

Bioerosion in the Late Devonian placoderm remains from the Holy Cross Mountains, Poland
PATRYCJA G. DWORCZAK, PIOTR SZREK, and OLGA WILK
Dworczak, P.G., Szrek, P., and Wilk, O. 2025. Bioerosion in the Late Devonian placoderm remains from the Holy Cross Mountains, Poland. Acta Palaeontologica Polonica 70 (4): 629–639.
We report the first occurrence of trace fossils in placoderm bones from the Upper Devonian of the Holy Cross Mountains. A taxonomic analysis of three ichnogenera revealed the earliest evidence of Sulculites (ie., Sulculites bellus), characterised by curved grooves known from the tortoise shells, the potential Osteocallis? isp. consists of irregular and shallow grooves, which were first described on dinosaur bones from continental deposits, while Karethraichnus? isp. being a deep and cylindrical boring, recognized for the first time in turtle shells. They show the evidence of post mortem erosional activity by organisms (e.g., worms) that penetrated the decaying carcasses to search for nutritional particles, graze microbial mats and colonize the osteic substrate. The massive dermal bones of placoderms seem to be appealing to scavengers, albeit rather as an opportunistic behaviour. Nevertheless, this uncommon finding may be caused by the relatively limited attention that has been paid to this phenomenon. The described traces also represent the oldest evidence of macrobioerosion in osteic substrate, as well as the oldest documented occurrence of these forms that have been known so far.
Key words: Placodermi, trace fossils, Sulculites, Famennian, Devonian, Holy Cross Mountains, Poland.
Patrycja G. Dworczak [patrycja.g.dworczak@fau.de; ORCID: https://orcid.org/0000-0003-0885-7546 ], Polish Geological Institute-National Research Institute, Rakowiecka 4 Street, 00-975 Warsaw, Poland; GeoZentrum Nordbayern, Friedrich-Alexander University Erlangen-Nuremberg, Loewenichstrasse 28, 91054 Erlangen, Germany.
Piotr Szrek [pszr@pgi.gov.pl; ORCID:https://orcid.org/0000-0001-9855-2003 ], Polish Geological Institute-National Research Institute, Rakowiecka 4 Street, 00-975 Warsaw, Poland.
Olga Wilk [olga.wilk@nhm.uio.no; ORCID:https://orcid.org/0000-0002-6199-4518 ], Natural History Museum, University of Oslo, Sars’ gate 1, 0562 Oslo, Norway.
Received 29 October 2024, accepted 12 August 2025, published online 30 October 2025 .
Copyright © 2025 P.G. Dworczak et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Bioerosion structures, representing the results of chemical and/or mechanical processing (e.g., Neumann 1966; Bromley 1992; Taylor and Wilson 2003; Pirrone et al. 2014), have the significant importance in the analysis of taphonomy and are essential to understanding the relationships among ancient organisms and the structure of hard ground communities (Knaust and Bromley 2012; Vinn et al. 2015; Vinn and Toom 2016; Höpner and Bertling 2017; Wisshak et al. 2019). Trace fossils in osteic substrates are rare in the fossil record, however, a recent study by Jaminson-Todd et al. (2023) has significantly expanded the documented occurrences of invertebrate bioerosion in the Mesozoic vertebrate remains. This phenomenon has been observed in both marine (e.g., Bystrov 1956; Tapanila et al. 2004; Muñiz et al. 2010; Belaústegui et al. 2012; Karl et al. 2012; Collareta et al. 2022; Jamison-Todd et al. 2024) and terrestrial environnements (e.g., Paik 2000; Roberts et al. 2007; Britt et al. 2008; Paes Neto et al. 2016; Cunha et al. 2024). The bioerosional activity of invertebrates is responsible for the formation of various trace fossils on osteic substrates, including etchings, grooves, lesions, and pits (e.g., Kaiser 2000; Paik 2000; Britt et al. 2008; Roberts et al. 2007; Zonneveld et al. 2022a). The organisms bored into a structurally varied substrate composed of alternating layers of compact and spongy hydroxyapatite (Zonneveld et al. 2016, 2022a). The primary functions of this behaviour are the extraction of nutrients both from the soft body and the hydroxyapatite, grazing on microbial mats or algae developed on the osteic substrate, the founding of combined protection from predators, and also the colonisation of hard substrate on the seafloor (e.g., Wisshak et al. 2019; Jaminson-Todd et al. 2023, 2024). Since trace makers rarely reach the endoskeletons of living vertebrates, such a form of bioerosion occurring on osteic substrates is typically interpreted as a part of the post mortem degradation process (Kaiser 2000; Roberts et al. 2007; Sato and Jenkins 2020).
Herein, we provide the first report of the bioerosion traces in the Late Devonian placoderm bones from the Holy Cross Mountains, Poland. Among the hundreds of collected Devonian vertebrate macrofossils in the Holy Cross Mountains, placoderm remains are most common and they provide a wealth of data on their morphology, distribution and taphonomy (e.g., Gorizdro-Kulczycka 1934; Kulczycki 1957; Ivanov and Ginter 1997; Szrek 2009, 2020; Dworczak and Szrek 2016; Grygorczyk et al. 2024). Placoderms (armoured fish) were the most common and diverse group of the Devonian fishes, which are known from freshwater, brackish, and marine environments (e.g., Dupret 2008; Young 2010). They had robust dermal bones that formed the armour at the anterior part of the body. Additionally, the group Antiarcha possessed the armour that also covered the pectoral fins (e.g., Young 2010; Béchard et al. 2013). Despite the massive plate bones and the frequent occurrence of placoderms in the Devonian rocks from the Holy Cross Mountains, the trace fossils in their remains escaped attention of the researchers so far. Owing to the fact that borings in placoderm remains are considered a rare phenomenon, the evaluation of significance, origin, and frequency of trace fossils in placoderm skeletons is still pending.
The aim of this study is to describe a suite of bioerosional traces on the placoderm bones collected from the Upper Devonian deposits of the Holy Cross Mountains, Poland. Additionally, an attempt has been made to distinguish if borings were produced during the host’s lifetime (syn vivo) or after its death (post mortem). The presented material significantly expands the known stratigraphic range and diversity of the hard ground communities.
Institutional abbreviations.—Muz. PGI-NRI, Geological Museum of the Polish Geological Institute-National Research Institute in Warsaw, Poland; MWG UW, Faculty of Geology, University of Warsaw, Poland.
Geological setting
In the Late Devonian, the southern region of the Holy Cross Mountains, the Kielce region, was situated in a subtropical zone at the southern margin of Laurussia. The region constituted a wide shallow carbonate platform (Szulczewski 1995). During Frasnian, the platform was divided into blocks and stepwisely drowned (Szulczewski 1989, 1995). The submersion of the carbonate platform ended during the Visean (Mississippian) and caused the development of a deep-sea pelagic environment (Szulczewski 1989, 1995; Szulczewski et al. 1996). The deepening of the region was a result of synsedimentary block tectonics (Szulczewski 1995; Narkiewicz et al. 2006).
The Wietrznia quarry in the city of Kielce, is located in the western part of the Holy Cross Mountains (about 190 km south of Warsaw), in a chain of hills known as the Kadzielnia Range (Fig. 1). The range is built up of Upper Devonian rocks and belongs structurally to the western part of the Kielce-Łagów Synclinorium. The details of the Late Devonian facies evolution of the present-day Kadzielnia Range area remain under discussion. Synsedimentary block tectonics resulted in a wide variety of marine environments in this small area, mainly during the Frasnian Stage (Szulczewski 1971, 1973, 1995; Racki 1993; Racki and Bultynck 1993; Racki and Narkiewicz 2000; Pisarzowska et al. 2006). The succession shows progressing stepwise drowning of the carbonate platform, expressed by an increasingly finer sediment fraction with nektonic fauna. However, the horizons of carbonate sediments of coarse-grained sediments appear with brachiopods, fragments of stromatoporoids and rugose corals, and crinoid segments. The age of the studied placoderm bones was determined based on brachiopods (Gorizdro-Kulczycka 1934). Their age in standard conodont zonation may be ranged from the Palmatolepis falsiovalis Zone to the early Palmatolepis triangularis Zone according to Szulczewski (1971) and Ivanov and Ginter (1997). The specimens described herein have been found in such a kind of high-energy event horizon. Among other fauna arthrodires, coelacanths, dipnoans, porolepiforms, ptyctodonts, and sharks were identified from Wietrznia and constitute one of the better preserved and diverse assemblages of the Late Devonian vertebrates from Poland (Gorizdro-Kulczycka 1934, 1950; Kulczycki 1957; Liszkowski and Racki 1993, Ginter 1994, 2004; Ivanov and Ginter 1997; Szrek 2007, 2020).
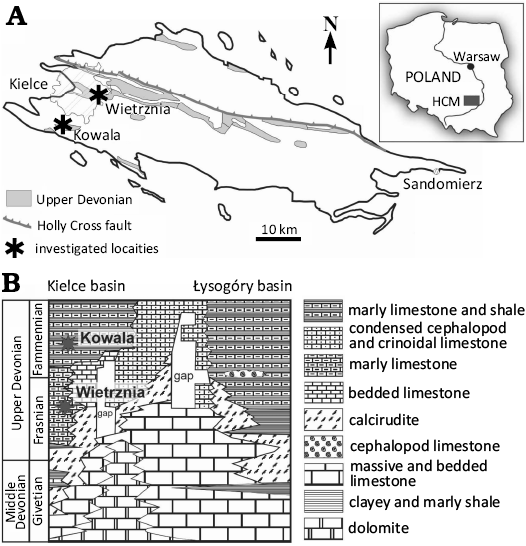
Fig. 1. Location of the investigated outcrop in the Holy Cross Mountains. A. Location map of the Holy Cross Mountains (HCM), central Poland (modified from Kowalczewski 1971 and Szrek 2020). B. Schematic cross-section through the Holy Cross Mountains from the Givetian to the top of the Upper Devonian (after Szulczewski 1995, modified) with the probable position of the localities investigated.
The Kowala quarry is a large, active open-pit limestone and marl quarry located in the eastern part of a syncline (Fig. 1). The quarry exposes a profile of sediments including Givetian–lower Carboniferous, developed in typical hemipelagic non reef phases. A detailed description of the profile exposed both in the quarry itself and in the adjacent railroad cutting has been the subject of many works, including i.e., Czermiński (1960), Szulczewski (1971), Racki (1993) and Racki et al. (2002), Marynowski and Filipiak (2007), Kaźmierczak et al. (2012), Malec (2014) and Zatoń et al. (2014). The profile exposed in the quarry reflects in its fundamental features the tectonic evolution of the entire region. It records the gradual development of increasingly deeper-water facies, but in a more continuous and complete manner than in the Kielce area. The manifestations of seismic activity in the form of synsedimentary folds, have been documented elsewhere (Szulczewski 1971). No significant sedimentation breaks were found in this profile, which made it possible to study the global events that took place in the Late Devonian, and which were documented at this site, i.e., the Kellwasser (Racki et al. 2002), the Annulata (Racka et al. 2010), the Dasberg (Marynowski et al. 2010; Pisarzowska et al. 2024) and the Hangenberg (Marynowski et al. 2012; Myrow et al. 2014) events. This applies in particular to the most prominent of these, the so-called “Great Extinction” at the Frasnian/Famennian boundary, the geochemical aspects of which have been studied in detail at Kowala (Bond et al. 2004; Racki et al. 2002). In recent years, the site has become again intensively studied, and in particular the Devonian/Carboniferous boundary well-exposed in the northern part of the quarry (see Filipiak and Racki 2005; Racki 2006; Rakociński et al. 2021).
Material and methods
Among over 200 of investigated placoderm bone specimens, 17 displayed borings on their surfaces, which were sporadically filled with sediment. Most of these bones are poorly preserved, but four have features that are valuable for further research and have been studied in detail. The best-preserved traces were found on the external surfaces of unidentified plate fragments of arthrodiran placoderms from the Kowala quarry (MWG UW ZI/43/0070 and Muz. PGI-NRI 1809.II.30) and the Wietrznia quarry (Muz. PGI-NRI 1809.II.31 and 36). The MWG UW ZI/43/0070 and Muz. PGI-NRI 1809.II.36 (Fig. 2) display deep, cylindrical borings with rounded, hemispherical termini. Additionally, Muz. PGI-NRI 1809.II.36 was cut perpendicularly to the boring with a diamond blade, grinding and polishing by the using aluminium oxide in the lab at the Friedrich-Alexander University (FAU) in Erlangen, Germany. Muz. PGI-NRI 1809.II.30 (Fig. 3) shows elongated and shallow grooves, which cross each other, whereas Muz. PGI-NRI 1809.II.31 (Fig. 4) displays numerous elongated, narrow and straight to curved structures. All studied specimens were photographed using an Axiocam digital camera attached to a ZEISS AXIO Zoom-V16 microscope with ZEISS ZEN-2-core software at the Friedrich-Alexander University (FAU) in Erlangen, Germany. The specimens are housed in the Geological Museum of the Polish Geological Institute in Warsaw and at the Faculty of Geology, University of Warsaw.
Systematic palaeoichnology
Ichnogenus Karethraichnus Zonneveld et al., 2016
Type ichnospecies: Karethraichus lakkos Zonneveld et al., 2016, the Green River Basin, Wyoming (USA), Eocene.
Emended diagnosis.—Circular to subcircular non-penetrative and penetrative holes bored into osteic substrates. The boring that terminates within the substrate, forms a shallow, bowl-shaped pit. If the holes are deeper, they display a deeper shaft with a rounded to flattened terminus. Penetrative holes usually have straight or convex vertical edges.
Remarks.—Karethraichnus displays similar morphology and dimensions to the ichnogenus Sedilichnus Müller, 1977. However, Karethraichnus shows a higher morphological variability in the circularity of the external burrow opening, the symmetry of their cross-sectional profile (Zonneveld et al. 2016). Additionally, the two ichnogenera also vary in a specific type of substrate. Sedilichnus occurs within carbonate substrates (usually invertebrate shells), whereas Karethraichnus is known within phosphatic substrates such as carapace of armadillos and turtles (Zonneveld et al. 2016; Moura et al. 2021).
Stratigraphic and geographic range.—Karethraichnus was previously described from the Upper Cretaceous of Japan (Sato and Jenkins 2020) and Egypt (El Hedeny et al. 2023), the middle and upper Eocene of the USA (Zonneveld et al. 2016; Adrian et al. 2021; Zonneveld and Bartels 2023), the Miocene of Peru and Egypt (Collareta et al. 2022; Zonneveld et al. 2022a), the upper Pliocene of Italy (Collareta et al. 2023), the Upper Pleistocene of Brasil (Moura et al. 2021), and the Recent (Zonneveld et al. 2022b). This documentation may extend the stratigraphic range of the ichnotaxon into the Upper Devonian, representing the first known occurrence from Poland.
Karethraichnus? isp.
Fig. 2.
Material.—Two specimens from the Upper Devonian of Poland: MWG UW ZI/43/0070, the Kowala quarry, Palmatolepis crepida–Palmatolepis postera zones, Famennian, and Muz. PGI-NRI 1809.II.36, the Wietrznia quarry, Frasnian.
Description.—Rounded borings 2–10 mm in diameter and 3–5 mm in depth, located on the dermal bone from the Kowala quarry (MWG UW ZI/43/0070, Fig. 2A) and on the dermal bone from the Wietrznia quarry (Muz. PGI-NRI 1809.II.36, Fig. 2B). The precise measurements of the depths of the original borings of both specimens are difficult to ascertain due to the presence of sediment at their bottoms, as well as from the erosional surface of the bone. They terminate in the bone and have rounded, flattened termini. The traces are hemispherical cavities with a circular transversal section and smooth walls. In longitudinal section, they are almost cylindrical-shape and their axis approximately perpendicular to the substrate surface. They appear only on the one side on both specimens, and are irregularly arranged on the surface, spaced from each other in distances of 5–10 mm increment.

Fig. 2. Dermal bones of placoderm with Karethraichnus? isp. from the Upper Devonian of the Holy Cross Mountains, Poland. A. MWG UW ZI/43/0070, external side of unidentified plate fragment of anarthrodiran placoderm indicates rounded borings (arrows), Famennian, Kowala quarry. B. Muz. PGI-NRI 1809.II.36, polished slab displays cross section through the boring, Frasnian, Wietrznia quarry. The rectangle indicates the location of the boring within the bone.
Remarks.—The studied borings in the Late Devonian placoderm bones are interpreted as a species of Karethraichnus? Zonneveld et al., 2016. The oldest known occurrence of this genus was described from fossil turtle remains of the Late Cretaceous (Sato and Jenkins 2020). Karethraichnus are considerably variable in size and circularity. Karethraichnus kulindros Zonneveld et al., 2016, displays deep, cylindrical, non-penetrative wholes with smooth margins and rounded to flattened, hemispherical terminus. Other non-penetrative ichnospecies, Karethraichnus lakkos Zonneveld et al., 2016, displays shallow hemispherical pits with rounded termini; Karethraichnus minimum Moura et al., 2021, shows cone-shaped profiles with circular to semicircular outline and Karethraichnus zaraton Sato & Jenkins, 2020, shows clavate-shaped holes with a smooth surface. Only Karethraichnus fiale Zonneveld et al., 2016 (deep, cylindrical holes with smooth margins) passes through the bony substrates (Sato and Jenkins 2020; Zonneveld and Bartles 2023).
Ichnogenus Osteocallis Roberts et al., 2007
Type ichnospecies: Osteocallis mandibulus Roberts et al., 2007, Maevarano Formation, Madagascar, Maastrichtian (Upper Cretaceous).
Emended diagnosis.—Shallow, curved or straight grooves bored into external bone surfaces. They may occur in pairs or as meandering trails. The latter may form a network of randomly overlapping trails.
Remarks.—Osteocallis has been observed in bones from both continental and marine settings. Osteocallis differs from etchings in shells and rocks, such as Gnathichnus Bromley, 1975 (radially arranged scratches) and Radulichnus Voigt, 1977 (linear traces forming small meandering clusters), which are also known from osteic substrates, by having accurate, often paired scratches (e.g., Jagt 2003; Mudler et al. 2005; Janssen et al. 2013). Although Osteocallis can resemble the ichnogenera Paleoscolytus Walker, 1938, described as branched channels in fossil wood, it lacks the shallow grooves or scratches that are diagnostic of Osteocallis (Collareta et al. 2023).
Stratigraphic and geographic range.—Ichnogenus Osteocalis was previously documented from the Upper Triassic of Brazil (Paes Neto et al. 2016; Cunha et al. 2024), the Upper Jurassic of USA (McHugh et al. 2020), the Upper Cretaceous of Madagascar, USA (Roberts et al. 2007) and Egypt (El Hedeny et al. 2023), and the lower Pliocene of Italy (Collareta et al. 2023). This determination may extend the stratigraphic range of Osteocalis up into the Late Devonian, providing the first occurrence from Poland.
?Osteocallis isp.
Fig. 3.
Material.—Muz. PGI-NRI 1809.II.30, the Kowala quarry, Palmatolepis crepida–Palmatolepis postera Zones, Famennian, Upper Devonian.
Description.—On the surface of the placoderm dermal bone (Muz. PGI-NRI 1809.II.30, Fig. 3) traces have been preserved as abundant, although irregular grooves, which are shallow in depth (0.5–1 mm). Their diameter varies from 0.4 to 1 mm and the length ranges from 1 to 3 mm. The grooves occur in dense cluster. They often overlap perpendicular or parallel other grooves. The ridges between them are partially invisible.
Remarks.—This specimen was interpreted by Szrek (2009, 2020) as a supposed bryozoan etching traces, but its morphology, especially regarding no multiple ramifications and a smaller diameter of single traces render this identification rather unlikely. These bioerosional marks closely correspond to Osteocallis Roberts et al., 2007, already known from vertebrate fossils. Osteocallis mandibulus shows a network of shallow randomly overlapping trails or paired grooves, creating long paths (Roberts et al. 2007: fig. 4) and Osteocallis infestans meandering trails of generally straight overlapping grooves which were excavated into the bone surfaces. Both ichnospecies are randomly oriented and cross each other which are either perpendicular or parallel to one another (Peas Neto et al. 2016: fig. 6), whereas Osteocallis leonardii has a characteristic arrangement in pairs “side-by-side” (Collareta et al. 2023: fig. 3).
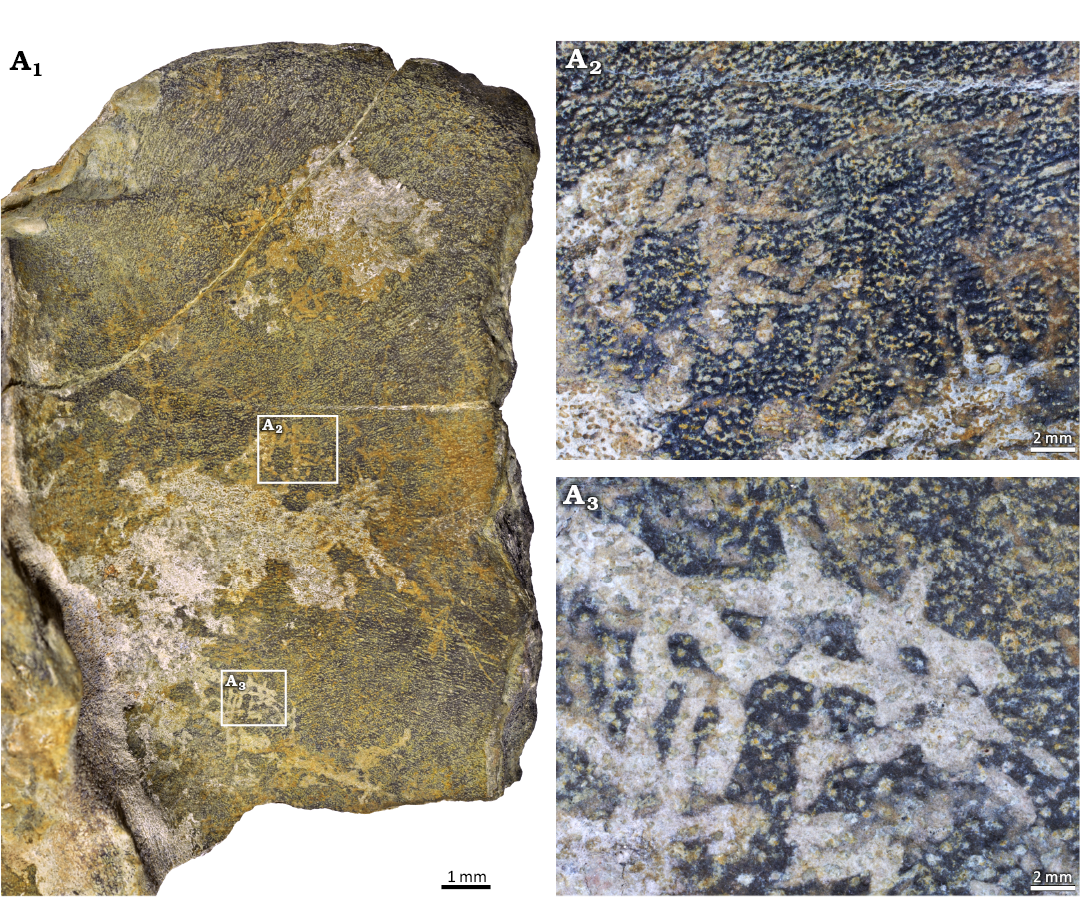
Fig. 3. The external surface of bioeroded unidentified arthrodiran placoderm bone fragment demonstrating Osteocallis? isp. (Muz. PGI-NRI 1809.II.30) from Famennian, Upper Devonian, Kowala quarry, Holy Cross Mountains, Poland (A1); cluster of shallow borings filled with sediment (A2); cluster of trace fossils overlapping each other (A3).
Ichnogenus Sulculites Vialov & Nessov, 1974
Type ichnospecies: Sulculites bellus Vialov & Nessov, 1974, the south-eastern Fergana, Uzbekistan, Albian (Lower Cretaceous).
Emended diagnosis.—Shallow, straight to curved grooves. They occur as a single furrow or create chains of parallel traces. Their bottom is flat and has 0.18–0.5 mm in diameter (usually 0.3‒0.4 mm). The ends of the furrows either gradually narrow or abruptly break off. The long, continuous and parallel grooves may occur close to each other and be oriented in various directions. The short furrows are usually curved and may intertwine the longer traces (after Vialov and Nessov 1974).
Remarks.—Sulculites has been recognized in osteic substrates—the Cretaceous turtle carapaces (Vialov and Nessov 1974; El Hedeny et al. 2023). It is morphologically similar to Cubiculum Roberts et al., 2007, which occurs also as isolated grooves and forms dense, sometimes overlapping groups of grooves. However, Cubiculum is typically much larger (7–20 mm in length and 2–6 mm in diameter; Roberts et al. 2007). The ichnogenus Gunnellichnus Zonneveld et al., 2022b, is considerably wider than Sulculites and creates branching structures that are not observed in Sulculites.
Stratigraphic and geographic range.—As for the monotypic type species.
Sulculites bellus Vialov & Nessov, 1974
Fig. 4.
Material.—Muz. PGI-NRI 1809.II.31 (Fig. 4), the Wietrznia quarry, Palmatolepis falsiovalis–lower Palmatolepis hassi zones, Frasnian, Upper Devonian.
Description.—The external side of a placoderm bone (Muz. PGI-NRI 1809.II.31, Fig. 4) exhibits elongated, narrow and shallow grooves (Fig. 4). Their diameters are 0.2–0.4 mm and their lengths 0.5–3 mm. The furrows are straight to curved and do not branch. They occur as either single furrows or form clusters of intersected grooves and pairs of long, parallel, closely spaced traces (Fig. 4A2). The groove ends gently taper or abruptly sever (Fig. 4A4, A5). The furrows cross each other in a different angle (Fig. 4A3, A5), creating complicated intertwines. The presence of thin, curved wrinkles is observed on the bottom of furrows (Fig. 4A5).
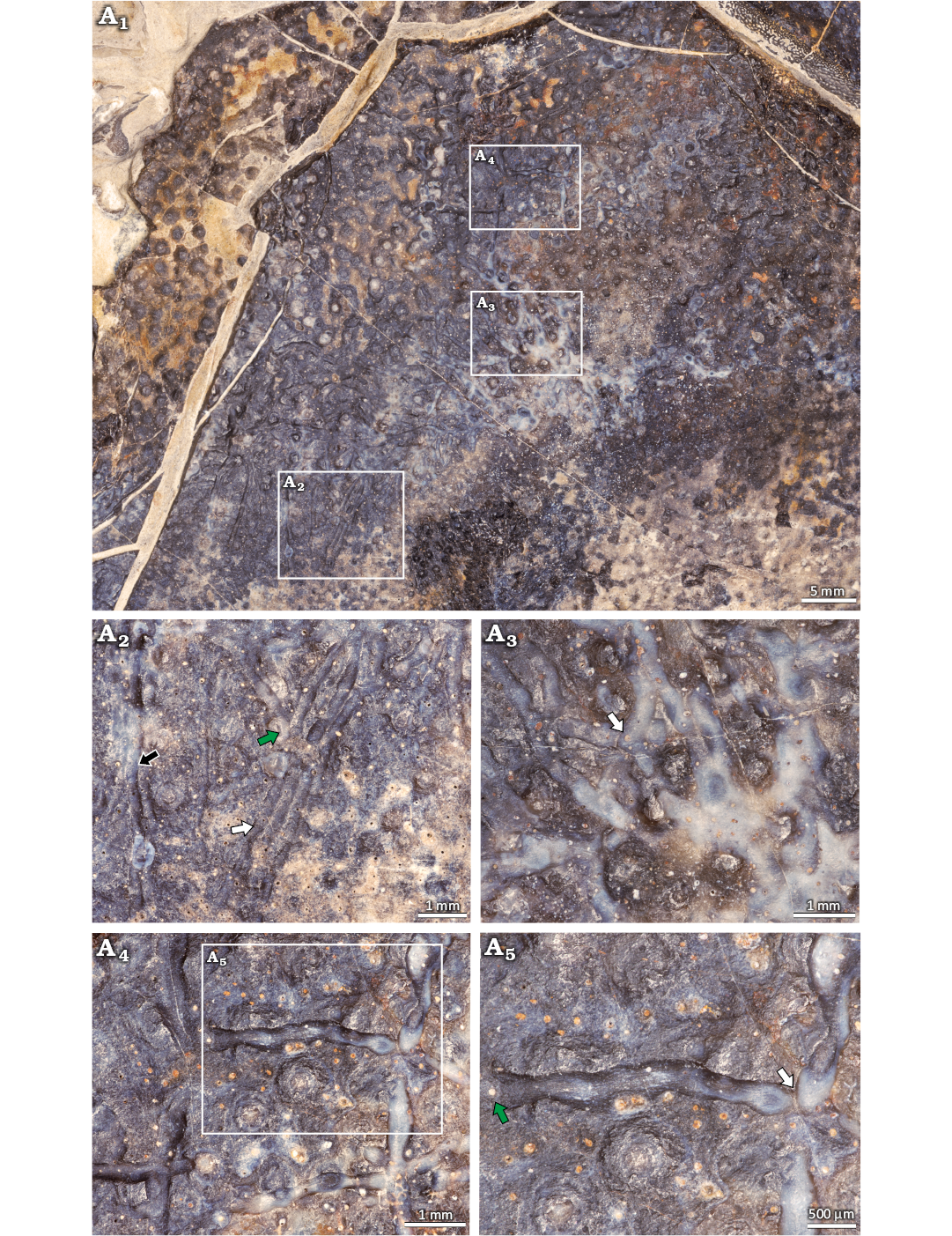
Fig. 4. Traces of Sulculites bellus Vialov & Nessov, 1974, on the external surface of a placoderm bone; Frasnian, Upper Devonian, Wietrznia quarry, Holy Cross Mountains, Poland. Muz. PGI-NRI 1809.II.31. A1, general view of the fragment showing long traces; A2, traces occur as single furrows (black arrow) or form clusters of intersected grooves (green arrow) and pairs of long, parallel, closely spaced traces (white arrow); A3, the elongated borings cross each other in different angle (white arrow); A4, A5 show furrows with thin, curved wrinkles on the bottom, green arrow shows where the groove ends abruptly, white arrow indicates the ends where the grooves gently taper.
Remarks.—The trace fossils occur on the well-preserved surface of the placoderm bone. They were interpreted as Sulculites bellus Vialov & Nessov, 1974. The traces exhibit both straight and curved furrows, which appear solitarily or in pairs and chains. They cross each other, creating complicated intertwines and have thin, curved wrinkles on the bottom, similar to the traces described by Vialov and Nessov (1974) and El Hedeny et al. (2023).
Stratigraphic and geographic range.—Sulculites bellus was previously reported from the Lower Cretaceous (Albian) of Uzbekistan (Vialov and Nessov 1974), and the Upper Cretaceous (Campanian) of Egypt (El Hedeny et al. 2023). This study expands the stratigraphic range of the ichnotaxon up into the Upper Devonian, thereby providing the first documented examples from Poland.
Discussion
The bioerosion traces reported from the dermal bone of placoderms from the Upper Devonian of Poland represent the earliest known record of the macrobioerosion in the osteic substrates. Hitherto, one case of microbioerosion (Mycelites ossifragus) in placoderm remains has only been reported by Bystrov (1956). Furthermore, two examples of epibionts on placoderm remains have been reported until present, one from the Middle Devonian of Australia (White 1978; BMNH P.50313) with the tabulate coral Aulopora sp., and one from the Holy Cross Mountains as possible bryozoans (Szrek 2009, 2020: fig. 6A, B). Consequently, these finds provide the oldest evidence for Sulculites bellus, as well as the earliest potential records of the ichnogenera Karethraichnus? and Osteocallis?. Judging by the numbers of bones studied here, it can be inferred that this low number of finds likely results from the limited attention that has been paid to this phenomenon. Contrary to previous suggestions, traces of bioerosion may be present on a considerable number of specimens. During the Middle Paleozoic, the diversity of bioerosion increased but has hardly been recorded from vertebrate remains (e.g., Taylor and Wilson 2003; Wilson 2007). Our research shows that bioerosional activity on phosphate substrates, such as placoderm bones, was much more common in the Late Devonian than previously reported.
So far, the oldest evidence of Karethraichnus was found in sea turtles remains of the Late Cretaceous age (Sato and Jenkins 2020). The bioerosion is known from both marine (Sato and Jenkins 2020; Collareta et al. 2022; Zonneveld et al. 2022b; this paper) and nonmarine environments (e.g., Moura et al. 2021; Zonneveld et al. 2022a; Zonneveld and Bartles 2023; El Hadeny et al. 2023). The ichnotaxon has been produced by arthropods such as dipterans and ixodid arachnids in terrestrial environment, and by leeches in freshwater settings (e.g., Zonneveld et al. 2016, 2022a; Zonneveld and Bartles 2022, 2023). In marine environments, Karethraichnus can be produced by bivalves and barnacles (see Sato and Jenkins 2020; Zonneveld et al. 2022b). The organisms mechanically bore into the substrates, thereby producing dwelling structures. Although barnacles commonly settle on mobile substrates, such as sea turtles and crabs (e.g., Seilacher 2005), they are also capable of boring into vertebrate remains, suggesting that their involvement in the traces on the placoderm remains cannot be entirely excluded. Additionally, the investigated borings occur on only one surface of the bones, which excluded predation.
The ichnogenus Osteocallis is known both from marine and especially nonmarine environments (e.g., Roberts et al. 2007; Peas Neto et al. 2016; Collareta et al. 2023). The oldest confirmed occurrence of this ichnogenus was found in the Upper Triassic rhynchosaur carcass (Cunha et al. 2024). Osteocallis is a grazing trace typically produced by osteophagous silphid and histerid beetles (e.g., Cunha et al. 2024), or alternatively by polyplacophoran trace makers (Collareta et al. 2023). In the marine environment, chitons are considered to be the producers of Osteocallis. Chitons possess a hard radula with teeth arranged in two elongated rows (Voigt 1977; Collareta et al. 2023). The radula abrades the substrate to enable feeding on embedded algae or microbial mats. The process results in the formation of grazing traces on the bones. The large placoderm plate could indicate the development and distribution of algae and microbial mats on the bone forming a secondary hard substrate on the muddy seafloor. Furthermore, the traces are similar to borings Gibbula isp. illustrated by Thompson et al. (1997) and Gibert et al. (2007), which create groups of shallow, short and curve scratches. Additionally, we observe two sizes of these traces, indicating that two different organisms could graze on the placoderm bone.
Previously, Sulculites bellus was known from the Cretaceous turtle carapaces from Eastern Uzbekistan (Vialov and Nessov 1974) and Egypt (El Hedeny et al. 2023). The bioerosion has been exclusively observed in osteic substrates, and it has been produced by invertebrates, most likely vermiform organism or larvae of marine organisms, such as arthropods (Vialov and Nessov 1974; El Hedeny et al. 2023; Zonneveld and Bartles 2023). These traces are rare and none of them appears to have caused the death of the host organisms (e.g., El Hedeny et al. 2023). The previous studies (Vialov and Nessov 1974; El Hedeny et al. 2023) suggest post mortem colonization of placoderm bones by scavengers, which bored into the bones to search for nutrients.
The study of bioerosional activity in vertebrate remains offers insights into the relationship between organisms, the taphonomic history of carcasses, as well as the development of hard ground communities (e.g., Kelley and Hansen 2006; Roberts et al. 2007). Bioerosion in osteic material is typically described as predation, parasitism, or scavenging, and may be part of the post mortem degradation process (Kaiser 2000; Roberts et al. 2007; Sato and Jenkins 2020). Our studies show that all of the investigated dermal bones have been affected post mortem. In case of Karethraichnus, many authors have indicated that fossilized turtle and armadillo bones display evidences of healing of the borings, which suggest a parasitic relationship (e.g., Zonneveld et al. 2022a, b). In the Muz. PGI-NRI 1809.II.36 (Fig. 2B), no malformations of bone were observed that would indicate a syn vivo interaction between the placoderm and the trace maker. Therefore, the studied bioerosion most likely resulted from opportunistic behaviour of the trace makers. It is unclear whether the placoderm bones were chosen selectively by trace makers as desirable phosphatic clasts or just have represented the only available hard substrate on the seafloor (e.g., Tapanila et al. 2004; Jaminson-Todd et al. 2023). Additionally, the findings suggest a temporally low energy depositional environment of the placoderm remains with low sedimentation rates. The organism which produced Sulculites bellus which has apparently mined for the nutrients contained in the bone and the remains of the soft tissue, had to settle on the bone immediately after their deposition. However, the presence of Osteocalis isp., whose trace maker fed on algae or microbial mats, indicates a temporal window during which microbes were capable of producing bio-mats on the bone surfaces after their deposition on the seafloor.
Conclusions
The trace fossils described herein represent the first documented example of bioerosion in placoderm bones from the Upper Devonian of the Holy Cross Mountains, Poland. Our results reveal the earliest occurrence of the ichnospecies Sulculites bellus and the potential ichnogenera Karethraichnus? and Osteocallis? in placoderm remains, significantly extending their stratigraphic range into the Late Devonian. The traces constitute the earliest appearance of macrobioerosion in placoderm bones, as well as in osteic substrates. All studied bioerosion show the post mortem erosional activity of the organisms that bored into the bones either to search for nutritional particles, to colonize the osteic substrate for shelter, or to graze on microbial mats that had developed on the bone surfaces.
Acknowledgements
We are very grateful to Alfred Uchman (Jagiellonian University, Cracow, Poland) for constructive discussion and comments, and to Birgit Leipner-Mata (Friedrich-Alexander University, Erlangen, Germany) for assist with laboratory work. We thank Michał Poros (Geonatura, Kielce, Poland) and Błażej Dziewięcki (Kielce, Poland) for providing specimens (Muz. PGI-NRI 1809.II.36 and Muz. PGI-NRI 1809.II.31 respectively) used in our studies. The manuscript was improved by the constructive reviews of Michał Zatoń (University of Silesia, Sosnowiec, Poland) and two anonymous reviewers. The authors express their sincere gratitude for these insightful contributions.
Editor: Andrzej Kaim
References
Adrian, B., Smith, H.F., Hutchison, J.H., and Townsend, K.E.B. 2021. Geometric morphometrics and anatomical network analyses reveal ecospace partitioning among geoemydid turtles from the Uinta Formation, Utah. The Anatomical Record 305: 1359–1393. Crossref
Belaústegui, Z., Gibert, J.M. de, Domènech, R., Muñiz, F., and Martinell, J. 2012. Clavate borings in a Miocene cetacean skeleton from Tarragona (NE Spain) and the fossil record of marine bone bioerosion. Palaeogeography, Palaeoclimatology, Palaeoecology 323: 68–74. Crossref
Béchard, I., Arsenault, F., Cloutier, R., and Kerr, J. 2013. The Devonian placoderm fish Bothriolepis canadensis revisited with three-dimensional digital imagery. Palaeontologia Electronica 17: 1–19. Crossref
Bond, D., Wignall, P.B., and Racki, G. 2004. Extent and duration of marine anoxia during the Frasnian–Famennian (Late Devonian) mass extinction in Poland, Germany, Austria and France. Geological Magazine 141: 173–193. Crossref
Britt, B.B., Scheetz, R.D., and Dangerfield, A. 2008. A suite of dermestid beetle traces on dinosaur bone from the Upper Jurassic Morrison Formation, Wyoming, USA. Ichnos 15: 59–71. Crossref
Bromley, R.G. 1975. Comparative analysis of fossils and recent echinoid bioerosion. Palaeontology 18: 725–739.
Bromley, R.G. 1992. Bioerosion: eating rocks for fun and profit. Short Courses in Paleontology 5: 121–129. Crossref
Bystrov, A.P. 1956. On the demage to the skeleton of fossil animals by fungi [in Russian]. Vestnik Leningradskogo universiteta (Geologiâ i Geografiâ) 11: 30–46.
Collareta, A., Merella, M., Casati, S., Di Cencio, A., Tinelli, C., and Bianucci, G. 2023. Polyplacophoran feeding traces on Mediterranean Pliocene sirenian bones: Insights on the role of grazing bioeroders in shallow-marine vertebrate falls. Life 13 (2): 327. Crossref
Collareta, A., Varas-Malca, R., Bosio, G., Urbina, M., and Coletti, G. 2022. Ghosts of the Holobiont: borings on a Miocene turtle carapace from the Pisco Formation (Peru) as witnesses of ancient symbiosis. Journal of Marine Science and Engineering 11: 45. Crossref
Cunha, L.S., Dentzien-Dias, P., and Francischini, H. 2024. New bioerosion traces in rhynchosaur bones from the Upper Triassic of Brazil and the oldest occurrence of the ichnogenera Osteocallis and Amphifaoichnus. Acta Palaeontologica Polonica 69: 1–21.
Czermiński, J. 1960. Rozwój litologiczny serii węglanowej dewonu południowej części Gór Świętokrzyskich. Prace Instytutu Geologicznego 30 (2): 31–104.
Dupret, V. 2008. A new wuttagoonaspid (Placodermi, Arthrodira) from the Lower Devonian of Yunnan (South China): origin, dispersal, and paleobiogeographic significance. Journal of Vertebrate Paleontology 28: 12–20. Crossref
Dworczak, P.G. and Szrek, P. 2016. The Late Devonian placoderm Aspidichthys Newberry, 1873 from the Holy Cross Mountains, Poland. Fossil Record 20: 9–19. Crossref
El Hedeny, M., Mohesn, S., Tantawy, A.A., El-Sabbagh, A., AbdelGawad, M., and El-Kheir, G.A. 2023. Bioerosion traces on the Campanian turtle remains: New data from the lagoonal deposits of the Quseir Formation, Kharga Oasis, Egypt. Palaeontologia Electronica 26: 1–21. Crossref
Filipiak, P. and Racki, G. 2005. Unikatowy zapis dewońskich zdarzeń beztlenowych w profilu kamieniołomu Kowala k. Kielc. Przegląd Geologiczny 53: 846–847.
Gibert, J.M. de Domènech, R., and Martinell, J. 2007. Bioerosion in shell beds from the Pliocene Roussillon Basin, France: Implications for the (macro) bioerosion ichnofacies model. Acta Palaeontologica Polonica 52: 783–798.
Ginter, M. 1994. Ichtiolity dewońskie z Polski i Uralu, oraz ich znaczenie stratygraficzne. 134 pp. Unpublished Ph.D. Dissertation, Uniwersytet Warszawski, Wydział Geologii, Warszawa.
Ginter, M. 2004. Devonian sharks and the origin of Xenacanthiformes. In: G. Arratia, M.V.H. Wilson, and R. Cloutier (eds.), Recent Advances in the Origin and Early Radiation of Vertebrates. Honoring Hans-Peter Schultze, 473–486. Friedrich Pfeil, München.
Gorizdro-Kulczycka, Z. 1934. Ptyctodontidae górnodewońskie z Gór Świętokrzyskich. Prace Państwowego Instytutu Geologicznego 3: 1–38.
Gorizdro-Kulczycka, Z. 1950. Dwudyszne ryby dewońskie Gór Świętokrzyskich. Acta Geologica Polonica 1: 53–82.
Grygorczyk, K., Wilk, O., and Szrek, P. 2024. Revision of the Late Devonian ptyctodonts (Vertebrata, Placodermi) from southern Poland. Estonian Journal of Earth Sciences 73: 1–14. Crossref
Höpner, S. and Bertling, M. 2017. Holes in bones: ichnotaxonomy of bone borings. Ichnos 24: 259–282. Crossref
Ivanov, A. and Ginter, M. 1997. Comments on the Late Devonian placoderms from the Holy Cross Mountains (Poland). Acta Palaeontologica Polonica 42: 413–426.
Jagt, J.W.M. 2003. The ichnofossil genera Radulichnus and Renichnus in the Maastrichtian of The Netherlands and Belgium. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique Sciences de la Terre 73: 175–184.
Jamison-Todd, S., Mannion, P. D., Glover, A. G., and Upchurch, P. 2024. New occurrences of the bone-eating worm Osedax from Late Cretaceous marine reptiles and implications for its biogeography and diversification. Proceedings of the Royal Society B 291 (2020): 20232830.
Jamison-Todd, S., Upchurch, P., and Mannion, P.D. 2023. The prevalence of invertebrate bioerosion on Mesozoic marinereptile bone from the Jurassic and Cretaceous of the United Kingdom: new data andimplications for taphonomy and environment. Geological Magazine 160: 1701–1710.
Janssen, R., Van Baal, R.R., and Schulp, A.S. 2013. Bone damage in Allopleuron hofmanni (Cheloniidae, Late Cretaceous). Netherlands Journal of Geosciences 92: 153–157.
Kaiser, T.M. 2000. Proposed modification to fossil mammalian bone from Plio-Pleistocene hominid-bearing deposits of Laetoli (northern Tanzania). Annals of the Entomological Society of America 93: 693–700.
Karl, H.V., Lindow, B.E., and Tütken, T. 2012. Miocene leatherback turtle material of the genus Psephophorus (Testudines: Dermochelyoidea) from the Gram Formation (Denmark). Studia Palaeocheloniologica 4: 205–216.
Kaźmierczak, J., Kremer B., and Racki, G. 2012. Late Devonian marine anoxia challenged by benthic cyanobacterial mats. Geobiology 10: 371–383.
Kelley, P.H. and Hansen, T.A. 2006. Comparisons of class- and lower taxon-level patterns in naticid gastropod predation, Cretaceous to Pleistocene of the US Coastal Plain. Palaeogeography, Palaeoclimatology, Palaeoecology 236: 302–320.
Knaust, D. and Bromley R.G. 2012. Trace Fossils as Indicators of Sedimentary Environments. 924 pp. Elsevier, Amsterdam.
Kowalczewski, Z. 1971. Podstawowe problemy geologiczne dewonu dolnego Gór Świętokrzyskich. Geological Quarterly 15: 263–283.
Kulczycki, J. 1957. Upper Devonian fishes from the Holy Cross Mountains (Poland). Acta Palaeontologica Polonica 2: 285–380.
Liszkowski, J. and Racki, G. 1993. Ichthyolits and deepening events in the Devonian carbonate platform of the Holy Cross Mountains. Acta Palaeontologica Polonica 37: 407–426.
Malec, J. 2014. The Devonian/Carboniferous boundary in the Holy Cross Mountains. Geological Quarterly 58: 217–234.
Marynowski, L. and Filipiak, P. 2007. Water column euxinia and wildfire evidence during deposition of the Upper Famennian Hangenberg event horizon from the Holy Cross Mountains (central Poland). Geological Magazine 144: 569–595.
Marynowski, L., Filipiak, P., and Zatoń, M. 2010. Geochemical and palynological study of the Upper Famennian Dasberg event horizon from the Holy Cross Mountains (central Poland). Geological Magazine 147: 527–550.
Marynowski, L., Zatoń, M., Rakociński, M., Filipiak, P., Kurkiewicz, S., and Pearce, T.J. 2012. Deciphering the upper Famennian Hangenberg Black Shale depositional environments based on multi-proxy record. Palaeogeography, Palaeoclimatology, Palaeoecology 346: 66–86.
McHugh, J.B., Drumheller, S.K., Riedel, A., and Kane, M. 2020. Decomposition of dinosaurian remains inferred by invertebrate traces on vertebrate bone reveal new insights into Late Jurassic ecology, decay, and climate inwestern Colorado. PeerJ 8: e9510.
Moura, F., Nascimento, C.S.I., de Barros, G.E., Robbi, B., and Fernandes, M.A. 2021. Damaged armour: ichnotaxonomy and paleoparasitology of bioerosion lesions in osteoderms of Quaternary extinct armadillos. Journal of South American Earth Sciences 109: 103255.
Mudler, E. W., John, W.M., and Schulp, A.S. 2005. Another record of hadrosaurid dinosaur from the Maastrichtian type area. Bulletin de l’institut royal des sciences naturelles de belgique sciences de la terre 75: 201–206.
Müller, A.H. 1977. Zur Ichnologie der subherzynen Oberkreide(Campan). Zeitschrift für geologische Wissenschaften 5: 881–897.
Muñiz, F., Gilbert J.M.de, and Esperante, R. 2010. First trace-fossil evidence of bone-eating worms in whale carcasses. Palaios 25: 269–273.
Myrow, P.M., Ramezani, J., Hanson, A.E., Bowring, S.A., Racki, G., and Rakociński, M. 2014. High‐precision U-Pb age and duration of the latest Devonian (Famennian) Hangenberg event, and its implications. Terra Nova 26: 222–229.
Narkiewicz, M., Racki, G., Skompski, S., and Szulczewski, M. 2006. Zapis procesów i zdarzeń w dewonie i karbonie Gór Świętokrzyskich. In: A. Żylińska (ed.), XX Konferencja Naukowa Paleobiologów i Biostratygrafów PTG, Granice Paleontologii, Św. Katarzyna pod Łysicą, 51–77. Wydzial Geologii UW, Warszawa.
Neumann, A.C. 1966. Observations on coastal erosion in Bermuda and measurements of the boring rate of the sponge, Cliona LAMPA 1, 2. Limnology and Oceanography 11: 92–108.
Paes Neto, V.D., Parkinson, A.H., Pretto, F.A., Soares, M.B., Schwanke, C., Schultz, C.L., and Kellner, A.W. 2016. Oldest evidence of osteophagic behavior by insects from the Triassic of Brazil. Palaeogeography, Palaeoclimatology, Palaeoecology 453: 30–41.
Paik, I.S. 2000. Bone chip-filled burrows associated with bored dinosaur bone in floodplain paleosols of the Cretaceous Hasandong Formation, Korea. Palaeogeography, Palaeoclimatology, Palaeoecology 157: 213–225.
Pirrone, C.A., Buatois, L.A., and Bromley, R.G. 2014. Ichnotaxobases for bioerosion trace fossils in bones. Journal of Paleontology 88: 195–203.
Pisarzowska, A., Kondas, M., Zatoń, M., Rakociński, M., Szczerba, M., Krzątała, A., and Radzikowska, M. 2024. Anatomy of the late Famennian Dasberg event in a deep shelf of southern Euramerica: Oxygenation and productivity in a restricted basin during a progressive long-term cooling. Marine and Petroleum Geology 168: 107032.
Pisarzowska, A., Sobstel, M., and Racki, G. 2006. Conodont-based event stratigraphy of the Early–Middle Frasnian transition on the South Polish carbonate shelf. Acta Palaeontologica Polonica 51: 609–646.
Racka, M., Marynowski, L., Filipiak, P., Sobstel, M., Pisarzowska, A., and Bond, D.P. 2010. Anoxic Annulata events in the Late Famennian of the Holy Cross Mountains (Southern Poland): geochemical and palaeontological record. Palaeogeography, Palaeoclimatology, Palaeoecology 297: 549–575.
Racki, G. 1993. Evolution of the bank to reef complex in the Devonian of the Holy Cross Mountains. Acta Palaeontologica Polonica 37: 87–182.
Racki, G. 2006. Świętokrzyski zapis globalnych zdarzeń biotycznych. In: S. Skompski and A. Żylińska (eds.), Procesy i zdarzenia w historii geologicznej Gór Świętokrzyskich. 77. Zjazd Naukowy PTG, Ameliówka k. Kielc, 63–64. Panstwowy Instytut Geologiczny, Warszawa.
Racki, G. and Bultynck, P. 1993. Conodont biostratigraphy of the Middle to Upper Devonian boundary beds in the Kielce area of the Holy Cross Mountains. Acta Geologica Polonica 43: 1–26.
Racki, G. and Narkiewicz, M. 2000. Tectonic versus eustatic controls of sedimentary development of the Devonian in the Holy Cross Moutains, Central Poland. Przegląd Geologiczny 48: 65–76.
Racki, G., Racka, M., Matyja, H., and Devleeschouwer, X. 2002. The Frasnian/Famennian boundary interval in the South Polish-Moravian shelf basins: integrated event-stratigraphical approach. Palaeogeography, Palaeoclimatology, Palaeoecology 181: 251–297.
Rakociński M., Marynowski L., Pisarzowska A., Bełdowski J., Siedlewicz G., Zatoń M., Perri M.C., Spalletta C., and Schönlaub H.P. 2021. Volcanic related methylmercury poisoning as the possible driver of the end-Devonian Mass Extinction. Scientific Reports 10: 7344.
Roberts, E.M., Rogers, R.R., and Foreman, B.Z. 2007. Continental insect borings in dinosaur bone: examples from the Late Cretaceous of Madagascar and Utah. Journal of Paleontology 81: 201–208.
Sato, K. and Jenkins, R.G. 2020. Mobile home for pholadoid boring bivalves: first example from a Late Cretaceous sea turtle in Hokkaido Japan. Palaios 35: 228–236.
Seilacher, A. 2005. Whale barnacles: exaptational access to a forbidden paradise. Paleobiology 31: 27–35.
Szrek, P. 2007. Coelacanths (Actinistia, Sarcopterygii) from the Famennian (Upper Devonian) of Kadzielnia Chain, Holy Cross Mountains, Poland. Acta Geologica Polonica 57: 403–413.
Szrek, P. 2009. Placoderm Fishes from the Devonian of the Holy Cross Mountains. 144 pp. Unpublished Ph.D. Dissertation, University of Warsaw, Warsaw.
Szrek, P. 2020. Comments on distribution and taphonomy of Devonian placoderms in the Holy Cross Mountains, Poland. Bulletin of Geosciences 95: 1–17.
Szulczewski, M. 1971. Upper Devonian conodonts, stratigraphy and facial development in the Holy Cross Mts. Acta Geolologica Polonica 21: 1–129.
Szulczewski, M. 1973. Famennian–Tournaisian neptunian dykes and their conodont fauna from Dalnia in the Holy Cross Mountains. Acta Geologica Polonica 23: 15–59.
Szulczewski, M. 1989. Światowe i regionalne zdarzenia w zapisie stratygraficznympogranicza franu i famenu Gór Świętokrzyskich. Przegląd Geologiczny 37: 551–557.
Szulczewski, M. 1995. Depositional evolution of the Holy Cross Mountains (Poland) in the Devonian and Carboniferous—a review. Geological Quarterly 39: 471–488.
Szulczewski, M., Bełka, Z., and Skompski, S. 1996. The drowning of a carbonate platform: an example from the Devonian–Carboniferous of the southwestern Holy Cross Mountains, Poland. Sedimentary Geology 106: 21–49.
Tapanila, L., Roberts, E.M., Bouare, M.L., Sissoko, F., and O’Leary, M.A. 2004. Bivalve borings in phosphatic coprolites and bone, Cretaceous–Paleogene, north-eastern Mali. Palaios 19: 565–573.
Taylor, P.D. and Wilson, M.A. 2003. Palaeoecology and evolution of marine hard substrate communities. Earth-Science Reviews 62: 1–103.
Thompson, R.C., Johnson, L.E., and Hawkins, S.J. 1997. A method for spatial and temporal assessment of gastropod grazing intensity in the field: the use of radula scrapes on wax surfaces. Journal of Experimental Marine Biology and Ecology 218: 63–76.
Vialov, O.S. and Nessov, L.A. 1974. Post-mortem injuries of shells of some Early Cretaceous turtles by bone-damaging organisms [in Russian, with English summary]. Paleontologičeskij Sbornik 11: 99–103.
Vinn, O. and Toom, U. 2016. Borings in phosphatized Cambrian siltstone pebbles, Estonia (Baltica). Geological Magazine 153: 635–642.
Vinn, O., Wilson, M.A., and Toom, U. 2015. Bioerosion of inorganic hard substrates in the Ordovician of Estonia (Baltica). PLOS ONE 10 (7): e0134279.
Voigt, E. 1977. On grazing traces produced by the radula of fossil and Recent gastropods and chitons. Geological Journal Special Issue 9: 335–346.
Walker, M.V. 1938. Evidence of Triassic insects in the Petrified Forest National Monument, Arizona. Proceedings of the United States National Museum 137–141.
White, E.I. 1978. The larger arthrodiran fishes from the area of the Burrinjuck Dam, NSW. The Transactions of the Zoological Society of London 34: 149–262.
Wilson, M.A. 2007. Macroborings and the evolution of marine bioerosion. In: W. Miller III (ed.), Trace Fossils: Concepts, Problems, Prospects, 356–367. Elsevier, Amsterdam.
Wisshak, M., Knaust, D., and Bertling, M. 2019. Bioerosion ichnotaxa: review and annotated list. Facies 65: art. 24.
Young, G.C. 2010. Placoderms (armored fish): dominant vertebrates of the Devonian period. Annual Review of Earth and Planetary Sciences 38: 523–550.
Zatoń, M., Filipiak, P., Rakociński, M., and Krawczyński, W. 2014. Kowala Lagerstätte: Late Devonian arthropods and non-biomineralized algae from Poland. Lethaia 47: 352–364.
Zonneveld, J.P. and Bartels, W.S. 2022. The occurrence of bone modification features in the carapace and plastron of the extant red-eared slider Trachemys scripta elegans (Wied-Neuwied, 1839): implications for paleoecological analyses of fossil turtle assemblages. Palaios 37: 499–519.
Zonneveld, J.P. and Bartels, W.S. 2023. The occurrence and significance of ulcerative shell disease (USD) on geoemydid turtles, Middle Eocene, Southwestern Wyoming, United States. Palaios 38: 453–473.
Zonneveld, J.P., AbdelGawad, M.K., and Miller, E.R. 2022a. Ectoparasite borings, mesoparasite borings, and scavenging traces in early Miocene turtle and tortoise shell: Moghra Formation, Wadi Moghra, Egypt. Journal of Paleontology 96: 304–322.
Zonneveld, J.P., Bartels, W.S., Gunnell, G.F., and McHugh, L.P. 2016. Borings in early Eocene turtle shell from the Wasatch Formation, South Pass, Wyoming. Journal of Paleontology 89: 802–820.
Zonneveld, JP., Zonneveld, Z.E.E., Bartels, W.S., Gingras, M.K., and Head, J.J. 2022b. Bone modification features resulting from barnacle attachment on the bones of Loggerhead sea turtles (Caretta caretta), Cumberland Island, Georgia, USA: implications for the paleoecological and taphonomic analyses of fossil sea turtles. Palaios 37: 650–670.
Acta Palaeontol. Pol. 70 (4): 629–639, 2025
https://doi.org/10.4202/app.01220.2024