

New materials of multicellular algae from the earliest Cambrian Kuanchuanpu biota in South China
QING-QIN TANG, YA-JUAN ZHENG, SONG QIN, YIN-CHU WANG, XIAO-GUANG YANG, XING WANG, JIE SUN, KENTARO UESUGI, TSUYOSHI KOMIYA, and JIAN HAN
Tang, Q.-Q., Zheng, Y.-J., Qin, S.,Wang, Y.-C., Yang, X.-G., Wang, X., Sun, J., Uesugi, K., Komiya, T., and Han, J. 2022. New materials of multicellular algae from the earliest Cambrian Kuanchuanpu biota in South China. Acta Palaeontologica Polonica 67 (2): 317–327.
The Kuanchuanpu biota (ca. 535 Ma) from the basal Cambrian in South China yields various phosphatized, three-dimensionally preserved microscopic fossils, such as animal embryos, cyanobacteria, and algae. This provides an exceptional window for investigating the rapid divergent evolution of animal phyla in the early Cambrian ocean. However, the fossil eukaryotic algae are poorly documented and are not properly incorporated into the conceptual food web of the Cambrian ocean ecosystem. In this study, we report a new taxon of calathiform alga, Calathophycus irregulatus Tang gen. et sp. nov., and an indeterminate alga, from the Kuanchuanpu biota. Calathophycus irregulatus can be distinguished from other reported phosphatized multicellular algal fossils from the Kuanchuanpu biota and the Ediacaran Weng’an biota (Guizhou Province, China), based on the external morphology and features of cell clump aggregation. The observation of C. irregulatus and the indeterminate alga indicates a certain diversity of algae at the beginning of the Cambrian. Concerning the regularly or irregularly shaped multicellular algal fossils from the Kuanchuanpu Formation, co-occurring spherical fossils, previously interpreted as “cleavage stage” of animal embryos, should be reconsidered in affinity.
Key words: Algal fossils, bowl-shaped fossils, early Cambrian, Kuanchuanpu biota.
Qing-Qin Tang [tangqingqin@stumail.nwu.edu.cn], Xiao-guang Yang [lqzy0301@gmail.com], Jie Sun [shuibaobei@vip.qq.com], and Jian Han [elihanj@nwu.edu.cn], Shaanxi Key Laboratory of Early Life and Environments (SKLELE), State Key Laboratory of Continental Dynamics (SKLCD), Department of Geology, Northwest University, Xi’an 710069, China.
Ya-Juan Zheng [yajuanzheng@126.com], School of Earth Science and Resources, Key Laboratory of Western China’s Mineral Resources and Geological Engineering, Ministry of Education, Chang’an University, Xi’an 710054, China.
Song Qin [sqin@yic.ac.cn] and Yin-Chu Wang [ycwang@yic.ac.cn], Yantai Institute of Coastal Zone Research, Chinese Academy of Science, Yantai, 264003, China.
Xing Wang [wx5432813@126.com], Qingdao Institute of Marine Geology, China Geological Survey, Qingdao 266071, China.
Kentaro Uesugi [ueken@spring8.or.jp], Japan Synchrotron Radiation Research Institute (JASRI), Hyogo, Japan.
Tsuyoshi Komiya [komiya@ea.c.u-tokyo.ac.jp], Department of Earth Science and Astronomy, Graduate School of Arts and Sciences, The University of Tokyo, Tokyo, Japan.
Received 22 September 2021, accepted 28 January 2022, available online 14 June 2022.
Copyright © 2022 Q.-Q. Tang et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Algae are one of the important primary producers in the Earth’s ecosystem and the influence of their evolution on early Earth has been extensively studied. Eukaryotic multicellular algae appeared, based on the fossil record, before the Mesoproterozoic, when cyanobacteria were still dominant in the ocean (Butterfield 2000; Bengtson et al. 2017; Tang et al. 2020). Biomarker evidence suggests that the ecological rise of algae occurred between 659 and 645 Ma, following which the algae broke the incumbency of phototrophic bacteria as the principal marine primary producers (Brocks et al. 2017).
Algal fossils, exceptionally preserved in the Ediacaran strata of South China, provide solid evidence for macroalgae reaching unprecedented morphological complexity and taxonomic diversity during the Ediacaran period (Xiao 2013). These fossils are grouped into two major taphonomic modes, based on their chemical compositions (carbon or phosphate). A remarkable amount of macroscopic algae is preserved as carbonaceous compressions in the Lantian biota (Yuan et al. 2013, 2016; Wan et al. 2016), Miaohe biota (Chen et al. 1994; Ding et al. 1996; Xiao et al. 1998a, 2002; Yuan et al. 2002; Ye et al. 2017), and Wenghui biota (Zhao et al. 2004; Wang et al. 2016). The phosphatized microalgae with cellular structure are represented in the Weng’an biota (Zhang 1989; Zhang and Yuan 1992; Xiao et al. 1998b, 2004), in the Ediacaran Doushantuo Formation in Guizhou Province.
Following the Ediacaran, the characteristics of the Cambrian marine ecosystem changed completely; the modern-type ocean food webs driven by prey-predator arm race were gradually established (Vannier et al. 2007). Eukaryotic algae, as vital oxygen and nutrients providers to various animals, however, have been rarely reported from the lower Cambrian (Guo et al. 2008, 2010; Hou et al. 2017; Fu et al. 2019; Wang et al. 2022).
The Kuanchuanpu biota, from the lowermost Cambrian in South China, comprises a variety of three-dimensional preserved phosphatized microscopic organisms. With the exception of the various hard parts (i.e., tubes, sclerites, or shells) of Small Shelly Fossils (SSFs) (Qian 1977; Steiner et al. 2004), the Kuanchuanpu biota consists of soft-bodied fossils, such as scalidophoran worms (Liu et al. 2014b, 2019; Zhang et al. 2015; Wang et al. 2019, 2020) and animal embryos mostly being assigned to cnidarians (Bengtson and Yue 1997; Dong et al. 2013; Han et al. 2013; Wang et al. 2017). In addition, various cyanobacteria (Ding 1986; Yin 1987; Qian et al. 2007), characterized by colonial coccoids (Cui et al. 2020) or microscopic, directional, sessile bundles (Liu et al. 2014a), were reported from the same biota. Therefore, the Kuanchuanpu biota provides possibilities to investigate the interactions among the major components of a shallow, euphotic tidal zone marine ecosystem in the early Cambrian. However, the eukaryotic algae in the Kuanchuanpu biota, which are the primary producers of the local marine ecosystem, are poorly understood in terms of palaeontology, evolution, and autoecology. A few investigations have revealed column-like algal fossils (Liu et al. 2009), sub-spherical fossils with tetrad cell structure (Zheng et al. 2017), and membrane-bearing multicellular aggregate fossils (Zheng et al. 2021). The membrane-bearing aggregate fossils show characteristics consistent with those of multicellular algae in the Weng’an biota in terms of morphology and organization. However, assigning a formal taxonomy to these fossils is difficult, owing to their wide scope of morphological variation (Zheng et al. 2021).
Here we describe two new taxa (Calathophycus irregulatus Tang gen. et sp. nov. and an indeterminate alga) of eukaryotic algae from the Kuanchuanpu Biota. This contributes to the diversity of algae in the beginning of the Cambrian. Unlike the previously reported forms, these two taxa exhibit relatively definite shape and patterns of cellular organizations.
Institutional abbreviations.—SKLELE, Shaanxi Key Laboratory of Early Life and Environments, Xi’an, China; SKLCD, State Key Laboratory of Continental Dynamics, Xi’an, China; JASRI, Japan Synchrotron Radiation Research Institute, Hyogo, Japan.
Other abbreviations.—EDS, energy dispersive X-ray spectrometer; Micro-CT, micro-computed tomography; SRXTM, synchrotron radiation X-ray tomographic microscopy; SSFs, Small Shelly Fossils; s.d., standard deviation; x , X-bar, mean value.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in Plant Fossil Names Registry (PFNR): urn:lsid:plantfossilnames.org:act:2870 and urn:lsid:plantfossilnames.org:act:2871.
Geological setting
The specimens were collected from the Shizhonggou Section in the lower Cambrian Kuanchuanpu Formation in Ningqiang County, southern Shaanxi, China (Fig. 1). This region comprises a series of shelf carbonate deposits of the Yangtze Platform, which is in turn primarily composed of dark grey or black phosphorus limestone interbedded with thin-layered cherts. In this section, the Kuanchuanpu Formation unconformably overlies the gray-white dolomite at the top of the Ediacaran Dengying Formation and is unconformably overlied by the siliciclastic lower Cambrian Guojiaba Formation composed of dark-gray silty shale. The Kuanchuanpu Formation is approximately 60–70 m thick and it is rich in SSF and animal embryos. The fossil assemblage is chronologically equivalent to the first assemblage zone of SSF (i.e., the Anabarites trisulcatus–Protohertzina anabarica Zone) of the Fortunian Stage (Terreneuvian Series) (see Steiner et al. 2004: fig. 2).
In the cherts of the Kuanchuanpu Formation, condensed mat fabrics with quartz grains indicates for microbial mat layers (see Cui et al. 2020: fig. 13a). Tabular cross beddings are common in the phosphorus limestones (SOM: fig. 1, Supplementary Online Material available at http://app.pan.pl/SOM/app67-Tang_etal_SOM.pdf). Taken together with evidence of sorting and transporting of SSFs (Steiner et al. 2007) and cyanobacteria (Ding 1986; Yin 1987; Qian et al. 2007), it indicates a shallow marine depositional setting in a fluctuating tidal environment above wave base for the Kuanchuanpu biota (see Yin et al. 1999).
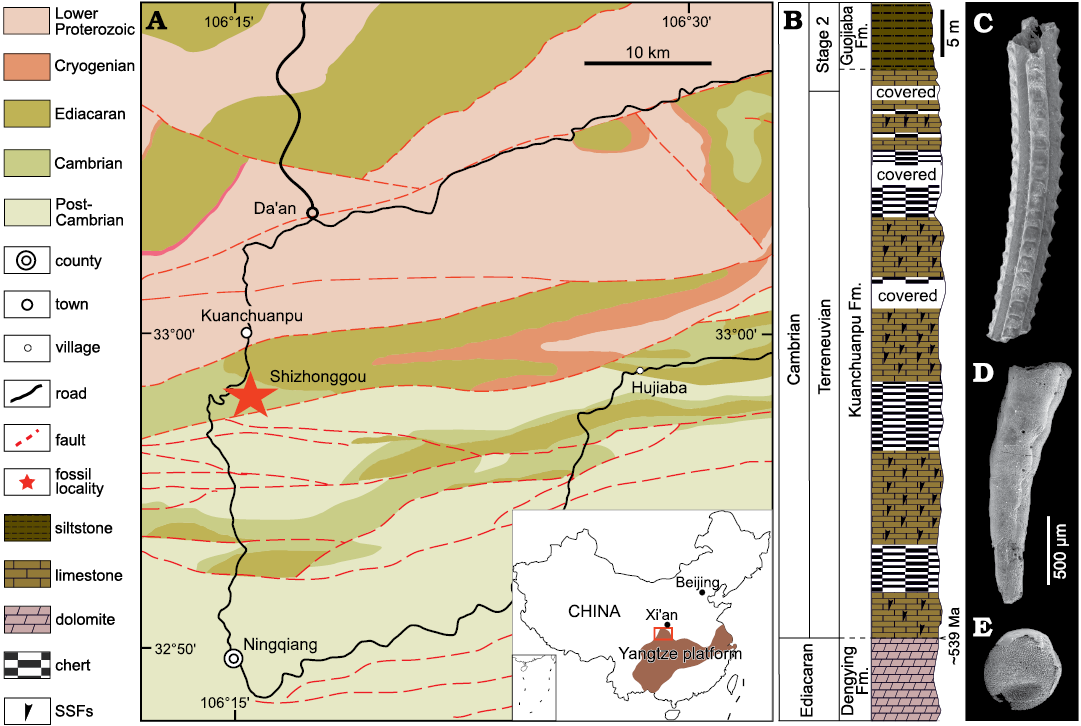
Fig. 1. Geological map (A; modified from Han et al. 2016), stratigraphic column (B), and typical fossils (C–E) from Cambrian the Shizhonggou section in Ningqiang County, Shaanxi Province, China. C. Carinachitid Carinachites spinatus Qian, 1977. D. Anabaritid Anabarites trisulcatus Missarzhevsky in Voronova and Missarzhevsky, 1969. E. Olivooid Olivooides multisulcatus Qian, 1977.
Material and methods
Fifty-six specimens were obtained from the Shizhonggou section of the Kuanchuanpu Formation. Approximately 5 tons of rock samples were macerated in 8–10% acetic acid solution. Fossil samples were selected manually from the insoluble residuals using standard binocular microscopy. The specimens showing well-preserved cellular structure were examined using an FEI Quanta 400F scanning electron microscope (SEM), equipped with an Oxford Instruments energy dispersive X-ray spectrometer (EDS), micro-computed tomography (Micro-CT) (Xradia520 Versa) at State Key Laboratory of Continental Dynamics (SKLCD), and using synchrotron radiation X-ray tomographic microscopy (SRXTM) at SPring-8 (Hyogo, Japan) (Uesugi et al. 2012). All Micro-CT data were processed with VG Studio 3.2 Max for tomographic analysis and 3D visualization. Fossil size measurements and statistical analysis were performed using Image J and Origin 2019, respectively. All analyzed specimens are deposited in the Early Life Institute, Northwest University, Xi’an, China.
Systematic palaeontology
Phylum, class, order, and family uncertain
Genus Calathophycus Tang nov.
PFNR: urn:lsid:plantfossilnames.org:act:2870
Type species: Calathophycus irregulatus Tang sp. nov.; by monotypy, see below.
Etymology: From Latin calathus, bowl; with reference to the calathiform shape of the fossils; and Latin phycus, sea weed. The name is assigned based on its morphology and presumed affinity. Gender feminine.
Diagnosis.—As for the type species by monotypy.
Calathophycus irregulatus Tang sp. nov.
Figs. 2, 3, 5; SOM 1, 2.
PFNR: urn:lsid:plantfossilnames.org:act:2871
Etymology: From Latin irregulatus, irregular; with reference to the irregular morphology of the fossils.
Holotype: A nearly complete specimen preserving cells and cell clumps, ELISN72-52 (Fig. 3A1, A2; SOM 1).
Type locality: Shizhonggou section, Ningqiang County, southern Shaanxi, China.
Type horizon: Kuanchuanpu Formation, Fortunian, Terreneuvian, Cambrian.
Material.—Type material and fifty-three specimens. All from the type locality and horizon.
Diagnosis.—Calathiform thallus, 0.45–2.12 mm in length, with a deep or shallow central concavity. Thallus enveloped by a thin, tightly attached membrane; comprised of multiple cell clumps with nearly uniform cells. Cells are irregularly arranged, polygon-shaped, 20–35 μm in length.
Description.—The specimens of Calathophycus irregulatus Tang gen. et sp. nov. are superficially calathiform or disc-shaped with a shallow (Fig. 2) or deep (Fig. 3) central concavity, and display a cauliflower-like, irregular bulging surface (Figs. 2, 3; SOM 1, 2). The specimens are, in length and width, respectively, 0.58–2.12 mm (x = 1.14 mm, s.d. = 0.35, n = 54) and 0.45–1.7 mm (x = 0.91 mm, s.d. = 0.30, n = 54; Fig. 4). The central concavities are 0.13–1.23 mm in length (x = 0.64 mm, s.d. = 0.28, n = 54) and 0.04–1.11 mm in width (x = 0.44 mm, s.d. = 0.23, n = 54; Fig. 4). Externally, these specimens are wrapped by a thin, close-fitting membrane (1–2 μm), which mostly falls off the surface; it is barely visible in the concave area of poorly preserved specimens (Figs. 2B–D and 5B6, equilateral arrowheads). EDS spectrums show that the specimens are enriched in calcium and phosphorus (SOM: fig. 2), suggesting an apatite composition.
The specimens consist of several cell clumps (100–500 μm) (Figs. 2, 3, 5, white arrows), which are loosely or tightly packed. These cell clumps contribute to the cauliflower-like external surface and the gross thickness of the specimens. Externally, the cell clumps are much evident at the peripheral margin of the thallus in larger specimens, displaying a lobed appearance (Figs. 2, 3). The inter-clump gaps are wider and easier to identify in some areas (Figs. 2, 3, 5A4, B6, B7, black arrows). X-ray tomography revealed that some larger cell clumps contain several smaller cell clumps (Fig. 5A5, B7). These cell clumps, which are honeycomb-like in virtual sections, are composed of tightly packed polygonal cells (Figs. 2E1, E2, 3A1, D, 5), which are 20–50 μm long and exhibit distinct gaps in-between (Fig. 5A4, B5, B6, white arrowheads). In addition, there are clear gaps between the cells and clumps (Fig. 5, black arrowheads), indicating the external membrane of cell clumps. However, cell structures are not well preserved in some cell clumps (Figs. 2C, 3A2).
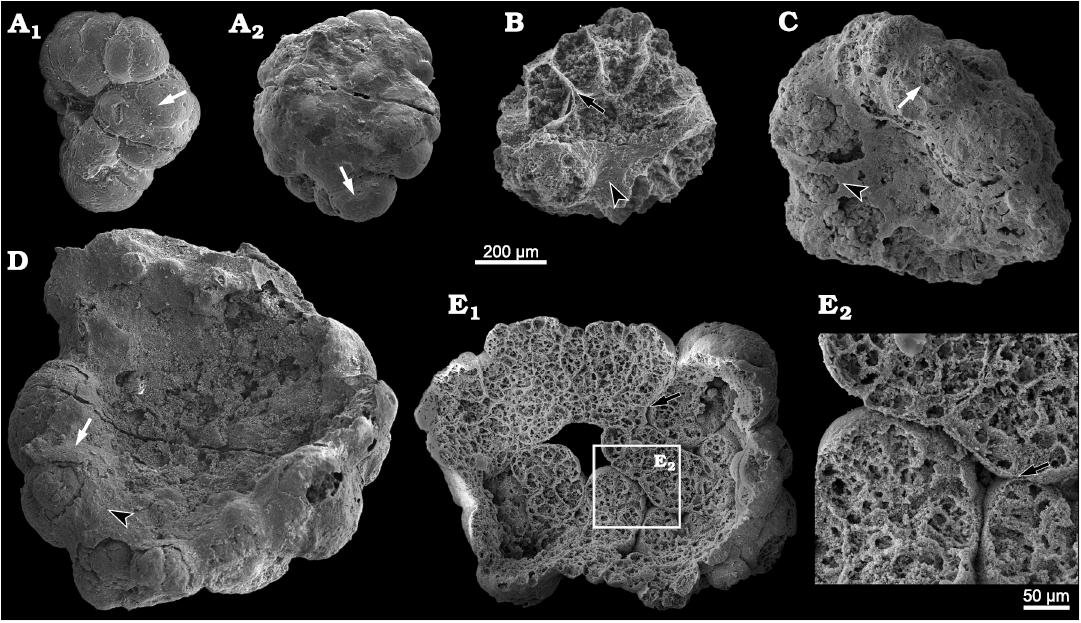
Fig. 2. SEM images of problematic alga Calathophycus irregulatus Tang gen. et sp. nov. with a shallow central concavity; Kuanchuanpu Formation, Fortunian, Terreneuvian, Cambrian in Ningqiang County, Shaanxi Province, China. A. ELISN80-91; A1, front view; A2, back view. B–D. ELISN85-39, ELISN163-455, ELISN164-15, respectively. E. ELISN164-271; E1, front view; E2, close-up view. Cell clumps, white arrows (A, C, D); gaps between cell clumps, black arrows (B, E); external membrane, equilateral arrowheads (B, C, D). The specimens are selected and arranged in order of size so as to indicate a possible sequence of growth and development.
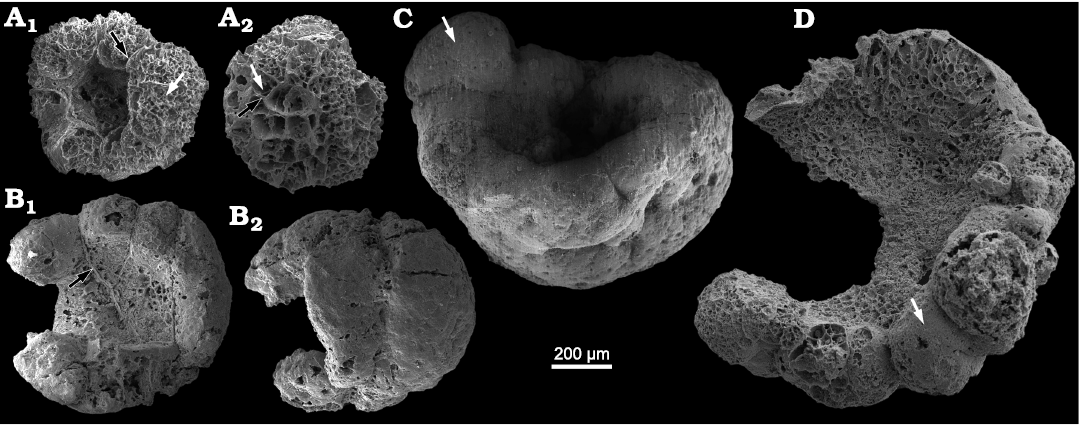
Fig. 3. SEM images of problematic alga Calathophycus irregulatus Tang gen. et sp. nov. with a deep central concavity; Kuanchuanpu Formation, Fortunian, Terreneuvian, Cambrian in Ningqiang County, Shaanxi Province, China. A. ELISN72-52, holotype; A1, front view; A2, back view. B. ELISN163-729; B1, front view; B2, back view. C. ELISN133-60. D. ELISN163-339. Cell clumps, white arrows (A, C, D); gaps between cell clumps, black arrows (A, B1). Fossils are arranged in order of size, suggesting a possible sequence of growth and development.
Remarks.—In Calathophycus irregulatus Tang gen. et sp. nov., membrane-bearing cell clumps are wrapped by a thin membrane. It is superficially similar to the nested individual and common colloid sheaths of the cyanobacteria. Morphologically, however, cyanobacteria are generally colonies of hemispherical to spherical shaped individuals, or branched, unbranched filaments, tubes and trichome, which can be seen both in living species (Lee 2008) and fossils, such as Myxococcoides sp. (Cui et al. 2020), Gloeodiniopsis lamellose Knoll and Golubic, 1979, Palaeosiphonella sp. (Cui et al. 2020), and Siphonophycus typium Hermann, 1974 (Butterfield et al. 1994). It is quite different from the polygonal cells and calathiform shape of C. irregulatus. Besides, the size of individuals of the coccoidal fossils mostly ranges 15–30 μm, smaller than cells in C. irregulatus; although a few vesicles of cyanobacteria reach about 60 μm in diameter, they consist of 2–6 cells and occur as dyads, triads, tetrads, and hexads (Cui et al. 2020). Taken together with the differences in size, morphology of cells and cell colonies, it is reasonable to infer that C. irregulatus is not cyanobacterium.
The external morphology of C. irregulatus is quite similar to the multicellular aggregate algae fossils from the Kuanchuanpu biota (Zheng et al. 2021), which exhibit some similarities with C. irregulatus: (i) Both possess an external membrane (Figs. 2, 3; see Zheng et al. 2021: fig. 2) and are composed of several cell clumps. (ii) The maximal length and maximal width of the two kinds of fossils are 0.2–2.5 mm. However, the following differences exist: (i) The multicellular aggregate fossils are irregularly spherical or nearly spherical in shape, and devoid of a central concavity (see Zheng et al. 2021). (ii) The multicellular aggregate fossils exhibit a distinct two-layered cell differentiation, with spherical cells with a diameter of about 5 μm inside, and polygonal cells with a length of about 50 μm and a width of about 15 μm outside (see Zheng et al. 2021: fig. 5). (iii) The hypothesized life cycle of multicellular algae in Zheng et al. (2021) shows that at middle of expansion stage, some outer cells develop as bump structures by proliferation and do not start to differentiate (see Zheng et al. 2021: fig. 9); its amorphous external morphology formed by randomly allometric growth is apparently different from the fixed and specific shape of C. irregulatus, although the cell configuration at this stage is similar to that of C. irregulatus. Therefore, they are not exactly equivalent to C. irregulatus.
Calathophycus irregulatus Tang gen. et sp. nov. resembles the invaginated, gastrula-like algae (Yin and Zhu 2011) from the Weng’an biota in central concavity and morphological characteristic. However, the invaginated algae (Yin and Zhu 2011) exhibit small outer cells and Paramecia-like inner cells, contrasting with the nearly uniform cells in C. irregulatus. In addition, the difference in cell morphology and arrangement (i.e., pseudoparenchymatous) can distinguish C. irregulatus from Weng’an algae, such as Thallophyca ramosa Zhang, 1989, Gremiphyca corymbiata Zhang, Yin, Xiao, and Knoll, 1998, Thallophycoides phloeatus Zhang and Yuan, 1992.
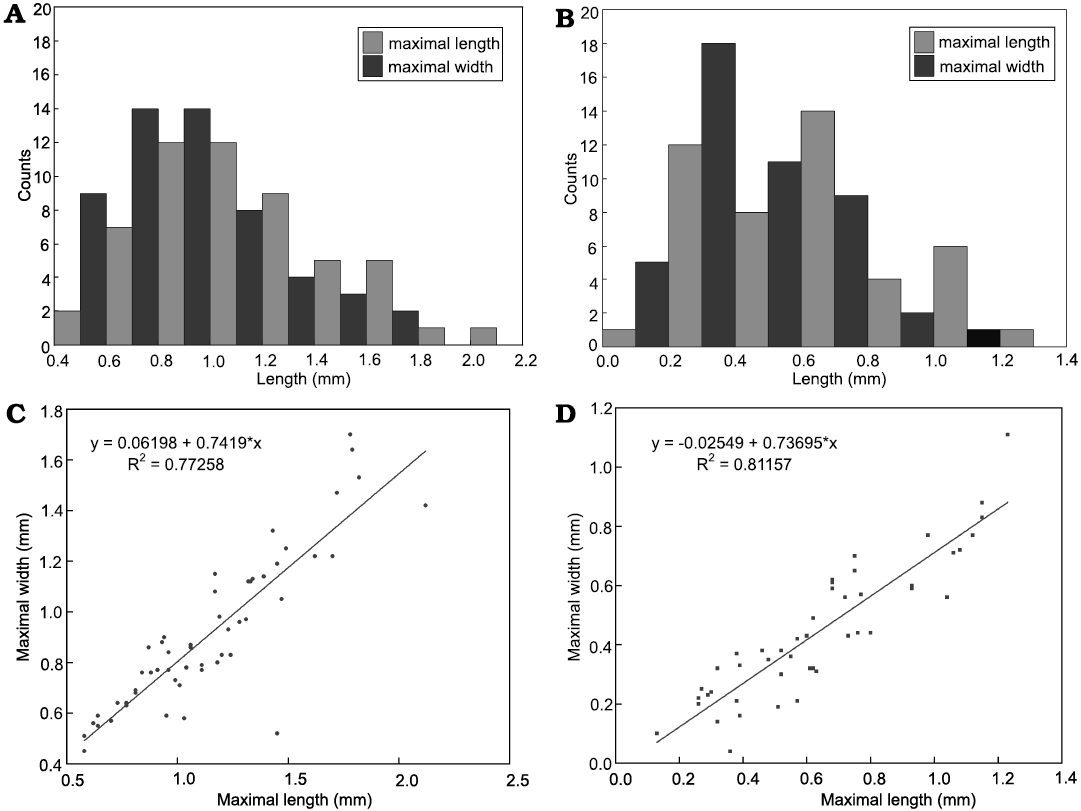
Fig. 4. Measurements of problematic alga Calathophycus irregulatus Tang gen. et sp. nov. and concave opening. A. Histogram of maximal length and width of bowl-shaped fossils. B. Histogram of maximal length and width of concave opening. C. Scatter diagram indicating a linear relationship between the maximal length and width of C. irregulatus. D. A linear relationship between the maximal length and width of the concave opening.
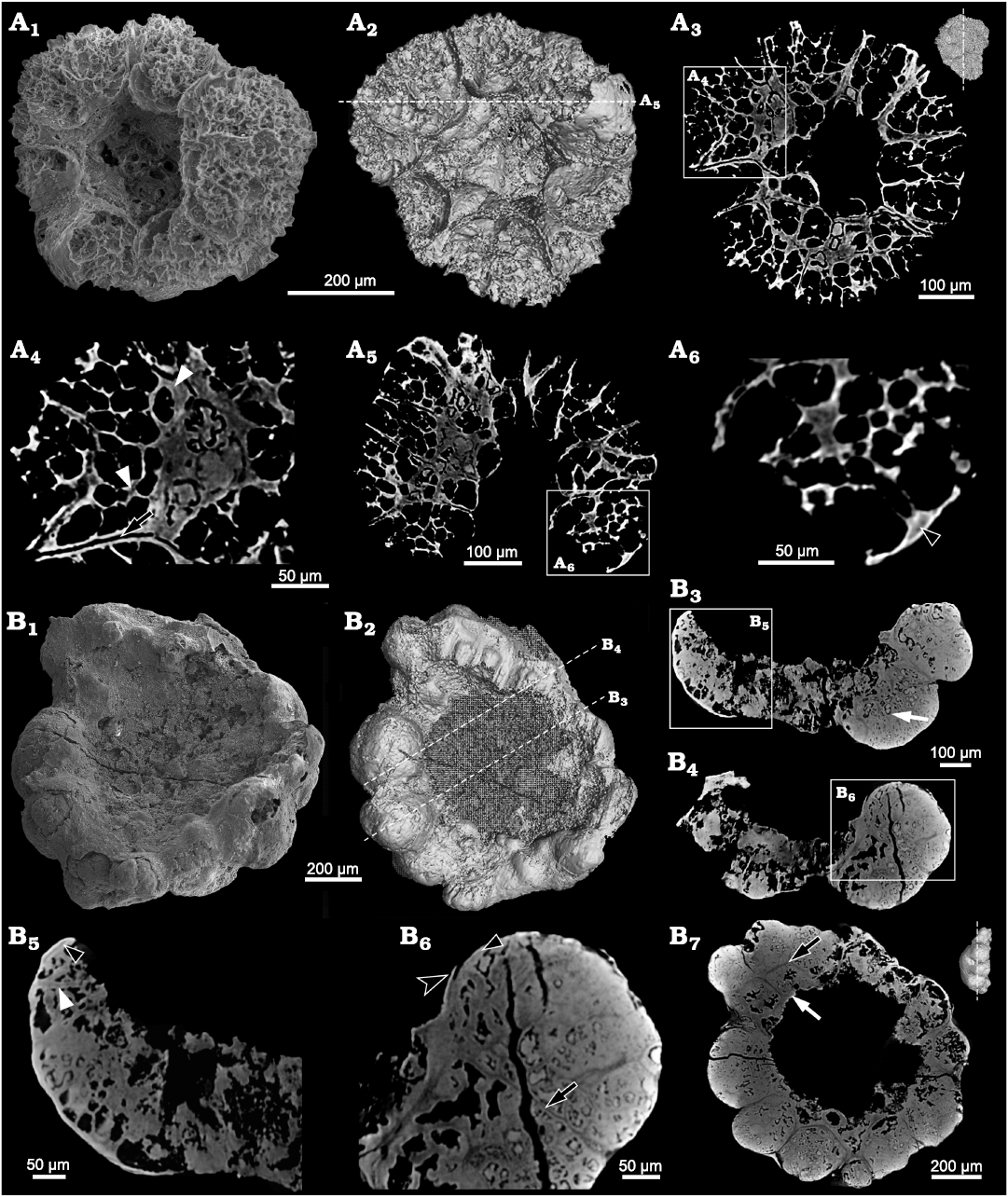
Fig. 5. Internal structure of problematic alga Calathophycus irregulatus Tang gen. et sp. nov. revealed by Micro-CT; Kuanchuanpu Formation, Fortunian, Terreneuvian, Cambrian in Ningqiang County, Shaanxi Province, China. A. ELISN72-52, holotype; A1, SEM image; A2, 3D rendering; A3, A5, virtual sections; A4, A6, close-up views. B. ELISN164-15; B1, SEM image; B2, 3D rendering; B3, B4, B7, virtual sections; B5, B6, close-up views. Gap between cell clumps, black arrows (A4, B6, B7); gaps between cells, white arrowhead (A4); interval between polygonal cells, white arrowhead (B5); interval between cells and clumps, black arrowheads (A6, B5, B6); cell clumps, white arrows (B3, B7); external membrane, equilateral arrowhead (B6).
Comparisons with the co-occurring multicellular algae (Zheng et al. 2021) and Weng’an algae indicated that C. irregulatus is likely a kind of eukaryotic multicellular algae. Morphologically, C. irregulatus is similar to modern species of Dictyosphaeria (class Ulvophyceae, order Cladophorales, family Siphonocladaceae), which are solid spheroid and in growth changing to hollow spheroid then breaking apically to form irregularly bowl shape (SOM: fig. 3; Alves et al. 2012). Species of Dictyosphaeria have rhizoids (SOM: fig. 3a) for sessile life, which are not found on C. irregulatus (Figs. 2A2, 3A2, B2). In addition, C. irregulatus (0.45–2.12 mm in shape and 20–35 μm in cell diameter), is much smaller than the species of Dictyosphaeria (several millimeters to centimeters in shape and 300–1000 μm in cell diameter) at different growth stages (Zeng and Zhang 1962; Chen and Zhou 1980; Alves et al. 2012). The calathiform shape and size range indicate that C. irregulatus can be inferred as a benthic alga, which may have been disturbed, broken into fragments, or transported frequently by waves before burial. Therefore, it cannot be ruled out that C. irregulatus is fragment of Dictyosphaeria-like algae. Nevertheless, it is always been highly speculative to correlate Ediacaran–Cambrian fossil algae to modern algae merely based on the shape and cell configurations, without any primary chemical composition. Therefore, continuing discovery and analysis could be helpful to refine our ideas about the biological affinity of C. irregulatus.
Stratigraphic and geographic range.—Type locality and horizon only.
Indeterminate alga
Fig. 6; SOM 3.
Material.—Two specimens (ELISN103-361 and ELISN111-78; 1.07 and 0.7 mm in maximal length, respectively) from from the Kuanchuanpu Formation, Fortunian, Terreneuvian, Cambrian in the Shizhonggou section, Ningqiang County, southern Shaanxi, China.
Description.—The available specimens exhibit a smooth surface and superficially an urn-shaped or cup-shaped profile. Unlike in Calahophycus irregulatus Tang gen. et sp. nov., only a few cell clumps (100–200 μm) are prominent at the urn margin (Fig. 6B2). SEM and SRXTM revealed that the cells are tightly packed and arranged in two different ways. Some of them are aggregated in cell clumps (Fig. 6B4, B6, B7; SOM 3), in which the cells near the central concavity are smaller (Fig. 6B4, B7). The other cells are irregularly arranged in two layers (Fig. 6A2, B2, B4–B8). The outer cells (50–100 μm high and about 20–35 μm in length and width) away from the central concavity are much bigger than the inner cells (20–35 μm), thus indicating a cell differentiation. The distinct gaps between polygonal cells indicate the separation of neighboring cell walls (Fig. 6A4, B2, B6). In addition, there are gaps between the polygonal cell walls and the external membrane of whole specimen (Fig. 6B2, B8).
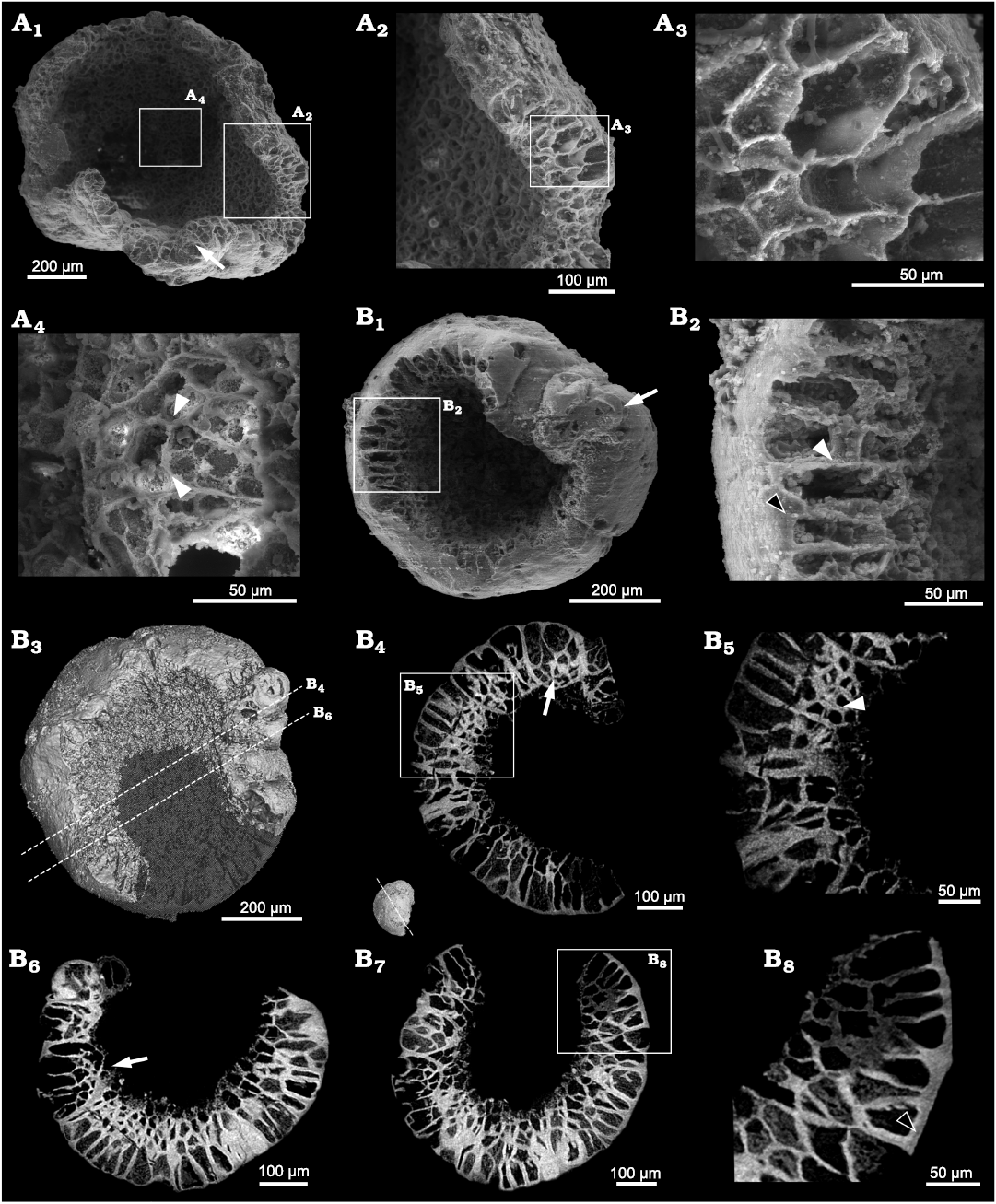
Fig. 6. SEM and SRXTM images of the indeterminate alga; Kuanchuanpu Formation, Fortunian, Terreneuvian, Cambrian in Ningqiang County, Shaanxi Province, China. A. ELISN103-361; A1, SEM image; A2, A3, A4, close-up views of polygonal cells. B. ELISN111-78; B1, SEM image; B3, 3D rendering; B4, B6, B7, virtual sections; B5, B8, close-up views of B4 and B7 (respectively). Intervals between cells, white arrowheads (A4, B2, B5); cell clumps, white arrows (B1, B4, B6); intervals between cell and membrane, black arrowheads (B2, B8).
Remarks.—Both Calathophycus irregulatus Tang gen. et sp. nov. and the indeterminate alga exhibit a central concavity; however, the indeterminate alga differs from the co-occurring C. irregulatus in terms of cell configuration. The cells of the indeterminate alga differentiate into irregular layers, some of which aggregate as cell clumps; these are not seen in C. irregulatus. There is another possibility that cells of C. irregulatus re-arranged to decrease the thickness and differentiate into irregular layers like the indeterminate alga; hence, these two types of fossils may belong to different stages of same species. However, it is debatable as the specimens of the indeterminate alga are much smaller than C. irregulatus. Therefore, more specimens are needed to clarify the nature of the indeterminate alga and its relationship with C. irregulatus.
Discussion
Many tiny spherical microfossils from the Kuanchuanpu Formation, which were previously treated as the cleavage stage of the life cycle of animal embryos (see Bengtson and Yue 1997: fig. 1A–D; Dong et al. 2013: fig. 1a), should be reassessed for their affinity. Firstly, none of them exhibits an envelope. Secondly, these cleavage fossils, much smaller than subsequent embryos with egg envelope, are inconsistent with the classic palintomic division. Thirdly, these fossils are quite similar to the spherical cell clumps hosted by the algal fossils (this study and Zheng et al. 2021) in the Kuanchuanpu biota in size, shape and cell configuration. Lastly, concerning the boundaries and gaps among spherical cell clumps, Calathophycus irregulatus Tang gen. et sp. nov. and the co-occurring multicellular algae (Zheng et al. 2021) may be fragmented into several clumps, which may develop into a new, independent individual, by some phytophagous invertebrates or water flows in a tidal setting from the Kuanchuanpu Formation (see Zheng et al. 2021: fig. 9). Therefore, further work is necessary to ascertain the reliable differences between these Cambrian animal embryos and algae.
Conclusions
The three-dimensional preservation of phosphatized microscopic fossils from the Kuanchuanpu Formation provides an exceptional window to investigate the cell configuration of organisms at a cell-level resolution; and therefore, it is critical for clarifying the affinity of organisms. Based on the external morphology and the cell organization, Calathophycus irregulatus Tang gen. et sp. nov. is more likely to be interpreted as an eukaryotic multicellular alga, rather than as animal gastrula or cyanobacterium. Although difficult to determine the biological affinity of C. irregulatus and the indeterminate alga in phylum level, this study sheds lights on the diversity of algae at the beginning of the Cambrian.
Authors’ contributions
JH and QS conceived the project. Q-QT, Y-JZ and JH found the specimens, interpreted the fossil material and wrote early drafts of the paper. XW, X-GY, Y-CW, and Q-QT prepared photographs. JS, KU, and TK undertook computerized tomography restoration. All authors discussed results, and developed observations and conclusion.
Acknowledgements
The authors thank Hu-Jun Gong, Juan Luo, and Na Liu (all SKLCD) for their assistance in both field and lab work. The authors thank Stefan Bengtson (Swedish Museum of Natural History, Stockholm, Sweden), Nicholas Butterfield (University of Cambridge, Cambridge, UK), and an anonymous reviewer for their helpful comments and suggestions. We thank authors of Alves et al. (2012) and the editors of Sitientibus Série Ciencias Biologicas for providing the figures of modern Dictyosphaeria. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB26000000), the Natural Science Foundation of China (Nos. 41902012, 41772010, 41621003, 41890844, 41720104002), 111 Project of the Ministry of Education of China (No. D17013, D163107), and the State Key Laboratory of Paleobiology and Stratigraphy (Nanjing Institute of Geology and Palaeontology, CAS No.203122, 203106).
References
Alves, A., Gestinari, L.M.S., and Moura, C. 2012. Flora da Bahia: Siphonocladaceae. Sitientibus Série Ciencias Biologicas 12: 167–177. Crossref
Bengtson, S. and Yue, Z. 1997. Fossilized metazoan embryos from the earliest Cambrian. Science 277: 1645–1648. Crossref
Bengtson, S., Sallstedt, T., Belivanova, V., and Whitehouse, M. 2017. Three-dimensional preservation of cellular and subcellular structures suggests 1.6 billion-year-old crown-group red algae. PLoS Biology 15: e2000735. Crossref
Brocks, J.J., Jarrett, A.J.M., Sirantoine, E., Hallmann, C., Hoshino, Y., and Liyanage, T. 2017. The rise of algae in Cryogenian oceans and the emergence of animals. Nature 548: 578–581. Crossref
Butterfield, N.J. 2000. Bangiomorpha pubescens n. gen., n. sp.: Implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology 26: 386–404. Crossref
Butterfield, N.J., Knoll, A.H., and Swett, K. 1994. Paleobiology of the Neoproterozoic Svanbergfjellet Formation, Spitsbergen. Lethaia 34: 1–84. Crossref
Chen, M., Xiao, Z., and Yuan, X. 1994. A new assemblage of megafossils—Miaohe biota from upper Sinian Doushantuo Formation Yangtze Gorges [in Chinese with English abstract]. Acta Palaeontologica Sinica 33: 391–403.
Chen, Z. and Zhou, Z. 1980. A new species of Dictyosphacria from Fujian Province [in Chinese with English abstract]. Journal of Fujian Normal University (Natural Science Edition) 1980: 93–98.
Cui, L., Liu, W., and Zhang, X. 2020. Phosphatized microbial fossils from the lowest Cambrian of South China and their ecological and environmental implications for the Kuanchuanpu biota. Precambrian Research 338: 105560. Crossref
Ding, L. 1986. On the occurrence of Obruchevella from the Formation in Early Cambrian of south Shaanxi and its stratigraphical significance [in Chinese with English abstract]. Journal of Xi’an College of Geology 8: 23–26.
Ding, L., Li, Y., Hu, X., Xiao, Y., Su, C., and Huang, J. 1996. Sinian Miaohe Biota [in Chinese]. 221 pp. Geological Publishing House, Beijing.
Dong, X., Cunningham, J.A., Bengtson, S., Thomas, C.W., Liu, J., Stampanoni, M., and Donoghue, P.C.J. 2013. Embryos, polyps and medusae of the Early Cambrian scyphozoan Olivooides. Proceedings of the Royal Society B: Biological Sciences 280: 20130071. Crossref
Fu, D., Tong, G., Dai, T., Liu, W., Yang, Y., Zhang, Y., Cui, L., Li, L., Yun, H., Wu, Y., Sun, A., Liu, C., Pei, W., Gaines, R.R., and Zhang, X. 2019. The Qingjiang biota—a Burgess Shale-type fossil Lagerstätte from the early Cambrian of South China. Science 363: 1338–1342. Crossref
Guo, J., Li, Y., Han, J., Zhang, X., Zhang, Z., Ou, Q., Liu, J., Shu, D., Maruyama, S., and Komiya, T. 2008. Fossil association from the Lower Cambrian Yanjiahe Formation in the Yangtze Gorges Area, Hubei, South China. Acta Geologica Sinica, English Edition 82: 1124–1132. Crossref
Guo, J., Li, Y., and Shu, D. 2010. Fossil macroscopic algae from the Yanjiahe Formation, Terreneuvian of the Three Gorges area, South China [in Chinese with English abstract]. Acta Palaeontologica Sinica 49: 336–342.
Han, J., Kubota, S., Li, G., Yao, X., Yang, X., Shu, D., Li, Y., Kinoshita, S., Sasaki, O., Komiya, T., and Yan, G. 2013. Early Cambrian pentamerous cubozoan embryos from South China. PLoS One 8: e70741. Crossref
Han, J., Li, G., Kubota, S., Ou, Q., Toshino, S., Wang, X., Yang, X., Uesugi, K., Masato, H., Sasaki, O., Kano, H., Sato, T., and Komiya, T. 2016. Internal microanatomy and zoological affinity of the Early Cambrian Olivooides. Acta Geologica Sinica, English Edition 90: 38–65. Crossref
Hermann, T.N. 1974. Discovery of massive clusters of trichomes in the Riphean [in Russian]. In: B.V. Timofeev (eds.), Mikrofitofossilii Proterozoâ i Rannego Paleozoâ SSSR, 6–10. Nauka, Leningrad.
Hou, X., Siveter, David. J., Siveter, Derek. J., Aldridge, R.J., Cong, P., Gabbott, S.E., Ma, X., Purnell, M.A., and Williams, M. 2017. The Cambrian Fossils of Chengjiang, China—The Flowering of Early Animal Life. 2nd Edition. 316 pp. John Wiley & Sons, Hoboken. Crossref
Knoll, A.H. and Golubic S. 1979. Anatomy and taphonomy of a precambrian algal stromatolite. Precambrian Research 10: 115–151. Crossref
Lee, R.E. 2008. Phycology. 4th Edition. 560 pp. Cambridge University Press, Cambridge.
Liu, Y., Li, Y., Shao, T, Hu, H., Bai, J., Zhang, W., Ren, M., and Duan, S. 2014a. The new record of phosphatized Cambricodium fossils from the basal Cambrian of the Southern Shaanxi, China [in Chinese with English abstract]. Acta Micropalaeontologica Sinica 31: 98–103.
Liu, Y., Qin, J., Wang, Q., Maas, A., Duan, B., Zhang, Y., Zhang, H., Shao, T., and Zhang, H. 2019. New armoured scalidophorans (Ecdysozoa, Cycloneuralia) from the Cambrian Fortunian Zhangjiagou Lagerstätte, South China. Papers in Palaeontology 5: 241–260. Crossref
Liu, Y., Shao, T., Fang, Q., Feng, J., and Zheng, X. 2009. Discovery on the column-like fossils from the early Cambrian of the South Shaanxi [in Chinese with English abstract]. Acta Micropalaeontologica Sinica 26: 291–296.
Liu, Y., Xiao, S., Shao, T., Broce, J., and Zhang, H. 2014b. The oldest known priapulid-like scalidophoran animal and its implications for the early evolution of cycloneuralians and ecdysozoans. Evolution & Development 16: 155–165. Crossref
Qian, Y. 1977. Hyolitha and some problematica from the Lower Cambrian Meishucun Stage in central and SW China [in Chinese with English abstract]. Acta Palaeontologica Sinica 16: 255–275.
Qian, Y., Li, G., Jiang, Z., Chen, M., and Yang, A. 2007. Some phosphatized cyanobacterian fossils from the basal Cambrian of China [in Chinese with English abstract]. Acta Micropalaeontologica Sinica 24: 222–228.
Steiner, M., Li, G., Qian, Y., and Zhu, M. 2004. Lower Cambrian Small Shelly Fossils of northern Sichuan and southern Shaanxi (China), and their biostratigraphic importance. Geobios 37: 259–275. Crossref
Steiner, M., Li, G., Qian, Y., Zhu, M., and Erdtmann, B.D. 2007. Neoproterozoic to early Cambrian small shelly fossil assemblages and a revised biostratigraphic correlation of the Yangtze Platform (China). Palaeogeography, Palaeoclimatology, Palaeoecology 254: 67–99. Crossref
Tang, Q., Pang, K., Yuan, X., and Xiao, S. 2020. A one-billion-year-old multicellular chlorophyte. Nature Ecology & Evolution 4: 543–549. Crossref
Uesugi, K., Hoshino, M., Takeuchi, A., Suzuki, Y., and Yagi, N. 2012. Development of fast and high throughput tomography using CMOS image detector at SPring-8. In: S.R. Stock (ed.), Developments in X-Ray Tomography VIII. SPIE Proceedings 8506: 85060I. Crossref
Vannier, J., Steiner, M., Renvoisé, E., Hu, S., and Casanova, J.P. 2007. Early Cambrian origin of modern food webs: evidence from predator arrow worms. Proceedings of the Royal Society B. Biological Sciences 274: 627–633. Crossref
Voronova, L.G and Missarzhevsky, V.V. 1969. Finds of algae and worm tubes in Precambrian–Cambrian boundary beds in the northern Siberian platform [in Russian]. Doklady AN SSSR 184: 207–210.
Wan, B., Yuan, X., Chen, Z., Guan, C., Pang, K., Tang, Q., Xiao, S., and McIlroy, D. 2016. Systematic description of putative animal fossils from the early Ediacaran Lantian Formation of South China. Palaeontology 59: 515–532. Crossref
Wang, D., Vannier, J., Schumann, I., Wang, X., Yang, X., Komiya, T., Uesugi, K., Sun, J., and Han, J. 2019. Origin of ecdysis: fossil evidence from 535-million-year-old scalidophoran worms. Proceedings of the Royal Society B: Biological Sciences 286: 20190791. Crossref
Wang, D., Vannier, J., Yang, X., Sun, J., Sun, Y., Hao, W., Tang, Q., Liu, P., and Han, J. 2020. Cuticular reticulation replicates the pattern of epidermal cells in lowermost Cambrian scalidophoran worms. Proceedings of the Royal Society B: Biological Sciences 287: 20200470. Crossref
Wang, P., LoDuca, S.T., Wu, M., Tang, Y., and Sun, Z. 2022. Benthic primary producers in exceptionally preserved Cambrian biotas of North China. Palaeoworld 31: 14–29. Crossref
Wang, X., Han, J., Vannier, J., Ou, Q., Yang, X., Uesugi, K., Sasaki, O., and Komiya, T. 2017. Anatomy and affinities of a new 535-million-year-old medusozoan from the Kuanchuanpu Formation, South China. Palaeontology 60: 853–867. Crossref
Wang, Y., Wang, Y., and Du, W. 2016. The long-ranging macroalga Grypania spiralis from the Ediacaran Doushantuo Formation, Guizhou, South China. Alcheringa: An Australasian Journal of Palaeontology 40: 303–312. Crossref
Xiao, S. 2013. Written in stone: The fossil record of early eukaryotes. In: G. Trueba and C. Montúfar (eds.), Evolution from the Galapagos. Social and Ecological Interactions in the Galapagos Islands, 107–124. Springer, New York. Crossref
Xiao, S., Knoll, A.H., and Yuan, X. 1998a. Morphological reconstruction of Miaohephyton bifurcatum, a possible brown alga from the Neoproterozoic Doushantuo Formation, South China. Journal of Paleontology 72: 1072–1086. Crossref
Xiao, S., Knoll, A.H., Yuan, X, and Pueschel, C.M. 2004. Phosphatized multicellular algae in the Neoproterozoic Doushantuo Formation, China, and the early evolution of florideophyte red algae. American Journal of Botany 91: 214–227. Crossref
Xiao, S., Yuan, X., Steiner, M., and Knoll, A.H. 2002. Macroscopic carbonaceous compressions in a terminal Proterozoic shale: A systematic reassessment of the Miaohe biota, South China. Journal of Paleontology 76: 347–376. Crossref
Xiao, S., Zhang, Y., and Knoll, A.H. 1998b. Three-dimensional preservation of algae and animal embryos in a Neoproterozoic phosphorite. Nature 391: 553–558. Crossref
Ye, Q., Tong, J., An, Z., Hu, J., Tian, L., Guan, K., and Xiao, S. 2017. A systematic description of new macrofossil material from the upper Ediacaran Miaohe Member in South China. Journal of Systematic Palaeontology 17: 183–238. Crossref
Yin, G., He, T., Qian, Y., and Xiao, B. 1999. Geological and geographical distribution of SSF, with discussion on Early Cambrian geographical provinces [in Chinese with English abstract]. In: Y. Qian (ed.), Taxonomy and Biostratigraphy of Small Shelly Fossils in China, 234–240. Science Press of China, Beijing.
Yin, L. 1987. New data of microfossils from Precambrian–Cambrian cherts in Ningqiang, southern Shaanxi [in Chinese with English abstract]. Acta Palaeontologica Sinica 26: 87–195.
Yin, Z. and Zhu, M. 2011. Critical reappraisal of Ediacaran Doushantuo animal gastrula fossils: new insights from Synchrotron X-ray Microtomographic Anatomic study [in Chinese with English abstract]. Science & Technology Review 29: 18–24.
Yuan, X., Chen, Z., Xiao, S., Wan, B., Guan, C., Wang, W., Zhou, C., and Hua, H. 2013. The Lantian biota: A new window onto the origin and early evolution of multicellular organisms. Chinese Science Bulletin 58: 701–707. Crossref
Yuan, X., Wan, B., Guan, C., Chen, Z., Zhou, C., Xiao, S., Wang, W., Pang, K., Tang, Q., and Hua, H. 2016. Lantian Biota [in Chinese]. 132 pp. Shanghai Scientific & Technical Publishers, Shanghai.
Yuan, X., Xiao, S., Yin, L., Knoll, A.H., Zhou, C., and Mu, X. 2002. Doushantuo Fossils: Life on the Eve of Animal Radiation [in Chinese]. 171 pp. China University of Science and Technology Press, Hefei.
Zeng, C. and Zhang, J. 1962. Studies on Chinese species of Dictyosphacria [in Chinese with English abstract]. Journal of Integrative Plant Biology 10: 120–132.
Zhang, H., Xiao, S., Liu, Y., Yuan, X., Wan, B., Muscente, A.D., Shao, T., Gong, H., and Cao, G. 2015. Armored kinorhynch-like scalidophoran animals from the early Cambrian. Scientific Reports 5: 16521. Crossref
Zhang, Y. 1989. Multicellular thallophytes with differentiated tissues from Late Proterozoic phosphate rocks of South China. Lethaia 22: 113–132. Crossref
Zhang, Y. and Yuan, X. 1992. New data on multicellular thallophytes and fragments of cellular tissues from Late Proterozoic phosphate rocks, South China. Lethaia 25: 1–18. Crossref
Zhang, Y., Yin, L., Xiao, S., and Knoll, A.H. 1998. Permineralized fossils from the terminal Proterozoic Doushantuo Formation, south China. Journal of Paleontology 72: 1–52. Crossref
Zhao, Y., Chen, M., Peng, J., Yu, M., He, M., Wang, Y., Yang, R., Wang, P., and Zhang, Z. 2004. Discovery of a Miaohe-type Biota from the Neoproterozoic Doushantuo Formation in Jiangkou County, Guizhou Province, China. Chinese Science Bulletin 49: 2224–2226. Crossref
Zheng, Y., Tang, Q., Liu, P., Sun, J., Yao, X., Hao, W., Yang, X., Guo, J., Uesugi, K., Komiya, T., and Han, J. 2021. Characterization of the multicellular membrane-bearing algae from the Kuanchuanpu biota (Cambrian: Terreneuvian). Journal of Geophysical Research: Biogeosciences 126: e2020JG006102. Crossref
Zheng, Y., Yao, X., Han, J., and Guo, J. 2017. Microscopic fossils with multi-level tetrad cell structures from the Cambrian Kuanchuanpu Formation in Southern Shaanxi [in Chinese with English abstract]. Acta Palaeontologica Sinica 56: 440–448.
Acta Palaeontol. Pol. 67 (2): 317–327, 2022
https://doi.org/10.4202/app.00946.2021