


Paedomorphosis and neurocranial ossification in two Devonian lungfishes
MARIE BOIROT, TOM CHALLANDS, and RICHARD CLOUTIER
Boirot, M., Challands, T., and Cloutier, R. 2022. Paedomorphosis and neurocranial ossification in two Devonian lungfishes. Acta Palaeontologica Polonica 67 (2): 283–295.
Lungfishes are one of the few early vertebrate clades with a rich 410-million-years-old fossil record. Lungfishes are characterized by a low evolutionary rate assumed to be associated with paedomorphosis since the Late Devonian. Lungfish paedomorphic trends include a reduction of the number of median fins, reduction of the number of cranial dermal bones, and reduction of the degree of neurocranial ossification. This neurocranial trait has evolved from heavily ossified in Devonian species to completely cartilaginous in post-Devonian species. Neurocranial conditions among derived Devonian lungfishes are needed to have a better understanding of paedomorphosis as a driving force during lungfish evolution. The neurocrania of two Devonian species, Scaumenacia curta (middle Frasnian, Escuminac Formation, eastern Canada) and Pentlandia macroptera (Givetian, Orcadian Basin, Scotland), have been micro-CT-scanned. These species were assumed to have a cartilaginous neurocranium like other “phaneropleurids” and “fleurantids”. Juvenile (or sub-adult) and adult specimens of S. curta possess cartilaginous neurocrania, whereas P. macroptera is now recognized to have a poorly ossified neurocranium. Pyrite filled neurocranial cavities preserving some endocranial structures (e.g., olfactory bulbs, semicircular canals) allow us to code for phylogenetic endocranial characters in S. curta. This unique mode of preservation suggests that occasionally pyrite is a preservative rather than a destructive diagenetic agent. In the evolutionary gap between Pentlandia and Scaumenacia, paedomorphosis had already resulted in reduction of neurocranial ossification while little changes occurred in cranial dermal bones.
Key words: Dipnoi, heterochrony, ontogeny, phylogeny, pyritization, Escuminac Formation, Orcadian Basin.
Marie Boirot [marie.boirot@uqar.ca], Laboratoire de Paléontologie et Biologie évolutive, Université du Québec à Rimouski, 300 allée des Ursulines, Rimouski, Québec, G5L 3A1, Canada.
Tom Challands [Tom.Challands@ed.ac.uk], University of Edinburgh, School of Geosciences, Grant Institute of Earth Sciences, James Hutton Road, Edinburgh, EH9 3FE, Scotland, UK.
Richard Cloutier [richard_cloutier@uqar.ca], Laboratoire de Paléontologie et Biologie évolutive, Université du Québec à Rimouski, 300 allée des Ursulines, Rimouski, Québec, G5L 3A1, Canada.
Received 15 October 2020, accepted 2 March 2022, available online 17 June 2022.
Copyright © 2022 M. Boirot et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Lungfishes, the living sister-group of tetrapods (Cloutier and Ahlberg 1996; Irisarri and Meyer 2016), form a monophyletic group that originated approximately 410 million years ago (Chang and Yu 1984). An adaptive radiation led to a great diversity of species during the Devonian (Lloyd et al. 2011; Friedman and Sallan 2012). There are only six living species: the Australian lungfish Neoceratodus forsteri, the South-American Lepidosiren paradoxa and four species of the Protopterus living in Africa, but more than 280 species have been identified in the fossil record (Schultze 1992; Cloutier and Ahlberg 1996; Nelson et al. 2016). Lungfishes have been studied for more than 150 years, but the phylogenetic relationships among the stem-group are not agreed upon (Miles 1977; Marshall 1986; Campbell and Barwick 1990; Schultze and Marshall 1993; Krupina and Reisz 2000; Schultze 2001; Ahlberg et al. 2006; Friedman 2007; Clack et al. 2011; Lloyd et al. 2011; Challands 2015).
Lungfishes are known primarily from their characteristic tooth plates and skull roofing bones (Campbell and Barwick 1990; Cloutier and Ahlberg 1996; Ahlberg et al. 2006; Friedman 2007). The number, arrangement and connection of skull roofing bones, particularly during the Devonian, present a great deal of intraspecific variation and ontogenetic changes (White 1965; Cloutier 1997; Kemp 1999), which might potentially result in coding atypical conditions (in particular supernumerary bones) as a standard (or normal) condition in phylogenetic analyses. Characters coded from structures less subject to intraspecific variation can potentially minimize the number of atypical conditions coded as standard in the phylogeny. This problem combined with the study of few or partially preserved specimens is frequently encountered in palaeontology. The neurocranium is considered to be more stable anatomically than the dermatocranium because it is more integrated with the nervous system (Lieberman et al. 2000; Richtsmeier et al. 2006; Willmore et al. 2007), and thus could be a key element to increasing the resolution of unclear phylogenetic relationships. Unfortunately, the neurocranium is rarely 3D-preserved in fossils. First, the ossification of the neurocranium (endochondral and/or perichondral bone) is more fragile than the dermal ossification of the skull roof (de Beer 1937; Schaeffer and Scott 1971; Miles 1977; Bemis 1984). Second, this differential preservation is also a result of lungfish evolutionary history, which comprises a great reduction in ossification degree for some structures (Clack et al. 2011; Clement and Ahlberg 2014). A preserved neurocranium has only been found in one post-Devonian lungfish, Persephonichthys chtonica (Pardo et al. 2014).
This neurocranial reductive evolutionary trend present in lungfishes, associated with other morphological changes, has been repeatedly inferred to result from paedomorphosis or neoteny (Moy-Thomas and Miles 1971; Gardiner 1973; Smith 1977; Bemis 1984; Joss 1998, 2006). Paedomorphosis, a reduction of development rate, is a retention of ancestral juvenile characters at sexual maturity for the adult descendants, while neoteny, a type of paedomorphosis, is produced by the retardation of somatic development (Gould 1977; Alberch et al. 1979; McNamara 1982). For lungfishes, paedomorphosis, presumably due to their increased genome size (Joss 2006) resulting in a longer cell cycle, affects numerous developmental aspects, and particularly ossification of endochondral bones of the skull, pectoral girdle, snout, and visceral skeleton. Post-Devonian lungfishes have a cartilaginous neurocranium and snout (Bemis 1984; Clack et al. 2011; Clement and Ahlberg 2014; Clement et al. 2015; Criswell 2015).
For the past 20 years, tomography (e.g., CT-scan, nano-CT-scan, micro-CT-scan, synchrotron) allowed non-invasive studies of internal cranial structures in palaeontology (Sutton 2008; Walsh and Knoll 2011; Walsh et al. 2013). The decreasing degree of neurocranial ossification throughout the early evolution of lungfishes is more easily observable as a result of new visualization technologies. Primitive lungfishes possessing ossified neurocrania have been previously scanned and their neurocrania virtually 3D-reconstructed using micro-CT-scanning. These include: Dipnorhynchus sussmilchi (Emsian, Early Devonian; Clement et al. 2016), Dipterus valenciennesi (Eifelian–Givetian, Middle Devonian; Challands 2015), Chirodipterus australis (Frasnian, Late Devonian; Henderson and Challands 2018), Rhinodipterus kimberleyensis (Frasnian, Late Devonian; Clement and Ahlberg 2014) and the living species Neoceratodus forsteri (Clement et al. 2015). The fossil species mentioned above possess quite heavily ossified neurocrania, a condition considered to be an early and primitive feature (Bemis 1984; Clement and Ahlberg 2014). The pivotal point in lungfish evolution between an ossified and cartilaginous neurocranium is not precisely known, but it is frequently assumed to have occurred during the Late Devonian, associated with a general decrease in ossification (Bemis 1984). No CT-scan study has been performed on more derived extinct lungfishes other than the Permian form Persephonichthys cthonica (Pardo et al. 2014). Two fairly well-preserved Middle–Late Devonian species, Scaumenacia curta (Whiteaves, 1881) and Pentlandia macroptera (Traquair, 1888) are potential candidates to be CT-scanned in order to determine the condition of their neurocrania.
Scaumenacia curta, from the Upper Devonian (middle Frasnian), comes from the Escuminac Formation, Miguasha, Québec, Canada. It is one of the most abundant lower vertebrate species of the Escuminac Formation assemblage (Cloutier 2013). More than 3000 specimens of S. curta have been discovered, including larvae, juveniles, and adults preserved in three dimensions. It is one of the best-known fossil species of lungfish (Cloutier 1996). Scaumenacia curta neurocrania is mentioned in various research (Stensiö 1947; Cloutier 1996, Challands and den Blaauwen 2016) but it has never been studied nor scanned.
Pentlandia macroptera is a Givetian (Middle Devonian) species found in North-East Scotland, in various localities and stratigraphic formations of the Orcadian Basin. Pentlandia macroptera was poorly studied before the comprehensive work of Jude et al. (2014) and Challands and den Blaauwen (2016). The neurocranium has not been studied but it is assumed to be cartilaginous (Jude et al. 2014; Challands and den Blaauwen 2016). Pentlandia macroptera is considered to be the most derived lungfish species from the Middle Devonian.
Scaumenacia curta and Pentlandia macroptera lived at a time of high rate of morphological evolution and high species diversity within lungfishes (Lloyd et al. 2011, Challands and den Blaauwen 2016). Both Devonian species share derived palatal characters found later in post-Devonian species (Cloutier 1996; Challands and den Blaauwen 2016). Because of their age and hypothesized advanced phylogenetic position among Devonian lungfishes (Thomson and Campbell 1971; Miles 1977; Campbell and Barwick 1990; Cloutier 1996, 1997; Lloyd et al. 2011; Challands and den Blaauwen 2016; Challands et al. 2019), the joint study of S. curta and P. macroptera could help to identify patterns or processes associated to the transition between ossified and cartilaginous neurocrania, and by extension the impact of paedomorphosis on endoskeletal ossification. Since the precise phylogenetic position of the two taxa remains questionable, our assessment of the neurocrania of these two species will help understanding the condition near the transition rather than documenting the transition itself. S. curta and P. macroptera are expected to possess cartilaginous, or lightly ossified neurocrania, confirming previous hypotheses. We also analyze neurocranial characters phylogenetically, retrieved from the CT-scan and 3D reconstructions, using primarily the matrix of Clement et al. (2016) and modified by Henderson and Challands (2018).
Institutional abbreviations.—MHNM, Musée d’Histoire Naturelle de Miguasha, Miguasha National Park, Miguasha, Canada; NMS, National Museums Scotland, Edinburgh, Scotland, UK.
Other abbreviations.—CI, consistency index; RI, retention index; SkL, skull length; TL, total length.
Material and methods
Scanning and segmentation.—Five 3D-preserved specimens of Scaumenacia curta of different sizes (i.e., MHNM 04-267, SkL: 26.1 mm; MHNM 04-243, SkL: 27.2 mm; MHNM 04-898B, SkL: 28.6 mm; MHNM 04-916, SkL: 31.6 mm; and MHNM 04-728A, SkL: 118 mm) were scanned and reconstructed using a High-Resolution X-ray Computed Tomography Skyscan 1173 (Skyscan NV 2011) at the Université du Québec à Rimouski. Specimens were selected based on the presence of complete articulated dermatocranium, 3D preservation of the skull and lower jaw, absence of pyrite (FeS2) damaging the outer surface of the dermatocranium, and the size of the sample that fit the CT-scan chamber.
The larger specimen (MHNM 04-728A, Fig. 1) was scanned using the following parameters: 130 kV, 61 μA, brass filter, 3000 ms exposure time, 3674 projections with an angular step of 0.2°, voxel resolution of 33.05 µm. The reconstruction has been made using NRecon (Skyscan NV 2011) and generated Tiff (16 bytes) images. Drishti Import and Drishti 2.6.4 and 2.6.5 (beta version) (Limaye 2012) were used for manual segmentation of the neurocranium. After segmentation, individual parts were reconstructed using Windows 3D objects and the final model was smoothed by a factor of two before export. The four smaller specimens were scanned using the same parameters except the resolution, which changed with the specimen size (voxel resolution of 34.8 µm for MHNM 04-243, 30.20 µm for MHNM 04-916, 22.04 µm for MHNM 04-267 and 21.33 µm for MHNM 04-898B).
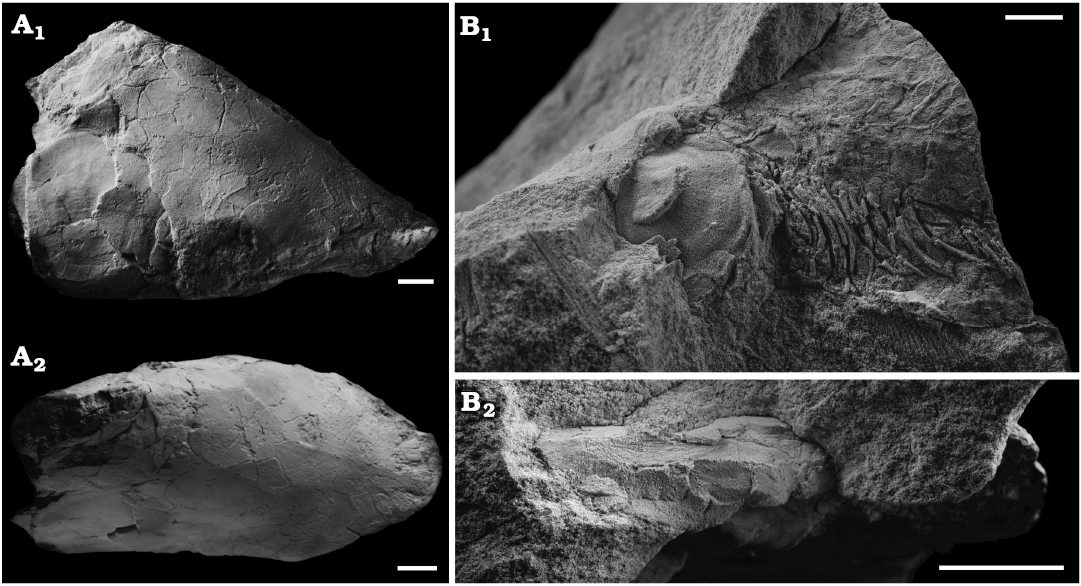
Fig. 1. Skulls of the lungfish Scaumenacia curta (Whiteaves, 1881) from the Escuminac Formation, middle Frasnian, Upper Devonian at Miguasha, Quebec, Canada. A. MHNM 04-728A, right side in lateral view (A1) and dorsal view (A2); whitened with ammonium chloride. B. MHNM 04-243, left side in lateral view (B1) and dorsal view (B2). Scale bars 10 mm.
The specimen NMS G.2022.10.436.1 of Pentlandia macroptera is from a private collection donated to National Museums Scotland and is currently awaiting an accession number (due to Covid restrictions). It has been scanned with a Nikon XT H 225 ST CT-scanner in the Department of Life Sciences at Bristol University (UK). The scan parameters were: 210 kV, 180 µA, 0.5 mm copper filter, 2500 projection with an angular step of 0.14°, voxel resolution of 36.5 µm. For the manual segmentation, Drishti Import and Drishti Paint 2.6.4 and 2.6.5 (beta version) (Limaye 2012) have been used. Drishti Render 2.6.5 has been used for the visualisation of structures.
Phylogenetic analysis.—The matrix of Henderson and Challands (2018) (modified version of Clement et al.’s 2016 matrix) was used and compiled in Mesquite version 3.11 (Maddison and Maddison 2018). Changes to characters are listed in the SOM 2 (Supplementary Online Material available at http://app.pan.pl/SOM/app67-Boirot_etal_SOM.pdf). Maximum parsimony trees were analysed with PAUP* Version 4.0a (Swofford 2003). Youngolepis praecursor, Diplocercides kayseri, Gogonasus andrewsae, Eusthenopteron foordi, and Qingmenodus yui were used as outgroups. All characters were weighted equally.
Systematic palaeontology
Osteichthyes Huxley, 1880
Sarcopterygii Romer, 1955
Dipnomorpha Ahlberg, 1991
Dipnoi Müller, 1845
Family Phaneropleuridae Huxley, 1861
Genus Scaumenacia Traquair, 1893
Type species: Phaneropleuron curtum Whiteaves, 1881; Miguasha, Québec, Canada; middle Frasnian, Upper Devonian.
Scaumenacia curta (Whiteaves, 1881)
Figs. 1–4.
Material.—3D skulls (MHNM 04-243, 04-267, 04-898B, and 04-916) are preserved in siltstone and come from Unit VI (beds 356–361), precise position of a large 3D skull (MHNM 04-728A) is unknown; however, it is most likely from Unit VIII, middle Frasnian, Devonian, Escuminac Formation, Miguasha, Quebec, Canada.
More than 30 specimens of Scaumenacia curta have been found in Unit VI (Parent and Cloutier 1996; Cloutier 1997; Cloutier et al. 2011). Numerous fishes from this horizon have been found inside large carbonate concretions in fine siltstone. Scaumenacia curta specimens found in beds 356–361 of the Escuminac Formation are 3D preserved and present external and internal layers of pyrite, especially in the skull (Parent and Cloutier 1996). The precise stratigraphic horizon of specimen MHNM 04-728A is unknown; however, it is most likely from Unit VIII of the Escuminac Formation, where some large 3D preserved S. curta specimens have been found (Parent and Cloutier 1996).
Description.—MHNM 04-728A of S. curta (Fig. 1) had an estimated TL of 465 mm (based on the regression line of TL from the bone B width from Cloutier 1997); this is the largest specimen of S. curta studied herein. Dermal bones are well preserved and articulated, although the skull is slightly compressed laterally (Fig. 1A2). MHNM 04-243, 04-267, 04-898B, and 04-916 include skulls filled with amorphous and aggregated pyrite. These specimens measured approximately 180 mm in TL (based on the regression line of TL from the bone B width Cloutier 1997). These four specimens do not present any lateral displacement of bones despite some lateral compression. Dermal skull roof bones, opercular series and scales are preserved. Some skull roof bones, like the postparietals and bones C, collapsed internally in the physical space where the neurocranium and brain would have been (Fig. 2A1, A2). The opercular and gular series can be seen in situ on the 3D reconstructed specimen MHNM 04-243 (Fig. 2). The parasphenoid and entopterygoids are well preserved and can be seen in the CT-scan data of all specimens (Fig. 2).
Pyrite is found in these four skulls; however, MHNM 04-267 has the greatest amount of pyrite (see SOM 3), whereas MHNM 04-243 has the smallest amount of pyrite. The quantity and distribution of pyrite in MHNM 04-898B and 04-916 are similar.
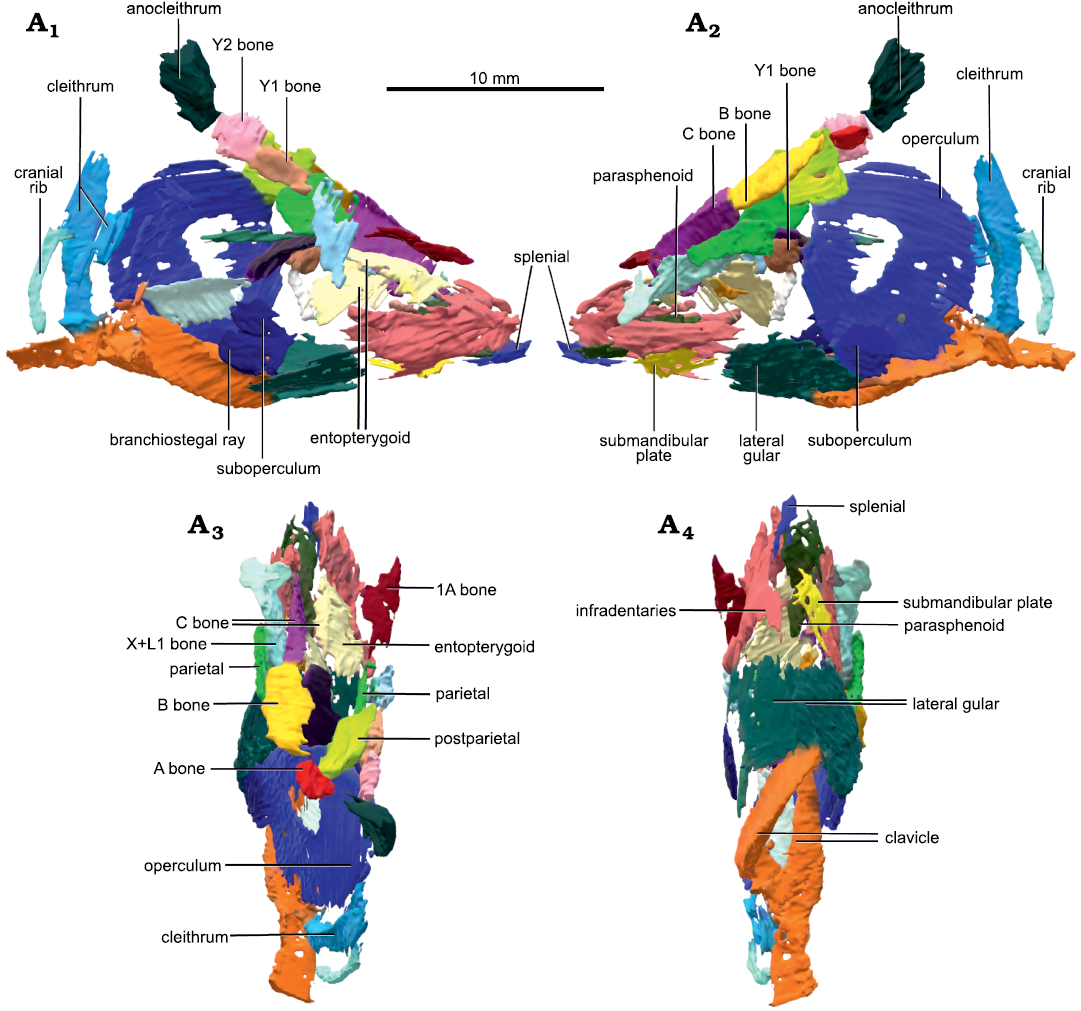
Fig. 2. Micro-CT-scan 3D model of the skull and pectoral girdle of the lungfish Scaumenacia curta (Whiteaves, 1881) (MHNM 04-243) from the Escuminac Formation, middle Frasnian, Upper Devonian at Miguasha, Quebec, Canada. Right (A1) and left (A2) side in lateral view; dorsal (A3) and ventral (A4) views.
Numerous pyrite nodules (>1 cm diameter) are observed on CT-scan images inside the skull of MHNM 04-728A (Figs. 3, 4).
Based on the CT-scan data of MHNM 04-243 (Fig. 2), MHNM 04-267, 04-898B, and 04-916, no ossified neurocranium is present. The parasphenoid, entopterygoids, entopterygoid tooth plates, and lower jaws of MHNM 04-728A are extremely well preserved as shown by the micro-CT-scan data (Figs. 3, 4). Although no mineralized neurocranium is visible in MHNM 04-728A, we segmented the pyrite concretions above the palate after seeing on the CT-scan data two symmetric pyrite concretions above the entopterygoid toothplates which could correspond to pyritized olfactory structures (Figs. 3A2). A 3D model of the pyrite concretions was created including the parasphenoid, entopterygoid tooth plates and entopterygoids (Figs. 3, 4). No segmentation was done posteriorly to the narrowing of the parasphenoid, because the neurocranium does not extend past this point in lungfishes (Miles 1977). From the snout to the parasphenoid narrowing, the neurocranium is estimated to be 80 mm long (out of the 118 mm SkL).
The snout is not ossified (Fig. 3). Two symmetrical structures are located above the tooth plates (Fig. 3A1, A2). They are interpreted as structures associated with the olfactory system because of their position in the skull (at about 1/3 of SkL, posterior to the snout), their length and shape. There are two possible interpretations: they could be (i) olfactory bulbs or (ii) part of the telencephalon (Figs. 3A2, 4A2). In the first case, olfactory bulbs are contained in the anterior part of the telencephalon and therefore, sessile. But on the anterior left side of the skull, there is one pyritized region posterior to the nasal capsules, which could also be interpreted as an olfactory bulb. In this interpretation (ii), olfactory bulbs are pedunculated and linked to the telencephalon by a narrow olfactory tract like in Neoceratodus forsteri (Clement and Ahlberg 2014: fig. 1; Fig. 3A2). Therefore, the symmetric structures are more likely to be telencephalic structures linked to olfaction. Since this cannot be interpreted unambiguously it is not coded in the matrix (character 16).
There is little pyrite in the median part of the skull. There is a pyritized nodule between the two olfactory structures, which could correspond to the pineal/parapineal recess, or just part of the telencephalon.
Posterolaterally on the right side, there are large pyritized structures. We can distinguish three tubular structures forming a circle. They are linked anteriorly and posteriorly but there is a gap between the three tubes, as well as between the two laterally. Because of their lateral position, circular shape, and relative size, we interpreted these structures as the semicircular canals. Two smaller canals separated by a gap are located anteriorly to the semicircular canals. Compared to other dipnoan endocasts, the two smaller canals are located too anteriorly to be identified as semicircular canals. Similar canals located anteriorly to the labyrinth have been observed in Chirodipterus australis (Henderson and Challands 2018) and Dipnorhynchus sussmilchi (Clement et al. 2016). From the shape, position and comparison to other dipnoan species, these structures could be canals for the mandibular and maxillary nerves (nV2 and nV3; Figs. 3, 4) (Clement et al. 2016; Henderson and Challands 2018). Posterior to the semicircular canals, there is a large pyritized structure, extending posteriorly to the parasphenoid stalk. Because of its posterior position, this is not interpreted to be part of the neurocranium and was not segmented further.
There is one large curved tubular pyritized structure latero-ventral to the left entopterygoid (Fig. 4). It extends dorsal to the dermal bones near the anterior margin of the parasphenoid. Given its position and topology we interpret it as the Meckel’s cartilage. The left side of the specimen is slightly crushed, and the left operculum is missing; therefore, internal structures are not preserved on the posterior left side.
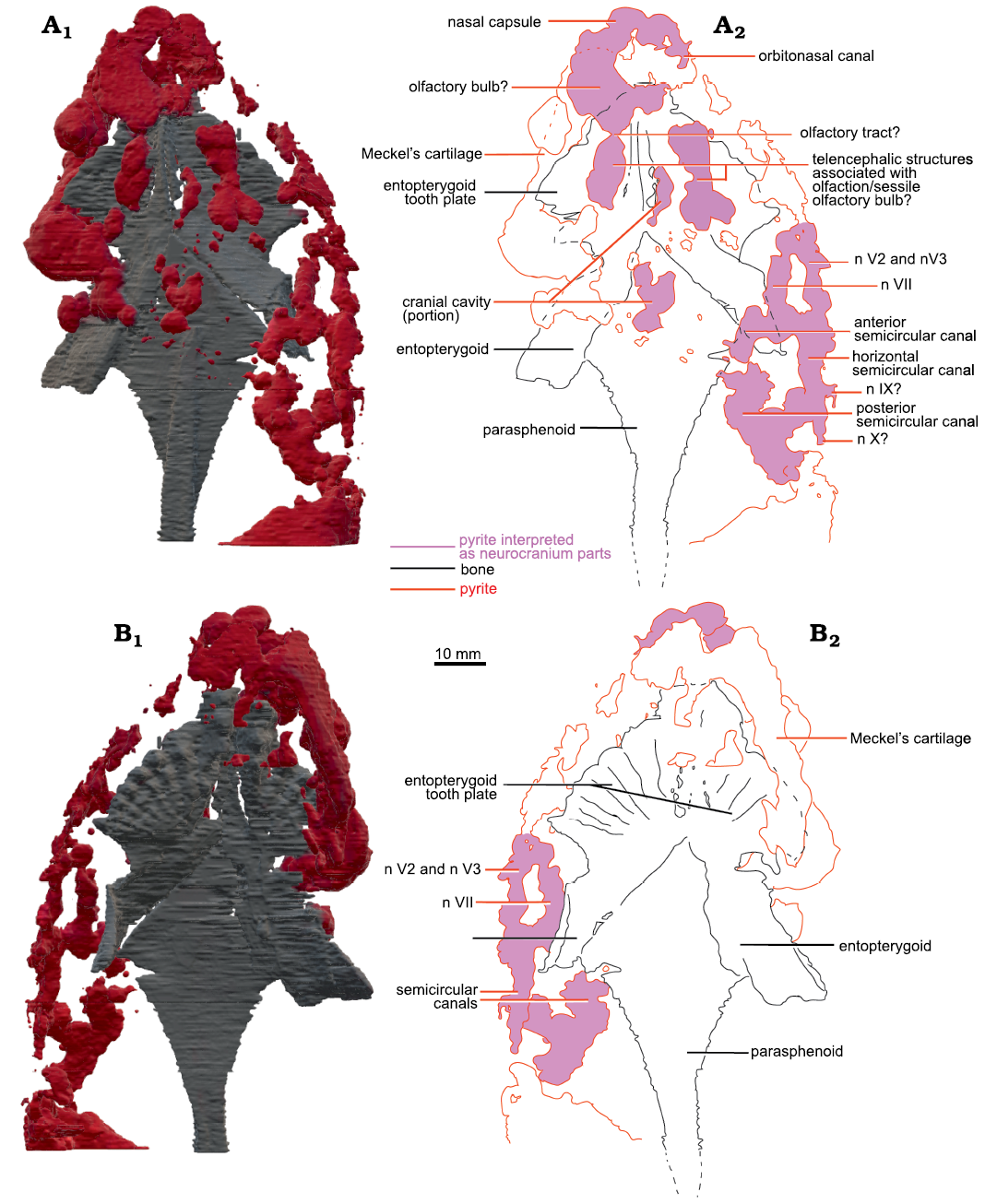
Fig. 3. Micro-CT-scan 3D model of the palate and neurocranial cavities (preserved as pyrite concretions) of the lungfish Scaumenacia curta (Whiteaves, 1881) (MHNM 04-728A) from the Escuminac Formation, middle Frasnian, Upper Devonian at Miguasha, Quebec, Canada. A. 3D model in dorsal view (A1), interpretative drawing (A2). B. 3D model in ventral view (B1), interpretative drawing (B2). Black lines, dermal bones outlines; red lines, pyrite concretions; pink infilling, interpreted as neurocranial parts.
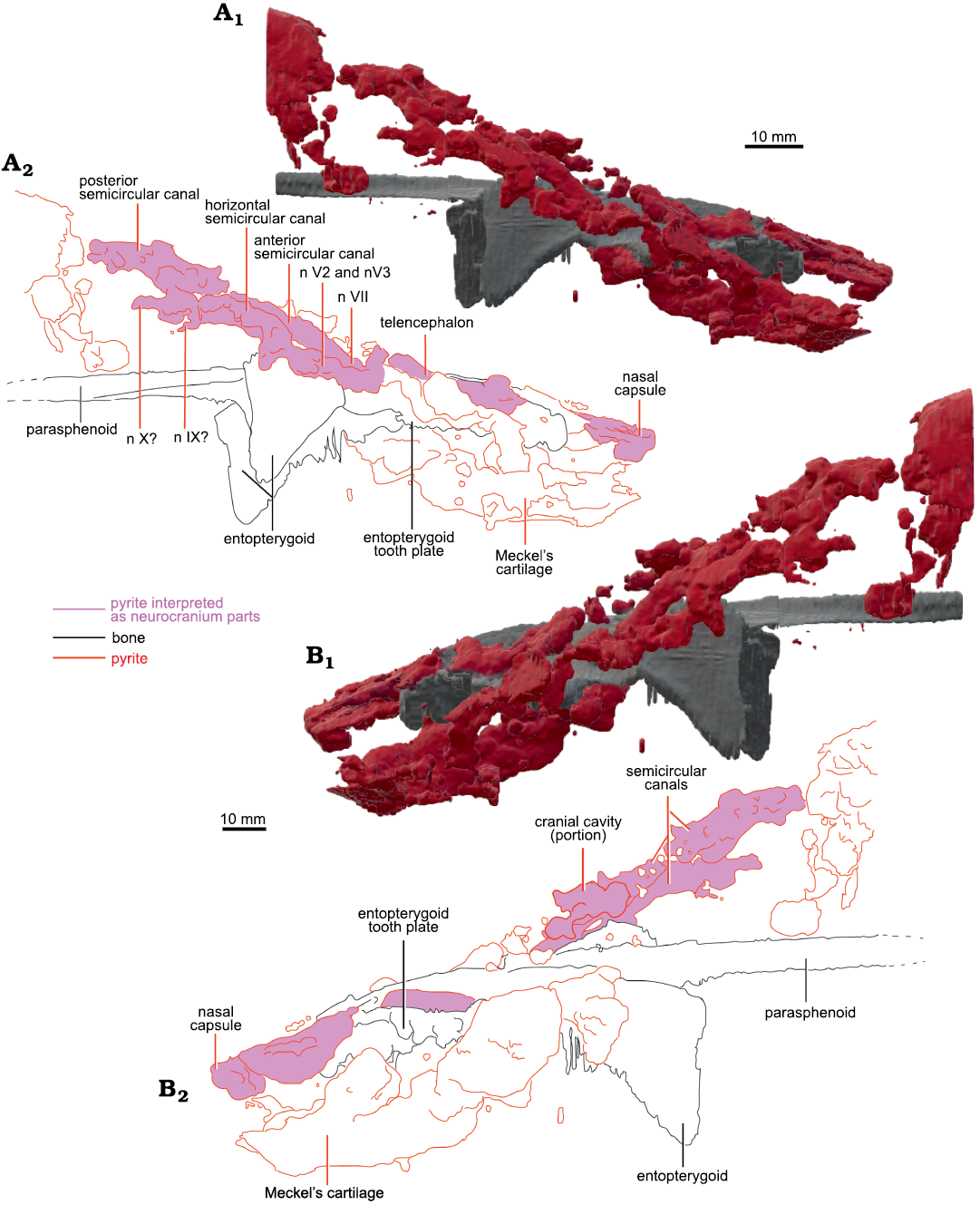
Fig. 4. Micro-CT-scan 3D model of the palate and neurocranial cavities (preserved as pyrite concretions) of the lungfish Scaumenacia curta (Whiteaves, 1881) (MHNM 04-728A) from the Escuminac Formation, middle Frasnian, Upper Devonian at Miguasha, Quebec, Canada. A. 3D model in right lateral view (A1), interpretative drawing (A2). B. 3D model in left lateral view (B1), interpretative drawing (B2).
Remarks.—No ossified neurocranial structure has been found in our ontogenetic series. We propose four taphonomical and ontogenetic arguments justifying that the absence of an ossified neurocranium in S. curta is not a preservation artefact but a real biological signal (Pardo et al. 2014). First, the absence of ossified neurocranial elements is not a preservation bias caused by the peculiar paleoenvironmental conditions in the Escuminac Formation. Some sarcopterygians of the Escuminac fauna (e.g., the porolepiform Quebecius quebecensis, see Cloutier and Schultze 1996, the osteolepiform Eusthenopteron foordi, see Jarvik 1996, and the “elpistostegalian” Elpistostege watsoni, see Schultze and Arsenault 1985) with well-preserved ossified neurocrania have been found in similar and different taphonomic conditions within the Escuminac Formation. Since other sarcopterygians from the Escuminac fauna are found with preserved neurocrania, it would be unlikely that the conditions of preservation of similar anatomical structures would differ in Scaumenacia. The four smaller specimens of S. curta do not come from the same stratigraphic unit as the larger MHNM 04-728A; thus, it is unlikely a taphonomic bias unique to a single stratigraphic bed.
Second, the absence of neurocranial structures does not result from a pyrite replacement of the braincase because some specimens have a small amount of pyrite in the same anatomical region (e.g., MHNM 04-243 and 04-898B) and they still do not show sign of neurocranial ossification.
Third, the absence of a preserved neurocranium does not result from the decay of the neurocranium. In MHNM 04-243, some dermal skull roof bones collapsed internally in the physical space occupied in vivo by the neurocranium and brain during the early decay of the individual. The sagging of skull roof bones would not have been possible if there was an ossified neurocranium beneath them, even if the neurocranium was more lightly ossified than the dermal bones.
Finally, the absence of ossified neurocranial structures in S. curta does not result from an ontogenetic bias of sampling. Dermal ossification (e.g., palatal and skull roof bones) is initiated earlier in ontogeny and is less fragile than neurocranial ossification (formed by endochondral and/or perichondral ossification) (de Beer 1937; Schaeffer and Scott 1971; Bemis 1984). Scamenacia curta larvae do not have ossified neurocrania but they do have dermal bones in development (Cloutier 2009). Herein, none of the four young adults (ca. 180 mm in TL) and the unambiguous adult (MHNM 04-728A, ca. 460 mm in TL) show signs of ossified neurocranial structures. Thus, the four arguments strongly suggest that S. curta does have a completely cartilaginous neurocranium, which confirms previous hypotheses and remove doubts about the potential preservation bias of neurocranial structures (Stensiö 1947; Cloutier 1996; Pardo et al. 2014).
Despite the absence of ossified structures, the pyrite found in specimen MHNM 04-728A could be considered as a “pyrite mold” allowing us to infer neurocranial information. This type of preservation is rare but has been found in other taxa like the Cambrian arthropod Fuxianhuia protensa or Cretaceous ornithomimosaurs (Ma et al. 2015 and Goedert 2017 respectively). However, the information provided by the pyrite mold is not entirely accurate because pyrite continues to expand during diagenesis outside the canals and soft tissues, as long as sulfate, iron and organic matter are provided (Hudson 1982). Due to this specific preservation, morpho-functional information could not be inferred from pyritized structures of S. curta.
Stratigraphic and geographic range.—Escuminac Formation, middle Frasnian, Upper Devonian; Miguasha, Quebec, Canada.
Genus Pentlandia Watson and Day, 1916
Type species: Dipterus macropterus Traquair, 1888; “John O’Groats fish bed”, Last House Formation, John O’Groats Sandstone Group, John O’Groats, Caithness, Scotland, UK.
Pentlandia macroptera (Traquair, 1888)
Fig. 5.
Material.—NMS G.2022.10.436.1, partly disarticulated skull (Fig. 5) is approximately 40 mm long and dorso-ventrally crushed. It was found in the Eday Flags, Givetian, Middle Devonian, Herston Taing, South Ronaldsay, Orkney, UK.
Description.—Most of NMS G.2022.10.436.1 dermatocranium is visible on the surface (Fig. 5A, B). Part of the lower jaw, the parasphenoid, entopterygoids and entopterygoid tooth plates are preserved and can be seen with the micro CT-scan data (Fig. 5C). The anterior part of the postcranial skeleton is also preserved. There is a lateral shift between the cranium and lower jaws, and between the skull roof and the palate. There are obvious signs of decay before burial. Some of the dermal cranial bones have been weathered, revealing internal structures (Fig. 5A, B). There are two pyrite concretions in the skull (between 6.0–8.5 mm long each).
One of the C-bones is broken revealing a less dense structure underneath (Fig. 5B). CT-scan data indicates that the structure is denser than the sediment and the texture is different from skull roof bones (see SOM 4). We interpret it to represent ossified tissue. It is located between the skull roof and the palate and is therefore identified as part of a crushed ossified neurocranium. Trabecular bone is apparent in parts of the tissue beneath the dermal bones indicative of neurocranium. Some vessels or nerve canals are visible on the neurocranium, but the specimen is too crushed to allow further detailed anatomical identification.

Fig. 5. Skull and anterior part of postcranial skeleton of the lungfish Pentlandia macroptera (Traquair, 1888) (NMS G.2022.10.436.1) from Eday Flags, Givetian (Middle Devonian) at Herston Taing, South Ronaldsay, Orkney, UK. Specimen in dorsal view (A1), external view of the skull roof and part of the ossified neurocranium (A2; micro-CT-scan rendering, viewed in Drishti Render).
Remarks.—In contrast to Challands and den Blaauwen (2016), we observed neurocranial ossification in one specimen of Pentlandia macroptera. Challands and den Blaauwen (2016) asserted that all “phaneropleurids” lack a fully ossified neurocranium, which is only partially confirmed with our study, given that we do not know the extent of the neurocranial ossification for P. macroptera.
The neurocranium of Howidipterus donnae and Barwickia downunda (Mount Howitt, Australia) is unknown, but their snouts are unossified and their dermal bones lack cosmine (Long 1992), features that could be indicative of paedomorphic traits (Bemis 1984). Mount Howitt fossils are preserved as natural moulds of flattened carcasses in black shale (Long 1992); this type of fossilisation most likely prevents the preservation of neurocrania mainly if such structures would have been poorly ossified (RC, personal observation).
Stratigraphic and geographic range.—John O’Groats Sandstone Group and Eday Flags Formation, Givetian, Middle Devonian; Caithness, Deerness and Orkney, Scotland, UK.
Phylogenetic results
The matrix includes 13 taxa (five of which are outgroups) and 21 characters (see SOM 1). We added Scaumenacia curta and Griphognathus whitei to the matrix used by Henderson and Challands (2018) which is a modification of the matrix used by Friedman (2007) and Clement et al. (2016). Pentlandia macroptera NMS G.2022.10.436.1 was too crushed to code for neurocranial characters. We included character 21 to this matrix that is a modification of character 155 of Challands et al. (2019). Character 155 (ossification of the neurocranium) of Challands et al. (2019) included two character-states: completely ossified (0); poorly-ossified/cartilaginous (1). Our character 21 is defined as follows: neurocranium ossification: ossified (0), cartilaginous (1). The branch-and-bound search produced three equally parsimonious trees at 39 steps (CI = 0.6154, RI = 0.6809). The strict consensus, 50% majority rule consensus and Adams consensus trees are identical, with S. curta being considered as the sister group to Neoceratodus forsteri. Dipnoans are grouped together in more than 70% of the cases (Fig. 6). Unlike Henderson and Challands (2018), Dipterus valenciennesi is resolved basal to Chirodipterus australis. The relationships among Chirodipterus wildungensis, Giphognathus whitei and a clade including more derived lungfishes are unresolved. Rhinodipterus kimberleyensis is sister-group to S. curta and Neoceratodus forsteri.
There was a difference regarding the coding of character 20 for Chirodipterus wildungensis between Clement et al. (2016) and Henderson and Challands (2018). In the Clement et al. (2016) analysis, the canal for the anterior cerebral vein is coded as a single canal in the cranial cavity, whereas Henderson and Challands (2018) coded it as missing data. We kept the coding of Henderson and Challands (2018); nevertheless, we ran the analysis with both possibilities (i.e., 1 or ?). Both codings did not change the tree topology nor the CI, only the RI slightly changed (from 0.6809 [Fig. 6] to 0.6875]. The neurocranium is at least partly ossified for all species except for S. curta and Neoceratodus forsteri, for which it is cartilaginous.
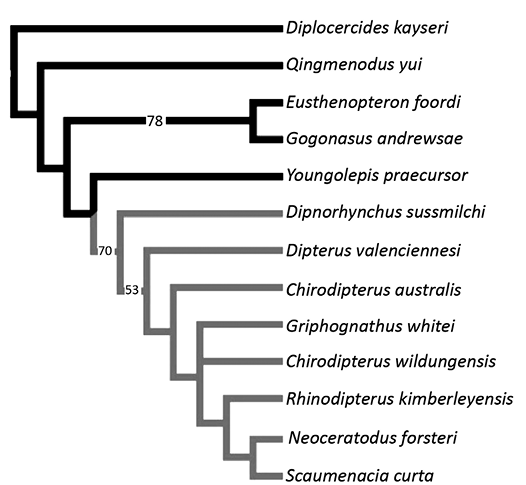
Fig. 6. Phylogenetic relationships of lungfishes. 50% majority rule consensus of three equally parsimonious trees at 39 steps (CI = 0.6154, RI = 0.6809) with bootstrap values on branches. The lungfish group has grey branches and other Devonian sarcopterygians (outgroups) have black branches.
Discussion
Our micro-CT-scan analyses of the neurocranial region of two Middle–Late Devonian lungfishes, Scaumenacia curta and Pentlandia macroptera, allowed us to validate some neurocranial characters for two Devonian lungfish taxa in which the neurocranium is poorly preserved. Our results show that (i) the neurocranium of S. curta remains cartilaginous throughout ontogeny, (ii) the neurocranium of P. macroptera is ossified, (iii) pyritization of cranial cavities could reveal gross braincase anatomy, (iv) among the Devonian lungfishes having neurocranial data, S. curta is the most derived.
Phylogeny, paedomorphosis, and lungfish evolution.—As a character-rich structure less subject to intraspecific variation than skull roof bones (Friedman 2007; Clement et al. 2016), the neurocranium is an interesting anatomical complex that has already provided promising phylogenetic information of various early vertebrates (Patterson 1973; Gardiner 1973; Coates and Sequeira 1998; Coates 1999; Maisey and Anderson 2001; Friedman 2007). However, it is better to combine neurocranial characters and other structures, to compensate for the incompleteness of neuro-anatomical information for numerous lungfish species. Moreover, some neurocranial characters have been described recently only from a few specimens and their polarity is still debated (Clement et al. 2016; Henderson and Challands 2018). In our analysis, S. curta is considered the sister-group to the living Neoceratodus forsteri. This phylogenetic position reflects the derived condition of the cartilaginous neurocranium rather than a true phylogenetic proximity between the two taxa. Moreover, it shows clearly that S. curta is more derived than the other taxa included in the analysis because the neurocranium remains cartilaginous in adults, as in Neoceratodus forsteri. Neurocranial studies require preserved structures, almost nonexistent for post-Devonian lungfish species, because of the reduction in ossification (Clack et al. 2011; Clement and Ahlberg 2014).
Paedomorphosis has been suggested as a driving force in lungfish and tetrapod evolution (Gould 1977; Bemis 1984; Roth et al. 1993; Schoch and Fröbisch 2006). Early lungfishes, like Dipnorhynchus sussmilchi (Clement et al. 2016) or Chirodipterus australis (Henderson and Challands 2018), had heavily ossified neurocrania and endoskeleton (Bertmar 1968; Bemis 1984) and Rhinodipterus kimberleyensis is one of the most derived lungfishes to still have an ossified neurocranium (Clement and Ahlberg 2014). Pentlandia macroptera has an ossified neurocranium. In our analysis, S. curta, more derived than P. macroptera, has a completely cartilaginous neurocranium, like all post-Devonian species included in this analysis (Clement and Ahlberg 2014; Clement et al. 2015; Criswell 2015). Paedomorphosis had already resulted in the reduction of ossification between P. macroptera and S. curta at least for the neurocranium (Bemis 1984; Friedman 2007). Neurocranium ossification occurs late in ontogeny (de Beer 1937; Bemis 1984). If somatic development is delayed compared to sexual maturity because of paedomorphosis the neurocranium will not have time to ossify before maturity and will therefore remain cartilaginous over time and evolution. Regarding S. curta, this hypothesis seems to be verified and paedomorphosis confirmed by our results, at least for the neurocranium and general degree of ossification. The decreasing ossification of neurocranial structures, coupled with other simultaneous anatomical changes (e.g., reduction of the number of median fins, loss of cosmine, global ossification decrease) during lungfish evolution is supporting the paedomorphic hypothesis (Bemis 1984).
Pyritization and preservation of “soft tissues”.—Pyritization is most frequently considered as a destructive process of soft parts rather than a preservation agent (Becherini et al. 2018). Pyrite is often an indicator of environmental and burial conditions (El Albani et al. 2001) but rarely a proxy for soft structures which would not have been preserved otherwise (Seilacher et al. 1985, Allison 1988a, Canfield and Raiswell 1991, Briggs et al. 1996).
Early diagenetic pyrite is known for helping preservation of soft structures and 3D preservation by cementing soft tissues before decay (Allison 1988b). Pyrite is formed in cavities close to pore water, and associated with rapid burial primarily in cavities like canals and structures occupied by soft tissues (Allison 1988b; Trueman et al. 2003). Cavities left empty after decay (e.g., semicircular canals and nasal capsules) are an ideal environment for pyrite formation (Hudson 1982; Brett and Baird 1986).
Pyrite is present in nearly all stratigraphic beds of the Escuminac Formation (El Albani et al. 2001; Cloutier et al. 2011). The abundance and structure of the pyrite are variable among the palaeoestuary facies of the Escuminac Formation. Pyrite occurs either associated around fossils, inside fossils (disseminated or cavity infilling) or replacing anatomical structures (pyritization) (Cloutier et al. 2011). The pyrite found in MHNM 04-728A could be considered as a “pyrite mold” allowing us to infer neurocranial information despite the absence of ossified structures.
Conclusions
CT-scan data on the neurocrania of Pentlandia macroptera and Scaumenacia curta allowed us to observe their degree of ossification. Contrary to previous hypotheses, the neurocranium of P. macroptera is ossified, at least partially. S. curta has a cartilaginous neurocranium. This discovery helps refine the pivotal point in dipnoan evolution where paedomorphosis impacts the ossification degree. It is important to keep analysing internal structures like the neurocranium using new technologies to reinforce the phylogeny and the hypothesis of an evolutionary paedomorphic trend among dipnoans. MHNM 04-728A of S. curta shows a unique type of endocranial preservation. Herein, pyritization acted like a preservation agent, a condition rarely seen in vertebrates. This makes us consider the value of diagenetic agents and their role in structure preservation. Some pyritized vertebrate structures may need re-evaluation in the light of our findings: maybe pyrite has preserved more information than previously expected.
Further investigation is required on the braincase in lungfishes, but early lungfishes with preserved braincases are ideal to conduct research on morpho-functional comparative anatomy, using the braincase to infer brain morphology (Campbell and Barwick 1982; Clement et al. 2015). The lungfish stratigraphic extent and rich fossil record offer a unique field of study for the evolution of structures associated with ecological and ontogenetic modifications over time (Clack et al. 2011). Combined with dermatocranial and postcranial characters, the neurocranium is a good candidate to resolve polytomies in the phylogeny, particularly for early dipnoans where the heavily ossified endocast can be almost perfectly conserved (Friedman 2007; Clement and Ahlberg 2014; Clement et al. 2016).
Acknowledgements
This article was written as a M.Sc. Thesis (MB) under the supervision of Richard Cloutier, at the Université du Québec à Rimouski, Québec, Canada (UQAR). We thank Alice Clement (Flinders University, Adelaide, Australia), Jorge Mondéjar-Fernández (Senckenberg Forschungsinstitut und Naturmuseum Frankfurt, Frankfurt am Main, Germany), and Christian Nozais (UQAR) for their constructive comments. We thank Johanne Kerr (MHNM) for the loan of the Scaumenacia curta specimens. We thank the UQAR for the Trottier’s Family Foundation Excellence Research Fellowship (MB) and the Discovery Grant from Natural Sciences and Engineering Research Council (RC).
References
Alberch, P., Gould, S.J., Oster, G.F., and Wake, D.B. 1979. Size and shape in ontogeny and phylogeny. Paleobiology 5: 296–317. Crossref
Ahlberg, P.E., Smith, M.M., and Johanson, Z. 2006. Developmental plasticity and disparity in early dipnoan (lungfish) dentitions. Evolution & Development 8: 331–349. Crossref
Allison, P.A. 1988a. Konservat-Lagerstätten: cause and classification. Paleobiology 14: 331–344. Crossref
Allison, P.A. 1988b. The role of anoxia in the decay and mineralization of proteinaceous macro-fossils. Paleobiology 14: 139–154. Crossref
Becherini, F., Del Favero, L., Fornasiero, M., Guastoni, A., and Bernardi, A. 2018. Pyrite decay of large fossils: The case study of the Hall of Palms in Padova, Italy. Minerals 8: e40. Crossref
Bemis, W.E. 1984. Paedomorphosis and the evolution of the Dipnoi. Paleobiology 10: 293–307. Crossref
Bertmar, G. 1968. Phylogeny and evolution in lungfishes. Acta Zoologica 49: 189–201. Crossref
Brett, C.E. and Baird, G.C. 1986. Comparative taphonomy: a key to paleoenvironmental interpretation based on fossil preservation. Palaios 1: 207–227. Crossref
Briggs, D.E., Raiswell, R., Bottrell, S., Hatfield, D.T., and Bartels, C. 1996. Controls on the pyritization of exceptionally preserved fossils; an analysis of the Lower Devonian Hunsrueck Slate of Germany. American Journal of Science 296: 633–663. Crossref
Skyscan NV 2011. NRecon User Manual. 23 pp. Bruker, Kontich.
Campbell, K. and Barwick, R. 1982. The neurocranium of the primitive dipnoan Dipnorhynchus sussmilchi (Etheridge). Journal of Vertebrate Paleontology 2: 286–327. Crossref
Campbell, K. and Barwick, R. 1990. Paleozoic dipnoan phylogeny: functional complexes and evolution without parsimony. Paleobiology 16: 143–169. Crossref
Canfield, D. and Raiswell, R. 1991. Pyrite formation and pyrite preservation. In: P.A. Allison and D.E.G. Briggs (eds.), Taphonomy: Releasing the Data Locked in the Fossil Record 9, 337–387. Plenum Press, New York. Crossref
Challands, T. 2015. The cranial endocast of the Middle Devonian dipnoan Dipterus valenciennesi and a fossilized dipnoan otoconial mass. Papers in Palaeontology 1: 289–317. Crossref
Challands, T. and den Blaauwen, J. 2016. A redescription of the Middle Devonian dipnoan Pentlandia macroptera Traquair, 1889, and an assessment of the Phaneropleuridae. Zoological Journal of the Linnean Society 180: 414–460. Crossref
Challands, T., Smithson, T.R., Clack, J.A., Bennett, C.E., Marshall, J.E., Wallace-Johnson, S.M., and Hill, H. 2019. A lungfish survivor of the end-Devonian extinction and an Early Carboniferous dipnoan radiation. Journal of Systematic Palaeontology 17: 1825–1846. Crossref
Chang, M.-M. and Yu, X. 1984. Structure and phylogenetic significance of Diabolichthys speratus gen. et sp. nov., a new dipnoan-like form from the Lower Devonian of eastern Yunnan, China. Proceedings of the Linnean Society of New South Wales 107: 171–184.
Clack, J.A., Sharp, E.L., and Long, J.A. 2011. The fossil record of lungfishes. In: J.M. Jorgensen and J. Joss (eds.), The Biology of Lungfishes, 1–42. CRC Press, New York. Crossref
Clement, A.M. and Ahlberg, P.E. 2014. The first virtual cranial endocast of a lungfish (Sarcopterygii: Dipnoi). PLoS One 9: e113898. Crossref
Clement, A.M., Challands, T.J., Long, J.A., and Ahlberg, P.E. 2016. The cranial endocast of Dipnorhynchus sussmilchi (Sarcopterygii: Dipnoi) and the interrelationships of stem-group lungfishes. PeerJ 4: e2539. Crossref
Clement, A.M., Nysjo, J., Strand, R., and Ahlberg, P.E. 2015. Brain—endocast relationship in the Australian lungfish, Neoceratodus forsteri, elucidated from tomographic data (Sarcopterygii: Dipnoi). PLoS One 10: e0141277. Crossref
Cloutier, R. 1996. Dipnoi (Akinetia: Sarcopterygii). In: H.-P. Schultze and R. Cloutier (eds.), Devonian Fishes and Plants of Miguasha, Quebec, Canada, 198–226. Verlag Dr. Friedrich Pfeil, München.
Cloutier, R. 1997. Morphologie et variations du toit crânien du Dipneuste Scaumenacia curta (Whiteaves)(Sarcopterygii), du Dévonien supérieur du Québec. Geodiversitas 19: 61–105.
Cloutier, R. 2009. The fossil record of fish ontogenies: insights into developmental patterns and processes. Seminars in Cell and Developmental Biology 21: 400–13. Crossref
Cloutier, R. 2013. Great Canadian Lagerstätten 4. The Devonian Miguasha biota (Québec): UNESCO World Heritage Site and a time capsule in the early history of vertebrates. Geoscience Canada 40: 149–163. Crossref
Cloutier, R. and Ahlberg, P.E. 1996. Morphology, characters, and the interrelationships of basal sarcopterygians. In: M.L.J. Stiassny, L.R. Parenti, and G.D. Johnson (eds.), Interrelationships of Fishes, 445–479. Academic Press, San Diego. Crossref
Cloutier, R. and Schultze, H.-P. 1996. Porolepiform fishes (Sarcopterygii). In: H.-P. Schultze and R. Cloutier (eds.), Devonian Fishes and Plants of Miguasha, Quebec, Canada, 248–270. Verlag Dr. Friedrich Pfeil, München.
Cloutier, R., Proust, J.-N., and Tessier, B. 2011. The Miguasha Fossil-Fish-Lagerstätte: A consequence of the Devonian land–sea interactions. Palaeobiodiversity and Palaeoenvironments 91: 293–323. Crossref
Coates, M. 1999. Endocranial preservation of a Carboniferous actinopterygian from Lancashire, UK, and the interrelationships of primitive actinopterygians. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 354: 435–462. Crossref
Coates, M. and Sequeira, S. 1998. The braincase of a primitive shark. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 89: 63–85. Crossref
Criswell, K.E. 2015. The comparative osteology and phylogenetic relationships of African and South American lungfishes (Sarcopterygii: Dipnoi). Zoological Journal of the Linnean Society 174: 801–858. Crossref
de Beer, G.R. 1937. The Development of the Vertebrate Skull. 552 pp. Oxford University Press, Oxford.
El Albani, A., Cloutier, R., and Candilier, A.-M. 2001. Early diagenesis of the Upper Devonian Escuminac Formation in the Gaspé Peninsula, Québec: Sedimentological and geochemical evidence. Sedimentary Geology 146: 209–223. Crossref
Friedman, M. 2007. The interrelationships of Devonian lungfishes (Sarcopterygii: Dipnoi) as inferred from neurocranial evidence and new data from the genus Soederberghia Lehman, 1959. Zoological Journal of the Linnean Society 151: 115–171. Crossref
Friedman, M. and Sallan, L.C. 2012. Five hundred million years of extinction and recovery: a Phanerozoic survey of large-scale diversity patterns in fishes. Palaeontology 55: 707–742. Crossref
Gardiner, B. 1973. Interrelationships of teleostomes. In: P.H. Greenwood, R.S. Miles, and C. Patterson (eds.), Interrelationships of Fishes, 105–135. Academic Press, London.
Goedert, J. 2017. Écologie des premiers tétrapodes dévoilée par la composition isotopique du soufre (34S/32S) de leurs squelettes. 222 pp. Ph.D. Thesis, Université de Lyon, Lyon.
Gould, S.J. 1977. Ontogeny and Phylogeny. 501 pp. Harvard University Press, Cambridge.
Henderson, S.A.C. and Challands, T.J. 2018. The cranial endocast of the Upper Devonian dipnoan ‘Chirodipterus’ australis. PeerJ 6: e5148. Crossref
Hudson, J. 1982. Pyrite in ammonite‐bearing shales from the Jurassic of England and Germany. Sedimentology 29: 639–667. Crossref
Irisarri, I. and Meyer, A. 2016. The identification of the closest living relative(s) of tetrapods: Phylogenomic lessons for resolving short ancient internodes. Systematic Biology 65: 1057–1075. Crossref
Jarvik, E. 1996. The evolutionary importance of Eusthenopteron foordi (Osteolepiformes). In: H.-P. Schultze and R. Cloutier (eds.), Devonian Fishes and Plants of Miguasha, Quebec, Canada, 285–313. Verlag Dr. Friedrich Pfeil, München.
Joss, J.M.P. 1998. Are extant lungfish neotenic? Clinical and Experimental Pharmacology and Physiology 25: 133–135. Crossref
Joss, J.M.P. 2006. Lungfish evolution and development. General and Comparative Endocrinology 148: 285–289. Crossref
Jude, E., Johanson, Z., Kearsley, A., and Friedman, M. 2014. Early evolution of the lungfish pectoral-fin endoskeleton: evidence from the Middle Devonian (Givetian) Pentlandia macroptera. Frontiers in Earth Science 2: e18. Crossref
Kemp, A. 1999. Anomalies in skull bones of the Australian lungfish, Neoceratodus forsteri, compared with aberrations in fossil dipnoan skulls. Journal of Vertebrate Paleontology 19: 407–429. Crossref
Krupina, N. and Reisz, R. 2000. Phylogeny of Devonian dipnoans, with special emphasis on forms from the Russian platform. Paleontological Journal 34: S147–S153.
Lieberman, D.E., Pearson, O.M., and Mowbray, K.M. 2000. Basicranial influence on overall cranial shape. Journal of Human Evolution 38: 291–315. Crossref
Limaye, A. 2012. Drishti: a volume exploration and presentation tool. In: S.R. Stock (ed.), Developments in X-ray Tomography VIII, 85060X. International Society for Optics and Photonics, San Diego. Crossref
Lloyd, G.T., Wang, S.C., and Brusatte, S.L. 2011. Identifying heterogeneity in rates of morphological evolution: discrete character change in the evolution of lungfish (Sarcopterygii; Dipnoi). Evolution 66: 330–48. Crossref
Long, J.A. 1992. Cranial anatomy of two new Late Devonian lungfishes (Pisces: Dipnoi) from Mount Howitt, Victoria. Records of the Australian Museum 44: 299–318. Crossref
Ma, X., Edgecombe, G.D., Hou, X., Goral, T., and Strausfeld, N.J. 2015. Preservational pathways of corresponding brains of a Cambrian euarthropod. Current Biology 25: 2969–2975. Crossref
McNamara, K.J. 1982. Heterochrony and phylogenetic trends. Paleobiology 8: 130–142. Crossref
Maddison, W. and Maddison, D. 2018. Mesquite: a Modular System for Evolutionary Analysis. Version 3.51
Maisey, J.G. and Anderson, M.E. 2001. A primitive chondrichthyan braincase from the Early Devonian of South Africa. Journal of Vertebrate Paleontology 21: 702–713. Crossref
Marshall, C.R. 1986. Lungfish: phylogeny and parsimony. Journal of Morphology, Supplement 1: 151–162. Crossref
Miles, R.S. 1977. Dipnoan (lungfish) skulls and the relationships of the group: a study based on new species from the Devonian of Australia. Zoological Journal of the Linnean Society 61: 1–328. Crossref
Moy-Thomas, J.A. and Miles, R.S. 1971. Palaeozoic Fishes. 2nd edition. 259 pp. Chapman and Hall Ltd., London. Crossref
Nelson, J.S., Grande, T.C., and Wilson, M.V.A. 2016. Fishes of the World. 707 pp. John Wiley & Sons, Hoboken. Crossref
Pardo, J.D., Huttenlocker, A.K., and Small, B.J. 2014. An exceptionally preserved transitional lungfish from the Lower Permian of Nebraska, USA, and the origin of modern lungfishes. PloS One 9 (9): e108542. Crossref
Parent, N. and Cloutier, R. 1996. Distribution and preservation of fossils in the Escuminac Formation. In: H.-P. Schultze and R. Cloutier (eds), Devonian Fishes and Plants of Miguasha, Quebec, Canada, 54–78. Verlag Dr. Friedrich Pfeil, München.
Patterson, C. 1973. Interrelationships of holosteans. In: P.H. Greenwood, R.S. Miles, and C. Patterson (eds.) Interrelationships of Fishes, 233–305. Academic Press, London.
Richtsmeier, J.T., Aldridge, K., DeLeon, V.B., Panchal, J., Kane, A.A., Marsh, J.L., Yan, P., and Cole, T.M. 2006. Phenotypic integration of neurocranium and brain. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 306: 360–378. Crossref
Roth, G., Nishikawa, K.C., Naujoks-Manteuffel, C., Schmidt, A., and Wake, D.B. 1993. Paedomorphosis and simplification in the nervous system of salamanders. Brain, Behaviour and Evolution 42: 137–152. Crossref
Schaeffer, B. and Scott, G. 1971. The braincase of the holostean fish Macrepistius, with comments on neurocranial ossification in the Actinopterygii. American Museum Novitates 2459: 1–34.
Schoch, R.R. and Fröbisch, N. 2006. Metamorphosis and neoteny: Alternative pathways in an extinct amphibian clade. Evolution 60: 1467–1475. Crossref
Schultze, H.-P. 1992. Fossilium Catalogus: Animalia, Pars 131, Dipnoi. 464 pp. Kugler Publications, Amsterdam.
Schultze, H.-P. 2001. Melanognathus, a primitive dipnoan from the Lower Devonian of the Canadian Arctic and the interrelationships of Devonian dipnoans. Journal of Vertebrate Paleontology 21: 781–794. Crossref
Schultze, H.P. and Arsenault, M. 1985. The panderichthyid fish Elpistostege: a close relative of tetrapods? Palaeontology 28, 293–309.
Schultze, H.-P., and Marshall, C.R. 1993. Contrasting the use of functional complexes and isolated characters in lungfish evolution. Memoirs of the Association of Australasian Palaeontologists 15: 211–224.
Seilacher, A., Reif, W.-E., and Westphal, F. 1985. Sedimentological, ecological and temporal patterns of fossil Lagerstätten. Philosophical Transactions of the Royal Society of London. B, Biological Sciences 311: 5–24. Crossref
Smith, M.M. 1977. The microstructure of the dentition and dermal ornament of three dipnoans from the Devonian of Western Australia: A contribution towards dipnoan interrelations, and morphogenesis, growth and adaption of the skeletal tissues. Philosophical Transactions of the Royal Society of London. B, Biological Sciences 281: 29–72. Crossref
Stensiö, E.A. 1947. The sensory lines and dermal bones of the cheek in fishes and amphibians. Kungliga Svenska Vetenskapsakademiens Handlingar 24: 1–195.
Sutton, M.D. 2008. Tomographic techniques for the study of exceptionally preserved fossils. Proceedings of the Royal Society B Biological Science 275: 1587–1593. Crossref
Swofford, D.L. 2003. PAUP*: Phylogenetic Analysis Using Parsimony (* and other methods), Version 4.0 a147. Sinauer, Sunderland.
Thomson, K.S. and Campbell, K.S.W. 1971. The Structure and Relationships of the Primitive Devonian Lungfish—Dipnorhynchus sussmilchi (Etheridge). 109 pp. Peabody Museum of Natural History, Yale University, New Heaven.
Trueman, C.N., Benton, M.J., and Palmer, M.R. 2003. Geochemical taphonomy of shallow marine vertebrate assemblages. Palaeogeography, Palaeoclimatology, Palaeoecology 197: 151–169. Crossref
Walsh, S.A. and Knoll, M.A. 2011. Directions in palaeoneurology. Special Papers in Palaeontology 86: 263–279.
Walsh, S.A., Luo, Z.-X., and Barrett, P.M. 2013. Modern imaging techniques as a window to prehistoric auditory worlds. In: C. Köppl, G.A. Manley, A.N. Popper, and R.R. Fay (eds.), Insights from Comparative Hearing Research. Springer Handbook of Auditory Research 49: 227–261. Crossref
White, E.I. 1965. The head of Dipterus valenciennesi Sedgwick & Murchison. Bulletin of the British Museum (Natural History), Geology 11: 3–45.
Willmore, K.E., Young, N.M., and Richtsmeier, J.T. 2007. Phenotypic variability: Its components, measurement and underlying developmental processes. Evolutionary Biology 34: 99–120. Crossref
Acta Palaeontol. Pol. 67 (2): 283–295, 2022
https://doi.org/10.4202/app.00841.2020