

Taxonomy and paleobiogeography of some Late Cretaceous desmoceratine ammonoids from the northwest Pacific province
TOMOHIRO NISHIMURA and HARUYOSHI MAEDA
Nishimura, T. and Maeda, H. 2025. Taxonomy and paleobiogeography of some Late Cretaceous desmoceratine ammonoids from the northwest Pacific province. Acta Palaeontologica Polonica 70 (2): 259–283.
Taxonomy of the Late Cretaceous desmoceratine ammonoid genus “Damesites” and allied taxa is revised and updated based on newly collected population samples from the Yezo Group, Hokkaido, Japan, together with their type specimens. Previously known seven species and subspecies of “Damesites” from the upper Turonian to lower Campanian in the northwest Pacific province are reassigned to one revised species of Damesites and two species of Paradamesites gen. nov. based on clear differences in developmental patterns of ribs and growth lines. The genus Damesites should be strictly applied to the north Pacific Realm’s species like Damesites damesi. On the other hand, the long-established species “Ammonites sugata” (= “Damesites sugata”), ranging from the middle Turonian to Santonian in the African-Indian, European, northwest and northeast Pacific provinces, is re-assigned here to a new genus Paradamesites forming new combination Paradamesites sugata. Late Turonian “Damesites ainuanus” and Coniacian “Damesites sp.” from the Yezo Group are junior synonyms of P. sugata. Paradamesites rectus gen. et sp. nov., previously called “Damesites sugata” from the Santonian–Campanian strata of the Yezo Group is a descendant of P. sugata. The revised species of Damesites and species of Paradamesites gen. nov. co-occur sympatrically in the Late Cretaceous of north Pacific Realm. Both the revised Damesites and its ancestral genus Tragodesmoceroides originated in the north Pacific Realm, and most species during the Turonian–Campanian were endemic to the north Pacific Realm. Paradamesites, in contrast, originated in the African-Indian province and became widespread. Paradamesites sugata appears to have migrated from the African-Indian province to the north Pacific Realm during the latest Turonian–Coniacian and subsequently dispersed as a cosmopolitan species. Similar evolutionary patterns are also recognized in some other ammonoid families (e.g., Gaudryceratidae, Tetragonitidae, Desmoceratidae, and Kossmaticeratidae) during the Late Cretaceous of the northwest Pacific province.
Key words: Damesites, Paradamesites, migration, worldwide distribution, Yezo Group, Cretaceous.
Tomohiro Nishimura [tomohiro-nishimura@indigo.plala.or.jp; ORCID: https://orcid.org/0000-0003-4382-9807 ], Hobetsu Museum, 80-6, Hobetsu, Mukawa, Hokkaido, 054-0211, Japan. Hokkaido University Museum, Kita 10, Nishi 8, Kita-ku, Sapporo, Hokkaido, 060-0810 Japan.
Haruyoshi Maeda [maeda@museum.kyushu-ac.jp; ORCID: https://orcid.org/0009-0000-0571-2419 ], The Kyushu University Museum, 6-10-1, Hakozaki, Higashi-ku, Fukuoka, 812-8581 Japan.
Received 4 September 2024, accepted 13 February 2025, published online 28 May 2025.
Copyright © 2025 T. Nishimura and H. Maeda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Cretaceous desmoceratine ammonoids are widespread (Arkell et al. 1957; Wright et al. 1996) and particularly abundant in the Cenomanian–Campanian successions of the northwest Pacific province including the locality of the Yezo Group in Hokkaido, Japan, and Sakhalin, Russia (Fig. 1). Desmoceras (Pseudouhligella) japonicum (Yabe, 1904), for example, often constitutes up to 90% of the total number of individuals in Cenomanian ammonoid assemblages in offshore muddy facies (Maeda 1991). Among desmoceratines, the keeled genus “Damesites” (Figs. 2–11) erected by Matsumoto (1942a) has been used as a key for biostratigraphic correlation of the Upper Cretaceous Series in the northwest Pacific province (e.g., Matsumoto 1954, 1959b; Toshimitsu et al. 1995; Yazykova 2004). Until now, five species and one subspecies were described from the Yezo Group in Hokkaido and Sakhalin (Matsumoto 1954, 1957; Matsumoto and Obata 1955; Saito and Matsumoto 1956).

Fig. 1. Maps of southern Sakhalin and Hokkaido. A. Map of southern Sakhalin and Hokkaido. B. Geological map showing Yezo Group deposits and location of the study area.
On the other hand, “Damesites sugata (Forbes, 1846)”, which was first described from South India as “Ammonites sugata” (Figs. 2D, 5C), shows a global distribution, e.g., from Madagascar, Angola, Spain, Hokkaido-Sakhalin (Yezo Group), and British Columbia (Forbes 1846; Stoliczka 1865; Kossmat 1895–1898; Matsumoto 1942a; Matsumoto and Obata 1955; Collignon 1961, 1983; Haggart 1989; Kennedy and Henderson 1991; Ludvigsen and Berard 1997; Cooper 2003b). “Damesites ainuanus Matsumoto, 1957” (Fig. 3) also shows worldwide distribution extending from Hokkaido-Sakhalin (Matsumoto 1957; Jagt-Yazykova 2011) to Angola (Howarth 1968; Cooper 2003a).
After the major taxonomic studies of the 1950s (Matsumoto 1954; Matsumoto and Obata 1955), large numbers of “Damesites” specimens have been reported and listed by various geological and paleontological studies (e.g., Tanabe et al. 1977; Wani and Hirano 2000; Okamoto et al. 2003; Tsujino 2009). However, many individuals do not completely match previous generic and/or specific diagnoses so that recent papers have been obliged to list them vaguely as “Damesites sp.” (e.g., Sekine et al. 1985; Okamoto et al. 2003). Besides the Yezo Group, “Damesites spp.” were also described from other regions worldwide (e.g., Haggart 1989; Szász 1981) but their identifications suffer from similar taxonomic issues. This ambiguity is mostly related to a wide intraspecific variation in their shell morphology including diagnostic features (Nishimura et al. 2010).
To resolve the taxonomic problems in “Damesites”, we analysed material from the Yezo Group since it provides rich material both in quantity and quality, i.e., the abundant occurrence of a total six species and one subspecies from the continuous successions and the good preservation of the shells allowing detailed observation of ontogenetic shell-growth from the embryo to the terminal shell stage. Nishimura et al. (2010) re-evaluated the morphological features that had been regarded as taxonomic criteria for classification in the “Damesites” group. They stressed that the “Damesites” group was better diagnosed taxonomically by certain patterns of ontogenetic change, e.g., the modes of rib-coarsening during growth, rather than any single typological feature.
Adopting the results of Nishimura et al. (2010), we have now revised the previous “Damesites” and allied taxa from the Yezo Group on the basis of newly collected population samples together with their type specimens.
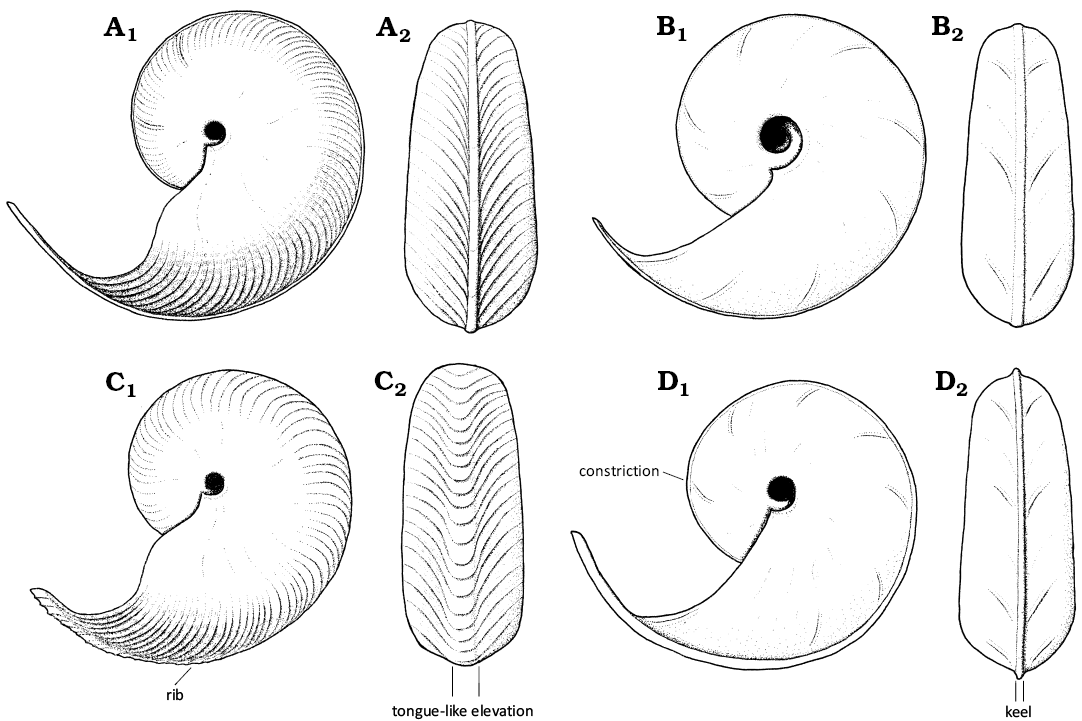
Fig. 2. Schematic illustration of “Damesites” and Tragodesmoceroides shells. A. Damesites damesi. B. Paradamesites rectus sp. nov. C. Tragodesmoceroides subcostatus. D. Paradamesites sugata. In left lateral (A1–D1) and ventral (A2–D2) views.
Institutional abbreviations.—CAS, California Academy of Science, San Francisco, USA; GK. H, Kyushu University Museum, Fukuoka, Japan; HMG, Hobetsu Museum, Mukawa, Hokkaido, Japan; KUM MM TN, Kyoto University Museum, Japan; MCM, Mikasa City Museum, Hokkaido, Japan; NHM (formerly BMNH), Natural History Museum, London, UK; NMNS (formerly NSM), National Museum of Nature and Science, Tsukuba, Japan; UMUT MM (formerly GT I-), University Museum, University of Tokyo, Japan.
Other abbreviations.—B, whorl breadth; D, diameter; FAD, first appearance datum; H, whorl height; LAD, last appearance datum; U, width of umbilicus; w, whorl expansion ratio; (Rn/Rn-1)2, where Rn is radius from the center of initial chamber at nπ stage (Raup 1966).
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:C40A461E-092F-4D80-B2A4-757D890BB558.
Material and methods
For the sake of convenience, previous nominal taxonomic names are regarded as morphotypes, using inverted double commas, e.g., “Damesites”, differentiating them in that way from the updated taxa after the revision.
A few type specimens of “Damesites” species originate from float concretions whose exact stratigraphic levels remain uncertain in previous studies. Through the recent field survey, the stratigraphic distribution of “Damesites” and allied taxa has been re-examined in detail by TN in the well-exposed Turonian to lower Campanian strata in the Haboro-Tappu area, northwest Hokkaido, Japan (Nishimura et al. 2010: fig. 2; Fig. 1B, SOM: fig. S1 in Supplementary Online Material available at http://app.pan.pl/SOM/app70-Nishimura_Maeda_SOM.pdf). In the course of the field survey, 244 population samples (i.e., in situ calcareous concretions) were collected from various horizons (Figs. 12–14, SOM: figs. S2, S3). The stratigraphic scheme in the Haboro-Tappu area follows Tanaka (1963). The biostratigraphic scheme is basically followed by Toshimitsu et al. (1995) combined with the Campanian subdivision by Shigeta et al. (2016, 2019). Mid- to Late Cretaceous paleobiogeographic provinces and realms follow Jagt-Yazykova (2011).
Type specimens of (“Damesites damesi damesi Matsumoto, 1942a”, “D. damesi intermedius Matsumoto, 1954”, “D. semicostatus Matsumoto, 1942a”, “D. laticarinatus Saito and Matsumoto, 1956”, and “D. ainuanus Matsumoto, 1957” from the Yezo Group, and previously studied specimens (e.g., Jimbo 1894; Matsumoto 1942a, 1954; Matsumoto and Obata 1955) were examined in UMUT and GK. “Damesites” specimens investigated by Ikeda and Wani (2012) from the Kotanbetsu area, Hokkaido were examined at the MCM. The plastolectotype, plastoparatype, and topotype of the long-established Indian species “Damesites sugata” (= “Ammonites sugata”, Paradamesites sugata, see below) in the collections of the NHM and the other Indian specimens, described in Stoliczka (1865) and Kossmat (1895–1898) housed in the GSI, were observed. In addition, types of two northeast Pacific province’s species of “Damesites”, i.e., Kotoceras richardsoni Anderson, 1958, and K. frazierenze Anderson, 1958, both which were subsequently treated as synonyms of “Damesites damesi intermedius” by Matsumoto (1959a), housed at CAS were also re-examined.
Results
Summary of stratigraphic occurrence of Damesites and Paradamesites species in the northwest (the Yezo Group) and northeast Pacific provinces.—Based on a detailed field survey by TN, stratigraphic occurrences of “Damesites” species in the Yezo Group of the northwest Pacific province are established below (Fig. 14). Stratigraphic horizons of each morphotype including type species also shown in Fig. 14. “Damesites ainuanus” occurs in the uppermost Turonian (Matsumoto 1954; Futakami et al. 1980). “Damesites sp.”, characterized by a siphonal keel (Nishimura et al. 2010), occurs from the upper Coniacian (Ud–e unit in Haboro-Tappu area). The type specimen of “Damesites damesi intermedius” occurs from the middle Campanian (GK. H 3269, holotype from the Ur2β’ unit of Matsumoto 1942b, which was previously correlated to the Sphenoceramus orientalis Zone that is Santonian in Matsumoto 1942b). The stratigraphic range of this “subspecies” was said to be Coniacian to Santonian (Matsumoto and Obata 1955), however, its last appearance datum (LAD) of the Sphenoceramus orientalis Zone is treated as the middle Campanian in recent studies (e.g., Shigeta et al. 2019). A similar morphotype (Fig. 7B, KUM MM TN 145) is also found from the lowest Santonian (Ud–e unit). This morphotype does not continuously occur from the lower Santonian to middle Campanian and shows discontinuous and sporadic occurrence from the two horizons: lower Santonian and middle Campanian. “Damesites damesi damesi” occurs from the lower Santonian to the lower Campanian sequence (Uf to Ui–j units) in the Haboro, Kotanbetsu and Tappu areas, and in the middle Campanian (Sphenoceramus schmidti Zone) in the Nakagawa area, while “Damesites semicostatus” is known from the lower Santonian (Uf unit). “Damesites sugata” from Yezo Group, which appears to be different from the Indian species and be assigned to Paradamesites rectus gen. et sp. nov. herein, occurs from the Santonian to the lower Campanian (Uf to Ui–j units). “Damesites damesi” and “Damesites sugata” from the Yezo group (= P. rectus gen. et sp. nov. herein) share similar stratigraphic ranges, though the former is more abundant.
Based on the updated stratigraphic data and revised systematics (Nishimura et al. 2010 and Systematic Paleontology herein), the following biostratigraphic conclusions can be drawn regarding the Cretaceous Yezo Group: Paradamesites sugata (Forbes, 1846) ranges from the uppermost Turonian to the Coniacian, while P. rectus gen. et sp. nov. and Damesites damesi (Jimbo, 1894) range from the Santonian to the middle Campanian (Fig. 14). Details of localities and provenance of the newly collected specimens were described in SOM: figs. S1–S4.
The northeast Pacific province (e.g., British Columbia and California) would have been inhabited by the same desmoceratine species found in the northwest Pacific province. In British Columbia, Canada, D. damesi range from the Santonian–lower Campanian (Haggart 1989; described in detail in systematic chapter). In California, USA, three “species” of desmoceratine species were described from the lower part of the “Senonian (= Coniacian to Maastrichtian)” by Anderson (1958) under “Kotôceras richardsoni sp. nov.”, “K. frazierense sp. nov.”, and “K. subsugatum sp. nov.” Re-examination of type specimens of nominal species suggests that “K. richardsoni” is identical to P. sugata, while “K. frazierense” is identical to D. damesi. “Kotôceras subsugatum” resembles P. sugata though the holotype of the former species was lost in the San Francisco fire of 1906 (see the Systematic Palaeontology section herein) and could not been re-examined.
Revised Paradamesites sugata (Forbes, 1846) in northwest Pacific province and South India (type region).—The name of “Damesites sugata” (formerly “Ammonites sugata”) has been misleadingly used in the northwest Pacific province’s populations (see Systematic palaeontology for details). Matsumoto and Obata (1955: 128–133) recognized a Santonian–lower Campanian keeled desmoceratine specimen having a compressed whorl with parallel flanks, a wider umbilicus, and a low keel. They assigned it to the previously known Indian species “Ammonites sugata.” Simultaneously, the species was assigned to the keeled genus Damesites (Matsumoto and Obata 1955: 128). However, there is an inconsistency in its stratigraphic range because the Indian population including the types appeared earlier. Our taxonomic reexamination of large population samples demonstrated that the true Paradamesites sugata includes “D. ainuanus” and “Damesites sp.” from the upper Turonian–Coniacian of the Yezo Group, and “D. sugata” from the Southern Hemisphere and Europe. “Damesites sugata” from the Santonian–lower Campanian of the Yezo Group (Matsumoto and Obata 1955) should be removed from the true P. sugata and assigned to its descendant species: P. rectus gen. et sp. nov.
Paradamesites sugata is a key taxon for understanding the desmoceratine evolution during the Turonian–middle Campanian, because of its worldwide distribution. Tragodemoceroides subcostatus Matsumoto, 1942a, native to northwest Pacific province is stratigraphically succeeded by P. sugata in the upper Turonian of the province (Fig. 14). Both species continuously occur without stratigraphic overlap, while there is no direct phylogenetic relationship between the two species.
Two parallel groups of Turonian–Campanian desmoceratines.—“Damesites spp.” and allied taxa are classified into two contemporaneous parallel lineages, namely the S-group and C-group, on the basis of the development of growth lines on the shell surface whorl expansion ratio (w), and shape of growth line (Nishimura et al. 2010). The difference in w of both groups was also supported by statistical analysis of the w (Nishimura et al. 2010: 52).
The S (sigmoid)-group shows flexuous, sigmoid (adorally biconcave) growth lines (Figs. 2A, 7–9, 14) and a greater w (Nishimura et al. 2010: fig. 17E, F). Damesites damesi (Jimbo, 1894) (= “D. damesi damesi [Jimbo, 1894]”, “D. damesi intermedius Matsumoto, 1954”, “D. semicostatus Matsumoto, 1942a”, and “D. laticarinatus Saito & Matsumoto, 1956”) belong to the S-group. Turonian Tragodesmoceroides also possesses these characters and belongs to this group.
The C (concave)-group is excluded from the S-group by having adorally concave simple growth lines (Figs. 2B, D, 3–6, 14) and a much lower w (Nishimura et al. 2010: fig. 17E, F). This group includes cosmopolitan Paradamesites sugata (Forbes, 1846) (Figs. 3–5) (including Indian “D. sugata”, “Damesites ainuanus, Matsumoto, 1957”, and Coniacian “Damesites sp.”) and Santonian–early Campanian P. rectus gen. et sp. nov. (Figs. 6, 13). The individuals of the latter species known from the Yezo Group had previously been erroneously assigned to “D. sugata” (e.g., Matsumoto and Obata 1955: 128).
As described in detail in the discussion, this subdivision is concordant with their paleobiogeographical distribution. The S-group is native to north Pacific Realm, and the C-group distributes mainly in the African-Indian province and shows worldwide distribution.
Systematic paleontology
Stages of desmoceratine shell growth are divided into the early (D < 30 mm), middle (D range 30–60 mm), and late stages (D > 60 mm) (Nishimura et al. 2010). Morphological terms follow Nishimura et al. (2010). Whorl volution is expressed by π scale (= every 180 degrees in rotation angle) from the initial chamber (Maeda 1993).
Order Ammonoidea Zittel, 1884
Suborder Ammonitina Hyatt, 1889
Superfamily Desmoceratoidea Zittel, 1895
Subfamily Desmoceratinae Zittel, 1895
Genus Damesites Matsumoto, 1942a
Type species: Desmoceras damesi Jimbo, 1894, by original designation of Matsumoto (1942a). Tappu area, Hokkaido, Japan, probably Santonian, a float.
Species included: Damesites damesi (Jimbo, 1894) and Damesites hetonaiensis Matsumoto, 1954.
Emended diagnosis.—Desmoceratine having sigmoidal growth lines, crater-like narrow umbilicus, very sharp or moderate to obtuse ventral keel in the adult stage. Single concave or weakly sigmoidal constrictions develop particularly in the early to middle growth stages.
Remarks.—Damesites Matsumoto, 1942a: 24 (1938: 193, nom. nud.), ICZN Opinion 555, 1959, Generic Name No. 1349.
Damesites damesi (Jimbo, 1894)
Figs. 2A, 7–9.
1890 Desmoceras sugata Forbes, 1846; Yokoyama 1890: 185, pl. 20: 11.
1894 Desmoceras Damesi sp. nov.; Jimbo 1894: 172, pl. 1: 2, 3.
1932 Desmoceras (Kotôceras) semicostatus Yabe M.S.; Nagao 1932: 178.
1942 Damesites damesi (Jimbo, 1894); Matsumoto 1942a: 27, text-fig. 1e.
1942 Damesites semicostatus (Yabe M.S.); Matsumoto 1942a: 27, text-fig. 1h.
1954 Damesites damesi (Jimbo, 1894); Matsumoto 1954: 267, pl. 5: 1–3.
1954 Damesites damesi intermedia subsp. nov.; Matsumoto 1954: 270, pl. 6: 4.
1955 Damesites semicostatus (Yabe M.S.) Matsumoto, 1942a; Matsumoto and Obata 1955: 126, pl. 25: 1–5; pl. 26: 1–3; pl. 30: 6.
1955 Damesites damesi intermedius Matsumoto, 1955; Matsumoto and Obata 1955: 131, pl. 27: 1a–b.
1956 Damesites laticarinatus sp. nov.; Saito and Matsumoto 1956: 192–193, text-fig. 1a–c.
1958 Kotôceras frazierense sp. nov.; Anderson 1958: 217, pl. 40: 5, 5a.
1960 Damesites damesi (Jimbo, 1894); Obata 1960: fig. 4b, pl. 15: 4.
1963 Damesites semicostatus (Yabe M.S.) Matsumoto, 1942a; Tanaka 1963: pl. 1: 3a–b.
1978 Damesites semicostatus Matsumoto, 1942a; Obata et al. 1978: fig. 3, pl. 2: 1a–c
1987 Damesites semicostatus Matsumoto, 1942a; Tanabe and Shigeta 1987: figs. 2, 5A, B.
1989 Damesites sugata (Forbes, 1846); Haggart 1989: 195–196, text-figs. 14–23, pl. 8.4.
1993 Damesites sugata (Forbes, 1846); Alabushev and Wiedmann 1993: fig. 1A–E.
?1996 Damesites damesi (Jimbo, 1894); Cooper and Greying 1996: fig. 7I–L.
1996 Damesites semicostatus Matsumoto, 1942a; Wright et al. 1996: fig. 64: 2c–e.
2000 Damesites damesi intermedius Matsumoto, 1954; Seki et al. 2000: pl. 1: 4.
2000 Damesites semicostatus Matsumoto, 1942a; Seki et al. 2000: pl. 1: 5–6.
2000 Damesites sugata (Forbes, 1846); Seki et al. 2000: pl. 1: 7–8.
2002 Damesites damesi (Jimbo, 1894); Henderson et al. 2002: fig. 3A–C.
2003 Damesites damesi (Jimbo, 1894); Nishimura 2003: fig. 2C–F.
2003 Damesites semicostatus Matsumoto, 1942a; Nishimura 2003: fig. 2A, B.
?2005 Damesites cf. sugata (Forbes, 1846); Maeda et al. 2005:102, 104, fig. 53: 1–6.
2009 Damesites sugata (Forbes, 1846); Tsujino 2009: fig. 6A.
2010 “Damesites damesi (Jimbo, 1894)”; Nishimura et al. 2010: figs. 4A–C, 8A–D, 9F, 11B, D, 12D, 13D–F, 15B, D, 16A–B.
2010 “Damesites damesi intermedius Matsumoto, 1955”; Nishimura et al. 2010: figs. 1D, E, 16C, E.
2010 “Damesites semicostatus Matsumoto, 1942a”; Nishimura et al. 2010: figs. 4F–H, 8E–F, 9E, 12A, 13A–C.
2010 “Damesites laticarinatus Saito and Matsumoto, 1956”; Nishimura et al. 2010: figs. 6E–G, 16D.
2011 Damesites damesi (Jimbo, 1894); Honda et al. 2011: fig. 9B, C.
?2019 Damesites sp.; Shigeta 2019 in Shigeta et al. 2019: fig. 27A–AN.
2023 Damesites aff. sugata (Forbes, 1846); Tanabe and Misaki 2023: fig. 7.
2025 Damesites damesi (Jimbo, 1894), Nishimura 2025: fig. 20B–D.
Lectotype: UMUT MM 7500 (GT. I-91), originally described a middle-sized specimen by Jimbo (1894) without designation of the type. Referred to as the holotype by Matsumoto (1954: 267) but should be treated as the lectotype.
Type locality: Float in Obirashibe-gawa River in Tappu area, Hokkaido, Japan.
Type horizon: Most likely Santonian.
Material.—In addition to the lectotype, more than 100 specimens in GK, UMUT from various Santonian–Campanian localities in Hokkaido and Sakhalin, as well as 134 specimens (KUM MM TN 145–279) from the Haboro-Tappu and Nakagawa areas collected by us (Table 1).
Table 1. List of the studied material. All material from Japan, except otherwise indicated.
|
Damesites damesi |
|
|
lower to middle Campanian, Yezo Group |
KUM MM TN 264–279; UMUT MM 6761, 6739 previously listed to “Damesites damesi intermedius”: GK. H 3269 previously included to Desmophyllites diphylloides: UMUT MM 5552, 5553, 5557, 5575, 5578 |
|
upper Santonian, Yezo Group |
KUM MM TN 218–263; UMUT MM 6737, 6738, 6740, 6741, 6744, 7689, 7890 previously listed to “Damesites sugata”: UMUT MM 5598; GK. H 3272, 3273, 3319 previously listed to “Damesites
laticarinatus”: GK. H 20026
(holotype) |
|
lower Santonian, Yezo Group |
KUM MM TN 145–217; UMUT MM 7500
(lectotype); UMUT MM 6735, 6743, 6745, 7504, 7630; |
|
Paradamesites sugata |
|
|
South India, possibly Coniacian |
BMNH C22674 (lectotype); BMNH C 22675 (paralectotype); BMNH C 24196a, b (paralectotypes); KUM MM TN 292 (topotype) |
|
upper Coniacian, Yezo Group |
KUM MM TN 109–144 |
|
lower Coniacian, Yezo Group |
KUM MM TN 101–108 previously listed to “Damesites damesi”: UMUT MM 6764 , 6748–3753, 6756, 6757 |
|
uppermost Turonian, |
KUM MM TN 099, 100 |
|
Paradamesites rectus sp. nov. |
|
|
Santonian to lower Campanian, Yezo Group |
KUM MM TN 281(holotype); KUM MM TN
280, 282–291 (paratypes) |
Emended diagnosis.—Sigmoidal ribs vary from very weak to coarse, the w becoming large in the middle to late growth stages. Moderate to obtuse keel developing on adult venter. Faint furrows sometimes may appear on both sides of the keel. The w increasing with growth. U/D ratio decreasing with growth. Suture line complex, bifid external lobe and trifid lateral lobe and umbilical lobes.
Measurements.—See Table 2.
Table 2. Dimensions (in mm) of Damesites damesi and two species of Paradamesites gen. nov. Abbreviations: B, whorl breadth; D, shell diameter; H, whorl height; U, width of umbilicus; w, whorl expansion rate.
| |
|
D |
U |
B |
H |
U/D |
B/H |
w |
|
Damesites damesi |
UMUT MM 7500 (lectotype) |
63.68 |
5.38 |
25.56 |
35.96 |
0.0845 |
0.71 |
2.59 |
|
KUM MM TN 159 |
49.59 |
3.59 |
22.11 |
28.21 |
0.0724 |
0.78 |
2.52 |
|
|
KUM MM TN 168 |
25.17 |
1.98 |
12.51 |
13.76 |
0.0786 |
0.91 |
2.11 |
|
|
KUM MM TN 244 |
30.00 |
2.89 |
13.46 |
16.57 |
0.0962 |
0.81 |
2.46 |
|
|
UMUT MM 5579 (holotype of “D. semicostatus”) |
40.27 |
3.40 |
19.08 |
22.74 |
0.0843 |
0.84 |
2.59 |
|
|
GK. H 3269 (holotype of “D. damesi intermedius”) |
48.63 |
4.12 |
22.68 |
27.43 |
0.0847 |
0.83 |
2.58 |
|
|
KUM MM TN 168 |
47.72 |
3.84 |
22.81 |
26.89 |
0.0805 |
0.85 |
2.51 |
|
|
Paradamesites sugata |
BMNH C22674 (lectotype) |
37.40 |
4.09 |
14.67 |
19.21 |
0.1088 |
0.76 |
2.09 |
|
GK. H 4198 (holotype of “D. ainuanus”) |
79.17 |
9.16 |
27.47 |
41.31 |
0.1157 |
0.66 |
2.07 |
|
|
KUM MM TN 129 |
71.02 |
6.87 |
28.31 |
37.84 |
0.0967 |
0.75 |
2.07 |
|
|
KUM MM TN 109 |
39.34 |
3.19 |
16.42 |
19.34 |
0.0810 |
0.83 |
1.39 |
|
|
Paradamesites rectus sp. nov. |
KUM MM TN 281 (holotype) |
59.28 |
9.11 |
29.22 |
22.81 |
0.1536 |
0.78 |
1.94 |
|
KUM MM TN 285 (paratype) |
65.15 |
10.83 |
23.40 |
32.96 |
0.1660 |
0.71 |
2.38 |
|
|
KUM MM TN 287 (paratype) |
21.28 |
2.58 |
8.40 |
10.99 |
0.1210 |
0.76 |
2.04 |
Description.—In early growth stage (e.g., D 1 mm), whorl is depressed (B/H = 2.0) (Fig. 10A, B, F), and the whorl becomes compressed with shell growth. In middle to late growth stage, whorl is moderately compressed (B/H is about 0.75 at D 50 mm) (Fig. 10A, B; see Nishimura et al. 2010: fig. 17B, D). Umbilical shoulder appearing in 3–4π stage (about D 1.5 mm) and a sharp umbilical break appears in 5–6π stage (about 2.5 mm in D) (Fig. 10F). U/D ratio decreasing with growth (Fig. 10D; see Nishimura et al. 2010: fig. 17C), changing from 0.3 (at 1 mm in D) to 0.08 (at 40 mm in D) (Fig. 10D). Moderate to obtuse keel appearing at 10 to 40 mm in D (Figs. 8D2, 9D2). Faint furrows sometimes appear on both sides of the keel particularly in individual with both shoulders inflated (Figs. 7A2, 8A1; lectotype figured in Nishimura et al. 2010: fig. 4C). The w increasing with growth, starting at about 2.0 in the early growth stage (about 20 mm in D), and increasing to 2.5 in the middle to late growth stage (about 50 mm in D) (Fig. 10E; see Nishimura et al. 2010: fig. 17F).
The curvature of growth line changing from single concave to sigmoidal at 15 mm to 50 mm in D (Figs. 8C2, 9B1; see Nishimura et al. 2010: fig. 13B, E). The single concave growth line runs nearly straight on both flanks and becomes gently projected forwards in the ventral periphery (Figs. 8D1, E1, 9C2, D1). The succeeding sigmoidal growth line is slightly biconvex in lateral view, weakly or strongly flexuous on both flanks and strongly projected forward at the venter (Figs. 7A1, B1, 8A2, B, C2, F2, 9A1, B1; see Nishimura et al. 2010: figs. 4B, 13C, F). A rostrum appears at mid-venter in the middle to adult stage (Fig. 9B2).
Frequent desmoceratid constrictions occur as sharp grooves on internal mould in the early to middle growth stages (< 30 mm in D), while these are almost indiscernible on the shell surface, while exception of the ventrolateral shoulder (Figs. 8D1, E1, 9C2, D1, E1). Constrictions retain a single concave or weakly sigmoidal curvature throughout growth (Fig. 9A1). Unlike ancestral Desmoceras (Pseudouhligella) spp., the curvature of constrictions does not completely match that of growth lines, particularly in the middle to late growth stages. The growth lines obliquely converge to the constrictions in the present species (Nishimura et al. 2010: fig. 11B, D).
The curvature pattern of constriction is single concave or weakly sigmoidal; in contrast, the growth line is strongly sigmoidal in the middle to late growth stages. On the ventrolateral shoulder, patterns of constriction and growth line become similar (Nishimura et al. 2010: fig. 11B, D).
Longitudinal striation crossing ribs gradually appears on the shell surface in the middle to late growth stages (Figs. 8A2, B, C2, 9C2, D1). It is easily discernible in individuals with widely interspaced ribs but is indiscernible in specimens with narrowly interspaced ribs (Nishimura et al. 2010: fig. 12).
Phase 1 in surface of shell ornament is characterized by almost smooth with very fine growth lines developing in the early to middle growth stages, at about 2–30 mm H (5–50 mm in D) (Figs. 7B3, 8D1, E1, 9A1, B1, C2, D1, E1). The concave or slightly sigmoidal fine growth line is weakly convex on both flanks and moderately projected on the venter (Figs. 8D1, E1, 9A1, C2, D1). These appear very weak in relief on the shell surface, while the internal mold is smooth (Fig. 9E1). The growth line is very faint, particularly on the inner flanks (Figs. 8D1, E1, 9A1, C2, D1).
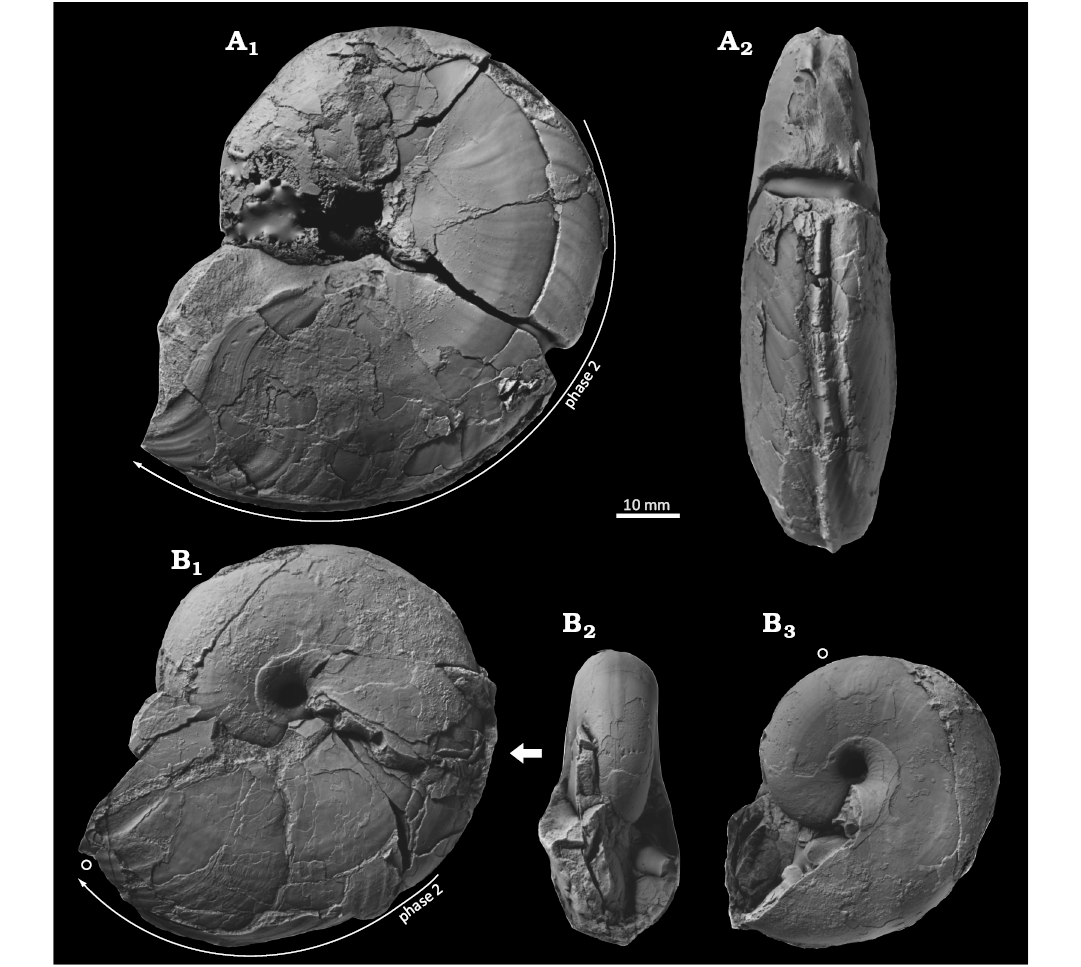
Fig. 7. Desmoceratine ammonoid Damesites damesi (Jimbo, 1894) from the lowest Santonian (Unit Ud–e) of the Yezo Group, Hokkaido, Japan. A. KUM MM TN 146 (deformed and compressed laterally), loc. OT1601, in left lateral (A1) and ventral (A2) views. B. KUM MM TN 145, loc. TK4111, in left lateral (B1, B3) and apertural (B2) views. An open circle marks the position of constrictions. The bold arrow show the ontogenetic change of a single specimen.
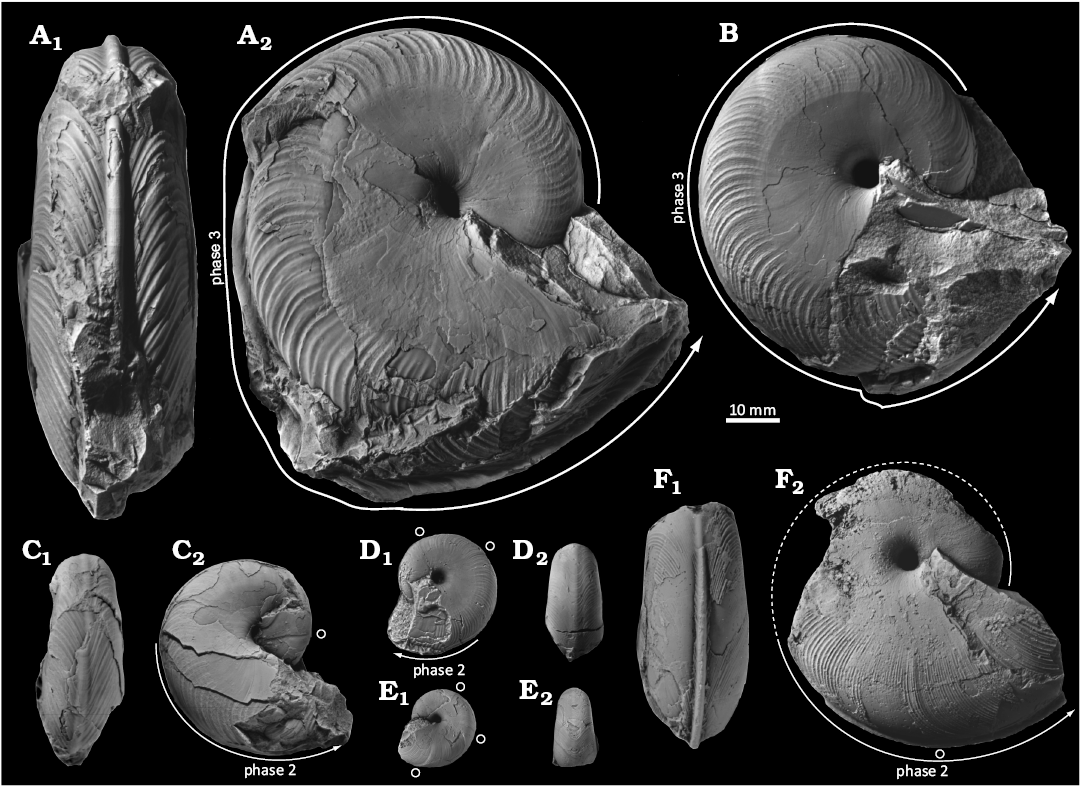
Fig. 8. Desmoceratine ammonoid Damesites damesi (Jimbo, 1894) from the lower Santonian (Unit Uf) of the Yezo Group, Hokkaido, Japan. A. KUM MM TN 158, loc. OT7027, in ventral (A1) and right lateral (A2) views. B. KUM MM TN 193, loc. OT1429 (see Nishimura 2003: fig. 2D–F), in right lateral view. C. KUM MM TN 166, loc. OT7027, in ventral (C1) and right lateral (C2) views. D. KUM MM TN 189, loc. OT7028, in left lateral (D1) and ventral (D2) views. E. KUM MM TN 183, loc. OT7028, in left lateral (E1) and ventral (E2) views. F. KUM MM TN 200, loc. OT1599, in ventral (F1) and right lateral (F2) views. An open circle marks the position of constrictions.
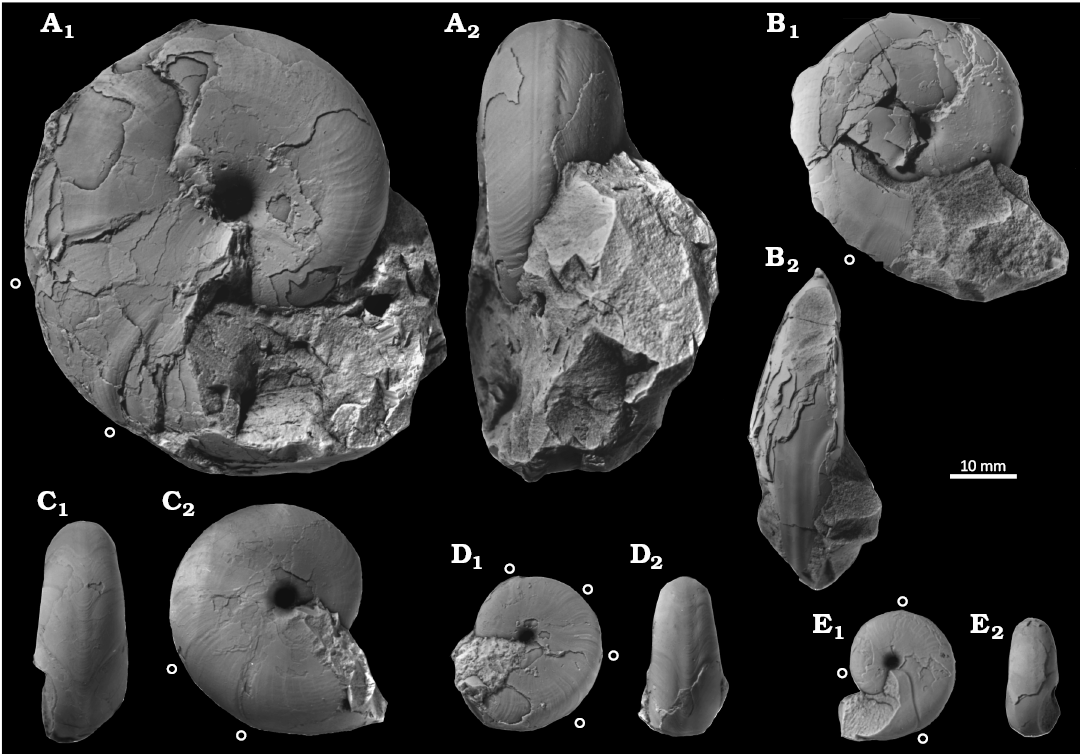
Fig. 9. Desmoceratine ammonoid Damesites damesi (Jimbo, 1894) from the upper Santonian (Unit Ug–h) (A, D) and middle Campanian, Osoushinai Formation, Nakagawa area (B, C, E) of the Yezo Group, Hokkaido, Japan. A. KUM MM TN 218, loc. TK1043 (Unit Ug), in right lateral (A1) and apertural (A2) views. B. KUM MM TN 264, in right lateral (B1) and ventral (B2) views. C. KUM MM TN 265, loc. NS1009 of Matsuda and Ubukata (1999), Sphenoceramus orientalis Zone, in ventral (C1) and right lateral (C2) views. D. KUM MM TN 239, loc. TK1019 (Unit Ug), in left lateral (D1) and ventral (D2) views. E. KUM MM TN 272, a float near loc. NS1007 of Matsuda and Ubukata (1999), in left lateral (E1) and ventral (E2) views. An open circle marks the position of constriction.
Phase 2 is characterized by weak ribbing on the shell surface. This phase follows phase 1 in the middle to late growth stages, although, the timing of change of phases 1 to 2 is variable among specimens (Figs. 7A1, B1, 8C2, D1). The change from phase 1 to phase 2 is rather abrupt. The weak ribs, running parallel to fine growth lines, are moderately interspaced, sigmoidal, weakly convex on both flanks and moderately or strongly projected forward on the venter as on the rostrum (Figs. 7A1, B1, 8C2, D1, F2). The rib profile is rounded to acute and most elevated on the ventrolateral shoulders and venter (Figs. 7A1, B1, 8C2, D1, F2). Rib height decreases at the umbilical seam (Figs. 7B1, 8C2, D1, F2). Phase 3 is characterized by sharp and coarse ribbing (Fig. 8A2, B; see Nishimura et al. 2010: fig. 13C). This phase follows phase 2 (weak ribbing), and appears in the middle to late growth stages, at about 15–25 mm H (25–45 mm in D) (Nishimura et al. 2010: fig. 13C). The transition from phase 2 is rather abrupt. The coarse ribs are narrowly interspaced, sigmoidal, weakly convex on both flanks and strongly projected forwards on the venter (Fig. 8A2, B; see Nishimura et al. 2010: fig. 13C). The rib profile is rounded to acute (Fig. 8A2, B; see Nishimura et al. 2010: figs. 4B, 13C). The coarse ribs run parallel to growth lines. They are most elevated on the ventrolateral shoulders (Fig. 8A2, B; see Nishimura et al. 2010: figs. 4B, 13C).
The coarse ribs at phase 3 also differ from weak ribs at phase 2 in the rib-height/H ratio. In the coarse ribs of phase 3, this ratio exceeds 0.0005 at the ventrolateral point at which the ribs are most elevated. In contrast, the ratio is less than 0.0005 in the weak ribs of phase 2.
The suture line is a typical Desmoceras pattern (Matsumoto 1954: figs. 3–6), with a consisting of E, L, U2, U4, U5, U6 (= S), U3, U2, and I lobes and corresponding saddles. Trifid lateral and umbilical lobes are symmetrical. The U2 lobe is slightly asymmetrical. Saddles show the bifid pattern. Lobes are deeply incised and make lobules (Fig. 11A; see Matsumoto 1954: figs. 10, 11).
Remarks.—Similar to Turonian Tragodesmoceroides subcostatus Matsumoto, 1942a, from Hokkaido (see Nishimura et al. 2006), the shell ornament of this species changes during growth. Shell surface ornament changes from phase 1 (= stage 1 in Nishimura et al. 2010) (i.e., almost smooth with very fine growth lines) through phase 2 (weak rib) to phase 3 (coarse rib) as growth progresses (Fig. 12; see Nishimura et al. 2010: fig. 13D–F).
Unlike ancestral T. subcostatus (Nishimura et al. 2006), the constriction exists even in phase 2 (<15 mm in D) in Damesites damesi (Fig. 12; see Nishimura et al. 2006: fig. 11), although frequent constrictions in the early growth stage are a common feature of D. damesi and T. subcostatus (Fig. 12; see Nishimura et al. 2006: fig. 11). Three phases of shell surface ornament appear in a common order, which seems to be ontogenetically fixed in D. damesi as well as in T. subcostatus (Fig. 12; see Nishimura et al. 2006: figs. 8, 11; 2010: fig. 13A–C).

Fig. 12. Schematic diagram showing ontogenetic development of shell ornament change in late Turonian–early Campanian desmoceratine ammonoids (S-group, Damesites damesi) from the northwest Pacific province. Stratigraphic and morphological dates are collected from the Haboro-Tappu and Pombetsu areas, Hokkaido. Detailed ontogenetic development of Turonian Tragodesmoceroides subcostatus of S-group is shown in Nishimura et al. (2006). Each population sample and its horizon showing a–m. Ontogenetic development stages; phases 2 and 3 are recognized in Damesites damesi. Horizontal dashed lines show localities of molluscan fossil occurrence (see SOM: fig. S4).

Fig. 13. Schematic diagram showing ontogenetic development of shell ornament change in late Turonian–early Campanian desmoceratine ammonoids (C-group, Paradamesites spp.) from the northwest Pacific province. Paradamesites spp. exhibit only phase 1 (i.e., almost smooth shell surface) with frequent constrictions throughout growth. Detailed stratigraphic horizons of population samples (a–g) are shown in Fig. 12.
The interspacing of ribs becomes narrower in the last 10° whorl volution of the outer whorl of some large-sized specimens (KUM MM TN 193, Nishimura 2003: fig. 1D). This might be a sign of maturity. In the last growth stage, constrictions almost disappear, while a strong forwardly projected rostrum and strongly sigmoidal growth line develop.
In a smooth-surfaced specimen (KUM MM TN 218, Fig. 9A), exceptionally weak ribs are developed on the inner shell.
Shell form and shell ornament of the present species change stratigraphically (Figs. 12, 14). Specimens from lower Santonian (units Ud–e and Uf in the Haboro-Tappu area; see Figs. 7, 8) are large when adult (100 mm in D) (Figs. 7A1, 8B; see Nishimura 2003: fig. 2D) and have a great whorl breadth (B/H = 0.85 in 30 mm in D) (e.g., Fig. 7B2), an earlier appearance of a keel (1.0–1.5 mm in D) (Fig. 8D2), and an elevated keel (Figs. 7A2, 8A1). The adult shell ornament reaches phase 3 (Figs. 8A2, B, 12). On the other hand, specimens from upper Santonian (units Ug and Uh in the Haboro-Tappu area, Fig. 9) and the lower Campanian (units Ui–j and Uk in the Haboro-Tappu area) have a smaller shell size (40–60 mm in D), moderately compressed whorl (B/H = 0.8 in 30 mm in D; e.g., Fig. 9C2), retarded appearance of a keel (at 20–30 mm in D; Fig. 9C1) and a low, obtuse keel (Fig. 9B2, C1). The shell ornament remains in phase 1 even in adulthood (Fig. 9). However, this morphological trend appears to be intraspecific, judging from the presence of intermediate forms such as an upper Santonian individual from unit Ug with an intermediate adult shell size (ca. 60 mm D; Fig. 9A, B). Most specimens from the middle Campanian (Sphenoceramus orientalis Zone and S. schmidti Zone) individuals from Osoushinai Formation in the Nakagawa area (Fig. 9B, E) are small size, less than 30 mm D. These specimens are similar to the lower Campanian individuals (units Ui–j and Uk in the Haboro-Tappu area). On the contrary to these juvenile specimens, middle sized specimen about 48 mm D (holotype of “Damesites damesi intermedius”, Matsumoto 1954: pl. 6: 4a, b; Nishimura et al. 2010: fig. 4D, E) from the middle Campanian (S. orientalis Zone) possess a slightly depressed whorl (B/H = 0.8 in 48 mm in D) (Table 2). The shell ornament remains in phase 1 even in adulthood.
Ikeda and Wani (2012) subdivided the Santonian desmoceratines into three related groups on the basis of “bathymetry” of an upward-shallowing succession in the Kotanbetsu area. Although their taxonomic treatment is out of date, their observation is generally concordant with our data. For example, the B of the Santonian and lower Campanian Damesites damesi becomes compressed in upward sequence. Such temporal change of D. damesi is not merely a local phenomenon because a similar pattern has been also observed in the other sections (e.g., the Naiba area, Sakhalin). In addition, the population sample of “Damesites sugata” in Ikeda and Wani (2012) includes not only specimens of P rectus gen. et sp. nov. (Ikeda and Wani 2012: fig. 1.1) but also many specimens of D. damesi.
As described briefly in Shigeta et al. (2016), small desmoceratines previously identified as “Desmophyllites diphilloides (Forbes, 1846)” from the Osoushinai Formation (Sphenoceramus schmidti Zone; middle Campanian), Nakagawa area (e.g., Matsumoto and Obata 1955), should be considered as juvenile individuals of Damesites damesi before the development of a keel because they have much wider umbilicus (U/D = 0.08 in 20 mm in D) than Desmophyllites diphylloides (U/D = 0.06 in 20 mm in D).
“Damesites sp.” in Shigeta et al. (2019) from the middle Campanian (Sphenoceramus orientalis Zone) of the Ribira area, Hokkaido, and “Damesites cf. sugata” in Maeda et al. (2005) from the middle Campanian (Sphenoceramus orientalis Zone) of the Makarov area, southern Sakhalin, should probably be included to Damesites damesi having sigmoid constriction in early growth stage. These specimens are quite similar to middle Campanian D. damesi from the Nakagawa area. However, it is difficult to accurately identify this specimen, because of early growth stage or outer shell is peeled off.
“Desmoceras sugata” in Yokoyama (1890: pl. 20: 11a–c) is here reassigned to the upper Santonian or lower to middle Campanian form of D. damesi.
Some individuals of “Damesites sugata” have been reported from the Santonian to the lower Campanian of British Columbia, Canada by Haggart (1989). These are here reassigned to Damesites damesi because of their sigmoid growth lines and shell form.
As for the northeast Pacific province’s species, Coniacian or Santonian (lower Senonian in Anderson 1958) Kotoceras frazierense Anderson, 1958, is characterized by very compressed shell form, but this character is in fact the result of post-burial compaction. Sigmoidal weak ribs and shell shape show this “species” to belong to Damesites damesi.
A specimen of “Damesites sp.” from the upper Cenomanian (?) of the Western Interior, North America (Saito and Matsumoto 1956, without illustration; a plaster cast, GK. H 20027 is deposited at GK.) is assignable to Tragodesmoceroides sp. on account of its much wider umbilicus and tongue-like elevation on the venter instead of keel.
As discussed in Nishimura et al. (2010), “Damesites damesi”, “D. damesi intermedius”, “D. semicostatus”, and “D. laticarinatus” should be lumped to a single biological species. For example, “D. damesi” and “D. semicostatus” co-occurring in the lower Santonian cannot be discriminated from each other because of the presence of numerous intermediate forms (Nishimura et al. 2010: figs. 9, 10, 12).
“Damesites laticarinatus” (see Saito and Matsumoto 1956: text-fig. 1a–c; Nishimura et al. 2010: fig. 6E–G) is a monotypic species defined by almost smooth shell surface and diagnostic broad keel. The fragmentary holotype (GK. H 20026) was recovered from a river float. Saito and Matsumoto (1956) presumed that the holotype came from the lower Cenomanian (?), but this stratigraphic assignment appears to be questionable by the following reason. Wide continuous variation in the profile of the keel of “D. damesi” suggests that it is difficult to discriminate “D. laticarinatus” from the other forms on the basis of the keel (Nishimura et al. 2010: fig. 16). “Damesites laticarinatus” has sigmoidal growth lines and narrowly umbilicate (U/D is about 0.07 at 50 mm in D) compressed whorls (B/H is about 0.7) without constrictions in the late growth stage. These are common features in the late Santonian forms of D. damesi (e.g., KUM MM TN 218, 229, see Fig. 9A; compare Nishimura et al. 2010: fig. 13D–F). The preservation of the shell casts doubt about a lower Cenomanian origin of “D. laticarinatus” holotype because dark brown color of both shell tests and sparry calcite in the camera are dissimilar to those of the other ammonoids from lower Cenomanian in the Ikushunbetsu area (white sparry calcite in camera). Therefore, the Cenomanian affinity for “D. laticarinatus” is apparently erroneous, and it should be assigned to the upper Santonian form of D. damesi (Fig. 9).
“Damesites damesi intermedius” (see Matsumoto 1954: pl. 6: 4a, b; Nishimura et al. 2010: fig. 4D, E) recorded from Japan, Sakhalin, and California, was said to discriminated from “D. damesi damesi” by its weakly sigmoidal constrictions (Matsumoto 1954, 1959a). “Damesites damesi intermedius” was also assumed as a morphologic mosaic for having “D. damesi”-like whorl shape and “D. sugata” (= P. rectus gen. et sp. nov. herein)-like nearly concave constrictions from the Yezo Group (Matsumoto 1954; Matsumoto and Obata 1955). However, discrimination of ”D. damesi damesi” and “D. damesi intermedius” on the basis of the constriction curvature is difficult because in the former it is also weakly sigmoidal (Nishimura et al. 2010: fig. 11D, E). Constriction curvature of “D. damesi intermedius” is more closely similar to that of “D. damesi damesi” than that of “D. sugata” from the Yezo Group (= P. rectus gen. et sp. nov. herein) (Nishimura et al. 2010: fig. 11). Matsumoto and Obata (1955) treat D. damesi intermedius as an intermediate form between D. damesi damesi and “D. sugata” from the Yezo Group by shape of constriction, however, shape of constriction, shape of growth line, w, etc. do not show an ancestor-descendant relationship between these two subspecies. Diagnostic feature of the holotype of “D. damesi intermedius” (GK. H 3269) from the middle Campanian of the Urakawa area (Matsumoto 1954) is its greater B. Most similar specimens occur in the lower Santonian (KUM MM TN 145, Fig. 7B). This, “Damesites damesi intermedius” occur from around first appearance datum (FAD) and last appearance datum (LAD) of “Damesites damesi”; lower Santonian and middle Campanian. In addition, the difference of B/H ratio between the two subspecies of D. damesi is slight, and an intermediate form exists between them as in other morphological characters (Nishimura et al. 2010). Considering those evidences, “D. damesi intermedius” should be lumped with D. damesi. Another specimen, UMUT MM 6747 (previously listed as “D. damesi intermedius”), has a moderately compressed whorl, and resembles the lower Santonian–lower Campanian forms of D. damesi.
Damesites damesi resembles Tragodesmoceroides subcostatus Matsumoto, 1942a, in whorl shape (ca. 0.8 in B/H ratio at 30 mm in D), curvature of growth lines, three-phased ontogenetic development of surface ornament and frequently developed constrictions in early growth stage (see Nishimura et al. 2006: figs. 9C, 10, 11). Damesites damesi possesses a keel from the juvenile to middle growth stages (i.e., 10–50 mm in D) (Figs. 8D2, 9D2). Tragodesmoceroides subcostatus, in contrast, possesses no keel except for a blunt tongue-like ventral elevation (Fig. 2C2; compare Nishimura et al. 2006: fig. 4B). The latter species has a wider umbilicus at the juvenile to middle growth stages (U/D ratio 0.11, at 30 mm in D) compared to the present species (U/D = 0.08, at 30 mm in D) (Nishimura et al. 2010: fig. 17C).
Damesites damesi also resembles the lower Maastrichtian D. hetonaiensis Matsumoto, 1954, in its sigmoidal growth lines, frequently developed constrictions in the early growth stage, and possession of a keel. The B is also similar in the two specimens (B/H = 0.80 in 30 mm in D). However, D. damesi differs from D. hetonaiensis in having a wider keel (Matsumoto 1954: pl. 4, fig. 1c). The umbilicus of D. hetonaiensis is much narrower (U/D = 0.06 in 50 mm in D) than that of D. damesi (U/D = 0.08 in 50 mm in D).
There are similarities among D. damesi, P. sugata, and P. rectus gen. et sp. nov.; all have a keel. However, D. damesi is discriminated from Paradamesites spp. by having sigmoidal growth lines (Figs. 7A1, B1, 8A2, B, F2), a larger w (Nishimura et al. 2010: fig. 17F) and appearance of ribs in the late growth stage (phase 3) (Fig. 12).
Stratigraphic and geographic range.—Santonian–middle Campanian, north Pacific Realm.
Genus Paradamesites nov.
ZooBank LSID: urn:lsid:zoobank.org:act:891736E2-4827-40AB-B0D9- 631F181F53D0.
Etymology: Form Greek para, parallel; parallel lineage of genus Damesites.
Type species: Ammonites sugata Forbes, 1846, from South India, Coniacian or Santonian.
Species included: Paradamesites sugata (Forbes, 1846), P. compactus (Hoepen, 1921), P. rabei (Collignon, 1961), P. tsianalokyensis (Collignon, 1961), and P. rectus sp. nov.
Diagnosis.—Superficially similar to Damesites but having single concave growth lines, much compressed whorls, an obtuse to acute keel, and a smaller whorl expansion rate. Shell surface is smooth throughout growth except for fine growth lines and periodic constrictions. Suture line is complex, bifid external lobe and trifid lateral, umbilical, and internal lobes.
Remarks.—Another lineage of Damesites-like forms with concave growth lines is discriminated from the true Damesites. Newly established Paradamesites gen. nov., comprised P. sugata (originally Indian populations) and P. rectus gen. et sp. nov. In addition, this new genus includes numerous individuals which have previously been mistakenly assigned for their generic position to “Damesites”.
Stratigraphic and geographic range.—Middle Turonian–Campanian, Upper Cretaceous, African-Indian province, European province, north and south Pacific Realms.
Paradamesites sugata (Forbes, 1846) comb. nov.
Figs. 2D, 3–5.
1846 Ammonites Sugata sp. nov.; Forbes 1846: 113: pl. 10: 2a–c.
1865 Ammonites Sugata Forbes, 1846; Stoliczka 1865: 60, pl. 32: 4–6; pl. 33: 1, 2.
1898 Desmoceras Sugata Forbes, 1846; Kossmat 1895–1898: 111 (176), pl. 18 (24): 11; pl. 19 (25): 1.
1921 Hauericeras? sugata (Forbes, 1846); Spath 1921: 46, pl. 6: 3.
?1921 Desmoceras compactum n. sp.; Hoepen 1921: 21–23, pl. 4: 5–7.
1931 Desmoceras Sugata (Forbes 1846); Basse 1931: 21, pl. 2: 19, 20.
non 1955 Damesites sugata (Forbes, 1846); Matsumoto and Obata 1955: 128, pl. 26: 4, pl. 27: 3, 4.
1957 Damesites sugata (Forbes, 1846); Arkell et al. 1957: L370, fig. 482: 4.
1957 Damesites ainuanus sp. nov.; Matsumoto 1957: 86, pl. 15: 1, 2.
1958 Kotȏceras richardsoni sp. nov.; Anderson 1958: 217, pl. 36: 3, 3a.
?1958 Kotȏceras subsugatum Anderson, 1902; Anderson 1958: 217–218, pl. 35: 2, 2a.
1961 Desmoceras sugatus (Forbes, 1846); Collignon 1961: 67, pl. 27: 1, 2, text-fig. 7.
1965 Desmoceras sugata (Forbes, 1846); Collignon 1965: 20, pl. 421: 1751.
1968 Damesites ainuanus Matsumoto, 1957; Howarth 1968: 222, pl. 1: 3–5.
?1968 Damesites sugata (Forbes, 1846); Pauliuc 1968: 103, pl. 29: 2, 3.
1969 Damesites petrobrasia sp. nov.; Beurlen 1969: 149–150, pl. 2: 1, pl.4: 1a, 1b.
1980 Damesites ainuanus Matsumoto, 1957; Futakami et al. 1980: pl. 1: 3.
?1980 Damesites compactus (Hoepen 1921); Summesberger 1980: 278–280, pl. 1: 3, 4.
?1981 Damesites spp.; Szász 1981: 101, pl. 2: 3a, 3b.
?1983 Damesites sugatus (Forbes, 1846); Collignon 1983: 190, pl. 2: 4.
1987 Damesites damesi Jimbo, 1894; Poyarkova 1987: pl. 24, fig. 5a–c.
1991 Damesites sugata (Forbes. 1846); Kennedy and Henderson 1991: 471, figs. 1A–I, 2A–G.
1996 Damesites sugata (Forbes, 1846); Wright et al. 1996: 84, fig. 64: 2a, b.
2003 Damesites ainuanus Matsumoto, 1957; Cooper 2003a: 109–110, fig. 3C, D
2003 Damesites sugata (Forbes, 1846); Cooper 2003b: 155–157, fig. 6E, F
2010 “Damesites ainuanus Matsumoto, 1957”; Nishimura et al. 2010: figs. 5A–B, 11A, 14D–F, 16H.
2010 “Damesites sp.”; Nishimura et al. 2010: figs. 5C–F, 7C–F, 15A, 16F–G.
2011 Damesites ainuanus Matsumoto, 1957; Jagt-Yazykova 2011: pl. 4: 2–6.
2012 Damesites aff. sugata (Forbes, 1846); Tanabe et al. 2012: fig. 5A–C.
2013 Damesites sugata (Forbes, 1846); Kennedy and Klinger 2013: fig. 7A–J.
2020 Damesites sugata (Forbes, 1846); Kennedy and Gale 2020: fig. 9A–I.
Type material: Lectotype BMNH C22674, the original of Forbes (1846) is small-sized specimen from South India. Paralectotypes: BMNH C24169a, b and BMNH C22675 are small-sized specimens as lectotype. These type specimens are said to be collected from Ariyalur Group of Vridachellum, South India. Lectotype and paralectotypes are designated by Matsumoto (1942a).
Type locality: Vridachellum (Verdachellum) in Madras state, South India.
Type horizon: Coniacian or Santonian.
Material.—Five specimens (BMNH C22674, C27169a, b, C22675, KUM MM TN 292) from South India including the type series of Forbes (1846). Five specimens (GSI 145–148, 14870) from South India of Stoliczka (1865) and Kossmat (1895–1898). One specimen was collected probably from Coniacian of the Varagur in Madras state, South India (KUM MM TN 292; collected from 11°15’16.3”N, 79°02’51.5”E; Fig. 5B). About 10 registered specimens from the Yezo Group in various areas of Hokkaido and Sakhalin are deposited in the UMUT and GK. Additionally, 45 specimens from the uppermost Turonian–Coniacian in the Pombetsu and Haboro-Tappu areas in the Yezo Group are HMG-1623, 1794, 1795, and KUM MM TN 099–144 (Table 1).
Emended diagnosis.—Narrowly umbilicate whorl with a moderate to acute high keel. Constriction is concave and becoming weakly flexuous on both flanks in later growth stage. The relative umbilical width versus shell D gradually increases during ontogeny (see Nishimura et al. 2010: fig. 17C). Suture line is complex, bifid external lobe and trifid lateral, umbilical, and internal lobes.
Measurements.—Shown in Table 2.
Description.—An acute, narrow, and high ventral keel develops after the early growth stage at < 30 mm in D in the lectotype and paralectotype collected from South India (BMNH C22674, 22645 and 24196a, b). Both flanks of the whorl are flat and nearly parallel. The umbilicus is crater-like rounded in umbilical edge and slightly wider (U is 3.5 mm, 37 mm in D) than that in the species of the revised genus Damesites. The shell surface is almost smooth except for fine growth lines and periodic constrictions. The curvature of constriction is moderately prorsiradiate and single concave to the aperture.
Compared with the Indian types, specimens from the Yezo Group are better preserved in general and retain details of morphological change through growth as follows.
Whorls become more compressed during growth (Fig. 10B, H). In the early growth stage (1 mm in D), the whorl is depressed (B/H = 1.7), while it is moderately compressed in the middle to late growth stage (B/H is about 0.7 at 50 mm in D) (Fig. 10B, H; see Nishimura et al. 2010: fig. 17D).
The umbilical shoulder appears in the 3–4π stage and a desmoceratid umbilical break (Maeda 1993) appears in the 5–6π stage (Fig. 10H). The U/D ratio decreases with growth, changing from 0.2 (1 mm in D) to 0.08 (30 mm in D) (Fig. 10D; see Nishimura et al. 2010: fig. 17C, H).
The w is small (about 2.2 at 50 mm in D) and is stable throughout growth (Fig. 10E; see Nishimura et al. 2010: fig. 17F).
The shell of the late growth stage attaining 80–90 mm in D consists of 6–7 whorl volutions (Fig. 10H). At venter, prorsiradiate and simple concave growth lines flex strongly forwards to form a projected rostrum (Figs. 3A1, 4A2, 5A2; see Nishimura et al. 2010: fig. 5E). Shell surface ornament is almost smooth except for periodic constrictions throughout growth (phase 1, Fig. 13). The constrictions become frequent in the last 30° of the outer whorl in some specimens (GK. H 4198, KUM MM TN 129, Nishimura et al. 2010: fig. 5B, E). This may be a sign of maturity.
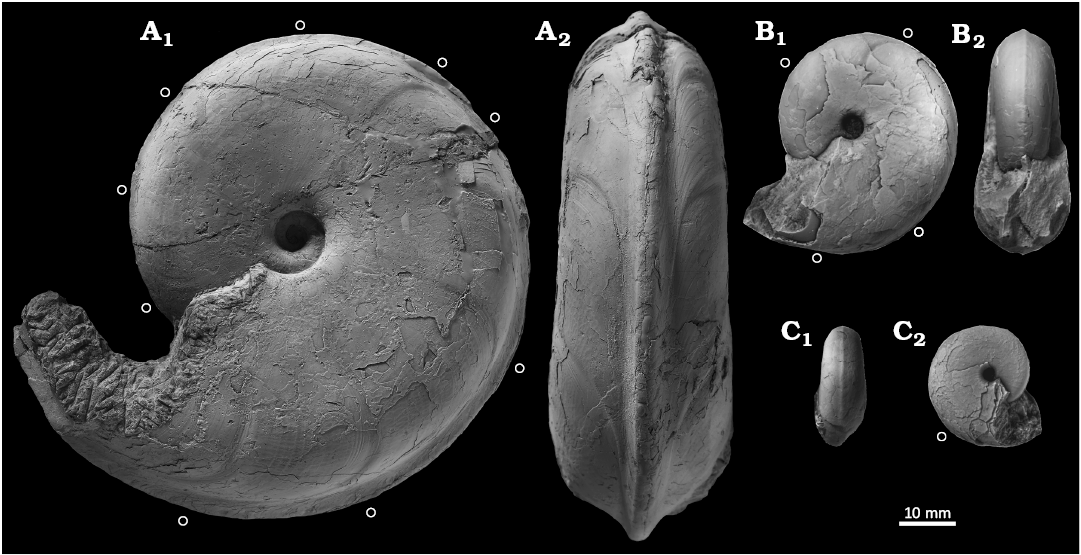
Fig. 3. Desmoceratine ammonoid Paradamesites sugata (Forbes, 1846) from the uppermost Turonian of the Yezo Group in Hokkaido, Japan (= “Damesites ainuanus Matsumoto, 1957”). A. HMG-1623, topotype of “Damesites ainuanus” from the type locality (loc. IK 2013), in left lateral (A1) and ventral (A2) views. B. GK. H 4200, paratype of “Damesites ainuanus” (loc. IK 2013), in left lateral (B1) and apertural (B2) views. C. GK. H 4198, paratype of “Damesites ainuanus” (loc. IK 2013), in right lateral (C1) and ventral (C2) views. An open circle marks the position of constrictions.
Growth lines are prorsiradiate and single concave (Figs. 3–5). Only in the late growth stage weakly sigmoidal growth lines restrictedly appear on the periphery of constrictions although they retain a single concave pattern in most areas (Nishimura et al. 2010: fig. 15A). The curvature of constriction changes from single concave to weakly sigmoid in the late growth stage (about 60 mm in D). Constrictions are well developed in the inner shell, and show a very weak rise in the outer shell. For example, the specimen with shell D of 80 mm; GSI 148 (Stoliczka 1865: pl. 33: 1) has periodic constrictions showing weakly sigmoid curvature without growth lines, because of the weathered surface of the shell.
The suture line is a typical Desmoceras pattern (Matsumoto 1954: 248), with a formula of [E, L, U2, U4, U5, U6 (= S), U3, U2, I]. Trifid lateral, umbilical, and internal lobes are symmetrical. The U2 lobe is slightly asymmetrical. Saddles show the bifid pattern. Lobes are deeply incised and make many lobules (Fig. 11B).
Remarks.—In profile, the keel becomes gradually higher in an upward sequence in the Yezo Group of the northwest Pacific province; i.e., the latest Turonian “D. ainuanus” (Fig. 3) via early Coniacian forms (from Unit Ua, Ub–c; Fig. 4) to late Coniacian forms (= “Damesites sp.” from Unit Ud–e; Fig. 5A). The height of keel changes from 0.2 mm (latest Turonian) to 0.6 mm (late Coniacian) via 0.4 mm (early Coniacian) at 20 mm H. Late Coniacian forms are most similar to South Indian P. sugata, inclusive of the types of Forbes (1846).
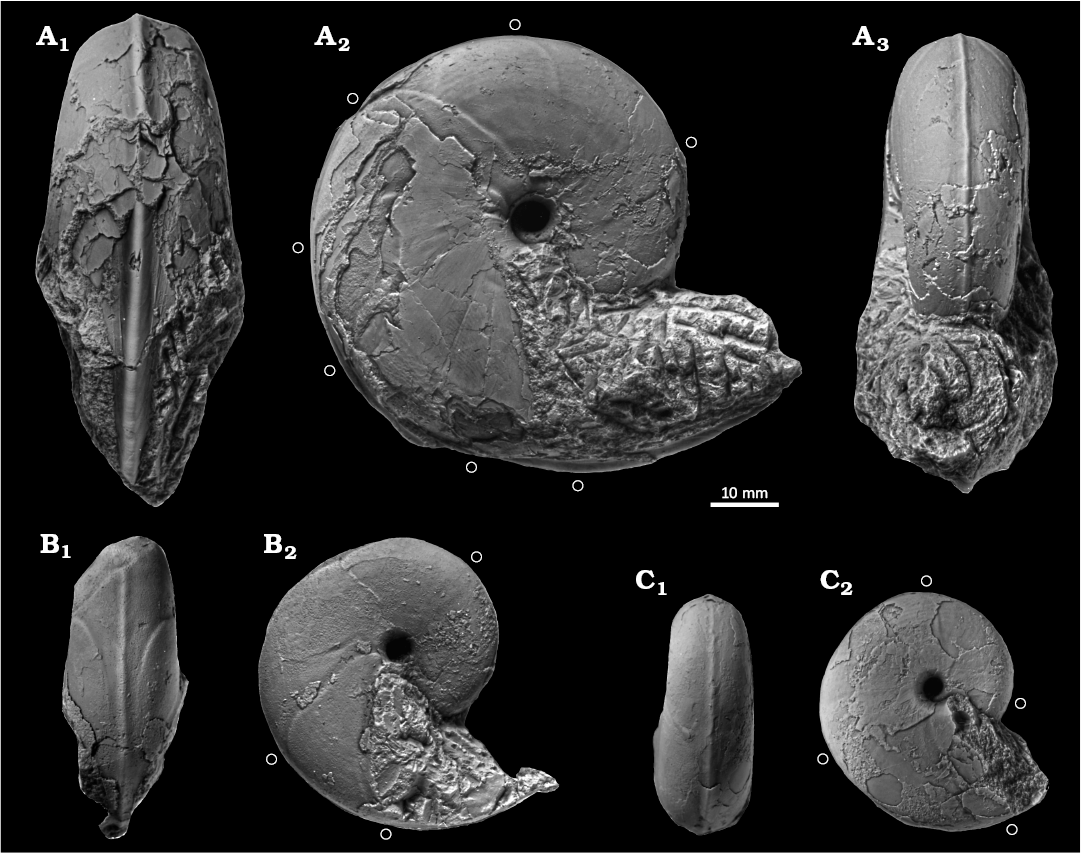
Fig. 4. Desmoceratine ammonoid Paradamesites sugata (Forbes, 1846) from lower Coniacian of the Yezo Group in Hokkaido, Japan. A. KUM MM TN 103 from loc. OT1000, in ventral (A1), right lateral (A2), and apertural (A3) views. B. KUM MM TN 106 from loc. OT1402, in ventral (B1) and right lateral (B2) views. C. KUM MM TN 108 from loc. OT1402, in ventral (C1) and right lateral (C2) views. An open circle marks the position of constrictions.
The morphology of the keel changes stratigraphically within the single biospecies P. sugata. This trend is confirmed by biostratigraphic observations in the northwest Pacific and African-Indian provinces. “Damesites ainuanus”, an early form with a narrow and low keel is reported not only from the upper Turonian of Hokkaido (Matsumoto 1957) and Sakhalin (Jagt-Yazykova 2011) but also found from the middle and upper Turonian of Angola (Howarth 1968; Cooper 2003a). Paradamesites sugata-like specimens were also reported from the lower Coniacian of South India (Kennedy and Gale 2020). These specimens are identified as “Damesites sugata,” however, they possess a narrow and low keel. One of the specimens, OUM KY 2218 possesses a slightly broader and lower keel. This specimen should be identified as “Damesites ainuanus” in the previous scheme.
Paradamesites sugata was first described by Forbes (1846) as Ammonites Sugata. Many specimens from various areas have been identified as the present species (Stoliczka 1865; Kossmat 1895–1898; Spath 1921; Collignon 1961, 1965, 1983) or listed without illustration (Chiplonkar et al. 1985). Kennedy and Henderson (1991) first photographically documented the lectotype, paralectotypes, and topotypic adult shell of Paradamesites sugata. According to Kennedy and Henderson (1991), Forbes’ (1846) specimens (lectotype and paralectotypes) are said to have been collected from the Ariyalur Group of Vridachellum (Verdachellum) in Madras state, South India. Kossmat (1895–1898) reported this species from the upper part of the Trichinopoly Group and the lower part of the Ariyalur Group, both of which were correlated to the Coniacian–Santonian. In Madagascar, this species ranges from the Coniacian to Santonian (Collignon 1961), whereas in Angola, it occurs throughout the Santonian (Cooper 2003b).
UMUT MM 27833 (Damesites sugata in Tanabe and Landman 2002; Damesites aff. sugata in Tanabe et al. 2012) with a narrow and moderate high keel is identified as the early Coniacian form of P. sugata (e.g., Fig. 4).
The holotype and the other examples of the northeast Pacific province’s species Coniacian “Kotôceras subsugatum Anderson, 1902” (figured in Anderson 1958: pl. 35: 2, 2a for the holotype) were lost in the San Francisco Fire of 1906. Judging from illustrations of the holotype, however, due to its almost smooth shell surface, slightly wider umbilicus, moderate height, and slightly narrower keel it resembles those of P. sugata.
The monotypic species of “Damesites pterobrasia Beurlen, 1969” was reported from the Coniacian strata of Brazil. Judging from Beurlen’s (1969) photograph and illustration, the large shell (100 mm D), small w, slightly wider umbilicus, almost smooth shell surface and concave growth line suggest this “species” to be conspecific to P. sugata.
“Damesites sugatus” from Santonian of Spain, as recorded by Collignon (1983) has a slightly wider umbilicus (U/D = 0.12 in 67 mm D) and a narrow keel. These characteristics match those of P. sugata. However, we treated them as Paradamesites cf. sugata, because, Collignon (1983) does not show a photograph of the lateral view.
“Damesites sugata” was also recorded from the upper Turonian of Romania (Pauliuc 1968). Based on photographs of two specimens, the small w (w = 1.96 at 23.9 mm in D and w = 2.03 at 44.8 mm in D), moderate or slightly wider umbilicus, and almost smooth shell surface ornamentation match diagnostic features of Paradamesites gen. nov. It is, however, difficult to identify species because of the only lateral view of photographs (Pauliuc 1968). Thus, these Romanian specimens should be treated as Paradamesites cf. sugata according to our present knowledge.
“Damesites spp.” reported from the Coniacian of Romania (Szász 1981), have a siphonal keel and a moderately wide umbilicus, and are similar to Paradamesites sugata. However, precise identification is difficult because of the small size and fragmentary nature of these specimens.
The shape of the keel in “Damesites sp.” from the “upper Cenomanian” in Germany (Immel et al. 1981: fig. 2.2a, b) resembles that of the Coniacian forms of P. sugata. This record is important to reconstruct the evolutionary processes of the Damesites group, although the specimen is fragmentary. In addition, there is inconsistency in the biostratigraphic correlation between ammonoids (Subprionocyclus Zone suggesting the upper Turonian) and planktonic foraminifera (Helventogrobotruncana helvetica suggesting the lower and middle Turonian) for this German section. A more detailed interpretation of the German record will be postponed until the stratigraphic correlation is determined.
As far as northeast Pacific province’s species, Coniacian or Santonian “Kotôceras richardsoni Anderson, 1958” is characterized by a small w, a slightly large umbilicus, frequent sigmoidal constrictions, a siphonal rostrum, and a siphonal keel. Although shell surface ornamentations in the holotype of this “species” have been abraded (see Anderson 1958: pl. 36: 3, 3a), what can be seen is identical to that of the Coniacian form of P. sugata.
Lower Campanian “Damesites damesi” from South Africa (Cooper and Greyling 1996) is conspecific with P. sugata, because of its almost smooth shell ornament and siphonal keel. Cooper and Greyling (1996) described that the South African “Damesites damesi” has a biconcave peristome in the internal mold of the adult shell (ca. 92 mm in D). Around the constrictions, the shape of the growth lines of P. sugata of C-group exceptionally appears to be biconcave.
Kennedy and Klinger (2013) regarded “Damesites compactus (Hoepen, 1921) ” as a probable synonym of P. sugata. The present study agrees with Kennedy and Klinger (2013), and the differences between “Damesites compactus” and P. sugata are slight.
Compared with the Indian types, a detailed examination of specimens from the Yezo Group has revealed that “Damesites sp.” from the upper Coniacian (Fig. 5A; see Nishimura et al. 2010: fig. 5C–F) should be identified as true P. sugata. These specimens possess all diagnostic characteristics of Paradamesites sugata such as the development of an acute keel from an earlier growth stage onwards. In contrast, the identification of Santonian “D. sugata” from the Yezo Group (Fig. 6; see Nishimura et al. 2010: fig. 6A–D) is erroneous; and these specimens should be reassigned to P. rectus gen. et sp. nov. The stratigraphic range of the true P. sugata seems to be the middle Turonian to Santonian in India, Madagascar, and Angola (Forbes 1846; Collignon 1961, 1965; Cooper 2003b; Chiplonkar et al. 1985).
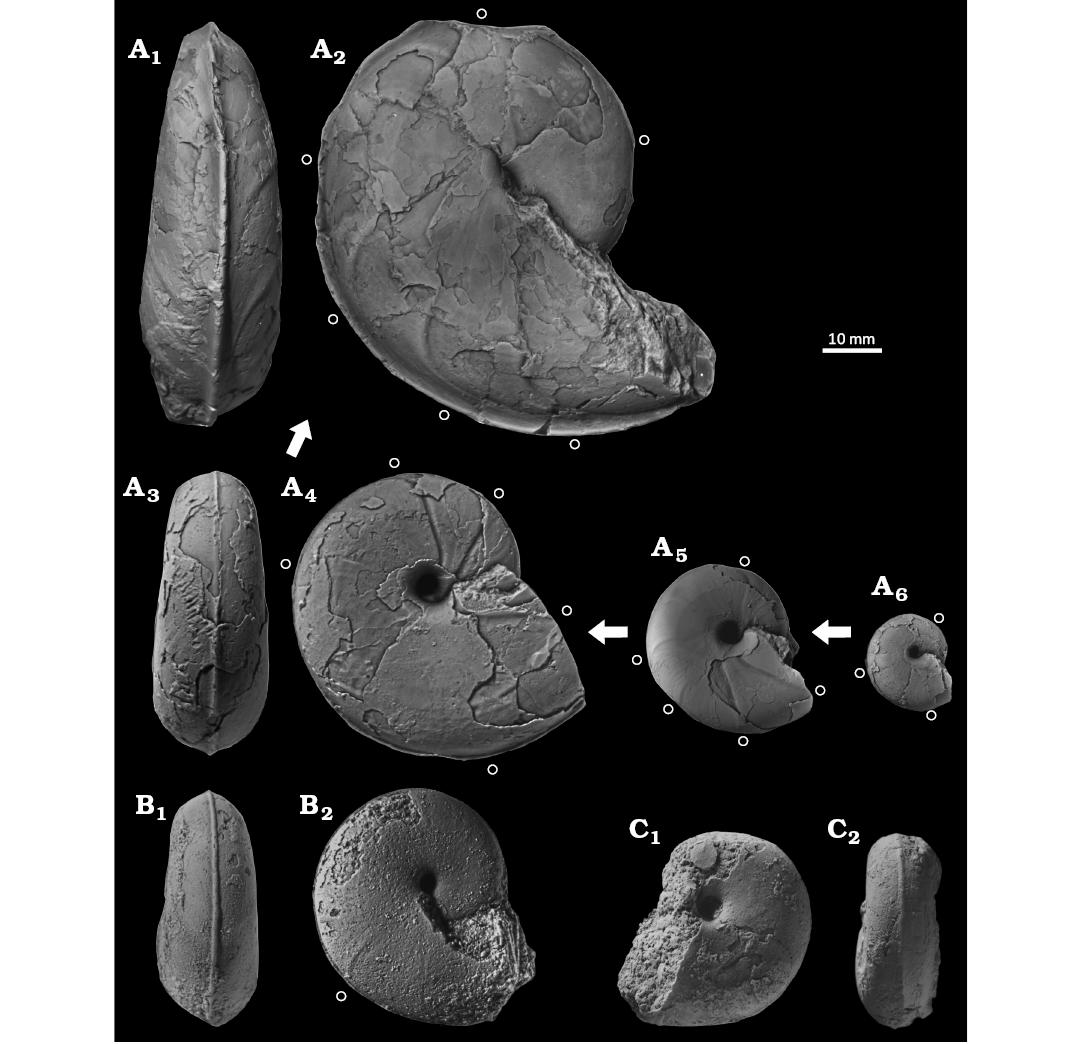
Fig. 5. Desmoceratine ammonoid Paradamesites sugata (Forbes, 1846) from the upper Coniacian of the Yezo Group, Hokkaido, Japan (A), and from the South India (B, C). A. KUM MM TN 129, loc. HB1028, in ventral (A1, A3) and right lateral (A2, A4, A5, A6) views. B. KUM MM TN 292, ?Coniacian of Varagur in Madras state, India, in ventral (B1) and right lateral (B2) views. C. BMNH C22674, plaster cast of the lectotype, Coniacian or Santonian of Vridachellum (Verdachellum) in Madras state, India, in left lateral (C1) and ventral (C2) views. An open circle marks the position of constrictions. Arrows illustrate ontogenetic change of a single specimen.
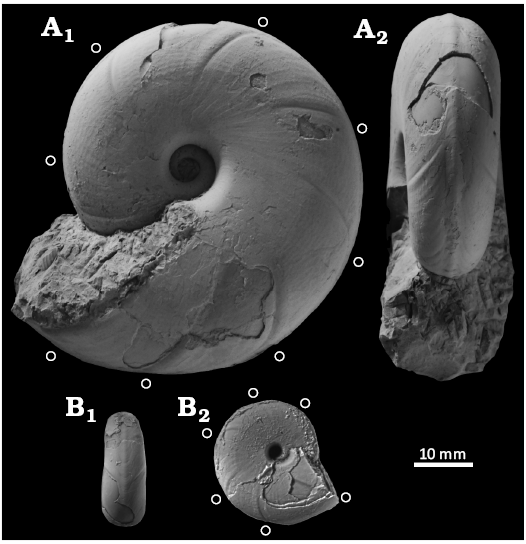
Fig. 6. Desmoceratine ammonoid Paradamesites rectus gen. et sp. nov. (= “Damesites sugata” from the Yezo Group) from the Santonian–lower Campanian of the Yezo Group, Hokkaido, Japan. A. KUM MM TN 281 holotype, loc. OT5034, in left lateral (A1) and apertural (A2) views. B. KUM MM TN 287 paratype, loc. TK1019, in ventral (B1) and right lateral (B2) views. An open circle marks the position of constriction.
Paradamesites sugata superficially resembles Damesites damesi in features of whorls and keel, but differs from the latter in having a smaller w (2.2 in the former compared to 3.0 in the latter at 50 mm in D), a slightly wider umbilicus (U/D is 0.09 in the former and 0.08 in the latter), frequently developed periodic constrictions in later growth stage and almost smooth shell surface ornament.
Stratigraphic and geographic range.—Uppermost Turonian–Coniacian of Hokkaido and Sakhalin; middle Turonian–Santonian of South India, Madagascar, South Africa, Angola, and Spain.
Paradamesites rectus sp. nov.
Figs. 2B, 6.
1927 Kotôceras damesi (Jimbo, 1894); Yabe 1927: pl. 7 (5): 9a, b.
1942 Damesites sugatus (Forbes, 1846); Matsumoto 1942a: 27, text-fig. 1f.
1955 Damesites sugata (Forbes, 1846); Matsumoto and Obata 1955: 128, pl. 26: 4, pl. 27: 3, 4.
2003 Damesites sugata (Forbes, 1846); Nishimura 2003: fig. 2G, H.
2010 “Damesites sugata (Forbes, 1846)”; Nishimura et al. 2010: figs. 6A–D, 7A, 7B, 11C, 14A–C, 15C.
2012 Damesites sugata (Forbes, 1846); Ikeda and Wani 2012: fig 1.1.
ZooBank LSID: urn:lsid:zoobank.org:act:2CDA43CC-434B-4E06-AB 6B-8DB5D6B6E1D6.
Etymology: From Latin rectus, straight; after simple concave, nearly straight shape of growth line in mid flank.
Type material: Holotype KUM MM TN 281 (Fig. 6A) is a medium-sized specimen. Paratype KUM MM TN 281 (Nishimura et al. 2010: fig. 6C, D) is associated with the holotype in the same calcareous concretion. Paratypes (KUM MM. TN 280, 282–291 (Fig. 6), see Nishimura et al. 2010: figs. 6C, D, 7A, B, 11C, 14A–C, 15C) are variously sized shells collected from the Santonian–Campanian in the Haboro-Tappu and Nakagawa areas.
Type locality: Akanosawa Creek (locality OT5034), Tappu area, Hokkaido, Japan (SOM: fig. S3).
Type horizon: Santonian.
Material.—The type material and ten additional specimens from the Santonian–Campanian strata of the Yezo Group at various localities in Hokkaido, Japan and Sakhalin, Russia. These are deposited in the UMUT and the GK collections (Table 1).
Diagnosis.—Widely umbilicate shell for desmoceratines with an obtuse keel. Growth lines and constrictions are less flexuous and simple concave in shape. Suture line is complex, bifid external lobe and trifid lateral and umbilical lobes.
Measurements.—See Table 2.
Description.—Whorl becomes compressed as growth progresses. B/H ratio changes from 2.0 (1 mm in D) to 0.75 (60 mm in D) (Fig. 10B; see Nishimura et al. 2010: fig. 17B, D).
The umbilical shoulder appears in the 3–4π stage and a desmoceratid umbilical break appears in the 6–7π stage (Fig. 10G). U/D ratio decreases during 5π to 10π stage (0.2 to 1.0, about 0.6 mm to 10 mm in D), increases again after 10π stage (U/D = 0.15 in 50 mm in D) (Fig. 10D). The w is small (about 2.2 at 50 mm in D) (Fig. 10E; see also Nishimura et al. 2010: fig. 17E) and is stable throughout growth. An obtuse keel develops at the venter after 20 mm in D (Fig. 6B1; Nishimura et al. 2010: fig. 6C).
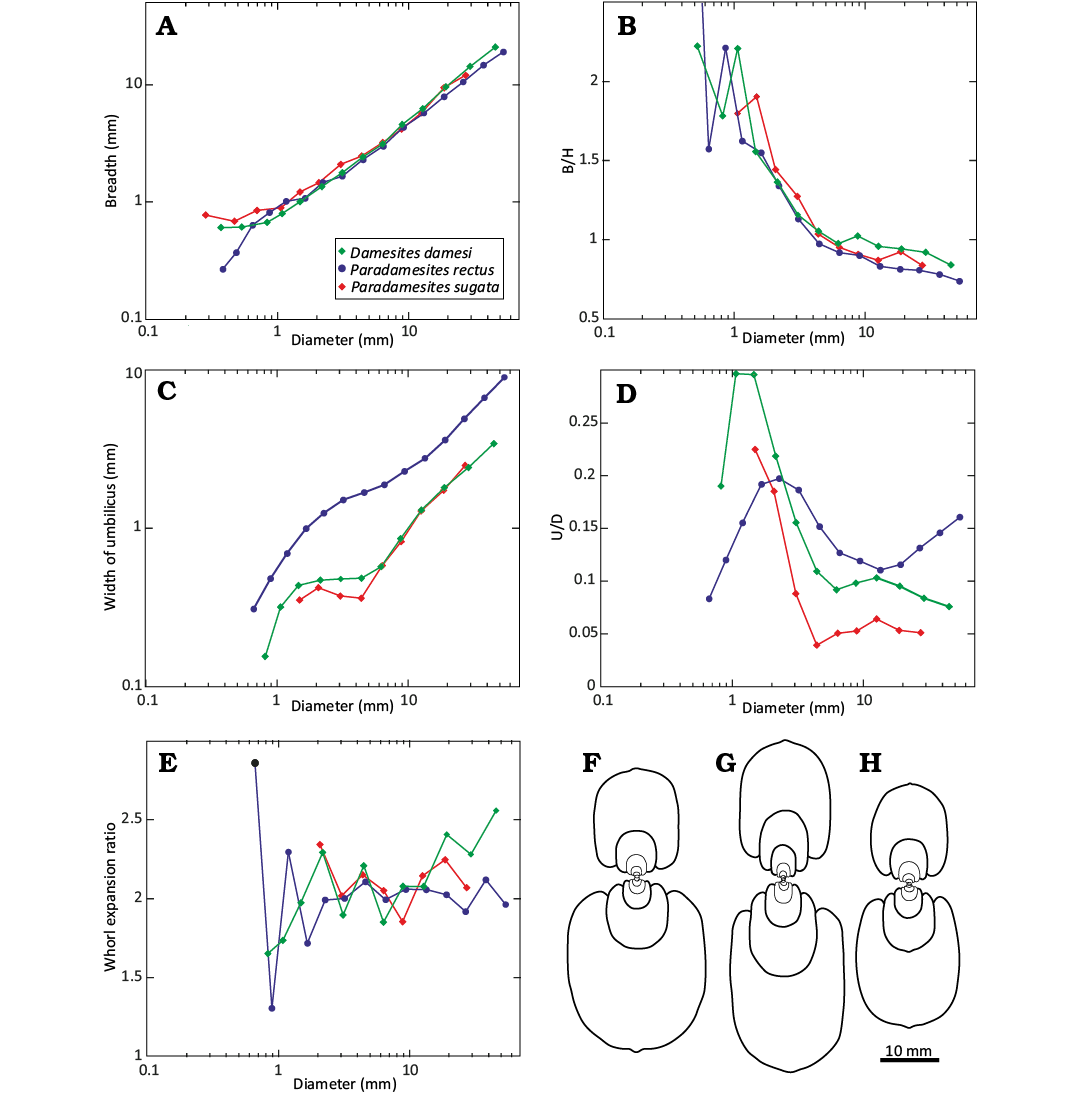
Fig. 10. Ontogenetic changes of whorl dimensions (A, C) and whorl shape (B, D, and E) in relation to shell D, and diagrammatic drawings of median shell cross section (F–H) for representative specimens of desmoceratine ammonoid species. F. Damesites damesi (KUM MM TN 206, loc. OT1600), G. Paradamesites rectus gen. et sp. nov. (KUM MM TN 288, a float from Kotanbetsu River). H. Paradamesites sugata (KUM MM TN 134, loc. HB1028). Abbreviations: B/H, ratio of whorl breadth (B) to whorl height (H); U/D, ratio of width of umbilicus (U) to diameter (D).
During the late growth stage, the shell attains 90–100 mm D and consists of 7–8 whorls (Fig. 10G; see also Nishimura et al. 2010: fig. 6A–B). The whorl is compressed (B/H ratio is 0.75 at 50 mm in D) (Fig. 10B; see also Nishimura et al. 2010: fig. 17D). The umbilicus is wide (U/D ratio is 0.15 in 50 mm in D) (Fig. 10D; see also Nishimura et al. 2010: fig. 17C). Curvature of growth line is prorsiradiate and single concave throughout growth (Fig. 6). Periodic constrictions develop frequently throughout growth (the number per volution is 3 or 4) and become very frequent in the late growth stage in some specimens (KUM MM TN 285, see Nishimura et al. 2010: fig. 6A, B). This may represent a sign of maturity in the present species.
The suture line is a typical Desmoceras pattern (Matsumoto 1954: 248), with a formula of (E, L, U2, U4, U5, U6[= S]). Trifid lateral and umbilical lobes are symmetrical. The U2 lobe is slightly asymmetrical. Saddles show the bifid pattern. Lobes are deeply incised and make lobules (Fig. 11C).
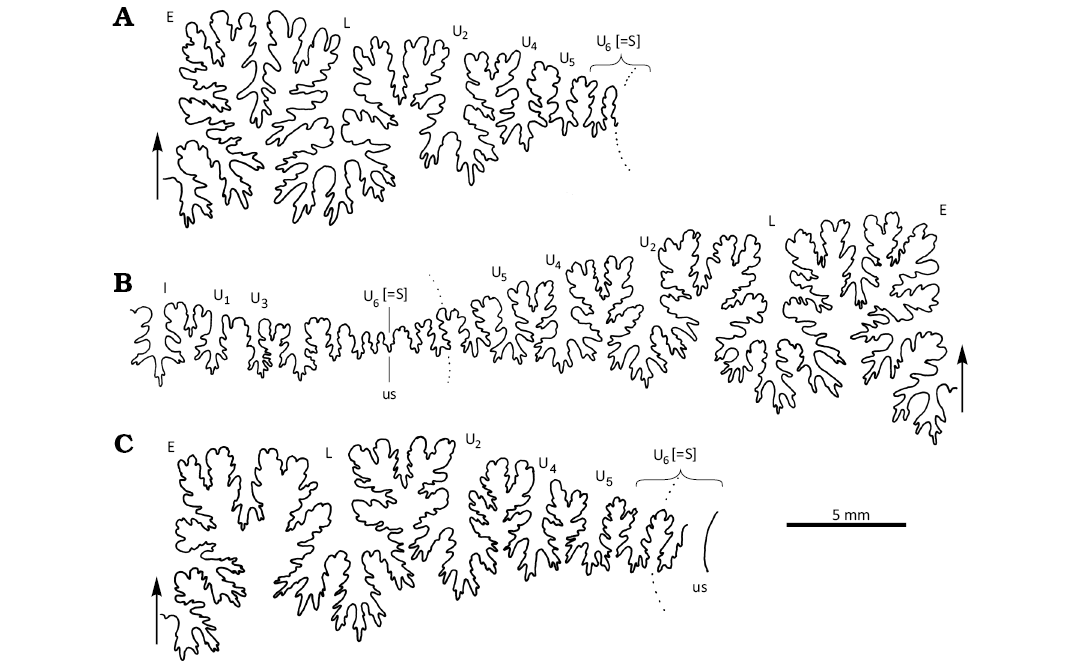
Fig. 11. Suture line of desmoceratine ammonoids from Yezo Group, Hokkaido, Japan. A. Paradamesites rectus gen. et sp. nov. KUM MM TN 284, at the 19.50 mm H, Ui–j Unit of the tributary of Akanosawa-Creek, Tappu area. B. Padradamesites sugata (Forbes, 1846). KUM MM TN 129, at the 19.65 mm H, the Nakafutamata-zawa Creek, Haboro (same specimen as Fig. 5A). C. Damesites damesi (Jimbo, 1894). KUM MM TN 164, at the 17.15 mm H, the Jugosen-zawa Creek, Tappu area. Broken line shows umbilical edge. Arrows show position of siphuncle and adoral direction. Abbreviations: E, L, U, external (E), lateral (L), and umbilical (U) lobes; us, umbilicus.
Temporal trends of morphological variation are not observable in this species.
Remarks.—Specimens illustrated under the name of “Damesites sugata” in Ikeda and Wani (2012: fig. 1.1) and Santonian populations previously assigned to “Damesites sugata” by numerous authors should mostly be transferred to Paradamesites rectus gen. et sp. nov.
Paradamesites rectus gen. et sp. nov. (Fig. 6) was described as “Damesites sugata” by Matsumoto (1942: 27, 28) and Matsumoto and Obata (1955: 128), on the basis of specimens from the Yezo Group. However, the FAD is apparently higher than that of the true P. sugata (Forbes, 1846), and these Santonian populations in the Yezo Group can be classified to P. rectus gen. et sp. nov.
“Damesites sugata” from the Yezo Group (Nishimura et al. 2010) is here assigned to a new species of the new genus Paradamesites, P. rectus gen. et sp. nov. So far as we can determine at the moment, this species is endemic to the northwest Pacific province.
Paradamesites rectus gen. et sp. nov. resembles P. sugata in many morphological aspects. Shell surface ornament is almost smooth in both species except for frequent periodic constrictions (Fig. 6). The number of constrictions similarly increases in their late growth stage (Fig. 6A1). Paradamesites rectus gen. et sp. nov. and P. sugata also share relatively compressed whorl shape (B/H ratio is about 0.75 at 50 mm in D; see Fig. 10B and compare Nishimura et al. 2010: fig. 17D) and a small w (about 2.0; see Fig. 10E, and compare Nishimura et al. 2010: fig. 17F). However, P. rectus gen. et sp. nov. has a much wider umbilicus (U/D is about 0.15 in 50 mm in D) than P. sugata (U/D ratio is about 0.09 at 50 mm in D) (Fig. 10D; Nishimura et al. 2010: fig. 17C). The curvature of constriction in P. rectus gen. et sp. nov. is single concave throughout growth (Fig. 6) whereas that of P. sugata changes from single concave to weakly sigmoidal in late growth stage (< 60 mm in D) (Nishimura et al. 2010: fig. 15A).
This species is similar to Tragodesmoceroides subcostatus in having a wide umbilicus in early growth stage (< 10 mm D; Nishimura et al. 2010: fig. 17C). Although relative umbilical width (U/D) changes ontogenetically, P. rectus gen. et sp. nov. possesses a much wider umbilicus, and its U/D ratio considerably increases during growth. In contrast, the U of T. subcostatus remains smaller in middle to late growth stages (Nishimura et al. 2006: fig. 9D; Nishimura et al. 2010: fig. 17C).
Stratigraphic and geographic range.—Santonian–lower Campanian of Hokkaido, Japan, and Sakhalin, Russia.
Discussion
The revised genus Damesites classified to the S-group is endemic to the north Pacific Realm (the northwest, Boreal, and northeast Pacific provinces). This genus is presumed to have originated from Turonian Tragodesmoceroides of S-group that flourished in the northwest Pacific province (e.g., Nishimura et al. 2006). Desmoceratine fauna in the northeast Pacific province (i.e., the specimens from the British Columbia, Canada and California, USA) is similar in species composition to that of the northwest Pacific province (i.e., the specimens from the Yezo Group, Hokkaido and Sakhalin; Fig. 14). In the northeast Pacific province, Damesites damesi occurs from the Coniacian (?) or Santonian to the lower Campanian.
On the other hand, Paradamesites sugata of C-group shows worldwide distribution among Turonian–middle Campanian desmoceratines. It widely occurs in South India, Madagascar, South Africa, Angola, Spain, Brazil, California, Hokkaido, and Sakhalin (Forbes 1846; Stoliczka 1865; Kossmat 1895–1898; Anderson 1958; Collignon 1961, 1983; Beurlen 1969; Kennedy and Henderson 1991; Cooper 2003b; present study). In northeast Pacific province (i.e., British Columbia, Canada and California, USA), P. sugata belonging to the C-group occurs from the Coniacian (= “Kotȏceras subsugatum”) and Coniacian or Santonian (= “Kotȏceras frazierense”).
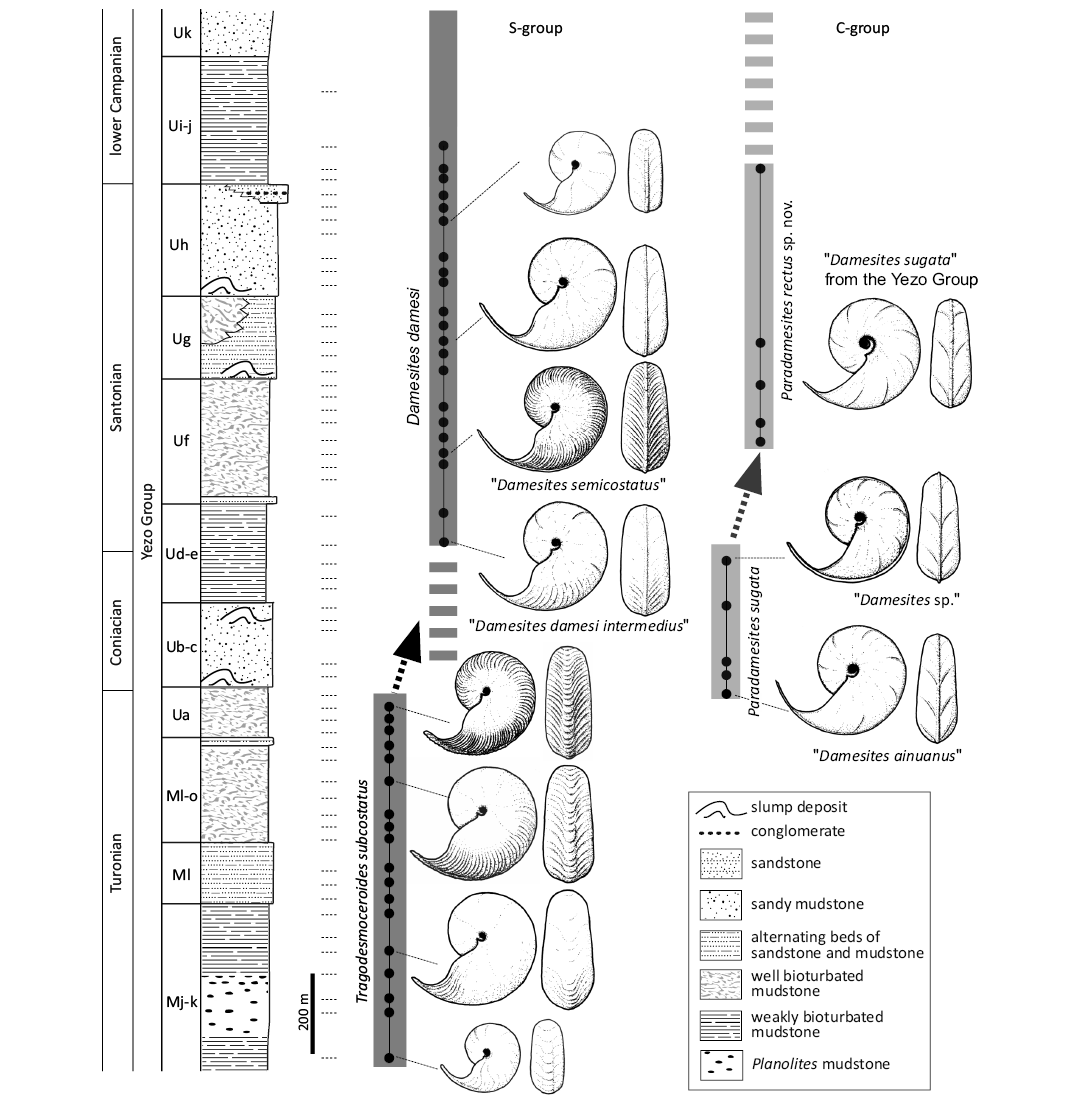
Fig. 14. Revised stratigraphic occurrence, morphological change in single species and phylogenetic relationship of the Turonian–early Campanian desmoceratine ammonoids in the northwest Pacific province. Desmoceratine ammonoids comprise two different lineages (S- and C-groups). Stratigraphic occurrences of Tragodesmoceroides subcostatus are based on Nishimura et al. (2006). The last occurrence of Damesites damesi is confirmed the middle Campanian Sphenoceramus schmidti Inoceramid Zone, Osoushinai Formation, Nakagawa area. This inoceramid zone does not exist in the Haboro-Tappu area and is located in more upper horizon of Uk unit. The origin of C-group Paradamesites spp. is presumed in outside of the northwest Pacific province and Paradamesites sugata migrated in the latest Turonian to the Coniacian. Horizontal dashed lines show localities of molluscan fossil occurrence (see SOM: fig. S4).
The possible occurrence of Paradamesites sugata are also recorded from Romania (Szász 1981). Besides, three other species of Santonian–Campanian C-group; “Damesites compactus (Hoepen, 1921)” from South Africa, and “Damesites tsianalokyensis Collignon, 1961” and “Damesites Rabei Collignon, 1961” from Madagascar are recognized. These “species” belong to Paradamesites and possibly allied to Santonian P. sugata of C-group.
The biogeographic distribution of desmoceratines from the Turonian–early Campanian is shown on Fig. 15. Paradamesites sugata belonging to the C-group is a cosmopolitan species and distributed mainly in African-Indian province. The first appearance of P. sugata in the African-Indian province is slightly earlier than that of the northwest Pacific province. Paradamesites sugata appeared already from the middle–late Turonian of Angola, suggesting its origin to the African-Indian province. On the other hand, Paradamesites sugata is absent from the middle–late Turonian (except the latest Turonian) in northwest Pacific province. Instead, endemic Tragodesmoceroides subcostatus belonging to the S-group is flourished in this interval. Therefore, desmoceratine distribution was clearly vicariant during the Turonian (Fig. 15C). During the latest Turonian to Coniacian, P. sugata migrated northward and expanded the distribution not only in the African-Indian province but also to the north Pacific Realm and European province (Fig. 15B). Populations of P. sugata seemed to be not isolated between the northwest Pacific province and African-Indian province because common evolutionary trends are recognized contemporarily in both bioprovinces; e.g., the keel of P. sugata becomes sharper in shape and stratigraphically distributed from the middle or latest Turonian to upper Coniacian (see systematic description of P. sugata). On the other hand, the endemic lineage (S-group) also survived in the northwest Pacific province but declined in numbers at this interval. Damesites damesi, succeeding Tragodesmoceroides subcostatus, coexisted with P. sugata. Therefore, desmoceratine fauna was composed of a mixture of endemic- and cosmopolitan species in the Coniacian (Fig. 15B).

Fig. 15. Paleobiogeography of desmoceratine ammonoids during the Turonian–early Campanian (A–C) and their presumed migratory patterns. S-group was distributed only in the north Pacific Realm. The C-group species Paradamesites sugata migrated to the north Pacific Realm in the latest Turonian. Unclear occurrence of Damesites damesi in the northwest Pacific and the northeast Pacific province are “Damesites semicostatus” holotype from the Coniacian (?), and “Kotȏceras frazierense” and “Kotȏceras richardsoni” holotypes from the Coniacian or Santonian, respectively. Paradamesites cf. sugata are reported from the upper Turonian–Coniacian of Romania in Pauliuc (1968) and Szász (1981). Late Cretaceous map based on Konishi et al. (2016). Migration patterns are estimated based on Late Cretaceous oceanic circulation as estimated by Hay (2009).
During Santonian–early Campanian, faunal exchange between north Pacific Realm and other provinces declined, and the desmoceratine fauna became isolated again like before the middle Turonian (Fig. 15A). Endemic Damesites damesi of the S-group abundantly occur in the north Pacific Realm, while P. rectus gen. et sp. nov. originated from P. sugata appeared and coexisted with D. damesi. Paradamesites rectus gen. et sp. nov. is native to the northwest Pacific province and shares the common morphological characters of the C-group, i.e., small w, almost smooth shell surface, and frequent development of constrictions in early to late growth stages. These lines of evidence suggest that P. rectus gen. et sp. nov. is a descendant and relict species of cosmopolitan P. sugata. In the same interval, P. sugata still survived in the African-Indian province and European province (Fig. 15A). In addition, several descendants of P. sugata (i.e., Paradamesites compactus [Hoepen, 1921], P. tsianalokyensis [Collignon, 1961] and P. rabei [Collignon, 1961]) of C-group exist in African-Indian province in the Santonian–Campanian (Fig. 15A).
Similar temporal changing patterns of biogeographic distribution are also recognized in several other ammonoids in the Yezo Group. Jagt-Yazykova (2011) reviewed the faunal endemism, provincialism, and turnovers of ammonoids in the northwestern Pacific province in the mid- to Late Cretaceous, mainly on the evidence from the Yezo Group in Sakhalin. She showed that some ammonoid taxa (e.g., Pseudophyllites, Zelandites, Desmophyllites, Hauericeras, Marshalites, Forresteria, Peroniceras, etc.) migrated to the northwest Pacific province, via Tethyan Realm or Arctic province through mid- to Late Cretaceous. Besides this review, similar temporal changing patterns have been reported by the other studies in the Yezo Group. The cosmopolitan Kossmaticeras migrated north from the African-Indian, southwest Pacific, and/or southeast Pacific provinces in the latest Turonian–Coniacian interval, and its endemic descendant Yokoyamaoceras survived during the Santonian–early Campanian in the northwest Pacific province (Maeda 1993). The gabbioceratid ammonoids of European province origin migrated to the northwest Pacific province where they coexisted with the endemic fauna in the late Alban–early Cenomanian age (Shigeta et al. 2012). The Santonian and late Maastrichtian tetragonitid ammonoid Pseudophyllites indra, which had originated in the African-Indian province, extended its distribution to the northwest Pacific province in the late Campanian (Shigeta 1992; Maeda et al. 2005). The Santonian–middle Campanian nostoceratid ammonoid Amapondella amapondense that was distributed in European province, Tethyan Realm, and African-Indian province migrated to the northwest Pacific province in the middle Campanian (Shigeta and Izukura 2022). During a short interval in the Maastrichtian, cosmopolitan fauna comprised of Pachydiscus subcompressus, Gaudryceras seymouriense, and Zelandites varuna migrated to the northwest Pacific province (Kodama et al. 2002; Shigeta and Maeda 2023), where they contrasted strikingly to the endemic ammonoids such as Pachydiscus flexuosus, which were restrictedly distributed in the northwest Pacific province (Maeda et al. 2005). Therefore, knowledge of the repeated northward migration of cosmopolitan species is crucial to our understanding of ammonoid evolution, paleoecology, and paleobiogeography in the northwest Pacific province.
Conclusions
Adopting new key characteristics, such as the shape of growth lines, whorl expansion ratio, and ontogenetic sequence of ornament of shell surface, allowed to propose a new taxonomic scheme of Damesites and allied groups. The lineage with sigmoidal growth lines (S-group) consists of Tragodesmoceroides subcostatus and its descendant true Damesites damesi. It is endemic to the north Pacific Realm. Another lineage with concave growth lines (C-group) is clearly distinguishable from Damesites as Paradamesites gen. nov., consisting of Paradamesites sugata and its descendant Paradamesites rectus gen. et sp. nov. Paradamesites sugata shows the much wider distribution in the African-Indian province. It migrated to the northwest Pacific province during the latest Turonian–Coniacian time, as the endemic S-group decreased in number. Descendants of both groups coexisted in the Santonian–early Campanian time throughout the northwest Pacific province. Besides desmoceratines, similar temporal patterns of ammonoid distribution are often observed in the northwest Pacific province. Thus, repeated northward migration of cosmopolitan species from the African-Indian province is crucial to understand the evolution of Cretaceous ammonoids in the northwest Pacific province.
Acknowledgements
We thank Elena Jagt-Yazykova (University of Opole, Poland) and an anonymous referee for constructive comments that helped us to improve the manuscript. We thank Gengo Tanaka (Kumamoto University, Japan), Terufumi Ohno (Kyoto University Museum, Japan), Yasunari Shigeta (NMNS), Takao Ubukata (Kyoto University, Japan), Kumar Ayyasami (Geological Survey of India, Hyderabad, India), Masataka Izukura (Sapporo, Hokkaido, Japan), Hajime Naruse (Kyoto University, Japan), Shin-ichi Nomura (Chiba prefectural General Education Center, Japan), Taro Kumagae (JAPEX, Indonesia), Takeshi Matsunaga (Osaka Prefectural Semboku Senir High School, Japan), Yasuyuki Tsujino (Tokushima Prefectural Museum, Japan), Yoshinori Hikida (Nakagawa Museum of Natural History, Hokkaido, Japan), Kyo Tanoue (Fukuoka University, Japan), Koji Hasegawa (Mikasa, Hokkaido, Japan), Sho’ichi Matsuda (Iwamizawa, Hokkaido, Japan), Harunobu Yamato (Sapporo, Hokkaido, Japan), Ryoichi Sato (Chitose City, Hokkaido, Japan), Tomoki Karasawa (Mikasa City Museum, Hokkaido, Japan), Sho Iwade (Kaijo Junior and Senior High School, Tokyo, Japan), Tamiko Nasu (Kyoto University, Japan), Fumko Mirakami (Haboro, Hokkaido, Japan), the late Kazuo Kobayashi (1935–2018), and Nobuko Kobayashi (Mikasa, Hokkaido, Japan) for their kind assistance during this study. We are also grateful to Takenori Sasaki (University Museum of the University of Tokyo, Japan), Hiroyoshi Sano (Kyushu University, Fukuoka, Japan), Simone Wells (Natural Historical Museum, London, UK), Jean DeMouthe (California Academy of Science, San Francisco, USA), and Paritosh Bhowmick (Geological Survey of India, Kolkata, India) for the access to the type and museum specimens. This study was supported by the Sasakawa Scientific Grant from the Japan Society (no. 22-234) to TN.
Editor: Andrzej Kaim.
References
Anderson, F.M. 1902. Cretaceous deposits of the Pacific Coast, Proceedings of the California Academy of Science, Third Series, Geology 2: 1–131.
Anderson, F.M. 1958. Upper Cretaceous of the Pacific Coast. Geological Society of America, Special Paper 16: 1–339. Crossref
Alabushev, A. and Wiedmann, J. 1993. On the ontogeny of Damesites Matsumoto (Ammonoidea, Upper Cretaceous). Neues Jahrbuch für Geologie und Paläontologie Monatshefte 1993 (9): 553–560. Crossref
Arkell, W.J., Furnish, W.M., Kummel, B., Miller, A.K., Moore, R.C., Schindewolf, O.H., Sylvester-Bradley, P.C., and Wright, C.W. 1957. Treatise on Invertebrate Paleontology, Part L Mollusca 4, Cephalopoda, Ammonoidea, L1–L490. Geological Society of America and University of Kansas Press, Lawrence, Kansas.
Basse, E. 1931. Monographie paléontologique du Crétacé de la Province de Maintirano (Madagascar). Mémoires géologiques du Service des Mines 1931: 1–86.
Beurlen, G. 1969. Uma nova Fauna de amonóides da Formação Sapucari / Laranjeiras (Cretáceo de Sergipe) – Considerações sôbre sua bioestratigrafia. Boletim Técnico Petrobras 12: 147–169.
Chiplonkar, G.W., Ghare, M.A., and Oka, S.M. 1985. Desmocerated ammonoids from Upper Cretaceous rock formation of South India. In: A.K. Bhattacharya, M.S. Srinivasan, R.K. Lal, and V.K. Gairola (eds.), Proceedings of Indian Geological IVth Session Congress, Varanasi, 1982. Current Trends in Geology 7–8: 27–48.
Collignon, M. 1961. Ammonites néocrétacées du Menabe (Madagascar). VII, Les Desmoceratidae. Annales Géologique du Service des Mines (de Madagascar) 31: 1–115.
Collignon, M. 1965. Atlas des fossiles caractéristiques de Madagascar (Ammonites), XIII (Coniacien). xii + 88 pp. Service Géologique, Tananarive.
Collignon, M. 1983. Les faunas d’ammonites du Santonien. Documents des Laboratoires de l’Université Lyon, hors série 6: 97–174.
Cooper, M.R. 2003a. Upper Cretaceous (Turonian–Coniacian) ammonites from São Nicolau, Angola. Annals of the South African Museum 110: 89–146.
Cooper, M. R. 2003b. Stratigraphy and Palaeontology of the Upper Cretaceous (Santonian) Baba Formation at São Nicolau, Angola. Annals of the South African Museum 110: 147–170.
Cooper, M.R. and Greyling, E.H. 1996. Stratigraphy and palaeontology of a temporary exposure of the Mzamba Formation (Upper Cretaceous, Lower Campanian) in the Eastern Cape, South Africa. Durban Museum Novitates 21: 11–24.
Forbes, E. 1846. Report on the fossil invertebrates from Southern India, collected by Mr. Kaye and Mr. Cunliffe. Transactions of the Geological Society of London, Second series 7: 97–174. Crossref
Futakami, M., Tanabe, K., and Obata, I. 1980. Characteristics of the megafossil assemblages in the Upper Turonian (Cretaceous) of the Pombetsu area, Hokkaido [in Japanese, with English abstract]. Bulletin of the Kitakyushu Museum of Natural History 2: 1–13.
Haggart, J.W. 1989. New and revised ammonites from the Upper Cretaceous Nanaimo Group of British Colombia and Washington State. Bulletin of the Geological Survey of Canada 396: 181–221. Crossref
Hay, W.W. 2009. Cretaceous oceans and ocean modeling. In: X. Hu, C, Wang, R.W. Scott, M, Wagreich, and L. Jansa (eds.), Cretaceous Oceanic Red Beds: Stratigraphy, Composition, Origins, and Paleoceanographic and Paleoclimatic Significance. SEPM Special Publication 91: 73–88.
Henderson, R.A., Kennedy, W.J., and Cobban, W.A. 2002. Perspectives of ammonite paleobiology from shell abnormalities in the genus Baculites. Lethaia 35: 215–230. Crossref
Hoepen, E.C.N. van 1921. Cretaceous Cephalopods from Pondoland. Annals of the Transvaal Museum 8: 1–48.
Honda, B., Takahashi, A., and Hirano, H. 2011. Megafossil biostratigraphy of the Upper Cretaceous Yezo Group in the northern Hobetsu area, Hokkaido, Japan [in Japanese, with English abstract]. Journal of the Geological Society of Japan 117: 599–616. Crossref
Howarth, M.K. 1968. A mid-Turonian ammonite fauna from the Moçâmedes desert, Angola. Garcia de Orta 14: 217–228.
Hyatt, A. 1889. Genesis of the Arietidae. Memoirs of the Museum of Comparative Zoology at Harvard College 16: i–xi, 1–238.
Ikeda, Y. and Wani, R. 2012. Different modes of migration among Late Cretaceous ammonoids in north-western Hokkaido, Japan: evidence from the analyses of shell whorls. Journal of Paleontology 86: 605–615. Crossref
Immel, H., Rochow, H. von, and Wiedich, K.F. 1981. Erste Ammoniten-funde aus dem oberen Cenoman und Turon der nördlichen Kalkalpen und ihre paläogeographische Bedeutung. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 1981 (8): 463–474. Crossref
Jagt-Yazykova, E.A. 2011. Palaeobiogeographical and palaeobiological aspect of mid- and Late Cretaceous ammonite evolution and bio-events in the Russian Pacific. Scripta Geologica 14: 15–121.
Jimbo, K. 1894. Beiträge zur Kenntniss der Fauna Kreideformation von Hokkaidō. Paläontologische Abhandlungen 2: 149–194.
Kennedy, W.J. and Gale, A.S. 2020. A lower Coniacian ammonite fauna from the Cauvery Basin Tamil Nadu south-east India. Proceedings of the Geologists’ Association 131: 397–412. Crossref
Kennedy, W.J. and Henderson, R.A. 1991. A note of Ammonites Sugata Forbes, 1846 (Cretaceous Ammonoidea). Neues Jahrbuch für Geologie und Paläontologie. Monatshefte 1991 (8): 470–476. Crossref
Kennedy, W.J. and Klinger, H.C. 2013. Cretaceous faunas from Zululand and Natal, South Africa. The ammonite subfamily Desmoceratinae Zittel, 1895. African Natural History 9: 39–54.
Kodama, K., Maeda, H., Shigeta, Y., Kase, T., and Takeuchi, T. 2002. Integrated biostratigraphy and magnetostratigraphy of the upper Cretaceous System along the River Naiba in southern Sakhalin, Russia [in Japanese, with English abstract]. Journal of the Geological Society of Japan 108 (6): 366–384. Crossref
Konishi, T., Caldwell, M.W., Nishimura, T., Sakurai, K., and Tanoue, K. 2016. A new halisaurine mosasaur (Squamata: Halisaurinae) from Japan: the first record in the western Pacific realm and the first-documented binocular vision in mosasaurs. Journal of Systematic Palaeontology 14: 809–839. Crossref
Kossmat, F. 1895–1898. Untersuchungen üder die Südindische Kreideformation, Beiträge zur Paläontologie Oesterreich-Ungarns 11: 89–152.
Ludvigsen, R. and Beard, G. 1991. West Coast Fossils. A Guide to the Ancient Life of Vancouver Island. 216 pp. Harbour Publishing, British Columbia, Manderia Park.
Maeda, H. 1991. Sheltered preservation: a peculiar mode of ammonite occurrence in the Cretaceous Yezo Group, Hokkaido, north Japan. Lethaia 24: 69–82. Crossref
Maeda, H. 1993. Dimorphism of Late Cretaceous false-puzosiine ammonites, Yokoyamaoceras Wright and Matsumoto, 1954 and Neopuzosia Matsumoto, 1954. Transactions and Proceedings of the Palaeontological Society of Japan, New Series 169: 97–128.
Maeda, H., Shigeta, Y., Fernando, A.G.S., and Okada, H. 2005. Stratigraphy and fossil assemblages of the Upper Cretaceous System in the Makarov area, southern Sakhalin, Russian Far East. In: Y. Shigeta and H. Maeda (eds.), The Cretaceous System in the Makarov Area, Southern Sakhalin, Russian Far East. National Science Museum Monographs 31: 25–120.
Matsuda, M. and Ubukata, T. 1999. Variation of the shell sculpture in a Cretaceous bivalve Sphenoceramus (Inoceramidae) [in Japanese, with English abstract]. Geoscience Reports of Shizuoka University 26: 1–15.
Matsumoto, T. 1938. A biostratigraphic study on the Cretaceous deposits of the Naibuti valley, Southern Karahuto. Proceedings of the Imperial Academy of Japan, Tokyo 14: 190–194. Crossref
Matsumoto [= Matumoto], T. 1942a. A note on the Japanese Cretaceous ammonites belonging to the subfamily Desmoceratinae. Proceedings of the Imperial Academy of Japan 18: 24–29. Crossref
Matsumoto [=Matumoto], T. 1942b. Fundamentals in the Cretaceous stratigraphy of Japan, Part 1. Memoirs of the Faculty of Science, Kyushu Imperial University, Series D 1: 130–280.
Matsumoto, T. 1954. Selected Cretaceous leading ammonites in Hokkaido and Saghalien. In: T. Matsumoto (ed.), Cretaceous System in the Japanese Islands, 242–313. The Japanese Society for the Promotion of Science, Tokyo.
Matsumoto, T. 1957. A Turonian Damesites from Hokkaido, Japan. Transactions and Proceedings of the Palaeontological Society of Japan, New Series 27: 86–88.
Matsumoto, T. 1959a. Upper Cretaceous ammonites of California. Part II. Memoirs of the Faculty of Science, Kyushu University, Series D, Special Volume 1: 172 pp. Crossref
Matsumoto, T. 1959b. Zonation of the Upper Cretaceous in Japan. Memoirs of the Faculty of Science, Kyushu University, Series D 9: 55–93.
Matsumoto, T. and Obata, I. 1955. Some Upper Cretaceous desmoceratids from Hokkaido and Saghalien. Memoirs of the Faculty of Science, Kyushu University, Series D 5: 119–151.
Nagao, T. 1932. Discovery of a Desmoceras-operculum. Proceedings of the Imperial Academy of Tokyo 8: 175–178. Crossref
Nishimura, T. 2003. Cladistic analysis of desmoceratine ammonoids from the Cretaceous System (Albian–Santonian) in Japan [in Japanese, with English abstract]. Bulletin of the Mikasa City Museum 7: 19–26.
Nishimura, T. 2025. Megafossil biostratigraphy of the Upper Cretaceous deposits in the Sanushube River upstream area, Hobetsu, Hokkaido, Japan. Bulletin of the Hobetsu Museum 40: 1–32.
Nishimura, T., Maeda, H., and Shigeta, Y. 2006. Ontogenetic shell development of a Cretaceous desmoceratine ammonoid “Tragodesmoceroides subcostatus” Matsumoto, 1942 from Hokkaido, Japan. Paleontological Research 10: 11–28. Crossref
Nishimura, T., Maeda, H., Tanaka, G., and Ohno, T. 2010. Taxonomic evaluation of various morphologic characters in the Late Cretaceous desmoceratine polyphyletic genus “Damesites” from the Yezo Group, Hokkaido and Sakhalin. Paleontological Research 14: 33–55. Crossref
Obata, I. 1960. Spirale de quelques Ammonites. Memoirs of Faculty of Science, Kyushu University, Series D (Geology) 9: 151–163. Crossref
Obata, I., Futakami, M., Kawashita, Y., and Takahashi T. 1978. Apertural features in some Cretaceous ammonites from Hokkaido. Bulletin of the National Science Museum, Tokyo (Series C) 4, 139–155.
Okamoto, T., Matsunaga, T., and Okada, M. 2003. Restudy of the Upper Cretaceous stratigraphy in the Haboro area, northwestern Hokkaido [in Japanese, with English abstract]. Journal of the Geological Society of Japan 109: 363–382. Crossref
Pauliuc, S. 1968. Studiul geologic al perşanilor centrali cu privire speciala la cretacicul superior. Romȃnia institutul Geologic, Studii Tehnice şi Economice, Seria J (Stratigrafie) 4: 1–131.
Poyarkova, Z.N. (ed.) 1987. Reference section of Cretaceous deposits in Sakhalin (Naiba section). Transactions of Academy of Science of USSR, Leningrad 16: 1–197.
Raup, D.M. 1966. Geometric analysis of shell coiling: general problems. Journal of Paleontology 40: 1178–1190.
Saito, R. and Matsumoto, T. 1956. A new species of Damesites from Cenomanian of Hokkaido, Japan. Transactions and Proceedings of the Palaeontological Society of Japan, New Series 22: 191–194.
Seki, K., Tanabe, K., Landman, N.H., and Jacobs, D.K. 2000. Hydrodynamic analysis of Late Cretaceous desmoceratine ammonites. Revue de Paléobiologie, Genève 8: 141–155.
Sekine, H., Takagi, A., and Hirano, H. 1985. Biostratigraphical study of the Upper Cretaceous of the north-eastern part of the Obira area, Hokkaido [in Japanese, with English abstract]. Fossils 38: 1–15.
Shigeta, Y. 1992. A record of Pseudophyllites indra (Lytoceratina, Tetragonitidae) from the Upper Cretaceous of Hokkaido and Sakhalin. Transactions and Proceedings of the Palaeontological Society of Japan, New Series 166: 1157–1163.
Shigeta, Y. and Izukura, M. 2022. Discovery of the heteromorph ammonoid Amapondella amapodense in the middle Campanian of Hokkaido. Paleontological Research 26: 369–377. Crossref
Shigeta, Y., Futakami, M., and Hoffmann, R. 2012. Two new ammonoid genera of the subfamily Gabbioceratinae from the Upper Albian (Lower Cretaceous) of Hokkaido, Japan. Paleontological Research 16: 208–218. Crossref
Shigeta, Y., Izukura, M., Nishimura, T., and Tsutsumi, Y. 2016. Middle and late Campanian (Late Cretaceous) ammonoids from the Urakawa area, Hokkaido, northern Japan. Paleontological Research 20: 322–366. Crossref
Shigeta, Y., Izukura, M., and Nishimura T. 2019. Campanian (Late Cretaceous) ammonoids and inoceramids from the Ribira area, Hokkaido, northern Japan. National Museum of Nature and Science Monographs 50: 1–139.
Shigeta, Y. and Maeda, H. 2023. Late Maastrichtian (latest Cretaceous) ammonoids from the Naiba area, southern Sakhalin, Russian Far East. Paleontological Research 27: 277–309. Crossref
Spath, L.F. 1921. On Upper Cretaceous ammonoidea from Pondoland. Annals of the Durban Museum 3: 39–57.
Stoliczka, F. 1865. The fossil Cephalopoda of the Cretaceous rocks of southern India. Ammonitidae with revision of the Nautilidae etc. Memoirs of the Geological Survey of India, Palaeontologia Indica, ser. 1–3 7: 107–130.
Summersberger, V.H. 1980. Neue ammoniten aus der sandkalkbank der Hochmoosschichten (Obersanton; Gosau, Österrech). Annalen des naturhistorischen Museums, Wien 83: 275– 283.
Szász, L. 1981. Contributions à l’études ammonites coniaciens de la Roumanie. Institutul de Geologie şi Geofizică, Bucureşti, Memoiriile 30: 93–114.
Tanabe, K., Hirano, H., Matsumoto, T., and Miyata, Y. 1977. Stratigraphy of the Upper Cretaceous deposits in the Obira area, northwestern Hokkaido [in Japanese, with English abstract]. Science Reports of the Department of Geology, Kyushu University 12: 203–214.
Tanabe, K, and Landman, N. H. 2002. Morphological diversity of the jaws of Cretaceous Ammonoidea. Abhandlungen der Geologischen Bundesanstalt 57: 157–165.
Tanabe, K. and Misaki, A. 2023. Upper Cretaceous record of non-belemnitid coleoid jaws from Hokkaido, Japan, and its evolutionary implications. Cretaceous Research 151: 105624. Crossref
Tanabe, K. and Shigeta, Y. 1987. Ontogenetic shell variation and streaming of some Cretaceous ammonites. Transactions and Proceedings of the Palaeontological Society of Japan, New Series 147: 165–179.
Tanabe, K., Landman, N. H., and Kruta, I. 2012. Microstructure and mineralogy of the outer calcareous layer in the lower jaws of Cretaceous Tetragonitoidea and Desmoceratoidea (Ammonoidea). Lethaia 45: 191–199. Crossref
Tanaka, K. 1963. A study of Cretaceous sedimentation in Hokkaido, Japan. Report of the Geological Survey of Japan 197: 1–122.
Toshimitsu, S., Matsumoto, T., Noda, M., Nishida, T., and Maiya, S. 1995. Towards an integrated mega-, micro- and magneto-stratigraphy of the Upper Cretaceous in Japan [in Japanese, with English abstract]. Journal of the Geological Society of Japan 101: 19–29. Crossref
Tsujino, Y. 2009. The Upper Cretaceous Hakobuchi Formation of the Yezo Group in the Kotanbetsu area, Hokkaido, northeast Japan [in Japanese, with English abstract]. Journal of the Geological Society of Japan 115: 122–129. Crossref
Wani, R. and Hirano, H. 2000. Upper Cretaceous biostratigraphy in the Kotanbetsu area, northwestern Hokkaido [in Japanese, with English abstract]. Journal of the Geological Society of Japan 106: 171–188. Crossref
Wright, C.W., Calloman, J.H., and Howarth, M.K. 1996. Treatise on Invertebrate Paleontology. Part L, Mollusca 4 Revised: Vol. 4: Cretaceous Ammonoidea, 362 pp. Geological Society of America and University of Kansas Press, Boulder, Colorado and Lawrence, Kansas. Crossref
Yabe, H. 1904. Cretaceous cephalopoda from the Hokkaido. Part 2. The Journal of the College of Science, Imperial University of Tokyo, Japan 20: 1–45.
Yabe, H. 1927. Cretaceous stratigraphy of the Japanese Islands. Science Reports of the Tohoku Imperial University. 2nd series, Geology 11: 27–100.
Yazykova, E., 2004. Ammonite biozonation and litho-/chronostratigraphy of the Cretaceous in Sakhalin and adjacent territories of Far East Russia. Acta Geologica Polonica 54: 273–312.
Yokoyama, M. 1890. Versteinerungen aus der japanischen Kreide. Palaeontographica 36: 159–202.
Zittel, K.A. von 1884. Cephalopoda. In: K.A. Zittel (ed.). Handbuch der Palaeontologie, Vol. 1, 329–522. Oldenburg, Munich.
Zittel, K.A. von 1895. Grundzüge der Paläontologie (Paläozoologie). 972 pp. Oldenburg, München.
Acta Palaeontol. Pol. 70 (2): 259–283, 2025
https://doi.org/10.4202/app.01207.2024