

Phylogenetic relationships in Nothofagus: The role of Antarctic fossil leaves
BÁRBARA VENTO, FEDERICO AGRAÍN, GABRIELA G. PUEBLA, and DIEGO PINZÓN
Vento, B., Agraín, F., Puebla, G.G., and Pinzón, D. 2023. Phylogenetic relationships in Nothofagus: The role of Antarctic fossil leaves. Acta Palaeontologica Polonica 68 (1): 175–183.
The Nothofagus (southern beech) has a rich fossil record and a number of living species distributed exclusively in the Southern Hemisphere. Many attempts have been made to clarify the phylogenetic relationships in Nothofagus but only a few works have included fossil specimens in a phylogenetic framework for a more accurate resolution. Fossil leaves play an important role in deciphering of the evolutionary processes and are a necessary complement in phylogenetic studies. Fossils of Nothofagus have been found in sediments of Antarctica, Australia, New Zealand, New Caledonia, Papua New Guinea, and South America. Here, we performed a phylogenetic analysis including fossils from these areas and examined the character evolution, especially those referred to the morphology of the leaf. Fossil leaves from Antarctica were revised and included in the analysis for the first time. Our results support the monophyly of the four currently recognized subgenera, and novel relationships between extinct and living taxa are discussed. Morphological features of fossil leaves were expressed differently, especially in the teeth shape, size, and secondary venation pattern, when compared to the extant taxa probably related to past climate conditions. The most ancient leaves were recorded in the Upper Cretaceous of Antarctica and placed in subgenera Lophozonia and Fuscospora. Brassospora and Nothofagus are younger clades with distinctive plesiomorphic leaf morphological features. The morphological leaf characters proposed herein, and the inclusion of a considerable number of fossils in our analysis allowed us to provide a study of the evolutionary history of Nothofagus with more precise resolution.
Key words: Nothofagaceae, Nothofagus, fossil, living, evolution, Cretaceous, Antarctica.
Bárbara Vento [bvento@mendoza-conicet.gov.ar, ORCID: https://orcid.org/0000-0002-5470-6290 ], and Federico A. Agraín [fagrain@mendoza-conicet.gov.ar, ORCID: https://orcid.org/0000-0001-7871-9355 ], Instituto Argentino de Investigaciones en Zonas Áridas, IADIZA, CCT-CONICET, Adrián Ruíz Leal s/n, Parque General San Martín, 5500 Mendoza, Argentina.
Gabriela G. Puebla [gpuebla@mendoza-conicet.gov.ar, ORCID: https://orcid.org/0000-0002-0084-2422 ] and Diego Pinzón [dpinzon@mendoza-conicet.gov.ar, ORCID: https://orcid.org/0000-0001-8582-2756 ], Instituto Argentino de Nivología, Glaciología y Ciencias Ambientales, IANIGLA, CCT-CONICET, Adrián Ruíz Leal s/n, Parque General San Martín, 5500 Mendoza, Argentina.
Received 5 October 2022, accepted 25 January 2023, available online 13 February 2023.
Copyright © 2023 B. Vento et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Fossil plants are an important component in the reconstructions of past environments. They bring new insights into the clarification of relationships among extant taxa and contribute to understanding of the evolutionary processes on the Earth (Carpenter et al. 2014; Donogue et al. 1989; Vento and Agraín 2018). Over the past decades, a major progress has been achieved in paleobotany as several new findings contributed important data to a more accurate reconstruction of the evolutionary history of plant ecosystems in high southern latitudes (Carpenter et al. 2014; Dutra and Batten 2000; Hill 1992).
The family Nothofagaceae is a monogeneric family represented by the Nothofagus Blume, 1851 (southern beech), which is distributed exclusively in the Southern Hemisphere. This genus is a keystone for the reconstructions of past history because it is one of the few living genera in the Southern Hemisphere with both a well-researched phylogeny and a rich fossil record (Jordan and Hill 1994; Swenson et al. 2001). The identification of new fossil taxa has led to considerable progress in clarifying the relationships among taxa of Nothofagaceae (Carpenter et al. 2014; Jordan and Hill 1999; Vento et al. 2017; Vento and Agraín 2018). The macro and microfossil record indicates that the past diversity of Nothofagus was probably much higher than its current diversity (Dettmann et al. 1990). Given this, the available evidence on both extinct and extant species has to be taken into consideration while assessing phylogenetic relationships (Donoghue et al. 1989; Vento and Agraín 2018).
Relationships among living species of Nothofagus are well understood due to previous molecular studies and particularly valuable are those merging both morphological and molecular data (Cook and Crisp 2005; Hill and Jordan 1993; Jordan 1999; Manos 1997; Martin and Dowd 1993; Premoli et al. 2011; Sauquet et al. 2012). However, the molecular clock estimations can only be verified with the fossil record (Cantrill and Poole 2012). Fossil data have long been overlooked, most likely due to the small number of well-preserved specimens available (Heads 2006), however, their addition to phylogenetic analysis improves the resolution of the results (Vento and Agraín 2018). A great diversity of fossil leaves and reproductive structures of Nothofagus have been discovered during the last decades (e.g., Birkenmajer and Zastawniak 1989; Carpenter et al. 2014; Dutra and Batten 2000; Hill 1983a, b, 1991; Hünicken 1967; Jordan 1999; Paull and Hill 2003; Romero and Dibbern 1985; Zastawniak 1981, 1994). Yet most of the findings lack the organic connection or reproductive structures and were assigned to separate fossil species (Hill 1991). For this reason, their inclusion in a phylogenetic framework produces controversial results. Fossil leaves may help in deciphering the evolution and relationships within the Nothofagus (Hill 1994) as the variations in leaf shape and size of these plants could have been directly influenced by the surrounding climate and environmental conditions (Peppe et al. 2011; Vento et al. 2021; Wilf et al. 1998). The differences in leaf morphology are important characters in delimiting the species of Nothofagus and they also bring information about the past environmental and climate conditions as well as about the evolution and adaptation of species (Premoli 1996).
The Antarctic Peninsula plays an important role in understanding the evolutionary history of Nothofagaceae (Dutra and Batten 2000; Vento et al. 2022a). Currently, there are no living representatives of Nothofagus on this continent but the presence of the oldest fossil leaves from the Cretaceous has been reported (Dutra 2001; Dutra and Batten 2000; Hayes et al. 2006; Kvaček and Vodrážka 2016; Leppe et al. 2016; Romero et al. 2019; Zastawniak 1994). Many attempts to clarify the phylogenetic relationships of Nothofagus have been made and a few works included fossil species in the analysis (Carpenter et al. 2014; Jordan and Hill 1999; Vento and Agraín 2018). However, none of these works included fossils from Antarctica. Recently, a novel biogeographic analysis including fossil leaves from Antarctica Peninsula was performed but the relationships between extinct and living species were not discussed (Vento et al. 2022a).
The macrofossil record of Nothofagus is fragmentary and mainly composed of isolated leaves (Hill 1991; Romero 1986). However, morphological features of fossil leaves are relevant traits and in most cases the only available information to be coded for a phylogenetic framework (Vento and Agraín 2018). Even though some archaic characters are found in fossil specimens, their morphological features can be related to living species (Dutra and Batten 2000).
The goal of this work is to update knowledge on the phylogenetic relationships and evolutionary patterns of the Nothofagus. Coding fossil leaves from Antarctica that have never been included in a phylogenetic framework before. We sought novel evolutionary insights into one of the most important plant families distributed in the Southern Hemisphere. We studied the evolution of morphological leaf characters among plants with particular emphasis on understanding how leaf features shape the dynamics of evolutionary change.
Institutional abbreviations.—HRL, Ruíz Leal Herbarium, Mendoza, Argentina; MACN, Museo Argentino de Ciencias Naturales, Buenos Aires, Argentina.
Other abbreviations.—EIW, extended implied weighting analysis, EW, Equal Weight; IW, Implied Weight; MPTs, Most Parsimonious Trees; TBR, tree bisection reconnection; RAM, random access memory.
Material and methods
Taxon sampling: Extinct and living material.—We added a total of 23 fossil taxa of the subgenus Nothofagus from Australia, Antarctica, New Zealand, Tasmania, and southern South America as terminal taxa, expanding a former dataset performed by Vento and Agraín (2018). Fossil leaves of Nothofagus from southern South America previously studied and described by several authors were also considered (Berry 1937; Dusén 1899; 1908; Fiori 1939; Romero and Dibbern 1985; Tanai 1986; Vento et al. 2017; Vento and Prámparo 2018).
Additionally, literature sources with fossil leave descriptions from Antarctica and the most complete and the best-preserved specimens were revisited and included in the phylogenetic analysis (Dutra 2001, 2004; Dutra and Batten 2000; Hill et al. 1996; HaoMin and ZheKun 2007; Zastawniak 1981, 1994; Zastawniak et al. 1985). We also included fossils from Australia, New Zealand, Papua New Guinea, and New Caledonia to improve the resolution of the phylogenetic relationships among members of Nothofagaceae. There are many fossil taxa from these places in the literature; however, some of them were not depicted clear enough to observe the diagnostic characters necessary for our analysis. We only considered those that presented clearly descriptions of the anatomical and morphological characters and illustrations to minimize the missing data. We coded taxa following the taxonomic descriptions and codification made by Jordan and Hill (1999) and Carpenter et al. (2014). We included fossils of subgenera Nothofagus, Fuscospora, Brassospora, and Lophozonia previously recognized and described (Carpenter et al. 2014; Hill 1983b, 1984, 1991; Jordan 1999; Paull and Hill 2003).
The character codification of living species was based on observations from the previous work made by Vento and Agraín (2018) in which living species were examined from herbarium sheets held at HRL, MACN, and the herbarium catalogs of the Royal Botanical Garden available online at www.kew.org and the Royal Botanical Garden of Edinburgh available at www.data.rbge.org.uk.
The phylogenetic analysis included both extinct and extant taxa of the genus Nothofagus (excluding extant hybrids) as follows: from Antarctica (only fossils), southern South America (Argentina and Chile; fossil and extant), Australasia (southern Australia, New Caledonia, New Guinea, New Zealand, and Tasmania; fossil and extant). Species of Nothofagus were previously included as members of the Fagaceae (Hill and Jordan 1993; Jordan and Hill 1999; Manos 1997) but were later considered in Nothofagaceae which were more closely related to Betulaceae (Dutra 1997; Jones 1986; Nixon 1982, 1989). Therefore, we selected two outgroups: Fagus Linnaeus, 1753 (Fagaceae) and Betulaceae represented by Betula pendula Roth, 1788 (SOM 1, Supplementary Online Material available at http://app.pan.pl/SOM/app68-Vento_etal_SOM.pdf).
Character sampling: Morphological evidence.—Morphological characters 0–35, including fruit, flower, and pollen features of the living species were coded directly from Heenan and Smissen (2013); characters based on leaf morphology (36–45) were coded for both fossil material and living species following Vento and Agraín (2018). Fossil morphological characters scored in this contribution were based on the leaf margin and the venation pattern, such as type of leaf margin, teeth simple or composite, tooth spacing, teeth size, primary vein, number of secondary veins, secondary vein ending, and apex morphology (SOM 2).
Phylogenetic analysis.—The data matrix comprises a total of 46 morphological characters and 45 terminal taxa. All characters were informative and treated as non-additive. The phylogenetic analysis was performed using TNT v.1.5 parsimony software (Goloboff et al. 2008). We used the extended implied weighting analysis approach (EIW), exploring the topologies of the strict consensus trees resulting from a range (1–30) of concavity constants (k) (Goloboff 1993). We set the command “xpiwe (*” to avoid the missing entries generating too much homoplasy of the observed characters during the optimization of the most parsimonious trees MPTs (i.e., to receive a high fit). This command is comparable to using different values of k for each character according to its percentage of missing entries. Missing entries are assumed to have 50% of the homoplasy of observed entries as part of the EIW functions of TNT, which allow us to assign lower values of k to those characters with more missing data (Goloboff 2014). We performed the EIW analysis using a traditional heuristic search that was performed on the base of Wagner trees with 3000 random addition sequences, followed by the tree bisection reconnection (TBR) swapping algorithm, saving ten trees per replicate, and collapsing trees after the search. This was followed by a branch and bound search based on these trees from RAM. The evaluation of branch support was performed using symmetric resampling (Goloboff et al. 2003) with a change probability of 0.33 (default) and 500 replicates with values indicated as a frequency difference. Winclada v.1.00.08 (Nixon 2002) was used to map the non-exclusive (ambiguous) and exclusive (unambiguous) character states supporting each node. The homoplasy in the cladogram was marked by character state, considering only discontinuous states as homoplasious.
Results
The results using EIW were: 6 MPTs for k1, 1 MPT for k2 to k5, 2 MPTs for k6 to k8, and 1 MPT for k9 to k30. Most of the changes observed in the topology of these trees affect the relationships among the species within polytomies. Phylogenetic relationships among and within the subgenera will be discussed based on the k=9 selection tree with a fit of 6.21. Even though the support values were low, several nodes have a support value of more than 50 (SOM 3). The genus Nothofagus is supported by the exclusive synapomorphies absence of giant stomata on veins (171) and composite teeth (371). The fossil taxa included in our analysis were placed into four differentiated clades that corresponded to the subgenera Lophozonia, Fuscospora, Brassospora, and Nothofagus. Many relationships between fossils and living species were clear and largely resolved (Fig. 1). Lophozonia is the sister clade of the other three subgenera. Subgenera Brassospora + Nothofagus + Fuscospora are grouped by four exclusive synapomorphies (71, 131, 270, 320). Subgenus Lophozonia is supported by six synapomorphies, five of which are exclusive (51, 83, 231, 281, 351). Brassospora is supported by 12 synapomorphies, with seven exclusive (03, 82, 192, 221, 282, 301, 312). Subgenus Nothofagus is supported by three synapomorphies, of which only 161 is exclusive, while Fuscospora is supported by one non-exclusive synapomorphy 120.
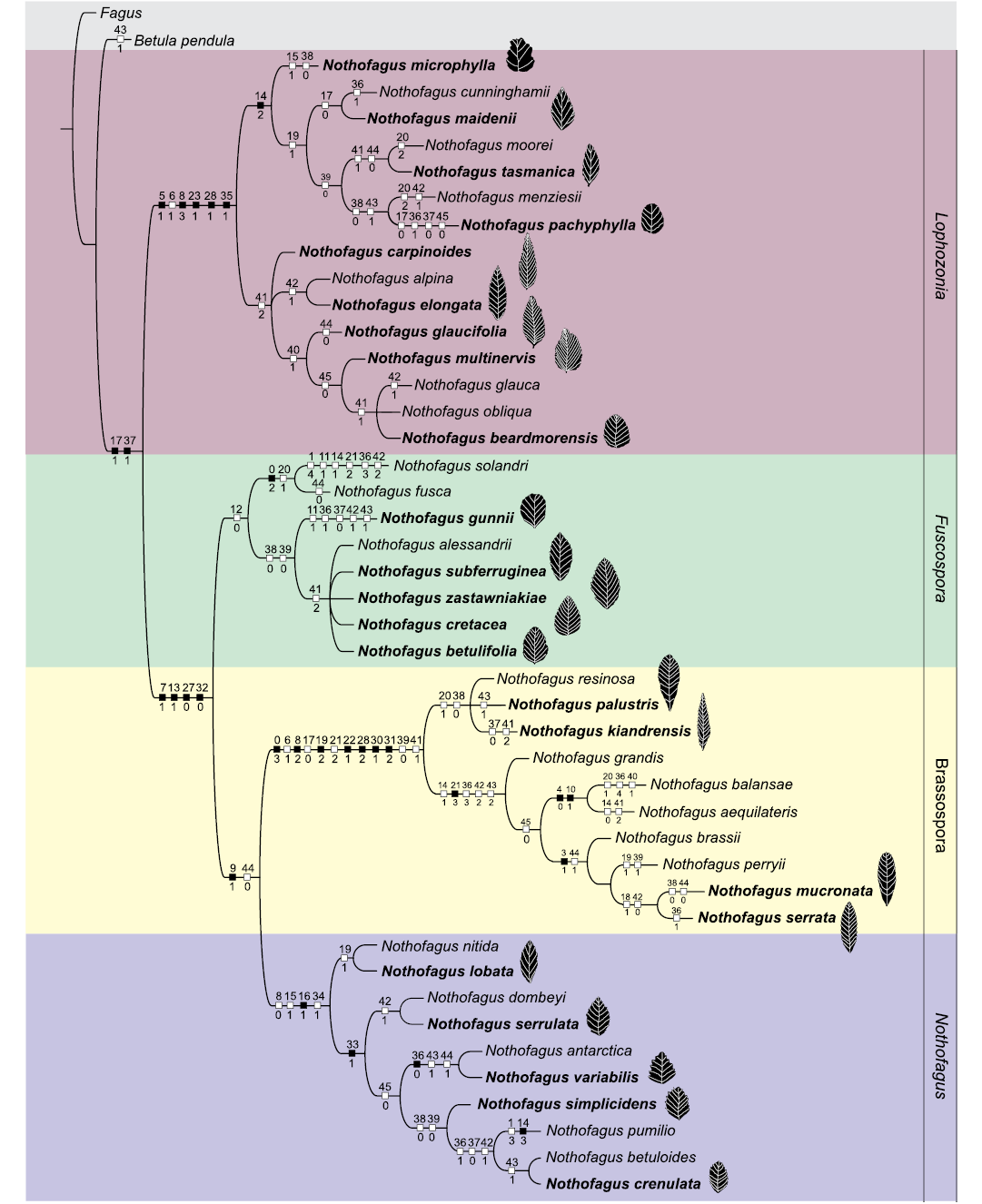
Fig. 1. Cladogram showing the phylogenetic relationships among extant and extinct species of Nothofagus. Result from EIW (k = 9) analysis. Bold indicate fossil species. Black squares represent exclusive (unambiguous or derived “unique” character states), white squares are non-exclusive (ambiguous character states).
The clade Lophozonia is split into two main clades. One clade is represented by the association of species from Australasia. This clade includes a branch with the fossil Nothofagus microphylla Hill, 1991. The other branch includes the fossil only taxa Nothofagus maidenii (Deane, 1902) Pole, 1993 + Nothofagus cunninghamii (Hooker, 1844) Oersted, 1871 + Nothofagus tasmanica Hill, 1983b + Nothofagus pachyphylla Jordan, 1999. The other main clade is represented by species from the Antarctic Peninsula, Transantarctic Mountains, and southern South America. It shows a polytomy with three branches where the fossil taxa Nothofagus carpinoides HaoMin and ZheKun, 2007 + Nothofagus elongata (Dusén, 1899) Romero and Dibbern, 1985. The last branch shows the association of the fossil only taxa Nothofagus glaucifolia Dutra and Batten, 2000, Nothofagus multinervis HaoMin and ZheKun, 2007, and Nothofagus beardmorensis Hill, Harwood, and Webb, 1996.
The clade Fuscospora contains the fossil only taxa Nothofagus betulifolia Dutra, 2000 + Nothofagus cretacea Zastawniak, 1994 + Nothofagus subferruginea (Dusén, 1899) Tanai, 1986 + N. zastawniakiae Dutra and Batten, 2000, grouped in a polytomy, and the species with a long temporal range, Nothofagus gunnii (Hooker, 1844) Oersted, 1871.
The clade Brassospora is split into two sister groups. One has the fossil only taxa Nothofagus palustris Carpenter, Bannister, Lee, and Jordan, 2014 + Nothofagus kiandrensis Paull and Hill, 2003. The other sister group has the fossil species Nothofagus serrata Hill, 1991 + Nothofagus mucronata Hill, 1991.
The clade Nothofagus is split into two sister clades; one of them contains the fossil only taxon Nothofagus lobata Hill, 1991. The fossil only Nothofagus serrulata Dusén, 1899 + Nothofagus variabilis Dusén, 1899 + Nothofagus crenulata Dusén, 1899 + Nothofagus simplicidens Dusén, 1899, are grouped in the other sister clade.
All the foliar characters (36–45) proposed in our analysis are present with their different states as non-exclusive synapomorphies both in living and fossil taxa. The extinct taxa associated with each subgenus show leaf morphological variations in the margin type, teeth, venation pattern, apex, and base. Only one leaf character (360) that corresponds to the dentate margin, is an exclusive synapomorphy for the living Nothofagus antarctica (Forster, 1789) Oersted, 1871, and the extinct N. variabilis. The rest of the characters (0–35) were only coded in living species, except for some anatomical characters that were available for nine Australasian extinct taxa, and they are shown in the cladogram as non-exclusive and exclusive synapomorphies. These characters support the division of the four subgenera. Variations in leaf and apex morphology (43–44) are observed in different members of the main clades as also in the venation pattern (40–42, and 45). Changes in the number of secondary veins (41) are shown with a reduction of veins in fossil representatives of subgenus Nothofagus compared with members of Lophozonia, restricted to Antarctica-South America, and of Fuscospora.
Discussion
On the importance of fossils.—The fossil record of Nothofagus has proven to be useful for reconstructing past distributions and evolutionary events of this genus. Moreover, it appears to be extremely conservative which is potentially important for past vegetation reconstruction and climate interpretations (Hill 1992). The addition of extinct taxa occasionally results in a high degree of homoplasy which makes it difficult to resolve some particular relationships. In our cladogram, unresolved polytomies can be observed. However, most of the nodes resulted in resolved relationships (SOM 3). Jordan and Hill (1999) added extinct taxa integrating a molecular and morphological data set into a phylogenetic framework but they only included seven extinct taxa from Australasia due to the preservation and the observable characters that could be coded. Carpenter et al. (2014) added extinct taxa in a phylogeny with anatomical and morphological characters but only considered four extinct species of subgenus Brassospora. Ancient records of Nothofagus have provided relevant clues for reconstructing past distribution and evolutionary events (Hayes et al. 2006; Hill 1994, 2001). It appears that the diagnostic characteristics of Nothofagus were established early in the history of the group and were maintained during its evolution (Dutra and Batten 2000).
The inclusion of extinct taxa into a phylogenetic framework (Fig. 1) agrees with the previous analyses where both living and extinct representatives were put together (Carpenter et al. 2014; Jordan and Hill 1999; Vento and Agraín 2018) and some novelties were observed. For example, N. mucronata and N. serrata were considered a single terminal taxon in previous works because the characters that distinguish them were autapomorphic (Jordan and Hill 1999). However, there is a clear distinction between them in our analysis (Fig. 1).
The morphological and molecular evidence re-affirm the monophyly of the four subgenera with the endemic subgenus Brassospora, now restricted to New Caledonia and New Guinea and Nothofagus restricted to southern South America (Hill and Read 1991; Hill and Jordan 1993; Manos 1997; Martin and Dowd 1993; Premoli et al. 2011; Vento and Agraín 2018). We recovered four deeply divergent clades which are coincident with the four subgenera of Nothofagus (Fig. 1). We detected phylogenetic associations within Nothofagus that help to infer the evolutionary history of this genus and the relationships between extinct and living taxa.
Leaf changes through time.—Here, we provide an overview of the evolutionary changes in leaf morphology to better understand the possible evolutionary drivers of diversification in leaf shape and the functional significance in the genus Nothofagus. Fossil evidence suggests that evolutionary changes within Nothofagus have been slow. Ancient fossil remains have been assigned to living species for example the leaves of N. gunnii found in the upper Oligocene of west Antarctica and lower Eocene–Oligocene of Tasmania (Hill 1984, 1991). A large number of fossil leaf shapes previously described indicated the leaf morphology in Nothofagus is a conservative character and clear differences may be detected in new phylogenetic analyzes (Bastos et al. 2013). Plant leaves are sensitive to environmental and climate changes and provide reliable information about the surrounding area where they developed. The physiognomy of an individual leaf reflects the microclimate conditions and may reveal the adaptive strategies adopted by plants in different habitats or environments (Davis and Taylor 1980; McPherson et al. 2004). Leaf shape and size of both living and extinct angiosperms have been strongly related to temperature and rainfall conditions respectively (Peppe et al. 2011; Vento et al. 2022b; Wilf et al. 1998; Wright et al. 2017). Extinct and living species of Nothofagaceae are mainly characterized by the presence of compound teeth, a feature that distinguishes the Fagaceae from the Betulaceae (Fig. 1). Nothofagus species have distinctly multiple teeth while a few of them have single teeth (Jordan and Hill 1999). A exception is a few living species with untoothed margins such as Nothofagus solandri (Hooker, 1844) Oersted, 1871, Nothofagus grandis Steenis, 1952, Nothofagus balansae (Baillon, 1874) Steenis, 1954, and Nothofagus aequilateralis (Baumann-Bodenheim, 1953) Steenis, 1954 (Carpenter et al. 2014; Jordan and Hill 1999). The smooth versus the toothed margin is putatively a reflection of temperature (Wolfe 1993). The fossil only N. mucronata has a smooth margin with apical serrations. However, N. serrata possesses a crenate margin. Even though both these extinct taxa were recorded in Tasmania, changes in margin shape could reflect a transition toward a leaf adaptation (since Miocene) to warmer and humid climate conditions in New Caledonia and Papua New Guinea (Hill 1992). On the other hand, deciduous taxa are more likely to be toothed than evergreen taxa, and the leaf life-span (deciduous or evergreen) influences on size and number of teeth (Peppe et al. 2011; Royer et al. 2005).
The most ancient fossil leaves of Nothofagaceae are recorded in sediments of Antarctica (Zastawniak 1994; Dutra and Batten 2000; Vento et al. 2022a). These fossil leaves are placed in subgenera Fuscospora and Lophozonia and have leaf features such as big size, toothed margins, spaced teeth, and a large number of secondary veins. Fossil leaves of N. zastawniakiae, N. glaucifolia, N. cretacea, N. betulifolia, and N. subferruginea have mostly more than 12 pairs of secondary veins and larger size compared with those of taxa included in the subgenus Nothofagus (Fig. 1). Among the living species, the close representative for the mentioned fossils is Nothofagus alessandrii Espinosa, 1928, sharing some morphological features such as the leaf shape, number of secondary veins, and teeth (Kellner et al. 2007) and it is considered the most primitive living taxon (Hill and Jordan 1993; Vento et al. 2022a).
The venation pattern is a leaf feature that seems to have experienced some changes through time as the reduction of the number of secondary veins from ancient to more recent species. The close disposition of the secondary veins and the plicate vernation character found in species of Fuscospora have also been detected in leaves of N. subferruginea from Antarctica, re-classified as Nothofagus hillii Dutra and Batten, 2000 (Keller et al. 2007) and also in records of southern South America (Vento and Prámparo 2018). The leaf features of N. glaucifolia as the teeth shape could represent an ancestral form of the living N. glauca (Philippi, 1858) Krasser, 1896 (Dutra and Batten 2000), an endemic species of southernmost Chile (Moya et al. 2017). The fossil taxon N. beardmorensis has a leaf margin and venation pattern similar to the living deciduous species, N. alessandrii and N. obliqua (Mirbel 1827) Oersted, 1871 (Hill et al. 1996). According to our cladogram, it is placed in the clade Lophozonia and is closely related to N. glauca and N. obliqua sharing features such as the number of secondary veins and margin type (Fig. 1).
Ancestral species presented less pronounced, spaced, and bigger teeth as can be observed in members of subgenera Lophozonia and Fuscospora. In contrast, more recent species (from Paleocene–Eocene to present) showed a reduction in the secondary veins, less spaced teeth, and deeper serrations as for example the species of subgenus Nothofagus (Fig. 1). Leaves from the Oligocene species were more deeply serrate, and as the subgenus adapted to warmer conditions the serrations, as in the species of Brassospora, were reduced and completely lost. Moreover, toothed species recorded changes in the venation pattern from craspedodromous to semicraspedodromous, and finally, to species with smooth and brochidodromous venation pattern (Hill 1991). On the other hand, plicate vernation is an indicator of deciduousness (Hill et al. 1996). This character is sometimes hardly being coded for fossils. However, N. beardmorensis, from Pliocene sediments in the Transantarctic Mountains, showed a plicate vernation similar to its living relatives (Fig. 1). The winter deciduous habit in the Pliocene suggests different temperature conditions in Antarctica than present (Hill et al. 1996).
The leaf morphology of the genus Nothofagus may reflect local environmental and climate conditions (Hill 1991; Hinojosa et al. 2016; Jordan and Hill 1994; Read 1990). Differences have been observed between environmental and climate requirements of particular species as in the case of the subtropical subgenus Brassospora with smooth margin leaf and the remaining clades from cooler climates, which are characterized by a serrate margin (Troncoso and Encinas 2006). Leaf size is an important feature related to temperature conditions (Peppe et al. 2011; Vento et al. 2021; Wolfe 1993). In this sense, a larger leaf size could indicate warmer conditions. Many living species of New Guinea, New Caledonia, and New Zealand have large leaves with a smooth or crenate margin that can be related to temperate and tropical conditions (Carpenter et al. 2014; Jordan and Hill 1999; Steenis 1971). Since the end of the Cretaceous global climate at high latitudes became warmer and more humid until the beginning of the middle Eocene (Birkenmajer and Zastawniak 1989; Cantrill and Poole 2012; Prámparo et al. 2018). These conditions can be reflected in the large fossil leaves from Antarctica (Fig. 1). The occurrence of larger leaves is also related to the availability of nutrients and water for their development (Wolfe 1993). Smaller leaves are probably indicative of a climate deterioration with cooling events during the late Eocene–Oligocene and changes in the rainfall patterns (Dutra 2004; Vento et al. 2021). Many extinct and living species show a reduction in size over time. For example, fossil leaves of the predecessor of N. cunninghamii from older sediments (Eocene–Oligocene) in Tasmania showed a larger length variation than fossil leaves of younger sediments (Hill 1983a; Jordan and Hill 1994). The decrease in leaf size during the glacial and interglacial periods is probably a response to cooling events (Hill 1983b). Present cool-temperate climate conditions are characterized by a higher proportion of small leaves in species of Nothofagus. The preponderance of small-toothed fossil leaves in Antarctica in the middle Miocene probably indicates a transition toward cooling climate conditions (Barton 1964). Living winter deciduous species of subgenus Nothofagus distributed in southern South America have small leaves (Vento and Agraín 2018) except for N. glauca, N. obliqua, and N. alessandrii endemic to southernmost Chile placed on slopes and exposed to humid conditions (Amigo and Rodríguez 2011). From the late Eocene to present leaves placed in the southern South American clade showed a smaller size, fewer secondary veins, and less pronounced teeth (Fig. 1).
Finally, the morphology of the leaf apex can be related to humid environmental conditions (Premoli 1996). As the growing season becomes drier in microthermal climates, the apex becomes sharp and finally rounded. Therefore, acute apices are found typically in wet, microthermal climates, whereas rounded apices predominate in dry megathermal to microthermal climates (Wolfe 1993). Fossil only taxa placed in the four subgenera showed variations in their apexes with acute or attenuate and rounded shapes that probably reflect the local past climate conditions (Fig. 1).
Conclusions
The discovery of new fossils of subgenus Nothofagus is crucial for understanding its past distribution and possible ancestors of the crown group. The findings from the Antarctic Peninsula are the most ancient ones found to date. They have not been coded and included in a phylogenetic framework previously. Our analysis reveals clear relationships between these extinct and living species of subgenus Nothofagus. The use of morphological leaf characters proposed herein, for fossil and living species, allows us to infer clear relationships among taxa and helps to better understand the evolutionary history of Nothofagus. The integration of morphological, anatomical, and molecular data in future research can bring more accurate results for supporting extinct and living relationships.
The first records of Nothofagus leaves are of Late Cretaceous age and have been placed in the subgenera Fuscospora and Lophozonia. Our analysis supports the hypothesis that ancient taxa possessed larger leaf size, spaced and fewer teeth, and more secondary veins, probably adapted to warmer and humid conditions. Additionally, most of these leaves have a plicate vernation which indicates a winter deciduous habit. Contrary to these, species placed in younger clades as subgenus Nothofagus have smaller leaf sizes, more toothed leaves, and less secondary veins probably as an adaptation to cooler conditions. Changes in leaf morphological features in Nothofagaceae can be strongly related to the climate and the environmental conditions that allow its adaptation and survival through time.
Acknowledgments
We kindly thank Robert Hill (University of Adelaide, Australia) for his valuable suggestions and the comments from an anonymous reviewer that contributed to improving our work. We thank the Willi Hennig Society for making the TNT program freely available. This work was funded by ANPCyT (Agencia Nacional de Promoción Científica y Técnica, Argentina) PICT-2019-03121, and CONICET PIP 2021-2023 (11220200102638CO).
References
Amigo, J. and Rodríguez, M.A. 2011. Bioclimatic and phytosociological diagnosis of the species of the Nothofagus genus (Nothofagaceae) in South America. International Journal of Geobotanical Research 1: 1–120. Crossref
Baillon, H.E. 1874. Nouvelles observations sur les Euphorbiacées. Adansonia 11: 72–138.
Barton, C.M. 1964. Significance of the Tertiary fossil floras of King George Island, South Shetland Islands. In: R.J. Adie (ed.), Antarctic Geology: Proceedings of the First International Symposium on Antarctic Geology, 604–608. North-Holland Publishing Company, Cape Town.
Bastos, B.L., Dutra, T.L., Wilberger, T.P., and Trevisan, C. 2013. Uma flora do final do Cretáceo na Ilha Nelson, Ilhas Shetland do Sul, Península Antártica. Revista Brasileira de Paleontologia 16: 441–464. Crossref
Baumann-Bodenheim, M.G. 1953. Fagacées de la Nouvelle Caledonie. Bulletin du Muséum National d’Histoire Naturelle, Series 2 25: 419–421.
Berry, E.W. 1937. Eocene plants from Río Turbio in the territory of Santa Cruz, Patagonia. Johns Hopkins University Studies in Geology 12: 91–98.
Birkenmajer, K. and Zastawniak E. 1989. Late Cretaceous–early Tertiary floras of King George Island, West Antarctica: their stratigraphic distribution and palaeoclimatic significance. Geological Society, London, Special Publications 47: 227–240. Crossref
Blume, C.L. von 1851. Ord. Cupulifereae. Museum Botanicum Lugduno-Batavum 1: 305–320.
Cantrill, D.J. and Poole, I. 2012. The origin of southern temperate ecosystems. In: D.J. Cantrill and I. Poole (eds.), The Vegetation of Antarctica Through Geological Time, 249–307. Cambridge University Press, Cambridge. Crossref
Carpenter, R.J., Bannister, J.M., Lee, D.E., and Jordan, G.J. 2014. Nothofagus subgenus Brassospora (Nothofagaceae) leaf fossils from New Zealand: A link to Australia and New Guinea? Botanical Journal of the Linnean Society 174: 503–515. Crossref
Cook, L.G. and Crisp, M.D. 2005. Not so ancient: The living crown group of Nothofagus represents a post-Gondwanan radiation. Proceedings of the Royal Society B: Biological Sciences 272: 2535–2544. Crossref
Davis, J.M. and Taylor, S.E. 1980 Leaf physiognomy and climate: a multivariate analysis. Quaternary Research 14: 337–348. Crossref
Deane, H. 1902. Notes on the fossil flora of Berwick. Records of the Geological Survey of Victoria 1: 21–32.
Dettmann, M.E., Pocknall, D.T., Romero, E.J., and Zamaloa, M.C. 1990. Nothofagidites Erdtman ex Potonie, 1960; a catalogue of species with notes on the paleogeographic distribution of Nothofagus Bl. (southern beech). New Zealand Geological Survey Paleontological Bulletin 60: 1–79.
Donoghue, M.J., Doyle, J.A., Gauthier, J., Kluge, A.G., and Rowe, T. 1989. The importance of fossils in phylogeny reconstruction. Annual Review of Ecology and Systematics 20: 431–460. Crossref
Dusén, P. 1899. Über die tertiäre Flora der Magallans-Länder. In: O. Nordenskjöld (ed.), Wissenschaftliche Ergebnisse der Schwedischen Expedition nach den Megallansländer 1895–97. Band I. Geologie, Geographie und Anthropologie, 87–108. Lithographisches Institut des Generalstabs, Stockholm.
Dusén, P. 1908. Über die tertiäre Flora der Seymour Insel. In: O. Nordenskjöld (ed.), Wissenschaftliche Ergebnisse der Schwedischen Südpolar Expedition 1901–1903, Geologie und Paläontologie 3, 1–27. Norstedt and Söner, Stockholm.
Dutra, T.L. 1997. Primitive leaves of Nothofagus (Nothofagaceae) in Antarctic Peninsula: An Upper Campanian record and a betulaceous more than fagaceous morphological character. Congreso Geológico Chileno, Antofagasta, Chile 9: 24–29.
Dutra, T.L. 2001. Paleoflora da ilha 25 de Mayo, Península Antártica: contribuição à paleogeografia, paleoclima e para a evolução de Nothofagus. Asociación Paleontológica Argentina, Publicación Especial 8: 29–37.
Dutra, T.L. 2004. Paleofloras da Antártica e sua relação com os eventos tectônicos e paleoclimáticos nas altas latitudes do sul. Revista Brasileira de Geociências 34: 401–410. Crossref
Dutra, T.L. and Batten, D.J. 2000. Upper Cretaceous floras of King George Island, West Antarctica, and their palaeoenvironmental and phytogeographic implication. Cretaceous Research 21: 181–209. Crossref
Espinosa, M.R. 1928. Dos especies nuevas de Nothofagus. Revista Chilena Historia de Natural 32: 171–197.
Fiori, A. 1939. Fillite terziarie della Patagonia. II. Fillite del Río Ñirihuau. Giornale di Geologia 13: 1–27.
Forster, G. 1789. Fasciculus Plantarum Magellanicum. Commentationes Societatis Regiae Scientiarum Gottingensis 9: 13–45.
Goloboff, P.A. 1993. Estimating character weights during tree search. Cladistics 9: 83–91. Crossref
Goloboff, P.A. 2014. Extended implied weighting. Cladistics 30: 20–272. Crossref
Goloboff, P.A., Farris, J.S., and Nixon, K.C. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24: 774–786. Crossref
Goloboff, P.A., Farris, J.S., Kallersjo, M., Oxelman, B., Ramírez, M.J., and Szumik, C.A. 2003. Improvements to resampling measures of group support. Cladistics 19: 324–332. Crossref
HaoMin, L. and ZheKun, Z. 2007. Fossil nothofagaceous leaves from the Eocene of western Antarctica and their bearing on the origin, dispersal and systematics of Nothofagus. Science in China Series D: Earth Sciences 50: 1525–1535. Crossref
Hayes, P.A., Francis, J.E., Cantrill, D.J., and Crame, J.A. 2006. Palaeoclimate analysis of Late Cretaceous angiosperm leaf floras, James Ross Island, Antarctica. Geological Society, London, Special Publications 58: 49–62. Crossref
Heads, M. 2006. Panbiogeography of Nothofagus (Nothofagaceae): analysis of the main species massings. Journal of Biogeography 33: 1066–1075. Crossref
Heenan, P.B. and Smissen, R.D. 2013. Revised circumscription of Nothofagus and recognition of the segregate genera Fuscospora, Lophozonia, and Trisyngyne (Nothofagaceae). Phytotaxa 146: 1–31. Crossref
Hill, R.S. 1983a. Evolution of Nothofagus cunninghamii and its relationship to N. moorei as inferred from Tasmanian macrofossils. Australian Journal of Botany 31: 453–465. Crossref
Hill, R.S. 1983b. Nothofagus macrofossils from the Tertiary of Tasmania. Alcheringa 7: 169–183. Crossref
Hill, R.S. 1984. Tertiary Nothofagus macrofossils from Cethana, Tasmania, Alcheringa 8: 81–86. Crossref
Hill, R.S. 1991. Tertiary Nothofagus (Fagaceae) macrofossils from Tasmania and Antarctica and their bearing on the evolution of the genus. Botanical Journal of the Linnean Society 105: 73–112. Crossref
Hill, R.S. 1992. Nothofagus: evolution from a southern perspective. Trends in Ecology and Evolution 7: 190–194. Crossref
Hill, R.S. 1994. History of selected Australian taxa. In: R.S. Hill (ed.), History of the Australian Vegetation: Cretaceous to Recent, 390–419. Cambridge University Press, Cambridge. Crossref
Hill, R.S. 2001 Biogeography, evolution and palaeoecology of Nothofagus (Nothofagaceae): The contribution of the fossil record. Australian Journal of Botany 49: 321–332. Crossref
Hill, R.S. and Jordan, G.J. 1993. The evolutionary history of Nothofagus (Nothofagaceae). Australian Systematic Botany 6: 111–126. Crossref
Hill, R.S. and Read, J. 1991. A revised infrageneric classification of Nothofagus (Fagaceae). Botanical Journal of the Linnean Society 105: 37–72. Crossref
Hill, R.S., Harwood, D.M., and Webb, P.N. 1996. Nothofagus beardmorensis (Nothofagaceae), a new species based on leaves from the Pliocene Sirius Group, Transantarctic Mountains, Antarctica. Review of Palaeobotany and Palynology 94: 11–24. Crossref
Hinojosa, L.F., Gaxiola, A., Pérez, M.F., Carvajal, F., Campano, M.F., Quattrocchio, M., Nishida, H., Uemura, K., Yabe, A., Bustamante, R., and Arroyo, M.T. 2016. Non-congruent fossil and phylogenetic evidence on the evolution of climatic niche in the Gondwana genus Nothofagus. Journal of Biogeography 43: 555–567. Crossref
Hooker, J.D. 1844. Icones plantarum; or figures, with brief descriptive characters and remarks, of new or rare plants, selected from the authors herbarium, 601–690 pls. Baillière, London.
Hünicken, M. 1967. Flora terciaria de los estratos de Río Turbio, Santa Cruz (Niveles plantíferos del Arroyo Santa Flavia). Revista de la Facultad de Ciencias Exactas, Físicas y Naturales de la Universidad de Córdoba. Serie Ciencias Naturales 56: 139–262.
Jones, J.H. 1986. Evolution of the Fagaceae: the implications of foliar features. Annals of the Missouri Botanical Garden 73: 228–275. Crossref
Jordan, G. 1999. A new Early Pleistocene species of Nothofagus and the climatic implications of co-occurring Nothofagus fossils. Australian Systematic Botany 12: 757–76. Crossref
Jordan, G. and Hill, R.S. 1994. Past and present variability in leaf length of evergreen members of Nothofagus subgenus Lophozonia related to ecology and population dynamics. New Phytologist 127: 377–390. Crossref
Jordan, G. and Hill, R.S. 1999. The phylogenetic affinities of Nothofagus (Nothofagaceae) leaf fossils based on combined molecular and morphological data. International Journal of Plant Science 160: 1177–1188. Crossref
Krasser, F. 1896. Bemerkungen zur Systematik der Buchen. Annalen des Naturhístoríschen Hofmuseums Wien 11: 155–163.
Kvaček, J. and Vodrážka, R. 2016. Late Cretaceous flora of the Hidden Lake Formation, James Ross Island (Antarctica), its biostratigraphy and palaeoecological implications. Cretaceous Research 58: 183–201. Crossref
Kellner, A.W.A., Dutra, T.L., de Araújo Carvalho, M., Rodriguez, R., Ramos, C., Grillo, O.N., Helder de, P.S., Douglas, R., and Romano, P.S.R. 2007. First record of fossil leaves from the Keller Peninsula, King George Island, Antarctica. In: I. de Souza Carvalho (ed.), Paleontologia: Cenários de Vida, 627–635. Editora Interciencia, Rio de Janeiro.
Leppe, M., Stinnesbeck, W., Mansilla, H., Mansilla, C., Kaempfe, S., Manriquez, L., González, E., Ortuya, M., Fernández, R., Garrido, S., et al. 2016. El complejo Cerro Guido-Las Chinas y el fin del Cretácico austral. Abstracts, V Simposio de Paleontología de Chile, 41–44. Universidad Andres Bello, Concepcion.
Linnaeus, C. 1753. Species plantarum, exhibentes plantas rite cognitas, ad genera relatas, cum differentiis specificis, nominibus trivialibus, synonymis selectis, locis natalibus, secundum systema sexuale digestas. Tomus 1 and 2. xii+1200 pp. Laurentius Salvius, Stockholm. Crossref
Manos, P.S. 1997. Systematics of Nothofagus (Nothofagaceae) based on rDNA spacer sequences (ITS): taxonomic congruence with morphology and plastid sequences. American Journal of Botany 84: 1137–1137. Crossref
Martin, P.G. and Dowd, J.M. 1993. Using sequences of rbcL to study phylogeny and biogeography of Nothofagus species. Australian Systematic Botany 6: 441–447. Crossref
McPherson, S., Eamus, D., and Murray, B.R. 2004 Seasonal impacts on leaf attributes of several tree species growing in three diverse ecosystems of south-eastern Australia. Australian Journal of Botany 52: 293–301. Crossref
Mirbel, C.F.B. 1827. Recherches sur la distribution géographique des Végétaux phanérogames de l’Ancien-Monde, depuis l’équateur jusqu’au pôle arctique; suivies de la description de neuf espèces de la famille des Amentacées. Mémoires du Museum d’histoire naturelle de Paris 14: 349–474.
Moya, R.S., Meza, S.E., Díaz, C.M., Ariza, A.C., Calderón, S.D., and Peña-Rojas, K. 2017. Variability in seed germination and seedling growth at the intra-and inter-provenance levels of Nothofagus glauca (Lophozonia glauca), an endemic species of Central Chile. New Zealand Journal of Forestry Science 47: 1–9. Crossref
Nixon, K.C. 1982. In support of the Nothofagaceae Kuprianova. Botanical Society of America Miscellaneous Series 162: 102.
Nixon, K.C. 1989. Origins of Fagaceae. In: P.R Crane and S. Blackmore (eds.), Evolution, Systematics, and Fossil History of the Hamamelidae, 23–43. Clarendon Press, Oxford.
Nixon, K.C. 2002. WinClada ver. 1.00.08. Published by the author, Ithaca.
Oersted, S.A. 1871. Bidrag til Kundskab om Egefamilien i Nutid og Fortid. Kongelige Danske Videnskabernes Selskabs Skrifter 9: 31–538. Crossref
Paull, R. and Hill, R.S. 2003. Nothofagus kiandrensis (Nothofagaceae subgenus Brassospora), a new macrofossil leaf species from Miocene sediments at Kiandra, New South Wales. Australian Systematic Botany 16: 549–559. Crossref
Peppe, D.J., Royer, D.L., Cariglino, B., Oliver, S.Y., Newman, S., Leight, E., Grisha E., Fernández, M., Herreras F., Adams J., Correa E., Curranos E., Erickson M., Hinojosa L., Hoganson J., Iglesias A., Jaramillo C., Johnson K., Jordan G., Kraf N., Lovelock E. et al. 2011. Sensitivity of leaf size and shape to climate: global patterns and paleoclimatic applications. New Phytologist 190: 724–739. Crossref
Philippi, R.A. 1858. Plantarum novarum Chilensium: Centuria quarta. Linnaea 29: 1–47.
Pole, M.S., Hill, R.S., Green, N., and Macphail, M.K. 1993 The Miocene Berwick Quarry flora: rainforest in a drying climate. Australian Systematic Botany 6: 399–428. Crossref
Prámparo, M.B., Vento, B., Narvaez, P.L., Mego, N., and Puebla, G.G. 2018. Cretaceous climatic reconstruction from Argentina based on palynological data. Boletín Geológico y Minero 129: 615–632 Crossref
Premoli, A.C. 1996. Leaf architecture of South American Nothofagus (Nothofagaceae) using traditional and new methods in morphometrics. Botanical Journal of the Linnean Society 121: 25–40. Crossref
Premoli, A.C., Mathiasen, P., Acosta, M.C., and Ramos, V.A. 2011. Phylogeographically concordant chloroplast DNA divergence in sympatric Nothofagus s.s. How deep can it be? New Phytologist 193: 261–275. Crossref
Read, J. 1990. Some effects of acclimation temperature on net photosynthesis in some tropical and extra-tropical Australasian Nothofagus species. The Journal of Ecology 78: 100–112. Crossref
Romero, E.J. 1986. Fossil evidence regarding the evolution of Nothofagus Blume. Annals of the Missouri Botanical Garden 73: 276–283. Crossref
Romero, E.J. and Dibbern, M.C. 1985. A review of the species described as Fagus and Nothofagus by Dusén. Palaeontographica B 197: 123–137.
Royer, D.L., Wilf, P., Janesko, D.A., Kowalski, E.A., and Dilcher, D.L. 2005. Correlations of climate and plant ecology to leaf size and shape: potential proxies for the fossil record. American Journal of Botany 92: 1141–1151. Crossref
Romero, E.J., Rodriguez Amenabar, C., Zamaloa, M.C., and Concheyro, G. 2019. Nothofagus and the associated palynoflora from the Late Cretaceous of Vega Island, Antarctic Peninsula. Polish Polar Research 40: 227–253.
Roth, A.W. 1788. Tentamen Florae Germanieae. Vol. 1. 560 pp. I.G. Mülleriano, Leipzig.
Sauquet, H., Ho, S.Y., Gandolfo, M.A., Jordan, G.J., Wilf, P., Cantrill, D., Bayly, M.J., Bromham, L., Brown, G., Carpenter, R., Lee, D., Murphy, D., Kale Sniderman, J.M., and Udovicic, F. 2012. Testing the impact of calibration on molecular divergence times using a fossil-rich group: the case of Nothofagus (Fagales). Systematic Biology 61: 289–313. Crossref
Steenis, C.G.G.J. van 1971. Nothofagus, key genus of plant geography, in time and space, living and fossil, ecology and phylogeny. Blumea: Biodiversity, Evolution and Biogeography of Plants 19: 65–98.
Steenis, C.G.G.J. van 1952. Preliminary account of Papuan Nothofagus. Blumea: Biodiversity, Evolution and Biogeography of Plants 7: 146–147.
Steenis, C.G.G.J. van 1954. Additional note on Nothofagus. Journal of the Arnold Arboretum 35: 266–267. Crossref
Swenson, U., Hill, R.S., and McLoughlin, S. 2001. Biogeography of Nothofagus supports the sequence of Gondwana break‐up. Taxon 50: 1025–1041. Crossref
Tanai, T. 1986. Phytogeographic and phylogenetic history of the genus Nothofagus Bl. (Fagaceae) in the southern hemisphere. Journal of the Faculty of Science, Hokkaido University, Series 4: Geology and Mineralogy 21: 505–582.
Troncoso, A. and Encinas, A. 2006. La tafoflora de cerro Centinela (Chile, VI Región): vegetación y clima de Chile central a fines del Mioceno-comienzos del Plioceno. Ameghiniana 43: 171–180.
Vento, B. and Agraín, F.A. 2018. Phylogenetic relationships and time-calibration of the South American fossil and living species of southern beeches (Nothofagus). Acta Palaeontologica Polonica 63: 815–825. Crossref
Vento, B. and Prámparo, M. 2018. Angiosperm association from the Río Turbio Formation (Eocene–?Oligocene) Santa Cruz, Argentina: Revision of Hünicken’s (1955) fossil leaves collection. Alcheringa: An Australasian Journal of Palaeontology 42: 125–153. Crossref
Vento, B., Agraín, F. and Puebla, G. 2022a. Ancient
Antarctica: the early evolutionary history of
Nothofagus. Historical Biology 1–11. Crossref
Vento, B., Gandolfo, A., Nixon, K., and Prámparo, M. 2017. Paleofloristic assemblage from the Paleogene Río Guillermo Formation, Argentina: Preliminary results of phylogenetic relationships of Nothofagus in South America. Historical Biology 29: 93–107. Crossref
Vento, B., Puebla, G.G., Pinzon, D., and Prámparo, M. 2021. Paleoclimate estimates for the Paleogene–Neogene in southern South America using fossil leaves as proxies. Comptes Rendus Palevol 20: 29–48. Crossref
Vento, B., Rivera, J., Ontivero, M., and Carretero, E.M. 2022b. Insights into the relationships between morphological traits of Larrea divaricata and climate variables in southern South America. International Journal of Plant Sciences 183: 220–234. Crossref
Wilf, P., Wing, S.L., Greenwood, D.R., and Greenwood, C.L. 1998 Using fossil leaves as paleoprecipitation indicators: An Eocene example. Geology 26: 203–206. Crossref
Wolfe, J.A. 1993 A method of obtaining climatic parameters from leaf assemblages. US Geological Survey Bulletin 2040: 1–71.
Wright, I.J., Dong, N., Maire, V., Prentice, I.C., Westoby, M., Díaz, S., Gallagher, R.V., Jacobs, B.F., Kooyman, R., Law, E.A., Leishman, M.R., Niinemets, Ü., Reich, P.B., Sack, L., Villar, R., Wang, H., and Wilf, P. 2017. Global climatic drivers of leaf size. Science 357: 917–921. Crossref
Zastawniak, E. 1981. Tertiary leaf flora from the Point Hennequin Group of King George Island (South Shetland Islands, Antarctica), Preliminary report. Studia Geologica Polonica 72: 97–108.
Zastawniak E. 1994. Upper Cretaceous leaf flora from the Blaszyk Moraine [Zamek Formation], King George Island, South Shetland Islands, West Antarctica. Acta Palaeobotanica 34: 119–163.
Zastawniak, E., Wrona, R., Gaździcki, A., and Birkenmajer, K. 1985. Plant remains from the top part of the point Hennequin Group (Upper Oligocene), King George (South Shetland islands, Antarctica), Studia Geologica Polonica 31: 143–164.
Acta Palaeontol. Pol. 68 (1): 175–183, 2023
https://doi.org/10.4202/app.01029.2022