbacho, 2008; Van Roy et al. 2015a: 546.
2015 Lehua velai Corbacho, 2008; Van Roy et al. 2015a: 546.
?2015 Parapilekia sp.; Van Roy et al. 2015a: 546.
2015 Lehua adserai Corbacho and Vela, 2007; Valent and Corbacho 2015: 51.
2015 Lehua corbachoi Vela, 2007; Valent and Corbacho 2015: 51.
2015 Lehua tahirii Corbacho, 2008; Valent and Corbacho 2015: 51.
2015 Lehua velai Corbacho, 2008; Valent and Corbacho 2015: 51.
2016 Anacheirurus adserai (Vela and Corbacho, 2007); Martin et al. 2016b: 149, figs. 2, 3, 5, table 1.
2018 Lehua corbachoi Vela, 2007; Lebrun 2018: 107, fig. D.
Type material: Holotype: GMSB 73845, complete specimen (selected by Vela and Corbacho 2007). Paratype: MGB 46755, complete specimen (selected by Vela and Corbacho 2007).
Type locality: Fezouata Shale in the central Anti-Atlas of Morocco, presumably located north of the city of Zagora. The original material doubtfully assigned to the locality “Tanssikhte” (see section Remarks for details).
Type horizon: Fezouata Shale, Upper Tremadocian (Tr3) (Araneograptus murrayi Zone), Lower Ordovician. The type locality was incorrectly assigned by Vela and Corbacho (2007) stratigraphically to the Upper Fezouata Shale, “lower–middle Arenig” (= Floian age). See section Remarks for details.
Material.—Assigned specimens MGL 102153, MGL 102170, MGL 102172, MGL 102179, MGL 102225, MGL 103863, MGL 104146, MGL 104533, YPM 226573, YPM 517074, YPM 522182, YPM 525125, YPM530933, ML20-269198. All the material come from the Fezouata Shale, Upper Tremadocian (Tr3), Araneograptus murrayi Zone, Lower Ordovician.
Description.—Exoskeleton: Exoskeleton micropygous (Fig. 1A, B, C), ovoid to sub-ovoid in outline (excluding spines) (Fig. 1A1, C). The sagittal length of the observed holaspid individuals ranges from 5.6 mm (MGL 101262) to 35 mm (YPM 522182). Cephalon semi-elliptical in outline, relatively short (sag.), length/width ratio ranges from 1.04 to 0.88. Glabella sub-quadratic in shape, parallel-sided, slightly tapering anteriorly. Anterior glabellar margin bowed anteriorly. Posterior glabellar margin slightly bowed anteriorly medially. Three pairs of well-defined lateral glabellar furrows (Fig. 1C). S1 deeper, longer (tr.), and wider (sag.) than S2 and S3, directed inwards and slightly backwards, adaxially strongly curved backwards, not connected with the occipital furrow. S2 and S3 sub-parallel, directed inwards and slightly backwards. L1 sub-oval in shape, L2 and L3 rectangular in shape. Frontal lobe wider (tr.) than longer (sag.), with an inverted triangular shape. Occipital ring sub-rectangular in shape, slightly narrower (tr.) than the glabella, longer (sag.) in medial part, tapering distally, the medial part of anterior margin slightly convex anteriorly. Anterior border short (sag.), slightly bowed anteriorly almost transversal. Anterior border furrow narrow (sag.), relatively shallow, of the same depth as the axial furrow. Posterior border furrow long (sag.), deeply incised. Posterior border short (sag.) proximally, expanding (sag.) gradually distally, expanding abruptly beyond the medial region of the fixigenal field, wider at the genal angle, distally curving forward and slightly inward. Lateral border widest (tr.) next to the genal angle, tapering forward. Genal spines developed at a genal angle, relatively long, directed backwards, thick at the base, and tapering backward. Fixigenal field sub-triangular in shape, with narrow (tr.) anterior part triangular in shape and wide (tr.) rectangular posterior part. The anterior part of the fixigenal field crossed by the eye ridge. Librigenal field small with an elongated triangular shape, outer margin convex outwardly. Eye lobe semi-circular in shape, outwardly convex, narrow (Fig. 1A1). Fixigenal and librigenal fields densely sculpted by small pits equally distributed around the surface. Small palpebral lobe, elongated, oval in shape, not differentiated from the eye ridge, posterior tip opposite (exsag.) to anterior part of L2. Palpebral furrow deeply incised, narrow, going from the posterior tip of the palpebral lobe through eye ridge to the axial furrow. Eye ridge prominent, directed slightly obliquely forward, reaching the axial furrow on the S3. Cephalic doublure is not preserved.
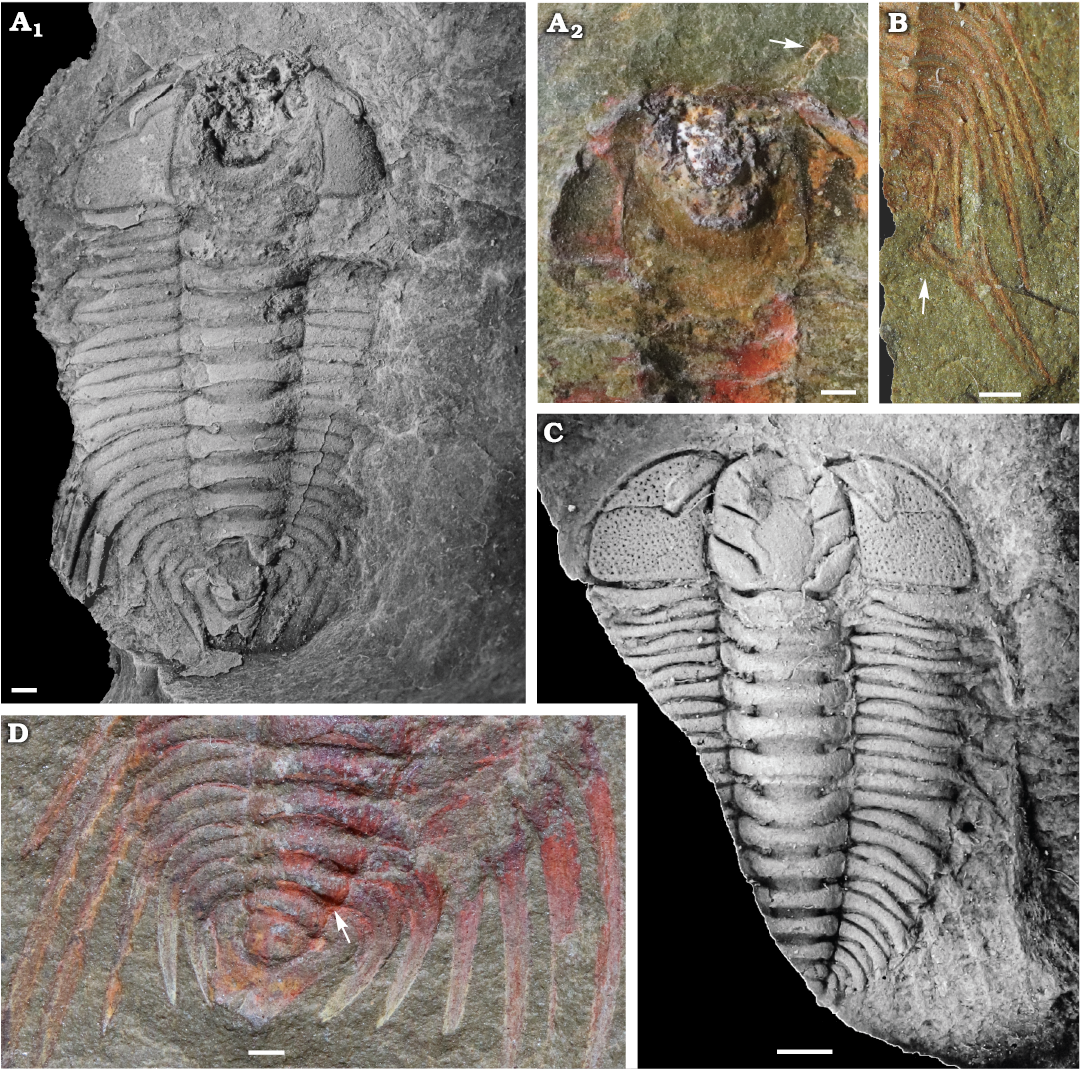
Fig. 1. Cheirurid trilobite Anacheirurus adserai (Vela and Corbacho, 2007) from the Fezouata Shale, Araneograptus murrayi Zone, Tremadocian, Lower Ordovician, near Beni Zouli, Zagora Province, Morocco. A. MGL 102179, complete specimen (A1) and anterior region of the counterpart (A2); arrow pointing the antennae. B. MGL 102170, close-up of the posterior region of the trunk; arrow pointing the disarticulated pygidium. C. ML20-269198, latex cast of the counterpart. D. YPM 525125, close up of the posterior region of the trunk; arrow pointing the articulation between the thorax and the pygidium. Specimens in A1 and C coated with ammonium chloride. Scale bars 1 mm.
Proparian facial suture. Anterior branch shorter than posterior, running posteriorly slightly outwardly, lightly curved. Posterior branch mostly transverse, almost perpendicular to the sagittal axis, turning posteriorly abruptly beyond the border furrow.
Conterminant hypostome (Figs. 1A2, 2A). Anterior part not well preserved. Anterior margin almost transversal (Fig. 2A). Middle body oval in outline with an anterior lobe oval in shape, ventrally vaulted. Posterior lobe shorter (sag.) than the anterior lobe, U-shaped. Posterior border expanded, gently curved posteriorly, smooth without spines.
Thorax composed of eleven tergites (e.g., Fig. 2B). Thoracic axial ring sub-rectangular in outline, distal tips lightly rounded, slightly shorter (sag.) medially, width (tr.) less than one-third of the pleural width, gradually narrowing (tr.) in posterior tergites. Articulation half-ring with sagittal length half of the axial ring length, longer medially, tapering distally, anterior margin bowed anteriorly. Thoracic pleurae unconstrained, narrow flange in the anterior and posterior margin, carrying a deep, oblique pleural furrow that divides the pleura into an anterior and posterior band. Fulcrum subtle, situated in the distal second third of the pleura. Anterior band with the same length (sag.) as the posterior, but narrower (tr.). The posterior band extended beyond the fulcrum in the pleural spine, needle-like and sub-circular in cross-section. Five anterior tergites with short pleural spines that are directed posterolaterally, and increasing subtly in length from anterior toward posterior tergites. Six posterior tergites with long, thick, rather posteriorly directed pleural spines (Figs. 1C, D, 2A). Pleural spine of the sixth tergite longer and thicker than the rest, curved outward and backward, and extending (exsag.) beyond the pygidial terminal piece. Pleural spines in posterior trunk decreasing in size gradually from the sixth to the eleventh tergite.
Pygidium reduced with two axial rings plus terminal piece and one pair of pleural spines (Fig. 1B, D). Articulating half-ring short (sag.) with half of the length of the first axial ring, longer (sag.) medially, tapering distally, anterior margin anteriorly bowed. First pygidial axial ring rectangular in outline, longer (sag.) and wider (tr.) than the second pygidial axial ring. Terminal piece narrower (tr.) than pygidial axial rings, similar in length (sag.) to second axial ring; anterior margin transversal, posterior margin slightly bowed posteriorly. One pleural tergite strongly curved backwards, with a distinct pleural furrow dividing the pleura in anterior and posterior bands, the posterior band extended in a pair of long pleural spines directed backwards.
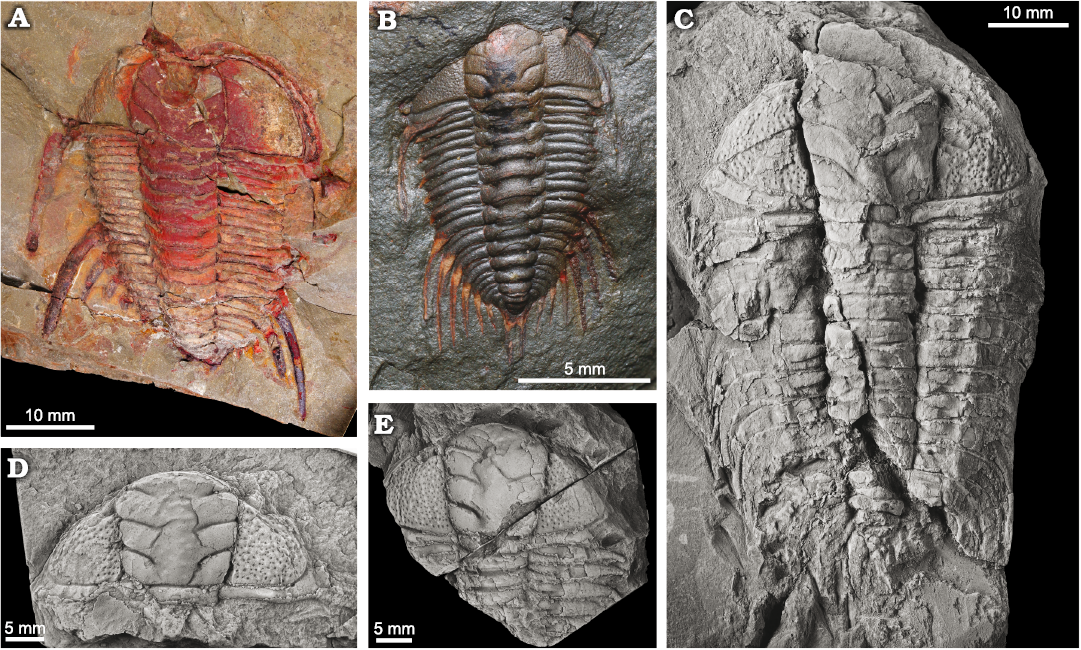
Fig. 2. Cheirurid trilobites Anacheirurus adserai (Vela and Corbacho, 2007) from the Fezouata Shale, Araneograptus murrayi Zone, Tremadocian, Lower Ordovician, near Beni Zouli, Zagora Province, Morocco (A, B) and Lehua vinculum (Barrande, 1852) from the Dobrotivá Formation, upper Darriwilian to lowermost Sandbian, Zaječov-Svatá Dobrotivá, Czech Republic (C–E). A. YPM 522182, part, complete holaspid with hypostome impressed. B. YPM 530933, complete holaspid. C. NM L19066, complete holaspid. D. NM L19075, complete cranidium . E. JV1607, anterior half of an holaspid specimen. Specimens in C–E covered by ammonium chloride.
Ontogeny: The smallest recorded specimen (MGL 104146) is a late meraspid stage that is 2.6 mm long (Fig. 3A). The cephalon of this juvenile stage is generally identical to the cephala of the adult individuals, only the genal spines are proportionally longer (such longer genal spines are evident also in some small holaspid specimens, Fig. 3B, C). The trunk bears at least ten or eleven axial rings, posteriors of which are difficult to recognize, and twelve pairs of pleural spines. In contrast to the holaspids, the pleural spines of this small individual show a slightly different pattern in respect to their mutual sizes. The spines of tergites one to seven are quite long with equal or nearly equal length. The spines on tergites one to five are not significantly smaller than the rest, as is the case for holaspids. From the seventh tergite, the spine sizes decrease in length rapidly, such that the tenth to twelve pairs are minute. The equal number of pleural spines between this juvenile specimen and the adults suggests that this juvenile might have already reached the total number of trunk segments characteristic for A. adserai, despite the posterior-most axial rings not being recognizable. It is not clear whether the last pre-terminal tergite (no. 13) was present, for this one does not bear any spines in the holaspid individuals. Seven anterior trunk tergites are separated by articulating structures, forming the thorax. The posterior tergites, bearing five pairs of pleural spines, are still fused, forming a meraspid pygidium. This tergite-rich meraspid pygidium suggests that the A. adserai holaspid pygidium developed into its final form by depletion of the conjoined tergites of the thorax, as is known in many other trilobites (e.g., Hughes et al. 2006, Laibl et al. 2014). MGL 101262 (5.6 mm long) and MGL 104533 (5.9 mm long) are the smallest holaspids found (Fig. 3B, C). The morphology of the pleural spines is similar to the morphology of the adult specimens. The only differences to fully-grown individuals are proportionally longer genal spines and the slightly larger size of the first five thoracic pleural spines.
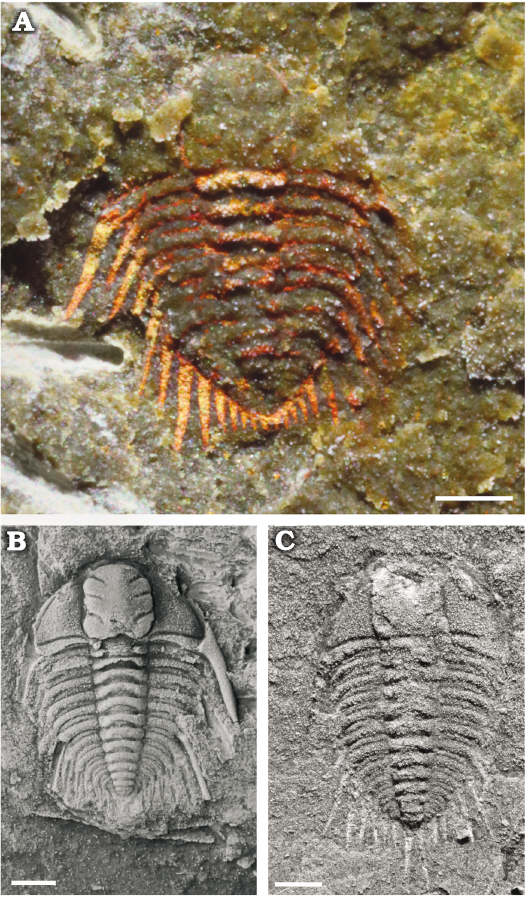
Fig. 3. Juveniles of the cheirurid trilobite Anacheirurus adserai from the Fezouata Shale, Araneograptus murrayi Zone, Tremadocian, Lower Ordovician, near Beni Zouli, Zagora Province, Morocco. A. MGL 104146, late meraspid stage. B. MGL 102153, early holaspid stage. C. MGL 104533, early holaspid stage. Specimens in B, C covered by ammonium chloride. Scale bars 1 mm.
Appendages: Antennae: A uniramous antenna is partially visible in the counterpart of MGL 102179 (Fig. 1A2, white arrow). The proximal part of the right antenna is preserved in the front of the cephalon. The individual podomeres are poorly recognizable but seem to be rather short, and quadratic in outline. The total number of podomeres and the total length of the antenna are not known because the distal tip is missing.
Protopodites: The protopodite is not preserved in any of the studied specimens. In general, the appendages in the Fezouata Shale trilobites are visible in the parts where the exoskeleton has been removed. The protopodite should be located below the axial region, which usually is not removed. As a consequence, the proximal parts of the appendages are not visible. In MGL 10225 and YPM 226573 some structures can be interpreted as a part of the protopodite (Figs. 4B, 5A2, A4), however, the nature of the structures is not clear and not enough information is available to interpret the morphology or the presence of a gnathobase.
Endopodites: The endopodites are preserved in the MGL 103863 (Fig. 4A), MGL 10225 (Fig. 4B), and YPM 226573 (Fig. 5). The proximal parts of the endopodites are poorly preserved, and the attachment point to the protopodite is not visible in any of the specimens. The three anterior-most endopodites belong to the cephalon (Fig. 4A2, B2), corresponding with attachment sites on S2, S1, and SO (Fig. 4A, B). In the thorax, each tergite bears a pair of endopodites (Fig. 4B). The posterior thoracic (behind the tenth tergite) and the pygidial endopodites are not preserved.
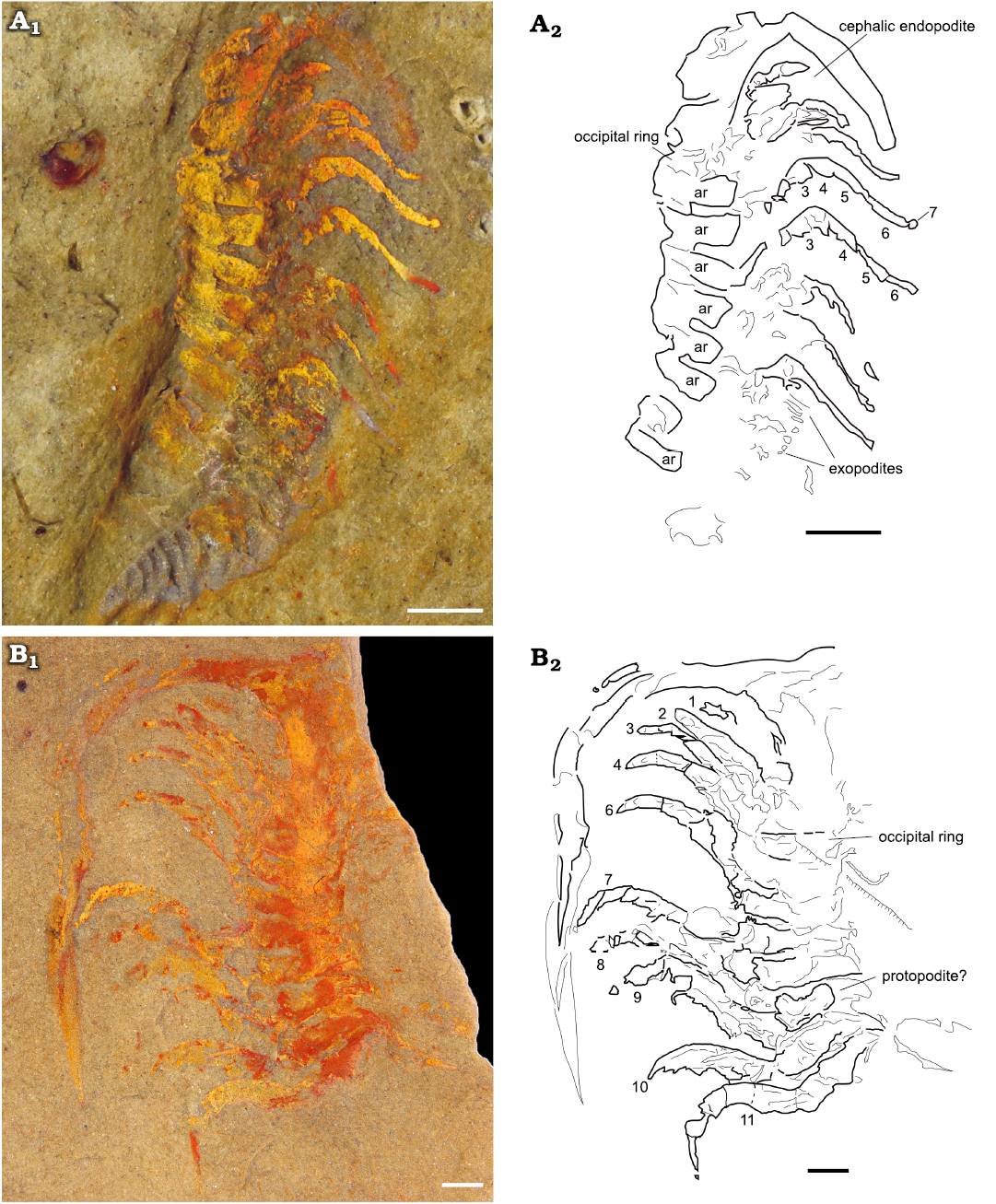
Fig. 4. Endopodites of the cheirurid trilobite Anacheirurus adserai (Vela and Corbacho, 2007) from the Fezouata Shale, Araneograptus murrayi Zone, Tremadocian, Lower Ordovician, near Beni Zouli, Zagora Province, Morocco. A. MGL 103863, photograph under alcohol with polarized light (A1), explanatory drawing with podomere numbers (A2). B. MGL 102225, photograph under alcohol with polarized light (B1), explanatory drawing with endopodite numbers (B2). Scale bars 1 mm. Abbreviations: ar, axial ring.
The endopodites are divided into seven podomeres (numbered in Fig. 5A2, A4). The most proximal podomeres 1–3 have a rectangular shape and are more robust than the rest of the podomeres. The podomere 3 is just slightly shorter and thinner than the previous ones (1 and 2). The following podomeres (4–6) are much narrower and more elongated in shape. They gradually decrease in width and increase in length distally, with the podomere 6 being the narrowest and most elongated (Fig. 5A2, A4). The podomeres 5 and 6 are both nearly twice as long as the most proximal podomeres. The podomere 7 is reduced, forming a tripartite claw, with a fork shape (Fig. 5A3, A5).
The morphology of the endopodites is consistent along the body, at least up to the tenth thoracic endopodite. The cephalic endopodites are shorter than the thoracic ones, with thicker proximal podomeres (Fig. 4A, B). Large endites are visible in the posterior endopodites, but no endites are visible in the anterior endopodites. In YPM 226573, one of the posterior endopodites bears two large endites on podomeres 2 and 3 (Fig. 5B4, B6, B5). The endopodites are usually flexed at a joint between the third and the fourth endopodite. In most of the specimens, the proximal part of each endopodite is directed anterolaterally while the distal region beyond the joint is directed posterolaterally.
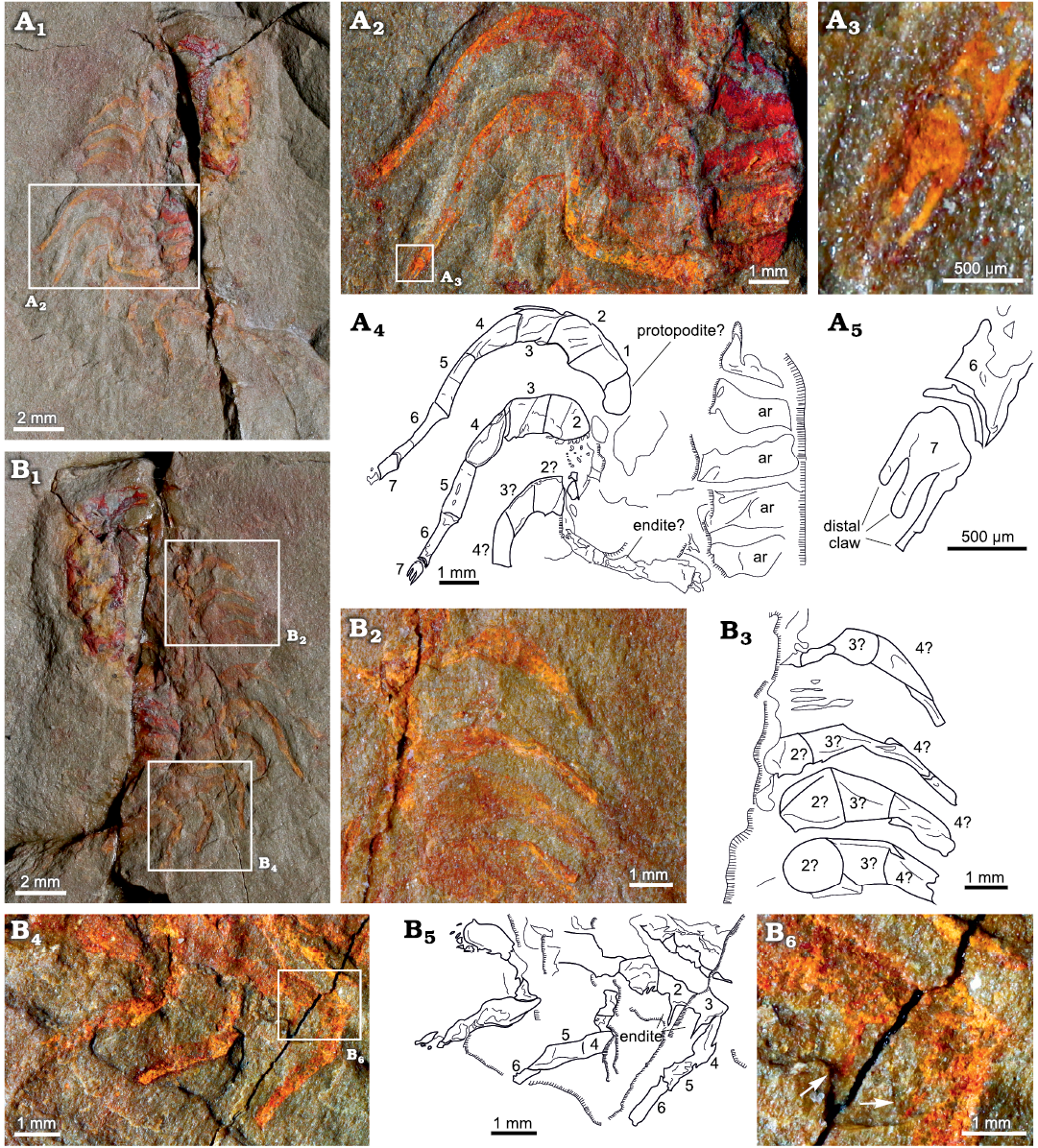
Fig. 5. Endopodites of the cheirurid trilobite Anacheirurus adserai (Vela and Corbacho, 2007) from the Fezouata Shale, Araneograptus murrayi Zone, Tremadocian, Lower Ordovician, near Beni Zouli, Zagora Province, Morocco. A. Part of YPM 226573, general view (A1), close-up of the endopodites (A2) and explanatory drawing (A4), close-up of the distal claw (A3) and explanatory drawing (A5). B. Counterpart of YPM 226573, general view (B1), close up of the endopodites (B2) and explanatory drawing (B3), close up of the endopodites (B4) and explanatory drawing (B5), close up of the area pointed out in B4 (B6), arrows showing long endites on podomere 2 and 3. Numbers represent podomere number. Abbreviations: ar, axial ring.
Exopodites: The exopodites are preserved in MGL 102172 (Fig. 6) and YMP 517074 (Fig. 7), with the latter preserving the only complete exopodite (ninth thoracic exopodite; Fig. 7A4, A5). The rest of the exopodites are partially covered in the anterior region, obscuring the complete shape. The exopodite consists of a flat, elongated, wider (tr.) than longer (sag.) flap. Around the flap, there is a reinforced marginal rim (Fig. 7A5) showing a smooth surface. The anterior margin of the flap is slightly concave, while the posterior is almost straight, with the distal part curving forward. The flap is divided into two separate lobes by an articulation that extends from the anterior to the posterior region in an almost straight line, perpendicular to the main axis of the flap (Fig. 7A4). The proximal lobe is sub-rectangular in outline, longer (tr.) and wider (exsag.) than the distal lobe. Attached to the posterior rim, there are long and flat imbricated lamellae (Figs. 6A2, A3, 7A2–A5). The distal lobe is sub-oval in outline, also with bristles attached to the posterior margin. The lamellae on the proximal lobe are directed posteriorly, reaching the posterior tergite border, and are wider in the transversal section and longer than the bristles on the distal lobe, which are shorter and thinner. The bristles on the distal lobe are directed slightly outwards. The joint between the protopodite and the exopodite is not visible; it is covered by the thoracic axial rings. The three anterior exopodites in YPM 517074 display a similar morphology, bearing long and robust lamellae attached to the posterior rim of the flap, however, the anterior region of the flaps is overlapped by the lamella of the next most anterior exopodite (Fig. 7A2, A3). The exopodites show different spatial distributions between specimens. In YMP 517074 the posterior exopodite is oriented transversally (Fig. 7), perpendicular to the sagittal axis, with the lamellae pointing backward. In MGL 102172 the exopodite is forwardly and slightly outwardly oriented with the lamellae pointing transversally to the sagittal axis (Fig. 6).
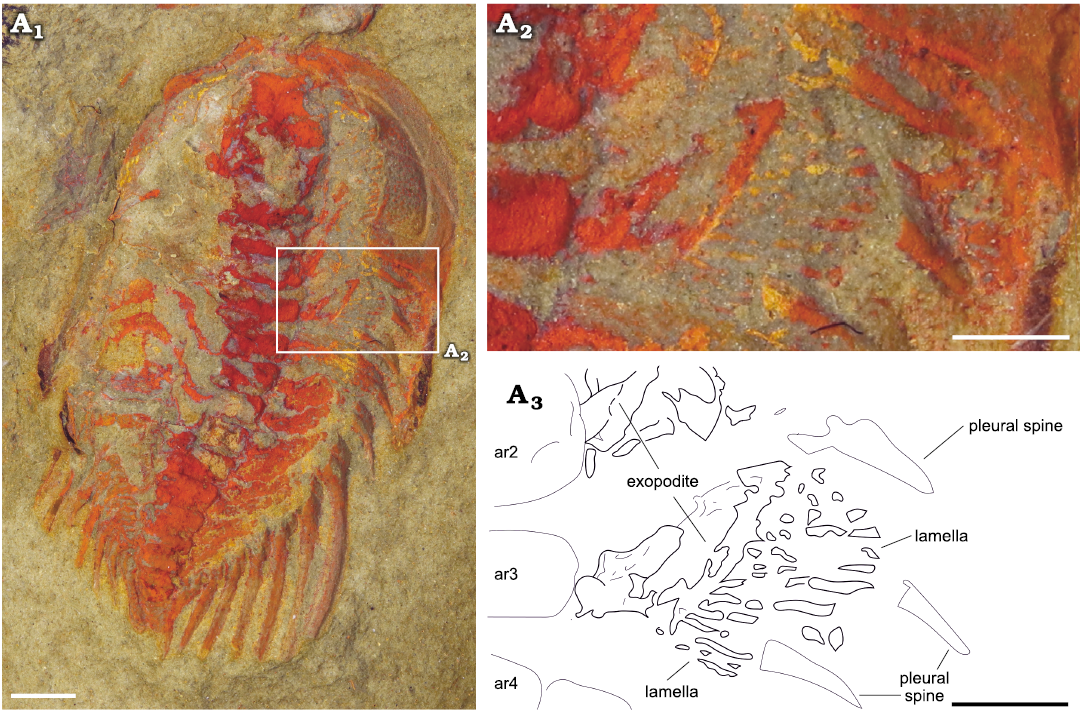
Fig. 6. Cheirurid trilobite Anacheirurus adserai (Vela and Corbacho, 2007) (MGL 102172) from the Fezouata Shale, Araneograptus murrayi Zone, Tremadocian, Lower Ordovician, near Beni Zouli, Zagora Province, Morocco. Photographs under alcohol: general view (A1), close-up of the exopodites (A2) and explanatory drawing (A3). Scale bars 1 mm. Abbreviations: ar, axial ring.
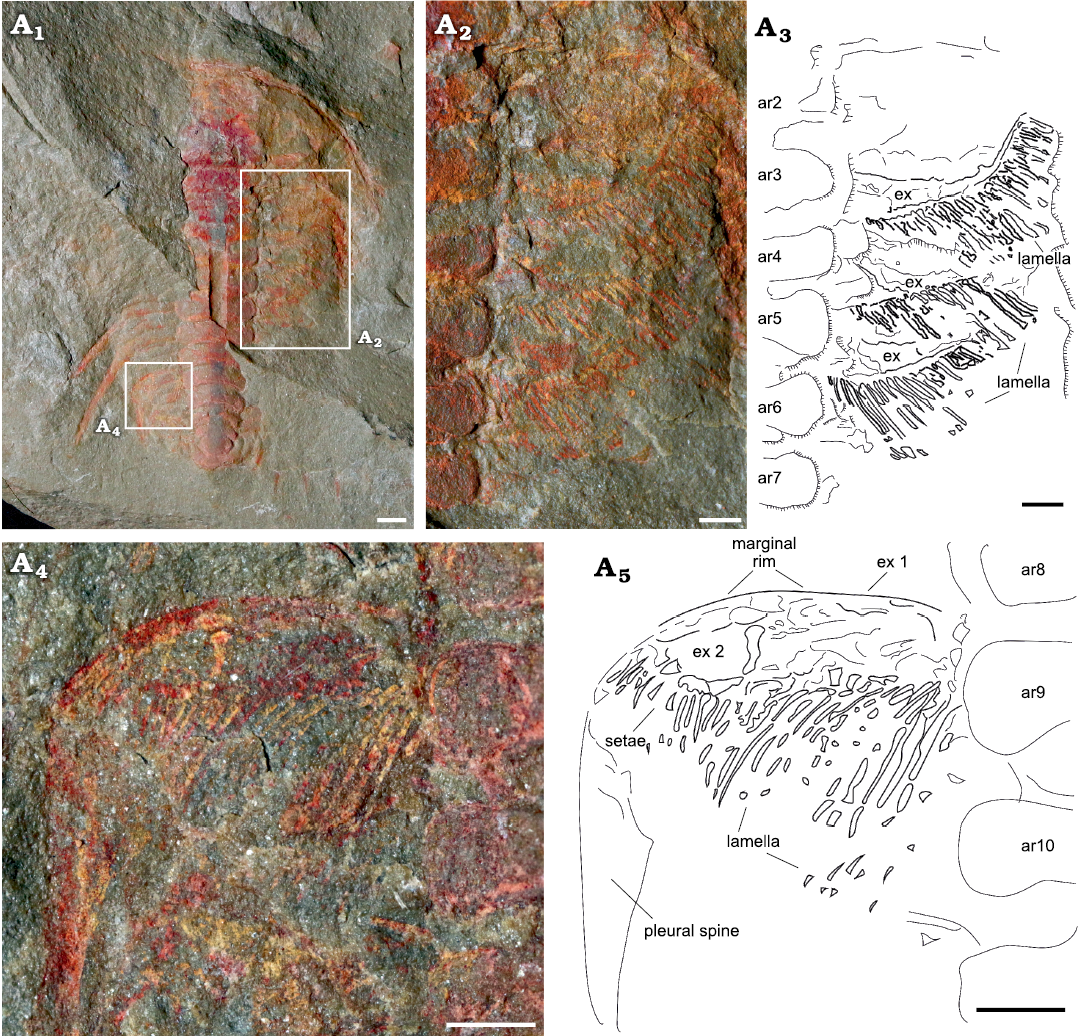
Fig. 7. Exopodities of the cheirurid trilobite Anacheirurus adserai (Vela and Corbacho, 2007) (YPM 517074) from the Fezouata Shale, Araneograptus murrayi Zone, Tremadocian, Lower Ordovician, near Beni Zouli, Zagora Province, Morocco. Photographs: general view (A1), close-up of the exopodites (A2) and explanatory drawing (A3), close up to the ninth exopodite (A4) and explanatory drawing (A5). Scale bars 1 mm. Abbreviations: ar, axial ring; ex, exopodite.
Remarks.—The species discussed herein was originally described by Vela and Corbacho (2007) as Lehua adserai. These authors assigned this species to the genus Lehua Barton, 1915, but unfortunately did not provide any justification or discussion why they did so. The Lehua was erected by Barton (1915). The characters defining Lehua, as presented by Barton (1915), are: glabella not tumid; eyes absent or extremely rudimentary; genal spines present; frontal lobe much less than one-third of the glabella; pleura with transverse constriction or node; the inner portion cut by a diagonal furrow and pygidium not differentiated from the thorax. Later, Prantl and Přibyl (1947: 18), amended the description of Lehua as having: “… 11 thoracic segments and six-lobate pygidium. Free cheeks small, shifted forward. Facial suture forming a small arc; at first, it runs close along the margin of the glabella and at the level of the first lateral glabellar lobe it turns back in an arc to the lateral margin of the cephalon. Neither visual organs nor palpebral lobes are developed. Pygidium with raised subtriangular axis composed of four rings, the fourth being completely stunted. Three pairs of sword-like curved, free, flat pleurae of unequal length. The lower margin of the pygidium is sharply cut off in a straight line perpendicular to the axis.” These diagnostic features of Lehua are not present in the species described by Vela and Corbacho (2007) as Lehua adserai, making this generic assignment questionable. On the contrary, L. adserai shows numerous similarities with the type species of the Anacheirurus (A. frederici) and should be therefore placed within this genus. Both A. adserai and A. frederici are morphologically nearly identical. Anacheirurus adserai differs from A. frederici in having a shorter (sag.) anterior border, an eye ridge not adjacent to facial suture, by the absence of S4, and by the number of pygidial spines with probably two pairs in A. frederici and one pair in A. adserai.
Whittard (1967: 285) pointed out that the morphological differences between Lehua and Anacheirurus are not enough to warrant two different genera, suggesting Lehua should be considered as a junior synonym of Anacheirurus, an argument later followed by Martin et al. (2016b) in their review of Fezouata Shale trilobites. Based on the new material studied herein, we advocate for the idea formulated by Lane (1971) that Lehua is a valid genus different from Anacheirurus. Lehua vinculum (Fig. 2C–E), shows several major morphological differences when compared to A. frederici. First, the glabella of L. vinculum is slightly expanded in the front, while in A. frederici it is sub-parallel with the median part slightly wider. Second, the posterior margin of the cephalon in L. vinculum is not transverse; the more distal parts are curved slightly forward. In A. frederici, on contrary, the distal part of the posterior margin is curved backward, creating a slightly expanded posterior border close to the genal spines. Third, the palpebral lobes and eye ridges are not present in L. vinculum, whereas in A. frederici they are prominent and well developed (however the lack of eyes in Lehua could be taphonomic, as the anterior part of the fixigena is not well preserved in the type species). Finally, the main difference between L. vinculum and A. frederici is related to the morphology and arrangement of the thoracic pleurae. In L. vinculum, all the thoracic and pygidial pleural spines are of the same size (only slightly decreasing in length posteriorly on the pygidium), contrary to A. frederici, which has posterior thoracic and pygidial spines that are longer than the anterior thoracic pleural spines. The thoracic pleurae of L. vinculum are divided into an inner and outer parts, with the inner part consisting of one-quarter of the length of the pleura and the outer part three quarters. Only the inner part of the pleura in L. vinculum bears an oblique pleural furrow. The pleurae in A. frederici are not divided into an inner and an outer part, instead, the pleural furrow is deep, oblique, and traversing the whole width (tr.) of the pleura dividing it into anterior and posterior parts of similar size.
Since the morphology of the pleural furrow is relevant for the classification of different groups inside the family Cheiruridae (e.g., Barrande 1852; Salter 1864; Schmidt 1881; Reed 1896; Barton 1915; Öpik 1937; Lane 1971), this character has a broader implication for the systematic position of A. adserai. Anacheirurus adserai displays a pleural morphology diagnostic of the subfamily Pilekiinae (Lee and Chatterton 1997), whereas L. vinculum displays a pleural morphology typical of the subfamily Cheirurinae (Lane 1971). Lehua is a more derived taxon belonging to a late diverging group that contains highly derived taxa such as Cheirurus and Crotalocephalus.
Some authors (Bulman and Rushton 1973; Přibyl et al. 1985; Sdzuy et al. 2001) suggested that Parapilekia sougyi (Destombes in Destombes et al., 1969), described from the Zemmour locality (Mauritania) by Destombes in Destombes et al. (1969) should also belong to the Anacheirurus. The morphology of the cephalon of P. sougyi and A. adserai is generally identical, with the only notable difference in glabellar furrows, which are not connected to the axial furrow in Parapilekia sougyi. This character might be, however, caused by differences in the preservation. Destombes et al. (1969) also described a fragmentary pygidium with three axial rings (two rings and a terminal piece?) and one pair of spines directed backward. Such a morphology corresponds with what is seen in Anacheirurus adserai. This suggests that Parapilekia sougyi is a species of Anacheirurus, possibly even synonymous with A. adserai (in which case the name A. adserai would be a junior subjective synonym of A. sougyi). However, the type material of A. sougyi does not allow more detailed comparison, due to its fragmentary nature. Until more complete specimens of A. sougyi from the type and/or nearby localities are available, we recommend keeping A. sougyi and A. adserai as two separate species.
Seven species of Anacheirurus, assigned previously to Lehua, were originally described in the area of Zagora, Morocco (Vela and Corbacho 2007; Vela 2007; Corbacho 2008). In the following years, Vela and Corbacho (2011) considered three of them (A. colli, A. ponti, and A. iannacconnei) as junior synonym of A. corbachoi, keeping four different species of Anacheirurus from the Fezouata Shale. Their characters separating individual species are in general related to the shape of the cephalon and the glabella, the presence and morphology of the anterior notch in the cephalon, and the number and shape of posterior pygidial spines. These species are based on specimens that were heavily prepared, a process that may modify or exaggerate the original morphology, and can lead to misinterpretations of anatomical characters (Fortey 2009; Gutiérrez-Marco et al. 2017; Gutiérrez-Marco and García-Bellido 2018). Martin et al. (2016b) in a study about the trilobite community in the Fezouata Shale concluded that there is only one morphotype of pilekiid, and it was assigned to Anacheirurus adserai which is the oldest species of Anacheirurus described. Detailed observations of numerous specimens from Fezouata Shale, which had not undergone the same degree of preparation suggest, in concordance with Martin et al. (2016b), that all the species may be junior subjective synonyms of A. adserai for the following reasons.
Firstly, the anterior cephalic notch that is variously developed in some specimens (Vela and Corbacho 2011: pl. 1: 2) represents the dorsal arching of the cephalon. Such arching is expressed to a higher or lower degree on the compressed specimens, ranging from an indistinct or distinct notch to a protrusion, depending on the original orientation of the cephalon to the bedding plane. Hughes and Rushton (1990) explained how similar variability in the pygidial shape of trilobite Cermatops discoidalis (Salter, 1866) is caused by different depositional orientations of the pygidium. Similarly, Hughes (1995) listed morphological characters in trilobite Bailiella lantenoisi Mansuy, 1916, that can be a subject of taphonomic variation when preserved in shales. Four of these characters (frontal area length, border length, preglabellar field depression, and anterior border furrow) are generally related to the shape of the anterior cephalic margin (see Hughes 1995: table 1), corroborating this part of the trilobite exoskeleton is prone to taphonomic variation when preserved in shales. These examples demonstrate that certain types of variation in trilobite body parts, when preserved in shales, should not be considered as real biological features, but taphonomic artifacts.
Secondly, the exact number of pygidial spines is generally hard to determine in complete individuals of Anancheirurus, as the boundary between the thorax and the pygidium is difficult to identify owing to the similarity of thoracic and pygidial pleurae. Especially in species where both thoracic and pygidial pleurae bear spines but otherwise do not show any major morphological differences between thorax and pygidium, the last articulation structure is not easy to discern (see Esteve et al. 2017 for a similar issue). The variation in the number of pygidial spines in individual species described by Corbacho and Vela (2011) may be subject to such a bias, especially in heavily prepared specimens.
There is also an uncertainty regarding the exact stratigraphic position of several Anacheirurus species from the Anti-Atlas region. The type locality of A. adserai, A. corbachoi (and its subjective synonyms A. colli, A. ponti, A. iannacconnei), A. tahirii, and A. velai was named as “Tanssikhte”, west of Zagora (Vela and Corbacho 2007: fig. 1; Vela 2007: fig. 1). The type locality was assigned stratigraphically to the Upper Fezouata Shales, “lower–middle Arenig” (= Floian age). Thirty additional trilobite species were identified, with some of them typical for Floian strata (belonging to genera Foulonia, Pateraspis, or Ormathops), but others typical for Tremadocian (such as Bavarilla zemmourensis) (Vela and Corbacho 2007; Vela 2007). However, the latest review of trilobite stratigraphic distribution in the Fezouata Shale made by Martin et al. (2016b) shows that Anacheirurus adserai is restricted to Araneograptus murrayi Zone of the upper Tremadocian. We agree with Martin et al. (2016b) statement and we question the original locality and stratigraphic assignement Anacheirurus adserai.
Stratigraphic and geographic range.—Anti-Atlas of Morocco and possibly also northern Mauritania (= A. sougyi, see section Remarks for details). Fezouata Shale, Araneograptus murrayi Zone, Tremadocian, Lower Ordovician.
Discussion
Morphological disparity of Pilekiinae.—Trilobites of the subfamily Pilekiinae represent the earliest radiation of Cheiruridae, which is one of the major post-Cambrian trilobite groups. During the Tremadocian, pilekiines reached a high global diversity (over 50 species) being a common worldwide faunal component of shallow marine communities. From the morphological perspective, it is interesting to analyze how the cranidia and pygidia vary in shape within the group. In general, the cephalic morphology of Pilekiinae is characterized by the following features: glabella sub-rectangular to expanded posteriorly and tapering forwards, anterior border short (sag.), eyes in anterior position, small palpebral lobe, thick well-defined eye-ridge, reduced librigenal field, fixigenal field sculpted by small pits. The main differences found between the cephala of various taxa are small variations in the shape of the glabella, length of the genal spines, shape of the anterior border and small changes in the position of palpebral lobes (Fig. 8A1, B1, C1, D1, E1). Some early putative pliomerids (for example the Rossaspis Harrington, 1957; see Adrain et al. 2014: fig. 20) also share a cephalic morphology similar to pilekiines. In contrast, the pygidia of pilekiines show a broader range of morphologies. This is evident, especially when comparing the pygidia of various pilekiid species (Fig. 8A2, B2, C2, D2, E2). The main differences are the overall shape, the number of axial rings, the number and length of pleural spines, and the size and shape of the terminal piece. For example, the pygidium of Tesselacauda depressa displays four broad and short adjacent pygidial pleurae with rounded tips, (Fig. 8C2; e.g., see Adrain and Karim 2019: pl. 6), whereas the pygidium of Parapilekia olesnaensis has the pygidial spines arranged in a radial disposition with a space between them (Fig. 8B2; see Mergl 2006: fig. 19K). The pygidium of Anacheirurus adserai (Fig. 8A2) is unique with its extremely long pleural spines that project posteriorly from the end of the pygidium. The pygidium of Macrogrammus rafi has the pygidial spines strongly curved backward and the terminal piece extremely reduced (Fig. 8E2; see Edgecombe et al. 1999: fig. 8), whereas the pygidium of Landyia elizabethae has a prominent terminal piece and four pleurae with a pleural furrow that do not reach the pleural margin and very short pygidial spines (Fig. 8D2; see Jell 1985: pl. 31: 2B). The number of pygidial segments, as well as the number of pleural spines, also differ considerably among the group. The range of axial rings goes from two axial rings plus terminal piece (A. adserai; Fig. 8A2) to five axial rings plus terminal piece (e.g., Parapilekia speciosa; see Ebbestad 1999: fig. 79; Fig. 8B2), with all the intermediate cases. The range of pygidial spines goes from one pair of pygidial spines (A. adserai; Fig. 8A2) to four pairs of pygidial spines, with examples of two pairs (Seisonia sphericaudata) and three pairs (e.g., Anacheirurus nanus) and multiple cases of four pairs (e.g., Macrogrammus rafi). The pygidium of A. adserai is the most reduced pygidium (in terms of segmentation) recorded among Pilekiinae. Anacheirurus frederici may have also a reduced pygidium, however, it is not well known how many segments are allocated in its pygidium, as the boundary between the thorax and pygidium is obscure (owing to the strong similarity between the thoracic and the pygidial morphology and the tectonic deformation of the holotype).
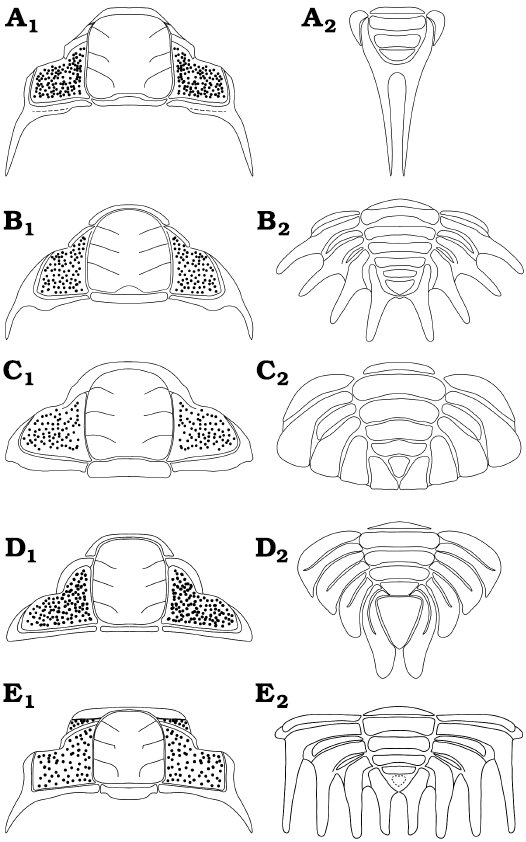
Fig. 8. Reconstructions of cranidia (A1–E1) and pygidia (A2–E2) of different members of the subfamily Pilekiinae. A. Anacheirurus adserai (Vela and Corbacho, 2008). B. Parapilekia olesnaensis (Růžička, 1935), based on Mergl (2006). C. Tesselacauda depressa Ross, 1951, based on Adrain and Karim (2019). D. Landyia elizabethae Jell, 1985, based on Jell (1985). E. Macrogrammus rafi Edgecombe, Chatterton, Vaccari, and Waisfeld, 1999, based on Edgecombe et al. (1999).
The variable number of pygidial segments in Pilekiinae contrasts with the rest of cheirurid subfamilies. Cheirurinae displays a stable configuration with three segments plus a terminal piece. Deiphoninae display three to four segments plus terminal piece. Acanthoparyphinae varies from two to three pygidial segments. Sphaerexochinae display three pygidial segments plus a terminal piece. Heliomerinae shows two pygidial segments. Eccoptochilinae shows from three to four pygidial segments plus a terminal piece.
Pilekiines display a homonomous trunk (i.e., the thoracic and pygidial tergites are generally of equal morphology; see Hughes 2003, 2005). In some younger members of Cheiruridae the pygidium is clearly differentiated from the thorax (heteronomous trunk), for example in the genera Ceraurinella, Ceraurus, Hemisphaerocoryphe, Sphaerocoryphe, Deiphon, Holia, or Nieszkowskia. The degree of differentiation varies, with some taxa having less differentiated trunk than the aforementioned examples, e.g., Actinopeltis. Such trunk differentiation could drive the fixation of the number of pygidial tergites. Another factor that has been claimed as a constraint for the variation in the number of trunk segments is enrollment (Hughes et al. 1999; Hughes 2003, 2005; Esteve et al. 2011, 2013). Pilekiines display a thoracic articulation composed of a short (exsag.) flange in the anterior margin of the pleura and a less-developed fulcrum positioned far from the axis anteriorly and progressively positioned closer to the axis posteriorly. The articulating facet is not developed in pilekiines. No evidence of coaptative structures has been found. The simple flange-hinged articulation and the lack of articulating facets and coaptative structures suggest that the enrollment of pilekiines was non-encapsulated, similar to those seen in some Cambrian trilobites (Ortega‐Hernández et al. 2013; Esteve and Yuan 2017). In contrast, most of the other members of Cheiruridae have developed thoracic devices to achieve spheroidal (and likely also encapsulated) enrollment. These taxa show a well-developed fulcrum that is situated closer (adaxially) to the axis, a fulcral process and socket (e.g., Cheirurinae), or a faceted outer pleural part (e.g., Kawina). In addition, some taxa have developed coaptative structures as a pair of spines in the rostral plate in Cyrtometopus clavifrons (Dalman, 1827), which fit between the pygidial spines when fully enrolled (see Lane 2002 for more information) or a furrow in the doublure in the genus Sphaerexochus (see Chatterton and Ludvigsen 1976: pl. 13: 35, 36) for accommodating the first thoracic pleura. It is believed that spheroidal enrollment requires a precisely coordinated scaling of the body proportions (Hughes 2007), therefore the stable number of pygidial tergites in stratigraphically young cheirurids might be an adaptation to this particular enrollment style.
Comparison of trilobite appendages.—Broadly speaking, the general morphology of the appendages of A. adserai is similar to the appendages of other trilobites. They consist of anterior pair of uniramous antennae composed of multiple podomeres followed by a series of homonomous biramous limbs (cf. Scholtz and Edgecombe 2005, 2006; Ortega-Hernández et al. 2017). The number of appendages belonging to the pygidium is unknown. The protopodite and the junction between the protopodite and the ventral part of the body are not preserved in any specimen of A. adserai, so its morphology and structure cannot be described. Each endopodite is composed of seven podomeres, with the most distal reduced (Fig. 9). The exopodite is a flap bearing long filaments (Fig. 9).
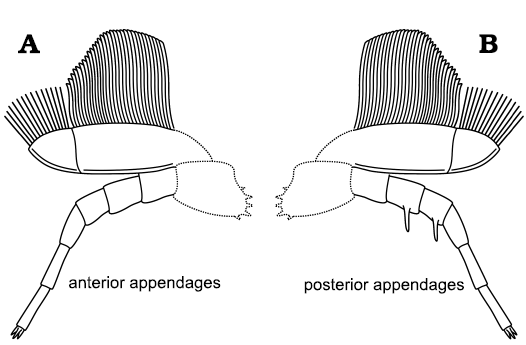
Fig. 9. Biramous appendages reconstruction for the cheirurid trilobite Anacheirurus adserai, showing the anterior (A) and the posterior (B) morphology. Dashed lines indicate inferred proximal parts of the appendages.
The detailed appendage morphology of A. adserai shows several important differences when compared to known appendages of other trilobites. One of the unique characteristics of A. adserai is the elongated podomere 5 and 6 of the endopodite (Fig. 9), which are approximately two times longer than the first four podomeres. In other trilobites, the podomeres are of equal or nearly equal length (e.g., Holmes et al. 2020), or they gradually shorten distally (e.g., Whittington 1975: Whittington and Almond 1987: Zeng et al. 2017). Similar elongated distal podomeres, as in A. adserai, can be found in other non-trilobite arthropods such as Emeraldella brocki (Stein and Selden 2012), where the fourth and sixth podomeres are elongated, or in the second cephalic endopodite in Mollisonia plenovenatrix, which has an elongated sixth podomere (Aria and Caron 2019).
The cephalic endopodites in A. adserai are shorter than those of the thorax. The overall pattern of the endopodite length is similar to Olenoides serratus, which has the longest endopodites between the fourth and thirteenth appendage (Whittington 1975), or Triarthrus eatoni, which displays a progressive increase in size from the second to the fourth endopodite, after which the size remains constant until an abrupt reduction in endopodite size in the most posterior part of the thorax and the pygidium (Whittington and Almond 1987). Megistaspis hammondi, also from the Fezouata Shale, shows a different pattern, with the anterior endopodites being more robust and more spinose than the posterior ones (Gutiérrez-Marco et al. 2017). Such heteropody is not present in A. adserai, although there might be a slight difference in the spinosity between the anterior and posterior endopodites. Indeed, some posterior endopodites show long endites in proximal podomeres (Fig. 9), while anterior endopodites seem to lack these endites. Whether this is a real morphological feature or just a difference in endite preservation is uncertain. Some other trilobites, such as Olenoides serratus, Triarthrus eatoni, or Hongshiyanaspis yiliangensis preserve endites on all the endopodites (Whittington 1980; Whittington and Almond 1987; Zeng et al. 2017). In these trilobites, the endites are also more prominent in proximal podomeres, as in A. adserai. Contrary to all the previous examples, Redlichia rex has no endites on the endopodites (Holmes et al. 2020). In Triarthrus eatoni, the more posterior endopodites have more prominent endites (Whittington and Almond 1987). This might also explain why we observe endites only on the posterior endopodites of A. adserai.
The morphology of the exopodites of A. adserai resembles those of Cambrian trilobites, namely Olenoides serratus, Hongshiyanaspis yiliangensis, Eoredlichia intermediata, Redlichia rex, Kootenia burgessensis, or Kuanyangia pustulosa (Whittington 1975; Hou et al. 2008; Zeng et al. 2017; Holmes et al. 2020; Fig. 10A–D). Their exopodites consist of an elongated flap, with long imbricated lamellae that are attached to the posterior margin of the flap, and with shorter bristles in its distal part. The flap itself can be bipartite (e.g., in Olenoides serratus, Eoredlichia intermediata, Kuanyangia pustulosa, Kootenia burgessensis), or tripartite (e.g., in Redlichia rex, Hongshiyanaspis yiliangensis). In both cases, the overall shape of the flap is similar, with the most proximal lobe being bigger and bearing long lamellae and the most distal lobe being reduced in size and bearing bristles. The exopodite morphology of A. adserai resembles these Cambrian taxa as the flap is wide and flat, and has a bipartite morphology, with longer lamellae in the proximal part and shorter bristles in the distal part.
The exopodite morphology from post-Cambrian trilobites is less well known, but the available data suggest a different morphology than that seen in Cambrian trilobites. The Ordovician taxa Ceraurus pleurexanthemus, Cryptolithus bellulus, and Triarthrus eatoni (Størmer 1939; Bergström 1972; Whittington and Almond 1987), and the Devonian Chotecops fernandi (Bruton and Haas 1999) are the best-studied examples (Fig. 10F–I). Cryptolithus bellulus and Thriarthus eatoni both come from Beecher’s Trilobite Bed in the Frankfort Formation, Upper Ordovician, New York. The exopodite of T. eatoni consists of a slender shaft annulated by 15 oblique grooves with the filaments attached to the upper side of the shaft, and a small lobe at the tip of the shaft (Cisne 1975, 1981; Whittington and Almond 1987; Fig. 10G). In Cryptolithus bellulus the morphology is unclear. All of the different interpretations, however, agree that it has a series of bristles attached to a slender shaft (Raymond 1920; Størmer 1939; Bergström 1972, 1973; Campbell 1975; Fig. 10H), more similar to the morphology present in the species of Triarthrus than to the flap-shaped exopodites present in Cambrian taxa and the species of Anacheirurus. The exopodites of Ceraurus pleurexanthemus from the Trenton Limestone, Upper Ordovician, New York, are preserved inside the body cavity of enrolled specimens, by authigenic mineralisation with calcite (Walcott 1918; Raymond 1920; Størmer 1939, 1951), and can be examined only in thin section. The overall morphology of the appendages of Ceraurus pleurexanthemus remains difficult to determine. Størmer (1939, 1951) reconstructed an exopodite divided into five segments with long bristles only on the last segment (Fig. 10F).
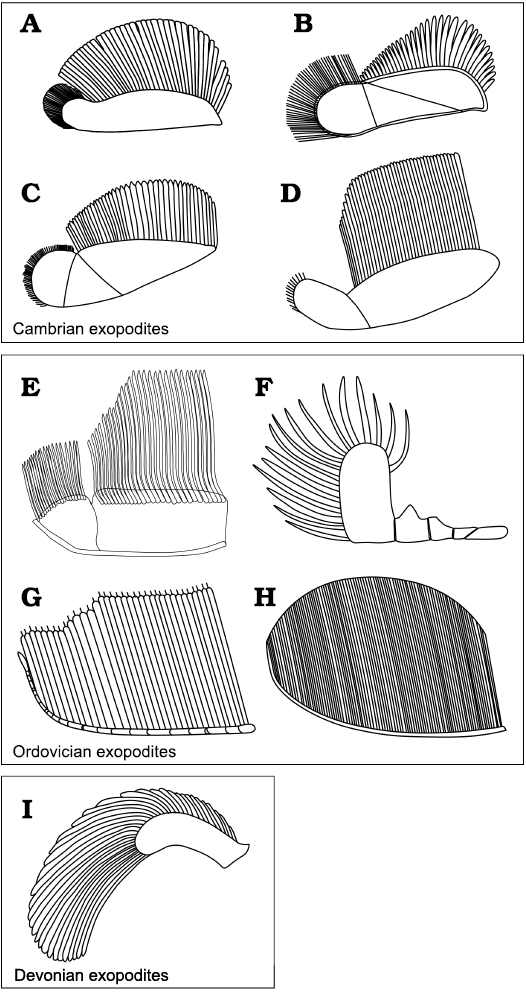
Fig. 10. Trilobite exopodite reconstructions grouped by geological age. A. Eoredlichia intermediata (Lu, 1940), based on Ramsköld and Edgecombe (1996). B. Hongshiyanaspis yiliangensis Zhang and Lin in Zhang et al., 1980, based on Zeng et al. (2017). C. Redlichia rex Holmes, 2019, based on Holmes et al. (2019). D. Olenoides serratus (Rominger, 1887), based on Whittington (1980). E. Anacheirurus adserai (Vela and Corbacho, 2007). F. Ceraurus pleurexanthemus Green, 1832, based on Størmer (1951). G. Triarthrus eatoni (Hall, 1838), based on Whittington and Almond (1987). H. Cryptolithus bellulus (Ulrich, 1879), based on Campbell (1975). I. Chotecops ferdinandi (Kayser, 1880), based on Bruton and Haas (1999).
The appendage morphology described above does not resemble that seen in Anacheirurus adserai, a difference that is striking because C. pleurexanthemus and A. adserai are both members of the family Cheiruridae. The similarity between Cambrian trilobites and A. adserai exopods on the one side, and the difference between the exopods within Cheiruridae on the other, can be explained by two hypotheses. Either Anacheirurus retained the plesiomorphic Cambrian exopodite morphology, which was subsequently modified within Cheiruridae, or the exopodite of Anacheirurus convergently evolved into a similar form to that seen in Cambrian taxa owing to similar ecological pressures. Zeng et al. (2017) argued that the variations in the morphology of exopodites might be the result of different ecological adaptations, which would rather favor the latter hypothesis.
The last example of trilobite appendages is Chotecops ferdinandi from the Hunsrück Slate, Lower Devonian, Germany, which has been studied mainly using the X-rays (Stürmer 1968, 1970; Stürmer and Bergström 1973; Bruton and Haas 1999). The exopodite remains of C. ferdinandi are fragmentary and the general morphology is not known in detail. Bruton and Haas (1999) suggested there is an exopodite with broad filaments attached along the margin of a thinner smooth shaft (Fig. 10I). Bruton and Haas (1999) have not found any evidence of a bipartite shaft with different bristles in the distal region.
Implications for evolutionary trends and the Ordovician biodiversification.—During the Ordovician major changes in trilobite faunal composition took place (Adrain et al. 1998, 2004) which were a part of the Ordovician biodiversification and the Great Ordovician Biodiversification Event (sensu Stigall et al. 2019, 2020). Changes in the morphology of trilobite appendages (especially exopodites) also happened during this time interval. Interestingly, these changes in appendage morphology are coincident with a major diversification of the ichnogenus Cruziana during the Early Ordovician.
Adrain et al. (1998, 2004) recognized several diversifications of Ordovician trilobite faunas, with the major diversification occurring during the Middle Ordovician. Within Cheiruridae, pilekiines diversified during the Tremadocian and reduced drastically in diversity during the Floian (Fig. 11). Hence, pilekiines are not part of this Middle Ordovician trilobite diversification (Adrain et al. 1998). In contrast, the rest of the family Cheiruridae diversified from the Floian to the Sandbian (Fig. 11), better matching the aforementioned diversification (Adrain et al. 1998, 2004) as well as the Great Ordovician Biodiversification Event (sensu Stigall et al. 2019, 2020).
Although trilobite appendage data from the Ordovician are sparse, the Ordovician diversification of trilobites might explain higher post-Cambrian variability of trilobite exopodites. If the exopodite morphology was indeed driven by ecological adaptations, as Zeng et al. (2017) suggested, then the appendage data follows the increasing complexity of Middle Ordovician ecosystems (Bambach 1983; Signor and Vermeij 1994; Droser and Finnegan 2003; Stigall et al. 2019). This would also explain why the “Cambrian-type” exopodites are present in Anacheirurus, an Early Ordovician member of Pilekiinae.
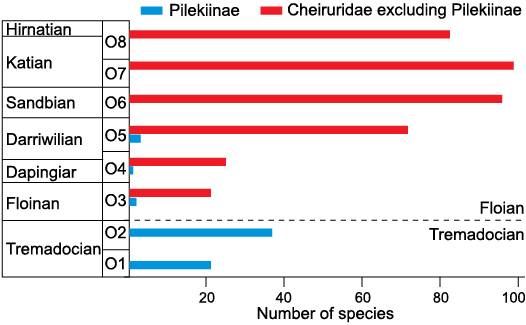
Fig. 11. Ordovician diversity of Cheiruridae species across individual time slices, as defined in Adrain (2013). The dashed line represents the boundary between Tremadocian and Floian; blue, members of the subfamily Pilekiinae; red, members of the rest of Cheiruridae subfamilies excluding pilekiids. Data based on Adrain (2013, personal communication 2020).
The sparse appendage data from the Ordovician can be supplemented by examining the trace fossil record, in particular the changes observed within the ichnogenus Cruziana. Cruziana records the imprints of trilobite appendages as they move through and over the sediments, therefore a change in Cruziana morphology can reflect a change in the appendage morphology. Different morphologies and ichnospecies of Cruziana have been identified across the Paleozoic and clustered in groups (Seilacher 1970, 1991, 1994). In the transition from the Cambrian to the Ordovician, two groups of Cruziana ichnospecies are identified. The Cruziana semiplicata group ranges from the Lower Cambrian to the Tremadocian (Seilacher 1970, 1991) and is characterized by prominent lateral lobes defined by exopodal brushings flanked by marginal thin ridges (Seilacher 1970, 1991; Crimes 1975). The Cruziana rugosa group from the upper Tremadocian to Darriwilian (Seilacher 1970, 1994; Baldwin 1975, 1977; Buatois and Mángano 2011) is characterized by an absence of the external lobe (exopodal markings), and an internal pattern composed by sharp scratches from up to twelve subequal claws (Seilacher 1970, 1991; Crimes 1975). Both groups with different patterns overlap in the Tremadocian. This Early Ordovician change in the Cruziana morphology may record the diversification and the specialization of trilobite appendages (including the exopodites). Although Cruziana is hard to link to exact trilobite taxa, the change in trilobite faunal composition and the change in appendages morphologies are coincident in time with the change in traces.
Conclusions
Only one species of Anacheirurus, Anacheirurus adserai, is present in the Fezouata Shale, and is restricted to the upper Tremadocian strata. This species is diagnosed by eleven thoracic segments in the thorax, pygidium reduced with two axial rings plus terminal piece and one pair of pleural spines and seven posterior trunk pleural spines elongated.
Anacheirurus is a member of Pilekiinae, a subfamily of Cheiruridae characterized by bearing a wide pleural furrow that divides the thoracic pleura in the anterior and posterior part. Pilekiinae have rather conservative cranidia but highly variable pygidia. The number of pygidial tergites and the morphology of pygidial spines are the major characters that vary across the group.
The endopodites of Anacheirurus adserai have seven podomeres, with a reduced distal podomere carrying a tripartite claw, and elongated podomeres 5 and 6. The exopodites consist of a bipartite flap, with a proximal lobe carrying elongated lamellae in its posterior margin and a smaller distal lobe carrying shorter bristles. The overall morphology of the exopodite is similar to “Cambrian-type” and dissimilar to “post-Cambrian” trilobite appendages.
The changes in the Ordovician trilobite diversity are linked with major changes in trilobite exopodite morphology and with the diversification of the ichnogenus Cruziana.
Acknowledgements
We are grateful to the reviewers Juan Carlos Gutiérrez-Marco (Instituto de Geociencias, CSIC, UCM, Madrid, Spain) and Jorge Esteve (Universidad de los Andes, Bogotá, Colombia) for their comments which improved the manuscript. Specimen access was facilitated by Antoine Pictet (MGL); Susan Butts and Jessica Utrup (YPM); Bertrand Lefebvre (University of Lyon, France). We also gratefully acknowledge Jonathan Adrain (University of Iowa, Iowa City, USA) and Jorge Esteve for valuable discussions. This research was funded by the Swiss National Science Foundation, grant number 205321_179084 entitled “Arthropod Evolution during the Ordovician Radiation: Insights from the Fezouata Biota” and awarded to ACD. LL’s research was funded by the Czech Science Foundation (grant no. 20-23550Y), by the Center for Geosphere Dynamics (UNCE/SCI/006), and was conducted within institutional support RVO 67985831 of the Institute of Geology of the Czech Academy of Sciences.
References
Adrain, J.M. 1998. Systematics of the Acanthoparyphinae (Trilobita), with species from the Silurian of Arctic Canada. Journal of Paleontology 72: 698–718. Crossref
Adrain, J.M. 2011. Class Trilobita Walch, 1771. In: Z.-Q. Zhang (ed.), Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa 3148: 104–109. Crossref
Adrain, J.M. 2013. A synopsis of Ordovician trilobite distribution and diversity. Geological Society, London, Memoirs 38: 297–336. Crossref
Adrain, J.M. and Karim, T.S. 2019. Revision of the Early Ordovician (late Tremadocian; Stairsian) cheirurid trilobite Tesselacauda Ross, with species from the Great Basin, western USA. Zootaxa 4661: 201–255. Crossref
Adrain, J.M., Edgecombe, G.D., Fortey, R.A., Hammer, Ø., Laurie, J.R., McCormick, T., Owen, A.W., Waisfeld, B.G., Webby, B.D., and Westrop, S.R. 2004. Trilobites. In: B.D. Webby, M.L. Droser, F. Paris, and I.G. Percival (eds.), The Great Ordovician Biodiversification Event, 231–254. Columbia University Press, New York. Crossref
Adrain, J.M., Fortey, R.A., and Westrop, S.R. 1998. Post-Cambrian trilobite diversity and evolutionary faunas. Science 280: 1922–1925. Crossref
Adrain, J.M., Westrop, S.R., Karim, T.S., and Landing, E. 2014. Trilobite biostratigraphy of the Stairsian Stage (upper Tremadocian) of the Ibexian Series, Lower Ordovician, western United States. Memoirs of the Association of Australasian Palaeontologists 45: 167–214.
Antcliffe, J.B. and Brasier, M.D. 2011. Fossils with little relief: using lasers to conserve, image, and analyze the Ediacara biota. In: M. Laflamme, J.D. Schiffbauer, and S.Q. Dornbos (eds.), Quantifying the Evolution of Early Life, 223–240. Springer, Washington. Crossref
Aria, C. and Caron, J.-B. 2019. A middle Cambrian arthropod with chelicerae and proto-book gills. Nature 573: 586–589. Crossref
Baldwin, C.T. 1975. The stratigraphy of the Cabos Series in the section between Cadavedo and Luarca, province of Oviedo, NW of Spain. Breviora Geologica Asturica 19: 4–9.
Baldwin, C.T. 1977. Internal structures of trilobite trace fossils indicative of an open surface furrow origin. Palaeogeography, Palaeoclimatology, Palaeoecology 21: 273–284. Crossref
Bambach, R.K. 1983. Ecospace utilization and guilds in marine communities through the Phanerozoic. In: M.J.S. Tevesz and P.L. McCall (eds.), Biotic Interactions in Recent and Fossil Benthic Communities, 719–746. Plenum Press, New York. Crossref
Barrande, J. 1852. Systême silurien du centre de la Bohême. 1ère partie: Recherches Paléontologiques. Vol. I. Crustacés: Trilobites. 935 pp. Chez l’auteur et éditeur, Prague. Crossref
Barton, D.C. 1915. A revision of the Cheirurinae, with notes on their evolution. St Louis Washington University Studies 3: 101–151.
Beecher, C.E. 1893. On the thoracic legs of Triarthrus. American Journal of Science 3: 467–470. Crossref
Bergström, J. 1972. Appendage morphology of the trilobite Cryptolithus and its implications. Lethaia 5: 85–94.
Bergström, J. 1973. Organization, life, and systematics of trilobites. Fossils and Strata 2: 69. Crossref
Billings, E. 1870. Notes on some specimens of lower Silurian trilobites. Quarterly Journal of the Geological Society 26 (1–2): 479–486. Crossref
Bonino, E. and Kier, C. 2010. The Back to the Past Museum Guide to Trilobites. 494 pp. Editrice Marca, Bargazzo.
Broili, F. 1930. Weitere Funde von Trilobiten mit Gliedmassen aus dem rheinischen Unterdevon. Neues Jahrbuch für Mineralogie, Geologie und Paläontologie B 64: 293–306.
Bruton, D. and Haas, W. 1999. The anatomy and functional morphology of Phacops (Trilobita) from the Hunsrück Slate (Devonian). Palaeontographica A 253: 29–75.
Buatois, L.A. and Mángano, M.G. 2011. Ichnology: Organism-substrate Interactions in Space and Time. 358 pp. Cambridge University Press, Cambridge. Crossref
Bulman, O. and Rushton, A. 1973. Tremadoc faunas from boreholes in central England. Bulletin of the Geological Survey of Great Britain 43: 1–40.
Butterfield, N.J. 1995. Secular distribution of Burgess‐Shale‐type preservation. Lethaia 28: 1–13. Crossref
Butterfield, N.J. 2003. Exceptional fossil preservation and the Cambrian explosion. Integrative and Comparative Biology 43: 166–177. Crossref
Campbell, K.S. 1975. The functional morphology of Cryptolithus. Fossils and Strata 4: 65–86.
Chatterton, B. and Ludvigsen, R. 1976. Silicified Middle Ordovician trilobites from the South Nahanni River area, District of Mackenzie, Canada. Palaeontographica A 154: 1–106.
Choubert, G., Termier, H., and Termier, G. 1947. Sur la stratigraphie de l’Ordovicien marocain. Comptes Rendus Sommaires de la Société Géologique de France 16: 335–337.
Cisne, J.L. 1975. Anatomy of Triarthrus and the relationships of the Trilobita. Fossils and Strata 4: 45–63.
Cisne, J.L. 1981. Triarthrus eatoni (Trilobita): Anatomy of its exoskeletal, skeletomuscular, and digestive systems. Palaeontographica Americana 9: 99–140.
Congreve, C.R. and Lieberman, B.S. 2010. Phylogenetic and biogeographic analysis of deiphonine trilobites. Journal of Paleontology 84: 128–136. Crossref
Congreve, C.R. and Lieberman, B.S. 2011. Phylogenetic and biogeographic analysis of sphaerexochine trilobites. PloS ONE 6: e21304. Crossref
Corbacho, J. 2008. Nuevas especies de Lehua del Ordovíco inderior del Valle del Dra (Marruecos). Scripta Musei Geologici Seminarii Barcinonensis 5: 3–13.
Corbacho, J. and Vela, J.A. 2011. Revisión de las especies de Lehua de la región de Zagora, Marruecos. Batalleria 16: 46–49.
Crimes, T. 1975. The stratigraphical significance of trace fossils. In: R.W. Frey (ed.), The Study of Trace Fossils, 109–130. Springer, New York. Crossref
Daley, A.C., Antcliffe, J.B., Drage, H.B., and Pates, S. 2018. Early fossil record of Euarthropoda and the Cambrian Explosion. Proceedings of the National Academy of Sciences 115: 5323–5331. Crossref
Dalman, J.W. 1827. Om palaeaderna, eller de så kallade trilobiterna. Kungliga Svenska Vetenskapsakademiens Handlingar 1826: 113–294.
Dekay, J.E. 1824. Observations on the structure of trilobites, and descriptions of an apparently new genus. With notes on the geology of Trenton Falls by J. Renwick. Annals of the Lyceum of Natural History of New York 1: 174–189. Crossref
Demeter, E.J. 1973. Lower Ordovician pliomerid trilobites from western Utah. Brigham Young University Geology Studies 20: 37–65.
Destombes, J. 2006. Carte géologique au 1/200 000 de l’Anti‐Atlas marocain. Paléozoïque inférieur: Cambrien moyen et supérieur – Ordovicien – base du Silurien. Sommaire général sur les mémoires explicatifs des cartes géologiques au 1/200 000 de l’Anti‐Atlas marocain. Notes et Mémoires du Service Géologique du Maroc 515: 1–150.
Destombes, J., Hollard, H., and Willefert, S. 1985. Lower Palaeozoic rocks of Morocco. In: C. H. Holland (ed.), Lower Palaeozoic Rocks of the World: Lower Palaeozoic Rocks of Northwestern and West-Central Africa, 91–136. John Wiley & Sons, Chichester.
Destombes, J., Sougy, J., and Willefert, S. 1969. Révisions et découvertes paléontologiques (Brachiopodes, Trilobites et Graptolites) dans le Cambro-Ordovicien du Zemmour (Mauritanie septentrionale). Bulletin de la Société geologique de France 6: 185–206. Crossref
Droser, M.L. and Finnegan, S. 2003. The Ordovician Radiation: a follow-up to the Cambrian explosion? Integrative and Comparative Biology 43: 178–184. Crossref
Ebbestad, J.O.R. 1999. Trilobites of the Tremadoc Bjørkåsholmen Formation in the Oslo Region, Norway. Fossils and Strata 47: 1–188.
Edgecombe, G.D., Chatterton, B.D., Vaccari, N.E., and Waisfeld, B.G. 1999. Ordovician cheirurid trilobites from the Argentine Precordillera. Journal of Paleontology: 1155–1175. Crossref
Egorova, L.I., Lomovitskaya, M.P. [Lomovitskaâ, M.P.], Poletaeva, O.K., and Sivov, A.G. 1955. Klass Trilobita, trilobites [in Russian]. In: L.L. Kalfin (ed.), Atlas indeksnyh form iskopaemyh faun i flor zapadnyh Sibir’, 102–145. Gosgeoltexizdat, Moskva.
Esteve, J. and Yuan J.-L. 2017. Palaeoecology and evolutionary implications of enrolled trilobites from the Kushan Formation, Guzhangian of North China. Historical Biology 29: 328–340. Crossref
Esteve J., Hughes N.C, and Zamora, S. 2011. Purujosa trilobite assemblage and the evolution of trilobite enrollment. Geology 39: 575–578. Crossref
Esteve, J., Hughes, N.C., and Zamora, S. 2013. Thoracic structure and enrolment style in middle Cambrian Eccaparadoxides pradoanus presages caudalization of the derived trilobite trunk. Palaeontology 56: 589–601. Crossref
Esteve, J., Zhao, Y., and Peng, J. 2017. Morphological assessment of the Cambrian trilobites Oryctocephalus indicus (Reed 1910) from China and Oryctocephalus “reticulatus” (Lermontova 1940) from Siberia. Lethaia 50: 175–193. Crossref
Feist, R. 1991. The Late Devonian trilobite crises. Historical Biology 5: 197–214. Crossref
Fortey, R. 1980. The Ordovician trilobites of Spitsbergen. Part 3, remaining trilobites of the Valhallfonna Formation. Norsk Polarinstitutt Skrifter 171: 1–161.
Fortey, R.A. 2009. A new giant Asaphid trilobite from the Lower Ordovician of Morocco. Memoirs of the Association of Australasian Palaeontologists 37: 9–16.
Gaines, R.R., Briggs, D.E., and Yuanlong, Z. 2008. Cambrian Burgess Shale-type deposits share a common mode of fossilization. Geology 36: 755–758. Crossref
Gapp, I.W., Congreve, C.R., and Lieberman, B.S. 2012. Unraveling the phylogenetic relationships of the Eccoptochilinae, an enigmatic array of Ordovician cheirurid trilobites. PLoS ONE 7: e49115. Crossref
Green, J. 1832. A Monograph of the Trilobites of North America: With Coloured Models of the Species. 93 pp. J. Brano, Philadelphia. Crossref
Gutiérrez-Marco, J.C. and García-Bellido, D.C. 2018. The international fossil trade from the Paleozoic of the Anti-Atlas, Morocco. Geological Society, London, Special Publications 485: 1–28. Crossref
Gutiérrez-Marco, J.C. and Martin, E.L. 2016. Biostratigraphy and palaeoecology of Lower Ordovician graptolites from the Fezouata Shale (Moroccan Anti-Atlas). Palaeogeography, Palaeoclimatology, Palaeoecology 460: 35–49. Crossref
Gutiérrez-Marco, J.C., García-Bellido, D.C., Rábano, I., and Sá, A.A. 2017. Digestive and appendicular soft-parts, with behavioural implications, in a large Ordovician trilobite from the Fezouata Lagerstätte, Morocco. Scientific Reports 7: 39728. Crossref
Hall, J. 1838. Descriptions of two species of Trilobites belonging to the genus Paradoxides. American Journal of Science and Arts 13: 139–142.
Hammann, W. 1971. Stratigraphische Einteilung des spanischen Ordoviziums nach Dalmanitacea und Cheirurina (Trilobita). Mémoires. Bureau de Recherches Géologique et Minières 73: 265–272.
Harrington, H.J. 1938. Sobre las faunas del Ordovicico inferior del norte Argentino. Revista del Museo de La Plata, Paleontologia 4: 109–289.
Harrington, H.J. 1957. Notes on new genera of Pliomeridae (Trilobita). Journal of Paleontology 31: 811–812.
Harrington, H.J. and Leanza, A.F. 1957. Ordovician trilobites of Argentina. 276 pp. University of Kansas Press, Lawrence.
Hawle, I. and Corda, A.J. 1847. Prodrom einer Monographie der böhmischen Trilobiten. 176 pp. JG Calve, Prague.
Holmes, J.D., Paterson, J.R., and García-Bellido, D.C. 2020. The trilobite Redlichia from the lower Cambrian Emu Bay Shale Konservat-Lagerstätte of South Australia: systematics, ontogeny and soft-part anatomy. Journal of Systematic Palaeontology 18: 295–334. Crossref
Hou, X., Clarkson, E.N.K., Yang, J., Zhang, X., Wu, G., and Yuan, Z. 2008. Appendages of early Cambrian Eoredlichia (Trilobita) from the Chengjiang biota, Yunnan, China. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 99: 213–223. Crossref
Hughes, N.C. 1995. Trilobite taphonomy and taxonomy: a problem and some implications. Palaios 10: 283–285. Crossref
Hughes, N.C. 2003. Trilobite body patterning and the evolution of arthropod tagmosis. BioEssays 25: 386–395. Crossref
Hughes, N.C. 2005. Trilobite construction: building a bridge across the micro and macroevolutionary divide. In: D. Briggs (ed.), Evolving Form and Function: Fossils and Development, 138–158. Peabody Museum of Natural History, Yale University, New Haven.
Hughes, N.C. 2007. The evolution of trilobite body patterning. Annual Review of Earth and Planetary Sciences 35: 401–434. Crossref
Hughes, N.C. and Rushton, A.A. 1990. Computer-aided restoration of a Late Cambrian ceratopygid trilobite from Wales, and its phylogenetic implications. Palaeontology 33: 429–445.
Hughes, N.C., Chapman, R.E., and Adrain, J.M. 1999. The stability of thoracic segmentation in trilobites: a case study in developmental and ecological constraints. Evolution & Development 1: 24–35. Crossref
Hughes, N.C., Minelli, A., and Fusco, G. 2006. The ontogeny of trilobite segmentation: a comparative approach. Paleobiology 32: 602–627. Crossref
Jell, P. 1985. Tremadoc trilobites of the Digger Island Formation, Waratah Bay, Victoria. Memoirs of the Museum of Victoria 46: 53–88. Crossref
Jell, P. and Adrain, J.M. 2002. Available generic names for trilobites. Memoirs-Queensland Museum 48: 331–552.
Kayser, E. 1880. Dechenella, eine devonische Gruppe der Gattung Phillipsia. Zeitschrift der Deutschen Geologischen Gesellschaft 32: 703–707.
Kobayashi, T. 1934. The Cambro-Ordovician formations and faunas of South Chosen. Palaeontology. Part I, Middle Ordovician faunas. Journal of the Faculty of Science, Imperial University of Tokyo, Section 2 3: 329–519.
Laibl, L., Fatka, O., Crônier, C., and Budil, P. 2014. Early ontogeny of the Cambrian trilobite Sao hirsuta from the Skryje-Týřovice Basin, Barrandian area, Czech Republic. Bulletin of Geosciences 89: 291–207. Crossref
Landing, E., Antcliffe, J.B., Geyer, G., Kouchinsky, A., Bowser, S.S., and Andreas, A. 2018. Early evolution of colonial animals (Ediacaran Evolutionary Radiation–Cambrian Evolutionary Radiation–Great Ordovician Biodiversification Interval). Earth-Science Reviews 178: 105–135. Crossref
Lane, P.D. 2002. The taxonomic position and coaptative structures of the Lower Ordovician trilobite Cyrtometopus. Special Papers in Palaeontology 67: 153–170.
Lane, P.D. 1971. British Cheiruridae (Trilobita). Palaeontographical Society Monopgraph 530: 1–95.
Lebrun, P. 2018. Fossils From Morocco. Volume I. Emblematic Localities From the Palaeozoic Of the Anti-Atlas. 298 pp. Saint-Julien-du-Pinet, France.
Lee, D.-C. and Chatterton, B.D. 1997. Ontogenies of trilobites from the Lower Ordovician Garden City Formation of Idaho and their implications for the phylogeny of the Cheirurina. Journal of Paleontology 71: 683–702. Crossref
Lefebvre, B., Gutiérrez‐Marco, J.C., Lehnert, O., Martin, E.L., Nowak, H., Akodad, M., El Hariri, K., and Servais, T. 2018. Age calibration of the Lower Ordovician Fezouata Lagerstätte, Morocco. Lethaia 51: 296–311. Crossref
Lehnert, O., Nowak, H., Sarmiento, G.N., Gutiérrez-Marco, J.C., Akodad, M., and Servais, T. 2016. Conodonts from the Lower Ordovician of Morocco—contributions to age and faunal diversity of the Fezouata Lagerstätte and peri-Gondwana biogeography. Palaeogeography, Palaeoclimatology, Palaeoecology 460: 50–61. Crossref
Lu, Y.H., Chu, C.L., Chien, Y.Y., Zhou, Z.Y., Chen, J.Y., Liu, G.W., Yu, W., Chen, X., and Xu, H.K. 1976. Ordovician biostratigraphy and palaeozoogeography of China. Nanjing Institute of Geology and Palaeontology, Academia Sinica, Memoir 7: 1–83.
Mansuy, H. 1916. Faunes Cambriennes de l’Extrême-Orient méridional Mémoires du Service Géologique de L’Indochine 5 1: 1–44.
Martin, E.L., Pittet, B., Gutiérrez-Marco, J.-C., Vannier, J., El Hariri, K., Lerosey-Aubril, R., Masrour, M., Nowak, H., Servais, T., and Vandenbroucke, T.R. 2016a. The Lower Ordovician Fezouata Konservat-Lagerstätte from Morocco: age, environment and evolutionary perspectives. Gondwana Research 34: 274–283. Crossref
Martin, E.L., Vidal, M., Vizcaïno, D., Vaucher, R., Sansjofre, P., Lefebvre, B., and Destombes, J. 2016b. Biostratigraphic and palaeoenvironmental controls on the trilobite associations from the Lower Ordovician Fezouata Shale of the central Anti-Atlas, Morocco. Palaeogeography, Palaeoclimatology, Palaeoecology 460: 142–154. Crossref
Mergl, M. 1984. Fauna of the upper Tremadocian of central Bohemia. Sborník geologickćh věd Paleontologie 26: 9–46.
Mergl, M. 2006. Tremadocian trilobites of the Prague Basin, Czech Republic. Acta Musei Nationalis Pragae, Series B, Historia Naturalis 62: 1–70.
Moore, R. (ed.) 1959. Treatise on Invertebrate Paleontology, Part O, Arthropoda 1. 560 pp. Geological Society of America and University of Kansas Press, Lawrence.
Moork, H., Servais, T., Pittet, B., Vaucher, R., Akodad, M., Gaines, R.R., and Vandenbroucke, T.R. 2016. Palynomorphs of the Fezouata Shale (Lower Ordovician, Morocco): age and environmental constraints of the Fezouata Biota. Palaeogeography, Palaeoclimatology, Palaeoecology 460: 62–74.
Nowak, H., Servais, T., Pittet, B., Vaucher, R., Akodad, M., Gaines, R.R., and Vandenbroucke, T.R. 2016. Palynomorphs of the Fezouata Shale (Lower Ordovician, Morocco): age and environmental constraints of the Fezouata Biota. Palaeogeography, Palaeoclimatology, Palaeoecology 460: 62-74.Crossref
Öpik, A. 1937. Trilobiten aus Estland. Acta et Commentationes Universitatis Tartuensis, A 32: 1–163.
Ortega-Hernández, J., Janssen, R., and Budd, G.E. 2017. Origin and evolution of the panarthropod head—a palaeobiological and developmental perspective. Arthropod Structure & Development 46: 354–379. Crossref
Ortega‐Hernández, J., Legg, D.A., and Braddy, S.J. 2013. The phylogeny of aglaspidid arthropods and the internal relationships within Artiopoda. Cladistics 29: 15–45. Crossref
Pärnaste, H. 2004. Early Ordovician Trilobites of Suborder Cheirurina in Estonia and NW Russia: Systematics, Evolution and Distribution. 58 pp. Tartu University Press, Tartu.
Peng, S. 1984. Cambrian–Ordovician boundary in the Cili-Taoyuan border area, northwestern Hunan. In: Nanjing Institute of Geology and Palaeontology, Academia Sinica (ed.), Stratigraphy and Paleontology of Systemic Boundaries in China, Cambrian and Ordovician Boundary, 285–405. Anhui Science and Technology Publishing House, Hefei.
Peng, S. 1990. Tremadoc stratigraphy and trilobite faunas of northwestern Hunan. Trilobites from the Panjiazui Formation and the Madaoyu Formation in Jiangnan Slope Belt. Beringeria 2: 55–171.
Pérez-Peris, F., Laibl, L., Lustri, L., Gueriau, P., Antcliffe, J.B., Enright, O.G.B., and Daley, A.C. 2021. A new nektaspid euarthropod from the Lower Ordovician strata of Morocco. Geological Magazine 158: 509–517. Crossref
Prantl, F. and Přibyl, A. 1947. Classification of some Bohemian Cheiruridae (Trilobitae). Sborník Národního Musea Praze 3: 1–44.
Přibyl, A., Vaněk, J., and Pek, I. 1985. Phylogeny and taxonomy of family Cheiruridae (Trilobita). Acta Universitatis Palackianae Olomucensis Facultas rerum naturalium 83: 107–193.
Ramsköld, L. and Edgecombe, G. 1996. Trilobite appendage structure—Eoredlichia reconsidered. Alcheringa 20: 269–276. Crossref
Raymond, P.E. 1920. The appendages, anatomy, and relationships of trilobites. Memoirs of the Connecticut Academy of Arts and Sciences 7: 1–169. Crossref
Reed, F.C. 1896. Notes on the evolution of the genus Cheirurus. Geological Magazine 3: 117–123. Crossref
Rominger, C. 1887. Description of primordial fossils from Mount Stephens, N.W. Territories of Canada. Proceedings of the Academy of Natural Sciences of Philadelphia: 12–19.
Rosova, A. 1960. Upper Cambrian trilobites of Salair [in Russian]. Trudy Instituta Geologii i Geofiziki. Sibirskoe Otdelenie, Akademiâ Nauk SSSR 5: 1–116.
Ross, R.J. 1951. Stratigraphy of the Garden City Formation in northeastern Utah, and its trilobite faunas. Bulletin of Peabody Muesum of Natural History 6: 1–116.
Růžička, R. 1926. Fauna vrstev Eulomových rudního ložiska u Holoubkova (v Ouzkém). Část I. Trilobiti. Rozpravy České akademie věd a umění 35 (39): 1–26.
Saleh, F., Antcliffe, J.B., Lefebvre, B., Pittet, B., Laibl, L., Peris, F.P., Lustri, L., Gueriau, P., and Daley, A.C. 2020a. Taphonomic bias in exceptionally preserved biotas. Earth and Planetary Science Letters 529: 115873. Crossref
Saleh, F., Vaucher, R., Antcliffe, J.B., Daley, A.C., El Hariri, K., Kouraiss, K., Lefebvre, B., Martin, E.L., Perrillat, J.P., and Sansjofre, P. 2020b. Insights into soft-part preservation from the Early Ordovician Fezouata Biota. Earth-Science Reviews 213: 103464. Crossref
Salter, J.W. 1864. A monograph of British trilobites. Part I. Monographs of the Palaeontographical Society 16 (67): 1–83.
Salter, J.W. 1866. On the fossils of North Wales. Memoirs of the Geological Survey of Great Britain 3: 1–381.
Schmidt, J.G. 1881. Ueber die Einwirkung von Aceton auf Furfurol und auf Bittermandelöl bei Gegenwart von Alkalilauge. Berichte der deutschen chemischen Gesellschaft 14: 1459–1461. Crossref
Scholtz, G. and Edgecombe, G.D. 2005. Heads, hox and the phylogenetic position of trilobites. Crustacean Issues 16: 139. Crossref
Scholtz, G. and Edgecombe, G.D. 2006. The evolution of arthropod heads: reconciling morphological, developmental and palaeontological evidence. Development Genes and Evolution 216: 395–415. Crossref
Sdzuy, K. 1955. Die Fauna der Leimitz-Schiefer (Tremadoc). Abhandlungen der senckenbergischen naturforschenden Gesellschaft 492: 1–74.
Sdzuy, K., Hammann, W., and Villas, E. 2001. The upper Tremadoc fauna from Vogtendorf and the Bavarian Ordovician of the Frankenwald (Germany). Senckenbergiana lethaea 81: 207–261. Crossref
Seilacher, A. 1962. Form und funktion des trilobiten-daktylus. Paläontologische Zeitschrift 36: 218–227. Crossref
Seilacher, A. 1970. Cruziana stratigraphy of “non-fossiliferous” Palaeozoic sandstones. In: T.P. Crimes and J.C. Harper (eds.), Trace Fossils, 447–476. The Seel House Press, Liverpool.
Seilacher, A. 1991. An updated Cruziana stratigraphy of Gondwana Palaeozoic sandstones. In: M.J. Salem (ed.), Symposium on the Geology of Libya, 1565–1581. Elsevier, Amsterdam.
Seilacher, A. 1994. How valid is Cruziana stratigraphy? Geologische Rundschau 83: 752–758. Crossref
Servais, T. and Harper, D.A. 2018. The great Ordovician Biodiversification Event (GOBE): definition, concept and duration. Lethaia 51: 151–164. Crossref
Servais, T., Owen, A.W., Harper, D.A., Kröger, B., and Munnecke, A. 2010. The Great Ordovician Biodiversification Event (GOBE): the palaeoecological dimension. Palaeogeography, Palaeoclimatology, Palaeoecology 294: 99–119. Crossref
Signor, P.W. and Vermeij, G.J. 1994. The plankton and the benthos: origins and early history of an evolving relationship. Paleobiology 20: 297–319. Crossref
Stein, M. and Selden, P.A. 2012. A restudy of the Burgess Shale (Cambrian) arthropod Emeraldella brocki and reassessment of its affinities. Journal of Systematic Palaeontology 10: 361–383. Crossref
Stigall, A.L., Edwards, C.T., Freeman, R.L., and Rasmussen, C.M.Ø. 2019. Coordinated biotic and abiotic change during the Great Ordovician Biodiversification Event: Darriwilian assembly of early Paleozoic building blocks. Palaeogeography, Palaeoclimatology, Palaeoecology 530: 249–270. Crossref
Stigall, A.L., Freeman, R.L., Edwards, C.T., and Rasmussen, C.M. 2020. A multidisciplinary perspective on the Great Ordovician Biodiversification Event and the development of the early Paleozoic world. Palaeogeography, Palaeoclimatology, Palaeoecology 543: 109521. Crossref
Størmer, L. 1939. Studies on trilobite morphology. Part I. The thoracic appendages and their phylogenetic significance. Norsk Geologisk Tidsskrift 19: 143–273.
Størmer, L. 1951. Studies on trilobite morphology. Part III. The ventral cephalic sutures with remarks on the zoological position of the trilobites. Norsk Geologisk Tidsskrift 29: 108–158.
Stürmer, W. 1968. Einige Beobachtungen an devonischen Fossilien mit Röntgenstrahlen. Natur und Museum 98: 413–417.
Stürmer, W. 1970. Soft parts of cephalopods and trilobites: some surprising results of X-ray examinations of Devonian slates. Science 170: 1300–1302. Crossref
Stürmer, W. and Bergström, J. 1973. New discoveries on trilobites by X-rays. Paläontologische Zeitschrift 47: 104–141. Crossref
Ulrich, E.O. 1879. Descriptions of new genera and species of fossils from the Lower Silurian about Cincinnati. Journal of the Cincinnati Society of Natural History 2: 8–30.
Van Roy, P., Briggs, D.E., and Gaines, R.R. 2015a. The Fezouata fossils of Morocco; an extraordinary record of marine life in the Early Ordovician. Journal of the Geological Society 172: 541–549. Crossref
Van Roy, P., Daley, A.C., and Briggs, D.E. 2015b. Anomalocaridid trunk limb homology revealed by a giant filter-feeder with paired flaps. Nature 522: 77–80. Crossref
Van Roy, P., Orr, P.J., Botting, J.P., Muir, L.A., Vinther, J., Lefebvre, B., El Hariri, K., and Briggs, D.E. 2010. Ordovician faunas of Burgess Shale type. Nature 465: 215–218. Crossref
Vaněk, J. 1965. Die Trilobiten des mittelböhmischen Tremadoc. Senckenbergiana lethaea 46: 263–308.
Vaucher, R., Martin, E.L., Hormière, H., and Pittet, B. 2016. A genetic link between Konzentrat-and Konservat-Lagerstätten in the Fezouata Shale (lower Ordovician, Morocco). Palaeogeography, Palaeoclimatology, Palaeoecology 460: 24–34. Crossref
Vela, J.A. 2007. Three new species of Lehua from the Lower Ordovician of Dra Valley (Morocco). Scripta Musei Geologici Seminarii Barcinonensis (Serries Palaeontologica) 4: 24–37.
Vela, J.A. and Corbacho, J. 2007. A new species of Lehua from Lower Ordovician of Dra Valley of Morocco. Batalleria 13: 75–80.
Valent, M. and Corbacho, J. 2015. Pauxillites thaddei a new Lower Ordovician hyolith from Morocco. Sborník Národního Muzea v Praze B 71: 51–54. Crossref
Walcott, C.D. 1912. Cambrian Geology and Paleontology II. Middle Cambrian Branchiopoda, Malacostraca, Trilobita and Merostomata. Smithsonian Miscellaneous Collections 57: 145–228.
Walcott, C.D. 1918. Cambrian Geology and Paleontology IV. Appendages of trilobites. Smithsonian Miscellaneous Collections 67: 115–216.
Whittard, W.F. 1966. The Ordovician trilobites of the Shelve Inlier, west Shropshire. Part VIII. Monographs of the Palaeontographical Society 508: 265–306.
Whittard, W. 1967. The trilobite Anacheirurus frederici (Salter) from the Tremadoc series of north Wales. Geological Magazine 104: 284–288. Crossref
Whittington, H.B. 1961. Middle Ordovician Pliomeridae (Trilobita) from Nevada, New York, Quebec, Newfoundland. Journal of Paleontology 31: 911–922.
Whittington, H.B. 1975. Trilobites with appendages from the middle Cambrian, Burgess Shale, British Columbia. Fossils and Strata 4: 97–136.
Whittington, H.B. 1980. Exoskeleton, moult stage, appendage morphology, and habits of the Middle Cambrian trilobite Olenoides serratus. Palaeontology 23: 171–204.
Whittington, H.B. and Almond, J. 1987. Appendages and habits of the Upper Ordovician trilobite Triarthrus eatoni. Philosophical Transactions of the Royal Society of London. B, Biological Sciences 317 (1182): 1–46. Crossref
Yin, G. and Li, S. 1978. Trilobita [in Chinese]. In: Guizhou Working Group of Stratigraphy and Paleontology (ed.), Paleontological Atlas of Southwest China. Guizhou Volume (1) Cambrian–Devonian, 143–192. Geological Publishing House, Beijing.
Zeng, H., Zhao, F., Yin, Z., and Zhu, M. 2017. Appendages of an early Cambrian metadoxidid trilobite from Yunnan, SW China support mandibulate affinities of trilobites and artiopods. Geological Magazine 154: 1306–1328. Crossref
Zhang, W.T. and Fan, J. 1960. Ordovician and Silurian trilobites of the Chilian Mountains [in Chinese]. In: T. Yin (ed.), Geological Gazetter of the Chilian Mountains 4, 83–148. Science Press, Beijing.
Zhang, W.T., Lu, Y.H., Zhu, Z.L., Qian, Y.Y., Lin, H. L., Zhou, Z.Y., Zhang, S.G., and Yuan, J.L. 1980. Cambrian Trilobite Faunas of Southwestern China [in Chinese with English summary]. 497 pp. Science Press, Beijing.
Zhou, T.M., Liu, Y.R., Meng, X.S., and Sun, Z.H. 1977. Trilobita [in Chinese]. In: X.F. Wang and Y.Q. Jin (eds.), Palaeontological Atlas of Central and South China 1, 104–266. Geological Publishing House, Beijing.
Acta Palaeontol. Pol. 66 (4): 857–877, 2021
https://doi.org/10.4202/app.00902.2021