

A new Early Triassic crinoid from Nevada questions the origin and palaeobiogeographical history of dadocrinids
THOMAS SAUCÈDE, CHRISTOPHER SMITH, NICOLAS OLIVIER, CHRISTOPHE DURLET, PIERRE GUERIAU, MATHIEU THOURY, EMMANUEL FARA, GILLES ESCARGUEL, and ARNAUD BRAYARD
Saucède, T., Smith, C., Olivier, N., Durlet, C., Gueriau, P., Thoury, M., Fara, E., Escarguel, G., and Brayard, A. 2023. A new Early Triassic crinoid from Nevada questions the origin and palaeobiogeographical history of dadocrinids. Acta Palaeontologica Polonica 68 (1): 155–166.
Knowledge of the early evolution of post-Palaeozoic crinoids mainly relies on the well-preserved and abundant material sampled in Triassic Konservat-Lagerstätten such as those from the Anisian Muschelkalk (Middle Triassic) of the Germanic Basin. These crinoid-bearing Lagerstätten have been central to understanding the rapid evolution and diversification of crinoids after the dramatic Permian/Triassic Boundary biological crisis that led the class to near-extinction. The Encrinida are the emblematic crinoids of the Triassic. They are mainly known from rich fossil deposits where their abundant ossicles are at the origin of the extensive crinoidal limestone beds of the German Upper Muschelkalk. So far, they were first represented in the Middle Triassic by the family Dadocrinidae and genus Dadocrinus. In the present work, a new species Dadocrinus montellonis sp. nov., is described based on a well-preserved, almost complete articulated specimen from the Spathian (Lower Triassic) of Nevada (USA). The new species differs from other species of Dadocrinus by its palaeobiogeographic position but also by its earlier stratigraphic occurrence and ancestral morphology. It represents the first reported occurrence of Dadocrinus outside the Germanic Basin prior to the Middle Triassic and also the oldest firm evidence of its presence in the Early Triassic (middle–late Spathian). This discovery sheds new light on the origin of post-Palaeozoic crinoids. It suggests a much wider distribution than commonly assumed for the genus Dadocrinus and implies that the first dadocrinids originated either in the Panthalassa or Tethys oceans, and then dispersed over long distances in a relative short period of time.
Key words: Crinoidea, Articulata, Dadocrinus, Early Triassic, Spathian, Nevada, USA.
Thomas Saucède [thomas.saucede@u-bourgogne.fr; ORCID: https://orcid.org/ 0000-0001-6056-4447 ], Christopher Smith [christopher.smith@u-bourgogne.fr; ORCID: https://orcid.org/0000-0002-7909-1165 ], Christophe Durlet [christophe.durlet@u-bourgogne.fr; ORCID: https://orcid.org/0000-0003-2834-1715 ], Emmanuel Fara [emmanuel.fara@u-bourgogne.fr; ORCID: https://orcid.org/0000-0002-7991-4467 ], and Arnaud Brayard [arnaud.brayard@u-bourgogne.fr; ORCID: https://orcid.org/0000-0003-1304-6553 ], Biogéosciences, UMR 6282, CNRS, Université de Bourgogne, 6 boulevard Gabriel, F-21000 Dijon, France.
Nicolas Olivier [ nicolas.olivier@uca.fr; ORCID: https://orcid.org/0000-0001-6119-3561 ], Université Clermont Auvergne, CNRS, IRD, Laboratoire Magmas et Volcans, F-63000 Clermont-Ferrand, France.
Pierre Guériau [pierre.gueriau@synchrotron-soleil.fr; ORCID: https://orcid.org/0000-0002-7529-3456 ], and Mathieu Thoury [ mathieu.thoury@synchrotron-soleil.fr; ORCID: https://orcid.org/0000-0002-8641-3791 ], Université Paris-Saclay, CNRS, ministère de la Culture, UVSQ, MNHN, Institut photonique d’analyse non destructive européen des matériaux anciens UAR3461, 91190 Saint-Aubin, France.
Gilles Escarguel [ gilles.escarguel@univ-lyon1.fr, ORCID: https://orcid.org/0000-0003-0985-6369 ], Univ Lyon, Université Claude Bernard Lyon 1, CNRS, ENTPE, UMR 5023 LEHNA, F-69622, Villeurbanne, France.
Received 15 November 2022, accepted 21 January 2023, available online 21 February 2023.
Copyright © 2023 T. Saucède et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
With over 6000 fossil species reported, against about 650 extant ones, crinoids are a class of echinoderms with a rich fossil record (Pawson 2007). They are common and abundant components of fossil assemblages, being regularly observed in Palaeozoic and Mesozoic epicontinental marine deposits, most often as disarticulated ossicles. However, exceptionally well-preserved and articulated specimens have been extensively studied and described in Konservat-Lagerstätten such as those from the Anisian Muschelkalk of the Germanic Basin, Tatra Mountains, and the late Ladinian/early Carnian Cassian Formation in the Southern Alps (Lefeld 1958; Hagdorn 1996, 2011, 2020), among others (Hess and Messing 2011). Studies of Triassic Lagerstätten have been central to understanding the evolution and diversification of the group after the dramatic Permian/Triassic Boundary (PTB) biological crisis that led the class to the point of near-extinction (Paul 1988; Benton and Twitchett 2003; Hagdorn 2011; Hess and Messing 2011). During the Triassic, post-Palaeozoic crinoids evolved many innovations, diversified, and (re)colonised most known Palaeozoic crinoid habitats (Hagdorn 2011). By the Middle Triassic, crinoid diversity had partially recovered and many ecological niches occupied during the Palaeozoic were filled up again (Hagdorn 2011).
Congruent fossil and molecular evidence indicates that post-Palaeozoic crinoids belong to the single monophyletic subclass Articulata and that they radiated from a small clade (Pawson 2007; Hagdorn 2011; Hess and Messing 2011; Rouse et al. 2013; Wright et al. 2017), which gave rise to all subsequent forms including present-day comatulids and stem-crinoids (Rouse et al. 2013; Oji and Twitchett 2015). Palaeontological studies also agree on a scenario of rapid evolution and diversification of post-Palaeozoic crinoids into two distinct lineages, the Encrinida, which are the emblematic crinoids of the Triassic, and the Holocrinida/Isocrinida (Webster and Jell 1999; Benton and Twitchett 2003; Kashiyama and Oji 2004; Twitchett and Oji 2005; Hagdorn and Göncüoglu 2007; Webster and Lane 2007; Baumiller et al. 2010; Hagdorn 2011, 2020; Hess and Messing 2011; Salamon et al. 2012, 2015; Rouse et al. 2013; Oji and Twitchett 2015; Cohen and Pisera 2017; Brosse et al. 2019; Saucède et al. 2019). The Encrinida are mainly known from rich fossil deposits of the western Tethys domain where their abundant ossicles are at the origin of the extensive crinoidal limestones of the German Upper Muschelkalk (Middle Triassic) (Hagdorn 2011). They were unknown from the Lower Triassic so far and did not appear in the fossil record from the rocks older than early Anisian (Middle Triassic) in the Germanic Basin (Hagdorn 1999, 2011, 2020), or potentially latest Early Triassic (Nawrocki and Szulc 2000). In contrast, first representatives of the Holocrinida/Isocrinida lineage are reported from the rocks as old as middle Griesbachian (lower Induan; Early Triassic) from Oman (Oji and Twitchett 2015; Brosse et al. 2019).
The Encrinida are first represented by the family Dadocrinidae Lowenstam, 1942, and the genus Dadocrinus von Meyer, 1847 (Hagdorn 2011; Hess and Messing 2011). Dadocrinus is mostly known from the well-preserved and abundant material sampled in Konservat-Lagerstätten of the eastern part of the Germanic Basin (Poland, Germany, Switzerland, Austria, and Hungary) and of the Southern Alps (northern Italy) and Tatra Mountains (southern Poland) (Lefeld 1958; Hagdorn 1996, 2011, 2020; Hess and Messing 2011). It is also reported from upper Anisian of Turkey and New Zealand (Eagle 2003, 2004). The well-preserved and abundant material of Dadocrinus makes it an important taxon for the basal Triassic biostratigraphy of the Germanic Basin (Hagdorn 1999, 2020) where it was identified as a marker of the uppermost Lower–lowermost Middle Triassic transition (Kozur 1974; Nawrocki and Szulc 2000; Niedźwiedzki and Salamon 2006).
In the present work, a new species of the genus Dadocrinus is described based on a new specimen from the Spathian (upper Olenekian, Lower Triassic) of Nevada (USA). The early temporal position and unprecedented geographic location of this new Dadocrinus species makes it of particular interest to document the origin of Encrinida, but also more generally of the early evolution of post-Palaeozoic crinoids.
Institutional abbreviations.—UBGD, Université de Bourgogne, Géologie Dijon, France.
Other abbreviations.—PTB, Permian/Triassic Boundary.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:75391A18-D9AA-4D9F-B5D4-99A9E9EC868E
Geological setting
The crinoid specimen has been found in Montello Canyon, northeastern Nevada, which during the Early Triassic was situated on the eastern side of the intertropical, epicontinental sea of the Western USA Basin on the eastern margin of the Panthalassa (Fig. 1A). Lower Triassic marine sedimentary deposits presently crop out in Utah, northern Arizona, eastern Nevada, southeastern Idaho, western Wyoming, and southwestern Montana (e.g., Paull and Paull 1993). These marine deposits generally correspond to the classical shale-limestone alternations of the Thaynes Group (sensu Lucas et al. 2007) representing relatively shallow water habitats. In northeastern Nevada, the Thaynes Group documents most of the Early Triassic, basal beds corresponding to the Griesbachian (lower Induan, Lower Triassic) and Dienerian (upper Induan, Lower Triassic) Dinwoody Formation (e.g., Jenks et al. 2021). The Montello Canyon locality is located ~10 km west to the small town of Montello. Permian and Lower Triassic exposures in Montello Canyon and the neighbouring Immigrant Canyon were initially described by Clark (1957) and later mapped in detail by Mullen (1985). Within this area, Mullen (1985) identified the classical Permian Gerster Formation and three Lower Triassic units lying unconformably above it. These three units consist of the Dinwoody Formation, an unnamed siltstone unit and the typical thick alternating calcareous shale-limestone beds of the Thaynes Group, in the ascending order (Fig. 1D). They all represent an open shelf marine environment as testified by the finely laminated shale alternating with lenticular bioclastic limestone beds, deposited below the fair-weather wave base, as well as by the sampled macroinvertebrate faunas with abundant, complete specimens of cephalopods and various benthic organisms (Mullen 1985; Smith et al. 2021).
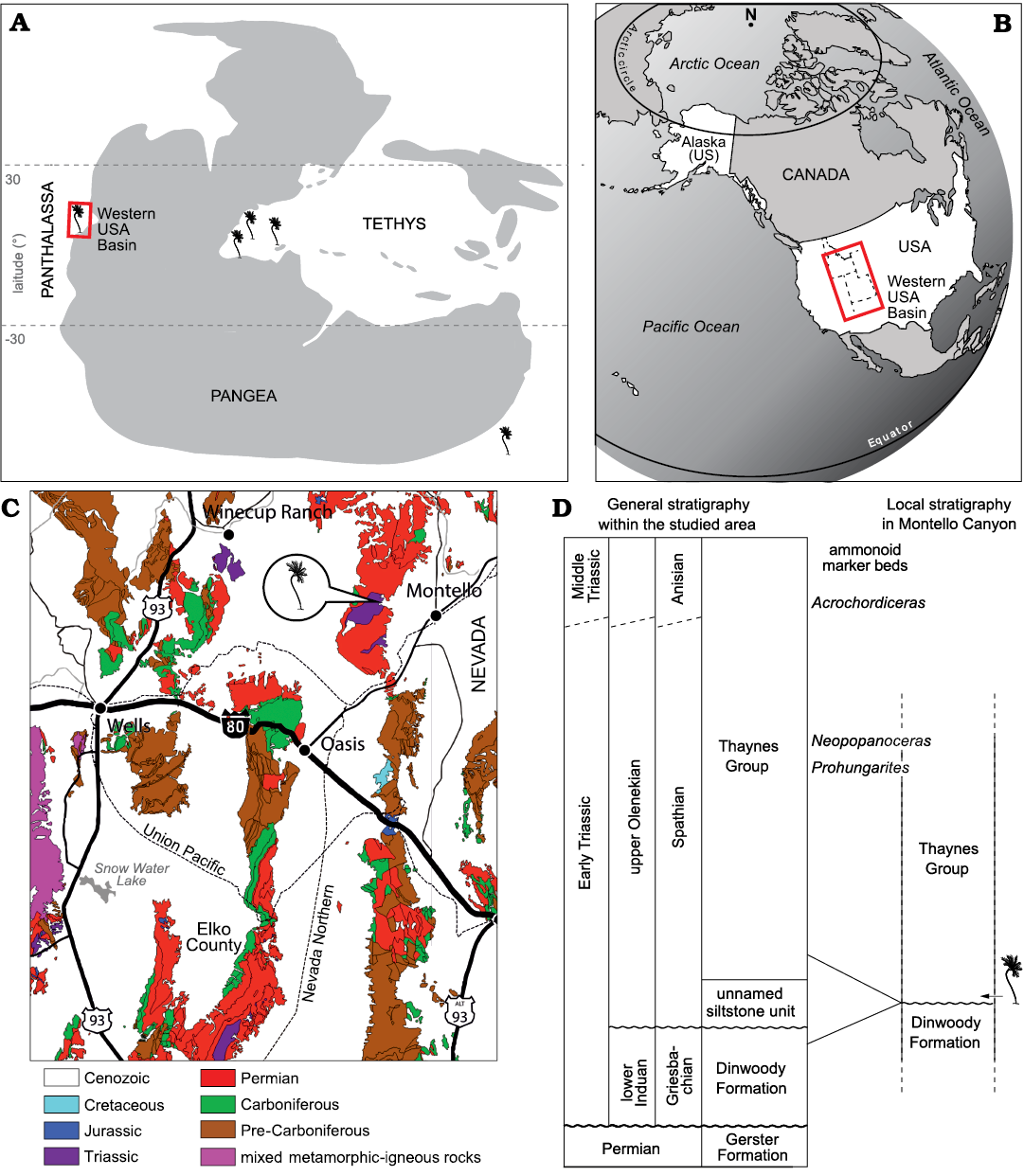
Fig. 1. Location, geological and stratigraphic setting of the Thaynes Group where Dadocrinus montellonis sp. nov. was found. A. Palaeogeographic map of the late Early/early Middle Triassic showing the occurrence record of Dadocrinus species. B. Present-day map showing the location of the Western USA Basin. C. Simplified geological map of north-eastern Nevada with location of Montello Canyon. D. Stratigraphic position of Dadocrinus montellonis sp. nov. Maps modified after Brayard et al. (2017) and Smith et al. (2021).
Mullen (1985) reported the occurrence of Griesbachian (early Induan, Early Triassic) conodonts in the Dinwoody Formation and middle–late Spathian-aged (late Olenekian, Early Triassic) conodonts in the unnamed siltstone unit and Thaynes Group, meaning that part of the Dienerian, the entire Smithian and part of the lower Spathian were locally removed by thrust faulting and erosion. For instance, the iconic middle Smithian (lower Olenekian, Lower Triassic) regional marker beds, the Meekoceras beds (e.g., Jattiot et al. 2017; Jenks and Brayard 2018), are consistently absent from the studied area. Our recent field investigations agree with Mullen’s (1985) conclusions and the discovery of successive ammonoid faunas in the Thaynes Group of Immigrant Canyon confirms a middle–late Spathian age (late Olenekian, Early Triassic) for the lower part of this unit (see Smith et al. 2021 for a schematic preliminary biostratigraphic zonation of the area). In addition, these new ammonoid faunas indicate that the upper part is actually Anisian (Middle Triassic), as already hypothesized by Mullen (1985) but without biostratigraphic evidence. At the sample site in Montello Canyon, no ammonoids or other marine organisms were found thus far. The Thaynes Group also directly overlies the Dinwoody Formation, implying that both units are probably incomplete. However, by comparison with the geographically close Immigrant Canyon, most of the basal part of the Thaynes Group was preserved in Montello Canyon indicating that these exposures are middle–upper Spathian (thicker limestone beds of the upper part are also found above in the section). The sampled slab was located on the slope of a hill where the lower part of the Thaynes Group outcrops, just above the contact between the Dinwoody Formation and the Thaynes Group. It is thus middle–late Spathian in age (late Olenekian, Early Triassic).
From Montello Canyon, Twitchett et al. (2005) also reported the presence of complete Early Triassic ophiuroid specimens, but without precisions on their sampled sites and thus, on their exact ages. Taking into account the complex tectonic structure of the studied area, these specimens might also be early Anisian, instead of Early Triassic. Smith et al. (2022) described various exceptionally-preserved specimens of leptomitid sponges, crustacean decapods, brachiopods, ophiuroids, as well as vertebrate remains from the middle–upper Spathian (upper Olenekian, Lower Triassic) of the Thaynes Group in the close Immigrant Canyon. These organisms are associated to the remarkably diversified and complex Spathian Paris Biota found at various places and time intervals within the Western USA Basin (Brayard et al. 2017; Smith et al. 2021). The newly described and well-preserved Dadocrinus specimen may therefore be related to the Paris Biota, enlarging the number of clades occurring in it.
Material and methods
A single specimen was collected in Montello Canyon (41°17’21.30”N, 114°19’36.30”W), Elko County, northeastern Nevada (Fig. 1), from a calcareous shale slab in an exceptional good state of preservation with most ossicles of stem, crown and arms in connection. Unfortunately, the surficial weathering of the specimen hinders observation of articulations between skeletal elements. After delicate manual preparation of the crinoid-bearing slab, the anatomy of the specimen was examined under a binocular microscope equipped with a camera lucida for drawing the detailed plate architecture (Fig. 2).
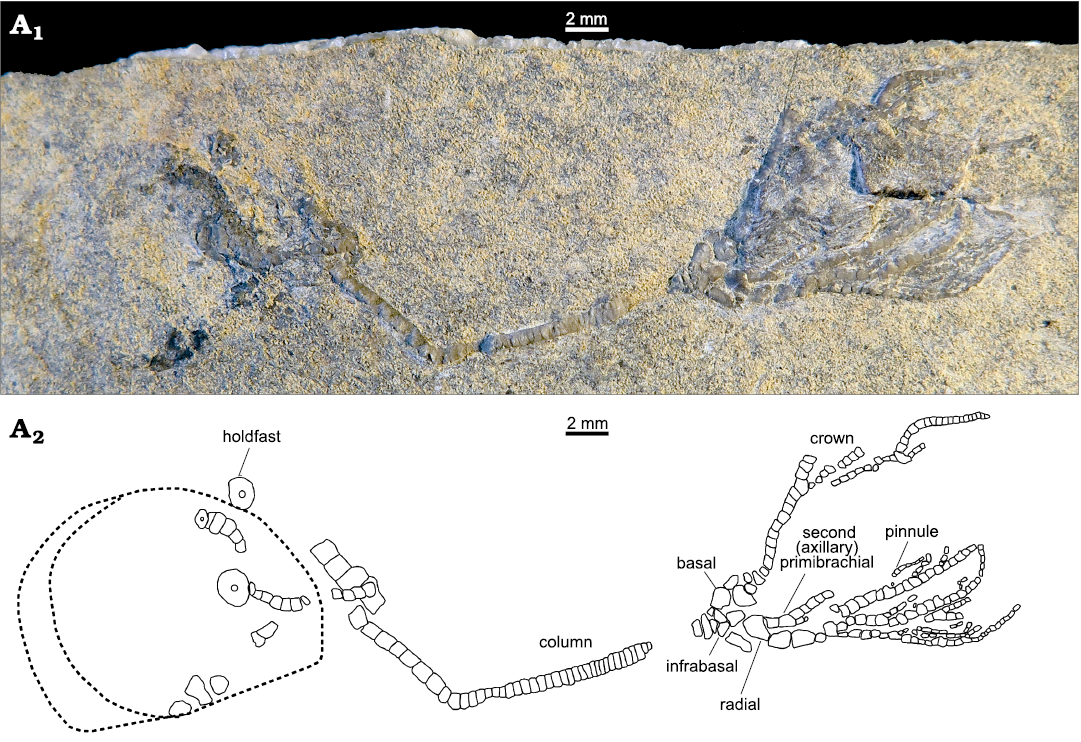
Fig. 2. Overall view of the holotype of the encrinid crinoid Dadocrinus montellonis sp. nov. (UBGD 292410), Spathian, Lower Triassic, Montello Canyon, Nevada, USA. Natural light photography (A1) and camera lucida drawing (A2). The dashed line shows the outline of a shell imprint.
Elemental maps were acquired using a BRUKER M4 Tornado micro-X-ray fluorescence (XRF) 2D scanner at the Biogeosciences laboratory (GISMO Platform, Université de Bourgogne, Dijon, France). Equipped with a chromium polycapillary X-ray tube (25 µm spot size) and with two XFlash silicon drift detectors working together, this system permits chemical mapping of the whole Dadocrinus specimen with a scan pitch of 25 µm, but also zooms with a scan pitch of 12 µm. Under a vacuum of 7 mbar, several chemical elements were mapped (e.g., Ca, Mg, Si, Al, K, Sr, Fe, Ti) but the best contrast between the fossil and the matrix was achieved with calcium (Fig. 3A1), an element obviously abundant in the calcite test of the crinoid but less abundant in the clay-rich and quartz-rich matrix of the fossil.
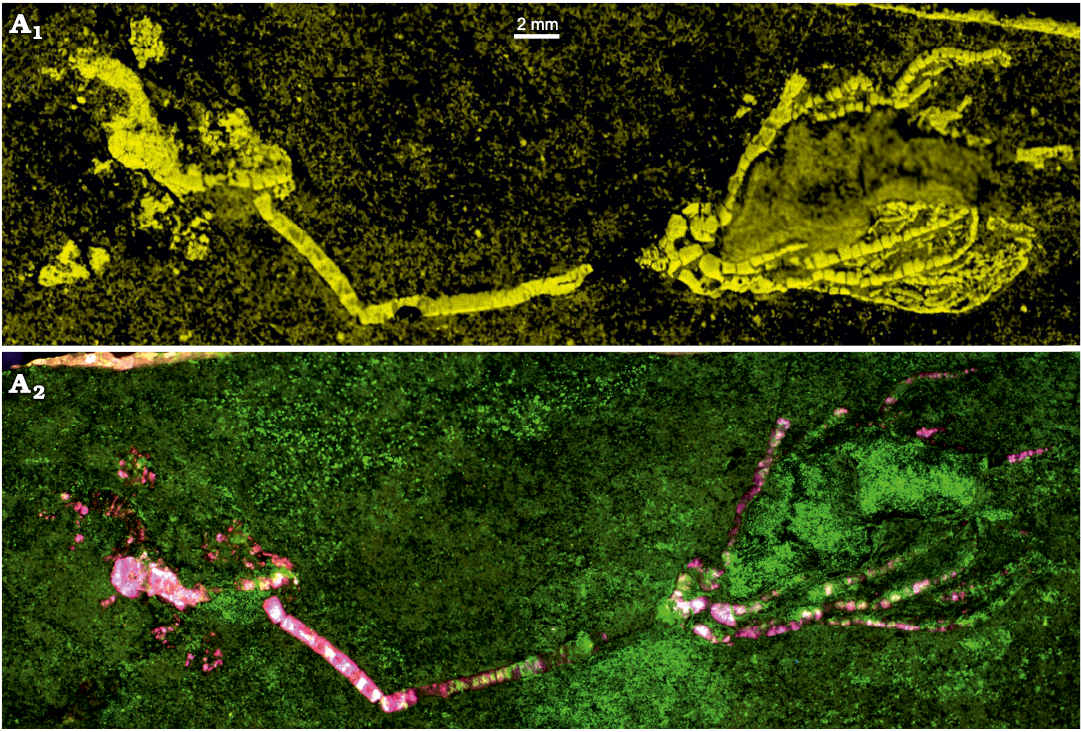
Fig. 3. Advanced imaging of the holotype of the encrinid crinoid Dadocrinus montellonis sp. nov. (UBGD 292410), Spathian, Lower Triassic, Montello Canyon, Nevada, USA. A1, Ca map; the colour scale goes from dark to light yellow for low to higher relative concentration of calcium (Ca is abundant in the calcite crinoid pieces, but rarer in the fine clastic sediment hosting the fossil). A2, UV-excited luminescence composite image; ilumination/detection couples: red 385/732 nm; green 385/571 nm; blue 385/835 nm.
UV-visible and X-ray excited optical luminescence images were acquired at the IPANEMA Laboratory (Gif-sur-Yvette, France) and at the PUMA beamline of the SOLEIL synchrotron (Gif-sur-Yvette, France), respectively (Figs. 3A2, 4D). UV-visible illumination was provided by a 16-LED light source (CoolLED pE-4000) coupled to a liquid light-guide fibre fitted with a fibre-optic ring light-guide (full setup described in Brayard et al. 2019). At PUMA, the specimen was illuminated with a 12.5 keV monochromatic X-ray beam. A series of images with 1 mm movement were recorded to extend laterally and vertically the field of view (1.3 × 1.3 mm2) and image most of the crown of the specimen (Fig. 4D). In both cases, the luminescence signal was detected in narrow spectral ranges using interference band-pass filters (Semrock) positioned in front of a high-sensitivity and low-noise camera. Greyscale images obtained under different illumination/detection spectral ranges (detailed in the figure captions) were combined into false colour RGB composite images using ImageJ, after contrast enhancement of each image using the auto “Brightness/Contrast” tool.
Systematics and terminology for morphological descriptions follow the revised version of the Treatise of Invertebrate Paleontology, Crinoidea, volume 3 (Hess and Messing 2011) as well as Hagdorn (2020).
Systematic palaeontology
Phylum Echinodermata Klein, 1778
Class Crinoidea Miller, 1821
Subclass Articulata Zittel, 1879
Order Encrinida Matsumoto, 1929
Family Dadocrinidae Lowenstam, 1942
Genus Dadocrinus von Meyer, 1847
Type species: Dadocrinus gracilis (von Buch, 1845), from the Formazione à gracilis, upper Olenekian–upper Anisian, Recoaro, Italy.
Dadocrinus montellonis sp. nov.
Figs. 2–5.
ZooBank LSID: urn:lsid:zoobank.org:act:ECA27511-2CA8-4BA7-A689-CE2CEEE0EFD9
Etymology: From the geographical name Montello Canyon, the place where the specimen originates (genitive singular case).
Holotype: One near complete and articulated specimen UBGD 292410 preserved on a fine sandstone slab along with holdfasts and distal columnals of two other specimens.
Type locality: Montello Canyon, Elko County, northeastern Nevada, USA (Fig. 1B, C).
Type horizon: Middle–upper Spathian (upper Olenekian, Lower Triassic) of the Thaynes Group (sensu Lucas et al. 2007) (Fig. 1C, D).
Material.—Holotype only.
Diagnosis.—Small dicyclic species of Dadocrinus with a contiguous circle of connected and well-developed infrabasals, clearly visible in lateral view. Primibrachials 1 and 2 very thin, higher than wide, only half as wide as radials. Pinnules stem from every third brachial.
Description.—The almost complete specimen consists of articulated arms, cup and stem. The total length of the specimen, from the holdfast to the preserved extremity of arms is 46.5 mm. The crown itself is 16.5 mm long (but entire arms are not preserved) and has 3 radials, 3 basals and 3 infrabasals visible (Figs. 2, 3).
Crown: Dicyclic cup low, conical in shape (Fig. 4). Circle of infrabasals contiguous, infrabasals very well developed and clearly visible in lateral view. They are wider than long, 0.6 mm long, and 0.8 mm wide, almost rectangular in shape and articulate in between basals with a curved, convex suture. Basals and radials high and thin. Basals are twice as long as wide and insert with a roof-shaped suture between radials. Radials are as long as wide. From the lateral view only, it can be deduced that radial articular facets with primibrachials are muscular and sloping outward. Infrabasals, basals and radials seem to be connected by synostoses with deep ligament pits visible on basals. Surface of infrabasals, basals, and radials smooth.
Arms: Ten arms, uniserial throughout, branching at the second primibrachials (Fig. 4A2). Brachials relatively high and rectangular in shape (not wedge-shaped). Primibrachials 1 and 2 very thin, higher than wide, only half as wide as radials. First secondibrachials thin and higher than wide, then following ones as high as wide, progressively thinner and narrower distally. At least 28 articulated secondibrachials can be counted on one arm but arms are very likely longer. Dorsal side of all brachials rounded and even, but not flat, sharp-edged or keeled. Pinnulars smooth and thin, about three times as long as wide, pinnules widely spaced, branching on every third brachial. First pinnular twice shorter than the following ones. Terminal pinnulars not visible.
Stem: Total length is 30 mm. Column cylindrical with no cirri. No differentiation in size between nodals and internodals is visible, but proximal and proxistele are only partially preserved and the shape of proximal columnars is hardly discernible. Typically, the proximal however appears a bit enlarged but not higher as compared to the following columnals. In the mesistele, all columnals are equally sized and circular, about twice as wide as high. Columnals become barrel-shaped in the dististele; they are about three times as high as medial columnals. Articulations between columnars symplexial with multiradiate crenulation visible in the mesistele. Terminal columnal with discoid, encrusting holdfast.
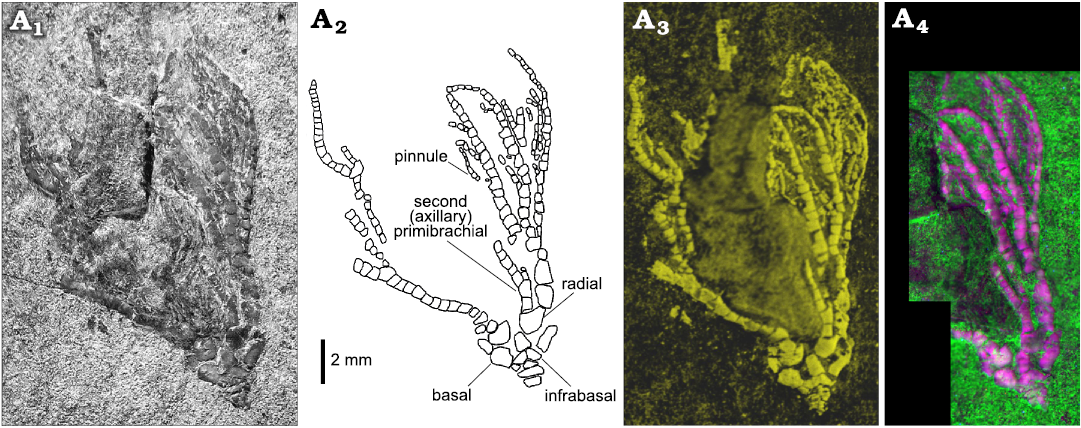
Fig. 4. Close-up views of the crown of the encrinid crinoid Dadocrinus montellonis sp. nov. (UBGD 292410), Spathian, Lower Triassic, Montello Canyon, Nevada, USA. Natural light photography (A1), camera lucida drawing (A2), Ca map (A3), and X-ray and visible excited luminescence composite image (A4). Illumination/detection couples: red, natural light/650 nm; green, X-ray/571 nm; blue, X-ray/650 nm.
Remarks.—Dadocrinus montellonis sp. nov. shows the presence of a conical dicyclic to cryptodicyclic cup with a circle of contiguous basals, radial articular facets sloping outward, and a cylindrical column with no cirri ending in a terminal disk, which are diagnostic features of the family Dadocrinidae (Hagdorn 2011; Hess and Messing 2011). Unfortunately, the preservation of the specimen, only visible in lateral view, and surficial weathering hinder observation of articulations between skeletal elements. Three nominal species of Dadocrinus have been described so far, all reported from the Anisian (Middle Triassic) of Central Europe (Poland, Germany, Switzerland, Austria, Hungary, northern Italy), Turkey, and New Zealand: Dadocrinus gracilis (Buch, 1845), Dadocrinus grundeyi Langenhan, 1903, and Dadocrinus kunischi Wachsmuth and Springer, 1887 (Lefeld 1958; Eagle 2003, 2004; Hagdorn 1996, 2011, 2020; Hess and Messing 2011).
All species of Dadocrinus are characterised by the presence of high and thin basals and radials, ten arms uniserial throughout branching at the second primibrachials, with brachials relatively high and rectangular, second primibrachials axillary with two almost equally wide muscular facets, and smooth pinnulars (Hess and Messing 2011; Hagdorn 2020). Species of Dadocrinus differ with each other mainly in overall body size, in the position, development, and visibility of infrabasals in the cup, and in the morphology of proximal columnals (Hess and Messing 2011; Hagdorn 2011, 2020). However, these characters may vary within a single fossil assemblage, and the three nominal species have been regarded as distinct ontogenetic stages or ecophenotypes of one single species (Głuchowski 1986; Hagdorn et al. 1996; Eagle 2003; Hess and Messing 2011). D. montellonis sp. nov. differs from the three nominal species of Dadocrinus by its smaller size, the circle of contiguous infrabasals, the thin primibrachials, and pinnules stemming from every third brachial and not on alternate consecutive brachials starting from the second secondibrachials (Eagle 2003).
In D. gracilis, infrabasals are rarely visible, the proximal part of arms is slightly rounded then V-shaped, and in the column, proximal nodals are a bit thicker and higher than internodals.
Juveniles of D. grundeyi show a dicyclic cup but infrabasals are not contiguous and are partly covered by the proximal columnal (Lefeld 1958). Moreover, in D. grundeyi, the dorsal side of proximal arms is weakly rounded, then more rounded distally, pinnules articulate on alternate side of brachials (Hagdorn 1996: pl. 3), and axillary primibrachials are the same size as radials.
In D. kunischi, individuals grow larger (up to 7 cm for the crown, 20 cm in total), infrabasals are not visible, and pinnules articulate on every brachial (Hagdorn 1996: pl. 2). In the proxistele, nodals and first internodals are wider and higher.
Hagdorn (2020) recently described a new genus and species of the family Dadocrinidae, Aszulcicrinus pentebrachiatus Hagdorn, 2020, from the lower Anisian (Middle Triassic) of Upper Silesia (Poland). Dadocrinus montellonis sp. nov. differs from A. pentebrachiatus by a complete circle of contiguous infrabasals, ten arms branching at the second primibrachial (against five in A. pentebrachiatus), the dorsal side of radials and brachials rounded (not sharp-edged, keeled or V-shaped) and the cup is low cone-shaped.
Based on small isolated columnals, Oji and Twitchett (2015) described a new genus and species from the lower Induan (Griesbachian, Lower Triassic) of Oman, Baudicrinus krystyni Oji and Twitchett, 2015, which they attributed to the family Dadocrinidae, thereby pushing back the origin of the family to the very early Triassic. However, new material from the middle Griesbachian of Oman was revisited by Brosse et al. (2019) who re-assigned B. krystyni to the family Holocrinidae Jackel, 1918, based on the presence of sub-pentagonal proximal columnals, bifurcated culmina on columnals, and cylindrical cirri. These new interpretations support the hypothesis of early representatives of Holocrinidae as the oldest post-Palaeozoic crinoids (Hagdorn 2020).
Discussion
Biostratigraphy.—Crinoid diversity was profoundly affected by the severe mass extinction at the PTB (Paul 1988; Benton and Twitchett 2003), when most echinoderms went extinct. As soon as the Early Triassic, they rapidly diversified from two distinct lineages, the holocrinids and the dadocrinids (Simms 1988; Hagdorn 1995, 2020; Twitchett and Oji 2005; Hess and Messing 2011; Oji and Twitchett 2015; Brosse et al. 2019; Saucède et al. 2019). The first representatives of the holocrinids differentiated rapidly after the PTB, during the early Griesbachian (Oji and Twitchett 2015; Brosse et al. 2019) and then markedly diversified during the Triassic (Hagdorn 2011). So far, dadocrinids were first represented in the fossil record by the genus Dadocrinus from the early Anisian-aged deposits of the Germanic Basin (Hagdorn 1999, 2011, 2020) and potentially from rocks as old as the latest Early Triassic (see Nawrocki and Szulc 2000), but this age remains to be confirmed based on firm biostratigraphic evidence.
Five species were assigned to the small crinoid family Dadocrinidae so far: the five-armed species A. pentebrachiatus (early Anisian), the 10-armed nominal species D. gracilis (late Olenekian to late Anisian), D. grundeyi (early Anisian), D. kunischi (early Anisian), and the 20-armed species Carnallicrinus carnalli (middle Anisian) (Hagdorn 2011, 2020). The Dadocrinus was widely distributed in the South Alpine and Germanic basins in the late Olenekian and early Anisian (Hagdorn 1999; Niedźwiedzki and Salamon 2006) (Fig. 1A). The morphology and stratigraphic distribution of Dadocrinus species have been extensively studied and detailed in many palaeontological and stratigraphic works based on the abundant and exceptionally well-preserved material from the Lower Muschelkalk Lagerstätten of Poland (Upper Silesia, North-Sudetic Basin, and Tatra Mountains), eastern Germany, and South Alpine Italy (Vicentinian Prealps) (Lefeld 1958; Hagdorn 1996, 2011, 2020; Hess and Messing 2011). Along with the associated benthic fauna, studies of this exceptional material significantly improved our understanding of Triassic crinoid evolution and palaeocology (Hagdorn 2011; Hess and Messing 2011).
The well-preserved and abundant material of Dadocrinus also makes it the most important crinoid genus for the basal Triassic stratigraphy of the Germanic Basin due to the scarcity of otherwise common index taxa such as ammonoids and conodonts (see Hagdorn 1999, 2020 for review). Restricted to the Upper Bundsandstein and Lower Muschelkalk, the genus was used for regional and inter-basin correlations (Niedźwiedzki and Salamon 2006; Hagdorn 2020). In the crinoid biostratigraphic zonation of the Muschelkalk, established based on data from Germanic Lagerstätten, the Dadocrinus biozone was recognized in the lower Gogolin Formation of Silesia (Poland), Röt Formation (Germany) (Hagdorn and Głuchowski 1993), North-Sudetic Basin (Głuchowski and Salamon 2005) and Tatra Mountains (Niedźwiedzki and Salamon 2006). The Dadocrinus biozone is an indicator of the lower Anisian and also potentially of the very uppermost Olenekian (Hagdorn and Głuchowski 1993; Hagdorn 1999; Nawrocki and Szulc 2000). Apart from Europe, Dadocrinus was also reported from the upper Anisian of Turkey and New Zealand (Eagle 2003, 2004; Niedźwiedzki and Salamon 2006; Hagdorn 2011) (Fig. 1A). Thus, D. montellonis sp. nov. corresponds to the first reported occurrence of the genus Dadocrinus outside the Germanic Basin prior to the Anisian (Brosse et al. 2019; Hagdorn 2020) and also to the oldest firm evidence of its presence in the Early Triassic (middle–late Spathian). This new occurrence therefore suggests a much wider distribution than commonly assumed for the genus already during the Spathian, both in the Tethys and Panthalassa (Fig. 1A). It also questions the centre of origin for dadocrinids that was hypothesized to be in the western Tethys so far (Eagle 2004).
Taphonomy and palaeocology.—Like crinoid specimens from the Lower Muschelkalk Lagerstätten of the Germanic Basin, the specimen of D. montellonis sp. nov. from Montello Canyon is exceptionally well-preserved, almost complete and entirely articulated. The near complete articulation of the entire skeleton along with the preservation of the holdfast (Figs. 2, 3, 5) in close proximity to distal columnals suggest a rapid in situ smothering of the individual and its preservation under fair weather conditions; occasional storms being responsible for a rapid input of sedimentary material. Two additional holdfasts are also preserved on the same slab, along with isolated distal columnals from at least two supplementary specimens that were not preserved on the slab (Figs. 2, 3). On the well-preserved specimen of D. montellonis sp. nov., plates of the cup are partially displaced relative to each other probably as a result of the sedimentary load, a common taphonomic pattern observed in other dadocrinids and which is thought to have been facilitated by the smooth and flat surface of synostosial articulations between apposed facets of cup plates (Hagdorn 2011; Hess and Messing 2011).
The fine detrital nature of the sediment (a fine quartz sandstone with a marly matrix) also likely favoured the partial preservation of a fossil shell outline imprint that is clearly visible on the slab when observed with raking light (Fig. 5A1). The presence of the three holdfasts preserved in close proximity to each other and associated to the extremity of the shell imprint suggests that specimens may have lived anchored as epibionts on the shell, which probably constituted a hard substratum amongst the soft-bottom habitat offered by the fine sandstone. Unfortunately, the entire dissolution of the shell, as indicated by the low concentration of calcium in micro-XRF scans (Fig. 3A1), precludes an unambiguous identification of the organism at the origin of the cast, which can be either a bivalve or a brachiopod. This type of encrusting life style and attachment as sessile epizoans on shell parts has only been reported on few occasions for Early Triassic organisms (Fraiser 2011; Brayard et al. 2011, 2015, 2017; Hautmann et al. 2017; Zatoń et al. 2018), but never so far for crinoids. The specimen of D. montellonis sp. nov. is therefore all the more important as it confirms the existence of Early Triassic attached, erected, suspension-feeders other than, e.g., bryozoans (Schäfer et al. 2003; Baud et al. 2008), at least as soon as the middle–late Spathian.
Post-Palaeozoic crinoids diversified in two major lineages during the inception of the so-called “Mesozoic Marine Revolution”: the Encrinida (including the Dadocrinidae) and the Holocrinida/Isocrinida (Baumiller et al. 2010). The two lineages followed radically distinct evolutionary trajectories: while Encrinida inherited from their Palaeozoic ancestor a passive anchored mode of life with permanent attachment to hard substrates, Holocrinida and Isocrinida evolved the capacity of autotomy and flexible re-attachment after stem rupture associated with active motility (Baumiller et al. 2010; Hagdorn 2011; Gorzelak 2018).
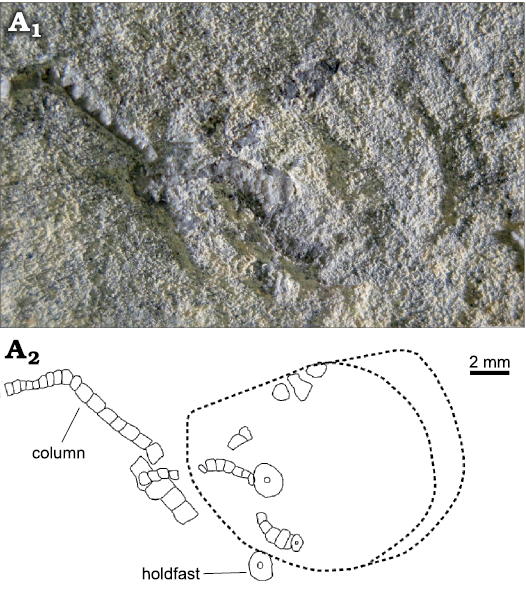
Fig. 5. Close-up view of holdfast of the encrinid crinoid Dadocrinus montellonis sp. nov. (UBGD 292410), Spathian, Lower Triassic, Montello Canyon, Nevada, USA, also showing the outline of a shell imprint (dashed line) for possible crinoid anchorage. Natural light photograph (A1) and camera lucida drawing (A2).
Dadocrinus species likely did not move as an adult and had inherited from their hypothetical “proto-Articulata” ancestor a discoid holdfast as a result of the specialisation of terminal columnals for attachment to hard substrates (Hagdorn 2011). The presence of a terminal discoid holdfast in species of Dadocrinus implies that they needed a solid anchoring ground to settle and live, yet sedimentological and palaeoecological evidences from Germanic Lagerstätten show that they likely inhabited soft-bottom habitats (Hagdorn 1996, 2011). This is in line with the facies of the slab in which the specimen of D. montellonis sp. nov. was discovered. The specimen was fossilised in situ and likely lived in a soft-bottom environment with sediments characterised by some bioclasts but dominated by fine detrital particles deposited under relatively low hydrodynamic conditions. In Lagerstätten from the Germanic Basin, specimens of D. kunischi were described settled on the posterior side of endobyssate bivalve shells (i.e., the part of the shell emerging above the sediment-water interface), such as the bakevelliid Gervillella sp., or stalks of other crinoid individuals; the fixation of specimens to hardgrounds has also been seldom observed (Hagdorn 1996, 1999). Specimens of D. gracilis from the upper Anisian of New Zealand were also found attached to the shells of athyridid brachiopods (Eagle 2003). It has been hypothesized that living as epibionts on mud-sticking bivalves or brachiopods allowed species of Dadocrinus to colonise quiet soft-bottom environments and gain access to a higher position in the water column, leading to an improved filter-feeding efficiency (Eagle 2003). The relative, small size of species Dadocrinus, as compared to other encrinids, is also thought to have influenced its evolutionary success in early Anisian soft bottom communities of the Germanic Basin (Hagdorn 1996), because endobenthic bivalves may not have stood large crinoids in muddy environments (Hagdorn 2011). This markedly contrasts with later living conditions documented for late Anisian encrinids that encrusted patches of terquemiid bivalves and formed bioherms of up to 2–3 m in diameter and 1.5 m thick. Late Anisian encrinids were frame builders, their holdfasts forming solid crusts and the stems of adults also forming solid anchors for juveniles.
Evolutionary trends in Dadocrinidae.—Over the last two decades, several palaeontological studies investigated the origin, evolution and early diversification of post-Palaeozoic crinoids (Benton and Twitchett 2003; Twitchett and Oji 2005; Oji and Twitchett 2015; Baumiller et al. 2010; Hagdorn 2011, 2020; Hess and Messing 2011; Rouse et al. 2013; Brosse et al. 2019; Saucède et al. 2019; among others). They all converged towards a scenario of rapid evolution and intense morphological diversification of the subclass Articulata as soon as the earliest Triassic (Webster and Jell 1999; Twitchett and Oji 2005; Webster and Lane 2007; Oji and Twitchett 2015; Salamon et al. 2015; Brosse et al. 2019), although the alternative scenario of a Permian origin of the Articulata cannot be excluded (Cohen and Pisera 2017). Dadocrinus montellonis sp. nov. is among the oldest representatives of the order Encrinida to be reported in the fossil record so far, as fossil evidence from the Griesbachian (lower Induan) of Oman and Svalbard suggests that only holocrinids and isocrinids differentiated before the Olenekian (Oji and Twitchett 2015; Salamon et al. 2015; but see Brosse et al. 2019; Hagdorn 2020). The early occurrence of D. montellonis sp. nov. is also well in line with the predominance of ancestral dadocrinid characters featured by the small size of the specimen as well as by the morphology and plate arrangement of the stem, cup and arms, and by pinnule density.
Hagdorn (2011) embodied all ancestral characters inherited from the likely Palaeozoic cladid root of all Articulata into an Early Triassic hypothetical “proto-Articulata” ancestor. This ancestral proto-articulate crinoid would include a dicyclic cup with three complete and contiguous plate circles made of infrabasals, basals, and radials. The family Dadocrinidae would have subsequently evolved a low-cone cup shape with progressively concealed infrabasal, the basal and radial plates only remaining visible in lateral view (Hagdorn 2011). The family also shows a phylogenetic trend towards an increase in body size, arm length, arm number and pinnule density, the co-evolution of these characters being associated with the improvement of filter-feeding efficiency through time (Hagdorn 2011, 2020).
Size is one of the main features that distinguish D. montellonis sp. nov. from other species of Dadocrinus. In the studied specimen of D. montellonis, the crown is less than 20 mm long. In contrast, it is 25 mm long in D. gracilis, 40 mm in D. grundeyi and up to 70 mm long in D. kunischi (Hagdorn 1996). The five-arm species Aszulcicrinus pentebrachiatus is also larger than D. montellonis sp. nov. with a crown of up to 30 mm long (Hagdorn 2020). As a consequence, the relative small size of D. montellonis sp. nov. can be interpreted as ancestral and indicative of the species preference for soft-bottom habitats (Hagdorn 1999).
The dicyclic plate pattern of D. montellonis sp. nov. with a circle of contiguous infrabasals can be also interpreted as ancestral within the Dadocrinidae, in which the remodelling of the cup evolved toward a decreasing size of infrabasals. In the early Anisian, the cup is dicyclic in juveniles of D. grundeyi, but infrabasals are not contiguous and are partly covered by the proximal columnal (Lefeld 1958). It is cryptodicyclic in D. gracilis and D. kunischi. In the middle Anisian, the cup is monocyclic in C. carnalli with infrabasals concealed in stem pits (Lefeld 1958; Hagdorn 2011). In D. montellonis sp. nov., arms are uniserial throughout with widely spaced pinnules stemming from every third brachial but not from alternate consecutive brachials as in other Dadocrinidae. This is representative of a first stage in the evolution of the Encrinida that later evolved towards a biserial plate pattern and denser pinnulation for improvement of filter-feeding efficiency (Hagdorn 2011).
With five arms, the early Anisian Aszulcicrinus was interpreted as representative of an ancestral stage in the evolution of the Dadocrinidae that evolved ten arms in the genus Dadocrinus, and then twenty arms in the genus Carnallicrinus (Hess and Messing 2011; Hagdorn 2020). However, Hagdorn (2020) also pointed out that the five-arm number, which is diagnostic of the genus Aszulcicrinus, could well be interpreted as a secondary reduction of the arm number from a ten-armed ancestor (Hagdorn 2011). The lower stratigraphic position of the ten-armed D. montellonis sp. nov. supports this second hypothesis of a secondary reduction in arm number from Dadocrinus to Aszulcicrinus.
Palaeobiogeography.—The genus Dadocrinus is mostly known from the western Tethys in the Anisian. Its distribution covers the eastern part of the Germanic Basin (Poland, Germany, Switzerland, Austria, and Hungary) and the Alpine realm (northern Italy and Tatra Mountains) (Lefeld 1958; Hagdorn 1996, 2011, 2020; Hess and Messing 2011). The genus first occurred during the Muschelkalk transgression that potentially brought Tethyan elements of the marine faunas from the Asiatic palaeobiogeographic province into the Germanic Basin through the northern Palaeotethys (Kozur 1974; Hagdorn 1999, 2020). Dadocrinus gracilis is also reported from upper Anisian deposits of Turkey and New Zealand (Eagle 2003, 2004). Considering the abundant material and wide distribution of Dadocrinus in the western Tethys as soon as the early Anisian, Eagle (2003, 2004) suggested a Tethyan origin of the genus followed by a late migration to the southwestern Panthalassa (i.e., New Zealand) along a Tethyan route, before the genus went extinct in the late Anisian.
The discovery of a specimen of D. montellonis sp. nov. in the Spathian of Nevada thus questions this former palaeobiogeographic dispersal scenario favouring the Tethys as a centre of origin for Dadocrinus. During the Early Triassic and Anisian, Montello Canyon was situated at the opposite side of the Pangea from the Germanic Basin, where much material of Dadocrinus has been described so far. It was also very distant from the southwestern Panthalassa, from where New Zealand materials of D. gracilis were reported (Eagle 2003, 2004). The newly reported occurrence of D. montellonis sp. nov. thus considerably enlarges the known palaeogeographic distribution of the genus, being represented in intertropical settings across both the Tethys and the Panthalassa. No marine corridor has ever been reported, however, across the Pangea between the Tethys and the eastern Panthalassa for the considered time interval (Fig. 1), strongly suggesting that the genus has been able of long transoceanic dispersals, especially through the large Panthalassa Ocean (at larval stages?) during its evolutionary history. Long-distance transoceanic faunal exchanges across the entire Panthalassa have already been documented for some marine Early Triassic and Anisian organisms such as ammonoids (Brayard et al. 2007, 2009; Jenks et al. 2010; Monnet et al. 2013; Shigeta and Kumagae 2015; Jenks and Brayard 2018).
Dadocrinus montellonis sp. nov. differs from other species of Dadocrinus by its palaeobiogeographic position but also by its early stratigraphic occurrence and ancestral morphology. Altogether, these palaeobiogeographic, stratigraphic and morphological evidences may point to alternative palaeobiogeographic scenarios for the origin of Dadocrinidae. Potentially, such scenarios would imply an ancient origin of the Dadocrinidae, well before the early Anisian, and question the Tethyan origin of Dadocrinus which is the earliest representative of the family Dadocrinidae and order Encrinida. An Early Triassic evolution and differentiation of dadocrinids is congruent with the occurrence of early representatives of holocrinids as soon as the Griesbachian (Oji and Twitchett 2015; Brosse et al. 2019), also considering that the diversity of early post-Palaeozoic crinoids has long been under-estimated (Kashiyama and Oji 2004; Hagdorn and Göncüoglu 2007; Oji 2009; Hagdorn 2011, 2020; Salamon et al. 2012; Saucède et al. 2019). This also implies that the first dadocrinids originated either in the Panthalassa or Tethys oceans, and then dispersed over long distances in a relative short period of time, good dispersal capabilities being well in line with the wide distribution of Dadocrinus (Eagle 2004; Niedźwiedzki and Salamon 2006) and its local, endemic species or ecophenotypes (Hess and Messing 2011; Hagdorn 2011, 2020; Niedźwiedzki and Salamon 2006). However, such interpretations are in need of more data and additional material to support robust palaeobiogeographic scenarios, and the discovery of new specimens from other places may well change again the current state of knowledge on the early evolution of post-Palaeozoic crinoids.
Conclusions
The discovery of a new species of Dadocrinus, D. montellonis sp. nov., from the middle–late Spathian (late Olenekian, Early Triassic) of Nevada sheds new light on the origin of the family Dadocrinidae and order Encrinida, one of the two lineages of early post-Palaeozoic crinoids that ensured the recovery of crinoid diversity following the Permian/Triassic Boundary mass extinction. The early stratigraphic position of the species is congruent with its small size and ancestral morphology that match the evolutionary trend formerly documented for the family (Hagdorn 2011). It also confirms that dadocrinids were already differentiated by the Spathian (Nawrocki and Szulc 2000; Hagdorn 2020) and were widely distributed (Niedźwiedzki and Salamon 2006). However, D. montellonis sp. nov. questions the commonly assumed Tethyan origin and proposed palaeobiogeographic history of the family Dadocrinidae, which is mainly known from the Lower Muschelkalk of the Germanic Basin (Eagle 2004). It suggests that dadocrinids originated either in the Panthalassa or Tethys and then dispersed over long distances.
Acknowledgements
We acknowledge the GISMO platform for the XRF acquisition and the SOLEIL synchrotron for provision of beamtime and we would like to thank Sebastian Schöder (SOLEIL synchrotron, Paris-Saclay, France) for assistance at the PUMA beamline. This work was supported by the ANR project AFTER (ANR-13-JS06-0001-01), the French “Investissements d’Avenir” program (project ISITE-BFC: ANR-15-IDEX-03), and also received funding from the programme TelluS of the Institut National des Sciences de l’Univers, CNRS. We thank James F. Jenks (West Jordan, Utah, USA) and Kevin G. Bylund (Spanish Fork, Utah, USA) for their constant support in our Triassic projects and for their invaluable help in the field. The Winecup Gamble Ranch (Montello, Nevada, USA) is also thanked for allowing access to the Montello Canyon site. Sample management and valorisation benefited from the ANR project RECOLNAT (ANR-11-INBS-0004). We also acknowledge Przemysław Gorzelak (Insitute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland) and Tatsuo Oji (University Museum, Nagoya University, Japan) for their constructive comments on an earlier version of the manuscript.
References
Baud, A., Nakrem, H.A., Beauchamp, B., Beatty, T.W., Embry, A.F., and Henderson, C.M. 2008. Lower Triassic bryozoan beds from Ellesmere Island, High Arctic, Canada. Polar Research 27: 428–440. Crossref
Baumiller, T.K., Salamon, M.A., Gorzelak, P., Mooi, R., Messing, C.G., and Gahn, F.J. 2010. Post-Paleozoic crinoid radiation in response to benthic predation preceded the Mesozoic marine revolution. Proceedings of the National Academy of Sciences 107: 5893–5896. Crossref
Benton, M.J. and Twitchett, R.J. 2003. How to kill (almost) all life: the end-Permian extinction event. Trends in Ecology and Evolution 18: 358–365. Crossref
Brayard, A., Escarguel, G., and Bucher, H. 2007. The biogeography of Early Triassic ammonoid faunas: Clusters, gradients, and networks. Geobios 40: 749–765. Crossref
Brayard, A., Escarguel, G., Bucher, H., and Brühwiler, T. 2009. Smithian and Spathian (Early Triassic) ammonoid assemblages from terranes: Paleoceanographic and paleogeographic implications. Journal of Asian Earth Sciences 36: 420–433. Crossref
Brayard, A., Gueriau, P., Thoury, M., Escarguel, G., and the Paris Biota team. 2019. Glow in the dark: Use of synchrotron μXRF trace elemental mapping and multispectral macro-imaging on fossils from the Paris Biota (Bear Lake County, Idaho, USA). Geobios 54: 71–79. Crossref
Brayard, A., Krumenacker, L.J., Botting, J.P., Jenks, J.F., Bylund, K.G., Fara, E., Vennin, E., Olivier, N., Goudemand, N., Saucède, T., Charbonnier, S., Romano, C., Doguzhaeva, L., Thuy, B., Hautmann, M., Stephen, D.A., Thomazo, C., and Escarguel, G. 2017. Unexpected Early Triassic marine ecosystem and the rise of the Modern evolutionary fauna. Science Advances 3 (2): e1602159. Crossref
Brayard, A., Meier, M., Escarguel, G., Fara, E., Nützel, A., Olivier, N., Bylund, K.G., Jenks, J.F., Stephen, D.A., Hautmann, M., Vennin, E., and Bucher, H. 2015. Early Triassic Gulliver gastropods: Spatio-temporal distribution and significance for biotic recovery after the end-Permian mass extinction. Earth-Science Reviews 146: 31–64. Crossref
Brayard, A., Vennin, E., Olivier, N., Bylund, K.G., Jenks, J., Stephen, D.A., Bucher, H., Hofmann, R., Goudemand, N., and Escarguel, G. 2011. Transient metazoan reefs in the aftermath of the end-Permian mass extinction. Nature Geoscience 4: 693–697. Crossref
Brosse, M., Bucher, H., Baud, A., Frisk, A.M., Goudemand, N., Hagdorn, H., Nützel, A., Ware, D., and Hautmann, M. 2019. New data from Oman indicate benthic high biomass productivity coupled with low taxonomic diversity in the aftermath of the Permian–Triassic Boundary mass extinction. Lethaia 52: 165–187. Crossref
Clark, D.L. 1957. Marine Triassic stratigraphy in Eastern Great Basin. AAPG Bulletin 41: 2192–2222. Crossref
Cohen, B.L. and Pisera, A. 2017. Crinoid phylogeny: new interpretation of the main Permo-Triassic divergence, comparisons with echinoids and brachiopods, and EvoDevo interpretations of major morphological variations. Biological Journal of the Linnean Society 120: 38–53. Crossref
Eagle, M.K. 2003. Etalian and Kaihikuan (Middle Triassic) Crinoidea (Echinodermata: Articulata) from Caroline Cutting, Oreti valley, Southland, New Zealand. Journal of the Royal Society of New Zealand 33: 269–299. Crossref
Eagle, M.K. 2004. Tethyan Crinoids in the Panthalassa Ocean. Gondwana Research 7: 193–197. Crossref
Fraiser, M.L. 2011. Paleoecology of secondary tierers from Western Pangean tropical marine environments during the aftermath of the end-Permian mass extinction. Palaeogeography, Palaeoclimatology, Palaeoecology 308: 181–189. Crossref
Głuchowski, E. 1986. Crinoids from the Lower Gogolin Beds (Lower Muschelkalk) of the North-Eastern Part of Upper Silesia. Bulletin of the Polish Academy of Sciences, Earth Sciences 34: 179–187.
Głuchowski, E. and Salamon, M.A. 2005. The lower Muschelkalk crinoids from Raciborowice, north-Sudetic Basin, SW Poland. Geological Quarterly 49: 83–92.
Gorzelak, P. 2018. Microstructural evidence for stalk autotomy in Holocrinus—the oldest stem-group isocrinid. Palaeogeography, Palaeoclimatology, Palaeoecology 506: 202–207. Crossref
Hagdorn, H. 1995. Literaturbericht—Triassic crinoids. Zentralblatt für Geologie und Paläontologie, Teil II 1–2: 1–22.
Hagdorn, H. 1996. Palökologie der Trias-Seelilie Dadocrinus. Geologisch-paläontologische Mitteilungen Innsbruck 21: 18–38.
Hagdorn, H. 1999. Triassic Muschelkalk of Central Europe. In: H. Hess, W. Ausich, C. Brett, and M. Simms (eds.), Fossil Crinoids, 164–176. Cambridge University Press, Cambridge. Crossref
Hagdorn, H. 2011. Triassic: the crucial period of post-Palaeozoic crinoid diversification. Swiss Journal of Palaeontology 130: 91–112. Crossref
Hagdorn, H. 2020. Aszulcicrinus, a new genus of the Triassic crinoid family Dadocrinidae (Articulata; Encrinida) from Poland. Annales Societatis Geologorum Poloniae 90: 381–390. Crossref
Hagdorn, H. and Głuchowski, E. 1993. Palaeobiogeography and stratigraphy of Muschelkalk echinoderms (Crinoidea, Echinoidea) in Upper Silesia. In: H. Hagdorn and A. Seilacher (eds.), Muschelkalk. Schöntaler Symposium 1991. Sonderbände der Gesellschaft für Naturkunde in Württemberg 2: 165–176.
Hagdorn, H. and Göncüoglu, M.C. 2007. Early–Middle Triassic echinoderm remains from the Istranca Massif, Turkey. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 246: 235–245. Crossref
Hagdorn, H., Głuchowski, E., and Boczarowski, A. 1996. The crinoid fauna of the Diplopora Dolomite (Middle Muschelkalk, Upper Anisian) at Piekary Śląskie in Upper Silesia. Geologisch-Paläontologische Mitteilungen Innsbruck 21: 47–87.
Hautmann, M., Ware, D., and Bucher, H. 2017. Geologically oldest oysters were epizoans on Early Triassic ammonoids. Journal of Molluscan Studies 83: 253–260. Crossref
Hess, H. and Messing, C.G. 2011. Treatise on Invertebrate Paleontology. Part T, Echinodermata 2 Revised, Crinoidea 3. 261 pp. University of Kansas Press, Lawrence. Crossref
Jattiot, R., Bucher, H., Brayard, A., Brosse, M., Jenks, J.F., and Bylund, K. 2017. Smithian ammonoid faunas from northeastern Nevada: implications for Early Triassic biostratigraphy and correlation within the western USA basin. Palaeontographica A 309: 1–89. Crossref
Jenks, J.F. and Brayard, A. 2018. Smithian (Early Triassic) Ammonoids from Crittenden Springs, Elko County, Nevada: Taxomony, Biostratigraphy and Biogeography. New Mexico Museum of Natural History and Science 78: 1–175.
Jenks, J.F., Brayard, A., Brühwiler, T., and Bucher, H. 2010. New Smithian (Early Triassic) ammonoids from Crittenden Springs, Elko County, Nevada: Implications for taxonomy, biostratigraphy and biogeography. New Mexico Museum of Natural History and Science 48: 1–41.
Jenks, J.F., Maekawa, T., Ware, D., Shigeta, Y., Brayard, A., and Bylund, K.G. 2021. Late Griesbachian (Early Triassic) ammonoids and nautiloids from the Dinwoody Formation at Crittenden Springs, Elko County, Nevada. New Mexico Museum of Natural History and Science 86: 1–23.
Kashiyama, Y. and Oji, T. 2004. Low diversity shallow marine benthic fauna from the Smithian of Northeast Japan: paleoecologic and paleobiogeographic implications. Paleontological Research 8: 199–218. Crossref
Kozur, H. 1974. Biostratigraphie der germanischen Mitteltrias. Freiberger Forschungshefte C 280 (1): 1–86, (2) 1–71.
Lefeld, J. 1958. Dadocrinus grundeyi Langenhan (Crinoidea) from the High-Tatric Middle Triassic in the Tatra Mountains (Poland). Acta Palaeontologica Polonica 3: 59–74.
Lucas, S.G., Krainer, K., and Milner, A.R. 2007. The type section and age of the Timpoweap Member and stratigraphic nomenclature of the Triassic Moenkopi Group in Southwestern Utah. New Mexico Museum of Natural History and Science Bulletin 40: 109–117.
Monnet, C., Bucher, H., Brayard, A., and Jenks, J.F. 2013. Globacrochordiceras gen. nov. (Acrochordiceratidae, late Early Triassic) and its significance for stress-induced evolutionary jumps in ammonoid lineages (cephalopods). Fossil Record 16: 197–215. Crossref
Mullen, C.E. 1985. Structure and Stratigraphy of Triassic Rocks in the Immigrant Canyon Area, Northeast Elko County, Nevada. 78 pp. Unpublished M.Sc. Thesis, The University of Wisconsin, Milwaukee.
Nawrocki, J. and Szulc, J. 2000. The Middle Triassic magnetostratigraphy from the Peri-Tethys basin of Poland. Earth and Planetary Science Letters 182: 77– 92. Crossref
Niedźwiedzki, R. and Salamon, M.A. 2006. Triassic crinoids from the Tatra Mountains and their stratigraphic significance (Poland). Geologica Carpathica 57: 69–77.
Oji, T. 2009. Crinoids. In: Y. Shigeta, Y.D. Zakaharov, H. Maeda, and A.M. Popov (eds.), The fauna of the Abrek Bay (Far East Russia): a new window to the aftermath of the end-Permian mass extinction event. National Museum of Nature and Science Monographs 38: 180–181.
Oji, T. and Twitchett, R.J. 2015. The oldest Post-Palaeozoic crinoid and Permian–Triassic origins of the Articulata (Echinodermata). Zoological Science 32: 211–215. Crossref
Paul, C.R.C. 1988. Extinction and survival in the echinoderms. In: G.P. Larwood (ed.), Extinction and Survival in the Fossil Record. Systematics Association Special Volume 34: 155–170.
Paull, R.A. and Paull, R.K. 1993. Interpretation of Early Triassic nonmarine-marine relations, Utah, USA. New Mexico Museum of Natural History and Science Bulletin 3: 403–409.
Pawson, D.L. 2007. Phylum Echinodermata. Zootaxa 1668: 749–764. Crossref
Rouse, G.W., Jermiin, L.S., Wilson, N.G., Eeckhaut, I., Lanterbecq, D., Oji, T., Young, C.M., Browning, T., Cisternas, P., Heigen, L.E., Stuckey, M., and Messing, C.G. 2013. Fixed, free, and fixed: the fickle phylogeny of extant Crinoidea (Echinodermata) and their Permian–Triassic origin. Molecular Phylogenetics and Evolution 66: 161–181. Crossref
Salamon, M., Aghababalou, B., Gorzelak, P., and Niedźwiedzki, R. 2012. Intriguing crinoid remains from the Rhaetian of Iran and their possible implications for the mid-Carnian crinoid extinction event. Geobios 45: 479–484. Crossref
Salamon, M.A., Gorzelak, P., and Hanken, N.-M. 2015. Crinoids from Svalbard in the aftermath of the end-Permian mass extinction. Polish Polar Research 36: 225–238. Crossref
Saucède, T., Vennin, E., Fara, E., Olivier, N., and the Paris Biota Team 2019. A new holocrinid (Articulata) from the Paris Biota (Bear Lake County, Idaho, USA) highlights the high diversity of Early Triassic crinoids. Geobios 54: 45–53. Crossref
Schäfer, P., Cuffey, R.J., and Albert, R.Y. 2003. New trepostome Bryozoa from the Early Triassic (Smithian/Spathian) of Nevada. Paläontologische Zeitschrift 77: 323–340. Crossref
Shigeta, Y. and Kumagae, T. 2015. Churkites, a Trans-Panthalassic Early Triassic Ammonoid Genus from South Primorye, Russian Far East. Paleontological Research 19: 219–236. Crossref
Simms, M.J. 1988. The phylogeny of post-Palaeozoic crinoids. In: C.R.C. Paul and A.B. Smith (eds.), Echinoderm Phylogeny and Evolutionary Biology, 269–284. Clarendon Press, Oxford.
Smith, C.P.A., Charbonnier, S., Jenks, J.F., Bylund, K.G., Escarguel, G., Olivier, N., Fara, E., and Brayard, A. 2022. The Paris Biota decapod (Arthropoda) fauna and the diversity of Triassic decapods. Journal of Paleontology 96: 1–29. Crossref
Smith, C.P.A., Laville, T., Fara, E., Escarguel, G., Olivier, N., Vennin, E., Goudemand, N., Bylund, K.G., Jenks, J.F., Stephen, D.A., Hautmann, M., Charbonnier, S., Krumenacker, L.J., and Brayard, A. 2021. Exceptional fossil assemblages confirm the existence of complex Early Triassic ecosystems during the early Spathian. Scientific Reports 11: 19657. Crossref
Twitchett, R.J. and Oji, T. 2005. Early Triassic recovery of echinoderms. Comptes Rendus Palevol 4: 531–542. Crossref
Twitchett, R.J., Feinberg, J.M., O’Connor, D.D., Alavarez, W., and McCollum, L.B. 2005. Early Triassic Ophiuroids: Their Paleoecology, Taphonomy, and Distribution. Palaios 20: 213–223. Crossref
Webster, G.D. and Jell, P.A. 1999. New Permian crinoids from Australia. Memoirs of the Queensland Museum 43: 279–339.
Webster, G.D. and Lane, N.G. 2007. New Permian crinoids from the Battleship Wash patch reef in southern Nevada. Journal of Paleontology 81: 951–965. Crossref
Wright, D.F., Ausich, W.I., Cole, S.R., Peter, M.E., and Rhenberg, E.C. 2017. Phylogenetic taxonomy and classification of the Crinoidea (Echinodermata). Journal of Paleontology 91: 829–846. Crossref
Zatoń, M., Niedźwiedzki, G., Rakociński, M., Blom, H., and Kear, B.P. 2018. Earliest Triassic metazoan bioconstructions from East Greenland reveal a pioneering benthic community in the immediate aftermath of the end-Permian mass extinction. Global and Planetary Change 167: 87–98. Crossref
Acta Palaeontol. Pol. 68 (1): 155–166, 2023
https://doi.org/10.4202/app.01042.2022