


Early Miocene coastal taphonomy: piddock and barnacle inclusions from Chiapas amber
BŁAŻEJ BOJARSKI, KAROLINA CIEROCKA, and JACEK SZWEDO
Bojarski, B., Cierocka, K., and Szwedo, J. 2025. Early Miocene coastal taphonomy: piddock and barnacle inclusions from Chiapas amber. Acta Palaeontologica Polonica 70 (3): 495–505.
Piddocks (Pholadidae) are bivalves adapted for boring in substrates such as wood, rocks, and fossil resins. While the trace fossils associated with their boring behavior (Teredolites and Apectoichnus) are well documented, their body fossils are much rarer in the fossil record, particularly as amber inclusions. This work reports inclusions of fossilized piddocks and the first known barnacle inclusions from the Early Miocene simojovelite (Chiapas amber). We examined seven amber specimens containing over 90 piddock inclusions, representing at least five distinct morphotypes. The amber preserved various developmental stages, from juveniles with open pedal gaps to fully mature individuals with well-developed accessory plates. Taphonomic analysis, observed orientations, and the presence of diagenetically altered inclusions suggest that these piddocks inhabited waterlogged wood of Hymenaea and possibly semi-hardened resin before entrapment. This study highlights fossil resins as an overlooked preservational medium for marine invertebrates, providing new data on piddock behavior, fossil resin taphonomy, and the Miocene coastal environments of the Chiapas region.
Key words: Bivalvia, Martesiinae, Pholadidae, Cirripedia, piddocks, barnacles, ichnofossils, taphonomy, Chiapas amber, simojovelite, Miocene.
Błażej Bojarski [blazej.bojarski@ug.edu.pl; ORCID: https://orcid.org/0000-0001-6301-7959 ] and Jacek Szwedo [jacek.szwedo@ug.edu.pl; ORCID: https://orcid.org/0000-0002-2796-9538 ], Laboratory of Evolutionary Entomology and Museum of Amber Inclusions, Faculty of Biology, University of Gdańsk, 59 Wita Stwosza St., 80-309 Gdańsk, Poland.
Karolina Cierocka [karolina.cierocka@ug.edu.pl; ORCID: https://orcid.org/0000-0003-2615-4609 ]; Laboratory of Parasitology and General Zoology, Faculty of Biology, University of Gdańsk, 59 Wita Stwosza St., 80-309 Gdańsk, Poland.
Received 16 August 2024, accepted 1 July 2025, published online 10 September 2025.
Copyright © 2025 B. Bojarski et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Piddocks, members of the family Pholadidae (Pholadoidea) are a widely distributed group of marine bivalves. They are known for their boring behaviour, which allows them to excavate substrates such as rocks, bones, shells, corals, and xylic material (wood, seeds, and fossil resins). Piddocks bore primarily for shelter, whereas other representatives of the superfamily, such as the members of the families Teredinidae and Xylophagaidae, use xylic materials primarily as a food source. These bivalves can attack not only floating or sunken wood but also living mangrove (Bartsch and Rehder 1945; Turner 1966; Ansell and Nair 1969a, b; Kennedy1974; Tapanila et al. 2004; Alyakrinskaya 2005; Monari 2009; Borges 2014; Voight 2015; Wisshak et al. 2019). The burrows they leave behind in xylic substrates are preserved as ichnofossils and are frequently included into ichnogenera Teredolites and Apectoichnus, which are significant for reconstructing marine paleoenvironments. These ichnofossil types have been present in the fossil record since the Jurassic (Evans 1999; Donovan 2018; Buntin et al. 2022).
Among the most abundant borings found in fossil resins are those attributed to bivalves belonging to the extant genus Martesia Sowerby, 1824 (Smith and Ross 2016; Kocsis et al. 2020; Bolotov et al. 2021; Castañeda-Posadas et al. 2021). The genus Martesia includes 19 described species, 12 of which are known exclusively from the fossil record (Maury 1917; MolluscaBase 2025). These bivalves range in size from less than 12 mm to 60 mm (Brearley et al. 2003). Although Martesia frequently burrows into wood and other plant materials, it lacks the ability to digest them (Srinivasan 1960). Its burrows serve exclusively as shelter, and excavation is completed once the bivalve reaches maturity (Pournou 2020).
Currently, only two species of piddocks, both assigned to the subfamily Martesiinae, have been discovered in fossilized resins, preserved as inclusions. Martesia mazantica Castañeda-Posadas et al., 2021, was described from Early Miocene Chiapas amber. Palaeolignopholas kachinensis Bolotov et al., 2021, assigned to a monospecific extinct genus, was reported from Cenomanian Kachin amber. Palaeoenvironmental reconstructions of the taphocoenoses of the Chiapas and Kachin ambers suggest that both species inhabited waterlogged or floating wood in brackish environments, such as estuaries and mangroves (Bolotov et al. 2021; Castañeda-Posadas et al. 2021). Another fossil piddock specimen, also from Chiapas amber, was misidentified as a fossil puffball fungus and described as Lycoperdites tertiarius Poinar, 2001 (Poinar 2001; Smith and Ross 2016). The holotype specimen shows well-preserved valves with rasping teeth at its anterior slope, a callum, and a mesoplax, indicating it is a bivalve instead. However, the taxonomic status of L. tertiarius needs revision, as the specimen may in fact represent a species of Martesia. Additionally, the original work mentions immature forms of L. tertiarus which appear to be borings Teredolites clavatus Leymerie, 1842. Similar borings were likely misinterpreted as entomopathogenic fungi belonging to the order Hypocreales Lindau, 1897, described as growing on termites in Chiapas amber (Arroyo-Sánchez et al. 2023). Likewise, T. clavatus borings were erroneously identified as neonates of the snail Cretatortulosa gignens Jochum et al., 2021, and the earliest known fossilized evidence of vivipary, found in Kachin amber (Jochum and Neubauer 2021). The borings in question, however, exhibit bioglyphs consistent with piddock morphology found in respective fossil resins.
The simojovelite (commonly known as Chiapas amber or Mexican amber) deposits are located near the cities of Simojovel, Totolapa, and Estrella de Belén in Chiapas, Mexico. These amber-bearing beds are intercalated with limestone, sandstone, siltstone, marl, shale, and lignite (Riquelme et al. 2014, 2025). The mined simojovelite originates from the Mazantic Shale Formation, dated to 23–24 million years old and Early Miocene (García-Villafuerte et al. 2022; Riquelme et al. 2025).
The original resin of simojovelite was produced by an extinct species of Hymenaea, a genus of leguminous trees (Fabaceae), closely related to extant members of the genus (Poinar and Brown 2002; Solórzano-Kraemer 2010; Penney 2010; Riquelme et al. 2014). Modern species of Hymenaea still grow in forests near mangrove environments in Chiapas (Solórzano-Kraemer 2010). Extant Martesia and Lignopholas inhabit floating wood in shallow, warm, littoral and brackish waters, including lagoons, estuaries, and mangroves (Turner and Santhakumaran 1989). It is hypothesized that extinct Lignopholas, Palaeolignopholas, and Martesia, found as inclusions in amber, were likewise associated with estuarine and mangrove environments (Smith and Ross 2016; Bolotov et al. 2021; Castañeda-Posadas et al. 2021), the type of brackish waters where wood is most abundant (Voight 2015).
In this study, we present new findings supporting this hypothesis and describe possible mechanisms of piddock entrapment in amber. Additionally, we report extraordinary barnacle inclusions, which expand the taphonomic interpretation of the Miocene Chiapas coastal ecosystem.
Institutional abbreviations.—MAIG, Museum of Amber Inclusions University of Gdańsk, Poland.
Material and methods
The study focuses on seven pieces of simojovelite from the Montecristo amber mine in the Simojovel region (Mexico), located near the town of Simojovel de Allende (Fig. 1A–C). The fossil resin excavated from this mine originates from the Miocene Mazantic Shale Formation (Fig. 1D).
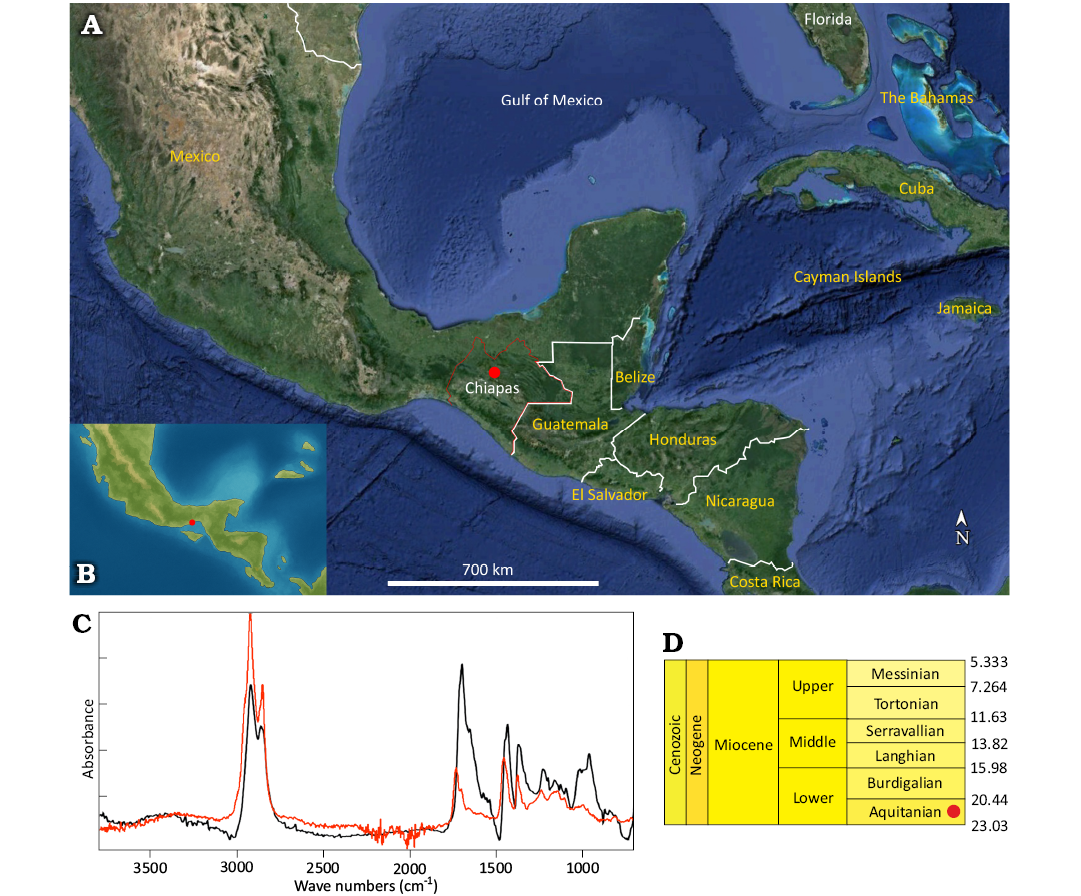
Fig. 1. Detailed information on the studied simojovelite (Chiapas amber). A, B. Maps of Mexico and the Gulf of Mexico. A. Modern geographic placement of Simojovel de Allende (Chiapas, Mexico) and the Montecristo Mine, marked with a red dot (map based on Google Earth Pro 7.3.6.9796). B. Paleogeographic reconstruction of the region approximately 20 million years ago (after Blakey 2006). C. Infrared (IR) spectra of simojovelite, based on MAIG 6820. The black line represents simojovelite (after Riquelme et al. 2014), while the red line indicates the matching spectrum of the studied amber specimen. D. Miocene chronostratigraphy with the age of simojovelite marked by a red dot.
The specimens are housed in the Museum of Amber Inclusions University of Gdańsk under the catalogue numbers MAIG 6820, 7070–7073, 7189, and 7190.
To enhance specimen visibility, the amber pieces were cut, ground, and polished. The preparation process utilized the following equipment: a water-cooled HITECH 22-321 table saw with a diamond plate, water-cooled AVALON SW1mini and SW1F grinding machines, and a custom-built AVALON polishing machine. Manual finishing and separating cuts were performed on MAIG 6820 using a razor blade.
The specimens were examined under transmitted and reflected light using a Leica M205A stereoscopic microscope, equipped with a Leica DFC 495 digital camera and operated via Leica Application Suite 3.7 software. Photographic documentation was obtained using the same Leica microscope and Canon EOS 90D DSLR camera. Helicon 8.2.2 software was used for focus stacking, while Photoshop 25.7 was employed for color, texture, and light corrections.
Results
More than 90 fossil piddock inclusions were identified, representing five distinct morphotypes. Each specimen is assigned to the Pholadidae family based on valve morphology and the presence of accessory plates and callum. In this work the labels M1–5 are used solely as provisional morphotypes, that group together specimens showing the same suite of valve characters (outline of the umbonal-ventral sulcus, degree of callum development, and presence/shape of accessory plates). This allows to describe and compare the numerous, often fragmentary inclusions and to trace their taphonomic distribution within the studied pieces without prematurely assigning formal taxonomic names (Table 1).
Table 1. Characteristics of fossil piddocks inclusions in studied simojovelite. Abbreviations: a, absent; j, juvenile; m, matured; Mx, morphotype x; p, present; u, unknown.
|
Collection number |
MAIG 6820 |
MAIG 7070 |
MAIG 7072 |
MAIG 7073 |
MAIG 7189 |
MAIG 7190 |
|
|
Assigned taxa |
Martesiinae |
Martesiinae |
Martesiinae |
Pholadidae: Martesiinae? |
Martesiinae |
Martesiinae, |
|
|
Number of specimens |
2 (3?) |
2 |
10 |
2 |
~70 |
6 |
|
|
Development stage |
j (M1); |
m (M4); |
m |
m? |
m and j(?) |
m and j(?) |
|
|
Orientation |
same |
same |
same |
same |
same |
same |
|
|
Morphotype |
M1? and M2 |
M4 and M3? |
M1 |
M5 |
M3 |
M3 |
|
|
Additional plates |
Protoplax |
a (M1); u (M2) |
u (M4, M3?) |
u |
u |
a |
a |
|
Mesoplax |
a (M1); p (M2) |
p (M4); u (M3?) |
p |
p |
p |
p |
|
|
Metaplax |
a (M1); u (M2) |
u (M4, M3?) |
u |
u |
a |
a |
|
|
Hypoplax |
a (M1, M2) |
u (M4, M3?) |
a |
a |
a |
a |
|
|
Siphonoplax |
u (M1, M2) |
u (M4, M3?) |
p |
a |
a |
a |
|
|
Callum |
a (M1); u (M2) |
p (M4); a (M3?) |
p |
a |
p |
u |
|
|
Apophysis |
p (M1, M2) |
u (M4, M3?) |
u |
u |
u |
u |
|
MAIG 6820
Fig. 2A.
Identified taxa: Martesiinae, juvenile of morphotype 1 (M1?) and morphotype 2 (M2); Pholadidae (?).
Description.—This amber specimen contains a fully preserved juvenile and a damaged mature representative of Martesiinae. The juvenile measures 12.7 mm in length, is widely gaping, and lacks a callum, fully developed mesoplax and other accessory plates (Fig. 2A1). The second piddock, positioned close to the juvenile, covers its dorsal side. This specimen preserves the full length of the posterior slope and part of the anterior slope, including the umbonal area, but lacks the beak and measures 6.5 mm. It shows a partially preserved dorsal extension of the callum and partially visible mesoplax and metaplax. Both specimens display an umbonal-ventral sulcus (groove), rasping teeth on the anterior slope, and apophyses (Fig. 2A2). A third possible specimen is either a highly compressed young pholadid consists of fragments of the juvenile valve, but its preservation does not allow further identification.
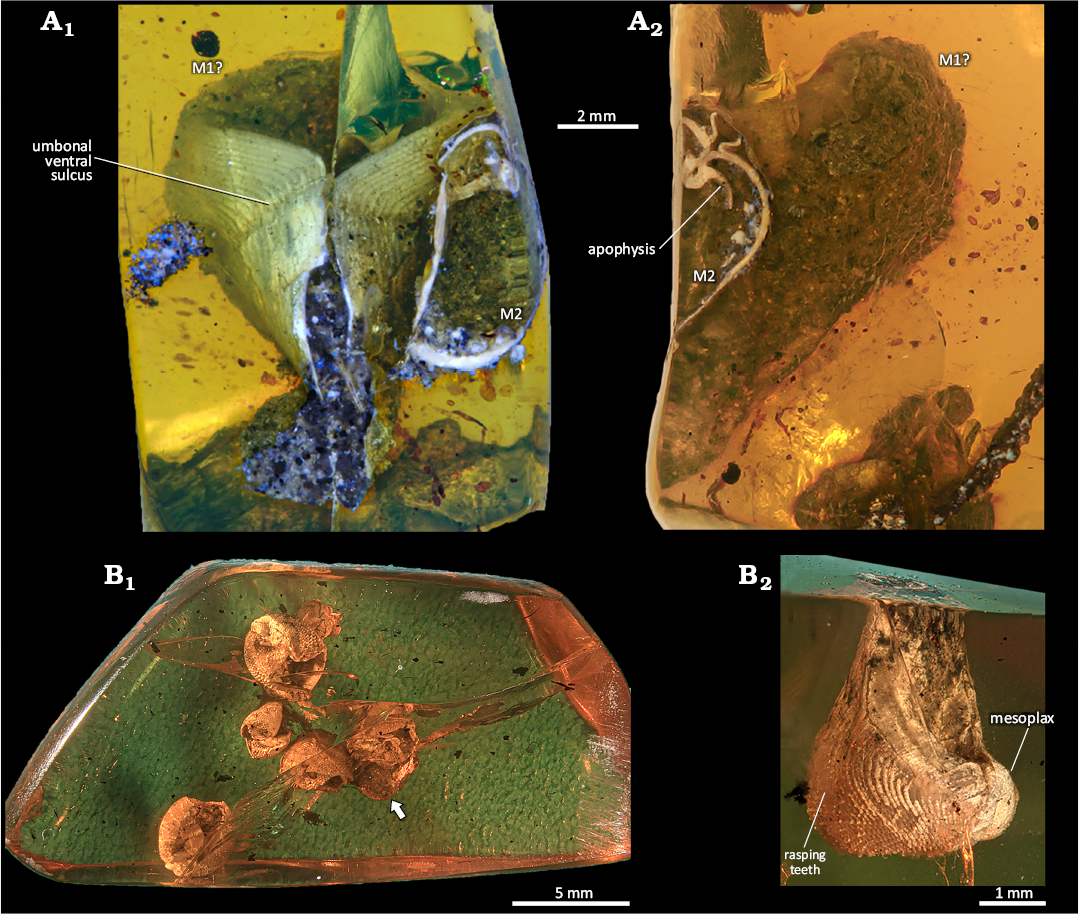
Fig. 2. General view of amber specimens from Simojovel de Allende, Chiapas, Mexico, Aquitanian showing piddock inclusions. A. MAIG 6820 with Martesiinae bivalves morphotypes M1? and M2. A1, ventral view of the larger, juvenile M1? and a partially preserved M2; A2, lateral view, showing organic debris covering the valve of M1 and the inner shell morphology of M2. B. MAIG 7190 with Martesiinae bivalves morphotype M3. B1, general view of the beak area, with the boring Teredolites clavatus Leymerie, 1842, marked by an arrow; B2, dorsal-lateral view of a mature piddock.
Remarks.—The inclusions are covered by organic debris resulting from the bivalve body’s decomposition. Two additional puzzling structures are preserved. The first structure, located above the juvenile specimen, resembles a third, strongly compressed pholadid valve with preserved anterior and posterior slopes. However, its position suggests it might be the displaced periostracum of the juvenile. The second structure, partially preserved at the edge of the amber, appears calcareous, significantly bulkier than the preserved valves, and resembles the overlapping plates of the siphonoplax. The presence of compressed gas bubbles in the resin matrix suggests high pressure and potential heating during fossil diagenesis. Both specimens are oriented in the same direction.
MAIG 7190
Fig. 2B.
Identified taxa: Martesiinae, morphotype 3 (M3) and ichnospecies Teredolites clavatus Leymerie, 1842.
Description.—This amber specimen preserves six piddocks (Fig. 2B1), four of them most likely mature, based on their lenght (2.6 mm and 3 mm for the two fully preserved valves). Two other specimens considered juveniles as they are smaller, approximately 1 mm in length. The anterior slopes are slightly damaged due to compression, making it difficult to determine the presence of a callum, but its rasping teeth are observable (Fig. 2B2). Both adult specimens have a mesoplax, and the posterior and anterior slopes are separated by a single umbonal-ventral sulcus in both individuals.
Remarks.—This amber contains six piddocks and one boring assigned to ichnotaxon T. clavatus (Fig. 2B1). It is the only amber piece in this study that preserves both shells and trace fossils of piddocks. All fossils are oriented in the same direction.
MAIG 7189
Fig. 3.
Identified taxa: Martesiinae, morphotype 3 (M3).
Description.—The valve morphology closely matches that of the inclusions in MAIG 7190, supporting their classification as the same morphotype. Yet, the inclusions differs with regard to their size—the largest valve reaches a length of 5 mm and presence of the callum (Fig. 3A1, A2). The degree of callum development varies, ranging from fully open pedal gapes specimens to completely closed by callum (Fig. 3A2). The amber contains piddocks at different developmental stages.
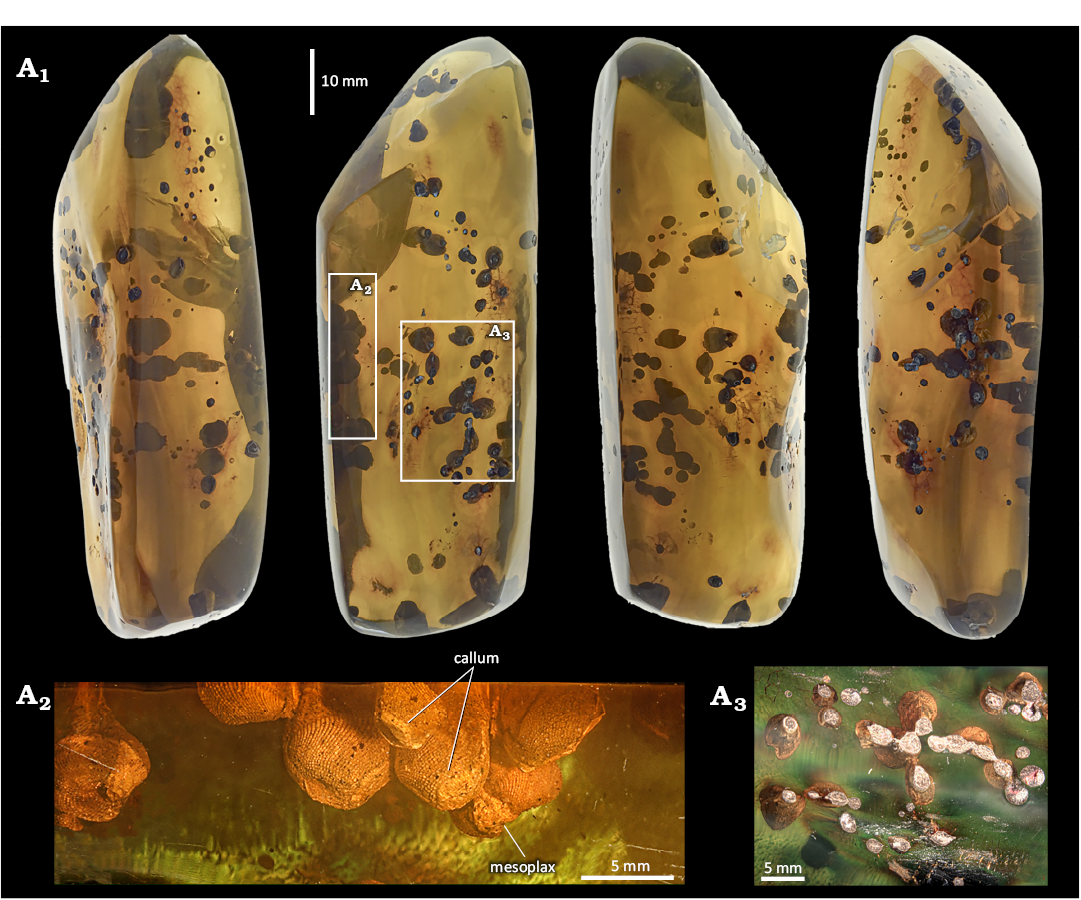
Fig. 3. General view of amber specimen from Simojovel de Allende, Chiapas, Mexico, Aquitanian showing piddock inclusions. MAIG 7189 with Martesiinae bivalves morphotype M3. A1, arrangement of piddock inclusions in the amber specimen; A2, lateral view of the anterior slope and beak of mature piddocks; A3, surface of the amber specimen, showing piddock inclusions.
Remarks.—This amber piece contains over 70 individual specimens, covering all surfaces of the amber (Fig. 3A1). All piddock inclusions are positioned near the surface (Fig. 3A3), with none embedded in deeper layers. This high density of inclusions hampers measurement and detailed analysis, as cutting or grinding would damage the specimens.
MAIG 7070
Fig. 4A.
Identified taxa: Martesiinae, morphotype 4 (M4) and possible morphotype 3 (M3?).
Description.—A fully mature Martesiinae inclusion, measuring 22.7 mm in length, is preserved in this amber specimen (Fig. 4A1, A2). It has a broad, rounded mesoplax (Fig. 4A1) and a fully developed callum (Fig. 4A2, A3). The posterior and anterior slopes are separated by a shallow umbonal-ventral sulcus, with rasping teeth on the anterior slope. The right valve is intact but covered with decomposition debris, while the left valve has collapsed at the posterior slope, likely due to compression (Fig. 4A2). The decayed, fossilized soft body is preserved inside the shell. A second inclusion consists of a partially preserved anterior slope of a valve, resembling morphotype M3 (Fig. 4A2).
Remarks.—Debris obscures details of the sculpture, the umbonal-ventral sulcus, and the rasping teeth, though some areas of the right valve remain visible. This is the largest specimen studied, alongside a fully developed callum and a mesoplax, confirming its mature status.
MAIG 7072
Fig. 4B.
Identified taxa: Martesiinae, morphotype 1 (M1).
Description.—This amber specimen contains piddock inclusions showing a various degrees of damage, with two shells entirely collapsed (Fig. 4B1). Two piddocks have at least one fully preserved valve, with anterior and posterior slopes intact. The distinct umbonal-ventral sulcus is clearly visible in one inclusion (Fig. 4B2). All piddocks belong to the same morphotype, with a wide, divided mesoplax and a callum that does not extend over the beak (Fig. 4B3). At least four of the inclusions preserve a siphonoplax (Fig. 4B1). This and the presence of a mesoplax and callum indicate full maturity. The most complete specimen measures 14.5 mm in length (without siphonoplax).
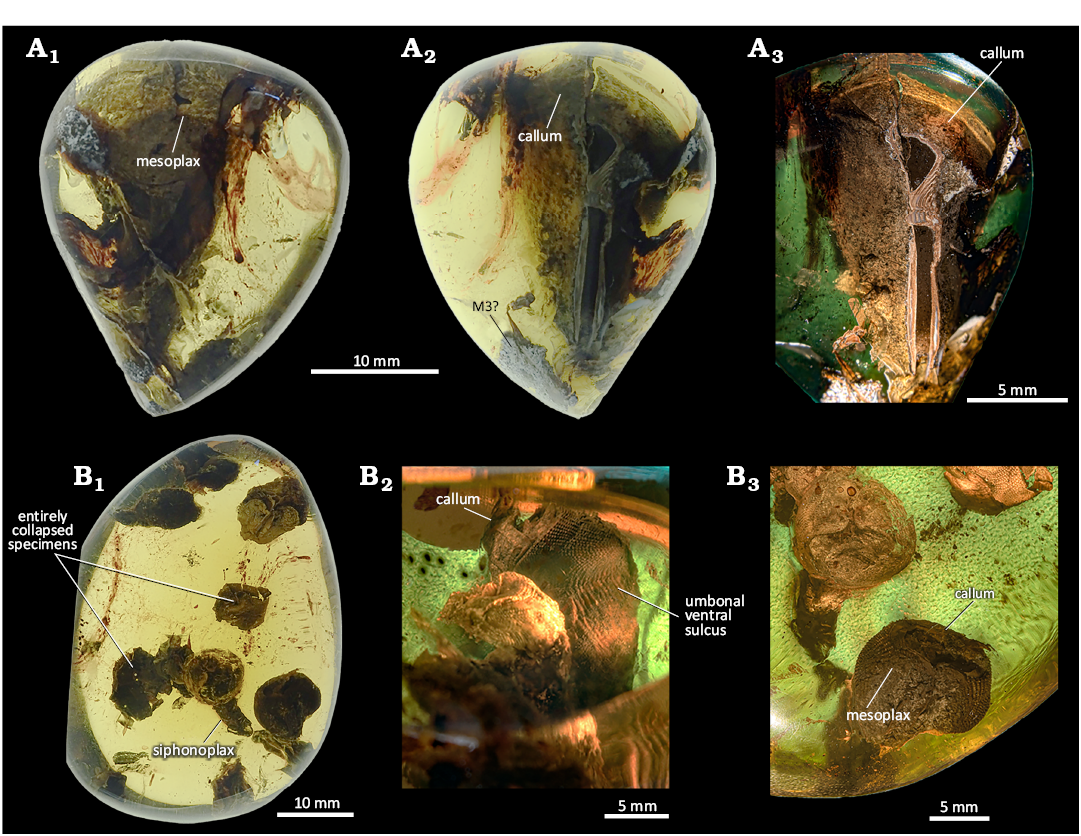
Fig. 4. General view of amber specimens from Simojovel de Allende, Chiapas, Mexico, Aquitanian showing piddock inclusions. A. MAIG 7070 with Martesiinae bivalves morphotype M4 and a partially preserved M3?. A1, dorsal view of M4; A2, ventral view of M4, with the position of M3?; A3, detailed ventral view of M4. B. MAIG 7072 with Martesiinae bivalves morphotype M1. B1, arrangement of inclusions within the amber specimen; B2, lateral view of a mature piddock; B3, close-up view of the beak area.
Remarks.—The amber contains ten piddock inclusions, with different levels of damage caused by compression. All specimens are oriented in the same direction, with their posterior slopes reaching the amber surface.
MAIG 7073
Fig. 5A.
Identified taxa: Pholadidae: Martesiinae?, morphotype 5 (M5).
Description.—Two inclusions are present in this amber piece (Fig. 5A1, A2). One piddock is partially damaged, missing approximately the posterior third of the valve (Fig. 5A1). The remains of this piddock are preserved in a highly altered state (see remarks). The second valve appears intact, with a shell length of 13.5 mm (Fig. 5A2, A3). The umbonal-ventral sulcus is visible, and the partially preserved mesoplax is the only accessory plate observed (Fig. 5A3). This and the missing callum prevents from precise classification. By comparison of the pedal gape shape, specimens are considered to represent the same morphotype.
Remarks.—This bivalve specimen is not preserved in its original mineralogy (Fig. 5A1, A2), but shows altered shell material. The shell was either recrystallized or dissolved and replaced by sedimentary infill (pseudomorphosis). This is evident from the clear difference in colour and texture between the internal sediment (or fossilized soft body) and the material that replaced the shell. Both structures face the same direction and reach the surface of the amber.
MAIG 7071
Fig. 5B.
Identified taxa: Balanomorpha.
Description.—This amber piece contains nine inclusions of balanomorph cirripedes (Fig. 5B1). Two of the specimens show fully preserved carinae (Fig. 5B2), measuring 7.4 mm and 6.9 mm in base width or height (the aperture is not visible, and basal plates are moved so it is difficult to determine their arrangement). Seven barnacles are partially preserved. All specimens lack scuta and terga.

Fig. 5. General view of amber specimens from Simojovel de Allende, Chiapas, Mexico, Aquitanian showing piddock inclusions. A. MAIG 7073 with Martesiinae bivalves morphotype M5. A1, arrangement of piddock inclusions. A2, lateral view of the best-preserved valve. A3, beak area, showing displaced callum. B. MAIG 7071 with Balanomorpha inclusions. B1, overview of the amber specimen; B2, barnacle carina submerged in sediment; B3, barnacles preserved partially in resin and sediment, marked by an arrows; B4, quartz sandstone sediment.
Remarks.—The studied amber specimen is unique, featuring an in situ resin-sediment boundary. This boundary lacks any features of weathering, and some of the fossils are preserved within partly the resin and the surrounding sandstone (Fig. 5B3). The resin flow likely covered the original sandy substrate, trapping balanomorph plates that were lying on the surface. Notably, each observed barnacle inclusion has its aperture submerged in the sediment, suggesting that the organisms were already dead when they were encapsulated in the resin. The shells are partially infilled with sediment (Fig. 5B2), further supporting the interpretation that the barnacles were embedded post-mortem. The sediment surrounding the amber is composed of micaceous quartz sandstone with shell fragments (Fig. 5B4), including a single tergum or scutum.
Discussion
Growth-stage assessment.—An underdeveloped callum, a wide posterior gape that is partially covered by a chitinous hood, or underdeveloped mesoplax indicate that a specimen is a juvenile Martesiinae in the “working stage”, a phase during which young piddocks actively drill into wood. In contrast, mature piddocks enter the “resting stage”, where they inhabit a chamber at the end of their boring, developing a callum and accessory plates. Only, the mesoplax is an obligatory accessory plate that is present from the juvenile stage onward (Lloyd 1897; Turner 1955; Kennedy 1974). The studied specimens are therefore mature or maturing, as indicated by the presence of a mesoplax, a partially or fully developed callum, and, in some cases, a siphonoplax and other accessory plates.
Most of the amber specimens examined contain at least one piddock with juvenile characters and one specimen with adult characteristics of the Martesiinae, with the exception of MAIG 7070 and 7073, where preservation was too poor for reliable interpretation. In specimens where multiple individuals of the same morphotype co-occur, differences in callum development, size, and shape are observed. The callum, when preserved, appears to grow from the anterior margin of the shell toward the center of the pedal gape, eventually closing it—a pattern seen in MAIG 7189, which contains both juveniles and mature specimens.
The estimated growth rate for species of Martesia is approximately 0.383 mm/day (Turner 1954; Smith and Ross 2016), with a minimum rate of 0.19 mm/day during the first 30 days after larval settlement (Mann and Gallager 1984). This rate is sufficient for the smallest species to develop a callum, marking their transition from the working to the resting stage (Turner 1954). Based on these growth rates, the estimated ages (since metamorphosis) at the time of entrapment in fossil resin are: 2–5 and 2–4 weeks, for morphotypes M2 and M3, respectively; 5–11 weeks old for morphotypes M1 and M5; and 8–17 weeks for morphotype M4. These findings suggest that morphotypes M1, M4, and M5 may have had higher growth rates than modern representatives of Martesia. These morphotypes could have developed on a floating log of wood, given that waterlogged wood can remain afloat for approximately 6 to 17 months (Voight 2015). However, this scenario is unlikely, as the amber specimens were preserved within the same taphocoenosis, suggesting that the wooden material likely originated from the same sedimentary source (Kennedy 1974).
Taphonomy.—Evidence from studied simojovelite shows pholadid inclusions preserved inside fossil resin pieces that are essentially free of terrestrial syninclusions, consistent with resin that originated in internal tree cavities rather than as external flows (Bojarski et al. 2025). Unlike tree exudates running down a trunk, resin sequestered inside wood forms isolated pockets that remained relatively inaccessible to typical forest-floor organisms but became available to aquatic wood-borers once the wood was moved to the water body. Taphonomic patterns in various fossil resins support this scenario. For example, Kachin amber contains abundant club-shaped borings and numerous piddock shells “floating” in the resin, indicating that these bivalves colonized semi-liquid resin and were trapped alive (Smith and Ross 2016; Bolotov et al. 2021). In contrast, ambers where resin had fully hardened prior to colonization (e.g., some Eocene Baltic amber) preserve only the borings and not the borers (Maksoud et al. 2017; Mayoral et al. 2020). This reflects that semi-liquid resin was required to trap the borer and once solidified, the resin would only record its excavation, not the organism. The dominance of pholadids in fossil resins as inclusions alongside the absence of xylophagous teredinid “shipworms” or limnoriid gribble isopods may reflect ecological and preservational factors.
The studied piddock inclusions are considered wood borers. However, alternatively their substrate may have included drifting pieces of semi-hardened Hymenaea resin. This scenario is particularly supported by the distribution pattern observed in MAIG 7189, where piddocks are present on all surfaces of the specimen, suggesting that the larvae had access to the entire piece of resin. This would indicate the resin was solid enough to serve as a substrate for the piddocks while still sufficiently viscous to encapsulate the bivalves without preserving excavation traces (bioglyphs). In nearly all studied amber specimens, except MAIG 7189 and 7190, the presence of gas bubbles in the resin matrix suggests that the resin underwent compression. Most of the bubbles are squashed, elongated, and curved, with some forming worm-like structures rather than having collapsed completely. This form of liquid inclusion preservation likely resulted from diagenetic pressure applied to the semi-fossilized resin while the matrix was still semi-liquid. These diagenetic changes may be linked to volcanic activity associated with the Mazantic Shale Formation, which is particularly evident in the Totolapa area, where volcanic tuffs and sandstones have been observed above the amber-bearing beds, indicating that the volcanic activity coincided with or followed the deposition of the Mazantic Shale Fm. (Breton et al. 2014; Riquelme et al. 2025). The pressure and/or thermal alteration of the resin may have significantly influenced the preservation state of the inclusions.
The piddocks preserved in amber are all oriented in the same direction, with their posterior ends directed towards the amber surface and the anterior end of the shell facing inward. This uniform arrangement, along with the aforementioned taphonomic observations, suggests that the piddocks were trapped as a direct result of their excavation activity, rather than being entrapped post-mortem by resin secreted from a plant stem or immobilized in a semi-liquid resinous pebble, as observed in the case of the barnacle inclusions (Kennedy 1974). This scenario is consistent with a brackish-water environment, given that entrapment of aquatic organisms in resin has been documented from modern paralic swamps (Schmidt and Dilcher 2007).
The observed abundance of mature piddocks in amber may be linked to xylic substrate degradation. Martesiinae are incapable of relocating once they begin excavating a piece of wood. However, upon exposure of their burrow to external conditions, they are known to resume drilling to cover themselves (Turner 1955; Ansell and Nair 1969b; Nishimoto et al. 2015; Buntin et al. 2022; Pournou 2020). In a waterlogged Hymenaea logs this exposure would have followed successive colonisation by other wood-borers such as additional piddocks, teredinid shipworms, gribbles, and pill-bugs that progressively removed the outer layers. Bioerosion, alongside mechanical abrasion, would erase the shallow traces of the latest pholads, which occupied the wood surface but never reached the inner resin pockets.
The presence of wood-boring pholadids as inclusions raises the question how they were entrapped. Given that these bivalves inhabited brackish waters, the resin-producing tree or its remains had to be present nearby to allow the larvae to develop on a Hymenaea-derived resin substrate. The resin producing tree must have grown in proximity to such an environment to ensure that its resin retained the ability to trap aquatic organisms in waterlogged stems. The tree could have reached this area via rivers, encountering piddocks in a river estuary.
Plant remains may also have been accessible to boring organisms in the open sea, but only if the wood was not waterlogged for an extended period before the resin stabilized (Nishimoto et al. 2015; Buntin et al. 2022). Long-drifting or sunken wood with hardened resin would have lost its ability to preserve these organisms as inclusions, as the piddocks would have continued to bore through the resin, leaving behind the same characteristic boring patterns found in other xylic substrates.
Both extinct and extant Martesiinae leave conical excavations with a rounded bottom (Kennedy 1974; Kelly and Bromley 1984; Kelly 1988), typically formed near the surface of submerged wood. Over time, as the wood degrades, these borings become less abundant, while teredinid borers progressively dominate, leaving characteristic borings with calcitic linings in the substrate (Kelly and Bromley 1984; Kelly 1988; Nishimoto et al. 2015; Voight 2015; Buntin et al. 2022). The findings presented in this study, along with previous reports of T. clavatus in Chiapas amber (Ross et al. 2016; Mayoral et al. 2020), further support the presence of Martesiinae in this paleoenvironment.
The abundance of piddock inclusions in the studied amber, alongside specimens of Martesia mazantica, which were earlier reported from the same type of fossil resin, the studied barnacle inclusions and the other fossil mollusks known from the Mazantic Shale Formation in the same region (del Carmen Perrilliat et al. 2010), supports the hypothesis of a shallow, warm lagoon and river estuary surrounded by mangrove forest.
Conclusions
Simojovelite (Chiapas amber) presents a relatively abundant concentration of wood-boring pholadids (Martesiinae) inclusions. The high proportion of mature piddocks in simojovelite most likely reflects progressive degradation of the waterlogged wood of Hymenaea. Matured and maturing piddocks that bored into this wood reached semi-liquid resin pockets and were immobilised there are therefore over-represented in the studied amber assemblage.
Lithological, palaeontological and taphonomic evidence together show that this process took place in a shallow, warm, brackish-water lagoon or river-mouth estuary bordered by mangrove forest. The amber-bearing Mazantic Shale Formation is interbedded with sandstone, siltstone and lignite, which can preserve fossils such as balanomorph barnacles formed across a resin-sand interface, suggesting that this amber-bearing bed was not redeposited.
These findings support the palaeoenvironmental model for the simojovelite and illustrate how resin, especially inner-wood resin pockets, can capture near-shore aquatic communities. Future work comparing the ratio of borings to body fossils across different fossil resins may further clarify their fossil record and the taphonomical history they underwent.
Acknowledgements
We would like to appreciate Doug Lundberg (Amberica West, Colorado Springs, USA), for providing access and supplying amber specimens, his contribution has been invaluable in facilitating the data collection process and enabling the analysis conducted in this study. We would like to give heartfelt appreciation to two anonymous peer reviewers who generously dedicated their time and expertise to review and provide constructive feedback on this paper.
Editor: Andrzej Kaim
References
Alyakrinskaya, I. 2005. Functional significance and weight properties of the shell in some mollusks. Biology Bulletin 32: 397–418. Crossref
Ansell, A.D., and Nair, N.B. 1969a. A comparative study of bivalves which bore mainly by mechanical means. American Zoologist 9: 857–868. Crossref
Ansell, A.D. and Nair, N.B. 1969b. The mechanisms of boring in Martesia striata Linné (Bivalvia: Pholadidae) and Xylophaga dorsalis Turton (Bivalvia: Xylophaginidae). Proceedings of the Royal Society of London. Series B, Biological Sciences 174: 123–133. Crossref
Arroyo-Sánchez, D.K., Víctor, J., Córdova-Tabares, V.M., Hernández-Damián, A.L., and Estrada-Ruiz, E. 2023. Entomopathogenic fungi (Ascomycota: Hypocreales) on a termite (Blattodea: Mastotermitidae) trapped in Miocene amber from Chiapas, Mexico. Historical Biology 37: 113–119. Crossref
Bartsch, P. and Rehder, H.A. 1945. The west Atlantic boring mollusks of the genus Martesia. Smithsonian Miscellaneous Collections 104: 1–16.
Blakey, R. 2006. Global Paleogeographic Views of Earth History: Late Precambrian to Recent [accessed at: https://earth.google.com/gallery/kmz/global_paleogeographic_n.kmz on 2025-06-27].
Bojarski, B., Cierocka, K., and Szwedo, J. 2025. Ichnotaxonomy of new boring taxa: linking insect activity and fossil resins formation. Palaeoentomology 8: 147–156. Crosssref
Bolotov, I.N., Aksenova, O.V., Vikhrev, I.V., Konopleva, E.S., Chapurina, Y.E., and Kondakov, A.V. 2021. A new fossil piddock (Bivalvia: Pholadidae) may indicate estuarine to freshwater environments near Cretaceous amber-producing forests in Myanmar. Scientific Reports 11: 6646. Crossref
Borges, L.M.S. 2014. Biodegradation of wood exposed in the marine environment: Evaluation of the hazard posed by marine wood-borers in fifteen European sites. International Biodeterioration and Biodegradation 96: 97–104. Crossref
Brearley, A., Chalermwat, K., and Kakhai, N. 2003. Pholadidae and Teredinidae (Mollusca: Bivalvia) collected from mangrove habitats on the Burrup Peninsula, Western Australia. In: F.E. Wells, D.I. Walkers, and D.S. Jones (eds.), The marine flora and fauna of Dampier, Western Australia. Western Australian Museum, Perth 2: 345–361.
Breton, G., Serrano-Sánchez, M.D.L., and Vega, F.J. 2014. Filamentous micro-organisms, inorganic inclusions and pseudo-fossils in the Miocene amber from Totolapa (Chiapas, Mexico): taphonomy and systematics. Boletín de la Sociedad Geológica Mexicana 66: 199–214. Crossref
Buntin, R.C.C., Hasiotis, S.T., and Flaig, P.P. 2022. Evaluating the ichnofossil Teredolites as an indicator of salinity and paleoenvironment. Palaios 37: 53–72. Crossref
Castañeda-Posadas, C., Trujillo-Hernández, A., and Zúñiga-Mijangos, L.A. 2021. Especie nueva del género Martesia (Bivalvia: Pholadidae) incluida en el ámbar de Chiapas como indicador de un ambiente estuarino. Revista Mexicana de Biodiversidad 92: 1–6. Crossref
del Carmen Perrilliat, M., Vega, F.J., and Coutiño, M.A. 2010. Miocene mollusks from the Simojovel area in Chiapas, southwestern Mexico. Journal of South American Earth Sciences 30: 111–119. Crossref
Donovan, S.K. 2018. A new ichnogenus for Teredolites longissimus Kelly and Bromley. Swiss Journal of Palaeontology 137: 95–98. Crossref
Evans, S. 1999. Wood-boring bivalves and boring linings. Bulletin of the Geological Society of Denmark 45: 130–134. Crossref
García-Villafuerte, M.Á., Carbot-Chanona, G., Rivera-Velázquez, G., de Bonilla, E.P.D., and Matamoros, W.A. 2022. The first fossil record of the genus Phycosoma (Araneae, Theridiidae) from the lower Miocene Mexican amber, with the description of a new species. Journal of Paleontology 96: 1346–1353. Crossref
Jochum, A., Yu, T., and Neubauer, T.A. 2021. Mother snail labors for posterity in bed of mid-Cretaceous amber. Gondwana Research 97: 68–72. Crossref
Kelly, S.R.A. 1988. Cretaceous wood-boring bivalves from western Antarctica with a review of the Mesozoic Pholadidae. Palaeontology 31: 341–372.
Kelly, S.R.A. and Bromley, R.G. 1984. Ichnological, nomenclature of clavate borings. Palaeontology 27: 793–807.
Kennedy, G.L. 1974. West American Cenozoic Pholadidae (Mollusca: Bivalvia). San Diego Society of Natural History Memoirs 8: 2–127.
Kocsis, L., Usman, A., Jourdan, A.L., Jumat, N., Daud, D., Briguglio, A., Hassan, S.H., Jumat, N., Slik, F., Rinyu, L., and Futó, I. 2020. The Bruneian record of “Borneo Amber”: A regional review of fossil tree resins in the Indo-Australian Archipelago. Earth-Science Reviews 201: 103005.
Leymerie, A. 1842. Suite de mémoire sur le terrain Crétacé du Département de l’Aube. Second partie. Mémoires de la Société Géologique de France 5: 1–34.
Lindau, G. 1897. Hypocreales. In: A. Engler and K. Prantl (eds.), Die natürlichen Pflanzenfamilien. Teil I, Abteilung 1, 343–372. Verlag Wilhelm Engelmann, Leipzig.
Lloyd, F.E. 1897. On the mechanisms in certain lamellibranch boring molluscs. Transactions of the New York Academy of Sciences 16: 307–316.
Maksoud, S., Azar, D., Granier, B., and Gèze, R. 2017. New data on the age of the Lower Cretaceous amber outcrops of Lebanon. Palaeoworld 26: 331–338. Crossref
Mann, R. and Gallager, S.M. 1984. Physiology of the wood boring mollusc Martesia cuneiformis Say. The Biological Bulletin 166: 167–177. Crossref
Maury, C.J. 1917. Santo Domingo type sections and fossils. Part I. Mollusca. Bulletins of American Paleontology 5: 165–415.
Mayoral, E., Santos, A., Vintaned, J.G., Wisshak, M., Neumann, C., Uchman, A., and Nel, A. 2020. Bivalve bioerosion in Cretaceous–Neogene amber around the globe, with implications for the ichnogenera Teredolites and Apectoichnus. Palaeogeography, Palaeoclimatology, Palaeoecology 538: 109410. Crossref
MolluscaBase (Editorial Board) 2025. MolluscaBase [accessed at https://www.molluscabase.org on 2025-06-27].
Monari, S. 2009. Phylogeny and biogeography of pholadid bivalve Barnea (Anchomasa) with considerations on the phylogeny of Pholadoidea. Acta Palaeontologica Polonica 54: 315–335. Crossref
Nishimoto, A., Haga, T., Asakura, A., and Shirayama, Y. 2015. An experimental approach for understanding the process of wood fragmentation by marine wood borers in shallow temperate waters. Marine Ecology Progress Series 538: 53–65. Crossref
Penney, D. 2010. Dominican amber. In: D. Penney (ed.), Biodiversity of Fossils in Amber from the Major World Deposits, 22–41. Siri Scientific Press, Manchester.
Poinar, Jr. G. 2001. Fossil puffballs (Gasteromycetes: Lycoperdales) in Mexican amber. Historical Biology 15: 219–222. Crossref
Poinar, Jr. G. and Brown, A.E. 2002. Hymenaea mexicana sp. nov. (Leguminosae: Caesalpinioideae) from Mexican amber indicates Old World connections. Botanical Journal of the Linnean Society 139: 125–132. Crossref
Pournou, A. 2020. Biodeterioration of Wooden Cultural Heritage. Organisms and Decay Mechanisms in Aquatic and Terrestrial Ecosystems. xv+538 pp. Springer International Publishing, Cham. Crossref
Riquelme, F., Ortega-Flores, B., Estrada-Ruiz, E., and Córdova-Tabares, V. 2025. Zircon U-Pb ages of the Chiapas Amber-Lagerstätte in the uppermost Simojovel Formation, southwest Mexico. Canadian Journal of Earth Sciences 62: 1088–1103. Crossref
Riquelme, F., Ruvalcaba-Sil, J.L., Alvarado-Ortega, J., Estrada-Ruiz, E., Galicia-Chávez, M., Porras-Múzquiz, H., Stojanoff, V., Siddons, D.P., and Miller, L. 2014. Amber from México: coahuilite, simojovelite and bacalite. MRS Online Proceedings Library 1618: 169–180. Crossref
Ross, A.J., Mellish, C.J., Crighton, B., and York, P.V. 2016. A catalogue of the collections of Mexican amber at the Natural History Museum, London and National Museums Scotland, Edinburgh, UK. Boletín de la Sociedad Geológica Mexicana 68: 45–55. Crossref
Schmidt, A.R. and Dilcher, D.L. 2007. Aquatic organisms as amber inclusions and examples from a modern swamp forest. Proceedings of the National Academy of Sciences 104: 16581–16585. Crossref
Smith, R.D. and Ross, A.J. 2016. Amberground pholadid bivalve borings and inclusions in Burmese amber: implications for proximity of resin-producing forests to brackish waters, and the age of the amber. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 107: 239–247. Crossref
Solórzano Kraemer, M.M. 2010. Mexican amber. In: D. Penney (ed.), Biodiversity of Fossils in Amber from the Major World Deposits, 42–56. Siri Scientific Press, Manchester.
Sowerby I, G.B. 1821–1834. The Genera of Recent and Fossil Shells, for the Use of Students, in Conchology and Geology. Published in 42 parts. Vol. 1, pls. 1–126 [1821–1825]; Vol. 2, pls. 127–262 + text (unpaginated) [1825–1834]. George Brettingham Sowerby I, London.
Srinivasan, V.V. 1960. Ciliary currents and associated organs of Martesia fragilis, a wood boring pholad of Madras. Journal of the Marine Biological Association of India 2: 186–193.
Tapanila, L., Roberts, E.M., Bouare, M.L., Sissoko, F., and O’Leary, M.A. 2004. Bivalve borings in phosphatic coprolites and bone, Cretaceous–Paleogene, northeastern Mali. Palaios 19: 565–573. Crossref
Turner, R.D. 1954. The family Pholadidae in the western Atlantic and the eastern Pacific. Part I—Pholadinae. Johnsonia 3: 1–63.
Turner, R.D. 1955. The family Pholadidae in the western Atlantic and the eastern Pacific. Part II—Martesiinae, Jouannetiinae and Xylophaginae. Johnsonia 3: 65–160.
Turner, R.D. 1966. A survey and illustrated
catalogue of the Teredinidae (Mollusca: Bivalvia). Museum
of Comparative Zoology, Harvard University, Cambridge, Mass. Crossref
Turner, R.D. and Santhakumaran, L.N. 1989. The genera Martesia and Lignopholas in the Indo-Pacific (Mollusca: Bivalvia: Pholadidae). Ophelia 30: 155–186. Crossref
Voight, J.R. 2015. Xylotrophic bivalves: aspects of their biology and the impacts of humans. Journal of Molluscan Studies 81: 175–186. Crossref
Wisshak, M., Knaust, D., and Bertling, M. 2019. Bioerosion ichnotaxa: review and annotated list. Facies 65: 24. Crossref
Acta Palaeontol. Pol. 70 (3): 495–505, 2025
https://doi.org/10.4202/app.01200.2024