

The palaeoneurology of a new specimen of the Middle Triassic dicynodont synapsid Kombuisia frerensis
JULIEN BENOIT and JAGANMOY JODDER
The pineal eye is a photoreceptive organ, sometimes called the “third eye”, housed within the parietal foramen of some ectothermic vertebrates (Eakin 1973; Quay 1979). It is amongst the most enigmatic organs, and accordingly, concerns relating to its origin and evolution have long fascinated palaeontologists (Eakin 1973; Roth and Roth 1980; Benoit et al. 2016). In dicynodont synapsids, a parietal foramen is almost always present, with a few noticeable exceptions that have, so far, eluded explanations (Benoit et al. 2016; Kammerer 2019). Kombuisia frerensis is one such exception. There are two recognised species of Kombuisia: K. frerensis, from South Africa, and K. antarctica, from Antarctica. The two species are virtually undistinguishable except for the absence of a parietal foramen in the former, whereas the latter retains a slit-like opening on the skull roof (Fröbisch et al. 2010). While describing K. frerensis, Hotton (1974) refrained from including the absence of a parietal foramen in the diagnosis of the species as very little was known about the intraspecific variability of this character. Intraspecific variation of the parietal foramen has since been documented in modern reptiles (e.g., Gundy and Wurst 1976; Roth and Roth 1980) and other synapsids (Benoit et al. 2016). As a result, the absence of a parietal foramen in K. frerensis has been treated with caution by subsequent authors (e.g., Kammerer 2019). In 2023, our research team found a new specimen referrable to K. frerensis: an almost undeformed skull with articulated lower jaw and associated postcrania (Fig. 1A). This new specimen confirms that the absence of a parietal foramen is diagnostic for K. frerensis. We here explore the physiological implications of this condition and propose that it may be the result of latitudinal gradient separating the two species of Kombuisia.
Material and methods
Specimen BP/1/9600 (field number 39-09-2023) was found during an Evolutionary Studies Institute (ESI) fieldtrip in September 2023 by JJ at GPS coordinates S30°46.4868’; E26°17.8113’ on farm Luiperdkop (or Luiperdskop), in the Eastern Cape Province, Walter Sisulu local municipality (South Africa). Outcrops at Luiperdkop expose the Trirachodon–Kannemeyeria Subzone of the Cynognathus Assemblage Zone (Burgersdorp Formation), which is consistent with the age of the type locality of Kombuisia frerensis (Fröbisch 2007; Botha-Brink and Smith 2011; Hancox et al. 2020). BP/1/9600 was preserved in an ex-situ calcareous, slightly haematitic nodule (Fig. 1A).

Fig. 1. Dorsal view of BP/1/9600, dicynodont synapsid Kombuisia frerensis Hotton, 1974, from Luiperdkop (Eastern Cape, South Africa), Burgersdorp Formation, Anisian (Middle Triassic). Picture taken at the moment of its discovery by JJ (A1); close up of the sagittal crest area (after preparation) (A2).
The fossil was prepared by Charlton Dube (Evolutionary Studies Institute, Johannesburg, South Africa) using tungsten-carbide tipped air-scribes. It was CT-scanned at the ESI scanning facility of the University of the Witwatersrand, in Johannesburg, using a Nikon Metrology XTH 225/320 LC dual source CT system, with a voxel size of 0.0458 mm. The endocast was reconstructed using manual segmentation in Avizo 2021 (Thermo Fisher Scientific, Hillsborough, USA). Attempts at scanning BP/1/430, the holotype skull of K. frerensis, failed to provide enough contrast between the bone and matrix.
The body mass of BP/1/9600 was estimated using skull length following the method provided by Benoit et al. (2017). The synapsid encephalization quotient (SEQ) was calculated following Benoit et al. (2023). A complete description of the cranial and postcranial anatomy of BP/1/9600 is currently in preparation (Christian Kammerer, personal communication 2025). In this contribution, we focus primarily on the endocranial anatomy aspect.
Institutional abbreviations.—BP, Evolutionary Studies Institute (formerly, Bernard Price), Johannesburg, South Africa.
Other abbreviations.—SEQ, synapsid encephalization quotient.
Systematic palaeontology
Synapsida Osborn, 1903
Dicynodontia Owen, 1859
Genus Kombuisia Hotton, 1974
Type species: Kombuisia frerensis Hotton, 1974; Lady Frere, Eastern Cape Province, South Africa, Anisian.
Kombuisia frerensis Hotton, 1974
Figs. 1, 2.
Material.—BP/1/9600 (field number 39-09-2023), skull from the Anisian, Middle Triassic, Burgersdorp Formation, farm Luiperdkop (or Luiperdskop), Eastern Cape Province, Walter Sisulu local municipality, South Africa.
Description.—The skull is 75 mm long. Its identification as Kombuisia frerensis is supported by its small size, relatively short and rounded snout in dorsal view, the absence of tusks, inverted triangular shape of the interparietal, absence of a parietal foramen, and the two postorbitals that almost meet at the midline dorsal to the orbits (Fig. 1B). The small size and absence of a parietal foramen preclude its identification as a kannemeyeriiform. The occlusion of the mandibular fenestra and presence of a large lateral dentary shelf also supports its identification as a species of Kombuisia. The cranial sutures, though still visible, are mostly closed, so it is not a juvenile of a larger taxon. The two postorbitals joining at the midline is a unique diagnostic trait of K. frerensis, whereas these two bones are relatively more separated in the Antarctic species, Kombuisa antarctica Frobïsch et al., 2010 (Fröbisch et al. 2010). BP/1/9600 is thus safely referrable to K. frerensis regardless of the parietal foramen condition.
The endocast of the brain cavity shows no sign of a pineal tube nor body (Fig. 2C). The overall outline of the endocast aligns with the generalised Permian dicynodont condition, as it is mostly linearly arranged (Hopson et al. 1979; Laaß 2015b; George et al. 2024). In contrast, Triassic species belonging to Lystrosaurus and the kannemeyeriiforms have strongly flexed, anteroposteriorly compressed endocasts (Lehman 1961; Cluver 1971; Hopson et al. 1979). Anteriorly, the olfactory bulbs appear narrower than the cerebral hemispheres. The volume of the olfactory bulbs (defined as the slight impression at the base of the olfactory tracts) is 0.161 cm3, which is similar to those of Rastodon (0.102 cm3, Simão-Oliveira et al. 2020), but a lot smaller than in Cistecephalus (1.31 cm3, Macungo et al. 2023; although it exceeds 50% of that of the total endocast, so it must be overestimated). The cerebral hemispheres are dorsoventrally flat and slightly expanded mediolaterally. This condition is also present in Rastodon (Simão-Oliveira et al. 2020). Mediolaterally expanded cerebral hemispheres are also observed in Cistecephalus, Kembawacela, and Kawingasaurus; however, these cistecephalids differ from Kombuisia in having more globular, rather than tubular, cerebral hemispheres (Laaß and Kaestner 2017; Araujo et al. 2022b; Macungo et al. 2023). Unlike Kombuisia, Niassodon has very narrow hemispheres (Castanhinha et al. 2013). The dorsal surface of the endocast is smooth as no sagittal sulcus is visible on the olfactory bulbs and hemispheres (Fig. 2C1). In contrast, species of Pristerodon, Cistecephalus, and Kembawacela have a well-developed sulcus that separates the olfactory bulbs medially (Laaß 2015b). A sagittal sulcus splits the cerebral hemispheres in Kawingasaurus (Laaß and Kaestner 2017). Posteriorly, the unossified zone is small dorsally, and the floccular lobes are so reduced they are almost invisible (Fig. 2C). This is similar to the condition in Rastodon, Kembawacela, and Gordonia, whereas most other dicynodonts have rather prominent floccular lobes (Benoit et al. 2017; Araujo et al. 2022; George et al. 2024). The optic chiasma and hypophyseal fossa are salient ventrally (Fig. 2C2). The complete volume of the endocast (including the olfactory bulbs) is 2.65 cm3 which, given a body mass of 1317 g, gives a SEQ of 1.43. This is about twice as much as in Rastodon (0.72), Niassodon (0.81), and Lystrosaurus (0.74), but still within the range of dicynodonts, as it is below that of Kawingasaurus (2.00) (Benoit et al. 2023). The SEQ value of Kombuisia is most similar to that of Pristerodon (1.09) (Benoit et al. 2023).
Stratigraphic and geographic range.—Anisian, Burgersdorp Formation, South African Main Karoo Basin.
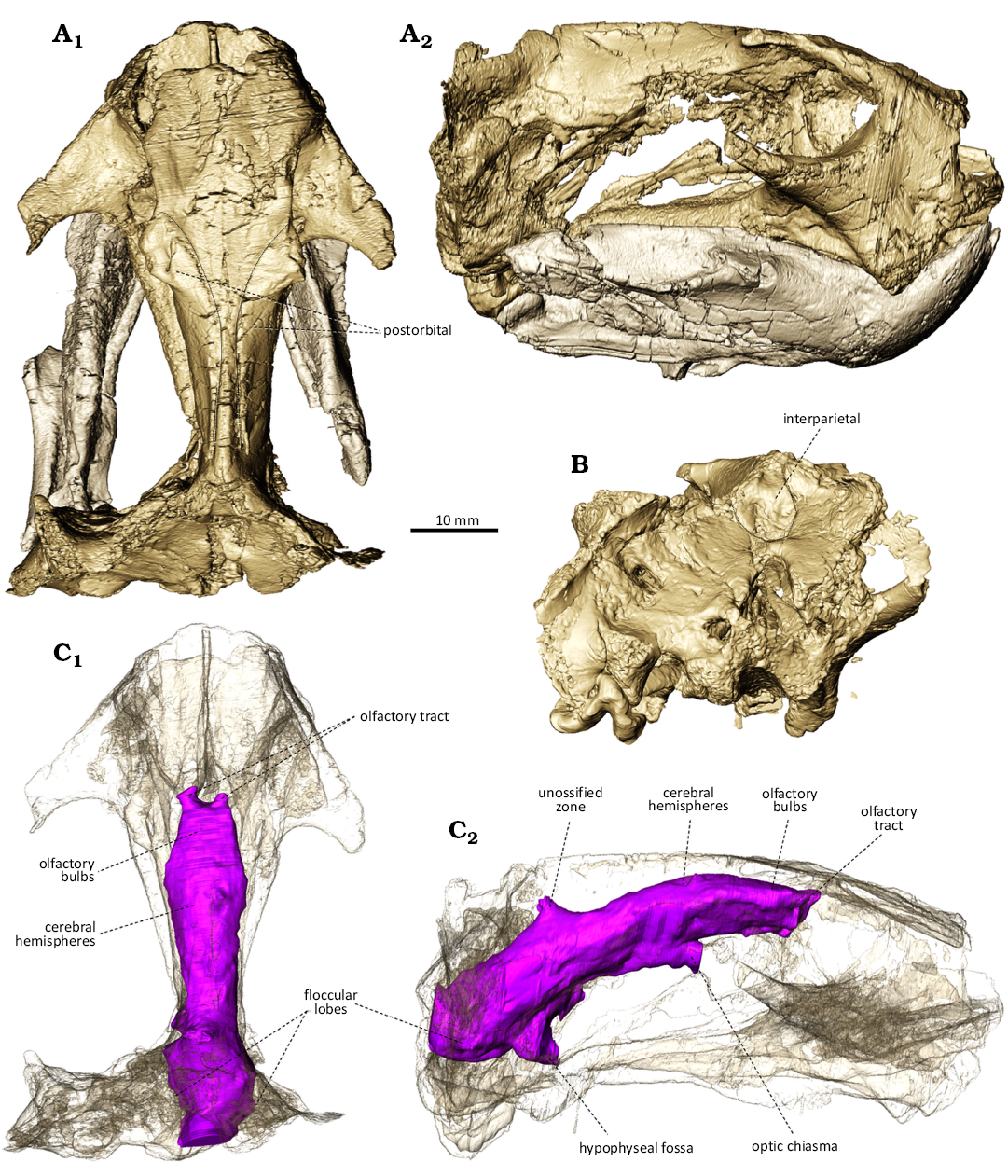
Fig. 2. Digitally prepared skull and mandible of BP/1/9600, dicynodont synapsid Kombuisia frerensis Hotton, 1974, from Luiperdkop (Eastern Cape, South Africa), Burgersdorp Formation, Anisian (Middle Triassic). A. Skull with lower jaw in dorsal (A1) and right lateral (A2) views. B. Occipital view of the skull (lower jaw removed). C. Endocast (skull made transparent), in dorsal (C1) and right lateral (C2) views.
Discussion
The new specimen of Kombuisia frerensis supports that the absence of a parietal foramen is a genuine diagnostic character of the South African species of this genus. The complete absence of a cavity for the pineal body and tube in K. frerensis (Fig. 2C) suggests that (i) the foramen had regressed beyond possible intraspecific variability (ontogenetic or pathologic, see Benoit et al. 2015, 2016; Medina et al. 2025) and (ii) the organ had lost its photoreceptive function (Davenport et al. 2014).
Kombuisia antarctica is likely from the Induan, whereas K. frerensis is younger, from the early Anisian, which gave the latter two to three million years to lose the parietal foramen and pineal eye (Fröbisch 2007; Sidor et al. 2023). The reasons may be related to the multiple biological functions of the pineal eye. The pineal eye is a photoreceptor that can only detect light levels (light and darkness), and based on the duration of daylight, it regulates hormone secretion by the pineal gland (Eakin 1973; Tosini 1997). These hormones are involved in synchronising life cycles such as sleep and reproduction, but also other functions such as orientation and behavioural thermoregulation (Quay 1979; Roth and Roth 1980; Foà et al. 2009).
Life in a low light environment may diminish the function of the pineal eye, potentially leading to the loss of the photoreceptive organ and its corresponding foramen (Policarpo et al. 2021; Benoit et al. 2023). Adaptation to fossoriality, a common trait amongst dicynodonts, would render the photoreceptive function of the pineal eye redundant, making the structure expensive to maintain given its low selective value (Benoit et al. 2016). For example, Kawingasaurus fossilis was uniquely adapted to a low-light environment (i.e., obligate fossoriality), which accounts well for the loss of its parietal foramen (Benoit et al. 2023). The reduced size of the floccular fossa in K. frerensis is a character found in fossorial rodents, such as mountain beavers (Bertrand et al. 2021), so it is possible that K. frerensis was fossorial, but there is no evidence to suggest that K. antarctica would have been any different in this respect. The skulls of both species equally lack the adaptations to fossoriality encountered in cistecephalids (Laaß 2015a). Moreover, modern fossorial reptiles do not tend to lose their pineal eye compared to their non fossorial counterparts (Gundy and Wurst 1976).
Adaptation to nocturnality may have the same effect (Emerling 2017). There is no sclerotic ring preserved in any of the known Kombuisia specimens belonging to both species, to test this hypothesis, but given that no dicynodont is reconstructed as nocturnal, including Kombuisia’s close relative Emydops (Angielczyk and Schmitz 2014), it does not appear likely.
It has long been proposed that endothermy could explain the loss of the pineal eye and parietal foramen in synapsids (Roth and Roth 1980; Benoit et al. 2016). Some recent isotopic analyses and osteohistological studies even supported that Early Triassic dicynodonts may have evolved endothermy, although they did not include Kombuisia (Olivier et al. 2017; Rey et al. 2017; Faure-Brac and Cubo 2020; Faure-Brac et al. 2024). Under this scenario, the absence of a parietal foramen in the South African species would imply that K. frerensis had a more elevated metabolism than its sister taxon K. antarctica, which is unlikely. Future works on the inner ear of the new specimen could address this possibility (Araujo et al. 2022a).
The Transantarctic Basin was situated at a higher latitude (about 75°S) than the Main Karoo Basin (about 60°S) during the Middle Triassic (Zharkov and Chumakov 2001; Romano et al. 2020). This latitudinal gradient accounts better for the differing parietal foramen conditions in both species of Kombuisia. Modern ectothermic species living at lower latitude rely less on behavioural thermoregulation as temperatures are warmer on average. In addition, the duration of nights and days is less seasonally contrasted in low latitudes, making reliance upon these cues by the pineal eye less crucial for monitoring life cycles (Gundy et al. 1975; Ralph 1975). As a result, the parietal foramen is more often absent in families of squamates that live closer to the equator than in their close relatives from higher latitudes (Gundy et al. 1975; Ralph 1975). Similarly, rodents from higher latitudes have a larger pineal gland (Quay 1980). Given that the Transantarctic and Main Karoo basins were under two different climatic zones (temperate and arid, respectively) and separated by some 10–15° latitudinal difference in the Early Triassic (Zharkov and Chumakov 2001; Romano et al. 2020), it has been shown that seasonality had more adverse effects on the dicynodonts of Antarctica than those from the Karoo (Whitney and Sidor 2020). It is possible that the evolutionary pressure to maintain a parietal foramen and pineal eye differed significantly between K. frerensis and K. antarctica. Compared to its South African relative, K. antarctica would have been exposed to more pronounced seasonal differences in sunlight and colder winter days, which are both crucial inputs to the pineal gland for timing the reproductive cycle and monitoring behavioural thermoregulatory decisions. Kingoriids already having a tendency towards reducing or losing their parietal foramen (Kammerer 2019), the pressure for K. frerensis to maintain a pineal eye under the lower South African latitudes may not have been sufficient.
The degree to which this latitudinal explanation can be generalised to other dicynodonts is uncertain given that the presence of a pineal foramen is ubiquitous in most other Triassic taxa regardless of latitude. In Lystrosaurus and Prolacerta, the presence of a parietal foramen is more variable in southern Africa than in Antarctica (Colbert 1987; Miller-Camp 2010; Benoit et al. 2016; Spiekman 2018). This variability could also be a consequence of the latitudinal gradient. Consistently with the above, in the late Permian, Kawingasaurus is the cistecephalid with the most northern palaeolatitude and the only one to lose its parietal foramen completely (Benoit et al. 2016).
More specimens of Kombuisia and other Triassic taxa represented by high and low latitude specimens will be necessary to address this hypothesis. As this evolutionary trend is observed in modern ectothermic species only, its systematic study in Triassic taxa may shed some light on the ongoing debates about the origins of endothermy in synapsids.
Acknowledgements.—To the ESI students and staff, and other researchers not mentioned in this work who contributed fieldwork. To Johann de Buin (Luiperdkop, South Africa), landowner of the farm Luiperdkop for authorising access to his property. To Brandon Peecoock (Idaho State University, Pocatello, USA) and Kenneth Angielczyk (Field Museum, Chicago, USA) for reviewing this manuscript. To ECPHRA for issuing excavation permit n° 35/4/23/05/002. To the THRINAKS2023 contributors, DSI-NRF African Origins Platform (AOP240418214774), DSI-NRF GENUS and PAST for financial support.
Editor: Eli Amson.
References
Angielczyk, K.D. and Schmitz, L. 2014. Nocturnality in synapsids predates the origin of mammals by over 100 million years. Proceedings of the Royal Society B: Biological Sciences 281 (1793): 20141642. Crossref
Araujo, R., David, R., Benoit, J., Lungmus, J.K., Stoessel, A., Barrett, P.M., Maisano, J.A., Ekdale, E., Orliac, M., Luo, Z.-X., Martinelli, A.G., Hoffman, E.A., Sidor, C.A., Martins, R.M.S., Spoor, F., and Angielczyk, K.D. 2022a. Inner ear biomechanics reveals a Late Triassic origin for mammalian endothermy. Nature 607: 726–731. Crossref
Araujo, R., Macungo, Z., Fernandez, V., Chindebvu, E.G., and Jacobs, L.L. 2022b. Kembawacela yajuwayeyi n. sp., a new cistecephalid species (Dicynodontia: Emydopoidea) from the Upper Permian of Malawi. Journal of African Earth Sciences 196: 104726. Crossref
Benoit, J., Abdala, F., Manger, P., and Rubidge, B. 2016. The sixth sense in mammalians forerunners: variability of the parietal foramen and the evolution of the pineal eye in South African Permo-Triassic eutheriodont therapsids. Acta Palaeontologica Polonica 61: 777–789. Crossref
Benoit, J., Fernandez, V., Manger, P.R., and Rubidge, B.S. 2017. Endocranial casts of pre-mammalian therapsids reveal an unexpected neurological diversity at the deep evolutionary root of mammals. Brain Behavior and Evolution 90: 311–333. Crossref
Benoit, J., Dollman, K.N., Smith, R.M.H., and Manger, P.R. 2023. At the root of the mammalian mind: The sensory organs, brain and behavior of pre-mammalian synapsids. Progress in Brain Research 275: 25–72. Crossref
Benoit, J., Abdala, F., Van den Brandt, M.J., Manger, P.R., and Rubidge, B.S. 2015. Physiological implications of the abnormal absence of the parietal foramen in a late Permian cynodont (Therapsida). The Science of Nature 102 (11): 69. Crossref
Bertrand, O.C., Püschel, H.P., Schwab, J.A., Silcox, M.T., and Brusatte, S.L. 2021. The impact of locomotion on the brain evolution of squirrels and close relatives. Communications Biology 4 (1): 1–15. Crossref
Botha-Brink, J. and Smith, R.M.H. 2011. Osteohistology of the Triassic archosauromorphs Prolacerta, Proterosuchus, Euparkeria, and Erythrosuchus from the Karoo Basin of South Africa. Journal of Vertebrate Paleontology 31: 1238–1254.
Castanhinha, R., Araújo, R., Júnior, L.C., Angielczyk, K.D., Martins, G.G., Martins, R.M.S., Chaouiya, C., Beckmann, F., and Wilde, F. 2013. Bringing dicynodonts back to life: Paleobiology and anatomy of a new emydopoid genus from the upper Permian of Mozambique. PLoS ONE 8 (12): e80974. Crossref
Cluver, M.A. 1971. The cranial morphology of the dicynodont genus Lystrosaurus. Annals of The South African Museum 56 (5): 155–274.
Colbert, E.H. 1987. The Triassic reptile Prolacerta in Antarctica. American Museum Novitates 2882: 1–19.
Davenport, J., Jones, T.T., Work, T.M., and Balazs, G.H. 2014. Pink spot, white spot: The pineal skylight of the leatherback turtle (Dermochelys coriacea Vandelli 1761) skull and its possible role in the phenology of feeding migrations. Journal of Experimental Marine Biology and Ecology 461: 1–6. Crossref
Eakin, R.M. 1973. The Third Eye. 168 pp. University of California Press, Berkeley. Crossref
Emerling, C.A. 2017. Archelosaurian Color Vision, Parietal Eye Loss, and the Crocodylian Nocturnal Bottleneck. Molecular Biology and Evolution 34 (3): 666–676. Crossref
Faure-Brac, M.G. and Cubo, J. 2020. Were the synapsids primitively endotherms? A palaeohistological approach using phylogenetic eigenvector maps. Philosophical Transactions of the Royal Society B: Biological Sciences 375 (1793): 20190138. Crossref
Faure-Brac, M.G., Woodward, H.N., Aubier, P., and Cubo, J. 2024. On the origins of endothermy in amniotes. iScience 27 (4): 109375. Crossref
Foà, A., Basaglia, F., Beltrami, G., Carnacina, M., Moretto, E., and Bertolucci, C. 2009. Orientation of lizards in a Morris water-maze: roles of the sun compass and the parietal eye. Journal of Experimental Biology 212: 2918–2924. Crossref
Fröbisch, J. 2007. The cranial anatomy of Kombuisia frerensis Hotton (Synapsida, Dicynodontia) and a new phylogeny of anomodont therapsids. Zoological Journal of the Linnean Society 150: 117–144. Crossref
Fröbisch, J., Angielczyk, K.D., and Sidor, C.A. 2010. The Triassic dicynodont Kombuisia (Synapsida, Anomodontia) from Antarctica, a refuge from the terrestrial Permian–Triassic mass extinction. Naturwissenschaften 97 (2): 187–196. Crossref
George, H., Kammerer, C.F., Foffa, D., Clark, N.D.L., and Brusatte, S.L. 2024. Micro-CT data reveal new information on the craniomandibular and neuroanatomy of the dicynodont Gordonia (Therapsida: Anomodontia) from the late Permian of Scotland. Zoological Journal of the Linnean Society: zlae065. Crossref
Gundy, G.C. and Wurst, G.Z. 1976. Parietal eye-pineal morphology in lizards and its physiological implications. The Anatomical Record 185 (4): 419–431. Crossref
Gundy, G.C., Ralph, C.L., and Wurst, G.Z. 1975. Parietal eyes in lizards: zoogeographical correlates. Science 190: 671–673. Crossref
Hancox, P.J., Neveling, J., and Rubidge, B.S. 2020. Biostratigraphy of the Cynognathus Assemblage Zone (Beaufort Group, Karoo Supergroup), South Africa. South African Journal of Geology 123: 217–238. Crossref
Hopson, J.A., Gans, C., Northcutt, R.G., and Ulinski, P. 1979. Paleoneurology. In: C. Gans (ed.), Biology of the Reptilia, 39–146. Academic Press, New York.
Hotton, N.I. 1974. A new dicynodont (Reptilia, Therapsida) from Cynognathus Zone deposits of South Africa. Annals of the South African Museum 64: 157–165.
Kammerer, C.F. 2019. A new dicynodont (Anomodontia: Emydopoidea) from the terminal Permian of KwaZulu-Natal, South Africa. Palaeontologia Africana 53: 179–191.
Laaß, M. 2015a. Bone-conduction hearing and seismic sensitivity of the late Permian anomodont Kawingasaurus fossilis. Journal of Morphology 276: 121–143. Crossref
Laaß, M. 2015b. Virtual reconstruction and description of the cranial endocast of Pristerodon mackayi (Therapsida, Anomodontia). Journal of Morphology 276: 1089–1099. Crossref
Laaß, M. and Kaestner, A. 2017. Evidence for convergent evolution of a neocortex-like structure in a late Permian therapsid. Journal of Morphology 278: 1033–1057. Crossref
Lehman, J.P. 1961. Dicynodontia. In: J.P. Piveteau (ed.), Traité de Paléontologie, VI, Mammifères. Origine Reptilienne Evolution, Vol. 1, 287–351. Masson et Cie, Paris, France.
Macungo, Z., Araujo, R., Browning, C., Smith, R.M.H., David, R., Angielczyk, K.D., Massingue, A., Ferreira-Cardoso, S., and Kortje, D.J.P. 2023. Novel anatomy and paleobiological insights on Cistecephalus microrhinus (Synapsida: Dicynodontia). In: Y.N. Lee (ed.), Windows into Sauropsid and Synapsid Evolution, Essays in Honor of Louis Jacobs, 1–65. Dinosaur Science Center Press, Hwaseong City.
Medina, T.G.M., Martinelli, A.G., Gaetano, L.C., Roese-Miron, L., Tartaglione, A., Backs, A., Novas, F.E., and Kerber, L. 2025. Revisiting the neuroanatomy of Massetognathus pascuali (Eucynodontia: Cynognathia) from the early Late Triassic of South America using neutron tomography. The Science of Nature 112 (1): 7. Crossref
Miller-Camp, J. 2010. Morphological Variation and Disparity in Lystrosaurus (Therapsida: Dicynodontia). 141 pp. Masters Thesis, The University of Iowa, Iowa City.
Olivier, C., Houssaye, A., Jalil, N.-E., and Cubo, J. 2017. First palaeohistological inference of resting metabolic rate in an extinct synapsid, Moghreberia nmachouensis (Therapsida: Anomodontia). Biological Journal of the Linnean Society 121: 409–419. Crossref
Osborn, H.F. 1903. On the primary division of the Reptilia into two sub-classes, Synapsida and Diapsida. Science 17: 275–276. Crossref
Owen, R. 1859. On the orders of fossil and recent Reptilia and their distribution in time. Report of the British Association for the Advancement of Science 1859: 153–166.
Policarpo, M., Fumey, J., Lafargeas, P., Naquin, D., Thermes, C., Naville, M., Dechaud, C., Volff, J.-N., Cabau, C., Klopp, C., Møller, P.R., Bernatchez, L., García-Machado, E., Rétaux, S., and Casane, D. 2021. Contrasting gene decay in subterranean vertebrates: insights from cavefishes and fossorial mammals. Molecular Biology and Evolution 38 (2): 589–605. Crossref
Quay, W.B. 1979. The parietal eye-pineal complex. In: C. Gans, R.G. Northcutt, and P. Ulinski (eds.), Biology of the Reptilia, 245–406. Academic Press, New York.
Quay, W.B. 1980. Greater pineal volume at higher latitudes in Rodentia: Exponential relationship and its biological interpretation, General and Comparative Endocrinology 41: 340–348. Crossref
Ralph, C.L. 1975. The pineal gland and geographical distribution of animals. International Journal of Biometeorology 19: 289–303. Crossref
Rey, K., Amiot, R., Fourel, F., Abdala, F., Fluteau, F., Jalil, N.-E., Liu, J., Rubidge, B.S., Smith, R.M., Steyer, J.S., Viglietti, P.A., Wang, X., and Lécuyer, C. 2017. Oxygen isotopes suggest elevated thermometabolism within multiple Permo-Triassic therapsid clades. eLife 6: e28589. Crossref
Romano, M., Bernardi, M., Petti, F.M., Rubidge, B., Hancox, J., and Benton, M.J. 2020. Early Triassic terrestrial tetrapod fauna: a review. Earth-Science Reviews 210: 103331. Crossref
Roth, J.J. and Roth, E.C. 1980. The parietal-pineal complex among paleovertebrates, evidence for temperature regulation. In: R.D.K. Thomas and D.E.C. Olson (eds.), A Cold Look at the Warm-Blooded Dinosaurs, 189–231. Westview Press, Boulder.
Sidor, C.A., McIntosh, J.A., Gee, B.M., Hammer, W.R., Makovicky, P.J., Smith, N.D., Smith, R.M.H., Tabor, N.J., Whitney, M.R., and Woolley, C.H. 2023. The Fremouw Formation of Antarctica: Updated vertebrate fossil record and reevaluation of high-latitude Permian–Triassic paleoenvironments. Earth-Science Reviews 246: 104587. Crossref
de Simão-Oliveira, D., Kerber, L., and L. Pinheiro, F. 2020. Endocranial morphology of the Brazilian Permian dicynodont Rastodon procurvidens (Therapsida: Anomodontia). Journal of Anatomy 236 (3): 384–397. Crossref
Spiekman, S.N.F. 2018. A new specimen of Prolacerta broomi from the lower Fremouw Formation (Early Triassic) of Antarctica, its biogeographical implications and a taxonomic revision. Scientific Reports 8 (1): 17996. Crossref
Tosini, G. 1997. The pineal complex of reptiles: physiological and behavioral roles. Ethology Ecology & Evolution 9: 313–333. Crossref
Whitney, M.R. and Sidor, C.A. 2020. Evidence of torpor in the tusks of Lystrosaurus from the Early Triassic of Antarctica. Communications Biology 3 (1): 1–6. Crossref
Zharkov, M.A. and Chumakov, N.M. 2001. Paleogeography and sedimentation settings during Permian–Triassic reorganizations in biosphere. Stratigraphy and Geological Correlation 9 (4): 340–363.
Julien Benoit [julien.benoit@wits.ac.za; ORCID: https://orcid.org/0000-0001-5378-3940 ], Evolutionary Studies Institute and School of Geosciences, University of the Witwatersrand, Private Bag 3, WITS 2050, Johannesburg, South Africa.
Jaganmoy Jodder [jaganmoyj@gmail.com; ORCID: https://orcid.org/0000-0003-3993-003X ], Evolutionary Studies Institute and School of Geosciences, University of the Witwatersrand, Private Bag 3, WITS 2050, Johannesburg, South Africa. Centre for Planetary Habitability (PHAB), University of Oslo, Oslo, Norway.
Received 3 February 2025, accepted 22 May 2025, published online 30 June 2025.
Copyright © 2025 J. Benoit and J. Jodder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Acta Palaeontol. Pol. 70 (2): 369–374, 2025
https://doi.org/10.4202/app.01247.2025