

Second specimen of Corriebaatar marywaltersae from the Lower Cretaceous of Australia confirms its multituberculate affinities
THOMAS H. RICH, DAVID W. KRAUSE, PETER TRUSLER, MATT A. WHITE, LESLEY KOOL, ALISTAIR R. EVANS, STEVEN MORTON, and PATRICIA VICKERS-RICH
Rich, T.H., Krause, D.W., Trusler, P., White, M.A., Kool, L., Evans, A.R., Morton, S., and Vickers-Rich, P. 2022. Second specimen of Corriebaatar marywaltersae from the Lower Cretaceous of Australia confirms its multituberculate affinities. Acta Palaeolontologica Polonica 67 (1): 115–134.
A second specimen of the Australian cimolodontan multituberculate Corriebaatar marywaltersae from the same locality (Flat Rocks) as the holotype and previously only known specimen, reveals far more anatomical information about the species. The new specimen, composed of most of a dentary containing a complete p4 and alveoli for the lower incisor and the lower first and second molars, exhibits a suite of features consistent with allocation of Corriebaatar to Cimolodonta and further confirms the presence of multituberculates on Gondwana during the Mesozoic. The revised (older) age of the Flat Rocks locality to latest Barremian (mid-Early Cretaceous) establishes C. marywaltersae as the oldest currently known cimolodontan. This has profound biogeographic implications for the distribution of multituberculates on Gondwana as well as globally, particularly in light of the fact that Corriebaatar appears to be a relatively derived member of Cimolodonta.
Key words: Mammalia, Multituberculata, Cimolodonta, Cretaceous, Gondwana, Australia.
Thomas H. Rich [trich@museum.vic.gov.au] and Lesley Kool [koolasuchas@bigpond.com], Museums Victoria, P.O. Box 666, Melbourne, Victoria 3001, Australia.
David W. Krause [David.Krause@dmns.org], Department of Earth Sciences, Denver Museum of Nature & Science, 2001 Colorado Boulevard, Denver, Colorado 80205, USA; and Department of Anatomical Sciences, Stony Brook University, Stony Brook, New York 11794-8081, USA.
Peter Trusler [peter@petertrusler.com.au], School of Earth, Atmosphere & Environment, 9 Rainforest Walk, Monash University, 3800,Victoria, Australia.
Matt A. White [fossilised@hotmail.com], University of New England, Armidale, NSW 2350, Australia; and Australian Age of Dinosaurs Museum of Natural History, 1 Dinosaur Way, Winton, Queensland 4735, Australia.
Alistair R. Evans [alistair.evans@monash.edu], School of Biological Sciences, Monash University, Clayton, Victoria 3800, Australia; and Museums Victoria, P.O. Box 666, Melbourne, Victoria 3001, Australia.
Steven Morton [steven.morton@monash.edu], School of Physics and Astronomy, Monash University, Victoria 3800, Australia.
Patricia Vickers-Rich [pat.rich@monash.edu; prich@swin.edu.au], School of Earth, Atmosphere and Environment, Monash University, Victoria 3800; and Department of Chemistry and Biotechnology, Swinburne University of Technology, Hawthorn, Victoria 3122, Australia.
Received 2 July 2021, accepted 24 January 2022, available online 30 March 2022.
Copyright © 2022 T.H. Rich et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Corriebaatar marywaltersae Rich, Vickers-Rich, Flannery, Kear, Cantrill, Komarower, Kool, Pickering, Trusler, Morton, van Klaveren, and Fitzgerald, 2009, was initially based on a well-preserved, little-worn, plagiaulacoid lower fourth premolar (p4) attached to a very small fragment of dentary (Figs. 1, 2). This new genus and species was placed in a new family (Corriebaataridae) and hypothesized to represent a cimolodontan multituberculate rather than a previously unrecognized mammalian lineage. Beck (2017: 335) has since suggested a third possibility, that it may be “a representative of the gondwanatherian family Ferugliotheriidae.” This specimen, NMV P216655, is the holotype and represented the only known remains of the species. It was recovered from the Flat Rocks fossil site (Victoria, Australia), now thought to be of latest Barremian (mid-Early Cretaceous) age (Wagstaff et al. 2020). The site is renowned for its collection of other early mammals, including three possible eutherians/australosphenidans (Ausktribosphenos nyktos, Bishops whitmorei, and Kryoparvus gerriti), and the monotreme Teinolophos trusleri, as well as a host of fungi, plants, palynomorphs, invertebrates, and other vertebrates (see overview in Poropat et al. 2018). Rich et al. (2009) suggested that NMV P216655 was the first record of Multituberculata in Australia and one of the very few records of that clade from the entire southern supercontinent of Gondwana, the other three being at the time questionable records from the Early Cretaceous of Morocco and the Late Cretaceous of Argentina and Madagascar (see reviews in Krause et al. 2017, Rougier et al. 2021, and “Biogeographic implications” section below).
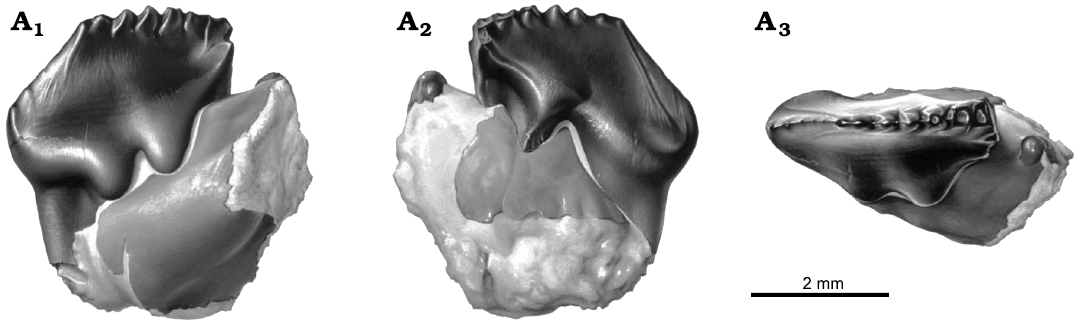
Fig. 1. Reconstruction of left p4 in dentary fragment, NMV P216655, holotype of the cimolodontan multituberculate Corriebaatar marywaltersae Rich, Vickers-Rich, Flannery, Kear, Cantrill, Komarower, Kool, Pickering, Trusler, Morton, van Klaveren, and Fitzgerald, 2009, Flat Rocks locality, Eumeralla Formation, Barremian, Lower Cretaceous of Victoria, Australia, in buccal (A1), lingual (A2), and occlusal (A3) views. Artwork by Peter Trusler.

Fig. 2. Left p4 in dentary fragment, NMV P216655, holotype of the cimolodontan multituberculate Corriebaatar marywaltersae Rich, Vickers-Rich, Flannery, Kear, Cantrill, Komarower, Kool, Pickering, Trusler, Morton, van Klaveren, and Fitzgerald, 2009, Flat Rocks locality, Eumeralla Formation, Barremian, Lower Cretaceous of Victoria, Australia; stereopairs in buccal (A1) and lingual (A2) views.
A second specimen of C. marywaltersae was discovered in 2017 at the same Flat Rocks site as the holotype, and initially reported by Poropat et al. (2018). The new specimen, NMV P252730, consists of the horizontal ramus and anterior half of the ascending ramus of a left dentary preserving the lower incisor alveolus, a complete p4, and alveoli for the roots of m1 and m2 (Figs. 3–5). It is, therefore, much more complete than the holotype and reveals far more anatomical information about this species. Our objectives in this paper are to (i) describe and illustrate this new specimen in detail, (ii) compare it with the dentaries of a diverse array of multituberculates and other allotherian mammals in order to test the working hypothesis that Corriebaatar is a cimolodontan multituberculate, and (iii) assess its biogeographic significance in the context of other reported discoveries of Gondwanan multituberculates, including those made since the first specimen of C. marywaltersae was described in 2009.
Institutional abbreviations.—MACN-RN, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Colección Río Negro, Buenos Aires, Argentina; MPEF, Museo Paleontológico “Egidio Feruglio”, Trelew, Argentina; NMV, Palaeontological Collection, Museums Victoria, Australia; RRBP, Rukwa Rift Basin Project, Tanzania Antiquities Unit, Dar es Salaam, Tanzania; UA, Université d’Antananarivo, Antananarivo, Madagascar.
Other abbreviations.—We follow standard convention in abbreviating tooth families as I, C, P, and M, with upper and lower case letters referring to upper and lower teeth, respectively.
Geological settings
Both specimens of Corriebaatar marywaltersae were recovered from sediments at the Flat Rocks fossil site on the marine shore platform in the Bunurong Marine Park to the west of Inverloch, Victoria, Australia (38°39′40±02″ S, 145°40′52±03″ E, World Geodetic System 1984). Previously, strata at the Flat Rocks site were assigned to the “Wonthaggi Formation” in the “Strzelecki Group” (Rich et al. 1997). However, Vandenberg et al. (2006) suggested extending application of the name Eumeralla Formation (Otway Group) from the Otway Basin to encompass equivalent deposits dominated by feldspathic sandstones in the Gippsland Basin, including those cropping out in the Flat Rocks area. Based on palynological evidence, the age of the fossil site is now considered to be latest Barremian, or mid-Early Cretaceous (Wagstaff et al. 2020). Specifically, the spore/pollen content places the Flat Rocks sediments low in the Pilosisporites notensis Zone. A discussion of the taphonomy of the Flat Rocks site can be found in Rich et al. (2016).
Material and methods
The partial dentary, NMV P252730, was discovered on 11 November 2017 by Wendy Jane White, who split a rock and exposed two fragments, a small anterior section (Fig. 3A1) and a much larger posterior section (Fig. 3A2). Due to the small size (roughly 12 mm in total length), fragile nature of the specimen, and how it was discovered (i.e., split obliquely into part and counterpart), minimal mechanical preparation was carried out; it could only safely be prepared from the medial side of the anterior section and the lateral side of the posterior section.

Fig. 3. Partial left dentary with p4 in situ in referred specimen, NMV P252730, of the cimolodontan multituberculate Corriebaatar marywaltersae Rich, Vickers-Rich, Flannery, Kear, Cantrill, Komarower, Kool, Pickering, Trusler, Morton, van Klaveren, and Fitzgerald, 2009, Flat Rocks locality, Eumeralla Formation, Barremian, Lower Cretaceous of Victoria, Australia, photographed as embedded in rock matrix. Medial view of small anterior section (A1) and lateral view of large posterior section (A2, stereopair).
The two fragments were microCT-scanned separately using an Xradia Versa 520 XRM microCT at the Monash University X-ray Microscopy Facility for Imaging Geomaterials using 140 kV, 72 μA, 10 W, ×0.39 optical magnification, HE1 filter, 1.5 sec exposure, isometric voxel size = 15.167 μm, and 1601 projections. The resulting slices were exported as 16 bit TIFs.
The image stacks were imported into Dragonfly v. 2020 (Object Research Systems Inc., Montreal, Canada). The bone region and the internal voids were masked separately. Noise due to low contrast between the bone and matrix was reduced by using the Smooth function in Dragonfly, as well as some manual editing. This depicted the voids more clearly, enabling void features (e.g., alveoli, mandibular canal) to be visually differentiated from diagenetically deformed regions. 3D meshes created from the masks were exported as object files (OBJ) and imported into the CAD software Rhinoceros 5 (Robert McNeel and Associates, Seattle, USA). Individual mesh objects were edited and exported separately.
The two 3D meshes from each of the two parts of NMV P252730 were imported into the 3D graphic design package Zbrush (Pixologic Inc, Los Angeles, USA) to undertake digital reassembly, to colour and export images for Figs. 4 and 6, and to create the 3D video of the specimen (SOM, Supplementary Online Material, available at http://app.pan.pl/SOM/app67-Rich_etal_SOM.pdf). 3D PDFs were created using Simulation Lab Software 10.21.8 (Amman, Jordan).
Systematic palaeontology
Allotheria Marsh, 1880
Multituberculata Cope, 1884
Cimolodonta McKenna, 1975
Family Corriebaataridae Rich, Vickers-Rich, Flannery, Kear, Cantrill, Komarower, Kool, Pickering, Trusler, Morton, van Klaveren, and Fitzgerald, 2009
Genus Corriebaatar Rich, Vickers-Rich, Flannery, Kear, Cantrill, Komarower, Kool, Pickering, Trusler, Morton, van Klaveren, and Fitzgerald, 2009
Type species: Corriebaatar marywaltersae Rich, Vickers-Rich, Flannery, Kear, Cantrill, Komarower, Kool, Pickering, Trusler, Morton, van Klaveren, and Fitzgerald, 2009; monotypic, see below.
Corriebaatar marywaltersae Rich, Vickers-Rich, Flannery, Kear, Cantrill, Komarower, Kool, Pickering, Trusler, Morton, van Klaveren, and Fitzgerald, 2009
Figs. 1–6.
Holotype: NMV P216655, small fragment of left dentary bearing complete plagiaulacoid p4 and mesial root of m1.
Type locality: Flat Rocks fossil site on the marine shore platform in the Bunurong Marine Park to the west of Inverloch, Victoria, Australia (38°39′40±02″ S, 145°40′52±03″ E, World Geodetic System 1984).
Type horizon: Eumeralla Formation (Otway Group), latest Barremian (mid-Early Cretaceous) in age (Wagstaff et al. 2020).
Stratigraphic and geographic range.—Type locality and horizon only.
Material.—Holotype and NMV P252730, horizontal ramus and anterior half of ascending ramus of left dentary with alveolus for root of lower incisor, complete p4 in situ, and alveoli for roots of m1 and m2, also from the type locality and horizon.
Emended diagnosis.—Differs from “plagiaulacidan” multituberculates in possessing a large, robust, laterally compressed, and erect lower incisor (all except Glirodon); absence of p1, p2, and p3; mesial root more robust than distal root on p4; presence of interradicular crest between mesial and distal roots of p4; absence of buccal row of cusps (or cuspules) on p4; presence of accessory roots on molars; m1 only one-half the length of p4; and m2 shorter than m1. Differs from most cimolodontans in absence of p3 and in possession of extraordinarily short buccal and lingual ridges descending from serrations along apical crest of p4. Differs from all cimolodontans in possessing two, sharply divided exodaenodont lobes (mesial and distal) on buccal aspect of p4 (incipiently developed in Ferugliotherium) and in long, straight buccal and lingual ridges descending ventromesially from first serration.
Description.—NMV P252730 is a left dentary fragment composed of the tooth-bearing horizontal ramus and the anterior portion of the ascending ramus (Figs. 3–5; video of specimen presented in SOM). The dentary itself is much more complete than the tiny fragment attached to the roots of p4 and the mesial root of m1 on the holotype, NMV P216655 (Rich et al. 2009: figs. 1, 2). The horizontal ramus of the new specimen reveals for the first time that the dental formula of Corriebaatar marywaltersae is likely 1.0.1.2, including a large, laterally compressed lower incisor, a plagiaulacoid p4, and two molars. The p4 is complete in NMV P252730, whereas the lower incisor and two molars are represented only by alveoli (except for small fragments of roots inside the alveolus for the lower incisor and the mesial alveolus for m1).
The alveolus for the lower incisor is incomplete, with small portions of the rim broken away (particularly a V-shaped piece ventrolaterally), but most of it is preserved. In lateral view, the mesial (medial) side of the rim was positioned dorsal to that on the distal (lateral) side. The portions that are preserved indicate clearly that the alveolus hosted a large, mediolaterally compressed tooth, one that was approximately 2.2 mm tall and 0.7 mm wide and therefore over three times taller than wide, although this may be exaggerated because of mediolateral compression. The alveolus extends to beneath the mesial edge of the distal root of p4 and does not taper significantly, suggesting that the root may have been ever-growing, or at least grew late into life. In dorsal view, the alveolus passed posteriorly in a plane medial to the mesial root of p4. The angle of the alveolus in lateral view suggests that the incisor was implanted at a steep angle and that the crown would have been quite erect, projecting anterodorsally at approximately 116° relative to the ventral margin of the dentary.
A short diastema is present (1.62 mm) between the lower incisor and p4 (measured as a straight line from the distal rim of the incisor alveolus to the mesial rim of the mesial alveolus of p4). In lateral view, this diastema is strongly concave dorsally, representing approximately one-third of a circle, with its mesial end being at about the same height as its distal end. There is no trace of a p3 (or alveolus for it), even as a diminutive peg-like tooth, and we therefore regard it to be absent in this taxon.
The single preserved cheektooth in NMV P252730 is strikingly similar to the equivalent tooth in the holotype, NMV 216655, and is here interpreted as the p4; the crown and root morphology of this tooth, coupled with features of the dentary, show strong resemblance to multituberculates in general and Cimolodonta in particular. We therefore assume, as a working hypothesis based on available material, that this tooth is the p4, and that the other, more mesial premolar positions have been lost in the lineage leading to C. marywaltersae. Although there is a slight difference in size, we have little doubt that the two specimens belong to the same species. The p4 in NMV P252730 is complete and well preserved but considerably more worn than that of the holotype, NMV P216655, indicating a more mature individual. The new specimen measures 3.62 mm long (as measured along the baseline from the apex of the mesial projection of the crown to the point where the distobuccal ledge intersects the distal margin; see Krause 1987: fig. 1) and 1.52 mm wide (the greatest buccolingual dimension) whereas the holotype p4 is 3.7 mm long and 1.6 mm wide. The length of the holotype is given as 3.4 mm in Rich et al. (2009) where it was evidently measured in a slightly different manner. On the mesial half of the crown, on the slope ascending to the first serration, there are three faint “pseudoserrations” (sensu Krause 1982a) and associated short, faint buccal and lingual ridges, as on the holotype; the first pseudoserration is more prominent than the second, and the second is more prominent than the third. Whereas seven serrations are present along the apical crest of the distal half of the p4 crown in the holotype, the number is more difficult to discern on NMV P216655 because of wear, although there is no evidence of a significant difference. Particularly striking is the presence of long narrow ridges descending ventromesially from the first serration, one on the buccal side and one on the lingual side of the crown, much better preserved on the less worn holotype. The lingual ridge is longer, extending to nearly the base of the crown. The ridges descending from the remaining serrations on both the buccal and lingual sides are extraordinarily short. Also much better preserved on the holotype is the distobuccal ledge. There is no trace of a buccal row of cusps (or cuspules) near the base of the crown (as is present in most plagiaulacidans). As on the holotype, there are two prominent but mesiodistally constricted exodaenodont lobes at the base of the crown on the buccal side, one on the mesial half and another on the distal half, with a strong dorsally directed concavity between them. There are two large roots supporting the crown, the larger mesial root being roughly triangular in cross section (approximating an isosceles triangle with the sharpest angle located mesially and a vertical groove located distally) and the smaller distal root being more rounded but also roughly triangular in cross section (but with the sharpest angle located distally and a vertical groove located mesially). A low interradicular crest spans the distance between the distal margin of the mesial root and the mesial margin of the distal root. The roots are approximately as long as the crown is tall and extend to roughly mid-height of the horizontal ramus.
Some wear features can be discerned on p4 of NMV P252730, but COVID restrictions precluded access to a scanning electron microscope that would have allowed adequate imaging of wear striations. Some wear appears to be present along the ascending mesial slope of the crown, the surface of which faces mesiobuccally. We assume that this was formed during the orthal slicing-crushing phase of mastication (Krause 1982b). A large, prominent, well-circumscribed wear facet is present on the buccal side of the distal slope, demarcated with a dashed white line in Fig. 5A4. On this facet there are very slightly curved wear striations (visible using light microscopy) roughly parallel to the ventral margin of the dentary; the orientation of these striations and the direction of p4 movement relative to the upper cheekteeth are also highlighted in Fig. 5A4. If a comparison to jaw movement in species of the ptilodontoid multituberculate Ptilodus (Paleocene, North America) is valid, these striations were formed during the palinally-directed power stroke of the grinding cycle as p4 slid distally past the distolingual part of P4 (Krause 1982b).
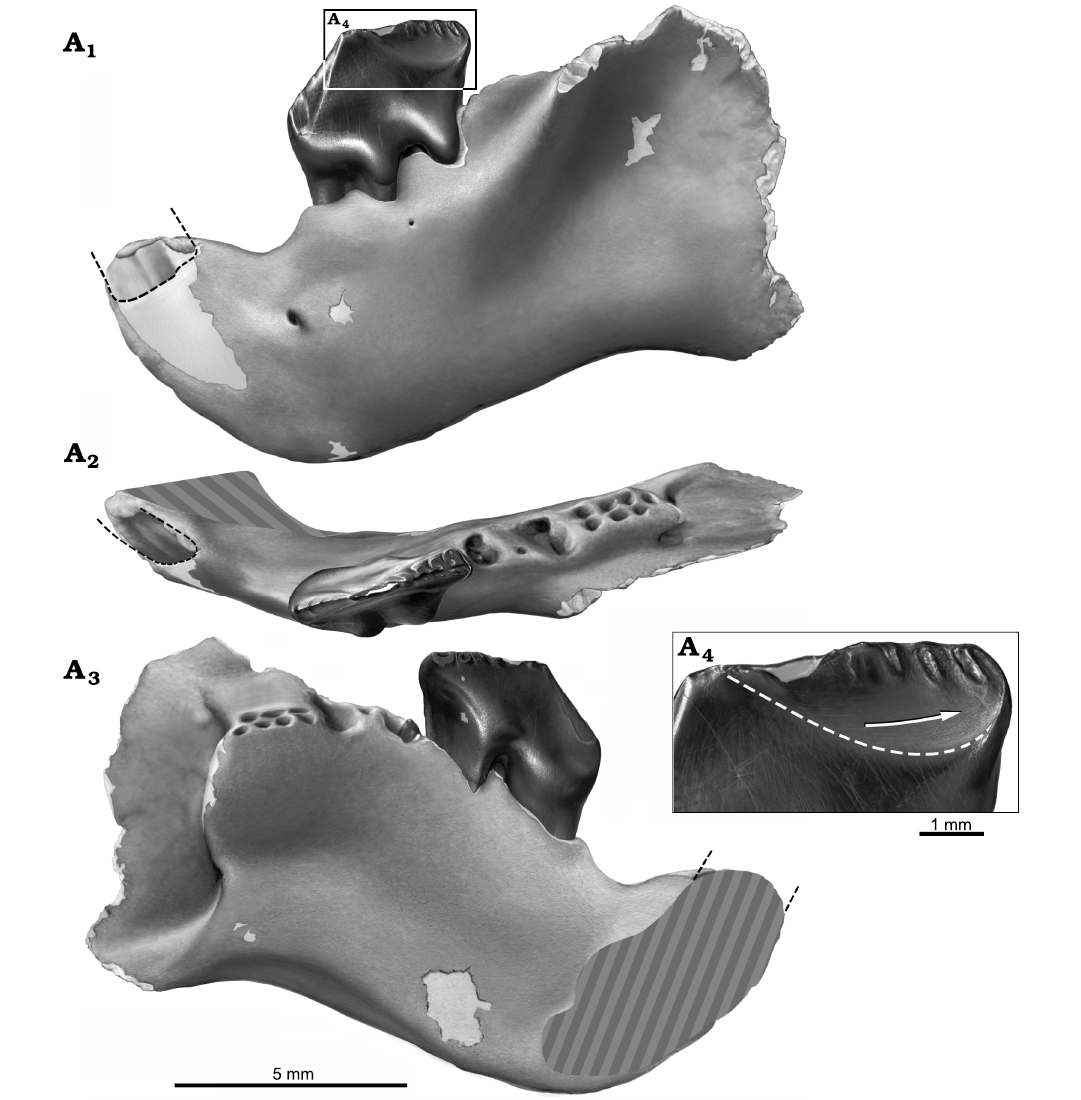
Fig. 5. Reconstruction of partial left dentary with p4 in situ, NMV P252730, of the cimolodontan multituberculate Corriebaatar marywaltersae Rich, Vickers-Rich, Flannery, Kear, Cantrill, Komarower, Kool, Pickering, Trusler, Morton, van Klaveren, and Fitzgerald, 2009, Flat Rocks locality, Eumeralla Formation, Barremian, Lower Cretaceous of Victoria, Australia. Specimen in lateral (A1), dorsal/occlusal (A2), and medial (A3) views. Size and shape of base of crown of lower incisor indicated by black dashed lines. Estimate of size and position of symphyseal area indicated by gray hatching in A2 and A3. Buccal view of distal portion of p4 (A4), illustrating abraded distobuccal wear facet, the lower limit of which is indicated by white dashed line. Buccal ridges are preserved as elliptical troughs of enamel surrounded by the abraded wear surface. Arrow indicates the slightly curved orientation of wear striations and the direction of palinal movement of p4 relative to the upper dentition. Artwork by Peter Trusler.
The molars are not preserved in NMV P252730, except for a small fragment of a root in the mesial alveolus of m1. However, although the alveoli of the molar roots are not completely preserved and somewhat deformed, we were able to effectively visualize some basic aspects of their structure by using the microCT scans to digitally fill the alveolar voids to simulate the roots (Fig. 6).
Lying immediately distal to p4 are two large alveoli, one behind the other. We interpret these two alveoli to be for the primary mesial and distal roots of m1. The medial portions of the dorsal rims of both alveoli are broken away and not preserved, but that for the distal alveolus shows the most breakage. The shapes of these alveoli are not reliably discernible, but it appears that the mesial root was oval (the longest dimension oriented mesiolingually–distobuccally), whereas the distal root was larger and approximately in the shape of the numeral 8 in occlusal view (the longest dimension also oriented mesiolingually–distobuccally). Digitally infilling the alveoli (simulating mold-making compound) reveals that the two parts of this distal root diverged toward the root apices, with one part positioned mesiolingually and the other part distobuccally. As a rough estimate, judging from these two alveoli, m1 would have been about one-half the length of p4. In addition to the two primary alveoli, there is a much smaller alveolus, circular in cross section, for an accessory root (= intercalary root sensu Butler 1956) between the two primary alveoli and toward the buccal side.
Distal to the distal alveolus for m1 are several closely spaced, partial alveoli that we interpret to be for m2 based on the amount of space occupied. These alveoli lie slightly farther medially and ventrally than do the alveoli of m1 but there is some indication that deformation disrupted the alignment of m1 and m2. Relative sizes of any of the alveoli are difficult to reliably assess. The best-preserved alveolus for m2 is that for the mesiolingual root; this alveolus is bisected vertically, with the buccal half still fully intact and the lingual half broken away. Distal to the mesiolingual alveolus are at least two alveoli, aligned mesiodistally, with only their ventral, cup-shaped tips preserved. The ventral tips lie at approximately the same level as the ventral tip of the mesiolingual alveolus. There is probably an additional ventral tip in this row, indicative of a fourth alveolus preserved farther distally (indicated by dashed lines in Fig. 6A1, A2), but poor preservation precludes a definitive assessment. The void-filling exercise revealed the presence of an alveolus buccal to the mesiolingual alveolus, its full length likely not preserved. Distal to this mesiobuccal alveolus is one, perhaps two, additional alveoli but they are too poorly preserved to fully evaluate. Nonetheless, the parts of m2 alveoli preserved indicate that there were two rows of roots, one lying buccally and consisting of two or perhaps three roots and one lingually consisting of three or probably four roots. Despite the incompleteness of these alveoli, it is clear that those in the lingual row taper to the root terminus, thus indicating that the roots were “closed” and not ever-growing.
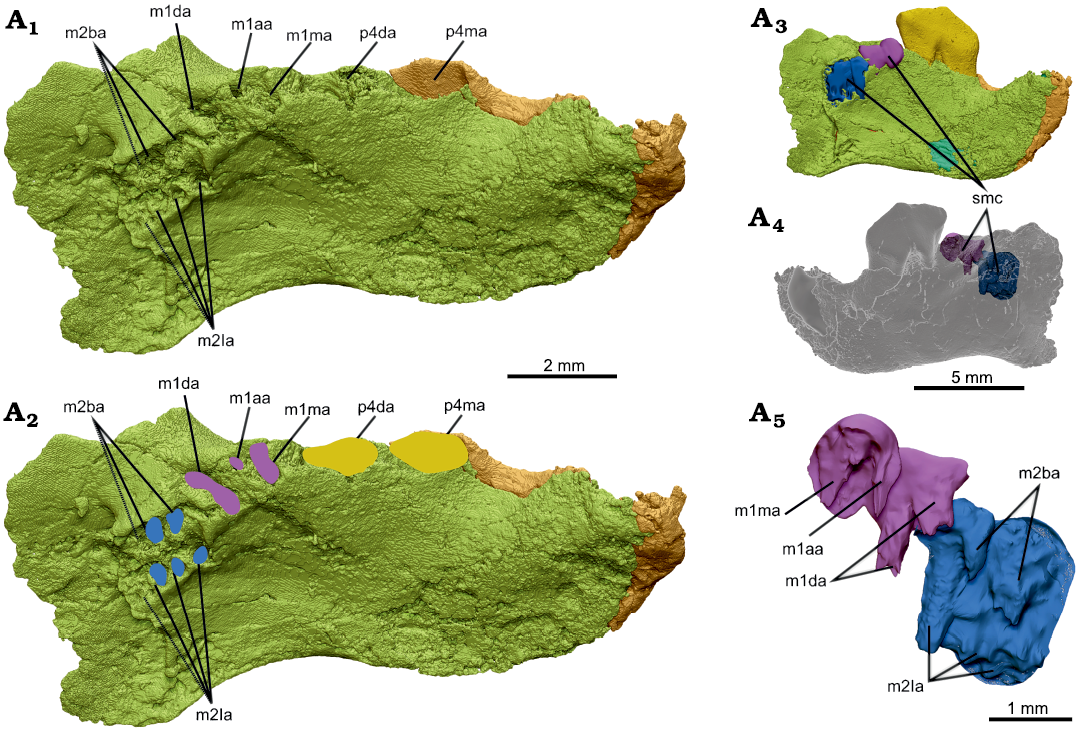
Fig 6. Interpretation of molar alveolar structure in dentary, NMV P252730, of the cimolodontan multituberculate Corriebaatar marywaltersae Rich, Vickers-Rich, Flannery, Kear, Cantrill, Komarower, Kool, Pickering, Trusler, Morton, van Klaveren, and Fitzgerald, 2009, Flat Rocks locality, Eumeralla Formation, Barremian, Lower Cretaceous of Victoria, Australia. Partial left dentary in dorsomedial view with p4 digitally extracted (A1), outlines of interpreted alveoli for p4 (yellow), m1 (purple), and m2 (blue) (A2). Medial (A3) and lateral (A4) views of dentary with computer-simulated mold-making compound overlying the partially preserved alveoli of m1 and m2 and filling the voids. A3 and A4 are views of entire specimen to provide context, dentary semi-transparent in A4. Simulated mold-making compound enlarged (A5) showing the parts that filled the voids of the partially preserved alveoli. Abbreviations: m1aa, m1 accessory alveolus; m1da, m1 distal alveolus; m1ma, m1 mesial alveolus; m2ba, m2 buccal alveoli; m2la, m2 lingual alveoli; p4da, p4 distal alveolus; p4ma, p4 mesial alveolus; smc, simulated mold-making compound. Note that m1da divides as it descends ventrally (contrast view in A1, A2 with that in A5).
Another interpretation we considered was that there are two molars distal to m1. We do not, however, observe any change in size and orientation of the roots as there is between those of m1 and m2, and all of the roots distal to m1 are very closely spaced (Fig. 6). Furthermore, the bone supporting the toothrow appears to naturally end distal to this series of roots, which is roughly aligned with the posterior terminus of the temporal groove. We therefore regard the more parsimonious interpretation to be that there is only one molar behind m1 and that, tentatively, the lower dental formula is 1.0.1.2. Assuming that C. marywaltersae had only two molars and acknowledging that poor preservation precludes accurate measurement, we estimate the length of m1 to have been approximately 1.86 mm and the length of m2 to have been approximately 1.74 mm. It thus appears that m2 was approximately 94% the length of m1.
The dentary of NMV P252730 is rodentiform and includes virtually all of the horizontal ramus and anterior parts of the ascending process. As such, it does not preserve the posterior aspects of the masseteric fossa laterally or the pterygoid fossa medially, most of the coronoid process (dorsally and posteriorly), the pterygoid fovea, the condylar process, or the condyle. In lateral outline, in addition to the dorsally concave diastema described above, it is noteworthy that the ventral margin of the dentary is sinuous, being strongly convex anteroventrally (anteroventral to the lower incisor alveolus) and markedly concave beneath the molars (the apex of the concavity situated beneath m2), with the beginnings of a convexity that formed the ventral margin of the ascending process posteriorly. Although the strongly downturned aspect posteriorly gives the visual impression of a potential angular process more posteriorly, we conclude that an angular process was not present because: (i) the area posterior to the downturned area is clearly broken and the more posterior regions of the ascending ramus are not preserved; (ii) whereas most Mesozoic mammaliaforms exhibit a flat or convex ventral margin, such a strong concavity ventral to the cheekteeth is known to occur in species of several other Mesozoic and Paleogene mammaliaform genera (e.g., Galulatherium, Krause et al. 2003; O’Connor et al. 2019; Volaticotherium, Meng et al. 2006), particularly multituberculates (e.g., Allocosmodon, Fox 2005; Heishanobaatar, Kusuhashi et al. 2010; Meniscoessus, Archibald 1982; Microcosmodon, Fox 2005; Taeniolabis, Granger and Simpson 1929; Zofiabaatar, Carpenter 1998); and (iii) it would be unprecedented among Mammaliaformes to have an angular process positioned so far anteriorly on the dentary, at a dorsoventral level immediately posterior to the cheekteeth (Grossnickle et al. 2021).
The lateral side of the dentary exhibits a number of features (Fig. 4A). A single mental foramen is present just above mid-height of the horizontal ramus and below the nadir of the diastema. A much smaller foramen, most likely a nutrient foramen, is present posterior to the proximal part of the mesial root of p4. A masseteric foramen (see discussion of homologies in Krause et al. 2020b) in the anterior part of the masseteric fossa is absent. The anterior root of the coronoid process is represented by a prominent rounded ridge lateral to m1 that bulges laterally and extends anteroventrally–posterodorsally. A portion of this anterior root of the coronoid process is also observable on the holotype specimen, NMV P216655 (Rich et al. 2009: figs. 1A, 2B). The height and posterior extent of the coronoid process cannot be established on either specimen, because these areas are broken away. It is likewise not possible to reasonably estimate the angle between the anterior root and the alveolar plane of the molars, a character frequently used in phylogenetic analyses of mammaliaforms (e.g., Luo et al. 2015, 2017; Han et al. 2017; Krause et al. 2014, 2020a; Wang et al. 2021), because the coronoid process is so incomplete and the preserved alveoli are too deformed and broken.
As in other mammals, the anterior root of the coronoid process forms the anterodorsal border of the masseteric fossa. The masseteric fossa, as a whole, however, is not well delimited, in part because the anterodorsal border of the coronoid process becomes much less prominent as it descends anteroventrally. Nonetheless, it clearly extends anteriorly to below p4, perhaps as far as to the area below the mesial root. The masseteric crest (sensu Gambaryan and Kielan-Jaworowska 1995), defining the anteroventral border of the masseteric fossa, however, is represented only by a low, faint, rounded ridge; it is almost indiscernible, perhaps a result of deformation. Similarly, a masseteric fovea (sensu Gambaryan and Kielan-Jaworowska 1995) anterior to the masseteric fossa and a masseteric line (sensu Gambaryan and Kielan-Jaworowska 1995) extending posteriorly from the masseteric crest along the ventral border of the ascending ramus are not in evidence.
Medial to the anterior root of the coronoid process in the new specimen is a well-developed temporal groove (sulcus temporalis of Gambaryan and Kielan-Jaworowska 1995), best seen in dorsal view (Fig. 4C). It passes from lateral to m1 to almost as far posteriorly as the distal end of m2. At this point it terminates abruptly, because it intersects nearly perpendicular to the anterior end of the pterygoid fossa. The surface of the temporal groove is dorsally convex from anterior to posterior, whereas it is dorsally concave from lateral to medial. There is no trace in this region of a coronoid bone, rudimentary or otherwise.
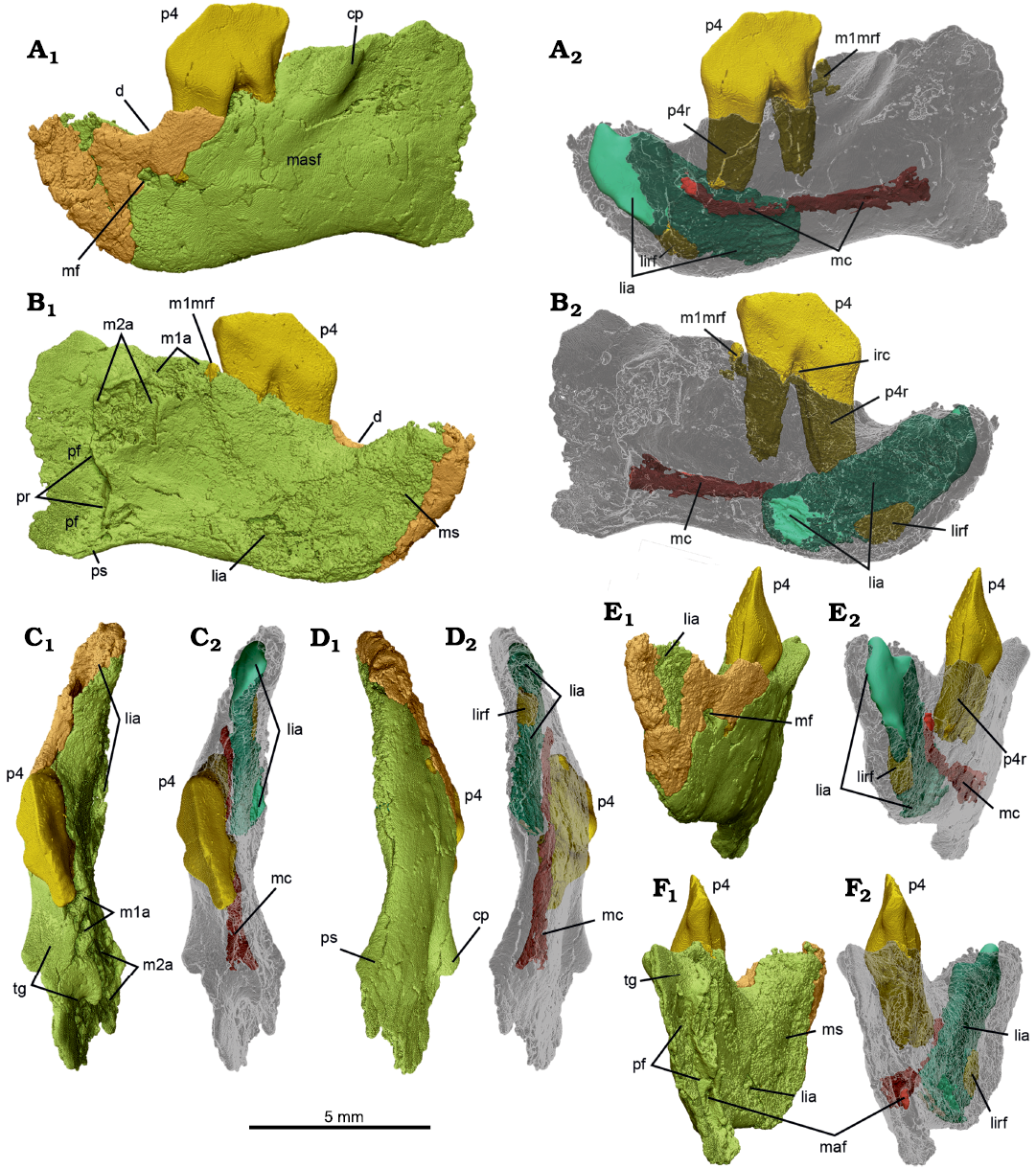
Fig. 4. MicroCT scans of left dentary with p4 in situ, NMV P252730, of the cimolodontan multituberculate Corriebaatar marywaltersae Rich, Vickers-Rich, Flannery, Kear, Cantrill, Komarower, Kool, Pickering, Trusler, Morton, van Klaveren, and Fitzgerald, 2009, Flat Rocks locality, Eumeralla Formation, Barremian, Lower Cretaceous of Victoria, Australia. The two parts (anterior fragment in brown, posterior fragment in lime green) that compose the specimen, in lateral (A), medial (B), dorsal (C), ventral (D), anterior (E), and posterior (F) views, with dentary semi-transparent in A2–F2. Abbreviations: cp, coronoid process; d, diastema; irc, interradicular crest; lia, lower incisor alveolus (turquoise); lirf, root fragment (dark yellow) in lower incisor alveolus; m1a, lower first molar alveoli; m1mrf, root fragment (dark yellow) in mesial alveolus of m1; m2a, lower second molar alveoli; maf, mandibular foramen; masf, masseteric fossa; mc, mandibular canal (deep red); mf, mental foramen; ms, mandibular symphysis; p4, lower fourth premolar (bright yellow); p4r, lower fourth premolar root (dark yellow); pr, pterygoid rim; pf, pterygoid fossa; ps, pterygoid shelf; tg, temporal groove.
On the medial side of the dentary (Fig. 4B) there is a large planar area (flat medially) anteriorly that represents the mandibular symphysis, which was unfused. Although the relatively poor preservation of this side precludes precise delimitation of the margins of the symphysis, it appears to have extended posteriorly to at least the level of the posterior part of the diastema, and probably as far back as the mesial root of p4. Posterior to the symphysis, the medial side of the dentary is gently concave, but some aspects of this may be owing to mediolateral compression of the specimen. Immediately posterior to the distal alveoli of m2, the medial side of the dentary is interrupted by a strong crest that descends essentially vertically. This crest, termed the “pterygoid rim” here (because the term “pterygoid crest” has occasionally been used as a synonym for pterygoid shelf), is in precisely the position and orientation that one would expect for multituberculates and marks the anteromedial boundary of the pterygoid fossa, for insertion of the large medial pterygoid muscle that is so typical of multituberculates (Gambaryan and Kielan-Jaworowska 1995). The presence of a large pterygoid fossa is also clearly visible in posterior view (Fig. 4F), where mostly smooth (unbroken) bone is preserved, as a vertically elongate anterior concavity lateral to the pterygoid rim. Only a small part of the lateral wall of the pterygoid fossa is preserved, but it extends posteriorly well beyond the level of the pterygoid rim. The identity of the pterygoid fossa is further confirmed by the clear presence of a large oval (taller than wide) hole, the mandibular foramen (inferior dental foramen of Miao 1988).
A pterygoid shelf, forming the base of the pterygoid fossa, is not readily apparent although the anterior beginnings of it appear to be preserved because the ventral floor of the fossa is slightly wider than the area above it. Miao (1988) and Kielan-Jaoworowska et al. (2004, 2005) have summarized different terms employed for the pterygoid shelf of multituberculates. We restrict the term here to the mediolaterally wide structure that forms the floor of the pterygoid fossa. By contrast, we restrict the term “pterygoid rim” to refer to the more-or-less vertical crest that descends ventrally from posterior to m2, intersecting the pterygoid shelf along the ventral margin of the dentary. In other words, the pterygoid rim forms the lateral edge of the anterior wall of the pterygoid fossa, even though the anterior–most aspect of the wall may be situated slightly farther anteriorly than the rim itself, creating a vertically aligned pocket.
There is no evidence of a postdentary trough or attachment of postdentary bones in Corriebaatar marywaltersae; absence of these features seems highly probable in NMV P252730 even without complete preservation of the most posterior aspects of the ascending ramus. A Meckelian sulcus and a dental lamina are also absent.
The microCT scans also reveal several features internally, in addition to the roots of p4 and the alveoli of the lower incisor and the molars described above (Fig. 4A2–F2). There is, for instance, a small fragment of the lower incisor root located deep within the alveolus anteroventrally. Correspondingly, there is a small root fragment preserved within the mesial alveolus of m1. Neither of these fragments is large enough to yield any meaningful insights into the morphology of either of the roots or the crowns of these teeth. Also revealed on the scans is a small interradicular crest between the mesial and distal roots of p4. Most significantly, a canal, extending anteriorly and then anterodorsally, passes from the mandibular foramen inside the pterygoid fossa to the mental foramen on the lateral side of the dentary, below the diastema. This is the mandibular canal, which is more or less complete and coherent save for a few vertical fractures and a few connections to other voids in the horizontal ramus. The canal served as passage for the inferior alveolar neurovascular bundle.
Discussion
The concept of the mammalian subclass Allotheria is not stable and has expanded from the two main groups (Haramiyida and Multituberculata) included in the most recent comprehensive compendium of Mesozoic mammaliamorphs by Kielan-Jaworwoska et al. (2004) to also include two additional major clades, Gondwanatheria and Euharamiyida (see recent review in Hoffmann et al. 2020). Since Allotheria was first proposed by Marsh (1880), several other taxa (e.g., Tritylodontidae, Cope 1884; Kollikodontidae, Musser 2005; Musser et al. 2019) have also been suggested to belong to the group but are not currently included. In all phylogenetic analyses by Hoffmann et al. (2020) in which Allotheria was resolved as a clade, the enigmatic Cifelliodon was also included, but this taxon is not considered further here because it is not represented by lower jaw material or any lower teeth (Huttenlocker et al. 2018). In this context, we compare the known anatomy of Corriebaatar with that of haramiyidans, multituberculates, euharamiyidans, and gondwanatherians. For the purposes here, we do not include the enigmatic and highly controversial Ferugliotheriidae in Gondwanatheria and consider it separately.
Haramiyida.—Placement of Haramiyida outside of Mammaliaformes or within Allotheria is controversial, the history of which has been recently reviewed by Hoffmann et al. (2020), who recovered Haramiyida outside of Mammaliaformes in their parsimony and tip-dated Bayesian analyses but in a polytomy with other allotherians in their undated Bayesian analyses. Also controversial is the composition of Haramiyida but, pending resolution of these controversies, we include here a comparison of the lower jaw of Corriebaatar marywaltersae with that of the only two known unquestioned haramiyidans represented by lower jaw material, the Late Triassic Haramiyavia clemmenseni (see Jenkins et al. 1997; Luo et al. 2015) and Kalaallitkigun jenkinsi (see Sulej et al. 2020).
The lower jaws of Corriebaatar on the one hand and Haramiyavia and Kalaallitkigun on the other have little in common in that those of the latter exhibit a host of relatively plesiomorphic characteristics that are not present in Corriebaatar. Collectively, these include a lower dental formula of 3.1.4.3 (and associated absence of a sizeable diastema), a long and shallow horizontal ramus, a prominent postdentary trough and Meckelian sulcus, presence of a coronoid bone (as indicated by a scar), a coronoid process that originates almost fully posterior to the cheektooth row and is not laterally separated from it by a wide temporal groove, a masseteric fossa that does not extend anteriorly onto the horizontal ramus, and absence of a pterygoid fossa and associated pterygoid shelf and rim. Furthermore, the mode of chewing in haramiyidans is inferred to have retained a significant orthal component rather than being strictly palinal (e.g., Butler 2000; Butler and Hooker 2005; Luo et al. 2015). A few attributes are shared with Corriebaatar (e.g., absence of dental lamina groove, unfused mandibular symphysis, absence of masseteric foramen) but these are features that are either lost near the stem of the mammaliamorph phylogenetic tree or are highly variable in their occurrence across the tree (e.g., Hoffmann et al. 2020). It should also be noted that Haramiyavia clemmenseni, in the only known specimen, has one (left) or two (right) mental foramina placed at mid-height or below mid-height on the horizontal ramus whereas C. marywaltersae, in the only specimen in which the condition can be documented, has only one placed above mid-height. This area is not preserved in the dentary of Kalaallitkigun jenkinsi.
The dentition of C. marywaltersae differs markedly from that of haramiyidans. In addition to the fact that there are far fewer teeth in C. marywaltersae (lower dental formula 1.0.1.2 vs. 3.1.4.3), the lower central incisor is relatively much enlarged and more laterally compressed, and p4 is fully plagiaulacoid (i.e., laterally compressed and blade-like), which is not the case in H. clemmenseni and other haramiyidans (Jenkins et al. 1997; Hahn and Hahn 2006; Luo et al. 2015). These teeth are not preserved in the specimen of K. jenkinsi. In sum, the overwhelming balance of morphological differences makes it extremely unlikely that Corriebaatar is a haramiyidan.
Multituberculata.—Multituberculata is the core group of Allotheria, phylogenetically defined by Sereno (2006) as the most inclusive clade including Taeniolabis taoensis but not Mus musculus and Ornithorhynchus anatinus, and has been since Marsh (1880) erected the subclass over 140 years ago. Multituberculates are arguably the most successful of early mammaliaforms, with a temporal range extending from the Middle Jurassic to the late Eocene (Kielan-Jaworowska et al. 2004; Butler and Hooker 2005; Schumaker and Kihm 2006; Dawson and Constenius 2018) and were also among the most speciose and diverse mammals in the northern supercontinent Laurasia, particularly North America, in the Late Cretaceous and early Paleogene (Krause 1986; Cifelli et al. 2004; Kielan-Jaworowska et al. 2004; Weil and Krause 2008). Documentation of their presence in Gondwana, by contrast, has been challenging and controversial.
Unfortunately, the posterior end of the ascending ramus in NMV P252730 is not preserved and cannot be evaluated for phylogenetic character states. However, in comparison to other mammaliaforms, the suite of morphological features exhibited by the new dentary of Corriebaatar marywaltersae is consistent with placement in Multituberculata, as originally concluded by Rich et al. (2009) on the basis of p4 morphology alone. These features include: (i) absence of the postdentary trough and attached postdentary bones; (ii) absence of the Meckelian sulcus; (iii) absence of a groove for dental lamina; (iv) absence of a coronoid bone (the paulchoffatiid Kuehneodon is the only multituberculate known to retain a remnant of this element, Hahn 1977); (v) a large pterygoid fossa with an associated strong pterygoid rim (defined in the “Description” section; see Fig. 4B1) and presence of a pterygoid shelf (tentatively identified in NMV P252730 based on its anterior beginning); (vi) position of the mandibular foramen inside the pterygoid fossa and below the alveolar plane; (vii) a distinct anterior root of the coronoid process beginning far anteriorly (lateral to the molars and separated from them by a distinct temporal groove) and forming the anterodorsal border of the masseteric fossa; (viii) anterior extension of the masseteric fossa onto the horizontal ramus up to level of at least m1; (ix) absence of a masseteric foramen; (x) presence of a distinct diastema between the lower incisor and the first cheektooth; (xi) presence of a single mental foramen, situated ventral to the diastema; (xii) horizontal ramus short and deep; and (xiii) longitudinal axis of the incisor crown and its root positioned oblique to the longitudinal axis of the cheektooth row, resulting in a marked external angle between anterior and posterior parts of the horizontal ramus in dorsal (or ventral) view (the paulchoffatiid Paulchoffatia is unique among multituberculates in not exhibiting such an obliquity between the longitudinal axes of the incisor and the postcanine tooth row, Hahn 1971). With the stated exceptions, these features are almost ubiquitously present in multituberculates, including early-branching forms such as the paulchoffatiids Rugosodon (Yuan et al. 2013), Paulchoffatia (Hahn 1969), and Kuehneodon (Hahn 1969); the plagiaulacid Plagiaulax (Simpson 1928, Ride 1957); and the eobaatarids Sinobaatar (Kusuhashi et al. 2009) and Jeholbaatar (Wang et al. 2019). The exceptions (coronoid bone in Kuehneodon, lack of oblique angle between anterior and posterior parts of horizontal ramus in Paulchoffatia) support a higher position on the multituberculate phylogenetic tree for Corriebaatar than these early-branching paulchoffatiids.
Two features of the dentary stated to be characteristic of multituberculates (Gambaryan and Kielan-Jaworowska 1995) are not strongly developed in Corriebaatar: (i) a distinct masseteric crest forming the anteroventral border of the masseteric fossa (and continuing posteriorly as a masseteric line along the ventral margin of the ascending process), and (ii) a masseteric fovea, anterior to the masseteric crest, and, according to Gambaryan and Kielan-Jaworowska (1995), serving as the insertion area for the masseter medialis pars anterior. A broader survey of multituberculates, however, reveals that these two features are variably developed in this group. A masseteric crest is only faintly visible in NMV P252730, presenting as a low, rounded ridge, but such is also the case in several other multituberculates (e.g., Cimexomys minor, Sloan and Van Valen 1965: fig. 2G; Nessovbaatar multicostatus, Kielan-Jaworowska and Hurum 1997). A masseteric fovea is strongly delimited in a few genera like Chulsanbaatar, Catopsbaatar, Guibaatar, Guimarotodon, Meketibolodon, and Stygimys, but it is not ubiquitous in multituberculates and, indeed, is poorly developed “in most genera” and confluent with the masseteric fossa (Gambaryan and Kielan-Jaworowska 1995: 54; Hahn and Hahn 1998; Kielan-Jaworowska et al. 2004, 2005; Wible et al. 2019). We tentatively consider the poor development of these features to not preclude assignment of Corriebaatar to Multituberculata.
The dentition of Corriebaatar remains poorly known, limited to p4 and what can be gleaned from the alveoli for the lower incisor and lower molars. However, all that is known of the dentition is also consistent with placement in Multituberculata and, more specifically, in Cimolodonta. The presence of a single lower incisor separated from p3 or p4 by a distinct diastema is ubiquitous across Cimolodonta. The fact that the lower incisor of Corriebaatar is large, robust, and significantly compressed mediolaterally (and likely ever-growing) is a derived feature (more derived than in all “plagiaulacidans”, with the possible exception of Glirodon; Engelmann and Callison 1999) and is characteristic of a host of cimolodontan multituberculates, including djadochtatherioids, taeniolabidoids, microcosmodontids, and eucosmodontids. Furthermore, the erect angle of the incisor is not unlike that of Lambdopsalis, Taeniolabis, and various djadochtatherioids such as Sloanbaatar, Kryptobaatar, Djadochtatherium, Catopsbaatar, and Mangasbaatar (Rougier et al. 2016: fig. 31).
The absence of p3 (as well as p1 and p2) is an apomorphic trait and is also indicative of cimolodontan affinities: p3 is present in all “plagiaulacidans” for which the condition is known and in most but certainly not all cimolodontans; p3 is absent in all taeniolabidids and lambdopsalids, in some and perhaps all kogaionids (see Smith et al. 2021), and in some neoplagiaulacids, eucosmodontids, and microcosmodontids (Krause et al. 2020c: table 4).
Aside from a couple of features that can be considered autapomorphies for Corriebaatar, and therefore diagnostic of the taxon, its p4 is typical of that in multituberculates in being laterally compressed and blade-like, with a row of serrations and associated buccal and lingual ridges, and anchored by two long, stout roots, the mesial one being more robust than the distal one, a condition regarded as typical for cimolodontans but not plagiaulacidans (Kielan-Jaworowska et al. 2007). The roots are also connected by a low interradicular crest, which is frequently encountered in cimolodontan p4s (e.g., Anconodon, Cimexomys, Cimolomys, Ectypodus, Meniscoessus, Mesodma, Neoplagiaulax, Ptilodus, Tashtykia, and Uzbekbaatar, Sahni 1972; Kielan-Jaworowska and Nessov 1992; Eberle and Lillegraven 1998; Scott 2005; Scott and Krause 2006, Averianov et al. 2021). The shape of p4 of Corriebaatar is unusual for a multituberculate in that it exhibits a peaked apex on the crown at approximately mid-length (rather than more mesially), but this is not unprecedented among multituberculates, both basal (Kermackodon, Butler and Hooker 2005) and derived (e.g., Fractinus, Higgins 2003; Xanclomys, Rigby 1980; Lofgren et al. 2017). Corriebaatar lacks a basal row of cusps (or cuspules) buccally, which is present in most plagiaulacidans (Kielan-Jaworowska and Hurum 2001; Hahn and Hahn 2004, Averianov et al. 2021). The buccal and lingual ridges descending from the serrations along the apical crest of p4 of Corriebaatar are unusually short (all except those descending from the first serration), in contrast to the much longer ones present in most cimolodontans, but several other cimolodontan taxa also exhibit short ridges (e.g., Barbatodon, Microcosmodon, and Yubaatar, Csiki et al. 2005; Fox 2005; Xu et al. 2015; Smith et al. 2021), and ridges are entirely absent or rudimentary in cimolodontans with much-reduced p4s (e.g., Catopsalis, Lambdopsalis, Prionessus, Shenopsalis, Taeniolabis, and Valenopsalis, Granger and Simpson 1929; Sloan and Van Valen 1965; Miao 1986; Meng et al. 1998; Williamson et al. 2016). The short p4 ridges of Corriebaatar are also quite similar to those on some “plagiaulacidans” (see examples in Hahn and Hahn 2004: fig. 13a–d, f, g, l). Finally, the position of the wear facets on p4 and the wear striations within them are consistent with the palinal direction of jaw movement inferred for multituberculates (e.g., Krause 1982b, Gambaryan and Kielan-Jaworowska 1995).
The molars are not preserved in NMV P252730, but the alveoli for them demonstrate three aspects that are also consistent with inclusion of Corriebaatar in Multituberculata and further suggestive of cimolodontan affinities. First, m1 and m2 are each supported by more than just two roots. Accessory roots supporting the molars are not uncommon in multituberculates and have been documented for several cimolodontan genera (e.g., Acheronodon, Allocosmodon, Catopsalis, Cimolodon, Cimolomys, Essonodon, Filikomys, Meniscoessus, Microcosmodon, Paressonodon, Pentacosmodon, and Taeniolabis, Clemens 1964; Holtzman and Wolberg 1977; Krause 1977; Archibald 1982; Middleton 1982; Simmons 1987; Hunter et al. 1997; Fox 2005; Wilson et al. 2010; Scott et al. 2018; Weaver et al. 2020). Although the molar roots of “plagiaulacidans” appear to be less well known (or documented), Butler (2000: 336) stated that “the possession of three roots … has not been observed in Jurassic multituberculates”. Consistent with that generalization, Hahn (1969, 1971) indicated that paulchoffatiids do not have accessory roots but, instead, two main roots that often coalesce (Hahn 1969, 1971). Similarly, Simpson (1928) made note of a plagiaulacid molar with only two roots. Finally, accessory molar roots were not noted by Hahn and Hahn (2004) in their review of “plagiaulacidan” dentitions or in descriptions of recently described, early branching forms like Rugosodon (Yuan et al. 2013) and Jeholbaatar (Wang et al. 2019). We have found reports of only two exceptions of more than two roots in “plagiaulacidan” molars but it must be noted that these both pertain to upper, not lower, molars: (i) three probable roots (“due to division of the mesial root”) in an M2 of the hahnotheriid Hahnotherium (Butler and Hooker 2005: 199); and (ii) an M1 of the pinheirodontid Teutonodon bears a small accessory root between the two primary roots (Martin et al. 2016). Second, although the length of m1 can only be roughly estimated in NMV P252730 based on its preserved alveoli, it appears to have been only about one-half (~0.51 times) the length of p4; this ratio is present only in some of the more “specialized” multituberculates (e.g., Arginbaatar, Anconodon, Eucosmodon, Prochetodon, and Ptilodus) that have a relatively large p4 (Eaton and Cifelli 2001: table 2; Weil and Krause 2008). Third, the preserved molar alveoli in NMV P252730 of Corriebaatar indicate that m2 was slightly shorter than m1 (~0.94 times). With only a few exceptions (e.g., some members of the “Paracimexomys group” and the highly derived Sphenopsalis), m2 is shorter than m1 in cimolodontan multituberculates whereas m2 is approximately equal in length or longer than m1 in “plagiaulacidans” (Krause et al. 1992: table 2; Eaton and Cifelli 2001: table 2; Mao et al. 2016: table 2).
We conclude that the preserved morphology of the two known specimens of C. marywaltersae is consistent with assignment to Multituberculata and, more specifically, to Cimolodonta.
Non-ferugliotheriid Gondwanatheria.—Based on current evidence, Gondwanatheria is a strictly Late Cretaceous–early Paleogene Gondwanan clade. Although higher-order relationships of allotherians are beyond the scope of this study, it must be noted that the position of Gondwanatheria within Allotheria is far from established; in the recent phylogenetic analyses of Mammaliaformes by Hoffmann et al. (2020: 213), gondwanatherians were resolved “either as sister to Multituberculata, nested within Multituberculata, or as sister to Cifelliodon (and Euharamiyida), or in a polytomy with other allotherians.” Furthermore, as stated above, the Ferugliotheriidae (including Ferugliotherium, Trapalcotherium, and, tentatively, Magallanodon) are considered separately below. Although variations in placement occur, the non-ferugliotheriid gondwanatherians are currently thought to include the adalatheriid Adalatherium, the enigmatic Galulatherium (not firmly established as a gondwanatherian), and the sudamericids Sudamerica, Gondwanatherium, Vintana, Greniodon, Bharattherium, and Lavanify, with the last two genera being more closely related to one another than to any other gondwanatherian (Hoffmann et al. 2020).
The lower jaw of non-ferugliotheriid gondwanatherians is known from virtually complete dentaries of Adalatherium hui (see Krause et al. 2020a, b) and partial dentaries of Sudamerica ameghinoi (see Pascual et al. 1999), cf. Sudamerica ameghinoi (see Goin et al. 2006), Gondwanatherium patagonicum (see Bonaparte 1990; Gurovich 2001, 2006), and Galulatherium jenkinsi (see Krause et al. 2003; O’Connor et al. 2019). The specimens of cf. Sudamerica ameghinoi and Gondwanatherium patagonicum are too fragmentary to be particularly informative in the current context, and identification of the latter as pertaining to Gondwanatherium has been questioned (Pascual et al. 1999; Rougier et al. 2021). Our comparisons of the lower jaw morphology of Corriebaatar marywaltersae are, therefore, restricted to the dentaries of Adalatherium hui (UA 9030), Sudamerica ameghinoi (MPEFCH 534), and Galulatherium jenkinsi (RRBP 02067).
It is important to underscore that the dentary characteristics listed above as being consistent with referral of Corriebaatar to Multituberculata do not all ineluctably exclude the possibility of referral to other allotherian taxa, including Gondwanatheria; indeed, many of these features may be plesiomorphic for Allotheria, or at least for a clade that unites multituberculates, gondwanatherians, and euharamiyidans. As such, the dentary specimens of Adalatherium, Sudamerica, and Galulatherium, collectively, exhibit almost all of the same dentary features listed above as being shared between Corriebaatar and multituberculates. There are two exceptions: (i) the anterior root of the coronoid process does not extend as far anteriorly relative to the cheekteeth as in Corriebaatar and, (ii) related to the previous condition, the masseteric fossa does not extend as far anteriorly on the horizontal ramus. Another difference is that the diastema is quite asymmetrical (more L-shaped) in the gondwanatherians Adalatherium and Sudamerica (but not Galulatherium) compared to that in Corriebaatar (more U-shaped), although it must be noted that some derived multituberculates also exhibit marked asymmetry in this regard (e.g., Catopsbaatar and Taeniolabis).
Whereas the morphology of the dentary of Corriebaatar is consistent with assignment to Multituberculata and/or Gondwanatheria, the lower dentition does not support gondwanatherian affinities. The morphology of p4, which is large, laterally compressed, and blade-like in Corriebaatar, is as in multituberculates but unlike any tooth in the lower dentition of non-ferugliotheriid gondwanatherians (also see discussion of Ferugliotheriidae below). The alveoli preserved in NMV P252730 are inferred to reveal the presence of two molariform cheekteeth distal to p4. Most gondwanatherians, by contrast, exhibit four molariform cheekteeth (Pascual et al. 1999; Krause et al. 2014, 2020c), although their homologies are debated (e.g., Pascual et al. 1999; Pascual and Ortiz-Jaureguizar 2007; Gurovich and Beck 2009). Galulatherium is the known exception in that it possesses four, columnar, enamel-less, ever-growing cheekteeth (O’Connor et al. 2019). Nonetheless, no non-ferugliotheriid gondwanatherian bears a plagiaulacoid cheektooth. Finally, the roots of the cheekteeth were not ever-growing in Corriebaatar like those in most non-ferugliotheriid gondwanatherians (those of Adalatherium and Vintana providing exceptions in this regard).
Euharamiyida.—Euharamiyidans are a clade of allotherian mammals with disputed members and taxonomic names (variously also referred to as Eleutherodonta, Eleutherodontida, and Eleutherodontidae) that were represented by isolated teeth and are now known from additional relatively complete material, including lower jaws, from the Middle–Late Jurassic of China. Undoubted euharamiyidans represented by lower jaw material include Arboroharamiya (Zheng et al. 2013; Meng et al. 2014, 2018; Han et al. 2017), Shenshou (Bi et al. 2014), Xianshou (Bi et al. 2014), Vilevolodon (Luo et al. 2017; Wang et al. 2021), and Qishou (Mao and Meng 2019a). Megaconus, from the Middle Jurassic of China (Zhou et al. 2013), is a problematic taxon and is not considered here because of lingering controversies concerning its morphology (e.g., Bi et al. 2014; Meng et al. 2014; Han et al. 2017; Wang et al. 2021) and because it is unstable in recent phylogenies (e.g., Bi et al. 2014; Luo et al. 2017; Huttenlocker et al. 2018; Hoffman et al. 2020, Wang et al. 2021).
Like multituberculates and/or gondwanatherians, the lower jaws of euharamiyidans share a number of features with that of Corriebaatar. These include: dentary short and deep; lower dental formula 1.0.1.2; absence of postdentary trough, postdentary bones, and Meckelian sulcus (for clarification, see Wang et al. 2021); presence of diastema between lower incisor and cheekteeth; absence of groove for dental lamina; presence of prominent pterygoid fossa with associated pterygoid shelf and rim; masseteric fossa extending far anteriorly on horizontal ramus; a mandibular foramen situated in pterygoid fossa, below alveolar plane; absence of masseteric foramen; and unfused mandibular symphysis. The lower jaws of euharamiyidans are plesiomorphic relative to those of Corriebaatar in a few features: presence of vestigial coronoid bone (but apparently absent in Vilevolodon, Wang et al. 2021), long axis of incisor aligned with cheektooth row (see Mao and Meng 2019a: fig. 2), and retention of at least some orthal component in the movement of the lower jaw during chewing (Luo et al. 2017; Mao and Meng 2019b). They are also specialized in their own right in possessing a dentary that is extraordinarily thin (mediolaterally) and gracile and in having an angular process that may or may not be homologous with that of other mammaliaforms. Somewhat controversial is the position of the mental foramen in euharamiyidans, scored as “in the canine and anterior premolar (premolariform) region” in Arboroharamiya, Shenshou, Xianshou, and Vilevolodon by Luo et al. (2015, 2017), “at the ultimate premolar and the first molar junction” by Han et al. (2017: 37, supplementary information), and illustrated as below the diastema (between the lower incisor and p4) in Vilevolodon by Wang et al. (2021: extended data fig. 3a, d) and in Qishou, which has two foramina, by Mao and Meng (2019a: fig. 2). Overall, lower jaws of genera traditionally included in Euharamiyida and of Corriebaatar are similar in some respects, but those of the former are more plesiomorphic in some aspects of their morphology and more specialized in others.
The dentitions of Euharamiyida and Corriebaatar are quite different. The lower incisor in euharamiyidans is large and has a long root but is not strongly compressed mediolaterally nor robust like that of Corriebaatar. Furthermore, the lower incisor root in euharamiyidans is tapered, indicating finite growth, which is likely not the case for Corriebaatar. The tooth identified as a p4 in euharamiyidans is, like that of Corriebaatar, supported by two stout roots and has a crown that is tall, laterally compressed, and considerably longer than m1. There are two important differences, however, in that the p4 crown in euharamiyidans rises to a single, tall mesial cusp followed distally by a basin or basin-like structure bearing several minor cusps on its rim. By contrast, the crown of Corriebaatar is arched, has multiple serrations (coupled with short buccal and lingual ridges), and no basin. Finally, the lower molars of euharamiyidans appear to have multiple roots, which is also the condition in Corriebaatar, but they are all fused proximally and were housed in a single large alveolus (Bi et al. 2014; Meng et al. 2014; Luo et al. 2017), thus differing dramatically from the multiple, small, separate alveoli for fully divided roots supporting m1 and m2 in Corriebaatar.
Ferugliotheriidae.—Ferugliotherium windhauseni from the latest Cretaceous (Maastrichtian) of Argentina includes two junior synonyms, Vucetichia gracilis (see Krause 1993) and Argentodites coloniensis (see Gurovich and Beck 2009; Brown et al. 2020; Rougier et al. 2021). Whether or not the similarly sized Trapalcotherium matuastensis, known only from a single incomplete m1 from the Allen Formation (Rougier et al. 2009), is a distinct genus and species is debatable and awaits the recovery of more complete material. Also debatable is whether or not the relatively large but low-crowned Magallanodon baikashkenke, known from several molariform teeth, an upper incisor, and a small fragment of dentary, should be included in Ferugliotheriidae (Goin et al. 2020; Chimento et al. 2020, 2021; Rougier et al. 2021). It is noteworthy that the molariform teeth of Magallanodon, like those of Ferugliotherium and Corriebaatar, have more than two roots (the number of roots on the molariform of Trapalcotherium is unknown). The dentary fragment of Magallanodon, consisting of an anterior portion of the horizontal ramus, demonstrates the presence of an unfused mandibular symphysis, a large, laterally compressed, long-rooted incisor, a dorsally concave diastema, a single, dorsally placed mental foramen, and a masseteric fossa that extends far anteriorly (Chimento et al. 2021). Although the molariform teeth are distinctive, these features of the dentary do not meaningfully differentiate Magallanodon from those of gondwanatherians, multituberculates, euharamiyidans, or Corriebaatar; Magallanodon is therefore not considered further here.
The affinities of Ferugliotherium, whether a multituberculate or an early branching gondwanatherian, are controversial (see review in Rougier et al. 2021). A recent analysis by Hoffmann et al. (2020) found conflicting support: parsimony analyses recovered ferugliotheriids within Gondwanatheria (and outside of Multituberculata), tip-dated Bayesian analyses nested ferugliotheriids within Multituberculata (and outside of Gondwanatheria), and undated Bayesian analyses found Gondwanatheria (including Ferugliotherium and Trapalcotherium) within Multituberculata. Brown et al. (2020), however, on the basis of new gnathic and dental material from the La Colonia Formation (not included in the analysis by Hoffmann et al. 2020) were able to formally synonymize Argentodites with Ferugliotherium and argued persuasively for multituberculate affinities. Correspondingly, Rougier et al. (2021: 303) argued that the morphology of the dentary and the presence of a large incisor, a sizeable diastema, a plagiaulacoid premolar, and two molariforms in Ferugliotherium, “is congruent with that of cimolodont multituberculates.” We eagerly await the description of the new material but, for our purposes here, provisionally accept the conclusion that Ferugliotherium is a cimolodontan multituberculate. However, we also acknowledge the conundrum presented by the pattern of cusps on the molariforms; it is similar to that of more hypsodont gondwanatherians, as first established by Krause and Bonaparte (1993) and recently reemphasized by Goin et al. (2020).
With the exception of the as-yet-undescribed dentaries containing p4 announced by Brown et al. (2020), knowledge of the lower jaw morphology of ferugliotheriids is restricted to the single small dentary fragment (MACN-RN 975) tentatively referred to Ferugliotherium windhauseni by Kielan-Jaworowska and Bonaparte (1996). Accepting the conclusion that the ferugliotheriid molariforms are associated with the plagiaulacoid premolars (Brown et al. 2020; Rougier et al. 2021), Ferugliotherium exhibits the following features that are shared with Corriebaatar: small size (p4 length 4.15–4.8 mm, Kielan-Jaworowska and Bonaparte 1996; Kielan-Jaworowska et al. 2007); lower dental formula of 1.0.1.2 (and therefore absence of p3); large, laterally compressed lower incisor, the root of which extends posteriorly to the level of p4; short diastema (shorter than the length of p4); plagiaulacoid p4 with arcuate dorsal outline (contra Kielan-Jaworowska et al. 2007; see Gurovich and Beck 2009), low number of serrations (7 in Corriebaatar, 8 in Ferugliotherium), and mesial root more robust than distal root; two lower molariform teeth with multiple, non-hypsodont roots; single mental foramen situated above mid-height on horizontal ramus; distinct angle between anterior and posterior parts of the horizontal ramus; and palinal stroke of grinding cycle (Krause et al. 1992; Krause and Bonaparte 1993; Kielan-Jaworowska and Bonaparte 1996; Kielan-Jaworowska et al. 2007; Brown et al. 2020). Despite these similarities, they are quite different in details, primarily in that the ridges descending from the serrations of p4 in Ferugliotherium are long (more typical of cimolodontans in general) and those of Corriebaatar, except those descending from the first serration, are extraordinarily short. In this context, the short ridges of Corriebaatar are considered to be autapomorphic. It is, however, worth observing that p4 of Ferugliotherium appears to have at least incipiently developed the double exodaenodont lobes (Kielan-Jaworowska et al. 2007) that are so characteristic of Corriebaatar. The implications of this observation can only be evaluated with more complete remains of both C. marywaltersae and F. windhauseni (or new, closely related taxa) but current evidence leads us to formulate the working hypothesis that these two taxa may belong to the same lineage of cimolodontan multituberculates.
Concluding remarks
Phylogenetic placement.—Although the character states of Corriebaatar marywaltersae revealed by the dentary in NMV P252730 and p4 in both NMV P216655 (the holotype) and NMV P252730 support the conclusion that it is a cimolodontan multituberculate, we eagerly look forward to recovering more material that would allow us to more meaningfully incorporate the genus into a phylogenetic analysis and thus more precisely understand its relationships within Cimolodonta. Indeed, cimolodontan interrelationships are, in general, poorly understood (e.g., Cifelli 2001; Martin 2018) and, although most workers (e.g., Simmons 1993; Kielan-Jaworowska and Hurum 2001; Kielan-Jaworowska et al. 2004) regard the group as monophyletic, even this has been questioned (Hahn and Hahn 1999; Fox 2005). We summarize here our interpretations of phylogenetic placement of Corriebaatar marywaltersae within Allotheria based on the limited information available from the holotype and referred specimens.
The lower jaw morphology exhibited by the new specimen described in this report, NMV P252730, reveals a plethora of characters that are seen in multituberculates, gondwanatherians, and euharamiyidans and are much more derived than those of haramiyidans. Whereas features of the dentary are entirely consistent with assignation to Cimolodonta, the dentary of Corriebaatar differs from those of non-ferugliotheriid gondwanatherians in possessing a coronoid process that originates relatively far anteriorly and, correspondingly, a masseteric fossa that extends far anteriorly onto the horizontal ramus beyond the level of the molars. Similarly, the lower jaw of Corriebaatar differs from those of euharamiyidans in being generally much more robust, in that the long axis of the incisor is not aligned with the long axis of the cheektooth row, in lacking any trace of a Meckelian sulcus, and in having a fully palinal power stroke of the grinding cycle.
The dentition of Corriebaatar, consisting of a large, mediolaterally compressed lower incisor separated from a blade-like p4 by a distinct diastema and the presence of two multi-rooted molars with proportions typical of derived multituberculates confirms the cimolodontan affinities of Corriebaatar, as first concluded by Rich et al. (2009) on the basis of p4 morphology alone. The dentition of Corriebaatar differs from those of non-ferugliotheriid gondwanatherians in possessing a plagiaulacoid p4 followed by two molars; non-ferugliotheriid gondwanatherians have four molariform teeth that are not preceded by a plagiaulacoid tooth. It also differs from those of euharamiyidans in having a much more robust and more strongly compressed (mediolaterally) lower incisor (which was likely ever-growing), a fully plagiaulacoid p4 with multiple serrations and no basin, and molars with multiple roots that are not coalesced. Finally, features of the p4 crown (double exodaenodont lobes, long buccal and lingual ridges descending from first serration but extremely short ridges on more distal serrations) are distinctive and diagnostic for Corriebaatar relative to other multituberculates, including Ferugliotherium.
All available evidence revealed by the two known specimens of Corriebaatar confirms that it is a multituberculate firmly nested within Cimolodonta and that, although well separated in time and space, it may be closely related to Ferugliotherium.
Biogeographic implications.—Including the holotype specimen of Corriebaatar marywaltersae described by Rich et al. (2009), the disputed and undisputed records of multituberculates from the southern supercontinent Gondwana were recently reviewed by Krause et al. (2017). Since that compendium, further doubts have been expressed concerning the original referral of isolated hahnodontid teeth from the Early Cretaceous of Morocco by Sigogneau-Russell (1991) to Multituberculata. Huttenlocker et al. (2018) have argued, in agreement with Butler and Hooker (2005) and Meng et al. (2014), but not with Hahn and Hahn (2006), that the Hahnodontidae are not multituberculates and should be assigned to the Haramiyida, a conclusion that we tentatively accept. Conversely, recent discoveries of dentary fragments of ferugliotheriids from the Upper Cretaceous La Colonia Formation of Argentina support the conclusion that ferugliotheriids are multituberculates rather than gondwanatherians (Brown et al. 2020; for fuller discussion of ferugliotheriid affinities, see Rougier et al. 2021). Multituberculates have also been identified in the Late Cretaceous of Madagascar based on two specimens, a molar fragment (Krause 2013) and a proximal femur (Krause et al. 2017). Finally, Parmar et al. (2013) assigned an isolated tooth, the holotype of Indobaatar zofiae, from the Early–Middle Jurassic of India to Multituberculata. These occurrences are depicted in Fig. 7, a paleogeographic map of Gondwana in the Barremian (125 Ma; Scotese 2021), the approximate time at which Corriebaatar lived.
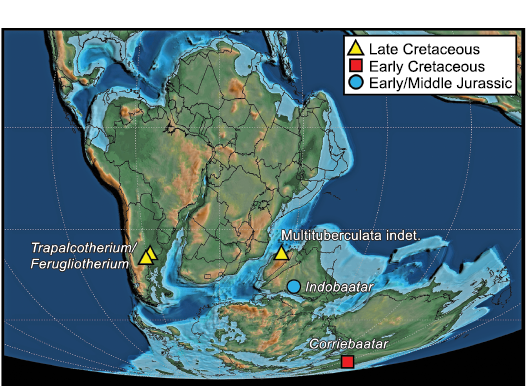
Fig. 7. Known distribution of Mesozoic multituberculates on Gondwana. Base map at 125 m.y.a. (latest Barremian) from Scotese (2021).
Given the extremely poor record of Gondwanan multituberculates from the Mesozoic (and the fact that none are known from the Cenozoic), there are profound limitations in formulating testable biogeographic hypotheses. Nonetheless, it must be noted that, if the specimen of Indobaatar zofiae from India (Parmar et al. 2013) is verified as that of an eobaatarid multituberculate and its age is verified as Early/Middle Jurassic, its presence informs the possibilities for biogeographic interpretation because it existed at a time prior to the separation of Laurasia and Gondwana and prior to the fragmentation of the latter (Beck 2017).
?Paracimexomys crossi Cifelli, 1997 (modified to cf. P. crossi by Eaton and Cifelli 2001) is generally accepted as the earliest occurrence of cimolodontan multituberculates (e.g., Cifelli et al. 2014; Weaver et al. 2019). cf. P. crossi derives from the Antlers Formation in Oklahoma, which has been dated to near the Aptian/Albian boundary (Jacobs and Winkler 1998; Jacobs et al. 2005), currently regarded as at 113.2 Ma (Gale et al. 2020). Laterally equivalent strata of the Antlers Formation in north-central Texas have also yielded multituberculates, which, although not yet described in detail, also appear to be cimolodontans of the “Paracimexomys group” (Krause et al. 1990).
When Rich et al. (2009: 3) described Corriebaatar marywaltersae on the basis of only the holotype specimen, they suggested from p4 morphology alone that it was a cimolodontan “as a working hypothesis”. The new, much more complete specimen, NMV P252730, can be taken as strong evidence supporting that hypothesis. The combined evidence from the two specimens, coupled with a revised age for the Flat Rocks site as latest Barremian (Wagstaff et al. 2020), establishes that C. marywaltersae is the earliest known cimolodontan. The Barremian/Aptian boundary is currently placed at 121.4 Ma (Gale et al. 2020). Furthermore, C. marywaltersae, as a cimolodontan from the latest Barremian of Australia, has additional implications for the origin and early distribution of that group on Gondwana but, given the poor fossil record of multituberculates on the supercontinent (and in the early Mesozoic of Laurasia), we refrain from speculating that its presence indicates an origin for cimolodontans there. Current knowledge, extremely limited by the poor fossil record of early mammalian evolution on Gondwana, allows for multiple scenarios, including both dispersal and vicariance. Nonetheless, Corriebaatar establishes that cimolodontans were present on Gondwana during the earlier stages of supercontinental breakup, although separation between West Gondwana (South America and Afro-Arabia) and East Gondwana (Antarctica, Australia, Indian subcontinent, and Madagascar) was already underway (Fig. 7). Of particular note is the highly derived nature of Corriebaatar (e.g., absence of p3, morphology of p4), which suggests a ghost lineage for Cimolodonta extending back earlier in time. Furthermore, the presence of multituberculates in the latest Cretaceous of South America and Madagascar could therefore be viewed as vicariant relicts of an earlier presence on Gondwana. Alternatively, it cannot be discounted (but see Rougier et al. 2021) that the South American record is a result of a later dispersal from North America along with other terrestrial vertebrate taxa during the “First American Biotic Interchange” (Goin et al. 2012; see review in Krause et al. 2019) near the end of the Cretaceous, as suggested by Kielan-Jaworowska et al. (2007).
We eagerly look forward to the recovery of more multituberculate specimens from the Mesozoic of Gondwana to shed further light on their distribution in space and time. Eaton and Cifelli (2001: 454) opined that a “major problem in understanding of multituberculate phylogeny and biogeography is the origin of cimolodontans” and, correspondingly, Kielan-Jaworowska and Hurum (2001: 403) bluntly stated that the origin of Cimolodonta “remains a mystery”; that is still the case over two decades later and is perhaps even more of an enigma in light of the presence of Corriebaatar in the mid-Early Cretaceous of Australia.
Authors’ contributions
LK meticulously prepared NMV P252730, AE microCT-scanned the specimen, SM provided the photographs in Fig. 3, PT created the scientific illustrations in Figs. 1 and 5 and provided detailed visual assessments of the specimen that assisted in its description, and MW developed the 3D reconstruction, the 3D PDFs, and the video in SOM. PV-R and THR wrote the original manuscript. Assisted by them, and including contributions from AE and MW to the Material and methods section, DK wrote the second draft.
Acknowledgements
We are delighted to be able to contribute to this festschrift volume honoring Richard L. Cifelli (Oklahoma Museum of Natural History, Norman, USA). Richard Cifelli was an author on a number of important papers cited in this study and participated in the seminal synthesis of knowledge about Mesozoic mammals generally for the benefit of all workers in the field. We thank Brian M. Davis (University of Louisville, USA) for the invitation to contribute to this issue and his skilled editing of our manuscript. We also thank Robin M.D. Beck (University of Salford, UK), Louis L. Jacobs (Southern Methodist University, Dallas, USA), and Lucas N. Weaver (University of Michigan, Ann Arbor, USA) for stimulating correspondence about the subject matter of this article and both Robin M.D. Beck and Guillermo W. Rougier (University of Louisville, USA) for their insights and helpful comments on the revised manuscript. We also thank Wendy J. White (Monash University, Clayton, Australia) for her eagle-eye editorial efforts to improve the manuscript. Making possible the discovery of this new specimen and the original Corriebaatar marywaltersae specimen were the many volunteers who have taken part in the Dinosaur Dreaming program along the south coast of Victoria over years, the funding provided by the National Geographic Society and Swinburne and Monash universities, and the grace of the Bunurong People upon whose land this material was collected. Parks Victoria Permits to PV-R supported this work over the years. DK’s effort was supported by U.S. National Science Foundation grant EAR-1664432.
References
Archibald, J.D. 1982. A study of Mammalia and geology across the Cretaceous–Tertiary boundary in Garfield County, Montana. University of California Publications in Geological Sciences 122: 1–286.
Averianov, A.O., Martin, T., Lopatin, A.V., Schultz, J.A., Schellhorn, R., Krasnolutskii, S., Skutschas, P., and Ivantsov, S. 2021. Multituberculate mammals from the Middle Jurassic of western Siberia, Russia, and the origin of Multituberculata. Papers in Palaeontology 7: 769–787. Crossref
Beck, R.M.D. 2017. The biogeographical history of non-marine mammaliaforms in the Sahul region. In: M.C. Ebach (ed.), Handbook of Austalasian Biogeography, 329–366. CRC Press, Boca Raton.
Bi, S., Wang, Y., Guan, J., Sheng, X., and Meng, J. 2014. Three new Jurassic euharamiyidan species reinforce early divergence of mammals. Nature 514: 579–584. Crossref
Bonaparte, J.F. 1990. New Late Cretaceous mammals from the Los Alamitos Formation, northern Patagonia. National Geographic Research 6: 63–93.
Brown, C.E., Cardozo, M.S., Kuwabara, N., and Rougier, G.W. 2020. New Late Cretaceous mammals from La Colonia Formation, Patagonia, Argentina support multituberculate affinities for ferugliotheriids. In: Society of Vertebrate Paleontology Annual Meeting Program and Abstracts.
Butler, P.M. 1956. The ontogeny of molar pattern. Biological Reviews 31: 30–70. Crossref
Butler, P.M. 2000. Review of the early allotherian mammals. Acta Palaeontologica Polonica 45: 317–342.
Butler, P.M. and Hooker, J.J. 2005. New teeth of allotherian mammals from the English Bathonian, including the earliest multituberculates. Acta Palaeontologica Polonica 50: 185–207.
Carpenter, K. 1998. Redescription of the multituberculate, Zofiabaatar and the paurodont, Foxraptor, from Pine Tree Ridge, Wyoming. Modern Geology 23: 393–405.
Chimento, N.R., Agnolin, F.L., Tsuihiji, T., Manabe, M., and Novas, F.E. 2020. New record of a Mesozoic gondwanatherian mammaliaform from southern Patagonia. The Science of Nature 107: 49. Crossref
Chimento, N.R., Agnolin, F.L., Tsuihiji, T., Manabe, M., and Novas, F.E. 2021. New gondwanatherian (Mammaliaformes) remains from the Chorrillo Formation (Upper Cretaceous) of southern Patagonia, Argentina. Cretaceous Research 127: 104947. Crossref
Cifelli, R.L. 1997. First notice on Mesozoic mammals from Oklahoma. Oklahoma Geology Notes, Oklahoma Geological Survey 57: 4–17.
Cifelli, R.L. 2001. Early mammalian radiations. Journal of Paleontology 75: 1214–1226. Crossref
Cifelli, R.L., Davis, B.M., and Sames, B. 2014. Earliest Cretaceous mammals from the United States. Acta Palaeontologica Polonica 59: 31–52.
Cifelli, R.L., Eberle, J.J., Lofgren, D.L., Lillegraven, J.A., and Clemens, W.A. 2004. Mammalian biochronology of the latest Cretaceous. In: M.O. Woodburne (ed.), Late Cretaceous and Cenozoic Mammals of North America, 21–42. Columbia University Press, New York. Crossref
Clemens, W.A., Jr. 1964. Fossil mammals from the type Lance Formation, Wyoming. Part I. Introduction and Multituberculata. University of California Publications in Geological Sciences 48: 1–105.
Cope, E.D. 1884. The Tertiary Marsupialia. American Naturalist 18: 686–697. Crossref
Csiki, Z., Grigorescu, D., and Rücklin, M. 2005. A new multituberculate specimen from the Maastrichtian of Pui, Romania and reassessment of affinities of Barbatodon. Acta Palaeontologica Romaniae 5: 73–86.
Dawson, M.R. and Constenius, K.N. 2018. Mammalian fauna of the middle Eocene Kishenehn Formation, Middle Fork of the Flathead River, Montana. Annals of Carnegie Museum 85: 25–60. Crossref
Dragonfly 2020. Object Research Systems Inc, Montreal, Canada, 2020. .
Eaton, J.G. and Cifelli, R.L. 2001. Multituberculate mammals from near the Early–Late Cretaceous boundary, Cedar Mountain Formation, Utah. Acta Palaeontologica Polonica 46: 453–518.
Eberle, J.J. and Lillegraven, J.A. 1998. A new important record of earliest Cenozoic mammalian history: geologic setting, Multituberculata, and Peradectia. Rocky Mountain Geology 33 (1): 3–47.
Engelmann, G.F. and Callison, G. 1999. Glirodon grandis, a new multituberculate mammal from the Upper Jurassic Morrison Formation. In: D.D. Gillette (ed.), Vertebrate Paleontology in Utah. Utah Geological Survey, Miscellaneous Publication 99-1: 161–177.
Fox, R.C. 2005. Microcosmodontid multituberculates (Allotheria, Mammalia) from the Paleocene and Late Cretaceous of western Canada. Palaeontographica Canadiana 23: 1–109.
Gale, A.S., Mutterlose, J., and Batenburg, S. 2020. The Cretaceous period. In: F.M. Gradstein, J.G. Ogg, M.D. Schmitz, and G.M. Ogg (eds.), Geologic Time Scale 2020, volume 2, 1023–1086. Elsevier, Amsterdam. Crossref
Gambaryan, P.P. and Kielan-Jaworowska, Z. 1995. Masticatory musculature of Asian taeniolabidoid multituberculate mammals. Acta Palaeontologica Polonica 40: 45–108.
Goin, F.J., Gelfo, N.F., Chornogubsky, L., Woodburne, M.O., and Martin ,T. 2012. Origins, radiations, and distribution of South American mammals: from greenhouse to icehouse worlds. In: B.D. Patterson and L.P. Costa (eds.), Bones, Clones, and Biomes: An 80-Million Year History of Recent Neotropical Mammals, 20–50. University of Chicago Press, Chicago. Crossref
Goin, F.J., Martinelli, A.G., Soto-Acuña, S., Vieytes, E.C., Manríquez, L.M.E., Fernández, R.A., Pino, J.P., Trevisan, C., Kaluza, J., Reguero, M.A., Leppe, M., Ortiz, H., Rubilar-Rogers, D., and Vargas, A.O. 2020. First Mesozoic mammal from Chile: the southernmost record of a Late Cretaceous gondwanatherian. Boletín del Museo Nacional de Historia Natural, Chile 69: 5–31.
Goin, F.J., Reguero, M.A., Pascual, R., von Koenigswald, W., Woodburne, M.O., Case, J.A., Marenssi, S.A., Vieytes, C., and Vizcaíno, S.F. 2006. First gondwanatherian mammal from Antarctica. In: J.E. Francis, D. Pirrie, and J.A. Crame (eds.), Cretaceous–Tertiary High-latitude Palaeoenvironments, James Ross Basin, Antarctica. The Geological Society, London, Special Publications 258: 135–144. Crossref
Granger, W. and Simpson, G.G. 1929. A revision of the Tertiary Multituberculata. Bulletin of the American Museum of Natural History 56: 601–676.
Grossnickle, D.M., Weaver, L.N., Jäger, K.R.K., and Schultz, J.A. 2021. The evolution of anteriorly directed molar occlusion in mammals. Zoological Journal of the Linnean Society [published online https://doi.org/10.1093/zoolinnean/zlab039]. Crossref
Gurovich, Y. 2001. Redescription of a dentary of Gondwanatherium patagonicum (Mammalia, Gondwanatheria) and comparisons with dentaries of other gondwanathere mammals of Argentina. Journal of Vertebrate Paleontology 21(Supplement 3): 57A.
Gurovich, Y. 2006. Bio-evolutionary Aspects of Mesozoic Mammals: Description, Phylogenetic Relationships and Evolution of the Gondwanatheria (Late Cretaceous and Paleocene of Gondwana). 621 pp. Ph.D. dissertation, Universidad Nacional de Buenos Aires, Buenos Aires.
Gurovich, Y. and Beck, R. 2009. The phylogenetic affinities of the enigmatic mammalian clade Gondwanatheria. Journal of Mammalian Evolution 16: 25–49. Crossref
Hahn, G. 1969. Beiträge zur Fauna der Grube Guimarota Nr. 3. Die Multituberculata. Palaeontographica A 133: 1–100.
Hahn, G. 1971. The dentition of the Paulchoffatiidae (Multituberculata, Upper Jurassic). Memória dos Serviços Geológicos de Portugal 17: 7–39.
Hahn, G. 1977. Das Coronoid der Paulchoffatiidae (Multituberculata; Ober-Jura). Paläontologische Zeitschrift 51: 246–253. Crossref
Hahn, G. and Hahn, R. 1998. Neue Beobachtungen an Plagiaulacoidea (Multituberculata) des Ober-Juras 2. Zum Bau des Unterkiefers und des Gebisses bei Meketibolodon und bei Guimarotodon. Berliner Geowissenschaftliche Abhandlungen E 28: 9–37.
Hahn, G., and Hahn. R. 1999. Pinheirodontidae n. fam. (Multituberculata) (Mammalia) aus der tiefen Unter-Kreide Portugals. Palaeontographica A 253: 77–222. Crossref
Hahn, G. and Hahn, R. 2004. The dentition of the Plagiaulacida (Multituberculata, Late Jurassic to Early Cretaceous). Geologica et Palaeontologica 38: 119–159.
Hahn, G. and Hahn, R. 2006. Evolutionary tendencies and systematic arrangement in the Haramiyida (Mammalia). Geologica et Palaeontologica 40: 173–193.
Han, G., Mao, F., Bi, S., Wang, Y., and Meng, J. 2017. A Jurassic gliding euharamiyidan mammal with an ear of five auditory bones. Nature 551: 451–456. Crossref
Higgins, P. 2003. A new species of Paleocene multituberculate (Mammalia: Allotheria) from the Hanna Basin, south-central Wyoming. Journal of Vertebrate Paleontology 23: 468–470. Crossref
Hoffmann, S., Beck, R.M.D., Wible, J.R., Rougier, G.W., and Krause, D.W. 2020. Phylogenetic placement of Adalatherium hui (Mammalia, Gondwanatheria) from the Late Cretaceous of Madagascar: implications for allotherian relationships. Journal of Vertebrate Paleontology 40 (Supplement 2): 214–235. Crossref
Holtzman, R.C. and Wolberg, D.L. 1977. The Microcosmodontinae and Microcosmodon woodi, new Multituberculata taxa (Mammalia) from the Late Paleocene of North America. Scientific Publications of the Science Museum of Minnesota 4 (1): 1–13.
Hunter, J.P., Hartman, J.H., and Krause, D.W. 1997. Mammals and mollusks across the Cretaceous–Tertiary boundary from Makoshika State Park and vicinity (Williston Basin), Montana. Contributions to Geology, University of Wyoming 32: 61–114.
Huttenlocker, A.K., Grossnickle, D.M., Kirkland, J.I., Schultz, J.A., and Luo, Z.-X. 2018. Late-surviving stem mammal links the lowermost Cretaceous of North America and Gondwana. Nature 588: 108–112. Crossref
Jacobs, L.L. and Winkler, D.A. 1998. Mammals, archosaurs, and the Early to Late Cretaceous transition in north-central Texas. In: Y. Tomida, L.J. Flynn, and L.L. Jacobs (eds.), Advances in Vertebrate Paleontology and Geochronology. National Science Museum Monographs 14: 253–280.
Jacobs, L.L., Ferguson K., Polcyn, M.J., and Rennison, C. 2005. Cretaceous δ13C stratigraphy and the age of dolichosaurs and early mosasaurs. Netherlands Journal of Geosciences 84: 257–268. Crossref
Jenkins, F.A., Jr., Gatesy, S.M., Shubin, N.H., and Amaral, W.M. 1997. Haramiyids and Triassic mammalian evolution. Nature 385: 715–718. Crossref
Kielan-Jaworowska, Z. and Bonaparte, J.F. 1996. Partial dentary of a multituberculate mammal from the Late Cretaceous of Argentina and its taxonomic implications. Revista del Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” 145: 1–9.
Kielan-Jaworowska, Z. and Hurum, J.H. 1997. Djadochtatheria—a new suborder of multituberculate mammals. Acta Palaeontologica Polonica 42: 201–242.
Kielan-Jaworowska, Z. and Hurum, J.H. 2001. Phylogeny and systematics of multituberculate mammals. Palaeontology 44: 389–429. Crossref
Kielan-Jaworowska, Z. and Nessov, L.A. 1992. Multituberculate mammals from the Cretaceous of Uzbekistan. Acta Palaeontologica Polonica 37: 1–17.
Kielan-Jaworowska, Z., Cifelli, R.L., and Luo, Z.-X. 2004. Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure. 630 pp. Columbia University Press, New York. Crossref
Kielan-Jaworowska, Z., Hurum, J.H., and Lopatin, A.V. 2005. Skull structure in Catopsbaatar and the zygomatic ridges in multituberculate mammals. Acta Palaeontologica Polonica 50: 487–512.
Kielan-Jaworowska, Z., Ortiz-Jaureguizar, E., Vieytes, E.C., Pascual, R., and Goin, F.J. 2007. First ?cimolodontan multituberculate mammal from South America. Acta Palaeontologica Polonica 52: 257–262.
Krause, D.W. 1977. Paleocene multituberculates (Mammalia) of the Roche Percée local fauna, Ravenscrag Formation, Saskatchewan, Canada. Palaeontographica A 159: 1–36.
Krause, D.W. 1982a. Evolutionary History and Paleobiology of Early Cenozoic Multituberculata (Mammalia), with Emphasis on the Family Ptilodontidae. 555 pp. Ph.D. Dissertation, University of Michigan, Ann Arbor.
Krause, D.W. 1982b. Jaw movement, dental function, and diet in the Paleocene multituberculate Ptilodus. Paleobiology 8: 265–281. Crossref
Krause, D.W. 1986. Competitive exclusion and taxonomic displacement in the fossil record: the case of rodents and multituberculates in North America. In: K.M. Flanagan and J.A. Lillegraven (eds.), Vertebrates, Phylogeny, and Philosophy: a Tribute to George Gaylord Simpson. Contributions to Geology, University of Wyoming, Special Paper 3: 95–117. Crossref
Krause, D.W. 1987. Baiotomeus, a new ptilodontid multituberculate (Mammalia) from the middle Paleocene of western North America. Journal of Paleontology 61: 595–603. Crossref
Krause, D.W. 1993. Vucetichia (Gondwanatheria) is a junior synonym of Ferugliotherium (Multituberculata). Journal of Paleontology 67: 321–324. Crossref
Krause, D.W. 2013. Gondwanatheria and ?Multituberculata (Mammalia) from the Late Cretaceous of Madagascar. Canadian Journal of Earth Sciences 50: 324e340. Crossref
Krause, D.W. and Bonaparte, J.F. 1993. Superfamily Gondwanatherioidea: a previously unrecognized radiation of multituberculate mammals in South America. Proceedings of the National Academy of Sciences of the United States of America 90: 9379–9383. Crossref
Krause, D.W., Gottfried, M.D., O’Connor, P.M., and Roberts E.M. 2003. A Cretaceous mammal from Tanzania. Acta Palaeontologica Polonica 48: 321–330.
Krause, D.W., Hoffmann, S., and Werning, S. 2017. First postcranial remains of Multituberculata (Allotheria, Mammalia) from Gondwana. Cretaceous Research 80: 91–100. Crossref
Krause, D.W., Hoffmann, S., Hu, Y., Wible, J.R., Rougier, G.W., Kirk, E.C., Groenke, J.R., Rogers, R.R., Rossie, J.B., Schultz, J.A., Evans, A.R., Koenigswald, W. von, and Rahantarisoa, L.J. 2020a. Skeleton of Cretaceous gondwanatherian mammal from Madagascar reflects long-term insularity. Nature 581: 421–427. Crossref
Krause, D.W., Hoffmann, S., Wible, J.R., and Rougier, G.W. 2020b. Lower jaw morphology of Adalatherium hui (Mammalia, Gondwanatheria) from the Late Cretaceous of Madagascar. Journal of Vertebrate Paleontology 40 (Supplement 2): 81–96. Crossref
Krause, D.W., Hoffmann, S., Wible, J.R., Kirk, E.C., Schultz, J.A., Koenigswald, W. von, Groenke, J.R., Rossie, J.B., O’Connor, P.M., Seiffert, E.R., Dumont, E.R., Holloway, W.L., Rogers, R.R., Rahantarisoa, L.J., Kemp, A.D., and Andriamialison H. 2014. First cranial remains of gondwanatherian mammal reveal remarkable mosaicism. Nature 515: 512–517. Crossref
Krause, D.W., Hu, Y., Hoffmann, S., Groenke, J.R., Schultz, J.A., Evans, A., Koenigswald, W. von, and Rougier, G.W. 2020c. Dental morphology of Adalatherium hui (Mammalia, Gondwanatheria) from the Late Cretaceous of Madagascar. Journal of Vertebrate Paleontology 40 (Supplement 2): 97–132. Crossref
Krause, D.W., Kielan-Jaworowska, Z., and Bonaparte, J.F. 1992. Ferugliotherium Bonaparte, the first known multituberculate from South America. Journal of Vertebrate Paleontology 12: 351–376. Crossref
Krause, D.W., Kielan-Jaworowska, Z., and Turnbull, W.D. 1990. Early Cretaceous Multituberculata (Mammalia) from the Antlers Formation, Trinity Group, of north-central Texas. Journal of Vertebrate Paleontology 10 (Supplement): 109.
Krause, D.W., Sertich, J.J.W., O’Connor, P.M., Curry Rogers, K.A., and Rogers, R.R. 2019. The Mesozoic biogeographic history of Gondwanan terrestrial vertebrates: insights from Madagascar’s fossil record. Annual Review of Earth and Planetary Sciences 47: 519–553. Crossref
Kusuhashi, N., Hu, Y., Wang, Y., Setoguchi, T., and Matsuoka, H. 2009. Two eobaatarid (Multituberculata; Mammalia) genera from the Lower Cretaceous Shahai and Fuxin formations, northeastern China. Journal of Vertebrate Paleontology 29: 1264–1288. Crossref
Kusuhashi, N., Hu, Y., Wang, Y., Setoguchi, T., and Matsuoka, H. 2010. New multituberculate mammals from the Lower Cretaceous (Shahai and Fuxin formations), northeastern China. Journal of Vertebrate Paleontology 30: 1501–1514. Crossref
Lofgren, D., Gosney, G., Soltis, C., Oney, K., and West, L. 2017. New records of Kimbetohia campi and Xanclomys mcgrewi (Mammalia, Multituberculata) from the Fort Union Formation of the Great Divide Basin, Wyoming. Paludicola 11: 43–50.
Luo, Z.-X., Gatesy, S.M., Jenkins, F.A., Jr., Amaral, W.W., and Shubin, N.H. 2015. Mandibular and dental characteristics of Late Triassic mammaliaform Haramiyavia and their ramifications for basal mammal evolution. Proceedings of the National Academy of Sciences of the United States of America 112: E7101–E7109. Crossref
Luo, Z.-X., Meng, Q.-J., Grossnickle, D.M., Liu, D., Neander, A.I., Zhang, Y.-G., and Ji, Q. 2017. New evidence for mammaliaform ear evolution and feeding adaptation in a Jurassic ecosystem. Nature 548: 326–329. Crossref
Mao, F.-Y., and Meng, J. 2019a. A new haramiyidan mammal from the Jurassic Yanliao Biota and comparisons with other haramiyidans. Zoological Journal of the Linnean Society 186: 529–552. Crossref
Mao, F.-Y. and Meng, J. 2019b. Tooth microwear and occlusal modes of euharamiyidans from the Jurassic Yanliao Biota reveal mosaic tooth evolution in Mesozoic allotherian mammals. Palaeontology 62: 639–660. Crossref
Mao, F.-Y., Wang, Y.-Q., and Meng, J. 2016. New specimens of the multituberculate mammal Sphenopsalis from China: implications for phylogeny and biology of taeniolabidoids. Acta Palaeontologica Polonica 61: 429–454. Crossref
Marsh, O.C. 1880. Notice on Jurassic mammals representing two new orders. American Journal of Science 20: 235–239. Crossref
Martin, T. 2018. Mesozoic mammals—early mammalian diversity and ecomorphological adaptations. In: F.E. Zachos and R.J. Asher (eds.), Handbook of Zoology, Mammalia. Mammalian Evolution, Diversity and Systematics, 199–299. De Gruyter, Berlin. Crossref
Martin, T., Schultz, J.A., Schwermann, A.H., and Wings, O. 2016. First Jurassic mammals of Germany: multituberculate teeth from Langenberg Quarry (Lower Saxony). Palaeontologia Polonica 67: 171–179.
McKenna, M.C. 1975. Toward a phylogenetic classification of the Mammalia. In: P. Luckett and F.S. Szalay (eds.), Phylogeny of the Primates, 21–46. Cambridge University Press, Cambridge. Crossref
Meng, J., Bi, S., Wang, Y., Zheng, X., and Wang, X. 2014. Dental and mandibular morphologies of Arboroharamiya (Haramiyida, Mammalia): a comparison with other haramiyidans and Megaconus and implications for mammalian evolution. PLoS ONE 9 (12): e113847. Crossref
Meng, J., Bi., S., Zheng, X., and Wang, X. 2018. Ear ossicle morphology of the Jurassic euharamiyidan Arboroharamiya and evolution of the mammalian middle ear. Journal of Morphology 279: 441–457. Crossref
Meng, J., Hu, Y., Wang, Y., Wang, X., and Li, C. 2006. A Mesozoic gliding mammal from northeastern China. Nature 444: 889–893. Crossref
Meng, J., Zhai, R., and Wyss, A.R. 1998. The late Paleocene Bayan Ulan fauna of Inner Mongolia, China. Bulletin of Carnegie Museum of Natural History 34: 148–185.
Miao, D. 1986. Dental anatomy and ontogeny of Lambdopsalis bulla (Mammalia, Multituberculata). Contributions to Geology, University of Wyoming 24: 65–76.
Miao, D. 1988. Skull morphology of Lambdopsalis bulla (Mammalia, Multituberculata) and its implications to mammalian evolution. Contributions to Geology, The University of Wyoming, Special Paper 4: 1–104.
Middleton, M.D. 1982. A new species and additional material of Catopsalis (Mammalia, Multituberculata) from the Western Interior of North America. Journal of Paleontology 56: 1197–1206.
Musser, A.M. 2005. Investigations into the Evolution of Australian Mammals with a Focus on Monotremata. 291 pp. Ph.D. Dissertation, University of New South Wales, Sydney. Crossref
Musser, A.M., Lamanna, M.C., Martinelli, A.G., Salisbury, S.W., Ahyong, S., and Jones, R. 2019. The first non-mammalian cynodonts from Australia and the unusual nature of Australian Cretaceous continental tetrapod faunas. In: Society of Vertebrate Paleontology Annual Meeting, Program and Abstracts, 157. Society of Vertebrate Paleontology, Brisbane.
O’Connor, P.M., Krause, D.W., Stevens, N.J., Groenke, J.R., MacPhee, R.D.E., Kalthoff, D.C., and Roberts, E.M. 2019. A new mammal from the Turonian–Campanian (Upper Cretaceous) Galula Formation, southwestern Tanzania. Acta Palaeontologica Polonica 64: 65–84. Crossref
Parmar, V., Prasad, G.V.R., and Kumar, D. 2013. The first multituberculate mammal from India. Naturwissenschaften 100: 515–523. Crossref
Pascual, R. and Ortiz-Jaureguizar, E. 2007. The Gondwanan and South American episodes: two major and unrelated moments in the history of the South American mammals. Journal of Mammalian Evolution 14: 75–137. Crossref
Pascual, R., Goin, F.J., Krause, D.W., Ortiz-Jaureguizar, E., and Carlini, A.A. 1999. The first gnathic remains of Sudamerica: implications for gondwanathere relationships. Journal of Vertebrate Paleontology 19: 373–382. Crossref
Poropat, S.F., Martin, S.K., Tosolini, A.-M.P., Wagstaff, B.E., Bean, L.B., Kear, B.P., Vickers-Rich, P., and Rich, T. 2018. Early Cretaceous polar biotas of Victoria, southern Australia—an overview of research to date. Alcheringa 42: 158–230. Crossref
Rich, T.H., Hopson, J.A., Gill, P.G., Trusler, P., Rogers-Davidson, S., Morton, S., Cifelli, R.L., Pickering, D., Kool, L., Siu, K., Burgmann, F.A., Senden, T., Evans, A.R., Wagstaff, B.E., Seegets-Villiers, D., Corfe, I.J., Flannery, T.F., Walker, K., Musser, A.M., Archer, M., Pian, R., and Vickers-Rich, P. 2016. The mandible and dentition of the Early Cretaceous monotreme Teinolophos trusleri. Alcheringa 40: 475–501. Crossref
Rich, T.H., Vickers-Rich, P., Constantine, A., Flannery, T.F., Kool, L., and van Klaveren, N. 1997. A tribosphenic mammal from the Mesozoic of Australia. Science 278: 1438–1442. Crossref
Rich, T.H., Vickers-Rich, P., Flannery, T.F., Kear, B.P., Cantrill, D., Komarower, P., Kool, L., Pickering, D., Trusler, P., Morton, S., van Klaveren, N. and Fitzgerald, E.M.G. 2009. An Australian multituberculate and its palaeobiogeographic implications. Acta Paleontologica Polonica 54: 1–5. Crossref
Ride, W.D.L. 1957. The affinities of Plagiaulax (Multituberculata). Proceedings of the Zoological Society of London 128: 397–402. Crossref
Rigby, J.K., Jr. 1980. Swain Quarry of the Fort Union Formation, middle Paleocene (Torrejonian), Carbon County, Wyoming: geologic setting and mammalian fauna. Evolutionary Monographs 3: 1–179.
Rougier, G.W., Chornogubsky, L., Casadio, S., Arango, N.P., and Giallombardo, A. 2009. Mammals from the Allen Formation, Late Cretaceous, Argentina. Cretaceous Research 30: 223–238. Crossref
Rougier, G.W., Martinelli, A.G., and Forasiepi, A.M. 2021. Mesozoic Mammals from South America and Their Forerunners. 388 pp. Springer, Cham. Crossref
Rougier, G.W., Sheth, A.S., Spurlin, B.K., Bolortsetseg, M., and Novacek M.J. 2016. Craniodental anatomy of a new Late Cretaceous multituberculate mammal from Udan Sayr, Mongolia. Palaeontologia Polonica 67: 197–248.
Sahni, A. 1972. The vertebrate fauna of the Judith River Formation, Montana. Bulletin of the American Museum of Natural History 147: 321–412.
Schumaker, K.K. and Kihm, A.J. 2006. Multituberculates from the Medicine Pole Hills local fauna (Chadronian) of Bowman County, North Dakota. Paludicola 6: 9–21.
Scotese, C.R. 2021. An atlas of Phanerozoic paleogeographic maps: the seas come in and the seas go out. Annual Review of Earth and Planetary Sciences 49: 679–728. Crossref
Scott, C.S. 2005. New neoplagiaulacid multituberculates (Mammalia: Allotheria) from the Paleocene of Alberta, Canada. Journal of Paleontology 79: 1189–1213. Crossref
Scott, C.S. and Krause, D.W. 2006. Multituberculates (Mammalia, Allotheria) from the earliest Tiffanian (late Paleocene) Douglass Quarry, eastern Crazy Mountains Basin, Montana. Contributions from the Museum of Paleontology, The University of Michigan 31 (10): 211–243.
Scott, C.S., Weil, A., and Theodor, J.M. 2018. A new, diminutive species of Catopsalis (Mammalia, Multituberculata, Taeniolabidoidea) from the early Paleocene of southwestern Alberta, Canada. Journal of Paleontology 92: 896–910. Crossref
Sereno, P.C. 2006. Shoulder girdle and forelimb in multituberculates: evolution of parasagittal forelimb posture in mammals. In: M.T. Carrano, T.J. Gaudin, R.W. Blob, and J.R. Wible (eds.), Amniote Paleobiology: Perspectives on the Evolution of Mammals, Birds, and Reptiles, 315–366. University of Chicago Press, Chicago.
Sigogneau-Russell, D. 1991. First evidence of Multituberculata (Mammalia) in the Mesozoic of Africa. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 1991: 119–125. Crossref
Simmons, N.B. 1987. A revision of Taeniolabis (Mammalia: Multituberculata), with a new species from the Puercan of eastern Montana. Journal of Paleontology 61: 794–808. Crossref
Simmons, N.B. 1993. Phylogeny of Multituberculata. In: F.S. Szalay, M.J. Novacek, and M.C. McKenna (eds.), Mammal Phylogeny: Mesozoic Differentiation, Multituberculates, Monotremes, Early Therians, and Marsupials, 146–164. Springer-Verlag, New York.
Simpson, G.G. 1928. A Catalogue of the Mesozoic Mammalia in the Geological Department of the British Museum. 215 pp. British Museum (Natural History), London.
Sloan, R.E. and Van Valen, L. 1965. Cretaceous mammals from Montana. Science 148: 220–227. Crossref
Smith, T., Codrea, V.A., Devillet, G., and Soloman, A.A. 2021. A new mammal skull from the Late Cretaceous of Romania and phylogenetic affinities of kogaionid multituberculates. Journal of Mammalian Evolution [published online, https://doi.org/10.1007/s10914-021-09564-7]. Crossref
Sulej, T., Krzesiński, G., Tałanda, M., Wolniewicz, A.S., Błażejowski, B., Bonde, N., Gutowski, P., Sienkiewicz, M., and Niedźwiedzki, G. 2020. The earliest-known mammaliaform fossil from Greenland sheds light on origin of mammals. Proceedings of the National Academy of Sciences of the United States of America 117: 26861–26867. Crossref
Vandenberg, A.H.M., Cayley, R.A., Willman, C.E., Morand, V.J., Seymon, A.R., Osborne, C.R., Taylor, D.H., Haydon, S.J., Mclean, M., Quinn, C., Jackson, P., and Sandford, A.C. 2006. Walhalla-Woods Point-Tallangallook special map area. Geological Survey of Victoria Report 127: 1–448.
Wagstaff, B.E., Gallagher, S.J., Hall, W.M., Korasidis, V.A., Rich, T.H., Seegets-Villiers, D.E., and Vickers-Rich, P.A. 2020. Palynological-age determination of Early Cretaceous vertebrate-bearing beds along the south Victorian coast of Australia, with implications for the spore-pollen biostratigraphy of the region. Alcheringa: An Australasian Journal of Palaeontology 44: 460–474. Crossref
Wang, H., Meng, J., and Wang, Y. 2019. Cretaceous fossil reveals a new pattern in mammalian middle ear evolution. Nature 576: 102–105. Crossref
Wang, J., Wible, J.R., Guo, B., Shelley, S.L., Hu, H., and Bi, S. 2021. A monotreme-like auditory apparatus in a Middle Jurassic haramiyidan. Nature 590: 279–283. Crossref
Weaver, L.N., Varricchio, D.J., Sargis, E.J., Chen, M., Freimuth, W.J., and Wilson Mantilla, G.P. 2020. Early mammalian social behaviour revealed by multituberculates from a dinosaur nesting site. Nature Ecology & Evolution 5: 32–37. Crossref
Weaver, L.N., Wilson, G.P., Krumenacker, L.J., McLaughlin, K., Moore, J.R., and Varricchio, D.L. 2019. New multituberculate mammals from the mid-Cretaceous (lower Cenomanian) Wayan Formation of southeastern Idaho and implications for the early evolution of Cimolodonta. Journal of Vertebrate Paleontology 39: e1604532. Crossref
Weil, A. and Krause, D.W. 2008. Multituberculata (Chapter 2). In: C.M. Janis, G.F. Gunnell, and M.D. Uhen (eds.), Evolution of Tertiary Mammals of North America. Volume 2: Small Mammals, Xenarthrans, and Marine Mammals, 19–38. Cambridge University Press, Cambridge. Crossref
Wible, J.R., Shelley, S.L., and Bi, S. 2019. New genus and species of djadochtatheriid multituberculate (Allotheria, Mammalia) from the Upper Cretaceous Bayan Mandahu Formation of Inner Mongolia. Annals of Carnegie Museum 85 (4): 285–327. Crossref
Williamson, T.E., Brusatte, S.L., Secord, R., and Shelley, S. 2016. A new taeniolabidoid multituberculates (Mammalia) from the middle Puercan of the Nacimiento Formation, New Mexico, and a revision of taeniolabidoid systematics and phylogeny. Zoological Journal of the Linnean Society 177: 183–208. Crossref
Wilson, G.P., Dechesne, M., and Anderson, I.R. 2010. New latest Cretaceous mammals from northeastern Colorado with biochronologic and biogeographic implications. Journal of Vertebrate Paleontology 30: 499–520. Crossref
Xu, L., Zhang, X., Pu, H., Jia, S., Zhang, J., Lü, J., and Meng, J. 2015. Largest known Mesozoic multituberculate from Eurasia and implications for multituberculate evolution and biology. Scientific Reports 5: 14950. Crossref
Yuan, C.-X., Ji, Q., Meng, Q.-J., Tabrum, A.R., and Luo, Z.-X. 2013. Earliest evolution of multituberculate mammals revealed by a new Jurassic fossil. Science 241: 779–783. Crossref
Zheng, X., Bi, S., Wang, X., and Meng, J. 2013. A new arboreal haramiyid shows the diversity of crown mammals in the Jurassic period. Nature 500: 199–202. Crossref
Zhou, C.-F., Wu, S., Martin, T., and Luo, Z.-X. 2013. A Jurassic mammaliaform and the earliest mammalian evolutionary adaptations. Nature 500: 163–167. Crossref
Acta Palaeontol. Pol. 67 (1): 115–134, 2022
https://doi.org/10.4202/app.00924.2021