

Revisiting the choristodere and stem-lepidosaur specimens of the Guimarota Beds (Kimmeridgian, Portugal): taxonomic implications
ALEXANDRE R.D. GUILLAUME, EDUARDO PUÉRTOLAS-PASCUAL, and MIGUEL MORENO-AZANZA
Guillaume, A.R.D. Puértolas-Pascual, E., and Moreno-Azanza, M. 2025. Revisiting the choristodere and stem-lepidosaur specimens of the Guimarota Beds (Kimmeridgian, Portugal): taxonomic implications. Acta Palaeontologica Polonica 70 (1): 77–96.
The Guimarota beds (Kimmeridgian, Portugal) constitute one of the richest microvertebrate assemblages for the Upper Jurassic, which include a diverse fauna of small reptiles. Among others, was described a new species of a small choristodere, “Cteniogenys reedi”. The genus, also known from the Morrison Formation (Upper Jurassic, USA) and the Kirtlington Mammal Bed (Middle Jurassic, UK), constitutes one of the oldest and most basal forms of this aquatic reptile lineage considered to be ecologically similar to crocodylomorphs. However, later works considered this species to be a junior synonym, and challenged the assignment of some of this material, ascribing them to the aquatic stem-lepidosaur Marmoretta. Here, we provided a revision of the published material from the Guimarota beds assigned to Cteniogenys, together with unreported and mislabelled specimens. We confirmed that the Portuguese specimens are probably non-conspecific with the taxa described in the Upper Jurassic of North America and in the Middle Jurassic of England. Unfortunately, the lack of diagnostic features from the only valid species prevented to confirm the original description as a distinct new species. Therefore, we only referred it to Cteniogenys aff. C. antiquus. We further supported the presence of Marmoretta in the Upper Jurassic of Portugal, and erected a new species, Marmoretta drescherae. Those occurrences support original palaeoenvironmental interpretations of the Guimarota beds as a wetland, probably close to mangrove-like, with important freshwater inputs. The presence of Cteniogenys in Portugal further supports faunal interchanges between North America, Europe, and potentially Northwestern Africa during the Jurassic/Cretaceous transition, if later occurrences are to be confirmed. The presence of Marmoretta also extend the temporal range of this relict reptile lineage at a time where squamates were radiating. However, its absence in other contemporary Jurassic localities, notably in the Lourinhã and Morrison formations, could hint towards ecological differences between those assemblages.
Key words: Reptilia, Diapsida, palaeobiogeography, systematic, Lusitanian Basin.
Alexandre R. D. Guillaume [alexandre.guillaume.763@gmail.com; ORCID: https://orcid.org/0000-0002-9005-9916 ], GEOBIOTEC, Department of Earth Sciences, NOVA School of Science and Technology, Universidade Nova de Lisboa, Campus de Caparica, P-2829 516 Caparica, Portugal.
Eduardo Puértolas-Pascual [eduardo.puertolas@gmail.com; ORCID: https://orcid.org/0000-0003-0759-7105 ] and Miguel Moreno-Azanza [mmazanza@gmail.com; ORCID: https://orcid.org/0000-0002-7210-1033 ], GEOBIOTEC, Department of Earth Sciences, NOVA School of Science and Technology, Universidade Nova de Lisboa, Campus de Caparica, P-2829 516 Caparica, Portugal; Aragosaurus: Recursos Geológicos y Paleoambientes – IUCA. Departamento de Ciencias de la Tierra, Universidad de Zaragoza, Calle Pedro Cerbuna 12, 50009, Spain.
Received 23 August 2024, accepted 2 December 2024, published online 14 March 2025.
Copyright © 2025 A.R.D. Guillaume et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Guimarota beds are one of the richest vertebrate microfossil assemblages for the Upper Jurassic, yielding thousands remains from various groups of vertebrates (Martin and Krebs 2000), including a vast diversity of “lizards”, despite only a few works focusing on them (Seiffert 1973; Broschinski 2000). Among them, a choristodere has been described and a putative stem-lepidosaur has been reported (Seiffert 1973; Evans 1989, 1991a).
The Choristodera Cope, 1884, are an extinct group of enigmatic freshwater amphibious diapsids, distributed through Laurasia (Matsumoto and Evans 2010; Matsumoto et al. 2019; Dong et al. 2020; Matsumoto et al. 2022b), with putative occurrences in Gondwana (Haddoumi et al. 2016; Lasseron et al. 2020). They range from the Middle Jurassic of UK and Kyrgyzstan to the Miocene of Europe (Evans 1990; Evans and Klembara 2005; Averianov et al. 2006; Matsumoto et al. 2013; Panciroli et al. 2020). They displayed various morphotypes, from brevirostrine to longirostrine, and from short-necked to long-necked, with size-range from that of small lizards to large crocodiles (Matsumoto and Evans 2010). They can be distinguished between Neochoristodera, the informal “allochoristoderes”, and more primitive forms such as Cteniogenys (Matsumoto et al. 2019; Dong et al. 2020; Dudgeon et al. 2024).
Despite being consistently recovered as monophyletic, the phylogenetic position of Choristodera within Diapsida remains uncertain (Matsumoto and Evans 2010; Matsumoto et al. 2019). They have been recovered as basal Archosauromorpha (Evans 1988; Gauthier et al. 1988), as sister group of Archosauromorpha + Lepidosauromorpha (Evans 1988; Dilkes 1998; Ezcurra et al. 2021), as basal diapsids (Gao and Fox 1998), within an expanded Lepidosauromorpha clade (Müller 2004), constrained to the base of either Lepidosauromorpha or Archosauromorpha (Ezcurra 2016), as basal Neodiapsida (Simões et al. 2018), or within Archosauromorpha (Simões et al. 2018; Griffiths et al. 2021; Jiang et al. 2023).
Cteniogenys was first recognized as an undetermined reptile (Gilmore 1928), later associated with “eolacertilians” based on the tooth implantation which was originally described as pleurothecodont (Seiffert 1973; Estes 1983), before being recognized as a Choristodera based on new material from the Bathonian of England (Evans 1989). Since then, it has been consistently described as the most basal taxon (Gao and Fox 1998; Matsumoto and Evans 2010; Matsumoto et al. 2013, 2019; Dong et al. 2020; Dudgeon et al. 2024) and one of the oldest occurrences of the group (Evans 1989, 1990; Averianov et al. 2006). Cteniogenys antiquus Gilmore, 1928, is currently the only valid species, and was initially described only by dentaries from North America.
Dentaries have been found in Europe, but could only be referred to Cteniogenys sp., due to the lack of diagnostic features differing from the C. antiquus material (Estes 1983; Evans 1989; Chure and Evans 1998). The Bathonian Kirtlington Mammal Bed (UK) yielded a more extensive cranial and post-cranial material, allowing a better characterization of Cteniogenys (Evans 1990, 1991b). More jaw and vertebral elements assigned to Cteniogenys sp. or referred to cf. Cteniogenys have been since reported in the Kimmeridgian–Tithonian of North America and from the Tithonian–Berriasian of western Europe (Chure and Evans 1998; Foster and Trujillo 2000; Foster 2001; Vullo et al. 2014; Foster et al. 2020; Allain et al. 2022), as well as allegedly in the Bathonian and Berriasian of Morocco (Haddoumi et al. 2016; Lasseron et al. 2020) and the Bathonian of Scotland (Panciroli et al. 2020). More recently, scarce choristodere material was reported from the Lourinhã Formation (Guillaume et al. 2023a), but it is pending further description.
A new species, “Cteniogenys reedi”, was described from the Guimarota beds (Seiffert 1973), but it was later considered as a junior synonym of the type species (Estes 1983), as the characters originally used could be included within the range of intraspecific variation observed in the American material (Estes 1983; Evans 1989; Chure and Evans 1998). Furthermore, some specimens’ identification has been considered questionable (Evans 1989); while others have been since then reattributed to the stem-lepidosaur Marmoretta (Evans 1991a).
Occurrences of Marmoretta oxoniensis Evans, 1991b, the only described species, are restricted to the Middle Jurassic of England (Evans 1991a, 1992; Evans and Milner 1994; Evans 1998a; Panciroli et al. 2020; Griffiths et al. 2021). Additional associated cranial and postcranial material are known in the Middle Jurassic of Scotland (Waldman and Evans 1994; Griffiths et al. 2021). It was originally described as a non-lepidosaurian lepidosauromorph, outside the crown-group of Lepidosauria (Evans 1991a; Conrad 2008), and subsequent material and analyses confirmed this original hypothesis (Waldman and Evans 1994; Evans 2009; Evans and Borsuk-Białynicka 2009; Evans and Jones 2010; Jones et al. 2013). The latest phylogenies also recovered Marmoretta as a stem-lepidosaur (Bolet et al. 2022), and as sister-taxon of Fraxinisaura from the Ladinian of Germany (Schoch and Sues 2018; Griffiths et al. 2021), despite being significantly younger than its counterpart. Other studies recovered it as a stem-squamate within Lepidosauria, along with either Megachirella or Huehuecuetzpalli (Simões et al. 2018; Sobral et al. 2020). A fragmented maxilla similar to Fraxinisaura and Marmoretta was reported in the upper Bathonian Peski locality (Moskvoretskaya Formation, Russia), but its preservation does not permit further comparison (Skutschas et al. 2022). Although not described, the Portuguese material has not been considered conspecific with the British specimens (Evans 1991a; Evans and Milner 1994).
Here, we review the material from the Guimarota beds originally attributed to Cteniogenys. We provide new descriptions as well as material previously unpublished or unreported. However, the material available does not permit us to confirm the validity of the species described by Seiffert (1973). Part of this material also confirms the presence of Marmoretta in the Guimarota beds, with new fossils previously misidentified, unpublished, or unreported, and provides support for a new lepidosauromorph species in the Upper Jurassic of Portugal.
Institutional abbreviations.—IPFUB, Institut für Paläontologie, Freie Universität Berlin, Germany; MG, Museu Geológico, Lisbon, Portugal; NMS, National Museums of Scotland, Edinburgh, UK.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the International Commission on Zoological Nomenclature (ICZN): urn:lsid:zoobank.org: act:127D1CEC-EFEA-4CCB-984C-8E6C759C7AC9.
Geological setting
The Institut für Paläontologie, Freie Universität Berlin (IPFUB) carried out two camapigns in the Guimarota beds, one in the early 1960s and a second one through the 1970s. Thousands of microfossil remains were recovered then (Martin and Krebs, 2000). The Guimarota beds are located in Guimarota, a neighbourhood of Leiria over 100 km north-east of Lisbon (Fig. 1). Since the first attempts to determine their age, the Guimarota beds have consistently been considered as part of the Alcobaça Formation (Ribeiro et al. 1979; Mateus et al. 2017; Fürsich et al. 2022), and have been given a Kimmeridgian age based on ammonites, charophytes, ostracods, pollens, lithostratigraphic correlation, and isotopic analyses (Ribeiro et al. 1979; Leinfelder and Wilson 1989; Schudack et al. 1998; Schudack 2000a; Fürsich et al. 2022). The fossiliferous series consists of two coal seams (Fig. 1D), with intercalation of lignitic marls occasionally rich in bivalve shells, separated by a single 5 m-thick layer of limestone (Schudack 2000b).
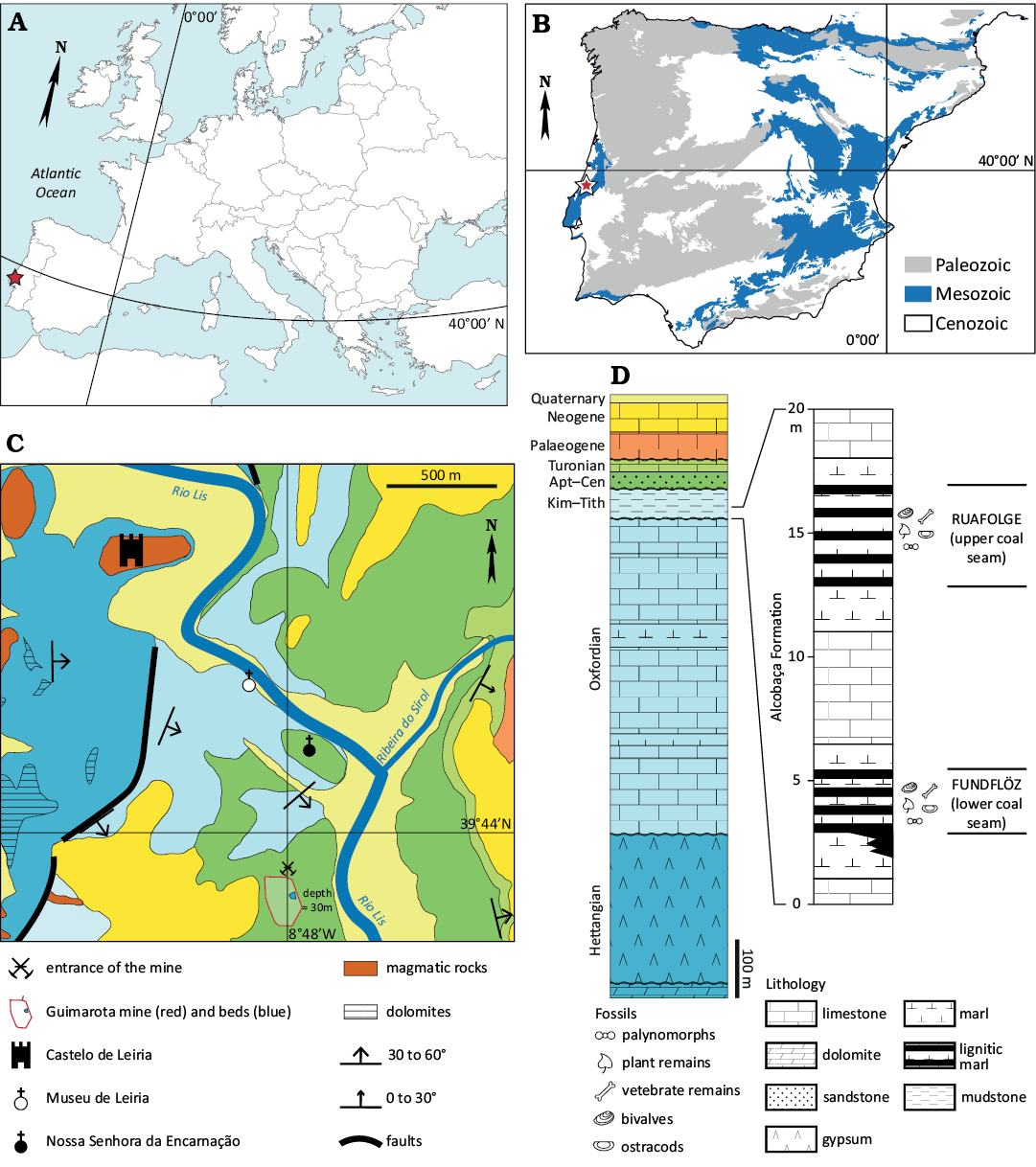
Fig. 1. Geographical and geological context of the vertebrate microfossil assemblages localities from the Alcobaça Formation. A. Map of Europe (modified from Erin Dill <fr.vecteezy.com>). B. Geological sketch of the Iberian Peninsula, showing the location of the study area (star). C. Geological sketch of the Leiria area, showing the location of the assemblage (modified from Teixeira et al. 1966). The entrance of the Guimarota mine, together with the outline of the pit and the approximate location of the fossil beds (blue area) are shown. Note that the fossil locality is located 30 meters below the surface, in the Upper Jurassic formations overlain by the Cretaceous. D. Stratigraphic position of the Guimarota beds and stratigraphic log of the Guimarota beds. General stratigraphic sketch derived from data in Teixeira et al. 1968. The colours for each stage correspond to the ones used in the map. Detailed stratigraphic log of the Alcobaça Formation from Schudack (2000a). Abbreviations: Apt–Cen, Aptian–Cenomanian; Kim–Tith, Kimmeridgian–Tithonian.
Due to the mining activity in Guimarota mine, the exact stratigraphic position of the specimens is unknown (Gloy 2000; Krebs 2000). However, it is our understanding that material here reviewed comes from specimens collected during both expeditions, and therefore from the Alcobaça Formation (Kimmeridgian). Based on its similarity with other brown coals from the Eocene and its geological settings, Guimarota has been regarded as a terrestrial to lagoonal environment similar to modern mangroves, with occasional freshwater influx and saltwater flooding (Gloy 2000; Martin 2000).
Material and methods
All specimens revised come from the excavations of the Guimarota beds by IPFUB. The specimen numbers attributed by IPFUB during the excavations were changed when the specimens were returned to the Museu Geológico of Lisbon, where they are now housed, following the agreements signed at the time of the excavations. Therefore, they are here published with their final specimen numbers from the Museu Geológico. However, it must be noted that specimen numbers are given to sample boxes or specimen holders, and that some boxes may yield more than one element, sometimes dozens of fragments. Because of the quantity of the material that represent the fossils from Guimarota, we focus our effort on specimens that were stored with the material described by Seiffert (1973) or mentioned in later works (Evans 1989, 1991a). Only the specimens that could be safely attributed either to Cteniogenys aff. Cteniogenys antiquus (77 specimen numbers) or Marmoretta drescherae sp. nov. (73 specimen numbers) are here described (see SOM: table S1.1, Supplementary Online Material available at http://app.pan.pl/SOM/app70-Guillaume_etal_SOM.pdf). Most relevant specimens were photographed using a DinoLite AM7013MZT, using DinoCapture 2.0 software.
Systematic palaeontology
Reptilia Linnaeus, 1758
Diapsida Osborn, 1903
Choristodera Cope, 1884
Genus Cteniogenys Gilmore, 1928
Type species: Cteniogenys antiquus Gilmore, 1928, monotypic; Quarry 9, Como Bluff (Wyoming, USA); Kimmeridgian (Trujillo et al. 2015).
Diagnosis.—Small choristoderes that differ from other members of the order by the following character states: contact between premaxillae and nasals absent; orbits significantly narrow and elongat relative to skull dimensions; frontals subrectangular, anterior border of which retracted slightly posterior to anterior level of orbits; dorsal process of maxilla prominent, confined to anterior part of maxilla, and slightly inrolled dorsally; prefrontal short, wide, having elongate palatal process. The genus is further distinguished from more derived neochoristoderes in having separate postorbital and postfrontal, both entering orbital rim; quadrate with weakly developed quadratojugal process; marginal teeth short, with striated crowns; tooth bases having large replacement pits, but lacking basal plicidentine infolding (modified from Gao and Fox 1998).
Stratigraphic and geographic range.—Kilmaluag Formation from the Isle of Skye (Bathonian), Middle Jurassic of Scotland (Panciroli et al. 2020); Kirtlington Mammal Bed (Bathonian), Middle Jurassic of England (Evans 1990); Guimarota beds (Kimmeridgian) (Seiffert 1973; Evans 1990) and Lourinhã Formation (Kimmeridgian–Tithonian) (Guillaume et al. 2023a), Upper Jurassic of Portugal; Como Bluff and others localities from Wyoming (Gilmore 1928; Foster and Trujillo 2000), Dinosaur National Monument in Utah (Chure and Evans 1998), and Wonderland Quarry in South Dakota (Foster and Trujillo 2000), Morrison Formation (Kimmeridgian), Upper Jurassic of USA; Charisson bonebeds (Tithonian), Upper Jurassic of France (Vullo et al. 2014); Angeac-Charente Lagerstätte (Berriasian), Lower Cretaceous of France (Allain et al. 2022); and Oldman and Dinosaur Park formations (mid-Campanian), Upper Cretaceous of Alberta, Canada (Gao and Fox 1998).
Cteniogenys aff. Cteniogenys antiquus Gilmore, 1928
Figs. 2, 3.
1973 Cteniogenys reedi; Seiffert 1973: 14, text-figs. 3–8.
1983 Cteniogenys reedi Seiffert, 1973; Estes 1983: nomen dubium.
Material.—One premaxilla, MG27600; 2 maxillae, MG 27417, 27419; 8 highly fragmented specimens; 18 dentaries, MG27605, 27609, 27627, 27628, 27638, 27646, 27652, 27653, 27662, 27664, 27666, 28761, 28826, 28831, 28843, 28882, 28901, 28914; 33 specimen holders with highly fragmented specimens; 2 surangulars, MG28420, 28423; 5 vertebra, MG28284, 28287, 28512, 28514, 28552; 2 humeri, MG28890, 28928; 5 ilia, MG28282, 28288–28791; block of associated bones, MG28792, vertebra, frontal, and putative jugal and postorbitofrontal (Evans 1991b). All from Guimarota beds (District of Leiria); Alcobaça Formation (Kimmeridgian), Upper Jurassic of Portugal (Seiffert 1973; Evans 1990).
Description.—Premaxilla (Fig. 2A): Only one specimen, a left premaxilla, was identified, but the very short anteromedial sutured border suggests it was paired in the live animal. The border surface is rather smooth, with two small pits (Fig. 2A2). The dorsal process indents the anterodorsal margin of the external nares (Fig. 2A1, A2). It is anteroposteriorly elongated, projecting strongly with an acute posterior apex. Although posteriorly broken, it extends up to 2.5 tooth loci (Fig. 2A3). Its smooth dorsal margin is bevelled and diverges medially from the anterior midline of the bone. The alveolar border displays three tooth loci (Fig. 2A3). Locus 1 and 3 preserved the root and part of the crown. The teeth are subcircular, subthecodont with the labial wall higher than the lingual wall. Locus 2 is empty and seems larger than the other ones. Although only three tooth loci are preserved, the bone seems to be almost complete. Indeed, the premaxilla displays a strong posterior suture to articulate with the maxilla, as well as a deep, short lateral facet (Fig. 2A1). The palatal shelf is not preserved. In lateral view, the premaxilla is relatively low, without anterior extension. Instead, it is subvertical, facing anteroventrally (Fig. 2A1). Two anterior sensory foramina are preserved, one dorsal and one ventral, followed posteriorly by fainter foramina organized in line.
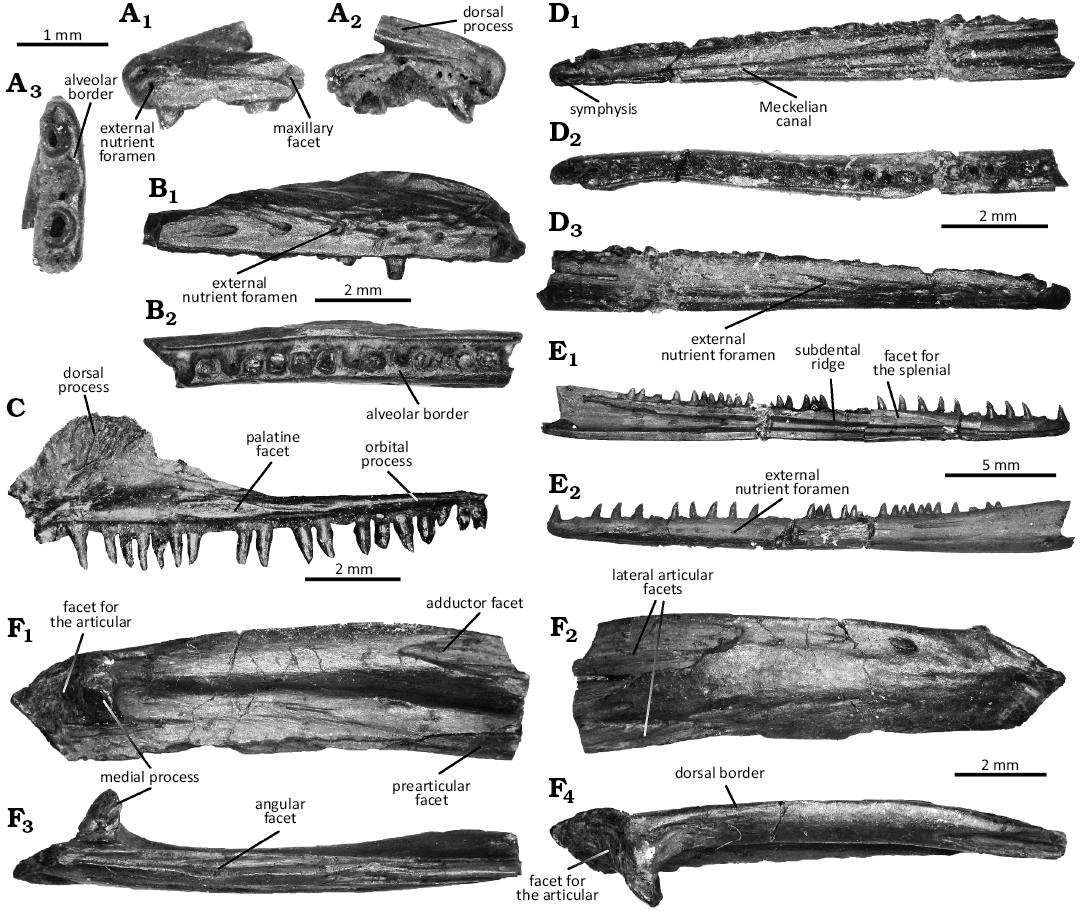
Fig. 2. Jaws of the choristoderan diapsid Cteniogenys aff. C. antiquus Gilmore, 1928, from the Guimarota beds (Guimarota mine, Leiria; Kimmeridgian). A. Left premaxilla MG27600, in labial (A1), lingual (A2), and ventral (A3) views. B. Right maxilla MG27417, in lateral (B1) and ventral (B2) views. C. Right maxilla MG27419 (Seiffert 1973: Gui L. 234, fig. 4), in lingual (C1) view. D. Right dentary MG27628 (holotype, Seiffert 1973: Gui A33, fig. 3), in lingual (D1), dorsal (D2), and labial (D3) views. E. Left dentary MG28514, in lingual (E1) and labial (E2) views. F. Left surangular MG27423, in medial (F1), lateral (F2), ventral (F3), and dorsal (F4) views.
Maxilla (Fig. 2B, C): Maxillary elements are mostly fragments from the posterior ramus. The maxilla is a long, slender bone with at least 21 teeth based on MG27419 (Fig. 2C). The dorsal process is flabellate, like a crest, with a dorsomedial facet ornamented by striae (Fig. 2C1). The labial surface displays heavy ornamentations similar to the dentary (Fig. 2B1): nutritive foramina extending into short anteroposterior striae. The anterior process is not preserved in MG27419, despite what was previously illustrated (Seiffert 1973), and could not be identified among other fragments. The bone tapers posteriorly into a long, low orbital process, forming a dorsoventrally compressed ramus, as a plate (Fig. 2C1). It displays a very shallow posterodorsal facet, which extends across the full width. The alveolar border exhibits a broad, elongated medial facet for the vomer (Fig. 2C1), with a large gap between vomerine and palatine facets to form the internal nares. It is associated with a large foramen connected to a deep anteroposterior groove, posterior to the dorsal process, for the opening of the superior alveolar canal.
Dentary (Fig. 2D, E): Dentaries are by far the most common bone, although most of the specimens consist of highly fragmented small elements of which any assessment cannot be safely confirmed. The dentary is a long, slender, mostly straight bone. Due to the preservation of the specimens, the tooth row displays between 24 and 33 teeth. The symphyseal surface is posteriorly elongated, dorsally and ventrally encapsulating the Meckelian canal (Fig. 2D1, E1). Its surface is rugose, but not ornamented. The facet for the splenial is visible, posteriorly to the symphysis (Fig. 2E1). Its anterior apex is acute. It participates ventrally, then medially, to the subdental shelf, with a bevelled rim. The facet keeps the Meckelian canal open dorsally and extends to the end of the tooth row based on MG28914 (Fig. 2E1). The subdental ridge is strong and robust, more marked than the ventral part of the dentary. Its mediolateral surface is blunt anteriorly but becomes posteriorly a horizontal shelf which separates the coronoid facet to the ventral prearticular facet.
The Meckelian canal is open all along the dentary, although the opening becomes wider towards the posterior end of the tooth row (Fig. 2D1, E1). MG28914 does not display an angular facet, but a posterior alveolar opening on the ventral wall. The labial surface is highly ornamented with anteroposterior short striae extending nutritive foramina in a double line (Fig. 2D3, E2). The teeth are conical, with an acute apex, and subthecodont. They are striated mostly on their lingual surface, although this is restricted to the top of the crown. The striations are absent in MG28901, of which teeth are more triangular with a blunt apex, but they are well pronounced in MG28914. The teeth have a cylindric base, with a slight anteroposterior compression. The anterior-most teeth are slightly curved distally.
Surangular (Fig. 2F): Two elements could be identified as surangulars, with MG27420 being still articulated with the posterior part of a dentary. It is a robust, elongated, subrectangular bone. The lateral surface is broad, with few foramina extending into short grooves in its anterior section. In MG27423, the large articular facets have an acute posterior apex (Fig. 2F2). The dorsal facet is obscured in MG27420 because of the dentary, and the ventral one could not be confirmed. The ventral margin displays a deep anteroposterior groove, the angular facet (Fig. 2F3). Its medial flange is shorter and can be seen in medial view. The posterior edge is acute to blunt, displaying a small process (Fig. 2F1).
The surangular exhibits a medially extended process, which is anteroposteriorly compressed to form a prominent prong. This process delimits a posterior, more rugose fossa for the articular (Fig. 2F1). Dorsally, it is connected to a strong wide ridge forming the dorsal border of the bone (Fig. 2F4). It participates in forming the thick, round adductor region and opens anteriorly into a deep slot with an acute posterior apex (Fig. 2F1). Ventrally, the process extends into a shallow ridge participating in the ventral border of the surangular with the angular facet (Fig. 2F1). It displays anteriorly a triangular facet for the prearticular, which is less marked than the dorsal slot.
Dorsal/sacral vertebra (Fig. 3A, C): MG28512 and 28552 both possess a short amphicoelous to platycoelous centrum, with subcircular anterior and posterior articular surfaces. The notochordal canal can be seen in MG28552 (Fig. 3C4, C5), suggesting it is an immature individual. Relatively square-shaped in dorsoventral view, the centrum is wider than long. Its dorsal surface is excavated on either side of strong median crest (Fig. 3A1, C1). Although the excavation is clogged by coal in MG28512, the dorsal ridge of the median crest, as well as the margin of the excavation, can be clearly seen.
Both vertebrae display large, raised lateral articular facets. These facets are roughly triangular, with the apex projecting laterally. In MG28512, it dips lateroventrally, with the apex more ventral than the base (Fig. 3A2). The facets are immediately posterior to the anterior margin of the centrum, being separated by a shallow groove. Neither ventral surface exhibits a sharp, midventral keel, as they are rather convex. However, MG28512 is smooth and broad, which would suggest it might be more likely dorsal than sacral (Fig. 3A3); while MG28552 is smooth, but narrower, almost constricted (Fig. 3C3), suggesting it might be sacral or transitional. However, the lack of material to compare with, and the fact that MG28552 seems to be more immature, prevent us from confirming the exact identification.
Caudal vertebra (Fig. 3B, D): Only three caudal vertebrae could be safely identified. The morphology of MG28514 (Fig. 3B) suggests it is an anterior caudal vertebra. It displays a short amphicoelous to platycoelous centrum, relative square-shaped in dorsal and ventral views (Fig. 3B1, B3). The opening of the notochordal canal is visible (Fig. 3B4, B5), suggesting it is an immature specimen. The anterior and posterior articular surfaces are subcircular. The dorsal surface of the centrum is excavated on either side of a strong median crest (Fig. 3B1); while the ventral surface displays a large, deep midventral groove with paired ventral flanges (Fig. 3B3). The morphology of MG28284 and 28287 suggests they were caudal and more posterior than MG28514, maybe mid-caudal. They share an amphicoelous to platycoelous subcircular centrum, slightly compressed laterally (Fig. 3D2). They display a deep ventral groove flanked by two lateral flanges extending anteroposteriorly (Fig. 3D2). They are preserved with the neural arch (Fig. 3D1), but the dorsal surface is obscured by sediments. The neural arch exhibits a basal longitudinal thin crest. The neural spine and the zygapophyses are not preserved.
Humerus (Fig. 3E): Two left humeri could be identified, although they are not complete. Based on overlapping material, the humerus displayed a broad proximal and distal head for a slender shaft that looks dense in cross-section and rotates around 90°. The proximal head is anteroposteriorly flattened. It extends on the proximal ridge as a convex bar, with a faint condyle in the middle. The ectotuberosity is well marked on the radial border (Fig. 3E1). It is separated from the head by a long proximodistal crest. The mediodorsal border displays a small peg, as well as a long groove on the dorsal surface where it joins the shaft in MG28928. The fossa is well marked. In medial view, along the radial border and distal to a shallow surface, a small dorsoradial rough area can be observed (Fig. 3E2).
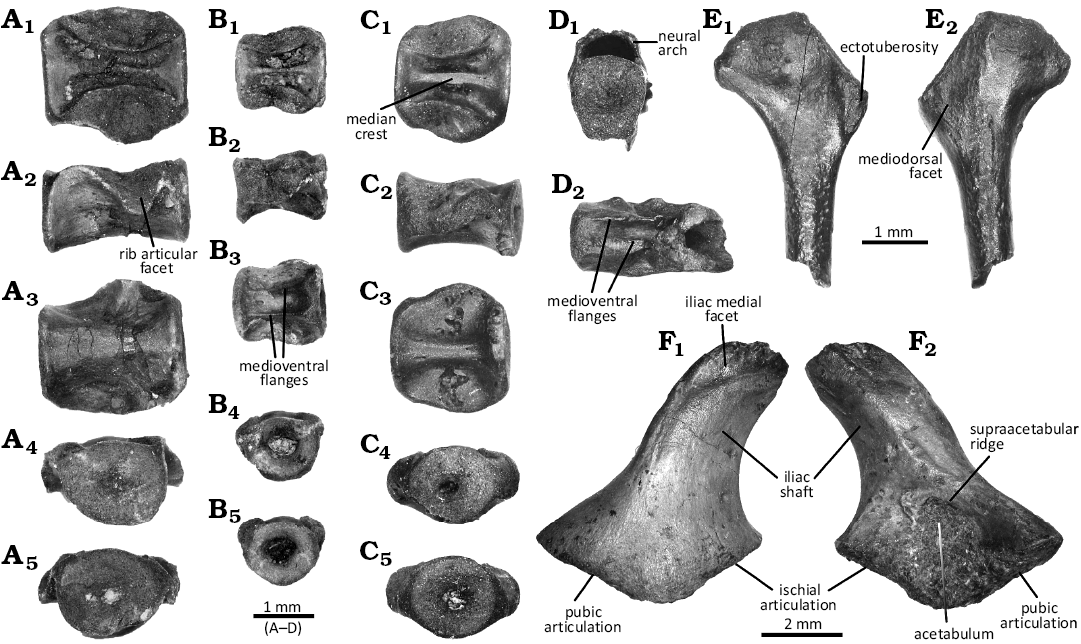
Fig. 3. Axial and appendicular skeleton of the choristoderan diapsid Cteniogenys aff. C. antiquus Gilmore, 1928, from the Guimarota beds (Guimarota mine, Leiria; Kimmeridgian). A. Dorsal vertebra MG28512, in dorsal (A1), lateral (A2), ventral (A3), anterior (A4), and posterior (A5) views. B. Anterior caudal vertebra MG28514, in dorsal (B1), lateral (B2), ventral (B3), anterior (B4), and posterior (B5) views. C. Dorsal/sacral vertebra MG28552, in dorsal (C1), lateral (C2), ventral (C3), anterior (C4), and posterior (C5) views. D. Mid-caudal vertebra MG28287 in anterior (D1) and ventral (D2) views. E. Left humerus MG28928, in lateral (E1) and medial (E2) views. F. Right ilium MG28289, in medial (F1) and lateral (F2) views.
The distal head is mediolaterally flattened and flares dorsoventrally, although the epicondyle is poorly developed as no clear differentiation of articular facet can be observed. The distal head also exhibits a deep but narrow ectepicondylar groove in dorsal view. The lateral supinator region barely extends, and is almost not separated from the rest of the bone. There is no sign of an entepicondylar groove. Both ventral and dorsal surfaces are smooth. The latter displays a shallow fossa, while the former displays a fossa which has a strong anterior rim and becomes fainter posteriorly.
Ilium (Fig. 3F): Only the ventralmost part of the ilia has been preserved. The acetabular region, fully preserved only in MG28289, is mediolaterally flattened. The anterior and posterior margins flare out, making a triangular general outline. The ventral border is wide V-shaped, highly flattened and obtuse, with a bulbous apex. Both ischial and pubic articular facets are preserved, the former being hooked in MG28289 (seemingly more mature than other specimens). The acetabulum is subovoid to triangular, rather shallow, no matter the size of the specimens, but with a strong rim in MG28289 (Fig. 3F2).
The supraacetabular ridge thickens in the middle, creating a distinct boss which merges with the ridge running along the iliac shaft; and is bordered ventrally in MG28282 by a round cavity that may yield a small foramen. The ilium narrows dorsally into a posteriorly curved blade, with a lateral shallow ridge running along. There is no sign of a dorsal tuberosity. The iliac shaft is mediolaterally flattened with a sharp posterior edge and a blunter anterior edge. Its width remains similar in all the sections, but the dorsalmost part is not preserved. It bears a medial facet (Fig. 3E1). However, its ventral margin can be either convex, making it like a strip, or concave, making it circular.
Remarks.—The dentaries here described display the typical morphology and ornamentation previously observed in the Cteniogenys sp. material from Kirtlington in the UK (Evans 1989, 1990), as well as in the material found in the Morrison Formation (Chure and Evans 1998; Foster and Trujillo 2000; Foster et al. 2020) and to the type species Cteniogenys antiquus (Gilmore 1928; Evans 1989), based on holotype and referred material currently housed in the Smithsonian National Museum of Natural History and the Yale Peabody Museum (USA; ARDG, personal observation 2022). They display the characteristic double row of nutritive foramina extended in striae on the labial surface, visible splenial facets tapered anteriorly, the medially directed symphysis, the broad conical teeth with subthecodont implantation—the base of each tooth is sitting in a shallow, rounded socket, but there is not root—and striae restricted to the apical part of the lingual surface of the tooth crown (Seiffert 1973; Evans 1989, 1990).
In his original work, Seiffert (1973) used the depth of the subdental ridge and the compression of the tooth crowns to distinguish the Guimarota taxon as C. antiquus Gilmore, 1928. However, it was later argued that the designated holotype MG27628 (Gui A. 33 in the original publication, see Fig. 2D) does not exhibit any significant difference in depth, neither does it preserve any tooth crown (Estes 1983); and being relatively small, it could belong to a juvenile (Evans 1989). Our examination of the Portuguese material, as well as holotype and referred material of C. antiquus, confirmed the limitation of the dentary to distinguish the species.
Nevertheless, previous work on the material from Guimarota and Kirtlington (Seiffert 1973; Evans 1989, 1990, 1991b) allows us identification of more elements with affinity between both localities, suggesting they were indeed sharing this faunal component. Notably, Seiffert (1973) could identify premaxillae and maxillae aside from the dentaries, although some of the premaxillae were later referred to the lepidosauromorph Marmoretta (Evans 1991a; Griffiths et al. 2021). Although the attribution of the maxilla MG27419 to Cteniogenys (Gui L. 234 in the original publication, see Fig. 2C) was questioned (Evans 1989), the other maxillae (MG27417, and other fragmentary specimens) described in this work could confirm the original assessment of Seiffert (1973) regarding MG27419: the maxilla displays the characteristic prominent dorsal process, confined to the anterior part of the maxilla, and slightly inrolled dorsally (Evans 1989, 1990; Gao and Fox 1998).
No complete maxillae were found associated with C. antiquus material in North America, and fragments were only referred to Cteniogenys sp. (ARDG personal observation, 2022). None seem to have preserved the flabellate dorsal process, but the material would require a deeper revision to confirm this statement as the isolated specimens are still embeded in their matrix. Nevertheless, their robustness, their ornamentation, and their tooth implantation match the European material.
Only one premaxilla (MG27600) assignment to Cteniogenys could be safely confirmed, based on its differences with other premaxillae attributed to Marmoretta and the presence of the dorsal process observed in the Cteniogenys sp. from Kirtlington (Evans 1990, 1991a). However, the Portuguese material slightly differs from the English material: the dorsal process is more acute, projecting not only further posteriorly (2.5 tooth loci instead of 1.5) but also more posteriorly rather than dorsally. The alveolar border from the Guimarota specimen is similar to the one observed in Kirtlington (Evans 1990: fig. 5A), although it preserves only three tooth loci instead of four and seems rather complete, suggesting it bore fewer teeth than the Kirtlington specimen. Finally, the Kirtlington premaxillae display a small anterior extension to the dorsal process, that can form a small ventral sickle (Evans 1990: fig. 5C, D); but that feature is not observed in Guimarota specimen. The well-preserved anterior section of the bone is instead subvertical, facing anteroventrally.
In addition to these bones, here we report for the first time the presence of surangulars, isolated vertebrae, humeri, and ilia. All of them display strong affinities with the material from Kirtlington. Surangulars were never reported previously, and they were actually labelled as Albanerpetontidae in the Guimarota collection. Albanerpetontids are an enigmatic group of extinct lissamphibians (Fox and Naylor 1982), but the surangulars from Guimarota do not resemble to any bone elements known in this group (Guillaume et al. 2023b), while their morphology is strikingly similar to the surangulars from Kirtlington (Evans 1990).
The vertebrae MG28512 and 28514 were also labelled as Albanerpetontidae. However, they display the dorsal excavations in either side of the median crest that is absent in albanerpetontids, and albanerpetontid vertebrae are hourglass shaped (Guillaume et al. 2023b). On the contrary, the square overall morphology, the dorsal excavations in either side of the median crest, the morphology of the articular facet, and the presence of facets to articulate with the rib match what has been described in dorsal and sacral vertebrae from Kirtlington (Evans 1991b). MG28552 was only labelled as Amphibia, but the square overall morphology, the dorsal excavations in either side of the median crest, the morphology of the articular facet, and ventral groove flanked by paired flanges also fit the description of caudal vertebrae from Kirtlington (Evans 1991b). The vertebra MG28287 was labelled as “Anura? or Cteniogenys?” but was not reported by Seiffert (1973). It closely resembles the vertebra MG28284, labelled Cteniogenys sp., although the latter was not reported by Seiffert (1973) either. Furthermore, MG28287 resembles other mid-caudal vertebrae from Kirtlington (Evans 1991b), and it is seemingly more elongated than MG28514.
The humerus MG28890 is labelled as Cteniogenys sp. but not mentioned by Seiffert (1973); while the humerus MG28928 was labelled as Squamata and neither referred in Seiffert work (1973). Regarding the ilia, only MG28289 and 28290 were labelled as Cteniogenys sp., while MG28291 was labelled as Anura, and MG28282 and 28288 as “Anura? or Cteniogenys?”; but none was mentioned nor reported previously. However, none of the five ilia displays the dorsal tuberosity characteristic of anurans. Rather, their morphology shares strong similarities with other choristoderes, especially Cteniogenys (Evans 1991b; Matsumoto et al. 2009). Furthermore, even if the ilia from the Guimarota beds exhibit different stages of preservation, they can be assigned to the same taxon.
However, some differences can be noted between the Portuguese and the English material, which would suggest there were several Cteniogenys species. The surangulars from the Guimarota beds display a small process on the posterior edge of the ventral margin which was not reported in the Kirtlington specimens (Evans 1990). Also, the bones from Guimarota seem longer than the ones from Kirtlington, although this could be due to a preservation bias in the English material. The ectotuberosity in the proximal head of the humerus appears relatively lower than the one observed in Kirtlington specimens. Furthermore, the ectotuberosity in Kirtlington is separated from the humeral head by a groove (Evans 1991b), not a crest as observed in Guimarota. The humeri from Kirtlington also display a proximal roughened region (Evans 1991b), but this one is absent in Guimarota specimens. Finally, the ectepicondylar grove is less marked in the Portuguese specimens than in the English ones (Evans 1991b).
Previous statements regarding the validity of the Guimarota species were correct (Estes 1983; Evans 1989; Chure and Evans 1998), as the dentaries alone do not allow differentiation of species from North America and Europe. A deeper revision of the material from the type locality of C. antiquus (Quarry 9, Como Bluff, Wyoming), as well as a more complete redescription of the type species with associated material, is needed to confirm, thus preventing us from erecting a new species or supporting previous taxonomic claims of its condition as a new species (Seiffert 1973). Nevertheless, our study confirms that although they are similar, the Portuguese and English species are different, hinting toward a higher diversity of early choristoderes in the Jurassic.
Lepidosauromorpha Gauthier et al., 1988
Genus Marmoretta Evans, 1991a
Type species: Marmoretta oxoniensis Evans, 1991a; Old Cement Works Quarry, Kirtlington, (Oxfordshire, England, UK); upper Bathonian.
Diagnosis.—Small lepidosauromorph; large upper and lower temporal fenestrae; premaxillae paired, each with deep posterolateral maxillary facet; specialized maxillary/premaxillary overlap; small posteroventral process of the jugal; narrow fused frontals; fused parietal forming a broad parietal table, parietal foramen absent, large midline sagittal crest; dorsoventrally wide posterior (squamosal) process of the postorbital that overlaps on to a broad shallow facet on the squamosal; palatine with small teeth that decrease in size medially from a larger row along the medial choana margin to smaller scattered teeth on the ventral surface; pterygoids bear three rows of teeth which radiate anteriorly; long and slender dentary with subpleurodont teeth; coronoid with prominent coronoid process having a smooth concave posterior surface that emerges through the lower temporal fenestra (from Griffiths et al. 2021).
Stratigraphic and geographic range.—Kilmaluag Fm from the Isle of Skye (Bathonian), Middle Jurassic of Scotland (Waldman and Evans 1994; Griffiths et al. 2021); Kirtlington Old Cement Works and Leigh Delamere (all Bathonian), Middle Jurassic of England, UK (Evans 1991a; Evans and Milner 1994; Evans 1998a); and Guimarota beds (Kimmeridgian), Upper Jurassic of Portugal (Evans 1991a).
Marmoretta drescherae sp. nov.
Figs. 4, 5.
ZooBank LSID: urn:lsid:zoobank.org:act:127D1CEC-EFEA-4CCB-984C-8E6C759C7AC9.
Etymology: In honour of Ellen Eggert (born Drescher), the German preparator from the IPFUB who oversaw and prepared the thousands of specimens from the Guimarota beds. Her tremendous and priceless work allowed and keeps allowing researchers to study one of the most important parts of the Portuguese palaeontological heritage.
Type material: Holotype: MG28841, right maxilla (Fig. 4B). Paratypes: 2 premaxillae, MG27588, 27644; 6 maxillae, MG28771, 28816, 28842, 28907, 28908, 28925; 3 dentaries, MG28785, 28794, 28910; 4 frontals, MG28740–28743.
Type locality: Guimarota mine, Leiria municipality, central Portugal.
Type horizon: Guimarota beds; Alcobaça Formation, Kimmeridgian, Upper Jurassic.
Material.—Type material and one premaxillae, MG27593; 6 maxillae, MG28751, 28757, 28798, 28815, 28887, 28888; 50 dentaries, MG27626, 27633, 27641, 27643, 27659, 27665, 27667, 28763, 28778, 28779, 28784, 28791, 28797, 28803, 28828, 28833, 28857, 28874, 28905, 28942, and 30 specimen holders and sample boxes with highly fragmented specimens. All from the type locality and horizon.
Diagnosis.—Small lepidosauromorph differing from Marmoretta oxoniensis by having highly constricted margins of the maxillary facet in the paired premaxillae; a more curved anterior rim of the dorsal process in the maxilla, making it rise more sharply; a lacrimal facet not extending dorsally on the dorsal process of the maxilla; maxillary teeth without apicolingual curvature; postfrontal facets not meeting posterodorsally in the fused frontals.
Description.—Premaxilla (Fig. 4A): None of the three premaxillae is complete, as the specimens from the Guimarota beds only preserved the anterior part. The alveolar tooth bearing plate is curved and formed by the alveolar border and crest. The premaxilla did not bear more than five teeth. Although none is preserved, the alveolar border suggests they were pleurodont, being attached via the labial wall only by the root, and were subcircular, being mesiodistally constricted (Fig. 4A1). MG27644 does not preserve the maxillary facet. In other specimens, the facet is located in the posterolateral region of the premaxilla and is anteroposteriorly elongated, with an acute anterior apex (Fig. 4A2). It is ventrally deep and V-shaped, with margins highly constricted, making it look like a groove or a slot. In MG27588 (Fig. 4A), the facet exhibits a shallow, small anterodorsal emargination which is not present in other specimens. The narial process extends posteriorly from the anteromedial part of the bone and participates in the alveolar tooth bearing plate, but it is poorly preserved (Fig. 4A). None of the specimens preserves the anterodorsal process (or nasal process sensu Evans 1991b).
Maxilla (Fig. 4B): The maxilla is a long, slender bone, tapered posteriorly. It is overall quite gracile, but its anterior section appears more robust. It is mediolaterally constricted, forming a long crest in dorsal view. The long, bulked anterior process is anteriorly acute with a round apex (Fig. 4B2, B4). It is best preserved in MG28841 and 28908, and partially in MG28771, 28816, 28907, and 28925; although, based on the shape of the maxillary facets observed in the premaxillae, it seems that no specimens fully preserved the anterior tip. The pars dentalis anteriorly extends up to 1/3 of the process (Fig. 4B4). MG28841 and 28908 do not display a clear premaxillary facet, but MG28925 displays a narrow slot, although its medial surface is convex. The dorsal surface of the anterior process is rather smooth, with faint anteroposterior ridges extending to the dorsal process. The dorsal margin itself is a thin crest (Fig. 4B1).

Fig. 4. Jaws of the lepidosauromorph diapsid Marmoretta drescherae sp. nov. from the Guimarota beds (Guimarota mine, Leiria; Kimmeridgian). A. Right premaxilla MG27588, in ventral (A1) and dorsal (A2) views. B. Right maxilla, holotype MG28841, in dorsal (B1), lateral (B2), ventral (B3), and medial views (B4). C. Right dentary MG28785, in dorsal (C1), medial (C2), and lateral views (C3); the specimen is broken, but both parts could be put together for the lateral and medial views.
The tip of the dorsal process (or facial process sensu Evans 1991b) is preserved in none of the Portuguese specimens, as it is always dorsally broken (Fig. 4B2, B4). Therefore, the development of the apex is unknown. Only the ventral section has been preserved in some specimens, allowing some overlapping material for that part. The base is rather large and its unparallel anterior and posterior edges suggest the dorsal process was either triangular or trapezoidal, but not much developed. Also, it does not seem to be medially inclined (Fig. 4B1). The anterior rim is thicker than the posterior rim, which looks more like a crest. The anterior rim outline is concave, being extremely marked in MG28816, 28841, and 28842. Medially, there is a small foramen at the base of the dorsal process. The lacrimal facet is not clearly marked: there is a deep medial depression posterior to the dorsal process, but it is ventrally restricted and does not extend dorsally.
The long posterodorsal jugal facet exhibits an acute anterior apex on the medial surface. The margin is well marked. It is located in the groove formed by the rim and the alveolar shelf. The palatine facet is well preserved only in the medial surface MG28908. It displays an anterodorsal-posteroventral shallow groove with two facets on the alveolar shelf: the dorsal facet is rather short while the ventral one is longer. It is ventral to the posterior rim of the dorsal process and is pierced by two foramina in its dorsal facet. The lateral surface of the bone is rather smooth. MG28842 and 28908 display occasional nutritive foramina. Posteriorly, the midline of the surface can be marked by a deep anteroposterior grove. MG28841 exhibits a tooth row with 17 teeth but is not complete and is missing its anterior section. Teeth are tubular to conical, with an acute apex. They are pleurodont, being attached only via the labial wall only by the root. No apicolingual curvature could be observed. The enamel covers the entire crown and can be slightly apically striated on the lingual side.
Dentary (Fig. 4C): Most of the specimens are broken, although several preserved all the parts, making MG28794 and 28910 the only ones seemingly complete and not broken. However, the former is embedded in coal matrix, and part of the anterior section of the latter is obscured. The dentary is a long, slender bone, relatively gracile. It deepens dorsoventrally in its posterior section. The labial surface is relatively smooth, although it can be covered by small nutritive foramina (Fig. 4C3). They are usually aligned, restricted to the anterodorsal part of the dental parapet. The anterior symphysis is small and short, forming a subvertical wall (Fig. 4C2). Lingually, the symphysis is divided in an upper and a lower rim by the Meckelian canal. The symphyseal surface is mostly flat, either in right or left specimens, although more rugose than the rest of the bone, and is U to V-shaped, the apex being more or less round. Also, the dentary is medially curved in that part (Fig. 4C1).
The lingual surface of the dentary is divided in two parts by the Meckelian canal: the dorsal subdental ridge and the ventral lip (Fig. 4C2). This canal is anteriorly widely opened at the symphysis and immediately posteriorly. Then, around the 4th and 5th tooth loci, the canal narrows drastically, to the point to form a thin groove or even contacting both the subdental ridges and the ventral lips, making a fold. However, some specimens can be dorsoventrally crushed, making one part looks like it overlaps the other. Posteriorly, the canal reopens broadly, with an acuminate apex. It broadens posteriorly up to the full height of the bone. Due to various preservations and intraspecific variation from one specimen to another, it is difficult to safely determine where this posterior opening starts, but it seems to be around seven or eight tooth loci before the end of the tooth row.
The tooth row is supported by the alveolar border. Posteriorly, where the Meckelian canal fully opens, it becomes a shelf. By the posterior end of the tooth row, it becomes weaker and bears a shallow facet for the coronoid (Fig. 4C1, C2). Teeth are tubular to conical, sharply pointed, and more apically elongated than in the maxilla. They are pleurodont, being attached via the taller labial wall only by the root; although, the implantation seems weak. The enamel covers most of the crown, with the apex being striated on the lingual surface. Based on specimens with tooth row the most complete, there were at least 19 teeth and up to 26.
Frontal (Fig. 5): All frontal bones are fused, suggesting they are all from mature individuals (Evans 1991a). MG28740 is broken midway in two pieces, and MG28743 did not preserve the anterior part. In dorsoventral view, their general outline is trapezoidal to subrectangular, with the posterior border being wider than the anterior border, especially in MG28742 (Fig. 5B). The lateral margins are embayed for the orbits.
Despite having similar length, MG28741 is broader than MG28742, probably due to intraspecific variation. Only MG28740 exhibits an ornamented dorsal surface, being sculptured by fine furrow and drumlin-shaped knobs. The fused frontals are anteroposteriorly convex in lateral views, being more marked in MG28742 but with upturned lateral edges (medial orbital margin) stronger in MG28741 (Fig. 5A3, A4, B3, B4). Unfortunately, the anterior border is not well preserved in any specimen. MG28742 retains a short process that could be the median process (Fig. 5B1, B2), but no anterolateral process can be seen.
In dorsal view, the anterior section of the dorsal surface is rather flat and shallow, while the posterior section appears more bulbous in its centre. The postfrontal facets are at the posterolateral corners of the bone (Fig. 5A3, A4, B3, B4). They are restricted to the lateral border, with a lenticular to triangular outline, and face lateroventrally. The blunt ventral vertex, where the anterior ridge of the facet meets the ventral surface of the bone, can be located at the midpoint of the facet to slightly posteriorly (MG28741, Fig. 5A3, and A4), or more anteriorly (MG28742, Fig. 5B3, B4 and MG28743, not figured).
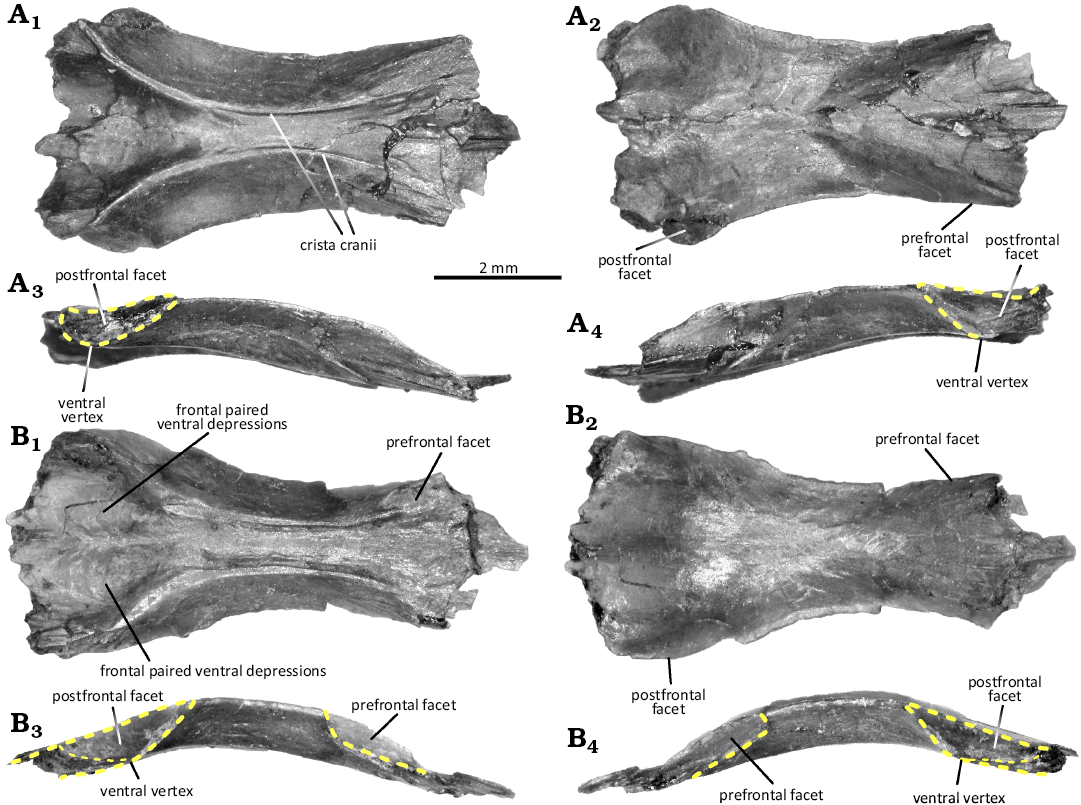
Fig. 5. Frontals of the lepidosauromorph diapsid Marmoretta drescherae sp. nov. from the Guimarota beds (Guimarota mine, Leiria; Kimmeridgian). A. MG28741, in ventral (A1), dorsal (A2), lateral right (A3), and lateral left (A4) views. B. MG28742, in ventral (B1), dorsal (B2), lateral right (B3), and lateral left (B4) views.
The ventral margin is more marked than the dorsal. The posterior margin of the fused frontals is not well preserved, but the postfrontal facets do not seem to meet posterodorsally (Fig. 5A1, A2, B1, B2). The anterolateral prefrontal facets participate to the anterior section of the fused frontal lateral edges (Fig. 5A3, A4, B3, B4). They are quite deep and wide, forming a posteroventral right angle, with posterior and ventral margins strongly marked. The ventral surface of the fused frontals bears distinct paired anteroposterior crista cranii (Fig. 5A1, B1), ventral extensions of the bone that act as dorsolateral walls for the olfactory tract. They lengthen the surface of the fused frontals’ lateral edges and participate in the orbits. They extend anteriorly after the lateral edges, and border the prefrontal facets ventrally. Both crista cranii form an hourglass outline, being lateromedially restricted at the centre of the bone. Their width is similar between all specimens. The ventral surface of the fused frontals displays a paired depression, separated by a ridge (Fig. 5A1, B1). The ridge can be marked, as in MG28742, or fainter, as in MG28741 and 28743, in which the crista cranii are posteriorly stronger. These paired depressions might house the dorsal region of the brain and would indicate that both cerebral hemispheres would be well differentiated by a sagittal sulcus (interhemispheric fissure sensu Barrios et al. 2023).
Remarks.—Thanks to the previous works on Marmoretta from the Middle Jurassic of the British Isles (Evans 1991a; Waldman and Evans 1994; Griffiths et al. 2021), more substantial material could be identified from the Guimarota beds and used to confirm the presence of the genus in the Upper Jurassic of Portugal. Indeed, all bones identified share similarities with Marmoretta oxoniensis material from Kirtlington and the Isle of Skye. The premaxillae are paired and exhibited a deep posterolateral maxillary facet, the maxilla displayed the characteristic anterior process to articulate with the premaxilla, the frontal bones are fused and narrow with shallow hourglass crista cranii, and the dentaries are long and slender with subpleurodont teeth.
However, some differences suggest the material from the Guimarota beds belongs to another species, as previously suggested (Evans 1991a; Evans and Milner 1994). Notably, the curvature of the anterior rim of the dorsal process in the maxilla in Marmoretta drescherae sp. nov. is more marked than in M. oxoniensis, where the rim rise smoothly instead (Evans 1991a; Waldman and Evans 1994; Griffiths et al. 2021). Marmoretta drescherae sp. nov. does not exhibit a dorsally extended lacrimal facet in the maxilla as represented in the reconstruction based on the specimens from Kirtlington (Evans 1991b: fig. 2D); and the maxillary teeth do not display apicolingual curvature as observed in the specimen from the Isle of Skye (Griffiths et al. 2021).
Furthermore, the dorsal process itself is not as medially inclined as observed in the Isle of Skye NMS G1992.47.1a, although this has been suggested to be a postmortem deformation (Griffiths et al. 2021). In the premaxilla, the characteristic maxillary facet exhibits margins more constricted than in M. oxoniensis, making the V-shaped facet narrower and acuter. Finally, the postfrontal facets do not meet posterodorsally as observed in the fused frontals from Kirtlington (Evans 1991a). When the anterior ridge of the postfrontal facets meets the ventral surface of the bone, they form a blunt ventral vertex. This vertex can be located at the midpoint of the facet, as observed in the specimen from the Isle of Skye (Griffiths et al. 2021) or in MG28741; but can also appear more anterior as in MG28742 and 28743. Unfortunately, the position of the vertex could not be confirmed in frontals from Kirtlington based on figured specimens, but it seems to be also located at the midpoint (Evans 1991a). It remains unclear if these different positions of the ventral vertex represent some intraspecific variation or are due to a preservational bias.
Stratigraphic and geographic range.—Type horizon and locality only.
Discussion
Although the Guimarota beds are known for their incredible diversity (Martin and Krebs 2000), and for yielding extraordinarily well-preserved specimens (Jäger et al. 2020), the material used in this study relies mostly on isolated, fragmented bones. On top of this, very few are complete, and even among those specimens, all are broken and were later glued back during their preparation at IPFUB in Berlin. Eventually, most of the specimens checked consisted of barely identifiable jaw fragments that could not even be distinguished between Cteniogenys and Marmoretta. For this reason, those were not included in this analysis and remain undetermined.
Cteniogenys from the Morrison Formation (Kimmeridgian–Tithonian, USA) was originally referred as a potential lizard but with uncertainty (Gilmore 1928), and later included within the informal squamate group “Eolacertilla” (Seiffert 1973). However, new and more complete material from the Kirtlington Mammal Bed (Bathonian, UK) allowed a better skull reconstruction, which highlighted a set of characters specific neither to squamates nor other basal lepidosauromorphs, but choristoderes instead, despite being an early-branching form: (i) external nares confluent; (ii) prefrontals elongated and meeting in the midline; (iii) pineal foramen absent; (iv) coarse striated dermal bones and complex skull sutures; (v) preorbital skull elongated; (vi) skull dorsoventrally flattened with inrolled maxilla; (vii) skull akinetic, braincase and quadrate firmly attached to the dermatocranium; (viii) diapsid, upper and lower temporal bars complete; (ix) lacrimal small, meets both maxilla and palatine; (x) postorbital large, meets jugal and ectopterygoid ventrally; (xi) vomer meets maxilla laterally, displacing the choana posteriorly; (xii) vomer, palatine and pterygoid separated by a small palatal foramen; (xiii) pterygoid with broad tooth battery in adult and teeth on the pterygoid flange; (xiv) ectopterygoid broadly overlaps pterygoid; (xv) basipterygoid processes reduced, sphenoid sutured to pterygoid; (xvi) hemispherical occipital condyle; (xvii) paroccipital process of opisthotic sutured to pterygoid flange of quadrate; (xviii) pterygoid and opisthotic facets separated by a third facet, probably for a neomorph; (xix) dentary elongate with double row of lateral foramina; (xx) dentary symphysis medial and elongated in mature specimens; (xxi) implantation subthecodont; and (xxii) coronoid long and low (Evans 1989). If the position of Choristedera within Diapsida remains uncertain, more recent findings confirmed the position of Cteniogenys as a basal member of this group (Matsumoto et al. 2019; Dong et al. 2020; Dudgeon et al. 2024). Furthermore, the Guimarota material shared clear affinity with the one from the Middle Jurassic of England and the Upper Jurassic of the US, more than any other reptiles known from the Upper Jurassic of Portugal and the Guimarota beds. No evidence was found that could challenge the original assignment of the specimens to Cteniogenys, nor that the genus could not be a choristodere.
As for Marmoretta, most of the material here described was previously mislabelled or misidentified. Evans (1991a) was the first to report that some of the specimens (see below) described by Seiffert (1973) as Cteniogenys should actually be referred as Marmoretta (Evans 1991a), but no further work on these specimens was done. Apart from Guimarota, Marmoretta is only known from the Middle Jurassic of England and Scotland, and was originally identified as a stem-lepidosaur (Evans 1991a; Griffiths et al. 2021). However, this has been challenged more recently (Simões et al. 2018; Sobral et al. 2020) and no consensus on the position of Marmoretta within Lepidosauromorpha has been reached yet. Marmoretta shares with lepidosauromorphs: (i) a reduced lacrimal; (ii) pleurodont implantation of maxillary and dentary dentitions; (iii) an anterior end of the dentaries split by the Meckelian canal; (iv) a quadratojugal foramen; and (v) an hourglass-shaped frontal (Schoch and Sues 2018; Simões et al. 2018; Griffiths et al. 2021). Furthermore, Marmoretta shares with several crown-lepidosaurs frontal bones fused to each other, but this feature is known to have appeared independently in other lepidosauromorphs (Schoch and Sues 2018) and repeatedly within lepidosaurs (Evans 1991a; Gauthier et al. 2012; Simões et al. 2018). However, Marmoretta lacks characters found in the crown-group lepidosaurs: (i) the presences of subolfactory processes of the frontals; and (ii) the lateral conch of the quadrate (Schoch and Sues 2018; Griffiths et al. 2021). Marmoretta further differs from squamates by not sharing most of their synapomorphies (Gauthier et al. 2012; Whiteside et al. 2022) and by having: (i) a quadratojugal; (ii) a lateral process of ectopterygoid; (iii) an anteroventral process of squamosal; (iv) a ventral exposure of the Vidian canal; and (v) lacking a notch for the squamosal on the cephalic head of the quadrate (Simões et al. 2018; Griffiths et al. 2021). Marmoretta further differs from rhynchocephalians by: (i) the absence of frontal tabs on the parietal; (ii) presence of a splenial; (iii) absence of a notochordal canal in adults; and (iv) a closed Vidian canal absent (Gauthier et al. 2012; Simões et al. 2018; Griffiths et al. 2021). Unfortunately, the specimens here described are too fragmentary and isolated to help in solving this controversy. Nevertheless, they clearly differ from all other reptile material known in the Guimarota beds, while presenting several affinities with the material from the Middle Jurassic of England. Therefore, we have found no evidence contradicting the original claim of Evans (1991a) to refer these specimens to Marmoretta and as a stem-lepidosaur reptile.
Reassigning specimens to Marmoretta.—Previous to this work, the only mention of Marmoretta from the Guimarota beds referred MG27644, originally described as “Cteniogenys reedi” sensu Seiffert 1973 (Seiffert 1973: fig. 5, Gui. 345), as a misidentified premaxilla (Evans 1991a). The revision of the material now stored in the Museu Geológico of Lisbon allowed us to identify at least 73 specimens and describe them as a new species.
Of the 73 specimens here described (see SOM: table S1.1), only 27 were formerly identified as Cteniogenys, and two more to Choristodera (MG28764 and 28785) in previous identifications in the MG catalogue; although Cteniogenys was the only choristodere species reported in the Guimarota beds. This concerns the three premaxillae, three maxillae, and 23 dentaries. As previously stated, the premaxillae of Marmoretta can be differentiated from Cteniogenys by the presence of the posterolateral maxillary facet and the absence of the small dorsal process indenting the anterior margin (Evans 1990, 1991a).
Dentary fragments can be hard to differentiate due to the similarities between both taxa, explaining the original misidentification by Seiffert (1973). However, the tooth implantation is subtly different between them: Marmoretta has a weak subpleurodont implantation (Evans 1991a; Griffiths et al. 2021), while it is rather subthecodont in Cteniogenys (Evans 1989, 1990). The enamel covers all the teeth in Marmoretta, while only the tip of the crown in Cteniogenys, and is strongly apically striated lingually in the latter, while the striae are smoother in the former (Evans 1990, 1991a; Griffiths et al. 2021). Both dentaries display an opened Meckelian canal, but in Marmoretta it is less open in the anterior section, and less regular (Evans 1991a; Griffiths et al. 2021). Another difference in the dentaries concerns the labial ornamentation, that is much more marked among Cteniogenys specimens (Evans 1990). Finally, when complete in mature specimens, the dentaries in Marmoretta drescherae sp. nov. are much shorter and less robust than the dentaries observed in Cteniogenys aff. C. antiquus from the Guimarota beds.
Similarly, maxillary fragments can be hard to differentiate between both taxa due to their similarities. Nevertheless, the tooth implantation and enamel also differ in the maxillae (Evans 1990, 1991a). The deep posterior groove observed in Marmoretta could not be confirmed in Cteniogenys aff. C. antiquus from the Guimarota beds. They also differ by the labial surface texture, the maxillae in Cteniogenys displaying similar ornamentation to the dentary, while they are rather smooth in Marmoretta (Evans 1990, 1991a; Griffiths et al. 2021). The main difference in complete specimens regards the long anterior process, absent in Cteniogenys, and the flabellate dorsal process, which is rather triangular or trapezoidal in Marmoretta (Evans 1990, 1991a).
It is worth noting that the four fused frontals were all identified as “Anura” but labelled as albanerpetontid frontals, while they clearly do not display the characteristic bell-shaped outline and the flabellate, bulbous internasal process observed and described in the other albanerpetontid material from Guimarota (Wiechmann 2003; Guillaume et al. 2022). Furthermore, these frontals differ from any frontal morphology currently known in albanerpetontids (Gardner 2002; Carrano et al. 2022; Guillaume et al. 2022), or usually observed in frogs. Instead, by being fused with lateral margins embayed for the orbits, anteroposteriorly convex, and exhibiting two marked ventral crista cranii, they strongly resemble the ones described in M. oxoniensisi (Evans 1991a; Griffiths et al. 2021). Two more maxillae (MG28751 and 28757) were also identified as albanerpetontid; but, here again, they are completely different to equivalent material known and described from the Guimarota beds (Guillaume et al. 2023b).
Three dentaries (MG28811, 28812, and 28861) were previously assigned to undetermined Reptilia, but they share the characteristics described above. One dentary was referred to “thecodont” (MG28854), but its anatomy more closely matches the one observed in the other Marmoretta drescherae sp. nov. dentaries, rather than other potential thecodont jaw-bearer known from the Guimarota assemblage (crocodylomorphs, dinosaurs, mammals). The remaining 34 specimens (8 maxillae and 26 dentaries) were assigned to Squamata, but their morphology fits better the one described for Marmoretta drescherae sp. nov. than in any other squamate species described from the diverse Guimarota assemblage (Seiffert 1973; Broschinski 2000). We acknowledge that Seiffert and subsequent researchers possibly identified these bones as different from other reptiles in the Guimarota assemblage but were not able to identify them properly.
Nevertheless, this work mostly focused on some cranial bones from Marmoretta and Cteniogenys, while Guimarota yields thousands of small diapsid fossils (Martin and Krebs 2000). Previous works already highlighted the significant Jurassic diversity for this group (Seiffert 1973; Broschinski 2000; Caldwell et al. 2015), but most of the remains are still unidentified or assigned only to higher taxonomic ranks. As exemplified by this work, it would not be surprising that more elements could be assigned to either Cteniogenys aff. C. antiquus, Marmoretta drescherae sp. nov., or another diapsid taxon with a deeper revision of the material.
Palaeobiogeographical implications.—During the Late Jurassic, the Iberian Plate was one of the largest islands of the European archipelago system, surrounded by warm, shallow epicontinental seas, with several transgressive/regressive transitions (Moore et al. 1992; Martinius and Gowland 2011; Myers et al. 2012). At the cross-road between the Tethys and the proto-Atlantic oceans, the Iberian Plate was both connected to North America (Dercourt et al. 2000; Scotese 2014; Brikiatis 2016) and close to the northwestern African shore (Gheerbrant and Rage 2006; Haddoumi et al. 2016). Potential continental bridges in the Late Jurassic could allow faunal interchanges with North America (Evans 1998b, 2003; Mateus 2006; Tennant et al. 2017; Lasseron et al. 2020; Allain et al. 2022). Meanwhile, the Tethys was rather a permeable barrier (Goodwin et al. 1999), and therefore could allow dispersal events between Laurasia and Gondwana through ephemeral land connections during the Jurassic/Cretaceous transition (Haddoumi et al. 2016; Lasseron et al. 2020; Allain et al. 2022).
As a primitive choristodere, Cteniogenys ranges from the Bathonian of UK (Evans 1989, 1990, 1991b) to the Berriasian of France (Allain et al. 2022), with occurrences in the Upper Jurassic (Kimmeridgian) Morrison Formation in North America (Gilmore 1928; Chure and Evans 1998; Foster and Trujillo 2000; Carrano and Velez-Juarbe 2006; Foster et al. 2020), the Upper Jurassic Alcobaça and Lourinhã formations (Seiffert 1973; Guillaume et al. 2023a), both dated from the Kimmeridgian–Tithonian, and the Tithonian of France (Vullo et al. 2014). More material has been attributed to the genus in the Upper Cretaceous of Alberta (Gao and Fox 1998), depsite the important chronological gap with Jurassic species. Notably, the bones used for this identification are extremely fragmented (ARDG personal observation, 2023), and have since been regarded as not sufficiently diagnostic to reach this identification and might represent another genus (Gao and Brinkman 2005; Matsumoto et al. 2009; Matsumoto and Evans 2010). The material is now considered as an undetermined choristodere (Matsumoto et al. 2009).
The fossil record of Cteniogenys further supports faunal interchanges between Europe and North America during the final stages of the Late Jurassic, where the Iberian Plate could have played a connecting role between both realms. Furthermore, fragmentated material from the Bathonian and the Berriasian of Morrocco was attributed to Choristodera, but the authors highlighted similarities with Cteniogenys (Haddoumi et al. 2016; Lasseron et al. 2020). If this identification is confirmed, it would extend considerably the geographic range of the genus. Furthermore, it would support the hypothesis for faunal interchanges between northwestern Africa, North America, and Europe via the Iberian Plate during the Jurassic/Cretaceous transition, as suggested by other small vertebrate groups such as albanerpetontids (although absent in North America), discoglossids, sphenodonts, scincomorph and anguimorph squamates, and docodont and dryolestid mammals (Evans 1993, 2003; Evans and Milner 1994; Broschinski 1999; Carrano and Velez-Juarbe 2006; Ortega 2009; Gardner and DeMar 2013; Foster et al. 2020; Lasseron et al. 2020; Guillaume et al. 2023a).
While choristoderes were emerging and dispersing in the Late Jurassic, “lizards” were going through major changes. Based on their fossil record and molecular clock analyses, lepidosauromorph diapsids emerged in the Permian or the Early Triassic (Evans 1993, 2003; Evans and Jones 2010; Jones et al. 2013; Simões et al. 2018; Sobral et al. 2020), before that lepidosaurs diversified into squamates and rhynchocephalians during the Triassic (Evans and Jones 2010; Bolet et al. 2022; Herrera-Flores et al. 2022). The latter remained dominant and more abundant in the fossil assemblages through all the Triassic and the Middle Jurassic before declining in the Early Cretaceous, while the former started radiating in the Middle to Late Jurassic (Estes 1983; Evans 1993, 1998b, 2003; Conrad 2008; Evans and Jones 2010; Gauthier et al. 2012; Caldwell et al. 2015; Simões et al. 2018; Bolet et al. 2022; Brownstein et al. 2022; Herrera-Flores et al. 2022).
Although Early Cretaceous assemblages may yield some archaic forms (Evans 1998b), most stem-lepidosaurs seem to have become extinct in the Triassic (Evans 2003; Gauthier et al. 2012; Schoch and Sues 2018; Simões et al. 2018; Sobral et al. 2020; Bolet et al. 2022). If recent analyses placing M. oxoniensis as a stem-lepidosaur are confirmed (Griffiths et al. 2021; Bolet et al. 2022), the new material would extend the temporal range of this relict lineage up to the Kimmeridgian. Nevertheless, its absence from the coeval Lourinhã Formation (Guillaume et al. 2023a), but also from other vertebrate assemblages in North America or Africa (Aberhan et al. 2002; Carrano and Velez-Juarbe 2006; Lasseron et al. 2020), would support a European endemism of stem-lepidosaurs by the end of the Late Jurassic during their final decline.
Palaeoecology of Cteniogenys and Marmoretta.—The structure of the skull in Cteniogenys and its postcranial skeleton display anatomical features usually associated with aquatic reptiles (Evans 1991b), and younger choristoderes could be associated to an aquatic to subaquatic lifestyle in freshwater environments (Matsumoto and Evans 2010; Matsumoto et al. 2022a; Wang et al. 2023). Both cranial and postcranial morphologies of Marmoretta reflect as well adaptions for an aquatic to amphibious lifestyle, or a burrowing one, to avoid competition with more terrestrial “lizards” that were rising in the Middle Jurassic (Evans 1991a; Evans and Milner 1994; Waldman and Evans 1994). The weaker tooth implantation compared to Cteniogenys would suggest a different diet (Evans 1991a), although the Marmoretta skull suggests that it could display comparatively strong bit-force for a small diapsid (Evans 1991a; King 1996; Pritchard et al. 2018; Griffiths et al. 2021).
The Morrison Formation’s Quarry 9, from where C. antiquus was first described, has been interpreted as a low-energy depositional environment, such as a pond or oxbow lake (Carrano and Velez-Juarbe 2006), which fits the palaeoenvironment of one locality of the Lourinhã Formation where new putative material has been reported (Guillaume et al. 2023a). More generally, Cteniogenys material is mostly concentrated into the northern and eastern parts of the Morrison Fm (Foster and Trujillo 2000), as other semi-aquatic animals with similar pattern such as crocodylomorphs, turtles, or the mammaliamorph Docodont (Foster et al. 2006; Foster and McMullen 2017); areas considered to have more abundant surface water (Turner and Peterson 2004). Meanwhile, the Kirtlington Mammal Bed has been interpreted as a shallow swampy coastal region with lagoons and freshwater lakes, similar to modern-days Everglades in Florida, dominated by aquatic and amphibious taxa (McKerrow et al. 1969; Freeman 1976, 1979; Sellwood 1978; Palmer 1979; Evans 1991a, b), which fits the interpretations for other localities in the Lourinhã Formation (Guillaume et al. 2023a), but also with a mangrove-like environment in which the Guimarota assemblage is thought to have deposited (Gloy 2000; Martin 2000).
The presence of Cteniogenys and Marmoretta material in the Guimarota beds and Kirtlington Mammal Bed support an important freshwater component in both ecosystems. However, the absence of Marmoretta remains in other Portuguese and North American localities where Cteniogenys remains have been reported (Carrano and Velez-Juarbe 2006; Guillaume et al. 2023a) could hint at true ecological differences between Guimarota beds/Kirtlington Mammal Bed and localities from the coeval Morrison and Lourinhã formations.
Furthermore, choristoderes seem to be less diverse and rarer in environments where crocodiles thrive (Matsumoto and Evans 2010), to which they are often considered as eco-analogues (Fox 1968; Erickson 1987; Evans and Hecht 1993; Matsumoto and Evans 2010; Matsumoto et al. 2022a). Such partitioning could be explained by climatic, depositional, and other abiotic factors; but it has also been suggested it could be the result of some preferences or competition for ecological niches between choristoderes and aquatic crocodiles and other freshwater animals of similar size (Matsumoto and Evans 2010). In the Morrison Formation, localities that produced Cteniogenys material also produced more abundant semi-aquatic crocodylomorph material, choristoderes remaining relatively scarce (Foster and Trujillo 2000; Foster and McMullen 2017). Moreover, until further revision of the North American material, choristodere and Cteniogenys are known by only one species; while semi-aquatic crocodylomorphs are represented by at least six species, but mostly goniopholids (Carrano and Velez-Juarbe 2006; Foster and McMullen 2017; Foster et al. 2020).
This pattern is congruent with the faunal composition of the Guimarota assemblage, in which crocodylomorphs are extremely diverse and common (Schwarz 2002; Schwarz and Fechner 2004, 2008; Schwarz and Salisbury 2005; Schwarz et al. 2017; Guillaume et al. 2020), whereas choristodere material is much rarer. Indeed, because of its small size, reconstructions suggest a total length of 30 cm (Evans 1991b), Cteniogenys was unlikely competing with the giant marine predator Machimosaurus; nor with generalist feeders, such as goniopholids, and other macrocarnivorous terrestrial crocodylomorphs known from the Guimarota beds and the Lourinhã Formation (Schwarz 2002; Guillaume et al. 2020; Puértolas-Pascual and Mateus 2020; López-Rojas et al. 2024).
On the other hand, Cteniogenys was from a similar size-range as bernissartiids (Sweetman 2016; Martin et al. 2020), atoposaurids (Schwarz and Salisbury 2005; Schwarz et al. 2017) and other small crocodylomorphs Lusitanisuchus and Lisboasaurus (Schwarz and Fechner 2004, 2008) reported in the Upper Jurassic of Portugal (Guillaume et al. 2020). The conical teeth observed in Cteniogenys suggest it was most likely not sharing with bernissartiids the durophagous diet implied by their molariform teeth (Guillaume et al. 2020). However, Cteniogenys could have competed with atoposaurids and other small crocodylomorphs, whose diet was based on small arthropods (insects, crustaceans, soft-bodied) and occasional small vertebrates such as mammals and amphibians (Guillaume et al. 2020). This may explain why Cteniogenys seems to be more common in the Late Jurassic of North America, where goniopholids were rather diverse but smaller semi-aquatic crocodylomorphs not so much (Foster and McMullen 2017), than in the Late Jurassic of Europe, where atoposaurids were thriving (Tennant and Mannion 2014).
Conclusions
Revision of material from the well-known Upper Jurassic Guimarota beds from Portugal provided support for a Cteniogenys taxon non-conspecific with the ones described from the Morrison Formation and the Kirtlington Mammal Bed, although the clade would require a revision to properly describe its species. It further supports faunal interchanges between North America, Northwestern Africa, and Europe through the Iberian Plate during the Jurassic/Cretaceous transition. Furthermore, specimens from Guimarota formerly attributed to Cteniogenys and other herpetofaunal clades were redescribed and gave support for a new stem-lepidosaur species. This new species further extends the temporal range of this relict lineage to the very end of the Jurassic period, at a time where other lizards were going through a major faunal shift. It also highlights a vestigial European endemism for stem-lepidosaurs, as well as hints toward true ecological differences with other localities in Europe and North America.
Acknowledgements
We thank Jorge Sequeira (Museu Geológico, Laboratório Nacional de Energia e Geologia, Lisbon, Portugal) and Museu Geológico de Lisboa for providing the access to the Guimarota collection; Mariana Valente (NOVA School of Science and Technology, Lisbon, Portugal) for the help in cataloguing the specimens from Guimarota, allowing to locate the new relevant material; and Susan Evans (Cell & Developmental Biology, University College London, London, UK) for her help in understanding the anatomy of both animals. We are grateful to both reviewers, Susan Evans and John Foster (Utah Field House of Natural History State Park Museum, Vernal, USA), for providing insightful comments and suggestions to improve the manuscript. ARDG wants to thank Matthew Carrano (Smithsonian National Museum of Natural History, Washington, USA), Daniel Brinkman and Vanessa Rhue (both Yale Peabody Museum of Natural History, New Haven, USA), Howard Gibbins and Michael Cadwell (both University of Alberta Laboratory of Vertebrate Palaeontology, Edmonton, Canada) and James Gardner (Royal Tyrell Museum of Palaeontology, Drumheller, Canada) for granting access to comparative material; as well as Anabela Veiga (Escola Superior de Tecnologia e Gestão of the Politécnico de Leiria, Portugal) and the Museu de Leiria to facilitate access to the Guimarota mine and give global context of the excavation. This work was supported by the Fundação para a Ciência e Tecnologia (FCT-MCTES) of Portugal (grant numbers SFRH/BD/144665/2019, PTDC/CTA-PAL/2217/2021, and Research Unit GeoBioCiências, GeoTecnologias e GeoEngenharias (GeoBioTec) UIDB/04035/2020 (href="https://doi.org/10.54499/UIDB/04035/2020%29">https://doi.org/10.54499/UIDB/04035/2020); Project PID2021-122612OB-I00, funded by MCIN/AEI/10.13039/501100011033; Grant RYC2021-034473-I funded by MCIN/AEI/10.13039/501100011033 and by the European Union “Next Generation EU”/PRTR”; and by the Aragon Regional Government (Grupo de referencia: E18_23R Aragosaurus: Recursos Geológicos y Paleoambientales). EPP was supported by a postdoctoral contract (María Zambrano) funded by the Ministry of Universities of the Government of Spain through the Next Generation EU funds of the European Union.
Authors’ contributions
ARDG: conceptualization, formal analysis, investigation, methodology writing—original draft; MMA, EPP: supervision; ARDG, MMA, EPP: writing—review & edit; MMA: funding acquisition.
Editor: Daniel Barta.
References
Aberhan, M., Bussert, R., Heinrich, W.-D., Schrank, E., Schultka, S., Sames, B., Kriwet, J., and Kapilima, S. 2002. Palaeoecology and depositional environments of the Tendaguru Beds (Late Jurassic to Early Cretaceous, Tanzania). Fossil Record 5: 19–44. Crossref
Allain, R., Vullo, R., Rozada, L., Anquetin, J., Bourgeais, R., Goedert, J., Martin, J., de Fabrègues, C.P., Royo-Torres, R., and Augier, D. 2022. Vertebrate paleobiodiversity of the Early Cretaceous (Berriasian) Angeac-Charente Lagerstätte (southwestern France): implications for continental faunal turnover at the J/K boundary. Geodiversitas 44: 683–752. Crossref
Averianov, A.O., Martin, T., Evans, S.E., and Bakirov, A.A. 2006. First Jurassic Choristodera from Asia. Naturwissenschaften 93: 46–50. Crossref
Barrios, F., Bona, P., Paulina-Carabajal, A., Leardi, J.M., Holliday, C.M., and Lessner, E.J. 2023. An overview on the Crocodylomorpha cranial neuroanatomy: variability, morphological patterns and paleobiological implications. In: M.T. Dozo, A. Paulina-Carabajal, T.E. Macrini, and S. Walsh (eds.), Paleoneurology of Amniotes: New Directions in the Study of Fossil Endocasts, 213–266. Springer International Publishing, Cham. Crossref
Bolet, A., Stubbs, T.L., Herrera-Flores, J.A., and Benton, M.J. 2022. The Jurassic rise of squamates as supported by lepidosaur disparity and evolutionary rates. eLife 11: e66511. Crossref
Brikiatis, L. 2016. Late Mesozoic North Atlantic land bridges. Earth-Science Reviews 159: 47–57. Crossref
Broschinski, A. 1999. Ein Lacertilier (Scincomorpha, Paramacellodidae) aus dem Oberen Jura von Tendaguru (Tansania). Fossil Record 2: 155–158. Crossref
Broschinski, A. 2000. The lizards from the Guimarota mine. In: T. Martin and B. Krebs (eds.), Guimarota: A Jurassic Ecosystem, 59–68. Verlag Dr. Friedrich Pfeil, Munich.
Brownstein, C.D., Meyer, D.L., Fabbri, M., Bhullar, B.-A.S., and Gauthier, J.A. 2022. Evolutionary origins of the prolonged extant squamate radiation. Nature Communications 13 (1): 7087. Crossref
Caldwell, M., Nydam, R., Palci, A., and Apesteguia, S. 2015. The oldest known snakes from the Middle Jurassic–Lower Cretaceous provide insights on snake evolution. Nature Communication 6: 5996. Crossref
Carrano, M.T. and Velez-Juarbe, J. 2006. Paleoecology of the Quarry 9 vertebrate assemblage from Como Bluff, Wyoming (Morrison Formation, Late Jurassic). Paleogeography, Paleoclimatology, Paleoecology 237: 147–159. Crossref
Carrano, M.T., Oreska, M.P.J., Murch, A., Trujillo, K.C., and Chamberlain, K.R. 2022. Vertebrate paleontology of the Cloverly Formation (Lower Cretaceous), III: a new species of Albanerpeton, with biogeographic and paleoecological implications. Journal of Vertebrate Paleontology: e2003372. Crossref
Chure, D.J. and Evans, S.E. 1998. A new occurrence of Cteniogenys, with comments on its distribution and abundance. Modern Geology 23: 49.
Conrad, J.L. 2008. Phylogeny and systematics of Squamata (Reptilia) based on morphology. Bulletin of the American Museum of Natural History 310: 1–182. Crossref
Cope, E.D. 1884. The Choristodera. American Naturalist 17: 815–817.
Dercourt, J., Gaetani, M., Vrielynck, B., Barrier, E., Biju-Duval, B., Brunet, M.-F., Cadet, J.-P., Crasquin, S., and Sandulescu, M. 2000. Atlas Peri-Tethys Palaeogeographical Maps. Commission de la carte géologique du monde, Paris.
Dilkes, D.W. 1998. The Early Triassic rhynchosaur Mesosuchus browni and the interrelationships of basal archosauromorph reptiles. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 353: 501–541. Crossref
Dong, L., Matsumoto, R., Kusuhashi, N., Wang, Y., Wang, Y., and Evans, S.E. 2020. A new choristodere (Reptilia: Choristodera) from an Aptian–Albian coal deposit in China. Journal of Systematic Palaeontology 18: 1223–1242. Crossref
Dudgeon, T.W., Mallon, J.C., and Evans, D.C. 2024. The first report of Champsosaurus lindoei (Choristodera: Champsosauridae) from the Campanian of the United States: anatomical, phylogenetic, and palaeoecological significance. Zoological Journal of the Linnean Society 200: 776–795. Crossref
Erickson, B.R. 1987. Simoedosaurus dakotensis, new species, a diapsid reptile (Archosauromorpha; Choristodera) from the Paleocene of North America. Journal of Vertebrate Paleontology 7: 237–251. Crossref
Estes, R. 1983. Sauria terrestria, Amphisbaenia. In: P. Wellnhofer (ed.), Handbuch der Paläoherpetologie. Part 10A. 249 pp. Gustav Fischer Verlag, Stuttgart.
Evans, S.E. 1988. The early history and relationships of the Diapsida. In: M.J. Benton (ed.), The Phylogeny and Classification of the Tetrapods, Vol. 1, 221–260. Clarendon Press, Oxford.
Evans, S.E. 1989. New material of Cteniogenys (Reptilia: Diapsida; Jurassic) and a reassessment of the phylogenetic position of the genus. Neues Jahrbuch für Geologie und Paläontologie-Monatshefte 1989 (10): 577–589. Crossref
Evans, S.E. 1990. The skull of Cteniogenys, a choristodere (Reptilia: Archosauromorpha) from the Middle Jurassic of Oxfordshire. Zoological Journal of the Linnean Society 99: 205–237. Crossref
Evans, S.E. 1991a. A new lizard-like reptile (Diapsida: Lepidosauromorpha) from the Middle Jurassic of England. Zoological Journal of the Linnean Society 103: 391–412. Crossref
Evans, S.E. 1991b. The postcranial skeleton of the choristodere Cteniogenys (Reptilia: Diapsida) from the Middle Jurassic of England. Geobios 24: 187–199. Crossref
Evans, S.E. 1992. Small reptiles and amphibians from the Forest Marble (Middle Jurassic) of Dorset. Proceedings of the Dorset Natural History and Archaeological Society 113: 201–202.
Evans, S.E. 1993. Jurassic lizard assemblages. Revue de Paléobiologie (Suisse) 7: 55–65.
Evans, S.E. 1998a. Crown group lizards from the Middle Jurassic. Palaeontographica A 250: 1–32. Crossref
Evans, S.E. 1998b. Lepidosaurian faunas from the Early Cretaceous: a clade in transition. In: S.G. Lucas, J.I. Kirkland, and J. Estep (eds.), Lower and Middle Cretaceous Continental Ecosystems, 195–200. New Mexico Museum of Natural History and Science, Albuquerque, NM.
Evans, S.E. 2003. At the feet of the dinosaurs: the early history and radiation of lizards. Biological Reviews 78: 513–551. Crossref
Evans, S.E. 2009. An early kuehneosaurid reptile from the Early Triassic of Poland. Palaeontologia Polonica 65: 145–178.
Evans, S.E. and Borsuk-Białynicka, M. 2009. The Early Triassic stemfrog Czatkobatrachus from Poland. Palaeontologica Polonica 65: 79–105.
Evans, S.E. and Hecht, M.K. 1993. A history of an extinct reptilian clade, the Choristodera: longevity, Lazarus-taxa, and the fossil record. In: M.K. Hecht, R.J. MacIntyre, and M.T. Clegg (eds.), Evolutionary Biology, 323–338. Springer US, Boston, MA. Crossref
Evans, S.E. and Jones, M.E. 2010. The origin, early history and diversification of lepidosauromorph reptiles. In: S. Bandyopadhyay (ed.), New Aspects of Mesozoic Biodiversity, 27–44. Springer, Berlin. Crossref
Evans, S.E. and Klembara, J. 2005. A choristoderan reptile (Reptilia: Diapsida) from the Lower Miocene of northwest Bohemia (Czech Republic). Journal of Vertebrate Paleontology 25: 171–184. Crossref
Evans, S.E. and Milner, A.R. 1994. Middle Jurassic microvertebrate assemblages from the British Isles. In: N.C. Fraser and S.E. Evans (eds.), In the Shadow of the Dinosaurs: Early Mesozoic Tetrapods, 303–321. Cambridge University Press, Cambridge.
Ezcurra, M.D. 2016. The phylogenetic relationships of basal archosauromorphs, with an emphasis on the systematics of proterosuchian archosauriforms. PeerJ 4: e1778. Crossref
Ezcurra, M.D., Bandyopadhyay, S., and Sen, K. 2021. A new faunistic component of the Lower Triassic Panchet Formation of India increases the continental non-archosauromorph neodiapsid record in the aftermath of the end-Permian mass extinction. Journal of Paleontology 96 (2): 428–438. Crossref
Foster, J.R. 2001. Taphonomy and paleoecology of a microvertebrate assemblage from the Morrison Formation (Upper Jurassic) of the Black Hills, Crook County, Wyoming. Brigham Young University Geology Studies 46: 13–34.
Foster, J.R. and McMullen, S.K. 2017. Paleobiogeographic distribution of Testudinata and neosuchian Crocodyliformes in the Morrison Formation (Upper Jurassic) of North America: Evidence of habitat zonation? Palaeogeography, Palaeoclimatology, Palaeoecology 468: 208–215. Crossref
Foster, J.R. and Trujillo, K.C. 2000. New occurrences of Cteniogenys (Reptilia, Choristodera) in the Late Jurassic of Wyoming and South Dakota. Brigham Young University Geology Studies 45: 11–18.
Foster, J., Pagnac, D., and Hunt-Foster, R. 2020. An unusually diverse northern biota from the Morrison Formation (Upper Jurassic), Black Hills, Wyoming. Geology of the Intermountain West 7: 29–67. Crossref
Foster, J.R., Trujillo, K.C., Madsen, S.K., and Martin, J.E. 2006. The Late Jurassic mammal Docodon, from the Morrison Formation of the Black Hills, Wyoming: implications for abundance and biogeography of the genus. New Mexico Museum of Natural History and Science Bulletin 36: 165–169.
Fox, R.C. 1968. Studies of Late Cretaceous Vertebrates I. The Braincase of Champsosaurus Cope (Reptilia: Eosuchia). Copeia 1968: 100–109. Crossref
Fox, R.C. and Naylor, B.G. 1982. A reconsideration of the relationships of the fossil amphibian Albanerpeton. Canadian Journal of Earth Sciences 19: 118–128. Crossref
Freeman, E.F. 1976. Mammal Teeth from the Forest Marble (Middle Jurassic) of Oxfordshire, England. Science 194: 1053–1055. Crossref
Freeman, E.F. 1979. A Middle Jurassic mammal bed from Oxfordshire. Palaeontology 22: 135–166.
Fürsich, F.T., Schneider, S., Werner, W., Lopez-Mir, B., and Pierce, C.S. 2022. Life at the continental-marine interface: palaeoenvironments and biota of the Alcobaça Formation (Late Jurassic, Central Portugal), with a formal definition of the unit appended. Palaeobiodiversity and Palaeoenvironments 102: 265–329. Crossref
Gao, K. and Brinkman, D.B. 2005. Choristoderes from the Park and Its Vicinity. In: P.J. Currie and E.B. Koppelhus (eds.), Dinosaur Provincial Park: A Spectacular Ancient Ecosystem Revealed, Vol. 1, 221–234. Indiana University Press, Bloomington, IN.
Gao, K. and Fox, R.C. 1998. New choristoderes (Reptilia: Diapsida) from the Upper Cretaceous and Palaeocene, Alberta and Saskatchewan, Canada, and phylogenetic relationships of Choristodera. Zoological Journal of the Linnean Society 124: 303–353. Crossref
Gardner, J.D. 2002. Monophyly and intra-generic relationships of Albanerpeton (Lissamphibia; Albanerpetontidae). Journal of Vertebrate Paleontology 22: 12–22. Crossref
Gardner, J.D. and DeMar, D.G. 2013. Mesozoic and Palaeocene lissamphibian assemblages of North America: a comprehensive review. Palaeobiodiversity and Palaeoenvironments 93: 459–515. Crossref
Gauthier, J.A., Kearney, M., Maisano, J.A., Rieppel, O., and Behlke, A.D. 2012. Assembling the squamate tree of life: perspectives from the phenotype and the fossil record. Bulletin of the Peabody Museum of Natural History 53: 3–308. Crossref
Gauthier, J.A., Kluge, A.G., and Rowe, T. 1988. Amniote phylogeny and the importance of fossils. Cladistics 4: 105–209. Crossref
Gheerbrant, E. and Rage, J.-C. 2006. Paleobiogeography of Africa: How distinct from Gondwana and Laurasia? Palaeogeography, Palaeoclimatology, Palaeoecology 241: 224–246. Crossref
Gilmore, C.W. 1928. Fossil lizards of North America. Memoirs of the National Academy of Sciences 22: 1–201.
Gloy, U. 2000. Taphonomy of the fossil lagerstatte Guimarota. In: T. Martin and B. Krebs (eds.), Guimarota: A Jurassic Ecosystem, 129–136. Verlag Dr. Friedrich Pfeil, Munich.
Goodwin, M.B., Clemens, W.A., Hutchison, J.H., Wood, C.B., Zavada, M.S., Kemp, A., Duffin, C.J., and Schaff, C.R. 1999. Mesozoic continental vertebrates with associated palynostratigraphic dates from the northwestern Ethiopian Plateau. Journal of Vertebrate Paleontology 19: 728–741. Crossref
Griffiths, E.F., Ford, D.P., Benson, R.B., and Evans, S.E. 2021. New information on the Jurassic lepidosauromorph Marmoretta oxoniensis. Papers in Palaeontology 7: 2255–2278. Crossref
Guillaume, A.R.D., Ezquerro, L., Moreno-Azanza, M., and Mateus, O. 2023a. Vertebrate microfossil assemblages from the Lourinhã Formation: a sneak peek on the paleoecology of the Late Jurassic in Portugal. In: R.K. Hunt-Foster, J.I. Kirkland, and M.A. Loewen (eds.), Special Issue: The 14th Symposium on Mesozoic Terrestrial Ecosystems and Biota. The Anatomical Record 306: 118–121. Crossref
Guillaume, A.R.D., Moreno-Azanza, M., and Mateus, O. 2023b. The Albanerpetontidae (Lissamphibia) from the Lourinhã Formation (Upper Jurassic, Portugal). In: R.K. Hunt-Foster, J.I. Kirkland, and M.A. Loewen (eds.), Special Issue: The 14th Symposium on Mesozoic Terrestrial Ecosystems and Biota. The Anatomical Record 306: 116–118. Crossref
Guillaume, A.R.D., Moreno-Azanza, M., Puértolas-Pascual, E., and Mateus, O. 2020. Palaeobiodiversity of crocodylomorphs from the Lourinhã Formation based on the tooth record: insights into the palaeoecology of the Late Jurassic of Portugal. Zoological Journal of the Linnean Society 189: 549–583.
Guillaume, A.R.D., Natário, C., Mateus, O., and Moreno-Azanza, M. 2022. Plasticity in the morphology of the fused frontals of Albanerpetontidae (Lissamphibia; Allocaudata). Historical Biology 35: 537–554. Crossref
Haddoumi, H., Allain, R., Meslouh, S., Metais, 8G., Monbaron, M., Pons, D., Rage, J.-C., Vullo, R., Zouhri, S., and Gheerbrant, E. 2016. Guelb el Ahmar (Bathonian, Anoual Syncline, eastern Morocco): first continental flora and fauna including mammals from the Middle Jurassic of Africa. Gondwana Research 29: 290–319. Crossref
Herrera-Flores, J.A., Elsler, A., Stubbs, T.L., and Benton, M.J. 2022. Slow and fast evolutionary rates in the history of lepidosaurs. Palaeontology 65 (1): e12579. Crossref
Jäger, K.R.K., Luo, Z.-X., and Martin, T. 2020. Postcranial Skeleton of Henkelotherium guimarotae (Cladotheria, Mammalia) and Locomotor Adaptation. Journal of Mammalian Evolution 27: 349–372. Crossref
Jiang, B., He, Y., Elsler, A., Wang, S., Keating, J.N., Song, J., Kearns, S.L., and Benton, M.J. 2023. Extended embryo retention and viviparity in the first amniotes. Nature Ecology & Evolution 7: 1131–1140. Crossref
Jones, M.E., Anderson, C.L., Hipsley, C.A., Müller, J., Evans, S.E., and Schoch, R.R. 2013. Integration of molecules and new fossils supports a Triassic origin for Lepidosauria (lizards, snakes, and tuatara). BMC Evolutionary Biology 13: art. 208. Crossref
King, G.M. 1996. Reptiles and Herbivory. 172 pp. Chapman & Hall, London.
Krebs, B. 2000. The excavations in the Guimarota mine. In: T. Martin and B. Krebs (eds.), Guimarota: A Jurassic Ecosystem, 9–20. Verlag Dr. Friedrich Pfeil, Munich.
Lasseron, M., Allain, R., Gheerbrant, E., Haddoumi, H., Jalil, N.-E., Métais, G., Rage, J.-C., Vullo, R., and Zouhri, S. 2020. New data on the microvertebrate fauna from the Upper Jurassic or lowest Cretaceous of Ksar Metlili (Anoual Syncline, eastern Morocco). Geological Magazine 157: 367–392. Crossref
Leinfelder, R.R. and Wilson, R.C. 1989. Seismic and sedimentologic features of Oxfordian–Kimmeridgian syn-rift sediments on the eastern margin of the Lusitanian Basin. Geologische Rundschau 78: 81–104. Crossref
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. 824 pp. Laurentius Salvius, Stockholm. Crossref
López-Rojas, V., Mateus, S., Marinheiro, J., Mateus, O., and Puértolas-Pascual, E. 2024. A new goniopholidid crocodylomorph from the Late Jurassic of Portugal. Palaeontologia Electronica 27 (1): 1–33. Crossref
Martin, J.E., Smith, T., Salaviale, C., Adrien, J., and Delfino, M. 2020. Virtual reconstruction of the skull of Bernissartia fagesii and current understanding of the neosuchian–eusuchian transition. Journal of Systematic Palaeontology 18: 1079–1101. Crossref
Martin, T. 2000. Overview over the Guimarota ecosystem. In: T. Martin and B. Krebs (eds.), Guimarota: A Jurassic Ecosystem, 143–146. Verlag Dr. Friedrich Pfeil, Munich.
Martin, T. and Krebs, B. 2000. Guimarota: A Jurassic Ecosystem. 155 pp. Verlag Dr. Friedrich Pfeil, Munich.
Martinius, A.W. and Gowland, S. 2011. Tide‐influenced fluvial bedforms and tidal bore deposits (late Jurassic Lourinhã Formation, Lusitanian Basin, Western Portugal). Sedimentology 58: 285–324. Crossref
Mateus, O. 2006. Late Jurassic Dinosaurs from the Morrison Formation (USA), the Lourinhã and Alcobaça Formations (Portugal), and the Tendaguru beds (Tanzania): A comparison. In: J.R. Foster and S.G. Lucas (eds.), Paleontology and Geology of the Upper Jurassic Morrison Formation. New Mexico Museum of Natural History and Science Bulletin 36: 223–231.
Mateus, O., Dinis, J., and Cunha, P. 2017. The Lourinhã Formation: the Upper Jurassic to lowermost Cretaceous of the Lusitanian Basin, Portugal—landscapes where dinosaurs walked. Ciências da Terra 19: 75–97. Crossref
Matsumoto, R. and Evans, S.E. 2010. Choristoderes and the freshwater assemblages of Laurasia/Coristoderos y las asociaciones de agua dulce de Laurasia. Journal of Iberian Geology 36: 253–274. Crossref
Matsumoto, R., Buffetaut, E., Escuillie, F., Hervet, S., and Evans, S.E. 2013. New material of the choristodere Lazarussuchus (Diapsida, Choristodera) from the Paleocene of France. Journal of Vertebrate Paleontology 33: 319–339. Crossref
Matsumoto, R., Dong, L., Wang, Y., and Evans, S.E. 2019. The first record of a nearly complete choristodere (Reptilia: Diapsida) from the Upper Jurassic of Hebei Province, People’s Republic of China. Journal of Systematic Palaeontology 17: 1031–1048. Crossref
Matsumoto, R., Fujiwara, S., and Evans, S.E. 2022a. Feeding behaviour and functional morphology of the neck in the long-snouted aquatic fossil reptile Champsosaurus (Reptilia: Diapsida) in comparison with the modern crocodilian Gavialis gangeticus. Journal of Anatomy 240: 893–913. Crossref
Matsumoto, R., Hirayama, R., Miyata, S., Yoshida, M., Mitsuzuka, S., Takisawa, T., and Evans, S.E. 2022b. The first choristoderan record from the Upper Cretaceous of Asia, Tamagawa Formation, Kuji Group, Japan. Cretaceous Research 129: 104999. Crossref
Matsumoto, R., Suzuki, S., Tsogtbaatar, K., and Evans, S.E. 2009. New material of the enigmatic reptile Khurendukhosaurus (Diapsida: Choristodera) from Mongolia. Naturwissenschaften 96: 233–242. Crossref
McKerrow, W.S., Johnson, R.T., and Jakobson, M.E. 1969. Palaeoecological studies in the Great Oolite at Kirtlington, Oxfordshire. Palaeontology 12: 56–83.
Moore, G.T., Hayashida, D.N., Ross, C.A., and Jacobson, S.R. 1992. Paleoclimate of the Kimmeridgian/Tithonian (Late Jurassic) world: I. Results using a general circulation model. Palaeogeography, Palaeoclimatology, Palaeoecology 93: 113–150. Crossref
Müller, J. 2004. The relationships among diapsid reptiles and the influence of taxon selection. In: G. Arratia, V.H. Wilson, and R. Cloutier (eds.), Recent Advances in the Origin and Early Radiation of Vertebrates, 379–408. Verlag Dr. Friedrich Pfeil, Munich.
Myers, T.S., Tabor, N.J., Jacobs, L.L., and Mateus, O. 2012. Palaeoclimate of the Late Jurassic of Portugal: comparison with the western United States. Sedimentology 59: 1695–1717. Crossref
Ortega, F. 2009. Faunas de répteis do Jurássico Superior de Portugal. Paleolusitana 1: 43–56.
Osborn, H.F. 1903. The reptilian subclasses Diapsida and Synapsida and the early history of the Diaptosauria. Memoirs of the American Museum of Natural History 1 (8): 449–507.
Palmer, T.J. 1979. The Hampen Marly and White Limestone formations: Florida-type carbonate lagoons in the Jurassic of central England. Palaeontology 22: 189–228.
Panciroli, E., Benson, R.B., Walsh, S., Butler, R.J., Castro, T.A., Jones, M.E., and Evans, S.E. 2020. Diverse vertebrate assemblage of the Kilmaluag Formation (Bathonian, Middle Jurassic) of Skye, Scotland. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 111 (3): 135–156. Crossref
Pritchard, A.C., Gauthier, J.A., Hanson, M., Bever, G.S., and Bhullar, B.-A.S. 2018. A tiny Triassic saurian from Connecticut and the early evolution of the diapsid feeding apparatus. Nature Communications 9: art. 1213. Crossref
Puértolas-Pascual, E. and Mateus, O. 2020. A three-dimensional skeleton of Goniopholididae from the Late Jurassic of Portugal: implications for the Crocodylomorpha bracing system. Zoological Journal of the Linnean Society 189: 521–548. Crossref
Ribeiro, A., Antunes, M.T., Ferreira, M.P., Rocha, R.B., Soares, A.F., Zbyszewski, G., De Almeida, F.M., De Carvalho, D., and Monteiro, J.H. 1979. Introduction à la géologie générale du Portugal. 114 pp. Serviços geológicos de Portugal, Lisboa.
Schoch, R.R. and Sues, H.-D. 2018. A new lepidosauromorph reptile from the Middle Triassic (Ladinian) of Germany and its phylogenetic relationships. Journal of Vertebrate Paleontology 38 (2): e1444619. Crossref
Schudack, M.E. 2000a. Geological setting and dating of the Guimarota beds. In: T. Martin and B. Krebs (eds.), Guimarota: A Jurassic Ecosystem, 21–26. Verlag Dr. Friedrich Pfeil, Munich.
Schudack, M.E. 2000b. Ostracodes and charophytes from the Guimarota beds. In: T. Martin and B. Krebs (eds.), Guimarota: A Jurassic Ecosystem, 33–36. Verlag Dr. Friedrich Pfeil, Munich.
Schudack, M.E., Turner, C.E., and Peterson, F. 1998. Biostratigraphy, paleoecology and biogeography of charophytes and ostracodes from the Upper Jurassic Morrison Formation, Western Interior, USA. Modern Geology 22: 379–414.
Schwarz, D. 2002. A new species of Goniopholis from the Upper Jurassic of Portugal. Paleontology 45: 185–208. Crossref
Schwarz, D. and Fechner, R. 2004. Lusitanisuchus, a new generic name for Lisboasaurus mitracostatus (Crocodylomorpha: Mesoeucrocodylia), with a description of new remains from the Upper Jurassic (Kimmeridgian) and Lower Cretaceous (Berriasian) of Portugal. Canadian Journal of Earth Sciences 41: 1259–1271. Crossref
Schwarz, D. and Fechner, R. 2008. The first dentary of Lisboasaurus (Crocodylomorpha, ?Mesoeucrocodylia) from the Lower Cretaceous (Barremian) of Uña, Cuenca Province, Spain. Journal of Vertebrate Paleontology 28: 264–268. Crossref
Schwarz, D. and Salisbury, S.W. 2005. A new species of Theriosuchus (Atoposauridae, Crocodylomorpha) from the Late Jurassic (Kimmeridgian) of Guimarota, Portugal. Geobios 38: 779–802. Crossref
Schwarz, D., Raddatz, M., and Wings, O. 2017. Knoetschkesuchus langenbergensis gen. nov. sp. nov., a new atoposaurid crocodyliform from the Upper Jurassic Langenberg Quarry (Lower Saxony, northwestern Germany), and its relationships to Theriosuchus. PLoS ONE 12 (2): e0160617. Crossref
Scotese, C.R. 2014. Atlas of Jurassic Paleogeographic Maps, PALEOMAP Atlas for ArcGIS, Volume 3, The Jurassic and Triassic, Maps 32–42, Mollweide Projection, PALEOMAP Project, Evanston, IL.
Seiffert, J. 1973. Contribução para o conhecimento da Fauna do Kimeridgiano da Mina de Lignito Guimarota (Leiria, Portugal) III Parte: V. Upper Jurassic lizards from Central Portugal. Memórias dos Serviços geológicos de Portugal (Nova Serie) 22: 7–85.
Sellwood, B.W. 1978. Jurassic. In: W.S. McKerrow (ed.), The Ecology of Fossils: An Illustrated Guide, 204–219. Duckworth, London.
Simões, T.R., Caldwell, M.W., Tałanda, M., Bernardi, M., Palci, A., Vernygora, O., Bernardini, F., Mancini, L., and Nydam, R.L. 2018. The origin of squamates revealed by a Middle Triassic lizard from the Italian Alps. Nature 557: 706–709. Crossref
Skutschas, P.P., Sennikov, A.G., Syromyatnikova, E.V., Vitenko, D.D., Parakhin, I.A., Bapinaev, R.A., and Evans, S.E. 2022. A lepidosauromorph specimen from the Middle Jurassic (Bathonian) Moskvoretskaya Formation of the Moscow Region, Russia. Historical Biology 34: 566–570. Crossref
Sobral, G., Simões, T.R., and Schoch, R.R. 2020. A tiny new Middle Triassic stem-lepidosauromorph from Germany: implications for the early evolution of lepidosauromorphs and the Vellberg fauna. Scientific Reports 10 (1): 2273. Crossref
Sweetman, S.C. 2016. A comparison of Barremian–early Aptian vertebrate assemblages from the Jehol Group, north-east China and the Wealden Group, southern Britain: the value of microvertebrate studies in adverse preservational settings. Palaeobiodiversity and Palaeoenvironments 96: 149–167. Crossref
Teixeira, C., Camarate França, J., Zbyszewski, G., Veiga Ferreira, O., and Manuppella, G. 1966. Folha 23-C – Leiria. 1ª Edição, 99 pp. Serviços Geológicos de Portugal, Lisboa.
Teixeira, C., Zbyszewski, G., Torre de Assunção, C., and Manuppella, G. 1968. Noticia explicativa da carta geológica de Portugal (esc. 1:50 000), folha 23-C – Leiria. 1ª Edição. 99 pp. Serviços Geológicos de Portugal, Lisboa.
Tennant, J.P. and Mannion, P.D. 2014. Revision of the Late Jurassic crocodyliform Alligatorellus, and evidence for allopatric speciation driving high diversity in western European atoposaurids. PeerJ 2: e599. Crossref
Tennant, J.P., Mannion, P.D., Upchurch, P., Sutton, M.D., and Price, G.D. 2017. Biotic and environmental dynamics through the Late Jurassic–Early Cretaceous transition: evidence for protracted faunal and ecological turnover. Biological Reviews 92: 776–814. Crossref
Trujillo, K.C., Carrano, M.T., and Chamberlain, K.R. 2015. A U-Pb zircon age for Reed’s Quarry 9, Upper Jurassic Morrison Formation, Albany County, WY. Proceedings of the 75th Annual Meeting of the Society of Vertebrate Paleontology. Programs and Abstracts, 226. Dallas, TX.
Turner, C.E. and Peterson, F. 2004. Reconstruction of the Upper Jurassic Morrison Formation extinct ecosystem—a synthesis. Sedimentary Geology 167: 309–355. Crossref
Vullo, R., Abit, D., Ballevre, M., Billon-Bruyat, J.P., Bourgeais, R., Buffetaut, E., Daviero-Gomez, V., Garcia, G., Gomez, B., Mazin, J.-M., Morel, S., Néraudeau, D., Pouech, J., Rage, J.-C., Schnyder, J., and Tong, H. 2014. Palaeontology of the Purbeck-type (Tithonian, Late Jurassic) bonebeds of Chassiron (Oléron Island, western France). Comptes Rendus Palevol 13: 421–441. Crossref
Waldman, M. and Evans, S.E. 1994. Lepidosauromorph reptiles from the Middle Jurassic of Skye. Zoological Journal of the Linnean Society 112: 135–150. Crossref
Wang, M., Xing, L., Niu, K., Liang, Q., and Evans, S.E. 2023. A new specimen of the Early Cretaceous long-necked choristodere Hyphalosaurus from Liaoning, China with exceptionally-preserved integument. Cretaceous Research 144: 105451. Crossref
Whiteside, D.I., Chambi-Trowell, S.A.V., and Benton, M.J. 2022. A Triassic crown squamate. Science Advances 8 (48): eabq8274. Crossref
Wiechmann, M.F. 2003. Albanerpetontidae (Lissamphibia) aus dem Mesozoikum der Iberischen Halbinsel und dem Neogen von Süddeutschland. 179 pp. Ph.D. Dissertation, Freie Universität Berlin, Berlin.
Acta Palaeontol. Pol. 70 (1): 77–96, 2025
https://doi.org/10.4202/app.01202.2024