

Terrestrial-aquatic wood-inhabiting ascomycete Potamomyces from the Miocene of Poland
GRZEGORZ WOROBIEC, ELŻBIETA WOROBIEC, PRZEMYSŁAW GEDL, JACEK R. KASIŃSKI, DANUTA PERYT, and MAREK WIDERA
Worobiec, G., Worobiec, E., Gedl, P., Kasiński, J.R., Peryt, D., and Widera, M. 2022. Terrestrial-aquatic wood-inhabiting ascomycete Potamomyces from the Miocene of Poland. Acta Palaeontologica Polonica 67 (3): 737–744.
We report fungal ascospores of Potamomyces affinities from four Miocene localities in Poland. The spores are similar to the ones known from extinct species of Potamomyces invaginatus, Potamomyces batii, and Potamomyces pontidiensis, as well as to living Potamomyces armatisporus. Living representatives of Potamomyces are saprophytic, and usually found on decaying wood. They are mainly found in a freshwater or brackish environment, and sometimes also on terrestrial, moist to damp substrates. Therefore, the species of Potamomyces can be classified as a facultative-aquatic or terrestrial-aquatic fungus. Both living and extinct species of Potamomyces prefer a tropical to subtropical, and usually humid climate as their past and recent distribution is mostly confined to the intertropical zone. Fossil record of the Potamomyces ranges from the Lower Miocene to the Holocene, covering all continents with the exception of the Antarctica. Miocene findings of Potamomyces from Poland represent the first known fossil record of this genus from Europe and confirm the warm temperate to subtropical and humid climate during Middle to Late Miocene of present Poland previously inferred from palaeobotanical investigations.
Key words: Fungi, palaeoecology, palaeoenvironment, non-pollen palynomorphs, Neogene.
Grzegorz Worobiec [g.worobiec@botany.pl] and Elżbieta Worobiec [e.worobiec@botany.pl], W. Szafer Institute of Botany, Polish Academy of Sciences, Lubicz 46, 31-512 Kraków, Poland.
Przemysław Gedl [p.gedl@ingpan.krakow.pl], Institute of Geological Sciences, Polish Academy of Sciences, Research Centre in Cracow, Senacka 1, 31-002 Kraków, Poland.
Jacek R. Kasiński [jacek.kasinski@pgi.gov.pl], Polish Geological Institute-National Research Institute, Rakowiecka 4, 00-975 Warszawa, Poland.
Danuta Peryt [d.peryt@twarda.pan.pl], Institute of Paleobiology, Polish Academy of Sciences, Twarda 51/55, 00-818 Warszawa, Poland.
Marek Widera [marek.widera@amu.edu.pl], Institute of Geology, Adam Mickiewicz University, Krygowskiego 12, 61-680 Poznań, Poland.
Received 21 January 2022, accepted 19 April 2022, available online 21 July 2022.
Copyright © 2022 G. Worobiec et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Fungi are one of the kingdoms of eukaryotic organisms that apparently appeared during the late Proterozoic (Krings et al. 2017; Loron et al. 2019; Bonneville et al. 2020) and their living species richness is estimated to be 2.2–3.8 million species (Hawksworth and Lücking 2017). They are usually terrestrial organisms, although some (about 3000–4000 species) have been reported from the aquatic environment (Shearer et al. 2007; Grossart and Rojas-Jimenez 2016). Fungi show many parasitic or symbiotic interactions with living plants (Krings et al. 2012), or they could be saprophytic on dead plant tissues (Dix and Webster 1995). The main ecological role of saprophytic fungi, both in terrestrial and aquatic environments, is the decomposition of organic matter including dead plant material (Dix and Webster 1995; Wong et al. 1998; Gessner et al. 2007; Tsui et al. 2016; Gulis et al. 2019). Especially freshwater ascomycetes prefer to colonize woody substrates (Shearer and Raja 2010). Remains of terrestrial-aquatic fungi that accompanied Miocene wetland plant associations were preserved in the Adamów lignite deposits, central Poland, as chitinous non-pollen palynomorphs (NPP) found during palynological investigations (Widera et al. 2021; Worobiec et al. 2021, 2022b). They are represented by species that were saprophytic on decaying wood and on fallen leaves in a very humid, periodically flooded environment (Worobiec et al. 2022b). Among the fungal remains a spore was found that is identical to those of living fungus Potamomyces Hyde, 1995. Comparable spores were also found in other Miocene localities from Poland (Babczyn, Bełchatów, and Komorniki). Similarly to many fossil fungi that have potential as a non-pollen palynomorph palaeoecological proxy (Lange 1978; Conran et al. 2016; Worobiec and Worobiec 2017; Worobiec et al. 2018), Potamomyces is considered useful for the reconstruction of palaeoenvironment and palaeoclimate (Nuñez Otaño et al. 2017). Considering this, the main goal of the present investigations was a detailed taxonomical and palaeoecological study of Potamomyces in the Miocene of Poland in terms of its importance as a palaeoecological proxy and the diversity of Miocene fossil ascomycetes.
Institutional abbreviations.—KRAM-P, Palaeobotanical collections of W. Szafer Institute of Botany, Polish Academy of Sciences, Kraków, Poland.
Other abbreviations.—NPP, non-pollen palynomorphs.
Material and methods
Ascospores of Potamomyces were collected from four Miocene localities from Poland (Fig. 1): the Middle Miocene of the Adamów lignite deposit, Upper Miocene from the Bełchatów Lignite Mine, the Middle Miocene of the Komorniki 97/72 borehole in the Ruja lignite deposit, and the Middle Miocene of the Babczyn 2 borehole. All of the ascospores were found during palynological studies.

Fig. 1. Location of the investigated Miocene localities in Poland (astersisks) where ascospores of Potamomyces were found.
The examined section of the lignite seam from the Adamów deposit was located in the north-western part of the Adamów opencast mine, central Poland (52°01’17”N 18°37’45”E). An ascospore belonging to Potamomyces genus was found in a palynological sample taken from the 3-m thick 1st mid-Polish lignite seam (Worobiec et al. 2022b). The age of the 1st mid-Polish seam was determined as the middle part of the Middle Miocene (Piwocki and Ziembińska-Tworzydło 1997; Kasiński and Słodkowska 2016; Worobiec et al. 2021, 2022b). Palynological analysis revealed that the Adamów area was overgrown by palustrine wetland communities similar in composition to modern pocosins (Worobiec et al. 2021).
Deposits with plant macroremains from Bełchatów, central Poland (sites KRAM-P 210 and KRAM-P 218) were found in the open pit of the Bełchatów Lignite Mine (51°14’42”N 19°17’14”E) in overburden sediments belonging to the clayey-coal unit (I-W) which are considered to be the latest Middle–Late Miocene age (Worobiec and Lesiak 1998; Worobiec and Worobiec 2016). According to Wilczyński (1992) and Krzyszkowski and Winter (1996) deposits of the lower part of the clayey-sandy unit were formed in a fluvial environment of braided to meandering rivers with dense vegetation along riverbanks.
The Komorniki 97/72 borehole in the Ruja lignite deposit was located in the southernmost part of the Legnica-Ścinawa lignite resource complex in Lower Silesia, SW Poland (51°11’N 16°23’E). About 100 samples were taken from the borehole core for palynological analysis. An ascospore of Potamomyces genus was found in a palynological sample taken from the 2nd Lusatian lignite seam. This seam developed during the Middle Miocene, in the warmest phase of the lignite origin of the Ruja deposit. In this phase, riparian, swamp and peat-bog vegetation dominated (Worobiec et al. 2022a).
The Babczyn 2 borehole (50°14’04.26”N 23°12’44.88”E) was located in the northern part of the Polish Carpathian Foredeep, SE Poland (Peryt et al. 2020). The palynologically studied section of the borehole core is composed of marine marls and clays. They came from the sediments lying on the upper Badenian Babczyn tuff (Peryt et al. 2021) and were radiometrically dated at 13.06±0.11 Ma (Serravalian, Middle Miocene) (Śliwiński et al. 2012; Peryt et al. 2020). The analysed samples yielded well preserved terrestrial (sporomorphs) and marine (dinoflagellate cysts) palynomorphs, along with fungal remains. The preliminary results of the palynological analysis revealed the presence of mesophytic and wetland vegetation along the Paratethys sea shoreline at the time of sedimentation.
The samples from the Adamów and Bełchatów mines outcrops and from the borehole Komorniki 97/72 were processed using successively 10% hydrochloric acid (HCl) to remove carbonates, 10% potassium hydroxide (KOH), 40% hydrofluoric acid (HF) for four days to remove silicates, and subsequently 10% hydrochloric acid (HCl) to remove silicofluorides (Moore et al. 1991). Additionally, the residuum was sieved at 5 µm on a nylon mesh. The samples from the Babczyn 2 borehole core were processed using 38% hydrochloric acid (HCl) followed by 40% hydrofluoric acid (HF), heavy liquid (ZnCl2 + HCl; density 2.0 g/cm3) separation, and sieving at 15 μm on a nylon mesh in and ultrasonic bath for 10–15 s (Peryt et al. 2020). Palynological slides from all of the localities were made using glycerine jelly as a mounting medium. They are housed in the W. Szafer Institute of Botany, Polish Academy of Sciences (Kraków). Microphotographs were taken with a Nikon Eclipse E400 microscope equipped with a Canon A640 digital camera.
The terminology for the morphology of Potamomyces ascospores follows Kirk et al. (2008), taxonomy follows Wijayawardene et al. (2017, 2018).
Systematic palaeontology
Kingdom Fungi Moore, 1980
Phyllum Ascomycota Cavalier-Smith, 1998
Family uncertain
Genus Potamomyces Hyde, 1995
Type species: Potamomyces armatisporus Hyde, 1995; Recent, North Queensland, Australia.
Potamomyces sp. ex gr. P. armatisporus Hyde, 1995
Fig. 2.
Material.—Adamów 2017: slide 0.1(1), one specimen; Bełchatów: KRAM-P 210/10(4), one specimen; KRAM-P 218/ 109B(5), one specimen; KRAM-P 218/85(1), one specimen; Babczyn 2 borehole: slide 2/31, one specimen; Komorniki borehole 97/72: slides from depth 104.7 m; four specimens. Nine specimens in total from Middle to Upper Miocene of Poland. Collections: Adamów 2017, KRAM-P 218, Babczyn 2, and Komorniki 97/72. Stored in the W. Szafer Institute of Botany, Polish Academy of Sciences, Kraków.
Description.—Ascospores distinctly biconical (rhomboid) to obovate, thick-walled, medium-brown to dark brown, more or less translucent, smooth, 52–62 µm long and 25–35 µm wide in the equatorial part. One end of the ascospore more or less rounded and one end usually more or less attenuated, while in the case of some ascospores both ends obtuse to nearly rounded. Mostly four, very rarely five, distinct verrucae arranged around the equator. The verrucae rounded, 3–5 µm high and 5–10 µm in diameter. An indistinct germ pore usually visible on one end of ascospore.
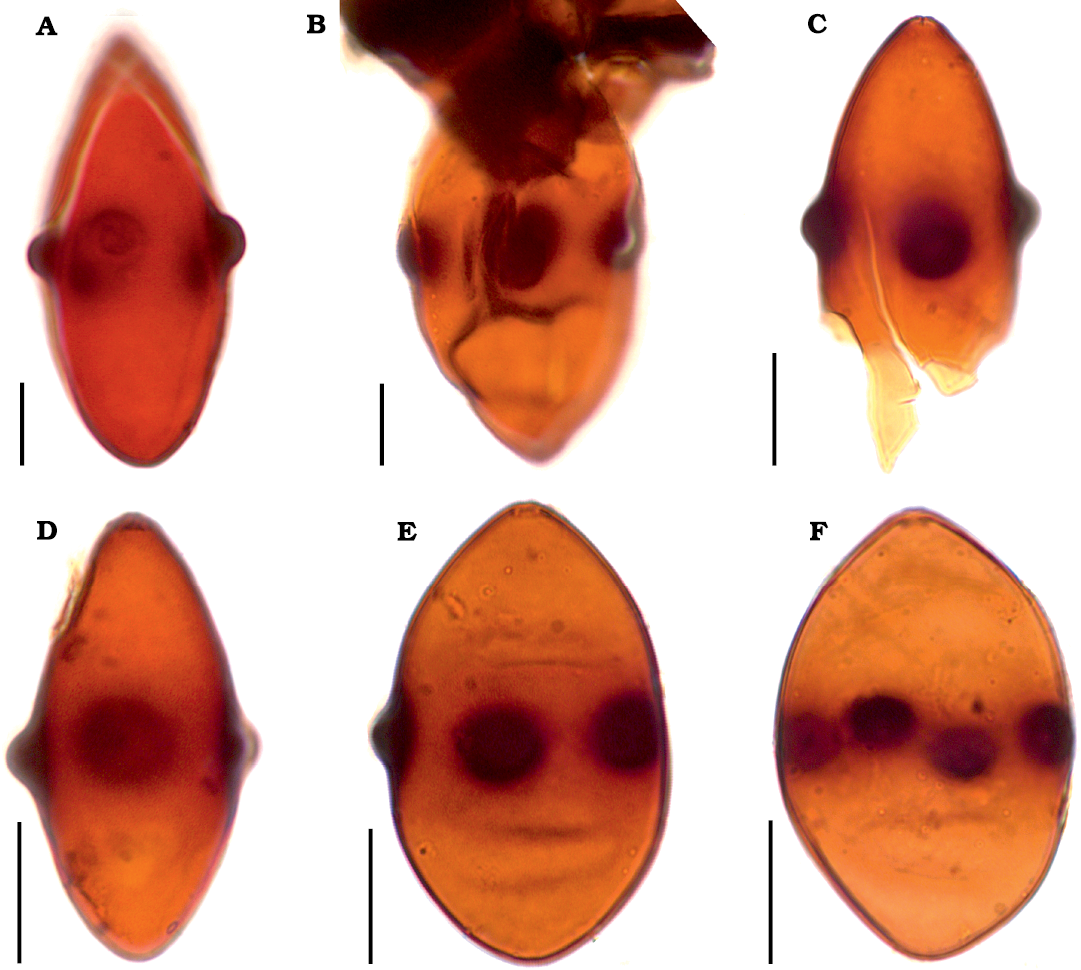
Fig. 2. Fossil ascospores of Potamomyces sp. ex gr. P. armatisporus Hyde, 1995, from Middle (A, B, D–F) and Middle/Upper (C) Miocene of Poland. A. Adamów 2017, slide Adamów 2017/0.1(1) (modified from Worobiec et al. 2022b: fig. 4c). B. Babczyn 2, slide Babczyn 2/2(31). C. Bełchatów KRAM-P 218, slide KRAM-P 218/85(1). D–F. Komorniki 97/72. D. Slide Komorniki 97/72/104.7(2). E. Slide Komorniki 97/72/104.7(3). F. Slide Komorniki 97/72/104.7(4). Scale bars 10 μm.
Remarks.—A biconical shape along with verrucae arranged around the equator of the above described fungal spores is rather unique among fungal spores. For the first time Muller (1959) reported such spores from the Recent sediments of the Orinoco Delta, Venezuela. Then, for many years, these spores were reported and informally classified as Mediaverrunites Jarzen and Elsik, 1986 or Mediaverrusporonites Elsik, 1976, without diagnostic features and without specified type specimens (Elsik 1976; Jarzen and Elsik 1986; Banerjee and Nandi 1992; Nandi and Sinha 2007). Only Nandi and Sinha (2007) formally described and validated the Mediaverrunites Jarzen and Elsik 1986 ex Nandi and Sinha, 2007. Schlütz and Shumilovskikh (2013) for the first time linked fossil spores of Mediaverrunites/Mediaverrusporonites with ascospores of living Potamomyces armatisporus Hyde, 1995, described earlier from submerged wood from Australia, Ecuador, and Malaysia (Hyde 1995). Furthermore, these authors described a new species Potamomyces nepalensis Schlütz and Shumilovskikh in Nuñez Otaño et al., 2017, and they compared living representatives of Potamomyces Hyde, 1995, with spores of the fossil-genus Mediaverrunites. However, according to Nuñez Otaño et al. (2017), the fungal taxonomical novelties of Schlütz and Shumilovskikh (2013) were published without any identifiers from a recognised repository and, thus, these names were invalid. Nuñez Otaño et al. (2017) validated the name Potamomyces nepalensis and made new combinations of the extinct species described in Mediaverrunites and re-assigned them to Potamomyces in accordance with the International Code of Nomenclature for algae, fungi and plants (Turland et al. 2018).
Potamomyces includes two living species (Potamomyces armatisporus Hyde, 1995, and Potamomyces nepalensis Schlütz and Shumilovskikh in Nuñez Otaño et al., 2017), and eight fossil-species (Potamomyces batii Sancay, 2014 ex Nuñez Otaño, di Pasquo, and Bianchinotti, 2017, Potamomyces elsikii (Nandi and Sinha, 2007) Nuñez Otaño, di Pasquo, and Bianchinotti, 2017, Potamomyces fournieri (Elsik and Jarzen, 2009) Nuñez Otaño, di Pasquo, and Bianchinotti, 2017, Potamomyces invaginatus (Elsik and Jarzen, 2009) Nuñez Otaño, di Pasquo, and Bianchinotti, 2017, Potamomyces magnus (Elsik and Jarzen, 2009) Nuñez Otaño, di Pasquo, and Bianchinotti, 2017, Potamomyces mulleri (Nandi and Sinha, 2007) Nuñez Otaño, di Pasquo, and Bianchinotti, 2017, Potamomyces pontidiensis Sancay, 2014 ex Nuñez Otaño, di Pasquo, and Bianchinotti, 2017 including subfossil Potamomyces palmarensis Nuñez Otaño, di Pasquo, and Bianchinotti, 2017) (MycoBank 2021). The fossil and living Potamomyces species can be divided into two morphological groups depending on the number of verrucae. The first group are taxa corresponding to the living P. armatisporus usually have 4, but sometimes 3–5 equatorial verrucae and besides living P. armatisporus this group includes the fossil-species P. invaginatus, P. batii, and P. pontidiensis. A second group are taxa close to living P. nepalensis and are represented by fossil-taxa having 6–8 verrucae and include P. palmarensis, P. elsikii, P. mulleri, and P. magnus. However, in the case of specimens illustrated by Nandi and Sinha (2007) as Mediaverrunites elsikii (= P. mulleri), and Mediaverrunites mulleri (= P. mulleri), it can be seen that contrary to the original description of Mediaverrunites elsikii (6–8 verrucae), and Mediaverrunites mulleri (4–8 verrucae), they have a maximum of 4–5 verrucae. Therefore, P. elsikii and P. mulleri should be included in the Potamomyces armatisporus group. Considering all fossil and living Potamomyces species they can be grouped into taxa close to P. armatisporus usually having 4 (3–5) verrucae (P. armatisporus, P. batii, P. elsikii, P. invaginatus, P. mulleri, and P. pontidiensis) and taxa corresponding to P. nepalensis having 6–8 verrucae (P. nepalensis, P. palmarensis, and P. magnus). The fossil-species of P. fournierii seems to be a transitional form between these groups having 5–6 verrucae (Nuñez Otaño et al. 2017). A peculiar feature of some fossil ascospores of Potamomyces is the presence of two bands of verrucae (Elsik and Jarzen 2009; Nuñez Otaño et al. 2017), additional verrucae near both poles in the case of P. nepalensis (Schlütz and Shumilovskikh 2013) or verrucae covering the whole surface of the ascospore, as in the case of the spore described as cf. Gliomastix-type (Jarzen and Elsik 1986: pl. 3: 7) that surely could be assigned to Potamomyces. These atypical features, however, could be teratological abnormalities which occurred during the formation of the ascospores. It seems especially true in the case of the above mentioned spore of Jarzen and Elsik (1986), with verrucae covering the whole surface of the ascospore. Other features of the morphology of Potamomyces ascospores (e.g., verrucae dimensions, and the presence or absence of an apical germ pore) are rather variable or sometimes hardly visible (compare Elsik and Jarzen 2009) and, thus, their taxonomical utility in these cases seems limited.
The shape and dimensions of ascospores are also variable, and in a given locality the same type of Potamomyces spores could be both biconical and wide elliptic. This concerns the ascospores of Potamomyces found in Poland as well. They correspond to the Potamomyces armatisporus group usually having 4 verrucae (the only specimen of Potamomyces from Adamów has 5 verrucae, Fig. 2A) and a biconical (Fig. 2A–D) to wide elliptic (Fig. 2E, F) shape. Fossil Potamomyces ascospores from Poland correspond to the fossil P. invaginatus, P. batii, and P. pontidiensis, and to living P. armatisporus. Potamomyces invaginatus differs with its smaller ascospores and larger verrucae (10–12 µm), when compared to the Potamomyces from the Miocene localities of Poland (Elsik and Jarzen 2009). Potamomyces batii and P. pontidiensis within the limits of variability, have a similar size and shape to our specimens of Potamomyces. However, they have 3–4 verrucae (Sancay 2014), contrary to Potamomyces from the Miocene localities of Poland, which mostly have 4 verrucae. Potamomyces pontidiensis is morphologically most similar to some of our specimens. Contemporary P. armatisporus differs with its smaller ascospores (Hyde 1995; Nuñez Otaño et al. 2017). As no fossil and living species of Potamomyces is identical to fossil ascospores from Poland, we decided to classify them as Potamomyces sp. ex gr. P. armatisporus Hyde, 1995.
It should be added that besides the Potamomyces, the fossil-genus Parapotamomyces O’Keefe, 2017, with fossil-species Parapotamomyces maydiformis O’Keefe, 2017, which is considered to be a fungal spore, has been described from the Miocene Heath Formation, Tumbes Province, Peru (O’Keefe 2017). However, Parapotamomyces maydiformis certainly should not be considered as a fungal spore but possibly represents plant remain resembling an isolated fragment of seed testa.
The ascospores of Potamomyces are rather similar to those of the living Biconiosporella Schaumann, 1972, represented by marine species Biconiosporella corniculata Schaumann, 1972, and are saprophytic on decayed wood (Hyde 1995; Jones et al. 2009). Both taxa posses biconical ascospores with equatorial verrucae (Hyde 1995). However, Potamomyces and Biconiosporella differ markedly as Biconiosporella corniculata has four-celled ascospores with 7–9 equatorial verrucae, while Potamomyces armatisporus is one-celled with 4 equatorial verrucae (Kohlmeyer and Kohlmeyer 1979; Hyde 1995). Moreover, Biconiosporella corniculata differs from all species of Potamomyces through its having two germ pores at each end of the ascospore (Huang et al. 2021). Amphisphaerella dispersella (Nylander, 1859) Eriksson, 1966, has ascospores with characteristic meridional germ pores that somewhat resemble verrucae that characterize all taxa of Potamomyces (Hyde 1995; Van Geel and Aptroot 2006; Schlütz and Shumilovskikh 2013). Ascorhombispora aquatica Cai and Hyde, 2007, found saprophytic on submerged bamboo culms, has a similar, more or less rhombic shape and a dark equatorial belt. It differs from fossil and living species of Potamomyces with its absence of verrucae (Cai and Hyde 2007). There is a similarity to ascospores of Potamomyces (with a biconical shape) also displayed in the conidia of some members of the family Beltraniaceae Nannizzi, 1934 (e.g., Beltrania rhombica Penzig, 1882, Pseudobeltrania cedrelae Hennings, 1902, Beltraniomyces lignicola Manoharachary, Agarwal, and Rao, 2003) but there is a clear difference in the absence of equatorial verrucae (Rajeshkumar et al. 2016).
Discussion
Geographical and stratigraphical distribution of Potamomyces.—The oldest fossil record of Potamomyces-like spores concerns a spore described as Diporicellaesporites sp. from the early Eocene (53–52 Ma) Vastan Lignite Mine, Gujarat, western India (Rao et al. 2013). As can be seen (Rao et al. 2013: pl. 3: 33) in the spore illustrated there is similar to species of Potamomyces in its having characteristic equatorial verrucae (two are visible) and in the shape. It differs markedly from species of Potamomyces, however, with two clearly visible germ pores on both poles. Contrary to spore from the Vastan Lignite Mine, ascospores of Potamomyces species have only one, sometimes hardly visible germ pore. This Eocene Potamomyces-like fungal spore could belong to an ancestor of Potamomyces or, through its having two germ pores, could be another fossil-genus displaying a similarity to ascospores of the living Biconiosporella Schaumann, 1972. All of the remaining fossil records of Potamomyces-like fungal spores range from the Lower Miocene to the Holocene (SOM: table 1, the Supplementary Online Material available at http://app.pan.pl/SOM/app67-Worobiec_etal_SOM.pdf). Neogene records come from Africa, Asia, and South America (i.e., Brazil, Colombia, India, Mexico, Nigeria, Peru, and Turkey), and Quaternary Potamomyces is known from Africa, Asia, North and South America, and Australia (i.e., Argentina, Australia, Brazil, Colombia, Dominican Republic, Fiji Islands, Gulf of Mexico, India, Jamaica, Marianas Islands, South Africa, and Turkey). Recent distribution of Potamomyces includes Africa, Asia, North and South America, and Australia (i.e., Australia, Brazil, Brunei, Colombia, Ecuador, Hong Kong, Malaysia, Nepal, USA, Venezuela, Zambia). Ascospores of Potamomyces, both fossil and living have not been known in Europe and Antarctica (compare SOM: table 1). Thus, the findings of Potamomyces sp. from the Miocene of Poland document the first fossil record of this fungus from Europe and represent the northernmost known occurrence of this genus.
Ecology of fossil and living Potamomyces.—Potamomyces armatisporus was found for the first time as a living saprophytic fungus isolated from submerged wood from Australia, Ecuador and Malaysia (Hyde 1995). Other records similarly suggest that Potamomyces occurs on decaying wood (Ho et al. 2001; Canto et al. 2020) or on driftwood in a brackish environment (Hyde 1988, 1995). Most fossil and modern findings of Potamomyces are confined to an aquatic, freshwater (riverine, lacustrine, swampy) or brackish (deltaic, coastal, mangrove) environment (SOM: table 1). Some modern findings, however, are not connected to an aquatic environment but rather to a terrestrial environment and there Potamomyces also prefers moist to damp substrates (Macphail 2006; Elsik and Jarzen 2009; Schlütz and Shumilovskikh 2013). These findings of Potamomyces (both aquatic and terrestrial localities) indicate that, ecologically, this fungus, by analogy with modern saprophytic facultative-aquatic hypomycetes (Goh 1997), could be classified as facultative-aquatic or terrestrial-aquatic fungus. Both fossil and living species of Potamomyces prefer a tropical to subtropical, and usually humid climate. Occasionally, Potamomyces is also found in warm temperate climate localities (SOM: table 1). Considering the data of past and modern distribution of Potamomyces (SOM: table 1), it is clear that this fungus is mostly confined to an intertropical zone.
Miocene findings of Potamomyces sp. from Poland (Adamów, Babczyn, Bełchatów, and Komorniki) are also associated with an aquatic, swampy, riverine or even marine (originally swampy or brackish) palaeoenvironment, and suggest its lignicolous habitat. Palynological analysis of the Middle Miocene lignite seam from Adamów revealed the presence of sporomorphs of peat-forming vegetation of palustrine wetland communities and mesophytic forests, along with fungal microremains of ascomycetes and hyphomycetes that were saprophytic on decaying wood and on fallen leaves in a very humid, periodically flooded environment and indicate that the climate was warm temperate and humid, with an estimated mean annual temperature at 15.7–18.0°C (Worobiec et al. 2021, 2022b). The floristic composition of Middle Miocene plant assemblages from the Komorniki borehole in the Ruja lignite deposit are characterized by dominance of wetland, swamp and riparian vegetation and suggest warm temperate climate conditions with mild winters (Worobiec et al. 2008), with a mean annual temperature in the range of 15.7–17.8°C (Worobiec et al. 2022a). In the Middle Miocene of Babczyn, the preliminary results of palynological analysis revealed the presence of remains of mesophytic and wetland plants and wood-inhabiting fungi in a warm-temperate to subtropical, mild and rather humid climate. Middle to Late Miocene fossil plant assemblages from Bełchatów were dominated by plant remains of wetland vegetation of swamp and riparian forests, along with taxa of mesophytic upland communities growing in a warm temperate climate with mild winters (Worobiec 2003; Worobiec and Lesiak 1998; Worobiec and Szynkiewicz 2016; Worobiec and Worobiec 2016). A mean annual temperature of 13.5–16.5°C for the KRAM-P 218 assemblage from Bełchatów was estimated (Worobiec and Szynkiewicz 2016). Therefore, the presence of ascospores of Potamomyces sp. in the Miocene of Poland fits well with warm temperate to subtropical and humid climate of Middle to Late Miocene of Poland inferred from palaeobotanical investigations and confirms that Potamomyces is a useful and credible non-pollen palynomorph proxy for reconstruction of a palaeoenvironment and palaeoclimate.
Conclusions
We found that ascospores of Potamomyces sp. from Poland correspond to Potamomyces armatisporus group. Ecologically, both fossil and living species of Potamomyces are usually found on decaying wood in an aquatic, freshwater, and sometimes brackish environment, but also in terrestrial, moist to damp settings. Potamomyces prefers a tropical to subtropical, usually humid climate and only occasionally it is also found in a warm temperate climate. Similar to modern saprophytic facultative-aquatic hypomycetes (Goh 1997), we consider Potamomyces to be a facultative-aquatic or terrestrial-aquatic fungus. The results of our investigations confirm that Potamomyces is a useful and credible non-pollen palynomorph proxy for reconstruction of a palaeoenvironment and palaeoclimate. Therefore, palynologists during palynological investigation of pre-Quaternary deposits should pay more attention to the various types of fungal non-pollen palynomorphs, including Potamomyces, as in many cases they are ignored by researches. More data on the stratigraphical range of Potamomyces obtained in this report could shed light on the problem of the time of emergence of Potamomyces and allow to determine the full palaeogeographical range of this genus, especially in the case of the Cenozoic of Europe. The results of our study may be informative not only for palaeobotanists and geologists, but also for mycologists regarding the calibration of the divergence time estimations in the phylogenetic trees of fungal lineages.
Acknowledgements
The authors are indebted to Michael Krings (Bayerische Staatssammlung für Paläontologie und Geologie, München, Germany) and Noelia Nuñez Otaño (Universidad Autónoma de Entre Ríos, Diamante, Argentina) for detailed revision and valuable suggestions that improved our manuscript. We would like to express special thanks to authority of the Adamów Lignite Mine for granting permission for our fieldwork in the mine. This work was supported by the W. Szafer Institute of Botany, Polish Academy of Sciences, through its statutory funds (GW and EW) and the National Science Centre, Poland, under grant 2017/27/B/ST10/00001 (to MW) and grant UMO-2017/27/B/ST10/01129 (to DP). This study is a contribution to the NECLIME—an open international network of scientists working on the Cenozoic climate evolution and related changes of continental ecosystems.
References
Banerjee, S. and Nandi, B. 1992. Fossil fungi from Miocene sediments of Mizoram and their environmental significance. Journal of Mycopathological Research 30: 81–90.
Bonneville, S., Delpomdor, F., Préat, A., Chevalier, C., Araki, T., Kazemian, M., Steele A., Schreiber, A., Wirth, R., and Benning, L.G. 2020. Molecular identification of fungi microfossils in a Neoproterozoic shale rock. Science Advances 6 (4): eaax7599. Crossref
Cai, L. and Hyde, K.D. 2007. Ascorhombispora aquatica gen. et sp. nov. from a freshwater habitat in China, and its phylogenetic placement based on molecular data. Cryptogamie Mycologie 28: 291.
Canto, E.S.M., Cortez, A.C.A., Monteiro, J.S., Barbosa, F.R., Zelski, S., and Souza, J.V.B.D. 2020. Composition and diversity of fungal decomposers of submerged wood in two lakes in the Brazilian Amazon State of Para. International Journal of Microbiology 2020: 6582514. Crossref
Cavalier-Smith, T. 1998. A revised six-kingdom system of life. Biological Reviews 73: 203–266. Crossref
Conran, J.G., Bannister, J.M., Reichgelt, T., and Lee, D.E. 2016. Epiphyllous fungi and leaf physiognomy suggest an ever-wet humid mesothermal (subtropical) climate in the late Eocene of southern New Zealand. Palaeogeography, Palaeoclimatology, Palaeoecology 452: 1–10. Crossref
Dix, N.J. and Webster, J.W. 1995. Fungal Ecology. 549 pp. Springer, Dordrecht. Crossref
Elsik, W.C. 1976. Microscopic fungal remains and Cenozoic palynostratigraphy. Geoscience and Man 15: 115–120. Crossref
Elsik, W.C. and Jarzen, D.M. 2009. New species of the Late Cenozoic fungal form genus Mediaverrunites Jarzen & Elsik 1986 ex Nandi & Sinha 2007. Palynology 33: 99–104. Crossref
Eriksson, O. 1966. On Anthostomella Sacc., Entosordaria (Sacc.) Höhn. and some related genera (Pyrenomycetes). Svensk Botanisk Tidskrift 60: 315–324.
Gessner, M.O., Gulis, V., Kuehn, K.A., Chauvet, E., and Suberkropp, K. 2007. Fungal decomposers of plant litter in aquatic ecosystems. In: C. Kubicek and I. Druzhinina (eds.), Environmental and Microbial Relationships. The Mycota 4: 301–324.
Goh, T.K. 1997. Tropical freshwater hyphomycetes. In: K.D. Hyde (ed.), Biodiversity of Tropical Microfungi, 189–227. Hong Kong University Press, Hong Kong.
Grossart, H.P. and Rojas-Jimenez, K. 2016. Aquatic fungi: targeting the forgotten in microbial ecology. Current Opinion in Microbiology 31: 140–145. Crossref
Gulis, V., Su, R., and Kuehn, K.A. 2019. Fungal decomposers in freshwater environments. In: C. Hurst (ed.), The Structure and Function of Aquatic Microbial Communities. Advances in Environmental Microbiology 7: 121–155. Crossref
Hawksworth, D.L. and Lücking, R. 2017. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiology Spectrum 5 (4): FUNK-0052-2016. Crossref
Hennings, P. 1902. Fungi S. Paulenses II. a cl. Puttemans Collecti. Hedwigia 41: 295–311.
Ho, W.H., Hyde, K.D. and Hodgkiss, I. J. 2001. Fungal communities on submerged wood from streams in Brunei, Hong Kong, and Malaysia. Mycological Research 105: 1492–1501. Crossref
Huang, S.K., Hyde, K.D., Mapook, A., Maharachchikumbura, S.S., Bhat, J.D., McKenzie, E.H., Jeewon, R., and Wen, T.C. 2021. Taxonomic studies of some often over-looked Diaporthomycetidae and Sordariomycetidae. Fungal Diversity 111: 443–572. Crossref
Hyde, K.D. 1988. Studies on the tropical marine fungi of Brunei. Botanical Journal of the Linnean Society 98: 135–151. Crossref
Hyde, K.D. 1995. Tropical Australasian fungi. VII. New genera and species of ascomycetes. Nova Hedwigia 61: 119–140.
Jarzen, D.M. and Elsik, W.C. 1986. Fungal palynomorphs recovered from recent river deposits, Luangwa Valley, Zambia. Palynology 10: 35–60. Crossref
Jones, E.B.G., Sakayaroj, J., Suetrong, S., Somrithipol, S., and Pang, K.L. 2009. Classification of marine Ascomycota, anamorphic taxa and Basidiomycota. Fungal Diversity 35: 1–187.
Kasiński, J.R. and Słodkowska, B. 2016. Factors controlling Cenozoic anthracogenesis in the Polish Lowlands. Geological Quarterly 60: 959–974.
Kirk, P.M., Cannon, P.F., Minter, D.W., and Stalpers, J.A. 2008. Ainsworth & Bisby’s Dictionary of the Fungi. 10th Edition. 771 рр. CAB International, Wallingford. Crossref
Kohlmeyer, J. and Kohlmeyer, E. 1979. Marine Mycology. The Higher Fungi. 704 pp. Academic Press, New York.
Krings, M., Taylor, T.N., and Dotzler, N. 2012. Fungal endophytes as a driving force in land plant evolution: evidence from the fossil record. In: D. Southworth (ed.), Biocomplexity of Plant-Fungal Interactions, First Edition, 5–28. John Wiley & Sons, Inc. Crossref
Krings, M., Taylor, T.N., and Harper, C.J. 2017. Early fungi: Evidence from the fossil record. In: J. Dighton and J.F. White (eds.), The Fungal Community, Its Organization and Role in the Ecosystem, 3rd Edition, 37–52. CRC Taylor and Francis, Boca Raton. Crossref
Krzyszkowski, D. and Winter, H. 1996. Stratigraphic position and sedimentary features of the Tertiary Uppermost Fluvial Member in the Kleszczów Graben, central Poland. Annales Societatis Geologorum Poloniae 66: 17–33.
Lange, R.T. 1978. Southern Australian Tertiary epiphyllous fungi, modern equivalents in the Australasian region, and habitat indicator value. Canadian Journal of Botany 56: 532–541. Crossref
Loron, C.C., François, C., Rainbird, R.H., Turner, E.C., Borensztajn, S., and Javaux, E.J. 2019. Early fungi from the Proterozoic era in Arctic Canada. Nature 570: 232–235. Crossref
Macphail, M.K. 2006. Pollen analysis of soil samples, 109–113 George Street, Parramatta. In: M. Casey and T. Lowe (eds.), Archaeological Investigation Report, Section 8.4, Pollen Report Vol. 2, 1–60. Casey & Lowe Pty Ltd., Marrickville.
Manoharachary, C., Agarwal, D.K., and Krishna Rao, N. 2003. Beltraniomyces, a new genus of Dematiaceous Hyphomycetes from India. Indian Phytopathology 56 (4): 418–421.
Moore, R.T. 1980. Taxonomic proposals for the classification of marine yeasts and other yeast-like fungi including the smuts. Botanica Marina 23: 361–373.
Moore, P.D., Webb, J.A., and Collinson, M.E. 1991. Pollen Analysis. 216 pp. Blackwell Scientific Publications, Oxford.
Muller, J. 1959. Palynology of Recent Orinoco delta and shelf sediments; reports of the Orinoco Shelf Expedition, Volume 5. Micropaleontology 5: 1–32. Crossref
MycoBank 2021. MycoBank Database, available at http://www.mycobank.org
Nandi, B. and Sinha, A. 2007. Validation of the Miocene fungal spore Mediaverrunites from Mizoram, India. Palynology 31: 95–100. Crossref
Nannizzi, A. 1934. Repertorio sistematico dei miceti dell’ uomo e degli animali. Trattato di micopatologia umana 4. 557 pp. Meini, Siena.
Nuñez Otaño, N., di Pasquo, M., and Bianchinotti, M.V. 2017. The occurrence of Potamomyces palmarensis sp. nov. in the Late Holocene of El Palmar National Park (Colón, Entre Ríos, Argentina) and transfer of fossil species of Mediaverrunites to Potamomyces. Palynology 41: 267–277. Crossref
Nylander, W. 1859. Herbarium musei Fennici. Förteckning öfver Finska Musei Växtsamling utgifven af Sällskapet pro Fauna et Flora Fennica, och uppgjord af W. Nylander och T. Sælan, etc. 112 pp. Finska litteratur-sällskapets tryckeri, Helsingfors.
O’Keefe, J.M.K. 2017. Fungal palynomorphs from the Miocene Heath Formation, Tumbes Province, Perú. Palynology 41: 309–326. Crossref
Penzig, O. 1882. Funghi agrumicoli. Contribuzione allo studio dei funghi parassiti degli agrumi. Michelia 2 (8): 385–508.
Peryt, D., Garecka, M., and Peryt, T.M. 2021. Foraminiferal and calcareous nannoplankton biostratigraphy of the upper Badenian–lower Sarmatian strata in the SE Polish Carpathian Foredeep. Geological Quarterly 65: 18. Crossref
Peryt, D., Gedl, P., and Peryt, T.M. 2020. Marine transgression(s) to evaporite basin: The case of middle Miocene (Badenian) gypsum in the Central Paratethys, SE Poland. Journal of Palaeogeography 9: 16. Crossref
Piwocki, M. and Ziembińska-Tworzydło, M. 1997. Neogene of the Polish Lowlands—litostratigraphy and pollen-spore zones. Geological Quarterly 41: 21–40.
Rajeshkumar, K.C., Crous, P.W., Groenewald, J.Z., and Seifert, K.A. 2016. Resolving the phylogenetic placement of Porobeltraniella and allied genera in the Beltraniaceae. Mycological Progress 15: 1119–1136. Crossref
Rao, M.R., Sahni, A., Rana, R.S., and Verma, P. 2013. Palynostratigraphy and depositional environment of Vastan lignite mine (Early Eocene), Gujarat, western India. Journal of Earth System Science 122: 289–307. Crossref
Sancay, R.H. 2014. The occurrence of Mediaverrunites in the Upper Miocene of the Black Sea, Turkey. Palynology 38: 28–37. Crossref
Schaumann, K. 1972. Biconiosporella corniculata nov. gen. et nov. spec., ein holzbesiedelnder Asomycet des marinen Litorals. Veröffentlichungen des Instituts für Meersforschung in Bremerhaven 14: 25–44.
Schlütz, F. and Shumilovskikh, L.S. 2013. On the relation of Potamomyces armatisporus to the fossil form-type Mediaverrunites and its taxonomical and ecological implications. Fungal Ecology 6: 309–315. Crossref
Shearer, C.A. and Raja, H.A. 2010. Freshwater Ascomycetes Database, available at http://fungi.life.illinois.edu/
Shearer, C.A., Descals, E., Kohlmeyer, B., Kohlmeyer, J., Marvanová, L., Padgett, D., Porter, D., Raja, H.A., Schmit, J.P., Thorton, H.A., and Voglymayr, H. 2007. Fungal biodiversity in aquatic habitats. Biodiversity and Conservation 16: 49–67. Crossref
Śliwiński, M., Bąbel, M., Nejbert, K., Olszewska-Nejbert, D., Gąsiewicz, A., Schreiber, B.C., Be-Nowitz, J.A., and Layer P. 2012. Badenian–Sarmatian chronostratigraphy in the Polish Carpathian Foredeep. Palaeogeography, Palaeoclimatology, Palaeoecology 326: 12–29. Crossref
Tsui, C.K., Baschien, C., and Goh, T.K. 2016. Biology and Ecology of Freshwater Fungi. In: D.W. Li (ed.), Biology of Microfungi, 285–313. Springer, Cham. Crossref
Turland, N.J., Wiersema, J.H., Barrie, F.R., Greuter, W., Hawksworth, D.L., Herendeen, P.S., Knapp, S., Kusber, W.-H., Li, D.-Z., Marhold, K., May, T.W., McNeill, J., Monro, A.M., Prado, J., Price, M.J., and Smith, G.F. (eds.) 2018. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. 254 pp. Koeltz Botanical Books, Glashütten. Crossref
Van Geel, B. and Aptroot, A. 2006. Fossil ascomycetes in Quaternary deposits. Nova Hedwigia 82: 313–330. Crossref
Widera, M., Bechtel, A., Chomiak, L., Maciaszek, P., Słodkowska, B., Wachocki, R., Worobiec, E., Worobiec, G., and Zieliński, T. 2021. Palaeoenvironmental reconstruction of the Konin Basin (central Poland) during lignite accumulation linked to the mid-Miocene climate optimum. Palaeogeography, Palaeoclimatology, Palaeoecology 568: 110307. Crossref
Wijayawardene, N.N., Hyde, K.D., Lumbsch, H.T., Liu, J.K., Maharachchikumbura, S.S., Ekanayaka, A.H., Tian, Q., and Phookamsak, R. 2018. Outline of Ascomycota: 2017. Fungal Diversity 88: 167–263. Crossref
Wijayawardene, N.N., Hyde, K.D., Rajeshkumar, K.C., Hawksworth, D.L., Madrid, H., Kirk, P.M. et al. 2017. Notes for genera: Ascomycota. Fungal Diversity 86: 1–594.
Wilczyński, R. 1992. Dotychczasowe wyniki badań podstawowych serii poznańskiej w świetle geologiczno-inżynierskich problemów prowadzenia robót górniczych w KWB “Bełchatów”. Acta Universitatis Wratislaviensis 1354, Prace Geologiczno-Mineralogiczne 24: 91–108.
Wong, M.K., Goh, T.K., Hodgkiss, I.J., Hyde, K.D., Ranghoo, V.M., Tsui, C.K., Ho, W.H., Wong, W.S.W., and Yuen, T.K. 1998. Role of fungi in freshwater ecosystems. Biodiversity & Conservation 7: 1187–1206. Crossref
Worobiec, E. and Worobiec, G. 2016. Miocene palynoflora from the KRAM-P 218 leaf assemblage from the Bełchatów Lignite Mine (Central Poland). Acta Palaeobotanica 56: 499–517. Crossref
Worobiec, E., Widera, M., Worobiec, G., and Kurdziel, B. 2021. Middle Miocene palynoflora from the Adamów lignite deposit, central Poland. Palynology 45: 59–71. Crossref
Worobiec, E., Worobiec, G., and Kasiński, J.R. 2022a. Decline of Neogene lignite formation as a result of vegetation and climate changes reflected in the middle Miocene palynoflora from the Ruja lignite deposit, SW Poland. Review of Palaeobotany and Palynology 298: 104593. Crossref
Worobiec, G. 2003. New fossil floras from Neogene deposits in the Bełchatów Lignite Mine. Acta Palaeobotanica Supplement 3: 3–133.
Worobiec, G. and Lesiak, M. 1998. Plant megafossils from the Neogene deposits of Stawek-1A (Bełchatów, Middle Poland). Review of Palaeobotany and Palynology 101: 179–208. Crossref
Worobiec, G. and Szynkiewicz, A. 2016. Neogene wetland vegetation based on a leaf assemblage from the Bełchatów Lignite Mine (Central Poland). Acta Palaeobotanica 56: 441–497. Crossref
Worobiec, G. and Worobiec, E. 2017. Epiphyllous fungi from Miocene deposits of the Bełchatów Lignite Mine (Central Poland). Mycosphere 8: 1003–1013. Crossref
Worobiec, G., Worobiec, E., and Kasiński, J. 2008. Plant assemblages of the drill cores from the Neogene Ruja lignite deposit near Legnica (Lower Silesia, Poland). Acta Palaeobotanica 48: 191–275.
Worobiec, G., Worobiec, E., and Liu, Y.C. 2018. Fungal remains from late Neogene deposits at the Gray Fossil Site, Tennessee, USA. Mycosphere 9: 1014–1024. Crossref
Worobiec, G., Worobiec, E., and Widera, M. 2022b. Middle Miocene wetland fungi from the Adamów Lignite Mine, central Poland. Historical Biology 34 (5): 841–856. Crossref
Acta Palaeontol. Pol. 67 (3): 737–744, 2022
https://doi.org/10.4202/app.00976.2022