

Reply to Bauer et al. (2022)
CHRISTOPHER R.C. PAUL
What’s in a name? That which we call a rose
By any other name would smell as sweet.
William Shakespeare,
“Romeo and Juliet”, act II, scene ii
Cladistic analyses will be inaccurate if based on inaccurate data. Most blastozoan taxa were defined before cladistics was proposed. Redescription of precladistic taxa should be a priority. Cladistics recognizes similarities between taxa, not differences. Differences always exist, so the two approaches are asymmetrical. Any suggested similarity or homology needs testing, including output of computer-based analyses. New interpretations of plate homology between glyptocystitoid and hemicosmitoid rhombiferans had already rendered input data obsolete before the pan-dichoporite paper was published. Universal Elemental Homology (UEH) is not a scientific hypothesis. It makes no testable predictions; no system of naming plates can.
Our approaches are so different that it is pointless to answer specific details, other than to confirm the factual error concerning Macurdablastus Broadhead, 1984. In Paul (2021: table 1: column 2) the family for Macurdablastus was left blank since neither Broadhead (1984) nor Bauer et al. (2019) assigned it to any family. The type setters took this to mean that the family Stephanocrinidae included both Stephanocrinus Conrad, 1842 and Macurdablastus. I did not notice this in the proofs but I am glad to confirm that Macurdablastus is not a coronate. I still believe it to be a eublastoid, but there is not space to argue that point here.
Abbreviations.—A–E, Carpenter’s (1884, 1891) ambulacral symbols; CO, COO, circumoral plates; PO, POO, perioral plates; UEH, Universal Elemental Homology (Sumrall 2010).
Background
If the Echinodermata is monophyletic, all its classes are related. These phylogenetic connections were established hundreds of millions of years ago; we are trying to find them. This can best be done using cladistsics, but cladistics is not a magic bullet. The biggest problem I see is that apart from a general rule that the more characters used the better, there is no guidance about how to select characters or which characters are useful and which not. Obviously using different characters will produce different phylogenies. So, cladistic analyses produce temporary phylogenetic hypotheses that themselves need testing.
Furthermore, cladistics is a relatively recent concept. Most blastozoan taxa were described without any reference to cladistics because it had not been thought of. Eight of the 14 genera in Paul (2021: table 1) were described in the 19th century. Thus, there is no guarantee that any major taxon (including the 20 extinct clades) is a valid concept or that the characters on which genera and species were defined are cladistically significant. Indeed, a case can be made for redescribing all these genera in cladistic terms.
Recently, such redescription has been my major goal and my personal experience reinforces the need to get accurate modern descriptions, especially of genera defined in the 19th century. Cystoblastus Volborth, 1867, is an excellent example. Comparison of Kesling’s (1968) plate diagrams with photographs of a real specimen on the “Geocollections of Estonia” website showed immediately that Kesling’s (1968) diagrams were inaccurate. Kesling (1968) showed a closed infralateral circlet (that is all the plates in contact) whereas the specimen showed that the infralateral circlet was interrupted in three places. Subsequent investigation revealed that all published descriptions and illustrations were based on Volborth’s (1867) original figures which were equally inaccurate. Whatever the inadequacies of my cladistic analysis, if such errors were included in a character analysis, the resulting phylogeny is unlikely to be accurate. So, at the moment I am trying to publish accurate descriptions of key taxa so that data input will be accurate. Others, who are better qualified can undertake the phylogenetic analyses.
In attempting to discover phylogenetic connections, similarities outweigh differences. Cladistics is based on the recognition of shared derived characters. Seeking similarities versus differences is an asymmetrical process. Differences always exist. Look closely enough and you will find them. Even identical twins have distinguishing marks. Furthermore, where connections do exist, their recognition will be more difficult if one emphasizes differences. Seeking similarities, on the other hand, helps recognize possible phylogenetic transformations between morphologically different structures that might be homologous. To me, this is a positive outcome and additional evidence should be sought that either confirms or refutes the suggested homology; that is possibilities should be tested.
I accept that my cladistic analysis in the pan-dichoporite paper (Paul 2021) was inadequate, to say the least. I am grateful for the improved versions. Regrettably, the data set used in both analyses is already out of date. Research on Cystoblastus (Paul and Toom 2021) not only enabled us to redescribe it accurately, but also to suggest possible homologies between the four lower plate circlets of glyptocystitoid rhombiferans and the three lower plate circlets of hemicosmitoid rhombiferans. Thus, if I were doing the same analysis now, I would include different characters and code others differently, thus inevitably producing a different phylogeny. This is a classic example of how new facts or interpretations can be used to test previous phylogenetic hypotheses.
Paul and Toom’s (2021) results have further ramifications. Previously, the alternative plate arrangements in the two rhombiferan superfamilies were regarded as fundamentally different and although the plate circlets had the same names the plates were not homologous. Now, we have suggested that all the plates are homologous, merely re-arranged in three rather than four circlets (or vice versa). The plates begin to grow before the respiratory dichopores that form the rhombs develop. Thus, it seems likely that a rhomb developed across the B2:IL2 plate suture in glyptocystitoids is homologous with a rhomb across the same suture in hemicosmitoids. If the plates are homologous, surely the rhomb must be too? This in turn, means the dichopores (endothecal canals) that form the rhombs in both superfamilies are probably homologous. Finally, that raises the possibility that all endothecal canals shared between two thecal plates might be homologous. All these ideas need testing, but that cannot be done unless someone suggests them in the first place.
Universal Elemental Homology
I am accused of ignoring UEH so I had better explain why. I doubt if my critics will be happy with the result. First, UEH is not a scientific hypothesis. It does not make testable predictions any more than any alternative plate terminology can. Rather, it is a series of assertions about the homology of plates in blastozoan and other early echinoderms. The “universal” aspect of UEH appears to be the hope that all echinoderm researchers will adopt UEH terminology. The aspiration of a universally-accepted, consistent terminology for homologous plates seems laudable, but a better approach might have been to seek consensus among echinoderm researchers first. Furthermore, an agreed terminology is likely to discourage alternative hypotheses on which scientific progress depends.
My second criticism of UEH concerns its application, which is decidedly selective. Only those examples that agree with the UEH are illustrated. Those that do not are either ignored (e.g., the family Aristocystitidae), their existence denied (e.g., the genus Brightonicystis Paul, 1971), or the morphology is forced to fit into the precepts of UEH (e.g., the oral plating of sphaeronitid diploporites). Alternative possible interpretations are not evaluated. Proponents of UEH emphasize the repeated existence of seven plates in the oral area. Parsley (1990) has shown that aristocystitid diploporites have eight plates forming the oral frame. Paul (2017: fig. 7) showed that this pattern is widespread within the family Aristocystitidae. So, my first challenge to proponents of UEH is to use it to explain the homologies of all eight aristocystitid oral plates.
Secondly, Sheffield and Sumrall (2015) and Sheffield et al. (2018) ignored the genus Brightonicystis when discussing the diploporite family Holocystitidae. Sheffield and Sumrall (2017: 756) denied that it exists. They freely admitted that they have never seen any specimens. This is particularly ironic, as, in my view, Sheffield et al. (2018) described a second species of Brightonicystis. Figure 1 presents their interpretation of the oral plating in “Holocystites” salmoensis Sheffield, Ausich, and Sumrall, 2018. Sheffield and Sumrall (2019: 743) stated that previous interpretations of Brightonicystis were “highly unlikely because it would prove to be the only echinoderm taxon known with more than seven oral plates”. This despite the fact that their own interpretation of the oral plating of “H.” salmoensis has at least ten oral plates (see below) and the Aristocystitidae consistently have eight. By this stage one could be forgiven for concluding that UEH has become a barrier to understanding echinoderm morphology and phylogeny, rather than an aid.
Finally, Sumrall (2010: 270) defined oral plates according to the UEH as “those plates and their descendant homologues that border the peristomial opening, are interradially positioned, and bear the proximal food grooves along their adjacent sutures”. Later, when the radially-positioned oral plates of sphaeronitid diploporites were “discovered”, it was claimed that sphaeronitid orals rotated 36° clockwise with respect to other blastozoans (e.g., Sumrall 2015: 170; Sheffield and Sumrall 2017: 746). No explanation was given as to how this came about. Indeed, rotating the plates clockwise does not derive the sphaeronitid condition in which ambulacral facets are present on plates that form the peristome border. Interradial orals never bear ambulacral facets. Furthermore, how do the food grooves move from lying along the sutures between interradial orals to a central position on radial orals? The suggested homology cannot be rejected outright, but it requires more changes than just rotating the “oral” circlet. A better explanation exists consistent with more facts (see next section). If I am right, then UEH is forcing the facts to fit the theory, not using exceptions to test any hypothesis.
An alternative approach
Figure 2 illustrates the oral areas of two holocystitid species (2a, 2c) compared with a sphaeronitid (2b) and parasphaeronitid (2d). There are numerous similarities between all four diagrams, but the most obvious difference is that the holocystitids have six interradial plates forming the mouth frame that are absent in the sphaeronitid and parasphaeronitid. In revising the holocystitids (Paul 1971) I was aware of these differences and thought that plates which were consistently radial in position were unlikely to be homologous with plates that were equally consistently interradial. So, to avoid implying unwarranted homologies, I introduced the term periorals for the interradial orals of holocystitids and circumorals for the radial orals of sphaeronitids. These terms are useful in discussing oral plate homologies.
The first point to make is that in the holocystitid plate diagrams (Fig. 2A, C), whatever they are called, circumoral and perioral plates cannot be homologous, according to the conjunction rule of Patterson (1988), because both occur in the same organism. Secondly, it becomes clear that the plate labelled CO6 in previous interpretations of sphaeronitids is homologous with plate PO7 of holocystitids. Furthermore, compare Fig. 1 with Figs. 2A, B. The outer plate circlet in Fig. 1 has exactly the same plate arrangement as seen in Trematocystis Jaekel, 1899 (Fig. 2C) except that Trematocystis lacks ambulacrum A and the other four facets are entirely within a single “facetal” plate. This strongly suggests that the outer plates in both genera are homologous, and again by the conjunction rule the plates of the inner circlet cannot be homologous with those of the outer circlet. In which case there is clear evidence that not all the plates inside the “facetal” circlet are preserved in all holocystitid genera. So, if some inner circlet plates can be lost between “Holocystites” salmoensis and other holocystitids, is it not more likely that all the inner circlet could also be lost? To me it is more probable that the sphaeronitid condition results from failure to develop all perioral plates.
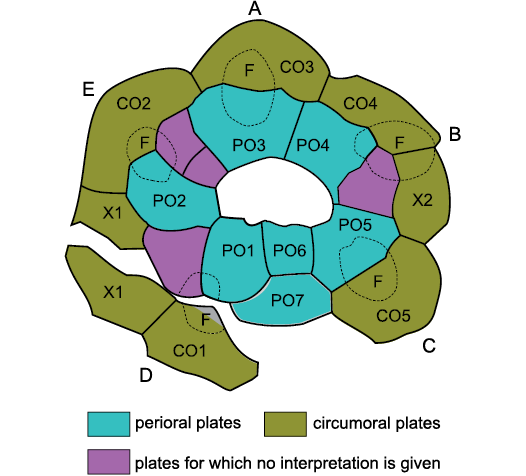
Fig. 1. Oral plating in “Holocystites” salmoensis Sheffield, Ausich, and Sumrall, 2018. A–E, ambulacra A–E (Carpenter 1884, 1891), CO1–CO5 facet-bearing circumorals, F ambulacral facets (dotted outlines), PO1–PO7 perioral plates, X1 and X2 extra lateral plates in the circumoral circlet. X1 has been split and CO1 detached from the other orals during preservation.
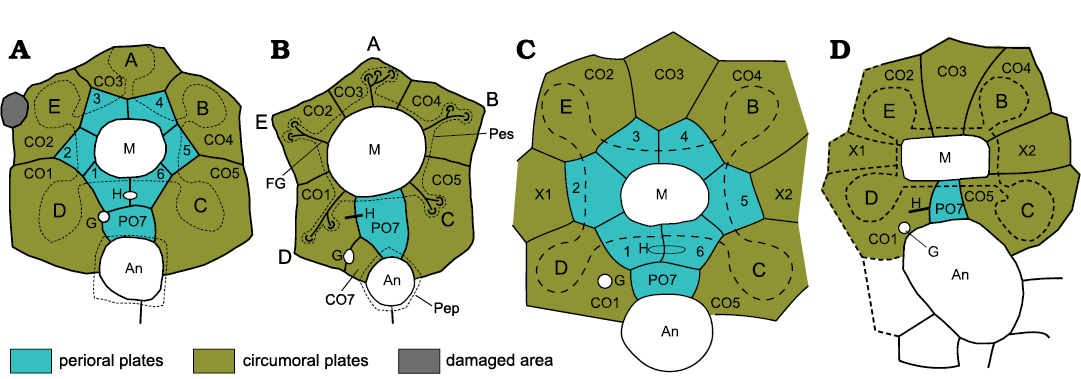
Fig. 2. Oral plating in holocystitids (A, C), sphaeronitids (B), and parasphaeronitids (D) to show similarities and differences. In holocystitids the mouth (M) is surrounded by six interradial plates (periorals, 1–6), two in the CD interambulacrum which share the hydropore (H) and one each in the other interambulacra. A seventh perioral (PO7) occurs in the CD interambulacrum and borders the anus (An). In Pustulocystis pentax Paul, 1971 (A) the perioral circlet is surrounded by five radial (circumoral) plates each of which bears a single ambulacral facet. In Eucystis angelini Regnéll, 1945 (B) six plates border the mouth, five of which are radial, bear ambulacral facets and are interpreted as circumorals, the sixth lies in the CD interradius and is interpreted as plate PO7. A small additional plate (CO7) shares the gonopore (G) with CO1. In Trematocystis globosus (Miller, 1878) (C) the perioral circlet is identical to that of Pustulocystis pentax, but is surrounded by seven circumorals (CO1–CO5, X1, X2) plus PO7. Trematocystis globosus also lacks the facet for ambulacrum A. In Pachycystis norvegica Bockelie, 1984 (D), as in Eucystis, the mouth frame of six periorals is absent, but the eight other plates are identical to those in the circumoral circlet of Trematocystis, including PO7. Pachycystis norvegica also lacks the facet in ambulacrum A. Two points are important. In holocystitids two plate circlets surround the mouth which, whatever they are called, cannot be homologous because they occur in the same individual. Secondly, if one accepts the plate homologies suggested here, the solitary interradial oral of sphaeronitids and parasphaeronitids must be PO7, not CO6 as it was originally labelled (Paul 1973: fig. 10B). A–E, ambulacra A–E; Pep, periproct (anal) border; Pes, peristome (mouth) border. Not to scale.
Does recognizing circum- and peri-oral plates help in interpreting oral plating that the UEH does not interpret? Most aristocystitid genera have four ambulacra and eight plates forming the oral frame (Paul 2017: 589, fig. 7). Four plates are directly radial have food grooves centrally and were accepted as circumorals. The other four are closer to the centre of the mouth, interradial and accepted as periorals. This is compatible with the fact that in some pentaradial blastozoans with a 2-1-2 pattern of ambulacra, only the same four periorals form the mouth frame. So, I had no problem in interpreting the oral plating of aristocystitids using the concept of circum- and peri-oral plates.
As usual, echinoderms have not read the literature and refuse to conform to mere human concepts of homology. Oretanocalix Guttiérrez-Marco, 2000, the only aristocystitid genus with all five ambulacra, also has eight plates forming its mouth frame (Fig. 3A). So, if facet-bearing orals are circumorals and those without facets are periorals, then Oretanocalix (and all other aristocystitids) have five circumorals and three periorals. This should not be unexpected. All tetraradiate diploporites lack ambulacrum A, but retain the associated circumoral (CO3). Why should aristocystitids be different? Now, examination of the plate diagram for Oretanocalix shows an unexpected pattern; neither circumoral plate in ambulacra B or D reaches the inner margin of the peristome, just as in “Holocystites” salmoensis (Fig. 1). I think this is another example of a “B D different” pattern of oral plating and reflects Lovén’s law (Paul and Hotchkiss 2020).
Recently, I have been testing a new, dynamic, plate terminology that reflects ambulacral growth. In this new scheme, plates around the mouth are attributed to their respective ambulacra and numbered according to the order of their growth. So, the first plate in ambulacrum A is A1, the second A2, etc. The oral plating in Oretanocalix, and other aristocystitid genera, may therefore be interpreted as in Fig. 3B, where BC1 is the first plate in the shared BC ambulacrum before division and DE1 similarly the first plate in the shared DE ambulacrum before division. Thus, the exclusion of B1 and D1 from the oral frame reflects the fact that when the shared ambulacra divided, ambulacrum C developed before ambulacrum B and similarly ambulacrum E before ambulacrum D. It remains to be seen whether or not this new system of plate numbering is of more use than the old, but I will happily abandon the old terminology if the new is a better explanation.
The main point here is that using the terms circumoral and perioral for radial and interradial orals, respectively enabled an interpretation of aristocystitid oral plating. The first interpretation was later shown to be inaccurate and modified. Although this approach reveals potential plate homologies, it does not explain how they came about. Consideration of growth patterns potentially provides an explanation of puzzling aspects of aristocystitid oral plating as a direct result of the order in which the plates were added and the lateral ambulacra divided. Repeated re-examination of previous interpretations improves our understanding.
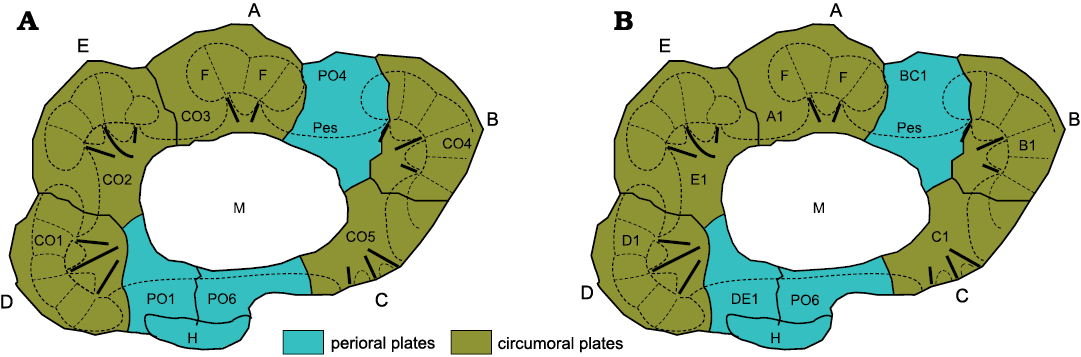
Fig. 3. Alternative interpretations of the oral plating of Oretanocalix sp. A. Oral plating in the aristocystitid Oretanocalix sp. using circumoral and perioral terminology The large mouth (M) is surrounded by eight plates, five of which bear ambulacral facets and are interpreted as circumorals (CO1–CO5). The other three are interpreted as periorals, PO4, PO6, and PO1. The latter two share the large hydropore (H). Note the although CO1 and CO4 reach the outer margin of the peristome (Pes), they do not reach its inner border. A–E, ambulacra A–E; F, ambulacral facet; bold black lines, food grooves within the peristome. Ambulacrum A has one facet less than ambulacra B–E. B. Oral plating in the aristocystitid Oretanocalix using dynamic growth terminology whereby PO4 and PO1 are regarded as the first plates formed in the combined B+C and D+E ambulacra, respectively. A1, B1, C1, etc., are the first plates formed in separate ambulacra A, B, C, etc.
Final comments
The fact that my critics concentrate on methods confirms my previous suspicion that they are more interested in techniques and terminology than in the fossils themselves. Sure, I made a pig’s ear of the cladistic analysis. I now regret including it (and I would have saved money had I left it out!). As my critics demonstrate, an inadequate analysis can be corrected and I am genuinely grateful for their efforts. So, read the paper without the cladistics section. Does this alter in any way the facts or suggestions included? Is it no longer true that Rhombifera Barrande, 1867, is the only glyptocystitid genus with ambulacral facets developed on radial rather than oral plates? Cambrocrinus Orłowski, 1968, has ornament on its proximal stem that spirals down the stem, as does Macrocystella Callaway, 1877. In Macrocystella this reflects the orientation of synarthrial articulations that also spiral down the stem making it extremely flexible. Does the absence of cladistic analysis make the inference that Cambrocrinus also had synarthrial articulations in its proximal stem any less likely? To be sure it needs confirmation, but note this is a testable hypothesis. Would Kesling’s (1962) exceptionally perceptive interpretation of the oral plating of Rhombifera become invalid if my cladistic analysis had been omitted? He made his interpretation without reference to cladistics.
Like Mary Bennett in Pride and Prejudice, I may draw this useful lesson. My scientific reputation will not be improved by publishing more inadequate cladistic analyses. Best leave them to those who understand what they are doing. Indeed, in an ideal world I would love to collaborate with a competent cladist. It is truly ironic that this particular cladistic analysis illustrates the biggest problem I see in cladistics: the choice of characters. The next project I pursued was the redescription of Cystoblastus (see Paul and Toom 2021). So, when the pan-dichoporite paper (Paul 2021) was at proof stage, I already knew that if I undertook the cladistic analysis again I would include additional characters and code others differently. It is too early to say whether or not the new homology scheme of rhombiferan plates is an improvement, but it would be useful to compare the two alternatives.
Just as natural selection requires a constant supply of new mutations from which to select, science requires new hypotheses to test. Furthermore, if a new hypothesis passes our tests, it is not proved; we can only disprove hypotheses. Progress is only made by hypotheses which fail tests. Thus, it seems to me a basic requirement to be a scientist is the willingness to admit that you got it wrong. New hypotheses may come from new discoveries or new interpretations. They can be tested by cladistic analysis, but the results of such analyses themselves need further testing. The process is continuous. I am just trying to ensure input data are accurate and to draw attention to potential similarities or homologies between taxa that also need testing. The pan-dichoporite paper (Paul 2021) predicts that Rhombifera Barrande, 1867 should be re-examined next.
Acknowledgements.—I thank Juan Carlos Guttiérrez Marco for agreeing to my inclusion of figures 3a, b from our unpublished research on Oretanocalix Guttiérrez Marco, 2000.
References
Barrande, J. 1867. Systême Silurien du centre de la Bohême. Première Partie: Recherches paléontologiques. Vol. 3, Classe des Mollusques. Ordre des Ptéropodes. xiv+179 pp. W. Waagen, Prague.
Bauer, J.E., Sheffield, S.L., Waters, J.A., and Sumrall, C.D. 2022. Echinoderm model systems, homology, and phylogenetic inference: Comment and reply to Paul (2021). Acta Palaeontologica Polonica 67: 465–468. Crossref
Bauer, J., Waters, J.A., and Sumrall, C.D. 2019. Redescription of Macurdablastus and redefinition of Eublastoidea as a clade of Blastoidea (Echinodermata). Palaeontology 62: 1003–1013. Crossref
Bockelie, J.F. 1984. The Diploporita of the Oslo Region, Norway. Palaeontology 27: 1–68.
Broadhead, T.W. 1984. Macurdablastus, a Middle Ordovician blastoid from the southern Appalachians. University of Kansas Paleontological Contributions Paper 110: 1–9.
Callaway, C. 1877. On a new area of Upper Cambrian rocks in south Shropshire, with a description of a new fauna. Quarterly Journal of the Geological Society of London 33: 652–672. Crossref
Carpenter, P.H. 1884. Report upon the Crinoidea collected during the voyage of HMS Challenger during the years 1873–76, Part 1. General morphology with descriptions of the stalked crinoids. Reports of the Scientific Results of the Voyage of HMS Challenger, Zoology 11: 1–442. Crossref
Carpenter, P.H. 1891. On certain points of the morphology of the Cystidea. Journal of the Linnean Society (Zoology) 34: 1–52. Crossref
Conrad, T.A. 1842. Observations on the Silurian and Devonian systems of the Unites States, with descriptions of new organic remains. Journal of the Academy of Natural Sciences, Philadelphia 8: 228–280.
Gutiérrez-Marco, J.C. 2000. Revisión taxonómica de “Echinosphaerites” murchisoni Verneuil y Barrande, 1855 (Echinodermata, Diploporita) del Ordovícico Medio centroibérica (España). Geogaceta 27: 83–86.
Jaekel, O. 1899. Stammesgeschichte der Pelmatozoen. 1. Thecoidea und Cystoidea. 442 pp. Julius Springer, Berlin. Crossref
Kesling, R.V. 1962. An interpretation of Rhombifera bohemica Barrande, 1867, an unusual hydrophoridean cystoid. Contributions from the Museum of Paleontology, University of Michigan 17: 277–289.
Kesling, R.V. 1968. Cystoids. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, Part S, Echinodermata 1 (1), S85–S267. Geological Society of America, Boulder and University of Kansas Press, Lawrence.
Miller, S.A. 1878. Descriptions of eight new species of Holocystites from the Niagara Group. Journal of the Cincinnati Society of Natural History 1: 129–136.
Orłowski, S. 1968. Upper Cambrian fauna of the Holy Cross Mountains. Acta Geologica Polonica 18: 257–291.
Parsley, R.L. 1990. Aristocystites, a recumbent diploporid (Echinodermata) from the Middle and Late Ordovician of Bohemia, ČSSR. Journal of Paleontology 64: 278–293. Crossref
Patterson, C. 1988. Homology in classical and molecular biology. Molecular Biology and Evolution 5: 603–625.
Paul, C.R.C. 1971. Revision of the Holocystities Fauna (Diploporita) of North America. Fieldiana: Geology 24: 1–166. Crossref
Paul, C.R.C. 1973. British Ordovician Cystoids, Part 1. Monographs of the Palaeontographical Society 127: 1–64.
Paul, C.R.C. 2017. Testing for homologies in the axial skeleton of primitive echinoderms. Journal of Paleontology 91: 582–603. Crossref
Paul, C.R.C 2021. New insights into the origin and relationships of blastoid echinoderms. Acta Palaeontologica Polonica 66: 41–62. Crossref
Paul, C.R.C. and Hotchkiss, F.H.C. 2020. Origin and significance of Lovén’s Law in echinoderms. Journal of Paleontology 94: 1089–1102. Crossref
Paul, C.R.C. and Toom, U. 2021. Cystoblastus and the origin of the Hemicosmitoida (Echinodermata, Blastozoa). Estonian Journal of Earth Sciences 21: 15–181. Crossref
Regnéll, G. 1945. Non-crinoid Pelmatozoa from the Paleozoic of Sweden. A taxonomic study. Meddelanden från Lunds Geologisk-Mineralogiska Institutionen 108: 1–255.
Sheffield, S.L. and Sumrall, C.D. 2015. A new interpretation of oral plating patterns of the Holocystites Fauna (Diploporita, Echinodermata. In: S. Zamora and I. Rabano (eds.), Progress in Echinoderm Palaeobiology. Cuadernos del Museo Geominero 19:159–162.
Sheffield, S.L. and Sumrall, C.D. 2017. Generic revision of the Holocystitidae of North America (Diploporita, Echinodermata) based on universal elemental homology. Journal of Paleontology 93: 755–766. Crossref
Sheffield, S.L. and Sumrall, C.D. 2019. The phylogeny of the Diploporita: a polyphyletic association of blastozoan echinoderms. Journal of Paleontology 91: 740–752. Crossref
Sheffield, S.L., Ausich, W.I., and Sumrall, C.D. 2018. Late Ordovician (Hirnantian) diploporitan fauna of Anticosti Island, Quebec, Canada: implications for evolutionary and biogeographic patterns. Canadian Journal of Earth Sciences 55: 1–7. Crossref
Sumrall, C.D. 2010. A model for elemental homology for the peristome and ambulacra in blastozoan echinoderms. In: L.G. Harris, S.A. Böttger, C.W. Walker, and M.P. Lesser (eds.), Echinoderms: Durham, 269–276. Taylor & Francis, London. Crossref
Sumrall, C.D. 2015. Understanding the oral area of derived stemmed echinoderms. In: S. Zamora and I. Rabano (eds.), Progress in Echinoderm Palaeobiology. Cuadernos del Museo Geominero 19: 169–173.
Volborth, A. von 1867. O tsistoblastah, novom rode morskih lilij ili krinoidej. 12 pp. Tipografiâ Imperatoskoj Akademii Nauk, St Petersburg.
Christopher R.C. Paul [glcrcp@bristol.ac.uk], School of Earth Sciences, University of Bristol, Wills Memorial Building, Bristol BS8 1RL, UK.
Received 12 March 2022, accepted 27 April 2022, available online 26 May 2022.
Copyright © 2022 C.R.C. Paul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Acta Palaeontol. Pol. 67 (2): 469–473, 2022
https://doi.org/10.4202/app.00995.2022