

First three-dimensional skull of the Middle Triassic mixosaurid ichthyosaur Phalarodon fraasi from Svalbard, Norway
AUBREY JANE ROBERTS, VICTORIA SJØHOLT ENGELSCHIØN, and JØRN HARALD HURUM
Roberts, A.J., Engelschiøn, V.S., and Hurum, J.H. 2022. First three-dimensional skull of the Middle Triassic mixosaurid ichthyosaur Phalarodon fraasi from Svalbard, Norway. Acta Palaeontologica Polonica 67 (1): 51–62.
The marine Middle Triassic sediments of Svalbard are rich in fossiliferous material and are particularly well-known for marine reptile fossils. Here, we present a new specimen of the small-bodied mixosaurid ichthyosaur Phalarodon fraasi from the Botneheia Formation. PMO 235.393 is unusual in being the first three-dimensional mixosaurid skull recovered from this formation, allowing us to use computed tomography to reconstruct the obscured right side of the cranium, resulting in the first 3D model available for a mixosaurid ichthyosaur. Although separated into different slabs, the specimen preserves most of the dermatocranium as well as some partial post-cranial elements. In particular, the rostrum, external naris, dentition, quadrate and sclerotic ring are well-preserved. This methodology gave new insights into the adaptations this taxon has to durophagy, as well as a detailed look at the heterodont dentition present in PMO 235.393. After comparing with other Phalarodon specimens, it was clear that the maxillary heterodonty of this genus is a synapomorphy. As such this was added as a new character in our phylogenetic analysis, supporting the separation of Phalarodon and Mixosaurus.
Key words: Ichthyosauria, Mixosauridae, Mixosaurus, Phalarodon, Triassic, Spitsbergen, Svalbard.
Aubrey Jane Roberts [a.j.roberts@nhm.uio.no], Natural History Museum, University of Oslo, box 1072 Blindern, 0318 Oslo, Norway; Natural History Museum, Cromwell Rd, South Kensington, London SW7 5BD, UK.
Victoria Sjøholt Engelschiøn [v.s.engelschion@nhm.uio.no] and Jørn Harald Hurum [j.h.hurum@nhm.uio.no] (corresponding author), Natural History Museum, University of Oslo, box 1072 Blindern, 0318 Oslo, Norway.
Received 2 June 2021, accepted 24 September 2021, available online 30 March 2022.
Copyright © 2022 A.J. Roberts et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Triassic Period saw the rise of multiple groups of marine reptiles including sauropterygians, thalattosaurians, and ichthyopterygians (Fröbisch et al. 2013; Scheyer et al. 2014; Pyenson et al. 2014; Kelley and Pyenson 2015; Jiang et al. 2016). As such, the period is the basis for our understanding of the early evolution and diversification of these reptiles (Scheyer et al. 2014; Stubbs and Benton 2016). The invertebrate diversification after the Permian–Triassic extinction gave rise to different types of amniote durophagous predators in the Triassic (Huang et al. 2020). One of these groups, the ichthyopterygians, have one of the longest temporal ranges of any marine reptile lineage (Moon and Stubbs 2020). Ichthyopterygians stem from terrestrial diapsid reptiles which over time adapted to a pelagic lifestyle and evolved its characteristic dolphin-like shape (Motani 2005b). One of the first semi-pelagic members of this group were the mixosaurid ichthyosaurs, with the oldest dorsal fin of any amniote (Renesto et al. 2020). This family of small-bodied ichthyosaurs has been found in Middle Triassic sediments from China, continental Europe, North America and Svalbard (Norway) (Sander and Bucher 1990; Nicholls and Brinkman 1995; Jiang et al. 2006; Hurum et al. 2014; Renesto et al. 2020). All mixosaurid taxa can be differentiated by dentition (Motani 1999; Liu et al. 2013). At least two genera, the durophagous Phalarodon and the more pelagic hunter Mixosaurus, can be recovered by phylogenetic analysis using cranial and postcranial characters (Jiang et al. 2007).
The earliest expeditions to the Svalbard archipelago to collect mixosaurid fossils were the Swedish Nordenskiöld expeditions in 1864 and 1868 (for a historical overview see Hurum et al. 2018). These expeditions resulted in the description of two new ichthyosaur species from this material; “Ichthyosaurus nordenskioeldii” and “Ichthyosaurus cornalianus” (Hulke 1873). These were later moved to the Mixosaurus by Baur (1887). Merriam (1910) established a North American genus Phalarodon, with close affinities to Mixosaurus nordenskioeldii. This spurred a discussion of the validity of the different genera and species (for further details see Økland et al. 2018). In general, the two genera are similar in morphology and size, but can be differentiated by their dentition and presence of a narial shelf in Phalarodon (Jiang et al. 2007). Phalarodon is known to have significant heterodonty between the anterior and posterior teeth. In Mixosaurus, this is less apparent and there is only a slight change between anterior and posterior teeth. Økland et al. (2018) proposed that both genera were co-existing in the Middle Triassic of Svalbard, based on the reassignment to Mixosaurus of a previously described Phalarodon sp. (PMO 219.250, Hurum et al. 2014), and a new Phalarodon fraasi (PMO 210.122).
There are currently two broadly supported genera in the mixosaurid family: Phalarodon and Mixosaurus. Three Mixosaurus species are currently recognized (Økland et al. 2018): M. cornalianus, M. kuhnschnyderi, and M. xindianensis. Phalarodon has three species (Økland et al. 2018): P. atavus, P. callawayi, and P. fraasi. Barracudasauroides panxianensis has been proposed as the outgroup of Mixosaurus and Phalarodon (Maisch 2010; Moon 2019), although this taxon has also been suggested to be synonymous with Mixosaurus (Jiang et al. 2006). We follow this interpretation.
This study focuses on a three-dimensional skull and associated anterior axial skeleton from the Middle Triassic of central Spitsbergen (Svalbard, Norway). PMO 235.393 is enclosed in a concretion. Computed tomography (CT) was utilised to describe the morphology and dentition in more detail. This specimen represents one of only a few three-dimensional crania from Mixosauridae and will form a basis for future studies on more compressed specimens.
Institutional abbreviations.—CCCGS, Chengdu Center of China Geological Survey, Chengdu, China; GMPKU, Geological Museum of Peking University, China; NHM-UiO, University of Oslo Natural History Museum, Norway; PMO, Paleontological Museum Oslo (part of the Natural History Museum in Oslo), Norway; TMP, Royal Tyrrell Museum of Paleontology, Drumheller, Canada.
Geological setting
The Svalbard archipelago forms an uplifted part of the Barents Shelf, and spans from 74° to 81° north in the Arctic Ocean. During the Triassic period, today’s Svalbard formed part of the seafloor of the Boreal Sea; a large bay at approximately 45°N opening to Panthalassa in the northwest (Vigran et al. 2014). The world’s continents were still gathered in the supercontinent Pangea, with the seaway to Tethys closed off in the latest Permian. Throughout the Middle Triassic, depositional environment in the Boreal Sea was shallow to deep marine, with periods of high surface water productivity and temporary anoxia (e.g., Mørk et al. 1982; Mørk and Bjorøy 1984; Krajewski 2008; Vigran et al. 2014). The resulting Botneheia Formation consists mainly of dark shale interbedded with siltstone. Today, these crop out on the islands of Spitsbergen, Barentsøya, Wilhelmøya, and Edgeøya, and continue further south into the Barents Sea.
The Botneheia Formation is divided into the Anisian Muen Member and the Ladinian Blanknuten Member (Krajewski 2008; Vigran et al. 2014). The Muen Member mainly consist of shale and mudstone with recurrent carbonate cementstone beds. Phosphate nodules are common in the upper part of the member (Krajewski 2008). The Blanknuten Member consists of fine-grained clastic deposits strongly enriched in organic carbon, with considerable contribution of macroscopic phosphate fraction in the lower and upper parts (Mørk and Bjorøy 1984; Krajewski 2008). It has a high TOC content (up to 10 %) and is characterised by abundant bivalve fossils and intervals of marine reptile remains (Hurum et al. 2014; Lundschien et al. 2014). The upper part of the Botneheia Formation has been known for its abundance of ichthyosaur specimens since the 19th century (Hulke 1873). Wiman (1910) described a particularly fossiliferous horizon (the Upper Saurian niveau, see discussion in Maxwell and Kear 2013). However, PMO 235.393 was collected as loose material. It is therefore not possible to establish whether it originated from a fossiliferous horizon.
Material and methods
The specimen described here (PMO 235.393) is housed in the palaeontology collections of the University of Oslo Natural History Museum (NHM-UiO). The concretion was collected as loose material approximately in the middle of the Blanknuten Member in the Isfjorden area on Spitsbergen (GPS 33x E515240 N8712225). Especially the middle and upper part of the member is known for its fossil richness. It was found close to similar concretions in situ and is therefore considered to be found close to its origin. The fossil is preserved in a calcareous concretion and was broken into different parts, as it was cracked during discovery.
The concretion consists of a main slab A (Fig. 1) and four counter slabs B–E (SOM: figs. S1–S4, Supplementary Online Material available at http://app.pan.pl/SOM/app67-Roberts_etal_SOM.pdf). A table over the different slabs and what they contain is available in the supplementary material (SOM: table S1). Some invertebrate fossils are preserved in the concretion; fragments of daonellid bivalves and a poorly preserved ammonoid with smooth ribs (?Aristoptychites sp.). The main part of the concretion (slab A) includes most of the skull, anterior section of the axial skeleton as well as a few invertebrates. Photographs were taken using a Nikon D850 and a Nikon Micro-Nikkor 60 mm lens. Image stacks were compiled using the Nikon D850 automatic function with ten stacking levels. Image processing and figures were completed using Affinity Designer, Affinity Photo, Adobe Photoshop and Adobe Illustrator.
After collection, the main part of the concretion (slab A), was cut and reduced in size to fit in the NIKON Metrology XT H 225 ST microfocus CT at the NHM-UiO. Cut parts that included invertebrate specimens or bone fragments were kept and documented. The scans were completed with settings of 225 keV, 340 W and a silver 1 mm filter. The first scan of the posterior region of the skull was made with 3016 projections, with two frames per projection. The remaining two parts were scanned using 3016 projections, with one frame per projection. The resulting voxel size was 0.07537 mm. The three scans were merged into a single file which was segmented manually using Avizo V.20.2. Three-dimensional surface models were exported from Avizo V.20.2 as surface (.stl) files.
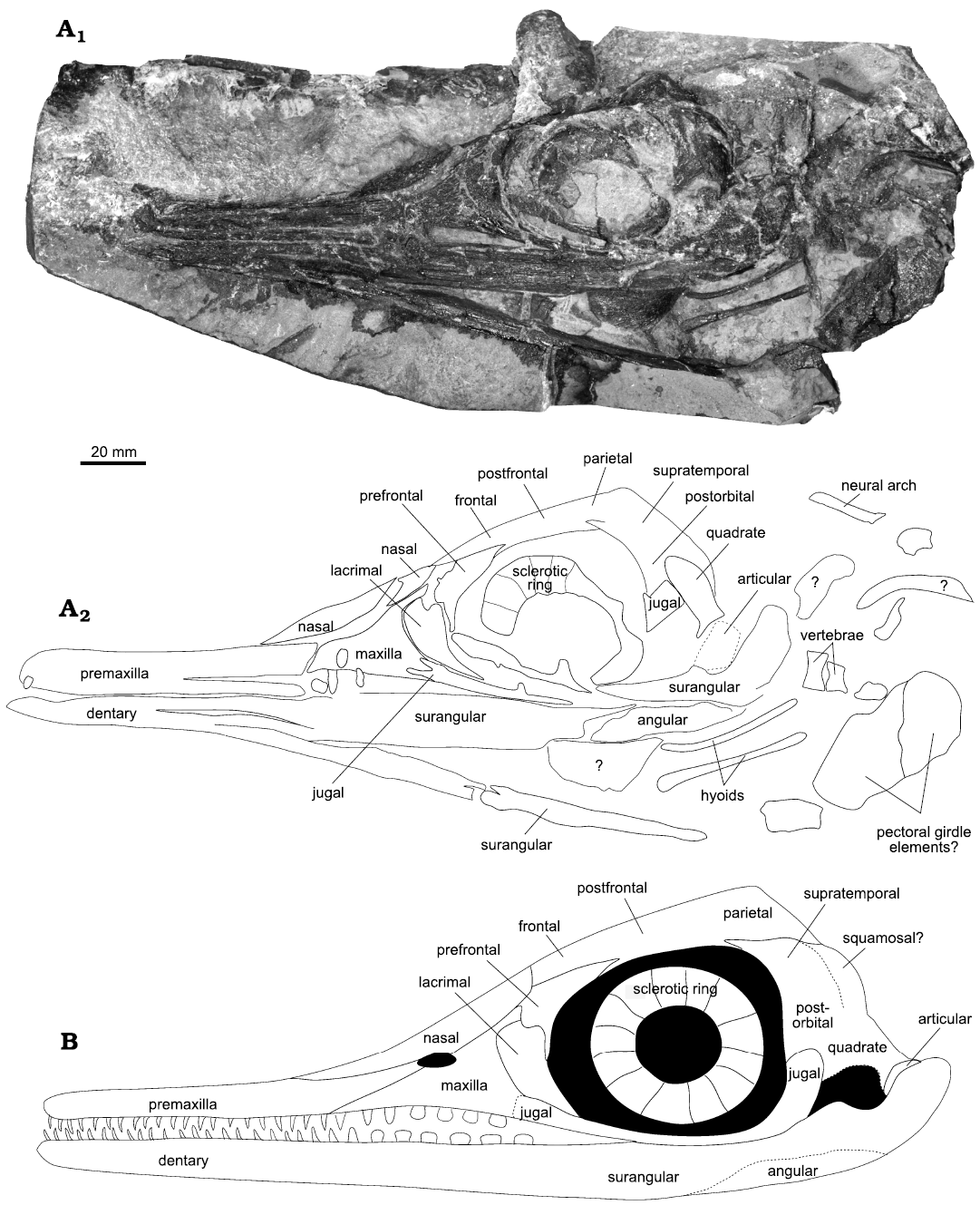
Fig. 1. Mixosaurid ichthyosaur Phalarodon fraasi (Merriam, 1910) PMO 235.393, from the Botneheia Formation, Middle Triassic of the Isfjorden area in Spitsbergen, Svalbard. A. Slab A in concretion, photograph (A1), line drawing (A2) showing the outline and identification of the visible bone elements. B. Hypothetical reconstruction of PMO 235.393.
A phylogenetic analysis was run in TNT V.1.5 (Goloboff and Catalano 2016) using the data matrix and character list provided by Økland et al. (2018), modified from Ji et al. (2015). This includes a total of 64 OTUs and 163 characters. We added an additional character (164) to describe the significant and unique maxillary heterodonty found in the different species of Phalarodon. This was recovered as a synapomorphy and has been added to genus diagnosis for Phalarodon. See supplementary for further information on the new character state. We followed a similar analysis to the original described by Økland et al. (2018), which attempted to replicate the analysis of Ji et al. (2015). More details on specific settings and scripts can be found in the supplementary materials. No taxa were pruned in this analysis.
Systematic palaeontology
Order Ichthyosauria Blainville, 1835
Family Mixosauridae Baur, 1887
Type genus: Mixosaurus Baur, 1887.
Included genera: Mixosaurus Baur, 1887 and Phalarodon Merriam, 1910.
Diagnosis.—Modified from Liu et al. (2013). Mixosauridae can be differentiated from other ichthyosaurs by the following combination of characters: premaxilla pointed posteriorly and lacking subnarial and supranarial processes; long sagittal crest which reaches the nasal; large anterior terrace of the upper temporal fenestra which reaches the nasal; pubis is significantly larger than the ischium; narrow and high caudal neural spines; large mid-caudal vertebral centra; heterodonty, posterior teeth more robust than anterior teeth; a pronounced notch present between the deltopectoral crest and the humeral head; distal carpal 1 larger than other carpals; three flattened middle metacarpals which are short and only have a slightly constricted shaft.
Genus Phalarodon Merriam, 1910
Type species: Phalaodon fraasi Merriam, 1910; West Humbolt Range, Nevada, USA; Middle Triassic.
Diagnosis.—Modified from Ji et al. (2015) and discussed in Økland et al. (2018). Premaxilla forms the anterior margin of external naris (also present in Mixosaurus panxianensis Jiang, Schmitz, Hao, and Sun, 2006); nasal region with a pronounced narial shelf; maxillary tooth implantation with sockets, dental groove absent posteriorly (completely absent in Phalarodon fraasi); significant heterodonty between anterior and posterior teeth in the maxilla (not extreme in Phalarodon atavus); teeth with laterally compressed horizontal section (circular in Phalarodon callawayi); posterior tooth crown flat (conical in P. atavus).
Phalarodon fraasi Merriam, 1910
Figs. 1–3.
Diagnosis.—Modified from Merriam (1910) and Ji et al. (2015). Marginal teeth ankylosed; significant heterodonty between anterior and posterior teeth in the maxilla; dental groove absent anteriorly and posteriorly (not clear in premaxilla of PMO 235.393); number of primary digits in forelimb increased to six; six distal carpals; metatarsal III gives rise to two digits.
Material.—PMO 235.393, skull, mandible and some axial and appendicular elements, the Isfjorden area (Spitsbergen), Middle Triassic.
Description.—The skull, mandible and some axial and appendicular elements are present. Using CT, it is possible to see that the skull is slightly distorted, with the right side positioned lower than the left side (Fig. 2). Ontogeny is not possible to determine as no bone surface is preserved. However, based on the size of the specimen and the relative size of the orbit, it is thought to be osteologically mature (Fernández et al. 2005). No braincase or palatal elements are visible for description on the specimen or by using CT. PMO 235.393 is referred to Mixosauridae based on the long sagittal crest which reaches the nasal; the large anterior terrace of the upper temporal fenestra; and posterior teeth more robust than anterior teeth. The specimen is referred to Phalarodon based on the presence of significant heterodonty a labial mandibular shelf and the lack of a dental groove in the maxillary region (Ji et al. 2015).
Skull: Premaxilla: Part of the premaxilla is damaged and missing on the left side. Using the CT scan, the right can be described in more detail. No over- or underbite is visible; however, the rostral tip is enclosed in matrix on slab D (SOM: fig. S4). Numerous teeth are set in the element (see dentition). The dentition cannot be clearly defined due to poor contrast, but there is some indication of ankylosed thecodonty (Fig. 3). This is likely a resolution and/or preservation issue, as the three-dimensional Phalarodon fraasi premaxilla (PMO 210.122) described by Økland et al. (2018) shows sockets. In PMO 210.122, the sockets are separated by alveolar walls and the length of the tooth crowns nearly equals the length of the roots. The slight compression and distortion of the specimen described herein might be enough to destroy the partitions as these are very thin. Økland et al. (2018) also observed that the alveolar walls became more robust and better preserved posteriorly. Small sockets are situated adjacent to larger sockets in PMO 210.122, which may have contained smaller teeth or demonstrate an uneven pattern of tooth replacement. These are not visible in PMO 235.393.
The presence of supra- or subnarial processes of the premaxilla of PMO 235.391, are not visible on the left side due to the preservation of this region. Using the CT scan data of the covered right side (Fig. 2), these processes appear absent as in all Phalarodon species (Schmitz et al. 2004; Jiang et al. 2007; Liu et al. 2013). Here, the posterior margin of the premaxilla only forms the anterior margin of the external naris (Jiang et al. 2007), a feature which is also present in PMO 235.391. This feature is also present in Mixosaurus panxianensis and may be a feature of all mixosaurids (Jiang et al. 2006).
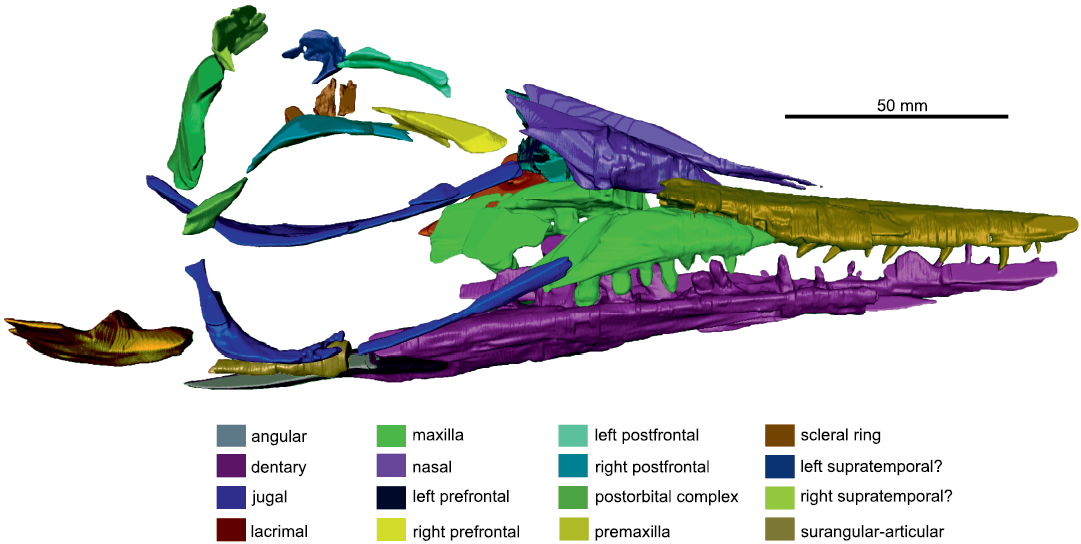
Fig. 2. The segmented model of right lateral side of the skull of mixosaurid ichthyosaur Phalarodon fraasi (Merriam, 1910) PMO 235.393, from the Botneheia Formation, Middle Triassic of the Isfjorden area in Spitsbergen, Svalbard.
Similar to the Phalarodon cf. fraasi specimen (GMPKU- P-1032) the premaxilla of PMO 235.391 borders the maxilla along a diagonal suture. The suture is visible within a small area, although distorted by teeth and not clear. Using CT data for the right side, the suture is shorter than maxilla-nasal suture, a feature differing from Mixosaurus panxianensis and Phalarodon avatus, where the sutures to the nasal and maxilla are near equal in length (Motani 1999, Ji et al. 2015). In the P. fraasi specimen PMO 210.122, Økland et al. (2018) described a three-dimensional premaxilla with anteroposteriorly-oriented coarse surface striations, which might also encompass foramina. This is not possible to see on PMO 235.393 due to preservation.
Maxilla: In lateral view, the maxilla is triangular with a long posterior process. It borders the premaxilla anteromedially, the nasal dorsally, and the lacrimal posteriorly. The anterior process of the maxilla appears short on the specimen, although CT analysis revealed this to be broken. The right side shows that maxilla terminates several centimetres anterior to the external naris and forms the entire ventral border of the external naris as in other specimens of Phalarodon fraasi and also Mixosaurus panxianensis (Jiang et al. 2007; Ji et al. 2015).
The maxilla articulates to the prefrontal posterodorsally, the lacrimal posteriorly, and the jugal posteroventrally. The posteriorly-pointed maxilla (common in mixosaurids, see Motani 1999 and Jiang et al. 2007) is often overlapped by the jugal just anteriorly to the orbital margin, as in other specimens of Phalarodon fraasi and also Phalarodon atavus. However, due to distortion in PMO 235.393, the jugal appears to have been pushed anteromedially and is therefore partially disarticulated. Although slightly displaced, the position of this suture differs from that seen in Mixosaurus panxianensis, where it is located just posterior to the anterior orbital margin (Ji et al. 2015).
From the CT scan, there are clear alveoli present in the maxilla. At least seven teeth are present in a single row on the better-preserved right maxilla. Replacement teeth are visible lingually. This confirms the observation in the Phalarodon fraasi specimen from Svalbard (PMO 210.122, Økland et al. 2018) of a single row of teeth in the maxilla as in the type specimen UCMP 9853 (Motani 2005a). Resultingly, we removed this character from the species diagnosis for P. fraasi (Schmitz et al. 2004). The posterior part of the maxilla bears significantly more robust and bulbous teeth compared to those in the premaxilla and the anterior part of the maxilla (see dentition). This is a dividing feature between Mixosaurus and Phalarodon, as Mixosaurus lacks true heterodont teeth which are entirely set in a dental groove (Ji et al. 2015; see also discussion in Økland et al. 2018). As such, PMO 235.393 shares these diagnostic features with Phalarodon species. The CT images also revealed the lingual surface of the maxilla (Fig. 3A5), which has a clear lingual shelf for articulation with the palate, likely the pterygoid. The maxilla is placed labially in respect to the dentary, so occlusion surfaces between the posterior maxillary teeth and posterior dentary teeth appear to be present (Fig. 3). This maxillary position also seems to be present in the type specimen of Phalarodon fraasi (Merriam 1910: fig. 2).
Nasal: The nasal of PMO 235.393 appears reduced in mediolateral extent on slab A. This is likely an artefact of the slightly skewed view and preservation, as the right nasal has a larger mediolateral extent seen in CT (Fig. 2). The nasal has a long anterior process which surpasses the anterior margin of the external naris and maxilla. The nasal forms the dorsal border of the external naris as in Phalarodon atavus (Liu et al. 2013). There is an indication of an anteroposteriorly orientated ridge on the CT scans, directly above the external naris. There is no descending process of the nasal. The posterior extent of the nasal is difficult to discern on the specimen or the CT scans, due to calcite crystal growth inside the concretion in that region. However, a contact between the prefrontal and nasal appears present, although its extent is not visible (Fig. 1). The nasal appears to terminate posterolaterally with a contact to the frontal where it forms the anteriormost part of the sagittal crest.
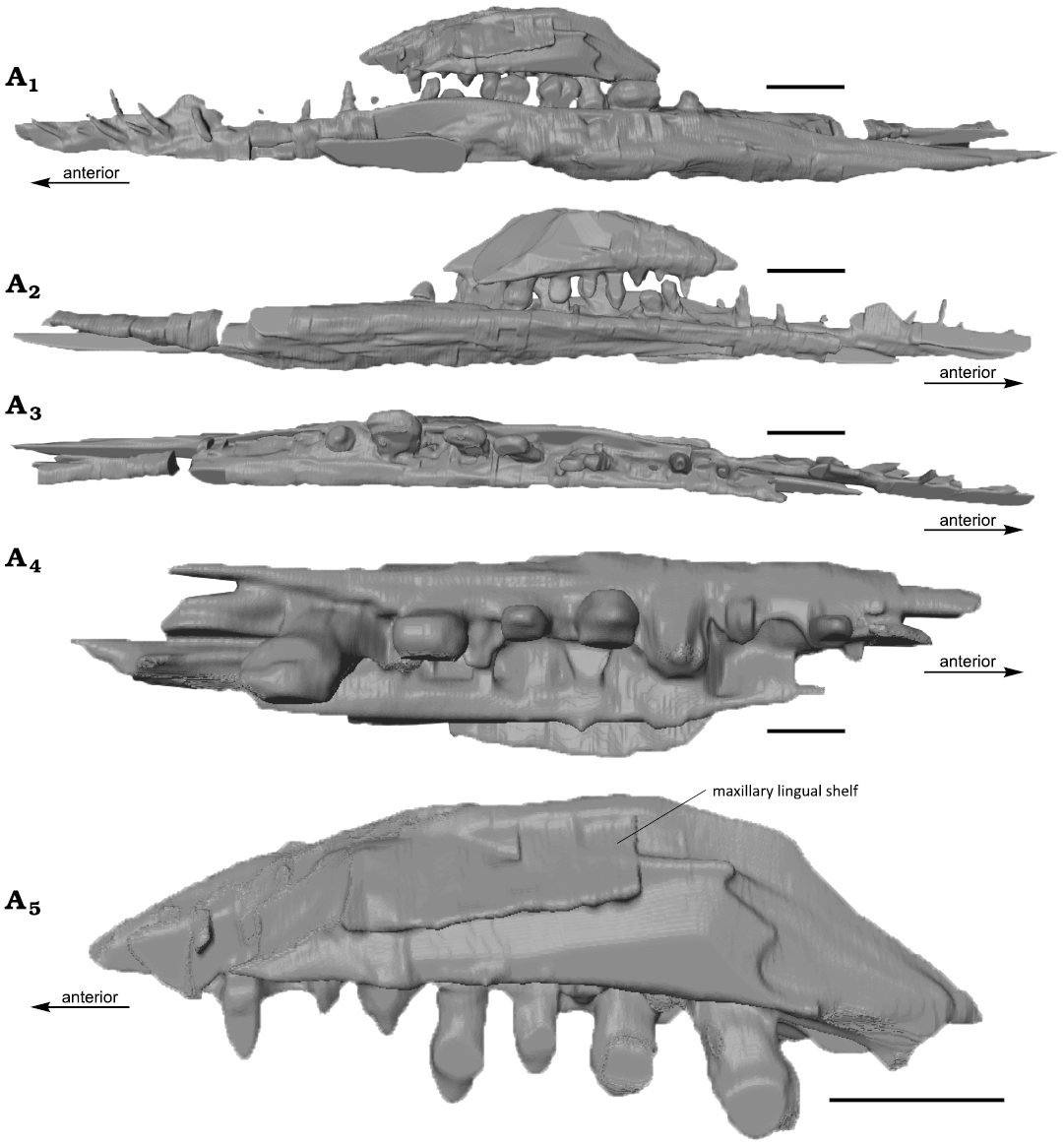
Fig. 3. The computed tomography rendered segmentation of rostral elements of mixosaurid ichthyosaur Phalarodon fraasi (Merriam, 1910) PMO 235.393, from the Botneheia Formation, Middle Triassic of the Isfjorden area in Spitsbergen, Svalbard. Right dentary and maxilla in lingual (A1) and labial (A2) views, right dentary (A3) in dorsal view, right maxilla in dorsal (A4) and lateral (A5) views. Note that the dorsal extent of the maxilla is not complete. Scale bars 10 mm.
Lacrimal: The lacrimal forms the anteroventral part of the orbit. Schmitz et al. (2004) described an antorbital ridge on the lacrimal of Phalarodon callawayi. In Phalarodon fraasi, this has been described as “prominent, triangular knob” in the type specimen (Merriam 1910: 387). This is more-clearly figured in Motani (2005a: fig. 2). No clear antorbital ridge is visible on PMO 235.393 due to matrix covering the surface in slab D (see SOM: fig. S4). However, in lateral view, a slight eminence is raised where the prefrontal overlaps with the lacrimal, indicating an antorbital ridge may be present. Similar to Phalarodon atavus (CCCGS LPV 30872, Liu et al. 2013), a small and narrow posteroventral process is overlapped by the jugal in lateral view. The anterior part of the orbit is not possible to describe in detail and the border between the prefrontal and the lacrimal is indiscernible. Based on observations from PMO 235.393 and descriptions of the Phalarodon fraasi type (Merriam 1910; Motani 2005a), we consider it unlikely that the lacrimal is as small as shown in Jiang et al. (2007) for Phalarodon fraasi GMPKU-P-1032. The element presented there as the prefrontal is more likely to be largely formed by the lacrimal.
Jugal: Visible on the CT segmentation (Fig. 2), the left jugal is slightly displaced and moved further anteriorly than in life. This would have likely fanned out and covered part of the maxilla and lacrimal as is visible on the well-segmented right side (Fig. 2). The left jugal is partly exposed on the left side in lateral view on slab A, with part of the posterior ramus preserved on counter slab C (SOM: fig. S3). It forms at least one third of the posterior margin of the orbit.
Prefrontal: The prefrontal forms the anterodorsal part of the orbit. A supraorbital crest is present on the prefrontal, although it is uncertain how far posteriorly this crest stretches due to preservation. This is feature is present in many mixosaurids and is clear on Mixosaurus kuhnschnyderi (Maisch and Matzke 2000), Mixosaurus cornalianus (Maisch and Matzke 1997, 1998) Phalarodon atavus and Phalarodon fraasi (Ji et al. 2015).
Orbit and sclerotic ring: The orbital margin is circular anteriorly and more irregular posteriorly. This differs from the more irregular orbits seen in some other Triassic ichthyosaurs (Fernández et al. 2005). The sclerotic ring is partially preserved on the left side, there are at least five elements forming the anterodorsal part of the ring. In cross-section, these curve inwards towards the socket and would have followed the shape of the eye itself, as described in Fernández et al. (2005).
Frontal: Most of the frontal is covered and not visible on the specimen or in the CT images. Only part of the left frontal is preserved on slab B (SOM: fig. S1) and as an impression on slab A in dorsal view. This preserved portion of the frontal is slightly concave and meets the prefrontal laterally. No part of the frontal-forming sagittal crest is visible.
Postfrontal: The postfrontal is only preserved as an impression on slab A, but is well preserved in dorsal view on slab C (SOM: fig. S2). In dorsal view, the postfrontal has an interdigitating suture with the prefrontal anteriorly (anterior third of orbit). Posteriorly, the postfrontal meets the anterodorsal process of the postorbital.
Postorbital: Most of the postorbital is visible in medial view on slab C (SOM: fig. S3). An impression and dorsal section of the element is present on slab A. Dorsally, the postorbital is overlapped by the supratemporal. Due to significant breakage in this area, the nature of this articulation and the extent of the border with the postfrontal is not entirely clear. Using slab C (SOM: fig. S2), there appears to be small overlap of the postfrontal at the anterodorsal part of the postorbital. Ventrally on slab C, a separate bone fragment which is likely a part of the jugal is present, although these do not clearly articulate (SOM: fig. S3).
Parietal and temporal fenestrae: The parietal is partly preserved as an impression on slab A (Fig. 1) and as an element on slab B (SOM: fig. S1). The temporal fenestra is visible on slab B, with parts of the parietal preserved along the margin. It is likely part of the sagittal crest, but the height and crest-forming elements are equivocal due to matrix cover and preservation.
Quadrate: The quadrate is preserved on part C and exposed in medial view, but partly encased in sediment (SOM: fig. S2). The outline of the element as shown in Fig. 1, is an impression from slab C preserved on slab A. The element is rather large, stretching to most of the dorsoventral height of the postorbital region. As in Phalarodon fraasi (GMPKU-P-1032), the quadrate condyle is massive (Jiang et al. 2007). In lateral view, most of this element would be covered by the postorbital, quadratojugal, squamosal and/or supratemporal.
Other cranial elements: No parts of the braincase, palate or quadratojugal are visible on the specimen or on the CT scan images due to the poor contrast in the posterior region of the skull. A possible part of the squamosal is marked in Fig. 1.
Mandible: Both lower jaws are preserved, although damaged, as a portion is preserved on counter slab D and E. There is no clear transition between the dentary and angular/surangular. In PMO 210.122, a small horizontal shelf of the dentary (labial mandibular shelf, Jiang et al. 2007) on the labial side offsets the teeth slightly towards the lingual margin (Økland et al. 2018). This is also preserved in PMO 235.393, but is more posteriorly pronounced. This is clearly visible in the segmented image of the maxilla and dentary (Fig. 3). This feature is also observed in other Phalarodon fraasi and Phalarodon callawayi specimens (Motani 2005a; Jiang et al. 2007).
Phalarodon fraasi has been described as having a dorsally inflected and posteriorly extending angular which is larger than the surangular (Jiang et al. 2007). The variation within Phalarodon is not known. The suture between the angular and surangular has not been described for Phalarodon callawayi (Schmitz et al. 2004). It is not possible in PMO 235.393 to determine the exact suture between the angular and surangular. However, the angular seems to have contributed to at least half the posterior portion of the mandible laterally. Part of the posterior section of the mandible including the articular and quadrate joint, is visible on slabs A, C (SOM: fig. S3). The element is skewed, making it appear more extensive than the actual extent. Part of the outline is exposed and shows the curved contact to the quadrate.
The dentition of the mandible is heterodont (Figs. 2, 3). Anteriorly, conical teeth match those in the premaxilla. Posteriorly, the teeth increase in size and become rounded crushing teeth. These are significantly larger than their counterparts in the maxilla. All teeth are preserved in separate alveoli as in PMO 210.122 (AJR personal observation).
Hyoids: Two hyoids are preserved in place, between the two lower jaws. These are only an impression on slab A, but are preserved on counter slab E. They are slightly medially convex with a larger anterior than posterior head.
Dentition: Although obscured on the specimen itself, teeth from PMO 235.393 are clearly visible in the CT scan (Figs. 2, 3). It is unclear how many teeth would have been present in the premaxilla and mandible.
The teeth on PMO 235.393 vary from conical to slightly anteroposteriorly elongated in the anterior region of the jaw. In the posterior region, they are strongly anteroposteriorly elongated with oval cross-sections. This differs from Phalarodon callawayi, which has circular teeth in cross-section in the anterior part of the jaw, and from Phalarodon atavus which has conical tooth crowns with a figure-of-eight cross-section in posterior maxillary teeth (Schmitz et al. 2004; Ji et al. 2015).
The dentition in the anterior part of the premaxilla and dentary consists of elongated piercing teeth which end in a sharp point (SOM: fig. S5). This differs significantly from the flattened and anteroposteriorly elongated crushing teeth in the posterior parts of the maxilla and dentary. The teeth in the posterior end of the dentary are twice the size of the teeth in the maxilla, both in height and anteroposterior length. An anteriorly positioned tooth in the maxilla is 3 mm anteroposteriorly long, which indicates a size around 5 mm in anteroposterior length for the more posterior teeth in the maxilla. Posterior teeth in the maxilla and in the dentary can be studied in CT scans. The posterior teeth in the dentary are approximately twice the size of the posterior teeth in the maxilla, approximately 10 mm. There are at least seven teeth preserved in the right maxilla.
There is a distinct difference between the tooth attachment in the anterior parts of the premaxilla and the dentary. From CT scans, the teeth are clearly visible in sockets in the dentary (Fig. 3). In the premaxilla, these are diffuse and hard to identify. This fits the description for Phalarodon fraasi by Motani (1997) as having subthecodont dentition anteriorly only in the upper jaw. In the maxilla, alveoli are clearly visible, and the dentition appears to be anchylosed thecodont as described by Motani (1997). This is not as clear posteriorly in the dentary.
Postcranial material: At least six vertebrae are seen on the different slabs, together with disarticulated neural arches. Many of these are better preserved on slab C. Several ribs are partially preserved. In addition, there are some broken appendicular elements, either the scapula or the coracoid. Due to the fragmented nature this is not possible to determine. For a better-preserved pectoral girdle of Phalarodon fraasi from Svalbard see Økland et al. (2018).
Stratigraphic and geographic range.—Middle Triassic, China, Central Europe, North-America, Svalbard.
Discussion
Phylogeny.—The phylogenetic analysis resulted in 9 most-parsimonious trees (MPTs) with 544 steps, a consistency index of 0.373 and retention index of 0.778. Although more steps were required, the consistency and retention indices are similar to those shown in Økland et al. (2018) and Ji et al. (2015). The overall tree topology (Fig. 4) is similar to that presented in Økland et al. (2018), with some taxa changing position in their respective groups (Gulosaurus). Unsurprisingly, our analysis received higher Bremer support values for Mixosauridae than Økland et al. (2018), this is due to the addition of the new character (164).
Our analysis clearly shows the position of PMO 235.393 within the Phalarodon and in an unresolved clade with Phalarodon callawayi and Phalarodon fraasi. However, based on the oval cross-section in the posterior teeth of PMO 235.393, this specimen is not referable to P. callawayi which has diagnostically circular teeth in cross-section (Økland et al. 2018). As such, the phylogenetic analysis confirms our referral of PMO 235.393 to Phalarodon fraasi.
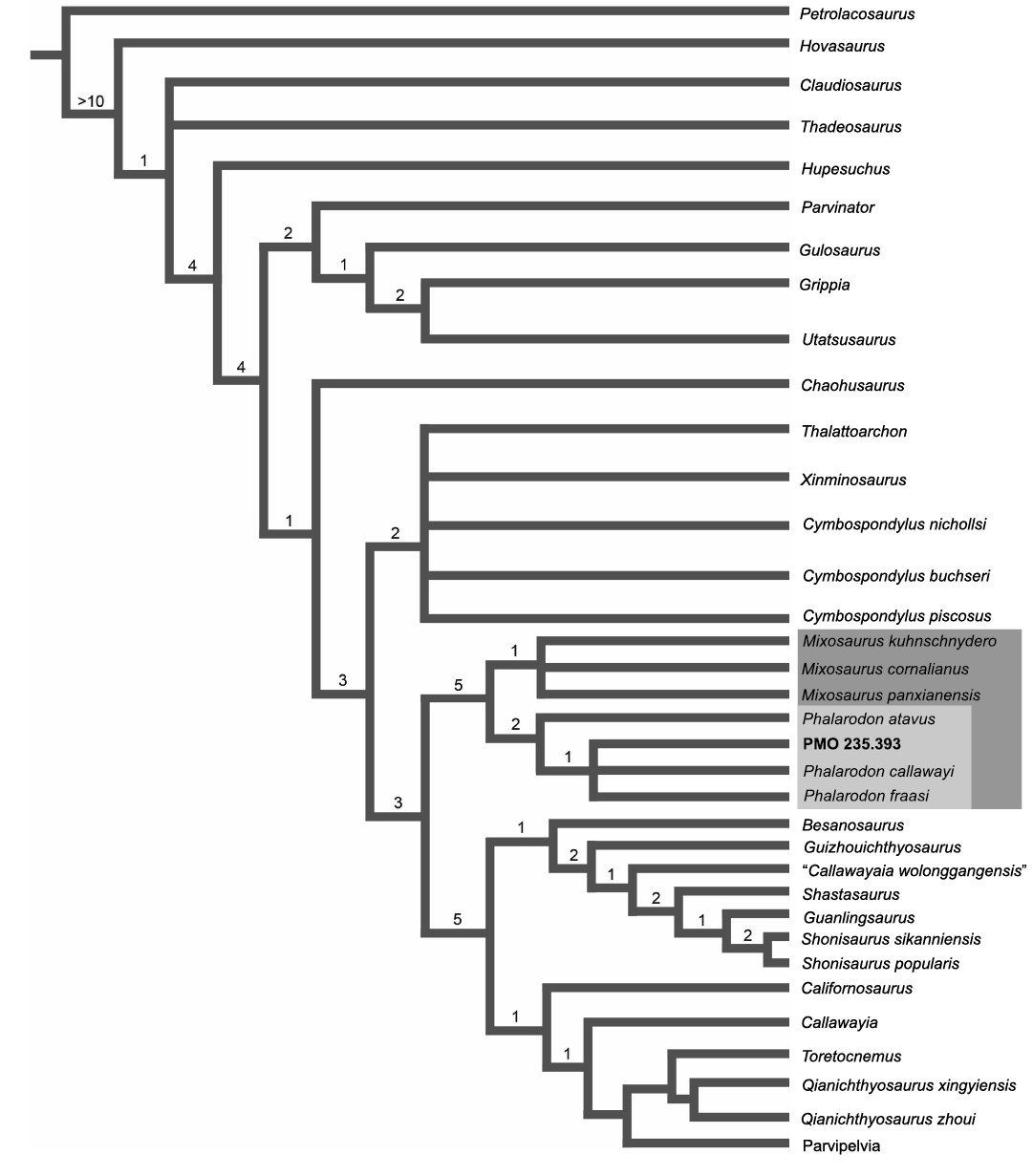
Fig. 4. The phylogenetic tree of Ichthyosauria and the placement of PMO 235.393. Note that Parvipelvia has collapsed as this part of the tree is not the focus of the paper. Consensus tree of 19 MPTs with a length of 544. Consistancy Index = 0.373, Rentention Index = 0.778. Bremer support values displayed above nodes.
Durophagy in Phalarodon.—Kelley et al. (2014) used several Middle and Late Triassic examples of dental morphology to classify Mesozoic marine reptile ecotypes. One of these examples was Phalarodon and was classified as a durophagous mixosaurid, living in a nearshore environment. In contrast, Kelley et al. (2014) interpreted Mixosaurus to be more pelagic than Phalarodon, as the dentition is more piercing/smashing than crushing (Massare 1987). This type of dentition is likely more suited for hunting soft-bodied and usually fast-swimming prey, such as fish or cephalopods (Massare 1987). Our study confirms the typically anteroposteriorly elongated posterior dentition in Phalarodon (except for Phalarodon callawayi) (Merriam 1910; Motani 1999, 2005a; Jiang et al. 2007). A novel observation in this study is the adaptation of the skull and lower jaw towards a more powerful shearing bite. Due to the three-dimensional preservation of the skull of PMO 235.393, it was possible to reconstruct in detail the maxilla and dentary using CT segmentation. The dentary forms a large area for the attachment of jaw musculature on the labial shelf in the posterior part of the tooth-bearing portion. This has also been described for the holotype of Phalarodon fraasi (Motani 2005a). A similar strong articulation in the form of a lingual shelf, is observed between the maxilla and the pterygoid.
Ichthyosauriforms with rounded posterior teeth (molariform) in the Early Triassic had small teeth with a diameter up to 3 mm, while those in the Middle Triassic were larger with a diameter of more than 10 mm (Huang et al. 2020). The occlusion between the elongated, posterior upper and lower teeth is hard to determine in PMO 235.393, as the skull is distorted. Motani (2005a) discussed that the presence of dental occlusion and wear of the posterior molariform teeth in the holotype of Phalarodon fraasi, as an adaptation for durophagy. In PMO 235.393, the posterior maxillary tooth row are placed labially to the posterior dental teeth, suggesting more of a cutting edge than a crushing surface. This supports the more “shearing” than crushing dentition observed in the holotype specimen (Motani 2005a). Additional specimens documenting dental wear is required to test this hypothesis.
Durophagy or the presence of crushing teeth has been described for other marine reptiles, such as placodonts (Neenan et al. 2013) and mosasaurs (Carinodens belgicus, Schulp 2005, Globidens sp., Russell 1975). Carinodens belgicus, a durophagous and possibly molluscivorous mosasaur differs from most other mosasaurs in having a heterodont dentition; with slender widely-spread teeth for gripping anteriorly and bulbous closely spaced teeth for crushing posteriorly (Schulp 2005). In mosasaurs, a more-bulbous tooth morphology decreases stress on the tooth, but also reduces bite pressure due to the increased surface area (Lingham-Soliar 1999). In addition, wrinkles on the surface of the tooth have been considered to strengthen the tooth as well as increase grip and bite pressure (Lingham-Soliar 1999; Schulp 2005). These have been described for multiple reptilian groups, many confirmed durophages by stomach content (Sander 1999). Although there are large anatomical differences between the jaw morphology of durophagous mosasaurs, placodonts and Phalarodon, there are some similarities in the dentition. The clear wrinkles seen in some globidensine mosasaur teeth and some placodont teeth (Gere et al. 2020) are absent or not visible in any Phalarodon teeth described from Svalbard. However, a sharp medial ridge on the posterior crushing teeth and slight midpoint constriction has previously been described for Phalarodon fraasi from Svalbard (Maxwell and Kear 2013). Wrinkled textures, furrows and a blunt crown ridge on the posterior dentition have been described for the type specimen of P. fraasi (Merriam 1910; Motani 2005a) and other Phalarodon specimens (P. fraasi and P. callawayi) from Canada (Nicholls et al. 1999). The uneven surface of the teeth may similarly increase dental strength and grip as in other marine reptiles (Lingham-Soliar 1999; Schulp 2005). In PMO 210.122, only weak lingual longitudinal ridges are present on the only preserved posterior tooth. These regional differences in tooth ornamentation may suggest a higher specific diversity differences in wear due to tooth age; or local dietary adaptations (Massare 1987). Based on the occlusion surfaces described for PMO 235.393, the reduced wrinkles on the Svalbard specimens may be an adaptation for cutting rather than crushing. Supporting the “shearing” hypothesis based on tooth wear for the holotype specimen of Phalarodon fraasi and PMO 235.393 (Motani 2005a).
A single functional tooth row is present in PMO 235.393, with a secondary row of replacement teeth. However, the posterior part of the dentary tooth row is similar in its general morphology and tooth spacing in the Canadian and Svalbard specimens (Nicholls et al. 1999: fig. 7). Schmitz (2005) found the syntype material of Mixosaurus nordenskioeldii described by Hulke (1873) to be undiagnostic and considered the species to be a nomen dubium, referring all specimens described by Nicholls et al. (1999) to either Phalarodon fraasi or Phalarodon callawayi.
Multiple tooth rows increase the functional surface of the jaw and is common in durophagous marine reptiles, such as placodonts (Neenan et al. 2013) and the enigmatic marine reptile Omphalosaurus (Ekeheien et al. 2018). The three irregular rows of teeth in a robust maxilla, as described by Nicholls et al. (1999) for a specimen (TMP 89.127.7) of “Phalarodon nordenskioeldii” (currently a nomen dubium), is not present in any of the referred Phalarodon fraasi specimens (Merriam 1910; Økland et al. 2018). As such, this feature has been removed from the species diagnosis of P. fraasi, as TMP 89.127.7 was not officially referred to P. fraasi (Schmitz 2005), and likely does not belong to this species.
Additional reinforcement of the jaw and jaw musculature is also present in durophagous mosasaurs to increase bite force/pressure (Schulp 2005). An example is the strengthening of the quadrate in all globidensine mosasaurs (Schulp 2005). We suggest that there is evidence for a reinforced quadrate in Phalarodon fraasi, based on the large size and extension of this element. Placodonts and globidensine mosasaurs both have large mandibular processes (mame, adductor mandibulae externus attachment) to support the jaw musculature. This differs from Phalarodon spp., which bear a prominent sagittal crest for muscle attachment stretching from the posterior margin of the skull, and past the anterior margin of the orbit (including the nasal). Although sagittal crests are common in most ichthyosaurs, those seen in all species of Phalarodon are significantly larger than those described for the contemporaneous Mixosaurus cornalianus (Motani 1999). As such, the large sagittal crest combined with anteroposteriorly elongated and flattened teeth in Phalarodon, may be associated trait to a more durophagous diet compared to the semi-pelagic Mixosaurus.
Conclusions
Acknowledgements
Rich Cifelli and I (JHH) are both the disciples of Zofia Kielan-Jaworowska. She sometimes nicknamed us her two extra sons. It is weird because we have never met. Rich and I alternated to be in Warsaw working on projects with Zofia for many years, never at the same time. His writing was always praised by Zofia as the best scientific writer she knew. Thank you to the Willi Hennig Society which supports the open access of TNT V1.5. We are grateful for the expertise and assistance given by Øyvind Hammer (Natural History Museum, University of Oslo, Norway) for the computed tomography (CT) acquisition. Thanks to Steve D. Torgersen (Svalbard explorer, Longyearbyen, Norway) for logistics and beverages. Benjamin Kear (Museum of Evolution, University of Uppsala, Sweden) and an anonymous reviewer are thanked for their comments on the manuscript. This research was funded by ARCEx partners and the Research Council of Norway (grant number 228107) for VSE.
References
Baur, G. 1887. On the morphology and origin of the Ichthyopterygia. American Naturalist 21: 837–840. Crossref
Blainville, H.M.D. 1835. Description de quelques espèces de reptiles de la Californie: précédée de l’analyse d’un systéme général d’erpétologie et d’amphibiologie. Nouvelles Annales du Muséum d’Histoire Naturelle 4: 233–296.
Ekeheien, C.P., Delsett, L.L., Roberts, A.J., and Hurum, J.H. 2018. Preliminary report on ichthyopterygian elements from the Early Triassic (Spathian) of Spitsbergen. Norwegian Journal of Geology 98: 219–238. Crossref
Fernández, M.S., Archuby, F., Talevi, M., and Ebner, R. 2005. Ichthyosaurian eyes: paleobiological information content in the sclerotic ring of Caypullisaurus (Ichthyosauria, Ophthalmosauria). Journal of Vertebrate Paleontology 25: 330–337. Crossref
Fröbisch, N.B., Fröbisch, J., Sander, P.M., Schmitz, L., and Rieppel, O. 2013. Macropredatory ichthyosaur from the Middle Triassic and the origin of modern trophic networks. Proceedings of the National Academy of Sciences 110: 1393–1397. Crossref
Gere, K., Scheyer, T.M., Makádi, L., and Ősi, A. 2020. Placodont remains (Sauropsida, Sauropterygia) from the Triassic of Hungary (Transdanubian Range and Villány Mountains). Palaeobiodiversity and Palaeoenvironments 100: 1047–1063. Crossref
Goloboff, P.A. and Catalano, S.A. 2016. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32: 221–238. Crossref
Huang, J.-D., Motani, R., Jiang, D.-Y., Ren, X.-X., Tintori, A., Rieppel, O., Zhou, M., Hu, Y.-C., and Zhang, R. 2020. Repeated evolution of durophagy during ichthyosaur radiation after mass extinction indicated by hidden dentition. Scientific Reports 10 (1): 1–10. Crossref
Hulke, J.W. 1873. Memorandum on some fossil vertebrate remains collected by the Swedisch expeditions to Spitzbergen in 1864 and 1868. Bihang till Kongliga Svenska Vetenskaps-Akademiens Handlingar 1 (9): 2–11.
Hurum, J.H., Engelschiøn, V.S., Økland, I., Bratvold, J., Ekeheien, C., Roberts, A.J., Delsett, L.L., Bolvig, B., Mørk, A., and Nakrem, H.A., Druckenmiller, P. S., and Hammer, Ø. 2018. The history of exploration and stratigraphy of the Early to Middle Triassic vertebrate bearing strata of Svalbard (Sassendalen Group, Spitsbergen). Norwegian Journal of Geology 2: 165–174. Crossref
Hurum, J.H., Roberts, A.J., Nakrem, H.A., Stenløkk, J.A., and Mørk, A. 2014. The first recovered ichthyosaur from the Middle Triassic of Edgeøya, Svalbard. Norwegian Petroleum Directorate Bulletin 11: 97–110.
Ji, C., Jiang, D.-Y., Motani, R., Rieppel, O., Hao, W.-C., and Sun, Z.-Y. 2015. Phylogeny of the Ichthyopterygia incorporating recent discoveries from South China. Journal of Vertebrate Paleontology 36: e1025956. Crossref
Jiang, D.-Y., Motani, R., Huang, J.-D., Tintori, A., Hu, Y.-C., Rieppel, O., Fraser, N.C., Ji, C., Kelley, N.P., Fu, W.-L., and Zhang, R. 2016. A large aberrant stem ichthyosauriform indicating early rise and demise of ichthyosauromorphs in the wake of the end-Permian extinction. Scientific Reports 6 (26232): 1–9. Crossref
Jiang, D.-Y., Schmitz, L., Hao, W.-C., and Sun, Y.-L. 2006. A new mixosaurid ichthyosaur from the Middle Triassic of China. Journal of Vertebrate Paleontology 26: 60–69. Crossref
Jiang, D.-Y., Schmitz, L., Motani, R., Hao, W.-C., and Sun, Y.-L. 2007. The mixosaurid ichthyosaur Phalarodon cf. P. fraasi from the Middle Triassic of Guizhou Province, China. Journal of Paleontology 81: 602–605. Crossref
Kelley, N.P. and Pyenson, N.D. 2015. Evolutionary innovation and ecology in marine tetrapods from the Triassic to the Anthropocene. Science 348: 3716–3716. Crossref
Kelley, N.P., Motani, R., Jiang, D.-Y., Rieppel, O., and Schmitz, L. 2014. Selective extinction of Triassic marine reptiles during long-term sea-level changes illuminated by seawater strontium isotopes. Palaeogeography, Palaeoclimatology, Palaeoecology 400: 9–16. Crossref
Krajewski, K.P. 2008. The Botneheia Formation (Middle Triassic) in Edgeøya and Barentsøya, Svalbard: lithostratigraphy, facies, phosphogenesis, paleoenvironment. Polish Polar Research 29: 319–364.
Lingham-Soliar, T. 1999. The durophagous mosasaurs (Lepidosauromorpha, Squamata) Globidens and Carinodens from the Upper Cretaceous of Belgium and The Netherlands. Paleontological Journal 33: 638–647.
Liu, J., Motani, R., Jiang, D.-Y., Hu, S.-X., Aitchison, J.C., Rieppel, O., Benton, M.J., Zhang, Q.-Y., and Zhou, C.-Y. 2013. The first specimen of the Middle Triassic Phalarodon atavus (Ichthyosauria: Mixosauridae) from South China, showing postcranial anatomy and peri-Tethyan distribution. Palaeontology 56: 849–866. Crossref
Lundschien, B.A., Høy, T., and Mørk, A. 2014. Triassic hydrocarbon potential in the Northern Barents Sea; integrating Svalbard and stratigraphic core data. Norwegian Petroleum Directorate Bulletin 11: 3–20.
Maisch, M.W. 2010. Phylogeny, systematics, and origin of the Ichthyosauria—the state of the art. Palaeodiversity 3: 151–214.
Maisch, M.W. and Matzke, A.T. 1997. Observations on Triassic ichthyosaurs. Part I. Structure of the palate and mode of tooth implantation in Mixosaurus cornalianus (Bassani, 1886). Neues Jahrbuch für Geologie und Paläontologie Monatshefte 1997 (12): 717–732. Crossref
Maisch, M.W. and Matzke, A.T. 1998. Observations on Triassic ichthyosaurs. Part III: A crested predatory mixosaurid from the Middle Triassic of the Germanic Basin. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 209: 105–134. Crossref
Maisch, M.W. and Matzke, A.T. 2000. The Ichthyosauria. Stuttgarter Beiträge zur Naturkunde, Serie B 298: 1–159.
Massare, J.A. 1987. Tooth morphology and prey preference of Mesozoic marine reptiles. Journal of Vertebrate Paleontology 7: 121–137. Crossref
Maxwell, E.E. and Kear, B.P. 2013. Triassic ichthyopterygian assemblages of the Svalbard archipelago: a reassessment of taxonomy and distribution. Journal of the Geological Society of Sweden 135: 85–94. Crossref
Merriam, J.C. 1910. The skull and dentition of a primitive ichthyosaurian from the Middle Triassic. University of California Publications, Bulletin of the Department of Geology 5: 381–390.
Moon, B.C. 2019. A new phylogeny of ichthyosaurs (Reptilia: Diapsida). Journal of Systematic Palaeontology 17: 129–155. Crossref
Moon, B.C. and Stubbs, T.L. 2020. Early high rates and disparity in the evolution of ichthyosaurs. Communications Biology 3: art. 68. Crossref
Motani, R. 1997. Temporal and spatial distribution of tooth implantations in ichthyosaurs. In: J.M. Callaway and E.L. Nicholls (eds.), Ancient Marine Reptiles, 81–103. Academic Press, San Diego. Crossref
Motani, R. 1999. The skull and taxonomy of Mixosaurus (Ichthyopterygia). Journal of Paleontology 73: 924–935. Crossref
Motani, R. 2005a. Detailed tooth morphology in a durophagous ichthyosaur captured by 3D laser scanner. Journal of Vertebrate Paleontology 25 (2): 462–465. Crossref
Motani, R. 2005b. Evolution of fish-shaped reptiles (Reptilia: Ichthyopterygia) in their physical environments and constraints. Annual Review of Earth and Planetary Sciences 33: 395–420. Crossref
Mørk, A. and Bjorøy, M. 1984. Mesozoic source rocks on Svalbard. In: A.M. Spencer (ed.), Petroleum Geology of the North European Margin, 371–382. Springer Netherlands, Dordrecht.
Mørk, A., Knarud, R., and Worsley, D. 1982. Depositional and diagenetic environments of the Triassic and Lower Jurassic succession of Svalbard. Arctic Geology and Geophysics: Proceedings of the Third International Symposium on Arctic Geology Memoir 8: 371–398.
Neenan, J.M., Klein, N., and Scheyer, T.M. 2013. European origin of placodont marine reptiles and the evolution of crushing dentition in Placodontia. Nature Communications 4 (1): 1621. Crossref
Nicholls, E.L. and Brinkman, D.B. 1995. A new ichthyosaur from the Triassic Sulphur Mountain formation of British Columbia. In: W.A.S. Sarjeant (ed.), Vertebrate fossils and the evolution of scientific concepts, 521–535. Gordon and Breach Publishers, Amsterdam.
Nicholls, E.L., Brinkman, D.B., and Callaway, J.M. 1999. New material of Phalarodon (Reptilia: Ichthyosauria) from the Triassic of British Columbia and its bearing on the interrelationships of mixosaurs. Palaeontographica A: 1–22. Crossref
Økland, I.H., Delsett, L.L., Roberts, A.J., and Hurum, J.H. 2018. A Phalarodon fraasi (Ichthyosauria: Mixosauridae) from the Middle Triassic of Svalbard. Norwegian Journal of Geology 98: 267–288. Crossref
Pyenson, N.D., Kelley, N.P., and Parham, J.F. 2014. Marine tetrapod macroevolution: Physical and biological drivers on 250 Ma of invasions and evolution in ocean ecosystems. Palaeogeography, Palaeoclimatology, Palaeoecology 400: 1–8. Crossref
Renesto, S., Dal Sasso, C., Fogliazza, F., and Ragni, C. 2020. New findings reveal that the Middle Triassic ichthyosaur Mixosaurus cornalianus is the oldest amniote with a dorsal fin. Acta Palaeontologica Polonica 65: 511–522. Crossref
Russell, D.A. 1975. A new species of Globidens from South Dakota, and a review of globidentine mosasaurs. Fieldiana 33: 235–256. Crossref
Sander, P.M. 1999. The Microstructure of Reptilian Tooth Enamel: Terminology, Function, and Phylogeny. 102 pp. Verlag Dr. Friedrich Pfeil, München.
Sander, P.M. and Bucher, H. 1990. On the presence of Mixosaurus (Ichthyopterygia: Reptilia) in the Middle Triassic of Nevada. Journal of Paleontology 64: 161–164. Crossref
Scheyer, T.M., Romano, C., Jenks, J., and Bucher, H. 2014. Early Triassic marine biotic recovery: The predators’ perspective. PLoS ONE 9 (3): e88987. Crossref
Schmitz, L. 2005. The taxonomic status of Mixosaurus nordenskioeldii (Ichthyosauria). Journal of Vertebrate Paleontology 25: 983–985. Crossref
Schmitz, L., Sander, P.M., Storrs, G.W., and Rieppel, O. 2004. New Mixosauridae (Ichthyosauria) from the Middle Triassic of the Augusta Mountains (Nevada, USA) and their implications for mixosaur taxonomy. Palaeontographica A 270 (4–6): 133–162. Crossref
Schulp, A.S. 2005. Feeding the mechanical mosasaur: what did Carinodens eat? Netherlands Journal of Geosciences 84: 345–357. Crossref
Stubbs, T.L. and Benton, M.J. 2016. Ecomorphological diversifications of Mesozoic marine reptiles: the roles of ecological opportunity and extinction. Paleobiology 42: 547–573. Crossref
Vigran, J.O., Mangerud, G., Mørk, A., Worsley, D., and Hochuli, P.A. 2014. Palynology and geology of the Triassic succession of Svalbard and the Barents Sea. Geological Survey of Norway Special Publication 14: 1–269.
Wiman, C. 1910. Ichthyosaurier aus der Trias Spitzbergens. Bulletin of the Geological Institution of the University of Upsala 10: 124–148.
Acta Palaeontol. Pol. 67 (1): 51–62, 2022
https://doi.org/10.4202/app.00915.2021