

A basal elasmosaurid plesiosaur from the upper Albian–lower Cenomanian? Cambridge Greensand
JOSE P. O’GORMAN and ROGER B.J. BENSON
O’Gorman, J.P. and Benson, R.B.J. 2025. A basal elasmosaurid plesiosaur from the upper Albian–lower Cenomanian? Cambridge Greensand. Acta Palaeontologica Polonica 70 (4): 613–628.
An historical indeterminate elasmosaurid specimen CAMSM X50356 (CAMSM = Sedgwick Museum) collected during the 19th century is described. The specimen comes from the Cenomanian Cambridge Greensand although the possibility of an Albian–lower Cenomanian age is discussed. CAMSM X50356 is recovered within the Elasmosauridae. Our phylogenetic analysis indicates that elongated cervical centra with absence of the lateral ridge are the basal condition of elasmosaurids. The lateral ridge is present in almost all other elasmosaurids with the exception of some taxa that have secondarily shortened the cervical centra such as the aristonectines and Nakonanectes bradti. The lack of correlation between cervical elongation and the lateral ridge is thus recorded here for the first time as CAMSM X50356 is the only elasmosaurid with cervical centra longer than high but without the lateral ridge.
Key words: Sauropterygia, Elasmosauridae, Cambridge Greensand, Albian, Cenomanian, Cretaceous.
Jose P.O’Gorman [joseogorman@fcnym.unlp.edu.ar; ORCID: https://orcid.org/0000-0001-9279-6314 ], División Paleontología Vertebrados, Museo de La Plata, Universidad Nacional de La Plata, La Plata, Argentina. CONICET, Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina.
Roger B.J. Benson [rbenson@amnh.org; ORCID: https://orcid.org/0000-0001-8244-6177 ], American Museum of Natural History, New York, New York, USA. Department of Earth Sciences, University of Oxford, Oxford, UK.
Received 25 October 2024, accepted 7 July 2025, published online 30 Octobeber 2025.
Copyright © 2025 J.P. O’Gorman and R.J.B. Benson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Plesiosaurians are a group of diapsid marine reptiles whose biochron extends from the Rhaetian (Late Triassic) to the K/Pg mass extinction event (Gasparini et al. 2003; Vincent et al. 2011; Wintrich et al. 2017). Throughout their long history, plesiosaurians showed substantial diversification in body proportions (O’Keefe 2002; Soul and Benson 2017; Gutarra et al. 2022), while retaining a conservative bauplan based on four-limbed propulsion and a stiff trunk. Several distinct intervals marked important episodes of diversification or turnover for plesiosaurians, including the initial diversification of the group in the earliest Jurassic (Bardet 1994; Benson et al. 2012), the diversification of thalassophonean pliosaurids and cryptoclidian plesiosaurians early in the Middle Jurassic (e.g., Sachs et al. 2023), and the rise of the xenopsarian groups Leptocleidia and Elasmosauridae around the Jurassic/Cretaceous transition (Benson and Druckenmiller 2014).
The transition from the Early to Late Cretaceous, around the Albian–early Turonian marked an important episode of faunal change that substantially altered the composition of marine reptile assemblages during a protracted interval of approximately 14 Ma. This interval saw the decline and final extinction of ichthyosaurs (Fischer et al. 2014; McGowan 1972) and the radiation of mosasaurs, beginning in the Early Cretaceous (Madzia and Cau 2020; Polcyn et al. 2014). It was also important for plesiosaurians, with the diversification of Euelasmosaurida, increases in polycotylid diversity, and the extinction of pliosaurids (Fischer et al. 2018; O’Gorman 2019). Understanding Albian–Cenomanian marine reptile faunas is extremely important in order to understand this process, but so far these are poorly understood compared to younger faunas.
Among the groups of plesiosaurians that radiated around the Albian–Cenomanian are Elasmosauridae (O’Gorman 2019). Elasmosaurids are characterized by an elongated neck and small skull (Benson and Druckenmiller 2014; Serratos et al. 2017; O’Gorman 2019). The cervical centra of elasmosaurids are usually elongate and bear a lateral keel but there are exceptions that lack both centrum elongation and lateral keel (O’Gorman 2016b, 2019; Serratos et al. 2017).
The Albian–early Cenomanian (see Geological setting for age discussion) vertebrate fauna of the Cambridge Greensand of the West Melbury Marly Chalk Formation is among the richest fossil vertebrate assemblages in England (Unwin 2001), and has great potential to shed light on this important interval of biotic turnover. Despite difficulties resulting from the fragmentary nature of the specimens and the complexity of their stratigraphy, revisions of several vertebrate groups, such as pterosaurs (Unwin 2001), turtles (Evers et al. 2019; Joyce 2025), ichthyosaurs (Fischer et al. 2014), and dinosaurs (Barrett and Bonsor 2021), have been undertaken in the past few decades. However a revision of plesiosaurians as a whole has not yet been undertaken.
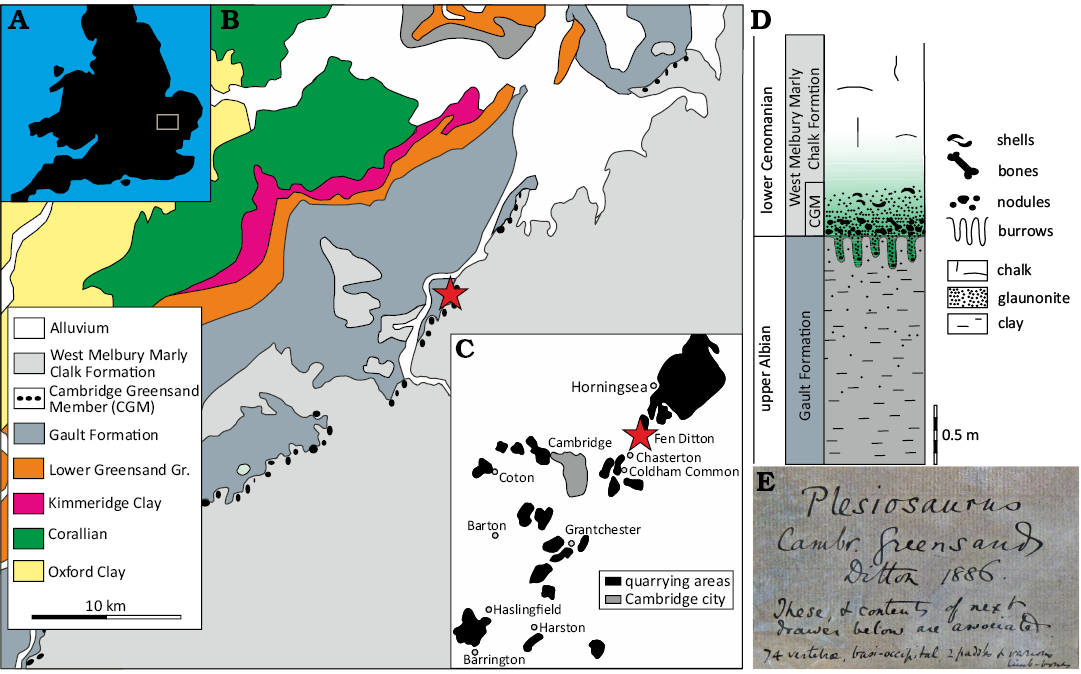
Fig. 1. A. Location of the study area in the UK. B. Geological map of eastern England showing the distribution of the Cambridge Greensand (red star). C. Cambridge Greensand phosphate quarrying areas near Cambridge (adapted from Unwin 2001; Grove 1976; Worssam and Taylor 1969). D. Lithologic and stratigraphic scheme of the Albian/Cenomanian boundary interval in Cambridgeshire (taken from Machalski 2018). E. Original inked label of CAMSM X50356.
Here, we describe CAMSM X50356, which is exceptionally complete compared to other plesiosaurians from the Cambridge Greensand. The specimen was collected and catalogued by Ditton (1886) as is seen in the original label (Fig. 1E).
We evaluate its phylogenetic relationships and discuss the implications for the early diversification of elasmosaurids.
Institutional abbreviations.—CAMSM, Sedgwick Museum, Cambridge, UK; NHMUK, Natural History Museum, London, UK; SCARB, Rotunda Museum, Scarborough, UK.
Other abbreviations.—CGS, Cambridge Greensand; cap, capitulum; cf, coracoid foramen; di, diapophysis; exf, exoccipital facet; opf, opisthotic facet; OTUs, operational taxonomic units; pa, parapophysis; pez, prezygapophysis; pf, pedicellar facet; pf+pa, pedicellar facet + parapophysis; poz, postzygapophysis; vf, ventral foramina; VLI, vertebral length index.
Geological setting
The Cambridge Greensand crops out in the region of Cambridge, extending to north to Soham and south to Harlington (Unwin 2001; Fig. 1A–C). The most representative outcrops of the Cambridge Greensand were exposed during phosphate quarrying in the nineteenth century, but were largely covered after the cessation of quarrying activity (Grove 1976) and are now inaccessible. This circumstance underscores the importance of the collections made at that time, which today constitute a significant part of the paleontological holdings of the Sedgwick Museum. It is also noteworthy that some specimens of the Cambridge Greensand have been recovered from other types of outcrops (Codrington 1864, 1908).
Stratigraphically the Cambridge Greensand is located at the basal level of the Lower Chalk (White 1932; Worssam and Taylor 1969), also called the West Melbury Marly Chalk Formation (Machalski 2018; Fig. 1D). The Cambridge Greensand is a thin unit (thickness between 0.6 m to 1.5 m, Worssam and Taylor 1969) of micaceous, glauconitic, silt marl with a basal lag of reworked phosphatic nodules usually associated with vertebrate fossils and exotic clasts, often encrusted in small oysters and other epibionts (Sedgwick 1846; Seeley 1866). The rich vertebrate fossil assemblage of the Cambridge Greensand may originate mostly as reworked material from the underlying Gault Formation (Reed 1897). Therefore this is a typical fossil “Konzentrat-Lagerstatte” (sensu Seilacher 1970). Regarding the stratigraphic age, the Cambridge Greensand were deposited very early in the Cenomanian (Hart 1973). However, most of the fossils may be reworked from the underlying Gault Clay Formation (Albian) (Unwin 2001; Gallois et al. 2016; Hart and Lyndsey 2020). The presence of some reworked phosphatised Cenomanian ammonites in recent studies indicates that at least some of the reworked fossils are also early Cenomanian in age (Gallois et al. 2016).
Material and methods
Measures.— Linear measurements were taken using a mechanical calliper with an accuracy of 0.1 mm. Both width and height were measured on the posterior articular surface of the vertebral centra. The vertebral indices considered in this contribution are those proposed by Welles (1952), which take into account the ratio between the centrum length length (L) and height (H) (HI = 100 * H/L) and the ratio between centrum width breadth (B) and length (BI = 100 * B/L). The ratio between the centrum breadth and height (BHI = 100 * B/H was also considered. The vertebral length index VLI = 100 * L/(0.5 * (H + B)) proposed by Brown (1981) was also used for comparison.
Taxon sampling and characters.—A phylogenetic analysis was conducted to clarify the evolutionary relationships of CAMSM X50356. This analysis makes use of the data set of Ketchum (2010) modified by Benson and Druckenmiller (2014) and Serratos et al. (2017), modified by rescoring and addition of new OTUs (O’Gorman 2019; O’Gorman et al. 2021; for references see O’Gorman et al. 2023). The resulting matrix includes 290 characters and 120 OTUs, including 33 elasmosaurid OTUs. The complete data set was compiled using Mesquite (Maddison and Maddison 2011) and given as SOM 1 (Supplementary Online Material available at http://app.pan.pl/SOM/app70-OGorman_Benson_SOM.pdf). The percentage of missing data of CAMSM X50356 is 88.3%.
Phylogenetic analysis.—The complete data set was analyzed with the TNT 1.5 software package (Goloboff and Catalano 2016). Only character 155 was considered ordered. Initial tree searches were conducted using new technology searches (1000 random addition sequence and default setting). The resulting trees were then reanalyzed using TBR (tree bisection-reconnection) to generate the full set of most parsimonious trees (MPTs).
To detect OTUs that act as wild card taxa, we used the command pcrprune of TNT.1.5 (Goloboff and Catalano 2016). To test clade robustness, Bremer Support values were calculated for some nodes using TNT.1.5 (Bremer 1994).
Systematic palaeontology
Subclass Sauropterygia Owen, 1860
Order Plesiosauria de Blainville, 1835
Clade Xenopsaria Benson & Druckenmiller, 2014
Family Elasmosauridae Cope, 1869
Elasmosauridae indet.
Figs. 2A–D, 4–8.
Material.—CAMSM X50356, 37 cervical centra, 3 pectoral centra, 20 dorsal centra, 5 sacral centra, 3 caudal centra, isolated neural arches, fragmentary ribs, part of a scapula, one almost complete propodial, and propodial fragments; from locality “Ditton”, probably referring to Fen Ditton, near Cambridge. Probably from the West Melbury Marly Chalk Formation, a unit that was historically referred to as the Cambridge Greensand (Joyce 2025). Most of the vertebrate materials from these levels are reworked from the underlying Gault Clay Formation that is upper Albian (Unwin 2001; Hart and Lyndsey 2020). However the specimen described could also be deposited simultaneously with the Cambridge Greensand matrix, representing the carcitanensis Subzone, at the very base of the Cenomanian (Hart 1973). Therefore the possible stratigraphic range is upper Albian–lower Cenomanian.
Description.—Taphonomic condition of the specimen: CAMSM X50356 is the disarticulated skeleton of an osteologically immature individual (sensu Araújo et al. 2015). Neural arches are detached from the vertebral centra, likely due to incomplete ossification during growth. The centra exhibit possible oyster encrustations (Fig. 2A–D). Some elements display signs of abrasion and fracturing, while the neural arches and girdles are fragmentarily preserved.
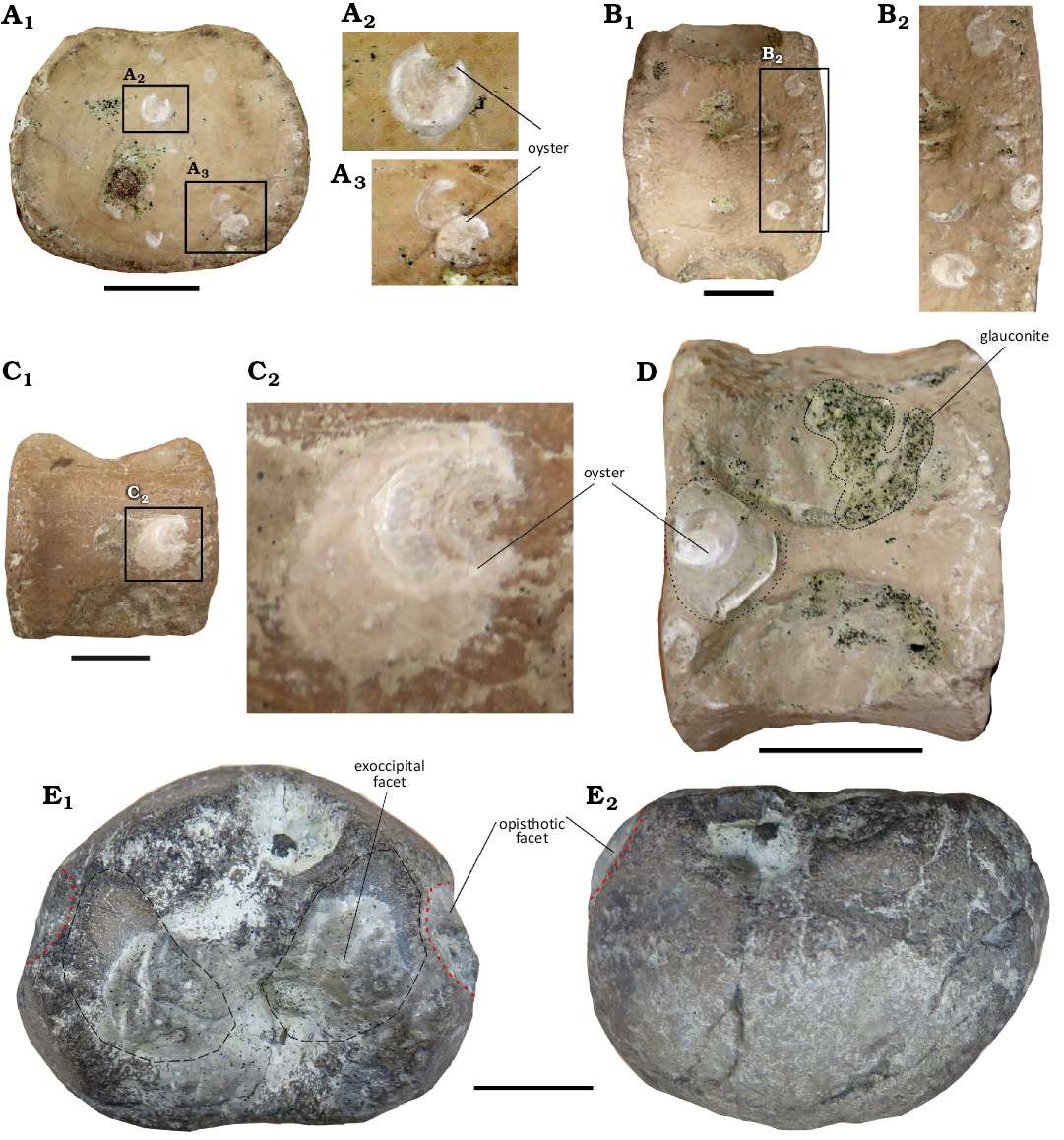
Fig. 2. A–D. Taphonomic features of four cervical centra of CAMSM X50356 (Elasmosauridae indet.) from Fen Ditton, near Cambridge, UK, upper Albian–lower Cenomanian, oyster encrustations. A1, cervical cetrum in posterior view; A2 and A3, details of incrusted oysters; B1, cervical centrum in ventral view; B2, detail of incrusted oyster; C1, cervical centrum in left lateral view; C2, detail of incrusted oyster. D. Cervical centrum in dorsal view. E. Platypterygiinae indet. CAMSM X50356 (Elasmosauridae indet.) from Fen Ditton, near Cambridge, UK, upper Albian–lower Cenomanian, basioccipital in dorsal (E1) and posterior (E2) views. Scale bars 20 mm.
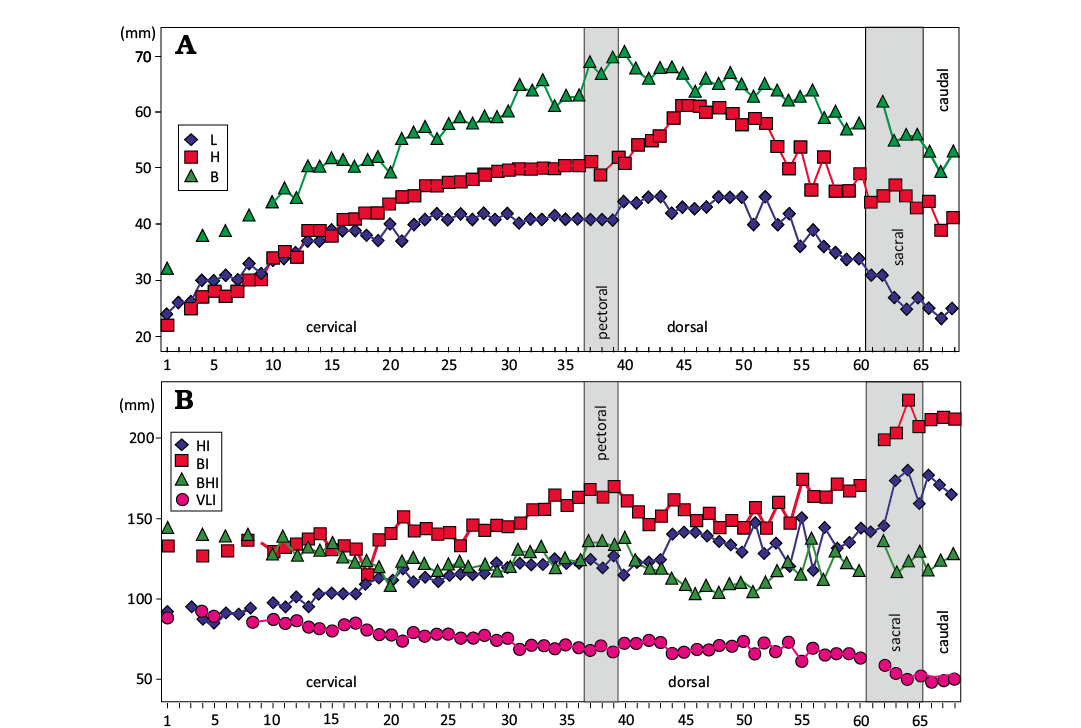
Fig. 3. CAMSM X50356 (Elasmosauridae indet.) from Fen Ditton, near Cambridge, UK, upper Albian–lower Cenomanian, vertebral centra measurements and indexes. A. Values (in mm) of length (L), height (H), and centrum width (B). B. Indexes HI (100*H/L), BI (100*B/L), BHI (100*B/H), VLI [100 *L/(0.5*(H + B))].
Cervical region: The cervical region comprises the centra of thirty-seven preserved vertebrae. The cervical centra are identified by the presence of ventrolateral parapophysys. However, the sequence is clearly not complete as it lacks the atlas, axis, and possibly other individual vertebrae. Therefore the original number of cervical vertebrae was at least thirty-nine, and possibly more. The cervical centra are proportionally wide, being broader mediolaterally than the diameters of their articular surfaces (Fig. 3, Table 1). The articular surfaces are elliptical and slightly concave. The vertebral centra are longer than high in anterior cervicals but higher than long in middle to posterior cervicals. VLI is less than 100 in all cervical centra (Fig. 3B). The pedicellar facets are elongated and strongly concave (Fig. 4). A pair of dorsal foramina is located on the medial limit of each pedicellar facet (Fig. 4A3–D3). The lateral surface lacks any trace of a lateral keel or ridge (Fig. 4A2–D2). The ventrolateral margins of anterior and medial vertebrae show deep parapophyses without any trace of cervical ribs attached. The parapophyses of the most posterior cervical centra are less concave or even slightly laterally projected (Fig. 4A2–D2). The ventral surface of each cervical centrum is pierced by a pair of subcentral foramina with a broad, almost flat surface between them (Fig. 4A4–D4).
Table 1. Measurements (in mm) of vertebrae (c, cervical; ca, caudal; d, dorsal; p, pectoral; s, sacaral) length (L), height (H), breadth (B) and indexes height/length ratio (HI = 100*H/L), breadth/length ratio (BI = 100*B/L), breadth/height ratio (BHI = 100*B/H) and vertebral length index [VLI = 100*L/(0.5*(H + B))]. The order given is relative because the skeleton was found disarticulated.
|
Vertebra |
L |
H |
B |
HI |
BI |
BHI |
VLI |
|
1c |
24 |
22 |
32 |
92 |
133 |
145 |
89 |
|
2c |
26 |
|
|
|
|
|
|
|
3c |
26 |
25 |
|
96 |
|
|
|
|
4c |
30 |
27 |
38 |
90 |
127 |
141 |
92 |
|
5c |
31 |
27 |
|
87 |
|
|
|
|
6c |
30 |
28 |
39 |
93 |
130 |
139 |
90 |
|
7c |
30 |
28 |
|
93 |
|
|
|
|
8c |
31 |
30 |
42 |
97 |
135 |
140 |
86 |
|
9c |
33 |
|
|
|
|
|
|
|
10c |
34 |
34 |
44 |
100 |
129 |
129 |
87 |
|
11c |
35 |
34 |
47 |
97 |
134 |
138 |
86 |
|
12c |
34 |
35 |
45 |
103 |
132 |
129 |
85 |
|
13c |
39 |
38 |
51 |
97 |
131 |
134 |
88 |
|
14c |
37 |
39 |
51 |
105 |
138 |
131 |
82 |
|
15c |
37 |
39 |
52 |
105 |
141 |
133 |
81 |
|
16c |
39 |
41 |
52 |
105 |
133 |
127 |
84 |
|
17c |
39 |
41 |
51 |
105 |
131 |
124 |
85 |
|
18c |
38 |
42 |
52 |
111 |
137 |
124 |
81 |
|
19c |
37 |
43 |
52 |
116 |
141 |
121 |
78 |
|
20c |
40 |
45 |
50 |
113 |
125 |
111 |
84 |
|
21c |
37 |
45 |
56 |
122 |
151 |
124 |
73 |
|
22c |
40 |
45 |
57 |
113 |
143 |
127 |
78 |
|
23c |
41 |
47 |
58 |
115 |
141 |
123 |
78 |
|
24c |
42 |
47 |
56 |
112 |
133 |
119 |
82 |
|
25c |
41 |
48 |
59 |
117 |
144 |
123 |
77 |
|
26c |
41 |
48 |
60 |
117 |
146 |
125 |
76 |
|
27c |
42 |
49 |
59 |
117 |
140 |
120 |
78 |
|
28c |
42 |
49 |
60 |
117 |
143 |
122 |
77 |
|
29c |
41 |
50 |
60 |
122 |
146 |
120 |
75 |
|
30c |
42 |
50 |
61 |
119 |
145 |
122 |
76 |
|
31c |
40 |
50 |
66 |
125 |
165 |
132 |
69 |
|
32c |
41 |
50 |
65 |
122 |
159 |
130 |
71 |
|
33c |
41 |
50 |
67 |
122 |
163 |
134 |
70 |
|
34c |
42 |
51 |
62 |
121 |
148 |
122 |
74 |
|
35c |
41 |
51 |
64 |
124 |
156 |
125 |
71 |
|
36c |
41 |
51 |
64 |
124 |
156 |
125 |
71 |
|
37c |
41 |
51 |
69 |
124 |
168 |
135 |
68 |
|
1p |
41 |
49 |
67 |
120 |
163 |
137 |
71 |
|
2p |
41 |
52 |
70 |
127 |
171 |
135 |
67 |
|
3p |
44 |
51 |
71 |
116 |
161 |
139 |
72 |
|
1d |
44 |
54 |
68 |
123 |
155 |
126 |
72 |
|
2d |
45 |
55 |
66 |
122 |
147 |
120 |
74 |
|
3d |
45 |
56 |
68 |
124 |
151 |
121 |
73 |
|
4d |
42 |
59 |
68 |
140 |
162 |
115 |
66 |
|
5d |
43 |
61 |
67 |
142 |
156 |
110 |
67 |
|
6d |
43 |
61 |
64 |
142 |
149 |
105 |
69 |
|
7d |
43 |
60 |
66 |
140 |
153 |
110 |
68 |
|
8d |
45 |
61 |
65 |
136 |
144 |
107 |
71 |
|
9d |
45 |
60 |
67 |
133 |
149 |
112 |
71 |
|
Vertebra |
L |
H |
B |
HI |
BI |
BHI |
VLI |
|
10d |
45 |
58 |
65 |
129 |
144 |
112 |
73 |
|
11d |
40 |
59 |
63 |
148 |
158 |
107 |
66 |
|
12d |
45 |
58 |
65 |
129 |
144 |
112 |
73 |
|
13d |
40 |
54 |
64 |
135 |
160 |
119 |
68 |
|
14d |
42 |
50 |
62 |
119 |
148 |
124 |
75 |
|
15d |
36 |
54 |
63 |
150 |
175 |
117 |
62 |
|
16d |
39 |
46 |
64 |
118 |
164 |
139 |
71 |
|
17d |
36 |
52 |
59 |
144 |
164 |
113 |
65 |
|
18d |
35 |
46 |
60 |
131 |
171 |
130 |
66 |
|
19d |
34 |
46 |
57 |
135 |
168 |
124 |
66 |
|
20d |
34 |
49 |
58 |
144 |
171 |
118 |
64 |
|
1s |
31 |
44 |
|
142 |
|
|
|
|
2s |
31 |
45 |
62 |
145 |
200 |
138 |
58 |
|
3s |
27 |
47 |
55 |
174 |
204 |
117 |
53 |
|
4s |
25 |
45 |
56 |
180 |
224 |
124 |
50 |
|
5s |
27 |
43 |
56 |
159 |
207 |
130 |
55 |
|
1ca |
25 |
44 |
53 |
176 |
212 |
120 |
52 |
|
2ca |
23 |
39 |
49 |
170 |
213 |
126 |
52 |
|
3ca |
25 |
41 |
53 |
164 |
212 |
129 |
53 |

Fig. 4. CAMSM X50356 (Elasmosauridae indet.) from Fen Ditton, near Cambridge, UK, upper Albian–lower Cenomanian, anterior and middle cervical centra. A. 10th cervical centrum in posterior (A1), right lateral (A2), dorsal (A3), and ventral (A4) views. B. 13th cervical centrum in posterior (B1), right lateral (B2), dorsal (B3), and ventral (B4) views. C. 23rd cervical centrum in posterior (C1), right lateral (C2), dorsal (C3), and ventral (C4) views. D. 30th cervical centrum in posterior (D1), right lateral (D2), dorsal (D3), and ventral (D4) views. Scale bars 20 mm.
The neural arches are not well preserved but some features are evident. The prezygapophyses are mediolaterally narrow compared to the centrum width. They have slightly curving articular surfaces that are inclined to face dorsomedially, and are fused in the midline, as are the postzygapophyses. A low median ridge is present between the prezygapophyseal facets (Fig. 5A2) that fits into a median sulcus between the postzygapophyses (Fig. 5A3).
Pectoral region: Three pectoral centra are preserved, identified based on the confluence of pedicellar and parapophyseal facets (Welles 1943). The proportions of the pectoral centra are similar to those of posterior cervical centra (Fig. 3). However, the ventral surfaces of pectoral centra are more convex and bear a pair of additional, smaller foramina (Fig. 6A3).

Fig. 5. CAMSM X50356 (Elasmosauridae indet.) from Fen Ditton, near Cambridge, UK, upper Albian–lower Cenomanian. Neural arches. A. Posterior cervical neural arches in left lateral (A1) and anterior (A2) views. B. Caudal? neural arch in anterior view. C. Caudal neural arches in posterior (C1) and left lateral (C2) views. D, E. Caudal neural arches in left alterl view; dash line indicate suposed morphology. F, G. Diapophyses. H. Proximal end of dorsal rib. Scale bars 20 mm.
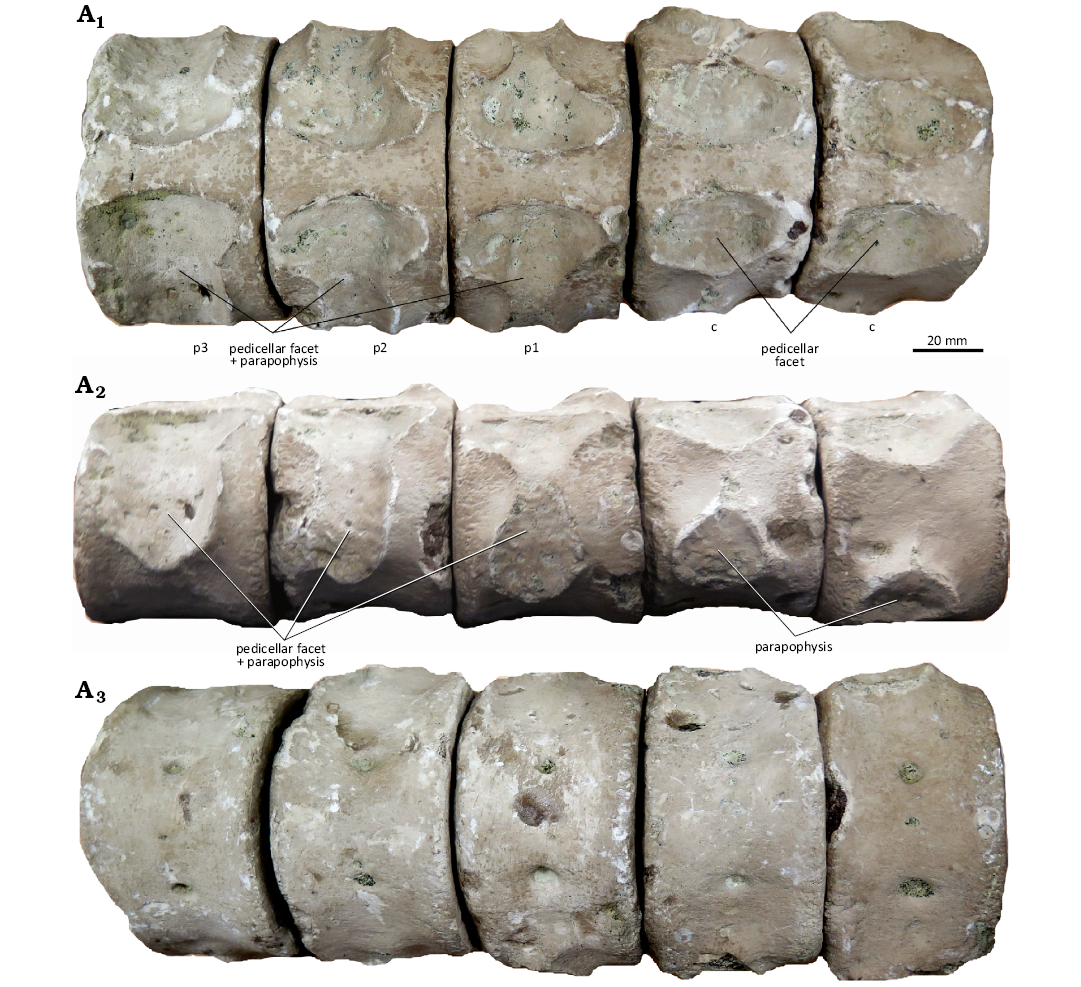
Fig. 6. CAMSM X50356 (Elasmosauridae indet.) from Fen Ditton, near Cambridge, UK, upper Albian–lower Cenomanian, two posteriormost cervical and the three pectoral centra in dorsal (A1), right lateral (A2), and ventral (A3) views. Abbreviations: c, cervical; p1, p2, p3, pectoral 1, 2, and 3.
Dorsal region: Twenty dorsal centra are preserved, identified based on the absence of parapophysis (Welles 1943). The pedicellar facets are deeply concave and elliptical in shape (Fig. 7). The dorsal centra are wider mediolaterally than high dorsoventrally, and are higher dorsoventrally than long anteroposteriorly. Anterior middle dorsal centra are larger than other vertebrae (Fig. 3). The lateral surfaces of dorsal centra are anteroposteriorly concave (Fig. 7). The ventrolateral and ventral surfaces are pierced by 2–4 foramina (Fig. 7A3). The diapophyses show a rounded cross section (Fig. 5F, G).
Sacral region: The sacral region comprises five vertebrae (Fig. 8A2–A4) identified by the presence of laterally projected transverse processes (Welles 1943). Their articular surfaces are more depressed than those of the dorsal and caudal vertebrae (Fig. 8A1). The ventral part of the rib facet is anteroposteriorly short and dorsoventrally elongated (Fig. 8A2).
Caudal region: Two caudal centra are preserved, identified by lateral parapophysis and haemal facets (Welles 1943). The caudal centra are broader than high and higher than long (Figs. 3A, 8B). The parapophyses are lateral in position and circular in shape. Ventrally there is one single foramen and low haemal facets are located both anteroventrally and posteroventrally (Fig. 8B3).
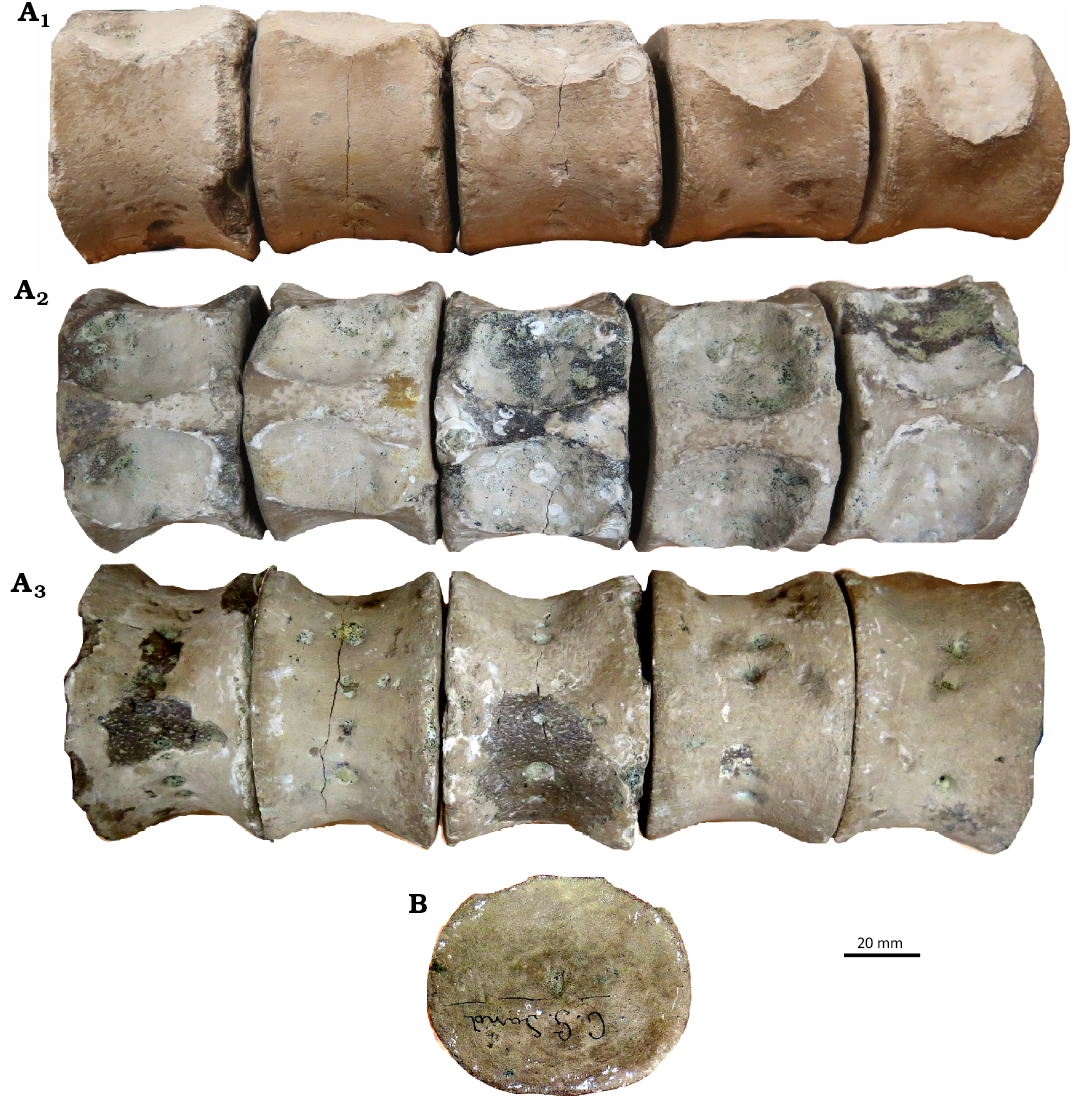
Fig. 7. CAMSM X50356 (Elasmosauridae indet.) from Fen Ditton, near Cambridge, UK, upper Albian–lower Cenomanian. A. 41–45th dorsal centra in right lateral (A1), dorsal (A2), and ventral (A3) views. B. Dorsal centrun in anterior view.

Fig. 8. CAMSM X50356 (Elasmosauridae indet.) from Fen Ditton, near Cambridge, UK, upper Albian–lower Cenomanian. A. Sacral centra in posterior (A1), right lateral (A2), ventral (A3), and dorsal (A4) views. B. Caudal centra in posterior (B1), left lateral (B2), ventral (B3), and dorsal (B4) views. Scale bars 20 mm.
Scapula: Only the glenoid ramus of the left scapula is preserved that shows the margin elevated and progressively displaced relative to the plane determined by the main axes of the coracoids and glenoid facet. This portion is interpreted as the proximal part of the dorsolateral process. The glenoid facet is as long as the coracoid facet in dorsal view (Fig. 9A).
Propodium: Two propodials are preserved. One is represented only by the proximal end (Fig. 9B) and the other preserves most of the element (Fig. 9C), with substantial damage. Due to the state of preservation, it is not possible to determine whether these propodials are humeri or femora. In both elements the fracture shows an internal structure that follows the limit between perichondral and endochondral ossification, a feature associated with specimens that have not achieved full osteological maturity as the absence of secondary remodelling has not affected the perichondral–endochondral boundary (O’Gorman et al. 2014; Fig. 9C3). The more complete of the two propodials provides more complete evidence of morphology. It is 205 mm in length and has more than 102 mm of distal expansion (complete distal expansion can not be measured due to incompleteness) (Fig. 9C). This is similar to the propodial proportions of many other elasmosaurids. The tuberosity/trochanter is not well developed but it is visible as a small lip in the ventral surface of the propodial (Fig. 9C2).
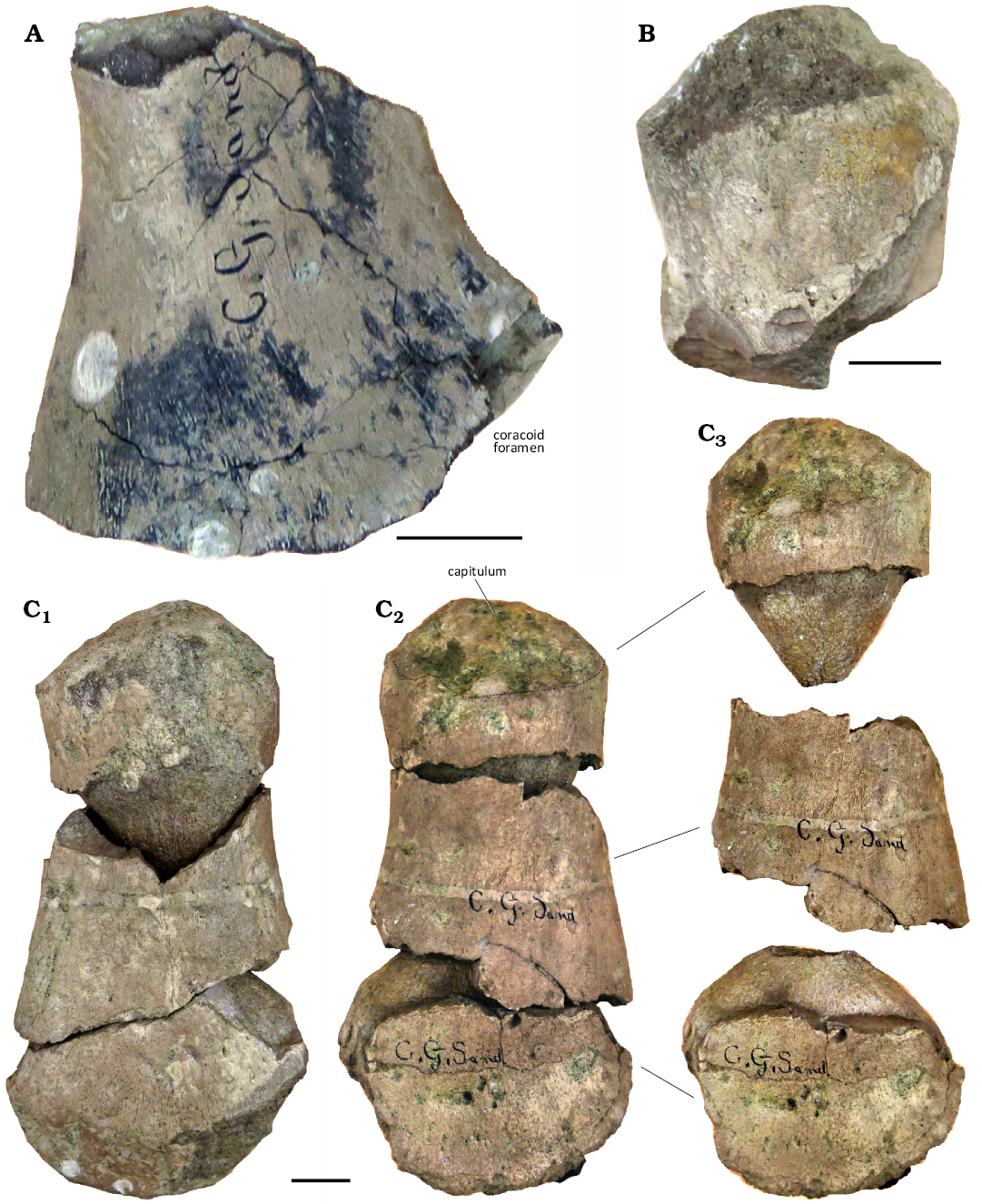
Fig. 9. CAMSM X50356 (Elasmosauridae indet.) from Fen Ditton, near Cambridge, UK, upper Albian–lower Cenomanian. A. Proximal half of left scapula. B. Part of propodium. C. Propodium in ventral (C1) and dorsal (C2) views; propodium separated in three sectors (C3), showing the separation between perichondral and endochondral ossification. Scale bars 20 mm.
Remarks.—The only element with the original elements that clearly does not belong to a plesiosaurian is a basioccipital bone, listed on the original label (Fig. 1E). The basioccipital differs from the morphology observed in elasmosaurids (Sato 2003; Zverkov et al. 2017; O’Gorman 2019, 2021) consistent with that of a platypterygiine ichthyosaur, based on the extremely reduced extracondylar area (Fig. 2E), which is not visible ventrally in posterior view (Fischer et al. 2012).
Phylogenetic results
Our initial tree search using new technologies recovered 50 MPTs of 1942 steps. After TBR branch swapping, a much larger set of 20 000 MPTs of 1942 steps was recovered. Three OTUs were recovered as wild card taxa Wapuskanectes betsynicholsae, Fluvionectes sloanae, and Alexandronectes zealandiensis. After pruning them from the consensus the CAMSM X50356 (“Cambridge Elasmosauridae”) is recovered as the basalmost member of Elasmosauridae (Fig. 10).
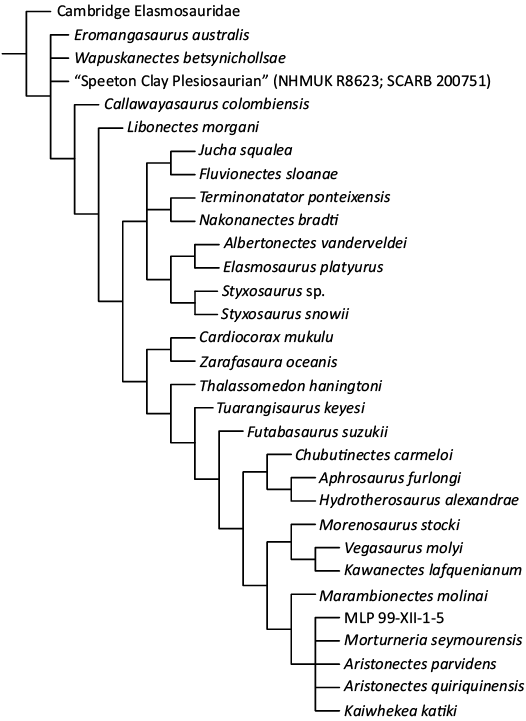
Fig. 10. Reduced consensus tree derived from 20 000 MPTs of length 1942 steps, after pruning Wapuskanectes betsynicholsae; Fluvionectes sloanae, and Alexandronectes zealandiensis. We show relationships within Elasmosauridae only, excluding other groups of plesiosaurs from this diagram. Bremer support below some nodes.
Discussion
The nature of CAMSM X50356.—It is very likely that most of the material accessioned as CAMSM X50356 belongs to a single individual (with the clear exception of the ichthyosaur basioccipital; Fig. 2E). Because no record of the original state of CAMSM X50356 is available the only evidence for this is indirect. However: (i) all elements of CAMSM X50356 described here are stored together, and are listed on the original specimen label, suggesting they represent a single episode of collection or acquisition (Fig. 1E); (ii) all vertebral elements have consistent and gradational morphology, and all elements bear features consistent with placement as an early-diverging elasmosaurid; (iii) all elements are consistent in size (Fig. 3); (iv) the vertebral sequence shows no anomalies in relative size; (v) all material belongs to an osteologically immature specimen, including vertebrae and propodials; (vi) there are no duplicated elements; and (vii) the preservation is consistent among all elements, showing abrasion and oyster encrustations. The only element that clearly does not belong to a plesiosaur is the basioccipital bone, listed on the original label (Fig. 1E) but more consisntent with the one of platypterygiine ichthyosaur (see above, Fischer et al. 2012).
Although most other vertebrate fossils from the Cambridge Greensand are isolated elements, associated remains were also found. For example, Seeley (1879: 592) reported seeing associated remains of plesiosaur and ichthyosaurs: “Then, in the cases of some Plesiosaurs and Ichthyosaurs, I have been present at the workings when associated portions of skeletons have been found, so that I can state from my own knowledge that naturally associated portions of single animals are met with; and often we have had to wait for months for the neck of an animal of which the body has been found, until the overlying rock was removed so that the bones could be collected”.
Therefore CAMSM X50356 is considered part of a single specimen. It is currently not clear how complete the vertebral sequence is, but the gradational nature of our measurements suggests that there are not large series of missing elements (Fig. 3A).
Phylogenetic analysis and comparison.—Our phylogenetic analysis recovered CAMSM X50356 in Elasmosauridae (sensu Benson and Druckenmiller 2014). Therefore detailed comparisons are made with members of this clade. The phylogeny of elasmosaurids have much attention recently (Vincent et al. 2011; O’Gorman et al. 2015; Serratos et al. 2017; Sachs et al. 2018; O’Gorman 2019; Fischer et al. 2021). The general results, the monophyly of Elasmosauridae and the recovering of Aristonectinae after removing the wild card taxa are shared by previous studies (O’Gorman et al. 2015; Serratos et al. 2017; O’Gorman 2019; Sachs et al. 2021).
The record of Albian–Cenomanian elasmosaurids is quite scarce and usually comprises isolated vertebrae (Bardet et al. 2016; Latil et al. 2021). However, a partially preserved elasmosaurids was collected from the Albian of the Marnes Bleues Formation, France. The specimen differs from CAMSM X50356 in having lateral keel and dorsal projections on dorsal surface of neural spines (Vincent et al. 2020).
First, CAMSM X50356 differs from the all the post Cenomanian elasmosaurids with the exception of Zarafasaura oceanis Vincent et al., 2011, and Traskasaura sandrae O’Keefe et al., 2025 (O’Gorman 2019) by lacking the ventral notch on articular surfaces of the cervical centra (Welles 1943, 1962; Cruickshank and Fordyce 2002; Sato 2003; Sato et al. 2006; O’Gorman et al. 2015; O’Gorman 2016a, b, 2019; Sachs and Kear 2017; Serratos et al. 2017; Otero et al. 2014). Additionally, CAMSM X50356 differs from the basal elasmosaurids such as the “Speeton Clay Plesiosaurian” from the Hauterivian of England, Jucha squalea Fischer et al., 2021, Eromangasaurus australis (Sachs, 2005b) Kear, 2007, and Callawayasaurus colombiensis Welles, 1962, by lacking the lateral keel on cervical centra (Welles 1962; Kear 2007; Benson and Druckenmiller 2014; Fischer et al. 2021). The lack of lateral keels on CAMSM X50356 is interpreted here as not related to the immature condition, as other immature elasmosaurids show well developed lateral keels (O’Gorman et al. 2018).
The cervical centra of CAMSM X50356 are relatively short compared to many elasmosaurids (Sato 2003; Sachs, 2005a; O’Keefe and Hiller 2006) , being only slightly longer than high, as long as high, or higher than long, depending on the location of the cervical region (Fig. 3). However the cervical centra of CAMSM X50356 are not markedly short along the neck as in Zarafasaura oceanis, the elasmosaurinae Nakonanectes bradti Serratos et al., 2017, and aristonectines (Lomax and Wahl 2013; Serratos et al. 2017; Otero et al. 2018; O’Gorman 2019). Additionally, because the specimen is not mature it is probable that the cervicals would have undergone at least a slight additional elongation during life. Therefore, it could be supposed that some cervicals of this specimen should become slightly more elongated in its adult form.
The minimum number of dorsal vertebrae of CAMSM X50356 is twenty, a dorsal vertebral count lower than those of Callawayasaurus colombiensis (23 dorsal vertebrae) and Zarafasaura oceanis (26 dorsal vertebrae). However, it is likely that there are some missing elements in CAMSM X50356, so the original number is not known but it was possibly more than 20. This is greater than the dorsal vertebral counts recorded for Kawanectes lafquenianum (Gasparini & Goñi, 1985) O’Gorman, 2016a (15 dorsal vertebrae), Morenosaurus stocki Welles, 1943 (17 dorsal vertebrae), Vegasaurus molyi O’Gorman et al., 2015 (17 dorsal vertebrae), and Albertonectes vanderveldei Kubo et al., 2012 (16 dorsal vertebrae) (Welles 1943; Kubo et al. 2012; O’Gorman et al. 2015; O’Gorman 2016a).
CAMSM X50356 shows 5 sacral centra differing from Zarafasaura oceanis, which has three sacral centra (Lomax and Wahl 2013) and more similar to the sacral vertebral count of the elasmosaurines Elasmosaurus platyurus Cope, 1869 (4); Terminonatator ponteixensis Sato, 2003 (4/+4); Albertonectes vanderveldei (5) and 5 sacral vertebrae in Fluvionectes sloanae Campbell et al., 2021 (Sato 2003; Sachs 2005a; Kubo et al. 2012; Campbell et al. 2021)
The caudal vertebrae of CAMSM X50356 bear a single ventral foramen, similar to many leptocleidians, and some elasmosaurids including Hydrotherosaurus alexandrae Welles, 1943, Elasmosaurus platyurus, Kawanectes lafquenianum, and Vegasaurus molyi (Welles 1943; O’Gorman et al. 2015; O’Gorman 2016a). This differs from the paired or multiple foramina present in many other plesiosaurians, including elasmosaurids such as Aristonectes spp. (Otero et al. 2018). Additionally it lacks a laterally projected parapophysis present in Kawanectes lafquenianum and Nakonanectes bradti (O’Gorman 2016a, b; Serratos et al. 2017; Otero et al. 2018).
Finally, CAMSM X50356 shows a glenoid facet of the scapula as long as the coracoid facet in dorsal view (Fig 9A), which differs from Wapuskanectes betsynichollsae Druckenmiller & Russell, 2006, in which the glenoid facet is shorter than the coracoid facet (Druckenmiller and Russell 2006: fig. 4).
Nomenclatural background.—Previously, material of the Cambridge Greensand with features similar to CAMSM X50356 has been referred to as “Plesiosaurus planus” (Owen, 1864), “Plesiosaurus euryspondilus” (Seeley, 1869), and “Stereosaurus platyomus” (Seeley, 1869), however, both are currently considered to be nomina dubia (Welles 1962). For details of collections numbers see of all the specimens mentioned see Seeley (1869).
It is interesting that Seeley (1869: xv), stated: “These names are only intended for the convenience of students using the Museum, and not necessarily to take rank as names of described species”. So, it is not clear if this were intended to be erected as formal species names.
Particularly interesting is that “Stereosaurus platyomus” is characterized by Seeley as having propodials without the trochanter developed (Seeley 1869); a feature that is now known to be usually present in juvenile specimens and that can be modified through ontogeny. (Brown 1981; Otero et al. 2014). We examined specimens at CAMSM that were historically labelled under these two names, specifically those stored at the holotype collection: CAMSM B56749, B56751, B56754, B56762, “Plesiosaurus planus” (Owen, 1864); CAMSM B56768–83, “Plesiosaurus euryspondilus” (Seeley, 1869); and CAMSM B57027–32, B57033–37, B27038–41, B57042–44, “Stereosaurus platyomus” (Seeley, 1869).
The morphology of these specimens is consistent with the general morphology of CAMSM X50356, including cervical vertebrae with centra as long as high or slightly longer, with flat ventral surfaces and absence of both the lateral ridge and ventral notch. However, we do not consider these specimens or CAMSM X50356 to have diagnostic features at the species level. Hence, the early-diverging elasmosaurid species represented by CAMSM X50356 and other specimens remains unnamed here.
Phylogeny and cervical features.—CAMSM X50356 is recovered as the basalmost elasmosaurid by our phylogenetic analysis. This position indicates that elongated cervical centra with absence of lateral ridge are the basal condition of elasmosaurids, consistent with the widespread occurrence of those states in other plesiosaurian groups. Previously the lateral ridge has been recorded in almost all other elasmosaurids (Sato 2003; Hiller et al. 2017; Serratos et al. 2017; O’Gorman 2019; Fischer et al. 2021) with the exception of taxa that have secondarily shortened the cervical centra such as Aristonectes parvidens Cabrera, 1941, Wunyelfia maulensis Otero & Soto Acuña, 2021, and Nakonanectes bradti Serratos et al., 2017 (O’Gorman 2016; Serratos et al. 2017; Otero and Soto-Acuña 2021). Therefore the lack of correlation between cervical elongation (present in CAMSM X50356) and the lateral ridge (absent in CAMSM X50356) is thus recorded here for the first time as CAMSM X50356 is the only elasmosaurid with cervical central longer than high but without lateral ridges, and its recovered phylogenetic position indicates this is the basal condition among elasmosaurids.
Acknowledgements
The authors thank to the curator Matt Riley (Sedgwick Museum, Cambridge, UK) for allowing the study of the specimens under their care. The authors thank Peggy Vincent (Muséum National d’Histoire Naturelle) and Lene Delsett (Natural history museum, University of Oslo). This contribution was supported by PICT (Proyecto de Investigación Científica y Tecnológica) 2015-0678 and PICT-2018-02443.
Editor: Daniel Barta
References
Araújo, R., Polcyn, M.J., Lindgren, J., Jacobs, L.L., Schulp, A.S., Mateus, O., Olimpio Gonçalves, A., and Morais, M.-L. 2015. New aristonectine elasmosaurid plesiosaur specimens from the Early Maastrichtian of Angola and comments on paedomorphism in plesiosaurs. Netherlands Journal of Geosciences 94: 1: 93–108. Crossref
Bardet, N. 1994. Extinction events among Mesozoic marine reptiles. Historical Biology 7: 313–324. Crossref
Bardet, N., Fischer, V., and Machalski, M. 2016. Large predatory marine reptiles from the Albian–Cenomanian of Annopol, Poland. Geological Magazine 153: 1–16. Crossref
Barrett, P.M. and Bonsor, J.A. 2021. A revision of the non-avian dinosaurs ‘Eucercosaurus tanyspondylus’ and ‘Syngonosaurus macrocercus’ from the Cambridge Greensand, UK. Cretaceous Research 118: 104638. Crossref
Benson, R.B.J. and Druckenmiller, P.S. 2014. Faunal turnover of marine tetrapods during the Jurassic–Cretaceous transition. Biological Reviews 89: 1–23. Crossref
Benson, R.B.J., Evans, M., and Druckenmiller, P.S. 2012. High diversity, low disparity and small body size in plesiosaurs (Reptilia, Sauropterygia) from the Triassic–Jurassic boundary. PLoS ONE 7 (3): e31838. Crossref
Benson, R.B.J., Evans, M., Smith, A.S., Sassoon, J., Moore-Faye, S., Ketchum, H.F., and Forrest, R. 2013. A giant pliosaurid skull from the Late Jurassic of England. PLOS ONE 8: e65989. Crossref
Bremer, K. 1994. Branch support and tree stability. Cladistics 10: 295–304. Crossref
Brown, D.S. 1981. The English Upper Jurassic Plesiosauroidea (Reptilia) and a review of the phylogeny and classification of the Plesiosauria. Bulletin of British Museum of Natural History (Geology) 35: 253–347.
Cabrera, A. 1941. Un plesiosaurio nuevo del Cretaceo del Chubut. Revista del Museode La Plata 2: 113–130.
Campbell, J.A., Mitchell, M.T., Ryan, M.J., and Anderson, J.S. 2021. A new elasmosaurid (Sauropterygia: Plesiosauria) from the non-marine to paralic Dinosaur Park Formation of southern Alberta, Canada. PeerJ 9: e10720.
Codrington, T. 1908. Note on the fossil remains of a Plesiosaurus from Savernake. Magazine of the Wiltshire Archaeological and Natural History Society 35: 114–114.
Codrington T. 1864. Geology of the Berks and Hants extension, any Marlborough railtops. Magazine of the Wiltshire Archaeological and Natural History Society 9: 167–193.
Cope, E.D. 1869. Synopsis of the extinct Batrachia, Reptilia and Aves of North America. Transactions of the American Philosophical Society (new series) 14: 1–252. Crossref
Cruickshank, A.R.I. and Fordyce, R.E. 2002. A new marine reptile (Sauropterygia) from New Zealand: further evidence for a Late Cretaceous Austral radiations of cryptoclidid plesiosaur. Palaeontology 45: 557–575. Crossref
de Blainville, H.D. 1835. Description de quelques espèces de reptiles de la Californie, precédée de l’analyse d’un système général d’herpetologie et d’amphibiologie. Nouvelles Archives du Museumd’Histoire Naturelle 4: 233–296.
Druckenmiller, P.S. and Russell, A.P. 2006. A new elasmosaurid plesiosaur (Reptilia: Sauropterygia) from the Lower Cretaceous Clearwater Formation, northeastern Alberta, Canada. Paludicola 5: 184–199.
Evers, S.W., Barrett, P.M., and Benson, R.B.J. 2019. Anatomy of Rhinochelys pulchriceps (Protostegidae) and marine adaptation during the early evolution of chelonioids. PeerJ 7: e6811. Crossref
Fischer, V., Bardet, N., Guiomar, M., and Godefroit, P. 2014. High diversity in Cretaceous ichthyosaurs from Europe prior to their extinction. PLOS ONE 9: e84709. Crossref
Fischer, V., Benson, R.B.J., Druckenmiller, P.S., Ketchum, H.F., and Bardet, N. 2018. The evolutionary history of polycotylid plesiosaurians. Royal Society Open Science 5 (3): 172177. Crossref
Fischer, V., Maisch, M.W., Naish, D., Liston, J., Kosma, R., Joger, U., Krüger, F.J., Pardo-Pérez, J., Tainsh, J., and Appleby, R.M. 2012. New ophthalmosaurids from the Early Cretaceous of Europe demonstrate extensive ichthyosaur survival across the Jurassic–Cretaceous boundary. PLOS ONE 7: e29234. Crossref
Fischer, V., Zverkov, N. G., Arkhangelsky, M.S., Stenshin, I.M., Blagovetshensky, I.V., and Uspensky, G.N. 2021. A new elasmosaurid plesiosaurian from the Early Cretaceous of Russia marks an early attempt at neck elongation. Zoological Journal of the Linnean Society 192: 1167–1194. Crossref
Gasparini, Z. and Goñi, R. 1985. Los plesiosaurios cretácicos de América del Sur y del continente antártico. Congresso Brasileirode Paleontología 8: 55–63.
Gasparini, Z., Martin, J.E., and Fernandez, M. 2003. The elasmosaurid plesiosaur Aristonectes Cabrera from the Latest Cretaceous of South America and Antarctica. Journal of Vertebrate Paleontology 23: 104–115. Crossref
Gallois, R., Morter, A.A., and Owen, H.G. 2016. The stratigraphy of the Gault Formation (Early Cretaceous, Albian) in East Anglia and south-east England. Proceedings of the Geologists’ Association 127: 606–628. Crossref
Goloboff, P.A. and Catalano, S.A. 2016. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32: 221–238. Crossref
Grove, R. 1976. The Cambridgeshire Coprolite Mining Rush. 51 pp. Oleander Press, Cambridge.
Gutarra, S., Stubbs, T.L., Moon, B.C., Palmer, C., and Benton, M.J. 2022. Large size in aquatic tetrapods compensates for high drag caused by extreme body proportions. Communications Biology 5: 380. Crossref
Hart, M.R. 1973. Foraminiferal inference for the age of the Cambridge Greensand. Proceedings of the Geologists’ Association 84: 65–82. Crossref
Hart, M.B. and Lyndsey, R.F. 2020. Micropalaeontology and stratigraphical setting of the Cambridge Greensand. Geological Society, London, Special Publications 498: 147–163. Crossref
Hiller, N., O’Gorman, J.P., Otero, R.A., and Mannering, A.A. 2017. A reappraisal of the Late Cretaceous Weddellian plesiosaur genus Mauisaurus Hector, 1874. New Zealand Journal of Geology and Geophysics 60 (2): 112–128.
Joyce, W.G. 2025. A review of helochelydrid shell material from late Albian to early Cenomanian greensands of Southern England, United Kingdom. The Anatomical Record 308: 1633–1645. Crossref
Kear, B.P. 2007. Taxonomic clarification of the Australian elasmosaurid Eromangasaurus, with reference to other austral elasmosaur taxa. Journal of Vertebrate Paleontology 27: 241–246. Crossref
Ketchum, H.F. and Benson, R.B .J. 2010. Global interrelationships of Plesiosauria (Reptilia, Sauropterygia) and the pivotal role of taxon sampling in determining the outcome of phylogenetic analyses. Biological Reviews 85: 361–392. Crossref
Kubo, T., Mitchell, M.T., and Henderson, D.M. 2012. Albertonectes vanderveldei, a new elasmosaur (Reptilia, Sauropterygia) from the Upper Cretaceous of Alberta. Journal of Vertebrate Paleontology 32: 557–572. Crossref
Latil, J.L., Jaillard, E., Bardet, N., Raisossadat, N., and Vincent, P. 2021. The Albian–Cenomanian transition in a shelf-basin transect: Biostratigraphy, sedimentology and paleontology of Jebel Mghila, Central Tunisia. Cretaceous Research 124: 104809. Crossref
Lomax, D.R. and Wahl, W.R. 2013. A new specimen of the elasmosaurid plesiosaur Zarafasaura oceanis from the Upper Cretaceous (Maastrichtian) of Morocco. Paludicola 9: 97–109.
Machalski, M. 2018. The Cenomanian ammonite Schloenbachia varians (J. Sowerby, 1817) from the Cambridge Greensand of eastern England: Possible sedimentological and taphonomic implications. Cretaceous Research 87: 120–125. Crossref
Maddison W.P. and Maddison D.R. 2011. Mesquite: A Modular System for Evolutionary Analysis. Version 2.75 [available from: http://mesquiteproject.org, last accessed December 1, 2023].
Madzia, D. and Cau, A. 2020. Estimating the evolutionary rates in mosasauroids and plesiosaurs: discussion of niche occupation in Late Cretaceous seas. PeerJ 8: e8941. Crossref
McGowan, C. 1972. The systematics of Cretaceous ichthyosaurs with particular reference to the material from North America. Rocky Mountain Geology 11: 9–29.
O’Gorman, J.P. 2016a. A small body sized Non-Aristonectine Elasmosaurid (Sauropterygia, Plesiosauria) from the late Cretaceous of Patagonia with comments on the relationships of the Patagonian and Antarctic Elasmosaurids. Ameghiniana 53: 245–268. Crossref
O’Gorman, J.P. 2016b. New insights on the Aristonectes parvidens (Plesiosauria, Elasmosauridae) holotype: news on an old specimen. Ameghiniana 53: 397–417. Crossref
O’Gorman, J.P. 2019. Elasmosaurid phylogeny and paleobiogeography, with a reappraisal of Aphrosaurus furlongi from the Maastrichtian of the Moreno Formation. Journal of Vertebrate Paleontology 39: e1692025. Crossref
O’Gorman, J.P. 2021. The most complete specimen of Kawanectes lafquenianum (Sauropterygia, Plesiosauria): New data on basicranial anatomy and possible sexual dimorphism in elasmosaurids. Cretaceous Research 125: 104836. Crossref
O’Gorman, J.P., Carignano, A.P., Calvo-Marcilese, L., and Panera, J.P.P. 2023. A new elasmosaurid (Sauropterygia, Plesiosauria) from the upper levels of the La Colonia Formation (upper Maastrichtian), Chubut Province, Argentina. Cretaceous Research 152: 105674. Crossref
O’Gorman, J.P., Coria, R.A., Reguero, M., Santillana, S., Mörs, T., and Cárdenas, M. 2018. The first non-aristonectine elasmosaurid (Sauropterygia; Plesiosauria) cranial material from Antarctica: New data on the evolution of the elasmosaurid basicranium and palate. Cretaceous Research 89: 248–263. Crossref
O’Gorman, J.P., Gasparini, Z., and Salgado, L. 2014. Reappraisal of Tuarangisaurus? cabazai (Elasmosauridae, Plesiosauria) from the upper Maastrichtian of northern Patagonia, Argentina. Cretaceous Research 47: 39–47. Crossref
O’Gorman, J.P., Salgado, L., Olivero, E.B., and Marenssi, S.A. 2015. Vegasaurus molyi, gen. et sp. nov. (Plesiosauria, Elasmosauridae), from the Cape Lamb Member (lower Maastrichtian) of the Snow Hill Island Formation, Vega Island, Antarctica, and remarks on Wedellian Elasmosauridae. Journal of Vertebrate Paleontology 35: e931285. Crossref
O’Keefe, F.R. 2002. The evolution of plesiosaur and pliosaur morphotypes in the Plesiosauria (Reptilia: Sauropterygia). Paleobiology 28: 101–112. Crossref
O’Keefe, F.R. and Hiller, N. 2006. Morphologic and ontogenetic patterns in elasmosaur neck length, with comments on the taxonomic utility of neck length variables. Paludicola 5: 206–229.
O’Keefe, F.R., Armour Smith, E., Clark, R.O., Otero, R.A., Perella, A., and Trask, P. 2025. A name for the Provincial Fossil of British Columbia: a strange new elasmosaur taxon from the Santonian of Vancouver Island. Journal of Systematic Palaeontology 23: 2489938. Crossref
Otero, R.A. and Soto-Acuña, S. 2021. Wunyelfia maulensis gen. et sp. nov., a new basal aristonectine (Plesiosauria, Elasmosauridae) from the Upper Cretaceous of central Chile. Cretaceous Research 118: 104651. Crossref
Otero, R.A., Soto-Acuña, S., and O’Keefe, F.R. 2018. Osteology of Aristonectes quiriquinensis (Elasmosauridae, Aristonectinae) from the upper Maastrichtian of central Chile. Journal of Vertebrate Paleontology 38: e1408638. Crossref
Otero, R.A., Soto-Acuña, S., O’Keefe, F.R., O’Gorman, J.P., Stinnesbeck, W., Suárez, M.E., Rubilar-Rogers, D., Salazar, C., and Quinzio-Sinn, L.A. 2014. Aristonectes quiriquinensis, sp. nov., a new highly derived elasmosaurid from the upper Maastrichtian of central Chile. Journal of Vertebrate Paleontology 34: 100–125. Crossref
Owen, R. 1860. On the orders of fossil and recent Reptilia, and their distribution in time. Reports of the British Association for the Advancement of Science 29: 153–166.
Owen, R. 1864. A Monograph on the Fossil Reptilia of the Cretaceous Formations, Supplement No. IV Sauropterygia (Plesiosaurus). 18 pp. Palaeontographical Society, London.
Polcyn, M.J., Jacobs L.L., Araújo, R., Schulp, A.S., and Mateus, O. 2014. Physical drivers of mosasaur evolution. Palaeogeography, Palaeoclimatology, Palaeoecology 400: 17–27. Crossref
Reed, F.R.C. 1897. A Handbook to the Geology of Cambridgeshire. x+276 pp. Cambridge University Press, Cambridge.
Sachs, S. 2005a. Redescription of Elasmosaurus platyurus Cope 1868 (Plesiosauria: Elasmosauridae) from the Upper Cretaceous (lower Campanian) of Kansas, USA. Paludicola 5: 92–106.
Sachs, S. 2005b. Tuarangisaurus australis sp. nov. (Plesiosauria: Elasmo-sauridae) from the Lower Cretaceous of northeastern Queensland, with additional notes on the phylogeny of the Elasmosauridae. Memoirs of the Queensland Museum 50: 425–440.
Sachs, S. and Kear, B.P. 2017. Redescription of the elasmosaurid plesiosaurian Libonectes atlasense from the Upper Cretaceous of Morocco. Cretaceous Research 74: 205–222. Crossref
Sachs, S., Lindgren, J., and Kear, B.P. 2018. Reassessment of the Styxosaurus snowii (Williston, 1890) holotype specimen and its implications for elasmosaurid plesiosaurian interrelationships. Alcheringa 42: 560–574. Crossref
Sachs, S., Lindgren, J., Madzia, D., and Kear, B.P. 2021. Cranial osteology of the mid-Cretaceous elasmosaurid Thalassomedon haningtoni from the Western Interior Seaway of North America. Cretaceous Research 123: 104769. Crossref
Sachs, S., Madzia, D., Thuy, B., and Kear, B.P. 2023. The rise of macropredatory pliosaurids near the Early–Middle Jurassic transition. Scientific Reports 13: 17558. Crossref
Sato, T. 2003. Terminonatator ponteixensis, a new elasmosaur (Reptilia; Sauropterygia) from the Upper Cretaceous of Saskatchewan. Journal of Vertebrate Paleontology 23: 89–103. Crossref
Sato, T., Hasegawa, Y., and Manakoto, M. 2006. A new elasmosaurid plesiosaur from the Upper Cretaceous of Fukushima. Japan. Paleontology 49: 467–484. Crossref
Sedgwick, A. 1846. On the geology of the neighbourhood of Cambridge. including the formations between the Chalk escarpment and the Great Bedford Ievel. Transactions of the British Association for the Advancement of Science 1845: 40–47.
Seeley, H.G. 1866. The rock of the Cambridge Greensand. Geolopical Magazine 3: 302–307. Crossref
Seeley, H.G. 1869. Index of the Fossil Remains of Aves, Ornithosauria and Reptilia, from the Secondary System of Strata Aranged in the Woodward Museum of the University of Cambridge. xxiii + 143 pp. Deighton, Bell, and Co. Press, Cambridge.
Seeley, H.G. 1879. On the Dinosauria of the Cambridge Greensand. Quarterly Journal of the Geological Society of London 35: 591–635. Crossref
Seilacher, A. 1970. Begriff und Bedeutung der Fossil-Lagerstatten. Neues Jahrbuch fur Geologie und Palontologie Monatshefte 1970: 34–39.
Serratos, D.J., Druckenmiller, P., and Benson, R.B. J. 2017. A new elasmosaurid (Sauropterygia, Plesiosauria) from the Bearpaw Shale (Late Cretaceous, Maastrichtian) of Montana demonstrates multiple evolutionary reductions of neck length within Elasmosauridae. Journal of Vertebrate Paleontology 37: e1278608. Crossref
Soul, L.C. and Benson, R.B. J. 2017. Developmental mechanisms of macroevolutionary change in the tetrapod axis: a case study of Sauropterygia. Evolution 71: 1164–1177. Crossref
Unwin, D.M. 2001. An overview of the pterosaur assemblage from the Cambridge Greensand (Cretaceous) of Eastern England. Fossil Record 4: 189–221. Crossref
Vincent, P., Bardet, N., Suberbiola, X.P., Bouya, B., Amaghzaz, M., and Meslouh, S. 2011. Zarafasaura oceanis, a new elasmosaurid (Reptilia: Sauropterygia) from the Maastrichtian phosphates of Morocco and the palaeobiogeography of latest Cretaceous plesiosaurs. Gondwana Research 9: 1062–1073. Crossref
Vincent, P., Grosjean, A.S., Bert, D., Ferreira, J., Suchéras-Marx, B., Suan, G., Guinot, G., Perrier, V., Janneau, K., Brazier, J.M., and Sarroca, E. 2020. Paleoenvironmental context and significance of a partial elasmosaurid skeleton from the Albian of Haute-Provence, France. Cretaceous Research 108: 104293. Crossref
Welles, S.P. 1943. Elasmosaurid plesiosaurs with description of newmaterial from California and Colorado. Memoirs of the University of California 13: 125–254.
Welles, S.P. 1952. A review of the North American Cretaceous elasmosaurs. University of California Publications in Geological Sciences 29: 46–144.
Welles, S.P. 1962. A new species of elasmosaur from the Aptian of Colombia and a review of the Cretaceous plesiosaurs. University of California Publications in the Geological Sciences 44: 1–96.
Wintrich, T., Hayashi, S., Houssaye, A., Nakajima, Y., and Sander, P.M. 2017. A Triassic plesiosaurian skeleton and bone histology inform on evolution of a unique body plan. Science Advances 3: e1701144. Crossref
White, H.J.O. 1932. The Geology of the Country near Saffron Walden. Memoirs of the Geological Survey England and Wales. Explanation of Sheet 205. xii+125 pp. Her Majesty’s Stationer Office, London.
Worssam B.C. and Taylor, J.H. 1969. Geology of the Country around Cambridge. Explanation of Sheet 188. Memoirs of the Geological Survey of Great Britain, England and Wales. 159 pp. Her Majesty’s Stationer Office, London.
Zverkov, N.G., Alexander O. Averianov, and Evgeny V.P. 2017. Basicranium of an elasmosaurid plesiosaur from the Campanian of European Russia. Alcheringa: An Australasian Journal of Palaeontology 42: 528–542. Crossref
Acta Palaeontol. Pol. 70 (4): 613–628, 2025
https://doi.org/10.4202/app.01219.2024