

Brachiopod fauna from uppermost Visean (Mississippian) mud mounds in Derbyshire, UK
ALESSANDRO P. CARNITI, GIOVANNA DELLA PORTA, VANESSA J. BANKS, MICHAEL H. STEPHENSON, and LUCIA ANGIOLINI
Carniti, A.P., Della Porta, G., Banks, V.J., Stephenson, M.H., and Angiolini, L. 2022. Brachiopod fauna from uppermost Visean (Mississippian) mud mounds in Derbyshire, UK. Acta Palaeontologica Polonica 67 (4): 865–915.
The systematic study of a brachiopod fauna collected from a Brigantian, uppermost Visean, Mississippian, mud mound complex on the Derbyshire Carbonate Platform (England, United Kingdom) recognises 45 species, representing 36 genera and seven orders (Productida, Orthotetida, Orthida, Rhynchonellida, Spiriferida, Spiriferinida, and Terebratulida). The mound complex is a decametre-scale lens-shaped buildup composed of three facies associations: the basal tabular unit made of skeletal packstone beds and tabular mounds, the complex core formed by metre-scale lens-shaped massive mounds and the surrounding inclined skeletal packstone flank beds. Brachiopods are widespread and very abundant in all three facies associations. Spinose, concavo–convex productides are dominant in the mud mound fauna, both in terms of the number of specimens, species, and biovolume. Productide success is related to scattered and scarce food resources, which they better exploited using their simple, unsupported feeding apparatus in comparison with that of the spiriferides. Spiriferides with a wide interarea are common and large in the basal tabular unit, but are rare and small in the complex core, probably due to greater availability of food resources during the deposition of the basal unit. Substrate type also played a role in controlling brachiopod diversity: varied substrates in the mound complex core allowed small-sized pedicle-attached rhynchonellides, spiriferides, and terebratulides to extensively colonise the seafloor, whereas they are rare in the basal unit.
Key words: Brachiopoda, mud mounds, Brigantian, Visean, Mississippian, Derbyshire, United Kingdom.
Alessandro Paolo Carniti [alessandro.carniti@unimi.it; ORCID: https://orcid.org/0000-0002-9590-1615], Giovanna Della Porta [giovanna.dellaporta@unimi.it; ORCID: https://orcid.org/0000-0003-3479-0592], and Lucia Angiolini [lucia.angiolini@unimi.it; ORCID: https://orcid.org/0000-0003-0778-5771], Dipartimento di Scienze della Terra ‘A. Desio’, Università degli Studi di Milano, Via Mangiagalli 34, 20133 Milano, Italy.
Vanessa J. Banks [vbanks@bgs.ac.uk; ORCID: https://orcid.org/0000-0001-6335-7080], British Geological Survey, Nicker Hill, NG12 5GG Keyworth, Nottinghamshire, UK.
Michael H. Stephenson [mikepalyno@me.com; ORCID: https://orcid.org/0000-0002-9881-1578], Stephenson Geoscience Consulting Ltd., NG12 5HU Keyworth, Nottinghamshire, UK and British Geological Survey, Nicker Hill, NG12 5GG Keyworth, Nottinghamshire, UK.
Received 3 January 2022, accepted 3 May 2022, available online 5 December 2022.
Copyright © 2022 A.P. Carniti et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
This paper systematically describes a brachiopod fauna from a Brigantian, uppermost Visean, mud mound complex in the Peak District, north-western Derbyshire (England, UK; Fig. 1A). The mound complex crops out at Ricklow Quarry (53°11’30’’ N, 01°45’17’’ W), near the village of Monyash (Fig. 1B), and it belongs to the Mississippian Derbyshire Carbonate Platform succession (Gutteridge 1990, 1995).
Brigantian mud mounds in the Peak District are metre- to decametre-scale carbonate buildups lacking a framework built by skeletal metazoans, but having many skeletal components associated with in-situ precipitation of carbonate mud by microbially mediated processes and marine cement-filled primary voids (Gutteridge 1995). Mud mounds usually show a massive, lens-shaped “core” laterally passing into inclined “flank” skeletal packstone beds. Flank beds represent the lateral transition to the adjacent equivalent, off-mound beds (Gutteridge 1990, 1995).
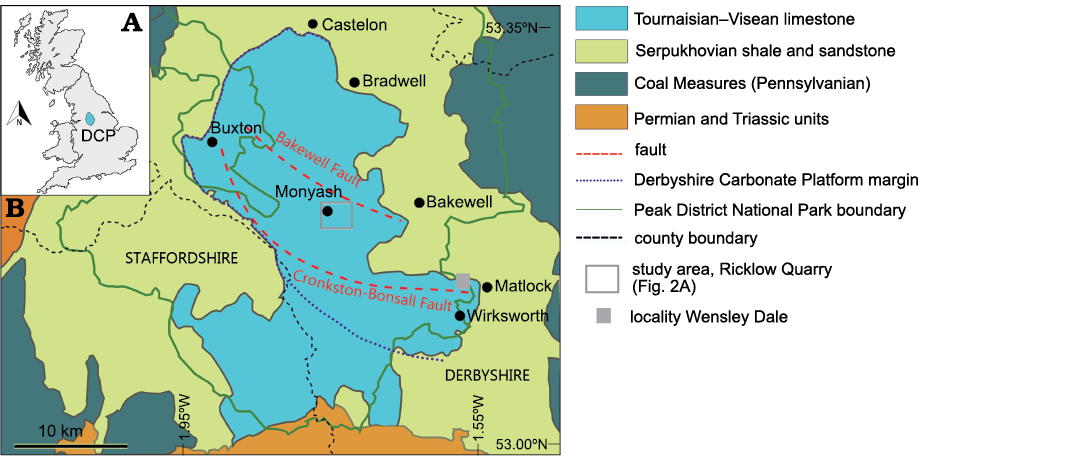
Fig. 1. A. Geographic position of the Derbyshire Carbonate Platform (DCP) within Great Britain. B. Geological map of the Peak District area and location of Ricklow Quarry near Monyash and Wensley Dale. Modified after Aitkenhead et al. (2002).
Despite the Derbyshire mounds hosting abundant and diverse brachiopod faunas, little work has been done on the taxonomic composition and palaeoecology of the faunas. Only faunal lists were provided by Smith et al. (1967: 46), Mitchell (1971: 140), and Gutteridge (1990: 31). In contrast, more studies were devoted to the Asbian, upper Visean, “Cracoean” reefs at the northern edge of the Derbyshire Carbonate Platform (e.g., Wolfenden 1958; Brunton and Mundy 1988; Brunton and Tilsley 1991) and to the Brigantian, uppermost Visean, gigantoproductins shell beds (Angiolini et al. 2012, 2019; Nolan et al. 2017). A deeper knowledge of the brachiopod fauna from the Derbyshire mounds is thus required, in particular, because mud mounds represent the relic of a microbial–metazoan reef community which was widespread in the Mississippian (e.g., Lees and Miller 1995; Bridges et al. 1995; Yao et al. 2019), but which is poorly known.
Very little is known about the brachiopod palaeoecology in Derbyshire mud mounds. Only Gutteridge (1990) provided an interpretation for the brachiopod spatial distribution in the mud mound complex of Ricklow Quarry. Along with the systematic description of the brachiopod fauna from the mud mounds, this study also aims to present palaeoecological reconstructions based on the fauna and a revised interpretation of the facies architecture of the mud mound complex.
Finally, this paper represents the first systematic description of a fauna from the Brigantian substage, uppermost Visean, of Great Britain and Ireland, as only faunal lists have been provided so far (Smith et al. 1967; Mitchell 1971; Wilson 1989; Gutteridge 1990).
Institutional abbreviations.—MPUM, Museum of Palaeontology University of Milan, Department of Earth Sciences “A. Desio”, Milan, Italy.
Other abbreviations.—FA, facies association; R, RCC, RCK, sample or specimen from Ricklow Quarry mud mound complex, Monyash, Derbyshire, UK; WI, sample or specimen from Wensley Dale, Wensley, Derbyshire, UK.
Geological setting
The Derbyshire Carbonate Platform succession consists of the Peak Limestone Group, part of the Carboniferous Limestone Supergroup (Waters et al. 2009). The Derbyshire Carbonate Platform crops out in the south of the Peak District, known as the “White Peak”, at the core of the Pennine Anticline (Fig. 1B; Stevenson and Gaunt 1971; Aitkenhead et al. 1985). The platform developed in the Tournaisian on the Derbyshire High, one of the many structural highs formed to the north of the Anglo-Brabant Massif (Pharaoh et al. 1987) between the end of the Devonian and the Mississippian (Haszeldine 1984, 1989; Stone et al. 2010). The Derbyshire High was at the time in a subtropical position (Pharaoh et al. 1987) and was subdivided by two normal faults, the Bakewell and Cronkston-Bonsall faults, into two south-dipping half-grabens with a fault terrace in-between (Fig. 1B; Gutteridge 1989). At first the structural high consisted of an irregular shallow marine area dominated by carbonate deposition with low-angle ramp margins. By the Asbian–Brigantian, late Visean, the carbonate system evolved into a flat-topped platform with framework reefs, mud mounds, and grainstone shoals at the margins (Stevenson and Gaunt 1971).
Carbonate strata deposited on the Derbyshire High in the Brigantian, latest Visean, belong to the Monsal Dale Limestone and Eyam Limestone formations (Fig. 2; Stevenson and Gaunt 1971; Aitkenhead and Chisholm 1982; Aitkenhead et al. 1985). The Monsal Dale Limestone is of early to middle Brigantian age and ranges in thickness commonly between 100 and 200 metres (Aitkenhead et al. 1985). The Eyam Limestone Formation is of late Brigantian age and has a thickness of 30–50 metres (Aitkenhead et al. 1985). The two formations are separated by a regional subaerial exposure surface (Aitkenhead and Chisholm 1982; Gutteridge 1991).
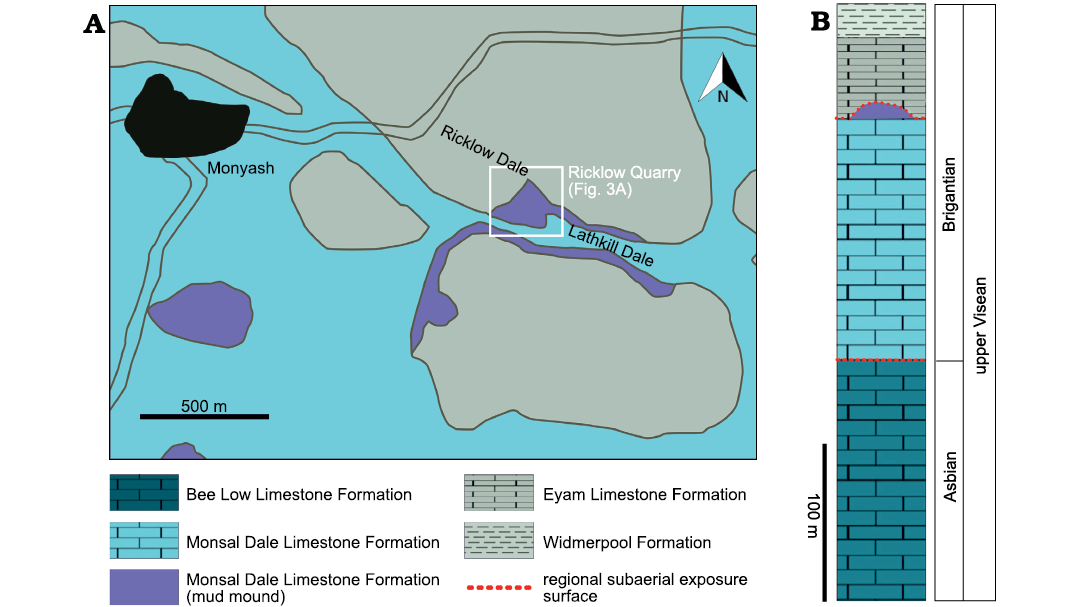
Fig. 2. Study area of Ricklow Quarry (Derbyshire, UK). A. Geological map of the surroundings of the village of Monyash and location of the site of Ricklow Quarry. Modified after British Geological Survey (1978). B. Stratigraphical column of the upper part of the Derbyshire Carbonate Platform succession in the study area around Monyash.
The depositional style of the Monsal Dale Limestone Formation was controlled by reactivation of basement faults in the Brigantian, leading to variation in thickness of the formation and the development of the intraplatform Ashford Basin to the north-east of the Bakewell Fault. By the end of the deposition of the Monsal Dale Limestone Formation, subsidence in-between the Bakewell and Cronkston-Bonsall faults (Fig. 1B) led to the development of an intraplatform ramp dipping to the east towards the intraplatform Stanton Basin (Gutteridge 1987, 1989). The Monsal Dale Limestone Formation is characterised on the platform interior by massive, cyclic, bioturbated skeletal wackestone–packstone and skeletal grainstone, with floatstone beds with gigantoproductins (Cox et al. 1977; Aitkenhead et al. 1985); on the margin, it comprises bioclastic, crinoid-dominated grainstone–rudstone interpreted as shoals (Gawthorpe and Gutteridge 1990; Harwood 2005). The intraplatform ramp and basin facies include bioturbated skeletal packstone, organic-rich wackestone, and very fine-grained, non-bioturbated packstone (Aitkenhead et al. 1985; Gutteridge 1989). Interbedded volcanic rocks (e.g., basaltic lava, hyaloclastite, and tuff) are common in the Monsal Dale Limestone Formation, forming useful marker beds (Aitkenhead et al. 1985). Mud mounds (sensu Bridges et al. 1995; Pratt 1995) developed at the platform margin, in the platform interior, and intraplatform ramps near the top of the Monsal Dale Limestone Formation, immediately beneath the regional subaerial exposure surface marking the boundary with the overlying Eyam Limestone Formation (Adams 1980; Gutteridge 1991, 1995). The studied Ricklow Quarry mound complex belongs to this group of mounds; its depositional setting was interpreted as an intraplatform inner ramp setting (sensu Burchette and Wright 1992) by Gutteridge (1995), and as middle ramp, below effective wave base, by Nolan et al. (2017). Brigantian mounds in Derbyshire consist of a massive “core” dominated by in situ precipitated micrite mediated by biologically induced and influenced processes associated with microbial mats (“automicrite” sensu Wolf 1965; Reitner and Neuweiler 1995; Reitner et al. 1995; Flügel 2004) with fenestellid bryozoan fronds and brachiopod shells (Bridges et al. 1995; Gutteridge 1995). These metre- to decametre-scale buildups formed decametre-scale “mud mound complexes” by lateral and vertical accretion (Gutteridge 1995). Gutteridge (1990) described the brachiopod fauna from the mud mound complex at Ricklow Quarry as mainly occurring in decimetre-scale “pockets”, surrounded by sparsely fossiliferous facies showing a less diverse fauna with only productides. Pockets were interpreted by Gutteridge (1990) as scoured by storm currents on the mud mound core surfaces. Inclined “flank” skeletal packstone beds with brachiopods and crinoids represented the transition to the lateral equivalent off-mound beds, dominated by crinoid ossicles (Gutteridge 1990, 1995). These mounds are considered as non-Waulsortian, type 3 buildups (crinoid–brachiopod–fenestellid bryozoans) in the classification scheme by Bridges et al. (1995) for Mississippian mounds.
The Eyam Limestone Formation comprises thin-beds of cherty skeletal packstone, fossiliferous beds dominated by gigantoproductins, corals, and crinoids, and a few dark calci-mudstone intercalations on the platform interior (Aitkenhead and Chisholm 1982; Aitkenhead et al. 1985); it consists of bioclastic crinoid-dominated grainstone–rudstone interpreted as shoals on the margins (Aitkenhead and Chisholm 1982; Aitkenhead et al. 1985; Gawthorpe and Gutteridge 1990). Sedimentation on the intraplatform ramps and basins consisted of crinoidal shoals with associated gigantoproductins in the shallow inner ramp portion, passing outward into packstone and wackestone with associated beds dominated by gigantoproductins of the middle ramp environment and then to outer ramp—intraplatform basin dark limestone units deposited by storms (Gutteridge 1984; Nolan et al. 2017). Mud mounds developed on the platform interior and intraplatform inner ramp settings during the deposition of the middle and upper portions of the Eyam Limestone Formation (British Geological Survey 1978; Gutteridge 1995).
The Derbyshire Platform carbonate deposition terminated in the early Serpukhovian, as delta systems advanced southwards burying it (Widmerpool Formation; Aitkenhead and Chisholm 1982; Guion and Fielding 1998).
Ricklow Quarry mud mound complex facies architecture.—Geological mapping and measurement of stratigraphic logs during fieldwork, along with petrographic and cathodoluminescence analysis of collected rock samples, allowed a revised model for the facies architecture of the mud mound complex cropping out at Ricklow Quarry (Fig. 3). The mound complex consists of a lens-shaped buildup formed by numerous metre-scale mounds, reaching a thickness of 20–25 m and a diameter of roughly 250 m. It is formed by several facies differing in texture and components, which were grouped into three facies associations (Fig. 3): a basal tabular unit (Facies Association 1), a massive lens-shaped core (Facies Association 2), and flank beds (Facies Association 3). Stratigraphically, the mound complex occurs at the top of the Monsal Dale Limestone Formation, and it is overlain by the basal beds of the Eyam Limestone Formation, onlapping against the mud mound top (Fig. 4A).
Facies Association 1 (FA1): This association characterises the basal unit of the mound complex. This unit is horizontal, 10 m thick, 250 m in diameter, and consists of an association of 3–5 cm thick nodular packstone beds and 5–100 cm thick tabular massive mud mounds (Fig. 4B). It has a sharp base overlying 1 m thick beds of fine-grained, well-sorted packstone–grainstone with peloids and bioclasts (foraminifers, crinoid ossicles). FA1 is lateral to beds of crinoidal grainstone/rudstone of the off-mound complex Monsal Dale Limestone Formation, while it gradually passes upward to the massive core of the mud mound complex (FA2). Packstone beds are dominated by brachiopods within a matrix made of detrital lime mud (“allomicrite”, sensu Wolf 1965; Reitner and Neuweiler 1995; Reitner et al. 1995; Flügel 2004) and mm-size automicrite patches (Fig. 5A). Packstone beds are irregularly interbedded and laterally transitional within a few metres to the tabular mounds, which consist of automicrite–fenestellid bryozoan boundstone with abundant brachiopod shells (Fig. 5B). Both packstone beds and tabular mounds contain common calcite-replaced siliceous sponge spicules, a few crinoid ossicles, ramose and fistuliporid bryozoans, ostracods, bivalves and rare foraminifers, gastropods, trilobite fragments, and fish scales. Skeletal components are usually not fragmented.
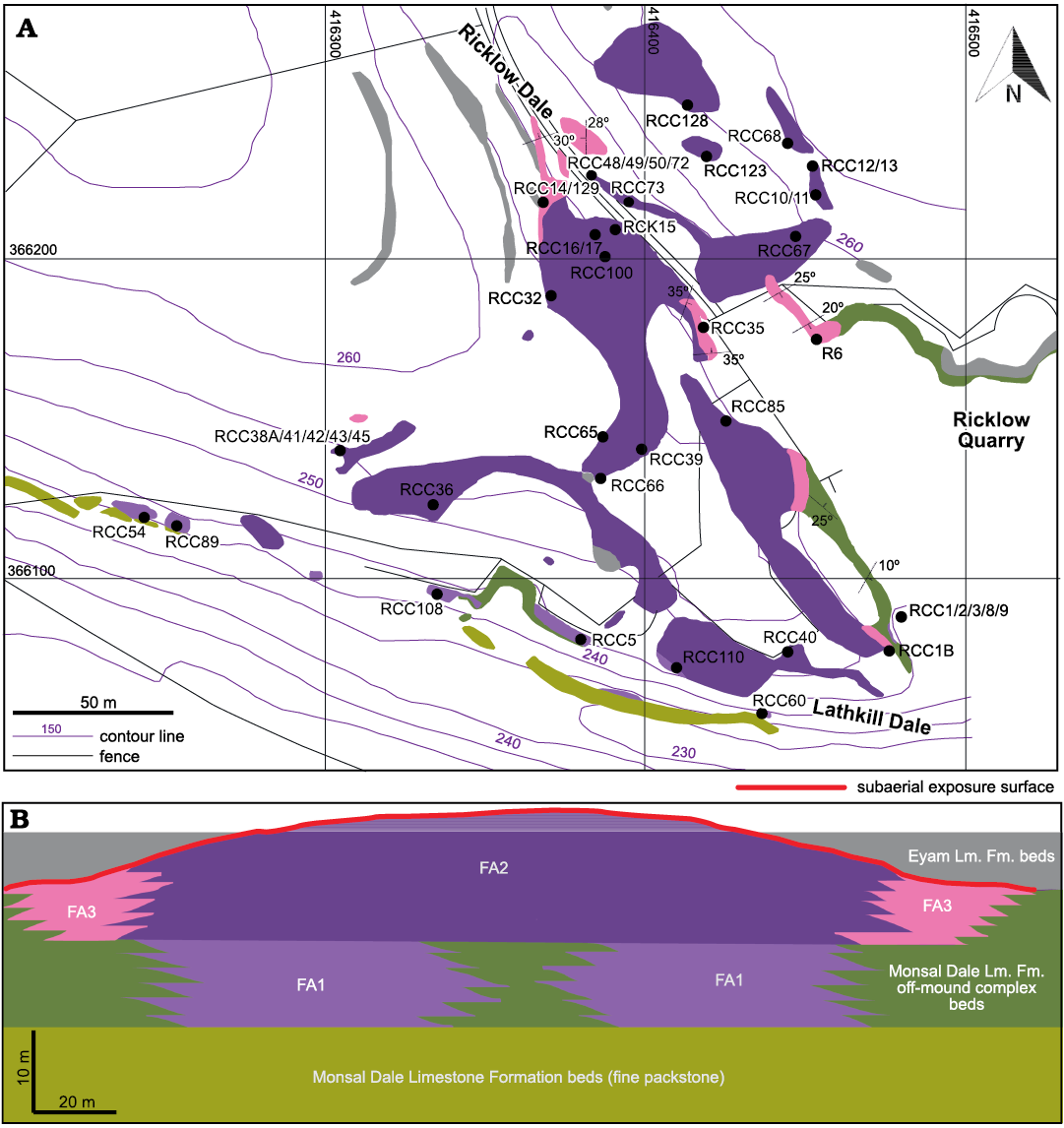
Fig. 3. Facies architecture of the Brigantian (uppermost Visean) mud mound complex of Ricklow Quarry. A. Facies association map (outcrops only) of the study area of Ricklow Quarry, with position of the collected fossil brachiopod assemblages and samples for thin sections. Map drawn from a geodatabase built with ESRI ArcGIS® software. Basemap by Ordnance Survey, OS MasterMap Topography Layer, 1:1250 series. Kilometric coordinate system: British National Grid (projection: Transverse Mercator; datum: OSGB 1936; units: metres). B. Interpretative stratigraphic scheme of the studied mud mound complex with spatial distribution of the distinguished facies associations. Scales are approximate with 2× vertical exaggeration. Flank beds are inclined with an angle of 20–44°. Abbreviations: Fm., Formation; Lm., Limestone;
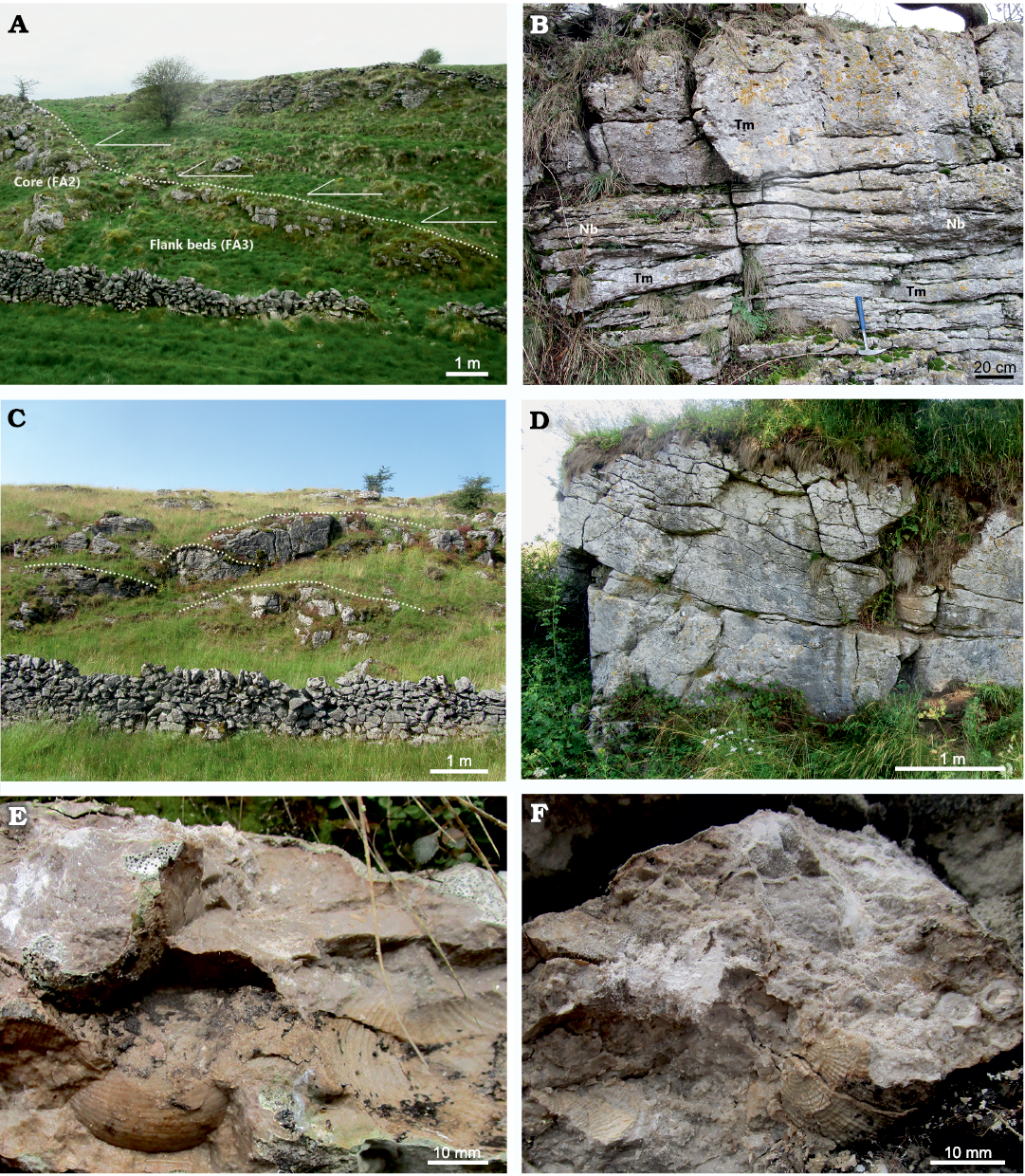
Fig. 4. Outcrop photographs from the Ricklow Quarry site, Monyash, England. A. Eyam Limestone Formation beds onlapping (solid line arrows) the mud mound complex capping surface (dotted line). The boundary is marked by subaerial exposure features. B. Nodular packstone beds (Nb) and tabular massive mounds (Tm) forming the basal facies association (FA1) of the mound complex. C. Lens-shape mounds accreting to form the mound complex core (FA2). Dotted lines indicate capping surface of mounds. D. Detail of inclined flank beds (FA3) dipping eastward (inclination 35–25°) on the south-eastern edge of the mound complex. E. A ventral valve of Latibrachythyris cf. crassa (De Koninck, 1843) (below) and reticulate productide in life position (above) in a nodular packstone bed from the basal FA1 unit of the mound complex. F. Dorsal valve of a species of Antiquatonia from the mud mound complex core (FA2). The valve is part of a specimen in life position. The specimen is surrounded by other brachiopod shells and crinoid ossicles.
Facies Association 2 (FA2): The basal FA1 unit passes gradually upward into an association of metre-scale, lens-shaped, massive mounds, which accreted to form the lens-shaped core of the mud mound complex (thickness 10–15 m, diameter 250 m; Fig. 4C). FA2 is lateral to inclined boundstone/packstone beds forming the flank facies (FA3). Mound cores are made of automicrite–fenestellid bryozoan boundstone–cementstone with abundant radiaxial fibrous calcite cement (Fig. 5C). Other skeletal components are brachiopods and calcite-replaced siliceous sponge spicules, sparse crinoid ossicles, ramose and fistuliporid bryozoans, ostracods, bivalves, rare foraminifers, and gastropods (Fig. 5D, E). Skeletal components are usually not fragmented.
Facies Association 3 (FA3): The massive mud mound complex core laterally passes to poorly to well-defined inclined beds (up to 44°) with a thickness of 10–100 cm (Fig. 4D). Beds consist of automicrite–fenestellid bryozoan boundstone grading downslope into skeletal packstone beds with brachiopods and crinoids, with a major proportion of allomicrite (Fig. 5F). These beds constitute the lateral flank of the mud mound complex core, and form an irregular wedge, 2 to 10 m thick, extending no more than 30 m around it. FA3 beds represent the transition from the core to horizontal crinoid-rich packstone/rudstone beds characterizing the areas lateral to the mound complex (Fig. 3).
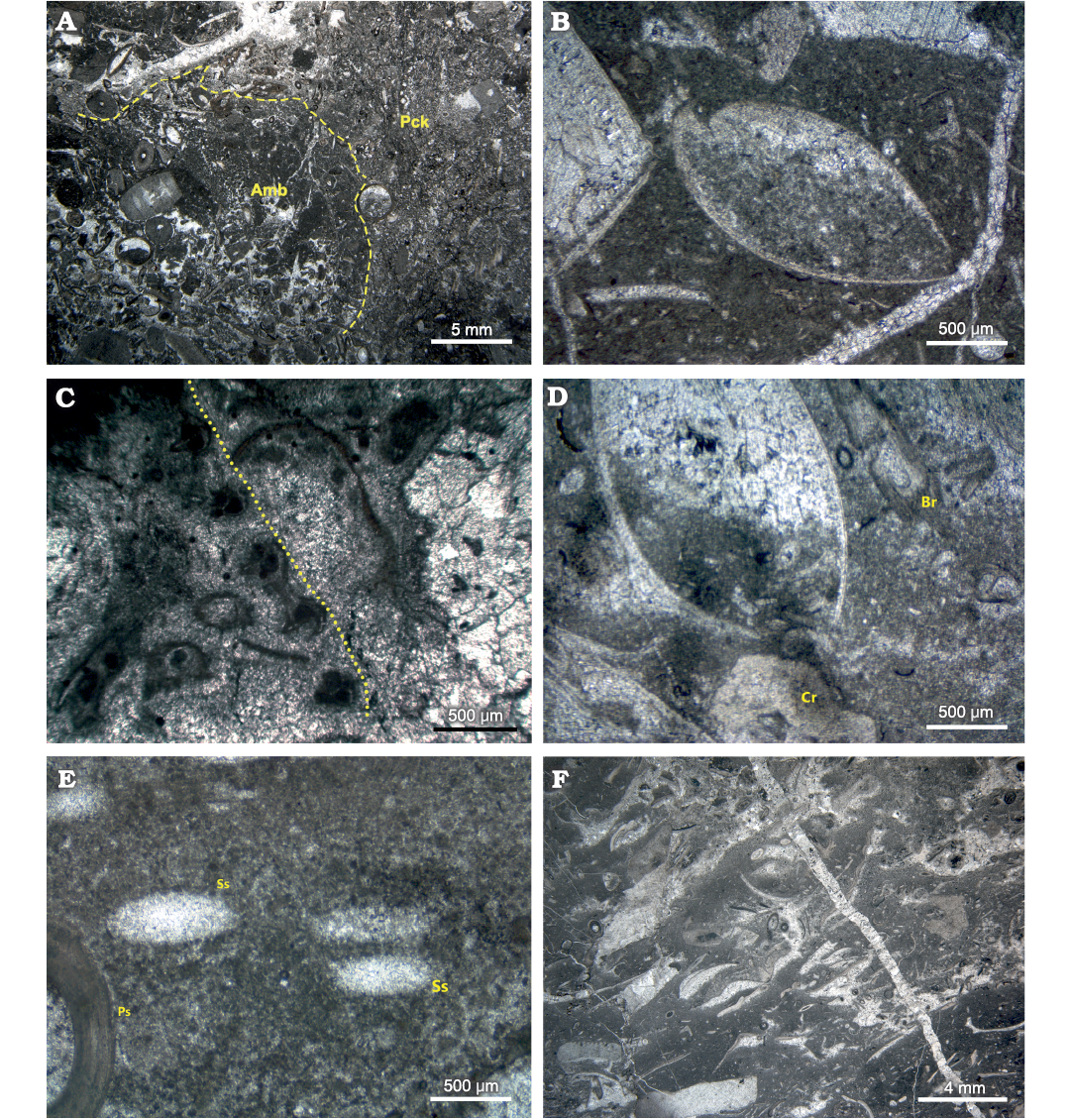
Fig. 5. Photomicrographs of petrographic thin sections from the Brigantian (uppermost Visean) mud mound complex of Ricklow Quarry (FA1, FA2, FA3), Monyash, England. A. Sample MPUM12295 (RCC89). Automicrite boundstone patch (Amb) within a packstone (Pck) with allomicrite, from a nodular packstone bed at the base of the mound complex (FA1). Note more heavily recrystallized allomicrite with respect to automicrite. Plane polarisers. B. Sample MPUM12295 (RCC5). Articulated brachiopod shells embedded in automicrite from a tabular mound from the basal unit of the mound complex (FA1). Plane polarisers. C. Sample MPUM12295 (RCC65). Automicrite‒fenestellid bryozoan boundstone with abundant radiaxial fibrous marine and equant burial calcite cements from the mound complex core (FA2). Fenestellid frond marked by dotted line. Plane polarisers. D. Sample MPUM12295 (RCC85). Articulated brachiopod shells, crinoid ossicles (Cr), and fenestellid bryozoan fronds (Br) in automicrite boundstone from the mound complex core (FA2). The biconvex brachiopod specimen is in life position and lived attached to the crinoid ossicle in the mud underneath its umbo. Plane polarisers. E. Sample MPUM12295 (RCC32B). Productide spine (Ps) and calcite-replaced siliceous sponge spicules (Ss) in automicrite boundstone from the mound complex core (FA2). Plane polarisers. F. Sample MPUM12295 (R6). Isoriented skeletal packstone with brachiopods and crinoids, with allo- and automicrite from the flank beds (FA3). Convex-up disarticulated brachiopod shells aligned along the flank beds forming shelter porosity, later filled by equant calcite cement. Plane polarisers.
Material and methods
In the mud mound complex of Ricklow Quarry (53°11’30’’ N, 01°45’17’’ W), three fossil brachiopod assemblages were collected from unit FA1 (RCC1, RCC2, RCC60) and six from FA2 (RCC10, RCC32, RCC49, RCC65G, RCC73, RCK15; Fig. 3A). Collection of a precise and standard volume of rock was not possible, although sampling continued until no new species were recovered, obtaining a minimum volume from each locality of about 1.5 dm3; the minimum volume contains at least 30–40 specimens (number of articulated specimens + number of valves of the most occurring type + half number of the other type of valve), alongside many fragments. Additionally 39 smaller assemblages were collected from FA1 (RCC3, RCC8, RCC9, RCC54, RCC108), FA2 (RCC11, RCC12, RCC12BIT, RCC13, RCC32B, RCC32C, RCC36, RCC38A, RCC39, RCC40, RCC41, RCC42, RCC43, RCC45, RCC48, RCC50, RCC65, RCC65T, RCC66, RCC67, RCC68, RCC72, RCC85, RCC110, RCC123, RCC128, RCK15A, RCK15B, RCK16, RCK17) and FA3 (RCC1B, RCC14, RCC35A, RCC129; Fig. 3A). A few specimens were collected from debris blocks of the mud mound complex (RCC300). In total, the collected fauna comprises more than 400 specimens and hundreds of fragments.
To facilitate description and identification of taxa, and to describe further material from the Brigantian, a small number of specimens (39) of a well-preserved fauna collected in Wensley Dale (53°08’41.7’’N, 01°35’58.4’’W; Matlock, Derbyshire; Fig. 1B) was included in the study (WI1). It comes from a poorly exposed, lenticular 30 cm-thick bed of skeletal packstone of probable mud mound affinity found above a gigantoproductins-dominated bed, in the Eyam Limestone Formation near the southern margin of the platform (Smith et al. 1967; British Geological Survey 2012).
The systematic study follows the classifications of Brunton et al. (2000) for the productidines, Williams and Brunton (2000) for the orthotetidines, Harper (2000) for the dalmanellidines, Savage et al. (2002) for the Pugnacoidea, Carter et al. (2006) for the spiriferides, Carter and Johnson (2006) for the spiriferinides, Lee et al. (2006) for the terebratulides. Morphological terminology follows Williams and Brunton (1997). Specimen measurements are given in SOM 1 (Supplementary Online material available at http://app.pan.pl/SOM/app67-Carniti_etal_SOM.pdf).
The palaeoecological analysis was performed classifying the studied brachiopod species into three groups based on their lifestyle and feeding mechanism and considering biovolume instead of the number of specimens. Biovolumes were calculated using the method of Powell and Stanton (1985) in assemblages having more than 20 specimens and for the total number of specimens collected from FA1 and FA2 (Table 1). Brachiopods were considered as half cylinders using the formula V = 1/2πr2h (r = half-width of the shell; h = length of the shell). Flank beds (FA3) were not included as insufficient specimens were available.
Table 1. Number of individuals for each brachiopod taxon in the fauna calculated for the two main lithofacies associations (FA1, FA2) forming the mound complex of Ricklow Quarry and in collected fossil assemblages with at least 20 individuals. Margalef diversity index, Shannon-Wiener diversity index, evenness and dominance were calculated using PAST software (Hammer et al. 2001). Percentages of brachiopod biovolume for the three ecological groups considered (seminfaunal productides, pedicle-attached brachiopods, pedicle-attached brachiopods with morphological adaptations to soft substrates) are reported as well for each facies association and assemblage. Biovolume was calculated using the method of Powell and Stanton (1985). Complete list of specimens in each assemblage is given in SOM 3.
|
Locality |
RCC1 |
RCC2 |
RCC60 |
FA1 |
RCC10 |
RCC32 |
RCC49 |
RCC65G |
RCC73 |
RCK15 |
FA2 |
|
Facies association |
FA1 |
FA1 |
FA1 |
FA2 |
FA2 |
FA2 |
FA2 |
FA2 |
FA2 |
||
|
Alitaria aff. triquetra |
2 |
5 |
3 |
10 |
0 |
4 |
0 |
0 |
0 |
0 |
11 |
|
Overtonia fimbriata |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
2 |
|
Avonia sp. |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
|
Quasiavonia aculeata |
3 |
1 |
0 |
5 |
1 |
4 |
7 |
2 |
2 |
2 |
29 |
|
?Quasiavonia sp. |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Krotovia spinulosa |
2 |
0 |
0 |
4 |
1 |
1 |
0 |
0 |
3 |
5 |
19 |
|
Breileenia radiata |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
14 |
|
Geniculifera keyserlingiana |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
1 |
|
Carringtonia cf. carringtoniana |
1 |
1 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Limbifera sp. |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Productus cf. productus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
|
Productus sp. |
1 |
0 |
1 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Eomarginifera cf. laqueata |
0 |
0 |
1 |
2 |
0 |
1 |
0 |
0 |
0 |
0 |
2 |
|
Antiquatonia hindi |
0 |
4 |
3 |
11 |
0 |
4 |
0 |
1 |
3 |
2 |
22 |
|
Antiquatonia aff. hindi |
0 |
1 |
0 |
2 |
0 |
2 |
0 |
0 |
0 |
0 |
8 |
|
Antiquatonia insculpta |
6 |
5 |
3 |
20 |
1 |
1 |
0 |
1 |
1 |
1 |
15 |
|
Dictyoclostus pinguis |
0 |
1 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
|
Pugilis cf. kilbridensis |
0 |
1 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
|
Pugilis cf. scotica |
0 |
1 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Buxtonia scabricula |
1 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Buxtonia sp. |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Buxtoniinae gen. et sp. indet. |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
|
Reticulate Productoidea gen. et sp. indet. |
4 |
5 |
12 |
28 |
2 |
0 |
0 |
0 |
2 |
2 |
16 |
|
Echinoconchus punctatus |
1 |
1 |
0 |
4 |
0 |
0 |
0 |
0 |
0 |
3 |
10 |
|
Pustula cf. pustulosa |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
|
Linoprotonia sp. |
0 |
0 |
1 |
1 |
0 |
0 |
0 |
0 |
1 |
0 |
2 |
|
Schellwienella sp. |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
|
?Serratocrista aff. dalriensis |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
2 |
|
Schizophoria (S.) resupinata |
0 |
0 |
1 |
2 |
1 |
0 |
0 |
0 |
0 |
0 |
3 |
|
Schizophoria (S.) cf. connivens |
0 |
0 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Pleuropugnoides pleurodon |
2 |
0 |
0 |
3 |
0 |
0 |
6 |
0 |
3 |
9 |
23 |
|
Propriopugnus pugnus |
0 |
1 |
3 |
5 |
1 |
2 |
0 |
1 |
1 |
0 |
8 |
|
Crurithyris urei |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
|
Martiniinae gen. et sp. indet. |
0 |
1 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
?Fusella sp. |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
|
Latibrachythyris cf. crassa |
3 |
0 |
0 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
|
Latibrachythyris rotundata |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
3 |
7 |
|
Brachythyrididae gen. et sp. indet. |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
4 |
|
?Reticularia cf. mesoloba |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
|
Phricodothyris paricosta |
0 |
1 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
3 |
4 |
|
Phricodothyris cf. periculosa |
2 |
1 |
2 |
6 |
0 |
0 |
0 |
0 |
1 |
1 |
2 |
|
Phricodothyris sp. |
1 |
0 |
1 |
4 |
0 |
0 |
1 |
0 |
0 |
1 |
3 |
|
Spiriferida gen. et sp. indet. |
0 |
1 |
0 |
1 |
0 |
1 |
0 |
0 |
0 |
1 |
5 |
|
?Punctospirifer sp. |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
1 |
|
Harttella oakleyi |
3 |
0 |
2 |
7 |
8 |
4 |
6 |
10 |
3 |
5 |
71 |
|
Beecheria sp. |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
3 |
|
?Balanoconcha sp. |
0 |
0 |
1 |
1 |
4 |
0 |
0 |
2 |
0 |
2 |
19 |
|
Terebratulida gen. et sp. indet. |
0 |
0 |
2 |
2 |
1 |
0 |
0 |
5 |
0 |
0 |
9 |
|
Specimens (total): |
32 |
31 |
37 |
140 |
22 |
25 |
21 |
23 |
23 |
44 |
329 |
|
Biovolume mm3 (total except Carringtonia cf. carringtoniana) |
227846.07 |
329274.79 |
158490.04 |
1206310.86 |
134752.23 |
88446.47 |
11842.28 |
25155.21 |
89108.23 |
192901.66 |
1238439.79 |
|
% biovolume seminfaunal productides |
62.06 |
99.32 |
73.69 |
81.49 |
20.26 |
98.78 |
36.73 |
86.73 |
82.62 |
93.07 |
76.58 |
|
% biovolume pedicle-attached |
1.91 |
0.68 |
2.46 |
1.20 |
1.57 |
1.22 |
63.27 |
11.08 |
3.91 |
6.08 |
3.69 |
|
% biovolume pedicle-attached with adaptations |
36.03 |
0.00 |
23.85 |
17.31 |
78.17 |
0.00 |
0.00 |
2.18 |
13.46 |
0.85 |
19.73 |
|
Margalef diversity index |
3.75 |
4.37 |
3.88 |
6.27 |
2.91 |
3.11 |
1.31 |
2.23 |
3.83 |
4.23 |
6.56 |
|
Shannon-Wiener diversity index |
2.68 |
2.72 |
2.53 |
3.03 |
2.16 |
2.42 |
1.47 |
1.82 |
2.70 |
2.77 |
3.03 |
|
Dominance |
0.07 |
0.08 |
0.12 |
0.08 |
0.16 |
0.09 |
0.24 |
0.23 |
0.06 |
0.07 |
0.08 |
|
Eveness |
1.04 |
0.95 |
0.83 |
0.65 |
0.87 |
1.02 |
0.87 |
0.77 |
1.15 |
0.94 |
0.53 |
Results
Brachiopod fauna composition and comparison with coeval faunas.—The studied brachiopod fauna comprises 45 species, representing 36 genera. Abundances and distribution of species in the different units of the mud mound complex are given in Table 2. Several poorly preserved specimens were identified as belonging to indeterminate species of taxa of Productoidea, Orthotetida, Spiriferida, and Terebratulida. Seven brachiopod orders are represented in the fauna: Productida, Orthotetida, Orthida, Rhynchonellida, Spiriferida, Spiriferinida, and Terebratulida.
Brachiopods from the Ricklow Quarry mud mound complex core had been previously listed by Gutteridge (1990) who reported a faunal list of 55 species of 35 genera. Our study revealed the list of Gutteridge (1990) was exhaustive regarding productide species (Table 2). However, the present study did not identify the productellid Acanthoplecta mesoloba (Phillips, 1836) and did not identify the high abundance of Productus productus (Martin, 1809) noted by Gutteridge (1990). Our study also suggested that Gutteridge (1990) underestimated orthotetide, spiriferide, and terebratulide diversity (Table 2).
Table 2. Range chart of the brachiopod fauna in the Ricklow mound complex (FA1, FA2, FA3). The table also shows the faunal composition of the fauna collected from Wensley Dale (WI1) and the one collected by Gutteridge (1990) in the Ricklow mound complex core, with synonymy and inferred abundance. Rare (r) <5%; sparse (s) 5–10%; common (c) 10–25%; abundant (a) >25%; ?, unkown location in the mound complex.
|
Species |
FA1 |
FA2 |
FA3 |
WI1 |
Gutteridge (1990) |
|
Alitaria aff. triquetra |
s |
r |
|
|
?Alitaria panderi; r |
|
Eomarginiferina trispina |
|
|
|
r |
|
|
Productina margaritacea |
|
|
|
|
r |
|
Overtonia fimbriata |
|
r |
|
|
r |
|
Avonia sp. |
|
r |
|
|
Avonia youngiana; s |
|
Quasiavonia aculeata |
r |
s |
r |
r |
Avonia aculeata; s |
|
?Quasiavonia sp. |
r |
|
|
|
|
|
Krotovia spinulosa |
r |
s |
|
|
s |
|
Breileenia radiata |
|
r |
r |
|
Avonia davidsoni; s |
|
Acanthoplecta mesoloba |
|
|
|
|
r |
|
Geniculifera keyserlingiana |
|
r |
|
|
|
|
Carringtonia cf. carringtoniana |
r |
|
|
|
|
|
Limbifera sp. |
r |
|
|
|
Sinuatella cf. sinuata; r |
|
Productus cf. productus |
|
r |
|
|
Productus productus; s |
|
Productus sp. |
r |
|
|
|
|
|
Eomarginifera cf. laqueata |
r |
r |
|
r |
Eomarginifera cf. lobata/E. longispinus group; r |
|
Antiquatonia hindi |
s |
s |
r |
c |
Antiquatonia cf. hindi; r |
|
Antiquatonia aff. hindi |
r |
r |
|
|
Antiquatonia sulcata; r |
|
Antiquatonia insculpta |
c |
r |
s |
|
Antiquatonia insculpta; r |
|
Leioproductinae gen. et sp. indet. |
|
|
|
|
Leioproductoid; r? |
|
Dictyoclostus pinguis |
r |
r |
r |
|
Dictyoclostus sp.; s |
|
Pugilis cf. kilbridensis |
r |
r |
|
|
Pugilis cf. pugilis/ Pugilis sp.; r |
|
Pugilis cf. scotica |
r |
|
|
|
|
|
Buxtonia scabricula |
r |
r |
s |
c |
Buxtonia sp.; r |
|
Buxtonia sp. |
r |
|
|
r |
Marginicinctus sp.; r |
|
Kochiproductus sp. |
|
|
|
|
r |
|
Buxtoniinae gen. et sp. indet. |
|
r |
|
|
Buxtonoid; r |
|
reticulate Productoidea gen. et sp. indet. |
c |
r |
r |
r |
Costate productoid indet.; s |
|
Echinoconchus punctatus |
r |
r |
c |
s |
r |
|
Echinoconchus subelegans |
|
|
|
|
r |
|
Pustula cf. pustulosa |
r |
r |
|
|
Pustula sp.; r |
|
Linoprotonia sp. |
r |
r |
|
|
r |
|
Fluctuaria undata |
? |
? |
? |
|
r |
|
Schellwienella sp. |
|
r |
|
|
|
|
?Serratocrista aff. dalriensis |
|
r |
|
|
|
|
Orthotetidina gen. et sp. indet. |
|
|
r |
|
Davidsonacean; r |
|
Rugosochonetes sp. |
|
|
|
|
r |
|
Schizophoria resupinata |
r |
r |
c |
|
Schizophoria sp.; s |
|
Schizophoria cf. connivens |
r |
|
r |
|
|
|
Rhipidiomella sp. |
|
|
|
|
r |
|
Pugnax acuminatus |
|
|
|
|
r |
|
Pugnax codiformis |
|
|
|
|
r |
|
Pugnax cf. pugnoides |
|
|
|
|
r |
|
Pleuropugnoides pleurodon |
r |
s |
r |
|
r |
|
Propriopugnus pugnus |
r |
r |
r |
r |
Pugnax pseudopugnus; r |
|
Coledium sp. |
|
|
|
|
r |
|
Crurithyris urei |
|
r |
|
|
|
|
Martiniinae gen. et sp. indet. |
r |
|
|
r |
Martinia sp., r |
|
Angiospirifer bisulcatus |
|
|
|
s |
Spirifer bisulcatus; r |
|
?Fusella sp. |
|
r |
|
|
Fusella sp./Spirifer triangularis; r |
|
Latibrachythyris cf. crassa |
r |
r |
|
|
Spirifer aff. planicostus; r |
|
Latibrachythyris rotundata |
r |
r |
s |
|
|
|
Brachythyrididae gen. et sp. indet. |
|
r |
|
|
Brachythyris ovalis; r |
|
?Reticularia cf. mesoloba |
|
r |
|
|
|
|
Georgethyris lobata |
|
|
|
r |
|
|
Phricodothyris paricosta |
r |
r |
|
|
|
|
Phricodothyris cf. periculosa |
s |
r |
|
s |
|
|
Phricodothyris sp. |
r |
r |
|
|
|
|
Nebenothyris sp. |
|
|
|
r |
|
|
Spiriferida gen. et sp. indet. |
r |
r |
|
|
Smooth spiriferoid; r |
|
?Punctospirifer sp. |
|
r |
|
|
|
|
Hustedia radialis |
|
|
|
|
r |
|
Harttella oakleyi |
s |
c |
|
|
Girtyella sacculus; a |
|
Beecheria sp. |
|
r |
|
|
|
|
?Balanoconcha sp. |
r |
r |
|
|
|
|
Terebratulida gen. et sp. indet. |
r |
r |
r |
|
|
The Ricklow Quarry fauna is similar to the one listed from other Brigantian mud mounds of the Derbyshire Carbonate Platform by Smith et al. (1967: 46) and Mitchell (1971: 140). Smith et al. (1967) based his study on mounds at the top of the Monsal Dale Limestone Formation at the eastern edge of the Derbyshire Platform, around Matlock, while Mitchell (1971) studied the mounds of the northern portion of the platform from the same stratigraphical level, near Intake Dale, Bradwell. The only differences are the absence in the fauna under study of the productellid Acanthoplecta mesoloba and the scarcity of specimens of Buxtonia scabricula (Sowerby, 1814), ?Fusella triangularis (Sowerby, 1827), small-sized species of Productus (e.g., Productus hispidus Muir-Wood, 1928), and Schizophoria resupinata (Martin, 1809).
Instead, the brachiopod assemblages from the mud mound complex of Ricklow Quarry are rather different from those typical of the platform interior of the Derbyshire Carbonate Platform in the late Visean (Wolfenden 1958). Platform interior taxa of Chonetidina (e.g., Daviesiella aff. comoides Sowerby, 1823a), Athyridida (Athyris expansa Phillips, 1836), and Spiriferinida (e.g., Davidsonina septosa Phillips, 1836) seem to be restricted to the platform interior (Wolfenden 1958) and were never found in the mud mound complex. Also, giant linoproductoid species (e.g., species of Gigantoproductus) were never found in the mound complex, though they are abundant in the beds onlapping the subaerial exposure surface capping the buildup (Nolan et al. 2017).
All brachiopod taxa collected in the mud mound complex were also found in upper Visean framework reef facies (Wolfenden 1958; Brunton and Tilsley 1991). However, the mud mound brachiopod fauna appears to be less diverse with greater dominance of a few taxa than that of the framework reef. The Craven Reef Belt in North Yorkshire yields assemblages with up to 40 species (Brunton and Tilsley 1991; 125 species in the entire belt). Many taxa typical of framework reefs were not found in the mud mound complex of Ricklow Quarry (e.g., species of Eomarginiferina, Angiospirifer, and Georgethyris), but most notable is the absence of taxa adapted to hard substrates, such as the shell-attached Strophalosioidea and Aulostegoidea which are widespread in Visean framework reef environments (Brunton and Mundy 1988; Brunton and Tilsley 1991).
Though most taxa in the mud mound complex were also identified in the Asbian reefs of Derbyshire and Yorkshire (Brunton and Tilsley 1991), the productellid Breileenia radiata Brunton in Brunton and Lazarev, 1997, the productids Eomarginifera laqueata (Martin, 1809) and Pugilis kilbridensis (Muir-Wood, 1928), and the terebratulide Harttella oakleyi Brunton, 1982, seem to be restricted to the Brigantian substage (Muir-Wood 1928; Mitchell 1971; Brunton and Tilsley 1991; Brunton et al. 1993). The rhynchonellide Pleuropugnoides pleurodon (Phillips, 1836), which is abundant in the fauna of the Ricklow Quarry mud mound complex, is considered typical of the upper Visean in Great Britain (Angiolini et al. 2021: fig. 5). The brachiopod fauna in the Ricklow Quarry mud mound complex thus confirms its Brigantian age, previously inferred from its stratigraphic position.
Brachiopod distribution in FA1, FA2, and FA3.—Brachiopods are widespread in the packstone beds and tabular mounds at the base of the mud mound complex of Ricklow Quarry (FA1). They are commonly articulated, but not in life position, although some may occur in life position at the base of beds (Fig. 4E). Productide spines frequently occur articulated to the shell, while only valves of large-sized spiriferides (Latibrachythyris cf. crassa De Koninck, 1843) occur disarticulated. Specimens are usually not fractured, though some are crushed by mechanical compaction. The ornamentation is usually well preserved with few signs of corrasion. Productides are dominant both in terms of species (19 in a total of 30; Fig. 6A1) and specimen number (71.4%; Fig. 6B1). Productidae are widespread, followed in abundance and diversity by Productellidae and a minor amount of Echinoconchidae and Monticuliferidae (Fig. 6C1). Also, spiriferides (13.6% of specimens) and terebratulides (7.1%) are common in the FA1 unit, while rhynchonellides and orthides are rare (Fig. 6A1, B1).
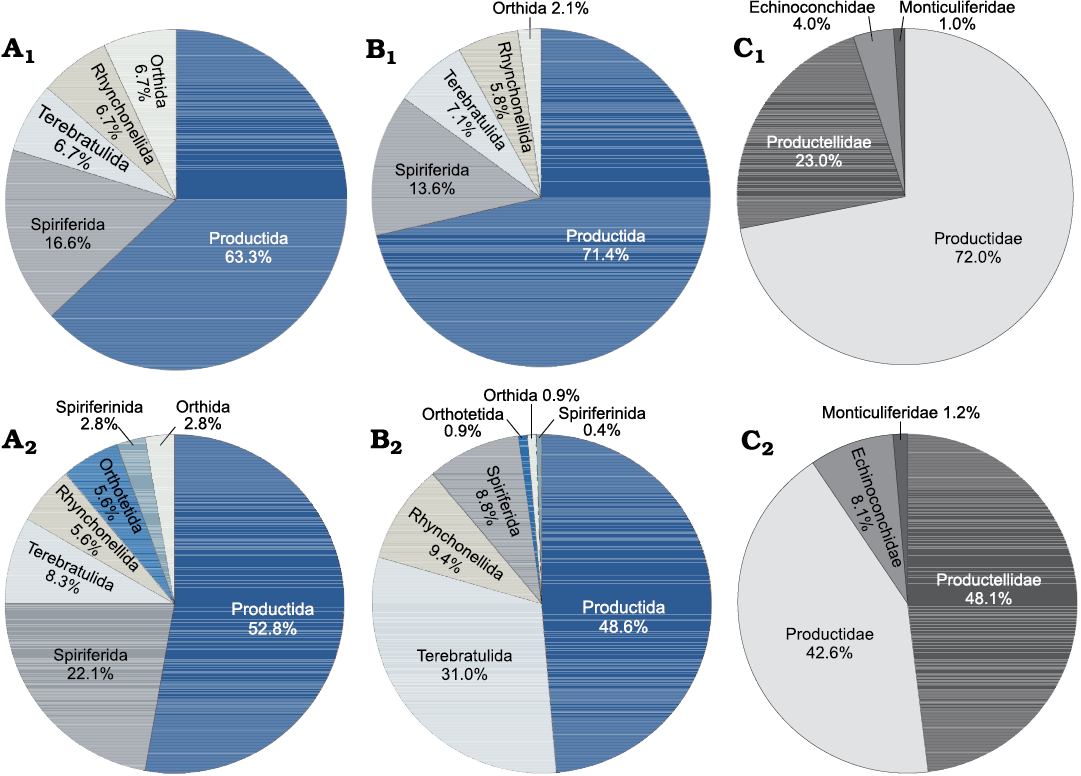
Fig. 6. Pie diagrams showing composition of the Brigantian (uppermost Visean) Ricklow Quarry mud mound complex brachiopod fauna. A. Species percentage distribution of the various brachiopod orders for unit FA1 (A1), FA2 (A2). B. Specimen percentage distribution of the various brachiopod orders for unit FA1 (B1), FA2 (B2). C. Specimen percentage distribution of the various families of the order Productida for unit FA1 (C1), FA2 (C2).
In the mud mound complex core (FA2) brachiopods are widespread and very abundant as well. Productides are commonly found in life position, frequently with some spines attached to the shell (Fig. 4F). Rhynchonellides, spiriferides, and terebratulides always occur articulated; valves of orthotetides occur disarticulated. Specimens are not fractured and show few signs of corrasion. Although productides (mainly Productidae and Productellidae) are still dominant (19 species in a total of 36; 48.6% specimens; Fig. 6A2, B2, C2), terebratulide specimens are more numerous than in FA1 (31.0%; Fig. 6B2). Spiriferides form a minor percentage of specimens with respect to FA1 (8.8%) but are more diverse (eight species in FA2, five in FA1). Rhynchonellides occur as well in small percentages, alongside orthotetides, orthides, and spiriferinides (Fig. 6A2, B2). Diversity indices and dominance are, however, similar between FA1 and FA2 (Table 1).
In the flank beds (FA3), brachiopods are abundant in the proximity of the mud mound complex core, whereas they become sparser away from it. They are similarly well preserved: some productides occur articulated but convex-up, not in life position. Productides are still dominant in terms of species (seven in a total of 14) and specimen number (47.6%). Orthides are also abundant (28.6% of specimens).
Based on their biostratinomic features (articulation, life-position, absence of fracturing, and corrasion), the brachiopod assemblages in FA1 and FA2 are interpreted as neighbourhood to life assemblages, i.e., that suffered limited or no transport (sensu Brenchley and Harper 1998). In FA3, the collected brachiopod assemblage is a neighbourhood one. As these beds had a depositional dip at the time, it is likely that gravity and faint currents moved down-slope and overturned some specimens living on the mud mound complex flank.
Our detailed study shows that brachiopods are much more homogeneously distributed in the mud mound complex both in terms of density of specimens and diversity with respect to the model proposed by Gutteridge (1990), who reported brachiopods from Ricklow Quarry mud mound core to occur only in decimetre-scale “pockets”. We did not find any discrete concentrations with a clearly distinguishable higher abundance and diversity of fossil brachiopods. This topic will be covered in more detail in a work in progress on the Ricklow Quarry mud mound complex, dealing with its facies architecture and depositional model.
Discussion
The neighbourhood to life brachiopod assemblages of the mud mound complex in Ricklow Quarry show a variety of lifestyles and trophic strategies that provide valuable insights when analysed in relation to the three facies associations.
Spinose, concavo-convex productides are dominant in the mud mound complex fauna. These have been commonly considered as adapted to live on soft muddy substrates, with a seminfaunal lifestyle (e.g., Muir-Wood and Cooper 1960; Thayer 1975; Brunton and Mundy 1988; Leighton et al. 1996). According to Mundy (1980), Productellidae and Echinoconchidae were shallow seminfaunal taxa, while long-trailed productoids such as species of Productus, Antiquatonia, and Dictyoclostus lived with more than one half of their valve inside the muddy substrate. Only species of Carringtonia may have lived differently, as they had a shallow corpus cavity and short trail, characters which were interpreted by Martinez-Chacon (2010) as adaptations to live on hard substrates with attachment by spines. Orthides, rhynchonellides, spiriferides, spiriferinides, and terebratulides were attached by pedicles and were thus adapted to a wider range of substrates (Brunton 1982, 1984, 1987; Harper and Jeffrey 1996; Richardson 1997). Though orthotetides had almost complete pseudodeltidia (McIntosh 1974), Grant (1980) described koskinoid perforations in some species of Schuchertella and Schellwienella; these perforations were likely openings for pedicle fibrils, used for attachment. Some pedicle-attached brachiopods in the Ricklow Quarry fauna show adaptations that can be interpreted as addressed to increase the surface-volume ratio to prevent sinking in soft muddy substrates. These brachiopods were ambitopic: they were initially fixed by their pedicle but when adult they lived free on the seafloor. These are orthides and orthotetides, with a wide flat shell (“snowshoe strategy”, Copper 1992), and some spiriferides and spiriferinides with a wide interarea (species of Fusella, Latibrachythyris, Punctospirifer; “ski strategy”, Copper 1992; Legrand-Blain 1986). Other spiriferides (Martiniinae, Reticularioidea), rhynchonellides, and terebratulides did not have a shell shape adapted to prevent sinking in muddy substrate and lived pedicle-attached to skeletal debris or on firm surfaces on the seafloor. However, Brunton (1987) and Harper and Jeffrey (1996) considered the rhynchonellide Pleuropugnoides pleurodon as ambitopic.
Brachiopod species in the fauna under study also show slightly different suspension-feeding strategies, based on their lophophore filtering efficiency. Productides had a simple feeding mechanism, the lophophore consisting of cilia attached to the dorsal brachial ridges (Muir-Wood and Cooper 1960; Perez-Huerta and Sheldon 2006). Orthotetides, orthides, and rhynchonellides had simple lophophores, while spiriferides and terebratulides had complex, supported spirolophe and plectolophe lophophores, respectively (Richardson 1997). Productides with their simple unsupported lophophore were able to generate multi-directional currents and were capable to filter food resources from a larger area around the shell with respect to more complex lophophore bearing brachiopods as spiriferides (Perez Huerta and Sheldon 2006; Angiolini 2007). Thus, productides are considered able to exploit environments with scarce and scattered food resources, while spiriferides were favoured and reached a large size in eutrophic settings by generating strong unidirectional inhalant currents (Perez Huerta and Sheldon 2006). Studies on modern plectolophe terebratulides Terebratulina septentrionalis (Couthouy, 1838), Liothyrella neozelanica Thomson, 1918, Neothyris lenticularis (Deshayes, 1839), and modern spirolophe rhychonellide Hemithiris psittacea (Gmelin, 1791) demonstrated that in environments with a high concentration of food particles, brachiopods with plectolophe lophophore are not as efficient as the ones with spirolophe lophophore (Rhodes and Thompson 1992, 1993; Peck et al. 1997). Therefore, terebratulides were likely better suited to mesotrophic environments.
Based on their lifestyle and feeding strategy, the brachiopods under study were classified into three groups: (i) productides, with a seminfaunal lifestyle and a simple, unsupported lophophore; (ii) pedicle-attached rhynchonellides, spiriferides, and terebratulides, with a generally efficient and complex lophophore; (iii) pedicle-attached/free-living orthotetides, orthides, spiriferides, and spiriferinides with morphological adaptation to softgrounds, with a low to high-efficiency feeding mechanism. Carringtonia cf. carringtoniana (Davidson, 1862a) was excluded from the count. To assess the distribution of these groups in the facies architecture of the mud mound complex, the biovolume was considered instead of the number of specimens. As biovolume is related to metabolic requirement, it is a better measure of the energy flow and functioning of a palaeocommunity (Powell and Stanton 1985; Angiolini 2007).
In the mud mound complex basal unit (FA1) productides and pedicle-attached brachiopods with adaptations to soft substrate dominate in terms of biovolume (Table 1; Fig. 7A). In fact, these are also the taxa that attain larger sizes in the assemblages; in particular specimens of Dictyoclostus pinguis (Muir-Wood, 1928), Echinoconchus punctatus (Sowerby, 1822) (up to 51 mm in width), Antiquatonia hindi (Muir-Wood, 1928) (up to 35.5 mm in width) among the productides, alongside the orthide Schizophoria resupinata (up to 56.6 mm in width) and the spiriferide Latibrachythyris cf. crassa (up to 50 mm in width). Spiriferides adapted to soft substrates alone represent 11.3% of the total biovolume in FA1. Pedicle-attached brachiopods with no adaptation to soft substrates represent a small percentage in biovolume because they have a limited number of specimens and are smaller in size.
Biovolume distribution in FA1 likely reflects the combined occurrence of abundant soft allomicrite substrates, as shown by facies analysis, and a mesotrophic environment with food resources sufficiently abundant to enable spiriferides to thrive alongside productides.
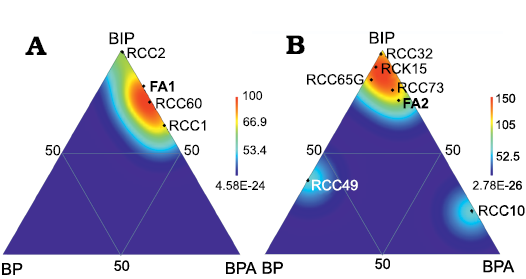
Fig. 7. Density maps in ternary diagrams showing percentages of brachiopod biovolume for the three different ecological groups (seminfaunal productides; pedicle-attached rhynchonellides, spiriferides, and terebratulides; pedicle-attached/free-living orthotetides, orthides, spiriferides, and spiriferinides with adaptation to softgrounds), calculated for FA1 (A), FA2 (B) and some collected assemblages with more than 20 specimens from FA1 (A) and FA2 (B) (see Table 2). Diagrams plotted with PAST software (Hammer et al. 2001). Abbreviations: BIP, biovolume of seminfaunal productides; BP, biovolume of pedicle attached rhynchonellides, spiriferides, and terebratulides (low to high efficiency); BPA, biovolume of pedicle attached/free living orthotetides, orthides, spiriferides, spiriferinides (low to high efficiency).
Productides dominate as well in terms of biovolume in the mound complex core (FA2), where there is a higher variability between assemblages (Table 1; Fig. 7B). Maximum dimensions are reached by the productides: Dictyoclostus pinguis (up to 67 mm in width), Echinoconchus punctatus (up to 59.5 mm in width), Linoprotonia sp. (up to 35 mm in width), and Antiquatonia hindi (up to 43 mm in width), with the only exception of the rare orthotetide Serratocrista aff. dalriensis McIntosh, 1974 (up to 52 mm) and a few Schizophoria resupinata specimens (up to 35 mm in width). Spiriferides with morphological adaptations to soft substrates are small-sized in the complex core, with a moderate maximum size reached by Latibrachythyris rotundata (Sowerby, 1825) (up to 19 mm in width) and Brachythyrididae gen. et sp. indet. (up to 26 mm in width); they represent a minor percentage of the total biovolume (4.5%). Pedicle-attached brachiopods with no adaptation to soft substrates, mainly rhynchonellides, Reticularioidea, and terebratulides are abundant in terms of specimen numbers in the mud mound complex core (Fig. 6B2), but do not occupy a major proportion of the biovolume as they are small. Survivorship curves (Thayer 1975) built for Quasiavonia aculeata (Sowerby, 1814) and Harttella oakleyi (SOM 3, 4) show that the mortality at the juvenile stage was low in two of the three groups considered.
The dominance of productides in terms of biovolume, coupled with the low juvenile mortality in FA2 and small dimensions of the spiriferides, suggests a stable environment with scarce and scattered food resources. Instead, the success of productides can not be related in the mound complex core to the presence of soft substrates, which were likely limited. In fact, the abundance of specimens of pedicle-attached rhynchonellides, spiriferides, and terebratulides is indicative of a more varied substrate with respect to FA1: microbial mats and in situ precipitated automicrite most probably formed firmgrounds; limited hardgrounds were represented by bryozoan fronds and brachiopod shells. The higher abundance and diversity of Productellidae in the mound complex core might be related as well to less soft muddy substrates. Firm substrates may have prevented also the abundance of orthides, which are few in the complex core, even though they were able to reach a large size. However, hardgrounds were not laterally distributed enough to allow colonization by shell-attached Strophalosioidea and Aulostegoidea, typical in the “Cracoean” framework reefs from the upper Visean of England (Brunton and Mundy 1988; Brunton and Tilsley 1991). Food resources shortage, coupled with more stable substrates, can be interpreted as related to lower hydrodynamic energy conditions in the mud mound complex core with respect to the basal FA1 unit.
In conclusion, productides are dominant in the two facies associations and were favoured in FA1 by moderate nutrient supply and soft substrates, in FA2 by scarce and scattered nutrient supply. Spiriferides adapted to soft substrates were not able to successfully adapt to the mud mound complex core (FA2) more varied substrates likely because food resources were limited. Rhynchonellides, Reticularioidea, and terebratulides distribution was mainly controlled by substrate, though food resources may have played a role in limiting the maximum size they reached. The distribution of orthides was mainly controlled by substrate type. Orthotetides are rare in the mud mound complex, but were able to attain a large size; apparently, they did not usually occur together with productides (McIntosh 1974) even though the reason for this is unclear.
Considering not only the brachiopods, but also the associated biota of bryozoans, siliceous sponges, bivalves, and crinoids, the palaeocommunities in the mud mound complex of Ricklow Quarry are dominated by suspension feeders; grazers such as gastropods and trilobites are very rare. The scarcity of bioturbation in the mound facies reflects the absence of infaunal deposit feeders. Few predation marks were detected on brachiopod shells, and goniatites and fish scales were rarely found in petrographic thin sections. This contrasts with other upper Visean localities, such as skeletal grainstone beds adjacent to mounds in Ireland (Brunton 1987) and framework reefs in North Yorkshire (Mundy 1982), where predation marks by gastropods and cephalopods are much more common. The reconstructed food chain for the mud mound complex of Ricklow Quarry is thus a suspension-feeding food chain with a variety of suspension feeders that collected food resources in different ways.
Epifaunal tiering in FA1 and FA2 likely consisted of three levels, roughly corresponding to the ones identified by Ausich and Bottjer (1982) for Phanerozoic suspension feeder communities in soft substrates. The lowest level (first 5 cm) was dominated by seminfaunal productides; large, long-trailed Productidae such as species of Antiquatonia and Dictyoclostus were capable of accessing resources at a higher level in comparison with small Productellidae such as species of Alitaria, Krotovia, and Quasiavonia. This level also hosted epifaunal bivalves. The intermediate tiering level (5–10 cm) was dominated by siliceous sponges and fenestellid bryozoans. Bryozoans were also capable of reaching the highest tiering level, up to 20 cm above the seafloor (Ausich and Bottjer 1982) alongside sparse crinoids. Some young productides were capable of attaching to crinoids and bryozoans with their larval-juvenile pedicle and “clasping spines” (Brunton 1966), thus inhabiting the intermediate and highest tiering level and avoiding competition from other brachiopods until they reached a larger size. This may have been also the case for some pedicle-attached brachiopods (e.g., Pleuropugnoides pleurodon, Phricodothyris paricosta George, 1932, Phricodothyris periculosa George, 1932, and Harttella oakleyi). Otherwise, these taxa inhabited the lowest tiering level.
In the flank beds (FA3) the lowest tiering level was also dominated by productides, alongside orthides (Schizophoria resupinata) which lived on the seafloor. Taxa found in the intermediate level (sponges and bryozoans) were scarce, while the highest tiering level (up to 20 cm height) was occupied by abundant crinoids.
Conclusions
The systematic study of the brachiopod fauna from the Brigantian, uppermost Visean (Mississippian) mud mound complex in Ricklow Quarry led to the recognition of 45 species, representing 36 genera and seven orders (Productida, Orthotetida, Orthida, Rhynchonellida, Spiriferida, Spiriferinida, and Terebratulida). The study revealed a fauna with a much higher orthotetide, spiriferide, and terebratulide diversity than previously known from faunal lists presented for the locality.
In the mound complex, brachiopods are not concentrated in “pockets” in the complex core as suggested by Gutteridge (1990), but are widespread and very abundant in all the three facies associations, representing one of the most important skeletal components of the mud mound palaeocommunities. Brachiopods are commonly found articulated and in life position, and are thus interpreted as life to neighbourhood assemblages. Productides are dominant in terms of species and specimen numbers both in the mound complex basal FA1 unit, the FA2 core, and FA3 flank beds.
Productides and spiriferides with a wide interarea dominate in terms of biovolume in the FA1 unit, while productides alone dominate in the FA2 mound complex core. The success of productides is interpreted as related to scarce and scattered food resources, which they were able to exploit with their simple, unsupported feeding mechanism with respect to spiriferides. This is in agreement with the intraplatform middle-ramp environment proposed for the mound complex by previous authors (Nolan et al. 2017). The abundance and large size of spiriferides with a wide interarea in FA1 may be due to the more widespread occurrence of soft muddy substrates and higher food resources. The presence of diverse rhynchonellides, Reticularioidea, and terebratulides in the FA2 mound complex core, though small-sized, is indicative of more varied, firmer substrates.
In conclusion, the mound ecosystem at Ricklow Quarry supported an abundant and diverse brachiopod fauna whose main distribution controlling factor was the availability of food resources. These were scarce and scattered, especially during deposition of the mound complex core. Substrate type also played a role in controlling brachiopod diversity: varied substrates led to the success of rhynchonellides and terebratulides in the FA2 mound complex core.
Systematic palaeontology
Phylum Brachiopoda Duméril, 1806
Subphylum Rhynchonelliformea Williams, Alwyn, Carlson, Brunton, Holmer, and Popov, 1996
Class Strophomenata Williams, Alwyn, Carlson, Brunton, Holmer, and Popov, 1996
Order Productida Sarycheva and Sokolskaya, 1959
Suborder Productidina Waagen, 1883
Superfamily Productoidea Gray, 1840
Family Productellidae Schuchert,1929
Subfamily Productininae Muir-Wood and Cooper, 1960
Tribe Paramarginiferini Lazarev, 1986
Genus Alitaria Cooper and Muir-Wood, 1967
Type species: Alifera konincki Muir-Wood and Cooper, 1960, from the Visé Formation, upper Tournaisian–Visean, Belgium.
Remarks.—Muir-Wood and Cooper (1960) described the genus as characterised by a cincture, an external narrow groove bordering the ventral corpus anteriorly. However, the cincture is poorly visible even in some of the specimens of Alitaria konincki figured by Muir-Wood and Cooper (1960: pl. 66: 6–12) from the Visean of Belgium.
Alitaria differs from Eomarginiferina Brunton, 1966, from the Visean of Ireland in having a ventral sulcus, larger ears and a different spine arrangement. It also differs from Eomarginifera Muir-Wood, 1930, of the Productidae Gray, 1840, from the Mississippian of Great Britain, in having much larger ears and a strong ventral subperipheral rim.
Alitaria aff. triquetra (Muir-Wood, 1928)
Fig. 8A–E.
Material.—Eleven articulated specimens: MPUM11860 (RCC2-13); MPUM11861 (RCC12-2); MPUM11862 (RCC60-2); MPUM11863 (RCC2-17, 24; RCC11-4; RCC32-1c, 28b; RCC32B-6; RCC60-29; RCC128-9). Five ventral valves: MPUM11864 (RCC2-40); MPUM11865 (RCC1-19, 57; RCC32C-1a; RCC300-11). Six dorsal valves: MPUM11866 (RCC128-13); MPUM11867 (RCC2-3; RCC11-2; RCC32-8a, 29; RCC60-18a). Five fragments: MPUM11868 (RCC10-4; RCC32-3; RCC32C-1d; RCC36-8; RCC49-25). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Small, concavo-convex shell with transversely subtriangular outline; cardinal margin corresponding to maximum width. Ears large, cylindrical, separated by a narrow groove. Cardinal angles right to slightly acute. Both valves geniculated; lateral profile spiral. Corpus cavity moderately deep. Anterior commissure broadly uniplicate. Ventral visceral disc moderately convex, geniculation angle 75°. Trail long, moderately convex. Sulcus starting from visceral disc mid-length, shallow, subrounded, increasing in width anteriorly up to geniculation and being uniform on the trail. Dorsal disc slightly concave, 10.0 mm long; geniculation angle 90°. Trail long. Low, rounded fold developed near umbo, extending on trail, 6.0 mm wide anteriorly.
Ornamentation consists of low, rounded ribs with wide interspaces on visceral discs, becoming prominent, with narrow interspaces on trails. They number 9–10 per 5.0 mm at the geniculation, 6–8 per 5.0 mm at the anterior margin. Sulcus-bounding costae becoming more prominent anteriorly and bifurcating immediately anterior to spines. Both ventral and dorsal discs are ornamented by low, subquadrate rugae, resulting in a reticulate ornamentation. Rugae number 6 per 5.0 mm and become obsolete on ears. Small spines scattered on ventral disc and cardinal margin. Two symmetrical spines on the ventral disc flanks, at mid-length. Two symmetrical large spines, posteriorly projected, at the cardinal extremities. Two symmetrical halteroid spines, laterally projected, on flanks at geniculation. Two symmetrical halteroid spines on ventral trail mid-length, arising from sulcus-bounding costae.
Remarks.—The specimens resemble Alitaria triquetra, erected by Muir-Wood (1928) based on specimens from the upper Visean of Derbyshire, in their dimensions and ornamentation. Also, they show a similar arrangement of the pair of spines at trail mid-length as the specimens of A. triquetra figured by Muir-Wood (1928: pl. 11: 7a–c; pl. 12: 18a–c). However, they have a flatter ventral visceral disc, a sulcus that persists on the trail and finer rib interspaces. Also, they lack the nasute anterior extension of the trail and the cincture which Muir-Wood (1928) claimed to be present in some specimens. The specimens differ from A. panderi (Muir-Wood and Cooper, 1960), from the upper Visean of Great Britain and Ireland, in being larger, in having a ventral sulcus and in having more distinct and coarser ribs.
Genus Eomarginiferina Brunton, 1966
Type species: Eomarginiferina trispina Brunton, 1966, from the middle Visean of County Fermanagh, Ireland.
Remarks.—Eomarginiferina differs from Eomarginifera in having three, rather than four, halteroid spines on the trail, different dorsal adductor scars and a dorsal subperipheral rim not reaching the anterior margin.
Eomarginiferina trispina Brunton, 1966
Fig. 8F.
1928 Productus minutus; Muir-Wood 1928: 195 (pars).
1966 Eomarginifera (Eomarginiferina) trispina; Brunton 1966: pl. 15: 9–22; pl. 16: 1–22; text-fig. 11.
1991 Eomarginiferina trispina Brunton, 1966; Brunton and Tilsley 1991: 191.
2000 Eomarginiferina trispina Brunton, 1966; Brunton et al. 2000: fig. 282: 2a–h.
Material.—One articulated specimen: MPUM11869 (WI1-31) from Wensley Dale, Matlock, Derbyshire, UK, Eyam Limestone Formation, upper Brigantian, upper Visean.
Description.—Small, concavo-convex shell with transversely semicircular outline; cardinal margin wide, almost corresponding to maximum width. Ears small, slightly convex, separated by a narrow groove. Cardinal angles right. Both valves geniculated; lateral profile tight spiral. Corpus cavity deep. Anterior commissure rectimarginate. Ventral visceral disc moderately convex; geniculation angle 100°. Trail long, strongly convex, nasute: anterior extension starting at 20.0 mm from geniculation, prominent. Dorsal disc slightly concave; geniculation angle 90°. Trail long, nasute.
Ornamentation consists of low, rounded ribs, becoming coarser anteriorly (1.2 mm); interspaces narrow. Ribs increase in number anteriorly by intercalation. They number seven per 5.0 mm at geniculation and on trail. Both ventral and dorsal discs ornamented by low, subquadrate rugae, resulting in reticulate ornamentation. They number eight per 5.0 mm length. Rugae absent on ears. One row of small spines bases on cardinal margin. Two symmetrical postero-laterally directed spines on ears, near the cardinal margin. Two symmetrical halteroid spines on flanks at the geniculation. One halteroid spine in the middle of the ventral trail.
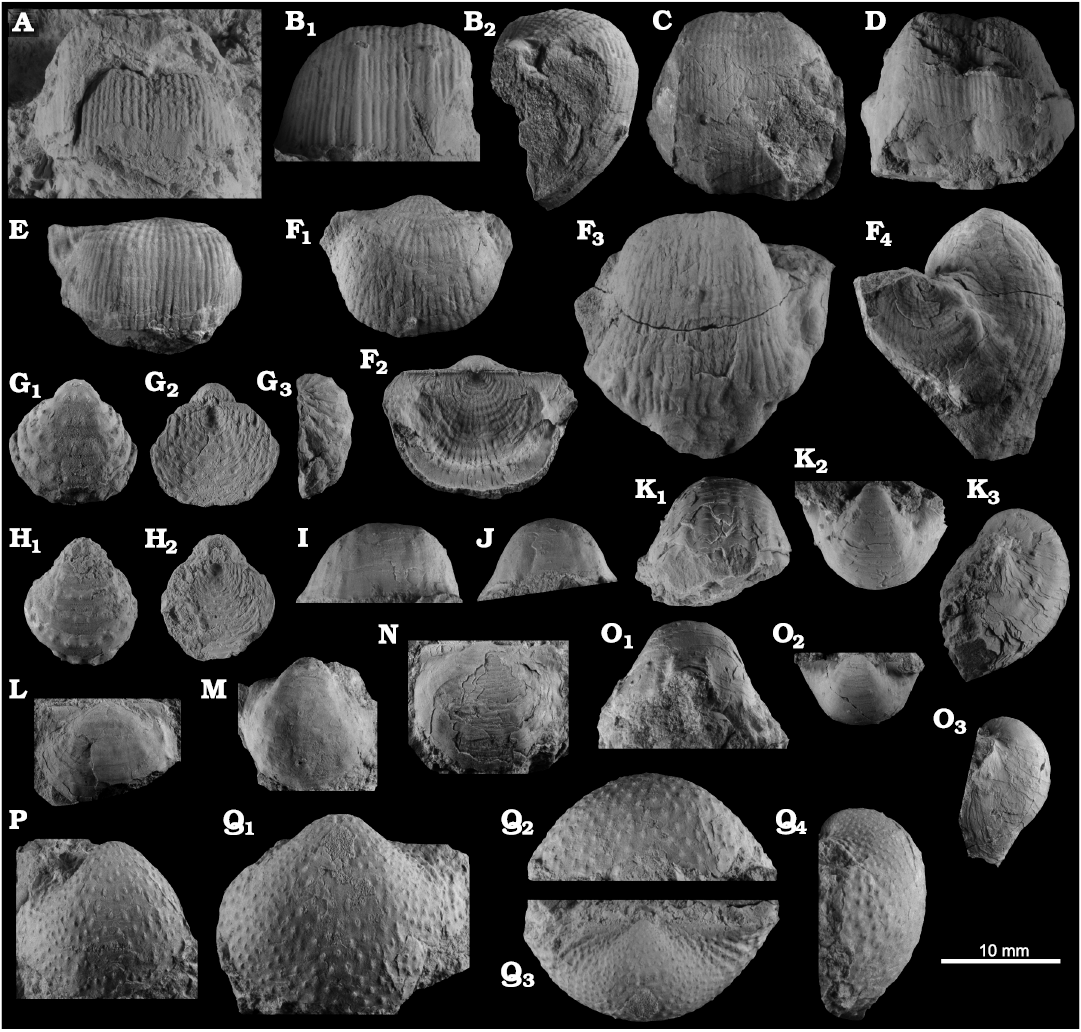
Fig. 8. Productellidae from the Brigantian (uppermost Visean) of Ricklow Quarry, near Monyash (localities RCC-, RCK-; see Fig. 3) and Wensley Dale, near Matlock (locality WI1; see Fig. 2), Derbyshire, UK. A–E. Alitaria aff. triquetra (Muir-Wood, 1928). A. MPUM11860 (RCC2-13) in anterior view. B. MPUM11861 (RCC12-2) in anterior (B1) and lateral (B2) views. C. MPUM11862 (RCC60-2) in antero-ventral view. D. MPUM11864 (RCC2-40), ventral valve in antero-ventral view. E. MPUM11866 (RCC128-13), dorsal valve in anterior view. F. Eomarginiferina trispina Brunton, 1966, MPUM11869 (WI1-31) in ventral (F1), dorsal (F2), anterior (F3), and lateral (F4) views. G, H. Overtonia fimbriata (Sowerby, 1824). G. MPUM11870 (RCK15-1b) in ventral (G1), dorsal (G2), and lateral (G3) views. H. MPUM11871 (RCK15-53) in ventral (H1) and dorsal (H2) views. I. Avonia sp., MPUM11872 (RCC128-4a), ventral valve in anterior view. J–N. Quasiavonia aculeata (Sowerby, 1814). J. MPUM11873 (RCC32-28a) in anterior view. K. MPUM11874 (RCC49-28) in anterior (K1), postero-ventral (K2), and lateral (K3) views. L. MPUM11877 (RCC49-24) dorsal valve interior and external cast. M. MPUM11875 (RCK17-2) in ventral view. N. MPUM11878 (RCK15-51) dorsal valve interior. O. ?Quasiavonia sp., MPUM11883 (RCC3-11); anterior (O1), posterior (O2), and lateral (O3) views. P, Q. Krotovia spinulosa (Sowerby, 1814). P. MPUM11884 (RCC54-17b) in ventral view. Q. MPUM11885 (RCC68-2) in ventral (Q1), anterior (Q2), posterior (Q3), and lateral (Q4) views.
Remarks.—The specimen resembles the specimens of Eomarginiferina trispina described by Brunton (1966) from the Visean of County Fermanagh, Ireland, in its nasute trail, absence of a ventral sulcus, ribs density, and halteroid spines on the trail. However, it differs in being larger, in having a slightly tighter spiral profile and in having spines posterolaterally directed on ears, near the cardinal margin, which are apparently absent in the County Fermanagh specimens (Brunton 1966).
Stratigraphic and geographic range.—Eomarginiferina trispina is recorded in the Asbian, upper Visean, of County Fermanagh in Ireland (Brunton 1966) and the Brigantian, uppermost Visean, of Derbyshire (this study).
Subfamily Overtoniinae Muir-Wood and Cooper, 1960
Tribe Overtoniini Muir-Wood and Cooper, 1960
Genus Overtonia Thomas, 1914
Type species: Producta fimbriata Sowerby, 1824, from the Mississippian of Derbyshire, England.
Remarks.—Overtonia differs from Avonia Thomas, 1914, and other genera of the Overtoniinae in having prominent concentric rugae, each bearing a single row of spine bases.
Overtonia fimbriata (Sowerby, 1824)
Fig. 8G, H.
1809 Conchyliolithus Anomites (punctatus); Martin 1809 (pars): pl. 37: 7–8, non 6.
1824 Producta fimbriata; Sowerby 1824: 85, pl. 549: 1.
1862 Productus fimbriatus Sowerby, 1824; Davidson 1862a: 171, pl. 33: 12–15; non pl. 44: 15. (cum syn.)
1914 Overtonia fimbriata (Sowerby, 1824); Thomas 1914: 259, 335, pl. 20: 12–17. (cum syn.)
1960 Overtonia fimbriata (Sowerby, 1824); Muir-Wood and Cooper 1960: 183, pl. 46: 10–20.
1965 Overtonia fimbriata (Sowerby, 1824); Muir-Wood 1965: fig. 339: 1: a–e.
1966 Overtonia fimbriata (Sowerby, 1824); Brunton 1966: 214–218, pl. 9: 1–13; pl. 10: 1–7; text-fig. 9.
1985 Overtonia fimbriata (Sowerby, 1824); Brunton 1985: fig. 9: a–d.
1991 Overtonia fimbriata (Sowerby 1824); Brunton and Tilsley 1991: 289.
2000 Overtonia fimbriata (Sowerby 1824); Brunton et al. 2000: fig. 283: 1a–g.
Material.—Two articulated specimens: MPUM11870 (RCK15-1b); MPUM11871 (RCK15-53) from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Small, concavo-convex shell with subtriangular outline; cardinal margin wide, corresponding to the maximum width. Corpus cavity deep. Anterior commissure rectimarginate. Dorsal valve moderately concave. Fold-sulcus absent, trail very short.
Ornamentation consists of low, subrounded rugae with broad interspaces, becoming closer and lamellose on flanks. Rugae number three per 7.0 mm length. Rugae bearing regularly spaced, coarse, elongated spine bases. Dorsal valve with lamellae with regularly spaced dimples. Regularly concentric growth lines on both valves.
Cardinal process stout, lateral ridges diverging anteriorly at an angle of 20° from the cardinal margin.
Remarks.—The specimens belong to Overtonia fimbriata for the characteristic ornamentation and the disposition of the lateral ridges (Muir-Wood and Cooper 1960; Brunton et al. 2000). They differ from O. laciniata (M’Coy, 1844), as the latter has a greater number of rugae and smaller, more numerous spine bases.
Stratigraphic and geographic range.—Overtonia fimbriata is recorded in several middle–upper Visean units in Great Britain and Ireland (e.g., Davidson 1862a; Thomas 1914; Wolfenden 1958; Brunton 1966; Mitchell 1971; Brunton and Tilsley 1991; Dean 2017; this study), Belgium (Davidson 1862a), and Germany (Winkler-Prins and Amler 2006).
Tribe Avoniini Sarycheva, 1960
Genus Avonia Thomas, 1914
Type species: Productus youngianus Davidson, 1860a, from the Visean of Scotland.
Remarks.—Avonia differs from Quasiavonia Brunton, 1966, in having narrow faint costae covering all valve surface except the umbonal region, instead of the broad, low ones restricted to the anterior portion of both valves which characterise the latter, and in having weaker and more regular lamellae. Moreover, Avonia is smaller in size, more elongated in outline and has a shallower corpus cavity. Avonia differs from Breileenia Brunton in Brunton and Lazarev, 1997, of the Marginiferinae Stehli, 1954, in being smaller, not geniculated, and in having fainter, narrower costae, but stronger lamellae. Avonia differs from Institifera Muir-Wood and Cooper, 1960, in having a markedly shallower corpus cavity, more elongated outline, less prominent delayed costae and in lacking a gutter anteriorly.
Avonia sp.
Fig. 8I.
Material.—One ventral valve internal cast: MPUM11872 (RCC128-4a) from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Remarks.—The specimen is an internal cast of a small, elongated ventral valve. The valve is enrolled, with no fold or sulcus. The trail bears faint delayed costation; costae are low and narrow (3–4 per 5.0 mm anteriorly), with wide, rounded interspaces. The shell morphology, dimensions and ornamentation are that typical of species of Avonia, but because of the poor preservation the specimen is left under open nomenclature.
Genus Quasiavonia Brunton, 1966
Type species: Productus aculeatus Sowerby, 1814, from Asbian–Brigantian, upper Visean, of Derbyshire, England.
Quasiavonia aculeata (Sowerby, 1814)
Fig. 8J–N.
1809 Conchyliolithus Anomites (aculeatus); Martin 1809: 8, pl. 37: 9–10.
1814 Productus aculeatus; Sowerby 1814: 156, pl. 68: 4.
1862 Productus aculeatus Sowerby, 1814; Davidson 1862a: 166, pl. 33: 16–17, ?18, non 19, ?20. (cum syn.)
1914 Pustula aculeata Sowerby, 1814; Thomas 1914: 320, pl. 19: 9–10, ?11. (cum syn.)
1951 Productus (?Krotovia) aculeatus Sowerby, 1814; Muir-Wood 1951: 101, pl. 3: 3.
non 1966 Avonia (Quasiavonia) aculeata (Sowerby, 1814); Brunton 1966: 220, pl. 10: 8–17; pl. 11: 1–21.
1991 Quasiavonia aculeata (Sowerby, 1814); Brunton and Tilsley 1991: 292.
2000 Quasiavonia aculeata (Sowerby, 1814); Brunton et al. 2000, fig. 284: 2a–e.
Material.—Twenty articulated specimens: MPUM11873 (RCC32-28a); MPUM11874 (RCC49-28); MPUM11875 (RCK17-2); MPUM11876 (RCC1-8e, 25; RCC2-12; RCC32-11; RCC41-24; RCC42-4a; RCC43-73; RCC48-13; RCC49-1, 42; RCC54-1; RCC65G-15a, 15b; RCC73-6, 23g; RCC85-3d; RCK15-55). Sixteen dorsal valves: MPUM11877 (RCC49-24); MPUM11878 (RCK15-51); MPUM11879 (RCC128-4b); MPUM11880 (RCC1-8d; RCC1B-20b; RCC10-13; RCC32-2, 17; RCC42-12a; RCC49-8, 10a, 10b; RCC68-51a, 51b; RCC85-2); MPUM11881 (WI1-27). Eight fragments: MPUM11882 (RCC10-39b; RCC14-8; RCC49-11; RCC65G-8c; RCC68-9; RCC128-23; RCC300-12; RCK15-35). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian (RCC-, RCK-), and Wensley Dale, Matlock, Derbyshire, UK, Eyam Limestone Formation, upper Brigantian, upper Visean (WI1).
Description.—Small, concavo-convex shell with subtriangular outline; cardinal margin wide; maximum width at shell mid-length. Ears small, convex; cardinal angles obtuse. Corpus cavity moderately deep. Anterior commissure rectimarginate. Ventral valve strongly and regularly convex. Dorsal valve concave. Fold-sulcus absent, trail long.
Ornamentation consists of irregular, imbricated lamellae, each 1.0–4.0 mm wide. Broad, faint delayed costae developed on ventral and dorsal trails, starting 8.0–9.0 mm from the umbo, with rounded interspaces. Lamellae cross ribs. Rounded spine bases roughly arranged in quincunx over ventral valve. Dorsal valve bearing similarly disposed rounded pits.
Remarks.—The specimens are close to the lectotype of Quasiavonia aculeata from the Brigantian, upper Visean, around Bakewell (Derbyshire), which was selected by Muir-Wood (1951: 102) and figured by Brunton et al. (2000). They are also similar to the specimens of Q. aculeata figured by Davidson (1862a) and Thomas (1914) from the Visean of Derbyshire and the Isle of Man respectively. They differ from Quasiavonia minranensis Brunton in Brunton and Lazarev, 1997, in having spine bases arranged quincuncially, rather than concentrically.
Stratigraphic and geographic range.—Quasiavonia aculeata is recorded in the Asbian–Brigantian, upper Visean, of Great Britain and Ireland (e.g., Thomas 1914; Gutteridge 1990; Brunton and Tilsley 1991; this study). The species is also recorded in the Visean of Germany (Winkler-Prins and Amler 2006).
?Quasiavonia sp.
Fig. 8O.
Material.—One articulated specimen: MPUM11883 (RCC3-11) from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Remarks.—The specimen is similar to the specimens assigned to Quasivonia aculeata from the same localities, but it differs in having a quadrate anterior nasute extension of the ventral trail, starting at 10.0 mm from the umbo.
Tribe Krotoviini Brunton, Lazarev, and Grant, 1995
Genus Krotovia Fredericks, 1928
Type species: Productus spinulosus Sowerby, 1814, from the Visean of Great Britain.
Remarks.—Krotovia differs from other genera in the Overtoniinae (e.g., Overtonia, Avonia, and Quasiavonia), by the lack of strongly developed rugae and lamellae.
Krotovia spinulosa (Sowerby, 1814)
Figs. 8P, Q, 9A–E.
1814 Productus spinulosus; Sowerby 1814: 155, pl. 68: 3.
1862 Productus spinulosus Sowerby, 1814; Davidson 1862a: 175, pl. 34: 18–19.
?1862 Productus granulosus Phillips, 1836; Davidson 1862a: 175, pl. 34: 20–21. (cum syn.)
1914 Pustula spinulosa Sowerby, 1814; Thomas 1914: 314, pl. 18: 7–9; pl. 19: 7, non 8. (cum syn.)
non 1943 Krotovia spinulosa (Sowerby, 1814); Prendergast 1943: 30, pl. 4: 19–24.
non 1957 Krotovia spinulosa (Sowerby, 1814); Coleman 1957: pl. 7: 19–24.
1960 Krotovia spinulosa (Sowerby, 1814); Muir-Wood and Cooper 1960: pl. 50: 1–5.
1965 Krotovia spinulosa (Sowerby, 1814); Muir-Wood 1965: fig. 340: 2a–d.
?1966 Krotovia spinulosa (Sowerby, 1814); Brunton 1966: 224, pl. 12: 1–18; pl. 13: 1–7. (cum syn.)
1989 Krotovia spinulosa (Sowerby, 1814); Wilson 1989: fig. 6d.
1991 Krotovia spinulosa (Sowerby, 1814); Brunton and Tilsley 1991: 289.
2000 Krotovia spinulosa (Sowerby, 1814); Brunton et al. 2000: fig. 287: 1a–e.
Material.—Sixteen articulated shells: MPUM11884 (RCC54-17b); MPUM11885 (RCC68-2); MPUM11886 (RCC73-22a); MPUM11887 (RCK15-10); MPUM11888 (RCC1-8f, 23a; RCC32-5a; RCC41-10a, 20a; RCC43-2a, 8a; RCC73-8, 11; RCC85-1b; RCK15-54; RCK16-1). Seven dorsal valves: MPUM11889 (RCC3-18); MPUM11891 (RCK15-16); MPUM11892 (RCK15-28); MPUM11893 (RCC10-24; RCC48-26a, 26b; RCK15-25). Seven fragments: MPUM11894 (RCC2-18; RCC12-4; RCC48-9; RCC49-29; RCC68-5; RCC73-23h; RCK15-44). All from from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Small, concavo-convex shell with transversely suboval outline; cardinal margin wide; maximum width attained at one-third shell length. Ears small, flat. Corpus cavity shallow. Anterior commissure rectimarginate. Dorsal valve strongly concave. Fold and sulcus absent. Trail long.
Rugae on umbonal slopes and ears and faint ribs on ventral valve. Ventral valve also covered by low, slightly elongated spine bases, quincuncially arranged, four in an area of 10.0 mm2 at 5.0 mm from umbo. Regularly spaced, small rounded spines on ears aligned parallel to cardinal margin. Dorsal valve with small circular dimples, quincuncially arranged. Both valves covered by dense irregularly concentric growth lines, occasionally poorly developed lamellae.
Remarks.—The specimens are very similar both in outline and ornamentation to the holotype of Krotovia spinulosa from West Lothian (Scotland), figured by Davidson (1862a), the specimens figured by Sarycheva and Sokolskaya (1952) from the Moscow Basin and those figured by Muir-Wood and Cooper (1960) from the Mississippian of Cumberland (England). They differ from the specimens of K. spinulosa described by Brunton (1966) from the Glencar Limestone of County Fermanagh, Ireland (Asbian, upper Visean), as these show strong lamellose ornamentation and should be revised.
Krotovia granulosa (Phillips, 1836) from the Visean of Great Britain and Ireland was considered as a junior synonym of K. spinulosa by both M’Coy (1844) and Davidson (1862a). Based on the specimens figured by Davidson (1862a) from Yorkshire and Scotland, K. granulosa seems to differ from K. spinulosa in having larger, more concentrically arranged pustules.
Stratigraphic and geographic range.—Krotovia spinulosa is recorded from the middle–upper Visean of Great Britain and Ireland (e.g., Sowerby 1814; Davidson 1862a; Parkinson 1926; Wolfenden 1958; Muir-Wood and Cooper 1960; Brunton 1966; Mitchell 1971; Wilson 1989; Brunton and Tilsley 1991; this study), Germany (Winkler-Prins and Amler 2006), Urals (Skompski et al. 2001), the Moscow Basin (Sarycheva and Sokolskaya 1952), Siberia (Sarycheva et al. 1963) and Morocco (Berkhli et al. 2001).
Subfamily Marginiferinae Stehli, 1954
Tribe Breileeniini Brunton in Brunton and Lazarev, 1997
Genus Breileenia Brunton in Brunton and Lazarev, 1997
Type species: Productus davidsoni Jarosz, 1917, from the Asbian, upper Visean, of Longnor, Derbyshire, England.
Remarks.—Small specimens of species of Breileenia may be confused with those of Institifera Muir-Wood and Cooper, 1960, which is characterised by having a gutter anteriorly in the adult stage. However, Breileenia has a shallower corpus cavity and some species with delayed costae starting near the umbo, rather than on trail.
Breileenia radiata Brunton in Brunton and Lazarev, 1997
Fig. 9F–K.
1997 Breileenia radiata; Brunton in Brunton and Lazarev 1997: 389, figs. 5, 12–21.
2000 Breileenia radiata Brunton in Brunton and Lazarev, 1997; Brunton et al. 2000: fig. 289: 1f–g.
Material.—Three articulated specimens: MPUM11895 (RCC65-1); MPUM11896 (RCC32-13; RCC42-30). Five ventral valve internal casts: MPUM11897 (RCC12-18a; RCC42-50; RCC43-2b; RCC68-10; RCC128-4f). Seven dorsal valve external casts: MPUM11898 (RCC12-1); MPUM11899 (RCC12-7); MPUM11900 (RCC12-15); MPUM11901 (RCC12-23a, 23b); MPUM11902 (RCC12-18b, 18c). Seven fragments: MPUM11903 (RCC38A-2); MPUM11904 (RCC12-22, 26, 28; RCC35A-9a, 9b; RCC300-32). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Medium-sized, elongated concavo-convex shell with subtriangular outline; cardinal margin corresponding to maximum width. Ears small, flat. Cardinal angles right. Corpus cavity shallow. Anterior commissure rectimarginate. Ventral visceral disc poorly convex; geniculation angle 60°. Trail long. Umbo enrolled. Dorsal valve concave, not geniculated, trail long. Fold and sulcus absent.
Ventral visceral disc ornamented by delayed, prominent, subrounded ribs starting 7.5 mm from the umbo, with narrow interspaces. Ribs increase in number anteriorly by bifurcation on flanks. They number 6–7 per 5.0 mm, 15.0 mm from the umbo. Widely spaced (1–1.5 mm) growth lamellae cover all of the valve surface, crossing ribs. Entire valve covered by spine bases, concentrically to quincuncially arranged. Dorsal valve with similar ornamentation, but with rounded dimples instead of spine bases, and ribs interspaces being wider than ribs anteriorly. Micro-ornamentation of sinuous growth lines.
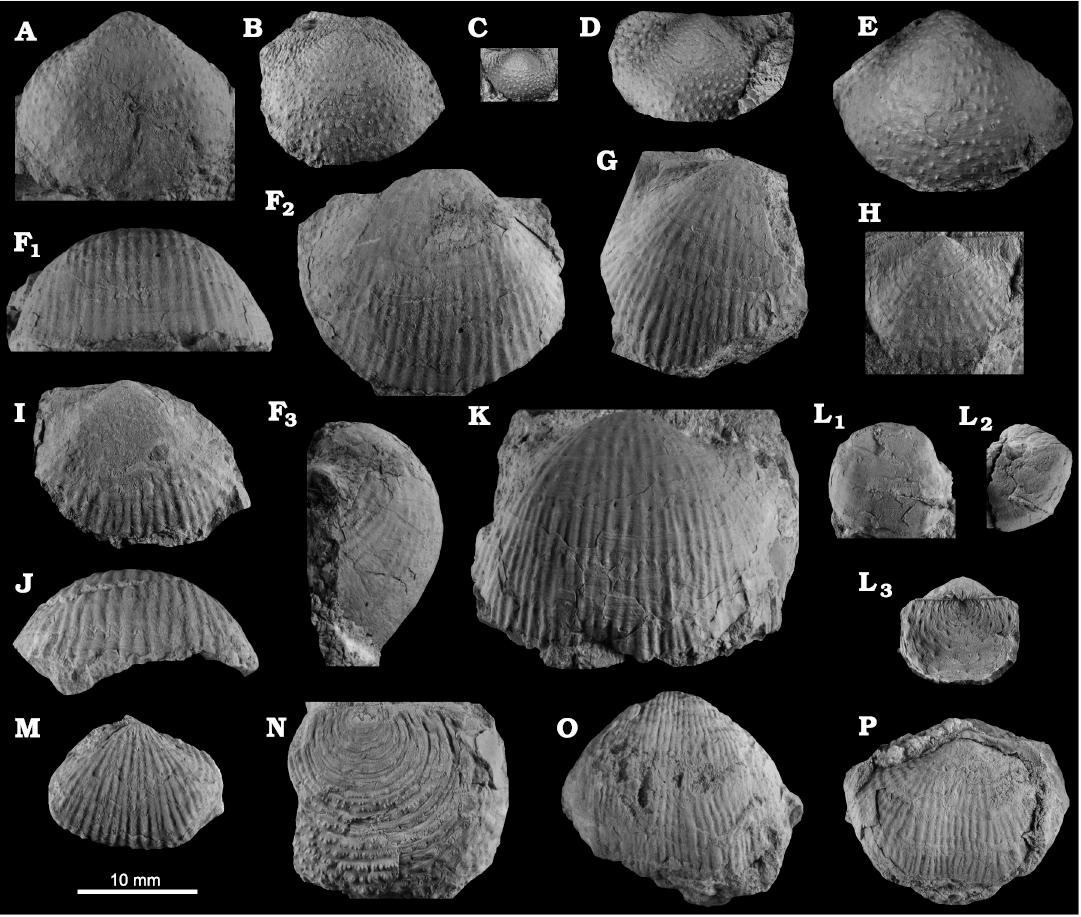
Fig. 9. Productellidae and Productidae from the Brigantian (uppermost Visean) of Ricklow Quarry, near Monyash, Derbyshire, UK (localities RCC-, RCK-; see Fig. 3). A–E. Krotovia spinulosa (Sowerby, 1814). A. MPUM11886 (RCC73-22a) in ventral view. B. MPUM11889 (RCC3-18) dorsal valve interior and external cast. C. MPUM11891 (RCK15-16) interior and external cast. D. MPUM11892 (RCK15-28) dorsal valve interior and external cast. E. MPUM11887 (RCK15-10) in ventral view. F–K. Breileenia radiata Brunton in Brunton and Lazarev, 1997. F. MPUM11895 (RCC65-1) in anterior (F1), ventral (F2), and lateral (F3) views. G. MPUM11898 (RCC12-1) dorsal valve external cast. H. MPUM11899 (RCC12-7) dorsal valve interior and external cast. I. MPUM11900 (RCC12-15) dorsal valve external cast. J. MPUM11903 (RCC38A-2) fragment in anterior view. K. MPUM11901 (RCC12-23b) dorsal valve external cast. L. Geniculifera keyserlingiana (De Koninck, 1843), MPUM11905 (RCC73-28) in antero-ventral (L1), lateral (L2), and dorsal (L3) views. M. Limbifera sp., MPUM11890 (RCC54-10b), ventral valve in ventral view. N. Carringtonia cf. carringtoniana (Davidson, 1862a), MPUM11906 (RCC2-51) dorsal valve interior and external cast. O. Productus sp., MPUM11908 (RCC1-61b) in ventral view. P. Productus sp., MPUM11909 (RCC60-6) dorsal valve external cast.
Remarks.—The specimens are close in their dimensions, morphology and ornamentation to the specimens of Breileenia radiata figured by Brunton and Lazarev (1997), including the holotype from the Brigantian, uppermost Visean, of Fall Hill Quarry (Derbyshire). Breileenia radiata had been confused with the closely allied Breileenia davidsoni (Jarosz, 1917), from the Asbian, upper Visean, of Great Britain and Ireland, until the revision of the species by Brunton (Brunton and Lazarev 1997). Brunton showed B. radiata to differ from B. davidsoni in having well-developed delayed costae starting near umbo, rather than being restricted anteriorly on trail, and denser spines. Spine bases are mostly not preserved in the specimens from Ricklow Quarry, but costae arise near umbo and are stronger than the ones shown by the specimens of B. davidsoni. Mitchell (1971) recorded specimens of B. davidsoni in the study area from the Monsal Dale and Eyam limestones formations which are likely specimens of B. radiata.
Stratigraphic and geographic range.—Breileenia radiata is recorded from the Brigantian, uppermost Visean of Derbyshire (Brunton and Lazarev 1997; this study).
Subfamily Plicatiferinae Muir-Wood and Cooper, 1960
Tribe Levitusiini Muir-Wood and Cooper, 1960
Genus Geniculifera Muir-Wood and Cooper, 1960
Type species: Avonia boonensi Branson, 1938, from the lower Tournaisian of Missouri, USA.
Geniculifera keyserlingiana (De Koninck, 1843)
Fig. 9L.
1843 Productus keyserlingiana; De Koninck 1843: 200, pl. 10: 8.
1847 Productus keyserlingianus De Koninck, 1843; De Koninck 1847: 239, pl. 14: 6.
1862 Productus keyserlingianus De Koninck, 1843; Davidson 1862a: 174, pl. 34: 15–16.
1991 Geniculifera keyserlingiana (De Koninck, 1843); Brunton and Tilsley 1991: 291.
1993 Geniculifera keyserlingiana (De Koninck, 1843); Brunton et al. 1993: 114, figs. 61–71.
2000 Geniculifera keyserlingiana (De Koninck, 1843); Brunton et al. 2000: fig. 304.
Material.—One articulated specimen: MPUM11905 (RCC 73-28) from from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Small, elongated concavo-convex shell with semi-elliptical outline; cardinal margin corresponding to the maximum width. Ears small, convex, poorly separate. Cardinal angles right. Both valves geniculated. Corpus cavity deep. Anterior commissure rectimarginate. Ventral visceral disc moderately convex; geniculation angle about 90°. Trail long; flanks steep. Dorsal visceral disc slightly concave posteriorly, more concave anteriorly. Flat ears. Geniculation angle 90°. Trail long. Fold and sulcus absent.
Ornamentation consists of low, rounded, circular spine bases covering the ventral visceral disc, quincuncially arranged. Broad (1.0 mm), low, rounded costae with flat wide interspaces on ventral trail. Dorsal valve covered by low, rounded rugae; they number four per 3.0 mm length. Micro-ornamentation of concentric growth lines on both valves.
Remarks.—The specimen differs from Geniculifera boonensis (Branson, 1938) in having more numerous prominent swollen spine bases on the ventral visceral disc and weaker concentric ornamentation.
Stratigraphic and geographic range.—Geniculifera keyserlingiana is recorded from the Asbian–lower Brigantian, upper Visean, of Visé in Belgium and of Derbyshire and Yorkshire in England (Brunton et al. 1993; this study).
Tribe Rugaurini Lazarev, 1990
Genus Carringtonia Brunton and Mundy, 1986
Type species: Productus carringtoniana Davidson, 1862a, from the Visean of Great Britain and Ireland.
Remarks.—The genus differs from other Mississippian productellids in having an ornamentation dominated by dense, irregular rugae. The genus differs from Orbinaria Muir-Wood and Cooper, 1960, from the Mississippian of USA, in having a more prominent concentric ornamentation and spines restricted to the ventral valve.
Carringtonia cf. carringtoniana (Davidson, 1862a)
Fig. 9N.
Material.—Two dorsal valves: MPUM11906 (RCC2-51); MPUM11907 (RCC1-11) from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Small concave dorsal valve. Convex geniculation, short trail. Dorsal valve covered by rounded rugae with wide rounded interspaces, eight per 5.0 mm length posteriorly, more prominent and scattered anteriorly. Trail with low, broad costae, 1.5 mm wide. Subcircular dimples scattered over visceral disc and trail, closely packed, aligned in rugae interspaces anteriorly.
Remarks.—The specimens are close to C. carringtoniana in their rugate ornamentation density (Brunton and Mundy 1986).
Tribe Semicostellini Nalivkin, 1979
Genus Limbifera Brunton and Mundy, 1988
Type species: Productus griffithianus De Koninck, 1847, from the upper Mississippian of Belgium.
Remarks.—The genus differs from the allied Cinctifera Muir-Wood and Cooper, 1960, from the Visean of Belgium, in lacking a cicatrix and cinctures, and having costae starting at the umbo.
Limbifera sp.
Fig. 9M.
Material.—One ventral valve: MPUM11890 (RCC54-10b) from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Small, transversely subrectangular ventral valve. Anterior commissure rectimarginate. Ventral visceral disc flat; geniculation angle about 100°. Very shallow sulcus.
Ornamentation consists of low, subrounded, bifurcating ribs with narrow interspaces, starting from umbo. They number 6–7 per 5.0 mm, 10.0 mm from the umbo. Subrounded rugae, 7–8 in 5.0 mm on ventral visceral disc, resulting in reticulate ornamentation.
Remarks.—The specimen is similar in the outline and ornamentation to Limbifera griffithiana from the upper Mississippian of England and Belgium, figured by Muir-Wood (1928: pl. 3: 1–3) and Brunton and Mundy (1988: figs. 5, 8.1–22, 9.1–7). However, the gutter anteriorly is not preserved, and it has stronger costae and a less evident concentric ornamentation.
Family Productidae Gray, 1840
Subfamily Productinae Gray, 1840
Tribe Productini Gray, 1840
Genus Productus Sowerby, 1814
Type species: Conchyliolithus Anomites (productus) Martin, 1809, from the upper Visean of Derbyshire, England.
Remarks.—The genus can be distinguished from others in the Productidae in having a straight, laterally spreading trail, a suboval rather than subquadrate corpus outline and in the absence of a ventral sulcus. Most species are characterised also by having spines arranged only in 2–3 rows on ears and scattered on visceral disc, and by the presence of a dorsal diaphragm.
Productus cf. productus (Martin, 1809)
Fig. 10A.
Material.—One articulated specimen: MPUM11919 (RCC41-3a) from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Medium-sized, elongated concavo-convex shell with suboval outline; cardinal margin wide. Ears large, convex. Both valves geniculated. Anterior commissure rectimarginate. Ventral visceral disc convex, slightly depressed anteriorly in the middle; geniculation angle 120°. Trail straight, long, laterally spreading anteriorly.
Ornamentation consists of low, rounded ribs with narrow subangular interspaces. Ribs increase in number anteriorly by intercalation. They number 12 per 10.0 mm at geniculation and on trail, where some become larger than average and sinuous. Subangular rugae on flanks, fainter on visceral disc, absent from trail. They number 5 per 5.0 mm length.
Remarks.—The specimen is close to P. productus based on the absence of a ventral sulcus, on the laterally spreading trail, and the lack of spine rows on flanks (Muir-Wood 1928). The density of ribs is similar to the one reported by Muir-Wood (1928: 41).
Productus sp.
Fig. 9O, P.
Material.—Two articulated specimens: MPUM11908 (RCC1-61b); MPUM11909 (RCC60-6) from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Medium-sized, elongated concavo-convex shell with suboval outline; cardinal margin straight. Ears large, convex. Both valves geniculated. Anterior commissure rectimarginate. Ventral visceral disc convex; geniculation angle about 90°. Trail straight, long, laterally spreading anteriorly. Dorsal valve geniculation angle 90°. Very low fold on trail.
Ornamentation consists of low, rounded ribs with narrow interspaces. Ribs increase in number anteriorly by intercalation. They number 14 per 10.0 mm at geniculation and on trail.
Remarks.—The specimens are close to Productus productus in lacking a ventral sulcus and in having a laterally spreading trail, but differ from it in having a shorter visceral disc and smaller overall dimensions. They may be similar to P. hispidus Muir-Wood, 1928, in being small-sized, but differ from it in having finer ornamentation.
Tribe Kozlowskiini Brunton, Lazarev, and Grant, 1995
Genus Eomarginifera Muir-Wood, 1930
Type species: Productus longispinus Sowerby, 1814, from the upper Visean of West Lothian, Scotland.
Remarks.—The genus was erected by Muir-Wood (1930) and then redefined by Muir-Wood and Cooper (1960) to include small posteriorly reticulate productides with moderate ears and six symmetrical halteroid spines on the ventral valve. It includes various species, many from the upper Visean of Great Britain and Ireland (Muir-Wood 1928, 1930), with a wide morphological variability.
The genus differs from Promarginifera Shiells, 1966, from the upper Visean of Scotland, assigned to the Retariini Muir-Wood and Cooper, 1960, in having a flat geniculated dorsal valve, less numerous, more regularly disposed spines on the ventral valve and in lacking dorsal spines. Moreover, Promarginifera has a diagnostic dorsal non-crenulate subperipheral rim dying out on the flanks.
Eomarginifera cf. laqueata (Muir-Wood, 1928)
Fig. 10B–E.
Material.—Four articulated specimens: MPUM11910 (RCC32-26); MPUM11911 (WI1-41); MPUM11912 (RCC128-16); MPUM11913 (WI1-5). Three dorsal valves: MPUM11914 (RCC60-35); MPUM11915 (RCC300-13); MPUM11916 (RCC 54-12). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian (RCC-, RCK-), and Wensley Dale, Matlock, Derbyshire, UK, Eyam Limestone Formation, upper Brigantian, upper Visean (WI1).
Description.—Small, elongated concavo-convex shell with transversely subtriangular outline, cardinal margin corresponding to maximum width. Ears small, convex, separated by a narrow groove. Both valves geniculated. Corpus cavity deep. Anterior commissure rectimarginate to gently uniplicate. Ventral visceral disc moderately convex; geniculation angle 70°. Trail long; flanks steep. Shallow sulcus starting from visceral disc mid-length. Dorsal visceral disc flat; geniculation angle 90°. Fold very low, rounded.
Ornamentation consists of low, subrounded ribs with narrow interspaces. Ribs number nine per 5.0 mm at the geniculation, eight per 5.0 mm on trail in the specimens from Ricklow (Brigantian; Monsal Dale Limestone Formation), 13 per 5.0 mm at geniculation, 10–12 per 5.0 mm on trail in the specimens from Wensley Dale (upper Brigantian; Eyam Limestone Formation). Visceral discs ornamented by subangular rugae, resulting in a reticulate ornamentation. On the ventral disc, rugae number five per 5.0 mm length anteriorly. Small spines on umbo. Two symmetrical halteroid spines on flanks near geniculation, laterally directed. Two symmetrical halteroid spines on trail at mid-length, bordering the sulcus, 8.0 mm apart from each other, anteriorly directed.
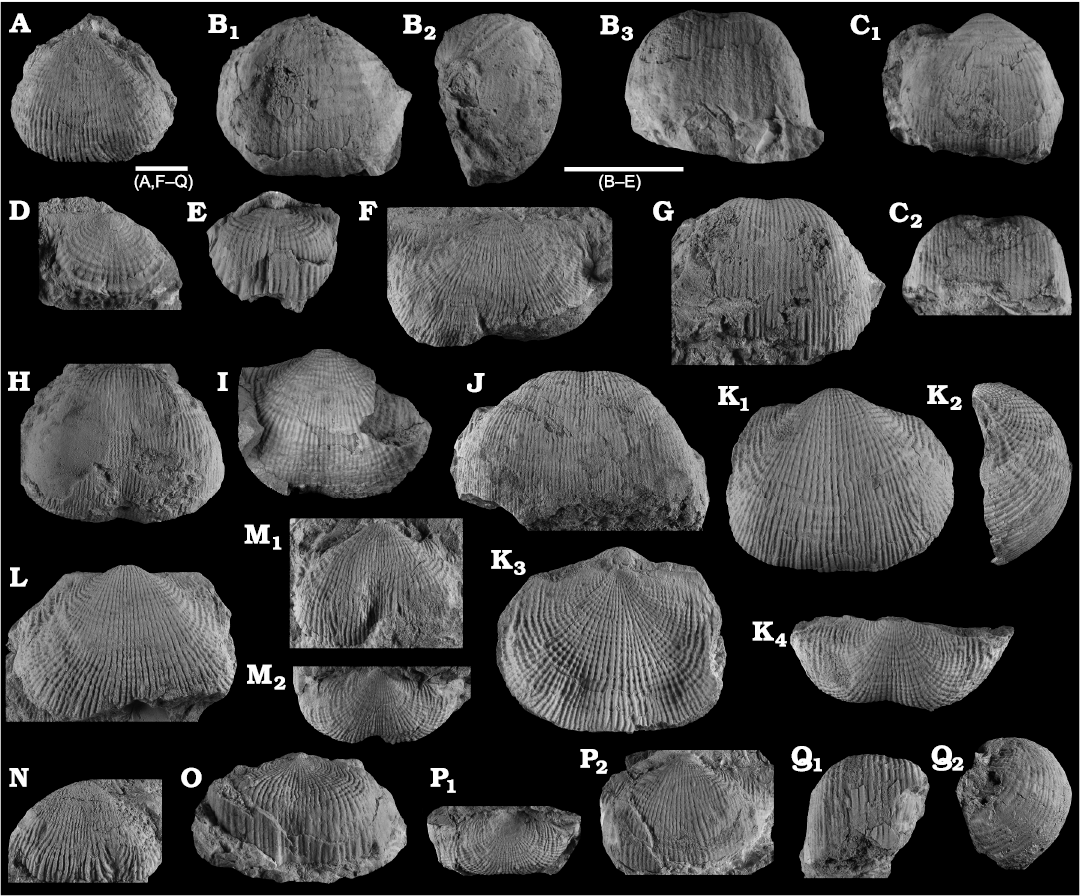
Fig. 10. Productidae from the Brigantian (uppermost Visean) of Ricklow Quarry, near Monyash (localities RCC-, RCK-; see Fig. 3) and Wensley Dale, near Matlock (locality WI1; see Fig. 2), Derbyshire, UK. A. Productus cf. productus (Martin, 1809), MPUM11919 (RCC41-3a) in ventral view. B–E. Eomarginifera cf. laqueata (Muir-Wood, 1928). B. MPUM11910 (RCC32-26) in ventral (B1), lateral (B2), and anterior (B3) views. C. MPUM11911 (WI1-41) in ventral (C1) and anterior (C2) views. D. MPUM11914 (RCC60-35) dorsal valve interior and external cast. E. MPUM11915 (RCC300-13) dorsal valve external cast. F–L. Antiquatonia hindi (Muir-Wood, 1928). F. MPUM11917 (RCC13-2) in ventral view. G. MPUM11918 (RCC50-1) in anterior view. H. MPUM11920 (RCC123-1) in ventral view. I. MPUM11921 (RCK15-7) in ventral view. J. MPUM11925 (RCC32-6) ventral valve in anterior view. K. MPUM11922 (WI1-19) in ventral (K1), lateral (K2), dorsal (K3), and posterior (K4) views. L. MPUM11923 (WI1-25) in ventral view. M–Q. Antiquatonia aff. hindi (Muir-Wood, 1928). M. MPUM11930 (RCC12-8) in ventral (M1) and posterior (M2) views. N. MPUM11934 (RCC39-19) ventral valve in ventral view. O. MPUM11935 (RCC2-8) dorsal valve external cast. P. MPUM11931 (RCC13-3a) in posterior (P1) and ventral (P2) views. Q. MPUM11932 (RCC32-19) in anterior (Q1) and lateral (Q2) views. Scale bars 10 mm.
Remarks.—The specimens are assigned to Eomarginifera cf. laqueata based on their convex ventral visceral disc with numerous spine bases, shallow ventral sulcus, and ribs density. They show a shallow ventral sulcus which is similar to the one of the specimens of E. lobata var. flexa (Muir-Wood, 1928) from the Brigantian, uppermost Visean, of Scotland (Muir-Wood 1928: pl. 11: 16a–b, 18), but they differ in having a convex, rather than flat, ventral visceral disc. They differ from E. lobata (Sowerby, 1821b) in having a weaker ventral sulcus and finer ribs; from E. longispina (Sowerby, 1814) in having a deeper sulcus, more convex ventral visceral disc and coarser radial ornamentation.
Tribe Retariini Muir-Wood and Cooper, 1960
Genus Antiquatonia Miloradovich, 1945
Type species: Productus antiquatus Sowerby, 1821b, from the Mississippian of Derbyshire, England.
Remarks.—Antiquatonia differs from Dictyoclostus Muir-Wood, 1930, in having a smaller-sized shell and a different arrangement of spines on the ventral valve: Dictyoclostus shows ventral spines arranged in a row on ears, in patches on flanks and near the anterior margin, rather than 1–2 rows on ears, one row on flanks and scattered over the visceral disc and trail as Antiquatonia.
Antiquatonia differs from Pugilis Sarycheva, 1949, in having a shallower corpus, less enrolled ventral valve, more prominent ribs on trail and a different ventral spine arrangement.
Antiquatonia hindi (Muir-Wood, 1928)
Fig. 10F–L.
1880 Productus semireticulatus (Martin, 1809); Davidson 1880: 307 (pars), pl. 35: 1–2.
1928 Productus hindi; Muir-Wood 1928: 108, pl. 6: 3, 4a–c, 5–6; text-figs. 1–5, 21.
1952 Antiquatonia hindi (Muir-Wood, 1928); Sarycheva and Sokolskaya 1952: 155, pl. 43.
1991 Antiquatonia hindi (Muir-Wood, 1928); Brunton and Tilsley 1991: 292.
2000 Antiquatonia hindi (Muir-Wood, 1928); Brunton et al. 2000: fig. 317: 2c–f.
Material.—Thirteen articulated specimens: MPUM11917 (RCC13-2); MPUM11918 (RCC50-1); MPUM11920 (RCC 123-1); MPUM11921 (RCK15-7); MPUM11922 (WI1-19); MPUM11923 (WI1-25); MPUM11924 (RCC2-48; RCC60-21; RCC65TOP-1; RCC73-20, 24; RCC110-3b; RCK15-35b). Thirteen ventral valves: MPUM11925 (RCC32-6); MPUM11927 (RCC1B-24b; RCC2-23b, 29; RCC3-12a, 16; RCC8-4; RCC11-1, 13; RCC32-1a, 1b; RCC73-7; RCC128-1). Twelve dorsal valves: MPUM11928 (RCC2-50; RCC3-4; RCC32-12b; RCC40-5a; RCC42-23, 32; RCC60-19a, 25; RCC65G-8b; RCC123-2; WI1-15a, 26b). Thirteen fragments: MPUM11929 (RCC1-43; RCC2-36; RCC3-15c, 23; RCC32-15, 21a, 21b; RCC40-18; RCC42-1b, 7; RCC300-8; RCK15-17; WI1-13). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian (RCC-, RCK-), and Wensley Dale, Matlock, Derbyshire, UK, Eyam Limestone Formation, upper Brigantian, upper Visean (WI1).
Description.—Medium-sized, concavo-convex shell with transversely subrectangular outline; cardinal margin wide, generally corresponding to the maximum width. Ears wide, flat, poorly separated. Both valves geniculated. Corpus cavity moderately deep. Anterior commissure gently uniplicate. Ventral visceral disc regularly convex; geniculation angle 120°. Trail long, tubiform. Sulcus starting 5.0 mm from umbo, widening and deepening anteriorly, becoming shallower on the trail. Dorsal visceral disc slightly concave; ears flat. Geniculation angle 90°. Low, rounded fold starting 7.0 mm from umbo, widening anteriorly.
Ornamentation consists of low, rounded ribs with narrow interspaces except for the dorsal anterior disc and trail where they may become wider than ribs. Ribs increasing in number by bifurcation, particularly common on trail flanks, and by rare intercalation on the ventral valve; by intercalation on the dorsal valve. They number 10–14 per 10.0 mm, 20.0 mm from the umbo. Visceral discs ornamented by subquadrate rugae, so that generally prominent reticulate ornamentation is formed; rugae number eight per 10.0 mm length. Rugae intersect the cardinal margin at 90°. Spines on ventral valve only: one row on each ear, diverging at a low angle from cardinal margin; one row of large spines diverging from cardinal margin at 30° and extending on trail flanks, set on a rounded ridge. Small spines on ribs scattered on umbonal flanks and trail. Dorsal valve bearing elongated pits in two rows starting near umbo, diverging from the cardinal margin at 30°, and scattered over the visceral disc. Micro-ornamentation of concentric growth lines on both valves.
Cardinal process stout, sessile, trifid, postero-ventrally directed.
Remarks.—The specimens are close in their morphology, dimensions and ornamentation to the specimens of Antiquatonia hindi figured by Muir-Wood (1928) and Brunton et al. (2000). Antiquatonia hindi var. wettonensis (Muir-Wood, 1928) from the Brigantian, uppermost Visean, of England and Wales, was distinguished by Muir-Wood (1928) based on its flatter visceral disc (Muir-Wood 1928: fig. 2a–c). Muir-Wood (1928) also stated the variety to be larger and with coarser ornamentation, but there is considerable overlapping in the shell and ornamentation measures given for A. hindi (Muir-Wood 1928: 110) and A. hindi var. wettonensis (Muir-Wood 1928: 113), so the distinctiveness of the variety seems not to be supported.
The specimens differ from Antiquatonia antiquata (Sowerby, 1821b) in having finer ornamentation and a spine-bearing ridge on flanks.
Stratigraphic and geographic range.—Antiquatonia hindi is recorded in the Asbian–Brigantian, upper Visean, to Serpukhovian of Great Britain and Ireland (Muir-Wood 1928; Mitchell 1971; Brunton and Tilsley 1991; this study) and in the upper Visean of the Moscow Basin (Sarycheva and Sokolskaya 1952).
Antiquatonia aff. hindi (Muir-Wood, 1928)
Fig. 10M–Q.
Material.—Five articulated specimens: MPUM11930 (RCC12-8); MPUM11931 (RCC13-3a); MPUM11932 (RCC32-19); MPUM11933 (RCC13-3e; RCC32-12a). One ventral valve: MPUM11934 (RCC39-19). Four dorsal valves: MPUM11935 (RCC2-8); MPUM11936 (RCC2-5; RCC12-3; RCC13-3b). Four fragments: MPUM11937 (RCC2-27; RCC11-9a, 11; RCC300-6). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Remarks.—The specimens are close to those assigned to A. hindi from the same localities, but differ in being smaller and in having more numerous and prominent spine bases on ribs, on the ventral visceral disc, and more numerous dimples on the dorsal visceral disc. They also have a weaker to absent ventral sulcus.
Antiquatonia insculpta (Muir-Wood, 1928)
Fig. 11A–F.
1860 Productus semireticulatus (Martin, 1809) var. sulcatus; Davidson 1860b: 109, pl. 3: 6–6a.
1928 Productus insculptus; Muir-Wood 1928: 89, pl. 3: 10a, 11a–c, 12.
1952 Antiquatonia insculpta (Muir-Wood, 1928); Sarycheva and Sokolskaya 1952: 157, pl. 42.
Material.—Eighteen articulated specimens: MPUM11938 (RCC1B-9); MPUM11939 (RCC3-15a); MPUM11940 (RCC 42-26); MPUM11941 (RCC110-1b); MPUM11944 (RCC2-25); MPUM11942 (RCC1-9, 31, 56a; RCC2-1, 4, 54; RCC3-13b; RCC11-5; RCC12-12; RCC41-4; RCC60-3, 4b; RCK15-56). Eight ventral valves: MPUM11943 (RCC1B-39); MPUM11945 (RCC1-35, 54; RCC2-53a, 53b; RCC65G-7; RCC73-29a; RCC 128-19). Thirteen dorsal valves: MPUM11947 (RCC128-2); MPUM11948 (RCC1-26; RCC3-2b, 5a, 5b, 15b; RCC10-19; RCC32-9; RCC43-1a; RCC60-1a, 11; RCC65TOP-40; RCC110-1c). Three fragments: MPUM11949 (RCC32-18; RCC43-1b; RCC73-1). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Medium-sized, elongated concavo-convex shell with transversely subtriangular outline; cardinal margin corresponding to maximum width. Ears large, concave, separated by a groove. Both valves geniculated. Anterior commissure uniplicate. Ventral visceral disc slightly convex to almost flat anteriorly; geniculation angle 90°. Trail very long, tubiform, convex. Ventral sulcus shallow, starting near umbo. Dorsal valve geniculated at 12.0 mm from the umbo; geniculation angle 45°. Very low fold starting posteriorly to geniculation, extending on trail.
Ornamentation consists of low, rounded ribs with narrow interspaces on visceral discs, wider on trails. Ribs increase by bifurcation on ventral trail, by intercalation on dorsal trail. They number 12 per 10.0 mm, 15.0 mm from the umbo. Ribs undulating anteriorly on ventral trail, may converge on sulcus. Ventral visceral disc bearing subrounded rugae, resulting in reticulate ornamentation. Rugae number five per 5.0 mm length. Rugae intersect the cardinal margin at 90°. Single row of spines on ears, almost parallel to the cardinal margin; one row of large spines on the umbonal slopes diverging from the cardinal margin at 30–45°, continuing on flanks on a low rounded ridge. Large spines scattered on ventral trail.
Cardinal process stout. Lateral ridges slightly diverge from the cardinal margin. Dorsal median septum longer than two-thirds valve length.
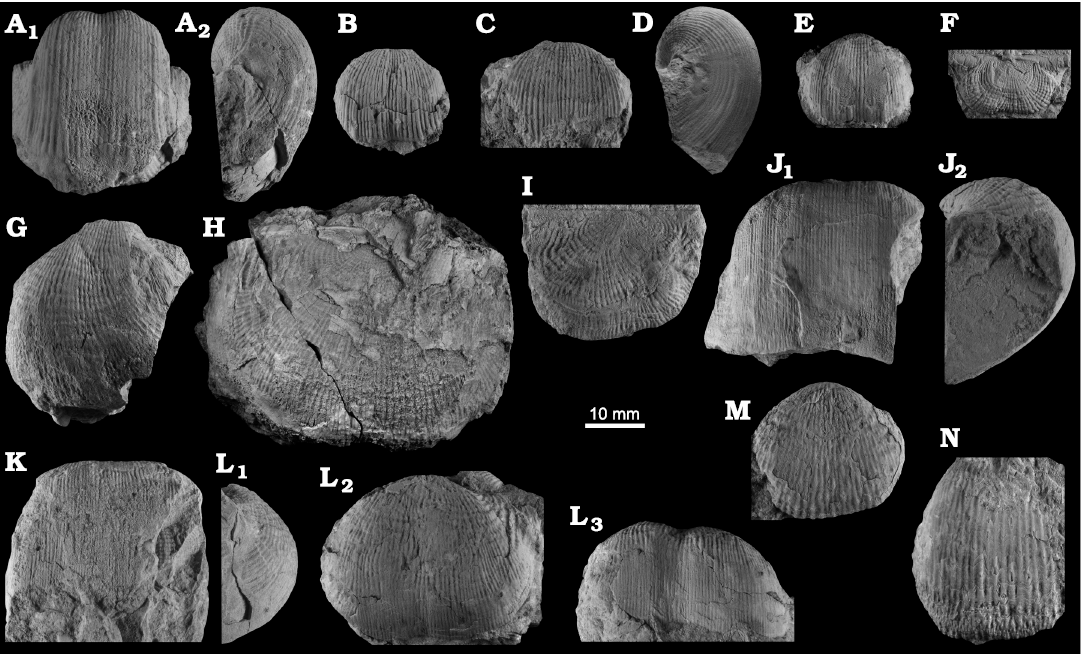
Fig. 11. Productidae from the Brigantian (uppermost Visean) of Ricklow Quarry, near Monyash, Derbyshire, UK (localities RCC-, RCK-; Fig. 3). A–F. Antiquatonia insculpta (Muir-Wood, 1928). A. MPUM11938 (RCC1B-9) in anterior (A1) and lateral (A2) views. B. MPUM11939 (RCC3-15a) in antero-ventral view. C. MPUM11940 (RCC42-26) in antero-ventral view. D. MPUM11941 (RCC110-1b) in lateral view. E. MPUM11944 (RCC2-25) ventral valve in ventral view. F. MPUM11947 (RCC128-2) dorsal valve interior and external cast. G, H. Dictyoclostus pinguis (Muir-Wood, 1928). G. MPUM11950 (RCC54-11) ventral valve in ventral view. H. MPUM11951 (RCK15-63a) dorsal valve external cast. I–K. Pugilis cf. kilbridensis (Muir-Wood, 1928). I. MPUM11956 (RCC128-3) dorsal valve interior and external cast. J. MPUM11955 (RCC54-4) ventral valve in anterior (J1) and lateral (J2) views. K. MPUM11954 (RCC2-47) ventral valve in anterior view. L. Pugilis cf. scotica (Sowerby, 1814), MPUM11958 (RCC2-41) in lateral (L1), ventral (L2), and anterior (L3) views. M, N. Buxtonia scabricula (Sowerby, 1814). M. MPUM11959 (RCC1B-13) in ventral view. N. MPUM11960 (RCC35A-11a) in ventral view.
Remarks.—The specimens are close in their morphology, dimensions and ornamentation to the specimens of Antiquatonia insculpta figured by Muir-Wood (1928). They differ from A. hindi from the same localities in having shorter visceral discs, smaller-sized adult shells and a lower angle of geniculation. Gutteridge (1990) reported the presence of A. sulcata (Sowerby, 1821b) from the mud mound complex in Ricklow. However, we can not confirm his report. The specimens of A. insculpta from Ricklow Quarry differ from A. sulcata in having a shorter visceral disc, smaller size and in having fainter ornamentation of narrower ribs.
Stratigraphic and geographic range.—Antiquatonia insculpta is recorded in the Brigantian, uppermost Visean, of England and Scotland (e.g., Muir-Wood 1928; Mitchell 1971; this study) and in the lower Serpukhovian of the Moscow Basin (Sarycheva and Sokolskaya 1952).
Subfamily Dictyoclostinae Stehli, 1954
Genus Dictyoclostus Muir-Wood, 1930
Type species: Conchyliolithus Anomites (semireticulatus) Martin, 1809, from the Visean of Great Britain and Ireland.
Remarks.—Dictyoclostus differs from Pugilis in its less elongated shell outline, stronger reticulation on the visceral disc and ribbing on the trail, and different ventral spine arrangement.
Dictyoclostus pinguis (Muir-Wood, 1928)
Fig. 11G–H.
1928 Productus pinguis; Muir-Wood 1928: 104, pl. 5: 1, 2a–d, 3; pl. 6: 1; text-fig. 20.
1952 Dictyoclostus pinguis (Muir-Wood, 1928); Sarycheva and Sokolskaya 1952: 142, pl. 38: 191.
1991 Dictyoclostus pinguis (Muir-Wood, 1928); Brunton and Tilsley 1991: 291.
2000 Dictyoclostus pinguis (Muir-Wood, 1928); Brunton et al. 2000: fig. 331: e.
?2016 Dictyoclostus pinguis (Muir-Wood, 1928); Tazawa et al. 2016: 53, fig. 5.3. (cum syn.)
Material.—One ventral valve: MPUM11950 (RCC54-11). Two dorsal valves: MPUM11951 (RCK15-63a); MPUM11952 (RCC2-23a). Two fragments: MPUM11953 (RCC1-10; RCC1B-23). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Large-sized, concavo-convex shell with transversely subrectangular outline; cardinal margin wide, almost corresponding to the maximum width. Anterior commissure rectimarginate. Ventral geniculation angle 90°. Visceral disc flattened medianly. Trail long, convex. Dorsal visceral disc flat posteriorly, gently concave anteriorly. Geniculation angle 70°. Low rounded fold starting at disc mid-length, becoming obsolete on trail.
Ornamentation consists of low, rounded ribs with narrow interspaces posteriorly, wide anteriorly. Ribs increase in number anteriorly by bifurcation on the ventral valve, by intercalation on the dorsal one. Ribs number 10–12 per 10.0 mm, 25.0 mm from the umbo. Ventral and dorsal visceral discs bearing low, rounded rugae, densely packed posteriorly (four per 5.0 mm length), lower and wider anteriorly (5–6 per 5.0 mm length). Intersection between ribs and rugae results in reticulate ornamentation.
Long and robust dorsal median septum, 1.0 mm wide, extending at least to one-third of visceral disc length.
Remarks.—The specimens are similar to the specimens of Dictyoclostus pinguis figured by Muir-Wood (1928) and Brunton et al. (2000). They differ from D. semireticulatus (Martin, 1809) in having a geniculated dorsal valve and finer ribbing.
Stratigraphic and geographic range.—Dictyoclostus pinguis is recorded in the Brigantian, uppermost Visean, of England and Scotland (e.g., Muir-Wood 1928; Mitchell 1971; this study), in the lower Visean of Tianshan–northern Junggar, north-western China (e.g., Shen et al. 2017) and the Serpukhovian of the Moscow Basin (Sarycheva and Sokolskaya 1952).
Genus Pugilis Sarycheva, 1949
Type species: Producta pugilis Phillips, 1836, from the Brigantian, uppermost Visean, of Yorkshire, England.
Pugilis cf. kilbridensis (Muir-Wood, 1928)
Fig. 11I–K.
Material.—Two ventral valves: MPUM11954 (RCC2-47); MPUM11955 (RCC54-4). One dorsal valve: MPUM11956 (RCC128-3). One fragment: MPUM11957 (RCC128-28b). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Medium-sized, elongated concavo-convex shell with suboval outline; cardinal margin corresponding to maximum width. Both valves geniculated. Corpus cavity deep. Anterior commissure rectimarginate. Ventral valve enrolled, visceral disc convex; geniculation angle 90°. Trail long, tubiform, straight. Dorsal visceral disc flat; geniculation angle 70°. Trail long, straight.
Ornamentation consists of subrounded ribs with narrow interspaces. Ribs increase in number anteriorly by bifurcation. They number 17 per 10.0 mm at geniculation and trail. Low, rounded rugae on visceral discs, six per 5.0 mm length, resulting in a reticulate ornamentation. Small spines scattered over the ventral visceral disc, on ribs.
Remarks.—The specimens are similar to Productus kilbridensis from the Brigantian, uppermost Visean, of Kilbride (Ayrshire, Scotland), figured by Muir-Wood (1928: pl. 7: 5a–c). The specimens are also close to P. scotica (Sowerby, 1814), from the upper Visean of England and Scotland, in their ornamentation, but they differ from it in having a longer, straight trail, a strongly geniculated dorsal valve and in lacking a sulcus (Muir-Wood 1928).
Pugilis cf. scotica (Sowerby, 1814)
Fig. 11L.
Material.—One articulated specimen: MPUM11958 (RCC2-41) from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Medium-sized, elongated concavo-convex shell with suboval outline. Cardinal margin wide, corresponding to maximum width. Both valves geniculated. Anterior commissure uniplicate. Ventral valve enrolled, visceral disc convex; geniculation angle 120°. Trail long, tubiform, straight. Low, rounded ventral sulcus, starting posteriorly to geniculation and extending on trail.
Ornamentation consists of subrounded ribs, with narrow interspaces on visceral discs, becoming fainter, thread like, with wide interspaces on trail. Ribs increase in number anteriorly by bifurcation. They number 16 per 10.0 mm at geniculation.
Remarks.—The specimen is close in its morphology and ornamentation to the specimens assigned to Pugilis cf. kilbridensis from the mud mound complex in Ricklow Quarry, but differs in having a less enrolled ventral valve, wider angle of geniculation and shallow ventral sulcus. These characters make it closer to Pugilis scotica, from the Asbian–Brigantian of England and Scotland (Muir-Wood 1928).
Subfamily Buxtoniinae Muir-Wood and Cooper, 1960
Tribe Buxtoniini Muir-Wood and Cooper, 1960
Genus Buxtonia Thomas, 1914
Type species: Productus scabriculus Sowerby, 1814, from the upper Visean of Derbyshire, England.
Remarks.—Buxtonia differs from the allied genus Marginicinctus Sutton, 1938, in being larger, in generally having a more convex ventral valve and in lacking a reflexed flange developed at the anterior margin; from the genus Setigerites Girty, 1939, in having a coarser radial ornamentation; from Kochiproductus Dunbar, 1955, in being smaller, in lacking a large anterolateral flange, in having larger spines and more regular rugation; moreover, Kochiproductus lacks an antron at the posterior end of the median septum.
Buxtonia scabricula (Sowerby, 1814)
Figs. 11M, N, 12A–C.
1809 Conchyliolithus Anomites (scabriculus); Martin 1809: 8, pl. 34: 5.
1814 Productus scabriculus; Sowerby 1814: pl. 69: 1.
1862 Productus scabriculus Sowerby, 1814; Davidson 1862a: 169, pl. 42: 5–8. (cum syn.)
1905 Productus scabriculus Sowerby, 1814 var. costata; Vaughan 1905: 293.
1951 Buxtonia scabricula (Sowerby, 1814); Muir-Wood 1951: fig. 358: 1a–e.
1952 Buxtonia scabricula (Sowerby, 1814); Sarycheva and Sokolskaya 1952: 96, pl. 114.
1960 Buxtonia scabricula (Sowerby, 1814); Muir-Wood and Cooper 1960: pl. 53: 10–11; pl. 74: 8–11.
1965 Buxtonia scabricula (Sowerby, 1814); Muir-Wood 1965: fig. 358: 1.
1991 Buxtonia scabricula (Sowerby, 1814); Brunton and Tilsley 1991: 291.
2000 Buxtonia scabricula (Sowerby, 1814); Brunton et al. 2000: fig. 338: 3a–c.
Material.—Seven articulated specimens: MPUM11959 (RCC1B-13); MPUM11960 (RCC35A-11a); MPUM11961 (WI1-14); MPUM11962 (WI1-38); MPUM11963 (WI1-40); MPUM11964 (WI1-24, 34). One ventral valve: MPUM11965 (RCC1-6). Two fragments: MPUM11966 (RCC128-10a); MPUM11967 (WI1-15b). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian (RCC-, RCK-), and Wensley Dale, Matlock, Derbyshire, UK, Eyam Limestone Formation, upper Brigantian, upper Visean (WI1).
Description.—Medium-sized, concavo-convex shell with transversely subrectangular outline. Cardinal margin wide, almost corresponding to the maximum width. Ears small, concave. Both valves geniculated. Corpus cavity deep. Anterior commissure rectimarginate. Ventral visceral disc convex. Geniculation angle 80°. Umbo straight, dorsally directed. Trail long, tubiform, convex. Shallow, broad sulcus starting 10.0 mm from the beak, widening anteriorly, becoming obsolete on trail. Dorsal valve slightly concave, with flat ears and low rounded fold starting near the cardinal margin, widening anteriorly. Geniculation angle 90°.
Ornamentation consists of low, rounded ribs, irregular in width and heigh, more or less continuous, undulating on the trail. Interspaces narrow posteriorly on visceral discs, becoming as wide as ribs anteriorly and on trail. Ribs increasing in number anteriorly by bifurcation on the ventral valve; commonly bifurcations occur immediately anterior to spine bases. They number eight per 10.0 mm, 20.0 mm from the umbo. Low, rounded rugae on the ventral valve; on ears and umbonal flanks, two per 5.0 mm length, with narrow interspaces, not extending around front of valve. Thinner rugae on dorsal ears, rarely extending on whole of disc; rugae intersect with the cardinal margin forming an angle of 45° near the umbo. Prostrate spines arranged in concentric rows on ventral rugae. Spines randomly distributed on ventral visceral disc, on ribs. Near the anterior commissure the ribs become obsolete and finer and at least three staggered rows of small rounded spine bases are developed. Dorsal valve bearing elongated to circular dimples, more frequent near geniculation; dimples concentrically arranged on rugae interspaces on the flanks, irregularly disposed on fold; four dimples per 5 mm2 at geniculation. Spine bases on ribs. Micro-ornamentation of concentric growth lines on both valves.
Cardinal process long-shafted, bilobed, quadrifid, apparently dorsally reflexed. Median septum short (6.0 mm long), thin, with small antron posteriorly.
Remarks.—The specimens resemble in their morphology and ornamentation the lectotype of Buxtonia scabricula figured by Brunton et al. (2000), from the upper Visean of Derbyshire, and the specimens figured by Muir-Wood and Cooper (1960) from the Visean of Flintshire, Wales. They differ from B. scabriculoides Paeckelmann, 1931, in being smaller, in lacking a clear reticulation on the ventral disc and in having spines arranged on all the valve, rather than being restricted posteriorly; from B. lichwini (Lisitzyn, 1909) from the upper Tournaisian of the Moscow Basin in being sulcate (Sarycheva and Sokolskaya 1952).
Stratigraphic and geographic range.—Buxtonia scabricula is common in upper Visean–lower Serpukhovian rocks of Great Britain and Ireland (Vaughan 1905; Muir-Wood and Cooper 1960; Brunton and Tilsley 1991; this study) and upper Visean rocks of Central Iran (Ruttner et al. 1968).
Buxtonia sp.
Fig. 12D–E.
Material.—Two ventral valves: MPUM11968 (RCC8-2a); MPUM11969 (WI1-2). One fragment: MPUM11970 (RCC1-39). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian (RCC-, RCK-), and Wensley Dale, Matlock, Derbyshire, UK, Eyam Limestone Formation, upper Brigantian, upper Visean (WI1).
Description.—Medium-sized, concavo-convex shell with transversely subtriangular outline; cardinal margin wide, maximum width attained at shell mid-length. Ears large, flat. Anterior commissure rectimarginate to gently uniplicate. Ventral valve moderately convex, not geniculated. Shallow sulcus starting near the umbo.
Ornamentation consists of low, rounded, ribs with narrow interspaces posteriorly on visceral discs, becoming wide anteriorly. Ribs increase in number anteriorly by bifurcation. They number 10–12 per 10.0 mm, 12.0 mm from the umbo. Prostrate, dense (5–6 in 5 mm2 at 16.0 mm from the umbo) spines on ribs, concentrically arranged on flanks, irregularly on visceral disc and trail. Ventral visceral disc covered by rugae, six per 10.0 mm length, more prominent on ears and umbonal flanks, increasing in number around front of valve by intercalation. Rugae intersect the cardinal margin forming a 40° angle with it.
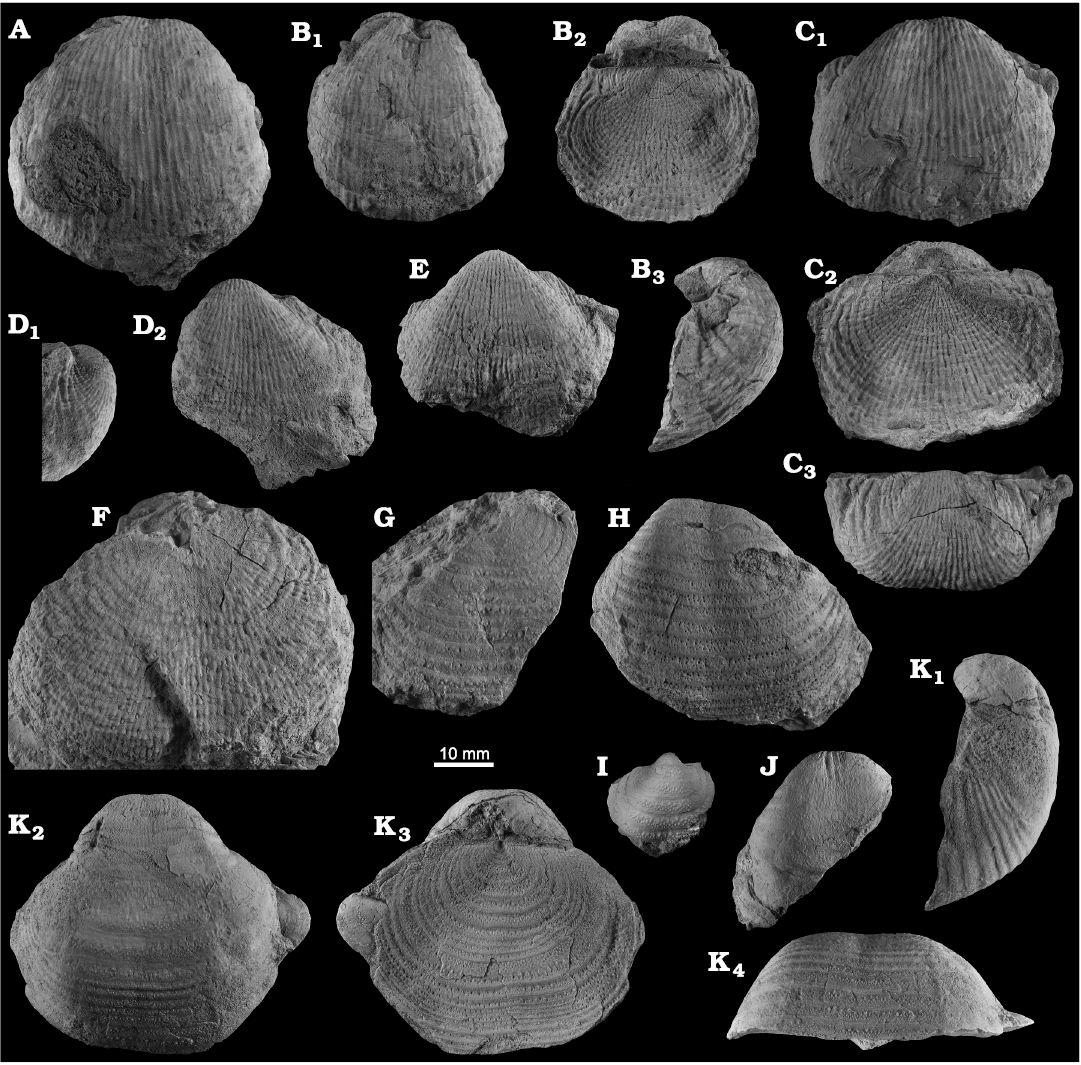
Fig. 12. Productidae and Echinoconchidae from the Brigantian (uppermost Visean) of Ricklow Quarry, near Monyash (localities RCC-, RCK-; see Fig. 3) and Wensley Dale, near Matlock (locality WI1; Fig. 2), Derbyshire, UK. A–C. Buxtonia scabricula (Sowerby, 1814). A. MPUM11963 (WI-40) in ventral view. B. MPUM11962 (WI1-38) in ventral (B1), dorsal (B2), and lateral (B3) views. C. MPUM11961 (WI1-14) in ventral (C1), dorsal (C2), and posterior (C3) views. D, E. Buxtonia sp. D. MPUM11968 (RCC8-2a) ventral valve in lateral (D1) and ventral (D2) views. E. MPUM11969 (WI1-2) ventral valve in ventral view. F. Buxtoniini gen. et sp. indet., MPUM11971 (RCK15B-1a) dorsal valve external cast. G–K. Echinoconchus punctatus (Sowerby, 1822). G. MPUM11983 (RCC1B-18) dorsal valve external cast. H. MPUM11980 (RCC54-7) ventral valve in ventral view. I. MPUM11975 (RCK15-21) in ventral view. J. MPUM11974 (RCC72-1) in ventral view. K. MPUM11977 (WI1-28) in lateral (K1), ventral (K2), dorsal (K3), and anterior (K4) views.
Remarks.—The specimens differ from the ones assigned to Buxtonia scabricula from the same localities in their less enrolled ventral valve and finer ornamentation. The gentle convexity of their ventral valve and the absence of a proper geniculation make them different from most other species of Buxtonia and closer to those of Marginicinctus, but they lack the anterior development of a gutter which is distinctive for the latter.
Buxtoniini gen. et sp. indet.
Fig. 12F.
Material.—Two dorsal valves: MPUM11971 (RCK15B-1a); MPUM11972 (RCC48-42). One fragment: MPUM11973 (RCC48-2a). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Remarks.—The specimens are poorly preserved dorsal valves with flat visceral discs and convex geniculation (geniculation angle 90°). They show a low, rounded fold starting from the umbo and an ornamentation of subangular rugae (three per 5.0 mm length) bearing irregular rows of elongated dimples (1.0 mm long; five per 5 mm2). Faint ribs with wide interspaces on trail, four per 5.0 mm. The specimens are attributed to the Buxtoniini based on their ornamentation of dense elongated dimples and irregular ribs anteriorly.
Superfamily Echinoconchoidea Stehli, 1954
Family Echinoconchidae Stehli, 1954
Subfamily Echinoconchinae Stehli, 1954
Tribe Echinoconchini Stehli, 1954
Genus Echinoconchus Weller, 1914
Type species: Productus punctatus Sowerby, 1822, from the upper Visean of Derbyshire, England.
Remarks.—Echinoconchus differs from Pustula Thomas, 1914, in the shape of muscle scars and in having stronger rugae bearing numerous rows of spines, the spines being of two series and concentrically arranged.
Echinoconchus punctatus (Sowerby, 1822)
Figs. 12G–K, 13A.
1809 Conchyliolithus Anomites (punctatus); Martin 1809: pl. 37: 6, non 7–8.
1822 Productus punctatus; Sowerby 1822: pl. 323: right and lower figure, ?left hand lower figure.
1844 Producta punctata Sowerby, 1822; M’Coy 1844: 113 (pars).
1862 Productus punctatus Sowerby, 1822; Davidson 1862a: 172 (pars), pl. 44: 9, 11, 16, 17. (cum syn.)
1914 Pustula punctata (Sowerby, 1822); Thomas 1914: 303, pl. 17: 16–19; text-fig. 11.
1951 Echinoconchus punctatus (Sowerby, 1822); Muir-Wood 1951: 102, pl. 4: 2.
1952 Echinoconchus punctatus (Sowery, 1822); Sarycheva and Sokolskaya 1952: 99, pl. 18: 120.
1960 Echinoconchus punctatus (Sowerby, 1822); Muir-Wood and Cooper 1960: pl. 66: 1–2; pl. 82: 8–10; pl. 83: 1–4; pl. 88: 11; pl. 125: 5.
1965 Echinoconchus punctatus (Sowerby, 1822); Muir-Wood 1965: fig. 353: 1a–d.
1971 Echinoconchus punctatus (Sowerby, 1822); Mitchell 1971: pl. 14: 3.
1991 Echinoconchus punctatus (Sowerby, 1822); Brunton and Tilsley 1991: 291.
2000 Echinoconchus punctatus (Sowerby, 1822); Brunton et al. 2000: fig. 349: a–e.
2016 Echinoconchus punctatus (Sowerby, 1822); Denayer et al. 2016: fig. 7: H–K.
2017 Echinoconchus punctatus (Sowerby, 1822); Tazawa 2017: 335, figs. 6.6, 6.7. (cum syn.)
Material.—Nine articulated specimen: MPUM11974 (RCC72-1); MPUM11975 (RCK15-21); MPUM11976 (RCK 15-47); MPUM11978 (RCC1B-1); MPUM11977 (WI1-28); MPUM11979 (WI1-22, 23, 29, 35). Seven ventral valves: MPUM11980 (RCC54-7); MPUM11981 (RCC1-24; RCC2-14a; RCC40-9; RCC48-12; RCC85-1a); MPUM11982 (WI1-7). Eight dorsal valves: MPUM11983 (RCC1B-18); MPUM11984 (RCC1B-15; RCC8-7; RCC12-13e; RCC13-3d; RCC68-1; RCK15-1c); MPUM11985 (WI1-12b). Nineteen fragments: MPUM11986 (RCC1-27a, 40a, 47, 61a, 61c; RCC10-39a; RCC12-11a, 13a; RCC12BIT-1; RCC40-4b; RCC41-17a; RCC54-1, 2, 10c, 13, 17d, 17e; RCK15-45; RCK16-2). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian (RCC-, RCK-), and Wensley Dale, Matlock, Derbyshire, UK, Eyam Limestone Formation, upper Brigantian, upper Visean (WI1).
Description.—Medium-sized, concavo-convex shell with suboval outline; cardinal margin wide, maximum width attained at shell mid-length. Ears small, flat. Rounded cardinal extremities. Corpus cavity deep. Anterior commissure uniplicate. Ventral valve poorly convex posteriorly, not geniculated. Shallow, rounded sulcus starting 10.0 mm from the umbo. Dorsal valve flat to concave anteriorly, not geniculated. Low, rounded fold starting 7.0–8.0 mm from the umbo.
Both valves covered by subangular rugae, 4–6 per 10.0 mm length, separated by broadly rounded interspaces, narrower on flanks. Each one bears one row of large spines posteriorly, then another row of spines, equal or smaller in diameter, and then 2–3 staggered rows of small spines, quincuncially arranged on the ruga crest. This pattern may vary considerably: some rugae may have only small quincuncially arranged spines, or only 1–2 rows of large spines, while wider rugae may be covered by quincuncially arranged large spines posteriorly with a narrower band of quincuncially arranged small spines anteriorly.
Cardinal process short-shafted, 2.0 mm long, bilobed.
Remarks.—The specimens are close in their morphology and ornamentation to Echinoconchus punctatus as described and figured by Thomas (1914), Muir-Wood and Cooper (1960) and Brunton (2000). They differ from E. defensus (Thomas, 1914) in having a poorly convex ventral valve and an almost flat, rather than concave, dorsal valve, and from E. eximius (Thomas, 1914) and E. subelegans (Thomas, 1914) in having a fold-sulcus.
Stratigraphic and geographic range.—Echinoconchus punctatus is common in the Visean of Great Britain and Ireland (Thomas 1914 and references therein; this study), the upper Tournaisian–upper Visean of Germany (Winkler-Prins and Amler 2006), the upper Visean of Belgium (e.g., Denayer et al. 2016), the upper Visean of the Badajoz Province, and the Bashkirian of Cantabrian Mountains in Spain (Winkler-Prins 1968; Martinez-Chacon 1979; Martinez-Chacon and Legrand-Blain 1992), the upper Visean of Poland (Żakowa 1966), the Visean–Serpukhovian of Russia, Kazakhstan, Uzbekistan, and Kyrgyzstan (e.g., Sarycheva and Sokolskaya 1952; Tazawa 2017 and references therein), the upper Visean of northern China, and the South Kitakami Belt, Japan (e.g., Tazawa et al. 2016).
Subfamily Pustulinae Waterhouse, 1981
Genus Pustula Thomas, 1914
Type species: Producta pustulosa Phillips, 1836, from the Mississippian of Bolland, Yorkshire, England, and Florence Court, Ireland.
Pustula cf. pustulosa (Phillips, 1836)
Fig. 13B–D.
Material.—Two articulated specimens: MPUM11987 (RCC41-1); MPUM11988 (RCC128-7). One dorsal valve: MPUM11989 (RCC128-17a). Four fragments: MPUM11990 (RCC1-18, 50; RCC41-10b, 27). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Medium-sized, planoconvex shell with subtriangular outline; cardinal margin straight, longer than two-thirds maximum width. Corpus cavity deep. Anterior commissure rectimarginate. Ventral valve regularly convex. Dorsal valve flat.
Ventral valve covered by elongated spine bases in irregular quincunx (10–12 per 5 mm2) except anteriorly on flanks where they are disposed on staggered rows on low, rounded rugae. Rugae are fainter on the remaining surface of the shell. Dorsal valve ornamented by concentric subrounded rugae, increasing in number by intercalation, three per 5.0 mm length. Slightly elongated dimples, distributed on rugae interspaces or quincuncially arranged (10–15 per 5 mm2).
Long prominent dorsal median septum.
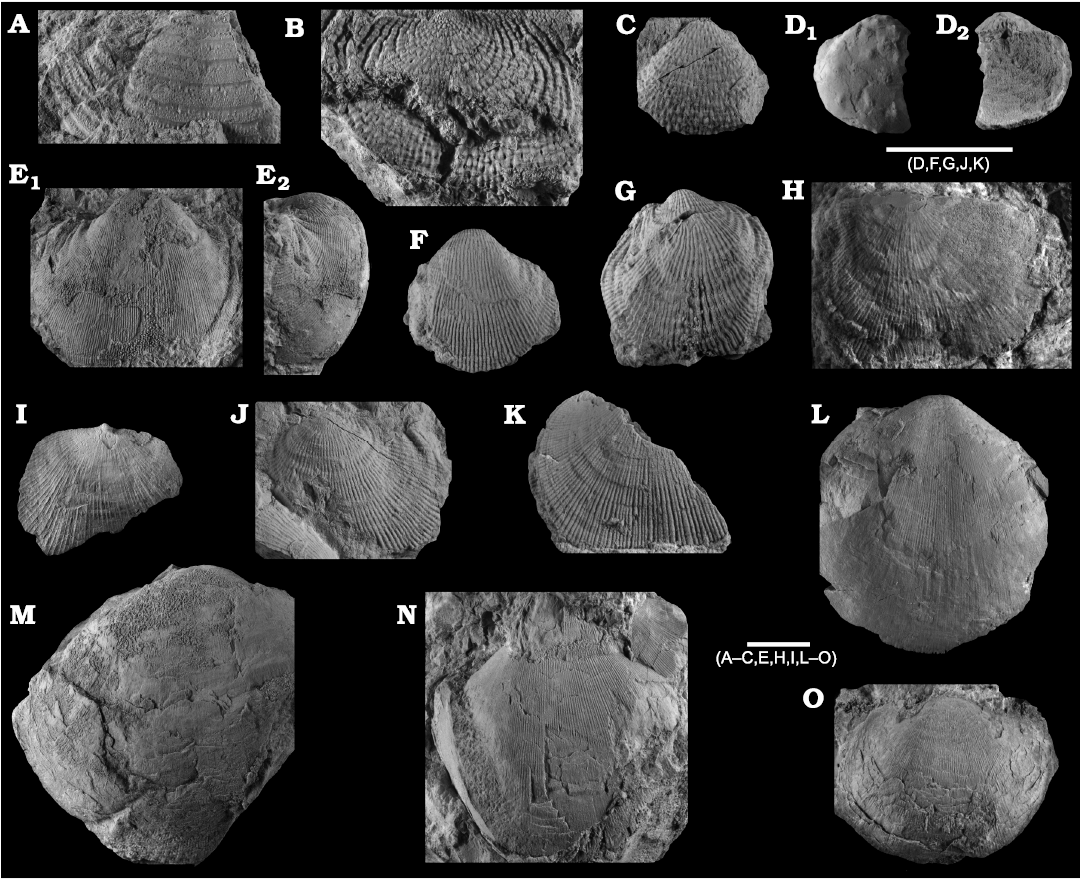
Fig. 13. Echinoconchidae, Linoproductidae, Monticuliferidae, Orthotetida, and Orthida from the Brigantian (uppermost Visean) of Ricklow Quarry, near Monyash, Derbyshire, UK (localities RCC-, RCK-; see Fig. 3). A. Echinoconchus punctatus (Sowerby, 1822), MPUM11976 (RCK15-47) in ventral view. B–D. Pustula cf. pustulosa (Phillips, 1836), B. MPUM11989 (RCC128-17a) dorsal valve external cast. C. MPUM11987 (RCC41-1) in ventral view. D. MPUM11988 (RCC128-7) in ventral (D1) and dorsal (D2) views. E, F. Linoprotonia sp. E. MPUM11991 (RCC42-17) in ventral (E1) and lateral (E2) views. F. MPUM11992 (RCC60-59) in ventral view. G. Fluctuaria undata (Defrance, 1826), MPUM11926 (RCC300-14) ventral valve in ventral view. H. ?Serratocrista aff. dalriensis McIntosh, 1974, MPUM11997 (RCC10-40) ventral valve in ventral view. I. Schellwienella sp., MPUM11995 (RCC36-12) ventral valve in ventral view. J, K. Schizophoria (Schizophoria) cf. connivens (Phillips, 1836). J. MPUM12009 (RCC60-48) in ventral view. K. MPUM12010 (RCC1B-7) dorsal valve in dorsal view. L–O. Schizophoria (Schizophoria.) resupinata (Martin, 1809). L. MPUM12003 (RCC1B-4) dorsal valve in dorsal view. M. MPUM11999 (RCC1B-26) in dorsal view. N. MPUM12004 (RCC1B-25) dorsal valve in dorsal view. O. MPUM12005 (RCC36-1a) dorsal valve in dorsal view. Scale bars 10 mm.
Remarks.—The specimens are close to Pustula pustolosa by the absence of a sulcus-fold and their ornamentation of slightly elongated spine bases-dimples. They differ from P. pyxidiformis (De Koninck, 1847), recorded by Brunton and Tilsley (1991) from the Asbian, upper Visean, of Treak Cliff, Derbyshire, in having regularly concentric rugae and spine bases not extending in length beyond a single ruga.
Superfamily Linoproductoidea Stehli, 1954
Family Linoproductidae Stehli, 1954
Subfamily Linoproductinae Stehli, 1954
Genus Fluctuaria Muir-Wood and Cooper, 1960
Type species: Productus undatus Defrance, 1826, from the upper Visean of Visé, Belgium.
Remarks.—The genus differs from other linoproductids by its small dimensions and in the development of strong, subangular rugae anteriorly. It is similar to Undaria Muir-Wood and Cooper, 1960, of the Monticuliferidae Muir-Wood and Cooper, 1960, but it differs from it in having prominent ribs crossing rugae and a smaller umbo.
Fluctuaria undata (Defrance, 1826)
Fig. 13G.
1826 Productus undatus; Defrance 1826: 354.
1844 Productus tortilis; M’Coy 1844: fig. 14.
1862 Productus undatus Defrance, 1826; Davidson 1862a: 161, pl. 34: 7–12, ?13. (cum syn.)
1960 Fluctuaria undata (Defrance, 1826); Muir-Wood and Cooper 1960: pl. 115: 11–20.
1965 Fluctuaria undata (Defrance, 1826); Muir-Wood 1965: fig. 373: 3.
1991 Fluctuaria undata (Defrance, 1826); Brunton and Tilsley 1991: 291.
2000 Fluctuaria undata (Defrance, 1826); Brunton et al. 2000: fig. 366: 2a–d.
2019 Fluctuaria undata (Defrance, 1826); Yuan et al. 2019: fig. 4E.
Material.—One ventral valve: MPUM11926 (RCC300-14) from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Small, suboval shell. Cardinal margin straight; maximum width at shell mid-length. Anterior commissure rectimarginate. Ventral valve regularly convex.
Ventral valve surface covered by fine, rounded ribs with narrow interspaces. Ribs increase anteriorly by intercalation, by bifurcation on flanks. They number 13 per 5.0 mm at the anterior commissure. Low, asymmetrical, subangular rugae on the entire ventral valve. Rugae number 13–14 in 5.0 mm near umbo, 4–5 in 5.0 mm near anterior commissure. Ribs cross rugae.
Remarks.—The specimen is assigned to Fluctuaria undata based on its small size and ornamentation of prominent thin ribs crossing subangular rugae. The density of ribs is similar to that reported by Davidson (1862a: 161).
Stratigraphic and geographic range.—Fluctuaria undata is recorded from the Mississippian of Northumberland (Davidson 1862a), the Visean of the Isle of Man (Muir-Wood and Cooper 1960), and the Asbian–Brigantian (upper Visean), of Derbyshire and Yorkshire (Brunton and Tilsley 1991; this study) in England. Davidson (1862a) also recorded some specimens from Scotland and Ireland. The species is also recorded from the upper Visean of Visé, Belgium (Muir-Wood and Cooper 1960), the Mississippian of Ukraine (Ohar 2021), the Serpukhovian of Kazakhstan, the Visean of Tibet, the upper Visean of the Urals region (Chen 2004), and the upper Visean–Serpukhovian of China (Chen 2004; Yuan et al. 2019; Shen et al. 2017).
Family Monticuliferidae Muir-Wood and Cooper, 1960
Subfamily Auricolispininae Waterhouse, 1986
Genus Linoprotonia Ferguson, 1971
Type species: Productus hemisphaericus Sowerby, 1822, from the Asbian, upper Visean, of Somerset, England, and Wales.
Remarks.—The genus was erected by Ferguson (1971) for Mississippian linoproductoid species formerly attributed to Linoproductus Chao, 1927, but differing from the upper Carboniferous–Permian species assigned to the latter in having internal characters similar to those of Gigantoproductus Prentice, 1950. Externally, Linoprotonia species differ from those of Gigantoproductus in being smaller, in having a shorter cardinal margin and less developed ears, a more enrolled umbo, thinner shell substance, finer radial ornamentation and in commonly lacking plicae on trail.
Linoprotonia sp.
Fig. 13E, F.
Material.—Three articulated specimens: MPUM11991 (RCC42-17); MPUM11992 (RCC60-59); MPUM11993 (RCC73-23f). Two fragments: MPUM11994 (RCC73- 30b; RCK15-60). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Medium-sized, transversely semicircular shell. Cardinal margin wide, almost corresponding to maximum width, which is attained anteriorly. Ears moderate, poorly separated. Anterior commissure rectimarginate. Ventral valve regularly convex, weakly geniculated. Trail convex, gently flaring laterally. Umbo strongly enrolled.
Ventral valve surface multicostellate. Ribs cylindrical, straight, with narrow interspaces, increasing in number anteriorly by intercalation. Some ribs may be thicker and wider than neighbouring ones. Ribs number 15 per 5.0 mm, 20.0 mm from the umbo. Three to four prominent, subquadrate, concentric rugae restricted to umbonal flanks. Two symmetrical spine bases scars on umbonal flanks, 7.0 mm from the umbo.
Remarks.—The specimens are similar to both Linoprotonia hemisphaerica from the Asbian, upper Visean, and its ancestor (Ferguson 1971) L. corrugatohemisphaerica (Vaughan, 1905) from the Holkerian, middle Visean, of Great Britain. The specimens are close to L. hemisphaerica in their dimensions and outline, but differ from it in having denser radial ornamentation. They share with L. corrugatohemisphaerica the radial ornamentation, though they are larger, they lack the characteristic pointed umbo displayed by L. corrugatohemisphaerica (Ferguson 1971: pl. 41: 8) and have a more convex ventral valve.
Order Orthotetida Waagen, 1884
Suborder Orthotetidina Waagen, 1884
Superfamily Orthotetoidea Waagen, 1884
Family Pulsiidae Cooper and Grant, 1974
Genus Schellwienella Thomas, 1910
Type species: Spirifera crenistria Phillips, 1836, from the Visean Pendleside Group of Bowland, Yorkshire, England.
Remarks.—The genus differs from Pulsia Ivanov, 1925, as this has parallel dental plates; from Meekella White and St. John, 1867, of the Meekellidae Stehli, 1954, as this shows dental plates converging on the valve floor.
Schellwienella sp.
Fig. 13I.
Material.—One ventral valve: MPUM11995 (RCC36-12). One fragment: MPUM11996 (RCC128-17b). Both from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Small, ventral shell moderately convex posteriorly, flat anteriorly. Cardinal margin straight, wide. Ventral umbo straight; interarea low, apsacline, asymmetrical. Convex, smooth pseudodeltidium, no monticulus; perideltidium evident. Apical angle 60°.
Ventral valve ornamented by two orders of ribs. Primary ribs have wide flat interspaces, and increase in number anteriorly by intercalation. One to two thinner secondary ribs are present between each pair of primary ribs, starting 10.0 mm from the umbo, increasing in number anteriorly by intercalation, becoming four between each pair of primary ribs at the anterior margin. Weak concentric micro-ornamentation of growth lines.
Short dental plates diverging from each other at 60°. Long bilobed cardinal process, directed postero-ventrally; lobes diverging from each other with an angle of 120°.
Remarks.—The specimen shows an ornamentation which is distinctive of a small number of Western European Schellwienella species: S. cylindrica (M’Coy, 1844) from the Visean of Castle Espie (Ireland), S. ornata Demanet, 1934, from the Visé Formation at Visé (Belgium), and S. scotica Reed, 1954, from the Visean of the Midland Valley of Scotland. The specimen differs from S. ornata by having a coarser ornamentation, fewer secondary ribs intercalated between primary ribs, a larger angle of divergence between the dental plates (25° in S. ornata; Mottequin and Simon 2017) and a broader pseudodeltidium. The specimen strongly resembles S. scotica specimens figured by McIntosh (1974: pl. I: I–N) both in rib density and dental plates divergence angle. However, it is also similar to S. cylindrica, which differs from S. scotica in having a resupinate ventral valve and strongly convex dorsal valve. As the specimen is a ventral valve broken anteriorly, no information about its outline and lateral profile is available. The specimen is thus left in open nomenclature.
Family Schuchertellidae Williams, 1953
Subfamily Schuchertellinae Williams, 1953
Genus Serratocrista Brunton, 1968
Type species: Serratocrista fistulosa Brunton, 1968, from the Asbian, upper Visean, of County Fermanagh, Ireland.
Remarks.—The genus was established by Brunton (1968) to include orthotetidines similar to Schuchertella Thomas, 1910, but lacking a median dorsal septum (though Serratocrista cylindricosta McIntosh, 1974, has a faint one). Serratocrista also differs from Schuchertella in having a vestigial chilidium, faint muscle scars and in lacking a myophragm.
The genus differs from Streptorhynchus King, 1850, of the Streptorhynchinae Stehli, 1954, in lacking a dorsal median septum, in having socket ridges diverging at a low angle from the cardinal margin, instead of diverging at a high angle, and in having a low, broad ventral interarea, rather than a high, narrow one.
?Serratocrista aff. dalriensis McIntosh, 1974
Fig. 13H.
Material.—Two ventral valves: MPUM11997 (RCC10-40); MPUM11998 (RCC10-2) from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Medium-sized ventral valve with a semicircular outline, flat posteriorly, convex anteriorly. Cardinal margin straight, corresponding to maximum width. Anterior commissure rectimarginate. Umbo moderate, straight. Ventral interarea low, broad, apsacline. Convex, smooth pseudodeltidium; no monticulus; perideltidium evident. Apical angle 60°.
Ventral valve covered by low, straight, rounded ribs with flat, commonly broad interspaces. Ribs increasing anteriorly by frequent intercalation. They number 7–10 per 5.0 mm, 30.0 mm from the umbo. Interspaces with faint concentric filae.
Dental plates and ventral median septum absent.
Remarks.—As the specimens are a disarticulated ventral valve and a fragment, no information about their dorsal internal features are available. Based on the absence of a ventral septum and dental plates the specimens seem to belong to a species of Schuchertellinae. They resemble Serratocrista dalriensis from the Namurian (Serpukhovian) of the Midland Valley of Scotland in their broad pseudodeltidium and ribs density. They can be also included in the size range of the type series of S. dalriensis (McIntosh 1974: 218), but differ from it by lacking branching costellae.
Class Rhynchonellata Williams, Alwyn, Carlson, Brunton, Holmer, and Popov, 1996
Order Orthida Schuchert and Cooper, 1932
Suborder Dalmanellidina Moore, 1952
Superfamily Enteletoidea Waagen, 1884
Family Schizophoriidae Schuchert and LeVene, 1929
Genus Schizophoria King, 1850
Subgenus Schizophoria King, 1850
Type species: Conchyliolithus Anomites (resupinatus) Martin, 1809, from the Visean of Derbyshire, England.
Remarks.—Schizophoria contains a large number of species from all over the World, with great morphological variability. Lazarev (1976) established two subgenera: Paraschizophoria and Pockockia but, as indicated by Harper (2000), the use of Lazarev’s subgenera in the literature is restricted.
Schizophoria is distinguished from Aulacophoria Schuchert and Cooper, 1931, from the Mississippian of Europe as this has a planoconvex profile, with a catacline ventral interarea.
Schizophoria (Schizophoria) resupinata (Martin, 1809)
Fig. 13L–O.
1809 Conchyliolithus Anomites (resupinatus); Martin 1809: pl. 49: 13.
1862 Orthis resupinata (Martin, 1809) var. connivens Phillips, 1836; Davidson 1862a: 131, pl. 29: 6,7; pl. 30: 1–5.
non 1862 Orthis resupinata Martin, 1809; Davidson 1862a: 130, pl. 29: 1–3; pl. 30: 1–5.
non 1862 Orthis resupinata Martin, 1809 var. gibbera Portlock, 1843; Davidson 1862a: 130, pl. 29: 5.
1965 Schizophoria resupinata (Martin, 1809); Wright 1965: H332, fig. 210: 5a–b.
1968 Schizophoria resupinata (Martin, 1809); Pocock 1968: 80, text-figs. 13–15, pl. 18: 7a–b. (cum syn.)
1991 Schizophoria resupinata (Martin, 1809); Brunton and Tilsley 1991: 289.
2000 Schizophoria (Schizophoria) resupinata (Martin, 1809); Harper 2000: fig. 612: a–b.
2000 Schizophoria (Schizophoria) resupinata (Martin, 1809) lata Stainbrook, 1940; Harper 2000: fig. 612: c–f.
2006 Schizophoria resupinata (Martin, 1809); Bassett and Bryant 2006: 504, pl. 6: 1–10; pl. 7: 1–16; text-figs. 6, 7.
non 2007 Schizophoria resupinata (Martin, 1809); Butts 2007: 55: 5.3–5.10.
2010 Schizophoria resupinata (Martin, 1809); Mottequin 2010: 259, pl. 2: 12–13; text-fig. 13.
2011 Schizophoria (Schizophoria) resupinata (Martin, 1809); Bahrammanesh et al. 2011: fig. 7: f–g.
?2014 Schizophoria resupinata (Martin, 1809); Ibaraki et al. 2014: 73, figs. 4.1, 4.2. (cum syn.)
2016 Schizophoria resupinata (Martin, 1809); Denayer et al. 2016: fig. 7M.
?2016 Schizophoria resupinata (Martin, 1809); Tazawa et al. 2016: 57: figs. 6.1, 6.2. (cum syn.)
2017 Schizophoria (Schizophoria) resupinata (Martin, 1809); Qiao et al. 2017: fig. 5e.
?2019 Schizophoria pinguis Demanet, 1934; Tazawa and Kurita 2019: 222, fig. 4b–c. (cum syn.)
Material.—Three articulated specimens: MPUM11999 (RCC1B-26); MPUM12000 (RCC1B-3; RCC60-1b). One ventral valves: MPUM12002 (RCC1B-20a). Six dorsal valves: MPUM12003 (RCC1B-4); MPUM12004 (RCC1B-25); MPUM12005 (RCC36-1a); MPUM12006 (RCC10-35a; RCC108-2; RCC110-1a). One unidentified valve: MPUM 12007 (RCC110-3a). Twenty-seven fragments: MPUM12008 (RCC1-3, 34b; RCC1B-2, 6, 8, 10, 11, 12a, 14, 17, 21, 24a, 27c, 27d, 28, 29; RCC32-8b; RCC36-2, 4, 5; RCC41-12; RCC42-31, 33; RCC60-18b; RCC128-14, 21; RCK15-60b). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Medium-sized, dorsibiconvex shell with transversely oval outline; cardinal margin straight, reaching more than two-thirds of maximum width, which is attained at one-third shell length. Anterior commissure rectimarginate. Ventral valve gently convex to slightly concave anteriorly. Ventral umbo straight. Interarea low, apsacline. Dorsal valve regularly convex. Dorsal umbo slightly curved. Shallow, flat sulcus may develop near the umbo, increasing in width, but becoming obsolete anteriorly.
Entire surface of both valves multicostellate. Ribs start at umbo, unless for rare intercalating costellae. Ribs rounded, very low, with narrow interspaces. Ribs number 3–4 per 1.0 mm, 22.0 mm from the umbo.
Remarks.—The specimens are assigned to Schizophoria (Schizophoria) resupinata because their morphology, size and ornamentation are comprised in the variability of the species as it was re-described by Pocock (1968), based on Mississippian specimens from Belgium, Great Britain, and Ireland. They also match the description for S. (S.) resupinata given by Bassett and Bryant (2006) and Bahrammanesh et al. (2011).
The specimens differ from S. connivens (Phillips, 1836) by being larger, by lacking a quadrate–uniplicate anterior commissure and by having a finer multicostellate ornamentation; from S. gibbera by lacking a high, rounded anterior uniplication; from S. woodi Bond, 1941, by lacking a narrow angular ventral sulcus and from S. linguata Quenstedt, 1871, and S. annectans Pocock, 1968, in being larger, in having a coarser multicostellate ornamentation and a rectimarginate commissure.
Stratigraphic and geographic range.—Schizophoria (Schizophoria) resupinata is recorded in the uppermost Famennian–Pennsylvanian of Great Britain and Ireland (e.g., Davidson 1862a; Parkinson 1954a; Wolfenden 1958; Brunton 1968; Mitchell 1971; Bassett and Bryant 2006; this study), Belgium (e.g., Pocock 1968), Germany (Winkler-Prins and Amler 2006), the Donets and Moscow basins in Ukraine and western Russia (e.g., Sarycheva and Sokolskaya 1952), Siberia (Kuznetsk Basin; e.g., Sarycheva et al. 1963), north Iran (Mobarak Formation; Bahrammanesh et al. 2011), Kazakhstan, Kirghizistan and China (e.g., Sun and Baliński 2008). It is abundant in the Visean of Eurasia (Pocock 1968).
Schizophoria (Schizophoria) cf. connivens (Phillips, 1836)
Fig. 13J, K.
Material.—One articulated specimen: MPUM12009 (RCC60-48). One dorsal valve: MPUM12010 (RCC1B-7). Both from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Remarks.—The specimens are a poorly convex valve and a fragment of the anterior part of an articulated specimen with a transverse oval outline and an inequibiconvex profile. Both specimens are covered by prominent cylindrical ribs with narrow interspaces, increasing in number anteriorly by intercalation. Ribs number 3 per 1.0 mm at the anterior commissure. Regularly concentric lamellae are developed, having a width of 2.0 mm.
The specimens differ from S. resupinata by having a more prominent ribbing, thus they are tentatively assigned to S. connivens, from the Mississippian of Yorkshire, which is small and characterised by prominent ribbing and concentric lamellae (Pocock 1968).
Order Rhynchonellida Kuhn, 1949
Superfamily Pugnacoidea Rzhonsnitskaia, 1956
Family Pugnacidae Rzhonsnitskaia, 1956
Genus Pleuropugnoides Ferguson, 1966
Type species: Terebratula pleurodon Phillips, 1836, from the lower Visean of England.
Remarks.—Savage et al. (2002) described Pleuropugnoides as having fold and sulcus starting at the umbones. However, it is evident from the published photographs (Savage et al. 2002: fig. 800: 1a–k) that in fact the fold and sulcus start from the mid-length.
The genus differs from Pugnoides Weller, 1910, Propriopugnus Brunton, 1984, and Pugnax Hall and Clarke, 1893, by the more pronounced ornamentation of costae covering the entire surface of both valves.
Pleuropugnoides pleurodon (Phillips, 1836)
Figs. 14A–G, 15A.
1836 Terebratula pleurodon; Phillips 1836: 222: pl. 12: 25–28.
1861 Rhynchonella pleurodon (Phillips, 1836); Davidson 1861a: 101, pl. 23: 1–6, non 7, 8–11, non 12–17, ?18, 19–21, non 22. (cum syn.)
1890 Rhynchonella pleurodon (Phillips, 1836); Walther 1890: 433, pl. 24: 9a–d.
1900 Rhynchonella pleurodon (Phillips, 1836); Frech and Arthaber 1900: 201, fig. 13: a–d.
non 1917 Rhynchonella (?pugnax) cf. pleurodon (Phillips, 1836); Trechmann 1917: 59, pl. 5: 6–8.
1952 Camarotoechia pleurodon (Phillips, 1836); Sarycheva and Sokolskaya 1952: 176, pl. 46.
non 1965 Coledium pleurodon (Phillips, 1836); Grant 1965: pl. 18: 3.
1966 Pleuropugnoides pleurodon (Phillips, 1836); Ferguson 1966: 355, pl. 23: 1–11.
1968 Camarotoechia pleurodon (Phillips, 1836); Korejwo and Teller 1968: pl. 20: 2–3.
1969 Pleuropugnoides pleurodon (Phillips, 1836); Parkinson 1969: pl. 19: 1–4; pl. 20: 1, 3, 4, non 2.
1974 Pleuropugnoides pleurodon (Phillips, 1836); Brunton and Champion 1974: 819, pl. 108: 1–4.
1984 Pleuropugnoides pleurodon (Phillips, 1836); Brunton 1984: 34–36, figs. 5–8.
1991 Pleuropugnoides pleurodon (Phillips, 1836); Brunton and Tilsley 1991: 289.
1991 Pleuropugnoides pleurodon (Phillips, 1836); Martinez-Chacon and Legrand-Blain 1992: 117, pl. 5: 5–9.
1995 Pleuropugnoides pleurodon (Phillips, 1836); Kora 1995: 3q.
2002 Pleuropugnoides pleurodon (Phillips, 1836); Savage et al. 2002: fig. 800: 1a–k.
?2011 Pleuropugnoides pleurodon (Phillips, 1836); El-Shazly 2011: pl. 3: 1, 4.
Material.—Twenty articulated shells: MPUM12011 (RCC49-22); MPUM12012 (RCC73-9); MPUM12013 (RCK15-4); MPUM12014 (RCK15-15); MPUM12015 (RCK15-19); MPUM12016 (RCK15-29); MPUM12017 (RCK15B-3); MPUM12018 (RCC1-23c; RCC49-10c, 17, 18, 44; RCC54-17c; RCC73-23a, 23b; RCC300-9; RCK15-1d, 1e, 46a, 46c). Three ventral valves: MPUM12019 (RCC1-27b; RCC129-2; RCK15-46b). Five dorsal valves: MPUM12020 (RCC41-17b; RCC48-26b, 40; RCC49-4; RCK17-3). Sixteen fragments: MPUM12020 (RCC1-8g; RCC1B-5b, 12b, 23b; RCC12-24; RCC41-17c; RCC42-38; RCC49-8b, 30, 43; RCC54-9; RCC65G-24; RCC73-23b; RCK15-8b, 41, 46d). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Small, dorsibiconvex shell with transversely suboval outline; cardinal margin curved; maximum width at shell mid-length. Anterior commissure strongly uniplicate with zig-zag profile; tongue high, trapezoid. Ventral valve flat posteriorly, gently concave anteriorly. Umbo slightly curved; palintrope orthocline. Foramen submesothyrid. Dorsal valve evenly convex. Dorsal umbo straight; dorsal palintrope orthocline, sealed by the ventral valve. Ventral sulcus and dorsal fold well developed, starting from shell mid-length.
Ornamentation consists of coarse, low, rounded costae becoming high and angular anteriorly, with narrow interspaces. Ventral sulcus usually with four costae; dorsal fold with five costae; five to seven lateral costae. Micro-ornamentation of faint growth lamellae.
There is variability in profile thickness and shape due to variation of the dorsal valve convexity (e.g., RCK15-15, Fig. 14E; RCK15-29; Fig. 14G). The number of lateral costae is variable, as it is their width: from four to six costae per 5.0 mm width at 5.0 mm from the dorsal umbo.
Juvenile shells are ovatotriangular in outline, equally biconvex, with a narrow body cavity and are ornamented by costae with narrow interspaces (e.g., RCK15-4, Fig. 15A). At a length of about 6.0 mm, an incipient tongue develops in the ventral valve (e.g., RCK15B-3, Fig. 14C), while an incipient fold in the dorsal one. During ontogeny, they change in shape, from equally biconvex to strongly dorsibiconvex.
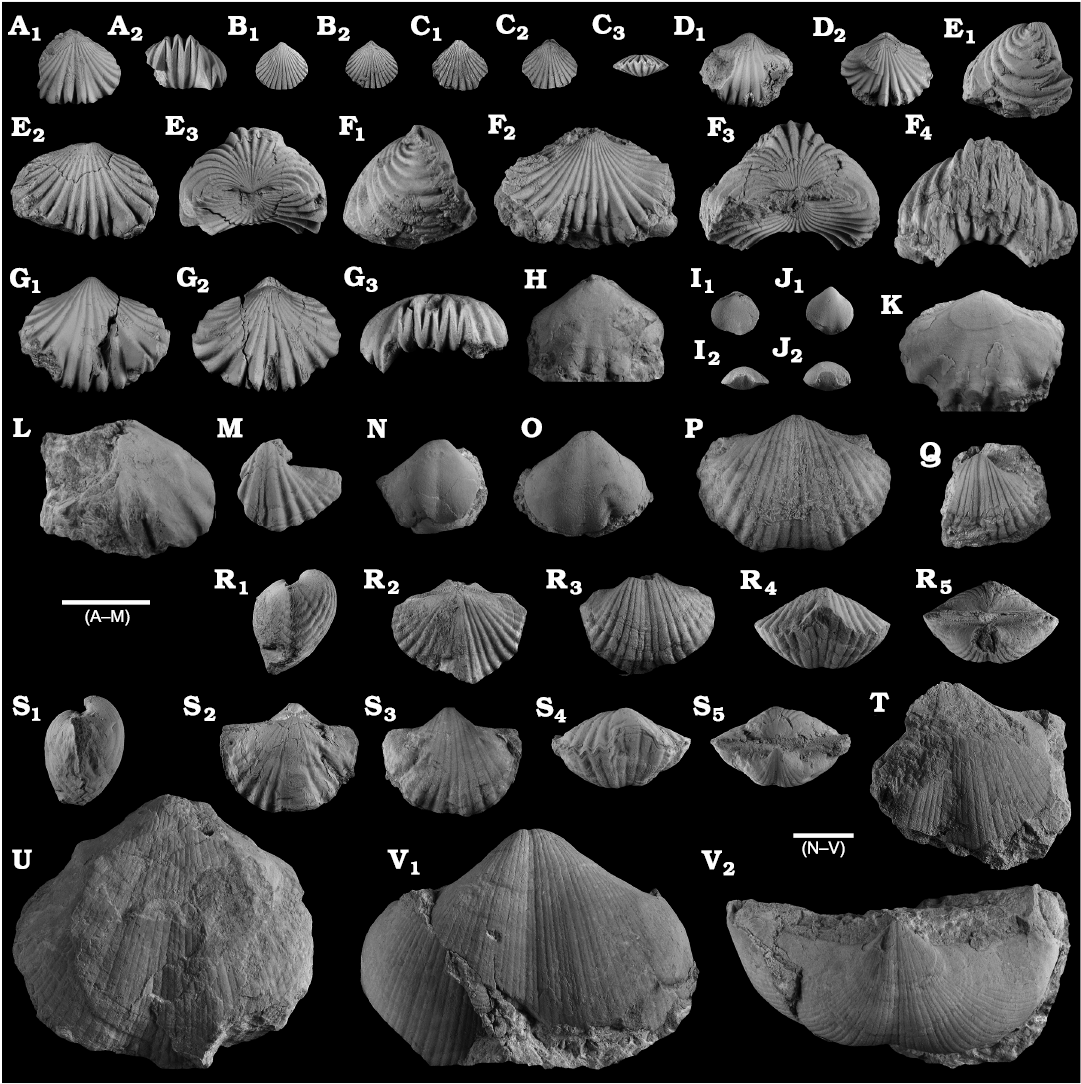
Fig. 14. Rhynchonellida and Spiriferida from the Brigantian (uppermost Visean) of Ricklow Quarry, near Monyash (localities RCC-, RCK-; Fig. 3) and Wensley Dale, near Matlock (locality WI1; Fig. 2), Derbyshire, UK. A–G. Pleuropugnoides pleurodon (Phillips, 1836). A. MPUM12011 (RCC49-22) in dorsal (A1) and anterior (A2) views. B. MPUM12013 (RCK15-4) in ventral (B1) and dorsal (B2) views. C. MPUM12017 (RCK15B-3) in ventral (C1), dorsal (C2), and anterior (C3) views. D. MPUM12015 (RCK15-19) in ventral (D1) and dorsal (D2) view. E. MPUM12014 (RCK15-15) in lateral (E1), ventral (E2), and posterior (E3) views. F. MPUM12012 (RCC73-9) in lateral (F1), ventral (F2), posterior (F3), and anterior (F4) views. G. MPUM12016 (RCK15-29) in ventral (G1), dorsal (G2), and anterior (G3) views. H–L. Propriopugnus pugnus (Martin, 1809). H. MPUM12021 (RCC2-55) in dorsal view. I. MPUM12022 (RCC3-7) in dorsal (I1) and anterior (I2) views. J. MPUM12024 (RCC73-22b) in ventral (J1) and anterior (J2) views. K. MPUM12026 (RCC60-4d) dorsal valve in dorsal view. L. MPUM12028 (WI1-33) in dorsal view. M. ?Fusella sp., MPUM12037 (RCC128-5) ventral valve in ventral view. N, O. Martiniinae gen. et sp. indet. N. MPUM12065 (RCC2-58a) ventral valve in ventral view. O. MPUM12066 (WI1-32) ventral valve in ventral view. P–S. Angiospirifer bisulcatus (Sowerby, 1825). P. MPUM12032 (WI1-12a) in ventral view. Q. MPUM12035 (WI1-11) ventral valve in ventral view. R. MPUM12031 (WI1-10) in lateral (R1), dorsal (R2), ventral (R3), anterior (R4), and posterior (R5) views. S. MPUM12033 (WI1-30) in lateral (S1), dorsal (S2), ventral (S3), anterior (S4), and posterior (S5) views. T–V. Latibrachythyris cf. crassa (De Koninck, 1843). T. MPUM12039 (RCC1-34a) ventral valve in ventral view. U. MPUM12038 (RCC1-21) ventral valve in ventral view. V. MPUM12041 (RCC54-17a) ventral valve in ventral (V1) and posterior (V2) views. Scale bars 10 mm.
Remarks.—The specimens have a more exposed palintrope and more angular costae with respect to the early Visean specimens of Pleuropugnoides pleurodon from Derbyshire (Thorpe Cloud) and Yorkshire (Bolland) on which Ferguson (1966) based his revision of the species. However, both Parkinson (1969) and Brunton and Champion (1974) illustrated specimens of P. pleurodon from the middle Visean of Yorkshire and Derbyshire and the lower Visean of the Manifold Valley (Staffordshire, England) respectively that have angular costae and a rather exposed palintrope.
Brigantian (uppermost Visean) specimens from Ricklow Quarry have four, rarely three costae in the sulcus; thus, they do not support the trend of increasing number of sulcal costae (from two to six) through the Visean envisaged for the species by Parkinson (1969) and recorded also by Brunton (1984).
Pleuropugnoides pleurodon differs from the allied species P. flexistria (Phillips, 1836) in lacking numerous intercalating and bifurcating costellae and narrower lateral costae with respect to the ones on the folds and sulcus, and from P. greenleightonensis Ferguson, 1966, as this species has a distinctive rhomboidal outline.
Stratigraphic and geographic range.—Pleuropugnoides pleurodon is recorded in the Visean of Great Britain and Ireland (e.g., Ferguson 1966; Parkinson 1969; Mitchell 1971; Brunton and Champion 1974; Brunton 1984; Brunton and Tilsley 1991; Harper and Jeffrey 1996; Dean 2017; this study), Germany (e.g., Nicolaus 1963), Spain (e.g., Martinez-Chacon and Legrand-Blain 1992), Belgium (e.g., Demanet 1923), the Silesian Basin, Poland and the Czech Republic (Ostrava Formation; e.g., Jirasek et al. 2012; Żakowa 1971), and North Africa (e.g., Pareyn 1961; Massa et al. 1974; Dedok and Hollard 1980; Kora 1995).
Genus Propriopugnus Brunton, 1984
Type species: Conchyliolithus Anomites (pugnus) Martin, 1809, from the upper Visean limestone of Castleton, Hope and Little-Longstone, Derbyshire, England.
Remarks.—The genus was erected by Brunton (1984) to include European rhynchonellides with a high fold and sulcus, a small septalium and a few costae originating in the posterior region of the shell. The specimens of P. pugnus figured by Davidson (1861a: pl. 22: 1–15), Parkinson (1954b: pl. 58: 1–15) and Savage et al. (2002: fig. 798: 1a–p) from the Visean of England have costae arising at one-third shell length and not at the umbones, as stated by Savage et al. (2002). Brunton and Tilsley (1991) included in Propriopugnus also Pugnax pseudopugnus (Parkinson, 1954b), from the upper Visean of England, which apparently lacks a dorsal median septum (Parkinson 1954b). Propriopugnus differs from Pugnax in having a lower, rounded fold and in having coarser costae.
Propriopugnus pugnus (Martin, 1809)
Fig. 14H–L.
1809 Conchyliolithus Anomites (pugnus); Martin 1809: pl. 22: 4–5.
1861 Rhynchonella pugnus (Martin, 1809); Davidson 1861a: 97, pl. 22: ?1, 2–15. (cum syn.)
1951 Pugnax pugnus (Martin, 1809); Muir-Wood 1951: pl. 4: 3a–c.
1952 Pugnax pugnus (Martin, 1809); Sarycheva and Sokolskaya 1952: 178, pl. 47.
1954 Pugnax pugnus (Martin, 1809); Parkinson 1954b: 564, pl. 58: 1–15.
1984 Propriopugnus pugnus (Martin, 1809); Brunton 1984: 32, figs. 3, 4.
1991 Propriopugnus pugnus (Martin, 1809); Brunton and Tilsley 1991: 289.
2002 Propriopugnus pugnus (Martin, 1809); Savage et al. 2002: fig. 798: 1a–p.
Material.—Thirteen articulated specimens: MPUM12021 (RCC2-55); MPUM12022 (RCC3-7); MPUM12023 (RCC60-44); MPUM12024 (RCC73-22b); MPUM12028 (WI1-33); MPUM12025 (RCC1B-40a; RCC10-1; RCC32-20; RCC41-18b; RCC42-25a, 39; RCC60-8b; RCC65G-15c). Two dorsal valves: MPUM12026 (RCC60-4d); MPUM12027 (RCC32-28c). One fragment: MPUM12029 (WI1-16). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian (RCC-, RCK-), and Wensley Dale, Matlock, Derbyshire, UK, Eyam Limestone Formation, upper Brigantian, upper Visean (WI1).
Description.—Small, dorsibiconvex shell with subpentagonal outline; cardinal margin curved; maximum width attained at shell mid-length. Anterior commissure strongly uniplicate with zig-zag profile; tongue high, trapezoid. Ventral umbo slightly curved; palintrope very low, orthocline to slightly apsacline. Delthyrium probably with deltidial plates. Sulcus developing at one-third shell length. Dorsal umbo straight; palintrope orthocline, sealed by the ventral valve. High fold developing from shell mid-length.
Three simple, rounded costae with broad rounded interspaces developing from shell mid-length on fold, becoming higher and subangular anteriorly. Two faint costae developed on sulcus from shell mid-length. Two poorly defined low coarse costae developing on each flank near to the commissure. Micro-ornamentation of faint concentric growth lines, sporadic lamellae developed.
Short dorsal median septum, approximately 0.3 mm in length.
The juvenile shell has an oval outline, less transverse with respect to the adult shell, an equally biconvex shape and narrow body cavity. The shell is smooth. Sulcus and subelliptical tongue start to develop approximately at 3.5 mm shell length; fold develops later in ontogeny. Tongue becoming subtrapezoidal later in ontogeny.
Remarks.—The specimens show mesial costae, which start at shell mid-length. This is not the most usual pattern of ornamentation in Propriopugnus pugnus that commonly shows mesial costae originating in the posterior half of the shell. However, many specimens attributed to P. pugnus in the literature, as the ones from Ireland figured by Sowerby (1825: pl. 497: 1–5) and some of those figured by Davidson (1861a: pl. 2: 2, 9, 10, 15), have mesial costae originating from shell mid-length, so this feature seems to be contained in the morphological variability of the species. The specimens differ from P. pseudopugnus in having more rounded costae, a higher average number of mesial costae and an incipient dorsal median septum (Parkinson 1954b).
Stratigraphic and geographic range.—Propriopugnus pugnus is widely reported from Visean units in Great Britain and Ireland (e.g., Davidson 1861a; Parkinson 1954b; Mitchell 1971; Brunton 1984; Brunton and Tilsley 1991; Brand 2011; Dean 2017; this study), Belgium and the eastern side of the Urals Mountains (Davidson 1861a).
Order Spiriferida Waagen, 1883
Suborder Spiriferidina Waagen, 1883
Superfamily Ambocoelioidea George, 1931
Family Ambocoeliidae George, 1931
Genus Crurithyris George, 1931
Type species: Spirifer urei Fleming, 1828, from the upper Visean of Strathaven, Lanarkshire (Scotland).
Remarks.—Crurithyris has no inner hinge plates (Brunton 1984), which allows to distinguish it from Ambothyris George, 1931, from the Middle Devonian of Devonshire.
Crurithyris urei (Fleming, 1828)
Fig. 15B.
1793 [unnamed]; Ure 1793: pl. 14: 12.
1828 Spirifer urei; Fleming 1828: 376.
1859 Spirifera urii Fleming, 1828; Davidson 1859: 58 (pars), pl. 12: 13–14.
1862 Spirifera urii Fleming, 1828; Davidson 1862a: pl. 54: 14.
?1899 Ambocoelia parva; Weller 1899: 20, pl. 4: 1–4.
1931 Crurithyris urei (Fleming, 1828); George 1931: 55, pl. 4: 1–4.
?1931 Crurithyris magnispina; George 1931: 50, pl. 5: 1–4.
1965 Crurithyris urei (Fleming, 1828); Pitrat 1965: fig. 546: 6a–d.
1984 Crurithyris urei (Fleming, 1828); Brunton 1984: 66, figs. 83, 85–92.
1991 Crurithyris urei (Fleming, 1828); Brunton and Tilsley 1991: 290.
?1991 Crurithyris magnispina George, 1931; Brunton and Tilsley 1991: 290.
2006 Crurithyris urei (Fleming, 1828); Johnson et al. 2006: fig. 1138: 4a–d.
Material.—One articulated specimen: MPUM12030 (RCC 42-25b) from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Small, ventribiconvex shell with suboval outline; cardinal margin straight, extending two-thirds of maximum width. Cardinal angles obtuse, cardinal extremities subangular. Anterior commissure unisulcate. Ventral interarea low, apsacline. Ventral sulcus absent. Dorsal valve evenly convex. Dorsal interarea very low, orthocline to gently anacline. Low, broad sulcus starting from one-third shell length. Shell smooth. Crural bases strong, almost parallel, 1.0 mm long, each forming an angle of 80° with the cardinal margin. Dorsal median septum absent.
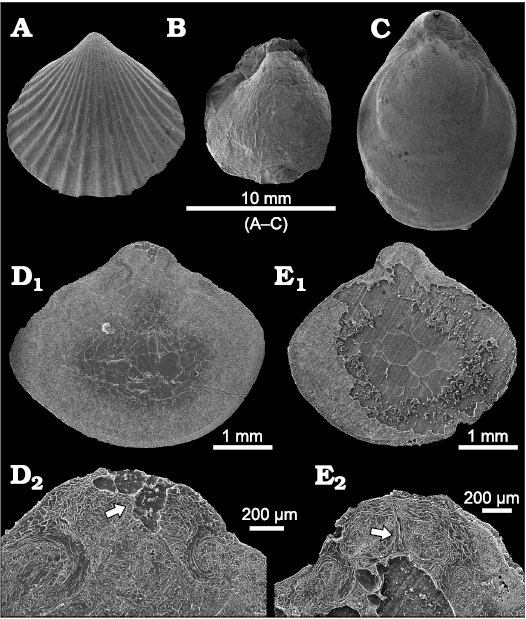
Fig. 15. SEM images of brachiopod specimens from the Brigantian (uppermost Visean) of Ricklow Quarry, near Monyash, Derbyshire, UK (localities RCC-, RCK-; Fig. 3). A. Pugnacid Pleuropugnoides pleurodon (Phillips, 1836), MPUM12013 (RCK15-4) in ventral view. B. Ambocoelid Crurithyris urei (Fleming, 1828), MPUM12030 (RCC42-25b) in ventral view. C. Gillediidid ?Balanoconcha sp., MPUM12094 (RCC10-15a) in dorsal view. D, E. Cranaenidid Harttella oakleyi Brunton, 1982. D. MPUM12088 (RCC65G-13), serial section 1.0 mm from the umbo. E. MPUM12088 (RCC48-31), serial section 1.6 mm from the umbo. Detail showing absence of dental plates (D1, E1), focus showing Y-shaped septalium (D2, E2).
Remarks.—The specimen is assigned to Crurithyris urei, instead of the closely allied species C. nastus Brunton and Champion, 1974, occurring in lower Visean strata of the Manifold Valley (Staffordshire), based on its regularly convex dorsal valve and crural bases. C. nastus shows a dorsal valve becoming flat anteriorly and shorter, diverging crural bases.
Crurithyris magnispina George, 1931, is very similar to C. urei and was considered as a synonym by Brunton (1984); later Brunton and Tilsley (1991) differentiated it only based on larger adult size. The specimen from Ricklow Quarry falls in the size range for C. urei.
Stratigraphic and geographic range.—Crurithyris urei is recorded in the Asbian–Brigantian (upper Visean) of northern England, Scotland, and Wales (e.g., George 1931; Brunton 1984; Wilson 1989; Brunton and Tilsley 1991; this study) and in the Tournaisian–upper Visean of the Moscow Basin (Sarycheva and Sokolskaya 1952).
Superfamily Martinioidea Waagen, 1883
Family Martiniidae Waagen, 1883
Subfamily Martiniinae Waagen, 1883
Martiniinae gen. et sp. indet.
Fig. 14N, O.
Material.—Two ventral valves: MPUM12065 (RCC2-58a); MPUM12066 (WI1-32) from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian (RCC-, RCK-), and Wensley Dale, Matlock, Derbyshire, UK, Eyam Limestone Formation, upper Brigantian, upper Visean (WI1).
Description.—Small shell with suboval outline; maximum width at shell mid-length. Anterior commissure uniplicate. Shallow, rounded sulcus develops at 10.0 mm from the umbo, extending into a tongue anteriorly. Few growth lamellae and growth lines. Ventral adminicula and septum absent.
Remarks.—The specimens are attributed to the Martiniinae based on their concentric ornamentation, shallow sulcus and absence of ventral adminicula. They are left under open nomenclature due to their poor preservation.
Superfamily Spiriferoidea King, 1846
Family Choristitidae Waterhouse, 1968
Subfamily Angiospiriferinae Legrand-Blain, 1985
Genus Angiospirifer Legrand-Blain, 1985
Type species: Conchyliolithus Anomites (trigonalis) Martin, 1809 from the Brigantian, uppermost Visean, of Scotland.
Remarks.—The genus differs from other Mississippian spiriferoids in having prominent simple costae and in lacking a strong transversely elongated outline. It differs from Brachythyrina Fredericks, 1929, from the Mississippian–Permian of Europe and Asia, in being larger, in having a broader sulcus-fold and ventral adminicula.
Angiospirifer bisulcatus (Sowerby, 1825)
Fig. 14P–S.
1825 Spirifer bisulcatus; Sowerby 1825: 152, pl. 494: 1–2.
1858 Spirifera bisulcatus Sowerby, 1825; Davidson 1858: 31 (pars), pl. 4: 1; pl. 5: 1; pl. 6: 1–9, 13–17; pl. 7: 4.
1991 Spirifer bisulcatus Sowerby, 1825; Brunton and Tilsley 1991: 293.
2008 Spirifer bisulcatus Sowerby, 1825; Legrand-Blain 2008: pl. 3: 1–2.
2011 Angiospirifer bisulcatus (Sowerby, 1825); Angiolini et al. 2011: 74, figs. 1, 2, 6A–I. (cum syn.)
Material.—Four articulated specimens: MPUM12031 (WI1-10); MPUM12032 (WI1-12a); MPUM12033 (WI1-30); MPUM12034 (WI1-39). Two ventral valves: MPUM12035 (WI1-11); MPUM12036 (WI1-36). All from Wensley Dale, Matlock, Derbyshire, UK, Eyam Limestone Formation, upper Brigantian, upper Visean.
Description.—Medium-sized, ventribiconvex shell with semicircular outline; cardinal margin straight, slightly less or corresponding to the maximum width. Cardinal angles acute to obtuse, cardinal extremities subangular. Anterior commissure uniplicate. Ventral interarea apsacline, denticulate. Low, rounded sulcus starting near umbo, widening and deepening anteriorly, extending in a subrounded tongue at the commissure. Dorsal interarea orthocline, very low. High, subrounded fold starting 5.0 mm from umbo, widening and increasing in height anteriorly.
Ornamentation consists of coarse, rounded, simple costae with narrow, rounded interspaces. Costae number 4–5 per 10.0 mm at the anterior commissure. Ventral sulcus with five ribs: one mesial costa widening anteriorly, not distinctly larger than lateral ones, which derive from bifurcation near the umbo of the sulcus-bounding costae. Fold with four indistinct ribs. Irregularly spaced lamellae covering both valves.
Remarks.—The specimens are close to Angiospirifer bisulcatus in their morphology and ornamentation. They differ from A. trigonalis in having a lower and more rounded dorsal fold, in having a regularly semicircular outline, rather than subtriangular, and in lacking a median sulcal costa widening and thickening anteriorly with respect to the lateral ones. However, the specimens lack the sulci bordering the fold which characterize most dorsal valves of A. bisulcatus.
Stratigraphic and geographic range.—Angiospirifer bisulcatus is recorded in the upper Tournaisian–Visean of England and Ireland and the Visean of Belgium (Brunton and Tilsley 1991; Angiolini et al. 2011; this study).
Superfamily Paeckelmanelloidea Ivanova, 1972
Family Strophopleuridae Carter, 1974
Subfamily Bashkiriinae Nalivkin, 1979
Genus Fusella M’Coy, 1844
Type species: Spirifera fusiformis Phillips, 1836, from the lower Visean of Bolland, Yorkshire, England.
Remarks.—Fusella was erected by M’Coy (1844) as a subgenus to include small spiriferides with a fusiform outline and very wide and high cardinal area. Buckman (1908) raised Fusella to genus level, and based on the type species Fusella fusiformis (Phillips, 1836), stated it to have a few faint costae in the sulcus. The internal characters of F. fusiformis are still unknown due to the rarity of the species. Brunton and Rissoné (1976) included in the genus Spirifer rhomboidea Phillips, 1836, from the lower–middle Visean of Great Britain and Ireland, describing its internal characters as an example to those for Fusella. However, S. rhomboidea has no costae in the sulcus. Also the allied Voiseyella Roberts, 1964, of the Strophopleurinae Carter, 1974, has no costae in the sulcus but is distinguished by disproportionally larger sulcus-bounding costae.
?Fusella sp.
Fig. 14M.
Material.—One ventral valve: MPUM12037 (RCC128-5) from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Small, strongly transverse shell. Maximum width at the cardinal margin. Cardinal angles strongly acute; cardinal extremities evolving from mucronate to subrounded during growth. Ventral interarea wider, apsacline. Sulcus starting as a furrow from the umbo, becoming larger and deeper towards the anterior commissure.
Costate: a median costa occurs in the sulcus, starting 6.5 mm from the umbo. Flanks covered by simple, subrounded costae with narrow interspaces, extending from the umbo, becoming lower and indistinct laterally. Four costae per 5.0 mm, 5.0 mm from the umbo. The two sulcus-bounding costae are only slightly wider than the other ones. Strong and irregular concentric ornamentation given by imbricated lamellae.
Remarks.—The specimen is tentatively assigned to Fusella as it shows a median sulcal costa and lacks disproportionately larger sulcus-bounding costae. It differs from F. fusiformis (re-figured by Waterhouse 1970: fig. 1a–f), which is ornamented by numerous weak costae, and from F. rhomboidea (Brunton and Rissoné 1976: pl. 11: 6–7; Brunton 1984: figs. 120–127), which lacks a median sulcal costa. The specimen has some similarities with Spirifer triangularis Sowerby, 1827, from the Visean of England and Belgium, assigned to Fusella by Muir-Wood (1951). However, F. triangularis is characterised by having a strongly transverse ventribiconvex shell with an elevated and narrow dorsal fold, extending anteriorly. The specimen is left under open nomenclature on the basis of its poor preservation.
Superfamily Brachythyridoidea Fredericks, 1924
Family Brachythyrididae Fredericks, 1924
Genus Latibrachythyris Angiolini, Long, and Davies, 2011
Type species: Spirifer pinguis Sowerby, 1821a, from the Tournaisian–Visean of Ireland and England.
Remarks.—Latibrachythyris is distinguishable from Skelidorygma Carter, 1974, of the Skelidorygmidae Carter, 1994, as it lacks a primary median rib in the sulcus and has a denticulate interarea. Spirifer crassus (De Koninck, 1843), from the Visean of Ireland, has wide, straight cardinal margin and an ornamentation of 50–70 low, flat ribs, unequal in width and irregularly bifurcating, with no sulcal median rib, as visible from the figures by Davidson (1858: pl. 6: 20–22). It is thus assigned to Latibrachythyris. Brachythyris hortonensi Carter, 1988, from the Mississippian of USA, was assigned to Latibrachythyris by Bahrammanesh et al. (2011), but as the species is characterised by a sulcal median rib, it is better included in Skelidorygma. Spirifer integricostus Phillips, 1836, was considered as a Skelidorygma species by Brunton (1984) and Brunton and Tilsley (1991). However, the figured specimens of S. integricostus from the Visean of Yorkshire (Davidson 1859: pl. 9: 18; Brunton 1984: fig. 129) and the upper Visean of County Fermanagh, Ireland (Brunton 1984: fig. 128) show a wide cardinal margin, wider than two-thirds maximum width, and no sulcal median rib. Legrand-Blain (1986) noted that S. integricostus has a weakly denticulate interarea, so it can be assigned to Latibrachythyris.
Latibrachythyris cf. crassa (De Koninck, 1843)
Figs. 14T–V, 16A.
Material.—Six ventral valves: MPUM12038 (RCC1-21); MPUM12039 (RCC1-34a); MPUM12040 (RCC54-10a); MPUM12041 (RCC54-17a); MPUM12042 (RCC1- 15a; RCC48-7). Five fragments: MPUM12087 (RCC1-8b, 17b; RCC8-3b; RCC39-7; RCK15-38). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Medium-sized shell with transversely subrectangular outline; cardinal margin straight, almost as wide as the maximum width. Cardinal angles obtuse; cardinal extremities subangular. Anterior commissure uniplicate. Ventral valve strongly convex. Ventral interarea low, apsacline, weakly denticulate. Sulcus starting as a furrow from umbo, becoming wider and broadly rounded anteriorly.
Ornamentation consists of low costae, almost flat, with narrow interspaces. At a variable distance from the umbo, commonly less than 10.0 mm, costae start to irregularly bifurcate giving rise to a distinctive pattern of ribs of variable width. Lateral ribs number four per 5.0 mm width at 2.2 mm from the umbo, six to seven at 30.0 mm. Sulcus ornamented by four ribs at 10.0 mm from the umbo, 16 ribs at 30.0 mm from the umbo. Micro-ornamentation of faint regularly concentric growth lines; sporadic lamellae.
Specimens with a length of approximately 25.0 mm have a less convex profile, with flatter lateral slopes, with respect to larger adult shells, which have steep, rounded lateral slopes resulting in a strongly convex shell with the maximum width at shell mid-length.
Remarks.—The specimens are similar to Latibrachythyris crassa for their convex valves with steep lateral slopes, moderate sulcus and fold and ornamentation. They differ from L. pinguis and L. rotundatus (Sowerby, 1825) in having a finer radial ornamentation of bifurcating ribs.
Latibrachythyris rotundata (Sowerby, 1825)
Fig. 16B–D.
1825 Spirifer rotundatus; Sowerby 1825: 89, pl. 461: 1.
1859 Spirifera pinguis Sowerby, 1821a; Davidson 1859: 50 (pars), pl. 10: 2, 4.
1859 Spirifera pinguis Sowerby, 1821a var. rotundata Sowerby, 1825; Davidson 1859: 50, pl. 10: 8–12.
2011 Latibrachythyris rotundatus (Sowerby, 1825); Angiolini et al. 2011: 79, figs. 5, 6N–O. (cum syn.)
Material.—Two articulated specimens: MPUM12043 (RCK 15-1a); MPUM12044 (RCK15-5). Six ventral valves: MPUM12045 (RCK15-40); MPUM12046 (RCC1B-26c; RCC43-6; RCC45-2; RCC48-24; RCC 129-1). One dorsal valve: MPUM12047 (RCC65G-12). One fragment: MPUM12048 (RCC60-50). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Small, ventribiconvex shell with suboval outline; cardinal margin straight, wide, two-thirds of the maximum width. Commissure gently uniplicate. Ventral umbo curved; interarea apsacline, low. Delthyrium lacking deltidial plates. Distinct median sulcus starting as a furrow from umbo, becoming low and broadly rounded anteriorly. Dorsal umbo straight; interarea orthocline to gently anacline, very low. Fold starting at one-third shell length, low, rounded.
Ventral valve covered by 12–14 simple, broad, rounded lateral costae extending from the umbo, with narrow interspaces. One faint pair of costae in the sulcus bifurcating from sulcus-bounding costae at 4.0–6.0 mm from the umbo; sulcal median costa absent. Dorsal valve covered by 10–12 simple lateral costae extending from the umbo. Fold smooth. Micro-ornamentation of faint irregular growth lamellae.
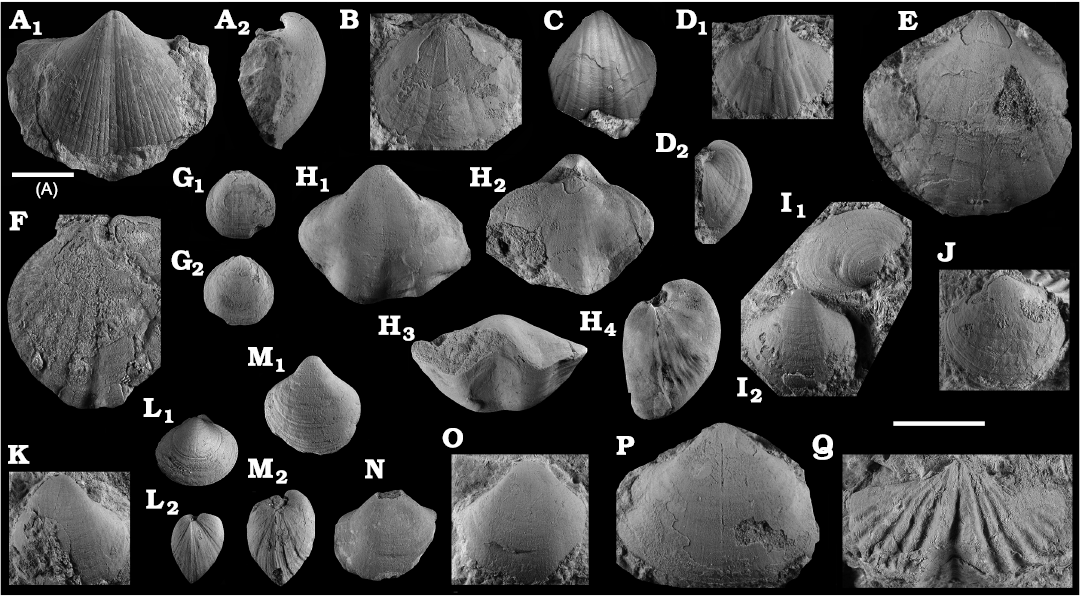
Fig. 16. Spiriferida and Spiriferinida from the Brigantian (uppermost Visean) of Ricklow Quarry, near Monyash (localities RCC-, RCK-; Fig. 3) and Wensley Dale, near Matlock (locality WI1; Fig. 2), Derbyshire, UK. A. Latibrachythyris cf. crassa (De Koninck, 1843), MPUM12040 (RCC54-10a) ventral valve in ventral (A1) and lateral (A2) views. B–D. Latibrachythyris rotundata (Sowerby, 1825). B. MPUM12047 (RCC65G-12) dorsal valve in dorsal view. C. MPUM12043 (RCK15-1a) in ventral view. D. MPUM12045 (RCK15-40) ventral valve in ventral (D1) and lateral (D2) views. E, F. Brachythyrididae gen. et sp. indet. E. MPUM12049 (RCC11-8) ventral valve in ventral view. F. MPUM12052 (RCC42-5a) dorsal valve in dorsal view. G. Reticularia cf. mesoloba (Phillips, 1836), MPUM12053 (RCK15-43) in ventral (G1) and dorsal (G2) views. H. Georgethyris lobata (Muir-Wood, 1926), MPUM12086 (WI1-9) in ventral (H1), dorsal (H2), anterior (H3) and lateral (H4) views. I–K. Phricodothyris paricosta George, 1932. I. MPUM12058 (RCK15-34a) dorsal valve (I1) and MPUM12055 (RCK15-34b) ventral valve (I2). J. MPUM12057 (RCK15-9) dorsal valve in dorsal view. K. MPUM12054 (RCC40-10a) ventral valve in ventral view. L–N. Phricodothyris cf. periculosa George, 1932. L. MPUM12061 (RCK15-3) in dorsal (L1) and lateral (L2) views. M. MPUM12060 (RCC73-30a) in ventral (M1) and lateral (M2) views. N. MPUM12064 (RCC2-37) ventral valve in ventral view. O. Phricodothyris sp., MPUM12071 (RCC60-4a) ventral valve in ventral view. P. Nebenothyris sp., MPUM12073 (WI1-26a) ventral valve in ventral view. Q. ?Punctospirifer sp., MPUM12074 (RCC73-23c) ventral valve in ventral view. Scale bars 10 mm.
Remarks.—The specimens differ from the ones of Latibrachythyris rotundata studied by Angiolini et al. (2011) in being smaller and in being strongly ventribiconvex. However, both the morphology and the ornamentation of the specimens are in the variability shown by L. rotundata, so they probably are juvenile specimens of the species. The specimens are not considered as juveniles of L. cf. crassa, as they lack the pattern of numerous ribs of different width that is described in the specimens from Ricklow Quarry assigned to it.
The specimens differ from L. pinguis in having a less convex and enrolled ventral valve, a less defined sulcus and in being more transverse. Moreover, in L. pinguis the cardinal margin is relatively wider and the interarea is more trapezoidal (Angiolini et al. 2011).
Stratigraphic and geographic range.—Latibrachythyris rotundata is recorded in the upper Tournaisian–Visean of Ireland and England and in the Tournaisian–Visean of Belgium (e.g., Davidson 1859; Angiolini et al. 2011; this study).
Brachythyrididae gen. et sp. indet.
Fig. 16E, F.
Material.—Two ventral valves: MPUM12049 (RCC11-8); MPUM12050 (RCC73-23i). Two dorsal valves: MPUM 12051 (RCC41-18a); MPUM12052 (RCC42-5a). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Remarks.—Specimen MPUM12049 is a medium-sized suboval ventral valve with a wide cardinal margin, shallow sulcus and ornamentation of eight broad simple flat lateral costae with narrow interspaces, reaching a width up to 4.0 mm anteriorly, and two faint costae in the sulcus. The specimen is thus very close to L. rotundata, although it lacks the median furrow in the umbonal region; in fact, the sulcus seems to occur only anteriorly.
Specimen MPUM12052 is a fragment of a suboval flat dorsal valve with five to six simple, broadly rounded lateral costae extending from the umbo and a smooth low fold. The cardinal margin is not fully preserved, but it is inferred to be narrow, suggesting a possible assignment to Brachythyris ovalis (Phillips, 1836), a species that shows a gently convex dorsal valve and a smooth fold.
Suborder Delthyridina Ivanova, 1972
Superfamily Reticularioidea Waagen, 1883
Family Reticulariidae Waagen, 1883
Subfamily Reticulariinae Waagen, 1883
Genus Reticularia M’Coy, 1844
Type species: ?Terebratula imbricata Sowerby, 1822, from the upper Visean of Yorkshire, England.
Remarks.—George (1932) emended the original description of Reticularia to include lamellose spiriferides bearing dental plates and a ventral septum, with ornamentation of uniramous spines. However, he included in the genus the species Reticularia alexandri George, 1932, which lacks a ventral septum. This led Minato (1953) to erect Georgethyris based on R. alexandri, characterised by the absence of a ventral septum and divergent dental plates. Minato (1953) also emended the description for Reticularia defining it as characterised by a ventral median septum and parallel dental plates. This left without generic assignment most species assigned to Reticularia by George (1932), characterised by a ventral median septum and divergent dental plates: R. acutiloba George, 1932, R. lobata Muir-Wood, 1926, R. mesoloba (Phillips, 1836), R. bellmanensis George, 1932, R. obtusa (Sowerby, 1820), R. profecta George, 1932. These species should all be included in Georgethyris if the divergent dental plates are considered to be a diagnostic character for the genus, regardless of the presence or absence of a septum. This was the view adopted by Brunton and Tilsley (1991) and Brunton and Mundy (1997: 229). The same species should be referred to Reticularia according to the view of Carter and Gourvennec (2006), who agreed with George (1932) in considering Reticularia as characterised by dental plates, regardless of their orientation, and a ventral median septum, whereas Georgethyris was defined as having “ventral interior seemingly lacking median ridge; otherwise similar to Reticularia” (Carter and Gourvennec 2006: 1848).
It is the authors’ opinion that species close in their morphology to Reticularia imbricata (R. bellmanensis, R. mesoloba, and R. profecta) should be referred to Reticularia, emended to include gently uniplicate shells with parallel to divergent dental plates and ventral median septum, while species closer to Georgethyris alexandri (R. acutiloba, R. lobata, and R. obtusa) should be referred to Georgethyris, emended to include transverse, strongly uniplicate to parasulcate shells with divergent dental plates and a variably developed to absent ventral median septum.
Reticularia cf. mesoloba (Phillips, 1836)
Fig. 16G.
Material.—One articulated specimen: MPUM12053 (RCK15-43). One fragment: MPUM11946 (RCC60-41). Both from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Small, ventribiconvex shell with suboval outline; cardinal margin straight, maximum width attained at shell mid-length. Anterior commissure rectimarginate. Rounded ventral sulcus developing from shell mid-length. Dorsal umbo straight; interarea orthocline. Both valves covered by subimbricated lamellae, numbering 40 in 5.0 mm length posteriorly, to 25 in 5.0 mm anteriorly. Dental plates divergent.
Remarks.—The specimens are close to Reticularia mesoloba, from the Mississippian of Bolland, Yorkshire, England (George 1932), based on its morphology, the presence of dental plates and dense lamellose ornamentation. George (1932) described the species as reaching 30.0 mm in length and with feeble fold-sulcus anteriorly; the specimens may then represent juveniles.
Genus Georgethyris Minato, 1953
Type species: Reticularia alexandri George, 1932, from the Brigantian, uppermost Visean, of Settle, Yorkshire, England.
Georgethyris lobata (Muir-Wood, 1926)
Fig. 16H.
1926 Reticularia lobata; Muir-Wood 1926: 242, pl. 16: 1a–c, 2.
1932 Reticularia lobata Muir-Wood, 1926; George 1932: 560.
1991 Georgethyris lobata (Muir-Wood, 1926); Brunton and Tilsley 1991: 293.
Material.—One articulated specimen: MPUM12086 (WI1-9) from Wensley Dale, Matlock, Derbyshire, UK, Eyam Limestone Formation, upper Brigantian, upper Visean.
Description.—Small, ventribiconvex shell with transversely sub-exagonal outline; cardinal margin straight, corresponding to half shell width; maximum width at shell mid-length. Anterior commissure paraplicate. Ventral umbo enrolled; interarea apsacline. Subangular sulcus starting 7.0 mm from the umbo, widening and deepening anteriorly, prolonged anteriorly as a subangular tongue; two rounded, low folds bordering the sulcus. Dorsal interarea apsacline, very low. Rounded dorsal fold starting 7.0 mm from the beak. Both valves covered by subimbricated lamellae, numbering 12 per 5.0 mm length. Dorsal adminicula and septum absent.
Remarks.—The specimen is assigned to Georgethyris lobata based on its paraplicate commissure, which distinguishes it from G. alexandri and G. obtusa, and the lower, broader fold with respect to G. acutiloba (George 1932: pl. 34: 2a–d). Moreover, G. acutiloba shows a more transverse outline. The specimen is smaller than the specimens of G. lobata recorded by Muir-Wood (1926) and George (1932).
Stratigraphic and geographic range.—Georgethyris lobata is recorded in the Chadian–Brigantian (Visean) of England (e.g., Mitchell 1971; Brunton and Tilsley 1991; this study).
Family Elythidae Fredericks, 1924
Subfamily Phricodothyridinae Carter, 1939
Genus Phricodothyris George, 1932
Type species: Phricodothyris lucerna George, 1932, from the Visean of Lowick, Northumberland, England.
Remarks.—The genus was erected by George (1932) and emended by Minato (1953) to include lamellose spiriferides with biramous spines showing no ventral adminicula nor median septa, whose absence allows to distinguish it from all other Mississippian Elythidae. Moreover, Phricodothyris differs from Nebenothyris Minato, 1953, from the Mississippian of Western Europe and Japan, in having a fainter, but denser concentric ornamentation.
Carter and Gourvennec (2006) described Phricodothyris as having no fold-sulcus: however, this is not always true, as Phricodothyris periculosa George, 1932, shows a shallow ventral sulcus.
Phricodothyris paricosta George, 1932
Fig. 16I–K.
1932 Phricodothyris paricosta; George 1932: 548, pl. 35: 4a–d.
Material.—Three ventral valves: MPUM12054 (RCC40-10a); MPUM12055 (RCK15-34b); MPUM12056 (RCC54-6). Three dorsal valves: MPUM12057 (RCK15-9); MPUM 12058 (RCK15-34a); MPUM12059 (RCC2-6a). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Small, ventribiconvex shell with transversely suboval outline. Cardinal margin straight; maximum width attained at shell mid-length. Anterior commissure rectimarginate. Ventral umbo straight to gently curved, interarea low, apsacline.
Ornamentation consists of regularly spaced (1.2–1.6 mm) subimbricated lamellae, each one bearing a single row of prominent, elongated spine bases. Spine bases on adjacent lamellae are aligned so that a ribbing-like pattern is developed. Spine bases number 6–8 per 5.0 mm width, 10.0 mm from umbo.
Ventral adminicula absent.
Remarks.—The specimens are included in Phricodothyris paricosta, from the Brigantian (uppermost Visean) of Northumberland, based on their rectimarginate commissure and ornamentation of widely spaced lamellae bearing coarse spine bases.
Stratigraphic and geographic range.—Phricodothyris paricosta is recorded in the Visean of Great Britain and Ireland (George 1932; Mitchell 1971).
Phricodothyris cf. periculosa George, 1932
Fig. 16L–N.
Material.—Six articulated specimens: MPUM12060 (RCC 73-30a); MPUM12061 (RCK15-3); MPUM12062 (RCC60-10, 56); MPUM12063 (WI1-8, 18). Four ventral valves: MPUM12064 (RCC2-37); MPUM12067 (RCC1-53a, 53b; RCC54-14). One fragment: MPUM12068 (RCC38A-1). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian (RCC-, RCK-), and Wensley Dale, Matlock, Derbyshire, UK, Eyam Limestone Formation, upper Brigantian, upper Visean (WI1).
Description.—Small, ventribiconvex to equally biconvex shell with suboval outline; cardinal margin straight; maximum width attained at shell mid-length. Anterior commissure rectimarginate to slightly uniplicate. Ventral interarea low, apsacline. Shallow sulcus develops at 10.0 mm from the umbo. Entire surface of both valves covered by lamellae, 10–12 per 5.0 mm length. Adminicula and median septa absent. Spiralia laterally directed.
Remarks.—The specimens are compared to Phricodothyris periculosa George, 1932, from the upper Visean–lower Serpukhovian of Yorkshire, England, which is characterised by shallow sulcus and an ornamentation of closely spaced lamellae bearing numerous fine spine bases. The specimens differ from P. paricosta from the same localities by the presence of a ventral sulcus developed from shell mid-length and by their denser lamellose ornamentation.
Phricodothyris sp.
Fig. 16O.
Material.—Three articulated specimens: MPUM12069 (RCC54-17b; RCC128-4c; RCK15B-4). Five ventral valves: MPUM12070 (RCC3-1); MPUM12071 (RCC60-4a); MPUM 12072 (RCC1-49; RCC49-35; RCK15-70). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Remarks.—The specimens are rectimarginate abraded Elythidae lacking ventral adminicula. Because their original lamellose ornamentation is not preserved, they are assigned to an undetermined species of Phricodothyris.
Genus Nebenothyris Minato, 1953
Type species: Nebenothyris lineatus Minato, 1953, from the upper Visean of the Hagen area in north-western Rheinische Schiefergebirge, Germany.
Nebenothyris sp.
Fig. 16P.
Material.—One ventral valve: MPUM12073 (WI1-26a) from Wensley Dale, Matlock, Derbyshire, UK, Eyam Limestone Formation, upper Brigantian, upper Visean.
Description.—Medium-sized, convex ventral valve with suboval outline; cardinal margin straight; maximum width attained at shell mid-length. Anterior commissure slightly uniplicate. Ventral umbo curved, interarea apsacline. Shell covered by subimbricated lamellae, spaced 1.0 mm in average. Ventral adminicula absent. Prominent ventral median septum, reaching two-thirds valve length.
Remarks.—The specimen is assigned to an undetermined species of Nebenothyris based on the long ventral median septum, ornamentation of broadly spaced lamellae and absence of ventral adminicula. It is close to N. lineata in its morphology, though limited information available on the taxon prevents its identification.
The specimen differs from Nebenothyris rhachis Brunton and Mundy, 1997, from the upper Visean of Yorkshire and Ireland, and N. tripustulosa (Demanet, 1938), from the upper Visean of Belgium, in having a more transverse outline; moreover N. rhachis shows a weak ventral sulcus developing from the umbo (Brunton and Mundy 1997: figs. 1.7, 1.13).
Order Spiriferinida Ivanova, 1972
Suborder Spiriferinidina Ivanova, 1972
Superfamily Pennospiriferinoidea Dagys, 1972
Family Punctospiriferidae Waterhouse, 1975
Genus Punctospirifer North, 1920
Type species: Punctospirifer scabricosta North, 1920, from the Visean of England.
Remarks.—Punctospirifer differs from the allied genus Spiriferellina Fredericks, 1924, of the Spiriferellinidae Ivanova, 1972, in having a lower ventral interarea, an angular ventral sulcus without coarse sulcus-bounding costae and a capillate, rather than pustulose, micro-ornamentation. European Carboniferous species assigned to Spiriferellina (e.g., S. insculpta Phillips, 1836, S. perplicata North, 1920) because of their pustulose micro-ornamentation, are characterised by an angular sulcus with sulcus-bounding costae not exceeding the width of lateral costae.
?Punctospirifer sp.
Fig. 16Q.
Material.—One ventral valve: MPUM12074 (RCC73-23c) from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Medium-sized, transverse shell. Cardinal margin corresponding to the maximum width. Cardinal extremities mucronate. Anterior commissure uniplicate. Shell substance punctate. Deep angular sulcus starting from umbo, widening and deepening anteriorly; 8.0 mm wide at anterior commissure.
Sulcus smooth. Flanks covered by coarse, simple, angular costae with narrow, subrounded interspaces, extending from the umbo, becoming lower and indistinct laterally. Six to seven costae on each flank.
Remarks.—The specimen is tentatively assigned to Punctospirifer based on its deep subangular sulcus and the absence of coarse sulcus-bounding costae. However, as the micro-ornamentation is not preserved, it is not possible to confirm the assignment.
Order Terebratulida Waagen, 1883
Suborder Terebratulidina Waagen, 1883
Superfamily Cryptonelloidea Thomson, 1926
Family Cranaenidae Cloud, 1942
Subfamily Girtyellinae Stehli, 1965
Genus Harttella Bell, 1929
Type species: Harttella parva Bell, 1929, from the Lower Windsor Group, Nova Scotia, Canada (upper Visean; Brunton 1982).
Remarks.—The genus was established by Bell (1929) to include terebratulides from Nova Scotia (Canada) lacking dental plates and having a high Y-shaped septalium. The absence of dental plates distinguishes Harttella from Girtyella Weller, 1911.
Harttella oakleyi Brunton, 1982
Figs. 15D–E, 17A–K.
1824 Terebratula saccula; Sowerby 1824: 65 (pars), pl. 446: 1 top left.
1858 Terebratula sacculus Sowerby, 1824; Davidson 1858: 14 (pars), pl. 1: 27, 29, ?30; pl. 54: 5.
1982 Harttella oakleyi; Brunton 1982: 50, figs. 5–7.
Material.—Sixty-five articulated specimens: MPUM12075 (RCC10-15b); MPUM12076 (RCC41-9); MPUM12077 (RCC41-22); MPUM12078 (RCC49-9); MPUM12079 (RCC49-12); MPUM12080 (RCC60-19b); MPUM12081 (RCC65G-2); MPUM12082 (RCC65G-8a); MPUM12083 (RCC65G-11); MPUM12084 (RCC65G-14); MPUM12085 (RCK15-14); MPUM12088 (RCC1-8a; RCC8-12; RCC9-1; RCC10-7a, 15c, 33, 37; RCC11-9b; RCC12-20; RCC32-5b, 28d, 36; RCC32B-1; RCC38A-4; RCC41-18c, 18d, 19b, 20b; RCC42-2, 9, 24; RCC45-4a, 5; RCC48-14, 31; RCC49-20, 34; RCC60-43; RCC65G-1, 5, 10, 13, 21, 22, 23; RCC65T-6; RCC67-1, 3; RCC73-23d, 23e, 29b; RCC85-1c, 3; RCC110-3f; RCC123-3; RCC128-8, 22; RCC300-2; RCK15-6, 27, 39a; RCK15A-1; RCK15B-2; RCK17-1). Twelve ventral valves: MPUM12089 (RCC1-19b, 38; RCC10-18a, 38a; RCC11-18; RCC41-20c; RCC42-27a; RCC49-33, 39; RCC128-6; RCC300-15; RCK15-20). Four dorsal valves: MPUM12090 (RCC10-7b, 29; RCC32-25; RCC41-21). One fragment: MPUM12091 (RCC12-10a). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Small, ventribiconvex shell with subpentagonal elongated outline; cardinal margin curved, maximum width at shell mid-length. Anterior commissure uniplicate to weakly lobate. Shell substance punctate. Ventral umbo strongly curved; palintrope anacline. Foramen permesothyrid. Shallow, broadly rounded sulcus starting at two-thirds shell length. Dorsal palintrope anacline. Dorsal sulcus absent to weakly developed near the commissure, rarely starting at shell mid-length.
Shell smooth, with regularly concentric growth lines. Sporadic lamellae developed.
Probable presence of a pedicle collar. Dental plates absent. Dorsal valve with convex hinge plates fusing medially to form a high Y-shaped septalium.
There is variability in the shape of the ventral sulcus: most specimens have a very broad rounded sulcus, whereas a few others show a narrower one, starting from shell mid-length. There is also large variability in convexity of both valves. Juvenile shells are almost as long as wide, with a distinct subpentagonal outline and rectimarginate commissure. When a length of 6.0–9.0 mm is attained, the ventral sulcus begins to develop.
Remarks.—The specimens are close both in their morphology and internal characters to the specimens of Harttella oakleyi figured by Brunton (1982). Brunton described the species as having a ventral sulcus starting posteriorly to shell mid-length and becoming narrow anteriorly, resulting in a sharply uniplicate commissure (Brunton 1982: 50). Most of the specimens from Ricklow Quarry commonly show a shallow sulcus starting anteriorly to shell mid-length, but there is a large morphological variability and some even show a narrow sulcus starting near umbo. The specimens are thus included in H. oakleyi and the species concept extended to include specimens with a shallow rounded ventral sulcus restricted anteriorly.
Externally, H. oakleyi resembles Balanoconcha saccula (Sowerby, 1824). None of the specimens from Ricklow Quarry could be associated to B. saccula, which is usually larger and reaches its maximum width at two-thirds shell length, resulting in a subtriangular, rather than subpentagonal, outline.
According to Brunton and Tilsley (1991) the specimens named Girtyella saccula (= Balanoconcha saccula) recorded by Gutteridge (1990) from the mound complex in Ricklow, are in fact H. oakleyi specimens. This is likely also the case for some of the specimens assigned by Mitchell (1971) to Dielasma hastata (Sowerby, 1824), in his study of Visean faunas from the study area.
Stratigraphic and geographic range.—Harttella oakleyi is recorded from the Brigantian (uppermost Visean) of Derbyshire, England (Brunton 1982; this study).
Superfamily Dielasmatoidea Schuchert, 1913
Family Beecheriidae Smirnova, 2004
Genus Beecheria Hall and Clarke, 1893
Type species: Beecheria davidsoni Hall and Clarke, 1893, from the upper Visean Windsor Group of Nova Scotia, Canada.
Remarks.—The genus was established by Hall and Clarke (1893) and then emended by Stehli (1956) to include large terebratulides with dental plates and inner hinge plates converging on a platform separated from the dorsal valve floor by a septum. Beecheria was recorded for the first time in Europe by Brunton (1982, 1984), who assigned to it Terebratula hastata Sowerby, 1824, from the Arundian (lower Visean) of Great Britain and Ireland, and established the new species Beecheria treakensis Brunton, 1984, from British Asbian rocks, upper Visean.
Another British species of Beecheria may be Terebratula gillingensis Davidson, 1857, from the Brigantian (uppermost Visean) of West Gilling, North Yorkshire. From the figures given by Davidson (1858) it is possible to see diverging outer hinge plates and a median septum.
Beecheria sp.
Fig. 17P.
Material.—Three articulated specimens: MPUM12092 (RCC49-31); MPUM12093 (RCC41-14; RCK16-3) from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Medium-sized shell, ventribiconvex posteriorly, dorsibiconvex anteriorly. Subpentagonal outline; cardinal margin curved, maximum width at shell mid-length. Anterior commissure uniplicate. Ventral valve flat posteriorly, convex anteriorly. Umbo curved; palintrope anacline. Shallow, rounded sulcus starting at two-thirds shell length. Dorsal valve gently convex posteriorly, flat anteriorly. Umbo straight; palintrope anacline.
Shell smooth. Lamellae occasionally developed, with a spacing of 1.0–2.0 mm.
Dental plates diverging anteriorly forming an angle of 60°, 3.0 mm long. Dorsal valve with divergent outer hinge plates, 1.5 mm long, forming an angle of 90°.
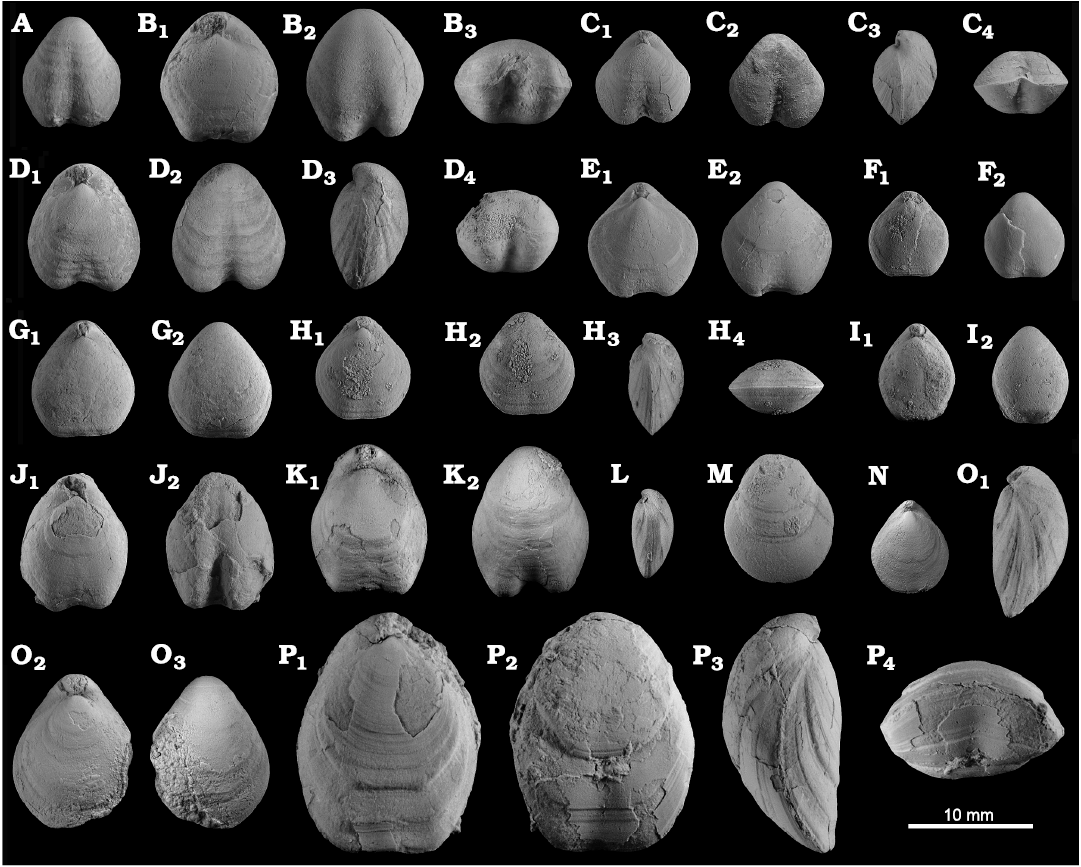
Fig. 17. Terebratulida from the Brigantian (uppermost Visean) of Ricklow Quarry, near Monyash, Derbyshire, UK (localities RCC-, RCK-; Fig. 3). A–K. Harttella oakleyi Brunton, 1982. A. MPUM12075 (RCC10-15b) in ventral view. B. MPUM12076 (RCC41-9) in dorsal (B1), ventral (B2), and anterior (B3) views. C. MPUM12077 (RCC41-22) in dorsal (C1), ventral (C2), lateral (C3), and anterior (C4) views. D. MPUM12078 (RCC49-9) in dorsal (D1), ventral (D2), lateral (D3), and anterior (D4) views. E. MPUM12080 (RCC60-19b) in dorsal (E1) and ventral (E2) views. F. MPUM12079 (RCC49-12) in dorsal (F1), and ventral (F2) views. G. MPUM12081 (RCC65G-2) in dorsal (G1) and ventral (G2) views. H. MPUM12082 (RCC65G-8a) ) in dorsal (H1), ventral (H2), lateral (H3), and anterior (H4) views. I. MPUM12083 (RCC65G-11) in dorsal (I1) and ventral (I2) views. J. MPUM12084 (RCC65G-14) in dorsal (J1) and ventral (J2) views. K. MPUM12085 (RCK15-14) in dorsal (K1) and ventral (K2) views. L–O. ?Balanoconcha sp. L. MPUM12094 (RCC10-15a) in lateral view. M. MPUM12095 (RCC300-1) in ventral view. N. MPUM12097 (RCK15-26) in dorsal view. O. MPUM12096 (RCK15-18) in lateral (O1), dorsal (O2), and ventral (O3) views. P. Beecheria sp., MPUM12092 (RCC49-31) in dorsal (P1), ventral (P2), lateral (P3), and anterior (P4) views.
Remarks.—The specimens are transitional in their morphology between the Asbian species Beecheria treakensis and the Brigantian Beecheria gillingensis. From the first species they differ in having a subpentagonal outline, an anteriorly flat dorsal valve and ventral sulcus, while from the latter they differ in having an elongated outline and a more convex, non-resupinate dorsal valve.
Family Gillediidae Campbell, 1965
Subfamily Gillediinae Campbell, 1965
Genus Balanoconcha Campbell, 1957
Type species: Balanoconcha elliptica Campbell, 1957, from the Watts District of New South Wales, Australia (middle–upper Visean).
Remarks.—The genus was recognised in Europe for the first time in England by Brunton (1982), who assigned to it Terebratula saccula from the Visean of Great Britain and Ireland on the basis of its internal characters.
?Balanoconcha sp.
Figs. 15C, 17L–O.
Material.—Fourteen articulated specimens: MPUM12094 (RCC10-15a); MPUM12095 (RCC300-1); MPUM12096 (RCK15-18); MPUM12097 (RCK15-26); MPUM12098 (RCC10-20; RCC39-2; RCC43-3, 4, 8b; RCC60-50b; RCC65G-3, 20; RCC66-2; RCC67-2). Two ventral valves: MPUM12099 (RCC10-10, 28). All from mud mound complex of Ricklow Quarry, Monyash, Derbyshire, UK, Monsal Dale Limestone Formation, Brigantian, upper Visean.
Description.—Small, elongated ventribiconvex shell with ovatotriangular outline; cardinal margin curved, maximum width at shell mid-length. Anterior commissure rectimarginate. Ventral umbo curved; palintrope anacline. Foramen permesothyrid. Dorsal palintrope anacline.
Shell smooth, with regularly concentric growth lines. Lamellae occasionally developed.
Dental plates and septalium absent. Crural bases diverging from umbo, extending to one-quarter shell length.
Remarks.—The specimens differ from Harttella oakleyi from the mound complex in Ricklow Quarry for their more elongated oval outline, less convex valves, and less recurved ventral umbo. Moreover, adult specimens completely lack a ventral or dorsal sulcus. The specimens are distinguishable from young specimens of Beecheria sp. from the same localities in having an elongate, suboval outline. They can be identified as species of Balanoconcha for their lack of both dental plates and septalium. However, no evidence of sessile hinge plates forming a V-shaped structure was found, so it is not possible to confirm the generic attribution.
Only one species assigned to Balanoconcha is known from the Visean of England: Terebratula saccula, assigned to Girtyella by Muir-Wood (1951) and later assigned to Balanoconcha by Brunton (1982, 1984) on the basis of its internal characters. The specimens here differ from B. saccula in being smaller, in lacking a ventral sulcus and in being less strongly biconvex.
Acknowledgements
We thank Giovanni Chiodi, Stefania Crespi, Ruben Marchesi, and Matteo Pegoraro (all University of Milan, Italy) for photographing the fossil specimens. We also thank Curzio Malinverno (University of Milan, Italy) for preparing thin sections for petrographic facies analyses and the British Geological Survey staff for hosting APC in their facilities and for sample packing to Italy. Finally, we wish to thank Natural England for study permits for the Ricklow Quarry site, part of the Lathkill Dale SSSI. The constructive comments of David Harper (Durham University, England) and an anonymous reviewer greatly helped to improve a first version of the manuscript.
Authors’ contributions
The study is based on fieldwork by APC, with the contribution of all co-authors. Study of the facies was carried out by APC and GDP, the systematic study of brachiopods by APC and LA. Palaeoecological interpretations were developed from discussions between APC, LA, and GDP. The manuscript was prepared by APC with contributions from all co-authors.
References
Adams, A.E. 1980. Calcrete profiles in the Eyam Limestone (Carboniferous) of Derbyshire: petrology and regional significance. Sedimentology 27: 651‒660. Crossref
Aitkenhead, N. and Chisholm, J.I. 1982. A standard nomenclature for the Dinantian formations of the Peak District of Derbyshire and Staffordshire. Institute of Geological Sciences Report 82/8: 1‒18.
Aitkenhead, N., Barclay, W.J., Brandon, A., Chadwick, R.A.J, Chisholm, I., Cooper, A.H., and Johnson, E.W. 2002. British Regional Geology: The Pennines and Adjacent Areas. Fourth edition. x + 206 pp. HMSO, London.
Aitkenhead, N., Chisholm, J.I., Stevenson, I.P., Mitchell, M., Strank, A.R.E, Cornwell, J.D., and Berridge, N.G. 1985. Geology of the Country Around Buxton, Leek and Bakewell: Memoir for 1:50.000 Geological Sheet 111 (England and Wales). 168 pp. HMSO, London.
Angiolini, L. 2007. Quantitative palaeoecology in the Pachycyrtella bed, Early Permian of interior Oman. Paleoword 16: 233‒245. Crossref
Angiolini, L., Cisterna, G.A., Mottequin, B., Shen, S., and Muttoni, G. 2021. Global Carboniferous brachiopod biostratigraphy. In: S. Lucas, J.W. Schneider, X. Wang, and S. Nikolaeva (eds.), The Carboniferous Timescale. Geological Society Special Publications 512: 497‒550. Crossref
Angiolini, L., Crippa, G., Azmy, K., Capitani, G., Confalonieri, G., Della Porta, G., Griesshaber, E., Harper, D.A.T., Leng, M., Nolan, L., Orlandi, M., Posenato, R., Schmahl, W.W, Banks, V., and Stephenson, M.H. 2019. The giants of the Phylum Brachiopoda: a matter of diet? Palaeontology 62: 889‒917. Crossref
Angiolini, L., Long, S., and Davies, L. 2011. Revision of Sowerby’s species Spirifer bisulcatus, Spirifer pinguis and Spirifer rotundatus from the late Tournaisian–Visean of Great Britain. Memoirs of the Association of Australasian Palaeontologists 41: 71‒85.
Angiolini, L., Stephenson, M.H., Leng, M.J., Jadoul, F., Millward, D., Aldridge, A., Andrews, J., Chenery, S., and Williams, G. 2012. Heterogenity, cyclicity and diagenesis in a Mississippian brachiopod shell of palaeoequatorial Britain. Terra Nova 24: 16‒26. Crossref
Ausich, W.I. and Bottjer, D.J. 1982. Tiering in suspension feeding communities on soft substrata throughout the Phanerozoic. Science 216: 173‒174. Crossref
Bahrammanesh, M., Angiolini, L., Antonelli, A.A., Aghababalou, B., and Gaetani, M. 2011. Tournaisian (Mississippian) brachiopods from the Mobarak Formation, North Iran. Geoarabia 16: 129‒192. Crossref
Bassett, M.G. and Bryant, C. 2006. A Tournaisian brachiopod fauna from South‒East Wales. Palaeontology 49: 485‒535. Crossref
Bell, W.A. 1929. Horton-Windsor District, Nova Scotia. Memoir of the Geological Survey of Canada 155: 1‒36. Crossref
Berkhli, M., Vachard, D., and Paicheler, J.L. 2001. Les series du Carbonifère inférieur de la région d’Adarouch, NE du Maroc central: lithologie et biostratigraphie. Journal of African Earth Sciences 32: 557‒571. Crossref
Brand, P.J. 2011. The Serpukhovian and Bashkirian (Carboniferous, Namurian and basal Westphalian) faunas of northern England. Proceedings of the Yorkshire Geological Society 58: 143‒165. Crossref
Brenchley, P.J. and Harper, D. 1998. Palaeoecology: Ecosystems, Environments, and Evolution. 402 pp. Chapman and Hall, London.
Bridges, P.H. and Chapman, A.J. 1988. The anatomy of a deep water mud-mound complex to the southwest of the Dinantian platform in Derbyshire, UK. Sedimentology 35: 139–162. Crossref
Bridges, P.H., Gutteridge, P., and Pickard, N.A.H. 1995. The environmental setting of Early Carboniferous mud-mounds. In: C.L. Monty, D.W.J. Bosence, P.H. Bridges, and B. Pratt (eds.), Carbonate Mud Mounds; Their Origin and Evolution. International Association of Sedimentologists Special Publication 23: 171‒190. Crossref
British Geological Survey 1978. Buxton. England and Wales Sheet 111. Bedrock and Superficial Deposits. 1:50.000. British Geological Survey, Keyworth, Nottingham.
British Geological Survey, 2012. Chesterfield. England and Wales Sheet 112. Bedrock and Superficial Deposits. 1:50.000. British Geological Survey, Keyworth, Nottingham.
Brunton, C.H.C. 1966. Silicified productoids from the Visean of County Fermanagh. Bulletin of the British Natural Museum (Natural History) ‒ Geology 12: 175‒243.
Brunton, C.H.C. 1968. Silicified brachiopods from the Visean of County Fermanagh (II). Bulletin of the British Natural Museum (Natural History) ‒ Geology 16: 3‒70.
Brunton, C.H.C. 1982. British Dinantian (Lower Carboniferous) Terebratulid brachiopods. Bulletin of the British Museum (Natural History) ‒ Geology 36: 45‒57.
Brunton, C.H.C. 1984. Silicified brachiopods from the Viséan of County Fermanagh, Ireland (III). Rhynchonellids, Spiriferids and Terebratulids. Bulletin of the British Natural Museum (Natural History) ‒ Geology 38: 28‒130.
Brunton, C.H.C. 1985. Growth and shell shape in productacean brachiopods. Bulletin of the British Natural Museum (Natural History) ‒ Geology 38: 175‒243.
Brunton, C.H.C. 1987. The Palaeoecology of brachiopods, and other faunas, of Lower Carboniferous (Asbian) limestones in west Fermanagh. Irish Journal of Earth Sciences 8: 97‒112.
Brunton, C.H.C. and Champion, C. 1974. A lower Carboniferous brachiopod fauna from the Manifold Valley, Staffordshire. Palaeontology 17: 811‒840.
Brunton, C.H.C. and Lazarev, S.S. 1997. Evolution and classification of the Productellidae (Productida), upper Paleozoic brachiopods. Journal of Palaeontology 7: 381‒394. Crossref
Brunton, C.H.C. and Mundy, D.C.J. 1986. Some Dinantian chonopectinid productaceans (Brachiopoda) from the British Isles. Proceedings of the Yorkshire Geological Society 46: 1‒10. Crossref
Brunton, C.H.C. and Mundy, D.C.J. 1988. Strophalosiacean and aulostegacean productoids (Brachiopoda) from the Craven Reef Belt (late Visean) of North Yorkshire. Proceedings of the Yorkshire Geological Society 47: 55‒58. Crossref
Brunton, C.H.C. and Mundy, D.C.J. 1997. A new elythid spiriferidae brachiopod from the Lower Carboniferous (Asbian) reef facies of the British Isles. Proceedings of the Yorkshire Geological Society 51: 229‒234. Crossref
Brunton, C.H.C. and Rissoné, A. 1976. Fusella M’Coy 1844, a problematic brachiopod genus from the Lower Carboniferous. Bulletin of the British Natural Museum (Natural History) ‒ Geology 27: 275‒284. Crossref
Brunton, C.H.C. and Tilsley J.W. 1991. A check list of brachiopods from Treak Cliff, Derbyshire, with reference to other Dinantian (Lower Carboniferous) localities. Proceedings of the Yorkshire Geological Society 48: 287‒295. Crossref
Brunton, C.H.C., Lazarev, S.S., Grant, R.E., and Yu-Gan, J. 2000. Productidina. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology. Part H: Brachiopoda. Revised. Volume 3: Linguliformea, Craniiformea, and Rhynchonelliformea (part), 424‒609. The Geological Society of America, Boulder and University of Kansas, Lawrence. Crossref
Brunton, C.H.C., Mundy, D.J.C., and Lazarev, S.S. 1993. Productellid and Plicatiferid (Productoid) brachiopods from the Lower Carboniferous of the Craven Reef Belt, North Yorkshire. Bulletin of the Natural History Museum London (Natural Sciences) ‒ Geology 49: 99‒119. Crossref
Buckman, S.S. 1908. Brachiopod homeomorphy: Spirifer glaber. Quarterly Journal of the Geological Society of London 64: 27‒33. Crossref
Burchette, T.P. and Wright, V.P. 1992. Carbonate ramp depositional systems. Sedimentary Geology 79: 3‒57. Crossref
Butts, S. 2007. Silicified Carboniferous (Chesterian) Brachiopoda of the Arco Hills Formation, Idaho. Journal of Paleontology 81: 48‒63. Crossref
Carter, J.L. and Gourvennec, R. 2006. Reticularioidea. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology. Part H: Brachiopoda. Revised. Volume 5: Rhynchonelliformea (part), 1848‒1870. The Geological Society of America, Boulder and University of Kansas, Lawrence. Crossref
Carter, J.L. and Johnson, J.G. 2006. Spiriferinida. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology. Part H: Brachiopoda. Revised. Volume 5: Rhynchonelliformea (part), 1877‒1937. The Geological Society of America, Boulder and University of Kansas, Lawrence.
Carter, J.L, Johnson, J.G., Gourvennec, R., and Hong-Fei, H. 2006. Spiriferida. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology. Part H: Brachiopoda. Revised. Volume 5: Rhynchonelliformea (part), 1689‒1876. The Geological Society of America, Boulder and University of Kansas, Lawrence. Crossref
Chen, Z. 2004. Devonian‒Carboniferous brachiopod zonation in the Tarim Basin, northwest China: implications for biostratigraphy and biogeography. Geological Journal 39: 431‒458. Crossref
Coleman, P.J. 1957. Permian productacea of Western Australia. Bureau of Mineral Resources Geology and Geophysics 40: 1‒189.
Copper, P. 1992. Paleoscene 14. Organisms and carbonate substrates in marine environments. Geoscience Canada 19: 97‒112.
Cox, F.C., Bridge, D.McC., Chisholm, J.I., and Aitkenhead, N. 1977. The limestone and dolomite resources of the country around Monyash, Derbyshire. Description of 1:25 000 resource sheet SK 16. Institute of Geological Sciences Mineral Assessment Report 26: 1‒138.
Davidson, T. (1857‒1862a) 1858‒1863a. Monograph of British Fossil Brachiopoda. Vol. II, Part. V: The Carboniferous Brachiopoda. Monograph of the Palaeontographical Society 10‒14 (42, 46, 54, 55, 59): 81‒280. Crossref
Davidson, T. 1860b‒1862b. Monographie of Scottish Carboniferous Brachiopoda. 76 pp. Geologists Office, London.
Davidson, T. 1880. Monograph of British Fossil Brachiopoda. Vol. IV, Part III: Supplement to the Permian and Carboniferous species. Monograph of the Palaeontographical Society 34: 243‒316. Crossref
Dean, M.T. 2017. An Upper Paleozoic palaeontological and biostratigraphical summary of Scotland Sheet 23E (Lanark). British Geological Survey Internal Report IR/01/17: 1–55.
Dedok, T.A. and Hollard, H. 1980. Brachiopodes du Carbonifère inférieur du Maroc central. Notes du Service géologique du Maroc 41: 185‒230.
Dedok, T.A. and Tschernjak, G.E. 1960. Lower Carboniferous brachiopods of the Taymir Peninsula [in Russian]. Trudy NIIGA 111: 52‒72.
Defrance, M.J.L. 1826. Art. Productus. Dictionnaire des Sciences Naturelles 43: 349–355.
De Koninck, L.G. 1842‒1844. Description des animaux fossils qui se trouvent dans le terrain Carbonifère de Belgique. 650 pp. H. Dessain, Liege.
De Koninck, L.G. 1847. Monographie du genre Productus. 278 pp. H. Dessain, Liege.
Demanet, F. (1921) 1923. Le Waulsortien de Sosoye et ses rapports fauniques avec le Waulsortien d’age tournaisien supérieur. Mémoire de l’Institute Géologique de Louvain 2: 392‒85.
Demanet, F. 1934. Les brachiopodes du Dinantien de la Belgique, Premier volume: Atremata, Neotremata, Protremata (Pars). Mémoires du Musée Royal d’Histoire Naturelle de Belgique 61: 1‒116.
Denayer, J., Aretz, M., Poty, E., and Mottequin, B. 2016. Royseaux: a palaeobiodiversity hotspot in the late Viséan (Carboniferous) of Belgium. Geologica Belgica 19: 7‒20. Crossref
El-Shazly, S.H. 2011. Late Carboniferous macrofauna from Wadi Araba, Eastern Desert, Egypt, and their paleoecological implications. Journal of African Earth Sciences 61: 309‒394. Crossref
Ferguson, J. 1966. Variation in two species of the Carboniferous brachiopod Pleuropugnoides. Proceedings of the Yorkshire Geological Society 35: 353‒374. Crossref
Ferguson, J. 1971. Linoprotonia, a new genus of Lower Carboniferous productoid. Proceedings of the Yorkshire Geological Society 38: 549‒564. Crossref
Fleming, J. 1828. A History of British Animals. xxiii + 265 pp. Bell and Bredfute, Edinburgh.
Flügel, E. 2004. Microfacies of Carbonate Rocks. xx + 976 pp. Springer-Verlag Press, Berlin. Crossref
Frech, F. and Arthaber, G.V. 1900. Über das Paläozoicum in Hocharmenien und Persien . Mit einem Anhang über die Kreide von Sirab in Persien. Beiträge zur Paläontologie und Geologie von Österreich-Ungarn und des Orients: Mitteilungen des Geologischen und Paläontologischen Institutes der Universität Wien 12: 161‒308.
Gawthorpe, R.L. and Gutteridge, P. 1990. Geometry and evolution of platform-margin bioclastic shoals, late Dinantian (Mississippian), Derbyshire, UK. Special Publications of the International Association of Sedimentologists 9: 39‒54. Crossref
George, T.N.G. 1931. Ambocoelia Hall and certain similar British spiriferidae. Quarterly Journal of the Geological Society 87: 30‒61. Crossref
George, T.N.G. 1932. The British Carboniferous reticulate spiriferidae. Quarterly Journal of the Geological Society 88: 516‒574. Crossref
Gmelin, J.F. 1791. Vermes. In: J.F. Gmelin (ed.), Caroli a Linnaei Systema Naturae per Regna Tria Naturae, Ed. 13. Tome 1 (6), 3021‒3910. G.E. Beer, Lipsiae.
Grant, R.E. 1965. The brachiopod superfamily Stenoscismatacea. Smithsonian Miscellanea Collections 148: 1‒192.
Grant, R.E. 1980. Koskinoid perforations in brachiopod shells: function and mode of formation. Lethaia 13: 313‒319. Crossref
Guion, P. and Fielding, C. 1988. Westphalian A and B sedimentation. In: B.M. Besly, and G. Kelling (eds.), Sedimentation in a Synorogenic Basin Complex; the Upper Carboniferous of NW Europe, 153–177. Blackie, Glasgow.
Gutteridge, P. 1984. Sedimentation of the Eyam Limestone Formation (Derbyshire). In: European Dinantian Environments 1st Meeting 1984. Abstracts, 128‒130. Department of Earth Sciences, Open University.
Gutteridge, P. 1987. Dinantian sedimentation and the basement structure of the Derbyshire Dome. Geological Journal 22: 25‒41. Crossref
Gutteridge, P. 1989. Controls on carbonate sedimentation in a Brigantian intrashelf basin (Derbyshire). In: R.S. Arthurton, P. Gutteridge, and S.C. Nolan (eds.), The role of tectonics and Carboniferous sedimentation in the British Isles. Proceedings of the Yorkshire Geolological Society Occasional Publications 6: 171–187.
Gutteridge, P. 1990. The origin and significance of the distribution of shelly macrofauna in late Dinantian carbonate mud mounds of Derbyshire. Proceedings of the Yorkshire Geological Society 48: 23‒32. Crossref
Gutteridge, P. 1991. Revision of the Monsal Dale / Eyam Limestone boundary (Dinantian) in Derbyshire. Mercian Geologist 12: 71‒78.
Gutteridge, P. 1995. Late Dinantian (Brigantian) carbonate mud mounds of the Derbyshire carbonate platform. In: C.L. Monty, D.W.J. Bosence, P.H. Bridges, and B. Pratt (eds.), Carbonate mud mounds; their origin and evolution. International Association of Sedimentologists Special Publication 23: 298‒307.
Hall, J. and Clarke, J.M. 1893. An introduction to the study of the genera of Palaeozoic Brachiopoda. In: J. Hall, G.B. Simpson, and J.M. Clarke (eds.), Palaeontology of New York, Vol. 8, Part 2, 1–317. Charles van Benthuysen and Sons, Albany.
Hammer, V., Harper, D.A.T., and Ryan, P.D. 2001. PAST: paleontological statistics software package for education and data analysis. Paleontologia Electronica 4: 1‒9.
Harper, D.A.T. 2000. Dalmanellidina. In: R.L. Kaesler, (ed.), Treatise on Invertebrate Paleontology. Part H: Brachiopoda. Revised. Volume 3: Linguliformea, Craniiformea, and Rhynchonelliformea (part), 782‒844. The Geological Society of America, Boulder and University of Kansas, Lawrence. Crossref
Harper, D.A.T. and Jeffrey, A.L. 1996. Mid-Dinantian brachiopod biofacies from western Ireland. In: P. Somerville, and G.L. Jones (eds.), Recent advances in Lower Carboniferous Geology. Geological Society Special Publication 107: 427‒436. Crossref
Harwood, M. 2005. Facies Architecture and Depositional Geometry of a Late Viséan Carbonate Platform Margin, Derbyshire, UK. 295 pp. PhD thesis, Cardiff University, Cardiff.
Haszeldine, R.S. 1984. Carboniferous North Atlantic palaeogeography: stratigraphic evidence for rifting, not megashear or subduction. Geological Magazine 121: 443‒463. Crossref
Haszeldine, R.S. 1989. Evidence against crustal stretching, north‒south tension and Hercynian collision, forming the British Carboniferous basins. In: R.S. Arthurton, P. Gutteridge, and S.C. Nolan (eds.), The role of tectonics and Carboniferous sedimentation in the British Isles. Yorkshire Geological Society Occasional Publication 6: 25‒33.
Ibaraki, Y., Miyake, Y., and Tazawa, J. 2014. Early Carboniferous (late Visean) brachiopods from the Koyama Limestone of Kamiotake in the Oga area, Okayama Prefecture, southwest Japan. Earth Science (Chikyu Kagaku) 68: 69‒79.
Jirasek, J., Hylova, L., Wlosok, J., and Sivek, M. 2012. Outcrops of the Carboniferous at Hladovy in Ostrava-Hostalkovice (Silesia, Czech Republic). Acta Museum Moroviae, Scientiarum Geologicarum 2: 85‒94.
Johnson, J.G., Carter, J.L., and Hong-fei, H. 2006. Ambocoelioidea. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology. Part H: Brachiopoda. Revised. Volume 5: Rhynchonelliformea (part), 1733‒1746. The Geological Society of America, Boulder and University of Kansas, Lawrence.
Kora, M. 1995. Carboniferous macrofauna from Sinai, Egypt: biostratigraphy and palaeogeography. Journal of African Earth Sciences 20: 37‒51. Crossref
Korejwo, K. and Teller, L. 1968. Stratygrafia Karbonu zachodniej czesci niecki lubelskiej. Acta Geologica Polonica 18: 153‒177.
Lazarev, S.S. 1976. Morphology and evolution of brachiopods (of the superfamily Enteletacea) [in Russian]. Akademiia Nauk SSSR, Paleontologičeskij Institut, Trudy 154: 1‒167.
Lee, D.E., Mackinnon, D.I., Smirnova, T.N., Baker, P.G., Yu-Gan, J., and Dong-li, S. 2006. Terebratulida. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology. Part H: Brachiopoda. Revised. Volume 5: Rhynchonelliformea (part), 1965‒2250. The Geological Society of America, Boulder and University of Kansas, Lawrence.
Lees, A. and Miller, J. 1995. Waulsortian banks. In: C.L. Monty, D.W.J. Bosence, P.H. Bridges, and B. Pratt (eds.), Carbonate Mud Mounds; Their Origin and Evolution. International Association of Sedimentologists Special Publication 23: 19‒271.
Legrand-Blain, M. 1986. Spiriferacea (Brachiopoda) Viséens et Serpukhoviens du Sahara algérien. Biostratigraphie du Paléozoique 5 (1): 1‒85.
Leighton, L.R., Savarese, M., Copper, P., and Jin, J. 1996. Functional and taphonomic implications of Ordovician strophomenid brachiopod valve morphology. In: P. Copper and J. Jin (eds.), Proceedings of the 3rd International Brachiopod Congress, Brachiopods, 161‒168. Balkema, Rotterdam. Crossref
M’Coy, F. 1844. A Synopsis of the Characters of the Carboniferous Limestone Fossils of Ireland. 207 pp. University Press, Dublin. Crossref
Martin, W. 1809. Petrefacta derbiensa; Figures and Descriptions of Petrefactions Collected in Derbyshire. ix + 28 pp. Wigan, London. Crossref
Martinez-Chacon, M.L. 1979. Braquiopodos carboniferos de la Cordillera Cantabrica (Orthida, Strophomenida y Rhynchonellida). Memoria del Instituto geológico y minero de Espaňa 96: 1‒285.
Martinez-Chacon, M.L. 2010. Adaptations to hard substrates in Pennsylvanian productides (Brachiopoda) from the Cantabrian mountains (North-west Spain). Special Papers in Palaeontology 84: 225‒241.
Martinez-Chacon, M.L. and Legrand-Blain, M. 1992. Braquiopodos. Coloquios de Paleontologia 44: 91‒144.
Massa, D., Termier, G., and Termier, H. 1974. Le Carbonifère de Libye occidentale. stratigraphie et paléontologie. Compagnie Francaise des Pétroles, Notes et Memoires 11: 139‒206.
McIntosh, M.J. 1974. Some Scottish Carboniferous davidsoniacean brachiopods. Scottish Journal of Geology 10: 199‒222. Crossref
Minato, M. 1953. On some reticulate spiriferidae. Transactions and Proceedings of the Palaeontological Society of Japan 11: 65‒73.
Mitchell, M. 1971. Stratigraphical palaeontology of the Carboniferous limestone series. In: I.P. Stevenson and G.D. Gaunt 1971. Geology of the Country Around Chapel-en-Frith. Memoir of the British Geological Survey. Sheet 99, 128‒154. Her Majesty’s Stationery Office, London.
Mottequin, B. 2010. Mississippian (Tournaisian) brachiopods from the Hook Head Formation, County Wexford (south-east Ireland). Special Papers in Palaeontology 84: 243‒285.
Mottequin, B. and Simon, E. 2017. New insights on Tournaisian‒Visean (Carboniferous, Mississippian) athyrididae, orthotetide, rhynchonellide and strophomenide brachiopods from southern Belgium. Palaeontologia Electronica 20 (2.28A): 1‒45. Crossref
Muir-Wood, H.M. 1926. Appendix: description of a new species of Spirifer and of one of Reticularia from the Lower Carboniferous near Clitheroe. Quarterly Journal of the Geological Society 82: 241‒249. Crossref
Muir-Wood, H.M. 1928. British Carboniferous Producti, 2. Productus sensu stricto semireticulatus and longispinus group. Memoir of the Geological Survey of U.K., Palaeontology 3: 3‒217.
Muir-Wood, H.M. 1930. The classification of the British Carboniferous brachiopod Subfamily Productinae. Annals and Magazine of Natural History 5: 100‒108. Crossref
Muir-Wood, H.M. 1951. The Brachiopoda of Martin’s Petrificata Derbiensia. Annals and Magazine of Natural History 4: 97‒118. Crossref
Muir-Wood, H.M. 1965. Productidina. In: R.L. Kaesler (ed.), Treatise on invertebrate paleontology, Part H: Brachiopoda, H439‒H510. The Geological Society of America, Boulder and University of Kansas, Lawrence.
Muir-Wood, H.M. and Cooper, G.A 1960. Morphology, classification and life history of the Productoidea. Memoir of the Geological Society of America 81: 1‒447. Crossref
Mundy, D.C.J. 1980. Aspects of the Palaeoecology of the Craven Reef Belt (Dinantian), of North Yorkshire. x+373 pp. Ph.D. Thesis, University of Manchester, Manchester.
Mundy, D.J.C. 1982. A note on the predation of brachiopods from the Dinantian reef limestones of Cracoe, North Yorkshire. Transactions of the Leeds Geological Association 9: 73‒83.
Nicolaus, H.J. 1963. Zur Stratigraphie und Fauna der crenistria‒Zoneim Kulm des Rheinischen Schiefergebirges. Beiheftezum Geologischen Jahrbuch 53: 1‒246.
Nolan, L.S.P., Angiolini, L., Jadoul, F., Della Porta, G., Davies, S.J., Banks, V.J., Stephenson, M.H., and Leng, M.J. 2017. Sedimentary context and palaeoecology of Gigantoproductus shell beds in the Mississippian Eyam Limestone Formation, Derbyshire carbonate platform, central England. Proceedings of the Yorkshire Geological Society 61: 239‒257. Crossref
Ohar, V. 2021. Carboniferous fauna from erratics in the Hradyzk area (Poltava region, Ukraine): paleo-ice streams indicator of the Dnipro glacial maximum. Historical Biology 33: 78‒87. Crossref
Pareyn, C. 1961. Les massifs Carbonifères du Sahara Sud‒oranais. T. II: Paléontologie strati‒ graphique. Publications du Centre de Recherches Sahariennes, Séries Géologie 1: 1‒244.
Parkinson, D. 1926. The faunal succession in the Carboniferous Limestone and Bowland Shales at Chlitheroe and Pendle Hill (Lancashire). Quarterly Journal of the Geological Society 82: 188‒240. Crossref
Parkinson, D. 1954a. Quantitative studies of brachiopods from the Lower Carboniferous reef limestones of England. I Schizophoria resupinata (Martin). Journal of Paleontology 28: 367‒381.
Parkinson, D. 1954b. Quantitative studies of brachiopods from the Lower Carboniferous reef limestones of England. II Pugnax pugnus (Martin) and P. pseudopugnus n. sp. Journal of Paleontology 28: 563‒574.
Parkinson, D. 1969. Relative growth, variation and evolutionary trends in a Carboniferous rhynchonellid brachiopod. Journal of Paleontology 43: 95‒110.
Peck, L.S., Rhodes, M.C., Curry, G.B., and Ansell, A.D. 1997. Physiology. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology. Part H: Brachiopoda. Revised. Volume 1: Introduction, 213‒242. The Geological Society of America, Boulder and University of Kansas, Lawrence.
Perez Huerta, A. and Sheldon, N.D. 2006. Pennsylvanian sea level cycles, nutrient availability and brachiopod palaeoecology. Palaeogeography, Palaeoclimatology, Palaeoecology 230: 264‒279. Crossref
Pharaoh, T.C., Merriman, R.J., Webb, P.C., and Beckinsale, R.D. 1987. The concealed Caledonides of eastern England: preliminary results of a multidisciplinary study. Proceedings of the Yorkshire Geological Society 46: 355‒369. Crossref
Phillips, J. 1836. Illustrations of the Geology of Yorkshire. Part II. The Mountain Limestone District. 243 pp. John Murray, London.
Pitrat, C.W. 1965. Spiriferidina. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology, Part H: Brachiopoda, H667‒H728. The Geological Society of America, Boulder and University of Kansas, Lawrence.
Pocock, Y.P. 1968. Carboniferous Schizophoriid brachiopods from Western Europe. Palaeontology 11: 64‒93.
Portlock, J.E. 1843. Report on the Geology of the County of Londonderry and of Parts of Tyrone and Fermanagh. 784 pp. Andrew Milliken, Dublin.
Powell, E.N. and Stanton, J.R.J. 1985. Estimating biomass and energy flow of molluscs in palaeocommunities. Palaeontology 28: 1‒35.
Pratt, B.R. 1995. The origin, biota and evolution of deep-water mud-mounds. In: C.L. Monty, D.W.J. Bosence, P.H. Bridges, and B. Pratt (eds.), Carbonate mud mounds; their origin and evolution. International Association of Sedimentologists Special Publication 23: 49‒123. Crossref
Prendergast, K.L. 1943. Permian Productinae and Strophalosiinae of Western Australia. Journal and Proceedings of the Royal Society of Western Austrialia 28: 1‒73.
Qiao, L., Falahatgar, M., and Shen, S. 2017. A lower Viséan (Carboniferous) brachiopod fauna from the eastern Albortz Mountains, northern Iran, and its palaeogeographical implications. Geological Journal 52: 317‒326. Crossref
Reed, F.R.C. 1954. Lower Carboniferous brachiopods from Scotland. Proceedings of the Leeds Phil. Lit. Society 5: 180‒190.
Reitner, J. and Neuweiler, F. 1995 (cords.). A polygenetic spectrum of fine-grained carbonate buildups. Facies 32: 1‒70. Crossref
Reitner, J., Gautret, P., Marin, F., and Neuweiler, F. 1995. Automicrites in a modern microbialite. Formation model via organic matrices (Lizard Island, Great Barrier Reef, Australia). Bulletin de l’Institut océanographique de Monaco, numerò special 13: 237‒263.
Rhodes, M.C. and Thompson, R.J. 1992. Clearance rate of the articulate brachiopod Neothyris lenticularis (Deshayes, 1839). Journal of Experimental Marine Biology and Ecology 163: 77‒89. Crossref
Rhodes, M.C. and Thompson, R.J. 1993. Comparative physiology of suspension feeding in living brachiopods and bivalves: evolutionary implications. Paleobiology 19: 322‒334. Crossref
Richardson, J.R. 1997. Ecology of articulated brachiopods. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology. Part H: Brachiopoda. Revised. Volume 1: Introduction, 441‒462. The Geological Society of America, Boulder and University of Kansas, Lawrence. Crossref
Rudwick, M.J.S. 1970. Living and Fossil Brachiopods. 199 pp. Hutchinson, London.
Ruttner, A., Nabavi, M., and Hajian, J. 1968. Geology of the Shirgesht Area (Tabas Area, East Iran). Geological Survey of Iran Report 4: 1‒133.
Savage, N., Manceñido, M.O., Owen, E.F., and Dagys, A.S. 2002. Pugnacoidea. In: A. Williams, C.H.C. Brunton, S.J. Carlson et al. (eds.), Treatise on Invertebrate Paleontology. Part H: Brachiopoda. Revised. Volume 4: Rhynchonelliformea (part), 1165‒1218. The Geological Society of America, Boulder and University of Kansas, Lawrence.
Sarycheva, T.G. 1949. Morphology, ecology and evolution of Carboniferous productids near Moscow (genera Dictyoclostus, Pugilis and Antiquatonia). Paleontological Institute of Academy of Sciences USSR Transactions 18: 1‒303.
Sarycheva, T.G. and Sokolskaya, A.N. 1952. An index to the Palaeozoic brachiopods of the Moscow Basin. Trudy Paleontological Institute 38: 1‒307.
Sarycheva, T.G., Sokolskaya, A.N., Beznosova, G.A., and Maxsimova, S.V. 1963. Brachiopods and Paleogeography of the Carboniferous of the Kuznetsk Basin. Academia Nauk USSR., Transactions of the Palaeontological Institute 95: 3‒448.
Shen, S.Z., Qiao, L., Zhang, Y., Sun, Y.L., and Jin, Y.G. 2017. Carboniferous Brachiopod Genera on Type Species of China. In: J.Y. Rong, Y.G. Jin., S.Z. Shen, and R.B. Zhan (eds.), Phanerozoic Brachiopod Genera of China, 563‒653. Science Press, Beijing.
Skompski, S., Paskowski, M., Krobicki, M., Kolkovin, K., Korn, D., Tomas, A., and Wrzolek, T. 2001. Depositional setting of the Devonian/Carboniferous biohermal Bol’shaya Nadota carbonate complex, Subpolar Urals. Acta Geologica Polonica 51: 217‒235.
Smith, E.G., Rhys, G.H., and Eden, R.A. 1967. Geology of the Country Around Chesterfield, Matlock and Mansfield. Memoir of the British Geological Survey. Sheet 112. viii + 430 pp. Her Majesty’s Stationary Office, Keyworth, Nottingham.
Sowerby, J. 1812–1815. The Mineral Conchology of Great Britain, Vol. 1. iv + 234 pp. Published by the author, London.
Sowerby, J. 1818–1821a. The Mineral Conchology of Great Britain, Vol. 3. 184 pp. Published by the author, London.
Sowerby, J. 1821b–1822. The Mineral Conchology of Great Britain, Vol. 4. 1–114. Published by the author, London.
Sowerby, J. de C. 1823b–1825. The Mineral Conchology of Great Britain, Vol. 5. 168 pp. Published by the author, London.
Stainbrook, M.A. 1940. Orthoid brachiopods of the Cedar Valley Limestone of Iowa. The American Midland Naturalist 23: 482–492. Crossref
Stehli, F.G. 1956. Dielasma and its external homeomorph Beecheria. Journal of Paleontology 30: 299‒302.
Stevenson, I.P. and Gaunt, G.D 1971. Geology of the country around Chapel-en-Frith. Memoir of the British Geological Survey. Sheet 99. xii + 444 pp. Her Majesty’s Stationery Office, London.
Stone, P., Millward, D., Young, B., Merritt, J.W., Clark, S.M., Mc Cormac, M., and Lawrence, D.J.D. 2010. British Regional Geology: Northern England, Fifth. 194 pp. British Geological Survey, Keyworth.
Sun, Y. and Baliński, A. 2008. Silicified Mississippian brachiopods from Muhua, southern China: lingulids, craniids, strophomenids, productoids, orthotetids and orthids. Acta Paleontologica Polonica 53: 485‒524. Crossref
Tazawa, J. 2017. An early Carboniferous (late Visean) brachiopod fauna from Tairagai in the Yokota area, South Kitakami Belt, Japan. Palaeontological Research 21: 329‒346. Crossref
Tazawa, J. and Ibaraki, Y. 2009. Linoprotonia and Gigantoproductus (Linoproductoidea, Brachiopoda) from the Lower Carboniferous in the Onimaru quarry, Hikoroichi, southern Kitakami Mountains, NE Japan. Science Report Niigata University (Geology) 24: 7‒19.
Tazawa, J. and Kurita, H. 2019. Early Carboniferous (late Tournaisian) brachiopod fauna from the Shittkazawa Formation in the Okuhinotsuchi area, South Kitakami Belt, Japan. Journal of the Geological Society of Japan 3: 219‒225. Crossref
Tazawa, J., Ibaraki, Y., and Nishikawa, I. 2016. Early Carboniferous (Visean) brachiopods from the Hina Limestone, Okayama Prefecture, SW Japan, and their palaeogeographical implications. Science Report Niigata Uniersity. (Geology) 31: 45‒68.
Thayer, C.W. 1975. Strength of pedicle attachment in articulate brachiopods: ecologic and palaeoecologic significance. Palaeobiology 1: 388‒399. Crossref
Thomas, I. 1910. The British Carboniferous Orthotetinae. Memoirs of the Geological Survey of Great Britain, Palaeontology 1: 83‒134.
Thomas, I. 1914. British Carboniferous Producti ‒ Pustula and Overtonia. Memoirs of the Geological Survey U.K., Palaeontology 1: 197‒366.
Trechmann, S. 1917. The age of the Maitai Series of New Zealand. Geological Magazine 4: 53‒64. Crossref
Ure, D. 1793. The History of Rutherglenand, East-Kilbride. 334 pp. Published by the author, Glasgow.
Vachard, D., Cózar, P., Aretz, M., and Izart, A. 2016. Late Viséan‒Serpukhovian foraminifers in the Montagne Noire (France): biostratigraphic revision and correlation with the Russian substages. Geobios 49: 469‒498. Crossref
Vaughan, A. 1905. The palaeontological sequence in the Carboniferous limestone of the Bristol area. Quarterly Journal of the Geological Society 61: 181‒307. Crossref
Walther, J.K. 1890. Ueber eine Kohlenkalk fauna aus der Agyptisch-arabischen W‘uste. Zeitschrift der Deutschen Geologischen Gesellschaft 42: 419‒449.
Waterhouse, J.B. 1970. The Lower Carboniferous brachiopod Genus Fusella M’Coy 1844. Life Sciences Occasional Papers of the Royal Ontario Museum 15: 1‒12.
Waters, C.N., Waters, R.A., Barclay, W.J., and Davies, J.R. 2009. Lithostratigraphical framework for Carboniferous successions of Southern Great Britain (Onshore). British Geological Survey Report RR/09/01: 1‒184.
Weller, S. 1899. Kinderhook faunal studies. 1. The fauna of the Vermicular Sandstone at Northview, Webster County, Missouri. Transactions of the Academy of Sciences of St Louis 9: 9‒51.
Williams, A. and Brunton, C.H.C. 1997. Morphological and anatomical terms applied to brachiopods. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology. Part H: Brachiopoda. Revised. Volume 1: Introduction, 423‒440. The Geological Society of America, Boulder and University of Kansas, Lawrence.
Williams, A. and Brunton, C.H.C. 2000. Orthotetidina. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology. Part H: Brachiopoda. Revised. Volume 3: Linguliformea, Craniiformea, and Rhynchonelliformea (part), 644‒681. The Geological Society of America, Boulder and University of Kansas, Lawrence.
Williams, A., Brunton, C.H.C., and MacKinnon, D.I. 1997. Morphology. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology. Part H: Brachiopoda. Revised. Volume 1: Introduction, 321‒422. The Geological Society of America, Boulder and University of Kansas, Lawrence. Crossref
Wilson, R.B. 1989. A study of the Dinantian marine macrofossils of central Scotland. Transactions of the Royal Society of Edinburgh Earth Sciences 89: 91‒126. Crossref
Winkler-Prins, C.F. 1968. Carboniferous Productidina and Chonetidina of the Cantabrian Mountains (NW Spain): systematics, stratigraphy and palaeontology. Leidse Geologische Mededelingen 43: 41‒155.
Winkler-Prins, C.F. and Amler, M.R.W. 2006. Brachiopoden. In: Deutsche Stratigraphische Kommission (ed.), Stratigraphie von Deutschland VI. Unterkarbon (Mississippian). Schriftenreihe der Deutschen Gesellschaft für Geowissenschaften 41: 89‒100.
Wolf, K.H. 1965. Gradational sedimentary products of calcareous algae. Sedimentology 5: 1‒37. Crossref
Wolfenden, E.B. 1958. Paleoecology of the Carboniferous reef complex and shelf limestones in North-West Derbyshire, England. Bulletin of the Geological Society of America 60: 871‒898. Crossref
Wright, A.D. 1965. Superfamily Enteletacea. In: R.L. Kaesler (ed.), Treatise on invertebrate paleontology, Part H: Brachiopoda, H328‒H346. The Geological Society of America, Boulder and University of Kansas, Lawrence.
Yao, L., Aretz, M., Wignall, P.B, Chen, J., Vachard, D.Y., Qi, S.S., and Wang, X. 2019. The longest delay: re-emergence of coral reef ecosystems after the Late Devonian extinctions. Earth Science Reviews 203. Crossref
Yuan, Z., Sun, Y., Shen, B., Xing, C., Liu, W., Yang, R., Qin, S., and Baliński, A. 2019. An in-situ preserved early Carboniferous (Serpukhovian) brachiopod community in southern Guizhou, China. Rivista Italiana di Paleontologia e Stratigrafia 125: 689‒710.
Żakowa, H. 1966. Zone Goniatites crenistria Phill in the vicinity of the Sowie Góry Mountains (Central Sudetes). Instytut Geologiczny Prace 43: 1‒197.
Żakowa, H. 1971. Zone Goniatites granosus in the Gałęzice Syncline (Góry Świętokrzyskie). Instytut Geologiczny Prace 60: 1‒137.
Acta Palaeontol. Pol. 67 (4): 865–915, 2022
https://doi.org/10.4202/app.00972.2022